User login
More AF patients need anticoagulants, guidelines suggest

Results of a large analysis suggest the latest guidelines for atrial fibrillation (AF) recommend anticoagulant therapy for nearly all women with AF and AF patients older than 65.
In 2014, the American Heart Association, American College of Cardiology, and Heart Rhythm Society issued broader guidelines for the use of anticoagulants in AF patients.
A group of researchers wanted to assess how these guidelines would change the use of anticoagulant therapy.
So they evaluated patients enrolled in the ORBIT-AF study, comparing how recommendations from the 2011 AF guidelines and the guidelines issued in 2014 would affect these patients.
Emily O’Brien, PhD, of the Duke Clinical Research Institute in Durham, North Carolina, and her colleagues conducted this research and described their findings in a letter to JAMA Internal Medicine.
The ORBIT-AF study included 10,132 AF patients from 176 sites across the US. Available data included patients’ age, gender, and risk factors such as prior congestive heart failure, high blood pressure, diabetes, and prior stroke.
The researchers found the overall proportion of AF patients recommended for anticoagulants increased from about 72% of patients with the 2011 guidelines to 91% with the newer guidelines.
A similar increase occurred for women with AF. Under the previous guidelines, anticoagulants would have been recommended for about 77% of female AF patients in the study population. Under the new guidelines, 98% of women in the sample population would have enough risk factors to qualify for treatment.
The 2014 guidelines also lower the age at which patients are considered at risk for stroke from 75 to 65.
In the study population, this meant that anticoagulant therapy would be recommended for almost 99% of patients with AF who were older than 65, compared to roughly 80% whose stroke risk was severe enough under the previous criteria.
“The full adoption of the guidelines could reclassify nearly 1 million people with AFib who previously weren’t recommended for treatment with blood thinners,” Dr O’Brien said.
“What we don’t know yet is the extent to which doctors in community practice will incorporate the guidelines into their clinical routines and what that will mean for the long-term outcomes for those patients. That will be the next step for our study.” ![]()

Results of a large analysis suggest the latest guidelines for atrial fibrillation (AF) recommend anticoagulant therapy for nearly all women with AF and AF patients older than 65.
In 2014, the American Heart Association, American College of Cardiology, and Heart Rhythm Society issued broader guidelines for the use of anticoagulants in AF patients.
A group of researchers wanted to assess how these guidelines would change the use of anticoagulant therapy.
So they evaluated patients enrolled in the ORBIT-AF study, comparing how recommendations from the 2011 AF guidelines and the guidelines issued in 2014 would affect these patients.
Emily O’Brien, PhD, of the Duke Clinical Research Institute in Durham, North Carolina, and her colleagues conducted this research and described their findings in a letter to JAMA Internal Medicine.
The ORBIT-AF study included 10,132 AF patients from 176 sites across the US. Available data included patients’ age, gender, and risk factors such as prior congestive heart failure, high blood pressure, diabetes, and prior stroke.
The researchers found the overall proportion of AF patients recommended for anticoagulants increased from about 72% of patients with the 2011 guidelines to 91% with the newer guidelines.
A similar increase occurred for women with AF. Under the previous guidelines, anticoagulants would have been recommended for about 77% of female AF patients in the study population. Under the new guidelines, 98% of women in the sample population would have enough risk factors to qualify for treatment.
The 2014 guidelines also lower the age at which patients are considered at risk for stroke from 75 to 65.
In the study population, this meant that anticoagulant therapy would be recommended for almost 99% of patients with AF who were older than 65, compared to roughly 80% whose stroke risk was severe enough under the previous criteria.
“The full adoption of the guidelines could reclassify nearly 1 million people with AFib who previously weren’t recommended for treatment with blood thinners,” Dr O’Brien said.
“What we don’t know yet is the extent to which doctors in community practice will incorporate the guidelines into their clinical routines and what that will mean for the long-term outcomes for those patients. That will be the next step for our study.” ![]()

Results of a large analysis suggest the latest guidelines for atrial fibrillation (AF) recommend anticoagulant therapy for nearly all women with AF and AF patients older than 65.
In 2014, the American Heart Association, American College of Cardiology, and Heart Rhythm Society issued broader guidelines for the use of anticoagulants in AF patients.
A group of researchers wanted to assess how these guidelines would change the use of anticoagulant therapy.
So they evaluated patients enrolled in the ORBIT-AF study, comparing how recommendations from the 2011 AF guidelines and the guidelines issued in 2014 would affect these patients.
Emily O’Brien, PhD, of the Duke Clinical Research Institute in Durham, North Carolina, and her colleagues conducted this research and described their findings in a letter to JAMA Internal Medicine.
The ORBIT-AF study included 10,132 AF patients from 176 sites across the US. Available data included patients’ age, gender, and risk factors such as prior congestive heart failure, high blood pressure, diabetes, and prior stroke.
The researchers found the overall proportion of AF patients recommended for anticoagulants increased from about 72% of patients with the 2011 guidelines to 91% with the newer guidelines.
A similar increase occurred for women with AF. Under the previous guidelines, anticoagulants would have been recommended for about 77% of female AF patients in the study population. Under the new guidelines, 98% of women in the sample population would have enough risk factors to qualify for treatment.
The 2014 guidelines also lower the age at which patients are considered at risk for stroke from 75 to 65.
In the study population, this meant that anticoagulant therapy would be recommended for almost 99% of patients with AF who were older than 65, compared to roughly 80% whose stroke risk was severe enough under the previous criteria.
“The full adoption of the guidelines could reclassify nearly 1 million people with AFib who previously weren’t recommended for treatment with blood thinners,” Dr O’Brien said.
“What we don’t know yet is the extent to which doctors in community practice will incorporate the guidelines into their clinical routines and what that will mean for the long-term outcomes for those patients. That will be the next step for our study.” ![]()
3D-printed devices can deliver drugs in vitro
ATLANTA—Interventional radiologists say they’ve successfully used 3D printers to develop personalized medical devices that can deliver antibiotics and chemotherapy in a targeted manner in vitro.
Researchers and engineers collaborated to print catheters, stents, and filaments that were bioactive, giving these devices the ability to deliver antibiotics and chemotherapeutic medications to a targeted area in cell cultures.
Horacio R. D’Agostino, MD, of Louisiana State University Health Sciences Center in Shreveport, discussed this work at the 2015 Society of Interventional Radiology’s Annual Scientific Meeting (abstract 13).
“3D printing allows for tailor-made materials for personalized medicine,” Dr D’Agostino said. “It gives us the ability to construct devices that meet patients’ needs, from their unique anatomy to specific medicine requirements. And as tools in interventional radiology, these devices are part of treatment options that are less invasive than traditional surgery.”
Using 3D printing technology and resorbable bioplastics, Dr D’Agostino and his colleagues developed bioactive filaments, chemotherapy beads, and catheters and stents containing antibiotics or chemotherapeutic agents.
The team then tested these devices in cell cultures. They found the antibiotic-containing catheters inhibited E coli growth, and filaments carrying chemotherapeutic agents inhibited the growth of osteosarcoma cells.
“We treat a wide variety of patients and, with some patients, the current one-size-fits-all devices are not an option,” Dr D’Agostino noted. “3D printing gives us the ability to craft devices that are better suited for certain patient populations that are traditionally tough to treat, such as children and the obese, who have different anatomy. There’s limitless potential to be explored with this technology.”
The researchers were also able to print biodegradable filaments, catheters, and stents that contain antibiotics and chemotherapeutic agents. These devices might help patients avoid the need to undergo a second procedure or treatment to remove or destroy the delivery vehicle.
Dr D’Agostino said this early success with 3D-printed instruments in the lab warrants further studies, with the goal of receiving approval to use these devices in humans. He also sees an opportunity to collaborate with other medical specialties to deliver higher-quality, personalized care to all types of patients. ![]()

ATLANTA—Interventional radiologists say they’ve successfully used 3D printers to develop personalized medical devices that can deliver antibiotics and chemotherapy in a targeted manner in vitro.
Researchers and engineers collaborated to print catheters, stents, and filaments that were bioactive, giving these devices the ability to deliver antibiotics and chemotherapeutic medications to a targeted area in cell cultures.
Horacio R. D’Agostino, MD, of Louisiana State University Health Sciences Center in Shreveport, discussed this work at the 2015 Society of Interventional Radiology’s Annual Scientific Meeting (abstract 13).
“3D printing allows for tailor-made materials for personalized medicine,” Dr D’Agostino said. “It gives us the ability to construct devices that meet patients’ needs, from their unique anatomy to specific medicine requirements. And as tools in interventional radiology, these devices are part of treatment options that are less invasive than traditional surgery.”
Using 3D printing technology and resorbable bioplastics, Dr D’Agostino and his colleagues developed bioactive filaments, chemotherapy beads, and catheters and stents containing antibiotics or chemotherapeutic agents.
The team then tested these devices in cell cultures. They found the antibiotic-containing catheters inhibited E coli growth, and filaments carrying chemotherapeutic agents inhibited the growth of osteosarcoma cells.
“We treat a wide variety of patients and, with some patients, the current one-size-fits-all devices are not an option,” Dr D’Agostino noted. “3D printing gives us the ability to craft devices that are better suited for certain patient populations that are traditionally tough to treat, such as children and the obese, who have different anatomy. There’s limitless potential to be explored with this technology.”
The researchers were also able to print biodegradable filaments, catheters, and stents that contain antibiotics and chemotherapeutic agents. These devices might help patients avoid the need to undergo a second procedure or treatment to remove or destroy the delivery vehicle.
Dr D’Agostino said this early success with 3D-printed instruments in the lab warrants further studies, with the goal of receiving approval to use these devices in humans. He also sees an opportunity to collaborate with other medical specialties to deliver higher-quality, personalized care to all types of patients. ![]()

ATLANTA—Interventional radiologists say they’ve successfully used 3D printers to develop personalized medical devices that can deliver antibiotics and chemotherapy in a targeted manner in vitro.
Researchers and engineers collaborated to print catheters, stents, and filaments that were bioactive, giving these devices the ability to deliver antibiotics and chemotherapeutic medications to a targeted area in cell cultures.
Horacio R. D’Agostino, MD, of Louisiana State University Health Sciences Center in Shreveport, discussed this work at the 2015 Society of Interventional Radiology’s Annual Scientific Meeting (abstract 13).
“3D printing allows for tailor-made materials for personalized medicine,” Dr D’Agostino said. “It gives us the ability to construct devices that meet patients’ needs, from their unique anatomy to specific medicine requirements. And as tools in interventional radiology, these devices are part of treatment options that are less invasive than traditional surgery.”
Using 3D printing technology and resorbable bioplastics, Dr D’Agostino and his colleagues developed bioactive filaments, chemotherapy beads, and catheters and stents containing antibiotics or chemotherapeutic agents.
The team then tested these devices in cell cultures. They found the antibiotic-containing catheters inhibited E coli growth, and filaments carrying chemotherapeutic agents inhibited the growth of osteosarcoma cells.
“We treat a wide variety of patients and, with some patients, the current one-size-fits-all devices are not an option,” Dr D’Agostino noted. “3D printing gives us the ability to craft devices that are better suited for certain patient populations that are traditionally tough to treat, such as children and the obese, who have different anatomy. There’s limitless potential to be explored with this technology.”
The researchers were also able to print biodegradable filaments, catheters, and stents that contain antibiotics and chemotherapeutic agents. These devices might help patients avoid the need to undergo a second procedure or treatment to remove or destroy the delivery vehicle.
Dr D’Agostino said this early success with 3D-printed instruments in the lab warrants further studies, with the goal of receiving approval to use these devices in humans. He also sees an opportunity to collaborate with other medical specialties to deliver higher-quality, personalized care to all types of patients. ![]()
Nighttime Clinical Encounters
For hospitalized patients, restrictions on resident duty hours and the hospitalist movement have led to fragmentation in care.[1] After 2003 duty‐hour regulations were implemented, one study estimated an increase of 11% in care transfers for a given patient, whereas another study reported that an individual intern participated in 40% more handoffs.[2, 3] Although these changes have represented an improvement in safety with reduced provider fatigue and increased expertise in inpatient care, tradeoffs in safety may occur. Communication breakdown during care transfers has been implicated in many medical errors,[4, 5, 6] and the ability to safely transfer a patient's care has been identified as a necessary clinical skill.[7] The Accreditation Council on Graduate Medical Education has mandated that training programs include education to ensure effective handoff processes.[8] The Joint Commission has developed a toolset for improving handoffs.[9] Taking cues from the military and other industries that operate continuously, approaches designed to standardize handoffs have been developed.[3, 10, 11, 12]
The use of handoff tools has been reported to reduce the time required to transfer care from one provider to another,[13] but evidence that these handoff tools improve quality of care is limited.[14, 15] Concern that patients have poorer outcomes in care transitions remains, particularly at night when many patients are cared for by covering or night float providers.[6] Studies regarding the outcomes of patients at night have had mixed results.[16, 17, 18] Uncertainty is inherent in the trajectories of individual patients and in the systems in which they receive care.[19] The recognition of uncertainty reframes care transitions from a problem of improving information transfer to a problem of navigating uncertainty, or making sense. Sensemaking is an activity through which providers come to understand what is happening with a patient, in a way that allows them to take action.[20]
We sought to better understand how to support providers' ability to make sense and act in uncertain situations, focusing on night float resident physicians. We hoped to better understand overnight encounters and the information needed to navigate them. We approached the issue in two ways: first, through assessing resident attitudes and perceptions of handoffs using survey methodology, and second, through assessing actual calls night float residents receive and strategies they use to navigate these scenarios. We focused on handoffs between the primary team and covering nighttime providers. Our goal was to use this information to understand what approaches could better support care transitions and handoff practices.
METHODS
General Approach
We surveyed residents regarding handoffs. We also collected self‐reported information about calls received by night float postgraduate year (PGY) 1 (intern) residents and the strategies they used to address these calls.
Setting
Our study was conducted in the internal medicine residency program at the University of Texas Health Science Center at San Antonio, which has approximately 90 residents, 76 of whom are categorical. Residents work at 2 primary teaching hospitals: the Audie L. Murphy Veterans Affairs Hospital (ALMVAH), the 220‐bed acute care hospital for the South Texas Veterans Health Care System, and University Hospital (UH), the 614‐bed county hospital for Bexar County.
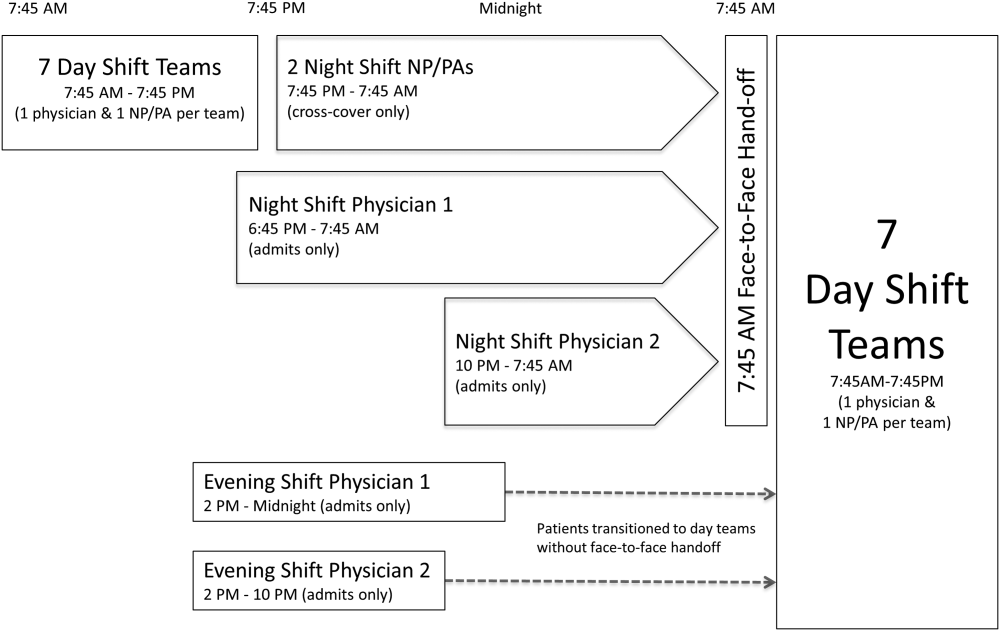
The residency program implemented a night float system in 1992. Daytime care is performed by multiple teams, which are comprised of one attending, one resident, and two to three interns. These teams sign out to the on‐call team in the late afternoon to early evening. The on‐call team in turn signs out to a night intern who is supervised by a resident and on‐site faculty member. The night float intern is responsible for all patient care on five inpatient teams until 7 am the following day, but is not responsible for admitting patients. In the morning, the night intern discusses overnight events with the day teams as they arrive.
Sign‐out consists of verbal and written communication. At ALMVAH, written documentation is created within the electronic medical record. Basic information is prepopulated, and clinical information is modifiable. At UH, written documentation is created in word processing software and maintained within a document saved electronically. It is expected that the day team update the modifiable information within these documents on a daily basis. The written documentation is printed and given to the covering interns (see Supporting Information, Appendix 1, in the online version of this article showing the sign‐out tools used by our program.).
The day team is responsible for the content and level of detail in the written sign‐out. There are three domains including: main diagnosis, clinical history and course, and plans of care. The clinical history and course is a synopsis of the patient presentation including current clinical status. The plans of care are reserved for expectant management or conditional statements.
Survey Development
A survey regarding resident experiences and perceptions of handoffs was developed by the Department of Surgery, and we adapted it to the internal medicine residency program. The survey contained 48 questions focused on the following areas: attitudes toward night float, communication content, and night float behaviors (see Supporting Information, Appendix 2, in the online version of this article for the full survey). Some responses were recorded in a 5‐point Likert‐type format, in a range of strongly disagree to strongly agree. Others were recorded on a 4‐point frequency scale from never to always. Paper and online survey versions were created, and residents could respond using either modality.
Survey Administration
All residents were asked to participate in the survey. Paper versions were distributed in March 2012. All residents also received an e‐mail soliciting participation. Responses were collected anonymously. Reminders were sent on a biweekly basis for six weeks. Survey administration was concluded by May 2012, and no incentive was offered for completion.
Overnight Call Data
We asked the night interns at both hospitals to self‐report activities in real time during their shift. To minimize respondent burden and obtain a representative sample, they collected data on their activities over 2‐hour periods. On any given night, a predetermined period was assigned, and all periods were sampled equally over the duration of data collection. A total of six interns at both hospitals were asked to participate over 18 nights during a 3‐month period in 2011. Convenience sampling was used, and participants were identified based on clinical schedules.
The tool allowed interns to record unique encounters initiated as a phone call or page. Open‐ended responses were permitted for caller identification and encounter reason. The interns categorized the source of background information and were permitted to select more than one for any given encounter. Similarly, the interns were asked to categorize the type of action required to respond (see Supporting Information, Appendix 3, in the online version of this article for the self‐report tool).
Overnight encounters were categorized as clinical, administrative, or pain related. Clinical encounters consisted of calls related to clinical conditions that would require clinical assessment and decision making, for example, a patient with new fever. Administrative encounters consisted of contact for reasons that would require only acknowledgement from a physician. An example of an administrative encounter is restraint renewal. Pain‐related calls consisted of calls for patients experiencing pain or requests for new or additional pain medications.
Analysis
Frequency and percentages were calculated for each category of encounter, including callers and reasons for calls. Comparisons were made between reasons for the encounter, the sources of background information utilized, and actions taken in response. Survey data were analyzed using Microsoft Excel (Microsoft Corp., Redmond, WA).
RESULTS
Encounter Data
Data from 299 encounters were recorded, and 96.7% (289/299) encounters were complete. Clinical encounters were most frequent at 54.7% (158/289), whereas administrative notifications or pain‐related encounters were 32.9% (158/289) and 12.5% (36/289), respectively. Nurses initiated 94.8% (274/289) of encounters.
