User login
College students with depressive symptoms with and without fatigue: Differences in functioning, suicidality, anxiety, and depressive severity
Nyer et al examined whether fatigue was associated with greater symptomatic burden and functional impairment in 287 college students with depressive symptoms using data from the self-report Beck Depression Inventory (BDI). Students endorsing significant symptoms of depression (BDI score ≥13) were grouped into 3 levels: no fatigue, mild fatigue, or moderate/severe fatigue. Researchers compared the 3 levels of fatigue across a battery of psychiatric and functional outcome measures.
The study found that depressed college students with symptoms of fatigue demonstrated functional impairment and symptomatic burden that worsened with increasing levels of fatigue. The authors call for more attention to assessing and treating symptoms of fatigue within this population.
Nyer et al examined whether fatigue was associated with greater symptomatic burden and functional impairment in 287 college students with depressive symptoms using data from the self-report Beck Depression Inventory (BDI). Students endorsing significant symptoms of depression (BDI score ≥13) were grouped into 3 levels: no fatigue, mild fatigue, or moderate/severe fatigue. Researchers compared the 3 levels of fatigue across a battery of psychiatric and functional outcome measures.
The study found that depressed college students with symptoms of fatigue demonstrated functional impairment and symptomatic burden that worsened with increasing levels of fatigue. The authors call for more attention to assessing and treating symptoms of fatigue within this population.
Nyer et al examined whether fatigue was associated with greater symptomatic burden and functional impairment in 287 college students with depressive symptoms using data from the self-report Beck Depression Inventory (BDI). Students endorsing significant symptoms of depression (BDI score ≥13) were grouped into 3 levels: no fatigue, mild fatigue, or moderate/severe fatigue. Researchers compared the 3 levels of fatigue across a battery of psychiatric and functional outcome measures.
The study found that depressed college students with symptoms of fatigue demonstrated functional impairment and symptomatic burden that worsened with increasing levels of fatigue. The authors call for more attention to assessing and treating symptoms of fatigue within this population.
Mortality Rates Associated With Odontoid and Subaxial Cervical Spine Fractures
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
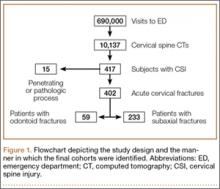
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
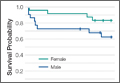
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
Mortality rate is an important indicator of the severity of traumatic injuries, and these values have been described for different orthopedic injuries and fractures. Studies have identified 3 distinct trends in patient survival when compared with the age- and sex-matched uninjured population:
1. Hip fractures bring about a transient increase in mortality relative to age-matched controls that normalizes after a few months to 1 year.1-10
2. Thoracic and lumbar compression fractures are associated with an ongoing, lifelong increase in mortality rate relative to age-matched controls without an initial marked upswing.11-15
3. Certain injuries such as isolated rib or wrist fractures do not adversely affect survival relative to age-matched controls.12,16-18
Understanding the mortality patterns after these injuries can help guide management and even facilitate the development of appropriate treatment algorithms.19-21 While studies have examined mortality in specific odontoid fracture types,22 such mortality trends have not been broadly established in persons with cervical spine fractures.
Cervical spine fractures are common: 60% of spine fractures localize to this region,23-26 and this equates to 2% to 3% of all blunt-trauma patients.27,28 These injuries can lead to devastating consequences, including neurologic compromise, permanent disability, and death.29-31
Studies have estimated that up to 20% of cervical fractures involve the odontoid process.23-26 These injuries are more common among the elderly population because of their greater prevalence of osteoporosis and likelihood of falling.32 Because of demographic similarities to those of the hip fracture population, a survival analysis of all odontoid fractures is particularly interesting. Published odontoid mortality rates vary significantly, with reports ranging from 13% to 44%.22,33-35 Unfortunately, these studies largely evaluated survival rates specific to an individual treatment modality, such as nonoperative compared with operative, or specific to certain odontoid fracture types (eg, type II). Additionally, studies have generally only considered survivorship during initial hospitalization, have been specific to a constrained age group, or have been based solely on inpatient records that do not permit the longer-term follow-up critical to determining the effect of odontoid fractures on overall mortality.36-39
Likewise, mortality rates after fractures of the subaxial spine (ie, the motion segments between C3 and C7) have yet to be established. In 1 study, the mortality risk of a cohort of elderly patients with cervical fractures appeared to be elevated for the first 6 to 12 months after the traumatic event.40 However, the sample size was too small to examine mortality beyond 1 year.
In this context, the purpose of the current study was to determine the mortality rates at several time points (3 months, 1 year, and 2 years) of patients 50 years or older (start of the second mode of the bimodal age distribution of odontoid fractures41-44) with fractures of the odontoid and subaxial cervical spine. A secondary purpose of this study was to compare survival rates of these 2 cohorts relative to each other and to the general population.
Materials and Methods
Identification of Cervical Fractures and Collection of Demographic Information
This protocol was approved by the human investigation committee of our institution. Every computed tomography (CT) scan of the cervical spine performed in the emergency department (ED) of an academic hospital between November 27, 1997, and December 31, 2006, was identified. Since the threshold for obtaining a CT scan of a patient with suspected cervical spine trauma is relatively low, it was assumed that virtually all acute cervical spine fractures during this time period would be successfully identified through this approach.
Radiology reports for all identified CT scans were reviewed for any findings consistent with acute fractures and/or dislocations of the cervical spine (Figure 1). Every study noted to be positive or equivocal for cervical trauma was directly visualized, including those that did not specifically mention the presence or absence of an injury. Scans with no signs of acute trauma or that showed fractures caused by a pathologic process or penetrating mechanism (eg, metastatic lesions or gunshot wounds) were omitted from this series. Finally, relevant demographic information, such as the medical record number, age, gender, and date of study, was recorded for every subject in this group.
Fracture Classification
Next, the level and the type of cervical injury were documented for each patient. Fractures were segregated according to their involvement with the odontoid or the subaxial vertebrae.
Odontoid fractures were categorized into type I (limited to the tip), type II (across the base of the process) and type III (through the base with extension into the C2 vertebral body).45,46 Since many systems for classifying subaxial cervical spine trauma require a subjective inference of the injury mechanism, which is difficult to ascertain from imaging studies alone, all of these fractures were pooled together.
A preliminary survey of the data indicated that the odontoid fractures appeared to exhibit a bimodal age distribution, with the beginning of the second cluster occurring around age 50 years (Figure 2). As noted above, this has been shown in previous studies.41-44 As a consequence, the mortalities of those older than 50 years became the focus of this study. To control for comorbid conditions, mechanism of injury, and to allow for more direct comparison with the odontoid fractures in this study, the same age demarcation was used for subaxial cervical fractures.
Mortality Data
The mortality status of every patient diagnosed with an acute cervical injury at our institution between November 27, 1997, and December 31, 2006, was determined by referencing the National Death Index (NDI). The NDI is a computerized database of death records maintained by the National Center for Health Statistics (NCHS). The time window for the current study was selected because we had access to NDI information only through 2007 at the time of this study. Social Security numbers (SSNs), which were available for approximately half of the subjects, were used to search the NDI catalog. For individuals whose SSNs were unavailable, patient names and birthdates were considered to be sufficient to confirm a true match. Our center’s medical records of this cohort were also examined to verify whether any had died during their initial hospitalizations and to substantiate the NDI data. Finally, patient deaths were categorized as trauma (eg, motor vehicle accident, fall from a height) or medical comorbidity (eg, diabetes mellitus, cancer, congestive heart failure), based on information in the NDI listing.
Age- and Sex-Matched Controls
Age- and sex-matched controls were determined from the Wide-ranging Online Data for Epidemiologic Research (WONDER) application distributed by the Centers for Disease Control and Prevention (http://wonder.cdc.gov). Composite mortality data from the state in which the study was performed was obtained for the years between 1999 and 2007, and this information was further stratified according to gender and age to estimate the mortality rates and construct survival curves for each group. Controls were used to establish a standardized mortality ratio (SMR) for subjects 50 years and older, a value that compares the number of observed deaths with the figure expected for matched populations from the general population.
Statistical Methods
Statistical analyses were performed by using both SAS 9.2 (SAS Institute Inc., Cary, North Carolina) and R (version 2.9; www.r-project.org, Auckland, New Zealand). Relevant comparisons were planned, and all tests were 2-sided. The Wilcoxon rank sum test was applied to compare the survival times of patients with odontoid fractures with different documented causes of death, and Pearson χ2 test was used to compare the age distributions of odontoid and subaxial fractures. Survival rates at 3 months, 1 year, and 2 years were estimated from Kaplan-Meier curves. The relative survival of these cohorts was compared by completing a 2-sample log-rank test. In addition, a 1-sample log-rank test was implemented to compare the mortality from either odontoid or subaxial cervical spine fractures with that of the age- and gender-matched general population. Statistical significance was defined as a 2-sided α error of less than 0.05 (P < .05).
Results
Fifty-nine patients were diagnosed with odontoid fractures (28 men, 31 women), and 233 patients were diagnosed with subaxial cervical spine fractures (168 men, 65 women).
Odontoid Fracture Patients
Odontoid fracture patients exhibited a distinct bimodal age distribution (Figure 2). In the younger population, there were 14 subjects, 3 of whom died within days of the injury (mean, 12 days; 78.6% survival). At 2-year follow-up, there were no further deaths. The fractures that caused death were high-energy injuries, and only early deaths occurred in these cases.
Because of the significant bimodal age distribution, it was believed these cohorts could not be directly compared. As a result, the remaining analysis focused on the older age group. In the older population mode (50 years and older) were 45 patients with odontoid fractures. Of the 12 subjects who died after odontoid fracture, 5 were assigned a trauma code as the cause of death, while a medical comorbidity code was assigned for the remaining 7. Mean survival time of those who died secondary to trauma was significantly shorter than the medical comorbidity group (P = .025).
In the cohort of subjects older than 50 years, 3-month, 1-year, and 2-year survival rates were 84.4%, 82.2%, and 72.9%, respectively. Figure 2 shows the 1- and 2-year follow-up data by age group.
Analysis was performed relative to gender. Of male patients (n = 22), the 3-month, 1-year, and 2-year survival rates were 72.7%, 72.7%, and 62.7%, respectively. Among women (n = 23), the 3-month, 1-year, and 2-year survival rates were 95.7%, 91.3%, and 82.6%, respectively.
Figure 3 shows the Kaplan-Meier survival curves of the older patients with odontoid fractures. A comparison of the curves for each gender showed no significant disparities between the male and female survival (Figure 3A, P = .124). Compared with age-matched male counterparts, the survival of male subjects with odontoid fractures was significantly worse (Figure 3B, P < .001). Men experienced an initial acute decline in survival, with the remainder of the survival curve matching that of the general male population. In contrast, odontoid fractures did not adversely affect female survival compared with the matched population (Figure 3C, P = .568).
The 2-year SMR of 2.98 for men showed that odontoid fractures led to greater mortality compared with a sex- and age-matched population. This means that men older than 50 years who sustained an odontoid fracture had nearly 3 times the mortality rate after 2 years compared with a normal, matched population; this increase is attributed to the 3-month time point that subsequently normalized. The female rate was 1.33 times that of a matched population, a difference that is not statistically significant.
Subaxial Fracture Patients
Of the 91 patients older than 50 years with subaxial fractures, 3-month, 1-year, and 2-year survival rates were 87.9%, 85.7%, and 85.7%, respectively. Figure 4 shows the 1- and 2-year follow-up data by age group.
Gender-specific analysis was performed. For men (n = 58), the 3-month, 1-year, and 2-year survival rates were 87.9%, 84.5%, and 84.5%, respectively. Among women (n = 33), 4 deaths were recorded at all time points (87.9% survival).
Figure 5 shows Kaplan-Meier survival curves for the older population with subaxial fractures. A comparison of the curves between genders again showed no significant differences between male and female survival (P = .683, Figure 5A). Compared with age- and gender-matched counterparts, men showed decreased relative survival (P < .0001, Figure 5B), whereas subaxial fractures did not decrease female survival (P = .554, Figure 5C).
The 2-year SMR of 2.90 for men showed higher mortality rates relative to sex- and age-matched controls. Men who were both 50 years old and sustained a subaxial fracture were 2.9 times as likely to die within 2 years of follow-up compared with their counterparts. Similar to odontoid fractures, this increase occurred by the 3-month time point and subsequently normalized. The female rate, which was 1.34 times that of the uninjured population, was not statistically significant.
Comparison of Odontoid and Subaxial Fracture Patients
The survival of subaxial injuries was not significantly different from that of odontoid fractures (P = .113, Figure 6A). When analyzed by gender and controlled for age, the rates in both male (P = .347, Figure 6B) and female (P = .643, Figure 6C) patients did not differ between fracture types.
Discussion
The US population is aging rapidly, with the demographic older than 65 years predicted to more than double in size between 2010 and 2050.47 As our elderly population grows, the incidence of age-related injuries will rise accordingly. An understanding of mortality risks associated with different fractures will not only assist practitioners in advising patients regarding prognosis but may also lead to improvements in clinical care.19,48-50 While we know cervical spine trauma is associated with significant morbidity,29-31 little is known about associated moderate-term mortality rates that can be compared with other known injury patterns, such as hip fractures or osteoporotic compression fractures.
An interesting finding of the present study is the bimodal age distribution of the 59 odontoid fractures (Figure 2). The 14 patients younger than 50 years included 3 individuals who died, all within days of their presentation from severe multisystem trauma. This is consistent with the determination that high-energy forces are required to fracture the odontoid process in younger individuals.38,45,46,51,52 Given the severity of their nonspinal injuries, the cause of death was likely not primarily related to their odontoid fractures. Also in line with previous studies, the majority (76%) of odontoid fractures were documented in subjects older than 50 years.32,53,54 Within our cohort older than 50 years, the deaths appear to be spread evenly across age groups and do not seem to be skewed by the oldest portion of the population (Figure 2).
Our gender-specific analyses revealed that older men with odontoid injuries exhibited higher mortality compared with an age-matched male cohort, with 6 of the 8 deaths occurring within 3 months. However, after this exaggerated decline in survival, the rate normalized towards general population mortality rates (Figure 3B). As in the younger cohort, these earlier deaths were largely attributable to multisystem trauma, whereas medical comorbidities were implicated in those who died later. In contrast, the Kaplan-Meier curve of older women with odontoid fractures closely approximates that of age-matched women at every time point (Figure 3C), indicating that these injuries do not decrease survival as they do in their male counterparts.
When comparing the survival of older patients with subaxial cervical spine fractures with that of gender- and age-matched controls, the mortality rates of women were, once again, essentially equivalent. However, the survival of older men was significantly compromised by these injuries. In men, 7 of the 9 deaths were within 3 months, with the remaining 2 deaths occurring within 7 months. Nevertheless, beyond this initial period of elevated mortality, the survival curve again stabilized and paralleled that of the general population. As with odontoid fractures, there was no sustained increase in the mortality of male patients who lived at least 3 months after injury.
The mortality rates of odontoid and subaxial fractures were also compared in the older population. When controlled for age, there was no difference in mortality rates between these 2 groups. When individually analyzed in both men and women, the mortality rates of both fracture types matched those of the general population at all time points.
It is useful to contextualize our findings alongside the mortality of older individuals with other fracture types. Based on our results, we believe that the survival curves of geriatric men with odontoid or subaxial cervical spine fractures most closely resemble the characteristic pattern seen in hip fractures. Hip fractures have shown an early spike in mortality by as much as 8% to 49% in the first 6 to 12 months that returns to baseline after 1 year.1-10 This presumably reflects the natural history of these injuries in response to appropriate therapeutic interventions. Interestingly, the male mortality rates for both odontoid and subaxial cervical spine fractures in this study are largely analogous to those reported by various hip fracture surveys.1,5,55-58 In contrast, similar to prior studies of rib or wrist fractures, older women with these cervical spine fractures did not show a survival decrease after their injuries.12,16-18
While the reasons underlying the differential effects of cervical fractures on the mortality of men and women have not been established, one explanation is that the female geriatric population is relatively more osteoporotic; thus, cervical injuries may occur after lower-energy forces, leading to less severe associated trauma that could otherwise decrease survival. Another explanation is that men are more likely to be involved in high-energy accidents,59,60 thus decreasing their overall survival after injury.
This investigation is not without limitations. Our primary concern is the determination of survival. The NDI maintained by the NCHS is an extremely reliable tool regularly employed by epidemiologists to collect mortality data. However, it is possible that deaths may have been missed. We believe this number would be small, because the NDI database provided multiple probable matches that were carefully compared with supplemental personal information. It is also possible that deaths that were not appropriately registered with the NDI are not represented in this series. Another limitation lies in the determination of controls. As with any case–control study, the patients sustaining these odontoid fractures may differ in some significant way from the average population. A final limitation is that a small portion of patients in the study have only 1-year follow-up, because patient data was collected through 2006, although access to NDI data ended in 2007.
Conclusion
Our results indicate that the survival of older men with either odontoid or subaxial cervical spine fractures shares many of the same mortality characteristics as hip fractures, with diminished survival in the first 3 months that normalizes to the survival rate of the age-matched population. Interestingly, and perhaps because of disparate rates of osteoporosis and traumatic forces, the mortality rates in the female cohort were similar to that of the age-matched general population at all time points. These trends were nearly identical for both odontoid and subaxial cervical fractures.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
1. Gennarelli TA, Champion HR, Sacco WJ, Copes WS, Alves WM. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma. 1989;29(9):1193-1201; discussion 1201-1202.
2. George GH, Patel S. Secondary prevention of hip fracture. Rheumatology (Oxford). 2000;39(4):346-349.
3. Gerrelts BD, Petersen EU, Mabry J, Petersen SR. Delayed diagnosis of cervical spine injuries. J Trauma. 1991;31(12):1622-1626.
4. Giannoudis PV, Mehta SS, Tsiridis E. Incidence and outcome of whiplash injury after multiple trauma. Spine. 2007;32(7):776-781.
5. Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38(1):17-21.
6. Grauer JN, Shafi B, Hilibrand AS, et al. Proposal of a modified, treatment-oriented classification of odontoid fractures. Spine J. 2005;5(2):123-129.
7. Greene KA, Dickman CA, Marciano FF, Drabier JB, Hadley MN, Sonntag VK. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine. 1997;22(16):1843-1852.
8. Gulli B, Templeman D. Compartment syndrome of the lower extremity. Orthop Clin North Am. 1994;25(4):677-684.
9. Guthkelch AN, Fleischer AS. Patterns of cervical spine injury and their associated lesions. West J Med. 1987;147(4):428-431.
10. Hackl W, Hausberger K, Sailer R, Ulmer H, Gassner R. Prevalence of cervical spine injuries in patients with facial trauma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(4):370-376.
11. Doruk H, Mas MR, Yildiz C, Sonmez A, Kyrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185.
12. Garabige V, Giraud P, De Rycke Y, et al. [Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful?]. Cancer Radiother. 2007;11(3):111-116.
13. Garbuz DS, Leitch K, Wright JG. The treatment of supracondylar fractures in children with an absent radial pulse. J Pediatr Orthop. 1996;16(5):594-596.
14. Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16(2):128-131.
15. Henrikson B. Supracondylar fracture of the humerus in children. A late review of end-results with special reference to the cause of deformity, disability and complications. Acta Chir Scand Suppl. 1966;369:1-72.
16. De Boeck H, De Smet P, Penders W, De Rydt D. Supracondylar elbow fractures with impaction of the medial condyle in children. J Pediatr Orthop. 1995;15(4):444-448.
17. Gelberman RH, Panagis JS, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part I: The extraosseous vascularity. J Hand Surg Am. 1983;8(4):367-375.
18. Hu J, Liao Q, Long W. Diagnosis and treatment of multiple-level noncontiguous spinal fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(6):424-426.
19. Eleraky MA, Theodore N, Adams M, Rekate HL, Sonntag VK. Pediatric cervical spine injuries: report of 102 cases and review of the literature. J Neurosurg. 2000;92(1 suppl):12-17.
20. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181(5):265-271.
21. Husby J, Sorensen KH. Fracture of the odontoid process of the axis. Acta Orthop Scand. 1974;45(2):182-192.
22. Schoenfeld AJ, Bono CM, Reichmann WM, et al. Type II odontoid fractures of the cervical spine: do treatment type and medical comorbidities affect mortality in elderly patients? Spine. 2011;36(11):879-885.
23. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48(3):241-249.
24. Fassett DR, Dailey AT, Vaccaro AR. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21(4):252-258.
25. Ippolito E, Caterini R, Scola E. Supracondylar fractures of the humerus in children. Analysis at maturity of fifty-three patients treated conservatively. J Bone Joint Surg Am. 1986;68(3):333-344.
26. Spence KF Jr, Decker S, Sell KW. Bursting atlantal fracture associated with rupture of the transverse ligament. J Bone Joint Surg Am. 1970;52(3):543-549.
27. Irwin ZN, Arthur M, Mullins RJ, Hart RA. Variations in injury patterns, treatment, and outcome for spinal fracture and paralysis in adult versus geriatric patients. Spine. 2004;29(7):796-802.
28. Ismail AA, O’Neill TW, Cooper C, et al. Mortality associated with vertebral deformity in men and women: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int. 1998;8(3):291-297.
29. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
30. Jackson AP, Haak MH, Khan N, Meyer PR. Cervical spine injuries in the elderly: acute postoperative mortality. Spine. 2005;30(13):1524-1527.
31. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147-1150.
32. Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2(2):116-122.
33. Chapman J, Smith JS, Kopjar B, et al. The AOSpine North America Geriatric Odontoid Fracture Mortality Study: a retrospective review of mortality outcomes for operative versus nonoperative treatment of 322 patients with long-term follow-up. Spine. 2013;38:1098-1104.
34. Denault A, Bains I, Moghadam K, Hu RW, Swamy G. Evaluation of mortality following an odontoid fracture in the octogenarian population. J Bone Joint Surg Br. 2011;93(Supp IV):585.
35. Molinari WJ III, Molinari RW, Khera OA, Gruhn WL. Functional outcomes, morbidity, mortality, and fracture healing in 58 consecutive patients with geriatric odontoid fracture treated with cervical collar or posterior fusion. Global Spine J. 2013;3(1):21-32.
36. Hanigan WC, Powell FC, Elwood PW, Henderson JP. Odontoid fractures in elderly patients. J Neurosurg. 1993;78(1):32-35.
37. Korres DS, Boscainos PJ, Papagelopoulos PJ, Psycharis I, Goudelis G, Nikolopoulos K. Multiple level noncontiguous fractures of the spine. Clin Orthop. 2003;411:95-102.
38. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
39. Leone A, Cerase A, Colosimo C, Lauro L, Puca A, Marano P. Occipital condylar fractures: a review. Radiology. 2000;216(3):635-644.
40. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799-1809.
41. Müller EJ, Wick M, Russe O, Muhr G. Management of odontoid fractures in the elderly. Eur Spine J. 1999;8(5):360-365.
42. Pepin JW, Bourne RB, Hawkins RJ. Odontoid fractures, with special reference to the elderly patient. Clin Orthop. 1985;193:178-183.
43. Ryan MD, Henderson JJ. The epidemiology of fractures and fracture-dislocations of the cervical spine. Injury. 1992;23(1):38-40.
44. Butler JS, Dolan RT, Burbridge M, et al. The long-term functional outcome of type II odontoid fractures managed non-operatively. Eur Spine J. 2010;19(10):1635-1642.
45. Levine AM, Edwards CC. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217-226.
46. Lowery DW, Wald MM, Browne BJ, Tigges S, Hoffman JR, Mover WR; NEXUS Group. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38(1):12-16.
47. Jacobsen LA, Kent M, Lee M, Mather M. America’s aging population. Popul Bull. 2011;66(1):1-16. http://www.prb.org/pdf11/aging-in-america.pdf. Published February 2011. Accessed April 22, 2015.
48. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96(3 suppl):285-291.
49. Holmes JF, Akkinepalli R. Computed tomography versus plain radiography to screen for cervical spine injury: a meta-analysis. J Trauma. 2005;58(5):902-905.
50. Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg. 1999;33(4):423-426.
51. Lu-Yao G, Baron Ja, Barrett Ja, Fisher Es. Treatment and survival among elderly Americans with hip fractures: a population-based study. Am J Public Health. 1994;84(8):1287-1291.
52. Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15-25.
53. Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215-1220.
54. Levine AM, Edwards CC. Fractures of the atlas. J Bone Joint Surg Am. 1991;73(5):680-691.
55. Maak TG, Grauer JN. The contemporary treatment of odontoid injuries. Spine. 2006;31(11 Suppl):S53-S60; discussion S61.
56. Magaziner J, Fredman L, Hawkes W, et al. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157(11):1023-1031.
57. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55(9):M498-M507.
58. Malham GM, Ackland HM, Jones R, Williamson OD, Varma DK. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol. 2009;16(4):291-297.
59. Probst C, Zelle B, Panzica M, et al. Clinical re-examination 10 or more years after polytrauma: is there a gender related difference? J Trauma. 2010;68(3):706-711.
60. Holbrook TL, Hoyt DB, Anderson JP. The importance of gender on outcome after major trauma: functional and psychologic outcomes in women versus men. J Trauma. 2001;50(2):270-273.
AUA: Testosterone may not deserve its reputation as a cardiovascular culprit
NEW ORLEANS – Evidence seems to be mounting that the link between testosterone replacement therapy and increased hematocrit doesn’t lead to more cardiac or thrombotic events in men.
The association between testosterone and secondary erythrocytosis has been known for some time, Dr. Wayne J. G. Hellstrom said at the annual meeting of the American Urological Association. An increase in hematocrit almost invariably follows testosterone supplementation. “The question is, is there a causal relation between testosterone replacement therapy–induced erythrocytosis and venous thromboembolism or major cardiac events?” said Dr. Hellstrom of Tulane Medical Center, New Orleans. “The available evidence doesn’t support this claim.”
Erythrocytosis is defined as a packed red blood cell volume exceeding 125% of the age-predicted mass. This may be primary – an intrinsic alteration of the hematopoietic stem cells – or secondary. “And it may actually be a physiologically appropriate response to something, as in anemia,” Dr. Hellstrom said. “In fact, some anemias are primarily treated with testosterone.”
In the presence of exogenous testosterone, the condition may be due to a couple of things, he noted, such as:
• An overall increase in the erythropoietin set point.
• Increased availability of iron in the liver.
• The conversion of testosterone to estradiol, which tends to stimulate the bone marrow.
Erythrocytosis, obviously then, increases blood viscosity – and this is the primary concern for cardiovascular events.
Intramuscular testosterone is the only form that significantly increases hematocrit above normal levels. However, it does so strongly, with up to a 6% change from baseline. The runner-up is testosterone gel, with an average increase of 2.5% over baseline levels.
But despite concerns – which in March prompted the FDA to require on labeling a warning about the risk of cardiovascular events – the relationship has never been thoroughly investigated, Dr. Hellstrom said.
“We only have retrospective data, primarily extrapolating from the nephrology literature. When we look at the renal literature, we see that 10%-20% of kidney transplant patients develop polycythemia – an increase of both red and white cells, with hematocrit values of more than 51% or 52%.”
This has led to a recommendation by the American Society of Nephrology for frequent complete blood cell counts in the year after transplant and annual measurements thereafter.
The highest-quality mortality data for kidney transplant patients come from a 2013 study of 365 patients; the investigators found that those with polycythemia were 2.7 times more likely to die over 4 years. “But this is a true primary polycythemia,” which is often accompanied by procoagulative changes. It is not the secondary condition induced by testosterone, Dr. Hellstrom said.
Older studies suggested a significant link between increased hematocrit and cardiovascular or thrombotic events, especially after surgery. But prospective data from the Atherosclerosis Risk in Communities and Cardiovascular Health Studies have found no increased risk of cardiovascular death by increasing tertiles of either hematocrit or hemoglobin, with respective cut points of 43% and 14.5 g/dL.
In fact, a recent transgenic mouse model with hematopoietic overexpression, reaching an 85% hematocrit, found no evidence of either lung or cardiovascular thromboses. “This seems to be related to a reduction in clot strength and increased osmotic fragility in the presence of increasing hematocrit. It seems to mechanically deter the interaction of platelets and fibrin in the extravascular space and endothelium.”
He referred to an in-press mouse study showing that a short course of high-dose testosterone did raise whole blood viscosity and hematocrit. “But over time, this returned to normal, even with supraphysiolgic testosterone levels, so it seems likely that there is an adaptive mechanism that occurs in these animals.”
Additionally, he said, men who live at high altitudes develop naturally high hematocrits as a response to decreased oxygen in the atmosphere. “We routinely see men from these locations with hematocrits of 57% and 59% who have no problems at all.”
Extrapolating all these data to the testosterone/thrombosis link is confusing. The most recent study, however, provided some measure of reassurance. The large meta-analysis comprised 75 randomized, placebo-controlled trials involving about 5,500 men; they all examined cardiovascular risk and testosterone therapy.
“Our analyses, performed on the largest number of studies collected so far, indicate that testosterone supplementation is not related to any increase in cardiovascular risk, even when composite or single adverse events were considered,” wrote Dr. Giovanni Corona of the Maggiore-Bellaria Hospital, Bologna, Italy. “In randomized trials performed in subjects with metabolic derangements, a protective effect … was observed. … Our results are in agreement with a large body of literature from the last 20 years supporting testosterone supplementation of hypogonadal men as a valuable strategy in improving a patient’s metabolic profile, reducing body fat, and increasing lean muscle mass, which would ultimately reduce the risk of heart disease
“There is a definite need for large multicenter, randomized trials to determine the true risk,” Dr. Hellstrom said. However, in light of the current evidence, he recommends what he called a “conservative” approach to testosterone prescribing:
• Before prescribing, get a baseline complete blood count.
• If the baseline hematocrit is more than 47%, consider alternative treatments, but proceed if testosterone replacement therapy seems to be the best clinical option. Repeat testing at 3 and 12 months after therapy initiation and then annually.
• If hematocrit increases above 54%, discontinue treatment until there is a further clinical assessment, as detailed by the Endocrine Society.
• Closely monitor any new diagnoses of hypertension.
• If hematocrit does rise precipitously, phlebotomy rapidly resolved the problem.
Dr Hellstrom made the following financial disclosures: consultant, advisor, or leadership position for Abbvie, Allergan, American Medical Systems, Antares, Astellas, Auxilim, Allergan, Coloplast, Endo, Lilly, New England Research Institutes Inc. Pfizer, Promescent, Reros Therapeutics, and Theralogix.
NEW ORLEANS – Evidence seems to be mounting that the link between testosterone replacement therapy and increased hematocrit doesn’t lead to more cardiac or thrombotic events in men.
The association between testosterone and secondary erythrocytosis has been known for some time, Dr. Wayne J. G. Hellstrom said at the annual meeting of the American Urological Association. An increase in hematocrit almost invariably follows testosterone supplementation. “The question is, is there a causal relation between testosterone replacement therapy–induced erythrocytosis and venous thromboembolism or major cardiac events?” said Dr. Hellstrom of Tulane Medical Center, New Orleans. “The available evidence doesn’t support this claim.”
Erythrocytosis is defined as a packed red blood cell volume exceeding 125% of the age-predicted mass. This may be primary – an intrinsic alteration of the hematopoietic stem cells – or secondary. “And it may actually be a physiologically appropriate response to something, as in anemia,” Dr. Hellstrom said. “In fact, some anemias are primarily treated with testosterone.”
In the presence of exogenous testosterone, the condition may be due to a couple of things, he noted, such as:
• An overall increase in the erythropoietin set point.
• Increased availability of iron in the liver.
• The conversion of testosterone to estradiol, which tends to stimulate the bone marrow.
Erythrocytosis, obviously then, increases blood viscosity – and this is the primary concern for cardiovascular events.
Intramuscular testosterone is the only form that significantly increases hematocrit above normal levels. However, it does so strongly, with up to a 6% change from baseline. The runner-up is testosterone gel, with an average increase of 2.5% over baseline levels.
But despite concerns – which in March prompted the FDA to require on labeling a warning about the risk of cardiovascular events – the relationship has never been thoroughly investigated, Dr. Hellstrom said.
“We only have retrospective data, primarily extrapolating from the nephrology literature. When we look at the renal literature, we see that 10%-20% of kidney transplant patients develop polycythemia – an increase of both red and white cells, with hematocrit values of more than 51% or 52%.”
This has led to a recommendation by the American Society of Nephrology for frequent complete blood cell counts in the year after transplant and annual measurements thereafter.
The highest-quality mortality data for kidney transplant patients come from a 2013 study of 365 patients; the investigators found that those with polycythemia were 2.7 times more likely to die over 4 years. “But this is a true primary polycythemia,” which is often accompanied by procoagulative changes. It is not the secondary condition induced by testosterone, Dr. Hellstrom said.
Older studies suggested a significant link between increased hematocrit and cardiovascular or thrombotic events, especially after surgery. But prospective data from the Atherosclerosis Risk in Communities and Cardiovascular Health Studies have found no increased risk of cardiovascular death by increasing tertiles of either hematocrit or hemoglobin, with respective cut points of 43% and 14.5 g/dL.
In fact, a recent transgenic mouse model with hematopoietic overexpression, reaching an 85% hematocrit, found no evidence of either lung or cardiovascular thromboses. “This seems to be related to a reduction in clot strength and increased osmotic fragility in the presence of increasing hematocrit. It seems to mechanically deter the interaction of platelets and fibrin in the extravascular space and endothelium.”
He referred to an in-press mouse study showing that a short course of high-dose testosterone did raise whole blood viscosity and hematocrit. “But over time, this returned to normal, even with supraphysiolgic testosterone levels, so it seems likely that there is an adaptive mechanism that occurs in these animals.”
Additionally, he said, men who live at high altitudes develop naturally high hematocrits as a response to decreased oxygen in the atmosphere. “We routinely see men from these locations with hematocrits of 57% and 59% who have no problems at all.”
Extrapolating all these data to the testosterone/thrombosis link is confusing. The most recent study, however, provided some measure of reassurance. The large meta-analysis comprised 75 randomized, placebo-controlled trials involving about 5,500 men; they all examined cardiovascular risk and testosterone therapy.
“Our analyses, performed on the largest number of studies collected so far, indicate that testosterone supplementation is not related to any increase in cardiovascular risk, even when composite or single adverse events were considered,” wrote Dr. Giovanni Corona of the Maggiore-Bellaria Hospital, Bologna, Italy. “In randomized trials performed in subjects with metabolic derangements, a protective effect … was observed. … Our results are in agreement with a large body of literature from the last 20 years supporting testosterone supplementation of hypogonadal men as a valuable strategy in improving a patient’s metabolic profile, reducing body fat, and increasing lean muscle mass, which would ultimately reduce the risk of heart disease
“There is a definite need for large multicenter, randomized trials to determine the true risk,” Dr. Hellstrom said. However, in light of the current evidence, he recommends what he called a “conservative” approach to testosterone prescribing:
• Before prescribing, get a baseline complete blood count.
• If the baseline hematocrit is more than 47%, consider alternative treatments, but proceed if testosterone replacement therapy seems to be the best clinical option. Repeat testing at 3 and 12 months after therapy initiation and then annually.
• If hematocrit increases above 54%, discontinue treatment until there is a further clinical assessment, as detailed by the Endocrine Society.
• Closely monitor any new diagnoses of hypertension.
• If hematocrit does rise precipitously, phlebotomy rapidly resolved the problem.
Dr Hellstrom made the following financial disclosures: consultant, advisor, or leadership position for Abbvie, Allergan, American Medical Systems, Antares, Astellas, Auxilim, Allergan, Coloplast, Endo, Lilly, New England Research Institutes Inc. Pfizer, Promescent, Reros Therapeutics, and Theralogix.
NEW ORLEANS – Evidence seems to be mounting that the link between testosterone replacement therapy and increased hematocrit doesn’t lead to more cardiac or thrombotic events in men.
The association between testosterone and secondary erythrocytosis has been known for some time, Dr. Wayne J. G. Hellstrom said at the annual meeting of the American Urological Association. An increase in hematocrit almost invariably follows testosterone supplementation. “The question is, is there a causal relation between testosterone replacement therapy–induced erythrocytosis and venous thromboembolism or major cardiac events?” said Dr. Hellstrom of Tulane Medical Center, New Orleans. “The available evidence doesn’t support this claim.”
Erythrocytosis is defined as a packed red blood cell volume exceeding 125% of the age-predicted mass. This may be primary – an intrinsic alteration of the hematopoietic stem cells – or secondary. “And it may actually be a physiologically appropriate response to something, as in anemia,” Dr. Hellstrom said. “In fact, some anemias are primarily treated with testosterone.”
In the presence of exogenous testosterone, the condition may be due to a couple of things, he noted, such as:
• An overall increase in the erythropoietin set point.
• Increased availability of iron in the liver.
• The conversion of testosterone to estradiol, which tends to stimulate the bone marrow.
Erythrocytosis, obviously then, increases blood viscosity – and this is the primary concern for cardiovascular events.
Intramuscular testosterone is the only form that significantly increases hematocrit above normal levels. However, it does so strongly, with up to a 6% change from baseline. The runner-up is testosterone gel, with an average increase of 2.5% over baseline levels.
But despite concerns – which in March prompted the FDA to require on labeling a warning about the risk of cardiovascular events – the relationship has never been thoroughly investigated, Dr. Hellstrom said.
“We only have retrospective data, primarily extrapolating from the nephrology literature. When we look at the renal literature, we see that 10%-20% of kidney transplant patients develop polycythemia – an increase of both red and white cells, with hematocrit values of more than 51% or 52%.”
This has led to a recommendation by the American Society of Nephrology for frequent complete blood cell counts in the year after transplant and annual measurements thereafter.
The highest-quality mortality data for kidney transplant patients come from a 2013 study of 365 patients; the investigators found that those with polycythemia were 2.7 times more likely to die over 4 years. “But this is a true primary polycythemia,” which is often accompanied by procoagulative changes. It is not the secondary condition induced by testosterone, Dr. Hellstrom said.
Older studies suggested a significant link between increased hematocrit and cardiovascular or thrombotic events, especially after surgery. But prospective data from the Atherosclerosis Risk in Communities and Cardiovascular Health Studies have found no increased risk of cardiovascular death by increasing tertiles of either hematocrit or hemoglobin, with respective cut points of 43% and 14.5 g/dL.
In fact, a recent transgenic mouse model with hematopoietic overexpression, reaching an 85% hematocrit, found no evidence of either lung or cardiovascular thromboses. “This seems to be related to a reduction in clot strength and increased osmotic fragility in the presence of increasing hematocrit. It seems to mechanically deter the interaction of platelets and fibrin in the extravascular space and endothelium.”
He referred to an in-press mouse study showing that a short course of high-dose testosterone did raise whole blood viscosity and hematocrit. “But over time, this returned to normal, even with supraphysiolgic testosterone levels, so it seems likely that there is an adaptive mechanism that occurs in these animals.”
Additionally, he said, men who live at high altitudes develop naturally high hematocrits as a response to decreased oxygen in the atmosphere. “We routinely see men from these locations with hematocrits of 57% and 59% who have no problems at all.”
Extrapolating all these data to the testosterone/thrombosis link is confusing. The most recent study, however, provided some measure of reassurance. The large meta-analysis comprised 75 randomized, placebo-controlled trials involving about 5,500 men; they all examined cardiovascular risk and testosterone therapy.
“Our analyses, performed on the largest number of studies collected so far, indicate that testosterone supplementation is not related to any increase in cardiovascular risk, even when composite or single adverse events were considered,” wrote Dr. Giovanni Corona of the Maggiore-Bellaria Hospital, Bologna, Italy. “In randomized trials performed in subjects with metabolic derangements, a protective effect … was observed. … Our results are in agreement with a large body of literature from the last 20 years supporting testosterone supplementation of hypogonadal men as a valuable strategy in improving a patient’s metabolic profile, reducing body fat, and increasing lean muscle mass, which would ultimately reduce the risk of heart disease
“There is a definite need for large multicenter, randomized trials to determine the true risk,” Dr. Hellstrom said. However, in light of the current evidence, he recommends what he called a “conservative” approach to testosterone prescribing:
• Before prescribing, get a baseline complete blood count.
• If the baseline hematocrit is more than 47%, consider alternative treatments, but proceed if testosterone replacement therapy seems to be the best clinical option. Repeat testing at 3 and 12 months after therapy initiation and then annually.
• If hematocrit increases above 54%, discontinue treatment until there is a further clinical assessment, as detailed by the Endocrine Society.
• Closely monitor any new diagnoses of hypertension.
• If hematocrit does rise precipitously, phlebotomy rapidly resolved the problem.
Dr Hellstrom made the following financial disclosures: consultant, advisor, or leadership position for Abbvie, Allergan, American Medical Systems, Antares, Astellas, Auxilim, Allergan, Coloplast, Endo, Lilly, New England Research Institutes Inc. Pfizer, Promescent, Reros Therapeutics, and Theralogix.
EXPERT ANALYSIS FROM THE AUA ANNUAL MEETING
Hodgkin lymphoma incidence on the decline worldwide

