User login
Unassigned, Undocumented Inpatients Present Challenges; Some Hospitalists Have Solutions
Hospitalists are charged with giving the best of care and treatment, regardless of whether or not a patient is insured or has a PCP to transition to after discharge. But patients who do not have insurance or a PCP pose many challenges to hospitalists, as well as the healthcare systems they work in. Although some hospitals and health systems have found ways to address these challenges, issues persist, with high costs to care for these patients topping the list. In 2013, the cost of community hospitals’ uncompensated care climbed to $46.4 billion.1
Typically, undocumented and unassigned patients face many social and economic challenges. Many of these patients are unemployed or work as independent contractors without employer-offered health insurance. Some have multiple jobs, can’t take time off from work for doctor appointments, or are undocumented workers.
More patients have acquired health insurance in recent years as a result of the Affordable Care Act (ACA) and Medicaid expansion; however, some eligible people never complete the necessary forms.
With or without insurance, some patients don’t establish primary care because they have been healthy, have difficulty navigating the healthcare system, lack transportation, or desire more culturally tailored care. Some Medicare and Medicaid patients don’t have a PCP in their community who accepts these programs.
Treatment Challenges
Uninsured patients often are sicker and have more complex conditions than those with insurance, according to Beth Feldpush, DrPH, senior vice president of policy and advocacy at the nonprofit trade group America’s Essential Hospitals, which is based in Washington, D.C., and represents 250 safety net hospitals throughout the U.S.
“Because they can’t afford regular preventive and primary care, they forgo needed healthcare services until their conditions worsen and they require costly hospital care,” says Dr. Feldpush. Uninsured patients often lack the resources for follow-up care to help them recover and stay well. She says more than half of all inpatient discharges and outpatient visits at her groups’ hospitals are for uninsured or Medicaid patients.
When an uninsured patient is discharged from the hospital, finding follow-up care can be difficult.
“Their ability to get an appointment to see a PCP is extremely limited, because many providers don’t see patients without health insurance,” says Scott Sears, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians. Dr. Sears notes that in some hospitalist programs, as many as 40% of hospitalized patients lack insurance. “But without secured follow-up care, hospitalists are hesitant to send patients home, because they could relapse.”
Typically, these patients are not completely well and should be transferred to a skilled nursing or hospice facility; however, many facilities won’t accept them without insurance. Often, these patients need a PCP to monitor them with laboratory tests and other follow-up tests, to prescribe and monitor medications, and to ensure that they are following their plan of care.
At some medical facilities, subspecialists who consult on patients may screen them and refuse to see anyone without health insurance.
“So even though some patients may need subspecialty support, they may not have access to it,” Dr. Sears says. “While some patients without insurance qualify for Medicaid or other programs, due to the amount of paperwork and time to enroll, they end up staying in the hospital even though they are ready for discharge.”
Transitional Challenges
Most patients admitted to the hospital either have exacerbations of chronic conditions or a new diagnosis. “It’s rare to hospitalize a patient with a discrete illness that wouldn’t need care after discharge, so having a robust PCP partner is critical to a patient’s health,” says Honora Englander, MD, medical director of the Care Transitions Innovation (C-TRAIN) program at Oregon Health and Science University (OHSU) in Portland. For many patients, psychosocial complexity complicates their transition out of the hospital. An effective system needs to address a patient’s mental health, housing, and other social needs.
It may take four to six weeks for a patient without an established PCP to get a new patient appointment. “This is a huge impediment, as the patient won’t have anyone to ensure that he or she continues along the proper care path,” Dr. Sears says.
“Studies estimate that more than half of medication errors that patients experience occur during transitions and after discharge,” Dr. Sears says.2 “Intervention with a healthcare provider who can review proper use after discharge can dramatically reduce errors and [improve] patient outcomes.”
Rates of patients without a PCP vary by region for Sound Physicians. In the northwest region, about 25% of admitted patients lack a PCP; in the gulf region, the figure can be as high as 60%.
“In Texas, there is a large number of patients and not as many PCPs,” he adds. “There is also a larger percentage of patients without health insurance. Sometimes patients have coverage but have never established care with a PCP.”
As a result of not having a PCP to transition to, some patients return to the hospital soon after discharge, Dr. Feldpush notes.
Tips for Treating Uninsured Patients
Some facilities have found successful ways to help hospitalized patients without health insurance. Dr. Sears says that hospitalists can investigate which clinics accept uninsured patients or which local physician groups are willing to see them after discharge, in exchange for hospitalists taking care of them in the hospital. They also can investigate the community-based insurance programs that are available.
Teresa Coker, MSN, ARNP, FNP-BC, a Sound Physicians program manager at Mercy Medical Center in Cedar Rapids, Iowa, says that when patients lack insurance at her hospital, an organization will review the patient’s case, determine insurance eligibility, and assist the patient in completing the appropriate paperwork. When patients are not eligible, they are instructed to inquire about the hospital’s charity care program if they receive a bill they are unable to pay.
In addition, the community has a free health clinic that serves those without insurance. “Patients are given the address and hours prior to discharge, because it is walk-in only,” Coker says. “All patients are recommended to follow up within one week, or sooner if medications are needed.”
Dr. Englander advocates that physicians take into account medication costs, transportation, and other social considerations when planning care after hospitalization. The team at OHSU developed a low-cost formulary (based in part on widely available $4 plans from national pharmacy chains), and OHSU provides medications for uninsured patients in the program for up to 30 days following discharge.
For patients who can’t afford the $4 drug plan, case managers offer coupons for $4 prescriptions, says Malik Merchant, MD, area medical officer for the Schumachergroup in Harker Heights, Texas. He says that as many as 30% of the patients in his area are undocumented or unassigned. For more expensive medications, a social worker offers pharmaceutical company coupons when they are available. The institution also has a small budget to pay for drugs.
Dr. Merchant has found the biggest challenge to be the transition of care from inpatient to outpatient.
“Case managers and social workers prepare a financial worksheet that provides the possibility of overall cost savings for the institution, if patients are willing to participate in some upfront cost,” he says. “When our parent institution came on board, we developed contracts with local pharmacies, [a] skilled nursing facility, and PCPs to take these patients until they recovered from an acute illness. Our institution paid for these services at a reduced rate but saved money by reducing the length of their hospital stay.”
Dr. Feldpush says her group’s hospitals work hard to reach the uninsured. South Florida’s Memorial Healthcare System (MHS) created the Health Intervention with Targeted Services (HITS) Program, an outreach initiative that links patients with insurance programs or medical homes.3 The HITS team used a geographic information system map to target 15 neighborhoods with the highest rates of hospitalized, uninsured patients. Over a six-month period, the team approached these neighborhoods using various outreach strategies, such as health fairs, educational workshops, and door-to-door visits.3
Approximately 6,910 HITS participants have been enrolled in Medicaid, Florida’s children’s health insurance program, or an MHS community health center. Over a three-year study period, MHS saved $284,856 in the ED, about $2.8 million in inpatient costs, and roughly $4 million overall.3
Barriers to Follow-Up Care
Whether you are looking to help uninsured patients, those without a PCP, or both, the key is to try to fill in the gaps.
“As hospitalists, we need to work with pharmacists, case managers and social workers, and others to identify affordable and effective ways to provide care,” Dr. Englander says. “Interprofessional team members, community partners, and family members can help hospitalists understand patient and population health needs and available resources.”
In an effort to close transitional care gaps, OHSU developed the C-TRAIN program, a multi-component transitional care intervention that includes four main elements:
- Transitional care nurse who sees patients in the hospital, makes home visits, and helps coordinate care 30 days post-discharge;
- Inpatient pharmacy consultation and prescription medications at discharge from a low-cost, value-based formulary;
- Medical home linkages, whereby OHSU partners with and provides payment to three community clinics to provide primary care for uninsured patients; and
- Monthly implementation team meetings that convene diverse healthcare stakeholders to integrate elements of the healthcare system and engage in ongoing quality improvement.
The Schumachergroup has also found an effective solution.
“The department head of our case managers and social workers made an agreement with a local multispecialty group,” Dr. Merchant says. “The group agreed to take all discharged patients and be their PCP for 30 days, even if the patient couldn’t pay, in exchange for receiving all patients who had good insurance but did not have an established PCP.
“This has worked well. Every patient discharged from our facility has a PCP listed at discharge, and the unit clerk makes an appointment and documents it in the electronic medical record.”
Sound Physicians has set up a service line, called transitional care services, to smooth transitions of care after discharge for up to 90 days, depending on their clinical needs. It hires providers who work in post-acute facilities and who can also visit patients at home. After discharge, a nurse practitioner will visit the patient, connect him or her with a PCP, and get the patient access to care.
“Smaller hospitalist groups could set up post-discharge clinics,” Dr. Sears suggests, “so when they discharge a patient without a PCP, [the patient] could return to see one of the hospitalists there.”
Mount Carmel East Hospital, a Sound Physicians’ hospital in Columbus, Ohio, has a financial assistance program.
“The case management department provides community health resources to patients who are insured but have no PCP,” says Shelli Morris, RN. “We also have a hotline that patients can call for a list of PCPs that are accepting new patients.”
When a patient lacks insurance or a PCP, Morris is contacted by the physician or case management to provide a referral to a neighborhood health clinic. “Then, as a courtesy, we set up a post-hospital follow-up appointment,” she says.
By working with other care team members at facilities such as outpatient clinics and pharmacies, hospitalists and other staff have been able to improve care for patients without insurance or a PCP after discharge. Knowing the funding that is available, as well as programs to help these patients, is also integral.
Karen Appold is a medical writer in Pennsylvania.
References
- American Hospital Association. American Hospital Association Uncompensated Hospital Care Cost Fact Sheet. Accessed October 8, 2015.
- The Office of the National Coordinator for Health Information Technology. Health IT in long-term and post acute care: issue brief. March 15, 2013. Accessed October 8, 2015.
- Addison E. Gage award winner HITS the streets to connect with the uninsured. America’s Essential Hospitals. July 22, 2014. Accessed October 8, 2015.
- DeNavas C, Proctor BD, Smith JC. Income, poverty, and health insurance coverage in the United States: 2010. United States Census Bureau. September 2011. Accessed October 8, 2015.
- United States Census Bureau. People without health insurance coverage by selected characteristics: 2010 and 2011. Accessed October 8, 2015.
- Coughlin TA, Holahan J, Caswell K, McGrath M. Uncompensated care for the uninsured in 2013: a detailed examination. The Henry J. Kaiser Family Foundation. May 30, 2014. Accessed October 8, 2015.
Hospitalists are charged with giving the best of care and treatment, regardless of whether or not a patient is insured or has a PCP to transition to after discharge. But patients who do not have insurance or a PCP pose many challenges to hospitalists, as well as the healthcare systems they work in. Although some hospitals and health systems have found ways to address these challenges, issues persist, with high costs to care for these patients topping the list. In 2013, the cost of community hospitals’ uncompensated care climbed to $46.4 billion.1
Typically, undocumented and unassigned patients face many social and economic challenges. Many of these patients are unemployed or work as independent contractors without employer-offered health insurance. Some have multiple jobs, can’t take time off from work for doctor appointments, or are undocumented workers.
More patients have acquired health insurance in recent years as a result of the Affordable Care Act (ACA) and Medicaid expansion; however, some eligible people never complete the necessary forms.
With or without insurance, some patients don’t establish primary care because they have been healthy, have difficulty navigating the healthcare system, lack transportation, or desire more culturally tailored care. Some Medicare and Medicaid patients don’t have a PCP in their community who accepts these programs.
Treatment Challenges
Uninsured patients often are sicker and have more complex conditions than those with insurance, according to Beth Feldpush, DrPH, senior vice president of policy and advocacy at the nonprofit trade group America’s Essential Hospitals, which is based in Washington, D.C., and represents 250 safety net hospitals throughout the U.S.
“Because they can’t afford regular preventive and primary care, they forgo needed healthcare services until their conditions worsen and they require costly hospital care,” says Dr. Feldpush. Uninsured patients often lack the resources for follow-up care to help them recover and stay well. She says more than half of all inpatient discharges and outpatient visits at her groups’ hospitals are for uninsured or Medicaid patients.
When an uninsured patient is discharged from the hospital, finding follow-up care can be difficult.
“Their ability to get an appointment to see a PCP is extremely limited, because many providers don’t see patients without health insurance,” says Scott Sears, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians. Dr. Sears notes that in some hospitalist programs, as many as 40% of hospitalized patients lack insurance. “But without secured follow-up care, hospitalists are hesitant to send patients home, because they could relapse.”
Typically, these patients are not completely well and should be transferred to a skilled nursing or hospice facility; however, many facilities won’t accept them without insurance. Often, these patients need a PCP to monitor them with laboratory tests and other follow-up tests, to prescribe and monitor medications, and to ensure that they are following their plan of care.
At some medical facilities, subspecialists who consult on patients may screen them and refuse to see anyone without health insurance.
“So even though some patients may need subspecialty support, they may not have access to it,” Dr. Sears says. “While some patients without insurance qualify for Medicaid or other programs, due to the amount of paperwork and time to enroll, they end up staying in the hospital even though they are ready for discharge.”
Transitional Challenges
Most patients admitted to the hospital either have exacerbations of chronic conditions or a new diagnosis. “It’s rare to hospitalize a patient with a discrete illness that wouldn’t need care after discharge, so having a robust PCP partner is critical to a patient’s health,” says Honora Englander, MD, medical director of the Care Transitions Innovation (C-TRAIN) program at Oregon Health and Science University (OHSU) in Portland. For many patients, psychosocial complexity complicates their transition out of the hospital. An effective system needs to address a patient’s mental health, housing, and other social needs.
It may take four to six weeks for a patient without an established PCP to get a new patient appointment. “This is a huge impediment, as the patient won’t have anyone to ensure that he or she continues along the proper care path,” Dr. Sears says.
“Studies estimate that more than half of medication errors that patients experience occur during transitions and after discharge,” Dr. Sears says.2 “Intervention with a healthcare provider who can review proper use after discharge can dramatically reduce errors and [improve] patient outcomes.”
Rates of patients without a PCP vary by region for Sound Physicians. In the northwest region, about 25% of admitted patients lack a PCP; in the gulf region, the figure can be as high as 60%.
“In Texas, there is a large number of patients and not as many PCPs,” he adds. “There is also a larger percentage of patients without health insurance. Sometimes patients have coverage but have never established care with a PCP.”
As a result of not having a PCP to transition to, some patients return to the hospital soon after discharge, Dr. Feldpush notes.
Tips for Treating Uninsured Patients
Some facilities have found successful ways to help hospitalized patients without health insurance. Dr. Sears says that hospitalists can investigate which clinics accept uninsured patients or which local physician groups are willing to see them after discharge, in exchange for hospitalists taking care of them in the hospital. They also can investigate the community-based insurance programs that are available.
Teresa Coker, MSN, ARNP, FNP-BC, a Sound Physicians program manager at Mercy Medical Center in Cedar Rapids, Iowa, says that when patients lack insurance at her hospital, an organization will review the patient’s case, determine insurance eligibility, and assist the patient in completing the appropriate paperwork. When patients are not eligible, they are instructed to inquire about the hospital’s charity care program if they receive a bill they are unable to pay.
In addition, the community has a free health clinic that serves those without insurance. “Patients are given the address and hours prior to discharge, because it is walk-in only,” Coker says. “All patients are recommended to follow up within one week, or sooner if medications are needed.”
Dr. Englander advocates that physicians take into account medication costs, transportation, and other social considerations when planning care after hospitalization. The team at OHSU developed a low-cost formulary (based in part on widely available $4 plans from national pharmacy chains), and OHSU provides medications for uninsured patients in the program for up to 30 days following discharge.
For patients who can’t afford the $4 drug plan, case managers offer coupons for $4 prescriptions, says Malik Merchant, MD, area medical officer for the Schumachergroup in Harker Heights, Texas. He says that as many as 30% of the patients in his area are undocumented or unassigned. For more expensive medications, a social worker offers pharmaceutical company coupons when they are available. The institution also has a small budget to pay for drugs.
Dr. Merchant has found the biggest challenge to be the transition of care from inpatient to outpatient.
“Case managers and social workers prepare a financial worksheet that provides the possibility of overall cost savings for the institution, if patients are willing to participate in some upfront cost,” he says. “When our parent institution came on board, we developed contracts with local pharmacies, [a] skilled nursing facility, and PCPs to take these patients until they recovered from an acute illness. Our institution paid for these services at a reduced rate but saved money by reducing the length of their hospital stay.”
Dr. Feldpush says her group’s hospitals work hard to reach the uninsured. South Florida’s Memorial Healthcare System (MHS) created the Health Intervention with Targeted Services (HITS) Program, an outreach initiative that links patients with insurance programs or medical homes.3 The HITS team used a geographic information system map to target 15 neighborhoods with the highest rates of hospitalized, uninsured patients. Over a six-month period, the team approached these neighborhoods using various outreach strategies, such as health fairs, educational workshops, and door-to-door visits.3
Approximately 6,910 HITS participants have been enrolled in Medicaid, Florida’s children’s health insurance program, or an MHS community health center. Over a three-year study period, MHS saved $284,856 in the ED, about $2.8 million in inpatient costs, and roughly $4 million overall.3
Barriers to Follow-Up Care
Whether you are looking to help uninsured patients, those without a PCP, or both, the key is to try to fill in the gaps.
“As hospitalists, we need to work with pharmacists, case managers and social workers, and others to identify affordable and effective ways to provide care,” Dr. Englander says. “Interprofessional team members, community partners, and family members can help hospitalists understand patient and population health needs and available resources.”
In an effort to close transitional care gaps, OHSU developed the C-TRAIN program, a multi-component transitional care intervention that includes four main elements:
- Transitional care nurse who sees patients in the hospital, makes home visits, and helps coordinate care 30 days post-discharge;
- Inpatient pharmacy consultation and prescription medications at discharge from a low-cost, value-based formulary;
- Medical home linkages, whereby OHSU partners with and provides payment to three community clinics to provide primary care for uninsured patients; and
- Monthly implementation team meetings that convene diverse healthcare stakeholders to integrate elements of the healthcare system and engage in ongoing quality improvement.
The Schumachergroup has also found an effective solution.
“The department head of our case managers and social workers made an agreement with a local multispecialty group,” Dr. Merchant says. “The group agreed to take all discharged patients and be their PCP for 30 days, even if the patient couldn’t pay, in exchange for receiving all patients who had good insurance but did not have an established PCP.
“This has worked well. Every patient discharged from our facility has a PCP listed at discharge, and the unit clerk makes an appointment and documents it in the electronic medical record.”
Sound Physicians has set up a service line, called transitional care services, to smooth transitions of care after discharge for up to 90 days, depending on their clinical needs. It hires providers who work in post-acute facilities and who can also visit patients at home. After discharge, a nurse practitioner will visit the patient, connect him or her with a PCP, and get the patient access to care.
“Smaller hospitalist groups could set up post-discharge clinics,” Dr. Sears suggests, “so when they discharge a patient without a PCP, [the patient] could return to see one of the hospitalists there.”
Mount Carmel East Hospital, a Sound Physicians’ hospital in Columbus, Ohio, has a financial assistance program.
“The case management department provides community health resources to patients who are insured but have no PCP,” says Shelli Morris, RN. “We also have a hotline that patients can call for a list of PCPs that are accepting new patients.”
When a patient lacks insurance or a PCP, Morris is contacted by the physician or case management to provide a referral to a neighborhood health clinic. “Then, as a courtesy, we set up a post-hospital follow-up appointment,” she says.
By working with other care team members at facilities such as outpatient clinics and pharmacies, hospitalists and other staff have been able to improve care for patients without insurance or a PCP after discharge. Knowing the funding that is available, as well as programs to help these patients, is also integral.
Karen Appold is a medical writer in Pennsylvania.
References
- American Hospital Association. American Hospital Association Uncompensated Hospital Care Cost Fact Sheet. Accessed October 8, 2015.
- The Office of the National Coordinator for Health Information Technology. Health IT in long-term and post acute care: issue brief. March 15, 2013. Accessed October 8, 2015.
- Addison E. Gage award winner HITS the streets to connect with the uninsured. America’s Essential Hospitals. July 22, 2014. Accessed October 8, 2015.
- DeNavas C, Proctor BD, Smith JC. Income, poverty, and health insurance coverage in the United States: 2010. United States Census Bureau. September 2011. Accessed October 8, 2015.
- United States Census Bureau. People without health insurance coverage by selected characteristics: 2010 and 2011. Accessed October 8, 2015.
- Coughlin TA, Holahan J, Caswell K, McGrath M. Uncompensated care for the uninsured in 2013: a detailed examination. The Henry J. Kaiser Family Foundation. May 30, 2014. Accessed October 8, 2015.
Hospitalists are charged with giving the best of care and treatment, regardless of whether or not a patient is insured or has a PCP to transition to after discharge. But patients who do not have insurance or a PCP pose many challenges to hospitalists, as well as the healthcare systems they work in. Although some hospitals and health systems have found ways to address these challenges, issues persist, with high costs to care for these patients topping the list. In 2013, the cost of community hospitals’ uncompensated care climbed to $46.4 billion.1
Typically, undocumented and unassigned patients face many social and economic challenges. Many of these patients are unemployed or work as independent contractors without employer-offered health insurance. Some have multiple jobs, can’t take time off from work for doctor appointments, or are undocumented workers.
More patients have acquired health insurance in recent years as a result of the Affordable Care Act (ACA) and Medicaid expansion; however, some eligible people never complete the necessary forms.
With or without insurance, some patients don’t establish primary care because they have been healthy, have difficulty navigating the healthcare system, lack transportation, or desire more culturally tailored care. Some Medicare and Medicaid patients don’t have a PCP in their community who accepts these programs.
Treatment Challenges
Uninsured patients often are sicker and have more complex conditions than those with insurance, according to Beth Feldpush, DrPH, senior vice president of policy and advocacy at the nonprofit trade group America’s Essential Hospitals, which is based in Washington, D.C., and represents 250 safety net hospitals throughout the U.S.
“Because they can’t afford regular preventive and primary care, they forgo needed healthcare services until their conditions worsen and they require costly hospital care,” says Dr. Feldpush. Uninsured patients often lack the resources for follow-up care to help them recover and stay well. She says more than half of all inpatient discharges and outpatient visits at her groups’ hospitals are for uninsured or Medicaid patients.
When an uninsured patient is discharged from the hospital, finding follow-up care can be difficult.
“Their ability to get an appointment to see a PCP is extremely limited, because many providers don’t see patients without health insurance,” says Scott Sears, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians. Dr. Sears notes that in some hospitalist programs, as many as 40% of hospitalized patients lack insurance. “But without secured follow-up care, hospitalists are hesitant to send patients home, because they could relapse.”
Typically, these patients are not completely well and should be transferred to a skilled nursing or hospice facility; however, many facilities won’t accept them without insurance. Often, these patients need a PCP to monitor them with laboratory tests and other follow-up tests, to prescribe and monitor medications, and to ensure that they are following their plan of care.
At some medical facilities, subspecialists who consult on patients may screen them and refuse to see anyone without health insurance.
“So even though some patients may need subspecialty support, they may not have access to it,” Dr. Sears says. “While some patients without insurance qualify for Medicaid or other programs, due to the amount of paperwork and time to enroll, they end up staying in the hospital even though they are ready for discharge.”
Transitional Challenges
Most patients admitted to the hospital either have exacerbations of chronic conditions or a new diagnosis. “It’s rare to hospitalize a patient with a discrete illness that wouldn’t need care after discharge, so having a robust PCP partner is critical to a patient’s health,” says Honora Englander, MD, medical director of the Care Transitions Innovation (C-TRAIN) program at Oregon Health and Science University (OHSU) in Portland. For many patients, psychosocial complexity complicates their transition out of the hospital. An effective system needs to address a patient’s mental health, housing, and other social needs.
It may take four to six weeks for a patient without an established PCP to get a new patient appointment. “This is a huge impediment, as the patient won’t have anyone to ensure that he or she continues along the proper care path,” Dr. Sears says.
“Studies estimate that more than half of medication errors that patients experience occur during transitions and after discharge,” Dr. Sears says.2 “Intervention with a healthcare provider who can review proper use after discharge can dramatically reduce errors and [improve] patient outcomes.”
Rates of patients without a PCP vary by region for Sound Physicians. In the northwest region, about 25% of admitted patients lack a PCP; in the gulf region, the figure can be as high as 60%.
“In Texas, there is a large number of patients and not as many PCPs,” he adds. “There is also a larger percentage of patients without health insurance. Sometimes patients have coverage but have never established care with a PCP.”
As a result of not having a PCP to transition to, some patients return to the hospital soon after discharge, Dr. Feldpush notes.
Tips for Treating Uninsured Patients
Some facilities have found successful ways to help hospitalized patients without health insurance. Dr. Sears says that hospitalists can investigate which clinics accept uninsured patients or which local physician groups are willing to see them after discharge, in exchange for hospitalists taking care of them in the hospital. They also can investigate the community-based insurance programs that are available.
Teresa Coker, MSN, ARNP, FNP-BC, a Sound Physicians program manager at Mercy Medical Center in Cedar Rapids, Iowa, says that when patients lack insurance at her hospital, an organization will review the patient’s case, determine insurance eligibility, and assist the patient in completing the appropriate paperwork. When patients are not eligible, they are instructed to inquire about the hospital’s charity care program if they receive a bill they are unable to pay.
In addition, the community has a free health clinic that serves those without insurance. “Patients are given the address and hours prior to discharge, because it is walk-in only,” Coker says. “All patients are recommended to follow up within one week, or sooner if medications are needed.”
Dr. Englander advocates that physicians take into account medication costs, transportation, and other social considerations when planning care after hospitalization. The team at OHSU developed a low-cost formulary (based in part on widely available $4 plans from national pharmacy chains), and OHSU provides medications for uninsured patients in the program for up to 30 days following discharge.
For patients who can’t afford the $4 drug plan, case managers offer coupons for $4 prescriptions, says Malik Merchant, MD, area medical officer for the Schumachergroup in Harker Heights, Texas. He says that as many as 30% of the patients in his area are undocumented or unassigned. For more expensive medications, a social worker offers pharmaceutical company coupons when they are available. The institution also has a small budget to pay for drugs.
Dr. Merchant has found the biggest challenge to be the transition of care from inpatient to outpatient.
“Case managers and social workers prepare a financial worksheet that provides the possibility of overall cost savings for the institution, if patients are willing to participate in some upfront cost,” he says. “When our parent institution came on board, we developed contracts with local pharmacies, [a] skilled nursing facility, and PCPs to take these patients until they recovered from an acute illness. Our institution paid for these services at a reduced rate but saved money by reducing the length of their hospital stay.”
Dr. Feldpush says her group’s hospitals work hard to reach the uninsured. South Florida’s Memorial Healthcare System (MHS) created the Health Intervention with Targeted Services (HITS) Program, an outreach initiative that links patients with insurance programs or medical homes.3 The HITS team used a geographic information system map to target 15 neighborhoods with the highest rates of hospitalized, uninsured patients. Over a six-month period, the team approached these neighborhoods using various outreach strategies, such as health fairs, educational workshops, and door-to-door visits.3
Approximately 6,910 HITS participants have been enrolled in Medicaid, Florida’s children’s health insurance program, or an MHS community health center. Over a three-year study period, MHS saved $284,856 in the ED, about $2.8 million in inpatient costs, and roughly $4 million overall.3
Barriers to Follow-Up Care
Whether you are looking to help uninsured patients, those without a PCP, or both, the key is to try to fill in the gaps.
“As hospitalists, we need to work with pharmacists, case managers and social workers, and others to identify affordable and effective ways to provide care,” Dr. Englander says. “Interprofessional team members, community partners, and family members can help hospitalists understand patient and population health needs and available resources.”
In an effort to close transitional care gaps, OHSU developed the C-TRAIN program, a multi-component transitional care intervention that includes four main elements:
- Transitional care nurse who sees patients in the hospital, makes home visits, and helps coordinate care 30 days post-discharge;
- Inpatient pharmacy consultation and prescription medications at discharge from a low-cost, value-based formulary;
- Medical home linkages, whereby OHSU partners with and provides payment to three community clinics to provide primary care for uninsured patients; and
- Monthly implementation team meetings that convene diverse healthcare stakeholders to integrate elements of the healthcare system and engage in ongoing quality improvement.
The Schumachergroup has also found an effective solution.
“The department head of our case managers and social workers made an agreement with a local multispecialty group,” Dr. Merchant says. “The group agreed to take all discharged patients and be their PCP for 30 days, even if the patient couldn’t pay, in exchange for receiving all patients who had good insurance but did not have an established PCP.
“This has worked well. Every patient discharged from our facility has a PCP listed at discharge, and the unit clerk makes an appointment and documents it in the electronic medical record.”
Sound Physicians has set up a service line, called transitional care services, to smooth transitions of care after discharge for up to 90 days, depending on their clinical needs. It hires providers who work in post-acute facilities and who can also visit patients at home. After discharge, a nurse practitioner will visit the patient, connect him or her with a PCP, and get the patient access to care.
“Smaller hospitalist groups could set up post-discharge clinics,” Dr. Sears suggests, “so when they discharge a patient without a PCP, [the patient] could return to see one of the hospitalists there.”
Mount Carmel East Hospital, a Sound Physicians’ hospital in Columbus, Ohio, has a financial assistance program.
“The case management department provides community health resources to patients who are insured but have no PCP,” says Shelli Morris, RN. “We also have a hotline that patients can call for a list of PCPs that are accepting new patients.”
When a patient lacks insurance or a PCP, Morris is contacted by the physician or case management to provide a referral to a neighborhood health clinic. “Then, as a courtesy, we set up a post-hospital follow-up appointment,” she says.
By working with other care team members at facilities such as outpatient clinics and pharmacies, hospitalists and other staff have been able to improve care for patients without insurance or a PCP after discharge. Knowing the funding that is available, as well as programs to help these patients, is also integral.
Karen Appold is a medical writer in Pennsylvania.
References
- American Hospital Association. American Hospital Association Uncompensated Hospital Care Cost Fact Sheet. Accessed October 8, 2015.
- The Office of the National Coordinator for Health Information Technology. Health IT in long-term and post acute care: issue brief. March 15, 2013. Accessed October 8, 2015.
- Addison E. Gage award winner HITS the streets to connect with the uninsured. America’s Essential Hospitals. July 22, 2014. Accessed October 8, 2015.
- DeNavas C, Proctor BD, Smith JC. Income, poverty, and health insurance coverage in the United States: 2010. United States Census Bureau. September 2011. Accessed October 8, 2015.
- United States Census Bureau. People without health insurance coverage by selected characteristics: 2010 and 2011. Accessed October 8, 2015.
- Coughlin TA, Holahan J, Caswell K, McGrath M. Uncompensated care for the uninsured in 2013: a detailed examination. The Henry J. Kaiser Family Foundation. May 30, 2014. Accessed October 8, 2015.
Expert panel issues guidelines for treatment of hematologic cancers in pregnancy
Consensus guidelines for the perinatal management of hematologic malignancies detected during pregnancy have been issued by a panel of international experts.
The guidelines, published online in the Journal of Clinical Oncology, aim to ensure that timely treatment of the cancers is not delayed in pregnant women (doi: 10.1200/JCO.2015.62.4445).
While rare, hematologic malignancies in pregnancy introduce clinical, social, ethical, and moral dilemmas. Evidence-based data are scarce, according to the researchers, who note the International Network on Cancer, Infertility and Pregnancy registers all cancers occurring during gestation.
“Patient accrual is ongoing and essential, because registration of new cases and long-term follow-up will improve clinical knowledge and increase the level of evidence,” Dr. Michael Lishner of Tel Aviv University and Meir Medical Center, Kfar Saba, Israel, and his fellow panelists wrote.
Hodgkin lymphoma
The researchers note that Hodgkin lymphoma is the most common hematologic cancer in pregnancy, and the prognosis for these patients is excellent. When diagnosed during the first trimester, a regimen based on vinblastine monotherapy has been used. ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy can be used postpartum and has been used in cases of progression during pregnancy, the panelists wrote.
“The limited data available suggest that ABVD may be administered safely and effectively during the latter phases of pregnancy,” the panel wrote. “Although it may be associated with prematurity and lower birth weights, studies have not reported significant disadvantages.”
Non-Hodgkin lymphoma
The second most common cancer in pregnancy is non-Hodgkin lymphoma. In the case of indolent disease, watchful waiting is possible, with the intent to treat with monoclonal antibodies – with or without chemotherapy – if symptoms or evidence of disease progression are noted. Steroids can be administered during the first trimester as a bridge to the second trimester, when chemotherapy can be used with relatively greater safety, the panelists noted.
Aggressive lymphomas diagnosed before 20 weeks’ gestation warrant pregnancy termination and treatment, they recommend. When diagnosed after 20 weeks, therapy should be comparable to that given a nonpregnant woman, including monoclonal antibodies (R-CHOP).
Chronic myeloid leukemia
Chronic myeloid leukemia occurs in approximately 1 in 100,000 pregnancies and is typically diagnosed during routine blood testing in an asymptomatic patient. As a result, treatment may not be needed until the patient’s white count or platelet count have risen to levels associated with the onset of symptoms. An approximate guideline is a white cell count greater than 100 X 109/L and a platelet count greater than 500 X 109/L.
Therapeutic approaches in pregnancy include interferon-a (INF-a), which does not inhibit DNA synthesis or readily cross the placenta, and leukapheresis, which is frequently required two to three times per week during the first and second trimesters. Counts tend to drop during the third trimester, allowing less frequent intervention.
Consideration should be given to adding aspirin or low-molecular-weight heparin (LMWH) when the platelet count exceeds 1,000 X 109/L.
Myeloproliferative neoplasms
The most common myeloproliferative neoplasm seen in women of childbearing age is essential thrombocytosis.
“A large meta-analysis of pregnant women with essential thrombocytosis reported a live birth rate of 50%-70%, first trimester loss in 25%-40%, late pregnancy loss in 10%, placental abruption in 3.6%, and intrauterine growth restriction in 4.5%. Maternal morbidity is rare, but stroke has been reported,” according to the panelists.
Limited literature suggests similar outcomes for pregnant women with polycythemia vera and primary myelofibrosis.
In low-risk pregnancies, aspirin (75 mg/day) should be offered unless clearly contraindicated. For women with polycythemia vera, venesection may be continued when tolerated to maintain the hematocrit within the gestation-appropriate range.
Fetal ultrasound scans should be performed at 20, 26, and 34 weeks of gestation and uterine artery Doppler should be performed at 20 weeks’ gestation. If the mean pulsatility index exceeds 1.4, the pregnancy may be considered high risk, and treatment and monitoring should be increased.
In high-risk pregnancies, additional treatment includes cytoreductive therapy with or without LMWH. If cytoreductive therapy is required, INF-a should be titrated to maintain a platelet count of less than 400 X 109/L and hematocrit within appropriate range.
Local protocols regarding interruption of LMWH should be adhered to during labor, and dehydration should be avoided. Platelet count and hematocrit may increase postpartum, requiring cytoreductive therapy. Thromboprophylaxis should be considered at 6 weeks’ postpartum because of the increased risk of thrombosis, the guidelines note.
Acute leukemia
“The remarkable anemia, thrombocytopenia, and neutropenia that characterize acute myeloid and lymphoblastic leukemia” require prompt treatment. Leukapheresis in the presence of clinically significant evidence of leukostasis can be considered, regardless of gestational stage. When patients are diagnosed with acute myeloid leukemia during the first trimester, pregnancy termination followed by conventional induction therapy (cytarabine/anthracycline) is recommended.
Those diagnosed later in pregnancy can receive conventional induction therapy, although this seems to be associated with increased risk of fetal growth restriction and even fetal loss. “Notably, neonates rarely experience neutropenia and cardiac impairment unless exposed to lipophilic idarubicin, which should not be used,” the panelists wrote.
When acute promyelocytic leukemia is diagnosed in the first trimester, pregnancy termination is recommended before initiating conventional ATRA-anthracycline therapy. Later in pregnancy, the regimen demonstrates low teratogenicity and can be used in women diagnosed after that stage. Arsenic treatment is highly teratogenic and is prohibited throughout gestation.
Acute lymphocytic leukemia (ALL) requires prophylactic CNS therapy, including methotrexate and L-asparaginase, which are fetotoxic. Methotrexate interferes with organogenesis and is prohibited before week 20 of gestation. L-asparaginase may increase the high risk for thromboembolic events attributed to the combination of pregnancy and malignancy.
Notably, tyrosine kinase inhibitors, essential for patients with Philadelphia chromosome–positive ALL, are teratogenic. Given these limitations, women diagnosed with ALL before 20 weeks’ gestation should undergo termination of the pregnancy and start conventional treatment. After week 20, conventional chemotherapy can be administered during pregnancy. Tyrosine kinase inhibitors can be initiated postpartum.
The guidelines also contain recommendations on diagnostic testing and radiotherapy, maternal supportive care, and perinatal and pediatric aspects of hematologic malignancies in pregnancy. An online appendix offers recommendations on the treatment of rare hematologic malignancies, including hairy cell leukemia, multiple myeloma, and myelodysplastic syndromes.
Dr. Lishner and nine of his coauthors had no financial relationships to disclose. Three coauthors received honoraria and research funding or are consultants to a wide variety of drug makers.
On Twitter @maryjodales
Consensus guidelines for the perinatal management of hematologic malignancies detected during pregnancy have been issued by a panel of international experts.
The guidelines, published online in the Journal of Clinical Oncology, aim to ensure that timely treatment of the cancers is not delayed in pregnant women (doi: 10.1200/JCO.2015.62.4445).
While rare, hematologic malignancies in pregnancy introduce clinical, social, ethical, and moral dilemmas. Evidence-based data are scarce, according to the researchers, who note the International Network on Cancer, Infertility and Pregnancy registers all cancers occurring during gestation.
“Patient accrual is ongoing and essential, because registration of new cases and long-term follow-up will improve clinical knowledge and increase the level of evidence,” Dr. Michael Lishner of Tel Aviv University and Meir Medical Center, Kfar Saba, Israel, and his fellow panelists wrote.
Hodgkin lymphoma
The researchers note that Hodgkin lymphoma is the most common hematologic cancer in pregnancy, and the prognosis for these patients is excellent. When diagnosed during the first trimester, a regimen based on vinblastine monotherapy has been used. ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy can be used postpartum and has been used in cases of progression during pregnancy, the panelists wrote.
“The limited data available suggest that ABVD may be administered safely and effectively during the latter phases of pregnancy,” the panel wrote. “Although it may be associated with prematurity and lower birth weights, studies have not reported significant disadvantages.”
Non-Hodgkin lymphoma
The second most common cancer in pregnancy is non-Hodgkin lymphoma. In the case of indolent disease, watchful waiting is possible, with the intent to treat with monoclonal antibodies – with or without chemotherapy – if symptoms or evidence of disease progression are noted. Steroids can be administered during the first trimester as a bridge to the second trimester, when chemotherapy can be used with relatively greater safety, the panelists noted.
Aggressive lymphomas diagnosed before 20 weeks’ gestation warrant pregnancy termination and treatment, they recommend. When diagnosed after 20 weeks, therapy should be comparable to that given a nonpregnant woman, including monoclonal antibodies (R-CHOP).
Chronic myeloid leukemia
Chronic myeloid leukemia occurs in approximately 1 in 100,000 pregnancies and is typically diagnosed during routine blood testing in an asymptomatic patient. As a result, treatment may not be needed until the patient’s white count or platelet count have risen to levels associated with the onset of symptoms. An approximate guideline is a white cell count greater than 100 X 109/L and a platelet count greater than 500 X 109/L.
Therapeutic approaches in pregnancy include interferon-a (INF-a), which does not inhibit DNA synthesis or readily cross the placenta, and leukapheresis, which is frequently required two to three times per week during the first and second trimesters. Counts tend to drop during the third trimester, allowing less frequent intervention.
Consideration should be given to adding aspirin or low-molecular-weight heparin (LMWH) when the platelet count exceeds 1,000 X 109/L.
Myeloproliferative neoplasms
The most common myeloproliferative neoplasm seen in women of childbearing age is essential thrombocytosis.
“A large meta-analysis of pregnant women with essential thrombocytosis reported a live birth rate of 50%-70%, first trimester loss in 25%-40%, late pregnancy loss in 10%, placental abruption in 3.6%, and intrauterine growth restriction in 4.5%. Maternal morbidity is rare, but stroke has been reported,” according to the panelists.
Limited literature suggests similar outcomes for pregnant women with polycythemia vera and primary myelofibrosis.
In low-risk pregnancies, aspirin (75 mg/day) should be offered unless clearly contraindicated. For women with polycythemia vera, venesection may be continued when tolerated to maintain the hematocrit within the gestation-appropriate range.
Fetal ultrasound scans should be performed at 20, 26, and 34 weeks of gestation and uterine artery Doppler should be performed at 20 weeks’ gestation. If the mean pulsatility index exceeds 1.4, the pregnancy may be considered high risk, and treatment and monitoring should be increased.
In high-risk pregnancies, additional treatment includes cytoreductive therapy with or without LMWH. If cytoreductive therapy is required, INF-a should be titrated to maintain a platelet count of less than 400 X 109/L and hematocrit within appropriate range.
Local protocols regarding interruption of LMWH should be adhered to during labor, and dehydration should be avoided. Platelet count and hematocrit may increase postpartum, requiring cytoreductive therapy. Thromboprophylaxis should be considered at 6 weeks’ postpartum because of the increased risk of thrombosis, the guidelines note.
Acute leukemia
“The remarkable anemia, thrombocytopenia, and neutropenia that characterize acute myeloid and lymphoblastic leukemia” require prompt treatment. Leukapheresis in the presence of clinically significant evidence of leukostasis can be considered, regardless of gestational stage. When patients are diagnosed with acute myeloid leukemia during the first trimester, pregnancy termination followed by conventional induction therapy (cytarabine/anthracycline) is recommended.
Those diagnosed later in pregnancy can receive conventional induction therapy, although this seems to be associated with increased risk of fetal growth restriction and even fetal loss. “Notably, neonates rarely experience neutropenia and cardiac impairment unless exposed to lipophilic idarubicin, which should not be used,” the panelists wrote.
When acute promyelocytic leukemia is diagnosed in the first trimester, pregnancy termination is recommended before initiating conventional ATRA-anthracycline therapy. Later in pregnancy, the regimen demonstrates low teratogenicity and can be used in women diagnosed after that stage. Arsenic treatment is highly teratogenic and is prohibited throughout gestation.
Acute lymphocytic leukemia (ALL) requires prophylactic CNS therapy, including methotrexate and L-asparaginase, which are fetotoxic. Methotrexate interferes with organogenesis and is prohibited before week 20 of gestation. L-asparaginase may increase the high risk for thromboembolic events attributed to the combination of pregnancy and malignancy.
Notably, tyrosine kinase inhibitors, essential for patients with Philadelphia chromosome–positive ALL, are teratogenic. Given these limitations, women diagnosed with ALL before 20 weeks’ gestation should undergo termination of the pregnancy and start conventional treatment. After week 20, conventional chemotherapy can be administered during pregnancy. Tyrosine kinase inhibitors can be initiated postpartum.
The guidelines also contain recommendations on diagnostic testing and radiotherapy, maternal supportive care, and perinatal and pediatric aspects of hematologic malignancies in pregnancy. An online appendix offers recommendations on the treatment of rare hematologic malignancies, including hairy cell leukemia, multiple myeloma, and myelodysplastic syndromes.
Dr. Lishner and nine of his coauthors had no financial relationships to disclose. Three coauthors received honoraria and research funding or are consultants to a wide variety of drug makers.
On Twitter @maryjodales
Consensus guidelines for the perinatal management of hematologic malignancies detected during pregnancy have been issued by a panel of international experts.
The guidelines, published online in the Journal of Clinical Oncology, aim to ensure that timely treatment of the cancers is not delayed in pregnant women (doi: 10.1200/JCO.2015.62.4445).
While rare, hematologic malignancies in pregnancy introduce clinical, social, ethical, and moral dilemmas. Evidence-based data are scarce, according to the researchers, who note the International Network on Cancer, Infertility and Pregnancy registers all cancers occurring during gestation.
“Patient accrual is ongoing and essential, because registration of new cases and long-term follow-up will improve clinical knowledge and increase the level of evidence,” Dr. Michael Lishner of Tel Aviv University and Meir Medical Center, Kfar Saba, Israel, and his fellow panelists wrote.
Hodgkin lymphoma
The researchers note that Hodgkin lymphoma is the most common hematologic cancer in pregnancy, and the prognosis for these patients is excellent. When diagnosed during the first trimester, a regimen based on vinblastine monotherapy has been used. ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) therapy can be used postpartum and has been used in cases of progression during pregnancy, the panelists wrote.
“The limited data available suggest that ABVD may be administered safely and effectively during the latter phases of pregnancy,” the panel wrote. “Although it may be associated with prematurity and lower birth weights, studies have not reported significant disadvantages.”
Non-Hodgkin lymphoma
The second most common cancer in pregnancy is non-Hodgkin lymphoma. In the case of indolent disease, watchful waiting is possible, with the intent to treat with monoclonal antibodies – with or without chemotherapy – if symptoms or evidence of disease progression are noted. Steroids can be administered during the first trimester as a bridge to the second trimester, when chemotherapy can be used with relatively greater safety, the panelists noted.
Aggressive lymphomas diagnosed before 20 weeks’ gestation warrant pregnancy termination and treatment, they recommend. When diagnosed after 20 weeks, therapy should be comparable to that given a nonpregnant woman, including monoclonal antibodies (R-CHOP).
Chronic myeloid leukemia
Chronic myeloid leukemia occurs in approximately 1 in 100,000 pregnancies and is typically diagnosed during routine blood testing in an asymptomatic patient. As a result, treatment may not be needed until the patient’s white count or platelet count have risen to levels associated with the onset of symptoms. An approximate guideline is a white cell count greater than 100 X 109/L and a platelet count greater than 500 X 109/L.
Therapeutic approaches in pregnancy include interferon-a (INF-a), which does not inhibit DNA synthesis or readily cross the placenta, and leukapheresis, which is frequently required two to three times per week during the first and second trimesters. Counts tend to drop during the third trimester, allowing less frequent intervention.
Consideration should be given to adding aspirin or low-molecular-weight heparin (LMWH) when the platelet count exceeds 1,000 X 109/L.
Myeloproliferative neoplasms
The most common myeloproliferative neoplasm seen in women of childbearing age is essential thrombocytosis.
“A large meta-analysis of pregnant women with essential thrombocytosis reported a live birth rate of 50%-70%, first trimester loss in 25%-40%, late pregnancy loss in 10%, placental abruption in 3.6%, and intrauterine growth restriction in 4.5%. Maternal morbidity is rare, but stroke has been reported,” according to the panelists.
Limited literature suggests similar outcomes for pregnant women with polycythemia vera and primary myelofibrosis.
In low-risk pregnancies, aspirin (75 mg/day) should be offered unless clearly contraindicated. For women with polycythemia vera, venesection may be continued when tolerated to maintain the hematocrit within the gestation-appropriate range.
Fetal ultrasound scans should be performed at 20, 26, and 34 weeks of gestation and uterine artery Doppler should be performed at 20 weeks’ gestation. If the mean pulsatility index exceeds 1.4, the pregnancy may be considered high risk, and treatment and monitoring should be increased.
In high-risk pregnancies, additional treatment includes cytoreductive therapy with or without LMWH. If cytoreductive therapy is required, INF-a should be titrated to maintain a platelet count of less than 400 X 109/L and hematocrit within appropriate range.
Local protocols regarding interruption of LMWH should be adhered to during labor, and dehydration should be avoided. Platelet count and hematocrit may increase postpartum, requiring cytoreductive therapy. Thromboprophylaxis should be considered at 6 weeks’ postpartum because of the increased risk of thrombosis, the guidelines note.
Acute leukemia
“The remarkable anemia, thrombocytopenia, and neutropenia that characterize acute myeloid and lymphoblastic leukemia” require prompt treatment. Leukapheresis in the presence of clinically significant evidence of leukostasis can be considered, regardless of gestational stage. When patients are diagnosed with acute myeloid leukemia during the first trimester, pregnancy termination followed by conventional induction therapy (cytarabine/anthracycline) is recommended.
Those diagnosed later in pregnancy can receive conventional induction therapy, although this seems to be associated with increased risk of fetal growth restriction and even fetal loss. “Notably, neonates rarely experience neutropenia and cardiac impairment unless exposed to lipophilic idarubicin, which should not be used,” the panelists wrote.
When acute promyelocytic leukemia is diagnosed in the first trimester, pregnancy termination is recommended before initiating conventional ATRA-anthracycline therapy. Later in pregnancy, the regimen demonstrates low teratogenicity and can be used in women diagnosed after that stage. Arsenic treatment is highly teratogenic and is prohibited throughout gestation.
Acute lymphocytic leukemia (ALL) requires prophylactic CNS therapy, including methotrexate and L-asparaginase, which are fetotoxic. Methotrexate interferes with organogenesis and is prohibited before week 20 of gestation. L-asparaginase may increase the high risk for thromboembolic events attributed to the combination of pregnancy and malignancy.
Notably, tyrosine kinase inhibitors, essential for patients with Philadelphia chromosome–positive ALL, are teratogenic. Given these limitations, women diagnosed with ALL before 20 weeks’ gestation should undergo termination of the pregnancy and start conventional treatment. After week 20, conventional chemotherapy can be administered during pregnancy. Tyrosine kinase inhibitors can be initiated postpartum.
The guidelines also contain recommendations on diagnostic testing and radiotherapy, maternal supportive care, and perinatal and pediatric aspects of hematologic malignancies in pregnancy. An online appendix offers recommendations on the treatment of rare hematologic malignancies, including hairy cell leukemia, multiple myeloma, and myelodysplastic syndromes.
Dr. Lishner and nine of his coauthors had no financial relationships to disclose. Three coauthors received honoraria and research funding or are consultants to a wide variety of drug makers.
On Twitter @maryjodales
FROM JOURNAL OF CLINICAL ONCOLOGY
Pediatric heart transplant results not improving
A 25-year study of heart transplants in children with congenital heart disease (CHD) at one institution has found that results haven’t improved over time despite advances in technology and techniques. To improve outcomes, transplant surgeons may need to do a better job of selecting patients and matching patients and donors, according to study in the December issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1455-62).
“Strategies to improve outcomes in CHD patients might need to address selection criteria, transplantation timing, pretransplant and posttransplant care,” noted Dr. Bahaaldin Alsoufi, of the division of cardiothoracic surgery, Children’s Healthcare of Atlanta, Emory University. “The effect of donor/recipient race mismatch warrants further investigation and might impact organ allocation algorithms or immunosuppression management,” wrote Dr. Alsoufi and his colleagues.
The researchers analyzed results of 124 children with CHD who had heart transplants from 1988 to 2013 at Emory University and Children’s Healthcare of Atlanta. Median age was 3.8 years; 61% were boys. Ten years after heart transplantation, 44% (54) of patients were alive without a second transplant, 13% (17) had a second transplant and 43% (53) died without a second transplant. After the second transplant, 9 of the 17 patients were alive, but 3 of them had gone onto a third transplant. Overall 15-year survival following the first transplant was 41% (51).
The study cited data from the Registry of the International Society for Heart and Lung Transplantation that reported more than 11,000 pediatric heart transplants worldwide in 2013, and CHD represents about 54% of all heart transplants in infants.
A multivariate analysis identified the following risk factors for early mortality after transplant: age younger than 12 months (hazard ration [HR] 7.2) and prolonged cardiopulmonary bypass (HR 5). Late-phase mortality risk factors were age younger than 12 months (HR 3) and donor/recipient race mismatch (HR 2.2).
“Survival was not affected by era, underlying anomaly, prior Fontan, sensitization or pulmonary artery augmentation,” wrote Dr. Alsoufi and his colleagues.
Among the risk factors, longer bypass times may be a surrogate for a more complicated operation, the authors said. But where prior sternotomy is a risk factor following a heart transplant in adults, the study found no such risk in children. Another risk factor previous reports identified is pulmonary artery augmentation, but, again, this study found no risk in the pediatric group.
The researchers looked at days on the waiting list, with a median wait of 39 days in the study group. In all, 175 children were listed for transplants, but 51 did not go through for various reasons. Most of the children with CHD who had a heart transplant had previous surgery; only 13% had a primary heart transplant, mostly in the earlier phase of the study.
Dr. Alsoufi and coauthors also identified African American race as a risk factor for lower survival, which is consistent with other reports. But this study agreed with a previous report that donor/recipient race mismatch was a significant risk factor in white and African American patients (Ann Thorac Surg. 2009;87:204-9). “While our finding might be anecdotal and specific to our geographic population, this warrants some investigation and might have some impact on future organ allocation algorithms and immunosuppression management,” the researchers wrote.
The authors had no relevant disclosures. Emory University School of Medicine, Children’s Healthcare of Atlanta provided study funding.
In his invited commentary, Dr. Robert D.B. Jaquiss of Duke University, Durham, N.C., took issue with the study authors’ “distress” at the lack of improvement in survival over the 25-year term of the study (J Thorac Cardiovasc Surg. 2015;150:1463-4) . Using the year 2000 as a demarcation line for early and late-phase results, Dr. Jaquiss said, “It must be pointed out that in the latter period recipients were much more ill.” He noted that 89% of post-2000 heart transplant patients had UNOS status 1 vs. 49% in the pre-2000 period.

|
Dr. Robert Jaquiss |
“Considering these between-era differences, an alternative, less ‘discouraging’ interpretation is that excellent outcomes were maintained despite the trend toward transplantation in sicker patients, undergoing more complex transplants, with longer ischemic times,” he said.
Dr. Jaquiss also cited “remarkably outstanding outcomes” in Fontan patients, reporting only one operative death in 33 patients. He found the lower survival for African-American patients in the study group “more sobering,” but also controversial because, among other reasons, “a complete mechanistic explanation remains elusive.” How these findings influence pediatric heart transplant practice “requires thoughtful and extensive investigation and discussion,” he said.
Wait-list mortality and mechanical bridge to transplant also deserve mention, he noted. “Though they are only briefly mentioned, the patients who died prior to transplant provide mute testimony to the lack of timely access to suitable donors,” Dr. Jaquiss said. Durable mechanical circulatory support can provide a bridge for these patients, but was not available through the majority of the study period.
“It is striking that no patient in this report was supported by a ventricular assist device (VAD), and only a small number (5%) had been on [extracorporeal membrane oxygenation] support,” Dr. Jaquiss said. “This is an unfortunate and unavoidable weakness of this report, given the recent introduction of VADs for pediatric heart transplant candidates.” The use of VAD in patients with CHD is “increasing rapidly,” he said.
Dr. Jaquiss had no disclosures.
In his invited commentary, Dr. Robert D.B. Jaquiss of Duke University, Durham, N.C., took issue with the study authors’ “distress” at the lack of improvement in survival over the 25-year term of the study (J Thorac Cardiovasc Surg. 2015;150:1463-4) . Using the year 2000 as a demarcation line for early and late-phase results, Dr. Jaquiss said, “It must be pointed out that in the latter period recipients were much more ill.” He noted that 89% of post-2000 heart transplant patients had UNOS status 1 vs. 49% in the pre-2000 period.

|
Dr. Robert Jaquiss |
“Considering these between-era differences, an alternative, less ‘discouraging’ interpretation is that excellent outcomes were maintained despite the trend toward transplantation in sicker patients, undergoing more complex transplants, with longer ischemic times,” he said.
Dr. Jaquiss also cited “remarkably outstanding outcomes” in Fontan patients, reporting only one operative death in 33 patients. He found the lower survival for African-American patients in the study group “more sobering,” but also controversial because, among other reasons, “a complete mechanistic explanation remains elusive.” How these findings influence pediatric heart transplant practice “requires thoughtful and extensive investigation and discussion,” he said.
Wait-list mortality and mechanical bridge to transplant also deserve mention, he noted. “Though they are only briefly mentioned, the patients who died prior to transplant provide mute testimony to the lack of timely access to suitable donors,” Dr. Jaquiss said. Durable mechanical circulatory support can provide a bridge for these patients, but was not available through the majority of the study period.
“It is striking that no patient in this report was supported by a ventricular assist device (VAD), and only a small number (5%) had been on [extracorporeal membrane oxygenation] support,” Dr. Jaquiss said. “This is an unfortunate and unavoidable weakness of this report, given the recent introduction of VADs for pediatric heart transplant candidates.” The use of VAD in patients with CHD is “increasing rapidly,” he said.
Dr. Jaquiss had no disclosures.
In his invited commentary, Dr. Robert D.B. Jaquiss of Duke University, Durham, N.C., took issue with the study authors’ “distress” at the lack of improvement in survival over the 25-year term of the study (J Thorac Cardiovasc Surg. 2015;150:1463-4) . Using the year 2000 as a demarcation line for early and late-phase results, Dr. Jaquiss said, “It must be pointed out that in the latter period recipients were much more ill.” He noted that 89% of post-2000 heart transplant patients had UNOS status 1 vs. 49% in the pre-2000 period.

|
Dr. Robert Jaquiss |
“Considering these between-era differences, an alternative, less ‘discouraging’ interpretation is that excellent outcomes were maintained despite the trend toward transplantation in sicker patients, undergoing more complex transplants, with longer ischemic times,” he said.
Dr. Jaquiss also cited “remarkably outstanding outcomes” in Fontan patients, reporting only one operative death in 33 patients. He found the lower survival for African-American patients in the study group “more sobering,” but also controversial because, among other reasons, “a complete mechanistic explanation remains elusive.” How these findings influence pediatric heart transplant practice “requires thoughtful and extensive investigation and discussion,” he said.
Wait-list mortality and mechanical bridge to transplant also deserve mention, he noted. “Though they are only briefly mentioned, the patients who died prior to transplant provide mute testimony to the lack of timely access to suitable donors,” Dr. Jaquiss said. Durable mechanical circulatory support can provide a bridge for these patients, but was not available through the majority of the study period.
“It is striking that no patient in this report was supported by a ventricular assist device (VAD), and only a small number (5%) had been on [extracorporeal membrane oxygenation] support,” Dr. Jaquiss said. “This is an unfortunate and unavoidable weakness of this report, given the recent introduction of VADs for pediatric heart transplant candidates.” The use of VAD in patients with CHD is “increasing rapidly,” he said.
Dr. Jaquiss had no disclosures.
A 25-year study of heart transplants in children with congenital heart disease (CHD) at one institution has found that results haven’t improved over time despite advances in technology and techniques. To improve outcomes, transplant surgeons may need to do a better job of selecting patients and matching patients and donors, according to study in the December issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1455-62).
“Strategies to improve outcomes in CHD patients might need to address selection criteria, transplantation timing, pretransplant and posttransplant care,” noted Dr. Bahaaldin Alsoufi, of the division of cardiothoracic surgery, Children’s Healthcare of Atlanta, Emory University. “The effect of donor/recipient race mismatch warrants further investigation and might impact organ allocation algorithms or immunosuppression management,” wrote Dr. Alsoufi and his colleagues.
The researchers analyzed results of 124 children with CHD who had heart transplants from 1988 to 2013 at Emory University and Children’s Healthcare of Atlanta. Median age was 3.8 years; 61% were boys. Ten years after heart transplantation, 44% (54) of patients were alive without a second transplant, 13% (17) had a second transplant and 43% (53) died without a second transplant. After the second transplant, 9 of the 17 patients were alive, but 3 of them had gone onto a third transplant. Overall 15-year survival following the first transplant was 41% (51).
The study cited data from the Registry of the International Society for Heart and Lung Transplantation that reported more than 11,000 pediatric heart transplants worldwide in 2013, and CHD represents about 54% of all heart transplants in infants.
A multivariate analysis identified the following risk factors for early mortality after transplant: age younger than 12 months (hazard ration [HR] 7.2) and prolonged cardiopulmonary bypass (HR 5). Late-phase mortality risk factors were age younger than 12 months (HR 3) and donor/recipient race mismatch (HR 2.2).
“Survival was not affected by era, underlying anomaly, prior Fontan, sensitization or pulmonary artery augmentation,” wrote Dr. Alsoufi and his colleagues.
Among the risk factors, longer bypass times may be a surrogate for a more complicated operation, the authors said. But where prior sternotomy is a risk factor following a heart transplant in adults, the study found no such risk in children. Another risk factor previous reports identified is pulmonary artery augmentation, but, again, this study found no risk in the pediatric group.
The researchers looked at days on the waiting list, with a median wait of 39 days in the study group. In all, 175 children were listed for transplants, but 51 did not go through for various reasons. Most of the children with CHD who had a heart transplant had previous surgery; only 13% had a primary heart transplant, mostly in the earlier phase of the study.
Dr. Alsoufi and coauthors also identified African American race as a risk factor for lower survival, which is consistent with other reports. But this study agreed with a previous report that donor/recipient race mismatch was a significant risk factor in white and African American patients (Ann Thorac Surg. 2009;87:204-9). “While our finding might be anecdotal and specific to our geographic population, this warrants some investigation and might have some impact on future organ allocation algorithms and immunosuppression management,” the researchers wrote.
The authors had no relevant disclosures. Emory University School of Medicine, Children’s Healthcare of Atlanta provided study funding.
A 25-year study of heart transplants in children with congenital heart disease (CHD) at one institution has found that results haven’t improved over time despite advances in technology and techniques. To improve outcomes, transplant surgeons may need to do a better job of selecting patients and matching patients and donors, according to study in the December issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015;150:1455-62).
“Strategies to improve outcomes in CHD patients might need to address selection criteria, transplantation timing, pretransplant and posttransplant care,” noted Dr. Bahaaldin Alsoufi, of the division of cardiothoracic surgery, Children’s Healthcare of Atlanta, Emory University. “The effect of donor/recipient race mismatch warrants further investigation and might impact organ allocation algorithms or immunosuppression management,” wrote Dr. Alsoufi and his colleagues.
The researchers analyzed results of 124 children with CHD who had heart transplants from 1988 to 2013 at Emory University and Children’s Healthcare of Atlanta. Median age was 3.8 years; 61% were boys. Ten years after heart transplantation, 44% (54) of patients were alive without a second transplant, 13% (17) had a second transplant and 43% (53) died without a second transplant. After the second transplant, 9 of the 17 patients were alive, but 3 of them had gone onto a third transplant. Overall 15-year survival following the first transplant was 41% (51).
The study cited data from the Registry of the International Society for Heart and Lung Transplantation that reported more than 11,000 pediatric heart transplants worldwide in 2013, and CHD represents about 54% of all heart transplants in infants.
A multivariate analysis identified the following risk factors for early mortality after transplant: age younger than 12 months (hazard ration [HR] 7.2) and prolonged cardiopulmonary bypass (HR 5). Late-phase mortality risk factors were age younger than 12 months (HR 3) and donor/recipient race mismatch (HR 2.2).
“Survival was not affected by era, underlying anomaly, prior Fontan, sensitization or pulmonary artery augmentation,” wrote Dr. Alsoufi and his colleagues.
Among the risk factors, longer bypass times may be a surrogate for a more complicated operation, the authors said. But where prior sternotomy is a risk factor following a heart transplant in adults, the study found no such risk in children. Another risk factor previous reports identified is pulmonary artery augmentation, but, again, this study found no risk in the pediatric group.
The researchers looked at days on the waiting list, with a median wait of 39 days in the study group. In all, 175 children were listed for transplants, but 51 did not go through for various reasons. Most of the children with CHD who had a heart transplant had previous surgery; only 13% had a primary heart transplant, mostly in the earlier phase of the study.
Dr. Alsoufi and coauthors also identified African American race as a risk factor for lower survival, which is consistent with other reports. But this study agreed with a previous report that donor/recipient race mismatch was a significant risk factor in white and African American patients (Ann Thorac Surg. 2009;87:204-9). “While our finding might be anecdotal and specific to our geographic population, this warrants some investigation and might have some impact on future organ allocation algorithms and immunosuppression management,” the researchers wrote.
The authors had no relevant disclosures. Emory University School of Medicine, Children’s Healthcare of Atlanta provided study funding.
Key clinical point: Pediatric heart transplantation outcomes for congenital heart disease haven’t improved in the current era, indicating ongoing challenges.
Major finding: Ten years following heart transplantation, 13% of patients had undergone retransplantation, 43% had died without retransplantation, and 44% were alive without retransplantation.
Data source: A review of 124 children with congenital heart disease who had heart transplantation at a single center.
Disclosures: The study authors had no relationships to disclose.
LISTEN NOW: Scott Sears, MD, Discusses Hospitalist Challenges with Unassigned, Uninsured Patients
Use of isolation in juvenile detention centers
Isolation in juvenile detention centers persists despite ample data demonstrating the traumatizing consequences to youth who often already have been traumatized. I recently visited a detention center as part of my pediatric residency’s advocacy rotation. There, I learned that youth are kept in isolation for several days, with a vague definition by staff on the limit of “several days.” Multiple words were used for confinement, the most stunning and horrific of which was “segregation” – “He got into a fight and was placed in segregation.” While in isolation or segregation – whatever it is called – mental illness and posttraumatic stress disorder are exacerbated. Youth do not participate in school classes, and they are barred from the daily hour of physical activity. In extreme cases, they go from complete isolation one day to complete freedom the next.
The vibe word in the facility I visited was “evidence-based strategies,” stressed by the new administration. But evidence-based strategies do not include isolation. They include educating staff about the pervasive effects of trauma in children. They include communication interventions, conflict resolution, and the implementation of rewards such as extra visitation, computer time, or the use of an adolescent’s own personal hygiene items or clothing. They include knowledge of the adolescent brain, and how the use of isolation in juvenile centers has led to increased suicide rates in those children.
Lindsay M. Hayes, author of the National Center on Institutions and Alternatives’ 2004 report “Juvenile Suicide in Confinement: A National Study,” wrote: ”Although room confinement remains a staple in most juvenile facilities, it is a sanction that can have deadly consequences. … More than 50% of all youths’ suicides in juvenile facilities occurred while young people were isolated alone in their rooms, and … more than 60% of young people who committed suicide in custody had a history of being held in isolation.”
The United Nations has called on all countries to absolutely prohibit solitary confinement for juveniles, as has the American Academy of Child and Adolescent Psychiatry. Thus, extreme isolation should not be another tool for juvenile detention centers.
Currently, 20 states have banned solitary confinement in juvenile detention facilities. The major barriers are from staff, who state it would remove a tool, put staff in danger, and allow youth to run the facilities. None of these has been shown to be true. Some juvenile detention centers have changed the traditional meaning of isolation – youth will have a minimum of 8 hours away from isolation when confined for a day or longer. “During that 8 hours, they have the opportunity to talk to and be in the company of staff,” said Adam Schwartz, a lawyer for the American Civil Liberties Union of Illinois, whose lawsuit drastically limited solitary confinement practices in Illinois’s juvenile detention centers. The policy also requires that inmates in isolation continue to receive education and mental health services.
There is a human dignity that not even detainees deserve to lose. President Obama has discussed this, as has the 2012 Report of the Attorney General’s National Task Force on Children Exposed to Violence, which concluded: “Nowhere is the damaging impact of incarceration on vulnerable children more obvious than when it involves solitary confinement.” I am writing this article to raise awareness about this underreported problem in hopes that new legislation will lead to change that is in the best interest of our children.
Dr. Raffa is in postgraduate year 2 in her pediatric residency at Vanderbilt Children’s Hospital in Nashville, Tenn.
Isolation in juvenile detention centers persists despite ample data demonstrating the traumatizing consequences to youth who often already have been traumatized. I recently visited a detention center as part of my pediatric residency’s advocacy rotation. There, I learned that youth are kept in isolation for several days, with a vague definition by staff on the limit of “several days.” Multiple words were used for confinement, the most stunning and horrific of which was “segregation” – “He got into a fight and was placed in segregation.” While in isolation or segregation – whatever it is called – mental illness and posttraumatic stress disorder are exacerbated. Youth do not participate in school classes, and they are barred from the daily hour of physical activity. In extreme cases, they go from complete isolation one day to complete freedom the next.
The vibe word in the facility I visited was “evidence-based strategies,” stressed by the new administration. But evidence-based strategies do not include isolation. They include educating staff about the pervasive effects of trauma in children. They include communication interventions, conflict resolution, and the implementation of rewards such as extra visitation, computer time, or the use of an adolescent’s own personal hygiene items or clothing. They include knowledge of the adolescent brain, and how the use of isolation in juvenile centers has led to increased suicide rates in those children.
Lindsay M. Hayes, author of the National Center on Institutions and Alternatives’ 2004 report “Juvenile Suicide in Confinement: A National Study,” wrote: ”Although room confinement remains a staple in most juvenile facilities, it is a sanction that can have deadly consequences. … More than 50% of all youths’ suicides in juvenile facilities occurred while young people were isolated alone in their rooms, and … more than 60% of young people who committed suicide in custody had a history of being held in isolation.”
The United Nations has called on all countries to absolutely prohibit solitary confinement for juveniles, as has the American Academy of Child and Adolescent Psychiatry. Thus, extreme isolation should not be another tool for juvenile detention centers.
Currently, 20 states have banned solitary confinement in juvenile detention facilities. The major barriers are from staff, who state it would remove a tool, put staff in danger, and allow youth to run the facilities. None of these has been shown to be true. Some juvenile detention centers have changed the traditional meaning of isolation – youth will have a minimum of 8 hours away from isolation when confined for a day or longer. “During that 8 hours, they have the opportunity to talk to and be in the company of staff,” said Adam Schwartz, a lawyer for the American Civil Liberties Union of Illinois, whose lawsuit drastically limited solitary confinement practices in Illinois’s juvenile detention centers. The policy also requires that inmates in isolation continue to receive education and mental health services.
There is a human dignity that not even detainees deserve to lose. President Obama has discussed this, as has the 2012 Report of the Attorney General’s National Task Force on Children Exposed to Violence, which concluded: “Nowhere is the damaging impact of incarceration on vulnerable children more obvious than when it involves solitary confinement.” I am writing this article to raise awareness about this underreported problem in hopes that new legislation will lead to change that is in the best interest of our children.
Dr. Raffa is in postgraduate year 2 in her pediatric residency at Vanderbilt Children’s Hospital in Nashville, Tenn.
Isolation in juvenile detention centers persists despite ample data demonstrating the traumatizing consequences to youth who often already have been traumatized. I recently visited a detention center as part of my pediatric residency’s advocacy rotation. There, I learned that youth are kept in isolation for several days, with a vague definition by staff on the limit of “several days.” Multiple words were used for confinement, the most stunning and horrific of which was “segregation” – “He got into a fight and was placed in segregation.” While in isolation or segregation – whatever it is called – mental illness and posttraumatic stress disorder are exacerbated. Youth do not participate in school classes, and they are barred from the daily hour of physical activity. In extreme cases, they go from complete isolation one day to complete freedom the next.
The vibe word in the facility I visited was “evidence-based strategies,” stressed by the new administration. But evidence-based strategies do not include isolation. They include educating staff about the pervasive effects of trauma in children. They include communication interventions, conflict resolution, and the implementation of rewards such as extra visitation, computer time, or the use of an adolescent’s own personal hygiene items or clothing. They include knowledge of the adolescent brain, and how the use of isolation in juvenile centers has led to increased suicide rates in those children.
Lindsay M. Hayes, author of the National Center on Institutions and Alternatives’ 2004 report “Juvenile Suicide in Confinement: A National Study,” wrote: ”Although room confinement remains a staple in most juvenile facilities, it is a sanction that can have deadly consequences. … More than 50% of all youths’ suicides in juvenile facilities occurred while young people were isolated alone in their rooms, and … more than 60% of young people who committed suicide in custody had a history of being held in isolation.”
The United Nations has called on all countries to absolutely prohibit solitary confinement for juveniles, as has the American Academy of Child and Adolescent Psychiatry. Thus, extreme isolation should not be another tool for juvenile detention centers.
Currently, 20 states have banned solitary confinement in juvenile detention facilities. The major barriers are from staff, who state it would remove a tool, put staff in danger, and allow youth to run the facilities. None of these has been shown to be true. Some juvenile detention centers have changed the traditional meaning of isolation – youth will have a minimum of 8 hours away from isolation when confined for a day or longer. “During that 8 hours, they have the opportunity to talk to and be in the company of staff,” said Adam Schwartz, a lawyer for the American Civil Liberties Union of Illinois, whose lawsuit drastically limited solitary confinement practices in Illinois’s juvenile detention centers. The policy also requires that inmates in isolation continue to receive education and mental health services.
There is a human dignity that not even detainees deserve to lose. President Obama has discussed this, as has the 2012 Report of the Attorney General’s National Task Force on Children Exposed to Violence, which concluded: “Nowhere is the damaging impact of incarceration on vulnerable children more obvious than when it involves solitary confinement.” I am writing this article to raise awareness about this underreported problem in hopes that new legislation will lead to change that is in the best interest of our children.
Dr. Raffa is in postgraduate year 2 in her pediatric residency at Vanderbilt Children’s Hospital in Nashville, Tenn.
AHA: Three measures risk stratify acute heart failure
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.
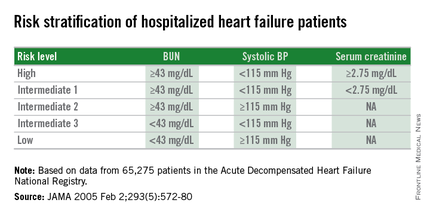
The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.

The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.

The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Dichotomous cutoffs for BUN, systolic BP, and serum creatinine together robustly risk stratified patients hospitalized for acute decompensated heart failure.
Major finding: Three baseline measures together stratified patients over an eightfold range for 30-day mortality following hospital discharge.
Data source: Application of the risk-stratification formula to 3,628 heart failure patients hospitalized at one U.S. center during 2000-2013.
Disclosures: Dr. Win had no disclosures.
Electronic Health Records Key Driver of Physician Burnout
Half of U.S. physicians are experiencing some of the symptoms of burnout, with even higher rates for general internists. Implementation of the electronic health record (EHR) has been cited as the biggest driver of physician job dissatisfaction, said Christine Sinsky, MD, a former hospitalist and currently vice president of professional satisfaction at the American Medical Association (AMA), to attendees at the 19th Management of the Hospitalized Patient Conference, presented by the University of California-San Francisco.1
Dr. Sinsky deemed physician discontent “the canary in the coal mine” for a dysfunctional healthcare system. After visiting 23 high-functioning medical teams, Dr. Sinsky said she had found that 70% to 80% of physician work output could be considered waste, defined as work that doesn’t need to be done and doesn’t add value to the patient. The AMA, she said, has made a commitment to addressing physicians’ dissatisfaction and burnout.
Dr. Sinsky offered a number of suggestions for physicians and the larger system. Among them was the idea of medical teams employing a documentation specialist, or scribe, to accompany physicians on patient rounds to help with the clerical tasks that divert physicians from patient care. She also cited David Reuben, MD, a gerontologist at UCLA whose JAMA Internal Medicine study documented his training of physician “practice partners,” often medical or nursing students, who help queue up orders in the EHR, and the improved patient satisfaction that resulted.2
“Be bold,” she advised hospitalists. “The patient care delivery modes of the future can’t be met with staffing models from the past.”
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Friedberg M, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. 2013. Accessed November 3, 2015.
- Reuben DB, Knudsen J, Senelick W, Glazier E, Koretz BK. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174(7):1190-1193.
Half of U.S. physicians are experiencing some of the symptoms of burnout, with even higher rates for general internists. Implementation of the electronic health record (EHR) has been cited as the biggest driver of physician job dissatisfaction, said Christine Sinsky, MD, a former hospitalist and currently vice president of professional satisfaction at the American Medical Association (AMA), to attendees at the 19th Management of the Hospitalized Patient Conference, presented by the University of California-San Francisco.1
Dr. Sinsky deemed physician discontent “the canary in the coal mine” for a dysfunctional healthcare system. After visiting 23 high-functioning medical teams, Dr. Sinsky said she had found that 70% to 80% of physician work output could be considered waste, defined as work that doesn’t need to be done and doesn’t add value to the patient. The AMA, she said, has made a commitment to addressing physicians’ dissatisfaction and burnout.
Dr. Sinsky offered a number of suggestions for physicians and the larger system. Among them was the idea of medical teams employing a documentation specialist, or scribe, to accompany physicians on patient rounds to help with the clerical tasks that divert physicians from patient care. She also cited David Reuben, MD, a gerontologist at UCLA whose JAMA Internal Medicine study documented his training of physician “practice partners,” often medical or nursing students, who help queue up orders in the EHR, and the improved patient satisfaction that resulted.2
“Be bold,” she advised hospitalists. “The patient care delivery modes of the future can’t be met with staffing models from the past.”
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Friedberg M, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. 2013. Accessed November 3, 2015.
- Reuben DB, Knudsen J, Senelick W, Glazier E, Koretz BK. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174(7):1190-1193.
Half of U.S. physicians are experiencing some of the symptoms of burnout, with even higher rates for general internists. Implementation of the electronic health record (EHR) has been cited as the biggest driver of physician job dissatisfaction, said Christine Sinsky, MD, a former hospitalist and currently vice president of professional satisfaction at the American Medical Association (AMA), to attendees at the 19th Management of the Hospitalized Patient Conference, presented by the University of California-San Francisco.1
Dr. Sinsky deemed physician discontent “the canary in the coal mine” for a dysfunctional healthcare system. After visiting 23 high-functioning medical teams, Dr. Sinsky said she had found that 70% to 80% of physician work output could be considered waste, defined as work that doesn’t need to be done and doesn’t add value to the patient. The AMA, she said, has made a commitment to addressing physicians’ dissatisfaction and burnout.
Dr. Sinsky offered a number of suggestions for physicians and the larger system. Among them was the idea of medical teams employing a documentation specialist, or scribe, to accompany physicians on patient rounds to help with the clerical tasks that divert physicians from patient care. She also cited David Reuben, MD, a gerontologist at UCLA whose JAMA Internal Medicine study documented his training of physician “practice partners,” often medical or nursing students, who help queue up orders in the EHR, and the improved patient satisfaction that resulted.2
“Be bold,” she advised hospitalists. “The patient care delivery modes of the future can’t be met with staffing models from the past.”
Larry Beresford is a freelance writer in Alameda, Calif.
References
- Friedberg M, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. 2013. Accessed November 3, 2015.
- Reuben DB, Knudsen J, Senelick W, Glazier E, Koretz BK. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174(7):1190-1193.
Claims Data Misclassify Cardiovascular Disease Event Rates
NEW YORK - Diagnostic codes from administrative claims data underestimate cardiovascular disease event rates, researchers report.
"Increasingly, the diagnostic codes from administrative claims data are being used to measure clinical outcomes," Dr. Bruce M. Psaty from the University of Washington, Seattle, said by email. "The methods of using only claims data as outcomes nonetheless influence the results. Methods that seek to avoid misclassification tend to underestimate event rates, and methods that attempt to include all events tend to include misclassified events such as non-event hospitalizations as part of the outcome."
Dr. Psaty's team used data from the Cardiovascular Health Study (CHS) to evaluate the degree of both misclassification and underestimation of event rates for cardiovascular disease outcomes identified solely from claims data compared with those identified through active surveillance.
An ICD9 code of 410 in the first position had a 90.6% positive predictive value (PPV) for MI, but this code only identified 53.8% of the incident MIs ascertained by active surveillance. Inclusion of this code as a secondary diagnosis identified an additional 16.6% of MI events.
Similarly, main stroke codes in the first position had an 80.4% PPV for stroke, but identified only 63.8% of the incident stroke events. For heart failure, main diagnostic codes had a high PPV of 93.2%, but identified only 27.2% of heart failure events.
Estimates of disease incidence differed markedly according to whether the incidence rates were determined by CHS surveillance, a first-position diagnostic code, or a diagnostic code in any position.
In general, misclassified events in the administrative claims data appeared to have little effect on the magnitude of associations for most cardiovascular disease risk factors, the researchers report in Circulation, online Nov. 4.
"No study is perfect, and some incomplete identification of events in a study is common," Dr. Psaty explained. "The effect depends on both the type of study and the degree of incompleteness. In a clinical trial, if there is no bias, the relative-risk comparison between the two groups remains valid even if some of the events were missed. If, on the other hand, the goal is the development of a model for the prediction of event rates to decide on whether to start a cholesterol lowering therapy, the incomplete identification of events introduces a bias in the model that is directly related to the degree of incompleteness in the identification of events."
"Events data collection should be appropriate for the study," Dr. Psaty concluded. "Published studies need to provide sufficient detail so that readers can judge whether the methods were indeed fit for purpose."
NEW YORK - Diagnostic codes from administrative claims data underestimate cardiovascular disease event rates, researchers report.
"Increasingly, the diagnostic codes from administrative claims data are being used to measure clinical outcomes," Dr. Bruce M. Psaty from the University of Washington, Seattle, said by email. "The methods of using only claims data as outcomes nonetheless influence the results. Methods that seek to avoid misclassification tend to underestimate event rates, and methods that attempt to include all events tend to include misclassified events such as non-event hospitalizations as part of the outcome."
Dr. Psaty's team used data from the Cardiovascular Health Study (CHS) to evaluate the degree of both misclassification and underestimation of event rates for cardiovascular disease outcomes identified solely from claims data compared with those identified through active surveillance.
An ICD9 code of 410 in the first position had a 90.6% positive predictive value (PPV) for MI, but this code only identified 53.8% of the incident MIs ascertained by active surveillance. Inclusion of this code as a secondary diagnosis identified an additional 16.6% of MI events.
Similarly, main stroke codes in the first position had an 80.4% PPV for stroke, but identified only 63.8% of the incident stroke events. For heart failure, main diagnostic codes had a high PPV of 93.2%, but identified only 27.2% of heart failure events.
Estimates of disease incidence differed markedly according to whether the incidence rates were determined by CHS surveillance, a first-position diagnostic code, or a diagnostic code in any position.
In general, misclassified events in the administrative claims data appeared to have little effect on the magnitude of associations for most cardiovascular disease risk factors, the researchers report in Circulation, online Nov. 4.
"No study is perfect, and some incomplete identification of events in a study is common," Dr. Psaty explained. "The effect depends on both the type of study and the degree of incompleteness. In a clinical trial, if there is no bias, the relative-risk comparison between the two groups remains valid even if some of the events were missed. If, on the other hand, the goal is the development of a model for the prediction of event rates to decide on whether to start a cholesterol lowering therapy, the incomplete identification of events introduces a bias in the model that is directly related to the degree of incompleteness in the identification of events."
"Events data collection should be appropriate for the study," Dr. Psaty concluded. "Published studies need to provide sufficient detail so that readers can judge whether the methods were indeed fit for purpose."
NEW YORK - Diagnostic codes from administrative claims data underestimate cardiovascular disease event rates, researchers report.
"Increasingly, the diagnostic codes from administrative claims data are being used to measure clinical outcomes," Dr. Bruce M. Psaty from the University of Washington, Seattle, said by email. "The methods of using only claims data as outcomes nonetheless influence the results. Methods that seek to avoid misclassification tend to underestimate event rates, and methods that attempt to include all events tend to include misclassified events such as non-event hospitalizations as part of the outcome."
Dr. Psaty's team used data from the Cardiovascular Health Study (CHS) to evaluate the degree of both misclassification and underestimation of event rates for cardiovascular disease outcomes identified solely from claims data compared with those identified through active surveillance.
An ICD9 code of 410 in the first position had a 90.6% positive predictive value (PPV) for MI, but this code only identified 53.8% of the incident MIs ascertained by active surveillance. Inclusion of this code as a secondary diagnosis identified an additional 16.6% of MI events.
Similarly, main stroke codes in the first position had an 80.4% PPV for stroke, but identified only 63.8% of the incident stroke events. For heart failure, main diagnostic codes had a high PPV of 93.2%, but identified only 27.2% of heart failure events.
Estimates of disease incidence differed markedly according to whether the incidence rates were determined by CHS surveillance, a first-position diagnostic code, or a diagnostic code in any position.
In general, misclassified events in the administrative claims data appeared to have little effect on the magnitude of associations for most cardiovascular disease risk factors, the researchers report in Circulation, online Nov. 4.
"No study is perfect, and some incomplete identification of events in a study is common," Dr. Psaty explained. "The effect depends on both the type of study and the degree of incompleteness. In a clinical trial, if there is no bias, the relative-risk comparison between the two groups remains valid even if some of the events were missed. If, on the other hand, the goal is the development of a model for the prediction of event rates to decide on whether to start a cholesterol lowering therapy, the incomplete identification of events introduces a bias in the model that is directly related to the degree of incompleteness in the identification of events."
"Events data collection should be appropriate for the study," Dr. Psaty concluded. "Published studies need to provide sufficient detail so that readers can judge whether the methods were indeed fit for purpose."
Movers and Shakers in Hospital Medicine December 2015
John Carlile, MD, is the new medical director of pediatric hospitalist and intensivist services at Summerville (S.C.) Medical Center. Most recently, Dr. Carlile worked as a pediatric critical care specialist at Cox Medical Center in Springfield, Mo.


Elaine McElveen, RN, has been named director of the hospitalist program at McLeod Medical Center Dillon in Dillon, S.C. In her new role, McElveen also will oversee the case management department. She has been a practicing nurse for the McLeod Health system for 28 years.

Christine Wentt, PA, an adult hospitalist physician assistant, has been inducted into the VIP Woman of the Year Circle for 2015-2016 by the National Association of Professional Women (NAPW). Wentt works for Physicians Inpatient Care Specialists (MDICS), a private hospitalist group in Glen Burnie, Md. Wentt also serves as an adjunct professor in Drexel University’s physician assistant program.
Robert Zurcher, MD, has been appointed chief medical officer for emergency medicine at HNI Healthcare (formerly Hospitalists Now, Inc.), based in Austin, Texas. Dr. Zurcher comes to HNI Healthcare from IPC Healthcare, where he served as the administrative director for their Northwest Region. Dr. Zurcher has 27 years of practice experience and has founded and managed physician services companies in both hospital medicine and emergency medicine.
Business Moves
North Hollywood, Calif.-based IPC Healthcare recently announced its acquisition of Hospital Medicine Consultants, LLC, in Elgin, Ill., a locally owned practice serving several hospitals in the Chicago suburbs. IPC staffs hospitalist providers in more than 400 hospitals across the country.
The Schumacher Group, a private hospitalist and emergency medicine provider, announced the implementation of a new telehospitalist program at Abrom Kaplan Memorial and Acadia General Hospitals, both in Lafayette, La. The program will allow physicians to provide overnight care to the two facilities from a remote location. The Schumacher Group was founded in 1994 and currently serves more than four million patients per year.
Mike O’Neal is a freelance writer in New York City.
John Carlile, MD, is the new medical director of pediatric hospitalist and intensivist services at Summerville (S.C.) Medical Center. Most recently, Dr. Carlile worked as a pediatric critical care specialist at Cox Medical Center in Springfield, Mo.


Elaine McElveen, RN, has been named director of the hospitalist program at McLeod Medical Center Dillon in Dillon, S.C. In her new role, McElveen also will oversee the case management department. She has been a practicing nurse for the McLeod Health system for 28 years.

Christine Wentt, PA, an adult hospitalist physician assistant, has been inducted into the VIP Woman of the Year Circle for 2015-2016 by the National Association of Professional Women (NAPW). Wentt works for Physicians Inpatient Care Specialists (MDICS), a private hospitalist group in Glen Burnie, Md. Wentt also serves as an adjunct professor in Drexel University’s physician assistant program.
Robert Zurcher, MD, has been appointed chief medical officer for emergency medicine at HNI Healthcare (formerly Hospitalists Now, Inc.), based in Austin, Texas. Dr. Zurcher comes to HNI Healthcare from IPC Healthcare, where he served as the administrative director for their Northwest Region. Dr. Zurcher has 27 years of practice experience and has founded and managed physician services companies in both hospital medicine and emergency medicine.
Business Moves
North Hollywood, Calif.-based IPC Healthcare recently announced its acquisition of Hospital Medicine Consultants, LLC, in Elgin, Ill., a locally owned practice serving several hospitals in the Chicago suburbs. IPC staffs hospitalist providers in more than 400 hospitals across the country.
The Schumacher Group, a private hospitalist and emergency medicine provider, announced the implementation of a new telehospitalist program at Abrom Kaplan Memorial and Acadia General Hospitals, both in Lafayette, La. The program will allow physicians to provide overnight care to the two facilities from a remote location. The Schumacher Group was founded in 1994 and currently serves more than four million patients per year.
Mike O’Neal is a freelance writer in New York City.
John Carlile, MD, is the new medical director of pediatric hospitalist and intensivist services at Summerville (S.C.) Medical Center. Most recently, Dr. Carlile worked as a pediatric critical care specialist at Cox Medical Center in Springfield, Mo.


Elaine McElveen, RN, has been named director of the hospitalist program at McLeod Medical Center Dillon in Dillon, S.C. In her new role, McElveen also will oversee the case management department. She has been a practicing nurse for the McLeod Health system for 28 years.

Christine Wentt, PA, an adult hospitalist physician assistant, has been inducted into the VIP Woman of the Year Circle for 2015-2016 by the National Association of Professional Women (NAPW). Wentt works for Physicians Inpatient Care Specialists (MDICS), a private hospitalist group in Glen Burnie, Md. Wentt also serves as an adjunct professor in Drexel University’s physician assistant program.
Robert Zurcher, MD, has been appointed chief medical officer for emergency medicine at HNI Healthcare (formerly Hospitalists Now, Inc.), based in Austin, Texas. Dr. Zurcher comes to HNI Healthcare from IPC Healthcare, where he served as the administrative director for their Northwest Region. Dr. Zurcher has 27 years of practice experience and has founded and managed physician services companies in both hospital medicine and emergency medicine.
Business Moves
North Hollywood, Calif.-based IPC Healthcare recently announced its acquisition of Hospital Medicine Consultants, LLC, in Elgin, Ill., a locally owned practice serving several hospitals in the Chicago suburbs. IPC staffs hospitalist providers in more than 400 hospitals across the country.
The Schumacher Group, a private hospitalist and emergency medicine provider, announced the implementation of a new telehospitalist program at Abrom Kaplan Memorial and Acadia General Hospitals, both in Lafayette, La. The program will allow physicians to provide overnight care to the two facilities from a remote location. The Schumacher Group was founded in 1994 and currently serves more than four million patients per year.
Mike O’Neal is a freelance writer in New York City.
Healthcare Policies Affecting Hospitalists, Hospitalized Patients Advance in 2015
Laws and regulations regularly impact hospitalists and hospitalized patients, which is why SHM’s Advocacy Department and Public Policy Committee (PPC) work on behalf of SHM membership, the hospital medicine movement, and its patients.
This year has seen significant positive movement within several key policy areas for all of these groups. Some of these issues have made headlines, while others happened under the radar, but SHM and its members have played an important role within each of them.
In general, observation care and related issues have received increased attention from lawmakers in no small part as a result of SHM’s efforts in this area. Early in the summer, the Centers for Medicare and Medicaid Services (CMS) responded to SHM’s efforts by proposing changes to the two-midnight rule—softening yet still retaining the rule. SHM commented positively on the proposed changes but signaled to both CMS and Congress that still more needs to be done to address problems inherent within observation policy.
As CMS and Congress work on a long-term solution to issues related to observation stays, SHM will play an important role in both proposing solutions and providing feedback for potential changes.
In July 2015, CMS proposed to pay for advance care planning (ACP). This proposal, assuming it is finalized at press time, is one that SHM has advocated for and supported for almost two years. Hospitalists should consider it a victory. The PPC, SHM members, and staff have consistently advocated for improved ACP policies in urging Medicare to recognize the value in ACP and reimburse for these critical services.
Hospitalists have also played a major part in the advocacy efforts on the Congressional level, dispelling misconceptions surrounding ACP by educating members of Congress and their staff and explaining the importance of these conversations within the healthcare system. Consistent advocacy and hospitalist involvement have led to a positive response that has been a long time coming.
These are just a few examples of areas in which SHM has seen tangible accomplishments, but there are also issues that have yet to play out and require SHM’s efforts to remain consistent in the coming years.
One of these issues is a hospitalist-specific problem within the meaningful use program. Hospital-based physicians are exempt from meaningful use and associated penalties based on their percentages of inpatient services; however, due to the way the law was written and changes in the healthcare landscape since passage, an unintended consequence has arisen.
Hospitalists who care for significant numbers of “observation” and skilled nursing facility patients (coded as outpatient services) do not qualify as hospital-based under the law and are finding themselves subject to unfair penalties. In response to SHM advocacy, CMS provided a limited hardship exception for hospitalists in 2015 and extended it into 2016. This exception saved numerous hospitalist groups an estimated $2,000 to $3,000 per hospitalist per year.
The temporary exception was limited in scope, however, and a legislative fix is needed to ensure a permanent solution. SHM will continue to meet with legislators to discuss this issue and garner interest in what we hope will result in a permanent resolution to this issue.
Finally, passage of the Medicare Access and CHIP Reauthorization Act (MACRA) in April 2015 was one of the most important laws impacting physicians to pass in years. While it finally repealed the broken sustainable growth rate, its passage was just the beginning of SHM’s advocacy efforts on the legislation.
MACRA will fundamentally change the way in which physicians are compensated and will accelerate the transition away from fee-for-service (FFS) by encouraging alternative payment model (APM) participation; however, many details remain unclear and are in need of hospitalist-specific clarification. SHM has already begun engaging with federal agencies and lawmakers as the regulations for MACRA are developed, including initiating multi-stakeholder conversations about problems facing facility-based providers in pay-for-performance programs.
SHM’s Performance Measurement and Reporting Committee has been working closely with the PPC to devise concrete proposals that will allow physician/hospital alignment within mandated quality reporting programs, and the PPC has launched an APM workgroup that is exploring the most effective avenues for hospitalists to move away from FFS and take advantage of incentives that will be available under MACRA.
Successful advocacy efforts often take time, persistence, and most importantly, patience; these victories demonstrate that endurance pays off.
SHM is clearly making headway on behalf of hospitalists and patients, and will build off of the momentum that these victories have generated. As SHM Public Policy Committee chair, Ron Greeno, stated after SHM’s most recent victories, “There are times when we all ask if our efforts are all worth it, and the clear message that we have heard in the past few weeks is a resounding ‘Yes!’”
Success does take time, however, and help from SHM members is a critical part of the equation. Your voice does make a difference.
To stay up to date and get involved with SHM’s advocacy efforts, connect with SHM’s Grassroots Network at www.hospitalmedicine.org/grassroots, and join the policy discussions in the Advocacy and Public Policy community on HMX.
Josh Boswell is SHM’s director of government relations.
Laws and regulations regularly impact hospitalists and hospitalized patients, which is why SHM’s Advocacy Department and Public Policy Committee (PPC) work on behalf of SHM membership, the hospital medicine movement, and its patients.
This year has seen significant positive movement within several key policy areas for all of these groups. Some of these issues have made headlines, while others happened under the radar, but SHM and its members have played an important role within each of them.
In general, observation care and related issues have received increased attention from lawmakers in no small part as a result of SHM’s efforts in this area. Early in the summer, the Centers for Medicare and Medicaid Services (CMS) responded to SHM’s efforts by proposing changes to the two-midnight rule—softening yet still retaining the rule. SHM commented positively on the proposed changes but signaled to both CMS and Congress that still more needs to be done to address problems inherent within observation policy.
As CMS and Congress work on a long-term solution to issues related to observation stays, SHM will play an important role in both proposing solutions and providing feedback for potential changes.
In July 2015, CMS proposed to pay for advance care planning (ACP). This proposal, assuming it is finalized at press time, is one that SHM has advocated for and supported for almost two years. Hospitalists should consider it a victory. The PPC, SHM members, and staff have consistently advocated for improved ACP policies in urging Medicare to recognize the value in ACP and reimburse for these critical services.
Hospitalists have also played a major part in the advocacy efforts on the Congressional level, dispelling misconceptions surrounding ACP by educating members of Congress and their staff and explaining the importance of these conversations within the healthcare system. Consistent advocacy and hospitalist involvement have led to a positive response that has been a long time coming.
These are just a few examples of areas in which SHM has seen tangible accomplishments, but there are also issues that have yet to play out and require SHM’s efforts to remain consistent in the coming years.
One of these issues is a hospitalist-specific problem within the meaningful use program. Hospital-based physicians are exempt from meaningful use and associated penalties based on their percentages of inpatient services; however, due to the way the law was written and changes in the healthcare landscape since passage, an unintended consequence has arisen.
Hospitalists who care for significant numbers of “observation” and skilled nursing facility patients (coded as outpatient services) do not qualify as hospital-based under the law and are finding themselves subject to unfair penalties. In response to SHM advocacy, CMS provided a limited hardship exception for hospitalists in 2015 and extended it into 2016. This exception saved numerous hospitalist groups an estimated $2,000 to $3,000 per hospitalist per year.
The temporary exception was limited in scope, however, and a legislative fix is needed to ensure a permanent solution. SHM will continue to meet with legislators to discuss this issue and garner interest in what we hope will result in a permanent resolution to this issue.
Finally, passage of the Medicare Access and CHIP Reauthorization Act (MACRA) in April 2015 was one of the most important laws impacting physicians to pass in years. While it finally repealed the broken sustainable growth rate, its passage was just the beginning of SHM’s advocacy efforts on the legislation.
MACRA will fundamentally change the way in which physicians are compensated and will accelerate the transition away from fee-for-service (FFS) by encouraging alternative payment model (APM) participation; however, many details remain unclear and are in need of hospitalist-specific clarification. SHM has already begun engaging with federal agencies and lawmakers as the regulations for MACRA are developed, including initiating multi-stakeholder conversations about problems facing facility-based providers in pay-for-performance programs.
SHM’s Performance Measurement and Reporting Committee has been working closely with the PPC to devise concrete proposals that will allow physician/hospital alignment within mandated quality reporting programs, and the PPC has launched an APM workgroup that is exploring the most effective avenues for hospitalists to move away from FFS and take advantage of incentives that will be available under MACRA.
Successful advocacy efforts often take time, persistence, and most importantly, patience; these victories demonstrate that endurance pays off.
SHM is clearly making headway on behalf of hospitalists and patients, and will build off of the momentum that these victories have generated. As SHM Public Policy Committee chair, Ron Greeno, stated after SHM’s most recent victories, “There are times when we all ask if our efforts are all worth it, and the clear message that we have heard in the past few weeks is a resounding ‘Yes!’”
Success does take time, however, and help from SHM members is a critical part of the equation. Your voice does make a difference.
To stay up to date and get involved with SHM’s advocacy efforts, connect with SHM’s Grassroots Network at www.hospitalmedicine.org/grassroots, and join the policy discussions in the Advocacy and Public Policy community on HMX.
Josh Boswell is SHM’s director of government relations.
Laws and regulations regularly impact hospitalists and hospitalized patients, which is why SHM’s Advocacy Department and Public Policy Committee (PPC) work on behalf of SHM membership, the hospital medicine movement, and its patients.
This year has seen significant positive movement within several key policy areas for all of these groups. Some of these issues have made headlines, while others happened under the radar, but SHM and its members have played an important role within each of them.
In general, observation care and related issues have received increased attention from lawmakers in no small part as a result of SHM’s efforts in this area. Early in the summer, the Centers for Medicare and Medicaid Services (CMS) responded to SHM’s efforts by proposing changes to the two-midnight rule—softening yet still retaining the rule. SHM commented positively on the proposed changes but signaled to both CMS and Congress that still more needs to be done to address problems inherent within observation policy.
As CMS and Congress work on a long-term solution to issues related to observation stays, SHM will play an important role in both proposing solutions and providing feedback for potential changes.
In July 2015, CMS proposed to pay for advance care planning (ACP). This proposal, assuming it is finalized at press time, is one that SHM has advocated for and supported for almost two years. Hospitalists should consider it a victory. The PPC, SHM members, and staff have consistently advocated for improved ACP policies in urging Medicare to recognize the value in ACP and reimburse for these critical services.
Hospitalists have also played a major part in the advocacy efforts on the Congressional level, dispelling misconceptions surrounding ACP by educating members of Congress and their staff and explaining the importance of these conversations within the healthcare system. Consistent advocacy and hospitalist involvement have led to a positive response that has been a long time coming.
These are just a few examples of areas in which SHM has seen tangible accomplishments, but there are also issues that have yet to play out and require SHM’s efforts to remain consistent in the coming years.
One of these issues is a hospitalist-specific problem within the meaningful use program. Hospital-based physicians are exempt from meaningful use and associated penalties based on their percentages of inpatient services; however, due to the way the law was written and changes in the healthcare landscape since passage, an unintended consequence has arisen.
Hospitalists who care for significant numbers of “observation” and skilled nursing facility patients (coded as outpatient services) do not qualify as hospital-based under the law and are finding themselves subject to unfair penalties. In response to SHM advocacy, CMS provided a limited hardship exception for hospitalists in 2015 and extended it into 2016. This exception saved numerous hospitalist groups an estimated $2,000 to $3,000 per hospitalist per year.
The temporary exception was limited in scope, however, and a legislative fix is needed to ensure a permanent solution. SHM will continue to meet with legislators to discuss this issue and garner interest in what we hope will result in a permanent resolution to this issue.
Finally, passage of the Medicare Access and CHIP Reauthorization Act (MACRA) in April 2015 was one of the most important laws impacting physicians to pass in years. While it finally repealed the broken sustainable growth rate, its passage was just the beginning of SHM’s advocacy efforts on the legislation.
MACRA will fundamentally change the way in which physicians are compensated and will accelerate the transition away from fee-for-service (FFS) by encouraging alternative payment model (APM) participation; however, many details remain unclear and are in need of hospitalist-specific clarification. SHM has already begun engaging with federal agencies and lawmakers as the regulations for MACRA are developed, including initiating multi-stakeholder conversations about problems facing facility-based providers in pay-for-performance programs.
SHM’s Performance Measurement and Reporting Committee has been working closely with the PPC to devise concrete proposals that will allow physician/hospital alignment within mandated quality reporting programs, and the PPC has launched an APM workgroup that is exploring the most effective avenues for hospitalists to move away from FFS and take advantage of incentives that will be available under MACRA.
Successful advocacy efforts often take time, persistence, and most importantly, patience; these victories demonstrate that endurance pays off.
SHM is clearly making headway on behalf of hospitalists and patients, and will build off of the momentum that these victories have generated. As SHM Public Policy Committee chair, Ron Greeno, stated after SHM’s most recent victories, “There are times when we all ask if our efforts are all worth it, and the clear message that we have heard in the past few weeks is a resounding ‘Yes!’”
Success does take time, however, and help from SHM members is a critical part of the equation. Your voice does make a difference.
To stay up to date and get involved with SHM’s advocacy efforts, connect with SHM’s Grassroots Network at www.hospitalmedicine.org/grassroots, and join the policy discussions in the Advocacy and Public Policy community on HMX.
Josh Boswell is SHM’s director of government relations.