Sources of information used by interns varied by reason for the call and are shown in Table 1. Responding to clinical requests, interns most frequently interacted with a nurse alone or in combination with the chart (51.3%, 81/158). Responding to administrative notifications, the interns most frequently spoke to only the nurse as the primary source of information (44.2%, 42/95). In pain‐related notifications, the nurse alone as a source of information accounted for 33.3% (12/36) of encounters. The sign‐out tool was not used in 72.3% (209/289) of encounters.
| Information Source | Encounter Type | |||
|---|---|---|---|---|
| Clinical* | Administrative | Pain Related | All | |
| ||||
| Only tool | 2 (1.3%) | 6 (6.3%) | 2 (5.6%) | 10 (3.5%) |
| Only nurse | 30 (19.0%) | 42 (44.2%) | 12 (33.3%) | 84 (29.1%) |
| Only chart‖ | 28 (17.7%) | 14 (14.7%) | 5 (13.9%) | 47 (16.3%) |
| Only miscellaneous | 4 (2.5%) | 2 (2.1%) | 0 (0.0%) | 6 (2.1%) |
| Tool+nurse | 10 (6.3%) | 8 (8.4%) | 5 (13.9%) | 23 (8.0%) |
| Tool+chart‖ | 10 (6.3%) | 5 (5.3%) | 1 (2.8%) | 16 (5.5%) |
| Nurse+chart‖ | 51 (32.3%) | 12 (12.6%) | 5 (13.9%) | 68 (23.5%) |
| Nurse+miscellaneous | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Chart+miscellaneous | 3 (1.9%) | 0 (0.0%) | 0 (0.0%) | 3 (1.0%) |
| Tool, nurse,+chart‖ | 19 (12.0%) | 6 (6.3%) | 6 (16.7%) | 31 (10.7%) |
Use of miscellaneous information sources was infrequent; removing these left 279 encounters with complete information. To better assess the instances in which the handoff tool was used, we combined categories for information sources. These data are summarized in Table 2.
| Information Source | Request Type | ||
|---|---|---|---|
| Clinicala | Administrativeb | Pain Relatedc | |
| |||
| Only tool | 2 (1.3%) | 6 (6.5%) | 2 (5.6%) |
| Only nurse | 30 (20.0%) | 42 (45.2%) | 12 (33.3%) |
| Only chart | 28 (18.7%) | 14 (15.1%) | 5 (13.9%) |
| Any combination with tool | 39 (26.0%) | 19 (20.4%) | 12 (33.3%) |
| Any combination without tool | 51 (34.0%) | 12 (12.9%) | 5 (13.9%) |
The actions taken by interns varied by reason for the call. Clinical encounters had the most variety of actions taken, with 55.1% (87/158) resulting in a new medication order and 49.9% (78/158) handled over the phone. Bedside evaluations occurred in 23.4% (37/158) of the encounters, and 3.8% (6/158) were documented in the electronic medical record. Administrative encounter responses were more homogeneous; 96.8% (92/95) were handled entirely over the phone. Responses to pain‐related requests were similarly less varied than clinical encounters; 63.9% (23/36) were handled over the phone and 66.7% (24/36) resulted in a new medication order. Neither administrative nor pain notifications resulted in documentation in the electronic medical records. These data are summarized in Table 3. Despite the availability of a resident and attending overnight, only 6.3% (10/150) of the clinical requests led to a discussion with them; none of the administrative or pain‐related notifications involved discussion with either the resident or the attending.
| Actions Taken | Encounter Type | ||
|---|---|---|---|
| Clinicala | Administrativeb | Pain Relatedc | |
| |||
| Handled over the phone | 78 (49.4%) | 92 (96.8%) | 23 (63.9%) |
| Evaluated the patient at the bedside | 37 (23.4%) | 2 (2.1%) | 2 (5.6%) |
| Reviewed previously ordered labs or imaging | 43 (27.2%) | 12 (12.6%) | 0 (0.0%) |
| Ordered new lab or imaging | 44 (27.8%) | 2 (2.1%) | 0 (0.0%) |
| Ordered new medication | 87 (55.1%) | 1 (1.1%) | 24 (66.7%) |
| Wrote cross‐cover note | 6 (3.8%) | 0 (0.0%) | 0 (0.0%) |
| Conferred with supervising physician | 10 (6.3%) | 0 (0.0%) | 0 (0.0%) |
| Called consult | 3 (1.9%) | 0 (0.0%) | 0 (0.0%) |
| Upgraded level of care | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) |
Survey Data
Fifty‐three residents completed surveys, for an overall response rate of 59.6% (53/89). All PGYs were represented; PGY‐3s had a response rate of 68.0% (17/25), PGY‐2s had a 58.3% response rate (14/24), and PGY‐1s had a 55% response rate (22/40).
A night float intern was perceived to be safer than an on‐call team performing the same job by 73.6% (39/89) of respondents. The written sign‐out was considered a time saver by 66% (35/53) of respondents. The sign‐out procedure was thought to be frequently or always safe by 73.6% (39/89). Overnight documentation within the electronic medical record was reported to be frequently or always completed by 58.5% (31/53).
Furthermore, 20.7% (11/53) of respondents reported receiving a do not do list frequently or always, and 43.4% (23/53) of respondents reported giving a do not do list frequently or always. Conditional statements were reported as frequently or always given by 90.4% (47/52). A standardized verbal checkout was considered safer by 71.7% (38/53), standardized written documentation was considered beneficial by 94.3% (50/53), and a checklist to go over was considered beneficial by 84.9% (45/53).
DISCUSSION
Our goal was to understand how to better support care transitions and handoff processes. Our residents report that current approaches to care transitions are safe and useful. Although this perception is reassuring, it is difficult to know whether this reflects the actual delivery of safe care. A minority of residents report giving and receiving do not do lists, which are important aspects of care when giving guidance to a covering physician. Also, we find discrepancies between our survey results and nighttime call collection data in important areas. Although residents report that the written sign‐out is useful, it was deemed useful for resolving a clinical issue only 27% of the time. Previous reports have found variable and conflicting rates of written sign‐out utilization, as well as variable quality of a written sign‐out,[21, 22, 23] and our data support infrequent usage. Residents were much more likely to access the electronic medical record than they were to use the handoff tool. Additionally, although residents report documentation, very little actual documentation occurred. The high rates of calls for routine and pain‐related notifications are notable and should be examined further for areas of potential improvement. Preemptive orders for routine, common, and benign conditions are often not employed as strategy and their omission can lead to higher workloads for nighttime physicians. Additionally, education and training may be necessary to help housestaff understand how such a strategy is safely implemented, such as a specific regimen for mild pain, and why it is helpful beyond reducing nighttime workload, such as a proactive approach to clinical care.
Several important insights emerge from our results. First, the electronic health record is accessible, and providers use it frequently. This raises the question of the need for a handoff tool for information transfer. When data can be easily accessed, their presence in a physical tool may be less important. Because electronic health records can easily be leveraged to populate handoff tools, having a brief tool that minimizes information transfer but better supports clinical reasoning may be more effective.
Second, our data highlight the need to focus on the handback, or providing information back to the returning day team. Our experience and previous studies support that this process is not adequately developed.[24, 25] There is little opportunity for communication between the covering and primary providers, and there is little documentation. In our observations, 3.8% of calls resulted in documentation, whereas the majority of respondents to the survey state it is performed frequently or always. The reason for this discrepancy is unclear, but fostering more of a mentality that considers all of the providers involved in patient care to be part of the same team may help address this issue.
Third, clinical services assume providers have what they need to provide care in the form of the handoff instrument. In fact, providers have handoff instruments, but whether they need them is unclear. Based on these observations, overnight physicians are able to provide care in the vast majority of cases without the use of the handoff tool.
Fourth, our data demonstrate the social or relational nature of providing clinical coverage. The single most frequent action taken by covering residents was speaking to the nurse. This may not be surprising; however, when we reframe transitions of care and handoffs as a relational issue, we are forced to reframe potential strategies to improve these transitions. The problem we need to address is not only of information transfer; it is also of making sense of what is happening.
How do we make handoff tools more effective sensemaking tools? More focus on contingency statements might be an approach. These have the dual benefit of helping the covering provider to make sense using the primary team's reasoning, as well as improving the primary team's reasoning by making the potential complications more explicit. Another approach could be to reinforce relational actions, through providing guidance on who to call if there is a change in the status of the patient. We found that the night intern rarely discussed care with supervising physicians, indicating weak integration of the night team. The handoff tool could thus strengthen the network of providers caring for the patient. A tool that emphasizes sensemaking may be a tool that captures the nonroutine aspects of care that are not already documented in the health record.
Our data are limited in that they were collected in a single institution over few nights with few interns. Our processes may not be representative, and our expectations for provider communication may not be the norm. Although a night float system of coverage is not the only model of providing care, it is common, and our handoff tool is similar to those reported in the literature. One area of concern is that our handback expectations may be less robust than other institutions. Despite this limitation, the larger issues of information transfer and sensemaking are generally applicable. Although we collected data over only 18 nights, we did obtain information on almost 300 calls, giving us a robust sample of actual issues that residents were called to resolve. Interns are the most involved in actually providing night coverage. Their response rate was 55%, slightly below our overall response rate of 59.6%, but representing the majority of interns. A 2‐step process of sign‐out may have ramifications on care transitions; however, these data were collected at night. Because the handoff tool information is the day team's responsibility, the process may have less impact on these results.
Coverage and care transfers are part of the inpatient landscape, and it may be unreasonable to expect care to be delivered by a group of providers who know the patient with the same level of depth at all hours of the day. By understanding that fostering effective care for patients requires providers to pay attention to not only how they transfer information, but also how they collectively make sense of what is happening, we will enable safer care.
Disclosures: The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (CDA 07‐022). Investigator salary support was provided through this funding, and through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Parts of these data were presented at the 2013 SGIM National Meeting in Denver, Colorado. The authors report no conflicts of interest.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–358.
- , , , . Transfers of patient care between house staff on internal medicine wards: a national survey. Arch Intern Med. 2006;166(11):1173–1177.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , , , . The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458–471.
- “Improving America's Hospitals.” The Joint Commission's Annual Report on Quality and Safety. Available at: http://www.jointcommission.org/Improving_Americas_Hospitals_The_Joint_Commissions_Annual_Report_on_Quality_and_Safety_‐_2007. Published 2007. Accessed November 17, 2014.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , . Resident handoffs: appreciating them as a critical competency. J Gen Intern Med. 2012;27(3):270–272.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/Common_Program_Requirements_07012011%5B2%5D.pdf. Published July 1, 2011. Accessed November 17, 2014.
- Joint Commission Center for Transforming Healthcare. Hand‐off communications. Available at: http://www.centerfortransforminghealthcare.org/projects/detail.aspx?Project=1. Accessed November 17, 2014.
- , . Handover and note‐keeping: the SBAR approach. Clin Risk. 2010;16(5):173–175.
- , , , et al. A structured handoff program for interns. Acad Med. 2009;84(3):347–352.
- , , , et al. I‐pass, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- , , . SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32(3):167–175.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , , , . Systematic review: effects of resident work hours on patient safety. Ann Intern Med. 2004;141(11):851–857.
- , . Mortality in out‐of‐hours emergency medical admissions—more than just a weekend effect. J R Coll Physicians Edinb. 2010;40(2):115–118.
- , , , , , . The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10–14.
- , , . Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31(6):828–838.
- . Sensemaking in Organizations. Thousand Oaks, CA: Sage Publications; 1995.
- , , , , . Answering questions on call: pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , . Effectiveness of written hospitalist sign‐outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609–614.
- , , . Assessment of internal medicine trainee sign‐out quality and utilization habits. Intern Emerg Med. 2014;9(5):529–535.
- , , , , . Morning handover of on‐call issues: opportunities for improvement. JAMA Intern Med. 2014;174(9):1479–1485.
- , , , et al. Improving resident morning sign‐out by use of daily events reports [published online ahead of print February 11, 2014]. J Patient Saf. doi: 10.1097/PTS.0b013e31829e4f56
For hospitalized patients, restrictions on resident duty hours and the hospitalist movement have led to fragmentation in care.[1] After 2003 duty‐hour regulations were implemented, one study estimated an increase of 11% in care transfers for a given patient, whereas another study reported that an individual intern participated in 40% more handoffs.[2, 3] Although these changes have represented an improvement in safety with reduced provider fatigue and increased expertise in inpatient care, tradeoffs in safety may occur. Communication breakdown during care transfers has been implicated in many medical errors,[4, 5, 6] and the ability to safely transfer a patient's care has been identified as a necessary clinical skill.[7] The Accreditation Council on Graduate Medical Education has mandated that training programs include education to ensure effective handoff processes.[8] The Joint Commission has developed a toolset for improving handoffs.[9] Taking cues from the military and other industries that operate continuously, approaches designed to standardize handoffs have been developed.[3, 10, 11, 12]
The use of handoff tools has been reported to reduce the time required to transfer care from one provider to another,[13] but evidence that these handoff tools improve quality of care is limited.[14, 15] Concern that patients have poorer outcomes in care transitions remains, particularly at night when many patients are cared for by covering or night float providers.[6] Studies regarding the outcomes of patients at night have had mixed results.[16, 17, 18] Uncertainty is inherent in the trajectories of individual patients and in the systems in which they receive care.[19] The recognition of uncertainty reframes care transitions from a problem of improving information transfer to a problem of navigating uncertainty, or making sense. Sensemaking is an activity through which providers come to understand what is happening with a patient, in a way that allows them to take action.[20]
We sought to better understand how to support providers' ability to make sense and act in uncertain situations, focusing on night float resident physicians. We hoped to better understand overnight encounters and the information needed to navigate them. We approached the issue in two ways: first, through assessing resident attitudes and perceptions of handoffs using survey methodology, and second, through assessing actual calls night float residents receive and strategies they use to navigate these scenarios. We focused on handoffs between the primary team and covering nighttime providers. Our goal was to use this information to understand what approaches could better support care transitions and handoff practices.
METHODS
General Approach
We surveyed residents regarding handoffs. We also collected self‐reported information about calls received by night float postgraduate year (PGY) 1 (intern) residents and the strategies they used to address these calls.
Setting
Our study was conducted in the internal medicine residency program at the University of Texas Health Science Center at San Antonio, which has approximately 90 residents, 76 of whom are categorical. Residents work at 2 primary teaching hospitals: the Audie L. Murphy Veterans Affairs Hospital (ALMVAH), the 220‐bed acute care hospital for the South Texas Veterans Health Care System, and University Hospital (UH), the 614‐bed county hospital for Bexar County.
The residency program implemented a night float system in 1992. Daytime care is performed by multiple teams, which are comprised of one attending, one resident, and two to three interns. These teams sign out to the on‐call team in the late afternoon to early evening. The on‐call team in turn signs out to a night intern who is supervised by a resident and on‐site faculty member. The night float intern is responsible for all patient care on five inpatient teams until 7 am the following day, but is not responsible for admitting patients. In the morning, the night intern discusses overnight events with the day teams as they arrive.
Sign‐out consists of verbal and written communication. At ALMVAH, written documentation is created within the electronic medical record. Basic information is prepopulated, and clinical information is modifiable. At UH, written documentation is created in word processing software and maintained within a document saved electronically. It is expected that the day team update the modifiable information within these documents on a daily basis. The written documentation is printed and given to the covering interns (see Supporting Information, Appendix 1, in the online version of this article showing the sign‐out tools used by our program.).
The day team is responsible for the content and level of detail in the written sign‐out. There are three domains including: main diagnosis, clinical history and course, and plans of care. The clinical history and course is a synopsis of the patient presentation including current clinical status. The plans of care are reserved for expectant management or conditional statements.
Survey Development
A survey regarding resident experiences and perceptions of handoffs was developed by the Department of Surgery, and we adapted it to the internal medicine residency program. The survey contained 48 questions focused on the following areas: attitudes toward night float, communication content, and night float behaviors (see Supporting Information, Appendix 2, in the online version of this article for the full survey). Some responses were recorded in a 5‐point Likert‐type format, in a range of strongly disagree to strongly agree. Others were recorded on a 4‐point frequency scale from never to always. Paper and online survey versions were created, and residents could respond using either modality.
Survey Administration
All residents were asked to participate in the survey. Paper versions were distributed in March 2012. All residents also received an e‐mail soliciting participation. Responses were collected anonymously. Reminders were sent on a biweekly basis for six weeks. Survey administration was concluded by May 2012, and no incentive was offered for completion.
Overnight Call Data
We asked the night interns at both hospitals to self‐report activities in real time during their shift. To minimize respondent burden and obtain a representative sample, they collected data on their activities over 2‐hour periods. On any given night, a predetermined period was assigned, and all periods were sampled equally over the duration of data collection. A total of six interns at both hospitals were asked to participate over 18 nights during a 3‐month period in 2011. Convenience sampling was used, and participants were identified based on clinical schedules.
The tool allowed interns to record unique encounters initiated as a phone call or page. Open‐ended responses were permitted for caller identification and encounter reason. The interns categorized the source of background information and were permitted to select more than one for any given encounter. Similarly, the interns were asked to categorize the type of action required to respond (see Supporting Information, Appendix 3, in the online version of this article for the self‐report tool).
Overnight encounters were categorized as clinical, administrative, or pain related. Clinical encounters consisted of calls related to clinical conditions that would require clinical assessment and decision making, for example, a patient with new fever. Administrative encounters consisted of contact for reasons that would require only acknowledgement from a physician. An example of an administrative encounter is restraint renewal. Pain‐related calls consisted of calls for patients experiencing pain or requests for new or additional pain medications.
Analysis
Frequency and percentages were calculated for each category of encounter, including callers and reasons for calls. Comparisons were made between reasons for the encounter, the sources of background information utilized, and actions taken in response. Survey data were analyzed using Microsoft Excel (Microsoft Corp., Redmond, WA).
RESULTS
Encounter Data
Data from 299 encounters were recorded, and 96.7% (289/299) encounters were complete. Clinical encounters were most frequent at 54.7% (158/289), whereas administrative notifications or pain‐related encounters were 32.9% (158/289) and 12.5% (36/289), respectively. Nurses initiated 94.8% (274/289) of encounters.
