User login
American Psychological Association investigation proves value of dissent
In 2005, the American Psychological Association formed a task force to address the role of psychologists in the interrogation of Central Intelligence Agency and Department of Defense detainees. The task force was formed at the request of psychologists consulting with both entities and their supervisors to address the ethics of psychologist involvement in shaping interrogation practices. The task force concluded that psychologist participation was allowed in order to ensure that the process was “safe, legal, ethical, and effective.” The Presidential Task Force on Psychological Ethics and National Security (PENS) report drew immediate objection from within the organization and triggered a member-initiated movement to rescind the findings. Members alleged that those involved in the creation of the PENS report had conflicts of interest and that the task force was heavily weighted with psychologists who were already consulting with national security organizations.
These concerns were substantiated in a book entitled Pay Any Price: Greed, Power, and Endless War (New York: Houghton Mifflin Harcourt, 2014) by New York Times reporter James Risen. In the spirit of the Watergate investigation, Risen “followed the money” as it flowed from the U.S. government to national security and defense agencies, and into the pockets of select psychologists who took active roles in harsh interrogation techniques.
Armed with this new evidence, the American Psychological Association initiated an independent investigation of the association’s role in November 2014. The results of that investigation were published recently in a 542-page report synopsized by James Risen in the New York Times. Also known as the Hoffman report, it confirmed numerous conflicts of interest between psychologists involved in the 2005 revision of the ethics guidelines, and both the C.I.A. and the D.O.D. The full implications of the Hoffman report remain to be seen, but the history and process of this problem are informative.
Both the American Medical Association and the American Psychiatric Association have position statements against participation in interrogation and torture. In 2006, the American Psychiatric Association issued a position statement in which it held that “that psychiatrists should not participate in, or otherwise assist or facilitate, the commission of torture of any person.” The American Medical Association similarly codified a prohibition against participation in interrogation in its ethical guidelines: “Physicians must oppose and must not participate in torture for any reason. Participation in torture includes, but is not limited to, providing or withholding any services, substances, or knowledge to facilitate the practice of torture. Physicians must not be present when torture is used or threatened.” Members who become aware of the practices are called upon to report them and to adhere to professional ethical standards.
But before psychiatry congratulates itself for its moral fortitude, we would do well to remember that the Department of Homeland Security’s Office of University Programs awarded $39 million to 12 academic institutions in 2011, to create “centers of excellence” related to cybersecurity, counterintelligence measures, disaster preparedness, prevention of terrorism, and research into the sociologic and psychological causes of radicalization. All mental health professionals should ensure that any research-related national security issues abide by international, ethical, and humanitarian standards, and should refrain from areas of investigation that target vulnerable individuals or groups.
The Hoffman report reminds us that everything that is legal is not necessarily ethical. It highlights the necessity of bright line standards over issues related to essential human rights and well-being. For these concerns, we should value internal challenge and dissent, and we must continually ask ourselves if we are on the right path. We must also create and protect avenues for our members who discover and report violations, even if this protection is given to the detriment of our organization. The purpose of a professional organization is to ensure the quality and integrity of its members and to protect the public from those members who fall short in either domain. As professionals, we are each responsible for ensuring that our organization carries out these duties.
Dr. Hanson is a forensic psychiatrist and coauthor of Shrink Rap: Three Psychiatrists Explain Their Work. The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
In 2005, the American Psychological Association formed a task force to address the role of psychologists in the interrogation of Central Intelligence Agency and Department of Defense detainees. The task force was formed at the request of psychologists consulting with both entities and their supervisors to address the ethics of psychologist involvement in shaping interrogation practices. The task force concluded that psychologist participation was allowed in order to ensure that the process was “safe, legal, ethical, and effective.” The Presidential Task Force on Psychological Ethics and National Security (PENS) report drew immediate objection from within the organization and triggered a member-initiated movement to rescind the findings. Members alleged that those involved in the creation of the PENS report had conflicts of interest and that the task force was heavily weighted with psychologists who were already consulting with national security organizations.
These concerns were substantiated in a book entitled Pay Any Price: Greed, Power, and Endless War (New York: Houghton Mifflin Harcourt, 2014) by New York Times reporter James Risen. In the spirit of the Watergate investigation, Risen “followed the money” as it flowed from the U.S. government to national security and defense agencies, and into the pockets of select psychologists who took active roles in harsh interrogation techniques.
Armed with this new evidence, the American Psychological Association initiated an independent investigation of the association’s role in November 2014. The results of that investigation were published recently in a 542-page report synopsized by James Risen in the New York Times. Also known as the Hoffman report, it confirmed numerous conflicts of interest between psychologists involved in the 2005 revision of the ethics guidelines, and both the C.I.A. and the D.O.D. The full implications of the Hoffman report remain to be seen, but the history and process of this problem are informative.
Both the American Medical Association and the American Psychiatric Association have position statements against participation in interrogation and torture. In 2006, the American Psychiatric Association issued a position statement in which it held that “that psychiatrists should not participate in, or otherwise assist or facilitate, the commission of torture of any person.” The American Medical Association similarly codified a prohibition against participation in interrogation in its ethical guidelines: “Physicians must oppose and must not participate in torture for any reason. Participation in torture includes, but is not limited to, providing or withholding any services, substances, or knowledge to facilitate the practice of torture. Physicians must not be present when torture is used or threatened.” Members who become aware of the practices are called upon to report them and to adhere to professional ethical standards.
But before psychiatry congratulates itself for its moral fortitude, we would do well to remember that the Department of Homeland Security’s Office of University Programs awarded $39 million to 12 academic institutions in 2011, to create “centers of excellence” related to cybersecurity, counterintelligence measures, disaster preparedness, prevention of terrorism, and research into the sociologic and psychological causes of radicalization. All mental health professionals should ensure that any research-related national security issues abide by international, ethical, and humanitarian standards, and should refrain from areas of investigation that target vulnerable individuals or groups.
The Hoffman report reminds us that everything that is legal is not necessarily ethical. It highlights the necessity of bright line standards over issues related to essential human rights and well-being. For these concerns, we should value internal challenge and dissent, and we must continually ask ourselves if we are on the right path. We must also create and protect avenues for our members who discover and report violations, even if this protection is given to the detriment of our organization. The purpose of a professional organization is to ensure the quality and integrity of its members and to protect the public from those members who fall short in either domain. As professionals, we are each responsible for ensuring that our organization carries out these duties.
Dr. Hanson is a forensic psychiatrist and coauthor of Shrink Rap: Three Psychiatrists Explain Their Work. The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
In 2005, the American Psychological Association formed a task force to address the role of psychologists in the interrogation of Central Intelligence Agency and Department of Defense detainees. The task force was formed at the request of psychologists consulting with both entities and their supervisors to address the ethics of psychologist involvement in shaping interrogation practices. The task force concluded that psychologist participation was allowed in order to ensure that the process was “safe, legal, ethical, and effective.” The Presidential Task Force on Psychological Ethics and National Security (PENS) report drew immediate objection from within the organization and triggered a member-initiated movement to rescind the findings. Members alleged that those involved in the creation of the PENS report had conflicts of interest and that the task force was heavily weighted with psychologists who were already consulting with national security organizations.
These concerns were substantiated in a book entitled Pay Any Price: Greed, Power, and Endless War (New York: Houghton Mifflin Harcourt, 2014) by New York Times reporter James Risen. In the spirit of the Watergate investigation, Risen “followed the money” as it flowed from the U.S. government to national security and defense agencies, and into the pockets of select psychologists who took active roles in harsh interrogation techniques.
Armed with this new evidence, the American Psychological Association initiated an independent investigation of the association’s role in November 2014. The results of that investigation were published recently in a 542-page report synopsized by James Risen in the New York Times. Also known as the Hoffman report, it confirmed numerous conflicts of interest between psychologists involved in the 2005 revision of the ethics guidelines, and both the C.I.A. and the D.O.D. The full implications of the Hoffman report remain to be seen, but the history and process of this problem are informative.
Both the American Medical Association and the American Psychiatric Association have position statements against participation in interrogation and torture. In 2006, the American Psychiatric Association issued a position statement in which it held that “that psychiatrists should not participate in, or otherwise assist or facilitate, the commission of torture of any person.” The American Medical Association similarly codified a prohibition against participation in interrogation in its ethical guidelines: “Physicians must oppose and must not participate in torture for any reason. Participation in torture includes, but is not limited to, providing or withholding any services, substances, or knowledge to facilitate the practice of torture. Physicians must not be present when torture is used or threatened.” Members who become aware of the practices are called upon to report them and to adhere to professional ethical standards.
But before psychiatry congratulates itself for its moral fortitude, we would do well to remember that the Department of Homeland Security’s Office of University Programs awarded $39 million to 12 academic institutions in 2011, to create “centers of excellence” related to cybersecurity, counterintelligence measures, disaster preparedness, prevention of terrorism, and research into the sociologic and psychological causes of radicalization. All mental health professionals should ensure that any research-related national security issues abide by international, ethical, and humanitarian standards, and should refrain from areas of investigation that target vulnerable individuals or groups.
The Hoffman report reminds us that everything that is legal is not necessarily ethical. It highlights the necessity of bright line standards over issues related to essential human rights and well-being. For these concerns, we should value internal challenge and dissent, and we must continually ask ourselves if we are on the right path. We must also create and protect avenues for our members who discover and report violations, even if this protection is given to the detriment of our organization. The purpose of a professional organization is to ensure the quality and integrity of its members and to protect the public from those members who fall short in either domain. As professionals, we are each responsible for ensuring that our organization carries out these duties.
Dr. Hanson is a forensic psychiatrist and coauthor of Shrink Rap: Three Psychiatrists Explain Their Work. The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Clustered Vesicles in a Blaschkoid Pattern
The Diagnosis: Linear Vesiculobullous Keratosis Follicularis (Darier Disease)
Darier disease (DD), or keratosis follicularis, is typically an autosomal-dominant disorder that is characterized by greasy hyperkeratotic papules that coalesce into warty plaques with a predilection for seborrheic areas. The lesions usually are pruritic; malodorous; and may be exacerbated by sunlight, heat, or sweating. Darier disease may be accompanied by oral mucosal involvement including fine white papules on the palate.1 The condition also can be accompanied by hand and nail involvement (95% of cases) including palmar pitting, punctate keratoses, hemorrhagic macules, palmoplantar keratoderma, and acrokeratosis verruciformis–like lesions on the dorsal aspects of the hands and feet. Nail changes predominately occur on the fingers, manifesting as longitudinal splitting, subungual hyperkeratosis, or characteristic white and red longitudinal bands with V-shaped nicks at the free margin of the nail. In linear DD, hand and nail involvement is rare and, when present, ipsilateral to the primary lesions.1,2
The clinical variants of DD are classified by lesion morphology or distribution, or both. Morphological variants include vesiculobullous, cornified, erosive, acral hemorrhagic, and guttate leukodermic macular.1,2 The clinical features of chronic relapsing vesicular lesions and histologic findings described in this case are consistent with vesiculobullous DD, though genetic testing was not performed.3,4 As in our case, some patients lack a family history and the disease is thought to be the result of genetic mosaicism or somatic postzygotic mutations that affect a limited number of cells. These mosaic variants are named by their cutaneous distribution (ie, linear, segmental, unilateral, localized) and tend to course along the Blaschko lines, most commonly on the trunk. Studies have shown various types of mutations specific to the ATP2A2 gene in the affected tissue but not in the unaffected skin.5 This gene encodes for sarcoplasmic/endoplasmic reticulum ATPase SERCA2, which is responsible for intracellular calcium signaling. These mosaic forms of DD are unlikely to be inherited by offspring, in contrast to patients with mosaic epidermal nevi with epidermolytic hyperkeratosis who have a high likelihood of having children with generalized epidermolytic hyperkeratosis.6
Darier disease is a chronic incurable disease. Topical corticosteroid, retinoid, 5-fluorouracil, keratolytics, and laser ablation or excision are used in mild and limited disease with mixed outcomes.7 Oral retinoids are effective in severe or systemic cases of DD by inhibiting hyperkeratosis.8 Individuals with DD are predisposed to infection, warranting regular surveillance and use of antimicrobials and bleach baths. In addition to prophylaxis for bacterial superinfection, patients also are predisposed to getting disseminated herpes simplex virus in the form of eczema herpeticum.
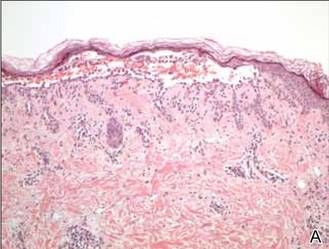
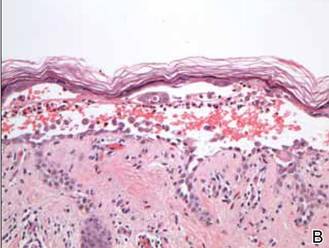
In our patient, the diagnosis was confirmed by performing a punch biopsy from one of the vesicular lesions. Histopathologic examination revealed suprabasal acantholytic dyskeratosis with superficial perivascular and interstitial inflammation with eosinophils (Figure). Immunohistochemical staining showed no evidence of varicella-zoster virus or herpes simplex virus type 1 or type 2.
Histopathology revealed suprabasal acantholysis forming an intraepidermal cleft with superficial perivascular inflammation (A)(H&E, original magnification ×100) and acantholysis with dyskeratotic keratinocytes floating within the intraepidermal cleft (B)(H&E, original magnification ×200). |
Our patient was prescribed tretinoin cream 0.1% daily and was advised to use sun protection and stop valacyclovir. At follow-up she noted decreased frequency of outbreaks after starting the tretinoin cream and the patient has now been free of any outbreaks for 8 months.
1. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
2. Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105.
3. Kakar B, Kabir S, Garg VK, et al. A case of bullous Darier’s disease histologically mimicking Hailey-Hailey disease. Dermatol Online J. 2007;13:28.
4. Telfer NR, Burge SM, Ryan TJ. Vesiculo-bullous Darier’s disease. Br J Dermatol. 1990;122:831-834.
5. Sakuntabhai A, Dhitavat J, Burge SM, et al. Mosaicism for ATP2A2 mutations causes segmental Darier’s disease. J Invest Dermatol. 2000;115:1144-1147.
6. O’Malley MP, Haake A, Goldsmith L, et al. Localized Darier disease. implications for genetic studies. Arch Dermatol. 1997;133:1134-1138.
7. Le Bidre E, Delage M, Celerier P, et al. Efficacy and risks of topical 5-fluorouracil in Darier’s disease. Ann Dermatol Venereol. 2010;137:455-459.
8. Abe M, Yasuda M, Yokoyama Y, et al. Successful treatment of combination therapy with tacalcitol lotion associated with sunscreen for localized Darier’s disease. J Dermatol. 2010;37:718-721.
The Diagnosis: Linear Vesiculobullous Keratosis Follicularis (Darier Disease)
Darier disease (DD), or keratosis follicularis, is typically an autosomal-dominant disorder that is characterized by greasy hyperkeratotic papules that coalesce into warty plaques with a predilection for seborrheic areas. The lesions usually are pruritic; malodorous; and may be exacerbated by sunlight, heat, or sweating. Darier disease may be accompanied by oral mucosal involvement including fine white papules on the palate.1 The condition also can be accompanied by hand and nail involvement (95% of cases) including palmar pitting, punctate keratoses, hemorrhagic macules, palmoplantar keratoderma, and acrokeratosis verruciformis–like lesions on the dorsal aspects of the hands and feet. Nail changes predominately occur on the fingers, manifesting as longitudinal splitting, subungual hyperkeratosis, or characteristic white and red longitudinal bands with V-shaped nicks at the free margin of the nail. In linear DD, hand and nail involvement is rare and, when present, ipsilateral to the primary lesions.1,2
The clinical variants of DD are classified by lesion morphology or distribution, or both. Morphological variants include vesiculobullous, cornified, erosive, acral hemorrhagic, and guttate leukodermic macular.1,2 The clinical features of chronic relapsing vesicular lesions and histologic findings described in this case are consistent with vesiculobullous DD, though genetic testing was not performed.3,4 As in our case, some patients lack a family history and the disease is thought to be the result of genetic mosaicism or somatic postzygotic mutations that affect a limited number of cells. These mosaic variants are named by their cutaneous distribution (ie, linear, segmental, unilateral, localized) and tend to course along the Blaschko lines, most commonly on the trunk. Studies have shown various types of mutations specific to the ATP2A2 gene in the affected tissue but not in the unaffected skin.5 This gene encodes for sarcoplasmic/endoplasmic reticulum ATPase SERCA2, which is responsible for intracellular calcium signaling. These mosaic forms of DD are unlikely to be inherited by offspring, in contrast to patients with mosaic epidermal nevi with epidermolytic hyperkeratosis who have a high likelihood of having children with generalized epidermolytic hyperkeratosis.6
Darier disease is a chronic incurable disease. Topical corticosteroid, retinoid, 5-fluorouracil, keratolytics, and laser ablation or excision are used in mild and limited disease with mixed outcomes.7 Oral retinoids are effective in severe or systemic cases of DD by inhibiting hyperkeratosis.8 Individuals with DD are predisposed to infection, warranting regular surveillance and use of antimicrobials and bleach baths. In addition to prophylaxis for bacterial superinfection, patients also are predisposed to getting disseminated herpes simplex virus in the form of eczema herpeticum.
In our patient, the diagnosis was confirmed by performing a punch biopsy from one of the vesicular lesions. Histopathologic examination revealed suprabasal acantholytic dyskeratosis with superficial perivascular and interstitial inflammation with eosinophils (Figure). Immunohistochemical staining showed no evidence of varicella-zoster virus or herpes simplex virus type 1 or type 2.
Histopathology revealed suprabasal acantholysis forming an intraepidermal cleft with superficial perivascular inflammation (A)(H&E, original magnification ×100) and acantholysis with dyskeratotic keratinocytes floating within the intraepidermal cleft (B)(H&E, original magnification ×200). |
Our patient was prescribed tretinoin cream 0.1% daily and was advised to use sun protection and stop valacyclovir. At follow-up she noted decreased frequency of outbreaks after starting the tretinoin cream and the patient has now been free of any outbreaks for 8 months.
The Diagnosis: Linear Vesiculobullous Keratosis Follicularis (Darier Disease)
Darier disease (DD), or keratosis follicularis, is typically an autosomal-dominant disorder that is characterized by greasy hyperkeratotic papules that coalesce into warty plaques with a predilection for seborrheic areas. The lesions usually are pruritic; malodorous; and may be exacerbated by sunlight, heat, or sweating. Darier disease may be accompanied by oral mucosal involvement including fine white papules on the palate.1 The condition also can be accompanied by hand and nail involvement (95% of cases) including palmar pitting, punctate keratoses, hemorrhagic macules, palmoplantar keratoderma, and acrokeratosis verruciformis–like lesions on the dorsal aspects of the hands and feet. Nail changes predominately occur on the fingers, manifesting as longitudinal splitting, subungual hyperkeratosis, or characteristic white and red longitudinal bands with V-shaped nicks at the free margin of the nail. In linear DD, hand and nail involvement is rare and, when present, ipsilateral to the primary lesions.1,2
The clinical variants of DD are classified by lesion morphology or distribution, or both. Morphological variants include vesiculobullous, cornified, erosive, acral hemorrhagic, and guttate leukodermic macular.1,2 The clinical features of chronic relapsing vesicular lesions and histologic findings described in this case are consistent with vesiculobullous DD, though genetic testing was not performed.3,4 As in our case, some patients lack a family history and the disease is thought to be the result of genetic mosaicism or somatic postzygotic mutations that affect a limited number of cells. These mosaic variants are named by their cutaneous distribution (ie, linear, segmental, unilateral, localized) and tend to course along the Blaschko lines, most commonly on the trunk. Studies have shown various types of mutations specific to the ATP2A2 gene in the affected tissue but not in the unaffected skin.5 This gene encodes for sarcoplasmic/endoplasmic reticulum ATPase SERCA2, which is responsible for intracellular calcium signaling. These mosaic forms of DD are unlikely to be inherited by offspring, in contrast to patients with mosaic epidermal nevi with epidermolytic hyperkeratosis who have a high likelihood of having children with generalized epidermolytic hyperkeratosis.6
Darier disease is a chronic incurable disease. Topical corticosteroid, retinoid, 5-fluorouracil, keratolytics, and laser ablation or excision are used in mild and limited disease with mixed outcomes.7 Oral retinoids are effective in severe or systemic cases of DD by inhibiting hyperkeratosis.8 Individuals with DD are predisposed to infection, warranting regular surveillance and use of antimicrobials and bleach baths. In addition to prophylaxis for bacterial superinfection, patients also are predisposed to getting disseminated herpes simplex virus in the form of eczema herpeticum.
In our patient, the diagnosis was confirmed by performing a punch biopsy from one of the vesicular lesions. Histopathologic examination revealed suprabasal acantholytic dyskeratosis with superficial perivascular and interstitial inflammation with eosinophils (Figure). Immunohistochemical staining showed no evidence of varicella-zoster virus or herpes simplex virus type 1 or type 2.
Histopathology revealed suprabasal acantholysis forming an intraepidermal cleft with superficial perivascular inflammation (A)(H&E, original magnification ×100) and acantholysis with dyskeratotic keratinocytes floating within the intraepidermal cleft (B)(H&E, original magnification ×200). |
Our patient was prescribed tretinoin cream 0.1% daily and was advised to use sun protection and stop valacyclovir. At follow-up she noted decreased frequency of outbreaks after starting the tretinoin cream and the patient has now been free of any outbreaks for 8 months.
1. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
2. Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105.
3. Kakar B, Kabir S, Garg VK, et al. A case of bullous Darier’s disease histologically mimicking Hailey-Hailey disease. Dermatol Online J. 2007;13:28.
4. Telfer NR, Burge SM, Ryan TJ. Vesiculo-bullous Darier’s disease. Br J Dermatol. 1990;122:831-834.
5. Sakuntabhai A, Dhitavat J, Burge SM, et al. Mosaicism for ATP2A2 mutations causes segmental Darier’s disease. J Invest Dermatol. 2000;115:1144-1147.
6. O’Malley MP, Haake A, Goldsmith L, et al. Localized Darier disease. implications for genetic studies. Arch Dermatol. 1997;133:1134-1138.
7. Le Bidre E, Delage M, Celerier P, et al. Efficacy and risks of topical 5-fluorouracil in Darier’s disease. Ann Dermatol Venereol. 2010;137:455-459.
8. Abe M, Yasuda M, Yokoyama Y, et al. Successful treatment of combination therapy with tacalcitol lotion associated with sunscreen for localized Darier’s disease. J Dermatol. 2010;37:718-721.
1. Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50.
2. Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105.
3. Kakar B, Kabir S, Garg VK, et al. A case of bullous Darier’s disease histologically mimicking Hailey-Hailey disease. Dermatol Online J. 2007;13:28.
4. Telfer NR, Burge SM, Ryan TJ. Vesiculo-bullous Darier’s disease. Br J Dermatol. 1990;122:831-834.
5. Sakuntabhai A, Dhitavat J, Burge SM, et al. Mosaicism for ATP2A2 mutations causes segmental Darier’s disease. J Invest Dermatol. 2000;115:1144-1147.
6. O’Malley MP, Haake A, Goldsmith L, et al. Localized Darier disease. implications for genetic studies. Arch Dermatol. 1997;133:1134-1138.
7. Le Bidre E, Delage M, Celerier P, et al. Efficacy and risks of topical 5-fluorouracil in Darier’s disease. Ann Dermatol Venereol. 2010;137:455-459.
8. Abe M, Yasuda M, Yokoyama Y, et al. Successful treatment of combination therapy with tacalcitol lotion associated with sunscreen for localized Darier’s disease. J Dermatol. 2010;37:718-721.
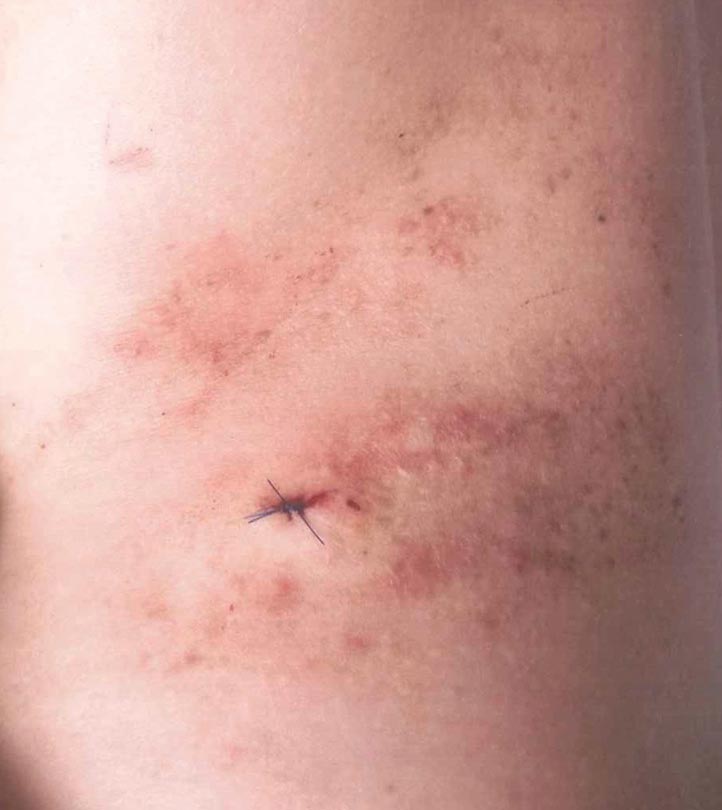
A 42-year-old woman presented with an intermittent nontender and minimally pruritic rash localized to the left side of the trunk of 20 years’ duration. Four to 6 times per year blisters would develop and then resolve after 1 to 2 weeks with mildly pruritic brown patches. These patches would resolve within approximately 4 weeks. Most notably, the condition was exacerbated by sunlight and heat, though stress sometimes led to an outbreak of vesicles. The patient reported that the eruption, which she was told was recurrent shingles, would improve with oral valacyclovir 1 g twice daily and did not improve with topical steroid usage. She never had antecedent or concurrent fevers, shortness of breath, arthralgia, or cold sores. There was no family history of any blistering skin conditions such as epidermolysis bullosa, pemphigus, Darier disease, or bullous pemphigoid. Her partner also did not have a history of similar rashes, and the patient denied any history of travel outside of England and the southwestern United States. Initial physical examination revealed clustered vesicles surrounded by brown-pink patches in a blaschkoid pattern spanning from the anterior to posterior aspects of the left flank. Notably, the patient had no oral lesions and no changes of the hair or nails.
A new way to treat ITP?

Photo courtesy of
St. Michael’s Hospital
New research appears to explain why symptoms and treatment responses vary in patients with immune thrombocytopenia (ITP). The work has also revealed a new potential treatment option.
Researchers previously thought that all ITP antibodies lead platelets to the spleen for destruction.
But the new study, published in Nature Communications, has shown that some ITP antibodies destroy platelets in the liver.
“Every existing treatment for ITP has been dedicated to stopping antibodies from destroying platelets in the spleen, but we’ve discovered that some antibodies actually destroy platelets in the liver,” said study author Heyu Ni, MD, of St. Michael’s Hospital in Toronto, Ontario, Canada.
The discovery was made by analyzing mice treated with two monoclonal antibodies, each targeting a different protein on the surface of platelets—GPIb or GPIIbIIIa.
The researchers found that antibodies targeting GPIb lead to platelet destruction in the liver, and those targeting GPIIbIIIa cause platelet destruction in the spleen.
