User login
Newer oral and noninsulin therapies to treat type 2 diabetes mellitus
Type 2 diabetes mellitus (DM) is caused by hyperglycemia and metabolic alterations due to abnormalities in insulin secretion or insulin action, or both. To achieve desired glycemic targets, different antihyperglycemic drugs are used alone or in combination with other agents, including insulin. First-line options for diabetes treatment are weight loss, lifestyle modification, and metformin. The American Diabetes Association and the European Association for the Study of Diabetes recommend a patient-specific treatment approach to enhance glycemic control while avoiding weight gain and hypoglycemia.1 This review will focus on the newer oral agents and injectable noninsulin agents that are used to achieve glycemic control. Table 1 lists the noninsulin drugs approved since 2005.
INCRETIN-BASED THERAPIES
The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are secreted by the gastrointestinal (GI) tract in response to food intake. Both GLP-1 and GIP stimulate beta cells of the pancreas, which contribute 60% of the insulin secretion after a meal. Type 2 DM is associated with decreased secretion of GLP-1 and lowered responsiveness to GIP. Benefits of the incretin hormones on glycemic control include enhanced satiety, decreased GI motility, increased glucose-dependent insulin secretion, reduced glucagon secretion, and decreased hepatic glucose release.2 Two incretin-based drug classes are used to treat patients with type 2 DM—oral dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists.
DPP-4 inhibitors
The oral DPP-4 inhibitors block the degradation of the enzyme DPP-4 active site and thus increase the GLP-1 and GIP concentrations by two to three times. Their primary effectiveness centers on controlling insulin and glucagon secretion without increasing weight.
Four DPP-4 inhibitors are approved by the US Food and Drug Administration (FDA) in once-daily oral formulations: sitagliptin, saxagliptin, linagliptin, and alogliptin. Another DPP-4 inhibitor, vildagliptin, is not licensed in the United States but is approved for use in Europe and Japan.3 Another DPP-4 inhibitor, teneligliptin, is also marketed in Japan.
The DPP-4 inhibitors are indicated for use as monotherapy or in combination with other agents such as metformin, sulfonylureas, thiazolidinediones, and insulin. Generally, DPP-4 inhibitors do not cause hypoglycemia when used as monotherapy.1 When adding a DPP-4 inhibitor to a sulfonylurea or insulin, it is recommended to decrease the sulfonylurea or insulin dose to reduce the risk of hypoglycemia. The potential of these agents to lower the hemoglobin A1c (HbA1c) when used as monotherapy and in combination with metformin, sulfonylureas, or thiazolidinediones is 0.3% to 0.71%.4
The DPP-4 inhibitors are not known to cause adverse GI effects. Sitagliptin, alogliptin, vildagliptin, and saxagliptin need dosing adjustments for renal insufficiency; however, linagliptin is not renally eliminated and does not require dosing adjustment. Common adverse events (> 5%) are nasopharyngitis, upper-respiratory infection, and headache. Sitagliptin and saxagliptin have been associated with urinary tract infection. Sitagliptin also has been associated with more extremity pain, back pain, and osteoarthritis.4
The DPP-4 inhibitors are primarily excreted by the renal or fecal route and, therefore, have few drug interactions. All DPP-4 inhibitors are partially metabolized through cytochrome P450 enzymes, except saxagliptin.4 Their pharmacokinetic profiles are shown on Table 2.
In keeping with the FDA guidelines, sitagliptin, saxagliptin, linagliptin, and alogliptin have been evaluated for cardiovascular (CV) outcomes. The SAVOR-TIMI 53 clinical trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) was a 2-year CV safety and efficacy trial.5 This trial demonstrated no statistically significant difference in the primary end point as a composite of CV death, myocardial infarction (MI), and ischemic stroke. Additionally, the secondary end points, including hospitalization for unstable angina, coronary revascularization, and heart failure, also did not show significant difference. However, based on a subgroup analysis, there was a statistically significant increase in patients in the saxagliptin group vs the placebo group who were hospitalized for heart failure.
A 2-year trial comparing linagliptin with glimepiride, a second-generation sulfonylurea, showed significantly fewer CV events with linagliptin.6 This trial found a relative risk reduction of 54% in the end points of CV death, nonfatal MI or stroke, and unstable angina during hospitalization.4,6 Another trial reviewed the incidence of CV events (CV death, nonfatal MI, and nonfatal stroke) in patients treated with alogliptin, placebo, or comparator antihyperglycemic drugs and found no increased incidence of major adverse CV events vs comparator therapies.7
The recently published TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin), reported no increase in major atherosclerotic CV events, no difference in all-cause mortality, and no difference in heart failure for hospitalization or other adverse events in patients with type 2 DM.8 Other clinical trials such as the CAROLINA study (linagliptin compared with glimepiride),9 the EXAMINE study (alogliptin),10 and the CARMALINA study (linagliptin)11 are also reviewing the CV safety of DPP-4 inhibitors in the United States.
Concern has been raised about the association between incretin-based therapies and adverse pancreatic effects. CV outcomes trials using saxagliptin and alogliptin found similar rates of pancreatitis and fewer pancreatic cancer cases in comparison with placebo.5,7,10 TECOS demonstrated that with sitagliptin, acute pancreatitis occurred more in the sitagliptin group, but there was no statistical significance reported. However, pancreatic cancer occurred more in the placebo group, although the difference was not statistically significant.8 Neither the FDA nor the European Medicines Agency (EMA) has reached a firm conclusion about the possible association between incretin-based therapies and pancreatitis or pancreatic cancer.12
GLP-1 agonists
The GLP-1 drugs mimic the action of native GLP-1. Several GLP-1 agents are available in the United States, and several more are in development.11,13 The drug class is divided into three groups:
- Short-acting (4–6 hours): exenatide, lixisenatide
- Intermediate-acting (24 hours): liraglutide
- Long-acting (7 days): exenatide extended-release (ER), dulaglutide, and albiglutide (semaglutide is in phase 3 study).
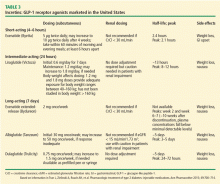
The GLP-1 receptor agonists heighten glucose homeostasis by the following mechanisms of action: stimulate insulin secretion, suppress glucagon secretion, directly and indirectly inhibit endogenous glucose production, promote satiety, heighten insulin sensitivity due to weight loss, and slow gastric emptying time. Table 3 lists dosing and pharmacokinetic profiles for GLP-1 agonists. When GLP-1 agonists are used as monotherapy, the HbA1c is reduced by 0.7% to 1.51%.13 When GLP-1 agonists are used in combination with metformin, sulfonylureas, thiazolidinediones, or as three-drug therapy with other oral antidiabetic medications, the HbA1c is lowered by 0.4% to 1.9%.13–17
A notable advantage of GLP-1 agonists is their effect on weight loss separate from GI side effects. Weight reductions of 0.2 to 3.6 kg in 26 weeks have been seen with the exenatide formulations, liraglutide, albiglutide, and dulaglutide.13,18 Liraglutide has demonstrated a greater weight reduction than exenatide, exenatide ER, or albiglutide.13,16,17 There were similar weight reductions of 1.5 kg in 26 weeks in a comparator trial involving liraglutide and dulaglutide (3.6 vs 2.9 kg).19
Common adverse effects of the GLP-1 agonists are nausea (8% to 44%), diarrhea (6% to 20%), and vomiting (4% to 18%), which may occur initially and diminish with continued use.13,14 There have been more GI side effects with liraglutide than with exenatide ER or albiglutide.20 Increased rates of injection site reactions, such as transient small nodule formations, were seen with exenatide ER (5.4% to 17.6%) and albiglutide, the once-weekly GLP-1 agonist therapies, vs exenatide, liraglutide, and insulin glargine.13,14 Dulaglutide, another once-weekly GLP-1 agonist, does not have this finding; however, there is enhanced patient satisfaction with the once-weekly preparations in comparison with the twice-daily preparations.14,16 Patients who received albiglutide have noted hypersensitivity reactions such as pruritus, rash, and dyspnea (10% to 18%).13 Hypoglycemia is not seen with the GLP-1 agonists, unless they are used in conjunction with a sulfonylurea or insulin.
Exenatide and exenatide ER are excreted by the renal route; therefore, it is not recommended to use these agonists in patients with renal impairment or end-stage renal disease (creatinine clearance [CrCl] < 30 mL/min). Liraglutide is not excreted by the renal route; however, it should be used with caution in patients with renal impairment.21 No renal dose adjustment is required when using albiglutide or dulaglutide.21
Clinical trials have demonstrated the short-term CV outcomes of GLP-1 agonists. The CV benefits include a decrease in blood pressure, reduction of lipid levels, enhanced endothelial function, and improved myocardial function.13 One meta-analysis reported a tendency for lowering the rate of major CV events, stroke, MI, CV mortality, and all-cause mortality.22 Several ongoing trials are evaluating the safety of GLP-1 agonists and CV safety: LEADER (liraglutide), EXSCEL (exenatide LR), ELIXA (lixisenatide), SUSTAIN 6 (semaglutide), and REWIND (dulaglutide).11
The GLP-1 agonists have been linked to an increased incidence of thyroid cancer. There was a potential increased risk of thyroid cancer in preclinical rodent studies involving liraglutide and exenatide ER, but this risk was not demonstrated for the exenatide twice-daily preparation.13 The FDA noted that the findings from rodent studies, which demonstrated a possible heightened risk for thyroid cancer, should not be conveyed to the outcomes for humans. Nevertheless, when liraglutide was approved in January 2010, the FDA issued a boxed warning about the risk of thyroid C-cell hyperplasia. The package inserts list a thyroid carcinoma risk for exenatide ER, liraglutide, albiglutide, and dulaglutide in those patients with a personal or family history of medullary thyroid cancer.
There is controversy about the incidence of pancreatitis and pancreatic cancer with the use of the incretin-based therapies. Published studies and case reports seem to support speculation that there is an increased incidence of acute pancreatitis associated with type 2 DM.21 The FDA and the EMA have independently reviewed postmarketing reports about pancreatitis and pancreatic cancer among more than 28,000 patients who received some form of incretin-based therapy.12 They independently agreed that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data.12 At this time, there is no final conclusion about a causal relationship between the use of incretin-based drugs and possible pancreatitis and pancreatic cancer.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
In 2013, canagliflozin became the first sodium-glucose cotransporter-2 (SGLT-2) inhibitor to be FDA-approved for treating patients with type 2 DM, followed in 2014 by dapagliflozin and empagliflozin. Several other drugs in this class are available outside the US or are currently undergoing clinical development, including ipragliflozin, luseogliflozin, tofogliflozin, and ertugliflozin. Currently, no SGLT-2 inhibitors are FDA-approved for type 1 DM, although they have been used off-label and in trials in this patient population.
These drugs work by targeting the SGLT-2 protein in the kidney. In healthy individuals, 99% of filtered glucose is reabsorbed by the kidney with a filtered load of approximately 180 g/day.23 Glucosuria occurs with glucose concentrations above this threshold. In patients with type 2 DM, this threshold and the ability to reabsorb glucose is increased, contributing to hyperglycemia.24 Located in the proximal tubule, the SGLT-2 protein is responsible for 80% to 90% of glucose reabsorption, with SGLT-1 responsible for the other 10% to 20%.25 Inhibition of SGLT-2 reduces the renal threshold for glucose, thus leading to glucosuria and reduction in serum glucose levels.24 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for the SGLT-2 inhibitors.
As a monotherapy, SGLT-2 inhibitors significantly reduce HbA1c levels by 0.4% to 1.1% when compared with placebo.26–28 Reductions may be more significant in patients with HbA1c levels greater than 8.5%, and even more so in patients with HbA1c levels above 10%.29 When compared with other therapeutic options for type 2 DM, SGLT-2 inhibitors have efficacy similar to metformin, sitagliptin, and glipizide; however, some studies have shown superiority to glimepiride and sitagliptin at reducing HbA1c, depending on the dose and duration of treatment.26,27
The SGLT-2 inhibitors do not rely on insulin activity, allowing for their use at any stage of type 2 DM and in combination with other therapies, including insulin. As an add-on medication, SGLT-2 inhibitors reduce HbA1c by 0.5% to 0.7%.27,28 Given that the mechanism of action depends on the filtered load of glucose, they are less effective in patients with a reduced glomerular filtration rate (GFR).
The SGLT-2 inhibitors have benefits beyond that of glycemic control. Studies report weight loss of 1 to 3 kg, which is maintained up to 104 weeks.27–31 Sustained weight loss is secondary to glucosuria, which amounts to a caloric loss of 200 to 300 kcal/day.30 Also, SGLT-2 inhibitors lead to modest reductions in systolic and diastolic blood pressure of approximately 3 to 6 mm Hg and 1 to 2 mm Hg, respectively, due to their diuretic effect.27,31 The risk of hypoglycemia is low—similar to that of metformin and DPP-4 inhibitors—and only slightly higher than placebo when used as monotherapy.26,31 When added to sulfonylureas or insulin, however, the risk of hypoglycemia is increased.26,29
Meta-analyses of SGLT-2 inhibitors showed rates of death and other serious adverse effects were no different than placebo.26,27 A 2015 study on the CV safety of empagliflozin showed lower rates of CV death (38% relative risk [RR] reduction), lower rates of hospitalization due to heart failure (35% RR reduction), and lower rates of all-cause mortality (32% RR reduction) when compared with placebo, with no difference in nonfatal stroke and MI.32 CV safety trials for dapagliflozin and canagliflozin are ongoing, although some trials have shown an increased incidence in CV events in the first 30 days of treatment with canagliflozin.4
Common side effects include genital infections, such as vaginitis and balanitis, as well as urinary tract infections. In a 2013 meta-analysis,27 genital infections carried an odds ratio of 3.5 for SGLT-2 inhibitors compared with placebo, while urinary tract infections carried a 1.34 odds ratio. The increased risk of infection is thought to be secondary to glucosuria combined with immune dysfunction and altered glycosylation uroepithelium cells.30
During clinical trials, more cases of bladder cancer were diagnosed in patients on dapagliflozin than on placebo, leading to a delay in FDA approval. No causal relationship was established, but dapagliflozin is not recommended in patients with active bladder cancer.30
Treatment with SGLT-2 inhibitors can lead to a decrease in GFR, likely secondary to the diuretic effect. In patients with GFR greater than 60 mL/min, this decrease is transient. In patients with GFR below 60 mL/min (moderate renal impairment) who were treated with dapagliflozin, GFR did not quite return to baseline, and they did not show an improvement in HbA1c relative to placebo.33 Canagliflozin at a dose of 300 mg/day caused renal-related adverse events with GFR 45 to 60 mL/min, but a lower dose of 100 mg/day did not.27 A decrease in GFR also occurred in patients with chronic kidney disease treated with empagliflozin, which returned to baseline after discontinuing the drug.31 Despite these findings, renal function stabilizes in patients on SGLT-2 inhibitors over time, whereas it continues to decrease with placebo, suggesting there may be a renal protective effect.30 Their diuretic effect can also lead to volume depletion in patients at risk such as elderly patients or those already taking diuretics.31
Some studies have shown mild increases in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels with no change in triglycerides, though long-term effects of this are unknown.31
There are case reports of euglycemic diabetic ketoacidosis occurring in patients with type 1 DM and type 2 DM treated with SGLT-2 inhibitors, which led the FDA in May 2015 to issue a warning that SGLT-2 inhibitors may increase the risk of ketoacidosis.34 There are several possible mechanisms for this increased risk. The SGLT-2 inhibitors may decrease renal clearance of ketones, stimulate glucagon secretion leading to hepatic ketogenesis, or suppress glucose-mediated insulin secretion leading practitioners to decrease insulin doses thus resulting in increased ketone production via lipolysis.34 More studies are needed, but patients and healthcare providers should be aware of potential euglycemic ketoacidosis associated with SGLT-2-inhibitors, as the lack of hyperglycemia can delay the diagnosis.
BILE ACID SEQUESTRANTS
Bile acid sequestrants have been used for years in hyperlipidemia to reduce LDL concentration; however, colesevelam is the only drug in this class approved (2009) for treating type 2 DM, after studies showed colesevelam improves glycemic control.35–37 Though several possibilities have been proposed, the precise mechanism of action for lowering blood glucose levels is unknown.35 Colesevelam is not absorbed systemically and does not affect endogenous insulin levels.4 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for colesevelam.
As monotherapy, studies have shown varying effectiveness in reducing HbA1c relative to placebo ranging from no statistical difference to 0.54% reduction.36,37 As an add-on to other diabetic medications, a Cochrane review of six randomized controlled trials showed a decrease in HbA1c by 0.3% to 0.5% and decrease in fasting glucose of 15 mg/dL.37 Additional benefits of colesevelam include low risk for hypoglycemia, weight neutrality, and reduction in LDL.4 No serious adverse events or deaths have been associated with colesevelam, including CV events; however, more trials on macrovascular outcomes are needed to clarify its side-effect profile.35
Common side effects include constipation, flatulence, and dyspepsia.35 Colesevelam has shown a statistically significant increase in triglycerides, so its use in patients with triglycerides above 500 mg/dL or with hypertriglyceridemia-induced pancreatitis is contraindicated.4 Caution should be used prior to starting treatment in patients with triglyceride levels above 200 mg/dL.35 Colesevelam is contraindicated in patients with a history of small-bowel obstruction, and caution is recommended in patients with decreased gastric motility. This drug may reduce absorption of fat-soluble vitamins and some medications.4
Although further research into the long-term effects of colesevelam is needed, its relatively good safety profile makes it a reasonable choice in diabetic patients with hyperlipidemia not controlled with statins.
DOPAMINE-RECEPTOR AGONIST
Bromocriptine, a dopamine-receptor agonist, was FDA-approved for the treatment of Parkinson disease, hyperprolactinemia, and acromegaly in the 1970s. In 2009, a quick-release formulation of bromocriptine (bromocriptine QR) was approved for treatment of type 2 DM. Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for bromocriptine.
The precise mechanism of action is unclear, but an American Association of Clinical Endocrinologists expert panel recommendation suggests that it may lower glucose levels by improving hypothalamic-mediated, postprandial insulin sensitivity via increasing morning dopaminergic activity (decreased in patients with type 2 DM) and by reducing hypothalamic adrenergic tone.38 It is not currently recommended as monotherapy, although a study of 154 patients showed monotherapy reduced HbA1c by 0.55%.39 When added to other diabetic medications, it reduced HbA1c by 0.4% to 0.7%.4,38
Bromocriptine QR is weight neutral and carries a low risk of hypoglycemia.4 A safety trial with 3,095 patients showed fewer adverse CV events in patients treated with bromocriptine QR compared with placebo, which may be secondary to reduced sympathetic tone or to reduced systemic inflammation.40 Some studies have shown reductions in blood pressure, free fatty acid levels, and triglycerides, with no change in LDL or HDL.38
Common side effects include nausea, headache, dizziness, diarrhea, and fatigue. Administration is recommended with food to reduce GI side effects. It is contraindicated in women who are nursing and those with syncopal migraines. Furthermore, it may be prudent to avoid this medication in patients with a history of psychosis, those currently treated with dopamine agonists or antagonists, or those at risk for hypotension.4
CONCLUSION
The pathophysiology of type 2 DM involves at least seven organs and tissues—the brain, liver, pancreas, intestines, kidneys, fat, and muscle—and no single medication addresses all seven of them. Most patients require more than one medication to adequately treat their diabetes, making availability and development of drugs with unique and complementary mechanisms of action of paramount importance. The medications described here—DPP-4 inhibitors, GLP-1 agonists, SGLT-2 inhibitors, colesevelam, and bromocriptine QR—provide therapeutic options with novel mechanisms of action, all while avoiding weight gain and providing a low risk of hypoglycemia. While not appropriate for every patient, these medications give healthcare providers additional options to individualize treatment and optimize care for patients.
Acknowledgments. The authors gratefully thank Julie Benke-Bennett for assistance with manuscript formatting and transcription. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association; European Association for the Study of Diabetes. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Ther Clin Risk Manag 2015; 11:621–632.
- Germino FW. Noninsulin treatment of type 2 diabetes mellitus in geriatric patients: a review. Clin Ther 2011; 12:1868–1882.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother 2015; 49:540–556.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomized, double-blind, non-inferiority trial. Lancet 2012; 380:475–483.
- White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15:668–673.
- Green JB, Bethel A, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013; 10:289–301.
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J 2011; 162:620–626.
- Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015; 36:2288–2296.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Eng J Med 2014; 370:794–797.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: injectable medications. Ann Pharmacother 2015; 49:700–714.
- Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab 2015; 6:109–134.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015; 6:19–28.
- Harris KB, McCarty DJ. Efficacy and tolerability of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2015; 6:3–18.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gludd LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analysis of randomized controlled trials. BMJ 2012; 344:d7771.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiority trial. Lancet 2014; 384:1349–1357.
- Pratley RE, Nauck MA, Barnett AH, et al; HARMONY 7 Study Group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomized, open-label, multicenter, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2:289–297.
- Neumiller JJ. Incretin-based therapies. Med Clin N Am 2015; 99:107–129.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:38–47.
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75:1272–1277.
- Defronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013; 36:3169–3176.
- Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 2012; 303:C348–C354.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:457–466.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014; 70:1149–1158.
- Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes 2015; 7:448–461.
- Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 2014; 5:124–136.
- Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context 2014; 3:212264.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Hinnen D. Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2015; 6:92–102.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Aggarwal W, Loomba RS, Arora RR. Efficacy of colesevelam on lowering glycemia and lipids. J Cardiovasc Pharmacol 2012; 59:198–205.
- Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam Hcl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 2008; 31:1479–1484.
- Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012; 12:CD009361.
- Garber AJ, Blonde L, Bloomgarden ZT, Handelsman Y, Dagogo-Jack S. The role of bromocriptine-QR in the management of type 2 diabetes expert panel recommendations. Endocr Pract 2013; 19:100–106.
- Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999; 8:1683–1707.
- Gaziano JM, Concotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
Type 2 diabetes mellitus (DM) is caused by hyperglycemia and metabolic alterations due to abnormalities in insulin secretion or insulin action, or both. To achieve desired glycemic targets, different antihyperglycemic drugs are used alone or in combination with other agents, including insulin. First-line options for diabetes treatment are weight loss, lifestyle modification, and metformin. The American Diabetes Association and the European Association for the Study of Diabetes recommend a patient-specific treatment approach to enhance glycemic control while avoiding weight gain and hypoglycemia.1 This review will focus on the newer oral agents and injectable noninsulin agents that are used to achieve glycemic control. Table 1 lists the noninsulin drugs approved since 2005.
INCRETIN-BASED THERAPIES
The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are secreted by the gastrointestinal (GI) tract in response to food intake. Both GLP-1 and GIP stimulate beta cells of the pancreas, which contribute 60% of the insulin secretion after a meal. Type 2 DM is associated with decreased secretion of GLP-1 and lowered responsiveness to GIP. Benefits of the incretin hormones on glycemic control include enhanced satiety, decreased GI motility, increased glucose-dependent insulin secretion, reduced glucagon secretion, and decreased hepatic glucose release.2 Two incretin-based drug classes are used to treat patients with type 2 DM—oral dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists.
DPP-4 inhibitors
The oral DPP-4 inhibitors block the degradation of the enzyme DPP-4 active site and thus increase the GLP-1 and GIP concentrations by two to three times. Their primary effectiveness centers on controlling insulin and glucagon secretion without increasing weight.
Four DPP-4 inhibitors are approved by the US Food and Drug Administration (FDA) in once-daily oral formulations: sitagliptin, saxagliptin, linagliptin, and alogliptin. Another DPP-4 inhibitor, vildagliptin, is not licensed in the United States but is approved for use in Europe and Japan.3 Another DPP-4 inhibitor, teneligliptin, is also marketed in Japan.
The DPP-4 inhibitors are indicated for use as monotherapy or in combination with other agents such as metformin, sulfonylureas, thiazolidinediones, and insulin. Generally, DPP-4 inhibitors do not cause hypoglycemia when used as monotherapy.1 When adding a DPP-4 inhibitor to a sulfonylurea or insulin, it is recommended to decrease the sulfonylurea or insulin dose to reduce the risk of hypoglycemia. The potential of these agents to lower the hemoglobin A1c (HbA1c) when used as monotherapy and in combination with metformin, sulfonylureas, or thiazolidinediones is 0.3% to 0.71%.4
The DPP-4 inhibitors are not known to cause adverse GI effects. Sitagliptin, alogliptin, vildagliptin, and saxagliptin need dosing adjustments for renal insufficiency; however, linagliptin is not renally eliminated and does not require dosing adjustment. Common adverse events (> 5%) are nasopharyngitis, upper-respiratory infection, and headache. Sitagliptin and saxagliptin have been associated with urinary tract infection. Sitagliptin also has been associated with more extremity pain, back pain, and osteoarthritis.4
The DPP-4 inhibitors are primarily excreted by the renal or fecal route and, therefore, have few drug interactions. All DPP-4 inhibitors are partially metabolized through cytochrome P450 enzymes, except saxagliptin.4 Their pharmacokinetic profiles are shown on Table 2.
In keeping with the FDA guidelines, sitagliptin, saxagliptin, linagliptin, and alogliptin have been evaluated for cardiovascular (CV) outcomes. The SAVOR-TIMI 53 clinical trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) was a 2-year CV safety and efficacy trial.5 This trial demonstrated no statistically significant difference in the primary end point as a composite of CV death, myocardial infarction (MI), and ischemic stroke. Additionally, the secondary end points, including hospitalization for unstable angina, coronary revascularization, and heart failure, also did not show significant difference. However, based on a subgroup analysis, there was a statistically significant increase in patients in the saxagliptin group vs the placebo group who were hospitalized for heart failure.
A 2-year trial comparing linagliptin with glimepiride, a second-generation sulfonylurea, showed significantly fewer CV events with linagliptin.6 This trial found a relative risk reduction of 54% in the end points of CV death, nonfatal MI or stroke, and unstable angina during hospitalization.4,6 Another trial reviewed the incidence of CV events (CV death, nonfatal MI, and nonfatal stroke) in patients treated with alogliptin, placebo, or comparator antihyperglycemic drugs and found no increased incidence of major adverse CV events vs comparator therapies.7
The recently published TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin), reported no increase in major atherosclerotic CV events, no difference in all-cause mortality, and no difference in heart failure for hospitalization or other adverse events in patients with type 2 DM.8 Other clinical trials such as the CAROLINA study (linagliptin compared with glimepiride),9 the EXAMINE study (alogliptin),10 and the CARMALINA study (linagliptin)11 are also reviewing the CV safety of DPP-4 inhibitors in the United States.
