User login
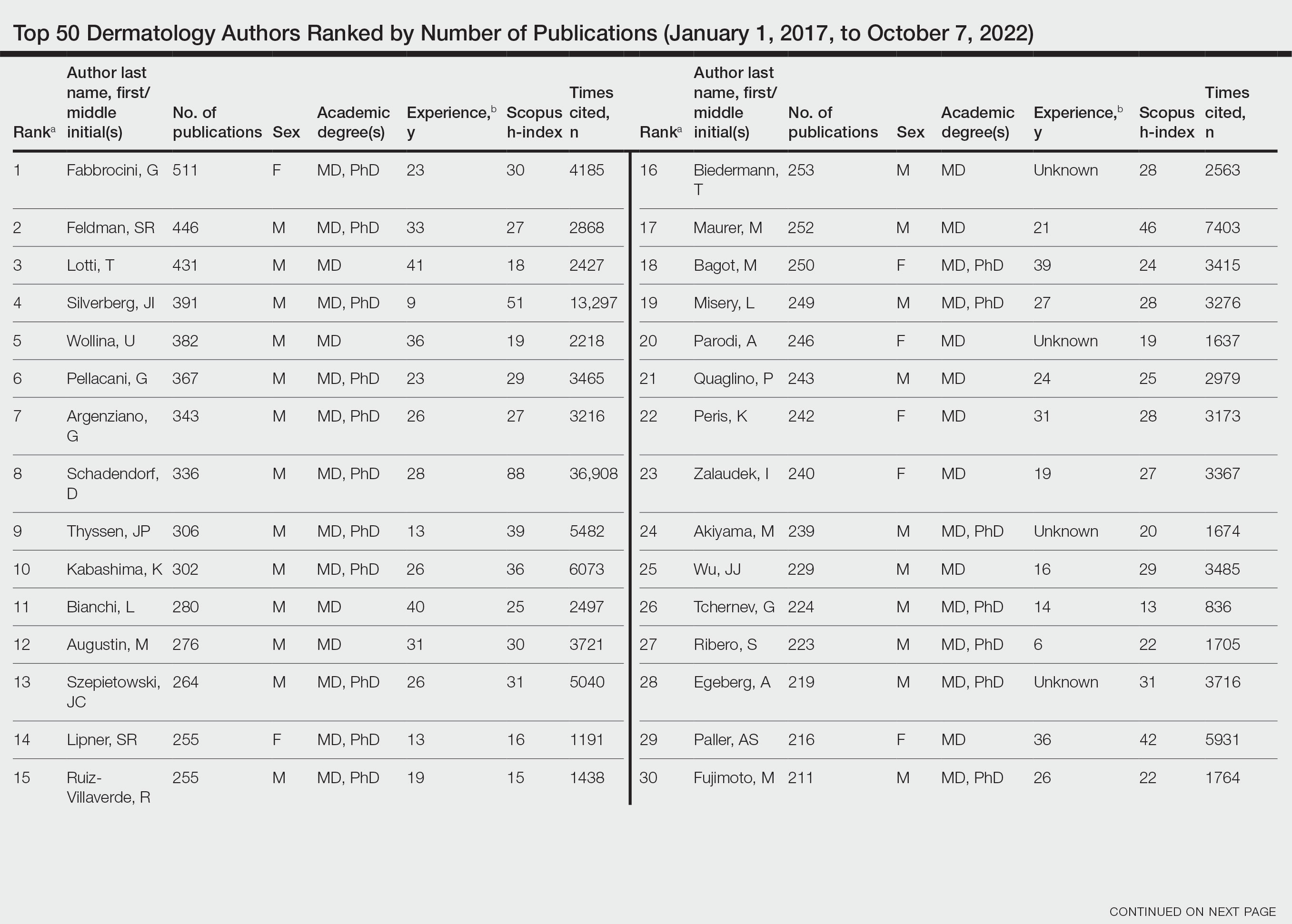
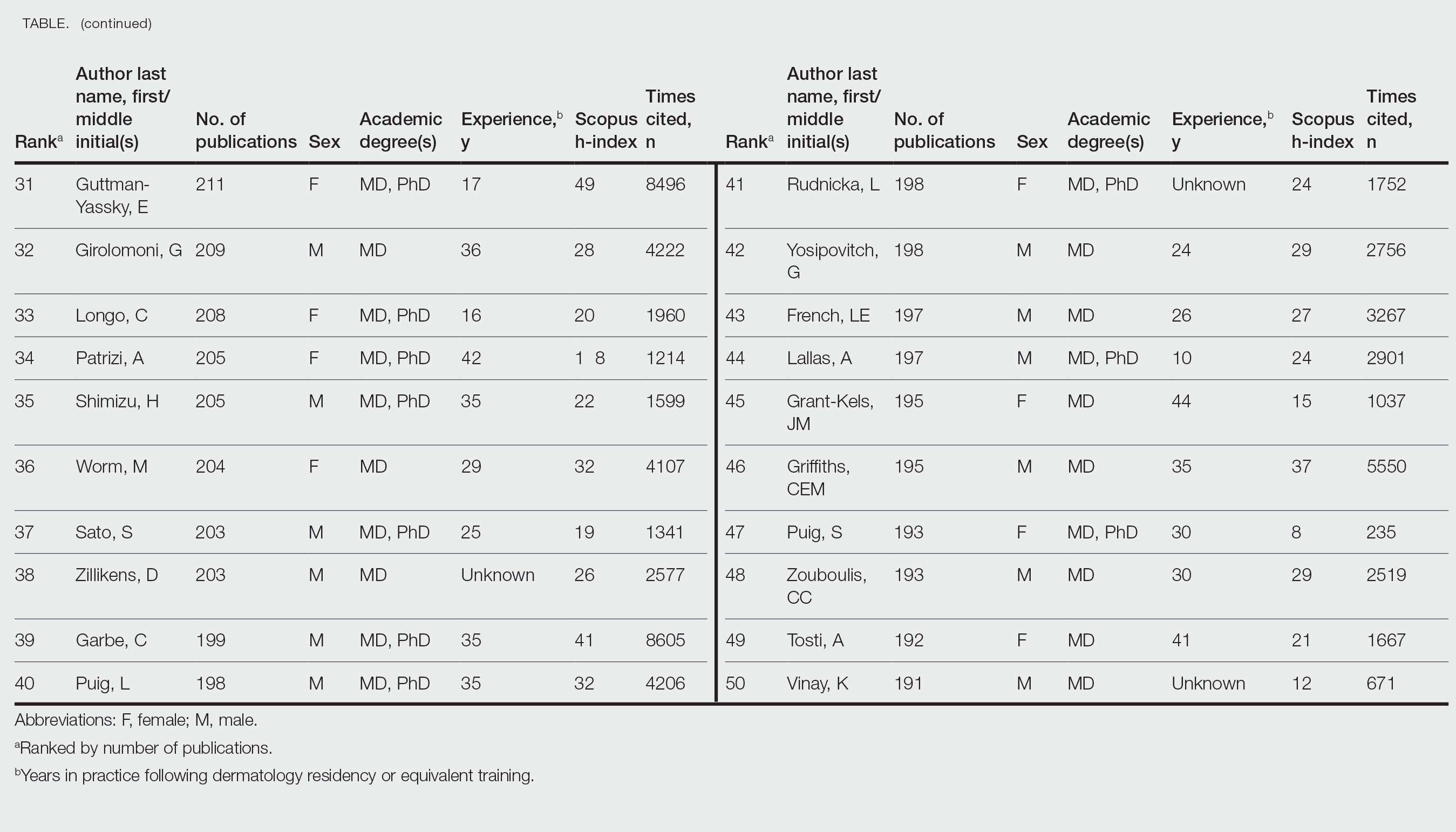
Top 50 Authors in Dermatology by Publication Rate (2017-2022)
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).
Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
To the Editor:
Citation number and Hirsch index (h-index) have long been employed as metrics of productivity for academic scholarship. The h-index is defined as the highest number of publications (the maximum h value) of an author who has published at least h papers, each cited by other authors at least h times.1 In a bibliometric analysis of the most frequently cited authors in dermatology from 1974 to 2019 (N=378,276), females comprised 12% of first and 11% of senior authors of the most cited publications, and 6 of the most cited authors in dermatology were women.2 In another study analyzing the most prolific dermatologic authors based on h-index, 0% from 1980 to 1989 and 19% from 2010 to 2019 were female (N=393,488).3 Because citation number and h-index favor longer-practicing dermatologists, we examined dermatology author productivity and gender trends by recent publication rates.
The Scopus database was searched for dermatology publications by using the field category “dermatology”from January 1, 2017, to October 7, 2022. Nondermatologists and authors with the same initials were excluded. Authors were ranked by number of publications, including original articles, case reports, letters, and reviews. Sex, degree, and years of experience were determined via a Google search of the author’s name. The h-index; number of citations; and percentages of first, middle, and last authorship were recorded.
Of the top 50 published dermatologists, 30% were female (n=15) and 56% (n=28) held both MD and PhD degrees (Table). The mean years of experience was 26.27 years (range, 6–44 years), with a mean of 29.23 years in females and 25.87 years in males. The mean h-index was 27.96 (range, 8–88), with 24.87 for females and 29.29 for males. The mean number of citations was 4032.64 (range, 235–36,908), with 2891.13 for females and 4521.86 for males. Thirty-one authors were most frequently middle authors, 18 were senior authors, and 1 was a first author. On average (SD), authors were senior or first author in 47.97% (20.08%) of their publications (range, 6.32%–94.93%).


Our study shows that females were more highly represented as top dermatology authors (30%) as measured by publication numbers from 2017 to 2022 than in studies measuring citation rate from 1974 to 2019 (12%)2 or h-index from 2010 to 2019 (19%).3 Similarly, in a study of dermatology authorship from 2009 to 2019, on average, females represented 51.06% first and 38.18% last authors.4
The proportion of females in the dermatology workforce has increased, with 3964 of 10,385 (38.2%) active dermatologists in 20075 being female vs 6372 of 12,505 (51.0%) in 2019.6 The lower proportion of practicing female dermatologists in earlier years likely accounts for the lower percentage of females in dermatology citations and h-index top lists during that time, given that citation and h-index metrics are biased to dermatologists with longer careers.
Although our data are encouraging, females still accounted for less than one-third of the top 50 authors by publication numbers. Gender inequalities persist, with only one-third of a total of 1292 National Institutes of Health dermatology grants and one-fourth of Research Project Grant Program (R01) grants being awarded to females in the years 2009 to 2014.7 Therefore, formal and informal mentorship, protected time for research, resources for childcare, and opportunities for funding will be critical in supporting female dermatologists to both publish highly impactful research and obtain research grants.
Limitations of our study include the omission of authors with identical initials and the inability to account for name changes. Furthermore, Scopus does not include all articles published by each author. Finally, publication number reflects quantity but may not reflect quality.
By quantitating dermatology author publication numbers, we found better representation of female authors compared with studies measuring citation number and h-index. With higher proportions of female dermatology trainees and efforts to increase mentorship and research support for female dermatologists, we expect improved equality in top lists of dermatology citations and h-index values.
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
- Dysart J. Measuring research impact and quality: h-index. Accessed July 11, 2023. https://libraryguides.missouri.edu/impact/hindex
- Maymone MBC, Laughter M, Vashi NA, et al. The most cited articles and authors in dermatology: a bibliometric analysis of 1974-2019. J Am Acad Dermatol. 2020;83:201-205. doi:10.1016/j.jaad.2019.06.1308
- Szeto MD, Presley CL, Maymone MBC, et al. Top authors in dermatology by h-index: a bibliometric analysis of 1980-2020. J Am Acad Dermatol. 2021;85:1573-1579. doi:10.1016/j.jaad.2020.10.087
- Laughter MR, Yemc MG, Presley CL, et al. Gender representation in the authorship of dermatology publications. J Am Acad Dermatol. 2022;86:698-700. doi:10.1016/j.jaad.2021.03.019
- Association of American Medical Colleges. 2008 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/media/33491/download
- Association of American Medical Colleges. 2019 physician specialty data report. Accessed July 11, 2023. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-and-specialty-2019
- Cheng MY, Sukhov A, Sultani H, et al. Trends in National Institutes of Health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
Practice Points
- Academic scholarship often is measured by number of citations and h-index. Using these measures, female dermatologists are infrequently represented on top author lists.
- Using the Scopus database to search for the 50 most published dermatology authors from January 1, 2017, to October 7, 2022, 30% were female.
- Higher proportions of female dermatology trainees as well as efforts to increase mentorship and research support for female dermatologists may improve equality in top lists of dermatology citations and h-index values.
Dermatologic care in Indian Country marked by unique challenges, opportunities
As a proud member of the Oglala Lakota Nation from the Pine Ridge Indian Reservation in southwestern South Dakota, Drew Hicks grew up with limited access to basic health care, let alone the luxury of scheduling an appointment with a dermatologist or another medical specialist.
The area – once home to the Lakota war leader Crazy Horse – encompasses nearly 47,000 residents scattered over about 2.2 million acres, larger than the size of Rhode Island, with land marked by rolling mixed grass prairie, sandhills, and badlands. Some of the Oglala Lakota people live in substandard housing and lack regular access to food, running water, and refrigeration, not to mention cell phone and Internet service. “It’s sparse,” said Mr. Hicks, the son of Tribal ranchers who now is a 3rd-year medical student at the Mayo Clinic College of Medicine and Science in Rochester, Minn., and has an early interest in pursuing dermatology. “There is a lot of territory and not a lot of health care serving the population.” From the Hicks home, the nearest place to receive health care is a family medicine practice in Martin, S.D. – about a 15-minute drive on gravel roads in the best of conditions, but in poor weather, it can be difficult, he said. “So, there are environmental challenges besides the limited number of health care providers.”