“By detecting the specific antibodies present in someone with ITP, we may be able to detect where and how the immune system will attack,” Dr Ni said. “And because we now know the liver’s immune response destroys platelets covered with GPIb, we may be able to design new therapies to stop this type of platelet destruction.”
Dr Ni noted that sialidase inhibitors such as oseltamivir phosphate (Tamiflu) may be able to inhibit the liver’s immune response to the platelets. In fact, he and his colleagues used human blood samples to test whether Tamiflu might inhibit antibodies targeting GPIb.
“Using healthy blood samples and ITP antibodies in a test tube, we showed that Tamiflu may impede platelet destruction for those with antibodies that target GPIb,” Dr Ni said.
Based on an early abstract of this research, some ITP patients around the world have been treated with oseltamivir phosphate. These patients were extremely resistant to existing treatments targeting the spleen, and their ITP was considered life-threatening.
Although these instances of experimental treatment have been successful, Dr Ni said more research is needed to verify the safety and efficacy of this approach. ![]()

Photo courtesy of
St. Michael’s Hospital
New research appears to explain why symptoms and treatment responses vary in patients with immune thrombocytopenia (ITP). The work has also revealed a new potential treatment option.
Researchers previously thought that all ITP antibodies lead platelets to the spleen for destruction.
But the new study, published in Nature Communications, has shown that some ITP antibodies destroy platelets in the liver.
“Every existing treatment for ITP has been dedicated to stopping antibodies from destroying platelets in the spleen, but we’ve discovered that some antibodies actually destroy platelets in the liver,” said study author Heyu Ni, MD, of St. Michael’s Hospital in Toronto, Ontario, Canada.
The discovery was made by analyzing mice treated with two monoclonal antibodies, each targeting a different protein on the surface of platelets—GPIb or GPIIbIIIa.
The researchers found that antibodies targeting GPIb lead to platelet destruction in the liver, and those targeting GPIIbIIIa cause platelet destruction in the spleen.
“By detecting the specific antibodies present in someone with ITP, we may be able to detect where and how the immune system will attack,” Dr Ni said. “And because we now know the liver’s immune response destroys platelets covered with GPIb, we may be able to design new therapies to stop this type of platelet destruction.”
Dr Ni noted that sialidase inhibitors such as oseltamivir phosphate (Tamiflu) may be able to inhibit the liver’s immune response to the platelets. In fact, he and his colleagues used human blood samples to test whether Tamiflu might inhibit antibodies targeting GPIb.
“Using healthy blood samples and ITP antibodies in a test tube, we showed that Tamiflu may impede platelet destruction for those with antibodies that target GPIb,” Dr Ni said.
Based on an early abstract of this research, some ITP patients around the world have been treated with oseltamivir phosphate. These patients were extremely resistant to existing treatments targeting the spleen, and their ITP was considered life-threatening.
Although these instances of experimental treatment have been successful, Dr Ni said more research is needed to verify the safety and efficacy of this approach. ![]()

Photo courtesy of
St. Michael’s Hospital
New research appears to explain why symptoms and treatment responses vary in patients with immune thrombocytopenia (ITP). The work has also revealed a new potential treatment option.
Researchers previously thought that all ITP antibodies lead platelets to the spleen for destruction.
But the new study, published in Nature Communications, has shown that some ITP antibodies destroy platelets in the liver.
“Every existing treatment for ITP has been dedicated to stopping antibodies from destroying platelets in the spleen, but we’ve discovered that some antibodies actually destroy platelets in the liver,” said study author Heyu Ni, MD, of St. Michael’s Hospital in Toronto, Ontario, Canada.
The discovery was made by analyzing mice treated with two monoclonal antibodies, each targeting a different protein on the surface of platelets—GPIb or GPIIbIIIa.
The researchers found that antibodies targeting GPIb lead to platelet destruction in the liver, and those targeting GPIIbIIIa cause platelet destruction in the spleen.
“By detecting the specific antibodies present in someone with ITP, we may be able to detect where and how the immune system will attack,” Dr Ni said. “And because we now know the liver’s immune response destroys platelets covered with GPIb, we may be able to design new therapies to stop this type of platelet destruction.”
Dr Ni noted that sialidase inhibitors such as oseltamivir phosphate (Tamiflu) may be able to inhibit the liver’s immune response to the platelets. In fact, he and his colleagues used human blood samples to test whether Tamiflu might inhibit antibodies targeting GPIb.
“Using healthy blood samples and ITP antibodies in a test tube, we showed that Tamiflu may impede platelet destruction for those with antibodies that target GPIb,” Dr Ni said.
Based on an early abstract of this research, some ITP patients around the world have been treated with oseltamivir phosphate. These patients were extremely resistant to existing treatments targeting the spleen, and their ITP was considered life-threatening.
Although these instances of experimental treatment have been successful, Dr Ni said more research is needed to verify the safety and efficacy of this approach. ![]()
Group creates mouse model of RUNX1-mutated AML

Researchers have developed a mouse model to help them understand why patients with RUNX1-mutated acute myeloid leukemia (AML) respond poorly to chemotherapy.
Approximately 15% of AML patients harbor a mutation in the RUNX1 gene.
In these patients, anthracycline/cytarabine-based chemotherapy does not eradicate AML cells from the bone marrow.
But scientists don’t fully understand the underlying mechanisms protecting these residual cells.
Jason H. Mendler, MD, PhD, of the University of Rochester Medical Center in Rochester, New York, and his colleagues have suggested that a genetically defined mouse model of RUNX1-mutated AML is the ideal platform to investigate the cellular mechanisms protecting residual AML cells in this disease subtype.
“Like all cancers, leukemia is not a one-size-fits-all, and, therefore, it’s important to find better ways to study high-risk subtypes of the disease,” Dr Mendler said. “We believe our mouse model will allow us to quickly define new ways to target this challenging disease.”
Dr Mendler and his colleagues described their model in PLOS ONE.
The researchers began with a patient-derived cell line of RUNX1-mutated, cytogenetically normal AML. They injected these cells into NOD-SCID-γ mice and observed leukemic engraftment in the bone marrow, spleen, and peripheral blood within 6 weeks.
When the researchers treated the mice with anthracycline/cytarabine-based chemotherapy, they saw AML clearance in the spleen and peripheral blood. But leukemic cells remained in the bone marrow.
Dr Mendler and his colleagues also found their mouse model contained mutations in 5 genes aside from RUNX1—ASXL1, CEBPA, GATA2, NRAS, and SETBP1.
The team said further investigation will be focused on identifying the interplay of genes and pathways that are critical to mediating chemotherapy resistance in this model. ![]()

Researchers have developed a mouse model to help them understand why patients with RUNX1-mutated acute myeloid leukemia (AML) respond poorly to chemotherapy.
Approximately 15% of AML patients harbor a mutation in the RUNX1 gene.
In these patients, anthracycline/cytarabine-based chemotherapy does not eradicate AML cells from the bone marrow.
But scientists don’t fully understand the underlying mechanisms protecting these residual cells.
Jason H. Mendler, MD, PhD, of the University of Rochester Medical Center in Rochester, New York, and his colleagues have suggested that a genetically defined mouse model of RUNX1-mutated AML is the ideal platform to investigate the cellular mechanisms protecting residual AML cells in this disease subtype.
“Like all cancers, leukemia is not a one-size-fits-all, and, therefore, it’s important to find better ways to study high-risk subtypes of the disease,” Dr Mendler said. “We believe our mouse model will allow us to quickly define new ways to target this challenging disease.”
Dr Mendler and his colleagues described their model in PLOS ONE.
The researchers began with a patient-derived cell line of RUNX1-mutated, cytogenetically normal AML. They injected these cells into NOD-SCID-γ mice and observed leukemic engraftment in the bone marrow, spleen, and peripheral blood within 6 weeks.
When the researchers treated the mice with anthracycline/cytarabine-based chemotherapy, they saw AML clearance in the spleen and peripheral blood. But leukemic cells remained in the bone marrow.
Dr Mendler and his colleagues also found their mouse model contained mutations in 5 genes aside from RUNX1—ASXL1, CEBPA, GATA2, NRAS, and SETBP1.
The team said further investigation will be focused on identifying the interplay of genes and pathways that are critical to mediating chemotherapy resistance in this model. ![]()

Researchers have developed a mouse model to help them understand why patients with RUNX1-mutated acute myeloid leukemia (AML) respond poorly to chemotherapy.
Approximately 15% of AML patients harbor a mutation in the RUNX1 gene.
In these patients, anthracycline/cytarabine-based chemotherapy does not eradicate AML cells from the bone marrow.
But scientists don’t fully understand the underlying mechanisms protecting these residual cells.
Jason H. Mendler, MD, PhD, of the University of Rochester Medical Center in Rochester, New York, and his colleagues have suggested that a genetically defined mouse model of RUNX1-mutated AML is the ideal platform to investigate the cellular mechanisms protecting residual AML cells in this disease subtype.
“Like all cancers, leukemia is not a one-size-fits-all, and, therefore, it’s important to find better ways to study high-risk subtypes of the disease,” Dr Mendler said. “We believe our mouse model will allow us to quickly define new ways to target this challenging disease.”
Dr Mendler and his colleagues described their model in PLOS ONE.
The researchers began with a patient-derived cell line of RUNX1-mutated, cytogenetically normal AML. They injected these cells into NOD-SCID-γ mice and observed leukemic engraftment in the bone marrow, spleen, and peripheral blood within 6 weeks.
When the researchers treated the mice with anthracycline/cytarabine-based chemotherapy, they saw AML clearance in the spleen and peripheral blood. But leukemic cells remained in the bone marrow.
Dr Mendler and his colleagues also found their mouse model contained mutations in 5 genes aside from RUNX1—ASXL1, CEBPA, GATA2, NRAS, and SETBP1.
The team said further investigation will be focused on identifying the interplay of genes and pathways that are critical to mediating chemotherapy resistance in this model. ![]()
Sepsis and Septic Shock Readmission Risk
Despite its decreasing mortality, sepsis remains a leading reason for intensive care unit (ICU) admission and is associated with crude mortality in excess of 25%.[1, 2] In the United States there are between 660,000 and 750,000 sepsis hospitalizations annually, with the direct costs surpassing $24 billion.[3, 4, 5] As mortality rates have begun to fall, attention has shifted to issues of morbidity and recovery, the intermediate and longer‐term consequences associated with survivorship, and how interventions made while the patient is acutely ill in the ICU alter later health outcomes.[3, 5, 6, 7, 8]
One area of particular interest is the need for healthcare utilization following an acute admission for sepsis, and specifically rehospitalization within 30 days of discharge. This outcome is important not just from the perspective of the patient's well‐being, but also from the point of view of healthcare financing. Through the establishment of Hospital Readmission Reduction Program, the Centers for Medicare and Medicaid Services have sharply reduced reimbursement to hospitals for excessive rates of 30‐day readmissions.[9]
For sepsis, little is known about such readmissions, and even less about how to prevent them. A handful of studies suggest that this rate is between 5% and 26%.[10, 11, 12, 13] Whereas some of these studies looked at some of the factors that impact readmissions,[11, 12] none examined the potential contribution of microbiology of sepsis to this outcome.
To explore these questions, we conducted a single‐center retrospective cohort study among critically ill patients admitted to the ICU with severe culture‐positive sepsis and/or septic shock and determined the rate of early posthospital discharge readmission. In addition, we sought to elucidate predictors of subsequent readmission.
METHODS
Study Design and Ethical Standards
We conducted a single‐center retrospective cohort study from January 2008 to December 2012. The study was approved by the Washington University School of Medicine Human Studies Committee, and informed consent was waived because the data collection was retrospective without any patient‐identifying information. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Aspects of our methodology have been previously published.[14]
Primary Endpoint
All‐cause readmission to an acute‐care facility in the 30 days following discharge after the index hospitalization with sepsis served as the primary endpoint. The index hospitalizations occurred at the Barnes‐Jewish Hospital, a 1200‐bed inner‐city academic institution that serves as the main teaching institution for BJC HealthCare, a large integrated healthcare system of both inpatient and outpatient care. BJC includes a total of 13 hospitals in a compact geographic region surrounding and including St. Louis, Missouri, and we included readmission to any of these hospitals in our analysis. Persons treated within this healthcare system are, in nearly all cases, readmitted to 1 of the system's participating 13 hospitals. If a patient who receives healthcare in the system presents to an out‐of‐system hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage.
Study Cohort
All consecutive adult ICU patients were included if (1) They had a positive blood culture for a pathogen (Cultures positive only for coagulase negative Staphylococcus aureus were excluded as contaminants.), (2) there was an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code corresponding to an acute organ dysfunction,[4] and (3) they survived their index hospitalization. Only the first episode of sepsis was included as the index hospitalization.
Definitions
All‐cause 30‐day readmission, was defined as a repeat hospitalization within 30 days of discharge from the index hospitalization among survivors of culture‐positive severe sepsis or septic shock. The definition of severe sepsis was based on discharge ICD‐9‐CM codes for acute organ dysfunction.[3] Patients were classified as having septic shock if vasopressors (norepinephrine, dopamine, epinephrine, phenylephrine, or vasopressin) were initiated within 24 hours of the blood culture collection date and time.
Initially appropriate antimicrobial treatment (IAAT) was deemed appropriate if the initially prescribed antibiotic regimen was active against the identified pathogen based on in vitro susceptibility testing and administered for at least 24 hours within 24 hours following blood culture collection. All other regimens were classified as non‐IAAT. Combination antimicrobial treatment was not required for IAAT designation.[15] Prior antibiotic exposure and prior hospitalization occurred within the preceding 90 days, and prior bacteremia within 30 days of the index episode. Multidrug resistance (MDR) among Gram‐negative bacteria was defined as nonsusceptibility to at least 1 antimicrobial agent from at least 3 different antimicrobial classes.[16] Both extended spectrum ‐lactamase (ESBL) organisms and carbapenemase‐producing Enterobacteriaceae were identified via molecular testing.
Healthcare‐associated (HCA) infections were defined by the presence of at least 1 of the following: (1) recent hospitalization, (2) immune suppression (defined as any primary immune deficiency or acquired immune deficiency syndrome or exposure within 3 prior months to immunosuppressive treatmentschemotherapy, radiation therapy, or steroids), (3) nursing home residence, (4) hemodialysis, (5) prior antibiotics. and (6) index bacteremia deemed a hospital‐acquired bloodstream infection (occurring >2 days following index admission date). Acute kidney injury (AKI) was defined according to the RIFLE (Risk, Injury, Failure, Loss, End‐stage) criteria based on the greatest change in serum creatinine (SCr).[17]
Data Elements
Patient‐specific baseline characteristics and process of care variables were collected from the automated hospital medical record, microbiology database, and pharmacy database of Barnes‐Jewish Hospital. Electronic inpatient and outpatient medical records available for all patients in the BJC HealthCare system were reviewed to determine prior antibiotic exposure. The baseline characteristics collected during the index hospitalization included demographics and comorbid conditions. The comorbidities were identified based on their corresponding ICD‐9‐CM codes. The Acute Physiology and Chronic Health Evaluation (APACHE) II and Charlson comorbidity scores were calculated based on clinical data present during the 24 hours after the positive blood cultures were obtained.[18] This was done to accommodate patients with community‐acquired and healthcare‐associated community‐onset infections who only had clinical data available after blood cultures were drawn. Lowest and highest SCr levels were collected during the index hospitalization to determine each patient's AKI status.
Statistical Analyses
Continuous variables were reported as means with standard deviations and as medians with 25th and 75th percentiles. Differences between mean values were tested via the Student t test, and between medians using the Mann‐Whitney U test. Categorical data were summarized as proportions, and the 2 test or Fisher exact test for small samples was used to examine differences between groups. We developed multiple logistic regression models to identify clinical risk factors that were associated with 30‐day all‐cause readmission. All risk factors that were significant at 0.20 in the univariate analyses, as well as all biologically plausible factors even if they did not reach this level of significance, were included in the models. All variables entered into the models were assessed for collinearity, and interaction terms were tested. The most parsimonious models were derived using the backward manual elimination method, and the best‐fitting model was chosen based on the area under the receiver operating characteristics curve (AUROC or the C statistic). The model's calibration was assessed with the Hosmer‐Lemeshow goodness‐of‐fit test. All tests were 2‐tailed, and a P value <0.05 represented statistical significance.
All computations were performed in Stata/SE, version 9 (StataCorp, College Station, TX).
Role of Sponsor
The sponsor had no role in the design, analyses, interpretation, or publication of the study.
RESULTS
Among the 1697 patients with severe sepsis or septic shock who were discharged alive from the hospital, 543 (32.0%) required a rehospitalization within 30 days. There were no differences in age or gender distribution between the groups (Table 1). All comorbidities examined were more prevalent among those with a 30‐day readmission than among those without, with the median Charlson comorbidity score reflecting this imbalance (5 vs 4, P<0.001). Similarly, most of the HCA risk factors were more prevalent among the readmitted group than the comparator group, with HCA sepsis among 94.2% of the former and 90.7% of the latter (P = 0.014).
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | ||||
|---|---|---|---|---|---|
| N = 543 | % = 32.00% | N = 1,154 | % = 68.00% | P Value | |
| |||||
| Baseline characteristics | |||||
| Age, y | |||||
| Mean SD | 58.5 15.7 | 59.5 15.8 | |||
| Median (25, 75) | 60 (49, 69) | 60 (50, 70) | 0.297 | ||
| Race | |||||
| Caucasian | 335 | 61.69% | 769 | 66.64% | 0.046 |
| African American | 157 | 28.91% | 305 | 26.43% | 0.284 |
| Other | 9 | 1.66% | 22 | 1.91% | 0.721 |
| Sex, female | 244 | 44.94% | 537 | 46.53% | 0.538 |
| Admission source | |||||
| Home | 374 | 68.88% | 726 | 62.91% | 0.016 |
| Nursing home, rehab, or LTAC | 39 | 7.81% | 104 | 9.01% | 0.206 |
| Transfer from another hospital | 117 | 21.55% | 297 | 25.74% | 0.061 |
| Comorbidities | |||||
| CHF | 131 | 24.13% | 227 | 19.67% | 0.036 |
| COPD | 156 | 28.73% | 253 | 21.92% | 0.002 |
| CLD | 83 | 15.29% | 144 | 12.48% | 0.113 |
| DM | 175 | 32.23% | 296 | 25.65% | 0.005 |
| CKD | 137 | 25.23% | 199 | 17.24% | <0.001 |
| Malignancy | 225 | 41.44% | 395 | 34.23% | 0.004 |
| HIV | 11 | 2.03% | 10 | 0.87% | 0.044 |
| Charlson comorbidity score | |||||
| Mean SD | 5.24 3.32 | 4.48 3.35 | |||
| Median (25, 75) | 5 (3, 8) | 4 (2, 7) | <0.001 | ||
| HCA RF | 503 | 94.19% | 1,019 | 90.66% | 0.014 |
| Hemodialysis | 65 | 12.01% | 114 | 9.92% | 0.192 |
| Immune suppression | 193 | 36.07% | 352 | 31.21% | 0.044 |
| Prior hospitalization | 339 | 65.07% | 620 | 57.09% | 0.002 |
| Nursing home residence | 39 | 7.81% | 104 | 9.01% | 0.206 |
| Prior antibiotics | 301 | 55.43% | 568 | 49.22% | 0.017 |
| Hospital‐acquired BSI* | 240 | 44.20% | 485 | 42.03% | 0.399 |
| Prior bacteremia within 30 days | 88 | 16.21% | 154 | 13.34% | 0.116 |
| Sepsis‐related parameters | |||||
| LOS prior to bacteremia, d | |||||
| Mean SD | 6.65 11.22 | 5.88 10.81 | |||
| Median (25, 75) | 1 (0, 10) | 0 (0, 8) | 0.250 | ||
| Surgery | |||||
| None | 362 | 66.67% | 836 | 72.44% | 0.015 |
| Abdominal | 104 | 19.15% | 167 | 14.47% | 0.014 |
| Extra‐abdominal | 73 | 13.44% | 135 | 11.70% | 0.306 |
| Status unknown | 4 | 0.74% | 16 | 1.39% | 0.247 |
| Central line | 333 | 64.41% | 637 | 57.80% | 0.011 |
| TPN at the time of bacteremia or prior to it during index hospitalization | 52 | 9.74% | 74 | 5.45% | 0.017 |
| APACHE II | |||||
| Mean SD | 15.08 5.47 | 15.35 5.43 | |||
| Median (25, 75) | 15 (11, 18) | 15 (12, 19) | 0.275 | ||
| Severe sepsis | 361 | 66.48% | 747 | 64.73% | 0.480 |
| Septic shock requiring vasopressors | 182 | 33.52% | 407 | 35.27% | |
| On MV | 104 | 19.22% | 251 | 21.90% | 0.208 |
| Peak WBC (103/L) | |||||
| Mean SD | 22.26 25.20 | 22.14 17.99 | |||
| Median (25, 75) | 17.1 (8.9, 30.6) | 16.9 (10, 31) | 0.654 | ||
| Lowest serum SCr, mg/dL | |||||
| Mean SD | 1.02 1.05 | 0.96 1.03 | |||
| Median (25, 75) | 0.68 (0.5, 1.06) | 0.66 (0.49, 0.96) | 0.006 | ||
| Highest serum SCr, mg/dL | |||||
| Mean SD | 2.81 2.79 | 2.46 2.67 | |||
| Median (25, 75) | 1.68 (1.04, 3.3) | 1.41 (0.94, 2.61) | 0.001 | ||
| RIFLE category | |||||
| None | 81 | 14.92% | 213 | 18.46% | 0.073 |
| Risk | 112 | 20.63% | 306 | 26.52% | 0.009 |
| Injury | 133 | 24.49% | 247 | 21.40% | 0.154 |
| Failure | 120 | 22.10% | 212 | 18.37% | 0.071 |
| Loss | 50 | 9.21% | 91 | 7.89% | 0.357 |
| End‐stage | 47 | 8.66% | 85 | 7.37% | 0.355 |
| Infection source | |||||
| Urine | 95 | 17.50% | 258 | 22.36% | 0.021 |
| Abdomen | 69 | 12.71% | 113 | 9.79% | 0.070 |
| Lung | 93 | 17.13% | 232 | 20.10% | 0.146 |
| Line | 91 | 16.76% | 150 | 13.00% | 0.038 |
| CNS | 1 | 0.18% | 16 | 1.39% | 0.012 |
| Skin | 51 | 9.39% | 82 | 7.11% | 0.102 |
| Unknown | 173 | 31.86% | 375 | 32.50% | 0.794 |
During the index hospitalization, 589 patients (34.7%) suffered from septic shock requiring vasopressors; this did not impact the 30‐day readmission risk (Table 1). Commensurately, markers of severity of acute illness (APACHE II score, mechanical ventilation, peak white blood cell count) did not differ between the groups. With respect to the primary source of sepsis, urine was less, whereas central nervous system was more likely among those readmitted within 30 days. Similarly, there was a significant imbalance between the groups in the prevalence of AKI (Table 1). Specifically, those who did require a readmission were slightly less likely to have sustained no AKI (RIFLE: None; 14.9% vs 18.5%, P = 0.073). Those requiring readmission were also less likely to be in the category RIFLE: Risk (20.6% vs 26.5%, P = 0.009). The direction of this disparity was reversed for the Injury and Failure categories. No differences between groups were seen among those with categories Loss and end‐stage kidney disease (ESKD) (Table 1).
The microbiology of sepsis did not differ in most respects between the 30‐day readmission groups, save for several organisms (Table 2). Most strikingly, those who required a readmission were more likely than those who did not to be infected with Bacteroides spp, Candida spp, an MDR or an ESBL organism (Table 2). As for the outcomes of the index hospitalization, those with a repeat admission had a longer overall and postonset of sepsis initial hospital length of stay, and were less likely to be discharged either home without home health care or transferred to another hospital at the end of their index hospitalization (Table 3).
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | P Value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| |||||
| 543 | 32.00% | 1,154 | 68.00% | ||
| Gram‐positive BSI | 260 | 47.88% | 580 | 50.26% | 0.376 |
| Staphylococcus aureus | 138 | 25.41% | 287 | 24.87% | 0.810 |
| MRSA | 78 | 14.36% | 147 | 12.74% | 0.358 |
| VISA | 6 | 1.10% | 9 | 0.78% | 0.580 |
| Streptococcus pneumoniae | 7 | 1.29% | 33 | 2.86% | 0.058 |
| Streptococcus spp | 34 | 6.26% | 81 | 7.02% | 0.606 |
| Peptostreptococcus spp | 5 | 0.92% | 15 | 1.30% | 0.633 |
| Clostridium perfringens | 4 | 0.74% | 10 | 0.87% | 1.000 |
| Enterococcus faecalis | 54 | 9.94% | 108 | 9.36% | 0.732 |
| Enterococcus faecium | 29 | 5.34% | 63 | 5.46% | 1.000 |
| VRE | 36 | 6.63% | 70 | 6.07% | 0.668 |
| Gram‐negative BSI | 231 | 42.54% | 515 | 44.63% | 0.419 |
| Escherichia coli | 54 | 9.94% | 151 | 13.08% | 0.067 |
| Klebsiella pneumoniae | 54 | 9.94% | 108 | 9.36% | 0.723 |
| Klebsiella oxytoca | 11 | 2.03% | 18 | 1.56% | 0.548 |
| Enterobacter aerogenes | 6 | 1.10% | 13 | 1.13% | 1.000 |
| Enterobacter cloacae | 21 | 3.87% | 44 | 3.81% | 1.000 |
| Pseudomonas aeruginosa | 28 | 5.16% | 65 | 5.63% | 0.733 |
| Acinetobacter spp | 8 | 1.47% | 27 | 2.34% | 0.276 |
| Bacteroides spp | 25 | 4.60% | 30 | 2.60% | 0.039 |
| Serratia marcescens | 14 | 2.58% | 21 | 1.82% | 0.360 |
| Stenotrophomonas maltophilia | 3 | 0.55% | 8 | 0.69% | 1.000 |
| Achromobacter spp | 2 | 0.37% | 3 | 0.17% | 0.597 |
| Aeromonas spp | 2 | 0.37% | 1 | 0.09% | 0.241 |
| Burkholderia cepacia | 0 | 0.00% | 6 | 0.52% | 0.186 |
| Citrobacter freundii | 2 | 0.37% | 15 | 1.39% | 0.073 |
| Fusobacterium spp | 7 | 1.29% | 10 | 0.87% | 0.438 |
| Haemophilus influenzae | 1 | 0.18% | 4 | 0.35% | 1.000 |
| Prevotella spp | 1 | 0.18% | 6 | 0.52% | 0.441 |
| Proteus mirabilis | 9 | 1.66% | 39 | 3.38% | 0.058 |
| MDR PA | 2 | 0.37% | 7 | 0.61% | 0.727 |
| ESBL | 10 | 6.25% | 8 | 2.06% | 0.017 |
| CRE | 2 | 1.25% | 0 | 0.00% | 0.028 |
| MDR Gram‐negative or Gram‐positive | 231 | 47.53% | 450 | 41.86% | 0.036 |
| Candida spp | 58 | 10.68% | 76 | 6.59% | 0.004 |
| Polymicrobal BSI | 50 | 9.21% | 111 | 9.62% | 0.788 |
| Initially inappropriate treatment | 119 | 21.92% | 207 | 17.94% | 0.052 |
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | ||||
|---|---|---|---|---|---|
| N = 543 | % = 32.00% | N = 1,154 | % = 68.00% | P Value | |
| |||||
| Hospital LOS, days | |||||
| Mean SD | 26.44 23.27 | 23.58 21.79 | 0.019 | ||
| Median (25, 75) | 19.16 (9.66, 35.86) | 17.77 (8.9, 30.69) | |||
| Hospital LOS following BSI onset, days | |||||
| Mean SD | 19.80 18.54 | 17.69 17.08 | 0.022 | ||
| Median (25, 75) | 13.9 (7.9, 25.39) | 12.66 (7.05, 22.66) | |||
| Discharge destination | |||||
| Home | 125 | 23.02% | 334 | 28.94% | 0.010 |
| Home with home care | 163 | 30.02% | 303 | 26.26% | 0.105 |
| Rehab | 81 | 14.92% | 149 | 12.91% | 0.260 |
| LTAC | 41 | 7.55% | 87 | 7.54% | 0.993 |
| Transfer to another hospital | 1 | 0.18% | 19 | 1.65% | 0.007 |
| SNF | 132 | 24.31% | 262 | 22.70% | 0.465 |
In a logistic regression model, 5 factors emerged as predictors of 30‐day readmission (Table 4). Having RIFLE: Injury or RIFLE: Failure carried an approximately 2‐fold increase in the odds of 30‐day rehospitalization (odds ratio: 1.95, 95% confidence interval: 1.302.93, P = 0.001) relative to having a RIFLE: None or RIFLE: Risk. Although having strong association with this outcome, harboring an ESBL organism or Bacteroides spp were both relatively infrequent events (3.3% ESBL and 3.2% Bacteroides spp). Infection with Escherichia coli and urine as the source of sepsis both appeared to be significantly protective against a readmission (Table 4). The model's discrimination was moderate (AUROC = 0.653) and its calibration adequate (Hosmer‐Lemeshow P = 0.907). (See Supporting Information, Appendix 1, in the online version of this article for the steps in the development of the final model.)
