User login
Homozygous Familial Hypercholesterolemia: Role of NPs and PAs in Achieving Optimal Outcomes Using Novel Therapeutic Interventions
Click here to read the supplement.
Click here to read the supplement.
Click here to read the supplement.
Case Studies in Toxicology: Double Take—Is Re-exposure Necessary to Explain Delayed Recurrent Opioid Toxicity?
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
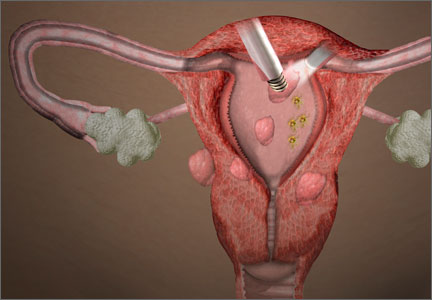
Tissue extraction at minimally invasive surgery: Where do we go from here?
The year 2014 marked a sea change in our approach to tissue extraction during minimally invasive surgery. The US Food and Drug Administration (FDA) initiated this transformation in April, when it issued a safety warning on the use of open power morcellation.1 A flurry of statements on the practice followed from professional societies, capped, in late November, with another statement from the FDA.2–4 The new bottom line: The use of open electromechanical (“power”) morcellation is contraindicated in perimenopausal and postmenopausal women, as well as in those who are known or suspected to have a malignancy.4
Most of the concern to date has centered on the risk that an occult leiomyosarcoma could be morcellated inadvertently during uterine surgery, an event that may worsen the prognosis for the patient. To get a gynecologic oncologist’s take on the controversy, OBG Management caught up with Amanda Nickles Fader, MD, director of the Kelly Gynecologic Oncology Service at Johns Hopkins University. Dr. Fader’s perspective is unique in that she treats a relatively high number of patients who have leiomyosarcoma and other uterine cancers.
In this Q&A, we discuss the patient population at Johns Hopkins; why Dr. Fader is especially qualified to speak to the future of electromechanical morcellation in gynecologic surgery; the benefits and risks of minimally invasive surgery, including tissue extraction; her recommendations for preoperative evaluation and counseling of patients undergoing uterine surgery; and guidance on how the specialty of gynecologic surgery should proceed in the future.
OBG Management: Dr. Fader, by way of introduction, could you characterize your patient population?
Amanda Nickles Fader, MD: Like most gynecologic oncologists, I primarily treat women with cancers of the uterus, ovary, cervix, and vulva. Many of us also have the opportunity to treat a number of women each year with complex benign gynecologic conditions that require surgery. As someone who is extremely interested in rare gynecologic tumors, I also treat a relatively high volume of women diagnosed with uterine sarcoma.
Approximately 75% of the women I see in my practice have preinvasive or invasive cancer, and 25% have a benign condition, such as enlarged fibroids or advanced-stage endometriosis.
OBG Management: How many cases of uterine sarcoma do you encounter on an annual basis?
Dr. Fader: Uterine sarcomas are very rare. They represent only 2% of all uterine cancers. Put into perspective, that means that about 0.4 cases of leiomyosarcoma occur in every 100,000 US women—most commonly postmenopausal women. Leiomyosarcoma is a biologically aggressive, high-grade malignancy that often is lethal.5
Endometrial stroma sarcoma is even less common—only 0.3 cases occur in every 100,000 US women. However, this tumor type is more indolent, often diagnosed at an earlier stage, and potentially curable with surgery (with or without hormonal therapy).6
As a referral center for rare uterine tumors, the Kelly Gynecologic Oncology Service and the Sarcoma Center at Johns Hopkins see approximately 35 to 40 new cases of uterine sarcoma annually for treatment of primary disease or recurrence. An additional 15 to 20 consult cases are reviewed from outside hospitals each year by our gynecologic pathology department.
Why a minimally invasive approach is vital
OBG Management: When it comes to uterine surgery for presumed benign conditions, why is a minimally invasive approach important?
Dr. Fader: Minimally invasive surgery clearly benefits women and is one of the greatest advances of the past half-century within our field. Randomized controlled trials and meta-analyses have demonstrated without question that women who undergo minimally invasive surgery for benign conditions or early-stage cancerous gynecologic conditions have superior clinical outcomes, compared with women who undergo surgery via laparotomy.7,8 These outcomes include fewer perioperative complications (including fewer cases of surgical site infection, venous thromboembolism, wound dehiscence, and hospital readmission), shorter hospital stays, less pain, faster recovery, and fewer adhesions. And when women with early-stage cancers undergo minimally invasive surgery, randomized controlled trials show, they have a stage-specific survival rate similar to that observed in women treated with laparotomy.9
Benefits and risks of tissue extraction in minimally invasive surgery
OBG Management: What are the main benefits of tissue extraction, including morcellation?
Dr. Fader: Tissue extraction is a practice that has allowed us to offer minimally invasive surgery to countless more women than we could have in the recent past. It is a technique in which a large specimen (typically a uterus or fibroid) is fragmented into smaller parts in order to remove it through a small laparoscopic incision or orifice (vagina, umbilicus). Without tissue-extraction practices, thousands of women who undergo myomectomy each year to conserve their fertility and hundreds of thousands of women who require hysterectomy potentially would have to undergo a more painful and risky surgery through a larger abdominal incision. That would not be desirable, as we know conclusively that laparotomy is associated with worse outcomes—and even an increased risk of mortality—compared with minimally invasive surgery performed by experienced surgeons.10
Tissue extraction can be approached in a variety of ways. It can be performed with a scalpel, with a resectoscope, or with an electromechanical morcellator. Tissue extraction can be performed within the uterine cavity, through the vagina, within the abdominal compartment, or within a containment system in any of those compartments.
OBG Management: What are the risks of tissue extraction?
Dr. Fader: The risks of tissue extraction with electromechanical morcellation include potential injury to intra-abdominal organs and vasculature and risk of dissemination of an occult (ie, undiagnosed) uterine cancer. A report by our research group also demonstrated the risks of dissemination of benign uterine tissue requiring subsequent surgery in the setting of open electromechanical morcellation.11 The risk of these events occurring in the hands of a thoughtful and experienced surgeon who conducts comprehensive preoperative patient evaluations is extremely low. However, recent evidence demonstrates that a handful of women worldwide are diagnosed with an occult uterine cancer each year during a morcellation procedure.12–14 Although it is a very rare event (given that most women undergoing hysterectomy and myomectomy procedures are of reproductive age and unlikely to have uterine cancer), this risk is a serious issue. There is an exigent need for the gynecologic surgical community to develop better approaches to tissue extraction that minimize preventable harm in women.
Needed: high-quality data on the risks of morcellation
OBG Management: How does the recent FDA statement urging caution with the use of open power morcellation factor into this equation? The most recent FDA statement noted that open power morcellation is contraindicated in perimenopausal and postmenopausal women.4
Dr. Fader: The FDA’s concern is legitimate. However, the magnitude of the risk of occult uterine sarcoma in women undergoing presumed benign gynecologic surgery has been scrutinized and debated. The FDA panel quoted a risk that roughly 1 in 350 women undergoing presumed benign gynecologic surgery for fibroids will have an occult leiomyosarcoma diagnosed. However, more recent systematic reviews and a review of the prospective published literature demonstrate that the risk is more likely on the order of 1 in 1,700 to 1 in 8,333 women.15 The risk may be even lower in gynecologic surgery practices that see a high volume of hysterectomy/myomectomy cases and utilize meticulous preoperative patient selection criteria to establish a woman’s candidacy for tissue-extraction procedures.
I am concerned with how “occult sarcoma” discovered during surgery for “presumed benign gynecologic disease is being defined in the literature. There is no uniform definition being used. A cancer in this setting is only truly occult or undiagnosed if the physician was thinking about it and made every effort to rule out cancer preoperatively, and the morcellation procedure was performed in a low-risk population (but cancer was still diagnosed on final pathology in this population). However, in the majority of the morcellation studies in the literature, it is not clear that thorough preoperative evaluations occurred in patients to rule out uterine malignancy—in fact, there is a paucity of published information regarding establishing appropriate patient candidacy for morcellation procedures. So we can’t derive any conclusions regarding whether “occult” sarcomas were truly undetectable or not in the published literature.
In addition, the literature is very clear that advancing age and postmenopausal status are risk factors for uterine malignancy.16 The vast majority of uterine sarcoma cases are diagnosed in postmenopausal women. Yet, in one large US population-based hysterectomy study, 20% of the morcellated cases (and the overwhelming majority of the “occult” morcellated uterine cancers) occurred in postmenopausal women!17
Further, in a more recent study by the same authors, again the risks of morcellating a uterine cancer in a population undergoing myomectomy was significantly higher in a postmenopausal population and occurred only rarely in women younger than age 40. But it should come as no surprise that a greater incidence of uterine cancer was identified in these older cohorts—cancer risk increases precipitously with age. That’s not “occult”; that’s basic cancer epidemiology.
In other words, we cannot assume from population-based administrative claims data that morcellation performed in inappropriate populations at higher risk of uterine malignancy (in which we do not know whether patients were properly screened for the procedure preoperatively or whether they had risk factors for uterine cancer but were presumably poor candidates for morcellation due to age alone) helps us define the true incidence of “occult” sarcoma or cancer in a population.
These studies are provocative, however, and do inform us that, as women get older, we are apt to see a greater incidence of uterine cancer. We cannot safely assume that a postmenopausal or elderly woman with symptomatic or enlarging fibroids has “presumed benign disease”—it is cancer until proven otherwise, and we need to be looking for it preoperatively. Therefore, we need to be particularly careful with our surgical practices in this population—ie, the basis for the FDA’s recommendation to avoid morcellation in older women. And I agree with the FDA that open electromechanical morcellation generally is contraindicated in postmenopausal women. However, we need better data from large prospective studies to inform our understanding of the true incidence of undiagnosed or “occult” uterine sarcoma in women undergoing surgery for presumed benign disease. These future studies are likely to demonstrate what we already know—that in young, well-screened, well-selected candidates for minimally invasive hysterectomy or myomectomy, the risk of occult cancer is going to be exceptionally low.
OBG Management: Which is greater—the risks or benefits of tissue extraction?
Dr. Fader: Assessment of risks and benefits in medicine has everything to do with the intervention in question as applied to an individual patient. At the end of the day, there are risks and benefits to every medical or surgical treatment offered to patients in every medical and surgical discipline. But the risk of an occult uterine sarcoma is extremely low in a woman of reproductive age who has been properly selected and comprehensively evaluated for minimally invasive surgery and tissue extraction prior to surgery. And this small—though not negligible—risk must be weighed against the much higher risk of harm that may be incurred with an open abdominal procedure, compared with minimally invasive surgery.
However, in many elderly women, the risks of tissue extraction with an electromechanical morcellator may outweigh the benefits. Even so, there are exceptions in which tissue extraction may be acceptable in postmenopausal women (ie, using alternative tissue-extraction methods in those undergoing minimally invasive supracervical hysterectomy and sacral colpopexy for pelvic organ prolapse).
Few of the data published on the risks of morcellation are of very high quality in terms of scientific rigor or methodology. The best thing we can do as a gynecologic surgical community is conduct sound quality-improvement (QI) programs, disseminate our QI results, publish our data, establish guidelines for best practices in uterine tissue extraction, and collaborate readily to increase the scholarly output on this issue so that national societies and government regulatory agencies have better-quality data to inform future policy on this issue.
A case-based approach
OBG Management: How would you approach tissue extraction in the following case?
CASE: A desire for myomectomy
A 35-year-old woman (G1P1) who delivered by cesarean has an 8-cm symptomatic intramural fibroid. She has regular heavy periods that have led to anemia (hemoglobin level = 10 mg/dL). Her medical history is negative for malignancy, pelvic radiation, or tamoxifen use, and she wants to preserve her fertility. Magnetic resonance imaging (MRI) confirms an 8-cm fibroid. Endometrial biopsy results are negative.
Dr. Fader: At Johns Hopkins, as at many other centers, we use well-defined criteria to determine whether a minimally invasive approach (and tissue extraction) might be appropriate. We also individualize treatment and surgical decision-making on a case-by-case basis. Any candidate for minimally invasive surgery and tissue extraction for uterine fibroids must undergo a thorough preoperative assessment.
Johns Hopkins preoperative assessment criteria include:
- endometrial biopsy
- imaging (often MRI)
- a detailed history and physical, with a comprehensive review of risk factors for malignancy, including family and genetic history or a personal history of malignancy, pelvic radiation, tamoxifen use, or BRCA or hereditary nonpolyposis colorectal cancer (HNPCC) deleterious mutation carrier status, among other things.
- In addition, all cases are discussed at a peer-reviewed, preoperative conference to ensure that a thorough work-up has been conducted and to verify the patient’s candidacy for a minimally invasive procedure with tissue extraction. As the FDA recommends, we conduct an enhanced informed consent process and counsel patients being considered for tissue extraction about the risk of occult sarcoma.
Our top priority is patient safety, so until more data are available, we no longer perform open electromechanical morcellation. We perform all tissue extraction within a containment system and under institutional review board protocol. We primarily perform tissue extraction via scalpel morcellation.
OBG Management: How do the patient’s wishes factor into the decision to perform minimally invasive surgery with morcellation?
Dr. Fader: Our patients make their own decisions regarding surgical approach and procedure after undergoing extensive counseling about the risks and benefits of the proposed procedures. I certainly would offer a patient like the one described in this case the opportunity for a minimally invasive approach (if, after thorough preoperative evaluation, she were deemed to be at very low risk for uterine malignancy). In my experience, most women opt for the minimally invasive approach in this setting; however, if a patient declines minimally invasive surgery, I respect her decision.
Tissue extraction in perimenopause
CASE: A desire for myomectomy at age 48
OBG Management: How would you approach the same case if the patient were a 48-year-old perimenopausal woman?
Dr. Fader: In perimenopausal women, we are more selective about performing morcellation, given the recent FDA safety statement, and because the incidence of occult cancer starts to increase slightly in this patient cohort (although it doesn’t precipitously increase until well into the postmenopausal period).
In addition, myomectomy may have less value in a 48-year-old, given the lower likelihood of achieving successful fertility, although there are exceptions. US cancer statistics and studies on morcellation demonstrate that the vast majority of women in their 30s and 40s have an extremely low risk for sarcomas and other uterine malignancies.2
In select cases in which a woman has undergone a comprehensive preoperative work-up, has a stable-appearing fibroid(s), and is well-educated and counseled about the pros and cons of morcellation, we would consider performing a procedure with contained tissue extraction. As a general matter, however, I would be more inclined to offer a 48-year-old in this situation a uterine artery embolization or minimally invasive hysterectomy than a myomectomy procedure, especially given the recent study by Wright and colleagues demonstrating the significantly increased risks of uterine cancer at myomectomy surgery for a woman in her late 40s or early 50s.18
Preoperative assessment should be comprehensive
OBG Management: What preoperative evaluation do you perform when tissue extraction, including morcellation, is an issue?
Dr. Fader: It is the policy at Johns Hopkins that all women being considered for minimally invasive surgery and tissue extraction must undergo a rigorous preoperative work-up that includes:
- a comprehensive history and physical (to exclude malignancy and risk for occult malignancy)
- an endometrial evaluation (most commonly, an endometrial biopsy)
- uterine imaging (with longitudinal evaluation of imaging findings if performed previously)
- discussion of each patient case at peer-reviewed, preoperative department conferences.
We have separate conferences for the gynecology and gynecologic oncology services and have employed this practice for many years at Hopkins. We are also studying the role of serum lactate dehydrogenase (LDH) isoenzyme levels in stratifying women with uterine fibroids versus cancer/sarcoma.19
OBG Management: Which patients would you exclude from the electromechanical morcellation option?
Dr. Fader: As the American College of Obstetricians and Gynecologists, the AAGL, the Society of Gynecologic Oncology, and the Society of Gynecologic Surgeons appear to agree:
- When utilized in select patients of reproductive age, minimally invasive surgery and morcellation are beneficial.
- Morcellation should categorically not be performed in any woman who has a known or suspected uterine (or other gynecologic) malignancy.2,3
At our institution, we have significantly curtailed the use of electromechanical morcellation at this time and especially do not perform it in women aged 50 or older or in those with confirmed postmenopausal status. We also do not perform electromechanical morcellation in women with a personal history of uterine, cervical, or ovarian preinvasive or invasive cancer, or in women with a strong family history of gynecologic malignancy.
Other populations we exclude are women with:
- a history of mitotically active or atypical fibroids (as determined at previous myomectomy)
- known BRCA or HNPCC deleterious mutation, hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, hereditary childhood retinoblastoma, or other genetic predisposition to uterine or ovarian cancer
- a history of pelvic radiation
- a history of tamoxifen use.
Counseling the patient
OBG Management: If tissue extraction is an issue, and morcellation will be necessary for a minimally invasive approach, how do you counsel the patient?
Dr. Fader: We have an informed consent protocol we use at Hopkins in this regard. We speak extensively to our patients about the fact that every procedure or intervention performed in medicine carries a benefit/risk ratio. We inform patients of the FDA morcellation safety statement—that their fibroid or fibroids may contain unexpected cancerous tissue and that laparoscopic electromechanical morcellation may spread the cancer and possibly worsen their prognosis. We also explain that, while the FDA quotes a risk of approximately 1 in 350 for occult sarcoma, that this data review was somewhat limited in scope and included postmenopausal women in the estimates. Based on the best available published systematic reviews and internal Hopkins data, we believe the risk of morcellating an occult uterine malignancy in a woman of reproductive age is more likely on the order of approximately 1 in 1,700 to 1 in 8,333.
We discuss our institution’s approach to tissue extraction (ie, that we no longer perform open electromechanical morcellation but instead perform contained tissue extraction on an institutional review board protocol). We tell patients that contained tissue-extraction practices are still experimental, although there is published literature preliminarily supporting the safety of the practice. In addition, we discuss the fact that contained tissue extraction has been used for years to safely remove large intra-abdominal specimens from laparoscopic incisions, from adnexal tissue to gallbladder, spleen, kidney, intestinal, and appendiceal specimens.
We also explain that, as physicians, we do our best to ensure that risks are minimized and reasonable in relation to anticipated benefits but that, even when we use the very best diagnostic measures, no test is 100% sensitive or specific to rule out malignancy in this setting (or in any setting, actually).
Finally, we discuss the fact that minimally invasive surgery and tissue extraction practices performed in appropriate patients by skilled surgeons conclusively benefit hundreds of thousands of women each year around the world. That is a narrative that has been somewhat lacking in the recent dialogue about tissue-extraction practices.
OBG Management: What do we know about outcomes when a leiomyosarcoma is inadvertently morcellated?
Dr. Fader: This is considered a “cut-through” procedure, in that a cancerous tumor that is potentially contained to an organ is not removed intact or with clean margins. The morcellation procedure effectively cuts through the occult cancer, which is not desirable. Intact surgical removal of uterine cancers or sarcomas is the mainstay of therapy for these malignancies, based on NCCN guidelines.22
OBG Management: Does a morcellated leiomyosarcoma carry a worse prognosis than an unmorcellated leiomyosarcoma?