Sources of information used by interns varied by reason for the call and are shown in Table 1. Responding to clinical requests, interns most frequently interacted with a nurse alone or in combination with the chart (51.3%, 81/158). Responding to administrative notifications, the interns most frequently spoke to only the nurse as the primary source of information (44.2%, 42/95). In pain‐related notifications, the nurse alone as a source of information accounted for 33.3% (12/36) of encounters. The sign‐out tool was not used in 72.3% (209/289) of encounters.
| Information Source | Encounter Type | |||
|---|---|---|---|---|
| Clinical* | Administrative | Pain Related | All | |
| ||||
| Only tool | 2 (1.3%) | 6 (6.3%) | 2 (5.6%) | 10 (3.5%) |
| Only nurse | 30 (19.0%) | 42 (44.2%) | 12 (33.3%) | 84 (29.1%) |
| Only chart‖ | 28 (17.7%) | 14 (14.7%) | 5 (13.9%) | 47 (16.3%) |
| Only miscellaneous | 4 (2.5%) | 2 (2.1%) | 0 (0.0%) | 6 (2.1%) |
| Tool+nurse | 10 (6.3%) | 8 (8.4%) | 5 (13.9%) | 23 (8.0%) |
| Tool+chart‖ | 10 (6.3%) | 5 (5.3%) | 1 (2.8%) | 16 (5.5%) |
| Nurse+chart‖ | 51 (32.3%) | 12 (12.6%) | 5 (13.9%) | 68 (23.5%) |
| Nurse+miscellaneous | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Chart+miscellaneous | 3 (1.9%) | 0 (0.0%) | 0 (0.0%) | 3 (1.0%) |
| Tool, nurse,+chart‖ | 19 (12.0%) | 6 (6.3%) | 6 (16.7%) | 31 (10.7%) |
Use of miscellaneous information sources was infrequent; removing these left 279 encounters with complete information. To better assess the instances in which the handoff tool was used, we combined categories for information sources. These data are summarized in Table 2.
| Information Source | Request Type | ||
|---|---|---|---|
| Clinicala | Administrativeb | Pain Relatedc | |
| |||
| Only tool | 2 (1.3%) | 6 (6.5%) | 2 (5.6%) |
| Only nurse | 30 (20.0%) | 42 (45.2%) | 12 (33.3%) |
| Only chart | 28 (18.7%) | 14 (15.1%) | 5 (13.9%) |
| Any combination with tool | 39 (26.0%) | 19 (20.4%) | 12 (33.3%) |
| Any combination without tool | 51 (34.0%) | 12 (12.9%) | 5 (13.9%) |
The actions taken by interns varied by reason for the call. Clinical encounters had the most variety of actions taken, with 55.1% (87/158) resulting in a new medication order and 49.9% (78/158) handled over the phone. Bedside evaluations occurred in 23.4% (37/158) of the encounters, and 3.8% (6/158) were documented in the electronic medical record. Administrative encounter responses were more homogeneous; 96.8% (92/95) were handled entirely over the phone. Responses to pain‐related requests were similarly less varied than clinical encounters; 63.9% (23/36) were handled over the phone and 66.7% (24/36) resulted in a new medication order. Neither administrative nor pain notifications resulted in documentation in the electronic medical records. These data are summarized in Table 3. Despite the availability of a resident and attending overnight, only 6.3% (10/150) of the clinical requests led to a discussion with them; none of the administrative or pain‐related notifications involved discussion with either the resident or the attending.
| Actions Taken | Encounter Type | ||
|---|---|---|---|
| Clinicala | Administrativeb | Pain Relatedc | |
| |||
| Handled over the phone | 78 (49.4%) | 92 (96.8%) | 23 (63.9%) |
| Evaluated the patient at the bedside | 37 (23.4%) | 2 (2.1%) | 2 (5.6%) |
| Reviewed previously ordered labs or imaging | 43 (27.2%) | 12 (12.6%) | 0 (0.0%) |
| Ordered new lab or imaging | 44 (27.8%) | 2 (2.1%) | 0 (0.0%) |
| Ordered new medication | 87 (55.1%) | 1 (1.1%) | 24 (66.7%) |
| Wrote cross‐cover note | 6 (3.8%) | 0 (0.0%) | 0 (0.0%) |
| Conferred with supervising physician | 10 (6.3%) | 0 (0.0%) | 0 (0.0%) |
| Called consult | 3 (1.9%) | 0 (0.0%) | 0 (0.0%) |
| Upgraded level of care | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) |
Survey Data
Fifty‐three residents completed surveys, for an overall response rate of 59.6% (53/89). All PGYs were represented; PGY‐3s had a response rate of 68.0% (17/25), PGY‐2s had a 58.3% response rate (14/24), and PGY‐1s had a 55% response rate (22/40).
A night float intern was perceived to be safer than an on‐call team performing the same job by 73.6% (39/89) of respondents. The written sign‐out was considered a time saver by 66% (35/53) of respondents. The sign‐out procedure was thought to be frequently or always safe by 73.6% (39/89). Overnight documentation within the electronic medical record was reported to be frequently or always completed by 58.5% (31/53).
Furthermore, 20.7% (11/53) of respondents reported receiving a do not do list frequently or always, and 43.4% (23/53) of respondents reported giving a do not do list frequently or always. Conditional statements were reported as frequently or always given by 90.4% (47/52). A standardized verbal checkout was considered safer by 71.7% (38/53), standardized written documentation was considered beneficial by 94.3% (50/53), and a checklist to go over was considered beneficial by 84.9% (45/53).
DISCUSSION
Our goal was to understand how to better support care transitions and handoff processes. Our residents report that current approaches to care transitions are safe and useful. Although this perception is reassuring, it is difficult to know whether this reflects the actual delivery of safe care. A minority of residents report giving and receiving do not do lists, which are important aspects of care when giving guidance to a covering physician. Also, we find discrepancies between our survey results and nighttime call collection data in important areas. Although residents report that the written sign‐out is useful, it was deemed useful for resolving a clinical issue only 27% of the time. Previous reports have found variable and conflicting rates of written sign‐out utilization, as well as variable quality of a written sign‐out,[21, 22, 23] and our data support infrequent usage. Residents were much more likely to access the electronic medical record than they were to use the handoff tool. Additionally, although residents report documentation, very little actual documentation occurred. The high rates of calls for routine and pain‐related notifications are notable and should be examined further for areas of potential improvement. Preemptive orders for routine, common, and benign conditions are often not employed as strategy and their omission can lead to higher workloads for nighttime physicians. Additionally, education and training may be necessary to help housestaff understand how such a strategy is safely implemented, such as a specific regimen for mild pain, and why it is helpful beyond reducing nighttime workload, such as a proactive approach to clinical care.
Several important insights emerge from our results. First, the electronic health record is accessible, and providers use it frequently. This raises the question of the need for a handoff tool for information transfer. When data can be easily accessed, their presence in a physical tool may be less important. Because electronic health records can easily be leveraged to populate handoff tools, having a brief tool that minimizes information transfer but better supports clinical reasoning may be more effective.
Second, our data highlight the need to focus on the handback, or providing information back to the returning day team. Our experience and previous studies support that this process is not adequately developed.[24, 25] There is little opportunity for communication between the covering and primary providers, and there is little documentation. In our observations, 3.8% of calls resulted in documentation, whereas the majority of respondents to the survey state it is performed frequently or always. The reason for this discrepancy is unclear, but fostering more of a mentality that considers all of the providers involved in patient care to be part of the same team may help address this issue.
Third, clinical services assume providers have what they need to provide care in the form of the handoff instrument. In fact, providers have handoff instruments, but whether they need them is unclear. Based on these observations, overnight physicians are able to provide care in the vast majority of cases without the use of the handoff tool.
Fourth, our data demonstrate the social or relational nature of providing clinical coverage. The single most frequent action taken by covering residents was speaking to the nurse. This may not be surprising; however, when we reframe transitions of care and handoffs as a relational issue, we are forced to reframe potential strategies to improve these transitions. The problem we need to address is not only of information transfer; it is also of making sense of what is happening.
How do we make handoff tools more effective sensemaking tools? More focus on contingency statements might be an approach. These have the dual benefit of helping the covering provider to make sense using the primary team's reasoning, as well as improving the primary team's reasoning by making the potential complications more explicit. Another approach could be to reinforce relational actions, through providing guidance on who to call if there is a change in the status of the patient. We found that the night intern rarely discussed care with supervising physicians, indicating weak integration of the night team. The handoff tool could thus strengthen the network of providers caring for the patient. A tool that emphasizes sensemaking may be a tool that captures the nonroutine aspects of care that are not already documented in the health record.
Our data are limited in that they were collected in a single institution over few nights with few interns. Our processes may not be representative, and our expectations for provider communication may not be the norm. Although a night float system of coverage is not the only model of providing care, it is common, and our handoff tool is similar to those reported in the literature. One area of concern is that our handback expectations may be less robust than other institutions. Despite this limitation, the larger issues of information transfer and sensemaking are generally applicable. Although we collected data over only 18 nights, we did obtain information on almost 300 calls, giving us a robust sample of actual issues that residents were called to resolve. Interns are the most involved in actually providing night coverage. Their response rate was 55%, slightly below our overall response rate of 59.6%, but representing the majority of interns. A 2‐step process of sign‐out may have ramifications on care transitions; however, these data were collected at night. Because the handoff tool information is the day team's responsibility, the process may have less impact on these results.
Coverage and care transfers are part of the inpatient landscape, and it may be unreasonable to expect care to be delivered by a group of providers who know the patient with the same level of depth at all hours of the day. By understanding that fostering effective care for patients requires providers to pay attention to not only how they transfer information, but also how they collectively make sense of what is happening, we will enable safer care.
Disclosures: The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (CDA 07‐022). Investigator salary support was provided through this funding, and through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Parts of these data were presented at the 2013 SGIM National Meeting in Denver, Colorado. The authors report no conflicts of interest.
For hospitalized patients, restrictions on resident duty hours and the hospitalist movement have led to fragmentation in care.[1] After 2003 duty‐hour regulations were implemented, one study estimated an increase of 11% in care transfers for a given patient, whereas another study reported that an individual intern participated in 40% more handoffs.[2, 3] Although these changes have represented an improvement in safety with reduced provider fatigue and increased expertise in inpatient care, tradeoffs in safety may occur. Communication breakdown during care transfers has been implicated in many medical errors,[4, 5, 6] and the ability to safely transfer a patient's care has been identified as a necessary clinical skill.[7] The Accreditation Council on Graduate Medical Education has mandated that training programs include education to ensure effective handoff processes.[8] The Joint Commission has developed a toolset for improving handoffs.[9] Taking cues from the military and other industries that operate continuously, approaches designed to standardize handoffs have been developed.[3, 10, 11, 12]
The use of handoff tools has been reported to reduce the time required to transfer care from one provider to another,[13] but evidence that these handoff tools improve quality of care is limited.[14, 15] Concern that patients have poorer outcomes in care transitions remains, particularly at night when many patients are cared for by covering or night float providers.[6] Studies regarding the outcomes of patients at night have had mixed results.[16, 17, 18] Uncertainty is inherent in the trajectories of individual patients and in the systems in which they receive care.[19] The recognition of uncertainty reframes care transitions from a problem of improving information transfer to a problem of navigating uncertainty, or making sense. Sensemaking is an activity through which providers come to understand what is happening with a patient, in a way that allows them to take action.[20]
We sought to better understand how to support providers' ability to make sense and act in uncertain situations, focusing on night float resident physicians. We hoped to better understand overnight encounters and the information needed to navigate them. We approached the issue in two ways: first, through assessing resident attitudes and perceptions of handoffs using survey methodology, and second, through assessing actual calls night float residents receive and strategies they use to navigate these scenarios. We focused on handoffs between the primary team and covering nighttime providers. Our goal was to use this information to understand what approaches could better support care transitions and handoff practices.
METHODS
General Approach
We surveyed residents regarding handoffs. We also collected self‐reported information about calls received by night float postgraduate year (PGY) 1 (intern) residents and the strategies they used to address these calls.
Setting
Our study was conducted in the internal medicine residency program at the University of Texas Health Science Center at San Antonio, which has approximately 90 residents, 76 of whom are categorical. Residents work at 2 primary teaching hospitals: the Audie L. Murphy Veterans Affairs Hospital (ALMVAH), the 220‐bed acute care hospital for the South Texas Veterans Health Care System, and University Hospital (UH), the 614‐bed county hospital for Bexar County.
The residency program implemented a night float system in 1992. Daytime care is performed by multiple teams, which are comprised of one attending, one resident, and two to three interns. These teams sign out to the on‐call team in the late afternoon to early evening. The on‐call team in turn signs out to a night intern who is supervised by a resident and on‐site faculty member. The night float intern is responsible for all patient care on five inpatient teams until 7 am the following day, but is not responsible for admitting patients. In the morning, the night intern discusses overnight events with the day teams as they arrive.
Sign‐out consists of verbal and written communication. At ALMVAH, written documentation is created within the electronic medical record. Basic information is prepopulated, and clinical information is modifiable. At UH, written documentation is created in word processing software and maintained within a document saved electronically. It is expected that the day team update the modifiable information within these documents on a daily basis. The written documentation is printed and given to the covering interns (see Supporting Information, Appendix 1, in the online version of this article showing the sign‐out tools used by our program.).
The day team is responsible for the content and level of detail in the written sign‐out. There are three domains including: main diagnosis, clinical history and course, and plans of care. The clinical history and course is a synopsis of the patient presentation including current clinical status. The plans of care are reserved for expectant management or conditional statements.
Survey Development
A survey regarding resident experiences and perceptions of handoffs was developed by the Department of Surgery, and we adapted it to the internal medicine residency program. The survey contained 48 questions focused on the following areas: attitudes toward night float, communication content, and night float behaviors (see Supporting Information, Appendix 2, in the online version of this article for the full survey). Some responses were recorded in a 5‐point Likert‐type format, in a range of strongly disagree to strongly agree. Others were recorded on a 4‐point frequency scale from never to always. Paper and online survey versions were created, and residents could respond using either modality.
Survey Administration
All residents were asked to participate in the survey. Paper versions were distributed in March 2012. All residents also received an e‐mail soliciting participation. Responses were collected anonymously. Reminders were sent on a biweekly basis for six weeks. Survey administration was concluded by May 2012, and no incentive was offered for completion.
Overnight Call Data
We asked the night interns at both hospitals to self‐report activities in real time during their shift. To minimize respondent burden and obtain a representative sample, they collected data on their activities over 2‐hour periods. On any given night, a predetermined period was assigned, and all periods were sampled equally over the duration of data collection. A total of six interns at both hospitals were asked to participate over 18 nights during a 3‐month period in 2011. Convenience sampling was used, and participants were identified based on clinical schedules.
The tool allowed interns to record unique encounters initiated as a phone call or page. Open‐ended responses were permitted for caller identification and encounter reason. The interns categorized the source of background information and were permitted to select more than one for any given encounter. Similarly, the interns were asked to categorize the type of action required to respond (see Supporting Information, Appendix 3, in the online version of this article for the self‐report tool).
Overnight encounters were categorized as clinical, administrative, or pain related. Clinical encounters consisted of calls related to clinical conditions that would require clinical assessment and decision making, for example, a patient with new fever. Administrative encounters consisted of contact for reasons that would require only acknowledgement from a physician. An example of an administrative encounter is restraint renewal. Pain‐related calls consisted of calls for patients experiencing pain or requests for new or additional pain medications.
Analysis
Frequency and percentages were calculated for each category of encounter, including callers and reasons for calls. Comparisons were made between reasons for the encounter, the sources of background information utilized, and actions taken in response. Survey data were analyzed using Microsoft Excel (Microsoft Corp., Redmond, WA).
RESULTS
Encounter Data
Data from 299 encounters were recorded, and 96.7% (289/299) encounters were complete. Clinical encounters were most frequent at 54.7% (158/289), whereas administrative notifications or pain‐related encounters were 32.9% (158/289) and 12.5% (36/289), respectively. Nurses initiated 94.8% (274/289) of encounters.
Sources of information used by interns varied by reason for the call and are shown in Table 1. Responding to clinical requests, interns most frequently interacted with a nurse alone or in combination with the chart (51.3%, 81/158). Responding to administrative notifications, the interns most frequently spoke to only the nurse as the primary source of information (44.2%, 42/95). In pain‐related notifications, the nurse alone as a source of information accounted for 33.3% (12/36) of encounters. The sign‐out tool was not used in 72.3% (209/289) of encounters.
| Information Source | Encounter Type | |||
|---|---|---|---|---|
| Clinical* | Administrative | Pain Related | All | |
| ||||
| Only tool | 2 (1.3%) | 6 (6.3%) | 2 (5.6%) | 10 (3.5%) |
| Only nurse | 30 (19.0%) | 42 (44.2%) | 12 (33.3%) | 84 (29.1%) |
| Only chart‖ | 28 (17.7%) | 14 (14.7%) | 5 (13.9%) | 47 (16.3%) |
| Only miscellaneous | 4 (2.5%) | 2 (2.1%) | 0 (0.0%) | 6 (2.1%) |
| Tool+nurse | 10 (6.3%) | 8 (8.4%) | 5 (13.9%) | 23 (8.0%) |
| Tool+chart‖ | 10 (6.3%) | 5 (5.3%) | 1 (2.8%) | 16 (5.5%) |
| Nurse+chart‖ | 51 (32.3%) | 12 (12.6%) | 5 (13.9%) | 68 (23.5%) |
| Nurse+miscellaneous | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) |
| Chart+miscellaneous | 3 (1.9%) | 0 (0.0%) | 0 (0.0%) | 3 (1.0%) |
| Tool, nurse,+chart‖ | 19 (12.0%) | 6 (6.3%) | 6 (16.7%) | 31 (10.7%) |
Use of miscellaneous information sources was infrequent; removing these left 279 encounters with complete information. To better assess the instances in which the handoff tool was used, we combined categories for information sources. These data are summarized in Table 2.
| Information Source | Request Type | ||
|---|---|---|---|
| Clinicala | Administrativeb | Pain Relatedc | |
| |||
| Only tool | 2 (1.3%) | 6 (6.5%) | 2 (5.6%) |
| Only nurse | 30 (20.0%) | 42 (45.2%) | 12 (33.3%) |
| Only chart | 28 (18.7%) | 14 (15.1%) | 5 (13.9%) |
| Any combination with tool | 39 (26.0%) | 19 (20.4%) | 12 (33.3%) |
| Any combination without tool | 51 (34.0%) | 12 (12.9%) | 5 (13.9%) |
The actions taken by interns varied by reason for the call. Clinical encounters had the most variety of actions taken, with 55.1% (87/158) resulting in a new medication order and 49.9% (78/158) handled over the phone. Bedside evaluations occurred in 23.4% (37/158) of the encounters, and 3.8% (6/158) were documented in the electronic medical record. Administrative encounter responses were more homogeneous; 96.8% (92/95) were handled entirely over the phone. Responses to pain‐related requests were similarly less varied than clinical encounters; 63.9% (23/36) were handled over the phone and 66.7% (24/36) resulted in a new medication order. Neither administrative nor pain notifications resulted in documentation in the electronic medical records. These data are summarized in Table 3. Despite the availability of a resident and attending overnight, only 6.3% (10/150) of the clinical requests led to a discussion with them; none of the administrative or pain‐related notifications involved discussion with either the resident or the attending.
| Actions Taken | Encounter Type | ||
|---|---|---|---|
| Clinicala | Administrativeb | Pain Relatedc | |
| |||
| Handled over the phone | 78 (49.4%) | 92 (96.8%) | 23 (63.9%) |
| Evaluated the patient at the bedside | 37 (23.4%) | 2 (2.1%) | 2 (5.6%) |
| Reviewed previously ordered labs or imaging | 43 (27.2%) | 12 (12.6%) | 0 (0.0%) |
| Ordered new lab or imaging | 44 (27.8%) | 2 (2.1%) | 0 (0.0%) |
| Ordered new medication | 87 (55.1%) | 1 (1.1%) | 24 (66.7%) |
| Wrote cross‐cover note | 6 (3.8%) | 0 (0.0%) | 0 (0.0%) |
| Conferred with supervising physician | 10 (6.3%) | 0 (0.0%) | 0 (0.0%) |
| Called consult | 3 (1.9%) | 0 (0.0%) | 0 (0.0%) |
| Upgraded level of care | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) |
Survey Data
Fifty‐three residents completed surveys, for an overall response rate of 59.6% (53/89). All PGYs were represented; PGY‐3s had a response rate of 68.0% (17/25), PGY‐2s had a 58.3% response rate (14/24), and PGY‐1s had a 55% response rate (22/40).
A night float intern was perceived to be safer than an on‐call team performing the same job by 73.6% (39/89) of respondents. The written sign‐out was considered a time saver by 66% (35/53) of respondents. The sign‐out procedure was thought to be frequently or always safe by 73.6% (39/89). Overnight documentation within the electronic medical record was reported to be frequently or always completed by 58.5% (31/53).
Furthermore, 20.7% (11/53) of respondents reported receiving a do not do list frequently or always, and 43.4% (23/53) of respondents reported giving a do not do list frequently or always. Conditional statements were reported as frequently or always given by 90.4% (47/52). A standardized verbal checkout was considered safer by 71.7% (38/53), standardized written documentation was considered beneficial by 94.3% (50/53), and a checklist to go over was considered beneficial by 84.9% (45/53).