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| ESBL | 4.503 | 1.42914.190 | 0.010 |
| RIFLE: Injury or Failure (reference: RIFLE: None or Risk) | 1.951 | 1.2972.933 | 0.001 |
| Bacteroides spp | 2.044 | 1.0583.948 | 0.033 |
| Source: urine | 0.583 | 0.3470.979 | 0.041 |
| Escherichia coli | 0.494 | 0.2700.904 | 0.022 |
DISCUSSION
In this single‐center retrospective cohort study, nearly one‐third of survivors of culture‐positive severe sepsis or septic shock required a rehospitalization within 30 days of discharge from their index admission. Factors that contributed to a higher odds of rehospitalization were having mild‐to‐moderate AKI (RIFLE: Injury or RIFLE: Failure) and infection with ESBL organisms or Bacteroides spp, whereas urine as the source of sepsis and E coli as the pathogen appeared to be protective.
A recent study by Hua and colleagues examining the New York Statewide Planning and Research Cooperative System for the years 2008 to 2010 noted a 16.2% overall rate of 30‐day rehospitalization among survivors of initial critical illness.[11] Just as we observed, Hua et al. concluded that development of AKI correlated with readmission. Because they relied on administrative data for their analysis, AKI was diagnosed when hemodialysis was utilized. Examining AKI using SCr changes, our findings add a layer of granularity to the relationship between AKI stages and early readmission. Specifically, we failed to detect any rise in the odds of rehospitalization when either very mild (RIFLE: Risk) or severe (RIFLE: Loss or RIFLE: ESKD) AKI was present. Only when either RIFLE: Injury or RIFLE: Failure developed did the odds of readmission rise. In addition to diverging definitions between our studies, differences in populations also likely yielded different results.[11] Although Hua et al. examined all admissions to the ICU regardless of the diagnosis or illness severity, our cohort consisted of only those ICU patients who survived culture‐positive severe sepsis/septic shock. Because AKI is a known risk factor for mortality in sepsis,[19] the potential for immortal time bias leaves a smaller pool of surviving patients with ESKD at risk for readmission. Regardless of the explanation, it may be prudent to focus on preventing AKI not only to improve survival, but also from the standpoint of diminishing the risk of an early readmission.
Four additional studies have examined the frequency of early readmissions among survivors of critical illness. Liu et al. noted 17.9% 30‐day rehospitalization rate among sepsis survivors.[12] Factors associated with the risk of early readmission included acute and chronic diseases burdens, index hospital LOS, and the need for the ICU in the index sepsis admission. In contrast to our cohort, all of whom were in the ICU during their index episode, less than two‐thirds of the entire population studied by Liu had required an ICU admission. Additionally, Liu's study did not specifically examine the potential impact of AKI or of microbiology on this outcome.
Prescott and coworkers examined healthcare utilization following an episode of severe sepsis.[13] Among other findings, they reported a 30‐day readmission rate of 26.5% among survivors. Although closer to our estimate, this study included all patients surviving a severe sepsis hospitalization, and not only those with a positive culture. These investigators did not examine predictors of readmission.[13]
Horkan et al. examined specifically whether there was an association between AKI and postdischarge outcomes, including 30‐day readmission risk, in a large cohort of patients who survived their critical illness.[20] In it they found that readmission risk ranged from 19% to 21%, depending on the extent of the AKI. Moreover, similar to our findings, they reported that in an adjusted analysis RIFLE: Injury and RIFLE: Failure were associated with a rise in the odds of a 30‐day rehospitalizaiton. In contrast to our study, Horkan et al. did detect an increase in the odds of this outcome associated with RIFLE: Risk. There are likely at least 3 reasons for this difference. First, we focused only on patients with severe sepsis or septic shock, whereas Horkan and colleagues included all critical illness survivors. Second, we were able to explore the impact of microbiology on this outcome. Third, Horkan's study included an order of magnitude more patients than did ours, thus making it more likely either to have the power to detect a true association that we may have lacked or to be more susceptible to type I error.
Finally, Goodwin and colleagues utilized 3 states' databases included in the Health Care and Utilization Project (HCUP) from the Agency for Healthcare Research and Quality to study frequency and risk factors for 30‐day readmission among survivors of severe sepsis.[21] Patients were identified based on the use of the severe sepsis (995.92) and septic shock (785.52). These authors found a 30‐day readmission rate of 26%. Although chronic renal disease, among several other factors, was associated with an increase in this risk, the data source did not permit these investigators to examine the impact of AKI on the outcomes. Similarly, HCUP data do not contain microbiology, a distinct difference from our analysis.
If clinicians are to pursue strategies to reduce the risk of an all‐cause 30‐day readmission, the key goal is not simply to identify all variables associated with readmission, but to focus on factors that are potentially modifiable. Although neither Hua nor Liu and their teams identified any additional factors that are potentially modifiable,[11, 12] in the present study, among the 5 factors we identified, the development of mild to moderate AKI during the index hospitalization may deserve stronger consideration for efforts at prevention. Although one cannot conclude automatically that preventing AKI in this population could mitigate some of the early rehospitalization risk, critically ill patients are frequently exposed to a multitude of nephrotoxic agents. Those caring for subjects with sepsis should reevaluate the risk‐benefit equation of these factors more cautiously and apply guideline‐recommended AKI prevention strategies more aggressively, particularly because a relatively minor change in SCr resulted in an excess risk of readmission.[22]
In addition to AKI, which is potentially modifiable, we identified several other clinical factors predictive of 30‐day readmission, which are admittedly not preventable. Thus, microbiology was predictive of this outcome, with E coli engendering fewer and Bacteroides spp and ESBL organisms more early rehospitalizations. Similarly, urine as the source of sepsis was associated with a lower risk for this endpoint.
Our study has a number of limitations. As a retrospective cohort, it is subject to bias, most notably a selection bias. Specifically, because the flagship hospital of the BJC HealthCare system is a referral center, it is possible that we did not capture all readmissions. However, generally, if a patient who receives healthcare within 1 of the BJC hospitals presents to a nonsystem hospital, that patient is nearly always transferred back into the integrated system because of issues of insurance coverage. Analysis of certain diagnosis‐related groups has indicated that 73% of all patients overall discharged from 4 of the large BJC system institutions who require a readmission within 30 days of discharge return to a BJC hospital (personal communication, Financial Analysis and Decision Support Department at BJC to Dr. Kollef May 12, 2015). Therefore, we may have misclassified the outcome in as many as 180 patients. The fact that our readmission rate was fully double that seen in Hua et al.'s and Liu et al.'s studies, and somewhat higher than that reported by Prescott et al., attests not only to the population differences, but also to the fact that we are unlikely to have missed a substantial percentage of readmissions.[11, 12, 13] Furthermore, to mitigate biases, we enrolled all consecutive patients meeting the predetermined criteria. Missing from our analysis are events that occurred between the index discharge and the readmission. Likewise, we were unable to obtain such potentially important variables as code status or outpatient mortality following discharge. These intervening factors, if included in subsequent studies, may increase the predictive power of the model. Because we relied on administrative coding to identify cases of severe sepsis and septic shock, it is possible that there is misclassification within our cohort. Recent studies indicate, however, that the Angus definition, used in our study, has high negative and positive predictive values for severe sepsis identification.[23] It is still possible that our cohort is skewed toward a more severely ill population, making our results less generalizable to the less severely ill septic patients.[24] The study was performed at a single healthcare system and included only cases of severe sepsis or septic shock that had a positive blood culture, and thus the findings may not be broadly generalizable either to patients without a positive blood culture or to institutions that do not resemble it.
In summary, we have demonstrated that survivors of culture‐positive severe sepsis or septic shock have a high rate of 30‐day rehospitalization. Because the US federal government's initiatives deem 30‐day readmissions to be a quality metric and penalize institutions with higher‐than average readmission rates, a high volume of critically ill patients with culture‐positive severe sepsis and septic shock may disproportionately put an institution at risk for such penalties. Unfortunately, not many of the determinants of readmission are amenable to prevention. As sepsis survival continues to improve, hospitals will need to concentrate their resources on coordinating care of these complex patients so as to improve both individual quality of life and the quality of care that they provide.
Disclosures
This study was supported by a research grant from Cubist Pharmaceuticals, Lexington, Massachusetts. Dr. Kollef's time was in part supported by the Barnes‐Jewish Hospital Foundation. The authors report no conflicts of interest.
- , , , et al; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353
- , , , et al. Death in the United States, 2007. NCHS Data Brief. 2009;26:1–8.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1548–1564.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310.
- , , , et al: Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalization. Crit Care Med. 2005;33:2555–2562.
- , , , et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250.
- , , , , . Two decades of mortality trends among patients with severe sepsis: a comparative meta‐analysis. Crit Care Med. 2014;42:625–631.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107.
- , . Trends in septicemia hospitalizations and readmissions in selected HCUP states, 2005 and 2010. HCUP Statistical Brief #161. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb161.pdf. Published September 2013, Accessed January 13, 2015.
- , , , . Early and late unplanned rehospitalizations for survivors of critical illness. Crit Care Med. 2015;43:430–438.
- , , , , , . Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9:502–507.
- , , , , . Increased 1‐year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190:62–69.
- , , , , . Multi‐drug resistance, inappropriate initial antibiotic therapy and mortality in Gram‐negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596.
- , , . Does combination antimicrobial therapy reduce mortality in Gram‐negative bacteraemia? A meta‐analysis. Lancet Infect Dis. 2004;4:519–527.
- , , , et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281.
- , , , , ; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- , , , et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73
- , , , , , . The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study. Crit Care Med. 2015;43:354–364.
- , , , . Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43:738–746.
- Acute Kidney Injury Work Group. Kidney disease: improving global outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf. Accessed March 4, 2015.
- , , , , , . Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19(1):139.
- , , , , , . Severe sepsis cohorts derived from claims‐based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41:945–953.
Despite its decreasing mortality, sepsis remains a leading reason for intensive care unit (ICU) admission and is associated with crude mortality in excess of 25%.[1, 2] In the United States there are between 660,000 and 750,000 sepsis hospitalizations annually, with the direct costs surpassing $24 billion.[3, 4, 5] As mortality rates have begun to fall, attention has shifted to issues of morbidity and recovery, the intermediate and longer‐term consequences associated with survivorship, and how interventions made while the patient is acutely ill in the ICU alter later health outcomes.[3, 5, 6, 7, 8]
One area of particular interest is the need for healthcare utilization following an acute admission for sepsis, and specifically rehospitalization within 30 days of discharge. This outcome is important not just from the perspective of the patient's well‐being, but also from the point of view of healthcare financing. Through the establishment of Hospital Readmission Reduction Program, the Centers for Medicare and Medicaid Services have sharply reduced reimbursement to hospitals for excessive rates of 30‐day readmissions.[9]
For sepsis, little is known about such readmissions, and even less about how to prevent them. A handful of studies suggest that this rate is between 5% and 26%.[10, 11, 12, 13] Whereas some of these studies looked at some of the factors that impact readmissions,[11, 12] none examined the potential contribution of microbiology of sepsis to this outcome.
To explore these questions, we conducted a single‐center retrospective cohort study among critically ill patients admitted to the ICU with severe culture‐positive sepsis and/or septic shock and determined the rate of early posthospital discharge readmission. In addition, we sought to elucidate predictors of subsequent readmission.
METHODS
Study Design and Ethical Standards
We conducted a single‐center retrospective cohort study from January 2008 to December 2012. The study was approved by the Washington University School of Medicine Human Studies Committee, and informed consent was waived because the data collection was retrospective without any patient‐identifying information. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Aspects of our methodology have been previously published.[14]
Primary Endpoint
All‐cause readmission to an acute‐care facility in the 30 days following discharge after the index hospitalization with sepsis served as the primary endpoint. The index hospitalizations occurred at the Barnes‐Jewish Hospital, a 1200‐bed inner‐city academic institution that serves as the main teaching institution for BJC HealthCare, a large integrated healthcare system of both inpatient and outpatient care. BJC includes a total of 13 hospitals in a compact geographic region surrounding and including St. Louis, Missouri, and we included readmission to any of these hospitals in our analysis. Persons treated within this healthcare system are, in nearly all cases, readmitted to 1 of the system's participating 13 hospitals. If a patient who receives healthcare in the system presents to an out‐of‐system hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage.
Study Cohort
All consecutive adult ICU patients were included if (1) They had a positive blood culture for a pathogen (Cultures positive only for coagulase negative Staphylococcus aureus were excluded as contaminants.), (2) there was an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code corresponding to an acute organ dysfunction,[4] and (3) they survived their index hospitalization. Only the first episode of sepsis was included as the index hospitalization.
Definitions
All‐cause 30‐day readmission, was defined as a repeat hospitalization within 30 days of discharge from the index hospitalization among survivors of culture‐positive severe sepsis or septic shock. The definition of severe sepsis was based on discharge ICD‐9‐CM codes for acute organ dysfunction.[3] Patients were classified as having septic shock if vasopressors (norepinephrine, dopamine, epinephrine, phenylephrine, or vasopressin) were initiated within 24 hours of the blood culture collection date and time.
Initially appropriate antimicrobial treatment (IAAT) was deemed appropriate if the initially prescribed antibiotic regimen was active against the identified pathogen based on in vitro susceptibility testing and administered for at least 24 hours within 24 hours following blood culture collection. All other regimens were classified as non‐IAAT. Combination antimicrobial treatment was not required for IAAT designation.[15] Prior antibiotic exposure and prior hospitalization occurred within the preceding 90 days, and prior bacteremia within 30 days of the index episode. Multidrug resistance (MDR) among Gram‐negative bacteria was defined as nonsusceptibility to at least 1 antimicrobial agent from at least 3 different antimicrobial classes.[16] Both extended spectrum ‐lactamase (ESBL) organisms and carbapenemase‐producing Enterobacteriaceae were identified via molecular testing.
Healthcare‐associated (HCA) infections were defined by the presence of at least 1 of the following: (1) recent hospitalization, (2) immune suppression (defined as any primary immune deficiency or acquired immune deficiency syndrome or exposure within 3 prior months to immunosuppressive treatmentschemotherapy, radiation therapy, or steroids), (3) nursing home residence, (4) hemodialysis, (5) prior antibiotics. and (6) index bacteremia deemed a hospital‐acquired bloodstream infection (occurring >2 days following index admission date). Acute kidney injury (AKI) was defined according to the RIFLE (Risk, Injury, Failure, Loss, End‐stage) criteria based on the greatest change in serum creatinine (SCr).[17]
Data Elements
Patient‐specific baseline characteristics and process of care variables were collected from the automated hospital medical record, microbiology database, and pharmacy database of Barnes‐Jewish Hospital. Electronic inpatient and outpatient medical records available for all patients in the BJC HealthCare system were reviewed to determine prior antibiotic exposure. The baseline characteristics collected during the index hospitalization included demographics and comorbid conditions. The comorbidities were identified based on their corresponding ICD‐9‐CM codes. The Acute Physiology and Chronic Health Evaluation (APACHE) II and Charlson comorbidity scores were calculated based on clinical data present during the 24 hours after the positive blood cultures were obtained.[18] This was done to accommodate patients with community‐acquired and healthcare‐associated community‐onset infections who only had clinical data available after blood cultures were drawn. Lowest and highest SCr levels were collected during the index hospitalization to determine each patient's AKI status.
Statistical Analyses
Continuous variables were reported as means with standard deviations and as medians with 25th and 75th percentiles. Differences between mean values were tested via the Student t test, and between medians using the Mann‐Whitney U test. Categorical data were summarized as proportions, and the 2 test or Fisher exact test for small samples was used to examine differences between groups. We developed multiple logistic regression models to identify clinical risk factors that were associated with 30‐day all‐cause readmission. All risk factors that were significant at 0.20 in the univariate analyses, as well as all biologically plausible factors even if they did not reach this level of significance, were included in the models. All variables entered into the models were assessed for collinearity, and interaction terms were tested. The most parsimonious models were derived using the backward manual elimination method, and the best‐fitting model was chosen based on the area under the receiver operating characteristics curve (AUROC or the C statistic). The model's calibration was assessed with the Hosmer‐Lemeshow goodness‐of‐fit test. All tests were 2‐tailed, and a P value <0.05 represented statistical significance.
All computations were performed in Stata/SE, version 9 (StataCorp, College Station, TX).
Role of Sponsor
The sponsor had no role in the design, analyses, interpretation, or publication of the study.
RESULTS
Among the 1697 patients with severe sepsis or septic shock who were discharged alive from the hospital, 543 (32.0%) required a rehospitalization within 30 days. There were no differences in age or gender distribution between the groups (Table 1). All comorbidities examined were more prevalent among those with a 30‐day readmission than among those without, with the median Charlson comorbidity score reflecting this imbalance (5 vs 4, P<0.001). Similarly, most of the HCA risk factors were more prevalent among the readmitted group than the comparator group, with HCA sepsis among 94.2% of the former and 90.7% of the latter (P = 0.014).
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | ||||
|---|---|---|---|---|---|
| N = 543 | % = 32.00% | N = 1,154 | % = 68.00% | P Value | |
| |||||
| Baseline characteristics | |||||
| Age, y | |||||
| Mean SD | 58.5 15.7 | 59.5 15.8 | |||
| Median (25, 75) | 60 (49, 69) | 60 (50, 70) | 0.297 | ||
| Race | |||||
| Caucasian | 335 | 61.69% | 769 | 66.64% | 0.046 |
| African American | 157 | 28.91% | 305 | 26.43% | 0.284 |
| Other | 9 | 1.66% | 22 | 1.91% | 0.721 |
| Sex, female | 244 | 44.94% | 537 | 46.53% | 0.538 |
| Admission source | |||||
| Home | 374 | 68.88% | 726 | 62.91% | 0.016 |
| Nursing home, rehab, or LTAC | 39 | 7.81% | 104 | 9.01% | 0.206 |
| Transfer from another hospital | 117 | 21.55% | 297 | 25.74% | 0.061 |
| Comorbidities | |||||
| CHF | 131 | 24.13% | 227 | 19.67% | 0.036 |
| COPD | 156 | 28.73% | 253 | 21.92% | 0.002 |
| CLD | 83 | 15.29% | 144 | 12.48% | 0.113 |
| DM | 175 | 32.23% | 296 | 25.65% | 0.005 |
| CKD | 137 | 25.23% | 199 | 17.24% | <0.001 |
| Malignancy | 225 | 41.44% | 395 | 34.23% | 0.004 |
| HIV | 11 | 2.03% | 10 | 0.87% | 0.044 |
| Charlson comorbidity score | |||||
| Mean SD | 5.24 3.32 | 4.48 3.35 | |||
| Median (25, 75) | 5 (3, 8) | 4 (2, 7) | <0.001 | ||
| HCA RF | 503 | 94.19% | 1,019 | 90.66% | 0.014 |
| Hemodialysis | 65 | 12.01% | 114 | 9.92% | 0.192 |
| Immune suppression | 193 | 36.07% | 352 | 31.21% | 0.044 |
| Prior hospitalization | 339 | 65.07% | 620 | 57.09% | 0.002 |
| Nursing home residence | 39 | 7.81% | 104 | 9.01% | 0.206 |
| Prior antibiotics | 301 | 55.43% | 568 | 49.22% | 0.017 |
| Hospital‐acquired BSI* | 240 | 44.20% | 485 | 42.03% | 0.399 |
| Prior bacteremia within 30 days | 88 | 16.21% | 154 | 13.34% | 0.116 |
| Sepsis‐related parameters | |||||
| LOS prior to bacteremia, d | |||||
| Mean SD | 6.65 11.22 | 5.88 10.81 | |||
| Median (25, 75) | 1 (0, 10) | 0 (0, 8) | 0.250 | ||
| Surgery | |||||
| None | 362 | 66.67% | 836 | 72.44% | 0.015 |
| Abdominal | 104 | 19.15% | 167 | 14.47% | 0.014 |
| Extra‐abdominal | 73 | 13.44% | 135 | 11.70% | 0.306 |
| Status unknown | 4 | 0.74% | 16 | 1.39% | 0.247 |
| Central line | 333 | 64.41% | 637 | 57.80% | 0.011 |
| TPN at the time of bacteremia or prior to it during index hospitalization | 52 | 9.74% | 74 | 5.45% | 0.017 |
| APACHE II | |||||
| Mean SD | 15.08 5.47 | 15.35 5.43 | |||
| Median (25, 75) | 15 (11, 18) | 15 (12, 19) | 0.275 | ||
| Severe sepsis | 361 | 66.48% | 747 | 64.73% | 0.480 |
| Septic shock requiring vasopressors | 182 | 33.52% | 407 | 35.27% | |
| On MV | 104 | 19.22% | 251 | 21.90% | 0.208 |
| Peak WBC (103/L) | |||||
| Mean SD | 22.26 25.20 | 22.14 17.99 | |||
| Median (25, 75) | 17.1 (8.9, 30.6) | 16.9 (10, 31) | 0.654 | ||
| Lowest serum SCr, mg/dL | |||||
| Mean SD | 1.02 1.05 | 0.96 1.03 | |||
| Median (25, 75) | 0.68 (0.5, 1.06) | 0.66 (0.49, 0.96) | 0.006 | ||
| Highest serum SCr, mg/dL | |||||
| Mean SD | 2.81 2.79 | 2.46 2.67 | |||
| Median (25, 75) | 1.68 (1.04, 3.3) | 1.41 (0.94, 2.61) | 0.001 | ||
| RIFLE category | |||||
| None | 81 | 14.92% | 213 | 18.46% | 0.073 |
| Risk | 112 | 20.63% | 306 | 26.52% | 0.009 |
| Injury | 133 | 24.49% | 247 | 21.40% | 0.154 |
| Failure | 120 | 22.10% | 212 | 18.37% | 0.071 |
| Loss | 50 | 9.21% | 91 | 7.89% | 0.357 |
| End‐stage | 47 | 8.66% | 85 | 7.37% | 0.355 |
| Infection source | |||||
| Urine | 95 | 17.50% | 258 | 22.36% | 0.021 |
| Abdomen | 69 | 12.71% | 113 | 9.79% | 0.070 |
| Lung | 93 | 17.13% | 232 | 20.10% | 0.146 |
| Line | 91 | 16.76% | 150 | 13.00% | 0.038 |
| CNS | 1 | 0.18% | 16 | 1.39% | 0.012 |
| Skin | 51 | 9.39% | 82 | 7.11% | 0.102 |
| Unknown | 173 | 31.86% | 375 | 32.50% | 0.794 |
During the index hospitalization, 589 patients (34.7%) suffered from septic shock requiring vasopressors; this did not impact the 30‐day readmission risk (Table 1). Commensurately, markers of severity of acute illness (APACHE II score, mechanical ventilation, peak white blood cell count) did not differ between the groups. With respect to the primary source of sepsis, urine was less, whereas central nervous system was more likely among those readmitted within 30 days. Similarly, there was a significant imbalance between the groups in the prevalence of AKI (Table 1). Specifically, those who did require a readmission were slightly less likely to have sustained no AKI (RIFLE: None; 14.9% vs 18.5%, P = 0.073). Those requiring readmission were also less likely to be in the category RIFLE: Risk (20.6% vs 26.5%, P = 0.009). The direction of this disparity was reversed for the Injury and Failure categories. No differences between groups were seen among those with categories Loss and end‐stage kidney disease (ESKD) (Table 1).
The microbiology of sepsis did not differ in most respects between the 30‐day readmission groups, save for several organisms (Table 2). Most strikingly, those who required a readmission were more likely than those who did not to be infected with Bacteroides spp, Candida spp, an MDR or an ESBL organism (Table 2). As for the outcomes of the index hospitalization, those with a repeat admission had a longer overall and postonset of sepsis initial hospital length of stay, and were less likely to be discharged either home without home health care or transferred to another hospital at the end of their index hospitalization (Table 3).
