User login
Trends in Thumb Carpometacarpal Interposition Arthroplasty in the United States, 2005–2011
A common entity, osteoarthritis (OA) at the base of the thumb is largely caused by the unique anatomy and biomechanics of the thumb carpometacarpal (CMC) joint.1 Radiographically evident CMC degeneration occurs in 40% of women and 25% of men over age 75 years, making the thumb CMC joint the most common site of surgical reconstruction for upper extremity OA.2,3
Over the past 40 years, numerous surgical techniques for managing thumb CMC-OA have been described. These include volar ligament reconstruction, first metacarpal osteotomy, CMC arthrodesis, CMC joint replacement, and trapeziectomy. Trapeziectomy can be performed in isolation or in combination with tendon interposition, ligament reconstruction, or ligament reconstruction and tendon interposition (LRTI).4-20 The authors of a recent systematic review concluded there is no evidence that any one surgical procedure for CMC-OA is superior to another in terms of pain, function, satisfaction, range of motion, or strength.4 Nevertheless, a recent survey found that 719 (62%) of 1156 US hand surgeons used LRTI as the treatment of choice for advanced CMC-OA.21
Our detailed literature search yielded no other database studies characterizing current trends in the practice patterns of US orthopedic surgeons who perform interposition arthroplasty for CMC arthritis. Analysis of these trends is important not only to patients but also to the broader orthopedic and health care community.22
We conducted a study to investigate current trends in CMC interposition arthroplasty across time, sex, age, and region of the United States; per-patient charges and reimbursements; and the association between this procedure and concomitantly performed carpal tunnel syndrome (CTS) and carpal tunnel release (CTR). In addition, we compared incidence of CMC interposition arthroplasty with that of CMC arthrodesis.
Patients and Methods
All data were derived from the PearlDiver Patient Records Database (PearlDiver Technologies), a publicly available database of patients. The database stores procedure volumes, demographics, and average charge information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the present study were drawn from the Medicare database within the PearlDiver records, which has a total of 179,094,296 patient records covering the period 2005–2011. This study did not require institutional review board approval, as it used existing, publicly available data without identifiers linked to subjects.
PearlDiver Technologies granted us database access for academic research. The database was stored on a password-protected server maintained by PearlDiver. ICD-9 and CPT codes can be searched in isolation or in combination. Search results yield number of patients with a searched code (or combination of codes) in each year, age group, or region of the United States, as well as mean charge and mean reimbursement for the code or combination of codes.
We used CPT code 25447 (arthroplasty, interposition, intercarpal, or CMC joints) to search the database for patients who underwent thumb CMC interposition arthroplasty. Although this code does not specify thumb, we are unaware of any procedure (other than thumb CMC interposition arthroplasty) typically given this code. Our search yielded procedure volumes, sex distribution, age distribution, region volumes, and mean per-patient charges and reimbursements for each CPT code. We then searched the resulting cohort for CTS (ICD-9 code 354.0), endoscopic CTR (CPT code 29848), and open CTR (CPT code 64721) to find CTR performed concomitantly with CMC interposition arthroplasty. Last, patients were tracked in the database past their surgery date to evaluate for postoperative physical or occupational therapy evaluations within 6 months (using CPT codes appearing in at least 1% of the cohort: 97001, 97003, 97004, 97110, 97112, 97124, 97140, 97150, 97350, 97535) and postoperative thumb, hand, or wrist radiographs within 6 months (using CPT codes appearing in at least 1% of the cohort: 73140, 73130, 73110). To ensure adequacy of 6-month postoperative data, we included in this portion of the study only those patients with surgery dates between 2005 and 2010.
For comparative purposes, we also searched the database for patients who underwent thumb CMC arthrodesis within the same period—using CPT codes 26841 and 26842 (arthrodesis CMC joint thumb, with or without internal fixation; with or without autograft) and CPT code 26820 (fusion in opposition, thumb, with autogenous graft).
Overall procedure volume data are reported as number of patients with the given CPT code in the database output in a given year. Age-group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who underwent the CPT code of interest that year. Mean charges and reimbursements are reported as results by the database for the code of interest (CPT 25447). Data for the region analysis are presented as an incidence, as there is an uneven distribution of patient volumes among regions. This incidence is calculated as number of patients in a particular region and year normalized to total number of patients in the database for that particular region or year. Regions are defined as Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-squared linear-by-linear association analysis was used to determine statistical significance with regard to trends over time in procedure volumes, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
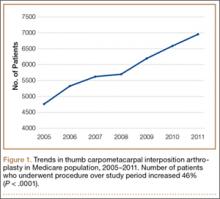
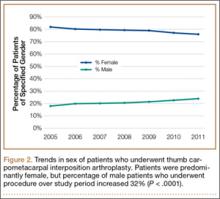
In the database, we identified 41,171 unique patients who underwent CMC interposition arthroplasty between 2005 and 2011. Over the 7-year study period, number of patients who had CMC interposition arthroplasty increased 46.2%, from 4761 in 2005 to 6960 in 2011 (P < .0001) (Table 1, Figure 1). Throughout this period, females underwent CMC interposition arthroplasty more frequently than males at all time points (P < .0001). Overall ratio of female to male patients, however, changed significantly. In 2005, 18.1% of all CMC interposition arthroplasties were performed on male patients; this increased to 23.9% of all procedures by 2011 (P < .0001) (Figure 2). Table 1 presents an age-group analysis. There were no significant differences in relative percentage of patients in any given age group who underwent CMC interposition arthroplasty over the study period.
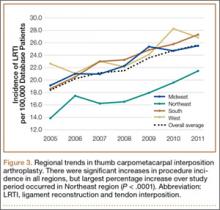
Analysis of overall procedure incidence by region revealed significant increases in all regions (P < .0001), ranging from 18.5% (West) to 54.5% (Northeast) (Figure 3). At all time points, the incidence of CMC interposition arthroplasty was significantly lower in the Northeast than in any other region and compared with the overall average.
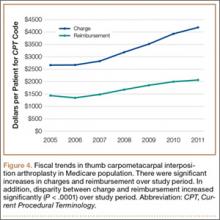
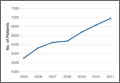
Between 2005 and 2011, there were significant increases in both per-patient charges and reimbursements for CMC interposition arthroplasty (Figure 4). Mean per-patient charge increased from $2676 in 2005 to $4181 in 2011 (P < .0001), and mean per-patient reimbursement increased from $1445 in 2005 to $2061 in 2011 (P < .0001). The discrepancy between charge and reimbursement increased throughout the study period: Reimbursement in 2005 was 54.0% of the charge; this decreased to 49.3% by 2011 but was not statistically significant (P = .08).
Overall, 40.9% of patients who underwent CMC interposition arthroplasty also had a CTS diagnosis. Between 15.5% and 17.3% of these patients had concomitant open or endoscopic CTR at time of CMC interposition arthroplasty (Table 2). Percentage of patients who underwent concomitant CTR did not change significantly from 2005 to 2011 (P = .139). Use of postoperative occupational and/or physical therapy increased significantly over the study period, from 33.5% of patients in 2005 to 50.7% of patients in 2010 (P < .0001). Use of postoperative thumb, hand, and/or wrist radiography also increased throughout the study period, from 7.4% of patients in 2005 to 18.7% of patients in 2010 (P < .0001).
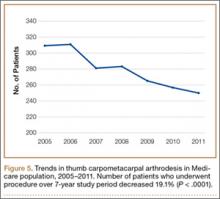
We identified 1916 unique patients who underwent thumb CMC arthrodesis between 2005 and 2011. Over the 7-year study period, there was a 19.1% decrease in number of patients who underwent CMC arthrodesis, from 309 in 2005 to 250 in 2011 (P < .0001) (Figure 5). Significantly fewer patients had CMC arthrodesis compared with CMC interposition arthroplasty at all time points, ranging from 6.5% (thumb CMC arthrodesis:CMC interposition arthroplasty) in 2005 to 3.6% in 2011 (P < .0001).
Discussion
Our results demonstrated a significant increase in use of thumb CMC interposition arthroplasty in a US Medicare population, with an increase of more than 46% from 2005 to 2011. This finding supports the findings of a recent cross-sectional survey-based study in which 719 (62%) of 1156 surveyed US hand surgeons reported performing trapeziectomy with LRTI for advanced thumb CMC-OA.21 A prior study had similar findings, with 692 (68%) of 1024 American Society for Surgery of the Hand (ASSH) members performing LRTI and 766 (75%) of 1024 performing some type of CMC interposition with trapeziectomy for advanced CMC-OA.23 This preference for CMC interposition arthroplasty prevails despite the fact that numerous studies have shown no superiority of any surgical procedure to another for CMC-OA in terms of pain, function, satisfaction, range of motion, and strength.7,15,18,19,24-34 Our data demonstrated that, not only does CMC interposition arthroplasty remain the most frequently used procedure for thumb CMC-OA, the incidence of CMC interposition arthroplasty continues to increase yearly.
The incidence of thumb CMC-OA is higher in women than in men, with more joint laxity a known contributor and subtle sex differences in trapezium geometry and congruence postulated as additional factors.3,35,36 This trend was confirmed in the present study, as females underwent significantly more CMC interposition arthroplasties at all time points. It is interesting that the overall ratio of female to male patients changed significantly over the study period, with the percentage of patients who were male increasing from 18.1% in 2005 to 23.9% in 2011. No previous studies have captured such a large cross section of the population to establish this trend. Although this trend is not necessarily intuitive, potential theories include increased acceptance of CMC interposition arthroplasty as a surgical option for male patients, and potentially a larger number of male patients seeking medical care for thumb CMC-OA in recent years.
Increases in procedure incidence were noted in all regions of the United States, but the largest percentage increase occurred in the Northeast. Despite this increase, the Northeast also had significantly lower CMC interposition arthroplasty incidence compared with all other regions and with the average procedure incidence throughout the study period—demonstrating some regional bias as to treatment of thumb CMC-OA. Unfortunately, because of database limitations and lack of specific CPT codes for other treatment options for thumb CMC-OA, we cannot ascertain if other types of surgery are more frequently used in the Northeast.
CTS and thumb CMC-OA often coexist.37 The estimated incidence of concomitant CTS in patients with CMC-OA is between 4% and 43%, but the rate of concomitant CTR and CMC interposition arthroplasty was not previously characterized in the literature.38,39 Results of the present study supported these findings; 41% of patients who underwent CMC interposition arthroplasty in our study also had a CTS diagnosis, compared with 43% in the 246-patient study by Florack and colleagues.38 We also found that 16% to 17% of patients who underwent CMC interposition arthroplasty underwent concomitant CTR; this rate remained consistent throughout the study period.
Our study demonstrated that, compared with CMC interposition arthroplasties, significantly fewer thumb CMC arthrodesis procedures were performed in the same Medicare population during the same period. Furthermore, the number of thumb CMC arthrodesis procedures declined yearly, with an overall decrease of 19% from 2005 to 2011. In a recent single-blinded, randomized trial, Vermeulen and colleagues40 compared thumb CMC arthrodesis and trapeziectomy with LRTI. They found superior patient satisfaction and significantly lower complication rates in women who underwent LRTI versus arthrodesis. The study was terminated prematurely because of these complications and thus was underpowered to determine differences in specific outcome measures. Previous studies comparing arthrodesis and interposition arthroplasties reported inconsistent outcomes. Hart and colleagues41 found no significant differences in pain or function between CMC arthrodesis and LRTI at a mean 7-year follow-up in a level II randomized controlled trial. Hartigan and colleagues15 reached similar conclusions in their retrospective comparison of the procedures. Without clear evidence supporting arthrodesis over interposition arthroplasty, the majority of surgeons favor interposition arthroplasty for thumb CMC-OA. Among Medicare patients, use of thumb CMC arthrodesis continues to fall.
This national database study had several limitations, which are common to all studies using the PearlDiver database22,42-47:
1. The power of the analysis depended on the quality of available data. Potential sources of error included accuracy of billing codes, and miscoding or noncoding by physicians.46
2. Although we used this database to try to accurately represent a large population of interest, we cannot guarantee the database represented a true cross section of the United States.
3. For the Medicare population, the PearlDiver database indexes data only in 7-year increments. Although the study period was long enough to detect significant trends, some data may not be accurately captured over a 7-year period.
4. Patients were not randomized to a treatment group.
5. The PearlDiver database does not include any clinical outcome data. Therefore, we cannot comment on the efficacy of the reported evaluations and interventions.
6. There is no specific CPT code for thumb CMC interposition arthroplasty. However, we are unaware of a CMC interposition arthroplasty performed for any area besides the thumb. Theoretically, the study population can include a negligible percentage of patients who had interposition arthroplasty of a CMC joint other than the thumb.
7. The database cannot be searched for use of thumb CMC-OA surgical techniques other than CMC interposition arthroplasty or arthrodesis, as isolated trapeziectomy, volar ligament reconstruction, implant arthroplasty, and metacarpal osteotomy lack specific CPT codes.
Conclusion
Thumb CMC-OA is a common entity among Medicare patients. There are numerous surgical options for cases that have failed conservative treatment. Despite the lack of evidence that thumb CMC interposition arthroplasty is superior to other surgical options, the number of patients who had this procedure increased 46% during the 2005–2011 study period. Although the majority of patients who undergo CMC interposition arthroplasty are female, the percentage of male patients has increased significantly. More than 40% of patients who have CMC interposition arthroplasty are also diagnosed with CTS, and 16% to 17% of patients who have CMC interposition arthroplasty will have a concomitant CTR. CMC arthrodesis is used in significantly fewer patients of Medicare age, and its use has been declining.
1. Hentz VR. Surgical treatment of trapeziometacarpal joint arthritis: a historical perspective. Clin Orthop Relat Res. 2014;472(4):1184-1189.
2. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341.
3. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16(3):140-151.
4. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
5. Bodin ND, Spangler R, Thoder JJ. Interposition arthroplasty options for carpometacarpal arthritis of the thumb. Hand Clin. 2010;26(3):339-350, v-vi.
6. Cooney WP, Linscheid RL, Askew LJ. Total arthroplasty of the thumb trapeziometacarpal joint. Clin Orthop Relat Res. 1987;(220):35-45.
7. De Smet L, Vandenberghe L, Degreef I. Long-term outcome of trapeziectomy with ligament reconstruction and tendon interposition (LRTI) versus prosthesis arthroplasty for basal joint osteoarthritis of the thumb. Acta Orthop Belg. 2013;79(2):146-149.
8. Dell PC, Muniz RB. Interposition arthroplasty of the trapeziometacarpal joint for osteoarthritis. Clin Orthop Relat Res. 1987;(220):27-34.
9. Dhar S, Gray IC, Jones WA, Beddow FH. Simple excision of the trapezium for osteoarthritis of the carpometacarpal joint of the thumb. J Hand Surg Br. 1994;19(4):485-488.
10. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55(8):1655-1666.
11. Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9(5):692-699.
12. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg Am. 1985;10(5):645-654.
13. Elfar JC, Burton RI. Ligament reconstruction and tendon interposition for thumb basal arthritis. Hand Clin. 2013;29(1):15-25.
14. Froimson AI. Tendon arthroplasty of the trapeziometacarpal joint. Clin Orthop Relat Res. 1970;70:191-199.
15. Hartigan BJ, Stern PJ, Kiefhaber TR. Thumb carpometacarpal osteoarthritis: arthrodesis compared with ligament reconstruction and tendon interposition. J Bone Joint Surg Am. 2001;83(10):1470-1478.
16. Kenniston JA, Bozentka DJ. Treatment of advanced carpometacarpal joint disease: arthrodesis. Hand Clin. 2008;24(3):285-294, vi-vii.
17. Kokkalis ZT, Zanaros G, Weiser RW, Sotereanos DG. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am. 2009;34(6):1029-1036.
18. Kriegs-Au G, Petje G, Fojtl E, Ganger R, Zachs I. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. Surgical technique. J Bone Joint Surg Am. 2005;87 suppl 1(Pt 1):78-85.
19. Park MJ, Lichtman G, Christian JB, et al. Surgical treatment of thumb carpometacarpal joint arthritis: a single institution experience from 1995–2005. Hand. 2008;3(4):304-310.
20. Park MJ, Lee AT, Yao J. Treatment of thumb carpometacarpal arthritis with arthroscopic hemitrapeziectomy and interposition arthroplasty. Orthopedics. 2012;35(12):e1759-e1764.
21. Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37(1):77-82.
22. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
23. Brunton LM, Wilgis EF. A survey to determine current practice patterns in the surgical treatment of advanced thumb carpometacarpal osteoarthrosis. Hand. 2010;5(4):415-422.
24. Belcher HJ, Nicholl JE. A comparison of trapeziectomy with and without ligament reconstruction and tendon interposition. J Hand Surg Br. 2000;25(4):350-356.
25. Davis TR, Pace A. Trapeziectomy for trapeziometacarpal joint osteoarthritis: is ligament reconstruction and temporary stabilisation of the pseudarthrosis with a Kirschner wire important? J Hand Surg Eur Vol. 2009;34(3):312-321.
26. Davis TR, Brady O, Dias JJ. Excision of the trapezium for osteoarthritis of the trapeziometacarpal joint: a study of the benefit of ligament reconstruction or tendon interposition. J Hand Surg Am. 2004;29(6):1069-1077.
27. De Smet L, Sioen W, Spaepen D, van Ransbeeck H. Treatment of basal joint arthritis of the thumb: trapeziectomy with or without tendon interposition/ligament reconstruction. Hand Surg. 2004;9(1):5-9.
28. Field J, Buchanan D. To suspend or not to suspend: a randomised single blind trial of simple trapeziectomy versus trapeziectomy and flexor carpi radialis suspension. J Hand Surg Eur Vol. 2007;32(4):462-466.
29. Gerwin M, Griffith A, Weiland AJ, Hotchkiss RN, McCormack RR. Ligament reconstruction basal joint arthroplasty without tendon interposition. Clin Orthop Relat Res. 1997;(342):42-45.
30. Jorheim M, Isaxon I, Flondell M, Kalen P, Atroshi I. Short-term outcomes of trapeziometacarpal Artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
31. Lehmann O, Herren DB, Simmen BR. Comparison of tendon suspension-interposition and silicon spacers in the treatment of degenerative osteoarthritis of the base of the thumb. Ann Chir Main Memb Super. 1998;17(1):25-30.
32. Nilsson A, Liljensten E, Bergstrom C, Sollerman C. Results from a degradable TMC joint spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
33. Schroder J, Kerkhoffs GM, Voerman HJ, Marti RK. Surgical treatment of basal joint disease of the thumb: comparison between resection-interposition arthroplasty and trapezio-metacarpal arthrodesis. Arch Orthop Trauma Surg. 2002;122(1):35-38.
34. Tagil M, Kopylov P. Swanson versus APL arthroplasty in the treatment of osteoarthritis of the trapeziometacarpal joint: a prospective and randomized study in 26 patients. J Hand Surg Br. 2002;27(5):452-456.
35. North ER, Rutledge WM. The trapezium-thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15(2):201-206.
36. Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25(6):591-607.
37. Sless Y, Sampson SP. Experience with transtrapezium approach for transverse carpal ligament release in patients with coexisted trapeziometacarpal joint osteoarthritis and carpal tunnel syndrome. Hand. 2007;2(3):151-154.
38. Florack TM, Miller RJ, Pellegrini VD, Burton RI, Dunn MG. The prevalence of carpal tunnel syndrome in patients with basal joint arthritis of the thumb. J Hand Surg Am. 1992;17(4):624-630.
39. Tsai TM, Laurentin-Perez LA, Wong MS, Tamai M. Ideas and innovations: radial approach to carpal tunnel release in conjunction with thumb carpometacarpal arthroplasty. Hand Surg. 2005;10(1):61-66.
