User login
The practice of caring
“To look deep into your child’s eyes and see in him both yourself and something utterly strange, and then to develop a zealous attachment to every aspect of him, is to achieve parenthood’s self-regarding, yet unselfish, abandon. It is astonishing how often such mutuality had been realized – how frequently parents who had supposed that they couldn’t care for an exceptional child discover that they can. The parental predisposition to love prevails in the most harrowing of circumstances. There is more imagination in the world than one might think.”
Andrew Solomon, “Far from the Tree: Parents, Children, and the Search for Identity” (New York: Scribner, 2012).
In his most recent book, writer and lecturer Andrew Solomon describes a deep love that leads to redemption. His case histories describe parents becoming virtuous through the practice of caring. Solomon records both their loving and their suffering. He does not see caring, necessarily, as an inherent trait but rather sees virtue emerging from the act of caring. The philosophical study of caring and virtue is known as the “ethics of care.” This column considers the ethics of care in relation to our patients and their families.
‘Ethics of care’ origins
Care ethics emerged as a distinct moral theory when psychologist Carol Gilligan, Ph.D., and philosopher Nel Noddings, Ph.D., labeled traditional moral theory as biased toward the male gender. They asserted the “voice of care” as a female alternative to Lawrence Kohlberg’s male “voice of justice.”
Originally, therefore, care ethics were described as a feminist ethic. To drive home this point, the suffragettes argued that granting voting rights to (white) women would lead to moral, social improvements! The naive assumption was that women by nature had traits of compassion, empathy, nurturance, and kindness, as exemplified by the good mother. This is known as feminist essentialism. Taking this gendered view further, Nel Noddings states that the domestic sphere is the originator and nurturer of justice, in the sense that the best social policies are identified, modeled, and sustained by practices in the “best families.” This is a difficult position: Who decides on the characteristics of “best families?”
Practice of caring vs. ethics
The practice of caring can be described as actions performed by the carer, or as a value, or as a disposition or virtue that resides in the person who is caring. The following points summarize the current positions of philosophers who identify themselves as care ethicists.
• Care reflects a specific type of moral reasoning. This is the Kohlberg-Gilligan argument of male vs. female reasoning. Although care and justice have evolved as distinct ethical practices and ideals, they are not necessarily incompatible. As gender roles soften and gender as a concept becomes more blurry, care and justice can be intertwined. Reasoning does not have to be either justice based or care based.
• Care is the practice of caring for someone (Andrew Solomon’s case histories). This stance does not romanticize the practice of caring. This stance does not consider caring as a trait or disposition. This stance acknowledges the suffering and hardship in caring that can coexist with love. This stance points to the potential for individual spiritual and personal growth that can accompany caregiving. Andrew Solomon would agree to the notion of stages of caring (“Moral Boundaries: A Political Argument for an Ethic of Care,” New York: Routledge, 1994). These stages are: (1) attentiveness, becoming aware of need; (2) responsibility, a willingness to respond and take care of need; (3) competence, the skill of providing good and successful care; and (4) responsiveness, consideration of the position of others as they see it and recognition of the potential for abuse in care. The practice of caring is more of a daily reality than the abstract virtue.
• Care is an inherent virtue. This stance includes both feminist essentialism and feminist care ethics. In feminist essentialism, the process of moral development follows gender roles. The prototypical caregiving mother and a care-receiving child romanticize and elevate motherhood to the ideal practice of care. Feminist care ethicists avoid this essentialism by situating caring practices in place and time. They describe care as the symbolic practice rather than actual practice of women. Feminist care ethicists explore care as a gender neutral activity, advancing a utopian vision of care as a gender-neutral activity and virtue. Cognitive capacities and virtues associated with mothering, (better described as being associated with parenting), are seen as essential to the concept of care. These virtues are preservative love (work of protection with cheerfulness and humility), fostering growth (sponsoring or nurturing a child’s unfolding), and training for social acceptability (a process of socialization that requires conscience and a struggle for authenticity). This position also is reflected in Solomon’s ethics of care.
• Care is an inherent character trait or disposition. This stance understands care ethics as a form of virtue ethics, with care being a central virtue. There is an emphasis on relationship as fundamental to being, and the parent-child relationship as paramount. Virtue ethics views emotions such as empathy, compassion, and sensitivity as prerequisites for moral development and the ethics of care.
• Care as social justice and political imperative. One of the earliest objections to an ethics of care was that it valorized the oppression of women. Nietzsche held that those who are oppressed develop moral theories that reaffirm subservient traits as virtues. Women who perform the work of care often perform this care to their own economic disadvantage. A social justice perspective implies that the voice of care is the voice of an oppressed person, and eschews the idea that moral maturity means self-sacrifice and self-effacement. Care ethics informed by a social justice perspective asks who is caring for whom and whether this relationship is just.
When care ethics are applied to domestic politics, economic justice, international relations, and culture, interesting ideas emerge. Governments and businesses become responsible for support in sickness, disability, old age, bad luck, and reversal of fortune, for providing protection, health care, and clean environments, and for upholding the rights of individuals. A focus on autonomy, independence, and self-determination, which traditionally are seen as male traits, devalues interdependence and relatedness, which traditionally are seen as female values. Care ethics suggest that we replace hierarchy and domination that is based on gender, class, race, and ethnicity with cooperation and attention to interdependency. Interdependency is ubiquitous, and care ethics is a political theory with universal application. The practice of caring has no political affiliation; however, if we had founding mothers instead of founding fathers, would the United States be based more on ethics of care?
•The caring professions. The practice of caring is a practice that helps individuals meet their basic needs, maintain capabilities, and alleviate pain and suffering, so they can survive and function in society. Using this definition, the practice of care does not require any emotional attachment. Using this definition, the activity itself is a virtuous moral position. The health care professions mostly provide “services” rather than “care.” Is empathy a necessary ingredient for the practice of care? Many people believe so, and organizations such as the Watson Caring Science Institute (watsoncaringscience.org) are dedicated to putting the caring back into health care.
Meaning for the psychiatrist
When caregivers of patients with dementia were asked how they felt about caregiving, they responded positively. Caregiving felt good. Here is a listing of some their responses:
“Feeling needed and responsible.”
“Feeling good inside, doing for someone what you want for yourself and knowing I’ve done my best.”
“Being able to help.”
“To brighten her days.”
“I know he is being cared for the way he is used to.”
“I feel that she is loved and not alone.”
These caregivers were mostly spouses (61%), with an average of 3.1 caregiving years. Caregivers reported that their relatives were moderately disabled, but they perceived more reward than burden (Int. J. Geriatr. Psychiatry, 2004;19:533-7). The caregivers’ quality of life also proved similar to those in an age-controlled normal community sample. So if caregiving can be carried out without significantly affecting quality of life, caregiving can be more rewarding than burdensome.
Questions for the family psychiatrist:
• How am I caring for my patients and their families?
• What does it mean to care rather than provide a service?
• How has my psychiatric training changed how I perceive caring? Do I now care in a different way?
• Has the way I care developed through my practice of caring?
• Where am I in the stages of caring?
• When does caring mean advocacy?
Questions to ask patients and their families:
• Do you experience reward in caregiving?
• Are there ways to sustain and enhance the satisfaction and reward of caring?
• How might you explore the practice of caring?
• Has caring been redeeming for you?
• Has caring brought individual growth for you, despite the hardships?
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Some of the research for this article came from The Internet Encyclopedia of Philosophy.
“To look deep into your child’s eyes and see in him both yourself and something utterly strange, and then to develop a zealous attachment to every aspect of him, is to achieve parenthood’s self-regarding, yet unselfish, abandon. It is astonishing how often such mutuality had been realized – how frequently parents who had supposed that they couldn’t care for an exceptional child discover that they can. The parental predisposition to love prevails in the most harrowing of circumstances. There is more imagination in the world than one might think.”
Andrew Solomon, “Far from the Tree: Parents, Children, and the Search for Identity” (New York: Scribner, 2012).
In his most recent book, writer and lecturer Andrew Solomon describes a deep love that leads to redemption. His case histories describe parents becoming virtuous through the practice of caring. Solomon records both their loving and their suffering. He does not see caring, necessarily, as an inherent trait but rather sees virtue emerging from the act of caring. The philosophical study of caring and virtue is known as the “ethics of care.” This column considers the ethics of care in relation to our patients and their families.
‘Ethics of care’ origins
Care ethics emerged as a distinct moral theory when psychologist Carol Gilligan, Ph.D., and philosopher Nel Noddings, Ph.D., labeled traditional moral theory as biased toward the male gender. They asserted the “voice of care” as a female alternative to Lawrence Kohlberg’s male “voice of justice.”
Originally, therefore, care ethics were described as a feminist ethic. To drive home this point, the suffragettes argued that granting voting rights to (white) women would lead to moral, social improvements! The naive assumption was that women by nature had traits of compassion, empathy, nurturance, and kindness, as exemplified by the good mother. This is known as feminist essentialism. Taking this gendered view further, Nel Noddings states that the domestic sphere is the originator and nurturer of justice, in the sense that the best social policies are identified, modeled, and sustained by practices in the “best families.” This is a difficult position: Who decides on the characteristics of “best families?”
Practice of caring vs. ethics
The practice of caring can be described as actions performed by the carer, or as a value, or as a disposition or virtue that resides in the person who is caring. The following points summarize the current positions of philosophers who identify themselves as care ethicists.
• Care reflects a specific type of moral reasoning. This is the Kohlberg-Gilligan argument of male vs. female reasoning. Although care and justice have evolved as distinct ethical practices and ideals, they are not necessarily incompatible. As gender roles soften and gender as a concept becomes more blurry, care and justice can be intertwined. Reasoning does not have to be either justice based or care based.
• Care is the practice of caring for someone (Andrew Solomon’s case histories). This stance does not romanticize the practice of caring. This stance does not consider caring as a trait or disposition. This stance acknowledges the suffering and hardship in caring that can coexist with love. This stance points to the potential for individual spiritual and personal growth that can accompany caregiving. Andrew Solomon would agree to the notion of stages of caring (“Moral Boundaries: A Political Argument for an Ethic of Care,” New York: Routledge, 1994). These stages are: (1) attentiveness, becoming aware of need; (2) responsibility, a willingness to respond and take care of need; (3) competence, the skill of providing good and successful care; and (4) responsiveness, consideration of the position of others as they see it and recognition of the potential for abuse in care. The practice of caring is more of a daily reality than the abstract virtue.
• Care is an inherent virtue. This stance includes both feminist essentialism and feminist care ethics. In feminist essentialism, the process of moral development follows gender roles. The prototypical caregiving mother and a care-receiving child romanticize and elevate motherhood to the ideal practice of care. Feminist care ethicists avoid this essentialism by situating caring practices in place and time. They describe care as the symbolic practice rather than actual practice of women. Feminist care ethicists explore care as a gender neutral activity, advancing a utopian vision of care as a gender-neutral activity and virtue. Cognitive capacities and virtues associated with mothering, (better described as being associated with parenting), are seen as essential to the concept of care. These virtues are preservative love (work of protection with cheerfulness and humility), fostering growth (sponsoring or nurturing a child’s unfolding), and training for social acceptability (a process of socialization that requires conscience and a struggle for authenticity). This position also is reflected in Solomon’s ethics of care.
• Care is an inherent character trait or disposition. This stance understands care ethics as a form of virtue ethics, with care being a central virtue. There is an emphasis on relationship as fundamental to being, and the parent-child relationship as paramount. Virtue ethics views emotions such as empathy, compassion, and sensitivity as prerequisites for moral development and the ethics of care.
• Care as social justice and political imperative. One of the earliest objections to an ethics of care was that it valorized the oppression of women. Nietzsche held that those who are oppressed develop moral theories that reaffirm subservient traits as virtues. Women who perform the work of care often perform this care to their own economic disadvantage. A social justice perspective implies that the voice of care is the voice of an oppressed person, and eschews the idea that moral maturity means self-sacrifice and self-effacement. Care ethics informed by a social justice perspective asks who is caring for whom and whether this relationship is just.
When care ethics are applied to domestic politics, economic justice, international relations, and culture, interesting ideas emerge. Governments and businesses become responsible for support in sickness, disability, old age, bad luck, and reversal of fortune, for providing protection, health care, and clean environments, and for upholding the rights of individuals. A focus on autonomy, independence, and self-determination, which traditionally are seen as male traits, devalues interdependence and relatedness, which traditionally are seen as female values. Care ethics suggest that we replace hierarchy and domination that is based on gender, class, race, and ethnicity with cooperation and attention to interdependency. Interdependency is ubiquitous, and care ethics is a political theory with universal application. The practice of caring has no political affiliation; however, if we had founding mothers instead of founding fathers, would the United States be based more on ethics of care?
•The caring professions. The practice of caring is a practice that helps individuals meet their basic needs, maintain capabilities, and alleviate pain and suffering, so they can survive and function in society. Using this definition, the practice of care does not require any emotional attachment. Using this definition, the activity itself is a virtuous moral position. The health care professions mostly provide “services” rather than “care.” Is empathy a necessary ingredient for the practice of care? Many people believe so, and organizations such as the Watson Caring Science Institute (watsoncaringscience.org) are dedicated to putting the caring back into health care.
Meaning for the psychiatrist
When caregivers of patients with dementia were asked how they felt about caregiving, they responded positively. Caregiving felt good. Here is a listing of some their responses:
“Feeling needed and responsible.”
“Feeling good inside, doing for someone what you want for yourself and knowing I’ve done my best.”
“Being able to help.”
“To brighten her days.”
“I know he is being cared for the way he is used to.”
“I feel that she is loved and not alone.”
These caregivers were mostly spouses (61%), with an average of 3.1 caregiving years. Caregivers reported that their relatives were moderately disabled, but they perceived more reward than burden (Int. J. Geriatr. Psychiatry, 2004;19:533-7). The caregivers’ quality of life also proved similar to those in an age-controlled normal community sample. So if caregiving can be carried out without significantly affecting quality of life, caregiving can be more rewarding than burdensome.
Questions for the family psychiatrist:
• How am I caring for my patients and their families?
• What does it mean to care rather than provide a service?
• How has my psychiatric training changed how I perceive caring? Do I now care in a different way?
• Has the way I care developed through my practice of caring?
• Where am I in the stages of caring?
• When does caring mean advocacy?
Questions to ask patients and their families:
• Do you experience reward in caregiving?
• Are there ways to sustain and enhance the satisfaction and reward of caring?
• How might you explore the practice of caring?
• Has caring been redeeming for you?
• Has caring brought individual growth for you, despite the hardships?
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Some of the research for this article came from The Internet Encyclopedia of Philosophy.
“To look deep into your child’s eyes and see in him both yourself and something utterly strange, and then to develop a zealous attachment to every aspect of him, is to achieve parenthood’s self-regarding, yet unselfish, abandon. It is astonishing how often such mutuality had been realized – how frequently parents who had supposed that they couldn’t care for an exceptional child discover that they can. The parental predisposition to love prevails in the most harrowing of circumstances. There is more imagination in the world than one might think.”
Andrew Solomon, “Far from the Tree: Parents, Children, and the Search for Identity” (New York: Scribner, 2012).
In his most recent book, writer and lecturer Andrew Solomon describes a deep love that leads to redemption. His case histories describe parents becoming virtuous through the practice of caring. Solomon records both their loving and their suffering. He does not see caring, necessarily, as an inherent trait but rather sees virtue emerging from the act of caring. The philosophical study of caring and virtue is known as the “ethics of care.” This column considers the ethics of care in relation to our patients and their families.
‘Ethics of care’ origins
Care ethics emerged as a distinct moral theory when psychologist Carol Gilligan, Ph.D., and philosopher Nel Noddings, Ph.D., labeled traditional moral theory as biased toward the male gender. They asserted the “voice of care” as a female alternative to Lawrence Kohlberg’s male “voice of justice.”
Originally, therefore, care ethics were described as a feminist ethic. To drive home this point, the suffragettes argued that granting voting rights to (white) women would lead to moral, social improvements! The naive assumption was that women by nature had traits of compassion, empathy, nurturance, and kindness, as exemplified by the good mother. This is known as feminist essentialism. Taking this gendered view further, Nel Noddings states that the domestic sphere is the originator and nurturer of justice, in the sense that the best social policies are identified, modeled, and sustained by practices in the “best families.” This is a difficult position: Who decides on the characteristics of “best families?”
Practice of caring vs. ethics
The practice of caring can be described as actions performed by the carer, or as a value, or as a disposition or virtue that resides in the person who is caring. The following points summarize the current positions of philosophers who identify themselves as care ethicists.
• Care reflects a specific type of moral reasoning. This is the Kohlberg-Gilligan argument of male vs. female reasoning. Although care and justice have evolved as distinct ethical practices and ideals, they are not necessarily incompatible. As gender roles soften and gender as a concept becomes more blurry, care and justice can be intertwined. Reasoning does not have to be either justice based or care based.
• Care is the practice of caring for someone (Andrew Solomon’s case histories). This stance does not romanticize the practice of caring. This stance does not consider caring as a trait or disposition. This stance acknowledges the suffering and hardship in caring that can coexist with love. This stance points to the potential for individual spiritual and personal growth that can accompany caregiving. Andrew Solomon would agree to the notion of stages of caring (“Moral Boundaries: A Political Argument for an Ethic of Care,” New York: Routledge, 1994). These stages are: (1) attentiveness, becoming aware of need; (2) responsibility, a willingness to respond and take care of need; (3) competence, the skill of providing good and successful care; and (4) responsiveness, consideration of the position of others as they see it and recognition of the potential for abuse in care. The practice of caring is more of a daily reality than the abstract virtue.
• Care is an inherent virtue. This stance includes both feminist essentialism and feminist care ethics. In feminist essentialism, the process of moral development follows gender roles. The prototypical caregiving mother and a care-receiving child romanticize and elevate motherhood to the ideal practice of care. Feminist care ethicists avoid this essentialism by situating caring practices in place and time. They describe care as the symbolic practice rather than actual practice of women. Feminist care ethicists explore care as a gender neutral activity, advancing a utopian vision of care as a gender-neutral activity and virtue. Cognitive capacities and virtues associated with mothering, (better described as being associated with parenting), are seen as essential to the concept of care. These virtues are preservative love (work of protection with cheerfulness and humility), fostering growth (sponsoring or nurturing a child’s unfolding), and training for social acceptability (a process of socialization that requires conscience and a struggle for authenticity). This position also is reflected in Solomon’s ethics of care.
• Care is an inherent character trait or disposition. This stance understands care ethics as a form of virtue ethics, with care being a central virtue. There is an emphasis on relationship as fundamental to being, and the parent-child relationship as paramount. Virtue ethics views emotions such as empathy, compassion, and sensitivity as prerequisites for moral development and the ethics of care.
• Care as social justice and political imperative. One of the earliest objections to an ethics of care was that it valorized the oppression of women. Nietzsche held that those who are oppressed develop moral theories that reaffirm subservient traits as virtues. Women who perform the work of care often perform this care to their own economic disadvantage. A social justice perspective implies that the voice of care is the voice of an oppressed person, and eschews the idea that moral maturity means self-sacrifice and self-effacement. Care ethics informed by a social justice perspective asks who is caring for whom and whether this relationship is just.
When care ethics are applied to domestic politics, economic justice, international relations, and culture, interesting ideas emerge. Governments and businesses become responsible for support in sickness, disability, old age, bad luck, and reversal of fortune, for providing protection, health care, and clean environments, and for upholding the rights of individuals. A focus on autonomy, independence, and self-determination, which traditionally are seen as male traits, devalues interdependence and relatedness, which traditionally are seen as female values. Care ethics suggest that we replace hierarchy and domination that is based on gender, class, race, and ethnicity with cooperation and attention to interdependency. Interdependency is ubiquitous, and care ethics is a political theory with universal application. The practice of caring has no political affiliation; however, if we had founding mothers instead of founding fathers, would the United States be based more on ethics of care?
•The caring professions. The practice of caring is a practice that helps individuals meet their basic needs, maintain capabilities, and alleviate pain and suffering, so they can survive and function in society. Using this definition, the practice of care does not require any emotional attachment. Using this definition, the activity itself is a virtuous moral position. The health care professions mostly provide “services” rather than “care.” Is empathy a necessary ingredient for the practice of care? Many people believe so, and organizations such as the Watson Caring Science Institute (watsoncaringscience.org) are dedicated to putting the caring back into health care.
Meaning for the psychiatrist
When caregivers of patients with dementia were asked how they felt about caregiving, they responded positively. Caregiving felt good. Here is a listing of some their responses:
“Feeling needed and responsible.”
“Feeling good inside, doing for someone what you want for yourself and knowing I’ve done my best.”
“Being able to help.”