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | P Value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| |||||
| 543 | 32.00% | 1,154 | 68.00% | ||
| Gram‐positive BSI | 260 | 47.88% | 580 | 50.26% | 0.376 |
| Staphylococcus aureus | 138 | 25.41% | 287 | 24.87% | 0.810 |
| MRSA | 78 | 14.36% | 147 | 12.74% | 0.358 |
| VISA | 6 | 1.10% | 9 | 0.78% | 0.580 |
| Streptococcus pneumoniae | 7 | 1.29% | 33 | 2.86% | 0.058 |
| Streptococcus spp | 34 | 6.26% | 81 | 7.02% | 0.606 |
| Peptostreptococcus spp | 5 | 0.92% | 15 | 1.30% | 0.633 |
| Clostridium perfringens | 4 | 0.74% | 10 | 0.87% | 1.000 |
| Enterococcus faecalis | 54 | 9.94% | 108 | 9.36% | 0.732 |
| Enterococcus faecium | 29 | 5.34% | 63 | 5.46% | 1.000 |
| VRE | 36 | 6.63% | 70 | 6.07% | 0.668 |
| Gram‐negative BSI | 231 | 42.54% | 515 | 44.63% | 0.419 |
| Escherichia coli | 54 | 9.94% | 151 | 13.08% | 0.067 |
| Klebsiella pneumoniae | 54 | 9.94% | 108 | 9.36% | 0.723 |
| Klebsiella oxytoca | 11 | 2.03% | 18 | 1.56% | 0.548 |
| Enterobacter aerogenes | 6 | 1.10% | 13 | 1.13% | 1.000 |
| Enterobacter cloacae | 21 | 3.87% | 44 | 3.81% | 1.000 |
| Pseudomonas aeruginosa | 28 | 5.16% | 65 | 5.63% | 0.733 |
| Acinetobacter spp | 8 | 1.47% | 27 | 2.34% | 0.276 |
| Bacteroides spp | 25 | 4.60% | 30 | 2.60% | 0.039 |
| Serratia marcescens | 14 | 2.58% | 21 | 1.82% | 0.360 |
| Stenotrophomonas maltophilia | 3 | 0.55% | 8 | 0.69% | 1.000 |
| Achromobacter spp | 2 | 0.37% | 3 | 0.17% | 0.597 |
| Aeromonas spp | 2 | 0.37% | 1 | 0.09% | 0.241 |
| Burkholderia cepacia | 0 | 0.00% | 6 | 0.52% | 0.186 |
| Citrobacter freundii | 2 | 0.37% | 15 | 1.39% | 0.073 |
| Fusobacterium spp | 7 | 1.29% | 10 | 0.87% | 0.438 |
| Haemophilus influenzae | 1 | 0.18% | 4 | 0.35% | 1.000 |
| Prevotella spp | 1 | 0.18% | 6 | 0.52% | 0.441 |
| Proteus mirabilis | 9 | 1.66% | 39 | 3.38% | 0.058 |
| MDR PA | 2 | 0.37% | 7 | 0.61% | 0.727 |
| ESBL | 10 | 6.25% | 8 | 2.06% | 0.017 |
| CRE | 2 | 1.25% | 0 | 0.00% | 0.028 |
| MDR Gram‐negative or Gram‐positive | 231 | 47.53% | 450 | 41.86% | 0.036 |
| Candida spp | 58 | 10.68% | 76 | 6.59% | 0.004 |
| Polymicrobal BSI | 50 | 9.21% | 111 | 9.62% | 0.788 |
| Initially inappropriate treatment | 119 | 21.92% | 207 | 17.94% | 0.052 |
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | ||||
|---|---|---|---|---|---|
| N = 543 | % = 32.00% | N = 1,154 | % = 68.00% | P Value | |
| |||||
| Hospital LOS, days | |||||
| Mean SD | 26.44 23.27 | 23.58 21.79 | 0.019 | ||
| Median (25, 75) | 19.16 (9.66, 35.86) | 17.77 (8.9, 30.69) | |||
| Hospital LOS following BSI onset, days | |||||
| Mean SD | 19.80 18.54 | 17.69 17.08 | 0.022 | ||
| Median (25, 75) | 13.9 (7.9, 25.39) | 12.66 (7.05, 22.66) | |||
| Discharge destination | |||||
| Home | 125 | 23.02% | 334 | 28.94% | 0.010 |
| Home with home care | 163 | 30.02% | 303 | 26.26% | 0.105 |
| Rehab | 81 | 14.92% | 149 | 12.91% | 0.260 |
| LTAC | 41 | 7.55% | 87 | 7.54% | 0.993 |
| Transfer to another hospital | 1 | 0.18% | 19 | 1.65% | 0.007 |
| SNF | 132 | 24.31% | 262 | 22.70% | 0.465 |
In a logistic regression model, 5 factors emerged as predictors of 30‐day readmission (Table 4). Having RIFLE: Injury or RIFLE: Failure carried an approximately 2‐fold increase in the odds of 30‐day rehospitalization (odds ratio: 1.95, 95% confidence interval: 1.302.93, P = 0.001) relative to having a RIFLE: None or RIFLE: Risk. Although having strong association with this outcome, harboring an ESBL organism or Bacteroides spp were both relatively infrequent events (3.3% ESBL and 3.2% Bacteroides spp). Infection with Escherichia coli and urine as the source of sepsis both appeared to be significantly protective against a readmission (Table 4). The model's discrimination was moderate (AUROC = 0.653) and its calibration adequate (Hosmer‐Lemeshow P = 0.907). (See Supporting Information, Appendix 1, in the online version of this article for the steps in the development of the final model.)
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| ESBL | 4.503 | 1.42914.190 | 0.010 |
| RIFLE: Injury or Failure (reference: RIFLE: None or Risk) | 1.951 | 1.2972.933 | 0.001 |
| Bacteroides spp | 2.044 | 1.0583.948 | 0.033 |
| Source: urine | 0.583 | 0.3470.979 | 0.041 |
| Escherichia coli | 0.494 | 0.2700.904 | 0.022 |
DISCUSSION
In this single‐center retrospective cohort study, nearly one‐third of survivors of culture‐positive severe sepsis or septic shock required a rehospitalization within 30 days of discharge from their index admission. Factors that contributed to a higher odds of rehospitalization were having mild‐to‐moderate AKI (RIFLE: Injury or RIFLE: Failure) and infection with ESBL organisms or Bacteroides spp, whereas urine as the source of sepsis and E coli as the pathogen appeared to be protective.
A recent study by Hua and colleagues examining the New York Statewide Planning and Research Cooperative System for the years 2008 to 2010 noted a 16.2% overall rate of 30‐day rehospitalization among survivors of initial critical illness.[11] Just as we observed, Hua et al. concluded that development of AKI correlated with readmission. Because they relied on administrative data for their analysis, AKI was diagnosed when hemodialysis was utilized. Examining AKI using SCr changes, our findings add a layer of granularity to the relationship between AKI stages and early readmission. Specifically, we failed to detect any rise in the odds of rehospitalization when either very mild (RIFLE: Risk) or severe (RIFLE: Loss or RIFLE: ESKD) AKI was present. Only when either RIFLE: Injury or RIFLE: Failure developed did the odds of readmission rise. In addition to diverging definitions between our studies, differences in populations also likely yielded different results.[11] Although Hua et al. examined all admissions to the ICU regardless of the diagnosis or illness severity, our cohort consisted of only those ICU patients who survived culture‐positive severe sepsis/septic shock. Because AKI is a known risk factor for mortality in sepsis,[19] the potential for immortal time bias leaves a smaller pool of surviving patients with ESKD at risk for readmission. Regardless of the explanation, it may be prudent to focus on preventing AKI not only to improve survival, but also from the standpoint of diminishing the risk of an early readmission.
Four additional studies have examined the frequency of early readmissions among survivors of critical illness. Liu et al. noted 17.9% 30‐day rehospitalization rate among sepsis survivors.[12] Factors associated with the risk of early readmission included acute and chronic diseases burdens, index hospital LOS, and the need for the ICU in the index sepsis admission. In contrast to our cohort, all of whom were in the ICU during their index episode, less than two‐thirds of the entire population studied by Liu had required an ICU admission. Additionally, Liu's study did not specifically examine the potential impact of AKI or of microbiology on this outcome.
Prescott and coworkers examined healthcare utilization following an episode of severe sepsis.[13] Among other findings, they reported a 30‐day readmission rate of 26.5% among survivors. Although closer to our estimate, this study included all patients surviving a severe sepsis hospitalization, and not only those with a positive culture. These investigators did not examine predictors of readmission.[13]
Horkan et al. examined specifically whether there was an association between AKI and postdischarge outcomes, including 30‐day readmission risk, in a large cohort of patients who survived their critical illness.[20] In it they found that readmission risk ranged from 19% to 21%, depending on the extent of the AKI. Moreover, similar to our findings, they reported that in an adjusted analysis RIFLE: Injury and RIFLE: Failure were associated with a rise in the odds of a 30‐day rehospitalizaiton. In contrast to our study, Horkan et al. did detect an increase in the odds of this outcome associated with RIFLE: Risk. There are likely at least 3 reasons for this difference. First, we focused only on patients with severe sepsis or septic shock, whereas Horkan and colleagues included all critical illness survivors. Second, we were able to explore the impact of microbiology on this outcome. Third, Horkan's study included an order of magnitude more patients than did ours, thus making it more likely either to have the power to detect a true association that we may have lacked or to be more susceptible to type I error.
Finally, Goodwin and colleagues utilized 3 states' databases included in the Health Care and Utilization Project (HCUP) from the Agency for Healthcare Research and Quality to study frequency and risk factors for 30‐day readmission among survivors of severe sepsis.[21] Patients were identified based on the use of the severe sepsis (995.92) and septic shock (785.52). These authors found a 30‐day readmission rate of 26%. Although chronic renal disease, among several other factors, was associated with an increase in this risk, the data source did not permit these investigators to examine the impact of AKI on the outcomes. Similarly, HCUP data do not contain microbiology, a distinct difference from our analysis.
If clinicians are to pursue strategies to reduce the risk of an all‐cause 30‐day readmission, the key goal is not simply to identify all variables associated with readmission, but to focus on factors that are potentially modifiable. Although neither Hua nor Liu and their teams identified any additional factors that are potentially modifiable,[11, 12] in the present study, among the 5 factors we identified, the development of mild to moderate AKI during the index hospitalization may deserve stronger consideration for efforts at prevention. Although one cannot conclude automatically that preventing AKI in this population could mitigate some of the early rehospitalization risk, critically ill patients are frequently exposed to a multitude of nephrotoxic agents. Those caring for subjects with sepsis should reevaluate the risk‐benefit equation of these factors more cautiously and apply guideline‐recommended AKI prevention strategies more aggressively, particularly because a relatively minor change in SCr resulted in an excess risk of readmission.[22]
In addition to AKI, which is potentially modifiable, we identified several other clinical factors predictive of 30‐day readmission, which are admittedly not preventable. Thus, microbiology was predictive of this outcome, with E coli engendering fewer and Bacteroides spp and ESBL organisms more early rehospitalizations. Similarly, urine as the source of sepsis was associated with a lower risk for this endpoint.
Our study has a number of limitations. As a retrospective cohort, it is subject to bias, most notably a selection bias. Specifically, because the flagship hospital of the BJC HealthCare system is a referral center, it is possible that we did not capture all readmissions. However, generally, if a patient who receives healthcare within 1 of the BJC hospitals presents to a nonsystem hospital, that patient is nearly always transferred back into the integrated system because of issues of insurance coverage. Analysis of certain diagnosis‐related groups has indicated that 73% of all patients overall discharged from 4 of the large BJC system institutions who require a readmission within 30 days of discharge return to a BJC hospital (personal communication, Financial Analysis and Decision Support Department at BJC to Dr. Kollef May 12, 2015). Therefore, we may have misclassified the outcome in as many as 180 patients. The fact that our readmission rate was fully double that seen in Hua et al.'s and Liu et al.'s studies, and somewhat higher than that reported by Prescott et al., attests not only to the population differences, but also to the fact that we are unlikely to have missed a substantial percentage of readmissions.[11, 12, 13] Furthermore, to mitigate biases, we enrolled all consecutive patients meeting the predetermined criteria. Missing from our analysis are events that occurred between the index discharge and the readmission. Likewise, we were unable to obtain such potentially important variables as code status or outpatient mortality following discharge. These intervening factors, if included in subsequent studies, may increase the predictive power of the model. Because we relied on administrative coding to identify cases of severe sepsis and septic shock, it is possible that there is misclassification within our cohort. Recent studies indicate, however, that the Angus definition, used in our study, has high negative and positive predictive values for severe sepsis identification.[23] It is still possible that our cohort is skewed toward a more severely ill population, making our results less generalizable to the less severely ill septic patients.[24] The study was performed at a single healthcare system and included only cases of severe sepsis or septic shock that had a positive blood culture, and thus the findings may not be broadly generalizable either to patients without a positive blood culture or to institutions that do not resemble it.
In summary, we have demonstrated that survivors of culture‐positive severe sepsis or septic shock have a high rate of 30‐day rehospitalization. Because the US federal government's initiatives deem 30‐day readmissions to be a quality metric and penalize institutions with higher‐than average readmission rates, a high volume of critically ill patients with culture‐positive severe sepsis and septic shock may disproportionately put an institution at risk for such penalties. Unfortunately, not many of the determinants of readmission are amenable to prevention. As sepsis survival continues to improve, hospitals will need to concentrate their resources on coordinating care of these complex patients so as to improve both individual quality of life and the quality of care that they provide.
Disclosures
This study was supported by a research grant from Cubist Pharmaceuticals, Lexington, Massachusetts. Dr. Kollef's time was in part supported by the Barnes‐Jewish Hospital Foundation. The authors report no conflicts of interest.
Despite its decreasing mortality, sepsis remains a leading reason for intensive care unit (ICU) admission and is associated with crude mortality in excess of 25%.[1, 2] In the United States there are between 660,000 and 750,000 sepsis hospitalizations annually, with the direct costs surpassing $24 billion.[3, 4, 5] As mortality rates have begun to fall, attention has shifted to issues of morbidity and recovery, the intermediate and longer‐term consequences associated with survivorship, and how interventions made while the patient is acutely ill in the ICU alter later health outcomes.[3, 5, 6, 7, 8]
One area of particular interest is the need for healthcare utilization following an acute admission for sepsis, and specifically rehospitalization within 30 days of discharge. This outcome is important not just from the perspective of the patient's well‐being, but also from the point of view of healthcare financing. Through the establishment of Hospital Readmission Reduction Program, the Centers for Medicare and Medicaid Services have sharply reduced reimbursement to hospitals for excessive rates of 30‐day readmissions.[9]
For sepsis, little is known about such readmissions, and even less about how to prevent them. A handful of studies suggest that this rate is between 5% and 26%.[10, 11, 12, 13] Whereas some of these studies looked at some of the factors that impact readmissions,[11, 12] none examined the potential contribution of microbiology of sepsis to this outcome.
To explore these questions, we conducted a single‐center retrospective cohort study among critically ill patients admitted to the ICU with severe culture‐positive sepsis and/or septic shock and determined the rate of early posthospital discharge readmission. In addition, we sought to elucidate predictors of subsequent readmission.
METHODS
Study Design and Ethical Standards
We conducted a single‐center retrospective cohort study from January 2008 to December 2012. The study was approved by the Washington University School of Medicine Human Studies Committee, and informed consent was waived because the data collection was retrospective without any patient‐identifying information. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Aspects of our methodology have been previously published.[14]
Primary Endpoint
All‐cause readmission to an acute‐care facility in the 30 days following discharge after the index hospitalization with sepsis served as the primary endpoint. The index hospitalizations occurred at the Barnes‐Jewish Hospital, a 1200‐bed inner‐city academic institution that serves as the main teaching institution for BJC HealthCare, a large integrated healthcare system of both inpatient and outpatient care. BJC includes a total of 13 hospitals in a compact geographic region surrounding and including St. Louis, Missouri, and we included readmission to any of these hospitals in our analysis. Persons treated within this healthcare system are, in nearly all cases, readmitted to 1 of the system's participating 13 hospitals. If a patient who receives healthcare in the system presents to an out‐of‐system hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage.
Study Cohort
All consecutive adult ICU patients were included if (1) They had a positive blood culture for a pathogen (Cultures positive only for coagulase negative Staphylococcus aureus were excluded as contaminants.), (2) there was an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) code corresponding to an acute organ dysfunction,[4] and (3) they survived their index hospitalization. Only the first episode of sepsis was included as the index hospitalization.
Definitions
All‐cause 30‐day readmission, was defined as a repeat hospitalization within 30 days of discharge from the index hospitalization among survivors of culture‐positive severe sepsis or septic shock. The definition of severe sepsis was based on discharge ICD‐9‐CM codes for acute organ dysfunction.[3] Patients were classified as having septic shock if vasopressors (norepinephrine, dopamine, epinephrine, phenylephrine, or vasopressin) were initiated within 24 hours of the blood culture collection date and time.
Initially appropriate antimicrobial treatment (IAAT) was deemed appropriate if the initially prescribed antibiotic regimen was active against the identified pathogen based on in vitro susceptibility testing and administered for at least 24 hours within 24 hours following blood culture collection. All other regimens were classified as non‐IAAT. Combination antimicrobial treatment was not required for IAAT designation.[15] Prior antibiotic exposure and prior hospitalization occurred within the preceding 90 days, and prior bacteremia within 30 days of the index episode. Multidrug resistance (MDR) among Gram‐negative bacteria was defined as nonsusceptibility to at least 1 antimicrobial agent from at least 3 different antimicrobial classes.[16] Both extended spectrum ‐lactamase (ESBL) organisms and carbapenemase‐producing Enterobacteriaceae were identified via molecular testing.
Healthcare‐associated (HCA) infections were defined by the presence of at least 1 of the following: (1) recent hospitalization, (2) immune suppression (defined as any primary immune deficiency or acquired immune deficiency syndrome or exposure within 3 prior months to immunosuppressive treatmentschemotherapy, radiation therapy, or steroids), (3) nursing home residence, (4) hemodialysis, (5) prior antibiotics. and (6) index bacteremia deemed a hospital‐acquired bloodstream infection (occurring >2 days following index admission date). Acute kidney injury (AKI) was defined according to the RIFLE (Risk, Injury, Failure, Loss, End‐stage) criteria based on the greatest change in serum creatinine (SCr).[17]
Data Elements
Patient‐specific baseline characteristics and process of care variables were collected from the automated hospital medical record, microbiology database, and pharmacy database of Barnes‐Jewish Hospital. Electronic inpatient and outpatient medical records available for all patients in the BJC HealthCare system were reviewed to determine prior antibiotic exposure. The baseline characteristics collected during the index hospitalization included demographics and comorbid conditions. The comorbidities were identified based on their corresponding ICD‐9‐CM codes. The Acute Physiology and Chronic Health Evaluation (APACHE) II and Charlson comorbidity scores were calculated based on clinical data present during the 24 hours after the positive blood cultures were obtained.[18] This was done to accommodate patients with community‐acquired and healthcare‐associated community‐onset infections who only had clinical data available after blood cultures were drawn. Lowest and highest SCr levels were collected during the index hospitalization to determine each patient's AKI status.
Statistical Analyses
Continuous variables were reported as means with standard deviations and as medians with 25th and 75th percentiles. Differences between mean values were tested via the Student t test, and between medians using the Mann‐Whitney U test. Categorical data were summarized as proportions, and the 2 test or Fisher exact test for small samples was used to examine differences between groups. We developed multiple logistic regression models to identify clinical risk factors that were associated with 30‐day all‐cause readmission. All risk factors that were significant at 0.20 in the univariate analyses, as well as all biologically plausible factors even if they did not reach this level of significance, were included in the models. All variables entered into the models were assessed for collinearity, and interaction terms were tested. The most parsimonious models were derived using the backward manual elimination method, and the best‐fitting model was chosen based on the area under the receiver operating characteristics curve (AUROC or the C statistic). The model's calibration was assessed with the Hosmer‐Lemeshow goodness‐of‐fit test. All tests were 2‐tailed, and a P value <0.05 represented statistical significance.
All computations were performed in Stata/SE, version 9 (StataCorp, College Station, TX).
Role of Sponsor
The sponsor had no role in the design, analyses, interpretation, or publication of the study.
RESULTS
Among the 1697 patients with severe sepsis or septic shock who were discharged alive from the hospital, 543 (32.0%) required a rehospitalization within 30 days. There were no differences in age or gender distribution between the groups (Table 1). All comorbidities examined were more prevalent among those with a 30‐day readmission than among those without, with the median Charlson comorbidity score reflecting this imbalance (5 vs 4, P<0.001). Similarly, most of the HCA risk factors were more prevalent among the readmitted group than the comparator group, with HCA sepsis among 94.2% of the former and 90.7% of the latter (P = 0.014).
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | ||||
|---|---|---|---|---|---|
| N = 543 | % = 32.00% | N = 1,154 | % = 68.00% | P Value | |
| |||||
| Baseline characteristics | |||||
| Age, y | |||||
| Mean SD | 58.5 15.7 | 59.5 15.8 | |||
| Median (25, 75) | 60 (49, 69) | 60 (50, 70) | 0.297 | ||
| Race | |||||
| Caucasian | 335 | 61.69% | 769 | 66.64% | 0.046 |
| African American | 157 | 28.91% | 305 | 26.43% | 0.284 |
| Other | 9 | 1.66% | 22 | 1.91% | 0.721 |
| Sex, female | 244 | 44.94% | 537 | 46.53% | 0.538 |
| Admission source | |||||
| Home | 374 | 68.88% | 726 | 62.91% | 0.016 |
| Nursing home, rehab, or LTAC | 39 | 7.81% | 104 | 9.01% | 0.206 |
| Transfer from another hospital | 117 | 21.55% | 297 | 25.74% | 0.061 |
| Comorbidities | |||||
| CHF | 131 | 24.13% | 227 | 19.67% | 0.036 |
| COPD | 156 | 28.73% | 253 | 21.92% | 0.002 |
| CLD | 83 | 15.29% | 144 | 12.48% | 0.113 |
| DM | 175 | 32.23% | 296 | 25.65% | 0.005 |
| CKD | 137 | 25.23% | 199 | 17.24% | <0.001 |
| Malignancy | 225 | 41.44% | 395 | 34.23% | 0.004 |
| HIV | 11 | 2.03% | 10 | 0.87% | 0.044 |
| Charlson comorbidity score | |||||
| Mean SD | 5.24 3.32 | 4.48 3.35 | |||
| Median (25, 75) | 5 (3, 8) | 4 (2, 7) | <0.001 | ||
| HCA RF | 503 | 94.19% | 1,019 | 90.66% | 0.014 |
| Hemodialysis | 65 | 12.01% | 114 | 9.92% | 0.192 |
| Immune suppression | 193 | 36.07% | 352 | 31.21% | 0.044 |
| Prior hospitalization | 339 | 65.07% | 620 | 57.09% | 0.002 |
| Nursing home residence | 39 | 7.81% | 104 | 9.01% | 0.206 |
| Prior antibiotics | 301 | 55.43% | 568 | 49.22% | 0.017 |
| Hospital‐acquired BSI* | 240 | 44.20% | 485 | 42.03% | 0.399 |
| Prior bacteremia within 30 days | 88 | 16.21% | 154 | 13.34% | 0.116 |
| Sepsis‐related parameters | |||||
| LOS prior to bacteremia, d | |||||
| Mean SD | 6.65 11.22 | 5.88 10.81 | |||
| Median (25, 75) | 1 (0, 10) | 0 (0, 8) | 0.250 | ||
| Surgery | |||||
| None | 362 | 66.67% | 836 | 72.44% | 0.015 |
| Abdominal | 104 | 19.15% | 167 | 14.47% | 0.014 |
| Extra‐abdominal | 73 | 13.44% | 135 | 11.70% | 0.306 |
| Status unknown | 4 | 0.74% | 16 | 1.39% | 0.247 |
| Central line | 333 | 64.41% | 637 | 57.80% | 0.011 |
| TPN at the time of bacteremia or prior to it during index hospitalization | 52 | 9.74% | 74 | 5.45% | 0.017 |
| APACHE II | |||||
| Mean SD | 15.08 5.47 | 15.35 5.43 | |||
| Median (25, 75) | 15 (11, 18) | 15 (12, 19) | 0.275 | ||
| Severe sepsis | 361 | 66.48% | 747 | 64.73% | 0.480 |
| Septic shock requiring vasopressors | 182 | 33.52% | 407 | 35.27% | |
| On MV | 104 | 19.22% | 251 | 21.90% | 0.208 |
| Peak WBC (103/L) | |||||
| Mean SD | 22.26 25.20 | 22.14 17.99 | |||
| Median (25, 75) | 17.1 (8.9, 30.6) | 16.9 (10, 31) | 0.654 | ||
| Lowest serum SCr, mg/dL | |||||
| Mean SD | 1.02 1.05 | 0.96 1.03 | |||
| Median (25, 75) | 0.68 (0.5, 1.06) | 0.66 (0.49, 0.96) | 0.006 | ||
| Highest serum SCr, mg/dL | |||||
| Mean SD | 2.81 2.79 | 2.46 2.67 | |||
| Median (25, 75) | 1.68 (1.04, 3.3) | 1.41 (0.94, 2.61) | 0.001 | ||
| RIFLE category | |||||
| None | 81 | 14.92% | 213 | 18.46% | 0.073 |
| Risk | 112 | 20.63% | 306 | 26.52% | 0.009 |
| Injury | 133 | 24.49% | 247 | 21.40% | 0.154 |
| Failure | 120 | 22.10% | 212 | 18.37% | 0.071 |
| Loss | 50 | 9.21% | 91 | 7.89% | 0.357 |
| End‐stage | 47 | 8.66% | 85 | 7.37% | 0.355 |
| Infection source | |||||
| Urine | 95 | 17.50% | 258 | 22.36% | 0.021 |
| Abdomen | 69 | 12.71% | 113 | 9.79% | 0.070 |
| Lung | 93 | 17.13% | 232 | 20.10% | 0.146 |
| Line | 91 | 16.76% | 150 | 13.00% | 0.038 |
| CNS | 1 | 0.18% | 16 | 1.39% | 0.012 |
| Skin | 51 | 9.39% | 82 | 7.11% | 0.102 |
| Unknown | 173 | 31.86% | 375 | 32.50% | 0.794 |
During the index hospitalization, 589 patients (34.7%) suffered from septic shock requiring vasopressors; this did not impact the 30‐day readmission risk (Table 1). Commensurately, markers of severity of acute illness (APACHE II score, mechanical ventilation, peak white blood cell count) did not differ between the groups. With respect to the primary source of sepsis, urine was less, whereas central nervous system was more likely among those readmitted within 30 days. Similarly, there was a significant imbalance between the groups in the prevalence of AKI (Table 1). Specifically, those who did require a readmission were slightly less likely to have sustained no AKI (RIFLE: None; 14.9% vs 18.5%, P = 0.073). Those requiring readmission were also less likely to be in the category RIFLE: Risk (20.6% vs 26.5%, P = 0.009). The direction of this disparity was reversed for the Injury and Failure categories. No differences between groups were seen among those with categories Loss and end‐stage kidney disease (ESKD) (Table 1).
The microbiology of sepsis did not differ in most respects between the 30‐day readmission groups, save for several organisms (Table 2). Most strikingly, those who required a readmission were more likely than those who did not to be infected with Bacteroides spp, Candida spp, an MDR or an ESBL organism (Table 2). As for the outcomes of the index hospitalization, those with a repeat admission had a longer overall and postonset of sepsis initial hospital length of stay, and were less likely to be discharged either home without home health care or transferred to another hospital at the end of their index hospitalization (Table 3).