Photo courtesy of NIH
In trying to estimate the global cancer burden, researchers found that cases of Hodgkin lymphoma (HL) have decreased worldwide over the last 2 decades.
The team studied 28 cancer types in 188 countries, and HL was the only malignancy whose incidence decreased from 1990 to 2013.
And the number of HL deaths in 2013 was comparatively low. When the researchers ranked cancers according to the number of global deaths, HL was 26th on the list of 28.
The researchers disclosed these results in JAMA Oncology.
The team collected data from cancer registries, vital records, verbal autopsy reports, and other sources to estimate the global cancer burden.
The data suggested that, in 2013, there were 14.9 million new cancer cases and 8.2 million cancer deaths worldwide. The proportion of cancer deaths as part of all deaths increased from 12% in 1990 to 15% in 2013.
The most common malignancy in men was prostate cancer, with 1.4 million cases in 2013. For women, it was breast cancer, with 1.8 million cases in 2013.
Tracheal, bronchus, and lung cancers were the leading cause of cancer death in men and women, with 1.6 million deaths in 2013.
Hematologic malignancies
Globally, the age-standardized incidence of HL per 100,000 people decreased by 34% during the time period studied. Cases of HL fell from about 103,000 in 1990 to 93,000 in 2013.
When the researchers ranked cancer types according to the number of global deaths in 2013, HL came in 26th. There were about 24,000 HL deaths in 2013—14,000 among men and 10,000 among women.
Non-Hodgkin lymphoma (NHL) came in 11th for global cancer deaths in 2013. There were about 226,000 NHL deaths—133,000 among men and 92,000 among women.
In addition, the incidence of NHL more than doubled from 1990 to 2013, rising from about 227,000 to 465,000. According to 2013 data, 1 in 103 men and 1 in 151 women developed NHL between birth and 79 years of age.
The researchers observed an increase in cases of multiple myeloma (MM) as well, from about 63,000 in 1990 to 117,000 in 2013.
In 2013, there were about 79,000 MM deaths—42,000 among men and 37,000 among women. MM ranked 19th on the list of global cancer deaths in 2013.
Leukemia ranked 9th on the list. There were about 265,000 leukemia deaths in 2013—149,000 among men and 116,000 among women.
Cases of leukemia increased from 297,000 in 1990 to 414,000 in 2013. According to 2013 data, 1 in 127 men and 1 in 203 women developed leukemia between birth and 79 years of age.
This research shows that cancer remains a major threat to people’s health around the world, said study author Christina Fitzmaurice, MD, of the University of Washington in Seattle.
“Cancer prevention, screening, and treatment programs are costly,” she noted, “and it is very important for countries to know which cancers cause the highest disease burden in order to allocate scarce resources appropriately.” ![]()

Photo courtesy of NIH
In trying to estimate the global cancer burden, researchers found that cases of Hodgkin lymphoma (HL) have decreased worldwide over the last 2 decades.
The team studied 28 cancer types in 188 countries, and HL was the only malignancy whose incidence decreased from 1990 to 2013.
And the number of HL deaths in 2013 was comparatively low. When the researchers ranked cancers according to the number of global deaths, HL was 26th on the list of 28.
The researchers disclosed these results in JAMA Oncology.
The team collected data from cancer registries, vital records, verbal autopsy reports, and other sources to estimate the global cancer burden.
The data suggested that, in 2013, there were 14.9 million new cancer cases and 8.2 million cancer deaths worldwide. The proportion of cancer deaths as part of all deaths increased from 12% in 1990 to 15% in 2013.
The most common malignancy in men was prostate cancer, with 1.4 million cases in 2013. For women, it was breast cancer, with 1.8 million cases in 2013.
Tracheal, bronchus, and lung cancers were the leading cause of cancer death in men and women, with 1.6 million deaths in 2013.
Hematologic malignancies
Globally, the age-standardized incidence of HL per 100,000 people decreased by 34% during the time period studied. Cases of HL fell from about 103,000 in 1990 to 93,000 in 2013.
When the researchers ranked cancer types according to the number of global deaths in 2013, HL came in 26th. There were about 24,000 HL deaths in 2013—14,000 among men and 10,000 among women.
Non-Hodgkin lymphoma (NHL) came in 11th for global cancer deaths in 2013. There were about 226,000 NHL deaths—133,000 among men and 92,000 among women.
In addition, the incidence of NHL more than doubled from 1990 to 2013, rising from about 227,000 to 465,000. According to 2013 data, 1 in 103 men and 1 in 151 women developed NHL between birth and 79 years of age.
The researchers observed an increase in cases of multiple myeloma (MM) as well, from about 63,000 in 1990 to 117,000 in 2013.
In 2013, there were about 79,000 MM deaths—42,000 among men and 37,000 among women. MM ranked 19th on the list of global cancer deaths in 2013.
Leukemia ranked 9th on the list. There were about 265,000 leukemia deaths in 2013—149,000 among men and 116,000 among women.
Cases of leukemia increased from 297,000 in 1990 to 414,000 in 2013. According to 2013 data, 1 in 127 men and 1 in 203 women developed leukemia between birth and 79 years of age.
This research shows that cancer remains a major threat to people’s health around the world, said study author Christina Fitzmaurice, MD, of the University of Washington in Seattle.
“Cancer prevention, screening, and treatment programs are costly,” she noted, “and it is very important for countries to know which cancers cause the highest disease burden in order to allocate scarce resources appropriately.” ![]()

Photo courtesy of NIH
In trying to estimate the global cancer burden, researchers found that cases of Hodgkin lymphoma (HL) have decreased worldwide over the last 2 decades.
The team studied 28 cancer types in 188 countries, and HL was the only malignancy whose incidence decreased from 1990 to 2013.
And the number of HL deaths in 2013 was comparatively low. When the researchers ranked cancers according to the number of global deaths, HL was 26th on the list of 28.
The researchers disclosed these results in JAMA Oncology.
The team collected data from cancer registries, vital records, verbal autopsy reports, and other sources to estimate the global cancer burden.
The data suggested that, in 2013, there were 14.9 million new cancer cases and 8.2 million cancer deaths worldwide. The proportion of cancer deaths as part of all deaths increased from 12% in 1990 to 15% in 2013.
The most common malignancy in men was prostate cancer, with 1.4 million cases in 2013. For women, it was breast cancer, with 1.8 million cases in 2013.
Tracheal, bronchus, and lung cancers were the leading cause of cancer death in men and women, with 1.6 million deaths in 2013.
Hematologic malignancies
Globally, the age-standardized incidence of HL per 100,000 people decreased by 34% during the time period studied. Cases of HL fell from about 103,000 in 1990 to 93,000 in 2013.
When the researchers ranked cancer types according to the number of global deaths in 2013, HL came in 26th. There were about 24,000 HL deaths in 2013—14,000 among men and 10,000 among women.
Non-Hodgkin lymphoma (NHL) came in 11th for global cancer deaths in 2013. There were about 226,000 NHL deaths—133,000 among men and 92,000 among women.
In addition, the incidence of NHL more than doubled from 1990 to 2013, rising from about 227,000 to 465,000. According to 2013 data, 1 in 103 men and 1 in 151 women developed NHL between birth and 79 years of age.
The researchers observed an increase in cases of multiple myeloma (MM) as well, from about 63,000 in 1990 to 117,000 in 2013.
In 2013, there were about 79,000 MM deaths—42,000 among men and 37,000 among women. MM ranked 19th on the list of global cancer deaths in 2013.
Leukemia ranked 9th on the list. There were about 265,000 leukemia deaths in 2013—149,000 among men and 116,000 among women.
Cases of leukemia increased from 297,000 in 1990 to 414,000 in 2013. According to 2013 data, 1 in 127 men and 1 in 203 women developed leukemia between birth and 79 years of age.
This research shows that cancer remains a major threat to people’s health around the world, said study author Christina Fitzmaurice, MD, of the University of Washington in Seattle.
“Cancer prevention, screening, and treatment programs are costly,” she noted, “and it is very important for countries to know which cancers cause the highest disease burden in order to allocate scarce resources appropriately.” ![]()
DART molecule proves active against AML