“To brighten her days.”
“I know he is being cared for the way he is used to.”
“I feel that she is loved and not alone.”
These caregivers were mostly spouses (61%), with an average of 3.1 caregiving years. Caregivers reported that their relatives were moderately disabled, but they perceived more reward than burden (Int. J. Geriatr. Psychiatry, 2004;19:533-7). The caregivers’ quality of life also proved similar to those in an age-controlled normal community sample. So if caregiving can be carried out without significantly affecting quality of life, caregiving can be more rewarding than burdensome.
Questions for the family psychiatrist:
• How am I caring for my patients and their families?
• What does it mean to care rather than provide a service?
• How has my psychiatric training changed how I perceive caring? Do I now care in a different way?
• Has the way I care developed through my practice of caring?
• Where am I in the stages of caring?
• When does caring mean advocacy?
Questions to ask patients and their families:
• Do you experience reward in caregiving?
• Are there ways to sustain and enhance the satisfaction and reward of caring?
• How might you explore the practice of caring?
• Has caring been redeeming for you?
• Has caring brought individual growth for you, despite the hardships?
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Some of the research for this article came from The Internet Encyclopedia of Philosophy.
Rapid INR reversal key in oral anticoagulant–associated intracerebral hemorrhage
Rapid reversal of international normalized ratio, along with systolic blood pressure reduction, in patients with oral anticoagulant–associated intracerebral hemorrhage can significantly reduce the rates of hematoma enlargement and in-hospital mortality, a retrospective cohort study has found.
In the German study of 1,176 patients with oral anticoagulant–associated intracerebral hemorrhage, patients whose INR was reduced below 1.3 with use of vitamin K agonists and whose systolic BP was reduced below 160 mm Hg within 4 hours of admission had a 72% reduction in the rates of hematoma enlargement and 40% reduction in in-hospital mortality, compared with patients who did not achieve those reductions in that time frame.
Researchers also showed that there were no significant increases in hemorrhagic complications, but fewer ischemic complications, among patients who resumed oral anticoagulation, according to the study published online Feb. 24 in JAMA.
“Consensus exists that elevated international normalized ratio (INR) levels should be reversed to minimize hematoma enlargement, yet mode, timing, and extent of INR reversal are unclear [and] valid data on safety and clinical benefit of [oral anticoagulant] resumption are missing and remain to be established,” wrote Dr. Joji B. Kuramatsu of the University of Erlangen-Nuremberg, Germany, and coauthors (JAMA 2015;313:824-836 [doi:10.1001/jama.2015.0846]).
The study was supported by the Johannes and Frieda Marohn Foundation. Authors declared a range of funding, grants, fees, and honoraria from the pharmaceutical industry.
Rapid reversal of international normalized ratio, along with systolic blood pressure reduction, in patients with oral anticoagulant–associated intracerebral hemorrhage can significantly reduce the rates of hematoma enlargement and in-hospital mortality, a retrospective cohort study has found.
In the German study of 1,176 patients with oral anticoagulant–associated intracerebral hemorrhage, patients whose INR was reduced below 1.3 with use of vitamin K agonists and whose systolic BP was reduced below 160 mm Hg within 4 hours of admission had a 72% reduction in the rates of hematoma enlargement and 40% reduction in in-hospital mortality, compared with patients who did not achieve those reductions in that time frame.
Researchers also showed that there were no significant increases in hemorrhagic complications, but fewer ischemic complications, among patients who resumed oral anticoagulation, according to the study published online Feb. 24 in JAMA.
“Consensus exists that elevated international normalized ratio (INR) levels should be reversed to minimize hematoma enlargement, yet mode, timing, and extent of INR reversal are unclear [and] valid data on safety and clinical benefit of [oral anticoagulant] resumption are missing and remain to be established,” wrote Dr. Joji B. Kuramatsu of the University of Erlangen-Nuremberg, Germany, and coauthors (JAMA 2015;313:824-836 [doi:10.1001/jama.2015.0846]).
The study was supported by the Johannes and Frieda Marohn Foundation. Authors declared a range of funding, grants, fees, and honoraria from the pharmaceutical industry.
Rapid reversal of international normalized ratio, along with systolic blood pressure reduction, in patients with oral anticoagulant–associated intracerebral hemorrhage can significantly reduce the rates of hematoma enlargement and in-hospital mortality, a retrospective cohort study has found.
In the German study of 1,176 patients with oral anticoagulant–associated intracerebral hemorrhage, patients whose INR was reduced below 1.3 with use of vitamin K agonists and whose systolic BP was reduced below 160 mm Hg within 4 hours of admission had a 72% reduction in the rates of hematoma enlargement and 40% reduction in in-hospital mortality, compared with patients who did not achieve those reductions in that time frame.
Researchers also showed that there were no significant increases in hemorrhagic complications, but fewer ischemic complications, among patients who resumed oral anticoagulation, according to the study published online Feb. 24 in JAMA.
“Consensus exists that elevated international normalized ratio (INR) levels should be reversed to minimize hematoma enlargement, yet mode, timing, and extent of INR reversal are unclear [and] valid data on safety and clinical benefit of [oral anticoagulant] resumption are missing and remain to be established,” wrote Dr. Joji B. Kuramatsu of the University of Erlangen-Nuremberg, Germany, and coauthors (JAMA 2015;313:824-836 [doi:10.1001/jama.2015.0846]).
The study was supported by the Johannes and Frieda Marohn Foundation. Authors declared a range of funding, grants, fees, and honoraria from the pharmaceutical industry.
FROM JAMA
Key clinical point: Rapid INR reversal and systolic blood pressure reduction in patients lowers the rates of hematoma enlargement and in-hospital mortality from oral anticoagulant–associated intracerebral hemorrhage.
Major finding: Patients whose INR was reduced below 1.3 and systolic blood pressure reduced below 160 mm Hg within 4 hours of admission had a 72% reduction in the rates of hematoma enlargement and 40% reduction in in-hospital mortality.
Data source: Retrospective cohort study of 1,176 patients with oral anticoagulant–associated intracerebral hemorrhage .
Disclosures: The study was supported by the Johannes and Frieda Marohn Foundation. Authors declared a range of funding, grants, fees, and honoraria from the pharmaceutical industry.
An Incidental Finding During Neuro Evaluation
ANSWER
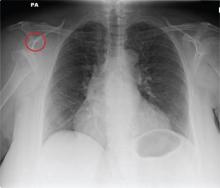
The radiograph shows a normal-appearing chest. Of note, though, is an anterior dislocation of the right shoulder. In addition, there is a fracture within the greater tuberosity of the right humerus.
Prompt orthopedic evaluation is obtained. In further discussion with the family, it was revealed that the patient had been experiencing falls recently; this injury was most likely the result of one.
ANSWER
The radiograph shows a normal-appearing chest. Of note, though, is an anterior dislocation of the right shoulder. In addition, there is a fracture within the greater tuberosity of the right humerus.
Prompt orthopedic evaluation is obtained. In further discussion with the family, it was revealed that the patient had been experiencing falls recently; this injury was most likely the result of one.
ANSWER
The radiograph shows a normal-appearing chest. Of note, though, is an anterior dislocation of the right shoulder. In addition, there is a fracture within the greater tuberosity of the right humerus.
Prompt orthopedic evaluation is obtained. In further discussion with the family, it was revealed that the patient had been experiencing falls recently; this injury was most likely the result of one.
A 65-year-old woman is transferred to your facility from an outlying hospital for evaluation of a brain tumor. Family members found the patient sitting on the sofa, with a decreased level of consciousness. There was reported “seizure-type activity.” When she arrived at the outlying hospital, the patient was noted to have right-side weakness. Stat CT of the head demonstrated a fairly large parasagittal mass, and the patient was urgently transferred to your facility for neurosurgical evaluation. Primary survey on arrival shows an older female who is awake, alert, and in no obvious distress. Vital signs are normal. She has fairly pronounced right upper extremity weakness, more proximally than distally. Otherwise, the exam grossly appears normal. The patient’s initial imaging studies were sent with her on a CD. As you are trying to view the images of the brain, a chest radiograph pops up on your screen. What is your impression?
What Does This Man Need (Besides Milk & Cookies)?
ANSWER
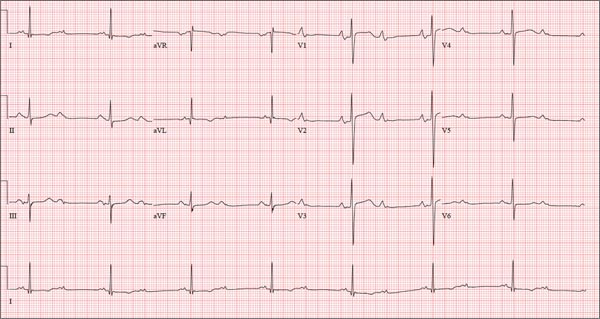
This ECG is representative of sinus rhythm with second-degree atrioventricular block with 2:1 conduction; possible left atrial enlargement; and ST-T wave abnormalities suspicious for lateral ischemia.
Sinus rhythm is evidenced by the P waves that march through at a rate that is consistently double that of the QRS rate (82 beats/min). The PR interval in the conducted beats remains constant, with every other P wave blocked from conducting into the ventricles.
The biphasic P wave seen in lead V1 does not meet criteria for left atrial enlargement (P wave in lead I ≥ 110 ms, terminal negative P wave in lead V1 ≥ 1 mm2) but is suspicious. Finally, ST-T wave elevations in leads V2-V4 are suspicious for ventricular septal ischemia.
The patient underwent placement of a dual-chamber permanent pacemaker. He has done well since.
ANSWER
This ECG is representative of sinus rhythm with second-degree atrioventricular block with 2:1 conduction; possible left atrial enlargement; and ST-T wave abnormalities suspicious for lateral ischemia.
Sinus rhythm is evidenced by the P waves that march through at a rate that is consistently double that of the QRS rate (82 beats/min). The PR interval in the conducted beats remains constant, with every other P wave blocked from conducting into the ventricles.
The biphasic P wave seen in lead V1 does not meet criteria for left atrial enlargement (P wave in lead I ≥ 110 ms, terminal negative P wave in lead V1 ≥ 1 mm2) but is suspicious. Finally, ST-T wave elevations in leads V2-V4 are suspicious for ventricular septal ischemia.
The patient underwent placement of a dual-chamber permanent pacemaker. He has done well since.
ANSWER
This ECG is representative of sinus rhythm with second-degree atrioventricular block with 2:1 conduction; possible left atrial enlargement; and ST-T wave abnormalities suspicious for lateral ischemia.
Sinus rhythm is evidenced by the P waves that march through at a rate that is consistently double that of the QRS rate (82 beats/min). The PR interval in the conducted beats remains constant, with every other P wave blocked from conducting into the ventricles.
The biphasic P wave seen in lead V1 does not meet criteria for left atrial enlargement (P wave in lead I ≥ 110 ms, terminal negative P wave in lead V1 ≥ 1 mm2) but is suspicious. Finally, ST-T wave elevations in leads V2-V4 are suspicious for ventricular septal ischemia.
The patient underwent placement of a dual-chamber permanent pacemaker. He has done well since.

A 74-year-old man has been a resident of a skilled nursing facility for seven years and is well known to the staff. This morning, when the medical assistant performed a routine vital sign check, she noticed the patient’s heart rate was in the 40s. This newly discovered bradycardia, coupled with a four-day history of lethargy, prompts the facility to transfer the patient to your emergency department for evaluation. The patient has a history of hypertension, hypothyroidism, chronic obstructive pulmonary disease, GERD, osteoarthritis, and dementia. Surgical history includes appendectomy, cholecystectomy, and left hip replacement. The patient’s multiple chronic conditions are well managed with medications, including a b-blocker, hydrochlorothiazide, levothyroxine, and an inhaler. He receives protein and vitamin supplements daily and is allergic to penicillin. There is a remote history of smoking (from his youth and tour of duty in the Korean War), although the patient says he hasn’t smoked in 30 years. He has “never touched” alcohol, because his father died of complications from alcoholism at age 45. The patient’s wife died of a stroke 11 years ago. His son (and family) visit him twice weekly, bringing chocolate milk and cookies that the patient anxiously awaits. The review of systems is remarkable for a recent cold (resolved), urinary retention, and loose stools. The patient’s appetite is intact. He also exhibits evidence of short-term memory loss; however, this is sporadic. Vital signs on arrival include a blood pressure of 158/88 mm Hg; pulse, 48 beats/min and regular; respiratory rate, 14 breaths/min; and temperature, 97.6°F. His weight is 174 lb and his height, 69 in. Pertinent findings on the physical exam include mild cataracts bilaterally, a right carotid bruit, no evidence of elevated neck veins, and late expiratory wheezes in both bases. The cardiac exam is remarkable for a regular rhythm with a heart rate of 42 beats/min. There is a grade II/VI early systolic murmur at the left upper sternal border but no radiation, extra heart sounds, or rubs. The abdomen is soft and nontender, with old surgical scars, and the abdominal aorta is easily palpable. The extremities exhibit full range of motion without peripheral edema, and osteoarthritic changes are evident in both hands. An ECG shows a ventricular rate of 43 beats/min; PR interval, 198 ms; QRS duration, 96 ms; QT/QTc interval, 464/392 ms; P axis, 60°; R axis, 4°; and T axis, 107°. What is your interpretation of this ECG?
Boy Has Had “Bald Spot” Since Birth
ANSWER
The answer is temporal triangular alopecia (choice “d”), an unusual form of permanent hair loss preferentially affecting the exact area depicted in this case.
Alopecia areata (choice “a”) involves localized hair loss. By contrast, this patient never had hair in this area to lose.
Nevus sebaceous (choice “b”) is a congenital hamartoma that is typically hairless; there are no follicles, and the bumpy, rough surface is composed of sebaceous globules.
Cutis aplasia (choice “c”) manifests with hairless lesions, but there is marked aplasia of the skin as well and no surface adnexae, let alone hairs or follicles.
DISCUSSION
Temporal triangular alopecia (TTA) is an unusual type of alopecia. Of unknown origin, it usually affects this area of the scalp—and usually unilaterally. Approximately one-third of TTA patients are born with the condition; the rest develop it in the first two to three years of life. As in this case, it is often wrongly attributed to the use of forceps but has nothing to do with trauma. One school of thought holds that TTA is probably an inherited condition—but others disagree.
TTA was originally known as congenital triangular alopecia. However, when enough cases had been accumulated to accurately determine the nature of the condition, it was realized that TTA is not always congenital or triangular. Thus, a new name was bestowed.
The hallmark of TTA is the normal number of hair follicles that only grow vellus hairs. The solitary peripheral tuft of terminal dark hairs is typical of TTA and thus a confirmatory finding.
TREATMENT/PROGNOSIS
TTA is by definition permanent. Since there’s no inflammation (a key difference from alopecia areata), steroids are useless. The only successful treatment for TTA, if any is attempted, is hair transplantation. As of this writing, the family is mulling this treatment option.
ANSWER
The answer is temporal triangular alopecia (choice “d”), an unusual form of permanent hair loss preferentially affecting the exact area depicted in this case.
Alopecia areata (choice “a”) involves localized hair loss. By contrast, this patient never had hair in this area to lose.
Nevus sebaceous (choice “b”) is a congenital hamartoma that is typically hairless; there are no follicles, and the bumpy, rough surface is composed of sebaceous globules.
Cutis aplasia (choice “c”) manifests with hairless lesions, but there is marked aplasia of the skin as well and no surface adnexae, let alone hairs or follicles.
DISCUSSION
Temporal triangular alopecia (TTA) is an unusual type of alopecia. Of unknown origin, it usually affects this area of the scalp—and usually unilaterally. Approximately one-third of TTA patients are born with the condition; the rest develop it in the first two to three years of life. As in this case, it is often wrongly attributed to the use of forceps but has nothing to do with trauma. One school of thought holds that TTA is probably an inherited condition—but others disagree.
TTA was originally known as congenital triangular alopecia. However, when enough cases had been accumulated to accurately determine the nature of the condition, it was realized that TTA is not always congenital or triangular. Thus, a new name was bestowed.
The hallmark of TTA is the normal number of hair follicles that only grow vellus hairs. The solitary peripheral tuft of terminal dark hairs is typical of TTA and thus a confirmatory finding.
TREATMENT/PROGNOSIS
TTA is by definition permanent. Since there’s no inflammation (a key difference from alopecia areata), steroids are useless. The only successful treatment for TTA, if any is attempted, is hair transplantation. As of this writing, the family is mulling this treatment option.
ANSWER
The answer is temporal triangular alopecia (choice “d”), an unusual form of permanent hair loss preferentially affecting the exact area depicted in this case.
Alopecia areata (choice “a”) involves localized hair loss. By contrast, this patient never had hair in this area to lose.
Nevus sebaceous (choice “b”) is a congenital hamartoma that is typically hairless; there are no follicles, and the bumpy, rough surface is composed of sebaceous globules.
Cutis aplasia (choice “c”) manifests with hairless lesions, but there is marked aplasia of the skin as well and no surface adnexae, let alone hairs or follicles.
DISCUSSION
Temporal triangular alopecia (TTA) is an unusual type of alopecia. Of unknown origin, it usually affects this area of the scalp—and usually unilaterally. Approximately one-third of TTA patients are born with the condition; the rest develop it in the first two to three years of life. As in this case, it is often wrongly attributed to the use of forceps but has nothing to do with trauma. One school of thought holds that TTA is probably an inherited condition—but others disagree.
TTA was originally known as congenital triangular alopecia. However, when enough cases had been accumulated to accurately determine the nature of the condition, it was realized that TTA is not always congenital or triangular. Thus, a new name was bestowed.
The hallmark of TTA is the normal number of hair follicles that only grow vellus hairs. The solitary peripheral tuft of terminal dark hairs is typical of TTA and thus a confirmatory finding.
TREATMENT/PROGNOSIS
TTA is by definition permanent. Since there’s no inflammation (a key difference from alopecia areata), steroids are useless. The only successful treatment for TTA, if any is attempted, is hair transplantation. As of this writing, the family is mulling this treatment option.
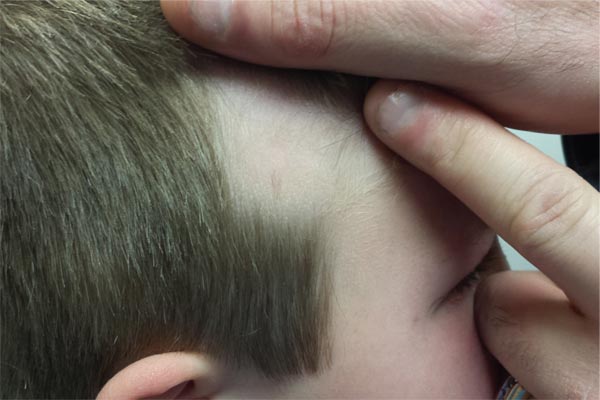
Since birth, this 8-year-old boy has had a “bald spot” on his scalp. The pediatrician who attended the birth suggested trauma as the cause, since forceps were used to facilitate delivery. But the problem has failed to resolve, leaving the boy an object of ridicule among his classmates. According to the patient’s parents, there has never been any broken skin or hair growth in the area. There is no family history of similar problems, and the child’s health history is unremarkable. The child’s current pediatrician, who made the referral to dermatology, suggested the lesion might be a form of nevus sebaceous. The affected site is roughly triangular, measures about 3 cm on each side, and is located just inside the temporal scalp. The hair loss in this sharply circumscribed area is almost complete, with a lone tuft of darker terminal hairs on the inferior aspect of the site. No redness or epidermal disturbance (eg, scaling) is noted. Dermatoscopic examination (with 10x magnification) reveals a normal number of follicles and hairs. The latter are vellus hairs, except for the aforementioned solitary tuft. The rest of the scalp, including the same location on the opposite side, is free of any significant changes.
Nanoparticles destroy blood clots faster
iron oxide (red), albumin
(gray), and tPA (green)
Image courtesy of
Paolo Decuzzi lab
Results of preclinical experiments suggest that nanoparticles made of iron oxide and coated with tissue plasminogen activator (tPA) and albumin can directly target blood clots and destroy them faster than free tPA injected into the bloodstream.
Researchers found these nanoparticles could destroy blood clots 100 times faster than free tPA. And when the nanoparticles were heated using alternating magnetic fields, they destroyed clots 1000 times faster than free tPA.
Paolo Decuzzi, PhD, of Fondazione Istituto Italiano di Tecnologia in Genoa, Italy, and his colleagues described these experiments in Advanced Functional Materials.
The researchers created iron oxide nanoparticles and coated them with tPA. Typically, a small volume of concentrated tPA is injected into a patient’s blood upstream of a confirmed or suspected clot. From there, some of the tPA reaches the clot, but much of it may travel past or around the clot, potentially ending up anywhere in the circulatory system.