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | P Value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| |||||
| 543 | 32.00% | 1,154 | 68.00% | ||
| Gram‐positive BSI | 260 | 47.88% | 580 | 50.26% | 0.376 |
| Staphylococcus aureus | 138 | 25.41% | 287 | 24.87% | 0.810 |
| MRSA | 78 | 14.36% | 147 | 12.74% | 0.358 |
| VISA | 6 | 1.10% | 9 | 0.78% | 0.580 |
| Streptococcus pneumoniae | 7 | 1.29% | 33 | 2.86% | 0.058 |
| Streptococcus spp | 34 | 6.26% | 81 | 7.02% | 0.606 |
| Peptostreptococcus spp | 5 | 0.92% | 15 | 1.30% | 0.633 |
| Clostridium perfringens | 4 | 0.74% | 10 | 0.87% | 1.000 |
| Enterococcus faecalis | 54 | 9.94% | 108 | 9.36% | 0.732 |
| Enterococcus faecium | 29 | 5.34% | 63 | 5.46% | 1.000 |
| VRE | 36 | 6.63% | 70 | 6.07% | 0.668 |
| Gram‐negative BSI | 231 | 42.54% | 515 | 44.63% | 0.419 |
| Escherichia coli | 54 | 9.94% | 151 | 13.08% | 0.067 |
| Klebsiella pneumoniae | 54 | 9.94% | 108 | 9.36% | 0.723 |
| Klebsiella oxytoca | 11 | 2.03% | 18 | 1.56% | 0.548 |
| Enterobacter aerogenes | 6 | 1.10% | 13 | 1.13% | 1.000 |
| Enterobacter cloacae | 21 | 3.87% | 44 | 3.81% | 1.000 |
| Pseudomonas aeruginosa | 28 | 5.16% | 65 | 5.63% | 0.733 |
| Acinetobacter spp | 8 | 1.47% | 27 | 2.34% | 0.276 |
| Bacteroides spp | 25 | 4.60% | 30 | 2.60% | 0.039 |
| Serratia marcescens | 14 | 2.58% | 21 | 1.82% | 0.360 |
| Stenotrophomonas maltophilia | 3 | 0.55% | 8 | 0.69% | 1.000 |
| Achromobacter spp | 2 | 0.37% | 3 | 0.17% | 0.597 |
| Aeromonas spp | 2 | 0.37% | 1 | 0.09% | 0.241 |
| Burkholderia cepacia | 0 | 0.00% | 6 | 0.52% | 0.186 |
| Citrobacter freundii | 2 | 0.37% | 15 | 1.39% | 0.073 |
| Fusobacterium spp | 7 | 1.29% | 10 | 0.87% | 0.438 |
| Haemophilus influenzae | 1 | 0.18% | 4 | 0.35% | 1.000 |
| Prevotella spp | 1 | 0.18% | 6 | 0.52% | 0.441 |
| Proteus mirabilis | 9 | 1.66% | 39 | 3.38% | 0.058 |
| MDR PA | 2 | 0.37% | 7 | 0.61% | 0.727 |
| ESBL | 10 | 6.25% | 8 | 2.06% | 0.017 |
| CRE | 2 | 1.25% | 0 | 0.00% | 0.028 |
| MDR Gram‐negative or Gram‐positive | 231 | 47.53% | 450 | 41.86% | 0.036 |
| Candida spp | 58 | 10.68% | 76 | 6.59% | 0.004 |
| Polymicrobal BSI | 50 | 9.21% | 111 | 9.62% | 0.788 |
| Initially inappropriate treatment | 119 | 21.92% | 207 | 17.94% | 0.052 |
| 30‐Day Readmission = Yes | 30‐Day Readmission = No | ||||
|---|---|---|---|---|---|
| N = 543 | % = 32.00% | N = 1,154 | % = 68.00% | P Value | |
| |||||
| Hospital LOS, days | |||||
| Mean SD | 26.44 23.27 | 23.58 21.79 | 0.019 | ||
| Median (25, 75) | 19.16 (9.66, 35.86) | 17.77 (8.9, 30.69) | |||
| Hospital LOS following BSI onset, days | |||||
| Mean SD | 19.80 18.54 | 17.69 17.08 | 0.022 | ||
| Median (25, 75) | 13.9 (7.9, 25.39) | 12.66 (7.05, 22.66) | |||
| Discharge destination | |||||
| Home | 125 | 23.02% | 334 | 28.94% | 0.010 |
| Home with home care | 163 | 30.02% | 303 | 26.26% | 0.105 |
| Rehab | 81 | 14.92% | 149 | 12.91% | 0.260 |
| LTAC | 41 | 7.55% | 87 | 7.54% | 0.993 |
| Transfer to another hospital | 1 | 0.18% | 19 | 1.65% | 0.007 |
| SNF | 132 | 24.31% | 262 | 22.70% | 0.465 |
In a logistic regression model, 5 factors emerged as predictors of 30‐day readmission (Table 4). Having RIFLE: Injury or RIFLE: Failure carried an approximately 2‐fold increase in the odds of 30‐day rehospitalization (odds ratio: 1.95, 95% confidence interval: 1.302.93, P = 0.001) relative to having a RIFLE: None or RIFLE: Risk. Although having strong association with this outcome, harboring an ESBL organism or Bacteroides spp were both relatively infrequent events (3.3% ESBL and 3.2% Bacteroides spp). Infection with Escherichia coli and urine as the source of sepsis both appeared to be significantly protective against a readmission (Table 4). The model's discrimination was moderate (AUROC = 0.653) and its calibration adequate (Hosmer‐Lemeshow P = 0.907). (See Supporting Information, Appendix 1, in the online version of this article for the steps in the development of the final model.)
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| ESBL | 4.503 | 1.42914.190 | 0.010 |
| RIFLE: Injury or Failure (reference: RIFLE: None or Risk) | 1.951 | 1.2972.933 | 0.001 |
| Bacteroides spp | 2.044 | 1.0583.948 | 0.033 |
| Source: urine | 0.583 | 0.3470.979 | 0.041 |
| Escherichia coli | 0.494 | 0.2700.904 | 0.022 |
DISCUSSION
In this single‐center retrospective cohort study, nearly one‐third of survivors of culture‐positive severe sepsis or septic shock required a rehospitalization within 30 days of discharge from their index admission. Factors that contributed to a higher odds of rehospitalization were having mild‐to‐moderate AKI (RIFLE: Injury or RIFLE: Failure) and infection with ESBL organisms or Bacteroides spp, whereas urine as the source of sepsis and E coli as the pathogen appeared to be protective.
A recent study by Hua and colleagues examining the New York Statewide Planning and Research Cooperative System for the years 2008 to 2010 noted a 16.2% overall rate of 30‐day rehospitalization among survivors of initial critical illness.[11] Just as we observed, Hua et al. concluded that development of AKI correlated with readmission. Because they relied on administrative data for their analysis, AKI was diagnosed when hemodialysis was utilized. Examining AKI using SCr changes, our findings add a layer of granularity to the relationship between AKI stages and early readmission. Specifically, we failed to detect any rise in the odds of rehospitalization when either very mild (RIFLE: Risk) or severe (RIFLE: Loss or RIFLE: ESKD) AKI was present. Only when either RIFLE: Injury or RIFLE: Failure developed did the odds of readmission rise. In addition to diverging definitions between our studies, differences in populations also likely yielded different results.[11] Although Hua et al. examined all admissions to the ICU regardless of the diagnosis or illness severity, our cohort consisted of only those ICU patients who survived culture‐positive severe sepsis/septic shock. Because AKI is a known risk factor for mortality in sepsis,[19] the potential for immortal time bias leaves a smaller pool of surviving patients with ESKD at risk for readmission. Regardless of the explanation, it may be prudent to focus on preventing AKI not only to improve survival, but also from the standpoint of diminishing the risk of an early readmission.
Four additional studies have examined the frequency of early readmissions among survivors of critical illness. Liu et al. noted 17.9% 30‐day rehospitalization rate among sepsis survivors.[12] Factors associated with the risk of early readmission included acute and chronic diseases burdens, index hospital LOS, and the need for the ICU in the index sepsis admission. In contrast to our cohort, all of whom were in the ICU during their index episode, less than two‐thirds of the entire population studied by Liu had required an ICU admission. Additionally, Liu's study did not specifically examine the potential impact of AKI or of microbiology on this outcome.
Prescott and coworkers examined healthcare utilization following an episode of severe sepsis.[13] Among other findings, they reported a 30‐day readmission rate of 26.5% among survivors. Although closer to our estimate, this study included all patients surviving a severe sepsis hospitalization, and not only those with a positive culture. These investigators did not examine predictors of readmission.[13]
Horkan et al. examined specifically whether there was an association between AKI and postdischarge outcomes, including 30‐day readmission risk, in a large cohort of patients who survived their critical illness.[20] In it they found that readmission risk ranged from 19% to 21%, depending on the extent of the AKI. Moreover, similar to our findings, they reported that in an adjusted analysis RIFLE: Injury and RIFLE: Failure were associated with a rise in the odds of a 30‐day rehospitalizaiton. In contrast to our study, Horkan et al. did detect an increase in the odds of this outcome associated with RIFLE: Risk. There are likely at least 3 reasons for this difference. First, we focused only on patients with severe sepsis or septic shock, whereas Horkan and colleagues included all critical illness survivors. Second, we were able to explore the impact of microbiology on this outcome. Third, Horkan's study included an order of magnitude more patients than did ours, thus making it more likely either to have the power to detect a true association that we may have lacked or to be more susceptible to type I error.
Finally, Goodwin and colleagues utilized 3 states' databases included in the Health Care and Utilization Project (HCUP) from the Agency for Healthcare Research and Quality to study frequency and risk factors for 30‐day readmission among survivors of severe sepsis.[21] Patients were identified based on the use of the severe sepsis (995.92) and septic shock (785.52). These authors found a 30‐day readmission rate of 26%. Although chronic renal disease, among several other factors, was associated with an increase in this risk, the data source did not permit these investigators to examine the impact of AKI on the outcomes. Similarly, HCUP data do not contain microbiology, a distinct difference from our analysis.
If clinicians are to pursue strategies to reduce the risk of an all‐cause 30‐day readmission, the key goal is not simply to identify all variables associated with readmission, but to focus on factors that are potentially modifiable. Although neither Hua nor Liu and their teams identified any additional factors that are potentially modifiable,[11, 12] in the present study, among the 5 factors we identified, the development of mild to moderate AKI during the index hospitalization may deserve stronger consideration for efforts at prevention. Although one cannot conclude automatically that preventing AKI in this population could mitigate some of the early rehospitalization risk, critically ill patients are frequently exposed to a multitude of nephrotoxic agents. Those caring for subjects with sepsis should reevaluate the risk‐benefit equation of these factors more cautiously and apply guideline‐recommended AKI prevention strategies more aggressively, particularly because a relatively minor change in SCr resulted in an excess risk of readmission.[22]
In addition to AKI, which is potentially modifiable, we identified several other clinical factors predictive of 30‐day readmission, which are admittedly not preventable. Thus, microbiology was predictive of this outcome, with E coli engendering fewer and Bacteroides spp and ESBL organisms more early rehospitalizations. Similarly, urine as the source of sepsis was associated with a lower risk for this endpoint.
Our study has a number of limitations. As a retrospective cohort, it is subject to bias, most notably a selection bias. Specifically, because the flagship hospital of the BJC HealthCare system is a referral center, it is possible that we did not capture all readmissions. However, generally, if a patient who receives healthcare within 1 of the BJC hospitals presents to a nonsystem hospital, that patient is nearly always transferred back into the integrated system because of issues of insurance coverage. Analysis of certain diagnosis‐related groups has indicated that 73% of all patients overall discharged from 4 of the large BJC system institutions who require a readmission within 30 days of discharge return to a BJC hospital (personal communication, Financial Analysis and Decision Support Department at BJC to Dr. Kollef May 12, 2015). Therefore, we may have misclassified the outcome in as many as 180 patients. The fact that our readmission rate was fully double that seen in Hua et al.'s and Liu et al.'s studies, and somewhat higher than that reported by Prescott et al., attests not only to the population differences, but also to the fact that we are unlikely to have missed a substantial percentage of readmissions.[11, 12, 13] Furthermore, to mitigate biases, we enrolled all consecutive patients meeting the predetermined criteria. Missing from our analysis are events that occurred between the index discharge and the readmission. Likewise, we were unable to obtain such potentially important variables as code status or outpatient mortality following discharge. These intervening factors, if included in subsequent studies, may increase the predictive power of the model. Because we relied on administrative coding to identify cases of severe sepsis and septic shock, it is possible that there is misclassification within our cohort. Recent studies indicate, however, that the Angus definition, used in our study, has high negative and positive predictive values for severe sepsis identification.[23] It is still possible that our cohort is skewed toward a more severely ill population, making our results less generalizable to the less severely ill septic patients.[24] The study was performed at a single healthcare system and included only cases of severe sepsis or septic shock that had a positive blood culture, and thus the findings may not be broadly generalizable either to patients without a positive blood culture or to institutions that do not resemble it.
In summary, we have demonstrated that survivors of culture‐positive severe sepsis or septic shock have a high rate of 30‐day rehospitalization. Because the US federal government's initiatives deem 30‐day readmissions to be a quality metric and penalize institutions with higher‐than average readmission rates, a high volume of critically ill patients with culture‐positive severe sepsis and septic shock may disproportionately put an institution at risk for such penalties. Unfortunately, not many of the determinants of readmission are amenable to prevention. As sepsis survival continues to improve, hospitals will need to concentrate their resources on coordinating care of these complex patients so as to improve both individual quality of life and the quality of care that they provide.
Disclosures
This study was supported by a research grant from Cubist Pharmaceuticals, Lexington, Massachusetts. Dr. Kollef's time was in part supported by the Barnes‐Jewish Hospital Foundation. The authors report no conflicts of interest.
- , , , et al; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353
- , , , et al. Death in the United States, 2007. NCHS Data Brief. 2009;26:1–8.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1548–1564.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310.
- , , , et al: Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalization. Crit Care Med. 2005;33:2555–2562.
- , , , et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250.
- , , , , . Two decades of mortality trends among patients with severe sepsis: a comparative meta‐analysis. Crit Care Med. 2014;42:625–631.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107.
- , . Trends in septicemia hospitalizations and readmissions in selected HCUP states, 2005 and 2010. HCUP Statistical Brief #161. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb161.pdf. Published September 2013, Accessed January 13, 2015.
- , , , . Early and late unplanned rehospitalizations for survivors of critical illness. Crit Care Med. 2015;43:430–438.
- , , , , , . Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9:502–507.
- , , , , . Increased 1‐year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190:62–69.
- , , , , . Multi‐drug resistance, inappropriate initial antibiotic therapy and mortality in Gram‐negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596.
- , , . Does combination antimicrobial therapy reduce mortality in Gram‐negative bacteraemia? A meta‐analysis. Lancet Infect Dis. 2004;4:519–527.
- , , , et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281.
- , , , , ; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- , , , et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73
- , , , , , . The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study. Crit Care Med. 2015;43:354–364.
- , , , . Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43:738–746.
- Acute Kidney Injury Work Group. Kidney disease: improving global outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf. Accessed March 4, 2015.
- , , , , , . Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19(1):139.
- , , , , , . Severe sepsis cohorts derived from claims‐based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41:945–953.
- , , , et al; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353
- , , , et al. Death in the United States, 2007. NCHS Data Brief. 2009;26:1–8.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1548–1564.
- , , , , , . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310.
- , , , et al: Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalization. Crit Care Med. 2005;33:2555–2562.
- , , , et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250.
- , , , , . Two decades of mortality trends among patients with severe sepsis: a comparative meta‐analysis. Crit Care Med. 2014;42:625–631.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107.
- , . Trends in septicemia hospitalizations and readmissions in selected HCUP states, 2005 and 2010. HCUP Statistical Brief #161. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb161.pdf. Published September 2013, Accessed January 13, 2015.
- , , , . Early and late unplanned rehospitalizations for survivors of critical illness. Crit Care Med. 2015;43:430–438.
- , , , , , . Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9:502–507.
- , , , , . Increased 1‐year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190:62–69.
- , , , , . Multi‐drug resistance, inappropriate initial antibiotic therapy and mortality in Gram‐negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596.
- , , . Does combination antimicrobial therapy reduce mortality in Gram‐negative bacteraemia? A meta‐analysis. Lancet Infect Dis. 2004;4:519–527.
- , , , et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281.
- , , , , ; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212.
- , , , . APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
- , , , et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73
- , , , , , . The association of acute kidney injury in the critically ill and postdischarge outcomes: a cohort study. Crit Care Med. 2015;43:354–364.
- , , , . Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43:738–746.
- Acute Kidney Injury Work Group. Kidney disease: improving global outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf. Accessed March 4, 2015.
- , , , , , . Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19(1):139.
- , , , , , . Severe sepsis cohorts derived from claims‐based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41:945–953.
© 2015 Society of Hospital Medicine
Dyspnea Assessment and Management Survey
Dyspnea, defined as a subjective experience of breathing discomfort,[1] is the seventh most frequent reason adult patients present to the emergency room and the most frequent cause for emergency room visits in patients 65 years or older.[2] Moreover, dyspnea is experienced by 49% of patients hospitalized with a medical condition[3, 4, 5] and by 70% of patients who are seriously ill.[6]
Based on evidence that patients are not treated consistently and effectively for relief of their shortness of breath, the American College of Chest Physicians (ACCP) statement on dyspnea management in patients with advanced lung or heart disease recommended that patients should be asked to rate their dyspnea, and the rating should be routinely documented in the medical record to guide management.[7] Although clinicians may question the utility of routine assessment of dyspnea using a standardized scale, studies have found that the prevalence of dyspnea reported from chart review is much lower than when patients are directly interviewed.[8] This may be the result of underrecognition of dyspnea or poor documentation by physicians, or that patients may not communicate their symptoms unless the physician specifically asks. As is the case with pain, routine assessment of dyspnea severity could lead to improved clinical management and greater patient‐centered care. However, unlike in the case of pain, regulatory bodies, such as the Joint Commission for Accreditation of Healthcare Organization, do not require routine dyspnea assessment.[9]
Currently, there are more than 40,000 hospitalists in the United States, and the vast majority of hospitals with >200 beds have a hospitalist group.[10] Hospitalists care for over 60% of inpatients[11] and play a major role in the management of patients with acute cardiopulmonary diseases. If standardized approaches for the assessment and documentation of dyspnea are to be implemented, hospitalists would be a key stakeholder group for utilizing enhanced clinical information about dyspnea. Therefore, we evaluated attitudes and practices of hospitalists in regard to the assessment and management of dyspnea, including the potential benefits and challenges related to the implementation of standardized assessment. We hypothesized that hospitalists would believe that a dyspnea scale for assessment of severity could improve their management of patients with cardiovascular diseases. Further, we hypothesized that physicians who agreed with the general statement that dyspnea is an important clinical problem would be more likely to believe that routine dyspnea assessment would be valuable.
METHODS
Study Sample
We invited 255 attending hospitalists from 9 geographically and structurally diverse hospitals to complete a survey about the assessment and management of dyspnea. The 9 hospitals represent range of practice environments including 4 academic medical centers, 2 community teaching and 3 nonteaching hospitals, 1 Veterans Administration hospital, and 2 staff‐model HMOs (see Supporting Table 1 in the online version of this article). The survey was distributed online using REDCap (Research Electronic Data Capture), a secure web‐based interface application.[12] A coinvestigator who was a pulmonary critical‐care physician at each site sent an initial email to their hospitalist groups that alerted them to expect a survey from the principal investigator. This notification was subsequently followed by an email invitation containing an informational cover letter and a link to the online survey. The cover letter stated that the completed surveys would not be stored at the local sites and that all the analyzed data would be deidentified. Nonrespondents were sent reminders at 2 and 4 weeks after the initial mailing. A $25 electronic gift card was provided as a gesture of appreciation for their time. The survey was conducted between September 2013 and December 2013.
The study was approved by the Baystate Health Institutional Review Board, Springfield, Massachusetts, with a waiver for written informed consent.
Questionnaire
We developed a 17‐item instrument based on a review of the dyspnea literature and a prior ACCP survey.[12] Questions were piloted with 4 hospitalists at a single institution and modified to improve face validity and clarity (see Supporting Information in the online version of this article for the full survey).
Hospitalists were asked to consider the care of patients admitted for acute cardiopulmonary disease, including heart failure, chronic obstructive pulmonary disease, and pneumonia. A series of 5‐point Likert scales were used to assess the respondents level of agreement with statements related to the following domains: the importance of dyspnea in clinical care, the potential benefits and challenges of routine dyspnea assessment (statements such as: Having a standardized assessment of dyspnea severity would be helpful in management of patients with cardiopulmonary diseases. Dyspnea assessment by a scale should be part of the vital signs for patients with cardiopulmonary diseases.), and management of dyspnea (questions regarding the use of opioids and other nonpharmacological therapies). Additional questions were asked about current assessment practices (questions such as: How often do you assess severity of dyspnea? What is your approach in assessing dyspnea? with options of choosing a categorical or numerical scale), if dyspnea is assessed in their institution by nurses and how often, and the influence of dyspnea severity assessment on their management. The survey had 1 question that solicited comments from the participants: If you don't think that it would be useful to have a standardized dyspnea assessment, please tell us why.
Data Analysis
Responses to survey questions were summarized via counts and percentages in each response category. Adopting the methodology used in the ACCP consensus statement, strongly agree and somewhat agree were combined into a single category of agreement. We also presented percentage of responses in the 2 levels of agreement (strongly agree and somewhat agree) for each question in a bar graph.
Associations between tertiles of physicians' time in practice and attitude toward dyspnea were evaluated via 2 or Fisher exact test.
To examine how answers to the first 2 questions, which assessed attitude toward importance of dyspnea in clinical care, affect answers to the remaining questions, we grouped respondents in 3 categories (strongly agree, agree to these questions, do not agree) and tested the associations using 2 or Fisher exact test.
All analyses were performed using SAS version 9.3 (SAS institute, Inc., Cary, NC) and Stata release 13.1 (StataCorp, College Station, TX).
RESULTS
Overall, 178 (69.8%) of 255 identified hospitalists completed the survey, and all 9 participating hospitals had a response rate greater than 50%. The median number of years in practice was 6 (range, 038 years). A majority (77.5%) of respondents agreed with the statement that dyspnea is 1 of the major symptoms of patients with cardiopulmonary disease, and that its treatment is central to the management of these patients (77.0%) (Figure 1).
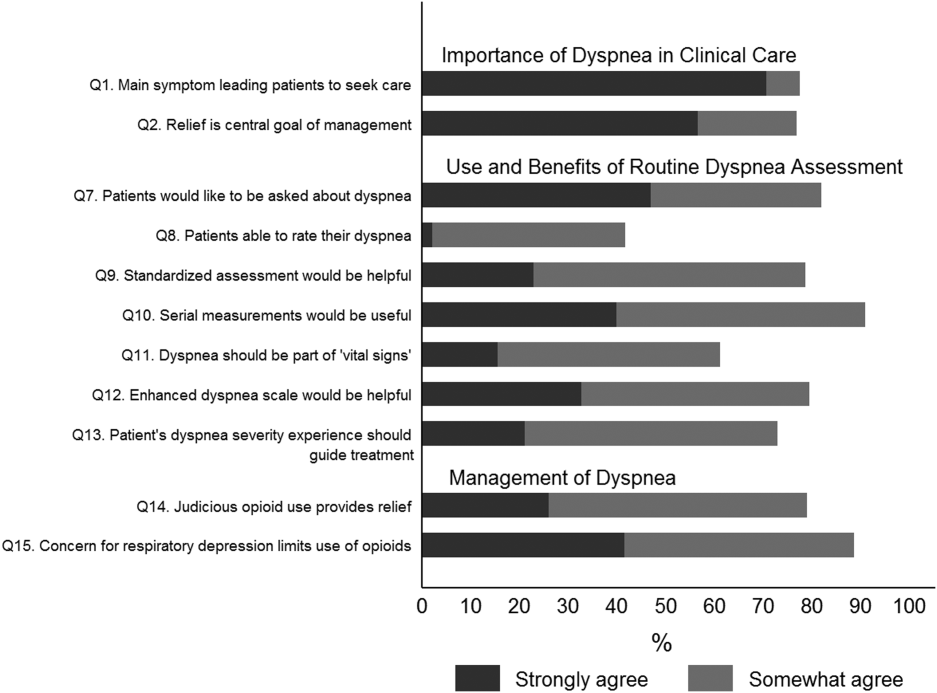
Attitude and Practices Surrounding Dyspnea Assessment
When asked about their current assessment of dyspnea, a majority (84.3%) of the hospitalists stated that they assess dyspnea on a daily basis; two‐thirds indicated that they use a categorical scale (ie, no shortness of breath, improved or worsened compared with a prior date), and one‐third indicated that they ask whether the patient is dyspneic or not. Fifty‐six percent of hospitalists stated that dyspnea is regularly assessed by nurses in their hospital.
The majority of respondents agreed (78.6%, 23.0% strongly and 55.6% somewhat agree) that standardized assessment of dyspnea severity, using a numeric scale and serial measurements as part of the vital signs, would benefit the management of patients with cardiopulmonary diseases. Furthermore, 79.6% (33.0% strongly and 46.6% somewhat agree) reported that using a dyspnea scale that included information to further characterize the patient‐reported experience, such as the level of distress associated with dyspnea, would be helpful in management.
Approximately 90% of the hospitalists indicated that awareness of dyspnea severity has an influence on clinical decision making, including whether to intensify treatment of underlying conditions, to pursue additional diagnostic testing, or to modify discharge timing. Additionally, two‐thirds of hospitalists agreed that awareness of dyspnea severity influences their decision to add opioids, whereas only one‐third prescribed nonpharmacologic symptom‐oriented treatment (Table 1).
| Frequency (%) | |
|---|---|
| |
| When caring for patients with acute cardiopulmonary diseases, how often do you assess severity of dyspnea?* | |
| At admission | 66 (37.1) |
| At discharge | 59 (33.2) |
| Daily until discharge | 150 (84.3) |
| More often than daily | 58 (32.6) |
| Which description best characterizes your approach to assessing dyspnea severity? | |
| I don't regularly ask about dyspnea severity | 3 (1.7) |
| I ask the patient whether or not they are having shortness of breath | 50 (28.3) |
| I ask the patient to rate the severity of shortness of breath using a numeric scale | 4 (2.3) |
| I ask the patient to rate the severity of shortness of breath using a categorical scale (eg, somewhat SOB, no SOB, improved or worsened compared with a prior date) | 120 (67.8) |
| When is dyspnea severity assessed and documented by nursing at your hospital?* | |
| Dyspnea is not routinely assessed | 60 (33.7) |
| At admission | 30 (16.9) |
| Daily | 43 (24.2) |
| Each shift | 64 (36.0) |
| Awareness of dyspnea severity affects my management by:* | |
| Influencing my decision to intensify treatment of the patient's underlying condition | 170 (95.5) |
| Influencing my decision to pursue additional diagnostic testing | 160 (89.9) |
| Influencing my decision to add pharmacologic‐based, symptom‐oriented treatment for dyspnea, such as opioids | 115 (64.6) |
| Influencing my decision to add nonpharmacologic‐based, symptom‐oriented treatment for dyspnea, such as fans or pursed lip breathing technique | 58 (32.6) |
| Influencing my decision regarding timing of discharge | 162 (91.0) |
| Which of the following nonpharmacologic therapies are effective for the relief of dyspnea?* | |
| Pursed lip breathing | 113 (63.5) |
| Relaxation techniques | 137 (77.0) |
| Noninvasive ventilation | 143 (80.3) |
| O2 for nonhypoxemic patients | 89 (50.0) |
| Cool air/fan | 125 (70.2) |
| Cognitive behavioral strategies | 101 (56.7) |
Forty‐two percent of the respondents agreed that patients are able to rate their dyspnea on a scale (2.3% strongly agree and 40.0% agree), and 73.0% indicated that patient experience of dyspnea should guide management independent of physiologic measures such as respiratory rate and oxygen saturation (Figure 1).
Several potential barriers were identified among the 18 participants who did not think that a standardized assessment of dyspnea would be beneficial, including concerns that (1) a dyspnea severity scale is too subjective and numerical scales are not useful for a subjective symptom (19.0%), (2) patients may overrate their symptom or will not be able to rate their dyspnea using a scale (31.0%), or (3) categorical description is sufficient (31.0%).
Practices in Dyspnea Management
Seventy‐nine percent of respondents agreed with the statement that judicious use of opioids can provide relief of dyspnea (26.1% strongly and 52.8% agreed), and 88.7% hospitalists identified the risk of respiratory depression as 1 of the barriers for the limited use of opioids. The majority of physicians (60%80%) considered nonpharmacologic therapies effective for symptomatic treatment of dyspnea, including in the order of agreement: noninvasive ventilation, relaxation techniques, cool air/fan, use of pursed lip breathing, and oxygen for nonhypoxemic patients (Table 1).
Physician Experience and Attitudes Toward Dyspnea Management
When we stratified hospitalists in tertiles of median years of time in practice (median [range]: 2 [04], 6 [58] and 15 [938]), we did not find an association with any of the responses to the questions.