DISCUSSION
Our goal was to understand how to better support care transitions and handoff processes. Our residents report that current approaches to care transitions are safe and useful. Although this perception is reassuring, it is difficult to know whether this reflects the actual delivery of safe care. A minority of residents report giving and receiving do not do lists, which are important aspects of care when giving guidance to a covering physician. Also, we find discrepancies between our survey results and nighttime call collection data in important areas. Although residents report that the written sign‐out is useful, it was deemed useful for resolving a clinical issue only 27% of the time. Previous reports have found variable and conflicting rates of written sign‐out utilization, as well as variable quality of a written sign‐out,[21, 22, 23] and our data support infrequent usage. Residents were much more likely to access the electronic medical record than they were to use the handoff tool. Additionally, although residents report documentation, very little actual documentation occurred. The high rates of calls for routine and pain‐related notifications are notable and should be examined further for areas of potential improvement. Preemptive orders for routine, common, and benign conditions are often not employed as strategy and their omission can lead to higher workloads for nighttime physicians. Additionally, education and training may be necessary to help housestaff understand how such a strategy is safely implemented, such as a specific regimen for mild pain, and why it is helpful beyond reducing nighttime workload, such as a proactive approach to clinical care.
Several important insights emerge from our results. First, the electronic health record is accessible, and providers use it frequently. This raises the question of the need for a handoff tool for information transfer. When data can be easily accessed, their presence in a physical tool may be less important. Because electronic health records can easily be leveraged to populate handoff tools, having a brief tool that minimizes information transfer but better supports clinical reasoning may be more effective.
Second, our data highlight the need to focus on the handback, or providing information back to the returning day team. Our experience and previous studies support that this process is not adequately developed.[24, 25] There is little opportunity for communication between the covering and primary providers, and there is little documentation. In our observations, 3.8% of calls resulted in documentation, whereas the majority of respondents to the survey state it is performed frequently or always. The reason for this discrepancy is unclear, but fostering more of a mentality that considers all of the providers involved in patient care to be part of the same team may help address this issue.
Third, clinical services assume providers have what they need to provide care in the form of the handoff instrument. In fact, providers have handoff instruments, but whether they need them is unclear. Based on these observations, overnight physicians are able to provide care in the vast majority of cases without the use of the handoff tool.
Fourth, our data demonstrate the social or relational nature of providing clinical coverage. The single most frequent action taken by covering residents was speaking to the nurse. This may not be surprising; however, when we reframe transitions of care and handoffs as a relational issue, we are forced to reframe potential strategies to improve these transitions. The problem we need to address is not only of information transfer; it is also of making sense of what is happening.
How do we make handoff tools more effective sensemaking tools? More focus on contingency statements might be an approach. These have the dual benefit of helping the covering provider to make sense using the primary team's reasoning, as well as improving the primary team's reasoning by making the potential complications more explicit. Another approach could be to reinforce relational actions, through providing guidance on who to call if there is a change in the status of the patient. We found that the night intern rarely discussed care with supervising physicians, indicating weak integration of the night team. The handoff tool could thus strengthen the network of providers caring for the patient. A tool that emphasizes sensemaking may be a tool that captures the nonroutine aspects of care that are not already documented in the health record.
Our data are limited in that they were collected in a single institution over few nights with few interns. Our processes may not be representative, and our expectations for provider communication may not be the norm. Although a night float system of coverage is not the only model of providing care, it is common, and our handoff tool is similar to those reported in the literature. One area of concern is that our handback expectations may be less robust than other institutions. Despite this limitation, the larger issues of information transfer and sensemaking are generally applicable. Although we collected data over only 18 nights, we did obtain information on almost 300 calls, giving us a robust sample of actual issues that residents were called to resolve. Interns are the most involved in actually providing night coverage. Their response rate was 55%, slightly below our overall response rate of 59.6%, but representing the majority of interns. A 2‐step process of sign‐out may have ramifications on care transitions; however, these data were collected at night. Because the handoff tool information is the day team's responsibility, the process may have less impact on these results.
Coverage and care transfers are part of the inpatient landscape, and it may be unreasonable to expect care to be delivered by a group of providers who know the patient with the same level of depth at all hours of the day. By understanding that fostering effective care for patients requires providers to pay attention to not only how they transfer information, but also how they collectively make sense of what is happening, we will enable safer care.
Disclosures: The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (CDA 07‐022). Investigator salary support was provided through this funding, and through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Parts of these data were presented at the 2013 SGIM National Meeting in Denver, Colorado. The authors report no conflicts of interest.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–358.
- , , , . Transfers of patient care between house staff on internal medicine wards: a national survey. Arch Intern Med. 2006;166(11):1173–1177.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , , , . The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458–471.
- “Improving America's Hospitals.” The Joint Commission's Annual Report on Quality and Safety. Available at: http://www.jointcommission.org/Improving_Americas_Hospitals_The_Joint_Commissions_Annual_Report_on_Quality_and_Safety_‐_2007. Published 2007. Accessed November 17, 2014.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , . Resident handoffs: appreciating them as a critical competency. J Gen Intern Med. 2012;27(3):270–272.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/Common_Program_Requirements_07012011%5B2%5D.pdf. Published July 1, 2011. Accessed November 17, 2014.
- Joint Commission Center for Transforming Healthcare. Hand‐off communications. Available at: http://www.centerfortransforminghealthcare.org/projects/detail.aspx?Project=1. Accessed November 17, 2014.
- , . Handover and note‐keeping: the SBAR approach. Clin Risk. 2010;16(5):173–175.
- , , , et al. A structured handoff program for interns. Acad Med. 2009;84(3):347–352.
- , , , et al. I‐pass, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- , , . SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32(3):167–175.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , , , . Systematic review: effects of resident work hours on patient safety. Ann Intern Med. 2004;141(11):851–857.
- , . Mortality in out‐of‐hours emergency medical admissions—more than just a weekend effect. J R Coll Physicians Edinb. 2010;40(2):115–118.
- , , , , , . The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10–14.
- , , . Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31(6):828–838.
- . Sensemaking in Organizations. Thousand Oaks, CA: Sage Publications; 1995.
- , , , , . Answering questions on call: pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , . Effectiveness of written hospitalist sign‐outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609–614.
- , , . Assessment of internal medicine trainee sign‐out quality and utilization habits. Intern Emerg Med. 2014;9(5):529–535.
- , , , , . Morning handover of on‐call issues: opportunities for improvement. JAMA Intern Med. 2014;174(9):1479–1485.
- , , , et al. Improving resident morning sign‐out by use of daily events reports [published online ahead of print February 11, 2014]. J Patient Saf. doi: 10.1097/PTS.0b013e31829e4f56
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–358.
- , , , . Transfers of patient care between house staff on internal medicine wards: a national survey. Arch Intern Med. 2006;166(11):1173–1177.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , , , . The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458–471.
- “Improving America's Hospitals.” The Joint Commission's Annual Report on Quality and Safety. Available at: http://www.jointcommission.org/Improving_Americas_Hospitals_The_Joint_Commissions_Annual_Report_on_Quality_and_Safety_‐_2007. Published 2007. Accessed November 17, 2014.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , . Resident handoffs: appreciating them as a critical competency. J Gen Intern Med. 2012;27(3):270–272.
- Accreditation Council for Graduate Medical Education. Common program requirements. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/Common_Program_Requirements_07012011%5B2%5D.pdf. Published July 1, 2011. Accessed November 17, 2014.
- Joint Commission Center for Transforming Healthcare. Hand‐off communications. Available at: http://www.centerfortransforminghealthcare.org/projects/detail.aspx?Project=1. Accessed November 17, 2014.
- , . Handover and note‐keeping: the SBAR approach. Clin Risk. 2010;16(5):173–175.
- , , , et al. A structured handoff program for interns. Acad Med. 2009;84(3):347–352.
- , , , et al. I‐pass, a mnemonic to standardize verbal handoffs. Pediatrics. 2012;129(2):201–204.
- , , . SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32(3):167–175.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , , , . Systematic review: effects of resident work hours on patient safety. Ann Intern Med. 2004;141(11):851–857.
- , . Mortality in out‐of‐hours emergency medical admissions—more than just a weekend effect. J R Coll Physicians Edinb. 2010;40(2):115–118.
- , , , , , . The association between night or weekend admission and hospitalization‐relevant patient outcomes. J Hosp Med. 2011;6(1):10–14.
- , , . Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. 2011;31(6):828–838.
- . Sensemaking in Organizations. Thousand Oaks, CA: Sage Publications; 1995.
- , , , , . Answering questions on call: pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , . Effectiveness of written hospitalist sign‐outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609–614.
- , , . Assessment of internal medicine trainee sign‐out quality and utilization habits. Intern Emerg Med. 2014;9(5):529–535.
- , , , , . Morning handover of on‐call issues: opportunities for improvement. JAMA Intern Med. 2014;174(9):1479–1485.
- , , , et al. Improving resident morning sign‐out by use of daily events reports [published online ahead of print February 11, 2014]. J Patient Saf. doi: 10.1097/PTS.0b013e31829e4f56
© 2015 Society of Hospital Medicine
Handoffs
In this issue of the Journal of Hospital Medicine, the results of 2 inpatient handoff studies further shape our evolving understanding of in‐hospital care transitions. Schouten and colleagues,[1] report no difference in adverse outcomes when admissions were handed off to the primary team using face‐to‐face compared to nonface‐to‐face interactions. Meanwhile, Hanson and colleagues[2] report that a written handoff tool is used infrequently by covering interns.
Schouten et al.'s study attempted to isolate the impact of the verbal portion of the handoff between admitting and accepting team by evaluating whether early adverse outcomes differed between patients whose teams performed a face‐to‐face handoffs compared to those who did not. Their study was a retrospective chart review, and no additional process changes, training, or instruction regarding handoffs were implemented or measured. Handoffs occurred primarily between advanced practice providers, hospitalists, and a small number of resident physicians, so generalizability of this study to other institutions may be limited. No difference in adverse events was noted between admissions with face‐to‐face compared to those without face‐to‐face handoffs (2.6% vs 3.2%). Unfortunately, this study was likely underpowered to detect significant changes in adverse events, with a sample size of 805 total patients with a 3% baseline rate of adverse events (by our estimate, over 5000 patients would be needed in each group10,000 overallto detect a 30% relative difference in event rates). Further, this study did not examine other outcomes that could be impacted by the handoff process such as provider efficiency or patient experience.
Face‐to‐face handoffs, the gold standard for handoffs between providers, was 1 of the sign‐out approaches examined in a study by Graham and colleagues.[3] This study, in contrast to the Schouten et al. study, prospectively evaluated adverse events before and after implementation of face‐to‐face handoffs, with structured written sign‐out from the primary team to nighttime covering physicians. Prior to implementation, handoffs consisted of a double handoff involving an intermediary physician and unstructured written sign‐out. Although no statistically significant reduction in adverse events was found in the Graham et al. study, significant improvements were noted in physician satisfaction, documentation of key elements in handoffs, and reduced data omissions; importantly, a trend of fewer near misses was noted comparing the pre‐ and postintervention periods. Although the Schouten et al. and Graham et al. studies suggest questionable benefit of face‐to‐face handoffs, we would caution that limitations in sample size and methodological sensitivity to detect adverse events in both studies could explain the lack of association between face‐to‐face handoffs and reduced adverse events. Furthermore, the promising findings of fewer data omissions and near misses in the intervention group in the Graham et al. study suggest benefit from a multipronged approach to improving handoffs including both face‐to‐face interactions and a structured written component.
In this issue, Hanson and colleagues also evaluated the use of a handoff tool by cross‐covering interns in a convenience sample of overnight clinical interactions. Despite finding that standard written documentation was considered beneficial by nearly all respondents (94.3%), the interns reported that the handoff tool was used in only 27.7% of encounters. This pales in comparison to the use of the nurse or chart in 94.4% of cross‐coverage encounters. The authors speculate that a handoff tool, for many years the only timely source of information, may not be as useful when information can be easily accessed in an electronic health record. Yet, in a prior systematic review that included 6 studies of computerized handoff tools, Li and colleagues found that computerized handoff tools may improve physician efficiency, enhance the completeness of handoff information, and even potentially reduce adverse events.[4]
The Schouten et al. and Hanson et al. studies raise important questions for the fields of hospital medicine and patient safety. Is it time to do away with the written and verbal portions of the handoff process? Should the handoff of patients simply consist of transferring a list of patients to covering providers? We do not believe this is the correct course of action. Rather, we recommend a more evolutionary, not revolutionary, interpretation of these results, especially when considered as part of a broader story of in‐hospital transitions of care.
For example, a recently published evaluation of a resident handoff‐improvement program in 9 hospitals and 10,740 patient admissions by Starmer and collegues[5] focused on a handoff bundle, I‐PASS, which is a pneumonic for Illness severity, Patient summary, Action items, Situation awareness and contingency planning, Synthesis by receiver. The authors report a reduction in medical errors and preventable adverse events without significant increases in the duration of oral handoff per patient. The handoff in this study included both oral and written elements in the I‐PASS format. Implementation was multipronged, and the I‐PASS bundle included (1) use of the I‐PASS pneumonic to standardize handoffs; (2) resident physician training in handoffs and communication through a 2‐hour workshop, followed by a 1‐hour role‐playing and simulation session, and a computer module for practice; (3) faculty development and observation with use of direct‐observation tools to provide structured feedback to residents; (4) active surveillance for errors (rather than relying on self‐report); and (5) a sustainability campaign to promote continuation of culture change. The complexity and robust nature of the I‐PASS handoff bundle suggests that having multiple structured components included in a handoff program with active, rather than retrospective, evaluation might increase the likelihood of improved, sustained outcomes. In addition, one might also conclude from the Starmer et al. study that it takes commitment from all levels, including residents, faculty, and administration, to improve handoffs between teams for inpatient care.
We commend Schouten et al. and Hanson et al. on their contributions to the literature, but believe that the story of the in‐hospital handoff has yet to be fully written. Although results from these 2 articles may cause speculation about the value of oral and written handoffs, we believe that the balance of evidence favors the use of a multipronged approach that involves both structured oral and written handoffs to improve the value and efficiency of handoffs. In addition, findings from the I‐PASS study support dedicated handoff training for providers, evaluation of handoffs using structured tools, and active surveillance for medical errors. Future areas of work should include a systematic review of the inpatient handoff literature and further evaluation of precisely which specific intervention components (eg, structured content of handoffs, sensemaking content) or modes of delivery (eg, face‐to‐face vs other) are most likely to reduce medical errors and improve patient outcomes. As the hospital medicine movement continues to grow, handoffs will continue to be paramount. Establishing the safest method to complete handoffs to promote patient safety should be a common goal for hospitalists.
The handoff story is still in evolution; as hospitalists, we are poised to be its author.
- , , , et al. Association of Face‐to‐Face Handoffs and Outcomes of Hospitalized Internal Medicine Patients. J Hosp Med. 2015;10(3):137–141.
- , , , et al. Nighttime Clinical Encounters: How Residents Perceive and Respond to Calls at Night. J Hosp Med. 2015;10(3):142–146.
- , , , , , . Effect of a systems intervention on the quality and safety of patient handoffs in an internal medicine residency program. J Gen Intern Med. 2013;28(8):986–993.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
In this issue of the Journal of Hospital Medicine, the results of 2 inpatient handoff studies further shape our evolving understanding of in‐hospital care transitions. Schouten and colleagues,[1] report no difference in adverse outcomes when admissions were handed off to the primary team using face‐to‐face compared to nonface‐to‐face interactions. Meanwhile, Hanson and colleagues[2] report that a written handoff tool is used infrequently by covering interns.
Schouten et al.'s study attempted to isolate the impact of the verbal portion of the handoff between admitting and accepting team by evaluating whether early adverse outcomes differed between patients whose teams performed a face‐to‐face handoffs compared to those who did not. Their study was a retrospective chart review, and no additional process changes, training, or instruction regarding handoffs were implemented or measured. Handoffs occurred primarily between advanced practice providers, hospitalists, and a small number of resident physicians, so generalizability of this study to other institutions may be limited. No difference in adverse events was noted between admissions with face‐to‐face compared to those without face‐to‐face handoffs (2.6% vs 3.2%). Unfortunately, this study was likely underpowered to detect significant changes in adverse events, with a sample size of 805 total patients with a 3% baseline rate of adverse events (by our estimate, over 5000 patients would be needed in each group10,000 overallto detect a 30% relative difference in event rates). Further, this study did not examine other outcomes that could be impacted by the handoff process such as provider efficiency or patient experience.
Face‐to‐face handoffs, the gold standard for handoffs between providers, was 1 of the sign‐out approaches examined in a study by Graham and colleagues.[3] This study, in contrast to the Schouten et al. study, prospectively evaluated adverse events before and after implementation of face‐to‐face handoffs, with structured written sign‐out from the primary team to nighttime covering physicians. Prior to implementation, handoffs consisted of a double handoff involving an intermediary physician and unstructured written sign‐out. Although no statistically significant reduction in adverse events was found in the Graham et al. study, significant improvements were noted in physician satisfaction, documentation of key elements in handoffs, and reduced data omissions; importantly, a trend of fewer near misses was noted comparing the pre‐ and postintervention periods. Although the Schouten et al. and Graham et al. studies suggest questionable benefit of face‐to‐face handoffs, we would caution that limitations in sample size and methodological sensitivity to detect adverse events in both studies could explain the lack of association between face‐to‐face handoffs and reduced adverse events. Furthermore, the promising findings of fewer data omissions and near misses in the intervention group in the Graham et al. study suggest benefit from a multipronged approach to improving handoffs including both face‐to‐face interactions and a structured written component.
In this issue, Hanson and colleagues also evaluated the use of a handoff tool by cross‐covering interns in a convenience sample of overnight clinical interactions. Despite finding that standard written documentation was considered beneficial by nearly all respondents (94.3%), the interns reported that the handoff tool was used in only 27.7% of encounters. This pales in comparison to the use of the nurse or chart in 94.4% of cross‐coverage encounters. The authors speculate that a handoff tool, for many years the only timely source of information, may not be as useful when information can be easily accessed in an electronic health record. Yet, in a prior systematic review that included 6 studies of computerized handoff tools, Li and colleagues found that computerized handoff tools may improve physician efficiency, enhance the completeness of handoff information, and even potentially reduce adverse events.[4]
The Schouten et al. and Hanson et al. studies raise important questions for the fields of hospital medicine and patient safety. Is it time to do away with the written and verbal portions of the handoff process? Should the handoff of patients simply consist of transferring a list of patients to covering providers? We do not believe this is the correct course of action. Rather, we recommend a more evolutionary, not revolutionary, interpretation of these results, especially when considered as part of a broader story of in‐hospital transitions of care.
For example, a recently published evaluation of a resident handoff‐improvement program in 9 hospitals and 10,740 patient admissions by Starmer and collegues[5] focused on a handoff bundle, I‐PASS, which is a pneumonic for Illness severity, Patient summary, Action items, Situation awareness and contingency planning, Synthesis by receiver. The authors report a reduction in medical errors and preventable adverse events without significant increases in the duration of oral handoff per patient. The handoff in this study included both oral and written elements in the I‐PASS format. Implementation was multipronged, and the I‐PASS bundle included (1) use of the I‐PASS pneumonic to standardize handoffs; (2) resident physician training in handoffs and communication through a 2‐hour workshop, followed by a 1‐hour role‐playing and simulation session, and a computer module for practice; (3) faculty development and observation with use of direct‐observation tools to provide structured feedback to residents; (4) active surveillance for errors (rather than relying on self‐report); and (5) a sustainability campaign to promote continuation of culture change. The complexity and robust nature of the I‐PASS handoff bundle suggests that having multiple structured components included in a handoff program with active, rather than retrospective, evaluation might increase the likelihood of improved, sustained outcomes. In addition, one might also conclude from the Starmer et al. study that it takes commitment from all levels, including residents, faculty, and administration, to improve handoffs between teams for inpatient care.
