User login
ObGyn Medicare and CPT coding changes that could affect your income in 2015
At least one, if not many, of the coding changes highlighted below is likely to modify the incomes of ObGyns in the upcoming year. Here, I outline the 2015 changes that are most likely to affect your practice to some degree.
Medicare changes kick off a melancholy 2015
Surgical global periods: A move to eliminate them is underway
I begin this article not with a new or revised code, but with an active proposal, which, if implemented, could adversely affect your surgical income in a few short years.
Starting in 2017, the Centers for Medicare and Medicaid Services (CMS) has indicated that it will change all surgical codes to 0-day global periods. They plan on starting by converting 10-day global codes to 0-day codes in 2017, and then move on to the conversion of 90-day global codes in 2018. This is being proposed because of an Office of Inspector General (OIG) finding that many surgeons are not providing the evaluation and management (E/M) services included in the surgical code; therefore, Medicare is reimbursing for surgical procedures at a higher rate than warranted. In addition, the number of assigned visits may no longer reflect current care protocols, which again may mean that Medicare is not paying appropriately.
The immediate effect of this proposal—which has been adopted in the final rule published in the November 13, 2014, Federal Register—would be a reduction in payment for the converted codes due to a decrease in the assigned relative value units (RVUs). In addition, surgeons would need to document and provide the level of service for all preoperative and postoperative care, which may lead some payers to begin scrutinizing both levels of service billed and frequency of visits before and after surgical procedures.
CMS is still looking for any additional comments from physicians on this conversion process and such comments can be submitted electronically through a link at www.regulations.gov. Reference the final rule as CMS-1612-FC in your reply.
Medicare reimbursements poised to decrease in April
The calendar year 2015 conversion factor will remain at $35.80 from January 1 through March 31, 2015, as mandated by section 101 of the Protecting Access to Medicare Act of 2014. This represents the amount that will be multiplied by the geographically adjusted RVU for a code to determine the final Medicare allowable per procedure or service billed. Effective April 1, 2015, the conversion factor based on the sustainable growth rate (SGR) formula will be only $28.22—representing a 21.2% decrease—unless Congress acts to override this mandate.
Code bundling leads to lost Medicare compensation
Hysterectomy bundling. Effective October 1, 2014, CMS began permanently bundling anterior/posterior colporrhaphy and colpopexy procedures into all vaginal and laparoscopic-assisted hysterectomy codes. By permanently, CMS means that no modifier can be used to report Current Procedural Terminology (CPT) code 57260 (anterior and posterior [A&P] repair) or codes 57280, 57282, 57283 (abdominal, and vaginal approach colpopexy procedures) separately when performed with a vaginal or laparoscopic-assisted hysterectomy.
The American Congress of Obstetricians and Gynecologists (ACOG) and the American Urogynecologic Society (AUGS) wrote to CMS in May with regard to these edits and objected to them strongly. These organizations will continue to work with CMS to get them removed. Until then, physicians who perform anterior/posterior colporrhaphy and colpopexy procedures with a vaginal or laparoscopic-assisted hysterectomy will need to clearly make a case in the operative report for the need to perform the additional procedures in order to add a modifier -22 (Increased procedural services) to the hysterectomy code for consideration of additional payment. If an A&P repair is performed, it would not be appropriate to bill only an anterior repair (CPT code 57240) or a posterior repair (CPT code 57250) to obtain some separate reimbursement as this would represent inaccurate coding.
Hysteroscopy bundling. Another edit that will affect ObGyns is the bundling of CPT code 58558 (Hysteroscopy, surgical; with sampling [biopsy] of endometrium and/or poly-pectomy, with or without D&C) into codes for hysteroscopic removal of a fibroid (58561) and the removal of an impacted foreign body (58562).
Previously, these codes could have been billed together, but now only the addition of a modifier -22 to the primary procedure (myomectomy or foreign body removal) presents any chance for additional reimbursement. In order to report the modifier, Medicare has indicated that the documentation must clearly support the additional work in accomplishing the primary procedure, including a statement of how much time it added to the normal procedure.
Awareness of new or revised CPT codes could benefit your earnings
The 2015 CPT code set includes several changes, including laboratory and vaccination codes, which may be of interest to your practice. Because of Health Insurance Portability and Accountability Act (HIPAA) requirements, insurers were required to accept new codes on January 1, 2015.
Added: Fetal chromosomal aneuploidy code for genomic sequencing
On January 1, 2014, CPT added a new code to report cell-free DNA to screen for fetal aneuploidy. This new code is 81507 (Fetal aneuploidy [trisomy 21, 18, and 13] DNA sequence analysis of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy), and it was added to report the Harmony™ Prenatal Test.
For 2015, another new code, 81420 (Fetal chromosomal aneuploidy [eg, trisomy 21, monosomy X] genomic sequence analysis panel, circulating cell-free fetal DNA in maternal blood, must include analysis of chromosomes 13, 18, and 21), was added. This code represents a more comprehensive analysis and would therefore not be reported or ordered with code 81507. This new code requires a genomic sequence analysis panel.
HPV revisions extend beyond new codes
The codes for HPV testing have been redefined. These codes have been deleted: 87620-87622 (Infectious agent detection by nucleic acid [DNA or RNA]; papillomavirus, human, direct probe technique/amplified probe technique/quantification). In their place are three new codes to choose from:
- 87623, Human papillomavirus (HPV), low-risk types (eg, 6, 11, 42, 43, 44)
- 87624, Human papillomavirus (HPV), high-risk types (eg, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68)
- 87625, Human papillomavirus (HPV), types 16 and 18 only, includes type 45, if performed.
This coding change may be significant for payment as some payers will cover testing for high-risk HPV types only, so be sure your practice management team is aware of the latest rules for ordering HPV testing for your patients. Otherwise, patients could be faced with unexpected out-of-pocket expenses.
Egg freezing recognized as mainstream
- Infertility laboratories will be pleased to learn that CPT has changed the status of the code for cryopreservation of oocytes from a Category III to a Category I code. This means that this technology has now proven itself as a mainstream procedure, warranting a Category I CPT code. The new code is 89337 (Cryopreservation, mature oocyte[s]), which replaces the deleted code 0059T (Cryopreservation; oocyte[s]).
Vaccination codes for 2015
Almost every year new codes are added, and 2015 is no different. This year, you will see codes for:
- 90651, Human papillomavirus vaccine types 6, 11, 16, 18, 31, 33, 45, 52, 58, nonavalent (HPV), 3 dose schedule, for intramuscular use
- 90630, Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, for intradermal use.
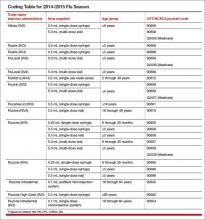
There is also a revision to the flu virus vaccine code 90654 to indicate that it represents a trivalent preservative-free vaccine. See the TABLE below for a complete list of all the vaccines by trade name and CPT/Medicare Healthcare Common Procedure Coding System (HCPCS) codes for the 2014−2015 flu season.
Keep in mind that reporting administration of the flu vaccine is different for Medicare than for private payers. Administration code G0008 and diagnosis code V04.81 (Need for prophylactic vaccination and inoculation against influenza) would be reported in conjunction with the appropriate vaccine code for Medicare, while CPT instructs you to report 90471 instead for the administration. When we switch to ICD-10 diagnostic coding on October 1, 2015, the code V04.81 becomes Z23 (Encounter for immunization).
Three new codes for anoscopy
The first two codes were formerly Category III codes representing new technology, but now have proven to be more mainstream.
- 46601, Anoscopy; diagnostic, with high-resolution magnification (HRA) (eg, colposcope, operating microscope) and chemical agent enhancement, including collection of specimen(s) by brushing or washing, when performed
- 46607, Anoscopy; diagnostic, with high-resolution magnification (HRA) (eg, colposcope, operating microscope) and chemical agent enhancement, with biopsy, single or multiple
- 0377T, Anoscopy with directed submucosal injection of bulking agent for fecal incontinence.
Replacement codes for vertebral fracture assessments
If your practice is performing or ordering vertebral assessments for patients, there are two new codes to report. The old code, 77082 (Dual-energy X-ray absorptiometry [DXA], bone density study, 1 or more sites; vertebral fracture assessment) for vertebral fracture assessment has been deleted and replaced with:
- 77085, Dual-energy X-ray absorptiometry (DXA), bone density study, 1 or more sites; axial skeleton (eg, hips, pelvis, spine), including vertebral fracture assessment
- 77086, Vertebral fracture assessment via dual-energy X-ray absorptiometry (DXA).
Coding for breast ultrasound and tomosynthesis get more descriptive—at least for private insurance
The CPT Editorial Panel created three codes to describe digital breast tomosynthesis services and two new codes for a breast ultrasound.
- 77061, Digital breast tomosynthesis; unilateral
- 77062, Digital breast tomosynthesis; bilateral
- 77063, Screening digital breast tomosynthesis, bilateral (List separately in addition to the code for the primary procedure.)
- 76641, Ultrasound, breast, unilateral, real time with image documentation, including axilla when performed; complete
- 76642, Ultrasound, breast, unilateral, … ; limited.
Medicare, on the other hand, has decided to create a G code for tomosynthesis, which is the only code that will be accepted for payment if the patient meets her high-risk criteria for performance of this test.
Under Medicare rules you would report/order the following codes for mammographic services:
- Film (use CPT codes)
- 77056, Mammography; bilateral
- 77057, Screening mammography, bilateral (2-view film study of each breast)
- 2D digital (use G0202, G0204, and G0206)
- G0204, Diagnostic mammography, producing direct digital image, bilateral, all views
- G0206, Diagnostic mammography, producing direct digital image, unilateral, all views
- 3D screening (use G0202 for 2D digital plus the new CPT code 77063 for 3D)
procedure.)
- 3D diagnostic (use G0204 or G0206 for the 2D digital plus the new G code G0279 for 3D)
Modifier 59 becomes more specific
Another Medicare coding change that may affect ObGyns is the addition of new Medicare modifiers that are intended to eventually replace the modifier -59. This new list of modifiers will need to be appended to bundled procedures to more clearly explain why the secondary procedures should be paid separately. At the most recent American Medical Association (AMA) CPT symposium in Chicago, Illinois, CMS medical directors indicated that the new modifiers should be used only when the clinician is given instructions to do so by the carrier. Until then, the modifier -59 should continue to be used by most clinicians.
Here are the new modifiers, with an example of their use with currently bundled procedures that allow a modifier -59 to be used under certain circumstances:
- -XE: Separate encounter (A service that is distinct because it occurred during a separate encounter.) For instance, a patient presents to the office in the morning to have an abscess on her labia near her urethra incised and drained (56405). She returns in the afternoon to have a temporary catheter inserted because she states she cannot urinate and you decide to put in a temporary Foley catheter (51702) until the swelling has gone down. Add the modifier XE to 51702 to indicate it was performed at a different patient encounter.
- -XP: Separate practitioner (A service that is distinct because it was performed by a different practitioner.) Normally, Medicare will reimburse an unaffiliated clinician for performing a procedure that is bundled, since the bundling edits apply to the billing surgeon. But when two physicians from the same practice each are performing a different procedure at the same operative session that would otherwise be bundled, this new modifier will make that clear.
For example, Dr. Bates is performing a laparoscopic paravaginal defect repair (57423) and calls Dr. Clark, a urogynecologist in his practice, to remove severe adhesions from the ureters. The claim should go in under the same tax ID number, with the code 50715 listed first (as it has greater RVUs) and code 57423 reported with the -XP modifier.
- -XS: Separate structure (A service that is distinct because it was performed on a separate organ/structure.) Dr. Scott is performing the removal of endometrial implants around the left fallopian tube and in the cul-de-sac and notices that the right fallopian tube appears closed. He performs chromotubation on the right fallopian tube and notes that the right tube is blocked. Billing in this case would be 58662, 58350-XS.
- -XU: Unusual non-overlapping service (The use of a service that is distinct because it does not overlap usual components of the main service.) Mary has Medicare coverage and presents at 20 weeks 4 days gestation with bleeding and labor pains. Her examination shows bulging membranes that rupture when you attempt to remove the cerclage suture. You note a large rent in the cervix, but cannot get to the cerclage sutures as the patient is in active labor and beginning to bear down. The fetus and placenta are delivered a short time later through the rent in the cervix. You repair the rent in the cervix following delivery.
In this case, code 59400-52 (reduced services since the patient delivered at 20 weeks and there were reduced antepartum services), and 57720-XU because the repair of the cervix is not part of the usual services for a vaginal delivery.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
At least one, if not many, of the coding changes highlighted below is likely to modify the incomes of ObGyns in the upcoming year. Here, I outline the 2015 changes that are most likely to affect your practice to some degree.
Medicare changes kick off a melancholy 2015
Surgical global periods: A move to eliminate them is underway
I begin this article not with a new or revised code, but with an active proposal, which, if implemented, could adversely affect your surgical income in a few short years.
Starting in 2017, the Centers for Medicare and Medicaid Services (CMS) has indicated that it will change all surgical codes to 0-day global periods. They plan on starting by converting 10-day global codes to 0-day codes in 2017, and then move on to the conversion of 90-day global codes in 2018. This is being proposed because of an Office of Inspector General (OIG) finding that many surgeons are not providing the evaluation and management (E/M) services included in the surgical code; therefore, Medicare is reimbursing for surgical procedures at a higher rate than warranted. In addition, the number of assigned visits may no longer reflect current care protocols, which again may mean that Medicare is not paying appropriately.
The immediate effect of this proposal—which has been adopted in the final rule published in the November 13, 2014, Federal Register—would be a reduction in payment for the converted codes due to a decrease in the assigned relative value units (RVUs). In addition, surgeons would need to document and provide the level of service for all preoperative and postoperative care, which may lead some payers to begin scrutinizing both levels of service billed and frequency of visits before and after surgical procedures.
CMS is still looking for any additional comments from physicians on this conversion process and such comments can be submitted electronically through a link at www.regulations.gov. Reference the final rule as CMS-1612-FC in your reply.
Medicare reimbursements poised to decrease in April
The calendar year 2015 conversion factor will remain at $35.80 from January 1 through March 31, 2015, as mandated by section 101 of the Protecting Access to Medicare Act of 2014. This represents the amount that will be multiplied by the geographically adjusted RVU for a code to determine the final Medicare allowable per procedure or service billed. Effective April 1, 2015, the conversion factor based on the sustainable growth rate (SGR) formula will be only $28.22—representing a 21.2% decrease—unless Congress acts to override this mandate.
Code bundling leads to lost Medicare compensation
Hysterectomy bundling. Effective October 1, 2014, CMS began permanently bundling anterior/posterior colporrhaphy and colpopexy procedures into all vaginal and laparoscopic-assisted hysterectomy codes. By permanently, CMS means that no modifier can be used to report Current Procedural Terminology (CPT) code 57260 (anterior and posterior [A&P] repair) or codes 57280, 57282, 57283 (abdominal, and vaginal approach colpopexy procedures) separately when performed with a vaginal or laparoscopic-assisted hysterectomy.
The American Congress of Obstetricians and Gynecologists (ACOG) and the American Urogynecologic Society (AUGS) wrote to CMS in May with regard to these edits and objected to them strongly. These organizations will continue to work with CMS to get them removed. Until then, physicians who perform anterior/posterior colporrhaphy and colpopexy procedures with a vaginal or laparoscopic-assisted hysterectomy will need to clearly make a case in the operative report for the need to perform the additional procedures in order to add a modifier -22 (Increased procedural services) to the hysterectomy code for consideration of additional payment. If an A&P repair is performed, it would not be appropriate to bill only an anterior repair (CPT code 57240) or a posterior repair (CPT code 57250) to obtain some separate reimbursement as this would represent inaccurate coding.
Hysteroscopy bundling. Another edit that will affect ObGyns is the bundling of CPT code 58558 (Hysteroscopy, surgical; with sampling [biopsy] of endometrium and/or poly-pectomy, with or without D&C) into codes for hysteroscopic removal of a fibroid (58561) and the removal of an impacted foreign body (58562).
Previously, these codes could have been billed together, but now only the addition of a modifier -22 to the primary procedure (myomectomy or foreign body removal) presents any chance for additional reimbursement. In order to report the modifier, Medicare has indicated that the documentation must clearly support the additional work in accomplishing the primary procedure, including a statement of how much time it added to the normal procedure.
Awareness of new or revised CPT codes could benefit your earnings
The 2015 CPT code set includes several changes, including laboratory and vaccination codes, which may be of interest to your practice. Because of Health Insurance Portability and Accountability Act (HIPAA) requirements, insurers were required to accept new codes on January 1, 2015.
Added: Fetal chromosomal aneuploidy code for genomic sequencing
On January 1, 2014, CPT added a new code to report cell-free DNA to screen for fetal aneuploidy. This new code is 81507 (Fetal aneuploidy [trisomy 21, 18, and 13] DNA sequence analysis of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy), and it was added to report the Harmony™ Prenatal Test.
For 2015, another new code, 81420 (Fetal chromosomal aneuploidy [eg, trisomy 21, monosomy X] genomic sequence analysis panel, circulating cell-free fetal DNA in maternal blood, must include analysis of chromosomes 13, 18, and 21), was added. This code represents a more comprehensive analysis and would therefore not be reported or ordered with code 81507. This new code requires a genomic sequence analysis panel.
HPV revisions extend beyond new codes
The codes for HPV testing have been redefined. These codes have been deleted: 87620-87622 (Infectious agent detection by nucleic acid [DNA or RNA]; papillomavirus, human, direct probe technique/amplified probe technique/quantification). In their place are three new codes to choose from:
- 87623, Human papillomavirus (HPV), low-risk types (eg, 6, 11, 42, 43, 44)
- 87624, Human papillomavirus (HPV), high-risk types (eg, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68)
- 87625, Human papillomavirus (HPV), types 16 and 18 only, includes type 45, if performed.
This coding change may be significant for payment as some payers will cover testing for high-risk HPV types only, so be sure your practice management team is aware of the latest rules for ordering HPV testing for your patients. Otherwise, patients could be faced with unexpected out-of-pocket expenses.
Egg freezing recognized as mainstream
- Infertility laboratories will be pleased to learn that CPT has changed the status of the code for cryopreservation of oocytes from a Category III to a Category I code. This means that this technology has now proven itself as a mainstream procedure, warranting a Category I CPT code. The new code is 89337 (Cryopreservation, mature oocyte[s]), which replaces the deleted code 0059T (Cryopreservation; oocyte[s]).
Vaccination codes for 2015
Almost every year new codes are added, and 2015 is no different. This year, you will see codes for:
- 90651, Human papillomavirus vaccine types 6, 11, 16, 18, 31, 33, 45, 52, 58, nonavalent (HPV), 3 dose schedule, for intramuscular use
- 90630, Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, for intradermal use.
There is also a revision to the flu virus vaccine code 90654 to indicate that it represents a trivalent preservative-free vaccine. See the TABLE below for a complete list of all the vaccines by trade name and CPT/Medicare Healthcare Common Procedure Coding System (HCPCS) codes for the 2014−2015 flu season.
Keep in mind that reporting administration of the flu vaccine is different for Medicare than for private payers. Administration code G0008 and diagnosis code V04.81 (Need for prophylactic vaccination and inoculation against influenza) would be reported in conjunction with the appropriate vaccine code for Medicare, while CPT instructs you to report 90471 instead for the administration. When we switch to ICD-10 diagnostic coding on October 1, 2015, the code V04.81 becomes Z23 (Encounter for immunization).
Three new codes for anoscopy
The first two codes were formerly Category III codes representing new technology, but now have proven to be more mainstream.
- 46601, Anoscopy; diagnostic, with high-resolution magnification (HRA) (eg, colposcope, operating microscope) and chemical agent enhancement, including collection of specimen(s) by brushing or washing, when performed
- 46607, Anoscopy; diagnostic, with high-resolution magnification (HRA) (eg, colposcope, operating microscope) and chemical agent enhancement, with biopsy, single or multiple
- 0377T, Anoscopy with directed submucosal injection of bulking agent for fecal incontinence.
Replacement codes for vertebral fracture assessments
If your practice is performing or ordering vertebral assessments for patients, there are two new codes to report. The old code, 77082 (Dual-energy X-ray absorptiometry [DXA], bone density study, 1 or more sites; vertebral fracture assessment) for vertebral fracture assessment has been deleted and replaced with:
- 77085, Dual-energy X-ray absorptiometry (DXA), bone density study, 1 or more sites; axial skeleton (eg, hips, pelvis, spine), including vertebral fracture assessment
- 77086, Vertebral fracture assessment via dual-energy X-ray absorptiometry (DXA).
Coding for breast ultrasound and tomosynthesis get more descriptive—at least for private insurance
The CPT Editorial Panel created three codes to describe digital breast tomosynthesis services and two new codes for a breast ultrasound.
- 77061, Digital breast tomosynthesis; unilateral
- 77062, Digital breast tomosynthesis; bilateral
- 77063, Screening digital breast tomosynthesis, bilateral (List separately in addition to the code for the primary procedure.)
- 76641, Ultrasound, breast, unilateral, real time with image documentation, including axilla when performed; complete
- 76642, Ultrasound, breast, unilateral, … ; limited.
Medicare, on the other hand, has decided to create a G code for tomosynthesis, which is the only code that will be accepted for payment if the patient meets her high-risk criteria for performance of this test.
Under Medicare rules you would report/order the following codes for mammographic services:
- Film (use CPT codes)
- 77056, Mammography; bilateral
- 77057, Screening mammography, bilateral (2-view film study of each breast)
- 2D digital (use G0202, G0204, and G0206)
- G0204, Diagnostic mammography, producing direct digital image, bilateral, all views
- G0206, Diagnostic mammography, producing direct digital image, unilateral, all views
- 3D screening (use G0202 for 2D digital plus the new CPT code 77063 for 3D)
procedure.)
- 3D diagnostic (use G0204 or G0206 for the 2D digital plus the new G code G0279 for 3D)
Modifier 59 becomes more specific
Another Medicare coding change that may affect ObGyns is the addition of new Medicare modifiers that are intended to eventually replace the modifier -59. This new list of modifiers will need to be appended to bundled procedures to more clearly explain why the secondary procedures should be paid separately. At the most recent American Medical Association (AMA) CPT symposium in Chicago, Illinois, CMS medical directors indicated that the new modifiers should be used only when the clinician is given instructions to do so by the carrier. Until then, the modifier -59 should continue to be used by most clinicians.
Here are the new modifiers, with an example of their use with currently bundled procedures that allow a modifier -59 to be used under certain circumstances:
- -XE: Separate encounter (A service that is distinct because it occurred during a separate encounter.) For instance, a patient presents to the office in the morning to have an abscess on her labia near her urethra incised and drained (56405). She returns in the afternoon to have a temporary catheter inserted because she states she cannot urinate and you decide to put in a temporary Foley catheter (51702) until the swelling has gone down. Add the modifier XE to 51702 to indicate it was performed at a different patient encounter.
- -XP: Separate practitioner (A service that is distinct because it was performed by a different practitioner.) Normally, Medicare will reimburse an unaffiliated clinician for performing a procedure that is bundled, since the bundling edits apply to the billing surgeon. But when two physicians from the same practice each are performing a different procedure at the same operative session that would otherwise be bundled, this new modifier will make that clear.
For example, Dr. Bates is performing a laparoscopic paravaginal defect repair (57423) and calls Dr. Clark, a urogynecologist in his practice, to remove severe adhesions from the ureters. The claim should go in under the same tax ID number, with the code 50715 listed first (as it has greater RVUs) and code 57423 reported with the -XP modifier.
- -XS: Separate structure (A service that is distinct because it was performed on a separate organ/structure.) Dr. Scott is performing the removal of endometrial implants around the left fallopian tube and in the cul-de-sac and notices that the right fallopian tube appears closed. He performs chromotubation on the right fallopian tube and notes that the right tube is blocked. Billing in this case would be 58662, 58350-XS.