Concern has been raised about the association between incretin-based therapies and adverse pancreatic effects. CV outcomes trials using saxagliptin and alogliptin found similar rates of pancreatitis and fewer pancreatic cancer cases in comparison with placebo.5,7,10 TECOS demonstrated that with sitagliptin, acute pancreatitis occurred more in the sitagliptin group, but there was no statistical significance reported. However, pancreatic cancer occurred more in the placebo group, although the difference was not statistically significant.8 Neither the FDA nor the European Medicines Agency (EMA) has reached a firm conclusion about the possible association between incretin-based therapies and pancreatitis or pancreatic cancer.12
GLP-1 agonists
The GLP-1 drugs mimic the action of native GLP-1. Several GLP-1 agents are available in the United States, and several more are in development.11,13 The drug class is divided into three groups:
- Short-acting (4–6 hours): exenatide, lixisenatide
- Intermediate-acting (24 hours): liraglutide
- Long-acting (7 days): exenatide extended-release (ER), dulaglutide, and albiglutide (semaglutide is in phase 3 study).
The GLP-1 receptor agonists heighten glucose homeostasis by the following mechanisms of action: stimulate insulin secretion, suppress glucagon secretion, directly and indirectly inhibit endogenous glucose production, promote satiety, heighten insulin sensitivity due to weight loss, and slow gastric emptying time. Table 3 lists dosing and pharmacokinetic profiles for GLP-1 agonists. When GLP-1 agonists are used as monotherapy, the HbA1c is reduced by 0.7% to 1.51%.13 When GLP-1 agonists are used in combination with metformin, sulfonylureas, thiazolidinediones, or as three-drug therapy with other oral antidiabetic medications, the HbA1c is lowered by 0.4% to 1.9%.13–17
A notable advantage of GLP-1 agonists is their effect on weight loss separate from GI side effects. Weight reductions of 0.2 to 3.6 kg in 26 weeks have been seen with the exenatide formulations, liraglutide, albiglutide, and dulaglutide.13,18 Liraglutide has demonstrated a greater weight reduction than exenatide, exenatide ER, or albiglutide.13,16,17 There were similar weight reductions of 1.5 kg in 26 weeks in a comparator trial involving liraglutide and dulaglutide (3.6 vs 2.9 kg).19
Common adverse effects of the GLP-1 agonists are nausea (8% to 44%), diarrhea (6% to 20%), and vomiting (4% to 18%), which may occur initially and diminish with continued use.13,14 There have been more GI side effects with liraglutide than with exenatide ER or albiglutide.20 Increased rates of injection site reactions, such as transient small nodule formations, were seen with exenatide ER (5.4% to 17.6%) and albiglutide, the once-weekly GLP-1 agonist therapies, vs exenatide, liraglutide, and insulin glargine.13,14 Dulaglutide, another once-weekly GLP-1 agonist, does not have this finding; however, there is enhanced patient satisfaction with the once-weekly preparations in comparison with the twice-daily preparations.14,16 Patients who received albiglutide have noted hypersensitivity reactions such as pruritus, rash, and dyspnea (10% to 18%).13 Hypoglycemia is not seen with the GLP-1 agonists, unless they are used in conjunction with a sulfonylurea or insulin.
Exenatide and exenatide ER are excreted by the renal route; therefore, it is not recommended to use these agonists in patients with renal impairment or end-stage renal disease (creatinine clearance [CrCl] < 30 mL/min). Liraglutide is not excreted by the renal route; however, it should be used with caution in patients with renal impairment.21 No renal dose adjustment is required when using albiglutide or dulaglutide.21
Clinical trials have demonstrated the short-term CV outcomes of GLP-1 agonists. The CV benefits include a decrease in blood pressure, reduction of lipid levels, enhanced endothelial function, and improved myocardial function.13 One meta-analysis reported a tendency for lowering the rate of major CV events, stroke, MI, CV mortality, and all-cause mortality.22 Several ongoing trials are evaluating the safety of GLP-1 agonists and CV safety: LEADER (liraglutide), EXSCEL (exenatide LR), ELIXA (lixisenatide), SUSTAIN 6 (semaglutide), and REWIND (dulaglutide).11
The GLP-1 agonists have been linked to an increased incidence of thyroid cancer. There was a potential increased risk of thyroid cancer in preclinical rodent studies involving liraglutide and exenatide ER, but this risk was not demonstrated for the exenatide twice-daily preparation.13 The FDA noted that the findings from rodent studies, which demonstrated a possible heightened risk for thyroid cancer, should not be conveyed to the outcomes for humans. Nevertheless, when liraglutide was approved in January 2010, the FDA issued a boxed warning about the risk of thyroid C-cell hyperplasia. The package inserts list a thyroid carcinoma risk for exenatide ER, liraglutide, albiglutide, and dulaglutide in those patients with a personal or family history of medullary thyroid cancer.
There is controversy about the incidence of pancreatitis and pancreatic cancer with the use of the incretin-based therapies. Published studies and case reports seem to support speculation that there is an increased incidence of acute pancreatitis associated with type 2 DM.21 The FDA and the EMA have independently reviewed postmarketing reports about pancreatitis and pancreatic cancer among more than 28,000 patients who received some form of incretin-based therapy.12 They independently agreed that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data.12 At this time, there is no final conclusion about a causal relationship between the use of incretin-based drugs and possible pancreatitis and pancreatic cancer.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
In 2013, canagliflozin became the first sodium-glucose cotransporter-2 (SGLT-2) inhibitor to be FDA-approved for treating patients with type 2 DM, followed in 2014 by dapagliflozin and empagliflozin. Several other drugs in this class are available outside the US or are currently undergoing clinical development, including ipragliflozin, luseogliflozin, tofogliflozin, and ertugliflozin. Currently, no SGLT-2 inhibitors are FDA-approved for type 1 DM, although they have been used off-label and in trials in this patient population.
These drugs work by targeting the SGLT-2 protein in the kidney. In healthy individuals, 99% of filtered glucose is reabsorbed by the kidney with a filtered load of approximately 180 g/day.23 Glucosuria occurs with glucose concentrations above this threshold. In patients with type 2 DM, this threshold and the ability to reabsorb glucose is increased, contributing to hyperglycemia.24 Located in the proximal tubule, the SGLT-2 protein is responsible for 80% to 90% of glucose reabsorption, with SGLT-1 responsible for the other 10% to 20%.25 Inhibition of SGLT-2 reduces the renal threshold for glucose, thus leading to glucosuria and reduction in serum glucose levels.24 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for the SGLT-2 inhibitors.
As a monotherapy, SGLT-2 inhibitors significantly reduce HbA1c levels by 0.4% to 1.1% when compared with placebo.26–28 Reductions may be more significant in patients with HbA1c levels greater than 8.5%, and even more so in patients with HbA1c levels above 10%.29 When compared with other therapeutic options for type 2 DM, SGLT-2 inhibitors have efficacy similar to metformin, sitagliptin, and glipizide; however, some studies have shown superiority to glimepiride and sitagliptin at reducing HbA1c, depending on the dose and duration of treatment.26,27
The SGLT-2 inhibitors do not rely on insulin activity, allowing for their use at any stage of type 2 DM and in combination with other therapies, including insulin. As an add-on medication, SGLT-2 inhibitors reduce HbA1c by 0.5% to 0.7%.27,28 Given that the mechanism of action depends on the filtered load of glucose, they are less effective in patients with a reduced glomerular filtration rate (GFR).
The SGLT-2 inhibitors have benefits beyond that of glycemic control. Studies report weight loss of 1 to 3 kg, which is maintained up to 104 weeks.27–31 Sustained weight loss is secondary to glucosuria, which amounts to a caloric loss of 200 to 300 kcal/day.30 Also, SGLT-2 inhibitors lead to modest reductions in systolic and diastolic blood pressure of approximately 3 to 6 mm Hg and 1 to 2 mm Hg, respectively, due to their diuretic effect.27,31 The risk of hypoglycemia is low—similar to that of metformin and DPP-4 inhibitors—and only slightly higher than placebo when used as monotherapy.26,31 When added to sulfonylureas or insulin, however, the risk of hypoglycemia is increased.26,29
Meta-analyses of SGLT-2 inhibitors showed rates of death and other serious adverse effects were no different than placebo.26,27 A 2015 study on the CV safety of empagliflozin showed lower rates of CV death (38% relative risk [RR] reduction), lower rates of hospitalization due to heart failure (35% RR reduction), and lower rates of all-cause mortality (32% RR reduction) when compared with placebo, with no difference in nonfatal stroke and MI.32 CV safety trials for dapagliflozin and canagliflozin are ongoing, although some trials have shown an increased incidence in CV events in the first 30 days of treatment with canagliflozin.4
Common side effects include genital infections, such as vaginitis and balanitis, as well as urinary tract infections. In a 2013 meta-analysis,27 genital infections carried an odds ratio of 3.5 for SGLT-2 inhibitors compared with placebo, while urinary tract infections carried a 1.34 odds ratio. The increased risk of infection is thought to be secondary to glucosuria combined with immune dysfunction and altered glycosylation uroepithelium cells.30
During clinical trials, more cases of bladder cancer were diagnosed in patients on dapagliflozin than on placebo, leading to a delay in FDA approval. No causal relationship was established, but dapagliflozin is not recommended in patients with active bladder cancer.30
Treatment with SGLT-2 inhibitors can lead to a decrease in GFR, likely secondary to the diuretic effect. In patients with GFR greater than 60 mL/min, this decrease is transient. In patients with GFR below 60 mL/min (moderate renal impairment) who were treated with dapagliflozin, GFR did not quite return to baseline, and they did not show an improvement in HbA1c relative to placebo.33 Canagliflozin at a dose of 300 mg/day caused renal-related adverse events with GFR 45 to 60 mL/min, but a lower dose of 100 mg/day did not.27 A decrease in GFR also occurred in patients with chronic kidney disease treated with empagliflozin, which returned to baseline after discontinuing the drug.31 Despite these findings, renal function stabilizes in patients on SGLT-2 inhibitors over time, whereas it continues to decrease with placebo, suggesting there may be a renal protective effect.30 Their diuretic effect can also lead to volume depletion in patients at risk such as elderly patients or those already taking diuretics.31
Some studies have shown mild increases in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels with no change in triglycerides, though long-term effects of this are unknown.31
There are case reports of euglycemic diabetic ketoacidosis occurring in patients with type 1 DM and type 2 DM treated with SGLT-2 inhibitors, which led the FDA in May 2015 to issue a warning that SGLT-2 inhibitors may increase the risk of ketoacidosis.34 There are several possible mechanisms for this increased risk. The SGLT-2 inhibitors may decrease renal clearance of ketones, stimulate glucagon secretion leading to hepatic ketogenesis, or suppress glucose-mediated insulin secretion leading practitioners to decrease insulin doses thus resulting in increased ketone production via lipolysis.34 More studies are needed, but patients and healthcare providers should be aware of potential euglycemic ketoacidosis associated with SGLT-2-inhibitors, as the lack of hyperglycemia can delay the diagnosis.
BILE ACID SEQUESTRANTS
Bile acid sequestrants have been used for years in hyperlipidemia to reduce LDL concentration; however, colesevelam is the only drug in this class approved (2009) for treating type 2 DM, after studies showed colesevelam improves glycemic control.35–37 Though several possibilities have been proposed, the precise mechanism of action for lowering blood glucose levels is unknown.35 Colesevelam is not absorbed systemically and does not affect endogenous insulin levels.4 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for colesevelam.
As monotherapy, studies have shown varying effectiveness in reducing HbA1c relative to placebo ranging from no statistical difference to 0.54% reduction.36,37 As an add-on to other diabetic medications, a Cochrane review of six randomized controlled trials showed a decrease in HbA1c by 0.3% to 0.5% and decrease in fasting glucose of 15 mg/dL.37 Additional benefits of colesevelam include low risk for hypoglycemia, weight neutrality, and reduction in LDL.4 No serious adverse events or deaths have been associated with colesevelam, including CV events; however, more trials on macrovascular outcomes are needed to clarify its side-effect profile.35
Common side effects include constipation, flatulence, and dyspepsia.35 Colesevelam has shown a statistically significant increase in triglycerides, so its use in patients with triglycerides above 500 mg/dL or with hypertriglyceridemia-induced pancreatitis is contraindicated.4 Caution should be used prior to starting treatment in patients with triglyceride levels above 200 mg/dL.35 Colesevelam is contraindicated in patients with a history of small-bowel obstruction, and caution is recommended in patients with decreased gastric motility. This drug may reduce absorption of fat-soluble vitamins and some medications.4
Although further research into the long-term effects of colesevelam is needed, its relatively good safety profile makes it a reasonable choice in diabetic patients with hyperlipidemia not controlled with statins.
DOPAMINE-RECEPTOR AGONIST
Bromocriptine, a dopamine-receptor agonist, was FDA-approved for the treatment of Parkinson disease, hyperprolactinemia, and acromegaly in the 1970s. In 2009, a quick-release formulation of bromocriptine (bromocriptine QR) was approved for treatment of type 2 DM. Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for bromocriptine.
The precise mechanism of action is unclear, but an American Association of Clinical Endocrinologists expert panel recommendation suggests that it may lower glucose levels by improving hypothalamic-mediated, postprandial insulin sensitivity via increasing morning dopaminergic activity (decreased in patients with type 2 DM) and by reducing hypothalamic adrenergic tone.38 It is not currently recommended as monotherapy, although a study of 154 patients showed monotherapy reduced HbA1c by 0.55%.39 When added to other diabetic medications, it reduced HbA1c by 0.4% to 0.7%.4,38
Bromocriptine QR is weight neutral and carries a low risk of hypoglycemia.4 A safety trial with 3,095 patients showed fewer adverse CV events in patients treated with bromocriptine QR compared with placebo, which may be secondary to reduced sympathetic tone or to reduced systemic inflammation.40 Some studies have shown reductions in blood pressure, free fatty acid levels, and triglycerides, with no change in LDL or HDL.38
Common side effects include nausea, headache, dizziness, diarrhea, and fatigue. Administration is recommended with food to reduce GI side effects. It is contraindicated in women who are nursing and those with syncopal migraines. Furthermore, it may be prudent to avoid this medication in patients with a history of psychosis, those currently treated with dopamine agonists or antagonists, or those at risk for hypotension.4
CONCLUSION
The pathophysiology of type 2 DM involves at least seven organs and tissues—the brain, liver, pancreas, intestines, kidneys, fat, and muscle—and no single medication addresses all seven of them. Most patients require more than one medication to adequately treat their diabetes, making availability and development of drugs with unique and complementary mechanisms of action of paramount importance. The medications described here—DPP-4 inhibitors, GLP-1 agonists, SGLT-2 inhibitors, colesevelam, and bromocriptine QR—provide therapeutic options with novel mechanisms of action, all while avoiding weight gain and providing a low risk of hypoglycemia. While not appropriate for every patient, these medications give healthcare providers additional options to individualize treatment and optimize care for patients.
Acknowledgments. The authors gratefully thank Julie Benke-Bennett for assistance with manuscript formatting and transcription. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Type 2 diabetes mellitus (DM) is caused by hyperglycemia and metabolic alterations due to abnormalities in insulin secretion or insulin action, or both. To achieve desired glycemic targets, different antihyperglycemic drugs are used alone or in combination with other agents, including insulin. First-line options for diabetes treatment are weight loss, lifestyle modification, and metformin. The American Diabetes Association and the European Association for the Study of Diabetes recommend a patient-specific treatment approach to enhance glycemic control while avoiding weight gain and hypoglycemia.1 This review will focus on the newer oral agents and injectable noninsulin agents that are used to achieve glycemic control. Table 1 lists the noninsulin drugs approved since 2005.
INCRETIN-BASED THERAPIES
The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are secreted by the gastrointestinal (GI) tract in response to food intake. Both GLP-1 and GIP stimulate beta cells of the pancreas, which contribute 60% of the insulin secretion after a meal. Type 2 DM is associated with decreased secretion of GLP-1 and lowered responsiveness to GIP. Benefits of the incretin hormones on glycemic control include enhanced satiety, decreased GI motility, increased glucose-dependent insulin secretion, reduced glucagon secretion, and decreased hepatic glucose release.2 Two incretin-based drug classes are used to treat patients with type 2 DM—oral dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists.
DPP-4 inhibitors
The oral DPP-4 inhibitors block the degradation of the enzyme DPP-4 active site and thus increase the GLP-1 and GIP concentrations by two to three times. Their primary effectiveness centers on controlling insulin and glucagon secretion without increasing weight.
Four DPP-4 inhibitors are approved by the US Food and Drug Administration (FDA) in once-daily oral formulations: sitagliptin, saxagliptin, linagliptin, and alogliptin. Another DPP-4 inhibitor, vildagliptin, is not licensed in the United States but is approved for use in Europe and Japan.3 Another DPP-4 inhibitor, teneligliptin, is also marketed in Japan.
The DPP-4 inhibitors are indicated for use as monotherapy or in combination with other agents such as metformin, sulfonylureas, thiazolidinediones, and insulin. Generally, DPP-4 inhibitors do not cause hypoglycemia when used as monotherapy.1 When adding a DPP-4 inhibitor to a sulfonylurea or insulin, it is recommended to decrease the sulfonylurea or insulin dose to reduce the risk of hypoglycemia. The potential of these agents to lower the hemoglobin A1c (HbA1c) when used as monotherapy and in combination with metformin, sulfonylureas, or thiazolidinediones is 0.3% to 0.71%.4
The DPP-4 inhibitors are not known to cause adverse GI effects. Sitagliptin, alogliptin, vildagliptin, and saxagliptin need dosing adjustments for renal insufficiency; however, linagliptin is not renally eliminated and does not require dosing adjustment. Common adverse events (> 5%) are nasopharyngitis, upper-respiratory infection, and headache. Sitagliptin and saxagliptin have been associated with urinary tract infection. Sitagliptin also has been associated with more extremity pain, back pain, and osteoarthritis.4
The DPP-4 inhibitors are primarily excreted by the renal or fecal route and, therefore, have few drug interactions. All DPP-4 inhibitors are partially metabolized through cytochrome P450 enzymes, except saxagliptin.4 Their pharmacokinetic profiles are shown on Table 2.
In keeping with the FDA guidelines, sitagliptin, saxagliptin, linagliptin, and alogliptin have been evaluated for cardiovascular (CV) outcomes. The SAVOR-TIMI 53 clinical trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) was a 2-year CV safety and efficacy trial.5 This trial demonstrated no statistically significant difference in the primary end point as a composite of CV death, myocardial infarction (MI), and ischemic stroke. Additionally, the secondary end points, including hospitalization for unstable angina, coronary revascularization, and heart failure, also did not show significant difference. However, based on a subgroup analysis, there was a statistically significant increase in patients in the saxagliptin group vs the placebo group who were hospitalized for heart failure.
A 2-year trial comparing linagliptin with glimepiride, a second-generation sulfonylurea, showed significantly fewer CV events with linagliptin.6 This trial found a relative risk reduction of 54% in the end points of CV death, nonfatal MI or stroke, and unstable angina during hospitalization.4,6 Another trial reviewed the incidence of CV events (CV death, nonfatal MI, and nonfatal stroke) in patients treated with alogliptin, placebo, or comparator antihyperglycemic drugs and found no increased incidence of major adverse CV events vs comparator therapies.7
The recently published TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin), reported no increase in major atherosclerotic CV events, no difference in all-cause mortality, and no difference in heart failure for hospitalization or other adverse events in patients with type 2 DM.8 Other clinical trials such as the CAROLINA study (linagliptin compared with glimepiride),9 the EXAMINE study (alogliptin),10 and the CARMALINA study (linagliptin)11 are also reviewing the CV safety of DPP-4 inhibitors in the United States.
Concern has been raised about the association between incretin-based therapies and adverse pancreatic effects. CV outcomes trials using saxagliptin and alogliptin found similar rates of pancreatitis and fewer pancreatic cancer cases in comparison with placebo.5,7,10 TECOS demonstrated that with sitagliptin, acute pancreatitis occurred more in the sitagliptin group, but there was no statistical significance reported. However, pancreatic cancer occurred more in the placebo group, although the difference was not statistically significant.8 Neither the FDA nor the European Medicines Agency (EMA) has reached a firm conclusion about the possible association between incretin-based therapies and pancreatitis or pancreatic cancer.12
GLP-1 agonists
The GLP-1 drugs mimic the action of native GLP-1. Several GLP-1 agents are available in the United States, and several more are in development.11,13 The drug class is divided into three groups:
- Short-acting (4–6 hours): exenatide, lixisenatide
- Intermediate-acting (24 hours): liraglutide
- Long-acting (7 days): exenatide extended-release (ER), dulaglutide, and albiglutide (semaglutide is in phase 3 study).
The GLP-1 receptor agonists heighten glucose homeostasis by the following mechanisms of action: stimulate insulin secretion, suppress glucagon secretion, directly and indirectly inhibit endogenous glucose production, promote satiety, heighten insulin sensitivity due to weight loss, and slow gastric emptying time. Table 3 lists dosing and pharmacokinetic profiles for GLP-1 agonists. When GLP-1 agonists are used as monotherapy, the HbA1c is reduced by 0.7% to 1.51%.13 When GLP-1 agonists are used in combination with metformin, sulfonylureas, thiazolidinediones, or as three-drug therapy with other oral antidiabetic medications, the HbA1c is lowered by 0.4% to 1.9%.13–17
A notable advantage of GLP-1 agonists is their effect on weight loss separate from GI side effects. Weight reductions of 0.2 to 3.6 kg in 26 weeks have been seen with the exenatide formulations, liraglutide, albiglutide, and dulaglutide.13,18 Liraglutide has demonstrated a greater weight reduction than exenatide, exenatide ER, or albiglutide.13,16,17 There were similar weight reductions of 1.5 kg in 26 weeks in a comparator trial involving liraglutide and dulaglutide (3.6 vs 2.9 kg).19
Common adverse effects of the GLP-1 agonists are nausea (8% to 44%), diarrhea (6% to 20%), and vomiting (4% to 18%), which may occur initially and diminish with continued use.13,14 There have been more GI side effects with liraglutide than with exenatide ER or albiglutide.20 Increased rates of injection site reactions, such as transient small nodule formations, were seen with exenatide ER (5.4% to 17.6%) and albiglutide, the once-weekly GLP-1 agonist therapies, vs exenatide, liraglutide, and insulin glargine.13,14 Dulaglutide, another once-weekly GLP-1 agonist, does not have this finding; however, there is enhanced patient satisfaction with the once-weekly preparations in comparison with the twice-daily preparations.14,16 Patients who received albiglutide have noted hypersensitivity reactions such as pruritus, rash, and dyspnea (10% to 18%).13 Hypoglycemia is not seen with the GLP-1 agonists, unless they are used in conjunction with a sulfonylurea or insulin.
Exenatide and exenatide ER are excreted by the renal route; therefore, it is not recommended to use these agonists in patients with renal impairment or end-stage renal disease (creatinine clearance [CrCl] < 30 mL/min). Liraglutide is not excreted by the renal route; however, it should be used with caution in patients with renal impairment.21 No renal dose adjustment is required when using albiglutide or dulaglutide.21
Clinical trials have demonstrated the short-term CV outcomes of GLP-1 agonists. The CV benefits include a decrease in blood pressure, reduction of lipid levels, enhanced endothelial function, and improved myocardial function.13 One meta-analysis reported a tendency for lowering the rate of major CV events, stroke, MI, CV mortality, and all-cause mortality.22 Several ongoing trials are evaluating the safety of GLP-1 agonists and CV safety: LEADER (liraglutide), EXSCEL (exenatide LR), ELIXA (lixisenatide), SUSTAIN 6 (semaglutide), and REWIND (dulaglutide).11
The GLP-1 agonists have been linked to an increased incidence of thyroid cancer. There was a potential increased risk of thyroid cancer in preclinical rodent studies involving liraglutide and exenatide ER, but this risk was not demonstrated for the exenatide twice-daily preparation.13 The FDA noted that the findings from rodent studies, which demonstrated a possible heightened risk for thyroid cancer, should not be conveyed to the outcomes for humans. Nevertheless, when liraglutide was approved in January 2010, the FDA issued a boxed warning about the risk of thyroid C-cell hyperplasia. The package inserts list a thyroid carcinoma risk for exenatide ER, liraglutide, albiglutide, and dulaglutide in those patients with a personal or family history of medullary thyroid cancer.
There is controversy about the incidence of pancreatitis and pancreatic cancer with the use of the incretin-based therapies. Published studies and case reports seem to support speculation that there is an increased incidence of acute pancreatitis associated with type 2 DM.21 The FDA and the EMA have independently reviewed postmarketing reports about pancreatitis and pancreatic cancer among more than 28,000 patients who received some form of incretin-based therapy.12 They independently agreed that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data.12 At this time, there is no final conclusion about a causal relationship between the use of incretin-based drugs and possible pancreatitis and pancreatic cancer.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
In 2013, canagliflozin became the first sodium-glucose cotransporter-2 (SGLT-2) inhibitor to be FDA-approved for treating patients with type 2 DM, followed in 2014 by dapagliflozin and empagliflozin. Several other drugs in this class are available outside the US or are currently undergoing clinical development, including ipragliflozin, luseogliflozin, tofogliflozin, and ertugliflozin. Currently, no SGLT-2 inhibitors are FDA-approved for type 1 DM, although they have been used off-label and in trials in this patient population.
These drugs work by targeting the SGLT-2 protein in the kidney. In healthy individuals, 99% of filtered glucose is reabsorbed by the kidney with a filtered load of approximately 180 g/day.23 Glucosuria occurs with glucose concentrations above this threshold. In patients with type 2 DM, this threshold and the ability to reabsorb glucose is increased, contributing to hyperglycemia.24 Located in the proximal tubule, the SGLT-2 protein is responsible for 80% to 90% of glucose reabsorption, with SGLT-1 responsible for the other 10% to 20%.25 Inhibition of SGLT-2 reduces the renal threshold for glucose, thus leading to glucosuria and reduction in serum glucose levels.24 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for the SGLT-2 inhibitors.
As a monotherapy, SGLT-2 inhibitors significantly reduce HbA1c levels by 0.4% to 1.1% when compared with placebo.26–28 Reductions may be more significant in patients with HbA1c levels greater than 8.5%, and even more so in patients with HbA1c levels above 10%.29 When compared with other therapeutic options for type 2 DM, SGLT-2 inhibitors have efficacy similar to metformin, sitagliptin, and glipizide; however, some studies have shown superiority to glimepiride and sitagliptin at reducing HbA1c, depending on the dose and duration of treatment.26,27
The SGLT-2 inhibitors do not rely on insulin activity, allowing for their use at any stage of type 2 DM and in combination with other therapies, including insulin. As an add-on medication, SGLT-2 inhibitors reduce HbA1c by 0.5% to 0.7%.27,28 Given that the mechanism of action depends on the filtered load of glucose, they are less effective in patients with a reduced glomerular filtration rate (GFR).
