User login
Celebrate Match Day, Future of Hospital Medicine Program
This year’s Match Day takes place on Friday, March 18.
Do you remember when you opened your letter? Do you know someone who is matching this year? Share your stories with SHM on Twitter @SHMLive and use the official Match Day 2016 hashtag, #Match2016, and #FutureofHospitalMedicine, plus encourage your students to do so as well. Follow along with the excitement and join in the conversation throughout the day.
It’s hard to believe, but Match Day 2017 is closer than you think. Fourth-year medical students can visit www.futureofhospitalmedicine.org for all of the resources needed to be successful, including:
- Residency match application checklist
- Application tool kit
- An overview of matching for fellowship applicants
- National Residency Matching Program FAQs
- Information on how to register to match
This year’s Match Day takes place on Friday, March 18.
Do you remember when you opened your letter? Do you know someone who is matching this year? Share your stories with SHM on Twitter @SHMLive and use the official Match Day 2016 hashtag, #Match2016, and #FutureofHospitalMedicine, plus encourage your students to do so as well. Follow along with the excitement and join in the conversation throughout the day.
It’s hard to believe, but Match Day 2017 is closer than you think. Fourth-year medical students can visit www.futureofhospitalmedicine.org for all of the resources needed to be successful, including:
- Residency match application checklist
- Application tool kit
- An overview of matching for fellowship applicants
- National Residency Matching Program FAQs
- Information on how to register to match
This year’s Match Day takes place on Friday, March 18.
Do you remember when you opened your letter? Do you know someone who is matching this year? Share your stories with SHM on Twitter @SHMLive and use the official Match Day 2016 hashtag, #Match2016, and #FutureofHospitalMedicine, plus encourage your students to do so as well. Follow along with the excitement and join in the conversation throughout the day.
It’s hard to believe, but Match Day 2017 is closer than you think. Fourth-year medical students can visit www.futureofhospitalmedicine.org for all of the resources needed to be successful, including:
- Residency match application checklist
- Application tool kit
- An overview of matching for fellowship applicants
- National Residency Matching Program FAQs
- Information on how to register to match
Q&A: Identifying gaps in the treatment of endometriosis
Despite the high prevalence of endometriosis – a condition that is estimated to affect about 10% of women of reproductive age – available treatments are inadequate for some women with chronic pain, and diagnosis is often delayed.
Stacey A. Missmer, Sc.D., the scientific director of the Boston Center for Endometriosis, is looking to advance understanding of the condition through the Women’s Health Study: From Adolescence to Adulthood. In an interview, Dr. Missmer, who is also the director of epidemiologic research in reproductive medicine at Brigham and Women’s Hospital in Boston, shared the progress of the study and her assessment of the treatment landscape.
Question: How would you rate the currently available treatments – both in terms of medications and surgery – in relieving the symptoms of endometriosis?
Dr. Missmer: The currently available treatments work well for some women and girls with pelvic pain, but it gets more difficult once that pain becomes chronic. If women have geographic and financial access to assisted reproductive treatment, then this works well for most women with endometriosis who also have subfertility. The great dilemma is that for pelvic pain, new nonhormonal/nonsurgical treatments have been slow to come for those who do not find relief with current treatments. These girls and young women are impacted just when they most need to be afforded a high quality of life that allows them to pursue all of their goals and dreams unimpeded by the symptoms associated with endometriosis.
Question: How does the Women’s Health Study: From Adolescence to Adulthood help advance treatment options?
Dr. Missmer: I am very proud of the Women’s Health Study: From Adolescence to Adulthood (A2A). Most studies of endometriosis have focused on women in their 30s and 40s, particularly those who present with infertility as their main health concern. However, we know that for women with endometriosis, the majority experience pain as their presenting symptom, and most report that endometriotic pain began during their teens and 20s. Uniquely, the A2A is a cohort of girl as young as 7 and young women, as well as those up to age 50. It is also uniquely a longitudinal cohort. By enrolling these girls and women now and following them into the future, we will be able to identify what characteristics in adolescence and young adulthood predicted who responds well to current treatments both in the short term and long into their reproductive and productive years.
We’ll also be able to discover the characteristics of those who did not respond as well, and we expect that this will drive us toward identifying unique groups of patients – and consequent biologic markers and patterns that are the basis for developing new treatments and prognostic tools for endometriosis.
Since November 2012, we have enrolled nearly 1,000 participants. Our initial enrollees are now completing their third year of lifestyle and symptom questionnaires, clinical details, and providing biologic samples. Their contributions are invaluable and are designed to be consistent with the harmonized data collection tools that we developed in collaboration with our national and international colleagues through the World Endometriosis Research Foundation (WERF). These Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) tools are publicly available and will allow us to combine data from our cohort with participants at other sites to begin to detect geographic and patient-specific treatment response differences across the globe.
Question: What other studies are you closely watching?
Dr. Missmer: I am thrilled about the opportunities that the WERF EPHect tools offer us and our respected colleagues across the globe. I’m quite excited to see what new collaborations are formed and what creative hypotheses are tested. We’re in the midst of a renaissance in endometriosis understanding. Despite its prevalence and potentially debilitating impact on girls and women, endometriosis research is dramatically underfunded. This impacts discovery itself, but it also diminishes the ability for young enthusiastic brilliant scientists to be drawn to and remain dedicated to our field.
Question: How far are we from a noninvasive test that physicians could use to diagnose endometriosis and monitor its progression?
Dr. Missmer: How far in terms of a time line is difficult to determine. Again, I stress that I believe wholeheartedly that we are in the midst of a renaissance for endometriosis. We now have the tools to advance multidisciplinary scientific discovery with the ability to conduct studies with large numbers of diverse girls and women and to rigorously detect changes in their symptoms across the life course. I have no doubt that this will lead to advancement of precision medicine that allows endometriosis-focused scientists to develop noninvasive diagnostics and novel treatments. The key is that everyone in the field of endometriosis work together with this goal in mind.
On Twitter @maryellenny
Despite the high prevalence of endometriosis – a condition that is estimated to affect about 10% of women of reproductive age – available treatments are inadequate for some women with chronic pain, and diagnosis is often delayed.
Stacey A. Missmer, Sc.D., the scientific director of the Boston Center for Endometriosis, is looking to advance understanding of the condition through the Women’s Health Study: From Adolescence to Adulthood. In an interview, Dr. Missmer, who is also the director of epidemiologic research in reproductive medicine at Brigham and Women’s Hospital in Boston, shared the progress of the study and her assessment of the treatment landscape.
Question: How would you rate the currently available treatments – both in terms of medications and surgery – in relieving the symptoms of endometriosis?
Dr. Missmer: The currently available treatments work well for some women and girls with pelvic pain, but it gets more difficult once that pain becomes chronic. If women have geographic and financial access to assisted reproductive treatment, then this works well for most women with endometriosis who also have subfertility. The great dilemma is that for pelvic pain, new nonhormonal/nonsurgical treatments have been slow to come for those who do not find relief with current treatments. These girls and young women are impacted just when they most need to be afforded a high quality of life that allows them to pursue all of their goals and dreams unimpeded by the symptoms associated with endometriosis.
Question: How does the Women’s Health Study: From Adolescence to Adulthood help advance treatment options?
Dr. Missmer: I am very proud of the Women’s Health Study: From Adolescence to Adulthood (A2A). Most studies of endometriosis have focused on women in their 30s and 40s, particularly those who present with infertility as their main health concern. However, we know that for women with endometriosis, the majority experience pain as their presenting symptom, and most report that endometriotic pain began during their teens and 20s. Uniquely, the A2A is a cohort of girl as young as 7 and young women, as well as those up to age 50. It is also uniquely a longitudinal cohort. By enrolling these girls and women now and following them into the future, we will be able to identify what characteristics in adolescence and young adulthood predicted who responds well to current treatments both in the short term and long into their reproductive and productive years.
We’ll also be able to discover the characteristics of those who did not respond as well, and we expect that this will drive us toward identifying unique groups of patients – and consequent biologic markers and patterns that are the basis for developing new treatments and prognostic tools for endometriosis.
Since November 2012, we have enrolled nearly 1,000 participants. Our initial enrollees are now completing their third year of lifestyle and symptom questionnaires, clinical details, and providing biologic samples. Their contributions are invaluable and are designed to be consistent with the harmonized data collection tools that we developed in collaboration with our national and international colleagues through the World Endometriosis Research Foundation (WERF). These Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) tools are publicly available and will allow us to combine data from our cohort with participants at other sites to begin to detect geographic and patient-specific treatment response differences across the globe.
Question: What other studies are you closely watching?
Dr. Missmer: I am thrilled about the opportunities that the WERF EPHect tools offer us and our respected colleagues across the globe. I’m quite excited to see what new collaborations are formed and what creative hypotheses are tested. We’re in the midst of a renaissance in endometriosis understanding. Despite its prevalence and potentially debilitating impact on girls and women, endometriosis research is dramatically underfunded. This impacts discovery itself, but it also diminishes the ability for young enthusiastic brilliant scientists to be drawn to and remain dedicated to our field.
Question: How far are we from a noninvasive test that physicians could use to diagnose endometriosis and monitor its progression?
Dr. Missmer: How far in terms of a time line is difficult to determine. Again, I stress that I believe wholeheartedly that we are in the midst of a renaissance for endometriosis. We now have the tools to advance multidisciplinary scientific discovery with the ability to conduct studies with large numbers of diverse girls and women and to rigorously detect changes in their symptoms across the life course. I have no doubt that this will lead to advancement of precision medicine that allows endometriosis-focused scientists to develop noninvasive diagnostics and novel treatments. The key is that everyone in the field of endometriosis work together with this goal in mind.
On Twitter @maryellenny
Despite the high prevalence of endometriosis – a condition that is estimated to affect about 10% of women of reproductive age – available treatments are inadequate for some women with chronic pain, and diagnosis is often delayed.
Stacey A. Missmer, Sc.D., the scientific director of the Boston Center for Endometriosis, is looking to advance understanding of the condition through the Women’s Health Study: From Adolescence to Adulthood. In an interview, Dr. Missmer, who is also the director of epidemiologic research in reproductive medicine at Brigham and Women’s Hospital in Boston, shared the progress of the study and her assessment of the treatment landscape.
Question: How would you rate the currently available treatments – both in terms of medications and surgery – in relieving the symptoms of endometriosis?
Dr. Missmer: The currently available treatments work well for some women and girls with pelvic pain, but it gets more difficult once that pain becomes chronic. If women have geographic and financial access to assisted reproductive treatment, then this works well for most women with endometriosis who also have subfertility. The great dilemma is that for pelvic pain, new nonhormonal/nonsurgical treatments have been slow to come for those who do not find relief with current treatments. These girls and young women are impacted just when they most need to be afforded a high quality of life that allows them to pursue all of their goals and dreams unimpeded by the symptoms associated with endometriosis.
Question: How does the Women’s Health Study: From Adolescence to Adulthood help advance treatment options?
Dr. Missmer: I am very proud of the Women’s Health Study: From Adolescence to Adulthood (A2A). Most studies of endometriosis have focused on women in their 30s and 40s, particularly those who present with infertility as their main health concern. However, we know that for women with endometriosis, the majority experience pain as their presenting symptom, and most report that endometriotic pain began during their teens and 20s. Uniquely, the A2A is a cohort of girl as young as 7 and young women, as well as those up to age 50. It is also uniquely a longitudinal cohort. By enrolling these girls and women now and following them into the future, we will be able to identify what characteristics in adolescence and young adulthood predicted who responds well to current treatments both in the short term and long into their reproductive and productive years.
We’ll also be able to discover the characteristics of those who did not respond as well, and we expect that this will drive us toward identifying unique groups of patients – and consequent biologic markers and patterns that are the basis for developing new treatments and prognostic tools for endometriosis.
Since November 2012, we have enrolled nearly 1,000 participants. Our initial enrollees are now completing their third year of lifestyle and symptom questionnaires, clinical details, and providing biologic samples. Their contributions are invaluable and are designed to be consistent with the harmonized data collection tools that we developed in collaboration with our national and international colleagues through the World Endometriosis Research Foundation (WERF). These Endometriosis Phenome and Biobanking Harmonisation Project (EPHect) tools are publicly available and will allow us to combine data from our cohort with participants at other sites to begin to detect geographic and patient-specific treatment response differences across the globe.
Question: What other studies are you closely watching?
Dr. Missmer: I am thrilled about the opportunities that the WERF EPHect tools offer us and our respected colleagues across the globe. I’m quite excited to see what new collaborations are formed and what creative hypotheses are tested. We’re in the midst of a renaissance in endometriosis understanding. Despite its prevalence and potentially debilitating impact on girls and women, endometriosis research is dramatically underfunded. This impacts discovery itself, but it also diminishes the ability for young enthusiastic brilliant scientists to be drawn to and remain dedicated to our field.
Question: How far are we from a noninvasive test that physicians could use to diagnose endometriosis and monitor its progression?
Dr. Missmer: How far in terms of a time line is difficult to determine. Again, I stress that I believe wholeheartedly that we are in the midst of a renaissance for endometriosis. We now have the tools to advance multidisciplinary scientific discovery with the ability to conduct studies with large numbers of diverse girls and women and to rigorously detect changes in their symptoms across the life course. I have no doubt that this will lead to advancement of precision medicine that allows endometriosis-focused scientists to develop noninvasive diagnostics and novel treatments. The key is that everyone in the field of endometriosis work together with this goal in mind.
On Twitter @maryellenny
Itraconazole targets basal cell carcinoma
WAIKOLOA, HAWAII – The familiar oral triazole antifungal agent itraconazole (Sporanox) is under active investigation for an unexpected use: as adjunctive therapy in patients with locally advanced or metastatic basal cell carcinoma.
“The promise of this drug is that the use of itraconazole with vismodegib or sonidegib may actually enhance the effectiveness of those drugs and also reduce the frequency of grade 2 toxicities by perhaps allowing a lower dose of vismodegib or sonidegib,” Dr. David L. Swanson said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Be looking for this drug that we use to treat toenail fungus as a potential drug for locally advanced or metastatic basal cell carcinoma,” advised Dr. Swanson, a dermatologist at the Mayo Clinic in Scottsdale, Ariz.
Vismodegib (Erivedge) has been a game changer for patients with inoperable locally advanced or metastatic breast cancer. “The response to this drug was amazing,” Dr. Swanson said of the landmark study which led to its approval in 2012 (N Engl J Med. 2012; 366[23]:2171-9).
Sonidegib (Odomzo), which like vismodegib inhibits the essential Hedgehog signaling pathway component known as Smoothened, was approved by the Food and Drug Administration in 2015 as the second oral drug in this novel class.
While the clinical benefits of these two drugs in patients with the most horrific basal cell carcinomas are extremely impressive, vismodegib and sonidegib have two major drawbacks: The tumors eventually develop resistance and commence growing again, and onerous grade 2 side effects requiring dose reduction are extremely common. The most frequent of these limiting side effects are a disturbed sense of taste, muscle spasms, alopecia, and weight loss.
The hope is that itraconazole may be of help with both issues, according to Dr. Swanson. It turns out that the antifungal agent is also an inhibitor of the Hedgehog pathway, and via a different mechanism than that of vismodegib and sonidegib.
He pointed to an international open-label exploratory phase II study led by Dr. Jean Y. Tang of Stanford (Calif.) University. The investigators treated 19 patients with a total of 90 basal cell carcinomas with oral itraconazole at 200 mg twice a day for 1 month or 100 mg twice a day for an average of 2.3 months.
The treatment reduced Hedgehog signaling pathway activity by 65%, Ki67 tumor cell proliferation by 45%, and tumor area by 24% (J Clin Oncol. 2014 Mar 10;32[8]:745-51).
These results aren’t as dramatic as what’s achieved using vismodegib or sonidegib. As stand-alone therapy, itraconazole doesn’t compare with those agents. However, the hope is that when itraconazole is prescribed in conjunction with vismodegib or sonidegib it will permit the latter drugs to be used at lower doses with no drop-off in efficacy, which would mean less grade 2 toxicity. Moreover, since itraconazole inhibits Hedgehog signaling through a mechanism that is different from that of the more potent agents, combination therapy might delay onset of tumor resistance, Dr. Swanson explained.
He reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The familiar oral triazole antifungal agent itraconazole (Sporanox) is under active investigation for an unexpected use: as adjunctive therapy in patients with locally advanced or metastatic basal cell carcinoma.
“The promise of this drug is that the use of itraconazole with vismodegib or sonidegib may actually enhance the effectiveness of those drugs and also reduce the frequency of grade 2 toxicities by perhaps allowing a lower dose of vismodegib or sonidegib,” Dr. David L. Swanson said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Be looking for this drug that we use to treat toenail fungus as a potential drug for locally advanced or metastatic basal cell carcinoma,” advised Dr. Swanson, a dermatologist at the Mayo Clinic in Scottsdale, Ariz.
Vismodegib (Erivedge) has been a game changer for patients with inoperable locally advanced or metastatic breast cancer. “The response to this drug was amazing,” Dr. Swanson said of the landmark study which led to its approval in 2012 (N Engl J Med. 2012; 366[23]:2171-9).
Sonidegib (Odomzo), which like vismodegib inhibits the essential Hedgehog signaling pathway component known as Smoothened, was approved by the Food and Drug Administration in 2015 as the second oral drug in this novel class.
While the clinical benefits of these two drugs in patients with the most horrific basal cell carcinomas are extremely impressive, vismodegib and sonidegib have two major drawbacks: The tumors eventually develop resistance and commence growing again, and onerous grade 2 side effects requiring dose reduction are extremely common. The most frequent of these limiting side effects are a disturbed sense of taste, muscle spasms, alopecia, and weight loss.
The hope is that itraconazole may be of help with both issues, according to Dr. Swanson. It turns out that the antifungal agent is also an inhibitor of the Hedgehog pathway, and via a different mechanism than that of vismodegib and sonidegib.
He pointed to an international open-label exploratory phase II study led by Dr. Jean Y. Tang of Stanford (Calif.) University. The investigators treated 19 patients with a total of 90 basal cell carcinomas with oral itraconazole at 200 mg twice a day for 1 month or 100 mg twice a day for an average of 2.3 months.
The treatment reduced Hedgehog signaling pathway activity by 65%, Ki67 tumor cell proliferation by 45%, and tumor area by 24% (J Clin Oncol. 2014 Mar 10;32[8]:745-51).
These results aren’t as dramatic as what’s achieved using vismodegib or sonidegib. As stand-alone therapy, itraconazole doesn’t compare with those agents. However, the hope is that when itraconazole is prescribed in conjunction with vismodegib or sonidegib it will permit the latter drugs to be used at lower doses with no drop-off in efficacy, which would mean less grade 2 toxicity. Moreover, since itraconazole inhibits Hedgehog signaling through a mechanism that is different from that of the more potent agents, combination therapy might delay onset of tumor resistance, Dr. Swanson explained.
He reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The familiar oral triazole antifungal agent itraconazole (Sporanox) is under active investigation for an unexpected use: as adjunctive therapy in patients with locally advanced or metastatic basal cell carcinoma.
“The promise of this drug is that the use of itraconazole with vismodegib or sonidegib may actually enhance the effectiveness of those drugs and also reduce the frequency of grade 2 toxicities by perhaps allowing a lower dose of vismodegib or sonidegib,” Dr. David L. Swanson said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Be looking for this drug that we use to treat toenail fungus as a potential drug for locally advanced or metastatic basal cell carcinoma,” advised Dr. Swanson, a dermatologist at the Mayo Clinic in Scottsdale, Ariz.
Vismodegib (Erivedge) has been a game changer for patients with inoperable locally advanced or metastatic breast cancer. “The response to this drug was amazing,” Dr. Swanson said of the landmark study which led to its approval in 2012 (N Engl J Med. 2012; 366[23]:2171-9).
Sonidegib (Odomzo), which like vismodegib inhibits the essential Hedgehog signaling pathway component known as Smoothened, was approved by the Food and Drug Administration in 2015 as the second oral drug in this novel class.
While the clinical benefits of these two drugs in patients with the most horrific basal cell carcinomas are extremely impressive, vismodegib and sonidegib have two major drawbacks: The tumors eventually develop resistance and commence growing again, and onerous grade 2 side effects requiring dose reduction are extremely common. The most frequent of these limiting side effects are a disturbed sense of taste, muscle spasms, alopecia, and weight loss.
The hope is that itraconazole may be of help with both issues, according to Dr. Swanson. It turns out that the antifungal agent is also an inhibitor of the Hedgehog pathway, and via a different mechanism than that of vismodegib and sonidegib.
He pointed to an international open-label exploratory phase II study led by Dr. Jean Y. Tang of Stanford (Calif.) University. The investigators treated 19 patients with a total of 90 basal cell carcinomas with oral itraconazole at 200 mg twice a day for 1 month or 100 mg twice a day for an average of 2.3 months.
The treatment reduced Hedgehog signaling pathway activity by 65%, Ki67 tumor cell proliferation by 45%, and tumor area by 24% (J Clin Oncol. 2014 Mar 10;32[8]:745-51).
These results aren’t as dramatic as what’s achieved using vismodegib or sonidegib. As stand-alone therapy, itraconazole doesn’t compare with those agents. However, the hope is that when itraconazole is prescribed in conjunction with vismodegib or sonidegib it will permit the latter drugs to be used at lower doses with no drop-off in efficacy, which would mean less grade 2 toxicity. Moreover, since itraconazole inhibits Hedgehog signaling through a mechanism that is different from that of the more potent agents, combination therapy might delay onset of tumor resistance, Dr. Swanson explained.
He reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
A New Approach to “Birthmarks”
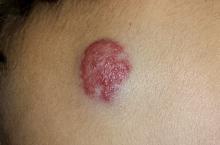
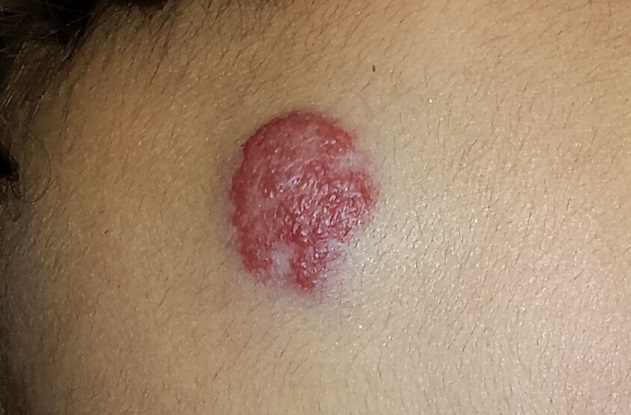
A 4-month-old boy is brought in by his mother for evaluation of a “birthmark” that has become more prominent with time. The child complains a bit when the lesion is touched.
His mother gives a history of a normal full-term pregnancy, with an uneventful delivery. Other than the skin lesion, there have been no other known problems with the child’s health.
EXAMINATION
The lesion in question is a 2-cm bright red nodule in the middle of the child’s forehead. There is a faint blue halo around it, but no other abnormalities are seen in the area or elsewhere on the child’s body. The patient otherwise appears well developed and well nourished; he is quite alert and reactive to verbal stimuli.
What is the diagnosis?
DISCUSSION
Most first-year medical students could tell you this was a case of an infantile hemangioma—but within the past five years, the categorization and treatment of hemangiomas have changed rapidly.
Hemangiomas are benign and usually self-involuting vascular tumors; they are distinct from the family of permanent congenital vascular lesions, such as port wine stains. About 80% occur on the face or neck, and they more commonly occur in female patients. Those that occur near the skin’s surface tend to be bright red, while those of deeper origin are more bluish. Hemangiomas can also occur in extracutaneous areas (eg, the liver); these are usually detected via imaging.
Most infantile hemangiomas appear in the first few weeks of life but begin to involute shortly after they reach maximal size (usually by age 12 months). Involution is complete by age 5 in about 50% of cases and by age 7 in 70% of them. The remainder usually resolve by age 12.
Until recently, the parents of an affected child would be told that the lesions were benign and would likely disappear on their own by the time the child was ages 7 to 10—all true enough. These lesions were treated only when they were large or symptomatic, or if their location blocked or otherwise interfered with the function of the eyes, ears, mouth, anus, or vagina. Unfortunately, this approach ignored the psychosocial aspects of having a prominent and fire-engine red lesion, which often becomes the object of unwanted attention, and even ridicule, at a crucial developmental age.
In the past, the main treatment options were destructive in nature (laser, excision) and often caused as many problems as they solved. Pharmacologic treatment with oral prednisone, and later with interferon, was also tried with mixed success.
In 2008, in a serendipitous observation, a French team came up with a groundbreaking approach: giving systemic propranolol multiple times over a 12 to 18–month period. This method proved to be both safe and effective when used in the right setting by specialists (pediatric dermatologists and/or pediatricians) experienced in this modality. It was found that the lesions responded best if treated before age 6 months.
This has worked so well and so safely that it has quickly become routine. But it will take time before it is sufficiently well known to become the actual standard of care for these common lesions.
TAKE-HOME LEARNING POINTS
• Infantile hemangiomas (IH) usually resolve (involute) by age 12 at the latest.
• IH, by definition, is different from permanent congenital vascular malformations, such as port wine stains.
• About 80% of IHs occur on the head and neck; they are often the object of unwanted attention or even ridicule.
• A new treatment approach, systemic propranolol, is now being used routinely and successfully. It works best when the patient is 6 months old or younger.
• Ideally, this new treatment approach should be overseen by the relevant specialist, be it in a pediatric or dermatologic setting.
A 4-month-old boy is brought in by his mother for evaluation of a “birthmark” that has become more prominent with time. The child complains a bit when the lesion is touched.
His mother gives a history of a normal full-term pregnancy, with an uneventful delivery. Other than the skin lesion, there have been no other known problems with the child’s health.
EXAMINATION
The lesion in question is a 2-cm bright red nodule in the middle of the child’s forehead. There is a faint blue halo around it, but no other abnormalities are seen in the area or elsewhere on the child’s body. The patient otherwise appears well developed and well nourished; he is quite alert and reactive to verbal stimuli.
What is the diagnosis?
DISCUSSION
Most first-year medical students could tell you this was a case of an infantile hemangioma—but within the past five years, the categorization and treatment of hemangiomas have changed rapidly.
Hemangiomas are benign and usually self-involuting vascular tumors; they are distinct from the family of permanent congenital vascular lesions, such as port wine stains. About 80% occur on the face or neck, and they more commonly occur in female patients. Those that occur near the skin’s surface tend to be bright red, while those of deeper origin are more bluish. Hemangiomas can also occur in extracutaneous areas (eg, the liver); these are usually detected via imaging.
Most infantile hemangiomas appear in the first few weeks of life but begin to involute shortly after they reach maximal size (usually by age 12 months). Involution is complete by age 5 in about 50% of cases and by age 7 in 70% of them. The remainder usually resolve by age 12.
Until recently, the parents of an affected child would be told that the lesions were benign and would likely disappear on their own by the time the child was ages 7 to 10—all true enough. These lesions were treated only when they were large or symptomatic, or if their location blocked or otherwise interfered with the function of the eyes, ears, mouth, anus, or vagina. Unfortunately, this approach ignored the psychosocial aspects of having a prominent and fire-engine red lesion, which often becomes the object of unwanted attention, and even ridicule, at a crucial developmental age.
In the past, the main treatment options were destructive in nature (laser, excision) and often caused as many problems as they solved. Pharmacologic treatment with oral prednisone, and later with interferon, was also tried with mixed success.
In 2008, in a serendipitous observation, a French team came up with a groundbreaking approach: giving systemic propranolol multiple times over a 12 to 18–month period. This method proved to be both safe and effective when used in the right setting by specialists (pediatric dermatologists and/or pediatricians) experienced in this modality. It was found that the lesions responded best if treated before age 6 months.
This has worked so well and so safely that it has quickly become routine. But it will take time before it is sufficiently well known to become the actual standard of care for these common lesions.
TAKE-HOME LEARNING POINTS
• Infantile hemangiomas (IH) usually resolve (involute) by age 12 at the latest.
• IH, by definition, is different from permanent congenital vascular malformations, such as port wine stains.
• About 80% of IHs occur on the head and neck; they are often the object of unwanted attention or even ridicule.
• A new treatment approach, systemic propranolol, is now being used routinely and successfully. It works best when the patient is 6 months old or younger.
• Ideally, this new treatment approach should be overseen by the relevant specialist, be it in a pediatric or dermatologic setting.
A 4-month-old boy is brought in by his mother for evaluation of a “birthmark” that has become more prominent with time. The child complains a bit when the lesion is touched.
His mother gives a history of a normal full-term pregnancy, with an uneventful delivery. Other than the skin lesion, there have been no other known problems with the child’s health.
EXAMINATION
The lesion in question is a 2-cm bright red nodule in the middle of the child’s forehead. There is a faint blue halo around it, but no other abnormalities are seen in the area or elsewhere on the child’s body. The patient otherwise appears well developed and well nourished; he is quite alert and reactive to verbal stimuli.
What is the diagnosis?
DISCUSSION
Most first-year medical students could tell you this was a case of an infantile hemangioma—but within the past five years, the categorization and treatment of hemangiomas have changed rapidly.
Hemangiomas are benign and usually self-involuting vascular tumors; they are distinct from the family of permanent congenital vascular lesions, such as port wine stains. About 80% occur on the face or neck, and they more commonly occur in female patients. Those that occur near the skin’s surface tend to be bright red, while those of deeper origin are more bluish. Hemangiomas can also occur in extracutaneous areas (eg, the liver); these are usually detected via imaging.
Most infantile hemangiomas appear in the first few weeks of life but begin to involute shortly after they reach maximal size (usually by age 12 months). Involution is complete by age 5 in about 50% of cases and by age 7 in 70% of them. The remainder usually resolve by age 12.
Until recently, the parents of an affected child would be told that the lesions were benign and would likely disappear on their own by the time the child was ages 7 to 10—all true enough. These lesions were treated only when they were large or symptomatic, or if their location blocked or otherwise interfered with the function of the eyes, ears, mouth, anus, or vagina. Unfortunately, this approach ignored the psychosocial aspects of having a prominent and fire-engine red lesion, which often becomes the object of unwanted attention, and even ridicule, at a crucial developmental age.
In the past, the main treatment options were destructive in nature (laser, excision) and often caused as many problems as they solved. Pharmacologic treatment with oral prednisone, and later with interferon, was also tried with mixed success.
In 2008, in a serendipitous observation, a French team came up with a groundbreaking approach: giving systemic propranolol multiple times over a 12 to 18–month period. This method proved to be both safe and effective when used in the right setting by specialists (pediatric dermatologists and/or pediatricians) experienced in this modality. It was found that the lesions responded best if treated before age 6 months.
This has worked so well and so safely that it has quickly become routine. But it will take time before it is sufficiently well known to become the actual standard of care for these common lesions.
TAKE-HOME LEARNING POINTS
• Infantile hemangiomas (IH) usually resolve (involute) by age 12 at the latest.
• IH, by definition, is different from permanent congenital vascular malformations, such as port wine stains.
• About 80% of IHs occur on the head and neck; they are often the object of unwanted attention or even ridicule.
• A new treatment approach, systemic propranolol, is now being used routinely and successfully. It works best when the patient is 6 months old or younger.
• Ideally, this new treatment approach should be overseen by the relevant specialist, be it in a pediatric or dermatologic setting.
Revisiting the ‘Key Principles and Characteristics of an Effective Hospital Medicine Group'
It has been two years since the “Key Characteristics” was published in the Journal of Hospital Medicine.1 The SHM board of directors envisions the Key Characteristics as a tool to improve the performance of hospital medicine groups (HMGs) and “raise the bar” for the specialty.
At SHM’s annual meeting (www.hospitalmedicine2016.org) next month in San Diego, the Key Characteristics will provide the framework for the Practice Management Pre-Course (Sunday, March 6). The pre-course faculty, of which I am a member, will address all 10 principles of the Key Characteristics (see Table 1), including case studies and practical ideas for performance improvement. As a preview, I will cover Principle 6 and provide a few practical tips that you can implement in your practice.
For a more comprehensive discussion of all the Key Characteristics and how to use them, visit the SHM website (visit www.hospitalmedicine.org, then click on the “Practice Management” icon at the top of the landing page).
Characteristic 6.1
The HMG has systems in place to ensure effective and reliable communication with the patient’s primary care physician and/or other provider(s) involved in the patient’s care in the non-acute-care setting.
Practical tip: Your practice probably has administrative procedures in place to notify PCPs that their patient has been admitted to the hospital, using the electronic health record or secure email, if available, or messaging by fax/phone. But are you receiving vital information from the PCP’s office or from the nursing facility? Establish a protocol for obtaining key history, medication, and diagnostic testing information from these sources. One approach is to request this information when notifying the PCP of the patient’s admission.
Practical tip: Use the “grocery store test” to determine when to contact the PCP during the hospital stay. For example, if the PCP were to run into a family member of the patient in the grocery store, would the PCP want to have learned of a change in the patient’s condition in advance of the family member encounter?
Practical tip: Because reaching skilling nursing facility (SNF) physicians/providers (SNFists) can be challenging, hold an annual social event so that they can meet the hospitalists in your practice face-to-face. At the event, exchange cellphone or beeper numbers with the SNFists, and establish an explicit understanding of how handoffs will occur, especially for high-risk patients.
Characteristic 6.2
The HMG contributes in meaningful ways to the hospital’s efforts to improve care transitions.
Because of readmissions penalties, every hospital in the country is concerned with care transitions and avoiding readmissions. But HMGs want to know which interventions reliably decrease readmissions. The Commonwealth Fund recently released the results of a study of 428 hospitals that participated in national efforts to reduce readmissions, including the State Action on Avoidable Rehospitalizations (STAAR) and Hospital to Home (H2H) initiatives. The study’s primary conclusions were as follows:
- The only strategy consistently associated with reduced risk-standardized readmissions was discharging patients with their appointments already made.2 No other single strategy was reliably associated with a reduction.
- Hospitals that implemented three or more readmission reduction strategies showed a significant decrease in risk-standardized readmissions versus those implementing fewer than three.
Practical tip: Ensure patients leave the hospital with a PCP follow-up appointment made and in hand.
Practical tip: Work with your hospital on at least three definitive strategies to reduce readmissions.
Implement to Improve Your HMG
The basic and updated 2015 versions of the “Key Principles and Characteristics of an Effective Hospital Medicine Group” can be downloaded from the SHM website (visit www.hospitalmedicine.org, then click on the “Practice Management” icon at the top of the landing page). The updated 2015 version provides definitions and requirements and suggested approaches to demonstrating the characteristic that enables the HMG to conduct a comprehensive self-assessment.
In addition, there is a new tool intended for use by hospitalist practice administrators that cross-references the Key Characteristics with another tool, The Core Competencies for a Hospitalist Practice Administrator. TH