Dr. Fader: When it comes to morcellated versus “intact” uterine leiomyosarcoma, we enter a largely data-free zone. We don’t know with certainty whether the outcome is worsened. If we’re being intellectually honest, we must admit the possibility that a morcellated cancer is more likely to be disseminated, rendering it potentially more difficult to treat. However, sarcomas primarily spread by hematogenous dissemination. It is quite possible that even the act of incising an intact fibroid in an open abdominal procedure or performing a supracervical or total hysterectomy without morcellation may still result in hematogenous cancer dissemination. So there is no indication yet that electromechanical morcellation poses a unique and higher risk of cancer upstaging or worse prognosis compared with techniques such as open myomectomy, supracervical hysterectomy, or hysteroscopic myomectomy.
In addition, the prognosis associated with even early-stage uterine leiomyosarcoma is uniformly poor. A published study from Hopkins that included 108 patients with uterine leiomyosarcoma suggests that the recurrence rate and survival of patients with early-stage, “intact” leiomyosarcoma are very poor and comparable to the survival rate documented in the literature for women with morcellated sarcomas.23
The few retrospective studies that exist suggesting worse outcomes with morcellation have limitations that preclude any definitive conclusions.2 These studies are marred by small numbers, heterogeneity in morcellation practices, poor follow-up times, and a lack of detail regarding how patients with morcellated versus unmorcellated sarcomas were treated. Nevertheless, a couple of these small retrospective reports indicate the possibility of worse outcomes in women with morcellated uterine sarcomas, compared with historical controls with intact sarcoma removal.
Is the morcellator at fault—or the user?
OBG Management: Some would argue that even one case of a morcellated uterine sarcoma is too many. How would you respond?
Dr. Fader: There is no doubt that a handful of women each year have been harmed by morcellation practices. Those women deserve our dedication and best efforts to learn how to better treat morcellated sarcomas—and more importantly—how to mitigate the risks associated with morcellation practices and reduce the risk of preventable harm for all women undergoing minimally invasive fibroid procedures. I think the single best thing we can do to mitigate risk is to be more conscientious about selecting our patients for tissue-extraction procedures (ie, strict selection criteria, appropriate preoperative work-ups). If we did this, we likely would reduce the number of oncologic morcellator mishaps by 50% to 80% without changing anything else.
When we closely scrutinize the literature, and when I reflect on the women with morcellated cancers that we have cared for at Hopkins, we observe that some (but not all) “occult” uterine cancers were not that hidden after all and may have been detected preoperatively if an effort had been made.
We have noted a number of patients in this setting who experienced harm not because a specific device was used to cut up their uterus but because they were never appropriate candidates for the procedure in the first place. For instance, I recently cared for an elderly woman with a morcellated uterine cancer who underwent a laparoscopic supracervical hysterectomy without an appropriate preoperative work-up (ie, no endometrial biopsy or imaging) or informed consent about the possibility that she might have cancer. If an elderly woman presents with concerning symptoms related to her uterus (ie, enlargement, bleeding), she must be evaluated and counseled regarding the considerable risk of potential malignancy. Even in the setting of a normal work-up, I don’t believe it is a good idea to perform electromechanical morcellation in higher-risk women, including elderly women. That does not mean that select, well-screened women cannot be considered for alternative tissue-extraction techniques, but the risks and benefits must be carefully weighed in each patient, and informed consent must be obtained.
By continuing to refine the safety features of the electromechanical morcellator devices and choosing patients more carefully for minimally invasive procedures and tissue extraction, we likely will reduce the risk of preventable harm in women undergoing gynecologic surgery.
Can leiomyosarcoma be detected preoperatively?
OBG Management: How can we improve preoperative detection of uterine malignancy, particularly leiomyosarcoma?
Dr. Fader: For starters, we can improve detection of uterine cancers by simply looking for them. Almost all epithelial uterine cancers will be detectable by endometrial sampling. A comprehensive history and physical and uterine imaging also may be helpful. And although they are more difficult to detect than epithelial uterine cancers, it is a myth that sarcomas cannot be diagnosed preoperatively. Investigators from Columbia University retrospectively evaluated the ability to preoperatively detect epithelial uterine cancers and uterine sarcomas on final pathology. In 72 women who were ultimately diagnosed with a sarcoma, preoperative endometrial sampling suggested an invasive tumor in 86% and predicted the correct histology in 64%. In fact, the rate of detection of invasive cancer by preoperative sampling was not statistically different among sarcomas than it was among epithelial uterine cancers, although there was less of a correlation with appropriate histology seen with endometrial biopsy.20
That being said, sarcomas can be missed, especially in younger women who have extremely large, degenerated, or necrotic-appearing fibroids. Improvements in diagnostic testing are desperately needed to help distinguish benign fibroids from sarcomas, as there are no reliable modalities to exclude a sarcoma at this time. MRI appears to be the most useful imaging modality, although it cannot definitively distinguish a fibroid from a sarcoma. However, a fairly constant finding in leiomyosarcomas is the absence of calcifications. Further, some studies also suggest that ill-defined margins are consistent with a sarcoma. Finally, several centers, including our own, are studying novel biologic markers and revisiting the utility of previously described markers such as LDH in the preoperative detection of uterine sarcoma.21
The way forward
OBG Management: You have said, “Keep patients informed and safe but avoid being too reactionary.” Could you expand on this statement?
Dr. Fader: Certainly. There are many examples throughout the history of medicine in which treatments have brought benefit to thousands or millions of individuals but may cause harm in a select few. We know that when controversial medical issues have arisen in the past, the pendulum has swung widely in terms of societal response.
For example, the landmark Women’s Health Initiative had an immediate and adverse impact on hormone replacement therapy (HRT) administration. The increased risk of cardiovascular disease and breast cancer observed with use of long-term combination therapy with conjugated equine estrogen and medroxyprogesterone acetate prompted many US health-care providers to abandon use of HRT—until more contemporary data demonstrated that, in younger, healthier postmenopausal women, these adverse events were very rare and the benefits of HRT outweighed the risks.
Can we avoid stroke, heart attack, and breast cancer in all younger women taking HRT? Of course not. But we counsel women about the benefit/risk ratio of the therapy and advise them that the likelihood of these events is rare.
Similarly, as with the HRT analogy, younger women have lower risks associated with surgery and morcellation, compared with older women, and are more likely to derive benefit from the procedure (after ensuring appropriate candidacy with a comprehensive preoperative evaluation and informed consent).
However, if the expectation is that no cases of harm will ever occur with a surgical device or procedure in order for it to be deemed acceptable and safe to use in practice, then that is simply an impossible standard to uphold. There is no device, medication, or intervention I know of in medicine that is completely risk-free.
OBG Management: Are there other examples of this type of benefit/risk assessment?
Dr. Fader: Yes. For instance, tamoxifen is a nonsteroidal anti-estrogen agent approved by the FDA for adjuvant treatment of breast cancer, treatment of metastatic breast cancer, and reduction of breast cancer incidence in high-risk women. Tamoxifen has effectively reduced breast cancer rates and significantly improved survival in select breast cancer patients. Yet, it is well known that long-term use of tamoxifen is associated with a twofold increased risk of uterine cancer and uterine sarcoma—a likely far more commonly occurring adverse event than an occult, morcellated uterine sarcoma during a minimally invasive gynecologic procedure.
Should the FDA ban or significantly curtail the use of tamoxifen? No, not likely, because the benefits far outweigh the risks in previvors and hormone-positive breast cancer survivors. “Keep patients informed and safe but avoid being too reactionary” means that we must do our due diligence as physicians by comprehensively counseling and obtaining informed consent from our patients before performing medical interventions. We also must closely scrutinize and improve upon practices that may cause harm.
However, what this also means is that while it may be prudent to restrict or limit a surgical practice in select higher-risk populations or modify it in some way to make it safer, we shouldn’t necessarily completely abandon or ban a practice that has benefited hundreds of thousands of patients at low risk of harm until we have objectively reviewed all of the available science and fully understand the implications of a practice change (ie, would the risks of preventable harm be even greater for women if more of them had to undergo open abdominal surgery?). We need continued cool heads and sound scientific reasoning to decide upon health-care policy changes or treatment paradigm shifts.
At the end of the day, however, it is paramount that we mitigate patient harm. The subject of tissue extraction during minimally invasive surgery is a complex and nuanced issue that merits continued study and open-minded and intelligent dialogue between patient stakeholders, clinicians, scientists, industry, ethicists, regulatory agencies, and the press. I think we can all appreciate how humbling and challenging the morcellation issue has been for many of us, especially our patients—particularly the unfortunate women who have been diagnosed with a uterine sarcoma.
Like many of my colleagues, I have been privileged to care for a number of women with uterine leiomyosarcoma. It is a devastating disease, and the prognosis is very poor, whether it is morcellated or removed intact. We are fortunate that the vast majority of women who undergo a minimally invasive procedure for fibroids or other presumed benign indications will not be at risk for an occult malignancy. But what can we do now to continue to offer the benefits of minimally invasive surgery and tissue extraction to women while simultaneously reducing the risk to the select few who will develop a rare uterine cancer?
We can all make a greater effort to more carefully select our patients for minimally invasive surgery and tissue extraction, to limit the performance of open electromechanical morcellation and collaborate in studying the role of refined tissue-extraction techniques and containment systems, to enhance the informed consent process, to develop improved diagnostic tests for preoperative cancer detection, and to conduct higher-quality studies on minimally invasive tissue-extraction techniques for regulatory agencies to review in the near future. It also goes without saying that we need more federal funding to study rare tumors (and gynecologic cancers in general) and develop better sarcoma treatments.
Although there is no medical treatment or surgical procedure that is completely risk-free, interventions such as HRT, tamoxifen, and uterine morcellation—when used in appropriate patients and for appropriate indications—will allow preventable harm to be minimized and make it possible for countless women to continue to derive tremendous benefit.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed February 3, 2014.
2. AAGL. Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed February 3, 2015.
3. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery: a special report. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery. Published May 2014. Accessed February 3, 2015.
4. US Food and Drug Administration. Updated Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Published November 24, 2014. Accessed February 2, 2015.
5. American Cancer Society. Uterine sarcoma: What is uterine sarcoma? http://www.cancer.org/cancer/uterinesarcoma/detailedguide/uterine-sarcoma-what-is-uterine-sarcoma. Updated January 12, 2015. Accessed February 3, 2015.
6. D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–139.
7. Chittawar B, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;10:CD004638. doi:10.1002/14651858.CD004638.pub3.
8. Mori KM, Neubauer NL. Minimally invasive surgery in gynecologic oncology. ISRN Obstet Gynecol. 2013; article ID 312982. http://dx.doi.org/10.1155/2013/312982. Accessed February 2, 2015.
9. Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib IIa cervical cancer. Gynecol Oncol. 2007;105(1):176–180.
10. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study Lap2. J Clin Oncol. 2009;27(32):5331–5336.
11. Ramos A, Fader AN, Long Roche K. Surgical cytoreduction for disseminated benign disease after open power uterine morcellation. Obstet Gynecol. 2014;125(1):99–102.
12. Theben J, Schellong A, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): an analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
13. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
14. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7(11):e50058.
15. Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33.
16. Wright JD, Tergas AI, Burke WM, et al. Prevalence of uterine pathology in women undergoing minimally invasive hysterectomy employing electric power morcellation. JAMA. 2014;312(12):1253–1255.
17. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312(12):1253–1255.
18. Wright JD, Tergas AI, Cul R, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncol. 2015; published online February 19, 2015. doi:10.1001/jamaoncol.2014.206.
19. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002; 12:354.
20. Ricci S, Giuntoli RL 2nd, Eisenhauer E, et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol Oncol. 2013;131(3):629–633.
21. Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008;110(1):43–48.
22. Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12(2):248–280.
23. Schwartz LB, Zawin M, Carcangiu ML, Lange R, McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 1998;70(3):580–587.
The year 2014 marked a sea change in our approach to tissue extraction during minimally invasive surgery. The US Food and Drug Administration (FDA) initiated this transformation in April, when it issued a safety warning on the use of open power morcellation.1 A flurry of statements on the practice followed from professional societies, capped, in late November, with another statement from the FDA.2–4 The new bottom line: The use of open electromechanical (“power”) morcellation is contraindicated in perimenopausal and postmenopausal women, as well as in those who are known or suspected to have a malignancy.4
Most of the concern to date has centered on the risk that an occult leiomyosarcoma could be morcellated inadvertently during uterine surgery, an event that may worsen the prognosis for the patient. To get a gynecologic oncologist’s take on the controversy, OBG Management caught up with Amanda Nickles Fader, MD, director of the Kelly Gynecologic Oncology Service at Johns Hopkins University. Dr. Fader’s perspective is unique in that she treats a relatively high number of patients who have leiomyosarcoma and other uterine cancers.
In this Q&A, we discuss the patient population at Johns Hopkins; why Dr. Fader is especially qualified to speak to the future of electromechanical morcellation in gynecologic surgery; the benefits and risks of minimally invasive surgery, including tissue extraction; her recommendations for preoperative evaluation and counseling of patients undergoing uterine surgery; and guidance on how the specialty of gynecologic surgery should proceed in the future.
OBG Management: Dr. Fader, by way of introduction, could you characterize your patient population?
Amanda Nickles Fader, MD: Like most gynecologic oncologists, I primarily treat women with cancers of the uterus, ovary, cervix, and vulva. Many of us also have the opportunity to treat a number of women each year with complex benign gynecologic conditions that require surgery. As someone who is extremely interested in rare gynecologic tumors, I also treat a relatively high volume of women diagnosed with uterine sarcoma.
Approximately 75% of the women I see in my practice have preinvasive or invasive cancer, and 25% have a benign condition, such as enlarged fibroids or advanced-stage endometriosis.
OBG Management: How many cases of uterine sarcoma do you encounter on an annual basis?
Dr. Fader: Uterine sarcomas are very rare. They represent only 2% of all uterine cancers. Put into perspective, that means that about 0.4 cases of leiomyosarcoma occur in every 100,000 US women—most commonly postmenopausal women. Leiomyosarcoma is a biologically aggressive, high-grade malignancy that often is lethal.5
Endometrial stroma sarcoma is even less common—only 0.3 cases occur in every 100,000 US women. However, this tumor type is more indolent, often diagnosed at an earlier stage, and potentially curable with surgery (with or without hormonal therapy).6
As a referral center for rare uterine tumors, the Kelly Gynecologic Oncology Service and the Sarcoma Center at Johns Hopkins see approximately 35 to 40 new cases of uterine sarcoma annually for treatment of primary disease or recurrence. An additional 15 to 20 consult cases are reviewed from outside hospitals each year by our gynecologic pathology department.
Why a minimally invasive approach is vital
OBG Management: When it comes to uterine surgery for presumed benign conditions, why is a minimally invasive approach important?
Dr. Fader: Minimally invasive surgery clearly benefits women and is one of the greatest advances of the past half-century within our field. Randomized controlled trials and meta-analyses have demonstrated without question that women who undergo minimally invasive surgery for benign conditions or early-stage cancerous gynecologic conditions have superior clinical outcomes, compared with women who undergo surgery via laparotomy.7,8 These outcomes include fewer perioperative complications (including fewer cases of surgical site infection, venous thromboembolism, wound dehiscence, and hospital readmission), shorter hospital stays, less pain, faster recovery, and fewer adhesions. And when women with early-stage cancers undergo minimally invasive surgery, randomized controlled trials show, they have a stage-specific survival rate similar to that observed in women treated with laparotomy.9
Benefits and risks of tissue extraction in minimally invasive surgery
OBG Management: What are the main benefits of tissue extraction, including morcellation?
Dr. Fader: Tissue extraction is a practice that has allowed us to offer minimally invasive surgery to countless more women than we could have in the recent past. It is a technique in which a large specimen (typically a uterus or fibroid) is fragmented into smaller parts in order to remove it through a small laparoscopic incision or orifice (vagina, umbilicus). Without tissue-extraction practices, thousands of women who undergo myomectomy each year to conserve their fertility and hundreds of thousands of women who require hysterectomy potentially would have to undergo a more painful and risky surgery through a larger abdominal incision. That would not be desirable, as we know conclusively that laparotomy is associated with worse outcomes—and even an increased risk of mortality—compared with minimally invasive surgery performed by experienced surgeons.10
Tissue extraction can be approached in a variety of ways. It can be performed with a scalpel, with a resectoscope, or with an electromechanical morcellator. Tissue extraction can be performed within the uterine cavity, through the vagina, within the abdominal compartment, or within a containment system in any of those compartments.
OBG Management: What are the risks of tissue extraction?
Dr. Fader: The risks of tissue extraction with electromechanical morcellation include potential injury to intra-abdominal organs and vasculature and risk of dissemination of an occult (ie, undiagnosed) uterine cancer. A report by our research group also demonstrated the risks of dissemination of benign uterine tissue requiring subsequent surgery in the setting of open electromechanical morcellation.11 The risk of these events occurring in the hands of a thoughtful and experienced surgeon who conducts comprehensive preoperative patient evaluations is extremely low. However, recent evidence demonstrates that a handful of women worldwide are diagnosed with an occult uterine cancer each year during a morcellation procedure.12–14 Although it is a very rare event (given that most women undergoing hysterectomy and myomectomy procedures are of reproductive age and unlikely to have uterine cancer), this risk is a serious issue. There is an exigent need for the gynecologic surgical community to develop better approaches to tissue extraction that minimize preventable harm in women.
Needed: high-quality data on the risks of morcellation
OBG Management: How does the recent FDA statement urging caution with the use of open power morcellation factor into this equation? The most recent FDA statement noted that open power morcellation is contraindicated in perimenopausal and postmenopausal women.4
Dr. Fader: The FDA’s concern is legitimate. However, the magnitude of the risk of occult uterine sarcoma in women undergoing presumed benign gynecologic surgery has been scrutinized and debated. The FDA panel quoted a risk that roughly 1 in 350 women undergoing presumed benign gynecologic surgery for fibroids will have an occult leiomyosarcoma diagnosed. However, more recent systematic reviews and a review of the prospective published literature demonstrate that the risk is more likely on the order of 1 in 1,700 to 1 in 8,333 women.15 The risk may be even lower in gynecologic surgery practices that see a high volume of hysterectomy/myomectomy cases and utilize meticulous preoperative patient selection criteria to establish a woman’s candidacy for tissue-extraction procedures.
I am concerned with how “occult sarcoma” discovered during surgery for “presumed benign gynecologic disease is being defined in the literature. There is no uniform definition being used. A cancer in this setting is only truly occult or undiagnosed if the physician was thinking about it and made every effort to rule out cancer preoperatively, and the morcellation procedure was performed in a low-risk population (but cancer was still diagnosed on final pathology in this population). However, in the majority of the morcellation studies in the literature, it is not clear that thorough preoperative evaluations occurred in patients to rule out uterine malignancy—in fact, there is a paucity of published information regarding establishing appropriate patient candidacy for morcellation procedures. So we can’t derive any conclusions regarding whether “occult” sarcomas were truly undetectable or not in the published literature.
In addition, the literature is very clear that advancing age and postmenopausal status are risk factors for uterine malignancy.16 The vast majority of uterine sarcoma cases are diagnosed in postmenopausal women. Yet, in one large US population-based hysterectomy study, 20% of the morcellated cases (and the overwhelming majority of the “occult” morcellated uterine cancers) occurred in postmenopausal women!17
Further, in a more recent study by the same authors, again the risks of morcellating a uterine cancer in a population undergoing myomectomy was significantly higher in a postmenopausal population and occurred only rarely in women younger than age 40. But it should come as no surprise that a greater incidence of uterine cancer was identified in these older cohorts—cancer risk increases precipitously with age. That’s not “occult”; that’s basic cancer epidemiology.