Clinicians in the practice “did have to be the point of care for everything from dermatologic issues to emergency medicine to delivering a baby, because the next-closest medical facility of any magnitude is 2 hours away,” he said.
Challenges of health literacy and limited access to comprehensive health care at Pine Ridge and other American Indian (AI) and Alaska Native (AN) reservations have long-term consequences. “My own mom struggled to control her blood pressure for years and now has chronic kidney disease,” Mr. Hicks said. “It’s not an uncommon story. Diabetes on the reservation is a big issue.” Then there’s his father, who survived two bouts with melanoma that was diagnosed at an advanced stage. “I think about how that has impacted him, and wonder, had we had a dermatologist who serviced our area, would we have caught things sooner?” he said. “I feel there is so much room for impactful health care deliveries to communities like Pine Ridge.” At the same time, he emphasized, “this isn’t poverty porn. We’re a resilient people. Any effort to engage with AIs or ANs should be from a perspective of a learner, having cultural humility, and seeking out community leaders to help lead you.”
According to the 2020 Census, there are 574 federally recognized sovereign tribal nations in the United States and federal- and state-recognized American Indian reservations in 35 states. AI/AN people make up about 2.9% of the total U.S. population, or 9.7 million, and their life expectancy is an average of 4.4 years less, compared with the general population (a mean of 73.7 vs. 78.1 years, respectively). Because of limited access to dermatologic care in these areas, the risk for developing significant skin conditions and diseases that may go undetected for long stretches of time is increased.
“That can mean advanced skin cancers like basal cell carcinomas that have become larger than what you would see in a typical metropolitan population,” said Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, who spent part of her dermatology residency rotating at the Chinle (Ariz.) Service Unit, an Indian Health Service facility, in 2017 and now provides teledermatology and regular in-person dermatology care at that clinic. “The climate there is dry, so you can see bad eczema and dry skin. There’s also a lot of acne and hidradenitis suppurativa. I think the acne and HS is due to the hyperglycemic index diet from the food deserts. Skin disease reflects the climate, the food desert, and the lack of close specialty care.”
Acne scarring common
Some published evidence suggests that acne is more prevalent and severe in AI/AN individuals. In a survey of 158 AI/AN individuals with a mean age of 32 years, 79.1% reported a history of acne, 55.1% reported acne scarring, and 31% reported having active lesions. “Looking back on my experience in high school, I definitely see that in myself and in my peers,” Mr. Hicks said. And, while there are limited published studies about the incidence of melanoma in this population, an analysis from 2006 found that the incidence was 3.1 per 100,000 between 2001 and 2005, which was an increase from 1.6 per 100,000 reported between 1992 and 2000.
There’s a lot to unpack for dermatologists caring for the AI/AN population besides the raw health disparities: a long history of distrust between AI/AN people and the federal government, structural racism, geographic isolation, health literacy challenges, and high rates of poverty and unemployment. And while individuals from federally recognized tribes have a legal right to receive health care provided by the Indian Health Service, a component of the Department of Health & Human Services, the U.S. Government Accountability Office found that in 2017 per capita spending available to the IHS was $4,078, compared with $8,109 for Medicaid, $10,692 for the Veterans Health Administration, and $13,185 for Medicare.
“Everyone deserves healthy skin and good health,” said Dr. Kohn, whose husband is AI and works in AI law. “Knowing that there are pockets of people who lack that access to care really bothers me. I think the American Indians are frequently overlooked. They’re just not even counted for in certain surveys,” she added, noting that categories are usually defined as Black, Hispanic, Asian, or White.
According to Dr. Kohn, who coauthored a chapter titled “Dermatology on American Indian and Alaska Native Reservations,” for the 2021 book “Dermatology in Rural Settings”, 70% of AIs live in urban areas, “so it’s not just people who live on reservations, though the disparity is greatest there.” To help deliver dermatologic care in the rural areas “where you’re on tribal lands, you must partner with the tribes,” she added. “You must get their permission, operate under their laws and regulations and their rules, learn the local customs, learn about the culture, learn the people, and learn their resources before you practice. That’s the only ethical way to practice.” This also means appreciating the fact that some AI/AN individuals may not understand what a dermatologist could do for them. “One of the bigger hurdles to overcome,” she said, is educating the population that dermatologists can cure skin diseases and that there are good medications for treating the diseases.
Shortcomings of teledermatology
Some dermatologists perform teledermatology visits for tribes, often from an office located in a different time zone. “And, they don’t have a sense of what resources are available for the people they’re serving,” Dr. Kohn said. “For example, if they diagnose a potential skin cancer on the face and say, ‘you need a biopsy,’ but the closest dermatologist is 4 hours away, is that really serving the patient? Or, if you tell a patient, ‘I want you to go out and buy Vanicream for your skin,’ but Vanicream costs $17 and the patient can’t even afford to buy food, are you really doing them a service?”
In a survey-based study of 238 AI individuals that is scheduled to be published in late 2023, Dr. Kohn and colleagues asked respondents at two regional powwows in Denver if they would be open to teledermatology – either in their home or in a primary care clinic. Most respondents (70%) lived in urban areas, the rest in rural settings. Nearly half of respondents (42%) “did not want to do teledermatology, even though they couldn’t access in-person dermatology,” Dr. Kohn said. “So, for people who think teledermatology is the answer [to improving access], the respondents to our survey weren’t interested in pursuing that as a solution. I was surprised by that.” When the researchers broke down the responses by age, teenage respondents were even less interested in teledermatology than adults were. “I think there’s something about having someone see you in person, knowing who you are,” she said.
Partnerships with tribes
To foster more sustainable change in the delivery of skin care beyond remote teledermatology and periodic visits from volunteers, some dermatology residencies have established partnerships with tribes, including Massachusetts General Hospital’s teaching partnership with the Rosebud Sioux tribe in Rosebud, S.D., and the University of Utah dermatology department’s resident continuity clinic with Navajo Nation in Montezuma Creek, Utah. In 2016, officials from the Utah Navajo Health System reached out to the University of Utah’s dermatology department to inquire about the potential for creating a teledermatology clinic to serve patients who receive primary care at the Montezuma Creek Community Health Center, located in Southeastern Utah on the northern tip of the Navajo Nation.
Stephanie Klein, MD, associate professor of dermatology at the university, spearheaded the clinic’s launch but soon encountered obstacles that ranged from not being able to visualize the patient’s skin clearly on her computer screen to difficulty making a personal connection with patients despite help from Navajo translators. “It was hard to build a relationship,” she said. A few years later, she drove down to meet with officials of the health system and posed the question: “What is the ideal thing you would want from dermatology?”
Continuity, they told her. “They said that a lot of the services they receive in the form of outreach are rotational, where someone might come in for a day, or a week, or five people may rotate throughout the year,” which did not serve them well, said Dr. Klein, who subsequently collaborated with Utah Navajo Health System clinicians to establish a resident continuity clinic, which launched in January 2021.
The arrangement also serves as a continuity clinic for Dr. Klein as an attending physician. Each month, she and one dermatology resident drive 6.5 hours from Salt Lake City to Montezuma Creek, where they spend 1 or 2 full days seeing about 25 patients referred by the primary care clinicians who work there. About one-quarter of the time they fly, thanks to financial support from a private donor. The flight takes about an hour, then it’s an hour-long drive to the actual clinic. “It’s a commitment,” Dr. Klein said. “A resident can come with me if they commit to the clinic for at least 1 year. This enables us to have continuity of care; it allows us to build relationships with the patients and with the care team there.” As for the prior teledermatology visits she had with residents, “I still do those, but now I do them in between the in-person visits, so I’m not meeting people over telehealth; I’m just following up with them.”

Situated in the high desert among rock formations, the estimated population of Montezuma Creek is just over 320 people. “It’s a beautiful place with otherworldly buttes and mesas, and the Blue Mountains rising up in the distance,” said Lowell Nicholson, MD, a dermatology resident at the University of Utah who is in his second year of a 2-year commitment to the clinic. “But the landscape can be harsh, and it is underserved from an infrastructure perspective,” with large areas with no cell phone service and limited access to running water and refrigeration. “People in general travel quite far to get their medical care and most of the roads are dirt or gravel, so after a big snowstorm or if it’s been raining, they can become impassable.”

Dermatologic conditions they often encounter include vitiligo, photodermatoses, hidradenitis suppurativa, eczema, psoriasis, and severe acne, often with lots of acne-associated scarring. “In general, we tend to see dramatic or advanced presentations of general dermatology diagnoses,” Dr. Nicholson said. “We see a lot of really extensive psoriasis, which can be socially stigmatizing.”
He recalled one middle-aged man who isolated himself from others because his psoriasis became unbearable. The man refused to leave his house, visit family members, or attend tribal meetups. “He tried to see his regular doctor about it and was given topicals, but his disease was just too extensive,” said Dr. Nicholson, who suggested trying a biologic but learned that the man did not have regular access to refrigeration. “That wasn’t going to work, but we started him on an oral medication, apremilast, which has completely cleared his skin,” he said. “He’s doing great. The last time we saw him he was re-engaged with his family, and he told us he was going on dates. We really improved his quality of life.”
Dr. Klein recalled seeing a 6-year-old girl at the clinic with atopic dermatitis so severe that it caused her to miss several days of school. “When she was in school, she was so distracted by the itching – it was so overwhelming,” she said. She was struggling with topical medicines that weren’t effective, but Dr. Klein got her on dupilumab, and during a follow-up visit the girl told her, “This is the first time in my life I can think about things” other than itching.
According to Dr. Nicholson, some patients seen at the Montezuma Creek clinic are on Medicare or carry standard insurance. “Others have a mix, and others are getting all their medications through the Montezuma Creek clinic or through the IHS clinics,” he said. “I have been surprised at the formulary and our ability to get relatively expensive medications for our patients, like biologics and TNF inhibitors. But it takes some creativity to know what is going to work for your patients’ living situation.”
Training more AI/AN dermatologists key
While efforts to increase the culturally respectful and sustainable dermatologic care for AI/AN individuals continue through programs like the continuity clinic at Montezuma Creek, sources interviewed for this story emphasized the importance of training more AI/AN dermatologists. “Of the people who graduate from high school, AIs have the lowest rate of going on to college,” said Dr. Kohn, who serves as a mentor to Mr. Hicks. “Let’s say they get all the way to medical school; it’s about good mentorship and support in what they’re pursuing. We are seeing more AIs in medical school now, something that I personally notice, and I notice it from what Chinle Service Unit tells me. They have received many requests from Native medical students and premed students who want to rotate at Chinle. Native trainees want the experience of being there.”
According to the Association of American Medical Colleges, the number of AI/AN applicants to medical schools increased from 72 in 2020-2021 to 105 in 2021-2022 but dipped slightly to 94 in 2022-2023. Inspired by a passion to serve Pine Ridge or a community like it, Mr. Hicks decided to apply for medical school. While he doesn’t want to “close any doors” on which medical specialty he ultimately chooses to practice, the current front-runner is dermatology, he said, largely because of the influence of Dr. Kohn and two Mayo dermatologists who have become mentors: Molly Lohman, MD, and Hafsa M. Cantwell, MD. “I didn’t see anyone from my background who was a doctor, so having those role models is so important for Native kids to think, ‘I can do this, too,’ and to pursue it,” he said.
As a proud member of the Oglala Lakota Nation from the Pine Ridge Indian Reservation in southwestern South Dakota, Drew Hicks grew up with limited access to basic health care, let alone the luxury of scheduling an appointment with a dermatologist or another medical specialist.
The area – once home to the Lakota war leader Crazy Horse – encompasses nearly 47,000 residents scattered over about 2.2 million acres, larger than the size of Rhode Island, with land marked by rolling mixed grass prairie, sandhills, and badlands. Some of the Oglala Lakota people live in substandard housing and lack regular access to food, running water, and refrigeration, not to mention cell phone and Internet service. “It’s sparse,” said Mr. Hicks, the son of Tribal ranchers who now is a 3rd-year medical student at the Mayo Clinic College of Medicine and Science in Rochester, Minn., and has an early interest in pursuing dermatology. “There is a lot of territory and not a lot of health care serving the population.” From the Hicks home, the nearest place to receive health care is a family medicine practice in Martin, S.D. – about a 15-minute drive on gravel roads in the best of conditions, but in poor weather, it can be difficult, he said. “So, there are environmental challenges besides the limited number of health care providers.”

Clinicians in the practice “did have to be the point of care for everything from dermatologic issues to emergency medicine to delivering a baby, because the next-closest medical facility of any magnitude is 2 hours away,” he said.
Challenges of health literacy and limited access to comprehensive health care at Pine Ridge and other American Indian (AI) and Alaska Native (AN) reservations have long-term consequences. “My own mom struggled to control her blood pressure for years and now has chronic kidney disease,” Mr. Hicks said. “It’s not an uncommon story. Diabetes on the reservation is a big issue.” Then there’s his father, who survived two bouts with melanoma that was diagnosed at an advanced stage. “I think about how that has impacted him, and wonder, had we had a dermatologist who serviced our area, would we have caught things sooner?” he said. “I feel there is so much room for impactful health care deliveries to communities like Pine Ridge.” At the same time, he emphasized, “this isn’t poverty porn. We’re a resilient people. Any effort to engage with AIs or ANs should be from a perspective of a learner, having cultural humility, and seeking out community leaders to help lead you.”
According to the 2020 Census, there are 574 federally recognized sovereign tribal nations in the United States and federal- and state-recognized American Indian reservations in 35 states. AI/AN people make up about 2.9% of the total U.S. population, or 9.7 million, and their life expectancy is an average of 4.4 years less, compared with the general population (a mean of 73.7 vs. 78.1 years, respectively). Because of limited access to dermatologic care in these areas, the risk for developing significant skin conditions and diseases that may go undetected for long stretches of time is increased.
“That can mean advanced skin cancers like basal cell carcinomas that have become larger than what you would see in a typical metropolitan population,” said Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, who spent part of her dermatology residency rotating at the Chinle (Ariz.) Service Unit, an Indian Health Service facility, in 2017 and now provides teledermatology and regular in-person dermatology care at that clinic. “The climate there is dry, so you can see bad eczema and dry skin. There’s also a lot of acne and hidradenitis suppurativa. I think the acne and HS is due to the hyperglycemic index diet from the food deserts. Skin disease reflects the climate, the food desert, and the lack of close specialty care.”
Acne scarring common
Some published evidence suggests that acne is more prevalent and severe in AI/AN individuals. In a survey of 158 AI/AN individuals with a mean age of 32 years, 79.1% reported a history of acne, 55.1% reported acne scarring, and 31% reported having active lesions. “Looking back on my experience in high school, I definitely see that in myself and in my peers,” Mr. Hicks said. And, while there are limited published studies about the incidence of melanoma in this population, an analysis from 2006 found that the incidence was 3.1 per 100,000 between 2001 and 2005, which was an increase from 1.6 per 100,000 reported between 1992 and 2000.
There’s a lot to unpack for dermatologists caring for the AI/AN population besides the raw health disparities: a long history of distrust between AI/AN people and the federal government, structural racism, geographic isolation, health literacy challenges, and high rates of poverty and unemployment. And while individuals from federally recognized tribes have a legal right to receive health care provided by the Indian Health Service, a component of the Department of Health & Human Services, the U.S. Government Accountability Office found that in 2017 per capita spending available to the IHS was $4,078, compared with $8,109 for Medicaid, $10,692 for the Veterans Health Administration, and $13,185 for Medicare.
“Everyone deserves healthy skin and good health,” said Dr. Kohn, whose husband is AI and works in AI law. “Knowing that there are pockets of people who lack that access to care really bothers me. I think the American Indians are frequently overlooked. They’re just not even counted for in certain surveys,” she added, noting that categories are usually defined as Black, Hispanic, Asian, or White.
According to Dr. Kohn, who coauthored a chapter titled “Dermatology on American Indian and Alaska Native Reservations,” for the 2021 book “Dermatology in Rural Settings”, 70% of AIs live in urban areas, “so it’s not just people who live on reservations, though the disparity is greatest there.” To help deliver dermatologic care in the rural areas “where you’re on tribal lands, you must partner with the tribes,” she added. “You must get their permission, operate under their laws and regulations and their rules, learn the local customs, learn about the culture, learn the people, and learn their resources before you practice. That’s the only ethical way to practice.” This also means appreciating the fact that some AI/AN individuals may not understand what a dermatologist could do for them. “One of the bigger hurdles to overcome,” she said, is educating the population that dermatologists can cure skin diseases and that there are good medications for treating the diseases.
Shortcomings of teledermatology
Some dermatologists perform teledermatology visits for tribes, often from an office located in a different time zone. “And, they don’t have a sense of what resources are available for the people they’re serving,” Dr. Kohn said. “For example, if they diagnose a potential skin cancer on the face and say, ‘you need a biopsy,’ but the closest dermatologist is 4 hours away, is that really serving the patient? Or, if you tell a patient, ‘I want you to go out and buy Vanicream for your skin,’ but Vanicream costs $17 and the patient can’t even afford to buy food, are you really doing them a service?”
In a survey-based study of 238 AI individuals that is scheduled to be published in late 2023, Dr. Kohn and colleagues asked respondents at two regional powwows in Denver if they would be open to teledermatology – either in their home or in a primary care clinic. Most respondents (70%) lived in urban areas, the rest in rural settings. Nearly half of respondents (42%) “did not want to do teledermatology, even though they couldn’t access in-person dermatology,” Dr. Kohn said. “So, for people who think teledermatology is the answer [to improving access], the respondents to our survey weren’t interested in pursuing that as a solution. I was surprised by that.” When the researchers broke down the responses by age, teenage respondents were even less interested in teledermatology than adults were. “I think there’s something about having someone see you in person, knowing who you are,” she said.
Partnerships with tribes
To foster more sustainable change in the delivery of skin care beyond remote teledermatology and periodic visits from volunteers, some dermatology residencies have established partnerships with tribes, including Massachusetts General Hospital’s teaching partnership with the Rosebud Sioux tribe in Rosebud, S.D., and the University of Utah dermatology department’s resident continuity clinic with Navajo Nation in Montezuma Creek, Utah. In 2016, officials from the Utah Navajo Health System reached out to the University of Utah’s dermatology department to inquire about the potential for creating a teledermatology clinic to serve patients who receive primary care at the Montezuma Creek Community Health Center, located in Southeastern Utah on the northern tip of the Navajo Nation.
Stephanie Klein, MD, associate professor of dermatology at the university, spearheaded the clinic’s launch but soon encountered obstacles that ranged from not being able to visualize the patient’s skin clearly on her computer screen to difficulty making a personal connection with patients despite help from Navajo translators. “It was hard to build a relationship,” she said. A few years later, she drove down to meet with officials of the health system and posed the question: “What is the ideal thing you would want from dermatology?”
Continuity, they told her. “They said that a lot of the services they receive in the form of outreach are rotational, where someone might come in for a day, or a week, or five people may rotate throughout the year,” which did not serve them well, said Dr. Klein, who subsequently collaborated with Utah Navajo Health System clinicians to establish a resident continuity clinic, which launched in January 2021.
The arrangement also serves as a continuity clinic for Dr. Klein as an attending physician. Each month, she and one dermatology resident drive 6.5 hours from Salt Lake City to Montezuma Creek, where they spend 1 or 2 full days seeing about 25 patients referred by the primary care clinicians who work there. About one-quarter of the time they fly, thanks to financial support from a private donor. The flight takes about an hour, then it’s an hour-long drive to the actual clinic. “It’s a commitment,” Dr. Klein said. “A resident can come with me if they commit to the clinic for at least 1 year. This enables us to have continuity of care; it allows us to build relationships with the patients and with the care team there.” As for the prior teledermatology visits she had with residents, “I still do those, but now I do them in between the in-person visits, so I’m not meeting people over telehealth; I’m just following up with them.”