40. Vermeulen GM, Brink SM, Slijper H, et al. Trapeziometacarpal arthrodesis or trapeziectomy with ligament reconstruction in primary trapeziometacarpal osteoarthritis: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(9):726-733.
41. Hart R, Janecek M, Siska V, Kucera B, Stipcak V. Interposition suspension arthroplasty according to Epping versus arthrodesis for trapeziometacarpal osteoarthritis. Eur Surg. 2006;38(6):433-438.
42. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005–2011. Am J Sports Med. 2013;41(10):2333-2339.
43. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665.
44. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443.
45. Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441.
46. Yeranosian MG, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. The costs associated with the evaluation of rotator cuff tears before surgical repair. J Shoulder Elbow Surg. 2013;22(12):1662-1666.
47. Daffner SD, Hymanson HJ, Wang JC. Cost and use of conservative management of lumbar disc herniation before surgical discectomy. Spine J. 2010;10(6):463-468.
A common entity, osteoarthritis (OA) at the base of the thumb is largely caused by the unique anatomy and biomechanics of the thumb carpometacarpal (CMC) joint.1 Radiographically evident CMC degeneration occurs in 40% of women and 25% of men over age 75 years, making the thumb CMC joint the most common site of surgical reconstruction for upper extremity OA.2,3
Over the past 40 years, numerous surgical techniques for managing thumb CMC-OA have been described. These include volar ligament reconstruction, first metacarpal osteotomy, CMC arthrodesis, CMC joint replacement, and trapeziectomy. Trapeziectomy can be performed in isolation or in combination with tendon interposition, ligament reconstruction, or ligament reconstruction and tendon interposition (LRTI).4-20 The authors of a recent systematic review concluded there is no evidence that any one surgical procedure for CMC-OA is superior to another in terms of pain, function, satisfaction, range of motion, or strength.4 Nevertheless, a recent survey found that 719 (62%) of 1156 US hand surgeons used LRTI as the treatment of choice for advanced CMC-OA.21
Our detailed literature search yielded no other database studies characterizing current trends in the practice patterns of US orthopedic surgeons who perform interposition arthroplasty for CMC arthritis. Analysis of these trends is important not only to patients but also to the broader orthopedic and health care community.22
We conducted a study to investigate current trends in CMC interposition arthroplasty across time, sex, age, and region of the United States; per-patient charges and reimbursements; and the association between this procedure and concomitantly performed carpal tunnel syndrome (CTS) and carpal tunnel release (CTR). In addition, we compared incidence of CMC interposition arthroplasty with that of CMC arthrodesis.
Patients and Methods
All data were derived from the PearlDiver Patient Records Database (PearlDiver Technologies), a publicly available database of patients. The database stores procedure volumes, demographics, and average charge information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the present study were drawn from the Medicare database within the PearlDiver records, which has a total of 179,094,296 patient records covering the period 2005–2011. This study did not require institutional review board approval, as it used existing, publicly available data without identifiers linked to subjects.
PearlDiver Technologies granted us database access for academic research. The database was stored on a password-protected server maintained by PearlDiver. ICD-9 and CPT codes can be searched in isolation or in combination. Search results yield number of patients with a searched code (or combination of codes) in each year, age group, or region of the United States, as well as mean charge and mean reimbursement for the code or combination of codes.
We used CPT code 25447 (arthroplasty, interposition, intercarpal, or CMC joints) to search the database for patients who underwent thumb CMC interposition arthroplasty. Although this code does not specify thumb, we are unaware of any procedure (other than thumb CMC interposition arthroplasty) typically given this code. Our search yielded procedure volumes, sex distribution, age distribution, region volumes, and mean per-patient charges and reimbursements for each CPT code. We then searched the resulting cohort for CTS (ICD-9 code 354.0), endoscopic CTR (CPT code 29848), and open CTR (CPT code 64721) to find CTR performed concomitantly with CMC interposition arthroplasty. Last, patients were tracked in the database past their surgery date to evaluate for postoperative physical or occupational therapy evaluations within 6 months (using CPT codes appearing in at least 1% of the cohort: 97001, 97003, 97004, 97110, 97112, 97124, 97140, 97150, 97350, 97535) and postoperative thumb, hand, or wrist radiographs within 6 months (using CPT codes appearing in at least 1% of the cohort: 73140, 73130, 73110). To ensure adequacy of 6-month postoperative data, we included in this portion of the study only those patients with surgery dates between 2005 and 2010.
For comparative purposes, we also searched the database for patients who underwent thumb CMC arthrodesis within the same period—using CPT codes 26841 and 26842 (arthrodesis CMC joint thumb, with or without internal fixation; with or without autograft) and CPT code 26820 (fusion in opposition, thumb, with autogenous graft).
Overall procedure volume data are reported as number of patients with the given CPT code in the database output in a given year. Age-group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who underwent the CPT code of interest that year. Mean charges and reimbursements are reported as results by the database for the code of interest (CPT 25447). Data for the region analysis are presented as an incidence, as there is an uneven distribution of patient volumes among regions. This incidence is calculated as number of patients in a particular region and year normalized to total number of patients in the database for that particular region or year. Regions are defined as Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-squared linear-by-linear association analysis was used to determine statistical significance with regard to trends over time in procedure volumes, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
In the database, we identified 41,171 unique patients who underwent CMC interposition arthroplasty between 2005 and 2011. Over the 7-year study period, number of patients who had CMC interposition arthroplasty increased 46.2%, from 4761 in 2005 to 6960 in 2011 (P < .0001) (Table 1, Figure 1). Throughout this period, females underwent CMC interposition arthroplasty more frequently than males at all time points (P < .0001). Overall ratio of female to male patients, however, changed significantly. In 2005, 18.1% of all CMC interposition arthroplasties were performed on male patients; this increased to 23.9% of all procedures by 2011 (P < .0001) (Figure 2). Table 1 presents an age-group analysis. There were no significant differences in relative percentage of patients in any given age group who underwent CMC interposition arthroplasty over the study period.
Analysis of overall procedure incidence by region revealed significant increases in all regions (P < .0001), ranging from 18.5% (West) to 54.5% (Northeast) (Figure 3). At all time points, the incidence of CMC interposition arthroplasty was significantly lower in the Northeast than in any other region and compared with the overall average.
Between 2005 and 2011, there were significant increases in both per-patient charges and reimbursements for CMC interposition arthroplasty (Figure 4). Mean per-patient charge increased from $2676 in 2005 to $4181 in 2011 (P < .0001), and mean per-patient reimbursement increased from $1445 in 2005 to $2061 in 2011 (P < .0001). The discrepancy between charge and reimbursement increased throughout the study period: Reimbursement in 2005 was 54.0% of the charge; this decreased to 49.3% by 2011 but was not statistically significant (P = .08).
Overall, 40.9% of patients who underwent CMC interposition arthroplasty also had a CTS diagnosis. Between 15.5% and 17.3% of these patients had concomitant open or endoscopic CTR at time of CMC interposition arthroplasty (Table 2). Percentage of patients who underwent concomitant CTR did not change significantly from 2005 to 2011 (P = .139). Use of postoperative occupational and/or physical therapy increased significantly over the study period, from 33.5% of patients in 2005 to 50.7% of patients in 2010 (P < .0001). Use of postoperative thumb, hand, and/or wrist radiography also increased throughout the study period, from 7.4% of patients in 2005 to 18.7% of patients in 2010 (P < .0001).
We identified 1916 unique patients who underwent thumb CMC arthrodesis between 2005 and 2011. Over the 7-year study period, there was a 19.1% decrease in number of patients who underwent CMC arthrodesis, from 309 in 2005 to 250 in 2011 (P < .0001) (Figure 5). Significantly fewer patients had CMC arthrodesis compared with CMC interposition arthroplasty at all time points, ranging from 6.5% (thumb CMC arthrodesis:CMC interposition arthroplasty) in 2005 to 3.6% in 2011 (P < .0001).
Discussion
Our results demonstrated a significant increase in use of thumb CMC interposition arthroplasty in a US Medicare population, with an increase of more than 46% from 2005 to 2011. This finding supports the findings of a recent cross-sectional survey-based study in which 719 (62%) of 1156 surveyed US hand surgeons reported performing trapeziectomy with LRTI for advanced thumb CMC-OA.21 A prior study had similar findings, with 692 (68%) of 1024 American Society for Surgery of the Hand (ASSH) members performing LRTI and 766 (75%) of 1024 performing some type of CMC interposition with trapeziectomy for advanced CMC-OA.23 This preference for CMC interposition arthroplasty prevails despite the fact that numerous studies have shown no superiority of any surgical procedure to another for CMC-OA in terms of pain, function, satisfaction, range of motion, and strength.7,15,18,19,24-34 Our data demonstrated that, not only does CMC interposition arthroplasty remain the most frequently used procedure for thumb CMC-OA, the incidence of CMC interposition arthroplasty continues to increase yearly.
The incidence of thumb CMC-OA is higher in women than in men, with more joint laxity a known contributor and subtle sex differences in trapezium geometry and congruence postulated as additional factors.3,35,36 This trend was confirmed in the present study, as females underwent significantly more CMC interposition arthroplasties at all time points. It is interesting that the overall ratio of female to male patients changed significantly over the study period, with the percentage of patients who were male increasing from 18.1% in 2005 to 23.9% in 2011. No previous studies have captured such a large cross section of the population to establish this trend. Although this trend is not necessarily intuitive, potential theories include increased acceptance of CMC interposition arthroplasty as a surgical option for male patients, and potentially a larger number of male patients seeking medical care for thumb CMC-OA in recent years.
Increases in procedure incidence were noted in all regions of the United States, but the largest percentage increase occurred in the Northeast. Despite this increase, the Northeast also had significantly lower CMC interposition arthroplasty incidence compared with all other regions and with the average procedure incidence throughout the study period—demonstrating some regional bias as to treatment of thumb CMC-OA. Unfortunately, because of database limitations and lack of specific CPT codes for other treatment options for thumb CMC-OA, we cannot ascertain if other types of surgery are more frequently used in the Northeast.
CTS and thumb CMC-OA often coexist.37 The estimated incidence of concomitant CTS in patients with CMC-OA is between 4% and 43%, but the rate of concomitant CTR and CMC interposition arthroplasty was not previously characterized in the literature.38,39 Results of the present study supported these findings; 41% of patients who underwent CMC interposition arthroplasty in our study also had a CTS diagnosis, compared with 43% in the 246-patient study by Florack and colleagues.38 We also found that 16% to 17% of patients who underwent CMC interposition arthroplasty underwent concomitant CTR; this rate remained consistent throughout the study period.
Our study demonstrated that, compared with CMC interposition arthroplasties, significantly fewer thumb CMC arthrodesis procedures were performed in the same Medicare population during the same period. Furthermore, the number of thumb CMC arthrodesis procedures declined yearly, with an overall decrease of 19% from 2005 to 2011. In a recent single-blinded, randomized trial, Vermeulen and colleagues40 compared thumb CMC arthrodesis and trapeziectomy with LRTI. They found superior patient satisfaction and significantly lower complication rates in women who underwent LRTI versus arthrodesis. The study was terminated prematurely because of these complications and thus was underpowered to determine differences in specific outcome measures. Previous studies comparing arthrodesis and interposition arthroplasties reported inconsistent outcomes. Hart and colleagues41 found no significant differences in pain or function between CMC arthrodesis and LRTI at a mean 7-year follow-up in a level II randomized controlled trial. Hartigan and colleagues15 reached similar conclusions in their retrospective comparison of the procedures. Without clear evidence supporting arthrodesis over interposition arthroplasty, the majority of surgeons favor interposition arthroplasty for thumb CMC-OA. Among Medicare patients, use of thumb CMC arthrodesis continues to fall.
This national database study had several limitations, which are common to all studies using the PearlDiver database22,42-47:
1. The power of the analysis depended on the quality of available data. Potential sources of error included accuracy of billing codes, and miscoding or noncoding by physicians.46
2. Although we used this database to try to accurately represent a large population of interest, we cannot guarantee the database represented a true cross section of the United States.
3. For the Medicare population, the PearlDiver database indexes data only in 7-year increments. Although the study period was long enough to detect significant trends, some data may not be accurately captured over a 7-year period.
4. Patients were not randomized to a treatment group.
5. The PearlDiver database does not include any clinical outcome data. Therefore, we cannot comment on the efficacy of the reported evaluations and interventions.
6. There is no specific CPT code for thumb CMC interposition arthroplasty. However, we are unaware of a CMC interposition arthroplasty performed for any area besides the thumb. Theoretically, the study population can include a negligible percentage of patients who had interposition arthroplasty of a CMC joint other than the thumb.
7. The database cannot be searched for use of thumb CMC-OA surgical techniques other than CMC interposition arthroplasty or arthrodesis, as isolated trapeziectomy, volar ligament reconstruction, implant arthroplasty, and metacarpal osteotomy lack specific CPT codes.
Conclusion
Thumb CMC-OA is a common entity among Medicare patients. There are numerous surgical options for cases that have failed conservative treatment. Despite the lack of evidence that thumb CMC interposition arthroplasty is superior to other surgical options, the number of patients who had this procedure increased 46% during the 2005–2011 study period. Although the majority of patients who undergo CMC interposition arthroplasty are female, the percentage of male patients has increased significantly. More than 40% of patients who have CMC interposition arthroplasty are also diagnosed with CTS, and 16% to 17% of patients who have CMC interposition arthroplasty will have a concomitant CTR. CMC arthrodesis is used in significantly fewer patients of Medicare age, and its use has been declining.
A common entity, osteoarthritis (OA) at the base of the thumb is largely caused by the unique anatomy and biomechanics of the thumb carpometacarpal (CMC) joint.1 Radiographically evident CMC degeneration occurs in 40% of women and 25% of men over age 75 years, making the thumb CMC joint the most common site of surgical reconstruction for upper extremity OA.2,3
Over the past 40 years, numerous surgical techniques for managing thumb CMC-OA have been described. These include volar ligament reconstruction, first metacarpal osteotomy, CMC arthrodesis, CMC joint replacement, and trapeziectomy. Trapeziectomy can be performed in isolation or in combination with tendon interposition, ligament reconstruction, or ligament reconstruction and tendon interposition (LRTI).4-20 The authors of a recent systematic review concluded there is no evidence that any one surgical procedure for CMC-OA is superior to another in terms of pain, function, satisfaction, range of motion, or strength.4 Nevertheless, a recent survey found that 719 (62%) of 1156 US hand surgeons used LRTI as the treatment of choice for advanced CMC-OA.21
Our detailed literature search yielded no other database studies characterizing current trends in the practice patterns of US orthopedic surgeons who perform interposition arthroplasty for CMC arthritis. Analysis of these trends is important not only to patients but also to the broader orthopedic and health care community.22
We conducted a study to investigate current trends in CMC interposition arthroplasty across time, sex, age, and region of the United States; per-patient charges and reimbursements; and the association between this procedure and concomitantly performed carpal tunnel syndrome (CTS) and carpal tunnel release (CTR). In addition, we compared incidence of CMC interposition arthroplasty with that of CMC arthrodesis.
Patients and Methods
All data were derived from the PearlDiver Patient Records Database (PearlDiver Technologies), a publicly available database of patients. The database stores procedure volumes, demographics, and average charge information for patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnoses and procedures or Current Procedural Terminology (CPT) codes. Data for the present study were drawn from the Medicare database within the PearlDiver records, which has a total of 179,094,296 patient records covering the period 2005–2011. This study did not require institutional review board approval, as it used existing, publicly available data without identifiers linked to subjects.
PearlDiver Technologies granted us database access for academic research. The database was stored on a password-protected server maintained by PearlDiver. ICD-9 and CPT codes can be searched in isolation or in combination. Search results yield number of patients with a searched code (or combination of codes) in each year, age group, or region of the United States, as well as mean charge and mean reimbursement for the code or combination of codes.
We used CPT code 25447 (arthroplasty, interposition, intercarpal, or CMC joints) to search the database for patients who underwent thumb CMC interposition arthroplasty. Although this code does not specify thumb, we are unaware of any procedure (other than thumb CMC interposition arthroplasty) typically given this code. Our search yielded procedure volumes, sex distribution, age distribution, region volumes, and mean per-patient charges and reimbursements for each CPT code. We then searched the resulting cohort for CTS (ICD-9 code 354.0), endoscopic CTR (CPT code 29848), and open CTR (CPT code 64721) to find CTR performed concomitantly with CMC interposition arthroplasty. Last, patients were tracked in the database past their surgery date to evaluate for postoperative physical or occupational therapy evaluations within 6 months (using CPT codes appearing in at least 1% of the cohort: 97001, 97003, 97004, 97110, 97112, 97124, 97140, 97150, 97350, 97535) and postoperative thumb, hand, or wrist radiographs within 6 months (using CPT codes appearing in at least 1% of the cohort: 73140, 73130, 73110). To ensure adequacy of 6-month postoperative data, we included in this portion of the study only those patients with surgery dates between 2005 and 2010.
For comparative purposes, we also searched the database for patients who underwent thumb CMC arthrodesis within the same period—using CPT codes 26841 and 26842 (arthrodesis CMC joint thumb, with or without internal fixation; with or without autograft) and CPT code 26820 (fusion in opposition, thumb, with autogenous graft).
Overall procedure volume data are reported as number of patients with the given CPT code in the database output in a given year. Age-group and sex analyses are reported as number of patients reported in the database output and as percentage of patients who underwent the CPT code of interest that year. Mean charges and reimbursements are reported as results by the database for the code of interest (CPT 25447). Data for the region analysis are presented as an incidence, as there is an uneven distribution of patient volumes among regions. This incidence is calculated as number of patients in a particular region and year normalized to total number of patients in the database for that particular region or year. Regions are defined as Midwest (IA, IL, IN, KS, MI, MN, MO, ND, NE, OH, SD, WI), Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, VT), South (AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, WY).
Chi-squared linear-by-linear association analysis was used to determine statistical significance with regard to trends over time in procedure volumes, sex, age group, and region. For all statistical comparisons, P < .05 was considered significant.
Results
In the database, we identified 41,171 unique patients who underwent CMC interposition arthroplasty between 2005 and 2011. Over the 7-year study period, number of patients who had CMC interposition arthroplasty increased 46.2%, from 4761 in 2005 to 6960 in 2011 (P < .0001) (Table 1, Figure 1). Throughout this period, females underwent CMC interposition arthroplasty more frequently than males at all time points (P < .0001). Overall ratio of female to male patients, however, changed significantly. In 2005, 18.1% of all CMC interposition arthroplasties were performed on male patients; this increased to 23.9% of all procedures by 2011 (P < .0001) (Figure 2). Table 1 presents an age-group analysis. There were no significant differences in relative percentage of patients in any given age group who underwent CMC interposition arthroplasty over the study period.
Analysis of overall procedure incidence by region revealed significant increases in all regions (P < .0001), ranging from 18.5% (West) to 54.5% (Northeast) (Figure 3). At all time points, the incidence of CMC interposition arthroplasty was significantly lower in the Northeast than in any other region and compared with the overall average.
Between 2005 and 2011, there were significant increases in both per-patient charges and reimbursements for CMC interposition arthroplasty (Figure 4). Mean per-patient charge increased from $2676 in 2005 to $4181 in 2011 (P < .0001), and mean per-patient reimbursement increased from $1445 in 2005 to $2061 in 2011 (P < .0001). The discrepancy between charge and reimbursement increased throughout the study period: Reimbursement in 2005 was 54.0% of the charge; this decreased to 49.3% by 2011 but was not statistically significant (P = .08).
Overall, 40.9% of patients who underwent CMC interposition arthroplasty also had a CTS diagnosis. Between 15.5% and 17.3% of these patients had concomitant open or endoscopic CTR at time of CMC interposition arthroplasty (Table 2). Percentage of patients who underwent concomitant CTR did not change significantly from 2005 to 2011 (P = .139). Use of postoperative occupational and/or physical therapy increased significantly over the study period, from 33.5% of patients in 2005 to 50.7% of patients in 2010 (P < .0001). Use of postoperative thumb, hand, and/or wrist radiography also increased throughout the study period, from 7.4% of patients in 2005 to 18.7% of patients in 2010 (P < .0001).
We identified 1916 unique patients who underwent thumb CMC arthrodesis between 2005 and 2011. Over the 7-year study period, there was a 19.1% decrease in number of patients who underwent CMC arthrodesis, from 309 in 2005 to 250 in 2011 (P < .0001) (Figure 5). Significantly fewer patients had CMC arthrodesis compared with CMC interposition arthroplasty at all time points, ranging from 6.5% (thumb CMC arthrodesis:CMC interposition arthroplasty) in 2005 to 3.6% in 2011 (P < .0001).
Discussion
Our results demonstrated a significant increase in use of thumb CMC interposition arthroplasty in a US Medicare population, with an increase of more than 46% from 2005 to 2011. This finding supports the findings of a recent cross-sectional survey-based study in which 719 (62%) of 1156 surveyed US hand surgeons reported performing trapeziectomy with LRTI for advanced thumb CMC-OA.21 A prior study had similar findings, with 692 (68%) of 1024 American Society for Surgery of the Hand (ASSH) members performing LRTI and 766 (75%) of 1024 performing some type of CMC interposition with trapeziectomy for advanced CMC-OA.23 This preference for CMC interposition arthroplasty prevails despite the fact that numerous studies have shown no superiority of any surgical procedure to another for CMC-OA in terms of pain, function, satisfaction, range of motion, and strength.7,15,18,19,24-34 Our data demonstrated that, not only does CMC interposition arthroplasty remain the most frequently used procedure for thumb CMC-OA, the incidence of CMC interposition arthroplasty continues to increase yearly.
The incidence of thumb CMC-OA is higher in women than in men, with more joint laxity a known contributor and subtle sex differences in trapezium geometry and congruence postulated as additional factors.3,35,36 This trend was confirmed in the present study, as females underwent significantly more CMC interposition arthroplasties at all time points. It is interesting that the overall ratio of female to male patients changed significantly over the study period, with the percentage of patients who were male increasing from 18.1% in 2005 to 23.9% in 2011. No previous studies have captured such a large cross section of the population to establish this trend. Although this trend is not necessarily intuitive, potential theories include increased acceptance of CMC interposition arthroplasty as a surgical option for male patients, and potentially a larger number of male patients seeking medical care for thumb CMC-OA in recent years.
Increases in procedure incidence were noted in all regions of the United States, but the largest percentage increase occurred in the Northeast. Despite this increase, the Northeast also had significantly lower CMC interposition arthroplasty incidence compared with all other regions and with the average procedure incidence throughout the study period—demonstrating some regional bias as to treatment of thumb CMC-OA. Unfortunately, because of database limitations and lack of specific CPT codes for other treatment options for thumb CMC-OA, we cannot ascertain if other types of surgery are more frequently used in the Northeast.
CTS and thumb CMC-OA often coexist.37 The estimated incidence of concomitant CTS in patients with CMC-OA is between 4% and 43%, but the rate of concomitant CTR and CMC interposition arthroplasty was not previously characterized in the literature.38,39 Results of the present study supported these findings; 41% of patients who underwent CMC interposition arthroplasty in our study also had a CTS diagnosis, compared with 43% in the 246-patient study by Florack and colleagues.38 We also found that 16% to 17% of patients who underwent CMC interposition arthroplasty underwent concomitant CTR; this rate remained consistent throughout the study period.
Our study demonstrated that, compared with CMC interposition arthroplasties, significantly fewer thumb CMC arthrodesis procedures were performed in the same Medicare population during the same period. Furthermore, the number of thumb CMC arthrodesis procedures declined yearly, with an overall decrease of 19% from 2005 to 2011. In a recent single-blinded, randomized trial, Vermeulen and colleagues40 compared thumb CMC arthrodesis and trapeziectomy with LRTI. They found superior patient satisfaction and significantly lower complication rates in women who underwent LRTI versus arthrodesis. The study was terminated prematurely because of these complications and thus was underpowered to determine differences in specific outcome measures. Previous studies comparing arthrodesis and interposition arthroplasties reported inconsistent outcomes. Hart and colleagues41 found no significant differences in pain or function between CMC arthrodesis and LRTI at a mean 7-year follow-up in a level II randomized controlled trial. Hartigan and colleagues15 reached similar conclusions in their retrospective comparison of the procedures. Without clear evidence supporting arthrodesis over interposition arthroplasty, the majority of surgeons favor interposition arthroplasty for thumb CMC-OA. Among Medicare patients, use of thumb CMC arthrodesis continues to fall.
This national database study had several limitations, which are common to all studies using the PearlDiver database22,42-47:
1. The power of the analysis depended on the quality of available data. Potential sources of error included accuracy of billing codes, and miscoding or noncoding by physicians.46
2. Although we used this database to try to accurately represent a large population of interest, we cannot guarantee the database represented a true cross section of the United States.
3. For the Medicare population, the PearlDiver database indexes data only in 7-year increments. Although the study period was long enough to detect significant trends, some data may not be accurately captured over a 7-year period.
4. Patients were not randomized to a treatment group.
5. The PearlDiver database does not include any clinical outcome data. Therefore, we cannot comment on the efficacy of the reported evaluations and interventions.
6. There is no specific CPT code for thumb CMC interposition arthroplasty. However, we are unaware of a CMC interposition arthroplasty performed for any area besides the thumb. Theoretically, the study population can include a negligible percentage of patients who had interposition arthroplasty of a CMC joint other than the thumb.
7. The database cannot be searched for use of thumb CMC-OA surgical techniques other than CMC interposition arthroplasty or arthrodesis, as isolated trapeziectomy, volar ligament reconstruction, implant arthroplasty, and metacarpal osteotomy lack specific CPT codes.
Conclusion
Thumb CMC-OA is a common entity among Medicare patients. There are numerous surgical options for cases that have failed conservative treatment. Despite the lack of evidence that thumb CMC interposition arthroplasty is superior to other surgical options, the number of patients who had this procedure increased 46% during the 2005–2011 study period. Although the majority of patients who undergo CMC interposition arthroplasty are female, the percentage of male patients has increased significantly. More than 40% of patients who have CMC interposition arthroplasty are also diagnosed with CTS, and 16% to 17% of patients who have CMC interposition arthroplasty will have a concomitant CTR. CMC arthrodesis is used in significantly fewer patients of Medicare age, and its use has been declining.
1. Hentz VR. Surgical treatment of trapeziometacarpal joint arthritis: a historical perspective. Clin Orthop Relat Res. 2014;472(4):1184-1189.
2. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341.
3. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16(3):140-151.
4. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
5. Bodin ND, Spangler R, Thoder JJ. Interposition arthroplasty options for carpometacarpal arthritis of the thumb. Hand Clin. 2010;26(3):339-350, v-vi.
6. Cooney WP, Linscheid RL, Askew LJ. Total arthroplasty of the thumb trapeziometacarpal joint. Clin Orthop Relat Res. 1987;(220):35-45.
7. De Smet L, Vandenberghe L, Degreef I. Long-term outcome of trapeziectomy with ligament reconstruction and tendon interposition (LRTI) versus prosthesis arthroplasty for basal joint osteoarthritis of the thumb. Acta Orthop Belg. 2013;79(2):146-149.
8. Dell PC, Muniz RB. Interposition arthroplasty of the trapeziometacarpal joint for osteoarthritis. Clin Orthop Relat Res. 1987;(220):27-34.
9. Dhar S, Gray IC, Jones WA, Beddow FH. Simple excision of the trapezium for osteoarthritis of the carpometacarpal joint of the thumb. J Hand Surg Br. 1994;19(4):485-488.
10. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55(8):1655-1666.
11. Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9(5):692-699.
12. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg Am. 1985;10(5):645-654.
13. Elfar JC, Burton RI. Ligament reconstruction and tendon interposition for thumb basal arthritis. Hand Clin. 2013;29(1):15-25.
14. Froimson AI. Tendon arthroplasty of the trapeziometacarpal joint. Clin Orthop Relat Res. 1970;70:191-199.
15. Hartigan BJ, Stern PJ, Kiefhaber TR. Thumb carpometacarpal osteoarthritis: arthrodesis compared with ligament reconstruction and tendon interposition. J Bone Joint Surg Am. 2001;83(10):1470-1478.
16. Kenniston JA, Bozentka DJ. Treatment of advanced carpometacarpal joint disease: arthrodesis. Hand Clin. 2008;24(3):285-294, vi-vii.
17. Kokkalis ZT, Zanaros G, Weiser RW, Sotereanos DG. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am. 2009;34(6):1029-1036.
18. Kriegs-Au G, Petje G, Fojtl E, Ganger R, Zachs I. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. Surgical technique. J Bone Joint Surg Am. 2005;87 suppl 1(Pt 1):78-85.
19. Park MJ, Lichtman G, Christian JB, et al. Surgical treatment of thumb carpometacarpal joint arthritis: a single institution experience from 1995–2005. Hand. 2008;3(4):304-310.
20. Park MJ, Lee AT, Yao J. Treatment of thumb carpometacarpal arthritis with arthroscopic hemitrapeziectomy and interposition arthroplasty. Orthopedics. 2012;35(12):e1759-e1764.
21. Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37(1):77-82.
22. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
23. Brunton LM, Wilgis EF. A survey to determine current practice patterns in the surgical treatment of advanced thumb carpometacarpal osteoarthrosis. Hand. 2010;5(4):415-422.
24. Belcher HJ, Nicholl JE. A comparison of trapeziectomy with and without ligament reconstruction and tendon interposition. J Hand Surg Br. 2000;25(4):350-356.
25. Davis TR, Pace A. Trapeziectomy for trapeziometacarpal joint osteoarthritis: is ligament reconstruction and temporary stabilisation of the pseudarthrosis with a Kirschner wire important? J Hand Surg Eur Vol. 2009;34(3):312-321.
26. Davis TR, Brady O, Dias JJ. Excision of the trapezium for osteoarthritis of the trapeziometacarpal joint: a study of the benefit of ligament reconstruction or tendon interposition. J Hand Surg Am. 2004;29(6):1069-1077.
27. De Smet L, Sioen W, Spaepen D, van Ransbeeck H. Treatment of basal joint arthritis of the thumb: trapeziectomy with or without tendon interposition/ligament reconstruction. Hand Surg. 2004;9(1):5-9.
28. Field J, Buchanan D. To suspend or not to suspend: a randomised single blind trial of simple trapeziectomy versus trapeziectomy and flexor carpi radialis suspension. J Hand Surg Eur Vol. 2007;32(4):462-466.
29. Gerwin M, Griffith A, Weiland AJ, Hotchkiss RN, McCormack RR. Ligament reconstruction basal joint arthroplasty without tendon interposition. Clin Orthop Relat Res. 1997;(342):42-45.
30. Jorheim M, Isaxon I, Flondell M, Kalen P, Atroshi I. Short-term outcomes of trapeziometacarpal Artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
31. Lehmann O, Herren DB, Simmen BR. Comparison of tendon suspension-interposition and silicon spacers in the treatment of degenerative osteoarthritis of the base of the thumb. Ann Chir Main Memb Super. 1998;17(1):25-30.
32. Nilsson A, Liljensten E, Bergstrom C, Sollerman C. Results from a degradable TMC joint spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
33. Schroder J, Kerkhoffs GM, Voerman HJ, Marti RK. Surgical treatment of basal joint disease of the thumb: comparison between resection-interposition arthroplasty and trapezio-metacarpal arthrodesis. Arch Orthop Trauma Surg. 2002;122(1):35-38.
34. Tagil M, Kopylov P. Swanson versus APL arthroplasty in the treatment of osteoarthritis of the trapeziometacarpal joint: a prospective and randomized study in 26 patients. J Hand Surg Br. 2002;27(5):452-456.
35. North ER, Rutledge WM. The trapezium-thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15(2):201-206.
36. Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25(6):591-607.
37. Sless Y, Sampson SP. Experience with transtrapezium approach for transverse carpal ligament release in patients with coexisted trapeziometacarpal joint osteoarthritis and carpal tunnel syndrome. Hand. 2007;2(3):151-154.
38. Florack TM, Miller RJ, Pellegrini VD, Burton RI, Dunn MG. The prevalence of carpal tunnel syndrome in patients with basal joint arthritis of the thumb. J Hand Surg Am. 1992;17(4):624-630.
39. Tsai TM, Laurentin-Perez LA, Wong MS, Tamai M. Ideas and innovations: radial approach to carpal tunnel release in conjunction with thumb carpometacarpal arthroplasty. Hand Surg. 2005;10(1):61-66.
40. Vermeulen GM, Brink SM, Slijper H, et al. Trapeziometacarpal arthrodesis or trapeziectomy with ligament reconstruction in primary trapeziometacarpal osteoarthritis: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(9):726-733.
41. Hart R, Janecek M, Siska V, Kucera B, Stipcak V. Interposition suspension arthroplasty according to Epping versus arthrodesis for trapeziometacarpal osteoarthritis. Eur Surg. 2006;38(6):433-438.
42. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005–2011. Am J Sports Med. 2013;41(10):2333-2339.
43. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665.
44. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443.
45. Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441.
46. Yeranosian MG, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. The costs associated with the evaluation of rotator cuff tears before surgical repair. J Shoulder Elbow Surg. 2013;22(12):1662-1666.
47. Daffner SD, Hymanson HJ, Wang JC. Cost and use of conservative management of lumbar disc herniation before surgical discectomy. Spine J. 2010;10(6):463-468.
1. Hentz VR. Surgical treatment of trapeziometacarpal joint arthritis: a historical perspective. Clin Orthop Relat Res. 2014;472(4):1184-1189.
2. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-341.
3. Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16(3):140-151.
4. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
5. Bodin ND, Spangler R, Thoder JJ. Interposition arthroplasty options for carpometacarpal arthritis of the thumb. Hand Clin. 2010;26(3):339-350, v-vi.
6. Cooney WP, Linscheid RL, Askew LJ. Total arthroplasty of the thumb trapeziometacarpal joint. Clin Orthop Relat Res. 1987;(220):35-45.
7. De Smet L, Vandenberghe L, Degreef I. Long-term outcome of trapeziectomy with ligament reconstruction and tendon interposition (LRTI) versus prosthesis arthroplasty for basal joint osteoarthritis of the thumb. Acta Orthop Belg. 2013;79(2):146-149.
8. Dell PC, Muniz RB. Interposition arthroplasty of the trapeziometacarpal joint for osteoarthritis. Clin Orthop Relat Res. 1987;(220):27-34.
9. Dhar S, Gray IC, Jones WA, Beddow FH. Simple excision of the trapezium for osteoarthritis of the carpometacarpal joint of the thumb. J Hand Surg Br. 1994;19(4):485-488.
10. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55(8):1655-1666.
11. Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9(5):692-699.
12. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg Am. 1985;10(5):645-654.
13. Elfar JC, Burton RI. Ligament reconstruction and tendon interposition for thumb basal arthritis. Hand Clin. 2013;29(1):15-25.
14. Froimson AI. Tendon arthroplasty of the trapeziometacarpal joint. Clin Orthop Relat Res. 1970;70:191-199.
15. Hartigan BJ, Stern PJ, Kiefhaber TR. Thumb carpometacarpal osteoarthritis: arthrodesis compared with ligament reconstruction and tendon interposition. J Bone Joint Surg Am. 2001;83(10):1470-1478.
16. Kenniston JA, Bozentka DJ. Treatment of advanced carpometacarpal joint disease: arthrodesis. Hand Clin. 2008;24(3):285-294, vi-vii.
17. Kokkalis ZT, Zanaros G, Weiser RW, Sotereanos DG. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am. 2009;34(6):1029-1036.
18. Kriegs-Au G, Petje G, Fojtl E, Ganger R, Zachs I. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. Surgical technique. J Bone Joint Surg Am. 2005;87 suppl 1(Pt 1):78-85.
19. Park MJ, Lichtman G, Christian JB, et al. Surgical treatment of thumb carpometacarpal joint arthritis: a single institution experience from 1995–2005. Hand. 2008;3(4):304-310.
20. Park MJ, Lee AT, Yao J. Treatment of thumb carpometacarpal arthritis with arthroscopic hemitrapeziectomy and interposition arthroplasty. Orthopedics. 2012;35(12):e1759-e1764.
21. Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37(1):77-82.
22. Zhang AL, Kreulen C, Ngo SS, Hame SL, Wang JC, Gamradt SC. Demographic trends in arthroscopic SLAP repair in the United States. Am J Sports Med. 2012;40(5):1144-1147.
23. Brunton LM, Wilgis EF. A survey to determine current practice patterns in the surgical treatment of advanced thumb carpometacarpal osteoarthrosis. Hand. 2010;5(4):415-422.
24. Belcher HJ, Nicholl JE. A comparison of trapeziectomy with and without ligament reconstruction and tendon interposition. J Hand Surg Br. 2000;25(4):350-356.
25. Davis TR, Pace A. Trapeziectomy for trapeziometacarpal joint osteoarthritis: is ligament reconstruction and temporary stabilisation of the pseudarthrosis with a Kirschner wire important? J Hand Surg Eur Vol. 2009;34(3):312-321.
26. Davis TR, Brady O, Dias JJ. Excision of the trapezium for osteoarthritis of the trapeziometacarpal joint: a study of the benefit of ligament reconstruction or tendon interposition. J Hand Surg Am. 2004;29(6):1069-1077.
27. De Smet L, Sioen W, Spaepen D, van Ransbeeck H. Treatment of basal joint arthritis of the thumb: trapeziectomy with or without tendon interposition/ligament reconstruction. Hand Surg. 2004;9(1):5-9.
28. Field J, Buchanan D. To suspend or not to suspend: a randomised single blind trial of simple trapeziectomy versus trapeziectomy and flexor carpi radialis suspension. J Hand Surg Eur Vol. 2007;32(4):462-466.
29. Gerwin M, Griffith A, Weiland AJ, Hotchkiss RN, McCormack RR. Ligament reconstruction basal joint arthroplasty without tendon interposition. Clin Orthop Relat Res. 1997;(342):42-45.
30. Jorheim M, Isaxon I, Flondell M, Kalen P, Atroshi I. Short-term outcomes of trapeziometacarpal Artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
31. Lehmann O, Herren DB, Simmen BR. Comparison of tendon suspension-interposition and silicon spacers in the treatment of degenerative osteoarthritis of the base of the thumb. Ann Chir Main Memb Super. 1998;17(1):25-30.
32. Nilsson A, Liljensten E, Bergstrom C, Sollerman C. Results from a degradable TMC joint spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
33. Schroder J, Kerkhoffs GM, Voerman HJ, Marti RK. Surgical treatment of basal joint disease of the thumb: comparison between resection-interposition arthroplasty and trapezio-metacarpal arthrodesis. Arch Orthop Trauma Surg. 2002;122(1):35-38.
34. Tagil M, Kopylov P. Swanson versus APL arthroplasty in the treatment of osteoarthritis of the trapeziometacarpal joint: a prospective and randomized study in 26 patients. J Hand Surg Br. 2002;27(5):452-456.
35. North ER, Rutledge WM. The trapezium-thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15(2):201-206.
36. Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25(6):591-607.