Attitude Regarding the Importance of Dyspnea in Clinical Care and Responses to Subsequent Questions
Respondents who strongly agree or agree that dyspnea is the primary presenting symptom in patients with cardiovascular condition and that dyspnea relief is central to the management of these patients were more likely to believe that patients would like to be asked about their dyspnea (61.2% vs 30.2% vs 29.7%). They also had a more positive attitude about the usefulness of a standardized assessment of dyspnea and the inclusion of the assessment of dyspnea by a scale in the vital signs (Table 2).
| Description | Do Not Agree, n (%) | Somewhat Agree, n (%) | Strongly Agree, n (%) | P Value* |
|---|---|---|---|---|
| ||||
| 37 (20.9) | 43 (24.3) | 97 (54.8) | ||
| Which description best characterizes your approach to assessing dyspnea severity? | 0.552 | |||
| I don't regularly ask about dyspnea severity | 0 (0) | 0 (0) | 3 (3.1) | |
| I ask the patient whether or not they are having shortness of breath | 11 (29.7) | 14 (32.6) | 25 (25.8) | |
| I ask the patient to rate the severity of shortness of breath using a numeric scale | 2 (5.4) | 1 (2.3) | 1 (1.0) | |
| I ask the patient to rate the severity of shortness of breath using a categorical scale (e.g., somewhat shortness of breath, no shortness of breath, improved or worsened compared with a prior date) | 24 (64.9) | 28 (65.1) | 68 (70.1) | |
| Patients would like me to ask them about their dyspnea. | <0.0001 | |||
| Somewhat agree | 9 (24.3) | 21 (48.8) | 32 (32.7) | |
| Strongly agree | 11 (29.7) | 13 (30.2) | 60 (61.2) | |
| Patients are able to rate their own dyspnea intensity on a scale of 0‐10. | 0.432 | |||
| Somewhat agree | 12 (32.4) | 16 (37.2) | 42 (43.3) | |
| Strongly agree | 2 (5.4) | 0 (0) | 2 (2.1) | |
| Having a standardized assessment of dyspnea severity would be helpful to me in management of patients with cardiopulmonary diseases. | 0.026 | |||
| Somewhat agree | 17 (46.0) | 25 (58.1) | 57 (58.2) | |
| Strongly agree | 7 (18.9) | 6 (14.0) | 28 (28.6) | |
| Serial measurements of dyspnea would be useful for assessing response to therapy. | 0.042 | |||
| Somewhat agree | 14 (37.8) | 28 (65.1) | 48 (49.5) | |
| Strongly agree | 16 (43.2) | 12 (27.9) | 43 (44.3) | |
| Dyspnea assessment by a scale should be part of the vital signs for patients with cardiopulmonary diseases. | 0.042 | |||
| Somewhat agree | 13 (35.1) | 17 (39.5) | 51 (52.0) | |
| Strongly agree | 4 (10.8) | 5 (11.6) | 19 (19.4) | |
| Using an enhanced dyspnea scale that includes information about the following 4 features 1) Current dyspnea severity, 2) Worst dyspnea ever, 3) Improvement of dyspnea since admission, 4) Acceptability of current level of dyspnea, would be more helpful for my management than a single question focused on dyspnea severity. | 0.03 | |||
| Somewhat agree | 14 (40.0) | 24 (55.8) | 44 (44.9) | |
| Strongly agree | 9 (25.7) | 9 (20.9) | 40 (40.8) | |
| The patients experience of dyspnea should be used to guide treatment decisions independent of objective measures such as respiratory rate and oxygen saturation. | 0.10 | |||
| Somewhat agree | 20 (54.0) | 21 (48.8) | 51 (52.0) | |
| Strongly agree | 5 (13.5) | 6 (14.0) | 27 (27.6) | |
| Judicious use of oral and/or parenteral opioids can provide relief of dyspnea. | 0.21 | |||
| Somewhat agree | 20 (54.0) | 23 (54.8) | 50 (51.6) | |
| Strongly agree | 10 (27.0) | 6 (14.3) | 30 (30.9) | |
| Limited use of opioids for relief of dyspnea in patients with advanced cardiopulmonary disorders is often due to concerns of respiratory depression. | 0.71 | |||
| Somewhat agree | 17 (46.0) | 23 (54.8) | 43 (43.9) | |
| Strongly agree | 15 (40.5) | 14 (33.3) | 45 (45.9) | |
DISCUSSION
In this survey of 178 most hospitalists from a diverse group of 9 US hospitals, we found that most indicate that severity of dyspnea has a profound influence on their clinical practice (including their decision whether to intensify treatments such as diuretics or bronchodilators, to pursue additional diagnostic testing, add opioids or other nonpharmacological treatments) and ultimately their decision regarding the timing of hospital discharge. More importantly, whereas less than half reported experience with standardized assessment of dyspnea severity, most stated that such data would be very useful in their practice.
Despite being a highly prevalent symptom in diverse patient populations, several studies have shown that documentation of dyspnea is sporadic and evaluation of dyspnea quality of care is not routinely performed.[13, 14, 15] Statements from a number of professional societies, including the ACCP, the American Thoracic Society and the Canadian Respiratory Society, recommend that dyspnea management should rely on patient reporting, and that dyspnea severity should be recorded.[1, 4, 7] Assessment is an essential step to guide interventions; however, simply asking about the presence or absence of dyspnea is insufficient.
Several rating scales have been validated and might be implementable in the acute care setting, including the Numerical Rating Scale and the Visual Assessment Scale.[16, 17, 18, 19, 20] Our survey shows that standardized documentation of dyspnea severity in clinical practice is uncommon. However, most hospitalists in our study believed that assessment of dyspnea, using a standardized scale, would positively impact their management of patients with cardiopulmonary disease.
There are a number of potential benefits of routine assessment of dyspnea in hospitalized patients. Implementation of a standardized approach to dyspnea measurement would result in more uniform assessment and documentation practices, and in turn greater awareness among members of the patient‐care team. Though not sufficient to improve care, measurement is necessary because physicians do not always recognize the severity of patients' dyspnea or may not recognize its presence. A retrospective study that assessed the prevalence of symptoms in 410 ambulatory patients showed that one‐quarter of patients had dyspnea, but only half of them told their doctor about it.[21] Two other studies of patients with cancer diagnoses found that 30%70% of patients had dyspnea, but the symptom was recognized in only half of them; even when recognized, dyspnea severity was frequently underrated by physicians.[21, 22] Importantly, underestimation appears to correlate with underutilization of symptomatic management of dyspnea.[8]
Although the results of our survey are encouraging, they highlight a number of potential barriers and misconceptions among hospitalists. For example, although dyspnea can be characterized only by the person experiencing it, only 42% of our survey respondents believed that patients are able to rate their dyspnea intensity on a scale. Some of these responses may be influenced by the fact that dyspnea scales are not currently available to patients under their care. Another explanation is that similar to the case for pain, some hospitalists may believe that patients will exaggerate dyspnea severity. Almost one‐third of the respondents stated that objective measures, such as respiratory rate or oxygen saturation, are more important than a patient's experience of dyspnea in guiding the treatment, and that dyspnea is a subjective symptom and not a vital sign itself. Hospitalists who appreciated the importance of dyspnea in clinical practice were more likely to support the implementation of a standardized dyspnea scale for dyspnea assessment.
Although the potential benefits of including routine measurement of dyspnea in standard hospital practice may seem obvious, evidence that implementing routine assessment improves patient care or outcomes is lacking. Even if hospitalists see the value of dyspnea assessment, asking nurses to collect and document additional information would represent a substantial change in hospital workflow. Finally, without specific protocols to guide care, it is unclear whether physicians will be able to use new information about dyspnea severity effectively. Future studies need to evaluate the impact of implementing routine dyspnea assessment on the management of patients with cardiopulmonary diseases including the use of evidence‐based interventions and reducing the use of less valuable care.
Most hospitalists agreed with the basic principles of dyspnea treatment in patients with advanced cardiopulmonary disease after the primary disease had been stabilized. Effective measures are available, and several guidelines endorse opioids in dyspnea management.[1, 4, 7] However, many clinicians are uncomfortable with this approach for dyspnea, and opioids remain underused. In our study, almost 90% of physicians recognized that concerns about respiratory depression limits opioids use as a treatment. A qualitative study that explored the physicians' perspective toward opioids showed that most physicians were reluctant to prescribe opioids for refractory dyspnea, describing a lack of related knowledge and experience, and fears related to the potential adverse effects. The findings of our study also outline the need to better educate residents and hospitalists on the assessment and management of dyspnea, including prescribing opioids for refractory dyspnea.[23]
Study Strengths and Limitations
This study has several strengths. To our knowledge, it is the first to explore hospitalists' perspectives on incorporating dyspnea assessment in their clinical practice. Hospitalists are the attending physicians for a large majority of inpatients and would be the main users of a dyspnea severity scale. Our questionnaire survey included a large number of hospitalists, from 9 geographically and structurally diverse hospitals, which increased the generalizability of the findings to other hospitals around the country.
The study also has several limitations that need be kept in mind in interpreting the study results. First, desirability bias may have exaggerated some of the positive views expressed by hospitalists toward implementation of routine assessment of dyspnea. Second, because this was a survey, the estimates of dyspnea assessment and documentation practices of both physicians and nurses were based on the respondents' perception and not an objective review of medical records, and the results may be different from actual practice. Third, this was not a population‐based random sample of hospitalists, and it may not be entirely representative; however, those surveyed were from a diverse set of sites with different geographical location, size, academic affiliation, and practice environment, and their time in practice varied widely. Last, we do not have information on nonrespondents, and there is a possibility of nonresponse bias, although the high response rate lessens the risk.
CONCLUSIONS
The results of this survey suggest that most hospitalists believe that routine assessment of dyspnea severity would enhance their clinical decision making and improve patient care. Standardized assessment of dyspnea might result in better awareness of this symptom among providers, reduce undertreatment and mistreatment, and ultimately result in better outcomes for patients. However, implementation of the routine assessment of dyspnea would change current clinical practices and may have a significant effect on existing nursing and physician workflows. Additional research is needed to determine the feasibility and impact on outcomes of routine dyspnea assessment.
Acknowledgements
The authors wish to acknowledge Ms. Anu Joshi for her help with editing the manuscript and assisting with table preparations.
Disclosures
Dr. Stefan is supported by grant K01HL114631‐01A1 from the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1RR025752. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. M.S.S. and P.K.L. conceived of the study. M.S.S. acquired the data with the help of all collaborators. M.S.S., P.K.L., P.S.P., and A.P. analyzed and interpreted the data. M.S.S. drafted the manuscript. All authors critically reviewed the manuscript for intellectual content. M.S.S., P.K.L., and A.P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M.S.S. is the guarantor for this article, and is responsible for the content of the article, including data and analysis. The authors report no conflicts of interest.
- , , , et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452.
- CDC/ National Center for Health Statistics. National Hospital Amulatory Medical Care Survey: 2011 Emergency Department Summary Tables. http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed May 15, 2015.
- , , , . Signs and symptoms of heart failure: are you asking the right questions? Am J Crit Care. 2010;19(5):443–452.
- , , , et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18(2):69–78.
- , . Prevalence of distressing symptoms in hospitalised patients on medical wards: A cross‐sectional study. BMC Palliat Care. 2008;7:16.
- , . Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234–236.
- , , , et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137(3):674–691.
- , . Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med. 1989;86(3):262–266.
- The Joint Commission. Facts about Pain Management. http://www.jointcommission.org/pain_management/. Accessed May, 15, 2015.
- . Hospitalist programs in the age of healthcare reform. J Healthc Manag. 2010;55(6):378–380.
- , , , . The Use of Hospitalists by Small Rural Hospitals: Results of a National Survey. Med Care Res Rev. 2014;71(4):356–366.
- Tufts CTSI. REDCap [Internet]. Tufts Clinical and Translational Science Institute. http://www.tuftsctsi.org/Services-and-Consultation/REDCap.aspx. Accessed May, 15, 2015.
- , . Multi‐dimensional Assessment of Dyspnea. Dyspnoea in Advanced Disease: A guide to clinical management; 2005.
- , , , et al. Cancer care quality measures: symptoms and end‐of‐life care. Evid Rep Technol Assess (Full Rep). 2006(137):1–77.
- . Defining and measuring quality palliative and end‐of‐life care in the intensive care unit. Crit Care Med. 2006;34(11 Suppl):S309–316.
- . Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325.
- . Can a self‐rating 0‐10 scale for dyspnea yield a common language that is understood by ED nurses, patients, and their families? J Emerg Nurs. 2000;26(3):233–234.
- , , . Measurement of dyspnea: word labeled visual analog scale vs. verbal ordinal scale. Respir Physiol Neurobiol. 2003;134(2):77–83.
- , , , , , . Verbal numerical scales are as reliable and sensitive as visual analog scales for rating dyspnea in young and older subjects. Respir Physiol Neurobiol. 2007;157(2‐3):360–365.
- , , , , . Validation of a three‐factor measurement model of dyspnea in hospitalized adults with heart failure. Heart Lung. 2011;41(1):44–56.
- , , . Patient reporting and doctor recognition of dyspnoea in a comprehensive cancer centre. Intern Med J. 2006;36(6):381–384.
- , , , . Lung cancer and dyspnea: the patient's perception. Oncol Nurs Forum. 1986;13(5):19–24.
- , , , . Opioids, respiratory function, and dyspnea. Am J Hosp Palliat Care. 2003;20(1):57–61.
Dyspnea, defined as a subjective experience of breathing discomfort,[1] is the seventh most frequent reason adult patients present to the emergency room and the most frequent cause for emergency room visits in patients 65 years or older.[2] Moreover, dyspnea is experienced by 49% of patients hospitalized with a medical condition[3, 4, 5] and by 70% of patients who are seriously ill.[6]
Based on evidence that patients are not treated consistently and effectively for relief of their shortness of breath, the American College of Chest Physicians (ACCP) statement on dyspnea management in patients with advanced lung or heart disease recommended that patients should be asked to rate their dyspnea, and the rating should be routinely documented in the medical record to guide management.[7] Although clinicians may question the utility of routine assessment of dyspnea using a standardized scale, studies have found that the prevalence of dyspnea reported from chart review is much lower than when patients are directly interviewed.[8] This may be the result of underrecognition of dyspnea or poor documentation by physicians, or that patients may not communicate their symptoms unless the physician specifically asks. As is the case with pain, routine assessment of dyspnea severity could lead to improved clinical management and greater patient‐centered care. However, unlike in the case of pain, regulatory bodies, such as the Joint Commission for Accreditation of Healthcare Organization, do not require routine dyspnea assessment.[9]
Currently, there are more than 40,000 hospitalists in the United States, and the vast majority of hospitals with >200 beds have a hospitalist group.[10] Hospitalists care for over 60% of inpatients[11] and play a major role in the management of patients with acute cardiopulmonary diseases. If standardized approaches for the assessment and documentation of dyspnea are to be implemented, hospitalists would be a key stakeholder group for utilizing enhanced clinical information about dyspnea. Therefore, we evaluated attitudes and practices of hospitalists in regard to the assessment and management of dyspnea, including the potential benefits and challenges related to the implementation of standardized assessment. We hypothesized that hospitalists would believe that a dyspnea scale for assessment of severity could improve their management of patients with cardiovascular diseases. Further, we hypothesized that physicians who agreed with the general statement that dyspnea is an important clinical problem would be more likely to believe that routine dyspnea assessment would be valuable.
METHODS
Study Sample
We invited 255 attending hospitalists from 9 geographically and structurally diverse hospitals to complete a survey about the assessment and management of dyspnea. The 9 hospitals represent range of practice environments including 4 academic medical centers, 2 community teaching and 3 nonteaching hospitals, 1 Veterans Administration hospital, and 2 staff‐model HMOs (see Supporting Table 1 in the online version of this article). The survey was distributed online using REDCap (Research Electronic Data Capture), a secure web‐based interface application.[12] A coinvestigator who was a pulmonary critical‐care physician at each site sent an initial email to their hospitalist groups that alerted them to expect a survey from the principal investigator. This notification was subsequently followed by an email invitation containing an informational cover letter and a link to the online survey. The cover letter stated that the completed surveys would not be stored at the local sites and that all the analyzed data would be deidentified. Nonrespondents were sent reminders at 2 and 4 weeks after the initial mailing. A $25 electronic gift card was provided as a gesture of appreciation for their time. The survey was conducted between September 2013 and December 2013.
The study was approved by the Baystate Health Institutional Review Board, Springfield, Massachusetts, with a waiver for written informed consent.
Questionnaire
We developed a 17‐item instrument based on a review of the dyspnea literature and a prior ACCP survey.[12] Questions were piloted with 4 hospitalists at a single institution and modified to improve face validity and clarity (see Supporting Information in the online version of this article for the full survey).
Hospitalists were asked to consider the care of patients admitted for acute cardiopulmonary disease, including heart failure, chronic obstructive pulmonary disease, and pneumonia. A series of 5‐point Likert scales were used to assess the respondents level of agreement with statements related to the following domains: the importance of dyspnea in clinical care, the potential benefits and challenges of routine dyspnea assessment (statements such as: Having a standardized assessment of dyspnea severity would be helpful in management of patients with cardiopulmonary diseases. Dyspnea assessment by a scale should be part of the vital signs for patients with cardiopulmonary diseases.), and management of dyspnea (questions regarding the use of opioids and other nonpharmacological therapies). Additional questions were asked about current assessment practices (questions such as: How often do you assess severity of dyspnea? What is your approach in assessing dyspnea? with options of choosing a categorical or numerical scale), if dyspnea is assessed in their institution by nurses and how often, and the influence of dyspnea severity assessment on their management. The survey had 1 question that solicited comments from the participants: If you don't think that it would be useful to have a standardized dyspnea assessment, please tell us why.
Data Analysis
Responses to survey questions were summarized via counts and percentages in each response category. Adopting the methodology used in the ACCP consensus statement, strongly agree and somewhat agree were combined into a single category of agreement. We also presented percentage of responses in the 2 levels of agreement (strongly agree and somewhat agree) for each question in a bar graph.
Associations between tertiles of physicians' time in practice and attitude toward dyspnea were evaluated via 2 or Fisher exact test.
To examine how answers to the first 2 questions, which assessed attitude toward importance of dyspnea in clinical care, affect answers to the remaining questions, we grouped respondents in 3 categories (strongly agree, agree to these questions, do not agree) and tested the associations using 2 or Fisher exact test.
All analyses were performed using SAS version 9.3 (SAS institute, Inc., Cary, NC) and Stata release 13.1 (StataCorp, College Station, TX).
RESULTS
Overall, 178 (69.8%) of 255 identified hospitalists completed the survey, and all 9 participating hospitals had a response rate greater than 50%. The median number of years in practice was 6 (range, 038 years). A majority (77.5%) of respondents agreed with the statement that dyspnea is 1 of the major symptoms of patients with cardiopulmonary disease, and that its treatment is central to the management of these patients (77.0%) (Figure 1).

Attitude and Practices Surrounding Dyspnea Assessment
When asked about their current assessment of dyspnea, a majority (84.3%) of the hospitalists stated that they assess dyspnea on a daily basis; two‐thirds indicated that they use a categorical scale (ie, no shortness of breath, improved or worsened compared with a prior date), and one‐third indicated that they ask whether the patient is dyspneic or not. Fifty‐six percent of hospitalists stated that dyspnea is regularly assessed by nurses in their hospital.
The majority of respondents agreed (78.6%, 23.0% strongly and 55.6% somewhat agree) that standardized assessment of dyspnea severity, using a numeric scale and serial measurements as part of the vital signs, would benefit the management of patients with cardiopulmonary diseases. Furthermore, 79.6% (33.0% strongly and 46.6% somewhat agree) reported that using a dyspnea scale that included information to further characterize the patient‐reported experience, such as the level of distress associated with dyspnea, would be helpful in management.
Approximately 90% of the hospitalists indicated that awareness of dyspnea severity has an influence on clinical decision making, including whether to intensify treatment of underlying conditions, to pursue additional diagnostic testing, or to modify discharge timing. Additionally, two‐thirds of hospitalists agreed that awareness of dyspnea severity influences their decision to add opioids, whereas only one‐third prescribed nonpharmacologic symptom‐oriented treatment (Table 1).
| Frequency (%) | |
|---|---|
| |
| When caring for patients with acute cardiopulmonary diseases, how often do you assess severity of dyspnea?* | |
| At admission | 66 (37.1) |
| At discharge | 59 (33.2) |
| Daily until discharge | 150 (84.3) |
| More often than daily | 58 (32.6) |
| Which description best characterizes your approach to assessing dyspnea severity? | |
| I don't regularly ask about dyspnea severity | 3 (1.7) |
| I ask the patient whether or not they are having shortness of breath | 50 (28.3) |
| I ask the patient to rate the severity of shortness of breath using a numeric scale | 4 (2.3) |
| I ask the patient to rate the severity of shortness of breath using a categorical scale (eg, somewhat SOB, no SOB, improved or worsened compared with a prior date) | 120 (67.8) |
| When is dyspnea severity assessed and documented by nursing at your hospital?* | |
| Dyspnea is not routinely assessed | 60 (33.7) |
| At admission | 30 (16.9) |
| Daily | 43 (24.2) |
| Each shift | 64 (36.0) |
| Awareness of dyspnea severity affects my management by:* | |
| Influencing my decision to intensify treatment of the patient's underlying condition | 170 (95.5) |
| Influencing my decision to pursue additional diagnostic testing | 160 (89.9) |
| Influencing my decision to add pharmacologic‐based, symptom‐oriented treatment for dyspnea, such as opioids | 115 (64.6) |
| Influencing my decision to add nonpharmacologic‐based, symptom‐oriented treatment for dyspnea, such as fans or pursed lip breathing technique | 58 (32.6) |
| Influencing my decision regarding timing of discharge | 162 (91.0) |
| Which of the following nonpharmacologic therapies are effective for the relief of dyspnea?* | |
| Pursed lip breathing | 113 (63.5) |
| Relaxation techniques | 137 (77.0) |
| Noninvasive ventilation | 143 (80.3) |
| O2 for nonhypoxemic patients | 89 (50.0) |
| Cool air/fan | 125 (70.2) |
| Cognitive behavioral strategies | 101 (56.7) |
Forty‐two percent of the respondents agreed that patients are able to rate their dyspnea on a scale (2.3% strongly agree and 40.0% agree), and 73.0% indicated that patient experience of dyspnea should guide management independent of physiologic measures such as respiratory rate and oxygen saturation (Figure 1).
Several potential barriers were identified among the 18 participants who did not think that a standardized assessment of dyspnea would be beneficial, including concerns that (1) a dyspnea severity scale is too subjective and numerical scales are not useful for a subjective symptom (19.0%), (2) patients may overrate their symptom or will not be able to rate their dyspnea using a scale (31.0%), or (3) categorical description is sufficient (31.0%).
Practices in Dyspnea Management
Seventy‐nine percent of respondents agreed with the statement that judicious use of opioids can provide relief of dyspnea (26.1% strongly and 52.8% agreed), and 88.7% hospitalists identified the risk of respiratory depression as 1 of the barriers for the limited use of opioids. The majority of physicians (60%80%) considered nonpharmacologic therapies effective for symptomatic treatment of dyspnea, including in the order of agreement: noninvasive ventilation, relaxation techniques, cool air/fan, use of pursed lip breathing, and oxygen for nonhypoxemic patients (Table 1).
Physician Experience and Attitudes Toward Dyspnea Management
When we stratified hospitalists in tertiles of median years of time in practice (median [range]: 2 [04], 6 [58] and 15 [938]), we did not find an association with any of the responses to the questions.
Attitude Regarding the Importance of Dyspnea in Clinical Care and Responses to Subsequent Questions
Respondents who strongly agree or agree that dyspnea is the primary presenting symptom in patients with cardiovascular condition and that dyspnea relief is central to the management of these patients were more likely to believe that patients would like to be asked about their dyspnea (61.2% vs 30.2% vs 29.7%). They also had a more positive attitude about the usefulness of a standardized assessment of dyspnea and the inclusion of the assessment of dyspnea by a scale in the vital signs (Table 2).
| Description | Do Not Agree, n (%) | Somewhat Agree, n (%) | Strongly Agree, n (%) | P Value* |
|---|---|---|---|---|
| ||||
| 37 (20.9) | 43 (24.3) | 97 (54.8) | ||
| Which description best characterizes your approach to assessing dyspnea severity? | 0.552 | |||
| I don't regularly ask about dyspnea severity | 0 (0) | 0 (0) | 3 (3.1) | |
| I ask the patient whether or not they are having shortness of breath | 11 (29.7) | 14 (32.6) | 25 (25.8) | |
| I ask the patient to rate the severity of shortness of breath using a numeric scale | 2 (5.4) | 1 (2.3) | 1 (1.0) | |
| I ask the patient to rate the severity of shortness of breath using a categorical scale (e.g., somewhat shortness of breath, no shortness of breath, improved or worsened compared with a prior date) | 24 (64.9) | 28 (65.1) | 68 (70.1) | |
| Patients would like me to ask them about their dyspnea. | <0.0001 | |||
| Somewhat agree | 9 (24.3) | 21 (48.8) | 32 (32.7) | |
| Strongly agree | 11 (29.7) | 13 (30.2) | 60 (61.2) | |
| Patients are able to rate their own dyspnea intensity on a scale of 0‐10. | 0.432 | |||
| Somewhat agree | 12 (32.4) | 16 (37.2) | 42 (43.3) | |
| Strongly agree | 2 (5.4) | 0 (0) | 2 (2.1) | |
| Having a standardized assessment of dyspnea severity would be helpful to me in management of patients with cardiopulmonary diseases. | 0.026 | |||
| Somewhat agree | 17 (46.0) | 25 (58.1) | 57 (58.2) | |
| Strongly agree | 7 (18.9) | 6 (14.0) | 28 (28.6) | |
| Serial measurements of dyspnea would be useful for assessing response to therapy. | 0.042 | |||
| Somewhat agree | 14 (37.8) | 28 (65.1) | 48 (49.5) | |
| Strongly agree | 16 (43.2) | 12 (27.9) | 43 (44.3) | |
| Dyspnea assessment by a scale should be part of the vital signs for patients with cardiopulmonary diseases. | 0.042 | |||
| Somewhat agree | 13 (35.1) | 17 (39.5) | 51 (52.0) | |
| Strongly agree | 4 (10.8) | 5 (11.6) | 19 (19.4) | |
| Using an enhanced dyspnea scale that includes information about the following 4 features 1) Current dyspnea severity, 2) Worst dyspnea ever, 3) Improvement of dyspnea since admission, 4) Acceptability of current level of dyspnea, would be more helpful for my management than a single question focused on dyspnea severity. | 0.03 | |||
| Somewhat agree | 14 (40.0) | 24 (55.8) | 44 (44.9) | |
| Strongly agree | 9 (25.7) | 9 (20.9) | 40 (40.8) | |
| The patients experience of dyspnea should be used to guide treatment decisions independent of objective measures such as respiratory rate and oxygen saturation. | 0.10 | |||
| Somewhat agree | 20 (54.0) | 21 (48.8) | 51 (52.0) | |
| Strongly agree | 5 (13.5) | 6 (14.0) | 27 (27.6) | |
| Judicious use of oral and/or parenteral opioids can provide relief of dyspnea. | 0.21 | |||
| Somewhat agree | 20 (54.0) | 23 (54.8) | 50 (51.6) | |
| Strongly agree | 10 (27.0) | 6 (14.3) | 30 (30.9) | |
| Limited use of opioids for relief of dyspnea in patients with advanced cardiopulmonary disorders is often due to concerns of respiratory depression. | 0.71 | |||
| Somewhat agree | 17 (46.0) | 23 (54.8) | 43 (43.9) | |
| Strongly agree | 15 (40.5) | 14 (33.3) | 45 (45.9) | |
DISCUSSION
In this survey of 178 most hospitalists from a diverse group of 9 US hospitals, we found that most indicate that severity of dyspnea has a profound influence on their clinical practice (including their decision whether to intensify treatments such as diuretics or bronchodilators, to pursue additional diagnostic testing, add opioids or other nonpharmacological treatments) and ultimately their decision regarding the timing of hospital discharge. More importantly, whereas less than half reported experience with standardized assessment of dyspnea severity, most stated that such data would be very useful in their practice.