We commend Schouten et al. and Hanson et al. on their contributions to the literature, but believe that the story of the in‐hospital handoff has yet to be fully written. Although results from these 2 articles may cause speculation about the value of oral and written handoffs, we believe that the balance of evidence favors the use of a multipronged approach that involves both structured oral and written handoffs to improve the value and efficiency of handoffs. In addition, findings from the I‐PASS study support dedicated handoff training for providers, evaluation of handoffs using structured tools, and active surveillance for medical errors. Future areas of work should include a systematic review of the inpatient handoff literature and further evaluation of precisely which specific intervention components (eg, structured content of handoffs, sensemaking content) or modes of delivery (eg, face‐to‐face vs other) are most likely to reduce medical errors and improve patient outcomes. As the hospital medicine movement continues to grow, handoffs will continue to be paramount. Establishing the safest method to complete handoffs to promote patient safety should be a common goal for hospitalists.
The handoff story is still in evolution; as hospitalists, we are poised to be its author.
In this issue of the Journal of Hospital Medicine, the results of 2 inpatient handoff studies further shape our evolving understanding of in‐hospital care transitions. Schouten and colleagues,[1] report no difference in adverse outcomes when admissions were handed off to the primary team using face‐to‐face compared to nonface‐to‐face interactions. Meanwhile, Hanson and colleagues[2] report that a written handoff tool is used infrequently by covering interns.
Schouten et al.'s study attempted to isolate the impact of the verbal portion of the handoff between admitting and accepting team by evaluating whether early adverse outcomes differed between patients whose teams performed a face‐to‐face handoffs compared to those who did not. Their study was a retrospective chart review, and no additional process changes, training, or instruction regarding handoffs were implemented or measured. Handoffs occurred primarily between advanced practice providers, hospitalists, and a small number of resident physicians, so generalizability of this study to other institutions may be limited. No difference in adverse events was noted between admissions with face‐to‐face compared to those without face‐to‐face handoffs (2.6% vs 3.2%). Unfortunately, this study was likely underpowered to detect significant changes in adverse events, with a sample size of 805 total patients with a 3% baseline rate of adverse events (by our estimate, over 5000 patients would be needed in each group10,000 overallto detect a 30% relative difference in event rates). Further, this study did not examine other outcomes that could be impacted by the handoff process such as provider efficiency or patient experience.
Face‐to‐face handoffs, the gold standard for handoffs between providers, was 1 of the sign‐out approaches examined in a study by Graham and colleagues.[3] This study, in contrast to the Schouten et al. study, prospectively evaluated adverse events before and after implementation of face‐to‐face handoffs, with structured written sign‐out from the primary team to nighttime covering physicians. Prior to implementation, handoffs consisted of a double handoff involving an intermediary physician and unstructured written sign‐out. Although no statistically significant reduction in adverse events was found in the Graham et al. study, significant improvements were noted in physician satisfaction, documentation of key elements in handoffs, and reduced data omissions; importantly, a trend of fewer near misses was noted comparing the pre‐ and postintervention periods. Although the Schouten et al. and Graham et al. studies suggest questionable benefit of face‐to‐face handoffs, we would caution that limitations in sample size and methodological sensitivity to detect adverse events in both studies could explain the lack of association between face‐to‐face handoffs and reduced adverse events. Furthermore, the promising findings of fewer data omissions and near misses in the intervention group in the Graham et al. study suggest benefit from a multipronged approach to improving handoffs including both face‐to‐face interactions and a structured written component.
In this issue, Hanson and colleagues also evaluated the use of a handoff tool by cross‐covering interns in a convenience sample of overnight clinical interactions. Despite finding that standard written documentation was considered beneficial by nearly all respondents (94.3%), the interns reported that the handoff tool was used in only 27.7% of encounters. This pales in comparison to the use of the nurse or chart in 94.4% of cross‐coverage encounters. The authors speculate that a handoff tool, for many years the only timely source of information, may not be as useful when information can be easily accessed in an electronic health record. Yet, in a prior systematic review that included 6 studies of computerized handoff tools, Li and colleagues found that computerized handoff tools may improve physician efficiency, enhance the completeness of handoff information, and even potentially reduce adverse events.[4]
The Schouten et al. and Hanson et al. studies raise important questions for the fields of hospital medicine and patient safety. Is it time to do away with the written and verbal portions of the handoff process? Should the handoff of patients simply consist of transferring a list of patients to covering providers? We do not believe this is the correct course of action. Rather, we recommend a more evolutionary, not revolutionary, interpretation of these results, especially when considered as part of a broader story of in‐hospital transitions of care.
For example, a recently published evaluation of a resident handoff‐improvement program in 9 hospitals and 10,740 patient admissions by Starmer and collegues[5] focused on a handoff bundle, I‐PASS, which is a pneumonic for Illness severity, Patient summary, Action items, Situation awareness and contingency planning, Synthesis by receiver. The authors report a reduction in medical errors and preventable adverse events without significant increases in the duration of oral handoff per patient. The handoff in this study included both oral and written elements in the I‐PASS format. Implementation was multipronged, and the I‐PASS bundle included (1) use of the I‐PASS pneumonic to standardize handoffs; (2) resident physician training in handoffs and communication through a 2‐hour workshop, followed by a 1‐hour role‐playing and simulation session, and a computer module for practice; (3) faculty development and observation with use of direct‐observation tools to provide structured feedback to residents; (4) active surveillance for errors (rather than relying on self‐report); and (5) a sustainability campaign to promote continuation of culture change. The complexity and robust nature of the I‐PASS handoff bundle suggests that having multiple structured components included in a handoff program with active, rather than retrospective, evaluation might increase the likelihood of improved, sustained outcomes. In addition, one might also conclude from the Starmer et al. study that it takes commitment from all levels, including residents, faculty, and administration, to improve handoffs between teams for inpatient care.
We commend Schouten et al. and Hanson et al. on their contributions to the literature, but believe that the story of the in‐hospital handoff has yet to be fully written. Although results from these 2 articles may cause speculation about the value of oral and written handoffs, we believe that the balance of evidence favors the use of a multipronged approach that involves both structured oral and written handoffs to improve the value and efficiency of handoffs. In addition, findings from the I‐PASS study support dedicated handoff training for providers, evaluation of handoffs using structured tools, and active surveillance for medical errors. Future areas of work should include a systematic review of the inpatient handoff literature and further evaluation of precisely which specific intervention components (eg, structured content of handoffs, sensemaking content) or modes of delivery (eg, face‐to‐face vs other) are most likely to reduce medical errors and improve patient outcomes. As the hospital medicine movement continues to grow, handoffs will continue to be paramount. Establishing the safest method to complete handoffs to promote patient safety should be a common goal for hospitalists.
The handoff story is still in evolution; as hospitalists, we are poised to be its author.
- , , , et al. Association of Face‐to‐Face Handoffs and Outcomes of Hospitalized Internal Medicine Patients. J Hosp Med. 2015;10(3):137–141.
- , , , et al. Nighttime Clinical Encounters: How Residents Perceive and Respond to Calls at Night. J Hosp Med. 2015;10(3):142–146.
- , , , , , . Effect of a systems intervention on the quality and safety of patient handoffs in an internal medicine residency program. J Gen Intern Med. 2013;28(8):986–993.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
- , , , et al. Association of Face‐to‐Face Handoffs and Outcomes of Hospitalized Internal Medicine Patients. J Hosp Med. 2015;10(3):137–141.
- , , , et al. Nighttime Clinical Encounters: How Residents Perceive and Respond to Calls at Night. J Hosp Med. 2015;10(3):142–146.
- , , , , , . Effect of a systems intervention on the quality and safety of patient handoffs in an internal medicine residency program. J Gen Intern Med. 2013;28(8):986–993.
- , , , , . Review of computerized physician handoff tools for improving the quality of patient care. J Hosp Med. 2013;8(8):456–463.
- , , , et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812.
Face‐to‐Face Handoffs and Outcomes
Handoffs are key events in the care of hospitalized patients whereby vital information is relayed between healthcare providers. Resident duty hour restrictions and the popularity of shift‐based work schedules have increased the frequency of inpatient handoffs.[1, 2] Failures in communication at the time of patient handoff have been implicated as contributing factors to preventable adverse events.[3, 4, 5, 6] With patient safety in mind, accreditation organizations and professional societies have made the standardization of hospital handoff procedures a priority.[7, 8] A variety of strategies have been utilized to standardize handoffs. Examples include the use of mnemonics,[9] electronic resources,[10, 11, 12] preformatted handoff sheets,[13, 14, 15, 16] and optimization of the handoff environment.[17] The primary outcomes for many of these studies center on the provider by measuring their retention of patient facts[18, 19] and completion of tasks[14, 16] after handoff, for example. Few studies examined patient‐centered outcomes such as transfer to a higher level of care,[20] length of stay,[11] mortality,[21] or readmission rate.[22] A study in the pediatric population found that implementation of a handoff bundle was associated with a decrease in medical errors and preventable adverse events.[23]
The Society of Hospital Medicine recommends that patient handoffs consist of both a written and verbal component.[8] Providers in our division work on 3 shifts: day, evening, and night. In 2009, we developed a face‐to‐face morning handoff, during which night‐shift providers hand off patient care to day‐shift providers incorporating an electronically generated service information list.[17] Given that the evening shift ends well before the day shift begins, the evening‐shift providers do not participate in this face‐to‐face handoff of care for patients they admit to day providers.
We wished to compare the clinical outcomes and adverse events of patients admitted by the night‐shift providers to those admitted by the evening‐shift providers. We hypothesized that transfer of care using a face‐to‐face handoff would be associated with fewer adverse events and improved clinical outcomes.
METHODS
The study was deemed exempt by the Mayo Clinic Institutional Review Board.
Study Population
Hospitalists at the study institution, a 1157‐bed academic tertiary referral hospital, admit general medical patients from the emergency department, as transfers from other institutions, and as direct admissions from outpatient offices. Patients included in the study were all adults admitted by evening‐ and night‐shift hospitalists from August 1, 2011 through August 1, 2012 between 6:45 pm and midnight. Our institution primarily uses 2 levels of care for adult inpatients on internal medicine services, including a general care floor for low‐acuity patients and an intensive care unit for high‐acuity patients. All of the patients in this study were triaged as low acuity at the time of admission and were initially admitted to general care units.
Setting
The division's shift schedule during the study period is depicted in Figure 1. Day‐shift providers included a physician and nurse practitioner (NP) or physician assistant (PA) on each of 7 teams. Each service had an average daily patient census between 10 and 15 patients with 3 to 4 new admissions every 24 hours, with 1 to 2 of these admissions occurring during the evening and night shifts, on average. The day shift started at 7:45 am and ended at 7:45 pm, at which time the day teams transitioned care of their patients to 1 of 2 overnight NP/PAs who provided cross‐cover for all teams through the night. The overnight NP/PAs then transitioned care back to the day teams at 7:45 am the following morning.
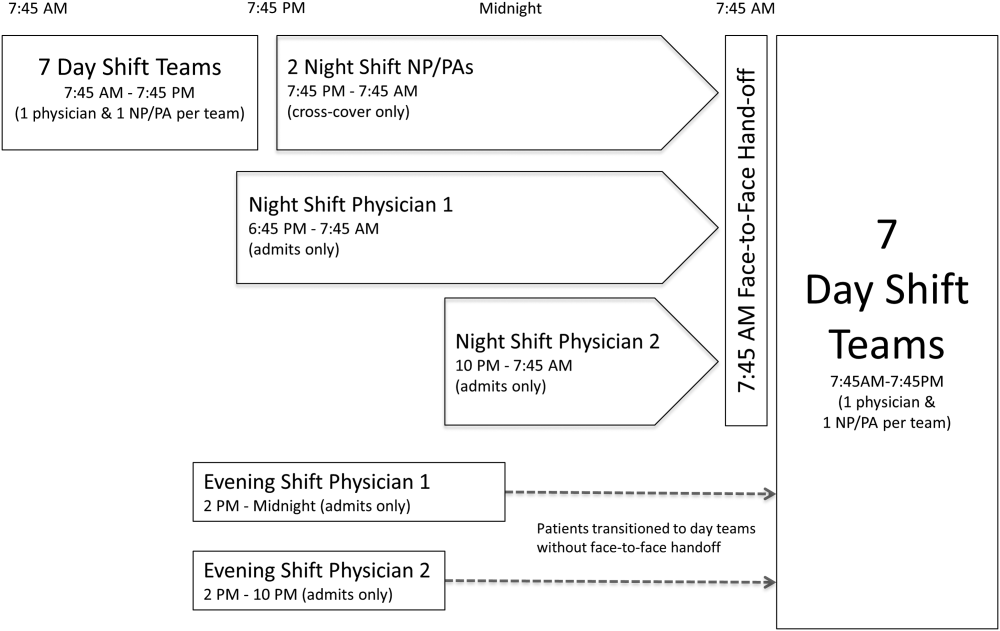
Two evening‐shift providers, both physicians, including a staff hospitalist and a hospital medicine fellowship trainee, admitted patients without any cross‐cover responsibility. Their shifts had the same start time, but staggered end times (2 pm10 pm and 2 pmmidnight). At the end of their shifts, the evening‐shift providers relayed concerns or items for follow‐up to the night cross‐cover NP/PAs; however, this handoff was nonstandardized and provider dependent. The cross‐cover providers could also choose to pass on any relevant information to day‐shift providers if thought to be necessary, but this, again, was not required or standardized. A printed electronic handoff tool (including the patient's problem list, medications, vital signs, laboratory results, and to do list as determined by the admitting provider) as well as all clinical notes generated since admission were made available to day‐shift providers who assumed care at 7:45 am; however, there was no face‐to‐face handoff between the evening‐ and day‐shift providers.
Two night‐shift physicians, including a moonlighting board‐eligible internal medicine physician and staff hospitalist, also started at staggered times, 6:45 pm and 10 pm, but their shifts both ended at 7:45 am. These physicians also admitted patients without cross‐cover responsibilities. At 7:45 am, in a face‐to‐face meeting, they transitioned care of patients admitted overnight to day‐shift providers. This handoff occurred at a predesignated place with assigned start times for each team. During the meeting, printed electronic documents, including the aforementioned electronic handoff tool as well as all clinical notes generated since admission, were made available to the oncoming day‐shift providers. The face‐to‐face interaction between night‐ and day‐shift providers lasted approximately 5 minutes and allowed for a brief presentation of the patient, review of the diagnostic testing and treatments performed so far, as well as anticipatory guidance regarding potential issues throughout the remainder of the hospitalization. Although inclusion of the above components was encouraged during the face‐to‐face handoff, the interaction was not scripted and topics discussed were at the providers' discretion.
Patients admitted during the evening and night shifts were assigned to day‐shift services primarily based on the current census of each team, so as to distribute the workload evenly.
Chart Review
Patients included in the study were admitted by evening‐ or night‐shift providers between 6:45 pm and midnight. This time period accounts for when the evening shift and night shift overlap, allowing for direct comparison of patients admitted during the same time of day, so as to avoid confounding factors. Patients were grouped by whether they were admitted by an evening‐shift provider or a night‐shift provider. Each study patient's chart was retrospectively reviewed and relevant demographic and clinical data were collected. Demographic information included age, gender, and race. Clinical information included medical comorbidities, Charlson Comorbidity Index score, rapid response team calls, code team calls, transfers to a higher level of care, death in hospital, 30‐day readmission rate, length of stay (LOS), and adverse events. The Charlson Comorbidity Index score[24] was determined from diagnoses in the institution's medical index database. The 30‐day readmission rate included observation stays and full hospital admissions that occurred at our institution in the 30 days following the patient's hospital discharge from the index admission. LOS was determined based on the time of admission and discharge, as reported in the hospital billing system, and is reported as the median and mean LOS in hours for all patients in each group.
The Global Trigger Tool (GTT) was used to identify adverse events, as defined within the GTT whitepaper to be unintended physical injury resulting from or contributed to by medical care that requires additional monitoring, treatment or hospitalization, or that results in death.[25] Developed by the Institute for Healthcare Improvement, the GTT uses triggers, clues in the medical record that suggest an adverse event may have occurred, to cue a more detailed chart review. Registered nurses trained in use of the GTT reviewed all of the included patients' electronic medical records. If a trigger was identified (such as a patient fall suffered in the hospital), further chart review was prompted to determine if patient harm occurred. If there was evidence of harm, an adverse event was determined to have occurred and was then categorized using the National Coordinating Council for Medication Error Reporting and Prevention Index for Categorizing Errors.[26] For example, in the case of a patient fall whereby the patient was determined to have fallen in the hospital and suffered a laceration requiring wound care, but the hospital stay was not prolonged, this adverse event was categorized as category E (an adverse event that caused the patient temporary harm necessitating intervention, without prolongation of the hospital stay).
Outcomes including rapid response team calls, code team calls, transfers to a higher level of care, death in the hospital, and adverse events, as identified using the GTT, were counted if they occurred between 7:45 am on the first morning of admission until 12 hours later at 7:45 pm, at the time of the first evening handoff of the admitted patients' care.
Statistical Methods
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Mayo Clinic.[27] When comparing outcomes between the 2 groups, Fisher exact test was used for categorical variables and Student t test was used for continuous variables. Global Trigger Tool data were analyzed using the SAS GENMOD procedure, assuming a negative binomial distribution. All the above analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC). Rates of adverse events were compared using MedCalc version 13 software (MedCalc Software, Ostend, Belgium).[28] A P value <0.05 was considered significant.
RESULTS
Of 805 patients admitted between 6:45 pm and midnight during the study period, 305 (37.9%) patients were handed off to day‐shift providers without face‐to‐face handoff, and 500 (62.1%) patients were transferred to the care of day‐shift providers with the use of a face‐to‐face handoff.
