User login
Switzerland approves drug to treat MCL

Credit: CDC
Swissmedic, the regulatory authority for Switzerland, has granted
approval for lenalidomide (Revlimid) to treat patients with
relapsed or refractory mantle cell lymphoma (MCL) after prior therapy
that included bortezomib and chemotherapy or rituximab.
This is the third approval of lenalidomide for MCL worldwide. The drug is also approved for this indication in the US and Israel.
Swissmedic’s decision to approve the drug was based on results of the phase 2 EMERGE study (MCL-001).
In this trial, researchers evaluated lenalidomide (25 mg once a day on days 1-21 of each 28-day cycle) in 134 MCL patients who had received prior treatment with rituximab, cyclophosphamide, an anthracycline (or mitoxantrone), and bortezomib alone or in combination.
The overall response rate (the primary endpoint) was 28% (37/134), and the complete response rate was 7% (10/134). The median duration of response was 16.6 months (95% CI, 7.7-26.7).
The most common grade 3/4 adverse events reported in at least 5% of patients were neutropenia (43%), thrombocytopenia (28%), anemia (11%), pneumonia (9%), fatigue (7%), leukopenia (7%), febrile neutropenia (6%), diarrhea (6%), and dyspnea (6%).
“MCL is a rare B-cell lymphoma of the elderly that usually responds quite well to first-line treatment,” said Christoph Renner, MD, of Onkozentrum Hirslanden Zürich.
“However, even intensive treatment does not prevent relapse in the majority of patients, and new therapeutic options are needed. Therefore, having access to lenalidomide, an immunomodulatory drug with a well-known safety profile, will definitely enrich our therapeutic armamentarium.”
Lenalidomide is already approved in Switzerland for use in combination
with dexamethasone to treat patients with multiple myeloma who have
received at least one previous treatment.
The drug is also
approved to treat patients with transfusion-dependent anemia due to low-
or intermediate-risk-1 myelodysplastic syndrome associated with a 5q
deletion, with or without additional cytogenetic abnormalities.
Lenalidomide is under development by Celgene International Sàrl, a wholly owned subsidiary of Celgene Corporation. ![]()

Credit: CDC
Swissmedic, the regulatory authority for Switzerland, has granted
approval for lenalidomide (Revlimid) to treat patients with
relapsed or refractory mantle cell lymphoma (MCL) after prior therapy
that included bortezomib and chemotherapy or rituximab.
This is the third approval of lenalidomide for MCL worldwide. The drug is also approved for this indication in the US and Israel.
Swissmedic’s decision to approve the drug was based on results of the phase 2 EMERGE study (MCL-001).
In this trial, researchers evaluated lenalidomide (25 mg once a day on days 1-21 of each 28-day cycle) in 134 MCL patients who had received prior treatment with rituximab, cyclophosphamide, an anthracycline (or mitoxantrone), and bortezomib alone or in combination.
The overall response rate (the primary endpoint) was 28% (37/134), and the complete response rate was 7% (10/134). The median duration of response was 16.6 months (95% CI, 7.7-26.7).
The most common grade 3/4 adverse events reported in at least 5% of patients were neutropenia (43%), thrombocytopenia (28%), anemia (11%), pneumonia (9%), fatigue (7%), leukopenia (7%), febrile neutropenia (6%), diarrhea (6%), and dyspnea (6%).
“MCL is a rare B-cell lymphoma of the elderly that usually responds quite well to first-line treatment,” said Christoph Renner, MD, of Onkozentrum Hirslanden Zürich.
“However, even intensive treatment does not prevent relapse in the majority of patients, and new therapeutic options are needed. Therefore, having access to lenalidomide, an immunomodulatory drug with a well-known safety profile, will definitely enrich our therapeutic armamentarium.”
Lenalidomide is already approved in Switzerland for use in combination
with dexamethasone to treat patients with multiple myeloma who have
received at least one previous treatment.
The drug is also
approved to treat patients with transfusion-dependent anemia due to low-
or intermediate-risk-1 myelodysplastic syndrome associated with a 5q
deletion, with or without additional cytogenetic abnormalities.
Lenalidomide is under development by Celgene International Sàrl, a wholly owned subsidiary of Celgene Corporation. ![]()

Credit: CDC
Swissmedic, the regulatory authority for Switzerland, has granted
approval for lenalidomide (Revlimid) to treat patients with
relapsed or refractory mantle cell lymphoma (MCL) after prior therapy
that included bortezomib and chemotherapy or rituximab.
This is the third approval of lenalidomide for MCL worldwide. The drug is also approved for this indication in the US and Israel.
Swissmedic’s decision to approve the drug was based on results of the phase 2 EMERGE study (MCL-001).
In this trial, researchers evaluated lenalidomide (25 mg once a day on days 1-21 of each 28-day cycle) in 134 MCL patients who had received prior treatment with rituximab, cyclophosphamide, an anthracycline (or mitoxantrone), and bortezomib alone or in combination.
The overall response rate (the primary endpoint) was 28% (37/134), and the complete response rate was 7% (10/134). The median duration of response was 16.6 months (95% CI, 7.7-26.7).
The most common grade 3/4 adverse events reported in at least 5% of patients were neutropenia (43%), thrombocytopenia (28%), anemia (11%), pneumonia (9%), fatigue (7%), leukopenia (7%), febrile neutropenia (6%), diarrhea (6%), and dyspnea (6%).
“MCL is a rare B-cell lymphoma of the elderly that usually responds quite well to first-line treatment,” said Christoph Renner, MD, of Onkozentrum Hirslanden Zürich.
“However, even intensive treatment does not prevent relapse in the majority of patients, and new therapeutic options are needed. Therefore, having access to lenalidomide, an immunomodulatory drug with a well-known safety profile, will definitely enrich our therapeutic armamentarium.”
Lenalidomide is already approved in Switzerland for use in combination
with dexamethasone to treat patients with multiple myeloma who have
received at least one previous treatment.
The drug is also
approved to treat patients with transfusion-dependent anemia due to low-
or intermediate-risk-1 myelodysplastic syndrome associated with a 5q
deletion, with or without additional cytogenetic abnormalities.
Lenalidomide is under development by Celgene International Sàrl, a wholly owned subsidiary of Celgene Corporation. ![]()
FDA grants drug orphan designation for aHUS

Credit: Kevin MacKenzie
The US Food and Drug Administration (FDA) has granted orphan drug designation for CCX168, an oral inhibitor targeting the receptor for the complement protein C5a, to treat atypical hemolytic uremic syndrome (aHUS).
This rare but life-threatening disease causes inflammation of the blood vessels and thrombus formation throughout the body.
Patients with aHUS are at constant risk of sudden and progressive damage to, and failure of, vital organs. Roughly 10% to 15% of patients die in the initial, acute phase of aHUS.
The majority of patients—up to 70%—develop end-stage kidney failure requiring dialysis. And 1 in 5 patients has aHUS affecting organs other than the kidneys, most commonly the brain or heart.
“Given the life-threatening nature of aHUS, we are very pleased with the granting of orphan drug designation for CCX168 in this disease,” said Thomas J. Schall, PhD, president and chief executive officer of ChemoCentryx, Inc., the company developing CCX168.
ChemoCentryx has generated positive preclinical data that suggest an important role of C5a receptor inhibition in reducing microvasculature thrombosis formation in aHUS.
The company plans to initiate a phase 2 proof-of-concept study in patients with aHUS by the end of 2014.
CCX168 is also in phase 2 development for the treatment of anti-neutrophil cytoplasmic antibody-associated vasculitis.
The orphan designation for CCX168 will provide ChemoCentryx with a 7-year period of US marketing exclusivity if the drug is approved to treat aHUS, tax credits for clinical research costs, the ability to apply for annual grant funding, clinical research trial design assistance, and the waiver of prescription drug user fees. ![]()

Credit: Kevin MacKenzie
The US Food and Drug Administration (FDA) has granted orphan drug designation for CCX168, an oral inhibitor targeting the receptor for the complement protein C5a, to treat atypical hemolytic uremic syndrome (aHUS).
This rare but life-threatening disease causes inflammation of the blood vessels and thrombus formation throughout the body.
Patients with aHUS are at constant risk of sudden and progressive damage to, and failure of, vital organs. Roughly 10% to 15% of patients die in the initial, acute phase of aHUS.
The majority of patients—up to 70%—develop end-stage kidney failure requiring dialysis. And 1 in 5 patients has aHUS affecting organs other than the kidneys, most commonly the brain or heart.
“Given the life-threatening nature of aHUS, we are very pleased with the granting of orphan drug designation for CCX168 in this disease,” said Thomas J. Schall, PhD, president and chief executive officer of ChemoCentryx, Inc., the company developing CCX168.
ChemoCentryx has generated positive preclinical data that suggest an important role of C5a receptor inhibition in reducing microvasculature thrombosis formation in aHUS.
The company plans to initiate a phase 2 proof-of-concept study in patients with aHUS by the end of 2014.
CCX168 is also in phase 2 development for the treatment of anti-neutrophil cytoplasmic antibody-associated vasculitis.
The orphan designation for CCX168 will provide ChemoCentryx with a 7-year period of US marketing exclusivity if the drug is approved to treat aHUS, tax credits for clinical research costs, the ability to apply for annual grant funding, clinical research trial design assistance, and the waiver of prescription drug user fees. ![]()

Credit: Kevin MacKenzie
The US Food and Drug Administration (FDA) has granted orphan drug designation for CCX168, an oral inhibitor targeting the receptor for the complement protein C5a, to treat atypical hemolytic uremic syndrome (aHUS).
This rare but life-threatening disease causes inflammation of the blood vessels and thrombus formation throughout the body.
Patients with aHUS are at constant risk of sudden and progressive damage to, and failure of, vital organs. Roughly 10% to 15% of patients die in the initial, acute phase of aHUS.
The majority of patients—up to 70%—develop end-stage kidney failure requiring dialysis. And 1 in 5 patients has aHUS affecting organs other than the kidneys, most commonly the brain or heart.
“Given the life-threatening nature of aHUS, we are very pleased with the granting of orphan drug designation for CCX168 in this disease,” said Thomas J. Schall, PhD, president and chief executive officer of ChemoCentryx, Inc., the company developing CCX168.
ChemoCentryx has generated positive preclinical data that suggest an important role of C5a receptor inhibition in reducing microvasculature thrombosis formation in aHUS.
The company plans to initiate a phase 2 proof-of-concept study in patients with aHUS by the end of 2014.
CCX168 is also in phase 2 development for the treatment of anti-neutrophil cytoplasmic antibody-associated vasculitis.
The orphan designation for CCX168 will provide ChemoCentryx with a 7-year period of US marketing exclusivity if the drug is approved to treat aHUS, tax credits for clinical research costs, the ability to apply for annual grant funding, clinical research trial design assistance, and the waiver of prescription drug user fees. ![]()
Public Quality Reporting
Few consumers would choose to dine at a restaurant if they knew the kitchen was infested with cockroaches. Few patients would choose to undergo a liver transplant in a hospital that was performing the procedure for the first time. In most sectors, consumers gather information about quality (and price) from the marketplace, where economic theory predicts that rational behavior and competition will lead to continuous improvement over time. However, for some goods and services, information is sparse and asymmetric between consumers and suppliers. In sectors where consumer health is at risk, society has often intervened to assure minimum standards. Yet sometimes these efforts have fallen short. In healthcare, physician licensure and hospital accreditation (eg, through the Joint Commission), although providing an important foundation to assure safety, have not come close to solving the widespread quality problems.[1] Basic regulatory requirements for restaurants have also proven inadequate to prevent food‐borne illness. Consumer trust, without information, can be a recipe (or prescription) for trouble.
In response, high‐profile efforts have been introduced to publicize the quality and safety of service providers. One example is Hospital Compare, Medicare's national quality reporting program for US hospitals.[2] The New York City sanitary grade inspection program is a parallel effort for restaurants. Although customers can judge how much they like the food from a restaurantor look up reviews at
The aims of Hospital Compare and the New York City sanitary inspection program are fundamentally similar. Both initiatives seek to address a common market failure resulting in the consumer's lack of information on quality and safety. By infusing the market with information, these programs enable consumers to make better choices and encourage service providers to improve quality and safety.[3] Despite the promise of these programs, a copious literature about the effects of public quality reporting in healthcare has found mixed results.[4, 5] Although the performance measures in any public reporting program must be valid and reliable, good measures are not sufficient to achieve the goals of public reporting. To engage patients, reported results must also be accessible, understandable, and meaningful. Both patients' lack of knowledge about the reports[6] and patients' inability to effectively use these data to make better decisions[7] are some reasons why public quality reporting has fallen short of its expectations. This article argues that the New York City program is much better structured to positively affect patient choice, and holds important lessons for public quality reporting in US hospitals.
CONTRASTS BETWEEN HOSPITAL COMPARE AND THE NEW YORK CITY RESTAURANT SANITARY INSPECTION PROGRAM
Hospital Compare reports performance for 108 separate quality indicators related to quality and patient safety for US hospitals (Table 1). These are a combination of structure measures (eg, hospital participation in a systematic database for cardiac surgery), process of care measures (eg, acute myocardial infarction patients receiving fibrinolytic therapy within 30 minutes of hospital arrival), outcomes (eg, 30‐day mortality and readmission), and patient experience measures (eg, how you would rate your communication with your physician). Hospital Compare data, frequently based on hospital quality performance 1 to 3 years prior to publication, are displayed on a website. Hospitals do not receive a summary measure of quality or safety.[8] Hospitals face financial incentives that are tied to measure reporting[9] and performance for some of the measures on Hospital Compare.[10, 11] Hospital accreditation is only loosely related to performance on these measures.
| Attribute | Hospital Compare | New York City Sanitary Inspection Program |
|---|---|---|
| Display of information | On a website ( |
On the front of the restaurant, with additional information also available on a website ( |
| Frequency of information update | Quarterly; data often lag by between 1 and 3 years. | Unannounced inspections occur at least annually. Grades are posted immediately after inspection. |
| Quality measures | Mix of measures pertaining to quality improvement activities (eg, hospital participation in a cardiac surgery registry or a quality improvement initiative), rates of adherence with evidence‐based medicine (eg, heart failure patients receiving discharge instructions, acute myocardial infarction patients receiving ‐blocker at arrival), and patient outcomes (eg, 30‐day mortality and 30‐day readmission for acute myocardial infarction, heart failure, and pneumonia). | Mix of measures pertaining to conditions of the facility (eg, improper sewage disposal system, improper food contact surface, evidence of live rats in the facility) and the treatment and handling of food (eg, food is unwrapped, appropriate thermometer not used to measure temperature of potentially hazardous foods, food not prepared to sufficiently high temperature). |
| Clarity and simplicity of information | 108 individual measures. No summary measure. | Single summary letter grade displayed on front of restaurant. Detailed data on individual violations (ie, measures) available on website. |
| Consequences of poor performance and mechanisms for enforcement | Hospitals are subject to financial penalties for not reporting certain measures and face financial incentives for performance on a subset of measures. | Restaurants are fined for violations, are subject to repeated inspections for poor performance, and are subject to closure for severe violations. |
| Consumer awareness | Limited | Widespread |
The New York City sanitation program regularly inspects restaurants and scores them on a standard set of indicators that correspond to critical violations (eg, food is contaminated by mouse droppings) or general violations (eg, garbage is not adequately covered).[12] Points are assigned to each type and severity of violation, and the sum of the points are converted into a summary grade of A, B, or C. Restaurants can dispute the grades, receiving a grade pending designation until the dispute is adjudicated. After inspection, sanitation grades are immediately posted on restaurants' front door or window, providing current information that is clearly visible to consumers before entering. More detailed information on sanitation violations is also available on a website. If restaurants receive an A grade, they face no additional inspections for 1 year, but poorly graded restaurants may receive monthly inspections. Restaurants face fines from violations and are subject to closure from severe violations. Recently proposed changes would decrease fines and give restaurants greater opportunities to appeal grades, but leave the program otherwise intact.[13]
IMPLICATIONS FOR PUBLIC QUALITY REPORTING IN HOSPITALS
Along with value‐based payment reforms, public quality reporting is one of the few major system‐level approaches that is being implemented in the US to improve quality and safety in healthcare. However, without a simple and understandable display of information that is available when a patient needs it, quality and safety information will likely go unused.[14] Hospital Compare leaves it up the patient to find the quality and safety information and does little to help patients understand and use the information effectively. Hospital Compare asks patients to do far more work, which is perhaps why it has been largely ignored by patients.[2, 15] The New York City sanitation inspection program evaluates restaurants, prominently displays an understandable summary result, and puts the scoring details in the background. Although peer‐reviewed evaluations of the New York City sanitation inspection program have not yet been published, internal data show that the program has decreased customer concern about getting sick, improved sanitary practices, and decreased salmonella.[16] Evidence from a similar program in Los Angeles County found that hygiene grades steered consumers toward restaurants with better sanitary conditions and decreased food‐borne illness.[17]
The nature of choice in healthcare, particularly the choice of hospital, is much different than it is for restaurants. In some areas, a single hospital may serve a large geographical area, severely limiting choice. Even when patients have the ability to receive care at different hospitals, choice may be limited because patients are referred to a specific hospital by their outpatient physician or are brought to a hospital during an emergency.[18] In these cases, quality grades on the front doors of hospitals would not affect patient decisions, at least for that admission. Nonetheless, if quality grades were posted on the front doors of hospitals, patients receiving both inpatient and outpatient care would see the grades, and could use the information to make future decisions. Posted grades may also lead patients to review more in‐depth quality information related to their condition on the Hospital Compare website. Posted quality grades would also increase the visibility of the grades for other stakeholdersincluding the media and boards of directorsmagnifying their salience and impact.
How quality information is displayed and summarized can make or break public reporting programs. The New York City sanitation inspection program displays summarized, composite measures in the form of widely understood letter grades. Hospital Compare, however, displays myriad, unrelated performance measures that are not summarized into a global quality or safety measure. This information display is at odds with best practice. Patients find it difficult to synthesize data from multiple performance indicators to determine the relative quality of healthcare providers or insurance plans.7 In many cases, more information can lead to worse decision making.[19] Patients' difficulty making optimal choices has been noted in numerous healthcare settings, including purchasing Medicare Part D plans[20] and choosing health plans.[21] Recent evidence suggests that Nursing Home Compare's shift from an unsummarized collection of disparate performance measures to a 5‐star rating system has led patients to choose higher‐ranked facilities.[22] The fact that commercial providers of product quality information, such as Consumer Reports[23] and US News and World Report,[24] publish global summary scores, in addition to component scores, is a hint that this style of reporting is more appealing to consumers. Reports suggest that Medicare is moving toward a 5‐star quality rating system for hospitals,[8] which is a welcome development.
Different types of patients may demand different types of quality information, and a single summary measure for Hospital Compare may not meet the needs of a diverse set of patients. Nonetheless, the benefits from an actionable, understandable, comprehensive, and appropriate summary measure likely outweigh the costs of a potential mismatch for certain types of patients. Many of the performance measures on Hospital Compare already apply broadly to diverse sets of patients (eg, the structure measures, patient experience, and surgical safety) and are not specific to certain disease areas. Global summary measures could be complemented by separate component scores (eg, by disease area or domain of quality) for patients who wanted information on different aspects of care.
The inspection regime that underlies the New York City sanitary inspection program has parallels in healthcare that could be extended to Hospital Compare. For instance, the Joint Commission performs surprise inspections of hospitals as part of its accreditation process. The publicly reported 5‐star ratings for nursing homes are also based, in part, on inspection results.[25] Results from these types of inspections can capture up‐to‐date information on important dimensions of quality and safety that are not available in standard administrative data sources. Incorporating inspection results into Hospital Compare could increase both the timeliness and validity of the reporting.
The New York City sanitation inspection program is not a panacea: the indicators may not capture all relevant aspects of restaurant sanitation, some research suggests that past sanitary grades do not predict future grades,[26] and sanitary grade inflation over time has the potential to mask meaningful differences in sanitary conditions that are related to food‐borne illness.[16, 26] However, by providing understandable and meaningful reports at the point of service, the New York City program is well designed to encourage sanitation improvement through both consumer and supplier behavior.
Where the New York City sanitation inspection program succeeds, Hospital Compare fails. Hospital Compare is not patient centered, and it is not working for patients. Medicare can learn from the New York City restaurant sanitation inspection program to enhance the effects of public reporting by presenting information to consumers that is relevant, easy to access and interpret, and up to date. The greater complexity of hospital product lines should not deter these efforts. Patients' lives, not just the health of their gastrointestinal tracts, are at stake.
ACKNOWLEDGEMENTS
The authors thank Kaveh G. Shojania, MD, and Edward E. Etchells, MD, MSc, University of Toronto, and Martin Roland, DM, University of Oxford and RAND Europe for their comments on an earlier draft of the manuscript. None were compensated for their contributions.
Disclosures: Nothing to report.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , . Medicare's public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585–592.
- , . Public reporting of hospital hand hygiene compliance—helpful or harmful? JAMA. 2010;304(10):1116–1117.
- . Do cardiac surgery report cards reduce mortality? Assessing the evidence. Med Care Res Rev. 2006;63(4):403–426.
- , . Quality and consumer decision making in the market for health insurance and health care services. Med Care Res Rev. 2009;66(1 suppl):28S–52S.
- , . Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA. 1998;279(20):1638–1642.
- , , . Informing consumer decisions in health care: implications from decision‐making research. Milbank Q. 1997;75(3):395–414.
- Centers for Medicare hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and proposed fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation. Fed Regist. 2013:27486–27823.
- , . Relationship between Medicare's hospital compare performance measures and mortality rates. JAMA. 2006;296(22):2694–2702.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- New York City Department of Health and Mental Hygiene. What to expect when you're inspected: a guide for food service operators. New York, NY: New York City Department of Health and Mental Hygiene; 2010.
- . In reprieve for restaurant industry, New York proposes changes to grading system. New York Times. March 22, 2014:A15.
- . Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux; 2011.
- , , . Public hospital quality report awareness: evidence from National and Californian Internet searches and social media mentions, 2012. BMJ Open. 2014;4(3):e004417.
- New York City Department of Health and Mental Hygiene. Restaurant Grading in New York City at 18 Months. New York, NY: New York City Department of Health and Mental Hygiene; 2013.
- , . The effect of information on product quality: evidence from restaurant hygiene grade cards. Q J Econ. 2003;118(2):409–451.
- , , , . Do high‐cost hospitals deliver better care? Evidence from ambulance referral patterns. National Bureau of Economic Research. Working paper no. 17936. Available at: http://www.nber.org/papers/w17936.pdf. Published March 2012. Accessed November 18, 2014.
- , , , , . Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2)169–190.
- and . Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. Amer Econ Rev. 2011;101(4)1180–1210.
- , , , . Strategies for reporting health plan performance information to consumers: evidence from controlled studies. Health Serv Res. 2002;37(2):291–313.
- , . Quality reporting and private prices: evidence from the nursing home industry. Paper presented at: American Society of Health Economists Annual Meeting; June 23, 2014; Los Angeles, CA.
- Consumer Reports. Best new care values. Available at: http://consumerreports.org/cro/2012/05/best-new-car-values/index.htm. Updated February 2014. Accessed November 18, 2014.
- . Best value schools methodology. US News and World Report. September 8, 2014. Available at: http://www.usnews.com/education/best-colleges/articles/2013/09/09/best-value-schools-methodology. Accessed November 18, 2014.
- Centers for Medicare 122:574–677.
Few consumers would choose to dine at a restaurant if they knew the kitchen was infested with cockroaches. Few patients would choose to undergo a liver transplant in a hospital that was performing the procedure for the first time. In most sectors, consumers gather information about quality (and price) from the marketplace, where economic theory predicts that rational behavior and competition will lead to continuous improvement over time. However, for some goods and services, information is sparse and asymmetric between consumers and suppliers. In sectors where consumer health is at risk, society has often intervened to assure minimum standards. Yet sometimes these efforts have fallen short. In healthcare, physician licensure and hospital accreditation (eg, through the Joint Commission), although providing an important foundation to assure safety, have not come close to solving the widespread quality problems.[1] Basic regulatory requirements for restaurants have also proven inadequate to prevent food‐borne illness. Consumer trust, without information, can be a recipe (or prescription) for trouble.
In response, high‐profile efforts have been introduced to publicize the quality and safety of service providers. One example is Hospital Compare, Medicare's national quality reporting program for US hospitals.[2] The New York City sanitary grade inspection program is a parallel effort for restaurants. Although customers can judge how much they like the food from a restaurantor look up reviews at
The aims of Hospital Compare and the New York City sanitary inspection program are fundamentally similar. Both initiatives seek to address a common market failure resulting in the consumer's lack of information on quality and safety. By infusing the market with information, these programs enable consumers to make better choices and encourage service providers to improve quality and safety.[3] Despite the promise of these programs, a copious literature about the effects of public quality reporting in healthcare has found mixed results.[4, 5] Although the performance measures in any public reporting program must be valid and reliable, good measures are not sufficient to achieve the goals of public reporting. To engage patients, reported results must also be accessible, understandable, and meaningful. Both patients' lack of knowledge about the reports[6] and patients' inability to effectively use these data to make better decisions[7] are some reasons why public quality reporting has fallen short of its expectations. This article argues that the New York City program is much better structured to positively affect patient choice, and holds important lessons for public quality reporting in US hospitals.
CONTRASTS BETWEEN HOSPITAL COMPARE AND THE NEW YORK CITY RESTAURANT SANITARY INSPECTION PROGRAM
Hospital Compare reports performance for 108 separate quality indicators related to quality and patient safety for US hospitals (Table 1). These are a combination of structure measures (eg, hospital participation in a systematic database for cardiac surgery), process of care measures (eg, acute myocardial infarction patients receiving fibrinolytic therapy within 30 minutes of hospital arrival), outcomes (eg, 30‐day mortality and readmission), and patient experience measures (eg, how you would rate your communication with your physician). Hospital Compare data, frequently based on hospital quality performance 1 to 3 years prior to publication, are displayed on a website. Hospitals do not receive a summary measure of quality or safety.[8] Hospitals face financial incentives that are tied to measure reporting[9] and performance for some of the measures on Hospital Compare.[10, 11] Hospital accreditation is only loosely related to performance on these measures.
| Attribute | Hospital Compare | New York City Sanitary Inspection Program |
|---|---|---|
| Display of information | On a website ( |
On the front of the restaurant, with additional information also available on a website ( |
| Frequency of information update | Quarterly; data often lag by between 1 and 3 years. | Unannounced inspections occur at least annually. Grades are posted immediately after inspection. |
| Quality measures | Mix of measures pertaining to quality improvement activities (eg, hospital participation in a cardiac surgery registry or a quality improvement initiative), rates of adherence with evidence‐based medicine (eg, heart failure patients receiving discharge instructions, acute myocardial infarction patients receiving ‐blocker at arrival), and patient outcomes (eg, 30‐day mortality and 30‐day readmission for acute myocardial infarction, heart failure, and pneumonia). | Mix of measures pertaining to conditions of the facility (eg, improper sewage disposal system, improper food contact surface, evidence of live rats in the facility) and the treatment and handling of food (eg, food is unwrapped, appropriate thermometer not used to measure temperature of potentially hazardous foods, food not prepared to sufficiently high temperature). |
| Clarity and simplicity of information | 108 individual measures. No summary measure. | Single summary letter grade displayed on front of restaurant. Detailed data on individual violations (ie, measures) available on website. |
| Consequences of poor performance and mechanisms for enforcement | Hospitals are subject to financial penalties for not reporting certain measures and face financial incentives for performance on a subset of measures. | Restaurants are fined for violations, are subject to repeated inspections for poor performance, and are subject to closure for severe violations. |
| Consumer awareness | Limited | Widespread |
The New York City sanitation program regularly inspects restaurants and scores them on a standard set of indicators that correspond to critical violations (eg, food is contaminated by mouse droppings) or general violations (eg, garbage is not adequately covered).[12] Points are assigned to each type and severity of violation, and the sum of the points are converted into a summary grade of A, B, or C. Restaurants can dispute the grades, receiving a grade pending designation until the dispute is adjudicated. After inspection, sanitation grades are immediately posted on restaurants' front door or window, providing current information that is clearly visible to consumers before entering. More detailed information on sanitation violations is also available on a website. If restaurants receive an A grade, they face no additional inspections for 1 year, but poorly graded restaurants may receive monthly inspections. Restaurants face fines from violations and are subject to closure from severe violations. Recently proposed changes would decrease fines and give restaurants greater opportunities to appeal grades, but leave the program otherwise intact.[13]
IMPLICATIONS FOR PUBLIC QUALITY REPORTING IN HOSPITALS
Along with value‐based payment reforms, public quality reporting is one of the few major system‐level approaches that is being implemented in the US to improve quality and safety in healthcare. However, without a simple and understandable display of information that is available when a patient needs it, quality and safety information will likely go unused.[14] Hospital Compare leaves it up the patient to find the quality and safety information and does little to help patients understand and use the information effectively. Hospital Compare asks patients to do far more work, which is perhaps why it has been largely ignored by patients.[2, 15] The New York City sanitation inspection program evaluates restaurants, prominently displays an understandable summary result, and puts the scoring details in the background. Although peer‐reviewed evaluations of the New York City sanitation inspection program have not yet been published, internal data show that the program has decreased customer concern about getting sick, improved sanitary practices, and decreased salmonella.[16] Evidence from a similar program in Los Angeles County found that hygiene grades steered consumers toward restaurants with better sanitary conditions and decreased food‐borne illness.[17]
The nature of choice in healthcare, particularly the choice of hospital, is much different than it is for restaurants. In some areas, a single hospital may serve a large geographical area, severely limiting choice. Even when patients have the ability to receive care at different hospitals, choice may be limited because patients are referred to a specific hospital by their outpatient physician or are brought to a hospital during an emergency.[18] In these cases, quality grades on the front doors of hospitals would not affect patient decisions, at least for that admission. Nonetheless, if quality grades were posted on the front doors of hospitals, patients receiving both inpatient and outpatient care would see the grades, and could use the information to make future decisions. Posted grades may also lead patients to review more in‐depth quality information related to their condition on the Hospital Compare website. Posted quality grades would also increase the visibility of the grades for other stakeholdersincluding the media and boards of directorsmagnifying their salience and impact.
How quality information is displayed and summarized can make or break public reporting programs. The New York City sanitation inspection program displays summarized, composite measures in the form of widely understood letter grades. Hospital Compare, however, displays myriad, unrelated performance measures that are not summarized into a global quality or safety measure. This information display is at odds with best practice. Patients find it difficult to synthesize data from multiple performance indicators to determine the relative quality of healthcare providers or insurance plans.7 In many cases, more information can lead to worse decision making.[19] Patients' difficulty making optimal choices has been noted in numerous healthcare settings, including purchasing Medicare Part D plans[20] and choosing health plans.[21] Recent evidence suggests that Nursing Home Compare's shift from an unsummarized collection of disparate performance measures to a 5‐star rating system has led patients to choose higher‐ranked facilities.[22] The fact that commercial providers of product quality information, such as Consumer Reports[23] and US News and World Report,[24] publish global summary scores, in addition to component scores, is a hint that this style of reporting is more appealing to consumers. Reports suggest that Medicare is moving toward a 5‐star quality rating system for hospitals,[8] which is a welcome development.
Different types of patients may demand different types of quality information, and a single summary measure for Hospital Compare may not meet the needs of a diverse set of patients. Nonetheless, the benefits from an actionable, understandable, comprehensive, and appropriate summary measure likely outweigh the costs of a potential mismatch for certain types of patients. Many of the performance measures on Hospital Compare already apply broadly to diverse sets of patients (eg, the structure measures, patient experience, and surgical safety) and are not specific to certain disease areas. Global summary measures could be complemented by separate component scores (eg, by disease area or domain of quality) for patients who wanted information on different aspects of care.
The inspection regime that underlies the New York City sanitary inspection program has parallels in healthcare that could be extended to Hospital Compare. For instance, the Joint Commission performs surprise inspections of hospitals as part of its accreditation process. The publicly reported 5‐star ratings for nursing homes are also based, in part, on inspection results.[25] Results from these types of inspections can capture up‐to‐date information on important dimensions of quality and safety that are not available in standard administrative data sources. Incorporating inspection results into Hospital Compare could increase both the timeliness and validity of the reporting.
The New York City sanitation inspection program is not a panacea: the indicators may not capture all relevant aspects of restaurant sanitation, some research suggests that past sanitary grades do not predict future grades,[26] and sanitary grade inflation over time has the potential to mask meaningful differences in sanitary conditions that are related to food‐borne illness.[16, 26] However, by providing understandable and meaningful reports at the point of service, the New York City program is well designed to encourage sanitation improvement through both consumer and supplier behavior.
Where the New York City sanitation inspection program succeeds, Hospital Compare fails. Hospital Compare is not patient centered, and it is not working for patients. Medicare can learn from the New York City restaurant sanitation inspection program to enhance the effects of public reporting by presenting information to consumers that is relevant, easy to access and interpret, and up to date. The greater complexity of hospital product lines should not deter these efforts. Patients' lives, not just the health of their gastrointestinal tracts, are at stake.
ACKNOWLEDGEMENTS
The authors thank Kaveh G. Shojania, MD, and Edward E. Etchells, MD, MSc, University of Toronto, and Martin Roland, DM, University of Oxford and RAND Europe for their comments on an earlier draft of the manuscript. None were compensated for their contributions.
