User login
TSH antibody levels predict Graves relapse after thionamides
BOSTON – Eighty-six percent of Graves disease patients with TSH receptor antibody levels of at least 2.0 mU/L at the end of thionamide therapy will relapse within 4 years, according to a British review.
TSH receptor antibody (TRAb) levels “are useful not only as a diagnostic tool but also as a prognostic tool. In patients where the risks of recurrent thyrotoxicosis are unacceptably high” – the elderly and those at risk for cardiovascular disease – “strong consideration should be given to primary radioiodine therapy” instead of thionamides, said investigator Nyo Nyo Tun of the Edinburgh Centre for Endocrinology and Diabetes.
Previous studies have suggested age and other risk factors for relapse after thionamides, but “have not [definitively] shown if elevation of TRAb levels” are predictive, she said at the annual meeting of the Endocrine Society.
Primary therapy with thionamides is more common in Europe than in the United States, where radioiodine tends to be the first choice. Part of the problem is that recurrence is known to be high after thionamides. The study suggests that using TRAb can help weed out patients who are likely to fail so that thionamides can be used with greater long-term success. Ms. Tun said the Edinburgh center routinely uses TRAb to guide Graves treatment; patients with high levels either stay on thionamide for prolonged periods or opt for radioiodine.
The investigators retrospectively studied 266 patients with a first presentation of Graves disease who completed a course of thionamide at two U.K. hospitals. In addition to TRAb levels at diagnosis and cessation of thionamide, they assessed age, sex, smoking status, free T4 levels, total T3, and time to normalization of thyroid function over 4 years of follow-up.
After thionamide cessation, thyrotoxicosis recurred in 31% of patients (82/266) at 1 year, 43% (111/261) at 2 years, 54% (125/232) at 3 years, and 66% (128/193) at 4 years.
Very high TRAb levels at diagnosis – those above 12 mU/L – were associated with a statistically significant 84% risk of recurrence over a 4-year period, compared with a 57% risk with diagnosis levels below 5mU/L (P = .002).
TRAb levels below 0.9 mU/L at cessation of an 18-month course of thionamide treatment were associated with a 22% risk of recurrence at 1 year and a 58% risk at 4 years. Those risks were significantly higher in patients whose TRAb levels were at least 2 mU/L at thionamide cessation, who had a 51% risk at 1 year and an 86% risk at 4 years (P less than 0.001). Relapse risk was highest in the first 18 months after cessation.
Younger age and time to TSH normalization also predicted relapse to some extent. Among patients who stayed in remission for 4 years, TSH normalized at a median of about 4 months after the start of drug treatment, but 6 months in those who relapsed. Similarly, patients who relapsed were a median of 39 years old at diagnosis; those who did not were a median of 47.
The investigators had no relevant financial disclosures.
The measurement of antibodies to the thyroid-stimulating hormone receptor is a useful clinical test that should be much more widely used in the United States. It’s a very accurate predictor of who’s going to get recurrent Grave’s disease after antithyroid drugs, but it’s misunderstood and not trusted.

|
Dr. Terry Davies |
When the test was first introduced, many major thyroid experts didn’t accept it and didn’t believe it was useful based on research at the time. The difference with the current study is that it was done carefully.
Dr. Terry Davies is the director of the division of endocrinology, diabetes, and bone diseases at the Mount Sinai Beth Israel Medical Center in New York. He moderated the presentation and was not involved in the work.
The measurement of antibodies to the thyroid-stimulating hormone receptor is a useful clinical test that should be much more widely used in the United States. It’s a very accurate predictor of who’s going to get recurrent Grave’s disease after antithyroid drugs, but it’s misunderstood and not trusted.

|
Dr. Terry Davies |
When the test was first introduced, many major thyroid experts didn’t accept it and didn’t believe it was useful based on research at the time. The difference with the current study is that it was done carefully.
Dr. Terry Davies is the director of the division of endocrinology, diabetes, and bone diseases at the Mount Sinai Beth Israel Medical Center in New York. He moderated the presentation and was not involved in the work.
The measurement of antibodies to the thyroid-stimulating hormone receptor is a useful clinical test that should be much more widely used in the United States. It’s a very accurate predictor of who’s going to get recurrent Grave’s disease after antithyroid drugs, but it’s misunderstood and not trusted.

|
Dr. Terry Davies |
When the test was first introduced, many major thyroid experts didn’t accept it and didn’t believe it was useful based on research at the time. The difference with the current study is that it was done carefully.
Dr. Terry Davies is the director of the division of endocrinology, diabetes, and bone diseases at the Mount Sinai Beth Israel Medical Center in New York. He moderated the presentation and was not involved in the work.
BOSTON – Eighty-six percent of Graves disease patients with TSH receptor antibody levels of at least 2.0 mU/L at the end of thionamide therapy will relapse within 4 years, according to a British review.
TSH receptor antibody (TRAb) levels “are useful not only as a diagnostic tool but also as a prognostic tool. In patients where the risks of recurrent thyrotoxicosis are unacceptably high” – the elderly and those at risk for cardiovascular disease – “strong consideration should be given to primary radioiodine therapy” instead of thionamides, said investigator Nyo Nyo Tun of the Edinburgh Centre for Endocrinology and Diabetes.
Previous studies have suggested age and other risk factors for relapse after thionamides, but “have not [definitively] shown if elevation of TRAb levels” are predictive, she said at the annual meeting of the Endocrine Society.
Primary therapy with thionamides is more common in Europe than in the United States, where radioiodine tends to be the first choice. Part of the problem is that recurrence is known to be high after thionamides. The study suggests that using TRAb can help weed out patients who are likely to fail so that thionamides can be used with greater long-term success. Ms. Tun said the Edinburgh center routinely uses TRAb to guide Graves treatment; patients with high levels either stay on thionamide for prolonged periods or opt for radioiodine.
The investigators retrospectively studied 266 patients with a first presentation of Graves disease who completed a course of thionamide at two U.K. hospitals. In addition to TRAb levels at diagnosis and cessation of thionamide, they assessed age, sex, smoking status, free T4 levels, total T3, and time to normalization of thyroid function over 4 years of follow-up.
After thionamide cessation, thyrotoxicosis recurred in 31% of patients (82/266) at 1 year, 43% (111/261) at 2 years, 54% (125/232) at 3 years, and 66% (128/193) at 4 years.
Very high TRAb levels at diagnosis – those above 12 mU/L – were associated with a statistically significant 84% risk of recurrence over a 4-year period, compared with a 57% risk with diagnosis levels below 5mU/L (P = .002).
TRAb levels below 0.9 mU/L at cessation of an 18-month course of thionamide treatment were associated with a 22% risk of recurrence at 1 year and a 58% risk at 4 years. Those risks were significantly higher in patients whose TRAb levels were at least 2 mU/L at thionamide cessation, who had a 51% risk at 1 year and an 86% risk at 4 years (P less than 0.001). Relapse risk was highest in the first 18 months after cessation.
Younger age and time to TSH normalization also predicted relapse to some extent. Among patients who stayed in remission for 4 years, TSH normalized at a median of about 4 months after the start of drug treatment, but 6 months in those who relapsed. Similarly, patients who relapsed were a median of 39 years old at diagnosis; those who did not were a median of 47.
The investigators had no relevant financial disclosures.
BOSTON – Eighty-six percent of Graves disease patients with TSH receptor antibody levels of at least 2.0 mU/L at the end of thionamide therapy will relapse within 4 years, according to a British review.
TSH receptor antibody (TRAb) levels “are useful not only as a diagnostic tool but also as a prognostic tool. In patients where the risks of recurrent thyrotoxicosis are unacceptably high” – the elderly and those at risk for cardiovascular disease – “strong consideration should be given to primary radioiodine therapy” instead of thionamides, said investigator Nyo Nyo Tun of the Edinburgh Centre for Endocrinology and Diabetes.
Previous studies have suggested age and other risk factors for relapse after thionamides, but “have not [definitively] shown if elevation of TRAb levels” are predictive, she said at the annual meeting of the Endocrine Society.
Primary therapy with thionamides is more common in Europe than in the United States, where radioiodine tends to be the first choice. Part of the problem is that recurrence is known to be high after thionamides. The study suggests that using TRAb can help weed out patients who are likely to fail so that thionamides can be used with greater long-term success. Ms. Tun said the Edinburgh center routinely uses TRAb to guide Graves treatment; patients with high levels either stay on thionamide for prolonged periods or opt for radioiodine.
The investigators retrospectively studied 266 patients with a first presentation of Graves disease who completed a course of thionamide at two U.K. hospitals. In addition to TRAb levels at diagnosis and cessation of thionamide, they assessed age, sex, smoking status, free T4 levels, total T3, and time to normalization of thyroid function over 4 years of follow-up.
After thionamide cessation, thyrotoxicosis recurred in 31% of patients (82/266) at 1 year, 43% (111/261) at 2 years, 54% (125/232) at 3 years, and 66% (128/193) at 4 years.
Very high TRAb levels at diagnosis – those above 12 mU/L – were associated with a statistically significant 84% risk of recurrence over a 4-year period, compared with a 57% risk with diagnosis levels below 5mU/L (P = .002).
TRAb levels below 0.9 mU/L at cessation of an 18-month course of thionamide treatment were associated with a 22% risk of recurrence at 1 year and a 58% risk at 4 years. Those risks were significantly higher in patients whose TRAb levels were at least 2 mU/L at thionamide cessation, who had a 51% risk at 1 year and an 86% risk at 4 years (P less than 0.001). Relapse risk was highest in the first 18 months after cessation.
Younger age and time to TSH normalization also predicted relapse to some extent. Among patients who stayed in remission for 4 years, TSH normalized at a median of about 4 months after the start of drug treatment, but 6 months in those who relapsed. Similarly, patients who relapsed were a median of 39 years old at diagnosis; those who did not were a median of 47.
The investigators had no relevant financial disclosures.
AT ENDO 2016
Key clinical point: Opt for radioiodine when Grave’s patients present with thyroid-stimulating hormone receptor antibody levels above 12 mU/L.
Major finding: Eighty-six percent of Grave’s disease patients with TSH receptor antibody levels of at least 2.0 mU/L at the end of thionamide therapy will relapse within 4 years.
Data source: A British review of 266 Grave’s patients treated with thionamides for 18 months.
Disclosures: The investigators had no relevant financial disclosures.
Tiotropium inhalation spray effective for asthma regardless of allergic status
LOS ANGELES – Once-daily tiotropium bromide inhalation spray as long-term, add-on maintenance therapy in patients with poorly controlled symptomatic asthma is similarly effective in both allergic and nonallergic asthma, according to Dr. Donald P. Tashkin.
In a series of analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, he and his coinvestigators showed that tiotropium bromide inhalation spray (Spiriva Respimat) given as add-on maintenance therapy resulted in significantly improved lung function, enhanced asthma symptom control, and fewer asthma exacerbations. The important new finding in these post hoc analyses was that the medication was similarly effective across the full range of baseline serum IgE and blood eosinophil levels, said Dr. Tashkin, director of the pulmonary function laboratories at the University of California, Los Angeles.
Last fall, the Food and Drug Administration approved an expanded indication for tiotropium bromide inhalation spray as long-term, add-on maintenance therapy of asthma in patients aged 12 and up who remain symptomatic despite taking other maintenance therapies. The once-daily medication had already been approved in the fall of 2014 for maintenance therapy of chronic obstructive pulmonary disease. Spiriva Respimat delivers a once-daily 2.5-mcg dose of tiotropium bromide via two 1.25-mcg puffs in a slow-moving, propellant-free mist designed to get the drug into the distal lungs independent of a patient’s skill in using a conventional metered-dose inhaler.