Situated in the high desert among rock formations, the estimated population of Montezuma Creek is just over 320 people. “It’s a beautiful place with otherworldly buttes and mesas, and the Blue Mountains rising up in the distance,” said Lowell Nicholson, MD, a dermatology resident at the University of Utah who is in his second year of a 2-year commitment to the clinic. “But the landscape can be harsh, and it is underserved from an infrastructure perspective,” with large areas with no cell phone service and limited access to running water and refrigeration. “People in general travel quite far to get their medical care and most of the roads are dirt or gravel, so after a big snowstorm or if it’s been raining, they can become impassable.”

Dermatologic conditions they often encounter include vitiligo, photodermatoses, hidradenitis suppurativa, eczema, psoriasis, and severe acne, often with lots of acne-associated scarring. “In general, we tend to see dramatic or advanced presentations of general dermatology diagnoses,” Dr. Nicholson said. “We see a lot of really extensive psoriasis, which can be socially stigmatizing.”
He recalled one middle-aged man who isolated himself from others because his psoriasis became unbearable. The man refused to leave his house, visit family members, or attend tribal meetups. “He tried to see his regular doctor about it and was given topicals, but his disease was just too extensive,” said Dr. Nicholson, who suggested trying a biologic but learned that the man did not have regular access to refrigeration. “That wasn’t going to work, but we started him on an oral medication, apremilast, which has completely cleared his skin,” he said. “He’s doing great. The last time we saw him he was re-engaged with his family, and he told us he was going on dates. We really improved his quality of life.”
Dr. Klein recalled seeing a 6-year-old girl at the clinic with atopic dermatitis so severe that it caused her to miss several days of school. “When she was in school, she was so distracted by the itching – it was so overwhelming,” she said. She was struggling with topical medicines that weren’t effective, but Dr. Klein got her on dupilumab, and during a follow-up visit the girl told her, “This is the first time in my life I can think about things” other than itching.
According to Dr. Nicholson, some patients seen at the Montezuma Creek clinic are on Medicare or carry standard insurance. “Others have a mix, and others are getting all their medications through the Montezuma Creek clinic or through the IHS clinics,” he said. “I have been surprised at the formulary and our ability to get relatively expensive medications for our patients, like biologics and TNF inhibitors. But it takes some creativity to know what is going to work for your patients’ living situation.”
Training more AI/AN dermatologists key
While efforts to increase the culturally respectful and sustainable dermatologic care for AI/AN individuals continue through programs like the continuity clinic at Montezuma Creek, sources interviewed for this story emphasized the importance of training more AI/AN dermatologists. “Of the people who graduate from high school, AIs have the lowest rate of going on to college,” said Dr. Kohn, who serves as a mentor to Mr. Hicks. “Let’s say they get all the way to medical school; it’s about good mentorship and support in what they’re pursuing. We are seeing more AIs in medical school now, something that I personally notice, and I notice it from what Chinle Service Unit tells me. They have received many requests from Native medical students and premed students who want to rotate at Chinle. Native trainees want the experience of being there.”
According to the Association of American Medical Colleges, the number of AI/AN applicants to medical schools increased from 72 in 2020-2021 to 105 in 2021-2022 but dipped slightly to 94 in 2022-2023. Inspired by a passion to serve Pine Ridge or a community like it, Mr. Hicks decided to apply for medical school. While he doesn’t want to “close any doors” on which medical specialty he ultimately chooses to practice, the current front-runner is dermatology, he said, largely because of the influence of Dr. Kohn and two Mayo dermatologists who have become mentors: Molly Lohman, MD, and Hafsa M. Cantwell, MD. “I didn’t see anyone from my background who was a doctor, so having those role models is so important for Native kids to think, ‘I can do this, too,’ and to pursue it,” he said.
As a proud member of the Oglala Lakota Nation from the Pine Ridge Indian Reservation in southwestern South Dakota, Drew Hicks grew up with limited access to basic health care, let alone the luxury of scheduling an appointment with a dermatologist or another medical specialist.
The area – once home to the Lakota war leader Crazy Horse – encompasses nearly 47,000 residents scattered over about 2.2 million acres, larger than the size of Rhode Island, with land marked by rolling mixed grass prairie, sandhills, and badlands. Some of the Oglala Lakota people live in substandard housing and lack regular access to food, running water, and refrigeration, not to mention cell phone and Internet service. “It’s sparse,” said Mr. Hicks, the son of Tribal ranchers who now is a 3rd-year medical student at the Mayo Clinic College of Medicine and Science in Rochester, Minn., and has an early interest in pursuing dermatology. “There is a lot of territory and not a lot of health care serving the population.” From the Hicks home, the nearest place to receive health care is a family medicine practice in Martin, S.D. – about a 15-minute drive on gravel roads in the best of conditions, but in poor weather, it can be difficult, he said. “So, there are environmental challenges besides the limited number of health care providers.”

Clinicians in the practice “did have to be the point of care for everything from dermatologic issues to emergency medicine to delivering a baby, because the next-closest medical facility of any magnitude is 2 hours away,” he said.
Challenges of health literacy and limited access to comprehensive health care at Pine Ridge and other American Indian (AI) and Alaska Native (AN) reservations have long-term consequences. “My own mom struggled to control her blood pressure for years and now has chronic kidney disease,” Mr. Hicks said. “It’s not an uncommon story. Diabetes on the reservation is a big issue.” Then there’s his father, who survived two bouts with melanoma that was diagnosed at an advanced stage. “I think about how that has impacted him, and wonder, had we had a dermatologist who serviced our area, would we have caught things sooner?” he said. “I feel there is so much room for impactful health care deliveries to communities like Pine Ridge.” At the same time, he emphasized, “this isn’t poverty porn. We’re a resilient people. Any effort to engage with AIs or ANs should be from a perspective of a learner, having cultural humility, and seeking out community leaders to help lead you.”
According to the 2020 Census, there are 574 federally recognized sovereign tribal nations in the United States and federal- and state-recognized American Indian reservations in 35 states. AI/AN people make up about 2.9% of the total U.S. population, or 9.7 million, and their life expectancy is an average of 4.4 years less, compared with the general population (a mean of 73.7 vs. 78.1 years, respectively). Because of limited access to dermatologic care in these areas, the risk for developing significant skin conditions and diseases that may go undetected for long stretches of time is increased.
“That can mean advanced skin cancers like basal cell carcinomas that have become larger than what you would see in a typical metropolitan population,” said Lucinda Kohn, MD, assistant professor of dermatology in the Centers for American Indian and Alaska Native Health at the University of Colorado at Denver, Aurora, who spent part of her dermatology residency rotating at the Chinle (Ariz.) Service Unit, an Indian Health Service facility, in 2017 and now provides teledermatology and regular in-person dermatology care at that clinic. “The climate there is dry, so you can see bad eczema and dry skin. There’s also a lot of acne and hidradenitis suppurativa. I think the acne and HS is due to the hyperglycemic index diet from the food deserts. Skin disease reflects the climate, the food desert, and the lack of close specialty care.”
Acne scarring common
Some published evidence suggests that acne is more prevalent and severe in AI/AN individuals. In a survey of 158 AI/AN individuals with a mean age of 32 years, 79.1% reported a history of acne, 55.1% reported acne scarring, and 31% reported having active lesions. “Looking back on my experience in high school, I definitely see that in myself and in my peers,” Mr. Hicks said. And, while there are limited published studies about the incidence of melanoma in this population, an analysis from 2006 found that the incidence was 3.1 per 100,000 between 2001 and 2005, which was an increase from 1.6 per 100,000 reported between 1992 and 2000.
There’s a lot to unpack for dermatologists caring for the AI/AN population besides the raw health disparities: a long history of distrust between AI/AN people and the federal government, structural racism, geographic isolation, health literacy challenges, and high rates of poverty and unemployment. And while individuals from federally recognized tribes have a legal right to receive health care provided by the Indian Health Service, a component of the Department of Health & Human Services, the U.S. Government Accountability Office found that in 2017 per capita spending available to the IHS was $4,078, compared with $8,109 for Medicaid, $10,692 for the Veterans Health Administration, and $13,185 for Medicare.
“Everyone deserves healthy skin and good health,” said Dr. Kohn, whose husband is AI and works in AI law. “Knowing that there are pockets of people who lack that access to care really bothers me. I think the American Indians are frequently overlooked. They’re just not even counted for in certain surveys,” she added, noting that categories are usually defined as Black, Hispanic, Asian, or White.
According to Dr. Kohn, who coauthored a chapter titled “Dermatology on American Indian and Alaska Native Reservations,” for the 2021 book “Dermatology in Rural Settings”, 70% of AIs live in urban areas, “so it’s not just people who live on reservations, though the disparity is greatest there.” To help deliver dermatologic care in the rural areas “where you’re on tribal lands, you must partner with the tribes,” she added. “You must get their permission, operate under their laws and regulations and their rules, learn the local customs, learn about the culture, learn the people, and learn their resources before you practice. That’s the only ethical way to practice.” This also means appreciating the fact that some AI/AN individuals may not understand what a dermatologist could do for them. “One of the bigger hurdles to overcome,” she said, is educating the population that dermatologists can cure skin diseases and that there are good medications for treating the diseases.
Shortcomings of teledermatology
Some dermatologists perform teledermatology visits for tribes, often from an office located in a different time zone. “And, they don’t have a sense of what resources are available for the people they’re serving,” Dr. Kohn said. “For example, if they diagnose a potential skin cancer on the face and say, ‘you need a biopsy,’ but the closest dermatologist is 4 hours away, is that really serving the patient? Or, if you tell a patient, ‘I want you to go out and buy Vanicream for your skin,’ but Vanicream costs $17 and the patient can’t even afford to buy food, are you really doing them a service?”
In a survey-based study of 238 AI individuals that is scheduled to be published in late 2023, Dr. Kohn and colleagues asked respondents at two regional powwows in Denver if they would be open to teledermatology – either in their home or in a primary care clinic. Most respondents (70%) lived in urban areas, the rest in rural settings. Nearly half of respondents (42%) “did not want to do teledermatology, even though they couldn’t access in-person dermatology,” Dr. Kohn said. “So, for people who think teledermatology is the answer [to improving access], the respondents to our survey weren’t interested in pursuing that as a solution. I was surprised by that.” When the researchers broke down the responses by age, teenage respondents were even less interested in teledermatology than adults were. “I think there’s something about having someone see you in person, knowing who you are,” she said.
Partnerships with tribes
To foster more sustainable change in the delivery of skin care beyond remote teledermatology and periodic visits from volunteers, some dermatology residencies have established partnerships with tribes, including Massachusetts General Hospital’s teaching partnership with the Rosebud Sioux tribe in Rosebud, S.D., and the University of Utah dermatology department’s resident continuity clinic with Navajo Nation in Montezuma Creek, Utah. In 2016, officials from the Utah Navajo Health System reached out to the University of Utah’s dermatology department to inquire about the potential for creating a teledermatology clinic to serve patients who receive primary care at the Montezuma Creek Community Health Center, located in Southeastern Utah on the northern tip of the Navajo Nation.
Stephanie Klein, MD, associate professor of dermatology at the university, spearheaded the clinic’s launch but soon encountered obstacles that ranged from not being able to visualize the patient’s skin clearly on her computer screen to difficulty making a personal connection with patients despite help from Navajo translators. “It was hard to build a relationship,” she said. A few years later, she drove down to meet with officials of the health system and posed the question: “What is the ideal thing you would want from dermatology?”
Continuity, they told her. “They said that a lot of the services they receive in the form of outreach are rotational, where someone might come in for a day, or a week, or five people may rotate throughout the year,” which did not serve them well, said Dr. Klein, who subsequently collaborated with Utah Navajo Health System clinicians to establish a resident continuity clinic, which launched in January 2021.
The arrangement also serves as a continuity clinic for Dr. Klein as an attending physician. Each month, she and one dermatology resident drive 6.5 hours from Salt Lake City to Montezuma Creek, where they spend 1 or 2 full days seeing about 25 patients referred by the primary care clinicians who work there. About one-quarter of the time they fly, thanks to financial support from a private donor. The flight takes about an hour, then it’s an hour-long drive to the actual clinic. “It’s a commitment,” Dr. Klein said. “A resident can come with me if they commit to the clinic for at least 1 year. This enables us to have continuity of care; it allows us to build relationships with the patients and with the care team there.” As for the prior teledermatology visits she had with residents, “I still do those, but now I do them in between the in-person visits, so I’m not meeting people over telehealth; I’m just following up with them.”

Situated in the high desert among rock formations, the estimated population of Montezuma Creek is just over 320 people. “It’s a beautiful place with otherworldly buttes and mesas, and the Blue Mountains rising up in the distance,” said Lowell Nicholson, MD, a dermatology resident at the University of Utah who is in his second year of a 2-year commitment to the clinic. “But the landscape can be harsh, and it is underserved from an infrastructure perspective,” with large areas with no cell phone service and limited access to running water and refrigeration. “People in general travel quite far to get their medical care and most of the roads are dirt or gravel, so after a big snowstorm or if it’s been raining, they can become impassable.”