Photo by Sakurai Midori
An artificial antibody that redirects T cells to target cancer cells shows promise for treating acute myeloid leukemia (AML), according to preclinical research.
The antibody, MGD006, induced tumor regression in mouse models of AML and was largely well-tolerated in cynomolgus monkeys.
Investigators say these results support clinical testing of MGD006 in AML, which is currently underway.
MGD006 is a humanized, dual-affinity re-targeting (DART) molecule that combines a portion of an antibody recognizing CD3, an activating molecule expressed by T cells, with an arm that recognizes CD123.
MGD006 redirects T cells to kill cells expressing CD123, which is upregulated in AML and other hematologic diseases.
Gurunadh Chichili, PhD, of MacroGenics, Inc., in Rockville, Maryland, and his colleagues described their work with MGD006 in Science Translational Medicine. MacroGenics, the company developing MGD006, funded this research.
Because MGD006 is designed to be cleared rapidly, it requires continuous delivery. So in mice, the investigators administered the molecule continuously for up to a week via peritoneally implanted osmotic pumps.
The NSG/b2m−/− mice had been reconstituted with human peripheral blood mononuclear cells and grafted with KG-1a cells, an AML-M0 line. The mice received MGD006 after tumors were allowed to grow to an average size of about 100 mm3.
Treated mice experienced significant tumor regression at all doses of MGD006 (P<0.005), but there was no activity in mice treated with a control DART molecule. The investigators found that 500 ng/kg of MGD006 per day was sufficient to completely eliminate leukemic cells.
The team also tested MGD006 in macaques and found the molecule binds to human and cynomolgus monkey antigens with similar affinities and redirects T cells from either species to kill CD123-expressing target cells.
The monkeys received continuous infusions of MGD006, starting at 0.1 mg/kg per day and escalating weekly to up to 1 mg/kg per day for a 4-week period. The treatment depleted circulating CD123-positive cells beginning at 72 hours and continuing throughout the infusion period.
The monkeys experienced cytokine release, but it was transient and most significant after the first dose of MDG006. After the first dose, IL-6 concentration returned to baseline by 72 hours, and the magnitude of IL-6 response decreased with each successive MGD006 infusion, even when the dose was increased.
The animals experienced reversible reductions in hematocrit and red cell mass at the highest doses of MDG006 but no neutropenia or thrombocytopenia.
“This research paved the way for our initiation of a phase 1 clinical study of MGD006 in 2014,” said Scott Koenig, MD, PhD, President and CEO of MacroGenics.
“MGD006 has demonstrated great promise as a T-cell-redirected cancer immunotherapy in preclinical studies. We are hopeful that these studies will translate into clinical trial results indicative of clinical improvement for patients with AML, myelodysplastic syndrome, and several other forms of leukemia and lymphoma.” ![]()

Photo by Sakurai Midori
An artificial antibody that redirects T cells to target cancer cells shows promise for treating acute myeloid leukemia (AML), according to preclinical research.
The antibody, MGD006, induced tumor regression in mouse models of AML and was largely well-tolerated in cynomolgus monkeys.
Investigators say these results support clinical testing of MGD006 in AML, which is currently underway.
MGD006 is a humanized, dual-affinity re-targeting (DART) molecule that combines a portion of an antibody recognizing CD3, an activating molecule expressed by T cells, with an arm that recognizes CD123.
MGD006 redirects T cells to kill cells expressing CD123, which is upregulated in AML and other hematologic diseases.
Gurunadh Chichili, PhD, of MacroGenics, Inc., in Rockville, Maryland, and his colleagues described their work with MGD006 in Science Translational Medicine. MacroGenics, the company developing MGD006, funded this research.
Because MGD006 is designed to be cleared rapidly, it requires continuous delivery. So in mice, the investigators administered the molecule continuously for up to a week via peritoneally implanted osmotic pumps.
The NSG/b2m−/− mice had been reconstituted with human peripheral blood mononuclear cells and grafted with KG-1a cells, an AML-M0 line. The mice received MGD006 after tumors were allowed to grow to an average size of about 100 mm3.
Treated mice experienced significant tumor regression at all doses of MGD006 (P<0.005), but there was no activity in mice treated with a control DART molecule. The investigators found that 500 ng/kg of MGD006 per day was sufficient to completely eliminate leukemic cells.
The team also tested MGD006 in macaques and found the molecule binds to human and cynomolgus monkey antigens with similar affinities and redirects T cells from either species to kill CD123-expressing target cells.
The monkeys received continuous infusions of MGD006, starting at 0.1 mg/kg per day and escalating weekly to up to 1 mg/kg per day for a 4-week period. The treatment depleted circulating CD123-positive cells beginning at 72 hours and continuing throughout the infusion period.
The monkeys experienced cytokine release, but it was transient and most significant after the first dose of MDG006. After the first dose, IL-6 concentration returned to baseline by 72 hours, and the magnitude of IL-6 response decreased with each successive MGD006 infusion, even when the dose was increased.
The animals experienced reversible reductions in hematocrit and red cell mass at the highest doses of MDG006 but no neutropenia or thrombocytopenia.
“This research paved the way for our initiation of a phase 1 clinical study of MGD006 in 2014,” said Scott Koenig, MD, PhD, President and CEO of MacroGenics.
“MGD006 has demonstrated great promise as a T-cell-redirected cancer immunotherapy in preclinical studies. We are hopeful that these studies will translate into clinical trial results indicative of clinical improvement for patients with AML, myelodysplastic syndrome, and several other forms of leukemia and lymphoma.” ![]()

Photo by Sakurai Midori
An artificial antibody that redirects T cells to target cancer cells shows promise for treating acute myeloid leukemia (AML), according to preclinical research.
The antibody, MGD006, induced tumor regression in mouse models of AML and was largely well-tolerated in cynomolgus monkeys.
Investigators say these results support clinical testing of MGD006 in AML, which is currently underway.
MGD006 is a humanized, dual-affinity re-targeting (DART) molecule that combines a portion of an antibody recognizing CD3, an activating molecule expressed by T cells, with an arm that recognizes CD123.
MGD006 redirects T cells to kill cells expressing CD123, which is upregulated in AML and other hematologic diseases.
Gurunadh Chichili, PhD, of MacroGenics, Inc., in Rockville, Maryland, and his colleagues described their work with MGD006 in Science Translational Medicine. MacroGenics, the company developing MGD006, funded this research.
Because MGD006 is designed to be cleared rapidly, it requires continuous delivery. So in mice, the investigators administered the molecule continuously for up to a week via peritoneally implanted osmotic pumps.
The NSG/b2m−/− mice had been reconstituted with human peripheral blood mononuclear cells and grafted with KG-1a cells, an AML-M0 line. The mice received MGD006 after tumors were allowed to grow to an average size of about 100 mm3.
Treated mice experienced significant tumor regression at all doses of MGD006 (P<0.005), but there was no activity in mice treated with a control DART molecule. The investigators found that 500 ng/kg of MGD006 per day was sufficient to completely eliminate leukemic cells.
The team also tested MGD006 in macaques and found the molecule binds to human and cynomolgus monkey antigens with similar affinities and redirects T cells from either species to kill CD123-expressing target cells.
The monkeys received continuous infusions of MGD006, starting at 0.1 mg/kg per day and escalating weekly to up to 1 mg/kg per day for a 4-week period. The treatment depleted circulating CD123-positive cells beginning at 72 hours and continuing throughout the infusion period.
The monkeys experienced cytokine release, but it was transient and most significant after the first dose of MDG006. After the first dose, IL-6 concentration returned to baseline by 72 hours, and the magnitude of IL-6 response decreased with each successive MGD006 infusion, even when the dose was increased.
The animals experienced reversible reductions in hematocrit and red cell mass at the highest doses of MDG006 but no neutropenia or thrombocytopenia.
“This research paved the way for our initiation of a phase 1 clinical study of MGD006 in 2014,” said Scott Koenig, MD, PhD, President and CEO of MacroGenics.
“MGD006 has demonstrated great promise as a T-cell-redirected cancer immunotherapy in preclinical studies. We are hopeful that these studies will translate into clinical trial results indicative of clinical improvement for patients with AML, myelodysplastic syndrome, and several other forms of leukemia and lymphoma.” ![]()
Urine test could reduce need for blood samples

Photo by Juan D. Alfonso
A new approach to urine testing could make the tests more versatile and therefore decrease the need for blood tests, according to researchers.
They believe the method could also reduce costs, produce results faster than current tests, and lower the volume of urine needed for a sample.
R. Kenneth Marcus, PhD, of Clemson University in South Carolina, and his colleagues described this method in Proteomics-Clinical Applications.
Dr Marcus noted that the trouble with testing urine is that it’s awash in salt, so it can be tricky to isolate the proteins that act as biomarkers.
To overcome this problem, he and his colleagues used a string made of capillary-channeled polymer fibers. The team packed the fibers into plastic tubes and then passed urine samples through the tubes by spinning them in a centrifuge for 30 seconds.
Then, the researchers ran de-ionized water through the tubes for a minute to wash off salt and other contaminants.
As proteins are hydrophobic, they remained stuck to the fibers. The team extracted the proteins by running a solvent through the tubes in the centrifuge for 30 seconds.
When this process was complete, the researchers were left with purified proteins that could be stored in a plastic vial and refrigerated until testing time.
The team was able to extract 12 samples in about 5 minutes, limited only by centrifuge capacity.
In addition to being faster and cheaper than current urine tests, the new testing method should also make it easier to test urine samples from infants, Dr Marcus said.
One of the challenges now is getting a large enough sample, but the new method requires only a few microliters of urine. ![]()

Photo by Juan D. Alfonso
A new approach to urine testing could make the tests more versatile and therefore decrease the need for blood tests, according to researchers.
They believe the method could also reduce costs, produce results faster than current tests, and lower the volume of urine needed for a sample.
R. Kenneth Marcus, PhD, of Clemson University in South Carolina, and his colleagues described this method in Proteomics-Clinical Applications.
Dr Marcus noted that the trouble with testing urine is that it’s awash in salt, so it can be tricky to isolate the proteins that act as biomarkers.
To overcome this problem, he and his colleagues used a string made of capillary-channeled polymer fibers. The team packed the fibers into plastic tubes and then passed urine samples through the tubes by spinning them in a centrifuge for 30 seconds.
Then, the researchers ran de-ionized water through the tubes for a minute to wash off salt and other contaminants.
As proteins are hydrophobic, they remained stuck to the fibers. The team extracted the proteins by running a solvent through the tubes in the centrifuge for 30 seconds.
When this process was complete, the researchers were left with purified proteins that could be stored in a plastic vial and refrigerated until testing time.
The team was able to extract 12 samples in about 5 minutes, limited only by centrifuge capacity.
In addition to being faster and cheaper than current urine tests, the new testing method should also make it easier to test urine samples from infants, Dr Marcus said.
One of the challenges now is getting a large enough sample, but the new method requires only a few microliters of urine. ![]()

Photo by Juan D. Alfonso
A new approach to urine testing could make the tests more versatile and therefore decrease the need for blood tests, according to researchers.
They believe the method could also reduce costs, produce results faster than current tests, and lower the volume of urine needed for a sample.
R. Kenneth Marcus, PhD, of Clemson University in South Carolina, and his colleagues described this method in Proteomics-Clinical Applications.
Dr Marcus noted that the trouble with testing urine is that it’s awash in salt, so it can be tricky to isolate the proteins that act as biomarkers.
To overcome this problem, he and his colleagues used a string made of capillary-channeled polymer fibers. The team packed the fibers into plastic tubes and then passed urine samples through the tubes by spinning them in a centrifuge for 30 seconds.
Then, the researchers ran de-ionized water through the tubes for a minute to wash off salt and other contaminants.
As proteins are hydrophobic, they remained stuck to the fibers. The team extracted the proteins by running a solvent through the tubes in the centrifuge for 30 seconds.
When this process was complete, the researchers were left with purified proteins that could be stored in a plastic vial and refrigerated until testing time.
The team was able to extract 12 samples in about 5 minutes, limited only by centrifuge capacity.
In addition to being faster and cheaper than current urine tests, the new testing method should also make it easier to test urine samples from infants, Dr Marcus said.
One of the challenges now is getting a large enough sample, but the new method requires only a few microliters of urine. ![]()
Understanding taste dysfunction in cancer
Image by Jonas Töle
Investigators have identified a molecular pathway that aids the renewal of taste buds, and they believe this discovery may have implications for cancer patients who suffer from an altered sense of taste during treatment.
“Taste dysfunction can . . . result from an alteration of the renewal capacities of taste buds and is often associated with psychological distress and malnutrition,” said Dany Gaillard, PhD, of the University of Colorado Anschutz Medical Campus in Aurora.
He and his colleagues decided to investigate this dysfunction using mouse models, and the group reported their findings in PLOS Genetics.
The investigators discovered that a protein in the Wnt pathway, ß-catenin, controls the renewal of taste cells by regulating separate stages of taste-cell turnover.
Previous research showed that Wnt/β-catenin signaling is crucial in developing taste buds in embryos and regulating the renewal of epithelial tissue in adults, including skin, hair follicles, the intestine, and the mouth.
“We show that activating this pathway directs the newly born cells to become, primarily, a specific taste- cell type whose role is to support the other taste cells and help them work efficiently,” said Linda Barlow, PhD, also of the University of Colorado Anschutz Medical Campus.
As chemotherapy destroys dividing precursor cells, including those that produce taste cells, the investigators believe that activating Wnt signaling may be a way to renew taste buds after chemotherapy.
New small-molecule drugs that specifically block the Wnt pathway are under development, and Drs Gaillard and Barlow predict these drugs could also cause taste dysfunction.
Dr Barlow said more research is needed to understand how taste is altered at the cellular level, but this research holds promise for developing new ways to improve cancer patients’ quality of life. ![]()

Image by Jonas Töle
Investigators have identified a molecular pathway that aids the renewal of taste buds, and they believe this discovery may have implications for cancer patients who suffer from an altered sense of taste during treatment.
“Taste dysfunction can . . . result from an alteration of the renewal capacities of taste buds and is often associated with psychological distress and malnutrition,” said Dany Gaillard, PhD, of the University of Colorado Anschutz Medical Campus in Aurora.
He and his colleagues decided to investigate this dysfunction using mouse models, and the group reported their findings in PLOS Genetics.
The investigators discovered that a protein in the Wnt pathway, ß-catenin, controls the renewal of taste cells by regulating separate stages of taste-cell turnover.
Previous research showed that Wnt/β-catenin signaling is crucial in developing taste buds in embryos and regulating the renewal of epithelial tissue in adults, including skin, hair follicles, the intestine, and the mouth.
“We show that activating this pathway directs the newly born cells to become, primarily, a specific taste- cell type whose role is to support the other taste cells and help them work efficiently,” said Linda Barlow, PhD, also of the University of Colorado Anschutz Medical Campus.
As chemotherapy destroys dividing precursor cells, including those that produce taste cells, the investigators believe that activating Wnt signaling may be a way to renew taste buds after chemotherapy.
New small-molecule drugs that specifically block the Wnt pathway are under development, and Drs Gaillard and Barlow predict these drugs could also cause taste dysfunction.
Dr Barlow said more research is needed to understand how taste is altered at the cellular level, but this research holds promise for developing new ways to improve cancer patients’ quality of life. ![]()

Image by Jonas Töle
Investigators have identified a molecular pathway that aids the renewal of taste buds, and they believe this discovery may have implications for cancer patients who suffer from an altered sense of taste during treatment.
“Taste dysfunction can . . . result from an alteration of the renewal capacities of taste buds and is often associated with psychological distress and malnutrition,” said Dany Gaillard, PhD, of the University of Colorado Anschutz Medical Campus in Aurora.
He and his colleagues decided to investigate this dysfunction using mouse models, and the group reported their findings in PLOS Genetics.
The investigators discovered that a protein in the Wnt pathway, ß-catenin, controls the renewal of taste cells by regulating separate stages of taste-cell turnover.
Previous research showed that Wnt/β-catenin signaling is crucial in developing taste buds in embryos and regulating the renewal of epithelial tissue in adults, including skin, hair follicles, the intestine, and the mouth.
“We show that activating this pathway directs the newly born cells to become, primarily, a specific taste- cell type whose role is to support the other taste cells and help them work efficiently,” said Linda Barlow, PhD, also of the University of Colorado Anschutz Medical Campus.
As chemotherapy destroys dividing precursor cells, including those that produce taste cells, the investigators believe that activating Wnt signaling may be a way to renew taste buds after chemotherapy.
New small-molecule drugs that specifically block the Wnt pathway are under development, and Drs Gaillard and Barlow predict these drugs could also cause taste dysfunction.
Dr Barlow said more research is needed to understand how taste is altered at the cellular level, but this research holds promise for developing new ways to improve cancer patients’ quality of life. ![]()
Verbal Communication at Discharge
Timely and reliable communication of important data between hospital‐based physicians and primary care physicians is critical for prevention of medical adverse events.[1, 2] Extrapolation from high‐performance organizations outside of medicine suggests that verbal communication is an important component of patient handoffs.[3, 4] Though the Joint Commission does not mandate verbal communication during handoffs per se, stipulating instead that handoff participants have an opportunity to ask and respond to questions,[5] there is some evidence that primary care providers prefer verbal handoffs at least for certain patients such as those with medical complexity.[6] Verbal communication offers the receiver the opportunity to ask questions, but in practice, 2‐way verbal communication is often difficult to achieve at hospital discharge.
At our institution, hospital medicine (HM) physicians serve as the primary inpatient providers for nearly 90% of all general pediatric admissions. When the HM service was established, primary care physicians (PCPs) and HM physicians together agreed upon an expectation for verbal, physician‐to‐physician communication at the time of discharge. Discharge communication is provided by either residents or attendings depending on the facility. A telephone operator service called Physician Priority Link (PPL) was made available to facilitate this communication. The PPL service is staffed 24/7 by operators whose only responsibilities are to connect providers inside and outside the institution. By utilizing this service, PCPs could respond in a nonemergent fashion to discharge phone calls.
Over the last several years, PCPs have observed high variation in the reliability of discharge communication phone calls. A review of PPL phone records in 2009 showed that only 52% of HM discharges had a record of a call initiated to the PCP on the day of discharge. The overall goal of this improvement project was to improve the completion of verbal handoffs from HM physicians (residents or attendings) to PCPs. The specific aim of the project was to increase the proportion of completed verbal handoffs from on‐call residents or attendings to PCPs within 24 hours of discharge to more than 90% within 18 months.
METHODS
Human Subjects Protection
Our project was undertaken in accordance with institutional review board (IRB) policy on systems improvement work and did not require formal IRB review.
Setting
This study included all patients admitted to the HM service at an academic children's hospital and its satellite campus.
Planning the Intervention
The project was championed by physicians on the HM service and supported by a chief resident, PPL administrators, and 2 information technology analysts.
At the onset of the project, the team mapped the process for completing a discharge call to the PCPs, conducted a modified failure mode and effects analysis,[7, 8] and examined the key drivers used to prioritize interventions (Figure 1). Through the modified failure modes effect analysis, the team was able to identify system issues that led to unsuccessful communication: failure of call initiation, absence of an identified PCP, long wait times on hold, failure of PCP to call back, and failure of the call to be documented. These failure modes informed the key drivers to achieving the study aim. Figure 2 depicts the final key drivers, which were revised through testing and learning.
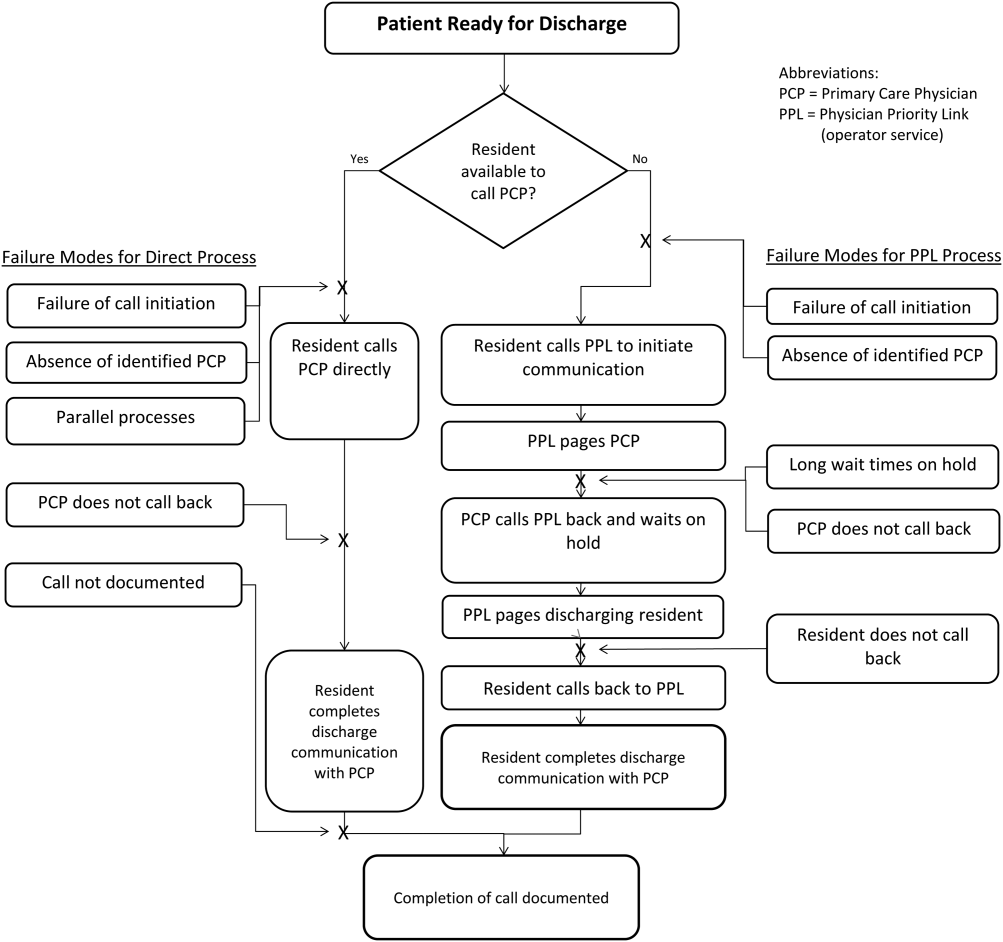

Interventions Targeting Key Stakeholder Buy‐in
To improve resident buy‐in and participation, the purpose and goals of the projects were discussed at resident morning report and during monthly team meetings by the pediatric chief resident on our improvement team. Resident physicians were interested in participating to reduce interruptions during daily rounds and to improve interactions with PCPs. The PPL staff was interested in standardizing the discharge call process to reduce confusion in identifying the appropriate contact when PCPs called residents back to discuss discharges. PCPs were interested in ensuring good communication at discharge, and individual PCPs were engaged through person‐to‐person contact by 1 of the HM physician champions.
Interventions to Standardization the Communication Process
To facilitate initiation of calls to PCPs at hospital discharge, the improvement team created a standard process using the PPL service (Figure 3). All patients discharged from the HM service were included in the process. Discharging physicians (who were usually but not always residents, depending on the facility), were instructed to call the PPL operator at the time of discharge. The PPL operator would then page the patient's PCP. It was the responsibility of the discharging physician to identify a PCP prior to discharge. Instances where no PCP was identified were counted as process failures because no phone call could be made. The expectation for the PCPs was that they would return the page within 20 minutes. PPL operators would then page back to the discharging physician to connect the 2 parties with the expectation that the discharging physician respond within 2 to 4 minutes to the PPL operator's page. Standardization of all calls through PPL allowed efficient tracking of incomplete calls and operators to reattempt calls that were not completed. This process also shifted the burden of following up on incomplete calls to PPL. The use of PPL to make the connection also allowed the physician to complete other work while awaiting a call back from the PCP.

