User login
Asthma severity, exacerbations increase with RV infection
TOPLINE:
Immunological and quantitative mRNA assays support a pathogenesis role for histamine-releasing factor (HRF), its interaction with HRF-reactive immunoglobulin E and rhinovirus (RV) in asthma severity and exacerbation.
METHODOLOGY:
- Clinical data for healthy controls (HCs) were compared with data from patients with asthma for three distinct cohorts recruited from programs located in Pittsburg, Boston, and Virginia.
- Cohorts differed primarily by total number of participants, median age, description of asthma severity, RV status, and longitudinal follow-up.
- Enzyme-linked immunoassay tests quantified for comparisons total IgE, IgGs, and IgG1 levels occurring in human sera samples and for HRF-reactive IgE, IgG1, and IgG2b in sera from mice inoculated with mouse .
- Anti-IgE stimulation experiments characterized bronchoalveolar lavage (BAL) cell supernatants for tryptase and PGD2 by ELISA and the mRNAs for tryptase and FCER1A
- Effect of inoculated RV infections and/or house dust mite allergen on stimulating HRF secretion from respiratory epithelial cells and in vitro–grown lung BEAS-2B cells was evaluated by Western blots.
TAKEAWAY:
- HRF-reactive IgGs and IgG1 levels in serum were lower in people with asthma than in HCs.
- People with asthma with high HRF-reactive IgE, compared with those with low levels, tended to release more tryptase prostaglandin D2 with anti-IgE stimulation of BAL cells.
- RV infection induced HFR secretions from both in vivo– and in vitro–grown respiratory epithelial cells and was associated with higher levels of HRF-IgE at the time of asthma exacerbations, compared with after resolution.
IN PRACTICE:
Inhibiting HRF and HRF-reactive IgE interactions “can be a preventative/therapeutic target” for severe and RV-induced exacerbated asthma conditions.
SOURCE:
The study led by Yu Kawakami, MD, of La Jolla Institute for Allergy & Immunology, California, and colleagues was published in the Journal of Allergy and Clinical Immunology
LIMITATIONS:
Small sample sizes, large median age differences between cohorts, and lack of data for other demographic traits and variant asthma phenotypes or endotypes in some cohorts are noted limitations that may affect result extrapolations and conclusions.
DISCLOSURES:
The authors report there are no conflicts of interest directly related to this study.
A version of this article first appeared on Medscape.com.
TOPLINE:
Immunological and quantitative mRNA assays support a pathogenesis role for histamine-releasing factor (HRF), its interaction with HRF-reactive immunoglobulin E and rhinovirus (RV) in asthma severity and exacerbation.
METHODOLOGY:
- Clinical data for healthy controls (HCs) were compared with data from patients with asthma for three distinct cohorts recruited from programs located in Pittsburg, Boston, and Virginia.
- Cohorts differed primarily by total number of participants, median age, description of asthma severity, RV status, and longitudinal follow-up.
- Enzyme-linked immunoassay tests quantified for comparisons total IgE, IgGs, and IgG1 levels occurring in human sera samples and for HRF-reactive IgE, IgG1, and IgG2b in sera from mice inoculated with mouse .
- Anti-IgE stimulation experiments characterized bronchoalveolar lavage (BAL) cell supernatants for tryptase and PGD2 by ELISA and the mRNAs for tryptase and FCER1A
- Effect of inoculated RV infections and/or house dust mite allergen on stimulating HRF secretion from respiratory epithelial cells and in vitro–grown lung BEAS-2B cells was evaluated by Western blots.
TAKEAWAY:
- HRF-reactive IgGs and IgG1 levels in serum were lower in people with asthma than in HCs.
- People with asthma with high HRF-reactive IgE, compared with those with low levels, tended to release more tryptase prostaglandin D2 with anti-IgE stimulation of BAL cells.
- RV infection induced HFR secretions from both in vivo– and in vitro–grown respiratory epithelial cells and was associated with higher levels of HRF-IgE at the time of asthma exacerbations, compared with after resolution.
IN PRACTICE:
Inhibiting HRF and HRF-reactive IgE interactions “can be a preventative/therapeutic target” for severe and RV-induced exacerbated asthma conditions.
SOURCE:
The study led by Yu Kawakami, MD, of La Jolla Institute for Allergy & Immunology, California, and colleagues was published in the Journal of Allergy and Clinical Immunology
LIMITATIONS:
Small sample sizes, large median age differences between cohorts, and lack of data for other demographic traits and variant asthma phenotypes or endotypes in some cohorts are noted limitations that may affect result extrapolations and conclusions.
