User login
Technique may predict progression in MM

Credit: Graham Colm
Scientists have found that a technique used in Earth science research may be able to help physicians predict the course of multiple myeloma (MM).
The team showed they could use calcium isotope analysis to predict whether MM patients are at risk for developing bone lesions.
The group believes this technique could be used to chart the progression or recurrence of MM and help tailor therapies to better protect patients’ bones.
“At present, there is no good way to track changes in bone balance, except retrospectively using X-ray methods,” said Ariel Anbar, PhD, of Arizona State University in Tempe. “By the time the X-rays show something, the damage has been done.”
“Right now, pain is usually the first indication that cancer is affecting the bones,” added Rafael Fonseca, MD, of the Mayo Clinic in Scottsdale, Arizona. “If we could detect it earlier by an analysis of urine or blood in high-risk patients, it could significantly improve their care.”
To explore this possibility, Drs Fonseca, Anbar, and their colleagues performed calcium isotope analysis using blood samples from MM patients. The team recounted their results in a letter to Leukemia.
As the name suggests, calcium isotope analysis measures calcium isotopes that are naturally present in blood. The technique makes use of a fact well known to Earth scientists but not normally used in biomedicine: different isotopes of a chemical element can react at slightly different rates.
An earlier study testing this technique in healthy subjects showed that when bones form, the lighter isotopes of calcium enter bone a little faster than the heavier isotopes. That difference, called isotope fractionation, is the key to the method.
In healthy, active humans, bone is forming at about the same rate as it resorbs. But if bone loss is occurring, the isotopic composition of blood becomes enriched in the lighter isotopes as bones resorb more quickly than they are formed.
The effect on calcium isotopes is very small, typically less than a 0.02% change in the isotope ratio. But even effects that small can be measured using precise mass spectrometry methods.
Using these methods, the researchers tested peripheral blood samples from 71 adult patients—55 with MM, 9 with smoldering MM, and 7 with monoclonal gammopathy of undetermined significance.
The researchers found an association between how active a patient’s disease was and the change in the isotope ratios. In fact, the isotope ratios predicted disease activity better than, and independent from, standard clinical variables.
Blood samples from patients with active disease had significantly lower mean calcium isotope compositions than samples from patients with non-active disease (mean δ44/42Ca=-0.8 vs -0.67, P=0.025), regardless of diagnosis.
For MM patients specifically, those with active disease had a significantly lower mean δ44/42Ca than patients with non-active disease (-0.79 vs -0.63, P=0.016), which was consistent with resorption of bone containing lighter 42Ca.
Dr Anbar noted that, although calcium isotope analysis has worked in this small set of patients, additional research is needed to verify these initial findings and improve the efficiency of analysis.
“If the method proves to be robust after more careful validation, it could provide earlier detection of bone involvement than presently possible,” he said, “and also provide the possibility to monitor the effectiveness of drugs to combat bone loss.” ![]()

Credit: Graham Colm
Scientists have found that a technique used in Earth science research may be able to help physicians predict the course of multiple myeloma (MM).
The team showed they could use calcium isotope analysis to predict whether MM patients are at risk for developing bone lesions.
The group believes this technique could be used to chart the progression or recurrence of MM and help tailor therapies to better protect patients’ bones.
“At present, there is no good way to track changes in bone balance, except retrospectively using X-ray methods,” said Ariel Anbar, PhD, of Arizona State University in Tempe. “By the time the X-rays show something, the damage has been done.”
“Right now, pain is usually the first indication that cancer is affecting the bones,” added Rafael Fonseca, MD, of the Mayo Clinic in Scottsdale, Arizona. “If we could detect it earlier by an analysis of urine or blood in high-risk patients, it could significantly improve their care.”
To explore this possibility, Drs Fonseca, Anbar, and their colleagues performed calcium isotope analysis using blood samples from MM patients. The team recounted their results in a letter to Leukemia.
As the name suggests, calcium isotope analysis measures calcium isotopes that are naturally present in blood. The technique makes use of a fact well known to Earth scientists but not normally used in biomedicine: different isotopes of a chemical element can react at slightly different rates.
An earlier study testing this technique in healthy subjects showed that when bones form, the lighter isotopes of calcium enter bone a little faster than the heavier isotopes. That difference, called isotope fractionation, is the key to the method.
In healthy, active humans, bone is forming at about the same rate as it resorbs. But if bone loss is occurring, the isotopic composition of blood becomes enriched in the lighter isotopes as bones resorb more quickly than they are formed.
The effect on calcium isotopes is very small, typically less than a 0.02% change in the isotope ratio. But even effects that small can be measured using precise mass spectrometry methods.
Using these methods, the researchers tested peripheral blood samples from 71 adult patients—55 with MM, 9 with smoldering MM, and 7 with monoclonal gammopathy of undetermined significance.
The researchers found an association between how active a patient’s disease was and the change in the isotope ratios. In fact, the isotope ratios predicted disease activity better than, and independent from, standard clinical variables.
Blood samples from patients with active disease had significantly lower mean calcium isotope compositions than samples from patients with non-active disease (mean δ44/42Ca=-0.8 vs -0.67, P=0.025), regardless of diagnosis.
For MM patients specifically, those with active disease had a significantly lower mean δ44/42Ca than patients with non-active disease (-0.79 vs -0.63, P=0.016), which was consistent with resorption of bone containing lighter 42Ca.
Dr Anbar noted that, although calcium isotope analysis has worked in this small set of patients, additional research is needed to verify these initial findings and improve the efficiency of analysis.
“If the method proves to be robust after more careful validation, it could provide earlier detection of bone involvement than presently possible,” he said, “and also provide the possibility to monitor the effectiveness of drugs to combat bone loss.” ![]()

Credit: Graham Colm
Scientists have found that a technique used in Earth science research may be able to help physicians predict the course of multiple myeloma (MM).
The team showed they could use calcium isotope analysis to predict whether MM patients are at risk for developing bone lesions.
The group believes this technique could be used to chart the progression or recurrence of MM and help tailor therapies to better protect patients’ bones.
“At present, there is no good way to track changes in bone balance, except retrospectively using X-ray methods,” said Ariel Anbar, PhD, of Arizona State University in Tempe. “By the time the X-rays show something, the damage has been done.”
“Right now, pain is usually the first indication that cancer is affecting the bones,” added Rafael Fonseca, MD, of the Mayo Clinic in Scottsdale, Arizona. “If we could detect it earlier by an analysis of urine or blood in high-risk patients, it could significantly improve their care.”
To explore this possibility, Drs Fonseca, Anbar, and their colleagues performed calcium isotope analysis using blood samples from MM patients. The team recounted their results in a letter to Leukemia.
As the name suggests, calcium isotope analysis measures calcium isotopes that are naturally present in blood. The technique makes use of a fact well known to Earth scientists but not normally used in biomedicine: different isotopes of a chemical element can react at slightly different rates.
An earlier study testing this technique in healthy subjects showed that when bones form, the lighter isotopes of calcium enter bone a little faster than the heavier isotopes. That difference, called isotope fractionation, is the key to the method.
In healthy, active humans, bone is forming at about the same rate as it resorbs. But if bone loss is occurring, the isotopic composition of blood becomes enriched in the lighter isotopes as bones resorb more quickly than they are formed.
The effect on calcium isotopes is very small, typically less than a 0.02% change in the isotope ratio. But even effects that small can be measured using precise mass spectrometry methods.
Using these methods, the researchers tested peripheral blood samples from 71 adult patients—55 with MM, 9 with smoldering MM, and 7 with monoclonal gammopathy of undetermined significance.
The researchers found an association between how active a patient’s disease was and the change in the isotope ratios. In fact, the isotope ratios predicted disease activity better than, and independent from, standard clinical variables.
Blood samples from patients with active disease had significantly lower mean calcium isotope compositions than samples from patients with non-active disease (mean δ44/42Ca=-0.8 vs -0.67, P=0.025), regardless of diagnosis.
For MM patients specifically, those with active disease had a significantly lower mean δ44/42Ca than patients with non-active disease (-0.79 vs -0.63, P=0.016), which was consistent with resorption of bone containing lighter 42Ca.
Dr Anbar noted that, although calcium isotope analysis has worked in this small set of patients, additional research is needed to verify these initial findings and improve the efficiency of analysis.
“If the method proves to be robust after more careful validation, it could provide earlier detection of bone involvement than presently possible,” he said, “and also provide the possibility to monitor the effectiveness of drugs to combat bone loss.” ![]()
Cancer survivors aren’t living healthy, study shows

Credit: Bill Branson
Childhood cancer survivors are no more likely than their cancer-free peers to adhere to healthy living guidelines, according to a study published in the Journal of Cancer Survivorship.
Survivors were less likely to be smokers and had a lower average body mass index (BMI).
But there were no significant differences between survivors and cancer-free control subjects with regard to overall diet, physical activity, or alcohol consumption.
Chloe Berdan, of Promedica in Toledo, Ohio, and her colleagues uncovered these results by examining data from the Chicago Healthy Living Study.
The team assessed adherence to American Cancer Society Guidelines on Nutrition and Physical Activity via interviews with 431 childhood cancer survivors and 361 control subjects who never had cancer. The survivors, ages 18 to 59, were all diagnosed with a malignant cancer before their 21st birthdays.
There were no significant differences in sex or race between survivors and controls. Survivors were younger than controls (28.4±7.8 vs 29.6± 8.3 years, P=0.04) and had less education (14.0±2.0 vs 14.4±2.0 years, P=0.01).
Overall, there was no significant difference between survivors and control subjects in adhering to the American Cancer Society guidelines.
Survivors and controls also had similar scores for several individual measures, including alcohol consumption, overall physical activity, overall diet, the servings of fruits/vegetables consumed, and the consumption of red/processed meat.
However, survivors were significantly less likely than controls to be smokers—11.4% vs 17.5% (P=0.02). Survivors had, on average, a BMI of about 1.2 kg/m² lower than controls (P=0.01). And survivors consumed significantly less fiber than controls—9.2±3.5 vs 9.7±3.8 kcal (P=0.05).
Only about 1 in 10 survivors (10.2%) met fiber recommendations, 17.7% ate 5 fruits or vegetables per day, and 46.2% met the red/processed meat recommendation of less than 18 oz per week. On average, survivors scored under 50% for the quality of their diets.
Survivors were better at meeting the goal of at least 5 hours of moderate activity per week (60.5%) than to sticking to any of the other guidelines.
About 36% of survivors were within a healthy BMI range, 2.9% were underweight, 28.9% were overweight, and 32.4% were obese.
The 0.7% of survivors who adhered fully to the guidelines tended to be women, non-smokers, and people with a good view of their own health.
“There is still much room for improvement in educating and encouraging survivors to follow healthier diets and lifestyles,” Berdan said. “Adopting such behavior during early adulthood may have a lasting impact on their quality of life and overall survival.” ![]()

Credit: Bill Branson
Childhood cancer survivors are no more likely than their cancer-free peers to adhere to healthy living guidelines, according to a study published in the Journal of Cancer Survivorship.
Survivors were less likely to be smokers and had a lower average body mass index (BMI).
But there were no significant differences between survivors and cancer-free control subjects with regard to overall diet, physical activity, or alcohol consumption.
Chloe Berdan, of Promedica in Toledo, Ohio, and her colleagues uncovered these results by examining data from the Chicago Healthy Living Study.
The team assessed adherence to American Cancer Society Guidelines on Nutrition and Physical Activity via interviews with 431 childhood cancer survivors and 361 control subjects who never had cancer. The survivors, ages 18 to 59, were all diagnosed with a malignant cancer before their 21st birthdays.
There were no significant differences in sex or race between survivors and controls. Survivors were younger than controls (28.4±7.8 vs 29.6± 8.3 years, P=0.04) and had less education (14.0±2.0 vs 14.4±2.0 years, P=0.01).
Overall, there was no significant difference between survivors and control subjects in adhering to the American Cancer Society guidelines.
Survivors and controls also had similar scores for several individual measures, including alcohol consumption, overall physical activity, overall diet, the servings of fruits/vegetables consumed, and the consumption of red/processed meat.
However, survivors were significantly less likely than controls to be smokers—11.4% vs 17.5% (P=0.02). Survivors had, on average, a BMI of about 1.2 kg/m² lower than controls (P=0.01). And survivors consumed significantly less fiber than controls—9.2±3.5 vs 9.7±3.8 kcal (P=0.05).
Only about 1 in 10 survivors (10.2%) met fiber recommendations, 17.7% ate 5 fruits or vegetables per day, and 46.2% met the red/processed meat recommendation of less than 18 oz per week. On average, survivors scored under 50% for the quality of their diets.
Survivors were better at meeting the goal of at least 5 hours of moderate activity per week (60.5%) than to sticking to any of the other guidelines.
About 36% of survivors were within a healthy BMI range, 2.9% were underweight, 28.9% were overweight, and 32.4% were obese.
The 0.7% of survivors who adhered fully to the guidelines tended to be women, non-smokers, and people with a good view of their own health.