Leveraging the Electronic Health Record for Process Initiation
To ensure reliable initiation of the discharge communication pathway, the improvement team introduced changes to the electronic health record (HER) (EpicCare Inpatient; Epic Systems Corp., Verona, WI), which generated a message to PPL operators whenever a discharge order was entered for an HM patient. The message contained the patient's name, medical record number, discharge date, discharging physician, and PCP name and phone number. A checklist was implemented by PPL to ensure that duplicate phone calls were not made. To initiate communication, the operator contacted the resident via text page to ensure they were ready to initiate the call. If the resident was ready to place a call, the operator then generated a phone call to the PCP. When the PCP returned the call, the operator connected the HM resident with the PCP for the handoff.
As the project progressed, several adaptations were made to address newly identified failure modes. To address confusion among PPL operators about which resident physicians should take discharge phone calls after the discharging resident was no longer available (for example, after a shift change), primary responsibility for discharge phone calls was reassigned to the daily on‐call resident rather than the resident who wrote the discharge order. Because the on‐call residents carry a single pager, the pager number listed on the automated discharge notification to PPL would never change and would always reach the appropriate team member. Second, to address the anticipated increase in interruption of resident workflow by calls back from PCPs, particularly during rounds, operators accessed information on pending discharge phone calls in batches at times of increased resident availability to minimize hold times for PCPs and work interruptions for the discharging physicians. Batch times were 1 pm and 4 pm to allow for completion of morning rounds, resident conference at noon, and patient‐care activities during the afternoon. Calls initiated after 4 pm were dispatched at the time of the discharge, and calls initiated after 10 pm were deferred to the following day.
Transparency of Data
Throughout the study, weekly failure data were generated from the EHR and emailed to improvement team members, enabling them to focus on near real‐time feedback of data to create a visible and more reliable system. With the standardization of all discharge calls directed to the PPL operators, the team was able to create a call record linked to the patient's medical record number. Team‐specific and overall results for the 5 HM resident teams were displayed weekly on a run chart in the resident conference room. As improvements in call initiation were demonstrated, completion rate data were also shared every several months with the attending hospitalists during a regularly scheduled divisional conference. This transparency of data gave the improvement team the opportunity to provide individual feedback to residents and attendings about failures. The weekly review of failure data allowed team leaders to learn from failures, identify knowledge gaps, and ensure accountability with the HM physicians.
Planning the Study of the Intervention
Data were collected prospectively from July 2011 to March 2014. A weekly list of patients discharged from the HM service was extracted from the EHR and compared to electronic call logs collected by PPL on the day of discharge. A standard sample size of 30 calls was audited separately by PPL and 1 of the physician leads to verify that the patients were discharged from the HM service and validate the percentage of completed and initiated calls.
The percentage of calls initiated within 24 hours of discharge was tracked as a process measure and served as the initial focus of improvement efforts. Our primary outcome measure was the percentage of calls completed to the PCP by the HM physician within 24 hours of discharge.
Methods of Evaluation and Analysis
We used improvement science methods and run charts to determine the percentage of patients discharged from the HM service with a call initiated to the PCP and completed within 24 hours of discharge. Data on calls initiated within 24 hours of discharge were plotted on a run chart to examine the impact of interventions over time. Once interventions targeted at call initiation had been implemented, we began tracking our primary outcome measure. A new run chart was created documenting the percentage of calls completed. For both metrics, the centerline was adjusted using established rules for special cause variation in run charts.[9, 10, 11, 12, 13]
RESULTS
From July 2011 to March 2014, there were 6313 discharges from the HM service. The process measure (percentage of calls initiated) improved from 50% to 97% after 4 interventions (Figure 4). Data for the outcome measure (percentage of calls completed) were collected starting in August 2012, shortly after linking the EHR discharge order to the discharge call. Over the first 8 weeks, our median was 80%, which increased to a median of 93% (Figure 5). These results were sustained for 18 months.
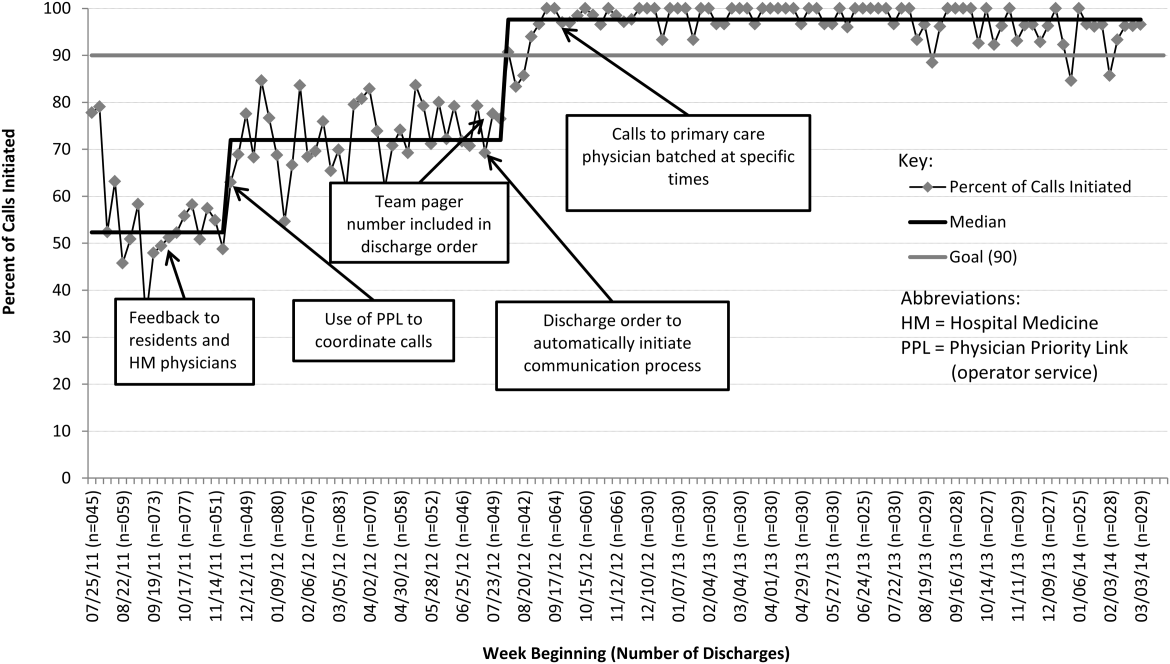

Several key interventions were identified that were critical to achievement of our goal. Standardization of the communication process through PPL was temporally associated with a shift in the median rate of call initiation from 52% to 72%. Use of the discharge order to initiate discharge communication was associated with an increase from 72% to 97%. Finally, the percentage of completed verbal handoffs increased to more than 93% following batching of phone calls to PCPs at specific times during the day.
DISCUSSION
We used improvement and reliability science methods to implement a successful process for improving verbal handoffs from HM physicians to PCPs within 24 hours of discharge to 93%. This result has been sustained for 18 months.
Utilization of the PPL call center for flexible call facilitation along with support for data analysis and leveraging the EHR to automate the process increased reliability, leading to rapid improvement. Prior to mandating the use of PPL to connect discharging physicians with PCPs, the exact rate of successful handoffs in our institution was not known. We do know, however, that only 52% of calls were initiated, so clearly a large gap was present prior to our improvement work. Data collection from the PPL system was automated so that accurate, timely, and sustainable data could be provided, greatly aiding improvement efforts. Flexibility in call‐back timing was also crucial, because coordinating the availability of PCPs and discharging physicians is often challenging. The EHR‐initiated process for discharge communication was a key intervention, and improvement of our process measure to 97% performance was associated with this implementation. Two final interventions: (1) assignment of responsibility for communication to a team pager held by a designated resident and (2) batching of calls to specific times streamlined the EHR‐initiated process and were associated with achievement of our main outcome goal of >90% completed verbal communication.
There are several reports of successful interventions to improve receipt or content of discharge summaries by PCPs following hospital discharge available in the literature.[14, 15, 16, 17, 18, 19, 20] Recently, Shen et al. reported on the success of a multisite improvement collaborative involving pediatric hospitalist programs at community hospitals whose aim was to improve the timely documentation of communication directed at PCPs.[21] In their report, all 7 hospital sites that participated in the collaborative for more than 4 months were able to demonstrate substantial improvement in documentation of some form of communication directed at PCPs (whether by e‐mail, fax, or telephone call), from a baseline of approximately 50% to more than 90%. A limitation of their study was that they were unable to document whether PCPs had received any information or by what method. A recent survey of PCPs by Sheu et al. indicated that for many discharges, information in addition to that present in the EHR was desirable to ensure a safe transition of care.[6] Two‐way communication, such as with a phone call, allows for senders to verify information receipt and for receivers to ask questions to ensure complete information. To our knowledge, there have been no previous reports describing processes for improving verbal communication between hospitalist services and PCPs at discharge.
It may be that use of the call system allowed PCPs to return phone calls regarding discharges at convenient stopping points in their day while allowing discharging physicians to initiate a call without having to wait on hold. Interestingly, though we anticipated the need for additional PPL resources during the course of this improvement, the final process was efficient enough that PPL did not require additional staffing to accommodate the higher call volume.
A key insight during our implementation was that relying on the EHR to initiate every discharge communication created disruption of resident workflow due to disregard of patient, resident, and PCP factors. This was reflected by the improvement in call initiation (our process measure) following this intervention, whereas at the same time call completion (our outcome measure) remained below goal. To achieve our goal of completing verbal communication required a process that was highly reliable yet flexible enough to allow discharging physicians to complete the call in the unpredictable environment of inpatient care. Ultimately, this was achieved by allowing discharging physicians to initiate the process when convenient, and allowing for the EHR‐initiated process to function as a backup strategy to identify and mitigate failures of initiation.
An important limitation of our study was the lack of PCPs on the improvement team, likely making the success of the project more difficult than it might have been. For example, during the study we did not measure the time PCPs spent on hold or how many reattempts were needed to complete the communication loop. Immediately following the completion of our study, it became apparent that physicians returning calls for our own institution's primary care clinic were experiencing regular workflow interruptions and occasional hold times more than 20 minutes, necessitating ongoing further work to determine the root causes and solutions to these problems. Though this work is ongoing, average PCP hold times measured from a sample of call reviews in 2013 to 2014 was 3 minutes and 15 seconds.
This study has several other limitations. We were unable to account for phone calls to PCPs initiated outside of the new process. It may be that PCPs were called more than 52% of the time at baseline due to noncompliance with the new protocol. Also, we only have data for call completion starting after implementation of the link between the discharge order and the discharge phone call, making the baseline appear artificially high and precluding any analysis of how earlier interventions affected our outcome metric. Communication with PCPs should ideally occur prior to discharge. An important limitation of our process is that calls could occur several hours after discharge between an on‐call resident and an on‐call outpatient physician rather than between the PCP and the discharging resident, limiting appropriate information exchange. Though verbal discharge communication is a desirable goal for many reasons, the current project did not focus on the quality of the call or the information that was transmitted to the PCP. Additionally, direct attending‐to‐attending communication may be valuable with medically or socially complex discharges, but we did not have a process to facilitate this. We also did not measure what effect our new process had on outcomes such as quality of patient and family transition from hospital or physician satisfaction. The existence of programs similar to our PPL subspecialty referral line may be limited to large institutions. However, it should be noted that although some internal resource reallocation was necessary within PPL, no actual staffing increases were required despite a large increase in call volume. It may be that any hospital operator system could be adapted for this purpose with modest additional resources. Finally, although our EHR system is widely utilized, there are many competing systems in the market, and our intervention required utilization of EHR capabilities that may not be present in all systems. However, our EHR intervention utilized existing functionality and did not require modification of the system.
This project focused on discharge phone calls to primary care physicians for patients hospitalized on the hospital medicine service. Because communication with the PCP should ideally occur prior to discharge, future work will include identifying a more proximal trigger than the discharge order to which to link the EHR trigger for discharge communication. Other next steps to improve handoff effectiveness and optimize the efficiency of our process include identifying essential information that should be transmitted to the primary care physician at the time of the phone call, developing processes to ensure communication of this information, measuring PCP satisfaction with this communication, and measuring the impact on patient outcomes. Finally, though expert opinion indicates that verbal handoffs may have safety advantages over nonverbal handoffs, studies comparing the safety and efficacy of verbal versus nonverbal handoffs at patient discharge are lacking. Studies establishing the relative efficacy and safety of verbal versus nonverbal handoffs at hospital discharge are needed. Knowledge gained from these activities could inform future projects centered on the spread of the process to other hospital services and/or other hospitals.
CONCLUSION
We increased the percentage of calls initiated to PCPs at patient discharge from 52% to 97% and the percentage of calls completed between HM physicians and PCPs to 93% through the use of a standardized discharge communication process coupled with a basic EHR messaging functionality. The results of this study may be of interest for further testing and adaptation for any institution with an electronic healthcare system.
Disclosure: Nothing to report.
- , , . Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S–39S.
- , , , , . Evaluating communication between pediatric primary care physicians and hospitalists. Clin Pediatr. 2011;50(10):923–928.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- Agency for Healthcare Research and Quality. Patient safety primers: handoffs and signouts. Available at: http://www.psnet.ahrq.gov/primer.aspx?primerID=9. Accessed March 19, 2014.
- , , , , . We need to talk: primary care provider communication at discharge in the era of a shared electronic medical record. J Hosp Med. 2015;10(5):307–310.
- , , . Failure mode and effects analysis: a novel approach to avoiding dangerous medication errors and accidents. Hosp Pharm. 1994;29:319–330.
- , , , . Using health care Failure Mode and Effect Analysis: the VA National Center for Patient Safety's prospective risk analysis system. Jt Comm J Qual Improv. 2002;28:248–267, 209.
- . Statistical quality control methods in infection control and hospital epidemiology, Part II: Chart use, statistical properties, and research issues. Infect Control Hosp Epidemiol. 1998;19(4):265–283.
- . Statistical quality control methods in infection control and hospital epidemiology, part I: Introduction and basic theory. Infect Control Hosp Epidemiol. 1998;19(3):194–214.
- , , . Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458–464.
- . The Improvement Guide: A Practical Approach to Enhancing Organizational +Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- , . The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey‐Bass; 2011.
- , . The initial hospital discharge note: send out with the patient or post? Health Trends. 1984;16(2):48.
- , . Which type of hospital discharge report reaches general practitioners most quickly? BMJ. 1989;298(6670):362–363.
- , . The application of a computer data base system to the generation of hospital discharge summaries. Obstet Gynecol. 1989;73(5 pt 1):803–807.
- . Hospital discharge medication: is seven days supply sufficient? Public Health. 1991;105(3):243–247.
- , , , et al. Electronic communication between providers of primary and secondary care. BMJ. 1992;305(6861):1068–1070.
- , , . Communication of discharge information for elderly patients in hospital. Ulster Med J. 1992;61(1):56–58.
- , , , , . A quality use of medicines program for continuity of care in therapeutics from hospital to community. Med J Aust. 2002;177(1):32–34.
- , , , , , . Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258–265.
Timely and reliable communication of important data between hospital‐based physicians and primary care physicians is critical for prevention of medical adverse events.[1, 2] Extrapolation from high‐performance organizations outside of medicine suggests that verbal communication is an important component of patient handoffs.[3, 4] Though the Joint Commission does not mandate verbal communication during handoffs per se, stipulating instead that handoff participants have an opportunity to ask and respond to questions,[5] there is some evidence that primary care providers prefer verbal handoffs at least for certain patients such as those with medical complexity.[6] Verbal communication offers the receiver the opportunity to ask questions, but in practice, 2‐way verbal communication is often difficult to achieve at hospital discharge.
At our institution, hospital medicine (HM) physicians serve as the primary inpatient providers for nearly 90% of all general pediatric admissions. When the HM service was established, primary care physicians (PCPs) and HM physicians together agreed upon an expectation for verbal, physician‐to‐physician communication at the time of discharge. Discharge communication is provided by either residents or attendings depending on the facility. A telephone operator service called Physician Priority Link (PPL) was made available to facilitate this communication. The PPL service is staffed 24/7 by operators whose only responsibilities are to connect providers inside and outside the institution. By utilizing this service, PCPs could respond in a nonemergent fashion to discharge phone calls.
Over the last several years, PCPs have observed high variation in the reliability of discharge communication phone calls. A review of PPL phone records in 2009 showed that only 52% of HM discharges had a record of a call initiated to the PCP on the day of discharge. The overall goal of this improvement project was to improve the completion of verbal handoffs from HM physicians (residents or attendings) to PCPs. The specific aim of the project was to increase the proportion of completed verbal handoffs from on‐call residents or attendings to PCPs within 24 hours of discharge to more than 90% within 18 months.
METHODS
Human Subjects Protection
Our project was undertaken in accordance with institutional review board (IRB) policy on systems improvement work and did not require formal IRB review.
Setting
This study included all patients admitted to the HM service at an academic children's hospital and its satellite campus.
Planning the Intervention
The project was championed by physicians on the HM service and supported by a chief resident, PPL administrators, and 2 information technology analysts.
At the onset of the project, the team mapped the process for completing a discharge call to the PCPs, conducted a modified failure mode and effects analysis,[7, 8] and examined the key drivers used to prioritize interventions (Figure 1). Through the modified failure modes effect analysis, the team was able to identify system issues that led to unsuccessful communication: failure of call initiation, absence of an identified PCP, long wait times on hold, failure of PCP to call back, and failure of the call to be documented. These failure modes informed the key drivers to achieving the study aim. Figure 2 depicts the final key drivers, which were revised through testing and learning.


Interventions Targeting Key Stakeholder Buy‐in
To improve resident buy‐in and participation, the purpose and goals of the projects were discussed at resident morning report and during monthly team meetings by the pediatric chief resident on our improvement team. Resident physicians were interested in participating to reduce interruptions during daily rounds and to improve interactions with PCPs. The PPL staff was interested in standardizing the discharge call process to reduce confusion in identifying the appropriate contact when PCPs called residents back to discuss discharges. PCPs were interested in ensuring good communication at discharge, and individual PCPs were engaged through person‐to‐person contact by 1 of the HM physician champions.
Interventions to Standardization the Communication Process
To facilitate initiation of calls to PCPs at hospital discharge, the improvement team created a standard process using the PPL service (Figure 3). All patients discharged from the HM service were included in the process. Discharging physicians (who were usually but not always residents, depending on the facility), were instructed to call the PPL operator at the time of discharge. The PPL operator would then page the patient's PCP. It was the responsibility of the discharging physician to identify a PCP prior to discharge. Instances where no PCP was identified were counted as process failures because no phone call could be made. The expectation for the PCPs was that they would return the page within 20 minutes. PPL operators would then page back to the discharging physician to connect the 2 parties with the expectation that the discharging physician respond within 2 to 4 minutes to the PPL operator's page. Standardization of all calls through PPL allowed efficient tracking of incomplete calls and operators to reattempt calls that were not completed. This process also shifted the burden of following up on incomplete calls to PPL. The use of PPL to make the connection also allowed the physician to complete other work while awaiting a call back from the PCP.

Leveraging the Electronic Health Record for Process Initiation
To ensure reliable initiation of the discharge communication pathway, the improvement team introduced changes to the electronic health record (HER) (EpicCare Inpatient; Epic Systems Corp., Verona, WI), which generated a message to PPL operators whenever a discharge order was entered for an HM patient. The message contained the patient's name, medical record number, discharge date, discharging physician, and PCP name and phone number. A checklist was implemented by PPL to ensure that duplicate phone calls were not made. To initiate communication, the operator contacted the resident via text page to ensure they were ready to initiate the call. If the resident was ready to place a call, the operator then generated a phone call to the PCP. When the PCP returned the call, the operator connected the HM resident with the PCP for the handoff.
As the project progressed, several adaptations were made to address newly identified failure modes. To address confusion among PPL operators about which resident physicians should take discharge phone calls after the discharging resident was no longer available (for example, after a shift change), primary responsibility for discharge phone calls was reassigned to the daily on‐call resident rather than the resident who wrote the discharge order. Because the on‐call residents carry a single pager, the pager number listed on the automated discharge notification to PPL would never change and would always reach the appropriate team member. Second, to address the anticipated increase in interruption of resident workflow by calls back from PCPs, particularly during rounds, operators accessed information on pending discharge phone calls in batches at times of increased resident availability to minimize hold times for PCPs and work interruptions for the discharging physicians. Batch times were 1 pm and 4 pm to allow for completion of morning rounds, resident conference at noon, and patient‐care activities during the afternoon. Calls initiated after 4 pm were dispatched at the time of the discharge, and calls initiated after 10 pm were deferred to the following day.
Transparency of Data
Throughout the study, weekly failure data were generated from the EHR and emailed to improvement team members, enabling them to focus on near real‐time feedback of data to create a visible and more reliable system. With the standardization of all discharge calls directed to the PPL operators, the team was able to create a call record linked to the patient's medical record number. Team‐specific and overall results for the 5 HM resident teams were displayed weekly on a run chart in the resident conference room. As improvements in call initiation were demonstrated, completion rate data were also shared every several months with the attending hospitalists during a regularly scheduled divisional conference. This transparency of data gave the improvement team the opportunity to provide individual feedback to residents and attendings about failures. The weekly review of failure data allowed team leaders to learn from failures, identify knowledge gaps, and ensure accountability with the HM physicians.
Planning the Study of the Intervention
Data were collected prospectively from July 2011 to March 2014. A weekly list of patients discharged from the HM service was extracted from the EHR and compared to electronic call logs collected by PPL on the day of discharge. A standard sample size of 30 calls was audited separately by PPL and 1 of the physician leads to verify that the patients were discharged from the HM service and validate the percentage of completed and initiated calls.
The percentage of calls initiated within 24 hours of discharge was tracked as a process measure and served as the initial focus of improvement efforts. Our primary outcome measure was the percentage of calls completed to the PCP by the HM physician within 24 hours of discharge.
Methods of Evaluation and Analysis
We used improvement science methods and run charts to determine the percentage of patients discharged from the HM service with a call initiated to the PCP and completed within 24 hours of discharge. Data on calls initiated within 24 hours of discharge were plotted on a run chart to examine the impact of interventions over time. Once interventions targeted at call initiation had been implemented, we began tracking our primary outcome measure. A new run chart was created documenting the percentage of calls completed. For both metrics, the centerline was adjusted using established rules for special cause variation in run charts.[9, 10, 11, 12, 13]
RESULTS
From July 2011 to March 2014, there were 6313 discharges from the HM service. The process measure (percentage of calls initiated) improved from 50% to 97% after 4 interventions (Figure 4). Data for the outcome measure (percentage of calls completed) were collected starting in August 2012, shortly after linking the EHR discharge order to the discharge call. Over the first 8 weeks, our median was 80%, which increased to a median of 93% (Figure 5). These results were sustained for 18 months.