DISCLOSURES:
The authors report there are no conflicts of interest directly related to this study.
A version of this article first appeared on Medscape.com.
TOPLINE:
Immunological and quantitative mRNA assays support a pathogenesis role for histamine-releasing factor (HRF), its interaction with HRF-reactive immunoglobulin E and rhinovirus (RV) in asthma severity and exacerbation.
METHODOLOGY:
- Clinical data for healthy controls (HCs) were compared with data from patients with asthma for three distinct cohorts recruited from programs located in Pittsburg, Boston, and Virginia.
- Cohorts differed primarily by total number of participants, median age, description of asthma severity, RV status, and longitudinal follow-up.
- Enzyme-linked immunoassay tests quantified for comparisons total IgE, IgGs, and IgG1 levels occurring in human sera samples and for HRF-reactive IgE, IgG1, and IgG2b in sera from mice inoculated with mouse .
- Anti-IgE stimulation experiments characterized bronchoalveolar lavage (BAL) cell supernatants for tryptase and PGD2 by ELISA and the mRNAs for tryptase and FCER1A
- Effect of inoculated RV infections and/or house dust mite allergen on stimulating HRF secretion from respiratory epithelial cells and in vitro–grown lung BEAS-2B cells was evaluated by Western blots.
TAKEAWAY:
- HRF-reactive IgGs and IgG1 levels in serum were lower in people with asthma than in HCs.
- People with asthma with high HRF-reactive IgE, compared with those with low levels, tended to release more tryptase prostaglandin D2 with anti-IgE stimulation of BAL cells.
- RV infection induced HFR secretions from both in vivo– and in vitro–grown respiratory epithelial cells and was associated with higher levels of HRF-IgE at the time of asthma exacerbations, compared with after resolution.
IN PRACTICE:
Inhibiting HRF and HRF-reactive IgE interactions “can be a preventative/therapeutic target” for severe and RV-induced exacerbated asthma conditions.
SOURCE:
The study led by Yu Kawakami, MD, of La Jolla Institute for Allergy & Immunology, California, and colleagues was published in the Journal of Allergy and Clinical Immunology
LIMITATIONS:
Small sample sizes, large median age differences between cohorts, and lack of data for other demographic traits and variant asthma phenotypes or endotypes in some cohorts are noted limitations that may affect result extrapolations and conclusions.
DISCLOSURES:
The authors report there are no conflicts of interest directly related to this study.
A version of this article first appeared on Medscape.com.
Rising patient costs tied to private equity ownership
The report was a collaboration of University of California, Berkeley, staff and researchers from two nonprofits, the American Antitrust Institute and the Washington Center for Equitable Growth. It provides “convincing evidence that incentives to put profits before patients have grown stronger with an increase in private equity ownership of physician practices,” lead author Richard Scheffler, PhD, of UC Berkeley said in a statement.
The report also noted that private equity acquisitions of physician groups have risen sixfold in just a decade, increasing from 75 deals in 2012 to 484 deals in 2021.
Separately, the American Medical Association earlier released a separate report on trends in physician practice arrangements, finding that the percentage of physicians working in private equity–owned groups was 4.5% in 2022, the same as in its previous 2020 report. The share of physicians working in private practices fell by 13 percentage points from 60.1% to 46.7% between 2012 and 2022, the AMA reported.
The Berkeley report and the AMA update come amid rising concerns about the effects of the decline of independent physician practices. The U.S. Senate Finance Committee, which oversees most federal health spending, held a June hearing examining the causes and consequences of increased corporate ownership in health care, including a look at physician practices.
“It’s increasingly clear that consolidation in health care is not lowering costs or increasing the quality of Americans’ health care,” Senate Finance Chairman Ron Wyden (D-Ore.) said in an email. “For private equity in health care in particular, there needs to be more transparency around ownership so the effect on these business relationships can be better understood.”
Federal and state agencies do not generally track acquisitions of physician practices.
The UC Berkeley report impressively documents the rising influence of private equity in health care, for which it’s tough to find good data, said Karen Joynt Maddox, MD, MPH, of Washington University in St. Louis. Dr. Maddox, a cardiologist and policy researcher who also has studied the effects of consolidation in health care, examined the new report at the request of this news organization.
“They did a great job with the data,” Dr. Maddox said. “One of the big issues around private equity, and in general, ‘corporatization’ and consolidation of health care, is that there’s not a great way to track ownership changes. It’s really difficult to study.”