In other words, we cannot assume from population-based administrative claims data that morcellation performed in inappropriate populations at higher risk of uterine malignancy (in which we do not know whether patients were properly screened for the procedure preoperatively or whether they had risk factors for uterine cancer but were presumably poor candidates for morcellation due to age alone) helps us define the true incidence of “occult” sarcoma or cancer in a population.
These studies are provocative, however, and do inform us that, as women get older, we are apt to see a greater incidence of uterine cancer. We cannot safely assume that a postmenopausal or elderly woman with symptomatic or enlarging fibroids has “presumed benign disease”—it is cancer until proven otherwise, and we need to be looking for it preoperatively. Therefore, we need to be particularly careful with our surgical practices in this population—ie, the basis for the FDA’s recommendation to avoid morcellation in older women. And I agree with the FDA that open electromechanical morcellation generally is contraindicated in postmenopausal women. However, we need better data from large prospective studies to inform our understanding of the true incidence of undiagnosed or “occult” uterine sarcoma in women undergoing surgery for presumed benign disease. These future studies are likely to demonstrate what we already know—that in young, well-screened, well-selected candidates for minimally invasive hysterectomy or myomectomy, the risk of occult cancer is going to be exceptionally low.
OBG Management: Which is greater—the risks or benefits of tissue extraction?
Dr. Fader: Assessment of risks and benefits in medicine has everything to do with the intervention in question as applied to an individual patient. At the end of the day, there are risks and benefits to every medical or surgical treatment offered to patients in every medical and surgical discipline. But the risk of an occult uterine sarcoma is extremely low in a woman of reproductive age who has been properly selected and comprehensively evaluated for minimally invasive surgery and tissue extraction prior to surgery. And this small—though not negligible—risk must be weighed against the much higher risk of harm that may be incurred with an open abdominal procedure, compared with minimally invasive surgery.
However, in many elderly women, the risks of tissue extraction with an electromechanical morcellator may outweigh the benefits. Even so, there are exceptions in which tissue extraction may be acceptable in postmenopausal women (ie, using alternative tissue-extraction methods in those undergoing minimally invasive supracervical hysterectomy and sacral colpopexy for pelvic organ prolapse).
Few of the data published on the risks of morcellation are of very high quality in terms of scientific rigor or methodology. The best thing we can do as a gynecologic surgical community is conduct sound quality-improvement (QI) programs, disseminate our QI results, publish our data, establish guidelines for best practices in uterine tissue extraction, and collaborate readily to increase the scholarly output on this issue so that national societies and government regulatory agencies have better-quality data to inform future policy on this issue.
A case-based approach
OBG Management: How would you approach tissue extraction in the following case?
CASE: A desire for myomectomy
A 35-year-old woman (G1P1) who delivered by cesarean has an 8-cm symptomatic intramural fibroid. She has regular heavy periods that have led to anemia (hemoglobin level = 10 mg/dL). Her medical history is negative for malignancy, pelvic radiation, or tamoxifen use, and she wants to preserve her fertility. Magnetic resonance imaging (MRI) confirms an 8-cm fibroid. Endometrial biopsy results are negative.
Dr. Fader: At Johns Hopkins, as at many other centers, we use well-defined criteria to determine whether a minimally invasive approach (and tissue extraction) might be appropriate. We also individualize treatment and surgical decision-making on a case-by-case basis. Any candidate for minimally invasive surgery and tissue extraction for uterine fibroids must undergo a thorough preoperative assessment.
Johns Hopkins preoperative assessment criteria include:
- endometrial biopsy
- imaging (often MRI)
- a detailed history and physical, with a comprehensive review of risk factors for malignancy, including family and genetic history or a personal history of malignancy, pelvic radiation, tamoxifen use, or BRCA or hereditary nonpolyposis colorectal cancer (HNPCC) deleterious mutation carrier status, among other things.
- In addition, all cases are discussed at a peer-reviewed, preoperative conference to ensure that a thorough work-up has been conducted and to verify the patient’s candidacy for a minimally invasive procedure with tissue extraction. As the FDA recommends, we conduct an enhanced informed consent process and counsel patients being considered for tissue extraction about the risk of occult sarcoma.
Our top priority is patient safety, so until more data are available, we no longer perform open electromechanical morcellation. We perform all tissue extraction within a containment system and under institutional review board protocol. We primarily perform tissue extraction via scalpel morcellation.
OBG Management: How do the patient’s wishes factor into the decision to perform minimally invasive surgery with morcellation?
Dr. Fader: Our patients make their own decisions regarding surgical approach and procedure after undergoing extensive counseling about the risks and benefits of the proposed procedures. I certainly would offer a patient like the one described in this case the opportunity for a minimally invasive approach (if, after thorough preoperative evaluation, she were deemed to be at very low risk for uterine malignancy). In my experience, most women opt for the minimally invasive approach in this setting; however, if a patient declines minimally invasive surgery, I respect her decision.
Tissue extraction in perimenopause
CASE: A desire for myomectomy at age 48
OBG Management: How would you approach the same case if the patient were a 48-year-old perimenopausal woman?
Dr. Fader: In perimenopausal women, we are more selective about performing morcellation, given the recent FDA safety statement, and because the incidence of occult cancer starts to increase slightly in this patient cohort (although it doesn’t precipitously increase until well into the postmenopausal period).
In addition, myomectomy may have less value in a 48-year-old, given the lower likelihood of achieving successful fertility, although there are exceptions. US cancer statistics and studies on morcellation demonstrate that the vast majority of women in their 30s and 40s have an extremely low risk for sarcomas and other uterine malignancies.2
In select cases in which a woman has undergone a comprehensive preoperative work-up, has a stable-appearing fibroid(s), and is well-educated and counseled about the pros and cons of morcellation, we would consider performing a procedure with contained tissue extraction. As a general matter, however, I would be more inclined to offer a 48-year-old in this situation a uterine artery embolization or minimally invasive hysterectomy than a myomectomy procedure, especially given the recent study by Wright and colleagues demonstrating the significantly increased risks of uterine cancer at myomectomy surgery for a woman in her late 40s or early 50s.18
Preoperative assessment should be comprehensive
OBG Management: What preoperative evaluation do you perform when tissue extraction, including morcellation, is an issue?
Dr. Fader: It is the policy at Johns Hopkins that all women being considered for minimally invasive surgery and tissue extraction must undergo a rigorous preoperative work-up that includes:
- a comprehensive history and physical (to exclude malignancy and risk for occult malignancy)
- an endometrial evaluation (most commonly, an endometrial biopsy)
- uterine imaging (with longitudinal evaluation of imaging findings if performed previously)
- discussion of each patient case at peer-reviewed, preoperative department conferences.
We have separate conferences for the gynecology and gynecologic oncology services and have employed this practice for many years at Hopkins. We are also studying the role of serum lactate dehydrogenase (LDH) isoenzyme levels in stratifying women with uterine fibroids versus cancer/sarcoma.19
OBG Management: Which patients would you exclude from the electromechanical morcellation option?
Dr. Fader: As the American College of Obstetricians and Gynecologists, the AAGL, the Society of Gynecologic Oncology, and the Society of Gynecologic Surgeons appear to agree:
- When utilized in select patients of reproductive age, minimally invasive surgery and morcellation are beneficial.
- Morcellation should categorically not be performed in any woman who has a known or suspected uterine (or other gynecologic) malignancy.2,3
At our institution, we have significantly curtailed the use of electromechanical morcellation at this time and especially do not perform it in women aged 50 or older or in those with confirmed postmenopausal status. We also do not perform electromechanical morcellation in women with a personal history of uterine, cervical, or ovarian preinvasive or invasive cancer, or in women with a strong family history of gynecologic malignancy.
Other populations we exclude are women with:
- a history of mitotically active or atypical fibroids (as determined at previous myomectomy)
- known BRCA or HNPCC deleterious mutation, hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, hereditary childhood retinoblastoma, or other genetic predisposition to uterine or ovarian cancer
- a history of pelvic radiation
- a history of tamoxifen use.
Counseling the patient
OBG Management: If tissue extraction is an issue, and morcellation will be necessary for a minimally invasive approach, how do you counsel the patient?
Dr. Fader: We have an informed consent protocol we use at Hopkins in this regard. We speak extensively to our patients about the fact that every procedure or intervention performed in medicine carries a benefit/risk ratio. We inform patients of the FDA morcellation safety statement—that their fibroid or fibroids may contain unexpected cancerous tissue and that laparoscopic electromechanical morcellation may spread the cancer and possibly worsen their prognosis. We also explain that, while the FDA quotes a risk of approximately 1 in 350 for occult sarcoma, that this data review was somewhat limited in scope and included postmenopausal women in the estimates. Based on the best available published systematic reviews and internal Hopkins data, we believe the risk of morcellating an occult uterine malignancy in a woman of reproductive age is more likely on the order of approximately 1 in 1,700 to 1 in 8,333.
We discuss our institution’s approach to tissue extraction (ie, that we no longer perform open electromechanical morcellation but instead perform contained tissue extraction on an institutional review board protocol). We tell patients that contained tissue-extraction practices are still experimental, although there is published literature preliminarily supporting the safety of the practice. In addition, we discuss the fact that contained tissue extraction has been used for years to safely remove large intra-abdominal specimens from laparoscopic incisions, from adnexal tissue to gallbladder, spleen, kidney, intestinal, and appendiceal specimens.
We also explain that, as physicians, we do our best to ensure that risks are minimized and reasonable in relation to anticipated benefits but that, even when we use the very best diagnostic measures, no test is 100% sensitive or specific to rule out malignancy in this setting (or in any setting, actually).
Finally, we discuss the fact that minimally invasive surgery and tissue extraction practices performed in appropriate patients by skilled surgeons conclusively benefit hundreds of thousands of women each year around the world. That is a narrative that has been somewhat lacking in the recent dialogue about tissue-extraction practices.
OBG Management: What do we know about outcomes when a leiomyosarcoma is inadvertently morcellated?
Dr. Fader: This is considered a “cut-through” procedure, in that a cancerous tumor that is potentially contained to an organ is not removed intact or with clean margins. The morcellation procedure effectively cuts through the occult cancer, which is not desirable. Intact surgical removal of uterine cancers or sarcomas is the mainstay of therapy for these malignancies, based on NCCN guidelines.22
OBG Management: Does a morcellated leiomyosarcoma carry a worse prognosis than an unmorcellated leiomyosarcoma?
Dr. Fader: When it comes to morcellated versus “intact” uterine leiomyosarcoma, we enter a largely data-free zone. We don’t know with certainty whether the outcome is worsened. If we’re being intellectually honest, we must admit the possibility that a morcellated cancer is more likely to be disseminated, rendering it potentially more difficult to treat. However, sarcomas primarily spread by hematogenous dissemination. It is quite possible that even the act of incising an intact fibroid in an open abdominal procedure or performing a supracervical or total hysterectomy without morcellation may still result in hematogenous cancer dissemination. So there is no indication yet that electromechanical morcellation poses a unique and higher risk of cancer upstaging or worse prognosis compared with techniques such as open myomectomy, supracervical hysterectomy, or hysteroscopic myomectomy.
In addition, the prognosis associated with even early-stage uterine leiomyosarcoma is uniformly poor. A published study from Hopkins that included 108 patients with uterine leiomyosarcoma suggests that the recurrence rate and survival of patients with early-stage, “intact” leiomyosarcoma are very poor and comparable to the survival rate documented in the literature for women with morcellated sarcomas.23
The few retrospective studies that exist suggesting worse outcomes with morcellation have limitations that preclude any definitive conclusions.2 These studies are marred by small numbers, heterogeneity in morcellation practices, poor follow-up times, and a lack of detail regarding how patients with morcellated versus unmorcellated sarcomas were treated. Nevertheless, a couple of these small retrospective reports indicate the possibility of worse outcomes in women with morcellated uterine sarcomas, compared with historical controls with intact sarcoma removal.
Is the morcellator at fault—or the user?
OBG Management: Some would argue that even one case of a morcellated uterine sarcoma is too many. How would you respond?
Dr. Fader: There is no doubt that a handful of women each year have been harmed by morcellation practices. Those women deserve our dedication and best efforts to learn how to better treat morcellated sarcomas—and more importantly—how to mitigate the risks associated with morcellation practices and reduce the risk of preventable harm for all women undergoing minimally invasive fibroid procedures. I think the single best thing we can do to mitigate risk is to be more conscientious about selecting our patients for tissue-extraction procedures (ie, strict selection criteria, appropriate preoperative work-ups). If we did this, we likely would reduce the number of oncologic morcellator mishaps by 50% to 80% without changing anything else.
When we closely scrutinize the literature, and when I reflect on the women with morcellated cancers that we have cared for at Hopkins, we observe that some (but not all) “occult” uterine cancers were not that hidden after all and may have been detected preoperatively if an effort had been made.
We have noted a number of patients in this setting who experienced harm not because a specific device was used to cut up their uterus but because they were never appropriate candidates for the procedure in the first place. For instance, I recently cared for an elderly woman with a morcellated uterine cancer who underwent a laparoscopic supracervical hysterectomy without an appropriate preoperative work-up (ie, no endometrial biopsy or imaging) or informed consent about the possibility that she might have cancer. If an elderly woman presents with concerning symptoms related to her uterus (ie, enlargement, bleeding), she must be evaluated and counseled regarding the considerable risk of potential malignancy. Even in the setting of a normal work-up, I don’t believe it is a good idea to perform electromechanical morcellation in higher-risk women, including elderly women. That does not mean that select, well-screened women cannot be considered for alternative tissue-extraction techniques, but the risks and benefits must be carefully weighed in each patient, and informed consent must be obtained.
By continuing to refine the safety features of the electromechanical morcellator devices and choosing patients more carefully for minimally invasive procedures and tissue extraction, we likely will reduce the risk of preventable harm in women undergoing gynecologic surgery.
Can leiomyosarcoma be detected preoperatively?
OBG Management: How can we improve preoperative detection of uterine malignancy, particularly leiomyosarcoma?
Dr. Fader: For starters, we can improve detection of uterine cancers by simply looking for them. Almost all epithelial uterine cancers will be detectable by endometrial sampling. A comprehensive history and physical and uterine imaging also may be helpful. And although they are more difficult to detect than epithelial uterine cancers, it is a myth that sarcomas cannot be diagnosed preoperatively. Investigators from Columbia University retrospectively evaluated the ability to preoperatively detect epithelial uterine cancers and uterine sarcomas on final pathology. In 72 women who were ultimately diagnosed with a sarcoma, preoperative endometrial sampling suggested an invasive tumor in 86% and predicted the correct histology in 64%. In fact, the rate of detection of invasive cancer by preoperative sampling was not statistically different among sarcomas than it was among epithelial uterine cancers, although there was less of a correlation with appropriate histology seen with endometrial biopsy.20
That being said, sarcomas can be missed, especially in younger women who have extremely large, degenerated, or necrotic-appearing fibroids. Improvements in diagnostic testing are desperately needed to help distinguish benign fibroids from sarcomas, as there are no reliable modalities to exclude a sarcoma at this time. MRI appears to be the most useful imaging modality, although it cannot definitively distinguish a fibroid from a sarcoma. However, a fairly constant finding in leiomyosarcomas is the absence of calcifications. Further, some studies also suggest that ill-defined margins are consistent with a sarcoma. Finally, several centers, including our own, are studying novel biologic markers and revisiting the utility of previously described markers such as LDH in the preoperative detection of uterine sarcoma.21
The way forward
OBG Management: You have said, “Keep patients informed and safe but avoid being too reactionary.” Could you expand on this statement?
Dr. Fader: Certainly. There are many examples throughout the history of medicine in which treatments have brought benefit to thousands or millions of individuals but may cause harm in a select few. We know that when controversial medical issues have arisen in the past, the pendulum has swung widely in terms of societal response.
For example, the landmark Women’s Health Initiative had an immediate and adverse impact on hormone replacement therapy (HRT) administration. The increased risk of cardiovascular disease and breast cancer observed with use of long-term combination therapy with conjugated equine estrogen and medroxyprogesterone acetate prompted many US health-care providers to abandon use of HRT—until more contemporary data demonstrated that, in younger, healthier postmenopausal women, these adverse events were very rare and the benefits of HRT outweighed the risks.
Can we avoid stroke, heart attack, and breast cancer in all younger women taking HRT? Of course not. But we counsel women about the benefit/risk ratio of the therapy and advise them that the likelihood of these events is rare.
Similarly, as with the HRT analogy, younger women have lower risks associated with surgery and morcellation, compared with older women, and are more likely to derive benefit from the procedure (after ensuring appropriate candidacy with a comprehensive preoperative evaluation and informed consent).
However, if the expectation is that no cases of harm will ever occur with a surgical device or procedure in order for it to be deemed acceptable and safe to use in practice, then that is simply an impossible standard to uphold. There is no device, medication, or intervention I know of in medicine that is completely risk-free.
OBG Management: Are there other examples of this type of benefit/risk assessment?
Dr. Fader: Yes. For instance, tamoxifen is a nonsteroidal anti-estrogen agent approved by the FDA for adjuvant treatment of breast cancer, treatment of metastatic breast cancer, and reduction of breast cancer incidence in high-risk women. Tamoxifen has effectively reduced breast cancer rates and significantly improved survival in select breast cancer patients. Yet, it is well known that long-term use of tamoxifen is associated with a twofold increased risk of uterine cancer and uterine sarcoma—a likely far more commonly occurring adverse event than an occult, morcellated uterine sarcoma during a minimally invasive gynecologic procedure.
Should the FDA ban or significantly curtail the use of tamoxifen? No, not likely, because the benefits far outweigh the risks in previvors and hormone-positive breast cancer survivors. “Keep patients informed and safe but avoid being too reactionary” means that we must do our due diligence as physicians by comprehensively counseling and obtaining informed consent from our patients before performing medical interventions. We also must closely scrutinize and improve upon practices that may cause harm.
However, what this also means is that while it may be prudent to restrict or limit a surgical practice in select higher-risk populations or modify it in some way to make it safer, we shouldn’t necessarily completely abandon or ban a practice that has benefited hundreds of thousands of patients at low risk of harm until we have objectively reviewed all of the available science and fully understand the implications of a practice change (ie, would the risks of preventable harm be even greater for women if more of them had to undergo open abdominal surgery?). We need continued cool heads and sound scientific reasoning to decide upon health-care policy changes or treatment paradigm shifts.
At the end of the day, however, it is paramount that we mitigate patient harm. The subject of tissue extraction during minimally invasive surgery is a complex and nuanced issue that merits continued study and open-minded and intelligent dialogue between patient stakeholders, clinicians, scientists, industry, ethicists, regulatory agencies, and the press. I think we can all appreciate how humbling and challenging the morcellation issue has been for many of us, especially our patients—particularly the unfortunate women who have been diagnosed with a uterine sarcoma.
Like many of my colleagues, I have been privileged to care for a number of women with uterine leiomyosarcoma. It is a devastating disease, and the prognosis is very poor, whether it is morcellated or removed intact. We are fortunate that the vast majority of women who undergo a minimally invasive procedure for fibroids or other presumed benign indications will not be at risk for an occult malignancy. But what can we do now to continue to offer the benefits of minimally invasive surgery and tissue extraction to women while simultaneously reducing the risk to the select few who will develop a rare uterine cancer?
