User login
Valve hemodynamic deterioration 2.5% at 1 year
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
CHICAGO – The incidence of valve hemodynamic deterioration in the first year after transcatheter aortic valve replacement is about 2.5%, but this event wasn’t clearly associated with adverse clinical outcomes out to 18 months of follow-up in an analysis of the large U.S. registry collaboratively maintained by the Society of Thoracic Surgeons and the American College of Cardiology.
“These findings, especially the patient and procedural predictors of valve hemodynamic deterioration we identified, may help to inform TAVR care, including patient selection, surveillance, and preventive strategies,” Dr. Sreekanth Vemulapalli reported at the annual meeting of the American College of Cardiology.
Recent reports have linked TAVR to subsequent development of leaflet abnormalities and valve thrombosis, with widely ranging estimates of incidence. Definitive answers as to the true rate of these adverse events and the underlying mechanisms will come from ongoing prospective studies using advanced imaging via four-dimensional CT or transesophageal echocardiography, but those studies will take years to complete, noted Dr. Vemulapalli of the Duke Clinical Research Institute in Durham, N.C.
In the meantime, he continued, the STS/ACC Transcatheter Valve Therapy Registry provides a unique opportunity to shed light on the incidence and consequences of valve hemodynamic deterioration (VHD) in real-world clinical practice. The registry includes all commercial TAVR procedures performed in the United States, with transthoracic echocardiograms obtained pre- and post-TAVR, at 30 days, and at 1 year after the procedure.
To examine the short- and longer-term rates of VHD, which Dr. Vemulapalli and his coinvestigators defined as an increase in the mean aortic valve gradient of 10 mm or more, the researchers assembled two separate patient cohorts. They comprised a short-term–risk group of 10,095 patients who underwent TAVR at 334 sites, with an incidence of VHD of 2.1% during the first 30 days after the procedure, and 3,175 patients at 254 sites, whose incidence of VHD from day 30 through 1 year post TAVR was 2.5%.
The combined rate of VHD and all-cause mortality during the first 30 days was 7.1%. For the long-term cohort, the combined endpoint rate from day 30 to 1 year was 23.5%.
Importantly, the occurrence of VHD was not associated with an excess of the composite endpoint of mortality, stroke, heart failure hospitalization, or aortic valve reintervention at 1 year in either the short- or long-term cohort. The same held true in an analysis covering the period of 12-18 months post TAVR, according to Dr. Vemulapalli.
In a multivariate analysis, the significant predictors of VHD in the short-term cohort were male sex; increased body mass index, with the risk rising stepwise with every additional 5 kg/m above normal weight; baseline severe chronic lung disease; a valve-in-valve procedure; a larger baseline aortic valve gradient; a TAVR valve size of 23 mm or less; and severe patient/prosthesis mismatch.
In the long-term cohort, the risk factors for VHD were hospital discharge on a factor Xa inhibitor and a larger predischarge aortic valve gradient.
Change in left ventricular ejection fraction over the course of the study bore no relation to VHD risk. Neither did which of the two commercially available TAVR valves a patient received.
This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. Vemulapalli reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
AT ACC 16
Key clinical point: The incidence of valve hemodynamic deterioration after transaortic valve replacement is 2.5% from day 30 through 12 months post procedure.
Major finding: Patients who experienced valve hemodynamic deterioration had a rate of adverse clinical outcomes similar to those without valve deterioration.
Data source: This was a retrospective study of 18-month outcomes in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, which covers all commercial transcatheter valve replacements done in the United States.
Disclosures: This study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. The presenter reported serving as a consultant to Novella and Premiere and receiving research grants from the Agency for Healthcare Research and Quality, Boston Scientific, Abbott Vascular, and the ACC.
Stand up for research benefiting our patients, and more
Focus on decreasing unintended pregnanciesI found the letters in response to Dr. Barbieri’s Editorial on inadequate contraception to be much overwrought. Dr. Will’s suggestion to have “automatic contraception … for all reproductive-age women including ‘children’ who are … menstruating” is excessive. Shouldn’t parents have the final decision making in their minor children’s health care?
An anonymous clinician ex-presses frustration with a Catholichealth care system for not allowing prescription of contraceptives, which does actually stay true to the religious beliefs of the institution, and proposes decreased reimbursements to these facilities across the board as a form of financial punishment for these practices. Not only would that be illegal and unconstitutional but it also demonstrates a lack of understanding of our First Amendment protections.
Overall, these letters and Dr. Barbieri’s response show a very narrow understanding of the issues involved. I think we can and should be focused on decreasing unintended pregnancies while also respecting the rights of all without resorting to Draconian and totalitarian solutions.
Myles Dotto, MD
Oradell, New Jersey
Dr. Barbieri respondsI share Dr. Dotto’s concern that government mandates regarding health care are potentially very dangerous. It is better for communities of clinicians and patients to develop optimal approaches to health care, without government interference.
“THE CRUSHING OF INNOVATION FOR TREATING FEMALE PELVIC FLOOR DISORDERS: A STORY OF ‘LEAD OR BE LED’”ANDREW CASSIDENTI, MD (GUEST EDITORIAL; APRIL 2016)Stand up for research benefiting our patientsI salute Dr. Cassidenti’s courage to call surgeons and the respective professional organizations to step up to defend the research and expose inappropriate expert testimony. We should be ashamed to be scattered like dogs because of fear and lack of courage to be advocates for what is in the best interest of our patients. Please continue the campaign to encourage physicians and surgeons to stand up.
Cleve Waters, MD
Chattanooga, Tennessee
Caving to class action litigation is a mistakeIn his Guest Editorial Dr. Cassidenti clarifies the importance of looking forward regarding mesh devices for pelvic organ prolapse (POP) treatment. As an advocate for women with POP and Founder/Executive Director of the Association for Pelvic Organ Prolapse Support—a US-based 501(c)(3)advocacy agency with global arms focused on generating awareness of POP and providing guidance and support to women navigating POP treatment—I found Endo International’s decision to close its Astora Women’s Health division extremely unsettling.
The nature of medicine is to continually advance, and that includes learning from experience and recognizing paths to evolution. Caving to class action litigation is a mistake. Research findings frequently indicate that up to half of the female population will experience POP and/or comorbid conditions.1 It is imperative that health care, industry, research, academia, policy, and advocacy agencies continue to shine a light on this much needed field in women’s health.
Sherrie Palm
Milwaukee, Wisconsin
Reference
- Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–1790.
Avoidance: the greatest tool to address shoulder dystociaAlthough avoiding endometrial injury at cesarean delivery, including the possibility of later pathologic implantation, can be attained with vaginal delivery, vaginal birth at all cost leads to a dangerous situation. The emergency environment of shoulder dystocia is not a preferable or safer stratagem.
It is granted that shoulder dystocia will happen at some point but avoidance, by employing cesarean delivery when it is indicated, is the greatest tool for addressing this very dangerous problem.
J. Michael Arnold, MD
Oconto Falls, Wisconsin
Another suggestion for shoulder dystociaMy senior partner taught me a technique that works well, although I do not know its name. After suprapubic and McRoberts maneuvers fail and the shoulders do not deliver with gentle downward guidance in one direction, I rotate the head 180° and try again. Usually this works. I have taught this technique to several midwives, and they swear by it.
Annette Fineberg, MD
Davis, California
Dr. Barbieri respondsI thank Drs. Arnold and Fineberg for sharing their perspective and experience with our readers. Dr. Arnold notes that recommending cesarean delivery in high-risk situations such as cases in which the mother has diabetes and the fetus is macrosomic would surely reduce the frequency of shoulder dystocia. I respect Dr. Fineberg’s recommendation, based on extensive clinical experience, that by rotating the fetal head the shoulder dystocia may be resolved. My concern with this technique is that the torque transmitted to the neck might cause fetal damage. I think that rotating the shoulders (Rubin or Wood maneuver) would be less likely to result in fetal injury.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Focus on decreasing unintended pregnanciesI found the letters in response to Dr. Barbieri’s Editorial on inadequate contraception to be much overwrought. Dr. Will’s suggestion to have “automatic contraception … for all reproductive-age women including ‘children’ who are … menstruating” is excessive. Shouldn’t parents have the final decision making in their minor children’s health care?
An anonymous clinician ex-presses frustration with a Catholichealth care system for not allowing prescription of contraceptives, which does actually stay true to the religious beliefs of the institution, and proposes decreased reimbursements to these facilities across the board as a form of financial punishment for these practices. Not only would that be illegal and unconstitutional but it also demonstrates a lack of understanding of our First Amendment protections.
Overall, these letters and Dr. Barbieri’s response show a very narrow understanding of the issues involved. I think we can and should be focused on decreasing unintended pregnancies while also respecting the rights of all without resorting to Draconian and totalitarian solutions.
Myles Dotto, MD
Oradell, New Jersey
Dr. Barbieri respondsI share Dr. Dotto’s concern that government mandates regarding health care are potentially very dangerous. It is better for communities of clinicians and patients to develop optimal approaches to health care, without government interference.
“THE CRUSHING OF INNOVATION FOR TREATING FEMALE PELVIC FLOOR DISORDERS: A STORY OF ‘LEAD OR BE LED’”ANDREW CASSIDENTI, MD (GUEST EDITORIAL; APRIL 2016)Stand up for research benefiting our patientsI salute Dr. Cassidenti’s courage to call surgeons and the respective professional organizations to step up to defend the research and expose inappropriate expert testimony. We should be ashamed to be scattered like dogs because of fear and lack of courage to be advocates for what is in the best interest of our patients. Please continue the campaign to encourage physicians and surgeons to stand up.
Cleve Waters, MD
Chattanooga, Tennessee
Caving to class action litigation is a mistakeIn his Guest Editorial Dr. Cassidenti clarifies the importance of looking forward regarding mesh devices for pelvic organ prolapse (POP) treatment. As an advocate for women with POP and Founder/Executive Director of the Association for Pelvic Organ Prolapse Support—a US-based 501(c)(3)advocacy agency with global arms focused on generating awareness of POP and providing guidance and support to women navigating POP treatment—I found Endo International’s decision to close its Astora Women’s Health division extremely unsettling.
The nature of medicine is to continually advance, and that includes learning from experience and recognizing paths to evolution. Caving to class action litigation is a mistake. Research findings frequently indicate that up to half of the female population will experience POP and/or comorbid conditions.1 It is imperative that health care, industry, research, academia, policy, and advocacy agencies continue to shine a light on this much needed field in women’s health.
Sherrie Palm
Milwaukee, Wisconsin
Reference
- Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–1790.
Avoidance: the greatest tool to address shoulder dystociaAlthough avoiding endometrial injury at cesarean delivery, including the possibility of later pathologic implantation, can be attained with vaginal delivery, vaginal birth at all cost leads to a dangerous situation. The emergency environment of shoulder dystocia is not a preferable or safer stratagem.
It is granted that shoulder dystocia will happen at some point but avoidance, by employing cesarean delivery when it is indicated, is the greatest tool for addressing this very dangerous problem.
J. Michael Arnold, MD
Oconto Falls, Wisconsin
Another suggestion for shoulder dystociaMy senior partner taught me a technique that works well, although I do not know its name. After suprapubic and McRoberts maneuvers fail and the shoulders do not deliver with gentle downward guidance in one direction, I rotate the head 180° and try again. Usually this works. I have taught this technique to several midwives, and they swear by it.
Annette Fineberg, MD
Davis, California
Dr. Barbieri respondsI thank Drs. Arnold and Fineberg for sharing their perspective and experience with our readers. Dr. Arnold notes that recommending cesarean delivery in high-risk situations such as cases in which the mother has diabetes and the fetus is macrosomic would surely reduce the frequency of shoulder dystocia. I respect Dr. Fineberg’s recommendation, based on extensive clinical experience, that by rotating the fetal head the shoulder dystocia may be resolved. My concern with this technique is that the torque transmitted to the neck might cause fetal damage. I think that rotating the shoulders (Rubin or Wood maneuver) would be less likely to result in fetal injury.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Focus on decreasing unintended pregnanciesI found the letters in response to Dr. Barbieri’s Editorial on inadequate contraception to be much overwrought. Dr. Will’s suggestion to have “automatic contraception … for all reproductive-age women including ‘children’ who are … menstruating” is excessive. Shouldn’t parents have the final decision making in their minor children’s health care?
An anonymous clinician ex-presses frustration with a Catholichealth care system for not allowing prescription of contraceptives, which does actually stay true to the religious beliefs of the institution, and proposes decreased reimbursements to these facilities across the board as a form of financial punishment for these practices. Not only would that be illegal and unconstitutional but it also demonstrates a lack of understanding of our First Amendment protections.
Overall, these letters and Dr. Barbieri’s response show a very narrow understanding of the issues involved. I think we can and should be focused on decreasing unintended pregnancies while also respecting the rights of all without resorting to Draconian and totalitarian solutions.
Myles Dotto, MD
Oradell, New Jersey
Dr. Barbieri respondsI share Dr. Dotto’s concern that government mandates regarding health care are potentially very dangerous. It is better for communities of clinicians and patients to develop optimal approaches to health care, without government interference.
“THE CRUSHING OF INNOVATION FOR TREATING FEMALE PELVIC FLOOR DISORDERS: A STORY OF ‘LEAD OR BE LED’”ANDREW CASSIDENTI, MD (GUEST EDITORIAL; APRIL 2016)Stand up for research benefiting our patientsI salute Dr. Cassidenti’s courage to call surgeons and the respective professional organizations to step up to defend the research and expose inappropriate expert testimony. We should be ashamed to be scattered like dogs because of fear and lack of courage to be advocates for what is in the best interest of our patients. Please continue the campaign to encourage physicians and surgeons to stand up.
Cleve Waters, MD
Chattanooga, Tennessee
Caving to class action litigation is a mistakeIn his Guest Editorial Dr. Cassidenti clarifies the importance of looking forward regarding mesh devices for pelvic organ prolapse (POP) treatment. As an advocate for women with POP and Founder/Executive Director of the Association for Pelvic Organ Prolapse Support—a US-based 501(c)(3)advocacy agency with global arms focused on generating awareness of POP and providing guidance and support to women navigating POP treatment—I found Endo International’s decision to close its Astora Women’s Health division extremely unsettling.
The nature of medicine is to continually advance, and that includes learning from experience and recognizing paths to evolution. Caving to class action litigation is a mistake. Research findings frequently indicate that up to half of the female population will experience POP and/or comorbid conditions.1 It is imperative that health care, industry, research, academia, policy, and advocacy agencies continue to shine a light on this much needed field in women’s health.
Sherrie Palm
Milwaukee, Wisconsin
Reference
- Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–1790.
Avoidance: the greatest tool to address shoulder dystociaAlthough avoiding endometrial injury at cesarean delivery, including the possibility of later pathologic implantation, can be attained with vaginal delivery, vaginal birth at all cost leads to a dangerous situation. The emergency environment of shoulder dystocia is not a preferable or safer stratagem.
It is granted that shoulder dystocia will happen at some point but avoidance, by employing cesarean delivery when it is indicated, is the greatest tool for addressing this very dangerous problem.
J. Michael Arnold, MD
Oconto Falls, Wisconsin
Another suggestion for shoulder dystociaMy senior partner taught me a technique that works well, although I do not know its name. After suprapubic and McRoberts maneuvers fail and the shoulders do not deliver with gentle downward guidance in one direction, I rotate the head 180° and try again. Usually this works. I have taught this technique to several midwives, and they swear by it.
Annette Fineberg, MD
Davis, California
Dr. Barbieri respondsI thank Drs. Arnold and Fineberg for sharing their perspective and experience with our readers. Dr. Arnold notes that recommending cesarean delivery in high-risk situations such as cases in which the mother has diabetes and the fetus is macrosomic would surely reduce the frequency of shoulder dystocia. I respect Dr. Fineberg’s recommendation, based on extensive clinical experience, that by rotating the fetal head the shoulder dystocia may be resolved. My concern with this technique is that the torque transmitted to the neck might cause fetal damage. I think that rotating the shoulders (Rubin or Wood maneuver) would be less likely to result in fetal injury.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Examining the fetal origins of obesity
The figures and trends behind the obesity epidemic are alarming: More than one-third of all adults in the United States are obese, as are 34% of women aged 20-39, and 17% of youth aged 2-19, according to data for 2011-2014 from the National Health and Nutrition Examination Survey.
In our ob.gyn. practices, many of us have witnessed the significant climb in national obesity rates over the past several decades. We’ve seen a continued increase in the prevalence of obesity among childbearing women, and a steady increase in the incidence of high-birth-weight babies. The percentage of women weighing 200 pounds has more than doubled since 1980, and up to 3-4 times as many children and teens in various age subsets are obese today as in the 1970s.
The obesity epidemic is often attributed to a high-fat and/or calorie-dense diet and decreased activity levels. However, this is only part of the picture. There has been growing recognition in recent years that obesity may be programmed by the in utero and newborn environment, particularly as it relates to nutritional permutations. We now have evidence, in fact, that developmental programming is likely a primary cause of the obesity epidemic.
Exposure to maternal obesity and being born with a low birth weight – especially a low birth weight paired with rapid catch-up growth – are both associated with a significantly increased risk of childhood and adult obesity.
Research has demonstrated that newborns may be programmed, in both of these scenarios, with an increased appetite and a predisposition to storing calories as fat. In addition, data are accumulating that exposure to bisphenol A and other endocrine-disruptive chemicals, other environmental toxins, and corticosteroids may exert similar programming effects.
This window into the origins of obesity has significant implications for the practice of ob.gyn., where we have the opportunity to address the programming effects of the in utero and early life environment. Most importantly, we must counsel women before pregnancy about the importance of losing weight, guide them during pregnancy to achieve optimal pregnancy nutrition and weight gain, and prepare them to adopt optimal newborn feeding strategies that will guard against overconsumption.
Programming of obesity
The current obesity epidemic is only minimally due to genetics. Although select genetic mutations may be associated with obesity, these mutations account for an exceedingly small proportion of the obese population. Instead, much of the obesity epidemic involves epigenetic change – in this case, largely epigenetic deregulation of gene expression – and more broadly what we call gestational, or developmental, programming.