Disclosures: Nothing to report.
Few consumers would choose to dine at a restaurant if they knew the kitchen was infested with cockroaches. Few patients would choose to undergo a liver transplant in a hospital that was performing the procedure for the first time. In most sectors, consumers gather information about quality (and price) from the marketplace, where economic theory predicts that rational behavior and competition will lead to continuous improvement over time. However, for some goods and services, information is sparse and asymmetric between consumers and suppliers. In sectors where consumer health is at risk, society has often intervened to assure minimum standards. Yet sometimes these efforts have fallen short. In healthcare, physician licensure and hospital accreditation (eg, through the Joint Commission), although providing an important foundation to assure safety, have not come close to solving the widespread quality problems.[1] Basic regulatory requirements for restaurants have also proven inadequate to prevent food‐borne illness. Consumer trust, without information, can be a recipe (or prescription) for trouble.
In response, high‐profile efforts have been introduced to publicize the quality and safety of service providers. One example is Hospital Compare, Medicare's national quality reporting program for US hospitals.[2] The New York City sanitary grade inspection program is a parallel effort for restaurants. Although customers can judge how much they like the food from a restaurantor look up reviews at
The aims of Hospital Compare and the New York City sanitary inspection program are fundamentally similar. Both initiatives seek to address a common market failure resulting in the consumer's lack of information on quality and safety. By infusing the market with information, these programs enable consumers to make better choices and encourage service providers to improve quality and safety.[3] Despite the promise of these programs, a copious literature about the effects of public quality reporting in healthcare has found mixed results.[4, 5] Although the performance measures in any public reporting program must be valid and reliable, good measures are not sufficient to achieve the goals of public reporting. To engage patients, reported results must also be accessible, understandable, and meaningful. Both patients' lack of knowledge about the reports[6] and patients' inability to effectively use these data to make better decisions[7] are some reasons why public quality reporting has fallen short of its expectations. This article argues that the New York City program is much better structured to positively affect patient choice, and holds important lessons for public quality reporting in US hospitals.
CONTRASTS BETWEEN HOSPITAL COMPARE AND THE NEW YORK CITY RESTAURANT SANITARY INSPECTION PROGRAM
Hospital Compare reports performance for 108 separate quality indicators related to quality and patient safety for US hospitals (Table 1). These are a combination of structure measures (eg, hospital participation in a systematic database for cardiac surgery), process of care measures (eg, acute myocardial infarction patients receiving fibrinolytic therapy within 30 minutes of hospital arrival), outcomes (eg, 30‐day mortality and readmission), and patient experience measures (eg, how you would rate your communication with your physician). Hospital Compare data, frequently based on hospital quality performance 1 to 3 years prior to publication, are displayed on a website. Hospitals do not receive a summary measure of quality or safety.[8] Hospitals face financial incentives that are tied to measure reporting[9] and performance for some of the measures on Hospital Compare.[10, 11] Hospital accreditation is only loosely related to performance on these measures.
| Attribute | Hospital Compare | New York City Sanitary Inspection Program |
|---|---|---|
| Display of information | On a website ( |
On the front of the restaurant, with additional information also available on a website ( |
| Frequency of information update | Quarterly; data often lag by between 1 and 3 years. | Unannounced inspections occur at least annually. Grades are posted immediately after inspection. |
| Quality measures | Mix of measures pertaining to quality improvement activities (eg, hospital participation in a cardiac surgery registry or a quality improvement initiative), rates of adherence with evidence‐based medicine (eg, heart failure patients receiving discharge instructions, acute myocardial infarction patients receiving ‐blocker at arrival), and patient outcomes (eg, 30‐day mortality and 30‐day readmission for acute myocardial infarction, heart failure, and pneumonia). | Mix of measures pertaining to conditions of the facility (eg, improper sewage disposal system, improper food contact surface, evidence of live rats in the facility) and the treatment and handling of food (eg, food is unwrapped, appropriate thermometer not used to measure temperature of potentially hazardous foods, food not prepared to sufficiently high temperature). |
| Clarity and simplicity of information | 108 individual measures. No summary measure. | Single summary letter grade displayed on front of restaurant. Detailed data on individual violations (ie, measures) available on website. |
| Consequences of poor performance and mechanisms for enforcement | Hospitals are subject to financial penalties for not reporting certain measures and face financial incentives for performance on a subset of measures. | Restaurants are fined for violations, are subject to repeated inspections for poor performance, and are subject to closure for severe violations. |
| Consumer awareness | Limited | Widespread |
The New York City sanitation program regularly inspects restaurants and scores them on a standard set of indicators that correspond to critical violations (eg, food is contaminated by mouse droppings) or general violations (eg, garbage is not adequately covered).[12] Points are assigned to each type and severity of violation, and the sum of the points are converted into a summary grade of A, B, or C. Restaurants can dispute the grades, receiving a grade pending designation until the dispute is adjudicated. After inspection, sanitation grades are immediately posted on restaurants' front door or window, providing current information that is clearly visible to consumers before entering. More detailed information on sanitation violations is also available on a website. If restaurants receive an A grade, they face no additional inspections for 1 year, but poorly graded restaurants may receive monthly inspections. Restaurants face fines from violations and are subject to closure from severe violations. Recently proposed changes would decrease fines and give restaurants greater opportunities to appeal grades, but leave the program otherwise intact.[13]
IMPLICATIONS FOR PUBLIC QUALITY REPORTING IN HOSPITALS
Along with value‐based payment reforms, public quality reporting is one of the few major system‐level approaches that is being implemented in the US to improve quality and safety in healthcare. However, without a simple and understandable display of information that is available when a patient needs it, quality and safety information will likely go unused.[14] Hospital Compare leaves it up the patient to find the quality and safety information and does little to help patients understand and use the information effectively. Hospital Compare asks patients to do far more work, which is perhaps why it has been largely ignored by patients.[2, 15] The New York City sanitation inspection program evaluates restaurants, prominently displays an understandable summary result, and puts the scoring details in the background. Although peer‐reviewed evaluations of the New York City sanitation inspection program have not yet been published, internal data show that the program has decreased customer concern about getting sick, improved sanitary practices, and decreased salmonella.[16] Evidence from a similar program in Los Angeles County found that hygiene grades steered consumers toward restaurants with better sanitary conditions and decreased food‐borne illness.[17]
The nature of choice in healthcare, particularly the choice of hospital, is much different than it is for restaurants. In some areas, a single hospital may serve a large geographical area, severely limiting choice. Even when patients have the ability to receive care at different hospitals, choice may be limited because patients are referred to a specific hospital by their outpatient physician or are brought to a hospital during an emergency.[18] In these cases, quality grades on the front doors of hospitals would not affect patient decisions, at least for that admission. Nonetheless, if quality grades were posted on the front doors of hospitals, patients receiving both inpatient and outpatient care would see the grades, and could use the information to make future decisions. Posted grades may also lead patients to review more in‐depth quality information related to their condition on the Hospital Compare website. Posted quality grades would also increase the visibility of the grades for other stakeholdersincluding the media and boards of directorsmagnifying their salience and impact.
How quality information is displayed and summarized can make or break public reporting programs. The New York City sanitation inspection program displays summarized, composite measures in the form of widely understood letter grades. Hospital Compare, however, displays myriad, unrelated performance measures that are not summarized into a global quality or safety measure. This information display is at odds with best practice. Patients find it difficult to synthesize data from multiple performance indicators to determine the relative quality of healthcare providers or insurance plans.7 In many cases, more information can lead to worse decision making.[19] Patients' difficulty making optimal choices has been noted in numerous healthcare settings, including purchasing Medicare Part D plans[20] and choosing health plans.[21] Recent evidence suggests that Nursing Home Compare's shift from an unsummarized collection of disparate performance measures to a 5‐star rating system has led patients to choose higher‐ranked facilities.[22] The fact that commercial providers of product quality information, such as Consumer Reports[23] and US News and World Report,[24] publish global summary scores, in addition to component scores, is a hint that this style of reporting is more appealing to consumers. Reports suggest that Medicare is moving toward a 5‐star quality rating system for hospitals,[8] which is a welcome development.
Different types of patients may demand different types of quality information, and a single summary measure for Hospital Compare may not meet the needs of a diverse set of patients. Nonetheless, the benefits from an actionable, understandable, comprehensive, and appropriate summary measure likely outweigh the costs of a potential mismatch for certain types of patients. Many of the performance measures on Hospital Compare already apply broadly to diverse sets of patients (eg, the structure measures, patient experience, and surgical safety) and are not specific to certain disease areas. Global summary measures could be complemented by separate component scores (eg, by disease area or domain of quality) for patients who wanted information on different aspects of care.
The inspection regime that underlies the New York City sanitary inspection program has parallels in healthcare that could be extended to Hospital Compare. For instance, the Joint Commission performs surprise inspections of hospitals as part of its accreditation process. The publicly reported 5‐star ratings for nursing homes are also based, in part, on inspection results.[25] Results from these types of inspections can capture up‐to‐date information on important dimensions of quality and safety that are not available in standard administrative data sources. Incorporating inspection results into Hospital Compare could increase both the timeliness and validity of the reporting.
The New York City sanitation inspection program is not a panacea: the indicators may not capture all relevant aspects of restaurant sanitation, some research suggests that past sanitary grades do not predict future grades,[26] and sanitary grade inflation over time has the potential to mask meaningful differences in sanitary conditions that are related to food‐borne illness.[16, 26] However, by providing understandable and meaningful reports at the point of service, the New York City program is well designed to encourage sanitation improvement through both consumer and supplier behavior.
Where the New York City sanitation inspection program succeeds, Hospital Compare fails. Hospital Compare is not patient centered, and it is not working for patients. Medicare can learn from the New York City restaurant sanitation inspection program to enhance the effects of public reporting by presenting information to consumers that is relevant, easy to access and interpret, and up to date. The greater complexity of hospital product lines should not deter these efforts. Patients' lives, not just the health of their gastrointestinal tracts, are at stake.
ACKNOWLEDGEMENTS
The authors thank Kaveh G. Shojania, MD, and Edward E. Etchells, MD, MSc, University of Toronto, and Martin Roland, DM, University of Oxford and RAND Europe for their comments on an earlier draft of the manuscript. None were compensated for their contributions.
Disclosures: Nothing to report.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , . Medicare's public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585–592.
- , . Public reporting of hospital hand hygiene compliance—helpful or harmful? JAMA. 2010;304(10):1116–1117.
- . Do cardiac surgery report cards reduce mortality? Assessing the evidence. Med Care Res Rev. 2006;63(4):403–426.
- , . Quality and consumer decision making in the market for health insurance and health care services. Med Care Res Rev. 2009;66(1 suppl):28S–52S.
- , . Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA. 1998;279(20):1638–1642.
- , , . Informing consumer decisions in health care: implications from decision‐making research. Milbank Q. 1997;75(3):395–414.
- Centers for Medicare hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and proposed fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation. Fed Regist. 2013:27486–27823.
- , . Relationship between Medicare's hospital compare performance measures and mortality rates. JAMA. 2006;296(22):2694–2702.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- New York City Department of Health and Mental Hygiene. What to expect when you're inspected: a guide for food service operators. New York, NY: New York City Department of Health and Mental Hygiene; 2010.
- . In reprieve for restaurant industry, New York proposes changes to grading system. New York Times. March 22, 2014:A15.
- . Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux; 2011.
- , , . Public hospital quality report awareness: evidence from National and Californian Internet searches and social media mentions, 2012. BMJ Open. 2014;4(3):e004417.
- New York City Department of Health and Mental Hygiene. Restaurant Grading in New York City at 18 Months. New York, NY: New York City Department of Health and Mental Hygiene; 2013.
- , . The effect of information on product quality: evidence from restaurant hygiene grade cards. Q J Econ. 2003;118(2):409–451.
- , , , . Do high‐cost hospitals deliver better care? Evidence from ambulance referral patterns. National Bureau of Economic Research. Working paper no. 17936. Available at: http://www.nber.org/papers/w17936.pdf. Published March 2012. Accessed November 18, 2014.
- , , , , . Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2)169–190.
- and . Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. Amer Econ Rev. 2011;101(4)1180–1210.
- , , , . Strategies for reporting health plan performance information to consumers: evidence from controlled studies. Health Serv Res. 2002;37(2):291–313.
- , . Quality reporting and private prices: evidence from the nursing home industry. Paper presented at: American Society of Health Economists Annual Meeting; June 23, 2014; Los Angeles, CA.
- Consumer Reports. Best new care values. Available at: http://consumerreports.org/cro/2012/05/best-new-car-values/index.htm. Updated February 2014. Accessed November 18, 2014.
- . Best value schools methodology. US News and World Report. September 8, 2014. Available at: http://www.usnews.com/education/best-colleges/articles/2013/09/09/best-value-schools-methodology. Accessed November 18, 2014.
- Centers for Medicare 122:574–677.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , . Medicare's public reporting initiative on hospital quality had modest or no impact on mortality from three key conditions. Health Aff (Millwood). 2012;31(3):585–592.
- , . Public reporting of hospital hand hygiene compliance—helpful or harmful? JAMA. 2010;304(10):1116–1117.
- . Do cardiac surgery report cards reduce mortality? Assessing the evidence. Med Care Res Rev. 2006;63(4):403–426.
- , . Quality and consumer decision making in the market for health insurance and health care services. Med Care Res Rev. 2009;66(1 suppl):28S–52S.
- , . Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA. 1998;279(20):1638–1642.
- , , . Informing consumer decisions in health care: implications from decision‐making research. Milbank Q. 1997;75(3):395–414.
- Centers for Medicare hospital inpatient prospective payment systems for acute care hospitals and the long‐term care hospital prospective payment system and proposed fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation. Fed Regist. 2013:27486–27823.
- , . Relationship between Medicare's hospital compare performance measures and mortality rates. JAMA. 2006;296(22):2694–2702.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- New York City Department of Health and Mental Hygiene. What to expect when you're inspected: a guide for food service operators. New York, NY: New York City Department of Health and Mental Hygiene; 2010.
- . In reprieve for restaurant industry, New York proposes changes to grading system. New York Times. March 22, 2014:A15.
- . Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux; 2011.
- , , . Public hospital quality report awareness: evidence from National and Californian Internet searches and social media mentions, 2012. BMJ Open. 2014;4(3):e004417.
- New York City Department of Health and Mental Hygiene. Restaurant Grading in New York City at 18 Months. New York, NY: New York City Department of Health and Mental Hygiene; 2013.
- , . The effect of information on product quality: evidence from restaurant hygiene grade cards. Q J Econ. 2003;118(2):409–451.
- , , , . Do high‐cost hospitals deliver better care? Evidence from ambulance referral patterns. National Bureau of Economic Research. Working paper no. 17936. Available at: http://www.nber.org/papers/w17936.pdf. Published March 2012. Accessed November 18, 2014.
- , , , , . Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2)169–190.
- and . Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. Amer Econ Rev. 2011;101(4)1180–1210.
- , , , . Strategies for reporting health plan performance information to consumers: evidence from controlled studies. Health Serv Res. 2002;37(2):291–313.
- , . Quality reporting and private prices: evidence from the nursing home industry. Paper presented at: American Society of Health Economists Annual Meeting; June 23, 2014; Los Angeles, CA.
- Consumer Reports. Best new care values. Available at: http://consumerreports.org/cro/2012/05/best-new-car-values/index.htm. Updated February 2014. Accessed November 18, 2014.
- . Best value schools methodology. US News and World Report. September 8, 2014. Available at: http://www.usnews.com/education/best-colleges/articles/2013/09/09/best-value-schools-methodology. Accessed November 18, 2014.
- Centers for Medicare 122:574–677.
Improving Notes in the EHR
There are described advantages to documenting in an electronic health record (EHR).[1, 2, 3, 4, 5] There has been, however, an unanticipated decline in certain aspects of documentation quality after implementing EHRs,[6, 7, 8] for example, the overinclusion of data (note clutter) and inappropriate use of copy‐paste.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17]
The objectives of this pilot study were to examine the effectiveness of an intervention bundle designed to improve resident progress notes written in an EHR (Epic Systems Corp., Verona, WI) and to establish the reliability of an audit tool used to assess the notes. Prior to this intervention, we provided no formal education for our residents about documentation in the EHR and had no policy governing format or content. The institutional review board at the University of Wisconsin approved this study.
METHODS
The Intervention Bundle
A multidisciplinary task force developed a set of Best Practice Guidelines for Writing Progress Notes in the EHR (see Supporting Information, Appendix 1, in the online version of this article). They were designed to promote cognitive review of data, reduce note clutter, promote synthesis of data, and discourage copy‐paste. For example, the guidelines recommended either the phrase, Vital signs from the last 24 hours have been reviewed and are pertinent for or a link that included minimum/maximum values rather than including multiple sets of data. We next developed a note template aligned with these guidelines (see Supporting Information, Appendix 2, in the online version of this article) using features and links that already existed within the EHR. Interns received classroom teaching about the best practices and instruction in use of the template.
Study Design
The study was a retrospective pre‐/postintervention. An audit tool designed to assess compliance with the guidelines was used to score 25 progress notes written by pediatric interns in August 2010 and August 2011 during the pre‐ and postintervention periods, respectively (see Supporting Information, Appendix 3, in the online version of this article).
Progress notes were eligible based on the following criteria: (1) written on any day subsequent to the admission date, (2) written by a pediatric intern, and (3) progress note from the previous day available for comparison. It was not required that 2 consecutive notes be written by the same resident. Eligible notes were identified using a computer‐generated report, reviewed by a study member to ensure eligibility, and assigned a number.
Notes were scored on a scale of 0 to 17, with each question having a range of possible scores from 0 to 2. Some questions related to inappropriate copy‐paste (questions 2, 9, 10) and a question related to discrete diagnostic language for abnormal labs (question 11) were weighted more heavily in the tool, as compliance with these components of the guideline was felt to be of greater importance. Several questions within the audit tool refer to clutter. We defined clutter as any additional data not endorsed by the guidelines or not explicitly stated as relevant to the patient's care for that day.
Raters were trained to score notes through practice sessions, during which they all scored the same note and compared findings. To rectify inter‐rater scoring discrepancies identified during these sessions, a reference manual was created to assist raters in scoring notes (see Supporting Information, Appendix 4, in the online version of this article). Each preintervention note was then systematically assigned to 2 raters, comprised of a physician and 3 staff from health information management. Each rater scored the note individually without discussion. The inter‐rater reliability was determined to be excellent, with kappa indices ranging from 88% to 100% for the 13 questions; each note in the postintervention period was therefore assigned to only 1 rater. Total and individual questions' scores were sent to the statistician for analysis.
Statistical Analysis
Inter‐rater reliability of the audit tool was evaluated by calculating the intraclass correlation (ICC) coefficient using a multilevel random intercept model to account for the rater effect.[18] The study was powered to detect an anticipated ICC of at least 0.75 at the 1‐sided 0.05 significance level, assuming a null hypothesis that the ICC is 0.4 or less. The total score was summarized in terms of means and standard deviation. Individual item responses were summarized using percentages and compared between the pre‐ and postintervention assessment using the Fisher exact test. The analysis of response patterns for individual item scores was considered exploratory. The Benjamini‐Hochberg false discovery rate method was utilized to control the false‐positive rate when comparing individual item scores.[19] All P values were 2‐sided and considered statistically significant at 0.05. Statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The ICC was 0.96 (95% confidence interval: 0.91‐0.98), indicating an excellent level of inter‐rater reliability. There was a significant improvement in the total score (see Supporting Information, Appendix 5, in the online version of this article) between the preintervention (mean 9.72, standard deviation [SD] 1.52) and postintervention (mean 11.72, SD 1.62) periods (P0.0001).
Table 1 shows the percentage of yes responses to each individual item in the pre‐ and postintervention periods. Our intervention had a significant impact on reducing vital sign clutter (4% preintervention, 84% postintervention, P0.0001) and other visual clutter within the note (0% preintervention, 28% postintervention, P=0.0035). We did not observe a significant impact on the reduction of input/output or lab clutter. There was no significant difference observed in the inclusion of the medication list. No significant improvements were seen in questions related to copy‐paste. The intervention had no significant impact on areas with an already high baseline performance: newly written interval histories, newly written physical exams, newly written plans, and the inclusion of discrete diagnostic language for abnormal labs.
| Question | Preintervention, N=25* | Postintervention, N=25 | P Value |
|---|---|---|---|
| |||
| 1. Does the note header include the name of the service, author, and training level of the author? | 0% | 68% | 0.0001 |
| 2. Does it appear that the subjective/emnterval history section of the note was newly written? (ie, not copied in its entirety from the previous note) | 100% | 96% | 0.9999 |
| 3. Is the vital sign section noncluttered? | 4% | 84% | 0.0001 |
| 4. Is the entire medication list included in the note? | 96% | 96% | 0.9999 |
| 5. Is the intake/output section noncluttered? | 0% | 16% | 0.3076 |
| 6. Does it appear that the physical exam was newly written? (ie, not copied in its entirety from the previous note) | 80% | 68% | 0.9103 |
| 7. Is the lab section noncluttered? | 64% | 44% | 0.5125 |
| 8. Is the imaging section noncluttered? | 100% | 100% | 0.9999 |
| 9. Does it appear that the assessment was newly written? | 48% | 28% | 0.5121 |
| 48% partial | 52% partial | 0.9999 | |
| 10. Does it appear that the plan was newly written or partially copied with new information added? | 88% | 96% | 0.9477 |
| 11. If the assessment includes abnormal lab values, is there also an accompanying diagnosis? (eg, inclusion of patient has hemoglobin of 6.2, also includes diagnosis of anemia) | 96% | 96% | 0.9999 |
| 12. Is additional visual clutter prevented by excluding other objective data found elsewhere in the chart? | 0% | 28% | 0.0035 |
| 13. Is the author's name and contact information (pager, cell) included at the bottom of the note? | 0% | 72% | 0.0001 |
DISCUSSION
Principal Findings
Improvements in electronic note writing, particularly in reducing note clutter, were achieved after the implementation of a bundled intervention. Because the intervention is a bundle, we cannot definitively identify which component had the greatest impact. Given the improvements seen in some areas with very low baseline performance, we hypothesize that these are most attributable to the creation of a compliant note template that (1) guided authors in using data links that were less cluttered and (2) eliminated the use of unnecessary links (eg, pain scores and daily weights). The lack of similar improvements in reducing input/output and lab clutter may be due to the fact that even with changes to the template suggesting a more narrative approach to these components, residents still felt compelled to use data links. Because our EHR does not easily allow for the inclusion of individual data elements, such as specific drain output or hemoglobin as opposed to a complete blood count, residents continued to use links that included more data than necessary. Although not significant findings, there was an observed decline in the proportion of notes containing a physical exam not entirely copied from the previous day and containing an assessment that was entirely new. These findings may be attributable to having a small sample of authors, a few of whom in the postintervention period were particularly prone to using copy‐paste.
Relationship to Other Evidence
The observed decline in quality of provider documentation after implementation of the EHR has led to a robust discussion in the literature about what really constitutes a quality provider note.[7, 8, 9, 10, 20] The absence of a defined gold standard makes research in this area challenging. It is our observation that when physicians refer to a decline in quality documentation in the EHR, they are frequently referring to the fact that electronically generated notes are often unattractive, difficult to read, and seem to lack clinical narrative.
Several publications have attempted to define note quality. Payne et al. described physical characteristics of electronically generated notes that were deemed more attractive to a reader, including a large proportion of narrative free text.[15] Hanson performed a qualitative study to describe outpatient clinical notes from the perspective of multiple stakeholders, resulting in a description of the characteristics of a quality note.[21] This formed the basis for the QNOTE, a validated tool to measure the quality of outpatient notes.[22] Similar work has not been done to rigorously define quality for inpatient documentation. Stetson did develop an instrument, the Physician Documentation Quality Instrument (PDQI‐9) to assess inpatient notes across 9 attributes; however, the validation method relied on a gold standard of a general impression score of 7 physician leaders.[23, 24]
Although these tools aim to address overall note quality, an advantage provided by our audit tool is that it directly addresses the problems most attributable to documenting in an EHR, namely note clutter and copy‐paste. A second advantage is that clinicians and nonclinicians can score notes objectively. The QNOTE and PDQI‐9 still rely on subjective assessment and require that the evaluator be a clinician.
There has also been little published about how to achieve notes of high quality. In 2013, Shoolin et al. did publish a consensus statement from the Association of Medical Directors of Information Systems outlining some guidelines for inpatient EHR documentation.[25] Optimal strategies for implementing such guidelines, however, and the overall impact such an implementation would have on improving note writing has not previously been studied. This study, therefore, adds to the existing body of literature by providing an example of an intervention that may lead to improvements in note writing.
Limitations
Our study has several limitations. The sample size of notes and authors was small. The short duration of the study and the assessment of notes soon after the intervention prevented an assessment of whether improvements were sustained over time.
Unfortunately, we were not evaluating the same group of interns in the pre‐ and postintervention periods. Interns were chosen as subjects as there was an existing opportunity to do large group training during new intern orientation. Furthermore, we were concerned that more note‐writing experience alone would influence the outcome if we examined the same interns later in the year.
The audit tool was also a first attempt at measuring compliance with the guidelines. Determination of an optimal score/weight for each item requires further investigation as part of a larger scale validation study. In addition, the cognitive review and synthesis of data encouraged in our guideline were more difficult to measure using the audit tool, as they require some clinical knowledge about the patient and an assessment of the author's medical decision making. We do not assert, therefore, that compliance with the guidelines or a higher total score necessarily translates into overall note quality, as we recognize these limitations of the tool.
Future Directions
In conclusion, this report is a first effort to improve the quality of note writing in the EHR. Much more work is necessary, particularly in improving the clinical narrative and inappropriate copy‐paste. The examination of other interventions, such as the impact of structured feedback to the note author, whether by way of a validated scoring tool and/or narrative comments, is a logical next step for investigation.
ACKNOWLEDGEMENTS
The authors acknowledge and appreciate the support of Joel Buchanan, MD, Ellen Wald, MD, and Ann Boyer, MD, for their contributions to this study and manuscript preparation. We also acknowledge the members of the auditing team: Linda Brickert, Jane Duckert, and Jeannine Strunk.
Disclosure: Nothing to report.
- , , . Use of computer‐based records, completeness of documentation, and appropriateness of documented clinical decisions. J Am Med Inform Assoc. 1999;6(3):245–251.
- , , , et al. An automated model to identify heart failure patients at risk for 30‐day readmission or death using electronic medical record data. Med Care. 2010;48(11):981–988.
- , , , , . Clinical information technologies and inpatient outcomes: a multiple hospital study. Arch Intern Med. 2009;169(2):108–114.
- , , , , . Identifying patients with diabetes and the earliest date of diagnosis in real time: an electronic health record case‐finding algorithm. BMC Med Inform Decis Mak. 2013;13:81.
- , , , et al. Relationship between use of electronic health record features and health care quality: results of a statewide survey. Med Care. 2010;48(3):203–209.
- , , , , , . Impacts of computerized physician documentation in a teaching hospital: perceptions of faculty and resident physicians. J Am Med Inform Assoc. 2004;11(4):300–309.
- , . Off the record—avoiding the pitfalls of going electronic. N Engl J Med. 2008;358(16):1656–1658.
- . A piece of my mind. Copy‐and‐paste. JAMA. 2006;295(20):2335–2336.
- , . Copy and paste: a remediable hazard of electronic health records. Am J Med. 2009;122(6):495–496.
- , , , , , . Physicians' attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63–68.
- . Improving the electronic health record—are clinicians getting what they wished for? JAMA. 2013;309(10):991–992.
- , , . Copying and pasting of examinations within the electronic medical record. Int J Med Inform. 2007;76(suppl 1):S122–S128.
- . The evolving medical record. Ann Intern Med. 2010;153(10):671–677.
- , , , , , . Direct text entry in electronic progress notes. An evaluation of input errors. Methods Inf Med. 2003;42(1):61–67.
- , , , . The physical attractiveness of electronic physician notes. AMIA Annu Symp Proc. 2010;2010:622–626.
- , . Copy‐and‐paste‐and‐paste. JAMA. 2006;296(19):2315; author reply 2315–2316.
- , , , . Are electronic medical records trustworthy? Observations on copying, pasting and duplication. AMIA Annu Symp Proc. 2003:269–273.
- , . Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Thousand Oaks, CA: Sage; 2002.
- , . Controlling the false discovery rate: a practical and powerful approach for multiple testing. J R Stat Soc Series B Stat Methodol 1995;57(1):289–300.
- , , . The role of copy‐and‐paste in the hospital electronic health record. JAMA Intern Med. 2014;174(8):1217–1218.
- , , , . Quality of outpatient clinical notes: a stakeholder definition derived through qualitative research. BMC Health Serv Res. 2012;12:407.
- , , , et al. QNOTE: an instrument for measuring the quality of EHR clinical notes. J Am Med Inform Assoc. 2014;21(5):910–916.
- , , , . Assessing electronic note quality using the physician documentation quality instrument (PDQI‐9). Appl Clin Inform. 2012;3(2):164–174.
- , , , . Preliminary development of the physician documentation quality instrument. J Am Med Inform Assoc. 2008;15(4):534–541.
- , , , . Association of Medical Directors of Information Systems consensus on inpatient electronic health record documentation. Appl Clin Inform. 2013;4(2):293–303.
There are described advantages to documenting in an electronic health record (EHR).[1, 2, 3, 4, 5] There has been, however, an unanticipated decline in certain aspects of documentation quality after implementing EHRs,[6, 7, 8] for example, the overinclusion of data (note clutter) and inappropriate use of copy‐paste.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17]
The objectives of this pilot study were to examine the effectiveness of an intervention bundle designed to improve resident progress notes written in an EHR (Epic Systems Corp., Verona, WI) and to establish the reliability of an audit tool used to assess the notes. Prior to this intervention, we provided no formal education for our residents about documentation in the EHR and had no policy governing format or content. The institutional review board at the University of Wisconsin approved this study.
METHODS
The Intervention Bundle
A multidisciplinary task force developed a set of Best Practice Guidelines for Writing Progress Notes in the EHR (see Supporting Information, Appendix 1, in the online version of this article). They were designed to promote cognitive review of data, reduce note clutter, promote synthesis of data, and discourage copy‐paste. For example, the guidelines recommended either the phrase, Vital signs from the last 24 hours have been reviewed and are pertinent for or a link that included minimum/maximum values rather than including multiple sets of data. We next developed a note template aligned with these guidelines (see Supporting Information, Appendix 2, in the online version of this article) using features and links that already existed within the EHR. Interns received classroom teaching about the best practices and instruction in use of the template.
Study Design
The study was a retrospective pre‐/postintervention. An audit tool designed to assess compliance with the guidelines was used to score 25 progress notes written by pediatric interns in August 2010 and August 2011 during the pre‐ and postintervention periods, respectively (see Supporting Information, Appendix 3, in the online version of this article).
Progress notes were eligible based on the following criteria: (1) written on any day subsequent to the admission date, (2) written by a pediatric intern, and (3) progress note from the previous day available for comparison. It was not required that 2 consecutive notes be written by the same resident. Eligible notes were identified using a computer‐generated report, reviewed by a study member to ensure eligibility, and assigned a number.
Notes were scored on a scale of 0 to 17, with each question having a range of possible scores from 0 to 2. Some questions related to inappropriate copy‐paste (questions 2, 9, 10) and a question related to discrete diagnostic language for abnormal labs (question 11) were weighted more heavily in the tool, as compliance with these components of the guideline was felt to be of greater importance. Several questions within the audit tool refer to clutter. We defined clutter as any additional data not endorsed by the guidelines or not explicitly stated as relevant to the patient's care for that day.
Raters were trained to score notes through practice sessions, during which they all scored the same note and compared findings. To rectify inter‐rater scoring discrepancies identified during these sessions, a reference manual was created to assist raters in scoring notes (see Supporting Information, Appendix 4, in the online version of this article). Each preintervention note was then systematically assigned to 2 raters, comprised of a physician and 3 staff from health information management. Each rater scored the note individually without discussion. The inter‐rater reliability was determined to be excellent, with kappa indices ranging from 88% to 100% for the 13 questions; each note in the postintervention period was therefore assigned to only 1 rater. Total and individual questions' scores were sent to the statistician for analysis.
Statistical Analysis
Inter‐rater reliability of the audit tool was evaluated by calculating the intraclass correlation (ICC) coefficient using a multilevel random intercept model to account for the rater effect.[18] The study was powered to detect an anticipated ICC of at least 0.75 at the 1‐sided 0.05 significance level, assuming a null hypothesis that the ICC is 0.4 or less. The total score was summarized in terms of means and standard deviation. Individual item responses were summarized using percentages and compared between the pre‐ and postintervention assessment using the Fisher exact test. The analysis of response patterns for individual item scores was considered exploratory. The Benjamini‐Hochberg false discovery rate method was utilized to control the false‐positive rate when comparing individual item scores.[19] All P values were 2‐sided and considered statistically significant at 0.05. Statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The ICC was 0.96 (95% confidence interval: 0.91‐0.98), indicating an excellent level of inter‐rater reliability. There was a significant improvement in the total score (see Supporting Information, Appendix 5, in the online version of this article) between the preintervention (mean 9.72, standard deviation [SD] 1.52) and postintervention (mean 11.72, SD 1.62) periods (P0.0001).