Dermatologic conditions they often encounter include vitiligo, photodermatoses, hidradenitis suppurativa, eczema, psoriasis, and severe acne, often with lots of acne-associated scarring. “In general, we tend to see dramatic or advanced presentations of general dermatology diagnoses,” Dr. Nicholson said. “We see a lot of really extensive psoriasis, which can be socially stigmatizing.”
He recalled one middle-aged man who isolated himself from others because his psoriasis became unbearable. The man refused to leave his house, visit family members, or attend tribal meetups. “He tried to see his regular doctor about it and was given topicals, but his disease was just too extensive,” said Dr. Nicholson, who suggested trying a biologic but learned that the man did not have regular access to refrigeration. “That wasn’t going to work, but we started him on an oral medication, apremilast, which has completely cleared his skin,” he said. “He’s doing great. The last time we saw him he was re-engaged with his family, and he told us he was going on dates. We really improved his quality of life.”
Dr. Klein recalled seeing a 6-year-old girl at the clinic with atopic dermatitis so severe that it caused her to miss several days of school. “When she was in school, she was so distracted by the itching – it was so overwhelming,” she said. She was struggling with topical medicines that weren’t effective, but Dr. Klein got her on dupilumab, and during a follow-up visit the girl told her, “This is the first time in my life I can think about things” other than itching.
According to Dr. Nicholson, some patients seen at the Montezuma Creek clinic are on Medicare or carry standard insurance. “Others have a mix, and others are getting all their medications through the Montezuma Creek clinic or through the IHS clinics,” he said. “I have been surprised at the formulary and our ability to get relatively expensive medications for our patients, like biologics and TNF inhibitors. But it takes some creativity to know what is going to work for your patients’ living situation.”
Training more AI/AN dermatologists key
While efforts to increase the culturally respectful and sustainable dermatologic care for AI/AN individuals continue through programs like the continuity clinic at Montezuma Creek, sources interviewed for this story emphasized the importance of training more AI/AN dermatologists. “Of the people who graduate from high school, AIs have the lowest rate of going on to college,” said Dr. Kohn, who serves as a mentor to Mr. Hicks. “Let’s say they get all the way to medical school; it’s about good mentorship and support in what they’re pursuing. We are seeing more AIs in medical school now, something that I personally notice, and I notice it from what Chinle Service Unit tells me. They have received many requests from Native medical students and premed students who want to rotate at Chinle. Native trainees want the experience of being there.”
According to the Association of American Medical Colleges, the number of AI/AN applicants to medical schools increased from 72 in 2020-2021 to 105 in 2021-2022 but dipped slightly to 94 in 2022-2023. Inspired by a passion to serve Pine Ridge or a community like it, Mr. Hicks decided to apply for medical school. While he doesn’t want to “close any doors” on which medical specialty he ultimately chooses to practice, the current front-runner is dermatology, he said, largely because of the influence of Dr. Kohn and two Mayo dermatologists who have become mentors: Molly Lohman, MD, and Hafsa M. Cantwell, MD. “I didn’t see anyone from my background who was a doctor, so having those role models is so important for Native kids to think, ‘I can do this, too,’ and to pursue it,” he said.
ALL: Excess weight linked to much worse outcomes
The study, published in Blood Advances, doesn’t identify a culprit behind the worse outcomes in obese and overweight patients. However, it does highlight the limits of asparaginase-containing pediatric regimens in these patients, said study senior author and Dana-Farber Cancer Institute leukemia specialist Marlise R. Luskin, MD, in an interview.
“Pediatric-inspired regimens can be applied safely in adults up to the age of 50 years, with those of normal BMI having particularly good outcomes. Further research is needed to determine the best approach to treating patients of all ages with elevated BMIs,” Dr. Luskin said.
ALL is an uncommon cancer that kills about 1,390 people in the United States each year, according to the American Cancer Society. Most cases are in children, but most deaths are in adults, according to the ACS.
Research over the past 20 years has shown that younger adults “with ALL have better outcomes when they treated with intensive pediatric-style chemotherapy regimens than when they are treated with traditional adult regimens,” Dr. Luskin said. “Still, too many young adults do not have good outcomes. Either their disease is resistant to chemotherapy, or they experience significant side effects from treatment. Our main motivation for this study was to better understand which adolescents and younger adults have good outcomes with pediatric-style chemotherapy, and which patients require improved approaches.”
The researchers retrospectively tracked 388 patients aged 15-50 who were treated with Dana-Farber Consortium regimens from 2001 to 2021. A total of 46.7% were overweight or obese as defined by BMI. Of the rest, 2.6% were underweight, and 50.7% had normal weight. The study defined this combined group (53.3%) as having normal weight.
Most patients were male (61.9%), the median age was 24 years, and 35% were aged 30-50. All were treated with asparaginase-based regimens, although the components of the regimens changed over time.
In a bit of good news, “the study remarkably found equivalent overall survival among younger (aged 15-29) and older (aged 30-50) patients with normal BMI – 83% versus 85%, respectively [P = .89],” said lead author and Dana-Farber Cancer Institute advanced leukemia fellow Shai Shimony, MD. “This is an incredibly important finding as many are hesitant to offer pediatric regimens to patients over 30 years merely because of their age.”
As for differences by weight, both the normal and overweight/obese groups had identical rates of remission (87%; P = .84). However, overweight or obese patients had higher 4-year non-relapse mortality (11.7% vs. 2.8%; P = .006), worse event-free 4-year survival (63% vs. 77%; P = .003), and worse overall 4-year survival (64% vs. 83%; P = .0001).
Older obese/overweight patients (aged 30-50) were especially vulnerable to death, with worse overall 4-year survival versus their younger counterparts (55% vs. 73%; P = .023).
In another finding, the researchers also found that high triglyceride levels were common in patients, and this was linked to improved survival and decreased risk of relapse. These higher levels are likely linked to the drug regimen and “are not in and of themselves harmful or a reason to discontinue treatment,” Dr. Luskin said.
As for treatment-related side effects, an analysis of 353 patients found that grade III/IV hepatotoxicity – defined as elevation of AST, ALT, and/or bilirubin – was higher in patients who were overweight/obese versus normal weight (60.7% vs. 42.2%; P = .0005). Grade III/IV hyperglycemias were also higher in the overweight/obese group vs. normal weight (36.4% vs. 24.4%; P = .014).
Why might excess weight lead to worse outcomes? “We found that BMI was associated with more nonrelapse mortality, meaning death due to treatment-related side effects,” said Dr. Shimony. “This may be because patients with higher BMI are less able to tolerate chemotherapy, possibly due to more hyperglycemia, infection, and less overall resilience in the setting of complications. Obesity may also be associated with intrinsic disease resistance. This may be because adipose tissue protects lymphoblasts from the effects of chemotherapy or is due to underdosing of chemotherapy drugs.”
The study authors noted limitations to their research such as its reliance on BMI at diagnosis, without details about weight changes over time, and the lack of a systematic evaluation of measurable residual disease.
In an interview, Gwen Nichols, MD, chief medical officer of the Leukemia & Lymphoma Society, said it’s indeed possible that heavier patients may be underdosed with chemotherapy – especially older ones who may have more excess weight than children.
Dr. Nichols, who praised the study, highlighted another theory. “What causes you metabolically to be overweight may be connected to some reason to less metabolization of chemotherapy drugs,” she said.
Going forward, Dr. Shimony said “clinicians treating younger adults with ALL should monitor their patients with elevated BMI very closely for response and side effects. We recommend these patients be enrolled in clinical trials whenever possible so that more can be learned about how this group of patients responds to novel treatment approaches. Importantly, it is not yet known how obesity is associated with outcomes in nonasparaginase regimens such as hyper-CVAD or those approaches that rely on novel agents.”
The Foley Family Research Fund funded the study. Dr. Luskin disclosed research support from Abbvie and Novartis, and some other study authors reported various disclosures. Dr. Shimony and Dr. Nichols have no disclosures.
The study, published in Blood Advances, doesn’t identify a culprit behind the worse outcomes in obese and overweight patients. However, it does highlight the limits of asparaginase-containing pediatric regimens in these patients, said study senior author and Dana-Farber Cancer Institute leukemia specialist Marlise R. Luskin, MD, in an interview.
“Pediatric-inspired regimens can be applied safely in adults up to the age of 50 years, with those of normal BMI having particularly good outcomes. Further research is needed to determine the best approach to treating patients of all ages with elevated BMIs,” Dr. Luskin said.
ALL is an uncommon cancer that kills about 1,390 people in the United States each year, according to the American Cancer Society. Most cases are in children, but most deaths are in adults, according to the ACS.
Research over the past 20 years has shown that younger adults “with ALL have better outcomes when they treated with intensive pediatric-style chemotherapy regimens than when they are treated with traditional adult regimens,” Dr. Luskin said. “Still, too many young adults do not have good outcomes. Either their disease is resistant to chemotherapy, or they experience significant side effects from treatment. Our main motivation for this study was to better understand which adolescents and younger adults have good outcomes with pediatric-style chemotherapy, and which patients require improved approaches.”
The researchers retrospectively tracked 388 patients aged 15-50 who were treated with Dana-Farber Consortium regimens from 2001 to 2021. A total of 46.7% were overweight or obese as defined by BMI. Of the rest, 2.6% were underweight, and 50.7% had normal weight. The study defined this combined group (53.3%) as having normal weight.
Most patients were male (61.9%), the median age was 24 years, and 35% were aged 30-50. All were treated with asparaginase-based regimens, although the components of the regimens changed over time.
In a bit of good news, “the study remarkably found equivalent overall survival among younger (aged 15-29) and older (aged 30-50) patients with normal BMI – 83% versus 85%, respectively [P = .89],” said lead author and Dana-Farber Cancer Institute advanced leukemia fellow Shai Shimony, MD. “This is an incredibly important finding as many are hesitant to offer pediatric regimens to patients over 30 years merely because of their age.”
As for differences by weight, both the normal and overweight/obese groups had identical rates of remission (87%; P = .84). However, overweight or obese patients had higher 4-year non-relapse mortality (11.7% vs. 2.8%; P = .006), worse event-free 4-year survival (63% vs. 77%; P = .003), and worse overall 4-year survival (64% vs. 83%; P = .0001).
Older obese/overweight patients (aged 30-50) were especially vulnerable to death, with worse overall 4-year survival versus their younger counterparts (55% vs. 73%; P = .023).
In another finding, the researchers also found that high triglyceride levels were common in patients, and this was linked to improved survival and decreased risk of relapse. These higher levels are likely linked to the drug regimen and “are not in and of themselves harmful or a reason to discontinue treatment,” Dr. Luskin said.
As for treatment-related side effects, an analysis of 353 patients found that grade III/IV hepatotoxicity – defined as elevation of AST, ALT, and/or bilirubin – was higher in patients who were overweight/obese versus normal weight (60.7% vs. 42.2%; P = .0005). Grade III/IV hyperglycemias were also higher in the overweight/obese group vs. normal weight (36.4% vs. 24.4%; P = .014).
Why might excess weight lead to worse outcomes? “We found that BMI was associated with more nonrelapse mortality, meaning death due to treatment-related side effects,” said Dr. Shimony. “This may be because patients with higher BMI are less able to tolerate chemotherapy, possibly due to more hyperglycemia, infection, and less overall resilience in the setting of complications. Obesity may also be associated with intrinsic disease resistance. This may be because adipose tissue protects lymphoblasts from the effects of chemotherapy or is due to underdosing of chemotherapy drugs.”
The study authors noted limitations to their research such as its reliance on BMI at diagnosis, without details about weight changes over time, and the lack of a systematic evaluation of measurable residual disease.
In an interview, Gwen Nichols, MD, chief medical officer of the Leukemia & Lymphoma Society, said it’s indeed possible that heavier patients may be underdosed with chemotherapy – especially older ones who may have more excess weight than children.
Dr. Nichols, who praised the study, highlighted another theory. “What causes you metabolically to be overweight may be connected to some reason to less metabolization of chemotherapy drugs,” she said.
Going forward, Dr. Shimony said “clinicians treating younger adults with ALL should monitor their patients with elevated BMI very closely for response and side effects. We recommend these patients be enrolled in clinical trials whenever possible so that more can be learned about how this group of patients responds to novel treatment approaches. Importantly, it is not yet known how obesity is associated with outcomes in nonasparaginase regimens such as hyper-CVAD or those approaches that rely on novel agents.”
The Foley Family Research Fund funded the study. Dr. Luskin disclosed research support from Abbvie and Novartis, and some other study authors reported various disclosures. Dr. Shimony and Dr. Nichols have no disclosures.
The study, published in Blood Advances, doesn’t identify a culprit behind the worse outcomes in obese and overweight patients. However, it does highlight the limits of asparaginase-containing pediatric regimens in these patients, said study senior author and Dana-Farber Cancer Institute leukemia specialist Marlise R. Luskin, MD, in an interview.
“Pediatric-inspired regimens can be applied safely in adults up to the age of 50 years, with those of normal BMI having particularly good outcomes. Further research is needed to determine the best approach to treating patients of all ages with elevated BMIs,” Dr. Luskin said.
ALL is an uncommon cancer that kills about 1,390 people in the United States each year, according to the American Cancer Society. Most cases are in children, but most deaths are in adults, according to the ACS.
Research over the past 20 years has shown that younger adults “with ALL have better outcomes when they treated with intensive pediatric-style chemotherapy regimens than when they are treated with traditional adult regimens,” Dr. Luskin said. “Still, too many young adults do not have good outcomes. Either their disease is resistant to chemotherapy, or they experience significant side effects from treatment. Our main motivation for this study was to better understand which adolescents and younger adults have good outcomes with pediatric-style chemotherapy, and which patients require improved approaches.”
The researchers retrospectively tracked 388 patients aged 15-50 who were treated with Dana-Farber Consortium regimens from 2001 to 2021. A total of 46.7% were overweight or obese as defined by BMI. Of the rest, 2.6% were underweight, and 50.7% had normal weight. The study defined this combined group (53.3%) as having normal weight.
Most patients were male (61.9%), the median age was 24 years, and 35% were aged 30-50. All were treated with asparaginase-based regimens, although the components of the regimens changed over time.
In a bit of good news, “the study remarkably found equivalent overall survival among younger (aged 15-29) and older (aged 30-50) patients with normal BMI – 83% versus 85%, respectively [P = .89],” said lead author and Dana-Farber Cancer Institute advanced leukemia fellow Shai Shimony, MD. “This is an incredibly important finding as many are hesitant to offer pediatric regimens to patients over 30 years merely because of their age.”
As for differences by weight, both the normal and overweight/obese groups had identical rates of remission (87%; P = .84). However, overweight or obese patients had higher 4-year non-relapse mortality (11.7% vs. 2.8%; P = .006), worse event-free 4-year survival (63% vs. 77%; P = .003), and worse overall 4-year survival (64% vs. 83%; P = .0001).
Older obese/overweight patients (aged 30-50) were especially vulnerable to death, with worse overall 4-year survival versus their younger counterparts (55% vs. 73%; P = .023).
In another finding, the researchers also found that high triglyceride levels were common in patients, and this was linked to improved survival and decreased risk of relapse. These higher levels are likely linked to the drug regimen and “are not in and of themselves harmful or a reason to discontinue treatment,” Dr. Luskin said.
As for treatment-related side effects, an analysis of 353 patients found that grade III/IV hepatotoxicity – defined as elevation of AST, ALT, and/or bilirubin – was higher in patients who were overweight/obese versus normal weight (60.7% vs. 42.2%; P = .0005). Grade III/IV hyperglycemias were also higher in the overweight/obese group vs. normal weight (36.4% vs. 24.4%; P = .014).
Why might excess weight lead to worse outcomes? “We found that BMI was associated with more nonrelapse mortality, meaning death due to treatment-related side effects,” said Dr. Shimony. “This may be because patients with higher BMI are less able to tolerate chemotherapy, possibly due to more hyperglycemia, infection, and less overall resilience in the setting of complications. Obesity may also be associated with intrinsic disease resistance. This may be because adipose tissue protects lymphoblasts from the effects of chemotherapy or is due to underdosing of chemotherapy drugs.”
The study authors noted limitations to their research such as its reliance on BMI at diagnosis, without details about weight changes over time, and the lack of a systematic evaluation of measurable residual disease.
In an interview, Gwen Nichols, MD, chief medical officer of the Leukemia & Lymphoma Society, said it’s indeed possible that heavier patients may be underdosed with chemotherapy – especially older ones who may have more excess weight than children.
Dr. Nichols, who praised the study, highlighted another theory. “What causes you metabolically to be overweight may be connected to some reason to less metabolization of chemotherapy drugs,” she said.
Going forward, Dr. Shimony said “clinicians treating younger adults with ALL should monitor their patients with elevated BMI very closely for response and side effects. We recommend these patients be enrolled in clinical trials whenever possible so that more can be learned about how this group of patients responds to novel treatment approaches. Importantly, it is not yet known how obesity is associated with outcomes in nonasparaginase regimens such as hyper-CVAD or those approaches that rely on novel agents.”
The Foley Family Research Fund funded the study. Dr. Luskin disclosed research support from Abbvie and Novartis, and some other study authors reported various disclosures. Dr. Shimony and Dr. Nichols have no disclosures.
FROM BLOOD ADVANCES
Review of 3 Comprehensive Anki Flash Card Decks for Dermatology Residents
Similar to medical school, residency is a time to drink out of the proverbial firehose of knowledge. Along with clinical duties, there is a plethora of information ranging from clinical management decisions to boards fodder that dermatology residents are expected to know, leaving residents to adopt study habits from medical school. Flash cards remain a popular study tool in the medical education community. The use of Anki, a web-based and mobile flash card application (app) that features custom and premade flash card decks made and shared by users, has become increasingly popular. In a 2021 study, Lu et al1 found that Anki flash card usage was associated with higher US Medical Licensing Examination scores. Herein, I provide an updated review of the top 3 most comprehensive premade Anki decks for dermatology residents, per my assessment.
COMPREHENSIVE DERMATOLOGY DECKS
Dolphin Dermatology
- Creator: Reddit user, Unknown2
- Date created: December 2020
- Last updated: April 2022
- Number of cards: 13,833
- Resources covered: Photographs of common dermatologic diagnoses from online sources such as VisualDx (https://www.visualdx.com/) and DermNet (https://dermnetnz.org/).
- Format of cards: One image or factoid per card.
- Card tags (allow separation of Anki decks into subcategories): Each general dermatology card is tagged by the diagnosis name. Pediatric dermatology cards are tagged by affected body location.
- Advantages: As you may glean by the sheer number of flash cards, this deck is a comprehensive review of clinical dermatology. Most cards feature clinical vignettes with clinical photographs of a dermatologic condition or histologic slide and ask what the diagnosis may be. It features photographs of pathology on a range of skin tones and many different images of each diagnosis. This is a great deck for residents who need to study clinical photographs of dermatologic diagnoses.
- Disadvantages: This deck does not cover dermatopathology, basic science, treatment options, or pharmacology in depth. Additionally, is difficult to find a link to download this resource.
- At the time of publication of this article, users are unable to download this deck.
vismo_djib’s Review of Dermatology Anki
- Creator: Reddit user vismo_djib3
- Date created: June 2020
- Last updated: February 2022
- Number of cards: 8454
- Resources covered: Alikhan and Hocker’s Review of Dermatology4 is the main resource with supplemental images from VisualDx, Bolognia et al’s Dermatology,5 Patterson’s Weedon’s Skin Pathology Essentials,6 Elston et al’s Dermatopathology,7 Soyer et al’s Dermoscopy: The Essentials,8 and Robinson et al’s Surgery of the Skin: Procedural Dermatology.9
- Format of cards: Cards mostly feature a diagnosis with color-coded categories including epidemiology, pathogenesis, clinical features, histopathology, and treatment.
- Card tags (allow separation of Anki decks into subcategories): Cards are tagged with chapter numbers from Alikhan and Hocker’s Review of Dermatology.4
- Advantages: This impressive comprehensive review of dermatology is a great option for residents studying for the American Board of Dermatology CORE examinations and users looking to solidify the information in Alikhan and Hocker’s Review of Dermatology,4 a frequently used resource among dermatology residents. It currently is my favorite deck because it features holistic information on diagnosis, epidemiology, pathogenesis, histopathology, and treatment with excellent clinical photographs.
- Disadvantages: For some purposes, this deck may be too lofty. For maximum benefit, it may require user customization including separating cards by tag and other add-ons that allow only 1 card per note, which will separate the information on each card into smaller increments. The mostly free-response format and lengthy slides may make it difficult to practice recall.
AnKingMed Dermki
- Creator: Reddit user AnKingMed10,11
- Date created: April 2023
- Last updated: This deck features a dynamic add-on and collaboration application called AnkiHub, which allows for real-time updates. At the time this article was written, the deck was last updated on June 19, 2023.
- Number of cards: 7889
- Resources covered: Currently 75% of Alikhan and Hocker’s Review of Dermatology4 with supplemental images from DermNet and Eleryan and Friedman’s The Full Spectrum of Dermatology: A Diverse and Inclusive Atlas.12
- Format of cards: Cards are in a fill-in-the-blank format.
- Card tags (allow separation of Anki decks into subcategories): Cards are tagged by chapter number and subsection of Alikhan and Hocker’s Review of Dermatology.4
- Advantages: As the newest contribution to the dermatology Anki card compendium, this deck is up to date, innovative, and dynamic. It features an optional add-on application—AnkiHub—which allows users to keep up with live updates and collaborations. The deck features a fill-in-the-blank format that may be preferred to a free-response format for information recall. It features Alikhan and Hocker’s Review of Dermatology,4 which is a high-yield review of clinical dermatology, dermatopathology, surgical dermatology, pharmacology, and histopathology for dermatology residents.
- Disadvantages: The deck is still currently in a development phase, covering 75% of Alikhan and Hocker’s Review of Dermatology4 with plans to add the remaining 25%. The add-on to access the most up-to-date version of the flashcards requires a paid monthly or annual subscription; however, the creator announced they will release periodic free updates of the deck.
Final Thoughts
As a collaborative platform, new flash card decks are always being added to Anki. This article is not comprehensive of all dermatologic flash card decks available. There are decks better suited for medical students covering topics such as the American Academy of Dermatology Basic Dermatology Curriculum, UWorld United States Medical Licensing Examination dermatology, and dermatology in internal medicine. Furthermore, specific study tools in dermatology may have their own accompanying Anki decks (ie, The Grenz Zone podcast, Dermnemonics). Flash cards can be a valuable study tool to trainees in medicine, and residents are immensely grateful to our peers who make them for our use.
- Lu M, Farhat JH, Beck Dallaghan GL. Enhanced learning and retention of medical knowledge using the mobile flash card application Anki. Med Sci Educ. 2021;31:1975-1981. doi:10.1007/s40670-021-01386-9
- Unknown. Dolphin Dermatology. Reddit website. Accessed July 19, 2023. https://www.reddit.com/r/medicalschoolanki/comments/116jbpc/dolphin_derm/
- vismo_djib. Review of dermatology Anki. Reddit website. Published June 13, 2020. Accessed June 22, 2023. https://www.reddit.com/r/DermApp/comments/h8gz3d/review_of_dermatology_anki/
- Alikhan A, Hocker TLH. Review of Dermatology. Elsevier; 2016.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier Health Sciences; 2017.
- Patterson JW. Weedon’s Skin Pathology Essentials. Elsevier Health Sciences; 2016.
- Elston D, Ferringer T, Ko CJ, et al. Dermatopathology. Elsevier Health Sciences; 2013.
- Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al. Dermoscopy: The Essentials. Elsevier Health Sciences; 2011.
- Robinson JK, Hanke CW, Siegel DM, et al. Surgery of the Skin: Procedural Dermatology. Elsevier Health Sciences; 2014.
- AnKingMed. Dermki: dermatology residency Anki deck. Reddit website. Published April 8, 2023. Accessed June 22, 2023. https://www.reddit.com/r/medicalschoolanki/comments/12fo9ji/dermki_dermatology_residency_anki_deck/
- Dermki deck for Dermatology Residents. Notion website. Accessed July 10, 2023. https://ankingmed.notion.site/Dermki-deck-for-Dermatology-Residents-9e0b8d8abc2a4bf7941903d80e5b01a2
- Eleryan M, Friedman A. The Full Spectrum of Dermatology: A Diverse and Inclusive Atlas. Sanovaworks; 2021.
Similar to medical school, residency is a time to drink out of the proverbial firehose of knowledge. Along with clinical duties, there is a plethora of information ranging from clinical management decisions to boards fodder that dermatology residents are expected to know, leaving residents to adopt study habits from medical school. Flash cards remain a popular study tool in the medical education community. The use of Anki, a web-based and mobile flash card application (app) that features custom and premade flash card decks made and shared by users, has become increasingly popular. In a 2021 study, Lu et al1 found that Anki flash card usage was associated with higher US Medical Licensing Examination scores. Herein, I provide an updated review of the top 3 most comprehensive premade Anki decks for dermatology residents, per my assessment.
COMPREHENSIVE DERMATOLOGY DECKS
Dolphin Dermatology
- Creator: Reddit user, Unknown2
- Date created: December 2020
- Last updated: April 2022
- Number of cards: 13,833
- Resources covered: Photographs of common dermatologic diagnoses from online sources such as VisualDx (https://www.visualdx.com/) and DermNet (https://dermnetnz.org/).
- Format of cards: One image or factoid per card.
- Card tags (allow separation of Anki decks into subcategories): Each general dermatology card is tagged by the diagnosis name. Pediatric dermatology cards are tagged by affected body location.
- Advantages: As you may glean by the sheer number of flash cards, this deck is a comprehensive review of clinical dermatology. Most cards feature clinical vignettes with clinical photographs of a dermatologic condition or histologic slide and ask what the diagnosis may be. It features photographs of pathology on a range of skin tones and many different images of each diagnosis. This is a great deck for residents who need to study clinical photographs of dermatologic diagnoses.
- Disadvantages: This deck does not cover dermatopathology, basic science, treatment options, or pharmacology in depth. Additionally, is difficult to find a link to download this resource.
- At the time of publication of this article, users are unable to download this deck.
vismo_djib’s Review of Dermatology Anki
- Creator: Reddit user vismo_djib3
- Date created: June 2020
- Last updated: February 2022
- Number of cards: 8454
- Resources covered: Alikhan and Hocker’s Review of Dermatology4 is the main resource with supplemental images from VisualDx, Bolognia et al’s Dermatology,5 Patterson’s Weedon’s Skin Pathology Essentials,6 Elston et al’s Dermatopathology,7 Soyer et al’s Dermoscopy: The Essentials,8 and Robinson et al’s Surgery of the Skin: Procedural Dermatology.9
- Format of cards: Cards mostly feature a diagnosis with color-coded categories including epidemiology, pathogenesis, clinical features, histopathology, and treatment.
- Card tags (allow separation of Anki decks into subcategories): Cards are tagged with chapter numbers from Alikhan and Hocker’s Review of Dermatology.4
- Advantages: This impressive comprehensive review of dermatology is a great option for residents studying for the American Board of Dermatology CORE examinations and users looking to solidify the information in Alikhan and Hocker’s Review of Dermatology,4 a frequently used resource among dermatology residents. It currently is my favorite deck because it features holistic information on diagnosis, epidemiology, pathogenesis, histopathology, and treatment with excellent clinical photographs.
- Disadvantages: For some purposes, this deck may be too lofty. For maximum benefit, it may require user customization including separating cards by tag and other add-ons that allow only 1 card per note, which will separate the information on each card into smaller increments. The mostly free-response format and lengthy slides may make it difficult to practice recall.
AnKingMed Dermki
- Creator: Reddit user AnKingMed10,11
- Date created: April 2023
- Last updated: This deck features a dynamic add-on and collaboration application called AnkiHub, which allows for real-time updates. At the time this article was written, the deck was last updated on June 19, 2023.
- Number of cards: 7889
- Resources covered: Currently 75% of Alikhan and Hocker’s Review of Dermatology4 with supplemental images from DermNet and Eleryan and Friedman’s The Full Spectrum of Dermatology: A Diverse and Inclusive Atlas.12
- Format of cards: Cards are in a fill-in-the-blank format.
- Card tags (allow separation of Anki decks into subcategories): Cards are tagged by chapter number and subsection of Alikhan and Hocker’s Review of Dermatology.4
- Advantages: As the newest contribution to the dermatology Anki card compendium, this deck is up to date, innovative, and dynamic. It features an optional add-on application—AnkiHub—which allows users to keep up with live updates and collaborations. The deck features a fill-in-the-blank format that may be preferred to a free-response format for information recall. It features Alikhan and Hocker’s Review of Dermatology,4 which is a high-yield review of clinical dermatology, dermatopathology, surgical dermatology, pharmacology, and histopathology for dermatology residents.
- Disadvantages: The deck is still currently in a development phase, covering 75% of Alikhan and Hocker’s Review of Dermatology4 with plans to add the remaining 25%. The add-on to access the most up-to-date version of the flashcards requires a paid monthly or annual subscription; however, the creator announced they will release periodic free updates of the deck.