- -XU: Unusual non-overlapping service (The use of a service that is distinct because it does not overlap usual components of the main service.) Mary has Medicare coverage and presents at 20 weeks 4 days gestation with bleeding and labor pains. Her examination shows bulging membranes that rupture when you attempt to remove the cerclage suture. You note a large rent in the cervix, but cannot get to the cerclage sutures as the patient is in active labor and beginning to bear down. The fetus and placenta are delivered a short time later through the rent in the cervix. You repair the rent in the cervix following delivery.
In this case, code 59400-52 (reduced services since the patient delivered at 20 weeks and there were reduced antepartum services), and 57720-XU because the repair of the cervix is not part of the usual services for a vaginal delivery.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
At least one, if not many, of the coding changes highlighted below is likely to modify the incomes of ObGyns in the upcoming year. Here, I outline the 2015 changes that are most likely to affect your practice to some degree.
Medicare changes kick off a melancholy 2015
Surgical global periods: A move to eliminate them is underway
I begin this article not with a new or revised code, but with an active proposal, which, if implemented, could adversely affect your surgical income in a few short years.
Starting in 2017, the Centers for Medicare and Medicaid Services (CMS) has indicated that it will change all surgical codes to 0-day global periods. They plan on starting by converting 10-day global codes to 0-day codes in 2017, and then move on to the conversion of 90-day global codes in 2018. This is being proposed because of an Office of Inspector General (OIG) finding that many surgeons are not providing the evaluation and management (E/M) services included in the surgical code; therefore, Medicare is reimbursing for surgical procedures at a higher rate than warranted. In addition, the number of assigned visits may no longer reflect current care protocols, which again may mean that Medicare is not paying appropriately.
The immediate effect of this proposal—which has been adopted in the final rule published in the November 13, 2014, Federal Register—would be a reduction in payment for the converted codes due to a decrease in the assigned relative value units (RVUs). In addition, surgeons would need to document and provide the level of service for all preoperative and postoperative care, which may lead some payers to begin scrutinizing both levels of service billed and frequency of visits before and after surgical procedures.
CMS is still looking for any additional comments from physicians on this conversion process and such comments can be submitted electronically through a link at www.regulations.gov. Reference the final rule as CMS-1612-FC in your reply.
Medicare reimbursements poised to decrease in April
The calendar year 2015 conversion factor will remain at $35.80 from January 1 through March 31, 2015, as mandated by section 101 of the Protecting Access to Medicare Act of 2014. This represents the amount that will be multiplied by the geographically adjusted RVU for a code to determine the final Medicare allowable per procedure or service billed. Effective April 1, 2015, the conversion factor based on the sustainable growth rate (SGR) formula will be only $28.22—representing a 21.2% decrease—unless Congress acts to override this mandate.
Code bundling leads to lost Medicare compensation
Hysterectomy bundling. Effective October 1, 2014, CMS began permanently bundling anterior/posterior colporrhaphy and colpopexy procedures into all vaginal and laparoscopic-assisted hysterectomy codes. By permanently, CMS means that no modifier can be used to report Current Procedural Terminology (CPT) code 57260 (anterior and posterior [A&P] repair) or codes 57280, 57282, 57283 (abdominal, and vaginal approach colpopexy procedures) separately when performed with a vaginal or laparoscopic-assisted hysterectomy.
The American Congress of Obstetricians and Gynecologists (ACOG) and the American Urogynecologic Society (AUGS) wrote to CMS in May with regard to these edits and objected to them strongly. These organizations will continue to work with CMS to get them removed. Until then, physicians who perform anterior/posterior colporrhaphy and colpopexy procedures with a vaginal or laparoscopic-assisted hysterectomy will need to clearly make a case in the operative report for the need to perform the additional procedures in order to add a modifier -22 (Increased procedural services) to the hysterectomy code for consideration of additional payment. If an A&P repair is performed, it would not be appropriate to bill only an anterior repair (CPT code 57240) or a posterior repair (CPT code 57250) to obtain some separate reimbursement as this would represent inaccurate coding.
Hysteroscopy bundling. Another edit that will affect ObGyns is the bundling of CPT code 58558 (Hysteroscopy, surgical; with sampling [biopsy] of endometrium and/or poly-pectomy, with or without D&C) into codes for hysteroscopic removal of a fibroid (58561) and the removal of an impacted foreign body (58562).
Previously, these codes could have been billed together, but now only the addition of a modifier -22 to the primary procedure (myomectomy or foreign body removal) presents any chance for additional reimbursement. In order to report the modifier, Medicare has indicated that the documentation must clearly support the additional work in accomplishing the primary procedure, including a statement of how much time it added to the normal procedure.
Awareness of new or revised CPT codes could benefit your earnings
The 2015 CPT code set includes several changes, including laboratory and vaccination codes, which may be of interest to your practice. Because of Health Insurance Portability and Accountability Act (HIPAA) requirements, insurers were required to accept new codes on January 1, 2015.
Added: Fetal chromosomal aneuploidy code for genomic sequencing
On January 1, 2014, CPT added a new code to report cell-free DNA to screen for fetal aneuploidy. This new code is 81507 (Fetal aneuploidy [trisomy 21, 18, and 13] DNA sequence analysis of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy), and it was added to report the Harmony™ Prenatal Test.
For 2015, another new code, 81420 (Fetal chromosomal aneuploidy [eg, trisomy 21, monosomy X] genomic sequence analysis panel, circulating cell-free fetal DNA in maternal blood, must include analysis of chromosomes 13, 18, and 21), was added. This code represents a more comprehensive analysis and would therefore not be reported or ordered with code 81507. This new code requires a genomic sequence analysis panel.
HPV revisions extend beyond new codes
The codes for HPV testing have been redefined. These codes have been deleted: 87620-87622 (Infectious agent detection by nucleic acid [DNA or RNA]; papillomavirus, human, direct probe technique/amplified probe technique/quantification). In their place are three new codes to choose from:
- 87623, Human papillomavirus (HPV), low-risk types (eg, 6, 11, 42, 43, 44)
- 87624, Human papillomavirus (HPV), high-risk types (eg, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68)
- 87625, Human papillomavirus (HPV), types 16 and 18 only, includes type 45, if performed.
This coding change may be significant for payment as some payers will cover testing for high-risk HPV types only, so be sure your practice management team is aware of the latest rules for ordering HPV testing for your patients. Otherwise, patients could be faced with unexpected out-of-pocket expenses.
Egg freezing recognized as mainstream
- Infertility laboratories will be pleased to learn that CPT has changed the status of the code for cryopreservation of oocytes from a Category III to a Category I code. This means that this technology has now proven itself as a mainstream procedure, warranting a Category I CPT code. The new code is 89337 (Cryopreservation, mature oocyte[s]), which replaces the deleted code 0059T (Cryopreservation; oocyte[s]).
Vaccination codes for 2015
Almost every year new codes are added, and 2015 is no different. This year, you will see codes for:
- 90651, Human papillomavirus vaccine types 6, 11, 16, 18, 31, 33, 45, 52, 58, nonavalent (HPV), 3 dose schedule, for intramuscular use
- 90630, Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, for intradermal use.
There is also a revision to the flu virus vaccine code 90654 to indicate that it represents a trivalent preservative-free vaccine. See the TABLE below for a complete list of all the vaccines by trade name and CPT/Medicare Healthcare Common Procedure Coding System (HCPCS) codes for the 2014−2015 flu season.
Keep in mind that reporting administration of the flu vaccine is different for Medicare than for private payers. Administration code G0008 and diagnosis code V04.81 (Need for prophylactic vaccination and inoculation against influenza) would be reported in conjunction with the appropriate vaccine code for Medicare, while CPT instructs you to report 90471 instead for the administration. When we switch to ICD-10 diagnostic coding on October 1, 2015, the code V04.81 becomes Z23 (Encounter for immunization).
Three new codes for anoscopy
The first two codes were formerly Category III codes representing new technology, but now have proven to be more mainstream.
- 46601, Anoscopy; diagnostic, with high-resolution magnification (HRA) (eg, colposcope, operating microscope) and chemical agent enhancement, including collection of specimen(s) by brushing or washing, when performed
- 46607, Anoscopy; diagnostic, with high-resolution magnification (HRA) (eg, colposcope, operating microscope) and chemical agent enhancement, with biopsy, single or multiple
- 0377T, Anoscopy with directed submucosal injection of bulking agent for fecal incontinence.
Replacement codes for vertebral fracture assessments
If your practice is performing or ordering vertebral assessments for patients, there are two new codes to report. The old code, 77082 (Dual-energy X-ray absorptiometry [DXA], bone density study, 1 or more sites; vertebral fracture assessment) for vertebral fracture assessment has been deleted and replaced with:
- 77085, Dual-energy X-ray absorptiometry (DXA), bone density study, 1 or more sites; axial skeleton (eg, hips, pelvis, spine), including vertebral fracture assessment
- 77086, Vertebral fracture assessment via dual-energy X-ray absorptiometry (DXA).
Coding for breast ultrasound and tomosynthesis get more descriptive—at least for private insurance
The CPT Editorial Panel created three codes to describe digital breast tomosynthesis services and two new codes for a breast ultrasound.
- 77061, Digital breast tomosynthesis; unilateral
- 77062, Digital breast tomosynthesis; bilateral
- 77063, Screening digital breast tomosynthesis, bilateral (List separately in addition to the code for the primary procedure.)
- 76641, Ultrasound, breast, unilateral, real time with image documentation, including axilla when performed; complete
- 76642, Ultrasound, breast, unilateral, … ; limited.
Medicare, on the other hand, has decided to create a G code for tomosynthesis, which is the only code that will be accepted for payment if the patient meets her high-risk criteria for performance of this test.
Under Medicare rules you would report/order the following codes for mammographic services:
- Film (use CPT codes)
- 77056, Mammography; bilateral
- 77057, Screening mammography, bilateral (2-view film study of each breast)
- 2D digital (use G0202, G0204, and G0206)
- G0204, Diagnostic mammography, producing direct digital image, bilateral, all views
- G0206, Diagnostic mammography, producing direct digital image, unilateral, all views
- 3D screening (use G0202 for 2D digital plus the new CPT code 77063 for 3D)
procedure.)
- 3D diagnostic (use G0204 or G0206 for the 2D digital plus the new G code G0279 for 3D)
Modifier 59 becomes more specific
Another Medicare coding change that may affect ObGyns is the addition of new Medicare modifiers that are intended to eventually replace the modifier -59. This new list of modifiers will need to be appended to bundled procedures to more clearly explain why the secondary procedures should be paid separately. At the most recent American Medical Association (AMA) CPT symposium in Chicago, Illinois, CMS medical directors indicated that the new modifiers should be used only when the clinician is given instructions to do so by the carrier. Until then, the modifier -59 should continue to be used by most clinicians.
Here are the new modifiers, with an example of their use with currently bundled procedures that allow a modifier -59 to be used under certain circumstances:
- -XE: Separate encounter (A service that is distinct because it occurred during a separate encounter.) For instance, a patient presents to the office in the morning to have an abscess on her labia near her urethra incised and drained (56405). She returns in the afternoon to have a temporary catheter inserted because she states she cannot urinate and you decide to put in a temporary Foley catheter (51702) until the swelling has gone down. Add the modifier XE to 51702 to indicate it was performed at a different patient encounter.
- -XP: Separate practitioner (A service that is distinct because it was performed by a different practitioner.) Normally, Medicare will reimburse an unaffiliated clinician for performing a procedure that is bundled, since the bundling edits apply to the billing surgeon. But when two physicians from the same practice each are performing a different procedure at the same operative session that would otherwise be bundled, this new modifier will make that clear.
For example, Dr. Bates is performing a laparoscopic paravaginal defect repair (57423) and calls Dr. Clark, a urogynecologist in his practice, to remove severe adhesions from the ureters. The claim should go in under the same tax ID number, with the code 50715 listed first (as it has greater RVUs) and code 57423 reported with the -XP modifier.
- -XS: Separate structure (A service that is distinct because it was performed on a separate organ/structure.) Dr. Scott is performing the removal of endometrial implants around the left fallopian tube and in the cul-de-sac and notices that the right fallopian tube appears closed. He performs chromotubation on the right fallopian tube and notes that the right tube is blocked. Billing in this case would be 58662, 58350-XS.
- -XU: Unusual non-overlapping service (The use of a service that is distinct because it does not overlap usual components of the main service.) Mary has Medicare coverage and presents at 20 weeks 4 days gestation with bleeding and labor pains. Her examination shows bulging membranes that rupture when you attempt to remove the cerclage suture. You note a large rent in the cervix, but cannot get to the cerclage sutures as the patient is in active labor and beginning to bear down. The fetus and placenta are delivered a short time later through the rent in the cervix. You repair the rent in the cervix following delivery.
In this case, code 59400-52 (reduced services since the patient delivered at 20 weeks and there were reduced antepartum services), and 57720-XU because the repair of the cervix is not part of the usual services for a vaginal delivery.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Product Update
PLASMA ELECTROSURGERY DEVICE
Bovie Medical Corporation's new J-Plasma® hand piece has a retractable cutting feature for use in soft-tissue coagulation and cutting during surgery. The J-Plasma stream is formed by passing inert helium over the retractable blade. The blade can be extended and used with or without energy or plasma, similar to a scalpel, for incisions and other cutting procedures. The hand piece is available in open and a new laparoscopic Pistol Grip configuration, with an accompanying Bovie Ultimate generator.
FOR MORE INFORMATION, VISIT www.boviemedical.com
EMBRYO VIABILITY TEST FOR IVF
The Eeva Test™ from Auxogyn is an automated, noninvasive test that uses time-lapse imaging to determine which embryos will have the highest developmental potential during in vitro fertilization. The Eeva Test uses proprietary software to analyze embryo development against scientific and clinical cell-division timing parameters.
FOR MORE INFORMATION, VISIT www.eevaivf.com
NATURAL REMEDIES FOR POSTPARTUM PAIN
Fairhaven Health says its Sitting Pretty products offer pain relief and help to increase the healing process for perineal tears, vaginal swelling and bruising, and hemorrhoids following delivery. A 2-oz Soothing Spray bottle contains witch hazel, peppermint, lavender, and tea tree oils with grapefruit seed and other herbal extracts. A 4-oz jar of Soothing Balm has shea butter, coconut oil, and other organic ingredients.
FOR MORE INFORMATION, VISIT www.fairhavenhealth.com
TREATMENT FOR LARGE AND SMALL SCARS
ScarAway Daily Discs are small, round discs (1.375” in diameter) used to treat small scars that result from biopsies, mole removal, or acne blemishes. ScarAway Flex Long Sheets are for treating longer scars that may result from cesarean deliveries and other operations, injuries, and burns. The Flex Long Sheets measure 1.5”x7”. ScarAway Scar Gel is a colorless, odorless, self-drying silicon gel that forms a flexible, breathable, waterproof cover over the affected area. Once dry, makeup and sunscreen can be applied.
FOR MORE INFORMATION, VISIT www.MyScarAway.com
PORTABLE COLPOSCOPE
The Gynocular™ is a lightweight, portable colposcope with smart-phone adapter. Gynius says the battery-operated handheld device can be used with a tripod and allows physicians to capture, store, and send high-quality digital images and videos via phone or Skype. Gynius is collaborating with Woman Care Global, a nonprofit health-care company, to market and distribute The Gynocular worldwide.
FOR MORE INFORMATION, VISIT www.gynocular.com
SUTURING IN ROBOT-ASSISTED SURGERY
StitchKit® Robotic-Assisted Surgery Suturing Technology, from Origami Surgical, is a sterile, single-use plastic canister preloaded with six ePTFE sutures (8” each), with a secure, see-through disposal compartment permitting needle counting during surgery. Origami Surgical says that each canister is intended to complete one sacrocolpopexy surgery.
FOR MORE INFORMATION, VISIT www.origamisurgical.com
COLLAGEN DRESSING FOR CHRONIC WOUNDS
Gentell has introduced four new collagen products designed to treat chronic nonhealing wounds. Gentell Collagen, derived from bovine collagen, is available in a powdery particle (1-g bottle), or as dressing sheets in 2”x2”, 4”x5.25”, and 8”x12” sizes. Gentell Collagen products can be used to treat aggressive wounds with minimal to heavy exudate, and partial- or full-thickness, granulating or necrotic, or second-degree burns.
FOR MORE INFORMATION, VISIT www.gentell.com
INTRAOPERATIVE RADIATION THERAPY
The Xoft® Axxent® Electronic Brachytherapy System (eBx®) delivers intraoperative brachytherapy to treat breast cancer. Intraoperative radiation therapy is delivered as a single radiation dose using a balloon and a miniature x-ray source within the surgical cavity at the time of breast-conserving surgery (lumpectomy). Axxent eBx is designed to minimize radiation exposure to surrounding healthy tissue.
FOR MORE INFORMATION, VISIT www.xoftinc.com
KEGEL EXERCISE SYSTEM
Juve is a biofeedback Kegel exercise system that helps women develop pelvic-floor muscle strength. A device inserted into the vagina has sensors that work with a Bluetooth smartphone app to monitor pelvic-floor muscle strength and provide instant feedback to the user. Six training programs are offered.
FOR MORE INFORMATION, VISIT www.thejuve.com
WEARABLE BIOSENSOR FOR CORE METRICS
Vital Connect announced FDA clearance of its HealthPatch™ MD Biosensor. The device can capture clinical-grade biometric measurements of core-health metrics: single-lead ECG; heart rate; heart-rate variability; respiratory rate; skin temperature; posture including fall detection and severity; and steps.
FOR MORE INFORMATION, VISIT www.vitalconnect.com
HORMONE-FREE CONTRACEPTION
FemCap is a reusable, hormone-free, latex-free cervical cap for birth control. Made of surgical-grade silicone in three sizes, it covers the cervix, preventing sperm from moving into the uterus. Used in conjunction with spermicide, it is more than 92% effective in the prevention of pregnancy, says FemCap, Inc.
FOR MORE INFORMATION, VISIT www.femcap.com
PLASMA ELECTROSURGERY DEVICE
Bovie Medical Corporation's new J-Plasma® hand piece has a retractable cutting feature for use in soft-tissue coagulation and cutting during surgery. The J-Plasma stream is formed by passing inert helium over the retractable blade. The blade can be extended and used with or without energy or plasma, similar to a scalpel, for incisions and other cutting procedures. The hand piece is available in open and a new laparoscopic Pistol Grip configuration, with an accompanying Bovie Ultimate generator.
FOR MORE INFORMATION, VISIT www.boviemedical.com
EMBRYO VIABILITY TEST FOR IVF
The Eeva Test™ from Auxogyn is an automated, noninvasive test that uses time-lapse imaging to determine which embryos will have the highest developmental potential during in vitro fertilization. The Eeva Test uses proprietary software to analyze embryo development against scientific and clinical cell-division timing parameters.
FOR MORE INFORMATION, VISIT www.eevaivf.com
NATURAL REMEDIES FOR POSTPARTUM PAIN
Fairhaven Health says its Sitting Pretty products offer pain relief and help to increase the healing process for perineal tears, vaginal swelling and bruising, and hemorrhoids following delivery. A 2-oz Soothing Spray bottle contains witch hazel, peppermint, lavender, and tea tree oils with grapefruit seed and other herbal extracts. A 4-oz jar of Soothing Balm has shea butter, coconut oil, and other organic ingredients.
FOR MORE INFORMATION, VISIT www.fairhavenhealth.com
TREATMENT FOR LARGE AND SMALL SCARS
ScarAway Daily Discs are small, round discs (1.375” in diameter) used to treat small scars that result from biopsies, mole removal, or acne blemishes. ScarAway Flex Long Sheets are for treating longer scars that may result from cesarean deliveries and other operations, injuries, and burns. The Flex Long Sheets measure 1.5”x7”. ScarAway Scar Gel is a colorless, odorless, self-drying silicon gel that forms a flexible, breathable, waterproof cover over the affected area. Once dry, makeup and sunscreen can be applied.
FOR MORE INFORMATION, VISIT www.MyScarAway.com
PORTABLE COLPOSCOPE
The Gynocular™ is a lightweight, portable colposcope with smart-phone adapter. Gynius says the battery-operated handheld device can be used with a tripod and allows physicians to capture, store, and send high-quality digital images and videos via phone or Skype. Gynius is collaborating with Woman Care Global, a nonprofit health-care company, to market and distribute The Gynocular worldwide.
FOR MORE INFORMATION, VISIT www.gynocular.com
SUTURING IN ROBOT-ASSISTED SURGERY
StitchKit® Robotic-Assisted Surgery Suturing Technology, from Origami Surgical, is a sterile, single-use plastic canister preloaded with six ePTFE sutures (8” each), with a secure, see-through disposal compartment permitting needle counting during surgery. Origami Surgical says that each canister is intended to complete one sacrocolpopexy surgery.
FOR MORE INFORMATION, VISIT www.origamisurgical.com
COLLAGEN DRESSING FOR CHRONIC WOUNDS
Gentell has introduced four new collagen products designed to treat chronic nonhealing wounds. Gentell Collagen, derived from bovine collagen, is available in a powdery particle (1-g bottle), or as dressing sheets in 2”x2”, 4”x5.25”, and 8”x12” sizes. Gentell Collagen products can be used to treat aggressive wounds with minimal to heavy exudate, and partial- or full-thickness, granulating or necrotic, or second-degree burns.
FOR MORE INFORMATION, VISIT www.gentell.com
INTRAOPERATIVE RADIATION THERAPY
The Xoft® Axxent® Electronic Brachytherapy System (eBx®) delivers intraoperative brachytherapy to treat breast cancer. Intraoperative radiation therapy is delivered as a single radiation dose using a balloon and a miniature x-ray source within the surgical cavity at the time of breast-conserving surgery (lumpectomy). Axxent eBx is designed to minimize radiation exposure to surrounding healthy tissue.
FOR MORE INFORMATION, VISIT www.xoftinc.com
KEGEL EXERCISE SYSTEM
Juve is a biofeedback Kegel exercise system that helps women develop pelvic-floor muscle strength. A device inserted into the vagina has sensors that work with a Bluetooth smartphone app to monitor pelvic-floor muscle strength and provide instant feedback to the user. Six training programs are offered.
FOR MORE INFORMATION, VISIT www.thejuve.com
WEARABLE BIOSENSOR FOR CORE METRICS
Vital Connect announced FDA clearance of its HealthPatch™ MD Biosensor. The device can capture clinical-grade biometric measurements of core-health metrics: single-lead ECG; heart rate; heart-rate variability; respiratory rate; skin temperature; posture including fall detection and severity; and steps.
FOR MORE INFORMATION, VISIT www.vitalconnect.com
HORMONE-FREE CONTRACEPTION
FemCap is a reusable, hormone-free, latex-free cervical cap for birth control. Made of surgical-grade silicone in three sizes, it covers the cervix, preventing sperm from moving into the uterus. Used in conjunction with spermicide, it is more than 92% effective in the prevention of pregnancy, says FemCap, Inc.
FOR MORE INFORMATION, VISIT www.femcap.com
PLASMA ELECTROSURGERY DEVICE
Bovie Medical Corporation's new J-Plasma® hand piece has a retractable cutting feature for use in soft-tissue coagulation and cutting during surgery. The J-Plasma stream is formed by passing inert helium over the retractable blade. The blade can be extended and used with or without energy or plasma, similar to a scalpel, for incisions and other cutting procedures. The hand piece is available in open and a new laparoscopic Pistol Grip configuration, with an accompanying Bovie Ultimate generator.
FOR MORE INFORMATION, VISIT www.boviemedical.com
EMBRYO VIABILITY TEST FOR IVF
The Eeva Test™ from Auxogyn is an automated, noninvasive test that uses time-lapse imaging to determine which embryos will have the highest developmental potential during in vitro fertilization. The Eeva Test uses proprietary software to analyze embryo development against scientific and clinical cell-division timing parameters.
FOR MORE INFORMATION, VISIT www.eevaivf.com
NATURAL REMEDIES FOR POSTPARTUM PAIN
Fairhaven Health says its Sitting Pretty products offer pain relief and help to increase the healing process for perineal tears, vaginal swelling and bruising, and hemorrhoids following delivery. A 2-oz Soothing Spray bottle contains witch hazel, peppermint, lavender, and tea tree oils with grapefruit seed and other herbal extracts. A 4-oz jar of Soothing Balm has shea butter, coconut oil, and other organic ingredients.
FOR MORE INFORMATION, VISIT www.fairhavenhealth.com
TREATMENT FOR LARGE AND SMALL SCARS
ScarAway Daily Discs are small, round discs (1.375” in diameter) used to treat small scars that result from biopsies, mole removal, or acne blemishes. ScarAway Flex Long Sheets are for treating longer scars that may result from cesarean deliveries and other operations, injuries, and burns. The Flex Long Sheets measure 1.5”x7”. ScarAway Scar Gel is a colorless, odorless, self-drying silicon gel that forms a flexible, breathable, waterproof cover over the affected area. Once dry, makeup and sunscreen can be applied.
FOR MORE INFORMATION, VISIT www.MyScarAway.com
PORTABLE COLPOSCOPE
The Gynocular™ is a lightweight, portable colposcope with smart-phone adapter. Gynius says the battery-operated handheld device can be used with a tripod and allows physicians to capture, store, and send high-quality digital images and videos via phone or Skype. Gynius is collaborating with Woman Care Global, a nonprofit health-care company, to market and distribute The Gynocular worldwide.
FOR MORE INFORMATION, VISIT www.gynocular.com
SUTURING IN ROBOT-ASSISTED SURGERY
StitchKit® Robotic-Assisted Surgery Suturing Technology, from Origami Surgical, is a sterile, single-use plastic canister preloaded with six ePTFE sutures (8” each), with a secure, see-through disposal compartment permitting needle counting during surgery. Origami Surgical says that each canister is intended to complete one sacrocolpopexy surgery.
FOR MORE INFORMATION, VISIT www.origamisurgical.com
COLLAGEN DRESSING FOR CHRONIC WOUNDS
Gentell has introduced four new collagen products designed to treat chronic nonhealing wounds. Gentell Collagen, derived from bovine collagen, is available in a powdery particle (1-g bottle), or as dressing sheets in 2”x2”, 4”x5.25”, and 8”x12” sizes. Gentell Collagen products can be used to treat aggressive wounds with minimal to heavy exudate, and partial- or full-thickness, granulating or necrotic, or second-degree burns.
FOR MORE INFORMATION, VISIT www.gentell.com
INTRAOPERATIVE RADIATION THERAPY
The Xoft® Axxent® Electronic Brachytherapy System (eBx®) delivers intraoperative brachytherapy to treat breast cancer. Intraoperative radiation therapy is delivered as a single radiation dose using a balloon and a miniature x-ray source within the surgical cavity at the time of breast-conserving surgery (lumpectomy). Axxent eBx is designed to minimize radiation exposure to surrounding healthy tissue.
FOR MORE INFORMATION, VISIT www.xoftinc.com
KEGEL EXERCISE SYSTEM
Juve is a biofeedback Kegel exercise system that helps women develop pelvic-floor muscle strength. A device inserted into the vagina has sensors that work with a Bluetooth smartphone app to monitor pelvic-floor muscle strength and provide instant feedback to the user. Six training programs are offered.
FOR MORE INFORMATION, VISIT www.thejuve.com
WEARABLE BIOSENSOR FOR CORE METRICS
Vital Connect announced FDA clearance of its HealthPatch™ MD Biosensor. The device can capture clinical-grade biometric measurements of core-health metrics: single-lead ECG; heart rate; heart-rate variability; respiratory rate; skin temperature; posture including fall detection and severity; and steps.
FOR MORE INFORMATION, VISIT www.vitalconnect.com
HORMONE-FREE CONTRACEPTION
FemCap is a reusable, hormone-free, latex-free cervical cap for birth control. Made of surgical-grade silicone in three sizes, it covers the cervix, preventing sperm from moving into the uterus. Used in conjunction with spermicide, it is more than 92% effective in the prevention of pregnancy, says FemCap, Inc.
FOR MORE INFORMATION, VISIT www.femcap.com
Shrink Rap News: Writing about involuntary treatment is harder than you might think
There are many controversies in psychiatry, but the most controversial issue continues to be that of involuntary treatment. Over the past year, I have been working on a book on forced care, along with Dr. Annette Hanson, the forensic psychiatrist who also writes this Shrink Rap News column. The work I’ve been doing for the manuscript – identifying and interviewing the people with a stake in the issue – has placed me in a funny role of being more journalist than physician. It’s a role I mostly love, and if you’ve hit the midpoint of your career and have an opportunity to dedicate some of your time to doing something completely different, I highly recommend shifting gears for a little while.
One of the things I have found most surprising is the reactions of people when I ask to include them. While most people are willing, almost all hesitate a little. Some are quite eager to participate, and one group in Richmond who uses involuntary electroconvulsive therapy heard of my project and extended an invitation for me to come visit, interview them, watch the procedure, and speak with one of their patients. Many people are proud of the work they do and eager to showcase it; they want to have their viewpoint included. With others, I have needed to ask repeatedly, listen to their concerns, and offer reassurances. I’ve taken to telling people they can read a draft of what I write about them – I learned early in the process that without this offer, few people would be willing to speak to me.
I wish I could say that I’ve noticed a pattern to who is comfortable speaking and who is not. Many of the psychiatrists and researchers I’ve approached have given their time willingly without hesitation ( or at least without that much hesitation), including Dr. E. Fuller Torrey; Dr. Paul Appelbaum; Jeffrey Swanson, Ph.D.; Dr. Steven Sharfstein; Dr. Daniel Fisher; Vermont Psychiatric Society President Margaret Bolton; Dr. Bruce Hershfield; and American Psychiatric Association President Paul Summergrad, to name just a few of the many people who have shared their time, experiences, and wisdom with me. It was not as easy for me to find a psychiatrist I could observe working on an inpatient unit, and the project might have been halted in its tracks if not for the enthusiasm of Johns Hopkins psychiatry chair Raymond DePaulo, who allowed me to shadow his team while they tended to inpatients. It was surprisingly easy to find a Crisis Intervention Team police officer to ride with, a little more effort to engage a mental health court judge.
Those who oppose forced treatment initially were more difficult to engage, but with perseverance, I was able to persuade the leadership of MindFreedom International to speak with me, and well, I ambushed a barely willing Scientologist while I was in New York for APA last May. In terms of patient participation, this hesitation did not hold true – many people volunteered to speak with me about how involuntary treatment harmed them, and it was much more difficult to find patients who felt helped by the treatment that was thrust upon them. Two absolutely wonderful women gave generously of themselves so that this project would have real voices to it: both “Lily” and “Eleanor” relived difficult experiences for me and allowed me to obtain their medical records and to speak with their families and their doctors. None of this was easy for them, but it did provide me with an education I would have gotten no other way as they reflected back on the meaning it had to them to have been committed to psychiatric units.
The subject of involuntary outpatient commitment has been particularly difficult to research, in part because I live in Maryland, a state which has no provision for this. I spoke with one family member in Arizona who initially was eager to be interviewed, along with her adult child who has come to feel that involuntary care has been very helpful. When I called back, the mom had changed her mind about participating: “You’re writing a book with a balanced view. There no balance or controversy here for me; it’s a medical problem that needs treatment. ” Unless I could promise to support, unequivocally, the views she held without qualification, she was not interested in participating in our book, even if it would provide an avenue to express her beliefs about the value of outpatient commitment. For similar reasons, I had to approach Ron Honberg, J.D., at the national office of the National Alliance on Mental Illness (NAMI); the Maryland NAMI members did not want me to speak with me after two articles I wrote for this Clinical Psychiatry News column suggested that I was not wholeheartedly in favor of outpatient commitment. I was told the topic was “too sensitive” for their members to discuss with me. So while I’ve learned that the topic inspires a great deal of emotion, there are still surprises with every step.
With all that as a prelude, I am continuing to look for people to interview who are the subjects of mandated civil outpatient treatment orders, as well as their family members. I thought perhaps readers might be able to help me, and I am interested in hearing both the good and the bad. If you, your patients, or their families would like to participate, by all means contact me at [email protected].
Dr. Miller is a coauthor of Shrink Rap: Three Psychiatrists Explain Their Work (Baltimore: Johns Hopkins University Press, 2011).
There are many controversies in psychiatry, but the most controversial issue continues to be that of involuntary treatment. Over the past year, I have been working on a book on forced care, along with Dr. Annette Hanson, the forensic psychiatrist who also writes this Shrink Rap News column. The work I’ve been doing for the manuscript – identifying and interviewing the people with a stake in the issue – has placed me in a funny role of being more journalist than physician. It’s a role I mostly love, and if you’ve hit the midpoint of your career and have an opportunity to dedicate some of your time to doing something completely different, I highly recommend shifting gears for a little while.
One of the things I have found most surprising is the reactions of people when I ask to include them. While most people are willing, almost all hesitate a little. Some are quite eager to participate, and one group in Richmond who uses involuntary electroconvulsive therapy heard of my project and extended an invitation for me to come visit, interview them, watch the procedure, and speak with one of their patients. Many people are proud of the work they do and eager to showcase it; they want to have their viewpoint included. With others, I have needed to ask repeatedly, listen to their concerns, and offer reassurances. I’ve taken to telling people they can read a draft of what I write about them – I learned early in the process that without this offer, few people would be willing to speak to me.
I wish I could say that I’ve noticed a pattern to who is comfortable speaking and who is not. Many of the psychiatrists and researchers I’ve approached have given their time willingly without hesitation ( or at least without that much hesitation), including Dr. E. Fuller Torrey; Dr. Paul Appelbaum; Jeffrey Swanson, Ph.D.; Dr. Steven Sharfstein; Dr. Daniel Fisher; Vermont Psychiatric Society President Margaret Bolton; Dr. Bruce Hershfield; and American Psychiatric Association President Paul Summergrad, to name just a few of the many people who have shared their time, experiences, and wisdom with me. It was not as easy for me to find a psychiatrist I could observe working on an inpatient unit, and the project might have been halted in its tracks if not for the enthusiasm of Johns Hopkins psychiatry chair Raymond DePaulo, who allowed me to shadow his team while they tended to inpatients. It was surprisingly easy to find a Crisis Intervention Team police officer to ride with, a little more effort to engage a mental health court judge.
Those who oppose forced treatment initially were more difficult to engage, but with perseverance, I was able to persuade the leadership of MindFreedom International to speak with me, and well, I ambushed a barely willing Scientologist while I was in New York for APA last May. In terms of patient participation, this hesitation did not hold true – many people volunteered to speak with me about how involuntary treatment harmed them, and it was much more difficult to find patients who felt helped by the treatment that was thrust upon them. Two absolutely wonderful women gave generously of themselves so that this project would have real voices to it: both “Lily” and “Eleanor” relived difficult experiences for me and allowed me to obtain their medical records and to speak with their families and their doctors. None of this was easy for them, but it did provide me with an education I would have gotten no other way as they reflected back on the meaning it had to them to have been committed to psychiatric units.
The subject of involuntary outpatient commitment has been particularly difficult to research, in part because I live in Maryland, a state which has no provision for this. I spoke with one family member in Arizona who initially was eager to be interviewed, along with her adult child who has come to feel that involuntary care has been very helpful. When I called back, the mom had changed her mind about participating: “You’re writing a book with a balanced view. There no balance or controversy here for me; it’s a medical problem that needs treatment. ” Unless I could promise to support, unequivocally, the views she held without qualification, she was not interested in participating in our book, even if it would provide an avenue to express her beliefs about the value of outpatient commitment. For similar reasons, I had to approach Ron Honberg, J.D., at the national office of the National Alliance on Mental Illness (NAMI); the Maryland NAMI members did not want me to speak with me after two articles I wrote for this Clinical Psychiatry News column suggested that I was not wholeheartedly in favor of outpatient commitment. I was told the topic was “too sensitive” for their members to discuss with me. So while I’ve learned that the topic inspires a great deal of emotion, there are still surprises with every step.
With all that as a prelude, I am continuing to look for people to interview who are the subjects of mandated civil outpatient treatment orders, as well as their family members. I thought perhaps readers might be able to help me, and I am interested in hearing both the good and the bad. If you, your patients, or their families would like to participate, by all means contact me at [email protected].
Dr. Miller is a coauthor of Shrink Rap: Three Psychiatrists Explain Their Work (Baltimore: Johns Hopkins University Press, 2011).
There are many controversies in psychiatry, but the most controversial issue continues to be that of involuntary treatment. Over the past year, I have been working on a book on forced care, along with Dr. Annette Hanson, the forensic psychiatrist who also writes this Shrink Rap News column. The work I’ve been doing for the manuscript – identifying and interviewing the people with a stake in the issue – has placed me in a funny role of being more journalist than physician. It’s a role I mostly love, and if you’ve hit the midpoint of your career and have an opportunity to dedicate some of your time to doing something completely different, I highly recommend shifting gears for a little while.
One of the things I have found most surprising is the reactions of people when I ask to include them. While most people are willing, almost all hesitate a little. Some are quite eager to participate, and one group in Richmond who uses involuntary electroconvulsive therapy heard of my project and extended an invitation for me to come visit, interview them, watch the procedure, and speak with one of their patients. Many people are proud of the work they do and eager to showcase it; they want to have their viewpoint included. With others, I have needed to ask repeatedly, listen to their concerns, and offer reassurances. I’ve taken to telling people they can read a draft of what I write about them – I learned early in the process that without this offer, few people would be willing to speak to me.
I wish I could say that I’ve noticed a pattern to who is comfortable speaking and who is not. Many of the psychiatrists and researchers I’ve approached have given their time willingly without hesitation ( or at least without that much hesitation), including Dr. E. Fuller Torrey; Dr. Paul Appelbaum; Jeffrey Swanson, Ph.D.; Dr. Steven Sharfstein; Dr. Daniel Fisher; Vermont Psychiatric Society President Margaret Bolton; Dr. Bruce Hershfield; and American Psychiatric Association President Paul Summergrad, to name just a few of the many people who have shared their time, experiences, and wisdom with me. It was not as easy for me to find a psychiatrist I could observe working on an inpatient unit, and the project might have been halted in its tracks if not for the enthusiasm of Johns Hopkins psychiatry chair Raymond DePaulo, who allowed me to shadow his team while they tended to inpatients. It was surprisingly easy to find a Crisis Intervention Team police officer to ride with, a little more effort to engage a mental health court judge.
Those who oppose forced treatment initially were more difficult to engage, but with perseverance, I was able to persuade the leadership of MindFreedom International to speak with me, and well, I ambushed a barely willing Scientologist while I was in New York for APA last May. In terms of patient participation, this hesitation did not hold true – many people volunteered to speak with me about how involuntary treatment harmed them, and it was much more difficult to find patients who felt helped by the treatment that was thrust upon them. Two absolutely wonderful women gave generously of themselves so that this project would have real voices to it: both “Lily” and “Eleanor” relived difficult experiences for me and allowed me to obtain their medical records and to speak with their families and their doctors. None of this was easy for them, but it did provide me with an education I would have gotten no other way as they reflected back on the meaning it had to them to have been committed to psychiatric units.
The subject of involuntary outpatient commitment has been particularly difficult to research, in part because I live in Maryland, a state which has no provision for this. I spoke with one family member in Arizona who initially was eager to be interviewed, along with her adult child who has come to feel that involuntary care has been very helpful. When I called back, the mom had changed her mind about participating: “You’re writing a book with a balanced view. There no balance or controversy here for me; it’s a medical problem that needs treatment. ” Unless I could promise to support, unequivocally, the views she held without qualification, she was not interested in participating in our book, even if it would provide an avenue to express her beliefs about the value of outpatient commitment. For similar reasons, I had to approach Ron Honberg, J.D., at the national office of the National Alliance on Mental Illness (NAMI); the Maryland NAMI members did not want me to speak with me after two articles I wrote for this Clinical Psychiatry News column suggested that I was not wholeheartedly in favor of outpatient commitment. I was told the topic was “too sensitive” for their members to discuss with me. So while I’ve learned that the topic inspires a great deal of emotion, there are still surprises with every step.
With all that as a prelude, I am continuing to look for people to interview who are the subjects of mandated civil outpatient treatment orders, as well as their family members. I thought perhaps readers might be able to help me, and I am interested in hearing both the good and the bad. If you, your patients, or their families would like to participate, by all means contact me at [email protected].
Dr. Miller is a coauthor of Shrink Rap: Three Psychiatrists Explain Their Work (Baltimore: Johns Hopkins University Press, 2011).
Hot flashes linked to increased hip fracture risk
Hot flashes are associated with a significant increase in the risk of hip fracture, regardless of age, body mass index, and other confounders such as smoking, according to analysis of data from the Women’s Health Study.
The prospective, observational study among 4,867 women aged 50-79 years found a 78% increase in the risk of hip fracture among women with moderate to severe menopausal vasomotor symptoms at baseline, compared with women with no symptoms.
Vasomotor symptom severity was also inversely associated with bone mineral density (BMD) at both the femoral neck and the spine. Compared with women who had no vasomotor symptoms, those with moderate or severe symptoms had 0.015 g/cm2 lower femoral neck BMD and 0.016 g/cm2 lower lumbar spine BMD, according to an analysis published on Dec. 18 in the Journal of Clinical Endocrinology and Metabolism (2014 [doi:10.1210/jc.2014-3062]).
“Despite being younger and heavier than asymptomatic women, characteristics associated with higher BMD, women with moderate/severe [vasomotor symptoms] had a higher risk of hip fractures that was also independent of other established risk factors for fractures,” wrote Dr. Carolyn J. Crandall of the University of California, Los Angeles, and her colleagues.
The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
Hot flashes are associated with a significant increase in the risk of hip fracture, regardless of age, body mass index, and other confounders such as smoking, according to analysis of data from the Women’s Health Study.
The prospective, observational study among 4,867 women aged 50-79 years found a 78% increase in the risk of hip fracture among women with moderate to severe menopausal vasomotor symptoms at baseline, compared with women with no symptoms.
Vasomotor symptom severity was also inversely associated with bone mineral density (BMD) at both the femoral neck and the spine. Compared with women who had no vasomotor symptoms, those with moderate or severe symptoms had 0.015 g/cm2 lower femoral neck BMD and 0.016 g/cm2 lower lumbar spine BMD, according to an analysis published on Dec. 18 in the Journal of Clinical Endocrinology and Metabolism (2014 [doi:10.1210/jc.2014-3062]).
“Despite being younger and heavier than asymptomatic women, characteristics associated with higher BMD, women with moderate/severe [vasomotor symptoms] had a higher risk of hip fractures that was also independent of other established risk factors for fractures,” wrote Dr. Carolyn J. Crandall of the University of California, Los Angeles, and her colleagues.
The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
Hot flashes are associated with a significant increase in the risk of hip fracture, regardless of age, body mass index, and other confounders such as smoking, according to analysis of data from the Women’s Health Study.
The prospective, observational study among 4,867 women aged 50-79 years found a 78% increase in the risk of hip fracture among women with moderate to severe menopausal vasomotor symptoms at baseline, compared with women with no symptoms.
Vasomotor symptom severity was also inversely associated with bone mineral density (BMD) at both the femoral neck and the spine. Compared with women who had no vasomotor symptoms, those with moderate or severe symptoms had 0.015 g/cm2 lower femoral neck BMD and 0.016 g/cm2 lower lumbar spine BMD, according to an analysis published on Dec. 18 in the Journal of Clinical Endocrinology and Metabolism (2014 [doi:10.1210/jc.2014-3062]).
“Despite being younger and heavier than asymptomatic women, characteristics associated with higher BMD, women with moderate/severe [vasomotor symptoms] had a higher risk of hip fractures that was also independent of other established risk factors for fractures,” wrote Dr. Carolyn J. Crandall of the University of California, Los Angeles, and her colleagues.
The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Key clinical point: The severity of vasomotor menopause symptoms is associated with risk of hip fracture.
Major finding: Women with moderate to severe vasomotor symptoms have a 78% increase in their risk of hip fracture.
Data source: A prospective, observational study among 4,867 women aged 50-79 years.
Disclosures: The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
Teams map paths to drug resistance in cancers, MPNs
Researchers say they’ve discovered key events that prompt drug resistance in myeloproliferative neoplasms (MPNs) and certain solid tumor malignancies.
By mapping the steps that myelofibrosis, melanoma, and breast cancer cells use to become resistant to drugs, the investigators believe they have identified better targets for blocking those pathways to ensure current therapies are effective.
The groups reported these findings in two papers published in Science Signaling.
“In our studies, we developed a screening technology that allows us to quickly identify the routes cells can use to become resistant, and, using that information, we were able to show that these mechanisms seen in the laboratory are actually also occurring in patients’ tumors,” said Kris Wood, PhD, an assistant professor at Duke University in Durham, North Carolina, and senior author of both studies.
In the first study, Dr Wood and his colleagues conducted a broad survey of the known cell-signaling pathways that, when activated, have the potential to trigger drug resistance.
Using this screening technology, the researchers were able to corroborate the results of earlier drug-resistance studies, while also finding pathways that had not previously been described.
The team identified the MAPK and PI3K pathways, which are known to mediate drug resistance. But they also found that activation of the Notch1 pathway caused drug resistance, and inhibiting Notch1 signaling restored drug efficacy.
“Interestingly, the mechanisms are quite similar among all 3 of the cancer types,” Dr Wood noted. “In breast cancer and melanoma, our findings suggest the same Notch1 pathway as a potential driver of resistance to a wide array of targeted therapies—a role that has not been widely acknowledged previously.”
The investigators found that Notch signaling mediated drug resistance to an estrogen receptor-targeted therapy used in breast cancer and to a kinase-targeted therapy used in melanoma. And tumors from some patients with relapsed breast cancer or melanoma had increased markers of Notch1 signaling.
In the second study, the researchers used the aforementioned pathway-centric screen to track a pair of separate signaling pathways downstream of RAS, a protein frequently activated in MPNs.
The team found the pathway mediated by RAS promoted drug resistance in hematopoietic cell lines containing an activating mutation in JAK2. And RAS signaling led to the phosphorylation-mediated inactivation of the pro-apoptotic protein BAD, which enabled cell survival.
Combining JAK inhibitors with inhibitors of kinases downstream of RAS signaling prompted apoptosis in cell lines that were resistant to JAK inhibitors. This suggests such combinations may be effective for treating drug-resistant MPNs.
“Together, these findings improve our ability to stratify patients into groups more and less likely to respond to therapy and design drug combinations that work together to block or delay resistance,” Dr Wood concluded. ![]()
Researchers say they’ve discovered key events that prompt drug resistance in myeloproliferative neoplasms (MPNs) and certain solid tumor malignancies.
By mapping the steps that myelofibrosis, melanoma, and breast cancer cells use to become resistant to drugs, the investigators believe they have identified better targets for blocking those pathways to ensure current therapies are effective.
The groups reported these findings in two papers published in Science Signaling.
“In our studies, we developed a screening technology that allows us to quickly identify the routes cells can use to become resistant, and, using that information, we were able to show that these mechanisms seen in the laboratory are actually also occurring in patients’ tumors,” said Kris Wood, PhD, an assistant professor at Duke University in Durham, North Carolina, and senior author of both studies.
In the first study, Dr Wood and his colleagues conducted a broad survey of the known cell-signaling pathways that, when activated, have the potential to trigger drug resistance.
Using this screening technology, the researchers were able to corroborate the results of earlier drug-resistance studies, while also finding pathways that had not previously been described.
The team identified the MAPK and PI3K pathways, which are known to mediate drug resistance. But they also found that activation of the Notch1 pathway caused drug resistance, and inhibiting Notch1 signaling restored drug efficacy.
“Interestingly, the mechanisms are quite similar among all 3 of the cancer types,” Dr Wood noted. “In breast cancer and melanoma, our findings suggest the same Notch1 pathway as a potential driver of resistance to a wide array of targeted therapies—a role that has not been widely acknowledged previously.”
The investigators found that Notch signaling mediated drug resistance to an estrogen receptor-targeted therapy used in breast cancer and to a kinase-targeted therapy used in melanoma. And tumors from some patients with relapsed breast cancer or melanoma had increased markers of Notch1 signaling.
In the second study, the researchers used the aforementioned pathway-centric screen to track a pair of separate signaling pathways downstream of RAS, a protein frequently activated in MPNs.
The team found the pathway mediated by RAS promoted drug resistance in hematopoietic cell lines containing an activating mutation in JAK2. And RAS signaling led to the phosphorylation-mediated inactivation of the pro-apoptotic protein BAD, which enabled cell survival.
Combining JAK inhibitors with inhibitors of kinases downstream of RAS signaling prompted apoptosis in cell lines that were resistant to JAK inhibitors. This suggests such combinations may be effective for treating drug-resistant MPNs.
“Together, these findings improve our ability to stratify patients into groups more and less likely to respond to therapy and design drug combinations that work together to block or delay resistance,” Dr Wood concluded. ![]()
Researchers say they’ve discovered key events that prompt drug resistance in myeloproliferative neoplasms (MPNs) and certain solid tumor malignancies.
By mapping the steps that myelofibrosis, melanoma, and breast cancer cells use to become resistant to drugs, the investigators believe they have identified better targets for blocking those pathways to ensure current therapies are effective.
The groups reported these findings in two papers published in Science Signaling.
“In our studies, we developed a screening technology that allows us to quickly identify the routes cells can use to become resistant, and, using that information, we were able to show that these mechanisms seen in the laboratory are actually also occurring in patients’ tumors,” said Kris Wood, PhD, an assistant professor at Duke University in Durham, North Carolina, and senior author of both studies.
In the first study, Dr Wood and his colleagues conducted a broad survey of the known cell-signaling pathways that, when activated, have the potential to trigger drug resistance.
Using this screening technology, the researchers were able to corroborate the results of earlier drug-resistance studies, while also finding pathways that had not previously been described.
The team identified the MAPK and PI3K pathways, which are known to mediate drug resistance. But they also found that activation of the Notch1 pathway caused drug resistance, and inhibiting Notch1 signaling restored drug efficacy.
“Interestingly, the mechanisms are quite similar among all 3 of the cancer types,” Dr Wood noted. “In breast cancer and melanoma, our findings suggest the same Notch1 pathway as a potential driver of resistance to a wide array of targeted therapies—a role that has not been widely acknowledged previously.”
The investigators found that Notch signaling mediated drug resistance to an estrogen receptor-targeted therapy used in breast cancer and to a kinase-targeted therapy used in melanoma. And tumors from some patients with relapsed breast cancer or melanoma had increased markers of Notch1 signaling.
In the second study, the researchers used the aforementioned pathway-centric screen to track a pair of separate signaling pathways downstream of RAS, a protein frequently activated in MPNs.
The team found the pathway mediated by RAS promoted drug resistance in hematopoietic cell lines containing an activating mutation in JAK2. And RAS signaling led to the phosphorylation-mediated inactivation of the pro-apoptotic protein BAD, which enabled cell survival.
Combining JAK inhibitors with inhibitors of kinases downstream of RAS signaling prompted apoptosis in cell lines that were resistant to JAK inhibitors. This suggests such combinations may be effective for treating drug-resistant MPNs.
“Together, these findings improve our ability to stratify patients into groups more and less likely to respond to therapy and design drug combinations that work together to block or delay resistance,” Dr Wood concluded. ![]()
Drug combats complications of SCD
SAN FRANCISCO—Pharmaceutical-grade L-glutamine can reduce the risk of complications in patients with sickle cell disease (SCD), according to new research.
In a phase 3 study of more than 200 patients, those who received L-glutamine saw a reduction in sickle cell crises, hospitalizations, and the incidence of acute chest syndrome (ACS) when compared to patients who received placebo.
In addition, L-glutamine appeared to have a synergistic effect with hydroxyurea.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, presented these results at the 2014 ASH Annual Meeting (abstract 86*). Emmaus Medical produced the pharmaceutical-grade L-glutamine used in the study.
Dr Niihara noted that nicotinamide adenine dinucleotide (NAD) is an important physiological antioxidant in red blood cells. But in sickled red blood cells, NAD redox potential is significantly compromised.
So he and his colleagues hypothesized that glutamine, a precursor for NAD, can improve NAD redox potential and modify the destructive effect of increased oxidation and the pathophysiology of SCD. They also theorized that glutamine will decrease the frequency of vaso-occlusive crises.
To test their theories, the researchers enrolled 230 patients from 31 different sites on a phase 3 trial. Patients had sickle cell anemia (SS) or sickle B0 thalassemia. They were 5 years of age or older, and some were receiving hydroxyurea.
Patients were randomized 2:1 to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day) or placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks. The researchers had discovered high variation in the quality of over-the-counter L-glutamine, so they used pharmaceutical-grade L-glutamine only.
In all, 152 patients received L-glutamine, and 78 received placebo. Demographics and baseline characteristics were similar between the two treatment arms.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% and 57.7%, respectively. And most patients in both arms had sickle cell anemia—89.5% and 91%, respectively.
Safety and efficacy
Dr Niihara said there were no serious adverse events attributable to L-glutamine and no dose modifications due to toxicity.
There were more gastrointestinal events (such as constipation and flatulence) in the L-glutamine arm (8.6%) than in the placebo arm (5.2%). But there was no difference in other adverse events between the two arms.
The study’s primary endpoint was the frequency of painful sickle cell crises. Patients in the L-glutamine arm had 25% fewer crises than patients in the placebo arm (P=0.008).
In patients aged 18 and younger, the median number of sickle cell crises was 3 in the L-glutamine arm and 4 in the placebo arm (P<0.001). The numbers were the same in patients older than 18 (P<0.001) and among patients receiving hydroxyurea (P<0.001).
“Those patients, even though they were on hydroxyurea, they have shown improvements [with L-glutamine],” Dr Niihara said. “And it appears there is some synergistic effect . . ., certainly more than just an additive effect.”
Dr Niihara also noted that patients who received placebo took less than 2 months to experience a first sickle cell crisis, but, for patients on L-glutamine, it took almost 3 months before they had a first crisis. The time to a first crisis was a median of 87 days in the L-glutamine arm and 54 days in the placebo arm (P=0.010).
The study’s secondary endpoint was the frequency of hospitalizations. Compared to patients in the placebo arm, those in the L-glutamine arm had a 33% reduction in hospitalizations (P=0.005) and a 41% reduction in the length of hospital stay (P=0.022).
The researchers also looked at the incidence of ACS and found that L-glutamine was associated with a 58% reduction in ACS. The incidence was 26.9% in the placebo arm and 11.9% in the L-glutamine arm (P=0.006).
“We observed this cascade improvement [with L-glutamine],” Dr Niihara noted. “And what we mean [by that] is that, with glutamine, there is less chance of having painful crises, but if patients have to have crises, they have less chance of being hospitalized. And if they have to be hospitalized, their stays in the hospital are shorter. And, again, if they have to be hospitalized, there’s less chance of them going to the ICU with acute chest syndrome.”
Dr Niihara said the researchers’ next steps are to investigate L-glutamine in a clinical setting, in patients younger than 5 years old, in pregnant women, and as part of combination therapy. ![]()
*Information in the abstract differs from that presented at the meeting.
SAN FRANCISCO—Pharmaceutical-grade L-glutamine can reduce the risk of complications in patients with sickle cell disease (SCD), according to new research.
In a phase 3 study of more than 200 patients, those who received L-glutamine saw a reduction in sickle cell crises, hospitalizations, and the incidence of acute chest syndrome (ACS) when compared to patients who received placebo.
In addition, L-glutamine appeared to have a synergistic effect with hydroxyurea.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, presented these results at the 2014 ASH Annual Meeting (abstract 86*). Emmaus Medical produced the pharmaceutical-grade L-glutamine used in the study.
Dr Niihara noted that nicotinamide adenine dinucleotide (NAD) is an important physiological antioxidant in red blood cells. But in sickled red blood cells, NAD redox potential is significantly compromised.
So he and his colleagues hypothesized that glutamine, a precursor for NAD, can improve NAD redox potential and modify the destructive effect of increased oxidation and the pathophysiology of SCD. They also theorized that glutamine will decrease the frequency of vaso-occlusive crises.
To test their theories, the researchers enrolled 230 patients from 31 different sites on a phase 3 trial. Patients had sickle cell anemia (SS) or sickle B0 thalassemia. They were 5 years of age or older, and some were receiving hydroxyurea.
Patients were randomized 2:1 to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day) or placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks. The researchers had discovered high variation in the quality of over-the-counter L-glutamine, so they used pharmaceutical-grade L-glutamine only.
In all, 152 patients received L-glutamine, and 78 received placebo. Demographics and baseline characteristics were similar between the two treatment arms.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% and 57.7%, respectively. And most patients in both arms had sickle cell anemia—89.5% and 91%, respectively.
Safety and efficacy
Dr Niihara said there were no serious adverse events attributable to L-glutamine and no dose modifications due to toxicity.
There were more gastrointestinal events (such as constipation and flatulence) in the L-glutamine arm (8.6%) than in the placebo arm (5.2%). But there was no difference in other adverse events between the two arms.
The study’s primary endpoint was the frequency of painful sickle cell crises. Patients in the L-glutamine arm had 25% fewer crises than patients in the placebo arm (P=0.008).
In patients aged 18 and younger, the median number of sickle cell crises was 3 in the L-glutamine arm and 4 in the placebo arm (P<0.001). The numbers were the same in patients older than 18 (P<0.001) and among patients receiving hydroxyurea (P<0.001).
“Those patients, even though they were on hydroxyurea, they have shown improvements [with L-glutamine],” Dr Niihara said. “And it appears there is some synergistic effect . . ., certainly more than just an additive effect.”
Dr Niihara also noted that patients who received placebo took less than 2 months to experience a first sickle cell crisis, but, for patients on L-glutamine, it took almost 3 months before they had a first crisis. The time to a first crisis was a median of 87 days in the L-glutamine arm and 54 days in the placebo arm (P=0.010).
The study’s secondary endpoint was the frequency of hospitalizations. Compared to patients in the placebo arm, those in the L-glutamine arm had a 33% reduction in hospitalizations (P=0.005) and a 41% reduction in the length of hospital stay (P=0.022).
The researchers also looked at the incidence of ACS and found that L-glutamine was associated with a 58% reduction in ACS. The incidence was 26.9% in the placebo arm and 11.9% in the L-glutamine arm (P=0.006).
“We observed this cascade improvement [with L-glutamine],” Dr Niihara noted. “And what we mean [by that] is that, with glutamine, there is less chance of having painful crises, but if patients have to have crises, they have less chance of being hospitalized. And if they have to be hospitalized, their stays in the hospital are shorter. And, again, if they have to be hospitalized, there’s less chance of them going to the ICU with acute chest syndrome.”
Dr Niihara said the researchers’ next steps are to investigate L-glutamine in a clinical setting, in patients younger than 5 years old, in pregnant women, and as part of combination therapy. ![]()
*Information in the abstract differs from that presented at the meeting.
SAN FRANCISCO—Pharmaceutical-grade L-glutamine can reduce the risk of complications in patients with sickle cell disease (SCD), according to new research.
In a phase 3 study of more than 200 patients, those who received L-glutamine saw a reduction in sickle cell crises, hospitalizations, and the incidence of acute chest syndrome (ACS) when compared to patients who received placebo.
In addition, L-glutamine appeared to have a synergistic effect with hydroxyurea.
Yutaka Niihara, MD, of Emmaus Medical, Inc., in Torrance, California, presented these results at the 2014 ASH Annual Meeting (abstract 86*). Emmaus Medical produced the pharmaceutical-grade L-glutamine used in the study.
Dr Niihara noted that nicotinamide adenine dinucleotide (NAD) is an important physiological antioxidant in red blood cells. But in sickled red blood cells, NAD redox potential is significantly compromised.
So he and his colleagues hypothesized that glutamine, a precursor for NAD, can improve NAD redox potential and modify the destructive effect of increased oxidation and the pathophysiology of SCD. They also theorized that glutamine will decrease the frequency of vaso-occlusive crises.
To test their theories, the researchers enrolled 230 patients from 31 different sites on a phase 3 trial. Patients had sickle cell anemia (SS) or sickle B0 thalassemia. They were 5 years of age or older, and some were receiving hydroxyurea.
Patients were randomized 2:1 to receive L-glutamine at 0.3 g/kg twice daily (maximum 30 g/day) or placebo (maltodextrin) at 0.3 g/kg twice daily for 48 weeks. The researchers had discovered high variation in the quality of over-the-counter L-glutamine, so they used pharmaceutical-grade L-glutamine only.
In all, 152 patients received L-glutamine, and 78 received placebo. Demographics and baseline characteristics were similar between the two treatment arms.
The median age was 19 (range, 5-57) in the L-glutamine arm and 17 (range, 5-58) in the placebo arm. There were more females than males in both arms—52% and 57.7%, respectively. And most patients in both arms had sickle cell anemia—89.5% and 91%, respectively.
Safety and efficacy
Dr Niihara said there were no serious adverse events attributable to L-glutamine and no dose modifications due to toxicity.
There were more gastrointestinal events (such as constipation and flatulence) in the L-glutamine arm (8.6%) than in the placebo arm (5.2%). But there was no difference in other adverse events between the two arms.
The study’s primary endpoint was the frequency of painful sickle cell crises. Patients in the L-glutamine arm had 25% fewer crises than patients in the placebo arm (P=0.008).
In patients aged 18 and younger, the median number of sickle cell crises was 3 in the L-glutamine arm and 4 in the placebo arm (P<0.001). The numbers were the same in patients older than 18 (P<0.001) and among patients receiving hydroxyurea (P<0.001).
“Those patients, even though they were on hydroxyurea, they have shown improvements [with L-glutamine],” Dr Niihara said. “And it appears there is some synergistic effect . . ., certainly more than just an additive effect.”
Dr Niihara also noted that patients who received placebo took less than 2 months to experience a first sickle cell crisis, but, for patients on L-glutamine, it took almost 3 months before they had a first crisis. The time to a first crisis was a median of 87 days in the L-glutamine arm and 54 days in the placebo arm (P=0.010).
The study’s secondary endpoint was the frequency of hospitalizations. Compared to patients in the placebo arm, those in the L-glutamine arm had a 33% reduction in hospitalizations (P=0.005) and a 41% reduction in the length of hospital stay (P=0.022).
The researchers also looked at the incidence of ACS and found that L-glutamine was associated with a 58% reduction in ACS. The incidence was 26.9% in the placebo arm and 11.9% in the L-glutamine arm (P=0.006).
“We observed this cascade improvement [with L-glutamine],” Dr Niihara noted. “And what we mean [by that] is that, with glutamine, there is less chance of having painful crises, but if patients have to have crises, they have less chance of being hospitalized. And if they have to be hospitalized, their stays in the hospital are shorter. And, again, if they have to be hospitalized, there’s less chance of them going to the ICU with acute chest syndrome.”
Dr Niihara said the researchers’ next steps are to investigate L-glutamine in a clinical setting, in patients younger than 5 years old, in pregnant women, and as part of combination therapy. ![]()
*Information in the abstract differs from that presented at the meeting.
Introducing Choosing Wisely®
In this issue of the Journal of Hospital Medicine, we introduce a new recurring feature, Choosing Wisely: Next Steps in Improving Healthcare Value, sponsored by the American Board of Internal Medicine Foundation. The Choosing Wisely campaign is a collaborative initiative led by the American Board of Internal Medicine Foundation, in which specialty societies develop priority lists of activities that physicians should question doing routinely. The program has been broadly embraced by both patient and provider stakeholder groups. More than 35 specialty societies have contributed 26 published lists, including the Society of Hospital Medicine, which published 2 lists, 1 for adults and 1 for pediatrics. These included suggestions such as avoiding urinary catheters for convenience or monitoring of output, avoiding stress ulcer prophylaxis for low‐ to medium‐risk patients, and avoiding routine daily laboratory testing in clinically stable patients. A recent study estimated that up to $5 billion might be saved if just the primary care‐related recommendations were implemented.[1]
THE NEED FOR CHANGE
The Choosing Wisely campaign has so far focused primarily on identifying individual treatments that are not beneficial and potentially harmful to patients. At the Journal of Hospital Medicine, we believe the discipline of hospital medicine is well‐positioned to advance the broader discussion about achieving the triple aim: better healthcare, better health, and better value. Inpatient care represents only 7% of US healthcare encounters but 29% of healthcare expenditures (over $375 billion annually).[2] Patients aged 65 years and over account for 41% of all hospital costs and 34% of all hospital stays. Accordingly, without a change in current utilization patterns, the aging of the baby boomer generation will have a marked impact on expenditures for hospital care. Healthcare costs are increasingly edging out discretionary federal and municipal spending on critical services such as education and scientific research. Historically, federal discretionary spending has averaged 8.3% of gross domestic product (GDP). In 2014, it dropped to 7.2% and is projected to decline to 5.1% in 2024. By comparison, federal spending for Medicare, Medicaid, and health insurance subsidies was 2.1% in 1990[3] but in 2014 is estimated at 4.8% of GDP, rising to 5.7% by 2024.[4]
In conjunction with the deleterious consequences of unchecked growth in healthcare costs on national fiscal health, hospitals are feeling intense and increasing pressure to improve quality and value. In fiscal year 2015, hospitals will be at risk for up to 5.5% of Medicare payments under the parameters of the Hospital Readmission Reduction Program (maximum penalty 3% of base diagnosis‐related group [DRG] payments), Value‐Based Purchasing (maximum withholding 1.5% of base DRG payments), and the Hospital Acquired Conditions Program (maximum penalty 1% of all payments). Simultaneously, long‐standing subsidies are being phased out, including payments to teaching hospitals or for disproportionate share of care delivered to uninsured populations. The challenge for hospital medicine will be to take a leadership role in defining national priorities for change, organizing and guiding a pivot toward lower‐intensity care settings and services, and most importantly, promoting innovation in hospital‐based healthcare delivery.
EXISTING INNOVATIONS
The passage of the Affordable Care Act gave the Centers for Medicare & Medicaid Services (CMS) a platform for spurring innovation in healthcare delivery. In addition to deploying the payment penalty programs described above, the CMS Center for Medicare & Medicaid Innovation has a $10 billion budget to test alternate models of care. Demonstration projects to date include Accountable Care Organization pilots (ACOs, encouraging hospitals to join with community clinicians to provide integrated and coordinated care), the Bundled Payment program (paying providers a lump fee for an extended episode of care rather than service volume), a Comprehensive End Stage Renal Disease Care Initiative, and a variety of other tests of novel delivery and payment models that directly involve hospital medicine.[5] Private insurers are following suit, with an increasing proportion of hospital contracts involving shared savings or risk.
Hospitals are already responding to this new era of cost sharing and cross‐continuum accountability in a variety of creative ways. The University of Utah has developed an award‐winning cost accounting system that integrates highly detailed patient‐level cost data with clinical information to create a value‐driven outcomes tool that enables the hospital to consider costs as they relate to the results of care delivery. In this way, the hospital can justify maintaining high cost/better outcome activities, while targeting high cost/worse outcome practices for improvement.[6] Boston Children's Hospital is leading a group of healthcare systems in the development and application of a series of Standardized Clinical Assessment and Management Plans (SCAMPs), designed to improve patient care while decreasing unnecessary utilization (particularly in cases where existing evidence or guidelines are insufficient or outdated). Unlike traditional clinical care pathways or clinical guidelines, SCAMPs are developed iteratively based on actual internal practices, especially deviations from the standard plan, and their relationship to outcomes.[7, 8]
Local innovations, however, are of limited national importance in bending the cost curve unless broadly disseminated. The last decade has brought a new degree of cross‐institution collaboration to hospital care. Regional consortiums to improve care have existed for years, often prompted by CMS‐funded quality improvement organizations and demonstration projects.[9, 10] CMS's Partnership for Patients program has aimed to reduce hospital‐acquired conditions and readmissions by enrolling hospitals in 26 regional Hospital Engagement Networks.[11] Increasingly, however, hospitals are voluntarily engaging in collaboratives to improve the quality and value of their care. Over 500 US hospitals participate in the American College of Surgeons National Surgical Quality Improvement Program to improve surgical outcomes, nearly 1000 joined the Door‐to‐Balloon Alliance to improve percutaneous catheterization outcomes, and over 1000 joined the Hospital2Home collaborative to improve care transitions.[12, 13, 14] In 2008, the Premier hospital alliance formed QUEST (Quality, Efficiency, Safety and Transparency), a collaborative of approximately 350 members committed to improving a wide range of outcomes, from cost and efficiency to safety and mortality. Most recently, the High Value Healthcare Collaborative was formed, encompassing 19 large healthcare delivery organizations and over 70 million patients, with the central objective of creating a true learning healthcare system. In principle, these boundary‐spanning collaboratives should accelerate change nationally and serve as transformational agents. In practice, outcomes from these efforts have been variable, largely depending on the degree to which hospitals are able to share data, evaluate outcomes, and identify generalizable improvement interventions that can be reliably adopted.
Last, the focus of hospital care has already begun to extend beyond inpatient care. Hospitals already care for more outpatients than they do inpatients, and that trend is expected to continue. In 2012, hospitals treated 34.4 million inpatient admissions, but cared for nearly 675 million outpatient visits, only a fraction of which were emergency department visits or observation stays. From 2011 to 2012, outpatient visits to hospitals increased 2.9%, whereas inpatient admissions declined 1.2%.[15] Hospitals are buying up outpatient practices, creating infusion centers to provide intravenous‐based therapy to outpatients, establishing postdischarge clinics to transition their discharged patients, chartering their own visiting nurse agencies, and testing a host of other outpatient‐focused activities. Combined with an enhanced focus on postacute transitions following an inpatient admission as part of the care continuum, this broadening reach of hospital medicine brings a host of new opportunities for innovation in care delivery and payment models.
CHOOSING WISELY: NEXT STEPS IN IMPROVING HEALTHCARE VALUE
This series will consider a wide range of ways in which hospital medicine can help drive improvements in healthcare value, both from a conceptual standpoint (what to do and why?), as well as demonstration of practical application of these principles (how?). A companion series, Choosing Wisely: Things We Do For No Reason, will focus more explicitly on services such as blood transfusions or diagnostic tests such as creatinine kinase that are commonly overutilized. Example topics of interest for Next Steps include:
- Best methodologies for improvement science in hospital settings, including Lean healthcare, behavioral economics, human factors engineering
- Strategies for reconciling system‐level standardization with the delivery of personalized, patient‐centered care
- Impacts of national policies on hospital‐based improvement efforts: how do ACOs, bundled payments, and medical homes alter hospital practice?
- Reports on creative new ideas to help achieve value: changes in clinical workflow or care pathways, radical physical plant redesign, electronic medical record innovations, payment incentives, provider accountability and more
- Results of models that move the reach of hospital medicine beyond the walls as an integrated part of the care continuum.
We welcome unsolicited proposals for series topics submitted as a 500‐word precis to: [email protected].
Disclosures
Choosing Wisely: Next Steps in Improving Healthcare Value is sponsored by the American Board of Internal Medicine Foundation. Dr. Horwitz is supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. The authors report no conflicts of interest.
- , , , . “Top 5” lists top $5 billion. Arch Intern Med. 2011;171(20):1858–1859.
- Healthcare Cost and Utilization Project. Statistical brief #146. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb146.pdf. Published January 2013. Accessed October 18, 2014.
- Centers for Medicare 32(5):911–920.
- Institute for Relevant Clinical Data Analytics, Inc. Relevant clinical data analytics I. SCAMPs mission statement. 2014. Available at: http://www.scamps.org/index.htm. Accessed October 18, 2014.
- , , , . The long‐term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366(17):1606–1615.
- . Effects of the Premier Hospital Quality Incentive Demonstration on Medicare patient mortality and cost. Health Serv Res. 2009;44(3):821–842.
- Centers for Medicare 1(1):97–104.
- American College of Cardiology. Quality Improvement for Institutions. Hospital to home. 2014. Available at: http://cvquality.acc.org/Initiatives/H2H.aspx. Accessed October 19, 2014.
- American College of Surgeons. National Surgical Quality Improvement Program. 2014. Available at: http://site.acsnsqip.org/. Accessed October 19, 2014.
- . Hospitals on the rebound, show stronger operating margins. Modern Healthcare website. Available at: http://www. modernhealthcare.com/article/20140103/NEWS/301039973. Published January 3, 2014. Accessed October 18, 2014.
In this issue of the Journal of Hospital Medicine, we introduce a new recurring feature, Choosing Wisely: Next Steps in Improving Healthcare Value, sponsored by the American Board of Internal Medicine Foundation. The Choosing Wisely campaign is a collaborative initiative led by the American Board of Internal Medicine Foundation, in which specialty societies develop priority lists of activities that physicians should question doing routinely. The program has been broadly embraced by both patient and provider stakeholder groups. More than 35 specialty societies have contributed 26 published lists, including the Society of Hospital Medicine, which published 2 lists, 1 for adults and 1 for pediatrics. These included suggestions such as avoiding urinary catheters for convenience or monitoring of output, avoiding stress ulcer prophylaxis for low‐ to medium‐risk patients, and avoiding routine daily laboratory testing in clinically stable patients. A recent study estimated that up to $5 billion might be saved if just the primary care‐related recommendations were implemented.[1]
THE NEED FOR CHANGE
The Choosing Wisely campaign has so far focused primarily on identifying individual treatments that are not beneficial and potentially harmful to patients. At the Journal of Hospital Medicine, we believe the discipline of hospital medicine is well‐positioned to advance the broader discussion about achieving the triple aim: better healthcare, better health, and better value. Inpatient care represents only 7% of US healthcare encounters but 29% of healthcare expenditures (over $375 billion annually).[2] Patients aged 65 years and over account for 41% of all hospital costs and 34% of all hospital stays. Accordingly, without a change in current utilization patterns, the aging of the baby boomer generation will have a marked impact on expenditures for hospital care. Healthcare costs are increasingly edging out discretionary federal and municipal spending on critical services such as education and scientific research. Historically, federal discretionary spending has averaged 8.3% of gross domestic product (GDP). In 2014, it dropped to 7.2% and is projected to decline to 5.1% in 2024. By comparison, federal spending for Medicare, Medicaid, and health insurance subsidies was 2.1% in 1990[3] but in 2014 is estimated at 4.8% of GDP, rising to 5.7% by 2024.[4]
In conjunction with the deleterious consequences of unchecked growth in healthcare costs on national fiscal health, hospitals are feeling intense and increasing pressure to improve quality and value. In fiscal year 2015, hospitals will be at risk for up to 5.5% of Medicare payments under the parameters of the Hospital Readmission Reduction Program (maximum penalty 3% of base diagnosis‐related group [DRG] payments), Value‐Based Purchasing (maximum withholding 1.5% of base DRG payments), and the Hospital Acquired Conditions Program (maximum penalty 1% of all payments). Simultaneously, long‐standing subsidies are being phased out, including payments to teaching hospitals or for disproportionate share of care delivered to uninsured populations. The challenge for hospital medicine will be to take a leadership role in defining national priorities for change, organizing and guiding a pivot toward lower‐intensity care settings and services, and most importantly, promoting innovation in hospital‐based healthcare delivery.
EXISTING INNOVATIONS
The passage of the Affordable Care Act gave the Centers for Medicare & Medicaid Services (CMS) a platform for spurring innovation in healthcare delivery. In addition to deploying the payment penalty programs described above, the CMS Center for Medicare & Medicaid Innovation has a $10 billion budget to test alternate models of care. Demonstration projects to date include Accountable Care Organization pilots (ACOs, encouraging hospitals to join with community clinicians to provide integrated and coordinated care), the Bundled Payment program (paying providers a lump fee for an extended episode of care rather than service volume), a Comprehensive End Stage Renal Disease Care Initiative, and a variety of other tests of novel delivery and payment models that directly involve hospital medicine.[5] Private insurers are following suit, with an increasing proportion of hospital contracts involving shared savings or risk.
Hospitals are already responding to this new era of cost sharing and cross‐continuum accountability in a variety of creative ways. The University of Utah has developed an award‐winning cost accounting system that integrates highly detailed patient‐level cost data with clinical information to create a value‐driven outcomes tool that enables the hospital to consider costs as they relate to the results of care delivery. In this way, the hospital can justify maintaining high cost/better outcome activities, while targeting high cost/worse outcome practices for improvement.[6] Boston Children's Hospital is leading a group of healthcare systems in the development and application of a series of Standardized Clinical Assessment and Management Plans (SCAMPs), designed to improve patient care while decreasing unnecessary utilization (particularly in cases where existing evidence or guidelines are insufficient or outdated). Unlike traditional clinical care pathways or clinical guidelines, SCAMPs are developed iteratively based on actual internal practices, especially deviations from the standard plan, and their relationship to outcomes.[7, 8]
Local innovations, however, are of limited national importance in bending the cost curve unless broadly disseminated. The last decade has brought a new degree of cross‐institution collaboration to hospital care. Regional consortiums to improve care have existed for years, often prompted by CMS‐funded quality improvement organizations and demonstration projects.[9, 10] CMS's Partnership for Patients program has aimed to reduce hospital‐acquired conditions and readmissions by enrolling hospitals in 26 regional Hospital Engagement Networks.[11] Increasingly, however, hospitals are voluntarily engaging in collaboratives to improve the quality and value of their care. Over 500 US hospitals participate in the American College of Surgeons National Surgical Quality Improvement Program to improve surgical outcomes, nearly 1000 joined the Door‐to‐Balloon Alliance to improve percutaneous catheterization outcomes, and over 1000 joined the Hospital2Home collaborative to improve care transitions.[12, 13, 14] In 2008, the Premier hospital alliance formed QUEST (Quality, Efficiency, Safety and Transparency), a collaborative of approximately 350 members committed to improving a wide range of outcomes, from cost and efficiency to safety and mortality. Most recently, the High Value Healthcare Collaborative was formed, encompassing 19 large healthcare delivery organizations and over 70 million patients, with the central objective of creating a true learning healthcare system. In principle, these boundary‐spanning collaboratives should accelerate change nationally and serve as transformational agents. In practice, outcomes from these efforts have been variable, largely depending on the degree to which hospitals are able to share data, evaluate outcomes, and identify generalizable improvement interventions that can be reliably adopted.
Last, the focus of hospital care has already begun to extend beyond inpatient care. Hospitals already care for more outpatients than they do inpatients, and that trend is expected to continue. In 2012, hospitals treated 34.4 million inpatient admissions, but cared for nearly 675 million outpatient visits, only a fraction of which were emergency department visits or observation stays. From 2011 to 2012, outpatient visits to hospitals increased 2.9%, whereas inpatient admissions declined 1.2%.[15] Hospitals are buying up outpatient practices, creating infusion centers to provide intravenous‐based therapy to outpatients, establishing postdischarge clinics to transition their discharged patients, chartering their own visiting nurse agencies, and testing a host of other outpatient‐focused activities. Combined with an enhanced focus on postacute transitions following an inpatient admission as part of the care continuum, this broadening reach of hospital medicine brings a host of new opportunities for innovation in care delivery and payment models.
CHOOSING WISELY: NEXT STEPS IN IMPROVING HEALTHCARE VALUE
This series will consider a wide range of ways in which hospital medicine can help drive improvements in healthcare value, both from a conceptual standpoint (what to do and why?), as well as demonstration of practical application of these principles (how?). A companion series, Choosing Wisely: Things We Do For No Reason, will focus more explicitly on services such as blood transfusions or diagnostic tests such as creatinine kinase that are commonly overutilized. Example topics of interest for Next Steps include:
- Best methodologies for improvement science in hospital settings, including Lean healthcare, behavioral economics, human factors engineering
- Strategies for reconciling system‐level standardization with the delivery of personalized, patient‐centered care
- Impacts of national policies on hospital‐based improvement efforts: how do ACOs, bundled payments, and medical homes alter hospital practice?
- Reports on creative new ideas to help achieve value: changes in clinical workflow or care pathways, radical physical plant redesign, electronic medical record innovations, payment incentives, provider accountability and more
- Results of models that move the reach of hospital medicine beyond the walls as an integrated part of the care continuum.
We welcome unsolicited proposals for series topics submitted as a 500‐word precis to: [email protected].
Disclosures
Choosing Wisely: Next Steps in Improving Healthcare Value is sponsored by the American Board of Internal Medicine Foundation. Dr. Horwitz is supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. The authors report no conflicts of interest.
In this issue of the Journal of Hospital Medicine, we introduce a new recurring feature, Choosing Wisely: Next Steps in Improving Healthcare Value, sponsored by the American Board of Internal Medicine Foundation. The Choosing Wisely campaign is a collaborative initiative led by the American Board of Internal Medicine Foundation, in which specialty societies develop priority lists of activities that physicians should question doing routinely. The program has been broadly embraced by both patient and provider stakeholder groups. More than 35 specialty societies have contributed 26 published lists, including the Society of Hospital Medicine, which published 2 lists, 1 for adults and 1 for pediatrics. These included suggestions such as avoiding urinary catheters for convenience or monitoring of output, avoiding stress ulcer prophylaxis for low‐ to medium‐risk patients, and avoiding routine daily laboratory testing in clinically stable patients. A recent study estimated that up to $5 billion might be saved if just the primary care‐related recommendations were implemented.[1]
THE NEED FOR CHANGE
The Choosing Wisely campaign has so far focused primarily on identifying individual treatments that are not beneficial and potentially harmful to patients. At the Journal of Hospital Medicine, we believe the discipline of hospital medicine is well‐positioned to advance the broader discussion about achieving the triple aim: better healthcare, better health, and better value. Inpatient care represents only 7% of US healthcare encounters but 29% of healthcare expenditures (over $375 billion annually).[2] Patients aged 65 years and over account for 41% of all hospital costs and 34% of all hospital stays. Accordingly, without a change in current utilization patterns, the aging of the baby boomer generation will have a marked impact on expenditures for hospital care. Healthcare costs are increasingly edging out discretionary federal and municipal spending on critical services such as education and scientific research. Historically, federal discretionary spending has averaged 8.3% of gross domestic product (GDP). In 2014, it dropped to 7.2% and is projected to decline to 5.1% in 2024. By comparison, federal spending for Medicare, Medicaid, and health insurance subsidies was 2.1% in 1990[3] but in 2014 is estimated at 4.8% of GDP, rising to 5.7% by 2024.[4]
In conjunction with the deleterious consequences of unchecked growth in healthcare costs on national fiscal health, hospitals are feeling intense and increasing pressure to improve quality and value. In fiscal year 2015, hospitals will be at risk for up to 5.5% of Medicare payments under the parameters of the Hospital Readmission Reduction Program (maximum penalty 3% of base diagnosis‐related group [DRG] payments), Value‐Based Purchasing (maximum withholding 1.5% of base DRG payments), and the Hospital Acquired Conditions Program (maximum penalty 1% of all payments). Simultaneously, long‐standing subsidies are being phased out, including payments to teaching hospitals or for disproportionate share of care delivered to uninsured populations. The challenge for hospital medicine will be to take a leadership role in defining national priorities for change, organizing and guiding a pivot toward lower‐intensity care settings and services, and most importantly, promoting innovation in hospital‐based healthcare delivery.
EXISTING INNOVATIONS
The passage of the Affordable Care Act gave the Centers for Medicare & Medicaid Services (CMS) a platform for spurring innovation in healthcare delivery. In addition to deploying the payment penalty programs described above, the CMS Center for Medicare & Medicaid Innovation has a $10 billion budget to test alternate models of care. Demonstration projects to date include Accountable Care Organization pilots (ACOs, encouraging hospitals to join with community clinicians to provide integrated and coordinated care), the Bundled Payment program (paying providers a lump fee for an extended episode of care rather than service volume), a Comprehensive End Stage Renal Disease Care Initiative, and a variety of other tests of novel delivery and payment models that directly involve hospital medicine.[5] Private insurers are following suit, with an increasing proportion of hospital contracts involving shared savings or risk.
Hospitals are already responding to this new era of cost sharing and cross‐continuum accountability in a variety of creative ways. The University of Utah has developed an award‐winning cost accounting system that integrates highly detailed patient‐level cost data with clinical information to create a value‐driven outcomes tool that enables the hospital to consider costs as they relate to the results of care delivery. In this way, the hospital can justify maintaining high cost/better outcome activities, while targeting high cost/worse outcome practices for improvement.[6] Boston Children's Hospital is leading a group of healthcare systems in the development and application of a series of Standardized Clinical Assessment and Management Plans (SCAMPs), designed to improve patient care while decreasing unnecessary utilization (particularly in cases where existing evidence or guidelines are insufficient or outdated). Unlike traditional clinical care pathways or clinical guidelines, SCAMPs are developed iteratively based on actual internal practices, especially deviations from the standard plan, and their relationship to outcomes.[7, 8]
Local innovations, however, are of limited national importance in bending the cost curve unless broadly disseminated. The last decade has brought a new degree of cross‐institution collaboration to hospital care. Regional consortiums to improve care have existed for years, often prompted by CMS‐funded quality improvement organizations and demonstration projects.[9, 10] CMS's Partnership for Patients program has aimed to reduce hospital‐acquired conditions and readmissions by enrolling hospitals in 26 regional Hospital Engagement Networks.[11] Increasingly, however, hospitals are voluntarily engaging in collaboratives to improve the quality and value of their care. Over 500 US hospitals participate in the American College of Surgeons National Surgical Quality Improvement Program to improve surgical outcomes, nearly 1000 joined the Door‐to‐Balloon Alliance to improve percutaneous catheterization outcomes, and over 1000 joined the Hospital2Home collaborative to improve care transitions.[12, 13, 14] In 2008, the Premier hospital alliance formed QUEST (Quality, Efficiency, Safety and Transparency), a collaborative of approximately 350 members committed to improving a wide range of outcomes, from cost and efficiency to safety and mortality. Most recently, the High Value Healthcare Collaborative was formed, encompassing 19 large healthcare delivery organizations and over 70 million patients, with the central objective of creating a true learning healthcare system. In principle, these boundary‐spanning collaboratives should accelerate change nationally and serve as transformational agents. In practice, outcomes from these efforts have been variable, largely depending on the degree to which hospitals are able to share data, evaluate outcomes, and identify generalizable improvement interventions that can be reliably adopted.
Last, the focus of hospital care has already begun to extend beyond inpatient care. Hospitals already care for more outpatients than they do inpatients, and that trend is expected to continue. In 2012, hospitals treated 34.4 million inpatient admissions, but cared for nearly 675 million outpatient visits, only a fraction of which were emergency department visits or observation stays. From 2011 to 2012, outpatient visits to hospitals increased 2.9%, whereas inpatient admissions declined 1.2%.[15] Hospitals are buying up outpatient practices, creating infusion centers to provide intravenous‐based therapy to outpatients, establishing postdischarge clinics to transition their discharged patients, chartering their own visiting nurse agencies, and testing a host of other outpatient‐focused activities. Combined with an enhanced focus on postacute transitions following an inpatient admission as part of the care continuum, this broadening reach of hospital medicine brings a host of new opportunities for innovation in care delivery and payment models.
CHOOSING WISELY: NEXT STEPS IN IMPROVING HEALTHCARE VALUE
This series will consider a wide range of ways in which hospital medicine can help drive improvements in healthcare value, both from a conceptual standpoint (what to do and why?), as well as demonstration of practical application of these principles (how?). A companion series, Choosing Wisely: Things We Do For No Reason, will focus more explicitly on services such as blood transfusions or diagnostic tests such as creatinine kinase that are commonly overutilized. Example topics of interest for Next Steps include:
- Best methodologies for improvement science in hospital settings, including Lean healthcare, behavioral economics, human factors engineering
- Strategies for reconciling system‐level standardization with the delivery of personalized, patient‐centered care
- Impacts of national policies on hospital‐based improvement efforts: how do ACOs, bundled payments, and medical homes alter hospital practice?
- Reports on creative new ideas to help achieve value: changes in clinical workflow or care pathways, radical physical plant redesign, electronic medical record innovations, payment incentives, provider accountability and more
- Results of models that move the reach of hospital medicine beyond the walls as an integrated part of the care continuum.
We welcome unsolicited proposals for series topics submitted as a 500‐word precis to: [email protected].
Disclosures
Choosing Wisely: Next Steps in Improving Healthcare Value is sponsored by the American Board of Internal Medicine Foundation. Dr. Horwitz is supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. The authors report no conflicts of interest.
- , , , . “Top 5” lists top $5 billion. Arch Intern Med. 2011;171(20):1858–1859.
- Healthcare Cost and Utilization Project. Statistical brief #146. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb146.pdf. Published January 2013. Accessed October 18, 2014.
- Centers for Medicare 32(5):911–920.
- Institute for Relevant Clinical Data Analytics, Inc. Relevant clinical data analytics I. SCAMPs mission statement. 2014. Available at: http://www.scamps.org/index.htm. Accessed October 18, 2014.
- , , , . The long‐term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366(17):1606–1615.
- . Effects of the Premier Hospital Quality Incentive Demonstration on Medicare patient mortality and cost. Health Serv Res. 2009;44(3):821–842.
- Centers for Medicare 1(1):97–104.
- American College of Cardiology. Quality Improvement for Institutions. Hospital to home. 2014. Available at: http://cvquality.acc.org/Initiatives/H2H.aspx. Accessed October 19, 2014.
- American College of Surgeons. National Surgical Quality Improvement Program. 2014. Available at: http://site.acsnsqip.org/. Accessed October 19, 2014.
- . Hospitals on the rebound, show stronger operating margins. Modern Healthcare website. Available at: http://www. modernhealthcare.com/article/20140103/NEWS/301039973. Published January 3, 2014. Accessed October 18, 2014.
- , , , . “Top 5” lists top $5 billion. Arch Intern Med. 2011;171(20):1858–1859.
- Healthcare Cost and Utilization Project. Statistical brief #146. Available at: http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb146.pdf. Published January 2013. Accessed October 18, 2014.
- Centers for Medicare 32(5):911–920.
- Institute for Relevant Clinical Data Analytics, Inc. Relevant clinical data analytics I. SCAMPs mission statement. 2014. Available at: http://www.scamps.org/index.htm. Accessed October 18, 2014.
- , , , . The long‐term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366(17):1606–1615.
- . Effects of the Premier Hospital Quality Incentive Demonstration on Medicare patient mortality and cost. Health Serv Res. 2009;44(3):821–842.
- Centers for Medicare 1(1):97–104.
- American College of Cardiology. Quality Improvement for Institutions. Hospital to home. 2014. Available at: http://cvquality.acc.org/Initiatives/H2H.aspx. Accessed October 19, 2014.
- American College of Surgeons. National Surgical Quality Improvement Program. 2014. Available at: http://site.acsnsqip.org/. Accessed October 19, 2014.
- . Hospitals on the rebound, show stronger operating margins. Modern Healthcare website. Available at: http://www. modernhealthcare.com/article/20140103/NEWS/301039973. Published January 3, 2014. Accessed October 18, 2014.
Impact of MC Intervention on QIs
Decompensated cirrhosis (DC) is defined as cirrhosis with at least 1 of the following complications: ascites, hepatocellular carcinoma, bleeding from portal hypertension, or hepatic encephalopathy. Patients with DC have a median survival estimated at 2 years compared to the 12‐year median survival of compensated cirrhotics.[1] In an era where quality of hospital care is being measured, and where progress is being made in the management of several conditions including congestive heart failure and nosocomial infections, little attention has been paid to DC. The burden of chronic liver failure is clear in the United States, where DC leads to more than 150,000 annual admissions to the hospital and accounts for 40,000 deaths annually.[2]
This burden of disease spurred quality improvement efforts in 2010, when a team of experts identified a set of literature‐based parameters or quality indicators (QI) for patients with cirrhosis.[3] We have demonstrated that adherence to these indicators fell far short of desired targets.[4] A year before their publication, an overall compliance of <50% with these metrics was measured at a single medical center.
We sought to improve the quality of care for patients with DC through implementation of mandatory consultation (MC) with a gastroenterologist for all patients admitted with DC. We assessed whether MC was associated with better care and improved outcomes (hospitalization length of stay [LOS], 30‐day readmission, and inpatient mortality) when compared to usual care (UC).[4]
MATERIALS AND METHODS
Design, Setting, and Patients
We conducted a cohort study comparing adherence to QI and outcomes of patients admitted with DC after the institution of an MC to a historical cohort of patients managed with UC (ie, before MC, adherence to QI for this group has been reported elsewhere).[4] Both cohorts included all patients aged >18 years with DC admitted to Baystate Medical Center, a tertiary care medical center in western Massachusetts. The UC cohort was collected between January 1, 2009 and December 31, 2009, and the MC cohort was assembled between June 1, 2011 and June 30, 2012.
As previously reported,[4] patients were considered for inclusion in the historical cohort if their International Classification of DiseasesNinth Revision discharge code pertained to chronic liver disease (see Supporting Information, Appendix 1, in the online version of this article). This list was broad by design to identify all patients with decompensated cirrhosis. A gastroenterologist (R.G.) then manually extracted charts from electronic medical records (EMRs) using a set of predefined clinical criteria, the same in both cohorts, to identify the patients with DC: cirrhosis with concomitant ascites, hepatic encephalopathy, or gastrointestinal (GI) bleeding secondary to portal hypertension. Other types of decompensated states, such as hepatocellular carcinoma, were not included as their management was not detailed in the QI.[3]
We included patients with suspected or established cirrhosis who had ascites confirmed radiographically or by exam, noting shifting dullness or fluid wave. However, patients were excluded if they lacked sufficient peritoneal fluid for bedside or image‐guided paracentesis. Cirrhotic patients were defined as having hepatic encephalopathy if the patient had altered mental status not secondary to seizures, cerebrovascular accident, or alcohol withdrawal. Finally, gastrointestinal bleeding in cirrhotic patients was defined as any upper or lower bleeding prompting hospital admission, or identified in the medical record as clinically significant by the attending physician.
The same QIs were measured in both cohorts. From the QI set,[3] we selected the 16 QIs that would apply to the management of inpatients (see Supporting Information, Appendix 2, in the online version of this article). Indicators developed for outpatient settings were not included. A quality score was calculated for each admission, defined as the proportion of QIs met divided by the number of QIs for which the patient was eligible. For example, a patient with hepatic encephalopathy but without GI bleeding or ascites would have a score calculated as the number of QIs met for hepatic encephalopathy and documentation of transplant evaluation divided by 3 (2 QIs for hepatic encephalopathy and 1 QI for transplant evaluation). If the patient met both QIs for hepatic encephalopathy, but the consultant failed to address liver transplant eligibility, the score would be 2/3=0.666.
After the institution of the MC, all inpatients with DC were identified within 24 hours of admission by a gastroenterologist (R.G., D.D.), who manually reviewed on a daily basis all admissions from EMRs. An author (R.G.) would then contact the admitting team (hospitalist or resident) to make sure that a gastroenterology consult was called and would then obtain the QI by manual extraction from the EMRs.
Of the 16 gastroenterologists who work at the hospital, 12 of them belong to several private practice groups, whereas 4 are employed by the hospital. As part of the intervention, all gastroenterologists were made aware of the intervention 1 month before the starting date, were provided with a checklist of the QIs of interest, and were encouraged to work with the hospitalist attendings to achieve compliance with the QIs. We reminded the gastroenterologists of the ongoing study during routine division meetings and regularly sought feedback from the hospitalists
The MC consisted of a systematic consultation by a gastroenterologist: any identified patient with DC would generate a mandatory GI consultation and would be assigned to a specialist depending on the roster coverage for that day. A close monitoring of the process allowed us to confirm that all patients admitted with DC were seen by a gastroenterologist. Patients were followed until their discharge, death, or readmission to our institution during the study period.
Outcomes
The primary outcome was defined as the rate of adherence to the QIs and overall QI score expressed as a proportion as noted above. Secondary outcomes included in‐hospital mortality, LOS, and 30‐day readmission rate. These parameters were abstracted from the medical record.
Covariates
The hospital EMR (Cerner Corporation, North Kansas City, MO) was used to extract patient demographic parameters such as gender, race, language, and age at time of admission. Other admission‐level details were extracted from the EMR including Model for End‐Stage Liver Disease (MELD) scores, documented comorbidities (including substance abuse, psychiatric diagnosis, diabetes mellitus, renal failure, congestive heart failure, coronary artery disease, and cancer), underlying etiology for cirrhosis, and reason for admission.
The study was approved by Baystate Medical Center's institutional review board.
Statistical Analysis
Summary statistics for outcomes and covariates were calculated as means/standard deviations (SDs), medians/emnterquartile range, and proportions. Univariable statistics (unpaired t tests, 1‐way analysis of variance, Fisher exact test, Spearman correlation) were used to identify possible demographic (eg, age, race) and clinical (eg, admission complaint) predictors of quality score and with 30‐day outcomes. For each admission, a composite quality score, also known as an opportunity model score,[5, 6] was calculated as a fraction (ie, the number of QIs met divided by the total number of possible QIs indicated by the patient's presentation). This fraction was then multiplied by 100 so as to express the QI score as a percent. Possible scores, therefore, ranged from 0 to 100%.
Calculation of the 30‐day incidence proportion of readmission after the first admission was restricted to patients whose readmission occurred in this hospital, and occurring up to 30‐days before study closure (June 1, 2012). In‐hospital death was examined as a function of QI score during that admission. To derive an unbiased, risk‐adjusted estimate of the association between quality score and outcomes, multiple linear regression (opportunity model score [OMS], LOS) or multiple Poisson regression models (30‐day readmission, in‐hospital death) were built. These included a dummy variable for the study period, as well as any potential confounder that was associated at P0.10, with both study period and the outcome in univariable analyses. Robust standard errors were specified to account for multiple admissions within patients. Marginal means or proportions were then estimated with 95% confidence intervals derived using the delta method. All analyses were performed using Stata 12.1 for Windows (StataCorp, College Station, TX).
RESULTS
A total of 303 patients were observed in 695 hospitalizations;149 patients in 379 admissions were observed in the UC cohort, and 154 patients in 316 admissions were observed in the MC cohort. Baseline demographics of all study admissions appear in Table 1. Patients seen in the MC cohort were younger, more likely to speak English, and less likely to be male or have comorbid diabetes mellitus. Most admissions (n=217, 57.2%; 95% confidence interval: 52.3%‐62.3%) were not evaluated by a gastroenterologist in the UC cohort but all were in the MC cohort.
| UC, N=379, N (%) or Mean/SD | MC, N=316, N (%) or Mean/SD | P Value* | |
|---|---|---|---|
| |||
| Age, y | 55.3/12.1 | 53.3/13.6 | 0.05 |
| English speaking | 261 (68.9%) | 261 (82.6%) | <0.001 |
| Male | 251 (66.2%) | 163 (53.5%) | 0.001 |
| Race | <0.001 | ||
| White | 301 (79.4%) | 262 (82.9%) | |
| Black | 31 (8.2%) | 40 (12.7%) | |
| Asian | 16 (4.2%) | 0 (0.0%) | |
| Other | 31 (8.2%) | 14 (4.4%) | |
| Comorbidities | |||
| Substance | 75 (19.8%) | 58 (18.4%) | 0.70 |
| abuse | |||
| Psychiatric | 123 (32.5%) | 103 (32.9%) | 0.94 |
| Diabetes mellitus | 175 (45.4%) | 115 (36.5%) | 0.02 |
| Renal failure | 74 (19.3%) | 55 (17.4%) | 0.50 |
| CHF | 38 (10.0%) | 24 (7.6%) | 0.35 |
| CAD | 26 (6.9%) | 17 (5.4%) | 0.43 |
| Cancer | 48 (12.7%) | 40 (12.7%) | 1.00 |
| Admission MELD | 15.6/6.9 | 17.0/7.0 | 0.006 |
| Serum creatinine | 1.43/1.94 | 1.42/1.30 | 0.91 |
| Reason for admission | |||
| Hepatology/GI | 318 (83.9%) | 257 (81.3%) | 0.42 |
| Renal failure | 85 (22.4%) | 90 (28.5%) | 0.08 |
| Encephalopathy | 151 (39.3%) | 113 (34.9%) | 0.24 |
| GI bleed | 78 (20.5%) | 57 (18.0%) | 1.00 |
| Abdominal pain | 116 (30.7%) | 114 (36.2%) | 0.15 |
| Ascites | 246 (64.9%) | 185 (58.5%) | 0.10 |
Admission Characteristics
The baseline clinical measures of all study admissions appear in Table 1. The UC and MC cohorts had similar characteristics, with the majority of patients with DC admitted for a gastrointestinal/hepatology‐related reason specifically for the management of ascites and hepatic encephalopathy. The patients in the MC cohort had a statistically higher MELD score on admission, which was not clinically relevant.
Quality Measures
Adherence to individual quality indices is shown in Table 2.
| Condition (Denominator) | Quality Indicator (Numerator) | UC (n=379), Met/Indicated | MC (n=316), Met/Indicated | P Value | |
|---|---|---|---|---|---|
| |||||
| Admissions with ascites | |||||
| 1 | Admissions to the hospital because of ascites or encephalopathy. | Diagnostic paracentesis during admission. | 77/193, 39.9%, (32.9%, 46.9%) | 111/135, 82.2% (75.7%, 88.8%) | <0.001 |
| 2 | No fibrinolysis or disseminated intravascular coagulation before paracentesis INR <2.5, >100,000 platelets. | No fresh frozen plasma or platelet replacement given. | 36/37, 97.3% (91.8%, 103.0%) | 41/42, 97.6% (92.8%, 102.4%) | 1.00 |
| 3 | All admissions with diagnostic paracentesis (not limited to admissions for ascites or hepatic encephalopathy). | Cell count differential, total protein, albumin, and culture/sensitivity all performed. | 31/49, 63.3% (49.3%‐77.3%) | 46/72 63.9% (52.7%, 75.0%) | 1.00 |
| 4 | Admissions with known portal hypertension‐related ascites receiving a paracentesis. | Ascitic fluid cell count and differential performed. | 15/104, 14.4% (7.6%‐ 21.3%) | 47/62, 75.8% (63.2%, 88.4%) | <0.001 |
| 5 | Serum sodium 110 mEq/L. | Fluid restriction and discontinuation of diuretics. | NA | NA | NA |
| 6 | Polymorphonuclear count of 250/mm3 in ascites. | Empiric antibiotics, 6 hours of results. | 10/13, 76.9% (50.4%‐ 103.4%) | 16/20, 80.0% (60.8%, 99.2%) | 1.00 |
| 7 | Ascitic fluid, total protein 1.1 gm/dL, serum bilirubin 2.5 mg/dL. | Prophylactic antibiotics. | 4/12, 33.3% (2.0%‐ 64.6%) | 18/30, 60.0%, (41.4%, 78.6%) | 0.18 |
| 8 | Normal renal function. | Salt restriction and diuretics (spironolactone and loop diuretics). | 57/186, 30.6%, (24.0%‐ 37.3%) | 81/122, 66.4%, (57.9%, 74.9%) | <0.001 |
| Total ascites subscore, mean/SD | 30%/36% | 67%/34% | <0.001 | ||
| GI bleeding | |||||
| 9 | Admissions with GI bleeding: variceal and nonvariceal, hematemesis and melena. | Upper endoscopy 24 hours of presentation. | 60/78, 76.9% (67.4%, 86.4%) | 52/57, 91.2% (83.7%, 98.8%) | 0.04 |
| 10 | Esophageal varices (active, stigmata of recent bleeding, or no other causes to explain bleeding). | Endoscopic variceal ligation/sublerotherapy. | 40/46, 87.0% (76.8%‐97.1%) | 30/32, 93.8% (84.9%, 100.0%) | 0.46 |
| 11 | Admissions with established/suspected upper GI bleeding. | Antibiotics within 24 hours of admission. | 27/69, 39.1% (27.3%‐ 50.9%) | 26/58, 44.8% (31.6%, 58.0%) | 0.59 |
| 12 | Admissions with established/suspected variceal bleeding. | Somatostatin/octreotide given within 12 hours of presentation. | 53/69, 76.8%, (66.6%‐ 87.0%) | 49/58, 84.5% (73.8%, 95.2%) | 0.37 |
| 13 | Recurrent bleeding within 72 hours of initial endoscopic hemostasis. | Repeat endoscopy or transjugular intrahepatic portosystemic shunt. | 5/5 100% | 2/3, 66.7% (76.8%, 210.0%) | 0.38 |
| Total GI subscore, mean/SD | 61%/38% | 74%/28% | 0.04 | ||
| Liver transplantation | |||||
| 14 | Admissions with MELD 15 or MELD 15 and decompensated status (ie, all admissions in our study). | Documented evaluation for liver transplantation. | 112/379, 29.6% (24.9%‐ 34.2%) | 231/316, 73.6% (68.7%, 78.5%) | <0.001 |
| Hepatic encephalopathy | |||||
| 15 | Admissions with hepatic encephalopathy. | Search for reversible factors documented. | 81/151, 53.6% (45.6%‐ 61.7 %) | 97/113, 85.8% (79.4%, 92.3%) | <0.001 |
| 16 | Admissions with hepatic encephalopathy. | Oral disaccharides/ rifaximin. | 144/151, 95.3% (91.9 %‐ 98.7 %) | 107/113, 94.7% (90.7%. 98.69%) | 1.00 |
| Total encephalopathy subscore, mean/SD | 75%/28% | 90%/24% | <0.001 | ||
Ascites
The management of ascites yielded 3 main differences between the 2 cohorts. Following the implementation of the MC, 82.2 % (111/135) of ascites‐related admissions led to a diagnostic paracentesis as compared to 39.9% (77/193) in the UC group (P<0.001).
In the MC cohort, 75.8% (47/62) of admissions with known portal hypertensionrelated ascites who received a paracentesis had an ascites cell count checked. In contrast, only 14.4% (15/104) in the UC group receiving paracentesis had a fluid cell count (P<0.001). The management of ascites in patients with normal renal function was optimal, with sodium restriction and diuretics combination in 66.4% (81/122) of the MC cohort, whereas this parameter in the UC cohort was only 30.6% (57/186) (P<0.001). There were no significant differences between the groups for the other QIs.
Variceal Bleeding
The MC group had a higher frequency of endoscopy within 24 hours of admissions than the UC group (91.2% [52/57] vs 76.9% [60/78], respectively; P<0.04). The rest had endoscopy later in the admission. Among admissions with bleeding from varices, banding was done 93.8% of the time for patients in the MC group (30/32), which was not statistically different than 87.0% (40/46) for patients seen in the UC group. In the remaining admissions, endoscopy only revealed nonbleeding large esophageal varices, and the endoscopist opted not to proceed with therapy. There were no statistically significant differences in the rest of the management.
Hepatic Encephalopathy
For hepatic encephalopathy, an empirical treatment was given to 95.3 % (144/151) patients in the UC group and 94.7% (107/113) of the patients in the MC group. We found better documentation of a search for underlying etiologies leading to hepatic encephalopathy in the MC cohort 85.8% (97/113) versus the UC cohort, which was only 53.6% (81/151) (P<0.001).
Evaluation for Liver Transplantation
Better documentation of evaluation for liver transplantation was seen in the MC group 73.6% (231/316) in comparison to the UC group 29.4% (111/379) (P<0.001).
Opportunity Score and Clinical Outcomes
As detailed above, care provided during the MC achieved a higher compliance with the QI shown with the QI score or OMS (Table 3). These improvements were not associated with statistically significant differences in in‐hospital death, LOS, or 30‐day readmission. To explore this further we also examined the direct association between the OMS and outcomes in the MC group by dividing patients into 2 groups: patients whose OMS was 80% and those whose OMS was <80% (see Supporting Information, Appendix 4, in the online version of this article). Although there were trends toward decreased in‐hospital death (6.4% vs 8.6%, P=0.26), increased 30‐day readmission (33.8% vs 23.0%, P=0.27), and decreased LOS (6.2 days vs 6.6 days, P=0.77), none of these differences achieved statistical significance.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| UC | MC | Difference | UC | MC | Difference | |
| ||||||
| Opportunity model score | 0.46 | 0.77 | +0.31 (0.24, 0.39) | 0.46 | 0.77 | +0.30(0.23, 0.37) |
| In‐hospital death | 7.1% | 8.5% | +1.4 (0.3, +5.6) | 7.5% | 7.9% | +0.4% (4.0%, +4.5%) |
| Readmission within 30 days | 39.6% | 32.6% | 7.0% (16.4%, +2.5%) | 40.0% | 31.8% | 8.2%(18.0%, +1.5%) |
| Length of stay | 6.1d | 6.2d | +0.1d (1.0 d, +1.2 d) | 6.1d | 6.2d | +0.1d (1.0 d, +1.2d) |
Mandatory Consultation Subgroups: Employed Versus Private Physicians
The type of employment of the gastroenterologist on consultation (employed by the hospital vs private practice) affected the management of the patients admitted with DC (see Supporting Information, Appendix 3, in the online version of this article). Patients seen by a hospital‐employed gastroenterologist were more likely to have a better documentation in regard to evaluation for liver transplantation and better management of ascites. Except for the prescription of antibiotics in patients presenting with GI bleeding, which were more often given by the employed physician (63% vs 23%, P=0.004), the management of hepatic encephalopathy and GI bleeding was similar between employed and private‐practice physicians.
DISCUSSION
In this evaluation of an MC intervention for patients with DC cared for at a large tertiary academic medical center, we found that the implementation of a routine consultation by a gastroenterologist led to greater adherence to recommended care processes when compared to UC. Overall, the management of ascites and the documentation of evaluation for liver transplantation were statistically superior in the intervention (MC) group. UC and MC were similar with respect to treatment of variceal bleeding and hepatic encephalopathy. Although we did not demonstrate changes in mortality, readmission, or LOS as a result of the MC intervention, our study was underpowered to detect clinically meaningful effects.
The gaps in care of patients with cirrhosis were reported before and after the publication of the formal QIs.[7, 8, 9, 10] These gaps remain relevant in the face of an increasing prevalence of DC along with a recent publication suggesting an underestimation of the burden of liver disease in the United States.[11] Ours is the first study to evaluate the impact on inpatients with DC of a liver service with a systematic, mandatory, specialist consultation. A previous study[12] had shown that a GI consultation would improve the care of patients with DC, but excluded patients with variceal bleeding, did not specifically measure the compliance with QIs, and more important, the GI consult was not mandatory.
Our study has several limitations that must be considered while weighing its findings. The patients were not randomly assigned but followed a pre‐established distribution depending on the call schedule. Some of the improvement we noted might be the result of secular trends; however, this remains unlikely given the lack of national initiatives or pay for performance programs. In the UC cohort, patients who were nonEnglish‐speaking were associated with a lower QI score, which could account for part of the improvement seen in the MC group that has a more prominent English‐speaking cohort. Readmissions could have occurred at other hospitals, and patients were not monitored in an outpatient setting. We did not observe a change in the secondary outcomes (30‐day readmission, LOS, in‐hospital death); however, our study was underpowered for that purpose. Given the complexity of the billing process we did not collect the costs of the MC, which is another limitation of our work. Future studies are needed to determine the cost‐effectiveness of the intervention.
This study shows that a dedicated team of physicians focused on compliance with QIs can achieve a rapid improvement, over a year, in providing higher‐quality care. This may be relevant at other institutions. The strength of our study is that our large tertiary academic medical center serves a large catchment area, with a mix of patients from both rural and urban communities. It is located in Massachusetts, where most of the population has had access to healthcare since 2006. Therefore, although this is a single‐center study, we expect our findings to be more generalizable and less subject to selection bias than other single‐center studies.
Importantly, the compliance with QIs was often far from being perfect in the MC group and was different across type of employment of providers, reflecting the challenges in changing practice among physicians.[13] In fact the QI scores of the private practice group did not change, and mirror the compliance observed at our institution in the previous study, before the implementation of the MC.[4] The difference in performance according to the type of employment of providers stems from 2 factors. First, a better documentation of the need of formal evaluation for liver transplantation by the employed gastroenterologists resulted in better compliance with this QI. Second, and more important, among the employed physicians, there was a readiness to assist the hospitalist with diagnostic/therapeutic paracentesis without relying on, for example, an interventional radiologist. This is reflected by the higher score in the management of ascites. Although our study was not designed to answer this directly, employed physicians may have been more engaged in the project and showed a greater willingness to change practice. In the future, linking reimbursement to quality of care will lead to improved accountability of consultants.
In this study we show that a direct involvement of a gastroenterologist improves the care of inpatients as measured by QIs. We theorize that a better coordination of the transition to outpatient care involving the specialist should lead to better outcomes, specifically a reduction in the 22% observed readmission rate within 30 days of patients with DC.[14, 15] As we move forward, a broader definition of outcomes should be addressed, taking into account patient‐related outcomes and preferences.[16] Future studies should define the relationship between the gastroenterologist and the hospitalist service, the role of physician assistants and nurse practitioners in implementing and monitoring compliance with QIs, and define how physicians and patients can be made accountable in the transition to the outpatient setting.
Disclosures
R.G.: Conception, data collection and interpretation, manuscript. J.F.: Data management, data analysis, manuscript. P.V.: Conception, data analysis, manuscript. P.L.: Conception, data interpretation, manuscript. T.L.: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Conception, data interpretation, manuscript. D.D.: Conception, data collection and interpretation, manuscript. A.B.: Data collection. J.S.: Data collection. Source of funding: internal. The authors report no conflicts of interest.
- , , . Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231.
- , , , . Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11:850–858.
- , , , et al. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717.
- , , , , , Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014:34:204–210.
- , . All‐or‐none measurement raises the bar on performance. JAMA. 2006;295:1168–1170.
- Joint Commission on Accreditation of Healthcare Organizations. Quality report user guide. Available at: http://www.jointcommission.org. Accessed May 30, 2011.
- , , , et al. Management of patients with cirrhosis in Southern California: results of a practitioner survey. J Clin Gastroenterol. 2006;40:156–161.
- , , , et al. Spanish Collaborative Study Group on Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–440.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012 143(1):70–77.
- , , , et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659.
- , , , , . Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145:375–382.
- , , , et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized with advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259.
- , , , et al. Hospital Readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252.
- . Patient‐reported outcomes of cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1043–1045.
Decompensated cirrhosis (DC) is defined as cirrhosis with at least 1 of the following complications: ascites, hepatocellular carcinoma, bleeding from portal hypertension, or hepatic encephalopathy. Patients with DC have a median survival estimated at 2 years compared to the 12‐year median survival of compensated cirrhotics.[1] In an era where quality of hospital care is being measured, and where progress is being made in the management of several conditions including congestive heart failure and nosocomial infections, little attention has been paid to DC. The burden of chronic liver failure is clear in the United States, where DC leads to more than 150,000 annual admissions to the hospital and accounts for 40,000 deaths annually.[2]
This burden of disease spurred quality improvement efforts in 2010, when a team of experts identified a set of literature‐based parameters or quality indicators (QI) for patients with cirrhosis.[3] We have demonstrated that adherence to these indicators fell far short of desired targets.[4] A year before their publication, an overall compliance of <50% with these metrics was measured at a single medical center.
We sought to improve the quality of care for patients with DC through implementation of mandatory consultation (MC) with a gastroenterologist for all patients admitted with DC. We assessed whether MC was associated with better care and improved outcomes (hospitalization length of stay [LOS], 30‐day readmission, and inpatient mortality) when compared to usual care (UC).[4]
MATERIALS AND METHODS
Design, Setting, and Patients
We conducted a cohort study comparing adherence to QI and outcomes of patients admitted with DC after the institution of an MC to a historical cohort of patients managed with UC (ie, before MC, adherence to QI for this group has been reported elsewhere).[4] Both cohorts included all patients aged >18 years with DC admitted to Baystate Medical Center, a tertiary care medical center in western Massachusetts. The UC cohort was collected between January 1, 2009 and December 31, 2009, and the MC cohort was assembled between June 1, 2011 and June 30, 2012.
As previously reported,[4] patients were considered for inclusion in the historical cohort if their International Classification of DiseasesNinth Revision discharge code pertained to chronic liver disease (see Supporting Information, Appendix 1, in the online version of this article). This list was broad by design to identify all patients with decompensated cirrhosis. A gastroenterologist (R.G.) then manually extracted charts from electronic medical records (EMRs) using a set of predefined clinical criteria, the same in both cohorts, to identify the patients with DC: cirrhosis with concomitant ascites, hepatic encephalopathy, or gastrointestinal (GI) bleeding secondary to portal hypertension. Other types of decompensated states, such as hepatocellular carcinoma, were not included as their management was not detailed in the QI.[3]
We included patients with suspected or established cirrhosis who had ascites confirmed radiographically or by exam, noting shifting dullness or fluid wave. However, patients were excluded if they lacked sufficient peritoneal fluid for bedside or image‐guided paracentesis. Cirrhotic patients were defined as having hepatic encephalopathy if the patient had altered mental status not secondary to seizures, cerebrovascular accident, or alcohol withdrawal. Finally, gastrointestinal bleeding in cirrhotic patients was defined as any upper or lower bleeding prompting hospital admission, or identified in the medical record as clinically significant by the attending physician.
The same QIs were measured in both cohorts. From the QI set,[3] we selected the 16 QIs that would apply to the management of inpatients (see Supporting Information, Appendix 2, in the online version of this article). Indicators developed for outpatient settings were not included. A quality score was calculated for each admission, defined as the proportion of QIs met divided by the number of QIs for which the patient was eligible. For example, a patient with hepatic encephalopathy but without GI bleeding or ascites would have a score calculated as the number of QIs met for hepatic encephalopathy and documentation of transplant evaluation divided by 3 (2 QIs for hepatic encephalopathy and 1 QI for transplant evaluation). If the patient met both QIs for hepatic encephalopathy, but the consultant failed to address liver transplant eligibility, the score would be 2/3=0.666.
After the institution of the MC, all inpatients with DC were identified within 24 hours of admission by a gastroenterologist (R.G., D.D.), who manually reviewed on a daily basis all admissions from EMRs. An author (R.G.) would then contact the admitting team (hospitalist or resident) to make sure that a gastroenterology consult was called and would then obtain the QI by manual extraction from the EMRs.
Of the 16 gastroenterologists who work at the hospital, 12 of them belong to several private practice groups, whereas 4 are employed by the hospital. As part of the intervention, all gastroenterologists were made aware of the intervention 1 month before the starting date, were provided with a checklist of the QIs of interest, and were encouraged to work with the hospitalist attendings to achieve compliance with the QIs. We reminded the gastroenterologists of the ongoing study during routine division meetings and regularly sought feedback from the hospitalists
The MC consisted of a systematic consultation by a gastroenterologist: any identified patient with DC would generate a mandatory GI consultation and would be assigned to a specialist depending on the roster coverage for that day. A close monitoring of the process allowed us to confirm that all patients admitted with DC were seen by a gastroenterologist. Patients were followed until their discharge, death, or readmission to our institution during the study period.
Outcomes
The primary outcome was defined as the rate of adherence to the QIs and overall QI score expressed as a proportion as noted above. Secondary outcomes included in‐hospital mortality, LOS, and 30‐day readmission rate. These parameters were abstracted from the medical record.
Covariates
The hospital EMR (Cerner Corporation, North Kansas City, MO) was used to extract patient demographic parameters such as gender, race, language, and age at time of admission. Other admission‐level details were extracted from the EMR including Model for End‐Stage Liver Disease (MELD) scores, documented comorbidities (including substance abuse, psychiatric diagnosis, diabetes mellitus, renal failure, congestive heart failure, coronary artery disease, and cancer), underlying etiology for cirrhosis, and reason for admission.
The study was approved by Baystate Medical Center's institutional review board.
Statistical Analysis
Summary statistics for outcomes and covariates were calculated as means/standard deviations (SDs), medians/emnterquartile range, and proportions. Univariable statistics (unpaired t tests, 1‐way analysis of variance, Fisher exact test, Spearman correlation) were used to identify possible demographic (eg, age, race) and clinical (eg, admission complaint) predictors of quality score and with 30‐day outcomes. For each admission, a composite quality score, also known as an opportunity model score,[5, 6] was calculated as a fraction (ie, the number of QIs met divided by the total number of possible QIs indicated by the patient's presentation). This fraction was then multiplied by 100 so as to express the QI score as a percent. Possible scores, therefore, ranged from 0 to 100%.
Calculation of the 30‐day incidence proportion of readmission after the first admission was restricted to patients whose readmission occurred in this hospital, and occurring up to 30‐days before study closure (June 1, 2012). In‐hospital death was examined as a function of QI score during that admission. To derive an unbiased, risk‐adjusted estimate of the association between quality score and outcomes, multiple linear regression (opportunity model score [OMS], LOS) or multiple Poisson regression models (30‐day readmission, in‐hospital death) were built. These included a dummy variable for the study period, as well as any potential confounder that was associated at P0.10, with both study period and the outcome in univariable analyses. Robust standard errors were specified to account for multiple admissions within patients. Marginal means or proportions were then estimated with 95% confidence intervals derived using the delta method. All analyses were performed using Stata 12.1 for Windows (StataCorp, College Station, TX).
RESULTS
A total of 303 patients were observed in 695 hospitalizations;149 patients in 379 admissions were observed in the UC cohort, and 154 patients in 316 admissions were observed in the MC cohort. Baseline demographics of all study admissions appear in Table 1. Patients seen in the MC cohort were younger, more likely to speak English, and less likely to be male or have comorbid diabetes mellitus. Most admissions (n=217, 57.2%; 95% confidence interval: 52.3%‐62.3%) were not evaluated by a gastroenterologist in the UC cohort but all were in the MC cohort.
| UC, N=379, N (%) or Mean/SD | MC, N=316, N (%) or Mean/SD | P Value* | |
|---|---|---|---|
| |||
| Age, y | 55.3/12.1 | 53.3/13.6 | 0.05 |
| English speaking | 261 (68.9%) | 261 (82.6%) | <0.001 |
| Male | 251 (66.2%) | 163 (53.5%) | 0.001 |
| Race | <0.001 | ||
| White | 301 (79.4%) | 262 (82.9%) | |
| Black | 31 (8.2%) | 40 (12.7%) | |
| Asian | 16 (4.2%) | 0 (0.0%) | |
| Other | 31 (8.2%) | 14 (4.4%) | |
| Comorbidities | |||
| Substance | 75 (19.8%) | 58 (18.4%) | 0.70 |
| abuse | |||
| Psychiatric | 123 (32.5%) | 103 (32.9%) | 0.94 |
| Diabetes mellitus | 175 (45.4%) | 115 (36.5%) | 0.02 |
| Renal failure | 74 (19.3%) | 55 (17.4%) | 0.50 |
| CHF | 38 (10.0%) | 24 (7.6%) | 0.35 |
| CAD | 26 (6.9%) | 17 (5.4%) | 0.43 |
| Cancer | 48 (12.7%) | 40 (12.7%) | 1.00 |
| Admission MELD | 15.6/6.9 | 17.0/7.0 | 0.006 |
| Serum creatinine | 1.43/1.94 | 1.42/1.30 | 0.91 |
| Reason for admission | |||
| Hepatology/GI | 318 (83.9%) | 257 (81.3%) | 0.42 |
| Renal failure | 85 (22.4%) | 90 (28.5%) | 0.08 |
| Encephalopathy | 151 (39.3%) | 113 (34.9%) | 0.24 |
| GI bleed | 78 (20.5%) | 57 (18.0%) | 1.00 |
| Abdominal pain | 116 (30.7%) | 114 (36.2%) | 0.15 |
| Ascites | 246 (64.9%) | 185 (58.5%) | 0.10 |
Admission Characteristics
The baseline clinical measures of all study admissions appear in Table 1. The UC and MC cohorts had similar characteristics, with the majority of patients with DC admitted for a gastrointestinal/hepatology‐related reason specifically for the management of ascites and hepatic encephalopathy. The patients in the MC cohort had a statistically higher MELD score on admission, which was not clinically relevant.
Quality Measures
Adherence to individual quality indices is shown in Table 2.
| Condition (Denominator) | Quality Indicator (Numerator) | UC (n=379), Met/Indicated | MC (n=316), Met/Indicated | P Value | |
|---|---|---|---|---|---|
| |||||
| Admissions with ascites | |||||
| 1 | Admissions to the hospital because of ascites or encephalopathy. | Diagnostic paracentesis during admission. | 77/193, 39.9%, (32.9%, 46.9%) | 111/135, 82.2% (75.7%, 88.8%) | <0.001 |
| 2 | No fibrinolysis or disseminated intravascular coagulation before paracentesis INR <2.5, >100,000 platelets. | No fresh frozen plasma or platelet replacement given. | 36/37, 97.3% (91.8%, 103.0%) | 41/42, 97.6% (92.8%, 102.4%) | 1.00 |
| 3 | All admissions with diagnostic paracentesis (not limited to admissions for ascites or hepatic encephalopathy). | Cell count differential, total protein, albumin, and culture/sensitivity all performed. | 31/49, 63.3% (49.3%‐77.3%) | 46/72 63.9% (52.7%, 75.0%) | 1.00 |
| 4 | Admissions with known portal hypertension‐related ascites receiving a paracentesis. | Ascitic fluid cell count and differential performed. | 15/104, 14.4% (7.6%‐ 21.3%) | 47/62, 75.8% (63.2%, 88.4%) | <0.001 |
| 5 | Serum sodium 110 mEq/L. | Fluid restriction and discontinuation of diuretics. | NA | NA | NA |
| 6 | Polymorphonuclear count of 250/mm3 in ascites. | Empiric antibiotics, 6 hours of results. | 10/13, 76.9% (50.4%‐ 103.4%) | 16/20, 80.0% (60.8%, 99.2%) | 1.00 |
| 7 | Ascitic fluid, total protein 1.1 gm/dL, serum bilirubin 2.5 mg/dL. | Prophylactic antibiotics. | 4/12, 33.3% (2.0%‐ 64.6%) | 18/30, 60.0%, (41.4%, 78.6%) | 0.18 |
| 8 | Normal renal function. | Salt restriction and diuretics (spironolactone and loop diuretics). | 57/186, 30.6%, (24.0%‐ 37.3%) | 81/122, 66.4%, (57.9%, 74.9%) | <0.001 |
| Total ascites subscore, mean/SD | 30%/36% | 67%/34% | <0.001 | ||
| GI bleeding | |||||
| 9 | Admissions with GI bleeding: variceal and nonvariceal, hematemesis and melena. | Upper endoscopy 24 hours of presentation. | 60/78, 76.9% (67.4%, 86.4%) | 52/57, 91.2% (83.7%, 98.8%) | 0.04 |
| 10 | Esophageal varices (active, stigmata of recent bleeding, or no other causes to explain bleeding). | Endoscopic variceal ligation/sublerotherapy. | 40/46, 87.0% (76.8%‐97.1%) | 30/32, 93.8% (84.9%, 100.0%) | 0.46 |
| 11 | Admissions with established/suspected upper GI bleeding. | Antibiotics within 24 hours of admission. | 27/69, 39.1% (27.3%‐ 50.9%) | 26/58, 44.8% (31.6%, 58.0%) | 0.59 |
| 12 | Admissions with established/suspected variceal bleeding. | Somatostatin/octreotide given within 12 hours of presentation. | 53/69, 76.8%, (66.6%‐ 87.0%) | 49/58, 84.5% (73.8%, 95.2%) | 0.37 |
| 13 | Recurrent bleeding within 72 hours of initial endoscopic hemostasis. | Repeat endoscopy or transjugular intrahepatic portosystemic shunt. | 5/5 100% | 2/3, 66.7% (76.8%, 210.0%) | 0.38 |
| Total GI subscore, mean/SD | 61%/38% | 74%/28% | 0.04 | ||
| Liver transplantation | |||||
| 14 | Admissions with MELD 15 or MELD 15 and decompensated status (ie, all admissions in our study). | Documented evaluation for liver transplantation. | 112/379, 29.6% (24.9%‐ 34.2%) | 231/316, 73.6% (68.7%, 78.5%) | <0.001 |
| Hepatic encephalopathy | |||||
| 15 | Admissions with hepatic encephalopathy. | Search for reversible factors documented. | 81/151, 53.6% (45.6%‐ 61.7 %) | 97/113, 85.8% (79.4%, 92.3%) | <0.001 |
| 16 | Admissions with hepatic encephalopathy. | Oral disaccharides/ rifaximin. | 144/151, 95.3% (91.9 %‐ 98.7 %) | 107/113, 94.7% (90.7%. 98.69%) | 1.00 |
| Total encephalopathy subscore, mean/SD | 75%/28% | 90%/24% | <0.001 | ||
Ascites
The management of ascites yielded 3 main differences between the 2 cohorts. Following the implementation of the MC, 82.2 % (111/135) of ascites‐related admissions led to a diagnostic paracentesis as compared to 39.9% (77/193) in the UC group (P<0.001).
In the MC cohort, 75.8% (47/62) of admissions with known portal hypertensionrelated ascites who received a paracentesis had an ascites cell count checked. In contrast, only 14.4% (15/104) in the UC group receiving paracentesis had a fluid cell count (P<0.001). The management of ascites in patients with normal renal function was optimal, with sodium restriction and diuretics combination in 66.4% (81/122) of the MC cohort, whereas this parameter in the UC cohort was only 30.6% (57/186) (P<0.001). There were no significant differences between the groups for the other QIs.
Variceal Bleeding
The MC group had a higher frequency of endoscopy within 24 hours of admissions than the UC group (91.2% [52/57] vs 76.9% [60/78], respectively; P<0.04). The rest had endoscopy later in the admission. Among admissions with bleeding from varices, banding was done 93.8% of the time for patients in the MC group (30/32), which was not statistically different than 87.0% (40/46) for patients seen in the UC group. In the remaining admissions, endoscopy only revealed nonbleeding large esophageal varices, and the endoscopist opted not to proceed with therapy. There were no statistically significant differences in the rest of the management.
Hepatic Encephalopathy
For hepatic encephalopathy, an empirical treatment was given to 95.3 % (144/151) patients in the UC group and 94.7% (107/113) of the patients in the MC group. We found better documentation of a search for underlying etiologies leading to hepatic encephalopathy in the MC cohort 85.8% (97/113) versus the UC cohort, which was only 53.6% (81/151) (P<0.001).
Evaluation for Liver Transplantation
Better documentation of evaluation for liver transplantation was seen in the MC group 73.6% (231/316) in comparison to the UC group 29.4% (111/379) (P<0.001).
Opportunity Score and Clinical Outcomes
As detailed above, care provided during the MC achieved a higher compliance with the QI shown with the QI score or OMS (Table 3). These improvements were not associated with statistically significant differences in in‐hospital death, LOS, or 30‐day readmission. To explore this further we also examined the direct association between the OMS and outcomes in the MC group by dividing patients into 2 groups: patients whose OMS was 80% and those whose OMS was <80% (see Supporting Information, Appendix 4, in the online version of this article). Although there were trends toward decreased in‐hospital death (6.4% vs 8.6%, P=0.26), increased 30‐day readmission (33.8% vs 23.0%, P=0.27), and decreased LOS (6.2 days vs 6.6 days, P=0.77), none of these differences achieved statistical significance.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| UC | MC | Difference | UC | MC | Difference | |
| ||||||
| Opportunity model score | 0.46 | 0.77 | +0.31 (0.24, 0.39) | 0.46 | 0.77 | +0.30(0.23, 0.37) |
| In‐hospital death | 7.1% | 8.5% | +1.4 (0.3, +5.6) | 7.5% | 7.9% | +0.4% (4.0%, +4.5%) |
| Readmission within 30 days | 39.6% | 32.6% | 7.0% (16.4%, +2.5%) | 40.0% | 31.8% | 8.2%(18.0%, +1.5%) |
| Length of stay | 6.1d | 6.2d | +0.1d (1.0 d, +1.2 d) | 6.1d | 6.2d | +0.1d (1.0 d, +1.2d) |
Mandatory Consultation Subgroups: Employed Versus Private Physicians
The type of employment of the gastroenterologist on consultation (employed by the hospital vs private practice) affected the management of the patients admitted with DC (see Supporting Information, Appendix 3, in the online version of this article). Patients seen by a hospital‐employed gastroenterologist were more likely to have a better documentation in regard to evaluation for liver transplantation and better management of ascites. Except for the prescription of antibiotics in patients presenting with GI bleeding, which were more often given by the employed physician (63% vs 23%, P=0.004), the management of hepatic encephalopathy and GI bleeding was similar between employed and private‐practice physicians.
DISCUSSION
In this evaluation of an MC intervention for patients with DC cared for at a large tertiary academic medical center, we found that the implementation of a routine consultation by a gastroenterologist led to greater adherence to recommended care processes when compared to UC. Overall, the management of ascites and the documentation of evaluation for liver transplantation were statistically superior in the intervention (MC) group. UC and MC were similar with respect to treatment of variceal bleeding and hepatic encephalopathy. Although we did not demonstrate changes in mortality, readmission, or LOS as a result of the MC intervention, our study was underpowered to detect clinically meaningful effects.
The gaps in care of patients with cirrhosis were reported before and after the publication of the formal QIs.[7, 8, 9, 10] These gaps remain relevant in the face of an increasing prevalence of DC along with a recent publication suggesting an underestimation of the burden of liver disease in the United States.[11] Ours is the first study to evaluate the impact on inpatients with DC of a liver service with a systematic, mandatory, specialist consultation. A previous study[12] had shown that a GI consultation would improve the care of patients with DC, but excluded patients with variceal bleeding, did not specifically measure the compliance with QIs, and more important, the GI consult was not mandatory.
Our study has several limitations that must be considered while weighing its findings. The patients were not randomly assigned but followed a pre‐established distribution depending on the call schedule. Some of the improvement we noted might be the result of secular trends; however, this remains unlikely given the lack of national initiatives or pay for performance programs. In the UC cohort, patients who were nonEnglish‐speaking were associated with a lower QI score, which could account for part of the improvement seen in the MC group that has a more prominent English‐speaking cohort. Readmissions could have occurred at other hospitals, and patients were not monitored in an outpatient setting. We did not observe a change in the secondary outcomes (30‐day readmission, LOS, in‐hospital death); however, our study was underpowered for that purpose. Given the complexity of the billing process we did not collect the costs of the MC, which is another limitation of our work. Future studies are needed to determine the cost‐effectiveness of the intervention.
This study shows that a dedicated team of physicians focused on compliance with QIs can achieve a rapid improvement, over a year, in providing higher‐quality care. This may be relevant at other institutions. The strength of our study is that our large tertiary academic medical center serves a large catchment area, with a mix of patients from both rural and urban communities. It is located in Massachusetts, where most of the population has had access to healthcare since 2006. Therefore, although this is a single‐center study, we expect our findings to be more generalizable and less subject to selection bias than other single‐center studies.
Importantly, the compliance with QIs was often far from being perfect in the MC group and was different across type of employment of providers, reflecting the challenges in changing practice among physicians.[13] In fact the QI scores of the private practice group did not change, and mirror the compliance observed at our institution in the previous study, before the implementation of the MC.[4] The difference in performance according to the type of employment of providers stems from 2 factors. First, a better documentation of the need of formal evaluation for liver transplantation by the employed gastroenterologists resulted in better compliance with this QI. Second, and more important, among the employed physicians, there was a readiness to assist the hospitalist with diagnostic/therapeutic paracentesis without relying on, for example, an interventional radiologist. This is reflected by the higher score in the management of ascites. Although our study was not designed to answer this directly, employed physicians may have been more engaged in the project and showed a greater willingness to change practice. In the future, linking reimbursement to quality of care will lead to improved accountability of consultants.
In this study we show that a direct involvement of a gastroenterologist improves the care of inpatients as measured by QIs. We theorize that a better coordination of the transition to outpatient care involving the specialist should lead to better outcomes, specifically a reduction in the 22% observed readmission rate within 30 days of patients with DC.[14, 15] As we move forward, a broader definition of outcomes should be addressed, taking into account patient‐related outcomes and preferences.[16] Future studies should define the relationship between the gastroenterologist and the hospitalist service, the role of physician assistants and nurse practitioners in implementing and monitoring compliance with QIs, and define how physicians and patients can be made accountable in the transition to the outpatient setting.
Disclosures
R.G.: Conception, data collection and interpretation, manuscript. J.F.: Data management, data analysis, manuscript. P.V.: Conception, data analysis, manuscript. P.L.: Conception, data interpretation, manuscript. T.L.: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Conception, data interpretation, manuscript. D.D.: Conception, data collection and interpretation, manuscript. A.B.: Data collection. J.S.: Data collection. Source of funding: internal. The authors report no conflicts of interest.
Decompensated cirrhosis (DC) is defined as cirrhosis with at least 1 of the following complications: ascites, hepatocellular carcinoma, bleeding from portal hypertension, or hepatic encephalopathy. Patients with DC have a median survival estimated at 2 years compared to the 12‐year median survival of compensated cirrhotics.[1] In an era where quality of hospital care is being measured, and where progress is being made in the management of several conditions including congestive heart failure and nosocomial infections, little attention has been paid to DC. The burden of chronic liver failure is clear in the United States, where DC leads to more than 150,000 annual admissions to the hospital and accounts for 40,000 deaths annually.[2]
This burden of disease spurred quality improvement efforts in 2010, when a team of experts identified a set of literature‐based parameters or quality indicators (QI) for patients with cirrhosis.[3] We have demonstrated that adherence to these indicators fell far short of desired targets.[4] A year before their publication, an overall compliance of <50% with these metrics was measured at a single medical center.
We sought to improve the quality of care for patients with DC through implementation of mandatory consultation (MC) with a gastroenterologist for all patients admitted with DC. We assessed whether MC was associated with better care and improved outcomes (hospitalization length of stay [LOS], 30‐day readmission, and inpatient mortality) when compared to usual care (UC).[4]
MATERIALS AND METHODS
Design, Setting, and Patients
We conducted a cohort study comparing adherence to QI and outcomes of patients admitted with DC after the institution of an MC to a historical cohort of patients managed with UC (ie, before MC, adherence to QI for this group has been reported elsewhere).[4] Both cohorts included all patients aged >18 years with DC admitted to Baystate Medical Center, a tertiary care medical center in western Massachusetts. The UC cohort was collected between January 1, 2009 and December 31, 2009, and the MC cohort was assembled between June 1, 2011 and June 30, 2012.
As previously reported,[4] patients were considered for inclusion in the historical cohort if their International Classification of DiseasesNinth Revision discharge code pertained to chronic liver disease (see Supporting Information, Appendix 1, in the online version of this article). This list was broad by design to identify all patients with decompensated cirrhosis. A gastroenterologist (R.G.) then manually extracted charts from electronic medical records (EMRs) using a set of predefined clinical criteria, the same in both cohorts, to identify the patients with DC: cirrhosis with concomitant ascites, hepatic encephalopathy, or gastrointestinal (GI) bleeding secondary to portal hypertension. Other types of decompensated states, such as hepatocellular carcinoma, were not included as their management was not detailed in the QI.[3]
We included patients with suspected or established cirrhosis who had ascites confirmed radiographically or by exam, noting shifting dullness or fluid wave. However, patients were excluded if they lacked sufficient peritoneal fluid for bedside or image‐guided paracentesis. Cirrhotic patients were defined as having hepatic encephalopathy if the patient had altered mental status not secondary to seizures, cerebrovascular accident, or alcohol withdrawal. Finally, gastrointestinal bleeding in cirrhotic patients was defined as any upper or lower bleeding prompting hospital admission, or identified in the medical record as clinically significant by the attending physician.
The same QIs were measured in both cohorts. From the QI set,[3] we selected the 16 QIs that would apply to the management of inpatients (see Supporting Information, Appendix 2, in the online version of this article). Indicators developed for outpatient settings were not included. A quality score was calculated for each admission, defined as the proportion of QIs met divided by the number of QIs for which the patient was eligible. For example, a patient with hepatic encephalopathy but without GI bleeding or ascites would have a score calculated as the number of QIs met for hepatic encephalopathy and documentation of transplant evaluation divided by 3 (2 QIs for hepatic encephalopathy and 1 QI for transplant evaluation). If the patient met both QIs for hepatic encephalopathy, but the consultant failed to address liver transplant eligibility, the score would be 2/3=0.666.
After the institution of the MC, all inpatients with DC were identified within 24 hours of admission by a gastroenterologist (R.G., D.D.), who manually reviewed on a daily basis all admissions from EMRs. An author (R.G.) would then contact the admitting team (hospitalist or resident) to make sure that a gastroenterology consult was called and would then obtain the QI by manual extraction from the EMRs.
Of the 16 gastroenterologists who work at the hospital, 12 of them belong to several private practice groups, whereas 4 are employed by the hospital. As part of the intervention, all gastroenterologists were made aware of the intervention 1 month before the starting date, were provided with a checklist of the QIs of interest, and were encouraged to work with the hospitalist attendings to achieve compliance with the QIs. We reminded the gastroenterologists of the ongoing study during routine division meetings and regularly sought feedback from the hospitalists
The MC consisted of a systematic consultation by a gastroenterologist: any identified patient with DC would generate a mandatory GI consultation and would be assigned to a specialist depending on the roster coverage for that day. A close monitoring of the process allowed us to confirm that all patients admitted with DC were seen by a gastroenterologist. Patients were followed until their discharge, death, or readmission to our institution during the study period.
Outcomes
The primary outcome was defined as the rate of adherence to the QIs and overall QI score expressed as a proportion as noted above. Secondary outcomes included in‐hospital mortality, LOS, and 30‐day readmission rate. These parameters were abstracted from the medical record.
Covariates
The hospital EMR (Cerner Corporation, North Kansas City, MO) was used to extract patient demographic parameters such as gender, race, language, and age at time of admission. Other admission‐level details were extracted from the EMR including Model for End‐Stage Liver Disease (MELD) scores, documented comorbidities (including substance abuse, psychiatric diagnosis, diabetes mellitus, renal failure, congestive heart failure, coronary artery disease, and cancer), underlying etiology for cirrhosis, and reason for admission.
The study was approved by Baystate Medical Center's institutional review board.
Statistical Analysis
Summary statistics for outcomes and covariates were calculated as means/standard deviations (SDs), medians/emnterquartile range, and proportions. Univariable statistics (unpaired t tests, 1‐way analysis of variance, Fisher exact test, Spearman correlation) were used to identify possible demographic (eg, age, race) and clinical (eg, admission complaint) predictors of quality score and with 30‐day outcomes. For each admission, a composite quality score, also known as an opportunity model score,[5, 6] was calculated as a fraction (ie, the number of QIs met divided by the total number of possible QIs indicated by the patient's presentation). This fraction was then multiplied by 100 so as to express the QI score as a percent. Possible scores, therefore, ranged from 0 to 100%.
Calculation of the 30‐day incidence proportion of readmission after the first admission was restricted to patients whose readmission occurred in this hospital, and occurring up to 30‐days before study closure (June 1, 2012). In‐hospital death was examined as a function of QI score during that admission. To derive an unbiased, risk‐adjusted estimate of the association between quality score and outcomes, multiple linear regression (opportunity model score [OMS], LOS) or multiple Poisson regression models (30‐day readmission, in‐hospital death) were built. These included a dummy variable for the study period, as well as any potential confounder that was associated at P0.10, with both study period and the outcome in univariable analyses. Robust standard errors were specified to account for multiple admissions within patients. Marginal means or proportions were then estimated with 95% confidence intervals derived using the delta method. All analyses were performed using Stata 12.1 for Windows (StataCorp, College Station, TX).
RESULTS
A total of 303 patients were observed in 695 hospitalizations;149 patients in 379 admissions were observed in the UC cohort, and 154 patients in 316 admissions were observed in the MC cohort. Baseline demographics of all study admissions appear in Table 1. Patients seen in the MC cohort were younger, more likely to speak English, and less likely to be male or have comorbid diabetes mellitus. Most admissions (n=217, 57.2%; 95% confidence interval: 52.3%‐62.3%) were not evaluated by a gastroenterologist in the UC cohort but all were in the MC cohort.
| UC, N=379, N (%) or Mean/SD | MC, N=316, N (%) or Mean/SD | P Value* | |
|---|---|---|---|
| |||
| Age, y | 55.3/12.1 | 53.3/13.6 | 0.05 |
| English speaking | 261 (68.9%) | 261 (82.6%) | <0.001 |
| Male | 251 (66.2%) | 163 (53.5%) | 0.001 |
| Race | <0.001 | ||
| White | 301 (79.4%) | 262 (82.9%) | |
| Black | 31 (8.2%) | 40 (12.7%) | |
| Asian | 16 (4.2%) | 0 (0.0%) | |
| Other | 31 (8.2%) | 14 (4.4%) | |
| Comorbidities | |||
| Substance | 75 (19.8%) | 58 (18.4%) | 0.70 |
| abuse | |||
| Psychiatric | 123 (32.5%) | 103 (32.9%) | 0.94 |
| Diabetes mellitus | 175 (45.4%) | 115 (36.5%) | 0.02 |
| Renal failure | 74 (19.3%) | 55 (17.4%) | 0.50 |
| CHF | 38 (10.0%) | 24 (7.6%) | 0.35 |
| CAD | 26 (6.9%) | 17 (5.4%) | 0.43 |
| Cancer | 48 (12.7%) | 40 (12.7%) | 1.00 |
| Admission MELD | 15.6/6.9 | 17.0/7.0 | 0.006 |
| Serum creatinine | 1.43/1.94 | 1.42/1.30 | 0.91 |
| Reason for admission | |||
| Hepatology/GI | 318 (83.9%) | 257 (81.3%) | 0.42 |
| Renal failure | 85 (22.4%) | 90 (28.5%) | 0.08 |
| Encephalopathy | 151 (39.3%) | 113 (34.9%) | 0.24 |
| GI bleed | 78 (20.5%) | 57 (18.0%) | 1.00 |
| Abdominal pain | 116 (30.7%) | 114 (36.2%) | 0.15 |
| Ascites | 246 (64.9%) | 185 (58.5%) | 0.10 |
Admission Characteristics
The baseline clinical measures of all study admissions appear in Table 1. The UC and MC cohorts had similar characteristics, with the majority of patients with DC admitted for a gastrointestinal/hepatology‐related reason specifically for the management of ascites and hepatic encephalopathy. The patients in the MC cohort had a statistically higher MELD score on admission, which was not clinically relevant.
Quality Measures
Adherence to individual quality indices is shown in Table 2.
| Condition (Denominator) | Quality Indicator (Numerator) | UC (n=379), Met/Indicated | MC (n=316), Met/Indicated | P Value | |
|---|---|---|---|---|---|
| |||||
| Admissions with ascites | |||||
| 1 | Admissions to the hospital because of ascites or encephalopathy. | Diagnostic paracentesis during admission. | 77/193, 39.9%, (32.9%, 46.9%) | 111/135, 82.2% (75.7%, 88.8%) | <0.001 |
| 2 | No fibrinolysis or disseminated intravascular coagulation before paracentesis INR <2.5, >100,000 platelets. | No fresh frozen plasma or platelet replacement given. | 36/37, 97.3% (91.8%, 103.0%) | 41/42, 97.6% (92.8%, 102.4%) | 1.00 |
| 3 | All admissions with diagnostic paracentesis (not limited to admissions for ascites or hepatic encephalopathy). | Cell count differential, total protein, albumin, and culture/sensitivity all performed. | 31/49, 63.3% (49.3%‐77.3%) | 46/72 63.9% (52.7%, 75.0%) | 1.00 |
| 4 | Admissions with known portal hypertension‐related ascites receiving a paracentesis. | Ascitic fluid cell count and differential performed. | 15/104, 14.4% (7.6%‐ 21.3%) | 47/62, 75.8% (63.2%, 88.4%) | <0.001 |
| 5 | Serum sodium 110 mEq/L. | Fluid restriction and discontinuation of diuretics. | NA | NA | NA |
| 6 | Polymorphonuclear count of 250/mm3 in ascites. | Empiric antibiotics, 6 hours of results. | 10/13, 76.9% (50.4%‐ 103.4%) | 16/20, 80.0% (60.8%, 99.2%) | 1.00 |
| 7 | Ascitic fluid, total protein 1.1 gm/dL, serum bilirubin 2.5 mg/dL. | Prophylactic antibiotics. | 4/12, 33.3% (2.0%‐ 64.6%) | 18/30, 60.0%, (41.4%, 78.6%) | 0.18 |
| 8 | Normal renal function. | Salt restriction and diuretics (spironolactone and loop diuretics). | 57/186, 30.6%, (24.0%‐ 37.3%) | 81/122, 66.4%, (57.9%, 74.9%) | <0.001 |
| Total ascites subscore, mean/SD | 30%/36% | 67%/34% | <0.001 | ||
| GI bleeding | |||||
| 9 | Admissions with GI bleeding: variceal and nonvariceal, hematemesis and melena. | Upper endoscopy 24 hours of presentation. | 60/78, 76.9% (67.4%, 86.4%) | 52/57, 91.2% (83.7%, 98.8%) | 0.04 |
| 10 | Esophageal varices (active, stigmata of recent bleeding, or no other causes to explain bleeding). | Endoscopic variceal ligation/sublerotherapy. | 40/46, 87.0% (76.8%‐97.1%) | 30/32, 93.8% (84.9%, 100.0%) | 0.46 |
| 11 | Admissions with established/suspected upper GI bleeding. | Antibiotics within 24 hours of admission. | 27/69, 39.1% (27.3%‐ 50.9%) | 26/58, 44.8% (31.6%, 58.0%) | 0.59 |
| 12 | Admissions with established/suspected variceal bleeding. | Somatostatin/octreotide given within 12 hours of presentation. | 53/69, 76.8%, (66.6%‐ 87.0%) | 49/58, 84.5% (73.8%, 95.2%) | 0.37 |
| 13 | Recurrent bleeding within 72 hours of initial endoscopic hemostasis. | Repeat endoscopy or transjugular intrahepatic portosystemic shunt. | 5/5 100% | 2/3, 66.7% (76.8%, 210.0%) | 0.38 |
| Total GI subscore, mean/SD | 61%/38% | 74%/28% | 0.04 | ||
| Liver transplantation | |||||
| 14 | Admissions with MELD 15 or MELD 15 and decompensated status (ie, all admissions in our study). | Documented evaluation for liver transplantation. | 112/379, 29.6% (24.9%‐ 34.2%) | 231/316, 73.6% (68.7%, 78.5%) | <0.001 |
| Hepatic encephalopathy | |||||
| 15 | Admissions with hepatic encephalopathy. | Search for reversible factors documented. | 81/151, 53.6% (45.6%‐ 61.7 %) | 97/113, 85.8% (79.4%, 92.3%) | <0.001 |
| 16 | Admissions with hepatic encephalopathy. | Oral disaccharides/ rifaximin. | 144/151, 95.3% (91.9 %‐ 98.7 %) | 107/113, 94.7% (90.7%. 98.69%) | 1.00 |
| Total encephalopathy subscore, mean/SD | 75%/28% | 90%/24% | <0.001 | ||
Ascites
The management of ascites yielded 3 main differences between the 2 cohorts. Following the implementation of the MC, 82.2 % (111/135) of ascites‐related admissions led to a diagnostic paracentesis as compared to 39.9% (77/193) in the UC group (P<0.001).
In the MC cohort, 75.8% (47/62) of admissions with known portal hypertensionrelated ascites who received a paracentesis had an ascites cell count checked. In contrast, only 14.4% (15/104) in the UC group receiving paracentesis had a fluid cell count (P<0.001). The management of ascites in patients with normal renal function was optimal, with sodium restriction and diuretics combination in 66.4% (81/122) of the MC cohort, whereas this parameter in the UC cohort was only 30.6% (57/186) (P<0.001). There were no significant differences between the groups for the other QIs.
Variceal Bleeding
The MC group had a higher frequency of endoscopy within 24 hours of admissions than the UC group (91.2% [52/57] vs 76.9% [60/78], respectively; P<0.04). The rest had endoscopy later in the admission. Among admissions with bleeding from varices, banding was done 93.8% of the time for patients in the MC group (30/32), which was not statistically different than 87.0% (40/46) for patients seen in the UC group. In the remaining admissions, endoscopy only revealed nonbleeding large esophageal varices, and the endoscopist opted not to proceed with therapy. There were no statistically significant differences in the rest of the management.
Hepatic Encephalopathy
For hepatic encephalopathy, an empirical treatment was given to 95.3 % (144/151) patients in the UC group and 94.7% (107/113) of the patients in the MC group. We found better documentation of a search for underlying etiologies leading to hepatic encephalopathy in the MC cohort 85.8% (97/113) versus the UC cohort, which was only 53.6% (81/151) (P<0.001).
Evaluation for Liver Transplantation
Better documentation of evaluation for liver transplantation was seen in the MC group 73.6% (231/316) in comparison to the UC group 29.4% (111/379) (P<0.001).
Opportunity Score and Clinical Outcomes
As detailed above, care provided during the MC achieved a higher compliance with the QI shown with the QI score or OMS (Table 3). These improvements were not associated with statistically significant differences in in‐hospital death, LOS, or 30‐day readmission. To explore this further we also examined the direct association between the OMS and outcomes in the MC group by dividing patients into 2 groups: patients whose OMS was 80% and those whose OMS was <80% (see Supporting Information, Appendix 4, in the online version of this article). Although there were trends toward decreased in‐hospital death (6.4% vs 8.6%, P=0.26), increased 30‐day readmission (33.8% vs 23.0%, P=0.27), and decreased LOS (6.2 days vs 6.6 days, P=0.77), none of these differences achieved statistical significance.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| UC | MC | Difference | UC | MC | Difference | |
| ||||||
| Opportunity model score | 0.46 | 0.77 | +0.31 (0.24, 0.39) | 0.46 | 0.77 | +0.30(0.23, 0.37) |
| In‐hospital death | 7.1% | 8.5% | +1.4 (0.3, +5.6) | 7.5% | 7.9% | +0.4% (4.0%, +4.5%) |
| Readmission within 30 days | 39.6% | 32.6% | 7.0% (16.4%, +2.5%) | 40.0% | 31.8% | 8.2%(18.0%, +1.5%) |
| Length of stay | 6.1d | 6.2d | +0.1d (1.0 d, +1.2 d) | 6.1d | 6.2d | +0.1d (1.0 d, +1.2d) |
Mandatory Consultation Subgroups: Employed Versus Private Physicians
The type of employment of the gastroenterologist on consultation (employed by the hospital vs private practice) affected the management of the patients admitted with DC (see Supporting Information, Appendix 3, in the online version of this article). Patients seen by a hospital‐employed gastroenterologist were more likely to have a better documentation in regard to evaluation for liver transplantation and better management of ascites. Except for the prescription of antibiotics in patients presenting with GI bleeding, which were more often given by the employed physician (63% vs 23%, P=0.004), the management of hepatic encephalopathy and GI bleeding was similar between employed and private‐practice physicians.
DISCUSSION
In this evaluation of an MC intervention for patients with DC cared for at a large tertiary academic medical center, we found that the implementation of a routine consultation by a gastroenterologist led to greater adherence to recommended care processes when compared to UC. Overall, the management of ascites and the documentation of evaluation for liver transplantation were statistically superior in the intervention (MC) group. UC and MC were similar with respect to treatment of variceal bleeding and hepatic encephalopathy. Although we did not demonstrate changes in mortality, readmission, or LOS as a result of the MC intervention, our study was underpowered to detect clinically meaningful effects.
The gaps in care of patients with cirrhosis were reported before and after the publication of the formal QIs.[7, 8, 9, 10] These gaps remain relevant in the face of an increasing prevalence of DC along with a recent publication suggesting an underestimation of the burden of liver disease in the United States.[11] Ours is the first study to evaluate the impact on inpatients with DC of a liver service with a systematic, mandatory, specialist consultation. A previous study[12] had shown that a GI consultation would improve the care of patients with DC, but excluded patients with variceal bleeding, did not specifically measure the compliance with QIs, and more important, the GI consult was not mandatory.
Our study has several limitations that must be considered while weighing its findings. The patients were not randomly assigned but followed a pre‐established distribution depending on the call schedule. Some of the improvement we noted might be the result of secular trends; however, this remains unlikely given the lack of national initiatives or pay for performance programs. In the UC cohort, patients who were nonEnglish‐speaking were associated with a lower QI score, which could account for part of the improvement seen in the MC group that has a more prominent English‐speaking cohort. Readmissions could have occurred at other hospitals, and patients were not monitored in an outpatient setting. We did not observe a change in the secondary outcomes (30‐day readmission, LOS, in‐hospital death); however, our study was underpowered for that purpose. Given the complexity of the billing process we did not collect the costs of the MC, which is another limitation of our work. Future studies are needed to determine the cost‐effectiveness of the intervention.
This study shows that a dedicated team of physicians focused on compliance with QIs can achieve a rapid improvement, over a year, in providing higher‐quality care. This may be relevant at other institutions. The strength of our study is that our large tertiary academic medical center serves a large catchment area, with a mix of patients from both rural and urban communities. It is located in Massachusetts, where most of the population has had access to healthcare since 2006. Therefore, although this is a single‐center study, we expect our findings to be more generalizable and less subject to selection bias than other single‐center studies.
Importantly, the compliance with QIs was often far from being perfect in the MC group and was different across type of employment of providers, reflecting the challenges in changing practice among physicians.[13] In fact the QI scores of the private practice group did not change, and mirror the compliance observed at our institution in the previous study, before the implementation of the MC.[4] The difference in performance according to the type of employment of providers stems from 2 factors. First, a better documentation of the need of formal evaluation for liver transplantation by the employed gastroenterologists resulted in better compliance with this QI. Second, and more important, among the employed physicians, there was a readiness to assist the hospitalist with diagnostic/therapeutic paracentesis without relying on, for example, an interventional radiologist. This is reflected by the higher score in the management of ascites. Although our study was not designed to answer this directly, employed physicians may have been more engaged in the project and showed a greater willingness to change practice. In the future, linking reimbursement to quality of care will lead to improved accountability of consultants.
In this study we show that a direct involvement of a gastroenterologist improves the care of inpatients as measured by QIs. We theorize that a better coordination of the transition to outpatient care involving the specialist should lead to better outcomes, specifically a reduction in the 22% observed readmission rate within 30 days of patients with DC.[14, 15] As we move forward, a broader definition of outcomes should be addressed, taking into account patient‐related outcomes and preferences.[16] Future studies should define the relationship between the gastroenterologist and the hospitalist service, the role of physician assistants and nurse practitioners in implementing and monitoring compliance with QIs, and define how physicians and patients can be made accountable in the transition to the outpatient setting.
Disclosures
R.G.: Conception, data collection and interpretation, manuscript. J.F.: Data management, data analysis, manuscript. P.V.: Conception, data analysis, manuscript. P.L.: Conception, data interpretation, manuscript. T.L.: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Conception, data interpretation, manuscript. D.D.: Conception, data collection and interpretation, manuscript. A.B.: Data collection. J.S.: Data collection. Source of funding: internal. The authors report no conflicts of interest.
- , , . Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231.
- , , , . Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11:850–858.
- , , , et al. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717.
- , , , , , Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014:34:204–210.
- , . All‐or‐none measurement raises the bar on performance. JAMA. 2006;295:1168–1170.
- Joint Commission on Accreditation of Healthcare Organizations. Quality report user guide. Available at: http://www.jointcommission.org. Accessed May 30, 2011.
- , , , et al. Management of patients with cirrhosis in Southern California: results of a practitioner survey. J Clin Gastroenterol. 2006;40:156–161.
- , , , et al. Spanish Collaborative Study Group on Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–440.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012 143(1):70–77.
- , , , et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659.
- , , , , . Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145:375–382.
- , , , et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized with advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259.
- , , , et al. Hospital Readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252.
- . Patient‐reported outcomes of cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1043–1045.
- , , . Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231.
- , , , . Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11:850–858.
- , , , et al. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717.
- , , , , , Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014:34:204–210.
- , . All‐or‐none measurement raises the bar on performance. JAMA. 2006;295:1168–1170.
- Joint Commission on Accreditation of Healthcare Organizations. Quality report user guide. Available at: http://www.jointcommission.org. Accessed May 30, 2011.
- , , , et al. Management of patients with cirrhosis in Southern California: results of a practitioner survey. J Clin Gastroenterol. 2006;40:156–161.
- , , , et al. Spanish Collaborative Study Group on Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–440.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012 143(1):70–77.
- , , , et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659.
- , , , , . Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145:375–382.
- , , , et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized with advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259.
- , , , et al. Hospital Readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252.
- . Patient‐reported outcomes of cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1043–1045.
© 2014 Society of Hospital Medicine
‘Acting out’ or pathological?
Test predicts response to GVHD treatment
A newly developed algorithm could enable risk-adapted therapy for graft-vs-host disease (GVHD), researchers have reported in Lancet Haematology.
The problem with treating GVHD, the team said, is that the severity of symptoms at disease onset does not accurately define risk.
Patients with low-risk GVHD are often overtreated and experience significant side effects, while patients with high-risk GVHD can be undertreated and see their GVHD progress.
“Our goal is to provide the right treatment for each patient,” said study author James L. M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai.
“We hope to identify those patients at higher risk and design an aggressive intervention while tailoring a less-aggressive approach for those with low-risk.”
With that goal in mind, Dr Ferrara and his colleagues developed and tested a scoring system for GVHD. They collected plasma from 492 patients newly diagnosed with varying grades of GVHD and randomly assigned them to training (n=328) and test (n=164) sets.
The team used the concentrations of 3 validated biomarkers—TNFR1, ST2, and Reg3α—to create an algorithm that calculated the probability of non-relapse mortality (NRM) 6 months after GVHD onset for patients in the training set.
The researchers ranked the probabilities and identified thresholds that created 3 NRM scores. They then tested the algorithm in the test set of patients and a validation cohort of 300 additional patients who were enrolled on trials of GVHD treatment.
Results showed the algorithm works. The cumulative incidence of 6-month NRM significantly increased as the Ann Arbor GVHD score increased, and the response to primary GVHD treatment within 28 days decreased as the GVHD score increased.
In the validation set, the incidence of NRM was 8% for score 1, 27% for score 2, and 46% for score 3 (P<0.0001). Treatment response measured 86% for score 1, 67% for score 2, and 46% for score 3 (P<0.0001).
“This new scoring system will help identify patient who may not respond to standard treatments and may require an experimental and more aggressive approach,” Dr Ferrara said. “And it will also help guide treatment for patients with lower-risk GVHD who may be overtreated. This will allow us to personalize treatment at the onset of the disease. Future algorithms will prove increasingly useful to develop precision medicine for all [stem cell transplant] patients.”
To capitalize on this discovery, Dr Ferrara created the Mount Sinai Acute GVHD International Consortium (MAGIC), which consists of a group of 10 stem cell transplant centers in the US and Europe that will collaborate to use this new scoring system to test new treatments for acute GVHD.
Dr Ferrara and his colleagues have also written a protocol to treat high-risk GVHD that has been approved by the US Food and Drug Administration. ![]()
A newly developed algorithm could enable risk-adapted therapy for graft-vs-host disease (GVHD), researchers have reported in Lancet Haematology.
The problem with treating GVHD, the team said, is that the severity of symptoms at disease onset does not accurately define risk.
Patients with low-risk GVHD are often overtreated and experience significant side effects, while patients with high-risk GVHD can be undertreated and see their GVHD progress.
“Our goal is to provide the right treatment for each patient,” said study author James L. M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai.
“We hope to identify those patients at higher risk and design an aggressive intervention while tailoring a less-aggressive approach for those with low-risk.”
With that goal in mind, Dr Ferrara and his colleagues developed and tested a scoring system for GVHD. They collected plasma from 492 patients newly diagnosed with varying grades of GVHD and randomly assigned them to training (n=328) and test (n=164) sets.
The team used the concentrations of 3 validated biomarkers—TNFR1, ST2, and Reg3α—to create an algorithm that calculated the probability of non-relapse mortality (NRM) 6 months after GVHD onset for patients in the training set.
The researchers ranked the probabilities and identified thresholds that created 3 NRM scores. They then tested the algorithm in the test set of patients and a validation cohort of 300 additional patients who were enrolled on trials of GVHD treatment.
Results showed the algorithm works. The cumulative incidence of 6-month NRM significantly increased as the Ann Arbor GVHD score increased, and the response to primary GVHD treatment within 28 days decreased as the GVHD score increased.
In the validation set, the incidence of NRM was 8% for score 1, 27% for score 2, and 46% for score 3 (P<0.0001). Treatment response measured 86% for score 1, 67% for score 2, and 46% for score 3 (P<0.0001).
“This new scoring system will help identify patient who may not respond to standard treatments and may require an experimental and more aggressive approach,” Dr Ferrara said. “And it will also help guide treatment for patients with lower-risk GVHD who may be overtreated. This will allow us to personalize treatment at the onset of the disease. Future algorithms will prove increasingly useful to develop precision medicine for all [stem cell transplant] patients.”
To capitalize on this discovery, Dr Ferrara created the Mount Sinai Acute GVHD International Consortium (MAGIC), which consists of a group of 10 stem cell transplant centers in the US and Europe that will collaborate to use this new scoring system to test new treatments for acute GVHD.
Dr Ferrara and his colleagues have also written a protocol to treat high-risk GVHD that has been approved by the US Food and Drug Administration. ![]()
A newly developed algorithm could enable risk-adapted therapy for graft-vs-host disease (GVHD), researchers have reported in Lancet Haematology.
The problem with treating GVHD, the team said, is that the severity of symptoms at disease onset does not accurately define risk.
Patients with low-risk GVHD are often overtreated and experience significant side effects, while patients with high-risk GVHD can be undertreated and see their GVHD progress.
“Our goal is to provide the right treatment for each patient,” said study author James L. M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai.
“We hope to identify those patients at higher risk and design an aggressive intervention while tailoring a less-aggressive approach for those with low-risk.”
With that goal in mind, Dr Ferrara and his colleagues developed and tested a scoring system for GVHD. They collected plasma from 492 patients newly diagnosed with varying grades of GVHD and randomly assigned them to training (n=328) and test (n=164) sets.
The team used the concentrations of 3 validated biomarkers—TNFR1, ST2, and Reg3α—to create an algorithm that calculated the probability of non-relapse mortality (NRM) 6 months after GVHD onset for patients in the training set.
The researchers ranked the probabilities and identified thresholds that created 3 NRM scores. They then tested the algorithm in the test set of patients and a validation cohort of 300 additional patients who were enrolled on trials of GVHD treatment.
Results showed the algorithm works. The cumulative incidence of 6-month NRM significantly increased as the Ann Arbor GVHD score increased, and the response to primary GVHD treatment within 28 days decreased as the GVHD score increased.
In the validation set, the incidence of NRM was 8% for score 1, 27% for score 2, and 46% for score 3 (P<0.0001). Treatment response measured 86% for score 1, 67% for score 2, and 46% for score 3 (P<0.0001).
“This new scoring system will help identify patient who may not respond to standard treatments and may require an experimental and more aggressive approach,” Dr Ferrara said. “And it will also help guide treatment for patients with lower-risk GVHD who may be overtreated. This will allow us to personalize treatment at the onset of the disease. Future algorithms will prove increasingly useful to develop precision medicine for all [stem cell transplant] patients.”
To capitalize on this discovery, Dr Ferrara created the Mount Sinai Acute GVHD International Consortium (MAGIC), which consists of a group of 10 stem cell transplant centers in the US and Europe that will collaborate to use this new scoring system to test new treatments for acute GVHD.
Dr Ferrara and his colleagues have also written a protocol to treat high-risk GVHD that has been approved by the US Food and Drug Administration. ![]()