The SGLT-2 inhibitors have benefits beyond that of glycemic control. Studies report weight loss of 1 to 3 kg, which is maintained up to 104 weeks.27–31 Sustained weight loss is secondary to glucosuria, which amounts to a caloric loss of 200 to 300 kcal/day.30 Also, SGLT-2 inhibitors lead to modest reductions in systolic and diastolic blood pressure of approximately 3 to 6 mm Hg and 1 to 2 mm Hg, respectively, due to their diuretic effect.27,31 The risk of hypoglycemia is low—similar to that of metformin and DPP-4 inhibitors—and only slightly higher than placebo when used as monotherapy.26,31 When added to sulfonylureas or insulin, however, the risk of hypoglycemia is increased.26,29
Meta-analyses of SGLT-2 inhibitors showed rates of death and other serious adverse effects were no different than placebo.26,27 A 2015 study on the CV safety of empagliflozin showed lower rates of CV death (38% relative risk [RR] reduction), lower rates of hospitalization due to heart failure (35% RR reduction), and lower rates of all-cause mortality (32% RR reduction) when compared with placebo, with no difference in nonfatal stroke and MI.32 CV safety trials for dapagliflozin and canagliflozin are ongoing, although some trials have shown an increased incidence in CV events in the first 30 days of treatment with canagliflozin.4
Common side effects include genital infections, such as vaginitis and balanitis, as well as urinary tract infections. In a 2013 meta-analysis,27 genital infections carried an odds ratio of 3.5 for SGLT-2 inhibitors compared with placebo, while urinary tract infections carried a 1.34 odds ratio. The increased risk of infection is thought to be secondary to glucosuria combined with immune dysfunction and altered glycosylation uroepithelium cells.30
During clinical trials, more cases of bladder cancer were diagnosed in patients on dapagliflozin than on placebo, leading to a delay in FDA approval. No causal relationship was established, but dapagliflozin is not recommended in patients with active bladder cancer.30
Treatment with SGLT-2 inhibitors can lead to a decrease in GFR, likely secondary to the diuretic effect. In patients with GFR greater than 60 mL/min, this decrease is transient. In patients with GFR below 60 mL/min (moderate renal impairment) who were treated with dapagliflozin, GFR did not quite return to baseline, and they did not show an improvement in HbA1c relative to placebo.33 Canagliflozin at a dose of 300 mg/day caused renal-related adverse events with GFR 45 to 60 mL/min, but a lower dose of 100 mg/day did not.27 A decrease in GFR also occurred in patients with chronic kidney disease treated with empagliflozin, which returned to baseline after discontinuing the drug.31 Despite these findings, renal function stabilizes in patients on SGLT-2 inhibitors over time, whereas it continues to decrease with placebo, suggesting there may be a renal protective effect.30 Their diuretic effect can also lead to volume depletion in patients at risk such as elderly patients or those already taking diuretics.31
Some studies have shown mild increases in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) levels with no change in triglycerides, though long-term effects of this are unknown.31
There are case reports of euglycemic diabetic ketoacidosis occurring in patients with type 1 DM and type 2 DM treated with SGLT-2 inhibitors, which led the FDA in May 2015 to issue a warning that SGLT-2 inhibitors may increase the risk of ketoacidosis.34 There are several possible mechanisms for this increased risk. The SGLT-2 inhibitors may decrease renal clearance of ketones, stimulate glucagon secretion leading to hepatic ketogenesis, or suppress glucose-mediated insulin secretion leading practitioners to decrease insulin doses thus resulting in increased ketone production via lipolysis.34 More studies are needed, but patients and healthcare providers should be aware of potential euglycemic ketoacidosis associated with SGLT-2-inhibitors, as the lack of hyperglycemia can delay the diagnosis.
BILE ACID SEQUESTRANTS
Bile acid sequestrants have been used for years in hyperlipidemia to reduce LDL concentration; however, colesevelam is the only drug in this class approved (2009) for treating type 2 DM, after studies showed colesevelam improves glycemic control.35–37 Though several possibilities have been proposed, the precise mechanism of action for lowering blood glucose levels is unknown.35 Colesevelam is not absorbed systemically and does not affect endogenous insulin levels.4 Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for colesevelam.
As monotherapy, studies have shown varying effectiveness in reducing HbA1c relative to placebo ranging from no statistical difference to 0.54% reduction.36,37 As an add-on to other diabetic medications, a Cochrane review of six randomized controlled trials showed a decrease in HbA1c by 0.3% to 0.5% and decrease in fasting glucose of 15 mg/dL.37 Additional benefits of colesevelam include low risk for hypoglycemia, weight neutrality, and reduction in LDL.4 No serious adverse events or deaths have been associated with colesevelam, including CV events; however, more trials on macrovascular outcomes are needed to clarify its side-effect profile.35
Common side effects include constipation, flatulence, and dyspepsia.35 Colesevelam has shown a statistically significant increase in triglycerides, so its use in patients with triglycerides above 500 mg/dL or with hypertriglyceridemia-induced pancreatitis is contraindicated.4 Caution should be used prior to starting treatment in patients with triglyceride levels above 200 mg/dL.35 Colesevelam is contraindicated in patients with a history of small-bowel obstruction, and caution is recommended in patients with decreased gastric motility. This drug may reduce absorption of fat-soluble vitamins and some medications.4
Although further research into the long-term effects of colesevelam is needed, its relatively good safety profile makes it a reasonable choice in diabetic patients with hyperlipidemia not controlled with statins.
DOPAMINE-RECEPTOR AGONIST
Bromocriptine, a dopamine-receptor agonist, was FDA-approved for the treatment of Parkinson disease, hyperprolactinemia, and acromegaly in the 1970s. In 2009, a quick-release formulation of bromocriptine (bromocriptine QR) was approved for treatment of type 2 DM. Table 4 lists dosing regimens, HbA1c effects, and side-effect profiles for bromocriptine.
The precise mechanism of action is unclear, but an American Association of Clinical Endocrinologists expert panel recommendation suggests that it may lower glucose levels by improving hypothalamic-mediated, postprandial insulin sensitivity via increasing morning dopaminergic activity (decreased in patients with type 2 DM) and by reducing hypothalamic adrenergic tone.38 It is not currently recommended as monotherapy, although a study of 154 patients showed monotherapy reduced HbA1c by 0.55%.39 When added to other diabetic medications, it reduced HbA1c by 0.4% to 0.7%.4,38
Bromocriptine QR is weight neutral and carries a low risk of hypoglycemia.4 A safety trial with 3,095 patients showed fewer adverse CV events in patients treated with bromocriptine QR compared with placebo, which may be secondary to reduced sympathetic tone or to reduced systemic inflammation.40 Some studies have shown reductions in blood pressure, free fatty acid levels, and triglycerides, with no change in LDL or HDL.38
Common side effects include nausea, headache, dizziness, diarrhea, and fatigue. Administration is recommended with food to reduce GI side effects. It is contraindicated in women who are nursing and those with syncopal migraines. Furthermore, it may be prudent to avoid this medication in patients with a history of psychosis, those currently treated with dopamine agonists or antagonists, or those at risk for hypotension.4
CONCLUSION
The pathophysiology of type 2 DM involves at least seven organs and tissues—the brain, liver, pancreas, intestines, kidneys, fat, and muscle—and no single medication addresses all seven of them. Most patients require more than one medication to adequately treat their diabetes, making availability and development of drugs with unique and complementary mechanisms of action of paramount importance. The medications described here—DPP-4 inhibitors, GLP-1 agonists, SGLT-2 inhibitors, colesevelam, and bromocriptine QR—provide therapeutic options with novel mechanisms of action, all while avoiding weight gain and providing a low risk of hypoglycemia. While not appropriate for every patient, these medications give healthcare providers additional options to individualize treatment and optimize care for patients.
Acknowledgments. The authors gratefully thank Julie Benke-Bennett for assistance with manuscript formatting and transcription. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association; European Association for the Study of Diabetes. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Ther Clin Risk Manag 2015; 11:621–632.
- Germino FW. Noninsulin treatment of type 2 diabetes mellitus in geriatric patients: a review. Clin Ther 2011; 12:1868–1882.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother 2015; 49:540–556.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomized, double-blind, non-inferiority trial. Lancet 2012; 380:475–483.
- White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15:668–673.
- Green JB, Bethel A, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013; 10:289–301.
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J 2011; 162:620–626.
- Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015; 36:2288–2296.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Eng J Med 2014; 370:794–797.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: injectable medications. Ann Pharmacother 2015; 49:700–714.
- Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab 2015; 6:109–134.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015; 6:19–28.
- Harris KB, McCarty DJ. Efficacy and tolerability of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2015; 6:3–18.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gludd LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analysis of randomized controlled trials. BMJ 2012; 344:d7771.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiority trial. Lancet 2014; 384:1349–1357.
- Pratley RE, Nauck MA, Barnett AH, et al; HARMONY 7 Study Group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomized, open-label, multicenter, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2:289–297.
- Neumiller JJ. Incretin-based therapies. Med Clin N Am 2015; 99:107–129.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:38–47.
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75:1272–1277.
- Defronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013; 36:3169–3176.
- Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 2012; 303:C348–C354.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:457–466.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014; 70:1149–1158.
- Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes 2015; 7:448–461.
- Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 2014; 5:124–136.
- Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context 2014; 3:212264.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Hinnen D. Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2015; 6:92–102.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Aggarwal W, Loomba RS, Arora RR. Efficacy of colesevelam on lowering glycemia and lipids. J Cardiovasc Pharmacol 2012; 59:198–205.
- Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam Hcl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 2008; 31:1479–1484.
- Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012; 12:CD009361.
- Garber AJ, Blonde L, Bloomgarden ZT, Handelsman Y, Dagogo-Jack S. The role of bromocriptine-QR in the management of type 2 diabetes expert panel recommendations. Endocr Pract 2013; 19:100–106.
- Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999; 8:1683–1707.
- Gaziano JM, Concotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association; European Association for the Study of Diabetes. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Cornell S. Continual evolution of type 2 diabetes: an update on pathophysiology and emerging treatment options. Ther Clin Risk Manag 2015; 11:621–632.
- Germino FW. Noninsulin treatment of type 2 diabetes mellitus in geriatric patients: a review. Clin Ther 2011; 12:1868–1882.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: oral medications. Ann Pharmacother 2015; 49:540–556.
- Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomized, double-blind, non-inferiority trial. Lancet 2012; 380:475–483.
- White WB, Pratley R, Fleck P, et al. Cardiovascular safety of the dipeptidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15:668–673.
- Green JB, Bethel A, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373:232–242.
- Rosenstock J, Marx N, Kahn SE, et al. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res 2013; 10:289–301.
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J 2011; 162:620–626.
- Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015; 36:2288–2296.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Eng J Med 2014; 370:794–797.
- Tran L, Zielinski A, Roach AH, et al. Pharmacologic treatment of type 2 diabetes: injectable medications. Ann Pharmacother 2015; 49:700–714.
- Tella SH, Rendell MS. Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab 2015; 6:109–134.
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015; 6:19–28.
- Harris KB, McCarty DJ. Efficacy and tolerability of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2015; 6:3–18.
- Vilsboll T, Christensen M, Junker AE, Knop FK, Gludd LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analysis of randomized controlled trials. BMJ 2012; 344:d7771.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiority trial. Lancet 2014; 384:1349–1357.
- Pratley RE, Nauck MA, Barnett AH, et al; HARMONY 7 Study Group. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomized, open-label, multicenter, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2:289–297.
- Neumiller JJ. Incretin-based therapies. Med Clin N Am 2015; 99:107–129.
- Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:38–47.
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009; 75:1272–1277.
- Defronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013; 36:3169–3176.
- Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 2012; 303:C348–C354.
- Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014; 16:457–466.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159:262–274.
- Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014; 70:1149–1158.
- Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes 2015; 7:448–461.
- Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 2014; 5:124–136.
- Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context 2014; 3:212264.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128.
- Hinnen D. Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes. Ther Adv Endocrinol Metab 2015; 6:92–102.
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015; 100:2849–2852.
- Aggarwal W, Loomba RS, Arora RR. Efficacy of colesevelam on lowering glycemia and lipids. J Cardiovasc Pharmacol 2012; 59:198–205.
- Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam Hcl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 2008; 31:1479–1484.
- Ooi CP, Loke SC. Colesevelam for type 2 diabetes mellitus. Cochrane Database Syst Rev 2012; 12:CD009361.
- Garber AJ, Blonde L, Bloomgarden ZT, Handelsman Y, Dagogo-Jack S. The role of bromocriptine-QR in the management of type 2 diabetes expert panel recommendations. Endocr Pract 2013; 19:100–106.
- Cincotta AH, Meier AH, Cincotta Jr M. Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs 1999; 8:1683–1707.
- Gaziano JM, Concotta AH, O’Connor CM, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010; 33:1503–1508.
KEY POINTS
- The US Food and Drug Administration has approved 14 noninsulin pharmaceuticals in five drug classes in the past decade for type 2 diabetes therapy.
- The noninsulin drug classes of dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, bile acid sequestrants, and dopamine-receptor agonists have different mechanisms of action and therapeutic effects.
- Successful management strategies require a balancing of multiple agents to achieve target glucose while avoiding adverse effects.
New insulin preparations: A primer for the clinician
The first isolation and successful extraction of insulin in 1921 opened an important chapter in the management of diabetes, especially for patients with profound insulin deficiency. At that time, a 14-year-old patient who was dying from type 1 diabetes received the first insulin injection—a canine pancreatic extract. It was a lifesaving treatment. Within a few months of insulin administration, the patient regained weight and health and went on to live another 13 years before succumbing to pneumonia and chronic complications of hyperglycemia.
While the introduction of regular insulin from animal extracts provided lifesaving therapy for patients with type 1 diabetes, it was the introduction of protaminated insulin in 1946 that provided more extended “basal” coverage to taper some of the large glycemic fluctuations that occurred with the administration of regular insulin two to three times daily. The use of a split-mix approach with twice-daily administration of a combination of regular insulin plus either insulin neutral protamine Hagedorn (NPH) or insulin lente provided overall better control with fewer episodes of hypoglycemia or severe hyperglycemia.
Insulin was the first protein to be sequenced (in 1955), and it became the first human protein to be manufactured through human recombinant technology. It was introduced into clinical practice in 1982 as synthetic “human” insulin, with the advantage of being less allergenic than animal insulin preparations. Human insulin eventually replaced all of the animal insulin preparations in the US market.
The pursuit of tight glycemic control as an effective strategy to prevent the chronic and devastating complications of the disease was confirmed in 1993 by publication of the Diabetes Control and Complications Trial (DCCT), which undeniably established the relationship between normalization of glycemia and prevention of microvascular complications in patients with type 1 diabetes.1 The UK Prospective Diabetes Study, demonstrating a similar relationship in type 2 diabetes, was soon to follow.2 In both trials, the follow-up observation periods further underscored the importance of early glycemic control by showing both sustained reductions in microvascular complications (retinopathy, nephropathy, and neuropathy) and statistically significant decreases in the risk of a cardiovascular event.3,4 Of note, it was the introduction in clinical practice of safer and more user-friendly insulin options that made these gains in glycemic control possible.
With the publication of the DCCT results,1 physiologic insulin replacement became the therapeutic standard in patients with advanced insulin deficiency, demonstrating that lowering hemoglobin A1c (HbA1c) and mitigating glycemic variability translated into microvascular risk reduction. The use of longer-acting insulin preparations, such as ultralente insulin, and the delivery of the basal component through continuous subcutaneous (SC) insulin infusion using an insulin pump further facilitated achievement of near-normal glycemia.
Insulin products continue to be refined and new formulations and molecular entities developed (Table 1). The following sections review the current insulin products, their pharmacologic profiles, and their clinical roles in diabetes practice.
INSULIN ANALOGUES
In 1996, the first short-acting insulin analogue (or insulin-receptor ligand), lispro, was brought to market. In lispro, the penultimate lysine and proline amino acids on the end of the C-terminal of the beta-chain of human insulin are reversed, facilitating faster absorption of the insulin through the greater availability of insulin monomers following SC depot injection.
The three short-acting insulin analogues—lispro, aspart, and glulisine—have similar pharmacokinetic and pharmacodynamic properties, with earlier onset and peak of biologic action, and shorter duration of activity than regular insulin. Potentially, these characteristics should translate into greater administration flexibility (patients can inject anywhere from 20 minutes before to 20 minutes after the start of the meal), better control of postprandial hyperglycemia, and less risk of late prandial hypoglycemia (3 to 6 hours after the meal). In a meta-analysis comparing short-acting analogues with human regular insulin, the most relevant difference reported was a lower risk of severe hypoglycemia with the analogue preparations5 (Table 2). There might be an advantage with regards to bedtime and overnight hypoglycemia when using short-acting analogues, especially if a protaminated insulin is used for overnight basal coverage.6
While short-acting analogues have been approved for administration following a meal, postprandial control is clearly better if these preparations are injected prior to the meal, ideally 15 to 20 minutes before, to allow time to enter the circulation.7 Additionally, the pharmacokinetics and biologic activity of short-acting insulin analogues appear to be very different when administered to obese, insulin-resistant patients with type 2 diabetes, in whom the onset of action is delayed and the biologic activity considerably reduced.8
INHALED INSULIN
A recent entry into the short-acting insulin marketplace—Technosphere oral-inhaled insulin (Afrezza)—was US Food and Drug Administration (FDA)-approved in 2014. Inhaled insulin has low bioavailability but is absorbed much more rapidly into the circulation than the current short-acting insulin analogues and has a shorter duration of biologic activity. However, the pharmacodynamics of inhaled insulin, when compared with insulin lispro, show only a slightly faster onset of action and a lower peak of biologic activity9 (Figure 1). Studies comparing the efficacy and safety of inhaled insulin with short-acting analogues or premix insulin have demonstrated equivalent or less effective blood glucose-lowering effect and equivalent or lower risk of hypoglycemia.10 For example, in trials of aspart insulin in patients with type 1 diabetes, inhaled insulin had statistically less reduction in HbA1c; in only one of the two trials did it show less hypoglycemia risk. Another trial comparing inhaled insulin plus basal insulin to premix aspart 70/30 showed equivalent HbA1c reductions, but less hypoglycemia with inhaled insulin.10
Inhaled insulin should not be used by smokers, patients with chronic lung disease (such as asthma and chronic obstructive pulmonary disease), and those with acute episodes of bronchospasm. In patients who have a history of or are at risk for lung cancer, the benefits of using inhaled insulin need to be carefully weighed against the potential risks, especially given the increase in lung cancer events in smokers that was observed with the prior inhaled-insulin preparation Exubera.11 Baseline and follow-up spirometry needs to be implemented for those using inhaled insulin to exclude clinically significant changes in forced expiratory volume in 1 second. Dosing of inhaled technosphere insulin is done via single-use cartridges of 4-, 8-, or 12-unit composition, making titration of smaller insulin increments more of a challenge. Patient reported outcomes in trials of inhaled insulin report variable effect (equivalent or favorable) on diabetes worries, health-related quality of life, or perceptions of insulin therapy, satisfaction, or preference.12,13
CONCENTRATED INSULIN
Concentrated insulin preparations have been available in clinical practice for many years and have been implemented with variable success. For example, Humulin R U-500 is a concentrated human regular insulin product (five times more concentrated than U-100) that has been used in patients requiring consistently high daily doses of insulin (usually > 200 U/day). Nonrandomized studies have shown significant improvement in glycemic control comparing pre- and post-intervention periods in patients switched from U-100 prandial insulin preparations to U-500 regular insulin.14 Given the slight differences in pharmacodynamic profiles between regular U-100 and U-500 insulin preparations,15 a randomized controlled trial comparing a U-500 insulin strategy with other currently available alternatives in very insulin-resistant patients will be needed to draw objective conclusions regarding the efficacy and safety of this concentrated insulin preparation.
For patients and providers who opt for a trial of regular U-500 insulin, a number of issues need to be considered to mitigate risks and optimize benefits of using this concentrated insulin. First and foremost is the frequent confusion in the communication between provider, pharmacy, and patient regarding the correct insulin dose to be administered. Because regular U-500 insulin is administered with U-100 insulin syringes, a 1-unit measure of U-500 corresponds to a 5-unit delivery of regular insulin.
To minimize confusion, regular U-500 prescriptions should be made out in volume rather than units, but this difference must be clearly explained to patients to avoid overdosing. For example, 0.01 mL of U-500 equates to 5 units of insulin, but it corresponds to the 1-unit mark of the standard U-100 insulin syringe. Newer concentrated insulin preparations on the market have avoided this confusion by providing measured doses in an insulin pen delivery system. For example, insulin lispro U-200 (Humalog U-200), a twofold concentration of insulin lispro U-100 with similar pharmacodynamics, is only available in a prefilled pen. Using insulin pen technology, a 1-unit dose of insulin actually corresponds to 1 unit of insulin, thereby removing any possible confusion regarding the prescription or administration of the correct insulin dose. Insulin lispro U-200 offers the convenience of holding more insulin per pen; it contains 600 units of insulin per pen compared with 300 units in the lispro U-100 pen.
BASAL INSULIN
Currently available basal insulin preparations include insulin NPH (Humulin N, Novolin N), insulin glargine U-100 (Lantus), insulin detemir (Levemir), and the 2015 FDA-approved formulations insulin glargine U-300 (Toujeo) and insulin degludec (Tresiba). The basal analogues introduced in the year 2000 with glargine U-100 were meant to fill the void left when the long-acting insulin ultralente animal preparations were pulled from the market in the early 1990s. The basal analogues have a longer duration of action than insulin NPH and, more importantly, have more stable and consistent biologic activity over a 24-hour period, resulting in more predictable glycemic levels and a lower risk of hypoglycemia.16–18
Three insulin analogue preparations—glargine U-300 and degludec (both FDA-approved) and pegylated lispro (currently in phase 3 trials)—have demonstrated longer protraction of biologic activity than glargine U-100, considered the current technical standard for basal insulin replacement. These three “second-generation” basal insulin analogues have pharmacodynamic activity that extends beyond 24 hours. When compared with glargine U-100 insulin, they exhibited fewer pronounced peaks of biologic activity and less pharmacokinetic variability, with similar glycemic control (as determined by HbA1c) but with an even lower risk of hypoglycemia, especially nocturnal hypoglycemia.19–21
The extended biologic activity raises concern for potential insulin stacking and subsequent hypoglycemia, which should be easily mitigated by restricting basal insulin dose adjustments to no more frequently than every 3 to 4 days, which corresponds to the time needed for these preparations to reach 90% or more of their effective steady state22 (Figure 2). Indeed, most of the clinical trials comparing these basal insulin preparations with glargine U-100 show a lower risk of hypoglycemia when basal dose adjustments are carried out weekly and no more frequently than every 3 days.23
Insulin glargine U-300 is essentially a threefold concentrated preparation of insulin glargine U-100 that results in a two-thirds volume reduction and a one-half reduction in depot surface following SC administration. The reduced depot surface area is presumed to account for much of the protracted absorption of glargine U-300 from the SC tissues. The metabolism and elimination of glargine U-300 is similar to that of the original compound, with formation of two active metabolites: M1 (the principal active moiety) and M2. Biologic steady state is achieved after 4 to 5 days of once-daily injections.24
When compared with glargine U-100 in patients with type 1 diabetes, insulin glargine U-300 at doses of 0.4 U/kg produced more stable insulin concentrations and glucose-lowering effect with a longer duration of action at steady state, as reflected by tight glucose control being maintained for about 5 hours longer (median of 30 hours).25 A meta-analysis of the EDITION I to III clinical trials in patients with type 2 diabetes at various stages of treatment found similar glucose-lowering effects for glargine U-300 compared with glargine U-100 but a lower rate of nonsevere hypoglycemia.20 Of note was the need for 10% to 15% more units of insulin for glargine U-300 in these clinical trials. Insulin glargine U-300 is available only in a 1.5 mL disposable prefilled pen, which contains 450 units of insulin. Because the dose counter on the pen window corresponds to the actual number of units of insulin to be injected, no dose recalculation is required by the patient or provider.
Insulin degludec is another ultra-long-acting basal insulin analogue with a half-life at steady state of greater than 25 hours.26 In comparison, the half-life of insulin glargine U-100 in that same study was reported as 12.1 hours. Further, insulin degludec exhibited flatter and more stable biologic activity, more evenly distributed over the course of a 24-hour period than insulin glargine U-100. The protraction mechanism is based on the formation of long strings of multihexamers, facilitated by a 16-carbon fatty acid chain linked via a glutamic acid spacer to the terminal end of the B-chain of the insulin molecule.27 In studies of patients with type 2 diabetes at various stages of treatment, insulin degludec also demonstrated lower risk of nonsevere hypoglycemia for an equivalent level of HbA1c control achieved.19
The flexibility of administration time for an ultra-long-acting insulin preparation such as degludec was tested by asking patients to alternate the injection of degludec between morning and evening, in effect creating administration intervals of up to 8 to 40 hours.28 Even within such drastic parameters, the efficacy and safety of insulin degludec were maintained when compared with insulin glargine U-100 injected at the same time of the day every day.
Because of an increase in major adverse cardiovascular events in phase 3 trials, degludec is undergoing a cardiovascular safety trial in patients with type 2 diabetes. The DEVOTE trial, which started in October 2013, will include 7,500 patients and will continue for up to 5 years. Interim results have recently been submitted to the FDA resulting in conditional approval of degludec in the US (Clinical Trials.gov Registration: NCT01959529). Degludec is available in disposable pen or cartridge format in U-100 and U-200 formulations.
COST
These new insulin preparations have introduced clinical options that have efficacy similar to that of available insulin products but, for the most part, have advantages of safety (less risk of nonsevere hypoglycemia) and patient convenience (flexibility in timing of insulin dose administration). While the latter is presumed to improve patient adherence, this has yet to be confirmed. Compared with synthetic human insulin preparations (regular insulin, NPH, and premix 70/30 insulin), which can be obtained in certain pharmacies at a discount (usually around 3 cents per unit of insulin), the currently available insulin analogues are considerably more expensive (around 16 to 27 cents per unit of insulin).
Within the guidelines for initiation and intensification of the insulin regimen using basal insulin formulations, the clinician will need to balance the potential benefits and current costs for the treatment of the individual patient. Clearly, as patients with diabetes are brought closer to their glycemic goals with insulin options, they stand to increasingly benefit from formulations that provide more consistent glycemic response and less risk of hypoglycemia. For those who are unable to afford the higher costs, especially if their glycemic control is far from the desired target, the use of synthetic human insulin formulations may be entirely appropriate. In this era of individualized care and prescriptions, clinicians have a range of insulin treatment options that will facilitate patients reaching appropriate goals.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2006; 19:CD003287.
- Gale EA. A randomized, controlled trial comparing insulin lispro with human soluble insulin in patients with type 1 diabetes on intensified insulin therapy. The UK Trial Group. Diabet Med 2000; 17:209–214.
- Cobry E, McFann K, Messer L, et al. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther 2010; 12:173–177.
- Gagnon-Auger M, du Souich P, Baillargeon JP, et al. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care 2010; 33:2502–2507.
- Afrezza (insulin human) inhalation powder [package insert]. Danbury, CT: MannKind Corp; 2014.
- Kugler AJ, Fabbio KL, Pham DQ, Nadeau DA. Inhaled technosphere insulin: a novel delivery system and formulation for the treatment of types 1 and 2 diabetes mellitus. Pharmacotherapy 2015; 35:298–314.
- Kling J. Inhaled insulin’s last gasp? Nat Biotechnol 2008; 26:479–480.