Final Thoughts
As a collaborative platform, new flash card decks are always being added to Anki. This article is not comprehensive of all dermatologic flash card decks available. There are decks better suited for medical students covering topics such as the American Academy of Dermatology Basic Dermatology Curriculum, UWorld United States Medical Licensing Examination dermatology, and dermatology in internal medicine. Furthermore, specific study tools in dermatology may have their own accompanying Anki decks (ie, The Grenz Zone podcast, Dermnemonics). Flash cards can be a valuable study tool to trainees in medicine, and residents are immensely grateful to our peers who make them for our use.
Similar to medical school, residency is a time to drink out of the proverbial firehose of knowledge. Along with clinical duties, there is a plethora of information ranging from clinical management decisions to boards fodder that dermatology residents are expected to know, leaving residents to adopt study habits from medical school. Flash cards remain a popular study tool in the medical education community. The use of Anki, a web-based and mobile flash card application (app) that features custom and premade flash card decks made and shared by users, has become increasingly popular. In a 2021 study, Lu et al1 found that Anki flash card usage was associated with higher US Medical Licensing Examination scores. Herein, I provide an updated review of the top 3 most comprehensive premade Anki decks for dermatology residents, per my assessment.
COMPREHENSIVE DERMATOLOGY DECKS
Dolphin Dermatology
- Creator: Reddit user, Unknown2
- Date created: December 2020
- Last updated: April 2022
- Number of cards: 13,833
- Resources covered: Photographs of common dermatologic diagnoses from online sources such as VisualDx (https://www.visualdx.com/) and DermNet (https://dermnetnz.org/).
- Format of cards: One image or factoid per card.
- Card tags (allow separation of Anki decks into subcategories): Each general dermatology card is tagged by the diagnosis name. Pediatric dermatology cards are tagged by affected body location.
- Advantages: As you may glean by the sheer number of flash cards, this deck is a comprehensive review of clinical dermatology. Most cards feature clinical vignettes with clinical photographs of a dermatologic condition or histologic slide and ask what the diagnosis may be. It features photographs of pathology on a range of skin tones and many different images of each diagnosis. This is a great deck for residents who need to study clinical photographs of dermatologic diagnoses.
- Disadvantages: This deck does not cover dermatopathology, basic science, treatment options, or pharmacology in depth. Additionally, is difficult to find a link to download this resource.
- At the time of publication of this article, users are unable to download this deck.
vismo_djib’s Review of Dermatology Anki
- Creator: Reddit user vismo_djib3
- Date created: June 2020
- Last updated: February 2022
- Number of cards: 8454
- Resources covered: Alikhan and Hocker’s Review of Dermatology4 is the main resource with supplemental images from VisualDx, Bolognia et al’s Dermatology,5 Patterson’s Weedon’s Skin Pathology Essentials,6 Elston et al’s Dermatopathology,7 Soyer et al’s Dermoscopy: The Essentials,8 and Robinson et al’s Surgery of the Skin: Procedural Dermatology.9
- Format of cards: Cards mostly feature a diagnosis with color-coded categories including epidemiology, pathogenesis, clinical features, histopathology, and treatment.
- Card tags (allow separation of Anki decks into subcategories): Cards are tagged with chapter numbers from Alikhan and Hocker’s Review of Dermatology.4
- Advantages: This impressive comprehensive review of dermatology is a great option for residents studying for the American Board of Dermatology CORE examinations and users looking to solidify the information in Alikhan and Hocker’s Review of Dermatology,4 a frequently used resource among dermatology residents. It currently is my favorite deck because it features holistic information on diagnosis, epidemiology, pathogenesis, histopathology, and treatment with excellent clinical photographs.
- Disadvantages: For some purposes, this deck may be too lofty. For maximum benefit, it may require user customization including separating cards by tag and other add-ons that allow only 1 card per note, which will separate the information on each card into smaller increments. The mostly free-response format and lengthy slides may make it difficult to practice recall.
AnKingMed Dermki
- Creator: Reddit user AnKingMed10,11
- Date created: April 2023
- Last updated: This deck features a dynamic add-on and collaboration application called AnkiHub, which allows for real-time updates. At the time this article was written, the deck was last updated on June 19, 2023.
- Number of cards: 7889
- Resources covered: Currently 75% of Alikhan and Hocker’s Review of Dermatology4 with supplemental images from DermNet and Eleryan and Friedman’s The Full Spectrum of Dermatology: A Diverse and Inclusive Atlas.12
- Format of cards: Cards are in a fill-in-the-blank format.
- Card tags (allow separation of Anki decks into subcategories): Cards are tagged by chapter number and subsection of Alikhan and Hocker’s Review of Dermatology.4
- Advantages: As the newest contribution to the dermatology Anki card compendium, this deck is up to date, innovative, and dynamic. It features an optional add-on application—AnkiHub—which allows users to keep up with live updates and collaborations. The deck features a fill-in-the-blank format that may be preferred to a free-response format for information recall. It features Alikhan and Hocker’s Review of Dermatology,4 which is a high-yield review of clinical dermatology, dermatopathology, surgical dermatology, pharmacology, and histopathology for dermatology residents.
- Disadvantages: The deck is still currently in a development phase, covering 75% of Alikhan and Hocker’s Review of Dermatology4 with plans to add the remaining 25%. The add-on to access the most up-to-date version of the flashcards requires a paid monthly or annual subscription; however, the creator announced they will release periodic free updates of the deck.

