User login
Genetic mutation identifies favorable prognosis MDS
Among patients with refractory anemia with ring sideroblasts, the presence of a common mutation in SF3B1 appears to be a marker for an indolent clinical course and favorable outcome compared to patients with wild-type SF3B1, European investigators reported.
The gene SF3B1, which encodes for a splicing factor subunit, is frequently mutated in cases of chronic lymphocytic leukemia and myelodysplastic syndromes.
“SF3B1 mutation is a major determinant of disease phenotype and clinical outcome in MDS [myelodysplastic syndrome] with ring sideroblasts. SF3B1-mutated MDS is characterized by homogeneous hematologic features, favorable prognosis, and restricted patterns of co-mutated genes and clonal evolution. Overall, these results strongly support the recognition of MDS associated with SF3B1 mutation as a distinct MDS subtype. Conversely, SF3B1-negative MDS with ring sideroblasts represents a subset with a high prevalence of TP53 mutations and worse outcome that should be taken into consideration in clinical decision-making,” the study authors conclude.
Dr. Luca Malcovati and his colleagues from the University of Pavia, Italy, and other European centers, conducted a mutational analysis of 293 patients with myeloid neoplasms and 1% or more ring sideroblasts. They found somatic mutations in SF3B1 in 129 of 159 patients with refractory anemia with ring sideroblasts (RARS) or refractory cytopenia with multilineage dysplasia and ring sideroblasts (RCMD-RS). In contrast, there was a significantly lower prevalence of SF3B1 mutations among 50 patients with myelodysplastic/myeloproliferative neoplasm (MDS/MPN), and among 84 additional patients with other myeloid diseases under the World Health Organization classification of disorders of hematopoietic and lymphoid tissues (P < .001).
In multivariable analyses controlling for demographic and disease-related factors, patients with SF3B1 mutations had significantly better overall survival (hazard ratio, 0.37; P = .003), as well as a lower cumulative incidence of disease progression (HR, 0.31; P = .018), compared with patients with wild-type SF3B1 (Blood 2015;126[2]:233-41).
Mutations in SF3B1 were predictive of better outcomes among patients with RARS, RCMD-RS, and in patients with MDS without excess blasts.
When they looked at other mutations, the investigators found that in patients with SF3B1 mutations, the mutations in DNA methylation genes were associated with the presence of multilineage dysplasia, but this association had no significant effect on clinical outcomes.
Among patients with wild-type SB3B1, mutations in TP53 were frequently seen, and these mutations were associated with poor outcomes.
Gene sequencing efforts in myeloid malignancies have largely charted the mutational “landscape.” This map allows us to (1) have some idea of the fundamental biology underlying the disease, (2) define potential drug targets, and (3) refine outcome expectations, especially when there are no “knockout” therapies (as in chronic myeloid leukemia). The consequence is also the further subclassification of myeloid malignancies, thus making relatively rare diseases into extremely rare ones. One obvious challenge is to cleverly design clinical studies given the myriad subcategories of disease. The higher bar is understanding the biology of how the various mutations and pathways merge to cause disease. The work Malcovati et al., along with the other fine studies noted above, gets us one step farther down the road to cures.
Dr. Jerald Radich of Fred Hutchinson Cancer Research Center, Seattle, made his comment in an accompanying editorial.
Gene sequencing efforts in myeloid malignancies have largely charted the mutational “landscape.” This map allows us to (1) have some idea of the fundamental biology underlying the disease, (2) define potential drug targets, and (3) refine outcome expectations, especially when there are no “knockout” therapies (as in chronic myeloid leukemia). The consequence is also the further subclassification of myeloid malignancies, thus making relatively rare diseases into extremely rare ones. One obvious challenge is to cleverly design clinical studies given the myriad subcategories of disease. The higher bar is understanding the biology of how the various mutations and pathways merge to cause disease. The work Malcovati et al., along with the other fine studies noted above, gets us one step farther down the road to cures.
Dr. Jerald Radich of Fred Hutchinson Cancer Research Center, Seattle, made his comment in an accompanying editorial.
Gene sequencing efforts in myeloid malignancies have largely charted the mutational “landscape.” This map allows us to (1) have some idea of the fundamental biology underlying the disease, (2) define potential drug targets, and (3) refine outcome expectations, especially when there are no “knockout” therapies (as in chronic myeloid leukemia). The consequence is also the further subclassification of myeloid malignancies, thus making relatively rare diseases into extremely rare ones. One obvious challenge is to cleverly design clinical studies given the myriad subcategories of disease. The higher bar is understanding the biology of how the various mutations and pathways merge to cause disease. The work Malcovati et al., along with the other fine studies noted above, gets us one step farther down the road to cures.
Dr. Jerald Radich of Fred Hutchinson Cancer Research Center, Seattle, made his comment in an accompanying editorial.
Among patients with refractory anemia with ring sideroblasts, the presence of a common mutation in SF3B1 appears to be a marker for an indolent clinical course and favorable outcome compared to patients with wild-type SF3B1, European investigators reported.
The gene SF3B1, which encodes for a splicing factor subunit, is frequently mutated in cases of chronic lymphocytic leukemia and myelodysplastic syndromes.
“SF3B1 mutation is a major determinant of disease phenotype and clinical outcome in MDS [myelodysplastic syndrome] with ring sideroblasts. SF3B1-mutated MDS is characterized by homogeneous hematologic features, favorable prognosis, and restricted patterns of co-mutated genes and clonal evolution. Overall, these results strongly support the recognition of MDS associated with SF3B1 mutation as a distinct MDS subtype. Conversely, SF3B1-negative MDS with ring sideroblasts represents a subset with a high prevalence of TP53 mutations and worse outcome that should be taken into consideration in clinical decision-making,” the study authors conclude.
Dr. Luca Malcovati and his colleagues from the University of Pavia, Italy, and other European centers, conducted a mutational analysis of 293 patients with myeloid neoplasms and 1% or more ring sideroblasts. They found somatic mutations in SF3B1 in 129 of 159 patients with refractory anemia with ring sideroblasts (RARS) or refractory cytopenia with multilineage dysplasia and ring sideroblasts (RCMD-RS). In contrast, there was a significantly lower prevalence of SF3B1 mutations among 50 patients with myelodysplastic/myeloproliferative neoplasm (MDS/MPN), and among 84 additional patients with other myeloid diseases under the World Health Organization classification of disorders of hematopoietic and lymphoid tissues (P < .001).
In multivariable analyses controlling for demographic and disease-related factors, patients with SF3B1 mutations had significantly better overall survival (hazard ratio, 0.37; P = .003), as well as a lower cumulative incidence of disease progression (HR, 0.31; P = .018), compared with patients with wild-type SF3B1 (Blood 2015;126[2]:233-41).
Mutations in SF3B1 were predictive of better outcomes among patients with RARS, RCMD-RS, and in patients with MDS without excess blasts.
When they looked at other mutations, the investigators found that in patients with SF3B1 mutations, the mutations in DNA methylation genes were associated with the presence of multilineage dysplasia, but this association had no significant effect on clinical outcomes.
Among patients with wild-type SB3B1, mutations in TP53 were frequently seen, and these mutations were associated with poor outcomes.
Among patients with refractory anemia with ring sideroblasts, the presence of a common mutation in SF3B1 appears to be a marker for an indolent clinical course and favorable outcome compared to patients with wild-type SF3B1, European investigators reported.
The gene SF3B1, which encodes for a splicing factor subunit, is frequently mutated in cases of chronic lymphocytic leukemia and myelodysplastic syndromes.
“SF3B1 mutation is a major determinant of disease phenotype and clinical outcome in MDS [myelodysplastic syndrome] with ring sideroblasts. SF3B1-mutated MDS is characterized by homogeneous hematologic features, favorable prognosis, and restricted patterns of co-mutated genes and clonal evolution. Overall, these results strongly support the recognition of MDS associated with SF3B1 mutation as a distinct MDS subtype. Conversely, SF3B1-negative MDS with ring sideroblasts represents a subset with a high prevalence of TP53 mutations and worse outcome that should be taken into consideration in clinical decision-making,” the study authors conclude.
Dr. Luca Malcovati and his colleagues from the University of Pavia, Italy, and other European centers, conducted a mutational analysis of 293 patients with myeloid neoplasms and 1% or more ring sideroblasts. They found somatic mutations in SF3B1 in 129 of 159 patients with refractory anemia with ring sideroblasts (RARS) or refractory cytopenia with multilineage dysplasia and ring sideroblasts (RCMD-RS). In contrast, there was a significantly lower prevalence of SF3B1 mutations among 50 patients with myelodysplastic/myeloproliferative neoplasm (MDS/MPN), and among 84 additional patients with other myeloid diseases under the World Health Organization classification of disorders of hematopoietic and lymphoid tissues (P < .001).
In multivariable analyses controlling for demographic and disease-related factors, patients with SF3B1 mutations had significantly better overall survival (hazard ratio, 0.37; P = .003), as well as a lower cumulative incidence of disease progression (HR, 0.31; P = .018), compared with patients with wild-type SF3B1 (Blood 2015;126[2]:233-41).
Mutations in SF3B1 were predictive of better outcomes among patients with RARS, RCMD-RS, and in patients with MDS without excess blasts.
When they looked at other mutations, the investigators found that in patients with SF3B1 mutations, the mutations in DNA methylation genes were associated with the presence of multilineage dysplasia, but this association had no significant effect on clinical outcomes.
Among patients with wild-type SB3B1, mutations in TP53 were frequently seen, and these mutations were associated with poor outcomes.
FROM BLOOD
Key clinical point: Mutations in SF3B1 identify a subset of patients with MDS with favorable prognosis.
Major finding: Patients with SF3B1 had a hazard ratio for death of 0.37, compared with patients with unmutated (wild-type) SF3B1.
Data source: Mutational analysis of 293 patients with myeloid neoplasms with 1% of more ring sideroblasts followed in centers in Italy, Sweden, and Denmark.
Disclosures: The study was supported by grants from Associazione Italiana per la Ricerca sul Cancro, Fondo per gli Investimenti della Ricerca di Base, and Ministero dell’Istruzione, dell’Università e della Ricerca PRIN 2010-2011, Fondazione Veronesi and Regione Lombardia/Fondazione Cariplo, and Associazione Italiana per la Ricerca sul Cancro IG. The authors and Dr. Radich reported no conflicts of interest.
SNP linked to poor survival in MM

Photo courtesy of NIGMS
Investigators have identified a single nucleotide polymorphism (SNP) that seems to confer shorter survival in patients with multiple myeloma (MM).
The group found a significant association between survival and a SNP near the gene FOPNL on chromosome 16p13.
On average, MM patients with this SNP (rs72773978) died 1 to 3 years sooner than patients without it.
The investigators pinpointed the SNP via genome-wide association studies and verified its impact on survival in patient populations from North America and Europe.
The research, which is published in Nature Communications, included 1635 MM patients.
“This is the largest study of inherited genetics and myeloma survival to date,” said Nicola Camp, PhD, of the Huntsman Cancer Institute in Salt Lake City, Utah.
“We were able to identify the FOPNL variant because it has quite a large effect on survival. With even larger collaborative studies, we hope to add to this. The ability to stratify patients based on their genetic make-up opens the door to personalizing their treatment and care.”
For this study, Dr Camp and her colleagues first conducted a meta-analysis of 306 MM patients treated at University of California, San Francisco and 239 patients treated at the Mayo Clinic.
The investigators found a significant association between rs72773978 and survival. Patients with the minor allele had an increased risk of mortality compared to patients who were homozygous for the major allele (hazard ratio=2.65).
The team then conducted a replication meta-analysis of 1090 MM cases, including 772 European patients from the IMMEnSE consortium and 318 from the Utah cohort. Again, there was a significant association between rs72773978 and survival (hazard ratio=1.34).
Although the investigators don’t yet understand why the SNP is associated with poor prognosis, there are clues that it could be involved in disease progression through centrosome amplification.
Analyses of the different MM patient datasets showed that individuals with the worst outcomes have abnormal amounts of FOPNL and carry another sign of poor prognosis—a high centrosome index. The implication is that disruptions in FOPNL could affect fundamental mechanisms controlling the distribution of genetic material to newly made cells.
“The results point us to a previously unrecognized gene as a determinant of myeloma prognosis,” said Elad Ziv, MD, of the University of California, San Francisco.
“If we understand what about this gene is causing poor prognosis, that may lead to a better fundamental grasp of the pathways that are important in multiple myeloma progression. Such knowledge could ultimately lead to better therapies.” ![]()

Photo courtesy of NIGMS
Investigators have identified a single nucleotide polymorphism (SNP) that seems to confer shorter survival in patients with multiple myeloma (MM).
The group found a significant association between survival and a SNP near the gene FOPNL on chromosome 16p13.
On average, MM patients with this SNP (rs72773978) died 1 to 3 years sooner than patients without it.
The investigators pinpointed the SNP via genome-wide association studies and verified its impact on survival in patient populations from North America and Europe.
The research, which is published in Nature Communications, included 1635 MM patients.
“This is the largest study of inherited genetics and myeloma survival to date,” said Nicola Camp, PhD, of the Huntsman Cancer Institute in Salt Lake City, Utah.
“We were able to identify the FOPNL variant because it has quite a large effect on survival. With even larger collaborative studies, we hope to add to this. The ability to stratify patients based on their genetic make-up opens the door to personalizing their treatment and care.”
For this study, Dr Camp and her colleagues first conducted a meta-analysis of 306 MM patients treated at University of California, San Francisco and 239 patients treated at the Mayo Clinic.
The investigators found a significant association between rs72773978 and survival. Patients with the minor allele had an increased risk of mortality compared to patients who were homozygous for the major allele (hazard ratio=2.65).
The team then conducted a replication meta-analysis of 1090 MM cases, including 772 European patients from the IMMEnSE consortium and 318 from the Utah cohort. Again, there was a significant association between rs72773978 and survival (hazard ratio=1.34).
Although the investigators don’t yet understand why the SNP is associated with poor prognosis, there are clues that it could be involved in disease progression through centrosome amplification.
Analyses of the different MM patient datasets showed that individuals with the worst outcomes have abnormal amounts of FOPNL and carry another sign of poor prognosis—a high centrosome index. The implication is that disruptions in FOPNL could affect fundamental mechanisms controlling the distribution of genetic material to newly made cells.
“The results point us to a previously unrecognized gene as a determinant of myeloma prognosis,” said Elad Ziv, MD, of the University of California, San Francisco.
“If we understand what about this gene is causing poor prognosis, that may lead to a better fundamental grasp of the pathways that are important in multiple myeloma progression. Such knowledge could ultimately lead to better therapies.” ![]()

Photo courtesy of NIGMS
Investigators have identified a single nucleotide polymorphism (SNP) that seems to confer shorter survival in patients with multiple myeloma (MM).
The group found a significant association between survival and a SNP near the gene FOPNL on chromosome 16p13.
On average, MM patients with this SNP (rs72773978) died 1 to 3 years sooner than patients without it.
The investigators pinpointed the SNP via genome-wide association studies and verified its impact on survival in patient populations from North America and Europe.
The research, which is published in Nature Communications, included 1635 MM patients.
“This is the largest study of inherited genetics and myeloma survival to date,” said Nicola Camp, PhD, of the Huntsman Cancer Institute in Salt Lake City, Utah.
“We were able to identify the FOPNL variant because it has quite a large effect on survival. With even larger collaborative studies, we hope to add to this. The ability to stratify patients based on their genetic make-up opens the door to personalizing their treatment and care.”
For this study, Dr Camp and her colleagues first conducted a meta-analysis of 306 MM patients treated at University of California, San Francisco and 239 patients treated at the Mayo Clinic.
The investigators found a significant association between rs72773978 and survival. Patients with the minor allele had an increased risk of mortality compared to patients who were homozygous for the major allele (hazard ratio=2.65).
The team then conducted a replication meta-analysis of 1090 MM cases, including 772 European patients from the IMMEnSE consortium and 318 from the Utah cohort. Again, there was a significant association between rs72773978 and survival (hazard ratio=1.34).
Although the investigators don’t yet understand why the SNP is associated with poor prognosis, there are clues that it could be involved in disease progression through centrosome amplification.
Analyses of the different MM patient datasets showed that individuals with the worst outcomes have abnormal amounts of FOPNL and carry another sign of poor prognosis—a high centrosome index. The implication is that disruptions in FOPNL could affect fundamental mechanisms controlling the distribution of genetic material to newly made cells.
“The results point us to a previously unrecognized gene as a determinant of myeloma prognosis,” said Elad Ziv, MD, of the University of California, San Francisco.
“If we understand what about this gene is causing poor prognosis, that may lead to a better fundamental grasp of the pathways that are important in multiple myeloma progression. Such knowledge could ultimately lead to better therapies.” ![]()
Team synthesizes compounds that induce rapid apoptosis in leukemia

apoptosis in cancer cells
For the first time, researchers have synthesized compounds that induce rapid apoptosis in leukemic cells.
The team synthesized several members of the family of dimeric nuphar alkaloids, which are compounds previously isolated from the yellow pond lily.
These structurally complex molecules have proven capable of inducing apoptosis in human leukemia cell lines faster than any other small molecule tested to date.
The researchers were also able to synthesize some related structures that they predict might exist in nature but have not yet been found.
Their work is published in Angewandte Chemie International Edition.
“We anticipate that these compounds will serve as useful tools for dissecting an important, but as yet undefined, step in the regulation of apoptosis,” said study author Jimmy Wu, PhD, of Dartmouth College in Hanover, New Hampshire.
The research also provides a means to a steady supply of the active compounds for further study.
Preliminary biological tests conducted by Alan Eastman, PhD, also of Dartmouth College, suggest the compounds, both the naturally occurring ones and those predicted to exist in nature, are capable of inducing extremely rapid apoptosis in leukemic cells.
“Studies to clarify the biological mechanism by which they operate are ongoing,” Dr Wu said.
He noted that there have been 2 reports that attempt to explain the molecules’ mechanism of action. But these are incomplete, and more research is required to fully reveal how these compounds work.
“A better understanding of the biological basis of how the dimeric nuphar alkaloids can so rapidly induce cell death may lead to novel points of intervention for the design of prospective therapeutics,” Dr Wu concluded. ![]()

apoptosis in cancer cells
For the first time, researchers have synthesized compounds that induce rapid apoptosis in leukemic cells.
The team synthesized several members of the family of dimeric nuphar alkaloids, which are compounds previously isolated from the yellow pond lily.
These structurally complex molecules have proven capable of inducing apoptosis in human leukemia cell lines faster than any other small molecule tested to date.
The researchers were also able to synthesize some related structures that they predict might exist in nature but have not yet been found.
Their work is published in Angewandte Chemie International Edition.
“We anticipate that these compounds will serve as useful tools for dissecting an important, but as yet undefined, step in the regulation of apoptosis,” said study author Jimmy Wu, PhD, of Dartmouth College in Hanover, New Hampshire.
The research also provides a means to a steady supply of the active compounds for further study.
Preliminary biological tests conducted by Alan Eastman, PhD, also of Dartmouth College, suggest the compounds, both the naturally occurring ones and those predicted to exist in nature, are capable of inducing extremely rapid apoptosis in leukemic cells.
“Studies to clarify the biological mechanism by which they operate are ongoing,” Dr Wu said.
He noted that there have been 2 reports that attempt to explain the molecules’ mechanism of action. But these are incomplete, and more research is required to fully reveal how these compounds work.
“A better understanding of the biological basis of how the dimeric nuphar alkaloids can so rapidly induce cell death may lead to novel points of intervention for the design of prospective therapeutics,” Dr Wu concluded. ![]()

apoptosis in cancer cells
For the first time, researchers have synthesized compounds that induce rapid apoptosis in leukemic cells.
The team synthesized several members of the family of dimeric nuphar alkaloids, which are compounds previously isolated from the yellow pond lily.
These structurally complex molecules have proven capable of inducing apoptosis in human leukemia cell lines faster than any other small molecule tested to date.
The researchers were also able to synthesize some related structures that they predict might exist in nature but have not yet been found.
Their work is published in Angewandte Chemie International Edition.
“We anticipate that these compounds will serve as useful tools for dissecting an important, but as yet undefined, step in the regulation of apoptosis,” said study author Jimmy Wu, PhD, of Dartmouth College in Hanover, New Hampshire.
The research also provides a means to a steady supply of the active compounds for further study.
Preliminary biological tests conducted by Alan Eastman, PhD, also of Dartmouth College, suggest the compounds, both the naturally occurring ones and those predicted to exist in nature, are capable of inducing extremely rapid apoptosis in leukemic cells.
“Studies to clarify the biological mechanism by which they operate are ongoing,” Dr Wu said.
He noted that there have been 2 reports that attempt to explain the molecules’ mechanism of action. But these are incomplete, and more research is required to fully reveal how these compounds work.
“A better understanding of the biological basis of how the dimeric nuphar alkaloids can so rapidly induce cell death may lead to novel points of intervention for the design of prospective therapeutics,” Dr Wu concluded. ![]()
Lipids aid engraftment of HSPCs
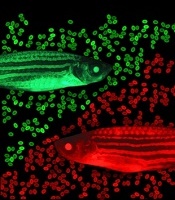
zebrafish used in the study
Image by Jonathan Henninger
and Vera Binder
Using zebrafish drug-screening models, researchers have identified a family of lipids that aid the engraftment of hematopoietic stem and progenitor cells (HSPCs).
The lipids, known as epoxyeicosatrienoic acids (EETs), boosted HSPC engraftment in zebrafish and mice.
The researchers therefore believe EETs could help make human HSPC transplants, particularly umbilical cord blood transplants, more efficient and effective.
“Ninety percent of cord blood units can’t be used because they’re too small,” said Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“If you add these chemicals, you might be able to use more units. Being able to get engraftment allows you to pick a smaller cord blood sample that might be a better match.”
Dr Zon and his colleagues described their work with EETs in Nature.
EETs appear to work by stimulating cell migration. They were among the top hits in a screen of 500 known compounds the researchers conducted.
In the past, such screens have led Dr Zon’s team to compounds that boost HSPC numbers, such as prostaglandin. But the new drug screen was designed to assess HSPCs’ transplantability and engraftment.
The screen was done in a lab-created strain of zebrafish called Casper. Because Casper is translucent, Dr Zon and his colleagues could visually compare the engraftment of transplanted HSPCs chemically tagged to glow green or red.
The researchers first used tagging to color the fishes’ marrow either red or green, then removed HSPCs for transplantation. The green cells were incubated with various chemicals, while the red cells were left untreated.
The team then injected a mixture of green and red HSPCs into other groups of zebrafish (10 fish per test chemical). And they visually tracked the cells’ activity, measuring the green-to-red ratio.
“The expectation was that if a chemical didn’t increase engraftment, all the fish would be equal parts red and green,” Dr Zon said. “But if it was effective, green marrow would predominate.”
That was the case for green marrow incubated with EETs, a finding that held up over thousands of transplants.
“In a mouse system, this experiment would cost $3 million,” Dr Zon noted. “In fish, it cost about $150,000.”
In a smaller-scale set of mouse experiments, the team confirmed EETs’ efficacy in promoting homing and engraftment of HSPCs.
EETs are chemical cousins of prostaglandin. Both are made from arachidonic acid, and both are made during inflammation. But EETs work in a different way, by activating the PI3K pathway. EETs also enhanced PI3K activity in human blood vessel cells in vitro.
After more studies in human cells to determine exactly how EETs work, Dr Zon hopes to begin clinical trials of EETs within the next 2 years, likely in the setting of cord blood transplant. The lab is also investigating its other top hits from the zebrafish screen.
“Every new pathway that we find has the chance of making stem cell engraftment and migration even better,” Dr Zon said. “I think we’ll end up being able to manipulate this process.” ![]()

zebrafish used in the study
Image by Jonathan Henninger
and Vera Binder
Using zebrafish drug-screening models, researchers have identified a family of lipids that aid the engraftment of hematopoietic stem and progenitor cells (HSPCs).
The lipids, known as epoxyeicosatrienoic acids (EETs), boosted HSPC engraftment in zebrafish and mice.
The researchers therefore believe EETs could help make human HSPC transplants, particularly umbilical cord blood transplants, more efficient and effective.
“Ninety percent of cord blood units can’t be used because they’re too small,” said Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“If you add these chemicals, you might be able to use more units. Being able to get engraftment allows you to pick a smaller cord blood sample that might be a better match.”
Dr Zon and his colleagues described their work with EETs in Nature.
EETs appear to work by stimulating cell migration. They were among the top hits in a screen of 500 known compounds the researchers conducted.
In the past, such screens have led Dr Zon’s team to compounds that boost HSPC numbers, such as prostaglandin. But the new drug screen was designed to assess HSPCs’ transplantability and engraftment.
The screen was done in a lab-created strain of zebrafish called Casper. Because Casper is translucent, Dr Zon and his colleagues could visually compare the engraftment of transplanted HSPCs chemically tagged to glow green or red.
The researchers first used tagging to color the fishes’ marrow either red or green, then removed HSPCs for transplantation. The green cells were incubated with various chemicals, while the red cells were left untreated.
The team then injected a mixture of green and red HSPCs into other groups of zebrafish (10 fish per test chemical). And they visually tracked the cells’ activity, measuring the green-to-red ratio.
“The expectation was that if a chemical didn’t increase engraftment, all the fish would be equal parts red and green,” Dr Zon said. “But if it was effective, green marrow would predominate.”
That was the case for green marrow incubated with EETs, a finding that held up over thousands of transplants.
“In a mouse system, this experiment would cost $3 million,” Dr Zon noted. “In fish, it cost about $150,000.”
In a smaller-scale set of mouse experiments, the team confirmed EETs’ efficacy in promoting homing and engraftment of HSPCs.
EETs are chemical cousins of prostaglandin. Both are made from arachidonic acid, and both are made during inflammation. But EETs work in a different way, by activating the PI3K pathway. EETs also enhanced PI3K activity in human blood vessel cells in vitro.
After more studies in human cells to determine exactly how EETs work, Dr Zon hopes to begin clinical trials of EETs within the next 2 years, likely in the setting of cord blood transplant. The lab is also investigating its other top hits from the zebrafish screen.
“Every new pathway that we find has the chance of making stem cell engraftment and migration even better,” Dr Zon said. “I think we’ll end up being able to manipulate this process.” ![]()

zebrafish used in the study
Image by Jonathan Henninger
and Vera Binder
Using zebrafish drug-screening models, researchers have identified a family of lipids that aid the engraftment of hematopoietic stem and progenitor cells (HSPCs).
The lipids, known as epoxyeicosatrienoic acids (EETs), boosted HSPC engraftment in zebrafish and mice.
The researchers therefore believe EETs could help make human HSPC transplants, particularly umbilical cord blood transplants, more efficient and effective.
“Ninety percent of cord blood units can’t be used because they’re too small,” said Leonard Zon, MD, of Boston Children’s Hospital in Massachusetts.
“If you add these chemicals, you might be able to use more units. Being able to get engraftment allows you to pick a smaller cord blood sample that might be a better match.”
Dr Zon and his colleagues described their work with EETs in Nature.
EETs appear to work by stimulating cell migration. They were among the top hits in a screen of 500 known compounds the researchers conducted.
In the past, such screens have led Dr Zon’s team to compounds that boost HSPC numbers, such as prostaglandin. But the new drug screen was designed to assess HSPCs’ transplantability and engraftment.
The screen was done in a lab-created strain of zebrafish called Casper. Because Casper is translucent, Dr Zon and his colleagues could visually compare the engraftment of transplanted HSPCs chemically tagged to glow green or red.
The researchers first used tagging to color the fishes’ marrow either red or green, then removed HSPCs for transplantation. The green cells were incubated with various chemicals, while the red cells were left untreated.
The team then injected a mixture of green and red HSPCs into other groups of zebrafish (10 fish per test chemical). And they visually tracked the cells’ activity, measuring the green-to-red ratio.
“The expectation was that if a chemical didn’t increase engraftment, all the fish would be equal parts red and green,” Dr Zon said. “But if it was effective, green marrow would predominate.”
That was the case for green marrow incubated with EETs, a finding that held up over thousands of transplants.
“In a mouse system, this experiment would cost $3 million,” Dr Zon noted. “In fish, it cost about $150,000.”
In a smaller-scale set of mouse experiments, the team confirmed EETs’ efficacy in promoting homing and engraftment of HSPCs.
EETs are chemical cousins of prostaglandin. Both are made from arachidonic acid, and both are made during inflammation. But EETs work in a different way, by activating the PI3K pathway. EETs also enhanced PI3K activity in human blood vessel cells in vitro.
After more studies in human cells to determine exactly how EETs work, Dr Zon hopes to begin clinical trials of EETs within the next 2 years, likely in the setting of cord blood transplant. The lab is also investigating its other top hits from the zebrafish screen.
“Every new pathway that we find has the chance of making stem cell engraftment and migration even better,” Dr Zon said. “I think we’ll end up being able to manipulate this process.” ![]()
Tests improve treatment of malaria

artemisinin-based combination
therapy. Photo courtesy of
The Global Fund
Introducing rapid diagnostic tests in drug shops can improve the treatment of malaria, according to research published in PLOS ONE.
Most of the 15,000 patients in this study, all of whom visited drug shops with a fever, chose to buy a rapid diagnostic test when offered one.
Test results showed that less than 60% of the patients had malaria, and vendors usually complied with the results, which reduced overprescription of malaria drugs by 73%.
Investigators conducted this study because the private sector is a common source of treatment in many malaria-endemic areas, especially where there is poor access to public health facilities.
Patients buy antimalarial drugs in shops to medicate themselves, but malaria is not always the cause of their fever, so inappropriate treatment is common.
“Our findings show that it is feasible to collaborate with the private health sector and introduce malaria rapid diagnostic tests in drug shops,” said study author Anthony Mbonye, PhD, of the Ugandan Ministry of Health in Kampala, Uganda.
“The next step is to refine the strategy and understand the cost implications of scaling it up in Uganda. Our long-term aim is to provide evidence to help the World Health Organization develop guidance to improve malaria treatment in the private sector.”
For this study, Dr Mbonye and his colleagues introduced rapid diagnostic tests in 10 clusters of drug shops in the Mukono district of central Uganda.
The team compared results at these shops to results at 10 other clusters of shops in the control arm, where treatment was given based on patients’ signs and symptoms.
The vendors’ decision of whether to treat a patient with artemisinin-based combination therapy was validated by confirming the presence of malaria parasites in the patient’s blood through microscopy carried out by the research team.
The rapid diagnostic tests reduced overdiagnosis and overprescription of malaria treatment by 73%, increasing appropriate treatment with artemisinin-based combination therapy by 36%.
“This study shows that rapid diagnostic tests can improve the use of artemisinin-based combination therapies—the most effective treatment for malaria—in drug shops, but it’s not without its challenges,” said Sian Clarke, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“These tests alone will not improve the treatment of other diseases. We now need to continue working with the Ministry of Health to investigate how to improve our approach and expand it to other common illnesses.”
An investigation conducted alongside this trial showed that, despite their popularity, rapid diagnostic tests for malaria were not a simple fix in the private sector.
Patients welcomed the rapid diagnostic tests as well as government involvement in improving drug shops. And vendors felt more akin to qualified health workers in the public sector for being allowed to test blood.
But investigators warn that this could give a false impression of vendors’ other skills and services, and regulation by authorities is needed.
This report was published in Critical Public Health. ![]()

artemisinin-based combination
therapy. Photo courtesy of
The Global Fund
Introducing rapid diagnostic tests in drug shops can improve the treatment of malaria, according to research published in PLOS ONE.
Most of the 15,000 patients in this study, all of whom visited drug shops with a fever, chose to buy a rapid diagnostic test when offered one.
Test results showed that less than 60% of the patients had malaria, and vendors usually complied with the results, which reduced overprescription of malaria drugs by 73%.
Investigators conducted this study because the private sector is a common source of treatment in many malaria-endemic areas, especially where there is poor access to public health facilities.
Patients buy antimalarial drugs in shops to medicate themselves, but malaria is not always the cause of their fever, so inappropriate treatment is common.
“Our findings show that it is feasible to collaborate with the private health sector and introduce malaria rapid diagnostic tests in drug shops,” said study author Anthony Mbonye, PhD, of the Ugandan Ministry of Health in Kampala, Uganda.
“The next step is to refine the strategy and understand the cost implications of scaling it up in Uganda. Our long-term aim is to provide evidence to help the World Health Organization develop guidance to improve malaria treatment in the private sector.”
For this study, Dr Mbonye and his colleagues introduced rapid diagnostic tests in 10 clusters of drug shops in the Mukono district of central Uganda.
The team compared results at these shops to results at 10 other clusters of shops in the control arm, where treatment was given based on patients’ signs and symptoms.
The vendors’ decision of whether to treat a patient with artemisinin-based combination therapy was validated by confirming the presence of malaria parasites in the patient’s blood through microscopy carried out by the research team.
The rapid diagnostic tests reduced overdiagnosis and overprescription of malaria treatment by 73%, increasing appropriate treatment with artemisinin-based combination therapy by 36%.
“This study shows that rapid diagnostic tests can improve the use of artemisinin-based combination therapies—the most effective treatment for malaria—in drug shops, but it’s not without its challenges,” said Sian Clarke, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“These tests alone will not improve the treatment of other diseases. We now need to continue working with the Ministry of Health to investigate how to improve our approach and expand it to other common illnesses.”
An investigation conducted alongside this trial showed that, despite their popularity, rapid diagnostic tests for malaria were not a simple fix in the private sector.
Patients welcomed the rapid diagnostic tests as well as government involvement in improving drug shops. And vendors felt more akin to qualified health workers in the public sector for being allowed to test blood.
But investigators warn that this could give a false impression of vendors’ other skills and services, and regulation by authorities is needed.
This report was published in Critical Public Health. ![]()

artemisinin-based combination
therapy. Photo courtesy of
The Global Fund
Introducing rapid diagnostic tests in drug shops can improve the treatment of malaria, according to research published in PLOS ONE.
Most of the 15,000 patients in this study, all of whom visited drug shops with a fever, chose to buy a rapid diagnostic test when offered one.
Test results showed that less than 60% of the patients had malaria, and vendors usually complied with the results, which reduced overprescription of malaria drugs by 73%.
Investigators conducted this study because the private sector is a common source of treatment in many malaria-endemic areas, especially where there is poor access to public health facilities.
Patients buy antimalarial drugs in shops to medicate themselves, but malaria is not always the cause of their fever, so inappropriate treatment is common.
“Our findings show that it is feasible to collaborate with the private health sector and introduce malaria rapid diagnostic tests in drug shops,” said study author Anthony Mbonye, PhD, of the Ugandan Ministry of Health in Kampala, Uganda.
“The next step is to refine the strategy and understand the cost implications of scaling it up in Uganda. Our long-term aim is to provide evidence to help the World Health Organization develop guidance to improve malaria treatment in the private sector.”
For this study, Dr Mbonye and his colleagues introduced rapid diagnostic tests in 10 clusters of drug shops in the Mukono district of central Uganda.
The team compared results at these shops to results at 10 other clusters of shops in the control arm, where treatment was given based on patients’ signs and symptoms.
The vendors’ decision of whether to treat a patient with artemisinin-based combination therapy was validated by confirming the presence of malaria parasites in the patient’s blood through microscopy carried out by the research team.
The rapid diagnostic tests reduced overdiagnosis and overprescription of malaria treatment by 73%, increasing appropriate treatment with artemisinin-based combination therapy by 36%.
“This study shows that rapid diagnostic tests can improve the use of artemisinin-based combination therapies—the most effective treatment for malaria—in drug shops, but it’s not without its challenges,” said Sian Clarke, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“These tests alone will not improve the treatment of other diseases. We now need to continue working with the Ministry of Health to investigate how to improve our approach and expand it to other common illnesses.”
An investigation conducted alongside this trial showed that, despite their popularity, rapid diagnostic tests for malaria were not a simple fix in the private sector.
Patients welcomed the rapid diagnostic tests as well as government involvement in improving drug shops. And vendors felt more akin to qualified health workers in the public sector for being allowed to test blood.
But investigators warn that this could give a false impression of vendors’ other skills and services, and regulation by authorities is needed.
This report was published in Critical Public Health. ![]()
Home VTE treatment with rivaroxaban safe and effective
Home treatment with rivaroxaban for patients with a low-risk first deep vein thrombosis or pulmonary embolism is associated with low rates of thrombosis recurrence or major bleeding, according to data from a prospective observational study.
The study enrolled 71 patients with low-risk deep vein thrombosis (DVT), 30 with pulmonary embolism, and five with both, all of whom were discharged with prescriptions for 15mg of rivaroxaban twice a day for 21 days and then 20 mg once per day for a further month.
There were no cases of thrombosis recurrence within the treatment period – although three patients had a recurrent DVT after stopping treatment – and no incidents of major or clinically relevant bleeding while on the therapy, as was reported in the July edition of Academic Emergency Medicine.
“This preliminary report provides data to support the initial outpatient treatment of low-risk ED patients with deep vein thrombosis and pulmonary embolism,” wrote Dr. Daren M. Beam and colleagues from the Indiana University School of Medicine (Academic Emergency Medicine 2015, 22:789–795 [doi:10.1111/acem.12711]).
The study was partly supported by the Lilly Physician Scientist Award. One author declared consultancies with Stago Diagnostica, Janssen, and Pfizer.
Home treatment with rivaroxaban for patients with a low-risk first deep vein thrombosis or pulmonary embolism is associated with low rates of thrombosis recurrence or major bleeding, according to data from a prospective observational study.
The study enrolled 71 patients with low-risk deep vein thrombosis (DVT), 30 with pulmonary embolism, and five with both, all of whom were discharged with prescriptions for 15mg of rivaroxaban twice a day for 21 days and then 20 mg once per day for a further month.
There were no cases of thrombosis recurrence within the treatment period – although three patients had a recurrent DVT after stopping treatment – and no incidents of major or clinically relevant bleeding while on the therapy, as was reported in the July edition of Academic Emergency Medicine.
“This preliminary report provides data to support the initial outpatient treatment of low-risk ED patients with deep vein thrombosis and pulmonary embolism,” wrote Dr. Daren M. Beam and colleagues from the Indiana University School of Medicine (Academic Emergency Medicine 2015, 22:789–795 [doi:10.1111/acem.12711]).
The study was partly supported by the Lilly Physician Scientist Award. One author declared consultancies with Stago Diagnostica, Janssen, and Pfizer.
Home treatment with rivaroxaban for patients with a low-risk first deep vein thrombosis or pulmonary embolism is associated with low rates of thrombosis recurrence or major bleeding, according to data from a prospective observational study.
The study enrolled 71 patients with low-risk deep vein thrombosis (DVT), 30 with pulmonary embolism, and five with both, all of whom were discharged with prescriptions for 15mg of rivaroxaban twice a day for 21 days and then 20 mg once per day for a further month.
There were no cases of thrombosis recurrence within the treatment period – although three patients had a recurrent DVT after stopping treatment – and no incidents of major or clinically relevant bleeding while on the therapy, as was reported in the July edition of Academic Emergency Medicine.
“This preliminary report provides data to support the initial outpatient treatment of low-risk ED patients with deep vein thrombosis and pulmonary embolism,” wrote Dr. Daren M. Beam and colleagues from the Indiana University School of Medicine (Academic Emergency Medicine 2015, 22:789–795 [doi:10.1111/acem.12711]).
The study was partly supported by the Lilly Physician Scientist Award. One author declared consultancies with Stago Diagnostica, Janssen, and Pfizer.
FROM ACADEMIC EMERGENCY MEDICINE
Key clinical point: Home treatment with rivaroxaban for patients with a low-risk first deep vein thrombosis or pulmonary embolism is safe and effective.
Major finding: Patients treated at home with rivaroxaban reported no cases of recurrent VTE or major bleeding during the treatment period.
Data source: A prospective observational study in 106 patients with deep vein thrombosis or pulmonary embolism.
Disclosures: The study was partly supported by the Lilly Physician Scientist Award. One author declared consultancies with Stago Diagnostica, Janssen, and Pfizer.
Pregnancy registries add to the clinical picture
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Most hospitals overestimate their door-to-needle performance
Personnel at most hospitals that treat acute stroke, particularly the lowest-performing hospitals, greatly overestimate their ability to deliver TPA to eligible patients within 1 hour, according to a report published online July 22 in Journal of the American Heart Association.
Overestimating the quality of care they actually provide may perpetuate this suboptimal performance, “whereas accurate measurements of current performance and realistic comparison to other, more successful, sites might provide the needed motivation to fuel quality improvement,” said Dr. Cheryl B. Lin of Tufts Medical Center Floating Hospital for Children, Boston, and her associates.
They compared stroke teams’ perceptions of their door-to-needle performance, as measured on survey questionnaires answered by nurses, neurologists, and other staff members, against the hospitals’ actual performance, which was recorded in a large stroke registry. The investigators focused on 141 hospitals that treated 48,201 stroke patients during a 1-year period. This included 49 top-performing, 52 average-performing, and 40 low-performing hospitals. The top category had door-to-needle rates of 45%-93%, while the bottom category had consistent door-to-needle rates of 0%. The middle category had door-to-needle rates of 16%-25%.
Regardless of their hospital’s performance category, 61% of the respondents overestimated how many eligible patients there actually received TPA within 1 hour. The lowest-performing hospitals had the most unrealistic estimates, with 68% of them guessing that 20% of their patients received timely TPA when in fact 0% of patients did so. Low-performing hospitals also overestimated their performance in comparison with other hospitals, with 85% of them characterizing their performance as average, above average, or even superior relative to other hospitals, when in fact it was very poor, Dr. Lin and her associates wrote (J. Am. Heart Assoc. 2015 July 22 [doi:10.1161/JAHA.114.001298]).
“Addressing misperceptions that one’s performance is average or above average when it actually is not is an important step in addressing motivation for change,” they added.
The study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Lin reported having no relevant financial disclosures; her associates reported ties to Genentech, Lilly, Johnson & Johnson, Bristol-Myers Squibb, Sanofi-Aventis, and Merck Schering-Plough.
Personnel at most hospitals that treat acute stroke, particularly the lowest-performing hospitals, greatly overestimate their ability to deliver TPA to eligible patients within 1 hour, according to a report published online July 22 in Journal of the American Heart Association.
Overestimating the quality of care they actually provide may perpetuate this suboptimal performance, “whereas accurate measurements of current performance and realistic comparison to other, more successful, sites might provide the needed motivation to fuel quality improvement,” said Dr. Cheryl B. Lin of Tufts Medical Center Floating Hospital for Children, Boston, and her associates.
They compared stroke teams’ perceptions of their door-to-needle performance, as measured on survey questionnaires answered by nurses, neurologists, and other staff members, against the hospitals’ actual performance, which was recorded in a large stroke registry. The investigators focused on 141 hospitals that treated 48,201 stroke patients during a 1-year period. This included 49 top-performing, 52 average-performing, and 40 low-performing hospitals. The top category had door-to-needle rates of 45%-93%, while the bottom category had consistent door-to-needle rates of 0%. The middle category had door-to-needle rates of 16%-25%.
Regardless of their hospital’s performance category, 61% of the respondents overestimated how many eligible patients there actually received TPA within 1 hour. The lowest-performing hospitals had the most unrealistic estimates, with 68% of them guessing that 20% of their patients received timely TPA when in fact 0% of patients did so. Low-performing hospitals also overestimated their performance in comparison with other hospitals, with 85% of them characterizing their performance as average, above average, or even superior relative to other hospitals, when in fact it was very poor, Dr. Lin and her associates wrote (J. Am. Heart Assoc. 2015 July 22 [doi:10.1161/JAHA.114.001298]).
“Addressing misperceptions that one’s performance is average or above average when it actually is not is an important step in addressing motivation for change,” they added.
The study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Lin reported having no relevant financial disclosures; her associates reported ties to Genentech, Lilly, Johnson & Johnson, Bristol-Myers Squibb, Sanofi-Aventis, and Merck Schering-Plough.
Personnel at most hospitals that treat acute stroke, particularly the lowest-performing hospitals, greatly overestimate their ability to deliver TPA to eligible patients within 1 hour, according to a report published online July 22 in Journal of the American Heart Association.
Overestimating the quality of care they actually provide may perpetuate this suboptimal performance, “whereas accurate measurements of current performance and realistic comparison to other, more successful, sites might provide the needed motivation to fuel quality improvement,” said Dr. Cheryl B. Lin of Tufts Medical Center Floating Hospital for Children, Boston, and her associates.
They compared stroke teams’ perceptions of their door-to-needle performance, as measured on survey questionnaires answered by nurses, neurologists, and other staff members, against the hospitals’ actual performance, which was recorded in a large stroke registry. The investigators focused on 141 hospitals that treated 48,201 stroke patients during a 1-year period. This included 49 top-performing, 52 average-performing, and 40 low-performing hospitals. The top category had door-to-needle rates of 45%-93%, while the bottom category had consistent door-to-needle rates of 0%. The middle category had door-to-needle rates of 16%-25%.
Regardless of their hospital’s performance category, 61% of the respondents overestimated how many eligible patients there actually received TPA within 1 hour. The lowest-performing hospitals had the most unrealistic estimates, with 68% of them guessing that 20% of their patients received timely TPA when in fact 0% of patients did so. Low-performing hospitals also overestimated their performance in comparison with other hospitals, with 85% of them characterizing their performance as average, above average, or even superior relative to other hospitals, when in fact it was very poor, Dr. Lin and her associates wrote (J. Am. Heart Assoc. 2015 July 22 [doi:10.1161/JAHA.114.001298]).
“Addressing misperceptions that one’s performance is average or above average when it actually is not is an important step in addressing motivation for change,” they added.
The study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Lin reported having no relevant financial disclosures; her associates reported ties to Genentech, Lilly, Johnson & Johnson, Bristol-Myers Squibb, Sanofi-Aventis, and Merck Schering-Plough.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: Personnel at most hospitals, particularly the lowest-performing hospitals, greatly overestimate their performance at giving stroke patients TPA within 1 hour of arrival.
Major finding: The lowest-performing hospitals had the most unrealistic estimates of their door-to-needle times, with 68% of them guessing that 20% of their patients received timely TPA when in fact 0% of their patients did so.
Data source: An analysis of data in a stroke registry regarding 141 hospitals that treated 48,201 patients during a 1-year period, plus a survey of stroke personnel at those hospitals.
Disclosures: This study was supported by the U.S. Agency for Healthcare Research and Quality. Dr. Lin reported having no relevant financial disclosures; her associates reported ties to Genentech, Lilly, Johnson & Johnson, Bristol-Myers Squibb, Sanofi-Aventis and Merck Schering-Plough.
Harnessing new data on immunotherapies
Immunotherapies once again took center stage at the 2015 annual meeting of the American Society for Clinical Oncology in Chicago, though many other groundbreaking clinical advances were also presented. The meeting’s theme, “Illumination and innovation: transforming data into learning,” captured the current focus, by both researchers and practicing oncologists, on the importance of being able to draw on new and enticing data and use it as the basis for improving the care of and outcomes for cancer patients.
CheckMate 067: Two immunotherapies better than one for advanced melanoma
Key clinical point Nivolumab alone or combined with ipilimumab significantly improves progression-free survival (PFS) and objective response rates (ORRs), compared with ipilimumab alone in previously untreated metastatic melanoma. Major finding Median PFS was 11.5 months with nivolumab plus ipilimumab, 6.9 months with nivolumab, and 2.9 months with ipilimumab. Data source Phase 3, double-blind randomized trial in 945 patients with previously untreated metastatic melanoma. Disclosures Bristol-Myers Squibb funded the trial. Dr Wolchok reported financial relationships with several firms including research funding from and consulting or advising for Bristol-Myers Squibb…
Click on the PDF icon at the top of this introduction to read the full article.
Immunotherapies once again took center stage at the 2015 annual meeting of the American Society for Clinical Oncology in Chicago, though many other groundbreaking clinical advances were also presented. The meeting’s theme, “Illumination and innovation: transforming data into learning,” captured the current focus, by both researchers and practicing oncologists, on the importance of being able to draw on new and enticing data and use it as the basis for improving the care of and outcomes for cancer patients.
CheckMate 067: Two immunotherapies better than one for advanced melanoma
Key clinical point Nivolumab alone or combined with ipilimumab significantly improves progression-free survival (PFS) and objective response rates (ORRs), compared with ipilimumab alone in previously untreated metastatic melanoma. Major finding Median PFS was 11.5 months with nivolumab plus ipilimumab, 6.9 months with nivolumab, and 2.9 months with ipilimumab. Data source Phase 3, double-blind randomized trial in 945 patients with previously untreated metastatic melanoma. Disclosures Bristol-Myers Squibb funded the trial. Dr Wolchok reported financial relationships with several firms including research funding from and consulting or advising for Bristol-Myers Squibb…
Click on the PDF icon at the top of this introduction to read the full article.
Immunotherapies once again took center stage at the 2015 annual meeting of the American Society for Clinical Oncology in Chicago, though many other groundbreaking clinical advances were also presented. The meeting’s theme, “Illumination and innovation: transforming data into learning,” captured the current focus, by both researchers and practicing oncologists, on the importance of being able to draw on new and enticing data and use it as the basis for improving the care of and outcomes for cancer patients.
CheckMate 067: Two immunotherapies better than one for advanced melanoma
Key clinical point Nivolumab alone or combined with ipilimumab significantly improves progression-free survival (PFS) and objective response rates (ORRs), compared with ipilimumab alone in previously untreated metastatic melanoma. Major finding Median PFS was 11.5 months with nivolumab plus ipilimumab, 6.9 months with nivolumab, and 2.9 months with ipilimumab. Data source Phase 3, double-blind randomized trial in 945 patients with previously untreated metastatic melanoma. Disclosures Bristol-Myers Squibb funded the trial. Dr Wolchok reported financial relationships with several firms including research funding from and consulting or advising for Bristol-Myers Squibb…
Click on the PDF icon at the top of this introduction to read the full article.
Oncogenic drivers and immunotherapy: staying one step ahead of lung cancer
The majority of newly diagnosed lung cancers are NSCLC, and about half of those are adenocarcinomas (Figure 1).2 Over the past decade there has been a significant evolution in the understanding and treatment of lung adenocarcinoma, mostly stemming from a greater appreciation of the distinct pathologies and unique molecular signatures of these tumors. Genomic characterization of the molecular signatures has led to the identification of numerous key genetic alterations that drive lung cancer. The dependency of lung tumors on these genetic drivers has enabled the pharmacological development of targeted therapies that exploit this vulnerability...
Click on the PDF icon at the top of this introduction to read the full article.
The majority of newly diagnosed lung cancers are NSCLC, and about half of those are adenocarcinomas (Figure 1).2 Over the past decade there has been a significant evolution in the understanding and treatment of lung adenocarcinoma, mostly stemming from a greater appreciation of the distinct pathologies and unique molecular signatures of these tumors. Genomic characterization of the molecular signatures has led to the identification of numerous key genetic alterations that drive lung cancer. The dependency of lung tumors on these genetic drivers has enabled the pharmacological development of targeted therapies that exploit this vulnerability...
Click on the PDF icon at the top of this introduction to read the full article.
The majority of newly diagnosed lung cancers are NSCLC, and about half of those are adenocarcinomas (Figure 1).2 Over the past decade there has been a significant evolution in the understanding and treatment of lung adenocarcinoma, mostly stemming from a greater appreciation of the distinct pathologies and unique molecular signatures of these tumors. Genomic characterization of the molecular signatures has led to the identification of numerous key genetic alterations that drive lung cancer. The dependency of lung tumors on these genetic drivers has enabled the pharmacological development of targeted therapies that exploit this vulnerability...
Click on the PDF icon at the top of this introduction to read the full article.