Several key interventions were identified that were critical to achievement of our goal. Standardization of the communication process through PPL was temporally associated with a shift in the median rate of call initiation from 52% to 72%. Use of the discharge order to initiate discharge communication was associated with an increase from 72% to 97%. Finally, the percentage of completed verbal handoffs increased to more than 93% following batching of phone calls to PCPs at specific times during the day.
DISCUSSION
We used improvement and reliability science methods to implement a successful process for improving verbal handoffs from HM physicians to PCPs within 24 hours of discharge to 93%. This result has been sustained for 18 months.
Utilization of the PPL call center for flexible call facilitation along with support for data analysis and leveraging the EHR to automate the process increased reliability, leading to rapid improvement. Prior to mandating the use of PPL to connect discharging physicians with PCPs, the exact rate of successful handoffs in our institution was not known. We do know, however, that only 52% of calls were initiated, so clearly a large gap was present prior to our improvement work. Data collection from the PPL system was automated so that accurate, timely, and sustainable data could be provided, greatly aiding improvement efforts. Flexibility in call‐back timing was also crucial, because coordinating the availability of PCPs and discharging physicians is often challenging. The EHR‐initiated process for discharge communication was a key intervention, and improvement of our process measure to 97% performance was associated with this implementation. Two final interventions: (1) assignment of responsibility for communication to a team pager held by a designated resident and (2) batching of calls to specific times streamlined the EHR‐initiated process and were associated with achievement of our main outcome goal of >90% completed verbal communication.
There are several reports of successful interventions to improve receipt or content of discharge summaries by PCPs following hospital discharge available in the literature.[14, 15, 16, 17, 18, 19, 20] Recently, Shen et al. reported on the success of a multisite improvement collaborative involving pediatric hospitalist programs at community hospitals whose aim was to improve the timely documentation of communication directed at PCPs.[21] In their report, all 7 hospital sites that participated in the collaborative for more than 4 months were able to demonstrate substantial improvement in documentation of some form of communication directed at PCPs (whether by e‐mail, fax, or telephone call), from a baseline of approximately 50% to more than 90%. A limitation of their study was that they were unable to document whether PCPs had received any information or by what method. A recent survey of PCPs by Sheu et al. indicated that for many discharges, information in addition to that present in the EHR was desirable to ensure a safe transition of care.[6] Two‐way communication, such as with a phone call, allows for senders to verify information receipt and for receivers to ask questions to ensure complete information. To our knowledge, there have been no previous reports describing processes for improving verbal communication between hospitalist services and PCPs at discharge.
It may be that use of the call system allowed PCPs to return phone calls regarding discharges at convenient stopping points in their day while allowing discharging physicians to initiate a call without having to wait on hold. Interestingly, though we anticipated the need for additional PPL resources during the course of this improvement, the final process was efficient enough that PPL did not require additional staffing to accommodate the higher call volume.
A key insight during our implementation was that relying on the EHR to initiate every discharge communication created disruption of resident workflow due to disregard of patient, resident, and PCP factors. This was reflected by the improvement in call initiation (our process measure) following this intervention, whereas at the same time call completion (our outcome measure) remained below goal. To achieve our goal of completing verbal communication required a process that was highly reliable yet flexible enough to allow discharging physicians to complete the call in the unpredictable environment of inpatient care. Ultimately, this was achieved by allowing discharging physicians to initiate the process when convenient, and allowing for the EHR‐initiated process to function as a backup strategy to identify and mitigate failures of initiation.
An important limitation of our study was the lack of PCPs on the improvement team, likely making the success of the project more difficult than it might have been. For example, during the study we did not measure the time PCPs spent on hold or how many reattempts were needed to complete the communication loop. Immediately following the completion of our study, it became apparent that physicians returning calls for our own institution's primary care clinic were experiencing regular workflow interruptions and occasional hold times more than 20 minutes, necessitating ongoing further work to determine the root causes and solutions to these problems. Though this work is ongoing, average PCP hold times measured from a sample of call reviews in 2013 to 2014 was 3 minutes and 15 seconds.
This study has several other limitations. We were unable to account for phone calls to PCPs initiated outside of the new process. It may be that PCPs were called more than 52% of the time at baseline due to noncompliance with the new protocol. Also, we only have data for call completion starting after implementation of the link between the discharge order and the discharge phone call, making the baseline appear artificially high and precluding any analysis of how earlier interventions affected our outcome metric. Communication with PCPs should ideally occur prior to discharge. An important limitation of our process is that calls could occur several hours after discharge between an on‐call resident and an on‐call outpatient physician rather than between the PCP and the discharging resident, limiting appropriate information exchange. Though verbal discharge communication is a desirable goal for many reasons, the current project did not focus on the quality of the call or the information that was transmitted to the PCP. Additionally, direct attending‐to‐attending communication may be valuable with medically or socially complex discharges, but we did not have a process to facilitate this. We also did not measure what effect our new process had on outcomes such as quality of patient and family transition from hospital or physician satisfaction. The existence of programs similar to our PPL subspecialty referral line may be limited to large institutions. However, it should be noted that although some internal resource reallocation was necessary within PPL, no actual staffing increases were required despite a large increase in call volume. It may be that any hospital operator system could be adapted for this purpose with modest additional resources. Finally, although our EHR system is widely utilized, there are many competing systems in the market, and our intervention required utilization of EHR capabilities that may not be present in all systems. However, our EHR intervention utilized existing functionality and did not require modification of the system.
This project focused on discharge phone calls to primary care physicians for patients hospitalized on the hospital medicine service. Because communication with the PCP should ideally occur prior to discharge, future work will include identifying a more proximal trigger than the discharge order to which to link the EHR trigger for discharge communication. Other next steps to improve handoff effectiveness and optimize the efficiency of our process include identifying essential information that should be transmitted to the primary care physician at the time of the phone call, developing processes to ensure communication of this information, measuring PCP satisfaction with this communication, and measuring the impact on patient outcomes. Finally, though expert opinion indicates that verbal handoffs may have safety advantages over nonverbal handoffs, studies comparing the safety and efficacy of verbal versus nonverbal handoffs at patient discharge are lacking. Studies establishing the relative efficacy and safety of verbal versus nonverbal handoffs at hospital discharge are needed. Knowledge gained from these activities could inform future projects centered on the spread of the process to other hospital services and/or other hospitals.
CONCLUSION
We increased the percentage of calls initiated to PCPs at patient discharge from 52% to 97% and the percentage of calls completed between HM physicians and PCPs to 93% through the use of a standardized discharge communication process coupled with a basic EHR messaging functionality. The results of this study may be of interest for further testing and adaptation for any institution with an electronic healthcare system.
Disclosure: Nothing to report.
Timely and reliable communication of important data between hospital‐based physicians and primary care physicians is critical for prevention of medical adverse events.[1, 2] Extrapolation from high‐performance organizations outside of medicine suggests that verbal communication is an important component of patient handoffs.[3, 4] Though the Joint Commission does not mandate verbal communication during handoffs per se, stipulating instead that handoff participants have an opportunity to ask and respond to questions,[5] there is some evidence that primary care providers prefer verbal handoffs at least for certain patients such as those with medical complexity.[6] Verbal communication offers the receiver the opportunity to ask questions, but in practice, 2‐way verbal communication is often difficult to achieve at hospital discharge.
At our institution, hospital medicine (HM) physicians serve as the primary inpatient providers for nearly 90% of all general pediatric admissions. When the HM service was established, primary care physicians (PCPs) and HM physicians together agreed upon an expectation for verbal, physician‐to‐physician communication at the time of discharge. Discharge communication is provided by either residents or attendings depending on the facility. A telephone operator service called Physician Priority Link (PPL) was made available to facilitate this communication. The PPL service is staffed 24/7 by operators whose only responsibilities are to connect providers inside and outside the institution. By utilizing this service, PCPs could respond in a nonemergent fashion to discharge phone calls.
Over the last several years, PCPs have observed high variation in the reliability of discharge communication phone calls. A review of PPL phone records in 2009 showed that only 52% of HM discharges had a record of a call initiated to the PCP on the day of discharge. The overall goal of this improvement project was to improve the completion of verbal handoffs from HM physicians (residents or attendings) to PCPs. The specific aim of the project was to increase the proportion of completed verbal handoffs from on‐call residents or attendings to PCPs within 24 hours of discharge to more than 90% within 18 months.
METHODS
Human Subjects Protection
Our project was undertaken in accordance with institutional review board (IRB) policy on systems improvement work and did not require formal IRB review.
Setting
This study included all patients admitted to the HM service at an academic children's hospital and its satellite campus.
Planning the Intervention
The project was championed by physicians on the HM service and supported by a chief resident, PPL administrators, and 2 information technology analysts.
At the onset of the project, the team mapped the process for completing a discharge call to the PCPs, conducted a modified failure mode and effects analysis,[7, 8] and examined the key drivers used to prioritize interventions (Figure 1). Through the modified failure modes effect analysis, the team was able to identify system issues that led to unsuccessful communication: failure of call initiation, absence of an identified PCP, long wait times on hold, failure of PCP to call back, and failure of the call to be documented. These failure modes informed the key drivers to achieving the study aim. Figure 2 depicts the final key drivers, which were revised through testing and learning.


Interventions Targeting Key Stakeholder Buy‐in
To improve resident buy‐in and participation, the purpose and goals of the projects were discussed at resident morning report and during monthly team meetings by the pediatric chief resident on our improvement team. Resident physicians were interested in participating to reduce interruptions during daily rounds and to improve interactions with PCPs. The PPL staff was interested in standardizing the discharge call process to reduce confusion in identifying the appropriate contact when PCPs called residents back to discuss discharges. PCPs were interested in ensuring good communication at discharge, and individual PCPs were engaged through person‐to‐person contact by 1 of the HM physician champions.
Interventions to Standardization the Communication Process
To facilitate initiation of calls to PCPs at hospital discharge, the improvement team created a standard process using the PPL service (Figure 3). All patients discharged from the HM service were included in the process. Discharging physicians (who were usually but not always residents, depending on the facility), were instructed to call the PPL operator at the time of discharge. The PPL operator would then page the patient's PCP. It was the responsibility of the discharging physician to identify a PCP prior to discharge. Instances where no PCP was identified were counted as process failures because no phone call could be made. The expectation for the PCPs was that they would return the page within 20 minutes. PPL operators would then page back to the discharging physician to connect the 2 parties with the expectation that the discharging physician respond within 2 to 4 minutes to the PPL operator's page. Standardization of all calls through PPL allowed efficient tracking of incomplete calls and operators to reattempt calls that were not completed. This process also shifted the burden of following up on incomplete calls to PPL. The use of PPL to make the connection also allowed the physician to complete other work while awaiting a call back from the PCP.

Leveraging the Electronic Health Record for Process Initiation
To ensure reliable initiation of the discharge communication pathway, the improvement team introduced changes to the electronic health record (HER) (EpicCare Inpatient; Epic Systems Corp., Verona, WI), which generated a message to PPL operators whenever a discharge order was entered for an HM patient. The message contained the patient's name, medical record number, discharge date, discharging physician, and PCP name and phone number. A checklist was implemented by PPL to ensure that duplicate phone calls were not made. To initiate communication, the operator contacted the resident via text page to ensure they were ready to initiate the call. If the resident was ready to place a call, the operator then generated a phone call to the PCP. When the PCP returned the call, the operator connected the HM resident with the PCP for the handoff.
As the project progressed, several adaptations were made to address newly identified failure modes. To address confusion among PPL operators about which resident physicians should take discharge phone calls after the discharging resident was no longer available (for example, after a shift change), primary responsibility for discharge phone calls was reassigned to the daily on‐call resident rather than the resident who wrote the discharge order. Because the on‐call residents carry a single pager, the pager number listed on the automated discharge notification to PPL would never change and would always reach the appropriate team member. Second, to address the anticipated increase in interruption of resident workflow by calls back from PCPs, particularly during rounds, operators accessed information on pending discharge phone calls in batches at times of increased resident availability to minimize hold times for PCPs and work interruptions for the discharging physicians. Batch times were 1 pm and 4 pm to allow for completion of morning rounds, resident conference at noon, and patient‐care activities during the afternoon. Calls initiated after 4 pm were dispatched at the time of the discharge, and calls initiated after 10 pm were deferred to the following day.
Transparency of Data
Throughout the study, weekly failure data were generated from the EHR and emailed to improvement team members, enabling them to focus on near real‐time feedback of data to create a visible and more reliable system. With the standardization of all discharge calls directed to the PPL operators, the team was able to create a call record linked to the patient's medical record number. Team‐specific and overall results for the 5 HM resident teams were displayed weekly on a run chart in the resident conference room. As improvements in call initiation were demonstrated, completion rate data were also shared every several months with the attending hospitalists during a regularly scheduled divisional conference. This transparency of data gave the improvement team the opportunity to provide individual feedback to residents and attendings about failures. The weekly review of failure data allowed team leaders to learn from failures, identify knowledge gaps, and ensure accountability with the HM physicians.
Planning the Study of the Intervention
Data were collected prospectively from July 2011 to March 2014. A weekly list of patients discharged from the HM service was extracted from the EHR and compared to electronic call logs collected by PPL on the day of discharge. A standard sample size of 30 calls was audited separately by PPL and 1 of the physician leads to verify that the patients were discharged from the HM service and validate the percentage of completed and initiated calls.
The percentage of calls initiated within 24 hours of discharge was tracked as a process measure and served as the initial focus of improvement efforts. Our primary outcome measure was the percentage of calls completed to the PCP by the HM physician within 24 hours of discharge.
Methods of Evaluation and Analysis
We used improvement science methods and run charts to determine the percentage of patients discharged from the HM service with a call initiated to the PCP and completed within 24 hours of discharge. Data on calls initiated within 24 hours of discharge were plotted on a run chart to examine the impact of interventions over time. Once interventions targeted at call initiation had been implemented, we began tracking our primary outcome measure. A new run chart was created documenting the percentage of calls completed. For both metrics, the centerline was adjusted using established rules for special cause variation in run charts.[9, 10, 11, 12, 13]
RESULTS
From July 2011 to March 2014, there were 6313 discharges from the HM service. The process measure (percentage of calls initiated) improved from 50% to 97% after 4 interventions (Figure 4). Data for the outcome measure (percentage of calls completed) were collected starting in August 2012, shortly after linking the EHR discharge order to the discharge call. Over the first 8 weeks, our median was 80%, which increased to a median of 93% (Figure 5). These results were sustained for 18 months.