“We have designed the nanoparticles so that they trap themselves at the site of the clot, which means they can quickly deliver a burst of the commonly used, clot-busting drug tPA where it is most needed,” Dr Decuzzi said.
He and his colleagues used iron oxide as the nanoparticles’ core so the particles can be used for magnetic resonance imaging, remote guidance with external magnetic fields, and for further accelerating clot dissolution with localized magnetic heating.
“We think it is possible to use a static magnetic field first to help guide the nanoparticles to the clot, then alternate the orientation of the field to increase the nanoparticles’ efficiency in dissolving clots,” Dr Decuzzi said.
The team also coated the nanoparticles in albumin, which provides a sort of camouflage. It gives the nanoparticles time to reach a clot before the immune system recognizes the particles as invaders and attacks them.
“The nanoparticle protects the drug from the body’s defenses, giving the tPA time to work,” said Alan Lumsden, MD, of Houston Methodist Hospital in Texas.
“But it also allows us to use less tPA, which could make hemorrhage less likely. We are excited to see if the technique works as phenomenally well for our patients as what we saw in these experiments.”
The researchers tested the nanoparticles using human tissue cultures to see where the tPA landed and how long it took to destroy fibrin-rich clots. They also introduced blood clots in mice, injected the nanoparticles into the bloodstream, and used optical microscopy to follow the dissolution of the clots.
The nanoparticles destroyed clots about 100 times faster than free tPA.
Although free tPA is usually injected at room temperature, a number of studies have suggested the drug is most effective at higher temperatures (40° C or about 104° F). The same seems to be true for tPA delivered via the iron oxide nanoparticles.
By exposing the nanoparticles to external, alternating magnetic fields, the researchers created friction and heat. Warmer tPA (42° C or about 108° F) was released faster and increased the rate of clot dissolution 10-fold (to 1000 times greater than free tPA).
Dr Decuzzi said the next steps with this research will be testing the nanoparticles’ safety and effectiveness in other animal models, with the ultimate goal of clinical trials. He said his group will continue to examine the feasibility of using magnetic fields to guide and heat the nanoparticles.
“We are optimistic because the [US Food and Drug Administration] has already approved the use of iron oxide as a contrast agent in MRIs,” Dr Decuzzi said. “And we do not anticipate needing to use as much of the iron oxide at concentrations higher than what’s already been approved. The other chemical aspects of the nanoparticles are natural substances you already find in the bloodstream.” ![]()

iron oxide (red), albumin
(gray), and tPA (green)
Image courtesy of
Paolo Decuzzi lab
Results of preclinical experiments suggest that nanoparticles made of iron oxide and coated with tissue plasminogen activator (tPA) and albumin can directly target blood clots and destroy them faster than free tPA injected into the bloodstream.
Researchers found these nanoparticles could destroy blood clots 100 times faster than free tPA. And when the nanoparticles were heated using alternating magnetic fields, they destroyed clots 1000 times faster than free tPA.
Paolo Decuzzi, PhD, of Fondazione Istituto Italiano di Tecnologia in Genoa, Italy, and his colleagues described these experiments in Advanced Functional Materials.
The researchers created iron oxide nanoparticles and coated them with tPA. Typically, a small volume of concentrated tPA is injected into a patient’s blood upstream of a confirmed or suspected clot. From there, some of the tPA reaches the clot, but much of it may travel past or around the clot, potentially ending up anywhere in the circulatory system.
“We have designed the nanoparticles so that they trap themselves at the site of the clot, which means they can quickly deliver a burst of the commonly used, clot-busting drug tPA where it is most needed,” Dr Decuzzi said.
He and his colleagues used iron oxide as the nanoparticles’ core so the particles can be used for magnetic resonance imaging, remote guidance with external magnetic fields, and for further accelerating clot dissolution with localized magnetic heating.
“We think it is possible to use a static magnetic field first to help guide the nanoparticles to the clot, then alternate the orientation of the field to increase the nanoparticles’ efficiency in dissolving clots,” Dr Decuzzi said.
The team also coated the nanoparticles in albumin, which provides a sort of camouflage. It gives the nanoparticles time to reach a clot before the immune system recognizes the particles as invaders and attacks them.
“The nanoparticle protects the drug from the body’s defenses, giving the tPA time to work,” said Alan Lumsden, MD, of Houston Methodist Hospital in Texas.
“But it also allows us to use less tPA, which could make hemorrhage less likely. We are excited to see if the technique works as phenomenally well for our patients as what we saw in these experiments.”
The researchers tested the nanoparticles using human tissue cultures to see where the tPA landed and how long it took to destroy fibrin-rich clots. They also introduced blood clots in mice, injected the nanoparticles into the bloodstream, and used optical microscopy to follow the dissolution of the clots.
The nanoparticles destroyed clots about 100 times faster than free tPA.
Although free tPA is usually injected at room temperature, a number of studies have suggested the drug is most effective at higher temperatures (40° C or about 104° F). The same seems to be true for tPA delivered via the iron oxide nanoparticles.
By exposing the nanoparticles to external, alternating magnetic fields, the researchers created friction and heat. Warmer tPA (42° C or about 108° F) was released faster and increased the rate of clot dissolution 10-fold (to 1000 times greater than free tPA).
Dr Decuzzi said the next steps with this research will be testing the nanoparticles’ safety and effectiveness in other animal models, with the ultimate goal of clinical trials. He said his group will continue to examine the feasibility of using magnetic fields to guide and heat the nanoparticles.
“We are optimistic because the [US Food and Drug Administration] has already approved the use of iron oxide as a contrast agent in MRIs,” Dr Decuzzi said. “And we do not anticipate needing to use as much of the iron oxide at concentrations higher than what’s already been approved. The other chemical aspects of the nanoparticles are natural substances you already find in the bloodstream.” ![]()

iron oxide (red), albumin
(gray), and tPA (green)
Image courtesy of
Paolo Decuzzi lab
Results of preclinical experiments suggest that nanoparticles made of iron oxide and coated with tissue plasminogen activator (tPA) and albumin can directly target blood clots and destroy them faster than free tPA injected into the bloodstream.
Researchers found these nanoparticles could destroy blood clots 100 times faster than free tPA. And when the nanoparticles were heated using alternating magnetic fields, they destroyed clots 1000 times faster than free tPA.
Paolo Decuzzi, PhD, of Fondazione Istituto Italiano di Tecnologia in Genoa, Italy, and his colleagues described these experiments in Advanced Functional Materials.
The researchers created iron oxide nanoparticles and coated them with tPA. Typically, a small volume of concentrated tPA is injected into a patient’s blood upstream of a confirmed or suspected clot. From there, some of the tPA reaches the clot, but much of it may travel past or around the clot, potentially ending up anywhere in the circulatory system.
“We have designed the nanoparticles so that they trap themselves at the site of the clot, which means they can quickly deliver a burst of the commonly used, clot-busting drug tPA where it is most needed,” Dr Decuzzi said.
He and his colleagues used iron oxide as the nanoparticles’ core so the particles can be used for magnetic resonance imaging, remote guidance with external magnetic fields, and for further accelerating clot dissolution with localized magnetic heating.
“We think it is possible to use a static magnetic field first to help guide the nanoparticles to the clot, then alternate the orientation of the field to increase the nanoparticles’ efficiency in dissolving clots,” Dr Decuzzi said.
The team also coated the nanoparticles in albumin, which provides a sort of camouflage. It gives the nanoparticles time to reach a clot before the immune system recognizes the particles as invaders and attacks them.
“The nanoparticle protects the drug from the body’s defenses, giving the tPA time to work,” said Alan Lumsden, MD, of Houston Methodist Hospital in Texas.
“But it also allows us to use less tPA, which could make hemorrhage less likely. We are excited to see if the technique works as phenomenally well for our patients as what we saw in these experiments.”
The researchers tested the nanoparticles using human tissue cultures to see where the tPA landed and how long it took to destroy fibrin-rich clots. They also introduced blood clots in mice, injected the nanoparticles into the bloodstream, and used optical microscopy to follow the dissolution of the clots.
The nanoparticles destroyed clots about 100 times faster than free tPA.
Although free tPA is usually injected at room temperature, a number of studies have suggested the drug is most effective at higher temperatures (40° C or about 104° F). The same seems to be true for tPA delivered via the iron oxide nanoparticles.
By exposing the nanoparticles to external, alternating magnetic fields, the researchers created friction and heat. Warmer tPA (42° C or about 108° F) was released faster and increased the rate of clot dissolution 10-fold (to 1000 times greater than free tPA).
Dr Decuzzi said the next steps with this research will be testing the nanoparticles’ safety and effectiveness in other animal models, with the ultimate goal of clinical trials. He said his group will continue to examine the feasibility of using magnetic fields to guide and heat the nanoparticles.
“We are optimistic because the [US Food and Drug Administration] has already approved the use of iron oxide as a contrast agent in MRIs,” Dr Decuzzi said. “And we do not anticipate needing to use as much of the iron oxide at concentrations higher than what’s already been approved. The other chemical aspects of the nanoparticles are natural substances you already find in the bloodstream.” ![]()
Older age doesn’t decrease HRQOL among PBSC donors
Photo courtesy of Canterbury
District Health Board
SAN DIEGO—New research indicates that older stem cell donors have somewhat poorer overall health before they donate, but their health-related quality of life (HRQOL) post-donation is similar to that of younger donors.
In fact, the older donors included in this study actually fared better than their younger counterparts in some respects.
Galen E. Switzer, PhD, of the University of Pittsburgh in Pennsylvania, presented these results at the 2015 BMT Tandem Meetings (abstract 27*).
“[Older donors] may be at greater physical and psychological risk because of their age and comorbid conditions,” Dr Switzer noted. “So it’s critical for us to understand the health-related quality of life experiences of these donors.”
With that in mind, he and his colleagues evaluated 163 subjects who donated peripheral blood stem cells (PBSCs) to relatives in need of a transplant. The team compared donors over the age of 60 (n=104, median age 66 years) to those aged 18 to 60 (n=59, median age 41 years).
The investigators collected data via structured telephone interviews 2 weeks before PBSC donation and at 4 weeks and 1 year post-donation.
A comparison of sociodemographic factors revealed that older PBSC donors were significantly less likely to be employed (P<0.001) but more likely be white (P=0.009), be married (P=0.044), and have children (P<0.001).
Pre- and post-donation HRQOL
Pre-donation, older donors had significantly poorer physical health (P=0.001) and better mental health (P=0.036) than younger donors. But there was no significant difference between the age groups with regard to the incidence of depression or anxiety.
Similarly, there were no significant differences with regard to ambivalence, satisfaction, or medical concerns about donation. However, older donors were more likely to consult their physician about donation (P=0.049), and they had fewer work/family concerns (P=0.049) than younger donors.
At 4 weeks post-donation, there were no significant differences between the age groups with regard to general physical health, mental health, or any of 12 donation-related symptoms. However, younger donors were significantly more likely to report that donation was painful (P=0.025).
Older donors were significantly less likely to report work/family concerns, such as missing work, family worry, or worry about what others would think (P=0.001). They were less likely to have other donation-related concerns as well, such as worrying about who would pay for the procedure (P=0.034). And they were less likely to say they would feel responsible if the transplant did not have a favorable outcome (P=0.022).
At 1 year post-donation, there were no significant differences between the age groups with regard to overall physical and mental health, depression, ambivalence, satisfaction, 11 of 12 donation side effects, physical difficulty, psychological difficulty, or “other concerns.”
However, older donors reported significantly less anxiety, fewer medical concerns, and fewer work/family concerns (P<0.05 for all). They were also less likely to feel responsible for transplant outcomes and less likely to have problems sleeping, which was 1 of the 12 donation side effects (P<0.05 for both).
“So the overall conclusion, I think, is really reassuring,” Dr Switzer said. “Despite having somewhat poorer overall general physical health at pre-donation, older donors experience similar—and, in some domains, better—donation-related health-related quality of life than younger donors. So they seem to be doing at least as well and, in some domains, better than their younger counterparts.” ![]()
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of Canterbury
District Health Board
SAN DIEGO—New research indicates that older stem cell donors have somewhat poorer overall health before they donate, but their health-related quality of life (HRQOL) post-donation is similar to that of younger donors.
In fact, the older donors included in this study actually fared better than their younger counterparts in some respects.
Galen E. Switzer, PhD, of the University of Pittsburgh in Pennsylvania, presented these results at the 2015 BMT Tandem Meetings (abstract 27*).
“[Older donors] may be at greater physical and psychological risk because of their age and comorbid conditions,” Dr Switzer noted. “So it’s critical for us to understand the health-related quality of life experiences of these donors.”
With that in mind, he and his colleagues evaluated 163 subjects who donated peripheral blood stem cells (PBSCs) to relatives in need of a transplant. The team compared donors over the age of 60 (n=104, median age 66 years) to those aged 18 to 60 (n=59, median age 41 years).
The investigators collected data via structured telephone interviews 2 weeks before PBSC donation and at 4 weeks and 1 year post-donation.
A comparison of sociodemographic factors revealed that older PBSC donors were significantly less likely to be employed (P<0.001) but more likely be white (P=0.009), be married (P=0.044), and have children (P<0.001).
Pre- and post-donation HRQOL
Pre-donation, older donors had significantly poorer physical health (P=0.001) and better mental health (P=0.036) than younger donors. But there was no significant difference between the age groups with regard to the incidence of depression or anxiety.
Similarly, there were no significant differences with regard to ambivalence, satisfaction, or medical concerns about donation. However, older donors were more likely to consult their physician about donation (P=0.049), and they had fewer work/family concerns (P=0.049) than younger donors.
At 4 weeks post-donation, there were no significant differences between the age groups with regard to general physical health, mental health, or any of 12 donation-related symptoms. However, younger donors were significantly more likely to report that donation was painful (P=0.025).
Older donors were significantly less likely to report work/family concerns, such as missing work, family worry, or worry about what others would think (P=0.001). They were less likely to have other donation-related concerns as well, such as worrying about who would pay for the procedure (P=0.034). And they were less likely to say they would feel responsible if the transplant did not have a favorable outcome (P=0.022).
At 1 year post-donation, there were no significant differences between the age groups with regard to overall physical and mental health, depression, ambivalence, satisfaction, 11 of 12 donation side effects, physical difficulty, psychological difficulty, or “other concerns.”
However, older donors reported significantly less anxiety, fewer medical concerns, and fewer work/family concerns (P<0.05 for all). They were also less likely to feel responsible for transplant outcomes and less likely to have problems sleeping, which was 1 of the 12 donation side effects (P<0.05 for both).
“So the overall conclusion, I think, is really reassuring,” Dr Switzer said. “Despite having somewhat poorer overall general physical health at pre-donation, older donors experience similar—and, in some domains, better—donation-related health-related quality of life than younger donors. So they seem to be doing at least as well and, in some domains, better than their younger counterparts.” ![]()
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of Canterbury
District Health Board
SAN DIEGO—New research indicates that older stem cell donors have somewhat poorer overall health before they donate, but their health-related quality of life (HRQOL) post-donation is similar to that of younger donors.
In fact, the older donors included in this study actually fared better than their younger counterparts in some respects.
Galen E. Switzer, PhD, of the University of Pittsburgh in Pennsylvania, presented these results at the 2015 BMT Tandem Meetings (abstract 27*).
“[Older donors] may be at greater physical and psychological risk because of their age and comorbid conditions,” Dr Switzer noted. “So it’s critical for us to understand the health-related quality of life experiences of these donors.”
With that in mind, he and his colleagues evaluated 163 subjects who donated peripheral blood stem cells (PBSCs) to relatives in need of a transplant. The team compared donors over the age of 60 (n=104, median age 66 years) to those aged 18 to 60 (n=59, median age 41 years).
The investigators collected data via structured telephone interviews 2 weeks before PBSC donation and at 4 weeks and 1 year post-donation.
A comparison of sociodemographic factors revealed that older PBSC donors were significantly less likely to be employed (P<0.001) but more likely be white (P=0.009), be married (P=0.044), and have children (P<0.001).
Pre- and post-donation HRQOL
Pre-donation, older donors had significantly poorer physical health (P=0.001) and better mental health (P=0.036) than younger donors. But there was no significant difference between the age groups with regard to the incidence of depression or anxiety.
Similarly, there were no significant differences with regard to ambivalence, satisfaction, or medical concerns about donation. However, older donors were more likely to consult their physician about donation (P=0.049), and they had fewer work/family concerns (P=0.049) than younger donors.
At 4 weeks post-donation, there were no significant differences between the age groups with regard to general physical health, mental health, or any of 12 donation-related symptoms. However, younger donors were significantly more likely to report that donation was painful (P=0.025).
Older donors were significantly less likely to report work/family concerns, such as missing work, family worry, or worry about what others would think (P=0.001). They were less likely to have other donation-related concerns as well, such as worrying about who would pay for the procedure (P=0.034). And they were less likely to say they would feel responsible if the transplant did not have a favorable outcome (P=0.022).
At 1 year post-donation, there were no significant differences between the age groups with regard to overall physical and mental health, depression, ambivalence, satisfaction, 11 of 12 donation side effects, physical difficulty, psychological difficulty, or “other concerns.”
However, older donors reported significantly less anxiety, fewer medical concerns, and fewer work/family concerns (P<0.05 for all). They were also less likely to feel responsible for transplant outcomes and less likely to have problems sleeping, which was 1 of the 12 donation side effects (P<0.05 for both).
“So the overall conclusion, I think, is really reassuring,” Dr Switzer said. “Despite having somewhat poorer overall general physical health at pre-donation, older donors experience similar—and, in some domains, better—donation-related health-related quality of life than younger donors. So they seem to be doing at least as well and, in some domains, better than their younger counterparts.” ![]()
*Information in the abstract differs from that presented at the meeting.
Treatment likely doesn’t increase risk of cancer
Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()

Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()

Pimecrolimus, a topical cream used to treat eczema in children, does not appear to confer an increased risk of hematologic and other cancers, according to researchers.
In 2001 and 2002, respectively, the US Food and Drug Administration and the European Medicines Agency approved pimecrolimus to treat eczema in children 2 years of age and older.
The product was approved with a black box warning describing a potential risk of malignancy.
To investigate this risk, and as part of the post-marketing commitments for the approval of pimecrolimus, researchers initiated the Pediatric Eczema Elective Registry (PEER) study in 2004.
David J. Margolis, MD, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues analyzed data from this study, comparing the incidence of malignancy among PEER subjects to expected cancer rates from the Surveillance, Epidemiology and End Results program.
The team reported their findings in JAMA Dermatology.
The PEER study enrolled 7457 children (26,792 person-years) who used an average of 793 g of pimecrolimus while on study.
As of May 2014, 5 malignancies were reported in these children: 2 leukemias, 2 lymphomas, and 1 osteosarcoma.
No skin cancers were reported, and none of the findings regarding cancer incidence were statistically significant.
The researchers therefore said it’s unlikely that topical pimecrolimus, as it was used in the PEER study, is associated with an increased risk of malignancy.
This research and the PEER study were both funded by Valeant Pharmaceuticals International, makers of pimecrolimus.
In a related editorial in JAMA Dermatology, Jon M. Hanifin, MD, of Oregon Health and Science University in Portland, said he hopes this research will improve the management of eczema by countering any concerns about pimecrolimus use that were raised by the treatment’s black box warning.
“The positive and optimistic report of pimecrolimus post-marketing surveillance by Margolis et al should help reduce the physician and pharmacist concerns that have restricted the use of these effective topical alternatives to corticosteroids,” Dr Hanifin wrote. “The interim results should help bring relief to a larger segment of the many young individuals with [eczema].” ![]()
FDA approves first HDAC inhibitor for MM

The US Food and Drug Administration (FDA) has granted accelerated approval for panobinostat (Farydak) to treat patients with multiple myeloma (MM).
Panobinostat is the first histone deacetylase (HDAC) inhibitor approved to treat MM.
The drug can now be used in combination with bortezomib and dexamethasone to treat patients who have received at least 2 prior standard therapies, including bortezomib and an immunomodulatory agent (IMiD).
Panobinostat was approved with a boxed warning alerting patients and healthcare professionals that severe diarrhea and severe and fatal cardiac events, arrhythmias, and electrocardiogram changes have occurred in patients receiving the drug.
Panobinostat was approved with a Risk Evaluation and Mitigation Strategy as well, which consists of a communication plan to inform healthcare professionals of these risks and how to minimize them.
Data supporting approval
In November 2014, the FDA’s Oncologic Drugs Advisory Committee advised the agency that, based on the data reviewed, the benefits of panobinostat did not outweigh its risks for patients with relapsed MM.