Table 1 shows the percentage of yes responses to each individual item in the pre‐ and postintervention periods. Our intervention had a significant impact on reducing vital sign clutter (4% preintervention, 84% postintervention, P0.0001) and other visual clutter within the note (0% preintervention, 28% postintervention, P=0.0035). We did not observe a significant impact on the reduction of input/output or lab clutter. There was no significant difference observed in the inclusion of the medication list. No significant improvements were seen in questions related to copy‐paste. The intervention had no significant impact on areas with an already high baseline performance: newly written interval histories, newly written physical exams, newly written plans, and the inclusion of discrete diagnostic language for abnormal labs.
| Question | Preintervention, N=25* | Postintervention, N=25 | P Value |
|---|---|---|---|
| |||
| 1. Does the note header include the name of the service, author, and training level of the author? | 0% | 68% | 0.0001 |
| 2. Does it appear that the subjective/emnterval history section of the note was newly written? (ie, not copied in its entirety from the previous note) | 100% | 96% | 0.9999 |
| 3. Is the vital sign section noncluttered? | 4% | 84% | 0.0001 |
| 4. Is the entire medication list included in the note? | 96% | 96% | 0.9999 |
| 5. Is the intake/output section noncluttered? | 0% | 16% | 0.3076 |
| 6. Does it appear that the physical exam was newly written? (ie, not copied in its entirety from the previous note) | 80% | 68% | 0.9103 |
| 7. Is the lab section noncluttered? | 64% | 44% | 0.5125 |
| 8. Is the imaging section noncluttered? | 100% | 100% | 0.9999 |
| 9. Does it appear that the assessment was newly written? | 48% | 28% | 0.5121 |
| 48% partial | 52% partial | 0.9999 | |
| 10. Does it appear that the plan was newly written or partially copied with new information added? | 88% | 96% | 0.9477 |
| 11. If the assessment includes abnormal lab values, is there also an accompanying diagnosis? (eg, inclusion of patient has hemoglobin of 6.2, also includes diagnosis of anemia) | 96% | 96% | 0.9999 |
| 12. Is additional visual clutter prevented by excluding other objective data found elsewhere in the chart? | 0% | 28% | 0.0035 |
| 13. Is the author's name and contact information (pager, cell) included at the bottom of the note? | 0% | 72% | 0.0001 |
DISCUSSION
Principal Findings
Improvements in electronic note writing, particularly in reducing note clutter, were achieved after the implementation of a bundled intervention. Because the intervention is a bundle, we cannot definitively identify which component had the greatest impact. Given the improvements seen in some areas with very low baseline performance, we hypothesize that these are most attributable to the creation of a compliant note template that (1) guided authors in using data links that were less cluttered and (2) eliminated the use of unnecessary links (eg, pain scores and daily weights). The lack of similar improvements in reducing input/output and lab clutter may be due to the fact that even with changes to the template suggesting a more narrative approach to these components, residents still felt compelled to use data links. Because our EHR does not easily allow for the inclusion of individual data elements, such as specific drain output or hemoglobin as opposed to a complete blood count, residents continued to use links that included more data than necessary. Although not significant findings, there was an observed decline in the proportion of notes containing a physical exam not entirely copied from the previous day and containing an assessment that was entirely new. These findings may be attributable to having a small sample of authors, a few of whom in the postintervention period were particularly prone to using copy‐paste.
Relationship to Other Evidence
The observed decline in quality of provider documentation after implementation of the EHR has led to a robust discussion in the literature about what really constitutes a quality provider note.[7, 8, 9, 10, 20] The absence of a defined gold standard makes research in this area challenging. It is our observation that when physicians refer to a decline in quality documentation in the EHR, they are frequently referring to the fact that electronically generated notes are often unattractive, difficult to read, and seem to lack clinical narrative.
Several publications have attempted to define note quality. Payne et al. described physical characteristics of electronically generated notes that were deemed more attractive to a reader, including a large proportion of narrative free text.[15] Hanson performed a qualitative study to describe outpatient clinical notes from the perspective of multiple stakeholders, resulting in a description of the characteristics of a quality note.[21] This formed the basis for the QNOTE, a validated tool to measure the quality of outpatient notes.[22] Similar work has not been done to rigorously define quality for inpatient documentation. Stetson did develop an instrument, the Physician Documentation Quality Instrument (PDQI‐9) to assess inpatient notes across 9 attributes; however, the validation method relied on a gold standard of a general impression score of 7 physician leaders.[23, 24]
Although these tools aim to address overall note quality, an advantage provided by our audit tool is that it directly addresses the problems most attributable to documenting in an EHR, namely note clutter and copy‐paste. A second advantage is that clinicians and nonclinicians can score notes objectively. The QNOTE and PDQI‐9 still rely on subjective assessment and require that the evaluator be a clinician.
There has also been little published about how to achieve notes of high quality. In 2013, Shoolin et al. did publish a consensus statement from the Association of Medical Directors of Information Systems outlining some guidelines for inpatient EHR documentation.[25] Optimal strategies for implementing such guidelines, however, and the overall impact such an implementation would have on improving note writing has not previously been studied. This study, therefore, adds to the existing body of literature by providing an example of an intervention that may lead to improvements in note writing.
Limitations
Our study has several limitations. The sample size of notes and authors was small. The short duration of the study and the assessment of notes soon after the intervention prevented an assessment of whether improvements were sustained over time.
Unfortunately, we were not evaluating the same group of interns in the pre‐ and postintervention periods. Interns were chosen as subjects as there was an existing opportunity to do large group training during new intern orientation. Furthermore, we were concerned that more note‐writing experience alone would influence the outcome if we examined the same interns later in the year.
The audit tool was also a first attempt at measuring compliance with the guidelines. Determination of an optimal score/weight for each item requires further investigation as part of a larger scale validation study. In addition, the cognitive review and synthesis of data encouraged in our guideline were more difficult to measure using the audit tool, as they require some clinical knowledge about the patient and an assessment of the author's medical decision making. We do not assert, therefore, that compliance with the guidelines or a higher total score necessarily translates into overall note quality, as we recognize these limitations of the tool.
Future Directions
In conclusion, this report is a first effort to improve the quality of note writing in the EHR. Much more work is necessary, particularly in improving the clinical narrative and inappropriate copy‐paste. The examination of other interventions, such as the impact of structured feedback to the note author, whether by way of a validated scoring tool and/or narrative comments, is a logical next step for investigation.
ACKNOWLEDGEMENTS
The authors acknowledge and appreciate the support of Joel Buchanan, MD, Ellen Wald, MD, and Ann Boyer, MD, for their contributions to this study and manuscript preparation. We also acknowledge the members of the auditing team: Linda Brickert, Jane Duckert, and Jeannine Strunk.
Disclosure: Nothing to report.
There are described advantages to documenting in an electronic health record (EHR).[1, 2, 3, 4, 5] There has been, however, an unanticipated decline in certain aspects of documentation quality after implementing EHRs,[6, 7, 8] for example, the overinclusion of data (note clutter) and inappropriate use of copy‐paste.[6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17]
The objectives of this pilot study were to examine the effectiveness of an intervention bundle designed to improve resident progress notes written in an EHR (Epic Systems Corp., Verona, WI) and to establish the reliability of an audit tool used to assess the notes. Prior to this intervention, we provided no formal education for our residents about documentation in the EHR and had no policy governing format or content. The institutional review board at the University of Wisconsin approved this study.
METHODS
The Intervention Bundle
A multidisciplinary task force developed a set of Best Practice Guidelines for Writing Progress Notes in the EHR (see Supporting Information, Appendix 1, in the online version of this article). They were designed to promote cognitive review of data, reduce note clutter, promote synthesis of data, and discourage copy‐paste. For example, the guidelines recommended either the phrase, Vital signs from the last 24 hours have been reviewed and are pertinent for or a link that included minimum/maximum values rather than including multiple sets of data. We next developed a note template aligned with these guidelines (see Supporting Information, Appendix 2, in the online version of this article) using features and links that already existed within the EHR. Interns received classroom teaching about the best practices and instruction in use of the template.
Study Design
The study was a retrospective pre‐/postintervention. An audit tool designed to assess compliance with the guidelines was used to score 25 progress notes written by pediatric interns in August 2010 and August 2011 during the pre‐ and postintervention periods, respectively (see Supporting Information, Appendix 3, in the online version of this article).
Progress notes were eligible based on the following criteria: (1) written on any day subsequent to the admission date, (2) written by a pediatric intern, and (3) progress note from the previous day available for comparison. It was not required that 2 consecutive notes be written by the same resident. Eligible notes were identified using a computer‐generated report, reviewed by a study member to ensure eligibility, and assigned a number.
Notes were scored on a scale of 0 to 17, with each question having a range of possible scores from 0 to 2. Some questions related to inappropriate copy‐paste (questions 2, 9, 10) and a question related to discrete diagnostic language for abnormal labs (question 11) were weighted more heavily in the tool, as compliance with these components of the guideline was felt to be of greater importance. Several questions within the audit tool refer to clutter. We defined clutter as any additional data not endorsed by the guidelines or not explicitly stated as relevant to the patient's care for that day.
Raters were trained to score notes through practice sessions, during which they all scored the same note and compared findings. To rectify inter‐rater scoring discrepancies identified during these sessions, a reference manual was created to assist raters in scoring notes (see Supporting Information, Appendix 4, in the online version of this article). Each preintervention note was then systematically assigned to 2 raters, comprised of a physician and 3 staff from health information management. Each rater scored the note individually without discussion. The inter‐rater reliability was determined to be excellent, with kappa indices ranging from 88% to 100% for the 13 questions; each note in the postintervention period was therefore assigned to only 1 rater. Total and individual questions' scores were sent to the statistician for analysis.
Statistical Analysis
Inter‐rater reliability of the audit tool was evaluated by calculating the intraclass correlation (ICC) coefficient using a multilevel random intercept model to account for the rater effect.[18] The study was powered to detect an anticipated ICC of at least 0.75 at the 1‐sided 0.05 significance level, assuming a null hypothesis that the ICC is 0.4 or less. The total score was summarized in terms of means and standard deviation. Individual item responses were summarized using percentages and compared between the pre‐ and postintervention assessment using the Fisher exact test. The analysis of response patterns for individual item scores was considered exploratory. The Benjamini‐Hochberg false discovery rate method was utilized to control the false‐positive rate when comparing individual item scores.[19] All P values were 2‐sided and considered statistically significant at 0.05. Statistical analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
The ICC was 0.96 (95% confidence interval: 0.91‐0.98), indicating an excellent level of inter‐rater reliability. There was a significant improvement in the total score (see Supporting Information, Appendix 5, in the online version of this article) between the preintervention (mean 9.72, standard deviation [SD] 1.52) and postintervention (mean 11.72, SD 1.62) periods (P0.0001).
Table 1 shows the percentage of yes responses to each individual item in the pre‐ and postintervention periods. Our intervention had a significant impact on reducing vital sign clutter (4% preintervention, 84% postintervention, P0.0001) and other visual clutter within the note (0% preintervention, 28% postintervention, P=0.0035). We did not observe a significant impact on the reduction of input/output or lab clutter. There was no significant difference observed in the inclusion of the medication list. No significant improvements were seen in questions related to copy‐paste. The intervention had no significant impact on areas with an already high baseline performance: newly written interval histories, newly written physical exams, newly written plans, and the inclusion of discrete diagnostic language for abnormal labs.
| Question | Preintervention, N=25* | Postintervention, N=25 | P Value |
|---|---|---|---|
| |||
| 1. Does the note header include the name of the service, author, and training level of the author? | 0% | 68% | 0.0001 |
| 2. Does it appear that the subjective/emnterval history section of the note was newly written? (ie, not copied in its entirety from the previous note) | 100% | 96% | 0.9999 |
| 3. Is the vital sign section noncluttered? | 4% | 84% | 0.0001 |
| 4. Is the entire medication list included in the note? | 96% | 96% | 0.9999 |
| 5. Is the intake/output section noncluttered? | 0% | 16% | 0.3076 |
| 6. Does it appear that the physical exam was newly written? (ie, not copied in its entirety from the previous note) | 80% | 68% | 0.9103 |
| 7. Is the lab section noncluttered? | 64% | 44% | 0.5125 |
| 8. Is the imaging section noncluttered? | 100% | 100% | 0.9999 |
| 9. Does it appear that the assessment was newly written? | 48% | 28% | 0.5121 |
| 48% partial | 52% partial | 0.9999 | |
| 10. Does it appear that the plan was newly written or partially copied with new information added? | 88% | 96% | 0.9477 |
| 11. If the assessment includes abnormal lab values, is there also an accompanying diagnosis? (eg, inclusion of patient has hemoglobin of 6.2, also includes diagnosis of anemia) | 96% | 96% | 0.9999 |
| 12. Is additional visual clutter prevented by excluding other objective data found elsewhere in the chart? | 0% | 28% | 0.0035 |
| 13. Is the author's name and contact information (pager, cell) included at the bottom of the note? | 0% | 72% | 0.0001 |
DISCUSSION
Principal Findings
Improvements in electronic note writing, particularly in reducing note clutter, were achieved after the implementation of a bundled intervention. Because the intervention is a bundle, we cannot definitively identify which component had the greatest impact. Given the improvements seen in some areas with very low baseline performance, we hypothesize that these are most attributable to the creation of a compliant note template that (1) guided authors in using data links that were less cluttered and (2) eliminated the use of unnecessary links (eg, pain scores and daily weights). The lack of similar improvements in reducing input/output and lab clutter may be due to the fact that even with changes to the template suggesting a more narrative approach to these components, residents still felt compelled to use data links. Because our EHR does not easily allow for the inclusion of individual data elements, such as specific drain output or hemoglobin as opposed to a complete blood count, residents continued to use links that included more data than necessary. Although not significant findings, there was an observed decline in the proportion of notes containing a physical exam not entirely copied from the previous day and containing an assessment that was entirely new. These findings may be attributable to having a small sample of authors, a few of whom in the postintervention period were particularly prone to using copy‐paste.
Relationship to Other Evidence
The observed decline in quality of provider documentation after implementation of the EHR has led to a robust discussion in the literature about what really constitutes a quality provider note.[7, 8, 9, 10, 20] The absence of a defined gold standard makes research in this area challenging. It is our observation that when physicians refer to a decline in quality documentation in the EHR, they are frequently referring to the fact that electronically generated notes are often unattractive, difficult to read, and seem to lack clinical narrative.
Several publications have attempted to define note quality. Payne et al. described physical characteristics of electronically generated notes that were deemed more attractive to a reader, including a large proportion of narrative free text.[15] Hanson performed a qualitative study to describe outpatient clinical notes from the perspective of multiple stakeholders, resulting in a description of the characteristics of a quality note.[21] This formed the basis for the QNOTE, a validated tool to measure the quality of outpatient notes.[22] Similar work has not been done to rigorously define quality for inpatient documentation. Stetson did develop an instrument, the Physician Documentation Quality Instrument (PDQI‐9) to assess inpatient notes across 9 attributes; however, the validation method relied on a gold standard of a general impression score of 7 physician leaders.[23, 24]
Although these tools aim to address overall note quality, an advantage provided by our audit tool is that it directly addresses the problems most attributable to documenting in an EHR, namely note clutter and copy‐paste. A second advantage is that clinicians and nonclinicians can score notes objectively. The QNOTE and PDQI‐9 still rely on subjective assessment and require that the evaluator be a clinician.
There has also been little published about how to achieve notes of high quality. In 2013, Shoolin et al. did publish a consensus statement from the Association of Medical Directors of Information Systems outlining some guidelines for inpatient EHR documentation.[25] Optimal strategies for implementing such guidelines, however, and the overall impact such an implementation would have on improving note writing has not previously been studied. This study, therefore, adds to the existing body of literature by providing an example of an intervention that may lead to improvements in note writing.
Limitations
Our study has several limitations. The sample size of notes and authors was small. The short duration of the study and the assessment of notes soon after the intervention prevented an assessment of whether improvements were sustained over time.
Unfortunately, we were not evaluating the same group of interns in the pre‐ and postintervention periods. Interns were chosen as subjects as there was an existing opportunity to do large group training during new intern orientation. Furthermore, we were concerned that more note‐writing experience alone would influence the outcome if we examined the same interns later in the year.
The audit tool was also a first attempt at measuring compliance with the guidelines. Determination of an optimal score/weight for each item requires further investigation as part of a larger scale validation study. In addition, the cognitive review and synthesis of data encouraged in our guideline were more difficult to measure using the audit tool, as they require some clinical knowledge about the patient and an assessment of the author's medical decision making. We do not assert, therefore, that compliance with the guidelines or a higher total score necessarily translates into overall note quality, as we recognize these limitations of the tool.
Future Directions
In conclusion, this report is a first effort to improve the quality of note writing in the EHR. Much more work is necessary, particularly in improving the clinical narrative and inappropriate copy‐paste. The examination of other interventions, such as the impact of structured feedback to the note author, whether by way of a validated scoring tool and/or narrative comments, is a logical next step for investigation.
ACKNOWLEDGEMENTS
The authors acknowledge and appreciate the support of Joel Buchanan, MD, Ellen Wald, MD, and Ann Boyer, MD, for their contributions to this study and manuscript preparation. We also acknowledge the members of the auditing team: Linda Brickert, Jane Duckert, and Jeannine Strunk.
Disclosure: Nothing to report.
- , , . Use of computer‐based records, completeness of documentation, and appropriateness of documented clinical decisions. J Am Med Inform Assoc. 1999;6(3):245–251.
- , , , et al. An automated model to identify heart failure patients at risk for 30‐day readmission or death using electronic medical record data. Med Care. 2010;48(11):981–988.
- , , , , . Clinical information technologies and inpatient outcomes: a multiple hospital study. Arch Intern Med. 2009;169(2):108–114.
- , , , , . Identifying patients with diabetes and the earliest date of diagnosis in real time: an electronic health record case‐finding algorithm. BMC Med Inform Decis Mak. 2013;13:81.
- , , , et al. Relationship between use of electronic health record features and health care quality: results of a statewide survey. Med Care. 2010;48(3):203–209.
- , , , , , . Impacts of computerized physician documentation in a teaching hospital: perceptions of faculty and resident physicians. J Am Med Inform Assoc. 2004;11(4):300–309.
- , . Off the record—avoiding the pitfalls of going electronic. N Engl J Med. 2008;358(16):1656–1658.
- . A piece of my mind. Copy‐and‐paste. JAMA. 2006;295(20):2335–2336.
- , . Copy and paste: a remediable hazard of electronic health records. Am J Med. 2009;122(6):495–496.
- , , , , , . Physicians' attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63–68.
- . Improving the electronic health record—are clinicians getting what they wished for? JAMA. 2013;309(10):991–992.
- , , . Copying and pasting of examinations within the electronic medical record. Int J Med Inform. 2007;76(suppl 1):S122–S128.
- . The evolving medical record. Ann Intern Med. 2010;153(10):671–677.
- , , , , , . Direct text entry in electronic progress notes. An evaluation of input errors. Methods Inf Med. 2003;42(1):61–67.
- , , , . The physical attractiveness of electronic physician notes. AMIA Annu Symp Proc. 2010;2010:622–626.
- , . Copy‐and‐paste‐and‐paste. JAMA. 2006;296(19):2315; author reply 2315–2316.
- , , , . Are electronic medical records trustworthy? Observations on copying, pasting and duplication. AMIA Annu Symp Proc. 2003:269–273.
- , . Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Thousand Oaks, CA: Sage; 2002.
- , . Controlling the false discovery rate: a practical and powerful approach for multiple testing. J R Stat Soc Series B Stat Methodol 1995;57(1):289–300.
- , , . The role of copy‐and‐paste in the hospital electronic health record. JAMA Intern Med. 2014;174(8):1217–1218.
- , , , . Quality of outpatient clinical notes: a stakeholder definition derived through qualitative research. BMC Health Serv Res. 2012;12:407.
- , , , et al. QNOTE: an instrument for measuring the quality of EHR clinical notes. J Am Med Inform Assoc. 2014;21(5):910–916.
- , , , . Assessing electronic note quality using the physician documentation quality instrument (PDQI‐9). Appl Clin Inform. 2012;3(2):164–174.
- , , , . Preliminary development of the physician documentation quality instrument. J Am Med Inform Assoc. 2008;15(4):534–541.
- , , , . Association of Medical Directors of Information Systems consensus on inpatient electronic health record documentation. Appl Clin Inform. 2013;4(2):293–303.
- , , . Use of computer‐based records, completeness of documentation, and appropriateness of documented clinical decisions. J Am Med Inform Assoc. 1999;6(3):245–251.
- , , , et al. An automated model to identify heart failure patients at risk for 30‐day readmission or death using electronic medical record data. Med Care. 2010;48(11):981–988.
- , , , , . Clinical information technologies and inpatient outcomes: a multiple hospital study. Arch Intern Med. 2009;169(2):108–114.
- , , , , . Identifying patients with diabetes and the earliest date of diagnosis in real time: an electronic health record case‐finding algorithm. BMC Med Inform Decis Mak. 2013;13:81.
- , , , et al. Relationship between use of electronic health record features and health care quality: results of a statewide survey. Med Care. 2010;48(3):203–209.
- , , , , , . Impacts of computerized physician documentation in a teaching hospital: perceptions of faculty and resident physicians. J Am Med Inform Assoc. 2004;11(4):300–309.
- , . Off the record—avoiding the pitfalls of going electronic. N Engl J Med. 2008;358(16):1656–1658.
- . A piece of my mind. Copy‐and‐paste. JAMA. 2006;295(20):2335–2336.
- , . Copy and paste: a remediable hazard of electronic health records. Am J Med. 2009;122(6):495–496.
- , , , , , . Physicians' attitudes towards copy and pasting in electronic note writing. J Gen Intern Med. 2009;24(1):63–68.
- . Improving the electronic health record—are clinicians getting what they wished for? JAMA. 2013;309(10):991–992.
- , , . Copying and pasting of examinations within the electronic medical record. Int J Med Inform. 2007;76(suppl 1):S122–S128.
- . The evolving medical record. Ann Intern Med. 2010;153(10):671–677.
- , , , , , . Direct text entry in electronic progress notes. An evaluation of input errors. Methods Inf Med. 2003;42(1):61–67.
- , , , . The physical attractiveness of electronic physician notes. AMIA Annu Symp Proc. 2010;2010:622–626.
- , . Copy‐and‐paste‐and‐paste. JAMA. 2006;296(19):2315; author reply 2315–2316.
- , , , . Are electronic medical records trustworthy? Observations on copying, pasting and duplication. AMIA Annu Symp Proc. 2003:269–273.
- , . Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Thousand Oaks, CA: Sage; 2002.
- , . Controlling the false discovery rate: a practical and powerful approach for multiple testing. J R Stat Soc Series B Stat Methodol 1995;57(1):289–300.
- , , . The role of copy‐and‐paste in the hospital electronic health record. JAMA Intern Med. 2014;174(8):1217–1218.
- , , , . Quality of outpatient clinical notes: a stakeholder definition derived through qualitative research. BMC Health Serv Res. 2012;12:407.
- , , , et al. QNOTE: an instrument for measuring the quality of EHR clinical notes. J Am Med Inform Assoc. 2014;21(5):910–916.
- , , , . Assessing electronic note quality using the physician documentation quality instrument (PDQI‐9). Appl Clin Inform. 2012;3(2):164–174.
- , , , . Preliminary development of the physician documentation quality instrument. J Am Med Inform Assoc. 2008;15(4):534–541.
- , , , . Association of Medical Directors of Information Systems consensus on inpatient electronic health record documentation. Appl Clin Inform. 2013;4(2):293–303.
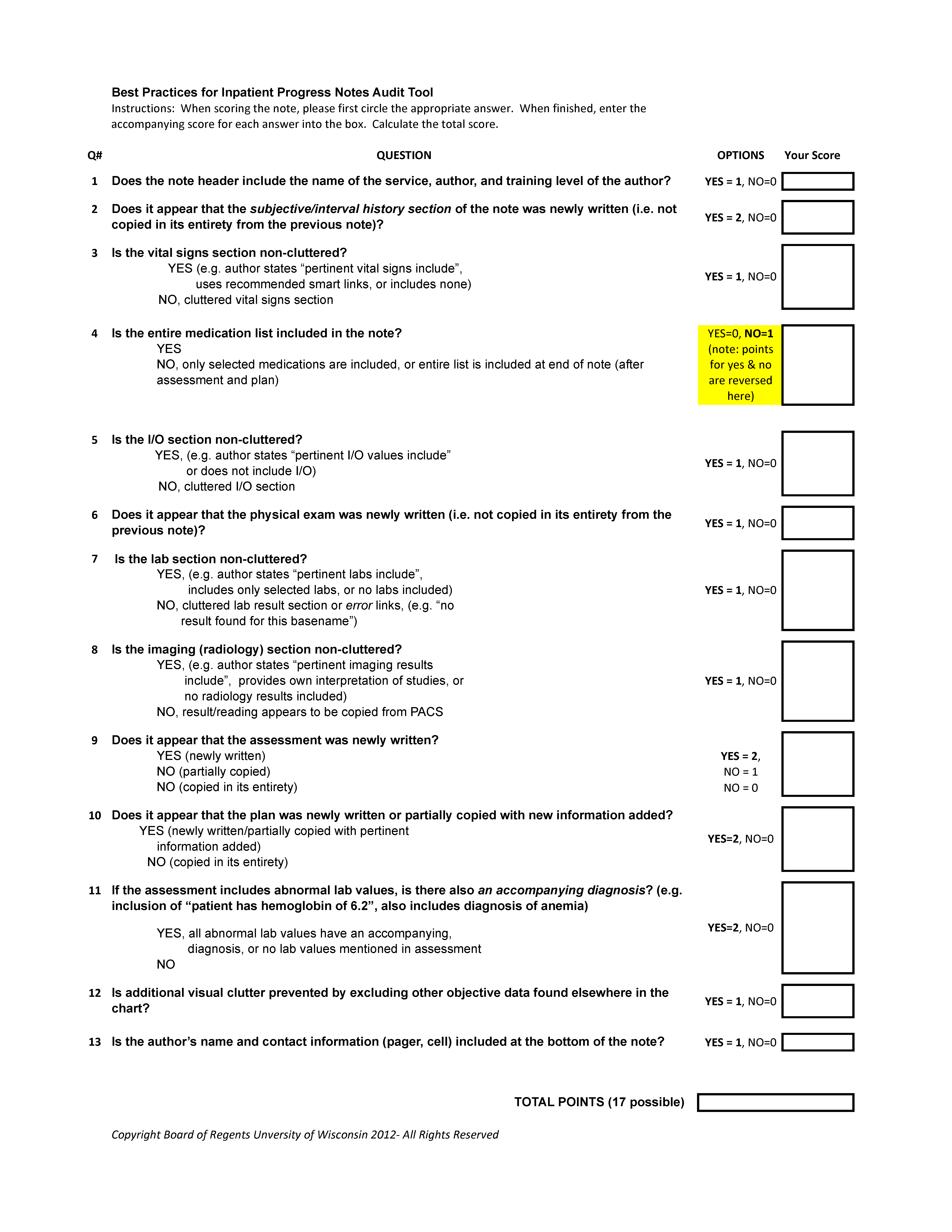
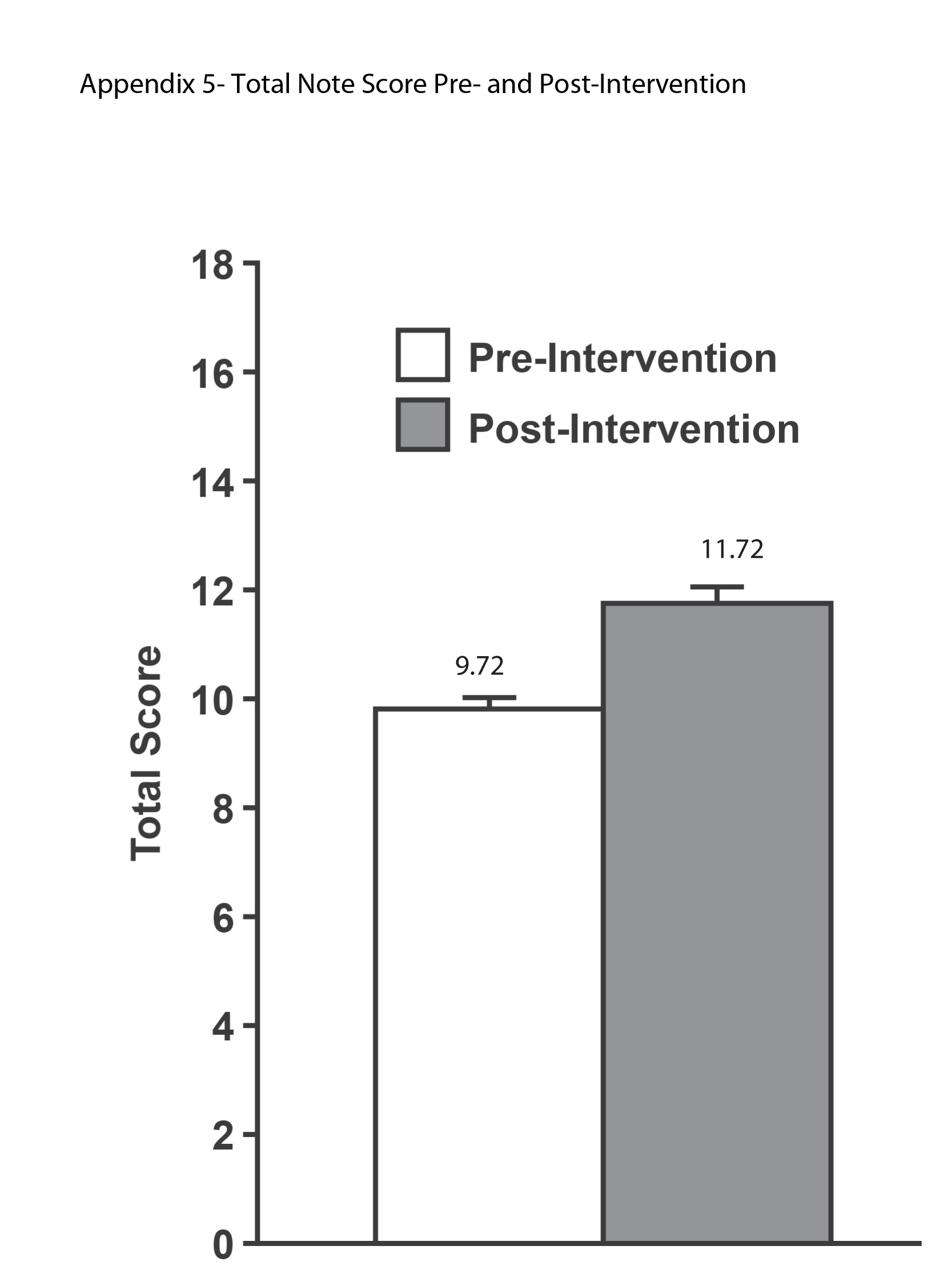
Severe‐Sepsis Screening Tool
Sepsis remains a significant healthcare burden and is the sixth most common reason for hospitalization in the United States. For patients presenting with severe sepsis, mortality rates are approximately 30%,[1, 2] and sepsis remains the most expensive reason for hospitalization. In 2009, septicemia accounted for nearly $15.4 billion in aggregate hospital costs.[2]
Early identification of sepsis and the timely implementation of goal‐directed therapy significantly decrease sepsis‐related mortality and are cost‐effective,[3, 4, 5] highlighting the need for new clinical strategies to aid in early diagnosis. To date, most studies have focused on the screening and management of sepsis in the emergency department and intensive care unit (ICU),[6, 7] and less is known about the benefits of screening in non‐ICU settings. In the non‐ICU setting, conditions may go unrecognized and treatments delayed. Evidence suggests that patients diagnosed with severe sepsis in the non‐ICU setting are almost twice as likely to die as those diagnosed in an emergency department.[8, 9]
Application of a sepsis screening tool to both medical and surgical patients poses an additional challenge that may impact the screen's performance. The specificity may be compromised by noninfectious causes of systemic inflammatory response syndrome (SIRS) commonly seen in the postsurgical patient. For example, the tachycardia and fever often seen in the postoperative patient are sufficient to qualify for SIRS, making the diagnosis of sepsis more challenging. The purpose of this study was to examine the performance of a nurse‐driven, simple sepsis screening tool in a mixed medical and surgical non‐ICU setting.
METHODS
Setting
This was an observational pilot study of prospectively screened patients admitted to a 26‐bed medical/surgical intermediate care unit with telemetry monitoring in a 613‐bed university tertiary referral hospital over a 1‐month time period. The surgical patient population of this floor consisted of cardiothoracic (50%), general (24%), and vascular surgery (17%) patients as well as a small number of trauma (7%) patients. The medical patient population admitted to this unit included pretransplant and complex medical patients requiring telemetry monitoring. Though the incidence of sepsis specific to this unit was unknown prior to the study, after an analysis of discharges the study team surmised there would be sufficient volume for testing of a nurse‐based screening tool.