Dr. Scheffler and colleagues used data from the commercial firm PitchBook to identify acquisitions of physician practices by private equity firms. They consulted IQVIA’s physician databases – OneKey and SK&A Office-Based Physicians Database – to learn about the location, size, and specialties of acquired practices. They also used data from the nonprofit Health Care Cost Institute, which tracks commercial health plan claims, to assess how private equity acquisitions affected prices.
The researchers then matched the findings for practices acquired by private equity firms from 2015 to 2021 against those for comparable physician practices that remained independent from 2012 to 2021.
The authors then tied private-equity ownership to the following price increases:
- Gastroenterology (14%; 95% confidence interval, 7.9%-20.4%
- Oncology (16.4%; 95% CI, 5.5%-28.4%)
- Dermatology (4.0%; 95% CI, 1%-7.1%)
- Ob.gyn. (8.8%; 95% CI, 3.8%-14%)
- Ophthalmology (8.7%; 95% CI, 5.1%-12.3%)
- Radiology (8.2%; 95% CI, 0.8%-16.1%)
- Orthopedics (7.1%; 95% CI, 2.2%-12.3%)
- Primary care (4.1%; 95% CI, 1.3%-7%)
The analysis also found higher prices for cardiology (8.7%; 95% CI, –6.4% to 26.1%) and urology (4.2%; 95% CI, –2.3% to 11.1%), but neither of these findings was statistically significant, one of the authors, Daniel R. Arnold, PhD, of UC Berkeley, said in an email. This was most likely caused by smaller sample sizes for these fields.
Factors driving consolidation
The two reports and the Senate Finance consolidation hearing raised similar issues, including calls to look at the factors driving more physicians out of independent practice, including Medicare reimbursement that may not keep up with rising inflation.
The Berkeley report authors called for Congress to add a broad inflation component to the Medicare physician fee schedule. It also called on Congress to add cases where Medicare, the biggest U.S. purchaser of health care, pays less for services when performed in independent practices than in hospital-affiliated ones.
Shawn Martin, executive vice president and CEO of the American Academy of Family Physicians, said his group appreciates how the report from UC Berkeley and nonprofit groups echoed recommendations many clinicians have made, including the call for a broad inflation adjustment for the fee schedule.
“To move the needle forward, Congress must advance site-neutral payment policies while also addressing the administrative requirements that take physicians away from the important work of caring for patients,” Mr. Martin said in an email.
Arnold Ventures provided funding for the report, which was a joint project of the American Antitrust Institute, the Nicholas C. Petris Center on Health Care Markets and Consumer Welfare, UC Berkeley, and the Washington Center for Equitable Growth.
A version of this article appeared on Medscape.com.
The report was a collaboration of University of California, Berkeley, staff and researchers from two nonprofits, the American Antitrust Institute and the Washington Center for Equitable Growth. It provides “convincing evidence that incentives to put profits before patients have grown stronger with an increase in private equity ownership of physician practices,” lead author Richard Scheffler, PhD, of UC Berkeley said in a statement.
The report also noted that private equity acquisitions of physician groups have risen sixfold in just a decade, increasing from 75 deals in 2012 to 484 deals in 2021.
Separately, the American Medical Association earlier released a separate report on trends in physician practice arrangements, finding that the percentage of physicians working in private equity–owned groups was 4.5% in 2022, the same as in its previous 2020 report. The share of physicians working in private practices fell by 13 percentage points from 60.1% to 46.7% between 2012 and 2022, the AMA reported.
The Berkeley report and the AMA update come amid rising concerns about the effects of the decline of independent physician practices. The U.S. Senate Finance Committee, which oversees most federal health spending, held a June hearing examining the causes and consequences of increased corporate ownership in health care, including a look at physician practices.
“It’s increasingly clear that consolidation in health care is not lowering costs or increasing the quality of Americans’ health care,” Senate Finance Chairman Ron Wyden (D-Ore.) said in an email. “For private equity in health care in particular, there needs to be more transparency around ownership so the effect on these business relationships can be better understood.”
Federal and state agencies do not generally track acquisitions of physician practices.
The UC Berkeley report impressively documents the rising influence of private equity in health care, for which it’s tough to find good data, said Karen Joynt Maddox, MD, MPH, of Washington University in St. Louis. Dr. Maddox, a cardiologist and policy researcher who also has studied the effects of consolidation in health care, examined the new report at the request of this news organization.
“They did a great job with the data,” Dr. Maddox said. “One of the big issues around private equity, and in general, ‘corporatization’ and consolidation of health care, is that there’s not a great way to track ownership changes. It’s really difficult to study.”