Final Thoughts
As a collaborative platform, new flash card decks are always being added to Anki. This article is not comprehensive of all dermatologic flash card decks available. There are decks better suited for medical students covering topics such as the American Academy of Dermatology Basic Dermatology Curriculum, UWorld United States Medical Licensing Examination dermatology, and dermatology in internal medicine. Furthermore, specific study tools in dermatology may have their own accompanying Anki decks (ie, The Grenz Zone podcast, Dermnemonics). Flash cards can be a valuable study tool to trainees in medicine, and residents are immensely grateful to our peers who make them for our use.
- Lu M, Farhat JH, Beck Dallaghan GL. Enhanced learning and retention of medical knowledge using the mobile flash card application Anki. Med Sci Educ. 2021;31:1975-1981. doi:10.1007/s40670-021-01386-9
- Unknown. Dolphin Dermatology. Reddit website. Accessed July 19, 2023. https://www.reddit.com/r/medicalschoolanki/comments/116jbpc/dolphin_derm/
- vismo_djib. Review of dermatology Anki. Reddit website. Published June 13, 2020. Accessed June 22, 2023. https://www.reddit.com/r/DermApp/comments/h8gz3d/review_of_dermatology_anki/
- Alikhan A, Hocker TLH. Review of Dermatology. Elsevier; 2016.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier Health Sciences; 2017.
- Patterson JW. Weedon’s Skin Pathology Essentials. Elsevier Health Sciences; 2016.
- Elston D, Ferringer T, Ko CJ, et al. Dermatopathology. Elsevier Health Sciences; 2013.
- Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al. Dermoscopy: The Essentials. Elsevier Health Sciences; 2011.
- Robinson JK, Hanke CW, Siegel DM, et al. Surgery of the Skin: Procedural Dermatology. Elsevier Health Sciences; 2014.
- AnKingMed. Dermki: dermatology residency Anki deck. Reddit website. Published April 8, 2023. Accessed June 22, 2023. https://www.reddit.com/r/medicalschoolanki/comments/12fo9ji/dermki_dermatology_residency_anki_deck/
- Dermki deck for Dermatology Residents. Notion website. Accessed July 10, 2023. https://ankingmed.notion.site/Dermki-deck-for-Dermatology-Residents-9e0b8d8abc2a4bf7941903d80e5b01a2
- Eleryan M, Friedman A. The Full Spectrum of Dermatology: A Diverse and Inclusive Atlas. Sanovaworks; 2021.
- Lu M, Farhat JH, Beck Dallaghan GL. Enhanced learning and retention of medical knowledge using the mobile flash card application Anki. Med Sci Educ. 2021;31:1975-1981. doi:10.1007/s40670-021-01386-9
- Unknown. Dolphin Dermatology. Reddit website. Accessed July 19, 2023. https://www.reddit.com/r/medicalschoolanki/comments/116jbpc/dolphin_derm/
- vismo_djib. Review of dermatology Anki. Reddit website. Published June 13, 2020. Accessed June 22, 2023. https://www.reddit.com/r/DermApp/comments/h8gz3d/review_of_dermatology_anki/
- Alikhan A, Hocker TLH. Review of Dermatology. Elsevier; 2016.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier Health Sciences; 2017.
- Patterson JW. Weedon’s Skin Pathology Essentials. Elsevier Health Sciences; 2016.
- Elston D, Ferringer T, Ko CJ, et al. Dermatopathology. Elsevier Health Sciences; 2013.
- Soyer HP, Argenziano G, Hofmann-Wellenhof R, et al. Dermoscopy: The Essentials. Elsevier Health Sciences; 2011.
- Robinson JK, Hanke CW, Siegel DM, et al. Surgery of the Skin: Procedural Dermatology. Elsevier Health Sciences; 2014.
- AnKingMed. Dermki: dermatology residency Anki deck. Reddit website. Published April 8, 2023. Accessed June 22, 2023. https://www.reddit.com/r/medicalschoolanki/comments/12fo9ji/dermki_dermatology_residency_anki_deck/
- Dermki deck for Dermatology Residents. Notion website. Accessed July 10, 2023. https://ankingmed.notion.site/Dermki-deck-for-Dermatology-Residents-9e0b8d8abc2a4bf7941903d80e5b01a2
- Eleryan M, Friedman A. The Full Spectrum of Dermatology: A Diverse and Inclusive Atlas. Sanovaworks; 2021.
Resident Pearl
- Publicly available Anki flashcard decks may aid dermatology residents in mastering the learning objectives required during training.
Pigmenting Purpuric Dermatoses: Striking But Not a Manifestation of COVID-19 Infection
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6
Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1
Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions, one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24
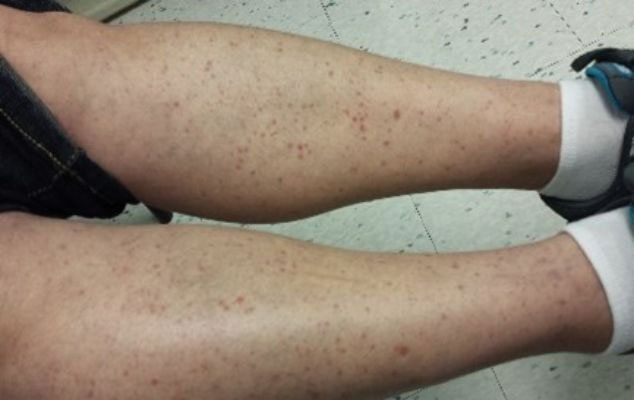
Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.
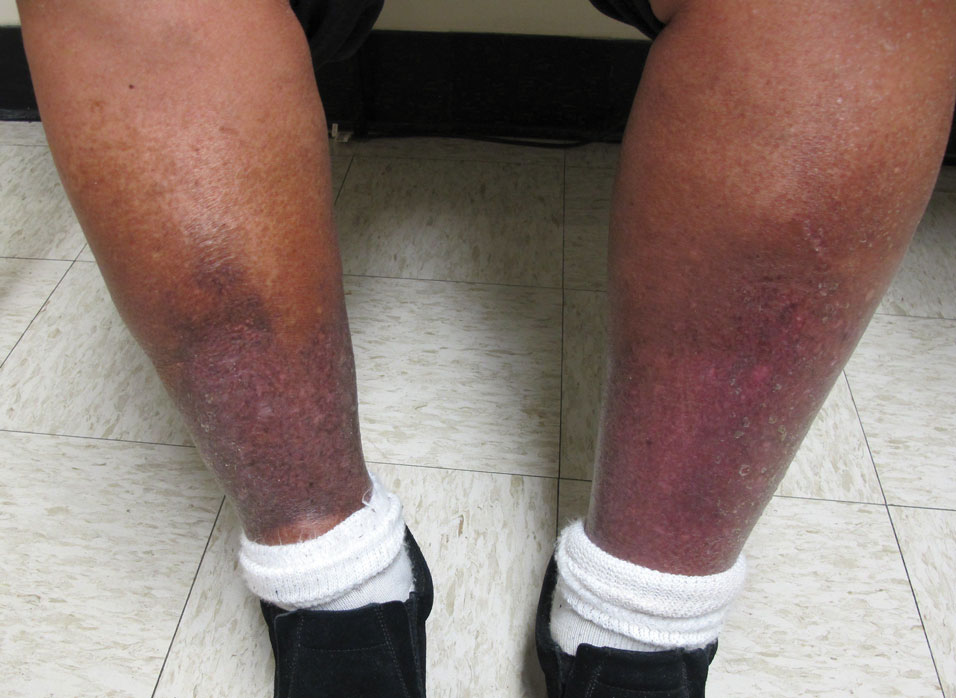
Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions, one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
Pigmented purpuric dermatoses (PPDs) are characterized by petechiae, dusky macules representative of postinflammatory hyperpigmentation and dermal hemosiderin, and purpura generally localized to the lower extremities. They typically represent a spectrum of lymphocytic capillaritis, variable erythrocyte extravasation from papillary dermal blood vessels, and deposition of hemosiderin, yielding the classic red to orange to golden-brown findings on gross examination. Clinical overlap exists, but variants include Schamberg disease (SD), Majocchi purpura, Gougerot-Blum purpura, eczematoid purpura of Doucas and Kapetanakis (DK), and lichen aureus.1 Other forms are rarer, including linear, granulomatous, quadrantic, transitory, and familial variants. It remains controversial whether PPD may precede or have an association with cutaneous T-cell lymphoma.2 Dermoscopy usually shows copper-red pigmentation in the background, oval red dots, linear vessels, brown globules, and follicular openings. Although these findings may be useful in PPD diagnosis, they are not applicable in differentiating among the variants.
Pigmented purpuric dermatoses can easily be mistaken for stasis dermatitis or cellulitis, as these may occur concomitantly or in populations at risk for all 3 conditions, such as women older than 50 years with recent trauma or infection in the affected area. Tissue biopsy and clinical laboratory evaluation may be required to differentiate between PPD from leukocytoclastic vasculitis or the myriad causes of retiform purpura. Importantly, clinicians also should differentiate PPD from the purpuric eruptions of the lower extremities associated with COVID-19 infection.
Pigmented Purpuric Dermatoses
Schamberg Disease—In 1901, Jay Frank Schamberg, a distinguished professor of dermatology in Philadelphia, Pennsylvania, described “a peculiar progressive pigmentary disease of the skin” in a 15-year-old adolescent boy.3 Schamberg disease is the most common PPD, characterized by pruritic spots resembling cayenne pepper (Figure 1) with orange-brown pigmented macules on the legs and feet.4 Although platelet dysfunction, coagulation deficiencies, or dermal atrophy may contribute to hemorrhaging that manifests as petechiae or ecchymoses, SD typically is not associated with any laboratory abnormalities, and petechial eruption is not widespread.5 Capillary fragility can be assessed by the tourniquet test, in which pressure is applied to the forearm with a blood pressure cuff inflated between systolic and diastolic blood pressure for 5 to 10 minutes. Upon removing the cuff, a positive test is indicated by 15 or more petechiae in an area 5 cm in diameter due to poor platelet function. A positive result may be seen in SD.6

Histologically, SD is characterized by patchy parakeratosis, mild spongiosis of the stratum Malpighi, and lymphoid capillaritis (Figure 2).7 In addition to CD3+, CD4+, CD8+, CD1a+, and CD36+ lymphocytes, histology also may contain dendritic cells and cellular adhesion molecules (intercellular adhesion molecule 1, epithelial cell adhesion molecule 1) within the superficial perivascular infiltrate.8 There is no definitive therapy, but first-line interventions include emollients, topical steroids, and oral antihistamines. Nonpharmacologic management includes compression or support stockings, elevation of the lower extremities, and avoidance of offending medications (if identifiable).1

Majocchi Purpura—Domenico Majocchi was a renowned Italian dermatologist who described an entity in 1898 that he called purpura annularis telangiectodes, now also known as Majocchi purpura.9 It is more common in females, young adults, and children. Majocchi purpura has rarely been reported in families with a possible autosomal-dominant inheritance.10 Typically, bluish-red annular macules with central atrophy surrounded by hyperpigmentation may be seen on the lower extremities, potentially extending to the upper extremities.1 Treatment of Majocchi purpura remains a challenge but may respond to narrowband UVB phototherapy. Emollients and topical steroids also are used as first-line treatments. Biopsy demonstrates telangiectasia, pericapillary infiltration of mononuclear lymphocytes, and papillary dermal hemosiderin.11
Gougerot-Blum Purpura—In 1925, French dermatologists Henri Gougerot and Paul Blum described a pigmented purpuric lichenoid dermatitis known as Gougerot-Blum purpura,12 a rare PPD characterized by lichenoid papules that eventually coalesce into plaques of various colors, along with red-brown hyperpigmentation.4 As with other PPD variants, the legs are most involved, with rare extension to the trunk or thighs. The plaques may resemble and be mistaken for Kaposi sarcoma, cutaneous vasculitis, traumatic purpura, or mycosis fungoides. Dermoscopic examination reveals small, polygonal or round, red dots underlying brown scaly patches.13 Gougerot-Blum purpura is found more commonly in adult men and rarely affects children.4 Histologically, a lichenoid and superficial perivascular infiltrate composed of lymphocytes and macrophages is seen. Various therapies have been described, including topical steroids, antihistamines, psoralen plus UVA phototherapy, and cyclosporin A.14
Eczematoid Purpura of Doucas and Kapetanakis—In 1949, Greek dermatologists Christopher Doucas and John Kapetanakis observed several cases of purpuric dermatosis similar in form to the “pigmented purpuric lichenoid dermatitis” of Gougerot-Blum purpura12 and to the “progressive pigmentary dermatitis” of Schamberg disease.3 After observing a gradual disappearance of the classic yellow color from hemosiderin deposition, Doucas and Kapetanakis described a new bright red eruption with lichenification.15 Eczematoid purpura of Doucas and Kapetanakis is rare and predominantly seen in middle-aged males. Hyperpigmented or dark brown macules may develop bilaterally on the legs, progressing to the thighs and upper extremities. Unlike the other types of PPD, DK is extensive and severely pruritic.4
Although most PPD can be drug induced, DK has shown the greatest tendency for pruritic erythematous plaques following drug usage including but not limited to amlodipine, aspirin, acetaminophen, thiamine, interferon alfa, chlordiazepoxide, and isotretinoin. Additionally, DK has been associated with a contact allergy to clothing dyes and rubber.4 On histology, epidermal spongiosis may be seen, correlating with the eczematoid clinical findings. Spontaneous remission also is more common compared to the other PPDs. Treatment consists of topical corticosteroids and antihistamines.16
Lichen Aureus—Lichen aureus was first observed by the dermatologist R.H. Martin in 1958.17 It is clinically characterized by closely aggregated purpuric papules with a distinctive golden-brown color more often localized to the lower extremities and sometimes in a dermatomal distribution. Lichen aureus affects males and females equally, and similar to Majocchi purpura can be seen in children.4 Histopathologic examination reveals a prominent lichenoid plus superficial and deep perivascular lymphocytic infiltrate, extravasated erythrocytes, papillary dermal edema, hemosiderophages, and an unaffected epidermis. In rare cases, perineural infiltrates may be seen. Topical steroids usually are ineffective in lichen aureus treatment, but responses to psoralen plus UVA therapy also have been noted.17
Differential Diagnosis
COVID-19–Related Cutaneous Changes—Because COVID-19–related pathology is now a common differential diagnosis for many cutaneous eruptions, one must be mindful of the possibility for patients to have PPD, cutaneous changes from underlying COVID-19, or both.18 The microvascular changes from COVID-19 infection can be variable.19 Besides the presence of erythema along a distal digit, manifestations can include reticulated dusky erythema mimicking livedoid vasculopathy or inflammatory purpura.19
Retiform Purpura—Retiform purpura may occur in the setting of microvascular occlusion and can represent the pattern of underlying dermal vasculature. It is nonblanching and typically stellate or branching.20 The microvascular occlusion may be a result of hypercoagulability or may be secondary to cutaneous vasculitis, resulting in thrombosis and subsequent vascular occlusion.21 There are many reasons for hypercoagulability in retiform purpura, including disseminated intravascular coagulation in the setting of COVID-19 infection.22 The treatment of retiform purpura is aimed at alleviating the underlying cause and providing symptomatic relief. Conversely, the PPDs generally are benign and require minimal workup.
Leukocytoclastic Vasculitis—The hallmark of leukocytoclastic vasculitis is palpable purpura, often appearing as nonblanchable papules, typically in a dependent distribution such as the lower extremities (Figure 3). Although it primarily affects children, Henoch-Schönlein purpura is a type of leukocytoclastic vasculitis with lesions potentially similar in appearance to those of PPD.23 Palpable purpura may be painful and may ulcerate but rarely is pruritic. Leukocytoclastic vasculitis represents perivascular infiltrates composed of neutrophils, lymphocytes, and occasionally eosinophils, along with karyorrhexis, luminal fibrin, and fibrinoid degeneration of blood vessel walls, often resulting from immune complex deposition. Leukocytoclastic vasculitis may affect blood vessels of any size and requires further clinical and laboratory evaluation for infection (including COVID-19), hypercoagulability, autoimmune disease, or medication-related reactions.24

Stasis Dermatitis—Stasis dermatitis, a chronic inflammatory condition stemming from retrograde venous flow due to incompetent venous valves, mimics PPD. Stasis dermatitis initially appears as demarcated erythematous plaques, fissures, and scaling of the lower legs bilaterally, usually involving the medial malleolus.25 With time, the affected region develops overlying brawny hyperpigmentation and fibrosis (Figure 4). Pruritus or pain are common features, while fissures and superficial erosions may heal and recur, leading to lichenification.