Developmental programming is a process by which a stress or stimulus at a critical or sensitive period of development has long-term effects. The major part of the developmental process pertaining to cell division occurs during intrauterine life; more than 90% of the cell divisions necessary to make an adult human occur before birth. Although there are important effects of the early newborn period, developmental programming is therefore largely gestational programming. Depending on when an in utero stress or perturbation occurs, it may permanently change cell number and/or cell differentiation, organ structure, metabolic set points, and gene expression.
The late physician Dr. David Barker got us thinking about in utero programming when he demonstrated an association between low birth weight, rapid weight gain in early life, and adult cardiovascular mortality. His theory about how nutrition and growth before birth may affect cardiovascular health later on, as well as other adult chronic diseases and conditions, became known as the Barker Hypothesis.
Many studies, both animal research and human epidemiological studies, have since confirmed and expanded our understanding of this phenomena. Research has demonstrated associations, for instance, between low birth weight and later risks of insulin resistance, diabetes, fatty liver, and the often-underlying metabolic syndrome.
Obesity is also central to the development of the metabolic syndrome, and we now have irrefutable evidence to show that low birth weight infants have a higher risk of obesity than do normal weight infants. We also know, as Dr. Barker and his colleagues had surmised, that the greatest risks occur when there is rapid catch-up growth of low-birth-weight infants in the early years of life.
Moreover, we now understand that maternal obesity has programming effects that are similar to those of an in utero environment of undernutrition and growth restriction. In the past several decades, the marked increase in maternal obesity has resulted in this programming process having an ever-increasing impact.
Both animal and human studies have shown that infants born to obese mothers have the same increased risks for adult chronic disease – including the risk of becoming obese – as those of low birth weight infants. This increased risk is often, but not always, associated with high birth weight, and it is independent of whether the mother has gestational diabetes mellitus (GDM). Having a high birth weight is more likely in the setting of maternal obesity and itself raises the risk of eventual obesity (as does GDM), but an infant’s exposure to maternal obesity in and of itself is a risk factor.
The mechanisms
The programming mechanisms that predispose offspring to obesity are similar in infants of obese mothers and intrauterine growth restricted newborns, though they involve different epigenetic signals. Both involve dysregulation of appetite/satiety and of adipogenesis.
Appetite is primarily controlled by a complex circuit of neurons in the hypothalamus of the brain called the hypothalamic arcuate nucleus. Some neurons are orexigenic and stimulate or increase appetite, while others are anorexigenic and suppress appetite by promoting satiety.
During fetal development, hypothalamic neural stem cells proliferate and differentiate into various cell types. Neurons destined for the arcuate nucleus then differentiate into these so-called appetite neurons and satiety neurons. Though there is continued neural development and maturation during newborn life, hypothalamic control of appetite and satiety is largely set during this period.
Differentiation to appetite or satiety neurons is regulated by a complex interplay of pathways that may be significantly altered by the nutrient environment. Research in our laboratory and others has shown that both limited and excess nutrition can program the structure and function of the arcuate nucleus – changing its wiring, in essence – such that there is an increased ratio of appetite to satiety neurons (Clin Obstet Gynecol. 2013 Sep;56[3]:529-36).
There also appears to be a programmed down-regulation in the reward pathway of the brain, and some studies have shown that children of obese mothers and children who were born with low birth weights have a higher preference for sweet and high-calorie foods. This all begins at the neural stem cell level.
With more appetite neurons and fewer satiety neurons, as well as a down-regulation of reward – and an abundance of available food – a newborn is at high risk of becoming obese. Eating for this child will not only be pleasurable; it will be driven by an enhanced appetite, an inability to feel full after reasonable amounts of food, and a down-regulation of reward (potentially requiring greater amounts of food or a shift in preference for high fat/sweet food to achieve the pleasure from eating).
In addition to alterations in appetite/satiety, the nutrition environment in utero can alter adipose tissue development and function.
Like neural development, adipogenesis – the process by which preadipocytes proliferate and differentiate into mature adipocytes – is tightly regulated by a cascade of transcription factors that are expressed in response to stimuli, including nutrients. In animal studies we have found an up-regulation of adipogenic and lipogenic transcription factors in intrauterine growth restricted offspring as well as in offspring of obese mothers (Reprod Sci. 2008 Oct;15[8]:785-96 and Curr Diab Rep. 2013 Feb;13[1]:27-33).
This up-regulation leads to greater proliferation of preadipocytes and greater lipid synthesis and storage in mature adipocytes. Not only will the newborn have an increased number of adipocytes, but he or she will have an increased number of hypertrophic lipid-filled fat cells. The enhanced adipogenesis will contribute to the newborn’s programmed propensity for obesity, and the directive to “just eat less” will likely be ineffective throughout childhood and beyond.
Programmed offspring are resistant to both central and peripheral effects of leptin and insulin, resulting in impaired satiety (i.e., overeating) and manifestations of GDM. Responses to an array of additional energy regulatory factors (e.g., ghrelin) demonstrate a similar programmed dysfunction.
In practice
There are several approaches that ob.gyns. can take to prevent childhood and lifelong obesity. Most importantly, we must counsel our obese patients to lose weight before pregnancy. In doing so, it may be meaningful and effective to ask the patient to think about her baby’s future as an obese adult.
Patients who have experienced the challenges of trying to lose weight, and who are told about the developmental origins of obesity and how obesity can be programmed, may be more motivated to lose weight to avoid passing on to their children the burden and challenges that they’ve experienced. We can tell obese patients that their children may well be predisposed through the current in utero environment to have an increased appetite and a propensity to store body fat, and that they subsequently will face higher risks of diabetes and other serious chronic conditions.
We should also appropriately counsel women on healthy weight gain during pregnancy, and urge them not to gain excessive weight.
Newborn feeding strategies are also important for babies exposed to gestational programming of obesity, but especially small babies given the high risk of obesity when there is rapid catch-up growth. We must encourage good growth of both the low-birth-weight and macrosomic infant during the newborn period, but not overgrowth.
The importance of breastfeeding cannot be overestimated, as it has been demonstrated to reduce the occurrence of excessive newborn weight gain and improve long term infant health. We should encourage breastfeeding for the natural opportunity it provides to avoid excessive feeding, in addition to its other benefits. And for newborns who are bottle fed, we should counsel the new mother on optimal feeding and strategies for comforting a crying baby, which will protect against overfeeding.
Regarding environmental exposures, this area of developmental programming is continuing to evolve at a rapid rate. Both animal research and epidemiological studies support the association of developmental exposure to BPA and other chemicals with obesity.
For the present, we should educate our patients regarding optimal nutrition prior to and during pregnancy, and the avoidance of potentially toxic or metabolically-active chemicals or drugs. We look forward to continued research into the mechanisms and preventive/therapeutic strategies for optimization of childhood and adult health.
Dr. Ross is professor of obstetrics and gynecology at the University of California, Los Angeles. Dr. Desai is assistant professor of ob.gyn. at the university. They reported having no relevant financial disclosures.
The figures and trends behind the obesity epidemic are alarming: More than one-third of all adults in the United States are obese, as are 34% of women aged 20-39, and 17% of youth aged 2-19, according to data for 2011-2014 from the National Health and Nutrition Examination Survey.
In our ob.gyn. practices, many of us have witnessed the significant climb in national obesity rates over the past several decades. We’ve seen a continued increase in the prevalence of obesity among childbearing women, and a steady increase in the incidence of high-birth-weight babies. The percentage of women weighing 200 pounds has more than doubled since 1980, and up to 3-4 times as many children and teens in various age subsets are obese today as in the 1970s.
The obesity epidemic is often attributed to a high-fat and/or calorie-dense diet and decreased activity levels. However, this is only part of the picture. There has been growing recognition in recent years that obesity may be programmed by the in utero and newborn environment, particularly as it relates to nutritional permutations. We now have evidence, in fact, that developmental programming is likely a primary cause of the obesity epidemic.
Exposure to maternal obesity and being born with a low birth weight – especially a low birth weight paired with rapid catch-up growth – are both associated with a significantly increased risk of childhood and adult obesity.
Research has demonstrated that newborns may be programmed, in both of these scenarios, with an increased appetite and a predisposition to storing calories as fat. In addition, data are accumulating that exposure to bisphenol A and other endocrine-disruptive chemicals, other environmental toxins, and corticosteroids may exert similar programming effects.
This window into the origins of obesity has significant implications for the practice of ob.gyn., where we have the opportunity to address the programming effects of the in utero and early life environment. Most importantly, we must counsel women before pregnancy about the importance of losing weight, guide them during pregnancy to achieve optimal pregnancy nutrition and weight gain, and prepare them to adopt optimal newborn feeding strategies that will guard against overconsumption.
Programming of obesity
The current obesity epidemic is only minimally due to genetics. Although select genetic mutations may be associated with obesity, these mutations account for an exceedingly small proportion of the obese population. Instead, much of the obesity epidemic involves epigenetic change – in this case, largely epigenetic deregulation of gene expression – and more broadly what we call gestational, or developmental, programming.
Developmental programming is a process by which a stress or stimulus at a critical or sensitive period of development has long-term effects. The major part of the developmental process pertaining to cell division occurs during intrauterine life; more than 90% of the cell divisions necessary to make an adult human occur before birth. Although there are important effects of the early newborn period, developmental programming is therefore largely gestational programming. Depending on when an in utero stress or perturbation occurs, it may permanently change cell number and/or cell differentiation, organ structure, metabolic set points, and gene expression.
The late physician Dr. David Barker got us thinking about in utero programming when he demonstrated an association between low birth weight, rapid weight gain in early life, and adult cardiovascular mortality. His theory about how nutrition and growth before birth may affect cardiovascular health later on, as well as other adult chronic diseases and conditions, became known as the Barker Hypothesis.
Many studies, both animal research and human epidemiological studies, have since confirmed and expanded our understanding of this phenomena. Research has demonstrated associations, for instance, between low birth weight and later risks of insulin resistance, diabetes, fatty liver, and the often-underlying metabolic syndrome.
Obesity is also central to the development of the metabolic syndrome, and we now have irrefutable evidence to show that low birth weight infants have a higher risk of obesity than do normal weight infants. We also know, as Dr. Barker and his colleagues had surmised, that the greatest risks occur when there is rapid catch-up growth of low-birth-weight infants in the early years of life.
Moreover, we now understand that maternal obesity has programming effects that are similar to those of an in utero environment of undernutrition and growth restriction. In the past several decades, the marked increase in maternal obesity has resulted in this programming process having an ever-increasing impact.
Both animal and human studies have shown that infants born to obese mothers have the same increased risks for adult chronic disease – including the risk of becoming obese – as those of low birth weight infants. This increased risk is often, but not always, associated with high birth weight, and it is independent of whether the mother has gestational diabetes mellitus (GDM). Having a high birth weight is more likely in the setting of maternal obesity and itself raises the risk of eventual obesity (as does GDM), but an infant’s exposure to maternal obesity in and of itself is a risk factor.
The mechanisms
The programming mechanisms that predispose offspring to obesity are similar in infants of obese mothers and intrauterine growth restricted newborns, though they involve different epigenetic signals. Both involve dysregulation of appetite/satiety and of adipogenesis.
Appetite is primarily controlled by a complex circuit of neurons in the hypothalamus of the brain called the hypothalamic arcuate nucleus. Some neurons are orexigenic and stimulate or increase appetite, while others are anorexigenic and suppress appetite by promoting satiety.
During fetal development, hypothalamic neural stem cells proliferate and differentiate into various cell types. Neurons destined for the arcuate nucleus then differentiate into these so-called appetite neurons and satiety neurons. Though there is continued neural development and maturation during newborn life, hypothalamic control of appetite and satiety is largely set during this period.
Differentiation to appetite or satiety neurons is regulated by a complex interplay of pathways that may be significantly altered by the nutrient environment. Research in our laboratory and others has shown that both limited and excess nutrition can program the structure and function of the arcuate nucleus – changing its wiring, in essence – such that there is an increased ratio of appetite to satiety neurons (Clin Obstet Gynecol. 2013 Sep;56[3]:529-36).
There also appears to be a programmed down-regulation in the reward pathway of the brain, and some studies have shown that children of obese mothers and children who were born with low birth weights have a higher preference for sweet and high-calorie foods. This all begins at the neural stem cell level.
With more appetite neurons and fewer satiety neurons, as well as a down-regulation of reward – and an abundance of available food – a newborn is at high risk of becoming obese. Eating for this child will not only be pleasurable; it will be driven by an enhanced appetite, an inability to feel full after reasonable amounts of food, and a down-regulation of reward (potentially requiring greater amounts of food or a shift in preference for high fat/sweet food to achieve the pleasure from eating).
In addition to alterations in appetite/satiety, the nutrition environment in utero can alter adipose tissue development and function.
Like neural development, adipogenesis – the process by which preadipocytes proliferate and differentiate into mature adipocytes – is tightly regulated by a cascade of transcription factors that are expressed in response to stimuli, including nutrients. In animal studies we have found an up-regulation of adipogenic and lipogenic transcription factors in intrauterine growth restricted offspring as well as in offspring of obese mothers (Reprod Sci. 2008 Oct;15[8]:785-96 and Curr Diab Rep. 2013 Feb;13[1]:27-33).
This up-regulation leads to greater proliferation of preadipocytes and greater lipid synthesis and storage in mature adipocytes. Not only will the newborn have an increased number of adipocytes, but he or she will have an increased number of hypertrophic lipid-filled fat cells. The enhanced adipogenesis will contribute to the newborn’s programmed propensity for obesity, and the directive to “just eat less” will likely be ineffective throughout childhood and beyond.
Programmed offspring are resistant to both central and peripheral effects of leptin and insulin, resulting in impaired satiety (i.e., overeating) and manifestations of GDM. Responses to an array of additional energy regulatory factors (e.g., ghrelin) demonstrate a similar programmed dysfunction.
In practice
There are several approaches that ob.gyns. can take to prevent childhood and lifelong obesity. Most importantly, we must counsel our obese patients to lose weight before pregnancy. In doing so, it may be meaningful and effective to ask the patient to think about her baby’s future as an obese adult.
Patients who have experienced the challenges of trying to lose weight, and who are told about the developmental origins of obesity and how obesity can be programmed, may be more motivated to lose weight to avoid passing on to their children the burden and challenges that they’ve experienced. We can tell obese patients that their children may well be predisposed through the current in utero environment to have an increased appetite and a propensity to store body fat, and that they subsequently will face higher risks of diabetes and other serious chronic conditions.
We should also appropriately counsel women on healthy weight gain during pregnancy, and urge them not to gain excessive weight.
Newborn feeding strategies are also important for babies exposed to gestational programming of obesity, but especially small babies given the high risk of obesity when there is rapid catch-up growth. We must encourage good growth of both the low-birth-weight and macrosomic infant during the newborn period, but not overgrowth.
The importance of breastfeeding cannot be overestimated, as it has been demonstrated to reduce the occurrence of excessive newborn weight gain and improve long term infant health. We should encourage breastfeeding for the natural opportunity it provides to avoid excessive feeding, in addition to its other benefits. And for newborns who are bottle fed, we should counsel the new mother on optimal feeding and strategies for comforting a crying baby, which will protect against overfeeding.
Regarding environmental exposures, this area of developmental programming is continuing to evolve at a rapid rate. Both animal research and epidemiological studies support the association of developmental exposure to BPA and other chemicals with obesity.
For the present, we should educate our patients regarding optimal nutrition prior to and during pregnancy, and the avoidance of potentially toxic or metabolically-active chemicals or drugs. We look forward to continued research into the mechanisms and preventive/therapeutic strategies for optimization of childhood and adult health.
Dr. Ross is professor of obstetrics and gynecology at the University of California, Los Angeles. Dr. Desai is assistant professor of ob.gyn. at the university. They reported having no relevant financial disclosures.
The figures and trends behind the obesity epidemic are alarming: More than one-third of all adults in the United States are obese, as are 34% of women aged 20-39, and 17% of youth aged 2-19, according to data for 2011-2014 from the National Health and Nutrition Examination Survey.
In our ob.gyn. practices, many of us have witnessed the significant climb in national obesity rates over the past several decades. We’ve seen a continued increase in the prevalence of obesity among childbearing women, and a steady increase in the incidence of high-birth-weight babies. The percentage of women weighing 200 pounds has more than doubled since 1980, and up to 3-4 times as many children and teens in various age subsets are obese today as in the 1970s.
The obesity epidemic is often attributed to a high-fat and/or calorie-dense diet and decreased activity levels. However, this is only part of the picture. There has been growing recognition in recent years that obesity may be programmed by the in utero and newborn environment, particularly as it relates to nutritional permutations. We now have evidence, in fact, that developmental programming is likely a primary cause of the obesity epidemic.
Exposure to maternal obesity and being born with a low birth weight – especially a low birth weight paired with rapid catch-up growth – are both associated with a significantly increased risk of childhood and adult obesity.
Research has demonstrated that newborns may be programmed, in both of these scenarios, with an increased appetite and a predisposition to storing calories as fat. In addition, data are accumulating that exposure to bisphenol A and other endocrine-disruptive chemicals, other environmental toxins, and corticosteroids may exert similar programming effects.
This window into the origins of obesity has significant implications for the practice of ob.gyn., where we have the opportunity to address the programming effects of the in utero and early life environment. Most importantly, we must counsel women before pregnancy about the importance of losing weight, guide them during pregnancy to achieve optimal pregnancy nutrition and weight gain, and prepare them to adopt optimal newborn feeding strategies that will guard against overconsumption.
Programming of obesity
The current obesity epidemic is only minimally due to genetics. Although select genetic mutations may be associated with obesity, these mutations account for an exceedingly small proportion of the obese population. Instead, much of the obesity epidemic involves epigenetic change – in this case, largely epigenetic deregulation of gene expression – and more broadly what we call gestational, or developmental, programming.
Developmental programming is a process by which a stress or stimulus at a critical or sensitive period of development has long-term effects. The major part of the developmental process pertaining to cell division occurs during intrauterine life; more than 90% of the cell divisions necessary to make an adult human occur before birth. Although there are important effects of the early newborn period, developmental programming is therefore largely gestational programming. Depending on when an in utero stress or perturbation occurs, it may permanently change cell number and/or cell differentiation, organ structure, metabolic set points, and gene expression.
The late physician Dr. David Barker got us thinking about in utero programming when he demonstrated an association between low birth weight, rapid weight gain in early life, and adult cardiovascular mortality. His theory about how nutrition and growth before birth may affect cardiovascular health later on, as well as other adult chronic diseases and conditions, became known as the Barker Hypothesis.
Many studies, both animal research and human epidemiological studies, have since confirmed and expanded our understanding of this phenomena. Research has demonstrated associations, for instance, between low birth weight and later risks of insulin resistance, diabetes, fatty liver, and the often-underlying metabolic syndrome.
Obesity is also central to the development of the metabolic syndrome, and we now have irrefutable evidence to show that low birth weight infants have a higher risk of obesity than do normal weight infants. We also know, as Dr. Barker and his colleagues had surmised, that the greatest risks occur when there is rapid catch-up growth of low-birth-weight infants in the early years of life.
Moreover, we now understand that maternal obesity has programming effects that are similar to those of an in utero environment of undernutrition and growth restriction. In the past several decades, the marked increase in maternal obesity has resulted in this programming process having an ever-increasing impact.
Both animal and human studies have shown that infants born to obese mothers have the same increased risks for adult chronic disease – including the risk of becoming obese – as those of low birth weight infants. This increased risk is often, but not always, associated with high birth weight, and it is independent of whether the mother has gestational diabetes mellitus (GDM). Having a high birth weight is more likely in the setting of maternal obesity and itself raises the risk of eventual obesity (as does GDM), but an infant’s exposure to maternal obesity in and of itself is a risk factor.
The mechanisms
The programming mechanisms that predispose offspring to obesity are similar in infants of obese mothers and intrauterine growth restricted newborns, though they involve different epigenetic signals. Both involve dysregulation of appetite/satiety and of adipogenesis.
Appetite is primarily controlled by a complex circuit of neurons in the hypothalamus of the brain called the hypothalamic arcuate nucleus. Some neurons are orexigenic and stimulate or increase appetite, while others are anorexigenic and suppress appetite by promoting satiety.
During fetal development, hypothalamic neural stem cells proliferate and differentiate into various cell types. Neurons destined for the arcuate nucleus then differentiate into these so-called appetite neurons and satiety neurons. Though there is continued neural development and maturation during newborn life, hypothalamic control of appetite and satiety is largely set during this period.
Differentiation to appetite or satiety neurons is regulated by a complex interplay of pathways that may be significantly altered by the nutrient environment. Research in our laboratory and others has shown that both limited and excess nutrition can program the structure and function of the arcuate nucleus – changing its wiring, in essence – such that there is an increased ratio of appetite to satiety neurons (Clin Obstet Gynecol. 2013 Sep;56[3]:529-36).
There also appears to be a programmed down-regulation in the reward pathway of the brain, and some studies have shown that children of obese mothers and children who were born with low birth weights have a higher preference for sweet and high-calorie foods. This all begins at the neural stem cell level.
With more appetite neurons and fewer satiety neurons, as well as a down-regulation of reward – and an abundance of available food – a newborn is at high risk of becoming obese. Eating for this child will not only be pleasurable; it will be driven by an enhanced appetite, an inability to feel full after reasonable amounts of food, and a down-regulation of reward (potentially requiring greater amounts of food or a shift in preference for high fat/sweet food to achieve the pleasure from eating).
In addition to alterations in appetite/satiety, the nutrition environment in utero can alter adipose tissue development and function.
Like neural development, adipogenesis – the process by which preadipocytes proliferate and differentiate into mature adipocytes – is tightly regulated by a cascade of transcription factors that are expressed in response to stimuli, including nutrients. In animal studies we have found an up-regulation of adipogenic and lipogenic transcription factors in intrauterine growth restricted offspring as well as in offspring of obese mothers (Reprod Sci. 2008 Oct;15[8]:785-96 and Curr Diab Rep. 2013 Feb;13[1]:27-33).
This up-regulation leads to greater proliferation of preadipocytes and greater lipid synthesis and storage in mature adipocytes. Not only will the newborn have an increased number of adipocytes, but he or she will have an increased number of hypertrophic lipid-filled fat cells. The enhanced adipogenesis will contribute to the newborn’s programmed propensity for obesity, and the directive to “just eat less” will likely be ineffective throughout childhood and beyond.
Programmed offspring are resistant to both central and peripheral effects of leptin and insulin, resulting in impaired satiety (i.e., overeating) and manifestations of GDM. Responses to an array of additional energy regulatory factors (e.g., ghrelin) demonstrate a similar programmed dysfunction.
In practice
There are several approaches that ob.gyns. can take to prevent childhood and lifelong obesity. Most importantly, we must counsel our obese patients to lose weight before pregnancy. In doing so, it may be meaningful and effective to ask the patient to think about her baby’s future as an obese adult.
Patients who have experienced the challenges of trying to lose weight, and who are told about the developmental origins of obesity and how obesity can be programmed, may be more motivated to lose weight to avoid passing on to their children the burden and challenges that they’ve experienced. We can tell obese patients that their children may well be predisposed through the current in utero environment to have an increased appetite and a propensity to store body fat, and that they subsequently will face higher risks of diabetes and other serious chronic conditions.
We should also appropriately counsel women on healthy weight gain during pregnancy, and urge them not to gain excessive weight.
Newborn feeding strategies are also important for babies exposed to gestational programming of obesity, but especially small babies given the high risk of obesity when there is rapid catch-up growth. We must encourage good growth of both the low-birth-weight and macrosomic infant during the newborn period, but not overgrowth.
The importance of breastfeeding cannot be overestimated, as it has been demonstrated to reduce the occurrence of excessive newborn weight gain and improve long term infant health. We should encourage breastfeeding for the natural opportunity it provides to avoid excessive feeding, in addition to its other benefits. And for newborns who are bottle fed, we should counsel the new mother on optimal feeding and strategies for comforting a crying baby, which will protect against overfeeding.
Regarding environmental exposures, this area of developmental programming is continuing to evolve at a rapid rate. Both animal research and epidemiological studies support the association of developmental exposure to BPA and other chemicals with obesity.
For the present, we should educate our patients regarding optimal nutrition prior to and during pregnancy, and the avoidance of potentially toxic or metabolically-active chemicals or drugs. We look forward to continued research into the mechanisms and preventive/therapeutic strategies for optimization of childhood and adult health.
Dr. Ross is professor of obstetrics and gynecology at the University of California, Los Angeles. Dr. Desai is assistant professor of ob.gyn. at the university. They reported having no relevant financial disclosures.
The far-reaching implications of weight gain in pregnancy
It is not surprising that a pregnant woman’s actions heavily influence her developing baby. Ob.gyns. advise patients to stop smoking or to stop using illicit drugs, and limit their alcohol consumption during pregnancy because we know that these substances can cause serious, even fatal, consequences for the fetus. Although we routinely provide nutrition information and guidelines on healthy weight gain in pregnancy, we may not stress the importance of healthy eating to the same degree as we may emphasize the need to eliminate tobacco use. But should we?
In 2011, a study by researchers at Yale University, the University of Texas, and Arizona State University suggested that food can have effects on the brain similar to those of addictive substances (Arch Gen Psychiatry. 2011 Aug;68[8]:808-16). Using MRI, the investigators examined which areas of the brain became active in response to the consumption of a chocolate milkshake, and compared these results to brain scans of people addicted to opioids. The study enrolled 48 women who were lean to obese, based on body mass index. The researchers found that people who were obese had brain activity patterns in response to food that were similar to patterns that people with drug addiction had in response to opioids. Although the sample size was small, the investigators showed, in essence, that food is a “drug.”
Ob.gyns. working with patients who are overweight or obese typically encourage weight loss prior to pregnancy, or suggest limited weight gain during gestation, because obesity increases complications for both the pregnant mother and her unborn baby. If, as the 2011 study suggests, we were to think of food addiction as we do any other drug addiction – tobacco, opioids, alcohol – that should be curbed out of concern for the developing baby, ob.gyns. might tell our patients to reduce or completely eliminate their “trans-fat food habit” before and during pregnancy.
Importantly, a mother’s nutrition, or lack thereof, may exert harmful effects on her child’s long-term health. This idea was intimated decades ago when Dr. David Barker proposed that a person’s future risk for disease began during pregnancy. Exactly how this type of early programming may occur remains to be determined. Therefore, this month we examine the fetal origins of obesity, and have invited Dr. Michael G. Ross, professor of obstetrics and gynecology, and Mina Desai, Ph.D., assistant professor of obstetrics and gynecology, at the University of California, Los Angeles, to discuss this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
It is not surprising that a pregnant woman’s actions heavily influence her developing baby. Ob.gyns. advise patients to stop smoking or to stop using illicit drugs, and limit their alcohol consumption during pregnancy because we know that these substances can cause serious, even fatal, consequences for the fetus. Although we routinely provide nutrition information and guidelines on healthy weight gain in pregnancy, we may not stress the importance of healthy eating to the same degree as we may emphasize the need to eliminate tobacco use. But should we?
In 2011, a study by researchers at Yale University, the University of Texas, and Arizona State University suggested that food can have effects on the brain similar to those of addictive substances (Arch Gen Psychiatry. 2011 Aug;68[8]:808-16). Using MRI, the investigators examined which areas of the brain became active in response to the consumption of a chocolate milkshake, and compared these results to brain scans of people addicted to opioids. The study enrolled 48 women who were lean to obese, based on body mass index. The researchers found that people who were obese had brain activity patterns in response to food that were similar to patterns that people with drug addiction had in response to opioids. Although the sample size was small, the investigators showed, in essence, that food is a “drug.”
Ob.gyns. working with patients who are overweight or obese typically encourage weight loss prior to pregnancy, or suggest limited weight gain during gestation, because obesity increases complications for both the pregnant mother and her unborn baby. If, as the 2011 study suggests, we were to think of food addiction as we do any other drug addiction – tobacco, opioids, alcohol – that should be curbed out of concern for the developing baby, ob.gyns. might tell our patients to reduce or completely eliminate their “trans-fat food habit” before and during pregnancy.
Importantly, a mother’s nutrition, or lack thereof, may exert harmful effects on her child’s long-term health. This idea was intimated decades ago when Dr. David Barker proposed that a person’s future risk for disease began during pregnancy. Exactly how this type of early programming may occur remains to be determined. Therefore, this month we examine the fetal origins of obesity, and have invited Dr. Michael G. Ross, professor of obstetrics and gynecology, and Mina Desai, Ph.D., assistant professor of obstetrics and gynecology, at the University of California, Los Angeles, to discuss this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
It is not surprising that a pregnant woman’s actions heavily influence her developing baby. Ob.gyns. advise patients to stop smoking or to stop using illicit drugs, and limit their alcohol consumption during pregnancy because we know that these substances can cause serious, even fatal, consequences for the fetus. Although we routinely provide nutrition information and guidelines on healthy weight gain in pregnancy, we may not stress the importance of healthy eating to the same degree as we may emphasize the need to eliminate tobacco use. But should we?
In 2011, a study by researchers at Yale University, the University of Texas, and Arizona State University suggested that food can have effects on the brain similar to those of addictive substances (Arch Gen Psychiatry. 2011 Aug;68[8]:808-16). Using MRI, the investigators examined which areas of the brain became active in response to the consumption of a chocolate milkshake, and compared these results to brain scans of people addicted to opioids. The study enrolled 48 women who were lean to obese, based on body mass index. The researchers found that people who were obese had brain activity patterns in response to food that were similar to patterns that people with drug addiction had in response to opioids. Although the sample size was small, the investigators showed, in essence, that food is a “drug.”
Ob.gyns. working with patients who are overweight or obese typically encourage weight loss prior to pregnancy, or suggest limited weight gain during gestation, because obesity increases complications for both the pregnant mother and her unborn baby. If, as the 2011 study suggests, we were to think of food addiction as we do any other drug addiction – tobacco, opioids, alcohol – that should be curbed out of concern for the developing baby, ob.gyns. might tell our patients to reduce or completely eliminate their “trans-fat food habit” before and during pregnancy.
Importantly, a mother’s nutrition, or lack thereof, may exert harmful effects on her child’s long-term health. This idea was intimated decades ago when Dr. David Barker proposed that a person’s future risk for disease began during pregnancy. Exactly how this type of early programming may occur remains to be determined. Therefore, this month we examine the fetal origins of obesity, and have invited Dr. Michael G. Ross, professor of obstetrics and gynecology, and Mina Desai, Ph.D., assistant professor of obstetrics and gynecology, at the University of California, Los Angeles, to discuss this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Understanding ovarian germ cell neoplasms
Germ cell neoplasms arise from primordial germ cells of the gonad that differentiate to embryonic and extraembryonic tissues. Approximately 20%-25% of ovarian neoplasms are germ cell in origin but they account for only 3%-5% of ovarian malignancies. Importantly, germ cell neoplasms encompass 70% of the ovarian neoplasms among girls and young women aged 10-30 years, of which approximately one-third are malignant.
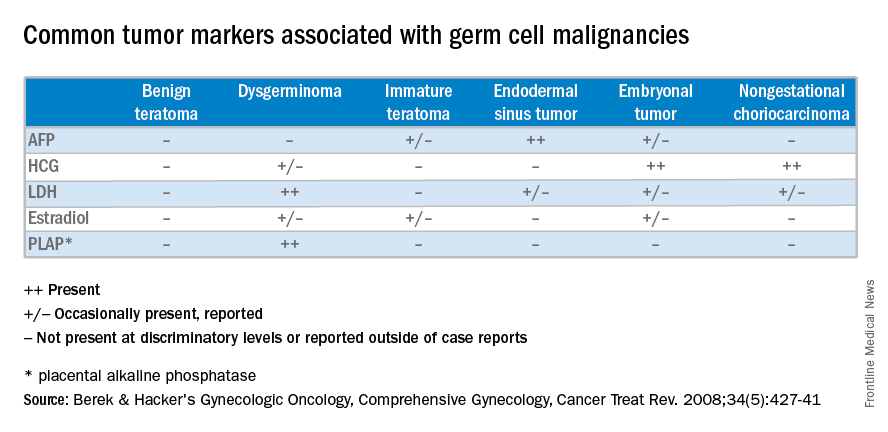
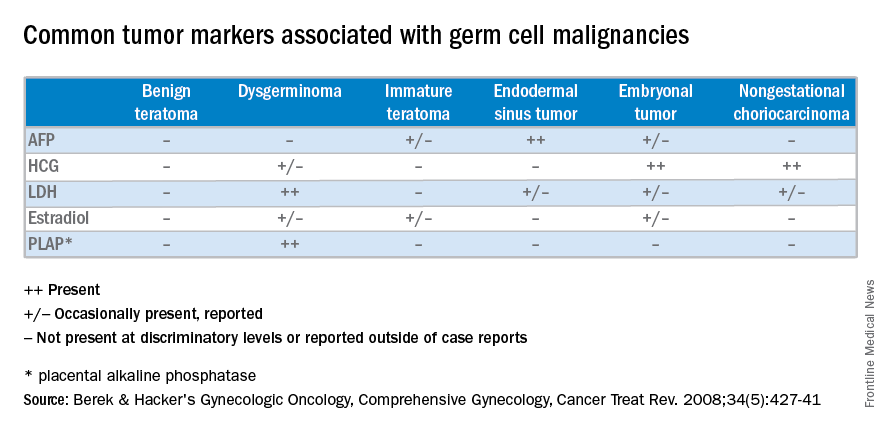
Unlike epithelial ovarian cancers, malignant germ cell neoplasms are typically diagnosed at early stages. They can present as a palpable mass or cause acute abdominal pain secondary to their rapid growth, penchant for necrosis, torsion, hemorrhage, infection, or rupture. Since malignant germ cell neoplasms can excrete hormonally-active tumor markers, such as human chorionic gonadotropin (HCG), many women present with menstrual irregularities.
Management of malignant germ cell neoplasms generally includes fertility-sparing surgery – with or without neoadjuvant combination bleomycin, etoposide, and cisplatin (BEP) – and is typically associated with a favorable prognosis (Cancer Treat Rev. 2008 Aug;34[5]:427-41).
Teratomas
Benign teratomas represent about a quarter of all ovarian neoplasms. Benign teratomas can be solid or cystic, and contain components representing all three cell layers: endoderm, mesoderm, and ectoderm. Due to the presence of differentiated adult tissues, benign teratomas have a characteristic radiographic appearance. They can appear as cystic echogenic masses with intense acoustic shadowing, homogenous nodules or bands, and rounded protuberances called “Rokitansky nodules.”
Treatment of benign teratomas in reproductive-age women includes ovarian cystectomy and careful inspection of the contralateral ovary as 10%-15% may be bilateral. In postmenopausal women, there is less than a 2% risk for malignant transformation of the associated cell lines, most commonly a squamous cell carcinoma. In these cases, spread beyond the ovarian capsule is associated with a poor prognosis and chemotherapy and/or radiation are indicated (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:140-4). Malignant monodermal teratomas are composed of single cell lines with malignant transformations of that tissue type; struma ovarii composed of thyroid tissue, for example, is exceedingly rare.
Dysgerminoma
Dysgerminomas are the most common malignant germ cell neoplasms, accounting for about one-third of cases. They typically present in girls and young women between 10 and 30 years of age, and rarely occur after 50 years of age. As a result, a quarter of cases are identified during pregnancy and another 5% are found in patients presenting with amenorrhea secondary to gonadal dysgenesis, such as is associated with Turner’s syndrome.
At diagnosis, lactate dehydrogenase (LDH) may be elevated and 10% may have an elevation of HCG. Ultrasound findings include a solid, mostly echoic, but heterogeneous mass with apparent lobulations. About two-thirds are stage I at the time of diagnosis, and 10%-15% are bilateral, making dysgerminomas the only malignant germ cell neoplasm with significant risk for bilaterality.
Treatment for early stage dysgerminoma is surgical; young women should have at least unilateral oophorectomy performed; if the contralateral ovary is spared there’s a 10% risk for recurrence over the next 2 years. Comprehensive fertility-sparing surgery is recommended with pelvic and para-aortic lymphadenectomy. Women with gonadal dysgenesis should have a bilateral salpingo-oophorectomy, and those beyond childbearing should undergo a total hysterectomy, with bilateral salpingo-oophorectomy and appropriate staging. BEP should be added for patients with advanced disease.
Other malignant germ cell neoplasms
Uncommon malignant germ cell neoplasms include immature teratomas and endodermal sinus tumors. Though uncommon, the majority of immature teratomas present between the ages of 10 and 20 years and account for nearly 30% of the ovarian cancer deaths in this age group. Immature teratomas usually have negative serum markers, though about one-third will excrete HCG. Immature teratomas are graded by the proportion of neuro-epithelium. Treatment includes unilateral oophorectomy, and surgical staging with adjuvant chemotherapy (BEP) for patients with greater than stage 1A grade 1 disease.
Endodermal sinus tumors are derived from the primitive yolk sac, are unilateral, and most will secrete alpha-fetoprotein (AFP). The median age of diagnosis of an endodermal sinus tumor is 18 years, thus treatment includes unilateral salpingo-oophorectomy and comprehensive fertility-sparing surgical staging followed by BEP.
Embryonal and nongestational choriocarcinomas are rare malignant germ cell neoplasms found in prepubertal girls to young women. Embryonal carcinomas can secrete estrogens, HCG and/or AFP, so patients may present with precocious puberty. Treatment is similar to that of endodermal sinus tumors. Nongestational choriocarcinomas, like the gestational forms, have a poor prognosis and are monitored and treated similarly.
Understanding presentation and treatments for malignant germ cell neoplasms is important in the evaluation of a young patient with a pelvic mass and should prompt the practicing gynecologist to test for AFP and HCG, with or without LDH and CA-125. If encountered inadvertently, every attempt should be made to preserve fertility in these young patients and expedient referral to a gynecologic oncologist, pediatric gynecologist, and/or a reproductive endocrinologist is warranted. Rarely, a second look laparotomy is indicated without obvious intraperitoneal spread and reproductive potential is preserved even in those requiring BEP. If managed appropriately, the overall prognosis remains good for these young women.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Germ cell neoplasms arise from primordial germ cells of the gonad that differentiate to embryonic and extraembryonic tissues. Approximately 20%-25% of ovarian neoplasms are germ cell in origin but they account for only 3%-5% of ovarian malignancies. Importantly, germ cell neoplasms encompass 70% of the ovarian neoplasms among girls and young women aged 10-30 years, of which approximately one-third are malignant.
Unlike epithelial ovarian cancers, malignant germ cell neoplasms are typically diagnosed at early stages. They can present as a palpable mass or cause acute abdominal pain secondary to their rapid growth, penchant for necrosis, torsion, hemorrhage, infection, or rupture. Since malignant germ cell neoplasms can excrete hormonally-active tumor markers, such as human chorionic gonadotropin (HCG), many women present with menstrual irregularities.

Management of malignant germ cell neoplasms generally includes fertility-sparing surgery – with or without neoadjuvant combination bleomycin, etoposide, and cisplatin (BEP) – and is typically associated with a favorable prognosis (Cancer Treat Rev. 2008 Aug;34[5]:427-41).
Teratomas
Benign teratomas represent about a quarter of all ovarian neoplasms. Benign teratomas can be solid or cystic, and contain components representing all three cell layers: endoderm, mesoderm, and ectoderm. Due to the presence of differentiated adult tissues, benign teratomas have a characteristic radiographic appearance. They can appear as cystic echogenic masses with intense acoustic shadowing, homogenous nodules or bands, and rounded protuberances called “Rokitansky nodules.”
Treatment of benign teratomas in reproductive-age women includes ovarian cystectomy and careful inspection of the contralateral ovary as 10%-15% may be bilateral. In postmenopausal women, there is less than a 2% risk for malignant transformation of the associated cell lines, most commonly a squamous cell carcinoma. In these cases, spread beyond the ovarian capsule is associated with a poor prognosis and chemotherapy and/or radiation are indicated (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:140-4). Malignant monodermal teratomas are composed of single cell lines with malignant transformations of that tissue type; struma ovarii composed of thyroid tissue, for example, is exceedingly rare.
Dysgerminoma
Dysgerminomas are the most common malignant germ cell neoplasms, accounting for about one-third of cases. They typically present in girls and young women between 10 and 30 years of age, and rarely occur after 50 years of age. As a result, a quarter of cases are identified during pregnancy and another 5% are found in patients presenting with amenorrhea secondary to gonadal dysgenesis, such as is associated with Turner’s syndrome.
At diagnosis, lactate dehydrogenase (LDH) may be elevated and 10% may have an elevation of HCG. Ultrasound findings include a solid, mostly echoic, but heterogeneous mass with apparent lobulations. About two-thirds are stage I at the time of diagnosis, and 10%-15% are bilateral, making dysgerminomas the only malignant germ cell neoplasm with significant risk for bilaterality.
Treatment for early stage dysgerminoma is surgical; young women should have at least unilateral oophorectomy performed; if the contralateral ovary is spared there’s a 10% risk for recurrence over the next 2 years. Comprehensive fertility-sparing surgery is recommended with pelvic and para-aortic lymphadenectomy. Women with gonadal dysgenesis should have a bilateral salpingo-oophorectomy, and those beyond childbearing should undergo a total hysterectomy, with bilateral salpingo-oophorectomy and appropriate staging. BEP should be added for patients with advanced disease.
Other malignant germ cell neoplasms
Uncommon malignant germ cell neoplasms include immature teratomas and endodermal sinus tumors. Though uncommon, the majority of immature teratomas present between the ages of 10 and 20 years and account for nearly 30% of the ovarian cancer deaths in this age group. Immature teratomas usually have negative serum markers, though about one-third will excrete HCG. Immature teratomas are graded by the proportion of neuro-epithelium. Treatment includes unilateral oophorectomy, and surgical staging with adjuvant chemotherapy (BEP) for patients with greater than stage 1A grade 1 disease.
Endodermal sinus tumors are derived from the primitive yolk sac, are unilateral, and most will secrete alpha-fetoprotein (AFP). The median age of diagnosis of an endodermal sinus tumor is 18 years, thus treatment includes unilateral salpingo-oophorectomy and comprehensive fertility-sparing surgical staging followed by BEP.
Embryonal and nongestational choriocarcinomas are rare malignant germ cell neoplasms found in prepubertal girls to young women. Embryonal carcinomas can secrete estrogens, HCG and/or AFP, so patients may present with precocious puberty. Treatment is similar to that of endodermal sinus tumors. Nongestational choriocarcinomas, like the gestational forms, have a poor prognosis and are monitored and treated similarly.
Understanding presentation and treatments for malignant germ cell neoplasms is important in the evaluation of a young patient with a pelvic mass and should prompt the practicing gynecologist to test for AFP and HCG, with or without LDH and CA-125. If encountered inadvertently, every attempt should be made to preserve fertility in these young patients and expedient referral to a gynecologic oncologist, pediatric gynecologist, and/or a reproductive endocrinologist is warranted. Rarely, a second look laparotomy is indicated without obvious intraperitoneal spread and reproductive potential is preserved even in those requiring BEP. If managed appropriately, the overall prognosis remains good for these young women.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Germ cell neoplasms arise from primordial germ cells of the gonad that differentiate to embryonic and extraembryonic tissues. Approximately 20%-25% of ovarian neoplasms are germ cell in origin but they account for only 3%-5% of ovarian malignancies. Importantly, germ cell neoplasms encompass 70% of the ovarian neoplasms among girls and young women aged 10-30 years, of which approximately one-third are malignant.
Unlike epithelial ovarian cancers, malignant germ cell neoplasms are typically diagnosed at early stages. They can present as a palpable mass or cause acute abdominal pain secondary to their rapid growth, penchant for necrosis, torsion, hemorrhage, infection, or rupture. Since malignant germ cell neoplasms can excrete hormonally-active tumor markers, such as human chorionic gonadotropin (HCG), many women present with menstrual irregularities.

Management of malignant germ cell neoplasms generally includes fertility-sparing surgery – with or without neoadjuvant combination bleomycin, etoposide, and cisplatin (BEP) – and is typically associated with a favorable prognosis (Cancer Treat Rev. 2008 Aug;34[5]:427-41).
Teratomas
Benign teratomas represent about a quarter of all ovarian neoplasms. Benign teratomas can be solid or cystic, and contain components representing all three cell layers: endoderm, mesoderm, and ectoderm. Due to the presence of differentiated adult tissues, benign teratomas have a characteristic radiographic appearance. They can appear as cystic echogenic masses with intense acoustic shadowing, homogenous nodules or bands, and rounded protuberances called “Rokitansky nodules.”
Treatment of benign teratomas in reproductive-age women includes ovarian cystectomy and careful inspection of the contralateral ovary as 10%-15% may be bilateral. In postmenopausal women, there is less than a 2% risk for malignant transformation of the associated cell lines, most commonly a squamous cell carcinoma. In these cases, spread beyond the ovarian capsule is associated with a poor prognosis and chemotherapy and/or radiation are indicated (Int J Gynecol Cancer. 2006 Jan-Feb;16[1]:140-4). Malignant monodermal teratomas are composed of single cell lines with malignant transformations of that tissue type; struma ovarii composed of thyroid tissue, for example, is exceedingly rare.
Dysgerminoma
Dysgerminomas are the most common malignant germ cell neoplasms, accounting for about one-third of cases. They typically present in girls and young women between 10 and 30 years of age, and rarely occur after 50 years of age. As a result, a quarter of cases are identified during pregnancy and another 5% are found in patients presenting with amenorrhea secondary to gonadal dysgenesis, such as is associated with Turner’s syndrome.
At diagnosis, lactate dehydrogenase (LDH) may be elevated and 10% may have an elevation of HCG. Ultrasound findings include a solid, mostly echoic, but heterogeneous mass with apparent lobulations. About two-thirds are stage I at the time of diagnosis, and 10%-15% are bilateral, making dysgerminomas the only malignant germ cell neoplasm with significant risk for bilaterality.
Treatment for early stage dysgerminoma is surgical; young women should have at least unilateral oophorectomy performed; if the contralateral ovary is spared there’s a 10% risk for recurrence over the next 2 years. Comprehensive fertility-sparing surgery is recommended with pelvic and para-aortic lymphadenectomy. Women with gonadal dysgenesis should have a bilateral salpingo-oophorectomy, and those beyond childbearing should undergo a total hysterectomy, with bilateral salpingo-oophorectomy and appropriate staging. BEP should be added for patients with advanced disease.
Other malignant germ cell neoplasms
Uncommon malignant germ cell neoplasms include immature teratomas and endodermal sinus tumors. Though uncommon, the majority of immature teratomas present between the ages of 10 and 20 years and account for nearly 30% of the ovarian cancer deaths in this age group. Immature teratomas usually have negative serum markers, though about one-third will excrete HCG. Immature teratomas are graded by the proportion of neuro-epithelium. Treatment includes unilateral oophorectomy, and surgical staging with adjuvant chemotherapy (BEP) for patients with greater than stage 1A grade 1 disease.
Endodermal sinus tumors are derived from the primitive yolk sac, are unilateral, and most will secrete alpha-fetoprotein (AFP). The median age of diagnosis of an endodermal sinus tumor is 18 years, thus treatment includes unilateral salpingo-oophorectomy and comprehensive fertility-sparing surgical staging followed by BEP.
Embryonal and nongestational choriocarcinomas are rare malignant germ cell neoplasms found in prepubertal girls to young women. Embryonal carcinomas can secrete estrogens, HCG and/or AFP, so patients may present with precocious puberty. Treatment is similar to that of endodermal sinus tumors. Nongestational choriocarcinomas, like the gestational forms, have a poor prognosis and are monitored and treated similarly.
Understanding presentation and treatments for malignant germ cell neoplasms is important in the evaluation of a young patient with a pelvic mass and should prompt the practicing gynecologist to test for AFP and HCG, with or without LDH and CA-125. If encountered inadvertently, every attempt should be made to preserve fertility in these young patients and expedient referral to a gynecologic oncologist, pediatric gynecologist, and/or a reproductive endocrinologist is warranted. Rarely, a second look laparotomy is indicated without obvious intraperitoneal spread and reproductive potential is preserved even in those requiring BEP. If managed appropriately, the overall prognosis remains good for these young women.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Establishing a Role for Polysomnography in Hospitalized Children
Clinical question: What is the role for inpatient polysomnograms for children with medical complexity?
Background: Sleep-disordered breathing is more common in certain pediatric populations. Children with neuromuscular disease, craniofacial or tracheobronchial malformations, or developmental delay have up to 10 times the rate of sleep-disordered breathing as compared to the general pediatric population, with a prevalence as high as 40%. It is recommended that patients with neuromuscular conditions get annual polysomnograms (PSGs). The medical complexity and requirement for nursing and respiratory care makes it challenging to obtain routine outpatient PSGs in this population. This study is the first of its kind to examine the characteristics of patients receiving inpatient PSGs and to determine the effects the findings of these studies had on the patients’ care.
Study design: Retrospective case series.
Setting: Single, large, academic medical center.
Synopsis: Eight-five PSGs were completed on 70 patients during the study period. These occurred primarily in the pediatric intensive care unit (50 patients) but also in the neonatal intensive care unit (five patients) and the general pediatric floor (15 patients). The mean age of patients was 6.5 years, and 60% were male.
The most common diagnoses in this group were airway obstruction due to craniofacial abnormalities or defects of the tracheobronchial tree (54%), chronic respiratory failure (34%), hypoxic ischemic encephalopathy (23%), and genetic syndromes (14%). All sleep studies were successfully completed using the center’s dedicated sleep technicians and PSG scoring staff. There were no complications associated with the PSGs.
The most common specific indications for obtaining the PSGs were chronic pulmonary failure with airway obstruction and ventilator requirement assessment. Eighty-nine percent of patients had some abnormality of their PSG. Obstructive sleep apnea, tachypnea and desaturation, and disorders of sleep architecture were the most commonly found abnormalities.
The most common interventions based upon the PSG results were adjustment of ventilator parameters (46%), ENT referral for upper airway assessment (31%), and initiation of positive pressure ventilation (CPAP or BiPAP, 25%). Follow-up PSGs after these interventions demonstrated statistically significant improvement in apnea-hypopnea index, arousal index, and lowest oxygen saturation.
Bottom line: Inpatient PSGs for children with medical complexity are safe and often have significant findings that alter care for the patient.
Citation: Tkachenko N, Singh K, Abreu N, et al. Establishing a role for polysomnography in hospitalized children. Pediatr Neurol. 2016;57:39-45.e1. doi:10.1016/j.pediatrneurol.2015.12.020.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. Dupont Hospital for Children in Wilmington, Del., and assistant professor of pediatrics at Thomas Jefferson Medical College in Philadelphia.
Clinical question: What is the role for inpatient polysomnograms for children with medical complexity?
Background: Sleep-disordered breathing is more common in certain pediatric populations. Children with neuromuscular disease, craniofacial or tracheobronchial malformations, or developmental delay have up to 10 times the rate of sleep-disordered breathing as compared to the general pediatric population, with a prevalence as high as 40%. It is recommended that patients with neuromuscular conditions get annual polysomnograms (PSGs). The medical complexity and requirement for nursing and respiratory care makes it challenging to obtain routine outpatient PSGs in this population. This study is the first of its kind to examine the characteristics of patients receiving inpatient PSGs and to determine the effects the findings of these studies had on the patients’ care.
Study design: Retrospective case series.
Setting: Single, large, academic medical center.
Synopsis: Eight-five PSGs were completed on 70 patients during the study period. These occurred primarily in the pediatric intensive care unit (50 patients) but also in the neonatal intensive care unit (five patients) and the general pediatric floor (15 patients). The mean age of patients was 6.5 years, and 60% were male.
The most common diagnoses in this group were airway obstruction due to craniofacial abnormalities or defects of the tracheobronchial tree (54%), chronic respiratory failure (34%), hypoxic ischemic encephalopathy (23%), and genetic syndromes (14%). All sleep studies were successfully completed using the center’s dedicated sleep technicians and PSG scoring staff. There were no complications associated with the PSGs.
The most common specific indications for obtaining the PSGs were chronic pulmonary failure with airway obstruction and ventilator requirement assessment. Eighty-nine percent of patients had some abnormality of their PSG. Obstructive sleep apnea, tachypnea and desaturation, and disorders of sleep architecture were the most commonly found abnormalities.
The most common interventions based upon the PSG results were adjustment of ventilator parameters (46%), ENT referral for upper airway assessment (31%), and initiation of positive pressure ventilation (CPAP or BiPAP, 25%). Follow-up PSGs after these interventions demonstrated statistically significant improvement in apnea-hypopnea index, arousal index, and lowest oxygen saturation.
Bottom line: Inpatient PSGs for children with medical complexity are safe and often have significant findings that alter care for the patient.
Citation: Tkachenko N, Singh K, Abreu N, et al. Establishing a role for polysomnography in hospitalized children. Pediatr Neurol. 2016;57:39-45.e1. doi:10.1016/j.pediatrneurol.2015.12.020.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. Dupont Hospital for Children in Wilmington, Del., and assistant professor of pediatrics at Thomas Jefferson Medical College in Philadelphia.
Clinical question: What is the role for inpatient polysomnograms for children with medical complexity?
Background: Sleep-disordered breathing is more common in certain pediatric populations. Children with neuromuscular disease, craniofacial or tracheobronchial malformations, or developmental delay have up to 10 times the rate of sleep-disordered breathing as compared to the general pediatric population, with a prevalence as high as 40%. It is recommended that patients with neuromuscular conditions get annual polysomnograms (PSGs). The medical complexity and requirement for nursing and respiratory care makes it challenging to obtain routine outpatient PSGs in this population. This study is the first of its kind to examine the characteristics of patients receiving inpatient PSGs and to determine the effects the findings of these studies had on the patients’ care.
Study design: Retrospective case series.
Setting: Single, large, academic medical center.
Synopsis: Eight-five PSGs were completed on 70 patients during the study period. These occurred primarily in the pediatric intensive care unit (50 patients) but also in the neonatal intensive care unit (five patients) and the general pediatric floor (15 patients). The mean age of patients was 6.5 years, and 60% were male.
The most common diagnoses in this group were airway obstruction due to craniofacial abnormalities or defects of the tracheobronchial tree (54%), chronic respiratory failure (34%), hypoxic ischemic encephalopathy (23%), and genetic syndromes (14%). All sleep studies were successfully completed using the center’s dedicated sleep technicians and PSG scoring staff. There were no complications associated with the PSGs.
The most common specific indications for obtaining the PSGs were chronic pulmonary failure with airway obstruction and ventilator requirement assessment. Eighty-nine percent of patients had some abnormality of their PSG. Obstructive sleep apnea, tachypnea and desaturation, and disorders of sleep architecture were the most commonly found abnormalities.
The most common interventions based upon the PSG results were adjustment of ventilator parameters (46%), ENT referral for upper airway assessment (31%), and initiation of positive pressure ventilation (CPAP or BiPAP, 25%). Follow-up PSGs after these interventions demonstrated statistically significant improvement in apnea-hypopnea index, arousal index, and lowest oxygen saturation.
Bottom line: Inpatient PSGs for children with medical complexity are safe and often have significant findings that alter care for the patient.
Citation: Tkachenko N, Singh K, Abreu N, et al. Establishing a role for polysomnography in hospitalized children. Pediatr Neurol. 2016;57:39-45.e1. doi:10.1016/j.pediatrneurol.2015.12.020.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. Dupont Hospital for Children in Wilmington, Del., and assistant professor of pediatrics at Thomas Jefferson Medical College in Philadelphia.
Study links radon and hematologic cancers in women

New research suggests there is a significant positive association between high levels of residential radon and the risk of hematologic malignancies in women.
The study is the first prospective, population-based study of residential radon exposure and hematologic malignancy risk.
Therefore, the researchers caution that it requires replication to better understand the association and whether it truly differs by sex.
Lauren Teras, PhD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in Environmental Research.
Radon is a naturally occurring byproduct of the decay of radium and is a known human lung carcinogen. It is the second-leading cause of lung cancer in the US.
Modeling studies have shown that radon delivers a non-negligible dose of alpha radiation to the bone marrow and therefore could increase the risk of hematologic malignancies. However, studies investigating the link between radon and hematologic malignancies have produced inconsistent results.
For the current study, Dr Teras and her colleagues used data from the American Cancer Society Cancer Prevention Study-II Nutrition Cohort to examine the association between county-level residential radon exposure and the risk of hematologic cancer.
The analysis included 140,652 participants, including 3019 who had hematologic malignancies during 19 years of follow-up (1992 to 2011).
The researchers found that women living in counties with the highest mean radon concentration (> 148 Bq/m3) had a significantly higher risk of developing a hematologic malignancy than women living in counties with the lowest radon levels (< 74 Bq/m3).
The adjusted hazard ratio (adjusted for age, race, family history of hematologic malignancy, etc.) was 1.63 (P=0.0010).
The researchers also found evidence of a dose-response relationship, with an adjusted hazard ratio of 1.38 (P=0.001).
The team said there was evidence of a positive exposure-response relationship between radon concentration and the risk of all lymphoid malignancy subtypes in women. But the highest risk was observed for follicular lymphoma, with an adjusted hazard ratio of 2.74 (P=0.02).
On the other hand, there was a non-significant inverse association between radon and myeloid leukemias in women.
There was no association between hematologic malignancy and radon exposure among the men.
The researchers said a possible explanation for this finding is that men may have a higher baseline risk of hematologic malignancy, possibly because of more exposure to occupational or other risk factors, which would reduce the impact of any additional risk from residential radon.
In women, who have a smaller baseline risk, residential radon exposure might be a larger contributor to overall risk.
Another reason for the sex difference observed in this study may be that the women of this generation spent more time in their homes, so they had more residential exposure than men.
“The overall lifetime risk of hematological cancers in the United States is about 2%, so even a 60% relative increase would still mean a relatively small absolute risk,” Dr Teras noted.
“Nonetheless, radon is already associated with lung cancer, and if other studies confirm the link to blood cancers, we think it would warrant strengthened public health efforts to mitigate residential radon risks.” ![]()

New research suggests there is a significant positive association between high levels of residential radon and the risk of hematologic malignancies in women.
The study is the first prospective, population-based study of residential radon exposure and hematologic malignancy risk.
Therefore, the researchers caution that it requires replication to better understand the association and whether it truly differs by sex.
Lauren Teras, PhD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in Environmental Research.
Radon is a naturally occurring byproduct of the decay of radium and is a known human lung carcinogen. It is the second-leading cause of lung cancer in the US.
Modeling studies have shown that radon delivers a non-negligible dose of alpha radiation to the bone marrow and therefore could increase the risk of hematologic malignancies. However, studies investigating the link between radon and hematologic malignancies have produced inconsistent results.
For the current study, Dr Teras and her colleagues used data from the American Cancer Society Cancer Prevention Study-II Nutrition Cohort to examine the association between county-level residential radon exposure and the risk of hematologic cancer.
The analysis included 140,652 participants, including 3019 who had hematologic malignancies during 19 years of follow-up (1992 to 2011).
The researchers found that women living in counties with the highest mean radon concentration (> 148 Bq/m3) had a significantly higher risk of developing a hematologic malignancy than women living in counties with the lowest radon levels (< 74 Bq/m3).
The adjusted hazard ratio (adjusted for age, race, family history of hematologic malignancy, etc.) was 1.63 (P=0.0010).
The researchers also found evidence of a dose-response relationship, with an adjusted hazard ratio of 1.38 (P=0.001).
The team said there was evidence of a positive exposure-response relationship between radon concentration and the risk of all lymphoid malignancy subtypes in women. But the highest risk was observed for follicular lymphoma, with an adjusted hazard ratio of 2.74 (P=0.02).
On the other hand, there was a non-significant inverse association between radon and myeloid leukemias in women.
There was no association between hematologic malignancy and radon exposure among the men.
The researchers said a possible explanation for this finding is that men may have a higher baseline risk of hematologic malignancy, possibly because of more exposure to occupational or other risk factors, which would reduce the impact of any additional risk from residential radon.
In women, who have a smaller baseline risk, residential radon exposure might be a larger contributor to overall risk.
Another reason for the sex difference observed in this study may be that the women of this generation spent more time in their homes, so they had more residential exposure than men.
“The overall lifetime risk of hematological cancers in the United States is about 2%, so even a 60% relative increase would still mean a relatively small absolute risk,” Dr Teras noted.
“Nonetheless, radon is already associated with lung cancer, and if other studies confirm the link to blood cancers, we think it would warrant strengthened public health efforts to mitigate residential radon risks.” ![]()

New research suggests there is a significant positive association between high levels of residential radon and the risk of hematologic malignancies in women.
The study is the first prospective, population-based study of residential radon exposure and hematologic malignancy risk.
Therefore, the researchers caution that it requires replication to better understand the association and whether it truly differs by sex.
Lauren Teras, PhD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in Environmental Research.
Radon is a naturally occurring byproduct of the decay of radium and is a known human lung carcinogen. It is the second-leading cause of lung cancer in the US.
Modeling studies have shown that radon delivers a non-negligible dose of alpha radiation to the bone marrow and therefore could increase the risk of hematologic malignancies. However, studies investigating the link between radon and hematologic malignancies have produced inconsistent results.
For the current study, Dr Teras and her colleagues used data from the American Cancer Society Cancer Prevention Study-II Nutrition Cohort to examine the association between county-level residential radon exposure and the risk of hematologic cancer.
The analysis included 140,652 participants, including 3019 who had hematologic malignancies during 19 years of follow-up (1992 to 2011).
The researchers found that women living in counties with the highest mean radon concentration (> 148 Bq/m3) had a significantly higher risk of developing a hematologic malignancy than women living in counties with the lowest radon levels (< 74 Bq/m3).
The adjusted hazard ratio (adjusted for age, race, family history of hematologic malignancy, etc.) was 1.63 (P=0.0010).
The researchers also found evidence of a dose-response relationship, with an adjusted hazard ratio of 1.38 (P=0.001).
The team said there was evidence of a positive exposure-response relationship between radon concentration and the risk of all lymphoid malignancy subtypes in women. But the highest risk was observed for follicular lymphoma, with an adjusted hazard ratio of 2.74 (P=0.02).
On the other hand, there was a non-significant inverse association between radon and myeloid leukemias in women.
There was no association between hematologic malignancy and radon exposure among the men.
The researchers said a possible explanation for this finding is that men may have a higher baseline risk of hematologic malignancy, possibly because of more exposure to occupational or other risk factors, which would reduce the impact of any additional risk from residential radon.
In women, who have a smaller baseline risk, residential radon exposure might be a larger contributor to overall risk.
Another reason for the sex difference observed in this study may be that the women of this generation spent more time in their homes, so they had more residential exposure than men.
“The overall lifetime risk of hematological cancers in the United States is about 2%, so even a 60% relative increase would still mean a relatively small absolute risk,” Dr Teras noted.
“Nonetheless, radon is already associated with lung cancer, and if other studies confirm the link to blood cancers, we think it would warrant strengthened public health efforts to mitigate residential radon risks.” ![]()
CHMP recommends approving drug to treat FL

The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that obinutuzumab (Gazyvaro), an anti-CD20 monoclonal antibody, be approved for use in patients with follicular lymphoma (FL).
The recommended indication is for obinutuzumab to be given first in combination with bendamustine and then as maintenance therapy in FL patients who did not respond to, progressed during, or progressed up to 6 months after treatment with rituximab or a rituximab-containing regimen.
Based on the CHMP’s recommendation, a final decision regarding the approval of obinutuzumab in FL is expected from the European Commission in the coming months.
Obinutuzumab is already approved in the European Union for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia and comorbidities that make them unsuitable for full-dose fludarabine-based therapy.
Obinutuzumab is being developed by Roche.
GADOLIN trial
The CHMP’s recommendation to approve obinutuzumab in FL is based on results from the phase 3 GADOLIN trial. The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()

The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that obinutuzumab (Gazyvaro), an anti-CD20 monoclonal antibody, be approved for use in patients with follicular lymphoma (FL).
The recommended indication is for obinutuzumab to be given first in combination with bendamustine and then as maintenance therapy in FL patients who did not respond to, progressed during, or progressed up to 6 months after treatment with rituximab or a rituximab-containing regimen.
Based on the CHMP’s recommendation, a final decision regarding the approval of obinutuzumab in FL is expected from the European Commission in the coming months.
Obinutuzumab is already approved in the European Union for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia and comorbidities that make them unsuitable for full-dose fludarabine-based therapy.
Obinutuzumab is being developed by Roche.
GADOLIN trial
The CHMP’s recommendation to approve obinutuzumab in FL is based on results from the phase 3 GADOLIN trial. The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()

The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that obinutuzumab (Gazyvaro), an anti-CD20 monoclonal antibody, be approved for use in patients with follicular lymphoma (FL).
The recommended indication is for obinutuzumab to be given first in combination with bendamustine and then as maintenance therapy in FL patients who did not respond to, progressed during, or progressed up to 6 months after treatment with rituximab or a rituximab-containing regimen.
Based on the CHMP’s recommendation, a final decision regarding the approval of obinutuzumab in FL is expected from the European Commission in the coming months.
Obinutuzumab is already approved in the European Union for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia and comorbidities that make them unsuitable for full-dose fludarabine-based therapy.
Obinutuzumab is being developed by Roche.
GADOLIN trial
The CHMP’s recommendation to approve obinutuzumab in FL is based on results from the phase 3 GADOLIN trial. The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()
FIRST reflections: Impact of ACGME duty hours on CT practitioners
Several months ago, Dr. Bilimoria and his colleagues published the long awaited study in NEJM essentially contradicting adverse effects of strict duty hours on patient outcomes (N Engl J Med. 2016 374:713-2). The study, known as the FIRST trial, was published in the March issue of Thoracic Surgery News. Although the study enrolled general surgery residents, its conclusion impacts no specialty more than cardiothoracic surgery, where frequent handoffs complicate tedious perioperative care of sick patients stall learning opportunities for young trainees.
As Dr. Shari Meyerson eloquently noted in her perspective piece for Thoracic Surgery News (March 2016, page 4), surgery training needs to adapt to meet the modern day needs of trainees to rest and spend time with family and friends, with those of exposure to complex clinical scenarios in a short residency period. Arguably, CT surgery trainees are some of the most motivated and driven; to limit their experience on a national level may be shortsighted. On the other hand, appropriate incorporation of advanced practice providers (APPs) may help allay some patient care challenges, free valuable family time, and allow thoracic residents to function well in a more flexible ACGME duty-hour paradigm. To add thoracic relevance to the findings of Dr. Bilimoria and his colleagues, the debate is brought to Thoracic Surgery News by our colleagues from different training pathways below:
Dr. Antonoff: “Due to the timing of my medical school matriculation, I completed my surgical clerkships in the preregulated duty-hour era. I had expectations of what my life would be like as a surgeon when I applied for the general surgery match, and frankly, my expectations prepared me for a life that would revolve around my education, my technical training, and my commitment to patient care. During my years as a junior resident, my surgical training program gradually adapted in recognition of the new guidelines, but it took time. I spent 3 years in the research lab, and, after I came out as a senior resident, I discovered that the rules of the game had totally changed. While duty hours for me, as a senior trainee, were still fairly open, I found that my interns and junior residents had to play by completely new rules. In some ways, on rare occasions, I felt frustrated and resentful of the fact that my duties as a chief resident and thoracic fellow included many of the tasks that I’d done as an intern, because my interns would ‘expire’ after 16 hours. However, much more often, I felt bad for those who came after me.
They seemed desperate to operate, eager to see their patients’ problems through to resolution, and embarrassed to have to end their days earlier than the more senior members of the team. I feel fortunate that I had years of frequent in-house call after long days in the operating room and followed by post-call days of more operating. I finished my junior years without fear of any sick patient in the hospital, I finished my general surgery training without fear of any emergent operation, and I finished my fellowship with confidence that I could get a patient through just about anything if I had access to a cannula.
My early experiences as an attending have certainly kept me humble, and I’ve spent many a night worried about my patients, rethinking choices that I’ve made and stitches that I’ve thrown. But I thank my lucky stars that I was exposed to phenomenal training, and that I had the privilege and opportunity to work the hours needed to reach a reasonable level of safety! I can only imagine that if I’d have spent fewer nights in the hospital, that I’d feel even more anxiety and nausea at this early stage of my surgical career.
As elaborated in the editorial by Dr. Birkmeyer (N Engl J Med. 2016;374:783-4), it is not surprising that patient outcomes did not immediately depend on whether the programs had adhered strictly to the ACGME duty-hour rules. Limited numbers of patients experience critical events during shift change, and hospitals are evolving to function with greater reliance on midlevel practitioners and attending physicians. I would not expect the short-term results of duty-hour flexibility to demonstrate any impact on patient care. However, I do fear that there will be a mid-term impact on trainee accountability and autonomy, which will ultimately impact the competence of the attending surgeons of the future, and downstream potential long-term impact on day-to-day patient outcomes.
As a wife and mother of 3, I recognize that we, as a specialty, need to find ways to support our trainees and their families and to help them live happy lives conducive to functioning outside the hospital. I believe that we can do this with support, mentorship, and advocacy; I do not believe that it requires cutting back on the training that we are all, in the end, so incredibly grateful to receive.”
Dr. Mara Antonoff is an assistant professor of thoracic and cardiovascular surgery at UT MD Anderson Cancer Center. She performed her General Surgery training at the University of Minnesota, 2004-2012, and her Thoracic Surgery Training at Washington University, as a traditional 2-year resident, 2012-2014.
Dr. Stephens: “There is nothing that replaces being bedside. Whether it be a postoperative patient struggling with low cardiac output syndrome overnight, or a patient with a high pressor requirement the etiology of which you are trying to uncover, or a patient you have been following who suddenly arrests, the value of seeing a patient’s trajectory longitudinally is critical to developing clinical acumen. When as an attending I will get called in the middle of the night about a postoperative patient not “doing well,” I will be drawing on my years of being on call and being bedside with my patients.
Patient care is the ultimate goal, and it is clear that overworked residents are at higher risk for making mistakes that jeopardize patient care, which nobody wants. However, the restrictions that duty hours place don’t allow the flexibility necessary for a specialty such as ours, and in fact strict adherence to such regulations inhibits opportunities for our learning. Also concerning is the “shift-work” mentality that seems to be increasingly pervasive with the implementation of duty hours. As has been well documented, and as I have seen personally, the constant patient handoffs that are requisite to implementation of duty hours pose their own perils in terms of patient safety.
Ultimately, these are our patients and we are responsible. Once we are attendings, those responsibilities will not be turned off after we have reached some prespecified hour limit.
The question then remains how best to implement a system across a wide variety of programs that ensures both patient safety and adequate clinical experience in the context of a culture of patient responsibility for the residents. As the NEJM study (N Engl J Med. 2016 374:713-2) shows, flexibility in implementation of duty hours did not result in increased complications, but resulted in improved resident satisfaction in continuity of care and handoffs. In my opinion, this study then encourages specialties such as ours to be more flexible in work hours, to encourage residents when there is a learning opportunity that previously they would be prohibited from taking part in to take hold of that opportunity, and to use this flexibility in implementation of duty hours to combat the invading “shift-work” mentality that will only jeopardize patient care.”
Dr. Elizabeth H. Stephens, MD, PhD is a Cardiothoracic Surgery, resident, PGY4, at Columbia University, New York, as an Integrated I-6 Resident.
Dr. Brown: “I took the traditional 5-year of General Surgery + 2 years of Cardiothoracic Surgery training route to becoming a General Thoracic Surgeon. My General Surgery experience was invaluable to my development as a surgeon. However, after all of those years of General Surgery cases and minimal exposure to Cardiothoracic Surgery cases, coupled with minimal overlap between the two specialties with regard to patient care, I found the learning curve in fellowship to be very steep. I was fortunate to train in a program with phenomenal physician extender support [APPs] in addition to top-notch colleagues in other specialties and excellent nursing, which allowed me to spend the majority of those 2 years in the operating room and completely focused on patient care. During that final phase of training, I welcomed flexibility with regard to the work-hour restrictions to ensure that I was acquiring the experience I needed prior to starting my own practice.”
Lisa M. Brown, MD, MAS, Assistant Professor of Thoracic Surgery, UC Davis Health System, Calif.; Training Institution: Washington University
Dr. Lee: “I started my surgical training in 2005, 2 years after the implementation of the 80-hour workweek restriction. Fortunately for my personal life, my training program took the restriction very seriously and strictly enforced it. As a result, I had scheduled periods off from work, and rarely worked more than 80 hours per week over the course of general surgery. On those occasions that I did, the next weeks, or preceding weeks would be shorter, to compensate. As a product of a 4+3 Thoracic Surgery residency in this environment, the 80-hour workweek extended to my subspecialty training. Our cardiac surgery time strictly enforced the go-home post-call policy. As a result, I believe my duty hours during Thoracic Surgery are likely shorter overall than many other programs.
Everyone hears the rumors of other programs lying about their duty hours. Fortunately, mine was not one of these. Despite this, or because of this, I received top-notch training. At the same time and more importantly, I started a family. I married my wife a week before my internship started, and am still married to the same person. We had two precious daughters during my Thoracic Surgery years. I don’t believe this would have been possible without duty-hours restrictions.
To create an environment where such a task was possible, my program hired an army of mid-level practitioners to deal with the day-to-day tasks of providing cardiothoracic surgical care to the patients, both in the intensive care unit and on the ward.
I rarely had to write a history and physical during Thoracic Surgery training. I consented fewer patients than I have fingers on my hands. I pulled even fewer chest tubes. I can now no longer remember having pulled a central line. I learned these tasks when I was a junior resident. What I did instead as a senior resident was perform over 900 cardiothoracic procedures as the primary surgeon. Now, as an attending surgeon, I still don’t write full histories and physicals by myself. I certainly don’t pull chest tubes and central lines. I consent patients by having a conversation with them, which I did as a resident, but I don’t bring a piece of paper with me in to the clinic room. I have a physician assistant who helps me fill in the gaps.
In 8 months of practice, I have performed over 15 thoracic organ transplants, repaired over 15 aortic dissections, half of whom required root replacements, performed more double-valve surgeries than straightforward single-valve replacements, started a minimally invasive atrial fibrillation surgical program, and applied almost every shred of knowledge and experience gained in 3 years of Thoracic Surgery Residency to a busy clinical practice. Most importantly, I continue to come home and watch my two daughters grow up.
With that perspective, I don’t believe the question should be whether or not programs should have the flexibility to enforce or not enforce duty-hours restrictions. It should be, how could every program find a way to effectively train residents to be good physicians and still allow them a personal life?”
Dr. Anson Lee is an Assistant Professor of Cardiac Surgery, Stanford University, Calif.; Training: 4+3 CT Residency, at Washington University, St. Louis.
Ms. Bohlman: “As a physician assistant in cardiac surgery, I represent the reality that physicians with a critical patient population appreciate consistency in their patient management. However, working in a university hospital setting also requires that surgical residents receive appropriate training. With the recently implemented duty restriction hours on resident training programs, advanced practice providers (APPs) have been utilized as an excellent solution for scheduling conflicts without compromising patient care. An example to this point is evident in my own place of work.
Approximately 1 year ago, our surgical intensive care unit transitioned from resident care to a combination of residents and APPs. At that time, the APPs were tasked with the complete care of all cardiac surgery patients. This change reduced the quantity and acuity of patients for which the residents were responsible and therefore allowed for more flexible hours along with a more manageable patient load. These changes, among others, have contributed to improved patient outcomes in the cardiac surgery patient population within our institution. With the increase in APPs that have training in various specialties, there comes an increasing ability to not only fill the gaps in scheduling but to do so with an extension of the providing physician. Although the NEJM article demonstrated no difference in patient outcomes between resident programs with restricted duty hours versus more flexible duty-hour policies, I foresee the future of medicine focusing on trained APPs as a complement to the care that the residents provide.”
Allison Bohlman is a Physician Assistant at Rush University Medical Center in the Integrated cardiovascular thoracic intensive care unit.
Several months ago, Dr. Bilimoria and his colleagues published the long awaited study in NEJM essentially contradicting adverse effects of strict duty hours on patient outcomes (N Engl J Med. 2016 374:713-2). The study, known as the FIRST trial, was published in the March issue of Thoracic Surgery News. Although the study enrolled general surgery residents, its conclusion impacts no specialty more than cardiothoracic surgery, where frequent handoffs complicate tedious perioperative care of sick patients stall learning opportunities for young trainees.
As Dr. Shari Meyerson eloquently noted in her perspective piece for Thoracic Surgery News (March 2016, page 4), surgery training needs to adapt to meet the modern day needs of trainees to rest and spend time with family and friends, with those of exposure to complex clinical scenarios in a short residency period. Arguably, CT surgery trainees are some of the most motivated and driven; to limit their experience on a national level may be shortsighted. On the other hand, appropriate incorporation of advanced practice providers (APPs) may help allay some patient care challenges, free valuable family time, and allow thoracic residents to function well in a more flexible ACGME duty-hour paradigm. To add thoracic relevance to the findings of Dr. Bilimoria and his colleagues, the debate is brought to Thoracic Surgery News by our colleagues from different training pathways below:
Dr. Antonoff: “Due to the timing of my medical school matriculation, I completed my surgical clerkships in the preregulated duty-hour era. I had expectations of what my life would be like as a surgeon when I applied for the general surgery match, and frankly, my expectations prepared me for a life that would revolve around my education, my technical training, and my commitment to patient care. During my years as a junior resident, my surgical training program gradually adapted in recognition of the new guidelines, but it took time. I spent 3 years in the research lab, and, after I came out as a senior resident, I discovered that the rules of the game had totally changed. While duty hours for me, as a senior trainee, were still fairly open, I found that my interns and junior residents had to play by completely new rules. In some ways, on rare occasions, I felt frustrated and resentful of the fact that my duties as a chief resident and thoracic fellow included many of the tasks that I’d done as an intern, because my interns would ‘expire’ after 16 hours. However, much more often, I felt bad for those who came after me.
They seemed desperate to operate, eager to see their patients’ problems through to resolution, and embarrassed to have to end their days earlier than the more senior members of the team. I feel fortunate that I had years of frequent in-house call after long days in the operating room and followed by post-call days of more operating. I finished my junior years without fear of any sick patient in the hospital, I finished my general surgery training without fear of any emergent operation, and I finished my fellowship with confidence that I could get a patient through just about anything if I had access to a cannula.
My early experiences as an attending have certainly kept me humble, and I’ve spent many a night worried about my patients, rethinking choices that I’ve made and stitches that I’ve thrown. But I thank my lucky stars that I was exposed to phenomenal training, and that I had the privilege and opportunity to work the hours needed to reach a reasonable level of safety! I can only imagine that if I’d have spent fewer nights in the hospital, that I’d feel even more anxiety and nausea at this early stage of my surgical career.
As elaborated in the editorial by Dr. Birkmeyer (N Engl J Med. 2016;374:783-4), it is not surprising that patient outcomes did not immediately depend on whether the programs had adhered strictly to the ACGME duty-hour rules. Limited numbers of patients experience critical events during shift change, and hospitals are evolving to function with greater reliance on midlevel practitioners and attending physicians. I would not expect the short-term results of duty-hour flexibility to demonstrate any impact on patient care. However, I do fear that there will be a mid-term impact on trainee accountability and autonomy, which will ultimately impact the competence of the attending surgeons of the future, and downstream potential long-term impact on day-to-day patient outcomes.
As a wife and mother of 3, I recognize that we, as a specialty, need to find ways to support our trainees and their families and to help them live happy lives conducive to functioning outside the hospital. I believe that we can do this with support, mentorship, and advocacy; I do not believe that it requires cutting back on the training that we are all, in the end, so incredibly grateful to receive.”
Dr. Mara Antonoff is an assistant professor of thoracic and cardiovascular surgery at UT MD Anderson Cancer Center. She performed her General Surgery training at the University of Minnesota, 2004-2012, and her Thoracic Surgery Training at Washington University, as a traditional 2-year resident, 2012-2014.
Dr. Stephens: “There is nothing that replaces being bedside. Whether it be a postoperative patient struggling with low cardiac output syndrome overnight, or a patient with a high pressor requirement the etiology of which you are trying to uncover, or a patient you have been following who suddenly arrests, the value of seeing a patient’s trajectory longitudinally is critical to developing clinical acumen. When as an attending I will get called in the middle of the night about a postoperative patient not “doing well,” I will be drawing on my years of being on call and being bedside with my patients.
Patient care is the ultimate goal, and it is clear that overworked residents are at higher risk for making mistakes that jeopardize patient care, which nobody wants. However, the restrictions that duty hours place don’t allow the flexibility necessary for a specialty such as ours, and in fact strict adherence to such regulations inhibits opportunities for our learning. Also concerning is the “shift-work” mentality that seems to be increasingly pervasive with the implementation of duty hours. As has been well documented, and as I have seen personally, the constant patient handoffs that are requisite to implementation of duty hours pose their own perils in terms of patient safety.
Ultimately, these are our patients and we are responsible. Once we are attendings, those responsibilities will not be turned off after we have reached some prespecified hour limit.
The question then remains how best to implement a system across a wide variety of programs that ensures both patient safety and adequate clinical experience in the context of a culture of patient responsibility for the residents. As the NEJM study (N Engl J Med. 2016 374:713-2) shows, flexibility in implementation of duty hours did not result in increased complications, but resulted in improved resident satisfaction in continuity of care and handoffs. In my opinion, this study then encourages specialties such as ours to be more flexible in work hours, to encourage residents when there is a learning opportunity that previously they would be prohibited from taking part in to take hold of that opportunity, and to use this flexibility in implementation of duty hours to combat the invading “shift-work” mentality that will only jeopardize patient care.”
Dr. Elizabeth H. Stephens, MD, PhD is a Cardiothoracic Surgery, resident, PGY4, at Columbia University, New York, as an Integrated I-6 Resident.
Dr. Brown: “I took the traditional 5-year of General Surgery + 2 years of Cardiothoracic Surgery training route to becoming a General Thoracic Surgeon. My General Surgery experience was invaluable to my development as a surgeon. However, after all of those years of General Surgery cases and minimal exposure to Cardiothoracic Surgery cases, coupled with minimal overlap between the two specialties with regard to patient care, I found the learning curve in fellowship to be very steep. I was fortunate to train in a program with phenomenal physician extender support [APPs] in addition to top-notch colleagues in other specialties and excellent nursing, which allowed me to spend the majority of those 2 years in the operating room and completely focused on patient care. During that final phase of training, I welcomed flexibility with regard to the work-hour restrictions to ensure that I was acquiring the experience I needed prior to starting my own practice.”
Lisa M. Brown, MD, MAS, Assistant Professor of Thoracic Surgery, UC Davis Health System, Calif.; Training Institution: Washington University
Dr. Lee: “I started my surgical training in 2005, 2 years after the implementation of the 80-hour workweek restriction. Fortunately for my personal life, my training program took the restriction very seriously and strictly enforced it. As a result, I had scheduled periods off from work, and rarely worked more than 80 hours per week over the course of general surgery. On those occasions that I did, the next weeks, or preceding weeks would be shorter, to compensate. As a product of a 4+3 Thoracic Surgery residency in this environment, the 80-hour workweek extended to my subspecialty training. Our cardiac surgery time strictly enforced the go-home post-call policy. As a result, I believe my duty hours during Thoracic Surgery are likely shorter overall than many other programs.
Everyone hears the rumors of other programs lying about their duty hours. Fortunately, mine was not one of these. Despite this, or because of this, I received top-notch training. At the same time and more importantly, I started a family. I married my wife a week before my internship started, and am still married to the same person. We had two precious daughters during my Thoracic Surgery years. I don’t believe this would have been possible without duty-hours restrictions.
To create an environment where such a task was possible, my program hired an army of mid-level practitioners to deal with the day-to-day tasks of providing cardiothoracic surgical care to the patients, both in the intensive care unit and on the ward.
I rarely had to write a history and physical during Thoracic Surgery training. I consented fewer patients than I have fingers on my hands. I pulled even fewer chest tubes. I can now no longer remember having pulled a central line. I learned these tasks when I was a junior resident. What I did instead as a senior resident was perform over 900 cardiothoracic procedures as the primary surgeon. Now, as an attending surgeon, I still don’t write full histories and physicals by myself. I certainly don’t pull chest tubes and central lines. I consent patients by having a conversation with them, which I did as a resident, but I don’t bring a piece of paper with me in to the clinic room. I have a physician assistant who helps me fill in the gaps.
In 8 months of practice, I have performed over 15 thoracic organ transplants, repaired over 15 aortic dissections, half of whom required root replacements, performed more double-valve surgeries than straightforward single-valve replacements, started a minimally invasive atrial fibrillation surgical program, and applied almost every shred of knowledge and experience gained in 3 years of Thoracic Surgery Residency to a busy clinical practice. Most importantly, I continue to come home and watch my two daughters grow up.
With that perspective, I don’t believe the question should be whether or not programs should have the flexibility to enforce or not enforce duty-hours restrictions. It should be, how could every program find a way to effectively train residents to be good physicians and still allow them a personal life?”
Dr. Anson Lee is an Assistant Professor of Cardiac Surgery, Stanford University, Calif.; Training: 4+3 CT Residency, at Washington University, St. Louis.
Ms. Bohlman: “As a physician assistant in cardiac surgery, I represent the reality that physicians with a critical patient population appreciate consistency in their patient management. However, working in a university hospital setting also requires that surgical residents receive appropriate training. With the recently implemented duty restriction hours on resident training programs, advanced practice providers (APPs) have been utilized as an excellent solution for scheduling conflicts without compromising patient care. An example to this point is evident in my own place of work.
Approximately 1 year ago, our surgical intensive care unit transitioned from resident care to a combination of residents and APPs. At that time, the APPs were tasked with the complete care of all cardiac surgery patients. This change reduced the quantity and acuity of patients for which the residents were responsible and therefore allowed for more flexible hours along with a more manageable patient load. These changes, among others, have contributed to improved patient outcomes in the cardiac surgery patient population within our institution. With the increase in APPs that have training in various specialties, there comes an increasing ability to not only fill the gaps in scheduling but to do so with an extension of the providing physician. Although the NEJM article demonstrated no difference in patient outcomes between resident programs with restricted duty hours versus more flexible duty-hour policies, I foresee the future of medicine focusing on trained APPs as a complement to the care that the residents provide.”
Allison Bohlman is a Physician Assistant at Rush University Medical Center in the Integrated cardiovascular thoracic intensive care unit.
Several months ago, Dr. Bilimoria and his colleagues published the long awaited study in NEJM essentially contradicting adverse effects of strict duty hours on patient outcomes (N Engl J Med. 2016 374:713-2). The study, known as the FIRST trial, was published in the March issue of Thoracic Surgery News. Although the study enrolled general surgery residents, its conclusion impacts no specialty more than cardiothoracic surgery, where frequent handoffs complicate tedious perioperative care of sick patients stall learning opportunities for young trainees.
As Dr. Shari Meyerson eloquently noted in her perspective piece for Thoracic Surgery News (March 2016, page 4), surgery training needs to adapt to meet the modern day needs of trainees to rest and spend time with family and friends, with those of exposure to complex clinical scenarios in a short residency period. Arguably, CT surgery trainees are some of the most motivated and driven; to limit their experience on a national level may be shortsighted. On the other hand, appropriate incorporation of advanced practice providers (APPs) may help allay some patient care challenges, free valuable family time, and allow thoracic residents to function well in a more flexible ACGME duty-hour paradigm. To add thoracic relevance to the findings of Dr. Bilimoria and his colleagues, the debate is brought to Thoracic Surgery News by our colleagues from different training pathways below:
Dr. Antonoff: “Due to the timing of my medical school matriculation, I completed my surgical clerkships in the preregulated duty-hour era. I had expectations of what my life would be like as a surgeon when I applied for the general surgery match, and frankly, my expectations prepared me for a life that would revolve around my education, my technical training, and my commitment to patient care. During my years as a junior resident, my surgical training program gradually adapted in recognition of the new guidelines, but it took time. I spent 3 years in the research lab, and, after I came out as a senior resident, I discovered that the rules of the game had totally changed. While duty hours for me, as a senior trainee, were still fairly open, I found that my interns and junior residents had to play by completely new rules. In some ways, on rare occasions, I felt frustrated and resentful of the fact that my duties as a chief resident and thoracic fellow included many of the tasks that I’d done as an intern, because my interns would ‘expire’ after 16 hours. However, much more often, I felt bad for those who came after me.
They seemed desperate to operate, eager to see their patients’ problems through to resolution, and embarrassed to have to end their days earlier than the more senior members of the team. I feel fortunate that I had years of frequent in-house call after long days in the operating room and followed by post-call days of more operating. I finished my junior years without fear of any sick patient in the hospital, I finished my general surgery training without fear of any emergent operation, and I finished my fellowship with confidence that I could get a patient through just about anything if I had access to a cannula.
My early experiences as an attending have certainly kept me humble, and I’ve spent many a night worried about my patients, rethinking choices that I’ve made and stitches that I’ve thrown. But I thank my lucky stars that I was exposed to phenomenal training, and that I had the privilege and opportunity to work the hours needed to reach a reasonable level of safety! I can only imagine that if I’d have spent fewer nights in the hospital, that I’d feel even more anxiety and nausea at this early stage of my surgical career.
As elaborated in the editorial by Dr. Birkmeyer (N Engl J Med. 2016;374:783-4), it is not surprising that patient outcomes did not immediately depend on whether the programs had adhered strictly to the ACGME duty-hour rules. Limited numbers of patients experience critical events during shift change, and hospitals are evolving to function with greater reliance on midlevel practitioners and attending physicians. I would not expect the short-term results of duty-hour flexibility to demonstrate any impact on patient care. However, I do fear that there will be a mid-term impact on trainee accountability and autonomy, which will ultimately impact the competence of the attending surgeons of the future, and downstream potential long-term impact on day-to-day patient outcomes.
As a wife and mother of 3, I recognize that we, as a specialty, need to find ways to support our trainees and their families and to help them live happy lives conducive to functioning outside the hospital. I believe that we can do this with support, mentorship, and advocacy; I do not believe that it requires cutting back on the training that we are all, in the end, so incredibly grateful to receive.”
Dr. Mara Antonoff is an assistant professor of thoracic and cardiovascular surgery at UT MD Anderson Cancer Center. She performed her General Surgery training at the University of Minnesota, 2004-2012, and her Thoracic Surgery Training at Washington University, as a traditional 2-year resident, 2012-2014.
Dr. Stephens: “There is nothing that replaces being bedside. Whether it be a postoperative patient struggling with low cardiac output syndrome overnight, or a patient with a high pressor requirement the etiology of which you are trying to uncover, or a patient you have been following who suddenly arrests, the value of seeing a patient’s trajectory longitudinally is critical to developing clinical acumen. When as an attending I will get called in the middle of the night about a postoperative patient not “doing well,” I will be drawing on my years of being on call and being bedside with my patients.
Patient care is the ultimate goal, and it is clear that overworked residents are at higher risk for making mistakes that jeopardize patient care, which nobody wants. However, the restrictions that duty hours place don’t allow the flexibility necessary for a specialty such as ours, and in fact strict adherence to such regulations inhibits opportunities for our learning. Also concerning is the “shift-work” mentality that seems to be increasingly pervasive with the implementation of duty hours. As has been well documented, and as I have seen personally, the constant patient handoffs that are requisite to implementation of duty hours pose their own perils in terms of patient safety.
Ultimately, these are our patients and we are responsible. Once we are attendings, those responsibilities will not be turned off after we have reached some prespecified hour limit.
The question then remains how best to implement a system across a wide variety of programs that ensures both patient safety and adequate clinical experience in the context of a culture of patient responsibility for the residents. As the NEJM study (N Engl J Med. 2016 374:713-2) shows, flexibility in implementation of duty hours did not result in increased complications, but resulted in improved resident satisfaction in continuity of care and handoffs. In my opinion, this study then encourages specialties such as ours to be more flexible in work hours, to encourage residents when there is a learning opportunity that previously they would be prohibited from taking part in to take hold of that opportunity, and to use this flexibility in implementation of duty hours to combat the invading “shift-work” mentality that will only jeopardize patient care.”
Dr. Elizabeth H. Stephens, MD, PhD is a Cardiothoracic Surgery, resident, PGY4, at Columbia University, New York, as an Integrated I-6 Resident.
Dr. Brown: “I took the traditional 5-year of General Surgery + 2 years of Cardiothoracic Surgery training route to becoming a General Thoracic Surgeon. My General Surgery experience was invaluable to my development as a surgeon. However, after all of those years of General Surgery cases and minimal exposure to Cardiothoracic Surgery cases, coupled with minimal overlap between the two specialties with regard to patient care, I found the learning curve in fellowship to be very steep. I was fortunate to train in a program with phenomenal physician extender support [APPs] in addition to top-notch colleagues in other specialties and excellent nursing, which allowed me to spend the majority of those 2 years in the operating room and completely focused on patient care. During that final phase of training, I welcomed flexibility with regard to the work-hour restrictions to ensure that I was acquiring the experience I needed prior to starting my own practice.”
Lisa M. Brown, MD, MAS, Assistant Professor of Thoracic Surgery, UC Davis Health System, Calif.; Training Institution: Washington University
Dr. Lee: “I started my surgical training in 2005, 2 years after the implementation of the 80-hour workweek restriction. Fortunately for my personal life, my training program took the restriction very seriously and strictly enforced it. As a result, I had scheduled periods off from work, and rarely worked more than 80 hours per week over the course of general surgery. On those occasions that I did, the next weeks, or preceding weeks would be shorter, to compensate. As a product of a 4+3 Thoracic Surgery residency in this environment, the 80-hour workweek extended to my subspecialty training. Our cardiac surgery time strictly enforced the go-home post-call policy. As a result, I believe my duty hours during Thoracic Surgery are likely shorter overall than many other programs.
Everyone hears the rumors of other programs lying about their duty hours. Fortunately, mine was not one of these. Despite this, or because of this, I received top-notch training. At the same time and more importantly, I started a family. I married my wife a week before my internship started, and am still married to the same person. We had two precious daughters during my Thoracic Surgery years. I don’t believe this would have been possible without duty-hours restrictions.
To create an environment where such a task was possible, my program hired an army of mid-level practitioners to deal with the day-to-day tasks of providing cardiothoracic surgical care to the patients, both in the intensive care unit and on the ward.
I rarely had to write a history and physical during Thoracic Surgery training. I consented fewer patients than I have fingers on my hands. I pulled even fewer chest tubes. I can now no longer remember having pulled a central line. I learned these tasks when I was a junior resident. What I did instead as a senior resident was perform over 900 cardiothoracic procedures as the primary surgeon. Now, as an attending surgeon, I still don’t write full histories and physicals by myself. I certainly don’t pull chest tubes and central lines. I consent patients by having a conversation with them, which I did as a resident, but I don’t bring a piece of paper with me in to the clinic room. I have a physician assistant who helps me fill in the gaps.
In 8 months of practice, I have performed over 15 thoracic organ transplants, repaired over 15 aortic dissections, half of whom required root replacements, performed more double-valve surgeries than straightforward single-valve replacements, started a minimally invasive atrial fibrillation surgical program, and applied almost every shred of knowledge and experience gained in 3 years of Thoracic Surgery Residency to a busy clinical practice. Most importantly, I continue to come home and watch my two daughters grow up.
With that perspective, I don’t believe the question should be whether or not programs should have the flexibility to enforce or not enforce duty-hours restrictions. It should be, how could every program find a way to effectively train residents to be good physicians and still allow them a personal life?”
Dr. Anson Lee is an Assistant Professor of Cardiac Surgery, Stanford University, Calif.; Training: 4+3 CT Residency, at Washington University, St. Louis.
Ms. Bohlman: “As a physician assistant in cardiac surgery, I represent the reality that physicians with a critical patient population appreciate consistency in their patient management. However, working in a university hospital setting also requires that surgical residents receive appropriate training. With the recently implemented duty restriction hours on resident training programs, advanced practice providers (APPs) have been utilized as an excellent solution for scheduling conflicts without compromising patient care. An example to this point is evident in my own place of work.
Approximately 1 year ago, our surgical intensive care unit transitioned from resident care to a combination of residents and APPs. At that time, the APPs were tasked with the complete care of all cardiac surgery patients. This change reduced the quantity and acuity of patients for which the residents were responsible and therefore allowed for more flexible hours along with a more manageable patient load. These changes, among others, have contributed to improved patient outcomes in the cardiac surgery patient population within our institution. With the increase in APPs that have training in various specialties, there comes an increasing ability to not only fill the gaps in scheduling but to do so with an extension of the providing physician. Although the NEJM article demonstrated no difference in patient outcomes between resident programs with restricted duty hours versus more flexible duty-hour policies, I foresee the future of medicine focusing on trained APPs as a complement to the care that the residents provide.”
Allison Bohlman is a Physician Assistant at Rush University Medical Center in the Integrated cardiovascular thoracic intensive care unit.
Vitamin C
Vitamin C (ascorbic acid) is one of the four most important ingredients in skin care products.
• It is proven to increase collagen production when applied topically to skin.
• It inhibits tyrosinase to even skin tone and has a strong antioxidant activity.
• It is absorbed well orally, but not enough gets to the skin.
• It is best absorbed at a pH of 2.0.
• It is unstable when exposed to light and air. Instruct patients to discard 6 months after opening.
In addition, the proper formulation is patented and expensive. Stick with brands you trust. Use vitamin C on skin prior to procedures to speed healing. It will sting when used on inflamed skin because of the low pH.
In my opinion, all patients need to be on the proper skin care regimen for their skin type. This includes a daily sun protection factor (SPF), a cleanser, a retinoid, and an antioxidant. Ascorbic acid is one of my favorite antioxidants because it is the only one shown to increase the production of collagen by fibroblasts and inhibit tyrosinase while scavenging free radicals. Sure it is expensive – but that is because formulating and packaging it properly is expensive. Unfortunately, many subpar brands have entered the market. Ask to see the company’s research data on its formulation before choosing to recommend or sell ascorbic acid/vitamin C in your practice.
An essential water-soluble nutrient for the development of bone and connective tissue, vitamin C is found in citrus fruits and green leafy vegetables. It is produced in most plants and animals, but a mutated gene in humans has resulted in a deficiency of L-gulono-gamma-lactone oxidase, the enzyme required for its production.1,2 Although ascorbic acid cannot be synthesized by the human body, dietary consumption renders it the most abundant antioxidant in human skin and blood, and vitamin C plays an important role in endogenous collagen production and the inhibition of collagen degradation.3-6 Ascorbic acid also is known to regenerate alpha-tocopherol (vitamin E) levels and, therefore, is thought to protect against diseases related to oxidative stress.7
Epidermal vitamin C can be depleted by sunlight and environmental pollution, such as ozone in urban pollution.8,9 Known to exhibit a wide range of biologic activities, ascorbic acid has been shown to deliver rejuvenating effects on skin wrinkles, texture, strength, and evenness of tone through its antioxidant, tyrosinase-inhibiting, and collagen production-promoting activities.10 Indeed, as a topical agent, vitamin C has been used to prevent photodamage, and to treat melasma, striae alba, and postoperative erythema in laser patients.11,12 It is regularly used to treat aging skin, and as a depigmenting agent.2,10,13 This column will discuss the antioxidant, antiaging, and depigmenting activity of vitamin C in the context of recent human studies.
Antioxidant and anti-aging activity
Vitamin C is unique among antioxidants because of its ability to increase collagen production in addition to its free radical scavenging antioxidant activity. Due to its capacity to interfere with the UV-induced generation of reactive oxygen species by reacting with the superoxide anion or the hydroxyl radical, vitamin C has become a popular addition to “after-sun” products,14,15 and been shown to be effective in mitigating the effects of UVB, such as erythema and signs of photoaging, on porcine and human skin.2,16-17
A 2001 study in 10 postmenopausal women by Nusgens et al. found that daily topical application of 5% L-ascorbic acid enhanced the levels of procollagen types I and III, their posttranslational maturation enzymes, and tissue inhibitor of matrix metalloproteinase.18 This led to increased levels of collagen in the skin.
In 2003, Humbert et al. conducted a 6-month, double-blind, vehicle-controlled trial with 20 healthy female volunteers showing that patients treated with 5% vitamin C cream experienced significant improvements in deep furrows on the neck and forearms.19
In a small study of nine adults with Fitzpatrick skin types II or III in 2008, Murray et al. studied whether a stable topical preparation of 15% L-ascorbic acid, 1% alpha-tocopherol, and 0.5% ferulic acid could protect human skin in vivo from UV-induced damage. They found that the antioxidant formulation supplemented the antioxidant pool of the skin and conferred significant photoprotection, guarding the skin against erythema and apoptosis as well as effectively suppressing p53 activation and reducing thymine dimer mutations known to be associated with skin cancer.13
In 2012, Xu et al. evaluated the efficacy and safety of topical 23.8% L-ascorbic acid on photoaged skin in a split-face study of 20 Chinese women. Significant improvements in fine lines, dyspigmentation, and surface roughness were observed, without adverse side effects.20
In a 2015 study of 60 healthy female subjects, Crisan et al. used high-frequency ultrasound to determine that the use of a topical vitamin C formulation yielded significant increases in collagen synthesis, revealing the solution to be an effective rejuvenation therapy.21
Skin lightening activity
Melasma
In 2004, Espinal-Perez et al. conducted a double-blind randomized trial of 5% ascorbic acid, compared with 4% hydroquinone (HQ) water–oil emulsion in 16 female patients with melasma, aged 23-43 years (mean 36 years). Of those treated with vitamin C, 62.5% exhibited good or excellent subjectively assessed skin lightening. There was no statistically significant difference in depigmenting activity in the HQ group, of which 68.7% experienced irritation whereas vitamin C was well tolerated.22
In a randomized, double-blind, placebo-controlled study, researchers used iontophoresis to enhance the penetration of vitamin C into the skin and significantly reduce pigmentation, compared with placebo.23
Although ascorbic acid is viewed by many as ineffective as a depigmenting agent alone, particularly in 5%-10% concentrations, when used in combination with other ingredients such as HQ, it is considered effective.24 In the magnesium-L-ascorbyl-2-phosphate esterified form, however, vitamin C is among the most popular prescribed depigmenting agents around the world, especially in countries where HQ and its derivatives are prohibited.25 In a 2009 16-week open-label study by Hwang et al. of 25% L-ascorbic acid and a chemical penetration enhancer for treating melasma in 40 patients, researchers observed significant reductions in pigmentation.26
In a small split-face study early in 2015, Lee et al. showed that the combination of 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet (QS-Nd:YAG) laser and ultrasonic application of vitamin C was more effective than was the laser treatment alone in achieving a cosmetically acceptable treatment for melasma.27
PIPA
Vitamin C can be used to diminish or prevent post-inflammatory pigment alteration (PIPA) after procedures because it inhibits tyrosinase, lowers inflammation, and quenches free radicals. In a study of 10 patients, the application of topical vitamin C 2 or more weeks after surgery reduced the duration and degree of erythema after skin resurfacing with a carbon dioxide laser.28
Stretch marks
The depigmenting effects of vitamin C can lighten the pigmentation associated with stretch marks and its anti-inflammatory activity can contribute to blunting related redness.12
Conclusion
Although orally administered ascorbic acid is readily bioavailable, ascorbic acid in the skin is quickly depleted and oral supplementation alone does not yield optimal skin levels. Therefore, topical use of vitamin C is desirable. In fact, I tell my patients to use it topically in the morning and add a vitamin C supplement to their diet. Numerous formulation considerations (e.g., packaging, exposure to air or light during use, skin sensitivity, and user preference) are involved in the stabilization and effective penetration of ascorbic acid into the skin, and the process of developing, manufacturing, and packaging of effective, stable vitamin C products is expensive.
Vitamin C, particularly when combined with other ingredients, has been shown to be an integral constituent in topical antioxidant, antiaging, and depigmenting formulations that show promise in the dermatologic armamentarium. It is a great choice for use in a prep-procedure skin care regimen to speed healing. Use after a procedure is prohibited by the stinging associated with the low pH of properly formulated products.
References
1. J Biol Chem. 1994 May 6;269(18):13685-8.
2. Dermatol Surg. 2001 Feb;27(2):137-42.
3. J Invest Dermatol. 1994 Jan;102(1):122-4.
4. Dermatol Surg. 2005 Jul;31(7 Pt 2):814-7.
5. Annu Rev Nutr. 1994;14:371-91.
6. J Drugs Dermatol. 2008 Jul;7(7 Suppl):s2-6.
7. J Am Acad Dermatol. 2003 Jun;48(6):866-74.
8. J Invest Dermatol. 1994 Apr;102(4):470-5.
9. Free Radic Biol Med. 1997;23:85-91.
10. J Drugs Dermatol. 2014 Oct;13(10):1208-13.
11. J Am Acad Dermatol. 1996 Jan;34(1):29-33.
12. Dermatol Surg. 1998 Aug;24(8):849-56.
13. J Am Acad Dermatol. 2008 Sep;59(3):418-25.
14. J Biol Chem. 1983 Jun 10;258(11):6695-7.
15. J Phys Chem. 1983;87:1809-12.
16. Br J Dermatol. 1992 Sep;127(3):247-53.
17. J Invest Dermatol. 1991;96:587.
18. J Invest Dermatol. 2001 Jun;116(6):853-9.
19. Exp Dermatol. 2003 Jun;12(3):237-44.
20. J Drugs Dermatol. 2012 Jan;11(1):51-6.
21. Clin Cosmet Investig Dermatol. 2015 Sep 2;8:463-70
22. Int J Dermatol. 2004 Aug;43(8):604-7.
23. Dermatology. 2003;206(4):316-20.
24. Am J Clin Dermatol. 2011 Apr 1;12(2):87-99.
25. Phytother Res. 2006 Nov;20(11):921-34.
26. J Cutan Med Surg. 2009 Mar-Apr;13(2):74-81.
27. Lasers Med Sci. 2015 Jan;30(1):159-63.
28. Dermatol Surg. 1998 Mar;24(3):331-4.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Vitamin C (ascorbic acid) is one of the four most important ingredients in skin care products.
• It is proven to increase collagen production when applied topically to skin.
• It inhibits tyrosinase to even skin tone and has a strong antioxidant activity.
• It is absorbed well orally, but not enough gets to the skin.
• It is best absorbed at a pH of 2.0.
• It is unstable when exposed to light and air. Instruct patients to discard 6 months after opening.