Dr. Scheffler and colleagues used data from the commercial firm PitchBook to identify acquisitions of physician practices by private equity firms. They consulted IQVIA’s physician databases – OneKey and SK&A Office-Based Physicians Database – to learn about the location, size, and specialties of acquired practices. They also used data from the nonprofit Health Care Cost Institute, which tracks commercial health plan claims, to assess how private equity acquisitions affected prices.
The researchers then matched the findings for practices acquired by private equity firms from 2015 to 2021 against those for comparable physician practices that remained independent from 2012 to 2021.
The authors then tied private-equity ownership to the following price increases:
- Gastroenterology (14%; 95% confidence interval, 7.9%-20.4%
- Oncology (16.4%; 95% CI, 5.5%-28.4%)
- Dermatology (4.0%; 95% CI, 1%-7.1%)
- Ob.gyn. (8.8%; 95% CI, 3.8%-14%)
- Ophthalmology (8.7%; 95% CI, 5.1%-12.3%)
- Radiology (8.2%; 95% CI, 0.8%-16.1%)
- Orthopedics (7.1%; 95% CI, 2.2%-12.3%)
- Primary care (4.1%; 95% CI, 1.3%-7%)
The analysis also found higher prices for cardiology (8.7%; 95% CI, –6.4% to 26.1%) and urology (4.2%; 95% CI, –2.3% to 11.1%), but neither of these findings was statistically significant, one of the authors, Daniel R. Arnold, PhD, of UC Berkeley, said in an email. This was most likely caused by smaller sample sizes for these fields.
Factors driving consolidation
The two reports and the Senate Finance consolidation hearing raised similar issues, including calls to look at the factors driving more physicians out of independent practice, including Medicare reimbursement that may not keep up with rising inflation.
The Berkeley report authors called for Congress to add a broad inflation component to the Medicare physician fee schedule. It also called on Congress to add cases where Medicare, the biggest U.S. purchaser of health care, pays less for services when performed in independent practices than in hospital-affiliated ones.
Shawn Martin, executive vice president and CEO of the American Academy of Family Physicians, said his group appreciates how the report from UC Berkeley and nonprofit groups echoed recommendations many clinicians have made, including the call for a broad inflation adjustment for the fee schedule.
“To move the needle forward, Congress must advance site-neutral payment policies while also addressing the administrative requirements that take physicians away from the important work of caring for patients,” Mr. Martin said in an email.
Arnold Ventures provided funding for the report, which was a joint project of the American Antitrust Institute, the Nicholas C. Petris Center on Health Care Markets and Consumer Welfare, UC Berkeley, and the Washington Center for Equitable Growth.
A version of this article appeared on Medscape.com.
The report was a collaboration of University of California, Berkeley, staff and researchers from two nonprofits, the American Antitrust Institute and the Washington Center for Equitable Growth. It provides “convincing evidence that incentives to put profits before patients have grown stronger with an increase in private equity ownership of physician practices,” lead author Richard Scheffler, PhD, of UC Berkeley said in a statement.
The report also noted that private equity acquisitions of physician groups have risen sixfold in just a decade, increasing from 75 deals in 2012 to 484 deals in 2021.
Separately, the American Medical Association earlier released a separate report on trends in physician practice arrangements, finding that the percentage of physicians working in private equity–owned groups was 4.5% in 2022, the same as in its previous 2020 report. The share of physicians working in private practices fell by 13 percentage points from 60.1% to 46.7% between 2012 and 2022, the AMA reported.
The Berkeley report and the AMA update come amid rising concerns about the effects of the decline of independent physician practices. The U.S. Senate Finance Committee, which oversees most federal health spending, held a June hearing examining the causes and consequences of increased corporate ownership in health care, including a look at physician practices.
“It’s increasingly clear that consolidation in health care is not lowering costs or increasing the quality of Americans’ health care,” Senate Finance Chairman Ron Wyden (D-Ore.) said in an email. “For private equity in health care in particular, there needs to be more transparency around ownership so the effect on these business relationships can be better understood.”
Federal and state agencies do not generally track acquisitions of physician practices.
The UC Berkeley report impressively documents the rising influence of private equity in health care, for which it’s tough to find good data, said Karen Joynt Maddox, MD, MPH, of Washington University in St. Louis. Dr. Maddox, a cardiologist and policy researcher who also has studied the effects of consolidation in health care, examined the new report at the request of this news organization.
“They did a great job with the data,” Dr. Maddox said. “One of the big issues around private equity, and in general, ‘corporatization’ and consolidation of health care, is that there’s not a great way to track ownership changes. It’s really difficult to study.”