Dr. Tashkin and his coinvestigators presented a series of post hoc analyses combining data on 3,012 participants in the two prospective PrimoTinA-asthma and two MezzoTinA-asthma clinical trials. These phase III, double-blind, placebo-controlled trials defined participants’ allergic phenotype on the basis of the conventional cut points of a serum IgE level above or below 430 mcg/mL or a blood eosinophil count above or below 600 cells/mcL.
“The question is, are these appropriate cut points? Can we be sure that somebody below those cut points doesn’t have atopy? To answer that question, we looked at the whole spectrum of eosinophils in the blood from 5/mcL to 2,000/mcL and serum IgE levels from 2 mcg/mL to 2,000 mcg/mL. We found that the efficacy was similar across the entire spectrum of these measures of allergy,” he said in an interview.
Thus, these new findings support the use of this novel maintenance therapy without any need for lab tests to determine whether an individual patient’s asthma is T helper 2–cell dominant or not, Dr. Tashkin added.
The PrimoTinA-asthma trials were 48-week studies of add-on Spiriva Respimat or placebo conducted in patients with symptomatic asthma despite treatment with an inhaled corticosteroid plus a long-acting beta-agonist. The MezzoTinA-asthma studies were 24 weeks long and focused on patients who remained symptomatic despite at least moderate-dose inhaled corticosteroid therapy. Key endpoints included improvement in asthma symptom control as measured by the seven-question Asthma Control Questionnaire, improved peak and trough forced expiratory volume in 1 second, and time to a first severe asthma exacerbation.
Dr. Tashkin reported serving as a paid speaker on behalf of Boehringer Ingelheim, which markets Spiriva Respimat and sponsored the studies.
LOS ANGELES – Once-daily tiotropium bromide inhalation spray as long-term, add-on maintenance therapy in patients with poorly controlled symptomatic asthma is similarly effective in both allergic and nonallergic asthma, according to Dr. Donald P. Tashkin.
In a series of analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, he and his coinvestigators showed that tiotropium bromide inhalation spray (Spiriva Respimat) given as add-on maintenance therapy resulted in significantly improved lung function, enhanced asthma symptom control, and fewer asthma exacerbations. The important new finding in these post hoc analyses was that the medication was similarly effective across the full range of baseline serum IgE and blood eosinophil levels, said Dr. Tashkin, director of the pulmonary function laboratories at the University of California, Los Angeles.
Last fall, the Food and Drug Administration approved an expanded indication for tiotropium bromide inhalation spray as long-term, add-on maintenance therapy of asthma in patients aged 12 and up who remain symptomatic despite taking other maintenance therapies. The once-daily medication had already been approved in the fall of 2014 for maintenance therapy of chronic obstructive pulmonary disease. Spiriva Respimat delivers a once-daily 2.5-mcg dose of tiotropium bromide via two 1.25-mcg puffs in a slow-moving, propellant-free mist designed to get the drug into the distal lungs independent of a patient’s skill in using a conventional metered-dose inhaler.
Dr. Tashkin and his coinvestigators presented a series of post hoc analyses combining data on 3,012 participants in the two prospective PrimoTinA-asthma and two MezzoTinA-asthma clinical trials. These phase III, double-blind, placebo-controlled trials defined participants’ allergic phenotype on the basis of the conventional cut points of a serum IgE level above or below 430 mcg/mL or a blood eosinophil count above or below 600 cells/mcL.
“The question is, are these appropriate cut points? Can we be sure that somebody below those cut points doesn’t have atopy? To answer that question, we looked at the whole spectrum of eosinophils in the blood from 5/mcL to 2,000/mcL and serum IgE levels from 2 mcg/mL to 2,000 mcg/mL. We found that the efficacy was similar across the entire spectrum of these measures of allergy,” he said in an interview.
Thus, these new findings support the use of this novel maintenance therapy without any need for lab tests to determine whether an individual patient’s asthma is T helper 2–cell dominant or not, Dr. Tashkin added.
The PrimoTinA-asthma trials were 48-week studies of add-on Spiriva Respimat or placebo conducted in patients with symptomatic asthma despite treatment with an inhaled corticosteroid plus a long-acting beta-agonist. The MezzoTinA-asthma studies were 24 weeks long and focused on patients who remained symptomatic despite at least moderate-dose inhaled corticosteroid therapy. Key endpoints included improvement in asthma symptom control as measured by the seven-question Asthma Control Questionnaire, improved peak and trough forced expiratory volume in 1 second, and time to a first severe asthma exacerbation.
Dr. Tashkin reported serving as a paid speaker on behalf of Boehringer Ingelheim, which markets Spiriva Respimat and sponsored the studies.
LOS ANGELES – Once-daily tiotropium bromide inhalation spray as long-term, add-on maintenance therapy in patients with poorly controlled symptomatic asthma is similarly effective in both allergic and nonallergic asthma, according to Dr. Donald P. Tashkin.
In a series of analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, he and his coinvestigators showed that tiotropium bromide inhalation spray (Spiriva Respimat) given as add-on maintenance therapy resulted in significantly improved lung function, enhanced asthma symptom control, and fewer asthma exacerbations. The important new finding in these post hoc analyses was that the medication was similarly effective across the full range of baseline serum IgE and blood eosinophil levels, said Dr. Tashkin, director of the pulmonary function laboratories at the University of California, Los Angeles.
Last fall, the Food and Drug Administration approved an expanded indication for tiotropium bromide inhalation spray as long-term, add-on maintenance therapy of asthma in patients aged 12 and up who remain symptomatic despite taking other maintenance therapies. The once-daily medication had already been approved in the fall of 2014 for maintenance therapy of chronic obstructive pulmonary disease. Spiriva Respimat delivers a once-daily 2.5-mcg dose of tiotropium bromide via two 1.25-mcg puffs in a slow-moving, propellant-free mist designed to get the drug into the distal lungs independent of a patient’s skill in using a conventional metered-dose inhaler.
Dr. Tashkin and his coinvestigators presented a series of post hoc analyses combining data on 3,012 participants in the two prospective PrimoTinA-asthma and two MezzoTinA-asthma clinical trials. These phase III, double-blind, placebo-controlled trials defined participants’ allergic phenotype on the basis of the conventional cut points of a serum IgE level above or below 430 mcg/mL or a blood eosinophil count above or below 600 cells/mcL.
“The question is, are these appropriate cut points? Can we be sure that somebody below those cut points doesn’t have atopy? To answer that question, we looked at the whole spectrum of eosinophils in the blood from 5/mcL to 2,000/mcL and serum IgE levels from 2 mcg/mL to 2,000 mcg/mL. We found that the efficacy was similar across the entire spectrum of these measures of allergy,” he said in an interview.
Thus, these new findings support the use of this novel maintenance therapy without any need for lab tests to determine whether an individual patient’s asthma is T helper 2–cell dominant or not, Dr. Tashkin added.
The PrimoTinA-asthma trials were 48-week studies of add-on Spiriva Respimat or placebo conducted in patients with symptomatic asthma despite treatment with an inhaled corticosteroid plus a long-acting beta-agonist. The MezzoTinA-asthma studies were 24 weeks long and focused on patients who remained symptomatic despite at least moderate-dose inhaled corticosteroid therapy. Key endpoints included improvement in asthma symptom control as measured by the seven-question Asthma Control Questionnaire, improved peak and trough forced expiratory volume in 1 second, and time to a first severe asthma exacerbation.
Dr. Tashkin reported serving as a paid speaker on behalf of Boehringer Ingelheim, which markets Spiriva Respimat and sponsored the studies.
EXPERT ANALYSIS FROM THE 2016 AAAAI ANNUAL MEETING
Resistant Hypertension? Time to Consider This Fourth-line Drug
PRACTICE CHANGER
When a triple regimen (ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic) fails to achieve the target blood pressure, try adding spironolactone.
Strength of recommendation
C: Based on a high-quality disease-oriented randomized controlled trial.1
Willie S, a 56-year-old man with chronic essential hypertension, has been on an optimally dosed three-drug regimen of an ACE inhibitor, a calcium channel blocker, and a thiazide diuretic for more than three months, but his blood pressure is still not at goal. What is the best antihypertensive agent to add to his regimen?
About 5% to 30% of those being treated for hypertension have resistant hypertension, defined as inadequate blood pressure (BP) control despite a triple regimen of an ACE inhibitor or angiotensin receptor blocker (ARB), calcium channel blocker (CCB), and thiazide diuretic.1,2Guidelines from the Eighth Joint National Committee (JNC-8) on the management of high BP recommend ß-blockers, α-blockers, or aldosterone antagonists (AAs) as equivalent choices for a fourth-line agent. The recommendation is based on expert opinion.3
Earlier hypertension guidelines from the UK’s National Institute for Health and Care Excellence recommend an AA if BP targets have not been met with the triple regimen. But this recommendation is based on lower-quality evidence, without comparison to ß-blockers, α-blockers, or other drug classes.4
More evidence since guideline’s release
A 2015 meta-analysis of 15 studies and a total of more than 1,200 participants (three randomized controlled trials [RCTs], one non-randomized placebo-controlled comparative trial, and 11 single-arm observational studies) demonstrated the effectiveness of the AAs spironolactone and eplerenone on resistant hypertension.5In the four comparative studies, AAs decreased office systolic blood pressure (SBP) by 24.3 mm Hg and diastolic blood pressure (DBP) by 7.8 mm Hg more than placebo. In the 11 single-arm studies, AAs reduced SBP by 22.74 mm Hg and DBP by 10.49 mm Hg.
Another RCT examined the effect of low-dose (25-mg) spironolactone, compared with placebo, in 161 patients with resistant hypertension.6At eight weeks, 73% of those receiving spironolactone reached a goal SBP < 140 mm Hg versus 41% of patients on placebo. The same proportion (73%) achieved a goal DBP < 90 mm Hg in the spironolactone group, compared with 63% of those in the placebo group. Ambulatory BP was also found to be significantly improved among those receiving spironolactone versus placebo, with a decrease in SBP of 9.8 mm Hg and in DSP of 3.2 mm Hg.6
Continue for the study summary >>
STUDY SUMMARY
Spironolactone vs other drugs
The placebo-controlled crossover RCT conducted in the UK by Williams et al was the first to directly compare spironolactone with other medications for the treatment of resistant hypertension in adults already taking triple therapy.1The trial randomized 335 individuals with a mean age of 61.4 (range, 18 to 79), 69% of whom were male; 314 were included in the intention-to-treat analysis.1
Enrollment criteria for resistant hypertension specified a clinic-recorded SBP of ≥ 140 mm Hg (or ≥ 135 mm Hg in those with diabetes) and home SBP (in 18 readings over four days) of ≥ 130 mm Hg.1 To ensure fidelity to treatment protocols, the investigators directly observed therapy, took tablet counts, measured serum ACE activity, and assessed BP measurement technique, with all participants adhering to a minimum of three months on a maximally dosed triple regimen.
Among subjects, 14% had diabetes and 7.8% reported tobacco use. Average weight was 93.5 kg (205.7 lbs).1 Because of the expected inverse relationship between plasma renin and response to AAs, plasma renin was measured at baseline to test whether resistant hypertension was primarily due to sodium retention.1
Four 12-week rotations
All participants began the trial with four weeks of placebo, followed by randomization to 12-week rotations of once-daily oral treatment with (1) spironolactone 25 to 50 mg, (2) doxazosin modified release 4 to 8 mg, (3) bisoprolol 5 to 10 mg, and (4) placebo.1 Six weeks after initiation of each study medication, participants were titrated to the higher dose. There was no washout period between cycles.
The primary outcome was mean SBP measured at home on four consecutive days prior to the study visits in weeks 6 and 12. Participants were required to have at least six BP measurements per each six-week period in order to establish a valid average. Primary endpoints included the difference in home SBP between spironolactone and placebo, the difference in home SBP between spironolactone and the mean of the other two drugs, and the difference in home SBP between spironolactone and each of the other two drugs.
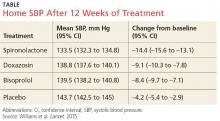
The results. Spironolactone lowered SBP more than placebo, doxazosin, and bisoprolol (see the Table).1 Clinic measurements were consistent with home BP readings.
Overall, 58% of participants achieved goal SBP < 135 mm Hg on spironolactone, compared with 42% on doxazosin, 44% on bisoprolol, and 24% on placebo.1 The effectiveness of spironolactone on SBP reduction was shown to exhibit an inverse relationship to plasma renin levels, a finding that was not apparent with the other two study drugs. However, spironolactone had a superior BP-lowering effect throughout nearly the entire renin distribution of the cohort.
The mean difference between spironolactone and placebo was –10.2 mm Hg; compared with the other drugs, spironolactone lowered SBP, on average, by 5.64 mm Hg more than bisoprolol and doxazosin; 5.3 mm Hg more than doxazosin alone; and 5.98 mm Hg more than bisoprolol alone.
Only 1% of trial participants had to discontinue spironolactone due to adverse events—the same proportion of withdrawals as that for bisoprolol and placebo and three times less than for doxazosin.1
Continue for what's new >>
WHAT’S NEW
Evidence of superiority
This is the first RCT to compare spironolactone with two other commonly used fourth-line antihypertensives—bisoprolol and doxazosin—in patients with resistant hypertension. The study demonstrated clear superiority of spironolactone in achieving carefully measured ambulatory and clinic-recorded BP targets versus a ß-blocker or an α-blocker.
CAVEATS
Findings not universal
Spironolactone is contraindicated in patients with severe renal impairment. Although multiple drug trials have demonstrated the medication’s safety and effectiveness, especially in patients with resistant hypertension, we should factor in the need for monitoring electrolytes and renal function within weeks of treatment initiation and periodically thereafter.7,8 In this study, spironolactone increased potassium levels, on average, by 0.45 mmol/L. No gynecomastia (typically seen in about 6% of men) was found in those taking spironolactone for a 12-week cycle.1
This single trial enrolled mostly Caucasian men with a mean age of 61. Although smaller observational studies that included African-American patients have shown promising results for spironolactone, the question of external validity or applicability to a diverse population has yet to be decisively answered.9
CHALLENGES TO IMPLEMENTATION
Potential for adverse reactions
The evidence supporting this change in practice has been accumulating for the past few years. However, clinicians who treat patients with resistant hypertension may have concerns about hyperkalemia, gynecomastia, and effects on renal function. More patient-oriented evidence is likewise needed to assist with the revision of guidelines and wider adoption of AAs by primary care providers.
References
1. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068.
2. Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 Study. Hypertension. 2015;65:407-413.
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507-520.
4. National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management (Clinical Guideline CG127). August 2011. https://www.nice.org.uk/guidance/cg127. Accessed March 4, 2016.
5. Dahal K, Kunwar S, Rijal J, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376-1385.
6. Václavík J, Sedlák R, Jarkovský J, et al. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine (Baltimore). 2014;93:e162.
7. Wei L, Struthers AD, Fahey T, et al. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768.
8. Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus. J Hypertens. 2013;31:2094-2102.
9. Nishizaka M, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925-930.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(4):266-268.
PRACTICE CHANGER
When a triple regimen (ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic) fails to achieve the target blood pressure, try adding spironolactone.
Strength of recommendation
C: Based on a high-quality disease-oriented randomized controlled trial.1
Willie S, a 56-year-old man with chronic essential hypertension, has been on an optimally dosed three-drug regimen of an ACE inhibitor, a calcium channel blocker, and a thiazide diuretic for more than three months, but his blood pressure is still not at goal. What is the best antihypertensive agent to add to his regimen?
About 5% to 30% of those being treated for hypertension have resistant hypertension, defined as inadequate blood pressure (BP) control despite a triple regimen of an ACE inhibitor or angiotensin receptor blocker (ARB), calcium channel blocker (CCB), and thiazide diuretic.1,2Guidelines from the Eighth Joint National Committee (JNC-8) on the management of high BP recommend ß-blockers, α-blockers, or aldosterone antagonists (AAs) as equivalent choices for a fourth-line agent. The recommendation is based on expert opinion.3
Earlier hypertension guidelines from the UK’s National Institute for Health and Care Excellence recommend an AA if BP targets have not been met with the triple regimen. But this recommendation is based on lower-quality evidence, without comparison to ß-blockers, α-blockers, or other drug classes.4
More evidence since guideline’s release
A 2015 meta-analysis of 15 studies and a total of more than 1,200 participants (three randomized controlled trials [RCTs], one non-randomized placebo-controlled comparative trial, and 11 single-arm observational studies) demonstrated the effectiveness of the AAs spironolactone and eplerenone on resistant hypertension.5In the four comparative studies, AAs decreased office systolic blood pressure (SBP) by 24.3 mm Hg and diastolic blood pressure (DBP) by 7.8 mm Hg more than placebo. In the 11 single-arm studies, AAs reduced SBP by 22.74 mm Hg and DBP by 10.49 mm Hg.
Another RCT examined the effect of low-dose (25-mg) spironolactone, compared with placebo, in 161 patients with resistant hypertension.6At eight weeks, 73% of those receiving spironolactone reached a goal SBP < 140 mm Hg versus 41% of patients on placebo. The same proportion (73%) achieved a goal DBP < 90 mm Hg in the spironolactone group, compared with 63% of those in the placebo group. Ambulatory BP was also found to be significantly improved among those receiving spironolactone versus placebo, with a decrease in SBP of 9.8 mm Hg and in DSP of 3.2 mm Hg.6
Continue for the study summary >>
STUDY SUMMARY
Spironolactone vs other drugs
The placebo-controlled crossover RCT conducted in the UK by Williams et al was the first to directly compare spironolactone with other medications for the treatment of resistant hypertension in adults already taking triple therapy.1The trial randomized 335 individuals with a mean age of 61.4 (range, 18 to 79), 69% of whom were male; 314 were included in the intention-to-treat analysis.1
Enrollment criteria for resistant hypertension specified a clinic-recorded SBP of ≥ 140 mm Hg (or ≥ 135 mm Hg in those with diabetes) and home SBP (in 18 readings over four days) of ≥ 130 mm Hg.1 To ensure fidelity to treatment protocols, the investigators directly observed therapy, took tablet counts, measured serum ACE activity, and assessed BP measurement technique, with all participants adhering to a minimum of three months on a maximally dosed triple regimen.
Among subjects, 14% had diabetes and 7.8% reported tobacco use. Average weight was 93.5 kg (205.7 lbs).1 Because of the expected inverse relationship between plasma renin and response to AAs, plasma renin was measured at baseline to test whether resistant hypertension was primarily due to sodium retention.1
Four 12-week rotations
All participants began the trial with four weeks of placebo, followed by randomization to 12-week rotations of once-daily oral treatment with (1) spironolactone 25 to 50 mg, (2) doxazosin modified release 4 to 8 mg, (3) bisoprolol 5 to 10 mg, and (4) placebo.1 Six weeks after initiation of each study medication, participants were titrated to the higher dose. There was no washout period between cycles.
The primary outcome was mean SBP measured at home on four consecutive days prior to the study visits in weeks 6 and 12. Participants were required to have at least six BP measurements per each six-week period in order to establish a valid average. Primary endpoints included the difference in home SBP between spironolactone and placebo, the difference in home SBP between spironolactone and the mean of the other two drugs, and the difference in home SBP between spironolactone and each of the other two drugs.
The results. Spironolactone lowered SBP more than placebo, doxazosin, and bisoprolol (see the Table).1 Clinic measurements were consistent with home BP readings.
Overall, 58% of participants achieved goal SBP < 135 mm Hg on spironolactone, compared with 42% on doxazosin, 44% on bisoprolol, and 24% on placebo.1 The effectiveness of spironolactone on SBP reduction was shown to exhibit an inverse relationship to plasma renin levels, a finding that was not apparent with the other two study drugs. However, spironolactone had a superior BP-lowering effect throughout nearly the entire renin distribution of the cohort.
The mean difference between spironolactone and placebo was –10.2 mm Hg; compared with the other drugs, spironolactone lowered SBP, on average, by 5.64 mm Hg more than bisoprolol and doxazosin; 5.3 mm Hg more than doxazosin alone; and 5.98 mm Hg more than bisoprolol alone.
Only 1% of trial participants had to discontinue spironolactone due to adverse events—the same proportion of withdrawals as that for bisoprolol and placebo and three times less than for doxazosin.1
Continue for what's new >>
WHAT’S NEW
Evidence of superiority
This is the first RCT to compare spironolactone with two other commonly used fourth-line antihypertensives—bisoprolol and doxazosin—in patients with resistant hypertension. The study demonstrated clear superiority of spironolactone in achieving carefully measured ambulatory and clinic-recorded BP targets versus a ß-blocker or an α-blocker.
CAVEATS
Findings not universal
Spironolactone is contraindicated in patients with severe renal impairment. Although multiple drug trials have demonstrated the medication’s safety and effectiveness, especially in patients with resistant hypertension, we should factor in the need for monitoring electrolytes and renal function within weeks of treatment initiation and periodically thereafter.7,8 In this study, spironolactone increased potassium levels, on average, by 0.45 mmol/L. No gynecomastia (typically seen in about 6% of men) was found in those taking spironolactone for a 12-week cycle.1
This single trial enrolled mostly Caucasian men with a mean age of 61. Although smaller observational studies that included African-American patients have shown promising results for spironolactone, the question of external validity or applicability to a diverse population has yet to be decisively answered.9
CHALLENGES TO IMPLEMENTATION
Potential for adverse reactions
The evidence supporting this change in practice has been accumulating for the past few years. However, clinicians who treat patients with resistant hypertension may have concerns about hyperkalemia, gynecomastia, and effects on renal function. More patient-oriented evidence is likewise needed to assist with the revision of guidelines and wider adoption of AAs by primary care providers.
References
1. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068.
2. Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 Study. Hypertension. 2015;65:407-413.
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507-520.
4. National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management (Clinical Guideline CG127). August 2011. https://www.nice.org.uk/guidance/cg127. Accessed March 4, 2016.
5. Dahal K, Kunwar S, Rijal J, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376-1385.
6. Václavík J, Sedlák R, Jarkovský J, et al. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine (Baltimore). 2014;93:e162.
7. Wei L, Struthers AD, Fahey T, et al. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768.
8. Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus. J Hypertens. 2013;31:2094-2102.
9. Nishizaka M, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925-930.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(4):266-268.
PRACTICE CHANGER
When a triple regimen (ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic) fails to achieve the target blood pressure, try adding spironolactone.
Strength of recommendation
C: Based on a high-quality disease-oriented randomized controlled trial.1
Willie S, a 56-year-old man with chronic essential hypertension, has been on an optimally dosed three-drug regimen of an ACE inhibitor, a calcium channel blocker, and a thiazide diuretic for more than three months, but his blood pressure is still not at goal. What is the best antihypertensive agent to add to his regimen?
About 5% to 30% of those being treated for hypertension have resistant hypertension, defined as inadequate blood pressure (BP) control despite a triple regimen of an ACE inhibitor or angiotensin receptor blocker (ARB), calcium channel blocker (CCB), and thiazide diuretic.1,2Guidelines from the Eighth Joint National Committee (JNC-8) on the management of high BP recommend ß-blockers, α-blockers, or aldosterone antagonists (AAs) as equivalent choices for a fourth-line agent. The recommendation is based on expert opinion.3
Earlier hypertension guidelines from the UK’s National Institute for Health and Care Excellence recommend an AA if BP targets have not been met with the triple regimen. But this recommendation is based on lower-quality evidence, without comparison to ß-blockers, α-blockers, or other drug classes.4
More evidence since guideline’s release
A 2015 meta-analysis of 15 studies and a total of more than 1,200 participants (three randomized controlled trials [RCTs], one non-randomized placebo-controlled comparative trial, and 11 single-arm observational studies) demonstrated the effectiveness of the AAs spironolactone and eplerenone on resistant hypertension.5In the four comparative studies, AAs decreased office systolic blood pressure (SBP) by 24.3 mm Hg and diastolic blood pressure (DBP) by 7.8 mm Hg more than placebo. In the 11 single-arm studies, AAs reduced SBP by 22.74 mm Hg and DBP by 10.49 mm Hg.
Another RCT examined the effect of low-dose (25-mg) spironolactone, compared with placebo, in 161 patients with resistant hypertension.6At eight weeks, 73% of those receiving spironolactone reached a goal SBP < 140 mm Hg versus 41% of patients on placebo. The same proportion (73%) achieved a goal DBP < 90 mm Hg in the spironolactone group, compared with 63% of those in the placebo group. Ambulatory BP was also found to be significantly improved among those receiving spironolactone versus placebo, with a decrease in SBP of 9.8 mm Hg and in DSP of 3.2 mm Hg.6
Continue for the study summary >>
STUDY SUMMARY
Spironolactone vs other drugs
The placebo-controlled crossover RCT conducted in the UK by Williams et al was the first to directly compare spironolactone with other medications for the treatment of resistant hypertension in adults already taking triple therapy.1The trial randomized 335 individuals with a mean age of 61.4 (range, 18 to 79), 69% of whom were male; 314 were included in the intention-to-treat analysis.1
Enrollment criteria for resistant hypertension specified a clinic-recorded SBP of ≥ 140 mm Hg (or ≥ 135 mm Hg in those with diabetes) and home SBP (in 18 readings over four days) of ≥ 130 mm Hg.1 To ensure fidelity to treatment protocols, the investigators directly observed therapy, took tablet counts, measured serum ACE activity, and assessed BP measurement technique, with all participants adhering to a minimum of three months on a maximally dosed triple regimen.
Among subjects, 14% had diabetes and 7.8% reported tobacco use. Average weight was 93.5 kg (205.7 lbs).1 Because of the expected inverse relationship between plasma renin and response to AAs, plasma renin was measured at baseline to test whether resistant hypertension was primarily due to sodium retention.1
Four 12-week rotations
All participants began the trial with four weeks of placebo, followed by randomization to 12-week rotations of once-daily oral treatment with (1) spironolactone 25 to 50 mg, (2) doxazosin modified release 4 to 8 mg, (3) bisoprolol 5 to 10 mg, and (4) placebo.1 Six weeks after initiation of each study medication, participants were titrated to the higher dose. There was no washout period between cycles.
The primary outcome was mean SBP measured at home on four consecutive days prior to the study visits in weeks 6 and 12. Participants were required to have at least six BP measurements per each six-week period in order to establish a valid average. Primary endpoints included the difference in home SBP between spironolactone and placebo, the difference in home SBP between spironolactone and the mean of the other two drugs, and the difference in home SBP between spironolactone and each of the other two drugs.
The results. Spironolactone lowered SBP more than placebo, doxazosin, and bisoprolol (see the Table).1 Clinic measurements were consistent with home BP readings.
Overall, 58% of participants achieved goal SBP < 135 mm Hg on spironolactone, compared with 42% on doxazosin, 44% on bisoprolol, and 24% on placebo.1 The effectiveness of spironolactone on SBP reduction was shown to exhibit an inverse relationship to plasma renin levels, a finding that was not apparent with the other two study drugs. However, spironolactone had a superior BP-lowering effect throughout nearly the entire renin distribution of the cohort.
The mean difference between spironolactone and placebo was –10.2 mm Hg; compared with the other drugs, spironolactone lowered SBP, on average, by 5.64 mm Hg more than bisoprolol and doxazosin; 5.3 mm Hg more than doxazosin alone; and 5.98 mm Hg more than bisoprolol alone.
Only 1% of trial participants had to discontinue spironolactone due to adverse events—the same proportion of withdrawals as that for bisoprolol and placebo and three times less than for doxazosin.1
Continue for what's new >>
WHAT’S NEW
Evidence of superiority
This is the first RCT to compare spironolactone with two other commonly used fourth-line antihypertensives—bisoprolol and doxazosin—in patients with resistant hypertension. The study demonstrated clear superiority of spironolactone in achieving carefully measured ambulatory and clinic-recorded BP targets versus a ß-blocker or an α-blocker.
CAVEATS
Findings not universal
Spironolactone is contraindicated in patients with severe renal impairment. Although multiple drug trials have demonstrated the medication’s safety and effectiveness, especially in patients with resistant hypertension, we should factor in the need for monitoring electrolytes and renal function within weeks of treatment initiation and periodically thereafter.7,8 In this study, spironolactone increased potassium levels, on average, by 0.45 mmol/L. No gynecomastia (typically seen in about 6% of men) was found in those taking spironolactone for a 12-week cycle.1
This single trial enrolled mostly Caucasian men with a mean age of 61. Although smaller observational studies that included African-American patients have shown promising results for spironolactone, the question of external validity or applicability to a diverse population has yet to be decisively answered.9
CHALLENGES TO IMPLEMENTATION
Potential for adverse reactions
The evidence supporting this change in practice has been accumulating for the past few years. However, clinicians who treat patients with resistant hypertension may have concerns about hyperkalemia, gynecomastia, and effects on renal function. More patient-oriented evidence is likewise needed to assist with the revision of guidelines and wider adoption of AAs by primary care providers.
References
1. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068.
2. Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 Study. Hypertension. 2015;65:407-413.
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507-520.
4. National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management (Clinical Guideline CG127). August 2011. https://www.nice.org.uk/guidance/cg127. Accessed March 4, 2016.
5. Dahal K, Kunwar S, Rijal J, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376-1385.
6. Václavík J, Sedlák R, Jarkovský J, et al. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine (Baltimore). 2014;93:e162.
7. Wei L, Struthers AD, Fahey T, et al. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768.
8. Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus. J Hypertens. 2013;31:2094-2102.
9. Nishizaka M, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925-930.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(4):266-268.
Diverticulitis recurs more with observation vs. elective resection
CHICAGO – Observation, compared with elective resection, was associated with significantly increased recurrence rates in a single-center randomized, controlled trial of patients who had successfully recovered via nonoperative management from their first episode of acute sigmoid diverticulitis with extraluminal air/abscess.
Recurrence rates in 111 patients randomized to observation or elective resection were 31% in the observation group and 7% in the resection group, at 15 and 18 months, respectively, Dr. Ryan Bendl of State University of New York, Stony Brook reported at the annual meeting of the American Surgical Association.
Patients in the two groups were comparable with respect to age, sex, body mass index, Colorectal Physiologic and Operative Severity Score for the Enumeration of Mortality and Morbidity (CR-POSSUM), and comorbidities, he noted.
Subjects included in the single-center study were adults admitted for a first episode of acute diverticulitis with abscess or extraluminal air who were managed nonoperatively with intravenous antibiotics, a period of nothing by mouth, drainage, and total parenteral nutrition followed by colonoscopy. They were randomized 3:1 to observation or resection, and 68% of the elective resection patients underwent minimally invasive surgery. The study’s primary endpoint was recurrent diverticulitis defined as an acute episode confirmed by computed tomography and requiring hospitalization with intravenous antibiotics.
Diverticulitis accounted for more than 300,000 hospital admissions in 2010 in the United States alone, and 10%-20% of patients had abscess formation. At one time, most patients were managed with immediate operative intervention, but medical and radiologic advances have led to a shift toward nonoperative management, Dr. Bendl said.
Some prior studies have suggested that recurrence rates are higher with nonoperative management, and the current study supports those data.
However, despite the significant increase in the recurrence rate with observation vs. resection, most patients in the observation group did not experience recurrence, and of those who did, none had peritonitis.
“All those with recurrences were successfully treated again using nonoperative management,” he said.
This study was supported in part by grants from Merck and Covidien. Dr. Bendl reported having no relevant financial disclosures.
CHICAGO – Observation, compared with elective resection, was associated with significantly increased recurrence rates in a single-center randomized, controlled trial of patients who had successfully recovered via nonoperative management from their first episode of acute sigmoid diverticulitis with extraluminal air/abscess.
Recurrence rates in 111 patients randomized to observation or elective resection were 31% in the observation group and 7% in the resection group, at 15 and 18 months, respectively, Dr. Ryan Bendl of State University of New York, Stony Brook reported at the annual meeting of the American Surgical Association.
Patients in the two groups were comparable with respect to age, sex, body mass index, Colorectal Physiologic and Operative Severity Score for the Enumeration of Mortality and Morbidity (CR-POSSUM), and comorbidities, he noted.
Subjects included in the single-center study were adults admitted for a first episode of acute diverticulitis with abscess or extraluminal air who were managed nonoperatively with intravenous antibiotics, a period of nothing by mouth, drainage, and total parenteral nutrition followed by colonoscopy. They were randomized 3:1 to observation or resection, and 68% of the elective resection patients underwent minimally invasive surgery. The study’s primary endpoint was recurrent diverticulitis defined as an acute episode confirmed by computed tomography and requiring hospitalization with intravenous antibiotics.
Diverticulitis accounted for more than 300,000 hospital admissions in 2010 in the United States alone, and 10%-20% of patients had abscess formation. At one time, most patients were managed with immediate operative intervention, but medical and radiologic advances have led to a shift toward nonoperative management, Dr. Bendl said.
Some prior studies have suggested that recurrence rates are higher with nonoperative management, and the current study supports those data.
However, despite the significant increase in the recurrence rate with observation vs. resection, most patients in the observation group did not experience recurrence, and of those who did, none had peritonitis.
“All those with recurrences were successfully treated again using nonoperative management,” he said.
This study was supported in part by grants from Merck and Covidien. Dr. Bendl reported having no relevant financial disclosures.
CHICAGO – Observation, compared with elective resection, was associated with significantly increased recurrence rates in a single-center randomized, controlled trial of patients who had successfully recovered via nonoperative management from their first episode of acute sigmoid diverticulitis with extraluminal air/abscess.
Recurrence rates in 111 patients randomized to observation or elective resection were 31% in the observation group and 7% in the resection group, at 15 and 18 months, respectively, Dr. Ryan Bendl of State University of New York, Stony Brook reported at the annual meeting of the American Surgical Association.
Patients in the two groups were comparable with respect to age, sex, body mass index, Colorectal Physiologic and Operative Severity Score for the Enumeration of Mortality and Morbidity (CR-POSSUM), and comorbidities, he noted.
Subjects included in the single-center study were adults admitted for a first episode of acute diverticulitis with abscess or extraluminal air who were managed nonoperatively with intravenous antibiotics, a period of nothing by mouth, drainage, and total parenteral nutrition followed by colonoscopy. They were randomized 3:1 to observation or resection, and 68% of the elective resection patients underwent minimally invasive surgery. The study’s primary endpoint was recurrent diverticulitis defined as an acute episode confirmed by computed tomography and requiring hospitalization with intravenous antibiotics.
Diverticulitis accounted for more than 300,000 hospital admissions in 2010 in the United States alone, and 10%-20% of patients had abscess formation. At one time, most patients were managed with immediate operative intervention, but medical and radiologic advances have led to a shift toward nonoperative management, Dr. Bendl said.
Some prior studies have suggested that recurrence rates are higher with nonoperative management, and the current study supports those data.
However, despite the significant increase in the recurrence rate with observation vs. resection, most patients in the observation group did not experience recurrence, and of those who did, none had peritonitis.
“All those with recurrences were successfully treated again using nonoperative management,” he said.
This study was supported in part by grants from Merck and Covidien. Dr. Bendl reported having no relevant financial disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point: Observation vs. elective resection was associated with significantly increased recurrence rates in patients who had recovered via nonoperative management from their first episode of acute sigmoid diverticulitis with extraluminal air/abscess.
Major finding: Recurrence rates in 111 patients randomized to observation or elective resection were 31% in the observation group and 7% in the resection group, at 15 and 18 months, respectively.
Data source: A randomized, controlled trial involving 111 patients.
Disclosures: This study was supported in part by grants from Merck and Covidien. Dr. Bendl reported having no relevant financial disclosures.
Kawasaki disease and infections aren’t mutually exclusive
CHICAGO – Kawasaki disease and concurrent bacterial or viral infection are by no means mutually exclusive, Dr. Cathie-Kim Le cautioned at the annual meeting of the American College of Cardiology.
“Recognizing that both can coexist will ensure timely IVIg [intravenous immunoglobulin] treatment and appropriate containment of coronary artery complications,” said Dr. Le of Sainte-Justine University Hospital in Montreal.
She presented a retrospective study of 128 patients, mean age 3.4 years, admitted to the pediatric academic tertiary care center with a discharge diagnosis of Kawasaki disease. During their hospitalization all of them underwent a work-up for bacterial and viral infectious diseases, which proved positive in 33% of cases. Roughly 40% of subjects had incomplete Kawasaki disease, meaning they lacked a sufficient number of signs of mucocutaneous inflammation to fulfill the epidemiologic case definition; however, their prevalence of concomitant infection was similar to that of the group with classic Kawasaki disease.
Among the most common types of infections in patients with Kawasaki disease were otitis media, which accounted for 17% of the infections; upper respiratory infections, 21%; and group A streptococcal pharyngitis and pneumonia, which accounted for 14% each.
There were no differences in clinical presentation or laboratory values between children with or without concomitant infection. Nor was myocardial profiling useful in differentiating patients with concomitant infection from those without: Ventricular shortening fraction scores and N-terminal probrain natriuretic peptide levels were similar in the two groups of Kawasaki disease patients.
Acute coronary dilatation occurred in 26% of Kawasaki disease patients with concomitant infection and similarly in 30% of those without infection. Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with infection and an identical proportion of the uninfected.
Almost all patients received IVIg. Resistance to the effects of IVIg occurred in 36% of patients with concomitant infection, a rate twice that seen in the group without infection. Coronary aneurysms and dilatations were significantly more common in IVIg-resistant patients, regardless of their infection status.
Dr. Le reported having no relevant financial conflicts.
CHICAGO – Kawasaki disease and concurrent bacterial or viral infection are by no means mutually exclusive, Dr. Cathie-Kim Le cautioned at the annual meeting of the American College of Cardiology.
“Recognizing that both can coexist will ensure timely IVIg [intravenous immunoglobulin] treatment and appropriate containment of coronary artery complications,” said Dr. Le of Sainte-Justine University Hospital in Montreal.
She presented a retrospective study of 128 patients, mean age 3.4 years, admitted to the pediatric academic tertiary care center with a discharge diagnosis of Kawasaki disease. During their hospitalization all of them underwent a work-up for bacterial and viral infectious diseases, which proved positive in 33% of cases. Roughly 40% of subjects had incomplete Kawasaki disease, meaning they lacked a sufficient number of signs of mucocutaneous inflammation to fulfill the epidemiologic case definition; however, their prevalence of concomitant infection was similar to that of the group with classic Kawasaki disease.
Among the most common types of infections in patients with Kawasaki disease were otitis media, which accounted for 17% of the infections; upper respiratory infections, 21%; and group A streptococcal pharyngitis and pneumonia, which accounted for 14% each.
There were no differences in clinical presentation or laboratory values between children with or without concomitant infection. Nor was myocardial profiling useful in differentiating patients with concomitant infection from those without: Ventricular shortening fraction scores and N-terminal probrain natriuretic peptide levels were similar in the two groups of Kawasaki disease patients.
Acute coronary dilatation occurred in 26% of Kawasaki disease patients with concomitant infection and similarly in 30% of those without infection. Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with infection and an identical proportion of the uninfected.
Almost all patients received IVIg. Resistance to the effects of IVIg occurred in 36% of patients with concomitant infection, a rate twice that seen in the group without infection. Coronary aneurysms and dilatations were significantly more common in IVIg-resistant patients, regardless of their infection status.
Dr. Le reported having no relevant financial conflicts.
CHICAGO – Kawasaki disease and concurrent bacterial or viral infection are by no means mutually exclusive, Dr. Cathie-Kim Le cautioned at the annual meeting of the American College of Cardiology.
“Recognizing that both can coexist will ensure timely IVIg [intravenous immunoglobulin] treatment and appropriate containment of coronary artery complications,” said Dr. Le of Sainte-Justine University Hospital in Montreal.
She presented a retrospective study of 128 patients, mean age 3.4 years, admitted to the pediatric academic tertiary care center with a discharge diagnosis of Kawasaki disease. During their hospitalization all of them underwent a work-up for bacterial and viral infectious diseases, which proved positive in 33% of cases. Roughly 40% of subjects had incomplete Kawasaki disease, meaning they lacked a sufficient number of signs of mucocutaneous inflammation to fulfill the epidemiologic case definition; however, their prevalence of concomitant infection was similar to that of the group with classic Kawasaki disease.
Among the most common types of infections in patients with Kawasaki disease were otitis media, which accounted for 17% of the infections; upper respiratory infections, 21%; and group A streptococcal pharyngitis and pneumonia, which accounted for 14% each.
There were no differences in clinical presentation or laboratory values between children with or without concomitant infection. Nor was myocardial profiling useful in differentiating patients with concomitant infection from those without: Ventricular shortening fraction scores and N-terminal probrain natriuretic peptide levels were similar in the two groups of Kawasaki disease patients.
Acute coronary dilatation occurred in 26% of Kawasaki disease patients with concomitant infection and similarly in 30% of those without infection. Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with infection and an identical proportion of the uninfected.
Almost all patients received IVIg. Resistance to the effects of IVIg occurred in 36% of patients with concomitant infection, a rate twice that seen in the group without infection. Coronary aneurysms and dilatations were significantly more common in IVIg-resistant patients, regardless of their infection status.
Dr. Le reported having no relevant financial conflicts.
AT ACC 16
Key clinical point: One-third of Kawasaki patients have a concomitant common infection that may delay timely diagnosis.
Major finding: Coronary aneurysm, the most serious complication of Kawasaki disease, occurred in 14% of patients with an infection and in 14% of the uninfected.
Data source: A retrospective study of 128 children with Kawasaki disease.
Disclosures: The study presenter reported having no relevant financial conflicts.
Advances in Hematology and Oncology (May 2016)
Table of Contents
- Introduction
- Consensus Statement Supporting the Recommendation for Single-Fraction Palliative Radiotherapy for Uncomplicated, Painful Bone Metastases
- The Availability of Advanced Radiation Oncology Technology Within VHA Radiation Oncology Centers
- γ-δ T-Cell Lymphoma With Disseminated Intravascular Coagulation and Autoimmune Hemolytic Anemia
- Use of Fluorodeoxyglucose-Positron Emission Tomography in the Diagnosis of Intravascular Diffuse Large B-Cell Lymphoma
- Prevalence of Hypogonadism in Low-Risk Prostate Cancer Survivors
- Molecular Profiles Guide Colorectal Cancer Treatment
- Treating VA Patients With Multiple Myeloma

Table of Contents
- Introduction
- Consensus Statement Supporting the Recommendation for Single-Fraction Palliative Radiotherapy for Uncomplicated, Painful Bone Metastases
- The Availability of Advanced Radiation Oncology Technology Within VHA Radiation Oncology Centers
- γ-δ T-Cell Lymphoma With Disseminated Intravascular Coagulation and Autoimmune Hemolytic Anemia
- Use of Fluorodeoxyglucose-Positron Emission Tomography in the Diagnosis of Intravascular Diffuse Large B-Cell Lymphoma
- Prevalence of Hypogonadism in Low-Risk Prostate Cancer Survivors
- Molecular Profiles Guide Colorectal Cancer Treatment
- Treating VA Patients With Multiple Myeloma

Table of Contents
- Introduction
- Consensus Statement Supporting the Recommendation for Single-Fraction Palliative Radiotherapy for Uncomplicated, Painful Bone Metastases
- The Availability of Advanced Radiation Oncology Technology Within VHA Radiation Oncology Centers
- γ-δ T-Cell Lymphoma With Disseminated Intravascular Coagulation and Autoimmune Hemolytic Anemia
- Use of Fluorodeoxyglucose-Positron Emission Tomography in the Diagnosis of Intravascular Diffuse Large B-Cell Lymphoma
- Prevalence of Hypogonadism in Low-Risk Prostate Cancer Survivors
- Molecular Profiles Guide Colorectal Cancer Treatment
- Treating VA Patients With Multiple Myeloma

Thrill-Seeking Hospitalist Alleviates Stress Through Scuba Diving
Not much intimidates Jasen Gundersen, MD, president of the acute care services division at TeamHealth, an outsourcer of hospital-based clinical and specialty services based in Knoxville, Tenn. Besides traveling 150,000 miles a year overseeing 2,500 hospitalists at 285 facilities, Dr. Gundersen has climbed frozen waterfalls in Vermont and New Hampshire, raced in bicycle competitions, and skied mountains towering 10,000 feet.
But his love for adventure is now focused below the surface. Over the years, he has spent many weekends diving in open waters surrounding southeast Florida; Cozumel, Mexico; Turks and Caicos; and the Cayman Islands. He believes there’s no place on Earth that is as peaceful, serene, or even magical as under the ocean.
Reclaimed Passion
Growing up in Connecticut, Dr. Gundersen and his family frequently vacationed in the Bahamas, where he was introduced to scuba diving.
“As a teenager, I really loved diving,” he recalls. “Every time we went to the Bahamas, I always tried to go diving or snorkeling.”
However, the harsh Connecticut winters and frigid Atlantic Ocean prevented him from diving. More delays followed, namely medical school. After graduating from the University of Connecticut School of Medicine in 2000, Dr. Gundersen completed his three-year residency in family medicine at the UMass Memorial Medical Center. During the next two years, he worked as a physician and hospitalist at the Family Health Center of Worcester, a federally qualified health center where he did everything from examining sore throats to delivering babies.
In 2005, he launched a small hospital medicine program at the University of Massachusetts that quickly grew and bumped up his title to division chief for hospital medicine. Then in January 2011, he accepted a new position as chief medical officer at TeamHealth, requiring him and his wife, Elizabeth, also a hospitalist, to move to Florida.
Within several weeks, the couple started diving near their home in Pompano Beach. He says Elizabeth, his “diving buddy,” was eager to learn and developed a passion for scuba diving that rivals his own.
“We did 80 to 90 dives in the first year we were down there,” Dr. Gundersen says, explaining that unlike many sports, diving doesn’t require athletic ability, size, or strength. “We normally did recreational diving, where you basically can always swim slowly straight to the surface. You don’t stay down long enough that you build up enough bubbles in your system that you have to stop on the way up.”
Sharks and Shipwrecks
Since then, Dr. Gundersen purchased a 38-foot powerboat, became a PADI (Professional Association of Diving Instructors) open-water scuba instructor, and earned a U.S. Coast Guard 50-ton master captain’s license. He and Elizabeth are certified for advanced nitrox and decompression diving, technical diving that requires the use of different gases to decompress when heading to the surface, and diving in overhead environments, such as caves or shipwrecks.
“One of our favorite wrecks is called the USS Spiegel Gove that sits on the ocean floor in Key Largo,” he says, adding that on occasion, they also swim with hammerhead sharks. “The walls of the ship go 30 feet up on each side. You can swim where they loaded the cargo and see the old crane above you. It’s spectacular.”
Among their favorite spots to dive is Eagle Ray Pass in Grand Cayman, where entire schools of spotted eagle rays live, he says, adding that 17 rays swam and floated around them during one dive.
Fortunately, after some initial costs, he says the sport isn’t too expensive, roughly around $1,500 to get started. Basic scuba gear costs approximately $1,000. Likewise, certifications can run $350 a piece. Boat trips range between $60 and $100, unless you prefer shore diving, where you park at the beach and simply swim into the ocean. Then add a few extra dollars to fill your tank with air.
Scary and Serene
Although the Gundersens are accomplished divers who prefer warm waters and flat seas, Dr. Gundersen says only one moment of one dive actually scared him.
Years ago, he, Elizabeth, and a friend were wreck diving. Diving protocol is based on follow the leader, where divers swim into wrecks one at a time, follow each other, and signal their turns. Somehow, their friend unintentionally swam in between Dr. Gundersen and his wife. Elizabeth and the friend then turned to see something inside the wreck, but the friend failed to signal to Dr. Gundersen that they were turning.
“I went a bit farther and turned around,” Dr. Gundersen recalls. “He and Elizabeth were gone. It gave me a moment of panic. I’m particularly careful about staying with my diving buddy and making sure we don’t get lost. It wasn’t dangerous but broke the cardinal rule of what you’re supposed to do when diving. I swam back and found them.”
While that was a rare experience, he says diving, when done properly, is the most peaceful and serene activity that people may experience. When under water, all you hear are your air bubbles. There are no cellphones ringing, emails or texts to respond to, or work issues to resolve.
“Work-life balance is a really big deal for me and my team to prevent burnout,” Dr. Gundersen says. “It allows me to have my personal time to enjoy and relax so when I’m back at work on Monday, my batteries are recharged. I’m ready to go.” TH
Carol Patton is a freelance writer in Las Vegas.
Not much intimidates Jasen Gundersen, MD, president of the acute care services division at TeamHealth, an outsourcer of hospital-based clinical and specialty services based in Knoxville, Tenn. Besides traveling 150,000 miles a year overseeing 2,500 hospitalists at 285 facilities, Dr. Gundersen has climbed frozen waterfalls in Vermont and New Hampshire, raced in bicycle competitions, and skied mountains towering 10,000 feet.
But his love for adventure is now focused below the surface. Over the years, he has spent many weekends diving in open waters surrounding southeast Florida; Cozumel, Mexico; Turks and Caicos; and the Cayman Islands. He believes there’s no place on Earth that is as peaceful, serene, or even magical as under the ocean.
Reclaimed Passion
Growing up in Connecticut, Dr. Gundersen and his family frequently vacationed in the Bahamas, where he was introduced to scuba diving.
“As a teenager, I really loved diving,” he recalls. “Every time we went to the Bahamas, I always tried to go diving or snorkeling.”
However, the harsh Connecticut winters and frigid Atlantic Ocean prevented him from diving. More delays followed, namely medical school. After graduating from the University of Connecticut School of Medicine in 2000, Dr. Gundersen completed his three-year residency in family medicine at the UMass Memorial Medical Center. During the next two years, he worked as a physician and hospitalist at the Family Health Center of Worcester, a federally qualified health center where he did everything from examining sore throats to delivering babies.
In 2005, he launched a small hospital medicine program at the University of Massachusetts that quickly grew and bumped up his title to division chief for hospital medicine. Then in January 2011, he accepted a new position as chief medical officer at TeamHealth, requiring him and his wife, Elizabeth, also a hospitalist, to move to Florida.
Within several weeks, the couple started diving near their home in Pompano Beach. He says Elizabeth, his “diving buddy,” was eager to learn and developed a passion for scuba diving that rivals his own.
“We did 80 to 90 dives in the first year we were down there,” Dr. Gundersen says, explaining that unlike many sports, diving doesn’t require athletic ability, size, or strength. “We normally did recreational diving, where you basically can always swim slowly straight to the surface. You don’t stay down long enough that you build up enough bubbles in your system that you have to stop on the way up.”
Sharks and Shipwrecks
Since then, Dr. Gundersen purchased a 38-foot powerboat, became a PADI (Professional Association of Diving Instructors) open-water scuba instructor, and earned a U.S. Coast Guard 50-ton master captain’s license. He and Elizabeth are certified for advanced nitrox and decompression diving, technical diving that requires the use of different gases to decompress when heading to the surface, and diving in overhead environments, such as caves or shipwrecks.
“One of our favorite wrecks is called the USS Spiegel Gove that sits on the ocean floor in Key Largo,” he says, adding that on occasion, they also swim with hammerhead sharks. “The walls of the ship go 30 feet up on each side. You can swim where they loaded the cargo and see the old crane above you. It’s spectacular.”
Among their favorite spots to dive is Eagle Ray Pass in Grand Cayman, where entire schools of spotted eagle rays live, he says, adding that 17 rays swam and floated around them during one dive.
Fortunately, after some initial costs, he says the sport isn’t too expensive, roughly around $1,500 to get started. Basic scuba gear costs approximately $1,000. Likewise, certifications can run $350 a piece. Boat trips range between $60 and $100, unless you prefer shore diving, where you park at the beach and simply swim into the ocean. Then add a few extra dollars to fill your tank with air.
Scary and Serene
Although the Gundersens are accomplished divers who prefer warm waters and flat seas, Dr. Gundersen says only one moment of one dive actually scared him.
Years ago, he, Elizabeth, and a friend were wreck diving. Diving protocol is based on follow the leader, where divers swim into wrecks one at a time, follow each other, and signal their turns. Somehow, their friend unintentionally swam in between Dr. Gundersen and his wife. Elizabeth and the friend then turned to see something inside the wreck, but the friend failed to signal to Dr. Gundersen that they were turning.
“I went a bit farther and turned around,” Dr. Gundersen recalls. “He and Elizabeth were gone. It gave me a moment of panic. I’m particularly careful about staying with my diving buddy and making sure we don’t get lost. It wasn’t dangerous but broke the cardinal rule of what you’re supposed to do when diving. I swam back and found them.”
While that was a rare experience, he says diving, when done properly, is the most peaceful and serene activity that people may experience. When under water, all you hear are your air bubbles. There are no cellphones ringing, emails or texts to respond to, or work issues to resolve.
“Work-life balance is a really big deal for me and my team to prevent burnout,” Dr. Gundersen says. “It allows me to have my personal time to enjoy and relax so when I’m back at work on Monday, my batteries are recharged. I’m ready to go.” TH
Carol Patton is a freelance writer in Las Vegas.
Not much intimidates Jasen Gundersen, MD, president of the acute care services division at TeamHealth, an outsourcer of hospital-based clinical and specialty services based in Knoxville, Tenn. Besides traveling 150,000 miles a year overseeing 2,500 hospitalists at 285 facilities, Dr. Gundersen has climbed frozen waterfalls in Vermont and New Hampshire, raced in bicycle competitions, and skied mountains towering 10,000 feet.
But his love for adventure is now focused below the surface. Over the years, he has spent many weekends diving in open waters surrounding southeast Florida; Cozumel, Mexico; Turks and Caicos; and the Cayman Islands. He believes there’s no place on Earth that is as peaceful, serene, or even magical as under the ocean.
Reclaimed Passion
Growing up in Connecticut, Dr. Gundersen and his family frequently vacationed in the Bahamas, where he was introduced to scuba diving.
“As a teenager, I really loved diving,” he recalls. “Every time we went to the Bahamas, I always tried to go diving or snorkeling.”
However, the harsh Connecticut winters and frigid Atlantic Ocean prevented him from diving. More delays followed, namely medical school. After graduating from the University of Connecticut School of Medicine in 2000, Dr. Gundersen completed his three-year residency in family medicine at the UMass Memorial Medical Center. During the next two years, he worked as a physician and hospitalist at the Family Health Center of Worcester, a federally qualified health center where he did everything from examining sore throats to delivering babies.
In 2005, he launched a small hospital medicine program at the University of Massachusetts that quickly grew and bumped up his title to division chief for hospital medicine. Then in January 2011, he accepted a new position as chief medical officer at TeamHealth, requiring him and his wife, Elizabeth, also a hospitalist, to move to Florida.
Within several weeks, the couple started diving near their home in Pompano Beach. He says Elizabeth, his “diving buddy,” was eager to learn and developed a passion for scuba diving that rivals his own.
“We did 80 to 90 dives in the first year we were down there,” Dr. Gundersen says, explaining that unlike many sports, diving doesn’t require athletic ability, size, or strength. “We normally did recreational diving, where you basically can always swim slowly straight to the surface. You don’t stay down long enough that you build up enough bubbles in your system that you have to stop on the way up.”
Sharks and Shipwrecks
Since then, Dr. Gundersen purchased a 38-foot powerboat, became a PADI (Professional Association of Diving Instructors) open-water scuba instructor, and earned a U.S. Coast Guard 50-ton master captain’s license. He and Elizabeth are certified for advanced nitrox and decompression diving, technical diving that requires the use of different gases to decompress when heading to the surface, and diving in overhead environments, such as caves or shipwrecks.
“One of our favorite wrecks is called the USS Spiegel Gove that sits on the ocean floor in Key Largo,” he says, adding that on occasion, they also swim with hammerhead sharks. “The walls of the ship go 30 feet up on each side. You can swim where they loaded the cargo and see the old crane above you. It’s spectacular.”
Among their favorite spots to dive is Eagle Ray Pass in Grand Cayman, where entire schools of spotted eagle rays live, he says, adding that 17 rays swam and floated around them during one dive.
Fortunately, after some initial costs, he says the sport isn’t too expensive, roughly around $1,500 to get started. Basic scuba gear costs approximately $1,000. Likewise, certifications can run $350 a piece. Boat trips range between $60 and $100, unless you prefer shore diving, where you park at the beach and simply swim into the ocean. Then add a few extra dollars to fill your tank with air.
Scary and Serene
Although the Gundersens are accomplished divers who prefer warm waters and flat seas, Dr. Gundersen says only one moment of one dive actually scared him.
Years ago, he, Elizabeth, and a friend were wreck diving. Diving protocol is based on follow the leader, where divers swim into wrecks one at a time, follow each other, and signal their turns. Somehow, their friend unintentionally swam in between Dr. Gundersen and his wife. Elizabeth and the friend then turned to see something inside the wreck, but the friend failed to signal to Dr. Gundersen that they were turning.
“I went a bit farther and turned around,” Dr. Gundersen recalls. “He and Elizabeth were gone. It gave me a moment of panic. I’m particularly careful about staying with my diving buddy and making sure we don’t get lost. It wasn’t dangerous but broke the cardinal rule of what you’re supposed to do when diving. I swam back and found them.”
While that was a rare experience, he says diving, when done properly, is the most peaceful and serene activity that people may experience. When under water, all you hear are your air bubbles. There are no cellphones ringing, emails or texts to respond to, or work issues to resolve.
“Work-life balance is a really big deal for me and my team to prevent burnout,” Dr. Gundersen says. “It allows me to have my personal time to enjoy and relax so when I’m back at work on Monday, my batteries are recharged. I’m ready to go.” TH
Carol Patton is a freelance writer in Las Vegas.
May 2016 Digital Edition
Click here to access the May 2016 Digital Edition.
Table of Contents
- Why VA Health Care Is Different
- Anterior Cervical Interbody Fusion Using Polyetheretherketone (PEEK) Cage Device and Local Autograft Bone
- Academic Reasonable Accommodations for Post-9/11 Veterans With Psychiatric Diagnoses, Part 2
- A Real Welcome Home: Permanent Housing for Homeless Veterans
- Veterans’ Satisfaction With Erectile Dysfunction Treatment
- VA PBM Academic Detailing Service: Implementation and Lessons Learned
- Adherence to Disease-Modifying Therapies in Patients With MS: A Retrospective Cohort Study
- The Role of Fidaxomicin in Clostridium difficile Infection

Click here to access the May 2016 Digital Edition.
Table of Contents
- Why VA Health Care Is Different
- Anterior Cervical Interbody Fusion Using Polyetheretherketone (PEEK) Cage Device and Local Autograft Bone
- Academic Reasonable Accommodations for Post-9/11 Veterans With Psychiatric Diagnoses, Part 2
- A Real Welcome Home: Permanent Housing for Homeless Veterans
- Veterans’ Satisfaction With Erectile Dysfunction Treatment
- VA PBM Academic Detailing Service: Implementation and Lessons Learned
- Adherence to Disease-Modifying Therapies in Patients With MS: A Retrospective Cohort Study
- The Role of Fidaxomicin in Clostridium difficile Infection

Click here to access the May 2016 Digital Edition.
Table of Contents
- Why VA Health Care Is Different
- Anterior Cervical Interbody Fusion Using Polyetheretherketone (PEEK) Cage Device and Local Autograft Bone
- Academic Reasonable Accommodations for Post-9/11 Veterans With Psychiatric Diagnoses, Part 2
- A Real Welcome Home: Permanent Housing for Homeless Veterans
- Veterans’ Satisfaction With Erectile Dysfunction Treatment
- VA PBM Academic Detailing Service: Implementation and Lessons Learned
- Adherence to Disease-Modifying Therapies in Patients With MS: A Retrospective Cohort Study
- The Role of Fidaxomicin in Clostridium difficile Infection

Cell-based strategy curbs constipation
Treatment at the nanomolecular level may become an alternative for various types of constipation, in particular for the opioid-induced constipation that is common after surgery, based on data from a proof of concept study involving mice and a small-molecule activator.
“Activation of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is the primary pathway that drives fluid secretion in the intestine, which maintains lubrication of luminal contents,” wrote Dr. Onur Cil of the University of California, San Francisco, and colleagues. The researchers examined whether direct activation of the CFTR would prompt fluid secretion and reverse stool dehydration when applied to constipated mice. The findings were published online in the May issue of the journal Cellular and Molecular Gastroenterology and Hepatology (2016. doi: 10.1016/j.jcmgh.2015.12.010).
The researchers identified a promising activator, the phenylquinoxalinone CFTRact-J027. Mice received up to 10 mg/kg CFTRact-J027 either orally or intraperitoneally (IP), with doses including 0.1 mg/kg, 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 10 mg/kg.
Overall, IP doses of CFTRact-J027 at 10 mg/kg normalized stool in the constipated mice, and dose-response studies showed a 50% effective dose of 2 mg/kg in these mice.
When given orally, CFTRact-J027 “normalized stool output and water content in a loperamide-induced mouse model of constipation with a 50% effective dose of approximately 0.5 mg/kg,” that was significantly lower than the IP administration, the researchers noted. An oral dose of 10 mg/kg CFTRact-J027 1 hour before inducing constipation also was effective in normalizing stool output and water content in loperamide-treated mice, with no effect in control nonconstipated mice.
The activator was not effective against constipation in cystic fibrosis mice that were missing a functional CFTR, they added.
The researchers used an in vivo closed loop model to specifically test the effects of CFTRact-J027 on intestinal fluid secretion and absorption and found that CFTRact-J027 caused “a 140% increase in loop weight/length ratio, indicating fluid secretion into the intestinal lumen in wild-type mice.” However, there was no effect in cystic fibrosis mice, further supporting the CFTR-selective mechanism of action, the researchers said.
As for potential toxic effects of the treatment, CFTRact-J027 showed no impact on the major serum chemistry and blood parameters of the mice after 7 days, and had no apparent impact on body weight. No accumulation of fluid (the most significant potential adverse effect) was noted in the airway or lungs of the treated mice.
Additional toxicity data are needed to continue preclinical development, the researchers said. However, “our data provide evidence for the prosecretory action of a CFTR activator in mouse intestine and proof of concept for its use in the treatment of various types of constipation, which could include opioid-induced constipation, chronic idiopathic constipation, and irritable bowel syndrome with constipation predominance,” they wrote. In addition, a CFTR activator similar to that used in this study may have clinical applications for other conditions including asthma, dry eye, cholestatic liver disease, chronic obstructive pulmonary disease and bronchitis, and cigarette smoke–induced lung dysfunction, they added.
Dr. Cil and two coauthors are inventors on a provisional patent filing, with rights owned by the University of California, San Francisco. The study was funded in part by several grants from organizations including the National Institutes of Health and the Cystic Fibrosis Foundation.
Treatment at the nanomolecular level may become an alternative for various types of constipation, in particular for the opioid-induced constipation that is common after surgery, based on data from a proof of concept study involving mice and a small-molecule activator.
“Activation of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is the primary pathway that drives fluid secretion in the intestine, which maintains lubrication of luminal contents,” wrote Dr. Onur Cil of the University of California, San Francisco, and colleagues. The researchers examined whether direct activation of the CFTR would prompt fluid secretion and reverse stool dehydration when applied to constipated mice. The findings were published online in the May issue of the journal Cellular and Molecular Gastroenterology and Hepatology (2016. doi: 10.1016/j.jcmgh.2015.12.010).
The researchers identified a promising activator, the phenylquinoxalinone CFTRact-J027. Mice received up to 10 mg/kg CFTRact-J027 either orally or intraperitoneally (IP), with doses including 0.1 mg/kg, 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 10 mg/kg.
Overall, IP doses of CFTRact-J027 at 10 mg/kg normalized stool in the constipated mice, and dose-response studies showed a 50% effective dose of 2 mg/kg in these mice.
When given orally, CFTRact-J027 “normalized stool output and water content in a loperamide-induced mouse model of constipation with a 50% effective dose of approximately 0.5 mg/kg,” that was significantly lower than the IP administration, the researchers noted. An oral dose of 10 mg/kg CFTRact-J027 1 hour before inducing constipation also was effective in normalizing stool output and water content in loperamide-treated mice, with no effect in control nonconstipated mice.
The activator was not effective against constipation in cystic fibrosis mice that were missing a functional CFTR, they added.
The researchers used an in vivo closed loop model to specifically test the effects of CFTRact-J027 on intestinal fluid secretion and absorption and found that CFTRact-J027 caused “a 140% increase in loop weight/length ratio, indicating fluid secretion into the intestinal lumen in wild-type mice.” However, there was no effect in cystic fibrosis mice, further supporting the CFTR-selective mechanism of action, the researchers said.
As for potential toxic effects of the treatment, CFTRact-J027 showed no impact on the major serum chemistry and blood parameters of the mice after 7 days, and had no apparent impact on body weight. No accumulation of fluid (the most significant potential adverse effect) was noted in the airway or lungs of the treated mice.
Additional toxicity data are needed to continue preclinical development, the researchers said. However, “our data provide evidence for the prosecretory action of a CFTR activator in mouse intestine and proof of concept for its use in the treatment of various types of constipation, which could include opioid-induced constipation, chronic idiopathic constipation, and irritable bowel syndrome with constipation predominance,” they wrote. In addition, a CFTR activator similar to that used in this study may have clinical applications for other conditions including asthma, dry eye, cholestatic liver disease, chronic obstructive pulmonary disease and bronchitis, and cigarette smoke–induced lung dysfunction, they added.
Dr. Cil and two coauthors are inventors on a provisional patent filing, with rights owned by the University of California, San Francisco. The study was funded in part by several grants from organizations including the National Institutes of Health and the Cystic Fibrosis Foundation.
Treatment at the nanomolecular level may become an alternative for various types of constipation, in particular for the opioid-induced constipation that is common after surgery, based on data from a proof of concept study involving mice and a small-molecule activator.
“Activation of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is the primary pathway that drives fluid secretion in the intestine, which maintains lubrication of luminal contents,” wrote Dr. Onur Cil of the University of California, San Francisco, and colleagues. The researchers examined whether direct activation of the CFTR would prompt fluid secretion and reverse stool dehydration when applied to constipated mice. The findings were published online in the May issue of the journal Cellular and Molecular Gastroenterology and Hepatology (2016. doi: 10.1016/j.jcmgh.2015.12.010).
The researchers identified a promising activator, the phenylquinoxalinone CFTRact-J027. Mice received up to 10 mg/kg CFTRact-J027 either orally or intraperitoneally (IP), with doses including 0.1 mg/kg, 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 10 mg/kg.
Overall, IP doses of CFTRact-J027 at 10 mg/kg normalized stool in the constipated mice, and dose-response studies showed a 50% effective dose of 2 mg/kg in these mice.
When given orally, CFTRact-J027 “normalized stool output and water content in a loperamide-induced mouse model of constipation with a 50% effective dose of approximately 0.5 mg/kg,” that was significantly lower than the IP administration, the researchers noted. An oral dose of 10 mg/kg CFTRact-J027 1 hour before inducing constipation also was effective in normalizing stool output and water content in loperamide-treated mice, with no effect in control nonconstipated mice.
The activator was not effective against constipation in cystic fibrosis mice that were missing a functional CFTR, they added.
The researchers used an in vivo closed loop model to specifically test the effects of CFTRact-J027 on intestinal fluid secretion and absorption and found that CFTRact-J027 caused “a 140% increase in loop weight/length ratio, indicating fluid secretion into the intestinal lumen in wild-type mice.” However, there was no effect in cystic fibrosis mice, further supporting the CFTR-selective mechanism of action, the researchers said.
As for potential toxic effects of the treatment, CFTRact-J027 showed no impact on the major serum chemistry and blood parameters of the mice after 7 days, and had no apparent impact on body weight. No accumulation of fluid (the most significant potential adverse effect) was noted in the airway or lungs of the treated mice.
Additional toxicity data are needed to continue preclinical development, the researchers said. However, “our data provide evidence for the prosecretory action of a CFTR activator in mouse intestine and proof of concept for its use in the treatment of various types of constipation, which could include opioid-induced constipation, chronic idiopathic constipation, and irritable bowel syndrome with constipation predominance,” they wrote. In addition, a CFTR activator similar to that used in this study may have clinical applications for other conditions including asthma, dry eye, cholestatic liver disease, chronic obstructive pulmonary disease and bronchitis, and cigarette smoke–induced lung dysfunction, they added.
Dr. Cil and two coauthors are inventors on a provisional patent filing, with rights owned by the University of California, San Francisco. The study was funded in part by several grants from organizations including the National Institutes of Health and the Cystic Fibrosis Foundation.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Treatment at the nanomolecular level may become an alternative for various types of constipation.
Major finding: Oral doses of CFTRact-J027 normalized stool output and water content in a loperamide-induced mouse model of constipation with a 50% effective dose of approximately 0.5 mg/kg.
Data source: A proof-of-concept study in which a cell-based screen was performed for 120,000 druglike, synthetic small molecules that were then tested in constipation-induced mice and control mice.
Disclosures: Dr. Cil and two coauthors are inventors on a provisional patent filing, with rights owned by the University of California, San Francisco. The study was funded in part by several grants from organizations including the National Institutes of Health and the Cystic Fibrosis Foundation.
Cirrhosis 30-day readmissions down 40% with quality improvement initiative
Using checklists and electronic decision support in an inpatient liver unit, quality improvement (QI) care protocols reduced 30-day readmissions of patients with cirrhosis by 40%, due mostly to a drop in readmissions for hepatic encephalopathy (HE) according to a report published in the May issue of Clinical Gastroenterology and Hepatology.
For patients initially admitted for overt HE, the 30-day readmission rate was 26.0% (27 of 104), compared with 48.9% (66 of 135) before implementation of QI. The proportion of total readmissions due to HE after QI was 9.6% (14 of 146), compared with 40.7% (79 of 194) before QI. In addition, length of stay for HE patients was significantly reduced (–1.34 days; 95% confidence interval, –2.38 to –0.32; P = .01). There were no significant changes in 90-day mortality.
Source: American Gastroenterological Association
“Our study advances the current literature on QI for patients with cirrhosis by presenting an inexpensive, easy to implement, and generalizable approach,” wrote Dr. Elliot Tapper of Beth Israel Deaconess Medical Center, Boston, and his colleagues. Previous studies have addressed readmission interventions among patients with cirrhosis, but the protocols required costly infrastructure, expertise, and institutional commitments. The current study supports the value of standard checklists and education, according to the investigators, “showing that outcomes improve further when checklist items are hard-wired into the ordering system.” (Clin Gastroenterol Hepatol. 2016 Apr 7. doi: 10.1016/j.cgh.2015.08.041).
The QI initiative encompassed several aspects of care. All HE patients were designated to receive rifaximin, and their lactulose dosing was adjusted to mental status using the Richmond Agitation and Sedation Scale. For patients with spontaneous bacterial peritonitis (SBP), timely administration of the correct dose of antibiotics and albumin was promoted, as were prophylactic measures for all patients, such as variceal hemorrhage prophylaxis and subcutaneous heparin for the prevention of venous thrombosis.
The three-part program entailed a run-in phase for preliminary checklist troubleshooting, a hand-held checklist phase, including the HE protocol, SBP treatment, and prophylactic measures, and a final electronic phase in which checklist items were incorporated into the hospital’s electronic provider order entry system using mandatory preset doses and linked medications.
Individual protocol items were demonstrated to affect the readmission rate. Rifaximin use for HE patients rose from 78.1% to 96.3%, and use of rifaximin was associated with lower adjusted odds of 30-day readmission (OR, 0.39; 95% CI, 0.16-0.87; P = .02). The dose/frequency of lactulose for HE patients increased, and patients who had 6 or more cups of lactulose on the day of their readmission had significantly lower adjusted length of stay (–2.36 days; 95% CI, –3.40 to –1.31; P less than .0001). Patients taking SBP prophylaxis had lower readmission rates (OR, 0.51; 95% CI, 0.31-0.83; P = .007).
The prospective study from 2011 to 2013 evaluated patients with cirrhosis who were admitted to the liver unit of Beth Israel Deaconess Medical Center, Boston. Patients were diagnosed with cirrhosis caused by hepatitis C (44.9%), alcoholic liver disease (34%), hepatitis B (5.4%), and biliary cirrhosis (1.8%). In total, 824 unique patients were admitted 1,720 times; 485 (58.9%) were admitted once, 268 (32.5%) were admitted 2-4 times, and 71 (8.6%) were admitted 5 or more times. The median length of stay for all patients was 4.0 days (interquartile range, 2.0-8.0).
Dr. Tapper and his coauthors reported having no disclosures.
Using checklists and electronic decision support in an inpatient liver unit, quality improvement (QI) care protocols reduced 30-day readmissions of patients with cirrhosis by 40%, due mostly to a drop in readmissions for hepatic encephalopathy (HE) according to a report published in the May issue of Clinical Gastroenterology and Hepatology.
For patients initially admitted for overt HE, the 30-day readmission rate was 26.0% (27 of 104), compared with 48.9% (66 of 135) before implementation of QI. The proportion of total readmissions due to HE after QI was 9.6% (14 of 146), compared with 40.7% (79 of 194) before QI. In addition, length of stay for HE patients was significantly reduced (–1.34 days; 95% confidence interval, –2.38 to –0.32; P = .01). There were no significant changes in 90-day mortality.
Source: American Gastroenterological Association
“Our study advances the current literature on QI for patients with cirrhosis by presenting an inexpensive, easy to implement, and generalizable approach,” wrote Dr. Elliot Tapper of Beth Israel Deaconess Medical Center, Boston, and his colleagues. Previous studies have addressed readmission interventions among patients with cirrhosis, but the protocols required costly infrastructure, expertise, and institutional commitments. The current study supports the value of standard checklists and education, according to the investigators, “showing that outcomes improve further when checklist items are hard-wired into the ordering system.” (Clin Gastroenterol Hepatol. 2016 Apr 7. doi: 10.1016/j.cgh.2015.08.041).
The QI initiative encompassed several aspects of care. All HE patients were designated to receive rifaximin, and their lactulose dosing was adjusted to mental status using the Richmond Agitation and Sedation Scale. For patients with spontaneous bacterial peritonitis (SBP), timely administration of the correct dose of antibiotics and albumin was promoted, as were prophylactic measures for all patients, such as variceal hemorrhage prophylaxis and subcutaneous heparin for the prevention of venous thrombosis.
The three-part program entailed a run-in phase for preliminary checklist troubleshooting, a hand-held checklist phase, including the HE protocol, SBP treatment, and prophylactic measures, and a final electronic phase in which checklist items were incorporated into the hospital’s electronic provider order entry system using mandatory preset doses and linked medications.
Individual protocol items were demonstrated to affect the readmission rate. Rifaximin use for HE patients rose from 78.1% to 96.3%, and use of rifaximin was associated with lower adjusted odds of 30-day readmission (OR, 0.39; 95% CI, 0.16-0.87; P = .02). The dose/frequency of lactulose for HE patients increased, and patients who had 6 or more cups of lactulose on the day of their readmission had significantly lower adjusted length of stay (–2.36 days; 95% CI, –3.40 to –1.31; P less than .0001). Patients taking SBP prophylaxis had lower readmission rates (OR, 0.51; 95% CI, 0.31-0.83; P = .007).
The prospective study from 2011 to 2013 evaluated patients with cirrhosis who were admitted to the liver unit of Beth Israel Deaconess Medical Center, Boston. Patients were diagnosed with cirrhosis caused by hepatitis C (44.9%), alcoholic liver disease (34%), hepatitis B (5.4%), and biliary cirrhosis (1.8%). In total, 824 unique patients were admitted 1,720 times; 485 (58.9%) were admitted once, 268 (32.5%) were admitted 2-4 times, and 71 (8.6%) were admitted 5 or more times. The median length of stay for all patients was 4.0 days (interquartile range, 2.0-8.0).
Dr. Tapper and his coauthors reported having no disclosures.
Using checklists and electronic decision support in an inpatient liver unit, quality improvement (QI) care protocols reduced 30-day readmissions of patients with cirrhosis by 40%, due mostly to a drop in readmissions for hepatic encephalopathy (HE) according to a report published in the May issue of Clinical Gastroenterology and Hepatology.
For patients initially admitted for overt HE, the 30-day readmission rate was 26.0% (27 of 104), compared with 48.9% (66 of 135) before implementation of QI. The proportion of total readmissions due to HE after QI was 9.6% (14 of 146), compared with 40.7% (79 of 194) before QI. In addition, length of stay for HE patients was significantly reduced (–1.34 days; 95% confidence interval, –2.38 to –0.32; P = .01). There were no significant changes in 90-day mortality.
Source: American Gastroenterological Association
“Our study advances the current literature on QI for patients with cirrhosis by presenting an inexpensive, easy to implement, and generalizable approach,” wrote Dr. Elliot Tapper of Beth Israel Deaconess Medical Center, Boston, and his colleagues. Previous studies have addressed readmission interventions among patients with cirrhosis, but the protocols required costly infrastructure, expertise, and institutional commitments. The current study supports the value of standard checklists and education, according to the investigators, “showing that outcomes improve further when checklist items are hard-wired into the ordering system.” (Clin Gastroenterol Hepatol. 2016 Apr 7. doi: 10.1016/j.cgh.2015.08.041).
The QI initiative encompassed several aspects of care. All HE patients were designated to receive rifaximin, and their lactulose dosing was adjusted to mental status using the Richmond Agitation and Sedation Scale. For patients with spontaneous bacterial peritonitis (SBP), timely administration of the correct dose of antibiotics and albumin was promoted, as were prophylactic measures for all patients, such as variceal hemorrhage prophylaxis and subcutaneous heparin for the prevention of venous thrombosis.
The three-part program entailed a run-in phase for preliminary checklist troubleshooting, a hand-held checklist phase, including the HE protocol, SBP treatment, and prophylactic measures, and a final electronic phase in which checklist items were incorporated into the hospital’s electronic provider order entry system using mandatory preset doses and linked medications.
Individual protocol items were demonstrated to affect the readmission rate. Rifaximin use for HE patients rose from 78.1% to 96.3%, and use of rifaximin was associated with lower adjusted odds of 30-day readmission (OR, 0.39; 95% CI, 0.16-0.87; P = .02). The dose/frequency of lactulose for HE patients increased, and patients who had 6 or more cups of lactulose on the day of their readmission had significantly lower adjusted length of stay (–2.36 days; 95% CI, –3.40 to –1.31; P less than .0001). Patients taking SBP prophylaxis had lower readmission rates (OR, 0.51; 95% CI, 0.31-0.83; P = .007).
The prospective study from 2011 to 2013 evaluated patients with cirrhosis who were admitted to the liver unit of Beth Israel Deaconess Medical Center, Boston. Patients were diagnosed with cirrhosis caused by hepatitis C (44.9%), alcoholic liver disease (34%), hepatitis B (5.4%), and biliary cirrhosis (1.8%). In total, 824 unique patients were admitted 1,720 times; 485 (58.9%) were admitted once, 268 (32.5%) were admitted 2-4 times, and 71 (8.6%) were admitted 5 or more times. The median length of stay for all patients was 4.0 days (interquartile range, 2.0-8.0).
Dr. Tapper and his coauthors reported having no disclosures.
Key clinical point: Care protocols implemented by electronic decision support reduced 30-day readmissions of patients with cirrhosis by 40% in an inpatient liver unit.
Major finding: The drop was likely driven by fewer readmissions for hepatic encephalopathy (HE): the 30-day HE readmission rate was 26.0% (27 of 104), compared with 48.9% (66 of 135) before implementation of quality improvement.
Data sources: The prospective study evaluated 824 patients who were admitted 1,720 times to the liver unit of Beth Israel Deaconess Medical Center, Boston.
Disclosures: Dr. Tapper and his coauthors reported having no disclosures.