After the meeting, Novartis, the company developing the HDAC inhibitor, submitted additional information supporting the use of panobinostat for a different indication: MM patients who have received at least 2 prior standard therapies, including bortezomib and an IMiD.
The FDA’s accelerated approval of panobinostat is based on that data—efficacy and safety results in a subgroup analysis of 193 patients enrolled in the phase 3 PANORAMA-1 trial. These patients had received prior treatment with both bortezomib and an IMiD.
In these patients, treatment with panobinostat, bortezomib, and dexamethasone resulted in superior progression-free survival, when compared to treatment with bortezomib, dexamethasone, and placebo—10.6 months and 5.8 months, respectively (hazard ratio=0.52).
The most common adverse events (incidence ≥ 20%) in clinical studies of panobinostat have been diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, and vomiting.
The most common non-hematologic laboratory abnormalities (incidence ≥ 40%) were hypophosphatemia, hypokalemia, hyponatremia, and increased creatinine. The most common hematologic laboratory abnormalities (incidence ≥ 60%) were thrombocytopenia, lymphopenia, leukopenia, neutropenia, and anemia.
Panobinostat can cause fatal and serious toxicities, including severe diarrhea and cardiac toxicities.
The most frequent (≥ 5%) treatment-emergent serious adverse events for patients treated with the HDAC inhibitor were pneumonia (18%), diarrhea (11%), thrombocytopenia (7%), fatigue (6%), and sepsis (6%). Additional serious adverse events included hemorrhage, myelosuppression, infections, hepatotoxicity, and embryo-fetal toxicity.
Panobinostat development
The FDA previously granted panobinostat priority review and orphan product designation. Priority review provides an expedited review of drugs that are intended to treat a serious disease or condition and may provide a significant improvement over available therapy. Orphan product designation is given to drugs intended to treat rare diseases.
Now, the FDA has granted panobinostat accelerated approval, which allows for conditional approval of a drug based on clinical data showing the drug has an effect on a surrogate endpoint reasonably likely to predict clinical benefit to patients.
Continued approval of panobinostat may be contingent upon verification of a clinical benefit in confirmatory trials conducted by Novartis. An improvement in overall survival or disease-related symptoms has not yet been established for the HDAC inhibitor.
For more details on panobinostat, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has granted accelerated approval for panobinostat (Farydak) to treat patients with multiple myeloma (MM).
Panobinostat is the first histone deacetylase (HDAC) inhibitor approved to treat MM.
The drug can now be used in combination with bortezomib and dexamethasone to treat patients who have received at least 2 prior standard therapies, including bortezomib and an immunomodulatory agent (IMiD).
Panobinostat was approved with a boxed warning alerting patients and healthcare professionals that severe diarrhea and severe and fatal cardiac events, arrhythmias, and electrocardiogram changes have occurred in patients receiving the drug.
Panobinostat was approved with a Risk Evaluation and Mitigation Strategy as well, which consists of a communication plan to inform healthcare professionals of these risks and how to minimize them.
Data supporting approval
In November 2014, the FDA’s Oncologic Drugs Advisory Committee advised the agency that, based on the data reviewed, the benefits of panobinostat did not outweigh its risks for patients with relapsed MM.
After the meeting, Novartis, the company developing the HDAC inhibitor, submitted additional information supporting the use of panobinostat for a different indication: MM patients who have received at least 2 prior standard therapies, including bortezomib and an IMiD.
The FDA’s accelerated approval of panobinostat is based on that data—efficacy and safety results in a subgroup analysis of 193 patients enrolled in the phase 3 PANORAMA-1 trial. These patients had received prior treatment with both bortezomib and an IMiD.
In these patients, treatment with panobinostat, bortezomib, and dexamethasone resulted in superior progression-free survival, when compared to treatment with bortezomib, dexamethasone, and placebo—10.6 months and 5.8 months, respectively (hazard ratio=0.52).
The most common adverse events (incidence ≥ 20%) in clinical studies of panobinostat have been diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, and vomiting.
The most common non-hematologic laboratory abnormalities (incidence ≥ 40%) were hypophosphatemia, hypokalemia, hyponatremia, and increased creatinine. The most common hematologic laboratory abnormalities (incidence ≥ 60%) were thrombocytopenia, lymphopenia, leukopenia, neutropenia, and anemia.
Panobinostat can cause fatal and serious toxicities, including severe diarrhea and cardiac toxicities.
The most frequent (≥ 5%) treatment-emergent serious adverse events for patients treated with the HDAC inhibitor were pneumonia (18%), diarrhea (11%), thrombocytopenia (7%), fatigue (6%), and sepsis (6%). Additional serious adverse events included hemorrhage, myelosuppression, infections, hepatotoxicity, and embryo-fetal toxicity.
Panobinostat development
The FDA previously granted panobinostat priority review and orphan product designation. Priority review provides an expedited review of drugs that are intended to treat a serious disease or condition and may provide a significant improvement over available therapy. Orphan product designation is given to drugs intended to treat rare diseases.
Now, the FDA has granted panobinostat accelerated approval, which allows for conditional approval of a drug based on clinical data showing the drug has an effect on a surrogate endpoint reasonably likely to predict clinical benefit to patients.
Continued approval of panobinostat may be contingent upon verification of a clinical benefit in confirmatory trials conducted by Novartis. An improvement in overall survival or disease-related symptoms has not yet been established for the HDAC inhibitor.
For more details on panobinostat, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has granted accelerated approval for panobinostat (Farydak) to treat patients with multiple myeloma (MM).
Panobinostat is the first histone deacetylase (HDAC) inhibitor approved to treat MM.
The drug can now be used in combination with bortezomib and dexamethasone to treat patients who have received at least 2 prior standard therapies, including bortezomib and an immunomodulatory agent (IMiD).
Panobinostat was approved with a boxed warning alerting patients and healthcare professionals that severe diarrhea and severe and fatal cardiac events, arrhythmias, and electrocardiogram changes have occurred in patients receiving the drug.
Panobinostat was approved with a Risk Evaluation and Mitigation Strategy as well, which consists of a communication plan to inform healthcare professionals of these risks and how to minimize them.
Data supporting approval
In November 2014, the FDA’s Oncologic Drugs Advisory Committee advised the agency that, based on the data reviewed, the benefits of panobinostat did not outweigh its risks for patients with relapsed MM.
After the meeting, Novartis, the company developing the HDAC inhibitor, submitted additional information supporting the use of panobinostat for a different indication: MM patients who have received at least 2 prior standard therapies, including bortezomib and an IMiD.
The FDA’s accelerated approval of panobinostat is based on that data—efficacy and safety results in a subgroup analysis of 193 patients enrolled in the phase 3 PANORAMA-1 trial. These patients had received prior treatment with both bortezomib and an IMiD.
In these patients, treatment with panobinostat, bortezomib, and dexamethasone resulted in superior progression-free survival, when compared to treatment with bortezomib, dexamethasone, and placebo—10.6 months and 5.8 months, respectively (hazard ratio=0.52).
The most common adverse events (incidence ≥ 20%) in clinical studies of panobinostat have been diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, and vomiting.
The most common non-hematologic laboratory abnormalities (incidence ≥ 40%) were hypophosphatemia, hypokalemia, hyponatremia, and increased creatinine. The most common hematologic laboratory abnormalities (incidence ≥ 60%) were thrombocytopenia, lymphopenia, leukopenia, neutropenia, and anemia.
Panobinostat can cause fatal and serious toxicities, including severe diarrhea and cardiac toxicities.
The most frequent (≥ 5%) treatment-emergent serious adverse events for patients treated with the HDAC inhibitor were pneumonia (18%), diarrhea (11%), thrombocytopenia (7%), fatigue (6%), and sepsis (6%). Additional serious adverse events included hemorrhage, myelosuppression, infections, hepatotoxicity, and embryo-fetal toxicity.
Panobinostat development
The FDA previously granted panobinostat priority review and orphan product designation. Priority review provides an expedited review of drugs that are intended to treat a serious disease or condition and may provide a significant improvement over available therapy. Orphan product designation is given to drugs intended to treat rare diseases.
Now, the FDA has granted panobinostat accelerated approval, which allows for conditional approval of a drug based on clinical data showing the drug has an effect on a surrogate endpoint reasonably likely to predict clinical benefit to patients.
Continued approval of panobinostat may be contingent upon verification of a clinical benefit in confirmatory trials conducted by Novartis. An improvement in overall survival or disease-related symptoms has not yet been established for the HDAC inhibitor.
For more details on panobinostat, see the full prescribing information. ![]()
Observation, Visit Status, and RAC Audits
Medicare patients are increasingly hospitalized as outpatients under observation. From 2006 to 2012, outpatient services grew nationally by 28.5%, whereas inpatient discharges decreased by 12.6% per Medicare beneficiary.[1] This increased use of observation stays for hospitalized Medicare beneficiaries and the recent Centers for Medicare & Medicaid Services (CMS) 2‐Midnight rule for determination of visit status are increasing areas of concern for hospitals, policymakers, and the public,[2] as patients hospitalized under observation are not covered by Medicare Part A hospital insurance, are subject to uncapped out‐of‐pocket charges under Medicare Part B, and may be billed by the hospital for certain medications. Additionally, Medicare beneficiaries hospitalized in outpatient status, which includes all hospitalizations under observation, do not qualify for skilled nursing facility care benefits after discharge, which requires a stay that spans at least 3 consecutive midnights as an inpatient.[3]
In contrast, the federal Recovery Audit program, previously called and still commonly referred to as the Recovery Audit Contractor (RAC) program, responsible for postpayment review of inpatient claims, has received relatively little attention. Established in 2006, and fully operationalized in federal fiscal year (FY) 2010,[4] RACs are private government contractors granted the authority to audit hospital charts for appropriate medical necessity, which can consider whether the care delivered was indicated and whether it was delivered in the appropriate Medicare visit status, outpatient or inpatient. Criteria for hospitalization status (inpatient vs outpatient) as defined in the Medicare Conditions of Participation, often allow for subjectivity (medical judgment) in determining which status is appropriate.[5] Hospitals may contest RAC decisions and payment denials through a preappeals discussion period, then through a 5‐level appeals process. Although early appeals occur between the hospital and private contractors, appeals reaching level 3 are heard by the Department of Health and Human Services (HHS) Office of Medicare Hearings and Appeals (OMHA) Administrative Law Judges (ALJ). Levels 4 (Medicare Appeals Council) and 5 (United States District Court) appeals are also handled by the federal government.[6]
Medicare fraud and abuse should not be tolerated, and systematic surveillance needs to be an integral part of the Medicare program.[4] However, there are increasing concerns that the RAC program has resulted in overaggressive denials.[7, 8] Unlike other Medicare contractors, RAC auditors are paid a contingency fee based on the percentage of hospital payment recouped for cases they audit and deny for improper payment.[4] RACs are not subject to any financial penalty for cases they deny but are overturned in the discussion period or in the appeals process. This may create an incentive system that financially encourages RACs to assert improper payment, and the current system lacks both transparency and clear performance metrics for auditors. Of particular concern are Medicare Part A complex reviews, the most fiscally impactful area of RAC activity. According to CMS FY 2013 data, 41.1% of all claims with collections were complex reviews, yet these claims accounted for almost all (95.2%) of total dollars recovered by the RACs, with almost all (96%) dollars recovered being from Part A claims.[9] Complex reviews involve an auditor retrospectively and manually reviewing a medical record and then using his or her clinical and related professional judgment to decide whether the care was medically necessary. This is compared to automated coding or billing reviews, which are based solely on claims data.
Increased RAC activity and the willingness of hospitals to challenge RAC findings of improper payment has led to an increase in appeals volume that has overloaded the appeals process. On March 13, 2013, CMS offered hospitals the ability to rebill Medicare Part B as an appeals alternative.[10] This did not temper level 3 appeals requests received by the OMHA, which increased from 1250 per week in January 2012 to over 15,000 per week by November 2013.[11] Citing an overwhelmingly increased rate of appeal submissions and the resultant backlog, the OMHA decided to freeze new hospital appeals assignments in December 2013.[11] In another attempt to clear the backlog, on August 29, 2014, CMS offered a settlement that would pay hospitals 68% of the net allowable amount of the original Part A claim (minus any beneficiary deductibles) if a hospital agreed to concede all of its eligible appeals.[12] Notably, cases settled under this agreement would remain officially categorized as denied for improper payment.
The HHS Office of Inspector General (OIG)[4] and the CMS[9, 13, 14] have produced recent reports of RAC auditing and appeals activity that contain variable numbers that conflict with hospital accounts of auditing and appeals activity.[15, 16] In addition to these conflicting reports, little is known about RAC auditing of individual programs over time, the length of time cases spend in appeals, and staff required to navigate the audit and appeals processes. Given these questions, and the importance of RAC auditing pressure in the growth of hospital observation care, we conducted a retrospective descriptive study of all RAC activity for complex Medicare Part A alleged overpayment determinations at the Johns Hopkins Hospital, the University of Utah, and University of Wisconsin Hospital and Clinics for calendar years 2010 to 2013.
METHODS
The University of Wisconsin‐Madison Health Sciences institutional review board (IRB) and the Johns Hopkins Hospital IRB did not require review of this study. The University of Utah received an exemption. All 3 hospitals are tertiary care academic medical centers. The University of Wisconsin Hospital and Clinics (UWHC) is a 592‐bed hospital located in Madison, Wisconsin,[17] the Johns Hopkins Hospital (JHH) is a 1145‐bed medical center located in Baltimore, Maryland,[18] and the University of Utah Hospital (UU) is a 770‐bed facility in Salt Lake City, Utah (information available upon request). Each hospital is under a different RAC, representing 3 of the 4 RAC regions, and each is under a different Medicare Administrative Contractor, contractors responsible for level 1 appeals. The 3 hospitals have the same Qualified Independent Contractor responsible for level 2 appeals.
For the purposes of this study, any chart or medical record requested for review by an RAC was considered a medical necessity chart request or an audit. The terms overpayment determinations and denials were used interchangeably to describe audits the RACs alleged did not meet medical necessity for Medicare Part A billing. As previously described, the term medical necessity specifically considered not only whether actual medical services were appropriate, but also whether the services were delivered in the appropriate status, outpatient or inpatient. Appeals and/or request for discussion were cases where the overpayment determination was disputed and challenged by the hospital.
All complex review Medicare Part A RAC medical record requests by date of RAC request from the official start of the RAC program, January 1, 2010,[4] to December 31, 2013, were included in this study. Medical record requests for automated reviews that related to coding and billing clarifications were not included in this study, nor were complex Medicare Part B reviews, complex reviews for inpatient rehabilitation facilities, or psychiatric day hospitalizations. Notably, JHH is a Periodic Interim Payment (PIP) Medicare hospital, which is a reimbursement mechanism where biweekly payments [are] made to a Provider enrolled in the PIP program, and are based on the hospital's estimate of applicable Medicare reimbursement for the current cost report period.[19] Because PIP payments are made collectively to the hospital based on historical data, adjustments for individual inpatients could not be easily adjudicated and processed. Due to the increased complexity of this reimbursement mechanism, RAC audits did not begin at JHH until 2012. In addition, in contrast to the other 2 institutions, all of the RAC complex review audits at JHH in 2013 were for Part B cases, such as disputing need for intensity‐modulated radiation therapy versus conventional radiation therapy, or contesting the medical necessity of blepharoplasty. As a result, JHH had complex Part A review audits only for 2012 during the study time period. All data were deidentified prior to review by investigators.
As RACs can audit charts for up to 3 years after the bill is submitted,[13] a chart request in 2013 may represent a 2010 hospitalization, but for purposes of this study, was logged as a 2013 case. There currently is no standard methodology to calculate time spent in appeals. The UWHC and JHH calculate time in discussion or appeals from the day the discussion or appeal was initiated by the hospital, and the UU calculates the time in appeals from the date of the findings letter from the RAC, which makes comparable recorded time in appeals longer at UU (estimated 510 days for 20112013 cases, up to 120 days for 2010 cases).Time in appeals includes all cases that remain in the discussion or appeals process as of June 30, 2014.
The RAC process is as follows (Tables 1 and 2):
- The RAC requests hospital claims (RAC Medical Necessity Chart Requests [Audits]).
- The RAC either concludes the hospital claim was compliant as filed/paid and the process ends or the RAC asserts improper payment and requests repayment (RAC Overpayment Determinations of Requested Charts [Denials]).
- The hospital makes an initial decision to not contest the RAC decision (and repay), or to dispute the decision (Hospital Disputes Overpayment Determination [Appeal/Discussion]). Prior to filing an appeal, the hospital may request a discussion of the case with an RAC medical director, during which the RAC medical director can overturn the original determination. If the RAC declines to overturn the decision in discussion, the hospital may proceed with a formal appeal. Although CMS does not calculate the discussion period as part of the appeals process,[12] overpayment determinations contested by the hospital in either discussion or appeal represent the sum total of RAC denials disputed by the hospital.
Contested cases have 1 of 4 outcomes:
Contested overpayment determinations can be decided in favor of the hospital (Discussion or Appeal Decided in Favor of Hospital or RAC Withdrew)
- Contested overpayment determinations can be decided in favor of the RAC during the appeal process, and either the hospital exhausts the appeal process or elects not to take the appeal to the next level. Although the appeals process has 5 levels, no cases at our 3 hospitals have reached level 4 or 5, so cases without a decision to date remain in appeals at 1 of the first 3 levels (Case Still in Discussion or Appeals).[4]
- Hospital may miss an appeal deadline (Hospital Missed Appeal Deadline at Any Level) and the case is automatically decided in favor of the RAC.