Despite being a highly prevalent symptom in diverse patient populations, several studies have shown that documentation of dyspnea is sporadic and evaluation of dyspnea quality of care is not routinely performed.[13, 14, 15] Statements from a number of professional societies, including the ACCP, the American Thoracic Society and the Canadian Respiratory Society, recommend that dyspnea management should rely on patient reporting, and that dyspnea severity should be recorded.[1, 4, 7] Assessment is an essential step to guide interventions; however, simply asking about the presence or absence of dyspnea is insufficient.
Several rating scales have been validated and might be implementable in the acute care setting, including the Numerical Rating Scale and the Visual Assessment Scale.[16, 17, 18, 19, 20] Our survey shows that standardized documentation of dyspnea severity in clinical practice is uncommon. However, most hospitalists in our study believed that assessment of dyspnea, using a standardized scale, would positively impact their management of patients with cardiopulmonary disease.
There are a number of potential benefits of routine assessment of dyspnea in hospitalized patients. Implementation of a standardized approach to dyspnea measurement would result in more uniform assessment and documentation practices, and in turn greater awareness among members of the patient‐care team. Though not sufficient to improve care, measurement is necessary because physicians do not always recognize the severity of patients' dyspnea or may not recognize its presence. A retrospective study that assessed the prevalence of symptoms in 410 ambulatory patients showed that one‐quarter of patients had dyspnea, but only half of them told their doctor about it.[21] Two other studies of patients with cancer diagnoses found that 30%70% of patients had dyspnea, but the symptom was recognized in only half of them; even when recognized, dyspnea severity was frequently underrated by physicians.[21, 22] Importantly, underestimation appears to correlate with underutilization of symptomatic management of dyspnea.[8]
Although the results of our survey are encouraging, they highlight a number of potential barriers and misconceptions among hospitalists. For example, although dyspnea can be characterized only by the person experiencing it, only 42% of our survey respondents believed that patients are able to rate their dyspnea intensity on a scale. Some of these responses may be influenced by the fact that dyspnea scales are not currently available to patients under their care. Another explanation is that similar to the case for pain, some hospitalists may believe that patients will exaggerate dyspnea severity. Almost one‐third of the respondents stated that objective measures, such as respiratory rate or oxygen saturation, are more important than a patient's experience of dyspnea in guiding the treatment, and that dyspnea is a subjective symptom and not a vital sign itself. Hospitalists who appreciated the importance of dyspnea in clinical practice were more likely to support the implementation of a standardized dyspnea scale for dyspnea assessment.
Although the potential benefits of including routine measurement of dyspnea in standard hospital practice may seem obvious, evidence that implementing routine assessment improves patient care or outcomes is lacking. Even if hospitalists see the value of dyspnea assessment, asking nurses to collect and document additional information would represent a substantial change in hospital workflow. Finally, without specific protocols to guide care, it is unclear whether physicians will be able to use new information about dyspnea severity effectively. Future studies need to evaluate the impact of implementing routine dyspnea assessment on the management of patients with cardiopulmonary diseases including the use of evidence‐based interventions and reducing the use of less valuable care.
Most hospitalists agreed with the basic principles of dyspnea treatment in patients with advanced cardiopulmonary disease after the primary disease had been stabilized. Effective measures are available, and several guidelines endorse opioids in dyspnea management.[1, 4, 7] However, many clinicians are uncomfortable with this approach for dyspnea, and opioids remain underused. In our study, almost 90% of physicians recognized that concerns about respiratory depression limits opioids use as a treatment. A qualitative study that explored the physicians' perspective toward opioids showed that most physicians were reluctant to prescribe opioids for refractory dyspnea, describing a lack of related knowledge and experience, and fears related to the potential adverse effects. The findings of our study also outline the need to better educate residents and hospitalists on the assessment and management of dyspnea, including prescribing opioids for refractory dyspnea.[23]
Study Strengths and Limitations
This study has several strengths. To our knowledge, it is the first to explore hospitalists' perspectives on incorporating dyspnea assessment in their clinical practice. Hospitalists are the attending physicians for a large majority of inpatients and would be the main users of a dyspnea severity scale. Our questionnaire survey included a large number of hospitalists, from 9 geographically and structurally diverse hospitals, which increased the generalizability of the findings to other hospitals around the country.
The study also has several limitations that need be kept in mind in interpreting the study results. First, desirability bias may have exaggerated some of the positive views expressed by hospitalists toward implementation of routine assessment of dyspnea. Second, because this was a survey, the estimates of dyspnea assessment and documentation practices of both physicians and nurses were based on the respondents' perception and not an objective review of medical records, and the results may be different from actual practice. Third, this was not a population‐based random sample of hospitalists, and it may not be entirely representative; however, those surveyed were from a diverse set of sites with different geographical location, size, academic affiliation, and practice environment, and their time in practice varied widely. Last, we do not have information on nonrespondents, and there is a possibility of nonresponse bias, although the high response rate lessens the risk.
CONCLUSIONS
The results of this survey suggest that most hospitalists believe that routine assessment of dyspnea severity would enhance their clinical decision making and improve patient care. Standardized assessment of dyspnea might result in better awareness of this symptom among providers, reduce undertreatment and mistreatment, and ultimately result in better outcomes for patients. However, implementation of the routine assessment of dyspnea would change current clinical practices and may have a significant effect on existing nursing and physician workflows. Additional research is needed to determine the feasibility and impact on outcomes of routine dyspnea assessment.
Acknowledgements
The authors wish to acknowledge Ms. Anu Joshi for her help with editing the manuscript and assisting with table preparations.
Disclosures
Dr. Stefan is supported by grant K01HL114631‐01A1 from the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1RR025752. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. M.S.S. and P.K.L. conceived of the study. M.S.S. acquired the data with the help of all collaborators. M.S.S., P.K.L., P.S.P., and A.P. analyzed and interpreted the data. M.S.S. drafted the manuscript. All authors critically reviewed the manuscript for intellectual content. M.S.S., P.K.L., and A.P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M.S.S. is the guarantor for this article, and is responsible for the content of the article, including data and analysis. The authors report no conflicts of interest.
Dyspnea, defined as a subjective experience of breathing discomfort,[1] is the seventh most frequent reason adult patients present to the emergency room and the most frequent cause for emergency room visits in patients 65 years or older.[2] Moreover, dyspnea is experienced by 49% of patients hospitalized with a medical condition[3, 4, 5] and by 70% of patients who are seriously ill.[6]
Based on evidence that patients are not treated consistently and effectively for relief of their shortness of breath, the American College of Chest Physicians (ACCP) statement on dyspnea management in patients with advanced lung or heart disease recommended that patients should be asked to rate their dyspnea, and the rating should be routinely documented in the medical record to guide management.[7] Although clinicians may question the utility of routine assessment of dyspnea using a standardized scale, studies have found that the prevalence of dyspnea reported from chart review is much lower than when patients are directly interviewed.[8] This may be the result of underrecognition of dyspnea or poor documentation by physicians, or that patients may not communicate their symptoms unless the physician specifically asks. As is the case with pain, routine assessment of dyspnea severity could lead to improved clinical management and greater patient‐centered care. However, unlike in the case of pain, regulatory bodies, such as the Joint Commission for Accreditation of Healthcare Organization, do not require routine dyspnea assessment.[9]
Currently, there are more than 40,000 hospitalists in the United States, and the vast majority of hospitals with >200 beds have a hospitalist group.[10] Hospitalists care for over 60% of inpatients[11] and play a major role in the management of patients with acute cardiopulmonary diseases. If standardized approaches for the assessment and documentation of dyspnea are to be implemented, hospitalists would be a key stakeholder group for utilizing enhanced clinical information about dyspnea. Therefore, we evaluated attitudes and practices of hospitalists in regard to the assessment and management of dyspnea, including the potential benefits and challenges related to the implementation of standardized assessment. We hypothesized that hospitalists would believe that a dyspnea scale for assessment of severity could improve their management of patients with cardiovascular diseases. Further, we hypothesized that physicians who agreed with the general statement that dyspnea is an important clinical problem would be more likely to believe that routine dyspnea assessment would be valuable.
METHODS
Study Sample
We invited 255 attending hospitalists from 9 geographically and structurally diverse hospitals to complete a survey about the assessment and management of dyspnea. The 9 hospitals represent range of practice environments including 4 academic medical centers, 2 community teaching and 3 nonteaching hospitals, 1 Veterans Administration hospital, and 2 staff‐model HMOs (see Supporting Table 1 in the online version of this article). The survey was distributed online using REDCap (Research Electronic Data Capture), a secure web‐based interface application.[12] A coinvestigator who was a pulmonary critical‐care physician at each site sent an initial email to their hospitalist groups that alerted them to expect a survey from the principal investigator. This notification was subsequently followed by an email invitation containing an informational cover letter and a link to the online survey. The cover letter stated that the completed surveys would not be stored at the local sites and that all the analyzed data would be deidentified. Nonrespondents were sent reminders at 2 and 4 weeks after the initial mailing. A $25 electronic gift card was provided as a gesture of appreciation for their time. The survey was conducted between September 2013 and December 2013.
The study was approved by the Baystate Health Institutional Review Board, Springfield, Massachusetts, with a waiver for written informed consent.
Questionnaire
We developed a 17‐item instrument based on a review of the dyspnea literature and a prior ACCP survey.[12] Questions were piloted with 4 hospitalists at a single institution and modified to improve face validity and clarity (see Supporting Information in the online version of this article for the full survey).
Hospitalists were asked to consider the care of patients admitted for acute cardiopulmonary disease, including heart failure, chronic obstructive pulmonary disease, and pneumonia. A series of 5‐point Likert scales were used to assess the respondents level of agreement with statements related to the following domains: the importance of dyspnea in clinical care, the potential benefits and challenges of routine dyspnea assessment (statements such as: Having a standardized assessment of dyspnea severity would be helpful in management of patients with cardiopulmonary diseases. Dyspnea assessment by a scale should be part of the vital signs for patients with cardiopulmonary diseases.), and management of dyspnea (questions regarding the use of opioids and other nonpharmacological therapies). Additional questions were asked about current assessment practices (questions such as: How often do you assess severity of dyspnea? What is your approach in assessing dyspnea? with options of choosing a categorical or numerical scale), if dyspnea is assessed in their institution by nurses and how often, and the influence of dyspnea severity assessment on their management. The survey had 1 question that solicited comments from the participants: If you don't think that it would be useful to have a standardized dyspnea assessment, please tell us why.
Data Analysis
Responses to survey questions were summarized via counts and percentages in each response category. Adopting the methodology used in the ACCP consensus statement, strongly agree and somewhat agree were combined into a single category of agreement. We also presented percentage of responses in the 2 levels of agreement (strongly agree and somewhat agree) for each question in a bar graph.
Associations between tertiles of physicians' time in practice and attitude toward dyspnea were evaluated via 2 or Fisher exact test.
To examine how answers to the first 2 questions, which assessed attitude toward importance of dyspnea in clinical care, affect answers to the remaining questions, we grouped respondents in 3 categories (strongly agree, agree to these questions, do not agree) and tested the associations using 2 or Fisher exact test.
All analyses were performed using SAS version 9.3 (SAS institute, Inc., Cary, NC) and Stata release 13.1 (StataCorp, College Station, TX).
RESULTS
Overall, 178 (69.8%) of 255 identified hospitalists completed the survey, and all 9 participating hospitals had a response rate greater than 50%. The median number of years in practice was 6 (range, 038 years). A majority (77.5%) of respondents agreed with the statement that dyspnea is 1 of the major symptoms of patients with cardiopulmonary disease, and that its treatment is central to the management of these patients (77.0%) (Figure 1).

Attitude and Practices Surrounding Dyspnea Assessment
When asked about their current assessment of dyspnea, a majority (84.3%) of the hospitalists stated that they assess dyspnea on a daily basis; two‐thirds indicated that they use a categorical scale (ie, no shortness of breath, improved or worsened compared with a prior date), and one‐third indicated that they ask whether the patient is dyspneic or not. Fifty‐six percent of hospitalists stated that dyspnea is regularly assessed by nurses in their hospital.
The majority of respondents agreed (78.6%, 23.0% strongly and 55.6% somewhat agree) that standardized assessment of dyspnea severity, using a numeric scale and serial measurements as part of the vital signs, would benefit the management of patients with cardiopulmonary diseases. Furthermore, 79.6% (33.0% strongly and 46.6% somewhat agree) reported that using a dyspnea scale that included information to further characterize the patient‐reported experience, such as the level of distress associated with dyspnea, would be helpful in management.
Approximately 90% of the hospitalists indicated that awareness of dyspnea severity has an influence on clinical decision making, including whether to intensify treatment of underlying conditions, to pursue additional diagnostic testing, or to modify discharge timing. Additionally, two‐thirds of hospitalists agreed that awareness of dyspnea severity influences their decision to add opioids, whereas only one‐third prescribed nonpharmacologic symptom‐oriented treatment (Table 1).
| Frequency (%) | |
|---|---|
| |
| When caring for patients with acute cardiopulmonary diseases, how often do you assess severity of dyspnea?* | |
| At admission | 66 (37.1) |
| At discharge | 59 (33.2) |
| Daily until discharge | 150 (84.3) |
| More often than daily | 58 (32.6) |
| Which description best characterizes your approach to assessing dyspnea severity? | |
| I don't regularly ask about dyspnea severity | 3 (1.7) |
| I ask the patient whether or not they are having shortness of breath | 50 (28.3) |
| I ask the patient to rate the severity of shortness of breath using a numeric scale | 4 (2.3) |
| I ask the patient to rate the severity of shortness of breath using a categorical scale (eg, somewhat SOB, no SOB, improved or worsened compared with a prior date) | 120 (67.8) |
| When is dyspnea severity assessed and documented by nursing at your hospital?* | |
| Dyspnea is not routinely assessed | 60 (33.7) |
| At admission | 30 (16.9) |
| Daily | 43 (24.2) |
| Each shift | 64 (36.0) |
| Awareness of dyspnea severity affects my management by:* | |
| Influencing my decision to intensify treatment of the patient's underlying condition | 170 (95.5) |
| Influencing my decision to pursue additional diagnostic testing | 160 (89.9) |
| Influencing my decision to add pharmacologic‐based, symptom‐oriented treatment for dyspnea, such as opioids | 115 (64.6) |
| Influencing my decision to add nonpharmacologic‐based, symptom‐oriented treatment for dyspnea, such as fans or pursed lip breathing technique | 58 (32.6) |
| Influencing my decision regarding timing of discharge | 162 (91.0) |
| Which of the following nonpharmacologic therapies are effective for the relief of dyspnea?* | |
| Pursed lip breathing | 113 (63.5) |
| Relaxation techniques | 137 (77.0) |
| Noninvasive ventilation | 143 (80.3) |
| O2 for nonhypoxemic patients | 89 (50.0) |
| Cool air/fan | 125 (70.2) |
| Cognitive behavioral strategies | 101 (56.7) |
Forty‐two percent of the respondents agreed that patients are able to rate their dyspnea on a scale (2.3% strongly agree and 40.0% agree), and 73.0% indicated that patient experience of dyspnea should guide management independent of physiologic measures such as respiratory rate and oxygen saturation (Figure 1).
Several potential barriers were identified among the 18 participants who did not think that a standardized assessment of dyspnea would be beneficial, including concerns that (1) a dyspnea severity scale is too subjective and numerical scales are not useful for a subjective symptom (19.0%), (2) patients may overrate their symptom or will not be able to rate their dyspnea using a scale (31.0%), or (3) categorical description is sufficient (31.0%).
Practices in Dyspnea Management
Seventy‐nine percent of respondents agreed with the statement that judicious use of opioids can provide relief of dyspnea (26.1% strongly and 52.8% agreed), and 88.7% hospitalists identified the risk of respiratory depression as 1 of the barriers for the limited use of opioids. The majority of physicians (60%80%) considered nonpharmacologic therapies effective for symptomatic treatment of dyspnea, including in the order of agreement: noninvasive ventilation, relaxation techniques, cool air/fan, use of pursed lip breathing, and oxygen for nonhypoxemic patients (Table 1).
Physician Experience and Attitudes Toward Dyspnea Management
When we stratified hospitalists in tertiles of median years of time in practice (median [range]: 2 [04], 6 [58] and 15 [938]), we did not find an association with any of the responses to the questions.
Attitude Regarding the Importance of Dyspnea in Clinical Care and Responses to Subsequent Questions
Respondents who strongly agree or agree that dyspnea is the primary presenting symptom in patients with cardiovascular condition and that dyspnea relief is central to the management of these patients were more likely to believe that patients would like to be asked about their dyspnea (61.2% vs 30.2% vs 29.7%). They also had a more positive attitude about the usefulness of a standardized assessment of dyspnea and the inclusion of the assessment of dyspnea by a scale in the vital signs (Table 2).
| Description | Do Not Agree, n (%) | Somewhat Agree, n (%) | Strongly Agree, n (%) | P Value* |
|---|---|---|---|---|
| ||||
| 37 (20.9) | 43 (24.3) | 97 (54.8) | ||
| Which description best characterizes your approach to assessing dyspnea severity? | 0.552 | |||
| I don't regularly ask about dyspnea severity | 0 (0) | 0 (0) | 3 (3.1) | |
| I ask the patient whether or not they are having shortness of breath | 11 (29.7) | 14 (32.6) | 25 (25.8) | |
| I ask the patient to rate the severity of shortness of breath using a numeric scale | 2 (5.4) | 1 (2.3) | 1 (1.0) | |
| I ask the patient to rate the severity of shortness of breath using a categorical scale (e.g., somewhat shortness of breath, no shortness of breath, improved or worsened compared with a prior date) | 24 (64.9) | 28 (65.1) | 68 (70.1) | |
| Patients would like me to ask them about their dyspnea. | <0.0001 | |||
| Somewhat agree | 9 (24.3) | 21 (48.8) | 32 (32.7) | |
| Strongly agree | 11 (29.7) | 13 (30.2) | 60 (61.2) | |
| Patients are able to rate their own dyspnea intensity on a scale of 0‐10. | 0.432 | |||
| Somewhat agree | 12 (32.4) | 16 (37.2) | 42 (43.3) | |
| Strongly agree | 2 (5.4) | 0 (0) | 2 (2.1) | |
| Having a standardized assessment of dyspnea severity would be helpful to me in management of patients with cardiopulmonary diseases. | 0.026 | |||
| Somewhat agree | 17 (46.0) | 25 (58.1) | 57 (58.2) | |
| Strongly agree | 7 (18.9) | 6 (14.0) | 28 (28.6) | |
| Serial measurements of dyspnea would be useful for assessing response to therapy. | 0.042 | |||
| Somewhat agree | 14 (37.8) | 28 (65.1) | 48 (49.5) | |
| Strongly agree | 16 (43.2) | 12 (27.9) | 43 (44.3) | |
| Dyspnea assessment by a scale should be part of the vital signs for patients with cardiopulmonary diseases. | 0.042 | |||
| Somewhat agree | 13 (35.1) | 17 (39.5) | 51 (52.0) | |
| Strongly agree | 4 (10.8) | 5 (11.6) | 19 (19.4) | |
| Using an enhanced dyspnea scale that includes information about the following 4 features 1) Current dyspnea severity, 2) Worst dyspnea ever, 3) Improvement of dyspnea since admission, 4) Acceptability of current level of dyspnea, would be more helpful for my management than a single question focused on dyspnea severity. | 0.03 | |||
| Somewhat agree | 14 (40.0) | 24 (55.8) | 44 (44.9) | |
| Strongly agree | 9 (25.7) | 9 (20.9) | 40 (40.8) | |
| The patients experience of dyspnea should be used to guide treatment decisions independent of objective measures such as respiratory rate and oxygen saturation. | 0.10 | |||
| Somewhat agree | 20 (54.0) | 21 (48.8) | 51 (52.0) | |
| Strongly agree | 5 (13.5) | 6 (14.0) | 27 (27.6) | |
| Judicious use of oral and/or parenteral opioids can provide relief of dyspnea. | 0.21 | |||
| Somewhat agree | 20 (54.0) | 23 (54.8) | 50 (51.6) | |
| Strongly agree | 10 (27.0) | 6 (14.3) | 30 (30.9) | |
| Limited use of opioids for relief of dyspnea in patients with advanced cardiopulmonary disorders is often due to concerns of respiratory depression. | 0.71 | |||
| Somewhat agree | 17 (46.0) | 23 (54.8) | 43 (43.9) | |
| Strongly agree | 15 (40.5) | 14 (33.3) | 45 (45.9) | |
DISCUSSION
In this survey of 178 most hospitalists from a diverse group of 9 US hospitals, we found that most indicate that severity of dyspnea has a profound influence on their clinical practice (including their decision whether to intensify treatments such as diuretics or bronchodilators, to pursue additional diagnostic testing, add opioids or other nonpharmacological treatments) and ultimately their decision regarding the timing of hospital discharge. More importantly, whereas less than half reported experience with standardized assessment of dyspnea severity, most stated that such data would be very useful in their practice.
Despite being a highly prevalent symptom in diverse patient populations, several studies have shown that documentation of dyspnea is sporadic and evaluation of dyspnea quality of care is not routinely performed.[13, 14, 15] Statements from a number of professional societies, including the ACCP, the American Thoracic Society and the Canadian Respiratory Society, recommend that dyspnea management should rely on patient reporting, and that dyspnea severity should be recorded.[1, 4, 7] Assessment is an essential step to guide interventions; however, simply asking about the presence or absence of dyspnea is insufficient.
Several rating scales have been validated and might be implementable in the acute care setting, including the Numerical Rating Scale and the Visual Assessment Scale.[16, 17, 18, 19, 20] Our survey shows that standardized documentation of dyspnea severity in clinical practice is uncommon. However, most hospitalists in our study believed that assessment of dyspnea, using a standardized scale, would positively impact their management of patients with cardiopulmonary disease.
There are a number of potential benefits of routine assessment of dyspnea in hospitalized patients. Implementation of a standardized approach to dyspnea measurement would result in more uniform assessment and documentation practices, and in turn greater awareness among members of the patient‐care team. Though not sufficient to improve care, measurement is necessary because physicians do not always recognize the severity of patients' dyspnea or may not recognize its presence. A retrospective study that assessed the prevalence of symptoms in 410 ambulatory patients showed that one‐quarter of patients had dyspnea, but only half of them told their doctor about it.[21] Two other studies of patients with cancer diagnoses found that 30%70% of patients had dyspnea, but the symptom was recognized in only half of them; even when recognized, dyspnea severity was frequently underrated by physicians.[21, 22] Importantly, underestimation appears to correlate with underutilization of symptomatic management of dyspnea.[8]
Although the results of our survey are encouraging, they highlight a number of potential barriers and misconceptions among hospitalists. For example, although dyspnea can be characterized only by the person experiencing it, only 42% of our survey respondents believed that patients are able to rate their dyspnea intensity on a scale. Some of these responses may be influenced by the fact that dyspnea scales are not currently available to patients under their care. Another explanation is that similar to the case for pain, some hospitalists may believe that patients will exaggerate dyspnea severity. Almost one‐third of the respondents stated that objective measures, such as respiratory rate or oxygen saturation, are more important than a patient's experience of dyspnea in guiding the treatment, and that dyspnea is a subjective symptom and not a vital sign itself. Hospitalists who appreciated the importance of dyspnea in clinical practice were more likely to support the implementation of a standardized dyspnea scale for dyspnea assessment.
Although the potential benefits of including routine measurement of dyspnea in standard hospital practice may seem obvious, evidence that implementing routine assessment improves patient care or outcomes is lacking. Even if hospitalists see the value of dyspnea assessment, asking nurses to collect and document additional information would represent a substantial change in hospital workflow. Finally, without specific protocols to guide care, it is unclear whether physicians will be able to use new information about dyspnea severity effectively. Future studies need to evaluate the impact of implementing routine dyspnea assessment on the management of patients with cardiopulmonary diseases including the use of evidence‐based interventions and reducing the use of less valuable care.
Most hospitalists agreed with the basic principles of dyspnea treatment in patients with advanced cardiopulmonary disease after the primary disease had been stabilized. Effective measures are available, and several guidelines endorse opioids in dyspnea management.[1, 4, 7] However, many clinicians are uncomfortable with this approach for dyspnea, and opioids remain underused. In our study, almost 90% of physicians recognized that concerns about respiratory depression limits opioids use as a treatment. A qualitative study that explored the physicians' perspective toward opioids showed that most physicians were reluctant to prescribe opioids for refractory dyspnea, describing a lack of related knowledge and experience, and fears related to the potential adverse effects. The findings of our study also outline the need to better educate residents and hospitalists on the assessment and management of dyspnea, including prescribing opioids for refractory dyspnea.[23]
Study Strengths and Limitations
This study has several strengths. To our knowledge, it is the first to explore hospitalists' perspectives on incorporating dyspnea assessment in their clinical practice. Hospitalists are the attending physicians for a large majority of inpatients and would be the main users of a dyspnea severity scale. Our questionnaire survey included a large number of hospitalists, from 9 geographically and structurally diverse hospitals, which increased the generalizability of the findings to other hospitals around the country.
The study also has several limitations that need be kept in mind in interpreting the study results. First, desirability bias may have exaggerated some of the positive views expressed by hospitalists toward implementation of routine assessment of dyspnea. Second, because this was a survey, the estimates of dyspnea assessment and documentation practices of both physicians and nurses were based on the respondents' perception and not an objective review of medical records, and the results may be different from actual practice. Third, this was not a population‐based random sample of hospitalists, and it may not be entirely representative; however, those surveyed were from a diverse set of sites with different geographical location, size, academic affiliation, and practice environment, and their time in practice varied widely. Last, we do not have information on nonrespondents, and there is a possibility of nonresponse bias, although the high response rate lessens the risk.
CONCLUSIONS
The results of this survey suggest that most hospitalists believe that routine assessment of dyspnea severity would enhance their clinical decision making and improve patient care. Standardized assessment of dyspnea might result in better awareness of this symptom among providers, reduce undertreatment and mistreatment, and ultimately result in better outcomes for patients. However, implementation of the routine assessment of dyspnea would change current clinical practices and may have a significant effect on existing nursing and physician workflows. Additional research is needed to determine the feasibility and impact on outcomes of routine dyspnea assessment.
Acknowledgements
The authors wish to acknowledge Ms. Anu Joshi for her help with editing the manuscript and assisting with table preparations.
Disclosures
Dr. Stefan is supported by grant K01HL114631‐01A1 from the National Heart, Lung, and Blood Institute of the National Institutes of Health, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1RR025752. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. M.S.S. and P.K.L. conceived of the study. M.S.S. acquired the data with the help of all collaborators. M.S.S., P.K.L., P.S.P., and A.P. analyzed and interpreted the data. M.S.S. drafted the manuscript. All authors critically reviewed the manuscript for intellectual content. M.S.S., P.K.L., and A.P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M.S.S. is the guarantor for this article, and is responsible for the content of the article, including data and analysis. The authors report no conflicts of interest.