Dr. Scheffler and colleagues used data from the commercial firm PitchBook to identify acquisitions of physician practices by private equity firms. They consulted IQVIA’s physician databases – OneKey and SK&A Office-Based Physicians Database – to learn about the location, size, and specialties of acquired practices. They also used data from the nonprofit Health Care Cost Institute, which tracks commercial health plan claims, to assess how private equity acquisitions affected prices.
The researchers then matched the findings for practices acquired by private equity firms from 2015 to 2021 against those for comparable physician practices that remained independent from 2012 to 2021.
The authors then tied private-equity ownership to the following price increases:
- Gastroenterology (14%; 95% confidence interval, 7.9%-20.4%
- Oncology (16.4%; 95% CI, 5.5%-28.4%)
- Dermatology (4.0%; 95% CI, 1%-7.1%)
- Ob.gyn. (8.8%; 95% CI, 3.8%-14%)
- Ophthalmology (8.7%; 95% CI, 5.1%-12.3%)
- Radiology (8.2%; 95% CI, 0.8%-16.1%)
- Orthopedics (7.1%; 95% CI, 2.2%-12.3%)
- Primary care (4.1%; 95% CI, 1.3%-7%)
The analysis also found higher prices for cardiology (8.7%; 95% CI, –6.4% to 26.1%) and urology (4.2%; 95% CI, –2.3% to 11.1%), but neither of these findings was statistically significant, one of the authors, Daniel R. Arnold, PhD, of UC Berkeley, said in an email. This was most likely caused by smaller sample sizes for these fields.
Factors driving consolidation
The two reports and the Senate Finance consolidation hearing raised similar issues, including calls to look at the factors driving more physicians out of independent practice, including Medicare reimbursement that may not keep up with rising inflation.
The Berkeley report authors called for Congress to add a broad inflation component to the Medicare physician fee schedule. It also called on Congress to add cases where Medicare, the biggest U.S. purchaser of health care, pays less for services when performed in independent practices than in hospital-affiliated ones.
Shawn Martin, executive vice president and CEO of the American Academy of Family Physicians, said his group appreciates how the report from UC Berkeley and nonprofit groups echoed recommendations many clinicians have made, including the call for a broad inflation adjustment for the fee schedule.
“To move the needle forward, Congress must advance site-neutral payment policies while also addressing the administrative requirements that take physicians away from the important work of caring for patients,” Mr. Martin said in an email.
Arnold Ventures provided funding for the report, which was a joint project of the American Antitrust Institute, the Nicholas C. Petris Center on Health Care Markets and Consumer Welfare, UC Berkeley, and the Washington Center for Equitable Growth.
A version of this article appeared on Medscape.com.
FDA approves quizartinib for newly diagnosed AML
On July 20 the FDA also approved the LeukoStrat CDx FLT3 Mutation Assay to determine whether patients have this mutation.
The agency granted quizartinib a first-line indication for use in combination with standard chemotherapy – cytarabine and anthracycline induction followed by cytarabine consolidation – and as maintenance monotherapy afterwards, in adults whose tumors express FLT3-ITD.
The FLT3 protein is a tyrosine kinase receptor found on hematopoietic stem cells. Wild-type FLT3 promotes cell survival, growth, and differentiation, but ITD (internal tandem duplication)-mutated FLT3, which quizartinib targets, is associated with a higher relapse risk and shorter survival. About a quarter of AML patients carry the mutation.
Approval was based on the phase 3 QuANTUM-First trial in over 500 patients with the mutation. Median overall survival among patients on standard chemotherapy randomly assigned to quizartinib was 31.9 months versus 15.1 months in patients randomly assigned to placebo, a 22.4% reduction in the risk of death (P = .0324).
Quizartinib is not indicated as maintenance monotherapy after allogeneic hematopoietic stem cell transplantation.
In a company press release, the drug’s manufacturer Daiichi Sankyo said quizartinib will be available in the United States soon.
Company executive Ken Takeshita, MD, called the approval “an important milestone, as patients with the FLT3-ITD subtype of AML can now be treated with the first-ever FLT3 inhibitor approved across the three phases of treatment these patients typically receive.”
The FDA’s original decision date was April 24, but the agency pushed it back 3 months to review updates Daiichi Sankyo made to quizartinib’s Risk Evaluation and Mitigation Strategies (REMS) program in response to an agency request.
Quizartinib carries a boxed warning of QT prolongation, torsades de pointes, and cardiac arrest. Because of these risks, it’s only available through a new program, dubbed “Vanflyta REMS.”
In the trial, the most common adverse with quizartinib included lymphopenia (60%), hypokalemia (59%), hypoalbuminemia (53%), hypophosphatemia (52%), alkaline phosphatase increased (51%), hypomagnesemia (44%), febrile neutropenia (44%), diarrhea (42%), mucositis (38%), nausea (34%), and hypocalcemia (33%), among others.
The most common grade 3/4 adverse events were febrile neutropenia (43% with quizartinib vs. 41% with placebo), neutropenia (18% vs. 9%), hypokalemia (19% vs. 16%), and pneumonia (11% both). Adverse events were fatal in 11.3% of patients receiving quizartinib versus 9.7% of patients on placebo, mostly caused by infections.
In 2019, the FDA rejected quizartinib for FLT3-ITD mutated relapsed/refractory AML monotherapy in adults, after most of its oncology advisers thought the risk of treatment outweighed the benefits in an earlier trial.
A version of this article first appeared on Medscape.com.
On July 20 the FDA also approved the LeukoStrat CDx FLT3 Mutation Assay to determine whether patients have this mutation.
The agency granted quizartinib a first-line indication for use in combination with standard chemotherapy – cytarabine and anthracycline induction followed by cytarabine consolidation – and as maintenance monotherapy afterwards, in adults whose tumors express FLT3-ITD.
The FLT3 protein is a tyrosine kinase receptor found on hematopoietic stem cells. Wild-type FLT3 promotes cell survival, growth, and differentiation, but ITD (internal tandem duplication)-mutated FLT3, which quizartinib targets, is associated with a higher relapse risk and shorter survival. About a quarter of AML patients carry the mutation.
Approval was based on the phase 3 QuANTUM-First trial in over 500 patients with the mutation. Median overall survival among patients on standard chemotherapy randomly assigned to quizartinib was 31.9 months versus 15.1 months in patients randomly assigned to placebo, a 22.4% reduction in the risk of death (P = .0324).
Quizartinib is not indicated as maintenance monotherapy after allogeneic hematopoietic stem cell transplantation.
In a company press release, the drug’s manufacturer Daiichi Sankyo said quizartinib will be available in the United States soon.
Company executive Ken Takeshita, MD, called the approval “an important milestone, as patients with the FLT3-ITD subtype of AML can now be treated with the first-ever FLT3 inhibitor approved across the three phases of treatment these patients typically receive.”
The FDA’s original decision date was April 24, but the agency pushed it back 3 months to review updates Daiichi Sankyo made to quizartinib’s Risk Evaluation and Mitigation Strategies (REMS) program in response to an agency request.
Quizartinib carries a boxed warning of QT prolongation, torsades de pointes, and cardiac arrest. Because of these risks, it’s only available through a new program, dubbed “Vanflyta REMS.”
In the trial, the most common adverse with quizartinib included lymphopenia (60%), hypokalemia (59%), hypoalbuminemia (53%), hypophosphatemia (52%), alkaline phosphatase increased (51%), hypomagnesemia (44%), febrile neutropenia (44%), diarrhea (42%), mucositis (38%), nausea (34%), and hypocalcemia (33%), among others.
The most common grade 3/4 adverse events were febrile neutropenia (43% with quizartinib vs. 41% with placebo), neutropenia (18% vs. 9%), hypokalemia (19% vs. 16%), and pneumonia (11% both). Adverse events were fatal in 11.3% of patients receiving quizartinib versus 9.7% of patients on placebo, mostly caused by infections.
In 2019, the FDA rejected quizartinib for FLT3-ITD mutated relapsed/refractory AML monotherapy in adults, after most of its oncology advisers thought the risk of treatment outweighed the benefits in an earlier trial.
A version of this article first appeared on Medscape.com.
On July 20 the FDA also approved the LeukoStrat CDx FLT3 Mutation Assay to determine whether patients have this mutation.
The agency granted quizartinib a first-line indication for use in combination with standard chemotherapy – cytarabine and anthracycline induction followed by cytarabine consolidation – and as maintenance monotherapy afterwards, in adults whose tumors express FLT3-ITD.
The FLT3 protein is a tyrosine kinase receptor found on hematopoietic stem cells. Wild-type FLT3 promotes cell survival, growth, and differentiation, but ITD (internal tandem duplication)-mutated FLT3, which quizartinib targets, is associated with a higher relapse risk and shorter survival. About a quarter of AML patients carry the mutation.
Approval was based on the phase 3 QuANTUM-First trial in over 500 patients with the mutation. Median overall survival among patients on standard chemotherapy randomly assigned to quizartinib was 31.9 months versus 15.1 months in patients randomly assigned to placebo, a 22.4% reduction in the risk of death (P = .0324).