Nurse Education
Registered nurses (RNs) working on the study unit had an average of 5 to 7 years of experience. The all‐RN unit was staffed predominantly at a 1:3 RN to patient ratio. RNs were supported by a clinical nurse specialist (CNS) and clinical educator (CE) RN who provided regular ongoing education about infection prevention and identification of common conditions that are seen on the unit.
In the 6 months prior to our sepsis screening initiative, nursing staff had been given more than 8 hours of education on infection‐ and sepsis‐related topics in 15‐ to 20‐minute blocks of time. This dedicated education took place during the nurses' shift in groups of 2 to 3, and was run by the CNS, assistant nurse manager, and CE RN. Nurses were also encouraged to attend an optional 8‐hour sepsis continuing medical education (CME) program. Approximately 20% of the nurses on the study unit attended.
Just prior to the pilot study, nursing staff completed a 1‐hour refresher self‐study module on severe sepsis stressing the importance of early identification. There was also a training month prior to the actual data collection time frame, where unit core trainers (RNs) or champions who had attended the optional 8‐hour sepsis CME conducted 1:1 follow‐up with each RN, reviewing at least 1 of their screens to validate understanding of screening concepts. Each RN was checked off after correctly completing a screen. During the study, unit educators and the CNS provided additional on‐unit in‐service training with screening tool completion instructions and advice on how to incorporate the tool into the RN's current assessment workflow. In addition, the charge nurses were asked to review the screens collected each shift and validate any that may have seemed inconsistent with the RN's verbal report of the patient's status.
The university's institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Screening Tool
A sepsis screening tool was developed as part of a broader initiative to improve sepsis‐related morbidity and mortality at our hospital. The screening tool was adapted from the severe sepsis screening tool created by the Surviving Sepsis Campaign and Institute for Healthcare Improvement,[10] and consisted of a simple 3‐tiered paper‐based screening assessment that was to be completed by the bedside RN (Figure 1). RNs on the pilot medical/surgical intermediate care unit performed the screening assessment with their regular patient assessment at the beginning of each shift.
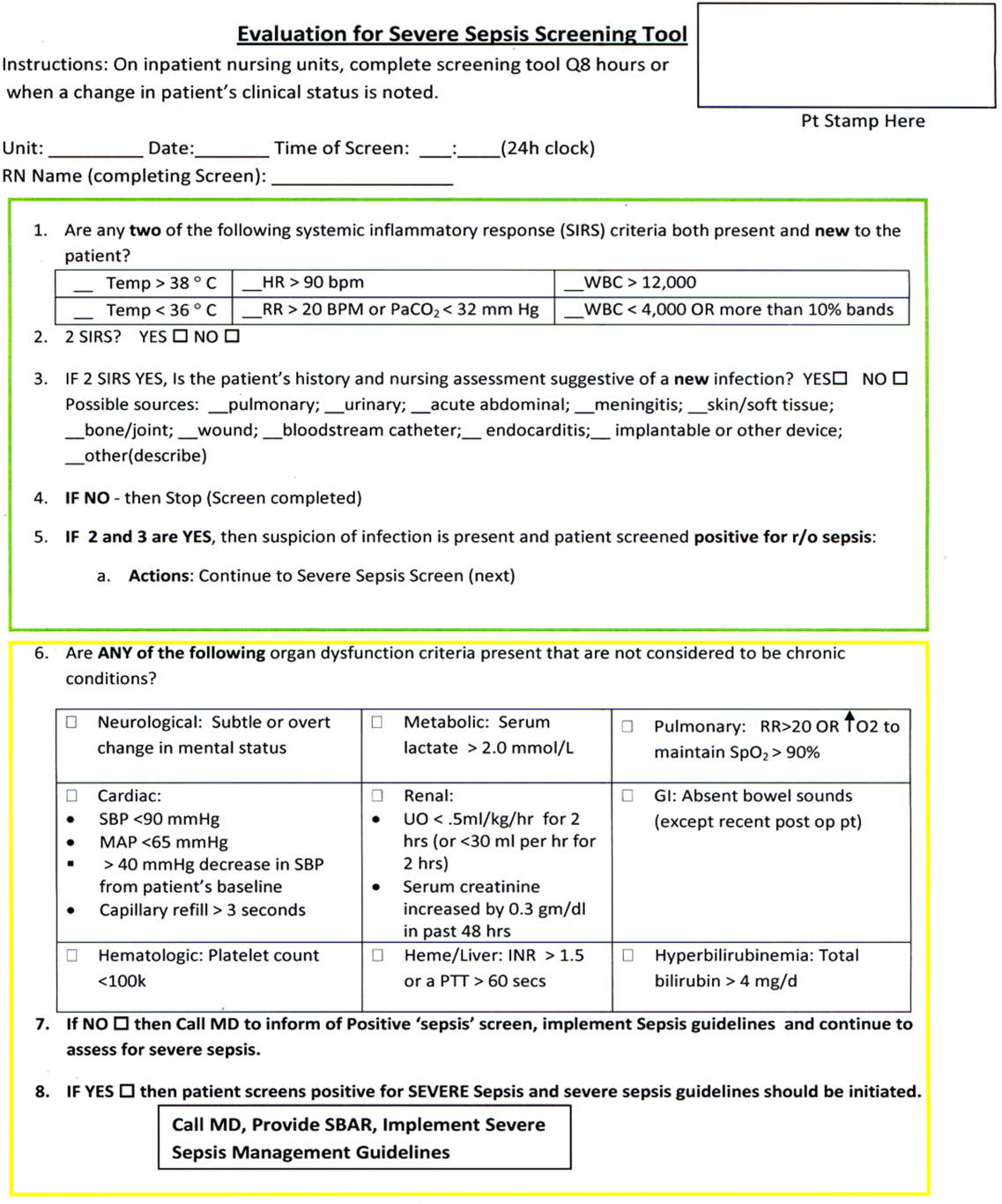
The first tier of the tool screened for the presence of SIRS. Positive parameters included heart rate >90, temperature >38C or <36C, white blood cell count >12,000 or<4000 or >10% bands, and/or respiratory rate >20 or partial pressure of carbon dioxide (PaCO2) <32 mm Hg. To decrease the number of false‐positive screens in patients whose abnormal vitals could already be attributed to a condition other than sepsis, these symptoms were only scored if they had emerged within the previous 8 hours.
If patients met 2 SIRS criteria, the nurse would move to the second tier of the tool, which involved consideration of possible infection as a contributor to a patient's clinical condition as well as a source of infection. If infection was not suspected, further screening was terminated. If infection was suspected, the patient then met criteria for a positive sepsis screen, and a third tier of screening involving assessment of organ dysfunction was initiated.
If the patient screened positive for sepsis (2 SIRS and suspicion for new infection) or severe sepsis (sepsis with end‐organ dysfunction), nurses were instructed to document this in the patient's electronic medical record (EMR) and call the primary team to initiate actions following the hospital‐wide sepsis guidelines. Any subsequent actions were recorded in the patient's EMR.
Data Collection
Completed sepsis screening forms during the month of October 2010 were reviewed by the authors (E.G., L.S., and P.M.). Data including age, gender, International Classification of Diseases, Ninth Revision (ICD‐9) admission and discharge diagnoses, vital signs, lab results, clinical interventions, and documented clinical decision processes by healthcare staff were collected on patients with a positive screen or those who did not screen positive but had an ICD‐9 code for sepsis, severe sepsis, or septic shock during their hospitalization or at discharge. We also collected demographic and clinical data for a random sample of patients who consistently screened negative for sepsis.
Performance Measurement
The sensitivity and specificity of the screening tool was determined by identifying true‐positive, false‐positive, true‐negative, and false‐negative results and calculating accordingly using a 2 2 contingency table. True positives were defined as cases where patients screened positive for sepsis and had a documented diagnosis of sepsis in their EMR within 24 hours of the positive screening or had an ICD‐9 billing code for sepsis. False‐positive cases were those in which patients screened positive for sepsis but did not have a diagnosis of sepsis by manual chart review nor was there an ICD‐9 code for sepsis for their hospital stay. True‐negative cases were those where patients screened negative and did not have an ICD‐9 code for sepsis. False negatives were cases where patients consistently screened negative for sepsis but had an ICD‐9 code for sepsis.
Clinical Activities
To examine the impact of a positive sepsis screen on subsequent clinical action, we assessed the frequency with which a treatment or diagnostic workup was initiated after a positive screen and compared this to clinical activity initiated after a negative screen. Specifically, the patient's EMR was reviewed for actions including measurement of lactate, blood cultures, administration of broad spectrum antibiotics, administration of fluid boluses, or consultation with or transfer to the ICU. These actions were chosen because they are part of the Surviving Sepsis Bundle, which has been demonstrated to improve mortality rates after diagnosis of severe sepsis or septic shock,[11, 12] and can be done outside of an ICU setting. Because screening was done every 8 hours, clinical activity was only attributed to a positive or negative sepsis screen if it occurred within 8 hours of the screening result. Patients were excluded if there were missing data points that precluded full analysis of their clinical course.
Statistical Analysis
To compare the performance of the screening tool between surgical and medical patients, we calculated 95% confidence intervals of screening test sensitivity and specificity. To test if performance was significantly different between these groups, we performed a nonparametric, 2‐sided, 2‐sample test of proportions. Though similar to a [2] test, the 2‐sided test of proportions allowed us to determine if there was a directional difference in test performance (ie, Does the screening tool perform better or worse in a certain patient group?). We also used the test of proportions to compare differences in the proportion of patients receiving sepsis‐related interventions before and after a positive or negative screening result. For comparisons of demographic variables we used nonparametric tests including the [2] test for categorical variables and the Kruskal‐Wallis test for continuous variables. We used SAS 9.3 (SAS Institute Inc., Cary, NC) to perform our analyses.
RESULTS
Over a 1‐month time period, 2143 screens were completed on 245 patients (169 surgical, 76 medical). The overall incidence of sepsis on the treatment unit during this time period was 9%. Surgical patients had an 8.9% incidence of sepsis, and medical patients had an incidence of 9.2%.
Screening tool performance is presented in Table 1. The screening tool had 95.5% sensitivity and 91.9% specificity, with no significant differences in performance between surgical and medical patients. The overall negative predictive value was 99.5%, also with comparable performance in both surgical and medical patients (P = 0.89). The overall positive predictive value (PPV) was 70% in medical patients and 48% in surgical patients (P = 0.12). Screening tool accuracy for medical and surgical patients was 92%.
| Overall, N = 245 (95% CI) | Surgery, N = 169 (95% CI) | Medicine, N = 76 (95% CI) | P Value* | |
|---|---|---|---|---|
| ||||
| Sensitivity | 95.5% (75%‐99.7%) | 93% (66%‐99.6%) | 100% (56%‐100%) | 0.17 |
| Specificity | 91.9% (87%‐95%) | 90% (84%‐94%) | 95% (87%‐99%) | 0.48 |
| NPV | 99.5% (81%‐100%) | 99.3% (71%‐100%) | 100% (67%‐100%) | 0.89 |
| PPV | 53.8% (39%‐70%) | 48% (23%‐73%) | 70% (30%‐100%) | 0.12 |
| LR+ | 11.8 | 9.3 | 20 | |
| LR | 0.05 | 0.08 | 0 | |
| Confirmed patient diagnosis, overall | ||||
| Sepsis | No sepsis | |||
| Screen positive | 21 (TP) | 18 (FP) | ||
| Screen negative | 1 (FN) | 205 (TN) | ||
| Confirmed patient diagnosis, medicine | ||||
| Sepsis | No sepsis | |||
| Screen positive | 7 (TP) | 3 (FP) | ||
| Screen negative | 0 (FN) | 66 (TN) | ||
| Confirmed patient diagnosis, surgery | ||||
| Sepsis | No sepsis | |||
| Screen positive | 14 (TP) | 15 (FP) | ||
| Screen negative | 1 (FN) | 139 (TN) | ||
Clinical Activities
Of the 39 patients who screened positive for sepsis, nurses classified 20 with sepsis and 19 with severe sepsis. Of these 39 patients, 33 were included in our descriptive analysis of the effect of positive screening results on clinical activity (3 were excluded for admission for sepsis and 3 for missing data). As a comparison, we randomly selected 30 patients of the 206 patients who screened negative for sepsis to evaluate clinical activity before and after a negative screen.
Characteristics of patients screening positive and negative for sepsis are reported in Table 2. We found no statistically significant differences in age, sex, length of hospital stay, or mortality amongst all groups.
| Patient Characteristics | Surgery (Positive) | Medicine (Positive) | Surgery (Negative) | Medicine (Negative) | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 26 | 7 | 20 | 10 | |
| Age, y, mean | 57.8 ( 16.5) | 72.4 ( 16.8) | 64.6 ( 19.4) | 63.6 ( 16.8) | 0.25 |
| % Male (no.) | 50% (13) | 57% (4) | 60% (12) | 60% (6) | 0.27 |
| Length of stay, d, median (IQR) | 9 (716.7) | 7 (5.511.5) | 11 (7.722) | 8 (421) | 0.38 |
| No. of PODs until first positive screen, d, median (IQR) | 2 (13) | N/A | N/A | N/A | |
| % Mortality (no.) | 0% | 14% (1) | 5% (1) | 10% (1) | 0.19 |
Figure 2 illustrates differences in the proportion of patients receiving a clinical action before and after a negative or positive screening test result. In the cohort of 33 patients screening positive for sepsis, clinical action after a positive screen was taken in 4 of the 7 (50%) medical patients and 11 of 26 (42%) surgical patients. In patients screening negative for sepsis we found only 1 incident in which a sepsis‐related action was taken after a negative screen. In this case the patient was admitted to the ICU within 8 hours of a negative screen, though there was no explicit documentation that sepsis was the reason for this admission.
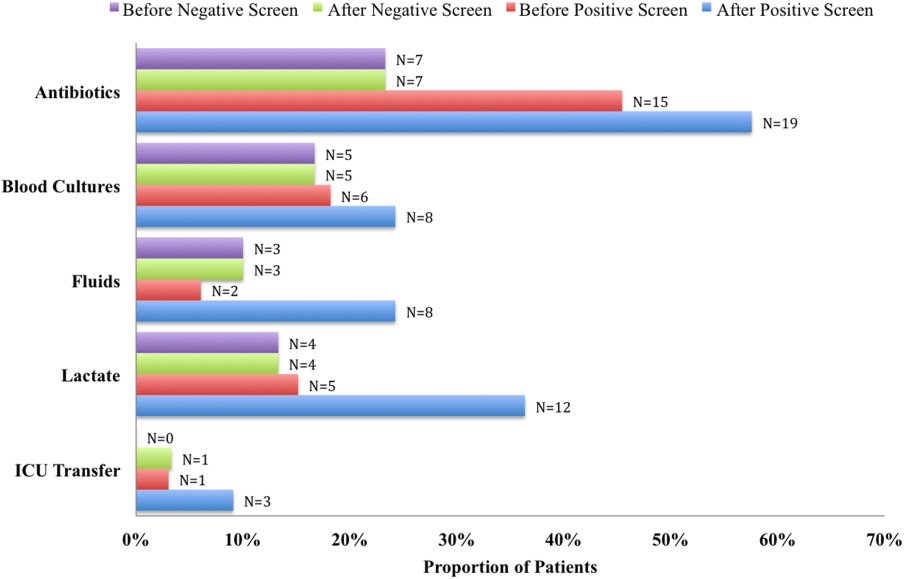
We compared the proportion of patients receiving sepsis‐related treatment before either a negative or positive screen and found no significant difference (Table 3). We then compared the proportion of patients receiving sepsis‐related actions after a positive or negative screening test result and found that the proportion of patients receiving antibiotics, blood cultures, and lactate measurement was significantly higher for patients with a positive sepsis screening result compared to those with a negative screening result (Table 3).
| Intervention and Group | Proportion | P Value |
|---|---|---|
| ||
| Before screening test | ||
| Antibiotics | 0.066 | |
| Positive screen | 45% | |
| Negative screen | 23% | |
| Lactate | 0.837 | |
| Positive screen | 15% | |
| Negative screen | 13% | |
| Blood culture | 0.181 | |
| Positive screen | 18% | |
| Negative screen | 17% | |
| Fluid administration | 0.564 | |
| Positive screen | 6% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.337 | |
| Positive screen | 3% | |
| Negative screen | 0% | |
| After screening test | ||
| Antibiotics | 0.006 | |
| Positive screen | 58% | |
| Negative screen | 23% | |
| Lactate | 0.018 | |
| Positive screen | 36% | |
| Negative screen | 13% | |
| Blood Culture | 0.002 | |
| Positive screen | 24% | |
| Negative screen | 17% | |
| Fluid administration | 0.112 | |
| Positive screen | 24% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.175 | |
| Positive screen | 9% | |
| Negative screen | 3% | |
DISCUSSION
Improving recognition and time to treatment of sepsis in a non‐ICU setting is an important step toward decreasing sepsis‐related mortality. Lundberg and colleagues found that mortality rates for patients diagnosed with septic shock on a general ward were higher than for patients diagnosed in the ICU, even though ward patients were younger and healthier at baseline.[8] For ward patients, treatment delays were most profound in initiating vasoactive therapies, and minor delays were encountered in initiating fluid resuscitation. In their international study on the impact of early goal‐directed therapy guidelines, Levy and colleagues found that patients diagnosed with severe sepsis on the wards were almost twice as likely to die as patients diagnosed with sepsis in the emergency department.[9]
We are the first to report about an accurate nurse‐driven SIRS‐based sepsis screening protocol that is effective in the early identification of sepsis in both medical and surgical patients in an intermediate care setting. We found no significant difference in the screening tool performance between the medical and surgical cohorts. This is an important comparison given that SIRS criteria alone can be nonspecific in the postoperative population, where it is common to have hemodynamic changes, elevation of inflammatory markers, and fevers from noninfectious sources.
Our sepsis screening tool was designed in 3 tiers to improve its specificity. The first tier was based strictly on SIRS criteria (eg, tachycardia or fever), whereas the second and third tiers served to increase the specificity of the screening tool by instructing the evaluator to assess possible sources of infection and assess for objective signs of organ dysfunction. We relied heavily on the nursing staff to assess for the presence or absence of infection and believe that the educational component prior to initiating the screening protocol was vital.
EMR‐based screening tools that rely purely on physiologic data have been considered for the early detection and management of sepsis, although they lack the specificity gained through the incorporation of clinical judgment.[13] Sawyer and colleagues report using a real‐time EMR‐based method for early sepsis detection in non‐ICU patients that is based solely on objective measures; however, their PPV was only 19.5%. The model we describe in this study is one that incorporates real‐time physiologic data available from an EMR coupled with the clinical judgment of a bedside registered nurse. As our data suggest, this provides a screen that is both sensitive and specific.
It is interesting to note that in our assessment of clinical action taken 8 hours after a positive screening test (the interval after which a new screening test was performed), the rate of diagnostic workup and/or treatment for sepsis was relatively low. One reason for this could have been that the treating team had suspicion for sepsis prior to a positive screen and had already initiated clinical action. Of the 51 recorded clinical actions taken around the time of a positive screen, the majority (67%) occurred before the screening result. It is also possible that clinical action was not pursued because the treatment team disagreed with a diagnosis of sepsis. Of all the false positive screening cases, manual chart review confirmed that these patients did not have sepsis, nor did they develop sepsis during their index hospital stay. Last, we only recorded clinical actions taken within 8 hours of the first positive screen for sepsis and measured 5 very specific actions. Thus, our analysis may have missed actions taken after 8 hours or actions that differed from the 5 we chose to assess.
Even with the apparently low levels of new clinical activity after a positive screen, when compared to patients who screened negative for sepsis, a significantly higher number of patients who had a positive screen received antibiotics, had lactate measured, and had blood cultures drawn. We did not find a significant difference in the proportion of patients receiving a sepsis‐related clinical action before a screening result (positive or negative), which suggests that a positive screening test may have led to increased clinical action.
A limitation of our study is its small size and that it was conducted in 1 pilot unit. Additionally, our retrospective analysis of clinical care inhibited our ability to fully understand a patient's clinical course or retrieve missing data points. A related limitation is that we could not ascertain how often the screening tool did not identify a case of sepsis before it was clinically diagnosed. Assessing the temporal performance of our screening tool is of great interest and may be more easily performed using an electronic version of the screening tool, which is currently in development.
Using ICD‐9 codes to determine the true‐negative cohort is another limitation of our study. It is well documented that use of administrative data can lead to inaccurate classification of patients.[14] To address this, we performed random audits of 30 test‐negative patients. In doing so we did not find any errors in classification.
Although we did not find a significant difference in screening tool performance between surgical and medical patients, the PPV of the tool was lower in the surgical population (48%) compared to the medical population (70%). The lower PPV observed in surgical patients could be attributable to an overall lower incidence of sepsis in this cohort as well as possible errors in initial assessment of infection, which can be difficult in postsurgical patients. Our retrospective analysis included data from the early months of the screening protocol, a time in which nursing staff was still developing clinical acumen in identifying sepsis. However, this could have led nurses to either overestimate or underestimate the presence of infection in either patient group.
Suspicion for infection is the cornerstone definition of sepsis, and in our screening protocol nurses were charged with making this decision based on their knowledge of the patient's clinical course and current status. Issues concerning nurses' recognition of infection symptoms are an area of opportunity for further research and education and could aid in improving PPV. Clinical judgment could be further bolstered by adding promising laboratory tests such as C‐reactive protein or procalcitonin as objective adjuncts to an initial assessment for sepsis,[15] which could potentially increase screening test PPV.
CONCLUSIONS
A simple screening tool for sepsis performed by the bedside nurse can provide a means to successfully identify sepsis early and lead to more timely diagnostics and treatment in both medical and surgical patients in an intermediate care setting.
ACKNOWLEDGEMENTS
The authors thank Eileen Pummer, quality manager for the sepsis team; Pauline Regner, patient care manager of the pilot study unit; and the nurses who contributed to the screening tool design team and data collection. The authors acknowledge Pooja Loftus for her statistical expertise, and Isabella Chu for her review of the manuscript. Disclosures: Presented as a poster at the 31st Annual Meeting of the Surgical Infection Society, Palm Beach, Florida, May 2011. The authors report no conflicts of interest.
- , , , , , Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. Published October 2011. Accessed on September 4, 2012.
- , , , Economic implications of an evidence‐based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35(5):1257–1262.
- , , , , , Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36(6):542–547.
- , , , , , The costs and cost‐effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36(4):1168–1174.
- , , A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98(11):813–820.
- , , , et al. Validation of a screening tool for the early identification of sepsis. J Trauma. 2009;66(6):1539–1546; discussion 1546–1547.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26(6):1020–1024.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- Institute of Healthcare Improvement. Evaluation for severe sepsis screening tool. Surviving Sepsis Campaign. Available at: http://www.survivingsepsis.org/About_the_Campaign/Documents/evaluationforseveresepsisscreeningtool.pdf. Accessed on September 30, 2012.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , , , et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29(9):621–628.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- , , , Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , , , Comparison of procalcitonin and C‐reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741.
Sepsis remains a significant healthcare burden and is the sixth most common reason for hospitalization in the United States. For patients presenting with severe sepsis, mortality rates are approximately 30%,[1, 2] and sepsis remains the most expensive reason for hospitalization. In 2009, septicemia accounted for nearly $15.4 billion in aggregate hospital costs.[2]
Early identification of sepsis and the timely implementation of goal‐directed therapy significantly decrease sepsis‐related mortality and are cost‐effective,[3, 4, 5] highlighting the need for new clinical strategies to aid in early diagnosis. To date, most studies have focused on the screening and management of sepsis in the emergency department and intensive care unit (ICU),[6, 7] and less is known about the benefits of screening in non‐ICU settings. In the non‐ICU setting, conditions may go unrecognized and treatments delayed. Evidence suggests that patients diagnosed with severe sepsis in the non‐ICU setting are almost twice as likely to die as those diagnosed in an emergency department.[8, 9]
Application of a sepsis screening tool to both medical and surgical patients poses an additional challenge that may impact the screen's performance. The specificity may be compromised by noninfectious causes of systemic inflammatory response syndrome (SIRS) commonly seen in the postsurgical patient. For example, the tachycardia and fever often seen in the postoperative patient are sufficient to qualify for SIRS, making the diagnosis of sepsis more challenging. The purpose of this study was to examine the performance of a nurse‐driven, simple sepsis screening tool in a mixed medical and surgical non‐ICU setting.
METHODS
Setting
This was an observational pilot study of prospectively screened patients admitted to a 26‐bed medical/surgical intermediate care unit with telemetry monitoring in a 613‐bed university tertiary referral hospital over a 1‐month time period. The surgical patient population of this floor consisted of cardiothoracic (50%), general (24%), and vascular surgery (17%) patients as well as a small number of trauma (7%) patients. The medical patient population admitted to this unit included pretransplant and complex medical patients requiring telemetry monitoring. Though the incidence of sepsis specific to this unit was unknown prior to the study, after an analysis of discharges the study team surmised there would be sufficient volume for testing of a nurse‐based screening tool.
Nurse Education
Registered nurses (RNs) working on the study unit had an average of 5 to 7 years of experience. The all‐RN unit was staffed predominantly at a 1:3 RN to patient ratio. RNs were supported by a clinical nurse specialist (CNS) and clinical educator (CE) RN who provided regular ongoing education about infection prevention and identification of common conditions that are seen on the unit.
In the 6 months prior to our sepsis screening initiative, nursing staff had been given more than 8 hours of education on infection‐ and sepsis‐related topics in 15‐ to 20‐minute blocks of time. This dedicated education took place during the nurses' shift in groups of 2 to 3, and was run by the CNS, assistant nurse manager, and CE RN. Nurses were also encouraged to attend an optional 8‐hour sepsis continuing medical education (CME) program. Approximately 20% of the nurses on the study unit attended.
Just prior to the pilot study, nursing staff completed a 1‐hour refresher self‐study module on severe sepsis stressing the importance of early identification. There was also a training month prior to the actual data collection time frame, where unit core trainers (RNs) or champions who had attended the optional 8‐hour sepsis CME conducted 1:1 follow‐up with each RN, reviewing at least 1 of their screens to validate understanding of screening concepts. Each RN was checked off after correctly completing a screen. During the study, unit educators and the CNS provided additional on‐unit in‐service training with screening tool completion instructions and advice on how to incorporate the tool into the RN's current assessment workflow. In addition, the charge nurses were asked to review the screens collected each shift and validate any that may have seemed inconsistent with the RN's verbal report of the patient's status.
The university's institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Screening Tool
A sepsis screening tool was developed as part of a broader initiative to improve sepsis‐related morbidity and mortality at our hospital. The screening tool was adapted from the severe sepsis screening tool created by the Surviving Sepsis Campaign and Institute for Healthcare Improvement,[10] and consisted of a simple 3‐tiered paper‐based screening assessment that was to be completed by the bedside RN (Figure 1). RNs on the pilot medical/surgical intermediate care unit performed the screening assessment with their regular patient assessment at the beginning of each shift.

The first tier of the tool screened for the presence of SIRS. Positive parameters included heart rate >90, temperature >38C or <36C, white blood cell count >12,000 or<4000 or >10% bands, and/or respiratory rate >20 or partial pressure of carbon dioxide (PaCO2) <32 mm Hg. To decrease the number of false‐positive screens in patients whose abnormal vitals could already be attributed to a condition other than sepsis, these symptoms were only scored if they had emerged within the previous 8 hours.
If patients met 2 SIRS criteria, the nurse would move to the second tier of the tool, which involved consideration of possible infection as a contributor to a patient's clinical condition as well as a source of infection. If infection was not suspected, further screening was terminated. If infection was suspected, the patient then met criteria for a positive sepsis screen, and a third tier of screening involving assessment of organ dysfunction was initiated.
If the patient screened positive for sepsis (2 SIRS and suspicion for new infection) or severe sepsis (sepsis with end‐organ dysfunction), nurses were instructed to document this in the patient's electronic medical record (EMR) and call the primary team to initiate actions following the hospital‐wide sepsis guidelines. Any subsequent actions were recorded in the patient's EMR.
Data Collection
Completed sepsis screening forms during the month of October 2010 were reviewed by the authors (E.G., L.S., and P.M.). Data including age, gender, International Classification of Diseases, Ninth Revision (ICD‐9) admission and discharge diagnoses, vital signs, lab results, clinical interventions, and documented clinical decision processes by healthcare staff were collected on patients with a positive screen or those who did not screen positive but had an ICD‐9 code for sepsis, severe sepsis, or septic shock during their hospitalization or at discharge. We also collected demographic and clinical data for a random sample of patients who consistently screened negative for sepsis.
Performance Measurement
The sensitivity and specificity of the screening tool was determined by identifying true‐positive, false‐positive, true‐negative, and false‐negative results and calculating accordingly using a 2 2 contingency table. True positives were defined as cases where patients screened positive for sepsis and had a documented diagnosis of sepsis in their EMR within 24 hours of the positive screening or had an ICD‐9 billing code for sepsis. False‐positive cases were those in which patients screened positive for sepsis but did not have a diagnosis of sepsis by manual chart review nor was there an ICD‐9 code for sepsis for their hospital stay. True‐negative cases were those where patients screened negative and did not have an ICD‐9 code for sepsis. False negatives were cases where patients consistently screened negative for sepsis but had an ICD‐9 code for sepsis.
Clinical Activities
To examine the impact of a positive sepsis screen on subsequent clinical action, we assessed the frequency with which a treatment or diagnostic workup was initiated after a positive screen and compared this to clinical activity initiated after a negative screen. Specifically, the patient's EMR was reviewed for actions including measurement of lactate, blood cultures, administration of broad spectrum antibiotics, administration of fluid boluses, or consultation with or transfer to the ICU. These actions were chosen because they are part of the Surviving Sepsis Bundle, which has been demonstrated to improve mortality rates after diagnosis of severe sepsis or septic shock,[11, 12] and can be done outside of an ICU setting. Because screening was done every 8 hours, clinical activity was only attributed to a positive or negative sepsis screen if it occurred within 8 hours of the screening result. Patients were excluded if there were missing data points that precluded full analysis of their clinical course.
Statistical Analysis
To compare the performance of the screening tool between surgical and medical patients, we calculated 95% confidence intervals of screening test sensitivity and specificity. To test if performance was significantly different between these groups, we performed a nonparametric, 2‐sided, 2‐sample test of proportions. Though similar to a [2] test, the 2‐sided test of proportions allowed us to determine if there was a directional difference in test performance (ie, Does the screening tool perform better or worse in a certain patient group?). We also used the test of proportions to compare differences in the proportion of patients receiving sepsis‐related interventions before and after a positive or negative screening result. For comparisons of demographic variables we used nonparametric tests including the [2] test for categorical variables and the Kruskal‐Wallis test for continuous variables. We used SAS 9.3 (SAS Institute Inc., Cary, NC) to perform our analyses.
RESULTS
Over a 1‐month time period, 2143 screens were completed on 245 patients (169 surgical, 76 medical). The overall incidence of sepsis on the treatment unit during this time period was 9%. Surgical patients had an 8.9% incidence of sepsis, and medical patients had an incidence of 9.2%.
Screening tool performance is presented in Table 1. The screening tool had 95.5% sensitivity and 91.9% specificity, with no significant differences in performance between surgical and medical patients. The overall negative predictive value was 99.5%, also with comparable performance in both surgical and medical patients (P = 0.89). The overall positive predictive value (PPV) was 70% in medical patients and 48% in surgical patients (P = 0.12). Screening tool accuracy for medical and surgical patients was 92%.
| Overall, N = 245 (95% CI) | Surgery, N = 169 (95% CI) | Medicine, N = 76 (95% CI) | P Value* | |
|---|---|---|---|---|
| ||||
| Sensitivity | 95.5% (75%‐99.7%) | 93% (66%‐99.6%) | 100% (56%‐100%) | 0.17 |
| Specificity | 91.9% (87%‐95%) | 90% (84%‐94%) | 95% (87%‐99%) | 0.48 |
| NPV | 99.5% (81%‐100%) | 99.3% (71%‐100%) | 100% (67%‐100%) | 0.89 |
| PPV | 53.8% (39%‐70%) | 48% (23%‐73%) | 70% (30%‐100%) | 0.12 |
| LR+ | 11.8 | 9.3 | 20 | |
| LR | 0.05 | 0.08 | 0 | |
| Confirmed patient diagnosis, overall | ||||
| Sepsis | No sepsis | |||
| Screen positive | 21 (TP) | 18 (FP) | ||
| Screen negative | 1 (FN) | 205 (TN) | ||
| Confirmed patient diagnosis, medicine | ||||
| Sepsis | No sepsis | |||
| Screen positive | 7 (TP) | 3 (FP) | ||
| Screen negative | 0 (FN) | 66 (TN) | ||
| Confirmed patient diagnosis, surgery | ||||
| Sepsis | No sepsis | |||
| Screen positive | 14 (TP) | 15 (FP) | ||
| Screen negative | 1 (FN) | 139 (TN) | ||
Clinical Activities
Of the 39 patients who screened positive for sepsis, nurses classified 20 with sepsis and 19 with severe sepsis. Of these 39 patients, 33 were included in our descriptive analysis of the effect of positive screening results on clinical activity (3 were excluded for admission for sepsis and 3 for missing data). As a comparison, we randomly selected 30 patients of the 206 patients who screened negative for sepsis to evaluate clinical activity before and after a negative screen.