- Peyrot M, Rubin RR. Patient-reported outcomes in adults with type 2 diabetes using mealtime inhaled technosphere insulin and basal insulin versus premixed insulin. Diabetes Technol Ther 2011; 13:1201–1206.
- Testa MA, Simonson DC. Satisfaction and quality of life with premeal inhaled versus injected insulin in adolescents and adults with type 1 diabetes. Diabetes Care 2007; 30:1399–1405.
- Reutrakul S, Wroblewski K, Brown RL. Clinical use of U-500 regular insulin: review and meta-analysis. J Diabetes Sci Technol 2012; 6:412–420.
- de la Peña A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects. Diabetes Care 2011; 34:2496–2501.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004; 53:1614–1620.
- Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006; 28:1569–1581.
- Einhorn D, Handelsman Y, Bode BW, Endahl LA, Mersebach H, King AB. Patients achieving good glycemic control (HbA1c <7%) experience a lower rate of hypoglycemia with insulin degludec than with insulin glargine: a meta-analysis of phase 3a trials. Endocr Pract 2015; 21:917–926.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015; 17:859–867.
- Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once-daily LY2605541, a novel long-acting basal insulin, versus insulin glargine in basal insulin-treated patients with type 2 diabetes. Diabetes Care 2012; 35:2140–2147.
- Heise T, Meneghini LF. Insulin stacking versus therapeutic accumulation: understanding the differences. Endocr Pract 2014; 20:75–83.
- Bolli GB, Riddle MC, Bergenstal RM, et al; on behalf of the EDITION 3 study investigators. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab 2015; 17:386–394.
- Steinstraesser A, Schmidt R, Bergmann K, Dahmen R, Becker RH. Investigational new insulin glargine 300 U/ml has the same metabolism as insulin glargine 100 U/ml. Diabetes Obes Metab 2014; 16:873–876.
- Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units⋅mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1. Diabetes Care 2015; 38:637–643.
- Heise T, Hövelmann U, Nosek L, Hermanski L, Bøttcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol 2015; 11:1193–1201.
- Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res 2012; 29:2104–2114.
- Meneghini L, Atkin SL, Gough SC, et al; NN1250-3668 (BEGIN FLEX) Trial Investigators. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care 2013; 36:858–864.
The first isolation and successful extraction of insulin in 1921 opened an important chapter in the management of diabetes, especially for patients with profound insulin deficiency. At that time, a 14-year-old patient who was dying from type 1 diabetes received the first insulin injection—a canine pancreatic extract. It was a lifesaving treatment. Within a few months of insulin administration, the patient regained weight and health and went on to live another 13 years before succumbing to pneumonia and chronic complications of hyperglycemia.
While the introduction of regular insulin from animal extracts provided lifesaving therapy for patients with type 1 diabetes, it was the introduction of protaminated insulin in 1946 that provided more extended “basal” coverage to taper some of the large glycemic fluctuations that occurred with the administration of regular insulin two to three times daily. The use of a split-mix approach with twice-daily administration of a combination of regular insulin plus either insulin neutral protamine Hagedorn (NPH) or insulin lente provided overall better control with fewer episodes of hypoglycemia or severe hyperglycemia.
Insulin was the first protein to be sequenced (in 1955), and it became the first human protein to be manufactured through human recombinant technology. It was introduced into clinical practice in 1982 as synthetic “human” insulin, with the advantage of being less allergenic than animal insulin preparations. Human insulin eventually replaced all of the animal insulin preparations in the US market.
The pursuit of tight glycemic control as an effective strategy to prevent the chronic and devastating complications of the disease was confirmed in 1993 by publication of the Diabetes Control and Complications Trial (DCCT), which undeniably established the relationship between normalization of glycemia and prevention of microvascular complications in patients with type 1 diabetes.1 The UK Prospective Diabetes Study, demonstrating a similar relationship in type 2 diabetes, was soon to follow.2 In both trials, the follow-up observation periods further underscored the importance of early glycemic control by showing both sustained reductions in microvascular complications (retinopathy, nephropathy, and neuropathy) and statistically significant decreases in the risk of a cardiovascular event.3,4 Of note, it was the introduction in clinical practice of safer and more user-friendly insulin options that made these gains in glycemic control possible.
With the publication of the DCCT results,1 physiologic insulin replacement became the therapeutic standard in patients with advanced insulin deficiency, demonstrating that lowering hemoglobin A1c (HbA1c) and mitigating glycemic variability translated into microvascular risk reduction. The use of longer-acting insulin preparations, such as ultralente insulin, and the delivery of the basal component through continuous subcutaneous (SC) insulin infusion using an insulin pump further facilitated achievement of near-normal glycemia.
Insulin products continue to be refined and new formulations and molecular entities developed (Table 1). The following sections review the current insulin products, their pharmacologic profiles, and their clinical roles in diabetes practice.
INSULIN ANALOGUES
In 1996, the first short-acting insulin analogue (or insulin-receptor ligand), lispro, was brought to market. In lispro, the penultimate lysine and proline amino acids on the end of the C-terminal of the beta-chain of human insulin are reversed, facilitating faster absorption of the insulin through the greater availability of insulin monomers following SC depot injection.
The three short-acting insulin analogues—lispro, aspart, and glulisine—have similar pharmacokinetic and pharmacodynamic properties, with earlier onset and peak of biologic action, and shorter duration of activity than regular insulin. Potentially, these characteristics should translate into greater administration flexibility (patients can inject anywhere from 20 minutes before to 20 minutes after the start of the meal), better control of postprandial hyperglycemia, and less risk of late prandial hypoglycemia (3 to 6 hours after the meal). In a meta-analysis comparing short-acting analogues with human regular insulin, the most relevant difference reported was a lower risk of severe hypoglycemia with the analogue preparations5 (Table 2). There might be an advantage with regards to bedtime and overnight hypoglycemia when using short-acting analogues, especially if a protaminated insulin is used for overnight basal coverage.6
While short-acting analogues have been approved for administration following a meal, postprandial control is clearly better if these preparations are injected prior to the meal, ideally 15 to 20 minutes before, to allow time to enter the circulation.7 Additionally, the pharmacokinetics and biologic activity of short-acting insulin analogues appear to be very different when administered to obese, insulin-resistant patients with type 2 diabetes, in whom the onset of action is delayed and the biologic activity considerably reduced.8
INHALED INSULIN
A recent entry into the short-acting insulin marketplace—Technosphere oral-inhaled insulin (Afrezza)—was US Food and Drug Administration (FDA)-approved in 2014. Inhaled insulin has low bioavailability but is absorbed much more rapidly into the circulation than the current short-acting insulin analogues and has a shorter duration of biologic activity. However, the pharmacodynamics of inhaled insulin, when compared with insulin lispro, show only a slightly faster onset of action and a lower peak of biologic activity9 (Figure 1). Studies comparing the efficacy and safety of inhaled insulin with short-acting analogues or premix insulin have demonstrated equivalent or less effective blood glucose-lowering effect and equivalent or lower risk of hypoglycemia.10 For example, in trials of aspart insulin in patients with type 1 diabetes, inhaled insulin had statistically less reduction in HbA1c; in only one of the two trials did it show less hypoglycemia risk. Another trial comparing inhaled insulin plus basal insulin to premix aspart 70/30 showed equivalent HbA1c reductions, but less hypoglycemia with inhaled insulin.10
Inhaled insulin should not be used by smokers, patients with chronic lung disease (such as asthma and chronic obstructive pulmonary disease), and those with acute episodes of bronchospasm. In patients who have a history of or are at risk for lung cancer, the benefits of using inhaled insulin need to be carefully weighed against the potential risks, especially given the increase in lung cancer events in smokers that was observed with the prior inhaled-insulin preparation Exubera.11 Baseline and follow-up spirometry needs to be implemented for those using inhaled insulin to exclude clinically significant changes in forced expiratory volume in 1 second. Dosing of inhaled technosphere insulin is done via single-use cartridges of 4-, 8-, or 12-unit composition, making titration of smaller insulin increments more of a challenge. Patient reported outcomes in trials of inhaled insulin report variable effect (equivalent or favorable) on diabetes worries, health-related quality of life, or perceptions of insulin therapy, satisfaction, or preference.12,13
CONCENTRATED INSULIN
Concentrated insulin preparations have been available in clinical practice for many years and have been implemented with variable success. For example, Humulin R U-500 is a concentrated human regular insulin product (five times more concentrated than U-100) that has been used in patients requiring consistently high daily doses of insulin (usually > 200 U/day). Nonrandomized studies have shown significant improvement in glycemic control comparing pre- and post-intervention periods in patients switched from U-100 prandial insulin preparations to U-500 regular insulin.14 Given the slight differences in pharmacodynamic profiles between regular U-100 and U-500 insulin preparations,15 a randomized controlled trial comparing a U-500 insulin strategy with other currently available alternatives in very insulin-resistant patients will be needed to draw objective conclusions regarding the efficacy and safety of this concentrated insulin preparation.
For patients and providers who opt for a trial of regular U-500 insulin, a number of issues need to be considered to mitigate risks and optimize benefits of using this concentrated insulin. First and foremost is the frequent confusion in the communication between provider, pharmacy, and patient regarding the correct insulin dose to be administered. Because regular U-500 insulin is administered with U-100 insulin syringes, a 1-unit measure of U-500 corresponds to a 5-unit delivery of regular insulin.
To minimize confusion, regular U-500 prescriptions should be made out in volume rather than units, but this difference must be clearly explained to patients to avoid overdosing. For example, 0.01 mL of U-500 equates to 5 units of insulin, but it corresponds to the 1-unit mark of the standard U-100 insulin syringe. Newer concentrated insulin preparations on the market have avoided this confusion by providing measured doses in an insulin pen delivery system. For example, insulin lispro U-200 (Humalog U-200), a twofold concentration of insulin lispro U-100 with similar pharmacodynamics, is only available in a prefilled pen. Using insulin pen technology, a 1-unit dose of insulin actually corresponds to 1 unit of insulin, thereby removing any possible confusion regarding the prescription or administration of the correct insulin dose. Insulin lispro U-200 offers the convenience of holding more insulin per pen; it contains 600 units of insulin per pen compared with 300 units in the lispro U-100 pen.
BASAL INSULIN
Currently available basal insulin preparations include insulin NPH (Humulin N, Novolin N), insulin glargine U-100 (Lantus), insulin detemir (Levemir), and the 2015 FDA-approved formulations insulin glargine U-300 (Toujeo) and insulin degludec (Tresiba). The basal analogues introduced in the year 2000 with glargine U-100 were meant to fill the void left when the long-acting insulin ultralente animal preparations were pulled from the market in the early 1990s. The basal analogues have a longer duration of action than insulin NPH and, more importantly, have more stable and consistent biologic activity over a 24-hour period, resulting in more predictable glycemic levels and a lower risk of hypoglycemia.16–18
Three insulin analogue preparations—glargine U-300 and degludec (both FDA-approved) and pegylated lispro (currently in phase 3 trials)—have demonstrated longer protraction of biologic activity than glargine U-100, considered the current technical standard for basal insulin replacement. These three “second-generation” basal insulin analogues have pharmacodynamic activity that extends beyond 24 hours. When compared with glargine U-100 insulin, they exhibited fewer pronounced peaks of biologic activity and less pharmacokinetic variability, with similar glycemic control (as determined by HbA1c) but with an even lower risk of hypoglycemia, especially nocturnal hypoglycemia.19–21
The extended biologic activity raises concern for potential insulin stacking and subsequent hypoglycemia, which should be easily mitigated by restricting basal insulin dose adjustments to no more frequently than every 3 to 4 days, which corresponds to the time needed for these preparations to reach 90% or more of their effective steady state22 (Figure 2). Indeed, most of the clinical trials comparing these basal insulin preparations with glargine U-100 show a lower risk of hypoglycemia when basal dose adjustments are carried out weekly and no more frequently than every 3 days.23
Insulin glargine U-300 is essentially a threefold concentrated preparation of insulin glargine U-100 that results in a two-thirds volume reduction and a one-half reduction in depot surface following SC administration. The reduced depot surface area is presumed to account for much of the protracted absorption of glargine U-300 from the SC tissues. The metabolism and elimination of glargine U-300 is similar to that of the original compound, with formation of two active metabolites: M1 (the principal active moiety) and M2. Biologic steady state is achieved after 4 to 5 days of once-daily injections.24
When compared with glargine U-100 in patients with type 1 diabetes, insulin glargine U-300 at doses of 0.4 U/kg produced more stable insulin concentrations and glucose-lowering effect with a longer duration of action at steady state, as reflected by tight glucose control being maintained for about 5 hours longer (median of 30 hours).25 A meta-analysis of the EDITION I to III clinical trials in patients with type 2 diabetes at various stages of treatment found similar glucose-lowering effects for glargine U-300 compared with glargine U-100 but a lower rate of nonsevere hypoglycemia.20 Of note was the need for 10% to 15% more units of insulin for glargine U-300 in these clinical trials. Insulin glargine U-300 is available only in a 1.5 mL disposable prefilled pen, which contains 450 units of insulin. Because the dose counter on the pen window corresponds to the actual number of units of insulin to be injected, no dose recalculation is required by the patient or provider.
Insulin degludec is another ultra-long-acting basal insulin analogue with a half-life at steady state of greater than 25 hours.26 In comparison, the half-life of insulin glargine U-100 in that same study was reported as 12.1 hours. Further, insulin degludec exhibited flatter and more stable biologic activity, more evenly distributed over the course of a 24-hour period than insulin glargine U-100. The protraction mechanism is based on the formation of long strings of multihexamers, facilitated by a 16-carbon fatty acid chain linked via a glutamic acid spacer to the terminal end of the B-chain of the insulin molecule.27 In studies of patients with type 2 diabetes at various stages of treatment, insulin degludec also demonstrated lower risk of nonsevere hypoglycemia for an equivalent level of HbA1c control achieved.19
The flexibility of administration time for an ultra-long-acting insulin preparation such as degludec was tested by asking patients to alternate the injection of degludec between morning and evening, in effect creating administration intervals of up to 8 to 40 hours.28 Even within such drastic parameters, the efficacy and safety of insulin degludec were maintained when compared with insulin glargine U-100 injected at the same time of the day every day.
Because of an increase in major adverse cardiovascular events in phase 3 trials, degludec is undergoing a cardiovascular safety trial in patients with type 2 diabetes. The DEVOTE trial, which started in October 2013, will include 7,500 patients and will continue for up to 5 years. Interim results have recently been submitted to the FDA resulting in conditional approval of degludec in the US (Clinical Trials.gov Registration: NCT01959529). Degludec is available in disposable pen or cartridge format in U-100 and U-200 formulations.
COST
These new insulin preparations have introduced clinical options that have efficacy similar to that of available insulin products but, for the most part, have advantages of safety (less risk of nonsevere hypoglycemia) and patient convenience (flexibility in timing of insulin dose administration). While the latter is presumed to improve patient adherence, this has yet to be confirmed. Compared with synthetic human insulin preparations (regular insulin, NPH, and premix 70/30 insulin), which can be obtained in certain pharmacies at a discount (usually around 3 cents per unit of insulin), the currently available insulin analogues are considerably more expensive (around 16 to 27 cents per unit of insulin).
Within the guidelines for initiation and intensification of the insulin regimen using basal insulin formulations, the clinician will need to balance the potential benefits and current costs for the treatment of the individual patient. Clearly, as patients with diabetes are brought closer to their glycemic goals with insulin options, they stand to increasingly benefit from formulations that provide more consistent glycemic response and less risk of hypoglycemia. For those who are unable to afford the higher costs, especially if their glycemic control is far from the desired target, the use of synthetic human insulin formulations may be entirely appropriate. In this era of individualized care and prescriptions, clinicians have a range of insulin treatment options that will facilitate patients reaching appropriate goals.
The first isolation and successful extraction of insulin in 1921 opened an important chapter in the management of diabetes, especially for patients with profound insulin deficiency. At that time, a 14-year-old patient who was dying from type 1 diabetes received the first insulin injection—a canine pancreatic extract. It was a lifesaving treatment. Within a few months of insulin administration, the patient regained weight and health and went on to live another 13 years before succumbing to pneumonia and chronic complications of hyperglycemia.
While the introduction of regular insulin from animal extracts provided lifesaving therapy for patients with type 1 diabetes, it was the introduction of protaminated insulin in 1946 that provided more extended “basal” coverage to taper some of the large glycemic fluctuations that occurred with the administration of regular insulin two to three times daily. The use of a split-mix approach with twice-daily administration of a combination of regular insulin plus either insulin neutral protamine Hagedorn (NPH) or insulin lente provided overall better control with fewer episodes of hypoglycemia or severe hyperglycemia.
Insulin was the first protein to be sequenced (in 1955), and it became the first human protein to be manufactured through human recombinant technology. It was introduced into clinical practice in 1982 as synthetic “human” insulin, with the advantage of being less allergenic than animal insulin preparations. Human insulin eventually replaced all of the animal insulin preparations in the US market.
The pursuit of tight glycemic control as an effective strategy to prevent the chronic and devastating complications of the disease was confirmed in 1993 by publication of the Diabetes Control and Complications Trial (DCCT), which undeniably established the relationship between normalization of glycemia and prevention of microvascular complications in patients with type 1 diabetes.1 The UK Prospective Diabetes Study, demonstrating a similar relationship in type 2 diabetes, was soon to follow.2 In both trials, the follow-up observation periods further underscored the importance of early glycemic control by showing both sustained reductions in microvascular complications (retinopathy, nephropathy, and neuropathy) and statistically significant decreases in the risk of a cardiovascular event.3,4 Of note, it was the introduction in clinical practice of safer and more user-friendly insulin options that made these gains in glycemic control possible.
With the publication of the DCCT results,1 physiologic insulin replacement became the therapeutic standard in patients with advanced insulin deficiency, demonstrating that lowering hemoglobin A1c (HbA1c) and mitigating glycemic variability translated into microvascular risk reduction. The use of longer-acting insulin preparations, such as ultralente insulin, and the delivery of the basal component through continuous subcutaneous (SC) insulin infusion using an insulin pump further facilitated achievement of near-normal glycemia.
Insulin products continue to be refined and new formulations and molecular entities developed (Table 1). The following sections review the current insulin products, their pharmacologic profiles, and their clinical roles in diabetes practice.
INSULIN ANALOGUES
In 1996, the first short-acting insulin analogue (or insulin-receptor ligand), lispro, was brought to market. In lispro, the penultimate lysine and proline amino acids on the end of the C-terminal of the beta-chain of human insulin are reversed, facilitating faster absorption of the insulin through the greater availability of insulin monomers following SC depot injection.
The three short-acting insulin analogues—lispro, aspart, and glulisine—have similar pharmacokinetic and pharmacodynamic properties, with earlier onset and peak of biologic action, and shorter duration of activity than regular insulin. Potentially, these characteristics should translate into greater administration flexibility (patients can inject anywhere from 20 minutes before to 20 minutes after the start of the meal), better control of postprandial hyperglycemia, and less risk of late prandial hypoglycemia (3 to 6 hours after the meal). In a meta-analysis comparing short-acting analogues with human regular insulin, the most relevant difference reported was a lower risk of severe hypoglycemia with the analogue preparations5 (Table 2). There might be an advantage with regards to bedtime and overnight hypoglycemia when using short-acting analogues, especially if a protaminated insulin is used for overnight basal coverage.6
While short-acting analogues have been approved for administration following a meal, postprandial control is clearly better if these preparations are injected prior to the meal, ideally 15 to 20 minutes before, to allow time to enter the circulation.7 Additionally, the pharmacokinetics and biologic activity of short-acting insulin analogues appear to be very different when administered to obese, insulin-resistant patients with type 2 diabetes, in whom the onset of action is delayed and the biologic activity considerably reduced.8
INHALED INSULIN
A recent entry into the short-acting insulin marketplace—Technosphere oral-inhaled insulin (Afrezza)—was US Food and Drug Administration (FDA)-approved in 2014. Inhaled insulin has low bioavailability but is absorbed much more rapidly into the circulation than the current short-acting insulin analogues and has a shorter duration of biologic activity. However, the pharmacodynamics of inhaled insulin, when compared with insulin lispro, show only a slightly faster onset of action and a lower peak of biologic activity9 (Figure 1). Studies comparing the efficacy and safety of inhaled insulin with short-acting analogues or premix insulin have demonstrated equivalent or less effective blood glucose-lowering effect and equivalent or lower risk of hypoglycemia.10 For example, in trials of aspart insulin in patients with type 1 diabetes, inhaled insulin had statistically less reduction in HbA1c; in only one of the two trials did it show less hypoglycemia risk. Another trial comparing inhaled insulin plus basal insulin to premix aspart 70/30 showed equivalent HbA1c reductions, but less hypoglycemia with inhaled insulin.10
Inhaled insulin should not be used by smokers, patients with chronic lung disease (such as asthma and chronic obstructive pulmonary disease), and those with acute episodes of bronchospasm. In patients who have a history of or are at risk for lung cancer, the benefits of using inhaled insulin need to be carefully weighed against the potential risks, especially given the increase in lung cancer events in smokers that was observed with the prior inhaled-insulin preparation Exubera.11 Baseline and follow-up spirometry needs to be implemented for those using inhaled insulin to exclude clinically significant changes in forced expiratory volume in 1 second. Dosing of inhaled technosphere insulin is done via single-use cartridges of 4-, 8-, or 12-unit composition, making titration of smaller insulin increments more of a challenge. Patient reported outcomes in trials of inhaled insulin report variable effect (equivalent or favorable) on diabetes worries, health-related quality of life, or perceptions of insulin therapy, satisfaction, or preference.12,13
CONCENTRATED INSULIN
Concentrated insulin preparations have been available in clinical practice for many years and have been implemented with variable success. For example, Humulin R U-500 is a concentrated human regular insulin product (five times more concentrated than U-100) that has been used in patients requiring consistently high daily doses of insulin (usually > 200 U/day). Nonrandomized studies have shown significant improvement in glycemic control comparing pre- and post-intervention periods in patients switched from U-100 prandial insulin preparations to U-500 regular insulin.14 Given the slight differences in pharmacodynamic profiles between regular U-100 and U-500 insulin preparations,15 a randomized controlled trial comparing a U-500 insulin strategy with other currently available alternatives in very insulin-resistant patients will be needed to draw objective conclusions regarding the efficacy and safety of this concentrated insulin preparation.
For patients and providers who opt for a trial of regular U-500 insulin, a number of issues need to be considered to mitigate risks and optimize benefits of using this concentrated insulin. First and foremost is the frequent confusion in the communication between provider, pharmacy, and patient regarding the correct insulin dose to be administered. Because regular U-500 insulin is administered with U-100 insulin syringes, a 1-unit measure of U-500 corresponds to a 5-unit delivery of regular insulin.
To minimize confusion, regular U-500 prescriptions should be made out in volume rather than units, but this difference must be clearly explained to patients to avoid overdosing. For example, 0.01 mL of U-500 equates to 5 units of insulin, but it corresponds to the 1-unit mark of the standard U-100 insulin syringe. Newer concentrated insulin preparations on the market have avoided this confusion by providing measured doses in an insulin pen delivery system. For example, insulin lispro U-200 (Humalog U-200), a twofold concentration of insulin lispro U-100 with similar pharmacodynamics, is only available in a prefilled pen. Using insulin pen technology, a 1-unit dose of insulin actually corresponds to 1 unit of insulin, thereby removing any possible confusion regarding the prescription or administration of the correct insulin dose. Insulin lispro U-200 offers the convenience of holding more insulin per pen; it contains 600 units of insulin per pen compared with 300 units in the lispro U-100 pen.
BASAL INSULIN
Currently available basal insulin preparations include insulin NPH (Humulin N, Novolin N), insulin glargine U-100 (Lantus), insulin detemir (Levemir), and the 2015 FDA-approved formulations insulin glargine U-300 (Toujeo) and insulin degludec (Tresiba). The basal analogues introduced in the year 2000 with glargine U-100 were meant to fill the void left when the long-acting insulin ultralente animal preparations were pulled from the market in the early 1990s. The basal analogues have a longer duration of action than insulin NPH and, more importantly, have more stable and consistent biologic activity over a 24-hour period, resulting in more predictable glycemic levels and a lower risk of hypoglycemia.16–18
Three insulin analogue preparations—glargine U-300 and degludec (both FDA-approved) and pegylated lispro (currently in phase 3 trials)—have demonstrated longer protraction of biologic activity than glargine U-100, considered the current technical standard for basal insulin replacement. These three “second-generation” basal insulin analogues have pharmacodynamic activity that extends beyond 24 hours. When compared with glargine U-100 insulin, they exhibited fewer pronounced peaks of biologic activity and less pharmacokinetic variability, with similar glycemic control (as determined by HbA1c) but with an even lower risk of hypoglycemia, especially nocturnal hypoglycemia.19–21
The extended biologic activity raises concern for potential insulin stacking and subsequent hypoglycemia, which should be easily mitigated by restricting basal insulin dose adjustments to no more frequently than every 3 to 4 days, which corresponds to the time needed for these preparations to reach 90% or more of their effective steady state22 (Figure 2). Indeed, most of the clinical trials comparing these basal insulin preparations with glargine U-100 show a lower risk of hypoglycemia when basal dose adjustments are carried out weekly and no more frequently than every 3 days.23
Insulin glargine U-300 is essentially a threefold concentrated preparation of insulin glargine U-100 that results in a two-thirds volume reduction and a one-half reduction in depot surface following SC administration. The reduced depot surface area is presumed to account for much of the protracted absorption of glargine U-300 from the SC tissues. The metabolism and elimination of glargine U-300 is similar to that of the original compound, with formation of two active metabolites: M1 (the principal active moiety) and M2. Biologic steady state is achieved after 4 to 5 days of once-daily injections.24
When compared with glargine U-100 in patients with type 1 diabetes, insulin glargine U-300 at doses of 0.4 U/kg produced more stable insulin concentrations and glucose-lowering effect with a longer duration of action at steady state, as reflected by tight glucose control being maintained for about 5 hours longer (median of 30 hours).25 A meta-analysis of the EDITION I to III clinical trials in patients with type 2 diabetes at various stages of treatment found similar glucose-lowering effects for glargine U-300 compared with glargine U-100 but a lower rate of nonsevere hypoglycemia.20 Of note was the need for 10% to 15% more units of insulin for glargine U-300 in these clinical trials. Insulin glargine U-300 is available only in a 1.5 mL disposable prefilled pen, which contains 450 units of insulin. Because the dose counter on the pen window corresponds to the actual number of units of insulin to be injected, no dose recalculation is required by the patient or provider.
Insulin degludec is another ultra-long-acting basal insulin analogue with a half-life at steady state of greater than 25 hours.26 In comparison, the half-life of insulin glargine U-100 in that same study was reported as 12.1 hours. Further, insulin degludec exhibited flatter and more stable biologic activity, more evenly distributed over the course of a 24-hour period than insulin glargine U-100. The protraction mechanism is based on the formation of long strings of multihexamers, facilitated by a 16-carbon fatty acid chain linked via a glutamic acid spacer to the terminal end of the B-chain of the insulin molecule.27 In studies of patients with type 2 diabetes at various stages of treatment, insulin degludec also demonstrated lower risk of nonsevere hypoglycemia for an equivalent level of HbA1c control achieved.19
The flexibility of administration time for an ultra-long-acting insulin preparation such as degludec was tested by asking patients to alternate the injection of degludec between morning and evening, in effect creating administration intervals of up to 8 to 40 hours.28 Even within such drastic parameters, the efficacy and safety of insulin degludec were maintained when compared with insulin glargine U-100 injected at the same time of the day every day.