Although both commonly appear on the lower extremities, duplex ultrasonography may be helpful to distinguish PPDs from stasis dermatitis since the latter occurs in the context of chronic venous insufficiency, varicose veins, soft tissue edema, and lymphedema.25 Additionally, pruritus, lichenification, and edema often are not seen in most PPD variants, although stasis dermatitis and PPD may occur in tandem. Conservative treatment involves elevation of the extremities, compression, and topical steroids for symptomatic relief.
Cellulitis—The key characteristics of cellulitis are redness, swelling, warmth, tenderness, fever, and leukocytosis. A history of trauma, such as a prior break in the skin, and pain in the affected area suggest cellulitis. Several skin conditions present similarly to cellulitis, including PPD, and thus approximately 30% of cases are misdiagnosed.26 Cellulitis rarely presents in a bilateral or diffusely scattered pattern as seen in PPDs. Rather, it is unilateral with smooth indistinct borders. Variables suggestive of cellulitis include immunosuppression, rapid progression, and previous occurrences. Hyperpigmented plaques or thickening of the skin are more indicative of a chronic process such as stasis dermatitis or lipodermatosclerosis rather than acute cellulitis. Purpura is not a typical finding in most cases of soft tissue cellulitis. Treatment may be case specific depending on severity, presence or absence of sepsis, findings on blood cultures, or other pathologic evaluation. Antibiotics are directed to the causative organism, typically Streptococcus and Staphylococcus species, although coverage against various gram-negative organisms may be indicated.27
Caution With Teledermatology
COVID-19 has established the value of telemedicine in providing access to health care services for at-risk or underserved individuals. The PPDs are benign, often asymptomatic, and potentially identifiable with teledermatology alone; however, they also can easily be mistaken for COVID-19–related eruptions, vasculitis, other types of purpura, stasis dermatitis, or other complications of lower extremity stasis and lymphedema, especially in an aging population. If tissue biopsy is required, as in the workup of vasculitis, the efficacy of telemedicine becomes more questionable. It is important to delineate the potentially confusing PPDs from other potentially dangerous or life-threatening inflammatory dermatoses.28
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
- Sardana K, Sarkar R , Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Çaytemel C, Baykut B, Ag˘ırgöl S¸, et al. Pigmented purpuric dermatosis: ten years of experience in a tertiary hospital and awareness of mycosis fungoides in differential diagnosis. J Cutan Pathol. 2021;48:611-616.
- Schamberg JF. A peculiar progressive pigmentary disease of the skin. Br J Dermatol. 1901;13:1-5.
- Martínez Pallás I, Conejero Del Mazo R, Lezcano Biosca V. Pigmented purpuric dermatosis: a review of the literature. Actas Dermosifiliogr (Engl Ed). 2020;111:196-204.
- Ozkaya DB, Emiroglu N, Su O, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584-587.
- Lava SAG, Milani GP, Fossali EF, et al. Cutaneous manifestations of small-vessel leukocytoclastic vasculitides in childhood. Clin Rev Allergy Immunol. 2017;53:439-451.
- Bonnet U, Selle C, Isbruch K, et al. Recurrent purpura due to alcohol-related Schamberg’s disease and its association with serum immunoglobulins: a longitudinal observation of a heavy drinker. J Med Case Rep. 2016;10:301.
- Zaldivar Fujigaki JL, Anjum F. Schamberg Disease. StatPearls Publishing; 2021.
- Majocchi J. Purpura annularis telangiectodes. Arch Dermatol Syph. 1898;43:447.
- Sethuraman G, Sugandhan S, Bansal A, et al. Familial pigmented purpuric dermatoses. J Dermatol. 2006;33:639-641.
- Miller K, Fischer M, Kamino H, et al. Purpura annularis telangiectoides. Dermatol Online J. 2012;18:5.
- Coulombe J, Jean SE, Hatami A, et al. Pigmented purpuric dermatosis: clinicopathologic characterization in a pediatric series. Pediatr Dermatol. 2015;32:358-362.
- Park MY, Shim WH, Kim JM, et al. Dermoscopic finding in pigmented purpuric lichenoid dermatosis of Gougerot-Blum: a useful tool for clinical diagnosis. Ann Dermatol. 2018;30:245-247.
- Risikesan J, Sommerlund M, Ramsing M, et al. Successful topical treatment of pigmented purpuric lichenoid dermatitis of Gougerot-Blum in a young patient: a case report and summary of the most common pigmented purpuric dermatoses. Case Rep Dermatol. 2017;9:169-176.
- Doucas C, Kapetanakis J. Eczematid-like purpura. Dermatologica. 1953;106:86-95.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Aung PP, Burns SJ, Bhawan J. Lichen aureus: an unusual histopathological presentation: a case report and a review of literature. Am J Dermatopathol. 2014;36:E1-E4.
- Singh P, Schwartz RA. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol Ther. 2020;33:E14053.
- Almutairi N, Schwartz RA. COVID-19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020;33:E13544.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Torregrosa Calatayud JL, Garcías Ladaria J, De Unamuno Bustos B, et al. Retiform purpura caused by the use of cocaine, that was probably adulterated with levamisole. Ann Dermatol. 2015;27:117-119.
- Keim CK, Schwartz RA, Kapila R. Levamisole-induced and COVID-19-induced retiform purpura: two overlapping, emerging clinical syndromes. Arch Dermatol Res. 2021;22:1-9.
- González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165.
- Yıldırım Bay E, Moustafa E, Semiz Y, et al. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. 2022;21:27-29.
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part I. lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.E1-E12; quiz 75-76.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleveland Clin J Med. 2012;79:547-552.
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816.
Practice Points
- Dermatologists should be aware of the clinical presentations of pigmenting purpuric dermatoses (PPDs).
- Certain PPDs may resemble the thromboembolic events seen in COVID-19. Clinicians should especially be aware of how to differentiate these benign pigmentary disorders from other serious conditions.
- Teledermatology is widely utilized, but caution may be prudent when evaluating erythematous or purpuric dermatoses, especially those of the lower extremities.
- Pigmenting purpuric dermatoses generally are benign and do not require immediate treatment.
Penile Herpes Vegetans in a Patient With Well-controlled HIV
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.
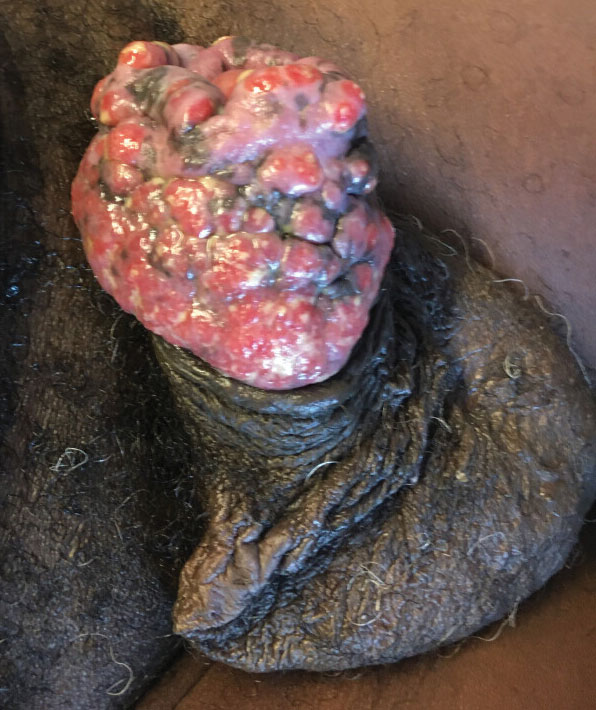
A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.
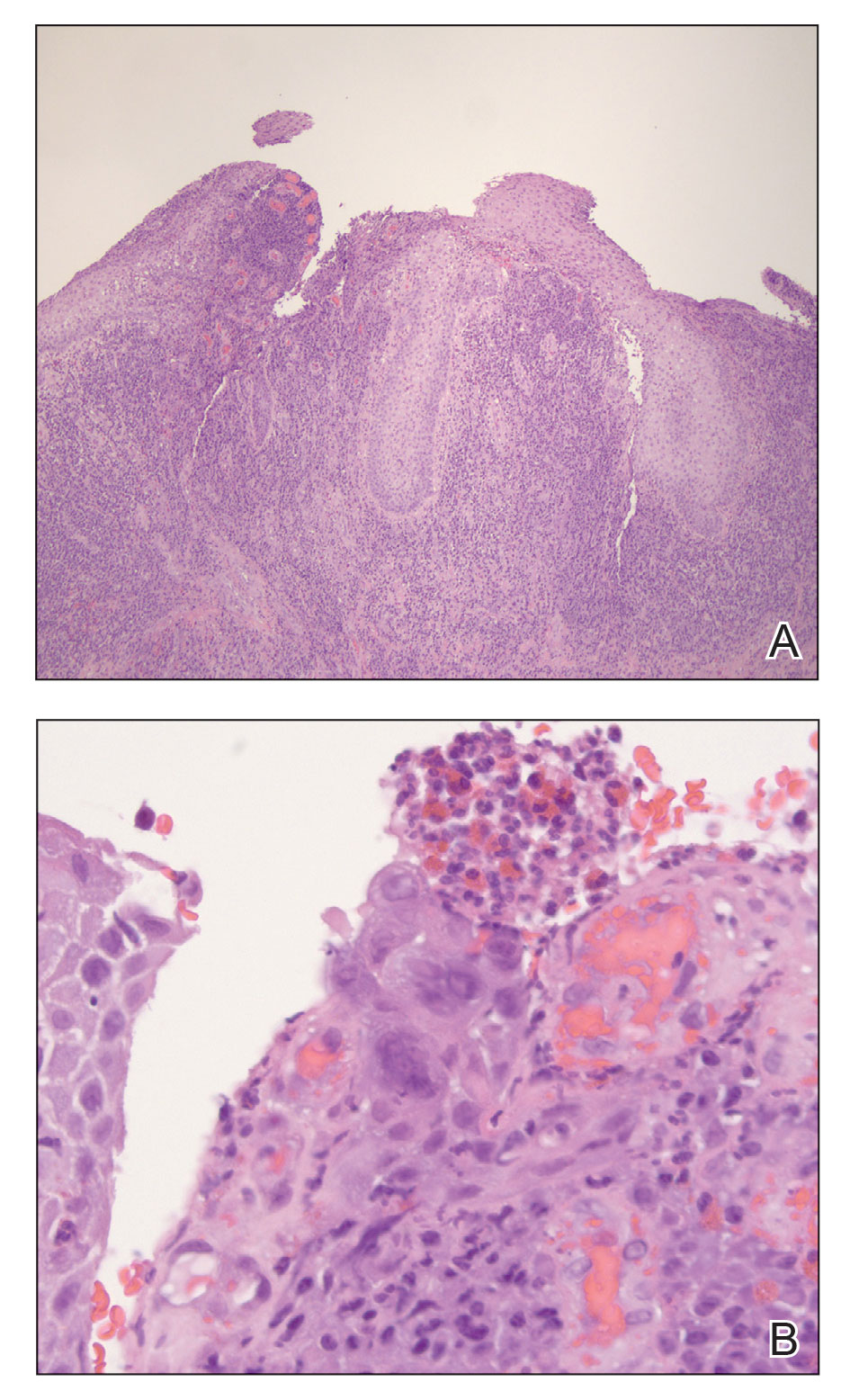
Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.
Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
Practice Points
- Maintain a high clinical suspicion for herpes vegetans (HV) in a patient who has a history of immunosuppression and presents with exophytic genital lesions.
- A history of resistance to acyclovir requires a multimodal approach to treatment of HV lesions, including medical and surgical therapies.
Treatment of an Unresectable Cutaneous Squamous Cell Carcinoma With ED&C and 5-FU
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.
Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.
Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
Practice Points
- In a subset of cases of cutaneous squamous cell carcinoma (cSCC), the tumor is not amenable to surgical resection or other standard treatment modalities.
- Electrodesiccation and curettage followed by topical 5-fluorouracil may be an effective option in eliminating unresectable primary cSCCs that do not respond to intralesional treatment.
U.S. mammogram update sparks concern, reignites debates
, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screening mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
“The study’s central finding that some women who have an abnormal result on a mammogram may not get appropriate follow-up because of cost is worrisome,” said Dr. Fendrick and Ilana B. Richman, MD, MHS, in an accompanying commentary to the JAMA analysis. “On an individual level, high out-of-pocket costs may directly contribute to worse health outcomes or require individuals to use scarce financial resources that may otherwise be used for critical items such as food or rent.”
For patients to fully benefit from early detection, the USPSTF would also need to make clear that follow-up diagnostic mammograms are covered, Dr. Fendrick said.
The ongoing debates
Concerns over the costs of potential follow-up tests are not the only issues experts have highlighted since USPSTF released its updated draft guidance on screening mammography.
The task force’s proposed update has also reignited questions and uncertainties surrounding when to screen, how often, and what types are best.
When it comes to frequency, the major organizations that provide screening guidance don’t see eye to eye. The USPSTF recommends breast cancer screening every other year, while the American College of Radiology recommends screening every year because that approach leads to saves “the most lives.”
At this time, the American College of Obstetricians and Gynecologists guidance currently teeters in the middle, suggesting either annual or biennial screening and highlighting the pros and cons of either approach. According to ACOG, “annual screening intervals appear to result in the least number of breast cancer deaths, particularly in younger women, but at the cost of additional callbacks and biopsies.”
When to begin screening represents another point of contention. While some experts, such as ACOG, agree with the task force’s decision to lower the screening start age to 40, others point to the need for greater nuance on setting the appropriate screening age. The main issue: the task force’s draft sets a uniform age to begin screening, but the risk for breast cancer and breast cancer mortality is not uniform across different racial and ethnic groups.
A recent study published in JAMA Network Open found that, among women aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years) followed by White women (15 deaths per 100,000 person-years). Based on a recommended screening age of 50, the authors suggested that Black women should start screening at age 42, whereas White women could start at 51.
“These findings suggest that health policy makers and clinicians could consider an alternative, race and ethnicity–adapted approach in which Black female patients start screening earlier,” writes Tianhui Chen, PhD, of China’s Zhejiang Cancer Hospital and coauthor of the study.
Weighing in on the guidance, the nonprofit National Center for Health Research urged the task force to consider suggesting different screening schedules based on race and ethnicity data. That would mean the recommendation to start at age 40 should only apply to Black women and other groups with higher-than-average risk for breast cancer at a younger age.
“Women are capable of understanding why the age to start mammography screening may be different for women with different risk factors,” the National Center for Health Research wrote in a comment to USPSTF, provided to this news organization by request. “What is confusing is when some physician groups recommend annual mammograms for all women starting at age 40, even though the data do not support that recommendation.”
While the ACR agreed with the task force’s recommendation to lower the screening age, the organization suggested starting risk assessments based on racial variations in breast cancer incidence and death even earlier. Specifically, the ACR recommended that high-risk groups, such as Black women, get risk assessments by age 25 to determine whether mammography before age 40 is needed.
Screening options for women with dense breasts may be some of the most challenging to weigh. Having dense breasts increases an individual’s risk for breast cancer, and mammography alone is not as effective at identifying breast cancer among these women. However, the evidence on the benefits vs. harms of additional screening beyond mammography remains mixed.
As a result, the task force decided to maintain its “I” grade on additional screening beyond mammography for these women – a grade that indicates insufficient evidence to determine the benefits and harms for a service.
The task force largely based its decision on the findings of two key reports. One report from the Cancer Intervention and Surveillance Modeling Network, which modeled potential outcomes of different screening strategies, indicated that extra screening might reduce breast cancer mortality in those with dense breasts, but at a cost of more false-positive reports.
The second report, a review from the Kaiser Permanente Evidence-based Practice Center, reaffirmed the benefits of routine mammography for reducing deaths from breast cancer, but found no solid evidence that different strategies – including supplemental screening in women with denser breasts – lowered breast cancer mortality or the risk of progression to advanced cancer. Further studies may show which approaches work best to reduce breast cancer deaths, the report said.
In this instance, ACOG agreed with USPSTF: “Based on the lack of data, ACOG does not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
Women with dense breasts should still be encouraged to receive regular screening mammography, even if the results they get may not be as accurate as those for women with less dense breasts, said Diana L. Miglioretti, PhD, of the University of California, Davis, who worked on a report for the USPSTF guidelines.
What’s next?
Despite ongoing debate and uncertainties surrounding some breast screening guidance, support for ending copay requirements for follow-up tests after a positive mammogram finding is widespread.
According to Dr. Fendrick, the USPSTF should expand coverage of follow-up testing after a positive mammogram to ensure people receive routine screening and any necessary diagnostic tests, as it did with colon cancer.
Before 2021, patients could face high costs for a colonoscopy following a positive stool-based Cologuard test. But in 2021, the USPSTF said that positive results on stool-based tests would require follow-up with colonoscopy, defining this follow-up as part of the screening benefit. In 2022, Medicare followed by setting a policy that ended the copay for these follow-up colonoscopies.
For breast screening, there are efforts underway in Congress to end copays for breast screening. In May, Rep. Rosa DeLauro (D-Conn.) introduced a bill, the Find It Early Act, that would require both private and government insurers to cover the out-of-pocket costs for many women receiving screening with ultrasound and MRI.
When the USPSTF finalizes its breast screening guidelines, the recommendations will be woven into discussions between primary care physicians and patients about breast cancer screening.
As guidelines and evidence evolve, “we’re learning to adjust” and communicate these changes to patients, said Tochi Iroku-Malize, MD, president of the American Academy of Family Physicians.
However, gaps in the guidance will leave some open-ended questions about optimal screening practices and how much screening may cost.
Given that, Dr. Iroku-Malize takes many factors into account when discussing screening options with her patients. Based on the new information and the patient’s information, she said she will tell her patients, “We’re going to adjust our guidance as to what you need.”
A version of this article first appeared on Medscape.com.
, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screening mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
“The study’s central finding that some women who have an abnormal result on a mammogram may not get appropriate follow-up because of cost is worrisome,” said Dr. Fendrick and Ilana B. Richman, MD, MHS, in an accompanying commentary to the JAMA analysis. “On an individual level, high out-of-pocket costs may directly contribute to worse health outcomes or require individuals to use scarce financial resources that may otherwise be used for critical items such as food or rent.”
For patients to fully benefit from early detection, the USPSTF would also need to make clear that follow-up diagnostic mammograms are covered, Dr. Fendrick said.
The ongoing debates
Concerns over the costs of potential follow-up tests are not the only issues experts have highlighted since USPSTF released its updated draft guidance on screening mammography.
The task force’s proposed update has also reignited questions and uncertainties surrounding when to screen, how often, and what types are best.
When it comes to frequency, the major organizations that provide screening guidance don’t see eye to eye. The USPSTF recommends breast cancer screening every other year, while the American College of Radiology recommends screening every year because that approach leads to saves “the most lives.”
At this time, the American College of Obstetricians and Gynecologists guidance currently teeters in the middle, suggesting either annual or biennial screening and highlighting the pros and cons of either approach. According to ACOG, “annual screening intervals appear to result in the least number of breast cancer deaths, particularly in younger women, but at the cost of additional callbacks and biopsies.”
When to begin screening represents another point of contention. While some experts, such as ACOG, agree with the task force’s decision to lower the screening start age to 40, others point to the need for greater nuance on setting the appropriate screening age. The main issue: the task force’s draft sets a uniform age to begin screening, but the risk for breast cancer and breast cancer mortality is not uniform across different racial and ethnic groups.
A recent study published in JAMA Network Open found that, among women aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years) followed by White women (15 deaths per 100,000 person-years). Based on a recommended screening age of 50, the authors suggested that Black women should start screening at age 42, whereas White women could start at 51.
“These findings suggest that health policy makers and clinicians could consider an alternative, race and ethnicity–adapted approach in which Black female patients start screening earlier,” writes Tianhui Chen, PhD, of China’s Zhejiang Cancer Hospital and coauthor of the study.
Weighing in on the guidance, the nonprofit National Center for Health Research urged the task force to consider suggesting different screening schedules based on race and ethnicity data. That would mean the recommendation to start at age 40 should only apply to Black women and other groups with higher-than-average risk for breast cancer at a younger age.
“Women are capable of understanding why the age to start mammography screening may be different for women with different risk factors,” the National Center for Health Research wrote in a comment to USPSTF, provided to this news organization by request. “What is confusing is when some physician groups recommend annual mammograms for all women starting at age 40, even though the data do not support that recommendation.”
While the ACR agreed with the task force’s recommendation to lower the screening age, the organization suggested starting risk assessments based on racial variations in breast cancer incidence and death even earlier. Specifically, the ACR recommended that high-risk groups, such as Black women, get risk assessments by age 25 to determine whether mammography before age 40 is needed.
Screening options for women with dense breasts may be some of the most challenging to weigh. Having dense breasts increases an individual’s risk for breast cancer, and mammography alone is not as effective at identifying breast cancer among these women. However, the evidence on the benefits vs. harms of additional screening beyond mammography remains mixed.
As a result, the task force decided to maintain its “I” grade on additional screening beyond mammography for these women – a grade that indicates insufficient evidence to determine the benefits and harms for a service.
The task force largely based its decision on the findings of two key reports. One report from the Cancer Intervention and Surveillance Modeling Network, which modeled potential outcomes of different screening strategies, indicated that extra screening might reduce breast cancer mortality in those with dense breasts, but at a cost of more false-positive reports.