Characteristics of patients screening positive and negative for sepsis are reported in Table 2. We found no statistically significant differences in age, sex, length of hospital stay, or mortality amongst all groups.
| Patient Characteristics | Surgery (Positive) | Medicine (Positive) | Surgery (Negative) | Medicine (Negative) | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 26 | 7 | 20 | 10 | |
| Age, y, mean | 57.8 ( 16.5) | 72.4 ( 16.8) | 64.6 ( 19.4) | 63.6 ( 16.8) | 0.25 |
| % Male (no.) | 50% (13) | 57% (4) | 60% (12) | 60% (6) | 0.27 |
| Length of stay, d, median (IQR) | 9 (716.7) | 7 (5.511.5) | 11 (7.722) | 8 (421) | 0.38 |
| No. of PODs until first positive screen, d, median (IQR) | 2 (13) | N/A | N/A | N/A | |
| % Mortality (no.) | 0% | 14% (1) | 5% (1) | 10% (1) | 0.19 |
Figure 2 illustrates differences in the proportion of patients receiving a clinical action before and after a negative or positive screening test result. In the cohort of 33 patients screening positive for sepsis, clinical action after a positive screen was taken in 4 of the 7 (50%) medical patients and 11 of 26 (42%) surgical patients. In patients screening negative for sepsis we found only 1 incident in which a sepsis‐related action was taken after a negative screen. In this case the patient was admitted to the ICU within 8 hours of a negative screen, though there was no explicit documentation that sepsis was the reason for this admission.

We compared the proportion of patients receiving sepsis‐related treatment before either a negative or positive screen and found no significant difference (Table 3). We then compared the proportion of patients receiving sepsis‐related actions after a positive or negative screening test result and found that the proportion of patients receiving antibiotics, blood cultures, and lactate measurement was significantly higher for patients with a positive sepsis screening result compared to those with a negative screening result (Table 3).
| Intervention and Group | Proportion | P Value |
|---|---|---|
| ||
| Before screening test | ||
| Antibiotics | 0.066 | |
| Positive screen | 45% | |
| Negative screen | 23% | |
| Lactate | 0.837 | |
| Positive screen | 15% | |
| Negative screen | 13% | |
| Blood culture | 0.181 | |
| Positive screen | 18% | |
| Negative screen | 17% | |
| Fluid administration | 0.564 | |
| Positive screen | 6% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.337 | |
| Positive screen | 3% | |
| Negative screen | 0% | |
| After screening test | ||
| Antibiotics | 0.006 | |
| Positive screen | 58% | |
| Negative screen | 23% | |
| Lactate | 0.018 | |
| Positive screen | 36% | |
| Negative screen | 13% | |
| Blood Culture | 0.002 | |
| Positive screen | 24% | |
| Negative screen | 17% | |
| Fluid administration | 0.112 | |
| Positive screen | 24% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.175 | |
| Positive screen | 9% | |
| Negative screen | 3% | |
DISCUSSION
Improving recognition and time to treatment of sepsis in a non‐ICU setting is an important step toward decreasing sepsis‐related mortality. Lundberg and colleagues found that mortality rates for patients diagnosed with septic shock on a general ward were higher than for patients diagnosed in the ICU, even though ward patients were younger and healthier at baseline.[8] For ward patients, treatment delays were most profound in initiating vasoactive therapies, and minor delays were encountered in initiating fluid resuscitation. In their international study on the impact of early goal‐directed therapy guidelines, Levy and colleagues found that patients diagnosed with severe sepsis on the wards were almost twice as likely to die as patients diagnosed with sepsis in the emergency department.[9]
We are the first to report about an accurate nurse‐driven SIRS‐based sepsis screening protocol that is effective in the early identification of sepsis in both medical and surgical patients in an intermediate care setting. We found no significant difference in the screening tool performance between the medical and surgical cohorts. This is an important comparison given that SIRS criteria alone can be nonspecific in the postoperative population, where it is common to have hemodynamic changes, elevation of inflammatory markers, and fevers from noninfectious sources.
Our sepsis screening tool was designed in 3 tiers to improve its specificity. The first tier was based strictly on SIRS criteria (eg, tachycardia or fever), whereas the second and third tiers served to increase the specificity of the screening tool by instructing the evaluator to assess possible sources of infection and assess for objective signs of organ dysfunction. We relied heavily on the nursing staff to assess for the presence or absence of infection and believe that the educational component prior to initiating the screening protocol was vital.
EMR‐based screening tools that rely purely on physiologic data have been considered for the early detection and management of sepsis, although they lack the specificity gained through the incorporation of clinical judgment.[13] Sawyer and colleagues report using a real‐time EMR‐based method for early sepsis detection in non‐ICU patients that is based solely on objective measures; however, their PPV was only 19.5%. The model we describe in this study is one that incorporates real‐time physiologic data available from an EMR coupled with the clinical judgment of a bedside registered nurse. As our data suggest, this provides a screen that is both sensitive and specific.
It is interesting to note that in our assessment of clinical action taken 8 hours after a positive screening test (the interval after which a new screening test was performed), the rate of diagnostic workup and/or treatment for sepsis was relatively low. One reason for this could have been that the treating team had suspicion for sepsis prior to a positive screen and had already initiated clinical action. Of the 51 recorded clinical actions taken around the time of a positive screen, the majority (67%) occurred before the screening result. It is also possible that clinical action was not pursued because the treatment team disagreed with a diagnosis of sepsis. Of all the false positive screening cases, manual chart review confirmed that these patients did not have sepsis, nor did they develop sepsis during their index hospital stay. Last, we only recorded clinical actions taken within 8 hours of the first positive screen for sepsis and measured 5 very specific actions. Thus, our analysis may have missed actions taken after 8 hours or actions that differed from the 5 we chose to assess.
Even with the apparently low levels of new clinical activity after a positive screen, when compared to patients who screened negative for sepsis, a significantly higher number of patients who had a positive screen received antibiotics, had lactate measured, and had blood cultures drawn. We did not find a significant difference in the proportion of patients receiving a sepsis‐related clinical action before a screening result (positive or negative), which suggests that a positive screening test may have led to increased clinical action.
A limitation of our study is its small size and that it was conducted in 1 pilot unit. Additionally, our retrospective analysis of clinical care inhibited our ability to fully understand a patient's clinical course or retrieve missing data points. A related limitation is that we could not ascertain how often the screening tool did not identify a case of sepsis before it was clinically diagnosed. Assessing the temporal performance of our screening tool is of great interest and may be more easily performed using an electronic version of the screening tool, which is currently in development.
Using ICD‐9 codes to determine the true‐negative cohort is another limitation of our study. It is well documented that use of administrative data can lead to inaccurate classification of patients.[14] To address this, we performed random audits of 30 test‐negative patients. In doing so we did not find any errors in classification.
Although we did not find a significant difference in screening tool performance between surgical and medical patients, the PPV of the tool was lower in the surgical population (48%) compared to the medical population (70%). The lower PPV observed in surgical patients could be attributable to an overall lower incidence of sepsis in this cohort as well as possible errors in initial assessment of infection, which can be difficult in postsurgical patients. Our retrospective analysis included data from the early months of the screening protocol, a time in which nursing staff was still developing clinical acumen in identifying sepsis. However, this could have led nurses to either overestimate or underestimate the presence of infection in either patient group.
Suspicion for infection is the cornerstone definition of sepsis, and in our screening protocol nurses were charged with making this decision based on their knowledge of the patient's clinical course and current status. Issues concerning nurses' recognition of infection symptoms are an area of opportunity for further research and education and could aid in improving PPV. Clinical judgment could be further bolstered by adding promising laboratory tests such as C‐reactive protein or procalcitonin as objective adjuncts to an initial assessment for sepsis,[15] which could potentially increase screening test PPV.
CONCLUSIONS
A simple screening tool for sepsis performed by the bedside nurse can provide a means to successfully identify sepsis early and lead to more timely diagnostics and treatment in both medical and surgical patients in an intermediate care setting.
ACKNOWLEDGEMENTS
The authors thank Eileen Pummer, quality manager for the sepsis team; Pauline Regner, patient care manager of the pilot study unit; and the nurses who contributed to the screening tool design team and data collection. The authors acknowledge Pooja Loftus for her statistical expertise, and Isabella Chu for her review of the manuscript. Disclosures: Presented as a poster at the 31st Annual Meeting of the Surgical Infection Society, Palm Beach, Florida, May 2011. The authors report no conflicts of interest.
Sepsis remains a significant healthcare burden and is the sixth most common reason for hospitalization in the United States. For patients presenting with severe sepsis, mortality rates are approximately 30%,[1, 2] and sepsis remains the most expensive reason for hospitalization. In 2009, septicemia accounted for nearly $15.4 billion in aggregate hospital costs.[2]
Early identification of sepsis and the timely implementation of goal‐directed therapy significantly decrease sepsis‐related mortality and are cost‐effective,[3, 4, 5] highlighting the need for new clinical strategies to aid in early diagnosis. To date, most studies have focused on the screening and management of sepsis in the emergency department and intensive care unit (ICU),[6, 7] and less is known about the benefits of screening in non‐ICU settings. In the non‐ICU setting, conditions may go unrecognized and treatments delayed. Evidence suggests that patients diagnosed with severe sepsis in the non‐ICU setting are almost twice as likely to die as those diagnosed in an emergency department.[8, 9]
Application of a sepsis screening tool to both medical and surgical patients poses an additional challenge that may impact the screen's performance. The specificity may be compromised by noninfectious causes of systemic inflammatory response syndrome (SIRS) commonly seen in the postsurgical patient. For example, the tachycardia and fever often seen in the postoperative patient are sufficient to qualify for SIRS, making the diagnosis of sepsis more challenging. The purpose of this study was to examine the performance of a nurse‐driven, simple sepsis screening tool in a mixed medical and surgical non‐ICU setting.
METHODS
Setting
This was an observational pilot study of prospectively screened patients admitted to a 26‐bed medical/surgical intermediate care unit with telemetry monitoring in a 613‐bed university tertiary referral hospital over a 1‐month time period. The surgical patient population of this floor consisted of cardiothoracic (50%), general (24%), and vascular surgery (17%) patients as well as a small number of trauma (7%) patients. The medical patient population admitted to this unit included pretransplant and complex medical patients requiring telemetry monitoring. Though the incidence of sepsis specific to this unit was unknown prior to the study, after an analysis of discharges the study team surmised there would be sufficient volume for testing of a nurse‐based screening tool.
Nurse Education
Registered nurses (RNs) working on the study unit had an average of 5 to 7 years of experience. The all‐RN unit was staffed predominantly at a 1:3 RN to patient ratio. RNs were supported by a clinical nurse specialist (CNS) and clinical educator (CE) RN who provided regular ongoing education about infection prevention and identification of common conditions that are seen on the unit.
In the 6 months prior to our sepsis screening initiative, nursing staff had been given more than 8 hours of education on infection‐ and sepsis‐related topics in 15‐ to 20‐minute blocks of time. This dedicated education took place during the nurses' shift in groups of 2 to 3, and was run by the CNS, assistant nurse manager, and CE RN. Nurses were also encouraged to attend an optional 8‐hour sepsis continuing medical education (CME) program. Approximately 20% of the nurses on the study unit attended.
Just prior to the pilot study, nursing staff completed a 1‐hour refresher self‐study module on severe sepsis stressing the importance of early identification. There was also a training month prior to the actual data collection time frame, where unit core trainers (RNs) or champions who had attended the optional 8‐hour sepsis CME conducted 1:1 follow‐up with each RN, reviewing at least 1 of their screens to validate understanding of screening concepts. Each RN was checked off after correctly completing a screen. During the study, unit educators and the CNS provided additional on‐unit in‐service training with screening tool completion instructions and advice on how to incorporate the tool into the RN's current assessment workflow. In addition, the charge nurses were asked to review the screens collected each shift and validate any that may have seemed inconsistent with the RN's verbal report of the patient's status.
The university's institutional review board notice of determination waived review for this study because it was classified as quality improvement.
Screening Tool
A sepsis screening tool was developed as part of a broader initiative to improve sepsis‐related morbidity and mortality at our hospital. The screening tool was adapted from the severe sepsis screening tool created by the Surviving Sepsis Campaign and Institute for Healthcare Improvement,[10] and consisted of a simple 3‐tiered paper‐based screening assessment that was to be completed by the bedside RN (Figure 1). RNs on the pilot medical/surgical intermediate care unit performed the screening assessment with their regular patient assessment at the beginning of each shift.

The first tier of the tool screened for the presence of SIRS. Positive parameters included heart rate >90, temperature >38C or <36C, white blood cell count >12,000 or<4000 or >10% bands, and/or respiratory rate >20 or partial pressure of carbon dioxide (PaCO2) <32 mm Hg. To decrease the number of false‐positive screens in patients whose abnormal vitals could already be attributed to a condition other than sepsis, these symptoms were only scored if they had emerged within the previous 8 hours.
If patients met 2 SIRS criteria, the nurse would move to the second tier of the tool, which involved consideration of possible infection as a contributor to a patient's clinical condition as well as a source of infection. If infection was not suspected, further screening was terminated. If infection was suspected, the patient then met criteria for a positive sepsis screen, and a third tier of screening involving assessment of organ dysfunction was initiated.
If the patient screened positive for sepsis (2 SIRS and suspicion for new infection) or severe sepsis (sepsis with end‐organ dysfunction), nurses were instructed to document this in the patient's electronic medical record (EMR) and call the primary team to initiate actions following the hospital‐wide sepsis guidelines. Any subsequent actions were recorded in the patient's EMR.
Data Collection
Completed sepsis screening forms during the month of October 2010 were reviewed by the authors (E.G., L.S., and P.M.). Data including age, gender, International Classification of Diseases, Ninth Revision (ICD‐9) admission and discharge diagnoses, vital signs, lab results, clinical interventions, and documented clinical decision processes by healthcare staff were collected on patients with a positive screen or those who did not screen positive but had an ICD‐9 code for sepsis, severe sepsis, or septic shock during their hospitalization or at discharge. We also collected demographic and clinical data for a random sample of patients who consistently screened negative for sepsis.
Performance Measurement
The sensitivity and specificity of the screening tool was determined by identifying true‐positive, false‐positive, true‐negative, and false‐negative results and calculating accordingly using a 2 2 contingency table. True positives were defined as cases where patients screened positive for sepsis and had a documented diagnosis of sepsis in their EMR within 24 hours of the positive screening or had an ICD‐9 billing code for sepsis. False‐positive cases were those in which patients screened positive for sepsis but did not have a diagnosis of sepsis by manual chart review nor was there an ICD‐9 code for sepsis for their hospital stay. True‐negative cases were those where patients screened negative and did not have an ICD‐9 code for sepsis. False negatives were cases where patients consistently screened negative for sepsis but had an ICD‐9 code for sepsis.
Clinical Activities
To examine the impact of a positive sepsis screen on subsequent clinical action, we assessed the frequency with which a treatment or diagnostic workup was initiated after a positive screen and compared this to clinical activity initiated after a negative screen. Specifically, the patient's EMR was reviewed for actions including measurement of lactate, blood cultures, administration of broad spectrum antibiotics, administration of fluid boluses, or consultation with or transfer to the ICU. These actions were chosen because they are part of the Surviving Sepsis Bundle, which has been demonstrated to improve mortality rates after diagnosis of severe sepsis or septic shock,[11, 12] and can be done outside of an ICU setting. Because screening was done every 8 hours, clinical activity was only attributed to a positive or negative sepsis screen if it occurred within 8 hours of the screening result. Patients were excluded if there were missing data points that precluded full analysis of their clinical course.
Statistical Analysis
To compare the performance of the screening tool between surgical and medical patients, we calculated 95% confidence intervals of screening test sensitivity and specificity. To test if performance was significantly different between these groups, we performed a nonparametric, 2‐sided, 2‐sample test of proportions. Though similar to a [2] test, the 2‐sided test of proportions allowed us to determine if there was a directional difference in test performance (ie, Does the screening tool perform better or worse in a certain patient group?). We also used the test of proportions to compare differences in the proportion of patients receiving sepsis‐related interventions before and after a positive or negative screening result. For comparisons of demographic variables we used nonparametric tests including the [2] test for categorical variables and the Kruskal‐Wallis test for continuous variables. We used SAS 9.3 (SAS Institute Inc., Cary, NC) to perform our analyses.
RESULTS
Over a 1‐month time period, 2143 screens were completed on 245 patients (169 surgical, 76 medical). The overall incidence of sepsis on the treatment unit during this time period was 9%. Surgical patients had an 8.9% incidence of sepsis, and medical patients had an incidence of 9.2%.
Screening tool performance is presented in Table 1. The screening tool had 95.5% sensitivity and 91.9% specificity, with no significant differences in performance between surgical and medical patients. The overall negative predictive value was 99.5%, also with comparable performance in both surgical and medical patients (P = 0.89). The overall positive predictive value (PPV) was 70% in medical patients and 48% in surgical patients (P = 0.12). Screening tool accuracy for medical and surgical patients was 92%.
| Overall, N = 245 (95% CI) | Surgery, N = 169 (95% CI) | Medicine, N = 76 (95% CI) | P Value* | |
|---|---|---|---|---|
| ||||
| Sensitivity | 95.5% (75%‐99.7%) | 93% (66%‐99.6%) | 100% (56%‐100%) | 0.17 |
| Specificity | 91.9% (87%‐95%) | 90% (84%‐94%) | 95% (87%‐99%) | 0.48 |
| NPV | 99.5% (81%‐100%) | 99.3% (71%‐100%) | 100% (67%‐100%) | 0.89 |
| PPV | 53.8% (39%‐70%) | 48% (23%‐73%) | 70% (30%‐100%) | 0.12 |
| LR+ | 11.8 | 9.3 | 20 | |
| LR | 0.05 | 0.08 | 0 | |
| Confirmed patient diagnosis, overall | ||||
| Sepsis | No sepsis | |||
| Screen positive | 21 (TP) | 18 (FP) | ||
| Screen negative | 1 (FN) | 205 (TN) | ||
| Confirmed patient diagnosis, medicine | ||||
| Sepsis | No sepsis | |||
| Screen positive | 7 (TP) | 3 (FP) | ||
| Screen negative | 0 (FN) | 66 (TN) | ||
| Confirmed patient diagnosis, surgery | ||||
| Sepsis | No sepsis | |||
| Screen positive | 14 (TP) | 15 (FP) | ||
| Screen negative | 1 (FN) | 139 (TN) | ||
Clinical Activities
Of the 39 patients who screened positive for sepsis, nurses classified 20 with sepsis and 19 with severe sepsis. Of these 39 patients, 33 were included in our descriptive analysis of the effect of positive screening results on clinical activity (3 were excluded for admission for sepsis and 3 for missing data). As a comparison, we randomly selected 30 patients of the 206 patients who screened negative for sepsis to evaluate clinical activity before and after a negative screen.
Characteristics of patients screening positive and negative for sepsis are reported in Table 2. We found no statistically significant differences in age, sex, length of hospital stay, or mortality amongst all groups.
| Patient Characteristics | Surgery (Positive) | Medicine (Positive) | Surgery (Negative) | Medicine (Negative) | P Value |
|---|---|---|---|---|---|
| |||||
| No. | 26 | 7 | 20 | 10 | |
| Age, y, mean | 57.8 ( 16.5) | 72.4 ( 16.8) | 64.6 ( 19.4) | 63.6 ( 16.8) | 0.25 |
| % Male (no.) | 50% (13) | 57% (4) | 60% (12) | 60% (6) | 0.27 |
| Length of stay, d, median (IQR) | 9 (716.7) | 7 (5.511.5) | 11 (7.722) | 8 (421) | 0.38 |
| No. of PODs until first positive screen, d, median (IQR) | 2 (13) | N/A | N/A | N/A | |
| % Mortality (no.) | 0% | 14% (1) | 5% (1) | 10% (1) | 0.19 |
Figure 2 illustrates differences in the proportion of patients receiving a clinical action before and after a negative or positive screening test result. In the cohort of 33 patients screening positive for sepsis, clinical action after a positive screen was taken in 4 of the 7 (50%) medical patients and 11 of 26 (42%) surgical patients. In patients screening negative for sepsis we found only 1 incident in which a sepsis‐related action was taken after a negative screen. In this case the patient was admitted to the ICU within 8 hours of a negative screen, though there was no explicit documentation that sepsis was the reason for this admission.

We compared the proportion of patients receiving sepsis‐related treatment before either a negative or positive screen and found no significant difference (Table 3). We then compared the proportion of patients receiving sepsis‐related actions after a positive or negative screening test result and found that the proportion of patients receiving antibiotics, blood cultures, and lactate measurement was significantly higher for patients with a positive sepsis screening result compared to those with a negative screening result (Table 3).
| Intervention and Group | Proportion | P Value |
|---|---|---|
| ||
| Before screening test | ||
| Antibiotics | 0.066 | |
| Positive screen | 45% | |
| Negative screen | 23% | |
| Lactate | 0.837 | |
| Positive screen | 15% | |
| Negative screen | 13% | |
| Blood culture | 0.181 | |
| Positive screen | 18% | |
| Negative screen | 17% | |
| Fluid administration | 0.564 | |
| Positive screen | 6% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.337 | |
| Positive screen | 3% | |
| Negative screen | 0% | |
| After screening test | ||
| Antibiotics | 0.006 | |
| Positive screen | 58% | |
| Negative screen | 23% | |
| Lactate | 0.018 | |
| Positive screen | 36% | |
| Negative screen | 13% | |
| Blood Culture | 0.002 | |
| Positive screen | 24% | |
| Negative screen | 17% | |
| Fluid administration | 0.112 | |
| Positive screen | 24% | |
| Negative screen | 10% | |
| ICU transfer/consult | 0.175 | |
| Positive screen | 9% | |
| Negative screen | 3% | |
DISCUSSION
Improving recognition and time to treatment of sepsis in a non‐ICU setting is an important step toward decreasing sepsis‐related mortality. Lundberg and colleagues found that mortality rates for patients diagnosed with septic shock on a general ward were higher than for patients diagnosed in the ICU, even though ward patients were younger and healthier at baseline.[8] For ward patients, treatment delays were most profound in initiating vasoactive therapies, and minor delays were encountered in initiating fluid resuscitation. In their international study on the impact of early goal‐directed therapy guidelines, Levy and colleagues found that patients diagnosed with severe sepsis on the wards were almost twice as likely to die as patients diagnosed with sepsis in the emergency department.[9]
We are the first to report about an accurate nurse‐driven SIRS‐based sepsis screening protocol that is effective in the early identification of sepsis in both medical and surgical patients in an intermediate care setting. We found no significant difference in the screening tool performance between the medical and surgical cohorts. This is an important comparison given that SIRS criteria alone can be nonspecific in the postoperative population, where it is common to have hemodynamic changes, elevation of inflammatory markers, and fevers from noninfectious sources.
Our sepsis screening tool was designed in 3 tiers to improve its specificity. The first tier was based strictly on SIRS criteria (eg, tachycardia or fever), whereas the second and third tiers served to increase the specificity of the screening tool by instructing the evaluator to assess possible sources of infection and assess for objective signs of organ dysfunction. We relied heavily on the nursing staff to assess for the presence or absence of infection and believe that the educational component prior to initiating the screening protocol was vital.
EMR‐based screening tools that rely purely on physiologic data have been considered for the early detection and management of sepsis, although they lack the specificity gained through the incorporation of clinical judgment.[13] Sawyer and colleagues report using a real‐time EMR‐based method for early sepsis detection in non‐ICU patients that is based solely on objective measures; however, their PPV was only 19.5%. The model we describe in this study is one that incorporates real‐time physiologic data available from an EMR coupled with the clinical judgment of a bedside registered nurse. As our data suggest, this provides a screen that is both sensitive and specific.
It is interesting to note that in our assessment of clinical action taken 8 hours after a positive screening test (the interval after which a new screening test was performed), the rate of diagnostic workup and/or treatment for sepsis was relatively low. One reason for this could have been that the treating team had suspicion for sepsis prior to a positive screen and had already initiated clinical action. Of the 51 recorded clinical actions taken around the time of a positive screen, the majority (67%) occurred before the screening result. It is also possible that clinical action was not pursued because the treatment team disagreed with a diagnosis of sepsis. Of all the false positive screening cases, manual chart review confirmed that these patients did not have sepsis, nor did they develop sepsis during their index hospital stay. Last, we only recorded clinical actions taken within 8 hours of the first positive screen for sepsis and measured 5 very specific actions. Thus, our analysis may have missed actions taken after 8 hours or actions that differed from the 5 we chose to assess.
Even with the apparently low levels of new clinical activity after a positive screen, when compared to patients who screened negative for sepsis, a significantly higher number of patients who had a positive screen received antibiotics, had lactate measured, and had blood cultures drawn. We did not find a significant difference in the proportion of patients receiving a sepsis‐related clinical action before a screening result (positive or negative), which suggests that a positive screening test may have led to increased clinical action.
A limitation of our study is its small size and that it was conducted in 1 pilot unit. Additionally, our retrospective analysis of clinical care inhibited our ability to fully understand a patient's clinical course or retrieve missing data points. A related limitation is that we could not ascertain how often the screening tool did not identify a case of sepsis before it was clinically diagnosed. Assessing the temporal performance of our screening tool is of great interest and may be more easily performed using an electronic version of the screening tool, which is currently in development.
Using ICD‐9 codes to determine the true‐negative cohort is another limitation of our study. It is well documented that use of administrative data can lead to inaccurate classification of patients.[14] To address this, we performed random audits of 30 test‐negative patients. In doing so we did not find any errors in classification.
Although we did not find a significant difference in screening tool performance between surgical and medical patients, the PPV of the tool was lower in the surgical population (48%) compared to the medical population (70%). The lower PPV observed in surgical patients could be attributable to an overall lower incidence of sepsis in this cohort as well as possible errors in initial assessment of infection, which can be difficult in postsurgical patients. Our retrospective analysis included data from the early months of the screening protocol, a time in which nursing staff was still developing clinical acumen in identifying sepsis. However, this could have led nurses to either overestimate or underestimate the presence of infection in either patient group.
Suspicion for infection is the cornerstone definition of sepsis, and in our screening protocol nurses were charged with making this decision based on their knowledge of the patient's clinical course and current status. Issues concerning nurses' recognition of infection symptoms are an area of opportunity for further research and education and could aid in improving PPV. Clinical judgment could be further bolstered by adding promising laboratory tests such as C‐reactive protein or procalcitonin as objective adjuncts to an initial assessment for sepsis,[15] which could potentially increase screening test PPV.
CONCLUSIONS
A simple screening tool for sepsis performed by the bedside nurse can provide a means to successfully identify sepsis early and lead to more timely diagnostics and treatment in both medical and surgical patients in an intermediate care setting.
ACKNOWLEDGEMENTS
The authors thank Eileen Pummer, quality manager for the sepsis team; Pauline Regner, patient care manager of the pilot study unit; and the nurses who contributed to the screening tool design team and data collection. The authors acknowledge Pooja Loftus for her statistical expertise, and Isabella Chu for her review of the manuscript. Disclosures: Presented as a poster at the 31st Annual Meeting of the Surgical Infection Society, Palm Beach, Florida, May 2011. The authors report no conflicts of interest.
- , , , , , Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. Published October 2011. Accessed on September 4, 2012.
- , , , Economic implications of an evidence‐based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35(5):1257–1262.
- , , , , , Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36(6):542–547.
- , , , , , The costs and cost‐effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36(4):1168–1174.
- , , A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98(11):813–820.
- , , , et al. Validation of a screening tool for the early identification of sepsis. J Trauma. 2009;66(6):1539–1546; discussion 1546–1547.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26(6):1020–1024.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- Institute of Healthcare Improvement. Evaluation for severe sepsis screening tool. Surviving Sepsis Campaign. Available at: http://www.survivingsepsis.org/About_the_Campaign/Documents/evaluationforseveresepsisscreeningtool.pdf. Accessed on September 30, 2012.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , , , et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29(9):621–628.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- , , , Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , , , Comparison of procalcitonin and C‐reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741.
- , , , , , Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310.
- , , Septicemia in U.S. hospitals, 2009. HCUP statistical brief #122. Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf. Published October 2011. Accessed on September 4, 2012.
- , , , Economic implications of an evidence‐based sepsis protocol: can we improve outcomes and lower costs? Crit Care Med. 2007;35(5):1257–1262.
- , , , , , Late compliance with the sepsis resuscitation bundle: impact on mortality. Shock. 2011;36(6):542–547.
- , , , , , The costs and cost‐effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36(4):1168–1174.
- , , A simple prediction algorithm for bacteraemia in patients with acute febrile illness. QJM. 2005;98(11):813–820.
- , , , et al. Validation of a screening tool for the early identification of sepsis. J Trauma. 2009;66(6):1539–1546; discussion 1546–1547.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26(6):1020–1024.
- , , , et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–374.
- Institute of Healthcare Improvement. Evaluation for severe sepsis screening tool. Surviving Sepsis Campaign. Available at: http://www.survivingsepsis.org/About_the_Campaign/Documents/evaluationforseveresepsisscreeningtool.pdf. Accessed on September 30, 2012.
- , , , et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three‐year follow‐up quasi‐experimental study. Crit Care Med. 2010;38(4):1036–1043.
- , , , et al. Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Reanim. 2010;29(9):621–628.
- , , , et al. Implementation of a real‐time computerized sepsis alert in nonintensive care unit patients. Crit Care Med. 2011;39(3):469–473.
- , , , Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328.
- , , , , , Comparison of procalcitonin and C‐reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741.
© 2014 Society of Hospital Medicine
USPSTF: Not enough evidence for vitamin D screening
The U.S. Preventive Services Task Force made no recommendation for or against primary care physicians screening asymptomatic adults for vitamin D deficiency, because the current evidence is insufficient to adequately assess the benefits and harms of doing so, according to a report published online Nov. 24 in Annals of Internal Medicine.
The USPSTF reviewed the evidence on screening and treatment for vitamin D deficiency, because the condition may contribute to fractures, falls, functional limitations, cancer, diabetes, cardiovascular disease, depression, and excess mortality.
In addition, testing of vitamin D levels has increased markedly in recent years. One national survey showed the annual rate of outpatient visits with a diagnosis code for vitamin D deficiency more than tripled between 2008 and 2010, and a 2009 survey of clinical laboratories reported that the testing increased by at least half in the space of just 1 year, said Dr. Michael L. LeFevre, chair of the task force and professor of family medicine at the University of Missouri, Columbia, and his associates.
The organization is a voluntary expert group tasked with making recommendations about specific preventive care services, devices, and medications for asymptomatic people, with a view to improving Americans’ general health.
The task force reviewed the evidence presented in 16 randomized trials, as well as nested case-control studies using data from the Women’s Health Initiative. They found that no study has directly examined the effects of vitamin D screening, compared with no screening, on clinical outcomes. There isn’t even any consensus about what constitutes vitamin D deficiency, or what the optimal circulating level of 25-hydroxyvitamin D is.
Many testing methods are available, including competitive protein binding, immunoassay, high-performance liquid chromatography, and mass spectrometry. But the sensitivity and specificity of these tests remains unknown, because there is no internationally recognized reference standard. Moreover, the USPSTF found that test results vary not just by which test is used, but even between laboratories using the same test.
Symptomatic vitamin D deficiency is known to affect health adversely, as is asymptomatic vitamin D deficiency in certain patient populations. But the evidence that deficiency contributes to adverse health outcomes in asymptomatic adults is inadequate. The evidence that screening for such deficiency and treating “low” vitamin D levels prevents adverse outcomes or simply improves general health also is inadequate, Dr. LeFevre and his associates said.
Similarly, no studies to date have directly examined possible harms of screening for and treating vitamin D deficiency. Although there are concerns that vitamin D supplements may lead to hypercalcemia, kidney stones, or gastrointestinal symptoms, there is no evidence of such effects in the asymptomatic patient population.
The USPSTF concluded that the harms of screening for and treating vitamin D deficiency are likely “small to none,” but it still is not possible to determine whether the benefits outweigh even that small amount of harm.
At present, no national primary care professional organization recommends screening of the general adult population for vitamin D deficiency. The American Academy of Family Physicians, the Endocrine Society, the American College of Obstetricians and Gynecologists, the American Geriatrics Society, and the National Osteoporosis Foundation all recommend screening for patients at risk for fractures or falls only. The Institute of Medicine has no formal guidelines regarding vitamin D screening, Dr. LeFevre and his associates noted.
The USPSTF summary report and the review of the evidence are available at www.uspreventiveservicestaskforce.org.
The USPSTF is focused on providing a firm evidential base for early detection and prevention of disease, noted Dr. Robert P. Heaney and Dr. Laura A. G. Armas in an accompanying editorial. But perhaps clinicians should have a different focus: full nutrient repletion in their patients, to optimize their health.
A strict disease-avoidance approach is too simplistic with regard to micronutrients, because they don’t directly cause the effects often attributed to them. Instead, when supplies of micronutrients are inadequate, cellular responses are blunted, Dr. Heaney and Dr. Armas noted. That is dysfunction, but not clinically manifest disease.
Such dysfunction may indeed lead ultimately to various diseases, they added, but disease prevention is a dull tool for discerning the defect. And a disease-prevention approach clearly doesn’t show whether there is enough of the nutrient present to enable appropriate physiological responses.