References
- Cawley P, Deitelzweig S, Flores L, et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123-128.
- Bradley EH, Brewster A, Curry L. National campaigns to reduce readmissions: what have we learned? The Commonwealth Fund website. Available at: commonwealthfund.org/publications/blog/2015/oct/national-campaigns-to-reduce-readmissions. Accessed December 28, 2015.
It has been two years since the “Key Characteristics” was published in the Journal of Hospital Medicine.1 The SHM board of directors envisions the Key Characteristics as a tool to improve the performance of hospital medicine groups (HMGs) and “raise the bar” for the specialty.
At SHM’s annual meeting (www.hospitalmedicine2016.org) next month in San Diego, the Key Characteristics will provide the framework for the Practice Management Pre-Course (Sunday, March 6). The pre-course faculty, of which I am a member, will address all 10 principles of the Key Characteristics (see Table 1), including case studies and practical ideas for performance improvement. As a preview, I will cover Principle 6 and provide a few practical tips that you can implement in your practice.
For a more comprehensive discussion of all the Key Characteristics and how to use them, visit the SHM website (visit www.hospitalmedicine.org, then click on the “Practice Management” icon at the top of the landing page).
Characteristic 6.1
The HMG has systems in place to ensure effective and reliable communication with the patient’s primary care physician and/or other provider(s) involved in the patient’s care in the non-acute-care setting.
Practical tip: Your practice probably has administrative procedures in place to notify PCPs that their patient has been admitted to the hospital, using the electronic health record or secure email, if available, or messaging by fax/phone. But are you receiving vital information from the PCP’s office or from the nursing facility? Establish a protocol for obtaining key history, medication, and diagnostic testing information from these sources. One approach is to request this information when notifying the PCP of the patient’s admission.
Practical tip: Use the “grocery store test” to determine when to contact the PCP during the hospital stay. For example, if the PCP were to run into a family member of the patient in the grocery store, would the PCP want to have learned of a change in the patient’s condition in advance of the family member encounter?
Practical tip: Because reaching skilling nursing facility (SNF) physicians/providers (SNFists) can be challenging, hold an annual social event so that they can meet the hospitalists in your practice face-to-face. At the event, exchange cellphone or beeper numbers with the SNFists, and establish an explicit understanding of how handoffs will occur, especially for high-risk patients.
Characteristic 6.2
The HMG contributes in meaningful ways to the hospital’s efforts to improve care transitions.
Because of readmissions penalties, every hospital in the country is concerned with care transitions and avoiding readmissions. But HMGs want to know which interventions reliably decrease readmissions. The Commonwealth Fund recently released the results of a study of 428 hospitals that participated in national efforts to reduce readmissions, including the State Action on Avoidable Rehospitalizations (STAAR) and Hospital to Home (H2H) initiatives. The study’s primary conclusions were as follows:
- The only strategy consistently associated with reduced risk-standardized readmissions was discharging patients with their appointments already made.2 No other single strategy was reliably associated with a reduction.
- Hospitals that implemented three or more readmission reduction strategies showed a significant decrease in risk-standardized readmissions versus those implementing fewer than three.
Practical tip: Ensure patients leave the hospital with a PCP follow-up appointment made and in hand.
Practical tip: Work with your hospital on at least three definitive strategies to reduce readmissions.
Implement to Improve Your HMG
The basic and updated 2015 versions of the “Key Principles and Characteristics of an Effective Hospital Medicine Group” can be downloaded from the SHM website (visit www.hospitalmedicine.org, then click on the “Practice Management” icon at the top of the landing page). The updated 2015 version provides definitions and requirements and suggested approaches to demonstrating the characteristic that enables the HMG to conduct a comprehensive self-assessment.
In addition, there is a new tool intended for use by hospitalist practice administrators that cross-references the Key Characteristics with another tool, The Core Competencies for a Hospitalist Practice Administrator. TH