“There is still much room for improvement in educating and encouraging survivors to follow healthier diets and lifestyles,” Berdan said. “Adopting such behavior during early adulthood may have a lasting impact on their quality of life and overall survival.” ![]()

Credit: Bill Branson
Childhood cancer survivors are no more likely than their cancer-free peers to adhere to healthy living guidelines, according to a study published in the Journal of Cancer Survivorship.
Survivors were less likely to be smokers and had a lower average body mass index (BMI).
But there were no significant differences between survivors and cancer-free control subjects with regard to overall diet, physical activity, or alcohol consumption.
Chloe Berdan, of Promedica in Toledo, Ohio, and her colleagues uncovered these results by examining data from the Chicago Healthy Living Study.
The team assessed adherence to American Cancer Society Guidelines on Nutrition and Physical Activity via interviews with 431 childhood cancer survivors and 361 control subjects who never had cancer. The survivors, ages 18 to 59, were all diagnosed with a malignant cancer before their 21st birthdays.
There were no significant differences in sex or race between survivors and controls. Survivors were younger than controls (28.4±7.8 vs 29.6± 8.3 years, P=0.04) and had less education (14.0±2.0 vs 14.4±2.0 years, P=0.01).
Overall, there was no significant difference between survivors and control subjects in adhering to the American Cancer Society guidelines.
Survivors and controls also had similar scores for several individual measures, including alcohol consumption, overall physical activity, overall diet, the servings of fruits/vegetables consumed, and the consumption of red/processed meat.
However, survivors were significantly less likely than controls to be smokers—11.4% vs 17.5% (P=0.02). Survivors had, on average, a BMI of about 1.2 kg/m² lower than controls (P=0.01). And survivors consumed significantly less fiber than controls—9.2±3.5 vs 9.7±3.8 kcal (P=0.05).
Only about 1 in 10 survivors (10.2%) met fiber recommendations, 17.7% ate 5 fruits or vegetables per day, and 46.2% met the red/processed meat recommendation of less than 18 oz per week. On average, survivors scored under 50% for the quality of their diets.
Survivors were better at meeting the goal of at least 5 hours of moderate activity per week (60.5%) than to sticking to any of the other guidelines.
About 36% of survivors were within a healthy BMI range, 2.9% were underweight, 28.9% were overweight, and 32.4% were obese.
The 0.7% of survivors who adhered fully to the guidelines tended to be women, non-smokers, and people with a good view of their own health.
“There is still much room for improvement in educating and encouraging survivors to follow healthier diets and lifestyles,” Berdan said. “Adopting such behavior during early adulthood may have a lasting impact on their quality of life and overall survival.” ![]()
A quicker way to manipulate malaria genes

(left) and Jeffrey Wagner
Credit: Bryce Vickmark
The gene-editing technique CRISPR can disrupt a single gene from the malaria parasite Plasmodium falciparum in a matter of weeks, a new study suggests.
Although CRISPR’s success rate ranged from 50% to 100%, the researchers believe the technique shows promise and could greatly speed up gene analysis.
At present, it can take up to a year to determine the function of a single gene in P falciparum, which can hinder efforts to develop drugs and vaccines.
“Even though we’ve sequenced the entire genome of Plasmodium falciparum, half of it still remains functionally uncharacterized,” said Jacquin Niles, MD, PhD, of the Massachusetts Institute of Technology in Cambridge.
“That’s about 2500 genes that, if only we knew what they did, we could think about novel therapeutics, whether it’s drugs or vaccines.”
Dr Niles and his colleagues described their use of CRISPR in P falciparum in Nature Methods.
The team noted that, in P falciparum, gene editing can take up to a year because it relies on homologous recombination, a type of genetic swapping that cells use to repair broken DNA strands and that occurs very rarely in the genome of the malaria parasite.
“You have to rely on this really inefficient process that occurs only if you have spontaneous DNA strand breaks that happen to fall within your region of interest,” Dr Niles said.
More recently, researchers have successfully used zinc finger nucleases to cut out specific genes, but this approach is costly because it requires a new nuclease to be designed for each gene target.
CRISPR exploits a set of bacterial proteins that protect microbes from viral infection. The system includes a DNA-cutting enzyme, Cas9, bound to a short RNA guide strand that is programmed to bind to a specific genome sequence, telling Cas9 where to make its cut. This approach allows scientists to target and delete any gene by simply changing the RNA guide strand sequence.
As soon as researchers proved this system could work in cells other than bacteria, Dr Niles started to think about using it to manipulate P falciparum.
To test this approach, he and his colleagues tried using CRISPR to disrupt 2 genes, kahrp and eba-175, that had previously been knocked out in malaria using traditional approaches.
The kahrp gene produces a protein that causes red blood cells to develop a knobby appearance when infected with malaria. Dr Niles’s team was able to disrupt this gene in 100% of parasites treated with the CRISPR system. The red blood cells infected by parasites remained smooth.
The other gene, eba-175, codes for a protein that binds to red blood cell receptors and helps the malaria parasite enter cells. The researchers were only able to disrupt this gene in 50% to 80% of parasites manipulated with CRISPR.
“We consider this to be a win,” Dr Niles said. “Compared to the efficiency with which P falciparum genetics have been done in the past, even 50% is pretty substantial.”
Now that CRISPR technology has been validated in P falciparum, Dr Niles expects many scientists will adopt it for genetic studies of the parasite. Such efforts could reveal more about how the parasite invades red blood cells and replicates inside cells, which could generate new drug and vaccine targets.
“I think the impact could be quite huge,” he said. “It lowers the barrier to really being more imaginative in terms of how we do experiments and the kinds of questions that we can ask.” ![]()

(left) and Jeffrey Wagner
Credit: Bryce Vickmark
The gene-editing technique CRISPR can disrupt a single gene from the malaria parasite Plasmodium falciparum in a matter of weeks, a new study suggests.
Although CRISPR’s success rate ranged from 50% to 100%, the researchers believe the technique shows promise and could greatly speed up gene analysis.
At present, it can take up to a year to determine the function of a single gene in P falciparum, which can hinder efforts to develop drugs and vaccines.
“Even though we’ve sequenced the entire genome of Plasmodium falciparum, half of it still remains functionally uncharacterized,” said Jacquin Niles, MD, PhD, of the Massachusetts Institute of Technology in Cambridge.
“That’s about 2500 genes that, if only we knew what they did, we could think about novel therapeutics, whether it’s drugs or vaccines.”
Dr Niles and his colleagues described their use of CRISPR in P falciparum in Nature Methods.
The team noted that, in P falciparum, gene editing can take up to a year because it relies on homologous recombination, a type of genetic swapping that cells use to repair broken DNA strands and that occurs very rarely in the genome of the malaria parasite.
“You have to rely on this really inefficient process that occurs only if you have spontaneous DNA strand breaks that happen to fall within your region of interest,” Dr Niles said.
More recently, researchers have successfully used zinc finger nucleases to cut out specific genes, but this approach is costly because it requires a new nuclease to be designed for each gene target.
CRISPR exploits a set of bacterial proteins that protect microbes from viral infection. The system includes a DNA-cutting enzyme, Cas9, bound to a short RNA guide strand that is programmed to bind to a specific genome sequence, telling Cas9 where to make its cut. This approach allows scientists to target and delete any gene by simply changing the RNA guide strand sequence.
As soon as researchers proved this system could work in cells other than bacteria, Dr Niles started to think about using it to manipulate P falciparum.
To test this approach, he and his colleagues tried using CRISPR to disrupt 2 genes, kahrp and eba-175, that had previously been knocked out in malaria using traditional approaches.
The kahrp gene produces a protein that causes red blood cells to develop a knobby appearance when infected with malaria. Dr Niles’s team was able to disrupt this gene in 100% of parasites treated with the CRISPR system. The red blood cells infected by parasites remained smooth.
The other gene, eba-175, codes for a protein that binds to red blood cell receptors and helps the malaria parasite enter cells. The researchers were only able to disrupt this gene in 50% to 80% of parasites manipulated with CRISPR.
“We consider this to be a win,” Dr Niles said. “Compared to the efficiency with which P falciparum genetics have been done in the past, even 50% is pretty substantial.”
Now that CRISPR technology has been validated in P falciparum, Dr Niles expects many scientists will adopt it for genetic studies of the parasite. Such efforts could reveal more about how the parasite invades red blood cells and replicates inside cells, which could generate new drug and vaccine targets.
“I think the impact could be quite huge,” he said. “It lowers the barrier to really being more imaginative in terms of how we do experiments and the kinds of questions that we can ask.” ![]()

(left) and Jeffrey Wagner
Credit: Bryce Vickmark
The gene-editing technique CRISPR can disrupt a single gene from the malaria parasite Plasmodium falciparum in a matter of weeks, a new study suggests.
Although CRISPR’s success rate ranged from 50% to 100%, the researchers believe the technique shows promise and could greatly speed up gene analysis.
At present, it can take up to a year to determine the function of a single gene in P falciparum, which can hinder efforts to develop drugs and vaccines.
“Even though we’ve sequenced the entire genome of Plasmodium falciparum, half of it still remains functionally uncharacterized,” said Jacquin Niles, MD, PhD, of the Massachusetts Institute of Technology in Cambridge.
“That’s about 2500 genes that, if only we knew what they did, we could think about novel therapeutics, whether it’s drugs or vaccines.”
Dr Niles and his colleagues described their use of CRISPR in P falciparum in Nature Methods.
The team noted that, in P falciparum, gene editing can take up to a year because it relies on homologous recombination, a type of genetic swapping that cells use to repair broken DNA strands and that occurs very rarely in the genome of the malaria parasite.
“You have to rely on this really inefficient process that occurs only if you have spontaneous DNA strand breaks that happen to fall within your region of interest,” Dr Niles said.
More recently, researchers have successfully used zinc finger nucleases to cut out specific genes, but this approach is costly because it requires a new nuclease to be designed for each gene target.
CRISPR exploits a set of bacterial proteins that protect microbes from viral infection. The system includes a DNA-cutting enzyme, Cas9, bound to a short RNA guide strand that is programmed to bind to a specific genome sequence, telling Cas9 where to make its cut. This approach allows scientists to target and delete any gene by simply changing the RNA guide strand sequence.
As soon as researchers proved this system could work in cells other than bacteria, Dr Niles started to think about using it to manipulate P falciparum.
To test this approach, he and his colleagues tried using CRISPR to disrupt 2 genes, kahrp and eba-175, that had previously been knocked out in malaria using traditional approaches.
The kahrp gene produces a protein that causes red blood cells to develop a knobby appearance when infected with malaria. Dr Niles’s team was able to disrupt this gene in 100% of parasites treated with the CRISPR system. The red blood cells infected by parasites remained smooth.
The other gene, eba-175, codes for a protein that binds to red blood cell receptors and helps the malaria parasite enter cells. The researchers were only able to disrupt this gene in 50% to 80% of parasites manipulated with CRISPR.
“We consider this to be a win,” Dr Niles said. “Compared to the efficiency with which P falciparum genetics have been done in the past, even 50% is pretty substantial.”
Now that CRISPR technology has been validated in P falciparum, Dr Niles expects many scientists will adopt it for genetic studies of the parasite. Such efforts could reveal more about how the parasite invades red blood cells and replicates inside cells, which could generate new drug and vaccine targets.
“I think the impact could be quite huge,” he said. “It lowers the barrier to really being more imaginative in terms of how we do experiments and the kinds of questions that we can ask.” ![]()
Hospitalists' Use of PPIs
Proton pump inhibitors (PPIs) are commonly used to treat acid‐related disorders but are associated with an increased risk of pneumonia and Clostridium difficile‐associated diarrhea.[1, 2] Initiation of PPIs in hospitalized patients should therefore be limited to specific clinical situations, such as upper gastrointestinal bleeding or stress ulcer prophylaxis in the critically ill.[3] Prior studies suggest significant overuse of PPIs in hospitalized patients exists,[4, 5, 6, 7] but these were published before the widespread implementation of local and national quality improvement efforts targeted at reducing PPI use in medical inpatients (eg, Society of Hospital Medicine's Choosing Wisely list[8]). We aimed to determine the frequency of inappropriate use of PPIs in a contemporary cohort of hospitalized patients in a tertiary care academic medical center.
METHODS
We conducted a retrospective cohort study of 297 patients admitted to a tertiary care center hospitalist service comprised of teaching and nonteaching medical patients who were not critically ill, were admitted between January 1, 2012 and March 31, 2012, and received a PPI during their hospital stay. Three internists used American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy and prior studies to develop criteria to identify appropriate and inappropriate PPI use (Table 1).[4, 5, 6, 7] Appropriate indications included gastrointestinal (GI) bleeding, esophagitis, gastritis, gastroesophageal reflux (GERD), and continuation of home PPI (abrupt discontinuation can trigger reflux symptoms).[9] We extracted the medical records of included patients, applying our prespecified criteria to determine whether use was appropriate. In patients in whom PPI was a continued home medication, we also extracted 2 years of data prior to the index date to determine if the medication was started during a prior hospital admission and, if so, whether this initiation was appropriate. We used descriptive statistics and [2] tests to compare patient characteristics and indications for PPI use.
| Appropriate PPI use | Inappropriate PPI use |
|---|---|
| |
| History of upper GI bleeding | No reason given |
| Endoscopic evidence of peptic ulcer disease | Unspecified GI prophylaxis |
| Esophagitis | Nonspecific abdominal pain |
| Gastritis and duodenitis | Heartburn (nonchronic) |
| Eradication of H pylori | Acute pancreatitis |
| GERD | Anemia |
| Barrett's esophagus | Heparin use for DVT prophylaxis |
| Continued on home PPI | Use of aspirin, NSAID, steroids or Coumadin (as a single agent) |
| Acute esophageal variceal bleeding | |
| NSAID used in patient >65 years‐old | |
| High‐risk groups; combination of 2 or more of aspirin, NSAID, clopidogrel, or Coumadin | |
RESULTS
Of 297 patients, the mean age was 64.4 years (standard deviation 16.3 years), most were white (69%), and 56% were women (Table 2). PPI use was appropriate in 231 (78%, 95% confidence interval: 72.6%‐82.4%) patients. Of these, a majority (172, 75%) of patients received a PPI because it was a continued home medication. Only 40 of the 172 patients had the medication started during a recent hospitalization, and in half of those cases (20) the PPI use was appropriate.
| Demographics | PPI Not Indicated, N=66 | PPI Indicated, N=231 | Total=297 | |||
|---|---|---|---|---|---|---|
| ||||||
| Age, y, mean (SD) | 62.5 | (16.2) | 64.9 | (16.3) | 64.4 | (16.3) |
| Sex, % No. | ||||||
| Female | 51.5% | 34 | 56.7% | 131 | 55.6% | 165 |
| Male | 48.5% | 32 | 43.3% | 100 | 44.4% | 132 |
| Race, % No. | ||||||
| Asian | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Black | 10.6% | 7 | 9.1% | 21 | 9.4% | 28 |
| Hispanic | 18.2% | 12 | 19.5% | 45 | 19.2% | 57 |
| Unknown | 0.0% | 0 | 2.2% | 5 | 1.7% | 5 |
| White | 71.2% | 47 | 68.4% | 158 | 69.0% | 205 |
| Insurance, % No. | ||||||
| Insured | 95.5% | 63 | 87.4% | 202 | 89.2% | 265 |
| Uninsured | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Unknown | 4.5% | 3 | 11.7% | 27 | 10.1% | 30 |
| Service, % No. | ||||||
| Teaching | 25.8% | 17 | 32.9% | 76 | 31.3% | 93 |
| Nonteaching | 74.2% | 49 | 66.7% | 154 | 68.4% | 203 |
| Unknown | 0.0% | 0 | 0.4% | 1 | 0.3% | 1 |
| Chronic disease, % No. | ||||||
| Cardiac disease | 16.7% | 11 | 13.4% | 31 | 14.1% | 42 |
| Pulmonary disease | 16.7% | 11 | 14.7% | 34 | 15.2% | 45 |
| Gastrointestinal disease | 13.6% | 9 | 19.5% | 45 | 18.2% | 54 |
| Hepatic disease | 7.6% | 5 | 3.9% | 9 | 4.7% | 14 |
| Stroke | 1.5% | 1 | 5.2% | 12 | 4.4% | 13 |
| Sepsis | 12.1% | 8 | 13.0% | 30 | 12.8% | 38 |
| Other | 33.3% | 22 | 29.4% | 68 | 30.3% | 90 |
| PPI status, % No. | ||||||
| Continued home PPI | 0.0% | 0 | 74.5% | 172 | 58.1% | 172 |
| Started on PPI in hospital | 100% | 65 | 25.5% | 59 | 41.9% | 124 |
| Discharged on AST, % No. | ||||||
| Yes | 36.4% | 24 | 89.6% | 207 | 22.2% | 231 |
| PPI | 87.5% | 21 | 96.6% | 200 | 95.7% | 221 |
| Brand | 52.4% | 11 | 59.5% | 119 | 58.8% | 130 |
| Generic | 47.6% | 10 | 40.5% | 81 | 41.2% | 91 |
| H2 blocker | 12.5% | 3 | 3.4% | 7 | 4.3% | 10 |
| Brand | 0.0% | 0 | 71.4% | 5 | 50.0% | 5 |
| Generic | 100.0% | 3 | 28.6% | 2 | 50.0% | 5 |
| Medications, % No. | ||||||
| Aspirin | 36.4% | 24 | 43.7% | 101 | 42.1% | 125 |
| NSAID | 10.6% | 4 | 6.5% | 15 | 6.4% | 19 |
| Corticosteroids | 13.6% | 9 | 16.9% | 39 | 16.2% | 48 |
| Warfarin | 0.0% | 5 | 19.0% | 44 | 16.5% | 49 |
| Clopidogrel | 12.1% | 8 | 10.8% | 25 | 11.1% | 33 |
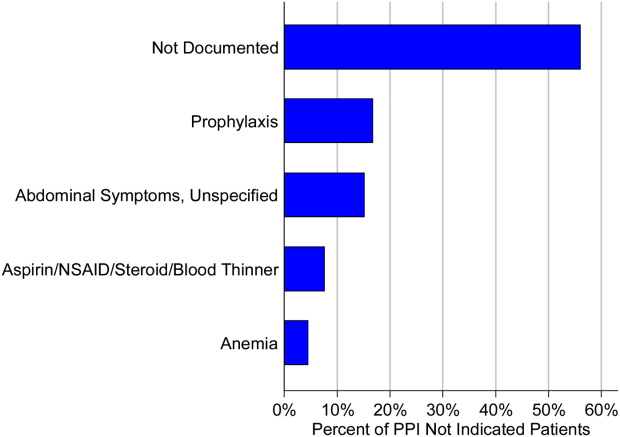
The second most common appropriate diagnosis was GERD (31%), followed by history of GI bleeding (19%) and treatment for esophagitis or gastritis (18%). Among the 66 patients receiving a PPI inappropriately, the majority of patients (56%) had no documented reason for PPI use, and only 11 patients (17%) were receiving PPI for stress ulcer prophylaxis (Figure 1). Five patients (8%) were treated prophylactically because of steroid or anticoagulant use. We observed no differences in age, gender, race, or reason for admission between the patients treated appropriately versus inappropriately.

DISCUSSION
In a contemporary cohort, chronic PPI use prior to admission was the most common reason PPIs were prescribed in the hospital. About 20% of hospitalized patients were started on a PPI for an inappropriate indication, the majority of whom lacked documentation concerning the reason for use. Among patients treated inappropriately, 36% were discharged on acid‐suppressive therapy.
The prior literature has reported a much higher percentages of unnecessary PPI use in hospitalized patients.[4, 5, 6, 7] Gupta et al. found that 70% of patients admitted to an internal medicine service received acid‐suppressive therapy, 73% of whom were treated unnecessarily.[5] Similarly, Nardino et al. found that 65% of acid‐suppressive therapy in hospitalized medical patients was not indicated.[4] If we had excluded patients on home PPIs from our study cohort, we would have found a higher rate of inappropriate use due to a smaller overall patient population. However, we chose to include these patients because they represented the vast majority of hospitalist‐prescribed PPIs. Notably, most of these prior prescriptions were not written during a recent hospital stay, indicating that the majority were initiated by outpatient physicians.
Our study is limited by its small sample size, single‐center design, and inability to determine the indications for outpatient PPI use. Still, it has important implications. Prior work has suggested that focusing efforts on PPI overuse may be premature in the absence of valid risk‐prediction models defining the patient populations that most benefit from PPI therapy.[10] Our work additionally suggests that hospital rates of inappropriate initiation may be relatively low, perhaps because hospitalist culture and practice have been affected by both local and national quality improvement efforts and by evidence dissemination.[8] Quality improvement efforts focused on reducing inpatient PPI use are likely to reveal diminishing returns, as admitting hospitalists are unlikely to abruptly discontinue PPIs prescribed in the outpatient setting.[9] Hospitalists should be encouraged to assess and document the need for PPIs during admission, hospitalization, and discharge processes. However, future efforts to reduce PPI overuse among hospitalized patients should predominately be focused on reducing inappropriate chronic PPI use in the outpatient setting.
Acknowledgements
The authors acknowledge Peter Lindenauer for his comments on an earlier draft of this manuscript.
Disclosures: The study was conducted with funding from the Department of Medicine at Baystate Medical Center. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Albugeaey had full access to all of the data in the study, and they take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Albugeaey, and Seiler conceived of the study. Drs. Albugeaey and Al Faraj acquired the data. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb analyzed and interpreted the data. Drs. Albugeaey and Lagu drafted the manuscript. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Albugeaey is a recipient of a scholarship from the Ministry of Higher Education, Kingdom of Saudi Arabia. The authors report no conflicts of interest.
- , , , , . Acid‐suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991–997.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , . Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360; quiz 361.
- , , . Overuse of acid‐suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118–3122.
- , , , et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207–211.
- , , , , , . Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421–425.
- , , , . Inappropriate utilization of intravenous proton pump inhibitors in hospital practice—a prospective study of the extent of the problem and predictive factors. QJM. 2010;103(5):327–335.
- Choosing Wisely, Society of Hospital Medicine, Adult Hospital Medicine. Available at:http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed April 11, 2014.
- , , , . Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol. 2010;16(19):2323–2330.
- , . Prophylaxis rates for venous thromboembolism and gastrointestinal bleeding in general medical patients: too low or too high? BMJ. 2012;344:e3248.
Proton pump inhibitors (PPIs) are commonly used to treat acid‐related disorders but are associated with an increased risk of pneumonia and Clostridium difficile‐associated diarrhea.[1, 2] Initiation of PPIs in hospitalized patients should therefore be limited to specific clinical situations, such as upper gastrointestinal bleeding or stress ulcer prophylaxis in the critically ill.[3] Prior studies suggest significant overuse of PPIs in hospitalized patients exists,[4, 5, 6, 7] but these were published before the widespread implementation of local and national quality improvement efforts targeted at reducing PPI use in medical inpatients (eg, Society of Hospital Medicine's Choosing Wisely list[8]). We aimed to determine the frequency of inappropriate use of PPIs in a contemporary cohort of hospitalized patients in a tertiary care academic medical center.
METHODS
We conducted a retrospective cohort study of 297 patients admitted to a tertiary care center hospitalist service comprised of teaching and nonteaching medical patients who were not critically ill, were admitted between January 1, 2012 and March 31, 2012, and received a PPI during their hospital stay. Three internists used American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy and prior studies to develop criteria to identify appropriate and inappropriate PPI use (Table 1).[4, 5, 6, 7] Appropriate indications included gastrointestinal (GI) bleeding, esophagitis, gastritis, gastroesophageal reflux (GERD), and continuation of home PPI (abrupt discontinuation can trigger reflux symptoms).[9] We extracted the medical records of included patients, applying our prespecified criteria to determine whether use was appropriate. In patients in whom PPI was a continued home medication, we also extracted 2 years of data prior to the index date to determine if the medication was started during a prior hospital admission and, if so, whether this initiation was appropriate. We used descriptive statistics and [2] tests to compare patient characteristics and indications for PPI use.
| Appropriate PPI use | Inappropriate PPI use |
|---|---|
| |
| History of upper GI bleeding | No reason given |
| Endoscopic evidence of peptic ulcer disease | Unspecified GI prophylaxis |
| Esophagitis | Nonspecific abdominal pain |
| Gastritis and duodenitis | Heartburn (nonchronic) |
| Eradication of H pylori | Acute pancreatitis |
| GERD | Anemia |
| Barrett's esophagus | Heparin use for DVT prophylaxis |
| Continued on home PPI | Use of aspirin, NSAID, steroids or Coumadin (as a single agent) |
| Acute esophageal variceal bleeding | |
| NSAID used in patient >65 years‐old | |
| High‐risk groups; combination of 2 or more of aspirin, NSAID, clopidogrel, or Coumadin | |
RESULTS
Of 297 patients, the mean age was 64.4 years (standard deviation 16.3 years), most were white (69%), and 56% were women (Table 2). PPI use was appropriate in 231 (78%, 95% confidence interval: 72.6%‐82.4%) patients. Of these, a majority (172, 75%) of patients received a PPI because it was a continued home medication. Only 40 of the 172 patients had the medication started during a recent hospitalization, and in half of those cases (20) the PPI use was appropriate.
| Demographics | PPI Not Indicated, N=66 | PPI Indicated, N=231 | Total=297 | |||
|---|---|---|---|---|---|---|
| ||||||
| Age, y, mean (SD) | 62.5 | (16.2) | 64.9 | (16.3) | 64.4 | (16.3) |
| Sex, % No. | ||||||
| Female | 51.5% | 34 | 56.7% | 131 | 55.6% | 165 |
| Male | 48.5% | 32 | 43.3% | 100 | 44.4% | 132 |
| Race, % No. | ||||||
| Asian | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Black | 10.6% | 7 | 9.1% | 21 | 9.4% | 28 |
| Hispanic | 18.2% | 12 | 19.5% | 45 | 19.2% | 57 |
| Unknown | 0.0% | 0 | 2.2% | 5 | 1.7% | 5 |
| White | 71.2% | 47 | 68.4% | 158 | 69.0% | 205 |
| Insurance, % No. | ||||||
| Insured | 95.5% | 63 | 87.4% | 202 | 89.2% | 265 |
| Uninsured | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Unknown | 4.5% | 3 | 11.7% | 27 | 10.1% | 30 |
| Service, % No. | ||||||
| Teaching | 25.8% | 17 | 32.9% | 76 | 31.3% | 93 |
| Nonteaching | 74.2% | 49 | 66.7% | 154 | 68.4% | 203 |
| Unknown | 0.0% | 0 | 0.4% | 1 | 0.3% | 1 |
| Chronic disease, % No. | ||||||
| Cardiac disease | 16.7% | 11 | 13.4% | 31 | 14.1% | 42 |
| Pulmonary disease | 16.7% | 11 | 14.7% | 34 | 15.2% | 45 |
| Gastrointestinal disease | 13.6% | 9 | 19.5% | 45 | 18.2% | 54 |
| Hepatic disease | 7.6% | 5 | 3.9% | 9 | 4.7% | 14 |
| Stroke | 1.5% | 1 | 5.2% | 12 | 4.4% | 13 |
| Sepsis | 12.1% | 8 | 13.0% | 30 | 12.8% | 38 |
| Other | 33.3% | 22 | 29.4% | 68 | 30.3% | 90 |
| PPI status, % No. | ||||||
| Continued home PPI | 0.0% | 0 | 74.5% | 172 | 58.1% | 172 |
| Started on PPI in hospital | 100% | 65 | 25.5% | 59 | 41.9% | 124 |
| Discharged on AST, % No. | ||||||
| Yes | 36.4% | 24 | 89.6% | 207 | 22.2% | 231 |
| PPI | 87.5% | 21 | 96.6% | 200 | 95.7% | 221 |
| Brand | 52.4% | 11 | 59.5% | 119 | 58.8% | 130 |
| Generic | 47.6% | 10 | 40.5% | 81 | 41.2% | 91 |
| H2 blocker | 12.5% | 3 | 3.4% | 7 | 4.3% | 10 |
| Brand | 0.0% | 0 | 71.4% | 5 | 50.0% | 5 |
| Generic | 100.0% | 3 | 28.6% | 2 | 50.0% | 5 |
| Medications, % No. | ||||||
| Aspirin | 36.4% | 24 | 43.7% | 101 | 42.1% | 125 |
| NSAID | 10.6% | 4 | 6.5% | 15 | 6.4% | 19 |
| Corticosteroids | 13.6% | 9 | 16.9% | 39 | 16.2% | 48 |
| Warfarin | 0.0% | 5 | 19.0% | 44 | 16.5% | 49 |
| Clopidogrel | 12.1% | 8 | 10.8% | 25 | 11.1% | 33 |
The second most common appropriate diagnosis was GERD (31%), followed by history of GI bleeding (19%) and treatment for esophagitis or gastritis (18%). Among the 66 patients receiving a PPI inappropriately, the majority of patients (56%) had no documented reason for PPI use, and only 11 patients (17%) were receiving PPI for stress ulcer prophylaxis (Figure 1). Five patients (8%) were treated prophylactically because of steroid or anticoagulant use. We observed no differences in age, gender, race, or reason for admission between the patients treated appropriately versus inappropriately.

DISCUSSION
In a contemporary cohort, chronic PPI use prior to admission was the most common reason PPIs were prescribed in the hospital. About 20% of hospitalized patients were started on a PPI for an inappropriate indication, the majority of whom lacked documentation concerning the reason for use. Among patients treated inappropriately, 36% were discharged on acid‐suppressive therapy.
The prior literature has reported a much higher percentages of unnecessary PPI use in hospitalized patients.[4, 5, 6, 7] Gupta et al. found that 70% of patients admitted to an internal medicine service received acid‐suppressive therapy, 73% of whom were treated unnecessarily.[5] Similarly, Nardino et al. found that 65% of acid‐suppressive therapy in hospitalized medical patients was not indicated.[4] If we had excluded patients on home PPIs from our study cohort, we would have found a higher rate of inappropriate use due to a smaller overall patient population. However, we chose to include these patients because they represented the vast majority of hospitalist‐prescribed PPIs. Notably, most of these prior prescriptions were not written during a recent hospital stay, indicating that the majority were initiated by outpatient physicians.
Our study is limited by its small sample size, single‐center design, and inability to determine the indications for outpatient PPI use. Still, it has important implications. Prior work has suggested that focusing efforts on PPI overuse may be premature in the absence of valid risk‐prediction models defining the patient populations that most benefit from PPI therapy.[10] Our work additionally suggests that hospital rates of inappropriate initiation may be relatively low, perhaps because hospitalist culture and practice have been affected by both local and national quality improvement efforts and by evidence dissemination.[8] Quality improvement efforts focused on reducing inpatient PPI use are likely to reveal diminishing returns, as admitting hospitalists are unlikely to abruptly discontinue PPIs prescribed in the outpatient setting.[9] Hospitalists should be encouraged to assess and document the need for PPIs during admission, hospitalization, and discharge processes. However, future efforts to reduce PPI overuse among hospitalized patients should predominately be focused on reducing inappropriate chronic PPI use in the outpatient setting.
Acknowledgements
The authors acknowledge Peter Lindenauer for his comments on an earlier draft of this manuscript.
Disclosures: The study was conducted with funding from the Department of Medicine at Baystate Medical Center. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Albugeaey had full access to all of the data in the study, and they take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Albugeaey, and Seiler conceived of the study. Drs. Albugeaey and Al Faraj acquired the data. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb analyzed and interpreted the data. Drs. Albugeaey and Lagu drafted the manuscript. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Albugeaey is a recipient of a scholarship from the Ministry of Higher Education, Kingdom of Saudi Arabia. The authors report no conflicts of interest.
Proton pump inhibitors (PPIs) are commonly used to treat acid‐related disorders but are associated with an increased risk of pneumonia and Clostridium difficile‐associated diarrhea.[1, 2] Initiation of PPIs in hospitalized patients should therefore be limited to specific clinical situations, such as upper gastrointestinal bleeding or stress ulcer prophylaxis in the critically ill.[3] Prior studies suggest significant overuse of PPIs in hospitalized patients exists,[4, 5, 6, 7] but these were published before the widespread implementation of local and national quality improvement efforts targeted at reducing PPI use in medical inpatients (eg, Society of Hospital Medicine's Choosing Wisely list[8]). We aimed to determine the frequency of inappropriate use of PPIs in a contemporary cohort of hospitalized patients in a tertiary care academic medical center.
METHODS
We conducted a retrospective cohort study of 297 patients admitted to a tertiary care center hospitalist service comprised of teaching and nonteaching medical patients who were not critically ill, were admitted between January 1, 2012 and March 31, 2012, and received a PPI during their hospital stay. Three internists used American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy and prior studies to develop criteria to identify appropriate and inappropriate PPI use (Table 1).[4, 5, 6, 7] Appropriate indications included gastrointestinal (GI) bleeding, esophagitis, gastritis, gastroesophageal reflux (GERD), and continuation of home PPI (abrupt discontinuation can trigger reflux symptoms).[9] We extracted the medical records of included patients, applying our prespecified criteria to determine whether use was appropriate. In patients in whom PPI was a continued home medication, we also extracted 2 years of data prior to the index date to determine if the medication was started during a prior hospital admission and, if so, whether this initiation was appropriate. We used descriptive statistics and [2] tests to compare patient characteristics and indications for PPI use.
| Appropriate PPI use | Inappropriate PPI use |
|---|---|
| |
| History of upper GI bleeding | No reason given |
| Endoscopic evidence of peptic ulcer disease | Unspecified GI prophylaxis |
| Esophagitis | Nonspecific abdominal pain |
| Gastritis and duodenitis | Heartburn (nonchronic) |
| Eradication of H pylori | Acute pancreatitis |
| GERD | Anemia |
| Barrett's esophagus | Heparin use for DVT prophylaxis |
| Continued on home PPI | Use of aspirin, NSAID, steroids or Coumadin (as a single agent) |
| Acute esophageal variceal bleeding | |
| NSAID used in patient >65 years‐old | |
| High‐risk groups; combination of 2 or more of aspirin, NSAID, clopidogrel, or Coumadin | |
RESULTS
Of 297 patients, the mean age was 64.4 years (standard deviation 16.3 years), most were white (69%), and 56% were women (Table 2). PPI use was appropriate in 231 (78%, 95% confidence interval: 72.6%‐82.4%) patients. Of these, a majority (172, 75%) of patients received a PPI because it was a continued home medication. Only 40 of the 172 patients had the medication started during a recent hospitalization, and in half of those cases (20) the PPI use was appropriate.
| Demographics | PPI Not Indicated, N=66 | PPI Indicated, N=231 | Total=297 | |||
|---|---|---|---|---|---|---|
| ||||||
| Age, y, mean (SD) | 62.5 | (16.2) | 64.9 | (16.3) | 64.4 | (16.3) |
| Sex, % No. | ||||||
| Female | 51.5% | 34 | 56.7% | 131 | 55.6% | 165 |
| Male | 48.5% | 32 | 43.3% | 100 | 44.4% | 132 |
| Race, % No. | ||||||
| Asian | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Black | 10.6% | 7 | 9.1% | 21 | 9.4% | 28 |
| Hispanic | 18.2% | 12 | 19.5% | 45 | 19.2% | 57 |
| Unknown | 0.0% | 0 | 2.2% | 5 | 1.7% | 5 |
| White | 71.2% | 47 | 68.4% | 158 | 69.0% | 205 |
| Insurance, % No. | ||||||
| Insured | 95.5% | 63 | 87.4% | 202 | 89.2% | 265 |
| Uninsured | 0.0% | 0 | 0.9% | 2 | 0.7% | 2 |
| Unknown | 4.5% | 3 | 11.7% | 27 | 10.1% | 30 |
| Service, % No. | ||||||
| Teaching | 25.8% | 17 | 32.9% | 76 | 31.3% | 93 |
| Nonteaching | 74.2% | 49 | 66.7% | 154 | 68.4% | 203 |
| Unknown | 0.0% | 0 | 0.4% | 1 | 0.3% | 1 |
| Chronic disease, % No. | ||||||
| Cardiac disease | 16.7% | 11 | 13.4% | 31 | 14.1% | 42 |
| Pulmonary disease | 16.7% | 11 | 14.7% | 34 | 15.2% | 45 |
| Gastrointestinal disease | 13.6% | 9 | 19.5% | 45 | 18.2% | 54 |
| Hepatic disease | 7.6% | 5 | 3.9% | 9 | 4.7% | 14 |
| Stroke | 1.5% | 1 | 5.2% | 12 | 4.4% | 13 |
| Sepsis | 12.1% | 8 | 13.0% | 30 | 12.8% | 38 |
| Other | 33.3% | 22 | 29.4% | 68 | 30.3% | 90 |
| PPI status, % No. | ||||||
| Continued home PPI | 0.0% | 0 | 74.5% | 172 | 58.1% | 172 |
| Started on PPI in hospital | 100% | 65 | 25.5% | 59 | 41.9% | 124 |
| Discharged on AST, % No. | ||||||
| Yes | 36.4% | 24 | 89.6% | 207 | 22.2% | 231 |
| PPI | 87.5% | 21 | 96.6% | 200 | 95.7% | 221 |
| Brand | 52.4% | 11 | 59.5% | 119 | 58.8% | 130 |
| Generic | 47.6% | 10 | 40.5% | 81 | 41.2% | 91 |
| H2 blocker | 12.5% | 3 | 3.4% | 7 | 4.3% | 10 |
| Brand | 0.0% | 0 | 71.4% | 5 | 50.0% | 5 |
| Generic | 100.0% | 3 | 28.6% | 2 | 50.0% | 5 |
| Medications, % No. | ||||||
| Aspirin | 36.4% | 24 | 43.7% | 101 | 42.1% | 125 |
| NSAID | 10.6% | 4 | 6.5% | 15 | 6.4% | 19 |
| Corticosteroids | 13.6% | 9 | 16.9% | 39 | 16.2% | 48 |
| Warfarin | 0.0% | 5 | 19.0% | 44 | 16.5% | 49 |
| Clopidogrel | 12.1% | 8 | 10.8% | 25 | 11.1% | 33 |
The second most common appropriate diagnosis was GERD (31%), followed by history of GI bleeding (19%) and treatment for esophagitis or gastritis (18%). Among the 66 patients receiving a PPI inappropriately, the majority of patients (56%) had no documented reason for PPI use, and only 11 patients (17%) were receiving PPI for stress ulcer prophylaxis (Figure 1). Five patients (8%) were treated prophylactically because of steroid or anticoagulant use. We observed no differences in age, gender, race, or reason for admission between the patients treated appropriately versus inappropriately.

DISCUSSION
In a contemporary cohort, chronic PPI use prior to admission was the most common reason PPIs were prescribed in the hospital. About 20% of hospitalized patients were started on a PPI for an inappropriate indication, the majority of whom lacked documentation concerning the reason for use. Among patients treated inappropriately, 36% were discharged on acid‐suppressive therapy.
The prior literature has reported a much higher percentages of unnecessary PPI use in hospitalized patients.[4, 5, 6, 7] Gupta et al. found that 70% of patients admitted to an internal medicine service received acid‐suppressive therapy, 73% of whom were treated unnecessarily.[5] Similarly, Nardino et al. found that 65% of acid‐suppressive therapy in hospitalized medical patients was not indicated.[4] If we had excluded patients on home PPIs from our study cohort, we would have found a higher rate of inappropriate use due to a smaller overall patient population. However, we chose to include these patients because they represented the vast majority of hospitalist‐prescribed PPIs. Notably, most of these prior prescriptions were not written during a recent hospital stay, indicating that the majority were initiated by outpatient physicians.
Our study is limited by its small sample size, single‐center design, and inability to determine the indications for outpatient PPI use. Still, it has important implications. Prior work has suggested that focusing efforts on PPI overuse may be premature in the absence of valid risk‐prediction models defining the patient populations that most benefit from PPI therapy.[10] Our work additionally suggests that hospital rates of inappropriate initiation may be relatively low, perhaps because hospitalist culture and practice have been affected by both local and national quality improvement efforts and by evidence dissemination.[8] Quality improvement efforts focused on reducing inpatient PPI use are likely to reveal diminishing returns, as admitting hospitalists are unlikely to abruptly discontinue PPIs prescribed in the outpatient setting.[9] Hospitalists should be encouraged to assess and document the need for PPIs during admission, hospitalization, and discharge processes. However, future efforts to reduce PPI overuse among hospitalized patients should predominately be focused on reducing inappropriate chronic PPI use in the outpatient setting.
Acknowledgements
The authors acknowledge Peter Lindenauer for his comments on an earlier draft of this manuscript.
Disclosures: The study was conducted with funding from the Department of Medicine at Baystate Medical Center. Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Drs. Lagu and Albugeaey had full access to all of the data in the study, and they take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Lagu, Albugeaey, and Seiler conceived of the study. Drs. Albugeaey and Al Faraj acquired the data. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb analyzed and interpreted the data. Drs. Albugeaey and Lagu drafted the manuscript. Drs. Lagu, Albugeaey, Al Faraj, Seiler, and Ms. Garb critically reviewed the manuscript for important intellectual content. Dr. Albugeaey is a recipient of a scholarship from the Ministry of Higher Education, Kingdom of Saudi Arabia. The authors report no conflicts of interest.
- , , , , . Acid‐suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991–997.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , . Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360; quiz 361.
- , , . Overuse of acid‐suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118–3122.
- , , , et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207–211.
- , , , , , . Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421–425.
- , , , . Inappropriate utilization of intravenous proton pump inhibitors in hospital practice—a prospective study of the extent of the problem and predictive factors. QJM. 2010;103(5):327–335.
- Choosing Wisely, Society of Hospital Medicine, Adult Hospital Medicine. Available at:http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed April 11, 2014.
- , , , . Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol. 2010;16(19):2323–2330.
- , . Prophylaxis rates for venous thromboembolism and gastrointestinal bleeding in general medical patients: too low or too high? BMJ. 2012;344:e3248.
- , , , , . Acid‐suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991–997.
- , , , . Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA. 2009;301(20):2120–2128.
- , . Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360; quiz 361.
- , , . Overuse of acid‐suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118–3122.
- , , , et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207–211.
- , , , , , . Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421–425.
- , , , . Inappropriate utilization of intravenous proton pump inhibitors in hospital practice—a prospective study of the extent of the problem and predictive factors. QJM. 2010;103(5):327–335.
- Choosing Wisely, Society of Hospital Medicine, Adult Hospital Medicine. Available at:http://www.choosingwisely.org/doctor‐patient‐lists/society‐of‐hospital‐medicine‐adult‐hospital‐medicine. Accessed April 11, 2014.
- , , , . Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol. 2010;16(19):2323–2330.
- , . Prophylaxis rates for venous thromboembolism and gastrointestinal bleeding in general medical patients: too low or too high? BMJ. 2012;344:e3248.
Society of Hospital Medicine (SHM) Calls for Overhaul of Medicare's Observation Status Rules
A hospitalist and SHM Public Policy Committee member is hopeful that SHM's recently released position paper recommending changes to the way the "observation status" designation is used for admitted hospital patients will help improve patient care.
The increasing use of the Centers for Medicare & Medicaid Services' (CMS) patient observation status designation—which grew 88% from 2006 to 2012—has frustrated hospitalists. Under the rule, patients are ineligible for skilled-nursing facility (SNF) care, may not claim insurance coverage for some medications, and may face uncertain cost-sharing and other financial liabilities for their hospitalization.
SHM outlined its concerns about the policy and suggested solutions in the report titled "The Observation Status Problem: Impact and Recommendations for Change."
CMS has attempted to address the issue by creating the "two-midnight rule." The report notes, however, that amid confusion on the application of the "two-midnight rule," Medicare auditing and enforcement have been pushed back several times, most recently to March 31, 2015.
"We still are unclear about what patient vulnerability is under this," says SHM Public Policy Committee member Ann Sheehy, MD, MS, FHM, a hospitalist at the University of Wisconsin School of Medicine and Public Health in Madison, who testified before Congress last May on observation status and other Medicare policies.
"We know that patients can't get SNF coverage when they're under observation," Dr. Sheehy says. "We know that patients are subject to unlimited co-pays when they're under observation, as opposed to when they're hospitalized as inpatients under Medicare Part A, which has a one-time deductible."
The SHM white paper outlines both short- and long-term fixes to the policy. In the near term, SHM recommends:
• Educating providers and patients on the purpose of observation status and raising confidence in when and how it should be applied;
• Changing SNF coverage rules to ensure patients’ eligibility; and
• Reforming the Medicare Recovery Audit Contractor program to improve RAC performance and reduce unintended pressures on admission decisions.
In the long term, the report suggests creating modifiers for diagnosis-related group (DRG) payments to assign to patients needing lower-acuity services, as well as crafting a list of DRGs to assign to patients needing short periods of inpatient care.
"The policy overall is very frustrating," Dr. Sheehy adds. "We hope that any rule change that comes out will address the core problems of observation so that patients can get the care they need with fair and appropriate insurance coverage." TH
Visit our website for more information about patient observation status.
A hospitalist and SHM Public Policy Committee member is hopeful that SHM's recently released position paper recommending changes to the way the "observation status" designation is used for admitted hospital patients will help improve patient care.
The increasing use of the Centers for Medicare & Medicaid Services' (CMS) patient observation status designation—which grew 88% from 2006 to 2012—has frustrated hospitalists. Under the rule, patients are ineligible for skilled-nursing facility (SNF) care, may not claim insurance coverage for some medications, and may face uncertain cost-sharing and other financial liabilities for their hospitalization.
SHM outlined its concerns about the policy and suggested solutions in the report titled "The Observation Status Problem: Impact and Recommendations for Change."
CMS has attempted to address the issue by creating the "two-midnight rule." The report notes, however, that amid confusion on the application of the "two-midnight rule," Medicare auditing and enforcement have been pushed back several times, most recently to March 31, 2015.
"We still are unclear about what patient vulnerability is under this," says SHM Public Policy Committee member Ann Sheehy, MD, MS, FHM, a hospitalist at the University of Wisconsin School of Medicine and Public Health in Madison, who testified before Congress last May on observation status and other Medicare policies.
"We know that patients can't get SNF coverage when they're under observation," Dr. Sheehy says. "We know that patients are subject to unlimited co-pays when they're under observation, as opposed to when they're hospitalized as inpatients under Medicare Part A, which has a one-time deductible."
The SHM white paper outlines both short- and long-term fixes to the policy. In the near term, SHM recommends:
• Educating providers and patients on the purpose of observation status and raising confidence in when and how it should be applied;
• Changing SNF coverage rules to ensure patients’ eligibility; and
• Reforming the Medicare Recovery Audit Contractor program to improve RAC performance and reduce unintended pressures on admission decisions.
In the long term, the report suggests creating modifiers for diagnosis-related group (DRG) payments to assign to patients needing lower-acuity services, as well as crafting a list of DRGs to assign to patients needing short periods of inpatient care.
"The policy overall is very frustrating," Dr. Sheehy adds. "We hope that any rule change that comes out will address the core problems of observation so that patients can get the care they need with fair and appropriate insurance coverage." TH
Visit our website for more information about patient observation status.
A hospitalist and SHM Public Policy Committee member is hopeful that SHM's recently released position paper recommending changes to the way the "observation status" designation is used for admitted hospital patients will help improve patient care.
The increasing use of the Centers for Medicare & Medicaid Services' (CMS) patient observation status designation—which grew 88% from 2006 to 2012—has frustrated hospitalists. Under the rule, patients are ineligible for skilled-nursing facility (SNF) care, may not claim insurance coverage for some medications, and may face uncertain cost-sharing and other financial liabilities for their hospitalization.
SHM outlined its concerns about the policy and suggested solutions in the report titled "The Observation Status Problem: Impact and Recommendations for Change."
CMS has attempted to address the issue by creating the "two-midnight rule." The report notes, however, that amid confusion on the application of the "two-midnight rule," Medicare auditing and enforcement have been pushed back several times, most recently to March 31, 2015.
"We still are unclear about what patient vulnerability is under this," says SHM Public Policy Committee member Ann Sheehy, MD, MS, FHM, a hospitalist at the University of Wisconsin School of Medicine and Public Health in Madison, who testified before Congress last May on observation status and other Medicare policies.
"We know that patients can't get SNF coverage when they're under observation," Dr. Sheehy says. "We know that patients are subject to unlimited co-pays when they're under observation, as opposed to when they're hospitalized as inpatients under Medicare Part A, which has a one-time deductible."
The SHM white paper outlines both short- and long-term fixes to the policy. In the near term, SHM recommends:
• Educating providers and patients on the purpose of observation status and raising confidence in when and how it should be applied;
• Changing SNF coverage rules to ensure patients’ eligibility; and
• Reforming the Medicare Recovery Audit Contractor program to improve RAC performance and reduce unintended pressures on admission decisions.
In the long term, the report suggests creating modifiers for diagnosis-related group (DRG) payments to assign to patients needing lower-acuity services, as well as crafting a list of DRGs to assign to patients needing short periods of inpatient care.
"The policy overall is very frustrating," Dr. Sheehy adds. "We hope that any rule change that comes out will address the core problems of observation so that patients can get the care they need with fair and appropriate insurance coverage." TH
Visit our website for more information about patient observation status.
Patient Safety Experts, Physicians Advocate for Prevention of Medical Errors
In a Senate subcommittee hearing last month, a panel of patient safety experts and physicians raised concerns about the problem of preventable medical errors, which they say can be linked to more than 1,000 patient deaths per day.
For their part, SHM Public Policy Committee member Bradley Flansbaum, DO, MPH, FHM, says hospitalists should demand that their hospitals report better data on patient outcomes.
"When you talk about a patient death, you're talking about the efforts of an entire hospital going into the death or survival of that patient," says Dr. Flansbaum, a hospitalist at Lenox Hill Hospital in New York. "If you ask, 'Where did something go wrong?' just looking at the mortality rate doesn't help. What service are you inquiring about? How can you get clean data that’s also useful to the clinician?"
In his testimony before the Subcommittee on Primary Health and Aging, hospitalist Ashish Jha, MD, MPH, referenced a landmark 1999 report from the Institute of Medicine (IOM), "To Err Is Human: Building a Safer Health System," [PDF] which estimated that between 44,000 and 98,000 deaths in the U.S. each year can be attributed to preventable medical errors.
Since the IOM report was published, little has been done to change the systems of care delivery that can lead providers to make errors, said Dr. Jha, an internist at the VA Boston Healthcare System and professor of health policy and management at the Harvard School of Public Health in Boston.
"When a physician orders the wrong medication because two drugs might sound alike, or when a patient develops a central-line infection because a rushed surgeon didn't use proper sterile technique, we now understand that we need to focus on the system that produced the errors," Dr. Jha told Senate subcommittee members.
Both Dr. Jha and panelist Peter Pronovost, MD, PhD, FCCM, senior vice president for patient safety and quality at Johns Hopkins Medicine in Baltimore, said the Centers for Disease Control and Prevention should expand its National Nosocomial Infections Surveillance Program to collect and report data on medical errors.
Several other speakers said hospitals should be mandated to publicly report medical errors.
"Public disclosure is a critical element to preventing these events from happening," said panelist Lisa McGiffert, director of the Consumers Union Safe Patient Project in Austin, Texas. "It informs people about healthcare outcomes and motivates healthcare providers to do more to prevent errors." TH
Visit our website for more information on the impact of medical errors.
In a Senate subcommittee hearing last month, a panel of patient safety experts and physicians raised concerns about the problem of preventable medical errors, which they say can be linked to more than 1,000 patient deaths per day.
For their part, SHM Public Policy Committee member Bradley Flansbaum, DO, MPH, FHM, says hospitalists should demand that their hospitals report better data on patient outcomes.
"When you talk about a patient death, you're talking about the efforts of an entire hospital going into the death or survival of that patient," says Dr. Flansbaum, a hospitalist at Lenox Hill Hospital in New York. "If you ask, 'Where did something go wrong?' just looking at the mortality rate doesn't help. What service are you inquiring about? How can you get clean data that’s also useful to the clinician?"
In his testimony before the Subcommittee on Primary Health and Aging, hospitalist Ashish Jha, MD, MPH, referenced a landmark 1999 report from the Institute of Medicine (IOM), "To Err Is Human: Building a Safer Health System," [PDF] which estimated that between 44,000 and 98,000 deaths in the U.S. each year can be attributed to preventable medical errors.
Since the IOM report was published, little has been done to change the systems of care delivery that can lead providers to make errors, said Dr. Jha, an internist at the VA Boston Healthcare System and professor of health policy and management at the Harvard School of Public Health in Boston.
"When a physician orders the wrong medication because two drugs might sound alike, or when a patient develops a central-line infection because a rushed surgeon didn't use proper sterile technique, we now understand that we need to focus on the system that produced the errors," Dr. Jha told Senate subcommittee members.
Both Dr. Jha and panelist Peter Pronovost, MD, PhD, FCCM, senior vice president for patient safety and quality at Johns Hopkins Medicine in Baltimore, said the Centers for Disease Control and Prevention should expand its National Nosocomial Infections Surveillance Program to collect and report data on medical errors.
Several other speakers said hospitals should be mandated to publicly report medical errors.
"Public disclosure is a critical element to preventing these events from happening," said panelist Lisa McGiffert, director of the Consumers Union Safe Patient Project in Austin, Texas. "It informs people about healthcare outcomes and motivates healthcare providers to do more to prevent errors." TH
Visit our website for more information on the impact of medical errors.
In a Senate subcommittee hearing last month, a panel of patient safety experts and physicians raised concerns about the problem of preventable medical errors, which they say can be linked to more than 1,000 patient deaths per day.
For their part, SHM Public Policy Committee member Bradley Flansbaum, DO, MPH, FHM, says hospitalists should demand that their hospitals report better data on patient outcomes.
"When you talk about a patient death, you're talking about the efforts of an entire hospital going into the death or survival of that patient," says Dr. Flansbaum, a hospitalist at Lenox Hill Hospital in New York. "If you ask, 'Where did something go wrong?' just looking at the mortality rate doesn't help. What service are you inquiring about? How can you get clean data that’s also useful to the clinician?"
In his testimony before the Subcommittee on Primary Health and Aging, hospitalist Ashish Jha, MD, MPH, referenced a landmark 1999 report from the Institute of Medicine (IOM), "To Err Is Human: Building a Safer Health System," [PDF] which estimated that between 44,000 and 98,000 deaths in the U.S. each year can be attributed to preventable medical errors.
Since the IOM report was published, little has been done to change the systems of care delivery that can lead providers to make errors, said Dr. Jha, an internist at the VA Boston Healthcare System and professor of health policy and management at the Harvard School of Public Health in Boston.
"When a physician orders the wrong medication because two drugs might sound alike, or when a patient develops a central-line infection because a rushed surgeon didn't use proper sterile technique, we now understand that we need to focus on the system that produced the errors," Dr. Jha told Senate subcommittee members.
Both Dr. Jha and panelist Peter Pronovost, MD, PhD, FCCM, senior vice president for patient safety and quality at Johns Hopkins Medicine in Baltimore, said the Centers for Disease Control and Prevention should expand its National Nosocomial Infections Surveillance Program to collect and report data on medical errors.
Several other speakers said hospitals should be mandated to publicly report medical errors.
"Public disclosure is a critical element to preventing these events from happening," said panelist Lisa McGiffert, director of the Consumers Union Safe Patient Project in Austin, Texas. "It informs people about healthcare outcomes and motivates healthcare providers to do more to prevent errors." TH
Visit our website for more information on the impact of medical errors.
Many surgical residents consider quitting during training
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
FROM JAMA Surgery
Major finding: More than half of survey respondents (58%) considered quitting their general surgery residency, an issue more persistent with female respondents.
Data source: Analysis of 288 responses to a survey of general surgery residents in 13 residency programs across different regions (West, Southwest, Midwest, and Northeast) and training centers (university programs, independent programs, or hybrid university-affiliated programs without an onsite university or medical school).
Disclosures: The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS. Report of the Guideline Development Subcommittee of the American Academy of Neurology
Screening and Diagnosis
What Clinical Evaluation Procedures and Screening and Diagnostic Tools Can Be Used to Accurately Identify Symptoms and Make Diagnoses of Emotional Disorders in Individuals with Multiple Sclerosis (MS)?
Conclusions and Recommendations
In individuals with MS, the Center for Neurologic Study Emotional Lability Scale (CNS-LS) is possibly effective and may be considered for screening for pseudobulbar affect (PBA) (Level C, 1 Class II study [Smith et al., 2004]). The General Health Questionnaire (GHQ) (Goldberg & Hillier, 1979) is possibly effective and may be considered for identifying individuals with broadly defined emotional disturbances (Level C, 1 Class II study [Rabins & Brooks, 1981]). The Beck Depression Inventory (BDI) (Beck et al., 1961) and a 2-question screen (Whooley et al., 1997) are possibly effective and may be considered for identifying individuals with major depressive disorder (MDD) (Level C, 1 Class II study each [Sullivan et al., 1995; Mohr et al., 2007]). There is insufficient evidence to support/refute using the Center for Epidemiologic Studies Depression Rating Scale (CES-D) (Radloff, 1977) to screen for depressive symptoms (Pandya, Metz, & Patten, 2005) or a single question to screen for MDD (Vahter et al., 2007) (Level U, 1 Class III study each); the possibility that somatic or neurovegetative symptoms negatively affect the accuracy of BDI results (Level U, 2 conflicting Class III studies) (Mohr et al., 1997; Randolph et al., 2000); and the use of specific instruments or clinical evaluation procedures to diagnose emotional disorders in individuals with MS (Level U).
Clinical Context
Because emotional disorders may be unrecognized in medical settings, validated screening tools might improve identification of individuals who could benefit from further evaluation and treatment. The true positive rate of a screening tool depends not only on its sensitivity but also on the point prevalence of the disorder in the population under study. Clinically, false-positive results are not a major concern because individuals with the conditions typically identified (e.g., adjustment and subthreshold depressive disorders) can benefit from further assessment. Administratively, however, screening tools with high false-positive rates unnecessarily increase resource use.
Treatments
What Are the Effective Treatments for Disorders of Mood in Individuals with MS?
Conclusion and Recommendations
For individuals with MS, a 16-week program of individual telephone-administered cognitive behavioral therapy (T-CBT) program is possibly effective and may be considered in treating depressive symptoms (Level C, 1 Class II study [Mohr et al., 2005], 1 Class III study [Mohr, et al., 2000]). There is insufficient evidence to support/refute the efficacy and use of 1) sertraline (Mohr et al., 2001), desipramine (Schiffer & Wineman, 1990), paroxetine (Ehde et al., 2008), individual in-person cognitive behavioral therapy (CBT) (Mohr et al., 2001), individual in-person CBT plus relaxation training (Foley et al., 1987), or CBT-based group therapy (Forman & Lincoln, 2010) for depressive symptoms; or 2) individual in-person CBT plus relaxation training (Foley et al., 1987), group relaxation and imagery (Maguire, 1996), or CBT-based group therapy (Forman & Lincoln, 2010) for anxiety (Level U, 1 Class III study each).
Clinical Context
There is evidence supporting the efficacy of pharmacologic and nonpharmacologic therapies for depressed mood and anxiety in individuals without MS. Despite the lack of evidence in individuals with MS, these therapies are frequently used to treat emotional disorders in this population.
What Are the Effective Treatments for Disorders of Affect in Individuals with MS?
Conclusion and Recommendations
Dextromethorphan and quinidine (DM/Q) is possibly effective and safe and may be considered for treating individuals with MS with PBA (Level C, 1 Class II study) (Panitch et al., 2006).
Clinical Context
DM/Q is the only drug approved by the US Food and Drug Administration for PBA treatment, although other drugs are used in clinical practice (e.g., selective serotonin reuptake inhibitors, tricyclic antidepressants). There are no randomized placebo-controlled trials of these other agents.
Definitions:
Classification of Evidence
Screening Articles
Class I: A statistical, population based sample of patients studied at a uniform point in time (usually early) during the course of the condition. All patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation that is masked to the patients' clinical presentations.
Class II: A statistical, non-referral clinic based sample of patients studied at a uniform point in time (usually early) during the course of the condition. Most patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation that is masked to the patients' clinical presentations.
Class III: A sample of patients studied during the course of the condition. Some patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation by someone other than the treating physician.
Class IV: Studies not meeting Class I, II, or III criteria, including consensus, expert opinion or a case report.
Diagnostic Articles
Class I: A cohort study with prospective data collection of a broad spectrum of persons with the suspected condition, using an acceptable reference standard for case definition. The diagnostic test is objective or performed and interpreted without knowledge of the patient's clinical status. Study results allow calculation of measures of diagnostic accuracy.
Class II: A case control study of a broad spectrum of persons with the condition established by an acceptable reference standard compared to a broad spectrum of controls or a cohort study where a broad spectrum of persons with the suspected condition where the data was collected retrospectively. The diagnostic test is objective or performed and interpreted without knowledge of disease status. Study results allow calculation of measures of diagnostic accuracy.
Class III: A case control study or cohort study where either persons with the condition or controls are of a narrow spectrum. The condition is established by an acceptable reference standard. The reference standard and diagnostic test are objective or performed and interpreted by different observers. Study results allow calculation of measures of diagnostic accuracy.
Class IV: Studies not meeting Class I, II, or III criteria including consensus, expert opinion, or a case report.
Therapeutic Articles
Class I: A randomized, controlled clinical trial of the intervention of interest with masked or objective outcome assessment, in a representative population. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences.
The following are also required:
- Concealed allocation
- Primary outcome(s) clearly defined
- Exclusion/inclusion criteria clearly defined
- Adequate accounting for dropouts (with at least 80% of enrolled subjects completing the study) and crossovers with numbers sufficiently low to have minimal potential for bias.
- For noninferiority or equivalence trials claiming to prove efficacy for one or both drugs, the following are also required*:
- The authors explicitly state the clinically meaningful difference to be excluded by defining the threshold for equivalence or noninferiority.
- The standard treatment used in the study is substantially similar to that used in previous studies establishing efficacy of the standard treatment (e.g., for a drug, the mode of administration, dose and dosage adjustments are similar to those previously shown to be effective).
- The inclusion and exclusion criteria for patient selection and the outcomes of patients on the standard treatment are comparable to those of previous studies establishing efficacy of the standard treatment.
- The interpretation of the results of the study is based upon a per protocol analysis that takes into account dropouts or crossovers.
Class II: A randomized controlled clinical trial of the intervention of interest in a representative population with masked or objective outcome assessment that lacks one criteria a–e above or a prospective matched cohort study with masked or objective outcome assessment in a representative population that meets b–e above. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences.
Class III: All other controlled trials (including well-defined natural history controls or patients serving as own controls) in a representative population, where outcome is independently assessed, or independently derived by objective outcome measurement.**
Class IV: Studies not meeting Class I, II, or III criteria including consensus or expert opinion.
*Note that numbers 1-3 in Class Ie are required for Class II in equivalence trials. If any one of the three is missing, the class is automatically downgraded to Class III.
**Objective outcome measurement: an outcome measure that is unlikely to be affected by an observer's (patient, treating physician, investigator) expectation or bias (e.g., blood tests, administrative outcome data).
Classification of Recommendations
Level A = Established as effective, ineffective or harmful (or established as useful/predictive or not useful/predictive) for the given condition in the specified population. (Level A rating requires at least two consistent Class I studies.)*
Level B = Probably effective, ineffective or harmful (or probably useful/predictive or not useful/predictive) for the given condition in the specified population. (Level B rating requires at least one Class I study or two consistent Class II studies.)
Level C = Possibly effective, ineffective or harmful (or possibly useful/predictive or not useful/predictive) for the given condition in the specified population. (Level C rating requires at least one Class II study or two consistent Class III studies.)
Level U = Data inadequate or conflicting; given current knowledge, treatment (test, predictor) is unproven.
* In exceptional cases, one convincing Class I study may suffice for an "A" recommendation if 1) all criteria are met, 2) the magnitude of effect is large (relative rate improved outcome >5 and the lower limit of the confidence interval is >2).
Screening and Diagnosis
What Clinical Evaluation Procedures and Screening and Diagnostic Tools Can Be Used to Accurately Identify Symptoms and Make Diagnoses of Emotional Disorders in Individuals with Multiple Sclerosis (MS)?
Conclusions and Recommendations
In individuals with MS, the Center for Neurologic Study Emotional Lability Scale (CNS-LS) is possibly effective and may be considered for screening for pseudobulbar affect (PBA) (Level C, 1 Class II study [Smith et al., 2004]). The General Health Questionnaire (GHQ) (Goldberg & Hillier, 1979) is possibly effective and may be considered for identifying individuals with broadly defined emotional disturbances (Level C, 1 Class II study [Rabins & Brooks, 1981]). The Beck Depression Inventory (BDI) (Beck et al., 1961) and a 2-question screen (Whooley et al., 1997) are possibly effective and may be considered for identifying individuals with major depressive disorder (MDD) (Level C, 1 Class II study each [Sullivan et al., 1995; Mohr et al., 2007]). There is insufficient evidence to support/refute using the Center for Epidemiologic Studies Depression Rating Scale (CES-D) (Radloff, 1977) to screen for depressive symptoms (Pandya, Metz, & Patten, 2005) or a single question to screen for MDD (Vahter et al., 2007) (Level U, 1 Class III study each); the possibility that somatic or neurovegetative symptoms negatively affect the accuracy of BDI results (Level U, 2 conflicting Class III studies) (Mohr et al., 1997; Randolph et al., 2000); and the use of specific instruments or clinical evaluation procedures to diagnose emotional disorders in individuals with MS (Level U).
Clinical Context
Because emotional disorders may be unrecognized in medical settings, validated screening tools might improve identification of individuals who could benefit from further evaluation and treatment. The true positive rate of a screening tool depends not only on its sensitivity but also on the point prevalence of the disorder in the population under study. Clinically, false-positive results are not a major concern because individuals with the conditions typically identified (e.g., adjustment and subthreshold depressive disorders) can benefit from further assessment. Administratively, however, screening tools with high false-positive rates unnecessarily increase resource use.
Treatments
What Are the Effective Treatments for Disorders of Mood in Individuals with MS?
Conclusion and Recommendations
For individuals with MS, a 16-week program of individual telephone-administered cognitive behavioral therapy (T-CBT) program is possibly effective and may be considered in treating depressive symptoms (Level C, 1 Class II study [Mohr et al., 2005], 1 Class III study [Mohr, et al., 2000]). There is insufficient evidence to support/refute the efficacy and use of 1) sertraline (Mohr et al., 2001), desipramine (Schiffer & Wineman, 1990), paroxetine (Ehde et al., 2008), individual in-person cognitive behavioral therapy (CBT) (Mohr et al., 2001), individual in-person CBT plus relaxation training (Foley et al., 1987), or CBT-based group therapy (Forman & Lincoln, 2010) for depressive symptoms; or 2) individual in-person CBT plus relaxation training (Foley et al., 1987), group relaxation and imagery (Maguire, 1996), or CBT-based group therapy (Forman & Lincoln, 2010) for anxiety (Level U, 1 Class III study each).
Clinical Context
There is evidence supporting the efficacy of pharmacologic and nonpharmacologic therapies for depressed mood and anxiety in individuals without MS. Despite the lack of evidence in individuals with MS, these therapies are frequently used to treat emotional disorders in this population.
What Are the Effective Treatments for Disorders of Affect in Individuals with MS?
Conclusion and Recommendations
Dextromethorphan and quinidine (DM/Q) is possibly effective and safe and may be considered for treating individuals with MS with PBA (Level C, 1 Class II study) (Panitch et al., 2006).
Clinical Context
DM/Q is the only drug approved by the US Food and Drug Administration for PBA treatment, although other drugs are used in clinical practice (e.g., selective serotonin reuptake inhibitors, tricyclic antidepressants). There are no randomized placebo-controlled trials of these other agents.
Definitions:
Classification of Evidence
Screening Articles
Class I: A statistical, population based sample of patients studied at a uniform point in time (usually early) during the course of the condition. All patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation that is masked to the patients' clinical presentations.
Class II: A statistical, non-referral clinic based sample of patients studied at a uniform point in time (usually early) during the course of the condition. Most patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation that is masked to the patients' clinical presentations.
Class III: A sample of patients studied during the course of the condition. Some patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation by someone other than the treating physician.
Class IV: Studies not meeting Class I, II, or III criteria, including consensus, expert opinion or a case report.
Diagnostic Articles
Class I: A cohort study with prospective data collection of a broad spectrum of persons with the suspected condition, using an acceptable reference standard for case definition. The diagnostic test is objective or performed and interpreted without knowledge of the patient's clinical status. Study results allow calculation of measures of diagnostic accuracy.
Class II: A case control study of a broad spectrum of persons with the condition established by an acceptable reference standard compared to a broad spectrum of controls or a cohort study where a broad spectrum of persons with the suspected condition where the data was collected retrospectively. The diagnostic test is objective or performed and interpreted without knowledge of disease status. Study results allow calculation of measures of diagnostic accuracy.
Class III: A case control study or cohort study where either persons with the condition or controls are of a narrow spectrum. The condition is established by an acceptable reference standard. The reference standard and diagnostic test are objective or performed and interpreted by different observers. Study results allow calculation of measures of diagnostic accuracy.
Class IV: Studies not meeting Class I, II, or III criteria including consensus, expert opinion, or a case report.
Therapeutic Articles
Class I: A randomized, controlled clinical trial of the intervention of interest with masked or objective outcome assessment, in a representative population. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences.
The following are also required:
- Concealed allocation
- Primary outcome(s) clearly defined
- Exclusion/inclusion criteria clearly defined
- Adequate accounting for dropouts (with at least 80% of enrolled subjects completing the study) and crossovers with numbers sufficiently low to have minimal potential for bias.
- For noninferiority or equivalence trials claiming to prove efficacy for one or both drugs, the following are also required*:
- The authors explicitly state the clinically meaningful difference to be excluded by defining the threshold for equivalence or noninferiority.
- The standard treatment used in the study is substantially similar to that used in previous studies establishing efficacy of the standard treatment (e.g., for a drug, the mode of administration, dose and dosage adjustments are similar to those previously shown to be effective).
- The inclusion and exclusion criteria for patient selection and the outcomes of patients on the standard treatment are comparable to those of previous studies establishing efficacy of the standard treatment.
- The interpretation of the results of the study is based upon a per protocol analysis that takes into account dropouts or crossovers.
Class II: A randomized controlled clinical trial of the intervention of interest in a representative population with masked or objective outcome assessment that lacks one criteria a–e above or a prospective matched cohort study with masked or objective outcome assessment in a representative population that meets b–e above. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences.
Class III: All other controlled trials (including well-defined natural history controls or patients serving as own controls) in a representative population, where outcome is independently assessed, or independently derived by objective outcome measurement.**
Class IV: Studies not meeting Class I, II, or III criteria including consensus or expert opinion.
*Note that numbers 1-3 in Class Ie are required for Class II in equivalence trials. If any one of the three is missing, the class is automatically downgraded to Class III.
**Objective outcome measurement: an outcome measure that is unlikely to be affected by an observer's (patient, treating physician, investigator) expectation or bias (e.g., blood tests, administrative outcome data).
Classification of Recommendations
Level A = Established as effective, ineffective or harmful (or established as useful/predictive or not useful/predictive) for the given condition in the specified population. (Level A rating requires at least two consistent Class I studies.)*
Level B = Probably effective, ineffective or harmful (or probably useful/predictive or not useful/predictive) for the given condition in the specified population. (Level B rating requires at least one Class I study or two consistent Class II studies.)
Level C = Possibly effective, ineffective or harmful (or possibly useful/predictive or not useful/predictive) for the given condition in the specified population. (Level C rating requires at least one Class II study or two consistent Class III studies.)
Level U = Data inadequate or conflicting; given current knowledge, treatment (test, predictor) is unproven.
* In exceptional cases, one convincing Class I study may suffice for an "A" recommendation if 1) all criteria are met, 2) the magnitude of effect is large (relative rate improved outcome >5 and the lower limit of the confidence interval is >2).
Screening and Diagnosis
What Clinical Evaluation Procedures and Screening and Diagnostic Tools Can Be Used to Accurately Identify Symptoms and Make Diagnoses of Emotional Disorders in Individuals with Multiple Sclerosis (MS)?
Conclusions and Recommendations
In individuals with MS, the Center for Neurologic Study Emotional Lability Scale (CNS-LS) is possibly effective and may be considered for screening for pseudobulbar affect (PBA) (Level C, 1 Class II study [Smith et al., 2004]). The General Health Questionnaire (GHQ) (Goldberg & Hillier, 1979) is possibly effective and may be considered for identifying individuals with broadly defined emotional disturbances (Level C, 1 Class II study [Rabins & Brooks, 1981]). The Beck Depression Inventory (BDI) (Beck et al., 1961) and a 2-question screen (Whooley et al., 1997) are possibly effective and may be considered for identifying individuals with major depressive disorder (MDD) (Level C, 1 Class II study each [Sullivan et al., 1995; Mohr et al., 2007]). There is insufficient evidence to support/refute using the Center for Epidemiologic Studies Depression Rating Scale (CES-D) (Radloff, 1977) to screen for depressive symptoms (Pandya, Metz, & Patten, 2005) or a single question to screen for MDD (Vahter et al., 2007) (Level U, 1 Class III study each); the possibility that somatic or neurovegetative symptoms negatively affect the accuracy of BDI results (Level U, 2 conflicting Class III studies) (Mohr et al., 1997; Randolph et al., 2000); and the use of specific instruments or clinical evaluation procedures to diagnose emotional disorders in individuals with MS (Level U).
Clinical Context
Because emotional disorders may be unrecognized in medical settings, validated screening tools might improve identification of individuals who could benefit from further evaluation and treatment. The true positive rate of a screening tool depends not only on its sensitivity but also on the point prevalence of the disorder in the population under study. Clinically, false-positive results are not a major concern because individuals with the conditions typically identified (e.g., adjustment and subthreshold depressive disorders) can benefit from further assessment. Administratively, however, screening tools with high false-positive rates unnecessarily increase resource use.
Treatments
What Are the Effective Treatments for Disorders of Mood in Individuals with MS?
Conclusion and Recommendations
For individuals with MS, a 16-week program of individual telephone-administered cognitive behavioral therapy (T-CBT) program is possibly effective and may be considered in treating depressive symptoms (Level C, 1 Class II study [Mohr et al., 2005], 1 Class III study [Mohr, et al., 2000]). There is insufficient evidence to support/refute the efficacy and use of 1) sertraline (Mohr et al., 2001), desipramine (Schiffer & Wineman, 1990), paroxetine (Ehde et al., 2008), individual in-person cognitive behavioral therapy (CBT) (Mohr et al., 2001), individual in-person CBT plus relaxation training (Foley et al., 1987), or CBT-based group therapy (Forman & Lincoln, 2010) for depressive symptoms; or 2) individual in-person CBT plus relaxation training (Foley et al., 1987), group relaxation and imagery (Maguire, 1996), or CBT-based group therapy (Forman & Lincoln, 2010) for anxiety (Level U, 1 Class III study each).
Clinical Context
There is evidence supporting the efficacy of pharmacologic and nonpharmacologic therapies for depressed mood and anxiety in individuals without MS. Despite the lack of evidence in individuals with MS, these therapies are frequently used to treat emotional disorders in this population.
What Are the Effective Treatments for Disorders of Affect in Individuals with MS?
Conclusion and Recommendations
Dextromethorphan and quinidine (DM/Q) is possibly effective and safe and may be considered for treating individuals with MS with PBA (Level C, 1 Class II study) (Panitch et al., 2006).
Clinical Context
DM/Q is the only drug approved by the US Food and Drug Administration for PBA treatment, although other drugs are used in clinical practice (e.g., selective serotonin reuptake inhibitors, tricyclic antidepressants). There are no randomized placebo-controlled trials of these other agents.
Definitions:
Classification of Evidence
Screening Articles
Class I: A statistical, population based sample of patients studied at a uniform point in time (usually early) during the course of the condition. All patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation that is masked to the patients' clinical presentations.
Class II: A statistical, non-referral clinic based sample of patients studied at a uniform point in time (usually early) during the course of the condition. Most patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation that is masked to the patients' clinical presentations.
Class III: A sample of patients studied during the course of the condition. Some patients undergo the intervention of interest. The outcome, if not objective, is determined in an evaluation by someone other than the treating physician.
Class IV: Studies not meeting Class I, II, or III criteria, including consensus, expert opinion or a case report.
Diagnostic Articles
Class I: A cohort study with prospective data collection of a broad spectrum of persons with the suspected condition, using an acceptable reference standard for case definition. The diagnostic test is objective or performed and interpreted without knowledge of the patient's clinical status. Study results allow calculation of measures of diagnostic accuracy.
Class II: A case control study of a broad spectrum of persons with the condition established by an acceptable reference standard compared to a broad spectrum of controls or a cohort study where a broad spectrum of persons with the suspected condition where the data was collected retrospectively. The diagnostic test is objective or performed and interpreted without knowledge of disease status. Study results allow calculation of measures of diagnostic accuracy.
Class III: A case control study or cohort study where either persons with the condition or controls are of a narrow spectrum. The condition is established by an acceptable reference standard. The reference standard and diagnostic test are objective or performed and interpreted by different observers. Study results allow calculation of measures of diagnostic accuracy.
Class IV: Studies not meeting Class I, II, or III criteria including consensus, expert opinion, or a case report.
Therapeutic Articles
Class I: A randomized, controlled clinical trial of the intervention of interest with masked or objective outcome assessment, in a representative population. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences.
The following are also required:
- Concealed allocation
- Primary outcome(s) clearly defined
- Exclusion/inclusion criteria clearly defined
- Adequate accounting for dropouts (with at least 80% of enrolled subjects completing the study) and crossovers with numbers sufficiently low to have minimal potential for bias.
- For noninferiority or equivalence trials claiming to prove efficacy for one or both drugs, the following are also required*:
- The authors explicitly state the clinically meaningful difference to be excluded by defining the threshold for equivalence or noninferiority.
- The standard treatment used in the study is substantially similar to that used in previous studies establishing efficacy of the standard treatment (e.g., for a drug, the mode of administration, dose and dosage adjustments are similar to those previously shown to be effective).
- The inclusion and exclusion criteria for patient selection and the outcomes of patients on the standard treatment are comparable to those of previous studies establishing efficacy of the standard treatment.
- The interpretation of the results of the study is based upon a per protocol analysis that takes into account dropouts or crossovers.
Class II: A randomized controlled clinical trial of the intervention of interest in a representative population with masked or objective outcome assessment that lacks one criteria a–e above or a prospective matched cohort study with masked or objective outcome assessment in a representative population that meets b–e above. Relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences.
Class III: All other controlled trials (including well-defined natural history controls or patients serving as own controls) in a representative population, where outcome is independently assessed, or independently derived by objective outcome measurement.**
Class IV: Studies not meeting Class I, II, or III criteria including consensus or expert opinion.
*Note that numbers 1-3 in Class Ie are required for Class II in equivalence trials. If any one of the three is missing, the class is automatically downgraded to Class III.
**Objective outcome measurement: an outcome measure that is unlikely to be affected by an observer's (patient, treating physician, investigator) expectation or bias (e.g., blood tests, administrative outcome data).
Classification of Recommendations
Level A = Established as effective, ineffective or harmful (or established as useful/predictive or not useful/predictive) for the given condition in the specified population. (Level A rating requires at least two consistent Class I studies.)*
Level B = Probably effective, ineffective or harmful (or probably useful/predictive or not useful/predictive) for the given condition in the specified population. (Level B rating requires at least one Class I study or two consistent Class II studies.)
Level C = Possibly effective, ineffective or harmful (or possibly useful/predictive or not useful/predictive) for the given condition in the specified population. (Level C rating requires at least one Class II study or two consistent Class III studies.)
Level U = Data inadequate or conflicting; given current knowledge, treatment (test, predictor) is unproven.
* In exceptional cases, one convincing Class I study may suffice for an "A" recommendation if 1) all criteria are met, 2) the magnitude of effect is large (relative rate improved outcome >5 and the lower limit of the confidence interval is >2).
OBJECTIVE: To make evidence-based recommendations for screening, diagnosing, and treating psychiatric disorders in individuals with multiple sclerosis (MS).
METHODS: We reviewed the literature (1950 to August 2011) and evaluated the available evidence.
Guidelines are copyright © 2014 American Academy of Neurology. All rights reserved. The summary is provided by the Agency for Healthcare Research and Quality.
Varenicline combined with nicotine replacement therapy ups smoking quit rates
An estimated 42 million (18.1%) of the U.S. adult population continues to smoke cigarettes. Effective treatments exist for patients who are willing to avail themselves of such assistance. Varenicline is one of the most effective medications that we have to combat tobacco dependence. However, it doesn’t work for everybody, and questions have remained about how safe and effective it is to combine varenicline with other smoking cessation therapies such as nicotine replacement therapy.
Varenicline binds to a specific nicotine receptor, thereby partially agonizing and blocking it. The result is decreased cravings for tobacco and increased smoking quit rates. Data from early studies conducted by our group suggested that nicotine replacement therapy (NRT) combined with varenicline was safe, but questions remained about its efficacy.
One group of researchers conducted a multicenter clinical trial evaluating the efficacy of combining varenicline and the nicotine patch for increasing smoking cessation rates. Smokers were eligible if they smoked at least 10 cigarettes per day, reported F.N. Coenraad, from the Stellenbosch University, Cape Town, South Africa, and associates. Participants were randomized to active 15-mg nicotine patches or placebo patches started 2 weeks before the target quit date. All participants received varenicline for a total of 14 weeks with a 1-week ramp-up and a 1-week taper. Use of the varenicline in combination with the nicotine patch resulted in increased rates of continuous abstinence from smoking at 12 weeks (no smoking from weeks 9 to 12: 55.4% vs. 40.9%; odds ratio, 1.85; 95% confidence interval, 1.19-2.89; P = .007) and at 24 weeks (no smoking from weeks 9 to 24: 49% vs. 32.6%; OR, 1.98; 95% CI, 1.25-3.14; P = .004) (JAMA 2014;312:155-61).
This is a fantastic study answering a lingering question in tobacco control. But what is the theoretical underpinning by which this combination works? Isn’t the NRT blocked by the varenicline? It is possible that the varenicline incompletely saturates the nicotine receptors, which are additionally saturated by the supplemented nicotine. The varenicline effect is mediated through the alpha-4 beta-2 nicotinic receptor, and it is also possible that nicotine binds to nicotine receptor types that varenicline does not bind to, which decreases withdrawal symptoms.
We aren’t exactly sure how this might be working, but a near doubling of the odds of quitting is not to be disregarded. We are also not sure whether the effect holds when one uses other types of NRT such as the nicotine inhaler, nicotine lozenge, nicotine nasal spray, and nicotine gum. In practice, I tend to lean toward a combination of varenicline with the nicotine inhaler since the inhaler can help with some of the behavioral aspects of smoking while the varenicline does its heavy lifting.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert reports receiving research support from Pfizer, manufacturer of varenicline and the nicotine inhaler, and consulting fees from GlaxoSmithKline, manufacturer of the nicotine patch. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
An estimated 42 million (18.1%) of the U.S. adult population continues to smoke cigarettes. Effective treatments exist for patients who are willing to avail themselves of such assistance. Varenicline is one of the most effective medications that we have to combat tobacco dependence. However, it doesn’t work for everybody, and questions have remained about how safe and effective it is to combine varenicline with other smoking cessation therapies such as nicotine replacement therapy.
Varenicline binds to a specific nicotine receptor, thereby partially agonizing and blocking it. The result is decreased cravings for tobacco and increased smoking quit rates. Data from early studies conducted by our group suggested that nicotine replacement therapy (NRT) combined with varenicline was safe, but questions remained about its efficacy.
One group of researchers conducted a multicenter clinical trial evaluating the efficacy of combining varenicline and the nicotine patch for increasing smoking cessation rates. Smokers were eligible if they smoked at least 10 cigarettes per day, reported F.N. Coenraad, from the Stellenbosch University, Cape Town, South Africa, and associates. Participants were randomized to active 15-mg nicotine patches or placebo patches started 2 weeks before the target quit date. All participants received varenicline for a total of 14 weeks with a 1-week ramp-up and a 1-week taper. Use of the varenicline in combination with the nicotine patch resulted in increased rates of continuous abstinence from smoking at 12 weeks (no smoking from weeks 9 to 12: 55.4% vs. 40.9%; odds ratio, 1.85; 95% confidence interval, 1.19-2.89; P = .007) and at 24 weeks (no smoking from weeks 9 to 24: 49% vs. 32.6%; OR, 1.98; 95% CI, 1.25-3.14; P = .004) (JAMA 2014;312:155-61).
This is a fantastic study answering a lingering question in tobacco control. But what is the theoretical underpinning by which this combination works? Isn’t the NRT blocked by the varenicline? It is possible that the varenicline incompletely saturates the nicotine receptors, which are additionally saturated by the supplemented nicotine. The varenicline effect is mediated through the alpha-4 beta-2 nicotinic receptor, and it is also possible that nicotine binds to nicotine receptor types that varenicline does not bind to, which decreases withdrawal symptoms.
We aren’t exactly sure how this might be working, but a near doubling of the odds of quitting is not to be disregarded. We are also not sure whether the effect holds when one uses other types of NRT such as the nicotine inhaler, nicotine lozenge, nicotine nasal spray, and nicotine gum. In practice, I tend to lean toward a combination of varenicline with the nicotine inhaler since the inhaler can help with some of the behavioral aspects of smoking while the varenicline does its heavy lifting.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert reports receiving research support from Pfizer, manufacturer of varenicline and the nicotine inhaler, and consulting fees from GlaxoSmithKline, manufacturer of the nicotine patch. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
An estimated 42 million (18.1%) of the U.S. adult population continues to smoke cigarettes. Effective treatments exist for patients who are willing to avail themselves of such assistance. Varenicline is one of the most effective medications that we have to combat tobacco dependence. However, it doesn’t work for everybody, and questions have remained about how safe and effective it is to combine varenicline with other smoking cessation therapies such as nicotine replacement therapy.
Varenicline binds to a specific nicotine receptor, thereby partially agonizing and blocking it. The result is decreased cravings for tobacco and increased smoking quit rates. Data from early studies conducted by our group suggested that nicotine replacement therapy (NRT) combined with varenicline was safe, but questions remained about its efficacy.
One group of researchers conducted a multicenter clinical trial evaluating the efficacy of combining varenicline and the nicotine patch for increasing smoking cessation rates. Smokers were eligible if they smoked at least 10 cigarettes per day, reported F.N. Coenraad, from the Stellenbosch University, Cape Town, South Africa, and associates. Participants were randomized to active 15-mg nicotine patches or placebo patches started 2 weeks before the target quit date. All participants received varenicline for a total of 14 weeks with a 1-week ramp-up and a 1-week taper. Use of the varenicline in combination with the nicotine patch resulted in increased rates of continuous abstinence from smoking at 12 weeks (no smoking from weeks 9 to 12: 55.4% vs. 40.9%; odds ratio, 1.85; 95% confidence interval, 1.19-2.89; P = .007) and at 24 weeks (no smoking from weeks 9 to 24: 49% vs. 32.6%; OR, 1.98; 95% CI, 1.25-3.14; P = .004) (JAMA 2014;312:155-61).
This is a fantastic study answering a lingering question in tobacco control. But what is the theoretical underpinning by which this combination works? Isn’t the NRT blocked by the varenicline? It is possible that the varenicline incompletely saturates the nicotine receptors, which are additionally saturated by the supplemented nicotine. The varenicline effect is mediated through the alpha-4 beta-2 nicotinic receptor, and it is also possible that nicotine binds to nicotine receptor types that varenicline does not bind to, which decreases withdrawal symptoms.
We aren’t exactly sure how this might be working, but a near doubling of the odds of quitting is not to be disregarded. We are also not sure whether the effect holds when one uses other types of NRT such as the nicotine inhaler, nicotine lozenge, nicotine nasal spray, and nicotine gum. In practice, I tend to lean toward a combination of varenicline with the nicotine inhaler since the inhaler can help with some of the behavioral aspects of smoking while the varenicline does its heavy lifting.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author. Dr. Ebbert reports receiving research support from Pfizer, manufacturer of varenicline and the nicotine inhaler, and consulting fees from GlaxoSmithKline, manufacturer of the nicotine patch. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician.
FDA approves ex vivo lung perfusion device that preserves donor organs
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lung to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating the lungs, "which oxygenates the cells and makes it possible for the transplant team to examine the lungs’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About one in five donor lungs meet the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage. "Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.
This is exciting news given the shortage of available lungs which meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in postoperative complications. Also, how significant will the financial impact be using the device? I look forward to the results of long-term studies and hopefully this will be a viable option for our patients.
Dr. Jennifer Cox is assistant professor of pulmonary and critical care medicine critical care selective, University of South Florida, Tampa.
This is exciting news given the shortage of available lungs which meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in postoperative complications. Also, how significant will the financial impact be using the device? I look forward to the results of long-term studies and hopefully this will be a viable option for our patients.
Dr. Jennifer Cox is assistant professor of pulmonary and critical care medicine critical care selective, University of South Florida, Tampa.
This is exciting news given the shortage of available lungs which meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in postoperative complications. Also, how significant will the financial impact be using the device? I look forward to the results of long-term studies and hopefully this will be a viable option for our patients.
Dr. Jennifer Cox is assistant professor of pulmonary and critical care medicine critical care selective, University of South Florida, Tampa.
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lung to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating the lungs, "which oxygenates the cells and makes it possible for the transplant team to examine the lungs’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About one in five donor lungs meet the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage. "Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lung to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating the lungs, "which oxygenates the cells and makes it possible for the transplant team to examine the lungs’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About one in five donor lungs meet the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage. "Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.