Dr. Heaney and Dr. Armas are at Creighton University in Omaha, Neb. Their remarks are drawn from an editorial accompanying the USPSTF reports.
The USPSTF is focused on providing a firm evidential base for early detection and prevention of disease, noted Dr. Robert P. Heaney and Dr. Laura A. G. Armas in an accompanying editorial. But perhaps clinicians should have a different focus: full nutrient repletion in their patients, to optimize their health.
A strict disease-avoidance approach is too simplistic with regard to micronutrients, because they don’t directly cause the effects often attributed to them. Instead, when supplies of micronutrients are inadequate, cellular responses are blunted, Dr. Heaney and Dr. Armas noted. That is dysfunction, but not clinically manifest disease.
Such dysfunction may indeed lead ultimately to various diseases, they added, but disease prevention is a dull tool for discerning the defect. And a disease-prevention approach clearly doesn’t show whether there is enough of the nutrient present to enable appropriate physiological responses.
Dr. Heaney and Dr. Armas are at Creighton University in Omaha, Neb. Their remarks are drawn from an editorial accompanying the USPSTF reports.
The USPSTF is focused on providing a firm evidential base for early detection and prevention of disease, noted Dr. Robert P. Heaney and Dr. Laura A. G. Armas in an accompanying editorial. But perhaps clinicians should have a different focus: full nutrient repletion in their patients, to optimize their health.
A strict disease-avoidance approach is too simplistic with regard to micronutrients, because they don’t directly cause the effects often attributed to them. Instead, when supplies of micronutrients are inadequate, cellular responses are blunted, Dr. Heaney and Dr. Armas noted. That is dysfunction, but not clinically manifest disease.
Such dysfunction may indeed lead ultimately to various diseases, they added, but disease prevention is a dull tool for discerning the defect. And a disease-prevention approach clearly doesn’t show whether there is enough of the nutrient present to enable appropriate physiological responses.
Dr. Heaney and Dr. Armas are at Creighton University in Omaha, Neb. Their remarks are drawn from an editorial accompanying the USPSTF reports.
The U.S. Preventive Services Task Force made no recommendation for or against primary care physicians screening asymptomatic adults for vitamin D deficiency, because the current evidence is insufficient to adequately assess the benefits and harms of doing so, according to a report published online Nov. 24 in Annals of Internal Medicine.
The USPSTF reviewed the evidence on screening and treatment for vitamin D deficiency, because the condition may contribute to fractures, falls, functional limitations, cancer, diabetes, cardiovascular disease, depression, and excess mortality.
In addition, testing of vitamin D levels has increased markedly in recent years. One national survey showed the annual rate of outpatient visits with a diagnosis code for vitamin D deficiency more than tripled between 2008 and 2010, and a 2009 survey of clinical laboratories reported that the testing increased by at least half in the space of just 1 year, said Dr. Michael L. LeFevre, chair of the task force and professor of family medicine at the University of Missouri, Columbia, and his associates.
The organization is a voluntary expert group tasked with making recommendations about specific preventive care services, devices, and medications for asymptomatic people, with a view to improving Americans’ general health.
The task force reviewed the evidence presented in 16 randomized trials, as well as nested case-control studies using data from the Women’s Health Initiative. They found that no study has directly examined the effects of vitamin D screening, compared with no screening, on clinical outcomes. There isn’t even any consensus about what constitutes vitamin D deficiency, or what the optimal circulating level of 25-hydroxyvitamin D is.
Many testing methods are available, including competitive protein binding, immunoassay, high-performance liquid chromatography, and mass spectrometry. But the sensitivity and specificity of these tests remains unknown, because there is no internationally recognized reference standard. Moreover, the USPSTF found that test results vary not just by which test is used, but even between laboratories using the same test.
Symptomatic vitamin D deficiency is known to affect health adversely, as is asymptomatic vitamin D deficiency in certain patient populations. But the evidence that deficiency contributes to adverse health outcomes in asymptomatic adults is inadequate. The evidence that screening for such deficiency and treating “low” vitamin D levels prevents adverse outcomes or simply improves general health also is inadequate, Dr. LeFevre and his associates said.
Similarly, no studies to date have directly examined possible harms of screening for and treating vitamin D deficiency. Although there are concerns that vitamin D supplements may lead to hypercalcemia, kidney stones, or gastrointestinal symptoms, there is no evidence of such effects in the asymptomatic patient population.
The USPSTF concluded that the harms of screening for and treating vitamin D deficiency are likely “small to none,” but it still is not possible to determine whether the benefits outweigh even that small amount of harm.
At present, no national primary care professional organization recommends screening of the general adult population for vitamin D deficiency. The American Academy of Family Physicians, the Endocrine Society, the American College of Obstetricians and Gynecologists, the American Geriatrics Society, and the National Osteoporosis Foundation all recommend screening for patients at risk for fractures or falls only. The Institute of Medicine has no formal guidelines regarding vitamin D screening, Dr. LeFevre and his associates noted.
The USPSTF summary report and the review of the evidence are available at www.uspreventiveservicestaskforce.org.
The U.S. Preventive Services Task Force made no recommendation for or against primary care physicians screening asymptomatic adults for vitamin D deficiency, because the current evidence is insufficient to adequately assess the benefits and harms of doing so, according to a report published online Nov. 24 in Annals of Internal Medicine.
The USPSTF reviewed the evidence on screening and treatment for vitamin D deficiency, because the condition may contribute to fractures, falls, functional limitations, cancer, diabetes, cardiovascular disease, depression, and excess mortality.
In addition, testing of vitamin D levels has increased markedly in recent years. One national survey showed the annual rate of outpatient visits with a diagnosis code for vitamin D deficiency more than tripled between 2008 and 2010, and a 2009 survey of clinical laboratories reported that the testing increased by at least half in the space of just 1 year, said Dr. Michael L. LeFevre, chair of the task force and professor of family medicine at the University of Missouri, Columbia, and his associates.
The organization is a voluntary expert group tasked with making recommendations about specific preventive care services, devices, and medications for asymptomatic people, with a view to improving Americans’ general health.
The task force reviewed the evidence presented in 16 randomized trials, as well as nested case-control studies using data from the Women’s Health Initiative. They found that no study has directly examined the effects of vitamin D screening, compared with no screening, on clinical outcomes. There isn’t even any consensus about what constitutes vitamin D deficiency, or what the optimal circulating level of 25-hydroxyvitamin D is.
Many testing methods are available, including competitive protein binding, immunoassay, high-performance liquid chromatography, and mass spectrometry. But the sensitivity and specificity of these tests remains unknown, because there is no internationally recognized reference standard. Moreover, the USPSTF found that test results vary not just by which test is used, but even between laboratories using the same test.
Symptomatic vitamin D deficiency is known to affect health adversely, as is asymptomatic vitamin D deficiency in certain patient populations. But the evidence that deficiency contributes to adverse health outcomes in asymptomatic adults is inadequate. The evidence that screening for such deficiency and treating “low” vitamin D levels prevents adverse outcomes or simply improves general health also is inadequate, Dr. LeFevre and his associates said.
Similarly, no studies to date have directly examined possible harms of screening for and treating vitamin D deficiency. Although there are concerns that vitamin D supplements may lead to hypercalcemia, kidney stones, or gastrointestinal symptoms, there is no evidence of such effects in the asymptomatic patient population.
The USPSTF concluded that the harms of screening for and treating vitamin D deficiency are likely “small to none,” but it still is not possible to determine whether the benefits outweigh even that small amount of harm.
At present, no national primary care professional organization recommends screening of the general adult population for vitamin D deficiency. The American Academy of Family Physicians, the Endocrine Society, the American College of Obstetricians and Gynecologists, the American Geriatrics Society, and the National Osteoporosis Foundation all recommend screening for patients at risk for fractures or falls only. The Institute of Medicine has no formal guidelines regarding vitamin D screening, Dr. LeFevre and his associates noted.
The USPSTF summary report and the review of the evidence are available at www.uspreventiveservicestaskforce.org.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: The USPSTF makes no recommendation for or against screening and treating asymptomatic adults for vitamin D deficiency, because the evidence regarding the benefits and harms is insufficient.
Major finding: Testing of vitamin D levels has increased markedly, with one national survey showing the annual rate of outpatient visits with a diagnosis code for vitamin D deficiency more than tripled between 2008 and 2010, and a 2009 survey of clinical laboratories reporting that the testing increased by at least half in the space of just 1 year.
Data source: A detailed review of the evidence and an expert consensus regarding screening asymptomatic adults for vitamin D deficiency to prevent fractures, cancer, CVD, and other adverse outcomes.
Disclosures: The USPSTF is an independent, voluntary group supported by the U.S. Agency for Healthcare Research and Quality to improve Americans’ health by making recommendations concerning preventive services such as screenings and medications. Dr. LeFevre and his associates reported having no relevant financial disclosures.
He’s been making new ‘friends’
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Seeing friends
Mr. B, age 91, presents to the emergency room (ER) for hip pain. As he is being evaluated, he asks a nurse to tell the “other people” around her to leave so that he can have privacy. As clarification, Mr. B reports visual hallucinations, which prompts the ER physician to request a psychiatry consult.
Mr. B is alert and oriented to time, place, and person when he is evaluated by the on-call psychiatry resident. He reports that he has been seeing several unusual things for the last 4 to 5 months. Asked to elaborate, Mr. B admits seeing colorful and vivid images of people around him. These people come and go as they like; rarely, they talk to him. He describes the conversations as “a constant chatter” in the background and adds that it is difficult to understand what they are talking about.
Mr. B states that he has been “seeing” a couple of people on a regular basis, and they are “sort of like my friends.” He endorses that these people often sing songs or dance for him. He states that, sometimes, these “friends” bring 3 or 4 friends and, although he could not make out their faces clearly, “they all are around me.” He describes the people he sees as “nice people” and does not report being scared or frightened by them.
Mr. B does not report paranoia, and denies command-type hallucinations. He and his family report no unusual changes in behavior in recent months. The medical history is remarkable for atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, age-related macular degeneration, and glaucoma.
Mr. B denies having any ongoing mood or anxiety symptoms. He states that he knows these people are “probably not real,” and they do not bother him and just keep him company.
What could be causing Mr. B’s hallucinations?
a) a stroke
b) late-onset schizophrenia
c) dementia
d) Charles Bonnet syndrome
The authors’ observations
Visual hallucinations among geriatric pa-tients are a common and confusing presentation. In addition to several medical causes for this presentation (Table 1), consider Charles Bonnet syndrome in patients with visual loss, presenting as visual hallucinations with intact insight and absence of a mental illness. Other conditions to consider in the differential diagnosis include Parkinson’s disease, dementia with Lewy bodies, schizophrenia, seizures, migraine, and stroke, including lesions of the thalamus or brain stem.
Charles Bonnet syndrome was first described by Swiss philosopher Charles Bonnet in the 18th century. He reported vivid visual hallucinations in his visually impaired grandfather (bilateral cataracts).1
It is important to recognize this syndrome because patients can present across different specialties, including psychiatry, ophthalmology, neurology, geriatric medicine, and family medicine.2 As life expectancy increases, this condition might be seen more often. It is prudent to identify, intervene, and refer as appropriate, in addition to educating patients and caregivers about the nature and course of the condition.
EVALUATION Not psychotic
Mr. B reports good sleep and appetite. He denies using alcohol or illicit drugs. He states he slipped in the bathroom the day before coming to the ER, but denies other recent falls or injuries. Other than hip pain, he has no other physical complaints. His medication regimen includes aspirin, lisinopril, lovastatin, and metoprolol.
The ER team diagnoses a hip fracture. Mr. B is transferred to the orthopedic service; the psychiatry consult team continues to follow him. Mental status examination is unremarkable other than the visual hallucinations. His speech is clear, non-pressured, with goal-directed thought processing. Mini-Mental State Examination score is 23/30 with Mr. B having difficulty with object drawing and 3-object recall. Brief cognitive examination in the ER is unremarkable.
The orthopedic team decides on conservative management of the hip fracture. There is no evidence of infection. Mr. B is afebrile with clear sensorium; complete blood cell count and normal liver function tests are normal; urinalysis and urine drug screen are negative; and chest radiography is unremarkable. CT and MRI of the head are unremarkable.
After 1 week in the hospital, Mr. B continues to experience vivid visual imagery. No signs of active infection are found. An ophthalmologist is consulted, who confirms Mr. B’s earlier diagnosis of glaucoma and age-related macular degeneration but does not recommend further treatment. Visual field test by confrontation is normal, with normal visual reflexes.
The authors’ observations
The reported prevalence of Charles Bonnet syndrome among visually impaired people varies from study to study—from as low as 0.4% to as high as 63%.3-6 The reason for such variation can be attributed to several variables:
• underdiagnosis
• misdiagnosis
• underreporting by patients because of the benign nature of the hallucinations
• patients’ reluctance to report visual hallucinations because of fear of being labeled “mentally ill.”7,8
Symptoms
There are no specific diagnostic criteria for Charles Bonnet syndrome (Table 2). However, the following are generally accepted for diagnosis9:
• grossly intact cognition, although mild cognitive impairment may be present in some cases10
• underlying visual disorder, usually acquired, such as glaucoma, age-related macular degeneration, diabetic retinopathy, central retinal artery occlusion, and optic neuritis3,4,11
• no hallucinations or perceptive difficulties in other sensory modalities
• generally intact insight
• absence of delusions
• absence of other neurologic, psychiatric, toxic, or metabolic conditions; medical causes of delirium must be ruled out.
Hallucinations might not be disturbing to the patient. Hallucinations could be simple (light flashes, lines, or geometric shapes) or complex (faces, figures, or scenes),12 and perceived as in color or in black and white. Hallucinations mostly are pleasant and rarely have any emotional impact or meaning. Although hallucinations are almost exclusively visual, they can be accompanied by noise or auditory hallucinations.13,14
Other characteristics of Charles Bonnet syndrome include:
• typical age of onset is approximately 72 years (range, 70 to 92 years)
• no sex distinction has been identified
• episodes can last from a few seconds to few hours; the syndrome may last a few days or a few years5
• it is not uncommon for episodes to occur in clusters, followed by symptom-free intervals and recurrences
• symptoms tend to fade away as patients progress to complete loss of sight.15
The course of Charles Bonnet syndrome is uncertain and unpredictable and the episodic nature can be frustrating for both patient and clinician. The syndrome could be misdiagnosed as a psychiatric condition.
Pathophysiology
The precise mechanism behind simple or complex vivid hallucinations in persons with Charles Bonnet syndrome is unclear. Several theories have been proposed.
Release theory proposes a loss of input to the primary visual areas, which decreases cortical inhibition and further causes disinhibition of visual association areas, thereby “releasing” visual hallucinations.16 Research suggests that this might be an attempt by surviving neurons to recover vision. Loss of input somehow causes surviving neurons to adapt by increased sensitivity to residual visual stimuli.
Deafferentation theory. This relatively new theory proposes deafferentation of the visual sensory pathway, which, in turn, causes disinhibition of neurons in the visual cortical regions, thereby causing them to fire spontaneously. This could cause a sensation analogous to phantom limb pain, which would be called “phantom vision presence of brain activity in the absence of an actual visual input.” Further, biochemical and molecular changes have been proposed to explain the deafferentation theory.17
Neurobiological evidence. Limited data are available for a neurobiological basis to visual hallucinations in Charles Bonnet syndrome. A few studies have used functional MRI and single-photon emission CT and reported possible association of visual hallucinations to specific visual areas.18,19
Risk factors
Social or physical isolation, loneliness, low extraversion, and shyness are risk factors for Charles Bonnet syndrome in visually impaired people.20 Sensory deprivation and low level of arousal favor the occurrence of hallucinations.5 Rate of vision loss—not the nature of pathology or severity of visual impairment—has been suggested to increase the risk of developing Charles Bonnet syndrome.21
What are the treatment options for Charles Bonnet syndrome?
a) begin an antipsychotic
b) do nothing; there is no cure
c) educate the patient about the nature of the hallucinations
d) refer the patient to an ophthalmologist for evaluation of vision loss
Treatment
There are several modalities to manage visual hallucinations in a patient with Charles Bonnet syndrome (Table 3). After ruling out medical and other psychiatric causes of visual hallucinations, treatment might not be indicated if the patient is not disturbed by the hallucinations. In most cases, reassurance and educating the patient and family about the benign nature of the visual hallucinations is all that is needed.
For patients who are disturbed by these visions or for whom there is a treatable cause, treatment could include cataract removal, medical therapy to reduce intraocular pressure in glaucoma, treatment of diabetic retinopathy, or laser photocoagulation. These treatments are associated with a reduction in hallucinations.22
In some cases, hallucinations disappear as visual acuity deteriorates. Psychotropics have been used to treat Charles Bonnet syndrome, including:
• antipsychotics, including haloperidol, risperidone, and olanzapine
• anticonvulsants, including valproic acid, gabapentin, and carbamazepine
• antidepressants, including mirtazapine and venlafaxine.23-30
Some experts recommend a conservative approach, which might be justified because some cases of Charles Bonnet syndrome are episodic and remit spontaneously.31 Again, however, consider pharmacotherapy if a patient is disturbed by hallucinations or if hallucinations impair overall functioning.
TREATMENT Education
After discussion with Mr. B and his family, he is started on risperidone, 1 mg at bedtime, and the psychiatric team provides information about the nature of Charles Bonnet syndrome. Mr. B reportedly takes this medication for a few days and then stops because he does not want the visual hallucinations to go away.
The psychiatry team sees Mr. B before discharge. He and his family are educated about the benign nature of the syndrome, the need for continued family support, and the fact that hallucinations will have minimal or no implications for his life.
The authors’ observations
It is important to remember that a visual description of hallucinations in Charles Bonnet syndrome can be quite vivid, and that the patient might not identify his hallucinations as such or consider them as a problem. Be careful not to dismiss the patient’s complaints as a primary psychiatric condition. It also is important to be mindful of the patient’s concerns with a psychiatric diagnosis; detailed discussion with the patient is helpful in most cases. A more comprehensive and empathetic approach to care could go a long way to sustain quality of life for these patients.
Bottom Line
Charles Bonnet syndrome is characterized by visual hallucinations in patients with visual impairment who have intact insight and an absence of mental illness. Taking a thorough history can help rule out medical and psychiatric causes of visual hallucinations. Educate patients and family about the nature of the hallucinations. In some cases, a psychotropic may be indicated.
Related Resources
• Nguyen ND, Osterweil D, Hoffman J. Charles Bonnet syndrome: treating nonpsychiatric hallucinations. Consult Pharm. 2013;28(3):184-188.
• Lapid MI, Burton MC, Chang MT, et al. Clinical phenomenology and mortality in Charles Bonnet syndrome. J Geriatr Psychiatry Neurol. 2013;26(1):3-9.
Drug Brand Names
Carbamazepine • Tegretol Mirtazapine • Remeron
Gabapentin • Neurontin Olanzapine • Zyprexa
Haloperidol • Haldol Risperidone • Risperdal
Lisinopril • Prinivil, Zestril Valproic acid • Depakene
Lovastatin • Mevacor Venlafaxine • Effexor
Metoprolol • Lopressor
Acknowledgement
The authors acknowledge Barry Liskow, MD, Vice Chair of Psychiatry, Kansas University Medical Center, Kansas City, Kansas, for providing both insight into the topic and useful feedback on the manuscript.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
1. Bonnet C. Essai analytique sur les facultes de l’ame. Copenhagen, Denmark: Chez le Ferres CI. & Ant. Philibert; 1760:426-429.
2. Plummer C, Kleinitz A, Vroomen P, et al. Of Roman chariots and goats in overcoats: the syndrome of Charles Bonnet. J Clin Neurosci. 2007;14(8):709-714.
3. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
4. Tan CS, Lim VS, Ho DY, et al. Charles Bonnet syndrome in Asian patients in a tertiary ophthalmic centre. Br J Ophthalmol. 2004;88(10):1325-1329.
5. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Visual hallucinations in psychologically normal people: Charles Bonnet’s syndrome. Lancet. 1996;347(9004):794-797.
6. Menon GJ. Complex visual hallucinations in the visually impaired: a structured history-taking approach. Arch Ophthalmol. 2005;123(3):349-355.
7. Hart CT. Formed visual hallucinations: a symptom of cranial arteritis. Br Med J. 1967;3(5566):643-644.
8. Norton-Wilson L, Munir M. Visual perceptual disorders resembling the Charles Bonnet syndrome. A study of 434 consecutive patients referred to a psychogeriatric unit. Fam Pract. 1987;4(1):27-35.
9. Eperjesi F, Akbarali N. Rehabilitation in Charles Bonnet syndrome: a review of treatment options. Clin Exp Optom. 2004;87(3):149-152.
10. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucinations in patients from an ophthalmology clinic and medical clinic population. J Nerv Ment Dis. 1994;182(5):273-276.
11. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(pt 10):1819-1840.
12. Kester EM. Charles Bonnet syndrome: case presentation and literature review. Optometry. 2009;80(7):360-366.
13. Hori H, Terao T, Nakamura JL. Charles Bonnet syndrome with auditory hallucinations: a diagnostic dilemma. Psychopathology. 2001;34(3):164-166.
14. Menon GJ, Rahman I, Menon SJ, et al. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48(1):58-72.
15. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis. 1997;185(3):195-200.
16. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150.
17. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002;73(5):535-541.
18. Ffytche DH, Howard RJ, Brammer MJ, et al. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1(8):738-742.
19. Adachi N, Watanabe T, Matsuda H, et al. Hyperperfusion in the lateral temporal cortex, the striatum and the thalamus during complex visual hallucinations: single photon emission computed tomography findings in patients with Charles Bonnet syndrome. Psychiatry Clin Neurosci. 2000;54(2):157-162.
20. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry. 1999;40(4):315-319.
21. Shiraishi Y, Terao T, Ibi K, et al. Charles Bonnet syndrome and visual acuity—the involvement of dynamic or acute sensory deprivation. Eur Arch Psychiatry Clin Neurosci. 2004;254(6):362-364.
22. Tueth MJ, Cheong JA, Samander J. The Charles Bonnet syndrome: a type of organic visual hallucinosis. J Geriatr Psychiatry Neurol. 1995;8(1):1-3.
23. Nguyen H, Le C, Nguyen H. Charles Bonnet syndrome in an elderly patient concurrent with acute cerebellar infarction treated successfully with haloperidol. J Am Geriatr Soc. 2011;59(4):761-762.
24. Campbell JJ, Ngo G. Risperidone treatment of complex hallucinations in a patient with posterior cortical atrophy. J Neuropsychiatry Clin Neurosci. 2008;20(3):378-379.
25. Colletti Moja M, Milano E, Gasverde S, et al. Olanzapine therapy in hallucinatory visions related to Bonnet syndrome. Neurol Sci. 2005;26(3):168-170.
26. Jang JW, Youn YC, Seok JW, et al. Hypermetabolism in the left thalamus and right inferior temporal area on positron emission tomography-statistical parametric mapping (PET-SPM) in a patient with Charles Bonnet syndrome resolving after treatment with valproic acid. J Clin Neurosci. 2011;18(8):1130-1132.
27. Paulig M, Mentrup H. Charles Bonnet’s syndrome; Complete remission of complex visual hallucinations treated by gabapentin. J Neurol Neurosurg Psychiatry. 2001;70(6):813-814.
28. Terao T. Effect of carbamazepine and clonazepam combination on Charles Bonnet syndrome: a case report. Hum Psychopharmacol. 1998;13(6):451-453.
29. Siddiqui Z, Ramaswmay S, Petty F. Mirtazapine for Charles Bonnet syndrome. Can J Psychiatry. 2004;49(11):787-788.
30. Lang UE, Stogowski D, Schulze D, et al. Charles Bonnet Syndrome: successful treatment of visual hallucinations due to vision loss with selective serotonin reuptake inhibitors. J Psychopharmacol. 2007;21(5):553-555.
31. Hartney KE, Catalano G, Catalano MC. Charles Bonnet syndrome: are medications necessary? J Psychiatr Pract. 2011;17(2):137-141.
How to modify psychotropic therapy for patients who have liver dysfunction
Police bring Ms. R, age 35, to the psychiatric ER after they find her asleep in a park. She is awake but drowsy, and states that she has a history of bipolar disorder. She claims that she had been stable on valproic acid (VPA), 1,500 mg/d, bupropion XL, 300 mg/d, quetiapine, 400 mg/d, and trazodone, 100 mg/d, until 2 weeks ago, when her best friend died and she stopped taking her medications all together. The previous evening, feeling “alone, hopeless, and sad,” she attempted suicide by ingesting a handful of VPA and clonazepam, obtained from a friend, and 2 liters of vodka. She complains of nausea, vomiting, and abdominal pain. Elevated laboratory chemistries included aspartate aminotransferase (AST), 220 U/L; alanine aminotransferase (ALT), 182 U/L; alkaline phosphatase (AP), 75 U/L; γ-glutamyltransferase (GGT), 104 U/L; total bilirubin, 1.4 mg/dL; and an elevated VPA serum concentration of 152 μg/mL.
Drug-induced hepatotoxicity accounts for approximately 50% of acute liver failure cases, and almost 10% of liver transplants in some facilities.1 The incidence of drug-induced hepatotoxicity is between 0.001% and 0.1% in patients on standard medication doses.2 Drug-induced hepatotoxicity is characterized by:
• abnormalities in laboratory parameters (hepatocellular, cholestatic, or mixed)
• mechanisms of toxicity (direct, immune-mediated, idiosyncratic, mitochondrial toxicity)
• liver biopsy histology (steatosis, sinusoidal obstruction syndrome).3
Liver function test results of hepatocellular injury are characterized by ALT elevation and minimal AP elevation, whereas cholestatic injury manifests as high AP. Table 13 categorizes psychotropics based on type of liver injury and how each injury manifest in liver function tests. Delayed idiosyncratic reactions occur after taking the drug, whereas direct toxicities are dose-dependent and more predictable. By definition, a clinically significant hepatotoxicity is associated with an ALT >3 times the upper limit of normal.3
VPA-induced liver injury occurs in approximately 1 in 37,000 persons taking the drug.4 Patients at an increased risk of VPA-induced liver injury include:
• children
• patients with mitochondrial enzyme deficiencies
• Reye’s syndrome
• Friedreich’s ataxia
• polypharmacy patients
• patients with a sibling who has experienced VPA toxicity.4
Benign enzyme elevations occur in approximately 20% of patients taking VPA.5 In Ms. R’s case, concomitant VPA, clonazepam, and alcohol may have led to elevations in ALT, AST, and GGT. Her nausea, vomiting, and abdominal pain are consistent with hepatic dysfunction.
Carnitine is effective in increasing survival of patients with VPA-induced hepatotoxicity.4 Because Ms. R is symptomatic, discontinuing VPA and administering IV L-carnitine is warranted.5 L-carnitine can be initiated at 100 mg/kg as an IV bolus, followed by 50 mg/kg as an IV infusion every 8 hours, with a maximum dosage of 3,000 mg.6 Patients may require several days of therapy based on symptoms. L-carnitine should be continued until a patient shows clinical improvement, such as decreases in ALT and AST.
Ms. R experienced a VPA-induced hepatotoxic reaction. However, continuous monitoring is appropriate for all patients who are prescribed any potentially hepatotoxic psychotropic, especially after hepatic injuries resolve. This includes mood stabilizers, antipsychotics, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors, especially when given concomitantly with other hepatotoxic agents.
Table 2 lists dosing recommendations for commonly used psychotropics in patients with hepatic impairment. Among mood stabilizers, carbamazepine and VPA are associated with the highest incidence of hepatotoxicity.2 A follow-up study of more than 1,000,000 VPA prescriptions found 29 cases of fatal hepatotoxicity in a 7-year period.7 Although there are case reports of hepatotoxicity with oxcarbazepine, it may have a better liver safety profile than carbamazepine.2 Hepatotoxicity with lamotrigine is rare, although fatal cases have been reported.5
When initiating an antipsychotic, a temporary, benign increase in liver enzymes can be expected, but typically discontinuation is unnecessary.2 Phenothiazines in particular can cause increases in liver enzymes in 20% of patients.2 Hepatotoxicity with benzodiazepines is infrequent, with a few cases of cholestatic injury reported with diazepam, chlordiazepoxide, and flurazepam.2
SSRIs are relatively safe; incidents of hepatic injury are rare. Among SSRIs, paroxetine is most frequently associated with hepatotoxicity. Abnormal liver function tests have been observed with fluoxetine (0.5% of long-term recipients) and other SSRIs.1,2,4
Among antidepressants with dual serotonergic action, nefazodone carries a black-box warning for hepatotoxicity and is used rarely, whereas trazodone is not regarded as hepatotoxic.2 Antidepressants with dual norepinephrine and serotonin reuptake inhibitor properties carry a higher risk of liver injury, especially duloxetine. Hepatocellular, cholestatic, and mixed types of hepatotoxicity are associated with duloxetine-induced hepatotoxicity.2
Monitoring recommendations
Before prescribing potentially hepatotoxic medications, order baseline liver function tests. During therapy, periodic liver function monitoring is recommended. Elevated transaminase concentrations (>3 × the upper limit of normal), bilirubin (>2 × the upper limit of normal), and prolonged prothrombin times are indicators of hepatic injury.2 Caution should be taken to prevent polypharmacy with multiple hepatotoxic medications and over-the-counter use of hepatotoxic drugs and supplements.
When choosing a psychotropic, take into account patient-specific factors, such as underlying liver disease and alcohol consumption. Patients on potentially hepatotoxic medications should be counseled to recognize and report symptoms of liver dysfunction, including nausea, vomiting, jaundice, and lower-extremity edema.2 If liver injury occurs, modify therapy with the potential offending agent and check liver function periodically.
Related Resourcesa
• Bleibel W, Kim S, D’Silva K, et al. Drug-induced liver injury: review article. Dig Dis Sci. 2007;52(10):2463-2471.
• U.S. National Library of Medicine. LiverTox. National Institute of Health. www.livertox.nih.gov.
Drug Brand Names
Amitriptyline • Elavil Lurasidone • Latuda
Molindone • Moban Molindone • Moban
Aripiprazole • Abilify Nefazodone • Serzone
Asenapine • Saphris Nortriptyline • Pamelor
Bupropion XL • Wellbutrin XL Olanzapine • Zyprexa
Citalopram • Celexa Oxcarbazepine • Trileptal
Carbamazepine • Tegretol Paroxetine • Paxil
Chlordiazepoxide • Librium Perphenazine • Trilafon
Chlorpromazine • Thorazine Phenobarbital • Luminal
Clonazepam • Klonopin Phenytoin • Dilantin
Clozapine • Clozaril Quetiapine • Seroquel
Desvenlafaxine • Pristiq Risperidone • Risperdal
Diazepam • Valium Sertraline • Zoloft
Duloxetine • Cymbalta Thiothixene • Navane
Escitalopram • Lexapro Trazodone • Desyrel
Fluoxetine • Prozac Trifluoperazine • Stelazine
Fluphenazine • Prolixin Topiramate • Topamax
Flurazepam • Dalmane Valproic acid • Depakote
Haloperidol • Haldol Venlafaxine • Effexor
Iloperidone • Fanapt Ziprasidone • Geodon
Lamotrigine • Lamictal
Levocarnitine • L-carnitine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pugh AJ, Barve AJ, Falkner K, et al. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13(2):277-294.
2. Sedky K, Nazir R, Joshi A, et al. Which psychotropic medications induce hepatotoxicity? Gen Hosp Psychiatry. 2012;34(1):53-61.
3. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10):1135-1151.
4. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
5. Murray KF, Hadzic N, Wirth S, et al. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47(4):395-405.
6. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293.
7. Bryant AE 3rd, Dreifuss FE. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46(2):465-469.
Police bring Ms. R, age 35, to the psychiatric ER after they find her asleep in a park. She is awake but drowsy, and states that she has a history of bipolar disorder. She claims that she had been stable on valproic acid (VPA), 1,500 mg/d, bupropion XL, 300 mg/d, quetiapine, 400 mg/d, and trazodone, 100 mg/d, until 2 weeks ago, when her best friend died and she stopped taking her medications all together. The previous evening, feeling “alone, hopeless, and sad,” she attempted suicide by ingesting a handful of VPA and clonazepam, obtained from a friend, and 2 liters of vodka. She complains of nausea, vomiting, and abdominal pain. Elevated laboratory chemistries included aspartate aminotransferase (AST), 220 U/L; alanine aminotransferase (ALT), 182 U/L; alkaline phosphatase (AP), 75 U/L; γ-glutamyltransferase (GGT), 104 U/L; total bilirubin, 1.4 mg/dL; and an elevated VPA serum concentration of 152 μg/mL.