References
- Cawley P, Deitelzweig S, Flores L, et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123-128.
- Bradley EH, Brewster A, Curry L. National campaigns to reduce readmissions: what have we learned? The Commonwealth Fund website. Available at: commonwealthfund.org/publications/blog/2015/oct/national-campaigns-to-reduce-readmissions. Accessed December 28, 2015.
It has been two years since the “Key Characteristics” was published in the Journal of Hospital Medicine.1 The SHM board of directors envisions the Key Characteristics as a tool to improve the performance of hospital medicine groups (HMGs) and “raise the bar” for the specialty.
At SHM’s annual meeting (www.hospitalmedicine2016.org) next month in San Diego, the Key Characteristics will provide the framework for the Practice Management Pre-Course (Sunday, March 6). The pre-course faculty, of which I am a member, will address all 10 principles of the Key Characteristics (see Table 1), including case studies and practical ideas for performance improvement. As a preview, I will cover Principle 6 and provide a few practical tips that you can implement in your practice.
For a more comprehensive discussion of all the Key Characteristics and how to use them, visit the SHM website (visit www.hospitalmedicine.org, then click on the “Practice Management” icon at the top of the landing page).
Characteristic 6.1
The HMG has systems in place to ensure effective and reliable communication with the patient’s primary care physician and/or other provider(s) involved in the patient’s care in the non-acute-care setting.
Practical tip: Your practice probably has administrative procedures in place to notify PCPs that their patient has been admitted to the hospital, using the electronic health record or secure email, if available, or messaging by fax/phone. But are you receiving vital information from the PCP’s office or from the nursing facility? Establish a protocol for obtaining key history, medication, and diagnostic testing information from these sources. One approach is to request this information when notifying the PCP of the patient’s admission.
Practical tip: Use the “grocery store test” to determine when to contact the PCP during the hospital stay. For example, if the PCP were to run into a family member of the patient in the grocery store, would the PCP want to have learned of a change in the patient’s condition in advance of the family member encounter?
Practical tip: Because reaching skilling nursing facility (SNF) physicians/providers (SNFists) can be challenging, hold an annual social event so that they can meet the hospitalists in your practice face-to-face. At the event, exchange cellphone or beeper numbers with the SNFists, and establish an explicit understanding of how handoffs will occur, especially for high-risk patients.
Characteristic 6.2
The HMG contributes in meaningful ways to the hospital’s efforts to improve care transitions.
Because of readmissions penalties, every hospital in the country is concerned with care transitions and avoiding readmissions. But HMGs want to know which interventions reliably decrease readmissions. The Commonwealth Fund recently released the results of a study of 428 hospitals that participated in national efforts to reduce readmissions, including the State Action on Avoidable Rehospitalizations (STAAR) and Hospital to Home (H2H) initiatives. The study’s primary conclusions were as follows:
- The only strategy consistently associated with reduced risk-standardized readmissions was discharging patients with their appointments already made.2 No other single strategy was reliably associated with a reduction.
- Hospitals that implemented three or more readmission reduction strategies showed a significant decrease in risk-standardized readmissions versus those implementing fewer than three.
Practical tip: Ensure patients leave the hospital with a PCP follow-up appointment made and in hand.
Practical tip: Work with your hospital on at least three definitive strategies to reduce readmissions.
Implement to Improve Your HMG
The basic and updated 2015 versions of the “Key Principles and Characteristics of an Effective Hospital Medicine Group” can be downloaded from the SHM website (visit www.hospitalmedicine.org, then click on the “Practice Management” icon at the top of the landing page). The updated 2015 version provides definitions and requirements and suggested approaches to demonstrating the characteristic that enables the HMG to conduct a comprehensive self-assessment.
In addition, there is a new tool intended for use by hospitalist practice administrators that cross-references the Key Characteristics with another tool, The Core Competencies for a Hospitalist Practice Administrator. TH

References
- Cawley P, Deitelzweig S, Flores L, et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123-128.
- Bradley EH, Brewster A, Curry L. National campaigns to reduce readmissions: what have we learned? The Commonwealth Fund website. Available at: commonwealthfund.org/publications/blog/2015/oct/national-campaigns-to-reduce-readmissions. Accessed December 28, 2015.
Regimen can reduce risk of malaria during pregnancy

Photo by Nina Matthews
A 2-drug prophylactic regimen can reduce the risk of Plasmodium falciparum malaria during pregnancy, according to a study published in NEJM.
Monthly treatment with the regimen, dihydroartemisinin-piperaquine (DP), reduced the rate of symptomatic malaria, placental malaria, and parasitemia, when compared to less frequent dosing with DP or treatment with sulfadoxine-pyrimethamine (SP), the current standard prophylactic regimen.
Researchers therefore believe DP may be a feasible alternative to SP, which has become less effective over time.
“The malaria parasite’s resistance to SP is widespread, especially in sub-Saharan Africa,” said study author Abel Kakuru, MD, of the Infectious Diseases Research Collaboration in Kampala, Uganda.
“But we are still using the same drugs because we have no better alternatives.”
In an attempt to identify a better alternative, Dr Kakuru and his colleagues studied 300 pregnant women from Tororo, Uganda, from June 2014 through October 2014. All of the subjects were age 16 or older and anywhere from 12 to 20 weeks pregnant.
The women were randomized to receive 1 of 3 regimens for malaria prophylaxis:
- DP once a month
- DP at 20, 28, and 30 weeks of pregnancy
- SP at 20, 28, and 30 weeks of pregnancy.
Participants had monthly checkups at the study clinic, where they received regular blood tests for malaria. The researchers also assessed malaria infection in the placenta.
Placental malaria was confirmed in 50% of women in the SP group, 34% in the 3-dose-DP group (P=0.03), and 27% in the monthly DP group (P=0.001).
Forty-one percent of the women on SP had malaria parasites in their blood, compared to 17% in the 3-dose DP group (P<0.001), and 5% in the monthly DP group (P<0.001).
None of the women on monthly DP had symptomatic malaria during pregnancy. But there were 41 episodes of malaria during pregnancy in the SP group and 12 episodes in the 3-dose DP group.
The researchers also evaluated the women and infants in the study for a composite adverse birth outcome of spontaneous abortion, stillbirth, low birth weight, preterm delivery, or birth defects.
The risk of any adverse birth outcome was 9% in the monthly DP group, 21% in the 3-dose DP group (P=0.02), and 19% in the SP group (P=0.05).
The researchers concluded that monthly dosing of DP provided the best protection against malaria and called for additional studies to determine if the regimen would provide an effective alternative treatment in other parts of Uganda and elsewhere in Africa. ![]()

Photo by Nina Matthews
A 2-drug prophylactic regimen can reduce the risk of Plasmodium falciparum malaria during pregnancy, according to a study published in NEJM.
Monthly treatment with the regimen, dihydroartemisinin-piperaquine (DP), reduced the rate of symptomatic malaria, placental malaria, and parasitemia, when compared to less frequent dosing with DP or treatment with sulfadoxine-pyrimethamine (SP), the current standard prophylactic regimen.
Researchers therefore believe DP may be a feasible alternative to SP, which has become less effective over time.
“The malaria parasite’s resistance to SP is widespread, especially in sub-Saharan Africa,” said study author Abel Kakuru, MD, of the Infectious Diseases Research Collaboration in Kampala, Uganda.
“But we are still using the same drugs because we have no better alternatives.”
In an attempt to identify a better alternative, Dr Kakuru and his colleagues studied 300 pregnant women from Tororo, Uganda, from June 2014 through October 2014. All of the subjects were age 16 or older and anywhere from 12 to 20 weeks pregnant.
The women were randomized to receive 1 of 3 regimens for malaria prophylaxis:
- DP once a month
- DP at 20, 28, and 30 weeks of pregnancy
- SP at 20, 28, and 30 weeks of pregnancy.
Participants had monthly checkups at the study clinic, where they received regular blood tests for malaria. The researchers also assessed malaria infection in the placenta.
Placental malaria was confirmed in 50% of women in the SP group, 34% in the 3-dose-DP group (P=0.03), and 27% in the monthly DP group (P=0.001).
Forty-one percent of the women on SP had malaria parasites in their blood, compared to 17% in the 3-dose DP group (P<0.001), and 5% in the monthly DP group (P<0.001).
None of the women on monthly DP had symptomatic malaria during pregnancy. But there were 41 episodes of malaria during pregnancy in the SP group and 12 episodes in the 3-dose DP group.
The researchers also evaluated the women and infants in the study for a composite adverse birth outcome of spontaneous abortion, stillbirth, low birth weight, preterm delivery, or birth defects.
The risk of any adverse birth outcome was 9% in the monthly DP group, 21% in the 3-dose DP group (P=0.02), and 19% in the SP group (P=0.05).
The researchers concluded that monthly dosing of DP provided the best protection against malaria and called for additional studies to determine if the regimen would provide an effective alternative treatment in other parts of Uganda and elsewhere in Africa. ![]()

Photo by Nina Matthews
A 2-drug prophylactic regimen can reduce the risk of Plasmodium falciparum malaria during pregnancy, according to a study published in NEJM.
Monthly treatment with the regimen, dihydroartemisinin-piperaquine (DP), reduced the rate of symptomatic malaria, placental malaria, and parasitemia, when compared to less frequent dosing with DP or treatment with sulfadoxine-pyrimethamine (SP), the current standard prophylactic regimen.
Researchers therefore believe DP may be a feasible alternative to SP, which has become less effective over time.
“The malaria parasite’s resistance to SP is widespread, especially in sub-Saharan Africa,” said study author Abel Kakuru, MD, of the Infectious Diseases Research Collaboration in Kampala, Uganda.
“But we are still using the same drugs because we have no better alternatives.”
In an attempt to identify a better alternative, Dr Kakuru and his colleagues studied 300 pregnant women from Tororo, Uganda, from June 2014 through October 2014. All of the subjects were age 16 or older and anywhere from 12 to 20 weeks pregnant.
The women were randomized to receive 1 of 3 regimens for malaria prophylaxis:
- DP once a month
- DP at 20, 28, and 30 weeks of pregnancy
- SP at 20, 28, and 30 weeks of pregnancy.
Participants had monthly checkups at the study clinic, where they received regular blood tests for malaria. The researchers also assessed malaria infection in the placenta.
Placental malaria was confirmed in 50% of women in the SP group, 34% in the 3-dose-DP group (P=0.03), and 27% in the monthly DP group (P=0.001).
Forty-one percent of the women on SP had malaria parasites in their blood, compared to 17% in the 3-dose DP group (P<0.001), and 5% in the monthly DP group (P<0.001).
None of the women on monthly DP had symptomatic malaria during pregnancy. But there were 41 episodes of malaria during pregnancy in the SP group and 12 episodes in the 3-dose DP group.
The researchers also evaluated the women and infants in the study for a composite adverse birth outcome of spontaneous abortion, stillbirth, low birth weight, preterm delivery, or birth defects.
The risk of any adverse birth outcome was 9% in the monthly DP group, 21% in the 3-dose DP group (P=0.02), and 19% in the SP group (P=0.05).
The researchers concluded that monthly dosing of DP provided the best protection against malaria and called for additional studies to determine if the regimen would provide an effective alternative treatment in other parts of Uganda and elsewhere in Africa. ![]()
Germline mutations linked to hematologic malignancies

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.” ![]()

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.” ![]()

A new study suggests mutations in the gene DDX41 occur in families where hematologic malignancies are common.
Previous research showed that both germline and acquired DDX41 mutations occur in families with multiple cases of late-onset myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
The new study, published in Blood, has linked germline mutations in DDX41 to chronic myeloid leukemia and lymphomas as well.
“This is the first gene identified in families with lymphoma and represents a major breakthrough for the field,” said study author Hamish Scott, PhD, of the University of Adelaide in South Australia.
“Researchers are recognizing now that genetic predisposition to blood cancer is more common than previously thought, and our study shows the importance of taking a thorough family history at diagnosis.”
To conduct this study, Dr Scott and his colleagues screened 2 cohorts of families with a range of hematologic disorders (malignant and non-malignant). One cohort included 240 individuals from 93 families in Australia. The other included 246 individuals from 198 families in the US.
In all, 9 of the families (3%) had germline DDX41 mutations.
Three families carried the recurrent p.D140Gfs*2 mutation, which was linked to AML.
One family carried a germline mutation—p.R525H, c.1574G.A—that was previously described only as a somatic mutation at the time of progression to MDS or AML. In the current study, the mutation was again linked to MDS and AML.
Five families carried novel DDX41 mutations.
One of these mutations was a germline substitution—c.435-2_435-1delAGinsCA—that was linked to MDS in 1 family.
Two families had a missense start-loss substitution—c.3G.A, p.M1I—that was linked to MDS, AML, chronic myeloid leukemia, and non-Hodgkin lymphoma.
One family had a DDX41 missense variant—c.490C.T, p.R164W. This was linked to Hodgkin and non-Hodgkin lymphoma (including 3 cases of follicular lymphoma). There was a possible link to multiple myeloma as well, but the diagnosis could not be confirmed.
And 1 family had a missense mutation in the helicase domain—p.G530D—that was linked to AML.
“DDX41 is a new type of cancer predisposition gene, and we are still investigating its function,” Dr Scott noted.
“But it appears to have dual roles in regulating the correct expression of genes in the cell and also enabling the immune system to respond to threats such as bacteria and viruses, as well as the development of cancer cells. Immunotherapy is a promising approach for cancer treatment, and our research to understand the function of DDX41 will help design better therapies.” ![]()
Intracranial bleeding in older adults on warfarin

Results of a large study suggest warfarin may pose a higher risk of traumatic intracranial bleeding than previously reported, at least among older patients with atrial fibrillation (AF).
The study included more than 30,000 US veterans with AF who were 75 years or older when starting warfarin.
These patients had a higher rate of traumatic intracranial bleeding than reported in previous trials.
John A. Dodson, MD, of the New York University School of Medicine in New York, New York, and his colleagues conducted this research and reported the results in JAMA Cardiology.
The researchers studied 31,951 subjects with AF who were 75 years or older and were new referrals to Veterans Affairs anticoagulation clinics for warfarin therapy between January 1, 2002, and December 31, 2012.
The patients had a mean age of 81.1, and 98.1% were male. Comorbidities included hypertension (82.5%), coronary artery disease (42.6%), and diabetes mellitus (33.8%).
The researchers found the incidence rate of hospitalization for any intracranial bleeding among these patients was 14.58 per 1000 person-years.
And the incidence rate of hospitalization for traumatic intracranial bleeding was 4.80 per 1000 person-years.
The researchers said this was “considerably higher” than reported in two previous trials, one published in The Lancet in 1996 and one published in JAMA Internal Medicine in 1998.
Dr Dodson and his colleagues also looked at factors associated with traumatic intracranial bleeding in their patient population.
In unadjusted analyses, the following factors were significant predictors of traumatic intracranial bleeding: dementia, fall within the past year, anemia, depression, abnormal renal or liver function, anticonvulsant use, labile international normalized ratio, and antihypertensive use.
However, when the researchers adjusted their analyses for potential confounders, fewer factors remained significant predictors. These were dementia (hazard ratio [HR]=1.76), anemia (HR=1.23), depression (HR=1.30), anticonvulsant use (HR=1.35), and labile international normalized ratio (HR=1.33).
The researchers noted that risk factors for traumatic intracranial bleeding were different from risk factors for ischemic stroke.
They also said the high overall rate of intracranial bleeding in this study suggests a need to more systematically evaluate the benefits and harms of warfarin in older adults. ![]()

Results of a large study suggest warfarin may pose a higher risk of traumatic intracranial bleeding than previously reported, at least among older patients with atrial fibrillation (AF).
The study included more than 30,000 US veterans with AF who were 75 years or older when starting warfarin.
These patients had a higher rate of traumatic intracranial bleeding than reported in previous trials.
John A. Dodson, MD, of the New York University School of Medicine in New York, New York, and his colleagues conducted this research and reported the results in JAMA Cardiology.
The researchers studied 31,951 subjects with AF who were 75 years or older and were new referrals to Veterans Affairs anticoagulation clinics for warfarin therapy between January 1, 2002, and December 31, 2012.
The patients had a mean age of 81.1, and 98.1% were male. Comorbidities included hypertension (82.5%), coronary artery disease (42.6%), and diabetes mellitus (33.8%).
The researchers found the incidence rate of hospitalization for any intracranial bleeding among these patients was 14.58 per 1000 person-years.
And the incidence rate of hospitalization for traumatic intracranial bleeding was 4.80 per 1000 person-years.
The researchers said this was “considerably higher” than reported in two previous trials, one published in The Lancet in 1996 and one published in JAMA Internal Medicine in 1998.
Dr Dodson and his colleagues also looked at factors associated with traumatic intracranial bleeding in their patient population.
In unadjusted analyses, the following factors were significant predictors of traumatic intracranial bleeding: dementia, fall within the past year, anemia, depression, abnormal renal or liver function, anticonvulsant use, labile international normalized ratio, and antihypertensive use.
However, when the researchers adjusted their analyses for potential confounders, fewer factors remained significant predictors. These were dementia (hazard ratio [HR]=1.76), anemia (HR=1.23), depression (HR=1.30), anticonvulsant use (HR=1.35), and labile international normalized ratio (HR=1.33).
The researchers noted that risk factors for traumatic intracranial bleeding were different from risk factors for ischemic stroke.
They also said the high overall rate of intracranial bleeding in this study suggests a need to more systematically evaluate the benefits and harms of warfarin in older adults. ![]()

Results of a large study suggest warfarin may pose a higher risk of traumatic intracranial bleeding than previously reported, at least among older patients with atrial fibrillation (AF).
The study included more than 30,000 US veterans with AF who were 75 years or older when starting warfarin.
These patients had a higher rate of traumatic intracranial bleeding than reported in previous trials.
John A. Dodson, MD, of the New York University School of Medicine in New York, New York, and his colleagues conducted this research and reported the results in JAMA Cardiology.
The researchers studied 31,951 subjects with AF who were 75 years or older and were new referrals to Veterans Affairs anticoagulation clinics for warfarin therapy between January 1, 2002, and December 31, 2012.
The patients had a mean age of 81.1, and 98.1% were male. Comorbidities included hypertension (82.5%), coronary artery disease (42.6%), and diabetes mellitus (33.8%).
The researchers found the incidence rate of hospitalization for any intracranial bleeding among these patients was 14.58 per 1000 person-years.
And the incidence rate of hospitalization for traumatic intracranial bleeding was 4.80 per 1000 person-years.
The researchers said this was “considerably higher” than reported in two previous trials, one published in The Lancet in 1996 and one published in JAMA Internal Medicine in 1998.
Dr Dodson and his colleagues also looked at factors associated with traumatic intracranial bleeding in their patient population.
In unadjusted analyses, the following factors were significant predictors of traumatic intracranial bleeding: dementia, fall within the past year, anemia, depression, abnormal renal or liver function, anticonvulsant use, labile international normalized ratio, and antihypertensive use.
However, when the researchers adjusted their analyses for potential confounders, fewer factors remained significant predictors. These were dementia (hazard ratio [HR]=1.76), anemia (HR=1.23), depression (HR=1.30), anticonvulsant use (HR=1.35), and labile international normalized ratio (HR=1.33).
The researchers noted that risk factors for traumatic intracranial bleeding were different from risk factors for ischemic stroke.
They also said the high overall rate of intracranial bleeding in this study suggests a need to more systematically evaluate the benefits and harms of warfarin in older adults. ![]()
FDA lifts partial clinical hold on pidilizumab

The US Food and Drug Administration (FDA) has lifted the partial clinical hold on the investigational new drug (IND) application for pidilizumab (MDV9300) in hematologic malignancies.
This means the phase 2 trial of pidilizumab in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), as well as other studies that cross-reference the IND for the drug, may now proceed.
The partial clinical hold on pidilizumab was not related to any safety concerns.
The FDA placed the hold because the company developing pidilizumab, Medivation Inc., determined that the drug is not an inhibitor of PD-1, as researchers previously thought.
The phase 2 trial of pidilizumab in DLBCL was launched in late 2015 but had not enrolled any patients before the FDA placed the partial clinical hold.
Patients who were receiving pidilizumab through investigator-sponsored trials have continued to receive treatment despite the hold, and those investigators have been told to update their protocols and informed consent documents to reflect that pidilizumab is not an anti-PD-1 antibody.
Medivation has likewise revised the investigator brochure, protocols, and informed consent documents related to the phase 2 trial of DLBCL patients.
The company said it is still trying to determine pidilizumab’s mechanism of action.
“We are delighted that the FDA has lifted the partial clinical hold and that we may proceed with our potentially pivotal trial in this area of high unmet medical need,” said David Hung, MD, founder, president, and chief executive officer of Medivation.
“As we move forward, we also are working to determine the compound’s exact binding mechanism which, we believe, modulates the body’s innate immune response and differentiates it from the heavily crowded immuno-oncology space that targets the adaptive side of immunity.”
Medivation said it intends to submit an amendment to the Chemistry, Manufacturing, and Controls section of the IND for pidilizumab to provide for larger manufacturing lot sizes to better support the current and planned clinical activities for the drug.
The company also said it plans to resume the phase 2 trial of pidilizumab in DLBLCL in the second half of this year.
The trial is expected to enroll approximately 180 patients who had an incomplete response to salvage therapy or autologous stem cell transplant for relapsed or refractory CD20+ DLBCL, transformed indolent lymphoma, or primary mediastinal B-cell lymphoma.
The patients will be assessed in 2 parallel cohorts of approximately 90 patients each. One cohort will enroll patients who have received an autologous stem cell transplant, and the other will enroll patients who have received salvage chemotherapy but are ineligible for transplant.
Pidilizumab will be given at a dose of 200 mg by intravenous infusion. The primary endpoint of the trial is best overall response rate. ![]()

The US Food and Drug Administration (FDA) has lifted the partial clinical hold on the investigational new drug (IND) application for pidilizumab (MDV9300) in hematologic malignancies.
This means the phase 2 trial of pidilizumab in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), as well as other studies that cross-reference the IND for the drug, may now proceed.
The partial clinical hold on pidilizumab was not related to any safety concerns.
The FDA placed the hold because the company developing pidilizumab, Medivation Inc., determined that the drug is not an inhibitor of PD-1, as researchers previously thought.
The phase 2 trial of pidilizumab in DLBCL was launched in late 2015 but had not enrolled any patients before the FDA placed the partial clinical hold.
Patients who were receiving pidilizumab through investigator-sponsored trials have continued to receive treatment despite the hold, and those investigators have been told to update their protocols and informed consent documents to reflect that pidilizumab is not an anti-PD-1 antibody.
Medivation has likewise revised the investigator brochure, protocols, and informed consent documents related to the phase 2 trial of DLBCL patients.
The company said it is still trying to determine pidilizumab’s mechanism of action.
“We are delighted that the FDA has lifted the partial clinical hold and that we may proceed with our potentially pivotal trial in this area of high unmet medical need,” said David Hung, MD, founder, president, and chief executive officer of Medivation.
“As we move forward, we also are working to determine the compound’s exact binding mechanism which, we believe, modulates the body’s innate immune response and differentiates it from the heavily crowded immuno-oncology space that targets the adaptive side of immunity.”
Medivation said it intends to submit an amendment to the Chemistry, Manufacturing, and Controls section of the IND for pidilizumab to provide for larger manufacturing lot sizes to better support the current and planned clinical activities for the drug.
The company also said it plans to resume the phase 2 trial of pidilizumab in DLBLCL in the second half of this year.
The trial is expected to enroll approximately 180 patients who had an incomplete response to salvage therapy or autologous stem cell transplant for relapsed or refractory CD20+ DLBCL, transformed indolent lymphoma, or primary mediastinal B-cell lymphoma.
The patients will be assessed in 2 parallel cohorts of approximately 90 patients each. One cohort will enroll patients who have received an autologous stem cell transplant, and the other will enroll patients who have received salvage chemotherapy but are ineligible for transplant.
Pidilizumab will be given at a dose of 200 mg by intravenous infusion. The primary endpoint of the trial is best overall response rate. ![]()

The US Food and Drug Administration (FDA) has lifted the partial clinical hold on the investigational new drug (IND) application for pidilizumab (MDV9300) in hematologic malignancies.
This means the phase 2 trial of pidilizumab in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), as well as other studies that cross-reference the IND for the drug, may now proceed.
The partial clinical hold on pidilizumab was not related to any safety concerns.
The FDA placed the hold because the company developing pidilizumab, Medivation Inc., determined that the drug is not an inhibitor of PD-1, as researchers previously thought.
The phase 2 trial of pidilizumab in DLBCL was launched in late 2015 but had not enrolled any patients before the FDA placed the partial clinical hold.
Patients who were receiving pidilizumab through investigator-sponsored trials have continued to receive treatment despite the hold, and those investigators have been told to update their protocols and informed consent documents to reflect that pidilizumab is not an anti-PD-1 antibody.
Medivation has likewise revised the investigator brochure, protocols, and informed consent documents related to the phase 2 trial of DLBCL patients.
The company said it is still trying to determine pidilizumab’s mechanism of action.
“We are delighted that the FDA has lifted the partial clinical hold and that we may proceed with our potentially pivotal trial in this area of high unmet medical need,” said David Hung, MD, founder, president, and chief executive officer of Medivation.
“As we move forward, we also are working to determine the compound’s exact binding mechanism which, we believe, modulates the body’s innate immune response and differentiates it from the heavily crowded immuno-oncology space that targets the adaptive side of immunity.”
Medivation said it intends to submit an amendment to the Chemistry, Manufacturing, and Controls section of the IND for pidilizumab to provide for larger manufacturing lot sizes to better support the current and planned clinical activities for the drug.
The company also said it plans to resume the phase 2 trial of pidilizumab in DLBLCL in the second half of this year.
The trial is expected to enroll approximately 180 patients who had an incomplete response to salvage therapy or autologous stem cell transplant for relapsed or refractory CD20+ DLBCL, transformed indolent lymphoma, or primary mediastinal B-cell lymphoma.
The patients will be assessed in 2 parallel cohorts of approximately 90 patients each. One cohort will enroll patients who have received an autologous stem cell transplant, and the other will enroll patients who have received salvage chemotherapy but are ineligible for transplant.
Pidilizumab will be given at a dose of 200 mg by intravenous infusion. The primary endpoint of the trial is best overall response rate. ![]()
Introducing the Hospitalist Morale Index
Explosive growth in hospital medicine has led to hospitalists having the option to change jobs easily. Annual turnover for all physicians is 6.8%, whereas that of hospitalists exceeds 14.8%.[1] Losing a single physician has significant financial and operational implications, with estimates of $20,000 to $120,000 in recruiting costs, and up to $500,000 in lost revenue that may take years to recoup due to the time required for new physician assimilation.[2, 3] In 2006, the Society of Hospital Medicine (SHM) appointed a career task force to develop retention recommendations, 1 of which includes monitoring hospitalists' job satisfaction.[4]
Studies examining physician satisfaction have demonstrated that high physician job satisfaction is associated with lower physician turnover.[5] However, surveys of hospitalists, including SHM's Hospital Medicine Physician Worklife Survey (HMPWS), have reported high job satisfaction among hospitalists,[6, 7, 8, 9, 10] suggesting that high job satisfaction may not be enough to overcome forces that pull hospitalists toward other opportunities.
Morale, a more complex construct related to an individual's contentment and happiness, might provide insight into reducing hospitalist turnover. Morale has been defined as the emotional or mental condition with respect to cheerfulness, confidence, or zeal and is especially relevant in the face of opposition or hardship.[11] Job satisfaction is 1 element that contributes to morale, but alone does not equate morale.[12] Morale, more than satisfaction, relates to how people see themselves within the group and may be closely tied to the concept of esprit de corps. To illustrate, workers may feel satisfied with the content of their job, but frustration with the organization may result in low morale.[13] Efforts focused on assessing provider morale may provide deeper understanding of hospitalists' professional needs and garner insight for retention strategies.
The construct of hospitalist morale and its underlying drivers has not been explored in the literature. Using literature within and outside of healthcare,[1, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22] and our own prior work,[23] we sought to characterize elements that contribute to hospitalist morale and develop a metric to measure it. The HMPWS found that job satisfaction factors vary across hospitalist groups.[9] We suspected that the same would hold true for factors important to morale at the individual level. This study describes the development and validation of the Hospitalist Morale Index (HMI), and explores the relationship between morale and intent to leave due to unhappiness.
METHODS
2009 Pilot Survey
To establish content validity, after reviewing employee morale literature, and examining qualitative comments from our 2007 and 2008 morale surveys, our expert panel, consisting of practicing hospitalists, hospitalist leaders, and administrative staff, identified 46 potential drivers of hospitalist morale. In May 2009, all hospitalists, including physicians, nurse practitioners (NPs), and physician assistants (PAs) from a single hospitalist group received invitations to complete the pilot survey. We asked hospitalists to assess on 5‐point Likert scales the importance of (not at all to tremendously) and contentment with (extremely discontent to extremely content) each of the 46 items as it relates to their work morale. Also included were demographic questions and general morale questions (including rating participants' own morale), investment, long‐term career plans, and intent to leave due to unhappiness.
Data Collection
To maintain anonymity and limit social desirability bias, a database manager, working outside the Division of Hospital Medicine and otherwise not associated with the research team, used Survey Monkey to coordinate survey distribution and data collection. Each respondent had a unique identifier code that was unrelated to the respondent's name and email address. Personal identifiers were maintained in a secure database accessible only to the database manager.
Establishing Internal Structure Validity Evidence
Response frequency to each question was examined for irregularities in distribution. For continuous variables, descriptive statistics were examined for evidence of skewness, outliers, and non‐normality to ensure appropriate use of parametric statistical tests. Upon ranking importance ratings by mode, 15 of 46 items were judged to be of low importance by almost all participants and removed from further consideration.
Stata 13.1 (StataCorp, College Station, TX) was used for exploratory factor analysis (EFA) of the importance responses for all 31 remaining items by principal components factoring. Eigenvalues >1 were designated as a cutoff point for inclusion in varimax rotation. Factor loading of 0.50 was the threshold for inclusion in a factor.
The 31 items loaded across 10 factors; however, 3 factors included 1 item each. After reviewing the scree plot and considering their face value, these items/factors were omitted. Repeating the factor analysis resulted in a 28‐item, 7‐factor solution that accounted for 75% variance. All items were considered informative as demonstrated by low uniqueness scores (0.050.38). Using standard validation procedures, all 7 factors were found to have acceptable factor loadings (0.460.98) and face validity. Cronbach's quantified internal reliability of the 7 factors with scores ranging from 0.68 to 0.92. We named the resultant solution the Hospitalist Morale Index (HMI).
Establishing Response Process Validity Evidence
In developing the HMI, we asked respondents to rate the importance of and their contentment with each variable as related to their work morale. From pilot testing, which included discussions with respondents immediately after completing the survey, we learned that the 2‐part consideration of each variable resulted in thoughtful reflection about their morale. Further, by multiplying the contentment score for each item (scaled from 15) by the corresponding importance score (scaled 01), we quantified the relative contribution and contentment of each item for each hospitalist. Scaling importance scores from 0 to 1 insured that items that were not considered important to the respondent did not affect the respondent's personal morale score. Averaging resultant item scores that were greater than 0 resulted in a personal morale score for each hospitalist. Averaging item scores >0 that constituted each factor resulted in factor scores.
May 2011 Survey
The refined survey was distributed in May 2011 to a convenience sample of 5 hospitalist programs at separate hospitals (3 community hospitals, 2 academic hospitals) encompassing 108 hospitalists in 3 different states. Responses to the 2011 survey were used to complete confirmatory factor analyses (CFA) and establish further validity and reliability evidence.
Based on the 28‐item, 7‐factor solution developed from the pilot study, we developed the theoretical model of factors constituting hospitalist morale. We used the structural equation modeling command in Stata 13 to perform CFA. Factor loading of 0.50 was the threshold for inclusion of an item in a factor. To measure internal consistency, we considered Cronbach's score of 0.60 acceptable. Iterative models were reviewed to find the optimal solution for the data. Four items did not fit into any of the 5 resulting factors and were evaluated in terms of mean importance score and face value. Three items were considered important enough to warrant being stand‐alone items, whereas 1 was omitted. Two additional items had borderline factor loadings (0.48, 0.49) and were included in the model as stand‐alone items due to their overall relevance. The resultant solution was a 5‐factor model with 5 additional stand‐alone items (Table 1).
| Factor | Cronbach's | |||||
|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | ||
| How much does the following item contribute to your morale? | ||||||
| Paperwork | 0.72 | 0.89 | ||||
| Relationship with patients | 0.69 | 0.90 | ||||
| Electronic medical system | 0.60 | 0.90 | ||||
| Intellectual stimulation | 0.59 | 0.90 | ||||
| Variety of cases | 0.58 | 0.90 | ||||
| Relationship with consultants | 0.51 | 0.89 | ||||
| No. of night shifts | 0.74 | 0.89 | ||||
| Patient census | 0.61 | 0.90 | ||||
| No. of shifts | 0.52 | 0.90 | ||||
| Fairness of leadership | 0.82 | 0.89 | ||||
| Effectiveness of leadership | 0.82 | 0.89 | ||||
| Leadership's receptiveness to my thoughts and suggestions | 0.78 | 0.89 | ||||
| Leadership as advocate for my needs | 0.77 | 0.89 | ||||
| Approachability of leadership | 0.77 | 0.89 | ||||
| Accessibility of leadership | 0.69 | 0.89 | ||||
| Alignment of the group's goals with my goals | 0.50 | 0.89 | ||||
| Recognition within the group | 0.82 | 0.89 | ||||
| Feeling valued within the institution | 0.73 | 0.89 | ||||
| Feeling valued within the group | 0.73 | 0.89 | ||||
| Feedback | 0.52 | 0.89 | ||||
| Pay | 0.99 | 0.90 | ||||
| Benefits | 0.56 | 0.89 | ||||
| Cronbach's | 0.78 | 0.65 | 0.89 | 0.78 | 0.71 | |
| How much does the following item contribute to your morale? | Single item indicators | |||||
| Family time | 0.90 | |||||
| Job security | 0.90 | |||||
| Institutional climate | 0.89 | |||||
| Opportunities for professional growth | 0.90 | |||||
| Autonomy | 0.89 | |||||
| Cronbach's | 0.90 | |||||
Establishing Convergent, Concurrent, and Discriminant Validity Evidence
To establish convergent, concurrent, and discriminant validity, linear and logistic regression models were examined for continuous and categorical data accordingly.
Self‐perceived overall work morale and perceived group morale, as assessed by 6‐point Likert questions with response options from terrible to excellent, were modeled as predictors for personal morale as calculated by the HMI.
Personal morale scores were modeled as predictors of professional growth, stress, investment in the group, and intent to leave due to unhappiness. While completing the HMI, hospitalists simultaneously completed a validated professional growth scale[24] and Cohen stress scale.[25] We hypothesized that those with higher morale would have more professional growth. Stress, although an important issue in the workplace, is a distinct construct from morale, and we did not expect a significant relationship between personal morale and stress. We used Pearson's r to assess the strength of association between the HMI and these scales. Participants' level of investment in their group was assessed on a 5‐point Likert scale. To simplify presentation, highly invested represents those claiming to be very or tremendously invested in the success of their current hospitalist group. Intent to leave due to unhappiness was assessed on a 5‐point Likert scale, I have had serious thoughts about leaving my current hospitalist group because I am unhappy, with responses from strongly disagree (1) to strongly agree (5). To simplify presentation, responses higher than 2 are considered to be consistent with intending to leave due to unhappiness.
Our institutional review board approved the study.
RESULTS
Respondents
In May 2009, 30 of the 33 (91%) invited hospitalists completed the original pilot morale survey; 19 (63%) were women. Eleven hospitalists (37%) had been part of the group 1 year or less, whereas 4 (13%) had been with the group for more than 5 years.
In May 2011, 93 of the 108 (86%) hospitalists from 5 hospitals completed the demographic and global parts of the survey. Fifty (53%) were from community hospitals; 47 (51%) were women. Thirty‐seven (40%) physicians and 6 (60%) NPs/PAs were from academic hospitals. Thirty‐nine hospitalists (42%) had been with their current group 1 year or less. Ten hospitalists (11%) had been with their current group over 5 years. Sixty‐three respondents (68%) considered themselves career hospitalists, whereas 5 (5%) did not; the rest were undecided.
Internal Structure Validity Evidence
The final CFA from the 2011 survey resulted in a 5‐factor plus 5stand‐alone‐items HMI. The solution with item‐level and factor‐level Cronbach's scores (range, 0.890.90 and range, 0.650.89, respectively) are shown in Table 1.
Personal Morale Scores and Factor Scores
Personal morale scores were normally distributed (mean = 2.79; standard deviation [SD] = 0.58), ranging from 1.23 to 4.22, with a theoretical low of 0 and high of 5 (Figure 1). Mean personal morale scores across hospitalist groups ranged from 2.70 to 2.99 (P > 0.05). Personal morale scores, factor sores and item scores for NPs and PAs did not significantly differ from those of physicians (P > 0.05 for all analyses). Personal morale scores were lower for those in their first 3 years with their current group, compared to those with greater institutional longevity. For every categorical increase in a participant's response to seeing oneself as a career hospitalist, the personal morale score rose 0.23 points (P < 0.001).
Factor scores for material reward and mean item scores for professional growth were significantly different across the 5 hospitalist groups (P = 0.03 and P < 0.001, respectively). Community hospitalists had significantly higher factor scores, despite having similar importance scores, for material rewards than academic hospitalists (diff. = 0.44, P = 0.02). Academic hospitalists had significantly higher scores for professional growth (diff. = 0.94, P < 0.001) (Table 2). Professional growth had the highest importance score for academic hospitalists (mean = 0.87, SD = 0.18) and the lowest importance score for community hospitalists (mean = 0.65, SD = 0.24, P < 0.001).
| Personal Morale Score | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | Family Time | Institutional Climate | Job Security | Autonomy | Professional Growth | |||
| ||||||||||||
| All participants | Mean | 2.79 | 2.54 | 2.78 | 3.18 | 2.58 | 2.48 | 3.05 | 2.67 | 2.92 | 3.00 | 2.76 |
| SD | 0.58 | 0.63 | 0.70 | 0.95 | 0.86 | 0.85 | 1.15 | 0.97 | 1.11 | 1.10 | 1.21 | |
| Academic A | Mean | 2.77 | 2.43 | 2.92 | 3.10 | 2.54 | 2.28 | 3.16 | 2.70 | 3.06 | 3.20 | 3.08 |
| SD | 0.57 | 0.62 | 0.64 | 0.92 | 0.84 | 0.77 | 1.19 | 0.95 | 1.08 | 1.12 | 1.24 | |
| Academic B | Mean | 2.99 | 2.58 | 2.99 | 3.88 | 2.69 | 2.00 | 2.58 | 2.13 | 1.65 | 3.29 | 4.33 |
| SD | 0.36 | 0.70 | 0.80 | 0.29 | 0.80 | 0.35 | 0.92 | 0.88 | 0.78 | 1.01 | 0.82 | |
| Community A | Mean | 2.86 | 2.61 | 2.51 | 3.23 | 2.73 | 3.03 | 2.88 | 2.84 | 2.95 | 3.23 | 2.66 |
| SD | 0.75 | 0.79 | 0.68 | 1.21 | 1.11 | 1.14 | 1.37 | 1.17 | 0.98 | 1.24 | 1.15 | |
| Community B | Mean | 2.86 | 2.74 | 2.97 | 3.37 | 2.67 | 2.44 | 3.28 | 2.35 | 2.70 | 2.50 | 2.25 |
| SD | 0.67 | 0.55 | 0.86 | 1.04 | 0.94 | 0.87 | 1.00 | 1.15 | 1.40 | 0.72 | 1.26 | |
| Community C | Mean | 2.70 | 2.56 | 2.64 | 2.99 | 2.47 | 2.53 | 3.03 | 2.79 | 3.07 | 2.68 | 2.15 |
| SD | 0.49 | 0.53 | 0.67 | 0.85 | 0.73 | 0.64 | 1.08 | 0.76 | 1.05 | 1.07 | 0.71 | |
| Academic combined | Mean | 2.80 | 2.45 | 2.93 | 3.22 | 2.56 | 2.24 | 3.07 | 2.62 | 2.88 | 3.21 | 3.28 |
| SD | 0.54 | 0.63 | 0.66 | 0.89 | 0.82 | 0.72 | 1.16 | 0.95 | 1.14 | 1.10 | 1.26 | |
| Community combined | Mean | 2.79 | 2.61 | 2.66 | 3.14 | 2.60 | 2.68 | 3.03 | 2.72 | 2.95 | 2.82 | 2.34 |
| SD | 0.62 | 0.62 | 0.72 | 1.01 | 0.90 | 0.90 | 1.15 | 0.99 | 1.09 | 1.09 | 1.00 | |
| P value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | 0.02 | >0.05 | >0.05 | >0.05 | >0.05 | <0.001 | |
Convergent, Concurrent, and Discriminant Validity Evidence
For every categorical increase on the question assessing overall morale, the personal morale score was 0.23 points higher (P < 0.001). For every categorical increase in a participant's perception of the group's morale, the personal morale score was 0.29 points higher (P < 0.001).
For every 1‐point increase in personal morale score, the odds of being highly invested in the group increased by 5 times (odds ratio [OR]: 5.23, 95% confidence interval [CI]: 1.91‐14.35, P = 0.001). The mean personal morale score for highly invested hospitalists was 2.92, whereas that of those less invested was 2.43 (diff. = 0.49, P < 0.001) (Table 3). Highly invested hospitalists had significantly higher importance factor scores for leadership (diff. = 0.08, P = 0.03) as well as appreciation and acknowledgement (diff. = 0.08, P = 0.02).
| Personal Morale Score | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | Family Time | Institutional Climate | Job Security | Autonomy | Professional Growth | ||
| |||||||||||
| Highly invested in success of current hospitalist group | |||||||||||
| Mean | 2.92 | 2.61 | 2.89 | 3.38 | 2.78 | 2.45 | 3.21 | 2.78 | 2.86 | 3.10 | 2.95 |
| SD | 0.55 | 0.59 | 0.68 | 0.92 | 0.88 | 0.77 | 1.11 | 1.00 | 1.09 | 1.06 | 1.25 |
| Less invested in success of current hospitalist group | |||||||||||
| Mean | 2.43 | 2.34 | 2.48 | 2.60 | 2.02 | 2.57 | 2.60 | 2.38 | 3.08 | 2.69 | 2.24 |
| SD | 0.52 | 0.69 | 0.69 | 0.81 | 0.49 | 1.04 | 1.17 | 0.83 | 1.18 | 1.19 | 0.94 |
| P value | <0.001 | >0.05 | 0.02 | 0.001 | <0.001 | >0.05 | 0.03 | >0.05 | >0.05 | >0.05 | 0.02 |
| Not intending to leave because unhappy | |||||||||||
| Mean | 2.97 | 2.67 | 2.89 | 3.48 | 2.77 | 2.52 | 3.24 | 2.85 | 3.05 | 3.06 | 3.01 |
| SD | 0.51 | 0.54 | 0.61 | 0.91 | 0.89 | 0.78 | 1.03 | 0.99 | 1.10 | 1.07 | 1.25 |
| Intending to leave current group because unhappy | |||||||||||
| Mean | 2.45 | 2.30 | 2.59 | 2.59 | 2.21 | 2.40 | 2.68 | 2.33 | 2.67 | 2.88 | 2.28 |
| SD | 0.56 | 0.72 | 0.82 | 0.74 | 0.68 | 0.97 | 1.29 | 0.83 | 1.11 | 1.17 | 0.97 |
| P value | <0.001 | 0.01 | >0.05 | <0.001 | 0.003 | >0.05 | 0.03 | 0.01 | >0.05 | >0.05 | 0.01 |
Every 1‐point increase in personal morale was associated with a rise of 2.27 on the professional growth scale (P = 0.01). The correlation between these 2 scales was 0.26 (P = 0.01). Every 1‐point increase in personal morale was associated with a 2.21 point decrease on the Cohen stress scale (P > 0.05). The correlation between these 2 scales was 0.21 (P > 0.05).
Morale and Intent to Leave Due to Unhappiness
Sixteen (37%) academic and 18 (36%) community hospitalists reported having thoughts of leaving their current hospitalist program due to unhappiness. The mean personal morale score for hospitalists with no intent to leave their current group was 2.97, whereas that of those with intent to leave was 2.45 (diff. = 0.53, P < 0.001). Each 1‐point increase in the personal morale score was associated with an 85% decrease (OR: 0.15, 95% CI: 0.05‐0.41, P < 0.001) in the odds of leaving because of unhappiness. Holding self‐perception of being a career hospitalist constant, each 1‐point increase in the personal morale score was associated with an 83% decrease (OR: 0.17, 95% CI: 0.05‐0.51, P = 0.002) in the odds of leaving because of unhappiness. Hospitalists who reported intent to leave had significantly lower factor scores for all factors and items except workload, material reward, and autonomy than those who did not report intent to leave (Table 3). Within the academic groups, those who reported intent to leave had significantly lower scores for professional growth (diff. = 1.08, P = 0.01). For community groups, those who reported intent to leave had significantly lower scores for clinical work (diff. = 0.54, P = 0.003), workload (diff. = 0.50, P = 0.02), leadership (diff. = 1.19, P < 0.001), feeling appreciated and acknowledged (diff. = 0.68, P = 0.01), job security (diff. = 0.70, P = 0.03), and institutional climate (diff. = 0.67, P = 0.02) than those who did not report intent to leave.
DISCUSSION
The HMI is a validated tool that objectively measures and quantifies hospitalist morale. The HMI's capacity to comprehensively assess morale comes from its breadth and depth in uncovering work‐related areas that may be sources of contentment or displeasure. Furthermore, the fact that HMI scores varied among groups of individuals, including those who are thinking about leaving their hospitalist group because they are unhappy and those who are highly invested in their hospitalist group, speaks to its ability to highlight and account for what is most important to hospitalist providers.
Low employee morale has been associated with decreased productivity, increased absenteeism, increased turnover, and decreased patient satisfaction.[2, 26, 27, 28] A few frustrated workers can breed group discontentment and lower the entire group's morale.[28] In addition to its financial impact, departures due to low morale can be sudden and devastating, leading to loss of team cohesiveness, increased work burden on the remaining workforce, burnout, and cascades of more turnover.[2] In contrast, when morale is high, workers more commonly go the extra mile, are more committed to the organization's mission, and are more supportive of their coworkers.[28]
While we asked the informants about plans to leave their job, there are many factors that drive an individual's intent and ultimate decision to make changes in his or her employment. Some factors are outside the control of the employer or practice leaders, such as change in an individual's family life or desire and opportunity to pursue fellowship training. Others variables, however, are more directly tied to the job or practice environment. In a specialty where providers are relatively mobile and turnover is high, it is important for hospitalist practices to cultivate a climate in which the sacrifices associated with leaving outweigh the promised benefits.[29]
Results from the HMPWS suggested the need to address climate and fairness issues in hospitalist programs to improve satisfaction and retention.[9] Two large healthcare systems achieved success by investing in multipronged physician retention strategies including recruiting advisors, sign‐on bonuses, extensive onboarding, family support, and the promotion of ongoing effective communication.[3, 30]
Our findings suggest that morale for hospitalists is a complex amalgam of contentment and importance, and that there may not be a one size fits all solution to improving morale for all. While we did not find a difference in personal morale scores across individual hospitalist groups, or even between academic and community groups, each group had a unique profile with variability in the dynamics between importance and contentment of different factors. If practice group leaders review HMI data for their providers and use the information to facilitate meaningful dialogue with them about the factors influencing their morale, such leaders will have great insight into allocating resources for the best return on investment.
While we believe that the HMI is providing unique perspective compared to other commonly used metrics, it may be best to employ HMI data as complementary measures alongside that of some of the benchmarked scales that explore job satisfaction, job fit, and burnout among hospitalists.[6, 9, 10, 31, 32, 33, 34, 35] Aggregate HMI data at the group level may allow for the identification of factors that are highly important to morale but scored low in contentment. Such factors deserve priority and attention such that the subgroups within a practice can collaborate to come to consensus on strategies for amelioration. Because the HMI generates a score and profile for each provider, we can imagine effective leaders using the HMI with individuals as part of an annual review to facilitate discussion about maximizing contentment at work. Being fully transparent and sharing an honest nonanonymous version of the HMI with a superior would require a special relationship founded on trust and mutual respect.
Several limitations of this study should be considered. First, the initial item reduction and EFA were based on a single‐site survey, and our overall sample size was relatively small. We plan on expanding our sample size in the future for further validation of our exploratory findings. Second, the data were collected at 2 specific times several years ago. In continuing to analyze the data from subsequent years, validity and reliability results remain stable, thereby minimizing the likelihood of significant historical bias. Third, there may have been some recall bias, in that respondents may have overlooked the good and perseverated over variables that disappointed them. Fourth, although intention to leave does not necessarily equate actual employee turnover, intention has been found to be a strong predictor of quitting a job.[36, 37] Finally, while we had high response rates, response bias may have existed wherein those with lower morale may have elected not to complete the survey or became apathetic in their responses.
The HMI is a validated instrument that evaluates hospitalist morale by incorporating each provider's characterization of the importance of and contentment with 27 variables. By accounting for the multidimensional and dynamic nature of morale, the HMI may help program leaders tailor retention and engagement strategies specific to their own group. Future studies may explore trends in contributors to morale and examine whether interventions to augment low morale can result in improved morale and hospitalist retention.
Acknowledgements
The authors are indebted to the hospitalists who were willing to share their perspectives about their work, and grateful to Ms. Lisa Roberts, Ms. Barbara Brigade, and Ms. Regina Landis for insuring confidentiality in managing the survey database.
Disclosures: Dr. Chandra had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Wright is a Miller‐Coulson Family Scholar through the Johns Hopkins Center for Innovative Medicine. Ethical approval has been granted for studies involving human subjects by a Johns Hopkins University School of Medicine institutional review board. The authors report no conflicts of interest.
- 2014 State of Hospital Medicine Report. Philadelphia, PA: Society of Hospital Medicine; 2014.
- , , . A review of physician turnover: rates, causes, and consequences. Am J Med Qual. 2004;19(2):56–66.
- . Physician retention plans help reduce costs and optimize revenues. Healthc Financ Manage. 1998;52(1):75–77.
- SHM Career Satisfaction Task Force. A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction.; 2006. Available at: www.hospitalmedicine.org. Accessed February 28, 2009.
- , . Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119–139.
- , , , , . Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851–858.
- . Doing the same and earning less: male and female physicians in a new medical specialty. Inquiry. 2004;41(3):301–315.
- . Physician satisfaction and communication. National findings and best practices. Available at: http://www.pressganey.com/files/clark_cox_acpe_apr06.pdf. Accessed October 10, 2010.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28–36.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- Morale | Define Morale at Dictionary.com. Morale | Define Morale at Dictionary.com. Morale | Define Morale at Dictionary.com. Available at: http://dictionary.reference.com/browse/morale. Accessed June 5, 2014.
- . Morale and satisfaction: a study in past‐future time perspective. Adm Sci Q. 1958:195–209.
- . Men and Women of the Corporation. 2nd ed. New York, NY: Basic Books; 1993.
- . The relation of morale to turnover among teachers. Am Educ Res J. 1965:163–173.
- . Structural and individual determinants of organization morale and satisfaction. Soc Forces. 1982;61:1088.
- , , . Morale matters: midlevel administrators and their intent to leave. J Higher Educ. 2000:34–59.
- . Factors influencing employee morale. Harv Bus Rev. 1950;28(1):61–73.
- . Dimensions of teacher morale. Am Educ Res J. 1970;7(2):221.
- , . The definition and measurement of employee morale. Adm Sci Q. 1958:157–184.
- , , , et al. Measuring physician job satisfaction in a changing workplace and a challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine. Med Care. 1999;37(11):1174–1182.
- . Structural and individual determinants of organization morale and satisfaction. Soc Forces. 1983;61(4):1088–1108.
- . Morale and its measurement. Am J Sociol. 1941;47(3):406–414.
- , , , . Following morale over time within an academic hospitalist division. J Clin Outcomes Manag. 2011;18(1):21–26.
- , , , et al. Personal growth and its correlates during residency training. Med Educ. 2006;40(8):737–745.
- , , . A global measure of perceived stress. J Health Soc Behav. 1983:385–396.
- , , . Morale matters: midlevel administrators and their intent to leave. J Higher Educ. 2000;71(1):34–59.
- , . Faculty members' morale and their intention to leave: a multilevel explanation. J Higher Educ. 2002;73(4):518–542.
- , . Employee Morale. New York, NY: Palgrave Macmillan; 2009.
- , , , , . Silence Kills. Silence Kills: The Seven Crucial Conversations® for Healthcare. VitalSmarts™ in association with the American Association of Critical Care Nurses, USA. 2005. Accessed October 10, 2014.
- , , . The lifelong iterative process of physician retention. J Healthc Manag. 2009;54(4):220–226.
- . Physicians' burnout. Rev Prat. 2004;54(7):753–754.
- , , , , , . Work stress and health in primary health care physicians and hospital physicians. Occup Environ Med. 2008;65(5):364–366.
- , , , et al. Understanding physicians' intentions to withdraw from practice: the role of job satisfaction, job stress, mental and physical health. 2001. Health Care Manage Rev. 2010;35(2):105–115.
- , , , , , . Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358–1367.
- , . Burnout and Hospitalists: Etiology and Prevention. In: What Exactly Does A Hospitalist Do? Best of the Best Hospital Medicine 2005: Strategies for Success. Society of Hospital Medicine; 2005:5.
- , . Using job involvement and organizational commitment interactively to predict turnover. J Manage. 1989;15(1):115–127.
- , , , et al. Nurse turnover: a literature review. Int J Nurs Stud. 2006;43(2):237–263.
Explosive growth in hospital medicine has led to hospitalists having the option to change jobs easily. Annual turnover for all physicians is 6.8%, whereas that of hospitalists exceeds 14.8%.[1] Losing a single physician has significant financial and operational implications, with estimates of $20,000 to $120,000 in recruiting costs, and up to $500,000 in lost revenue that may take years to recoup due to the time required for new physician assimilation.[2, 3] In 2006, the Society of Hospital Medicine (SHM) appointed a career task force to develop retention recommendations, 1 of which includes monitoring hospitalists' job satisfaction.[4]
Studies examining physician satisfaction have demonstrated that high physician job satisfaction is associated with lower physician turnover.[5] However, surveys of hospitalists, including SHM's Hospital Medicine Physician Worklife Survey (HMPWS), have reported high job satisfaction among hospitalists,[6, 7, 8, 9, 10] suggesting that high job satisfaction may not be enough to overcome forces that pull hospitalists toward other opportunities.
Morale, a more complex construct related to an individual's contentment and happiness, might provide insight into reducing hospitalist turnover. Morale has been defined as the emotional or mental condition with respect to cheerfulness, confidence, or zeal and is especially relevant in the face of opposition or hardship.[11] Job satisfaction is 1 element that contributes to morale, but alone does not equate morale.[12] Morale, more than satisfaction, relates to how people see themselves within the group and may be closely tied to the concept of esprit de corps. To illustrate, workers may feel satisfied with the content of their job, but frustration with the organization may result in low morale.[13] Efforts focused on assessing provider morale may provide deeper understanding of hospitalists' professional needs and garner insight for retention strategies.
The construct of hospitalist morale and its underlying drivers has not been explored in the literature. Using literature within and outside of healthcare,[1, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22] and our own prior work,[23] we sought to characterize elements that contribute to hospitalist morale and develop a metric to measure it. The HMPWS found that job satisfaction factors vary across hospitalist groups.[9] We suspected that the same would hold true for factors important to morale at the individual level. This study describes the development and validation of the Hospitalist Morale Index (HMI), and explores the relationship between morale and intent to leave due to unhappiness.
METHODS
2009 Pilot Survey
To establish content validity, after reviewing employee morale literature, and examining qualitative comments from our 2007 and 2008 morale surveys, our expert panel, consisting of practicing hospitalists, hospitalist leaders, and administrative staff, identified 46 potential drivers of hospitalist morale. In May 2009, all hospitalists, including physicians, nurse practitioners (NPs), and physician assistants (PAs) from a single hospitalist group received invitations to complete the pilot survey. We asked hospitalists to assess on 5‐point Likert scales the importance of (not at all to tremendously) and contentment with (extremely discontent to extremely content) each of the 46 items as it relates to their work morale. Also included were demographic questions and general morale questions (including rating participants' own morale), investment, long‐term career plans, and intent to leave due to unhappiness.
Data Collection
To maintain anonymity and limit social desirability bias, a database manager, working outside the Division of Hospital Medicine and otherwise not associated with the research team, used Survey Monkey to coordinate survey distribution and data collection. Each respondent had a unique identifier code that was unrelated to the respondent's name and email address. Personal identifiers were maintained in a secure database accessible only to the database manager.
Establishing Internal Structure Validity Evidence
Response frequency to each question was examined for irregularities in distribution. For continuous variables, descriptive statistics were examined for evidence of skewness, outliers, and non‐normality to ensure appropriate use of parametric statistical tests. Upon ranking importance ratings by mode, 15 of 46 items were judged to be of low importance by almost all participants and removed from further consideration.
Stata 13.1 (StataCorp, College Station, TX) was used for exploratory factor analysis (EFA) of the importance responses for all 31 remaining items by principal components factoring. Eigenvalues >1 were designated as a cutoff point for inclusion in varimax rotation. Factor loading of 0.50 was the threshold for inclusion in a factor.
The 31 items loaded across 10 factors; however, 3 factors included 1 item each. After reviewing the scree plot and considering their face value, these items/factors were omitted. Repeating the factor analysis resulted in a 28‐item, 7‐factor solution that accounted for 75% variance. All items were considered informative as demonstrated by low uniqueness scores (0.050.38). Using standard validation procedures, all 7 factors were found to have acceptable factor loadings (0.460.98) and face validity. Cronbach's quantified internal reliability of the 7 factors with scores ranging from 0.68 to 0.92. We named the resultant solution the Hospitalist Morale Index (HMI).
Establishing Response Process Validity Evidence
In developing the HMI, we asked respondents to rate the importance of and their contentment with each variable as related to their work morale. From pilot testing, which included discussions with respondents immediately after completing the survey, we learned that the 2‐part consideration of each variable resulted in thoughtful reflection about their morale. Further, by multiplying the contentment score for each item (scaled from 15) by the corresponding importance score (scaled 01), we quantified the relative contribution and contentment of each item for each hospitalist. Scaling importance scores from 0 to 1 insured that items that were not considered important to the respondent did not affect the respondent's personal morale score. Averaging resultant item scores that were greater than 0 resulted in a personal morale score for each hospitalist. Averaging item scores >0 that constituted each factor resulted in factor scores.
May 2011 Survey
The refined survey was distributed in May 2011 to a convenience sample of 5 hospitalist programs at separate hospitals (3 community hospitals, 2 academic hospitals) encompassing 108 hospitalists in 3 different states. Responses to the 2011 survey were used to complete confirmatory factor analyses (CFA) and establish further validity and reliability evidence.
Based on the 28‐item, 7‐factor solution developed from the pilot study, we developed the theoretical model of factors constituting hospitalist morale. We used the structural equation modeling command in Stata 13 to perform CFA. Factor loading of 0.50 was the threshold for inclusion of an item in a factor. To measure internal consistency, we considered Cronbach's score of 0.60 acceptable. Iterative models were reviewed to find the optimal solution for the data. Four items did not fit into any of the 5 resulting factors and were evaluated in terms of mean importance score and face value. Three items were considered important enough to warrant being stand‐alone items, whereas 1 was omitted. Two additional items had borderline factor loadings (0.48, 0.49) and were included in the model as stand‐alone items due to their overall relevance. The resultant solution was a 5‐factor model with 5 additional stand‐alone items (Table 1).
| Factor | Cronbach's | |||||
|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | ||
| How much does the following item contribute to your morale? | ||||||
| Paperwork | 0.72 | 0.89 | ||||
| Relationship with patients | 0.69 | 0.90 | ||||
| Electronic medical system | 0.60 | 0.90 | ||||
| Intellectual stimulation | 0.59 | 0.90 | ||||
| Variety of cases | 0.58 | 0.90 | ||||
| Relationship with consultants | 0.51 | 0.89 | ||||
| No. of night shifts | 0.74 | 0.89 | ||||
| Patient census | 0.61 | 0.90 | ||||
| No. of shifts | 0.52 | 0.90 | ||||
| Fairness of leadership | 0.82 | 0.89 | ||||
| Effectiveness of leadership | 0.82 | 0.89 | ||||
| Leadership's receptiveness to my thoughts and suggestions | 0.78 | 0.89 | ||||
| Leadership as advocate for my needs | 0.77 | 0.89 | ||||
| Approachability of leadership | 0.77 | 0.89 | ||||
| Accessibility of leadership | 0.69 | 0.89 | ||||
| Alignment of the group's goals with my goals | 0.50 | 0.89 | ||||
| Recognition within the group | 0.82 | 0.89 | ||||
| Feeling valued within the institution | 0.73 | 0.89 | ||||
| Feeling valued within the group | 0.73 | 0.89 | ||||
| Feedback | 0.52 | 0.89 | ||||
| Pay | 0.99 | 0.90 | ||||
| Benefits | 0.56 | 0.89 | ||||
| Cronbach's | 0.78 | 0.65 | 0.89 | 0.78 | 0.71 | |
| How much does the following item contribute to your morale? | Single item indicators | |||||
| Family time | 0.90 | |||||
| Job security | 0.90 | |||||
| Institutional climate | 0.89 | |||||
| Opportunities for professional growth | 0.90 | |||||
| Autonomy | 0.89 | |||||
| Cronbach's | 0.90 | |||||
Establishing Convergent, Concurrent, and Discriminant Validity Evidence
To establish convergent, concurrent, and discriminant validity, linear and logistic regression models were examined for continuous and categorical data accordingly.
Self‐perceived overall work morale and perceived group morale, as assessed by 6‐point Likert questions with response options from terrible to excellent, were modeled as predictors for personal morale as calculated by the HMI.
Personal morale scores were modeled as predictors of professional growth, stress, investment in the group, and intent to leave due to unhappiness. While completing the HMI, hospitalists simultaneously completed a validated professional growth scale[24] and Cohen stress scale.[25] We hypothesized that those with higher morale would have more professional growth. Stress, although an important issue in the workplace, is a distinct construct from morale, and we did not expect a significant relationship between personal morale and stress. We used Pearson's r to assess the strength of association between the HMI and these scales. Participants' level of investment in their group was assessed on a 5‐point Likert scale. To simplify presentation, highly invested represents those claiming to be very or tremendously invested in the success of their current hospitalist group. Intent to leave due to unhappiness was assessed on a 5‐point Likert scale, I have had serious thoughts about leaving my current hospitalist group because I am unhappy, with responses from strongly disagree (1) to strongly agree (5). To simplify presentation, responses higher than 2 are considered to be consistent with intending to leave due to unhappiness.
Our institutional review board approved the study.
RESULTS
Respondents
In May 2009, 30 of the 33 (91%) invited hospitalists completed the original pilot morale survey; 19 (63%) were women. Eleven hospitalists (37%) had been part of the group 1 year or less, whereas 4 (13%) had been with the group for more than 5 years.
In May 2011, 93 of the 108 (86%) hospitalists from 5 hospitals completed the demographic and global parts of the survey. Fifty (53%) were from community hospitals; 47 (51%) were women. Thirty‐seven (40%) physicians and 6 (60%) NPs/PAs were from academic hospitals. Thirty‐nine hospitalists (42%) had been with their current group 1 year or less. Ten hospitalists (11%) had been with their current group over 5 years. Sixty‐three respondents (68%) considered themselves career hospitalists, whereas 5 (5%) did not; the rest were undecided.
Internal Structure Validity Evidence
The final CFA from the 2011 survey resulted in a 5‐factor plus 5stand‐alone‐items HMI. The solution with item‐level and factor‐level Cronbach's scores (range, 0.890.90 and range, 0.650.89, respectively) are shown in Table 1.
Personal Morale Scores and Factor Scores
Personal morale scores were normally distributed (mean = 2.79; standard deviation [SD] = 0.58), ranging from 1.23 to 4.22, with a theoretical low of 0 and high of 5 (Figure 1). Mean personal morale scores across hospitalist groups ranged from 2.70 to 2.99 (P > 0.05). Personal morale scores, factor sores and item scores for NPs and PAs did not significantly differ from those of physicians (P > 0.05 for all analyses). Personal morale scores were lower for those in their first 3 years with their current group, compared to those with greater institutional longevity. For every categorical increase in a participant's response to seeing oneself as a career hospitalist, the personal morale score rose 0.23 points (P < 0.001).

Factor scores for material reward and mean item scores for professional growth were significantly different across the 5 hospitalist groups (P = 0.03 and P < 0.001, respectively). Community hospitalists had significantly higher factor scores, despite having similar importance scores, for material rewards than academic hospitalists (diff. = 0.44, P = 0.02). Academic hospitalists had significantly higher scores for professional growth (diff. = 0.94, P < 0.001) (Table 2). Professional growth had the highest importance score for academic hospitalists (mean = 0.87, SD = 0.18) and the lowest importance score for community hospitalists (mean = 0.65, SD = 0.24, P < 0.001).
| Personal Morale Score | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | Family Time | Institutional Climate | Job Security | Autonomy | Professional Growth | |||
| ||||||||||||
| All participants | Mean | 2.79 | 2.54 | 2.78 | 3.18 | 2.58 | 2.48 | 3.05 | 2.67 | 2.92 | 3.00 | 2.76 |
| SD | 0.58 | 0.63 | 0.70 | 0.95 | 0.86 | 0.85 | 1.15 | 0.97 | 1.11 | 1.10 | 1.21 | |
| Academic A | Mean | 2.77 | 2.43 | 2.92 | 3.10 | 2.54 | 2.28 | 3.16 | 2.70 | 3.06 | 3.20 | 3.08 |
| SD | 0.57 | 0.62 | 0.64 | 0.92 | 0.84 | 0.77 | 1.19 | 0.95 | 1.08 | 1.12 | 1.24 | |
| Academic B | Mean | 2.99 | 2.58 | 2.99 | 3.88 | 2.69 | 2.00 | 2.58 | 2.13 | 1.65 | 3.29 | 4.33 |
| SD | 0.36 | 0.70 | 0.80 | 0.29 | 0.80 | 0.35 | 0.92 | 0.88 | 0.78 | 1.01 | 0.82 | |
| Community A | Mean | 2.86 | 2.61 | 2.51 | 3.23 | 2.73 | 3.03 | 2.88 | 2.84 | 2.95 | 3.23 | 2.66 |
| SD | 0.75 | 0.79 | 0.68 | 1.21 | 1.11 | 1.14 | 1.37 | 1.17 | 0.98 | 1.24 | 1.15 | |
| Community B | Mean | 2.86 | 2.74 | 2.97 | 3.37 | 2.67 | 2.44 | 3.28 | 2.35 | 2.70 | 2.50 | 2.25 |
| SD | 0.67 | 0.55 | 0.86 | 1.04 | 0.94 | 0.87 | 1.00 | 1.15 | 1.40 | 0.72 | 1.26 | |
| Community C | Mean | 2.70 | 2.56 | 2.64 | 2.99 | 2.47 | 2.53 | 3.03 | 2.79 | 3.07 | 2.68 | 2.15 |
| SD | 0.49 | 0.53 | 0.67 | 0.85 | 0.73 | 0.64 | 1.08 | 0.76 | 1.05 | 1.07 | 0.71 | |
| Academic combined | Mean | 2.80 | 2.45 | 2.93 | 3.22 | 2.56 | 2.24 | 3.07 | 2.62 | 2.88 | 3.21 | 3.28 |
| SD | 0.54 | 0.63 | 0.66 | 0.89 | 0.82 | 0.72 | 1.16 | 0.95 | 1.14 | 1.10 | 1.26 | |
| Community combined | Mean | 2.79 | 2.61 | 2.66 | 3.14 | 2.60 | 2.68 | 3.03 | 2.72 | 2.95 | 2.82 | 2.34 |
| SD | 0.62 | 0.62 | 0.72 | 1.01 | 0.90 | 0.90 | 1.15 | 0.99 | 1.09 | 1.09 | 1.00 | |
| P value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | 0.02 | >0.05 | >0.05 | >0.05 | >0.05 | <0.001 | |
Convergent, Concurrent, and Discriminant Validity Evidence
For every categorical increase on the question assessing overall morale, the personal morale score was 0.23 points higher (P < 0.001). For every categorical increase in a participant's perception of the group's morale, the personal morale score was 0.29 points higher (P < 0.001).
For every 1‐point increase in personal morale score, the odds of being highly invested in the group increased by 5 times (odds ratio [OR]: 5.23, 95% confidence interval [CI]: 1.91‐14.35, P = 0.001). The mean personal morale score for highly invested hospitalists was 2.92, whereas that of those less invested was 2.43 (diff. = 0.49, P < 0.001) (Table 3). Highly invested hospitalists had significantly higher importance factor scores for leadership (diff. = 0.08, P = 0.03) as well as appreciation and acknowledgement (diff. = 0.08, P = 0.02).
| Personal Morale Score | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | Family Time | Institutional Climate | Job Security | Autonomy | Professional Growth | ||
| |||||||||||
| Highly invested in success of current hospitalist group | |||||||||||
| Mean | 2.92 | 2.61 | 2.89 | 3.38 | 2.78 | 2.45 | 3.21 | 2.78 | 2.86 | 3.10 | 2.95 |
| SD | 0.55 | 0.59 | 0.68 | 0.92 | 0.88 | 0.77 | 1.11 | 1.00 | 1.09 | 1.06 | 1.25 |
| Less invested in success of current hospitalist group | |||||||||||
| Mean | 2.43 | 2.34 | 2.48 | 2.60 | 2.02 | 2.57 | 2.60 | 2.38 | 3.08 | 2.69 | 2.24 |
| SD | 0.52 | 0.69 | 0.69 | 0.81 | 0.49 | 1.04 | 1.17 | 0.83 | 1.18 | 1.19 | 0.94 |
| P value | <0.001 | >0.05 | 0.02 | 0.001 | <0.001 | >0.05 | 0.03 | >0.05 | >0.05 | >0.05 | 0.02 |
| Not intending to leave because unhappy | |||||||||||
| Mean | 2.97 | 2.67 | 2.89 | 3.48 | 2.77 | 2.52 | 3.24 | 2.85 | 3.05 | 3.06 | 3.01 |
| SD | 0.51 | 0.54 | 0.61 | 0.91 | 0.89 | 0.78 | 1.03 | 0.99 | 1.10 | 1.07 | 1.25 |
| Intending to leave current group because unhappy | |||||||||||
| Mean | 2.45 | 2.30 | 2.59 | 2.59 | 2.21 | 2.40 | 2.68 | 2.33 | 2.67 | 2.88 | 2.28 |
| SD | 0.56 | 0.72 | 0.82 | 0.74 | 0.68 | 0.97 | 1.29 | 0.83 | 1.11 | 1.17 | 0.97 |
| P value | <0.001 | 0.01 | >0.05 | <0.001 | 0.003 | >0.05 | 0.03 | 0.01 | >0.05 | >0.05 | 0.01 |
Every 1‐point increase in personal morale was associated with a rise of 2.27 on the professional growth scale (P = 0.01). The correlation between these 2 scales was 0.26 (P = 0.01). Every 1‐point increase in personal morale was associated with a 2.21 point decrease on the Cohen stress scale (P > 0.05). The correlation between these 2 scales was 0.21 (P > 0.05).
Morale and Intent to Leave Due to Unhappiness
Sixteen (37%) academic and 18 (36%) community hospitalists reported having thoughts of leaving their current hospitalist program due to unhappiness. The mean personal morale score for hospitalists with no intent to leave their current group was 2.97, whereas that of those with intent to leave was 2.45 (diff. = 0.53, P < 0.001). Each 1‐point increase in the personal morale score was associated with an 85% decrease (OR: 0.15, 95% CI: 0.05‐0.41, P < 0.001) in the odds of leaving because of unhappiness. Holding self‐perception of being a career hospitalist constant, each 1‐point increase in the personal morale score was associated with an 83% decrease (OR: 0.17, 95% CI: 0.05‐0.51, P = 0.002) in the odds of leaving because of unhappiness. Hospitalists who reported intent to leave had significantly lower factor scores for all factors and items except workload, material reward, and autonomy than those who did not report intent to leave (Table 3). Within the academic groups, those who reported intent to leave had significantly lower scores for professional growth (diff. = 1.08, P = 0.01). For community groups, those who reported intent to leave had significantly lower scores for clinical work (diff. = 0.54, P = 0.003), workload (diff. = 0.50, P = 0.02), leadership (diff. = 1.19, P < 0.001), feeling appreciated and acknowledged (diff. = 0.68, P = 0.01), job security (diff. = 0.70, P = 0.03), and institutional climate (diff. = 0.67, P = 0.02) than those who did not report intent to leave.
DISCUSSION
The HMI is a validated tool that objectively measures and quantifies hospitalist morale. The HMI's capacity to comprehensively assess morale comes from its breadth and depth in uncovering work‐related areas that may be sources of contentment or displeasure. Furthermore, the fact that HMI scores varied among groups of individuals, including those who are thinking about leaving their hospitalist group because they are unhappy and those who are highly invested in their hospitalist group, speaks to its ability to highlight and account for what is most important to hospitalist providers.
Low employee morale has been associated with decreased productivity, increased absenteeism, increased turnover, and decreased patient satisfaction.[2, 26, 27, 28] A few frustrated workers can breed group discontentment and lower the entire group's morale.[28] In addition to its financial impact, departures due to low morale can be sudden and devastating, leading to loss of team cohesiveness, increased work burden on the remaining workforce, burnout, and cascades of more turnover.[2] In contrast, when morale is high, workers more commonly go the extra mile, are more committed to the organization's mission, and are more supportive of their coworkers.[28]
While we asked the informants about plans to leave their job, there are many factors that drive an individual's intent and ultimate decision to make changes in his or her employment. Some factors are outside the control of the employer or practice leaders, such as change in an individual's family life or desire and opportunity to pursue fellowship training. Others variables, however, are more directly tied to the job or practice environment. In a specialty where providers are relatively mobile and turnover is high, it is important for hospitalist practices to cultivate a climate in which the sacrifices associated with leaving outweigh the promised benefits.[29]
Results from the HMPWS suggested the need to address climate and fairness issues in hospitalist programs to improve satisfaction and retention.[9] Two large healthcare systems achieved success by investing in multipronged physician retention strategies including recruiting advisors, sign‐on bonuses, extensive onboarding, family support, and the promotion of ongoing effective communication.[3, 30]
Our findings suggest that morale for hospitalists is a complex amalgam of contentment and importance, and that there may not be a one size fits all solution to improving morale for all. While we did not find a difference in personal morale scores across individual hospitalist groups, or even between academic and community groups, each group had a unique profile with variability in the dynamics between importance and contentment of different factors. If practice group leaders review HMI data for their providers and use the information to facilitate meaningful dialogue with them about the factors influencing their morale, such leaders will have great insight into allocating resources for the best return on investment.
While we believe that the HMI is providing unique perspective compared to other commonly used metrics, it may be best to employ HMI data as complementary measures alongside that of some of the benchmarked scales that explore job satisfaction, job fit, and burnout among hospitalists.[6, 9, 10, 31, 32, 33, 34, 35] Aggregate HMI data at the group level may allow for the identification of factors that are highly important to morale but scored low in contentment. Such factors deserve priority and attention such that the subgroups within a practice can collaborate to come to consensus on strategies for amelioration. Because the HMI generates a score and profile for each provider, we can imagine effective leaders using the HMI with individuals as part of an annual review to facilitate discussion about maximizing contentment at work. Being fully transparent and sharing an honest nonanonymous version of the HMI with a superior would require a special relationship founded on trust and mutual respect.
Several limitations of this study should be considered. First, the initial item reduction and EFA were based on a single‐site survey, and our overall sample size was relatively small. We plan on expanding our sample size in the future for further validation of our exploratory findings. Second, the data were collected at 2 specific times several years ago. In continuing to analyze the data from subsequent years, validity and reliability results remain stable, thereby minimizing the likelihood of significant historical bias. Third, there may have been some recall bias, in that respondents may have overlooked the good and perseverated over variables that disappointed them. Fourth, although intention to leave does not necessarily equate actual employee turnover, intention has been found to be a strong predictor of quitting a job.[36, 37] Finally, while we had high response rates, response bias may have existed wherein those with lower morale may have elected not to complete the survey or became apathetic in their responses.
The HMI is a validated instrument that evaluates hospitalist morale by incorporating each provider's characterization of the importance of and contentment with 27 variables. By accounting for the multidimensional and dynamic nature of morale, the HMI may help program leaders tailor retention and engagement strategies specific to their own group. Future studies may explore trends in contributors to morale and examine whether interventions to augment low morale can result in improved morale and hospitalist retention.
Acknowledgements
The authors are indebted to the hospitalists who were willing to share their perspectives about their work, and grateful to Ms. Lisa Roberts, Ms. Barbara Brigade, and Ms. Regina Landis for insuring confidentiality in managing the survey database.
Disclosures: Dr. Chandra had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Wright is a Miller‐Coulson Family Scholar through the Johns Hopkins Center for Innovative Medicine. Ethical approval has been granted for studies involving human subjects by a Johns Hopkins University School of Medicine institutional review board. The authors report no conflicts of interest.
Explosive growth in hospital medicine has led to hospitalists having the option to change jobs easily. Annual turnover for all physicians is 6.8%, whereas that of hospitalists exceeds 14.8%.[1] Losing a single physician has significant financial and operational implications, with estimates of $20,000 to $120,000 in recruiting costs, and up to $500,000 in lost revenue that may take years to recoup due to the time required for new physician assimilation.[2, 3] In 2006, the Society of Hospital Medicine (SHM) appointed a career task force to develop retention recommendations, 1 of which includes monitoring hospitalists' job satisfaction.[4]
Studies examining physician satisfaction have demonstrated that high physician job satisfaction is associated with lower physician turnover.[5] However, surveys of hospitalists, including SHM's Hospital Medicine Physician Worklife Survey (HMPWS), have reported high job satisfaction among hospitalists,[6, 7, 8, 9, 10] suggesting that high job satisfaction may not be enough to overcome forces that pull hospitalists toward other opportunities.
Morale, a more complex construct related to an individual's contentment and happiness, might provide insight into reducing hospitalist turnover. Morale has been defined as the emotional or mental condition with respect to cheerfulness, confidence, or zeal and is especially relevant in the face of opposition or hardship.[11] Job satisfaction is 1 element that contributes to morale, but alone does not equate morale.[12] Morale, more than satisfaction, relates to how people see themselves within the group and may be closely tied to the concept of esprit de corps. To illustrate, workers may feel satisfied with the content of their job, but frustration with the organization may result in low morale.[13] Efforts focused on assessing provider morale may provide deeper understanding of hospitalists' professional needs and garner insight for retention strategies.
The construct of hospitalist morale and its underlying drivers has not been explored in the literature. Using literature within and outside of healthcare,[1, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22] and our own prior work,[23] we sought to characterize elements that contribute to hospitalist morale and develop a metric to measure it. The HMPWS found that job satisfaction factors vary across hospitalist groups.[9] We suspected that the same would hold true for factors important to morale at the individual level. This study describes the development and validation of the Hospitalist Morale Index (HMI), and explores the relationship between morale and intent to leave due to unhappiness.
METHODS
2009 Pilot Survey
To establish content validity, after reviewing employee morale literature, and examining qualitative comments from our 2007 and 2008 morale surveys, our expert panel, consisting of practicing hospitalists, hospitalist leaders, and administrative staff, identified 46 potential drivers of hospitalist morale. In May 2009, all hospitalists, including physicians, nurse practitioners (NPs), and physician assistants (PAs) from a single hospitalist group received invitations to complete the pilot survey. We asked hospitalists to assess on 5‐point Likert scales the importance of (not at all to tremendously) and contentment with (extremely discontent to extremely content) each of the 46 items as it relates to their work morale. Also included were demographic questions and general morale questions (including rating participants' own morale), investment, long‐term career plans, and intent to leave due to unhappiness.
Data Collection
To maintain anonymity and limit social desirability bias, a database manager, working outside the Division of Hospital Medicine and otherwise not associated with the research team, used Survey Monkey to coordinate survey distribution and data collection. Each respondent had a unique identifier code that was unrelated to the respondent's name and email address. Personal identifiers were maintained in a secure database accessible only to the database manager.
Establishing Internal Structure Validity Evidence
Response frequency to each question was examined for irregularities in distribution. For continuous variables, descriptive statistics were examined for evidence of skewness, outliers, and non‐normality to ensure appropriate use of parametric statistical tests. Upon ranking importance ratings by mode, 15 of 46 items were judged to be of low importance by almost all participants and removed from further consideration.
Stata 13.1 (StataCorp, College Station, TX) was used for exploratory factor analysis (EFA) of the importance responses for all 31 remaining items by principal components factoring. Eigenvalues >1 were designated as a cutoff point for inclusion in varimax rotation. Factor loading of 0.50 was the threshold for inclusion in a factor.
The 31 items loaded across 10 factors; however, 3 factors included 1 item each. After reviewing the scree plot and considering their face value, these items/factors were omitted. Repeating the factor analysis resulted in a 28‐item, 7‐factor solution that accounted for 75% variance. All items were considered informative as demonstrated by low uniqueness scores (0.050.38). Using standard validation procedures, all 7 factors were found to have acceptable factor loadings (0.460.98) and face validity. Cronbach's quantified internal reliability of the 7 factors with scores ranging from 0.68 to 0.92. We named the resultant solution the Hospitalist Morale Index (HMI).
Establishing Response Process Validity Evidence
In developing the HMI, we asked respondents to rate the importance of and their contentment with each variable as related to their work morale. From pilot testing, which included discussions with respondents immediately after completing the survey, we learned that the 2‐part consideration of each variable resulted in thoughtful reflection about their morale. Further, by multiplying the contentment score for each item (scaled from 15) by the corresponding importance score (scaled 01), we quantified the relative contribution and contentment of each item for each hospitalist. Scaling importance scores from 0 to 1 insured that items that were not considered important to the respondent did not affect the respondent's personal morale score. Averaging resultant item scores that were greater than 0 resulted in a personal morale score for each hospitalist. Averaging item scores >0 that constituted each factor resulted in factor scores.
May 2011 Survey
The refined survey was distributed in May 2011 to a convenience sample of 5 hospitalist programs at separate hospitals (3 community hospitals, 2 academic hospitals) encompassing 108 hospitalists in 3 different states. Responses to the 2011 survey were used to complete confirmatory factor analyses (CFA) and establish further validity and reliability evidence.
Based on the 28‐item, 7‐factor solution developed from the pilot study, we developed the theoretical model of factors constituting hospitalist morale. We used the structural equation modeling command in Stata 13 to perform CFA. Factor loading of 0.50 was the threshold for inclusion of an item in a factor. To measure internal consistency, we considered Cronbach's score of 0.60 acceptable. Iterative models were reviewed to find the optimal solution for the data. Four items did not fit into any of the 5 resulting factors and were evaluated in terms of mean importance score and face value. Three items were considered important enough to warrant being stand‐alone items, whereas 1 was omitted. Two additional items had borderline factor loadings (0.48, 0.49) and were included in the model as stand‐alone items due to their overall relevance. The resultant solution was a 5‐factor model with 5 additional stand‐alone items (Table 1).
| Factor | Cronbach's | |||||
|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | ||
| How much does the following item contribute to your morale? | ||||||
| Paperwork | 0.72 | 0.89 | ||||
| Relationship with patients | 0.69 | 0.90 | ||||
| Electronic medical system | 0.60 | 0.90 | ||||
| Intellectual stimulation | 0.59 | 0.90 | ||||
| Variety of cases | 0.58 | 0.90 | ||||
| Relationship with consultants | 0.51 | 0.89 | ||||
| No. of night shifts | 0.74 | 0.89 | ||||
| Patient census | 0.61 | 0.90 | ||||
| No. of shifts | 0.52 | 0.90 | ||||
| Fairness of leadership | 0.82 | 0.89 | ||||
| Effectiveness of leadership | 0.82 | 0.89 | ||||
| Leadership's receptiveness to my thoughts and suggestions | 0.78 | 0.89 | ||||
| Leadership as advocate for my needs | 0.77 | 0.89 | ||||
| Approachability of leadership | 0.77 | 0.89 | ||||
| Accessibility of leadership | 0.69 | 0.89 | ||||
| Alignment of the group's goals with my goals | 0.50 | 0.89 | ||||
| Recognition within the group | 0.82 | 0.89 | ||||
| Feeling valued within the institution | 0.73 | 0.89 | ||||
| Feeling valued within the group | 0.73 | 0.89 | ||||
| Feedback | 0.52 | 0.89 | ||||
| Pay | 0.99 | 0.90 | ||||
| Benefits | 0.56 | 0.89 | ||||
| Cronbach's | 0.78 | 0.65 | 0.89 | 0.78 | 0.71 | |
| How much does the following item contribute to your morale? | Single item indicators | |||||
| Family time | 0.90 | |||||
| Job security | 0.90 | |||||
| Institutional climate | 0.89 | |||||
| Opportunities for professional growth | 0.90 | |||||
| Autonomy | 0.89 | |||||
| Cronbach's | 0.90 | |||||
Establishing Convergent, Concurrent, and Discriminant Validity Evidence
To establish convergent, concurrent, and discriminant validity, linear and logistic regression models were examined for continuous and categorical data accordingly.
Self‐perceived overall work morale and perceived group morale, as assessed by 6‐point Likert questions with response options from terrible to excellent, were modeled as predictors for personal morale as calculated by the HMI.
Personal morale scores were modeled as predictors of professional growth, stress, investment in the group, and intent to leave due to unhappiness. While completing the HMI, hospitalists simultaneously completed a validated professional growth scale[24] and Cohen stress scale.[25] We hypothesized that those with higher morale would have more professional growth. Stress, although an important issue in the workplace, is a distinct construct from morale, and we did not expect a significant relationship between personal morale and stress. We used Pearson's r to assess the strength of association between the HMI and these scales. Participants' level of investment in their group was assessed on a 5‐point Likert scale. To simplify presentation, highly invested represents those claiming to be very or tremendously invested in the success of their current hospitalist group. Intent to leave due to unhappiness was assessed on a 5‐point Likert scale, I have had serious thoughts about leaving my current hospitalist group because I am unhappy, with responses from strongly disagree (1) to strongly agree (5). To simplify presentation, responses higher than 2 are considered to be consistent with intending to leave due to unhappiness.
Our institutional review board approved the study.
RESULTS
Respondents
In May 2009, 30 of the 33 (91%) invited hospitalists completed the original pilot morale survey; 19 (63%) were women. Eleven hospitalists (37%) had been part of the group 1 year or less, whereas 4 (13%) had been with the group for more than 5 years.
In May 2011, 93 of the 108 (86%) hospitalists from 5 hospitals completed the demographic and global parts of the survey. Fifty (53%) were from community hospitals; 47 (51%) were women. Thirty‐seven (40%) physicians and 6 (60%) NPs/PAs were from academic hospitals. Thirty‐nine hospitalists (42%) had been with their current group 1 year or less. Ten hospitalists (11%) had been with their current group over 5 years. Sixty‐three respondents (68%) considered themselves career hospitalists, whereas 5 (5%) did not; the rest were undecided.
Internal Structure Validity Evidence
The final CFA from the 2011 survey resulted in a 5‐factor plus 5stand‐alone‐items HMI. The solution with item‐level and factor‐level Cronbach's scores (range, 0.890.90 and range, 0.650.89, respectively) are shown in Table 1.
Personal Morale Scores and Factor Scores
Personal morale scores were normally distributed (mean = 2.79; standard deviation [SD] = 0.58), ranging from 1.23 to 4.22, with a theoretical low of 0 and high of 5 (Figure 1). Mean personal morale scores across hospitalist groups ranged from 2.70 to 2.99 (P > 0.05). Personal morale scores, factor sores and item scores for NPs and PAs did not significantly differ from those of physicians (P > 0.05 for all analyses). Personal morale scores were lower for those in their first 3 years with their current group, compared to those with greater institutional longevity. For every categorical increase in a participant's response to seeing oneself as a career hospitalist, the personal morale score rose 0.23 points (P < 0.001).