Baseline characteristics of both groups are depicted in Table 1. Demographic characteristics, including age, gender, and race, were not significantly different between groups. The mean Charlson Comorbidity Index score was not significantly different between the groups without and with a face‐to‐face handoff. In addition, the presence of medical comorbidities including type 2 diabetes mellitus, hypertension, coronary artery disease, hyperlipidemia, heart failure, body mass index (BMI) <18, active cancer, and current cigarette smoking were not significantly different between the 2 groups. There was a trend to a significantly increased proportion of patients with a BMI >30 in the group without face‐to‐face handoff (P=0.05).
| Without Face‐to‐Face Handoff, N=305 | With Face‐to‐Face Handoff, N=500 | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 65.8 (19.0) | 64.2 (20.0) | 0.25 |
| Sex, n (%) | 0.69 | ||
| Female | 166 (54%) | 265 (53%) | |
| Male | 139 (46%) | 235 (47%) | |
| Race, n (%) | 0.94 | ||
| White | 287 (95%) | 466 (93%) | |
| African American | 5 (2%) | 9 (2%) | |
| Arab/Middle Eastern | 3 (1%) | 8 (2%) | |
| Asian | 1 (0%) | 3 (1%) | |
| Indian subcontinental | 1 (0%) | 1 (0%) | |
| American Indian/Alaskan | 1 (0%) | 1 (0%) | |
| Other | 3 (1%) | 8 (2%) | |
| Unknown | 1 (0%) | 4 (1%) | |
| Charlson Comorbidity Index, mean ( SD) | 2.98 ( 3.73) | 2.93 ( 3.72) | 0.85 |
| Comorbidities, n (%) | |||
| Type 2 diabetes | 82 (27%) | 143 (29%) | 0.60 |
| Hypertension | 195 (64%) | 303 (61%) | 0.34 |
| Coronary artery disease | 76 (25%) | 137 (27%) | 0.44 |
| Hyperlipidemia | 122 (40%) | 206 (41%) | 0.74 |
| Heart failure | 30 (10%) | 66 (13%) | 0.15 |
| BMI >30 | 109 (36%) | 146 (29%) | 0.05 |
| BMI <18 | 7 (2%) | 12 (2%) | 0.92 |
| Active cancer | 29 (10%) | 46 (9%) | 0.88 |
| Current smoker | 49 (16%) | 90 (18%) | 0.48 |
Results for the outcomes of this study are depicted in Table 2. The frequency of rapid response team calls, code team calls, transfers to a higher level of care, and death in the hospital in the 12 hours following the first morning handoff of the admission were not significantly different between the 2 groups. Both 30‐day readmission rate and LOS (median and mean) were not significantly different between groups.
| Without Face‐to‐Face Handoff, N=305 | With Face‐to‐Face Handoff, N=500 | P Value | |
|---|---|---|---|
| |||
| Rapid response team call, n (%) | 4 (1%) | 5 (1%) | 0.68 |
| Code team call, n (%) | 0 (0%) | 1 (0%) | 0.43 |
| Transfer to higher level of care, n (%) | 7 (2%) | 11 (2%) | 0.93 |
| Patient death, n (%) | 0 (0%) | 0 (0%) | 1.00 |
| 30‐day readmission, n (%) | 50 (16%) | 67 (13%) | 0.23 |
| Hospital length of stay | |||
| Median, h (IQR) | 66.5 (41.3115.6) | 70.3 (41.9131.2) | 0.30 |
| Mean, h ( SD) | 102.0 ( 110.0) | 102.9 ( 94.0) | 0.90 |
| Adverse events (Global Trigger Tool) | |||
| Temporary harm and required intervention (E) | 4 | 7 | 0.92 |
| Temporary harm and required initial or prolonged hospitalization (F) | 7 | 8 | 0.53 |
| Permanent harm (G) | 0 | 1 | 0.44 |
| Intervention required to sustain life (H) | 0 | 6 | 0.14 |
| Death (I) | 0 | 0 | 1.00 |
| Total adverse events per 100 admissions | 3.61 | 4.40 | 0.59 |
| % of admissions with an adverse event | 2.6% | 3.2% | 0.64 |
There was no significant difference between the 2 groups in the frequency of adverse events resulting in harm for any of the categories (categories EI). Total adverse events between groups were also compared. Adverse events per 100 admissions were not significantly different between the group without face‐to‐face handoff compared to the group with face‐to‐face handoff. The percentage of admissions with an adverse event was also similar between groups.
DISCUSSION
We found no significant difference in the rate of rapid response team calls, code team calls, transfers to a higher level of care, death in hospital, or adverse events when comparing patients transitioned to the care of day‐shift providers with or without a face‐to‐face handoff. We hypothesize that a reason adverse events were no different between the 2 groups may be that providers were more vigilant when they did not receive a face‐to‐face handoff from the previous provider. As a result, providers may have dedicated additional time reviewing the medical record, speaking with the patients, and communicating with other healthcare providers to ensure a safe care transition. Similarly, other studies found no significant reduction in adverse events when using a standardized handoff.[10, 13, 29] This may be because patient handoff is 1 of a multitude of factors that impact the rate of adverse events, and a handoff may play a less vital role in a system where documentation of care for a given patient is readily accessible, uniform, and detailed. A face‐to‐face interaction itself in a patient handoff may be less pertinent if key information can be communicated through other channels, such as an electronic handoff tool, email, or phone.
Another potential explanation for the lack of a significant difference in patient outcomes with and without a face‐to‐face handoff is related to the study design and inherent rate of the events measured. With the exception of 30‐day readmission rate and LOS, the outcomes of the study were recorded only if they occurred in the 12 hours following the first morning handoff of the admission. This was done in an attempt to isolate the effect of the nonface‐to‐face versus face‐to‐face handoff on the first morning of the admission, and to avoid confounding effects by subsequent transitions of care later in the hospitalization. The frequency of hospital admissions in which an adverse event occurred during this relatively short 12‐hour window was approximately 3% for all patients in the study. With 805 total patients in the study, there may have been insufficient statistical power to detect a difference in the rate of outcomes, if a difference did exist, considering the event rate for both groups and the sample size.
There are several additional limitations to our study. First, the GTT was designed to be applied across the entirety of a hospitalization. By screening for adverse events over the span of only 12 hours for each hospitalization, the sensitivity of the tool may have been diminished, with a proportion of adverse events not captured, even when the sequence of events leading to patient harm began during the 12 hours in question. Second, this is a retrospective study, and all adverse events may not be documented in the medical record. Third, although not formally structured and infrequent, some evening‐shift providers did send an email or call the oncoming day‐shift provider to discuss patients admitted. This process, however, was provider dependent, unstructured, uncommon, and erratic, and thus we were not able to capture it from medical record review. Finally, the patients in this study were deemed low acuity upon triage prior to admission. A face‐to‐face handoff may be less important in ensuring patient safety when caring for low‐acuity compared to high‐acuity patients, considering the rapidity at which the critically ill can deteriorate.
Handoffs of patient care in the hospital have certainly increased in recent years. Consequently, communication among providers is undoubtedly important, with patient safety being the primary goal. Our work suggests that a face‐to‐face component of a handoff is not vital to ensure a safe care transition. Because of the increasing frequency of handoffs, providers' ability to do so face‐to‐face will likely be challenged by time and logistical constraints. Future work is needed to delineate the most effective components of the handoff so that we can design information transfer that promotes safe and efficient care, even without a face‐to‐face interaction.
Acknowledgements
The authors are grateful for support from the Mayo Clinic Department of Medicine Clinical Research Office, Ms. Donna Lawson, and Mr. Stephen Cha.
Disclosures: This publication was made possible by the Mayo Clinic Center for Clinical and Translational Science through grant number UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. The authors report no conflicts of interest.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- . 'Shift work': 24‐hour workdays are out as residents, hospitals deal with changes, mixed feelings on restrictions. Mod Healthc. 2011;41(30):6–7, 16, 1.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , . Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Archives of internal medicine. 2007;167(19):2030–2036.
- , , , et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204(4):533–540.
- Joint Commission International. Standard PC.02.02.01. 2013 Hospital Accreditation Standards. Oak Brook, IL: Joint Commission Resources; 2013.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , . Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196–204.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , , . Impact of a new electronic handover system in surgery. Int J Surg. 2011;9(3):217–220.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , . Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309–313.
- , , , et al. Simple standardized patient handoff system that increases accuracy and completeness. J Surg Educ. 2008;65(6):476–485.
- , , , et al. Enhancing patient safety in the trauma/surgical intensive care unit. J Trauma. 2009;67(3):430–433; discussion 433–435.
- , , . Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , , . Gaining efficiency and satisfaction in the handoff process. J Hosp Med. 2010;5(9):547–552.
- , , . Identification of patient information corruption in the intensive care unit: using a scoring tool to direct quality improvements in handover. Crit Care Med. 2009;37(11):2905–2912.
- . Examining the effects that manipulating information given in the change of shift report has on nurses' care planning ability. J Adv Nurs. 2001;33(6):836–846.
- , , , et al. Evaluation of an asynchronous physician voicemail sign‐out for emergency department admissions. Ann Emerg Med. 2009;54(3):368–378.
- , , , et al. Surgical team behaviors and patient outcomes. Am J Surg. 2009;197(5):678–685.
- , , , , , . The value of adding a verbal report to written handoffs on early readmission following prolonged respiratory failure. Chest. 2010;138(6):1475–1479.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , . IHI Global Trigger Tool for measuring adverse events (second edition). IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2009. Available at: http://www.ihi.org/resources/Pages/IHIWhitePapers/IHIGlobalTriggerToolWhitePaper.aspx. www.IHI.org). Accessed June 1, 2014.
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) index for categorizing errors. Available at: http://www.nccmerp.org/medErrorCatIndex.html. Accessed June 1, 2014.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , . Statistics in Epidemiology: Methods, Techniques, and Applications. Boca Raton, FL: CRC Press; 1996.
- , , , , , . Safety of using a computerized rounding and sign‐out system to reduce resident duty hours. Acad Med. 2010;85(7):1189–1195.
Handoffs are key events in the care of hospitalized patients whereby vital information is relayed between healthcare providers. Resident duty hour restrictions and the popularity of shift‐based work schedules have increased the frequency of inpatient handoffs.[1, 2] Failures in communication at the time of patient handoff have been implicated as contributing factors to preventable adverse events.[3, 4, 5, 6] With patient safety in mind, accreditation organizations and professional societies have made the standardization of hospital handoff procedures a priority.[7, 8] A variety of strategies have been utilized to standardize handoffs. Examples include the use of mnemonics,[9] electronic resources,[10, 11, 12] preformatted handoff sheets,[13, 14, 15, 16] and optimization of the handoff environment.[17] The primary outcomes for many of these studies center on the provider by measuring their retention of patient facts[18, 19] and completion of tasks[14, 16] after handoff, for example. Few studies examined patient‐centered outcomes such as transfer to a higher level of care,[20] length of stay,[11] mortality,[21] or readmission rate.[22] A study in the pediatric population found that implementation of a handoff bundle was associated with a decrease in medical errors and preventable adverse events.[23]
The Society of Hospital Medicine recommends that patient handoffs consist of both a written and verbal component.[8] Providers in our division work on 3 shifts: day, evening, and night. In 2009, we developed a face‐to‐face morning handoff, during which night‐shift providers hand off patient care to day‐shift providers incorporating an electronically generated service information list.[17] Given that the evening shift ends well before the day shift begins, the evening‐shift providers do not participate in this face‐to‐face handoff of care for patients they admit to day providers.
We wished to compare the clinical outcomes and adverse events of patients admitted by the night‐shift providers to those admitted by the evening‐shift providers. We hypothesized that transfer of care using a face‐to‐face handoff would be associated with fewer adverse events and improved clinical outcomes.
METHODS
The study was deemed exempt by the Mayo Clinic Institutional Review Board.
Study Population
Hospitalists at the study institution, a 1157‐bed academic tertiary referral hospital, admit general medical patients from the emergency department, as transfers from other institutions, and as direct admissions from outpatient offices. Patients included in the study were all adults admitted by evening‐ and night‐shift hospitalists from August 1, 2011 through August 1, 2012 between 6:45 pm and midnight. Our institution primarily uses 2 levels of care for adult inpatients on internal medicine services, including a general care floor for low‐acuity patients and an intensive care unit for high‐acuity patients. All of the patients in this study were triaged as low acuity at the time of admission and were initially admitted to general care units.
Setting
The division's shift schedule during the study period is depicted in Figure 1. Day‐shift providers included a physician and nurse practitioner (NP) or physician assistant (PA) on each of 7 teams. Each service had an average daily patient census between 10 and 15 patients with 3 to 4 new admissions every 24 hours, with 1 to 2 of these admissions occurring during the evening and night shifts, on average. The day shift started at 7:45 am and ended at 7:45 pm, at which time the day teams transitioned care of their patients to 1 of 2 overnight NP/PAs who provided cross‐cover for all teams through the night. The overnight NP/PAs then transitioned care back to the day teams at 7:45 am the following morning.

Two evening‐shift providers, both physicians, including a staff hospitalist and a hospital medicine fellowship trainee, admitted patients without any cross‐cover responsibility. Their shifts had the same start time, but staggered end times (2 pm10 pm and 2 pmmidnight). At the end of their shifts, the evening‐shift providers relayed concerns or items for follow‐up to the night cross‐cover NP/PAs; however, this handoff was nonstandardized and provider dependent. The cross‐cover providers could also choose to pass on any relevant information to day‐shift providers if thought to be necessary, but this, again, was not required or standardized. A printed electronic handoff tool (including the patient's problem list, medications, vital signs, laboratory results, and to do list as determined by the admitting provider) as well as all clinical notes generated since admission were made available to day‐shift providers who assumed care at 7:45 am; however, there was no face‐to‐face handoff between the evening‐ and day‐shift providers.
Two night‐shift physicians, including a moonlighting board‐eligible internal medicine physician and staff hospitalist, also started at staggered times, 6:45 pm and 10 pm, but their shifts both ended at 7:45 am. These physicians also admitted patients without cross‐cover responsibilities. At 7:45 am, in a face‐to‐face meeting, they transitioned care of patients admitted overnight to day‐shift providers. This handoff occurred at a predesignated place with assigned start times for each team. During the meeting, printed electronic documents, including the aforementioned electronic handoff tool as well as all clinical notes generated since admission, were made available to the oncoming day‐shift providers. The face‐to‐face interaction between night‐ and day‐shift providers lasted approximately 5 minutes and allowed for a brief presentation of the patient, review of the diagnostic testing and treatments performed so far, as well as anticipatory guidance regarding potential issues throughout the remainder of the hospitalization. Although inclusion of the above components was encouraged during the face‐to‐face handoff, the interaction was not scripted and topics discussed were at the providers' discretion.
Patients admitted during the evening and night shifts were assigned to day‐shift services primarily based on the current census of each team, so as to distribute the workload evenly.
Chart Review
Patients included in the study were admitted by evening‐ or night‐shift providers between 6:45 pm and midnight. This time period accounts for when the evening shift and night shift overlap, allowing for direct comparison of patients admitted during the same time of day, so as to avoid confounding factors. Patients were grouped by whether they were admitted by an evening‐shift provider or a night‐shift provider. Each study patient's chart was retrospectively reviewed and relevant demographic and clinical data were collected. Demographic information included age, gender, and race. Clinical information included medical comorbidities, Charlson Comorbidity Index score, rapid response team calls, code team calls, transfers to a higher level of care, death in hospital, 30‐day readmission rate, length of stay (LOS), and adverse events. The Charlson Comorbidity Index score[24] was determined from diagnoses in the institution's medical index database. The 30‐day readmission rate included observation stays and full hospital admissions that occurred at our institution in the 30 days following the patient's hospital discharge from the index admission. LOS was determined based on the time of admission and discharge, as reported in the hospital billing system, and is reported as the median and mean LOS in hours for all patients in each group.
The Global Trigger Tool (GTT) was used to identify adverse events, as defined within the GTT whitepaper to be unintended physical injury resulting from or contributed to by medical care that requires additional monitoring, treatment or hospitalization, or that results in death.[25] Developed by the Institute for Healthcare Improvement, the GTT uses triggers, clues in the medical record that suggest an adverse event may have occurred, to cue a more detailed chart review. Registered nurses trained in use of the GTT reviewed all of the included patients' electronic medical records. If a trigger was identified (such as a patient fall suffered in the hospital), further chart review was prompted to determine if patient harm occurred. If there was evidence of harm, an adverse event was determined to have occurred and was then categorized using the National Coordinating Council for Medication Error Reporting and Prevention Index for Categorizing Errors.[26] For example, in the case of a patient fall whereby the patient was determined to have fallen in the hospital and suffered a laceration requiring wound care, but the hospital stay was not prolonged, this adverse event was categorized as category E (an adverse event that caused the patient temporary harm necessitating intervention, without prolongation of the hospital stay).
Outcomes including rapid response team calls, code team calls, transfers to a higher level of care, death in the hospital, and adverse events, as identified using the GTT, were counted if they occurred between 7:45 am on the first morning of admission until 12 hours later at 7:45 pm, at the time of the first evening handoff of the admitted patients' care.
Statistical Methods
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Mayo Clinic.[27] When comparing outcomes between the 2 groups, Fisher exact test was used for categorical variables and Student t test was used for continuous variables. Global Trigger Tool data were analyzed using the SAS GENMOD procedure, assuming a negative binomial distribution. All the above analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC). Rates of adverse events were compared using MedCalc version 13 software (MedCalc Software, Ostend, Belgium).[28] A P value <0.05 was considered significant.
RESULTS
Of 805 patients admitted between 6:45 pm and midnight during the study period, 305 (37.9%) patients were handed off to day‐shift providers without face‐to‐face handoff, and 500 (62.1%) patients were transferred to the care of day‐shift providers with the use of a face‐to‐face handoff.
Baseline characteristics of both groups are depicted in Table 1. Demographic characteristics, including age, gender, and race, were not significantly different between groups. The mean Charlson Comorbidity Index score was not significantly different between the groups without and with a face‐to‐face handoff. In addition, the presence of medical comorbidities including type 2 diabetes mellitus, hypertension, coronary artery disease, hyperlipidemia, heart failure, body mass index (BMI) <18, active cancer, and current cigarette smoking were not significantly different between the 2 groups. There was a trend to a significantly increased proportion of patients with a BMI >30 in the group without face‐to‐face handoff (P=0.05).
| Without Face‐to‐Face Handoff, N=305 | With Face‐to‐Face Handoff, N=500 | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 65.8 (19.0) | 64.2 (20.0) | 0.25 |
| Sex, n (%) | 0.69 | ||
| Female | 166 (54%) | 265 (53%) | |
| Male | 139 (46%) | 235 (47%) | |
| Race, n (%) | 0.94 | ||
| White | 287 (95%) | 466 (93%) | |
| African American | 5 (2%) | 9 (2%) | |
| Arab/Middle Eastern | 3 (1%) | 8 (2%) | |
| Asian | 1 (0%) | 3 (1%) | |
| Indian subcontinental | 1 (0%) | 1 (0%) | |
| American Indian/Alaskan | 1 (0%) | 1 (0%) | |
| Other | 3 (1%) | 8 (2%) | |
| Unknown | 1 (0%) | 4 (1%) | |
| Charlson Comorbidity Index, mean ( SD) | 2.98 ( 3.73) | 2.93 ( 3.72) | 0.85 |
| Comorbidities, n (%) | |||
| Type 2 diabetes | 82 (27%) | 143 (29%) | 0.60 |
| Hypertension | 195 (64%) | 303 (61%) | 0.34 |
| Coronary artery disease | 76 (25%) | 137 (27%) | 0.44 |
| Hyperlipidemia | 122 (40%) | 206 (41%) | 0.74 |
| Heart failure | 30 (10%) | 66 (13%) | 0.15 |
| BMI >30 | 109 (36%) | 146 (29%) | 0.05 |
| BMI <18 | 7 (2%) | 12 (2%) | 0.92 |
| Active cancer | 29 (10%) | 46 (9%) | 0.88 |
| Current smoker | 49 (16%) | 90 (18%) | 0.48 |
Results for the outcomes of this study are depicted in Table 2. The frequency of rapid response team calls, code team calls, transfers to a higher level of care, and death in the hospital in the 12 hours following the first morning handoff of the admission were not significantly different between the 2 groups. Both 30‐day readmission rate and LOS (median and mean) were not significantly different between groups.
| Without Face‐to‐Face Handoff, N=305 | With Face‐to‐Face Handoff, N=500 | P Value | |
|---|---|---|---|
| |||
| Rapid response team call, n (%) | 4 (1%) | 5 (1%) | 0.68 |
| Code team call, n (%) | 0 (0%) | 1 (0%) | 0.43 |
| Transfer to higher level of care, n (%) | 7 (2%) | 11 (2%) | 0.93 |
| Patient death, n (%) | 0 (0%) | 0 (0%) | 1.00 |
| 30‐day readmission, n (%) | 50 (16%) | 67 (13%) | 0.23 |
| Hospital length of stay | |||
| Median, h (IQR) | 66.5 (41.3115.6) | 70.3 (41.9131.2) | 0.30 |
| Mean, h ( SD) | 102.0 ( 110.0) | 102.9 ( 94.0) | 0.90 |
| Adverse events (Global Trigger Tool) | |||
| Temporary harm and required intervention (E) | 4 | 7 | 0.92 |
| Temporary harm and required initial or prolonged hospitalization (F) | 7 | 8 | 0.53 |
| Permanent harm (G) | 0 | 1 | 0.44 |
| Intervention required to sustain life (H) | 0 | 6 | 0.14 |
| Death (I) | 0 | 0 | 1.00 |
| Total adverse events per 100 admissions | 3.61 | 4.40 | 0.59 |
| % of admissions with an adverse event | 2.6% | 3.2% | 0.64 |
There was no significant difference between the 2 groups in the frequency of adverse events resulting in harm for any of the categories (categories EI). Total adverse events between groups were also compared. Adverse events per 100 admissions were not significantly different between the group without face‐to‐face handoff compared to the group with face‐to‐face handoff. The percentage of admissions with an adverse event was also similar between groups.