Drug-induced hepatotoxicity accounts for approximately 50% of acute liver failure cases, and almost 10% of liver transplants in some facilities.1 The incidence of drug-induced hepatotoxicity is between 0.001% and 0.1% in patients on standard medication doses.2 Drug-induced hepatotoxicity is characterized by:
• abnormalities in laboratory parameters (hepatocellular, cholestatic, or mixed)
• mechanisms of toxicity (direct, immune-mediated, idiosyncratic, mitochondrial toxicity)
• liver biopsy histology (steatosis, sinusoidal obstruction syndrome).3
Liver function test results of hepatocellular injury are characterized by ALT elevation and minimal AP elevation, whereas cholestatic injury manifests as high AP. Table 13 categorizes psychotropics based on type of liver injury and how each injury manifest in liver function tests. Delayed idiosyncratic reactions occur after taking the drug, whereas direct toxicities are dose-dependent and more predictable. By definition, a clinically significant hepatotoxicity is associated with an ALT >3 times the upper limit of normal.3
VPA-induced liver injury occurs in approximately 1 in 37,000 persons taking the drug.4 Patients at an increased risk of VPA-induced liver injury include:
• children
• patients with mitochondrial enzyme deficiencies
• Reye’s syndrome
• Friedreich’s ataxia
• polypharmacy patients
• patients with a sibling who has experienced VPA toxicity.4
Benign enzyme elevations occur in approximately 20% of patients taking VPA.5 In Ms. R’s case, concomitant VPA, clonazepam, and alcohol may have led to elevations in ALT, AST, and GGT. Her nausea, vomiting, and abdominal pain are consistent with hepatic dysfunction.
Carnitine is effective in increasing survival of patients with VPA-induced hepatotoxicity.4 Because Ms. R is symptomatic, discontinuing VPA and administering IV L-carnitine is warranted.5 L-carnitine can be initiated at 100 mg/kg as an IV bolus, followed by 50 mg/kg as an IV infusion every 8 hours, with a maximum dosage of 3,000 mg.6 Patients may require several days of therapy based on symptoms. L-carnitine should be continued until a patient shows clinical improvement, such as decreases in ALT and AST.
Ms. R experienced a VPA-induced hepatotoxic reaction. However, continuous monitoring is appropriate for all patients who are prescribed any potentially hepatotoxic psychotropic, especially after hepatic injuries resolve. This includes mood stabilizers, antipsychotics, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors, especially when given concomitantly with other hepatotoxic agents.
Table 2 lists dosing recommendations for commonly used psychotropics in patients with hepatic impairment. Among mood stabilizers, carbamazepine and VPA are associated with the highest incidence of hepatotoxicity.2 A follow-up study of more than 1,000,000 VPA prescriptions found 29 cases of fatal hepatotoxicity in a 7-year period.7 Although there are case reports of hepatotoxicity with oxcarbazepine, it may have a better liver safety profile than carbamazepine.2 Hepatotoxicity with lamotrigine is rare, although fatal cases have been reported.5
When initiating an antipsychotic, a temporary, benign increase in liver enzymes can be expected, but typically discontinuation is unnecessary.2 Phenothiazines in particular can cause increases in liver enzymes in 20% of patients.2 Hepatotoxicity with benzodiazepines is infrequent, with a few cases of cholestatic injury reported with diazepam, chlordiazepoxide, and flurazepam.2
SSRIs are relatively safe; incidents of hepatic injury are rare. Among SSRIs, paroxetine is most frequently associated with hepatotoxicity. Abnormal liver function tests have been observed with fluoxetine (0.5% of long-term recipients) and other SSRIs.1,2,4
Among antidepressants with dual serotonergic action, nefazodone carries a black-box warning for hepatotoxicity and is used rarely, whereas trazodone is not regarded as hepatotoxic.2 Antidepressants with dual norepinephrine and serotonin reuptake inhibitor properties carry a higher risk of liver injury, especially duloxetine. Hepatocellular, cholestatic, and mixed types of hepatotoxicity are associated with duloxetine-induced hepatotoxicity.2
Monitoring recommendations
Before prescribing potentially hepatotoxic medications, order baseline liver function tests. During therapy, periodic liver function monitoring is recommended. Elevated transaminase concentrations (>3 × the upper limit of normal), bilirubin (>2 × the upper limit of normal), and prolonged prothrombin times are indicators of hepatic injury.2 Caution should be taken to prevent polypharmacy with multiple hepatotoxic medications and over-the-counter use of hepatotoxic drugs and supplements.
When choosing a psychotropic, take into account patient-specific factors, such as underlying liver disease and alcohol consumption. Patients on potentially hepatotoxic medications should be counseled to recognize and report symptoms of liver dysfunction, including nausea, vomiting, jaundice, and lower-extremity edema.2 If liver injury occurs, modify therapy with the potential offending agent and check liver function periodically.
Related Resourcesa
• Bleibel W, Kim S, D’Silva K, et al. Drug-induced liver injury: review article. Dig Dis Sci. 2007;52(10):2463-2471.
• U.S. National Library of Medicine. LiverTox. National Institute of Health. www.livertox.nih.gov.
Drug Brand Names
Amitriptyline • Elavil Lurasidone • Latuda
Molindone • Moban Molindone • Moban
Aripiprazole • Abilify Nefazodone • Serzone
Asenapine • Saphris Nortriptyline • Pamelor
Bupropion XL • Wellbutrin XL Olanzapine • Zyprexa
Citalopram • Celexa Oxcarbazepine • Trileptal
Carbamazepine • Tegretol Paroxetine • Paxil
Chlordiazepoxide • Librium Perphenazine • Trilafon
Chlorpromazine • Thorazine Phenobarbital • Luminal
Clonazepam • Klonopin Phenytoin • Dilantin
Clozapine • Clozaril Quetiapine • Seroquel
Desvenlafaxine • Pristiq Risperidone • Risperdal
Diazepam • Valium Sertraline • Zoloft
Duloxetine • Cymbalta Thiothixene • Navane
Escitalopram • Lexapro Trazodone • Desyrel
Fluoxetine • Prozac Trifluoperazine • Stelazine
Fluphenazine • Prolixin Topiramate • Topamax
Flurazepam • Dalmane Valproic acid • Depakote
Haloperidol • Haldol Venlafaxine • Effexor
Iloperidone • Fanapt Ziprasidone • Geodon
Lamotrigine • Lamictal
Levocarnitine • L-carnitine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Police bring Ms. R, age 35, to the psychiatric ER after they find her asleep in a park. She is awake but drowsy, and states that she has a history of bipolar disorder. She claims that she had been stable on valproic acid (VPA), 1,500 mg/d, bupropion XL, 300 mg/d, quetiapine, 400 mg/d, and trazodone, 100 mg/d, until 2 weeks ago, when her best friend died and she stopped taking her medications all together. The previous evening, feeling “alone, hopeless, and sad,” she attempted suicide by ingesting a handful of VPA and clonazepam, obtained from a friend, and 2 liters of vodka. She complains of nausea, vomiting, and abdominal pain. Elevated laboratory chemistries included aspartate aminotransferase (AST), 220 U/L; alanine aminotransferase (ALT), 182 U/L; alkaline phosphatase (AP), 75 U/L; γ-glutamyltransferase (GGT), 104 U/L; total bilirubin, 1.4 mg/dL; and an elevated VPA serum concentration of 152 μg/mL.
Drug-induced hepatotoxicity accounts for approximately 50% of acute liver failure cases, and almost 10% of liver transplants in some facilities.1 The incidence of drug-induced hepatotoxicity is between 0.001% and 0.1% in patients on standard medication doses.2 Drug-induced hepatotoxicity is characterized by:
• abnormalities in laboratory parameters (hepatocellular, cholestatic, or mixed)
• mechanisms of toxicity (direct, immune-mediated, idiosyncratic, mitochondrial toxicity)
• liver biopsy histology (steatosis, sinusoidal obstruction syndrome).3
Liver function test results of hepatocellular injury are characterized by ALT elevation and minimal AP elevation, whereas cholestatic injury manifests as high AP. Table 13 categorizes psychotropics based on type of liver injury and how each injury manifest in liver function tests. Delayed idiosyncratic reactions occur after taking the drug, whereas direct toxicities are dose-dependent and more predictable. By definition, a clinically significant hepatotoxicity is associated with an ALT >3 times the upper limit of normal.3
VPA-induced liver injury occurs in approximately 1 in 37,000 persons taking the drug.4 Patients at an increased risk of VPA-induced liver injury include:
• children
• patients with mitochondrial enzyme deficiencies
• Reye’s syndrome
• Friedreich’s ataxia
• polypharmacy patients
• patients with a sibling who has experienced VPA toxicity.4
Benign enzyme elevations occur in approximately 20% of patients taking VPA.5 In Ms. R’s case, concomitant VPA, clonazepam, and alcohol may have led to elevations in ALT, AST, and GGT. Her nausea, vomiting, and abdominal pain are consistent with hepatic dysfunction.
Carnitine is effective in increasing survival of patients with VPA-induced hepatotoxicity.4 Because Ms. R is symptomatic, discontinuing VPA and administering IV L-carnitine is warranted.5 L-carnitine can be initiated at 100 mg/kg as an IV bolus, followed by 50 mg/kg as an IV infusion every 8 hours, with a maximum dosage of 3,000 mg.6 Patients may require several days of therapy based on symptoms. L-carnitine should be continued until a patient shows clinical improvement, such as decreases in ALT and AST.
Ms. R experienced a VPA-induced hepatotoxic reaction. However, continuous monitoring is appropriate for all patients who are prescribed any potentially hepatotoxic psychotropic, especially after hepatic injuries resolve. This includes mood stabilizers, antipsychotics, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors, especially when given concomitantly with other hepatotoxic agents.
Table 2 lists dosing recommendations for commonly used psychotropics in patients with hepatic impairment. Among mood stabilizers, carbamazepine and VPA are associated with the highest incidence of hepatotoxicity.2 A follow-up study of more than 1,000,000 VPA prescriptions found 29 cases of fatal hepatotoxicity in a 7-year period.7 Although there are case reports of hepatotoxicity with oxcarbazepine, it may have a better liver safety profile than carbamazepine.2 Hepatotoxicity with lamotrigine is rare, although fatal cases have been reported.5
When initiating an antipsychotic, a temporary, benign increase in liver enzymes can be expected, but typically discontinuation is unnecessary.2 Phenothiazines in particular can cause increases in liver enzymes in 20% of patients.2 Hepatotoxicity with benzodiazepines is infrequent, with a few cases of cholestatic injury reported with diazepam, chlordiazepoxide, and flurazepam.2
SSRIs are relatively safe; incidents of hepatic injury are rare. Among SSRIs, paroxetine is most frequently associated with hepatotoxicity. Abnormal liver function tests have been observed with fluoxetine (0.5% of long-term recipients) and other SSRIs.1,2,4
Among antidepressants with dual serotonergic action, nefazodone carries a black-box warning for hepatotoxicity and is used rarely, whereas trazodone is not regarded as hepatotoxic.2 Antidepressants with dual norepinephrine and serotonin reuptake inhibitor properties carry a higher risk of liver injury, especially duloxetine. Hepatocellular, cholestatic, and mixed types of hepatotoxicity are associated with duloxetine-induced hepatotoxicity.2
Monitoring recommendations
Before prescribing potentially hepatotoxic medications, order baseline liver function tests. During therapy, periodic liver function monitoring is recommended. Elevated transaminase concentrations (>3 × the upper limit of normal), bilirubin (>2 × the upper limit of normal), and prolonged prothrombin times are indicators of hepatic injury.2 Caution should be taken to prevent polypharmacy with multiple hepatotoxic medications and over-the-counter use of hepatotoxic drugs and supplements.
When choosing a psychotropic, take into account patient-specific factors, such as underlying liver disease and alcohol consumption. Patients on potentially hepatotoxic medications should be counseled to recognize and report symptoms of liver dysfunction, including nausea, vomiting, jaundice, and lower-extremity edema.2 If liver injury occurs, modify therapy with the potential offending agent and check liver function periodically.
Related Resourcesa
• Bleibel W, Kim S, D’Silva K, et al. Drug-induced liver injury: review article. Dig Dis Sci. 2007;52(10):2463-2471.
• U.S. National Library of Medicine. LiverTox. National Institute of Health. www.livertox.nih.gov.
Drug Brand Names
Amitriptyline • Elavil Lurasidone • Latuda
Molindone • Moban Molindone • Moban
Aripiprazole • Abilify Nefazodone • Serzone
Asenapine • Saphris Nortriptyline • Pamelor
Bupropion XL • Wellbutrin XL Olanzapine • Zyprexa
Citalopram • Celexa Oxcarbazepine • Trileptal
Carbamazepine • Tegretol Paroxetine • Paxil
Chlordiazepoxide • Librium Perphenazine • Trilafon
Chlorpromazine • Thorazine Phenobarbital • Luminal
Clonazepam • Klonopin Phenytoin • Dilantin
Clozapine • Clozaril Quetiapine • Seroquel
Desvenlafaxine • Pristiq Risperidone • Risperdal
Diazepam • Valium Sertraline • Zoloft
Duloxetine • Cymbalta Thiothixene • Navane
Escitalopram • Lexapro Trazodone • Desyrel
Fluoxetine • Prozac Trifluoperazine • Stelazine
Fluphenazine • Prolixin Topiramate • Topamax
Flurazepam • Dalmane Valproic acid • Depakote
Haloperidol • Haldol Venlafaxine • Effexor
Iloperidone • Fanapt Ziprasidone • Geodon
Lamotrigine • Lamictal
Levocarnitine • L-carnitine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pugh AJ, Barve AJ, Falkner K, et al. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13(2):277-294.
2. Sedky K, Nazir R, Joshi A, et al. Which psychotropic medications induce hepatotoxicity? Gen Hosp Psychiatry. 2012;34(1):53-61.
3. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10):1135-1151.
4. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
5. Murray KF, Hadzic N, Wirth S, et al. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47(4):395-405.
6. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293.
7. Bryant AE 3rd, Dreifuss FE. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46(2):465-469.
1. Pugh AJ, Barve AJ, Falkner K, et al. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13(2):277-294.
2. Sedky K, Nazir R, Joshi A, et al. Which psychotropic medications induce hepatotoxicity? Gen Hosp Psychiatry. 2012;34(1):53-61.
3. Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10):1135-1151.
4. Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin Liver Dis. 2002;22(2):169-183.
5. Murray KF, Hadzic N, Wirth S, et al. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47(4):395-405.
6. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293.
7. Bryant AE 3rd, Dreifuss FE. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology. 1996;46(2):465-469.
Good, bad, and ugly: Prior authorization and medicolegal risk
Dear Dr. Mossman,
Where I practice, most health care plans won’t pay for certain medications without giving prior authorization (PA). Completing PA forms and making telephone calls take up time that could be better spent treating patients. I’m tempted to set a new policy of not doing PAs. If I do, might I face legal trouble?
Submitted by “Dr. A”
If you provide clinical care, you’ve probably dealt with third-party payers who require prior authorization (PA) before they will pay for certain treatments. Dr. A is not alone in feeling exasperated about the time it takes to complete a PA.1 After spending several hours each month waiting on hold and wading through stacks of paperwork, you may feel like Dr. A and consider refusing to do any more PAs.
But is Dr. A’s proposed solution a good idea? To address this question and the frustration that lies behind it, we’ll take a cue from Italian film director Sergio Leone and discuss:
• how PAs affect psychiatric care: the good, the bad, and the ugly
• potential exposure to professional liability and ethics complaints that might result from refusing or failing to seek PA
• strategies to reduce the burden of PAs while providing efficient, effective care.
The good
Recent decades have witnessed huge increases in spending on prescription medication. Psychotropics are no exception; state Medicaid spending for anti-psychotic medication grew from <$1 billion in 1995 to >$5.5 billion in 2005.2
Requiring a PA for expensive drugs is one way that third-party payers try to rein in costs and hold down insurance premiums. Imposing financial constraints often is just one aim of a pharmacy benefit management (PBM) program. Insurers also justify PBMs by pointing out that feedback to practitioners whose prescribing falls well outside the norm—in the form of mailed warnings, physician second opinions, or pharmacist consultation—can improve patient safety and encourage appropriate treatment options for enrolled patients.3,4 Examples of such benefits include reducing overuse of prescription opioids5 and antipsychotics among children,3 misuse of buprenorphine,6 and adverse effects from potentially inappropriate prescriptions.7
The bad
The bad news for doctors: Cost savings for payers come at the expense of providers and their practices, in the form of time spent doing paperwork and talking on the phone to complete PAs or contest PA decisions.8 Addressing PA requests costs an estimated $83,000 per physician per year. The total administrative burden for all 835,000 physicians who practice in the United States therefore is 868,000,000 hours, or $69 billion annually.9
To make matters worse, PA requirements may increase the overall cost of care. After Georgia Medicaid instituted PA requirements for second-generation antipsychotics (SGAs), average monthly per member drug costs fell $19.62, but average monthly outpatient treatment costs rose $31.59 per member.10 Pharmacy savings that result from requiring PAs for SGAs can be offset quickly by small increases in the hospitalization rate or emergency department visits.9,11
The ugly
Many physicians believe that the PA process undermines patient care by decreasing time devoted to direct patient contact, incentivizing suboptimal treatment, and limiting medication access.1,12,13 But do any data support this belief? Do PAs impede treatment for vulnerable persons with severe mental illnesses?
The answer, some studies suggest, is “Yes.” A Maine Medicaid PA policy slowed initiation of treatment for bipolar disorder by reducing the rate of starting non-preferred medications, although the same policy had no impact on patients already receiving treatment.14 Another study examined the effect of PA processes for inpatient psychiatry treatment and found that patients were less likely to be admitted on weekends, probably because PA review was not available on those days.15 A third study showed that PA requirements and resulting impediments to getting refills were correlated with medication discontinuation by patients with schizophrenia or bipolar disorder, which can increase the risk of decompensation, work-related problems, and hospitalization.16
Problems with PAs
Whether they are helpful or counterproductive, PAs are a practice reality. Dr. A’s proposed solution sounds appealing, but it might create ethical and legal problems.
Among the fundamental elements of ethical medical practice is physicians’ obligation to give patients “guidance … as to the optimal course of action” and to “advocate for patients in dealing with third parties when appropriate.”17 It’s fine for psychiatrists to consider prescribing treatments that patients’ health care coverage favors, but we also have to help patients weigh and evaluate costs, particularly when patients’ circumstances and medical interests militate strongly for options that third-party payers balk at paying for. Patients’ interests—not what’s expedient—are always physicians’ foremost concern.18
Beyond purely ethical considerations, you might face legal consequences if you refuse or fail to seek PAs for what you think is the proper medication. As Table 1 shows, one key factor is whether you are under contract with the patient’s insurance carrier; if you are, failure to seek a PA when appropriate may constitute a breach of the contract (Donna Vanderpool, written communication, October 5, 2014).
If the prescribed medication does not meet the standard of care and your patient suffers some harm, a licensing board complaint and investigation are possible. You also face exposure to a medical malpractice action. Although we do not know of any instances in which such an action has succeeded, 2 recent court decisions suggest that harm to a patient stemmed from failing to seek PA for a medication could constitute grounds for a lawsuit.19,20 Efforts to contain medical costs have been around for decades, and courts have held that physicians, third-party payers, and utilization review intermediaries are bound by “the standard of reasonable community practice”21 and should not let cost limitations “corrupt medical judgment.”22 Physicians who do not appeal limitations at odds with their medical judgment might bear responsibility for any injuries that occur.18,22
Managing PA requests
Given the inevitability of encountering PA requests and your ethical and professional obligations to help patients, what can you do (Table 29,23,27)?
Some practitioners charge patients for time spent completing PAs.23 Although physicians should “complete without charge the appropriate ‘simplified’ insurance claim form as a part of service to the patient;” they also may consider “a charge for more complex or multiple forms … in conformity with local custom.”24 Legally, physicians’ contracts with insurance panels may preclude charging such fees, but if a patient is being seen out of network, the physician does not have a contractual obligation and may charge.9 If your practice setting lets you choose which insurances you accept, the impact and burden of seeking PAs is a factor to consider when deciding whether to participate in a particular panel.23
In an interesting twist, an Ohio physician successfully sued a medical insurance administrator for the cost of his time responding to PA inquiries.25 Reasoning that the insurance administrator “should expect to pay for the reasonable value of” the doctor’s time because the PAs “were solely intended for the benefit of the insurance administrator” or parties whom the administrator served, the judge awarded the doctor $187.50 plus 8% interest.
Considerations that are more practical relate to how to triage and address the volume of PA requests. Some large medical practices centralize PAs and try to set up pre-approved plans of care or blanket approvals for frequently encountered conditions. Centralization also allows one key administrative assistant to develop skills in processing PA requests and to build relationships with payers.26
The administrative assistant also can compile lists of preferred alternative medications, PA forms, and payer Web sites. Using and submitting requests through payer Web sites can speed up PA processing, which saves time and money.27 As electronic health records improve, they may incorporate patients’ formularies and provide automatic alerts for required PAs.23
Patients should be involved, too. They can help to obtain relevant formulary information and to weigh alternative therapies. You can help them understand your role in the PA process, the reasoning behind your treatment recommendations, and the delays in picking up prescribed medications that waiting for PA approval can create.
It’s easy to get angry about PAs
Your best response, however, is to practice prudent and—within reason— cost-effective medicine. When generic or insurer-preferred medications are clinically appropriate and meet treatment guidelines, trying them first is sensible and defensible. If your patient fails the initial low-cost treatment, or if a low-cost choice isn’t appropriate, document this clearly and seek approval for a costlier treatment.9
BOTTOM LINE
Physicians have ethical and legal obligations to advocate for their patients’ needs and best interests. This sometimes includes completing prior authorization requests. Find strategies that minimize hassle and make sense in your practice, and seek efficient ways to document the medical necessity of requested tests, procedures, or therapies.
Acknowledgment
Drs. Marett and Mossman thanks Donna Vanderpool, MBA, JD, and Annette Reynolds, MD, for their helpful input in preparing this article.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Brown CM, Richards K, Rascati KL, et al. Effects of a psychotherapeutic drug prior authorization (PA) requirement on patients and providers: a providers’ perspective. Adm Policy Ment Health. 2008;35(3):181-188.
2. Law MR, Ross-Degnan D, Soumerai SB. Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatr Serv. 2008;59(5):540-546.
3. Stein BD, Leckman-Westin E, Okeke E, et al. The effects of prior authorization policies on Medicaid-enrolled children’s use of antipsychotic medications: evidence from two Mid-Atlantic states. J Child Adolesc Psychopharmacol. 2014;24(7):374-381.
4. Adams KT. Prior authorization–still used, still an issue. Biotechnol Healthc. 2010;7(4):28.
5. Garcia MM, Angelini MC, Thomas T, et al. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Pharm. 2014;20(5):447-454.
6. Clark RE, Baxter JD, Barton BA, et al. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence [published online July 9, 2014]. Health Serv Res. doi: 10.1111/1475-6773.12201.
7. Dunn RL, Harrison D, Ripley TL. The beers criteria as an outpatient screening tool for potentially inappropriate medications. Consult Pharm. 2011;26(10):754-763.
8. Lennertz MD, Wertheimer AI. Is prior authorization for prescribed drugs cost-effective? Drug Benefit Trends. 2008;20:136-139.
9. Bendix J. The prior authorization predicament. Med Econ. 2014;91(13)29-30,32,34-35.
10. Farley JF, Cline RR, Schommer JC, et al. Retrospective assessment of Medicaid step-therapy prior authorization policy for atypical antipsychotic medications. Clin Ther. 2008;30(8):1524-1539; discussion 1506-1507.
11. Abouzaid S, Jutkowitz E, Foley KA, et al. Economic impact of prior authorization policies for atypical antipsychotics in the treatment of schizophrenia. Popul Health Manag. 2010;13(5):247-254.
12. Brown CM, Nwokeji E, Rascati KL, et al. Development of the burden of prior authorization of psychotherapeutics (BoPAP) scale to assess the effects of prior authorization among Texas Medicaid providers. Adm Policy Ment Health. 2009;36(4):278-287.
13. Rascati KL, Brown CM. Prior authorization for antipsychotic medications—It’s not just about the money. Clin Ther. 2008;30(8):1506-1507.
14. Lu CY, Soumerai SB, Ross-Degnan D, et al. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar disorder. Med Care. 2010;48(1):4-9.
15. Stephens RJ, White SE, Cudnik M, et al. Factors associated with longer lengths of stay for mental health emergency department patients. J Emerg Med. 2014; 47(4):412-419.
16. Brown JD, Barrett A, Caffery E, et al. Medication continuity among Medicaid beneficiaries with schizophrenia and bipolar disorder. Psychiatr Serv. 2013;64(9):878-885.
17. American Medical Association. Opinion 10.01– Fundamental elements of the patient-physician relationship. http://www.ama-assn.org/ama/pub/ physician-resources/medical-ethics/code-medical-ethics/opinion1001.page?. Accessed October 11, 2014.
18. Hall RC. Ethical and legal implications of managed care. Gen Hosp Psychiatry. 1997;19(3):200-208.
19. Porter v Thadani, 2010 U.S. Dist. LEXIS 35145 (NH 2010).
20. NB ex rel Peacock v District of Columbia, 682 F3d 77 (DC Cir 2012).
21. Wilson v Blue Cross of Southern California, 222 Cal App 3d 660, 271 Cal Rptr 876 (1990).
22. Wickline v State of California, 192 Cal App 3d 1630, 239 Cal Rptr 810 (1986).
23. Terry K. Prior authorization made easier. Med Econ. 2007;84(20):34,38,40.
24. American Medical Association. Ethics Opinion 6.07– Insurance forms completion charges. http://www. ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion607.page? Updated June 1994. Accessed October 11, 2014.
25. Gibson v Medco Health Solutions, 06-CVF-106 (OH 2008).
26. Bendix J. Curing the prior authorization headache. Med Econ. 2013;90(19):24,26-27,29-31.
27. American Medical Association. Electronic prior authorization toolkit. Available at http://www.ama-assn.org/ama/pub/advocacy/topics/administrative-simplification-initiatives/electronic-transactions-toolkit/ prior-authorization.page. Accessed October 11, 2014.
Dear Dr. Mossman,
Where I practice, most health care plans won’t pay for certain medications without giving prior authorization (PA). Completing PA forms and making telephone calls take up time that could be better spent treating patients. I’m tempted to set a new policy of not doing PAs. If I do, might I face legal trouble?
Submitted by “Dr. A”
If you provide clinical care, you’ve probably dealt with third-party payers who require prior authorization (PA) before they will pay for certain treatments. Dr. A is not alone in feeling exasperated about the time it takes to complete a PA.1 After spending several hours each month waiting on hold and wading through stacks of paperwork, you may feel like Dr. A and consider refusing to do any more PAs.
But is Dr. A’s proposed solution a good idea? To address this question and the frustration that lies behind it, we’ll take a cue from Italian film director Sergio Leone and discuss:
• how PAs affect psychiatric care: the good, the bad, and the ugly
• potential exposure to professional liability and ethics complaints that might result from refusing or failing to seek PA
• strategies to reduce the burden of PAs while providing efficient, effective care.
The good
Recent decades have witnessed huge increases in spending on prescription medication. Psychotropics are no exception; state Medicaid spending for anti-psychotic medication grew from <$1 billion in 1995 to >$5.5 billion in 2005.2
Requiring a PA for expensive drugs is one way that third-party payers try to rein in costs and hold down insurance premiums. Imposing financial constraints often is just one aim of a pharmacy benefit management (PBM) program. Insurers also justify PBMs by pointing out that feedback to practitioners whose prescribing falls well outside the norm—in the form of mailed warnings, physician second opinions, or pharmacist consultation—can improve patient safety and encourage appropriate treatment options for enrolled patients.3,4 Examples of such benefits include reducing overuse of prescription opioids5 and antipsychotics among children,3 misuse of buprenorphine,6 and adverse effects from potentially inappropriate prescriptions.7
The bad
The bad news for doctors: Cost savings for payers come at the expense of providers and their practices, in the form of time spent doing paperwork and talking on the phone to complete PAs or contest PA decisions.8 Addressing PA requests costs an estimated $83,000 per physician per year. The total administrative burden for all 835,000 physicians who practice in the United States therefore is 868,000,000 hours, or $69 billion annually.9
To make matters worse, PA requirements may increase the overall cost of care. After Georgia Medicaid instituted PA requirements for second-generation antipsychotics (SGAs), average monthly per member drug costs fell $19.62, but average monthly outpatient treatment costs rose $31.59 per member.10 Pharmacy savings that result from requiring PAs for SGAs can be offset quickly by small increases in the hospitalization rate or emergency department visits.9,11
The ugly
Many physicians believe that the PA process undermines patient care by decreasing time devoted to direct patient contact, incentivizing suboptimal treatment, and limiting medication access.1,12,13 But do any data support this belief? Do PAs impede treatment for vulnerable persons with severe mental illnesses?
The answer, some studies suggest, is “Yes.” A Maine Medicaid PA policy slowed initiation of treatment for bipolar disorder by reducing the rate of starting non-preferred medications, although the same policy had no impact on patients already receiving treatment.14 Another study examined the effect of PA processes for inpatient psychiatry treatment and found that patients were less likely to be admitted on weekends, probably because PA review was not available on those days.15 A third study showed that PA requirements and resulting impediments to getting refills were correlated with medication discontinuation by patients with schizophrenia or bipolar disorder, which can increase the risk of decompensation, work-related problems, and hospitalization.16
Problems with PAs
Whether they are helpful or counterproductive, PAs are a practice reality. Dr. A’s proposed solution sounds appealing, but it might create ethical and legal problems.
Among the fundamental elements of ethical medical practice is physicians’ obligation to give patients “guidance … as to the optimal course of action” and to “advocate for patients in dealing with third parties when appropriate.”17 It’s fine for psychiatrists to consider prescribing treatments that patients’ health care coverage favors, but we also have to help patients weigh and evaluate costs, particularly when patients’ circumstances and medical interests militate strongly for options that third-party payers balk at paying for. Patients’ interests—not what’s expedient—are always physicians’ foremost concern.18
Beyond purely ethical considerations, you might face legal consequences if you refuse or fail to seek PAs for what you think is the proper medication. As Table 1 shows, one key factor is whether you are under contract with the patient’s insurance carrier; if you are, failure to seek a PA when appropriate may constitute a breach of the contract (Donna Vanderpool, written communication, October 5, 2014).
If the prescribed medication does not meet the standard of care and your patient suffers some harm, a licensing board complaint and investigation are possible. You also face exposure to a medical malpractice action. Although we do not know of any instances in which such an action has succeeded, 2 recent court decisions suggest that harm to a patient stemmed from failing to seek PA for a medication could constitute grounds for a lawsuit.19,20 Efforts to contain medical costs have been around for decades, and courts have held that physicians, third-party payers, and utilization review intermediaries are bound by “the standard of reasonable community practice”21 and should not let cost limitations “corrupt medical judgment.”22 Physicians who do not appeal limitations at odds with their medical judgment might bear responsibility for any injuries that occur.18,22
Managing PA requests
Given the inevitability of encountering PA requests and your ethical and professional obligations to help patients, what can you do (Table 29,23,27)?
Some practitioners charge patients for time spent completing PAs.23 Although physicians should “complete without charge the appropriate ‘simplified’ insurance claim form as a part of service to the patient;” they also may consider “a charge for more complex or multiple forms … in conformity with local custom.”24 Legally, physicians’ contracts with insurance panels may preclude charging such fees, but if a patient is being seen out of network, the physician does not have a contractual obligation and may charge.9 If your practice setting lets you choose which insurances you accept, the impact and burden of seeking PAs is a factor to consider when deciding whether to participate in a particular panel.23
In an interesting twist, an Ohio physician successfully sued a medical insurance administrator for the cost of his time responding to PA inquiries.25 Reasoning that the insurance administrator “should expect to pay for the reasonable value of” the doctor’s time because the PAs “were solely intended for the benefit of the insurance administrator” or parties whom the administrator served, the judge awarded the doctor $187.50 plus 8% interest.
Considerations that are more practical relate to how to triage and address the volume of PA requests. Some large medical practices centralize PAs and try to set up pre-approved plans of care or blanket approvals for frequently encountered conditions. Centralization also allows one key administrative assistant to develop skills in processing PA requests and to build relationships with payers.26
The administrative assistant also can compile lists of preferred alternative medications, PA forms, and payer Web sites. Using and submitting requests through payer Web sites can speed up PA processing, which saves time and money.27 As electronic health records improve, they may incorporate patients’ formularies and provide automatic alerts for required PAs.23
Patients should be involved, too. They can help to obtain relevant formulary information and to weigh alternative therapies. You can help them understand your role in the PA process, the reasoning behind your treatment recommendations, and the delays in picking up prescribed medications that waiting for PA approval can create.
It’s easy to get angry about PAs
Your best response, however, is to practice prudent and—within reason— cost-effective medicine. When generic or insurer-preferred medications are clinically appropriate and meet treatment guidelines, trying them first is sensible and defensible. If your patient fails the initial low-cost treatment, or if a low-cost choice isn’t appropriate, document this clearly and seek approval for a costlier treatment.9
BOTTOM LINE
Physicians have ethical and legal obligations to advocate for their patients’ needs and best interests. This sometimes includes completing prior authorization requests. Find strategies that minimize hassle and make sense in your practice, and seek efficient ways to document the medical necessity of requested tests, procedures, or therapies.
Acknowledgment
Drs. Marett and Mossman thanks Donna Vanderpool, MBA, JD, and Annette Reynolds, MD, for their helpful input in preparing this article.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dear Dr. Mossman,
Where I practice, most health care plans won’t pay for certain medications without giving prior authorization (PA). Completing PA forms and making telephone calls take up time that could be better spent treating patients. I’m tempted to set a new policy of not doing PAs. If I do, might I face legal trouble?