Several key interventions were identified that were critical to achievement of our goal. Standardization of the communication process through PPL was temporally associated with a shift in the median rate of call initiation from 52% to 72%. Use of the discharge order to initiate discharge communication was associated with an increase from 72% to 97%. Finally, the percentage of completed verbal handoffs increased to more than 93% following batching of phone calls to PCPs at specific times during the day.
DISCUSSION
We used improvement and reliability science methods to implement a successful process for improving verbal handoffs from HM physicians to PCPs within 24 hours of discharge to 93%. This result has been sustained for 18 months.
Utilization of the PPL call center for flexible call facilitation along with support for data analysis and leveraging the EHR to automate the process increased reliability, leading to rapid improvement. Prior to mandating the use of PPL to connect discharging physicians with PCPs, the exact rate of successful handoffs in our institution was not known. We do know, however, that only 52% of calls were initiated, so clearly a large gap was present prior to our improvement work. Data collection from the PPL system was automated so that accurate, timely, and sustainable data could be provided, greatly aiding improvement efforts. Flexibility in call‐back timing was also crucial, because coordinating the availability of PCPs and discharging physicians is often challenging. The EHR‐initiated process for discharge communication was a key intervention, and improvement of our process measure to 97% performance was associated with this implementation. Two final interventions: (1) assignment of responsibility for communication to a team pager held by a designated resident and (2) batching of calls to specific times streamlined the EHR‐initiated process and were associated with achievement of our main outcome goal of >90% completed verbal communication.
There are several reports of successful interventions to improve receipt or content of discharge summaries by PCPs following hospital discharge available in the literature.[14, 15, 16, 17, 18, 19, 20] Recently, Shen et al. reported on the success of a multisite improvement collaborative involving pediatric hospitalist programs at community hospitals whose aim was to improve the timely documentation of communication directed at PCPs.[21] In their report, all 7 hospital sites that participated in the collaborative for more than 4 months were able to demonstrate substantial improvement in documentation of some form of communication directed at PCPs (whether by e‐mail, fax, or telephone call), from a baseline of approximately 50% to more than 90%. A limitation of their study was that they were unable to document whether PCPs had received any information or by what method. A recent survey of PCPs by Sheu et al. indicated that for many discharges, information in addition to that present in the EHR was desirable to ensure a safe transition of care.[6] Two‐way communication, such as with a phone call, allows for senders to verify information receipt and for receivers to ask questions to ensure complete information. To our knowledge, there have been no previous reports describing processes for improving verbal communication between hospitalist services and PCPs at discharge.
It may be that use of the call system allowed PCPs to return phone calls regarding discharges at convenient stopping points in their day while allowing discharging physicians to initiate a call without having to wait on hold. Interestingly, though we anticipated the need for additional PPL resources during the course of this improvement, the final process was efficient enough that PPL did not require additional staffing to accommodate the higher call volume.
A key insight during our implementation was that relying on the EHR to initiate every discharge communication created disruption of resident workflow due to disregard of patient, resident, and PCP factors. This was reflected by the improvement in call initiation (our process measure) following this intervention, whereas at the same time call completion (our outcome measure) remained below goal. To achieve our goal of completing verbal communication required a process that was highly reliable yet flexible enough to allow discharging physicians to complete the call in the unpredictable environment of inpatient care. Ultimately, this was achieved by allowing discharging physicians to initiate the process when convenient, and allowing for the EHR‐initiated process to function as a backup strategy to identify and mitigate failures of initiation.
An important limitation of our study was the lack of PCPs on the improvement team, likely making the success of the project more difficult than it might have been. For example, during the study we did not measure the time PCPs spent on hold or how many reattempts were needed to complete the communication loop. Immediately following the completion of our study, it became apparent that physicians returning calls for our own institution's primary care clinic were experiencing regular workflow interruptions and occasional hold times more than 20 minutes, necessitating ongoing further work to determine the root causes and solutions to these problems. Though this work is ongoing, average PCP hold times measured from a sample of call reviews in 2013 to 2014 was 3 minutes and 15 seconds.
This study has several other limitations. We were unable to account for phone calls to PCPs initiated outside of the new process. It may be that PCPs were called more than 52% of the time at baseline due to noncompliance with the new protocol. Also, we only have data for call completion starting after implementation of the link between the discharge order and the discharge phone call, making the baseline appear artificially high and precluding any analysis of how earlier interventions affected our outcome metric. Communication with PCPs should ideally occur prior to discharge. An important limitation of our process is that calls could occur several hours after discharge between an on‐call resident and an on‐call outpatient physician rather than between the PCP and the discharging resident, limiting appropriate information exchange. Though verbal discharge communication is a desirable goal for many reasons, the current project did not focus on the quality of the call or the information that was transmitted to the PCP. Additionally, direct attending‐to‐attending communication may be valuable with medically or socially complex discharges, but we did not have a process to facilitate this. We also did not measure what effect our new process had on outcomes such as quality of patient and family transition from hospital or physician satisfaction. The existence of programs similar to our PPL subspecialty referral line may be limited to large institutions. However, it should be noted that although some internal resource reallocation was necessary within PPL, no actual staffing increases were required despite a large increase in call volume. It may be that any hospital operator system could be adapted for this purpose with modest additional resources. Finally, although our EHR system is widely utilized, there are many competing systems in the market, and our intervention required utilization of EHR capabilities that may not be present in all systems. However, our EHR intervention utilized existing functionality and did not require modification of the system.
This project focused on discharge phone calls to primary care physicians for patients hospitalized on the hospital medicine service. Because communication with the PCP should ideally occur prior to discharge, future work will include identifying a more proximal trigger than the discharge order to which to link the EHR trigger for discharge communication. Other next steps to improve handoff effectiveness and optimize the efficiency of our process include identifying essential information that should be transmitted to the primary care physician at the time of the phone call, developing processes to ensure communication of this information, measuring PCP satisfaction with this communication, and measuring the impact on patient outcomes. Finally, though expert opinion indicates that verbal handoffs may have safety advantages over nonverbal handoffs, studies comparing the safety and efficacy of verbal versus nonverbal handoffs at patient discharge are lacking. Studies establishing the relative efficacy and safety of verbal versus nonverbal handoffs at hospital discharge are needed. Knowledge gained from these activities could inform future projects centered on the spread of the process to other hospital services and/or other hospitals.
CONCLUSION
We increased the percentage of calls initiated to PCPs at patient discharge from 52% to 97% and the percentage of calls completed between HM physicians and PCPs to 93% through the use of a standardized discharge communication process coupled with a basic EHR messaging functionality. The results of this study may be of interest for further testing and adaptation for any institution with an electronic healthcare system.
Disclosure: Nothing to report.
- , , . Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S–39S.
- , , , , . Evaluating communication between pediatric primary care physicians and hospitalists. Clin Pediatr. 2011;50(10):923–928.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- Agency for Healthcare Research and Quality. Patient safety primers: handoffs and signouts. Available at: http://www.psnet.ahrq.gov/primer.aspx?primerID=9. Accessed March 19, 2014.
- , , , , . We need to talk: primary care provider communication at discharge in the era of a shared electronic medical record. J Hosp Med. 2015;10(5):307–310.
- , , . Failure mode and effects analysis: a novel approach to avoiding dangerous medication errors and accidents. Hosp Pharm. 1994;29:319–330.
- , , , . Using health care Failure Mode and Effect Analysis: the VA National Center for Patient Safety's prospective risk analysis system. Jt Comm J Qual Improv. 2002;28:248–267, 209.
- . Statistical quality control methods in infection control and hospital epidemiology, Part II: Chart use, statistical properties, and research issues. Infect Control Hosp Epidemiol. 1998;19(4):265–283.
- . Statistical quality control methods in infection control and hospital epidemiology, part I: Introduction and basic theory. Infect Control Hosp Epidemiol. 1998;19(3):194–214.
- , , . Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458–464.
- . The Improvement Guide: A Practical Approach to Enhancing Organizational +Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- , . The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey‐Bass; 2011.
- , . The initial hospital discharge note: send out with the patient or post? Health Trends. 1984;16(2):48.
- , . Which type of hospital discharge report reaches general practitioners most quickly? BMJ. 1989;298(6670):362–363.
- , . The application of a computer data base system to the generation of hospital discharge summaries. Obstet Gynecol. 1989;73(5 pt 1):803–807.
- . Hospital discharge medication: is seven days supply sufficient? Public Health. 1991;105(3):243–247.
- , , , et al. Electronic communication between providers of primary and secondary care. BMJ. 1992;305(6861):1068–1070.
- , , . Communication of discharge information for elderly patients in hospital. Ulster Med J. 1992;61(1):56–58.
- , , , , . A quality use of medicines program for continuity of care in therapeutics from hospital to community. Med J Aust. 2002;177(1):32–34.
- , , , , , . Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258–265.
- , , . Passing the clinical baton: 6 principles to guide the hospitalist. Am J Med. 2001;111(9B):36S–39S.
- , , , , . Evaluating communication between pediatric primary care physicians and hospitalists. Clin Pediatr. 2011;50(10):923–928.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- Agency for Healthcare Research and Quality. Patient safety primers: handoffs and signouts. Available at: http://www.psnet.ahrq.gov/primer.aspx?primerID=9. Accessed March 19, 2014.
- , , , , . We need to talk: primary care provider communication at discharge in the era of a shared electronic medical record. J Hosp Med. 2015;10(5):307–310.
- , , . Failure mode and effects analysis: a novel approach to avoiding dangerous medication errors and accidents. Hosp Pharm. 1994;29:319–330.
- , , , . Using health care Failure Mode and Effect Analysis: the VA National Center for Patient Safety's prospective risk analysis system. Jt Comm J Qual Improv. 2002;28:248–267, 209.
- . Statistical quality control methods in infection control and hospital epidemiology, Part II: Chart use, statistical properties, and research issues. Infect Control Hosp Epidemiol. 1998;19(4):265–283.
- . Statistical quality control methods in infection control and hospital epidemiology, part I: Introduction and basic theory. Infect Control Hosp Epidemiol. 1998;19(3):194–214.
- , , . Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458–464.
- . The Improvement Guide: A Practical Approach to Enhancing Organizational +Performance. 2nd ed. San Francisco, CA: Jossey‐Bass; 2009.
- , . The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey‐Bass; 2011.
- , . The initial hospital discharge note: send out with the patient or post? Health Trends. 1984;16(2):48.
- , . Which type of hospital discharge report reaches general practitioners most quickly? BMJ. 1989;298(6670):362–363.
- , . The application of a computer data base system to the generation of hospital discharge summaries. Obstet Gynecol. 1989;73(5 pt 1):803–807.
- . Hospital discharge medication: is seven days supply sufficient? Public Health. 1991;105(3):243–247.
- , , , et al. Electronic communication between providers of primary and secondary care. BMJ. 1992;305(6861):1068–1070.
- , , . Communication of discharge information for elderly patients in hospital. Ulster Med J. 1992;61(1):56–58.
- , , , , . A quality use of medicines program for continuity of care in therapeutics from hospital to community. Med J Aust. 2002;177(1):32–34.
- , , , , , . Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258–265.
© 2015 Society of Hospital Medicine
Baby Boomer HCV Screening and Care
INTRODUCTION
The baby boomer generation, born from 1945 to 1965, accounts for 75% of the estimated 2.7 to 3.9 million persons with chronic hepatitis C virus (HCV) infection in the US.[1, 2, 3] Most HCV‐infected baby boomers do not know that they are infected.[4] With the advent of better‐tolerated, more‐effective therapies to treat chronic HCV infection,[5] and to reduce rates of complications such as cirrhosis, liver failure, and hepatocellular carcinoma,[6] universal 1‐time screening of baby boomers has been endorsed by the Centers for Disease Control and Prevention (CDC) and the United States Preventive Services Task Force.[1, 7] Hospitalized baby boomers may offer an important target for HCV screening. Our group conducted an anonymous HCV seroprevalence study of nearly 800 patients on general medicine and trauma services of 2 Philadelphia hospitals, and found that 8% had undiagnosed HCV infection, and 8% had diagnosed HCV. [8]
Little is known about barriers and facilitators to implementation of universal HCV screening of baby boomers. Lessons from implementing HIV screening offer a useful guide.[9] First, limited clinician knowledge and confusion about screening guidelines necessitated convenient, well‐designed educational programs.[10] Second, burdensome consent procedures were reduced by opt‐out consent for screening supplemented by patient education.[9] Third, electronic medical record (EMR) algorithms minimized burdens on staff by efficiently identifying and flagging eligible persons for screening.[11] Fourth, ancillary staff support for patient education and linkage to follow‐up care increased screening rates compared with usual care by physicians/staff.[11] Finally, routine human immunodeficiency virus (HIV) testing of inpatients increased rates of diagnosis, especially compared with physician referral systems.[12]
This article describes how HIV screening strategies informed the development in a baby boomer HCV screening and linkage to a care program in a safety‐net hospital serving a majority Hispanic population. We report results of the first 14 months of the screening program and linkage to care for chronically HCV‐infected persons after a minimum 10 months follow‐up. We also estimate costs for program implementation and maintenance to inform hospital administrators, healthcare policymakers, and clinicians about resources that may be required to effectively screen hospitalized baby boomers for HCV.
METHODS
Study Setting
The HCV baby boomer screening program was pilot tested in November 2012 and launched December 1, 2012 in a 498‐bed academic‐affiliated hospital of a healthcare system serving the indigent population of South Texas.
Project Development Phase
From October 1, 2012 to November 30, 2012, project infrastructure development and provider/staff education were conducted. A half‐hour PowerPoint lecture (in person or online) was developed about HCV epidemiology, birth‐cohort HCV screening guidelines, newer treatment modalities, and screening program components. Lectures were delivered to departmental chairs at the affiliated medical school, departmental grand rounds, and the hospital's nursing supervisors. One‐on‐one informational meetings were also held with hospital administrators and staff.
With the hospital's information technology team, screens were developed to identify eligible baby boomers from up to 7 years of previous inpatient and outpatient encounters in the EMR from: birth year (19451965) and no prior diagnosis of HCV infection (070.41, 070.44, 070.51, 070.54, 070.7x, V02.62) or any type of completed test for HCV. The algorithm also excluded patients admitted to psychiatry due to lack of decision‐making capacity or patients with a poor prognosis such as metastatic cancer. An audit of 100 consecutive excluded patients identified all as legitimate.
A new laboratory order for HCV screening was developed by laboratory administrators and pathology faculty for an anti‐HCV antibody test followed by reflex HCV RNA testing for positive results per CDC recommendations.[13] The anti‐HCV test was performed on serum or ethylenediaminetetraacetic acid plasma using the Advia Centaur HCV Assay (Bayer HealthCare LLC, Tarrytown, NY). This assay has excellent sensitivity (99.9%) and specificity (97.5%).[14, 15] The HCV RNA assay was performed using quantitative real‐time polymerase chain reaction (PCR) using the COBAS AmpliPrep/COBAS TaqMan HCV test (Roche Molecular Systems, Pleasanton, CA). Use of plasma preparation tubes (PPTs) (BD Vacutainer PPT tubes; Becton, Dickinson and Co., Franklin Lakes, NJ) permitted both anti‐HCV antibody and HCV PCR testing to be performed on the same specimen when anti‐HCV antibody was detected, eliminating a second blood draw for the PCR test. For patients eligible for screening, an EMR algorithm was created to add an HCV screening order to over 50 different admission order sets.
To educate patients newly diagnosed with HCV infection, we developed an interactive, low‐literacy, educational program in Spanish and English for an electronic tablet device that addressed: HCV epidemiology, transmission prevention, factors that can accelerate chronic HCV infection, and management/treatment strategies. At several points in the program, the patient needed to answer questions correctly to continue. The tablet retained responses linked to a study identification about alcohol consumption, history of past and current illicit drug use, sexual risk behavior, and offered risk reduction messages. The tablet content and presentation reflected suggestions by Hispanic patient‐reviewers about cultural appropriateness and comprehension.
Project Implementation and Maintenance Phase
We report implementation of the program from December 1, 2012 to January 31, 2014. An automated EMR report classified all baby boomers admitted in the previous 24 hours as: (1) eligible with pending screening test order, (2) eligible without an order, (3) ineligible due to prior HCV test or diagnosis, or (4) ineligible due to comorbidity (eg, metastatic cancer). For approximately one‐third of eligible patients, a study team member placed an order after review of the daily admission report because the order had not been automatically placed.
Admitting nurses initially asked for consent from eligible patients for HCV screening, but this was ultimately deemed too onerous a task along with all of their other duties. We then instituted opt‐out consent with patient education about testing and opportunities to refuse via posters placed throughout the hospital and flyers in admission packets. A bilingual HCV counselor provided HCV screening test results to all patients. She counseled patients who screened positive for HCV with the educational program on an electronic tablet and developed a follow‐up care plan.
A bilingual promotora (community health worker) contacted patients newly diagnosed with chronic HCV infection after hospital discharge to address the following: obtaining insurance, access to primary care and HCV specialty care, scheduling appointments, and treatment for alcohol problems or drug abuse. After obtaining signed consent, the promotora sent test results and recommendations for follow‐up care (eg, hepatitis A and B immunization) to a designated outpatient physician and reminded patients about appointments and pending tests. The promotora received training in motivational interviewing skills to engage patients with needed care including alcohol treatment.
Study Data
A summary report was developed from the EMR with demographic, insurance, clinical, and HCV screening data for all admitted baby boomers. For patients diagnosed with chronic HCV infection, the promotora obtained data about follow‐up HCV care through December 10, 2014 from the EMR, outside provider records, and patient reports.
Study Variables
The 2 outcome measures were a positive anti‐HCV antibody test and positive HCV RNA test. Insurance status was categorized as insured (private, public, Veterans Administration, Department of Defense) or uninsured (self‐pay or county‐based financial assistance program). Problem drinking was identified from International Classification of Diseases, Ninth Revision, Clinical Modification codes for the admission, notes by clinicians describing alcohol abuse/dependence, or quantity/frequency meeting National Institute on Alcohol Abuse and Alcoholism criteria for alcohol problems of >14 drinks/week or >4 drinks/day for men and >7 drinks per week or >3 drinks per day for women.[16]
Implementation costs included informatics support, mobile app development, other patient educational materials, costs of screening tests for uninsured, and 0.3 full‐time equivalent (FTE) of a clinician for half a year. Maintenance costs included salaries for the study team, HCV testing costs, and postage.
Analysis
Demographics by HCV antibody test results are compared using [2] tests or Student t tests as appropriate. Among persons with a positive HCV antibody test, HCV RNA results are similarly compared. This implementation project was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board (HSC20130033N).
RESULTS
Within 14 months, 6410 unique baby boomers were admitted with a mean age 56.4 years (standard deviation [SD] 5.7), 55.9% men, 59.1% Hispanic, 8.2% nonwhite, and 46.7% uninsured (Table 1). Among admitted patients, 729 (11.4%) had a previous HCV diagnosis and 1904 (29.7%) had been tested for HCV (Figure 1). Anti‐HCV antibody testing was completed for 3168 (49.4% of all admitted patients and 83.9% of never‐tested patients). After exclusions such as significant comorbidity or psychiatric admission, 95% of eligible persons were tested. Of screened patients, 240 (7.6%) were positive; these patients were significantly younger (P<0.0001) and more likely to be men (P<0.0001) and uninsured (P=0.002) (Table 1). Notably, 10% of men were anti‐HCV positive versus 4% of women. In this predominantly Hispanic cohort, no significant difference appeared by race‐ethnicity, but African Americans had a higher prevalence (10.4%) than other groups.
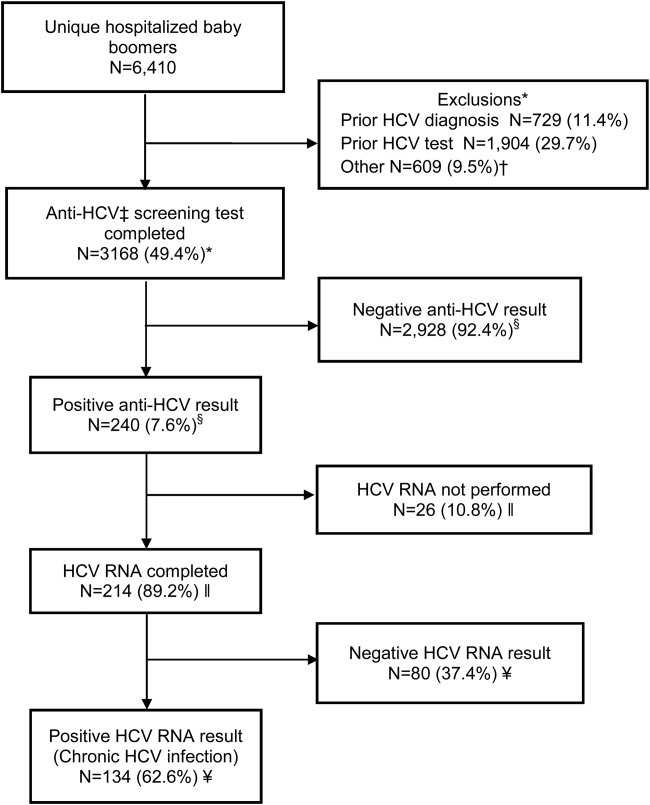
| Characteristic | All Screened Patients, No. | Anti‐HCV Antibody‐Positive Patients, No. (Row %) | P Value* |
|---|---|---|---|
| |||
| Overall | 3,168 | Total=240 (7.6) | |
| Age, mean (SD) | 57.0 (5.7) | 54.8 (5.0) | <0.0001 |
| Sex | |||
| Men | 1,771 | 185 (10.4) | <0.0001 |
| Women | 1,397 | 55 (3.9) | |
| Race | |||
| Non‐Hispanic white | 1,036 | 86 (8.3) | 0.12 |
| Hispanic | 1,872 | 134 (7.2) | |
| African American | 163 | 17 (10.4) | |
| Other | 97 | 3 (3.1) | |
| Insurance | |||
| Insured | 1,740 | 109 (6.3) | 0.002 |
| Uninsured | 1,428 | 131 (9.2) | |
HCV RNA testing was completed for 214 (89.2%) anti‐HCVpositive patients, of whom 134 (62.6%) had detectable RNA, indicating chronic HCV infection (Figure 1). Overall, 4.2% of all eligible patients tested for HCV were chronically infected. No characteristics were significantly associated with chronic HCV, but persons with chronic infection tended to be younger, uninsured, and African American (Table 2).
| Characteristics | All HCV RNA‐Tested Patients, No. | HCV RNA‐Positive Patients, No. (Row %) | P Value* |
|---|---|---|---|
| |||
| Overall | 214 | 134 (62.6) | |
| Age, y, mean (SD) | 54.6 (5.0) | 54.2 (5.1) | 0.09 |
| Sex | |||
| Men | 165 | 106 (64.2) | 0.37 |
| Women | 49 | 28 (57.1) | |
| Race | |||
| Non‐Hispanic white | 78 | 49 (62.8) | 0.65 |
| Hispanic | 118 | 73 (61.8) | |
| African American | 15 | 11 (73.3) | |
| Other | 3 | 1 (33.3) | |
| Insurance | |||
| Insured | 92 | 52 (56.5) | 0.11 |
| Uninsured | 122 | 82 (67.2) | |
Among patients with chronic HCV infection, 129 (96.3%) were counseled and follow‐up plans developed (Figure 2). By December 10, 2014, 108 (80.6%) patients had received follow‐up primary care, and 52 (38.8%) had care from a hepatologist. Five had initiated HCV‐specific treatment, but many others were awaiting approval for compassionate drug programs offering direct‐acting antivirals. Barriers to care included 82 (61.2%) uninsured, 45 (34%) problem drinkers, 22 (16%) homeless, and 25 (18.6%) incarcerated (not shown). The promotora addressed these issues by visiting homes or homeless shelters, assistance with obtaining county‐based or other types of insurance, offering alcohol risk‐reduction counseling, linking patients to alcohol‐treatment programs, and communicating with the county jail about follow‐up care.
Most of the developmental costs for the program were dedicated to developing EMR programs (Table 3). An optional cost was for the development of the tablet educational program about HCV. In regard to maintenance costs for the first 14 months, the majority was to support the program faculty, counseling/case management, and a nurse practitioner who helped with ordering tests. We also estimated costs for testing uninsured patients (45% of HCV antibody tested, 57% of HCV PCR tested, per Tables 1 and 2, respectively), as they must be borne by the hospital.
| Program Component | Monthly ($) | Total ($) |
|---|---|---|
| ||
| Development phase (2 months prior to start) | ||
| Personnel | ||
| Faculty physicians (0.3 FTE salary+benefits) | 6,641 | 13,282 |
| Role: Development educational materials, provider education, and pilot testing | ||
| Technology | ||
| Development of eligibility screen and order sets for electronic medical record | 41,171 | |
| HCV counseling educational program for tabletdevelopment and pilot testing (optional) | 15,000 | |
| Patient educational materials (posters, flyers) | 400 | |
| Total for development phase | 69,853 | |
| Maintenance phase (14 months) | ||
| Personnel | ||
| Faculty physicians (0.3 FTE, salary+benefits) | 6,641 | 92,974 |
| Role: Coordinate with hospital staff and faculty, liaison with laboratory, supervise study team, review all identified cases for eligibility and management plans | ||
| Inpatient counselor and outpatient case management (2 FTE, salary+benefits) | 6,343 | 88,802 |
| Role: Inpatient and outpatient counseling of HCV Ab+patients and facilitation of follow‐up care for patients with chronic HCV infection | ||
| Nurse practitioner ($35/hour @ 10 hours/month) | 350 | 4,900 |
| Role: Review daily list of admitted baby boomers and manually order HCV screening test for those missed by the automated order | ||
| Postage | 10 | 140 |
| Laboratory costs for uninsured (based on % in cohort) | ||
| HCV antibody in plasma preparation tubes ($13.41/test 1,423) | 19,082 | |
| HCV RNA PCR ($87.96/test 122) | 10,731 | |
| Total for maintenance phase | 216,629 | |
| Total program costs | 286,482 | |
DISCUSSION
Implementation of universal HCV screening and linkage to care for hospitalized baby boomers utilizes a multicomponent infrastructure that reflects lessons learned from similar HIV programs. Use of an EMR algorithm to identify eligible patients and programs to automatically order HCV screening was a linchpin of our high testing rate and averted testing those who did not require screening. Of all 6410 baby boomers admitted to our safety‐net hospital, the EMR screen identified over 40% as ineligible due to prior diagnosis of HCV infection or prior HCV tests. Most of the additional 609 patients who were not tested were excluded due to comorbidities or admission to psychiatry. Overall, the EMR programs, tests ordered by the team, and opt‐out screening with education resulted in screening 95% of eligible patients. However, this program carries substantial costs, nearly $300,000 for the first 2 years, for unreimbursed services in this safety‐net hospital. The new guidelines for HCV screening[1, 2] are not accompanied by financial support either for program implementation or for screening and linkage to care for the uninsured, creating significant financial hurdles to achieve guideline compliance within already overtaxed public healthcare systems.
The infrastructure implemented in this hospital succeeded in achieving a higher rate of HCV screening of baby boomers than reported by other programs. In an emergency department in Birmingham, Alabama, a screening program for baby boomers tested 66% of 2325 persons who were HCV‐unaware.[17] In an outpatient clinic for men who have sex with men, only 54% of 1329 patients were screened for HCV.[18]
Among 3168 screened patients in our cohort, 7.6% were anti‐HCV antibody positive, which is over twice the prevalence of 3.5% (95% confidence interval: 2.2%‐4.8%) for anti‐HCVpositive tests in baby boomers based on National Health and Nutrition Examination Survey (NHANES) data from 2001 to 2010.[19] However, the Alabama emergency department study found that 11% of tested patients were anti‐HCV positive.[17] Although that study lacked race‐ethnicity data for half of the subjects, among those with this information, 13% of black and 7% of white subjects tested anti‐HCV positive. Compared with the Alabama study, the anti‐HCV prevalence in our cohort was somewhat lower for blacks (10.4%) but higher for non‐Hispanic whites (8.3%). Hispanics in our cohort had the lowest anti‐HCV prevalence (7.2%), whereas the Alabama study did not report this figure. National studies also find that the prevalence of anti‐HCVpositive results is twice as high for blacks compared with non‐Hispanic whites and Hispanics, and nearly twice as high for men compared with women.[19] In our cohort, the proportion of men with anti‐HCVpositive results was nearly 3 times that for women.
Diagnosis of chronic HCV infection requires 2 tests, similar to performing a Western blot test after a positive enzyme‐linked immunoassay for HIV. In a Veterans Affairs study, only 64% of patients with a positive anti‐HCV antibody test had a HCV RNA performed when reflex testing was not performed, and patients had to come in for a second test versus >90% of patients in sites that offer reflex testing.[20] At a somewhat increased price due to using more expensive PPTs ($96/100 PPT tubes vs $6.50/100 for serum red top tubes), both tests were performed on the same blood sample, resulting in 89% of anti‐HCV antibody‐positive patients being tested for HCV RNA.
Overall, 62% of patients in our cohort with a positive anti‐HCV antibody test had HCV RNA detected (viremic) compared with 71% of persons aged 20 years in an NHANES study from 2003 to 2010.[21] Several factors may contribute to this lower rate of chronic infection. In a study of HCV seropositive blood donors, Hispanics and non‐Hispanic whites were significantly more likely to have spontaneously cleared HCV infection than Asians and non‐Hispanic blacks.[22] Spontaneous clearance of HCV has also been associated with younger age at infection and HCV genotype 1.[23] Poorly understood genetic factors may also play a role.[24] The high rate of HCV clearance in our cohort reinforces the need to perform HCV RNA testing.
Overall, 4.2% of our cohort had chronic HCV infection. According to CDC estimates from 1999 to 2008 NHANES data, 2.74 million (3.25%) of 84.2 million US baby boomers have been infected with HCV, and 2.04 million (2.4%) have chronic infection.[1] Therefore, our safety‐net cohort of never‐tested baby boomers had over twice the prevalence of chronic HCV infection than the national estimate for this age group. This high proportion of chronic HCV may reflect our predominantly low‐income patient population. An analysis conducted by Milliman, Inc. using 2010 data estimated that half of all persons with undiagnosed HCV infection are uninsured.[25] This finding reinforces the need to conduct HCV screening in acute‐care settings such as hospitals, because the uninsured have poor access to ambulatory care.
Our chronic HCV‐infected cohort had many barriers to follow‐up care because most were uninsured and 15% were homeless. Our counselors addressed socioeconomic barriers to care[26] and concerns about the disease.[27] Many patients also had problem drinking based on either self‐report or documented in the medical record. Even moderate alcohol use may increase the risk of overall and liver‐related mortality from chronic HCV infection,[28] so our team offered brief alcohol counseling and partnered with healthcare providers and local Alcoholics Anonymous programs to offer support.
We linked 80% of newly diagnosed patients to primary care or hepatology providers, aided by a county‐level financial assistance program for healthcare services for uninsured residents, but it still required patients to pay out of pocket for care. Access to newer, highly effective, all‐oral therapy treatment[5] was slowed while awaiting US Food and Drug Administration approval in the first year of this project, then treatment provided only after lengthy applications to drug company assistance programs with priority given to persons with compensated cirrhosis.
Our project raises serious concerns for policymakers and payers. Should universal baby boomer HCV testing be undertaken without taking into account the financial and personnel resources required to implement this screening program or the substantial expenditures necessary to treat chronically infected persons? Although the Centers for Medicaid and Medicare Services pay for HCV screening costs,[29] our hospital had to cover costs for uninsured persons. Admittedly, Texas has the highest proportion of residents who are uninsured in the nation, but even in other states, Medicaid and other insurance programs are wrestling with how to deal with the high cost of HCV therapy.[30]
We acknowledge several limitations of this project. First, it was undertaken in only 1 hospital. Yet, our challenges and solutions are likely to be applicable to other hospitals nationally, especially those serving vulnerable populations. Second, patients in our cohort were usually admitted for comorbidities that needed to be managed before HCV infection could be addressed. However, persons with a poor prognosis, such as metastatic cancer, were excluded. We did not attempt to exclude other persons with serious comorbidities such as congestive heart failure, because the guidelines do not currently recommend this, and there may be benefits for patients, their families, and providers from knowing that an individual is chronically HCV infected even if they are not eligible to be treated. Third, the cost of the program was supported in part by a grant and would otherwise have to be borne by the hospital. Fourth, the EMR used by our hospital allows hundreds of admission order sets to be created and made automated order entry hard to implement. This is unlikely to be the situation in other hospitals using different types of EMRs.
It remains to be seen whether safety‐net hospitals with populations at greater risk of HCV infection can afford to support HCV testing and linkage to care. In view of several cost‐effectiveness studies that find screening and treating chronic HCV‐infected baby boomers cost‐effective within standard thresholds,[31, 32, 33] it may be important for policymakers and payers to consider lessons from HIV programs. Because HIV‐infected persons could not afford life‐saving medication, vigorous advocacy efforts led to legislation approving the Ryan White program in 1990 to fill gaps in HIV care that were not covered by other sources of support.[34] HCV infection is the most common blood‐borne infection in the nation, with potentially devastating consequences if ignored, but the underlying premise that universal HCV testing will save lives is in question if most of the individuals who are diagnosed with chronic HCV are low income, uninsured, or underinsured with limited access to curative medications. A rigorous public policy debate regarding both the merits of screening and the availability of treatment to those who are diagnosed is essential to the success of these programs.
Disclosure
Funding for this study was received from the Centers for Disease Control and Prevention CDC PS12‐1209PPHF12. The authors report no conflicts of interest.
- , , , , , Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32.
- 2. Centers for Disease Control and Prevention. Vital signs: evaluation of hepatitis C virus infection testing and reporting–eight U.S. sites, 2005‐2011. MMWR Morb Mortal Wkly Rep. 2013;62:357–361.
- , , , , Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101.
- Institute of Medicine. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press; 2010.
- , Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917.
- , , , et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188.
- U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357.
- , , Undiagnosed hepatitis C on the general medicine and trauma services of two urban hospitals. J Infect. 2009;59:62–69.
- U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51–60.
- , , , et al. Factors affecting clinician educator encouragement of routine HIV testing among trainees. J Gen Intern Med. 2012;27:839–844.
- , , , et al. Counselor‐versus provider‐based HIV screening in the emergency department: Results from the universal screening for HIV infection in the emergency room (USHER) randomized controlled trial. Ann Emerg Med. 2011;58:S126–S132.e1–4.
- , , Approaching the CDC's guidelines on the HIV testing of inpatients: physician‐referral versus nonreferral‐based testing. AIDS Patient Care STDS. 2006;20:311–317.
- Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362–365.
- Advia Centaur Assay Manual. Malvern, PA: Siemens Medical Solutions Diagnostics; Pub# 07063235, Rev. C, 2005‐01.
- , , , Comparison of the ADVIA Centaur and Abbott AxSYM immunoassay systems for a routine diagnostic virology laboratory. J Clin Virol. 2004;30:S11–S15.
- National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Available at: http://www.niaaa.nih.gov/alcohol‐health/overview‐alcohol‐consumption/moderate‐binge‐drinking. Accessed June 12, 2014.
- , , , et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61:776–782.
- , , , et al. Low rates of hepatitis screening and vaccination of HIV‐infected MSM in HIV clinics. Sex Transm Dis. 2012;39:349–353.
- , , , , , The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60:691–698.
- , , , , Viral RNA testing in hepatitis C antibody‐positive veterans. Am J Prev Med. 2009;36:235–238.
- , , , et al. Chronic hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300.
- , , , et al. NHLBI Retrovirus Epidemiology Donor Study (REDS) Group. Correlates of hepatitis C virus (HCV) RNA negativity among HCV‐seropositive blood donors. Transfusion. 2006;46:469–475.
- , , , , , Spontaneous loss of hepatitis C virus RNA from serum is associated with genotype 1 and younger age at exposure. J Med Virol. 2011;83:1338–1344.
- , , , et al. Genetics of spontaneous clearance of hepatitis C virus infection: a complex topic with much to learn. Hepatology. 2014;60:2127–2128.
- , , , Health care reform and hepatitis C: a convergence of risk and opportunity. Available at: http://us.milliman.com/uploadedFiles/insight/2013/convergence‐of‐risk‐and‐opportunity.pdf. Accessed February 5, 2015.
- , , , Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009‐2010. Am J Public Health. 2013;103:112–119.
- , Barriers to hepatitis C treatment. Liver Int. 2012;32:151–156.
- , , , , Moderate, excessive or heavy alcohol consumption: Each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2013;37:703–709.
- Centers for Medicare and Medicaid Services. Proposed decision memo for screening for hepatitis c virus (HCV) in adults (CAG‐00436N). Available at: http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=272. Accessed June 2, 2014.
- , Therapy for hepatitis C—the costs of success. N Engl J Med. 2014;370:1552–1553.
- , , , et al. Economic model of a birth cohort screening program for hepatitis C. Hepatology. 2012;55:1344–1355.
- , , , Cost‐effectiveness analysis of risk‐factor guided and birth‐cohort screening for chronic hepatitis C infection in the United States. PLoS One. 2013;8:e58975.
- , , , et al. The cost‐effectiveness of birth‐cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270.
- U.S. Department of Health and Human Services. Health Resources and Services Administration: HIV/AIDS programs. Available at: http://hab.hrsa.gov/abouthab/legislation.html. Accessed April 8, 2015.
INTRODUCTION
The baby boomer generation, born from 1945 to 1965, accounts for 75% of the estimated 2.7 to 3.9 million persons with chronic hepatitis C virus (HCV) infection in the US.[1, 2, 3] Most HCV‐infected baby boomers do not know that they are infected.[4] With the advent of better‐tolerated, more‐effective therapies to treat chronic HCV infection,[5] and to reduce rates of complications such as cirrhosis, liver failure, and hepatocellular carcinoma,[6] universal 1‐time screening of baby boomers has been endorsed by the Centers for Disease Control and Prevention (CDC) and the United States Preventive Services Task Force.[1, 7] Hospitalized baby boomers may offer an important target for HCV screening. Our group conducted an anonymous HCV seroprevalence study of nearly 800 patients on general medicine and trauma services of 2 Philadelphia hospitals, and found that 8% had undiagnosed HCV infection, and 8% had diagnosed HCV. [8]
Little is known about barriers and facilitators to implementation of universal HCV screening of baby boomers. Lessons from implementing HIV screening offer a useful guide.[9] First, limited clinician knowledge and confusion about screening guidelines necessitated convenient, well‐designed educational programs.[10] Second, burdensome consent procedures were reduced by opt‐out consent for screening supplemented by patient education.[9] Third, electronic medical record (EMR) algorithms minimized burdens on staff by efficiently identifying and flagging eligible persons for screening.[11] Fourth, ancillary staff support for patient education and linkage to follow‐up care increased screening rates compared with usual care by physicians/staff.[11] Finally, routine human immunodeficiency virus (HIV) testing of inpatients increased rates of diagnosis, especially compared with physician referral systems.[12]
This article describes how HIV screening strategies informed the development in a baby boomer HCV screening and linkage to a care program in a safety‐net hospital serving a majority Hispanic population. We report results of the first 14 months of the screening program and linkage to care for chronically HCV‐infected persons after a minimum 10 months follow‐up. We also estimate costs for program implementation and maintenance to inform hospital administrators, healthcare policymakers, and clinicians about resources that may be required to effectively screen hospitalized baby boomers for HCV.
METHODS
Study Setting
The HCV baby boomer screening program was pilot tested in November 2012 and launched December 1, 2012 in a 498‐bed academic‐affiliated hospital of a healthcare system serving the indigent population of South Texas.
Project Development Phase
From October 1, 2012 to November 30, 2012, project infrastructure development and provider/staff education were conducted. A half‐hour PowerPoint lecture (in person or online) was developed about HCV epidemiology, birth‐cohort HCV screening guidelines, newer treatment modalities, and screening program components. Lectures were delivered to departmental chairs at the affiliated medical school, departmental grand rounds, and the hospital's nursing supervisors. One‐on‐one informational meetings were also held with hospital administrators and staff.
With the hospital's information technology team, screens were developed to identify eligible baby boomers from up to 7 years of previous inpatient and outpatient encounters in the EMR from: birth year (19451965) and no prior diagnosis of HCV infection (070.41, 070.44, 070.51, 070.54, 070.7x, V02.62) or any type of completed test for HCV. The algorithm also excluded patients admitted to psychiatry due to lack of decision‐making capacity or patients with a poor prognosis such as metastatic cancer. An audit of 100 consecutive excluded patients identified all as legitimate.
A new laboratory order for HCV screening was developed by laboratory administrators and pathology faculty for an anti‐HCV antibody test followed by reflex HCV RNA testing for positive results per CDC recommendations.[13] The anti‐HCV test was performed on serum or ethylenediaminetetraacetic acid plasma using the Advia Centaur HCV Assay (Bayer HealthCare LLC, Tarrytown, NY). This assay has excellent sensitivity (99.9%) and specificity (97.5%).[14, 15] The HCV RNA assay was performed using quantitative real‐time polymerase chain reaction (PCR) using the COBAS AmpliPrep/COBAS TaqMan HCV test (Roche Molecular Systems, Pleasanton, CA). Use of plasma preparation tubes (PPTs) (BD Vacutainer PPT tubes; Becton, Dickinson and Co., Franklin Lakes, NJ) permitted both anti‐HCV antibody and HCV PCR testing to be performed on the same specimen when anti‐HCV antibody was detected, eliminating a second blood draw for the PCR test. For patients eligible for screening, an EMR algorithm was created to add an HCV screening order to over 50 different admission order sets.
To educate patients newly diagnosed with HCV infection, we developed an interactive, low‐literacy, educational program in Spanish and English for an electronic tablet device that addressed: HCV epidemiology, transmission prevention, factors that can accelerate chronic HCV infection, and management/treatment strategies. At several points in the program, the patient needed to answer questions correctly to continue. The tablet retained responses linked to a study identification about alcohol consumption, history of past and current illicit drug use, sexual risk behavior, and offered risk reduction messages. The tablet content and presentation reflected suggestions by Hispanic patient‐reviewers about cultural appropriateness and comprehension.
Project Implementation and Maintenance Phase
We report implementation of the program from December 1, 2012 to January 31, 2014. An automated EMR report classified all baby boomers admitted in the previous 24 hours as: (1) eligible with pending screening test order, (2) eligible without an order, (3) ineligible due to prior HCV test or diagnosis, or (4) ineligible due to comorbidity (eg, metastatic cancer). For approximately one‐third of eligible patients, a study team member placed an order after review of the daily admission report because the order had not been automatically placed.
Admitting nurses initially asked for consent from eligible patients for HCV screening, but this was ultimately deemed too onerous a task along with all of their other duties. We then instituted opt‐out consent with patient education about testing and opportunities to refuse via posters placed throughout the hospital and flyers in admission packets. A bilingual HCV counselor provided HCV screening test results to all patients. She counseled patients who screened positive for HCV with the educational program on an electronic tablet and developed a follow‐up care plan.
A bilingual promotora (community health worker) contacted patients newly diagnosed with chronic HCV infection after hospital discharge to address the following: obtaining insurance, access to primary care and HCV specialty care, scheduling appointments, and treatment for alcohol problems or drug abuse. After obtaining signed consent, the promotora sent test results and recommendations for follow‐up care (eg, hepatitis A and B immunization) to a designated outpatient physician and reminded patients about appointments and pending tests. The promotora received training in motivational interviewing skills to engage patients with needed care including alcohol treatment.
Study Data
A summary report was developed from the EMR with demographic, insurance, clinical, and HCV screening data for all admitted baby boomers. For patients diagnosed with chronic HCV infection, the promotora obtained data about follow‐up HCV care through December 10, 2014 from the EMR, outside provider records, and patient reports.
Study Variables
The 2 outcome measures were a positive anti‐HCV antibody test and positive HCV RNA test. Insurance status was categorized as insured (private, public, Veterans Administration, Department of Defense) or uninsured (self‐pay or county‐based financial assistance program). Problem drinking was identified from International Classification of Diseases, Ninth Revision, Clinical Modification codes for the admission, notes by clinicians describing alcohol abuse/dependence, or quantity/frequency meeting National Institute on Alcohol Abuse and Alcoholism criteria for alcohol problems of >14 drinks/week or >4 drinks/day for men and >7 drinks per week or >3 drinks per day for women.[16]
Implementation costs included informatics support, mobile app development, other patient educational materials, costs of screening tests for uninsured, and 0.3 full‐time equivalent (FTE) of a clinician for half a year. Maintenance costs included salaries for the study team, HCV testing costs, and postage.
Analysis
Demographics by HCV antibody test results are compared using [2] tests or Student t tests as appropriate. Among persons with a positive HCV antibody test, HCV RNA results are similarly compared. This implementation project was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board (HSC20130033N).
RESULTS
Within 14 months, 6410 unique baby boomers were admitted with a mean age 56.4 years (standard deviation [SD] 5.7), 55.9% men, 59.1% Hispanic, 8.2% nonwhite, and 46.7% uninsured (Table 1). Among admitted patients, 729 (11.4%) had a previous HCV diagnosis and 1904 (29.7%) had been tested for HCV (Figure 1). Anti‐HCV antibody testing was completed for 3168 (49.4% of all admitted patients and 83.9% of never‐tested patients). After exclusions such as significant comorbidity or psychiatric admission, 95% of eligible persons were tested. Of screened patients, 240 (7.6%) were positive; these patients were significantly younger (P<0.0001) and more likely to be men (P<0.0001) and uninsured (P=0.002) (Table 1). Notably, 10% of men were anti‐HCV positive versus 4% of women. In this predominantly Hispanic cohort, no significant difference appeared by race‐ethnicity, but African Americans had a higher prevalence (10.4%) than other groups.