DISCUSSION
We found no significant difference in the rate of rapid response team calls, code team calls, transfers to a higher level of care, death in hospital, or adverse events when comparing patients transitioned to the care of day‐shift providers with or without a face‐to‐face handoff. We hypothesize that a reason adverse events were no different between the 2 groups may be that providers were more vigilant when they did not receive a face‐to‐face handoff from the previous provider. As a result, providers may have dedicated additional time reviewing the medical record, speaking with the patients, and communicating with other healthcare providers to ensure a safe care transition. Similarly, other studies found no significant reduction in adverse events when using a standardized handoff.[10, 13, 29] This may be because patient handoff is 1 of a multitude of factors that impact the rate of adverse events, and a handoff may play a less vital role in a system where documentation of care for a given patient is readily accessible, uniform, and detailed. A face‐to‐face interaction itself in a patient handoff may be less pertinent if key information can be communicated through other channels, such as an electronic handoff tool, email, or phone.
Another potential explanation for the lack of a significant difference in patient outcomes with and without a face‐to‐face handoff is related to the study design and inherent rate of the events measured. With the exception of 30‐day readmission rate and LOS, the outcomes of the study were recorded only if they occurred in the 12 hours following the first morning handoff of the admission. This was done in an attempt to isolate the effect of the nonface‐to‐face versus face‐to‐face handoff on the first morning of the admission, and to avoid confounding effects by subsequent transitions of care later in the hospitalization. The frequency of hospital admissions in which an adverse event occurred during this relatively short 12‐hour window was approximately 3% for all patients in the study. With 805 total patients in the study, there may have been insufficient statistical power to detect a difference in the rate of outcomes, if a difference did exist, considering the event rate for both groups and the sample size.
There are several additional limitations to our study. First, the GTT was designed to be applied across the entirety of a hospitalization. By screening for adverse events over the span of only 12 hours for each hospitalization, the sensitivity of the tool may have been diminished, with a proportion of adverse events not captured, even when the sequence of events leading to patient harm began during the 12 hours in question. Second, this is a retrospective study, and all adverse events may not be documented in the medical record. Third, although not formally structured and infrequent, some evening‐shift providers did send an email or call the oncoming day‐shift provider to discuss patients admitted. This process, however, was provider dependent, unstructured, uncommon, and erratic, and thus we were not able to capture it from medical record review. Finally, the patients in this study were deemed low acuity upon triage prior to admission. A face‐to‐face handoff may be less important in ensuring patient safety when caring for low‐acuity compared to high‐acuity patients, considering the rapidity at which the critically ill can deteriorate.
Handoffs of patient care in the hospital have certainly increased in recent years. Consequently, communication among providers is undoubtedly important, with patient safety being the primary goal. Our work suggests that a face‐to‐face component of a handoff is not vital to ensure a safe care transition. Because of the increasing frequency of handoffs, providers' ability to do so face‐to‐face will likely be challenged by time and logistical constraints. Future work is needed to delineate the most effective components of the handoff so that we can design information transfer that promotes safe and efficient care, even without a face‐to‐face interaction.
Acknowledgements
The authors are grateful for support from the Mayo Clinic Department of Medicine Clinical Research Office, Ms. Donna Lawson, and Mr. Stephen Cha.
Disclosures: This publication was made possible by the Mayo Clinic Center for Clinical and Translational Science through grant number UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. The authors report no conflicts of interest.
Handoffs are key events in the care of hospitalized patients whereby vital information is relayed between healthcare providers. Resident duty hour restrictions and the popularity of shift‐based work schedules have increased the frequency of inpatient handoffs.[1, 2] Failures in communication at the time of patient handoff have been implicated as contributing factors to preventable adverse events.[3, 4, 5, 6] With patient safety in mind, accreditation organizations and professional societies have made the standardization of hospital handoff procedures a priority.[7, 8] A variety of strategies have been utilized to standardize handoffs. Examples include the use of mnemonics,[9] electronic resources,[10, 11, 12] preformatted handoff sheets,[13, 14, 15, 16] and optimization of the handoff environment.[17] The primary outcomes for many of these studies center on the provider by measuring their retention of patient facts[18, 19] and completion of tasks[14, 16] after handoff, for example. Few studies examined patient‐centered outcomes such as transfer to a higher level of care,[20] length of stay,[11] mortality,[21] or readmission rate.[22] A study in the pediatric population found that implementation of a handoff bundle was associated with a decrease in medical errors and preventable adverse events.[23]
The Society of Hospital Medicine recommends that patient handoffs consist of both a written and verbal component.[8] Providers in our division work on 3 shifts: day, evening, and night. In 2009, we developed a face‐to‐face morning handoff, during which night‐shift providers hand off patient care to day‐shift providers incorporating an electronically generated service information list.[17] Given that the evening shift ends well before the day shift begins, the evening‐shift providers do not participate in this face‐to‐face handoff of care for patients they admit to day providers.
We wished to compare the clinical outcomes and adverse events of patients admitted by the night‐shift providers to those admitted by the evening‐shift providers. We hypothesized that transfer of care using a face‐to‐face handoff would be associated with fewer adverse events and improved clinical outcomes.
METHODS
The study was deemed exempt by the Mayo Clinic Institutional Review Board.
Study Population
Hospitalists at the study institution, a 1157‐bed academic tertiary referral hospital, admit general medical patients from the emergency department, as transfers from other institutions, and as direct admissions from outpatient offices. Patients included in the study were all adults admitted by evening‐ and night‐shift hospitalists from August 1, 2011 through August 1, 2012 between 6:45 pm and midnight. Our institution primarily uses 2 levels of care for adult inpatients on internal medicine services, including a general care floor for low‐acuity patients and an intensive care unit for high‐acuity patients. All of the patients in this study were triaged as low acuity at the time of admission and were initially admitted to general care units.
Setting
The division's shift schedule during the study period is depicted in Figure 1. Day‐shift providers included a physician and nurse practitioner (NP) or physician assistant (PA) on each of 7 teams. Each service had an average daily patient census between 10 and 15 patients with 3 to 4 new admissions every 24 hours, with 1 to 2 of these admissions occurring during the evening and night shifts, on average. The day shift started at 7:45 am and ended at 7:45 pm, at which time the day teams transitioned care of their patients to 1 of 2 overnight NP/PAs who provided cross‐cover for all teams through the night. The overnight NP/PAs then transitioned care back to the day teams at 7:45 am the following morning.

Two evening‐shift providers, both physicians, including a staff hospitalist and a hospital medicine fellowship trainee, admitted patients without any cross‐cover responsibility. Their shifts had the same start time, but staggered end times (2 pm10 pm and 2 pmmidnight). At the end of their shifts, the evening‐shift providers relayed concerns or items for follow‐up to the night cross‐cover NP/PAs; however, this handoff was nonstandardized and provider dependent. The cross‐cover providers could also choose to pass on any relevant information to day‐shift providers if thought to be necessary, but this, again, was not required or standardized. A printed electronic handoff tool (including the patient's problem list, medications, vital signs, laboratory results, and to do list as determined by the admitting provider) as well as all clinical notes generated since admission were made available to day‐shift providers who assumed care at 7:45 am; however, there was no face‐to‐face handoff between the evening‐ and day‐shift providers.
Two night‐shift physicians, including a moonlighting board‐eligible internal medicine physician and staff hospitalist, also started at staggered times, 6:45 pm and 10 pm, but their shifts both ended at 7:45 am. These physicians also admitted patients without cross‐cover responsibilities. At 7:45 am, in a face‐to‐face meeting, they transitioned care of patients admitted overnight to day‐shift providers. This handoff occurred at a predesignated place with assigned start times for each team. During the meeting, printed electronic documents, including the aforementioned electronic handoff tool as well as all clinical notes generated since admission, were made available to the oncoming day‐shift providers. The face‐to‐face interaction between night‐ and day‐shift providers lasted approximately 5 minutes and allowed for a brief presentation of the patient, review of the diagnostic testing and treatments performed so far, as well as anticipatory guidance regarding potential issues throughout the remainder of the hospitalization. Although inclusion of the above components was encouraged during the face‐to‐face handoff, the interaction was not scripted and topics discussed were at the providers' discretion.
Patients admitted during the evening and night shifts were assigned to day‐shift services primarily based on the current census of each team, so as to distribute the workload evenly.
Chart Review
Patients included in the study were admitted by evening‐ or night‐shift providers between 6:45 pm and midnight. This time period accounts for when the evening shift and night shift overlap, allowing for direct comparison of patients admitted during the same time of day, so as to avoid confounding factors. Patients were grouped by whether they were admitted by an evening‐shift provider or a night‐shift provider. Each study patient's chart was retrospectively reviewed and relevant demographic and clinical data were collected. Demographic information included age, gender, and race. Clinical information included medical comorbidities, Charlson Comorbidity Index score, rapid response team calls, code team calls, transfers to a higher level of care, death in hospital, 30‐day readmission rate, length of stay (LOS), and adverse events. The Charlson Comorbidity Index score[24] was determined from diagnoses in the institution's medical index database. The 30‐day readmission rate included observation stays and full hospital admissions that occurred at our institution in the 30 days following the patient's hospital discharge from the index admission. LOS was determined based on the time of admission and discharge, as reported in the hospital billing system, and is reported as the median and mean LOS in hours for all patients in each group.
The Global Trigger Tool (GTT) was used to identify adverse events, as defined within the GTT whitepaper to be unintended physical injury resulting from or contributed to by medical care that requires additional monitoring, treatment or hospitalization, or that results in death.[25] Developed by the Institute for Healthcare Improvement, the GTT uses triggers, clues in the medical record that suggest an adverse event may have occurred, to cue a more detailed chart review. Registered nurses trained in use of the GTT reviewed all of the included patients' electronic medical records. If a trigger was identified (such as a patient fall suffered in the hospital), further chart review was prompted to determine if patient harm occurred. If there was evidence of harm, an adverse event was determined to have occurred and was then categorized using the National Coordinating Council for Medication Error Reporting and Prevention Index for Categorizing Errors.[26] For example, in the case of a patient fall whereby the patient was determined to have fallen in the hospital and suffered a laceration requiring wound care, but the hospital stay was not prolonged, this adverse event was categorized as category E (an adverse event that caused the patient temporary harm necessitating intervention, without prolongation of the hospital stay).
Outcomes including rapid response team calls, code team calls, transfers to a higher level of care, death in the hospital, and adverse events, as identified using the GTT, were counted if they occurred between 7:45 am on the first morning of admission until 12 hours later at 7:45 pm, at the time of the first evening handoff of the admitted patients' care.
Statistical Methods
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Mayo Clinic.[27] When comparing outcomes between the 2 groups, Fisher exact test was used for categorical variables and Student t test was used for continuous variables. Global Trigger Tool data were analyzed using the SAS GENMOD procedure, assuming a negative binomial distribution. All the above analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC). Rates of adverse events were compared using MedCalc version 13 software (MedCalc Software, Ostend, Belgium).[28] A P value <0.05 was considered significant.
RESULTS
Of 805 patients admitted between 6:45 pm and midnight during the study period, 305 (37.9%) patients were handed off to day‐shift providers without face‐to‐face handoff, and 500 (62.1%) patients were transferred to the care of day‐shift providers with the use of a face‐to‐face handoff.
Baseline characteristics of both groups are depicted in Table 1. Demographic characteristics, including age, gender, and race, were not significantly different between groups. The mean Charlson Comorbidity Index score was not significantly different between the groups without and with a face‐to‐face handoff. In addition, the presence of medical comorbidities including type 2 diabetes mellitus, hypertension, coronary artery disease, hyperlipidemia, heart failure, body mass index (BMI) <18, active cancer, and current cigarette smoking were not significantly different between the 2 groups. There was a trend to a significantly increased proportion of patients with a BMI >30 in the group without face‐to‐face handoff (P=0.05).
| Without Face‐to‐Face Handoff, N=305 | With Face‐to‐Face Handoff, N=500 | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 65.8 (19.0) | 64.2 (20.0) | 0.25 |
| Sex, n (%) | 0.69 | ||
| Female | 166 (54%) | 265 (53%) | |
| Male | 139 (46%) | 235 (47%) | |
| Race, n (%) | 0.94 | ||
| White | 287 (95%) | 466 (93%) | |
| African American | 5 (2%) | 9 (2%) | |
| Arab/Middle Eastern | 3 (1%) | 8 (2%) | |
| Asian | 1 (0%) | 3 (1%) | |
| Indian subcontinental | 1 (0%) | 1 (0%) | |
| American Indian/Alaskan | 1 (0%) | 1 (0%) | |
| Other | 3 (1%) | 8 (2%) | |
| Unknown | 1 (0%) | 4 (1%) | |
| Charlson Comorbidity Index, mean ( SD) | 2.98 ( 3.73) | 2.93 ( 3.72) | 0.85 |
| Comorbidities, n (%) | |||
| Type 2 diabetes | 82 (27%) | 143 (29%) | 0.60 |
| Hypertension | 195 (64%) | 303 (61%) | 0.34 |
| Coronary artery disease | 76 (25%) | 137 (27%) | 0.44 |
| Hyperlipidemia | 122 (40%) | 206 (41%) | 0.74 |
| Heart failure | 30 (10%) | 66 (13%) | 0.15 |
| BMI >30 | 109 (36%) | 146 (29%) | 0.05 |
| BMI <18 | 7 (2%) | 12 (2%) | 0.92 |
| Active cancer | 29 (10%) | 46 (9%) | 0.88 |
| Current smoker | 49 (16%) | 90 (18%) | 0.48 |
Results for the outcomes of this study are depicted in Table 2. The frequency of rapid response team calls, code team calls, transfers to a higher level of care, and death in the hospital in the 12 hours following the first morning handoff of the admission were not significantly different between the 2 groups. Both 30‐day readmission rate and LOS (median and mean) were not significantly different between groups.
| Without Face‐to‐Face Handoff, N=305 | With Face‐to‐Face Handoff, N=500 | P Value | |
|---|---|---|---|
| |||
| Rapid response team call, n (%) | 4 (1%) | 5 (1%) | 0.68 |
| Code team call, n (%) | 0 (0%) | 1 (0%) | 0.43 |
| Transfer to higher level of care, n (%) | 7 (2%) | 11 (2%) | 0.93 |
| Patient death, n (%) | 0 (0%) | 0 (0%) | 1.00 |
| 30‐day readmission, n (%) | 50 (16%) | 67 (13%) | 0.23 |
| Hospital length of stay | |||
| Median, h (IQR) | 66.5 (41.3115.6) | 70.3 (41.9131.2) | 0.30 |
| Mean, h ( SD) | 102.0 ( 110.0) | 102.9 ( 94.0) | 0.90 |
| Adverse events (Global Trigger Tool) | |||
| Temporary harm and required intervention (E) | 4 | 7 | 0.92 |
| Temporary harm and required initial or prolonged hospitalization (F) | 7 | 8 | 0.53 |
| Permanent harm (G) | 0 | 1 | 0.44 |
| Intervention required to sustain life (H) | 0 | 6 | 0.14 |
| Death (I) | 0 | 0 | 1.00 |
| Total adverse events per 100 admissions | 3.61 | 4.40 | 0.59 |
| % of admissions with an adverse event | 2.6% | 3.2% | 0.64 |
There was no significant difference between the 2 groups in the frequency of adverse events resulting in harm for any of the categories (categories EI). Total adverse events between groups were also compared. Adverse events per 100 admissions were not significantly different between the group without face‐to‐face handoff compared to the group with face‐to‐face handoff. The percentage of admissions with an adverse event was also similar between groups.
DISCUSSION
We found no significant difference in the rate of rapid response team calls, code team calls, transfers to a higher level of care, death in hospital, or adverse events when comparing patients transitioned to the care of day‐shift providers with or without a face‐to‐face handoff. We hypothesize that a reason adverse events were no different between the 2 groups may be that providers were more vigilant when they did not receive a face‐to‐face handoff from the previous provider. As a result, providers may have dedicated additional time reviewing the medical record, speaking with the patients, and communicating with other healthcare providers to ensure a safe care transition. Similarly, other studies found no significant reduction in adverse events when using a standardized handoff.[10, 13, 29] This may be because patient handoff is 1 of a multitude of factors that impact the rate of adverse events, and a handoff may play a less vital role in a system where documentation of care for a given patient is readily accessible, uniform, and detailed. A face‐to‐face interaction itself in a patient handoff may be less pertinent if key information can be communicated through other channels, such as an electronic handoff tool, email, or phone.
Another potential explanation for the lack of a significant difference in patient outcomes with and without a face‐to‐face handoff is related to the study design and inherent rate of the events measured. With the exception of 30‐day readmission rate and LOS, the outcomes of the study were recorded only if they occurred in the 12 hours following the first morning handoff of the admission. This was done in an attempt to isolate the effect of the nonface‐to‐face versus face‐to‐face handoff on the first morning of the admission, and to avoid confounding effects by subsequent transitions of care later in the hospitalization. The frequency of hospital admissions in which an adverse event occurred during this relatively short 12‐hour window was approximately 3% for all patients in the study. With 805 total patients in the study, there may have been insufficient statistical power to detect a difference in the rate of outcomes, if a difference did exist, considering the event rate for both groups and the sample size.
There are several additional limitations to our study. First, the GTT was designed to be applied across the entirety of a hospitalization. By screening for adverse events over the span of only 12 hours for each hospitalization, the sensitivity of the tool may have been diminished, with a proportion of adverse events not captured, even when the sequence of events leading to patient harm began during the 12 hours in question. Second, this is a retrospective study, and all adverse events may not be documented in the medical record. Third, although not formally structured and infrequent, some evening‐shift providers did send an email or call the oncoming day‐shift provider to discuss patients admitted. This process, however, was provider dependent, unstructured, uncommon, and erratic, and thus we were not able to capture it from medical record review. Finally, the patients in this study were deemed low acuity upon triage prior to admission. A face‐to‐face handoff may be less important in ensuring patient safety when caring for low‐acuity compared to high‐acuity patients, considering the rapidity at which the critically ill can deteriorate.
Handoffs of patient care in the hospital have certainly increased in recent years. Consequently, communication among providers is undoubtedly important, with patient safety being the primary goal. Our work suggests that a face‐to‐face component of a handoff is not vital to ensure a safe care transition. Because of the increasing frequency of handoffs, providers' ability to do so face‐to‐face will likely be challenged by time and logistical constraints. Future work is needed to delineate the most effective components of the handoff so that we can design information transfer that promotes safe and efficient care, even without a face‐to‐face interaction.
Acknowledgements
The authors are grateful for support from the Mayo Clinic Department of Medicine Clinical Research Office, Ms. Donna Lawson, and Mr. Stephen Cha.
Disclosures: This publication was made possible by the Mayo Clinic Center for Clinical and Translational Science through grant number UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. The authors report no conflicts of interest.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- . 'Shift work': 24‐hour workdays are out as residents, hospitals deal with changes, mixed feelings on restrictions. Mod Healthc. 2011;41(30):6–7, 16, 1.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , . Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Archives of internal medicine. 2007;167(19):2030–2036.
- , , , et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204(4):533–540.
- Joint Commission International. Standard PC.02.02.01. 2013 Hospital Accreditation Standards. Oak Brook, IL: Joint Commission Resources; 2013.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , . Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196–204.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , , . Impact of a new electronic handover system in surgery. Int J Surg. 2011;9(3):217–220.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , . Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309–313.
- , , , et al. Simple standardized patient handoff system that increases accuracy and completeness. J Surg Educ. 2008;65(6):476–485.
- , , , et al. Enhancing patient safety in the trauma/surgical intensive care unit. J Trauma. 2009;67(3):430–433; discussion 433–435.
- , , . Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , , . Gaining efficiency and satisfaction in the handoff process. J Hosp Med. 2010;5(9):547–552.