Quizartinib is not indicated as maintenance monotherapy after allogeneic hematopoietic stem cell transplantation.
In a company press release, the drug’s manufacturer Daiichi Sankyo said quizartinib will be available in the United States soon.
Company executive Ken Takeshita, MD, called the approval “an important milestone, as patients with the FLT3-ITD subtype of AML can now be treated with the first-ever FLT3 inhibitor approved across the three phases of treatment these patients typically receive.”
The FDA’s original decision date was April 24, but the agency pushed it back 3 months to review updates Daiichi Sankyo made to quizartinib’s Risk Evaluation and Mitigation Strategies (REMS) program in response to an agency request.
Quizartinib carries a boxed warning of QT prolongation, torsades de pointes, and cardiac arrest. Because of these risks, it’s only available through a new program, dubbed “Vanflyta REMS.”
In the trial, the most common adverse with quizartinib included lymphopenia (60%), hypokalemia (59%), hypoalbuminemia (53%), hypophosphatemia (52%), alkaline phosphatase increased (51%), hypomagnesemia (44%), febrile neutropenia (44%), diarrhea (42%), mucositis (38%), nausea (34%), and hypocalcemia (33%), among others.
The most common grade 3/4 adverse events were febrile neutropenia (43% with quizartinib vs. 41% with placebo), neutropenia (18% vs. 9%), hypokalemia (19% vs. 16%), and pneumonia (11% both). Adverse events were fatal in 11.3% of patients receiving quizartinib versus 9.7% of patients on placebo, mostly caused by infections.
In 2019, the FDA rejected quizartinib for FLT3-ITD mutated relapsed/refractory AML monotherapy in adults, after most of its oncology advisers thought the risk of treatment outweighed the benefits in an earlier trial.
A version of this article first appeared on Medscape.com.
An Atypical Discussion of the Link Between Metabolic Syndrome and Type 2 Diabetes—and the Use of Precision Medicine to Treat the Whole Patient
Metabolic syndrome, type 2 diabetes mellitus (T2DM), and the “diabetes syndrome,” are interrelated, serious health conditions that share common risk factors and mechanisms. While they are each distinct conditions, a significant association exists between them, with metabolic syndrome often being considered a precursor to the development of typical T2DM.
Metabolic syndrome is a cluster of individual metabolic abnormalities that includes a combination of risk factors such as abdominal obesity, high blood pressure, elevated insulin levels, high triglyceride levels, and low levels of high-density lipoprotein (HDL) cholesterol related to genes and epigenetic changes associated with insulin resistance. These risk factors increase the likelihood of developing cardiovascular diseases, such as heart disease and stroke, and, when combined with significant damage to β -cell function and the influence of concordant environmental precipitants, result in hyperglycemia/overt diabetes—classically defined as T2DM.
It is estimated that there will be a staggering 3.1 billion people living with T2DM by 2050, according to a recent article in The Lancet. This devastating number will place a heavy burden on the health care system.
However, this typical pathophysiologic definition of T2DM is imprecise. Twenty percent of patients with T2DM have islet-cell antibodies that are typical of the immune destruction of β-cells in patients with type 1 diabetes mellitus (T1DM). Furthermore, approximately 40% of patients with T1DM have insulin resistance.
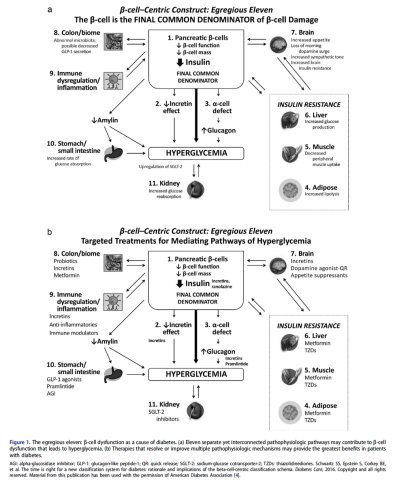
Thus, to better understand and distinguish the disease processes unique to each individual, we have defined a new beta cell classification for all forms of diabetes mellitus (DM). In this classification, there are 4 common pathophysiologic causes of all DM (including classic T2DM), with resultant damage to the β-cells (ie, genetic and epigenetic changes, inflammation, an abnormal environment, and insulin resistance), which results in 11 mechanisms of hyperglycemia, represented as “the egregious eleven” in Figure 1.