| Characteristic | All Screened Patients, No. | Anti‐HCV Antibody‐Positive Patients, No. (Row %) | P Value* |
|---|---|---|---|
| |||
| Overall | 3,168 | Total=240 (7.6) | |
| Age, mean (SD) | 57.0 (5.7) | 54.8 (5.0) | <0.0001 |
| Sex | |||
| Men | 1,771 | 185 (10.4) | <0.0001 |
| Women | 1,397 | 55 (3.9) | |
| Race | |||
| Non‐Hispanic white | 1,036 | 86 (8.3) | 0.12 |
| Hispanic | 1,872 | 134 (7.2) | |
| African American | 163 | 17 (10.4) | |
| Other | 97 | 3 (3.1) | |
| Insurance | |||
| Insured | 1,740 | 109 (6.3) | 0.002 |
| Uninsured | 1,428 | 131 (9.2) | |
HCV RNA testing was completed for 214 (89.2%) anti‐HCVpositive patients, of whom 134 (62.6%) had detectable RNA, indicating chronic HCV infection (Figure 1). Overall, 4.2% of all eligible patients tested for HCV were chronically infected. No characteristics were significantly associated with chronic HCV, but persons with chronic infection tended to be younger, uninsured, and African American (Table 2).
| Characteristics | All HCV RNA‐Tested Patients, No. | HCV RNA‐Positive Patients, No. (Row %) | P Value* |
|---|---|---|---|
| |||
| Overall | 214 | 134 (62.6) | |
| Age, y, mean (SD) | 54.6 (5.0) | 54.2 (5.1) | 0.09 |
| Sex | |||
| Men | 165 | 106 (64.2) | 0.37 |
| Women | 49 | 28 (57.1) | |
| Race | |||
| Non‐Hispanic white | 78 | 49 (62.8) | 0.65 |
| Hispanic | 118 | 73 (61.8) | |
| African American | 15 | 11 (73.3) | |
| Other | 3 | 1 (33.3) | |
| Insurance | |||
| Insured | 92 | 52 (56.5) | 0.11 |
| Uninsured | 122 | 82 (67.2) | |
Among patients with chronic HCV infection, 129 (96.3%) were counseled and follow‐up plans developed (Figure 2). By December 10, 2014, 108 (80.6%) patients had received follow‐up primary care, and 52 (38.8%) had care from a hepatologist. Five had initiated HCV‐specific treatment, but many others were awaiting approval for compassionate drug programs offering direct‐acting antivirals. Barriers to care included 82 (61.2%) uninsured, 45 (34%) problem drinkers, 22 (16%) homeless, and 25 (18.6%) incarcerated (not shown). The promotora addressed these issues by visiting homes or homeless shelters, assistance with obtaining county‐based or other types of insurance, offering alcohol risk‐reduction counseling, linking patients to alcohol‐treatment programs, and communicating with the county jail about follow‐up care.

Most of the developmental costs for the program were dedicated to developing EMR programs (Table 3). An optional cost was for the development of the tablet educational program about HCV. In regard to maintenance costs for the first 14 months, the majority was to support the program faculty, counseling/case management, and a nurse practitioner who helped with ordering tests. We also estimated costs for testing uninsured patients (45% of HCV antibody tested, 57% of HCV PCR tested, per Tables 1 and 2, respectively), as they must be borne by the hospital.
| Program Component | Monthly ($) | Total ($) |
|---|---|---|
| ||
| Development phase (2 months prior to start) | ||
| Personnel | ||
| Faculty physicians (0.3 FTE salary+benefits) | 6,641 | 13,282 |
| Role: Development educational materials, provider education, and pilot testing | ||
| Technology | ||
| Development of eligibility screen and order sets for electronic medical record | 41,171 | |
| HCV counseling educational program for tabletdevelopment and pilot testing (optional) | 15,000 | |
| Patient educational materials (posters, flyers) | 400 | |
| Total for development phase | 69,853 | |
| Maintenance phase (14 months) | ||
| Personnel | ||
| Faculty physicians (0.3 FTE, salary+benefits) | 6,641 | 92,974 |
| Role: Coordinate with hospital staff and faculty, liaison with laboratory, supervise study team, review all identified cases for eligibility and management plans | ||
| Inpatient counselor and outpatient case management (2 FTE, salary+benefits) | 6,343 | 88,802 |
| Role: Inpatient and outpatient counseling of HCV Ab+patients and facilitation of follow‐up care for patients with chronic HCV infection | ||
| Nurse practitioner ($35/hour @ 10 hours/month) | 350 | 4,900 |
| Role: Review daily list of admitted baby boomers and manually order HCV screening test for those missed by the automated order | ||
| Postage | 10 | 140 |
| Laboratory costs for uninsured (based on % in cohort) | ||
| HCV antibody in plasma preparation tubes ($13.41/test 1,423) | 19,082 | |
| HCV RNA PCR ($87.96/test 122) | 10,731 | |
| Total for maintenance phase | 216,629 | |
| Total program costs | 286,482 | |
DISCUSSION
Implementation of universal HCV screening and linkage to care for hospitalized baby boomers utilizes a multicomponent infrastructure that reflects lessons learned from similar HIV programs. Use of an EMR algorithm to identify eligible patients and programs to automatically order HCV screening was a linchpin of our high testing rate and averted testing those who did not require screening. Of all 6410 baby boomers admitted to our safety‐net hospital, the EMR screen identified over 40% as ineligible due to prior diagnosis of HCV infection or prior HCV tests. Most of the additional 609 patients who were not tested were excluded due to comorbidities or admission to psychiatry. Overall, the EMR programs, tests ordered by the team, and opt‐out screening with education resulted in screening 95% of eligible patients. However, this program carries substantial costs, nearly $300,000 for the first 2 years, for unreimbursed services in this safety‐net hospital. The new guidelines for HCV screening[1, 2] are not accompanied by financial support either for program implementation or for screening and linkage to care for the uninsured, creating significant financial hurdles to achieve guideline compliance within already overtaxed public healthcare systems.
The infrastructure implemented in this hospital succeeded in achieving a higher rate of HCV screening of baby boomers than reported by other programs. In an emergency department in Birmingham, Alabama, a screening program for baby boomers tested 66% of 2325 persons who were HCV‐unaware.[17] In an outpatient clinic for men who have sex with men, only 54% of 1329 patients were screened for HCV.[18]
Among 3168 screened patients in our cohort, 7.6% were anti‐HCV antibody positive, which is over twice the prevalence of 3.5% (95% confidence interval: 2.2%‐4.8%) for anti‐HCVpositive tests in baby boomers based on National Health and Nutrition Examination Survey (NHANES) data from 2001 to 2010.[19] However, the Alabama emergency department study found that 11% of tested patients were anti‐HCV positive.[17] Although that study lacked race‐ethnicity data for half of the subjects, among those with this information, 13% of black and 7% of white subjects tested anti‐HCV positive. Compared with the Alabama study, the anti‐HCV prevalence in our cohort was somewhat lower for blacks (10.4%) but higher for non‐Hispanic whites (8.3%). Hispanics in our cohort had the lowest anti‐HCV prevalence (7.2%), whereas the Alabama study did not report this figure. National studies also find that the prevalence of anti‐HCVpositive results is twice as high for blacks compared with non‐Hispanic whites and Hispanics, and nearly twice as high for men compared with women.[19] In our cohort, the proportion of men with anti‐HCVpositive results was nearly 3 times that for women.
Diagnosis of chronic HCV infection requires 2 tests, similar to performing a Western blot test after a positive enzyme‐linked immunoassay for HIV. In a Veterans Affairs study, only 64% of patients with a positive anti‐HCV antibody test had a HCV RNA performed when reflex testing was not performed, and patients had to come in for a second test versus >90% of patients in sites that offer reflex testing.[20] At a somewhat increased price due to using more expensive PPTs ($96/100 PPT tubes vs $6.50/100 for serum red top tubes), both tests were performed on the same blood sample, resulting in 89% of anti‐HCV antibody‐positive patients being tested for HCV RNA.
Overall, 62% of patients in our cohort with a positive anti‐HCV antibody test had HCV RNA detected (viremic) compared with 71% of persons aged 20 years in an NHANES study from 2003 to 2010.[21] Several factors may contribute to this lower rate of chronic infection. In a study of HCV seropositive blood donors, Hispanics and non‐Hispanic whites were significantly more likely to have spontaneously cleared HCV infection than Asians and non‐Hispanic blacks.[22] Spontaneous clearance of HCV has also been associated with younger age at infection and HCV genotype 1.[23] Poorly understood genetic factors may also play a role.[24] The high rate of HCV clearance in our cohort reinforces the need to perform HCV RNA testing.
Overall, 4.2% of our cohort had chronic HCV infection. According to CDC estimates from 1999 to 2008 NHANES data, 2.74 million (3.25%) of 84.2 million US baby boomers have been infected with HCV, and 2.04 million (2.4%) have chronic infection.[1] Therefore, our safety‐net cohort of never‐tested baby boomers had over twice the prevalence of chronic HCV infection than the national estimate for this age group. This high proportion of chronic HCV may reflect our predominantly low‐income patient population. An analysis conducted by Milliman, Inc. using 2010 data estimated that half of all persons with undiagnosed HCV infection are uninsured.[25] This finding reinforces the need to conduct HCV screening in acute‐care settings such as hospitals, because the uninsured have poor access to ambulatory care.
Our chronic HCV‐infected cohort had many barriers to follow‐up care because most were uninsured and 15% were homeless. Our counselors addressed socioeconomic barriers to care[26] and concerns about the disease.[27] Many patients also had problem drinking based on either self‐report or documented in the medical record. Even moderate alcohol use may increase the risk of overall and liver‐related mortality from chronic HCV infection,[28] so our team offered brief alcohol counseling and partnered with healthcare providers and local Alcoholics Anonymous programs to offer support.
We linked 80% of newly diagnosed patients to primary care or hepatology providers, aided by a county‐level financial assistance program for healthcare services for uninsured residents, but it still required patients to pay out of pocket for care. Access to newer, highly effective, all‐oral therapy treatment[5] was slowed while awaiting US Food and Drug Administration approval in the first year of this project, then treatment provided only after lengthy applications to drug company assistance programs with priority given to persons with compensated cirrhosis.
Our project raises serious concerns for policymakers and payers. Should universal baby boomer HCV testing be undertaken without taking into account the financial and personnel resources required to implement this screening program or the substantial expenditures necessary to treat chronically infected persons? Although the Centers for Medicaid and Medicare Services pay for HCV screening costs,[29] our hospital had to cover costs for uninsured persons. Admittedly, Texas has the highest proportion of residents who are uninsured in the nation, but even in other states, Medicaid and other insurance programs are wrestling with how to deal with the high cost of HCV therapy.[30]
We acknowledge several limitations of this project. First, it was undertaken in only 1 hospital. Yet, our challenges and solutions are likely to be applicable to other hospitals nationally, especially those serving vulnerable populations. Second, patients in our cohort were usually admitted for comorbidities that needed to be managed before HCV infection could be addressed. However, persons with a poor prognosis, such as metastatic cancer, were excluded. We did not attempt to exclude other persons with serious comorbidities such as congestive heart failure, because the guidelines do not currently recommend this, and there may be benefits for patients, their families, and providers from knowing that an individual is chronically HCV infected even if they are not eligible to be treated. Third, the cost of the program was supported in part by a grant and would otherwise have to be borne by the hospital. Fourth, the EMR used by our hospital allows hundreds of admission order sets to be created and made automated order entry hard to implement. This is unlikely to be the situation in other hospitals using different types of EMRs.
It remains to be seen whether safety‐net hospitals with populations at greater risk of HCV infection can afford to support HCV testing and linkage to care. In view of several cost‐effectiveness studies that find screening and treating chronic HCV‐infected baby boomers cost‐effective within standard thresholds,[31, 32, 33] it may be important for policymakers and payers to consider lessons from HIV programs. Because HIV‐infected persons could not afford life‐saving medication, vigorous advocacy efforts led to legislation approving the Ryan White program in 1990 to fill gaps in HIV care that were not covered by other sources of support.[34] HCV infection is the most common blood‐borne infection in the nation, with potentially devastating consequences if ignored, but the underlying premise that universal HCV testing will save lives is in question if most of the individuals who are diagnosed with chronic HCV are low income, uninsured, or underinsured with limited access to curative medications. A rigorous public policy debate regarding both the merits of screening and the availability of treatment to those who are diagnosed is essential to the success of these programs.
Disclosure
Funding for this study was received from the Centers for Disease Control and Prevention CDC PS12‐1209PPHF12. The authors report no conflicts of interest.
INTRODUCTION
The baby boomer generation, born from 1945 to 1965, accounts for 75% of the estimated 2.7 to 3.9 million persons with chronic hepatitis C virus (HCV) infection in the US.[1, 2, 3] Most HCV‐infected baby boomers do not know that they are infected.[4] With the advent of better‐tolerated, more‐effective therapies to treat chronic HCV infection,[5] and to reduce rates of complications such as cirrhosis, liver failure, and hepatocellular carcinoma,[6] universal 1‐time screening of baby boomers has been endorsed by the Centers for Disease Control and Prevention (CDC) and the United States Preventive Services Task Force.[1, 7] Hospitalized baby boomers may offer an important target for HCV screening. Our group conducted an anonymous HCV seroprevalence study of nearly 800 patients on general medicine and trauma services of 2 Philadelphia hospitals, and found that 8% had undiagnosed HCV infection, and 8% had diagnosed HCV. [8]
Little is known about barriers and facilitators to implementation of universal HCV screening of baby boomers. Lessons from implementing HIV screening offer a useful guide.[9] First, limited clinician knowledge and confusion about screening guidelines necessitated convenient, well‐designed educational programs.[10] Second, burdensome consent procedures were reduced by opt‐out consent for screening supplemented by patient education.[9] Third, electronic medical record (EMR) algorithms minimized burdens on staff by efficiently identifying and flagging eligible persons for screening.[11] Fourth, ancillary staff support for patient education and linkage to follow‐up care increased screening rates compared with usual care by physicians/staff.[11] Finally, routine human immunodeficiency virus (HIV) testing of inpatients increased rates of diagnosis, especially compared with physician referral systems.[12]
This article describes how HIV screening strategies informed the development in a baby boomer HCV screening and linkage to a care program in a safety‐net hospital serving a majority Hispanic population. We report results of the first 14 months of the screening program and linkage to care for chronically HCV‐infected persons after a minimum 10 months follow‐up. We also estimate costs for program implementation and maintenance to inform hospital administrators, healthcare policymakers, and clinicians about resources that may be required to effectively screen hospitalized baby boomers for HCV.
METHODS
Study Setting
The HCV baby boomer screening program was pilot tested in November 2012 and launched December 1, 2012 in a 498‐bed academic‐affiliated hospital of a healthcare system serving the indigent population of South Texas.
Project Development Phase
From October 1, 2012 to November 30, 2012, project infrastructure development and provider/staff education were conducted. A half‐hour PowerPoint lecture (in person or online) was developed about HCV epidemiology, birth‐cohort HCV screening guidelines, newer treatment modalities, and screening program components. Lectures were delivered to departmental chairs at the affiliated medical school, departmental grand rounds, and the hospital's nursing supervisors. One‐on‐one informational meetings were also held with hospital administrators and staff.
With the hospital's information technology team, screens were developed to identify eligible baby boomers from up to 7 years of previous inpatient and outpatient encounters in the EMR from: birth year (19451965) and no prior diagnosis of HCV infection (070.41, 070.44, 070.51, 070.54, 070.7x, V02.62) or any type of completed test for HCV. The algorithm also excluded patients admitted to psychiatry due to lack of decision‐making capacity or patients with a poor prognosis such as metastatic cancer. An audit of 100 consecutive excluded patients identified all as legitimate.
A new laboratory order for HCV screening was developed by laboratory administrators and pathology faculty for an anti‐HCV antibody test followed by reflex HCV RNA testing for positive results per CDC recommendations.[13] The anti‐HCV test was performed on serum or ethylenediaminetetraacetic acid plasma using the Advia Centaur HCV Assay (Bayer HealthCare LLC, Tarrytown, NY). This assay has excellent sensitivity (99.9%) and specificity (97.5%).[14, 15] The HCV RNA assay was performed using quantitative real‐time polymerase chain reaction (PCR) using the COBAS AmpliPrep/COBAS TaqMan HCV test (Roche Molecular Systems, Pleasanton, CA). Use of plasma preparation tubes (PPTs) (BD Vacutainer PPT tubes; Becton, Dickinson and Co., Franklin Lakes, NJ) permitted both anti‐HCV antibody and HCV PCR testing to be performed on the same specimen when anti‐HCV antibody was detected, eliminating a second blood draw for the PCR test. For patients eligible for screening, an EMR algorithm was created to add an HCV screening order to over 50 different admission order sets.
To educate patients newly diagnosed with HCV infection, we developed an interactive, low‐literacy, educational program in Spanish and English for an electronic tablet device that addressed: HCV epidemiology, transmission prevention, factors that can accelerate chronic HCV infection, and management/treatment strategies. At several points in the program, the patient needed to answer questions correctly to continue. The tablet retained responses linked to a study identification about alcohol consumption, history of past and current illicit drug use, sexual risk behavior, and offered risk reduction messages. The tablet content and presentation reflected suggestions by Hispanic patient‐reviewers about cultural appropriateness and comprehension.
Project Implementation and Maintenance Phase
We report implementation of the program from December 1, 2012 to January 31, 2014. An automated EMR report classified all baby boomers admitted in the previous 24 hours as: (1) eligible with pending screening test order, (2) eligible without an order, (3) ineligible due to prior HCV test or diagnosis, or (4) ineligible due to comorbidity (eg, metastatic cancer). For approximately one‐third of eligible patients, a study team member placed an order after review of the daily admission report because the order had not been automatically placed.
Admitting nurses initially asked for consent from eligible patients for HCV screening, but this was ultimately deemed too onerous a task along with all of their other duties. We then instituted opt‐out consent with patient education about testing and opportunities to refuse via posters placed throughout the hospital and flyers in admission packets. A bilingual HCV counselor provided HCV screening test results to all patients. She counseled patients who screened positive for HCV with the educational program on an electronic tablet and developed a follow‐up care plan.
A bilingual promotora (community health worker) contacted patients newly diagnosed with chronic HCV infection after hospital discharge to address the following: obtaining insurance, access to primary care and HCV specialty care, scheduling appointments, and treatment for alcohol problems or drug abuse. After obtaining signed consent, the promotora sent test results and recommendations for follow‐up care (eg, hepatitis A and B immunization) to a designated outpatient physician and reminded patients about appointments and pending tests. The promotora received training in motivational interviewing skills to engage patients with needed care including alcohol treatment.
Study Data
A summary report was developed from the EMR with demographic, insurance, clinical, and HCV screening data for all admitted baby boomers. For patients diagnosed with chronic HCV infection, the promotora obtained data about follow‐up HCV care through December 10, 2014 from the EMR, outside provider records, and patient reports.
Study Variables
The 2 outcome measures were a positive anti‐HCV antibody test and positive HCV RNA test. Insurance status was categorized as insured (private, public, Veterans Administration, Department of Defense) or uninsured (self‐pay or county‐based financial assistance program). Problem drinking was identified from International Classification of Diseases, Ninth Revision, Clinical Modification codes for the admission, notes by clinicians describing alcohol abuse/dependence, or quantity/frequency meeting National Institute on Alcohol Abuse and Alcoholism criteria for alcohol problems of >14 drinks/week or >4 drinks/day for men and >7 drinks per week or >3 drinks per day for women.[16]
Implementation costs included informatics support, mobile app development, other patient educational materials, costs of screening tests for uninsured, and 0.3 full‐time equivalent (FTE) of a clinician for half a year. Maintenance costs included salaries for the study team, HCV testing costs, and postage.
Analysis
Demographics by HCV antibody test results are compared using [2] tests or Student t tests as appropriate. Among persons with a positive HCV antibody test, HCV RNA results are similarly compared. This implementation project was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board (HSC20130033N).
RESULTS
Within 14 months, 6410 unique baby boomers were admitted with a mean age 56.4 years (standard deviation [SD] 5.7), 55.9% men, 59.1% Hispanic, 8.2% nonwhite, and 46.7% uninsured (Table 1). Among admitted patients, 729 (11.4%) had a previous HCV diagnosis and 1904 (29.7%) had been tested for HCV (Figure 1). Anti‐HCV antibody testing was completed for 3168 (49.4% of all admitted patients and 83.9% of never‐tested patients). After exclusions such as significant comorbidity or psychiatric admission, 95% of eligible persons were tested. Of screened patients, 240 (7.6%) were positive; these patients were significantly younger (P<0.0001) and more likely to be men (P<0.0001) and uninsured (P=0.002) (Table 1). Notably, 10% of men were anti‐HCV positive versus 4% of women. In this predominantly Hispanic cohort, no significant difference appeared by race‐ethnicity, but African Americans had a higher prevalence (10.4%) than other groups.