37. Sless Y, Sampson SP. Experience with transtrapezium approach for transverse carpal ligament release in patients with coexisted trapeziometacarpal joint osteoarthritis and carpal tunnel syndrome. Hand. 2007;2(3):151-154.
38. Florack TM, Miller RJ, Pellegrini VD, Burton RI, Dunn MG. The prevalence of carpal tunnel syndrome in patients with basal joint arthritis of the thumb. J Hand Surg Am. 1992;17(4):624-630.
39. Tsai TM, Laurentin-Perez LA, Wong MS, Tamai M. Ideas and innovations: radial approach to carpal tunnel release in conjunction with thumb carpometacarpal arthroplasty. Hand Surg. 2005;10(1):61-66.
40. Vermeulen GM, Brink SM, Slijper H, et al. Trapeziometacarpal arthrodesis or trapeziectomy with ligament reconstruction in primary trapeziometacarpal osteoarthritis: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(9):726-733.
41. Hart R, Janecek M, Siska V, Kucera B, Stipcak V. Interposition suspension arthroplasty according to Epping versus arthrodesis for trapeziometacarpal osteoarthritis. Eur Surg. 2006;38(6):433-438.
42. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005–2011. Am J Sports Med. 2013;41(10):2333-2339.
43. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665.
44. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443.
45. Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441.
46. Yeranosian MG, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. The costs associated with the evaluation of rotator cuff tears before surgical repair. J Shoulder Elbow Surg. 2013;22(12):1662-1666.
47. Daffner SD, Hymanson HJ, Wang JC. Cost and use of conservative management of lumbar disc herniation before surgical discectomy. Spine J. 2010;10(6):463-468.
Simultaneous Bilateral Functional Radiography in Ulnar Collateral Ligament Lesion of the Thumb: An Original Technique
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
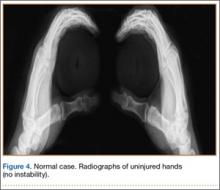
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
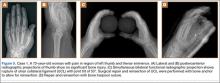
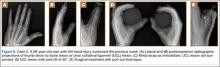
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
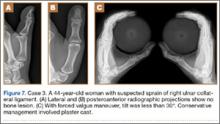
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
PHM15: New Quality Measures for Children with Medical Complexity
Pediatric Hospital Medicine 2015's keynote speaker, Rita Mangione-Smith, MD, MPH, reviewed quality measures being developed for medically complex patients by the Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN). As one of the most challenging groups to not only provide care but to determine if the management provided brings value, the importance of quality measures was emphasized.
Dr. Mangione-Smith, of Seattle Children’s Hospital, reviewed the need for quality measures, as well as the process of developing these measures. Quality measures help to quantify outcomes from care practices, stated Dr. Mangione-Smith, to compare similar settings, and also to set possible benchmarks. The processes range from identifying and prioritizing measures to how they are validated as true value added outcomes. Data sources, sample size, and reliability/validity of the measures are considered important components to ensure that answers or results acquired are applicable and relevant to the population. Another important component is to clearly define a child with medical complexity.
Some reasons why medically complex patients require this focus:
- The low amount of information about their quality of care, investment, and need for coordination;
- Lack of understanding of which care practices make the biggest differences on their outcomes; and
- Their high rate of resource utilization.
The objective was to see which areas of care, such as care coordination, have the highest benefit/improvement on outcomes so as to prioritize resources more effectively. Dr. Mangione-Smith also touched on some obstacles and challenges, such as lack of insurance coverage leading to use of emergency resources as their primary care and its effect on increasing resource utilization.
Measures were determined via a multi-component methodology. Surveys using a binary and linear mean scoring tool were used. This provided multiple types of information such as assessing family’s perception of care, their understanding of medical information and care plans, and their accessibility to medical care services or information about their child.
Currently there is very little evidence on which management methods have the most significant, or any, effect on children with medical complexity. The use of quality measures to help guide which practices may have the highest positive impact on their outcomes greatly adds to the challenging care of this population and can be “used to assess quality of care coordination over time.” TH
Dr. Alvarez is a pediatric hospitalist and medical director of community hospital services at Children’s National Health System in Washington, D.C.
Pediatric Hospital Medicine 2015's keynote speaker, Rita Mangione-Smith, MD, MPH, reviewed quality measures being developed for medically complex patients by the Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN). As one of the most challenging groups to not only provide care but to determine if the management provided brings value, the importance of quality measures was emphasized.
Dr. Mangione-Smith, of Seattle Children’s Hospital, reviewed the need for quality measures, as well as the process of developing these measures. Quality measures help to quantify outcomes from care practices, stated Dr. Mangione-Smith, to compare similar settings, and also to set possible benchmarks. The processes range from identifying and prioritizing measures to how they are validated as true value added outcomes. Data sources, sample size, and reliability/validity of the measures are considered important components to ensure that answers or results acquired are applicable and relevant to the population. Another important component is to clearly define a child with medical complexity.
Some reasons why medically complex patients require this focus:
- The low amount of information about their quality of care, investment, and need for coordination;
- Lack of understanding of which care practices make the biggest differences on their outcomes; and
- Their high rate of resource utilization.
The objective was to see which areas of care, such as care coordination, have the highest benefit/improvement on outcomes so as to prioritize resources more effectively. Dr. Mangione-Smith also touched on some obstacles and challenges, such as lack of insurance coverage leading to use of emergency resources as their primary care and its effect on increasing resource utilization.
Measures were determined via a multi-component methodology. Surveys using a binary and linear mean scoring tool were used. This provided multiple types of information such as assessing family’s perception of care, their understanding of medical information and care plans, and their accessibility to medical care services or information about their child.
Currently there is very little evidence on which management methods have the most significant, or any, effect on children with medical complexity. The use of quality measures to help guide which practices may have the highest positive impact on their outcomes greatly adds to the challenging care of this population and can be “used to assess quality of care coordination over time.” TH
Dr. Alvarez is a pediatric hospitalist and medical director of community hospital services at Children’s National Health System in Washington, D.C.
Pediatric Hospital Medicine 2015's keynote speaker, Rita Mangione-Smith, MD, MPH, reviewed quality measures being developed for medically complex patients by the Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN). As one of the most challenging groups to not only provide care but to determine if the management provided brings value, the importance of quality measures was emphasized.
Dr. Mangione-Smith, of Seattle Children’s Hospital, reviewed the need for quality measures, as well as the process of developing these measures. Quality measures help to quantify outcomes from care practices, stated Dr. Mangione-Smith, to compare similar settings, and also to set possible benchmarks. The processes range from identifying and prioritizing measures to how they are validated as true value added outcomes. Data sources, sample size, and reliability/validity of the measures are considered important components to ensure that answers or results acquired are applicable and relevant to the population. Another important component is to clearly define a child with medical complexity.
Some reasons why medically complex patients require this focus:
- The low amount of information about their quality of care, investment, and need for coordination;
- Lack of understanding of which care practices make the biggest differences on their outcomes; and
- Their high rate of resource utilization.
The objective was to see which areas of care, such as care coordination, have the highest benefit/improvement on outcomes so as to prioritize resources more effectively. Dr. Mangione-Smith also touched on some obstacles and challenges, such as lack of insurance coverage leading to use of emergency resources as their primary care and its effect on increasing resource utilization.
Measures were determined via a multi-component methodology. Surveys using a binary and linear mean scoring tool were used. This provided multiple types of information such as assessing family’s perception of care, their understanding of medical information and care plans, and their accessibility to medical care services or information about their child.
Currently there is very little evidence on which management methods have the most significant, or any, effect on children with medical complexity. The use of quality measures to help guide which practices may have the highest positive impact on their outcomes greatly adds to the challenging care of this population and can be “used to assess quality of care coordination over time.” TH
Dr. Alvarez is a pediatric hospitalist and medical director of community hospital services at Children’s National Health System in Washington, D.C.
How to prevent misuse of psychotropics among college students
Many college students suffer from mental illness (Table 1),1 which can have a negative impact on academic performance. Although psychotropic medications are an important part of treatment for many college students, the potential for misuse always is present. Drug misuse occurs when patients use medications for reasons inconsistent with legal or medical guidelines.2 For example, patients may take a medication that has not been prescribed for them or in a manner that is inconsistent with the prescriber’s instructions, including administration with other substances.3
Misuse of psychotropic drugs is prevalent among college students. A study of 14,175 students from 26 campuses reported that 14.7% of students taking a psychotropic are doing so without a prescription, including stimulants (52.6%), anxiolytics (38.4%), and antidepressants (17.4%).4 Another study states that more than one-third of responders reported misuse of >1 class of medication.5
Psychotropic misuse is concerning because it increases the risk of adverse events. Nearly one-half of medication errors are associated with writing and dispensing the prescription, which means that prescribers can work to reduce these errors.6 However, nonadherence, prescription misuse, and failure to disclose use of over-the-counter drugs, illicit drugs, and herbal products makes preventing most adverse events difficult, if not impossible, for prescribers.7,8
Psychotropic drug misuse among college students is highly variable and unpredictable. Students misuse medications, including stimulants, benzodiazepines, and antidepressants, for a variety of reasons, such as study enhancement, experimentation, intoxication, self-medication, relaxation, and stress management.8 One survey reported that >70% of students taking a psychotropic medication took it with alcohol or another illicit drug.9
However, <20% of those using a psychotropic medication with alcohol or other illicit drugs told their health care provider(s),9 making it impossible for clinicians to predict a patient’s risk of drug− drug interactions and subsequent adverse events. Additionally, additive effects could occur10 and changes in a patient’s presentation could be caused by a reaction to a combination of medications, rather than a new symptom of mental illness.
This article will examine common issues associated with drug misuse among college-age students and review prevention strategies (Table 2).
Stimulants
Stimulants have the highest rate of diversion; 61.7% of college students prescribed stimulants have shared or sold their medication.11 A survey of 115 students from 2 universities reported that the most common reason for stimulant misuse was to enhance academic performance.12 The same survey showed that some students take stimulants with Cannabis (17%) and alcohol (30%).12 As a result, in addition to lowering grade point average (GPA) and other academic difficulties,13 students misusing stimulants are at risk of drug interactions.14
It is critical to ascertain the route of drug administration, because non-oral routes, including crushing then snorting or injecting, are associated with additional health concerns, such as accidental death or blood-borne illnesses.15,16 Cardiac adverse effects of stimulants include hypertension, vasospasm, tachycardia, and dysrhythmia; psychiatric and other effects include serotonin syndrome, hallucinations, anxiety, paranoia, seizures, tics, hyperthermia, and tremor.17 Health care providers prescribing or caring for people taking a stimulant should monitor for these potential effects.
The risk of switch to mania might not be apparent to those who prescribe stimulants or to young people who take non-prescribed stimulants for academic enhancement or to achieve medication-induced euphoria. Adolescent stimulant use is associated with symptoms of early-onset bipolar disorder in patients who have attention-deficit/ hyperactivity disorder (ADHD) and undiagnosed bipolarity.18
The cardiovascular risk associated with stimulant use is debatable. Although several studies have been conducted,19-21 methodological factors limit their applicability. To minimize potential risks, several precautions should be taken before prescribing a stimulant to treat ADHD.
First, obtain a detailed personal and family medical history, asking about possible cardiovascular disease. Second, carefully scrutinize the patient’s cardiovascular system during the physical exam. Third, consider additional testing, such as an electrocardiogram, if the patient’s history or physical exam indicates possible risk.22
As a prescriber, you should be aware of the prevalence of stimulant use among students with and without ADHD, including those who could be feigning ADHD symptoms.15 Diversion could occur through sharing medications or selling them to friends and family.11 It also is possible that these medications may be used with other illicit substances, such as Cannabis, ecstasy, cocaine, and opiates.23 Students also could misuse stimulants by taking more than the prescribed dosage.24
Risk factors for misuse of stimulants include: heavy alcohol use, previous illicit drug use, white race, fraternity or sorority membership, low GPA, increased hyperactivity symptoms, and attendance at a competitive college or university.25-27
Benzodiazepines
Misuse of benzodiazepine is a significant component of prescription drug abuse and often occurs with other medications and alcohol.28 Additional methods of misuse include increased dosage and non-oral routes of administration.29
A 2001 national survey reported that 7.8% of college students have misused benzodiazepines.23 Common characteristics of benzodiazepine abusers include young age, male sex, personality characteristics of impulsivity and hopelessness, and abuse of other drugs, including cocaine and methadone.28,29
Benzodiazepines are prescribed for their anxiolytic and hypnotic properties and students could use these drugs with other agents to augment the euphoric effects or diminish withdrawal symptoms.30 Patients taking benzodiazepines for anxiety might self-medicate with alcohol, which increases sedation and depression, and can contribute to the risk for respiratory depression.10 Misuse of benzodiazepines can result in cognitive and psychomotor impairment and increase the risk of accidents and overdose.29,31
Although overdose with monotherapy is rare, the risk increases when a benzodiazepine is used with alcohol10 or another respiratory depressants, such as opioids, because combination use can produce additive effects.28 You should therefore avoid prescribing benzodiazepines to patients who have a history of significant substance abuse and consider using alternative, non-addictive agents, such as selective serotonin reuptake inhibitors, or non-pharmaceutical treatment when such patients present with an anxiety disorder. The risk of adverse effects of benzodiazepines can be reduced by limiting the dosing and the duration of the treatment, and by using longer-acting rather than the more addictive, shorter-acting, agents.
Antidepressants
Health care providers should be aware that, despite the relative absence of physically addictive properties, antidepressants from most classes are abusable agents sought by young people for non-medical use. In particular, the literature highlights monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and bupropion as the antidepressants most likely to be misused for their amphetamine-like euphoric effects or serotonin-induced dissociative effects.32 However, compared with other drug classes discussed in this article, the rate of antidepressant misuse is relatively low among college students.
Regardless of the antidepressant selected, clinicians should be concerned about alcohol use among college-age patients. Persons with depression are at increased risk of alcoholism compared with the general population.33 This combination can increase depressive symptoms and sedation, and decrease coordination, judgment, and reaction time.33
Excessive alcohol use can increase the risk of seizures in patients taking antidepressants such as buproprion.34 Employ caution when prescribing bupropion to patients who have a predisposing clinical factor that increases seizure risk, such as excessive alcohol use and abrupt cessation, use of other medications that may lower seizure threshold (eg, theophylline, amphetamines, phenothiazines), and a history of head trauma.34
To minimize the risk of seizures with bupropion, titrate up the dosage slowly. Furthermore, using a low dosage during dual therapy for antidepressant augmentation further decreases the risk of seizure.35 For these reasons, we recommend that you avoid bupropion in patients who are at risk of binge drinking, and give careful consideration to providing alternative therapies for them.
Prescribers and patients should also keep in mind that hypertensive crisis could occur if MAOIs are combined with certain types of alcoholic beverages containing tyramine, including some wines and draft beer.33
How you can identify and prevent misuse
Careful communication between health care provider and patient that is necessary to minimize the risk of adverse drug events with psychotropic medications often is lacking. For example, 24% of study college-age participants did not remember if their physician provided a diagnosis and 28.8% could not recall being informed about side effects and, perhaps as a result, many students did not take their medications as prescribed.9
Further, prescribers should ask college-age patients who are undergoing stimulant treatment if they believe that they are being adequately treated. They should inquire about how they are taking their medications.11 These questions can lead to discussion of the need for these medications and reevaluation of their perceived indication.11
Remind patients to take their medication only as directed.36 Highlight the need to:
• store medications in a discreet location
• properly dispose of unused medications
• keep tabs on the quantity of pills
• know how to resist requests for diversion from peers.
The Substance Abuse and Mental Health Services Administration offers additional useful strategies,37 and pharmacists also can be partners in substance use education and prevention.38 These are examples of how health care providers can take an active role in providing patients with a thorough and detailed understanding of (1) their conditions and (2) their prescribed medications to improve efficacy and safety while preventing misuse.8
A study found that the most common method of obtaining these medications without a prescription is acquiring them from peers; 54% of undergraduate patients with stimulant prescriptions have been approached by peers to give, trade, or sell their drugs.25 Other methods include purchasing medications online or faking prescriptions.39 Health care providers should remind patients of the legal ramifications of sharing or selling their prescribed medications. Finally, providers must be vigilant for students who may feign symptoms to obtain a prescription:
• be wary if symptom presentation sounds too “textbook”
• seek collateral history from family. Adults with ADHD should have shown symptoms during childhood
• use external verification such as neuropsychological testing for ADHD. A neuropsychologist can detect deception by analyzing the pattern of responses to questions.
Patient assessment is a key step to in preventing abuse of psychotropic medications. Gentle inquiry about school-related stress and other risk factors for misuse can help practitioners determine if students are at risk of diversion and if additional screening is necessary.
In response to these issues, Stone and Merlo8 have suggested that, in addition to the educational programs held on college campuses on alcohol, illicit drugs, and prescription painkillers, patients should be better informed on the appropriate use of prescription psychiatric medications, instructed to avoid sharing with family and friends, and assessed for abuse risk at regular intervals.
To further protect patients from adverse outcomes during treatment, you can employ conservative and safe prescribing techniques. One strategy might be to keep a personal formulary that lists key medications you use in everyday practice, including knowledge about each drug’s dosage, potential adverse effects, key warnings, and drug−drug interactions.40
Furthermore, maintain healthy caution about newly approved medications and carefully consider how they measure up to existing agents—in other words, practice evidence-based medicine, particularly when students request a particular agent.40,41 Prescribers should evaluate the risk of abuse before prescribing and attempt to prevent misuse by limiting quantities and minimizing polypharmacy.
Last, pharmacists can be key allies for consultation and appropriate medication selection.
Bottom Line
Psychotropic medications are necessary to treat the variety of conditions—anxiety, attention-deficit/hyperactivity disorder, depression, and panic disorder—common among college students. However, students are at risk of combining their prescribed medications with other medications, drugs, and alcohol or could sell or share their medication with peers. Proper counseling and identification of risk factors can be important tools for preventing such events.
Related Resources
• American College Health Association-National College Health Assessment. www.acha-ncha.org.
• Schwartz VI. College mental health: How to provide care for students in need. Current Psychiatry. 2011;10(12):22-29.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Methadone • Methadose, Dolophine
Theophylline • Theo-24, Theolair, Uniphyl
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American College Health Association. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2014. http://www.acha-ncha.org/docs/ACHA-NCHA-II_ReferenceGroup_ExecutiveSummary_ Spring2014.pdf. Published 2014. Accessed January 13, 2015.
2. World Health Organization. Management of substance abuse. http://www.who.int/substance_abuse/terminology/ abuse/en. Accessed June 4, 2015.
3. U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. http://www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm. Published July 28, 2010. Accessed June 18, 2014.
4. Eisenberg D, Hunt J, Speer N, et al. Mental health service utilization among college students in the United States. J Nerv Ment Dis. 2011;199(5):301-308.
5. Peralta RL, Steele JL. Nonmedical prescription drug use among US college students at a Midwest university: a partial test of social learning theory. Subst Use Misuse. 2010;45(6):865-887.
6. Agency for Healthcare Research and Quality. Reducing and preventing adverse drug events to decrease hospital costs: Research in action. http://www.ahrq.gov/research/ findings/factsheets/errors-safety/aderia/index.html. Updated March 2001. Accessed June 21, 2014.
7. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2010;24(7):595-609.
8. Stone AM, Merlo LJ. Attitudes of college students toward mental illness stigma and the misuse of psychiatric medications. J Clin Psychiatry. 2011;72(2):134-139.
9. Oberleitner LM, Tzilos GK, Zumberg KM, et al. Psychotropic drug use among college students: patterns of use, misuse, and medical monitoring. J Am Coll Health. 2011;59(7):658-661.
10. Linnoila MI. Benzodiazepines and alcohol. J Psychiatr Res. 1990;24(suppl 2):121-127.
11. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71(3):262-269.
