User login
Secondary Survey of Trauma Patient
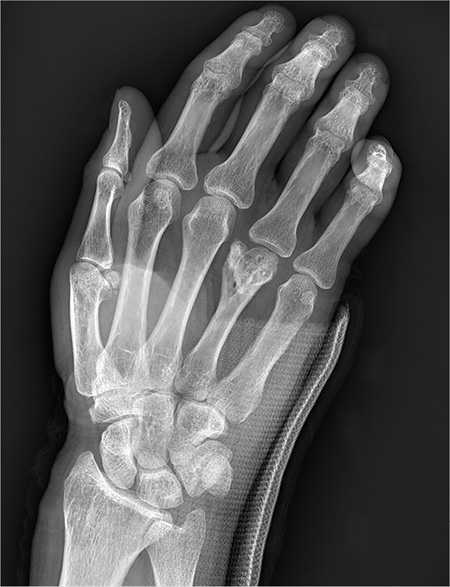
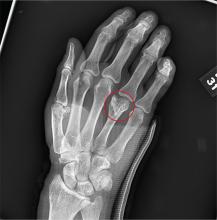
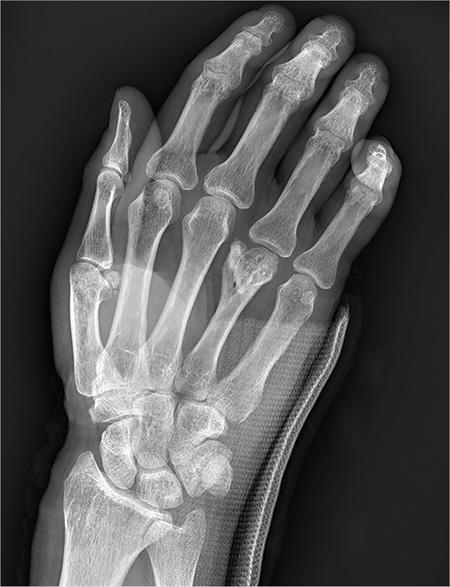
You are assisting in the evaluation and management of a trauma patient who was brought to your facility earlier today, following a motor vehicle collision. He is estimated to be in his 30s and is presumed to have been ejected, as he was found outside the vehicle. He was intubated in the field, and primary survey and resuscitation have been completed. History is otherwise unknown. The patient’s vital signs are currently stable. As you perform your secondary survey, you note that his right hand and wrist appear to be moderately swollen. He has been placed in a splint. You order a radiograph of the hand. What is your impression?
Steroids Beneficial as Adjunctive Treatment for Community-Acquired Pneumonia
Clinical question: Should steroids be used as adjunctive therapy for patients with community-acquired pneumonia?
Bottom line: Moderate-quality to high-quality evidence suggests that steroids, when added to antibiotics and usual care, can improve outcomes in the treatment of community-acquired pneumonia (CAP). Benefits include reduced hospital length of stay, decreased time to clinical stability, and lower rates of mechanical ventilation and acute respiratory distress syndrome. Steroids may also play a role in preventing deaths, especially in patients with severe CAP; however, the certainty of this evidence is not as clear. Given varying treatment regimens used in the individual studies, the appropriate steroid formulation, dose, and duration of steroids cannot be elucidated from the current set of data. (LOE = 1a)
Reference: Siemieniuk RAC, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia. Ann Intern Med. 2015;163(7):519-528.
Study design: Meta-analysis (randomized controlled trials)
Funding source: Self-funded or unfunded
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis: These authors searched MEDLINE, EMBASE, and the Cochrane Register to find randomized controlled trials that compared the use of steroids with placebo in adults with CAP. Two reviewers independently evaluated studies for eligibility, extracted data, and assessed the included studies for risk of bias. Five of the 13 included studies, whose population made up 70% of the total sample population, had low risk of bias. The treatment groups in the individual studies received different steroid preparations, routes of administration, dosages, and duration of treatment. All groups otherwise received antibiotics and usual care for CAP.
High-quality evidence showed that the use of steroids decreased hospital length of stay by 1 day (3 studies: mean difference: -1.0 day; 95% CI -1.79 to -0.21 days) and decreased time to clinical stability by 1.22 days (5 studies, mean difference: -1.22 days; -2.08 to -0.35 days). Moderate-quality evidence showed that the use of steroids decreased the need for mechanical ventilation (5 studies: relative risk [RR] = 0.45; 0.26-0.79) and the incidence of acute respiratory distress syndrome (4 studies: RR = 0.24; 0.10-0.56).
Finally, data from the 12 trials that assessed all-cause mortality revealed a trend toward decreased risk of death in the steroid group. The difference between the two groups for this end point became statistically significant when only the trials that met the criteria for severe pneumonia were included (6 studies: RR = 0.39; 0.20-0.77). Although steroid use, not surprisingly, increased the risk of significant hyperglycemia (6 studies: RR = 1.49;1.01-2.19), there were no differences detected in the rates of gastrointestinal bleeds, severe neuropsychiatric complications, or rehospitalizations.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Should steroids be used as adjunctive therapy for patients with community-acquired pneumonia?
Bottom line: Moderate-quality to high-quality evidence suggests that steroids, when added to antibiotics and usual care, can improve outcomes in the treatment of community-acquired pneumonia (CAP). Benefits include reduced hospital length of stay, decreased time to clinical stability, and lower rates of mechanical ventilation and acute respiratory distress syndrome. Steroids may also play a role in preventing deaths, especially in patients with severe CAP; however, the certainty of this evidence is not as clear. Given varying treatment regimens used in the individual studies, the appropriate steroid formulation, dose, and duration of steroids cannot be elucidated from the current set of data. (LOE = 1a)
Reference: Siemieniuk RAC, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia. Ann Intern Med. 2015;163(7):519-528.
Study design: Meta-analysis (randomized controlled trials)
Funding source: Self-funded or unfunded
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis: These authors searched MEDLINE, EMBASE, and the Cochrane Register to find randomized controlled trials that compared the use of steroids with placebo in adults with CAP. Two reviewers independently evaluated studies for eligibility, extracted data, and assessed the included studies for risk of bias. Five of the 13 included studies, whose population made up 70% of the total sample population, had low risk of bias. The treatment groups in the individual studies received different steroid preparations, routes of administration, dosages, and duration of treatment. All groups otherwise received antibiotics and usual care for CAP.