Because of an increase in major adverse cardiovascular events in phase 3 trials, degludec is undergoing a cardiovascular safety trial in patients with type 2 diabetes. The DEVOTE trial, which started in October 2013, will include 7,500 patients and will continue for up to 5 years. Interim results have recently been submitted to the FDA resulting in conditional approval of degludec in the US (Clinical Trials.gov Registration: NCT01959529). Degludec is available in disposable pen or cartridge format in U-100 and U-200 formulations.
COST
These new insulin preparations have introduced clinical options that have efficacy similar to that of available insulin products but, for the most part, have advantages of safety (less risk of nonsevere hypoglycemia) and patient convenience (flexibility in timing of insulin dose administration). While the latter is presumed to improve patient adherence, this has yet to be confirmed. Compared with synthetic human insulin preparations (regular insulin, NPH, and premix 70/30 insulin), which can be obtained in certain pharmacies at a discount (usually around 3 cents per unit of insulin), the currently available insulin analogues are considerably more expensive (around 16 to 27 cents per unit of insulin).
Within the guidelines for initiation and intensification of the insulin regimen using basal insulin formulations, the clinician will need to balance the potential benefits and current costs for the treatment of the individual patient. Clearly, as patients with diabetes are brought closer to their glycemic goals with insulin options, they stand to increasingly benefit from formulations that provide more consistent glycemic response and less risk of hypoglycemia. For those who are unable to afford the higher costs, especially if their glycemic control is far from the desired target, the use of synthetic human insulin formulations may be entirely appropriate. In this era of individualized care and prescriptions, clinicians have a range of insulin treatment options that will facilitate patients reaching appropriate goals.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2006; 19:CD003287.
- Gale EA. A randomized, controlled trial comparing insulin lispro with human soluble insulin in patients with type 1 diabetes on intensified insulin therapy. The UK Trial Group. Diabet Med 2000; 17:209–214.
- Cobry E, McFann K, Messer L, et al. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther 2010; 12:173–177.
- Gagnon-Auger M, du Souich P, Baillargeon JP, et al. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care 2010; 33:2502–2507.
- Afrezza (insulin human) inhalation powder [package insert]. Danbury, CT: MannKind Corp; 2014.
- Kugler AJ, Fabbio KL, Pham DQ, Nadeau DA. Inhaled technosphere insulin: a novel delivery system and formulation for the treatment of types 1 and 2 diabetes mellitus. Pharmacotherapy 2015; 35:298–314.
- Kling J. Inhaled insulin’s last gasp? Nat Biotechnol 2008; 26:479–480.
- Peyrot M, Rubin RR. Patient-reported outcomes in adults with type 2 diabetes using mealtime inhaled technosphere insulin and basal insulin versus premixed insulin. Diabetes Technol Ther 2011; 13:1201–1206.
- Testa MA, Simonson DC. Satisfaction and quality of life with premeal inhaled versus injected insulin in adolescents and adults with type 1 diabetes. Diabetes Care 2007; 30:1399–1405.
- Reutrakul S, Wroblewski K, Brown RL. Clinical use of U-500 regular insulin: review and meta-analysis. J Diabetes Sci Technol 2012; 6:412–420.
- de la Peña A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects. Diabetes Care 2011; 34:2496–2501.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004; 53:1614–1620.
- Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006; 28:1569–1581.
- Einhorn D, Handelsman Y, Bode BW, Endahl LA, Mersebach H, King AB. Patients achieving good glycemic control (HbA1c <7%) experience a lower rate of hypoglycemia with insulin degludec than with insulin glargine: a meta-analysis of phase 3a trials. Endocr Pract 2015; 21:917–926.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015; 17:859–867.
- Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once-daily LY2605541, a novel long-acting basal insulin, versus insulin glargine in basal insulin-treated patients with type 2 diabetes. Diabetes Care 2012; 35:2140–2147.
- Heise T, Meneghini LF. Insulin stacking versus therapeutic accumulation: understanding the differences. Endocr Pract 2014; 20:75–83.
- Bolli GB, Riddle MC, Bergenstal RM, et al; on behalf of the EDITION 3 study investigators. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab 2015; 17:386–394.
- Steinstraesser A, Schmidt R, Bergmann K, Dahmen R, Becker RH. Investigational new insulin glargine 300 U/ml has the same metabolism as insulin glargine 100 U/ml. Diabetes Obes Metab 2014; 16:873–876.
- Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units⋅mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1. Diabetes Care 2015; 38:637–643.
- Heise T, Hövelmann U, Nosek L, Hermanski L, Bøttcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol 2015; 11:1193–1201.
- Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res 2012; 29:2104–2114.
- Meneghini L, Atkin SL, Gough SC, et al; NN1250-3668 (BEGIN FLEX) Trial Investigators. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care 2013; 36:858–864.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database Syst Rev 2006; 19:CD003287.
- Gale EA. A randomized, controlled trial comparing insulin lispro with human soluble insulin in patients with type 1 diabetes on intensified insulin therapy. The UK Trial Group. Diabet Med 2000; 17:209–214.
- Cobry E, McFann K, Messer L, et al. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther 2010; 12:173–177.
- Gagnon-Auger M, du Souich P, Baillargeon JP, et al. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care 2010; 33:2502–2507.
- Afrezza (insulin human) inhalation powder [package insert]. Danbury, CT: MannKind Corp; 2014.
- Kugler AJ, Fabbio KL, Pham DQ, Nadeau DA. Inhaled technosphere insulin: a novel delivery system and formulation for the treatment of types 1 and 2 diabetes mellitus. Pharmacotherapy 2015; 35:298–314.
- Kling J. Inhaled insulin’s last gasp? Nat Biotechnol 2008; 26:479–480.
- Peyrot M, Rubin RR. Patient-reported outcomes in adults with type 2 diabetes using mealtime inhaled technosphere insulin and basal insulin versus premixed insulin. Diabetes Technol Ther 2011; 13:1201–1206.
- Testa MA, Simonson DC. Satisfaction and quality of life with premeal inhaled versus injected insulin in adolescents and adults with type 1 diabetes. Diabetes Care 2007; 30:1399–1405.
- Reutrakul S, Wroblewski K, Brown RL. Clinical use of U-500 regular insulin: review and meta-analysis. J Diabetes Sci Technol 2012; 6:412–420.
- de la Peña A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects. Diabetes Care 2011; 34:2496–2501.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004; 53:1614–1620.
- Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006; 28:1569–1581.
- Einhorn D, Handelsman Y, Bode BW, Endahl LA, Mersebach H, King AB. Patients achieving good glycemic control (HbA1c <7%) experience a lower rate of hypoglycemia with insulin degludec than with insulin glargine: a meta-analysis of phase 3a trials. Endocr Pract 2015; 21:917–926.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab 2015; 17:859–867.
- Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once-daily LY2605541, a novel long-acting basal insulin, versus insulin glargine in basal insulin-treated patients with type 2 diabetes. Diabetes Care 2012; 35:2140–2147.
- Heise T, Meneghini LF. Insulin stacking versus therapeutic accumulation: understanding the differences. Endocr Pract 2014; 20:75–83.
- Bolli GB, Riddle MC, Bergenstal RM, et al; on behalf of the EDITION 3 study investigators. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab 2015; 17:386–394.
- Steinstraesser A, Schmidt R, Bergmann K, Dahmen R, Becker RH. Investigational new insulin glargine 300 U/ml has the same metabolism as insulin glargine 100 U/ml. Diabetes Obes Metab 2014; 16:873–876.
- Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units⋅mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1. Diabetes Care 2015; 38:637–643.
- Heise T, Hövelmann U, Nosek L, Hermanski L, Bøttcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol 2015; 11:1193–1201.
- Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res 2012; 29:2104–2114.
- Meneghini L, Atkin SL, Gough SC, et al; NN1250-3668 (BEGIN FLEX) Trial Investigators. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care 2013; 36:858–864.
KEY POINTS
- Insulin extracted from an animal pancreas was first administered in 1921; the first insulin analogue was marketed in 1996.
- Insulin is considered the therapeutic standard in patients with advanced insulin deficiency.
- Types of available insulin products have differing onset, peak, and duration of action ranging from ultra-short-acting to ultra-long-acting.
- The US Food and Drug Administration approved an inhaled insulin product in 2014; all other products are administered subcutaneously.
- Concentrated insulin preparations provide an alternative for patients requiring consistently high daily doses of insulin.
Inpatient hyperglycemia management: A practical review for primary medical and surgical teams
Hyperglycemia in hospitalized patients, with or without diabetes, is associated with adverse outcomes including increased rates of infection and mortality and longer hospital length of stay.1–3 The rates of complications and mortality are even higher in hyperglycemic patients without a history of diabetes than in those with diabetes.1,2 Randomized clinical trials in critically ill and noncritically ill hyperglycemic patients demonstrate that improved glycemic control can reduce hospital complications, systemic infections, and hospitalization cost.4–6 However, intensive glycemic therapy is associated with increased risk of hypoglycemia, which is independently associated with increased morbidity and mortality in hospitalized patients. The concern about hypoglycemia has led to revised blood glucose target recommendations from professional organizations and a search for alternative treatment options.
This manuscript provides a review of updated recommendations for the management of inpatients with hyperglycemia in the critical care and general medical and surgical settings.
HYPERGLYCEMIA IN CRITICAL CARE SETTINGS
A substantial body of evidence links hyperglycemia in critically ill patients to higher rates of hospital complications, longer hospital stay, higher healthcare resource utilization, and greater hospital mortality.7,8 Although evidence from several cohort studies and randomized clinical trials suggests that tight glucose control can reduce hospital complications and mortality,9,10 this target has been difficult to achieve without increasing the risk of severe hypoglycemia. In addition, data from trials using intense glycemic control in patients in the intensive care unit (ICU) have failed to show a significant improvement in mortality and, in some instances, showed increased mortality risk associated with the therapy.11,12
The recommended target glucose levels are 140 to 180 mg/dL for most ICU patients.13 In agreement with this, the recent GLUCO-CABG trial reported no significant differences in the composite end points of complications and death between an intensive glucose target of 100 to 140 mg/dL and a conservative target of 141 to 180 mg/dL after cardiac surgery.14
HYPERGLYCEMIA IN NONCRITICAL CARE SETTINGS
In general medical and surgical patients, a strong association has been reported between hyperglycemia and prolonged hospital stay, infection, and disability after hospital discharge.1,15,16 For example, the risk of postoperative infections in patients undergoing general surgery was estimated to increase by 30% for every 40 mg/dL rise in glucose over normoglycemia (< 110 mg/dL).16 In general, appropriate glycemic control to maintain recommended glycemic levels in noncritically ill patients can reduce the risks and improve outcomes.
HYPOGLYCEMIA INCIDENCE
Hypoglycemia, defined as glucose less than 70 mg/dL, is a common complication of hyperglycemia treatment.17 Severe hypoglycemia is defined as glucose less than 40 mg/dL.18 The incidence of hypoglycemia in ICU trials ranged between 5% and 28%, depending on the intensity of glycemic control,19 and between 1% and 33% in non-ICU trials using subcutaneous (SC) insulin therapy.20 The most important hypoglycemia risk factors include older age, kidney failure, change in nutritional intake, interruption of glucose monitoring, previous insulin therapy, and failure to adjust therapy when glucose is trending down or steroid therapy is being tapered.21,22
In hospitalized patients with diabetes, hypoglycemia has been associated with poor outcomes, including a 66% increased risk of death within 1 year and 2.8 days longer hospital stay compared with patients without hypoglycemia.23 Hypoglycemia also has been associated with prolonged QT interval, ischemic electrocardiogram changes, angina, arrhythmias, and sudden death in patients with type 1 diabetes.24 Despite these observations, other studies have reported that the increased in-hospital mortality rate is limited to patients with spontaneous hypoglycemia rather than drug-associated hypoglycemia,25 raising the possibility that hypoglycemia may represent a marker of disease burden rather than be a direct cause of death.
INPATIENT ASSESSMENT OF HYPERGLYCEMIA
Clinical guidelines recommend glucose measurement in all patients admitted to the hospital.13,26 Patients with hyperglycemia (glucose > 140 mg/dL) and patients with a history of diabetes should undergo bedside point-of-care glucose testing before meals and at bedtime. Premeal testing should be done close to the time of the meal tray delivery and no longer than 1 hour before meals. For patients taking nothing by mouth or receiving continuous enteral nutrition, point-of-care testing is recommended every 4 to 6 hours.
Hemoglobin A1c (HbA1c) should be measured in patients with hyperglycemia and in those with diabetes if it has not been performed in the preceding 2 to 3 months. In hyperglycemic patients without a history of diabetes, an HbA1c of 6.5% or greater suggests that diabetes preceded hospitalization. In patients with diabetes, the HbA1c can help assess glycemic control prior to admission and tailor the treatment regimen at discharge.13,26
TARGET GLUCOSE LEVELS
Glycemic targets recommended by several organizations are shown in Table 1. For critically ill patients, most societies recommend glucose targets below 180 mg/dL, with the lower limit being anywhere from 110 to less than 150 mg/dL.
For patients in non-ICU settings, the Endocrine Society26 and the American Diabetes Association/American Association of Endocrinologists13 practice guidelines recommend premeal glucose levels below 140 mg/dL, and below 180 mg/dL if checked randomly. Higher glucose ranges (< 200 mg/dL) may be acceptable in terminally ill patients or in patients with severe comorbidities.26 Guidelines from the Joint British Diabetes Societies recommend targeting glucose levels between 108 and 180 mg/dL with an acceptable range of between 72 and 216 mg/dL.27
INPATIENT MANAGEMENT OF HYPERGLYCEMIA AND DIABETES
Insulin regimens in critical care settings
Insulin administration is the preferred way to control hyperglycemia in hospitalized patients. In critically ill patients, such as those with hypotension requiring pressor support, hyperglycemic crises, sepsis, or shock, insulin is best given via continuous intravenous (IV) infusion. The short half-life of IV insulin (< 15 minutes) allows flexibility in adjusting the infusion rate in the event of unpredicted changes in nutrition or the patient’s health. If the glucose level rises above 180 mg/dL, IV insulin infusion should be started to maintain levels below 180 mg/dL.13,26,28,29
A variety of infusion protocols have been shown to be effective in achieving glycemic control with a low rate of hypoglycemia. The ideal protocol should allow flexible rate adjustment taking into account current and previous glucose values as well as changes in infusion rate. Hourly glucose measurements until stable glycemic control is established, followed by point-of-care testing every 1 to 2 hours, is needed to assess response to therapy and prevent hypoglycemia.
Insulin regimens in noncritical care settings
For most patients in a general, non-ICU setting, SC insulin therapy with basal insulin administered once or twice daily, alone or in combination with prandial insulin, is effective and safe.13 Inhaled insulin is approved by the US Food and Drug Administration, but its use in the hospital has not been studied. The use of sliding-scale insulin is not acceptable as the single regimen in patients with diabetes, as it results in undesirable hypoglycemia and hyperglycemia.30Figure 1 presents an algorithm for selecting initial insulin treatment for patients with type 2 diabetes in the non-ICU setting.
Basal insulin prevents hyperglycemia during fasting states. Basal insulin is usually given as a once- or twice-daily long-acting insulin, such as glargine and detemir insulin. On occasion, twice-daily intermediate-acting insulin (neutral protamine Hagedorn; NPH) is used as a basal insulin.
Prandial insulin, also referred to as nutritional or bolus insulin, is given before meals as rapid-acting insulin (aspart, lispro, or glulisine) or short-acting insulin (regular) to prevent postmeal hyperglycemia. Rapid-acting insulin is preferred to regular insulin because of the faster onset and shorter duration of action, which may reduce the risk of hypoglycemia.
Correction or supplemental insulin is given to correct hyperglycemia when the glucose is above the goal. The same formulation is given together with prandial insulin.
Total daily dose of insulin is a measure that comprises basal and prandial insulin Figure 1 lists the recommended total daily dose for different clinical situations and patient populations.
Basal-bolus insulin usually refers to a regimen of long-acting basal insulin plus prandial insulin. In patients with adequate oral intake, the basal-bolus approach is preferred. The RABBIT 2 trial reported that basal-bolus regimens resulted in greater improvement in glucose control than sliding-scale regimens (correction insulin alone without basal or prandial components) in general medicine patients with type 2 diabetes.32 In general surgery patients, basal-bolus regimens significantly improved glucose control and reduced the numbers of postoperative complications, primarily wound infections compared with sliding-scale regimens.4
Multiple doses of NPH and regular insulin were compared with basal-bolus treatment with long-acting and rapid-acting insulin in two controlled trials in medical patients with type 2 diabetes.20,33 Both studies reported that treatment with NPH and regular insulin resulted in similar improvements in glycemic control and no difference in the rate of hypoglycemic events or in hospital length of stay, compared with basal-bolus insulin. Because NPH has a peak of action approximately 8 to 12 hours after injection, there is a risk of hypoglycemia in patients with poor oral intake.
In hospitalized patients who have reduced total caloric intake due to lack of appetite, acute illness, medical procedures, or surgical interventions, the Basal Plus trial34 reported that a single daily dose of glargine plus correction doses of rapid-acting insulin resulted in similar improvement in glycemic control and no difference in the frequency of hypoglycemia compared with a standard basal-bolus regimen. These results indicate that the basal-plus-correction regimen may be preferred for patients with poor or no oral intake, whereas an insulin regimen with basal, nutritional (basal-bolus), and correction components is preferred for patients with good nutritional intake.35
SC insulin dosing refers to insulin doses administered subcutaneously calculated based either on weight or on home insulin doses. For insulin-naive patients, the starting total daily dose of insulin can usually be computed as 0.4 to 0.5 U/kg/day. Higher starting doses are associated with greater odds of hypoglycemia than doses lower than 0.2 U/kg/day.36 In elderly patients and those with impaired renal function, lower initial daily doses (≤ 0.3 U/kg) may reduce the risk of hypoglycemia.26
In patients treated with insulin prior to admission, the total daily insulin dose at home can be given as half long-acting basal insulin and half prandial insulin. The dose can be reduced by 20% to 25% to prevent hypoglycemia, particularly in those with poor or uncertain caloric intake.31
Noninsulin therapies
The use of oral antidiabetic agents is generally not recommended in hospitalized patients due to the limited data available on their safety and efficacy, frequent contraindications, risk of hypoglycemia, and slow onset of action that may preclude achieving rapid glycemic control and daily dose adjustments. Table 3 lists the pros and cons of these agents in hospitalized patients.
The safety and efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the management of inpatient hyperglycemia was evaluated in a randomized pilot study in patients with type 2 diabetes treated at home with diet, oral antidiabetic agents, or a low daily insulin dose (≤ 0.4 U/kg/day).37 Patients were randomized to one of two treatments:
- Sitagliptin alone or with low-dose glargine insulin
- Basal-bolus insulin regimen plus supplemental doses of insulin lispro.
Both treatment regimens resulted in similar improvement in mean daily glucose concentrations. However, patients admitted to the hospital with glucose levels above 180 mg/dL in the sitagliptin group had higher mean daily glucose levels than patients treated with basal-bolus or sitagliptin plus glargine.
Transitioning from IV to SC insulin
When patients in critical care units are ready to be transferred to a general medical floor, appropriate transition from IV insulin to scheduled SC insulin is needed to prevent rebound hyperglycemia. This is imperative in patients with type 1 diabetes in whom just a few hours without insulin can result in diabetic ketoacidosis.
There are three general ways to calculate the SC insulin dose during the transition period. The first two methods are weight-based and based on the home dose, as previously discussed. The third method is to extrapolate from the IV insulin. A common way is to sum up the total IV insulin dose in the past 6 or 8 hours and multiply by 3 or 4, and then reduce by 20% to achieve the basal insulin dose, presuming the patient had no oral intake on the IV insulin infusion. This last method is preferred in hemodynamically stable patients with stable insulin requirements.
If long-acting insulin is chosen as basal insulin, it should be given 2 to 4 hours before discontinuation of the IV insulin infusion. Intermediate-acting insulin should be given 1 to 2 hours before IV insulin discontinuation.
SPECIFIC SITUATIONS AND POPULATIONS
Type 1 diabetes
Patients with type 1 diabetes have minimal to absent pancreatic beta cell function and rely on the exogenous administration of insulin to maintain glucose homeostasis. They have worse glycemic control and higher rates of acute kidney injury than patients with type 2 diabetes; however, the impact of inpatient glycemic control on clinical outcomes has not been determined in patients with type 1 diabetes. Insulin therapy must provide both basal and nutritional components to achieve the target goals. It is important to ask the patient directly to determine the times and doses of prescribed insulin, medication adherence, recent dietary habits (including changes in appetite), and level of physical activity. This information will be used to guide insulin therapy.
A systematic review of 16 clinical studies reported that patients who possess excellent self-management skills can be suitable for successful inpatient diabetes self-management.38 The American Diabetes Association supports patient self-management of diabetes in the hospital.39 However, the competence and readiness of each patient with type 1 diabetes need to be carefully determined in an individualized manner. Potential candidates for inpatient self-management are those with unaltered mental status, proven proficient outpatient skills (eg, carbohydrate counting, frequent glucose monitoring, strong knowledge related to the management of insulin pump or injection techniques), and who are tolerating oral intake.
Enteral nutrition and tube feeding
Accidental dislodgement of feeding tubes, temporary discontinuation of nutrition due to nausea or for diagnostic testing, and cycling of enteral nutrition with oral intake in patients with an inconsistent appetite pose unique challenges in the hospital. Although it may be tempting to give basal and nutritional requirements to these patients as a single dose of long-acting insulin, this is not recommended. Low-dose basal insulin plus scheduled doses of short-acting (regular) insulin (every 6 hours) or rapid-acting insulin (every 4 hours) with correction insulin is often used. Some providers prefer giving intermediate-acting (NPH) plus short-acting (regular) insulin every 8 hours or every 12 hours.
It is generally accepted that diabetic enteral formulas that are low in carbohydrate and high in monounsaturated fatty acids are preferable to standard high-carbohydrate formulas in hospitalized patients with diabetes. In a meta-analysis, the postprandial rise in glucose was reduced by 20 to 30 mg/dL with the low-carbohydrate high-fat formulations compared with standard formulations.40
Parenteral nutrition
The use of parenteral nutrition has been linked to aggravation of hyperglycemia independent of a history of diabetes as well as a higher risk of complications, infections, sepsis, and death.41 Regular insulin can be added to the parenteral solution at a starting dose of 0.1 U/g of dextrose in nondiabetic patients and at 0.15 U/g of dextrose in patients with diabetes.42
Alternatively, insulin can be given as a continuous IV infusion. Hemodynamically stable patients with mild to moderate hyperglycemia can be managed with basal insulin plus scheduled or as-needed doses of short-acting (regular) insulin every 6 hours. To prevent waste, it is better to underestimate the insulin added to parenteral nutrition so as to avoid having to discontinue it prematurely or add additional glucose.
Glucocorticoids
Glucocorticoids typically raise glucose starting 4 to 6 hours after administration. Low doses of glucocorticoids given in the morning tend to raise the late morning to evening glucose levels without affecting the fasting glucose. In this situation, the patient may be managed on prandial insulin without long-acting basal insulin or with intermediate-acting insulin given in the morning. Higher glucocorticoid doses may raise fasting glucose levels, in which case basal-bolus insulin would be appropriate, with the basal component comprising about 30% and the bolus about 70% of the daily dose.26
Insulin pump
Approximately 400,000 US patients with diabetes use an insulin pump.43 Successful management of inpatient diabetes with the continuation of insulin pump therapy has been previously demonstrated in select patients. Clear hospital policies, procedures, and physicians’ orders with specifics on the type of diet, frequency of point-of-care glucose testing, and insulin doses (ie, basal rates, carbohydrate ratios, and correction formulas) should be in place. An inpatient diabetes specialist should assist with the assessment and management of a patient with an insulin pump.
If pump use is contraindicated (Table 4)44 or if inpatient diabetes resources are not available, discontinuation of insulin pump and transition to a basal-bolus insulin regimen (“pump holiday”) may be the safest and most appropriate step. Most patients knowledgeable in insulin pump therapy are able to display in their pump screen the average total daily insulin used for the past few days. Based on this, safe estimations of basal, bolus, and supplemental insulin can be calculated. To avoid severe hyperglycemia or ketoacidosis from lack of basal insulin, it is important to administer the basal insulin component at least 2 hours before disconnecting the insulin pump.
Concentrated insulins
U-500 regular insulin is concentrated insulin that delivers the same amount of units in one-fifth the volume of conventional insulins, which are U-100. Whereas there are 100 units of insulin in 1 mL for conventional insulins, there are 500 units of U-500 regular insulin in 1 mL. Its onset of action is similar to that of regular insulin, and the peak and duration are similar to that of NPH insulin. Concentrated insulin is often administered in the outpatient setting to patients who are insulin resistant and require close to 200 units a day. The U-500 pen device was approved in January 2016 and was projected to be available in April 2016. For now, it can only be procured in the vial form. This causes confusion in its dosing since it is given either with the usual insulin syringes, which are designed for U-100 insulin administration, or a tuberculin syringe, which is not marked in units but in milliliters.
Because of its unique nature and providers’ lack of familiarity with U-500, certain institutions have a policy for its use. In many institutions, the doses are confirmed by pharmacy staff and delivered by pharmacy to the patient’s medication bin predrawn in a tuberculin syringe.45 In addition, a study reported that many patients on U-500 at home required significantly lower doses of insulin (average dose of 100 U/day) while hospitalized patients could be managed with conventional insulin formulations.45
There are newer concentrated insulins in the market, such as insulin glargine 300 U/mL (Toujeo) and insulin lispro 200 U/mL (Humalog). These insulins, so far, come only in the pen device form and not in vials, obviating the need for dose calculations using a U-100 insulin syringe or tuberculin syringe. The efficacy and safety of these insulin formulations have not been determined in the hospital setting.
Transitioning from home to hospital
Transition to an outpatient setting requires planning and coordination. Although insulin is used in the hospital for most patients with diabetes, many patients do not require insulin after discharge. On the other hand, diabetes regimens sometimes need intensification in other patients. One study showed that patients with acceptable diabetes control (HbA1c < 7.5%) near or on admission could be discharged on their prehospitalization treatment regimen, while those with HbA1c between 7.5% and 9% could be discharged on oral agents plus basal insulin at 50% of the hospital basal dose.46 Additionally, patients with an HbA1c of 9% to 10% should be discharged on a basal-bolus regimen or on a combination of oral agents plus basal insulin at 80% of hospital dose, with a reduction in HbA1c seen 12 weeks after discharge.