- , , , et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452.
- CDC/ National Center for Health Statistics. National Hospital Amulatory Medical Care Survey: 2011 Emergency Department Summary Tables. http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed May 15, 2015.
- , , , . Signs and symptoms of heart failure: are you asking the right questions? Am J Crit Care. 2010;19(5):443–452.
- , , , et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18(2):69–78.
- , . Prevalence of distressing symptoms in hospitalised patients on medical wards: A cross‐sectional study. BMC Palliat Care. 2008;7:16.
- , . Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234–236.
- , , , et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137(3):674–691.
- , . Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med. 1989;86(3):262–266.
- The Joint Commission. Facts about Pain Management. http://www.jointcommission.org/pain_management/. Accessed May, 15, 2015.
- . Hospitalist programs in the age of healthcare reform. J Healthc Manag. 2010;55(6):378–380.
- , , , . The Use of Hospitalists by Small Rural Hospitals: Results of a National Survey. Med Care Res Rev. 2014;71(4):356–366.
- Tufts CTSI. REDCap [Internet]. Tufts Clinical and Translational Science Institute. http://www.tuftsctsi.org/Services-and-Consultation/REDCap.aspx. Accessed May, 15, 2015.
- , . Multi‐dimensional Assessment of Dyspnea. Dyspnoea in Advanced Disease: A guide to clinical management; 2005.
- , , , et al. Cancer care quality measures: symptoms and end‐of‐life care. Evid Rep Technol Assess (Full Rep). 2006(137):1–77.
- . Defining and measuring quality palliative and end‐of‐life care in the intensive care unit. Crit Care Med. 2006;34(11 Suppl):S309–316.
- . Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325.
- . Can a self‐rating 0‐10 scale for dyspnea yield a common language that is understood by ED nurses, patients, and their families? J Emerg Nurs. 2000;26(3):233–234.
- , , . Measurement of dyspnea: word labeled visual analog scale vs. verbal ordinal scale. Respir Physiol Neurobiol. 2003;134(2):77–83.
- , , , , , . Verbal numerical scales are as reliable and sensitive as visual analog scales for rating dyspnea in young and older subjects. Respir Physiol Neurobiol. 2007;157(2‐3):360–365.
- , , , , . Validation of a three‐factor measurement model of dyspnea in hospitalized adults with heart failure. Heart Lung. 2011;41(1):44–56.
- , , . Patient reporting and doctor recognition of dyspnoea in a comprehensive cancer centre. Intern Med J. 2006;36(6):381–384.
- , , , . Lung cancer and dyspnea: the patient's perception. Oncol Nurs Forum. 1986;13(5):19–24.
- , , , . Opioids, respiratory function, and dyspnea. Am J Hosp Palliat Care. 2003;20(1):57–61.
- , , , et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452.
- CDC/ National Center for Health Statistics. National Hospital Amulatory Medical Care Survey: 2011 Emergency Department Summary Tables. http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf. Accessed May 15, 2015.
- , , , . Signs and symptoms of heart failure: are you asking the right questions? Am J Crit Care. 2010;19(5):443–452.
- , , , et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18(2):69–78.
- , . Prevalence of distressing symptoms in hospitalised patients on medical wards: A cross‐sectional study. BMC Palliat Care. 2008;7:16.
- , . Dyspnea in terminally ill cancer patients. Chest. 1986;89(2):234–236.
- , , , et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137(3):674–691.
- , . Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. Am J Med. 1989;86(3):262–266.
- The Joint Commission. Facts about Pain Management. http://www.jointcommission.org/pain_management/. Accessed May, 15, 2015.
- . Hospitalist programs in the age of healthcare reform. J Healthc Manag. 2010;55(6):378–380.
- , , , . The Use of Hospitalists by Small Rural Hospitals: Results of a National Survey. Med Care Res Rev. 2014;71(4):356–366.
- Tufts CTSI. REDCap [Internet]. Tufts Clinical and Translational Science Institute. http://www.tuftsctsi.org/Services-and-Consultation/REDCap.aspx. Accessed May, 15, 2015.
- , . Multi‐dimensional Assessment of Dyspnea. Dyspnoea in Advanced Disease: A guide to clinical management; 2005.
- , , , et al. Cancer care quality measures: symptoms and end‐of‐life care. Evid Rep Technol Assess (Full Rep). 2006(137):1–77.
- . Defining and measuring quality palliative and end‐of‐life care in the intensive care unit. Crit Care Med. 2006;34(11 Suppl):S309–316.
- . Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325.
- . Can a self‐rating 0‐10 scale for dyspnea yield a common language that is understood by ED nurses, patients, and their families? J Emerg Nurs. 2000;26(3):233–234.
- , , . Measurement of dyspnea: word labeled visual analog scale vs. verbal ordinal scale. Respir Physiol Neurobiol. 2003;134(2):77–83.
- , , , , , . Verbal numerical scales are as reliable and sensitive as visual analog scales for rating dyspnea in young and older subjects. Respir Physiol Neurobiol. 2007;157(2‐3):360–365.
- , , , , . Validation of a three‐factor measurement model of dyspnea in hospitalized adults with heart failure. Heart Lung. 2011;41(1):44–56.
- , , . Patient reporting and doctor recognition of dyspnoea in a comprehensive cancer centre. Intern Med J. 2006;36(6):381–384.
- , , , . Lung cancer and dyspnea: the patient's perception. Oncol Nurs Forum. 1986;13(5):19–24.
- , , , . Opioids, respiratory function, and dyspnea. Am J Hosp Palliat Care. 2003;20(1):57–61.
© 2015 Society of Hospital Medicine
SVS: Stroke reduction outweighs bleeding risk of dual antiplatelet therapy in CEA
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Strokes are less likely after CEA if patients are on perioperative clopidogrel and aspirin.
Major finding: On multivariate analysis, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62, P = .04); and stroke death (OR, 0.65; P = .03).
Data source: Review of more than 28,000 carotid endarterectomy patients
Disclosures: The presenter has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
FDA approves patch for treating hemorrhage
Image by James Weaver
The US Food and Drug Administration (FDA) has granted 501(k) clearance for the Hemogrip Patch, a product used to treat uncontrolled hemorrhage.
The patch is designed to control bleeding that occurs when accessing veins or arteries for various medical treatments and applications.
The Hemogrip platform technology is based on chitosan, a natural biopolymer found in the exoskeleton of crustaceans.
Chitosan is biocompatible, anti-microbial, and durable under a range of environmental conditions.
When applied to wounds, Hemogrip creates a nano-scale, 3-dimensional mesh, rapidly coagulating blood and stopping blood loss.
Hemogrip technology has proven effective in a study of swine, halting uncontrolled hemorrhage in a model of lethal arterial injury. Other in vivo research showed that Hemogrip’s hemostatic effects are reversible.
The Hemogrip Patch is under development by the medical device company Remedium Technologies, a spin-out of the University of Maryland. ![]()

Image by James Weaver
The US Food and Drug Administration (FDA) has granted 501(k) clearance for the Hemogrip Patch, a product used to treat uncontrolled hemorrhage.
The patch is designed to control bleeding that occurs when accessing veins or arteries for various medical treatments and applications.
The Hemogrip platform technology is based on chitosan, a natural biopolymer found in the exoskeleton of crustaceans.
Chitosan is biocompatible, anti-microbial, and durable under a range of environmental conditions.
When applied to wounds, Hemogrip creates a nano-scale, 3-dimensional mesh, rapidly coagulating blood and stopping blood loss.
Hemogrip technology has proven effective in a study of swine, halting uncontrolled hemorrhage in a model of lethal arterial injury. Other in vivo research showed that Hemogrip’s hemostatic effects are reversible.
The Hemogrip Patch is under development by the medical device company Remedium Technologies, a spin-out of the University of Maryland. ![]()

Image by James Weaver
The US Food and Drug Administration (FDA) has granted 501(k) clearance for the Hemogrip Patch, a product used to treat uncontrolled hemorrhage.
The patch is designed to control bleeding that occurs when accessing veins or arteries for various medical treatments and applications.
The Hemogrip platform technology is based on chitosan, a natural biopolymer found in the exoskeleton of crustaceans.
Chitosan is biocompatible, anti-microbial, and durable under a range of environmental conditions.
When applied to wounds, Hemogrip creates a nano-scale, 3-dimensional mesh, rapidly coagulating blood and stopping blood loss.
Hemogrip technology has proven effective in a study of swine, halting uncontrolled hemorrhage in a model of lethal arterial injury. Other in vivo research showed that Hemogrip’s hemostatic effects are reversible.
The Hemogrip Patch is under development by the medical device company Remedium Technologies, a spin-out of the University of Maryland. ![]()
HLA-matched sibling transplants provide best outcomes in infantile osteopetrosis
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
EHA: High-risk APL curable with chemo-free combo
VIENNA – Patients with high-risk newly diagnosed acute promyelocytic leukemia derive the same survival benefit from a chemotherapy-free combination as an anthracycline-containing standard of care, according to results of the AML17 APL study.
The 4-year overall survival rates in high-risk patients (WBC > 10 x 109/L) were 87% with arsenic trioxide plus all-trans retinoic acid and 84% with the standard all-trans retinoic acid and idarubicin schedule.
Relapse-free survival rates were superior with the chemotherapy-free combination (100% vs. 74%; P = .008), Dr. Alan Burnett reported at the annual congress of the European Hematology Association.
“One of our rationales for using arsenic as first-line (therapy) was to try and get at the early death that remains a major problem in this disease,” he said.
Arsenic trioxide and gemtuzumab ozogamicin are effective as single agents with the former approved for relapsed disease in patients with APL. The GIMEMA-AMLSG-SAL trial indicated that a daily schedule of arsenic trioxide plus all-trans retinoic acid was at least as effective and may be superior to all-trans retinoic acid plus chemotherapy in low to moderate risk APL patients (N. Engl. J. Med. 2013;369: 111-21).
The AML17 APL trial was designed by the U.K. National Cancer Research Institute with the aim of comparing all-trans retinoic acid and idarubicin with arsenic trioxide in an attenuated dosing schedule plus all-trans retinoic acid. Importantly, high-risk patients were included, with the option to receive a single dose of gemtuzumab ozogamicin (Mylotarg) 6 mg/m2 within the first 4 days of induction, said Dr. Burnett, who performed the research as head of hematology at Cardiff University in Wales and is now global lead for myeloid diseases at CTI BioPharma in Seattle. In the United States, gemtuzumab was withdrawn from the market in 2010 because of safety concerns.
From May 2009 to October 2013, 235 patients with molecularly confirmed APL were randomized at 81 centers to all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60 or complete remission plus arsenic trioxide 0.3 mg/kg on days 1-5 of week 1 and then 0.25 mg/kg twice per week for 7 weeks plus all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60 or complete remission or to idarubicin 12 mg/m2 on days 2, 4, 6, 8 plus all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60.
In the arsenic trioxide plus all-trans retinoic acid arm, this was followed by all-trans retinoic acid 45 mg/m2 as a divided daily dose 2 weeks on and 2 weeks off plus four consolidation courses of arsenic trioxide 0.3 mg/kg days 1-5 of week 1 and then 0.25 mg/kg twice per week for 3 weeks (total 63 days of arsenic trioxide).
Consolidation in the all-trans retinoic acid and idarubicin arm was all-trans retinoic acid 45 mg/m2 as a divided daily dose on days 1-15 plus idarubicin 5 mg/m2 days 1-4 in course 2, mitoxantrone 10mg/m2 days 1-4 in course 3, and idarubicin 12 mg/m2 day 1 in course 4.
No maintenance was given in either arm. The median patient age was 47 years, with about 20% of patients over age 60; over 20% of the patients were high-risk, and they were equally balanced in the two treatment groups.
At 4 years, the overall survival rate among all patients was comparable – 93% with arsenic trioxide plus all-trans retinoic acid and 89% with all-trans retinoic acid and idarubicin.
However, event-free survival was significantly better in the arsenic trioxide plus all-trans retinoic acid cohort (91% vs. 74%; hazard ratio, 0.36; P = .003), as were frank relapse-free survival (97% vs. 83%; HR, 0.24; P = .004) and molecular relapse-free survival (98% vs. 70%; HR, 0.17; P < .0001), Dr. Burnett said.
One patient on arsenic trioxide plus all-trans retinoic acid experienced frank relapse, compared with 13 on all-trans retinoic acid and idarubicin, plus a further 19 molecular relapses occurred on this arm (cumulative incidence of molecular and hematologic relapse 0% vs. 27%, HR, 0.12; P < .0001).
“Once a patient was in molecular remission there were no further relapses in patients on (arsenic trioxide plus all-trans retinoic acid),” he said.
Of the 30 high-risk patients allocated to the chemo-free arm, 28 received gemtuzumab ozogamicin as per protocol. The overall survival at 4 years for these patients was 89%. Of the two patients not treated with gemtuzumab ozogamicin, one died on day 12 due to causes unrelated to treatment.
Among the 49 patients older than 60 years, overall survival was 80% with arsenic trioxide plus all-trans retinoic acid and 74% with all-trans retinoic acid and idarubicin. Similarly, among good-risk patients, relapse-free survival was significantly improved (96% vs. 79%; HR, 0.33; P = .04). Also, overall survival was not inferior at 95% vs. 90%, “very much replicating the outcomes seen in the GIMEMA study,” Dr. Burnett said.
The benefits were also achieved with significantly less grade 3-4 liver toxicity than observed in the GIMEMA study (<10% vs 63%).
There was, however, an excess of cardiac toxicity in course 2 with arsenic trioxide plus all-trans retinoic acid, compared with all-trans retinoic acid and idarubicin (P = .001).
“We’re not totally sure what that’s all due to, but it doesn’t look to be due to a QC prolongation,” he said.
The arsenic trioxide plus all-trans retinoic acid regimen was associated with significant reductions in supportive care requirements including fewer blood and platelet transfusions, days on antibiotics, and days in hospital, with “many patients treated exclusively as outpatients,” he added.
The low risk of relapse with arsenic trioxide plus all-trans retinoic acid also negates the need for minimal residual disease monitoring.
Finally, compared with the GIMEMA study protocol, the attenuated arsenic dosing schedule in AML17 APL resulted in less frequent dosing of arsenic trioxide (63 doses vs. 140 doses) and less drug required (151 vials vs. 280 vials for a 70-kg patient). At an acquisition cost of £350 per vial, this represents a cost savings of £46,000 (nearly $72,000) per patient, not to mention the added convenience to patients, Dr. Burnett observed.
Cancer Research U.K. funded the study. Cephalon provided the arsenic trioxide. Dr. Burnett disclosed part-time employment with CTI LifeSciences and in the last 12 months serving on the advisory boards of Celgene, Agios, Pfizer, and Bristol-Myers Squibb.
VIENNA – Patients with high-risk newly diagnosed acute promyelocytic leukemia derive the same survival benefit from a chemotherapy-free combination as an anthracycline-containing standard of care, according to results of the AML17 APL study.
The 4-year overall survival rates in high-risk patients (WBC > 10 x 109/L) were 87% with arsenic trioxide plus all-trans retinoic acid and 84% with the standard all-trans retinoic acid and idarubicin schedule.
Relapse-free survival rates were superior with the chemotherapy-free combination (100% vs. 74%; P = .008), Dr. Alan Burnett reported at the annual congress of the European Hematology Association.
“One of our rationales for using arsenic as first-line (therapy) was to try and get at the early death that remains a major problem in this disease,” he said.
Arsenic trioxide and gemtuzumab ozogamicin are effective as single agents with the former approved for relapsed disease in patients with APL. The GIMEMA-AMLSG-SAL trial indicated that a daily schedule of arsenic trioxide plus all-trans retinoic acid was at least as effective and may be superior to all-trans retinoic acid plus chemotherapy in low to moderate risk APL patients (N. Engl. J. Med. 2013;369: 111-21).
The AML17 APL trial was designed by the U.K. National Cancer Research Institute with the aim of comparing all-trans retinoic acid and idarubicin with arsenic trioxide in an attenuated dosing schedule plus all-trans retinoic acid. Importantly, high-risk patients were included, with the option to receive a single dose of gemtuzumab ozogamicin (Mylotarg) 6 mg/m2 within the first 4 days of induction, said Dr. Burnett, who performed the research as head of hematology at Cardiff University in Wales and is now global lead for myeloid diseases at CTI BioPharma in Seattle. In the United States, gemtuzumab was withdrawn from the market in 2010 because of safety concerns.
From May 2009 to October 2013, 235 patients with molecularly confirmed APL were randomized at 81 centers to all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60 or complete remission plus arsenic trioxide 0.3 mg/kg on days 1-5 of week 1 and then 0.25 mg/kg twice per week for 7 weeks plus all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60 or complete remission or to idarubicin 12 mg/m2 on days 2, 4, 6, 8 plus all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60.
In the arsenic trioxide plus all-trans retinoic acid arm, this was followed by all-trans retinoic acid 45 mg/m2 as a divided daily dose 2 weeks on and 2 weeks off plus four consolidation courses of arsenic trioxide 0.3 mg/kg days 1-5 of week 1 and then 0.25 mg/kg twice per week for 3 weeks (total 63 days of arsenic trioxide).
Consolidation in the all-trans retinoic acid and idarubicin arm was all-trans retinoic acid 45 mg/m2 as a divided daily dose on days 1-15 plus idarubicin 5 mg/m2 days 1-4 in course 2, mitoxantrone 10mg/m2 days 1-4 in course 3, and idarubicin 12 mg/m2 day 1 in course 4.
No maintenance was given in either arm. The median patient age was 47 years, with about 20% of patients over age 60; over 20% of the patients were high-risk, and they were equally balanced in the two treatment groups.
At 4 years, the overall survival rate among all patients was comparable – 93% with arsenic trioxide plus all-trans retinoic acid and 89% with all-trans retinoic acid and idarubicin.
However, event-free survival was significantly better in the arsenic trioxide plus all-trans retinoic acid cohort (91% vs. 74%; hazard ratio, 0.36; P = .003), as were frank relapse-free survival (97% vs. 83%; HR, 0.24; P = .004) and molecular relapse-free survival (98% vs. 70%; HR, 0.17; P < .0001), Dr. Burnett said.
One patient on arsenic trioxide plus all-trans retinoic acid experienced frank relapse, compared with 13 on all-trans retinoic acid and idarubicin, plus a further 19 molecular relapses occurred on this arm (cumulative incidence of molecular and hematologic relapse 0% vs. 27%, HR, 0.12; P < .0001).
“Once a patient was in molecular remission there were no further relapses in patients on (arsenic trioxide plus all-trans retinoic acid),” he said.
Of the 30 high-risk patients allocated to the chemo-free arm, 28 received gemtuzumab ozogamicin as per protocol. The overall survival at 4 years for these patients was 89%. Of the two patients not treated with gemtuzumab ozogamicin, one died on day 12 due to causes unrelated to treatment.
Among the 49 patients older than 60 years, overall survival was 80% with arsenic trioxide plus all-trans retinoic acid and 74% with all-trans retinoic acid and idarubicin. Similarly, among good-risk patients, relapse-free survival was significantly improved (96% vs. 79%; HR, 0.33; P = .04). Also, overall survival was not inferior at 95% vs. 90%, “very much replicating the outcomes seen in the GIMEMA study,” Dr. Burnett said.
The benefits were also achieved with significantly less grade 3-4 liver toxicity than observed in the GIMEMA study (<10% vs 63%).
There was, however, an excess of cardiac toxicity in course 2 with arsenic trioxide plus all-trans retinoic acid, compared with all-trans retinoic acid and idarubicin (P = .001).
“We’re not totally sure what that’s all due to, but it doesn’t look to be due to a QC prolongation,” he said.
The arsenic trioxide plus all-trans retinoic acid regimen was associated with significant reductions in supportive care requirements including fewer blood and platelet transfusions, days on antibiotics, and days in hospital, with “many patients treated exclusively as outpatients,” he added.
The low risk of relapse with arsenic trioxide plus all-trans retinoic acid also negates the need for minimal residual disease monitoring.
Finally, compared with the GIMEMA study protocol, the attenuated arsenic dosing schedule in AML17 APL resulted in less frequent dosing of arsenic trioxide (63 doses vs. 140 doses) and less drug required (151 vials vs. 280 vials for a 70-kg patient). At an acquisition cost of £350 per vial, this represents a cost savings of £46,000 (nearly $72,000) per patient, not to mention the added convenience to patients, Dr. Burnett observed.
Cancer Research U.K. funded the study. Cephalon provided the arsenic trioxide. Dr. Burnett disclosed part-time employment with CTI LifeSciences and in the last 12 months serving on the advisory boards of Celgene, Agios, Pfizer, and Bristol-Myers Squibb.
VIENNA – Patients with high-risk newly diagnosed acute promyelocytic leukemia derive the same survival benefit from a chemotherapy-free combination as an anthracycline-containing standard of care, according to results of the AML17 APL study.
The 4-year overall survival rates in high-risk patients (WBC > 10 x 109/L) were 87% with arsenic trioxide plus all-trans retinoic acid and 84% with the standard all-trans retinoic acid and idarubicin schedule.
Relapse-free survival rates were superior with the chemotherapy-free combination (100% vs. 74%; P = .008), Dr. Alan Burnett reported at the annual congress of the European Hematology Association.
“One of our rationales for using arsenic as first-line (therapy) was to try and get at the early death that remains a major problem in this disease,” he said.
Arsenic trioxide and gemtuzumab ozogamicin are effective as single agents with the former approved for relapsed disease in patients with APL. The GIMEMA-AMLSG-SAL trial indicated that a daily schedule of arsenic trioxide plus all-trans retinoic acid was at least as effective and may be superior to all-trans retinoic acid plus chemotherapy in low to moderate risk APL patients (N. Engl. J. Med. 2013;369: 111-21).
The AML17 APL trial was designed by the U.K. National Cancer Research Institute with the aim of comparing all-trans retinoic acid and idarubicin with arsenic trioxide in an attenuated dosing schedule plus all-trans retinoic acid. Importantly, high-risk patients were included, with the option to receive a single dose of gemtuzumab ozogamicin (Mylotarg) 6 mg/m2 within the first 4 days of induction, said Dr. Burnett, who performed the research as head of hematology at Cardiff University in Wales and is now global lead for myeloid diseases at CTI BioPharma in Seattle. In the United States, gemtuzumab was withdrawn from the market in 2010 because of safety concerns.
From May 2009 to October 2013, 235 patients with molecularly confirmed APL were randomized at 81 centers to all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60 or complete remission plus arsenic trioxide 0.3 mg/kg on days 1-5 of week 1 and then 0.25 mg/kg twice per week for 7 weeks plus all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60 or complete remission or to idarubicin 12 mg/m2 on days 2, 4, 6, 8 plus all-trans retinoic acid 45 mg/m2 as a divided daily oral dose to day 60.
In the arsenic trioxide plus all-trans retinoic acid arm, this was followed by all-trans retinoic acid 45 mg/m2 as a divided daily dose 2 weeks on and 2 weeks off plus four consolidation courses of arsenic trioxide 0.3 mg/kg days 1-5 of week 1 and then 0.25 mg/kg twice per week for 3 weeks (total 63 days of arsenic trioxide).
Consolidation in the all-trans retinoic acid and idarubicin arm was all-trans retinoic acid 45 mg/m2 as a divided daily dose on days 1-15 plus idarubicin 5 mg/m2 days 1-4 in course 2, mitoxantrone 10mg/m2 days 1-4 in course 3, and idarubicin 12 mg/m2 day 1 in course 4.
No maintenance was given in either arm. The median patient age was 47 years, with about 20% of patients over age 60; over 20% of the patients were high-risk, and they were equally balanced in the two treatment groups.
At 4 years, the overall survival rate among all patients was comparable – 93% with arsenic trioxide plus all-trans retinoic acid and 89% with all-trans retinoic acid and idarubicin.
However, event-free survival was significantly better in the arsenic trioxide plus all-trans retinoic acid cohort (91% vs. 74%; hazard ratio, 0.36; P = .003), as were frank relapse-free survival (97% vs. 83%; HR, 0.24; P = .004) and molecular relapse-free survival (98% vs. 70%; HR, 0.17; P < .0001), Dr. Burnett said.
One patient on arsenic trioxide plus all-trans retinoic acid experienced frank relapse, compared with 13 on all-trans retinoic acid and idarubicin, plus a further 19 molecular relapses occurred on this arm (cumulative incidence of molecular and hematologic relapse 0% vs. 27%, HR, 0.12; P < .0001).
“Once a patient was in molecular remission there were no further relapses in patients on (arsenic trioxide plus all-trans retinoic acid),” he said.
Of the 30 high-risk patients allocated to the chemo-free arm, 28 received gemtuzumab ozogamicin as per protocol. The overall survival at 4 years for these patients was 89%. Of the two patients not treated with gemtuzumab ozogamicin, one died on day 12 due to causes unrelated to treatment.
Among the 49 patients older than 60 years, overall survival was 80% with arsenic trioxide plus all-trans retinoic acid and 74% with all-trans retinoic acid and idarubicin. Similarly, among good-risk patients, relapse-free survival was significantly improved (96% vs. 79%; HR, 0.33; P = .04). Also, overall survival was not inferior at 95% vs. 90%, “very much replicating the outcomes seen in the GIMEMA study,” Dr. Burnett said.
The benefits were also achieved with significantly less grade 3-4 liver toxicity than observed in the GIMEMA study (<10% vs 63%).
There was, however, an excess of cardiac toxicity in course 2 with arsenic trioxide plus all-trans retinoic acid, compared with all-trans retinoic acid and idarubicin (P = .001).
“We’re not totally sure what that’s all due to, but it doesn’t look to be due to a QC prolongation,” he said.
The arsenic trioxide plus all-trans retinoic acid regimen was associated with significant reductions in supportive care requirements including fewer blood and platelet transfusions, days on antibiotics, and days in hospital, with “many patients treated exclusively as outpatients,” he added.
The low risk of relapse with arsenic trioxide plus all-trans retinoic acid also negates the need for minimal residual disease monitoring.
Finally, compared with the GIMEMA study protocol, the attenuated arsenic dosing schedule in AML17 APL resulted in less frequent dosing of arsenic trioxide (63 doses vs. 140 doses) and less drug required (151 vials vs. 280 vials for a 70-kg patient). At an acquisition cost of £350 per vial, this represents a cost savings of £46,000 (nearly $72,000) per patient, not to mention the added convenience to patients, Dr. Burnett observed.
Cancer Research U.K. funded the study. Cephalon provided the arsenic trioxide. Dr. Burnett disclosed part-time employment with CTI LifeSciences and in the last 12 months serving on the advisory boards of Celgene, Agios, Pfizer, and Bristol-Myers Squibb.
AT THE EHA CONGRESS
Key clinical point: Arsenic trioxide plus all-trans retinoic acid is at least equivalent to all-trans retinoic acid and idarubicin in terms of overall survival in high-risk patients given gemtuzumab ozogamicin prophylaxis.
Major finding: At 4 years, the overall survival rate among all patients was comparable – 93% with arsenic trioxide plus all-trans retinoic acid and 89% with all-trans retinoic acid and idarubicin.
Data source: Prospective, randomized trial in 235 patients with acute promyelocytic leukemia.
Disclosures: Cancer Research U.K. funded the study. Cephalon provided the arsenic trioxide. Dr. Burnett disclosed part-time employment with CTI LifeSciences and in the last 12 months serving on the advisory boards of Celgene, Agios, Pfizer, and Bristol-Myers Squibb.