| Characteristic | All Screened Patients, No. | Anti‐HCV Antibody‐Positive Patients, No. (Row %) | P Value* |
|---|---|---|---|
| |||
| Overall | 3,168 | Total=240 (7.6) | |
| Age, mean (SD) | 57.0 (5.7) | 54.8 (5.0) | <0.0001 |
| Sex | |||
| Men | 1,771 | 185 (10.4) | <0.0001 |
| Women | 1,397 | 55 (3.9) | |
| Race | |||
| Non‐Hispanic white | 1,036 | 86 (8.3) | 0.12 |
| Hispanic | 1,872 | 134 (7.2) | |
| African American | 163 | 17 (10.4) | |
| Other | 97 | 3 (3.1) | |
| Insurance | |||
| Insured | 1,740 | 109 (6.3) | 0.002 |
| Uninsured | 1,428 | 131 (9.2) | |
HCV RNA testing was completed for 214 (89.2%) anti‐HCVpositive patients, of whom 134 (62.6%) had detectable RNA, indicating chronic HCV infection (Figure 1). Overall, 4.2% of all eligible patients tested for HCV were chronically infected. No characteristics were significantly associated with chronic HCV, but persons with chronic infection tended to be younger, uninsured, and African American (Table 2).
| Characteristics | All HCV RNA‐Tested Patients, No. | HCV RNA‐Positive Patients, No. (Row %) | P Value* |
|---|---|---|---|
| |||
| Overall | 214 | 134 (62.6) | |
| Age, y, mean (SD) | 54.6 (5.0) | 54.2 (5.1) | 0.09 |
| Sex | |||
| Men | 165 | 106 (64.2) | 0.37 |
| Women | 49 | 28 (57.1) | |
| Race | |||
| Non‐Hispanic white | 78 | 49 (62.8) | 0.65 |
| Hispanic | 118 | 73 (61.8) | |
| African American | 15 | 11 (73.3) | |
| Other | 3 | 1 (33.3) | |
| Insurance | |||
| Insured | 92 | 52 (56.5) | 0.11 |
| Uninsured | 122 | 82 (67.2) | |
Among patients with chronic HCV infection, 129 (96.3%) were counseled and follow‐up plans developed (Figure 2). By December 10, 2014, 108 (80.6%) patients had received follow‐up primary care, and 52 (38.8%) had care from a hepatologist. Five had initiated HCV‐specific treatment, but many others were awaiting approval for compassionate drug programs offering direct‐acting antivirals. Barriers to care included 82 (61.2%) uninsured, 45 (34%) problem drinkers, 22 (16%) homeless, and 25 (18.6%) incarcerated (not shown). The promotora addressed these issues by visiting homes or homeless shelters, assistance with obtaining county‐based or other types of insurance, offering alcohol risk‐reduction counseling, linking patients to alcohol‐treatment programs, and communicating with the county jail about follow‐up care.

Most of the developmental costs for the program were dedicated to developing EMR programs (Table 3). An optional cost was for the development of the tablet educational program about HCV. In regard to maintenance costs for the first 14 months, the majority was to support the program faculty, counseling/case management, and a nurse practitioner who helped with ordering tests. We also estimated costs for testing uninsured patients (45% of HCV antibody tested, 57% of HCV PCR tested, per Tables 1 and 2, respectively), as they must be borne by the hospital.
| Program Component | Monthly ($) | Total ($) |
|---|---|---|
| ||
| Development phase (2 months prior to start) | ||
| Personnel | ||
| Faculty physicians (0.3 FTE salary+benefits) | 6,641 | 13,282 |
| Role: Development educational materials, provider education, and pilot testing | ||
| Technology | ||
| Development of eligibility screen and order sets for electronic medical record | 41,171 | |
| HCV counseling educational program for tabletdevelopment and pilot testing (optional) | 15,000 | |
| Patient educational materials (posters, flyers) | 400 | |
| Total for development phase | 69,853 | |
| Maintenance phase (14 months) | ||
| Personnel | ||
| Faculty physicians (0.3 FTE, salary+benefits) | 6,641 | 92,974 |
| Role: Coordinate with hospital staff and faculty, liaison with laboratory, supervise study team, review all identified cases for eligibility and management plans | ||
| Inpatient counselor and outpatient case management (2 FTE, salary+benefits) | 6,343 | 88,802 |
| Role: Inpatient and outpatient counseling of HCV Ab+patients and facilitation of follow‐up care for patients with chronic HCV infection | ||
| Nurse practitioner ($35/hour @ 10 hours/month) | 350 | 4,900 |
| Role: Review daily list of admitted baby boomers and manually order HCV screening test for those missed by the automated order | ||
| Postage | 10 | 140 |
| Laboratory costs for uninsured (based on % in cohort) | ||
| HCV antibody in plasma preparation tubes ($13.41/test 1,423) | 19,082 | |
| HCV RNA PCR ($87.96/test 122) | 10,731 | |
| Total for maintenance phase | 216,629 | |
| Total program costs | 286,482 | |
DISCUSSION
Implementation of universal HCV screening and linkage to care for hospitalized baby boomers utilizes a multicomponent infrastructure that reflects lessons learned from similar HIV programs. Use of an EMR algorithm to identify eligible patients and programs to automatically order HCV screening was a linchpin of our high testing rate and averted testing those who did not require screening. Of all 6410 baby boomers admitted to our safety‐net hospital, the EMR screen identified over 40% as ineligible due to prior diagnosis of HCV infection or prior HCV tests. Most of the additional 609 patients who were not tested were excluded due to comorbidities or admission to psychiatry. Overall, the EMR programs, tests ordered by the team, and opt‐out screening with education resulted in screening 95% of eligible patients. However, this program carries substantial costs, nearly $300,000 for the first 2 years, for unreimbursed services in this safety‐net hospital. The new guidelines for HCV screening[1, 2] are not accompanied by financial support either for program implementation or for screening and linkage to care for the uninsured, creating significant financial hurdles to achieve guideline compliance within already overtaxed public healthcare systems.
The infrastructure implemented in this hospital succeeded in achieving a higher rate of HCV screening of baby boomers than reported by other programs. In an emergency department in Birmingham, Alabama, a screening program for baby boomers tested 66% of 2325 persons who were HCV‐unaware.[17] In an outpatient clinic for men who have sex with men, only 54% of 1329 patients were screened for HCV.[18]
Among 3168 screened patients in our cohort, 7.6% were anti‐HCV antibody positive, which is over twice the prevalence of 3.5% (95% confidence interval: 2.2%‐4.8%) for anti‐HCVpositive tests in baby boomers based on National Health and Nutrition Examination Survey (NHANES) data from 2001 to 2010.[19] However, the Alabama emergency department study found that 11% of tested patients were anti‐HCV positive.[17] Although that study lacked race‐ethnicity data for half of the subjects, among those with this information, 13% of black and 7% of white subjects tested anti‐HCV positive. Compared with the Alabama study, the anti‐HCV prevalence in our cohort was somewhat lower for blacks (10.4%) but higher for non‐Hispanic whites (8.3%). Hispanics in our cohort had the lowest anti‐HCV prevalence (7.2%), whereas the Alabama study did not report this figure. National studies also find that the prevalence of anti‐HCVpositive results is twice as high for blacks compared with non‐Hispanic whites and Hispanics, and nearly twice as high for men compared with women.[19] In our cohort, the proportion of men with anti‐HCVpositive results was nearly 3 times that for women.
Diagnosis of chronic HCV infection requires 2 tests, similar to performing a Western blot test after a positive enzyme‐linked immunoassay for HIV. In a Veterans Affairs study, only 64% of patients with a positive anti‐HCV antibody test had a HCV RNA performed when reflex testing was not performed, and patients had to come in for a second test versus >90% of patients in sites that offer reflex testing.[20] At a somewhat increased price due to using more expensive PPTs ($96/100 PPT tubes vs $6.50/100 for serum red top tubes), both tests were performed on the same blood sample, resulting in 89% of anti‐HCV antibody‐positive patients being tested for HCV RNA.
Overall, 62% of patients in our cohort with a positive anti‐HCV antibody test had HCV RNA detected (viremic) compared with 71% of persons aged 20 years in an NHANES study from 2003 to 2010.[21] Several factors may contribute to this lower rate of chronic infection. In a study of HCV seropositive blood donors, Hispanics and non‐Hispanic whites were significantly more likely to have spontaneously cleared HCV infection than Asians and non‐Hispanic blacks.[22] Spontaneous clearance of HCV has also been associated with younger age at infection and HCV genotype 1.[23] Poorly understood genetic factors may also play a role.[24] The high rate of HCV clearance in our cohort reinforces the need to perform HCV RNA testing.
Overall, 4.2% of our cohort had chronic HCV infection. According to CDC estimates from 1999 to 2008 NHANES data, 2.74 million (3.25%) of 84.2 million US baby boomers have been infected with HCV, and 2.04 million (2.4%) have chronic infection.[1] Therefore, our safety‐net cohort of never‐tested baby boomers had over twice the prevalence of chronic HCV infection than the national estimate for this age group. This high proportion of chronic HCV may reflect our predominantly low‐income patient population. An analysis conducted by Milliman, Inc. using 2010 data estimated that half of all persons with undiagnosed HCV infection are uninsured.[25] This finding reinforces the need to conduct HCV screening in acute‐care settings such as hospitals, because the uninsured have poor access to ambulatory care.
Our chronic HCV‐infected cohort had many barriers to follow‐up care because most were uninsured and 15% were homeless. Our counselors addressed socioeconomic barriers to care[26] and concerns about the disease.[27] Many patients also had problem drinking based on either self‐report or documented in the medical record. Even moderate alcohol use may increase the risk of overall and liver‐related mortality from chronic HCV infection,[28] so our team offered brief alcohol counseling and partnered with healthcare providers and local Alcoholics Anonymous programs to offer support.
We linked 80% of newly diagnosed patients to primary care or hepatology providers, aided by a county‐level financial assistance program for healthcare services for uninsured residents, but it still required patients to pay out of pocket for care. Access to newer, highly effective, all‐oral therapy treatment[5] was slowed while awaiting US Food and Drug Administration approval in the first year of this project, then treatment provided only after lengthy applications to drug company assistance programs with priority given to persons with compensated cirrhosis.
Our project raises serious concerns for policymakers and payers. Should universal baby boomer HCV testing be undertaken without taking into account the financial and personnel resources required to implement this screening program or the substantial expenditures necessary to treat chronically infected persons? Although the Centers for Medicaid and Medicare Services pay for HCV screening costs,[29] our hospital had to cover costs for uninsured persons. Admittedly, Texas has the highest proportion of residents who are uninsured in the nation, but even in other states, Medicaid and other insurance programs are wrestling with how to deal with the high cost of HCV therapy.[30]
We acknowledge several limitations of this project. First, it was undertaken in only 1 hospital. Yet, our challenges and solutions are likely to be applicable to other hospitals nationally, especially those serving vulnerable populations. Second, patients in our cohort were usually admitted for comorbidities that needed to be managed before HCV infection could be addressed. However, persons with a poor prognosis, such as metastatic cancer, were excluded. We did not attempt to exclude other persons with serious comorbidities such as congestive heart failure, because the guidelines do not currently recommend this, and there may be benefits for patients, their families, and providers from knowing that an individual is chronically HCV infected even if they are not eligible to be treated. Third, the cost of the program was supported in part by a grant and would otherwise have to be borne by the hospital. Fourth, the EMR used by our hospital allows hundreds of admission order sets to be created and made automated order entry hard to implement. This is unlikely to be the situation in other hospitals using different types of EMRs.
It remains to be seen whether safety‐net hospitals with populations at greater risk of HCV infection can afford to support HCV testing and linkage to care. In view of several cost‐effectiveness studies that find screening and treating chronic HCV‐infected baby boomers cost‐effective within standard thresholds,[31, 32, 33] it may be important for policymakers and payers to consider lessons from HIV programs. Because HIV‐infected persons could not afford life‐saving medication, vigorous advocacy efforts led to legislation approving the Ryan White program in 1990 to fill gaps in HIV care that were not covered by other sources of support.[34] HCV infection is the most common blood‐borne infection in the nation, with potentially devastating consequences if ignored, but the underlying premise that universal HCV testing will save lives is in question if most of the individuals who are diagnosed with chronic HCV are low income, uninsured, or underinsured with limited access to curative medications. A rigorous public policy debate regarding both the merits of screening and the availability of treatment to those who are diagnosed is essential to the success of these programs.
Disclosure
Funding for this study was received from the Centers for Disease Control and Prevention CDC PS12‐1209PPHF12. The authors report no conflicts of interest.
- , , , , , Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32.
- 2. Centers for Disease Control and Prevention. Vital signs: evaluation of hepatitis C virus infection testing and reporting–eight U.S. sites, 2005‐2011. MMWR Morb Mortal Wkly Rep. 2013;62:357–361.
- , , , , Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101.
- Institute of Medicine. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press; 2010.
- , Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917.
- , , , et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188.
- U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357.
- , , Undiagnosed hepatitis C on the general medicine and trauma services of two urban hospitals. J Infect. 2009;59:62–69.
- U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51–60.
- , , , et al. Factors affecting clinician educator encouragement of routine HIV testing among trainees. J Gen Intern Med. 2012;27:839–844.
- , , , et al. Counselor‐versus provider‐based HIV screening in the emergency department: Results from the universal screening for HIV infection in the emergency room (USHER) randomized controlled trial. Ann Emerg Med. 2011;58:S126–S132.e1–4.
- , , Approaching the CDC's guidelines on the HIV testing of inpatients: physician‐referral versus nonreferral‐based testing. AIDS Patient Care STDS. 2006;20:311–317.
- Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362–365.
- Advia Centaur Assay Manual. Malvern, PA: Siemens Medical Solutions Diagnostics; Pub# 07063235, Rev. C, 2005‐01.
- , , , Comparison of the ADVIA Centaur and Abbott AxSYM immunoassay systems for a routine diagnostic virology laboratory. J Clin Virol. 2004;30:S11–S15.
- National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Available at: http://www.niaaa.nih.gov/alcohol‐health/overview‐alcohol‐consumption/moderate‐binge‐drinking. Accessed June 12, 2014.
- , , , et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61:776–782.
- , , , et al. Low rates of hepatitis screening and vaccination of HIV‐infected MSM in HIV clinics. Sex Transm Dis. 2012;39:349–353.
- , , , , , The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60:691–698.
- , , , , Viral RNA testing in hepatitis C antibody‐positive veterans. Am J Prev Med. 2009;36:235–238.
- , , , et al. Chronic hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300.
- , , , et al. NHLBI Retrovirus Epidemiology Donor Study (REDS) Group. Correlates of hepatitis C virus (HCV) RNA negativity among HCV‐seropositive blood donors. Transfusion. 2006;46:469–475.
- , , , , , Spontaneous loss of hepatitis C virus RNA from serum is associated with genotype 1 and younger age at exposure. J Med Virol. 2011;83:1338–1344.
- , , , et al. Genetics of spontaneous clearance of hepatitis C virus infection: a complex topic with much to learn. Hepatology. 2014;60:2127–2128.
- , , , Health care reform and hepatitis C: a convergence of risk and opportunity. Available at: http://us.milliman.com/uploadedFiles/insight/2013/convergence‐of‐risk‐and‐opportunity.pdf. Accessed February 5, 2015.
- , , , Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009‐2010. Am J Public Health. 2013;103:112–119.
- , Barriers to hepatitis C treatment. Liver Int. 2012;32:151–156.
- , , , , Moderate, excessive or heavy alcohol consumption: Each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2013;37:703–709.
- Centers for Medicare and Medicaid Services. Proposed decision memo for screening for hepatitis c virus (HCV) in adults (CAG‐00436N). Available at: http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=272. Accessed June 2, 2014.
- , Therapy for hepatitis C—the costs of success. N Engl J Med. 2014;370:1552–1553.
- , , , et al. Economic model of a birth cohort screening program for hepatitis C. Hepatology. 2012;55:1344–1355.
- , , , Cost‐effectiveness analysis of risk‐factor guided and birth‐cohort screening for chronic hepatitis C infection in the United States. PLoS One. 2013;8:e58975.
- , , , et al. The cost‐effectiveness of birth‐cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270.
- U.S. Department of Health and Human Services. Health Resources and Services Administration: HIV/AIDS programs. Available at: http://hab.hrsa.gov/abouthab/legislation.html. Accessed April 8, 2015.
- , , , , , Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32.
- 2. Centers for Disease Control and Prevention. Vital signs: evaluation of hepatitis C virus infection testing and reporting–eight U.S. sites, 2005‐2011. MMWR Morb Mortal Wkly Rep. 2013;62:357–361.
- , , , , Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101.
- Institute of Medicine. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press; 2010.
- , Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917.
- , , , et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188.
- U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357.
- , , Undiagnosed hepatitis C on the general medicine and trauma services of two urban hospitals. J Infect. 2009;59:62–69.
- U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51–60.
- , , , et al. Factors affecting clinician educator encouragement of routine HIV testing among trainees. J Gen Intern Med. 2012;27:839–844.
- , , , et al. Counselor‐versus provider‐based HIV screening in the emergency department: Results from the universal screening for HIV infection in the emergency room (USHER) randomized controlled trial. Ann Emerg Med. 2011;58:S126–S132.e1–4.
- , , Approaching the CDC's guidelines on the HIV testing of inpatients: physician‐referral versus nonreferral‐based testing. AIDS Patient Care STDS. 2006;20:311–317.
- Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362–365.
- Advia Centaur Assay Manual. Malvern, PA: Siemens Medical Solutions Diagnostics; Pub# 07063235, Rev. C, 2005‐01.
- , , , Comparison of the ADVIA Centaur and Abbott AxSYM immunoassay systems for a routine diagnostic virology laboratory. J Clin Virol. 2004;30:S11–S15.
- National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Available at: http://www.niaaa.nih.gov/alcohol‐health/overview‐alcohol‐consumption/moderate‐binge‐drinking. Accessed June 12, 2014.
- , , , et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61:776–782.
- , , , et al. Low rates of hepatitis screening and vaccination of HIV‐infected MSM in HIV clinics. Sex Transm Dis. 2012;39:349–353.
- , , , , , The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60:691–698.
- , , , , Viral RNA testing in hepatitis C antibody‐positive veterans. Am J Prev Med. 2009;36:235–238.
- , , , et al. Chronic hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300.
- , , , et al. NHLBI Retrovirus Epidemiology Donor Study (REDS) Group. Correlates of hepatitis C virus (HCV) RNA negativity among HCV‐seropositive blood donors. Transfusion. 2006;46:469–475.
- , , , , , Spontaneous loss of hepatitis C virus RNA from serum is associated with genotype 1 and younger age at exposure. J Med Virol. 2011;83:1338–1344.
- , , , et al. Genetics of spontaneous clearance of hepatitis C virus infection: a complex topic with much to learn. Hepatology. 2014;60:2127–2128.
- , , , Health care reform and hepatitis C: a convergence of risk and opportunity. Available at: http://us.milliman.com/uploadedFiles/insight/2013/convergence‐of‐risk‐and‐opportunity.pdf. Accessed February 5, 2015.
- , , , Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009‐2010. Am J Public Health. 2013;103:112–119.
- , Barriers to hepatitis C treatment. Liver Int. 2012;32:151–156.
- , , , , Moderate, excessive or heavy alcohol consumption: Each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2013;37:703–709.
- Centers for Medicare and Medicaid Services. Proposed decision memo for screening for hepatitis c virus (HCV) in adults (CAG‐00436N). Available at: http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=272. Accessed June 2, 2014.
- , Therapy for hepatitis C—the costs of success. N Engl J Med. 2014;370:1552–1553.
- , , , et al. Economic model of a birth cohort screening program for hepatitis C. Hepatology. 2012;55:1344–1355.
- , , , Cost‐effectiveness analysis of risk‐factor guided and birth‐cohort screening for chronic hepatitis C infection in the United States. PLoS One. 2013;8:e58975.
- , , , et al. The cost‐effectiveness of birth‐cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270.
- U.S. Department of Health and Human Services. Health Resources and Services Administration: HIV/AIDS programs. Available at: http://hab.hrsa.gov/abouthab/legislation.html. Accessed April 8, 2015.
© 2015 Society of Hospital Medicine
Insomnia in the ICU—and after
Two recent studies—one conducted by researchers from Yale University in New Haven and one conducted by researchers from VA Puget Sound Health Care System in Seattle—suggest it is possible to help patients get better sleep, both in the intensive care unit (ICU) and after ICU discharge. However, protocol and policy changes—as well as education—are needed.
To read the full article, go to Federal Practitioner: http://www.fedprac.com/articles/the-latest/article/insomnia-in-the-icu-and-after/7823e1c993201366966884fa1863b588/ocregister.html.
Two recent studies—one conducted by researchers from Yale University in New Haven and one conducted by researchers from VA Puget Sound Health Care System in Seattle—suggest it is possible to help patients get better sleep, both in the intensive care unit (ICU) and after ICU discharge. However, protocol and policy changes—as well as education—are needed.
To read the full article, go to Federal Practitioner: http://www.fedprac.com/articles/the-latest/article/insomnia-in-the-icu-and-after/7823e1c993201366966884fa1863b588/ocregister.html.
Two recent studies—one conducted by researchers from Yale University in New Haven and one conducted by researchers from VA Puget Sound Health Care System in Seattle—suggest it is possible to help patients get better sleep, both in the intensive care unit (ICU) and after ICU discharge. However, protocol and policy changes—as well as education—are needed.
To read the full article, go to Federal Practitioner: http://www.fedprac.com/articles/the-latest/article/insomnia-in-the-icu-and-after/7823e1c993201366966884fa1863b588/ocregister.html.