12. Rabiner DL, Anastopoulos AD, Costello EJ, et al. The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord. 2009;13(2):144-153.
13. Arria AM. Nonmedical use of prescription stimulants and analgesics: associations with social and academic behaviors among college students. J Drug Issues. 2008; 38(4):1045-1060.
14. Arria AM, Caldeira KM, O’Grady KE, et al. Nonmedical use of prescription stimulants among college students: associations with attention-deficit-hyperactivity disorder and polydrug use. Pharmacotherapy. 2008;28(2):156-169.
15. Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep. 2013;15(7):375.
16. Sepúlveda DR, Thomas LM, McCabe SE, et al. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: preliminary analysis among college students. J Pharm Pract. 2011;24(6):551-560.
17. Greydanus DE. Stimulant misuse: strategies to manage a growing problem. http://www.acha.org/Continuing_ Education/docs/ACHA_Use_Misuse_of_Stimulants_ Article2.pdf. Accessed June 29, 2015.
18. Vergne D, Whitham E, Barroilhet S, et al. Adult ADHD and amphetamines: a new paradigm. Neuropsychiatry. 2011;1(6):591-598.
19. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
20. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
21. Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178-185.
22. U.S. Food and Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention-deficit/hyperactivity disorder (ADHD). http://www.fda.gov/Drugs/Drug Safety/PostmarketDrugSafetyInformationforPatients andProviders/DrugSafetyInformationforHeathcare Professionals/ucm165858.htm. Updated August 15, 2013. Accessed June 25, 2014.
23. McCabe SE, Knight JR, Teter CJ, et al. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96-106.
24. McNiel AD, Muzzin KB, DeWald JP, et al. The nonmedical use of prescription stimulants among dental and dental hygiene students. J Dent Educ. 2011;75(3):365-376.
25. McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38(1):43-56.
26. Arria AM, Garnier-Dykstra LM, Caldeira KM, et al. Persistent nonmedical use of prescription stimulants among college students: possible association with ADHD symptoms. J Atten Disord. 2011;15(5):347-356.
27. Teter CJ, McCabe SE, Boyd CJ, et al. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609-617.
28. Hernandez SH, Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther. 2010; 88(3):307-317.
29. McLarnon ME, Monaghan TL, Stewart SH, et al. Drug misuse and diversion in adults prescribed anxiolytics and sedatives. Pharmacotherapy. 2011;31(3):262-272.
30. Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44(2):151-347.
31. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45-58.
32. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
33. Hall-Flavin DK. Why is it bad to mix antidepressants and alcohol? http://www.mayoclinic.com/health/antidepressants-and-alcohol/AN01653. Updated June 12, 2014. Accessed June 20, 2014.
34. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline LLC; 2014.
35. Davidson J. Seizures and bupropion: a review. J Clin Psychiatry. 1989;50(7):256-261.
36. Maddox JC, Levi M, Thompson C. The compliance with antidepressants in general practice. J Psychopharmacol. 1994;8(1):48-52.
37. Substance Abuse and Mental Health Services Administration. You’re in control: using prescription medication responsibly. http://store.samhsa.gov/shin/content/SMA12-4678B3/SMA12-4678B3.pdf. Accessed June 5, 2015.
38. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health Syst Pharm. 2014;71(3):243-246.
39. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription drugs purchased through the internet: who are the end users? Drug Alcohol Depend. 2010;110(1-2):21-29.
40. Preskorn SH, Flockhart D. 2006 Guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
41. Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171(16): 1433-1440.
Many college students suffer from mental illness (Table 1),1 which can have a negative impact on academic performance. Although psychotropic medications are an important part of treatment for many college students, the potential for misuse always is present. Drug misuse occurs when patients use medications for reasons inconsistent with legal or medical guidelines.2 For example, patients may take a medication that has not been prescribed for them or in a manner that is inconsistent with the prescriber’s instructions, including administration with other substances.3
Misuse of psychotropic drugs is prevalent among college students. A study of 14,175 students from 26 campuses reported that 14.7% of students taking a psychotropic are doing so without a prescription, including stimulants (52.6%), anxiolytics (38.4%), and antidepressants (17.4%).4 Another study states that more than one-third of responders reported misuse of >1 class of medication.5
Psychotropic misuse is concerning because it increases the risk of adverse events. Nearly one-half of medication errors are associated with writing and dispensing the prescription, which means that prescribers can work to reduce these errors.6 However, nonadherence, prescription misuse, and failure to disclose use of over-the-counter drugs, illicit drugs, and herbal products makes preventing most adverse events difficult, if not impossible, for prescribers.7,8
Psychotropic drug misuse among college students is highly variable and unpredictable. Students misuse medications, including stimulants, benzodiazepines, and antidepressants, for a variety of reasons, such as study enhancement, experimentation, intoxication, self-medication, relaxation, and stress management.8 One survey reported that >70% of students taking a psychotropic medication took it with alcohol or another illicit drug.9
However, <20% of those using a psychotropic medication with alcohol or other illicit drugs told their health care provider(s),9 making it impossible for clinicians to predict a patient’s risk of drug− drug interactions and subsequent adverse events. Additionally, additive effects could occur10 and changes in a patient’s presentation could be caused by a reaction to a combination of medications, rather than a new symptom of mental illness.
This article will examine common issues associated with drug misuse among college-age students and review prevention strategies (Table 2).
Stimulants
Stimulants have the highest rate of diversion; 61.7% of college students prescribed stimulants have shared or sold their medication.11 A survey of 115 students from 2 universities reported that the most common reason for stimulant misuse was to enhance academic performance.12 The same survey showed that some students take stimulants with Cannabis (17%) and alcohol (30%).12 As a result, in addition to lowering grade point average (GPA) and other academic difficulties,13 students misusing stimulants are at risk of drug interactions.14
It is critical to ascertain the route of drug administration, because non-oral routes, including crushing then snorting or injecting, are associated with additional health concerns, such as accidental death or blood-borne illnesses.15,16 Cardiac adverse effects of stimulants include hypertension, vasospasm, tachycardia, and dysrhythmia; psychiatric and other effects include serotonin syndrome, hallucinations, anxiety, paranoia, seizures, tics, hyperthermia, and tremor.17 Health care providers prescribing or caring for people taking a stimulant should monitor for these potential effects.
The risk of switch to mania might not be apparent to those who prescribe stimulants or to young people who take non-prescribed stimulants for academic enhancement or to achieve medication-induced euphoria. Adolescent stimulant use is associated with symptoms of early-onset bipolar disorder in patients who have attention-deficit/ hyperactivity disorder (ADHD) and undiagnosed bipolarity.18
The cardiovascular risk associated with stimulant use is debatable. Although several studies have been conducted,19-21 methodological factors limit their applicability. To minimize potential risks, several precautions should be taken before prescribing a stimulant to treat ADHD.
First, obtain a detailed personal and family medical history, asking about possible cardiovascular disease. Second, carefully scrutinize the patient’s cardiovascular system during the physical exam. Third, consider additional testing, such as an electrocardiogram, if the patient’s history or physical exam indicates possible risk.22
As a prescriber, you should be aware of the prevalence of stimulant use among students with and without ADHD, including those who could be feigning ADHD symptoms.15 Diversion could occur through sharing medications or selling them to friends and family.11 It also is possible that these medications may be used with other illicit substances, such as Cannabis, ecstasy, cocaine, and opiates.23 Students also could misuse stimulants by taking more than the prescribed dosage.24
Risk factors for misuse of stimulants include: heavy alcohol use, previous illicit drug use, white race, fraternity or sorority membership, low GPA, increased hyperactivity symptoms, and attendance at a competitive college or university.25-27
Benzodiazepines
Misuse of benzodiazepine is a significant component of prescription drug abuse and often occurs with other medications and alcohol.28 Additional methods of misuse include increased dosage and non-oral routes of administration.29
A 2001 national survey reported that 7.8% of college students have misused benzodiazepines.23 Common characteristics of benzodiazepine abusers include young age, male sex, personality characteristics of impulsivity and hopelessness, and abuse of other drugs, including cocaine and methadone.28,29
Benzodiazepines are prescribed for their anxiolytic and hypnotic properties and students could use these drugs with other agents to augment the euphoric effects or diminish withdrawal symptoms.30 Patients taking benzodiazepines for anxiety might self-medicate with alcohol, which increases sedation and depression, and can contribute to the risk for respiratory depression.10 Misuse of benzodiazepines can result in cognitive and psychomotor impairment and increase the risk of accidents and overdose.29,31
Although overdose with monotherapy is rare, the risk increases when a benzodiazepine is used with alcohol10 or another respiratory depressants, such as opioids, because combination use can produce additive effects.28 You should therefore avoid prescribing benzodiazepines to patients who have a history of significant substance abuse and consider using alternative, non-addictive agents, such as selective serotonin reuptake inhibitors, or non-pharmaceutical treatment when such patients present with an anxiety disorder. The risk of adverse effects of benzodiazepines can be reduced by limiting the dosing and the duration of the treatment, and by using longer-acting rather than the more addictive, shorter-acting, agents.
Antidepressants
Health care providers should be aware that, despite the relative absence of physically addictive properties, antidepressants from most classes are abusable agents sought by young people for non-medical use. In particular, the literature highlights monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and bupropion as the antidepressants most likely to be misused for their amphetamine-like euphoric effects or serotonin-induced dissociative effects.32 However, compared with other drug classes discussed in this article, the rate of antidepressant misuse is relatively low among college students.
Regardless of the antidepressant selected, clinicians should be concerned about alcohol use among college-age patients. Persons with depression are at increased risk of alcoholism compared with the general population.33 This combination can increase depressive symptoms and sedation, and decrease coordination, judgment, and reaction time.33
Excessive alcohol use can increase the risk of seizures in patients taking antidepressants such as buproprion.34 Employ caution when prescribing bupropion to patients who have a predisposing clinical factor that increases seizure risk, such as excessive alcohol use and abrupt cessation, use of other medications that may lower seizure threshold (eg, theophylline, amphetamines, phenothiazines), and a history of head trauma.34
To minimize the risk of seizures with bupropion, titrate up the dosage slowly. Furthermore, using a low dosage during dual therapy for antidepressant augmentation further decreases the risk of seizure.35 For these reasons, we recommend that you avoid bupropion in patients who are at risk of binge drinking, and give careful consideration to providing alternative therapies for them.
Prescribers and patients should also keep in mind that hypertensive crisis could occur if MAOIs are combined with certain types of alcoholic beverages containing tyramine, including some wines and draft beer.33
How you can identify and prevent misuse
Careful communication between health care provider and patient that is necessary to minimize the risk of adverse drug events with psychotropic medications often is lacking. For example, 24% of study college-age participants did not remember if their physician provided a diagnosis and 28.8% could not recall being informed about side effects and, perhaps as a result, many students did not take their medications as prescribed.9
Further, prescribers should ask college-age patients who are undergoing stimulant treatment if they believe that they are being adequately treated. They should inquire about how they are taking their medications.11 These questions can lead to discussion of the need for these medications and reevaluation of their perceived indication.11
Remind patients to take their medication only as directed.36 Highlight the need to:
• store medications in a discreet location
• properly dispose of unused medications
• keep tabs on the quantity of pills
• know how to resist requests for diversion from peers.
The Substance Abuse and Mental Health Services Administration offers additional useful strategies,37 and pharmacists also can be partners in substance use education and prevention.38 These are examples of how health care providers can take an active role in providing patients with a thorough and detailed understanding of (1) their conditions and (2) their prescribed medications to improve efficacy and safety while preventing misuse.8
A study found that the most common method of obtaining these medications without a prescription is acquiring them from peers; 54% of undergraduate patients with stimulant prescriptions have been approached by peers to give, trade, or sell their drugs.25 Other methods include purchasing medications online or faking prescriptions.39 Health care providers should remind patients of the legal ramifications of sharing or selling their prescribed medications. Finally, providers must be vigilant for students who may feign symptoms to obtain a prescription:
• be wary if symptom presentation sounds too “textbook”
• seek collateral history from family. Adults with ADHD should have shown symptoms during childhood
• use external verification such as neuropsychological testing for ADHD. A neuropsychologist can detect deception by analyzing the pattern of responses to questions.
Patient assessment is a key step to in preventing abuse of psychotropic medications. Gentle inquiry about school-related stress and other risk factors for misuse can help practitioners determine if students are at risk of diversion and if additional screening is necessary.
In response to these issues, Stone and Merlo8 have suggested that, in addition to the educational programs held on college campuses on alcohol, illicit drugs, and prescription painkillers, patients should be better informed on the appropriate use of prescription psychiatric medications, instructed to avoid sharing with family and friends, and assessed for abuse risk at regular intervals.
To further protect patients from adverse outcomes during treatment, you can employ conservative and safe prescribing techniques. One strategy might be to keep a personal formulary that lists key medications you use in everyday practice, including knowledge about each drug’s dosage, potential adverse effects, key warnings, and drug−drug interactions.40
Furthermore, maintain healthy caution about newly approved medications and carefully consider how they measure up to existing agents—in other words, practice evidence-based medicine, particularly when students request a particular agent.40,41 Prescribers should evaluate the risk of abuse before prescribing and attempt to prevent misuse by limiting quantities and minimizing polypharmacy.
Last, pharmacists can be key allies for consultation and appropriate medication selection.
Bottom Line
Psychotropic medications are necessary to treat the variety of conditions—anxiety, attention-deficit/hyperactivity disorder, depression, and panic disorder—common among college students. However, students are at risk of combining their prescribed medications with other medications, drugs, and alcohol or could sell or share their medication with peers. Proper counseling and identification of risk factors can be important tools for preventing such events.
Related Resources
• American College Health Association-National College Health Assessment. www.acha-ncha.org.
• Schwartz VI. College mental health: How to provide care for students in need. Current Psychiatry. 2011;10(12):22-29.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Methadone • Methadose, Dolophine
Theophylline • Theo-24, Theolair, Uniphyl
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Many college students suffer from mental illness (Table 1),1 which can have a negative impact on academic performance. Although psychotropic medications are an important part of treatment for many college students, the potential for misuse always is present. Drug misuse occurs when patients use medications for reasons inconsistent with legal or medical guidelines.2 For example, patients may take a medication that has not been prescribed for them or in a manner that is inconsistent with the prescriber’s instructions, including administration with other substances.3
Misuse of psychotropic drugs is prevalent among college students. A study of 14,175 students from 26 campuses reported that 14.7% of students taking a psychotropic are doing so without a prescription, including stimulants (52.6%), anxiolytics (38.4%), and antidepressants (17.4%).4 Another study states that more than one-third of responders reported misuse of >1 class of medication.5
Psychotropic misuse is concerning because it increases the risk of adverse events. Nearly one-half of medication errors are associated with writing and dispensing the prescription, which means that prescribers can work to reduce these errors.6 However, nonadherence, prescription misuse, and failure to disclose use of over-the-counter drugs, illicit drugs, and herbal products makes preventing most adverse events difficult, if not impossible, for prescribers.7,8
Psychotropic drug misuse among college students is highly variable and unpredictable. Students misuse medications, including stimulants, benzodiazepines, and antidepressants, for a variety of reasons, such as study enhancement, experimentation, intoxication, self-medication, relaxation, and stress management.8 One survey reported that >70% of students taking a psychotropic medication took it with alcohol or another illicit drug.9
However, <20% of those using a psychotropic medication with alcohol or other illicit drugs told their health care provider(s),9 making it impossible for clinicians to predict a patient’s risk of drug− drug interactions and subsequent adverse events. Additionally, additive effects could occur10 and changes in a patient’s presentation could be caused by a reaction to a combination of medications, rather than a new symptom of mental illness.
This article will examine common issues associated with drug misuse among college-age students and review prevention strategies (Table 2).
Stimulants
Stimulants have the highest rate of diversion; 61.7% of college students prescribed stimulants have shared or sold their medication.11 A survey of 115 students from 2 universities reported that the most common reason for stimulant misuse was to enhance academic performance.12 The same survey showed that some students take stimulants with Cannabis (17%) and alcohol (30%).12 As a result, in addition to lowering grade point average (GPA) and other academic difficulties,13 students misusing stimulants are at risk of drug interactions.14
It is critical to ascertain the route of drug administration, because non-oral routes, including crushing then snorting or injecting, are associated with additional health concerns, such as accidental death or blood-borne illnesses.15,16 Cardiac adverse effects of stimulants include hypertension, vasospasm, tachycardia, and dysrhythmia; psychiatric and other effects include serotonin syndrome, hallucinations, anxiety, paranoia, seizures, tics, hyperthermia, and tremor.17 Health care providers prescribing or caring for people taking a stimulant should monitor for these potential effects.
The risk of switch to mania might not be apparent to those who prescribe stimulants or to young people who take non-prescribed stimulants for academic enhancement or to achieve medication-induced euphoria. Adolescent stimulant use is associated with symptoms of early-onset bipolar disorder in patients who have attention-deficit/ hyperactivity disorder (ADHD) and undiagnosed bipolarity.18
The cardiovascular risk associated with stimulant use is debatable. Although several studies have been conducted,19-21 methodological factors limit their applicability. To minimize potential risks, several precautions should be taken before prescribing a stimulant to treat ADHD.
First, obtain a detailed personal and family medical history, asking about possible cardiovascular disease. Second, carefully scrutinize the patient’s cardiovascular system during the physical exam. Third, consider additional testing, such as an electrocardiogram, if the patient’s history or physical exam indicates possible risk.22
As a prescriber, you should be aware of the prevalence of stimulant use among students with and without ADHD, including those who could be feigning ADHD symptoms.15 Diversion could occur through sharing medications or selling them to friends and family.11 It also is possible that these medications may be used with other illicit substances, such as Cannabis, ecstasy, cocaine, and opiates.23 Students also could misuse stimulants by taking more than the prescribed dosage.24
Risk factors for misuse of stimulants include: heavy alcohol use, previous illicit drug use, white race, fraternity or sorority membership, low GPA, increased hyperactivity symptoms, and attendance at a competitive college or university.25-27
Benzodiazepines
Misuse of benzodiazepine is a significant component of prescription drug abuse and often occurs with other medications and alcohol.28 Additional methods of misuse include increased dosage and non-oral routes of administration.29
A 2001 national survey reported that 7.8% of college students have misused benzodiazepines.23 Common characteristics of benzodiazepine abusers include young age, male sex, personality characteristics of impulsivity and hopelessness, and abuse of other drugs, including cocaine and methadone.28,29
Benzodiazepines are prescribed for their anxiolytic and hypnotic properties and students could use these drugs with other agents to augment the euphoric effects or diminish withdrawal symptoms.30 Patients taking benzodiazepines for anxiety might self-medicate with alcohol, which increases sedation and depression, and can contribute to the risk for respiratory depression.10 Misuse of benzodiazepines can result in cognitive and psychomotor impairment and increase the risk of accidents and overdose.29,31
Although overdose with monotherapy is rare, the risk increases when a benzodiazepine is used with alcohol10 or another respiratory depressants, such as opioids, because combination use can produce additive effects.28 You should therefore avoid prescribing benzodiazepines to patients who have a history of significant substance abuse and consider using alternative, non-addictive agents, such as selective serotonin reuptake inhibitors, or non-pharmaceutical treatment when such patients present with an anxiety disorder. The risk of adverse effects of benzodiazepines can be reduced by limiting the dosing and the duration of the treatment, and by using longer-acting rather than the more addictive, shorter-acting, agents.
Antidepressants
Health care providers should be aware that, despite the relative absence of physically addictive properties, antidepressants from most classes are abusable agents sought by young people for non-medical use. In particular, the literature highlights monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and bupropion as the antidepressants most likely to be misused for their amphetamine-like euphoric effects or serotonin-induced dissociative effects.32 However, compared with other drug classes discussed in this article, the rate of antidepressant misuse is relatively low among college students.
Regardless of the antidepressant selected, clinicians should be concerned about alcohol use among college-age patients. Persons with depression are at increased risk of alcoholism compared with the general population.33 This combination can increase depressive symptoms and sedation, and decrease coordination, judgment, and reaction time.33
Excessive alcohol use can increase the risk of seizures in patients taking antidepressants such as buproprion.34 Employ caution when prescribing bupropion to patients who have a predisposing clinical factor that increases seizure risk, such as excessive alcohol use and abrupt cessation, use of other medications that may lower seizure threshold (eg, theophylline, amphetamines, phenothiazines), and a history of head trauma.34
To minimize the risk of seizures with bupropion, titrate up the dosage slowly. Furthermore, using a low dosage during dual therapy for antidepressant augmentation further decreases the risk of seizure.35 For these reasons, we recommend that you avoid bupropion in patients who are at risk of binge drinking, and give careful consideration to providing alternative therapies for them.
Prescribers and patients should also keep in mind that hypertensive crisis could occur if MAOIs are combined with certain types of alcoholic beverages containing tyramine, including some wines and draft beer.33
How you can identify and prevent misuse
Careful communication between health care provider and patient that is necessary to minimize the risk of adverse drug events with psychotropic medications often is lacking. For example, 24% of study college-age participants did not remember if their physician provided a diagnosis and 28.8% could not recall being informed about side effects and, perhaps as a result, many students did not take their medications as prescribed.9
Further, prescribers should ask college-age patients who are undergoing stimulant treatment if they believe that they are being adequately treated. They should inquire about how they are taking their medications.11 These questions can lead to discussion of the need for these medications and reevaluation of their perceived indication.11
Remind patients to take their medication only as directed.36 Highlight the need to:
• store medications in a discreet location
• properly dispose of unused medications
• keep tabs on the quantity of pills
• know how to resist requests for diversion from peers.
The Substance Abuse and Mental Health Services Administration offers additional useful strategies,37 and pharmacists also can be partners in substance use education and prevention.38 These are examples of how health care providers can take an active role in providing patients with a thorough and detailed understanding of (1) their conditions and (2) their prescribed medications to improve efficacy and safety while preventing misuse.8
A study found that the most common method of obtaining these medications without a prescription is acquiring them from peers; 54% of undergraduate patients with stimulant prescriptions have been approached by peers to give, trade, or sell their drugs.25 Other methods include purchasing medications online or faking prescriptions.39 Health care providers should remind patients of the legal ramifications of sharing or selling their prescribed medications. Finally, providers must be vigilant for students who may feign symptoms to obtain a prescription:
• be wary if symptom presentation sounds too “textbook”
• seek collateral history from family. Adults with ADHD should have shown symptoms during childhood
• use external verification such as neuropsychological testing for ADHD. A neuropsychologist can detect deception by analyzing the pattern of responses to questions.
Patient assessment is a key step to in preventing abuse of psychotropic medications. Gentle inquiry about school-related stress and other risk factors for misuse can help practitioners determine if students are at risk of diversion and if additional screening is necessary.
In response to these issues, Stone and Merlo8 have suggested that, in addition to the educational programs held on college campuses on alcohol, illicit drugs, and prescription painkillers, patients should be better informed on the appropriate use of prescription psychiatric medications, instructed to avoid sharing with family and friends, and assessed for abuse risk at regular intervals.
To further protect patients from adverse outcomes during treatment, you can employ conservative and safe prescribing techniques. One strategy might be to keep a personal formulary that lists key medications you use in everyday practice, including knowledge about each drug’s dosage, potential adverse effects, key warnings, and drug−drug interactions.40
Furthermore, maintain healthy caution about newly approved medications and carefully consider how they measure up to existing agents—in other words, practice evidence-based medicine, particularly when students request a particular agent.40,41 Prescribers should evaluate the risk of abuse before prescribing and attempt to prevent misuse by limiting quantities and minimizing polypharmacy.
Last, pharmacists can be key allies for consultation and appropriate medication selection.
Bottom Line
Psychotropic medications are necessary to treat the variety of conditions—anxiety, attention-deficit/hyperactivity disorder, depression, and panic disorder—common among college students. However, students are at risk of combining their prescribed medications with other medications, drugs, and alcohol or could sell or share their medication with peers. Proper counseling and identification of risk factors can be important tools for preventing such events.
Related Resources
• American College Health Association-National College Health Assessment. www.acha-ncha.org.
• Schwartz VI. College mental health: How to provide care for students in need. Current Psychiatry. 2011;10(12):22-29.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Methadone • Methadose, Dolophine
Theophylline • Theo-24, Theolair, Uniphyl
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American College Health Association. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2014. http://www.acha-ncha.org/docs/ACHA-NCHA-II_ReferenceGroup_ExecutiveSummary_ Spring2014.pdf. Published 2014. Accessed January 13, 2015.
2. World Health Organization. Management of substance abuse. http://www.who.int/substance_abuse/terminology/ abuse/en. Accessed June 4, 2015.
3. U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. http://www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm. Published July 28, 2010. Accessed June 18, 2014.
4. Eisenberg D, Hunt J, Speer N, et al. Mental health service utilization among college students in the United States. J Nerv Ment Dis. 2011;199(5):301-308.
5. Peralta RL, Steele JL. Nonmedical prescription drug use among US college students at a Midwest university: a partial test of social learning theory. Subst Use Misuse. 2010;45(6):865-887.
6. Agency for Healthcare Research and Quality. Reducing and preventing adverse drug events to decrease hospital costs: Research in action. http://www.ahrq.gov/research/ findings/factsheets/errors-safety/aderia/index.html. Updated March 2001. Accessed June 21, 2014.
7. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2010;24(7):595-609.
8. Stone AM, Merlo LJ. Attitudes of college students toward mental illness stigma and the misuse of psychiatric medications. J Clin Psychiatry. 2011;72(2):134-139.
9. Oberleitner LM, Tzilos GK, Zumberg KM, et al. Psychotropic drug use among college students: patterns of use, misuse, and medical monitoring. J Am Coll Health. 2011;59(7):658-661.
10. Linnoila MI. Benzodiazepines and alcohol. J Psychiatr Res. 1990;24(suppl 2):121-127.
11. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71(3):262-269.
12. Rabiner DL, Anastopoulos AD, Costello EJ, et al. The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord. 2009;13(2):144-153.
13. Arria AM. Nonmedical use of prescription stimulants and analgesics: associations with social and academic behaviors among college students. J Drug Issues. 2008; 38(4):1045-1060.
14. Arria AM, Caldeira KM, O’Grady KE, et al. Nonmedical use of prescription stimulants among college students: associations with attention-deficit-hyperactivity disorder and polydrug use. Pharmacotherapy. 2008;28(2):156-169.
15. Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep. 2013;15(7):375.
16. Sepúlveda DR, Thomas LM, McCabe SE, et al. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: preliminary analysis among college students. J Pharm Pract. 2011;24(6):551-560.
17. Greydanus DE. Stimulant misuse: strategies to manage a growing problem. http://www.acha.org/Continuing_ Education/docs/ACHA_Use_Misuse_of_Stimulants_ Article2.pdf. Accessed June 29, 2015.
18. Vergne D, Whitham E, Barroilhet S, et al. Adult ADHD and amphetamines: a new paradigm. Neuropsychiatry. 2011;1(6):591-598.
19. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
20. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
21. Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178-185.
22. U.S. Food and Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention-deficit/hyperactivity disorder (ADHD). http://www.fda.gov/Drugs/Drug Safety/PostmarketDrugSafetyInformationforPatients andProviders/DrugSafetyInformationforHeathcare Professionals/ucm165858.htm. Updated August 15, 2013. Accessed June 25, 2014.
23. McCabe SE, Knight JR, Teter CJ, et al. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96-106.
24. McNiel AD, Muzzin KB, DeWald JP, et al. The nonmedical use of prescription stimulants among dental and dental hygiene students. J Dent Educ. 2011;75(3):365-376.
25. McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38(1):43-56.
26. Arria AM, Garnier-Dykstra LM, Caldeira KM, et al. Persistent nonmedical use of prescription stimulants among college students: possible association with ADHD symptoms. J Atten Disord. 2011;15(5):347-356.
27. Teter CJ, McCabe SE, Boyd CJ, et al. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609-617.
28. Hernandez SH, Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther. 2010; 88(3):307-317.
29. McLarnon ME, Monaghan TL, Stewart SH, et al. Drug misuse and diversion in adults prescribed anxiolytics and sedatives. Pharmacotherapy. 2011;31(3):262-272.
30. Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44(2):151-347.
31. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45-58.
32. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
33. Hall-Flavin DK. Why is it bad to mix antidepressants and alcohol? http://www.mayoclinic.com/health/antidepressants-and-alcohol/AN01653. Updated June 12, 2014. Accessed June 20, 2014.
34. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline LLC; 2014.
35. Davidson J. Seizures and bupropion: a review. J Clin Psychiatry. 1989;50(7):256-261.
36. Maddox JC, Levi M, Thompson C. The compliance with antidepressants in general practice. J Psychopharmacol. 1994;8(1):48-52.
37. Substance Abuse and Mental Health Services Administration. You’re in control: using prescription medication responsibly. http://store.samhsa.gov/shin/content/SMA12-4678B3/SMA12-4678B3.pdf. Accessed June 5, 2015.
38. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health Syst Pharm. 2014;71(3):243-246.
39. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription drugs purchased through the internet: who are the end users? Drug Alcohol Depend. 2010;110(1-2):21-29.
40. Preskorn SH, Flockhart D. 2006 Guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
41. Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171(16): 1433-1440.
1. American College Health Association. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2014. http://www.acha-ncha.org/docs/ACHA-NCHA-II_ReferenceGroup_ExecutiveSummary_ Spring2014.pdf. Published 2014. Accessed January 13, 2015.
2. World Health Organization. Management of substance abuse. http://www.who.int/substance_abuse/terminology/ abuse/en. Accessed June 4, 2015.
3. U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. http://www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm. Published July 28, 2010. Accessed June 18, 2014.
4. Eisenberg D, Hunt J, Speer N, et al. Mental health service utilization among college students in the United States. J Nerv Ment Dis. 2011;199(5):301-308.
5. Peralta RL, Steele JL. Nonmedical prescription drug use among US college students at a Midwest university: a partial test of social learning theory. Subst Use Misuse. 2010;45(6):865-887.
6. Agency for Healthcare Research and Quality. Reducing and preventing adverse drug events to decrease hospital costs: Research in action. http://www.ahrq.gov/research/ findings/factsheets/errors-safety/aderia/index.html. Updated March 2001. Accessed June 21, 2014.
7. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2010;24(7):595-609.
8. Stone AM, Merlo LJ. Attitudes of college students toward mental illness stigma and the misuse of psychiatric medications. J Clin Psychiatry. 2011;72(2):134-139.
9. Oberleitner LM, Tzilos GK, Zumberg KM, et al. Psychotropic drug use among college students: patterns of use, misuse, and medical monitoring. J Am Coll Health. 2011;59(7):658-661.
10. Linnoila MI. Benzodiazepines and alcohol. J Psychiatr Res. 1990;24(suppl 2):121-127.
11. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71(3):262-269.
12. Rabiner DL, Anastopoulos AD, Costello EJ, et al. The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord. 2009;13(2):144-153.
13. Arria AM. Nonmedical use of prescription stimulants and analgesics: associations with social and academic behaviors among college students. J Drug Issues. 2008; 38(4):1045-1060.
14. Arria AM, Caldeira KM, O’Grady KE, et al. Nonmedical use of prescription stimulants among college students: associations with attention-deficit-hyperactivity disorder and polydrug use. Pharmacotherapy. 2008;28(2):156-169.
15. Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep. 2013;15(7):375.
16. Sepúlveda DR, Thomas LM, McCabe SE, et al. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: preliminary analysis among college students. J Pharm Pract. 2011;24(6):551-560.
17. Greydanus DE. Stimulant misuse: strategies to manage a growing problem. http://www.acha.org/Continuing_ Education/docs/ACHA_Use_Misuse_of_Stimulants_ Article2.pdf. Accessed June 29, 2015.
18. Vergne D, Whitham E, Barroilhet S, et al. Adult ADHD and amphetamines: a new paradigm. Neuropsychiatry. 2011;1(6):591-598.
19. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
20. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
21. Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178-185.
22. U.S. Food and Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention-deficit/hyperactivity disorder (ADHD). http://www.fda.gov/Drugs/Drug Safety/PostmarketDrugSafetyInformationforPatients andProviders/DrugSafetyInformationforHeathcare Professionals/ucm165858.htm. Updated August 15, 2013. Accessed June 25, 2014.
23. McCabe SE, Knight JR, Teter CJ, et al. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96-106.
24. McNiel AD, Muzzin KB, DeWald JP, et al. The nonmedical use of prescription stimulants among dental and dental hygiene students. J Dent Educ. 2011;75(3):365-376.
25. McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38(1):43-56.
26. Arria AM, Garnier-Dykstra LM, Caldeira KM, et al. Persistent nonmedical use of prescription stimulants among college students: possible association with ADHD symptoms. J Atten Disord. 2011;15(5):347-356.
27. Teter CJ, McCabe SE, Boyd CJ, et al. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609-617.
28. Hernandez SH, Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther. 2010; 88(3):307-317.
29. McLarnon ME, Monaghan TL, Stewart SH, et al. Drug misuse and diversion in adults prescribed anxiolytics and sedatives. Pharmacotherapy. 2011;31(3):262-272.
30. Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44(2):151-347.
31. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45-58.
32. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
33. Hall-Flavin DK. Why is it bad to mix antidepressants and alcohol? http://www.mayoclinic.com/health/antidepressants-and-alcohol/AN01653. Updated June 12, 2014. Accessed June 20, 2014.
34. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline LLC; 2014.
35. Davidson J. Seizures and bupropion: a review. J Clin Psychiatry. 1989;50(7):256-261.
36. Maddox JC, Levi M, Thompson C. The compliance with antidepressants in general practice. J Psychopharmacol. 1994;8(1):48-52.
37. Substance Abuse and Mental Health Services Administration. You’re in control: using prescription medication responsibly. http://store.samhsa.gov/shin/content/SMA12-4678B3/SMA12-4678B3.pdf. Accessed June 5, 2015.
38. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health Syst Pharm. 2014;71(3):243-246.
39. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription drugs purchased through the internet: who are the end users? Drug Alcohol Depend. 2010;110(1-2):21-29.
40. Preskorn SH, Flockhart D. 2006 Guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
41. Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171(16): 1433-1440.
Early follicular lymphoma progression signals poor outcomes
For patients with follicular lymphoma treated with a rituximab-based combination chemotherapy regimen, early disease progression is associated with significantly worse overall survival, suggesting the need for additional interventions, according to results of a multicenter study.
Among 588 patients with stage 2-4 follicular lymphoma treated with first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and followed for a median of 7 years in the National LymphoCare Study, overall survival (OS) at 2 years was 68% for those who had disease progression within 2 years, compared with 97% for patients with no disease progression during that time.
Similarly, 5-year overall survival was 50% for patients with early progression of disease, compared with 90% for patients with no early progression, write Dr. Carla Casulo of the University of Rochester (N.Y.) Medical Center and colleagues. The study is in anearly online publication in the Journal of Clinical Oncology.
“Given our findings, early relapse after diagnosis in patients treated with first-line chemoimmunotherapy is a powerful prognostic indicator of outcome and should be used to stratify the risk of patients in studies of relapsed follicular lymphoma,” the authors wrote.
The findings were validated in an independent cohort of patients with follicular lymphoma treated with R-CHOP from the University of Iowa and Mayo Clinical Molecular Epidemiology Resource, and are consistent with findings from other studies of patients treated with different rituximab-based regimens, the investigators reported.
In unadjusted analysis, early disease progression was associated with a hazard ratio (HR) of 7.17 (95% confidence interval [CI] 4.83-10.65); the effect remained after adjustment for the Follicular Lymphoma International Prognostic Index (FLIPI) score (HR 6.44, 95% CI, 4.33-9.58).
Factors associated with early progression included age, Eastern Cooperative Oncology Group performance score, nodal sites, and disease stage.
Early use of aggressive salvage therapies or autologous stem-cell transplantation could improve outcomes in patients with early disease progression, the authors wrote. However, only 8 patients among the 110 with early progression went on to transplant, not a large enough sample for meaningful analysis, they added.
“This newly defined high-risk group of patients represents a distinct population in whom further study is warranted in both directed prospective clinical trials of follicular lymphoma biology and treatment. Moreover, we propose that 2-year progression-free survival may be a practical and meaningful clinical end point for trials involving a chemoimmunotherapy backbone,” they concluded.
If, in studying the immunologic and inflammatory host response to, and the genetic landscape of, these lymphomas, we are able to define this high-risk subgroup of patients with follicular lymphoma, the question becomes whether we could use this information to effectively treat these patients differently. Although high-dose chemotherapy and autologous stem-cell transplantation (HDC-ASCT) in first remission seems to have no effect on OS in all comers, results might be different for this cohort of high-risk patients. To study this would require an ability to identify these patients at diagnosis. Given that the efficacy of HDC-ASCT is maintained in the case of chemosensitive relapse, reserving HDC-ASCT for patients who relapse within the first 2 years of their initial therapy may be a more prudent strategy.
However, it may be that this is a particularly chemoresistant population and that, instead, attention should be paid to targeting the biologic and genetic factors that contribute to the poor prognosis of this group. Given the negative differential outcomes in patients with decreased tumor-infiltrating lymphocytes and increased monocyte/macrophage activation, immunologic approaches in the salvage setting, including immune checkpoint blockade drugs, chimeric antigen receptor T cells, and allogeneic transplantation may be biologically relevant.
Dr. Caron A. Jacobson and Dr. Arnold S. Freedman, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, made their remarks in an editorial accompanying the study.
If, in studying the immunologic and inflammatory host response to, and the genetic landscape of, these lymphomas, we are able to define this high-risk subgroup of patients with follicular lymphoma, the question becomes whether we could use this information to effectively treat these patients differently. Although high-dose chemotherapy and autologous stem-cell transplantation (HDC-ASCT) in first remission seems to have no effect on OS in all comers, results might be different for this cohort of high-risk patients. To study this would require an ability to identify these patients at diagnosis. Given that the efficacy of HDC-ASCT is maintained in the case of chemosensitive relapse, reserving HDC-ASCT for patients who relapse within the first 2 years of their initial therapy may be a more prudent strategy.
However, it may be that this is a particularly chemoresistant population and that, instead, attention should be paid to targeting the biologic and genetic factors that contribute to the poor prognosis of this group. Given the negative differential outcomes in patients with decreased tumor-infiltrating lymphocytes and increased monocyte/macrophage activation, immunologic approaches in the salvage setting, including immune checkpoint blockade drugs, chimeric antigen receptor T cells, and allogeneic transplantation may be biologically relevant.
Dr. Caron A. Jacobson and Dr. Arnold S. Freedman, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, made their remarks in an editorial accompanying the study.
If, in studying the immunologic and inflammatory host response to, and the genetic landscape of, these lymphomas, we are able to define this high-risk subgroup of patients with follicular lymphoma, the question becomes whether we could use this information to effectively treat these patients differently. Although high-dose chemotherapy and autologous stem-cell transplantation (HDC-ASCT) in first remission seems to have no effect on OS in all comers, results might be different for this cohort of high-risk patients. To study this would require an ability to identify these patients at diagnosis. Given that the efficacy of HDC-ASCT is maintained in the case of chemosensitive relapse, reserving HDC-ASCT for patients who relapse within the first 2 years of their initial therapy may be a more prudent strategy.
However, it may be that this is a particularly chemoresistant population and that, instead, attention should be paid to targeting the biologic and genetic factors that contribute to the poor prognosis of this group. Given the negative differential outcomes in patients with decreased tumor-infiltrating lymphocytes and increased monocyte/macrophage activation, immunologic approaches in the salvage setting, including immune checkpoint blockade drugs, chimeric antigen receptor T cells, and allogeneic transplantation may be biologically relevant.
Dr. Caron A. Jacobson and Dr. Arnold S. Freedman, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, made their remarks in an editorial accompanying the study.
For patients with follicular lymphoma treated with a rituximab-based combination chemotherapy regimen, early disease progression is associated with significantly worse overall survival, suggesting the need for additional interventions, according to results of a multicenter study.
Among 588 patients with stage 2-4 follicular lymphoma treated with first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and followed for a median of 7 years in the National LymphoCare Study, overall survival (OS) at 2 years was 68% for those who had disease progression within 2 years, compared with 97% for patients with no disease progression during that time.
Similarly, 5-year overall survival was 50% for patients with early progression of disease, compared with 90% for patients with no early progression, write Dr. Carla Casulo of the University of Rochester (N.Y.) Medical Center and colleagues. The study is in anearly online publication in the Journal of Clinical Oncology.
“Given our findings, early relapse after diagnosis in patients treated with first-line chemoimmunotherapy is a powerful prognostic indicator of outcome and should be used to stratify the risk of patients in studies of relapsed follicular lymphoma,” the authors wrote.
The findings were validated in an independent cohort of patients with follicular lymphoma treated with R-CHOP from the University of Iowa and Mayo Clinical Molecular Epidemiology Resource, and are consistent with findings from other studies of patients treated with different rituximab-based regimens, the investigators reported.
In unadjusted analysis, early disease progression was associated with a hazard ratio (HR) of 7.17 (95% confidence interval [CI] 4.83-10.65); the effect remained after adjustment for the Follicular Lymphoma International Prognostic Index (FLIPI) score (HR 6.44, 95% CI, 4.33-9.58).
Factors associated with early progression included age, Eastern Cooperative Oncology Group performance score, nodal sites, and disease stage.
Early use of aggressive salvage therapies or autologous stem-cell transplantation could improve outcomes in patients with early disease progression, the authors wrote. However, only 8 patients among the 110 with early progression went on to transplant, not a large enough sample for meaningful analysis, they added.
“This newly defined high-risk group of patients represents a distinct population in whom further study is warranted in both directed prospective clinical trials of follicular lymphoma biology and treatment. Moreover, we propose that 2-year progression-free survival may be a practical and meaningful clinical end point for trials involving a chemoimmunotherapy backbone,” they concluded.
For patients with follicular lymphoma treated with a rituximab-based combination chemotherapy regimen, early disease progression is associated with significantly worse overall survival, suggesting the need for additional interventions, according to results of a multicenter study.
Among 588 patients with stage 2-4 follicular lymphoma treated with first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and followed for a median of 7 years in the National LymphoCare Study, overall survival (OS) at 2 years was 68% for those who had disease progression within 2 years, compared with 97% for patients with no disease progression during that time.
Similarly, 5-year overall survival was 50% for patients with early progression of disease, compared with 90% for patients with no early progression, write Dr. Carla Casulo of the University of Rochester (N.Y.) Medical Center and colleagues. The study is in anearly online publication in the Journal of Clinical Oncology.
“Given our findings, early relapse after diagnosis in patients treated with first-line chemoimmunotherapy is a powerful prognostic indicator of outcome and should be used to stratify the risk of patients in studies of relapsed follicular lymphoma,” the authors wrote.
The findings were validated in an independent cohort of patients with follicular lymphoma treated with R-CHOP from the University of Iowa and Mayo Clinical Molecular Epidemiology Resource, and are consistent with findings from other studies of patients treated with different rituximab-based regimens, the investigators reported.
In unadjusted analysis, early disease progression was associated with a hazard ratio (HR) of 7.17 (95% confidence interval [CI] 4.83-10.65); the effect remained after adjustment for the Follicular Lymphoma International Prognostic Index (FLIPI) score (HR 6.44, 95% CI, 4.33-9.58).
Factors associated with early progression included age, Eastern Cooperative Oncology Group performance score, nodal sites, and disease stage.
Early use of aggressive salvage therapies or autologous stem-cell transplantation could improve outcomes in patients with early disease progression, the authors wrote. However, only 8 patients among the 110 with early progression went on to transplant, not a large enough sample for meaningful analysis, they added.
“This newly defined high-risk group of patients represents a distinct population in whom further study is warranted in both directed prospective clinical trials of follicular lymphoma biology and treatment. Moreover, we propose that 2-year progression-free survival may be a practical and meaningful clinical end point for trials involving a chemoimmunotherapy backbone,” they concluded.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Disease progression within 2 years of chemotherapy for follicular lymphoma is associated with poor outcomes.
Major finding: Five-year overall survival was 50% for patients with follicular lymphoma with disease progression within 2-years of R-CHOP, vs. 90% for patients with no early progression.
Data source: Retrospective review involving 588 patients in the longitudinal National LymphoCare Study.
Disclosures: Genentech and F. Hoffmann-La Roche supported the study. Dr. Casulo and Dr. Jacobson reported no relevant disclosures. Dr. Freedman reported ties with UpToDate, Axio, and Immunogen.
What do >700 letters to a mass murderer tell us about the people who wrote them?
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
Analysis reveals potential therapeutic target for AML

The protein tetraspanin3 (Tspan3) plays a critical role in the development and progression of acute myeloid leukemia (AML), according to research published in Cell Stem Cell.
Investigators found that Tspan3, a cell surface molecule, is expressed in hematopoietic stem and progenitor cells as well as in leukemic cells.
Deleting Tspan3 did not affect normal hematopoiesis, but it prevented AML self-renewal and propagation in vitro and in vivo.
Inhibiting Tspan3 in patient samples led to decreased colony formation in vitro and hindered leukemic growth in primary patient-derived xenografts.
“We found that blocking this molecule leads to a very profound inhibition of leukemia growth,” said study author Tannishtha Reya, PhD, of the University of California San Diego in La Jolla.
These findings build on earlier work by Dr Reya and her colleagues, in which they identified the RNA binding protein Musashi 2 (Msi2) as a critical stem cell signal that is hijacked in several hematologic malignancies.
“We had this idea that analysis of the molecular programs controlled by Musashi 2 may identify new genes important for these leukemias,” Dr Reya said.
So the investigators conducted a genome-wide expression analysis of Msi2-deficient cancer stem cells from blast-crisis chronic myelogenous leukemia and AML. This revealed genes commonly regulated by Msi2 in both leukemias.
Tspan3 was one of the core genes controlled by Msi2. The Tspan3 protein is part of a large family of membrane proteins (the tetraspanin family) that are active in diverse cellular processes, including cell adhesion and proliferation, hematopoietic stem cell function, and blood formation.
“We are particularly excited about this work because, to our knowledge, this is the first demonstration of a requirement for Tspan3 in any primary cancer,” Dr Reya said.
To explore the connection further, the investigators generated the first Tspan3 knockout mouse. In testing, the team found that Tspan3 deletion impaired leukemia stem cell self-renewal and disease propagation and markedly improved survival in the mice.
In patient samples, Tspan3 inhibition blocked the growth of AML, which suggests Tspan3 is also important in human disease.
Dr Reya said these findings are particularly important because AML often doesn’t respond to current therapies. And because Tspan3 is a surface molecule, it is of great translational interest as a target for antibody-mediated therapy.
“There’s been great progress in pediatric leukemia research and treatment over the last few years,” Dr Reya said. “But unfortunately, children with acute myeloid leukemia are often poor responders to current treatments. So identifying new approaches to target this disease remains critically important.” ![]()

The protein tetraspanin3 (Tspan3) plays a critical role in the development and progression of acute myeloid leukemia (AML), according to research published in Cell Stem Cell.
Investigators found that Tspan3, a cell surface molecule, is expressed in hematopoietic stem and progenitor cells as well as in leukemic cells.
Deleting Tspan3 did not affect normal hematopoiesis, but it prevented AML self-renewal and propagation in vitro and in vivo.
Inhibiting Tspan3 in patient samples led to decreased colony formation in vitro and hindered leukemic growth in primary patient-derived xenografts.
“We found that blocking this molecule leads to a very profound inhibition of leukemia growth,” said study author Tannishtha Reya, PhD, of the University of California San Diego in La Jolla.
These findings build on earlier work by Dr Reya and her colleagues, in which they identified the RNA binding protein Musashi 2 (Msi2) as a critical stem cell signal that is hijacked in several hematologic malignancies.
“We had this idea that analysis of the molecular programs controlled by Musashi 2 may identify new genes important for these leukemias,” Dr Reya said.
So the investigators conducted a genome-wide expression analysis of Msi2-deficient cancer stem cells from blast-crisis chronic myelogenous leukemia and AML. This revealed genes commonly regulated by Msi2 in both leukemias.
Tspan3 was one of the core genes controlled by Msi2. The Tspan3 protein is part of a large family of membrane proteins (the tetraspanin family) that are active in diverse cellular processes, including cell adhesion and proliferation, hematopoietic stem cell function, and blood formation.
“We are particularly excited about this work because, to our knowledge, this is the first demonstration of a requirement for Tspan3 in any primary cancer,” Dr Reya said.
To explore the connection further, the investigators generated the first Tspan3 knockout mouse. In testing, the team found that Tspan3 deletion impaired leukemia stem cell self-renewal and disease propagation and markedly improved survival in the mice.
In patient samples, Tspan3 inhibition blocked the growth of AML, which suggests Tspan3 is also important in human disease.
Dr Reya said these findings are particularly important because AML often doesn’t respond to current therapies. And because Tspan3 is a surface molecule, it is of great translational interest as a target for antibody-mediated therapy.
“There’s been great progress in pediatric leukemia research and treatment over the last few years,” Dr Reya said. “But unfortunately, children with acute myeloid leukemia are often poor responders to current treatments. So identifying new approaches to target this disease remains critically important.” ![]()

The protein tetraspanin3 (Tspan3) plays a critical role in the development and progression of acute myeloid leukemia (AML), according to research published in Cell Stem Cell.
Investigators found that Tspan3, a cell surface molecule, is expressed in hematopoietic stem and progenitor cells as well as in leukemic cells.
Deleting Tspan3 did not affect normal hematopoiesis, but it prevented AML self-renewal and propagation in vitro and in vivo.
Inhibiting Tspan3 in patient samples led to decreased colony formation in vitro and hindered leukemic growth in primary patient-derived xenografts.
“We found that blocking this molecule leads to a very profound inhibition of leukemia growth,” said study author Tannishtha Reya, PhD, of the University of California San Diego in La Jolla.
These findings build on earlier work by Dr Reya and her colleagues, in which they identified the RNA binding protein Musashi 2 (Msi2) as a critical stem cell signal that is hijacked in several hematologic malignancies.
“We had this idea that analysis of the molecular programs controlled by Musashi 2 may identify new genes important for these leukemias,” Dr Reya said.
So the investigators conducted a genome-wide expression analysis of Msi2-deficient cancer stem cells from blast-crisis chronic myelogenous leukemia and AML. This revealed genes commonly regulated by Msi2 in both leukemias.
Tspan3 was one of the core genes controlled by Msi2. The Tspan3 protein is part of a large family of membrane proteins (the tetraspanin family) that are active in diverse cellular processes, including cell adhesion and proliferation, hematopoietic stem cell function, and blood formation.
“We are particularly excited about this work because, to our knowledge, this is the first demonstration of a requirement for Tspan3 in any primary cancer,” Dr Reya said.
To explore the connection further, the investigators generated the first Tspan3 knockout mouse. In testing, the team found that Tspan3 deletion impaired leukemia stem cell self-renewal and disease propagation and markedly improved survival in the mice.
In patient samples, Tspan3 inhibition blocked the growth of AML, which suggests Tspan3 is also important in human disease.
Dr Reya said these findings are particularly important because AML often doesn’t respond to current therapies. And because Tspan3 is a surface molecule, it is of great translational interest as a target for antibody-mediated therapy.
“There’s been great progress in pediatric leukemia research and treatment over the last few years,” Dr Reya said. “But unfortunately, children with acute myeloid leukemia are often poor responders to current treatments. So identifying new approaches to target this disease remains critically important.” ![]()
Evolution drives cancer development, scientists say

Photo courtesy of University
of Colorado Cancer Center
Oncogenesis does not depend only on the accumulation of mutations but on evolutionary pressures acting on cell populations, according to a paper published in PNAS.
The authors say the ecosystem of a healthy tissue landscape lets healthy cells outcompete cells with cancerous mutations.
It is when the tissue ecosystem changes due to aging, smoking, or other stressors that cells with cancerous mutations can suddenly find themselves the most fit.
And this allows the cell population to expand over generations of natural selection.
This model of oncogenesis has profound implications for cancer therapy and drug design, according to the authors.
“We’ve been trying to make drugs that target mutations in cancer cells,” said author James DeGregori, PhD, of University of Colorado School of Medicine in Aurora.
“But if it’s the ecosystem of the body, and not only cancer-causing mutations, that allows the growth of cancer, we should also be prioritizing interventions and lifestyle choices that promote the fitness of healthy cells in order to suppress the emergence of cancer.”
The proposed model helps to answer a long-standing question known as Peto’s Paradox. If cancer is due to random activating mutation, larger animals with more cells should be at greater risk of developing cancer earlier in their lives. Why then do mammals of vastly different sizes and lifespans all seem to develop cancer mostly late in life?
“Blue whales have more than a million times more cells and live about 50 times longer than a mouse, but the whale has no more risk than a mouse of developing cancer over its lifespan,” Dr DeGregori noted.
The answer he and colleague Andrii Rozhok, PhD, propose is that, in addition to activating mutations, cancer may require age-associated changes to the tissue landscape in order for evolution to favor the survival and growth of cancer cells over the competition of healthy cells.
“Healthy cells are optimized for the ecosystem of the healthy body,” Dr DeGregori said. “But when the tissue ecosystem changes, such as with aging or smoking, cancer-causing mutations are often very good at exploiting the conditions of a damaged tissue landscape.”
This model is supported by studies showing that mutations that can cause cancer do not necessarily increase a cell’s fitness.
“In fact, healthy cells are so optimized to the healthy tissue landscape that almost any mutation makes them less fit,” Dr DeGregori said.
For example, some cancer cells mutate in a way that allows them to survive in the oxygen-poor tissue environments found in the center of developing tumors. But this adaptation only confers a fitness benefit in oxygen-poor tissues.
In a healthy, oxygen-rich tissue, this mutation would not confer this advantage. In healthy tissue, cells with this mutation lose the evolutionary race to the healthy cells. Cancer cells are outcompeted and die, or, at least, their population is held in check and remains insignificantly small.
But what happens when the tissue landscape changes?
“When the body changes due to aging, smoking, inherited genetic differences, or other factors, it changes the tissue ecosystem, allowing a new kind of cell to replace the healthy ones,” Dr DeGregori said.
Certainly, cancer development requires mutations and other genetic alterations. But how do these mutations cause cancer?
It may not be that these mutations create accidental “super cells” that immediately run amok. Instead, it may be that oncogenic mutations are often or always present in the body but are kept at bay by selection pressures set against them.
That is, until the tissue ecosystem and its pressures change in ways that make cells with cancerous mutations more likely to survive than healthy cells. Over time, this allows the population of cancer cells to overcome that of healthy cells.
People can avoid some of these tissue changes by lifestyle choices, Dr DeGregori noted, but aging cannot be stopped. Still, there may be features of the tissue landscape that, with new therapies and new understanding, could be reinforced in ways to resist cancer better for longer. ![]()

Photo courtesy of University
of Colorado Cancer Center
Oncogenesis does not depend only on the accumulation of mutations but on evolutionary pressures acting on cell populations, according to a paper published in PNAS.
The authors say the ecosystem of a healthy tissue landscape lets healthy cells outcompete cells with cancerous mutations.
It is when the tissue ecosystem changes due to aging, smoking, or other stressors that cells with cancerous mutations can suddenly find themselves the most fit.
And this allows the cell population to expand over generations of natural selection.
This model of oncogenesis has profound implications for cancer therapy and drug design, according to the authors.
“We’ve been trying to make drugs that target mutations in cancer cells,” said author James DeGregori, PhD, of University of Colorado School of Medicine in Aurora.
“But if it’s the ecosystem of the body, and not only cancer-causing mutations, that allows the growth of cancer, we should also be prioritizing interventions and lifestyle choices that promote the fitness of healthy cells in order to suppress the emergence of cancer.”
The proposed model helps to answer a long-standing question known as Peto’s Paradox. If cancer is due to random activating mutation, larger animals with more cells should be at greater risk of developing cancer earlier in their lives. Why then do mammals of vastly different sizes and lifespans all seem to develop cancer mostly late in life?
“Blue whales have more than a million times more cells and live about 50 times longer than a mouse, but the whale has no more risk than a mouse of developing cancer over its lifespan,” Dr DeGregori noted.
The answer he and colleague Andrii Rozhok, PhD, propose is that, in addition to activating mutations, cancer may require age-associated changes to the tissue landscape in order for evolution to favor the survival and growth of cancer cells over the competition of healthy cells.
“Healthy cells are optimized for the ecosystem of the healthy body,” Dr DeGregori said. “But when the tissue ecosystem changes, such as with aging or smoking, cancer-causing mutations are often very good at exploiting the conditions of a damaged tissue landscape.”
This model is supported by studies showing that mutations that can cause cancer do not necessarily increase a cell’s fitness.
“In fact, healthy cells are so optimized to the healthy tissue landscape that almost any mutation makes them less fit,” Dr DeGregori said.
For example, some cancer cells mutate in a way that allows them to survive in the oxygen-poor tissue environments found in the center of developing tumors. But this adaptation only confers a fitness benefit in oxygen-poor tissues.
In a healthy, oxygen-rich tissue, this mutation would not confer this advantage. In healthy tissue, cells with this mutation lose the evolutionary race to the healthy cells. Cancer cells are outcompeted and die, or, at least, their population is held in check and remains insignificantly small.
But what happens when the tissue landscape changes?
“When the body changes due to aging, smoking, inherited genetic differences, or other factors, it changes the tissue ecosystem, allowing a new kind of cell to replace the healthy ones,” Dr DeGregori said.
Certainly, cancer development requires mutations and other genetic alterations. But how do these mutations cause cancer?
It may not be that these mutations create accidental “super cells” that immediately run amok. Instead, it may be that oncogenic mutations are often or always present in the body but are kept at bay by selection pressures set against them.
That is, until the tissue ecosystem and its pressures change in ways that make cells with cancerous mutations more likely to survive than healthy cells. Over time, this allows the population of cancer cells to overcome that of healthy cells.
People can avoid some of these tissue changes by lifestyle choices, Dr DeGregori noted, but aging cannot be stopped. Still, there may be features of the tissue landscape that, with new therapies and new understanding, could be reinforced in ways to resist cancer better for longer. ![]()

Photo courtesy of University
of Colorado Cancer Center
Oncogenesis does not depend only on the accumulation of mutations but on evolutionary pressures acting on cell populations, according to a paper published in PNAS.
The authors say the ecosystem of a healthy tissue landscape lets healthy cells outcompete cells with cancerous mutations.
It is when the tissue ecosystem changes due to aging, smoking, or other stressors that cells with cancerous mutations can suddenly find themselves the most fit.
And this allows the cell population to expand over generations of natural selection.
This model of oncogenesis has profound implications for cancer therapy and drug design, according to the authors.
“We’ve been trying to make drugs that target mutations in cancer cells,” said author James DeGregori, PhD, of University of Colorado School of Medicine in Aurora.
“But if it’s the ecosystem of the body, and not only cancer-causing mutations, that allows the growth of cancer, we should also be prioritizing interventions and lifestyle choices that promote the fitness of healthy cells in order to suppress the emergence of cancer.”
The proposed model helps to answer a long-standing question known as Peto’s Paradox. If cancer is due to random activating mutation, larger animals with more cells should be at greater risk of developing cancer earlier in their lives. Why then do mammals of vastly different sizes and lifespans all seem to develop cancer mostly late in life?
“Blue whales have more than a million times more cells and live about 50 times longer than a mouse, but the whale has no more risk than a mouse of developing cancer over its lifespan,” Dr DeGregori noted.
The answer he and colleague Andrii Rozhok, PhD, propose is that, in addition to activating mutations, cancer may require age-associated changes to the tissue landscape in order for evolution to favor the survival and growth of cancer cells over the competition of healthy cells.
“Healthy cells are optimized for the ecosystem of the healthy body,” Dr DeGregori said. “But when the tissue ecosystem changes, such as with aging or smoking, cancer-causing mutations are often very good at exploiting the conditions of a damaged tissue landscape.”
This model is supported by studies showing that mutations that can cause cancer do not necessarily increase a cell’s fitness.
“In fact, healthy cells are so optimized to the healthy tissue landscape that almost any mutation makes them less fit,” Dr DeGregori said.
For example, some cancer cells mutate in a way that allows them to survive in the oxygen-poor tissue environments found in the center of developing tumors. But this adaptation only confers a fitness benefit in oxygen-poor tissues.
In a healthy, oxygen-rich tissue, this mutation would not confer this advantage. In healthy tissue, cells with this mutation lose the evolutionary race to the healthy cells. Cancer cells are outcompeted and die, or, at least, their population is held in check and remains insignificantly small.
But what happens when the tissue landscape changes?
“When the body changes due to aging, smoking, inherited genetic differences, or other factors, it changes the tissue ecosystem, allowing a new kind of cell to replace the healthy ones,” Dr DeGregori said.
Certainly, cancer development requires mutations and other genetic alterations. But how do these mutations cause cancer?
It may not be that these mutations create accidental “super cells” that immediately run amok. Instead, it may be that oncogenic mutations are often or always present in the body but are kept at bay by selection pressures set against them.
That is, until the tissue ecosystem and its pressures change in ways that make cells with cancerous mutations more likely to survive than healthy cells. Over time, this allows the population of cancer cells to overcome that of healthy cells.
People can avoid some of these tissue changes by lifestyle choices, Dr DeGregori noted, but aging cannot be stopped. Still, there may be features of the tissue landscape that, with new therapies and new understanding, could be reinforced in ways to resist cancer better for longer. ![]()
Team discovers ‘new avenue’ for TTP treatment

Image by Erhabor Osaro
Researchers say they have uncovered a new avenue for therapeutic intervention in thrombotic thrombocytopenic purpura (TTP).
The team discovered how autoimmune antibodies in a TTP patient recognize and bind to ADAMTS13.
They believe this knowledge could help them alter ADAMTS13 to produce a therapeutic enzyme that can elude recognition by autoimmune antibodies yet still retain its ability to cleave von Willebrand factor.
Such an enzyme could be given to TTP patients in the hospital to speed recovery and cut the cost of treatment.
Long Zheng, MD, PhD, of the University of Alabama at Birmingham, and his colleagues described this work in PNAS.
The researchers found that 5 small loops in ADAMTS13’s amino acid sequence are necessary for autoantibodies to bind to ADAMTS13.
Cutting or substituting several amino acids out of any of the 5 loops prevented binding. Small deletions in a loop also left the enzyme unable to cleave von Willebrand factor.
“This was really surprising,” Dr Zheng said. “It’s like a table with 5 legs. If you take 1 away, it should still stand, but, somehow, it collapsed. This suggests that you need the coordinated activity of all 5.”
Thus, it appears that the autoimmune antibodies in TTP patients inhibit the enzyme by physically blocking the recognition site of ADAMTS13 for von Willebrand factor.
Analyses of autoantibodies from 23 more TTP patients revealed that most use the same binding site. This suggests modifying the ADAMTS13 enzyme by protein engineering may be able to help a wide range of TTP patients.
Details of the research
Dr Zheng and his colleagues first isolated messenger RNAs that code single chains of variable regions of monoclonal antibodies from B cells collected from patients with acquired TTP.
The team used phage display to select the messenger RNAs that code specific antibodies that bind and inhibit ADAMTS13. These monoclonal antibodies were then expressed in E coli cells, purified, and biochemically characterized.
Three inhibitory monoclonal antibodies were selected for further study by hydrogen-deuterium exchange coupled with mass spectrometry. This technology uses amine hydrogen exchange with deuterium on each amino acid residue except proline.
After the reaction was stopped, the protein was cut into small pieces (or peptide fragments) and run through high-performance liquid chromatography for separation and mass spectrometry for identification.
Antibody binding sites were detected by their ability to block the hydrogen and deuterium exchange, as compared with ADAMTS13 that was unbound.
One of the 3 high-affinity probes selected by phage display was used for the competition experiments against polyclonal autoimmune antibodies from 23 TTP patients.
The results show that this particular binding epitope is common among patients with acquired autoimmune TTP. ![]()

Image by Erhabor Osaro
Researchers say they have uncovered a new avenue for therapeutic intervention in thrombotic thrombocytopenic purpura (TTP).
The team discovered how autoimmune antibodies in a TTP patient recognize and bind to ADAMTS13.
They believe this knowledge could help them alter ADAMTS13 to produce a therapeutic enzyme that can elude recognition by autoimmune antibodies yet still retain its ability to cleave von Willebrand factor.
Such an enzyme could be given to TTP patients in the hospital to speed recovery and cut the cost of treatment.
Long Zheng, MD, PhD, of the University of Alabama at Birmingham, and his colleagues described this work in PNAS.
The researchers found that 5 small loops in ADAMTS13’s amino acid sequence are necessary for autoantibodies to bind to ADAMTS13.
Cutting or substituting several amino acids out of any of the 5 loops prevented binding. Small deletions in a loop also left the enzyme unable to cleave von Willebrand factor.
“This was really surprising,” Dr Zheng said. “It’s like a table with 5 legs. If you take 1 away, it should still stand, but, somehow, it collapsed. This suggests that you need the coordinated activity of all 5.”
Thus, it appears that the autoimmune antibodies in TTP patients inhibit the enzyme by physically blocking the recognition site of ADAMTS13 for von Willebrand factor.
Analyses of autoantibodies from 23 more TTP patients revealed that most use the same binding site. This suggests modifying the ADAMTS13 enzyme by protein engineering may be able to help a wide range of TTP patients.
Details of the research
Dr Zheng and his colleagues first isolated messenger RNAs that code single chains of variable regions of monoclonal antibodies from B cells collected from patients with acquired TTP.
The team used phage display to select the messenger RNAs that code specific antibodies that bind and inhibit ADAMTS13. These monoclonal antibodies were then expressed in E coli cells, purified, and biochemically characterized.
Three inhibitory monoclonal antibodies were selected for further study by hydrogen-deuterium exchange coupled with mass spectrometry. This technology uses amine hydrogen exchange with deuterium on each amino acid residue except proline.
After the reaction was stopped, the protein was cut into small pieces (or peptide fragments) and run through high-performance liquid chromatography for separation and mass spectrometry for identification.
Antibody binding sites were detected by their ability to block the hydrogen and deuterium exchange, as compared with ADAMTS13 that was unbound.
One of the 3 high-affinity probes selected by phage display was used for the competition experiments against polyclonal autoimmune antibodies from 23 TTP patients.
The results show that this particular binding epitope is common among patients with acquired autoimmune TTP. ![]()

Image by Erhabor Osaro
Researchers say they have uncovered a new avenue for therapeutic intervention in thrombotic thrombocytopenic purpura (TTP).
The team discovered how autoimmune antibodies in a TTP patient recognize and bind to ADAMTS13.
They believe this knowledge could help them alter ADAMTS13 to produce a therapeutic enzyme that can elude recognition by autoimmune antibodies yet still retain its ability to cleave von Willebrand factor.
Such an enzyme could be given to TTP patients in the hospital to speed recovery and cut the cost of treatment.
Long Zheng, MD, PhD, of the University of Alabama at Birmingham, and his colleagues described this work in PNAS.
The researchers found that 5 small loops in ADAMTS13’s amino acid sequence are necessary for autoantibodies to bind to ADAMTS13.
Cutting or substituting several amino acids out of any of the 5 loops prevented binding. Small deletions in a loop also left the enzyme unable to cleave von Willebrand factor.
“This was really surprising,” Dr Zheng said. “It’s like a table with 5 legs. If you take 1 away, it should still stand, but, somehow, it collapsed. This suggests that you need the coordinated activity of all 5.”
Thus, it appears that the autoimmune antibodies in TTP patients inhibit the enzyme by physically blocking the recognition site of ADAMTS13 for von Willebrand factor.
Analyses of autoantibodies from 23 more TTP patients revealed that most use the same binding site. This suggests modifying the ADAMTS13 enzyme by protein engineering may be able to help a wide range of TTP patients.
Details of the research
Dr Zheng and his colleagues first isolated messenger RNAs that code single chains of variable regions of monoclonal antibodies from B cells collected from patients with acquired TTP.
The team used phage display to select the messenger RNAs that code specific antibodies that bind and inhibit ADAMTS13. These monoclonal antibodies were then expressed in E coli cells, purified, and biochemically characterized.
Three inhibitory monoclonal antibodies were selected for further study by hydrogen-deuterium exchange coupled with mass spectrometry. This technology uses amine hydrogen exchange with deuterium on each amino acid residue except proline.
After the reaction was stopped, the protein was cut into small pieces (or peptide fragments) and run through high-performance liquid chromatography for separation and mass spectrometry for identification.
Antibody binding sites were detected by their ability to block the hydrogen and deuterium exchange, as compared with ADAMTS13 that was unbound.
One of the 3 high-affinity probes selected by phage display was used for the competition experiments against polyclonal autoimmune antibodies from 23 TTP patients.
The results show that this particular binding epitope is common among patients with acquired autoimmune TTP. ![]()
Pediatric cancer specialist passes away

Photo courtesy of University
of Chicago Medicine
Charles M. Rubin, MD, an associate professor of pediatrics at the University of Chicago Medicine, has passed away at the age of 62.
Dr Rubin died while at work on July 17.
He had just arrived at the pediatric clinic at the University of Chicago Medicine Comprehensive Cancer Center at Silver Cross Hospital in New Lenox when his heart stopped.
Resuscitation efforts were unsuccessful.
An authority on pediatric cancers, Dr Rubin had a particular interest in brain tumors and cancer occurring in children with genetic syndromes. He combined experience in basic laboratory research on the genetics of cancer with broad clinical expertise.
“I can’t put into words how much I respected him,” said colleague Tara Henderson, MD, associate professor of pediatrics and director of the Childhood Cancer Survivors Center at the University of Chicago’s Comer Children’s Hospital.
“He was amazingly knowledgeable, compassionate, and thoughtful—traits at the core of our program. I take his influence with me as I care for my patients. He could also be funny, with a dry, quiet sense of humor. We never knew when it was coming. We miss him dearly.”
Dr Rubin was born February 10, 1953, in Long Branch, New Jersey. He earned his bachelor’s degree from the University of Pennsylvania in 1975 and his medical degree from Tufts University School of Medicine in 1979.
Dr Rubin completed his pediatric residency at the Children’s Hospital of Philadelphia in 1982, followed by a fellowship in pediatric hematology/oncology at the University of Minnesota in 1985.
He went to the University of Chicago in 1985 as a cytogenetics and molecular biology fellow in the laboratory of Janet Rowley, MD, an internationally recognized pioneer in understanding the genetics of cancer.
Dr Rubin joined the faculty as an assistant professor of pediatrics and medicine and a member of the university’s Cancer Research Center in 1987. In 1991, he and adult oncologist Funmi Olopade, MD, co-founded the university’s Cancer Risk Clinic.
“Chuck Rubin was one of the finest individuals I have ever known,” said Michelle Le Beau, PhD, a former colleague in the Rowley laboratory and now director of the University of Chicago Medicine Comprehensive Cancer Center.
“He was a consummate academician and physician who blended compassion and sensitivity with brilliant clinical acumen. His dedication to his family, his patients, and the University of Chicago was selfless and unparalleled. It was a privilege to work with him and an honor to learn from his example.”
Although Dr Rubin continued to work closely with his basic-science colleagues, contributing to more than 50 original reports in academic journals, his interests increasingly focused on patient care.
At the same time, he took on several administrative roles. He served as course director for pediatric grand rounds and the medical center’s pediatric tumor board.
He directed the pediatric hematology/oncology fellowship for 7 years and the pediatric neuro-oncology program for 10 years. He also volunteered for medical staff positions in various educational and rehabilitative summer camps for children with cancer.
Dr Rubin was a leader in the University of Chicago Medicine’s efforts to take a research-driven approach to pediatric cancer care into the community, serving as director of pediatric hematology/oncology outreach since 2008.
Dr Rubin is survived by his wife, Gretchen; their 4 daughters, Elizabeth, Jane, Lucy, and Claire; brothers Michael, Peter, and Richard; and many nieces and nephews. ![]()

Photo courtesy of University
of Chicago Medicine
Charles M. Rubin, MD, an associate professor of pediatrics at the University of Chicago Medicine, has passed away at the age of 62.
Dr Rubin died while at work on July 17.
He had just arrived at the pediatric clinic at the University of Chicago Medicine Comprehensive Cancer Center at Silver Cross Hospital in New Lenox when his heart stopped.
Resuscitation efforts were unsuccessful.
An authority on pediatric cancers, Dr Rubin had a particular interest in brain tumors and cancer occurring in children with genetic syndromes. He combined experience in basic laboratory research on the genetics of cancer with broad clinical expertise.
“I can’t put into words how much I respected him,” said colleague Tara Henderson, MD, associate professor of pediatrics and director of the Childhood Cancer Survivors Center at the University of Chicago’s Comer Children’s Hospital.
“He was amazingly knowledgeable, compassionate, and thoughtful—traits at the core of our program. I take his influence with me as I care for my patients. He could also be funny, with a dry, quiet sense of humor. We never knew when it was coming. We miss him dearly.”
Dr Rubin was born February 10, 1953, in Long Branch, New Jersey. He earned his bachelor’s degree from the University of Pennsylvania in 1975 and his medical degree from Tufts University School of Medicine in 1979.
Dr Rubin completed his pediatric residency at the Children’s Hospital of Philadelphia in 1982, followed by a fellowship in pediatric hematology/oncology at the University of Minnesota in 1985.
He went to the University of Chicago in 1985 as a cytogenetics and molecular biology fellow in the laboratory of Janet Rowley, MD, an internationally recognized pioneer in understanding the genetics of cancer.
Dr Rubin joined the faculty as an assistant professor of pediatrics and medicine and a member of the university’s Cancer Research Center in 1987. In 1991, he and adult oncologist Funmi Olopade, MD, co-founded the university’s Cancer Risk Clinic.
“Chuck Rubin was one of the finest individuals I have ever known,” said Michelle Le Beau, PhD, a former colleague in the Rowley laboratory and now director of the University of Chicago Medicine Comprehensive Cancer Center.
“He was a consummate academician and physician who blended compassion and sensitivity with brilliant clinical acumen. His dedication to his family, his patients, and the University of Chicago was selfless and unparalleled. It was a privilege to work with him and an honor to learn from his example.”
Although Dr Rubin continued to work closely with his basic-science colleagues, contributing to more than 50 original reports in academic journals, his interests increasingly focused on patient care.
At the same time, he took on several administrative roles. He served as course director for pediatric grand rounds and the medical center’s pediatric tumor board.
He directed the pediatric hematology/oncology fellowship for 7 years and the pediatric neuro-oncology program for 10 years. He also volunteered for medical staff positions in various educational and rehabilitative summer camps for children with cancer.
Dr Rubin was a leader in the University of Chicago Medicine’s efforts to take a research-driven approach to pediatric cancer care into the community, serving as director of pediatric hematology/oncology outreach since 2008.
Dr Rubin is survived by his wife, Gretchen; their 4 daughters, Elizabeth, Jane, Lucy, and Claire; brothers Michael, Peter, and Richard; and many nieces and nephews. ![]()

Photo courtesy of University
of Chicago Medicine
Charles M. Rubin, MD, an associate professor of pediatrics at the University of Chicago Medicine, has passed away at the age of 62.
Dr Rubin died while at work on July 17.
He had just arrived at the pediatric clinic at the University of Chicago Medicine Comprehensive Cancer Center at Silver Cross Hospital in New Lenox when his heart stopped.
Resuscitation efforts were unsuccessful.
An authority on pediatric cancers, Dr Rubin had a particular interest in brain tumors and cancer occurring in children with genetic syndromes. He combined experience in basic laboratory research on the genetics of cancer with broad clinical expertise.
“I can’t put into words how much I respected him,” said colleague Tara Henderson, MD, associate professor of pediatrics and director of the Childhood Cancer Survivors Center at the University of Chicago’s Comer Children’s Hospital.
“He was amazingly knowledgeable, compassionate, and thoughtful—traits at the core of our program. I take his influence with me as I care for my patients. He could also be funny, with a dry, quiet sense of humor. We never knew when it was coming. We miss him dearly.”
Dr Rubin was born February 10, 1953, in Long Branch, New Jersey. He earned his bachelor’s degree from the University of Pennsylvania in 1975 and his medical degree from Tufts University School of Medicine in 1979.
Dr Rubin completed his pediatric residency at the Children’s Hospital of Philadelphia in 1982, followed by a fellowship in pediatric hematology/oncology at the University of Minnesota in 1985.
He went to the University of Chicago in 1985 as a cytogenetics and molecular biology fellow in the laboratory of Janet Rowley, MD, an internationally recognized pioneer in understanding the genetics of cancer.
Dr Rubin joined the faculty as an assistant professor of pediatrics and medicine and a member of the university’s Cancer Research Center in 1987. In 1991, he and adult oncologist Funmi Olopade, MD, co-founded the university’s Cancer Risk Clinic.
“Chuck Rubin was one of the finest individuals I have ever known,” said Michelle Le Beau, PhD, a former colleague in the Rowley laboratory and now director of the University of Chicago Medicine Comprehensive Cancer Center.
“He was a consummate academician and physician who blended compassion and sensitivity with brilliant clinical acumen. His dedication to his family, his patients, and the University of Chicago was selfless and unparalleled. It was a privilege to work with him and an honor to learn from his example.”
Although Dr Rubin continued to work closely with his basic-science colleagues, contributing to more than 50 original reports in academic journals, his interests increasingly focused on patient care.
At the same time, he took on several administrative roles. He served as course director for pediatric grand rounds and the medical center’s pediatric tumor board.
He directed the pediatric hematology/oncology fellowship for 7 years and the pediatric neuro-oncology program for 10 years. He also volunteered for medical staff positions in various educational and rehabilitative summer camps for children with cancer.
Dr Rubin was a leader in the University of Chicago Medicine’s efforts to take a research-driven approach to pediatric cancer care into the community, serving as director of pediatric hematology/oncology outreach since 2008.
Dr Rubin is survived by his wife, Gretchen; their 4 daughters, Elizabeth, Jane, Lucy, and Claire; brothers Michael, Peter, and Richard; and many nieces and nephews. ![]()