We can all make a greater effort to more carefully select our patients for minimally invasive surgery and tissue extraction, to limit the performance of open electromechanical morcellation and collaborate in studying the role of refined tissue-extraction techniques and containment systems, to enhance the informed consent process, to develop improved diagnostic tests for preoperative cancer detection, and to conduct higher-quality studies on minimally invasive tissue-extraction techniques for regulatory agencies to review in the near future. It also goes without saying that we need more federal funding to study rare tumors (and gynecologic cancers in general) and develop better sarcoma treatments.
Although there is no medical treatment or surgical procedure that is completely risk-free, interventions such as HRT, tamoxifen, and uterine morcellation—when used in appropriate patients and for appropriate indications—will allow preventable harm to be minimized and make it possible for countless women to continue to derive tremendous benefit.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The year 2014 marked a sea change in our approach to tissue extraction during minimally invasive surgery. The US Food and Drug Administration (FDA) initiated this transformation in April, when it issued a safety warning on the use of open power morcellation.1 A flurry of statements on the practice followed from professional societies, capped, in late November, with another statement from the FDA.2–4 The new bottom line: The use of open electromechanical (“power”) morcellation is contraindicated in perimenopausal and postmenopausal women, as well as in those who are known or suspected to have a malignancy.4
Most of the concern to date has centered on the risk that an occult leiomyosarcoma could be morcellated inadvertently during uterine surgery, an event that may worsen the prognosis for the patient. To get a gynecologic oncologist’s take on the controversy, OBG Management caught up with Amanda Nickles Fader, MD, director of the Kelly Gynecologic Oncology Service at Johns Hopkins University. Dr. Fader’s perspective is unique in that she treats a relatively high number of patients who have leiomyosarcoma and other uterine cancers.
In this Q&A, we discuss the patient population at Johns Hopkins; why Dr. Fader is especially qualified to speak to the future of electromechanical morcellation in gynecologic surgery; the benefits and risks of minimally invasive surgery, including tissue extraction; her recommendations for preoperative evaluation and counseling of patients undergoing uterine surgery; and guidance on how the specialty of gynecologic surgery should proceed in the future.
OBG Management: Dr. Fader, by way of introduction, could you characterize your patient population?
Amanda Nickles Fader, MD: Like most gynecologic oncologists, I primarily treat women with cancers of the uterus, ovary, cervix, and vulva. Many of us also have the opportunity to treat a number of women each year with complex benign gynecologic conditions that require surgery. As someone who is extremely interested in rare gynecologic tumors, I also treat a relatively high volume of women diagnosed with uterine sarcoma.
Approximately 75% of the women I see in my practice have preinvasive or invasive cancer, and 25% have a benign condition, such as enlarged fibroids or advanced-stage endometriosis.
OBG Management: How many cases of uterine sarcoma do you encounter on an annual basis?
Dr. Fader: Uterine sarcomas are very rare. They represent only 2% of all uterine cancers. Put into perspective, that means that about 0.4 cases of leiomyosarcoma occur in every 100,000 US women—most commonly postmenopausal women. Leiomyosarcoma is a biologically aggressive, high-grade malignancy that often is lethal.5
Endometrial stroma sarcoma is even less common—only 0.3 cases occur in every 100,000 US women. However, this tumor type is more indolent, often diagnosed at an earlier stage, and potentially curable with surgery (with or without hormonal therapy).6
As a referral center for rare uterine tumors, the Kelly Gynecologic Oncology Service and the Sarcoma Center at Johns Hopkins see approximately 35 to 40 new cases of uterine sarcoma annually for treatment of primary disease or recurrence. An additional 15 to 20 consult cases are reviewed from outside hospitals each year by our gynecologic pathology department.
Why a minimally invasive approach is vital
OBG Management: When it comes to uterine surgery for presumed benign conditions, why is a minimally invasive approach important?
Dr. Fader: Minimally invasive surgery clearly benefits women and is one of the greatest advances of the past half-century within our field. Randomized controlled trials and meta-analyses have demonstrated without question that women who undergo minimally invasive surgery for benign conditions or early-stage cancerous gynecologic conditions have superior clinical outcomes, compared with women who undergo surgery via laparotomy.7,8 These outcomes include fewer perioperative complications (including fewer cases of surgical site infection, venous thromboembolism, wound dehiscence, and hospital readmission), shorter hospital stays, less pain, faster recovery, and fewer adhesions. And when women with early-stage cancers undergo minimally invasive surgery, randomized controlled trials show, they have a stage-specific survival rate similar to that observed in women treated with laparotomy.9
Benefits and risks of tissue extraction in minimally invasive surgery
OBG Management: What are the main benefits of tissue extraction, including morcellation?
Dr. Fader: Tissue extraction is a practice that has allowed us to offer minimally invasive surgery to countless more women than we could have in the recent past. It is a technique in which a large specimen (typically a uterus or fibroid) is fragmented into smaller parts in order to remove it through a small laparoscopic incision or orifice (vagina, umbilicus). Without tissue-extraction practices, thousands of women who undergo myomectomy each year to conserve their fertility and hundreds of thousands of women who require hysterectomy potentially would have to undergo a more painful and risky surgery through a larger abdominal incision. That would not be desirable, as we know conclusively that laparotomy is associated with worse outcomes—and even an increased risk of mortality—compared with minimally invasive surgery performed by experienced surgeons.10
Tissue extraction can be approached in a variety of ways. It can be performed with a scalpel, with a resectoscope, or with an electromechanical morcellator. Tissue extraction can be performed within the uterine cavity, through the vagina, within the abdominal compartment, or within a containment system in any of those compartments.
OBG Management: What are the risks of tissue extraction?
Dr. Fader: The risks of tissue extraction with electromechanical morcellation include potential injury to intra-abdominal organs and vasculature and risk of dissemination of an occult (ie, undiagnosed) uterine cancer. A report by our research group also demonstrated the risks of dissemination of benign uterine tissue requiring subsequent surgery in the setting of open electromechanical morcellation.11 The risk of these events occurring in the hands of a thoughtful and experienced surgeon who conducts comprehensive preoperative patient evaluations is extremely low. However, recent evidence demonstrates that a handful of women worldwide are diagnosed with an occult uterine cancer each year during a morcellation procedure.12–14 Although it is a very rare event (given that most women undergoing hysterectomy and myomectomy procedures are of reproductive age and unlikely to have uterine cancer), this risk is a serious issue. There is an exigent need for the gynecologic surgical community to develop better approaches to tissue extraction that minimize preventable harm in women.
Needed: high-quality data on the risks of morcellation
OBG Management: How does the recent FDA statement urging caution with the use of open power morcellation factor into this equation? The most recent FDA statement noted that open power morcellation is contraindicated in perimenopausal and postmenopausal women.4
Dr. Fader: The FDA’s concern is legitimate. However, the magnitude of the risk of occult uterine sarcoma in women undergoing presumed benign gynecologic surgery has been scrutinized and debated. The FDA panel quoted a risk that roughly 1 in 350 women undergoing presumed benign gynecologic surgery for fibroids will have an occult leiomyosarcoma diagnosed. However, more recent systematic reviews and a review of the prospective published literature demonstrate that the risk is more likely on the order of 1 in 1,700 to 1 in 8,333 women.15 The risk may be even lower in gynecologic surgery practices that see a high volume of hysterectomy/myomectomy cases and utilize meticulous preoperative patient selection criteria to establish a woman’s candidacy for tissue-extraction procedures.
I am concerned with how “occult sarcoma” discovered during surgery for “presumed benign gynecologic disease is being defined in the literature. There is no uniform definition being used. A cancer in this setting is only truly occult or undiagnosed if the physician was thinking about it and made every effort to rule out cancer preoperatively, and the morcellation procedure was performed in a low-risk population (but cancer was still diagnosed on final pathology in this population). However, in the majority of the morcellation studies in the literature, it is not clear that thorough preoperative evaluations occurred in patients to rule out uterine malignancy—in fact, there is a paucity of published information regarding establishing appropriate patient candidacy for morcellation procedures. So we can’t derive any conclusions regarding whether “occult” sarcomas were truly undetectable or not in the published literature.
In addition, the literature is very clear that advancing age and postmenopausal status are risk factors for uterine malignancy.16 The vast majority of uterine sarcoma cases are diagnosed in postmenopausal women. Yet, in one large US population-based hysterectomy study, 20% of the morcellated cases (and the overwhelming majority of the “occult” morcellated uterine cancers) occurred in postmenopausal women!17
Further, in a more recent study by the same authors, again the risks of morcellating a uterine cancer in a population undergoing myomectomy was significantly higher in a postmenopausal population and occurred only rarely in women younger than age 40. But it should come as no surprise that a greater incidence of uterine cancer was identified in these older cohorts—cancer risk increases precipitously with age. That’s not “occult”; that’s basic cancer epidemiology.
In other words, we cannot assume from population-based administrative claims data that morcellation performed in inappropriate populations at higher risk of uterine malignancy (in which we do not know whether patients were properly screened for the procedure preoperatively or whether they had risk factors for uterine cancer but were presumably poor candidates for morcellation due to age alone) helps us define the true incidence of “occult” sarcoma or cancer in a population.
These studies are provocative, however, and do inform us that, as women get older, we are apt to see a greater incidence of uterine cancer. We cannot safely assume that a postmenopausal or elderly woman with symptomatic or enlarging fibroids has “presumed benign disease”—it is cancer until proven otherwise, and we need to be looking for it preoperatively. Therefore, we need to be particularly careful with our surgical practices in this population—ie, the basis for the FDA’s recommendation to avoid morcellation in older women. And I agree with the FDA that open electromechanical morcellation generally is contraindicated in postmenopausal women. However, we need better data from large prospective studies to inform our understanding of the true incidence of undiagnosed or “occult” uterine sarcoma in women undergoing surgery for presumed benign disease. These future studies are likely to demonstrate what we already know—that in young, well-screened, well-selected candidates for minimally invasive hysterectomy or myomectomy, the risk of occult cancer is going to be exceptionally low.
OBG Management: Which is greater—the risks or benefits of tissue extraction?
Dr. Fader: Assessment of risks and benefits in medicine has everything to do with the intervention in question as applied to an individual patient. At the end of the day, there are risks and benefits to every medical or surgical treatment offered to patients in every medical and surgical discipline. But the risk of an occult uterine sarcoma is extremely low in a woman of reproductive age who has been properly selected and comprehensively evaluated for minimally invasive surgery and tissue extraction prior to surgery. And this small—though not negligible—risk must be weighed against the much higher risk of harm that may be incurred with an open abdominal procedure, compared with minimally invasive surgery.
However, in many elderly women, the risks of tissue extraction with an electromechanical morcellator may outweigh the benefits. Even so, there are exceptions in which tissue extraction may be acceptable in postmenopausal women (ie, using alternative tissue-extraction methods in those undergoing minimally invasive supracervical hysterectomy and sacral colpopexy for pelvic organ prolapse).
Few of the data published on the risks of morcellation are of very high quality in terms of scientific rigor or methodology. The best thing we can do as a gynecologic surgical community is conduct sound quality-improvement (QI) programs, disseminate our QI results, publish our data, establish guidelines for best practices in uterine tissue extraction, and collaborate readily to increase the scholarly output on this issue so that national societies and government regulatory agencies have better-quality data to inform future policy on this issue.
A case-based approach
OBG Management: How would you approach tissue extraction in the following case?
CASE: A desire for myomectomy
A 35-year-old woman (G1P1) who delivered by cesarean has an 8-cm symptomatic intramural fibroid. She has regular heavy periods that have led to anemia (hemoglobin level = 10 mg/dL). Her medical history is negative for malignancy, pelvic radiation, or tamoxifen use, and she wants to preserve her fertility. Magnetic resonance imaging (MRI) confirms an 8-cm fibroid. Endometrial biopsy results are negative.
Dr. Fader: At Johns Hopkins, as at many other centers, we use well-defined criteria to determine whether a minimally invasive approach (and tissue extraction) might be appropriate. We also individualize treatment and surgical decision-making on a case-by-case basis. Any candidate for minimally invasive surgery and tissue extraction for uterine fibroids must undergo a thorough preoperative assessment.
Johns Hopkins preoperative assessment criteria include:
- endometrial biopsy
- imaging (often MRI)
- a detailed history and physical, with a comprehensive review of risk factors for malignancy, including family and genetic history or a personal history of malignancy, pelvic radiation, tamoxifen use, or BRCA or hereditary nonpolyposis colorectal cancer (HNPCC) deleterious mutation carrier status, among other things.
- In addition, all cases are discussed at a peer-reviewed, preoperative conference to ensure that a thorough work-up has been conducted and to verify the patient’s candidacy for a minimally invasive procedure with tissue extraction. As the FDA recommends, we conduct an enhanced informed consent process and counsel patients being considered for tissue extraction about the risk of occult sarcoma.
Our top priority is patient safety, so until more data are available, we no longer perform open electromechanical morcellation. We perform all tissue extraction within a containment system and under institutional review board protocol. We primarily perform tissue extraction via scalpel morcellation.
OBG Management: How do the patient’s wishes factor into the decision to perform minimally invasive surgery with morcellation?
Dr. Fader: Our patients make their own decisions regarding surgical approach and procedure after undergoing extensive counseling about the risks and benefits of the proposed procedures. I certainly would offer a patient like the one described in this case the opportunity for a minimally invasive approach (if, after thorough preoperative evaluation, she were deemed to be at very low risk for uterine malignancy). In my experience, most women opt for the minimally invasive approach in this setting; however, if a patient declines minimally invasive surgery, I respect her decision.
Tissue extraction in perimenopause
CASE: A desire for myomectomy at age 48
OBG Management: How would you approach the same case if the patient were a 48-year-old perimenopausal woman?
Dr. Fader: In perimenopausal women, we are more selective about performing morcellation, given the recent FDA safety statement, and because the incidence of occult cancer starts to increase slightly in this patient cohort (although it doesn’t precipitously increase until well into the postmenopausal period).
In addition, myomectomy may have less value in a 48-year-old, given the lower likelihood of achieving successful fertility, although there are exceptions. US cancer statistics and studies on morcellation demonstrate that the vast majority of women in their 30s and 40s have an extremely low risk for sarcomas and other uterine malignancies.2
In select cases in which a woman has undergone a comprehensive preoperative work-up, has a stable-appearing fibroid(s), and is well-educated and counseled about the pros and cons of morcellation, we would consider performing a procedure with contained tissue extraction. As a general matter, however, I would be more inclined to offer a 48-year-old in this situation a uterine artery embolization or minimally invasive hysterectomy than a myomectomy procedure, especially given the recent study by Wright and colleagues demonstrating the significantly increased risks of uterine cancer at myomectomy surgery for a woman in her late 40s or early 50s.18
Preoperative assessment should be comprehensive
OBG Management: What preoperative evaluation do you perform when tissue extraction, including morcellation, is an issue?
Dr. Fader: It is the policy at Johns Hopkins that all women being considered for minimally invasive surgery and tissue extraction must undergo a rigorous preoperative work-up that includes:
- a comprehensive history and physical (to exclude malignancy and risk for occult malignancy)
- an endometrial evaluation (most commonly, an endometrial biopsy)
- uterine imaging (with longitudinal evaluation of imaging findings if performed previously)
- discussion of each patient case at peer-reviewed, preoperative department conferences.
We have separate conferences for the gynecology and gynecologic oncology services and have employed this practice for many years at Hopkins. We are also studying the role of serum lactate dehydrogenase (LDH) isoenzyme levels in stratifying women with uterine fibroids versus cancer/sarcoma.19
OBG Management: Which patients would you exclude from the electromechanical morcellation option?
Dr. Fader: As the American College of Obstetricians and Gynecologists, the AAGL, the Society of Gynecologic Oncology, and the Society of Gynecologic Surgeons appear to agree:
- When utilized in select patients of reproductive age, minimally invasive surgery and morcellation are beneficial.
- Morcellation should categorically not be performed in any woman who has a known or suspected uterine (or other gynecologic) malignancy.2,3
At our institution, we have significantly curtailed the use of electromechanical morcellation at this time and especially do not perform it in women aged 50 or older or in those with confirmed postmenopausal status. We also do not perform electromechanical morcellation in women with a personal history of uterine, cervical, or ovarian preinvasive or invasive cancer, or in women with a strong family history of gynecologic malignancy.
Other populations we exclude are women with:
- a history of mitotically active or atypical fibroids (as determined at previous myomectomy)
- known BRCA or HNPCC deleterious mutation, hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, hereditary childhood retinoblastoma, or other genetic predisposition to uterine or ovarian cancer
- a history of pelvic radiation
- a history of tamoxifen use.
Counseling the patient
OBG Management: If tissue extraction is an issue, and morcellation will be necessary for a minimally invasive approach, how do you counsel the patient?
Dr. Fader: We have an informed consent protocol we use at Hopkins in this regard. We speak extensively to our patients about the fact that every procedure or intervention performed in medicine carries a benefit/risk ratio. We inform patients of the FDA morcellation safety statement—that their fibroid or fibroids may contain unexpected cancerous tissue and that laparoscopic electromechanical morcellation may spread the cancer and possibly worsen their prognosis. We also explain that, while the FDA quotes a risk of approximately 1 in 350 for occult sarcoma, that this data review was somewhat limited in scope and included postmenopausal women in the estimates. Based on the best available published systematic reviews and internal Hopkins data, we believe the risk of morcellating an occult uterine malignancy in a woman of reproductive age is more likely on the order of approximately 1 in 1,700 to 1 in 8,333.
We discuss our institution’s approach to tissue extraction (ie, that we no longer perform open electromechanical morcellation but instead perform contained tissue extraction on an institutional review board protocol). We tell patients that contained tissue-extraction practices are still experimental, although there is published literature preliminarily supporting the safety of the practice. In addition, we discuss the fact that contained tissue extraction has been used for years to safely remove large intra-abdominal specimens from laparoscopic incisions, from adnexal tissue to gallbladder, spleen, kidney, intestinal, and appendiceal specimens.
We also explain that, as physicians, we do our best to ensure that risks are minimized and reasonable in relation to anticipated benefits but that, even when we use the very best diagnostic measures, no test is 100% sensitive or specific to rule out malignancy in this setting (or in any setting, actually).
Finally, we discuss the fact that minimally invasive surgery and tissue extraction practices performed in appropriate patients by skilled surgeons conclusively benefit hundreds of thousands of women each year around the world. That is a narrative that has been somewhat lacking in the recent dialogue about tissue-extraction practices.
OBG Management: What do we know about outcomes when a leiomyosarcoma is inadvertently morcellated?
Dr. Fader: This is considered a “cut-through” procedure, in that a cancerous tumor that is potentially contained to an organ is not removed intact or with clean margins. The morcellation procedure effectively cuts through the occult cancer, which is not desirable. Intact surgical removal of uterine cancers or sarcomas is the mainstay of therapy for these malignancies, based on NCCN guidelines.22
OBG Management: Does a morcellated leiomyosarcoma carry a worse prognosis than an unmorcellated leiomyosarcoma?
Dr. Fader: When it comes to morcellated versus “intact” uterine leiomyosarcoma, we enter a largely data-free zone. We don’t know with certainty whether the outcome is worsened. If we’re being intellectually honest, we must admit the possibility that a morcellated cancer is more likely to be disseminated, rendering it potentially more difficult to treat. However, sarcomas primarily spread by hematogenous dissemination. It is quite possible that even the act of incising an intact fibroid in an open abdominal procedure or performing a supracervical or total hysterectomy without morcellation may still result in hematogenous cancer dissemination. So there is no indication yet that electromechanical morcellation poses a unique and higher risk of cancer upstaging or worse prognosis compared with techniques such as open myomectomy, supracervical hysterectomy, or hysteroscopic myomectomy.
In addition, the prognosis associated with even early-stage uterine leiomyosarcoma is uniformly poor. A published study from Hopkins that included 108 patients with uterine leiomyosarcoma suggests that the recurrence rate and survival of patients with early-stage, “intact” leiomyosarcoma are very poor and comparable to the survival rate documented in the literature for women with morcellated sarcomas.23
The few retrospective studies that exist suggesting worse outcomes with morcellation have limitations that preclude any definitive conclusions.2 These studies are marred by small numbers, heterogeneity in morcellation practices, poor follow-up times, and a lack of detail regarding how patients with morcellated versus unmorcellated sarcomas were treated. Nevertheless, a couple of these small retrospective reports indicate the possibility of worse outcomes in women with morcellated uterine sarcomas, compared with historical controls with intact sarcoma removal.
Is the morcellator at fault—or the user?
OBG Management: Some would argue that even one case of a morcellated uterine sarcoma is too many. How would you respond?
Dr. Fader: There is no doubt that a handful of women each year have been harmed by morcellation practices. Those women deserve our dedication and best efforts to learn how to better treat morcellated sarcomas—and more importantly—how to mitigate the risks associated with morcellation practices and reduce the risk of preventable harm for all women undergoing minimally invasive fibroid procedures. I think the single best thing we can do to mitigate risk is to be more conscientious about selecting our patients for tissue-extraction procedures (ie, strict selection criteria, appropriate preoperative work-ups). If we did this, we likely would reduce the number of oncologic morcellator mishaps by 50% to 80% without changing anything else.
When we closely scrutinize the literature, and when I reflect on the women with morcellated cancers that we have cared for at Hopkins, we observe that some (but not all) “occult” uterine cancers were not that hidden after all and may have been detected preoperatively if an effort had been made.
We have noted a number of patients in this setting who experienced harm not because a specific device was used to cut up their uterus but because they were never appropriate candidates for the procedure in the first place. For instance, I recently cared for an elderly woman with a morcellated uterine cancer who underwent a laparoscopic supracervical hysterectomy without an appropriate preoperative work-up (ie, no endometrial biopsy or imaging) or informed consent about the possibility that she might have cancer. If an elderly woman presents with concerning symptoms related to her uterus (ie, enlargement, bleeding), she must be evaluated and counseled regarding the considerable risk of potential malignancy. Even in the setting of a normal work-up, I don’t believe it is a good idea to perform electromechanical morcellation in higher-risk women, including elderly women. That does not mean that select, well-screened women cannot be considered for alternative tissue-extraction techniques, but the risks and benefits must be carefully weighed in each patient, and informed consent must be obtained.
By continuing to refine the safety features of the electromechanical morcellator devices and choosing patients more carefully for minimally invasive procedures and tissue extraction, we likely will reduce the risk of preventable harm in women undergoing gynecologic surgery.
Can leiomyosarcoma be detected preoperatively?
OBG Management: How can we improve preoperative detection of uterine malignancy, particularly leiomyosarcoma?
Dr. Fader: For starters, we can improve detection of uterine cancers by simply looking for them. Almost all epithelial uterine cancers will be detectable by endometrial sampling. A comprehensive history and physical and uterine imaging also may be helpful. And although they are more difficult to detect than epithelial uterine cancers, it is a myth that sarcomas cannot be diagnosed preoperatively. Investigators from Columbia University retrospectively evaluated the ability to preoperatively detect epithelial uterine cancers and uterine sarcomas on final pathology. In 72 women who were ultimately diagnosed with a sarcoma, preoperative endometrial sampling suggested an invasive tumor in 86% and predicted the correct histology in 64%. In fact, the rate of detection of invasive cancer by preoperative sampling was not statistically different among sarcomas than it was among epithelial uterine cancers, although there was less of a correlation with appropriate histology seen with endometrial biopsy.20
That being said, sarcomas can be missed, especially in younger women who have extremely large, degenerated, or necrotic-appearing fibroids. Improvements in diagnostic testing are desperately needed to help distinguish benign fibroids from sarcomas, as there are no reliable modalities to exclude a sarcoma at this time. MRI appears to be the most useful imaging modality, although it cannot definitively distinguish a fibroid from a sarcoma. However, a fairly constant finding in leiomyosarcomas is the absence of calcifications. Further, some studies also suggest that ill-defined margins are consistent with a sarcoma. Finally, several centers, including our own, are studying novel biologic markers and revisiting the utility of previously described markers such as LDH in the preoperative detection of uterine sarcoma.21
The way forward
OBG Management: You have said, “Keep patients informed and safe but avoid being too reactionary.” Could you expand on this statement?
Dr. Fader: Certainly. There are many examples throughout the history of medicine in which treatments have brought benefit to thousands or millions of individuals but may cause harm in a select few. We know that when controversial medical issues have arisen in the past, the pendulum has swung widely in terms of societal response.
For example, the landmark Women’s Health Initiative had an immediate and adverse impact on hormone replacement therapy (HRT) administration. The increased risk of cardiovascular disease and breast cancer observed with use of long-term combination therapy with conjugated equine estrogen and medroxyprogesterone acetate prompted many US health-care providers to abandon use of HRT—until more contemporary data demonstrated that, in younger, healthier postmenopausal women, these adverse events were very rare and the benefits of HRT outweighed the risks.
Can we avoid stroke, heart attack, and breast cancer in all younger women taking HRT? Of course not. But we counsel women about the benefit/risk ratio of the therapy and advise them that the likelihood of these events is rare.
Similarly, as with the HRT analogy, younger women have lower risks associated with surgery and morcellation, compared with older women, and are more likely to derive benefit from the procedure (after ensuring appropriate candidacy with a comprehensive preoperative evaluation and informed consent).
However, if the expectation is that no cases of harm will ever occur with a surgical device or procedure in order for it to be deemed acceptable and safe to use in practice, then that is simply an impossible standard to uphold. There is no device, medication, or intervention I know of in medicine that is completely risk-free.
OBG Management: Are there other examples of this type of benefit/risk assessment?
Dr. Fader: Yes. For instance, tamoxifen is a nonsteroidal anti-estrogen agent approved by the FDA for adjuvant treatment of breast cancer, treatment of metastatic breast cancer, and reduction of breast cancer incidence in high-risk women. Tamoxifen has effectively reduced breast cancer rates and significantly improved survival in select breast cancer patients. Yet, it is well known that long-term use of tamoxifen is associated with a twofold increased risk of uterine cancer and uterine sarcoma—a likely far more commonly occurring adverse event than an occult, morcellated uterine sarcoma during a minimally invasive gynecologic procedure.
Should the FDA ban or significantly curtail the use of tamoxifen? No, not likely, because the benefits far outweigh the risks in previvors and hormone-positive breast cancer survivors. “Keep patients informed and safe but avoid being too reactionary” means that we must do our due diligence as physicians by comprehensively counseling and obtaining informed consent from our patients before performing medical interventions. We also must closely scrutinize and improve upon practices that may cause harm.
However, what this also means is that while it may be prudent to restrict or limit a surgical practice in select higher-risk populations or modify it in some way to make it safer, we shouldn’t necessarily completely abandon or ban a practice that has benefited hundreds of thousands of patients at low risk of harm until we have objectively reviewed all of the available science and fully understand the implications of a practice change (ie, would the risks of preventable harm be even greater for women if more of them had to undergo open abdominal surgery?). We need continued cool heads and sound scientific reasoning to decide upon health-care policy changes or treatment paradigm shifts.
At the end of the day, however, it is paramount that we mitigate patient harm. The subject of tissue extraction during minimally invasive surgery is a complex and nuanced issue that merits continued study and open-minded and intelligent dialogue between patient stakeholders, clinicians, scientists, industry, ethicists, regulatory agencies, and the press. I think we can all appreciate how humbling and challenging the morcellation issue has been for many of us, especially our patients—particularly the unfortunate women who have been diagnosed with a uterine sarcoma.
Like many of my colleagues, I have been privileged to care for a number of women with uterine leiomyosarcoma. It is a devastating disease, and the prognosis is very poor, whether it is morcellated or removed intact. We are fortunate that the vast majority of women who undergo a minimally invasive procedure for fibroids or other presumed benign indications will not be at risk for an occult malignancy. But what can we do now to continue to offer the benefits of minimally invasive surgery and tissue extraction to women while simultaneously reducing the risk to the select few who will develop a rare uterine cancer?
We can all make a greater effort to more carefully select our patients for minimally invasive surgery and tissue extraction, to limit the performance of open electromechanical morcellation and collaborate in studying the role of refined tissue-extraction techniques and containment systems, to enhance the informed consent process, to develop improved diagnostic tests for preoperative cancer detection, and to conduct higher-quality studies on minimally invasive tissue-extraction techniques for regulatory agencies to review in the near future. It also goes without saying that we need more federal funding to study rare tumors (and gynecologic cancers in general) and develop better sarcoma treatments.
Although there is no medical treatment or surgical procedure that is completely risk-free, interventions such as HRT, tamoxifen, and uterine morcellation—when used in appropriate patients and for appropriate indications—will allow preventable harm to be minimized and make it possible for countless women to continue to derive tremendous benefit.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed February 3, 2014.
2. AAGL. Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed February 3, 2015.
3. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery: a special report. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery. Published May 2014. Accessed February 3, 2015.
4. US Food and Drug Administration. Updated Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Published November 24, 2014. Accessed February 2, 2015.
5. American Cancer Society. Uterine sarcoma: What is uterine sarcoma? http://www.cancer.org/cancer/uterinesarcoma/detailedguide/uterine-sarcoma-what-is-uterine-sarcoma. Updated January 12, 2015. Accessed February 3, 2015.
6. D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–139.
7. Chittawar B, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;10:CD004638. doi:10.1002/14651858.CD004638.pub3.
8. Mori KM, Neubauer NL. Minimally invasive surgery in gynecologic oncology. ISRN Obstet Gynecol. 2013; article ID 312982. http://dx.doi.org/10.1155/2013/312982. Accessed February 2, 2015.
9. Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib IIa cervical cancer. Gynecol Oncol. 2007;105(1):176–180.
10. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study Lap2. J Clin Oncol. 2009;27(32):5331–5336.
11. Ramos A, Fader AN, Long Roche K. Surgical cytoreduction for disseminated benign disease after open power uterine morcellation. Obstet Gynecol. 2014;125(1):99–102.
12. Theben J, Schellong A, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): an analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
13. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
14. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7(11):e50058.
15. Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33.
16. Wright JD, Tergas AI, Burke WM, et al. Prevalence of uterine pathology in women undergoing minimally invasive hysterectomy employing electric power morcellation. JAMA. 2014;312(12):1253–1255.
17. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312(12):1253–1255.
18. Wright JD, Tergas AI, Cul R, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncol. 2015; published online February 19, 2015. doi:10.1001/jamaoncol.2014.206.
19. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002; 12:354.
20. Ricci S, Giuntoli RL 2nd, Eisenhauer E, et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol Oncol. 2013;131(3):629–633.
21. Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008;110(1):43–48.
22. Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12(2):248–280.
23. Schwartz LB, Zawin M, Carcangiu ML, Lange R, McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 1998;70(3):580–587.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed February 3, 2014.
2. AAGL. Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed February 3, 2015.
3. American College of Obstetricians and Gynecologists. Power morcellation and occult malignancy in gynecologic surgery: a special report. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery. Published May 2014. Accessed February 3, 2015.
4. US Food and Drug Administration. Updated Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Published November 24, 2014. Accessed February 2, 2015.
5. American Cancer Society. Uterine sarcoma: What is uterine sarcoma? http://www.cancer.org/cancer/uterinesarcoma/detailedguide/uterine-sarcoma-what-is-uterine-sarcoma. Updated January 12, 2015. Accessed February 3, 2015.
6. D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–139.
7. Chittawar B, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;10:CD004638. doi:10.1002/14651858.CD004638.pub3.
8. Mori KM, Neubauer NL. Minimally invasive surgery in gynecologic oncology. ISRN Obstet Gynecol. 2013; article ID 312982. http://dx.doi.org/10.1155/2013/312982. Accessed February 2, 2015.
9. Li G, Yan X, Shang H, Wang G, Chen L, Han Y. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib IIa cervical cancer. Gynecol Oncol. 2007;105(1):176–180.
10. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study Lap2. J Clin Oncol. 2009;27(32):5331–5336.
11. Ramos A, Fader AN, Long Roche K. Surgical cytoreduction for disseminated benign disease after open power uterine morcellation. Obstet Gynecol. 2014;125(1):99–102.
12. Theben J, Schellong A, Altgassen C, Kelling K, Schneider S, Grobe-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): an analysis of 1,584 LASH cases. Arch Gynecol Obstet. 2013;287(3):455–462.
13. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
14. Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7(11):e50058.
15. Pritts EA, Parker WH, Brown J, Olive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33.
16. Wright JD, Tergas AI, Burke WM, et al. Prevalence of uterine pathology in women undergoing minimally invasive hysterectomy employing electric power morcellation. JAMA. 2014;312(12):1253–1255.
17. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA. 2014;312(12):1253–1255.
18. Wright JD, Tergas AI, Cul R, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncol. 2015; published online February 19, 2015. doi:10.1001/jamaoncol.2014.206.
19. Goto A, Takeuchi S, Sugimura K, Maruo T. Usefulness of Gd-DTPA contrast-enhanced dynamic MRI and serum determination of LDH and its isozymes in the differential diagnosis of leiomyosarcoma from degenerated leiomyoma of the uterus. Int J Gynecol Cancer. 2002; 12:354.
20. Ricci S, Giuntoli RL 2nd, Eisenhauer E, et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol Oncol. 2013;131(3):629–633.
21. Bansal N, Herzog TJ, Burke W, Cohen CJ, Wright JD. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008;110(1):43–48.
22. Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw. 2014;12(2):248–280.
23. Schwartz LB, Zawin M, Carcangiu ML, Lange R, McCarthy S. Does pelvic magnetic resonance imaging differentiate among the histologic subtypes of uterine leiomyomata? Fertil Steril. 1998;70(3):580–587.
IN THIS ARTICLE
– A case-based approach
– Comprehensive preoperative assessment
– What is the prognosis when a leiomyosarcoma is morcellated?
Lost needle tip during hysterectomy
CASE: Lost needle tip
A 36-year-old woman (G3 P2012) with stress urinary incontinence (SUI) and abnormal uterine bleeding presented to a gynecologist. She had explored medical therapy for her SUI with no symptom improvement. She had a previous tubal ligation, and the gynecologist ordered urodynamic testing, the results of which led to a discussion of vaginal hysterectomy; anterior, posterior colporrhaphy; and mesh placement. It was felt that the patient had a number of risk factors for incontinence (including pregnancy with vaginal delivery, well-controlled diabetes mellitus, and obesity). She had a long-standing history of chronic pelvic pain, with an established diagnosis of diverticulosis with episodes of diverticulitis in the past.
The gynecologist had the patient keep a bladder diary for 1 week. When asked, the patient reported no problems with sexual dysfunction, stating that her quality of life was “fine” except for the vaginal bleeding and loss of urine refractory to medical therapy. The Urogenital Distress Inventory was administered, and it identified frequent urination, leakage, and incontinence related to activities. An Incontinence Impact Questionnaire also was administered. Physical examination included cotton-tipped swab urethral, or Q-tip, test and cough stress test as part of POP-Q (Pelvic Organ Prolapse Quantification system) evaluation. Urinary tract infection was ruled out. The gynecologist counseled the patient about possible medical therapies for urinary incontinence, and she requested definitive surgery.
The gynecologist obtained informed consent for surgery that included preoperative discussion of potential surgical complications, including bleeding, infection, trauma to surrounding structures, and the possibility of additional surgical procedures secondary to complications. The gynecologist also discussed transvaginal tape versus transobturator tape (TOT) placement, including potential complications and sequelae. The final planned procedure, which was performed by the gynecologist, included vaginal hysterectomy, anterior colporrhaphy, and TOT placement.
Intraoperatively, the patient was identified (upon entering the operating room [OR]); time-out occurred, and the gynecologist proceeded with surgery. During the procedure, the tip of a needle broke off. The gynecologist noted the broken tip as he removed the needle and handed it to the surgical technician. The gynecologist palpated the sidewall in the presumed area of the needle tip and felt it easily. He attempted to remove the tip, but his effort was fruitless. He made the intraoperative decision to leave the tip in situ. A needle and sponge count was performed, reported as correct, and it was felt there was no indication for imaging of the pelvis. The circulating nurse filled out an incident report immediately following the surgery, noting the missing needle tip. The occurrence was discussed by the surgical committee at the hospital.
Postoperatively, while the patient was in the hospital, she was informed of the intra-operative incident.
Three months later, the patient reported vaginal and pelvic pain on the sidewall in the area of the lost needle tip, with radiating pain down the involved extremity. A segment of the TOT was noted to be protruding into the vagina, and this was addressed in the OR with “trimming of such.”
Postoperatively, again the patient reported pain on the involved side. She sought the opinion of another gynecologist, who subsequently performed surgical intervention to remove the needle tip. Her symptoms improved.
The patient sued the original gynecologic surgeon, alleging pain and suffering from the surgery involving the lost needle tip.
What’s the verdict?
A defense verdict was awarded.
Medical teaching points
Medical evaluation seemed appropriate. Parity is associated with SUI (but not urge incontinence). In general, urinary incontinence is more commonly associated with a history of lower urinary tract infections. The patient in this case was asked about and evaluated for:
- stress incontinence (associated with loss of urine with sneezing, coughing, and exercise)
- urge incontinence (inability to reach the bathroom in time)
- frequency of urination, especially while sleeping
- overflow incontinence
- overall loss of bladder control.
Was information on the broken needle handled appropriately? This case explores the question of what, if any, obligation the surgeon and hospital system have to the patient when informing her of a broken needle and the intraoperative decision-making process that led to its staying in place. When such a situation occurs, which is very uncommon, should an intraoperative x-ray be performed to assess the location of the needle tip? Should the patient automatically be brought back to the OR for removal?
The surgeon’s concern was a legitimate one—that additional attempts at removal could lead to complications far worse than having a small segment of a needle left in place. After all, shrapnel, bullets, etc, remain lodged in various locations throughout the body without subsequent ill effects. He did discuss with the patient the fact that a needle segment was left in the muscle wall. But how do you assess postoperative pelvic pain in a patient who had preoperative chronic pelvic pain? These are questions we as clinicians ask. Clearly, there are no black-and-white answers, and we will call upon our legal consultants for their expertise in addressing these queries.
From the gynecologic perspective, however, it is of paramount importance to address the patient’s postoperative vaginal pain and determine the best management approach. In this case the TOT, and its association with a 21.5% complication rate, including reported vaginal extrusion, introduces a whole new set of concerns.1 The TOT use in itself raises the question of liability on the part of the surgeon. This mesh has more than 150 associated complications, including obturator nerve injuries, extensive blood loss, and ischiorectal fossa abscesses.2 Once a device comes upon the radar screen of the US Food and Drug Administration for significant complications, where does that leave the clinician in regard to litigation? Let’s look to our legal colleagues for their insight and expertise.
Legal considerations
Given the facts in this case, it is not surprising that it resulted in a defense verdict. The majority of cases filed are ultimately disposed of in favor of the medical defendants, and the majority of medical malpractice cases that go to trial result in defense verdicts.
Medical malpractice, or “professional negligence,” consists of a claim that a medical professional had a duty of care to the patient, a breach of that duty, injury to the patient, and a causal connection (“causation”) between the breach of duty and the injury. It is the obligation of the plaintiff to prove the elements of negligence by a preponderance of the evidence.
Were the surgeon’s actions in line with other surgeons’ expected actions? The issue of the breach of the duty of care essentially is the question of whether the physician acted similarly to a reasonably careful practitioner of the same specialty under the same circumstances. Doctors are not held to a standard of perfection. That is, not every injury or bad outcome is negligence—only those injuries that result from actions, or inactions, that were not within the level of care acceptable in the profession.
Why would this patient file a lawsuit? The injury was not trivial (it had both pain and cost associated with it), but it was not catastrophic, and the negligence was going to be difficult to prove. Furthermore, lawsuits are expensive in terms of time, energy, and emotional commitment—few people file them for the fun of it. We can only speculate on the answer to the question but, frequently, such claims are a search for the answer to “What happened, and why?” or a reaction to feeling ignored or disrespected. There is little in the case facts that we have to work with to indicate what the communication was between the gynecologist and the patient and her family. The statement of facts, however, leaves the impression that communication deteriorated as the postoperative pain endured.
Some additional areas of potential claims for liability in this case include:
- The explanation for the needle breaking during surgery is unclear from the brief statement of case facts. There might be malpractice liability if the surgeon was unreasonable in how the needle was used, used the wrong needle, or ignored defects in the needle.
- The surgeon tried unsuccessfully to retrieve the needle during the original surgery. If the surgeon’s failure to retrieve the needle was because of inadequate training, lack of care or the like, it might be seen as the “cause” of the patient’s injuries.
- The fact that a second surgeon was able to remove the needle tip, which resolved the patient’s pain, may raise the question of whether the first surgeon’s decision not to seek to remove it in response to the continuing pain was reasonable. If the first surgeon did not want to remove the needle tip, a question might be raised about whether that surgeon should have referred the patient to another surgeon. (The patient ultimately found another surgeon on her own.)
- Regarding use of TOT: A 21.5% complication rate ordinarily would be a significant factor to consider in a decision to use the tape. Physicians are responsible for keeping up with current developments in the devices and pharmaceuticals they use. Therefore, if information on the complication rate was available, the surgeon’s documentation should reflect the basis for choosing to use the tape. More important, the surgeon should document a conversation with the patient about the risks and benefits of using the TOT and the discussion of alternatives to its use.
What factors could have tipped the case toward the defense?
The defense verdict indicates that the jury determined there was no negligence, or that the patient could not prove any of these potential bases of liability. As noted above, what may have helped the defense is the fact that the surgeon documented the details of the informed consent conversation, including that “discussion was carried out regarding” the tape. The informed consent process is an important opportunity for communication with the patient, and a chance to make sure that expectations are reasonable. Liability for the failure of informed consent is not common. When something has gone wrong, however, it can matter whether the problem was something mentioned in the informed consent process. In addition, it was positive that postoperatively the patient was informed of the broken needle—although it is not clear who informed her about it.
A couple of other legal issues are worth noting. From our fact scenario we do not know what was documented in the incident report filed by the circulating nurse and reviewed by the surgical committee. We also do not know whether the plaintiff was privy to the incident report document. The surgical committee is likely a peer-review committee, and most states provide some privilege for such committees (to avoid disclosure of committee information for discovery or at trial). The deliberations and conclusions of the committee, therefore, were likely privileged. However, incident reports are frequently used for other purposes, such as administrative reports, that are not privileged—so the incident report often is determined to be discoverable depending on the interpretation of the state’s law.
No winner in this case
Despite the defense verdict, the physician was not really the “winner” after having spent a great deal of time, energy, money, and emotion defending this suit. Ultimately, the goal is not to win malpractice cases but to avoid them—in this case, among other things, by being frank with patients about expectations, keeping an open line of communication with patients when they are concerned with an outcome that is less than ideal, and referring a patient when it may be appropriate.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Bladder sling risks, complications and side effects. DrugWatch Web site. http://www.drugwatch.com/trans vaginal-mesh/bladder-sling/. Updated January 2, 2015. Accessed February 13, 2015.
2. Boyles SH, Edwards R, Gregory W, Clark A. Complications associated with transobturator sling procedures. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(1):19–22.
CASE: Lost needle tip
A 36-year-old woman (G3 P2012) with stress urinary incontinence (SUI) and abnormal uterine bleeding presented to a gynecologist. She had explored medical therapy for her SUI with no symptom improvement. She had a previous tubal ligation, and the gynecologist ordered urodynamic testing, the results of which led to a discussion of vaginal hysterectomy; anterior, posterior colporrhaphy; and mesh placement. It was felt that the patient had a number of risk factors for incontinence (including pregnancy with vaginal delivery, well-controlled diabetes mellitus, and obesity). She had a long-standing history of chronic pelvic pain, with an established diagnosis of diverticulosis with episodes of diverticulitis in the past.
The gynecologist had the patient keep a bladder diary for 1 week. When asked, the patient reported no problems with sexual dysfunction, stating that her quality of life was “fine” except for the vaginal bleeding and loss of urine refractory to medical therapy. The Urogenital Distress Inventory was administered, and it identified frequent urination, leakage, and incontinence related to activities. An Incontinence Impact Questionnaire also was administered. Physical examination included cotton-tipped swab urethral, or Q-tip, test and cough stress test as part of POP-Q (Pelvic Organ Prolapse Quantification system) evaluation. Urinary tract infection was ruled out. The gynecologist counseled the patient about possible medical therapies for urinary incontinence, and she requested definitive surgery.
The gynecologist obtained informed consent for surgery that included preoperative discussion of potential surgical complications, including bleeding, infection, trauma to surrounding structures, and the possibility of additional surgical procedures secondary to complications. The gynecologist also discussed transvaginal tape versus transobturator tape (TOT) placement, including potential complications and sequelae. The final planned procedure, which was performed by the gynecologist, included vaginal hysterectomy, anterior colporrhaphy, and TOT placement.
Intraoperatively, the patient was identified (upon entering the operating room [OR]); time-out occurred, and the gynecologist proceeded with surgery. During the procedure, the tip of a needle broke off. The gynecologist noted the broken tip as he removed the needle and handed it to the surgical technician. The gynecologist palpated the sidewall in the presumed area of the needle tip and felt it easily. He attempted to remove the tip, but his effort was fruitless. He made the intraoperative decision to leave the tip in situ. A needle and sponge count was performed, reported as correct, and it was felt there was no indication for imaging of the pelvis. The circulating nurse filled out an incident report immediately following the surgery, noting the missing needle tip. The occurrence was discussed by the surgical committee at the hospital.
Postoperatively, while the patient was in the hospital, she was informed of the intra-operative incident.
Three months later, the patient reported vaginal and pelvic pain on the sidewall in the area of the lost needle tip, with radiating pain down the involved extremity. A segment of the TOT was noted to be protruding into the vagina, and this was addressed in the OR with “trimming of such.”
Postoperatively, again the patient reported pain on the involved side. She sought the opinion of another gynecologist, who subsequently performed surgical intervention to remove the needle tip. Her symptoms improved.
The patient sued the original gynecologic surgeon, alleging pain and suffering from the surgery involving the lost needle tip.
What’s the verdict?
A defense verdict was awarded.
Medical teaching points
Medical evaluation seemed appropriate. Parity is associated with SUI (but not urge incontinence). In general, urinary incontinence is more commonly associated with a history of lower urinary tract infections. The patient in this case was asked about and evaluated for:
- stress incontinence (associated with loss of urine with sneezing, coughing, and exercise)
- urge incontinence (inability to reach the bathroom in time)
- frequency of urination, especially while sleeping
- overflow incontinence
- overall loss of bladder control.
Was information on the broken needle handled appropriately? This case explores the question of what, if any, obligation the surgeon and hospital system have to the patient when informing her of a broken needle and the intraoperative decision-making process that led to its staying in place. When such a situation occurs, which is very uncommon, should an intraoperative x-ray be performed to assess the location of the needle tip? Should the patient automatically be brought back to the OR for removal?
The surgeon’s concern was a legitimate one—that additional attempts at removal could lead to complications far worse than having a small segment of a needle left in place. After all, shrapnel, bullets, etc, remain lodged in various locations throughout the body without subsequent ill effects. He did discuss with the patient the fact that a needle segment was left in the muscle wall. But how do you assess postoperative pelvic pain in a patient who had preoperative chronic pelvic pain? These are questions we as clinicians ask. Clearly, there are no black-and-white answers, and we will call upon our legal consultants for their expertise in addressing these queries.
From the gynecologic perspective, however, it is of paramount importance to address the patient’s postoperative vaginal pain and determine the best management approach. In this case the TOT, and its association with a 21.5% complication rate, including reported vaginal extrusion, introduces a whole new set of concerns.1 The TOT use in itself raises the question of liability on the part of the surgeon. This mesh has more than 150 associated complications, including obturator nerve injuries, extensive blood loss, and ischiorectal fossa abscesses.2 Once a device comes upon the radar screen of the US Food and Drug Administration for significant complications, where does that leave the clinician in regard to litigation? Let’s look to our legal colleagues for their insight and expertise.
Legal considerations
Given the facts in this case, it is not surprising that it resulted in a defense verdict. The majority of cases filed are ultimately disposed of in favor of the medical defendants, and the majority of medical malpractice cases that go to trial result in defense verdicts.
Medical malpractice, or “professional negligence,” consists of a claim that a medical professional had a duty of care to the patient, a breach of that duty, injury to the patient, and a causal connection (“causation”) between the breach of duty and the injury. It is the obligation of the plaintiff to prove the elements of negligence by a preponderance of the evidence.
Were the surgeon’s actions in line with other surgeons’ expected actions? The issue of the breach of the duty of care essentially is the question of whether the physician acted similarly to a reasonably careful practitioner of the same specialty under the same circumstances. Doctors are not held to a standard of perfection. That is, not every injury or bad outcome is negligence—only those injuries that result from actions, or inactions, that were not within the level of care acceptable in the profession.
Why would this patient file a lawsuit? The injury was not trivial (it had both pain and cost associated with it), but it was not catastrophic, and the negligence was going to be difficult to prove. Furthermore, lawsuits are expensive in terms of time, energy, and emotional commitment—few people file them for the fun of it. We can only speculate on the answer to the question but, frequently, such claims are a search for the answer to “What happened, and why?” or a reaction to feeling ignored or disrespected. There is little in the case facts that we have to work with to indicate what the communication was between the gynecologist and the patient and her family. The statement of facts, however, leaves the impression that communication deteriorated as the postoperative pain endured.
Some additional areas of potential claims for liability in this case include:
- The explanation for the needle breaking during surgery is unclear from the brief statement of case facts. There might be malpractice liability if the surgeon was unreasonable in how the needle was used, used the wrong needle, or ignored defects in the needle.
- The surgeon tried unsuccessfully to retrieve the needle during the original surgery. If the surgeon’s failure to retrieve the needle was because of inadequate training, lack of care or the like, it might be seen as the “cause” of the patient’s injuries.
- The fact that a second surgeon was able to remove the needle tip, which resolved the patient’s pain, may raise the question of whether the first surgeon’s decision not to seek to remove it in response to the continuing pain was reasonable. If the first surgeon did not want to remove the needle tip, a question might be raised about whether that surgeon should have referred the patient to another surgeon. (The patient ultimately found another surgeon on her own.)
- Regarding use of TOT: A 21.5% complication rate ordinarily would be a significant factor to consider in a decision to use the tape. Physicians are responsible for keeping up with current developments in the devices and pharmaceuticals they use. Therefore, if information on the complication rate was available, the surgeon’s documentation should reflect the basis for choosing to use the tape. More important, the surgeon should document a conversation with the patient about the risks and benefits of using the TOT and the discussion of alternatives to its use.
What factors could have tipped the case toward the defense?
The defense verdict indicates that the jury determined there was no negligence, or that the patient could not prove any of these potential bases of liability. As noted above, what may have helped the defense is the fact that the surgeon documented the details of the informed consent conversation, including that “discussion was carried out regarding” the tape. The informed consent process is an important opportunity for communication with the patient, and a chance to make sure that expectations are reasonable. Liability for the failure of informed consent is not common. When something has gone wrong, however, it can matter whether the problem was something mentioned in the informed consent process. In addition, it was positive that postoperatively the patient was informed of the broken needle—although it is not clear who informed her about it.
A couple of other legal issues are worth noting. From our fact scenario we do not know what was documented in the incident report filed by the circulating nurse and reviewed by the surgical committee. We also do not know whether the plaintiff was privy to the incident report document. The surgical committee is likely a peer-review committee, and most states provide some privilege for such committees (to avoid disclosure of committee information for discovery or at trial). The deliberations and conclusions of the committee, therefore, were likely privileged. However, incident reports are frequently used for other purposes, such as administrative reports, that are not privileged—so the incident report often is determined to be discoverable depending on the interpretation of the state’s law.
No winner in this case
Despite the defense verdict, the physician was not really the “winner” after having spent a great deal of time, energy, money, and emotion defending this suit. Ultimately, the goal is not to win malpractice cases but to avoid them—in this case, among other things, by being frank with patients about expectations, keeping an open line of communication with patients when they are concerned with an outcome that is less than ideal, and referring a patient when it may be appropriate.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Lost needle tip
A 36-year-old woman (G3 P2012) with stress urinary incontinence (SUI) and abnormal uterine bleeding presented to a gynecologist. She had explored medical therapy for her SUI with no symptom improvement. She had a previous tubal ligation, and the gynecologist ordered urodynamic testing, the results of which led to a discussion of vaginal hysterectomy; anterior, posterior colporrhaphy; and mesh placement. It was felt that the patient had a number of risk factors for incontinence (including pregnancy with vaginal delivery, well-controlled diabetes mellitus, and obesity). She had a long-standing history of chronic pelvic pain, with an established diagnosis of diverticulosis with episodes of diverticulitis in the past.
The gynecologist had the patient keep a bladder diary for 1 week. When asked, the patient reported no problems with sexual dysfunction, stating that her quality of life was “fine” except for the vaginal bleeding and loss of urine refractory to medical therapy. The Urogenital Distress Inventory was administered, and it identified frequent urination, leakage, and incontinence related to activities. An Incontinence Impact Questionnaire also was administered. Physical examination included cotton-tipped swab urethral, or Q-tip, test and cough stress test as part of POP-Q (Pelvic Organ Prolapse Quantification system) evaluation. Urinary tract infection was ruled out. The gynecologist counseled the patient about possible medical therapies for urinary incontinence, and she requested definitive surgery.
The gynecologist obtained informed consent for surgery that included preoperative discussion of potential surgical complications, including bleeding, infection, trauma to surrounding structures, and the possibility of additional surgical procedures secondary to complications. The gynecologist also discussed transvaginal tape versus transobturator tape (TOT) placement, including potential complications and sequelae. The final planned procedure, which was performed by the gynecologist, included vaginal hysterectomy, anterior colporrhaphy, and TOT placement.
Intraoperatively, the patient was identified (upon entering the operating room [OR]); time-out occurred, and the gynecologist proceeded with surgery. During the procedure, the tip of a needle broke off. The gynecologist noted the broken tip as he removed the needle and handed it to the surgical technician. The gynecologist palpated the sidewall in the presumed area of the needle tip and felt it easily. He attempted to remove the tip, but his effort was fruitless. He made the intraoperative decision to leave the tip in situ. A needle and sponge count was performed, reported as correct, and it was felt there was no indication for imaging of the pelvis. The circulating nurse filled out an incident report immediately following the surgery, noting the missing needle tip. The occurrence was discussed by the surgical committee at the hospital.
Postoperatively, while the patient was in the hospital, she was informed of the intra-operative incident.
Three months later, the patient reported vaginal and pelvic pain on the sidewall in the area of the lost needle tip, with radiating pain down the involved extremity. A segment of the TOT was noted to be protruding into the vagina, and this was addressed in the OR with “trimming of such.”
Postoperatively, again the patient reported pain on the involved side. She sought the opinion of another gynecologist, who subsequently performed surgical intervention to remove the needle tip. Her symptoms improved.
The patient sued the original gynecologic surgeon, alleging pain and suffering from the surgery involving the lost needle tip.
What’s the verdict?
A defense verdict was awarded.
Medical teaching points
Medical evaluation seemed appropriate. Parity is associated with SUI (but not urge incontinence). In general, urinary incontinence is more commonly associated with a history of lower urinary tract infections. The patient in this case was asked about and evaluated for:
- stress incontinence (associated with loss of urine with sneezing, coughing, and exercise)
- urge incontinence (inability to reach the bathroom in time)
- frequency of urination, especially while sleeping
- overflow incontinence
- overall loss of bladder control.
Was information on the broken needle handled appropriately? This case explores the question of what, if any, obligation the surgeon and hospital system have to the patient when informing her of a broken needle and the intraoperative decision-making process that led to its staying in place. When such a situation occurs, which is very uncommon, should an intraoperative x-ray be performed to assess the location of the needle tip? Should the patient automatically be brought back to the OR for removal?
The surgeon’s concern was a legitimate one—that additional attempts at removal could lead to complications far worse than having a small segment of a needle left in place. After all, shrapnel, bullets, etc, remain lodged in various locations throughout the body without subsequent ill effects. He did discuss with the patient the fact that a needle segment was left in the muscle wall. But how do you assess postoperative pelvic pain in a patient who had preoperative chronic pelvic pain? These are questions we as clinicians ask. Clearly, there are no black-and-white answers, and we will call upon our legal consultants for their expertise in addressing these queries.
From the gynecologic perspective, however, it is of paramount importance to address the patient’s postoperative vaginal pain and determine the best management approach. In this case the TOT, and its association with a 21.5% complication rate, including reported vaginal extrusion, introduces a whole new set of concerns.1 The TOT use in itself raises the question of liability on the part of the surgeon. This mesh has more than 150 associated complications, including obturator nerve injuries, extensive blood loss, and ischiorectal fossa abscesses.2 Once a device comes upon the radar screen of the US Food and Drug Administration for significant complications, where does that leave the clinician in regard to litigation? Let’s look to our legal colleagues for their insight and expertise.
Legal considerations
Given the facts in this case, it is not surprising that it resulted in a defense verdict. The majority of cases filed are ultimately disposed of in favor of the medical defendants, and the majority of medical malpractice cases that go to trial result in defense verdicts.
Medical malpractice, or “professional negligence,” consists of a claim that a medical professional had a duty of care to the patient, a breach of that duty, injury to the patient, and a causal connection (“causation”) between the breach of duty and the injury. It is the obligation of the plaintiff to prove the elements of negligence by a preponderance of the evidence.
Were the surgeon’s actions in line with other surgeons’ expected actions? The issue of the breach of the duty of care essentially is the question of whether the physician acted similarly to a reasonably careful practitioner of the same specialty under the same circumstances. Doctors are not held to a standard of perfection. That is, not every injury or bad outcome is negligence—only those injuries that result from actions, or inactions, that were not within the level of care acceptable in the profession.
Why would this patient file a lawsuit? The injury was not trivial (it had both pain and cost associated with it), but it was not catastrophic, and the negligence was going to be difficult to prove. Furthermore, lawsuits are expensive in terms of time, energy, and emotional commitment—few people file them for the fun of it. We can only speculate on the answer to the question but, frequently, such claims are a search for the answer to “What happened, and why?” or a reaction to feeling ignored or disrespected. There is little in the case facts that we have to work with to indicate what the communication was between the gynecologist and the patient and her family. The statement of facts, however, leaves the impression that communication deteriorated as the postoperative pain endured.
Some additional areas of potential claims for liability in this case include:
- The explanation for the needle breaking during surgery is unclear from the brief statement of case facts. There might be malpractice liability if the surgeon was unreasonable in how the needle was used, used the wrong needle, or ignored defects in the needle.
- The surgeon tried unsuccessfully to retrieve the needle during the original surgery. If the surgeon’s failure to retrieve the needle was because of inadequate training, lack of care or the like, it might be seen as the “cause” of the patient’s injuries.
- The fact that a second surgeon was able to remove the needle tip, which resolved the patient’s pain, may raise the question of whether the first surgeon’s decision not to seek to remove it in response to the continuing pain was reasonable. If the first surgeon did not want to remove the needle tip, a question might be raised about whether that surgeon should have referred the patient to another surgeon. (The patient ultimately found another surgeon on her own.)
- Regarding use of TOT: A 21.5% complication rate ordinarily would be a significant factor to consider in a decision to use the tape. Physicians are responsible for keeping up with current developments in the devices and pharmaceuticals they use. Therefore, if information on the complication rate was available, the surgeon’s documentation should reflect the basis for choosing to use the tape. More important, the surgeon should document a conversation with the patient about the risks and benefits of using the TOT and the discussion of alternatives to its use.
What factors could have tipped the case toward the defense?
The defense verdict indicates that the jury determined there was no negligence, or that the patient could not prove any of these potential bases of liability. As noted above, what may have helped the defense is the fact that the surgeon documented the details of the informed consent conversation, including that “discussion was carried out regarding” the tape. The informed consent process is an important opportunity for communication with the patient, and a chance to make sure that expectations are reasonable. Liability for the failure of informed consent is not common. When something has gone wrong, however, it can matter whether the problem was something mentioned in the informed consent process. In addition, it was positive that postoperatively the patient was informed of the broken needle—although it is not clear who informed her about it.
A couple of other legal issues are worth noting. From our fact scenario we do not know what was documented in the incident report filed by the circulating nurse and reviewed by the surgical committee. We also do not know whether the plaintiff was privy to the incident report document. The surgical committee is likely a peer-review committee, and most states provide some privilege for such committees (to avoid disclosure of committee information for discovery or at trial). The deliberations and conclusions of the committee, therefore, were likely privileged. However, incident reports are frequently used for other purposes, such as administrative reports, that are not privileged—so the incident report often is determined to be discoverable depending on the interpretation of the state’s law.
No winner in this case
Despite the defense verdict, the physician was not really the “winner” after having spent a great deal of time, energy, money, and emotion defending this suit. Ultimately, the goal is not to win malpractice cases but to avoid them—in this case, among other things, by being frank with patients about expectations, keeping an open line of communication with patients when they are concerned with an outcome that is less than ideal, and referring a patient when it may be appropriate.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Bladder sling risks, complications and side effects. DrugWatch Web site. http://www.drugwatch.com/trans vaginal-mesh/bladder-sling/. Updated January 2, 2015. Accessed February 13, 2015.
2. Boyles SH, Edwards R, Gregory W, Clark A. Complications associated with transobturator sling procedures. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(1):19–22.
1. Bladder sling risks, complications and side effects. DrugWatch Web site. http://www.drugwatch.com/trans vaginal-mesh/bladder-sling/. Updated January 2, 2015. Accessed February 13, 2015.
2. Boyles SH, Edwards R, Gregory W, Clark A. Complications associated with transobturator sling procedures. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(1):19–22.
Three mesh cases: two defense verdicts; one large award
Transvaginal mesh not properly placed
In January 2007, polypropylene mesh (Gynecare Prolift Transvaginal Mesh; Ethicon) was inserted in a 57-year-old woman to treat bladder and rectal prolapse. The patient developed small-intestine obstruction, bladder contraction, and a large pelvic abscess. Surgical treatment of the complications included creation of a colostomy. She required daily self-catheterization. The patient died of unrelated causes after the suit was filed.
Estate’S CLAIM The gynecologist did not properly insert the mesh and did not fully inform the patient of possible complications.
PHYSICIAN’S DEFENSE The mesh was properly inserted. The patient developed an unpreventable adverse reaction to the mesh. Proper consent was obtained.
VERDICT A New York defense verdict was returned.
Polypropylene mesh removed due to pain
Polypropylene mesh (Obtryx Transobturator Midurethal Sling system, Boston Scientific Corporation [BSC]) was used to treat a woman’s stress urinary incontinence (SUI) in 2008. Following surgery, the patient reported pain. The mesh was partially removed in 2011. The patient has continuing pain and complications caused by remaining pieces of the mesh that the surgeon believes cannot be removed safely.
PATIENT’S CLAIM Although BSC warned that the material could oxidize and become brittle, the surgeon used it anyway. The mesh eroded through the urethra, causing permanent damage. BSC was negligent in the design, marketing, and instructions for Obtryx.
DEFENDANTS’ DEFENSE The surgeon read the instructions and felt the product was safe. BSC claimed the mesh is safe for SUI use. Directions for use clearly warn of possible erosion. A BSC engineer admitted that the tissue that surrounds the mesh can shrink, encapsulating nerves and causing chronic pain.
VERDICT A Massachusetts defense verdict was returned.
Abscesses, nerve damage: $73M
A 42-year-old woman reported SUI to her gynecologist. In January 2011, the gynecologist placed polypropylene pelvic mesh (Obtryx Trans-obturator Midurethal Sling system, BSC). Following surgery, the patient reported pain and fever; pelvic abscesses were found.
Multiple procedures partially removed the mesh and treated the infection. During one procedure, her femoral and obturator nerves were damaged; she walks with a limp. Dyspareunia and pain continue. Additional operations will be needed to remove more mesh and treat continuing infection.
PATIENT’S CLAIM BSC was negligent in the product’s design and marketing. Warnings for use were inadequate concerning the nature and extent of possible permanent injuries: groin and pelvic pain, dyspareunia, nerve damage, and chronic urinary tract infections. BSC withheld or concealed clinical trial information and did not perform and report proper post-market surveillance.
When pivotal study results were published in 2009, indicating that further research was needed to confirm that Obtryx was appropriate for treating SUI, the BSC sales department received an email telling them to not share this information with physicians.
At trial, BSC corporate executives knew little about system design and warnings regarding its use. Data that BSC provided to document the safety of Obtryx were not about that product.
MANUFACTURER’S DEFENSE Both sides agreed not to introduce discussion of the FDA and 510(k) process. BSC blamed a call-center for not passing along complaints from customers in a timely manner.
VERDICT A $73,465,000 Texas verdict was returned against BSC. The jury determined that the manufacturer displayed gross negligence; the design of the Obtryx system is faulty. The award included $50 million for exemplary damages, which the judge reduced to $11.2 million due to state caps, for a total award of $34.6 million.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Transvaginal mesh not properly placed
In January 2007, polypropylene mesh (Gynecare Prolift Transvaginal Mesh; Ethicon) was inserted in a 57-year-old woman to treat bladder and rectal prolapse. The patient developed small-intestine obstruction, bladder contraction, and a large pelvic abscess. Surgical treatment of the complications included creation of a colostomy. She required daily self-catheterization. The patient died of unrelated causes after the suit was filed.
Estate’S CLAIM The gynecologist did not properly insert the mesh and did not fully inform the patient of possible complications.
PHYSICIAN’S DEFENSE The mesh was properly inserted. The patient developed an unpreventable adverse reaction to the mesh. Proper consent was obtained.
VERDICT A New York defense verdict was returned.
Polypropylene mesh removed due to pain
Polypropylene mesh (Obtryx Transobturator Midurethal Sling system, Boston Scientific Corporation [BSC]) was used to treat a woman’s stress urinary incontinence (SUI) in 2008. Following surgery, the patient reported pain. The mesh was partially removed in 2011. The patient has continuing pain and complications caused by remaining pieces of the mesh that the surgeon believes cannot be removed safely.
PATIENT’S CLAIM Although BSC warned that the material could oxidize and become brittle, the surgeon used it anyway. The mesh eroded through the urethra, causing permanent damage. BSC was negligent in the design, marketing, and instructions for Obtryx.
DEFENDANTS’ DEFENSE The surgeon read the instructions and felt the product was safe. BSC claimed the mesh is safe for SUI use. Directions for use clearly warn of possible erosion. A BSC engineer admitted that the tissue that surrounds the mesh can shrink, encapsulating nerves and causing chronic pain.
VERDICT A Massachusetts defense verdict was returned.
Abscesses, nerve damage: $73M
A 42-year-old woman reported SUI to her gynecologist. In January 2011, the gynecologist placed polypropylene pelvic mesh (Obtryx Trans-obturator Midurethal Sling system, BSC). Following surgery, the patient reported pain and fever; pelvic abscesses were found.
Multiple procedures partially removed the mesh and treated the infection. During one procedure, her femoral and obturator nerves were damaged; she walks with a limp. Dyspareunia and pain continue. Additional operations will be needed to remove more mesh and treat continuing infection.
PATIENT’S CLAIM BSC was negligent in the product’s design and marketing. Warnings for use were inadequate concerning the nature and extent of possible permanent injuries: groin and pelvic pain, dyspareunia, nerve damage, and chronic urinary tract infections. BSC withheld or concealed clinical trial information and did not perform and report proper post-market surveillance.
When pivotal study results were published in 2009, indicating that further research was needed to confirm that Obtryx was appropriate for treating SUI, the BSC sales department received an email telling them to not share this information with physicians.
At trial, BSC corporate executives knew little about system design and warnings regarding its use. Data that BSC provided to document the safety of Obtryx were not about that product.
MANUFACTURER’S DEFENSE Both sides agreed not to introduce discussion of the FDA and 510(k) process. BSC blamed a call-center for not passing along complaints from customers in a timely manner.
VERDICT A $73,465,000 Texas verdict was returned against BSC. The jury determined that the manufacturer displayed gross negligence; the design of the Obtryx system is faulty. The award included $50 million for exemplary damages, which the judge reduced to $11.2 million due to state caps, for a total award of $34.6 million.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Transvaginal mesh not properly placed
In January 2007, polypropylene mesh (Gynecare Prolift Transvaginal Mesh; Ethicon) was inserted in a 57-year-old woman to treat bladder and rectal prolapse. The patient developed small-intestine obstruction, bladder contraction, and a large pelvic abscess. Surgical treatment of the complications included creation of a colostomy. She required daily self-catheterization. The patient died of unrelated causes after the suit was filed.
Estate’S CLAIM The gynecologist did not properly insert the mesh and did not fully inform the patient of possible complications.
PHYSICIAN’S DEFENSE The mesh was properly inserted. The patient developed an unpreventable adverse reaction to the mesh. Proper consent was obtained.
VERDICT A New York defense verdict was returned.
Polypropylene mesh removed due to pain
Polypropylene mesh (Obtryx Transobturator Midurethal Sling system, Boston Scientific Corporation [BSC]) was used to treat a woman’s stress urinary incontinence (SUI) in 2008. Following surgery, the patient reported pain. The mesh was partially removed in 2011. The patient has continuing pain and complications caused by remaining pieces of the mesh that the surgeon believes cannot be removed safely.
PATIENT’S CLAIM Although BSC warned that the material could oxidize and become brittle, the surgeon used it anyway. The mesh eroded through the urethra, causing permanent damage. BSC was negligent in the design, marketing, and instructions for Obtryx.
DEFENDANTS’ DEFENSE The surgeon read the instructions and felt the product was safe. BSC claimed the mesh is safe for SUI use. Directions for use clearly warn of possible erosion. A BSC engineer admitted that the tissue that surrounds the mesh can shrink, encapsulating nerves and causing chronic pain.
VERDICT A Massachusetts defense verdict was returned.
Abscesses, nerve damage: $73M
A 42-year-old woman reported SUI to her gynecologist. In January 2011, the gynecologist placed polypropylene pelvic mesh (Obtryx Trans-obturator Midurethal Sling system, BSC). Following surgery, the patient reported pain and fever; pelvic abscesses were found.
Multiple procedures partially removed the mesh and treated the infection. During one procedure, her femoral and obturator nerves were damaged; she walks with a limp. Dyspareunia and pain continue. Additional operations will be needed to remove more mesh and treat continuing infection.
PATIENT’S CLAIM BSC was negligent in the product’s design and marketing. Warnings for use were inadequate concerning the nature and extent of possible permanent injuries: groin and pelvic pain, dyspareunia, nerve damage, and chronic urinary tract infections. BSC withheld or concealed clinical trial information and did not perform and report proper post-market surveillance.
When pivotal study results were published in 2009, indicating that further research was needed to confirm that Obtryx was appropriate for treating SUI, the BSC sales department received an email telling them to not share this information with physicians.
At trial, BSC corporate executives knew little about system design and warnings regarding its use. Data that BSC provided to document the safety of Obtryx were not about that product.
MANUFACTURER’S DEFENSE Both sides agreed not to introduce discussion of the FDA and 510(k) process. BSC blamed a call-center for not passing along complaints from customers in a timely manner.
VERDICT A $73,465,000 Texas verdict was returned against BSC. The jury determined that the manufacturer displayed gross negligence; the design of the Obtryx system is faulty. The award included $50 million for exemplary damages, which the judge reduced to $11.2 million due to state caps, for a total award of $34.6 million.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Better stroke treatment moves tantalizingly within reach
Stroke is one of the most feared medical conditions, with the specter of suddenly finding oneself unable to talk, eat, walk, or live independently, according to study results.
In mid-February, results from three trials reported at the International Stroke Conference in Nashville, Tenn., changed the face of ischemic stroke treatment by proving that emergency endovascular catheterization to remove the embolus blocking cerebral blood flow produced better long-term outcomes than standard treatment with intravenous thrombolysis.
It wasn’t just that patients did better with endovascular embolectomy; it was how much they did better. In the two trials run in the United States and abroad, SWIFT PRIME and ESCAPE, the percentage of patients rated as not disabled (a modified Rankin Scale score of 0-1) when assessed after 90 days was 36% and 42% for patients treated with endovascular therapy in the two studies, compared with 17% and 19% in the two control arms. Embolectomy boosted the fraction of patients having the best stroke outcomes more than twofold, a breathtaking leap in efficacy.
Dr. Jeffrey L. Saver from UCLA, lead investigator for SWIFT PRIME, called it a “once-in-a-field” result, meaning that never again will stroke clinicians see this degree of incremental improvement by adding a new intervention.
The frustrating irony is how challenging delivery of this disease-altering treatment will be on a national scale. One problem is that it didn’t result from a single change in treatment, but from a careful mix of new diagnostic techniques with sophisticated CT imaging, new systems for expediting diagnosis, triage, transport, and treatment, in combination with new technology in the form of emboli-retrieving stents.
Stroke management specialists see a daunting series of issues to tackle as they attempt to roll out emergency endovascular interventions on a routine scale throughout much of the United States. Many more centers must open, modeled on the ones that succeeded in the trials. The centers need to be rationally positioned so they are close to patients but also give each center enough case volume to foster high interventional-skill levels. Staffing must be found for fast-moving stroke response teams that can make the diagnostics and interventions available around the clock and interpret the images to select appropriate patients. Ambulance systems have to be set up that take likely stroke patients to the centers that will best meet their treatment needs.
The stroke and public health communities will need to invest a lot of time, money, and leadership to make this happen, but it’s a clear mandate, given the promise endovascular treatment now holds to blunt the impact of one of medicine’s most feared maladies.
On Twitter @mitchelzoler
Stroke is one of the most feared medical conditions, with the specter of suddenly finding oneself unable to talk, eat, walk, or live independently, according to study results.
In mid-February, results from three trials reported at the International Stroke Conference in Nashville, Tenn., changed the face of ischemic stroke treatment by proving that emergency endovascular catheterization to remove the embolus blocking cerebral blood flow produced better long-term outcomes than standard treatment with intravenous thrombolysis.
It wasn’t just that patients did better with endovascular embolectomy; it was how much they did better. In the two trials run in the United States and abroad, SWIFT PRIME and ESCAPE, the percentage of patients rated as not disabled (a modified Rankin Scale score of 0-1) when assessed after 90 days was 36% and 42% for patients treated with endovascular therapy in the two studies, compared with 17% and 19% in the two control arms. Embolectomy boosted the fraction of patients having the best stroke outcomes more than twofold, a breathtaking leap in efficacy.
Dr. Jeffrey L. Saver from UCLA, lead investigator for SWIFT PRIME, called it a “once-in-a-field” result, meaning that never again will stroke clinicians see this degree of incremental improvement by adding a new intervention.
The frustrating irony is how challenging delivery of this disease-altering treatment will be on a national scale. One problem is that it didn’t result from a single change in treatment, but from a careful mix of new diagnostic techniques with sophisticated CT imaging, new systems for expediting diagnosis, triage, transport, and treatment, in combination with new technology in the form of emboli-retrieving stents.
Stroke management specialists see a daunting series of issues to tackle as they attempt to roll out emergency endovascular interventions on a routine scale throughout much of the United States. Many more centers must open, modeled on the ones that succeeded in the trials. The centers need to be rationally positioned so they are close to patients but also give each center enough case volume to foster high interventional-skill levels. Staffing must be found for fast-moving stroke response teams that can make the diagnostics and interventions available around the clock and interpret the images to select appropriate patients. Ambulance systems have to be set up that take likely stroke patients to the centers that will best meet their treatment needs.
The stroke and public health communities will need to invest a lot of time, money, and leadership to make this happen, but it’s a clear mandate, given the promise endovascular treatment now holds to blunt the impact of one of medicine’s most feared maladies.
On Twitter @mitchelzoler
Stroke is one of the most feared medical conditions, with the specter of suddenly finding oneself unable to talk, eat, walk, or live independently, according to study results.
In mid-February, results from three trials reported at the International Stroke Conference in Nashville, Tenn., changed the face of ischemic stroke treatment by proving that emergency endovascular catheterization to remove the embolus blocking cerebral blood flow produced better long-term outcomes than standard treatment with intravenous thrombolysis.
It wasn’t just that patients did better with endovascular embolectomy; it was how much they did better. In the two trials run in the United States and abroad, SWIFT PRIME and ESCAPE, the percentage of patients rated as not disabled (a modified Rankin Scale score of 0-1) when assessed after 90 days was 36% and 42% for patients treated with endovascular therapy in the two studies, compared with 17% and 19% in the two control arms. Embolectomy boosted the fraction of patients having the best stroke outcomes more than twofold, a breathtaking leap in efficacy.
Dr. Jeffrey L. Saver from UCLA, lead investigator for SWIFT PRIME, called it a “once-in-a-field” result, meaning that never again will stroke clinicians see this degree of incremental improvement by adding a new intervention.
The frustrating irony is how challenging delivery of this disease-altering treatment will be on a national scale. One problem is that it didn’t result from a single change in treatment, but from a careful mix of new diagnostic techniques with sophisticated CT imaging, new systems for expediting diagnosis, triage, transport, and treatment, in combination with new technology in the form of emboli-retrieving stents.
Stroke management specialists see a daunting series of issues to tackle as they attempt to roll out emergency endovascular interventions on a routine scale throughout much of the United States. Many more centers must open, modeled on the ones that succeeded in the trials. The centers need to be rationally positioned so they are close to patients but also give each center enough case volume to foster high interventional-skill levels. Staffing must be found for fast-moving stroke response teams that can make the diagnostics and interventions available around the clock and interpret the images to select appropriate patients. Ambulance systems have to be set up that take likely stroke patients to the centers that will best meet their treatment needs.
The stroke and public health communities will need to invest a lot of time, money, and leadership to make this happen, but it’s a clear mandate, given the promise endovascular treatment now holds to blunt the impact of one of medicine’s most feared maladies.
On Twitter @mitchelzoler
Diabetes therapy and cardiac risk
To the Editor: Recently, Drs. Zimmerman and Pantalone1 cited the Diabetes Control and Complications Trial (DCCT)2 and the United Kingdom Prospective Diabetes Study (UKPDS)3 as evidence that glycemic control lowers cardiac risk in type 2 diabetes. And in a related counterpoint article, Drs. Menon and Aggarwal4 also discussed the UKPDS.
These studies should not be cited in this context, since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available. The UKPDS was launched in 1977 and completed in 1997, and statins were not available until 1987. Indeed, the UKPDS showed that the strongest risk factor for myocardial infarction was an elevated level of low-density lipoprotein cholesterol, followed by a low level of high-density lipoprotein cholesterol.5 It is therefore not surprising that in the initial UKPDS report the incidence of myocardial infarction was not increased in the group with a 0.9% higher hemoglobin A1c, but that in the 10-year follow-up, when statins were probably used by most patients, myocardial infarction was reduced by a significant 15% (P = .01).3,6 As would be expected in the more modern studies, ie, the Action to Control Cardiovascular Risk (ACCORD),7 the Action in Diabetes and Vascular Disease (ADVANCE),8 and the Veteran Affairs Diabetes Trial (VADT),9 cardiovascular events were not reduced with improved glycemic control.
While the UKPDS clearly demonstrated a decrease in microvascular disease due to improved glycemic control, it should not be used as evidence that improved glycemic control in type 2 diabetes decreases cardiac events.3,6
- Zimmerman RS, Pantalone KM. Diabetes management: more than just cardiovascular risk? Cleve Clin J Med 2014; 81:672–676.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Menon V, Aggarwal B. Why are we doing cardiovascular outcome trials in type 2 diabetes? Cleve Clin J Med 2014; 81:665–671.
- Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316:823–828.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
To the Editor: Recently, Drs. Zimmerman and Pantalone1 cited the Diabetes Control and Complications Trial (DCCT)2 and the United Kingdom Prospective Diabetes Study (UKPDS)3 as evidence that glycemic control lowers cardiac risk in type 2 diabetes. And in a related counterpoint article, Drs. Menon and Aggarwal4 also discussed the UKPDS.
These studies should not be cited in this context, since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available. The UKPDS was launched in 1977 and completed in 1997, and statins were not available until 1987. Indeed, the UKPDS showed that the strongest risk factor for myocardial infarction was an elevated level of low-density lipoprotein cholesterol, followed by a low level of high-density lipoprotein cholesterol.5 It is therefore not surprising that in the initial UKPDS report the incidence of myocardial infarction was not increased in the group with a 0.9% higher hemoglobin A1c, but that in the 10-year follow-up, when statins were probably used by most patients, myocardial infarction was reduced by a significant 15% (P = .01).3,6 As would be expected in the more modern studies, ie, the Action to Control Cardiovascular Risk (ACCORD),7 the Action in Diabetes and Vascular Disease (ADVANCE),8 and the Veteran Affairs Diabetes Trial (VADT),9 cardiovascular events were not reduced with improved glycemic control.
While the UKPDS clearly demonstrated a decrease in microvascular disease due to improved glycemic control, it should not be used as evidence that improved glycemic control in type 2 diabetes decreases cardiac events.3,6
To the Editor: Recently, Drs. Zimmerman and Pantalone1 cited the Diabetes Control and Complications Trial (DCCT)2 and the United Kingdom Prospective Diabetes Study (UKPDS)3 as evidence that glycemic control lowers cardiac risk in type 2 diabetes. And in a related counterpoint article, Drs. Menon and Aggarwal4 also discussed the UKPDS.
These studies should not be cited in this context, since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available. The UKPDS was launched in 1977 and completed in 1997, and statins were not available until 1987. Indeed, the UKPDS showed that the strongest risk factor for myocardial infarction was an elevated level of low-density lipoprotein cholesterol, followed by a low level of high-density lipoprotein cholesterol.5 It is therefore not surprising that in the initial UKPDS report the incidence of myocardial infarction was not increased in the group with a 0.9% higher hemoglobin A1c, but that in the 10-year follow-up, when statins were probably used by most patients, myocardial infarction was reduced by a significant 15% (P = .01).3,6 As would be expected in the more modern studies, ie, the Action to Control Cardiovascular Risk (ACCORD),7 the Action in Diabetes and Vascular Disease (ADVANCE),8 and the Veteran Affairs Diabetes Trial (VADT),9 cardiovascular events were not reduced with improved glycemic control.
While the UKPDS clearly demonstrated a decrease in microvascular disease due to improved glycemic control, it should not be used as evidence that improved glycemic control in type 2 diabetes decreases cardiac events.3,6
- Zimmerman RS, Pantalone KM. Diabetes management: more than just cardiovascular risk? Cleve Clin J Med 2014; 81:672–676.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Menon V, Aggarwal B. Why are we doing cardiovascular outcome trials in type 2 diabetes? Cleve Clin J Med 2014; 81:665–671.
- Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316:823–828.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Zimmerman RS, Pantalone KM. Diabetes management: more than just cardiovascular risk? Cleve Clin J Med 2014; 81:672–676.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Menon V, Aggarwal B. Why are we doing cardiovascular outcome trials in type 2 diabetes? Cleve Clin J Med 2014; 81:665–671.
- Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316:823–828.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
In reply: Diabetes therapy and cardiac risk
In Reply: We appreciate Dr. Bell’s interest in and comments regarding our recent article. Dr. Bell contends that the DCCT1 and UKPDS2 studies should not be cited since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available.
While we can appreciate his point of view, we disagree with his interpretation of the available data. These studies, and their respective observational follow-up reports,3,4 provide evidence that early intervention may reduce cardiovascular risk, and that our approach to examining cardiovascular risk reduction in high-risk cardiovascular patients, as in ACCORD,5 ADVANCE,6 and VADT,7 may be short-sighted. There is an important difference between reducing long-term cardiovascular risk by treating younger and healthier patients with diabetes (type 1 or type 2) early in the disease course, before the development of complications (including cardiovascular disease), as was the case in DCCT and UKPDS, vs treating older patients with diabetes who have established cardiovascular disease or who have numerous risk factors substantially increasing their cardiovascular risk, as in ACCORD, ADVANCE, and VADT.
To his second point, that the UKPDS did not demonstrate cardiovascular risk reduction until after the 10-year follow-up when statins were probably utilized by the vast majority of patients, there would not have been a difference in cardiac events between treatment and control groups during this observational period if the statins were the cause of the reduced rate of cardiac events. The control and treatment groups would have had the same reduction in events. That was not the case. The finding of a lower risk of myocardial infarction at the completion of the follow-up period, despite ubiquitous statin use by both the treatment and control groups during this 10-year period, suggests another variable—ie, that the early differences in glycemic control achieved between the treatment and control groups during the UKPDS was responsible for the observed reduction in the risk of myocardial infarction.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
In Reply: We appreciate Dr. Bell’s interest in and comments regarding our recent article. Dr. Bell contends that the DCCT1 and UKPDS2 studies should not be cited since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available.
While we can appreciate his point of view, we disagree with his interpretation of the available data. These studies, and their respective observational follow-up reports,3,4 provide evidence that early intervention may reduce cardiovascular risk, and that our approach to examining cardiovascular risk reduction in high-risk cardiovascular patients, as in ACCORD,5 ADVANCE,6 and VADT,7 may be short-sighted. There is an important difference between reducing long-term cardiovascular risk by treating younger and healthier patients with diabetes (type 1 or type 2) early in the disease course, before the development of complications (including cardiovascular disease), as was the case in DCCT and UKPDS, vs treating older patients with diabetes who have established cardiovascular disease or who have numerous risk factors substantially increasing their cardiovascular risk, as in ACCORD, ADVANCE, and VADT.
To his second point, that the UKPDS did not demonstrate cardiovascular risk reduction until after the 10-year follow-up when statins were probably utilized by the vast majority of patients, there would not have been a difference in cardiac events between treatment and control groups during this observational period if the statins were the cause of the reduced rate of cardiac events. The control and treatment groups would have had the same reduction in events. That was not the case. The finding of a lower risk of myocardial infarction at the completion of the follow-up period, despite ubiquitous statin use by both the treatment and control groups during this 10-year period, suggests another variable—ie, that the early differences in glycemic control achieved between the treatment and control groups during the UKPDS was responsible for the observed reduction in the risk of myocardial infarction.
In Reply: We appreciate Dr. Bell’s interest in and comments regarding our recent article. Dr. Bell contends that the DCCT1 and UKPDS2 studies should not be cited since the DCCT is a study of type 1 and not type 2 diabetic patients, and the UKPDS was performed in an era when statins were not available.
While we can appreciate his point of view, we disagree with his interpretation of the available data. These studies, and their respective observational follow-up reports,3,4 provide evidence that early intervention may reduce cardiovascular risk, and that our approach to examining cardiovascular risk reduction in high-risk cardiovascular patients, as in ACCORD,5 ADVANCE,6 and VADT,7 may be short-sighted. There is an important difference between reducing long-term cardiovascular risk by treating younger and healthier patients with diabetes (type 1 or type 2) early in the disease course, before the development of complications (including cardiovascular disease), as was the case in DCCT and UKPDS, vs treating older patients with diabetes who have established cardiovascular disease or who have numerous risk factors substantially increasing their cardiovascular risk, as in ACCORD, ADVANCE, and VADT.
To his second point, that the UKPDS did not demonstrate cardiovascular risk reduction until after the 10-year follow-up when statins were probably utilized by the vast majority of patients, there would not have been a difference in cardiac events between treatment and control groups during this observational period if the statins were the cause of the reduced rate of cardiac events. The control and treatment groups would have had the same reduction in events. That was not the case. The finding of a lower risk of myocardial infarction at the completion of the follow-up period, despite ubiquitous statin use by both the treatment and control groups during this 10-year period, suggests another variable—ie, that the early differences in glycemic control achieved between the treatment and control groups during the UKPDS was responsible for the observed reduction in the risk of myocardial infarction.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- Nathan DM, Cleary PA, Backlund JY, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353:2643–2653.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
Epithelial Ovarian Cancer: Evaluation, Staging, Surgery, and Stage I and II Disease Management
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Ovarian cancer is the second most common gynecologic cancer among women in the United States. It is also the fifth leading cause of cancer mortality in women and the leading cause of death among women with gynecologic malignancies. The American Cancer Society statistics released in 2015 estimate that 21,290 new cases of ovarian cancer will occur during the year, with approximately 14,180 deaths. Globally, there were 238,719 new cases of ovarian cancer diagnosed in 2012, representing 3.6% of all cancers in women, and nearly 151,905 deaths. The highest incidence of ovarian cancer occurs in northern, central, and eastern Europe, followed by western Europe and North America, with the lowest incidence in parts of Africa and Asia. The majority of women presenting with ovarian cancer will present at an advanced stage, and the 5-year survival in this group is less than 30%.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Ovarian cancer is the second most common gynecologic cancer among women in the United States. It is also the fifth leading cause of cancer mortality in women and the leading cause of death among women with gynecologic malignancies. The American Cancer Society statistics released in 2015 estimate that 21,290 new cases of ovarian cancer will occur during the year, with approximately 14,180 deaths. Globally, there were 238,719 new cases of ovarian cancer diagnosed in 2012, representing 3.6% of all cancers in women, and nearly 151,905 deaths. The highest incidence of ovarian cancer occurs in northern, central, and eastern Europe, followed by western Europe and North America, with the lowest incidence in parts of Africa and Asia. The majority of women presenting with ovarian cancer will present at an advanced stage, and the 5-year survival in this group is less than 30%.
To read the full article in PDF:
Edited by: Arthur T. Skarin, MD, FACP, FCCP
Ovarian cancer is the second most common gynecologic cancer among women in the United States. It is also the fifth leading cause of cancer mortality in women and the leading cause of death among women with gynecologic malignancies. The American Cancer Society statistics released in 2015 estimate that 21,290 new cases of ovarian cancer will occur during the year, with approximately 14,180 deaths. Globally, there were 238,719 new cases of ovarian cancer diagnosed in 2012, representing 3.6% of all cancers in women, and nearly 151,905 deaths. The highest incidence of ovarian cancer occurs in northern, central, and eastern Europe, followed by western Europe and North America, with the lowest incidence in parts of Africa and Asia. The majority of women presenting with ovarian cancer will present at an advanced stage, and the 5-year survival in this group is less than 30%.
To read the full article in PDF:
Drug seems promising for kids with severe hemophilia B
Results of a phase 3 study suggest a recombinant factor IX Fc fusion protein (rFIXFc, also known as eftrenonacog alfa and Alprolix) is a feasible treatment option for children with severe hemophilia B.
rFIXFc effectively prevented and treated bleeding episodes, patients did not develop inhibitors, and there were no serious adverse events related to treatment.
Sobi and Biogen Idec, the companies developing rFIXFc, recently announced these results from the now-complete Kids B-LONG study.
They said the successful completion of this study supports applications for pediatric indications in several regions and is an important step in seeking marketing authorization for rFIXFc in Europe.
Interim results of the Kids B-LONG study helped support the US approval of rFIXFc for use in children.
In Kids B-LONG, researchers tested rFIXFc in 30 previously treated children younger than 12 who had severe hemophilia B. Patients had at least 50 prior exposure days to factor IX therapies.
Twenty-seven patients (90%) completed the study. The median time spent on study was 49.4 weeks, and 24 participants received rFIXFc injections on at least 50 separate days.
Children who received rFIXFc prophylactically had an overall median annualized bleeding rate (ABR) of 1.97. The median ABR for spontaneous joint bleeds was 0.
Approximately 33% of patients did not experience any bleeding episodes. About 92% of bleeding episodes were controlled by 1 or 2 injections of rFIXFc.
None of the patients developed inhibitors to rFIXFc. The terminal half-life of the product was 66.5 hours for children under 6 and 70.3 hours for children ages 6 to 11.
Researchers said there were no treatment-related serious adverse events and no cases of serious allergic reactions or vascular thrombotic events. None of the patients discontinued the study due to an adverse event.
One adverse event—decreased appetite occurring in 1 patient—was considered related to rFIXFc treatment.
The pattern of treatment-emergent adverse events in this study was generally consistent with results seen in adolescents and adults in the phase 3 B-LONG study. Common adverse reactions in that study were headache and oral paresthesia.
Additional analyses of the Kids B-LONG study are ongoing, and detailed results will be presented at a future scientific meeting, according to Sobi and Biogen Idec. ![]()
Results of a phase 3 study suggest a recombinant factor IX Fc fusion protein (rFIXFc, also known as eftrenonacog alfa and Alprolix) is a feasible treatment option for children with severe hemophilia B.
rFIXFc effectively prevented and treated bleeding episodes, patients did not develop inhibitors, and there were no serious adverse events related to treatment.
Sobi and Biogen Idec, the companies developing rFIXFc, recently announced these results from the now-complete Kids B-LONG study.
They said the successful completion of this study supports applications for pediatric indications in several regions and is an important step in seeking marketing authorization for rFIXFc in Europe.
Interim results of the Kids B-LONG study helped support the US approval of rFIXFc for use in children.
In Kids B-LONG, researchers tested rFIXFc in 30 previously treated children younger than 12 who had severe hemophilia B. Patients had at least 50 prior exposure days to factor IX therapies.
Twenty-seven patients (90%) completed the study. The median time spent on study was 49.4 weeks, and 24 participants received rFIXFc injections on at least 50 separate days.
Children who received rFIXFc prophylactically had an overall median annualized bleeding rate (ABR) of 1.97. The median ABR for spontaneous joint bleeds was 0.
Approximately 33% of patients did not experience any bleeding episodes. About 92% of bleeding episodes were controlled by 1 or 2 injections of rFIXFc.
None of the patients developed inhibitors to rFIXFc. The terminal half-life of the product was 66.5 hours for children under 6 and 70.3 hours for children ages 6 to 11.
Researchers said there were no treatment-related serious adverse events and no cases of serious allergic reactions or vascular thrombotic events. None of the patients discontinued the study due to an adverse event.
One adverse event—decreased appetite occurring in 1 patient—was considered related to rFIXFc treatment.
The pattern of treatment-emergent adverse events in this study was generally consistent with results seen in adolescents and adults in the phase 3 B-LONG study. Common adverse reactions in that study were headache and oral paresthesia.
Additional analyses of the Kids B-LONG study are ongoing, and detailed results will be presented at a future scientific meeting, according to Sobi and Biogen Idec. ![]()
Results of a phase 3 study suggest a recombinant factor IX Fc fusion protein (rFIXFc, also known as eftrenonacog alfa and Alprolix) is a feasible treatment option for children with severe hemophilia B.
rFIXFc effectively prevented and treated bleeding episodes, patients did not develop inhibitors, and there were no serious adverse events related to treatment.
Sobi and Biogen Idec, the companies developing rFIXFc, recently announced these results from the now-complete Kids B-LONG study.
They said the successful completion of this study supports applications for pediatric indications in several regions and is an important step in seeking marketing authorization for rFIXFc in Europe.
Interim results of the Kids B-LONG study helped support the US approval of rFIXFc for use in children.
In Kids B-LONG, researchers tested rFIXFc in 30 previously treated children younger than 12 who had severe hemophilia B. Patients had at least 50 prior exposure days to factor IX therapies.
Twenty-seven patients (90%) completed the study. The median time spent on study was 49.4 weeks, and 24 participants received rFIXFc injections on at least 50 separate days.
Children who received rFIXFc prophylactically had an overall median annualized bleeding rate (ABR) of 1.97. The median ABR for spontaneous joint bleeds was 0.
Approximately 33% of patients did not experience any bleeding episodes. About 92% of bleeding episodes were controlled by 1 or 2 injections of rFIXFc.
None of the patients developed inhibitors to rFIXFc. The terminal half-life of the product was 66.5 hours for children under 6 and 70.3 hours for children ages 6 to 11.
Researchers said there were no treatment-related serious adverse events and no cases of serious allergic reactions or vascular thrombotic events. None of the patients discontinued the study due to an adverse event.
One adverse event—decreased appetite occurring in 1 patient—was considered related to rFIXFc treatment.
The pattern of treatment-emergent adverse events in this study was generally consistent with results seen in adolescents and adults in the phase 3 B-LONG study. Common adverse reactions in that study were headache and oral paresthesia.
Additional analyses of the Kids B-LONG study are ongoing, and detailed results will be presented at a future scientific meeting, according to Sobi and Biogen Idec. ![]()