- As of March 13, 2013,[10] for appeals that meet certain criteria and involve dispute over the billing of hospital services under Part A, CMS allowed hospitals to withdraw an appeal and rebill Medicare Part B. Prior to this time, hospitals could rebill for a very limited list of ancillary Part B Only services, and only within the 1‐year timely filing period.[13] Due to the lengthy appeals process and associated legal and administrative costs, hospitals may not agree with the RAC determination but make a business decision to recoup some payment under this mechanism (Hospital Chose to Rebill as Part B During Discussion or Appeals Process).
| Totals | Johns Hopkins Hospital | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | All Years | 2010 | 2011 | 2012 | 2013 | All Years | ||
| University of Wisconsin Hospital and Clinics | University of Utah | ||||||||||
| 2010 | 2011 | 2012 | 2013 | All Years | 2010 | 2011 | 2012 | 2013 | All Years | ||
| |||||||||||
| Total no. of Medicare encounters | 24,400 | 24,998 | 25,370 | 27,094 | 101,862 | 11,212b | 11,750b | 11,842 | 12,674c | 47,478 | |
| RAC Medical Necessity Chart Requests (Audits) | 547 | 1,735 | 3,887 | 1,941 | 8,110 (8.0%) | 0 | 0 | 938 | 0 | 938 (2.0%) | |
| RAC Overpayment Determinations Of Requested Charts (Denials)d | 164 (30.0%) | 516 (29.7%) | 1,200 (30.9%) | 656 (33.8%) | 2,536 (31.3%) | 0 (0%) | 0 (0%) | 432 (46.1%) | 0 (0%) | 432 (46.1%) | |
| Hospital Disputes Overpayment Determination (Appeal/Discussion) | 128 (78.0%) | 409 (79.3%) | 1,129 (94.1%) | 643 (98.0%) | 2,309 (91.0% | 0 (0%) | 0 (0%) | 431 (99.8%) | 0 (0%) | 431 (99.8%) | |
| Outcome of Disputed Overpayment Determinatione | |||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (0.2%) | 13 (1.2%) | 4 (0.6%) | 18 (0.8%) | 0 (0%) | 0 (0%) | 0 (0.0%) | 0 (0%) | 0 (0.0%) | |
| Hospital Chose To Rebill as Part B During Discussion Or Appeals Process | 80 (62.5%) | 202 (49.4%) | 511 (45.3%) | 158 (24.6%) | 951 (41.2%) | 0 (0%) | 0 (0%) | 208 (48.3%) | 0 (0%) | 208 (48.3%) | |
| Discussion or Appeal Decided In Favor Of Hospital or RAC Withdrewf | 45 (35.2%) | 127 (31.1%) | 449 (39.8%) | 345 (53.7%) | 966 (41.8%) | 0 (0%) | 0 (0%) | 151 (35.0%) | 0 (0%) | 151 (35.0%) | |
| Case Still in Discussion or Appeals | 3 (2.3%) | 79 (19.3%) | 156 13.8%) | 136 (21.2%) | 374 (16.2%) | 0 (0%) | 0 (0%) | 72 (16.7%) | 0 (0%) | 72 (16.7%) | |
| Mean Time for Cases Still in Discussion or Appeals, d (SD) | 1208 (41) | 958 (79) | 518 (125) | 350 (101) | 555 (255) | N/A | N/A | 478 (164) | N/A | 478 (164) | |
| Total no. of Medicare encounters l | 8,096 | 8,038 | 8,429 | 9,086 | 33,649 | 5,092 | 5,210 | 5,099 | 5,334 | 20,735 | |
| RAC Medical Necessity Chart Requests (Audits) | 15 | 526 | 1,484 | 960 | 2,985 (8.9%) | 532 | 1,209 | 1,465 | 981 | 4,187 (20.2%) | |
| RAC Overpayment Determinations of Requested Charts (Denials)bd | 3 (20.0%) | 147 (27.9%) | 240 (16.2%) | 164 (17.1%) | 554 (18.6%) | 161 (30.3%) | 369 (30.5%) | 528 (36.0%) | 492 (50.2%) | 1,550 (37.0%) | |
| Hospital Disputes Overpayment Determination (Appeal/Discussion) | 1 (33.3%) | 71 (48.3%) | 170 (70.8%) | 151 (92.1%) | 393 (70.9%) | 127 (78.9%) | 338 (91.6%) | 528 (100.0%) | 492 (100.0%) | 1,485 (95.8%) | |
| Outcome of Disputed Overpayment Determinatione | |||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 4 (2.6%) | 5 (1.3%) | 0 (0.0%) | 0 (0.0%) | 13 (2.5%) | 0 (0.0%) | 13 (0.9%) | |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 1 (100%) | 3 (4.2%) | 13 (7.6%) | 3 (2.0%) | 20 (5.1%) | 79 (62.2%) | 199 (58.9%) | 290 (54.9%) | 155 (31.5%) | 723 (48.7%) | |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrewf | 0 (0.0%) | 44 (62.0%) | 123 (72.4%) | 93 (61.6%) | 260 (66.2%) | 45 (35.4%) | 83 (24.6%) | 175 (33.1%) | 252 (51.2%) | 555 (37.4%) | |
| Case Still in Discussion or Appeals | 0 0.0% | 23 (32.4%) | 34 (20.0%) | 51 (33.8%) | 108 (27.5%) | 3 (2.4%) | 56 (16.6%) | 50 (9.5%) | 85 (17.3%) | 194 (13.1%) | |
| Mean Time for Cases Still in Discussion or Appeals, d (SD) | N/A | 926 (70) | 564 (90) | 323 (134) | 528 (258) | 1,208 (41) | 970 (80) | 544 (25) | 365 (72) | 599 (273) | |
| 2010 | 2011 | 2012 | 2013 | All | 2010 | 2011 | 2012 | 2013 | All | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Appeals With Decisions | Johns Hopkins Hospital | |||||||||
| Total no. | 125 | 330 | 973 | 507 | 1,935 | 0 | 0 | 359 | 0 | 359 |
| ||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (0.3%) | 13 (1.3%) | 4 (0.8%) | 18 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 80 (64.0%) | 202 (61.2%) | 511 (52.5%) | 158 (31.2%) | 951 (49.1%) | 0 (0.0%) | 0 (0.0%) | 208 (57.9%) | 0 (0.0%) | 208 (57.9%) |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrew | 45 (36.0%) | 127 (38.5%) | 449 (46.1%) | 345 (68.0%) | 966 (49.9%) | 0 (0.0%) | 0 (0.0%) | 151 (42.1%) | 0 (0.0%) | 151 (42.1%) |
| Discussion Period and RAC Withdrawals | 0 (0.0%) | 59 (17.9%) | 351 (36.1%) | 235 (46.4%) | 645 (33.3%) | 0 (0.0%) | 0 (0.0%) | 139 (38.7%) | 0 (0.0%) | 139 (38.7%) |
| Level 1 Appeal | 10 (8.0%) | 22 (6.7%) | 60 (6.2%) | 62 (12.2%)1 | 154 (8.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.6%) | 0 (0.0%) | 2 (0.6%) |
| Level 2 Appeal | 22 (17.6%) | 36 (10.9%) | 38 (3.9%) | 48 (9.5%)1 | 144 (7.4%) | 0 (0.0%) | 0 (0.0%) | 10 (2.8%) | 0 (0.0%) | 10 (2.8%) |
| Level 3 Appealc | 13 (10.4%) | 10 (3.0%) | N/A (N/A) | N/A (N/A) | 23 (1.2%) | 0 (0.0%) | 0 (0.0%) | N/A (N/A) | 0 (0.0%) | 0 (0.0%) |
| 2010 | 2011 | 2012 | 2013 | All | 2010 | 2011 | 2012 | 2013 | All | |
| University of Wisconsin Hospital and Clinics | University of Utah | |||||||||
| Total no. | 1 | 48 | 136 | 100 | 285 | 124 | 282 | 478 | 407 | 1,291 |
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (2.1% | 0 (0.0%) | 4 (4.0%) | 5 (1.8%) | 0 (0.0%) | 0 (0.0%) | 13 (2.7%) | 0 (0.0%) | 13 (1.0%) |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 1 (100.0%) | 3 (6.3% | 13 (9.6%) | 3 (3.0%) | 20 (7.0%) | 79 (63.7%) | 199 (70.6%) | 290 (60.7%) | 155 (38.1%) | 723 (56.0%) |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrewb | 0 (0.0%) | 44 (91.7%) | 123 (90.4%) | 93 (93.0%) | 260 (91.2%) | 45 (36.3%) | 83 (29.4%) | 175 (36.6%) | 252 (61.9%) | 555 (43.0%) |
| Discussion Period and RAC Withdrawals | 0 (0.0%) | 38 (79.2%) | 66 (48.5%) | 44 (44.0%) | 148 (51.9% | 0 (0.0%) | 21 (7.4%) | 146 (30.5%) | 191 (46.9%) | 358 (27.7%) |
| Level 1 Appeal | 0 (0.0%) | 2 (4.2%) | 47 (34.6%) | 34 (34.0%) | 83 (29.1%) | 10 (8.1%) | 20 (7.1%) | 11 (2.3%) | 28 (6.9%) | 69 (5.3%) |
| Level 2 Appeal | 0 (0.0%) | 4 (8.3%) | 10 (7.4%) | 15 (15.0%) | 29 (10.2%) | 22 (17.7%) | 32 (11.3%) | 18 (3.8%) | 33 (8.1%) | 105 (8.1%) |
| Level 3 Appealc | 0 (0.0%) | N/A (N/A) | N/A (N/A) | N/A (N/A) | 0 (0.0%) | 13 (10.5%) | 10 (3.5%) | N/A (N/A) | N/A(N/A) | 23 (1.8%) |
The administration at each hospital provided labor estimates for workforce dedicated to the review process generated by the RACs based on hourly accounting of one‐quarter of work during 2012, updated to FY 2014 accounting (Table 3). Concurrent case management status determination work was not included in these numbers due to the difficulty in solely attributing concurrent review workforce numbers to the RACs, as concurrent case management is a CMS Condition of Participation irrespective of the RAC program.
| JHH | UWHC | UU | Mean | |
|---|---|---|---|---|
| ||||
| Physicians: assist with status determinations, audits, and appeals | 1.0 | 0.5 | 0.6 | 0.7 |
| Nursing administration: audit and appeal preparation | 0.9 | 0.2 | 1.9 | 1.0 |
| Legal counsel: assist with rules interpretation, audit, and appeal preparation | 0.2 | 0.3 | 0.1 | 0.2 |
| Data analyst: prepare and track reports of audit and appeals | 2.0 | 1.8 | 2.4 | 2.0 |
| Administration and other directors | 2.3 | 0.9 | 0.3 | 1.2 |
| Total FTE workforce | 6.4 | 3.7 | 5.3 | 5.1 |
Statistics
Descriptive statistics were used to describe the data. Staffing numbers are expressed as full‐time equivalents (FTE).
RESULTS
Yearly Medicare Encounters and RAC Activity of Part A Complex Reviews
RACs audited 8.0% (8110/101,862) of inpatient Medicare cases, alleged noncompliance (all overpayments) for 31.3% (2536/8110) of Part A complex review cases requested, and the hospitals disputed 91.0% (2309/2536) of these assertions. None of these cases of alleged noncompliance claimed the actual medical services were unnecessary. Rather, every Part A complex review overpayment determination by all 3 RACs contested medical necessity related to outpatient versus inpatient status. In 2010 and 2011, there were in aggregate fewer audits (2282), overpayment determinations (680), and appeals or discussion requests (537 of 680, 79.0%), compared to audits (5828), overpayment determinations (1856), and appeals or discussion requests (1772 of 1856, 95.5%) in 2012 and 2013. The hospitals appealed or requested discussion of a greater percentage each successive year (2010, 78.0%; 2011, 79.3%; 2012, 94.1%; and 2013, 98.0%). This increased RAC activity, and hospital willingness to dispute the RAC overpayment determinations equaled a more than 300% increase in appeals and discussion request volume related to Part A complex review audits in just 2 years.
The 16.2% (374/2309) of disputed cases still under discussion or appeal have spent an average mean of 555 days (standard deviation 255 days) without a decision, with time in appeals exceeding 900 days for cases from 2010 and 2011. Notably, the 3 programs were subject to Part A complex review audits at widely different rates (Table 1).
Yearly RAC Part A Complex Review Overpayment Determinations Disputed by Hospitals With Decisions
The hospitals won, either in discussion or appeal, a combined greater percentage of contested overpayment determinations annually, from 36.0% (45/125) in 2010, to 38.5% (127/330) in 2011, to 46.1% (449/973) in 2012, to 68.0% (345/507) in 2013. Overall, for 49.1% (951/1935) of cases with decisions, the hospitals withdrew or rebilled under Part B at some point in the discussion or appeals process to avoid the lengthy appeals process and/or loss of the amount of the entire claim. A total of 49.9% (966/1935) of appeals with decisions have been won in discussion or appeal over the 4‐year study period. One‐third of all resolved cases (33.3%, 645/1935) were decided in favor of the hospital in the discussion period, with these discussion cases accounting for two‐thirds (66.8%, 645/966) of all favorable resolved cases for the hospital. Importantly, if cases overturned in discussion were omitted as they are in federal reports, the hospitals' success rate would fall to 16.6% (321/1935), a number similar to those that appear in annual CMS reports.[9, 13, 14] The hospitals also conceded 18 cases (0.9%) by missing a filing deadline (Table 2).
Estimated Workforce Dedicated to Part A Complex Review Medical Necessity Audits and Appeals
The institutions each employ an average of 5.1 FTE staff to manage the audit and appeal process, a number that does not include concurrent case management staff who assist in daily status determinations (Table 3).
CONCLUSIONS
In this study of 3 academic medical centers, there was a more than 2‐fold increase in RAC audits and a nearly 3‐fold rise in overpayment determinations over the last 2 calendar years of the study, resulting in a more than 3‐fold increase in appeals or requests for discussion in 2012 to 2013 compared to 2010 to 2011. In addition, although CMS manually reviews less than 0.3% of submitted claims each year through programs such as the Recovery Audit Program,[9] at the study hospitals, complex Part A RAC audits occurred at a rate more than 25 times that (8.0%), suggesting that these types of claims are a disproportionate focus of auditing activity. The high overall complex Part A audit rate, accompanied by acceleration of RAC activity and the hospitals' increased willingness to dispute RAC overpayment determinations each year, if representative of similar institutions, would explain the appeals backlog, most notably at the ALJ (level 3) level. Importantly, none of these Part A complex review denials contested a need for the medical care delivered, demonstrating that much of the RAC process at the hospitals focused exclusively on the nuances of medical necessity and variation in interpretation of CMS guidelines that related to whether hospital care should be provided under inpatient or outpatient status.
These data also show continued aggressive RAC audit activity despite an increasing overturn rate in favor of the hospitals in discussion or on appeal each year (from 36.0% in 2010 to 68.0% in 2013). The majority of the hospitals' successful decisions occurred in the discussion period, when the hospital had the opportunity to review the denial with the RAC medical director, a physician, prior to beginning the official appeals process. The 33% overturn rate found in the discussion period represents an error rate by the initial RAC auditors that was internally verified by the RAC medical director. The RAC internal error rate was replicated at 3 different RACs, highlighting internal process problems across the RAC system. This is concerning, because the discussion period is not considered part of the formal appeals process, so these cases are not appearing in CMS or OIG reports of RAC activity, leading to an underestimation of the true successful overturned denial rates at the 3 study hospitals, and likely many other hospitals.
The study hospitals are also being denied timely due process and payments for services delivered. The hospitals currently face an appeals process that, on average, far exceeds 500 days. In almost half of the contested overpayment determinations, the hospitals withdrew a case or rebilled Part B, not due to agreement with a RAC determination, but to avoid the lengthy, cumbersome, and expensive appeals process and/or to minimize the risk of losing the amount of the entire Part A claim. This is concerning, as cases withdrawn in the appeals process are considered improper payments in federal reports, despite a large number of these cases being withdrawn simply to avoid an inefficient appeals process. Notably, Medicare is not adhering to its own rules, which require appeals to be heard in a timely manner, specifically 60 days for level 1 or 2 appeals, and 90 days for a level 3 appeal,[6, 20] even though the hospitals lost the ability to appeal cases when they missed a deadline. Even if hospitals agreed to the recent 68% settlement offer[12] from CMS, appeals may reaccumulate without auditing reform. As noted earlier, this recent settlement offer came more than a year after the enhanced ability to rebill denied Part A claims for Part B, yet the backlog remains.
This study also showed that a large hospital workforce is required to manage the lengthy audit and appeals process generated by RACs. These staff are paid with funds that could be used to provide direct patient care or internal process improvement. The federal government also directly pays for unchecked RAC activity through the complex appeals process. Any report of dollars that RACs recoup for the federal government should be considered in light of their administrative costs to hospitals and government contractors, and direct costs at the federal level.
This study also showed that RACs audited the 3 institutions differently, despite similar willingness of the hospitals to dispute overpayment determinations and similar hospital success rates in appeals or discussion, suggesting that hospital compliance with Medicare policy was not the driver of variable RAC activity. This variation may be due to factors not apparent in this study, such as variable RAC interpretation of federal policy, a decision of a particular RAC to focus on complex Medicare Part B or automated reviews instead of complex Part A reviews, or RAC workforce differences that are not specific to the hospitals. Regardless, the variation in audit activity suggests that greater transparency and accountability in RAC activity is merited.
Perhaps most importantly, this study highlights factors that may help explain differing auditing and appeals numbers reported by the OIG,[4] CMS,[9, 13, 14] and hospitals.[15, 16] Given the marked increase in RAC activity over the last 4 years, the 2010 and 2011 data included in a recent OIG report[4] likely do not represent current auditing and appeals practice. With regard to the CMS reports,[9, 13, 14] although CMS included FY 2013[9] activity in its most recent report, it did not account for denials overturned in the discussion period, as these are not technically appeals, even though these are contested cases decided in favor of the hospital. This most recent CMS report[9] uses overpayment determinations from FY 2013, yet counts appeals and decisions that occurred in 2013, with the comment that these decisions may be for overpayment determinations prior to 2013. The CMS reports also variably combine automated, semiautomated, complex Part A, and complex Part B claims in its reports, making interpretation challenging. Finally, although CMS reported an increase in improper payments recovered from FY 2011[14] ($939 million) to FY 2012[13] ($2.4 billion) to FY 2013[9] ($3.75 billion), this is at least partly a reflection of increased RAC activity as demonstrated in this study, and may reflect the fact that many hospitals do not have the resources to continually appeal or choose not to contest these cases based on a financial business decision. Importantly, these numbers now far exceed recoupment in other quality programs, such as the Readmissions Reduction Program (estimated $428 million next FY),[21] indicating the increased fiscal impact of the RAC program on hospital reimbursement.
To increase accuracy, future federal reports of auditing and appeals should detail and include cases overturned in the discussion period, and carefully describe the denominator of total audits and appeals given the likelihood that many appeals in a given year will not have a decision in that year. Percent of total Medicare claims subject to complex Part A audit should be stated. Reports should also identify and consider an alternative classification for complex Part A cases the hospital elects to rebill under Medicare Part B, and also detail on what grounds medical necessity is being contested (eg, whether the actual care delivered was not necessary or if it is an outpatient versus inpatient billing issue). Time spent in the appeals process must also be reported. Complex Part A, complex Part B, semiautomated, and automated reviews should also be considered separately, and dates of reported audits and appeals must be as current as possible in this rapidly changing environment.
In this study, RACs conducted complex Part A audits at a rate 25 times the CMS‐reported overall audit rate, confirming complex Part A audits are a particular focus of RAC activity. There was a more than doubling of RAC audits at the study hospitals from the years 2010 ‐ 2011 to 2012 ‐ 2013 and a nearly 3‐fold increase in overpayment determinations. Concomitantly, the more than 3‐fold increase in appeals and discussion volume over this same time period was consistent with the development of the current national appeals backlog. The 3 study hospitals won a greater percentage of contested cases each year, from approximately one‐third of cases in 2010 to two‐thirds of cases with decisions in 2013, but there was no appreciable decrease in RAC overpayment determinations over that time period. The majority of successfully challenged cases were won in discussion, favorable decisions for hospitals not appearing in federal appeals reports. Time in appeals exceeded 550 days, causing the hospitals to withdraw some cases to avoid the lengthy appeals process and/or to minimize the risk of losing the amount of the entire Part A claim. The hospitals also lost a small number of appeals by missing a filing deadline, yet there was no reciprocal case concession when the appeals system missed a deadline. RACs found no cases of care at the 3 hospitals that should not have been delivered, but rather challenged the status determination (inpatient vs outpatient) to dispute medical necessity of care delivered. Finally, an average of approximately 5 FTEs at each institution were employed in the audits and appeals process. These data support a need for systematic improvements in the RAC system so that fair, constructive, and cost‐efficient surveillance of the Medicare program can be realized.
Acknowledgements
The authors thank Becky Borchert, MS, RN BC, ACM, CPHQ, Program Manager for Medicare/Medicaid Utilization Review at the University of Wisconsin Hospital and Clinics; Carol Duhaney and Joan Kratz, RN, at Johns Hopkins Hospital; and Morgan Walker at the University of Utah for their assistance in data preparation and presentation. Without their meticulous work and invaluable assistance, this study would not have been possible. The authors also thank Josh Boswell, JD, for his critical review of the manuscript.
Disclosure: Nothing to report.
- Medicare Payment Advisory Commission. Hospital inpatient and observation services. 2014 Report to Congress. Medicare Payment Policy. Available at: http://www.medpac.gov/documents/reports/mar14_entirereport.pdf?sfvrsn=0. Accessed September 22, 2014.
- American Hospital Association “2‐midnight rule” lawsuit vs Department of Health and Human Services. Available at: http://www.aha.org/content/14/140414‐complaint‐2midnight.pdf. Accessed August 8, 2014.
- Centers for Medicare administrative law judge hearing program for Medicare claim appeals. Fed Regist. 2014;79(214): 65660 – 65663. Available at: http://www.hhs.gov/omha/files/omha_federal_register_notice_2014–26214.pdf. Accessed December 6, 2014.
- . Medicare fines 2,610 hospitals in third round of readmission penalties. Kaiser Health News. Available at: http://kaiserhealthnews.org/news/medicare‐readmissions‐penalties‐2015. Accessed November 30, 2014.
Medicare patients are increasingly hospitalized as outpatients under observation. From 2006 to 2012, outpatient services grew nationally by 28.5%, whereas inpatient discharges decreased by 12.6% per Medicare beneficiary.[1] This increased use of observation stays for hospitalized Medicare beneficiaries and the recent Centers for Medicare & Medicaid Services (CMS) 2‐Midnight rule for determination of visit status are increasing areas of concern for hospitals, policymakers, and the public,[2] as patients hospitalized under observation are not covered by Medicare Part A hospital insurance, are subject to uncapped out‐of‐pocket charges under Medicare Part B, and may be billed by the hospital for certain medications. Additionally, Medicare beneficiaries hospitalized in outpatient status, which includes all hospitalizations under observation, do not qualify for skilled nursing facility care benefits after discharge, which requires a stay that spans at least 3 consecutive midnights as an inpatient.[3]
In contrast, the federal Recovery Audit program, previously called and still commonly referred to as the Recovery Audit Contractor (RAC) program, responsible for postpayment review of inpatient claims, has received relatively little attention. Established in 2006, and fully operationalized in federal fiscal year (FY) 2010,[4] RACs are private government contractors granted the authority to audit hospital charts for appropriate medical necessity, which can consider whether the care delivered was indicated and whether it was delivered in the appropriate Medicare visit status, outpatient or inpatient. Criteria for hospitalization status (inpatient vs outpatient) as defined in the Medicare Conditions of Participation, often allow for subjectivity (medical judgment) in determining which status is appropriate.[5] Hospitals may contest RAC decisions and payment denials through a preappeals discussion period, then through a 5‐level appeals process. Although early appeals occur between the hospital and private contractors, appeals reaching level 3 are heard by the Department of Health and Human Services (HHS) Office of Medicare Hearings and Appeals (OMHA) Administrative Law Judges (ALJ). Levels 4 (Medicare Appeals Council) and 5 (United States District Court) appeals are also handled by the federal government.[6]
Medicare fraud and abuse should not be tolerated, and systematic surveillance needs to be an integral part of the Medicare program.[4] However, there are increasing concerns that the RAC program has resulted in overaggressive denials.[7, 8] Unlike other Medicare contractors, RAC auditors are paid a contingency fee based on the percentage of hospital payment recouped for cases they audit and deny for improper payment.[4] RACs are not subject to any financial penalty for cases they deny but are overturned in the discussion period or in the appeals process. This may create an incentive system that financially encourages RACs to assert improper payment, and the current system lacks both transparency and clear performance metrics for auditors. Of particular concern are Medicare Part A complex reviews, the most fiscally impactful area of RAC activity. According to CMS FY 2013 data, 41.1% of all claims with collections were complex reviews, yet these claims accounted for almost all (95.2%) of total dollars recovered by the RACs, with almost all (96%) dollars recovered being from Part A claims.[9] Complex reviews involve an auditor retrospectively and manually reviewing a medical record and then using his or her clinical and related professional judgment to decide whether the care was medically necessary. This is compared to automated coding or billing reviews, which are based solely on claims data.
Increased RAC activity and the willingness of hospitals to challenge RAC findings of improper payment has led to an increase in appeals volume that has overloaded the appeals process. On March 13, 2013, CMS offered hospitals the ability to rebill Medicare Part B as an appeals alternative.[10] This did not temper level 3 appeals requests received by the OMHA, which increased from 1250 per week in January 2012 to over 15,000 per week by November 2013.[11] Citing an overwhelmingly increased rate of appeal submissions and the resultant backlog, the OMHA decided to freeze new hospital appeals assignments in December 2013.[11] In another attempt to clear the backlog, on August 29, 2014, CMS offered a settlement that would pay hospitals 68% of the net allowable amount of the original Part A claim (minus any beneficiary deductibles) if a hospital agreed to concede all of its eligible appeals.[12] Notably, cases settled under this agreement would remain officially categorized as denied for improper payment.
The HHS Office of Inspector General (OIG)[4] and the CMS[9, 13, 14] have produced recent reports of RAC auditing and appeals activity that contain variable numbers that conflict with hospital accounts of auditing and appeals activity.[15, 16] In addition to these conflicting reports, little is known about RAC auditing of individual programs over time, the length of time cases spend in appeals, and staff required to navigate the audit and appeals processes. Given these questions, and the importance of RAC auditing pressure in the growth of hospital observation care, we conducted a retrospective descriptive study of all RAC activity for complex Medicare Part A alleged overpayment determinations at the Johns Hopkins Hospital, the University of Utah, and University of Wisconsin Hospital and Clinics for calendar years 2010 to 2013.
METHODS
The University of Wisconsin‐Madison Health Sciences institutional review board (IRB) and the Johns Hopkins Hospital IRB did not require review of this study. The University of Utah received an exemption. All 3 hospitals are tertiary care academic medical centers. The University of Wisconsin Hospital and Clinics (UWHC) is a 592‐bed hospital located in Madison, Wisconsin,[17] the Johns Hopkins Hospital (JHH) is a 1145‐bed medical center located in Baltimore, Maryland,[18] and the University of Utah Hospital (UU) is a 770‐bed facility in Salt Lake City, Utah (information available upon request). Each hospital is under a different RAC, representing 3 of the 4 RAC regions, and each is under a different Medicare Administrative Contractor, contractors responsible for level 1 appeals. The 3 hospitals have the same Qualified Independent Contractor responsible for level 2 appeals.
For the purposes of this study, any chart or medical record requested for review by an RAC was considered a medical necessity chart request or an audit. The terms overpayment determinations and denials were used interchangeably to describe audits the RACs alleged did not meet medical necessity for Medicare Part A billing. As previously described, the term medical necessity specifically considered not only whether actual medical services were appropriate, but also whether the services were delivered in the appropriate status, outpatient or inpatient. Appeals and/or request for discussion were cases where the overpayment determination was disputed and challenged by the hospital.
All complex review Medicare Part A RAC medical record requests by date of RAC request from the official start of the RAC program, January 1, 2010,[4] to December 31, 2013, were included in this study. Medical record requests for automated reviews that related to coding and billing clarifications were not included in this study, nor were complex Medicare Part B reviews, complex reviews for inpatient rehabilitation facilities, or psychiatric day hospitalizations. Notably, JHH is a Periodic Interim Payment (PIP) Medicare hospital, which is a reimbursement mechanism where biweekly payments [are] made to a Provider enrolled in the PIP program, and are based on the hospital's estimate of applicable Medicare reimbursement for the current cost report period.[19] Because PIP payments are made collectively to the hospital based on historical data, adjustments for individual inpatients could not be easily adjudicated and processed. Due to the increased complexity of this reimbursement mechanism, RAC audits did not begin at JHH until 2012. In addition, in contrast to the other 2 institutions, all of the RAC complex review audits at JHH in 2013 were for Part B cases, such as disputing need for intensity‐modulated radiation therapy versus conventional radiation therapy, or contesting the medical necessity of blepharoplasty. As a result, JHH had complex Part A review audits only for 2012 during the study time period. All data were deidentified prior to review by investigators.
As RACs can audit charts for up to 3 years after the bill is submitted,[13] a chart request in 2013 may represent a 2010 hospitalization, but for purposes of this study, was logged as a 2013 case. There currently is no standard methodology to calculate time spent in appeals. The UWHC and JHH calculate time in discussion or appeals from the day the discussion or appeal was initiated by the hospital, and the UU calculates the time in appeals from the date of the findings letter from the RAC, which makes comparable recorded time in appeals longer at UU (estimated 510 days for 20112013 cases, up to 120 days for 2010 cases).Time in appeals includes all cases that remain in the discussion or appeals process as of June 30, 2014.
The RAC process is as follows (Tables 1 and 2):
- The RAC requests hospital claims (RAC Medical Necessity Chart Requests [Audits]).
- The RAC either concludes the hospital claim was compliant as filed/paid and the process ends or the RAC asserts improper payment and requests repayment (RAC Overpayment Determinations of Requested Charts [Denials]).
- The hospital makes an initial decision to not contest the RAC decision (and repay), or to dispute the decision (Hospital Disputes Overpayment Determination [Appeal/Discussion]). Prior to filing an appeal, the hospital may request a discussion of the case with an RAC medical director, during which the RAC medical director can overturn the original determination. If the RAC declines to overturn the decision in discussion, the hospital may proceed with a formal appeal. Although CMS does not calculate the discussion period as part of the appeals process,[12] overpayment determinations contested by the hospital in either discussion or appeal represent the sum total of RAC denials disputed by the hospital.
Contested cases have 1 of 4 outcomes:
Contested overpayment determinations can be decided in favor of the hospital (Discussion or Appeal Decided in Favor of Hospital or RAC Withdrew)
- Contested overpayment determinations can be decided in favor of the RAC during the appeal process, and either the hospital exhausts the appeal process or elects not to take the appeal to the next level. Although the appeals process has 5 levels, no cases at our 3 hospitals have reached level 4 or 5, so cases without a decision to date remain in appeals at 1 of the first 3 levels (Case Still in Discussion or Appeals).[4]
- Hospital may miss an appeal deadline (Hospital Missed Appeal Deadline at Any Level) and the case is automatically decided in favor of the RAC.
- As of March 13, 2013,[10] for appeals that meet certain criteria and involve dispute over the billing of hospital services under Part A, CMS allowed hospitals to withdraw an appeal and rebill Medicare Part B. Prior to this time, hospitals could rebill for a very limited list of ancillary Part B Only services, and only within the 1‐year timely filing period.[13] Due to the lengthy appeals process and associated legal and administrative costs, hospitals may not agree with the RAC determination but make a business decision to recoup some payment under this mechanism (Hospital Chose to Rebill as Part B During Discussion or Appeals Process).
| Totals | Johns Hopkins Hospital | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | All Years | 2010 | 2011 | 2012 | 2013 | All Years | ||
| University of Wisconsin Hospital and Clinics | University of Utah | ||||||||||
| 2010 | 2011 | 2012 | 2013 | All Years | 2010 | 2011 | 2012 | 2013 | All Years | ||
| |||||||||||
| Total no. of Medicare encounters | 24,400 | 24,998 | 25,370 | 27,094 | 101,862 | 11,212b | 11,750b | 11,842 | 12,674c | 47,478 | |
| RAC Medical Necessity Chart Requests (Audits) | 547 | 1,735 | 3,887 | 1,941 | 8,110 (8.0%) | 0 | 0 | 938 | 0 | 938 (2.0%) | |
| RAC Overpayment Determinations Of Requested Charts (Denials)d | 164 (30.0%) | 516 (29.7%) | 1,200 (30.9%) | 656 (33.8%) | 2,536 (31.3%) | 0 (0%) | 0 (0%) | 432 (46.1%) | 0 (0%) | 432 (46.1%) | |
| Hospital Disputes Overpayment Determination (Appeal/Discussion) | 128 (78.0%) | 409 (79.3%) | 1,129 (94.1%) | 643 (98.0%) | 2,309 (91.0% | 0 (0%) | 0 (0%) | 431 (99.8%) | 0 (0%) | 431 (99.8%) | |
| Outcome of Disputed Overpayment Determinatione | |||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (0.2%) | 13 (1.2%) | 4 (0.6%) | 18 (0.8%) | 0 (0%) | 0 (0%) | 0 (0.0%) | 0 (0%) | 0 (0.0%) | |
| Hospital Chose To Rebill as Part B During Discussion Or Appeals Process | 80 (62.5%) | 202 (49.4%) | 511 (45.3%) | 158 (24.6%) | 951 (41.2%) | 0 (0%) | 0 (0%) | 208 (48.3%) | 0 (0%) | 208 (48.3%) | |
| Discussion or Appeal Decided In Favor Of Hospital or RAC Withdrewf | 45 (35.2%) | 127 (31.1%) | 449 (39.8%) | 345 (53.7%) | 966 (41.8%) | 0 (0%) | 0 (0%) | 151 (35.0%) | 0 (0%) | 151 (35.0%) | |
| Case Still in Discussion or Appeals | 3 (2.3%) | 79 (19.3%) | 156 13.8%) | 136 (21.2%) | 374 (16.2%) | 0 (0%) | 0 (0%) | 72 (16.7%) | 0 (0%) | 72 (16.7%) | |
| Mean Time for Cases Still in Discussion or Appeals, d (SD) | 1208 (41) | 958 (79) | 518 (125) | 350 (101) | 555 (255) | N/A | N/A | 478 (164) | N/A | 478 (164) | |
| Total no. of Medicare encounters l | 8,096 | 8,038 | 8,429 | 9,086 | 33,649 | 5,092 | 5,210 | 5,099 | 5,334 | 20,735 | |
| RAC Medical Necessity Chart Requests (Audits) | 15 | 526 | 1,484 | 960 | 2,985 (8.9%) | 532 | 1,209 | 1,465 | 981 | 4,187 (20.2%) | |
| RAC Overpayment Determinations of Requested Charts (Denials)bd | 3 (20.0%) | 147 (27.9%) | 240 (16.2%) | 164 (17.1%) | 554 (18.6%) | 161 (30.3%) | 369 (30.5%) | 528 (36.0%) | 492 (50.2%) | 1,550 (37.0%) | |
| Hospital Disputes Overpayment Determination (Appeal/Discussion) | 1 (33.3%) | 71 (48.3%) | 170 (70.8%) | 151 (92.1%) | 393 (70.9%) | 127 (78.9%) | 338 (91.6%) | 528 (100.0%) | 492 (100.0%) | 1,485 (95.8%) | |
| Outcome of Disputed Overpayment Determinatione | |||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 4 (2.6%) | 5 (1.3%) | 0 (0.0%) | 0 (0.0%) | 13 (2.5%) | 0 (0.0%) | 13 (0.9%) | |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 1 (100%) | 3 (4.2%) | 13 (7.6%) | 3 (2.0%) | 20 (5.1%) | 79 (62.2%) | 199 (58.9%) | 290 (54.9%) | 155 (31.5%) | 723 (48.7%) | |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrewf | 0 (0.0%) | 44 (62.0%) | 123 (72.4%) | 93 (61.6%) | 260 (66.2%) | 45 (35.4%) | 83 (24.6%) | 175 (33.1%) | 252 (51.2%) | 555 (37.4%) | |
| Case Still in Discussion or Appeals | 0 0.0% | 23 (32.4%) | 34 (20.0%) | 51 (33.8%) | 108 (27.5%) | 3 (2.4%) | 56 (16.6%) | 50 (9.5%) | 85 (17.3%) | 194 (13.1%) | |
| Mean Time for Cases Still in Discussion or Appeals, d (SD) | N/A | 926 (70) | 564 (90) | 323 (134) | 528 (258) | 1,208 (41) | 970 (80) | 544 (25) | 365 (72) | 599 (273) | |
| 2010 | 2011 | 2012 | 2013 | All | 2010 | 2011 | 2012 | 2013 | All | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Appeals With Decisions | Johns Hopkins Hospital | |||||||||
| Total no. | 125 | 330 | 973 | 507 | 1,935 | 0 | 0 | 359 | 0 | 359 |
| ||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (0.3%) | 13 (1.3%) | 4 (0.8%) | 18 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 80 (64.0%) | 202 (61.2%) | 511 (52.5%) | 158 (31.2%) | 951 (49.1%) | 0 (0.0%) | 0 (0.0%) | 208 (57.9%) | 0 (0.0%) | 208 (57.9%) |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrew | 45 (36.0%) | 127 (38.5%) | 449 (46.1%) | 345 (68.0%) | 966 (49.9%) | 0 (0.0%) | 0 (0.0%) | 151 (42.1%) | 0 (0.0%) | 151 (42.1%) |
| Discussion Period and RAC Withdrawals | 0 (0.0%) | 59 (17.9%) | 351 (36.1%) | 235 (46.4%) | 645 (33.3%) | 0 (0.0%) | 0 (0.0%) | 139 (38.7%) | 0 (0.0%) | 139 (38.7%) |
| Level 1 Appeal | 10 (8.0%) | 22 (6.7%) | 60 (6.2%) | 62 (12.2%)1 | 154 (8.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.6%) | 0 (0.0%) | 2 (0.6%) |
| Level 2 Appeal | 22 (17.6%) | 36 (10.9%) | 38 (3.9%) | 48 (9.5%)1 | 144 (7.4%) | 0 (0.0%) | 0 (0.0%) | 10 (2.8%) | 0 (0.0%) | 10 (2.8%) |
| Level 3 Appealc | 13 (10.4%) | 10 (3.0%) | N/A (N/A) | N/A (N/A) | 23 (1.2%) | 0 (0.0%) | 0 (0.0%) | N/A (N/A) | 0 (0.0%) | 0 (0.0%) |
| 2010 | 2011 | 2012 | 2013 | All | 2010 | 2011 | 2012 | 2013 | All | |
| University of Wisconsin Hospital and Clinics | University of Utah | |||||||||
| Total no. | 1 | 48 | 136 | 100 | 285 | 124 | 282 | 478 | 407 | 1,291 |
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (2.1% | 0 (0.0%) | 4 (4.0%) | 5 (1.8%) | 0 (0.0%) | 0 (0.0%) | 13 (2.7%) | 0 (0.0%) | 13 (1.0%) |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 1 (100.0%) | 3 (6.3% | 13 (9.6%) | 3 (3.0%) | 20 (7.0%) | 79 (63.7%) | 199 (70.6%) | 290 (60.7%) | 155 (38.1%) | 723 (56.0%) |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrewb | 0 (0.0%) | 44 (91.7%) | 123 (90.4%) | 93 (93.0%) | 260 (91.2%) | 45 (36.3%) | 83 (29.4%) | 175 (36.6%) | 252 (61.9%) | 555 (43.0%) |
| Discussion Period and RAC Withdrawals | 0 (0.0%) | 38 (79.2%) | 66 (48.5%) | 44 (44.0%) | 148 (51.9% | 0 (0.0%) | 21 (7.4%) | 146 (30.5%) | 191 (46.9%) | 358 (27.7%) |
| Level 1 Appeal | 0 (0.0%) | 2 (4.2%) | 47 (34.6%) | 34 (34.0%) | 83 (29.1%) | 10 (8.1%) | 20 (7.1%) | 11 (2.3%) | 28 (6.9%) | 69 (5.3%) |
| Level 2 Appeal | 0 (0.0%) | 4 (8.3%) | 10 (7.4%) | 15 (15.0%) | 29 (10.2%) | 22 (17.7%) | 32 (11.3%) | 18 (3.8%) | 33 (8.1%) | 105 (8.1%) |
| Level 3 Appealc | 0 (0.0%) | N/A (N/A) | N/A (N/A) | N/A (N/A) | 0 (0.0%) | 13 (10.5%) | 10 (3.5%) | N/A (N/A) | N/A(N/A) | 23 (1.8%) |
The administration at each hospital provided labor estimates for workforce dedicated to the review process generated by the RACs based on hourly accounting of one‐quarter of work during 2012, updated to FY 2014 accounting (Table 3). Concurrent case management status determination work was not included in these numbers due to the difficulty in solely attributing concurrent review workforce numbers to the RACs, as concurrent case management is a CMS Condition of Participation irrespective of the RAC program.
| JHH | UWHC | UU | Mean | |
|---|---|---|---|---|
| ||||
| Physicians: assist with status determinations, audits, and appeals | 1.0 | 0.5 | 0.6 | 0.7 |
| Nursing administration: audit and appeal preparation | 0.9 | 0.2 | 1.9 | 1.0 |
| Legal counsel: assist with rules interpretation, audit, and appeal preparation | 0.2 | 0.3 | 0.1 | 0.2 |
| Data analyst: prepare and track reports of audit and appeals | 2.0 | 1.8 | 2.4 | 2.0 |
| Administration and other directors | 2.3 | 0.9 | 0.3 | 1.2 |
| Total FTE workforce | 6.4 | 3.7 | 5.3 | 5.1 |
Statistics
Descriptive statistics were used to describe the data. Staffing numbers are expressed as full‐time equivalents (FTE).
RESULTS
Yearly Medicare Encounters and RAC Activity of Part A Complex Reviews
RACs audited 8.0% (8110/101,862) of inpatient Medicare cases, alleged noncompliance (all overpayments) for 31.3% (2536/8110) of Part A complex review cases requested, and the hospitals disputed 91.0% (2309/2536) of these assertions. None of these cases of alleged noncompliance claimed the actual medical services were unnecessary. Rather, every Part A complex review overpayment determination by all 3 RACs contested medical necessity related to outpatient versus inpatient status. In 2010 and 2011, there were in aggregate fewer audits (2282), overpayment determinations (680), and appeals or discussion requests (537 of 680, 79.0%), compared to audits (5828), overpayment determinations (1856), and appeals or discussion requests (1772 of 1856, 95.5%) in 2012 and 2013. The hospitals appealed or requested discussion of a greater percentage each successive year (2010, 78.0%; 2011, 79.3%; 2012, 94.1%; and 2013, 98.0%). This increased RAC activity, and hospital willingness to dispute the RAC overpayment determinations equaled a more than 300% increase in appeals and discussion request volume related to Part A complex review audits in just 2 years.
The 16.2% (374/2309) of disputed cases still under discussion or appeal have spent an average mean of 555 days (standard deviation 255 days) without a decision, with time in appeals exceeding 900 days for cases from 2010 and 2011. Notably, the 3 programs were subject to Part A complex review audits at widely different rates (Table 1).
Yearly RAC Part A Complex Review Overpayment Determinations Disputed by Hospitals With Decisions
The hospitals won, either in discussion or appeal, a combined greater percentage of contested overpayment determinations annually, from 36.0% (45/125) in 2010, to 38.5% (127/330) in 2011, to 46.1% (449/973) in 2012, to 68.0% (345/507) in 2013. Overall, for 49.1% (951/1935) of cases with decisions, the hospitals withdrew or rebilled under Part B at some point in the discussion or appeals process to avoid the lengthy appeals process and/or loss of the amount of the entire claim. A total of 49.9% (966/1935) of appeals with decisions have been won in discussion or appeal over the 4‐year study period. One‐third of all resolved cases (33.3%, 645/1935) were decided in favor of the hospital in the discussion period, with these discussion cases accounting for two‐thirds (66.8%, 645/966) of all favorable resolved cases for the hospital. Importantly, if cases overturned in discussion were omitted as they are in federal reports, the hospitals' success rate would fall to 16.6% (321/1935), a number similar to those that appear in annual CMS reports.[9, 13, 14] The hospitals also conceded 18 cases (0.9%) by missing a filing deadline (Table 2).
Estimated Workforce Dedicated to Part A Complex Review Medical Necessity Audits and Appeals
The institutions each employ an average of 5.1 FTE staff to manage the audit and appeal process, a number that does not include concurrent case management staff who assist in daily status determinations (Table 3).
CONCLUSIONS
In this study of 3 academic medical centers, there was a more than 2‐fold increase in RAC audits and a nearly 3‐fold rise in overpayment determinations over the last 2 calendar years of the study, resulting in a more than 3‐fold increase in appeals or requests for discussion in 2012 to 2013 compared to 2010 to 2011. In addition, although CMS manually reviews less than 0.3% of submitted claims each year through programs such as the Recovery Audit Program,[9] at the study hospitals, complex Part A RAC audits occurred at a rate more than 25 times that (8.0%), suggesting that these types of claims are a disproportionate focus of auditing activity. The high overall complex Part A audit rate, accompanied by acceleration of RAC activity and the hospitals' increased willingness to dispute RAC overpayment determinations each year, if representative of similar institutions, would explain the appeals backlog, most notably at the ALJ (level 3) level. Importantly, none of these Part A complex review denials contested a need for the medical care delivered, demonstrating that much of the RAC process at the hospitals focused exclusively on the nuances of medical necessity and variation in interpretation of CMS guidelines that related to whether hospital care should be provided under inpatient or outpatient status.
These data also show continued aggressive RAC audit activity despite an increasing overturn rate in favor of the hospitals in discussion or on appeal each year (from 36.0% in 2010 to 68.0% in 2013). The majority of the hospitals' successful decisions occurred in the discussion period, when the hospital had the opportunity to review the denial with the RAC medical director, a physician, prior to beginning the official appeals process. The 33% overturn rate found in the discussion period represents an error rate by the initial RAC auditors that was internally verified by the RAC medical director. The RAC internal error rate was replicated at 3 different RACs, highlighting internal process problems across the RAC system. This is concerning, because the discussion period is not considered part of the formal appeals process, so these cases are not appearing in CMS or OIG reports of RAC activity, leading to an underestimation of the true successful overturned denial rates at the 3 study hospitals, and likely many other hospitals.
The study hospitals are also being denied timely due process and payments for services delivered. The hospitals currently face an appeals process that, on average, far exceeds 500 days. In almost half of the contested overpayment determinations, the hospitals withdrew a case or rebilled Part B, not due to agreement with a RAC determination, but to avoid the lengthy, cumbersome, and expensive appeals process and/or to minimize the risk of losing the amount of the entire Part A claim. This is concerning, as cases withdrawn in the appeals process are considered improper payments in federal reports, despite a large number of these cases being withdrawn simply to avoid an inefficient appeals process. Notably, Medicare is not adhering to its own rules, which require appeals to be heard in a timely manner, specifically 60 days for level 1 or 2 appeals, and 90 days for a level 3 appeal,[6, 20] even though the hospitals lost the ability to appeal cases when they missed a deadline. Even if hospitals agreed to the recent 68% settlement offer[12] from CMS, appeals may reaccumulate without auditing reform. As noted earlier, this recent settlement offer came more than a year after the enhanced ability to rebill denied Part A claims for Part B, yet the backlog remains.
This study also showed that a large hospital workforce is required to manage the lengthy audit and appeals process generated by RACs. These staff are paid with funds that could be used to provide direct patient care or internal process improvement. The federal government also directly pays for unchecked RAC activity through the complex appeals process. Any report of dollars that RACs recoup for the federal government should be considered in light of their administrative costs to hospitals and government contractors, and direct costs at the federal level.
This study also showed that RACs audited the 3 institutions differently, despite similar willingness of the hospitals to dispute overpayment determinations and similar hospital success rates in appeals or discussion, suggesting that hospital compliance with Medicare policy was not the driver of variable RAC activity. This variation may be due to factors not apparent in this study, such as variable RAC interpretation of federal policy, a decision of a particular RAC to focus on complex Medicare Part B or automated reviews instead of complex Part A reviews, or RAC workforce differences that are not specific to the hospitals. Regardless, the variation in audit activity suggests that greater transparency and accountability in RAC activity is merited.
Perhaps most importantly, this study highlights factors that may help explain differing auditing and appeals numbers reported by the OIG,[4] CMS,[9, 13, 14] and hospitals.[15, 16] Given the marked increase in RAC activity over the last 4 years, the 2010 and 2011 data included in a recent OIG report[4] likely do not represent current auditing and appeals practice. With regard to the CMS reports,[9, 13, 14] although CMS included FY 2013[9] activity in its most recent report, it did not account for denials overturned in the discussion period, as these are not technically appeals, even though these are contested cases decided in favor of the hospital. This most recent CMS report[9] uses overpayment determinations from FY 2013, yet counts appeals and decisions that occurred in 2013, with the comment that these decisions may be for overpayment determinations prior to 2013. The CMS reports also variably combine automated, semiautomated, complex Part A, and complex Part B claims in its reports, making interpretation challenging. Finally, although CMS reported an increase in improper payments recovered from FY 2011[14] ($939 million) to FY 2012[13] ($2.4 billion) to FY 2013[9] ($3.75 billion), this is at least partly a reflection of increased RAC activity as demonstrated in this study, and may reflect the fact that many hospitals do not have the resources to continually appeal or choose not to contest these cases based on a financial business decision. Importantly, these numbers now far exceed recoupment in other quality programs, such as the Readmissions Reduction Program (estimated $428 million next FY),[21] indicating the increased fiscal impact of the RAC program on hospital reimbursement.
To increase accuracy, future federal reports of auditing and appeals should detail and include cases overturned in the discussion period, and carefully describe the denominator of total audits and appeals given the likelihood that many appeals in a given year will not have a decision in that year. Percent of total Medicare claims subject to complex Part A audit should be stated. Reports should also identify and consider an alternative classification for complex Part A cases the hospital elects to rebill under Medicare Part B, and also detail on what grounds medical necessity is being contested (eg, whether the actual care delivered was not necessary or if it is an outpatient versus inpatient billing issue). Time spent in the appeals process must also be reported. Complex Part A, complex Part B, semiautomated, and automated reviews should also be considered separately, and dates of reported audits and appeals must be as current as possible in this rapidly changing environment.
In this study, RACs conducted complex Part A audits at a rate 25 times the CMS‐reported overall audit rate, confirming complex Part A audits are a particular focus of RAC activity. There was a more than doubling of RAC audits at the study hospitals from the years 2010 ‐ 2011 to 2012 ‐ 2013 and a nearly 3‐fold increase in overpayment determinations. Concomitantly, the more than 3‐fold increase in appeals and discussion volume over this same time period was consistent with the development of the current national appeals backlog. The 3 study hospitals won a greater percentage of contested cases each year, from approximately one‐third of cases in 2010 to two‐thirds of cases with decisions in 2013, but there was no appreciable decrease in RAC overpayment determinations over that time period. The majority of successfully challenged cases were won in discussion, favorable decisions for hospitals not appearing in federal appeals reports. Time in appeals exceeded 550 days, causing the hospitals to withdraw some cases to avoid the lengthy appeals process and/or to minimize the risk of losing the amount of the entire Part A claim. The hospitals also lost a small number of appeals by missing a filing deadline, yet there was no reciprocal case concession when the appeals system missed a deadline. RACs found no cases of care at the 3 hospitals that should not have been delivered, but rather challenged the status determination (inpatient vs outpatient) to dispute medical necessity of care delivered. Finally, an average of approximately 5 FTEs at each institution were employed in the audits and appeals process. These data support a need for systematic improvements in the RAC system so that fair, constructive, and cost‐efficient surveillance of the Medicare program can be realized.
Acknowledgements
The authors thank Becky Borchert, MS, RN BC, ACM, CPHQ, Program Manager for Medicare/Medicaid Utilization Review at the University of Wisconsin Hospital and Clinics; Carol Duhaney and Joan Kratz, RN, at Johns Hopkins Hospital; and Morgan Walker at the University of Utah for their assistance in data preparation and presentation. Without their meticulous work and invaluable assistance, this study would not have been possible. The authors also thank Josh Boswell, JD, for his critical review of the manuscript.
Disclosure: Nothing to report.
Medicare patients are increasingly hospitalized as outpatients under observation. From 2006 to 2012, outpatient services grew nationally by 28.5%, whereas inpatient discharges decreased by 12.6% per Medicare beneficiary.[1] This increased use of observation stays for hospitalized Medicare beneficiaries and the recent Centers for Medicare & Medicaid Services (CMS) 2‐Midnight rule for determination of visit status are increasing areas of concern for hospitals, policymakers, and the public,[2] as patients hospitalized under observation are not covered by Medicare Part A hospital insurance, are subject to uncapped out‐of‐pocket charges under Medicare Part B, and may be billed by the hospital for certain medications. Additionally, Medicare beneficiaries hospitalized in outpatient status, which includes all hospitalizations under observation, do not qualify for skilled nursing facility care benefits after discharge, which requires a stay that spans at least 3 consecutive midnights as an inpatient.[3]
In contrast, the federal Recovery Audit program, previously called and still commonly referred to as the Recovery Audit Contractor (RAC) program, responsible for postpayment review of inpatient claims, has received relatively little attention. Established in 2006, and fully operationalized in federal fiscal year (FY) 2010,[4] RACs are private government contractors granted the authority to audit hospital charts for appropriate medical necessity, which can consider whether the care delivered was indicated and whether it was delivered in the appropriate Medicare visit status, outpatient or inpatient. Criteria for hospitalization status (inpatient vs outpatient) as defined in the Medicare Conditions of Participation, often allow for subjectivity (medical judgment) in determining which status is appropriate.[5] Hospitals may contest RAC decisions and payment denials through a preappeals discussion period, then through a 5‐level appeals process. Although early appeals occur between the hospital and private contractors, appeals reaching level 3 are heard by the Department of Health and Human Services (HHS) Office of Medicare Hearings and Appeals (OMHA) Administrative Law Judges (ALJ). Levels 4 (Medicare Appeals Council) and 5 (United States District Court) appeals are also handled by the federal government.[6]
Medicare fraud and abuse should not be tolerated, and systematic surveillance needs to be an integral part of the Medicare program.[4] However, there are increasing concerns that the RAC program has resulted in overaggressive denials.[7, 8] Unlike other Medicare contractors, RAC auditors are paid a contingency fee based on the percentage of hospital payment recouped for cases they audit and deny for improper payment.[4] RACs are not subject to any financial penalty for cases they deny but are overturned in the discussion period or in the appeals process. This may create an incentive system that financially encourages RACs to assert improper payment, and the current system lacks both transparency and clear performance metrics for auditors. Of particular concern are Medicare Part A complex reviews, the most fiscally impactful area of RAC activity. According to CMS FY 2013 data, 41.1% of all claims with collections were complex reviews, yet these claims accounted for almost all (95.2%) of total dollars recovered by the RACs, with almost all (96%) dollars recovered being from Part A claims.[9] Complex reviews involve an auditor retrospectively and manually reviewing a medical record and then using his or her clinical and related professional judgment to decide whether the care was medically necessary. This is compared to automated coding or billing reviews, which are based solely on claims data.
Increased RAC activity and the willingness of hospitals to challenge RAC findings of improper payment has led to an increase in appeals volume that has overloaded the appeals process. On March 13, 2013, CMS offered hospitals the ability to rebill Medicare Part B as an appeals alternative.[10] This did not temper level 3 appeals requests received by the OMHA, which increased from 1250 per week in January 2012 to over 15,000 per week by November 2013.[11] Citing an overwhelmingly increased rate of appeal submissions and the resultant backlog, the OMHA decided to freeze new hospital appeals assignments in December 2013.[11] In another attempt to clear the backlog, on August 29, 2014, CMS offered a settlement that would pay hospitals 68% of the net allowable amount of the original Part A claim (minus any beneficiary deductibles) if a hospital agreed to concede all of its eligible appeals.[12] Notably, cases settled under this agreement would remain officially categorized as denied for improper payment.
The HHS Office of Inspector General (OIG)[4] and the CMS[9, 13, 14] have produced recent reports of RAC auditing and appeals activity that contain variable numbers that conflict with hospital accounts of auditing and appeals activity.[15, 16] In addition to these conflicting reports, little is known about RAC auditing of individual programs over time, the length of time cases spend in appeals, and staff required to navigate the audit and appeals processes. Given these questions, and the importance of RAC auditing pressure in the growth of hospital observation care, we conducted a retrospective descriptive study of all RAC activity for complex Medicare Part A alleged overpayment determinations at the Johns Hopkins Hospital, the University of Utah, and University of Wisconsin Hospital and Clinics for calendar years 2010 to 2013.
METHODS
The University of Wisconsin‐Madison Health Sciences institutional review board (IRB) and the Johns Hopkins Hospital IRB did not require review of this study. The University of Utah received an exemption. All 3 hospitals are tertiary care academic medical centers. The University of Wisconsin Hospital and Clinics (UWHC) is a 592‐bed hospital located in Madison, Wisconsin,[17] the Johns Hopkins Hospital (JHH) is a 1145‐bed medical center located in Baltimore, Maryland,[18] and the University of Utah Hospital (UU) is a 770‐bed facility in Salt Lake City, Utah (information available upon request). Each hospital is under a different RAC, representing 3 of the 4 RAC regions, and each is under a different Medicare Administrative Contractor, contractors responsible for level 1 appeals. The 3 hospitals have the same Qualified Independent Contractor responsible for level 2 appeals.
For the purposes of this study, any chart or medical record requested for review by an RAC was considered a medical necessity chart request or an audit. The terms overpayment determinations and denials were used interchangeably to describe audits the RACs alleged did not meet medical necessity for Medicare Part A billing. As previously described, the term medical necessity specifically considered not only whether actual medical services were appropriate, but also whether the services were delivered in the appropriate status, outpatient or inpatient. Appeals and/or request for discussion were cases where the overpayment determination was disputed and challenged by the hospital.
All complex review Medicare Part A RAC medical record requests by date of RAC request from the official start of the RAC program, January 1, 2010,[4] to December 31, 2013, were included in this study. Medical record requests for automated reviews that related to coding and billing clarifications were not included in this study, nor were complex Medicare Part B reviews, complex reviews for inpatient rehabilitation facilities, or psychiatric day hospitalizations. Notably, JHH is a Periodic Interim Payment (PIP) Medicare hospital, which is a reimbursement mechanism where biweekly payments [are] made to a Provider enrolled in the PIP program, and are based on the hospital's estimate of applicable Medicare reimbursement for the current cost report period.[19] Because PIP payments are made collectively to the hospital based on historical data, adjustments for individual inpatients could not be easily adjudicated and processed. Due to the increased complexity of this reimbursement mechanism, RAC audits did not begin at JHH until 2012. In addition, in contrast to the other 2 institutions, all of the RAC complex review audits at JHH in 2013 were for Part B cases, such as disputing need for intensity‐modulated radiation therapy versus conventional radiation therapy, or contesting the medical necessity of blepharoplasty. As a result, JHH had complex Part A review audits only for 2012 during the study time period. All data were deidentified prior to review by investigators.
As RACs can audit charts for up to 3 years after the bill is submitted,[13] a chart request in 2013 may represent a 2010 hospitalization, but for purposes of this study, was logged as a 2013 case. There currently is no standard methodology to calculate time spent in appeals. The UWHC and JHH calculate time in discussion or appeals from the day the discussion or appeal was initiated by the hospital, and the UU calculates the time in appeals from the date of the findings letter from the RAC, which makes comparable recorded time in appeals longer at UU (estimated 510 days for 20112013 cases, up to 120 days for 2010 cases).Time in appeals includes all cases that remain in the discussion or appeals process as of June 30, 2014.
The RAC process is as follows (Tables 1 and 2):
- The RAC requests hospital claims (RAC Medical Necessity Chart Requests [Audits]).
- The RAC either concludes the hospital claim was compliant as filed/paid and the process ends or the RAC asserts improper payment and requests repayment (RAC Overpayment Determinations of Requested Charts [Denials]).
- The hospital makes an initial decision to not contest the RAC decision (and repay), or to dispute the decision (Hospital Disputes Overpayment Determination [Appeal/Discussion]). Prior to filing an appeal, the hospital may request a discussion of the case with an RAC medical director, during which the RAC medical director can overturn the original determination. If the RAC declines to overturn the decision in discussion, the hospital may proceed with a formal appeal. Although CMS does not calculate the discussion period as part of the appeals process,[12] overpayment determinations contested by the hospital in either discussion or appeal represent the sum total of RAC denials disputed by the hospital.
Contested cases have 1 of 4 outcomes:
Contested overpayment determinations can be decided in favor of the hospital (Discussion or Appeal Decided in Favor of Hospital or RAC Withdrew)
- Contested overpayment determinations can be decided in favor of the RAC during the appeal process, and either the hospital exhausts the appeal process or elects not to take the appeal to the next level. Although the appeals process has 5 levels, no cases at our 3 hospitals have reached level 4 or 5, so cases without a decision to date remain in appeals at 1 of the first 3 levels (Case Still in Discussion or Appeals).[4]
- Hospital may miss an appeal deadline (Hospital Missed Appeal Deadline at Any Level) and the case is automatically decided in favor of the RAC.
- As of March 13, 2013,[10] for appeals that meet certain criteria and involve dispute over the billing of hospital services under Part A, CMS allowed hospitals to withdraw an appeal and rebill Medicare Part B. Prior to this time, hospitals could rebill for a very limited list of ancillary Part B Only services, and only within the 1‐year timely filing period.[13] Due to the lengthy appeals process and associated legal and administrative costs, hospitals may not agree with the RAC determination but make a business decision to recoup some payment under this mechanism (Hospital Chose to Rebill as Part B During Discussion or Appeals Process).
| Totals | Johns Hopkins Hospital | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | All Years | 2010 | 2011 | 2012 | 2013 | All Years | ||
| University of Wisconsin Hospital and Clinics | University of Utah | ||||||||||
| 2010 | 2011 | 2012 | 2013 | All Years | 2010 | 2011 | 2012 | 2013 | All Years | ||
| |||||||||||
| Total no. of Medicare encounters | 24,400 | 24,998 | 25,370 | 27,094 | 101,862 | 11,212b | 11,750b | 11,842 | 12,674c | 47,478 | |
| RAC Medical Necessity Chart Requests (Audits) | 547 | 1,735 | 3,887 | 1,941 | 8,110 (8.0%) | 0 | 0 | 938 | 0 | 938 (2.0%) | |
| RAC Overpayment Determinations Of Requested Charts (Denials)d | 164 (30.0%) | 516 (29.7%) | 1,200 (30.9%) | 656 (33.8%) | 2,536 (31.3%) | 0 (0%) | 0 (0%) | 432 (46.1%) | 0 (0%) | 432 (46.1%) | |
| Hospital Disputes Overpayment Determination (Appeal/Discussion) | 128 (78.0%) | 409 (79.3%) | 1,129 (94.1%) | 643 (98.0%) | 2,309 (91.0% | 0 (0%) | 0 (0%) | 431 (99.8%) | 0 (0%) | 431 (99.8%) | |
| Outcome of Disputed Overpayment Determinatione | |||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (0.2%) | 13 (1.2%) | 4 (0.6%) | 18 (0.8%) | 0 (0%) | 0 (0%) | 0 (0.0%) | 0 (0%) | 0 (0.0%) | |
| Hospital Chose To Rebill as Part B During Discussion Or Appeals Process | 80 (62.5%) | 202 (49.4%) | 511 (45.3%) | 158 (24.6%) | 951 (41.2%) | 0 (0%) | 0 (0%) | 208 (48.3%) | 0 (0%) | 208 (48.3%) | |
| Discussion or Appeal Decided In Favor Of Hospital or RAC Withdrewf | 45 (35.2%) | 127 (31.1%) | 449 (39.8%) | 345 (53.7%) | 966 (41.8%) | 0 (0%) | 0 (0%) | 151 (35.0%) | 0 (0%) | 151 (35.0%) | |
| Case Still in Discussion or Appeals | 3 (2.3%) | 79 (19.3%) | 156 13.8%) | 136 (21.2%) | 374 (16.2%) | 0 (0%) | 0 (0%) | 72 (16.7%) | 0 (0%) | 72 (16.7%) | |
| Mean Time for Cases Still in Discussion or Appeals, d (SD) | 1208 (41) | 958 (79) | 518 (125) | 350 (101) | 555 (255) | N/A | N/A | 478 (164) | N/A | 478 (164) | |
| Total no. of Medicare encounters l | 8,096 | 8,038 | 8,429 | 9,086 | 33,649 | 5,092 | 5,210 | 5,099 | 5,334 | 20,735 | |
| RAC Medical Necessity Chart Requests (Audits) | 15 | 526 | 1,484 | 960 | 2,985 (8.9%) | 532 | 1,209 | 1,465 | 981 | 4,187 (20.2%) | |
| RAC Overpayment Determinations of Requested Charts (Denials)bd | 3 (20.0%) | 147 (27.9%) | 240 (16.2%) | 164 (17.1%) | 554 (18.6%) | 161 (30.3%) | 369 (30.5%) | 528 (36.0%) | 492 (50.2%) | 1,550 (37.0%) | |
| Hospital Disputes Overpayment Determination (Appeal/Discussion) | 1 (33.3%) | 71 (48.3%) | 170 (70.8%) | 151 (92.1%) | 393 (70.9%) | 127 (78.9%) | 338 (91.6%) | 528 (100.0%) | 492 (100.0%) | 1,485 (95.8%) | |
| Outcome of Disputed Overpayment Determinatione | |||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 4 (2.6%) | 5 (1.3%) | 0 (0.0%) | 0 (0.0%) | 13 (2.5%) | 0 (0.0%) | 13 (0.9%) | |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 1 (100%) | 3 (4.2%) | 13 (7.6%) | 3 (2.0%) | 20 (5.1%) | 79 (62.2%) | 199 (58.9%) | 290 (54.9%) | 155 (31.5%) | 723 (48.7%) | |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrewf | 0 (0.0%) | 44 (62.0%) | 123 (72.4%) | 93 (61.6%) | 260 (66.2%) | 45 (35.4%) | 83 (24.6%) | 175 (33.1%) | 252 (51.2%) | 555 (37.4%) | |
| Case Still in Discussion or Appeals | 0 0.0% | 23 (32.4%) | 34 (20.0%) | 51 (33.8%) | 108 (27.5%) | 3 (2.4%) | 56 (16.6%) | 50 (9.5%) | 85 (17.3%) | 194 (13.1%) | |
| Mean Time for Cases Still in Discussion or Appeals, d (SD) | N/A | 926 (70) | 564 (90) | 323 (134) | 528 (258) | 1,208 (41) | 970 (80) | 544 (25) | 365 (72) | 599 (273) | |
| 2010 | 2011 | 2012 | 2013 | All | 2010 | 2011 | 2012 | 2013 | All | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Appeals With Decisions | Johns Hopkins Hospital | |||||||||
| Total no. | 125 | 330 | 973 | 507 | 1,935 | 0 | 0 | 359 | 0 | 359 |
| ||||||||||
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (0.3%) | 13 (1.3%) | 4 (0.8%) | 18 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 80 (64.0%) | 202 (61.2%) | 511 (52.5%) | 158 (31.2%) | 951 (49.1%) | 0 (0.0%) | 0 (0.0%) | 208 (57.9%) | 0 (0.0%) | 208 (57.9%) |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrew | 45 (36.0%) | 127 (38.5%) | 449 (46.1%) | 345 (68.0%) | 966 (49.9%) | 0 (0.0%) | 0 (0.0%) | 151 (42.1%) | 0 (0.0%) | 151 (42.1%) |
| Discussion Period and RAC Withdrawals | 0 (0.0%) | 59 (17.9%) | 351 (36.1%) | 235 (46.4%) | 645 (33.3%) | 0 (0.0%) | 0 (0.0%) | 139 (38.7%) | 0 (0.0%) | 139 (38.7%) |
| Level 1 Appeal | 10 (8.0%) | 22 (6.7%) | 60 (6.2%) | 62 (12.2%)1 | 154 (8.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.6%) | 0 (0.0%) | 2 (0.6%) |
| Level 2 Appeal | 22 (17.6%) | 36 (10.9%) | 38 (3.9%) | 48 (9.5%)1 | 144 (7.4%) | 0 (0.0%) | 0 (0.0%) | 10 (2.8%) | 0 (0.0%) | 10 (2.8%) |
| Level 3 Appealc | 13 (10.4%) | 10 (3.0%) | N/A (N/A) | N/A (N/A) | 23 (1.2%) | 0 (0.0%) | 0 (0.0%) | N/A (N/A) | 0 (0.0%) | 0 (0.0%) |
| 2010 | 2011 | 2012 | 2013 | All | 2010 | 2011 | 2012 | 2013 | All | |
| University of Wisconsin Hospital and Clinics | University of Utah | |||||||||
| Total no. | 1 | 48 | 136 | 100 | 285 | 124 | 282 | 478 | 407 | 1,291 |
| Hospital Missed Appeal Deadline at Any Level | 0 (0.0%) | 1 (2.1% | 0 (0.0%) | 4 (4.0%) | 5 (1.8%) | 0 (0.0%) | 0 (0.0%) | 13 (2.7%) | 0 (0.0%) | 13 (1.0%) |
| Hospital Chose to Rebill as Part B During Discussion or Appeals Process | 1 (100.0%) | 3 (6.3% | 13 (9.6%) | 3 (3.0%) | 20 (7.0%) | 79 (63.7%) | 199 (70.6%) | 290 (60.7%) | 155 (38.1%) | 723 (56.0%) |
| Discussion or Appeal Decided in Favor of Hospital or RAC Withdrewb | 0 (0.0%) | 44 (91.7%) | 123 (90.4%) | 93 (93.0%) | 260 (91.2%) | 45 (36.3%) | 83 (29.4%) | 175 (36.6%) | 252 (61.9%) | 555 (43.0%) |
| Discussion Period and RAC Withdrawals | 0 (0.0%) | 38 (79.2%) | 66 (48.5%) | 44 (44.0%) | 148 (51.9% | 0 (0.0%) | 21 (7.4%) | 146 (30.5%) | 191 (46.9%) | 358 (27.7%) |
| Level 1 Appeal | 0 (0.0%) | 2 (4.2%) | 47 (34.6%) | 34 (34.0%) | 83 (29.1%) | 10 (8.1%) | 20 (7.1%) | 11 (2.3%) | 28 (6.9%) | 69 (5.3%) |
| Level 2 Appeal | 0 (0.0%) | 4 (8.3%) | 10 (7.4%) | 15 (15.0%) | 29 (10.2%) | 22 (17.7%) | 32 (11.3%) | 18 (3.8%) | 33 (8.1%) | 105 (8.1%) |
| Level 3 Appealc | 0 (0.0%) | N/A (N/A) | N/A (N/A) | N/A (N/A) | 0 (0.0%) | 13 (10.5%) | 10 (3.5%) | N/A (N/A) | N/A(N/A) | 23 (1.8%) |
The administration at each hospital provided labor estimates for workforce dedicated to the review process generated by the RACs based on hourly accounting of one‐quarter of work during 2012, updated to FY 2014 accounting (Table 3). Concurrent case management status determination work was not included in these numbers due to the difficulty in solely attributing concurrent review workforce numbers to the RACs, as concurrent case management is a CMS Condition of Participation irrespective of the RAC program.
| JHH | UWHC | UU | Mean | |
|---|---|---|---|---|
| ||||
| Physicians: assist with status determinations, audits, and appeals | 1.0 | 0.5 | 0.6 | 0.7 |
| Nursing administration: audit and appeal preparation | 0.9 | 0.2 | 1.9 | 1.0 |
| Legal counsel: assist with rules interpretation, audit, and appeal preparation | 0.2 | 0.3 | 0.1 | 0.2 |
| Data analyst: prepare and track reports of audit and appeals | 2.0 | 1.8 | 2.4 | 2.0 |
| Administration and other directors | 2.3 | 0.9 | 0.3 | 1.2 |
| Total FTE workforce | 6.4 | 3.7 | 5.3 | 5.1 |
Statistics
Descriptive statistics were used to describe the data. Staffing numbers are expressed as full‐time equivalents (FTE).
RESULTS
Yearly Medicare Encounters and RAC Activity of Part A Complex Reviews
RACs audited 8.0% (8110/101,862) of inpatient Medicare cases, alleged noncompliance (all overpayments) for 31.3% (2536/8110) of Part A complex review cases requested, and the hospitals disputed 91.0% (2309/2536) of these assertions. None of these cases of alleged noncompliance claimed the actual medical services were unnecessary. Rather, every Part A complex review overpayment determination by all 3 RACs contested medical necessity related to outpatient versus inpatient status. In 2010 and 2011, there were in aggregate fewer audits (2282), overpayment determinations (680), and appeals or discussion requests (537 of 680, 79.0%), compared to audits (5828), overpayment determinations (1856), and appeals or discussion requests (1772 of 1856, 95.5%) in 2012 and 2013. The hospitals appealed or requested discussion of a greater percentage each successive year (2010, 78.0%; 2011, 79.3%; 2012, 94.1%; and 2013, 98.0%). This increased RAC activity, and hospital willingness to dispute the RAC overpayment determinations equaled a more than 300% increase in appeals and discussion request volume related to Part A complex review audits in just 2 years.
The 16.2% (374/2309) of disputed cases still under discussion or appeal have spent an average mean of 555 days (standard deviation 255 days) without a decision, with time in appeals exceeding 900 days for cases from 2010 and 2011. Notably, the 3 programs were subject to Part A complex review audits at widely different rates (Table 1).
Yearly RAC Part A Complex Review Overpayment Determinations Disputed by Hospitals With Decisions
The hospitals won, either in discussion or appeal, a combined greater percentage of contested overpayment determinations annually, from 36.0% (45/125) in 2010, to 38.5% (127/330) in 2011, to 46.1% (449/973) in 2012, to 68.0% (345/507) in 2013. Overall, for 49.1% (951/1935) of cases with decisions, the hospitals withdrew or rebilled under Part B at some point in the discussion or appeals process to avoid the lengthy appeals process and/or loss of the amount of the entire claim. A total of 49.9% (966/1935) of appeals with decisions have been won in discussion or appeal over the 4‐year study period. One‐third of all resolved cases (33.3%, 645/1935) were decided in favor of the hospital in the discussion period, with these discussion cases accounting for two‐thirds (66.8%, 645/966) of all favorable resolved cases for the hospital. Importantly, if cases overturned in discussion were omitted as they are in federal reports, the hospitals' success rate would fall to 16.6% (321/1935), a number similar to those that appear in annual CMS reports.[9, 13, 14] The hospitals also conceded 18 cases (0.9%) by missing a filing deadline (Table 2).
Estimated Workforce Dedicated to Part A Complex Review Medical Necessity Audits and Appeals
The institutions each employ an average of 5.1 FTE staff to manage the audit and appeal process, a number that does not include concurrent case management staff who assist in daily status determinations (Table 3).
CONCLUSIONS
In this study of 3 academic medical centers, there was a more than 2‐fold increase in RAC audits and a nearly 3‐fold rise in overpayment determinations over the last 2 calendar years of the study, resulting in a more than 3‐fold increase in appeals or requests for discussion in 2012 to 2013 compared to 2010 to 2011. In addition, although CMS manually reviews less than 0.3% of submitted claims each year through programs such as the Recovery Audit Program,[9] at the study hospitals, complex Part A RAC audits occurred at a rate more than 25 times that (8.0%), suggesting that these types of claims are a disproportionate focus of auditing activity. The high overall complex Part A audit rate, accompanied by acceleration of RAC activity and the hospitals' increased willingness to dispute RAC overpayment determinations each year, if representative of similar institutions, would explain the appeals backlog, most notably at the ALJ (level 3) level. Importantly, none of these Part A complex review denials contested a need for the medical care delivered, demonstrating that much of the RAC process at the hospitals focused exclusively on the nuances of medical necessity and variation in interpretation of CMS guidelines that related to whether hospital care should be provided under inpatient or outpatient status.
These data also show continued aggressive RAC audit activity despite an increasing overturn rate in favor of the hospitals in discussion or on appeal each year (from 36.0% in 2010 to 68.0% in 2013). The majority of the hospitals' successful decisions occurred in the discussion period, when the hospital had the opportunity to review the denial with the RAC medical director, a physician, prior to beginning the official appeals process. The 33% overturn rate found in the discussion period represents an error rate by the initial RAC auditors that was internally verified by the RAC medical director. The RAC internal error rate was replicated at 3 different RACs, highlighting internal process problems across the RAC system. This is concerning, because the discussion period is not considered part of the formal appeals process, so these cases are not appearing in CMS or OIG reports of RAC activity, leading to an underestimation of the true successful overturned denial rates at the 3 study hospitals, and likely many other hospitals.
The study hospitals are also being denied timely due process and payments for services delivered. The hospitals currently face an appeals process that, on average, far exceeds 500 days. In almost half of the contested overpayment determinations, the hospitals withdrew a case or rebilled Part B, not due to agreement with a RAC determination, but to avoid the lengthy, cumbersome, and expensive appeals process and/or to minimize the risk of losing the amount of the entire Part A claim. This is concerning, as cases withdrawn in the appeals process are considered improper payments in federal reports, despite a large number of these cases being withdrawn simply to avoid an inefficient appeals process. Notably, Medicare is not adhering to its own rules, which require appeals to be heard in a timely manner, specifically 60 days for level 1 or 2 appeals, and 90 days for a level 3 appeal,[6, 20] even though the hospitals lost the ability to appeal cases when they missed a deadline. Even if hospitals agreed to the recent 68% settlement offer[12] from CMS, appeals may reaccumulate without auditing reform. As noted earlier, this recent settlement offer came more than a year after the enhanced ability to rebill denied Part A claims for Part B, yet the backlog remains.
This study also showed that a large hospital workforce is required to manage the lengthy audit and appeals process generated by RACs. These staff are paid with funds that could be used to provide direct patient care or internal process improvement. The federal government also directly pays for unchecked RAC activity through the complex appeals process. Any report of dollars that RACs recoup for the federal government should be considered in light of their administrative costs to hospitals and government contractors, and direct costs at the federal level.
This study also showed that RACs audited the 3 institutions differently, despite similar willingness of the hospitals to dispute overpayment determinations and similar hospital success rates in appeals or discussion, suggesting that hospital compliance with Medicare policy was not the driver of variable RAC activity. This variation may be due to factors not apparent in this study, such as variable RAC interpretation of federal policy, a decision of a particular RAC to focus on complex Medicare Part B or automated reviews instead of complex Part A reviews, or RAC workforce differences that are not specific to the hospitals. Regardless, the variation in audit activity suggests that greater transparency and accountability in RAC activity is merited.
Perhaps most importantly, this study highlights factors that may help explain differing auditing and appeals numbers reported by the OIG,[4] CMS,[9, 13, 14] and hospitals.[15, 16] Given the marked increase in RAC activity over the last 4 years, the 2010 and 2011 data included in a recent OIG report[4] likely do not represent current auditing and appeals practice. With regard to the CMS reports,[9, 13, 14] although CMS included FY 2013[9] activity in its most recent report, it did not account for denials overturned in the discussion period, as these are not technically appeals, even though these are contested cases decided in favor of the hospital. This most recent CMS report[9] uses overpayment determinations from FY 2013, yet counts appeals and decisions that occurred in 2013, with the comment that these decisions may be for overpayment determinations prior to 2013. The CMS reports also variably combine automated, semiautomated, complex Part A, and complex Part B claims in its reports, making interpretation challenging. Finally, although CMS reported an increase in improper payments recovered from FY 2011[14] ($939 million) to FY 2012[13] ($2.4 billion) to FY 2013[9] ($3.75 billion), this is at least partly a reflection of increased RAC activity as demonstrated in this study, and may reflect the fact that many hospitals do not have the resources to continually appeal or choose not to contest these cases based on a financial business decision. Importantly, these numbers now far exceed recoupment in other quality programs, such as the Readmissions Reduction Program (estimated $428 million next FY),[21] indicating the increased fiscal impact of the RAC program on hospital reimbursement.
To increase accuracy, future federal reports of auditing and appeals should detail and include cases overturned in the discussion period, and carefully describe the denominator of total audits and appeals given the likelihood that many appeals in a given year will not have a decision in that year. Percent of total Medicare claims subject to complex Part A audit should be stated. Reports should also identify and consider an alternative classification for complex Part A cases the hospital elects to rebill under Medicare Part B, and also detail on what grounds medical necessity is being contested (eg, whether the actual care delivered was not necessary or if it is an outpatient versus inpatient billing issue). Time spent in the appeals process must also be reported. Complex Part A, complex Part B, semiautomated, and automated reviews should also be considered separately, and dates of reported audits and appeals must be as current as possible in this rapidly changing environment.
In this study, RACs conducted complex Part A audits at a rate 25 times the CMS‐reported overall audit rate, confirming complex Part A audits are a particular focus of RAC activity. There was a more than doubling of RAC audits at the study hospitals from the years 2010 ‐ 2011 to 2012 ‐ 2013 and a nearly 3‐fold increase in overpayment determinations. Concomitantly, the more than 3‐fold increase in appeals and discussion volume over this same time period was consistent with the development of the current national appeals backlog. The 3 study hospitals won a greater percentage of contested cases each year, from approximately one‐third of cases in 2010 to two‐thirds of cases with decisions in 2013, but there was no appreciable decrease in RAC overpayment determinations over that time period. The majority of successfully challenged cases were won in discussion, favorable decisions for hospitals not appearing in federal appeals reports. Time in appeals exceeded 550 days, causing the hospitals to withdraw some cases to avoid the lengthy appeals process and/or to minimize the risk of losing the amount of the entire Part A claim. The hospitals also lost a small number of appeals by missing a filing deadline, yet there was no reciprocal case concession when the appeals system missed a deadline. RACs found no cases of care at the 3 hospitals that should not have been delivered, but rather challenged the status determination (inpatient vs outpatient) to dispute medical necessity of care delivered. Finally, an average of approximately 5 FTEs at each institution were employed in the audits and appeals process. These data support a need for systematic improvements in the RAC system so that fair, constructive, and cost‐efficient surveillance of the Medicare program can be realized.
Acknowledgements
The authors thank Becky Borchert, MS, RN BC, ACM, CPHQ, Program Manager for Medicare/Medicaid Utilization Review at the University of Wisconsin Hospital and Clinics; Carol Duhaney and Joan Kratz, RN, at Johns Hopkins Hospital; and Morgan Walker at the University of Utah for their assistance in data preparation and presentation. Without their meticulous work and invaluable assistance, this study would not have been possible. The authors also thank Josh Boswell, JD, for his critical review of the manuscript.
Disclosure: Nothing to report.
- Medicare Payment Advisory Commission. Hospital inpatient and observation services. 2014 Report to Congress. Medicare Payment Policy. Available at: http://www.medpac.gov/documents/reports/mar14_entirereport.pdf?sfvrsn=0. Accessed September 22, 2014.
- American Hospital Association “2‐midnight rule” lawsuit vs Department of Health and Human Services. Available at: http://www.aha.org/content/14/140414‐complaint‐2midnight.pdf. Accessed August 8, 2014.
- Centers for Medicare administrative law judge hearing program for Medicare claim appeals. Fed Regist. 2014;79(214): 65660 – 65663. Available at: http://www.hhs.gov/omha/files/omha_federal_register_notice_2014–26214.pdf. Accessed December 6, 2014.
- . Medicare fines 2,610 hospitals in third round of readmission penalties. Kaiser Health News. Available at: http://kaiserhealthnews.org/news/medicare‐readmissions‐penalties‐2015. Accessed November 30, 2014.
- Medicare Payment Advisory Commission. Hospital inpatient and observation services. 2014 Report to Congress. Medicare Payment Policy. Available at: http://www.medpac.gov/documents/reports/mar14_entirereport.pdf?sfvrsn=0. Accessed September 22, 2014.
- American Hospital Association “2‐midnight rule” lawsuit vs Department of Health and Human Services. Available at: http://www.aha.org/content/14/140414‐complaint‐2midnight.pdf. Accessed August 8, 2014.
- Centers for Medicare administrative law judge hearing program for Medicare claim appeals. Fed Regist. 2014;79(214): 65660 – 65663. Available at: http://www.hhs.gov/omha/files/omha_federal_register_notice_2014–26214.pdf. Accessed December 6, 2014.
- . Medicare fines 2,610 hospitals in third round of readmission penalties. Kaiser Health News. Available at: http://kaiserhealthnews.org/news/medicare‐readmissions‐penalties‐2015. Accessed November 30, 2014.
© 2015 Society of Hospital Medicine