Submitted by “Dr. A”
If you provide clinical care, you’ve probably dealt with third-party payers who require prior authorization (PA) before they will pay for certain treatments. Dr. A is not alone in feeling exasperated about the time it takes to complete a PA.1 After spending several hours each month waiting on hold and wading through stacks of paperwork, you may feel like Dr. A and consider refusing to do any more PAs.
But is Dr. A’s proposed solution a good idea? To address this question and the frustration that lies behind it, we’ll take a cue from Italian film director Sergio Leone and discuss:
• how PAs affect psychiatric care: the good, the bad, and the ugly
• potential exposure to professional liability and ethics complaints that might result from refusing or failing to seek PA
• strategies to reduce the burden of PAs while providing efficient, effective care.
The good
Recent decades have witnessed huge increases in spending on prescription medication. Psychotropics are no exception; state Medicaid spending for anti-psychotic medication grew from <$1 billion in 1995 to >$5.5 billion in 2005.2
Requiring a PA for expensive drugs is one way that third-party payers try to rein in costs and hold down insurance premiums. Imposing financial constraints often is just one aim of a pharmacy benefit management (PBM) program. Insurers also justify PBMs by pointing out that feedback to practitioners whose prescribing falls well outside the norm—in the form of mailed warnings, physician second opinions, or pharmacist consultation—can improve patient safety and encourage appropriate treatment options for enrolled patients.3,4 Examples of such benefits include reducing overuse of prescription opioids5 and antipsychotics among children,3 misuse of buprenorphine,6 and adverse effects from potentially inappropriate prescriptions.7
The bad
The bad news for doctors: Cost savings for payers come at the expense of providers and their practices, in the form of time spent doing paperwork and talking on the phone to complete PAs or contest PA decisions.8 Addressing PA requests costs an estimated $83,000 per physician per year. The total administrative burden for all 835,000 physicians who practice in the United States therefore is 868,000,000 hours, or $69 billion annually.9
To make matters worse, PA requirements may increase the overall cost of care. After Georgia Medicaid instituted PA requirements for second-generation antipsychotics (SGAs), average monthly per member drug costs fell $19.62, but average monthly outpatient treatment costs rose $31.59 per member.10 Pharmacy savings that result from requiring PAs for SGAs can be offset quickly by small increases in the hospitalization rate or emergency department visits.9,11
The ugly
Many physicians believe that the PA process undermines patient care by decreasing time devoted to direct patient contact, incentivizing suboptimal treatment, and limiting medication access.1,12,13 But do any data support this belief? Do PAs impede treatment for vulnerable persons with severe mental illnesses?
The answer, some studies suggest, is “Yes.” A Maine Medicaid PA policy slowed initiation of treatment for bipolar disorder by reducing the rate of starting non-preferred medications, although the same policy had no impact on patients already receiving treatment.14 Another study examined the effect of PA processes for inpatient psychiatry treatment and found that patients were less likely to be admitted on weekends, probably because PA review was not available on those days.15 A third study showed that PA requirements and resulting impediments to getting refills were correlated with medication discontinuation by patients with schizophrenia or bipolar disorder, which can increase the risk of decompensation, work-related problems, and hospitalization.16
Problems with PAs
Whether they are helpful or counterproductive, PAs are a practice reality. Dr. A’s proposed solution sounds appealing, but it might create ethical and legal problems.
Among the fundamental elements of ethical medical practice is physicians’ obligation to give patients “guidance … as to the optimal course of action” and to “advocate for patients in dealing with third parties when appropriate.”17 It’s fine for psychiatrists to consider prescribing treatments that patients’ health care coverage favors, but we also have to help patients weigh and evaluate costs, particularly when patients’ circumstances and medical interests militate strongly for options that third-party payers balk at paying for. Patients’ interests—not what’s expedient—are always physicians’ foremost concern.18
Beyond purely ethical considerations, you might face legal consequences if you refuse or fail to seek PAs for what you think is the proper medication. As Table 1 shows, one key factor is whether you are under contract with the patient’s insurance carrier; if you are, failure to seek a PA when appropriate may constitute a breach of the contract (Donna Vanderpool, written communication, October 5, 2014).
If the prescribed medication does not meet the standard of care and your patient suffers some harm, a licensing board complaint and investigation are possible. You also face exposure to a medical malpractice action. Although we do not know of any instances in which such an action has succeeded, 2 recent court decisions suggest that harm to a patient stemmed from failing to seek PA for a medication could constitute grounds for a lawsuit.19,20 Efforts to contain medical costs have been around for decades, and courts have held that physicians, third-party payers, and utilization review intermediaries are bound by “the standard of reasonable community practice”21 and should not let cost limitations “corrupt medical judgment.”22 Physicians who do not appeal limitations at odds with their medical judgment might bear responsibility for any injuries that occur.18,22
Managing PA requests
Given the inevitability of encountering PA requests and your ethical and professional obligations to help patients, what can you do (Table 29,23,27)?
Some practitioners charge patients for time spent completing PAs.23 Although physicians should “complete without charge the appropriate ‘simplified’ insurance claim form as a part of service to the patient;” they also may consider “a charge for more complex or multiple forms … in conformity with local custom.”24 Legally, physicians’ contracts with insurance panels may preclude charging such fees, but if a patient is being seen out of network, the physician does not have a contractual obligation and may charge.9 If your practice setting lets you choose which insurances you accept, the impact and burden of seeking PAs is a factor to consider when deciding whether to participate in a particular panel.23
In an interesting twist, an Ohio physician successfully sued a medical insurance administrator for the cost of his time responding to PA inquiries.25 Reasoning that the insurance administrator “should expect to pay for the reasonable value of” the doctor’s time because the PAs “were solely intended for the benefit of the insurance administrator” or parties whom the administrator served, the judge awarded the doctor $187.50 plus 8% interest.
Considerations that are more practical relate to how to triage and address the volume of PA requests. Some large medical practices centralize PAs and try to set up pre-approved plans of care or blanket approvals for frequently encountered conditions. Centralization also allows one key administrative assistant to develop skills in processing PA requests and to build relationships with payers.26
The administrative assistant also can compile lists of preferred alternative medications, PA forms, and payer Web sites. Using and submitting requests through payer Web sites can speed up PA processing, which saves time and money.27 As electronic health records improve, they may incorporate patients’ formularies and provide automatic alerts for required PAs.23
Patients should be involved, too. They can help to obtain relevant formulary information and to weigh alternative therapies. You can help them understand your role in the PA process, the reasoning behind your treatment recommendations, and the delays in picking up prescribed medications that waiting for PA approval can create.
It’s easy to get angry about PAs
Your best response, however, is to practice prudent and—within reason— cost-effective medicine. When generic or insurer-preferred medications are clinically appropriate and meet treatment guidelines, trying them first is sensible and defensible. If your patient fails the initial low-cost treatment, or if a low-cost choice isn’t appropriate, document this clearly and seek approval for a costlier treatment.9
BOTTOM LINE
Physicians have ethical and legal obligations to advocate for their patients’ needs and best interests. This sometimes includes completing prior authorization requests. Find strategies that minimize hassle and make sense in your practice, and seek efficient ways to document the medical necessity of requested tests, procedures, or therapies.
Acknowledgment
Drs. Marett and Mossman thanks Donna Vanderpool, MBA, JD, and Annette Reynolds, MD, for their helpful input in preparing this article.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Brown CM, Richards K, Rascati KL, et al. Effects of a psychotherapeutic drug prior authorization (PA) requirement on patients and providers: a providers’ perspective. Adm Policy Ment Health. 2008;35(3):181-188.
2. Law MR, Ross-Degnan D, Soumerai SB. Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatr Serv. 2008;59(5):540-546.
3. Stein BD, Leckman-Westin E, Okeke E, et al. The effects of prior authorization policies on Medicaid-enrolled children’s use of antipsychotic medications: evidence from two Mid-Atlantic states. J Child Adolesc Psychopharmacol. 2014;24(7):374-381.
4. Adams KT. Prior authorization–still used, still an issue. Biotechnol Healthc. 2010;7(4):28.
5. Garcia MM, Angelini MC, Thomas T, et al. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Pharm. 2014;20(5):447-454.
6. Clark RE, Baxter JD, Barton BA, et al. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence [published online July 9, 2014]. Health Serv Res. doi: 10.1111/1475-6773.12201.
7. Dunn RL, Harrison D, Ripley TL. The beers criteria as an outpatient screening tool for potentially inappropriate medications. Consult Pharm. 2011;26(10):754-763.
8. Lennertz MD, Wertheimer AI. Is prior authorization for prescribed drugs cost-effective? Drug Benefit Trends. 2008;20:136-139.
9. Bendix J. The prior authorization predicament. Med Econ. 2014;91(13)29-30,32,34-35.
10. Farley JF, Cline RR, Schommer JC, et al. Retrospective assessment of Medicaid step-therapy prior authorization policy for atypical antipsychotic medications. Clin Ther. 2008;30(8):1524-1539; discussion 1506-1507.
11. Abouzaid S, Jutkowitz E, Foley KA, et al. Economic impact of prior authorization policies for atypical antipsychotics in the treatment of schizophrenia. Popul Health Manag. 2010;13(5):247-254.
12. Brown CM, Nwokeji E, Rascati KL, et al. Development of the burden of prior authorization of psychotherapeutics (BoPAP) scale to assess the effects of prior authorization among Texas Medicaid providers. Adm Policy Ment Health. 2009;36(4):278-287.
13. Rascati KL, Brown CM. Prior authorization for antipsychotic medications—It’s not just about the money. Clin Ther. 2008;30(8):1506-1507.
14. Lu CY, Soumerai SB, Ross-Degnan D, et al. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar disorder. Med Care. 2010;48(1):4-9.
15. Stephens RJ, White SE, Cudnik M, et al. Factors associated with longer lengths of stay for mental health emergency department patients. J Emerg Med. 2014; 47(4):412-419.
16. Brown JD, Barrett A, Caffery E, et al. Medication continuity among Medicaid beneficiaries with schizophrenia and bipolar disorder. Psychiatr Serv. 2013;64(9):878-885.
17. American Medical Association. Opinion 10.01– Fundamental elements of the patient-physician relationship. http://www.ama-assn.org/ama/pub/ physician-resources/medical-ethics/code-medical-ethics/opinion1001.page?. Accessed October 11, 2014.
18. Hall RC. Ethical and legal implications of managed care. Gen Hosp Psychiatry. 1997;19(3):200-208.
19. Porter v Thadani, 2010 U.S. Dist. LEXIS 35145 (NH 2010).
20. NB ex rel Peacock v District of Columbia, 682 F3d 77 (DC Cir 2012).
21. Wilson v Blue Cross of Southern California, 222 Cal App 3d 660, 271 Cal Rptr 876 (1990).
22. Wickline v State of California, 192 Cal App 3d 1630, 239 Cal Rptr 810 (1986).
23. Terry K. Prior authorization made easier. Med Econ. 2007;84(20):34,38,40.
24. American Medical Association. Ethics Opinion 6.07– Insurance forms completion charges. http://www. ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion607.page? Updated June 1994. Accessed October 11, 2014.
25. Gibson v Medco Health Solutions, 06-CVF-106 (OH 2008).
26. Bendix J. Curing the prior authorization headache. Med Econ. 2013;90(19):24,26-27,29-31.
27. American Medical Association. Electronic prior authorization toolkit. Available at http://www.ama-assn.org/ama/pub/advocacy/topics/administrative-simplification-initiatives/electronic-transactions-toolkit/ prior-authorization.page. Accessed October 11, 2014.
1. Brown CM, Richards K, Rascati KL, et al. Effects of a psychotherapeutic drug prior authorization (PA) requirement on patients and providers: a providers’ perspective. Adm Policy Ment Health. 2008;35(3):181-188.
2. Law MR, Ross-Degnan D, Soumerai SB. Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatr Serv. 2008;59(5):540-546.
3. Stein BD, Leckman-Westin E, Okeke E, et al. The effects of prior authorization policies on Medicaid-enrolled children’s use of antipsychotic medications: evidence from two Mid-Atlantic states. J Child Adolesc Psychopharmacol. 2014;24(7):374-381.
4. Adams KT. Prior authorization–still used, still an issue. Biotechnol Healthc. 2010;7(4):28.
5. Garcia MM, Angelini MC, Thomas T, et al. Implementation of an opioid management initiative by a state Medicaid program. J Manag Care Pharm. 2014;20(5):447-454.
6. Clark RE, Baxter JD, Barton BA, et al. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence [published online July 9, 2014]. Health Serv Res. doi: 10.1111/1475-6773.12201.
7. Dunn RL, Harrison D, Ripley TL. The beers criteria as an outpatient screening tool for potentially inappropriate medications. Consult Pharm. 2011;26(10):754-763.
8. Lennertz MD, Wertheimer AI. Is prior authorization for prescribed drugs cost-effective? Drug Benefit Trends. 2008;20:136-139.
9. Bendix J. The prior authorization predicament. Med Econ. 2014;91(13)29-30,32,34-35.
10. Farley JF, Cline RR, Schommer JC, et al. Retrospective assessment of Medicaid step-therapy prior authorization policy for atypical antipsychotic medications. Clin Ther. 2008;30(8):1524-1539; discussion 1506-1507.
11. Abouzaid S, Jutkowitz E, Foley KA, et al. Economic impact of prior authorization policies for atypical antipsychotics in the treatment of schizophrenia. Popul Health Manag. 2010;13(5):247-254.
12. Brown CM, Nwokeji E, Rascati KL, et al. Development of the burden of prior authorization of psychotherapeutics (BoPAP) scale to assess the effects of prior authorization among Texas Medicaid providers. Adm Policy Ment Health. 2009;36(4):278-287.
13. Rascati KL, Brown CM. Prior authorization for antipsychotic medications—It’s not just about the money. Clin Ther. 2008;30(8):1506-1507.
14. Lu CY, Soumerai SB, Ross-Degnan D, et al. Unintended impacts of a Medicaid prior authorization policy on access to medications for bipolar disorder. Med Care. 2010;48(1):4-9.
15. Stephens RJ, White SE, Cudnik M, et al. Factors associated with longer lengths of stay for mental health emergency department patients. J Emerg Med. 2014; 47(4):412-419.
16. Brown JD, Barrett A, Caffery E, et al. Medication continuity among Medicaid beneficiaries with schizophrenia and bipolar disorder. Psychiatr Serv. 2013;64(9):878-885.
17. American Medical Association. Opinion 10.01– Fundamental elements of the patient-physician relationship. http://www.ama-assn.org/ama/pub/ physician-resources/medical-ethics/code-medical-ethics/opinion1001.page?. Accessed October 11, 2014.
18. Hall RC. Ethical and legal implications of managed care. Gen Hosp Psychiatry. 1997;19(3):200-208.
19. Porter v Thadani, 2010 U.S. Dist. LEXIS 35145 (NH 2010).
20. NB ex rel Peacock v District of Columbia, 682 F3d 77 (DC Cir 2012).
21. Wilson v Blue Cross of Southern California, 222 Cal App 3d 660, 271 Cal Rptr 876 (1990).
22. Wickline v State of California, 192 Cal App 3d 1630, 239 Cal Rptr 810 (1986).
23. Terry K. Prior authorization made easier. Med Econ. 2007;84(20):34,38,40.
24. American Medical Association. Ethics Opinion 6.07– Insurance forms completion charges. http://www. ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion607.page? Updated June 1994. Accessed October 11, 2014.
25. Gibson v Medco Health Solutions, 06-CVF-106 (OH 2008).
26. Bendix J. Curing the prior authorization headache. Med Econ. 2013;90(19):24,26-27,29-31.
27. American Medical Association. Electronic prior authorization toolkit. Available at http://www.ama-assn.org/ama/pub/advocacy/topics/administrative-simplification-initiatives/electronic-transactions-toolkit/ prior-authorization.page. Accessed October 11, 2014.
Is your patient using cocaine to self-medicate undiagnosed ADHD?
Attention-deficit/hyperactivity disorder (ADHD) often persists beyond childhood into adulthood. One of the therapeutic challenges of treating ADHD is identifying comorbidities, including underlying mood and anxiety disorders, and ongoing substance abuse. Effective treatment modalities tend to prioritize management of substance abuse, but the patient’s age may dictate the overall assessment plan.
So-called 'reward' center
Treating childhood ADHD with stimulants might reduce the risk for future drug abuse.1 It is estimated that approximately 10 million people with ADHD are undiagnosed in the United States2; characteristic ADHD symptoms—inattention, hyperactivity, impulsivity—can persist in adulthood, and affected persons might not meet societal expectations. Previously unidentified attention difficulties may emerge during early adulthood because of increasingly complex tasks at school and work.
Persons with undiagnosed ADHD might turn to potentially self-destructive means of placating inner tension. Cocaine has pharmacological properties in common with stimulants such as methylphenidate, which often is prescribed for ADHD. Cocaine and methylphenidate both work on altering brain chemistry with a similar mechanism of action, allowing for increased dopamine in the nucleus accumbens, also known as the “reward center” of the brain.
Adults with ADHD have a 300% higher risk of developing a substance use disorder than adults without ADHD.3 An estimated 15% to 25% of adults with substance abuse have comorbid ADHD. Although these patients abuse of a variety of substances including Cannabis and alcohol, cocaine is one of the most commonly abused substances among this population. These observations could point to a self-medication hypothesis.
Why self-medicate?
The self-medication hypothesis, formulated by Khantzian in 1985, was based on several clinical observations. Khantzian stated that an abuser’s drug of choice is not selected at random but, rather, by an inherent desire to suppress the attributes of the condition that seems to otherwise wreak havoc on his (her) life. Almost a century earlier, Freud mentioned that cocaine is an antidepressant. Among persons with ADHD who have not been given that diagnosis, or treated for the disorder, cocaine is a popular drug. Because of the antidepressant features of cocaine and its ability to produce a rapid increase of dopamine levels that exert a pro-euphoric effect, coupled with a seemingly paradoxical calming influence that leads to increased productivity, it is not surprising to find that cocaine is abused. Reportedly, persons who have not been treated because their ADHD is undiagnosed turn to cocaine because it improves attention, raises self-esteem, and allows users to harness a level of focus that they could not otherwise achieve.4
Mechanism of action
Methylphenidate reduces ADHD symptoms by increasing extracellular dopamine in the brain, acting by means of a mechanism that is similar to that of cocaine.5 By blocking reuptake of dopamine and allowing an extracellular surplus, users continue to experience the pleasurable effect the neuro-transmitter produces. Methylphenidate has been shown to be an even more potent inhibitor of the same autoreceptors. Injecting methylphenidate has been shown to produce a rapid release of dopamine similar to that of cocaine.5
However, methylphenidate causes a much slower increase in dopamine; its effect on the brain has been shown to be similar to that of cocaine without the increased abuse potential. Cocaine use remodels the brain by reconfiguring connections that are essential for craving and self-control.5 Therefore, substituting methylphenidate for cocaine could help ADHD patients by:
• improving overall executive functioning
• decreasing feelings of low self-worth
• increasing daily functioning
• minimizing craving and the risk of subsequent cocaine abuse.
Treatment recommendations
Carefully consider pharmacodynamics and pharmacokinetics when prescribing ADHD medication. In general, children and adolescents with ADHD respond more favorably to stimulants than adults do. In children, the mainstay of treatment is slow-dose stimulants such as methylphenidate; second-line treatments are immediate-release stimulants and atomoxetine, a selective norepinephrine reuptake inhibitor.6 Adults with ADHD might benefit from a nonstimulant, in part because of the presence of complex comorbidities.6 Modafinil often is prescribed for adults with ADHD.
Atomoxetine readily increases norepinephrine and dopamine in the prefrontal cortex as it bypasses the nucleus accumbens. Although atomoxetine is not a stimulant, the efficacy of the drug is based on its ability to increase norepinephrine through selective inhibition of the norepinephrine transporter. Norepinephrine modulates higher cortical functions—attention, executive function, arousal—that lead to a reduction in hyperactivity, inattention, and impulsivity.
Because dopamine is released in the prefrontal cortex—not in the nucleus accumbens—the addiction potential of atomoxetine is low.7 The drug might be an effective intervention for patients who are using cocaine to self-medicate. Stimulants such as methylphenidate have proven effective in safely mimicking the mechanism of action of cocaine. Nonstimulants, such as atomoxetine and modafinil, lack abuse potential and are excellent options for treating adults with ADHD.
Clinicians generally are advised to treat a patient’s underlying ADHD symptoms before addressing ongoing substance abuse. If a patient abruptly discontinues cocaine use before ADHD symptoms are properly controlled, her (his) condition might deteriorate further and the treatment plan might fail to progress. Some patients have experienced a reduction in craving for cocaine after they began stimulant therapy; these people no longer felt a need to self-medicate because their symptoms were being addressed.4
1. Jain S, Jain R, Islam J. Do stimulants for ADHD increase the risk of substance use disorders? Current Psychiatry. 2011;10(8):20-24.
2. Baskin S. Adult ADHD—A common disorder, often missed. http://www.stevebaskinmd.com/articles-about-adultadhd.html. Published 2009. Accessed November 5, 2014.
3. Tuzee M. Many adults who have ADHD go undiagnosed.
http://abclocal.go.com/kabc/story?section=news/health/your_health&id=7657326. Published September 8, 2010. Accessed October 9, 2014.
4. Plume D. The self medication hypothesis: ADHD & chronic cocaine abuse. A literature review. http://www.addcentre.co.uk/selfmedcocaine.htm. Published April 1995. Accessed October 9, 2014.
5. Searight HR, Burke JM. Adult attention deficit hyperactivity disorder. UpToDate. Updated Feb 2011. Accessed November 5, 2014.
6. Stahl SM. Attention deficit disorder and its treatment. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:884-897.
7. Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53(2):112-120.
Attention-deficit/hyperactivity disorder (ADHD) often persists beyond childhood into adulthood. One of the therapeutic challenges of treating ADHD is identifying comorbidities, including underlying mood and anxiety disorders, and ongoing substance abuse. Effective treatment modalities tend to prioritize management of substance abuse, but the patient’s age may dictate the overall assessment plan.
So-called 'reward' center
Treating childhood ADHD with stimulants might reduce the risk for future drug abuse.1 It is estimated that approximately 10 million people with ADHD are undiagnosed in the United States2; characteristic ADHD symptoms—inattention, hyperactivity, impulsivity—can persist in adulthood, and affected persons might not meet societal expectations. Previously unidentified attention difficulties may emerge during early adulthood because of increasingly complex tasks at school and work.
Persons with undiagnosed ADHD might turn to potentially self-destructive means of placating inner tension. Cocaine has pharmacological properties in common with stimulants such as methylphenidate, which often is prescribed for ADHD. Cocaine and methylphenidate both work on altering brain chemistry with a similar mechanism of action, allowing for increased dopamine in the nucleus accumbens, also known as the “reward center” of the brain.
Adults with ADHD have a 300% higher risk of developing a substance use disorder than adults without ADHD.3 An estimated 15% to 25% of adults with substance abuse have comorbid ADHD. Although these patients abuse of a variety of substances including Cannabis and alcohol, cocaine is one of the most commonly abused substances among this population. These observations could point to a self-medication hypothesis.
Why self-medicate?
The self-medication hypothesis, formulated by Khantzian in 1985, was based on several clinical observations. Khantzian stated that an abuser’s drug of choice is not selected at random but, rather, by an inherent desire to suppress the attributes of the condition that seems to otherwise wreak havoc on his (her) life. Almost a century earlier, Freud mentioned that cocaine is an antidepressant. Among persons with ADHD who have not been given that diagnosis, or treated for the disorder, cocaine is a popular drug. Because of the antidepressant features of cocaine and its ability to produce a rapid increase of dopamine levels that exert a pro-euphoric effect, coupled with a seemingly paradoxical calming influence that leads to increased productivity, it is not surprising to find that cocaine is abused. Reportedly, persons who have not been treated because their ADHD is undiagnosed turn to cocaine because it improves attention, raises self-esteem, and allows users to harness a level of focus that they could not otherwise achieve.4
Mechanism of action
Methylphenidate reduces ADHD symptoms by increasing extracellular dopamine in the brain, acting by means of a mechanism that is similar to that of cocaine.5 By blocking reuptake of dopamine and allowing an extracellular surplus, users continue to experience the pleasurable effect the neuro-transmitter produces. Methylphenidate has been shown to be an even more potent inhibitor of the same autoreceptors. Injecting methylphenidate has been shown to produce a rapid release of dopamine similar to that of cocaine.5
However, methylphenidate causes a much slower increase in dopamine; its effect on the brain has been shown to be similar to that of cocaine without the increased abuse potential. Cocaine use remodels the brain by reconfiguring connections that are essential for craving and self-control.5 Therefore, substituting methylphenidate for cocaine could help ADHD patients by:
• improving overall executive functioning
• decreasing feelings of low self-worth
• increasing daily functioning
• minimizing craving and the risk of subsequent cocaine abuse.
Treatment recommendations
Carefully consider pharmacodynamics and pharmacokinetics when prescribing ADHD medication. In general, children and adolescents with ADHD respond more favorably to stimulants than adults do. In children, the mainstay of treatment is slow-dose stimulants such as methylphenidate; second-line treatments are immediate-release stimulants and atomoxetine, a selective norepinephrine reuptake inhibitor.6 Adults with ADHD might benefit from a nonstimulant, in part because of the presence of complex comorbidities.6 Modafinil often is prescribed for adults with ADHD.
Atomoxetine readily increases norepinephrine and dopamine in the prefrontal cortex as it bypasses the nucleus accumbens. Although atomoxetine is not a stimulant, the efficacy of the drug is based on its ability to increase norepinephrine through selective inhibition of the norepinephrine transporter. Norepinephrine modulates higher cortical functions—attention, executive function, arousal—that lead to a reduction in hyperactivity, inattention, and impulsivity.
Because dopamine is released in the prefrontal cortex—not in the nucleus accumbens—the addiction potential of atomoxetine is low.7 The drug might be an effective intervention for patients who are using cocaine to self-medicate. Stimulants such as methylphenidate have proven effective in safely mimicking the mechanism of action of cocaine. Nonstimulants, such as atomoxetine and modafinil, lack abuse potential and are excellent options for treating adults with ADHD.
Clinicians generally are advised to treat a patient’s underlying ADHD symptoms before addressing ongoing substance abuse. If a patient abruptly discontinues cocaine use before ADHD symptoms are properly controlled, her (his) condition might deteriorate further and the treatment plan might fail to progress. Some patients have experienced a reduction in craving for cocaine after they began stimulant therapy; these people no longer felt a need to self-medicate because their symptoms were being addressed.4
Attention-deficit/hyperactivity disorder (ADHD) often persists beyond childhood into adulthood. One of the therapeutic challenges of treating ADHD is identifying comorbidities, including underlying mood and anxiety disorders, and ongoing substance abuse. Effective treatment modalities tend to prioritize management of substance abuse, but the patient’s age may dictate the overall assessment plan.
So-called 'reward' center
Treating childhood ADHD with stimulants might reduce the risk for future drug abuse.1 It is estimated that approximately 10 million people with ADHD are undiagnosed in the United States2; characteristic ADHD symptoms—inattention, hyperactivity, impulsivity—can persist in adulthood, and affected persons might not meet societal expectations. Previously unidentified attention difficulties may emerge during early adulthood because of increasingly complex tasks at school and work.
Persons with undiagnosed ADHD might turn to potentially self-destructive means of placating inner tension. Cocaine has pharmacological properties in common with stimulants such as methylphenidate, which often is prescribed for ADHD. Cocaine and methylphenidate both work on altering brain chemistry with a similar mechanism of action, allowing for increased dopamine in the nucleus accumbens, also known as the “reward center” of the brain.
Adults with ADHD have a 300% higher risk of developing a substance use disorder than adults without ADHD.3 An estimated 15% to 25% of adults with substance abuse have comorbid ADHD. Although these patients abuse of a variety of substances including Cannabis and alcohol, cocaine is one of the most commonly abused substances among this population. These observations could point to a self-medication hypothesis.
Why self-medicate?
The self-medication hypothesis, formulated by Khantzian in 1985, was based on several clinical observations. Khantzian stated that an abuser’s drug of choice is not selected at random but, rather, by an inherent desire to suppress the attributes of the condition that seems to otherwise wreak havoc on his (her) life. Almost a century earlier, Freud mentioned that cocaine is an antidepressant. Among persons with ADHD who have not been given that diagnosis, or treated for the disorder, cocaine is a popular drug. Because of the antidepressant features of cocaine and its ability to produce a rapid increase of dopamine levels that exert a pro-euphoric effect, coupled with a seemingly paradoxical calming influence that leads to increased productivity, it is not surprising to find that cocaine is abused. Reportedly, persons who have not been treated because their ADHD is undiagnosed turn to cocaine because it improves attention, raises self-esteem, and allows users to harness a level of focus that they could not otherwise achieve.4
Mechanism of action
Methylphenidate reduces ADHD symptoms by increasing extracellular dopamine in the brain, acting by means of a mechanism that is similar to that of cocaine.5 By blocking reuptake of dopamine and allowing an extracellular surplus, users continue to experience the pleasurable effect the neuro-transmitter produces. Methylphenidate has been shown to be an even more potent inhibitor of the same autoreceptors. Injecting methylphenidate has been shown to produce a rapid release of dopamine similar to that of cocaine.5
However, methylphenidate causes a much slower increase in dopamine; its effect on the brain has been shown to be similar to that of cocaine without the increased abuse potential. Cocaine use remodels the brain by reconfiguring connections that are essential for craving and self-control.5 Therefore, substituting methylphenidate for cocaine could help ADHD patients by:
• improving overall executive functioning
• decreasing feelings of low self-worth
• increasing daily functioning
• minimizing craving and the risk of subsequent cocaine abuse.
Treatment recommendations
Carefully consider pharmacodynamics and pharmacokinetics when prescribing ADHD medication. In general, children and adolescents with ADHD respond more favorably to stimulants than adults do. In children, the mainstay of treatment is slow-dose stimulants such as methylphenidate; second-line treatments are immediate-release stimulants and atomoxetine, a selective norepinephrine reuptake inhibitor.6 Adults with ADHD might benefit from a nonstimulant, in part because of the presence of complex comorbidities.6 Modafinil often is prescribed for adults with ADHD.
Atomoxetine readily increases norepinephrine and dopamine in the prefrontal cortex as it bypasses the nucleus accumbens. Although atomoxetine is not a stimulant, the efficacy of the drug is based on its ability to increase norepinephrine through selective inhibition of the norepinephrine transporter. Norepinephrine modulates higher cortical functions—attention, executive function, arousal—that lead to a reduction in hyperactivity, inattention, and impulsivity.
Because dopamine is released in the prefrontal cortex—not in the nucleus accumbens—the addiction potential of atomoxetine is low.7 The drug might be an effective intervention for patients who are using cocaine to self-medicate. Stimulants such as methylphenidate have proven effective in safely mimicking the mechanism of action of cocaine. Nonstimulants, such as atomoxetine and modafinil, lack abuse potential and are excellent options for treating adults with ADHD.
Clinicians generally are advised to treat a patient’s underlying ADHD symptoms before addressing ongoing substance abuse. If a patient abruptly discontinues cocaine use before ADHD symptoms are properly controlled, her (his) condition might deteriorate further and the treatment plan might fail to progress. Some patients have experienced a reduction in craving for cocaine after they began stimulant therapy; these people no longer felt a need to self-medicate because their symptoms were being addressed.4
1. Jain S, Jain R, Islam J. Do stimulants for ADHD increase the risk of substance use disorders? Current Psychiatry. 2011;10(8):20-24.
2. Baskin S. Adult ADHD—A common disorder, often missed. http://www.stevebaskinmd.com/articles-about-adultadhd.html. Published 2009. Accessed November 5, 2014.
3. Tuzee M. Many adults who have ADHD go undiagnosed.
http://abclocal.go.com/kabc/story?section=news/health/your_health&id=7657326. Published September 8, 2010. Accessed October 9, 2014.
4. Plume D. The self medication hypothesis: ADHD & chronic cocaine abuse. A literature review. http://www.addcentre.co.uk/selfmedcocaine.htm. Published April 1995. Accessed October 9, 2014.
5. Searight HR, Burke JM. Adult attention deficit hyperactivity disorder. UpToDate. Updated Feb 2011. Accessed November 5, 2014.
6. Stahl SM. Attention deficit disorder and its treatment. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:884-897.
7. Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53(2):112-120.
1. Jain S, Jain R, Islam J. Do stimulants for ADHD increase the risk of substance use disorders? Current Psychiatry. 2011;10(8):20-24.
2. Baskin S. Adult ADHD—A common disorder, often missed. http://www.stevebaskinmd.com/articles-about-adultadhd.html. Published 2009. Accessed November 5, 2014.
3. Tuzee M. Many adults who have ADHD go undiagnosed.
http://abclocal.go.com/kabc/story?section=news/health/your_health&id=7657326. Published September 8, 2010. Accessed October 9, 2014.
4. Plume D. The self medication hypothesis: ADHD & chronic cocaine abuse. A literature review. http://www.addcentre.co.uk/selfmedcocaine.htm. Published April 1995. Accessed October 9, 2014.
5. Searight HR, Burke JM. Adult attention deficit hyperactivity disorder. UpToDate. Updated Feb 2011. Accessed November 5, 2014.
6. Stahl SM. Attention deficit disorder and its treatment. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:884-897.
7. Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53(2):112-120.