- , , . Identification of patient information corruption in the intensive care unit: using a scoring tool to direct quality improvements in handover. Crit Care Med. 2009;37(11):2905–2912.
- . Examining the effects that manipulating information given in the change of shift report has on nurses' care planning ability. J Adv Nurs. 2001;33(6):836–846.
- , , , et al. Evaluation of an asynchronous physician voicemail sign‐out for emergency department admissions. Ann Emerg Med. 2009;54(3):368–378.
- , , , et al. Surgical team behaviors and patient outcomes. Am J Surg. 2009;197(5):678–685.
- , , , , , . The value of adding a verbal report to written handoffs on early readmission following prolonged respiratory failure. Chest. 2010;138(6):1475–1479.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , . IHI Global Trigger Tool for measuring adverse events (second edition). IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2009. Available at: http://www.ihi.org/resources/Pages/IHIWhitePapers/IHIGlobalTriggerToolWhitePaper.aspx. www.IHI.org). Accessed June 1, 2014.
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) index for categorizing errors. Available at: http://www.nccmerp.org/medErrorCatIndex.html. Accessed June 1, 2014.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , . Statistics in Epidemiology: Methods, Techniques, and Applications. Boca Raton, FL: CRC Press; 1996.
- , , , , , . Safety of using a computerized rounding and sign‐out system to reduce resident duty hours. Acad Med. 2010;85(7):1189–1195.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- . 'Shift work': 24‐hour workdays are out as residents, hospitals deal with changes, mixed feelings on restrictions. Mod Healthc. 2011;41(30):6–7, 16, 1.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , . Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Archives of internal medicine. 2007;167(19):2030–2036.
- , , , et al. Patterns of communication breakdowns resulting in injury to surgical patients. J Am Coll Surg. 2007;204(4):533–540.
- Joint Commission International. Standard PC.02.02.01. 2013 Hospital Accreditation Standards. Oak Brook, IL: Joint Commission Resources; 2013.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , . Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196–204.
- , , , , . Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improv. 1998;24(2):77–87.
- , , , , . Impact of a new electronic handover system in surgery. Int J Surg. 2011;9(3):217–220.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , . Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309–313.
- , , , et al. Simple standardized patient handoff system that increases accuracy and completeness. J Surg Educ. 2008;65(6):476–485.
- , , , et al. Enhancing patient safety in the trauma/surgical intensive care unit. J Trauma. 2009;67(3):430–433; discussion 433–435.
- , , . Standardized sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , , . Gaining efficiency and satisfaction in the handoff process. J Hosp Med. 2010;5(9):547–552.
- , , . Identification of patient information corruption in the intensive care unit: using a scoring tool to direct quality improvements in handover. Crit Care Med. 2009;37(11):2905–2912.
- . Examining the effects that manipulating information given in the change of shift report has on nurses' care planning ability. J Adv Nurs. 2001;33(6):836–846.
- , , , et al. Evaluation of an asynchronous physician voicemail sign‐out for emergency department admissions. Ann Emerg Med. 2009;54(3):368–378.
- , , , et al. Surgical team behaviors and patient outcomes. Am J Surg. 2009;197(5):678–685.
- , , , , , . The value of adding a verbal report to written handoffs on early readmission following prolonged respiratory failure. Chest. 2010;138(6):1475–1479.
- , , , et al. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013;310(21):2262–2270.
- , , , . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , . IHI Global Trigger Tool for measuring adverse events (second edition). IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2009. Available at: http://www.ihi.org/resources/Pages/IHIWhitePapers/IHIGlobalTriggerToolWhitePaper.aspx. www.IHI.org). Accessed June 1, 2014.
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) index for categorizing errors. Available at: http://www.nccmerp.org/medErrorCatIndex.html. Accessed June 1, 2014.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- , . Statistics in Epidemiology: Methods, Techniques, and Applications. Boca Raton, FL: CRC Press; 1996.
- , , , , , . Safety of using a computerized rounding and sign‐out system to reduce resident duty hours. Acad Med. 2010;85(7):1189–1195.
© 2015 Society of Hospital Medicine
ACP guidelines for preventing, treating pressure ulcers
Alternating-air and low-air-loss mattresses and overlays have little data to support their use for preventing or treating pressure ulcers, the Clinical Guidelines Committee of the American College of Physicians has concluded.
Many U.S. acute-care hospitals, home caregivers, and long-term nursing facilities use alternating-air and low-air-loss mattresses and overlays, even though the evidence in favor of using these surfaces is sparse and of poor quality, the guideline writers said.
The devices have not been show to actually reduce pressure ulcers. The harms have been poorly reported but could be significant. “Using these support systems is expensive and adds unnecessary burden on the health care system. Based on a review of the current evidence, lower-cost support surfaces should be the preferred approach to care,” Dr. Amir Qaseem, of the ACP, Philadelphia, and his associates wrote.
The committee performed an extensive review of the literature on pressure ulcers and compiled two Clinical Practice Guidelines – one concerning prevention (Ann. Intern. Med. 2015;162 [doi:10.7326/M14-1567]) and the other concerning treatment (Ann. Intern. Med. 2015;162 [doi:10.7326/M14-1568]) – in part because “a growing industry” has developed in recent years and aggressively pitches a wide array of products for this patient population. The guidelines present the available evidence on the comparative effectiveness of tools and strategies but state repeatedly that evidence regarding pressure ulcers is sparse and of poor quality.
The prevention guideline strongly recommends that clinicians choose advanced static mattresses or advanced static overlays rather than standard hospital mattresses for at-risk patients. Static mattresses and advanced static overlays provide a constant level of inflation or support and evenly distribute body weight. These products are among the few actually shown to reduce the incidence of pressure ulcers. They are also preferable to alternating-air mattresses and overlays, which change the distribution of pressure by inflating or deflating cells within the devices, and to low-air-loss mattresses and overlays, which use flowing air to regulate heat and humidity and adjust pressure.
Evidence is similarly poor or lacking concerning the use of other support surfaces such as heel supports or boots and a variety of wheelchair cushions. Also lacking evidence are other preventive interventions that extend beyond “usual care,” such as different types of repositioning schemes, a variety of leg elevations, various nutritional supplements, and a wide variety of skin care strategies and topical treatments.
The prevention guideline advises patient assessments to identify those at risk of developing pressure ulcers. However, there is not enough evidence to demonstrate that any one of the many risk assessment tools for this purpose is superior to the others, nor that any of these tools is superior to simple clinical judgment. Risk factors for pressure ulcers include older age; black race or Hispanic ethnicity; low body weight; cognitive impairment; physical impairments; and comorbid conditions that may affect soft-tissue integrity and healing, such as urinary or fecal incontinence, diabetes, edema, impaired microcirculation, hypoalbuminemia, and malnutrition, Dr. Qaseem and his associates wrote (Ann. Intern. Med. 2015 March 2 [doi:10.7326/M14-1567]).
The treatment guideline for patients who already have pressure ulcers similarly notes that the lack of evidence for advanced support surfaces such as alternating-air and low-air-loss mattresses and overlays. It similarly recommends advanced static mattresses or overlays for these patients.
The treatment guideline recommends protein or amino acid supplements as well as hydrocolloid or foam dressings to reduce wound size, and electrical stimulation to accelerate wound healing. The evidence for these recommendations is “weak” and of low- to moderate-quality, Dr. Qaseem and his associates said (Ann. Intern. Med. 2015 March 2 [doi:10.7326/M14-1568]).
The evidence for the safety and efficacy of hyperbaric oxygen therapy, even though it is often used to treat pressure ulcers in hospitals, is similarly inconclusive. Also lacking good-quality evidence are the use of alternating-air chair cushions, three-dimensional polyester overlays, zinc supplements, L-carnosine supplements, wound dressings other than the ones already discussed, debriding enzymes, topical phenytoin, maggot therapy, biological agents other than platelet-derived growth factor, or hydrotherapy in which wounds are cleaned using a whirlpool or pulsed lavage.
These guidelines emphasize the dire need for good science to guide both prevention and treatment of pressure ulcers. Despite the ubiquity of pressure ulcers and their potential to threaten life and limb, clinical management varies greatly. Most of the research in this field to date has been underpowered and focused on early signs of healing rather than on more definitive outcomes.
Joyce Black, Ph.D., R.N., is at the University of Nebraska Medical Center, Omaha. Her financial disclosures are available at www.acponline.org. Dr. Black made these remarks in an editorial accompanying the ACP Clinical Practice Guidelines on prevention and treatment of pressure ulcers (Ann. Intern. Med. 2015 March 2 [doi:10.1326/M15-0190]).
These guidelines emphasize the dire need for good science to guide both prevention and treatment of pressure ulcers. Despite the ubiquity of pressure ulcers and their potential to threaten life and limb, clinical management varies greatly. Most of the research in this field to date has been underpowered and focused on early signs of healing rather than on more definitive outcomes.
Joyce Black, Ph.D., R.N., is at the University of Nebraska Medical Center, Omaha. Her financial disclosures are available at www.acponline.org. Dr. Black made these remarks in an editorial accompanying the ACP Clinical Practice Guidelines on prevention and treatment of pressure ulcers (Ann. Intern. Med. 2015 March 2 [doi:10.1326/M15-0190]).
These guidelines emphasize the dire need for good science to guide both prevention and treatment of pressure ulcers. Despite the ubiquity of pressure ulcers and their potential to threaten life and limb, clinical management varies greatly. Most of the research in this field to date has been underpowered and focused on early signs of healing rather than on more definitive outcomes.
Joyce Black, Ph.D., R.N., is at the University of Nebraska Medical Center, Omaha. Her financial disclosures are available at www.acponline.org. Dr. Black made these remarks in an editorial accompanying the ACP Clinical Practice Guidelines on prevention and treatment of pressure ulcers (Ann. Intern. Med. 2015 March 2 [doi:10.1326/M15-0190]).
Alternating-air and low-air-loss mattresses and overlays have little data to support their use for preventing or treating pressure ulcers, the Clinical Guidelines Committee of the American College of Physicians has concluded.
Many U.S. acute-care hospitals, home caregivers, and long-term nursing facilities use alternating-air and low-air-loss mattresses and overlays, even though the evidence in favor of using these surfaces is sparse and of poor quality, the guideline writers said.
The devices have not been show to actually reduce pressure ulcers. The harms have been poorly reported but could be significant. “Using these support systems is expensive and adds unnecessary burden on the health care system. Based on a review of the current evidence, lower-cost support surfaces should be the preferred approach to care,” Dr. Amir Qaseem, of the ACP, Philadelphia, and his associates wrote.
The committee performed an extensive review of the literature on pressure ulcers and compiled two Clinical Practice Guidelines – one concerning prevention (Ann. Intern. Med. 2015;162 [doi:10.7326/M14-1567]) and the other concerning treatment (Ann. Intern. Med. 2015;162 [doi:10.7326/M14-1568]) – in part because “a growing industry” has developed in recent years and aggressively pitches a wide array of products for this patient population. The guidelines present the available evidence on the comparative effectiveness of tools and strategies but state repeatedly that evidence regarding pressure ulcers is sparse and of poor quality.
The prevention guideline strongly recommends that clinicians choose advanced static mattresses or advanced static overlays rather than standard hospital mattresses for at-risk patients. Static mattresses and advanced static overlays provide a constant level of inflation or support and evenly distribute body weight. These products are among the few actually shown to reduce the incidence of pressure ulcers. They are also preferable to alternating-air mattresses and overlays, which change the distribution of pressure by inflating or deflating cells within the devices, and to low-air-loss mattresses and overlays, which use flowing air to regulate heat and humidity and adjust pressure.
Evidence is similarly poor or lacking concerning the use of other support surfaces such as heel supports or boots and a variety of wheelchair cushions. Also lacking evidence are other preventive interventions that extend beyond “usual care,” such as different types of repositioning schemes, a variety of leg elevations, various nutritional supplements, and a wide variety of skin care strategies and topical treatments.
The prevention guideline advises patient assessments to identify those at risk of developing pressure ulcers. However, there is not enough evidence to demonstrate that any one of the many risk assessment tools for this purpose is superior to the others, nor that any of these tools is superior to simple clinical judgment. Risk factors for pressure ulcers include older age; black race or Hispanic ethnicity; low body weight; cognitive impairment; physical impairments; and comorbid conditions that may affect soft-tissue integrity and healing, such as urinary or fecal incontinence, diabetes, edema, impaired microcirculation, hypoalbuminemia, and malnutrition, Dr. Qaseem and his associates wrote (Ann. Intern. Med. 2015 March 2 [doi:10.7326/M14-1567]).
The treatment guideline for patients who already have pressure ulcers similarly notes that the lack of evidence for advanced support surfaces such as alternating-air and low-air-loss mattresses and overlays. It similarly recommends advanced static mattresses or overlays for these patients.
The treatment guideline recommends protein or amino acid supplements as well as hydrocolloid or foam dressings to reduce wound size, and electrical stimulation to accelerate wound healing. The evidence for these recommendations is “weak” and of low- to moderate-quality, Dr. Qaseem and his associates said (Ann. Intern. Med. 2015 March 2 [doi:10.7326/M14-1568]).
The evidence for the safety and efficacy of hyperbaric oxygen therapy, even though it is often used to treat pressure ulcers in hospitals, is similarly inconclusive. Also lacking good-quality evidence are the use of alternating-air chair cushions, three-dimensional polyester overlays, zinc supplements, L-carnosine supplements, wound dressings other than the ones already discussed, debriding enzymes, topical phenytoin, maggot therapy, biological agents other than platelet-derived growth factor, or hydrotherapy in which wounds are cleaned using a whirlpool or pulsed lavage.
Alternating-air and low-air-loss mattresses and overlays have little data to support their use for preventing or treating pressure ulcers, the Clinical Guidelines Committee of the American College of Physicians has concluded.
Many U.S. acute-care hospitals, home caregivers, and long-term nursing facilities use alternating-air and low-air-loss mattresses and overlays, even though the evidence in favor of using these surfaces is sparse and of poor quality, the guideline writers said.
The devices have not been show to actually reduce pressure ulcers. The harms have been poorly reported but could be significant. “Using these support systems is expensive and adds unnecessary burden on the health care system. Based on a review of the current evidence, lower-cost support surfaces should be the preferred approach to care,” Dr. Amir Qaseem, of the ACP, Philadelphia, and his associates wrote.
The committee performed an extensive review of the literature on pressure ulcers and compiled two Clinical Practice Guidelines – one concerning prevention (Ann. Intern. Med. 2015;162 [doi:10.7326/M14-1567]) and the other concerning treatment (Ann. Intern. Med. 2015;162 [doi:10.7326/M14-1568]) – in part because “a growing industry” has developed in recent years and aggressively pitches a wide array of products for this patient population. The guidelines present the available evidence on the comparative effectiveness of tools and strategies but state repeatedly that evidence regarding pressure ulcers is sparse and of poor quality.
The prevention guideline strongly recommends that clinicians choose advanced static mattresses or advanced static overlays rather than standard hospital mattresses for at-risk patients. Static mattresses and advanced static overlays provide a constant level of inflation or support and evenly distribute body weight. These products are among the few actually shown to reduce the incidence of pressure ulcers. They are also preferable to alternating-air mattresses and overlays, which change the distribution of pressure by inflating or deflating cells within the devices, and to low-air-loss mattresses and overlays, which use flowing air to regulate heat and humidity and adjust pressure.
Evidence is similarly poor or lacking concerning the use of other support surfaces such as heel supports or boots and a variety of wheelchair cushions. Also lacking evidence are other preventive interventions that extend beyond “usual care,” such as different types of repositioning schemes, a variety of leg elevations, various nutritional supplements, and a wide variety of skin care strategies and topical treatments.
The prevention guideline advises patient assessments to identify those at risk of developing pressure ulcers. However, there is not enough evidence to demonstrate that any one of the many risk assessment tools for this purpose is superior to the others, nor that any of these tools is superior to simple clinical judgment. Risk factors for pressure ulcers include older age; black race or Hispanic ethnicity; low body weight; cognitive impairment; physical impairments; and comorbid conditions that may affect soft-tissue integrity and healing, such as urinary or fecal incontinence, diabetes, edema, impaired microcirculation, hypoalbuminemia, and malnutrition, Dr. Qaseem and his associates wrote (Ann. Intern. Med. 2015 March 2 [doi:10.7326/M14-1567]).
The treatment guideline for patients who already have pressure ulcers similarly notes that the lack of evidence for advanced support surfaces such as alternating-air and low-air-loss mattresses and overlays. It similarly recommends advanced static mattresses or overlays for these patients.
The treatment guideline recommends protein or amino acid supplements as well as hydrocolloid or foam dressings to reduce wound size, and electrical stimulation to accelerate wound healing. The evidence for these recommendations is “weak” and of low- to moderate-quality, Dr. Qaseem and his associates said (Ann. Intern. Med. 2015 March 2 [doi:10.7326/M14-1568]).
The evidence for the safety and efficacy of hyperbaric oxygen therapy, even though it is often used to treat pressure ulcers in hospitals, is similarly inconclusive. Also lacking good-quality evidence are the use of alternating-air chair cushions, three-dimensional polyester overlays, zinc supplements, L-carnosine supplements, wound dressings other than the ones already discussed, debriding enzymes, topical phenytoin, maggot therapy, biological agents other than platelet-derived growth factor, or hydrotherapy in which wounds are cleaned using a whirlpool or pulsed lavage.
Patient fact sheet on the risks of pneumococcal disease
The National Foundation for Infectious Diseases offers basic information to share with patients about the risks of pneumococcal disease and the importance of vaccination in at-risk adults and adults over age 65. Get the facts and a handy information sheet for your patients at their web site.
The National Foundation for Infectious Diseases offers basic information to share with patients about the risks of pneumococcal disease and the importance of vaccination in at-risk adults and adults over age 65. Get the facts and a handy information sheet for your patients at their web site.
The National Foundation for Infectious Diseases offers basic information to share with patients about the risks of pneumococcal disease and the importance of vaccination in at-risk adults and adults over age 65. Get the facts and a handy information sheet for your patients at their web site.
Patient handout on the importance of vaccines
The Immunization Action Coalition offers a single-page handout for adult patients on the need for vaccinations and emphasizes that "getting immunized is a lifelong, life-protecting job." To access a copy of the handout for your adult patients, click here.
The Immunization Action Coalition offers a single-page handout for adult patients on the need for vaccinations and emphasizes that "getting immunized is a lifelong, life-protecting job." To access a copy of the handout for your adult patients, click here.
The Immunization Action Coalition offers a single-page handout for adult patients on the need for vaccinations and emphasizes that "getting immunized is a lifelong, life-protecting job." To access a copy of the handout for your adult patients, click here.
Adult vaccination record
The Centers for Disease Control and Prevention offer a handy adult immunization record that can be easily appended to medical charts. Click here to access a copy of the form.
The Centers for Disease Control and Prevention offer a handy adult immunization record that can be easily appended to medical charts. Click here to access a copy of the form.
The Centers for Disease Control and Prevention offer a handy adult immunization record that can be easily appended to medical charts. Click here to access a copy of the form.
Vaccine storage and handling toolkit
The Vaccine Storage and Handling Toolkit is based on the recommendations of the Advisory Committee on Immunization Practices (ACIP), the manufacturer's product information and studies from the National Institute for Scientific Technology (NIST). Click here to view or print a free copy of the Toolkit.
The Vaccine Storage and Handling Toolkit is based on the recommendations of the Advisory Committee on Immunization Practices (ACIP), the manufacturer's product information and studies from the National Institute for Scientific Technology (NIST). Click here to view or print a free copy of the Toolkit.
The Vaccine Storage and Handling Toolkit is based on the recommendations of the Advisory Committee on Immunization Practices (ACIP), the manufacturer's product information and studies from the National Institute for Scientific Technology (NIST). Click here to view or print a free copy of the Toolkit.