The second report, a review from the Kaiser Permanente Evidence-based Practice Center, reaffirmed the benefits of routine mammography for reducing deaths from breast cancer, but found no solid evidence that different strategies – including supplemental screening in women with denser breasts – lowered breast cancer mortality or the risk of progression to advanced cancer. Further studies may show which approaches work best to reduce breast cancer deaths, the report said.
In this instance, ACOG agreed with USPSTF: “Based on the lack of data, ACOG does not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
Women with dense breasts should still be encouraged to receive regular screening mammography, even if the results they get may not be as accurate as those for women with less dense breasts, said Diana L. Miglioretti, PhD, of the University of California, Davis, who worked on a report for the USPSTF guidelines.
What’s next?
Despite ongoing debate and uncertainties surrounding some breast screening guidance, support for ending copay requirements for follow-up tests after a positive mammogram finding is widespread.
According to Dr. Fendrick, the USPSTF should expand coverage of follow-up testing after a positive mammogram to ensure people receive routine screening and any necessary diagnostic tests, as it did with colon cancer.
Before 2021, patients could face high costs for a colonoscopy following a positive stool-based Cologuard test. But in 2021, the USPSTF said that positive results on stool-based tests would require follow-up with colonoscopy, defining this follow-up as part of the screening benefit. In 2022, Medicare followed by setting a policy that ended the copay for these follow-up colonoscopies.
For breast screening, there are efforts underway in Congress to end copays for breast screening. In May, Rep. Rosa DeLauro (D-Conn.) introduced a bill, the Find It Early Act, that would require both private and government insurers to cover the out-of-pocket costs for many women receiving screening with ultrasound and MRI.
When the USPSTF finalizes its breast screening guidelines, the recommendations will be woven into discussions between primary care physicians and patients about breast cancer screening.
As guidelines and evidence evolve, “we’re learning to adjust” and communicate these changes to patients, said Tochi Iroku-Malize, MD, president of the American Academy of Family Physicians.
However, gaps in the guidance will leave some open-ended questions about optimal screening practices and how much screening may cost.
Given that, Dr. Iroku-Malize takes many factors into account when discussing screening options with her patients. Based on the new information and the patient’s information, she said she will tell her patients, “We’re going to adjust our guidance as to what you need.”
A version of this article first appeared on Medscape.com.
, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screening mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
“The study’s central finding that some women who have an abnormal result on a mammogram may not get appropriate follow-up because of cost is worrisome,” said Dr. Fendrick and Ilana B. Richman, MD, MHS, in an accompanying commentary to the JAMA analysis. “On an individual level, high out-of-pocket costs may directly contribute to worse health outcomes or require individuals to use scarce financial resources that may otherwise be used for critical items such as food or rent.”
For patients to fully benefit from early detection, the USPSTF would also need to make clear that follow-up diagnostic mammograms are covered, Dr. Fendrick said.
The ongoing debates
Concerns over the costs of potential follow-up tests are not the only issues experts have highlighted since USPSTF released its updated draft guidance on screening mammography.
The task force’s proposed update has also reignited questions and uncertainties surrounding when to screen, how often, and what types are best.
When it comes to frequency, the major organizations that provide screening guidance don’t see eye to eye. The USPSTF recommends breast cancer screening every other year, while the American College of Radiology recommends screening every year because that approach leads to saves “the most lives.”
At this time, the American College of Obstetricians and Gynecologists guidance currently teeters in the middle, suggesting either annual or biennial screening and highlighting the pros and cons of either approach. According to ACOG, “annual screening intervals appear to result in the least number of breast cancer deaths, particularly in younger women, but at the cost of additional callbacks and biopsies.”
When to begin screening represents another point of contention. While some experts, such as ACOG, agree with the task force’s decision to lower the screening start age to 40, others point to the need for greater nuance on setting the appropriate screening age. The main issue: the task force’s draft sets a uniform age to begin screening, but the risk for breast cancer and breast cancer mortality is not uniform across different racial and ethnic groups.
A recent study published in JAMA Network Open found that, among women aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years) followed by White women (15 deaths per 100,000 person-years). Based on a recommended screening age of 50, the authors suggested that Black women should start screening at age 42, whereas White women could start at 51.
“These findings suggest that health policy makers and clinicians could consider an alternative, race and ethnicity–adapted approach in which Black female patients start screening earlier,” writes Tianhui Chen, PhD, of China’s Zhejiang Cancer Hospital and coauthor of the study.
Weighing in on the guidance, the nonprofit National Center for Health Research urged the task force to consider suggesting different screening schedules based on race and ethnicity data. That would mean the recommendation to start at age 40 should only apply to Black women and other groups with higher-than-average risk for breast cancer at a younger age.
“Women are capable of understanding why the age to start mammography screening may be different for women with different risk factors,” the National Center for Health Research wrote in a comment to USPSTF, provided to this news organization by request. “What is confusing is when some physician groups recommend annual mammograms for all women starting at age 40, even though the data do not support that recommendation.”
While the ACR agreed with the task force’s recommendation to lower the screening age, the organization suggested starting risk assessments based on racial variations in breast cancer incidence and death even earlier. Specifically, the ACR recommended that high-risk groups, such as Black women, get risk assessments by age 25 to determine whether mammography before age 40 is needed.
Screening options for women with dense breasts may be some of the most challenging to weigh. Having dense breasts increases an individual’s risk for breast cancer, and mammography alone is not as effective at identifying breast cancer among these women. However, the evidence on the benefits vs. harms of additional screening beyond mammography remains mixed.
As a result, the task force decided to maintain its “I” grade on additional screening beyond mammography for these women – a grade that indicates insufficient evidence to determine the benefits and harms for a service.
The task force largely based its decision on the findings of two key reports. One report from the Cancer Intervention and Surveillance Modeling Network, which modeled potential outcomes of different screening strategies, indicated that extra screening might reduce breast cancer mortality in those with dense breasts, but at a cost of more false-positive reports.
The second report, a review from the Kaiser Permanente Evidence-based Practice Center, reaffirmed the benefits of routine mammography for reducing deaths from breast cancer, but found no solid evidence that different strategies – including supplemental screening in women with denser breasts – lowered breast cancer mortality or the risk of progression to advanced cancer. Further studies may show which approaches work best to reduce breast cancer deaths, the report said.
In this instance, ACOG agreed with USPSTF: “Based on the lack of data, ACOG does not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
Women with dense breasts should still be encouraged to receive regular screening mammography, even if the results they get may not be as accurate as those for women with less dense breasts, said Diana L. Miglioretti, PhD, of the University of California, Davis, who worked on a report for the USPSTF guidelines.
What’s next?
Despite ongoing debate and uncertainties surrounding some breast screening guidance, support for ending copay requirements for follow-up tests after a positive mammogram finding is widespread.
According to Dr. Fendrick, the USPSTF should expand coverage of follow-up testing after a positive mammogram to ensure people receive routine screening and any necessary diagnostic tests, as it did with colon cancer.
Before 2021, patients could face high costs for a colonoscopy following a positive stool-based Cologuard test. But in 2021, the USPSTF said that positive results on stool-based tests would require follow-up with colonoscopy, defining this follow-up as part of the screening benefit. In 2022, Medicare followed by setting a policy that ended the copay for these follow-up colonoscopies.
For breast screening, there are efforts underway in Congress to end copays for breast screening. In May, Rep. Rosa DeLauro (D-Conn.) introduced a bill, the Find It Early Act, that would require both private and government insurers to cover the out-of-pocket costs for many women receiving screening with ultrasound and MRI.
When the USPSTF finalizes its breast screening guidelines, the recommendations will be woven into discussions between primary care physicians and patients about breast cancer screening.
As guidelines and evidence evolve, “we’re learning to adjust” and communicate these changes to patients, said Tochi Iroku-Malize, MD, president of the American Academy of Family Physicians.
However, gaps in the guidance will leave some open-ended questions about optimal screening practices and how much screening may cost.
Given that, Dr. Iroku-Malize takes many factors into account when discussing screening options with her patients. Based on the new information and the patient’s information, she said she will tell her patients, “We’re going to adjust our guidance as to what you need.”
A version of this article first appeared on Medscape.com.
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
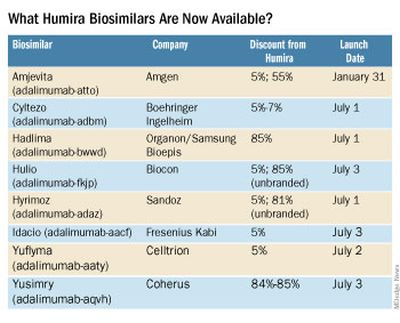
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
Verbal working memory deterioration predicts relapse in remitted psychosis
Previous research has suggested that cognitive impairments may predict recurrent psychotic episodes, but data on the association between specific cognitive deficits and relapse of psychosis over time are limited, wrote Tiffany J. Tao, MPhil, a PhD candidate at the University of Hong Kong, and colleagues.
In a naturalistic 1-year follow-up study published in Psychiatry Research , the researchers recruited psychosis patients with full remission for a least 6 months from two outpatient psychiatric clinics. The study population included adults aged 18-55 years, with an average age of 29.2 years; 62% were women. Relapse, defined as a recurrence of psychotic symptoms measured by the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression Scale, was assessed monthly via phone interviews with the use of a smartphone app. Cognitive decline was based on working memory deterioration, assessed monthly via the Visual Patterns Test (VPT) and the Letter-Number Sequencing (LNS) test, respectively, for visual and verbal working memory.
Overall, 18 patients (16%) experienced a relapse at 1 year. One-third of these (six patients) required hospitalization, with a median hospital stay of 23 days.
In a multivariate analysis, independent and significant predictors of relapse were verbal working memory deterioration 2 months prior to relapse (P = .029), worse medication adherence (P = .018), and less resilience (P = .014) with odds ratios of 9.445, 0.051, and 0.769, respectively.
“Specifically, declines in verbal working memory were observed beginning at 2 months prior to the relapse episode in both the univariate and multivariate models after controlling for other significant predictors,” the researchers wrote in their discussion.
The mechanism of action for the association remains unclear, but cognitive impairment might reflect dopamine dysregulation or other processes in the prefrontal cortex that could contribute to psychotic relapse, they said.
Other factors include the associations between cognitive impairment and medication nonadherence, and the impact of cognitive impairment on a patient’s ability to manage the stresses of daily living that could trigger a psychotic relapse, they added.
Notably, the current study identified verbal working memory, but not visual working memory, as a predictor of relapse, which is important given the different neurobiological bases for visual and verbal tasks, the researchers wrote.
The study findings were limited by several factors including the inability to identify weaker predictors of relapse given the low relapse rate, and potential lack of generalizability to other less homogeneous populations, and the exclusion of patients with illicit drug use, the researchers noted.
However, the results were strengthened by the prospective measurements that prevented recall bias, and the inclusion of other objective predictors of relapse. The findings highlight the potential for early intervention to prevent relapse based on cognitive assessment, which can be measured objectively in the clinical setting or remotely from home using digital technology, they concluded.
The study received no outside funding. Ms. Tao had no financial conflicts to disclose.
Previous research has suggested that cognitive impairments may predict recurrent psychotic episodes, but data on the association between specific cognitive deficits and relapse of psychosis over time are limited, wrote Tiffany J. Tao, MPhil, a PhD candidate at the University of Hong Kong, and colleagues.
In a naturalistic 1-year follow-up study published in Psychiatry Research , the researchers recruited psychosis patients with full remission for a least 6 months from two outpatient psychiatric clinics. The study population included adults aged 18-55 years, with an average age of 29.2 years; 62% were women. Relapse, defined as a recurrence of psychotic symptoms measured by the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression Scale, was assessed monthly via phone interviews with the use of a smartphone app. Cognitive decline was based on working memory deterioration, assessed monthly via the Visual Patterns Test (VPT) and the Letter-Number Sequencing (LNS) test, respectively, for visual and verbal working memory.
Overall, 18 patients (16%) experienced a relapse at 1 year. One-third of these (six patients) required hospitalization, with a median hospital stay of 23 days.
In a multivariate analysis, independent and significant predictors of relapse were verbal working memory deterioration 2 months prior to relapse (P = .029), worse medication adherence (P = .018), and less resilience (P = .014) with odds ratios of 9.445, 0.051, and 0.769, respectively.
“Specifically, declines in verbal working memory were observed beginning at 2 months prior to the relapse episode in both the univariate and multivariate models after controlling for other significant predictors,” the researchers wrote in their discussion.
The mechanism of action for the association remains unclear, but cognitive impairment might reflect dopamine dysregulation or other processes in the prefrontal cortex that could contribute to psychotic relapse, they said.
Other factors include the associations between cognitive impairment and medication nonadherence, and the impact of cognitive impairment on a patient’s ability to manage the stresses of daily living that could trigger a psychotic relapse, they added.
Notably, the current study identified verbal working memory, but not visual working memory, as a predictor of relapse, which is important given the different neurobiological bases for visual and verbal tasks, the researchers wrote.
The study findings were limited by several factors including the inability to identify weaker predictors of relapse given the low relapse rate, and potential lack of generalizability to other less homogeneous populations, and the exclusion of patients with illicit drug use, the researchers noted.
However, the results were strengthened by the prospective measurements that prevented recall bias, and the inclusion of other objective predictors of relapse. The findings highlight the potential for early intervention to prevent relapse based on cognitive assessment, which can be measured objectively in the clinical setting or remotely from home using digital technology, they concluded.
The study received no outside funding. Ms. Tao had no financial conflicts to disclose.
Previous research has suggested that cognitive impairments may predict recurrent psychotic episodes, but data on the association between specific cognitive deficits and relapse of psychosis over time are limited, wrote Tiffany J. Tao, MPhil, a PhD candidate at the University of Hong Kong, and colleagues.
In a naturalistic 1-year follow-up study published in Psychiatry Research , the researchers recruited psychosis patients with full remission for a least 6 months from two outpatient psychiatric clinics. The study population included adults aged 18-55 years, with an average age of 29.2 years; 62% were women. Relapse, defined as a recurrence of psychotic symptoms measured by the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression Scale, was assessed monthly via phone interviews with the use of a smartphone app. Cognitive decline was based on working memory deterioration, assessed monthly via the Visual Patterns Test (VPT) and the Letter-Number Sequencing (LNS) test, respectively, for visual and verbal working memory.
Overall, 18 patients (16%) experienced a relapse at 1 year. One-third of these (six patients) required hospitalization, with a median hospital stay of 23 days.
In a multivariate analysis, independent and significant predictors of relapse were verbal working memory deterioration 2 months prior to relapse (P = .029), worse medication adherence (P = .018), and less resilience (P = .014) with odds ratios of 9.445, 0.051, and 0.769, respectively.
“Specifically, declines in verbal working memory were observed beginning at 2 months prior to the relapse episode in both the univariate and multivariate models after controlling for other significant predictors,” the researchers wrote in their discussion.
The mechanism of action for the association remains unclear, but cognitive impairment might reflect dopamine dysregulation or other processes in the prefrontal cortex that could contribute to psychotic relapse, they said.
Other factors include the associations between cognitive impairment and medication nonadherence, and the impact of cognitive impairment on a patient’s ability to manage the stresses of daily living that could trigger a psychotic relapse, they added.
Notably, the current study identified verbal working memory, but not visual working memory, as a predictor of relapse, which is important given the different neurobiological bases for visual and verbal tasks, the researchers wrote.
The study findings were limited by several factors including the inability to identify weaker predictors of relapse given the low relapse rate, and potential lack of generalizability to other less homogeneous populations, and the exclusion of patients with illicit drug use, the researchers noted.
However, the results were strengthened by the prospective measurements that prevented recall bias, and the inclusion of other objective predictors of relapse. The findings highlight the potential for early intervention to prevent relapse based on cognitive assessment, which can be measured objectively in the clinical setting or remotely from home using digital technology, they concluded.
The study received no outside funding. Ms. Tao had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH