User login
App cuts alcohol intake in risky drinkers
The key to reducing problem drinking may just be an app away.
, researchers in Australia have found.
Participants in the randomized controlled trial tracked information about their alcohol consumption, including the quantity and frequency. The intervention then generated an impulsivity score and implications for their risk for alcohol-related disorders and diseases, hospitalization, and death. The findings were published in Alcohol: Clinical & Experimental Research.
Worldwide each year, alcohol consumption accounts for 5.3% of all deaths. In the United States, an estimated 29.5 million people older than 12 years had alcohol use disorder in 2021.
More than 60% of people with alcohol use problems never seek out in-person treatment. Many are deterred from doing so by fear of judgment, stigma, and embarrassment, especially those at the low end of the alcohol use severity spectrum, according to the Australian researchers. Such fear-based barriers, however, may be overcome through the anonymity of a smartphone app.
The researchers tested whether hazardous drinkers who receive personalized feedback about their alcohol consumption and level of self-control would reduce their problem drinking more than hazardous drinkers who received only personalized information about their alcohol consumption or no feedback at all would.
“I knew from my previous research that just putting in the information is not enough to change someone’s drinking: It seems that putting in the information and then having someone tell you, ‘You drank x number of drinks, and that level of drinking is high according to Australian or WHO [World Health Organization] standards’ seems to be the critical point,” said Antoinette Poulton, PhD, of the University of Melbourne, who developed the app and led the study.
The study was conducted among first-year psychology students at the University of Melbourne between 2020 and 2022.
Each of the 313 participants in the study (average age 21.7 years; 74% women) provided estimates of alcohol intake over 14 days. A subset of 178 individuals utilized Alcohol Capture, the validated smartphone app, which records alcohol intake in real-time and includes an online cognitive task assessing impulsivity.
Participants were categorized as “hazardous” or “nonharmful” drinkers according to guidelines from the World Health Organization and were divided into three groups. Members in the alcohol intake feedback (Alc) group were given personalized feedback about their alcohol consumption, including whether their drinking exceeded Australian and/or WHO guidelines. Others were assigned to the Alc plus cognitive feedback (AlcCog) group and received the same feedback plus details about their level of self-control and information about the links between poor self-control and vulnerability for transition to alcohol use disorder. The control group did not receive personalized feedback. After 8 weeks, alcohol intake was again recorded over 14 days.
Relative to hazardous drinkers in the control group, total alcohol consumption among risky drinkers in the Alc group fell by 32% (or 3.8 standard drinks per week) and by 35% (or 4.2 standard drinks per week) in the AlcCog group, according to the researchers. That difference was not statistically significant.
“Our brief electronic intervention had clear impact on the drinking behavior of hazardous drinkers,” the researchers reported. “In fact, following the intervention, hazardous drinkers did not differ from non-harmful ones on total alcohol intake, quantity of intake per drinking day, or frequency of six or more drinking occasions.”
Drinks per drinking day also decreased by 31% (or 1.6 standard drinks) and 32% (or 2.1 standard drinks) in the Alc and AlcCog groups, respectively, compared with the control group.
Alcohol use did not appear to change among nonharmful drinkers in any of the study groups.
“This is a nice study, because it shows that a simple, small intervention can really have a profound effect on hazardous drinking,” said Akhil Anand, MD, an addiction psychiatrist and Medical Director of the Alcohol and Drug Recovery Center at Cleveland Clinic. “It’s hard to say if this intervention would work on very severe cases, but I like it because it’s anonymous, it’s quick, it’s easily accessible, and it doesn’t take too much health care personnel power to apply it,” Dr. Anand added.
This research was supported by an Early Career Researcher grant from the University of Melbourne. Dr. Poulton and Dr. Anand reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
The key to reducing problem drinking may just be an app away.
, researchers in Australia have found.
Participants in the randomized controlled trial tracked information about their alcohol consumption, including the quantity and frequency. The intervention then generated an impulsivity score and implications for their risk for alcohol-related disorders and diseases, hospitalization, and death. The findings were published in Alcohol: Clinical & Experimental Research.
Worldwide each year, alcohol consumption accounts for 5.3% of all deaths. In the United States, an estimated 29.5 million people older than 12 years had alcohol use disorder in 2021.
More than 60% of people with alcohol use problems never seek out in-person treatment. Many are deterred from doing so by fear of judgment, stigma, and embarrassment, especially those at the low end of the alcohol use severity spectrum, according to the Australian researchers. Such fear-based barriers, however, may be overcome through the anonymity of a smartphone app.
The researchers tested whether hazardous drinkers who receive personalized feedback about their alcohol consumption and level of self-control would reduce their problem drinking more than hazardous drinkers who received only personalized information about their alcohol consumption or no feedback at all would.
“I knew from my previous research that just putting in the information is not enough to change someone’s drinking: It seems that putting in the information and then having someone tell you, ‘You drank x number of drinks, and that level of drinking is high according to Australian or WHO [World Health Organization] standards’ seems to be the critical point,” said Antoinette Poulton, PhD, of the University of Melbourne, who developed the app and led the study.
The study was conducted among first-year psychology students at the University of Melbourne between 2020 and 2022.
Each of the 313 participants in the study (average age 21.7 years; 74% women) provided estimates of alcohol intake over 14 days. A subset of 178 individuals utilized Alcohol Capture, the validated smartphone app, which records alcohol intake in real-time and includes an online cognitive task assessing impulsivity.
Participants were categorized as “hazardous” or “nonharmful” drinkers according to guidelines from the World Health Organization and were divided into three groups. Members in the alcohol intake feedback (Alc) group were given personalized feedback about their alcohol consumption, including whether their drinking exceeded Australian and/or WHO guidelines. Others were assigned to the Alc plus cognitive feedback (AlcCog) group and received the same feedback plus details about their level of self-control and information about the links between poor self-control and vulnerability for transition to alcohol use disorder. The control group did not receive personalized feedback. After 8 weeks, alcohol intake was again recorded over 14 days.
Relative to hazardous drinkers in the control group, total alcohol consumption among risky drinkers in the Alc group fell by 32% (or 3.8 standard drinks per week) and by 35% (or 4.2 standard drinks per week) in the AlcCog group, according to the researchers. That difference was not statistically significant.
“Our brief electronic intervention had clear impact on the drinking behavior of hazardous drinkers,” the researchers reported. “In fact, following the intervention, hazardous drinkers did not differ from non-harmful ones on total alcohol intake, quantity of intake per drinking day, or frequency of six or more drinking occasions.”
Drinks per drinking day also decreased by 31% (or 1.6 standard drinks) and 32% (or 2.1 standard drinks) in the Alc and AlcCog groups, respectively, compared with the control group.
Alcohol use did not appear to change among nonharmful drinkers in any of the study groups.
“This is a nice study, because it shows that a simple, small intervention can really have a profound effect on hazardous drinking,” said Akhil Anand, MD, an addiction psychiatrist and Medical Director of the Alcohol and Drug Recovery Center at Cleveland Clinic. “It’s hard to say if this intervention would work on very severe cases, but I like it because it’s anonymous, it’s quick, it’s easily accessible, and it doesn’t take too much health care personnel power to apply it,” Dr. Anand added.
This research was supported by an Early Career Researcher grant from the University of Melbourne. Dr. Poulton and Dr. Anand reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
The key to reducing problem drinking may just be an app away.
, researchers in Australia have found.
Participants in the randomized controlled trial tracked information about their alcohol consumption, including the quantity and frequency. The intervention then generated an impulsivity score and implications for their risk for alcohol-related disorders and diseases, hospitalization, and death. The findings were published in Alcohol: Clinical & Experimental Research.
Worldwide each year, alcohol consumption accounts for 5.3% of all deaths. In the United States, an estimated 29.5 million people older than 12 years had alcohol use disorder in 2021.
More than 60% of people with alcohol use problems never seek out in-person treatment. Many are deterred from doing so by fear of judgment, stigma, and embarrassment, especially those at the low end of the alcohol use severity spectrum, according to the Australian researchers. Such fear-based barriers, however, may be overcome through the anonymity of a smartphone app.
The researchers tested whether hazardous drinkers who receive personalized feedback about their alcohol consumption and level of self-control would reduce their problem drinking more than hazardous drinkers who received only personalized information about their alcohol consumption or no feedback at all would.
“I knew from my previous research that just putting in the information is not enough to change someone’s drinking: It seems that putting in the information and then having someone tell you, ‘You drank x number of drinks, and that level of drinking is high according to Australian or WHO [World Health Organization] standards’ seems to be the critical point,” said Antoinette Poulton, PhD, of the University of Melbourne, who developed the app and led the study.
The study was conducted among first-year psychology students at the University of Melbourne between 2020 and 2022.
Each of the 313 participants in the study (average age 21.7 years; 74% women) provided estimates of alcohol intake over 14 days. A subset of 178 individuals utilized Alcohol Capture, the validated smartphone app, which records alcohol intake in real-time and includes an online cognitive task assessing impulsivity.
Participants were categorized as “hazardous” or “nonharmful” drinkers according to guidelines from the World Health Organization and were divided into three groups. Members in the alcohol intake feedback (Alc) group were given personalized feedback about their alcohol consumption, including whether their drinking exceeded Australian and/or WHO guidelines. Others were assigned to the Alc plus cognitive feedback (AlcCog) group and received the same feedback plus details about their level of self-control and information about the links between poor self-control and vulnerability for transition to alcohol use disorder. The control group did not receive personalized feedback. After 8 weeks, alcohol intake was again recorded over 14 days.
Relative to hazardous drinkers in the control group, total alcohol consumption among risky drinkers in the Alc group fell by 32% (or 3.8 standard drinks per week) and by 35% (or 4.2 standard drinks per week) in the AlcCog group, according to the researchers. That difference was not statistically significant.
“Our brief electronic intervention had clear impact on the drinking behavior of hazardous drinkers,” the researchers reported. “In fact, following the intervention, hazardous drinkers did not differ from non-harmful ones on total alcohol intake, quantity of intake per drinking day, or frequency of six or more drinking occasions.”
Drinks per drinking day also decreased by 31% (or 1.6 standard drinks) and 32% (or 2.1 standard drinks) in the Alc and AlcCog groups, respectively, compared with the control group.
Alcohol use did not appear to change among nonharmful drinkers in any of the study groups.
“This is a nice study, because it shows that a simple, small intervention can really have a profound effect on hazardous drinking,” said Akhil Anand, MD, an addiction psychiatrist and Medical Director of the Alcohol and Drug Recovery Center at Cleveland Clinic. “It’s hard to say if this intervention would work on very severe cases, but I like it because it’s anonymous, it’s quick, it’s easily accessible, and it doesn’t take too much health care personnel power to apply it,” Dr. Anand added.
This research was supported by an Early Career Researcher grant from the University of Melbourne. Dr. Poulton and Dr. Anand reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ALCOHOL: CLINICAL & EXPERIMENTAL RESEARCH
How a heat wave affects glycemic control
TOPLINE:
, according to research published online May 17 in Science of The Total Environment.
METHODOLOGY:
Researchers in Spain analyzed data from 2,701 adults with type 1 diabetes who had been using intermittently scanned continuous glucose monitoring (CGM) devices during a 2022 heat wave (July 9-26) and 14 days after. Extreme heat claimed nearly 62,000 lives across Europe in the summer of 2022.
TAKEAWAY:
Time in range (between 70 mg/dL and 180 mg/dL of interstitial glucose) decreased by 4%, from 60.8% during the heat wave to 54.8% after (P < .001).
Patients who scanned their CGM results the most during the heat wave (more than 13 scans per day) scanned less often after the weather broke (1.8 fewer scans per day) and experienced the biggest drop in time in range (−5.4%).
More patients met all time-in-range recommendations during the heat wave (10.6% vs. 8.4%, P < .001).
IN PRACTICE:
“We hypothesized that people with diabetes, who are highly vulnerable, have more time for self-management as they spend more time indoors,” study author Jesús Moreno Fernández, MD, PhD, said in an interview. “During the COVID-19 pandemic, something similar was observed among people with diabetes.”
SOURCE:
Moreno Fernández, with the department of endocrinology and nutrition at Ciudad Real General University Hospital in Spain, is the study’s lead author.
LIMITATIONS:
The CGM data were anonymized, so researchers could not examine how individual patient factors like sex, education, or treatment type may have influenced outcomes. Temperatures remained higher than usual even after the heat wave. Worsening glycemic control could be interpreted as a lag effect of prolonged heat exposure, the researchers note.
DISCLOSURES:
The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to research published online May 17 in Science of The Total Environment.
METHODOLOGY:
Researchers in Spain analyzed data from 2,701 adults with type 1 diabetes who had been using intermittently scanned continuous glucose monitoring (CGM) devices during a 2022 heat wave (July 9-26) and 14 days after. Extreme heat claimed nearly 62,000 lives across Europe in the summer of 2022.
TAKEAWAY:
Time in range (between 70 mg/dL and 180 mg/dL of interstitial glucose) decreased by 4%, from 60.8% during the heat wave to 54.8% after (P < .001).
Patients who scanned their CGM results the most during the heat wave (more than 13 scans per day) scanned less often after the weather broke (1.8 fewer scans per day) and experienced the biggest drop in time in range (−5.4%).
More patients met all time-in-range recommendations during the heat wave (10.6% vs. 8.4%, P < .001).
IN PRACTICE:
“We hypothesized that people with diabetes, who are highly vulnerable, have more time for self-management as they spend more time indoors,” study author Jesús Moreno Fernández, MD, PhD, said in an interview. “During the COVID-19 pandemic, something similar was observed among people with diabetes.”
SOURCE:
Moreno Fernández, with the department of endocrinology and nutrition at Ciudad Real General University Hospital in Spain, is the study’s lead author.
LIMITATIONS:
The CGM data were anonymized, so researchers could not examine how individual patient factors like sex, education, or treatment type may have influenced outcomes. Temperatures remained higher than usual even after the heat wave. Worsening glycemic control could be interpreted as a lag effect of prolonged heat exposure, the researchers note.
DISCLOSURES:
The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to research published online May 17 in Science of The Total Environment.
METHODOLOGY:
Researchers in Spain analyzed data from 2,701 adults with type 1 diabetes who had been using intermittently scanned continuous glucose monitoring (CGM) devices during a 2022 heat wave (July 9-26) and 14 days after. Extreme heat claimed nearly 62,000 lives across Europe in the summer of 2022.
TAKEAWAY:
Time in range (between 70 mg/dL and 180 mg/dL of interstitial glucose) decreased by 4%, from 60.8% during the heat wave to 54.8% after (P < .001).
Patients who scanned their CGM results the most during the heat wave (more than 13 scans per day) scanned less often after the weather broke (1.8 fewer scans per day) and experienced the biggest drop in time in range (−5.4%).
More patients met all time-in-range recommendations during the heat wave (10.6% vs. 8.4%, P < .001).
IN PRACTICE:
“We hypothesized that people with diabetes, who are highly vulnerable, have more time for self-management as they spend more time indoors,” study author Jesús Moreno Fernández, MD, PhD, said in an interview. “During the COVID-19 pandemic, something similar was observed among people with diabetes.”
SOURCE:
Moreno Fernández, with the department of endocrinology and nutrition at Ciudad Real General University Hospital in Spain, is the study’s lead author.
LIMITATIONS:
The CGM data were anonymized, so researchers could not examine how individual patient factors like sex, education, or treatment type may have influenced outcomes. Temperatures remained higher than usual even after the heat wave. Worsening glycemic control could be interpreted as a lag effect of prolonged heat exposure, the researchers note.
DISCLOSURES:
The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM SCIENCE OF THE TOTAL ENVIRONMENT
Omega-3s and AFib: No added risk from eating fish but high-dose supplement questions persist
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Nurse practitioners sue state over right to use ‘doctor’ title
, saying it violates their first amendment right to use the honorific title without fear of regulatory repercussions.
The case highlights ongoing scope-creep battles as the American Medical Association tries to preserve the physician-led team model and nursing organizations and some lawmakers push for greater autonomy for allied professionals.
In the complaint filed in district court in June, plaintiffs Jacqueline Palmer, DNP, Heather Lewis, DNP, and Rodolfo Jaravata-Hanson, DNP, say they fear the state will sanction them. They note that “Doctor Sarah,” another DNP, was fined nearly $20,000 by the state last November for false advertising and fraud after using the moniker in her online advertising and social media accounts.
The fine was part of a settlement that the DNP, Sarah Erny, reached with the state to resolve allegations that she failed to identify her supervising physician and inform the public that she was not a medical doctor.
Under California’s Medical Practice Act, individuals cannot refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Instead, nurse practitioners certified by the California Board of Registered Nursing may use titles like “Certified Nurse Practitioner” and “Advanced Practice Registered Nurse,” corresponding letters such as APRN-CNP, RN, and NP, and phrases like pediatric nurse practitioner to identify specialization.
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
The nonprofit Pacific Legal Foundation represents the plaintiffs. In court records, its attorneys argue that after “years earning their advanced degrees and qualifications ... they should be able to speak truthfully about them in their workplaces, on their business cards, the Internet, and social media, so long as they clarify that they are nurse practitioners.”
State lawmakers’ attempts to clarify the roles of physicians and nurse practitioners have seen mixed results. Florida legislators recently passed a bill to prevent advanced practice nurses from using the honorific title, reserving it only for MDs and DOs. Gov. Ron DeSantis vetoed it last month.
In May, Georgia lawmakers passed the Health Care Practitioners Truth and Transparency Act. It requires advanced practice nurses and physician assistants with doctoral degrees who refer to themselves as doctors in a clinical setting to state they are not medical doctors or physicians.
Still, some health professionals say that the designation should only be used in academic settings or among peers, and that all doctoral degree holders should ditch the moniker at the bedside to ease patient communications.
Named as defendants in the suit are three state officials: California Attorney General Rob Bonta, state Medical Board President Kristina Lawson, and California Board of Registered Nursing Executive Officer Loretta Melby.
A version of this article first appeared on Medscape.com.
, saying it violates their first amendment right to use the honorific title without fear of regulatory repercussions.
The case highlights ongoing scope-creep battles as the American Medical Association tries to preserve the physician-led team model and nursing organizations and some lawmakers push for greater autonomy for allied professionals.
In the complaint filed in district court in June, plaintiffs Jacqueline Palmer, DNP, Heather Lewis, DNP, and Rodolfo Jaravata-Hanson, DNP, say they fear the state will sanction them. They note that “Doctor Sarah,” another DNP, was fined nearly $20,000 by the state last November for false advertising and fraud after using the moniker in her online advertising and social media accounts.
The fine was part of a settlement that the DNP, Sarah Erny, reached with the state to resolve allegations that she failed to identify her supervising physician and inform the public that she was not a medical doctor.
Under California’s Medical Practice Act, individuals cannot refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Instead, nurse practitioners certified by the California Board of Registered Nursing may use titles like “Certified Nurse Practitioner” and “Advanced Practice Registered Nurse,” corresponding letters such as APRN-CNP, RN, and NP, and phrases like pediatric nurse practitioner to identify specialization.
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
The nonprofit Pacific Legal Foundation represents the plaintiffs. In court records, its attorneys argue that after “years earning their advanced degrees and qualifications ... they should be able to speak truthfully about them in their workplaces, on their business cards, the Internet, and social media, so long as they clarify that they are nurse practitioners.”
State lawmakers’ attempts to clarify the roles of physicians and nurse practitioners have seen mixed results. Florida legislators recently passed a bill to prevent advanced practice nurses from using the honorific title, reserving it only for MDs and DOs. Gov. Ron DeSantis vetoed it last month.
In May, Georgia lawmakers passed the Health Care Practitioners Truth and Transparency Act. It requires advanced practice nurses and physician assistants with doctoral degrees who refer to themselves as doctors in a clinical setting to state they are not medical doctors or physicians.
Still, some health professionals say that the designation should only be used in academic settings or among peers, and that all doctoral degree holders should ditch the moniker at the bedside to ease patient communications.
Named as defendants in the suit are three state officials: California Attorney General Rob Bonta, state Medical Board President Kristina Lawson, and California Board of Registered Nursing Executive Officer Loretta Melby.
A version of this article first appeared on Medscape.com.
, saying it violates their first amendment right to use the honorific title without fear of regulatory repercussions.
The case highlights ongoing scope-creep battles as the American Medical Association tries to preserve the physician-led team model and nursing organizations and some lawmakers push for greater autonomy for allied professionals.
In the complaint filed in district court in June, plaintiffs Jacqueline Palmer, DNP, Heather Lewis, DNP, and Rodolfo Jaravata-Hanson, DNP, say they fear the state will sanction them. They note that “Doctor Sarah,” another DNP, was fined nearly $20,000 by the state last November for false advertising and fraud after using the moniker in her online advertising and social media accounts.
The fine was part of a settlement that the DNP, Sarah Erny, reached with the state to resolve allegations that she failed to identify her supervising physician and inform the public that she was not a medical doctor.
Under California’s Medical Practice Act, individuals cannot refer to themselves as “doctor, physician, or any other terms or letters indicating or implying that he or she is a physician and surgeon ... without having ... a certificate as a physician and surgeon.”
Instead, nurse practitioners certified by the California Board of Registered Nursing may use titles like “Certified Nurse Practitioner” and “Advanced Practice Registered Nurse,” corresponding letters such as APRN-CNP, RN, and NP, and phrases like pediatric nurse practitioner to identify specialization.
Individuals who misrepresent themselves are subject to misdemeanor charges and civil penalties.
The nonprofit Pacific Legal Foundation represents the plaintiffs. In court records, its attorneys argue that after “years earning their advanced degrees and qualifications ... they should be able to speak truthfully about them in their workplaces, on their business cards, the Internet, and social media, so long as they clarify that they are nurse practitioners.”
State lawmakers’ attempts to clarify the roles of physicians and nurse practitioners have seen mixed results. Florida legislators recently passed a bill to prevent advanced practice nurses from using the honorific title, reserving it only for MDs and DOs. Gov. Ron DeSantis vetoed it last month.
In May, Georgia lawmakers passed the Health Care Practitioners Truth and Transparency Act. It requires advanced practice nurses and physician assistants with doctoral degrees who refer to themselves as doctors in a clinical setting to state they are not medical doctors or physicians.
Still, some health professionals say that the designation should only be used in academic settings or among peers, and that all doctoral degree holders should ditch the moniker at the bedside to ease patient communications.
Named as defendants in the suit are three state officials: California Attorney General Rob Bonta, state Medical Board President Kristina Lawson, and California Board of Registered Nursing Executive Officer Loretta Melby.
A version of this article first appeared on Medscape.com.
Research points toward combination therapy for Lyme and improved diagnostics
Several recent developments in Lyme disease treatment and diagnosis may pave the way forward for combating disease that persists following missed or delayed diagnoses or remains following standard treatment. These include combination therapy to address “persister” bacteria and diagnostic tests that test directly for the pathogen and/or indirectly test for host response, according to experts who presented at a 2-day National Academies of Science, Engineering and Medicine workshop on infection-associated chronic illnesses.
Research has shown that 60% of people who are infected and not treated during the early or early disseminated stages of Lyme disease go on to develop late Lyme arthritis, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center in Baltimore. And in the real world, there’s an additional category of patients: Those who are misdiagnosed and develop infection-related persistent symptoms – such as fatigue, brain fog/cognitive dysfunction, and musculoskeletal problems – that don’t match the “textbook schematic” involving late Lyme arthritis and late neurologic disease.
Moreover, of patients who are treated with protocols recommended by the Infectious Diseases Society of America (IDSA), about 15% go on to develop persistent symptoms at 6 months – again, symptoms that don’t match textbook manifestations and do match symptoms of other infection-associated chronic illnesses. As a “research construct,” this has been coined posttreatment Lyme disease (PTLD), he said at the workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
(On a practical level, it is hard to know clinically who has early disseminated disease unless they have multiple erythema migrans rashes or neurologic or cardiac involvement, he said after the meeting.)
All this points to the need for tests that are sensitive and specific for diagnosis at all stages of infection and disease, he said in a talk on diagnostics. Currently available tests – those that fit into the widely used two-tiered enzyme-linked immunosorbent assay, Western Blot serology testing – have significant limitations in sensitivity and specificity, including for acute infection when the body has not generated enough antibodies, yet treatment is most likely to succeed.
Move toward combination therapy research
Lyme disease is most commonly treated with doxycycline, and that’s problematic because the antibiotic is a microstatic whose efficacy relies on immune clearance of static bacteria, said Monica E. Embers, PhD, director of vector-borne disease at the Tulane National Primate Research Center and associate professor of immunology at Tulane University, New Orleans.
“But we know that Borrelia burgdorferi has the capability to evade the host immune response in almost every way possible. Persistence is the norm in an immunocompetent host ... [and] dormant bacteria/persisters are more tolerant of microstatic antibiotics,” she said.
Other considerations for antibiotic efficacy include the fact that B. burgdorferi survives for many months inside ticks without nutrient replenishment or replication, “so dormancy is part of their life cycle,” she said. Moreover, the bacteria can be found deep in connective tissues and joints.
The efficacy of accepted regimens of antibiotic treatment has been “a very contentious issue,” she said, noting that guidelines from the International Lyme and Associated Diseases Society “leave open the possibility for antibiotic retreatment when a chronic infection is judged to be a possible cause [of ongoing symptoms].”
The development of persister B. burgdorferi in the presence of antibiotics has been well studied in vitro, which has limitations, Dr. Embers said. But her group specializes in animal models and has shown persistence of antimicrobial-tolerant B. burgdorferi in tick-inoculated rhesus macaques 8-9 months after treatment with oral doxycycline.
“We [also] saw persistence of mild-moderate inflammation in the brain, peripheral nerves, spinal cord, joints and skeletal muscle, and in the heart,” Dr. Embers said, who coauthored a 2022 review of B. burgdorferi antimicrobial-tolerant persistence in Lyme disease and PTLD.
Her work has also shown that ceftriaxone, which is recommended by IDSA for patients with clinically evident neurological and/or cardiac involvement, does not clear infection in mice. “In general, single drugs have not been capable of clearing the infection, yet combinations show promise,” she said.
Dr. Embers has combed large drug libraries looking for combinations of antibiotics that employ different mechanisms of action in hopes of eliminating persister spirochetes. Certain combinations have shown promise in mice and have been tested in her rhesus macaque model; data analyses are underway.
Other research teams, such as that of Ying Zhang, MD, PhD, at Johns Hopkins, have similarly been screening combinations of antibiotics and other compounds, identifying candidates for further testing.
During a question and answer period, Dr. Embers said her team is also investigating the pathophysiology and long-term effects of tick-borne coinfections, including Bartonella, and is pursuing a hypothesis that infection with Borrelia allows Bartonella to cause more extensive disease and persist longer. “I think Lyme is at the core because of its ability to evade and suppress the immune response so effectively.”
Diagnostic possibilities, biomarkers for PTLD
Direct diagnostic tests for microbial nucleic acid and proteins “are promising alternatives for indirect serologic tests,” Dr. Aucott said. For instance, in addition to polymerase chain reaction tests, which “are making advances,” it may be possible to target the B. burgdorferi peptidoglycan for antigen detection.
Researchers have shown that peptidoglycan, a component of the B. burgdorferi cell envelope, is a persistent antigen in the synovial fluid of patients with Lyme arthritis who have been treated with oral and intravenous antibiotics, and that it likely contributes to inflammation.
“Maybe the infection is gone but parts of the bacteria are still there that are driving inflammation,” said Dr. Aucott, also associate professor of medicine at John Hopkins.
Researchers have also been looking at the host response to B. burgdorferi – including cytokines, chemokines, and autoantibiodies – to identify biomarkers for PTLD and to identify patients during posttreatment follow-up who are at increased risk of developing PTLD, with the hope of someday intervening. Persistently high levels of interleukin-23, CCL19, and interferon-alpha have each been associated in different studies with persistent symptoms after treatment, Dr. Aucott said.
In addition, metabolomics research is showing that patients with PTLD have metabolic fingerprints that are different from those who return to good health after treatment, and it may be possible to identify an epigenetic signature for Lyme disease. A project sponsored by the Defense Advanced Research Projects Agency called ECHO (Epigenetic Characterization and Observation) aims to identify epigenetic signatures of exposures to various threats, including B. burgdorferi.
“At the very proximal end of [indirectly testing for host response], there are modifications of the DNA that can occur in response to infectious insults ... and that changed DNA changes RNA expression and protein synthesis,” Dr. Aucott explained. DARPA’s project is “exciting because their goal [at DARPA] is to have a diagnostic test quickly as a result of this epigenetics work.”
Imaging research is also fast offering diagnostic opportunities, Dr. Aucott said. Levels of microglial activation on brain PET imaging have been found to correlate with PTLD, and a study at Johns Hopkins of multimodal neuroimaging with functional MRI and diffusion tensor imaging has shown distinct changes to white matter activation within the frontal lobe of patients with PTLD, compared with controls.
The NASEM workshop did not collect or require disclosures of its participants.
Several recent developments in Lyme disease treatment and diagnosis may pave the way forward for combating disease that persists following missed or delayed diagnoses or remains following standard treatment. These include combination therapy to address “persister” bacteria and diagnostic tests that test directly for the pathogen and/or indirectly test for host response, according to experts who presented at a 2-day National Academies of Science, Engineering and Medicine workshop on infection-associated chronic illnesses.
Research has shown that 60% of people who are infected and not treated during the early or early disseminated stages of Lyme disease go on to develop late Lyme arthritis, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center in Baltimore. And in the real world, there’s an additional category of patients: Those who are misdiagnosed and develop infection-related persistent symptoms – such as fatigue, brain fog/cognitive dysfunction, and musculoskeletal problems – that don’t match the “textbook schematic” involving late Lyme arthritis and late neurologic disease.
Moreover, of patients who are treated with protocols recommended by the Infectious Diseases Society of America (IDSA), about 15% go on to develop persistent symptoms at 6 months – again, symptoms that don’t match textbook manifestations and do match symptoms of other infection-associated chronic illnesses. As a “research construct,” this has been coined posttreatment Lyme disease (PTLD), he said at the workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
(On a practical level, it is hard to know clinically who has early disseminated disease unless they have multiple erythema migrans rashes or neurologic or cardiac involvement, he said after the meeting.)
All this points to the need for tests that are sensitive and specific for diagnosis at all stages of infection and disease, he said in a talk on diagnostics. Currently available tests – those that fit into the widely used two-tiered enzyme-linked immunosorbent assay, Western Blot serology testing – have significant limitations in sensitivity and specificity, including for acute infection when the body has not generated enough antibodies, yet treatment is most likely to succeed.
Move toward combination therapy research
Lyme disease is most commonly treated with doxycycline, and that’s problematic because the antibiotic is a microstatic whose efficacy relies on immune clearance of static bacteria, said Monica E. Embers, PhD, director of vector-borne disease at the Tulane National Primate Research Center and associate professor of immunology at Tulane University, New Orleans.
“But we know that Borrelia burgdorferi has the capability to evade the host immune response in almost every way possible. Persistence is the norm in an immunocompetent host ... [and] dormant bacteria/persisters are more tolerant of microstatic antibiotics,” she said.
Other considerations for antibiotic efficacy include the fact that B. burgdorferi survives for many months inside ticks without nutrient replenishment or replication, “so dormancy is part of their life cycle,” she said. Moreover, the bacteria can be found deep in connective tissues and joints.
The efficacy of accepted regimens of antibiotic treatment has been “a very contentious issue,” she said, noting that guidelines from the International Lyme and Associated Diseases Society “leave open the possibility for antibiotic retreatment when a chronic infection is judged to be a possible cause [of ongoing symptoms].”
The development of persister B. burgdorferi in the presence of antibiotics has been well studied in vitro, which has limitations, Dr. Embers said. But her group specializes in animal models and has shown persistence of antimicrobial-tolerant B. burgdorferi in tick-inoculated rhesus macaques 8-9 months after treatment with oral doxycycline.
“We [also] saw persistence of mild-moderate inflammation in the brain, peripheral nerves, spinal cord, joints and skeletal muscle, and in the heart,” Dr. Embers said, who coauthored a 2022 review of B. burgdorferi antimicrobial-tolerant persistence in Lyme disease and PTLD.
Her work has also shown that ceftriaxone, which is recommended by IDSA for patients with clinically evident neurological and/or cardiac involvement, does not clear infection in mice. “In general, single drugs have not been capable of clearing the infection, yet combinations show promise,” she said.
Dr. Embers has combed large drug libraries looking for combinations of antibiotics that employ different mechanisms of action in hopes of eliminating persister spirochetes. Certain combinations have shown promise in mice and have been tested in her rhesus macaque model; data analyses are underway.
Other research teams, such as that of Ying Zhang, MD, PhD, at Johns Hopkins, have similarly been screening combinations of antibiotics and other compounds, identifying candidates for further testing.
During a question and answer period, Dr. Embers said her team is also investigating the pathophysiology and long-term effects of tick-borne coinfections, including Bartonella, and is pursuing a hypothesis that infection with Borrelia allows Bartonella to cause more extensive disease and persist longer. “I think Lyme is at the core because of its ability to evade and suppress the immune response so effectively.”
Diagnostic possibilities, biomarkers for PTLD
Direct diagnostic tests for microbial nucleic acid and proteins “are promising alternatives for indirect serologic tests,” Dr. Aucott said. For instance, in addition to polymerase chain reaction tests, which “are making advances,” it may be possible to target the B. burgdorferi peptidoglycan for antigen detection.
Researchers have shown that peptidoglycan, a component of the B. burgdorferi cell envelope, is a persistent antigen in the synovial fluid of patients with Lyme arthritis who have been treated with oral and intravenous antibiotics, and that it likely contributes to inflammation.
“Maybe the infection is gone but parts of the bacteria are still there that are driving inflammation,” said Dr. Aucott, also associate professor of medicine at John Hopkins.
Researchers have also been looking at the host response to B. burgdorferi – including cytokines, chemokines, and autoantibiodies – to identify biomarkers for PTLD and to identify patients during posttreatment follow-up who are at increased risk of developing PTLD, with the hope of someday intervening. Persistently high levels of interleukin-23, CCL19, and interferon-alpha have each been associated in different studies with persistent symptoms after treatment, Dr. Aucott said.
In addition, metabolomics research is showing that patients with PTLD have metabolic fingerprints that are different from those who return to good health after treatment, and it may be possible to identify an epigenetic signature for Lyme disease. A project sponsored by the Defense Advanced Research Projects Agency called ECHO (Epigenetic Characterization and Observation) aims to identify epigenetic signatures of exposures to various threats, including B. burgdorferi.
“At the very proximal end of [indirectly testing for host response], there are modifications of the DNA that can occur in response to infectious insults ... and that changed DNA changes RNA expression and protein synthesis,” Dr. Aucott explained. DARPA’s project is “exciting because their goal [at DARPA] is to have a diagnostic test quickly as a result of this epigenetics work.”
Imaging research is also fast offering diagnostic opportunities, Dr. Aucott said. Levels of microglial activation on brain PET imaging have been found to correlate with PTLD, and a study at Johns Hopkins of multimodal neuroimaging with functional MRI and diffusion tensor imaging has shown distinct changes to white matter activation within the frontal lobe of patients with PTLD, compared with controls.
The NASEM workshop did not collect or require disclosures of its participants.
Several recent developments in Lyme disease treatment and diagnosis may pave the way forward for combating disease that persists following missed or delayed diagnoses or remains following standard treatment. These include combination therapy to address “persister” bacteria and diagnostic tests that test directly for the pathogen and/or indirectly test for host response, according to experts who presented at a 2-day National Academies of Science, Engineering and Medicine workshop on infection-associated chronic illnesses.
Research has shown that 60% of people who are infected and not treated during the early or early disseminated stages of Lyme disease go on to develop late Lyme arthritis, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center in Baltimore. And in the real world, there’s an additional category of patients: Those who are misdiagnosed and develop infection-related persistent symptoms – such as fatigue, brain fog/cognitive dysfunction, and musculoskeletal problems – that don’t match the “textbook schematic” involving late Lyme arthritis and late neurologic disease.
Moreover, of patients who are treated with protocols recommended by the Infectious Diseases Society of America (IDSA), about 15% go on to develop persistent symptoms at 6 months – again, symptoms that don’t match textbook manifestations and do match symptoms of other infection-associated chronic illnesses. As a “research construct,” this has been coined posttreatment Lyme disease (PTLD), he said at the workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
(On a practical level, it is hard to know clinically who has early disseminated disease unless they have multiple erythema migrans rashes or neurologic or cardiac involvement, he said after the meeting.)
All this points to the need for tests that are sensitive and specific for diagnosis at all stages of infection and disease, he said in a talk on diagnostics. Currently available tests – those that fit into the widely used two-tiered enzyme-linked immunosorbent assay, Western Blot serology testing – have significant limitations in sensitivity and specificity, including for acute infection when the body has not generated enough antibodies, yet treatment is most likely to succeed.
Move toward combination therapy research
Lyme disease is most commonly treated with doxycycline, and that’s problematic because the antibiotic is a microstatic whose efficacy relies on immune clearance of static bacteria, said Monica E. Embers, PhD, director of vector-borne disease at the Tulane National Primate Research Center and associate professor of immunology at Tulane University, New Orleans.
“But we know that Borrelia burgdorferi has the capability to evade the host immune response in almost every way possible. Persistence is the norm in an immunocompetent host ... [and] dormant bacteria/persisters are more tolerant of microstatic antibiotics,” she said.
Other considerations for antibiotic efficacy include the fact that B. burgdorferi survives for many months inside ticks without nutrient replenishment or replication, “so dormancy is part of their life cycle,” she said. Moreover, the bacteria can be found deep in connective tissues and joints.
The efficacy of accepted regimens of antibiotic treatment has been “a very contentious issue,” she said, noting that guidelines from the International Lyme and Associated Diseases Society “leave open the possibility for antibiotic retreatment when a chronic infection is judged to be a possible cause [of ongoing symptoms].”
The development of persister B. burgdorferi in the presence of antibiotics has been well studied in vitro, which has limitations, Dr. Embers said. But her group specializes in animal models and has shown persistence of antimicrobial-tolerant B. burgdorferi in tick-inoculated rhesus macaques 8-9 months after treatment with oral doxycycline.
“We [also] saw persistence of mild-moderate inflammation in the brain, peripheral nerves, spinal cord, joints and skeletal muscle, and in the heart,” Dr. Embers said, who coauthored a 2022 review of B. burgdorferi antimicrobial-tolerant persistence in Lyme disease and PTLD.
Her work has also shown that ceftriaxone, which is recommended by IDSA for patients with clinically evident neurological and/or cardiac involvement, does not clear infection in mice. “In general, single drugs have not been capable of clearing the infection, yet combinations show promise,” she said.
Dr. Embers has combed large drug libraries looking for combinations of antibiotics that employ different mechanisms of action in hopes of eliminating persister spirochetes. Certain combinations have shown promise in mice and have been tested in her rhesus macaque model; data analyses are underway.
Other research teams, such as that of Ying Zhang, MD, PhD, at Johns Hopkins, have similarly been screening combinations of antibiotics and other compounds, identifying candidates for further testing.
During a question and answer period, Dr. Embers said her team is also investigating the pathophysiology and long-term effects of tick-borne coinfections, including Bartonella, and is pursuing a hypothesis that infection with Borrelia allows Bartonella to cause more extensive disease and persist longer. “I think Lyme is at the core because of its ability to evade and suppress the immune response so effectively.”
Diagnostic possibilities, biomarkers for PTLD
Direct diagnostic tests for microbial nucleic acid and proteins “are promising alternatives for indirect serologic tests,” Dr. Aucott said. For instance, in addition to polymerase chain reaction tests, which “are making advances,” it may be possible to target the B. burgdorferi peptidoglycan for antigen detection.
Researchers have shown that peptidoglycan, a component of the B. burgdorferi cell envelope, is a persistent antigen in the synovial fluid of patients with Lyme arthritis who have been treated with oral and intravenous antibiotics, and that it likely contributes to inflammation.
“Maybe the infection is gone but parts of the bacteria are still there that are driving inflammation,” said Dr. Aucott, also associate professor of medicine at John Hopkins.
Researchers have also been looking at the host response to B. burgdorferi – including cytokines, chemokines, and autoantibiodies – to identify biomarkers for PTLD and to identify patients during posttreatment follow-up who are at increased risk of developing PTLD, with the hope of someday intervening. Persistently high levels of interleukin-23, CCL19, and interferon-alpha have each been associated in different studies with persistent symptoms after treatment, Dr. Aucott said.
In addition, metabolomics research is showing that patients with PTLD have metabolic fingerprints that are different from those who return to good health after treatment, and it may be possible to identify an epigenetic signature for Lyme disease. A project sponsored by the Defense Advanced Research Projects Agency called ECHO (Epigenetic Characterization and Observation) aims to identify epigenetic signatures of exposures to various threats, including B. burgdorferi.
“At the very proximal end of [indirectly testing for host response], there are modifications of the DNA that can occur in response to infectious insults ... and that changed DNA changes RNA expression and protein synthesis,” Dr. Aucott explained. DARPA’s project is “exciting because their goal [at DARPA] is to have a diagnostic test quickly as a result of this epigenetics work.”
Imaging research is also fast offering diagnostic opportunities, Dr. Aucott said. Levels of microglial activation on brain PET imaging have been found to correlate with PTLD, and a study at Johns Hopkins of multimodal neuroimaging with functional MRI and diffusion tensor imaging has shown distinct changes to white matter activation within the frontal lobe of patients with PTLD, compared with controls.
The NASEM workshop did not collect or require disclosures of its participants.
FROM A NATIONAL ACADEMIES OF SCIENCE, ENGINEERING AND MEDICINE WORKSHOP
Functional MRI shows that empathetic remarks reduce pain
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Chronic constipation linked to cognitive decline
Compared with individuals who have a bowel movement once daily, adults with constipation who have a bowel movement every 3 days or more had significantly worse cognition that was commensurate with an additional 3 years of chronological cognitive aging, the investigators found.
“We should watch for symptoms of abnormal intestinal function, especially constipation, in older individuals, as these symptoms may hint at a higher risk of cognitive decline in the future,” study investigator Chaoran Ma, MD, PhD, former research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and current assistant professor at the University of Massachusetts Amherst, said in an interview.
The findings were presented at the Alzheimer’s Association International Conference.
Prevent constipation, improve brain health?
It’s estimated that 16% of the world’s population suffers from constipation. The problem is more common in older adults, owing to age-related factors such as a lack of dietary fiber and exercise and the use of constipating drugs to treat other medical conditions.
Chronic constipation – defined as having bowel movements every 3 days or more – has been associated with long-term health problems, such as inflammation, hormonal imbalances, anxiety, and depression.
However, few studies have investigated variations in intestinal motility and cognitive function.
“Our study provides first-of-its-kind evidence that examined a wide spectrum of bowel movement frequency, especially an analysis of the more frequent end, in relation to cognitive function,” Dr. Ma said.
The analysis involved data from 112,753 women and men from the Nurses’ Health Study (aged 30-55 years), the Nurses’ Health Study II (aged 25-42), and the Health Professionals Follow-up Study (aged 40-75).
Data on participants’ bowel movement frequency was collected between 2012 and 2013, and self-assessments of cognitive function were obtained from 2014 to 2017. A subgroup of 12,696 participants completed a standard neuropsychological test battery for objective cognitive assessment between 2014 and 2018.
The results show that bowel movement frequency was associated with overall objective cognitive function and learning and working memory in an inverse J-shape dose-response manner (both P for nonlinearity < .05).
Compared with adults who had one bowel movement daily, those who only had a bowel movement every 3 or more days had significantly worse cognition, equivalent to 3 years of additional aging (95% confidence interval, 1.2-4.7).
The researchers also observed similar J-shape dose-response relationships of bowel movement frequency with the odds of subjective cognitive decline and the likelihood of having more subjective cognitive complaints over time.
Compared with once-daily bowel movements, having bowel movements every 3 or more days was associated with a greater likelihood of subjective cognitive decline (odds ratio, 1.73; 95% CI, 1.60-1.86).
These relationships were generally consistent across the three cohorts and subgroups.
“These results stress the importance of clinicians discussing gut health, especially constipation, with their older patients,” senior investigator Dong Wang, MD, ScD, with Harvard Medical School and Brigham and Women’s Hospital, said in a conference statement.
“Interventions for preventing constipation and improving gut health include adopting healthy diets enriched with high-fiber and high-polyphenol foods such as fruits, vegetables, and whole grains; taking fiber supplementation; drinking plenty of water every day; and having regular physical activity,” Dr. Wang added.
The researchers also explored the role of the gut microbiome in the association between bowel movement frequency and cognitive function in a subgroup of 515 women and men.
They found that bowel movement frequency and subjective cognition were significantly associated with the overall variation of the gut microbiome (both P < .005) and specific microbial species.
“This research adds further evidence for a link between the microbiome and gastrointestinal function with cognitive function,” Dr. Ma said in an interview.
Interconnected systems
Commenting on the study in a conference statement, Heather M. Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that “our body systems are all interconnected. When one system is malfunctioning, it impacts other systems. When that dysfunction isn’t addressed, it can create a waterfall of consequences for the rest of the body.”
Dr. Snyder cautioned, however, that “there are a lot of unanswered questions about the connection between the health of our digestive system and our long-term cognitive function. Answering these questions may uncover novel therapeutic and risk-reduction approaches for Alzheimer’s and other dementias.”
In an interview, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, noted that the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk, is evaluating the impact of behavioral interventions on the gut-brain axis.
“We want to better understand how engaging in healthier habits can impact microorganisms in the gut and how changes in gut bacteria relate to brain health,” Dr. Griffin said.
The study was funded by the National Institutes of Health. Dr. Ma, Dr. Wang, Dr. Snyder, and Dr. Griffin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Compared with individuals who have a bowel movement once daily, adults with constipation who have a bowel movement every 3 days or more had significantly worse cognition that was commensurate with an additional 3 years of chronological cognitive aging, the investigators found.
“We should watch for symptoms of abnormal intestinal function, especially constipation, in older individuals, as these symptoms may hint at a higher risk of cognitive decline in the future,” study investigator Chaoran Ma, MD, PhD, former research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and current assistant professor at the University of Massachusetts Amherst, said in an interview.
The findings were presented at the Alzheimer’s Association International Conference.
Prevent constipation, improve brain health?
It’s estimated that 16% of the world’s population suffers from constipation. The problem is more common in older adults, owing to age-related factors such as a lack of dietary fiber and exercise and the use of constipating drugs to treat other medical conditions.
Chronic constipation – defined as having bowel movements every 3 days or more – has been associated with long-term health problems, such as inflammation, hormonal imbalances, anxiety, and depression.
However, few studies have investigated variations in intestinal motility and cognitive function.
“Our study provides first-of-its-kind evidence that examined a wide spectrum of bowel movement frequency, especially an analysis of the more frequent end, in relation to cognitive function,” Dr. Ma said.
The analysis involved data from 112,753 women and men from the Nurses’ Health Study (aged 30-55 years), the Nurses’ Health Study II (aged 25-42), and the Health Professionals Follow-up Study (aged 40-75).
Data on participants’ bowel movement frequency was collected between 2012 and 2013, and self-assessments of cognitive function were obtained from 2014 to 2017. A subgroup of 12,696 participants completed a standard neuropsychological test battery for objective cognitive assessment between 2014 and 2018.
The results show that bowel movement frequency was associated with overall objective cognitive function and learning and working memory in an inverse J-shape dose-response manner (both P for nonlinearity < .05).
Compared with adults who had one bowel movement daily, those who only had a bowel movement every 3 or more days had significantly worse cognition, equivalent to 3 years of additional aging (95% confidence interval, 1.2-4.7).
The researchers also observed similar J-shape dose-response relationships of bowel movement frequency with the odds of subjective cognitive decline and the likelihood of having more subjective cognitive complaints over time.
Compared with once-daily bowel movements, having bowel movements every 3 or more days was associated with a greater likelihood of subjective cognitive decline (odds ratio, 1.73; 95% CI, 1.60-1.86).
These relationships were generally consistent across the three cohorts and subgroups.
“These results stress the importance of clinicians discussing gut health, especially constipation, with their older patients,” senior investigator Dong Wang, MD, ScD, with Harvard Medical School and Brigham and Women’s Hospital, said in a conference statement.
“Interventions for preventing constipation and improving gut health include adopting healthy diets enriched with high-fiber and high-polyphenol foods such as fruits, vegetables, and whole grains; taking fiber supplementation; drinking plenty of water every day; and having regular physical activity,” Dr. Wang added.
The researchers also explored the role of the gut microbiome in the association between bowel movement frequency and cognitive function in a subgroup of 515 women and men.
They found that bowel movement frequency and subjective cognition were significantly associated with the overall variation of the gut microbiome (both P < .005) and specific microbial species.
“This research adds further evidence for a link between the microbiome and gastrointestinal function with cognitive function,” Dr. Ma said in an interview.
Interconnected systems
Commenting on the study in a conference statement, Heather M. Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that “our body systems are all interconnected. When one system is malfunctioning, it impacts other systems. When that dysfunction isn’t addressed, it can create a waterfall of consequences for the rest of the body.”
Dr. Snyder cautioned, however, that “there are a lot of unanswered questions about the connection between the health of our digestive system and our long-term cognitive function. Answering these questions may uncover novel therapeutic and risk-reduction approaches for Alzheimer’s and other dementias.”
In an interview, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, noted that the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk, is evaluating the impact of behavioral interventions on the gut-brain axis.
“We want to better understand how engaging in healthier habits can impact microorganisms in the gut and how changes in gut bacteria relate to brain health,” Dr. Griffin said.
The study was funded by the National Institutes of Health. Dr. Ma, Dr. Wang, Dr. Snyder, and Dr. Griffin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Compared with individuals who have a bowel movement once daily, adults with constipation who have a bowel movement every 3 days or more had significantly worse cognition that was commensurate with an additional 3 years of chronological cognitive aging, the investigators found.
“We should watch for symptoms of abnormal intestinal function, especially constipation, in older individuals, as these symptoms may hint at a higher risk of cognitive decline in the future,” study investigator Chaoran Ma, MD, PhD, former research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and current assistant professor at the University of Massachusetts Amherst, said in an interview.
The findings were presented at the Alzheimer’s Association International Conference.
Prevent constipation, improve brain health?
It’s estimated that 16% of the world’s population suffers from constipation. The problem is more common in older adults, owing to age-related factors such as a lack of dietary fiber and exercise and the use of constipating drugs to treat other medical conditions.
Chronic constipation – defined as having bowel movements every 3 days or more – has been associated with long-term health problems, such as inflammation, hormonal imbalances, anxiety, and depression.
However, few studies have investigated variations in intestinal motility and cognitive function.
“Our study provides first-of-its-kind evidence that examined a wide spectrum of bowel movement frequency, especially an analysis of the more frequent end, in relation to cognitive function,” Dr. Ma said.
The analysis involved data from 112,753 women and men from the Nurses’ Health Study (aged 30-55 years), the Nurses’ Health Study II (aged 25-42), and the Health Professionals Follow-up Study (aged 40-75).
Data on participants’ bowel movement frequency was collected between 2012 and 2013, and self-assessments of cognitive function were obtained from 2014 to 2017. A subgroup of 12,696 participants completed a standard neuropsychological test battery for objective cognitive assessment between 2014 and 2018.
The results show that bowel movement frequency was associated with overall objective cognitive function and learning and working memory in an inverse J-shape dose-response manner (both P for nonlinearity < .05).
Compared with adults who had one bowel movement daily, those who only had a bowel movement every 3 or more days had significantly worse cognition, equivalent to 3 years of additional aging (95% confidence interval, 1.2-4.7).
The researchers also observed similar J-shape dose-response relationships of bowel movement frequency with the odds of subjective cognitive decline and the likelihood of having more subjective cognitive complaints over time.
Compared with once-daily bowel movements, having bowel movements every 3 or more days was associated with a greater likelihood of subjective cognitive decline (odds ratio, 1.73; 95% CI, 1.60-1.86).
These relationships were generally consistent across the three cohorts and subgroups.
“These results stress the importance of clinicians discussing gut health, especially constipation, with their older patients,” senior investigator Dong Wang, MD, ScD, with Harvard Medical School and Brigham and Women’s Hospital, said in a conference statement.
“Interventions for preventing constipation and improving gut health include adopting healthy diets enriched with high-fiber and high-polyphenol foods such as fruits, vegetables, and whole grains; taking fiber supplementation; drinking plenty of water every day; and having regular physical activity,” Dr. Wang added.
The researchers also explored the role of the gut microbiome in the association between bowel movement frequency and cognitive function in a subgroup of 515 women and men.
They found that bowel movement frequency and subjective cognition were significantly associated with the overall variation of the gut microbiome (both P < .005) and specific microbial species.
“This research adds further evidence for a link between the microbiome and gastrointestinal function with cognitive function,” Dr. Ma said in an interview.
Interconnected systems
Commenting on the study in a conference statement, Heather M. Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that “our body systems are all interconnected. When one system is malfunctioning, it impacts other systems. When that dysfunction isn’t addressed, it can create a waterfall of consequences for the rest of the body.”
Dr. Snyder cautioned, however, that “there are a lot of unanswered questions about the connection between the health of our digestive system and our long-term cognitive function. Answering these questions may uncover novel therapeutic and risk-reduction approaches for Alzheimer’s and other dementias.”
In an interview, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, noted that the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk, is evaluating the impact of behavioral interventions on the gut-brain axis.
“We want to better understand how engaging in healthier habits can impact microorganisms in the gut and how changes in gut bacteria relate to brain health,” Dr. Griffin said.
The study was funded by the National Institutes of Health. Dr. Ma, Dr. Wang, Dr. Snyder, and Dr. Griffin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM AAIC 2023
Indian Health Service dermatologist: ‘I saw a real need to be of service’
After completing his dermatology residency at Johns Hopkins Hospital in 2010, Christopher Bengson, MD, MHS, then a Lieutenant Commander in the U.S. Public Health Service, accepted an offer to become a full-time dermatologist at Phoenix Indian Medical Center (PIMC) in Arizona, fulfilling a long desire to provide care for underserved individuals. Thirteen years later, .
As one of the largest hospitals in the IHS system, PIMC provides direct health care services to a population of more than 156,000, including tribal members from The Fort McDowell Yavapai Nation, the Salt River Pima-Maricopa Indian Community, and the San Lucy District of the Tohono O’odham Nation, the Tonto Apache Tribe, the Yavapai-Apache Indian Tribe, and the Yavapai-Prescott Indian Tribe. Dr. Bengson also cares for tribal members who travel to PIMC from as far away as Washington State and Hawaii to receive dermatologic care.
“There is a disproportionate number of Native American patients that come in with severe psoriasis, hidradenitis suppurativa, and dissecting cellulitis of the scalp compared to the general U.S. population, and I’ve been surprised by how many have nonmelanoma skin cancers and autoimmune connective tissue diseases like lupus, as the prevailing sentiment among his patients is that Native people do not get skin cancer,” he said in an interview. “Those who travel great distances are those who come see me for the surgical removal of skin cancers.”
Interesting cases he’s seen in his nearly 13 years on the job include Epstein-Barr virus-induced NK/T-cell lymphoma, anaplastic large cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, and necrobiotic xanthogranuloma, “tumors that have generally gone to tertiary care facilities for treatment, but we’ve been able to manage here.”
In 2017, Dr. Bengson was appointed as the IHS’s first chief clinical consultant for dermatology, a post that provides him the opportunity to interface with Native people and IHS-affiliated clinicians nationwide regarding skin-related questions and concerns. As the only full-time dermatologist employed by the IHS, he also views his role as providing an opportunity to change the perception that some Native Americans may still hold about federally delivered health care, “where there may be a cultural distrust of government health care in indigenous communities, driven by generational historical traumas that have come out of boarding schools, population relocation to desolate and isolated areas of the country, and contracts that were simply not honored,” he explained.
“While none of these issues are new, what has been great for me is that I’m going on 13 years of being at the same facility, and I’ve treated family members, their kids, and even their grandkids. In some ways the primary barrier of continuity of care – at least at PIMC – has been eliminated by me just being here for a long period of time.”
In Dr. Bengson’s opinion, efforts to improve access to attract more Native Americans to dermatology are laudable, including the American Academy of Dermatology’s Pathways Program, which aims to increase the number of dermatology residents from Black, Latino, and indigenous communities from approximately 100 residents to 250 residents by 2027, or by over 150%, through community-based engagement strategies that begin in high school.
“To have an objective benchmark is encouraging,” he said. However, he encourages dermatology residency program directors to rethink how they recruit Native Americans, many of whom hail from rural areas. “If you’re recruiting primarily from urban settings, you’re very unlikely to include Native Americans as a larger group of minorities,” he said. “When you look at the number of department chairs who are Native American, it’s on the order of 0.1%, [so] it’s no surprise that dermatologists coming out of a residency program don’t want to go to reservations to provide dermatologic care. We pay a lot of lip service to mentorship programs and things like that, but you need a mentor who follows you through the process – and it’s a long process.”
He believes that residency program directors should reconsider the metrics used to select dermatology residents and should consider the degree of adversity that a Native American applicant may have had to overcome to make it to the residency selection committees.
Despite obstacles to attracting young Native Americans to a career in medicine, Dr. Bengson sees encouraging signs ahead. Some of his Native American patients and family members of patients have enrolled in medical school and have asked to rotate with him at PIMC at the premedical and medical student level. “Some have moved on, not necessarily to dermatology, but to other specialties and careers in health care,” he said. “When you have such high rates of obesity, diabetes, hypertension, coronary artery disease, and stroke in Native American communities, nodulocystic acne and other skin conditions that are not threats to life and limb become less of a priority. We need to get more people in the pipeline to deliver medical services even if it may not be in dermatology, as the need for dedicated health care professionals is so great across all disciplines.”
After completing his dermatology residency at Johns Hopkins Hospital in 2010, Christopher Bengson, MD, MHS, then a Lieutenant Commander in the U.S. Public Health Service, accepted an offer to become a full-time dermatologist at Phoenix Indian Medical Center (PIMC) in Arizona, fulfilling a long desire to provide care for underserved individuals. Thirteen years later, .
As one of the largest hospitals in the IHS system, PIMC provides direct health care services to a population of more than 156,000, including tribal members from The Fort McDowell Yavapai Nation, the Salt River Pima-Maricopa Indian Community, and the San Lucy District of the Tohono O’odham Nation, the Tonto Apache Tribe, the Yavapai-Apache Indian Tribe, and the Yavapai-Prescott Indian Tribe. Dr. Bengson also cares for tribal members who travel to PIMC from as far away as Washington State and Hawaii to receive dermatologic care.
“There is a disproportionate number of Native American patients that come in with severe psoriasis, hidradenitis suppurativa, and dissecting cellulitis of the scalp compared to the general U.S. population, and I’ve been surprised by how many have nonmelanoma skin cancers and autoimmune connective tissue diseases like lupus, as the prevailing sentiment among his patients is that Native people do not get skin cancer,” he said in an interview. “Those who travel great distances are those who come see me for the surgical removal of skin cancers.”
Interesting cases he’s seen in his nearly 13 years on the job include Epstein-Barr virus-induced NK/T-cell lymphoma, anaplastic large cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, and necrobiotic xanthogranuloma, “tumors that have generally gone to tertiary care facilities for treatment, but we’ve been able to manage here.”
In 2017, Dr. Bengson was appointed as the IHS’s first chief clinical consultant for dermatology, a post that provides him the opportunity to interface with Native people and IHS-affiliated clinicians nationwide regarding skin-related questions and concerns. As the only full-time dermatologist employed by the IHS, he also views his role as providing an opportunity to change the perception that some Native Americans may still hold about federally delivered health care, “where there may be a cultural distrust of government health care in indigenous communities, driven by generational historical traumas that have come out of boarding schools, population relocation to desolate and isolated areas of the country, and contracts that were simply not honored,” he explained.
“While none of these issues are new, what has been great for me is that I’m going on 13 years of being at the same facility, and I’ve treated family members, their kids, and even their grandkids. In some ways the primary barrier of continuity of care – at least at PIMC – has been eliminated by me just being here for a long period of time.”
In Dr. Bengson’s opinion, efforts to improve access to attract more Native Americans to dermatology are laudable, including the American Academy of Dermatology’s Pathways Program, which aims to increase the number of dermatology residents from Black, Latino, and indigenous communities from approximately 100 residents to 250 residents by 2027, or by over 150%, through community-based engagement strategies that begin in high school.
“To have an objective benchmark is encouraging,” he said. However, he encourages dermatology residency program directors to rethink how they recruit Native Americans, many of whom hail from rural areas. “If you’re recruiting primarily from urban settings, you’re very unlikely to include Native Americans as a larger group of minorities,” he said. “When you look at the number of department chairs who are Native American, it’s on the order of 0.1%, [so] it’s no surprise that dermatologists coming out of a residency program don’t want to go to reservations to provide dermatologic care. We pay a lot of lip service to mentorship programs and things like that, but you need a mentor who follows you through the process – and it’s a long process.”
He believes that residency program directors should reconsider the metrics used to select dermatology residents and should consider the degree of adversity that a Native American applicant may have had to overcome to make it to the residency selection committees.
Despite obstacles to attracting young Native Americans to a career in medicine, Dr. Bengson sees encouraging signs ahead. Some of his Native American patients and family members of patients have enrolled in medical school and have asked to rotate with him at PIMC at the premedical and medical student level. “Some have moved on, not necessarily to dermatology, but to other specialties and careers in health care,” he said. “When you have such high rates of obesity, diabetes, hypertension, coronary artery disease, and stroke in Native American communities, nodulocystic acne and other skin conditions that are not threats to life and limb become less of a priority. We need to get more people in the pipeline to deliver medical services even if it may not be in dermatology, as the need for dedicated health care professionals is so great across all disciplines.”
After completing his dermatology residency at Johns Hopkins Hospital in 2010, Christopher Bengson, MD, MHS, then a Lieutenant Commander in the U.S. Public Health Service, accepted an offer to become a full-time dermatologist at Phoenix Indian Medical Center (PIMC) in Arizona, fulfilling a long desire to provide care for underserved individuals. Thirteen years later, .
As one of the largest hospitals in the IHS system, PIMC provides direct health care services to a population of more than 156,000, including tribal members from The Fort McDowell Yavapai Nation, the Salt River Pima-Maricopa Indian Community, and the San Lucy District of the Tohono O’odham Nation, the Tonto Apache Tribe, the Yavapai-Apache Indian Tribe, and the Yavapai-Prescott Indian Tribe. Dr. Bengson also cares for tribal members who travel to PIMC from as far away as Washington State and Hawaii to receive dermatologic care.
“There is a disproportionate number of Native American patients that come in with severe psoriasis, hidradenitis suppurativa, and dissecting cellulitis of the scalp compared to the general U.S. population, and I’ve been surprised by how many have nonmelanoma skin cancers and autoimmune connective tissue diseases like lupus, as the prevailing sentiment among his patients is that Native people do not get skin cancer,” he said in an interview. “Those who travel great distances are those who come see me for the surgical removal of skin cancers.”
Interesting cases he’s seen in his nearly 13 years on the job include Epstein-Barr virus-induced NK/T-cell lymphoma, anaplastic large cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, and necrobiotic xanthogranuloma, “tumors that have generally gone to tertiary care facilities for treatment, but we’ve been able to manage here.”
In 2017, Dr. Bengson was appointed as the IHS’s first chief clinical consultant for dermatology, a post that provides him the opportunity to interface with Native people and IHS-affiliated clinicians nationwide regarding skin-related questions and concerns. As the only full-time dermatologist employed by the IHS, he also views his role as providing an opportunity to change the perception that some Native Americans may still hold about federally delivered health care, “where there may be a cultural distrust of government health care in indigenous communities, driven by generational historical traumas that have come out of boarding schools, population relocation to desolate and isolated areas of the country, and contracts that were simply not honored,” he explained.
“While none of these issues are new, what has been great for me is that I’m going on 13 years of being at the same facility, and I’ve treated family members, their kids, and even their grandkids. In some ways the primary barrier of continuity of care – at least at PIMC – has been eliminated by me just being here for a long period of time.”
In Dr. Bengson’s opinion, efforts to improve access to attract more Native Americans to dermatology are laudable, including the American Academy of Dermatology’s Pathways Program, which aims to increase the number of dermatology residents from Black, Latino, and indigenous communities from approximately 100 residents to 250 residents by 2027, or by over 150%, through community-based engagement strategies that begin in high school.
“To have an objective benchmark is encouraging,” he said. However, he encourages dermatology residency program directors to rethink how they recruit Native Americans, many of whom hail from rural areas. “If you’re recruiting primarily from urban settings, you’re very unlikely to include Native Americans as a larger group of minorities,” he said. “When you look at the number of department chairs who are Native American, it’s on the order of 0.1%, [so] it’s no surprise that dermatologists coming out of a residency program don’t want to go to reservations to provide dermatologic care. We pay a lot of lip service to mentorship programs and things like that, but you need a mentor who follows you through the process – and it’s a long process.”
He believes that residency program directors should reconsider the metrics used to select dermatology residents and should consider the degree of adversity that a Native American applicant may have had to overcome to make it to the residency selection committees.
Despite obstacles to attracting young Native Americans to a career in medicine, Dr. Bengson sees encouraging signs ahead. Some of his Native American patients and family members of patients have enrolled in medical school and have asked to rotate with him at PIMC at the premedical and medical student level. “Some have moved on, not necessarily to dermatology, but to other specialties and careers in health care,” he said. “When you have such high rates of obesity, diabetes, hypertension, coronary artery disease, and stroke in Native American communities, nodulocystic acne and other skin conditions that are not threats to life and limb become less of a priority. We need to get more people in the pipeline to deliver medical services even if it may not be in dermatology, as the need for dedicated health care professionals is so great across all disciplines.”
Top 50 Authors in Dermatology by Publication Rate (2017-2022)
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).
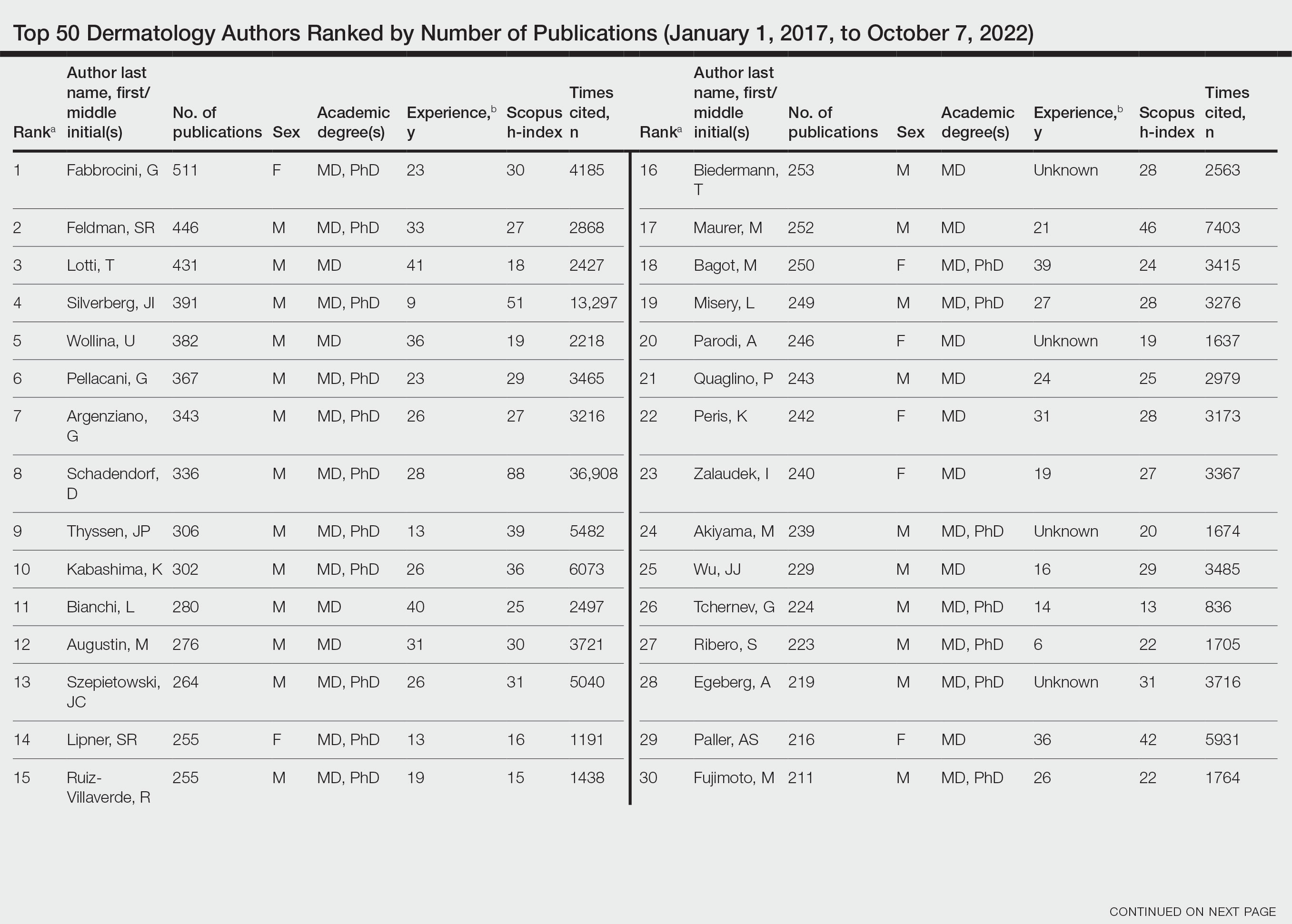
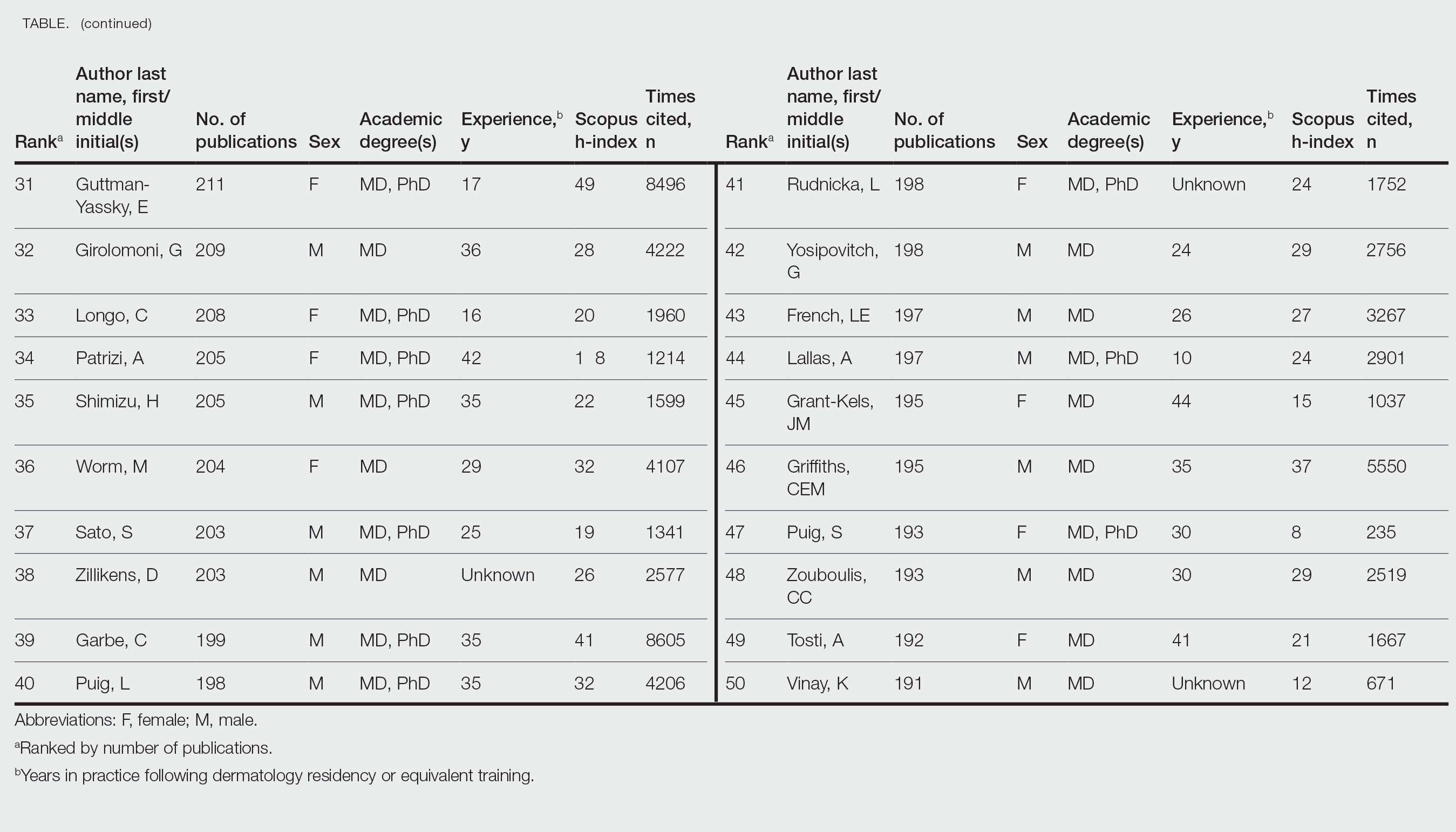
Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
Practice Points
- Academic scholarship often is measured by number of citations and h-index. Using these measures, female dermatologists are infrequently represented on top author lists.
- Using the Scopus database to search for the 50 most published dermatology authors from January 1, 2017, to October 7, 2022, 30% were female.
- Higher proportions of female dermatology trainees as well as efforts to increase mentorship and research support for female dermatologists may improve equality in top lists of dermatology citations and h-index values.
Dermatologic care in Indian Country marked by unique challenges, opportunities
As a proud member of the Oglala Lakota Nation from the Pine Ridge Indian Reservation in southwestern South Dakota, Drew Hicks grew up with limited access to basic health care, let alone the luxury of scheduling an appointment with a dermatologist or another medical specialist.
The area – once home to the Lakota war leader Crazy Horse – encompasses nearly 47,000 residents scattered over about 2.2 million acres, larger than the size of Rhode Island, with land marked by rolling mixed grass prairie, sandhills, and badlands. Some of the Oglala Lakota people live in substandard housing and lack regular access to food, running water, and refrigeration, not to mention cell phone and Internet service. “It’s sparse,” said Mr. Hicks, the son of Tribal ranchers who now is a 3rd-year medical student at the Mayo Clinic College of Medicine and Science in Rochester, Minn., and has an early interest in pursuing dermatology. “There is a lot of territory and not a lot of health care serving the population.” From the Hicks home, the nearest place to receive health care is a family medicine practice in Martin, S.D. – about a 15-minute drive on gravel roads in the best of conditions, but in poor weather, it can be difficult, he said. “So, there are environmental challenges besides the limited number of health care providers.”
Clinicians in the practice “did have to be the point of care for everything from dermatologic issues to emergency medicine to delivering a baby, because the next-closest medical facility of any magnitude is 2 hours away,” he said.
Challenges of health literacy and limited access to comprehensive health care at Pine Ridge and other American Indian (AI) and Alaska Native (AN) reservations have long-term consequences. “My own mom struggled to control her blood pressure for years and now has chronic kidney disease,” Mr. Hicks said. “It’s not an uncommon story. Diabetes on the reservation is a big issue.” Then there’s his father, who survived two bouts with melanoma that was diagnosed at an advanced stage. “I think about how that has impacted him, and wonder, had we had a dermatologist who serviced our area, would we have caught things sooner?” he said. “I feel there is so much room for impactful health care deliveries to communities like Pine Ridge.” At the same time, he emphasized, “this isn’t poverty porn. We’re a resilient people. Any effort to engage with AIs or ANs should be from a perspective of a learner, having cultural humility, and seeking out community leaders to help lead you.”
According to the 2020 Census, there are 574 federally recognized sovereign tribal nations in the United States and federal- and state-recognized American Indian reservations in 35 states. AI/AN people make up about 2.9% of the total U.S. population, or 9.7 million, and their life expectancy is an average of 4.4 years less, compared with the general population (a mean of 73.7 vs. 78.1 years, respectively). Because of limited access to dermatologic care in these areas, the risk for developing significant skin conditions and diseases that may go undetected for long stretches of time is increased.
“That can mean advanced skin cancers like basal cell carcinomas that have become larger than what you would see in a typical metropolitan population,” said Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, who spent part of her dermatology residency rotating at the Chinle (Ariz.) Service Unit, an Indian Health Service facility, in 2017 and now provides teledermatology and regular in-person dermatology care at that clinic. “The climate there is dry, so you can see bad eczema and dry skin. There’s also a lot of acne and hidradenitis suppurativa. I think the acne and HS is due to the hyperglycemic index diet from the food deserts. Skin disease reflects the climate, the food desert, and the lack of close specialty care.”
Acne scarring common
Some published evidence suggests that acne is more prevalent and severe in AI/AN individuals. In a survey of 158 AI/AN individuals with a mean age of 32 years, 79.1% reported a history of acne, 55.1% reported acne scarring, and 31% reported having active lesions. “Looking back on my experience in high school, I definitely see that in myself and in my peers,” Mr. Hicks said. And, while there are limited published studies about the incidence of melanoma in this population, an analysis from 2006 found that the incidence was 3.1 per 100,000 between 2001 and 2005, which was an increase from 1.6 per 100,000 reported between 1992 and 2000.
There’s a lot to unpack for dermatologists caring for the AI/AN population besides the raw health disparities: a long history of distrust between AI/AN people and the federal government, structural racism, geographic isolation, health literacy challenges, and high rates of poverty and unemployment. And while individuals from federally recognized tribes have a legal right to receive health care provided by the Indian Health Service, a component of the Department of Health & Human Services, the U.S. Government Accountability Office found that in 2017 per capita spending available to the IHS was $4,078, compared with $8,109 for Medicaid, $10,692 for the Veterans Health Administration, and $13,185 for Medicare.
“Everyone deserves healthy skin and good health,” said Dr. Kohn, whose husband is AI and works in AI law. “Knowing that there are pockets of people who lack that access to care really bothers me. I think the American Indians are frequently overlooked. They’re just not even counted for in certain surveys,” she added, noting that categories are usually defined as Black, Hispanic, Asian, or White.
According to Dr. Kohn, who coauthored a chapter titled “Dermatology on American Indian and Alaska Native Reservations,” for the 2021 book “Dermatology in Rural Settings”, 70% of AIs live in urban areas, “so it’s not just people who live on reservations, though the disparity is greatest there.” To help deliver dermatologic care in the rural areas “where you’re on tribal lands, you must partner with the tribes,” she added. “You must get their permission, operate under their laws and regulations and their rules, learn the local customs, learn about the culture, learn the people, and learn their resources before you practice. That’s the only ethical way to practice.” This also means appreciating the fact that some AI/AN individuals may not understand what a dermatologist could do for them. “One of the bigger hurdles to overcome,” she said, is educating the population that dermatologists can cure skin diseases and that there are good medications for treating the diseases.
Shortcomings of teledermatology
Some dermatologists perform teledermatology visits for tribes, often from an office located in a different time zone. “And, they don’t have a sense of what resources are available for the people they’re serving,” Dr. Kohn said. “For example, if they diagnose a potential skin cancer on the face and say, ‘you need a biopsy,’ but the closest dermatologist is 4 hours away, is that really serving the patient? Or, if you tell a patient, ‘I want you to go out and buy Vanicream for your skin,’ but Vanicream costs $17 and the patient can’t even afford to buy food, are you really doing them a service?”
In a survey-based study of 238 AI individuals that is scheduled to be published in late 2023, Dr. Kohn and colleagues asked respondents at two regional powwows in Denver if they would be open to teledermatology – either in their home or in a primary care clinic. Most respondents (70%) lived in urban areas, the rest in rural settings. Nearly half of respondents (42%) “did not want to do teledermatology, even though they couldn’t access in-person dermatology,” Dr. Kohn said. “So, for people who think teledermatology is the answer [to improving access], the respondents to our survey weren’t interested in pursuing that as a solution. I was surprised by that.” When the researchers broke down the responses by age, teenage respondents were even less interested in teledermatology than adults were. “I think there’s something about having someone see you in person, knowing who you are,” she said.
Partnerships with tribes
To foster more sustainable change in the delivery of skin care beyond remote teledermatology and periodic visits from volunteers, some dermatology residencies have established partnerships with tribes, including Massachusetts General Hospital’s teaching partnership with the Rosebud Sioux tribe in Rosebud, S.D., and the University of Utah dermatology department’s resident continuity clinic with Navajo Nation in Montezuma Creek, Utah. In 2016, officials from the Utah Navajo Health System reached out to the University of Utah’s dermatology department to inquire about the potential for creating a teledermatology clinic to serve patients who receive primary care at the Montezuma Creek Community Health Center, located in Southeastern Utah on the northern tip of the Navajo Nation.
Stephanie Klein, MD, associate professor of dermatology at the university, spearheaded the clinic’s launch but soon encountered obstacles that ranged from not being able to visualize the patient’s skin clearly on her computer screen to difficulty making a personal connection with patients despite help from Navajo translators. “It was hard to build a relationship,” she said. A few years later, she drove down to meet with officials of the health system and posed the question: “What is the ideal thing you would want from dermatology?”
Continuity, they told her. “They said that a lot of the services they receive in the form of outreach are rotational, where someone might come in for a day, or a week, or five people may rotate throughout the year,” which did not serve them well, said Dr. Klein, who subsequently collaborated with Utah Navajo Health System clinicians to establish a resident continuity clinic, which launched in January 2021.
The arrangement also serves as a continuity clinic for Dr. Klein as an attending physician. Each month, she and one dermatology resident drive 6.5 hours from Salt Lake City to Montezuma Creek, where they spend 1 or 2 full days seeing about 25 patients referred by the primary care clinicians who work there. About one-quarter of the time they fly, thanks to financial support from a private donor. The flight takes about an hour, then it’s an hour-long drive to the actual clinic. “It’s a commitment,” Dr. Klein said. “A resident can come with me if they commit to the clinic for at least 1 year. This enables us to have continuity of care; it allows us to build relationships with the patients and with the care team there.” As for the prior teledermatology visits she had with residents, “I still do those, but now I do them in between the in-person visits, so I’m not meeting people over telehealth; I’m just following up with them.”

Situated in the high desert among rock formations, the estimated population of Montezuma Creek is just over 320 people. “It’s a beautiful place with otherworldly buttes and mesas, and the Blue Mountains rising up in the distance,” said Lowell Nicholson, MD, a dermatology resident at the University of Utah who is in his second year of a 2-year commitment to the clinic. “But the landscape can be harsh, and it is underserved from an infrastructure perspective,” with large areas with no cell phone service and limited access to running water and refrigeration. “People in general travel quite far to get their medical care and most of the roads are dirt or gravel, so after a big snowstorm or if it’s been raining, they can become impassable.”
Dermatologic conditions they often encounter include vitiligo, photodermatoses, hidradenitis suppurativa, eczema, psoriasis, and severe acne, often with lots of acne-associated scarring. “In general, we tend to see dramatic or advanced presentations of general dermatology diagnoses,” Dr. Nicholson said. “We see a lot of really extensive psoriasis, which can be socially stigmatizing.”
He recalled one middle-aged man who isolated himself from others because his psoriasis became unbearable. The man refused to leave his house, visit family members, or attend tribal meetups. “He tried to see his regular doctor about it and was given topicals, but his disease was just too extensive,” said Dr. Nicholson, who suggested trying a biologic but learned that the man did not have regular access to refrigeration. “That wasn’t going to work, but we started him on an oral medication, apremilast, which has completely cleared his skin,” he said. “He’s doing great. The last time we saw him he was re-engaged with his family, and he told us he was going on dates. We really improved his quality of life.”
Dr. Klein recalled seeing a 6-year-old girl at the clinic with atopic dermatitis so severe that it caused her to miss several days of school. “When she was in school, she was so distracted by the itching – it was so overwhelming,” she said. She was struggling with topical medicines that weren’t effective, but Dr. Klein got her on dupilumab, and during a follow-up visit the girl told her, “This is the first time in my life I can think about things” other than itching.
According to Dr. Nicholson, some patients seen at the Montezuma Creek clinic are on Medicare or carry standard insurance. “Others have a mix, and others are getting all their medications through the Montezuma Creek clinic or through the IHS clinics,” he said. “I have been surprised at the formulary and our ability to get relatively expensive medications for our patients, like biologics and TNF inhibitors. But it takes some creativity to know what is going to work for your patients’ living situation.”
Training more AI/AN dermatologists key
While efforts to increase the culturally respectful and sustainable dermatologic care for AI/AN individuals continue through programs like the continuity clinic at Montezuma Creek, sources interviewed for this story emphasized the importance of training more AI/AN dermatologists. “Of the people who graduate from high school, AIs have the lowest rate of going on to college,” said Dr. Kohn, who serves as a mentor to Mr. Hicks. “Let’s say they get all the way to medical school; it’s about good mentorship and support in what they’re pursuing. We are seeing more AIs in medical school now, something that I personally notice, and I notice it from what Chinle Service Unit tells me. They have received many requests from Native medical students and premed students who want to rotate at Chinle. Native trainees want the experience of being there.”
According to the Association of American Medical Colleges, the number of AI/AN applicants to medical schools increased from 72 in 2020-2021 to 105 in 2021-2022 but dipped slightly to 94 in 2022-2023. Inspired by a passion to serve Pine Ridge or a community like it, Mr. Hicks decided to apply for medical school. While he doesn’t want to “close any doors” on which medical specialty he ultimately chooses to practice, the current front-runner is dermatology, he said, largely because of the influence of Dr. Kohn and two Mayo dermatologists who have become mentors: Molly Lohman, MD, and Hafsa M. Cantwell, MD. “I didn’t see anyone from my background who was a doctor, so having those role models is so important for Native kids to think, ‘I can do this, too,’ and to pursue it,” he said.
As a proud member of the Oglala Lakota Nation from the Pine Ridge Indian Reservation in southwestern South Dakota, Drew Hicks grew up with limited access to basic health care, let alone the luxury of scheduling an appointment with a dermatologist or another medical specialist.
The area – once home to the Lakota war leader Crazy Horse – encompasses nearly 47,000 residents scattered over about 2.2 million acres, larger than the size of Rhode Island, with land marked by rolling mixed grass prairie, sandhills, and badlands. Some of the Oglala Lakota people live in substandard housing and lack regular access to food, running water, and refrigeration, not to mention cell phone and Internet service. “It’s sparse,” said Mr. Hicks, the son of Tribal ranchers who now is a 3rd-year medical student at the Mayo Clinic College of Medicine and Science in Rochester, Minn., and has an early interest in pursuing dermatology. “There is a lot of territory and not a lot of health care serving the population.” From the Hicks home, the nearest place to receive health care is a family medicine practice in Martin, S.D. – about a 15-minute drive on gravel roads in the best of conditions, but in poor weather, it can be difficult, he said. “So, there are environmental challenges besides the limited number of health care providers.”
Clinicians in the practice “did have to be the point of care for everything from dermatologic issues to emergency medicine to delivering a baby, because the next-closest medical facility of any magnitude is 2 hours away,” he said.
Challenges of health literacy and limited access to comprehensive health care at Pine Ridge and other American Indian (AI) and Alaska Native (AN) reservations have long-term consequences. “My own mom struggled to control her blood pressure for years and now has chronic kidney disease,” Mr. Hicks said. “It’s not an uncommon story. Diabetes on the reservation is a big issue.” Then there’s his father, who survived two bouts with melanoma that was diagnosed at an advanced stage. “I think about how that has impacted him, and wonder, had we had a dermatologist who serviced our area, would we have caught things sooner?” he said. “I feel there is so much room for impactful health care deliveries to communities like Pine Ridge.” At the same time, he emphasized, “this isn’t poverty porn. We’re a resilient people. Any effort to engage with AIs or ANs should be from a perspective of a learner, having cultural humility, and seeking out community leaders to help lead you.”
According to the 2020 Census, there are 574 federally recognized sovereign tribal nations in the United States and federal- and state-recognized American Indian reservations in 35 states. AI/AN people make up about 2.9% of the total U.S. population, or 9.7 million, and their life expectancy is an average of 4.4 years less, compared with the general population (a mean of 73.7 vs. 78.1 years, respectively). Because of limited access to dermatologic care in these areas, the risk for developing significant skin conditions and diseases that may go undetected for long stretches of time is increased.
“That can mean advanced skin cancers like basal cell carcinomas that have become larger than what you would see in a typical metropolitan population,” said Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, who spent part of her dermatology residency rotating at the Chinle (Ariz.) Service Unit, an Indian Health Service facility, in 2017 and now provides teledermatology and regular in-person dermatology care at that clinic. “The climate there is dry, so you can see bad eczema and dry skin. There’s also a lot of acne and hidradenitis suppurativa. I think the acne and HS is due to the hyperglycemic index diet from the food deserts. Skin disease reflects the climate, the food desert, and the lack of close specialty care.”
Acne scarring common
Some published evidence suggests that acne is more prevalent and severe in AI/AN individuals. In a survey of 158 AI/AN individuals with a mean age of 32 years, 79.1% reported a history of acne, 55.1% reported acne scarring, and 31% reported having active lesions. “Looking back on my experience in high school, I definitely see that in myself and in my peers,” Mr. Hicks said. And, while there are limited published studies about the incidence of melanoma in this population, an analysis from 2006 found that the incidence was 3.1 per 100,000 between 2001 and 2005, which was an increase from 1.6 per 100,000 reported between 1992 and 2000.
There’s a lot to unpack for dermatologists caring for the AI/AN population besides the raw health disparities: a long history of distrust between AI/AN people and the federal government, structural racism, geographic isolation, health literacy challenges, and high rates of poverty and unemployment. And while individuals from federally recognized tribes have a legal right to receive health care provided by the Indian Health Service, a component of the Department of Health & Human Services, the U.S. Government Accountability Office found that in 2017 per capita spending available to the IHS was $4,078, compared with $8,109 for Medicaid, $10,692 for the Veterans Health Administration, and $13,185 for Medicare.
“Everyone deserves healthy skin and good health,” said Dr. Kohn, whose husband is AI and works in AI law. “Knowing that there are pockets of people who lack that access to care really bothers me. I think the American Indians are frequently overlooked. They’re just not even counted for in certain surveys,” she added, noting that categories are usually defined as Black, Hispanic, Asian, or White.
According to Dr. Kohn, who coauthored a chapter titled “Dermatology on American Indian and Alaska Native Reservations,” for the 2021 book “Dermatology in Rural Settings”, 70% of AIs live in urban areas, “so it’s not just people who live on reservations, though the disparity is greatest there.” To help deliver dermatologic care in the rural areas “where you’re on tribal lands, you must partner with the tribes,” she added. “You must get their permission, operate under their laws and regulations and their rules, learn the local customs, learn about the culture, learn the people, and learn their resources before you practice. That’s the only ethical way to practice.” This also means appreciating the fact that some AI/AN individuals may not understand what a dermatologist could do for them. “One of the bigger hurdles to overcome,” she said, is educating the population that dermatologists can cure skin diseases and that there are good medications for treating the diseases.
Shortcomings of teledermatology
Some dermatologists perform teledermatology visits for tribes, often from an office located in a different time zone. “And, they don’t have a sense of what resources are available for the people they’re serving,” Dr. Kohn said. “For example, if they diagnose a potential skin cancer on the face and say, ‘you need a biopsy,’ but the closest dermatologist is 4 hours away, is that really serving the patient? Or, if you tell a patient, ‘I want you to go out and buy Vanicream for your skin,’ but Vanicream costs $17 and the patient can’t even afford to buy food, are you really doing them a service?”
In a survey-based study of 238 AI individuals that is scheduled to be published in late 2023, Dr. Kohn and colleagues asked respondents at two regional powwows in Denver if they would be open to teledermatology – either in their home or in a primary care clinic. Most respondents (70%) lived in urban areas, the rest in rural settings. Nearly half of respondents (42%) “did not want to do teledermatology, even though they couldn’t access in-person dermatology,” Dr. Kohn said. “So, for people who think teledermatology is the answer [to improving access], the respondents to our survey weren’t interested in pursuing that as a solution. I was surprised by that.” When the researchers broke down the responses by age, teenage respondents were even less interested in teledermatology than adults were. “I think there’s something about having someone see you in person, knowing who you are,” she said.
Partnerships with tribes
To foster more sustainable change in the delivery of skin care beyond remote teledermatology and periodic visits from volunteers, some dermatology residencies have established partnerships with tribes, including Massachusetts General Hospital’s teaching partnership with the Rosebud Sioux tribe in Rosebud, S.D., and the University of Utah dermatology department’s resident continuity clinic with Navajo Nation in Montezuma Creek, Utah. In 2016, officials from the Utah Navajo Health System reached out to the University of Utah’s dermatology department to inquire about the potential for creating a teledermatology clinic to serve patients who receive primary care at the Montezuma Creek Community Health Center, located in Southeastern Utah on the northern tip of the Navajo Nation.
Stephanie Klein, MD, associate professor of dermatology at the university, spearheaded the clinic’s launch but soon encountered obstacles that ranged from not being able to visualize the patient’s skin clearly on her computer screen to difficulty making a personal connection with patients despite help from Navajo translators. “It was hard to build a relationship,” she said. A few years later, she drove down to meet with officials of the health system and posed the question: “What is the ideal thing you would want from dermatology?”
Continuity, they told her. “They said that a lot of the services they receive in the form of outreach are rotational, where someone might come in for a day, or a week, or five people may rotate throughout the year,” which did not serve them well, said Dr. Klein, who subsequently collaborated with Utah Navajo Health System clinicians to establish a resident continuity clinic, which launched in January 2021.
The arrangement also serves as a continuity clinic for Dr. Klein as an attending physician. Each month, she and one dermatology resident drive 6.5 hours from Salt Lake City to Montezuma Creek, where they spend 1 or 2 full days seeing about 25 patients referred by the primary care clinicians who work there. About one-quarter of the time they fly, thanks to financial support from a private donor. The flight takes about an hour, then it’s an hour-long drive to the actual clinic. “It’s a commitment,” Dr. Klein said. “A resident can come with me if they commit to the clinic for at least 1 year. This enables us to have continuity of care; it allows us to build relationships with the patients and with the care team there.” As for the prior teledermatology visits she had with residents, “I still do those, but now I do them in between the in-person visits, so I’m not meeting people over telehealth; I’m just following up with them.”

Situated in the high desert among rock formations, the estimated population of Montezuma Creek is just over 320 people. “It’s a beautiful place with otherworldly buttes and mesas, and the Blue Mountains rising up in the distance,” said Lowell Nicholson, MD, a dermatology resident at the University of Utah who is in his second year of a 2-year commitment to the clinic. “But the landscape can be harsh, and it is underserved from an infrastructure perspective,” with large areas with no cell phone service and limited access to running water and refrigeration. “People in general travel quite far to get their medical care and most of the roads are dirt or gravel, so after a big snowstorm or if it’s been raining, they can become impassable.”
Dermatologic conditions they often encounter include vitiligo, photodermatoses, hidradenitis suppurativa, eczema, psoriasis, and severe acne, often with lots of acne-associated scarring. “In general, we tend to see dramatic or advanced presentations of general dermatology diagnoses,” Dr. Nicholson said. “We see a lot of really extensive psoriasis, which can be socially stigmatizing.”
He recalled one middle-aged man who isolated himself from others because his psoriasis became unbearable. The man refused to leave his house, visit family members, or attend tribal meetups. “He tried to see his regular doctor about it and was given topicals, but his disease was just too extensive,” said Dr. Nicholson, who suggested trying a biologic but learned that the man did not have regular access to refrigeration. “That wasn’t going to work, but we started him on an oral medication, apremilast, which has completely cleared his skin,” he said. “He’s doing great. The last time we saw him he was re-engaged with his family, and he told us he was going on dates. We really improved his quality of life.”
Dr. Klein recalled seeing a 6-year-old girl at the clinic with atopic dermatitis so severe that it caused her to miss several days of school. “When she was in school, she was so distracted by the itching – it was so overwhelming,” she said. She was struggling with topical medicines that weren’t effective, but Dr. Klein got her on dupilumab, and during a follow-up visit the girl told her, “This is the first time in my life I can think about things” other than itching.
According to Dr. Nicholson, some patients seen at the Montezuma Creek clinic are on Medicare or carry standard insurance. “Others have a mix, and others are getting all their medications through the Montezuma Creek clinic or through the IHS clinics,” he said. “I have been surprised at the formulary and our ability to get relatively expensive medications for our patients, like biologics and TNF inhibitors. But it takes some creativity to know what is going to work for your patients’ living situation.”
Training more AI/AN dermatologists key
While efforts to increase the culturally respectful and sustainable dermatologic care for AI/AN individuals continue through programs like the continuity clinic at Montezuma Creek, sources interviewed for this story emphasized the importance of training more AI/AN dermatologists. “Of the people who graduate from high school, AIs have the lowest rate of going on to college,” said Dr. Kohn, who serves as a mentor to Mr. Hicks. “Let’s say they get all the way to medical school; it’s about good mentorship and support in what they’re pursuing. We are seeing more AIs in medical school now, something that I personally notice, and I notice it from what Chinle Service Unit tells me. They have received many requests from Native medical students and premed students who want to rotate at Chinle. Native trainees want the experience of being there.”
According to the Association of American Medical Colleges, the number of AI/AN applicants to medical schools increased from 72 in 2020-2021 to 105 in 2021-2022 but dipped slightly to 94 in 2022-2023. Inspired by a passion to serve Pine Ridge or a community like it, Mr. Hicks decided to apply for medical school. While he doesn’t want to “close any doors” on which medical specialty he ultimately chooses to practice, the current front-runner is dermatology, he said, largely because of the influence of Dr. Kohn and two Mayo dermatologists who have become mentors: Molly Lohman, MD, and Hafsa M. Cantwell, MD. “I didn’t see anyone from my background who was a doctor, so having those role models is so important for Native kids to think, ‘I can do this, too,’ and to pursue it,” he said.
As a proud member of the Oglala Lakota Nation from the Pine Ridge Indian Reservation in southwestern South Dakota, Drew Hicks grew up with limited access to basic health care, let alone the luxury of scheduling an appointment with a dermatologist or another medical specialist.
The area – once home to the Lakota war leader Crazy Horse – encompasses nearly 47,000 residents scattered over about 2.2 million acres, larger than the size of Rhode Island, with land marked by rolling mixed grass prairie, sandhills, and badlands. Some of the Oglala Lakota people live in substandard housing and lack regular access to food, running water, and refrigeration, not to mention cell phone and Internet service. “It’s sparse,” said Mr. Hicks, the son of Tribal ranchers who now is a 3rd-year medical student at the Mayo Clinic College of Medicine and Science in Rochester, Minn., and has an early interest in pursuing dermatology. “There is a lot of territory and not a lot of health care serving the population.” From the Hicks home, the nearest place to receive health care is a family medicine practice in Martin, S.D. – about a 15-minute drive on gravel roads in the best of conditions, but in poor weather, it can be difficult, he said. “So, there are environmental challenges besides the limited number of health care providers.”
Clinicians in the practice “did have to be the point of care for everything from dermatologic issues to emergency medicine to delivering a baby, because the next-closest medical facility of any magnitude is 2 hours away,” he said.
Challenges of health literacy and limited access to comprehensive health care at Pine Ridge and other American Indian (AI) and Alaska Native (AN) reservations have long-term consequences. “My own mom struggled to control her blood pressure for years and now has chronic kidney disease,” Mr. Hicks said. “It’s not an uncommon story. Diabetes on the reservation is a big issue.” Then there’s his father, who survived two bouts with melanoma that was diagnosed at an advanced stage. “I think about how that has impacted him, and wonder, had we had a dermatologist who serviced our area, would we have caught things sooner?” he said. “I feel there is so much room for impactful health care deliveries to communities like Pine Ridge.” At the same time, he emphasized, “this isn’t poverty porn. We’re a resilient people. Any effort to engage with AIs or ANs should be from a perspective of a learner, having cultural humility, and seeking out community leaders to help lead you.”
According to the 2020 Census, there are 574 federally recognized sovereign tribal nations in the United States and federal- and state-recognized American Indian reservations in 35 states. AI/AN people make up about 2.9% of the total U.S. population, or 9.7 million, and their life expectancy is an average of 4.4 years less, compared with the general population (a mean of 73.7 vs. 78.1 years, respectively). Because of limited access to dermatologic care in these areas, the risk for developing significant skin conditions and diseases that may go undetected for long stretches of time is increased.
“That can mean advanced skin cancers like basal cell carcinomas that have become larger than what you would see in a typical metropolitan population,” said Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, who spent part of her dermatology residency rotating at the Chinle (Ariz.) Service Unit, an Indian Health Service facility, in 2017 and now provides teledermatology and regular in-person dermatology care at that clinic. “The climate there is dry, so you can see bad eczema and dry skin. There’s also a lot of acne and hidradenitis suppurativa. I think the acne and HS is due to the hyperglycemic index diet from the food deserts. Skin disease reflects the climate, the food desert, and the lack of close specialty care.”
Acne scarring common
Some published evidence suggests that acne is more prevalent and severe in AI/AN individuals. In a survey of 158 AI/AN individuals with a mean age of 32 years, 79.1% reported a history of acne, 55.1% reported acne scarring, and 31% reported having active lesions. “Looking back on my experience in high school, I definitely see that in myself and in my peers,” Mr. Hicks said. And, while there are limited published studies about the incidence of melanoma in this population, an analysis from 2006 found that the incidence was 3.1 per 100,000 between 2001 and 2005, which was an increase from 1.6 per 100,000 reported between 1992 and 2000.
There’s a lot to unpack for dermatologists caring for the AI/AN population besides the raw health disparities: a long history of distrust between AI/AN people and the federal government, structural racism, geographic isolation, health literacy challenges, and high rates of poverty and unemployment. And while individuals from federally recognized tribes have a legal right to receive health care provided by the Indian Health Service, a component of the Department of Health & Human Services, the U.S. Government Accountability Office found that in 2017 per capita spending available to the IHS was $4,078, compared with $8,109 for Medicaid, $10,692 for the Veterans Health Administration, and $13,185 for Medicare.
“Everyone deserves healthy skin and good health,” said Dr. Kohn, whose husband is AI and works in AI law. “Knowing that there are pockets of people who lack that access to care really bothers me. I think the American Indians are frequently overlooked. They’re just not even counted for in certain surveys,” she added, noting that categories are usually defined as Black, Hispanic, Asian, or White.
According to Dr. Kohn, who coauthored a chapter titled “Dermatology on American Indian and Alaska Native Reservations,” for the 2021 book “Dermatology in Rural Settings”, 70% of AIs live in urban areas, “so it’s not just people who live on reservations, though the disparity is greatest there.” To help deliver dermatologic care in the rural areas “where you’re on tribal lands, you must partner with the tribes,” she added. “You must get their permission, operate under their laws and regulations and their rules, learn the local customs, learn about the culture, learn the people, and learn their resources before you practice. That’s the only ethical way to practice.” This also means appreciating the fact that some AI/AN individuals may not understand what a dermatologist could do for them. “One of the bigger hurdles to overcome,” she said, is educating the population that dermatologists can cure skin diseases and that there are good medications for treating the diseases.
Shortcomings of teledermatology
Some dermatologists perform teledermatology visits for tribes, often from an office located in a different time zone. “And, they don’t have a sense of what resources are available for the people they’re serving,” Dr. Kohn said. “For example, if they diagnose a potential skin cancer on the face and say, ‘you need a biopsy,’ but the closest dermatologist is 4 hours away, is that really serving the patient? Or, if you tell a patient, ‘I want you to go out and buy Vanicream for your skin,’ but Vanicream costs $17 and the patient can’t even afford to buy food, are you really doing them a service?”
In a survey-based study of 238 AI individuals that is scheduled to be published in late 2023, Dr. Kohn and colleagues asked respondents at two regional powwows in Denver if they would be open to teledermatology – either in their home or in a primary care clinic. Most respondents (70%) lived in urban areas, the rest in rural settings. Nearly half of respondents (42%) “did not want to do teledermatology, even though they couldn’t access in-person dermatology,” Dr. Kohn said. “So, for people who think teledermatology is the answer [to improving access], the respondents to our survey weren’t interested in pursuing that as a solution. I was surprised by that.” When the researchers broke down the responses by age, teenage respondents were even less interested in teledermatology than adults were. “I think there’s something about having someone see you in person, knowing who you are,” she said.
Partnerships with tribes
To foster more sustainable change in the delivery of skin care beyond remote teledermatology and periodic visits from volunteers, some dermatology residencies have established partnerships with tribes, including Massachusetts General Hospital’s teaching partnership with the Rosebud Sioux tribe in Rosebud, S.D., and the University of Utah dermatology department’s resident continuity clinic with Navajo Nation in Montezuma Creek, Utah. In 2016, officials from the Utah Navajo Health System reached out to the University of Utah’s dermatology department to inquire about the potential for creating a teledermatology clinic to serve patients who receive primary care at the Montezuma Creek Community Health Center, located in Southeastern Utah on the northern tip of the Navajo Nation.
Stephanie Klein, MD, associate professor of dermatology at the university, spearheaded the clinic’s launch but soon encountered obstacles that ranged from not being able to visualize the patient’s skin clearly on her computer screen to difficulty making a personal connection with patients despite help from Navajo translators. “It was hard to build a relationship,” she said. A few years later, she drove down to meet with officials of the health system and posed the question: “What is the ideal thing you would want from dermatology?”
Continuity, they told her. “They said that a lot of the services they receive in the form of outreach are rotational, where someone might come in for a day, or a week, or five people may rotate throughout the year,” which did not serve them well, said Dr. Klein, who subsequently collaborated with Utah Navajo Health System clinicians to establish a resident continuity clinic, which launched in January 2021.
The arrangement also serves as a continuity clinic for Dr. Klein as an attending physician. Each month, she and one dermatology resident drive 6.5 hours from Salt Lake City to Montezuma Creek, where they spend 1 or 2 full days seeing about 25 patients referred by the primary care clinicians who work there. About one-quarter of the time they fly, thanks to financial support from a private donor. The flight takes about an hour, then it’s an hour-long drive to the actual clinic. “It’s a commitment,” Dr. Klein said. “A resident can come with me if they commit to the clinic for at least 1 year. This enables us to have continuity of care; it allows us to build relationships with the patients and with the care team there.” As for the prior teledermatology visits she had with residents, “I still do those, but now I do them in between the in-person visits, so I’m not meeting people over telehealth; I’m just following up with them.”

Situated in the high desert among rock formations, the estimated population of Montezuma Creek is just over 320 people. “It’s a beautiful place with otherworldly buttes and mesas, and the Blue Mountains rising up in the distance,” said Lowell Nicholson, MD, a dermatology resident at the University of Utah who is in his second year of a 2-year commitment to the clinic. “But the landscape can be harsh, and it is underserved from an infrastructure perspective,” with large areas with no cell phone service and limited access to running water and refrigeration. “People in general travel quite far to get their medical care and most of the roads are dirt or gravel, so after a big snowstorm or if it’s been raining, they can become impassable.”
Dermatologic conditions they often encounter include vitiligo, photodermatoses, hidradenitis suppurativa, eczema, psoriasis, and severe acne, often with lots of acne-associated scarring. “In general, we tend to see dramatic or advanced presentations of general dermatology diagnoses,” Dr. Nicholson said. “We see a lot of really extensive psoriasis, which can be socially stigmatizing.”
He recalled one middle-aged man who isolated himself from others because his psoriasis became unbearable. The man refused to leave his house, visit family members, or attend tribal meetups. “He tried to see his regular doctor about it and was given topicals, but his disease was just too extensive,” said Dr. Nicholson, who suggested trying a biologic but learned that the man did not have regular access to refrigeration. “That wasn’t going to work, but we started him on an oral medication, apremilast, which has completely cleared his skin,” he said. “He’s doing great. The last time we saw him he was re-engaged with his family, and he told us he was going on dates. We really improved his quality of life.”
Dr. Klein recalled seeing a 6-year-old girl at the clinic with atopic dermatitis so severe that it caused her to miss several days of school. “When she was in school, she was so distracted by the itching – it was so overwhelming,” she said. She was struggling with topical medicines that weren’t effective, but Dr. Klein got her on dupilumab, and during a follow-up visit the girl told her, “This is the first time in my life I can think about things” other than itching.
According to Dr. Nicholson, some patients seen at the Montezuma Creek clinic are on Medicare or carry standard insurance. “Others have a mix, and others are getting all their medications through the Montezuma Creek clinic or through the IHS clinics,” he said. “I have been surprised at the formulary and our ability to get relatively expensive medications for our patients, like biologics and TNF inhibitors. But it takes some creativity to know what is going to work for your patients’ living situation.”
Training more AI/AN dermatologists key
While efforts to increase the culturally respectful and sustainable dermatologic care for AI/AN individuals continue through programs like the continuity clinic at Montezuma Creek, sources interviewed for this story emphasized the importance of training more AI/AN dermatologists. “Of the people who graduate from high school, AIs have the lowest rate of going on to college,” said Dr. Kohn, who serves as a mentor to Mr. Hicks. “Let’s say they get all the way to medical school; it’s about good mentorship and support in what they’re pursuing. We are seeing more AIs in medical school now, something that I personally notice, and I notice it from what Chinle Service Unit tells me. They have received many requests from Native medical students and premed students who want to rotate at Chinle. Native trainees want the experience of being there.”
According to the Association of American Medical Colleges, the number of AI/AN applicants to medical schools increased from 72 in 2020-2021 to 105 in 2021-2022 but dipped slightly to 94 in 2022-2023. Inspired by a passion to serve Pine Ridge or a community like it, Mr. Hicks decided to apply for medical school. While he doesn’t want to “close any doors” on which medical specialty he ultimately chooses to practice, the current front-runner is dermatology, he said, largely because of the influence of Dr. Kohn and two Mayo dermatologists who have become mentors: Molly Lohman, MD, and Hafsa M. Cantwell, MD. “I didn’t see anyone from my background who was a doctor, so having those role models is so important for Native kids to think, ‘I can do this, too,’ and to pursue it,” he said.