SUMMARY
Inpatient hyperglycemia is common and is associated with increased risk of hospital complications, higher healthcare resource utilization, and higher rates of in-hospital mortality. In the critically ill, IV insulin is most appropriate, with a starting threshold no higher than 180 mg/dL. Once IV insulin is started, the glucose level should be maintained between 140 and 180 mg/dL.
In noncritically ill patients, a basal-bolus regimen with basal, prandial, and correction components is preferred for patients with good nutritional intake. In contrast, a single dose of long-acting insulin plus correction insulin is preferred for patients with poor or no oral intake. Preliminary data indicate that incretin therapy has the potential to improve glycemic control in patients with mild to moderate hyperglycemia and a low risk of hypoglycemia.
Transition to an outpatient setting requires planning and coordination. Measuring HbA1c at admission is important to assess preadmission glycemic control and to tailor the treatment regimen at discharge. Patients with acceptable diabetes control could be discharged on their prehospitalization treatment regimen. Patients with suboptimal control should have more intensified therapy.
Dr. Umpierrez is supported in part by research grants from the American Diabetes Association (1-14-LLY-36), PHS grant UL1 RR025008 from the Clinical Translational Science Award Program (M01 RR-00039), and grants from the National Institutes of Health and the National Center for Research Resources. He has received unrestricted research support for inpatient studies (at Emory University) from Sanofi, Merck, Novo Nordisk, and Boehringer Ingelheim, and has received consulting fees and/or honoraria for membership on advisory boards from Novo Nordisk, Sanofi, Merck, Boehringer Ingelheim, and Regeneron.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87:978–982.
- Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009; 37:3001–3009.
- Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015; 261:97–103.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
- Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012; 97:49–58.
- Schroeder JE, Liebergall M, Raz I, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev 2012; 28:71–75.
- Clement S, Braithwaite SS, Magee MF, et al; American Diabetes Association in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27:553–591.
- Inzucchi SE. Clinical practice: Management of hyperglycemia in the hospital setting. N Engl J Med 2006; 355:1903–1911.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354:449–461.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009; 180:821–827.
- Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32:1119–1131.
- Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG Trial. Diabetes Care 2015; 38:1665–1672.
- Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA 2002; 288:2167–2169.
- Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 2008; 248:585–591.
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010; 33(suppl 1):S11–S61.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Current Opin Clin Nutr Metab Care 2010; 13:198–204.
- Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:564–569.
- Smith WD, Winterstein AG, Johns T, Rosenberg E, Sauer BC. Causes of hyperglycemia and hypoglycemia in adult inpatients. Am J Health Syst Pharm 2005; 62:714–719.
- Maynard G, Lee J, Phillips G, Fink E, Renvall M. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med 2009; 4:3–15.
- Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009; 32:1153–1157.
- Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the ‘dead in bed’ syndrome revisited. Diabetologia 2009; 52:42–45.
- Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 2011; 124:1028–1035.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012; 97:16–38.
- Dhatariya K, Levy N, Kilvert A, et al; Joint British Diabetes Societies. NHS diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 2012; 29:420–433.
- Lazar HL, McDonnell M, Chipkin SR, et al; Society of Thoracic Surgeons Blood Glucose Guideline Task Force. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87:663–669.
- Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012; 40:3251–3276.
- Hirsch IB. Sliding scale insulin—time to stop sliding. JAMA 2009; 301:213–214.
- Dobri GA, Lansang MC. How should we manage insulin therapy before surgery? Cleve Clin J Med 2013; 80:702–704.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Bueno E, Benitez A, Rufinelli JV, et al. Basal-bolus regimen with insulin analogs versus human insulin in medical patients with type 2 diabetes: a randomized controlled trial in Latin America. Endocr Pract 2015; 21:807–813.
- Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care 2013; 36:2169–2174.
- American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes 2015; 33:97–111.
- Rubin DJ, Rybin D, Doros G, McDonnell ME. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care 2011; 34:1723–1728.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013; 36:3430–3435.
- Munt R, Hutton A. Type 1 diabetes mellitus (T1DM) self management in hospital; is it possible? A literature review. Contemp Nurse 2012; 40:179–193.
- American Diabetes Association. Diabetes care in the hospital, nursing home, and skilled nursing facility. Diabetes Care 2015; 38(suppl):S80–S85.
- Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: a systematic review and meta-analysis. Diabetes Care 2005; 28:2267–2279.
- Pasquel FJ, Spiegelman R, McCauley M, et al. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care 2010; 33:739–741.
- Umpierrez GE, Spiegelman R, Zhao V, et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit Care Med 2012; 40:1792–1798.
- RNCOS Industry Research Solutions. US to dominate the global insulin pump market—April 28, 2011. www.rncos.com/Press_Releases/US-to-Dominate-the-Global-Insulin-Pump-Market.htm. Accessed March 16, 2016.
- Lansang MC, Modic MB, Sauvey R, et al. Approach to the adult hospitalized patient on an insulin pump. J Hosp Med 2013; 8:721–727.
- Tripathy PR, Lansang MC. U-500 regular insulin use in hospitalized patients. Endocr Pract 2015; 21:54–58.
- Umpierrez GE, Reyes D, Smiley D, et al. Hospital discharge algorithm based on admission HbA1c for the management of patients with type 2 diabetes. Diabetes Care 2014; 37:2934–2939.
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 154:260–267.
Hyperglycemia in hospitalized patients, with or without diabetes, is associated with adverse outcomes including increased rates of infection and mortality and longer hospital length of stay.1–3 The rates of complications and mortality are even higher in hyperglycemic patients without a history of diabetes than in those with diabetes.1,2 Randomized clinical trials in critically ill and noncritically ill hyperglycemic patients demonstrate that improved glycemic control can reduce hospital complications, systemic infections, and hospitalization cost.4–6 However, intensive glycemic therapy is associated with increased risk of hypoglycemia, which is independently associated with increased morbidity and mortality in hospitalized patients. The concern about hypoglycemia has led to revised blood glucose target recommendations from professional organizations and a search for alternative treatment options.
This manuscript provides a review of updated recommendations for the management of inpatients with hyperglycemia in the critical care and general medical and surgical settings.
HYPERGLYCEMIA IN CRITICAL CARE SETTINGS
A substantial body of evidence links hyperglycemia in critically ill patients to higher rates of hospital complications, longer hospital stay, higher healthcare resource utilization, and greater hospital mortality.7,8 Although evidence from several cohort studies and randomized clinical trials suggests that tight glucose control can reduce hospital complications and mortality,9,10 this target has been difficult to achieve without increasing the risk of severe hypoglycemia. In addition, data from trials using intense glycemic control in patients in the intensive care unit (ICU) have failed to show a significant improvement in mortality and, in some instances, showed increased mortality risk associated with the therapy.11,12
The recommended target glucose levels are 140 to 180 mg/dL for most ICU patients.13 In agreement with this, the recent GLUCO-CABG trial reported no significant differences in the composite end points of complications and death between an intensive glucose target of 100 to 140 mg/dL and a conservative target of 141 to 180 mg/dL after cardiac surgery.14
HYPERGLYCEMIA IN NONCRITICAL CARE SETTINGS
In general medical and surgical patients, a strong association has been reported between hyperglycemia and prolonged hospital stay, infection, and disability after hospital discharge.1,15,16 For example, the risk of postoperative infections in patients undergoing general surgery was estimated to increase by 30% for every 40 mg/dL rise in glucose over normoglycemia (< 110 mg/dL).16 In general, appropriate glycemic control to maintain recommended glycemic levels in noncritically ill patients can reduce the risks and improve outcomes.
HYPOGLYCEMIA INCIDENCE
Hypoglycemia, defined as glucose less than 70 mg/dL, is a common complication of hyperglycemia treatment.17 Severe hypoglycemia is defined as glucose less than 40 mg/dL.18 The incidence of hypoglycemia in ICU trials ranged between 5% and 28%, depending on the intensity of glycemic control,19 and between 1% and 33% in non-ICU trials using subcutaneous (SC) insulin therapy.20 The most important hypoglycemia risk factors include older age, kidney failure, change in nutritional intake, interruption of glucose monitoring, previous insulin therapy, and failure to adjust therapy when glucose is trending down or steroid therapy is being tapered.21,22
In hospitalized patients with diabetes, hypoglycemia has been associated with poor outcomes, including a 66% increased risk of death within 1 year and 2.8 days longer hospital stay compared with patients without hypoglycemia.23 Hypoglycemia also has been associated with prolonged QT interval, ischemic electrocardiogram changes, angina, arrhythmias, and sudden death in patients with type 1 diabetes.24 Despite these observations, other studies have reported that the increased in-hospital mortality rate is limited to patients with spontaneous hypoglycemia rather than drug-associated hypoglycemia,25 raising the possibility that hypoglycemia may represent a marker of disease burden rather than be a direct cause of death.
INPATIENT ASSESSMENT OF HYPERGLYCEMIA
Clinical guidelines recommend glucose measurement in all patients admitted to the hospital.13,26 Patients with hyperglycemia (glucose > 140 mg/dL) and patients with a history of diabetes should undergo bedside point-of-care glucose testing before meals and at bedtime. Premeal testing should be done close to the time of the meal tray delivery and no longer than 1 hour before meals. For patients taking nothing by mouth or receiving continuous enteral nutrition, point-of-care testing is recommended every 4 to 6 hours.
Hemoglobin A1c (HbA1c) should be measured in patients with hyperglycemia and in those with diabetes if it has not been performed in the preceding 2 to 3 months. In hyperglycemic patients without a history of diabetes, an HbA1c of 6.5% or greater suggests that diabetes preceded hospitalization. In patients with diabetes, the HbA1c can help assess glycemic control prior to admission and tailor the treatment regimen at discharge.13,26
TARGET GLUCOSE LEVELS
Glycemic targets recommended by several organizations are shown in Table 1. For critically ill patients, most societies recommend glucose targets below 180 mg/dL, with the lower limit being anywhere from 110 to less than 150 mg/dL.
For patients in non-ICU settings, the Endocrine Society26 and the American Diabetes Association/American Association of Endocrinologists13 practice guidelines recommend premeal glucose levels below 140 mg/dL, and below 180 mg/dL if checked randomly. Higher glucose ranges (< 200 mg/dL) may be acceptable in terminally ill patients or in patients with severe comorbidities.26 Guidelines from the Joint British Diabetes Societies recommend targeting glucose levels between 108 and 180 mg/dL with an acceptable range of between 72 and 216 mg/dL.27
INPATIENT MANAGEMENT OF HYPERGLYCEMIA AND DIABETES
Insulin regimens in critical care settings
Insulin administration is the preferred way to control hyperglycemia in hospitalized patients. In critically ill patients, such as those with hypotension requiring pressor support, hyperglycemic crises, sepsis, or shock, insulin is best given via continuous intravenous (IV) infusion. The short half-life of IV insulin (< 15 minutes) allows flexibility in adjusting the infusion rate in the event of unpredicted changes in nutrition or the patient’s health. If the glucose level rises above 180 mg/dL, IV insulin infusion should be started to maintain levels below 180 mg/dL.13,26,28,29
A variety of infusion protocols have been shown to be effective in achieving glycemic control with a low rate of hypoglycemia. The ideal protocol should allow flexible rate adjustment taking into account current and previous glucose values as well as changes in infusion rate. Hourly glucose measurements until stable glycemic control is established, followed by point-of-care testing every 1 to 2 hours, is needed to assess response to therapy and prevent hypoglycemia.
Insulin regimens in noncritical care settings
For most patients in a general, non-ICU setting, SC insulin therapy with basal insulin administered once or twice daily, alone or in combination with prandial insulin, is effective and safe.13 Inhaled insulin is approved by the US Food and Drug Administration, but its use in the hospital has not been studied. The use of sliding-scale insulin is not acceptable as the single regimen in patients with diabetes, as it results in undesirable hypoglycemia and hyperglycemia.30Figure 1 presents an algorithm for selecting initial insulin treatment for patients with type 2 diabetes in the non-ICU setting.
Basal insulin prevents hyperglycemia during fasting states. Basal insulin is usually given as a once- or twice-daily long-acting insulin, such as glargine and detemir insulin. On occasion, twice-daily intermediate-acting insulin (neutral protamine Hagedorn; NPH) is used as a basal insulin.
Prandial insulin, also referred to as nutritional or bolus insulin, is given before meals as rapid-acting insulin (aspart, lispro, or glulisine) or short-acting insulin (regular) to prevent postmeal hyperglycemia. Rapid-acting insulin is preferred to regular insulin because of the faster onset and shorter duration of action, which may reduce the risk of hypoglycemia.
Correction or supplemental insulin is given to correct hyperglycemia when the glucose is above the goal. The same formulation is given together with prandial insulin.
Total daily dose of insulin is a measure that comprises basal and prandial insulin Figure 1 lists the recommended total daily dose for different clinical situations and patient populations.
Basal-bolus insulin usually refers to a regimen of long-acting basal insulin plus prandial insulin. In patients with adequate oral intake, the basal-bolus approach is preferred. The RABBIT 2 trial reported that basal-bolus regimens resulted in greater improvement in glucose control than sliding-scale regimens (correction insulin alone without basal or prandial components) in general medicine patients with type 2 diabetes.32 In general surgery patients, basal-bolus regimens significantly improved glucose control and reduced the numbers of postoperative complications, primarily wound infections compared with sliding-scale regimens.4
Multiple doses of NPH and regular insulin were compared with basal-bolus treatment with long-acting and rapid-acting insulin in two controlled trials in medical patients with type 2 diabetes.20,33 Both studies reported that treatment with NPH and regular insulin resulted in similar improvements in glycemic control and no difference in the rate of hypoglycemic events or in hospital length of stay, compared with basal-bolus insulin. Because NPH has a peak of action approximately 8 to 12 hours after injection, there is a risk of hypoglycemia in patients with poor oral intake.
In hospitalized patients who have reduced total caloric intake due to lack of appetite, acute illness, medical procedures, or surgical interventions, the Basal Plus trial34 reported that a single daily dose of glargine plus correction doses of rapid-acting insulin resulted in similar improvement in glycemic control and no difference in the frequency of hypoglycemia compared with a standard basal-bolus regimen. These results indicate that the basal-plus-correction regimen may be preferred for patients with poor or no oral intake, whereas an insulin regimen with basal, nutritional (basal-bolus), and correction components is preferred for patients with good nutritional intake.35
SC insulin dosing refers to insulin doses administered subcutaneously calculated based either on weight or on home insulin doses. For insulin-naive patients, the starting total daily dose of insulin can usually be computed as 0.4 to 0.5 U/kg/day. Higher starting doses are associated with greater odds of hypoglycemia than doses lower than 0.2 U/kg/day.36 In elderly patients and those with impaired renal function, lower initial daily doses (≤ 0.3 U/kg) may reduce the risk of hypoglycemia.26
In patients treated with insulin prior to admission, the total daily insulin dose at home can be given as half long-acting basal insulin and half prandial insulin. The dose can be reduced by 20% to 25% to prevent hypoglycemia, particularly in those with poor or uncertain caloric intake.31
Noninsulin therapies
The use of oral antidiabetic agents is generally not recommended in hospitalized patients due to the limited data available on their safety and efficacy, frequent contraindications, risk of hypoglycemia, and slow onset of action that may preclude achieving rapid glycemic control and daily dose adjustments. Table 3 lists the pros and cons of these agents in hospitalized patients.
The safety and efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the management of inpatient hyperglycemia was evaluated in a randomized pilot study in patients with type 2 diabetes treated at home with diet, oral antidiabetic agents, or a low daily insulin dose (≤ 0.4 U/kg/day).37 Patients were randomized to one of two treatments:
- Sitagliptin alone or with low-dose glargine insulin
- Basal-bolus insulin regimen plus supplemental doses of insulin lispro.
Both treatment regimens resulted in similar improvement in mean daily glucose concentrations. However, patients admitted to the hospital with glucose levels above 180 mg/dL in the sitagliptin group had higher mean daily glucose levels than patients treated with basal-bolus or sitagliptin plus glargine.
Transitioning from IV to SC insulin
When patients in critical care units are ready to be transferred to a general medical floor, appropriate transition from IV insulin to scheduled SC insulin is needed to prevent rebound hyperglycemia. This is imperative in patients with type 1 diabetes in whom just a few hours without insulin can result in diabetic ketoacidosis.
There are three general ways to calculate the SC insulin dose during the transition period. The first two methods are weight-based and based on the home dose, as previously discussed. The third method is to extrapolate from the IV insulin. A common way is to sum up the total IV insulin dose in the past 6 or 8 hours and multiply by 3 or 4, and then reduce by 20% to achieve the basal insulin dose, presuming the patient had no oral intake on the IV insulin infusion. This last method is preferred in hemodynamically stable patients with stable insulin requirements.
If long-acting insulin is chosen as basal insulin, it should be given 2 to 4 hours before discontinuation of the IV insulin infusion. Intermediate-acting insulin should be given 1 to 2 hours before IV insulin discontinuation.
SPECIFIC SITUATIONS AND POPULATIONS
Type 1 diabetes
Patients with type 1 diabetes have minimal to absent pancreatic beta cell function and rely on the exogenous administration of insulin to maintain glucose homeostasis. They have worse glycemic control and higher rates of acute kidney injury than patients with type 2 diabetes; however, the impact of inpatient glycemic control on clinical outcomes has not been determined in patients with type 1 diabetes. Insulin therapy must provide both basal and nutritional components to achieve the target goals. It is important to ask the patient directly to determine the times and doses of prescribed insulin, medication adherence, recent dietary habits (including changes in appetite), and level of physical activity. This information will be used to guide insulin therapy.
A systematic review of 16 clinical studies reported that patients who possess excellent self-management skills can be suitable for successful inpatient diabetes self-management.38 The American Diabetes Association supports patient self-management of diabetes in the hospital.39 However, the competence and readiness of each patient with type 1 diabetes need to be carefully determined in an individualized manner. Potential candidates for inpatient self-management are those with unaltered mental status, proven proficient outpatient skills (eg, carbohydrate counting, frequent glucose monitoring, strong knowledge related to the management of insulin pump or injection techniques), and who are tolerating oral intake.
Enteral nutrition and tube feeding
Accidental dislodgement of feeding tubes, temporary discontinuation of nutrition due to nausea or for diagnostic testing, and cycling of enteral nutrition with oral intake in patients with an inconsistent appetite pose unique challenges in the hospital. Although it may be tempting to give basal and nutritional requirements to these patients as a single dose of long-acting insulin, this is not recommended. Low-dose basal insulin plus scheduled doses of short-acting (regular) insulin (every 6 hours) or rapid-acting insulin (every 4 hours) with correction insulin is often used. Some providers prefer giving intermediate-acting (NPH) plus short-acting (regular) insulin every 8 hours or every 12 hours.
It is generally accepted that diabetic enteral formulas that are low in carbohydrate and high in monounsaturated fatty acids are preferable to standard high-carbohydrate formulas in hospitalized patients with diabetes. In a meta-analysis, the postprandial rise in glucose was reduced by 20 to 30 mg/dL with the low-carbohydrate high-fat formulations compared with standard formulations.40
Parenteral nutrition
The use of parenteral nutrition has been linked to aggravation of hyperglycemia independent of a history of diabetes as well as a higher risk of complications, infections, sepsis, and death.41 Regular insulin can be added to the parenteral solution at a starting dose of 0.1 U/g of dextrose in nondiabetic patients and at 0.15 U/g of dextrose in patients with diabetes.42
Alternatively, insulin can be given as a continuous IV infusion. Hemodynamically stable patients with mild to moderate hyperglycemia can be managed with basal insulin plus scheduled or as-needed doses of short-acting (regular) insulin every 6 hours. To prevent waste, it is better to underestimate the insulin added to parenteral nutrition so as to avoid having to discontinue it prematurely or add additional glucose.
Glucocorticoids
Glucocorticoids typically raise glucose starting 4 to 6 hours after administration. Low doses of glucocorticoids given in the morning tend to raise the late morning to evening glucose levels without affecting the fasting glucose. In this situation, the patient may be managed on prandial insulin without long-acting basal insulin or with intermediate-acting insulin given in the morning. Higher glucocorticoid doses may raise fasting glucose levels, in which case basal-bolus insulin would be appropriate, with the basal component comprising about 30% and the bolus about 70% of the daily dose.26
Insulin pump
Approximately 400,000 US patients with diabetes use an insulin pump.43 Successful management of inpatient diabetes with the continuation of insulin pump therapy has been previously demonstrated in select patients. Clear hospital policies, procedures, and physicians’ orders with specifics on the type of diet, frequency of point-of-care glucose testing, and insulin doses (ie, basal rates, carbohydrate ratios, and correction formulas) should be in place. An inpatient diabetes specialist should assist with the assessment and management of a patient with an insulin pump.
If pump use is contraindicated (Table 4)44 or if inpatient diabetes resources are not available, discontinuation of insulin pump and transition to a basal-bolus insulin regimen (“pump holiday”) may be the safest and most appropriate step. Most patients knowledgeable in insulin pump therapy are able to display in their pump screen the average total daily insulin used for the past few days. Based on this, safe estimations of basal, bolus, and supplemental insulin can be calculated. To avoid severe hyperglycemia or ketoacidosis from lack of basal insulin, it is important to administer the basal insulin component at least 2 hours before disconnecting the insulin pump.
Concentrated insulins
U-500 regular insulin is concentrated insulin that delivers the same amount of units in one-fifth the volume of conventional insulins, which are U-100. Whereas there are 100 units of insulin in 1 mL for conventional insulins, there are 500 units of U-500 regular insulin in 1 mL. Its onset of action is similar to that of regular insulin, and the peak and duration are similar to that of NPH insulin. Concentrated insulin is often administered in the outpatient setting to patients who are insulin resistant and require close to 200 units a day. The U-500 pen device was approved in January 2016 and was projected to be available in April 2016. For now, it can only be procured in the vial form. This causes confusion in its dosing since it is given either with the usual insulin syringes, which are designed for U-100 insulin administration, or a tuberculin syringe, which is not marked in units but in milliliters.
Because of its unique nature and providers’ lack of familiarity with U-500, certain institutions have a policy for its use. In many institutions, the doses are confirmed by pharmacy staff and delivered by pharmacy to the patient’s medication bin predrawn in a tuberculin syringe.45 In addition, a study reported that many patients on U-500 at home required significantly lower doses of insulin (average dose of 100 U/day) while hospitalized patients could be managed with conventional insulin formulations.45
There are newer concentrated insulins in the market, such as insulin glargine 300 U/mL (Toujeo) and insulin lispro 200 U/mL (Humalog). These insulins, so far, come only in the pen device form and not in vials, obviating the need for dose calculations using a U-100 insulin syringe or tuberculin syringe. The efficacy and safety of these insulin formulations have not been determined in the hospital setting.
Transitioning from home to hospital
Transition to an outpatient setting requires planning and coordination. Although insulin is used in the hospital for most patients with diabetes, many patients do not require insulin after discharge. On the other hand, diabetes regimens sometimes need intensification in other patients. One study showed that patients with acceptable diabetes control (HbA1c < 7.5%) near or on admission could be discharged on their prehospitalization treatment regimen, while those with HbA1c between 7.5% and 9% could be discharged on oral agents plus basal insulin at 50% of the hospital basal dose.46 Additionally, patients with an HbA1c of 9% to 10% should be discharged on a basal-bolus regimen or on a combination of oral agents plus basal insulin at 80% of hospital dose, with a reduction in HbA1c seen 12 weeks after discharge.
SUMMARY
Inpatient hyperglycemia is common and is associated with increased risk of hospital complications, higher healthcare resource utilization, and higher rates of in-hospital mortality. In the critically ill, IV insulin is most appropriate, with a starting threshold no higher than 180 mg/dL. Once IV insulin is started, the glucose level should be maintained between 140 and 180 mg/dL.
In noncritically ill patients, a basal-bolus regimen with basal, prandial, and correction components is preferred for patients with good nutritional intake. In contrast, a single dose of long-acting insulin plus correction insulin is preferred for patients with poor or no oral intake. Preliminary data indicate that incretin therapy has the potential to improve glycemic control in patients with mild to moderate hyperglycemia and a low risk of hypoglycemia.
Transition to an outpatient setting requires planning and coordination. Measuring HbA1c at admission is important to assess preadmission glycemic control and to tailor the treatment regimen at discharge. Patients with acceptable diabetes control could be discharged on their prehospitalization treatment regimen. Patients with suboptimal control should have more intensified therapy.
Dr. Umpierrez is supported in part by research grants from the American Diabetes Association (1-14-LLY-36), PHS grant UL1 RR025008 from the Clinical Translational Science Award Program (M01 RR-00039), and grants from the National Institutes of Health and the National Center for Research Resources. He has received unrestricted research support for inpatient studies (at Emory University) from Sanofi, Merck, Novo Nordisk, and Boehringer Ingelheim, and has received consulting fees and/or honoraria for membership on advisory boards from Novo Nordisk, Sanofi, Merck, Boehringer Ingelheim, and Regeneron.
Hyperglycemia in hospitalized patients, with or without diabetes, is associated with adverse outcomes including increased rates of infection and mortality and longer hospital length of stay.1–3 The rates of complications and mortality are even higher in hyperglycemic patients without a history of diabetes than in those with diabetes.1,2 Randomized clinical trials in critically ill and noncritically ill hyperglycemic patients demonstrate that improved glycemic control can reduce hospital complications, systemic infections, and hospitalization cost.4–6 However, intensive glycemic therapy is associated with increased risk of hypoglycemia, which is independently associated with increased morbidity and mortality in hospitalized patients. The concern about hypoglycemia has led to revised blood glucose target recommendations from professional organizations and a search for alternative treatment options.
This manuscript provides a review of updated recommendations for the management of inpatients with hyperglycemia in the critical care and general medical and surgical settings.
HYPERGLYCEMIA IN CRITICAL CARE SETTINGS
A substantial body of evidence links hyperglycemia in critically ill patients to higher rates of hospital complications, longer hospital stay, higher healthcare resource utilization, and greater hospital mortality.7,8 Although evidence from several cohort studies and randomized clinical trials suggests that tight glucose control can reduce hospital complications and mortality,9,10 this target has been difficult to achieve without increasing the risk of severe hypoglycemia. In addition, data from trials using intense glycemic control in patients in the intensive care unit (ICU) have failed to show a significant improvement in mortality and, in some instances, showed increased mortality risk associated with the therapy.11,12
The recommended target glucose levels are 140 to 180 mg/dL for most ICU patients.13 In agreement with this, the recent GLUCO-CABG trial reported no significant differences in the composite end points of complications and death between an intensive glucose target of 100 to 140 mg/dL and a conservative target of 141 to 180 mg/dL after cardiac surgery.14
HYPERGLYCEMIA IN NONCRITICAL CARE SETTINGS
In general medical and surgical patients, a strong association has been reported between hyperglycemia and prolonged hospital stay, infection, and disability after hospital discharge.1,15,16 For example, the risk of postoperative infections in patients undergoing general surgery was estimated to increase by 30% for every 40 mg/dL rise in glucose over normoglycemia (< 110 mg/dL).16 In general, appropriate glycemic control to maintain recommended glycemic levels in noncritically ill patients can reduce the risks and improve outcomes.
HYPOGLYCEMIA INCIDENCE
Hypoglycemia, defined as glucose less than 70 mg/dL, is a common complication of hyperglycemia treatment.17 Severe hypoglycemia is defined as glucose less than 40 mg/dL.18 The incidence of hypoglycemia in ICU trials ranged between 5% and 28%, depending on the intensity of glycemic control,19 and between 1% and 33% in non-ICU trials using subcutaneous (SC) insulin therapy.20 The most important hypoglycemia risk factors include older age, kidney failure, change in nutritional intake, interruption of glucose monitoring, previous insulin therapy, and failure to adjust therapy when glucose is trending down or steroid therapy is being tapered.21,22
In hospitalized patients with diabetes, hypoglycemia has been associated with poor outcomes, including a 66% increased risk of death within 1 year and 2.8 days longer hospital stay compared with patients without hypoglycemia.23 Hypoglycemia also has been associated with prolonged QT interval, ischemic electrocardiogram changes, angina, arrhythmias, and sudden death in patients with type 1 diabetes.24 Despite these observations, other studies have reported that the increased in-hospital mortality rate is limited to patients with spontaneous hypoglycemia rather than drug-associated hypoglycemia,25 raising the possibility that hypoglycemia may represent a marker of disease burden rather than be a direct cause of death.
INPATIENT ASSESSMENT OF HYPERGLYCEMIA
Clinical guidelines recommend glucose measurement in all patients admitted to the hospital.13,26 Patients with hyperglycemia (glucose > 140 mg/dL) and patients with a history of diabetes should undergo bedside point-of-care glucose testing before meals and at bedtime. Premeal testing should be done close to the time of the meal tray delivery and no longer than 1 hour before meals. For patients taking nothing by mouth or receiving continuous enteral nutrition, point-of-care testing is recommended every 4 to 6 hours.
Hemoglobin A1c (HbA1c) should be measured in patients with hyperglycemia and in those with diabetes if it has not been performed in the preceding 2 to 3 months. In hyperglycemic patients without a history of diabetes, an HbA1c of 6.5% or greater suggests that diabetes preceded hospitalization. In patients with diabetes, the HbA1c can help assess glycemic control prior to admission and tailor the treatment regimen at discharge.13,26
TARGET GLUCOSE LEVELS
Glycemic targets recommended by several organizations are shown in Table 1. For critically ill patients, most societies recommend glucose targets below 180 mg/dL, with the lower limit being anywhere from 110 to less than 150 mg/dL.
For patients in non-ICU settings, the Endocrine Society26 and the American Diabetes Association/American Association of Endocrinologists13 practice guidelines recommend premeal glucose levels below 140 mg/dL, and below 180 mg/dL if checked randomly. Higher glucose ranges (< 200 mg/dL) may be acceptable in terminally ill patients or in patients with severe comorbidities.26 Guidelines from the Joint British Diabetes Societies recommend targeting glucose levels between 108 and 180 mg/dL with an acceptable range of between 72 and 216 mg/dL.27
INPATIENT MANAGEMENT OF HYPERGLYCEMIA AND DIABETES
Insulin regimens in critical care settings
Insulin administration is the preferred way to control hyperglycemia in hospitalized patients. In critically ill patients, such as those with hypotension requiring pressor support, hyperglycemic crises, sepsis, or shock, insulin is best given via continuous intravenous (IV) infusion. The short half-life of IV insulin (< 15 minutes) allows flexibility in adjusting the infusion rate in the event of unpredicted changes in nutrition or the patient’s health. If the glucose level rises above 180 mg/dL, IV insulin infusion should be started to maintain levels below 180 mg/dL.13,26,28,29
A variety of infusion protocols have been shown to be effective in achieving glycemic control with a low rate of hypoglycemia. The ideal protocol should allow flexible rate adjustment taking into account current and previous glucose values as well as changes in infusion rate. Hourly glucose measurements until stable glycemic control is established, followed by point-of-care testing every 1 to 2 hours, is needed to assess response to therapy and prevent hypoglycemia.
Insulin regimens in noncritical care settings
For most patients in a general, non-ICU setting, SC insulin therapy with basal insulin administered once or twice daily, alone or in combination with prandial insulin, is effective and safe.13 Inhaled insulin is approved by the US Food and Drug Administration, but its use in the hospital has not been studied. The use of sliding-scale insulin is not acceptable as the single regimen in patients with diabetes, as it results in undesirable hypoglycemia and hyperglycemia.30Figure 1 presents an algorithm for selecting initial insulin treatment for patients with type 2 diabetes in the non-ICU setting.
Basal insulin prevents hyperglycemia during fasting states. Basal insulin is usually given as a once- or twice-daily long-acting insulin, such as glargine and detemir insulin. On occasion, twice-daily intermediate-acting insulin (neutral protamine Hagedorn; NPH) is used as a basal insulin.
Prandial insulin, also referred to as nutritional or bolus insulin, is given before meals as rapid-acting insulin (aspart, lispro, or glulisine) or short-acting insulin (regular) to prevent postmeal hyperglycemia. Rapid-acting insulin is preferred to regular insulin because of the faster onset and shorter duration of action, which may reduce the risk of hypoglycemia.
Correction or supplemental insulin is given to correct hyperglycemia when the glucose is above the goal. The same formulation is given together with prandial insulin.
Total daily dose of insulin is a measure that comprises basal and prandial insulin Figure 1 lists the recommended total daily dose for different clinical situations and patient populations.
Basal-bolus insulin usually refers to a regimen of long-acting basal insulin plus prandial insulin. In patients with adequate oral intake, the basal-bolus approach is preferred. The RABBIT 2 trial reported that basal-bolus regimens resulted in greater improvement in glucose control than sliding-scale regimens (correction insulin alone without basal or prandial components) in general medicine patients with type 2 diabetes.32 In general surgery patients, basal-bolus regimens significantly improved glucose control and reduced the numbers of postoperative complications, primarily wound infections compared with sliding-scale regimens.4
Multiple doses of NPH and regular insulin were compared with basal-bolus treatment with long-acting and rapid-acting insulin in two controlled trials in medical patients with type 2 diabetes.20,33 Both studies reported that treatment with NPH and regular insulin resulted in similar improvements in glycemic control and no difference in the rate of hypoglycemic events or in hospital length of stay, compared with basal-bolus insulin. Because NPH has a peak of action approximately 8 to 12 hours after injection, there is a risk of hypoglycemia in patients with poor oral intake.
In hospitalized patients who have reduced total caloric intake due to lack of appetite, acute illness, medical procedures, or surgical interventions, the Basal Plus trial34 reported that a single daily dose of glargine plus correction doses of rapid-acting insulin resulted in similar improvement in glycemic control and no difference in the frequency of hypoglycemia compared with a standard basal-bolus regimen. These results indicate that the basal-plus-correction regimen may be preferred for patients with poor or no oral intake, whereas an insulin regimen with basal, nutritional (basal-bolus), and correction components is preferred for patients with good nutritional intake.35
SC insulin dosing refers to insulin doses administered subcutaneously calculated based either on weight or on home insulin doses. For insulin-naive patients, the starting total daily dose of insulin can usually be computed as 0.4 to 0.5 U/kg/day. Higher starting doses are associated with greater odds of hypoglycemia than doses lower than 0.2 U/kg/day.36 In elderly patients and those with impaired renal function, lower initial daily doses (≤ 0.3 U/kg) may reduce the risk of hypoglycemia.26
In patients treated with insulin prior to admission, the total daily insulin dose at home can be given as half long-acting basal insulin and half prandial insulin. The dose can be reduced by 20% to 25% to prevent hypoglycemia, particularly in those with poor or uncertain caloric intake.31
Noninsulin therapies
The use of oral antidiabetic agents is generally not recommended in hospitalized patients due to the limited data available on their safety and efficacy, frequent contraindications, risk of hypoglycemia, and slow onset of action that may preclude achieving rapid glycemic control and daily dose adjustments. Table 3 lists the pros and cons of these agents in hospitalized patients.
The safety and efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the management of inpatient hyperglycemia was evaluated in a randomized pilot study in patients with type 2 diabetes treated at home with diet, oral antidiabetic agents, or a low daily insulin dose (≤ 0.4 U/kg/day).37 Patients were randomized to one of two treatments:
- Sitagliptin alone or with low-dose glargine insulin
- Basal-bolus insulin regimen plus supplemental doses of insulin lispro.
Both treatment regimens resulted in similar improvement in mean daily glucose concentrations. However, patients admitted to the hospital with glucose levels above 180 mg/dL in the sitagliptin group had higher mean daily glucose levels than patients treated with basal-bolus or sitagliptin plus glargine.
Transitioning from IV to SC insulin
When patients in critical care units are ready to be transferred to a general medical floor, appropriate transition from IV insulin to scheduled SC insulin is needed to prevent rebound hyperglycemia. This is imperative in patients with type 1 diabetes in whom just a few hours without insulin can result in diabetic ketoacidosis.
There are three general ways to calculate the SC insulin dose during the transition period. The first two methods are weight-based and based on the home dose, as previously discussed. The third method is to extrapolate from the IV insulin. A common way is to sum up the total IV insulin dose in the past 6 or 8 hours and multiply by 3 or 4, and then reduce by 20% to achieve the basal insulin dose, presuming the patient had no oral intake on the IV insulin infusion. This last method is preferred in hemodynamically stable patients with stable insulin requirements.
If long-acting insulin is chosen as basal insulin, it should be given 2 to 4 hours before discontinuation of the IV insulin infusion. Intermediate-acting insulin should be given 1 to 2 hours before IV insulin discontinuation.
SPECIFIC SITUATIONS AND POPULATIONS
Type 1 diabetes
Patients with type 1 diabetes have minimal to absent pancreatic beta cell function and rely on the exogenous administration of insulin to maintain glucose homeostasis. They have worse glycemic control and higher rates of acute kidney injury than patients with type 2 diabetes; however, the impact of inpatient glycemic control on clinical outcomes has not been determined in patients with type 1 diabetes. Insulin therapy must provide both basal and nutritional components to achieve the target goals. It is important to ask the patient directly to determine the times and doses of prescribed insulin, medication adherence, recent dietary habits (including changes in appetite), and level of physical activity. This information will be used to guide insulin therapy.
A systematic review of 16 clinical studies reported that patients who possess excellent self-management skills can be suitable for successful inpatient diabetes self-management.38 The American Diabetes Association supports patient self-management of diabetes in the hospital.39 However, the competence and readiness of each patient with type 1 diabetes need to be carefully determined in an individualized manner. Potential candidates for inpatient self-management are those with unaltered mental status, proven proficient outpatient skills (eg, carbohydrate counting, frequent glucose monitoring, strong knowledge related to the management of insulin pump or injection techniques), and who are tolerating oral intake.
Enteral nutrition and tube feeding
Accidental dislodgement of feeding tubes, temporary discontinuation of nutrition due to nausea or for diagnostic testing, and cycling of enteral nutrition with oral intake in patients with an inconsistent appetite pose unique challenges in the hospital. Although it may be tempting to give basal and nutritional requirements to these patients as a single dose of long-acting insulin, this is not recommended. Low-dose basal insulin plus scheduled doses of short-acting (regular) insulin (every 6 hours) or rapid-acting insulin (every 4 hours) with correction insulin is often used. Some providers prefer giving intermediate-acting (NPH) plus short-acting (regular) insulin every 8 hours or every 12 hours.
It is generally accepted that diabetic enteral formulas that are low in carbohydrate and high in monounsaturated fatty acids are preferable to standard high-carbohydrate formulas in hospitalized patients with diabetes. In a meta-analysis, the postprandial rise in glucose was reduced by 20 to 30 mg/dL with the low-carbohydrate high-fat formulations compared with standard formulations.40
Parenteral nutrition
The use of parenteral nutrition has been linked to aggravation of hyperglycemia independent of a history of diabetes as well as a higher risk of complications, infections, sepsis, and death.41 Regular insulin can be added to the parenteral solution at a starting dose of 0.1 U/g of dextrose in nondiabetic patients and at 0.15 U/g of dextrose in patients with diabetes.42
Alternatively, insulin can be given as a continuous IV infusion. Hemodynamically stable patients with mild to moderate hyperglycemia can be managed with basal insulin plus scheduled or as-needed doses of short-acting (regular) insulin every 6 hours. To prevent waste, it is better to underestimate the insulin added to parenteral nutrition so as to avoid having to discontinue it prematurely or add additional glucose.
Glucocorticoids
Glucocorticoids typically raise glucose starting 4 to 6 hours after administration. Low doses of glucocorticoids given in the morning tend to raise the late morning to evening glucose levels without affecting the fasting glucose. In this situation, the patient may be managed on prandial insulin without long-acting basal insulin or with intermediate-acting insulin given in the morning. Higher glucocorticoid doses may raise fasting glucose levels, in which case basal-bolus insulin would be appropriate, with the basal component comprising about 30% and the bolus about 70% of the daily dose.26
Insulin pump
Approximately 400,000 US patients with diabetes use an insulin pump.43 Successful management of inpatient diabetes with the continuation of insulin pump therapy has been previously demonstrated in select patients. Clear hospital policies, procedures, and physicians’ orders with specifics on the type of diet, frequency of point-of-care glucose testing, and insulin doses (ie, basal rates, carbohydrate ratios, and correction formulas) should be in place. An inpatient diabetes specialist should assist with the assessment and management of a patient with an insulin pump.
If pump use is contraindicated (Table 4)44 or if inpatient diabetes resources are not available, discontinuation of insulin pump and transition to a basal-bolus insulin regimen (“pump holiday”) may be the safest and most appropriate step. Most patients knowledgeable in insulin pump therapy are able to display in their pump screen the average total daily insulin used for the past few days. Based on this, safe estimations of basal, bolus, and supplemental insulin can be calculated. To avoid severe hyperglycemia or ketoacidosis from lack of basal insulin, it is important to administer the basal insulin component at least 2 hours before disconnecting the insulin pump.
Concentrated insulins
U-500 regular insulin is concentrated insulin that delivers the same amount of units in one-fifth the volume of conventional insulins, which are U-100. Whereas there are 100 units of insulin in 1 mL for conventional insulins, there are 500 units of U-500 regular insulin in 1 mL. Its onset of action is similar to that of regular insulin, and the peak and duration are similar to that of NPH insulin. Concentrated insulin is often administered in the outpatient setting to patients who are insulin resistant and require close to 200 units a day. The U-500 pen device was approved in January 2016 and was projected to be available in April 2016. For now, it can only be procured in the vial form. This causes confusion in its dosing since it is given either with the usual insulin syringes, which are designed for U-100 insulin administration, or a tuberculin syringe, which is not marked in units but in milliliters.
Because of its unique nature and providers’ lack of familiarity with U-500, certain institutions have a policy for its use. In many institutions, the doses are confirmed by pharmacy staff and delivered by pharmacy to the patient’s medication bin predrawn in a tuberculin syringe.45 In addition, a study reported that many patients on U-500 at home required significantly lower doses of insulin (average dose of 100 U/day) while hospitalized patients could be managed with conventional insulin formulations.45
There are newer concentrated insulins in the market, such as insulin glargine 300 U/mL (Toujeo) and insulin lispro 200 U/mL (Humalog). These insulins, so far, come only in the pen device form and not in vials, obviating the need for dose calculations using a U-100 insulin syringe or tuberculin syringe. The efficacy and safety of these insulin formulations have not been determined in the hospital setting.
Transitioning from home to hospital
Transition to an outpatient setting requires planning and coordination. Although insulin is used in the hospital for most patients with diabetes, many patients do not require insulin after discharge. On the other hand, diabetes regimens sometimes need intensification in other patients. One study showed that patients with acceptable diabetes control (HbA1c < 7.5%) near or on admission could be discharged on their prehospitalization treatment regimen, while those with HbA1c between 7.5% and 9% could be discharged on oral agents plus basal insulin at 50% of the hospital basal dose.46 Additionally, patients with an HbA1c of 9% to 10% should be discharged on a basal-bolus regimen or on a combination of oral agents plus basal insulin at 80% of hospital dose, with a reduction in HbA1c seen 12 weeks after discharge.
SUMMARY
Inpatient hyperglycemia is common and is associated with increased risk of hospital complications, higher healthcare resource utilization, and higher rates of in-hospital mortality. In the critically ill, IV insulin is most appropriate, with a starting threshold no higher than 180 mg/dL. Once IV insulin is started, the glucose level should be maintained between 140 and 180 mg/dL.
In noncritically ill patients, a basal-bolus regimen with basal, prandial, and correction components is preferred for patients with good nutritional intake. In contrast, a single dose of long-acting insulin plus correction insulin is preferred for patients with poor or no oral intake. Preliminary data indicate that incretin therapy has the potential to improve glycemic control in patients with mild to moderate hyperglycemia and a low risk of hypoglycemia.
Transition to an outpatient setting requires planning and coordination. Measuring HbA1c at admission is important to assess preadmission glycemic control and to tailor the treatment regimen at discharge. Patients with acceptable diabetes control could be discharged on their prehospitalization treatment regimen. Patients with suboptimal control should have more intensified therapy.
Dr. Umpierrez is supported in part by research grants from the American Diabetes Association (1-14-LLY-36), PHS grant UL1 RR025008 from the Clinical Translational Science Award Program (M01 RR-00039), and grants from the National Institutes of Health and the National Center for Research Resources. He has received unrestricted research support for inpatient studies (at Emory University) from Sanofi, Merck, Novo Nordisk, and Boehringer Ingelheim, and has received consulting fees and/or honoraria for membership on advisory boards from Novo Nordisk, Sanofi, Merck, Boehringer Ingelheim, and Regeneron.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87:978–982.
- Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009; 37:3001–3009.
- Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015; 261:97–103.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
- Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012; 97:49–58.
- Schroeder JE, Liebergall M, Raz I, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev 2012; 28:71–75.
- Clement S, Braithwaite SS, Magee MF, et al; American Diabetes Association in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27:553–591.
- Inzucchi SE. Clinical practice: Management of hyperglycemia in the hospital setting. N Engl J Med 2006; 355:1903–1911.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354:449–461.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009; 180:821–827.
- Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32:1119–1131.
- Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG Trial. Diabetes Care 2015; 38:1665–1672.
- Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA 2002; 288:2167–2169.
- Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 2008; 248:585–591.
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010; 33(suppl 1):S11–S61.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Current Opin Clin Nutr Metab Care 2010; 13:198–204.
- Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:564–569.
- Smith WD, Winterstein AG, Johns T, Rosenberg E, Sauer BC. Causes of hyperglycemia and hypoglycemia in adult inpatients. Am J Health Syst Pharm 2005; 62:714–719.
- Maynard G, Lee J, Phillips G, Fink E, Renvall M. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med 2009; 4:3–15.
- Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009; 32:1153–1157.
- Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the ‘dead in bed’ syndrome revisited. Diabetologia 2009; 52:42–45.
- Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 2011; 124:1028–1035.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012; 97:16–38.
- Dhatariya K, Levy N, Kilvert A, et al; Joint British Diabetes Societies. NHS diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 2012; 29:420–433.
- Lazar HL, McDonnell M, Chipkin SR, et al; Society of Thoracic Surgeons Blood Glucose Guideline Task Force. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87:663–669.
- Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012; 40:3251–3276.
- Hirsch IB. Sliding scale insulin—time to stop sliding. JAMA 2009; 301:213–214.
- Dobri GA, Lansang MC. How should we manage insulin therapy before surgery? Cleve Clin J Med 2013; 80:702–704.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Bueno E, Benitez A, Rufinelli JV, et al. Basal-bolus regimen with insulin analogs versus human insulin in medical patients with type 2 diabetes: a randomized controlled trial in Latin America. Endocr Pract 2015; 21:807–813.
- Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care 2013; 36:2169–2174.
- American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes 2015; 33:97–111.
- Rubin DJ, Rybin D, Doros G, McDonnell ME. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care 2011; 34:1723–1728.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013; 36:3430–3435.
- Munt R, Hutton A. Type 1 diabetes mellitus (T1DM) self management in hospital; is it possible? A literature review. Contemp Nurse 2012; 40:179–193.
- American Diabetes Association. Diabetes care in the hospital, nursing home, and skilled nursing facility. Diabetes Care 2015; 38(suppl):S80–S85.
- Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: a systematic review and meta-analysis. Diabetes Care 2005; 28:2267–2279.
- Pasquel FJ, Spiegelman R, McCauley M, et al. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care 2010; 33:739–741.
- Umpierrez GE, Spiegelman R, Zhao V, et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit Care Med 2012; 40:1792–1798.
- RNCOS Industry Research Solutions. US to dominate the global insulin pump market—April 28, 2011. www.rncos.com/Press_Releases/US-to-Dominate-the-Global-Insulin-Pump-Market.htm. Accessed March 16, 2016.
- Lansang MC, Modic MB, Sauvey R, et al. Approach to the adult hospitalized patient on an insulin pump. J Hosp Med 2013; 8:721–727.
- Tripathy PR, Lansang MC. U-500 regular insulin use in hospitalized patients. Endocr Pract 2015; 21:54–58.
- Umpierrez GE, Reyes D, Smiley D, et al. Hospital discharge algorithm based on admission HbA1c for the management of patients with type 2 diabetes. Diabetes Care 2014; 37:2934–2939.
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 154:260–267.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87:978–982.
- Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 2009; 37:3001–3009.
- Kotagal M, Symons RG, Hirsch IB, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015; 261:97–103.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
- Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012; 97:49–58.
- Schroeder JE, Liebergall M, Raz I, et al. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev 2012; 28:71–75.
- Clement S, Braithwaite SS, Magee MF, et al; American Diabetes Association in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27:553–591.
- Inzucchi SE. Clinical practice: Management of hyperglycemia in the hospital setting. N Engl J Med 2006; 355:1903–1911.
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345:1359–1367.
- van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354:449–461.
- NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360:1283–1297.
- Griesdale DE, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009; 180:821–827.
- Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32:1119–1131.
- Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG Trial. Diabetes Care 2015; 38:1665–1672.
- Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA 2002; 288:2167–2169.
- Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 2008; 248:585–591.
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010; 33(suppl 1):S11–S61.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Current Opin Clin Nutr Metab Care 2010; 13:198–204.
- Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:564–569.
- Smith WD, Winterstein AG, Johns T, Rosenberg E, Sauer BC. Causes of hyperglycemia and hypoglycemia in adult inpatients. Am J Health Syst Pharm 2005; 62:714–719.
- Maynard G, Lee J, Phillips G, Fink E, Renvall M. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med 2009; 4:3–15.
- Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009; 32:1153–1157.
- Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the ‘dead in bed’ syndrome revisited. Diabetologia 2009; 52:42–45.
- Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 2011; 124:1028–1035.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012; 97:16–38.
- Dhatariya K, Levy N, Kilvert A, et al; Joint British Diabetes Societies. NHS diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med 2012; 29:420–433.
- Lazar HL, McDonnell M, Chipkin SR, et al; Society of Thoracic Surgeons Blood Glucose Guideline Task Force. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87:663–669.
- Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012; 40:3251–3276.
- Hirsch IB. Sliding scale insulin—time to stop sliding. JAMA 2009; 301:213–214.
- Dobri GA, Lansang MC. How should we manage insulin therapy before surgery? Cleve Clin J Med 2013; 80:702–704.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Bueno E, Benitez A, Rufinelli JV, et al. Basal-bolus regimen with insulin analogs versus human insulin in medical patients with type 2 diabetes: a randomized controlled trial in Latin America. Endocr Pract 2015; 21:807–813.
- Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care 2013; 36:2169–2174.
- American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes 2015; 33:97–111.
- Rubin DJ, Rybin D, Doros G, McDonnell ME. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care 2011; 34:1723–1728.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013; 36:3430–3435.
- Munt R, Hutton A. Type 1 diabetes mellitus (T1DM) self management in hospital; is it possible? A literature review. Contemp Nurse 2012; 40:179–193.
- American Diabetes Association. Diabetes care in the hospital, nursing home, and skilled nursing facility. Diabetes Care 2015; 38(suppl):S80–S85.
- Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: a systematic review and meta-analysis. Diabetes Care 2005; 28:2267–2279.
- Pasquel FJ, Spiegelman R, McCauley M, et al. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care 2010; 33:739–741.
- Umpierrez GE, Spiegelman R, Zhao V, et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit Care Med 2012; 40:1792–1798.
- RNCOS Industry Research Solutions. US to dominate the global insulin pump market—April 28, 2011. www.rncos.com/Press_Releases/US-to-Dominate-the-Global-Insulin-Pump-Market.htm. Accessed March 16, 2016.
- Lansang MC, Modic MB, Sauvey R, et al. Approach to the adult hospitalized patient on an insulin pump. J Hosp Med 2013; 8:721–727.
- Tripathy PR, Lansang MC. U-500 regular insulin use in hospitalized patients. Endocr Pract 2015; 21:54–58.
- Umpierrez GE, Reyes D, Smiley D, et al. Hospital discharge algorithm based on admission HbA1c for the management of patients with type 2 diabetes. Diabetes Care 2014; 37:2934–2939.
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2011; 154:260–267.
KEY POINTS
- Hyperglycemia in hospitalized patients, with or without diabetes, is associated with adverse outcomes.
- Measurement of hemoglobin A1c is recommended in all patients at hospital admission.
- Insulin administration is the preferred way to control hyperglycemia in hospitalized patients, with a starting threshold below 180 mg/dL then maintaining a level between 140 and 180 mg/dL.
Nighttime light radiance affects sleep
People who live in areas with more intense outdoor nighttime light radiance are more likely to go to bed after midnight, get fewer than 6 hours of sleep, and be dissatisfied with their sleep, compared with people who live in areas with less intense nighttime light radiance, according to research presented at the 68th Annual Meeting of the American Academy of Neurology. The researchers noted that if this association between outdoor nighttime light exposure and changes in sleep habits is confirmed by other studies, people may want to consider room darkening shades, sleep masks, or other options to reduce their exposure to nighttime light. An overview of the research is available at Neurology Reviews: http://www.neurologyreviews.com/the-publication/issue-single-view/nighttime-light-radiance-affects-sleep/d1f539419c140f8f9c536881cdb1d3d5.html.
People who live in areas with more intense outdoor nighttime light radiance are more likely to go to bed after midnight, get fewer than 6 hours of sleep, and be dissatisfied with their sleep, compared with people who live in areas with less intense nighttime light radiance, according to research presented at the 68th Annual Meeting of the American Academy of Neurology. The researchers noted that if this association between outdoor nighttime light exposure and changes in sleep habits is confirmed by other studies, people may want to consider room darkening shades, sleep masks, or other options to reduce their exposure to nighttime light. An overview of the research is available at Neurology Reviews: http://www.neurologyreviews.com/the-publication/issue-single-view/nighttime-light-radiance-affects-sleep/d1f539419c140f8f9c536881cdb1d3d5.html.