Factor scores for material reward and mean item scores for professional growth were significantly different across the 5 hospitalist groups (P = 0.03 and P < 0.001, respectively). Community hospitalists had significantly higher factor scores, despite having similar importance scores, for material rewards than academic hospitalists (diff. = 0.44, P = 0.02). Academic hospitalists had significantly higher scores for professional growth (diff. = 0.94, P < 0.001) (Table 2). Professional growth had the highest importance score for academic hospitalists (mean = 0.87, SD = 0.18) and the lowest importance score for community hospitalists (mean = 0.65, SD = 0.24, P < 0.001).
| Personal Morale Score | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | Family Time | Institutional Climate | Job Security | Autonomy | Professional Growth | |||
| ||||||||||||
| All participants | Mean | 2.79 | 2.54 | 2.78 | 3.18 | 2.58 | 2.48 | 3.05 | 2.67 | 2.92 | 3.00 | 2.76 |
| SD | 0.58 | 0.63 | 0.70 | 0.95 | 0.86 | 0.85 | 1.15 | 0.97 | 1.11 | 1.10 | 1.21 | |
| Academic A | Mean | 2.77 | 2.43 | 2.92 | 3.10 | 2.54 | 2.28 | 3.16 | 2.70 | 3.06 | 3.20 | 3.08 |
| SD | 0.57 | 0.62 | 0.64 | 0.92 | 0.84 | 0.77 | 1.19 | 0.95 | 1.08 | 1.12 | 1.24 | |
| Academic B | Mean | 2.99 | 2.58 | 2.99 | 3.88 | 2.69 | 2.00 | 2.58 | 2.13 | 1.65 | 3.29 | 4.33 |
| SD | 0.36 | 0.70 | 0.80 | 0.29 | 0.80 | 0.35 | 0.92 | 0.88 | 0.78 | 1.01 | 0.82 | |
| Community A | Mean | 2.86 | 2.61 | 2.51 | 3.23 | 2.73 | 3.03 | 2.88 | 2.84 | 2.95 | 3.23 | 2.66 |
| SD | 0.75 | 0.79 | 0.68 | 1.21 | 1.11 | 1.14 | 1.37 | 1.17 | 0.98 | 1.24 | 1.15 | |
| Community B | Mean | 2.86 | 2.74 | 2.97 | 3.37 | 2.67 | 2.44 | 3.28 | 2.35 | 2.70 | 2.50 | 2.25 |
| SD | 0.67 | 0.55 | 0.86 | 1.04 | 0.94 | 0.87 | 1.00 | 1.15 | 1.40 | 0.72 | 1.26 | |
| Community C | Mean | 2.70 | 2.56 | 2.64 | 2.99 | 2.47 | 2.53 | 3.03 | 2.79 | 3.07 | 2.68 | 2.15 |
| SD | 0.49 | 0.53 | 0.67 | 0.85 | 0.73 | 0.64 | 1.08 | 0.76 | 1.05 | 1.07 | 0.71 | |
| Academic combined | Mean | 2.80 | 2.45 | 2.93 | 3.22 | 2.56 | 2.24 | 3.07 | 2.62 | 2.88 | 3.21 | 3.28 |
| SD | 0.54 | 0.63 | 0.66 | 0.89 | 0.82 | 0.72 | 1.16 | 0.95 | 1.14 | 1.10 | 1.26 | |
| Community combined | Mean | 2.79 | 2.61 | 2.66 | 3.14 | 2.60 | 2.68 | 3.03 | 2.72 | 2.95 | 2.82 | 2.34 |
| SD | 0.62 | 0.62 | 0.72 | 1.01 | 0.90 | 0.90 | 1.15 | 0.99 | 1.09 | 1.09 | 1.00 | |
| P value | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | 0.02 | >0.05 | >0.05 | >0.05 | >0.05 | <0.001 | |
Convergent, Concurrent, and Discriminant Validity Evidence
For every categorical increase on the question assessing overall morale, the personal morale score was 0.23 points higher (P < 0.001). For every categorical increase in a participant's perception of the group's morale, the personal morale score was 0.29 points higher (P < 0.001).
For every 1‐point increase in personal morale score, the odds of being highly invested in the group increased by 5 times (odds ratio [OR]: 5.23, 95% confidence interval [CI]: 1.91‐14.35, P = 0.001). The mean personal morale score for highly invested hospitalists was 2.92, whereas that of those less invested was 2.43 (diff. = 0.49, P < 0.001) (Table 3). Highly invested hospitalists had significantly higher importance factor scores for leadership (diff. = 0.08, P = 0.03) as well as appreciation and acknowledgement (diff. = 0.08, P = 0.02).
| Personal Morale Score | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Workload | Leadership | Appreciation and Acknowledgement | Material Rewards | Family Time | Institutional Climate | Job Security | Autonomy | Professional Growth | ||
| |||||||||||
| Highly invested in success of current hospitalist group | |||||||||||
| Mean | 2.92 | 2.61 | 2.89 | 3.38 | 2.78 | 2.45 | 3.21 | 2.78 | 2.86 | 3.10 | 2.95 |
| SD | 0.55 | 0.59 | 0.68 | 0.92 | 0.88 | 0.77 | 1.11 | 1.00 | 1.09 | 1.06 | 1.25 |
| Less invested in success of current hospitalist group | |||||||||||
| Mean | 2.43 | 2.34 | 2.48 | 2.60 | 2.02 | 2.57 | 2.60 | 2.38 | 3.08 | 2.69 | 2.24 |
| SD | 0.52 | 0.69 | 0.69 | 0.81 | 0.49 | 1.04 | 1.17 | 0.83 | 1.18 | 1.19 | 0.94 |
| P value | <0.001 | >0.05 | 0.02 | 0.001 | <0.001 | >0.05 | 0.03 | >0.05 | >0.05 | >0.05 | 0.02 |
| Not intending to leave because unhappy | |||||||||||
| Mean | 2.97 | 2.67 | 2.89 | 3.48 | 2.77 | 2.52 | 3.24 | 2.85 | 3.05 | 3.06 | 3.01 |
| SD | 0.51 | 0.54 | 0.61 | 0.91 | 0.89 | 0.78 | 1.03 | 0.99 | 1.10 | 1.07 | 1.25 |
| Intending to leave current group because unhappy | |||||||||||
| Mean | 2.45 | 2.30 | 2.59 | 2.59 | 2.21 | 2.40 | 2.68 | 2.33 | 2.67 | 2.88 | 2.28 |
| SD | 0.56 | 0.72 | 0.82 | 0.74 | 0.68 | 0.97 | 1.29 | 0.83 | 1.11 | 1.17 | 0.97 |
| P value | <0.001 | 0.01 | >0.05 | <0.001 | 0.003 | >0.05 | 0.03 | 0.01 | >0.05 | >0.05 | 0.01 |
Every 1‐point increase in personal morale was associated with a rise of 2.27 on the professional growth scale (P = 0.01). The correlation between these 2 scales was 0.26 (P = 0.01). Every 1‐point increase in personal morale was associated with a 2.21 point decrease on the Cohen stress scale (P > 0.05). The correlation between these 2 scales was 0.21 (P > 0.05).
Morale and Intent to Leave Due to Unhappiness
Sixteen (37%) academic and 18 (36%) community hospitalists reported having thoughts of leaving their current hospitalist program due to unhappiness. The mean personal morale score for hospitalists with no intent to leave their current group was 2.97, whereas that of those with intent to leave was 2.45 (diff. = 0.53, P < 0.001). Each 1‐point increase in the personal morale score was associated with an 85% decrease (OR: 0.15, 95% CI: 0.05‐0.41, P < 0.001) in the odds of leaving because of unhappiness. Holding self‐perception of being a career hospitalist constant, each 1‐point increase in the personal morale score was associated with an 83% decrease (OR: 0.17, 95% CI: 0.05‐0.51, P = 0.002) in the odds of leaving because of unhappiness. Hospitalists who reported intent to leave had significantly lower factor scores for all factors and items except workload, material reward, and autonomy than those who did not report intent to leave (Table 3). Within the academic groups, those who reported intent to leave had significantly lower scores for professional growth (diff. = 1.08, P = 0.01). For community groups, those who reported intent to leave had significantly lower scores for clinical work (diff. = 0.54, P = 0.003), workload (diff. = 0.50, P = 0.02), leadership (diff. = 1.19, P < 0.001), feeling appreciated and acknowledged (diff. = 0.68, P = 0.01), job security (diff. = 0.70, P = 0.03), and institutional climate (diff. = 0.67, P = 0.02) than those who did not report intent to leave.
DISCUSSION
The HMI is a validated tool that objectively measures and quantifies hospitalist morale. The HMI's capacity to comprehensively assess morale comes from its breadth and depth in uncovering work‐related areas that may be sources of contentment or displeasure. Furthermore, the fact that HMI scores varied among groups of individuals, including those who are thinking about leaving their hospitalist group because they are unhappy and those who are highly invested in their hospitalist group, speaks to its ability to highlight and account for what is most important to hospitalist providers.
Low employee morale has been associated with decreased productivity, increased absenteeism, increased turnover, and decreased patient satisfaction.[2, 26, 27, 28] A few frustrated workers can breed group discontentment and lower the entire group's morale.[28] In addition to its financial impact, departures due to low morale can be sudden and devastating, leading to loss of team cohesiveness, increased work burden on the remaining workforce, burnout, and cascades of more turnover.[2] In contrast, when morale is high, workers more commonly go the extra mile, are more committed to the organization's mission, and are more supportive of their coworkers.[28]
While we asked the informants about plans to leave their job, there are many factors that drive an individual's intent and ultimate decision to make changes in his or her employment. Some factors are outside the control of the employer or practice leaders, such as change in an individual's family life or desire and opportunity to pursue fellowship training. Others variables, however, are more directly tied to the job or practice environment. In a specialty where providers are relatively mobile and turnover is high, it is important for hospitalist practices to cultivate a climate in which the sacrifices associated with leaving outweigh the promised benefits.[29]
Results from the HMPWS suggested the need to address climate and fairness issues in hospitalist programs to improve satisfaction and retention.[9] Two large healthcare systems achieved success by investing in multipronged physician retention strategies including recruiting advisors, sign‐on bonuses, extensive onboarding, family support, and the promotion of ongoing effective communication.[3, 30]
Our findings suggest that morale for hospitalists is a complex amalgam of contentment and importance, and that there may not be a one size fits all solution to improving morale for all. While we did not find a difference in personal morale scores across individual hospitalist groups, or even between academic and community groups, each group had a unique profile with variability in the dynamics between importance and contentment of different factors. If practice group leaders review HMI data for their providers and use the information to facilitate meaningful dialogue with them about the factors influencing their morale, such leaders will have great insight into allocating resources for the best return on investment.
While we believe that the HMI is providing unique perspective compared to other commonly used metrics, it may be best to employ HMI data as complementary measures alongside that of some of the benchmarked scales that explore job satisfaction, job fit, and burnout among hospitalists.[6, 9, 10, 31, 32, 33, 34, 35] Aggregate HMI data at the group level may allow for the identification of factors that are highly important to morale but scored low in contentment. Such factors deserve priority and attention such that the subgroups within a practice can collaborate to come to consensus on strategies for amelioration. Because the HMI generates a score and profile for each provider, we can imagine effective leaders using the HMI with individuals as part of an annual review to facilitate discussion about maximizing contentment at work. Being fully transparent and sharing an honest nonanonymous version of the HMI with a superior would require a special relationship founded on trust and mutual respect.
Several limitations of this study should be considered. First, the initial item reduction and EFA were based on a single‐site survey, and our overall sample size was relatively small. We plan on expanding our sample size in the future for further validation of our exploratory findings. Second, the data were collected at 2 specific times several years ago. In continuing to analyze the data from subsequent years, validity and reliability results remain stable, thereby minimizing the likelihood of significant historical bias. Third, there may have been some recall bias, in that respondents may have overlooked the good and perseverated over variables that disappointed them. Fourth, although intention to leave does not necessarily equate actual employee turnover, intention has been found to be a strong predictor of quitting a job.[36, 37] Finally, while we had high response rates, response bias may have existed wherein those with lower morale may have elected not to complete the survey or became apathetic in their responses.
The HMI is a validated instrument that evaluates hospitalist morale by incorporating each provider's characterization of the importance of and contentment with 27 variables. By accounting for the multidimensional and dynamic nature of morale, the HMI may help program leaders tailor retention and engagement strategies specific to their own group. Future studies may explore trends in contributors to morale and examine whether interventions to augment low morale can result in improved morale and hospitalist retention.
Acknowledgements
The authors are indebted to the hospitalists who were willing to share their perspectives about their work, and grateful to Ms. Lisa Roberts, Ms. Barbara Brigade, and Ms. Regina Landis for insuring confidentiality in managing the survey database.
Disclosures: Dr. Chandra had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Wright is a Miller‐Coulson Family Scholar through the Johns Hopkins Center for Innovative Medicine. Ethical approval has been granted for studies involving human subjects by a Johns Hopkins University School of Medicine institutional review board. The authors report no conflicts of interest.
- 2014 State of Hospital Medicine Report. Philadelphia, PA: Society of Hospital Medicine; 2014.
- , , . A review of physician turnover: rates, causes, and consequences. Am J Med Qual. 2004;19(2):56–66.
- . Physician retention plans help reduce costs and optimize revenues. Healthc Financ Manage. 1998;52(1):75–77.
- SHM Career Satisfaction Task Force. A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction.; 2006. Available at: www.hospitalmedicine.org. Accessed February 28, 2009.
- , . Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119–139.
- , , , , . Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851–858.
- . Doing the same and earning less: male and female physicians in a new medical specialty. Inquiry. 2004;41(3):301–315.
- . Physician satisfaction and communication. National findings and best practices. Available at: http://www.pressganey.com/files/clark_cox_acpe_apr06.pdf. Accessed October 10, 2010.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28–36.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- Morale | Define Morale at Dictionary.com. Morale | Define Morale at Dictionary.com. Morale | Define Morale at Dictionary.com. Available at: http://dictionary.reference.com/browse/morale. Accessed June 5, 2014.
- . Morale and satisfaction: a study in past‐future time perspective. Adm Sci Q. 1958:195–209.
- . Men and Women of the Corporation. 2nd ed. New York, NY: Basic Books; 1993.
- . The relation of morale to turnover among teachers. Am Educ Res J. 1965:163–173.
- . Structural and individual determinants of organization morale and satisfaction. Soc Forces. 1982;61:1088.
- , , . Morale matters: midlevel administrators and their intent to leave. J Higher Educ. 2000:34–59.
- . Factors influencing employee morale. Harv Bus Rev. 1950;28(1):61–73.
- . Dimensions of teacher morale. Am Educ Res J. 1970;7(2):221.
- , . The definition and measurement of employee morale. Adm Sci Q. 1958:157–184.
- , , , et al. Measuring physician job satisfaction in a changing workplace and a challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine. Med Care. 1999;37(11):1174–1182.
- . Structural and individual determinants of organization morale and satisfaction. Soc Forces. 1983;61(4):1088–1108.
- . Morale and its measurement. Am J Sociol. 1941;47(3):406–414.
- , , , . Following morale over time within an academic hospitalist division. J Clin Outcomes Manag. 2011;18(1):21–26.
- , , , et al. Personal growth and its correlates during residency training. Med Educ. 2006;40(8):737–745.
- , , . A global measure of perceived stress. J Health Soc Behav. 1983:385–396.
- , , . Morale matters: midlevel administrators and their intent to leave. J Higher Educ. 2000;71(1):34–59.
- , . Faculty members' morale and their intention to leave: a multilevel explanation. J Higher Educ. 2002;73(4):518–542.
- , . Employee Morale. New York, NY: Palgrave Macmillan; 2009.
- , , , , . Silence Kills. Silence Kills: The Seven Crucial Conversations® for Healthcare. VitalSmarts™ in association with the American Association of Critical Care Nurses, USA. 2005. Accessed October 10, 2014.
- , , . The lifelong iterative process of physician retention. J Healthc Manag. 2009;54(4):220–226.
- . Physicians' burnout. Rev Prat. 2004;54(7):753–754.
- , , , , , . Work stress and health in primary health care physicians and hospital physicians. Occup Environ Med. 2008;65(5):364–366.
- , , , et al. Understanding physicians' intentions to withdraw from practice: the role of job satisfaction, job stress, mental and physical health. 2001. Health Care Manage Rev. 2010;35(2):105–115.
- , , , , , . Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358–1367.
- , . Burnout and Hospitalists: Etiology and Prevention. In: What Exactly Does A Hospitalist Do? Best of the Best Hospital Medicine 2005: Strategies for Success. Society of Hospital Medicine; 2005:5.
- , . Using job involvement and organizational commitment interactively to predict turnover. J Manage. 1989;15(1):115–127.
- , , , et al. Nurse turnover: a literature review. Int J Nurs Stud. 2006;43(2):237–263.
- 2014 State of Hospital Medicine Report. Philadelphia, PA: Society of Hospital Medicine; 2014.
- , , . A review of physician turnover: rates, causes, and consequences. Am J Med Qual. 2004;19(2):56–66.
- . Physician retention plans help reduce costs and optimize revenues. Healthc Financ Manage. 1998;52(1):75–77.
- SHM Career Satisfaction Task Force. A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction.; 2006. Available at: www.hospitalmedicine.org. Accessed February 28, 2009.
- , . Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119–139.
- , , , , . Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851–858.
- . Doing the same and earning less: male and female physicians in a new medical specialty. Inquiry. 2004;41(3):301–315.
- . Physician satisfaction and communication. National findings and best practices. Available at: http://www.pressganey.com/files/clark_cox_acpe_apr06.pdf. Accessed October 10, 2010.
- , , , , . Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28–36.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- Morale | Define Morale at Dictionary.com. Morale | Define Morale at Dictionary.com. Morale | Define Morale at Dictionary.com. Available at: http://dictionary.reference.com/browse/morale. Accessed June 5, 2014.
- . Morale and satisfaction: a study in past‐future time perspective. Adm Sci Q. 1958:195–209.
- . Men and Women of the Corporation. 2nd ed. New York, NY: Basic Books; 1993.
- . The relation of morale to turnover among teachers. Am Educ Res J. 1965:163–173.
- . Structural and individual determinants of organization morale and satisfaction. Soc Forces. 1982;61:1088.
- , , . Morale matters: midlevel administrators and their intent to leave. J Higher Educ. 2000:34–59.
- . Factors influencing employee morale. Harv Bus Rev. 1950;28(1):61–73.
- . Dimensions of teacher morale. Am Educ Res J. 1970;7(2):221.
- , . The definition and measurement of employee morale. Adm Sci Q. 1958:157–184.
- , , , et al. Measuring physician job satisfaction in a changing workplace and a challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine. Med Care. 1999;37(11):1174–1182.
- . Structural and individual determinants of organization morale and satisfaction. Soc Forces. 1983;61(4):1088–1108.
- . Morale and its measurement. Am J Sociol. 1941;47(3):406–414.
- , , , . Following morale over time within an academic hospitalist division. J Clin Outcomes Manag. 2011;18(1):21–26.
- , , , et al. Personal growth and its correlates during residency training. Med Educ. 2006;40(8):737–745.
- , , . A global measure of perceived stress. J Health Soc Behav. 1983:385–396.
- , , . Morale matters: midlevel administrators and their intent to leave. J Higher Educ. 2000;71(1):34–59.
- , . Faculty members' morale and their intention to leave: a multilevel explanation. J Higher Educ. 2002;73(4):518–542.
- , . Employee Morale. New York, NY: Palgrave Macmillan; 2009.
- , , , , . Silence Kills. Silence Kills: The Seven Crucial Conversations® for Healthcare. VitalSmarts™ in association with the American Association of Critical Care Nurses, USA. 2005. Accessed October 10, 2014.
- , , . The lifelong iterative process of physician retention. J Healthc Manag. 2009;54(4):220–226.
- . Physicians' burnout. Rev Prat. 2004;54(7):753–754.
- , , , , , . Work stress and health in primary health care physicians and hospital physicians. Occup Environ Med. 2008;65(5):364–366.
- , , , et al. Understanding physicians' intentions to withdraw from practice: the role of job satisfaction, job stress, mental and physical health. 2001. Health Care Manage Rev. 2010;35(2):105–115.
- , , , , , . Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358–1367.
- , . Burnout and Hospitalists: Etiology and Prevention. In: What Exactly Does A Hospitalist Do? Best of the Best Hospital Medicine 2005: Strategies for Success. Society of Hospital Medicine; 2005:5.
- , . Using job involvement and organizational commitment interactively to predict turnover. J Manage. 1989;15(1):115–127.
- , , , et al. Nurse turnover: a literature review. Int J Nurs Stud. 2006;43(2):237–263.
© 2016 Society of Hospital Medicine