High-quality evidence showed that the use of steroids decreased hospital length of stay by 1 day (3 studies: mean difference: -1.0 day; 95% CI -1.79 to -0.21 days) and decreased time to clinical stability by 1.22 days (5 studies, mean difference: -1.22 days; -2.08 to -0.35 days). Moderate-quality evidence showed that the use of steroids decreased the need for mechanical ventilation (5 studies: relative risk [RR] = 0.45; 0.26-0.79) and the incidence of acute respiratory distress syndrome (4 studies: RR = 0.24; 0.10-0.56).
Finally, data from the 12 trials that assessed all-cause mortality revealed a trend toward decreased risk of death in the steroid group. The difference between the two groups for this end point became statistically significant when only the trials that met the criteria for severe pneumonia were included (6 studies: RR = 0.39; 0.20-0.77). Although steroid use, not surprisingly, increased the risk of significant hyperglycemia (6 studies: RR = 1.49;1.01-2.19), there were no differences detected in the rates of gastrointestinal bleeds, severe neuropsychiatric complications, or rehospitalizations.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Should steroids be used as adjunctive therapy for patients with community-acquired pneumonia?
Bottom line: Moderate-quality to high-quality evidence suggests that steroids, when added to antibiotics and usual care, can improve outcomes in the treatment of community-acquired pneumonia (CAP). Benefits include reduced hospital length of stay, decreased time to clinical stability, and lower rates of mechanical ventilation and acute respiratory distress syndrome. Steroids may also play a role in preventing deaths, especially in patients with severe CAP; however, the certainty of this evidence is not as clear. Given varying treatment regimens used in the individual studies, the appropriate steroid formulation, dose, and duration of steroids cannot be elucidated from the current set of data. (LOE = 1a)
Reference: Siemieniuk RAC, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia. Ann Intern Med. 2015;163(7):519-528.
Study design: Meta-analysis (randomized controlled trials)
Funding source: Self-funded or unfunded
Allocation: Uncertain
Setting: Inpatient (any location)
Synopsis: These authors searched MEDLINE, EMBASE, and the Cochrane Register to find randomized controlled trials that compared the use of steroids with placebo in adults with CAP. Two reviewers independently evaluated studies for eligibility, extracted data, and assessed the included studies for risk of bias. Five of the 13 included studies, whose population made up 70% of the total sample population, had low risk of bias. The treatment groups in the individual studies received different steroid preparations, routes of administration, dosages, and duration of treatment. All groups otherwise received antibiotics and usual care for CAP.
High-quality evidence showed that the use of steroids decreased hospital length of stay by 1 day (3 studies: mean difference: -1.0 day; 95% CI -1.79 to -0.21 days) and decreased time to clinical stability by 1.22 days (5 studies, mean difference: -1.22 days; -2.08 to -0.35 days). Moderate-quality evidence showed that the use of steroids decreased the need for mechanical ventilation (5 studies: relative risk [RR] = 0.45; 0.26-0.79) and the incidence of acute respiratory distress syndrome (4 studies: RR = 0.24; 0.10-0.56).
Finally, data from the 12 trials that assessed all-cause mortality revealed a trend toward decreased risk of death in the steroid group. The difference between the two groups for this end point became statistically significant when only the trials that met the criteria for severe pneumonia were included (6 studies: RR = 0.39; 0.20-0.77). Although steroid use, not surprisingly, increased the risk of significant hyperglycemia (6 studies: RR = 1.49;1.01-2.19), there were no differences detected in the rates of gastrointestinal bleeds, severe neuropsychiatric complications, or rehospitalizations.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Boy Wrestles With Scalp Problem
ANSWER
The correct answer is all of the above (choice “e”). This particular form of tinea capitis is called black dot tinea capitis (BDTC), a somewhat unusual dermatophytosis (superficial fungal infection) that mostly affects children. The causative organisms are anthropophilic—that is, acquired from human sources, such as other children, during activities that involve skin-to-skin contact (eg, sports).
The vast majority of these organisms are from the Trichophyton family, such as T tonsurans or T violaceum. They invade the hair shaft itself, leaving the hard covering (the cuticle) intact. The black dots represent the tips of broken-off hairs, themselves full of fungal elements, seen in the photomicrograph. The term endothrix is given to this kind of fungal infection, in which the organisms are contained within the hair shaft, which, as a result, becomes brittle and breaks off. This is a relatively common type of infection.
A more unusual form of tinea capitis is caused by zoophilic organisms, such as Microsporum canis (from dogs and cats), Microsporum gypseum (pigs or cows), or T equinum (horses). These infect the external surface of the hair shaft, breaking down the cuticle. This allows for identification of the infection by Wood’s lamp, which causes the affected area to turn a yellowish color. These infections also tend to provoke a more brisk inflammatory response in the victim and are more difficult to treat.
Diagnosis can be made from a combination of clinical findings, KOH prep (as in this patient), and/or fungal culture.
Treatment can entail griseofulvin or terbinafine; the case patient was treated with a two-month course of the latter (125 mg/d). Topical treatment is of limited usefulness.
ANSWER
The correct answer is all of the above (choice “e”). This particular form of tinea capitis is called black dot tinea capitis (BDTC), a somewhat unusual dermatophytosis (superficial fungal infection) that mostly affects children. The causative organisms are anthropophilic—that is, acquired from human sources, such as other children, during activities that involve skin-to-skin contact (eg, sports).
The vast majority of these organisms are from the Trichophyton family, such as T tonsurans or T violaceum. They invade the hair shaft itself, leaving the hard covering (the cuticle) intact. The black dots represent the tips of broken-off hairs, themselves full of fungal elements, seen in the photomicrograph. The term endothrix is given to this kind of fungal infection, in which the organisms are contained within the hair shaft, which, as a result, becomes brittle and breaks off. This is a relatively common type of infection.
A more unusual form of tinea capitis is caused by zoophilic organisms, such as Microsporum canis (from dogs and cats), Microsporum gypseum (pigs or cows), or T equinum (horses). These infect the external surface of the hair shaft, breaking down the cuticle. This allows for identification of the infection by Wood’s lamp, which causes the affected area to turn a yellowish color. These infections also tend to provoke a more brisk inflammatory response in the victim and are more difficult to treat.
Diagnosis can be made from a combination of clinical findings, KOH prep (as in this patient), and/or fungal culture.
Treatment can entail griseofulvin or terbinafine; the case patient was treated with a two-month course of the latter (125 mg/d). Topical treatment is of limited usefulness.
ANSWER
The correct answer is all of the above (choice “e”). This particular form of tinea capitis is called black dot tinea capitis (BDTC), a somewhat unusual dermatophytosis (superficial fungal infection) that mostly affects children. The causative organisms are anthropophilic—that is, acquired from human sources, such as other children, during activities that involve skin-to-skin contact (eg, sports).
The vast majority of these organisms are from the Trichophyton family, such as T tonsurans or T violaceum. They invade the hair shaft itself, leaving the hard covering (the cuticle) intact. The black dots represent the tips of broken-off hairs, themselves full of fungal elements, seen in the photomicrograph. The term endothrix is given to this kind of fungal infection, in which the organisms are contained within the hair shaft, which, as a result, becomes brittle and breaks off. This is a relatively common type of infection.
A more unusual form of tinea capitis is caused by zoophilic organisms, such as Microsporum canis (from dogs and cats), Microsporum gypseum (pigs or cows), or T equinum (horses). These infect the external surface of the hair shaft, breaking down the cuticle. This allows for identification of the infection by Wood’s lamp, which causes the affected area to turn a yellowish color. These infections also tend to provoke a more brisk inflammatory response in the victim and are more difficult to treat.
Diagnosis can be made from a combination of clinical findings, KOH prep (as in this patient), and/or fungal culture.
Treatment can entail griseofulvin or terbinafine; the case patient was treated with a two-month course of the latter (125 mg/d). Topical treatment is of limited usefulness.
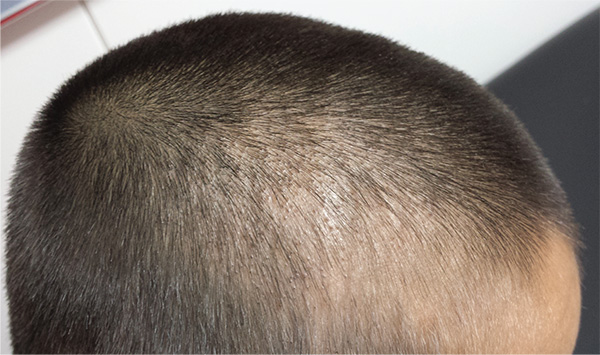
An 8-year-old boy is brought in by his mother for evaluation of a scalp condition that manifested several months ago. The first sign was hair loss in several locations, mostly on the sides, followed in a few weeks by faint scaling. As more hair came out, the scaling in the affected locations reduced, but uniformly spaced black dots began to appear. There has never been any redness. The boy was taken to a local urgent care center, where he was diagnosed with probable “ringworm” and given a prescription for topical antifungal cream (clotrimazole, bid application). This failed to help, so the family sought an appointment with dermatology. Additional history-taking reveals that the boy noticed the problem within a few weeks of starting wrestling at school. Examination of the scalp reveals several round areas of partial and uniform hair loss, averaging 3 cm in diameter. No redness or edema is seen, and only very faint scaling is observed on the surface of the skin. Distinct black dots are uniformly distributed within the lesions. A vigorous scrape of one of the areas is processed with potassium hydroxide 10% and examined under 10x magnification. The black dots are found to be broken-off hairs filled with hundreds of tiny round spheres. Several hyphae are seen adjacent to the hairs. Palpation reveals adenopathy in the adjacent nuchal scalp and neck. Wood’s lamp examination fails to highlight these areas.
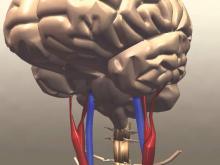
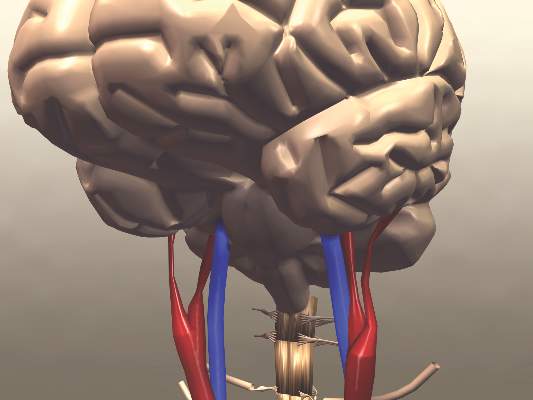
Functional Outcomes Better With Endovascular Thrombectomy for Acute Ischemic Stroke
Clinical question: Does endovascular thrombectomy improve clinical outcomes in patients presenting with acute ischemic stroke?
Bottom line: High-quality evidence shows that endovascular therapy using mechanical thrombectomy for the treatment of acute ischemic stroke leads to improved functional outcomes as compared with standard medical therapy with intravenous tissue plasminogen activator (tPA). You would have to treat 8 patients with endovascular therapy to achieve functional independence for 1 patient. (LOE = 1a)
Reference: Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015;314(17):1832–1843.
Study design: Meta-analysis (randomized controlled trials)
Funding source: Unknown/not stated
Allocation: Concealed
Setting: Inpatient (any location)
Synopsis: These investigators searched multiple databases including MEDLINE, EMBASE, Google Scholar, and the Cochrane Library to find randomized clinical trials that compared endovascular mechanical thrombectomy with tPA for the treatment of acute ischemic stroke. Three reviewers independently evaluated the studies for eligibility, extracted data from the included studies, and assessed study quality using the Cochrane Collaboration's tool for risk of bias. Ultimately, 8 studies with a total of 2423 patients were included in the review. Most studies used a time window of 6 hours from stroke onset for time to endovascular therapy. The primary outcome was the modified Rankin Scale (mRS) score, which measures the degree of functional disability (a scale of 0 to 6, where 0 = no symptoms, 1 = symptoms but no disability, 5 = severe disability, and 6 = death).
Endovascular thrombectomy led to reduced disability at 90 days (odds ratio = 1.56; 95% CI 1.14-2.13; P = .005). Furthermore, those who received endovascular thrombectomy were more likely to be functionally independent (mRS score of 0, 1, or 2) than those who received tPA (45% vs 32%; P = .005; number needed to treat = 8). There were no significant differences detected in mortality, symptomatic intracranial bleeds, or in-hospital medical complications. Given the high degree of heterogeneity noted in the primary end point, subgroup and sensitivity analyses were performed that showed better functional outcomes in patients with confirmed proximal arterial occlusion, those who received combined tPA and endovascular interventions, and those who had the newer retrievable stent devices used for thrombectomy. More recent studies, as compared with earlier studies, were also more likely to favor endovascular thrombectomy.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Does endovascular thrombectomy improve clinical outcomes in patients presenting with acute ischemic stroke?
Bottom line: High-quality evidence shows that endovascular therapy using mechanical thrombectomy for the treatment of acute ischemic stroke leads to improved functional outcomes as compared with standard medical therapy with intravenous tissue plasminogen activator (tPA). You would have to treat 8 patients with endovascular therapy to achieve functional independence for 1 patient. (LOE = 1a)
Reference: Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015;314(17):1832–1843.
Study design: Meta-analysis (randomized controlled trials)
Funding source: Unknown/not stated
Allocation: Concealed
Setting: Inpatient (any location)
Synopsis: These investigators searched multiple databases including MEDLINE, EMBASE, Google Scholar, and the Cochrane Library to find randomized clinical trials that compared endovascular mechanical thrombectomy with tPA for the treatment of acute ischemic stroke. Three reviewers independently evaluated the studies for eligibility, extracted data from the included studies, and assessed study quality using the Cochrane Collaboration's tool for risk of bias. Ultimately, 8 studies with a total of 2423 patients were included in the review. Most studies used a time window of 6 hours from stroke onset for time to endovascular therapy. The primary outcome was the modified Rankin Scale (mRS) score, which measures the degree of functional disability (a scale of 0 to 6, where 0 = no symptoms, 1 = symptoms but no disability, 5 = severe disability, and 6 = death).
Endovascular thrombectomy led to reduced disability at 90 days (odds ratio = 1.56; 95% CI 1.14-2.13; P = .005). Furthermore, those who received endovascular thrombectomy were more likely to be functionally independent (mRS score of 0, 1, or 2) than those who received tPA (45% vs 32%; P = .005; number needed to treat = 8). There were no significant differences detected in mortality, symptomatic intracranial bleeds, or in-hospital medical complications. Given the high degree of heterogeneity noted in the primary end point, subgroup and sensitivity analyses were performed that showed better functional outcomes in patients with confirmed proximal arterial occlusion, those who received combined tPA and endovascular interventions, and those who had the newer retrievable stent devices used for thrombectomy. More recent studies, as compared with earlier studies, were also more likely to favor endovascular thrombectomy.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Does endovascular thrombectomy improve clinical outcomes in patients presenting with acute ischemic stroke?
Bottom line: High-quality evidence shows that endovascular therapy using mechanical thrombectomy for the treatment of acute ischemic stroke leads to improved functional outcomes as compared with standard medical therapy with intravenous tissue plasminogen activator (tPA). You would have to treat 8 patients with endovascular therapy to achieve functional independence for 1 patient. (LOE = 1a)
Reference: Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015;314(17):1832–1843.
Study design: Meta-analysis (randomized controlled trials)
Funding source: Unknown/not stated
Allocation: Concealed
Setting: Inpatient (any location)
Synopsis: These investigators searched multiple databases including MEDLINE, EMBASE, Google Scholar, and the Cochrane Library to find randomized clinical trials that compared endovascular mechanical thrombectomy with tPA for the treatment of acute ischemic stroke. Three reviewers independently evaluated the studies for eligibility, extracted data from the included studies, and assessed study quality using the Cochrane Collaboration's tool for risk of bias. Ultimately, 8 studies with a total of 2423 patients were included in the review. Most studies used a time window of 6 hours from stroke onset for time to endovascular therapy. The primary outcome was the modified Rankin Scale (mRS) score, which measures the degree of functional disability (a scale of 0 to 6, where 0 = no symptoms, 1 = symptoms but no disability, 5 = severe disability, and 6 = death).
Endovascular thrombectomy led to reduced disability at 90 days (odds ratio = 1.56; 95% CI 1.14-2.13; P = .005). Furthermore, those who received endovascular thrombectomy were more likely to be functionally independent (mRS score of 0, 1, or 2) than those who received tPA (45% vs 32%; P = .005; number needed to treat = 8). There were no significant differences detected in mortality, symptomatic intracranial bleeds, or in-hospital medical complications. Given the high degree of heterogeneity noted in the primary end point, subgroup and sensitivity analyses were performed that showed better functional outcomes in patients with confirmed proximal arterial occlusion, those who received combined tPA and endovascular interventions, and those who had the newer retrievable stent devices used for thrombectomy. More recent studies, as compared with earlier studies, were also more likely to favor endovascular thrombectomy.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Derm Morphology, Part 2
1. Flat, distinct, discolored area usually less than 1 cm wide without change in skin texture or thickness.
Diagnosis: Macule
For more information on this case, see “Could Lesion Become a Pain in the Neck?” Clin Rev. 2015;25(7):W1
For the next photograph, proceed to the next page >>
2. Solid or cystic raised area less than 1 cm wide.
Diagnosis: Papule
For more information on this case, see “Irritated and Downright Painful.” Clin Rev. 2014;24(11):W3.
For the next photograph, proceed to the next page >>
3. Collection of pus in any part of the body typically accompanied by swelling and inflammation.
Diagnosis: Skin abscesses/Boils
For more information on this case, see “What Actually Qualifies as a ‘Boil’?” Clin Rev. 2013;23(8):W4.
For the next photograph, proceed to the next page >>
4. Resulting from chronic irritation from conditions like eczema or from continuous rubbing or scratching, the skin becomes thickened and leathery.
Diagnosis: Lichenification
For more information on Figure 4a, see “Leg Lesion Represents a Vicious Cycle.” Clin Rev. 2012;22(12):W5.
1. Flat, distinct, discolored area usually less than 1 cm wide without change in skin texture or thickness.
Diagnosis: Macule
For more information on this case, see “Could Lesion Become a Pain in the Neck?” Clin Rev. 2015;25(7):W1
For the next photograph, proceed to the next page >>
2. Solid or cystic raised area less than 1 cm wide.
Diagnosis: Papule
For more information on this case, see “Irritated and Downright Painful.” Clin Rev. 2014;24(11):W3.
For the next photograph, proceed to the next page >>
3. Collection of pus in any part of the body typically accompanied by swelling and inflammation.
Diagnosis: Skin abscesses/Boils
For more information on this case, see “What Actually Qualifies as a ‘Boil’?” Clin Rev. 2013;23(8):W4.
For the next photograph, proceed to the next page >>
4. Resulting from chronic irritation from conditions like eczema or from continuous rubbing or scratching, the skin becomes thickened and leathery.
Diagnosis: Lichenification
For more information on Figure 4a, see “Leg Lesion Represents a Vicious Cycle.” Clin Rev. 2012;22(12):W5.
1. Flat, distinct, discolored area usually less than 1 cm wide without change in skin texture or thickness.
Diagnosis: Macule
For more information on this case, see “Could Lesion Become a Pain in the Neck?” Clin Rev. 2015;25(7):W1
For the next photograph, proceed to the next page >>
2. Solid or cystic raised area less than 1 cm wide.
Diagnosis: Papule
For more information on this case, see “Irritated and Downright Painful.” Clin Rev. 2014;24(11):W3.
For the next photograph, proceed to the next page >>
3. Collection of pus in any part of the body typically accompanied by swelling and inflammation.
Diagnosis: Skin abscesses/Boils
For more information on this case, see “What Actually Qualifies as a ‘Boil’?” Clin Rev. 2013;23(8):W4.
For the next photograph, proceed to the next page >>
4. Resulting from chronic irritation from conditions like eczema or from continuous rubbing or scratching, the skin becomes thickened and leathery.
Diagnosis: Lichenification
For more information on Figure 4a, see “Leg Lesion Represents a Vicious Cycle.” Clin Rev. 2012;22(12):W5.
ACR: Don’t be fooled by contaminated synovial fluid
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
AT THE ACR ANNUAL MEETING
Key clinical point: It’s probably not really septic arthritis if patients have mild disease manifestations and slow-growing synovial fluid cultures.
Major finding: True cases of septic arthritis had substantially higher mean synovial fluid white blood cell counts than did false-positive cases (88,000 vs. 29,000).
Data source: Review of 450 patients with presumed septic arthritis.
Disclosures: The authors have no disclosures, and there was no outside funding for the work.
Mechanical thrombectomy improves stroke outcomes
Mechanical removal of a thrombus in addition to usual care is associated with significantly better functional outcomes than usual care in patients who have experienced an acute ischemic stroke, according to a meta-analysis published online Nov. 30 in Journal of the American College of Cardiology.
The analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients showed that those undergoing mechanical thrombectomy had a 45% greater likelihood of achieving a good functional outcome and a 67% greater chance of excellent functional outcome, defined respectively as a modified Rankin scale score of 0-2 or 0-1.
Researchers observed a nonsignificant trend toward lower all-cause mortality in the mechanical thrombectomy patients, which was also linked with improved recanalization, compared with usual care alone (risk ratio, 1.57; 95% confidence interval, 1.11-2.23; P = .01).
Mechanical thrombectomy did show a nonsignificant increase in the risk of recurrent stroke at 90 days, but this was largely driven by one study, and when that was excluded, the risk was similar between mechanical thrombectomy and no mechanical thrombectomy (J Am Coll Cardiol. 2015;66:2498-505).
“Although mechanical thrombectomy is beneficial, this procedure requires specialized centers of excellence; therefore, the widespread application of this therapy for acute ischemic stroke patients will likely remain limited for the foreseeable future,” wrote Dr. Islam Y. Elgendy of the University of Florida, Gainesville, and coauthors.
One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
In recent trials, removable devices consisting of self-expanding, clot-retrieving stents achieved higher rates of recanalization than did earlier methods of thrombus extraction, representing the first effective new treatment for stroke in nearly 20 years.
With absolute benefits substantially greater than systemic intravenous thrombolysis alone, the combination of intravenous tissue plasminogen activator and endovascular therapy have improved outcomes for selected patients who receive endovascular treatment within 6 hours of symptom onset, and further efforts to shorten the interval between emergency department arrival and treatment hold the promise of even better outcomes.
Dr. Gregory W. Albers of the Stanford Stroke Center at Stanford (Calif.) University, and Dr. Jonathan L. Halperin of the Cardiovascular Institute at the Mount Sinai Medical Center, New York, made these comments in an accompanying editorial (J Am Coll Cardiol. 2015;66:2506-9). Dr. Halperin has served as a consultant to Boston Scientific, Medtronic, and Johnson & Johnson. Dr. Albers has an equity interest in iSchemaView and is a consultant for iSchemaView and Medtronic.
In recent trials, removable devices consisting of self-expanding, clot-retrieving stents achieved higher rates of recanalization than did earlier methods of thrombus extraction, representing the first effective new treatment for stroke in nearly 20 years.
With absolute benefits substantially greater than systemic intravenous thrombolysis alone, the combination of intravenous tissue plasminogen activator and endovascular therapy have improved outcomes for selected patients who receive endovascular treatment within 6 hours of symptom onset, and further efforts to shorten the interval between emergency department arrival and treatment hold the promise of even better outcomes.
Dr. Gregory W. Albers of the Stanford Stroke Center at Stanford (Calif.) University, and Dr. Jonathan L. Halperin of the Cardiovascular Institute at the Mount Sinai Medical Center, New York, made these comments in an accompanying editorial (J Am Coll Cardiol. 2015;66:2506-9). Dr. Halperin has served as a consultant to Boston Scientific, Medtronic, and Johnson & Johnson. Dr. Albers has an equity interest in iSchemaView and is a consultant for iSchemaView and Medtronic.
In recent trials, removable devices consisting of self-expanding, clot-retrieving stents achieved higher rates of recanalization than did earlier methods of thrombus extraction, representing the first effective new treatment for stroke in nearly 20 years.
With absolute benefits substantially greater than systemic intravenous thrombolysis alone, the combination of intravenous tissue plasminogen activator and endovascular therapy have improved outcomes for selected patients who receive endovascular treatment within 6 hours of symptom onset, and further efforts to shorten the interval between emergency department arrival and treatment hold the promise of even better outcomes.
Dr. Gregory W. Albers of the Stanford Stroke Center at Stanford (Calif.) University, and Dr. Jonathan L. Halperin of the Cardiovascular Institute at the Mount Sinai Medical Center, New York, made these comments in an accompanying editorial (J Am Coll Cardiol. 2015;66:2506-9). Dr. Halperin has served as a consultant to Boston Scientific, Medtronic, and Johnson & Johnson. Dr. Albers has an equity interest in iSchemaView and is a consultant for iSchemaView and Medtronic.
Mechanical removal of a thrombus in addition to usual care is associated with significantly better functional outcomes than usual care in patients who have experienced an acute ischemic stroke, according to a meta-analysis published online Nov. 30 in Journal of the American College of Cardiology.
The analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients showed that those undergoing mechanical thrombectomy had a 45% greater likelihood of achieving a good functional outcome and a 67% greater chance of excellent functional outcome, defined respectively as a modified Rankin scale score of 0-2 or 0-1.
Researchers observed a nonsignificant trend toward lower all-cause mortality in the mechanical thrombectomy patients, which was also linked with improved recanalization, compared with usual care alone (risk ratio, 1.57; 95% confidence interval, 1.11-2.23; P = .01).
Mechanical thrombectomy did show a nonsignificant increase in the risk of recurrent stroke at 90 days, but this was largely driven by one study, and when that was excluded, the risk was similar between mechanical thrombectomy and no mechanical thrombectomy (J Am Coll Cardiol. 2015;66:2498-505).
“Although mechanical thrombectomy is beneficial, this procedure requires specialized centers of excellence; therefore, the widespread application of this therapy for acute ischemic stroke patients will likely remain limited for the foreseeable future,” wrote Dr. Islam Y. Elgendy of the University of Florida, Gainesville, and coauthors.
One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
Mechanical removal of a thrombus in addition to usual care is associated with significantly better functional outcomes than usual care in patients who have experienced an acute ischemic stroke, according to a meta-analysis published online Nov. 30 in Journal of the American College of Cardiology.
The analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients showed that those undergoing mechanical thrombectomy had a 45% greater likelihood of achieving a good functional outcome and a 67% greater chance of excellent functional outcome, defined respectively as a modified Rankin scale score of 0-2 or 0-1.
Researchers observed a nonsignificant trend toward lower all-cause mortality in the mechanical thrombectomy patients, which was also linked with improved recanalization, compared with usual care alone (risk ratio, 1.57; 95% confidence interval, 1.11-2.23; P = .01).
Mechanical thrombectomy did show a nonsignificant increase in the risk of recurrent stroke at 90 days, but this was largely driven by one study, and when that was excluded, the risk was similar between mechanical thrombectomy and no mechanical thrombectomy (J Am Coll Cardiol. 2015;66:2498-505).
“Although mechanical thrombectomy is beneficial, this procedure requires specialized centers of excellence; therefore, the widespread application of this therapy for acute ischemic stroke patients will likely remain limited for the foreseeable future,” wrote Dr. Islam Y. Elgendy of the University of Florida, Gainesville, and coauthors.
One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Mechanical thrombectomy and usual care are associated with significantly better functional outcomes after acute ischemic stroke than is usual care alone.
Major finding: Patients undergoing mechanical thrombectomy had a 67% greater likelihood of excellent functional outcome than patients having usual care alone.
Data source: Meta-analysis of nine trials of usual care with or without mechanical thrombectomy in 2,410 patients.
Disclosures: One author declared advisory board membership and research funding from private industry, but there were no other conflicts of interest declared.
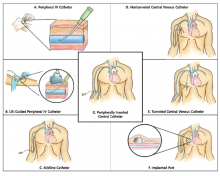
Guide Helps Hospitalists Choose Most Appropriate Catheter for Patients
The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) is a new resource designed to help clinicians select the safest and most appropriate peripherally inserted central catheter (PICC) for individual patients.
“How do you decide which catheter is best for your patient? Until now there wasn’t a guide bringing together all of the best available evidence,” says the guide’s lead author Vineet Chopra, MD, MSc, FHM, a hospitalist and assistant professor of medicine at the University of Michigan in Ann Arbor.
“These are among the most commonly performed procedures on any hospitalized patient, and yet, the least studied,” he adds. “We as hospitalists are the physicians who order most of these devices, especially PICCs.”
The guide includes algorithms and color-coded pocket cards to help physicians determine which PICC to choose. The cards can be freely downloaded and printed from the Improve PICC website at the University of Michigan.
The project to develop the guide brought together 15 leading international experts on catheters and their infections and complications, including the authors of existing guidelines, to brainstorm more than 600 clinical scenarios and best evidence-based practice for catheter use using the Rand/UCLA Appropriateness Method. “We also had a patient on the panel, which was important to the clinicians because this patient had actually used many of the devices being discussed,” Dr. Chopra explains.
The guidelines “have the potential to change the game for hospitalists,” Dr. Chopra adds. “There has never before been guidance on using IV devices in hospitalized medical patients, despite the fact that we use these devices every day. Now, for the first time, we not only have guidance but also a tool to benchmark the quality of care provided by doctors when it comes to venous access.”
The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) is a new resource designed to help clinicians select the safest and most appropriate peripherally inserted central catheter (PICC) for individual patients.
“How do you decide which catheter is best for your patient? Until now there wasn’t a guide bringing together all of the best available evidence,” says the guide’s lead author Vineet Chopra, MD, MSc, FHM, a hospitalist and assistant professor of medicine at the University of Michigan in Ann Arbor.
“These are among the most commonly performed procedures on any hospitalized patient, and yet, the least studied,” he adds. “We as hospitalists are the physicians who order most of these devices, especially PICCs.”
The guide includes algorithms and color-coded pocket cards to help physicians determine which PICC to choose. The cards can be freely downloaded and printed from the Improve PICC website at the University of Michigan.
The project to develop the guide brought together 15 leading international experts on catheters and their infections and complications, including the authors of existing guidelines, to brainstorm more than 600 clinical scenarios and best evidence-based practice for catheter use using the Rand/UCLA Appropriateness Method. “We also had a patient on the panel, which was important to the clinicians because this patient had actually used many of the devices being discussed,” Dr. Chopra explains.
The guidelines “have the potential to change the game for hospitalists,” Dr. Chopra adds. “There has never before been guidance on using IV devices in hospitalized medical patients, despite the fact that we use these devices every day. Now, for the first time, we not only have guidance but also a tool to benchmark the quality of care provided by doctors when it comes to venous access.”
The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) is a new resource designed to help clinicians select the safest and most appropriate peripherally inserted central catheter (PICC) for individual patients.
“How do you decide which catheter is best for your patient? Until now there wasn’t a guide bringing together all of the best available evidence,” says the guide’s lead author Vineet Chopra, MD, MSc, FHM, a hospitalist and assistant professor of medicine at the University of Michigan in Ann Arbor.
“These are among the most commonly performed procedures on any hospitalized patient, and yet, the least studied,” he adds. “We as hospitalists are the physicians who order most of these devices, especially PICCs.”
The guide includes algorithms and color-coded pocket cards to help physicians determine which PICC to choose. The cards can be freely downloaded and printed from the Improve PICC website at the University of Michigan.
The project to develop the guide brought together 15 leading international experts on catheters and their infections and complications, including the authors of existing guidelines, to brainstorm more than 600 clinical scenarios and best evidence-based practice for catheter use using the Rand/UCLA Appropriateness Method. “We also had a patient on the panel, which was important to the clinicians because this patient had actually used many of the devices being discussed,” Dr. Chopra explains.
The guidelines “have the potential to change the game for hospitalists,” Dr. Chopra adds. “There has never before been guidance on using IV devices in hospitalized medical patients, despite the fact that we use these devices every day. Now, for the first time, we not only have guidance but also a tool to benchmark the quality of care provided by doctors when it comes to venous access.”
Institute of Medicine Report Examines Medical Misdiagnoses
Authors of the IOM’s “Improving Diagnosis in Health Care” report cite problems in communication and limitations in electronic health records behind inaccurate and delayed diagnoses, concluding that the problem of diagnostic errors generally has not been adequately studied.1
“This problem is significant and serious. Yet we don’t know for sure how often it occurs, how serious it is, or how much it costs,” said the IOM committee’s chair, John Ball, MD, of the American College of Physicians, in a prepared statement. The report concludes there is no easy fix for the problem of diagnostic errors, which are a leading cause of adverse events in hospitals and of malpractice lawsuits for hospitalists, but calls for a major reassessment of the diagnostic process.2
Hospitalist Mangla Gulati, MD, FACP, SFHM, assistant chief medical officer at the University of Maryland Medical Center in Baltimore, says hospitalists would be remiss if they failed to take a closer look at the IOM report. “Diagnostic error is something we haven’t much talked about in medicine,” Dr. Gulati says. “Part of the goal of this report is to actually include the patient in those conversations.” Patients who are rehospitalized, she says, may have been given an incorrect initial diagnosis that was never rectified, or there may have been a failure to communicate important information.
“How many tests do we order where results come back after a patient leaves the hospital?” asks Kedar Mate, MD, senior vice president at the Institute for Healthcare Improvement and a hospitalist at Weill Cornell Medicine in New York City. “How many in-hospital diagnoses are made without all of the available information from outside providers?”
One simple intervention hospitalists could do immediately, he says, is to start tracking all important tests ordered for patients on a board in the medical team’s meeting room, only removing them from the board when results have been checked and communicated to the patient and outpatient provider.
References
- National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. 2015.
- Saber Tehrani AS, Lee HW, Mathews SC, et al. 25-year summary of U.S. malpractice claims for diagnostic errors 1986–2010: An analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013 Aug; 22(8):672–680.
Authors of the IOM’s “Improving Diagnosis in Health Care” report cite problems in communication and limitations in electronic health records behind inaccurate and delayed diagnoses, concluding that the problem of diagnostic errors generally has not been adequately studied.1
“This problem is significant and serious. Yet we don’t know for sure how often it occurs, how serious it is, or how much it costs,” said the IOM committee’s chair, John Ball, MD, of the American College of Physicians, in a prepared statement. The report concludes there is no easy fix for the problem of diagnostic errors, which are a leading cause of adverse events in hospitals and of malpractice lawsuits for hospitalists, but calls for a major reassessment of the diagnostic process.2
Hospitalist Mangla Gulati, MD, FACP, SFHM, assistant chief medical officer at the University of Maryland Medical Center in Baltimore, says hospitalists would be remiss if they failed to take a closer look at the IOM report. “Diagnostic error is something we haven’t much talked about in medicine,” Dr. Gulati says. “Part of the goal of this report is to actually include the patient in those conversations.” Patients who are rehospitalized, she says, may have been given an incorrect initial diagnosis that was never rectified, or there may have been a failure to communicate important information.
“How many tests do we order where results come back after a patient leaves the hospital?” asks Kedar Mate, MD, senior vice president at the Institute for Healthcare Improvement and a hospitalist at Weill Cornell Medicine in New York City. “How many in-hospital diagnoses are made without all of the available information from outside providers?”
One simple intervention hospitalists could do immediately, he says, is to start tracking all important tests ordered for patients on a board in the medical team’s meeting room, only removing them from the board when results have been checked and communicated to the patient and outpatient provider.
References
- National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. 2015.
- Saber Tehrani AS, Lee HW, Mathews SC, et al. 25-year summary of U.S. malpractice claims for diagnostic errors 1986–2010: An analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013 Aug; 22(8):672–680.
Authors of the IOM’s “Improving Diagnosis in Health Care” report cite problems in communication and limitations in electronic health records behind inaccurate and delayed diagnoses, concluding that the problem of diagnostic errors generally has not been adequately studied.1
“This problem is significant and serious. Yet we don’t know for sure how often it occurs, how serious it is, or how much it costs,” said the IOM committee’s chair, John Ball, MD, of the American College of Physicians, in a prepared statement. The report concludes there is no easy fix for the problem of diagnostic errors, which are a leading cause of adverse events in hospitals and of malpractice lawsuits for hospitalists, but calls for a major reassessment of the diagnostic process.2
Hospitalist Mangla Gulati, MD, FACP, SFHM, assistant chief medical officer at the University of Maryland Medical Center in Baltimore, says hospitalists would be remiss if they failed to take a closer look at the IOM report. “Diagnostic error is something we haven’t much talked about in medicine,” Dr. Gulati says. “Part of the goal of this report is to actually include the patient in those conversations.” Patients who are rehospitalized, she says, may have been given an incorrect initial diagnosis that was never rectified, or there may have been a failure to communicate important information.
“How many tests do we order where results come back after a patient leaves the hospital?” asks Kedar Mate, MD, senior vice president at the Institute for Healthcare Improvement and a hospitalist at Weill Cornell Medicine in New York City. “How many in-hospital diagnoses are made without all of the available information from outside providers?”
One simple intervention hospitalists could do immediately, he says, is to start tracking all important tests ordered for patients on a board in the medical team’s meeting room, only removing them from the board when results have been checked and communicated to the patient and outpatient provider.
References
- National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press. 2015.
- Saber Tehrani AS, Lee HW, Mathews SC, et al. 25-year summary of U.S. malpractice claims for diagnostic errors 1986–2010: An analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013 Aug; 22(8):672–680.
Quality Improvement Initiative Targets Sepsis
A quality improvement (QI) initiative at University Hospital in Salt Lake City aims to save lives and cut hospital costs by reducing inpatient sepsis mortality.
Program co-leaders, hospitalists Devin Horton, MD, and Kencee Graves, MD, of University Hospital, launched the initiative as a pilot program last October. They began by surveying hospital house staff and nurses on their ability to recognize and define six different sepsis syndromes from clinical vignettes. A total of 136 surveyed residents recognized the correct condition only 56% of the time, and 280 surveyed nurses only did so 17% of the time. The hospitalists determined that better education about sepsis was crucial.
“We developed a robust teaching program for nurses and residents using Septris, an online educational game from Stanford University,” Dr. Horton says. The team also developed technology that can recognize worsening vital signs in a patient and automatically trigger an alert to a charge nurse or rapid response team.
The team’s Modified Early Warning System (MEWS) for recognizing sepsis is similar to the Early Warning and Response System (EWRS) system used at the University of Pennsylvania Health System and the University of California San Diego, and draws on other hospitals’ sepsis systems. Dr. Horton says one difference in their system is the involvement of nursing aides who take vital signs, enter them real-time into electronic health records (EHR), and receive prompts from abnormal vital signs to retake all vitals and confirm abnormal results. It also incorporates EHR decision support tools, including links to pre-populated medical order panels, such as for the ordering of tests for lactate and blood cultures.
“Severe sepsis is often quoted as the number one cause of mortality among hospitalized patients, with a rate up to 10 times that of acute myocardial infarction,” Dr. Horton explains. “The one treatment that consistently decreases mortality is timely administration of antibiotics. But, in order for a patient to be given timely antibiotics, the nurse or resident must first recognize that the patient has sepsis.”
“This is one of the biggest and most far-reaching improvement initiatives that has been done at our institution,” says Robert Pendleton, MD, chief quality officer at University Hospital. Dr. Horton says he predicts the program will “save 50 lives and $1 million per year.”
For more information, contact him at: [email protected].
A quality improvement (QI) initiative at University Hospital in Salt Lake City aims to save lives and cut hospital costs by reducing inpatient sepsis mortality.
Program co-leaders, hospitalists Devin Horton, MD, and Kencee Graves, MD, of University Hospital, launched the initiative as a pilot program last October. They began by surveying hospital house staff and nurses on their ability to recognize and define six different sepsis syndromes from clinical vignettes. A total of 136 surveyed residents recognized the correct condition only 56% of the time, and 280 surveyed nurses only did so 17% of the time. The hospitalists determined that better education about sepsis was crucial.
“We developed a robust teaching program for nurses and residents using Septris, an online educational game from Stanford University,” Dr. Horton says. The team also developed technology that can recognize worsening vital signs in a patient and automatically trigger an alert to a charge nurse or rapid response team.
The team’s Modified Early Warning System (MEWS) for recognizing sepsis is similar to the Early Warning and Response System (EWRS) system used at the University of Pennsylvania Health System and the University of California San Diego, and draws on other hospitals’ sepsis systems. Dr. Horton says one difference in their system is the involvement of nursing aides who take vital signs, enter them real-time into electronic health records (EHR), and receive prompts from abnormal vital signs to retake all vitals and confirm abnormal results. It also incorporates EHR decision support tools, including links to pre-populated medical order panels, such as for the ordering of tests for lactate and blood cultures.
“Severe sepsis is often quoted as the number one cause of mortality among hospitalized patients, with a rate up to 10 times that of acute myocardial infarction,” Dr. Horton explains. “The one treatment that consistently decreases mortality is timely administration of antibiotics. But, in order for a patient to be given timely antibiotics, the nurse or resident must first recognize that the patient has sepsis.”
“This is one of the biggest and most far-reaching improvement initiatives that has been done at our institution,” says Robert Pendleton, MD, chief quality officer at University Hospital. Dr. Horton says he predicts the program will “save 50 lives and $1 million per year.”
For more information, contact him at: [email protected].
A quality improvement (QI) initiative at University Hospital in Salt Lake City aims to save lives and cut hospital costs by reducing inpatient sepsis mortality.
Program co-leaders, hospitalists Devin Horton, MD, and Kencee Graves, MD, of University Hospital, launched the initiative as a pilot program last October. They began by surveying hospital house staff and nurses on their ability to recognize and define six different sepsis syndromes from clinical vignettes. A total of 136 surveyed residents recognized the correct condition only 56% of the time, and 280 surveyed nurses only did so 17% of the time. The hospitalists determined that better education about sepsis was crucial.
“We developed a robust teaching program for nurses and residents using Septris, an online educational game from Stanford University,” Dr. Horton says. The team also developed technology that can recognize worsening vital signs in a patient and automatically trigger an alert to a charge nurse or rapid response team.
The team’s Modified Early Warning System (MEWS) for recognizing sepsis is similar to the Early Warning and Response System (EWRS) system used at the University of Pennsylvania Health System and the University of California San Diego, and draws on other hospitals’ sepsis systems. Dr. Horton says one difference in their system is the involvement of nursing aides who take vital signs, enter them real-time into electronic health records (EHR), and receive prompts from abnormal vital signs to retake all vitals and confirm abnormal results. It also incorporates EHR decision support tools, including links to pre-populated medical order panels, such as for the ordering of tests for lactate and blood cultures.
“Severe sepsis is often quoted as the number one cause of mortality among hospitalized patients, with a rate up to 10 times that of acute myocardial infarction,” Dr. Horton explains. “The one treatment that consistently decreases mortality is timely administration of antibiotics. But, in order for a patient to be given timely antibiotics, the nurse or resident must first recognize that the patient has sepsis.”
“This is one of the biggest and most far-reaching improvement initiatives that has been done at our institution,” says Robert Pendleton, MD, chief quality officer at University Hospital. Dr. Horton says he predicts the program will “save 50 lives and $1 million per year.”
For more information, contact him at: [email protected].