In addition, the proper formulation is patented and expensive. Stick with brands you trust. Use vitamin C on skin prior to procedures to speed healing. It will sting when used on inflamed skin because of the low pH.
In my opinion, all patients need to be on the proper skin care regimen for their skin type. This includes a daily sun protection factor (SPF), a cleanser, a retinoid, and an antioxidant. Ascorbic acid is one of my favorite antioxidants because it is the only one shown to increase the production of collagen by fibroblasts and inhibit tyrosinase while scavenging free radicals. Sure it is expensive – but that is because formulating and packaging it properly is expensive. Unfortunately, many subpar brands have entered the market. Ask to see the company’s research data on its formulation before choosing to recommend or sell ascorbic acid/vitamin C in your practice.
An essential water-soluble nutrient for the development of bone and connective tissue, vitamin C is found in citrus fruits and green leafy vegetables. It is produced in most plants and animals, but a mutated gene in humans has resulted in a deficiency of L-gulono-gamma-lactone oxidase, the enzyme required for its production.1,2 Although ascorbic acid cannot be synthesized by the human body, dietary consumption renders it the most abundant antioxidant in human skin and blood, and vitamin C plays an important role in endogenous collagen production and the inhibition of collagen degradation.3-6 Ascorbic acid also is known to regenerate alpha-tocopherol (vitamin E) levels and, therefore, is thought to protect against diseases related to oxidative stress.7
Epidermal vitamin C can be depleted by sunlight and environmental pollution, such as ozone in urban pollution.8,9 Known to exhibit a wide range of biologic activities, ascorbic acid has been shown to deliver rejuvenating effects on skin wrinkles, texture, strength, and evenness of tone through its antioxidant, tyrosinase-inhibiting, and collagen production-promoting activities.10 Indeed, as a topical agent, vitamin C has been used to prevent photodamage, and to treat melasma, striae alba, and postoperative erythema in laser patients.11,12 It is regularly used to treat aging skin, and as a depigmenting agent.2,10,13 This column will discuss the antioxidant, antiaging, and depigmenting activity of vitamin C in the context of recent human studies.
Antioxidant and anti-aging activity
Vitamin C is unique among antioxidants because of its ability to increase collagen production in addition to its free radical scavenging antioxidant activity. Due to its capacity to interfere with the UV-induced generation of reactive oxygen species by reacting with the superoxide anion or the hydroxyl radical, vitamin C has become a popular addition to “after-sun” products,14,15 and been shown to be effective in mitigating the effects of UVB, such as erythema and signs of photoaging, on porcine and human skin.2,16-17
A 2001 study in 10 postmenopausal women by Nusgens et al. found that daily topical application of 5% L-ascorbic acid enhanced the levels of procollagen types I and III, their posttranslational maturation enzymes, and tissue inhibitor of matrix metalloproteinase.18 This led to increased levels of collagen in the skin.
In 2003, Humbert et al. conducted a 6-month, double-blind, vehicle-controlled trial with 20 healthy female volunteers showing that patients treated with 5% vitamin C cream experienced significant improvements in deep furrows on the neck and forearms.19
In a small study of nine adults with Fitzpatrick skin types II or III in 2008, Murray et al. studied whether a stable topical preparation of 15% L-ascorbic acid, 1% alpha-tocopherol, and 0.5% ferulic acid could protect human skin in vivo from UV-induced damage. They found that the antioxidant formulation supplemented the antioxidant pool of the skin and conferred significant photoprotection, guarding the skin against erythema and apoptosis as well as effectively suppressing p53 activation and reducing thymine dimer mutations known to be associated with skin cancer.13
In 2012, Xu et al. evaluated the efficacy and safety of topical 23.8% L-ascorbic acid on photoaged skin in a split-face study of 20 Chinese women. Significant improvements in fine lines, dyspigmentation, and surface roughness were observed, without adverse side effects.20
In a 2015 study of 60 healthy female subjects, Crisan et al. used high-frequency ultrasound to determine that the use of a topical vitamin C formulation yielded significant increases in collagen synthesis, revealing the solution to be an effective rejuvenation therapy.21
Skin lightening activity
Melasma
In 2004, Espinal-Perez et al. conducted a double-blind randomized trial of 5% ascorbic acid, compared with 4% hydroquinone (HQ) water–oil emulsion in 16 female patients with melasma, aged 23-43 years (mean 36 years). Of those treated with vitamin C, 62.5% exhibited good or excellent subjectively assessed skin lightening. There was no statistically significant difference in depigmenting activity in the HQ group, of which 68.7% experienced irritation whereas vitamin C was well tolerated.22
In a randomized, double-blind, placebo-controlled study, researchers used iontophoresis to enhance the penetration of vitamin C into the skin and significantly reduce pigmentation, compared with placebo.23
Although ascorbic acid is viewed by many as ineffective as a depigmenting agent alone, particularly in 5%-10% concentrations, when used in combination with other ingredients such as HQ, it is considered effective.24 In the magnesium-L-ascorbyl-2-phosphate esterified form, however, vitamin C is among the most popular prescribed depigmenting agents around the world, especially in countries where HQ and its derivatives are prohibited.25 In a 2009 16-week open-label study by Hwang et al. of 25% L-ascorbic acid and a chemical penetration enhancer for treating melasma in 40 patients, researchers observed significant reductions in pigmentation.26
In a small split-face study early in 2015, Lee et al. showed that the combination of 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet (QS-Nd:YAG) laser and ultrasonic application of vitamin C was more effective than was the laser treatment alone in achieving a cosmetically acceptable treatment for melasma.27
PIPA
Vitamin C can be used to diminish or prevent post-inflammatory pigment alteration (PIPA) after procedures because it inhibits tyrosinase, lowers inflammation, and quenches free radicals. In a study of 10 patients, the application of topical vitamin C 2 or more weeks after surgery reduced the duration and degree of erythema after skin resurfacing with a carbon dioxide laser.28
Stretch marks
The depigmenting effects of vitamin C can lighten the pigmentation associated with stretch marks and its anti-inflammatory activity can contribute to blunting related redness.12
Conclusion
Although orally administered ascorbic acid is readily bioavailable, ascorbic acid in the skin is quickly depleted and oral supplementation alone does not yield optimal skin levels. Therefore, topical use of vitamin C is desirable. In fact, I tell my patients to use it topically in the morning and add a vitamin C supplement to their diet. Numerous formulation considerations (e.g., packaging, exposure to air or light during use, skin sensitivity, and user preference) are involved in the stabilization and effective penetration of ascorbic acid into the skin, and the process of developing, manufacturing, and packaging of effective, stable vitamin C products is expensive.
Vitamin C, particularly when combined with other ingredients, has been shown to be an integral constituent in topical antioxidant, antiaging, and depigmenting formulations that show promise in the dermatologic armamentarium. It is a great choice for use in a prep-procedure skin care regimen to speed healing. Use after a procedure is prohibited by the stinging associated with the low pH of properly formulated products.
References
1. J Biol Chem. 1994 May 6;269(18):13685-8.
2. Dermatol Surg. 2001 Feb;27(2):137-42.
3. J Invest Dermatol. 1994 Jan;102(1):122-4.
4. Dermatol Surg. 2005 Jul;31(7 Pt 2):814-7.
5. Annu Rev Nutr. 1994;14:371-91.
6. J Drugs Dermatol. 2008 Jul;7(7 Suppl):s2-6.
7. J Am Acad Dermatol. 2003 Jun;48(6):866-74.
8. J Invest Dermatol. 1994 Apr;102(4):470-5.
9. Free Radic Biol Med. 1997;23:85-91.
10. J Drugs Dermatol. 2014 Oct;13(10):1208-13.
11. J Am Acad Dermatol. 1996 Jan;34(1):29-33.
12. Dermatol Surg. 1998 Aug;24(8):849-56.
13. J Am Acad Dermatol. 2008 Sep;59(3):418-25.
14. J Biol Chem. 1983 Jun 10;258(11):6695-7.
15. J Phys Chem. 1983;87:1809-12.
16. Br J Dermatol. 1992 Sep;127(3):247-53.
17. J Invest Dermatol. 1991;96:587.
18. J Invest Dermatol. 2001 Jun;116(6):853-9.
19. Exp Dermatol. 2003 Jun;12(3):237-44.
20. J Drugs Dermatol. 2012 Jan;11(1):51-6.
21. Clin Cosmet Investig Dermatol. 2015 Sep 2;8:463-70
22. Int J Dermatol. 2004 Aug;43(8):604-7.
23. Dermatology. 2003;206(4):316-20.
24. Am J Clin Dermatol. 2011 Apr 1;12(2):87-99.
25. Phytother Res. 2006 Nov;20(11):921-34.
26. J Cutan Med Surg. 2009 Mar-Apr;13(2):74-81.
27. Lasers Med Sci. 2015 Jan;30(1):159-63.
28. Dermatol Surg. 1998 Mar;24(3):331-4.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Vitamin C (ascorbic acid) is one of the four most important ingredients in skin care products.
• It is proven to increase collagen production when applied topically to skin.
• It inhibits tyrosinase to even skin tone and has a strong antioxidant activity.
• It is absorbed well orally, but not enough gets to the skin.
• It is best absorbed at a pH of 2.0.
• It is unstable when exposed to light and air. Instruct patients to discard 6 months after opening.
In addition, the proper formulation is patented and expensive. Stick with brands you trust. Use vitamin C on skin prior to procedures to speed healing. It will sting when used on inflamed skin because of the low pH.
In my opinion, all patients need to be on the proper skin care regimen for their skin type. This includes a daily sun protection factor (SPF), a cleanser, a retinoid, and an antioxidant. Ascorbic acid is one of my favorite antioxidants because it is the only one shown to increase the production of collagen by fibroblasts and inhibit tyrosinase while scavenging free radicals. Sure it is expensive – but that is because formulating and packaging it properly is expensive. Unfortunately, many subpar brands have entered the market. Ask to see the company’s research data on its formulation before choosing to recommend or sell ascorbic acid/vitamin C in your practice.
An essential water-soluble nutrient for the development of bone and connective tissue, vitamin C is found in citrus fruits and green leafy vegetables. It is produced in most plants and animals, but a mutated gene in humans has resulted in a deficiency of L-gulono-gamma-lactone oxidase, the enzyme required for its production.1,2 Although ascorbic acid cannot be synthesized by the human body, dietary consumption renders it the most abundant antioxidant in human skin and blood, and vitamin C plays an important role in endogenous collagen production and the inhibition of collagen degradation.3-6 Ascorbic acid also is known to regenerate alpha-tocopherol (vitamin E) levels and, therefore, is thought to protect against diseases related to oxidative stress.7
Epidermal vitamin C can be depleted by sunlight and environmental pollution, such as ozone in urban pollution.8,9 Known to exhibit a wide range of biologic activities, ascorbic acid has been shown to deliver rejuvenating effects on skin wrinkles, texture, strength, and evenness of tone through its antioxidant, tyrosinase-inhibiting, and collagen production-promoting activities.10 Indeed, as a topical agent, vitamin C has been used to prevent photodamage, and to treat melasma, striae alba, and postoperative erythema in laser patients.11,12 It is regularly used to treat aging skin, and as a depigmenting agent.2,10,13 This column will discuss the antioxidant, antiaging, and depigmenting activity of vitamin C in the context of recent human studies.
Antioxidant and anti-aging activity
Vitamin C is unique among antioxidants because of its ability to increase collagen production in addition to its free radical scavenging antioxidant activity. Due to its capacity to interfere with the UV-induced generation of reactive oxygen species by reacting with the superoxide anion or the hydroxyl radical, vitamin C has become a popular addition to “after-sun” products,14,15 and been shown to be effective in mitigating the effects of UVB, such as erythema and signs of photoaging, on porcine and human skin.2,16-17
A 2001 study in 10 postmenopausal women by Nusgens et al. found that daily topical application of 5% L-ascorbic acid enhanced the levels of procollagen types I and III, their posttranslational maturation enzymes, and tissue inhibitor of matrix metalloproteinase.18 This led to increased levels of collagen in the skin.
In 2003, Humbert et al. conducted a 6-month, double-blind, vehicle-controlled trial with 20 healthy female volunteers showing that patients treated with 5% vitamin C cream experienced significant improvements in deep furrows on the neck and forearms.19
In a small study of nine adults with Fitzpatrick skin types II or III in 2008, Murray et al. studied whether a stable topical preparation of 15% L-ascorbic acid, 1% alpha-tocopherol, and 0.5% ferulic acid could protect human skin in vivo from UV-induced damage. They found that the antioxidant formulation supplemented the antioxidant pool of the skin and conferred significant photoprotection, guarding the skin against erythema and apoptosis as well as effectively suppressing p53 activation and reducing thymine dimer mutations known to be associated with skin cancer.13
In 2012, Xu et al. evaluated the efficacy and safety of topical 23.8% L-ascorbic acid on photoaged skin in a split-face study of 20 Chinese women. Significant improvements in fine lines, dyspigmentation, and surface roughness were observed, without adverse side effects.20
In a 2015 study of 60 healthy female subjects, Crisan et al. used high-frequency ultrasound to determine that the use of a topical vitamin C formulation yielded significant increases in collagen synthesis, revealing the solution to be an effective rejuvenation therapy.21
Skin lightening activity
Melasma
In 2004, Espinal-Perez et al. conducted a double-blind randomized trial of 5% ascorbic acid, compared with 4% hydroquinone (HQ) water–oil emulsion in 16 female patients with melasma, aged 23-43 years (mean 36 years). Of those treated with vitamin C, 62.5% exhibited good or excellent subjectively assessed skin lightening. There was no statistically significant difference in depigmenting activity in the HQ group, of which 68.7% experienced irritation whereas vitamin C was well tolerated.22
In a randomized, double-blind, placebo-controlled study, researchers used iontophoresis to enhance the penetration of vitamin C into the skin and significantly reduce pigmentation, compared with placebo.23
Although ascorbic acid is viewed by many as ineffective as a depigmenting agent alone, particularly in 5%-10% concentrations, when used in combination with other ingredients such as HQ, it is considered effective.24 In the magnesium-L-ascorbyl-2-phosphate esterified form, however, vitamin C is among the most popular prescribed depigmenting agents around the world, especially in countries where HQ and its derivatives are prohibited.25 In a 2009 16-week open-label study by Hwang et al. of 25% L-ascorbic acid and a chemical penetration enhancer for treating melasma in 40 patients, researchers observed significant reductions in pigmentation.26
In a small split-face study early in 2015, Lee et al. showed that the combination of 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet (QS-Nd:YAG) laser and ultrasonic application of vitamin C was more effective than was the laser treatment alone in achieving a cosmetically acceptable treatment for melasma.27
PIPA
Vitamin C can be used to diminish or prevent post-inflammatory pigment alteration (PIPA) after procedures because it inhibits tyrosinase, lowers inflammation, and quenches free radicals. In a study of 10 patients, the application of topical vitamin C 2 or more weeks after surgery reduced the duration and degree of erythema after skin resurfacing with a carbon dioxide laser.28
Stretch marks
The depigmenting effects of vitamin C can lighten the pigmentation associated with stretch marks and its anti-inflammatory activity can contribute to blunting related redness.12
Conclusion
Although orally administered ascorbic acid is readily bioavailable, ascorbic acid in the skin is quickly depleted and oral supplementation alone does not yield optimal skin levels. Therefore, topical use of vitamin C is desirable. In fact, I tell my patients to use it topically in the morning and add a vitamin C supplement to their diet. Numerous formulation considerations (e.g., packaging, exposure to air or light during use, skin sensitivity, and user preference) are involved in the stabilization and effective penetration of ascorbic acid into the skin, and the process of developing, manufacturing, and packaging of effective, stable vitamin C products is expensive.
Vitamin C, particularly when combined with other ingredients, has been shown to be an integral constituent in topical antioxidant, antiaging, and depigmenting formulations that show promise in the dermatologic armamentarium. It is a great choice for use in a prep-procedure skin care regimen to speed healing. Use after a procedure is prohibited by the stinging associated with the low pH of properly formulated products.
References
1. J Biol Chem. 1994 May 6;269(18):13685-8.
2. Dermatol Surg. 2001 Feb;27(2):137-42.
3. J Invest Dermatol. 1994 Jan;102(1):122-4.
4. Dermatol Surg. 2005 Jul;31(7 Pt 2):814-7.
5. Annu Rev Nutr. 1994;14:371-91.
6. J Drugs Dermatol. 2008 Jul;7(7 Suppl):s2-6.
7. J Am Acad Dermatol. 2003 Jun;48(6):866-74.
8. J Invest Dermatol. 1994 Apr;102(4):470-5.
9. Free Radic Biol Med. 1997;23:85-91.
10. J Drugs Dermatol. 2014 Oct;13(10):1208-13.
11. J Am Acad Dermatol. 1996 Jan;34(1):29-33.
12. Dermatol Surg. 1998 Aug;24(8):849-56.
13. J Am Acad Dermatol. 2008 Sep;59(3):418-25.
14. J Biol Chem. 1983 Jun 10;258(11):6695-7.
15. J Phys Chem. 1983;87:1809-12.
16. Br J Dermatol. 1992 Sep;127(3):247-53.
17. J Invest Dermatol. 1991;96:587.
18. J Invest Dermatol. 2001 Jun;116(6):853-9.
19. Exp Dermatol. 2003 Jun;12(3):237-44.
20. J Drugs Dermatol. 2012 Jan;11(1):51-6.
21. Clin Cosmet Investig Dermatol. 2015 Sep 2;8:463-70
22. Int J Dermatol. 2004 Aug;43(8):604-7.
23. Dermatology. 2003;206(4):316-20.
24. Am J Clin Dermatol. 2011 Apr 1;12(2):87-99.
25. Phytother Res. 2006 Nov;20(11):921-34.
26. J Cutan Med Surg. 2009 Mar-Apr;13(2):74-81.
27. Lasers Med Sci. 2015 Jan;30(1):159-63.
28. Dermatol Surg. 1998 Mar;24(3):331-4.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.