People who live in areas with more intense outdoor nighttime light radiance are more likely to go to bed after midnight, get fewer than 6 hours of sleep, and be dissatisfied with their sleep, compared with people who live in areas with less intense nighttime light radiance, according to research presented at the 68th Annual Meeting of the American Academy of Neurology. The researchers noted that if this association between outdoor nighttime light exposure and changes in sleep habits is confirmed by other studies, people may want to consider room darkening shades, sleep masks, or other options to reduce their exposure to nighttime light. An overview of the research is available at Neurology Reviews: http://www.neurologyreviews.com/the-publication/issue-single-view/nighttime-light-radiance-affects-sleep/d1f539419c140f8f9c536881cdb1d3d5.html.
Possible simeprevir/sofosbuvir-induced hepatic decompensation with acute kidney failure
In this case report, the authors describe their experience in caring for a 62-year-old man with hepatic decompensation and acute kidney injury that was caused by simeprevir/sofosbuvir initiation for chronic hepatitis C (HCV). In the article, which is available at http://www.fedprac.com/the-publication/issue-single-view/possible-simeprevirsofosbuvir-induced-hepatic-decompensation-with-acute-kidney-failure/4aa123f84bba6831d73a86fd67565a5e/ocregister.html, the authors advise that careful consideration is needed prior to the initiation of simeprevir/sofosbuvir—particularly in patients with advanced liver disease or known hepatocellular carcinoma and baseline renal impairment.
In this case report, the authors describe their experience in caring for a 62-year-old man with hepatic decompensation and acute kidney injury that was caused by simeprevir/sofosbuvir initiation for chronic hepatitis C (HCV). In the article, which is available at http://www.fedprac.com/the-publication/issue-single-view/possible-simeprevirsofosbuvir-induced-hepatic-decompensation-with-acute-kidney-failure/4aa123f84bba6831d73a86fd67565a5e/ocregister.html, the authors advise that careful consideration is needed prior to the initiation of simeprevir/sofosbuvir—particularly in patients with advanced liver disease or known hepatocellular carcinoma and baseline renal impairment.
In this case report, the authors describe their experience in caring for a 62-year-old man with hepatic decompensation and acute kidney injury that was caused by simeprevir/sofosbuvir initiation for chronic hepatitis C (HCV). In the article, which is available at http://www.fedprac.com/the-publication/issue-single-view/possible-simeprevirsofosbuvir-induced-hepatic-decompensation-with-acute-kidney-failure/4aa123f84bba6831d73a86fd67565a5e/ocregister.html, the authors advise that careful consideration is needed prior to the initiation of simeprevir/sofosbuvir—particularly in patients with advanced liver disease or known hepatocellular carcinoma and baseline renal impairment.
High-dose vitamin D improves heart structure, function in chronic heart failure
High-dose oral vitamin D supplements taken for one year significantly improved cardiac structure and function in patients with chronic heart failure (CHF) secondary to left ventricular systolic dysfunction, according to results from a new study published online in the Journal of the American College of Cardiology. However, the study, led by Dr. Klaus Witte of the University of Leeds (England), found that 6-minute walk-distance—the study’s primary outcome measure—was not improved after a year’s supplementation with vitamin D. For more on the study, go to Cardiology News: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/high-dose-vitamin-d-improves-heart-structure-function-in-chronic-heart-failure/ddee3f3aef17d20293b99a606045ef98.html.
High-dose oral vitamin D supplements taken for one year significantly improved cardiac structure and function in patients with chronic heart failure (CHF) secondary to left ventricular systolic dysfunction, according to results from a new study published online in the Journal of the American College of Cardiology. However, the study, led by Dr. Klaus Witte of the University of Leeds (England), found that 6-minute walk-distance—the study’s primary outcome measure—was not improved after a year’s supplementation with vitamin D. For more on the study, go to Cardiology News: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/high-dose-vitamin-d-improves-heart-structure-function-in-chronic-heart-failure/ddee3f3aef17d20293b99a606045ef98.html.
High-dose oral vitamin D supplements taken for one year significantly improved cardiac structure and function in patients with chronic heart failure (CHF) secondary to left ventricular systolic dysfunction, according to results from a new study published online in the Journal of the American College of Cardiology. However, the study, led by Dr. Klaus Witte of the University of Leeds (England), found that 6-minute walk-distance—the study’s primary outcome measure—was not improved after a year’s supplementation with vitamin D. For more on the study, go to Cardiology News: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/high-dose-vitamin-d-improves-heart-structure-function-in-chronic-heart-failure/ddee3f3aef17d20293b99a606045ef98.html.
Diabetes drug appears to reduce recurrent vascular events
Pioglitazone, which is used to treat type 2 diabetes, may reduce the risk of recurrent stroke and heart attacks by 24% in people who are insulin resistant but do not have diabetes, according to findings from the Insulin Resistance Intervention after Stroke (IRIS) trial. The study, supported by the National Institute of Neurological Disorders and Stroke, is the first to provide evidence that suggests a drug that targets cell metabolism may be protective against recurrent vascular events even before diabetes develops. More information on the study is available at Federal Practitioner: http://www.fedprac.com/the-publication/issue-single-view/diabetes-drug-reduces-recurrent-vascular-events/bd40f959f882ada3b88cfaa670c4fa44/ocregister.html.
Pioglitazone, which is used to treat type 2 diabetes, may reduce the risk of recurrent stroke and heart attacks by 24% in people who are insulin resistant but do not have diabetes, according to findings from the Insulin Resistance Intervention after Stroke (IRIS) trial. The study, supported by the National Institute of Neurological Disorders and Stroke, is the first to provide evidence that suggests a drug that targets cell metabolism may be protective against recurrent vascular events even before diabetes develops. More information on the study is available at Federal Practitioner: http://www.fedprac.com/the-publication/issue-single-view/diabetes-drug-reduces-recurrent-vascular-events/bd40f959f882ada3b88cfaa670c4fa44/ocregister.html.
Pioglitazone, which is used to treat type 2 diabetes, may reduce the risk of recurrent stroke and heart attacks by 24% in people who are insulin resistant but do not have diabetes, according to findings from the Insulin Resistance Intervention after Stroke (IRIS) trial. The study, supported by the National Institute of Neurological Disorders and Stroke, is the first to provide evidence that suggests a drug that targets cell metabolism may be protective against recurrent vascular events even before diabetes develops. More information on the study is available at Federal Practitioner: http://www.fedprac.com/the-publication/issue-single-view/diabetes-drug-reduces-recurrent-vascular-events/bd40f959f882ada3b88cfaa670c4fa44/ocregister.html.
Laser Best Practices for Darker Skin Types
What does your patient need to know at the first visit? Does it apply to patients of all genders and ages?
Before performing laser procedures on patients with richly pigmented skin (Fitzpatrick skin types IV–VI), patients need to be informed of the higher risk for pigmentary alterations as potential complications of the procedure. Specifically, hyperpigmentation or hypopigmentation can occur postprocedure, depending on the type of device used, the treatment settings, the technique, the underlying skin disorder being treated, and the patient’s individual response to treatment. Fortunately, these pigment alterations are in most cases self-limited but can last for weeks to months depending on the severity and the nature of the dyspigmentation.
What are your go-to treatments? What are the side effects?
Notwithstanding the higher risks for pigmentary alterations, lasers can be extremely useful for the management of numerous dermatologic concerns in patients with Fitzpatrick skin types IV to VI including laser hair removal for pseudofolliculitis barbae or nonablative fractional laser resurfacing for acne scarring and pigmentary disorders. My go-to treatments include the following: long-pulsed 1064-nm Nd:YAG laser for hair removal in Fitzpatrick skin types V to VI, 808-nm diode laser with linear scanning of hair removal in Fitzpatrick skin type IV or less, 1550-nm erbium-doped nonablative fractional laser for acne scarring in Fitzpatrick skin types IV to VI, and low-power diode 1927-nm fractional laser for melasma and postinflammatory hyperpigmentation in Fitzpatrick skin types IV to VI.
All of these procedures are performed with conservative treatment settings such as low fluences and longer pulse durations for laser hair removal and low treatment densities for fractional laser procedures. Prior to laser resurfacing, I recommend hydroquinone cream 4% twice daily starting 2 weeks before the first session and for 4 weeks posttreatment. These recommendations are based on published evidence (see Suggested Readings) as well as anecdotal experience.
How do you keep patients compliant with treatment?
Emphasizing the need for broad-spectrum sunscreen and avoidance of intense sun exposure before and after laser treatments is important during the initial consultation and prior to each treatment. I warn my patients of the higher risk for hyperpigmentation if the skin is tanned or has recently had intense sun exposure.
What do you do if they refuse treatment?
If patients refuse laser treatment or recommended precautions, then I will consider nonlaser treatment options.
What resources do you recommend to patients for more information?
I recommend patients visit the Skin of Color Society website (www.skinofcolorsociety.org).
Suggested Readings
- Alexis AF. Fractional laser resurfacing of acne scarring in patients with Fitzpatrick skin types IV-VI. J Drugs Dermatol. 2011;10(12 suppl):s6-s7.
- Alexis AF. Lasers and light-based therapies in ethnic skin: treatment options and recommendations for Fitzpatrick skin types V and VI. Br J Dermatol. 2013;169(suppl 3):91-97.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Battle EF, Hobbs LM. Laser-assisted hair removal for darker skin types. Dermatol Ther. 2004;17:177-183.
- Clark CM, Silverberg JI, Alexis AF. A retrospective chart review to assess the safety of nonablative fractional laser resurfacing in Fitzpatrick skin types IV to VI. J Drugs Dermatol. 2013;12:428-431.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
What does your patient need to know at the first visit? Does it apply to patients of all genders and ages?
Before performing laser procedures on patients with richly pigmented skin (Fitzpatrick skin types IV–VI), patients need to be informed of the higher risk for pigmentary alterations as potential complications of the procedure. Specifically, hyperpigmentation or hypopigmentation can occur postprocedure, depending on the type of device used, the treatment settings, the technique, the underlying skin disorder being treated, and the patient’s individual response to treatment. Fortunately, these pigment alterations are in most cases self-limited but can last for weeks to months depending on the severity and the nature of the dyspigmentation.
What are your go-to treatments? What are the side effects?
Notwithstanding the higher risks for pigmentary alterations, lasers can be extremely useful for the management of numerous dermatologic concerns in patients with Fitzpatrick skin types IV to VI including laser hair removal for pseudofolliculitis barbae or nonablative fractional laser resurfacing for acne scarring and pigmentary disorders. My go-to treatments include the following: long-pulsed 1064-nm Nd:YAG laser for hair removal in Fitzpatrick skin types V to VI, 808-nm diode laser with linear scanning of hair removal in Fitzpatrick skin type IV or less, 1550-nm erbium-doped nonablative fractional laser for acne scarring in Fitzpatrick skin types IV to VI, and low-power diode 1927-nm fractional laser for melasma and postinflammatory hyperpigmentation in Fitzpatrick skin types IV to VI.
All of these procedures are performed with conservative treatment settings such as low fluences and longer pulse durations for laser hair removal and low treatment densities for fractional laser procedures. Prior to laser resurfacing, I recommend hydroquinone cream 4% twice daily starting 2 weeks before the first session and for 4 weeks posttreatment. These recommendations are based on published evidence (see Suggested Readings) as well as anecdotal experience.
How do you keep patients compliant with treatment?
Emphasizing the need for broad-spectrum sunscreen and avoidance of intense sun exposure before and after laser treatments is important during the initial consultation and prior to each treatment. I warn my patients of the higher risk for hyperpigmentation if the skin is tanned or has recently had intense sun exposure.
What do you do if they refuse treatment?
If patients refuse laser treatment or recommended precautions, then I will consider nonlaser treatment options.
What resources do you recommend to patients for more information?
I recommend patients visit the Skin of Color Society website (www.skinofcolorsociety.org).
Suggested Readings
- Alexis AF. Fractional laser resurfacing of acne scarring in patients with Fitzpatrick skin types IV-VI. J Drugs Dermatol. 2011;10(12 suppl):s6-s7.
- Alexis AF. Lasers and light-based therapies in ethnic skin: treatment options and recommendations for Fitzpatrick skin types V and VI. Br J Dermatol. 2013;169(suppl 3):91-97.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Battle EF, Hobbs LM. Laser-assisted hair removal for darker skin types. Dermatol Ther. 2004;17:177-183.
- Clark CM, Silverberg JI, Alexis AF. A retrospective chart review to assess the safety of nonablative fractional laser resurfacing in Fitzpatrick skin types IV to VI. J Drugs Dermatol. 2013;12:428-431.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
What does your patient need to know at the first visit? Does it apply to patients of all genders and ages?
Before performing laser procedures on patients with richly pigmented skin (Fitzpatrick skin types IV–VI), patients need to be informed of the higher risk for pigmentary alterations as potential complications of the procedure. Specifically, hyperpigmentation or hypopigmentation can occur postprocedure, depending on the type of device used, the treatment settings, the technique, the underlying skin disorder being treated, and the patient’s individual response to treatment. Fortunately, these pigment alterations are in most cases self-limited but can last for weeks to months depending on the severity and the nature of the dyspigmentation.
What are your go-to treatments? What are the side effects?
Notwithstanding the higher risks for pigmentary alterations, lasers can be extremely useful for the management of numerous dermatologic concerns in patients with Fitzpatrick skin types IV to VI including laser hair removal for pseudofolliculitis barbae or nonablative fractional laser resurfacing for acne scarring and pigmentary disorders. My go-to treatments include the following: long-pulsed 1064-nm Nd:YAG laser for hair removal in Fitzpatrick skin types V to VI, 808-nm diode laser with linear scanning of hair removal in Fitzpatrick skin type IV or less, 1550-nm erbium-doped nonablative fractional laser for acne scarring in Fitzpatrick skin types IV to VI, and low-power diode 1927-nm fractional laser for melasma and postinflammatory hyperpigmentation in Fitzpatrick skin types IV to VI.
All of these procedures are performed with conservative treatment settings such as low fluences and longer pulse durations for laser hair removal and low treatment densities for fractional laser procedures. Prior to laser resurfacing, I recommend hydroquinone cream 4% twice daily starting 2 weeks before the first session and for 4 weeks posttreatment. These recommendations are based on published evidence (see Suggested Readings) as well as anecdotal experience.
How do you keep patients compliant with treatment?
Emphasizing the need for broad-spectrum sunscreen and avoidance of intense sun exposure before and after laser treatments is important during the initial consultation and prior to each treatment. I warn my patients of the higher risk for hyperpigmentation if the skin is tanned or has recently had intense sun exposure.
What do you do if they refuse treatment?
If patients refuse laser treatment or recommended precautions, then I will consider nonlaser treatment options.
What resources do you recommend to patients for more information?
I recommend patients visit the Skin of Color Society website (www.skinofcolorsociety.org).
Suggested Readings
- Alexis AF. Fractional laser resurfacing of acne scarring in patients with Fitzpatrick skin types IV-VI. J Drugs Dermatol. 2011;10(12 suppl):s6-s7.
- Alexis AF. Lasers and light-based therapies in ethnic skin: treatment options and recommendations for Fitzpatrick skin types V and VI. Br J Dermatol. 2013;169(suppl 3):91-97.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Battle EF, Hobbs LM. Laser-assisted hair removal for darker skin types. Dermatol Ther. 2004;17:177-183.
- Clark CM, Silverberg JI, Alexis AF. A retrospective chart review to assess the safety of nonablative fractional laser resurfacing in Fitzpatrick skin types IV to VI. J Drugs Dermatol. 2013;12:428-431.
- Ross EV, Cooke LM, Timko AL, et al. Treatment of pseudofolliculitis barbae in skin types IV, V, and VI with a long-pulsed neodymium:yttrium aluminum garnet laser. J Am Acad Dermatol. 2002;47:263-270.
Cardiovascular consequences of extreme prematurity persist into late adolescence
CHICAGO – The abnormal arterial hemodynamics identified in 11-year-olds with an extremely preterm birth persist at age 19, according to an update from the landmark longitudinal EPICure study.
“Given the implications of these significant findings, cardiovascular monitoring and risk prevention would be highly recommended for all individuals born extremely preterm,” Dr. Joanne Beckmann said in presenting the EPICure results on the long-term consequences of extreme prematurity at the annual meeting of the American College of Cardiology.
EPICure is a longitudinal study investigating health outcomes in a national cohort of babies born extremely preterm at 22-25 weeks’ gestation in the United Kingdom during 1995-1996. It is the longest such study conducted anywhere.
“Neonatal survival at the lowest gestations has improved significantly since the 1990s with the advancement in neonatal care treatments and the implementation of evidence-based practices. Therefore, long-term health outcomes following extremely preterm birth will have increasing relevance to adult physicians,” observed Dr. Beckmann of University College London.
She reported on the results of detailed cardiovascular assessments conducted in 130 extremely premature EPICure participants and 64 matched controls who made it to London for 2 days of health testing when they turned 19 years of age. The findings update the results of similar comprehensive examinations done at age 11 years.
The extremely premature birth (EP) subjects were shorter and weighed less than did the controls. The two groups had similar seated systolic and diastolic blood pressure, and cardiac index didn’t differ between the two groups. However, the EP group had significantly higher supine central systolic and diastolic blood pressure and a higher heart rate.
Moreover, the increases in aortic augmentation index – a composite of arterial stiffness and global wave reflections – and total peripheral resistance seen in the EP group at age 11 years persisted at the 19-year mark. It’s unclear whether the abnormal peripheral resistance in the EP group is structural or functional in nature. All hemodynamic differences between the two groups remained significant after adjustment for potential confounders.
Aortic pulse wave velocity was not significantly different between the two groups of 19-year-olds.
Data pertaining to other aspects of health in the 19-year-olds are now being analyzed. At the age-11 assessment, the EP group was found to have significantly impaired lung function (J Pediatr. 2012 Oct;161[4]:595-601.e2), high risk for neurodevelopmental disability (Pediatrics. 2009 Aug;124[2]:3249-57), a high rate of learning impairments, and an 18-fold increased risk of poor academic attainment compared to their matched peers (Arch Dis Child Fetal Neonatal Ed. 2009 Jul;94[4]:F283-9).
EPICure is funded by the Medical Research Council. Dr. Beckmann reported having no financial conflicts of interest.
CHICAGO – The abnormal arterial hemodynamics identified in 11-year-olds with an extremely preterm birth persist at age 19, according to an update from the landmark longitudinal EPICure study.
“Given the implications of these significant findings, cardiovascular monitoring and risk prevention would be highly recommended for all individuals born extremely preterm,” Dr. Joanne Beckmann said in presenting the EPICure results on the long-term consequences of extreme prematurity at the annual meeting of the American College of Cardiology.
EPICure is a longitudinal study investigating health outcomes in a national cohort of babies born extremely preterm at 22-25 weeks’ gestation in the United Kingdom during 1995-1996. It is the longest such study conducted anywhere.
“Neonatal survival at the lowest gestations has improved significantly since the 1990s with the advancement in neonatal care treatments and the implementation of evidence-based practices. Therefore, long-term health outcomes following extremely preterm birth will have increasing relevance to adult physicians,” observed Dr. Beckmann of University College London.
She reported on the results of detailed cardiovascular assessments conducted in 130 extremely premature EPICure participants and 64 matched controls who made it to London for 2 days of health testing when they turned 19 years of age. The findings update the results of similar comprehensive examinations done at age 11 years.
The extremely premature birth (EP) subjects were shorter and weighed less than did the controls. The two groups had similar seated systolic and diastolic blood pressure, and cardiac index didn’t differ between the two groups. However, the EP group had significantly higher supine central systolic and diastolic blood pressure and a higher heart rate.

Moreover, the increases in aortic augmentation index – a composite of arterial stiffness and global wave reflections – and total peripheral resistance seen in the EP group at age 11 years persisted at the 19-year mark. It’s unclear whether the abnormal peripheral resistance in the EP group is structural or functional in nature. All hemodynamic differences between the two groups remained significant after adjustment for potential confounders.
Aortic pulse wave velocity was not significantly different between the two groups of 19-year-olds.
Data pertaining to other aspects of health in the 19-year-olds are now being analyzed. At the age-11 assessment, the EP group was found to have significantly impaired lung function (J Pediatr. 2012 Oct;161[4]:595-601.e2), high risk for neurodevelopmental disability (Pediatrics. 2009 Aug;124[2]:3249-57), a high rate of learning impairments, and an 18-fold increased risk of poor academic attainment compared to their matched peers (Arch Dis Child Fetal Neonatal Ed. 2009 Jul;94[4]:F283-9).
EPICure is funded by the Medical Research Council. Dr. Beckmann reported having no financial conflicts of interest.
CHICAGO – The abnormal arterial hemodynamics identified in 11-year-olds with an extremely preterm birth persist at age 19, according to an update from the landmark longitudinal EPICure study.
“Given the implications of these significant findings, cardiovascular monitoring and risk prevention would be highly recommended for all individuals born extremely preterm,” Dr. Joanne Beckmann said in presenting the EPICure results on the long-term consequences of extreme prematurity at the annual meeting of the American College of Cardiology.
EPICure is a longitudinal study investigating health outcomes in a national cohort of babies born extremely preterm at 22-25 weeks’ gestation in the United Kingdom during 1995-1996. It is the longest such study conducted anywhere.
“Neonatal survival at the lowest gestations has improved significantly since the 1990s with the advancement in neonatal care treatments and the implementation of evidence-based practices. Therefore, long-term health outcomes following extremely preterm birth will have increasing relevance to adult physicians,” observed Dr. Beckmann of University College London.
She reported on the results of detailed cardiovascular assessments conducted in 130 extremely premature EPICure participants and 64 matched controls who made it to London for 2 days of health testing when they turned 19 years of age. The findings update the results of similar comprehensive examinations done at age 11 years.
The extremely premature birth (EP) subjects were shorter and weighed less than did the controls. The two groups had similar seated systolic and diastolic blood pressure, and cardiac index didn’t differ between the two groups. However, the EP group had significantly higher supine central systolic and diastolic blood pressure and a higher heart rate.

Moreover, the increases in aortic augmentation index – a composite of arterial stiffness and global wave reflections – and total peripheral resistance seen in the EP group at age 11 years persisted at the 19-year mark. It’s unclear whether the abnormal peripheral resistance in the EP group is structural or functional in nature. All hemodynamic differences between the two groups remained significant after adjustment for potential confounders.
Aortic pulse wave velocity was not significantly different between the two groups of 19-year-olds.
Data pertaining to other aspects of health in the 19-year-olds are now being analyzed. At the age-11 assessment, the EP group was found to have significantly impaired lung function (J Pediatr. 2012 Oct;161[4]:595-601.e2), high risk for neurodevelopmental disability (Pediatrics. 2009 Aug;124[2]:3249-57), a high rate of learning impairments, and an 18-fold increased risk of poor academic attainment compared to their matched peers (Arch Dis Child Fetal Neonatal Ed. 2009 Jul;94[4]:F283-9).
EPICure is funded by the Medical Research Council. Dr. Beckmann reported having no financial conflicts of interest.
AT ACC 16
Key clinical point: At age 19 years, persons born extremely premature still show significant abnormalities in arterial hemodynamics and peripheral resistance.
Major finding: The adjusted aortic augmentation index was 6.6% in 19-year-olds born at 22-25 weeks gestation compared with 0.3% in matched controls.
Data source: EPICure, a longitudinal study of health outcomes in a national cohort of babies born extremely preterm at 22-25 weeks gestation in the United Kingdom during 1995-1996.
Disclosures: EPICure is funded by the Medical Research Council. The presenter reported having no financial conflicts of interest.
Physicians, Residents, Students Can Learn High-Value, Cost-Conscious Care
Clinical question: What are the factors that promote education in delivering high-value, cost-conscious care?
Background: Healthcare costs are increasing, with most recent numbers showing U.S. expenditures on healthcare of more than $3 trillion, almost 18% of the gross domestic product. High-value care focuses on understanding the benefits, risks, and costs of care and promoting interventions that add value.
Study design: Systematic review.
Setting: Physicians, resident physicians, and medical students in North America, Asia, and Oceania.
Synopsis: Seventy-nine articles were included in the analysis, with 14 being RCTs. Most of the studies were conducted in North America (78.5%) and used a pre-post interventional design (58.2%). Practicing physicians (36.7%) made up the majority of participants in the study, with residents (15.2%) and medical students (6.3%) in smaller numbers. Analysis of the studies identified three factors for successful learning:
- effective transmission of knowledge about prices of services and general health economics, scientific evidence, and patient preferences;
- facilitation of reflective practice through feedback and/or stimulating reflection; and
- creation of a supportive environment.
Bottom line: The most-effective interventions in educating physicians, resident physicians, and medical students on high-value, cost-conscious care are effective transmission of knowledge, reflective practice, and supportive environment.
Citation: Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400.
Clinical question: What are the factors that promote education in delivering high-value, cost-conscious care?
Background: Healthcare costs are increasing, with most recent numbers showing U.S. expenditures on healthcare of more than $3 trillion, almost 18% of the gross domestic product. High-value care focuses on understanding the benefits, risks, and costs of care and promoting interventions that add value.
Study design: Systematic review.
Setting: Physicians, resident physicians, and medical students in North America, Asia, and Oceania.
Synopsis: Seventy-nine articles were included in the analysis, with 14 being RCTs. Most of the studies were conducted in North America (78.5%) and used a pre-post interventional design (58.2%). Practicing physicians (36.7%) made up the majority of participants in the study, with residents (15.2%) and medical students (6.3%) in smaller numbers. Analysis of the studies identified three factors for successful learning:
- effective transmission of knowledge about prices of services and general health economics, scientific evidence, and patient preferences;
- facilitation of reflective practice through feedback and/or stimulating reflection; and
- creation of a supportive environment.
Bottom line: The most-effective interventions in educating physicians, resident physicians, and medical students on high-value, cost-conscious care are effective transmission of knowledge, reflective practice, and supportive environment.
Citation: Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400.
Clinical question: What are the factors that promote education in delivering high-value, cost-conscious care?
Background: Healthcare costs are increasing, with most recent numbers showing U.S. expenditures on healthcare of more than $3 trillion, almost 18% of the gross domestic product. High-value care focuses on understanding the benefits, risks, and costs of care and promoting interventions that add value.
Study design: Systematic review.
Setting: Physicians, resident physicians, and medical students in North America, Asia, and Oceania.
Synopsis: Seventy-nine articles were included in the analysis, with 14 being RCTs. Most of the studies were conducted in North America (78.5%) and used a pre-post interventional design (58.2%). Practicing physicians (36.7%) made up the majority of participants in the study, with residents (15.2%) and medical students (6.3%) in smaller numbers. Analysis of the studies identified three factors for successful learning:
- effective transmission of knowledge about prices of services and general health economics, scientific evidence, and patient preferences;
- facilitation of reflective practice through feedback and/or stimulating reflection; and
- creation of a supportive environment.
Bottom line: The most-effective interventions in educating physicians, resident physicians, and medical students on high-value, cost-conscious care are effective transmission of knowledge, reflective practice, and supportive environment.
Citation: Stammen LA, Stalmeijer RE, Paternotte E, et al. Training physicians to provide high-value, cost-conscious care: a systematic review. JAMA. 2015;314(22):2384-2400.