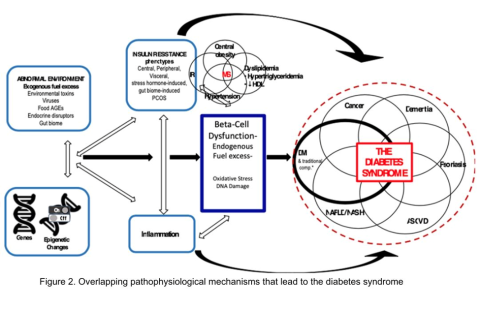
Additionally, Figure 2 illustrates the association between overlapping genes/epigenetic changes responsible for DM and the increased susceptibility to developing various microvascular complications commonly observed in all forms of DM, including classic T2DM. These complications, now recognized as components of the diabetes syndrome, encompass a range of conditions with shared interrelated pathophysiologic mechanisms, such as arteriosclerotic vascular disease (ASVD), dementia, some cancers, nonalcoholic fatty liver disease or nonalcoholic steatohepatitis (NAFLD/NASH), or psoriasis.
The likelihood of developing a specific type of DM, with classic complications or associated conditions, is contingent on an individual’s genes, epigenetic factors, inflammation, insulin resistance, and environmental exposures over time. It has now been postulated that these factors can be identified in a particular individual by a set of genomics, metabolomics, proteomics, and markers of these processes.
This more precise approach has the added benefit of giving rise to a more accurate individualization of therapy—precision medicine.
Precision medicine is an approach to healthcare that considers an individual's specific characteristics, such as genetic makeup, lifestyle, and environmental factors, to tailor medical treatments and interventions. In the context of this discussion on T2DM, precision medicine’s goal is to provide targeted therapies and interventions based on an individual's unique -omic profile to improve treatment outcomes and minimize side effects. An additional benefit of precision medicine use in diabetes syndrome is giving the diabetes specialist the opportunity to treat the whole patient, looking for complications and associated conditions earlier via defining the presence or absence of various markers of their individual pathophysiology. Additionally, we have come to recognize that many of the medications for treating T2DM (eg, glucagon-like peptide 1 receptor agonists [GLP-1 RA], dipeptidyl peptidase 4 inhibitors [DPP-4 inhibitors], sodium-glucose cotransporter-2 inhibitors [SGLT-2 inhibitors], metformin, Cycloset [bromocriptine mesylate]) can offer other benefits for the patient—treating not only multiple mechanisms of hyperglycemia (the egregious eleven: use the fewest number of agents in combination to treat the most number of mechanisms of hyperglycemia) but also recognize that they can prevent and treat the complications and associated conditions of the diabetes syndrome: cardiovascular, renal, liver, some cancers, psoriasis, and dementia.
The classic link between metabolic syndrome and T2DM is important to consider when applying precision medicine approaches to the management of T2DM. Here are some examples of how precision medicine is being applied in the management of T2DM:
Genetic testing: Genetic testing can help identify specific genetic variants or mutations that may influence an individual's risk of developing T2DM or their response to certain medications. By understanding a person's genetic predisposition, clinicians can make more informed decisions about treatment options and develop personalized strategies for their patients.
Pharmacogenomics: Certain genetic variations can impact how a person metabolizes and responds to specific diabetes medications. By analyzing an individual's genetic profile, medications that are more likely to be effective and have fewer adverse effects for that patient may be selected.
Continuous glucose monitoring (CGM): CGM devices provide real-time information about an individual’s blood glucose levels, allowing for more precise management of diabetes. By continuously monitoring glucose levels, patterns can be identified, allowing for adjustments to medication dosages, dietary recommendations, and lifestyle modifications on an individualized basis.
Lifestyle interventions: Precision medicine also recognizes that lifestyle factors play a crucial role in the development and management of T2DM. Lifestyle interventions, such as diet and exercise plans, based on an individual's preferences, metabolic profile, and response to different interventions can be personalized (ie, some individuals may benefit more from a low-carbohydrate diet, while others may respond better to a Mediterranean-style diet).
Predictive modeling and risk stratification: Precision medicine leverages data analytics and predictive modeling to assess an individual's risk of developing complications associated with T2DM. By analyzing various factors such as medical history, genetics, lifestyle, and biomarkers, individuals who are at a higher risk of developing complications can be identified, and their treatment plans can be tailored accordingly. Precision medicine enables early identification of individuals who are at a higher risk of developing T2DM based on their metabolic syndrome status.
In summary, precision medicine for T2DM considers the link between metabolic syndrome and diabetes syndrome to develop personalized approaches for prevention, early intervention, and treatment. By understanding an individual's metabolic and genetic profile, targeted strategies to optimize management and improve outcomes for patients with metabolic syndrome and those at risk of developing diabetes can be implemented.
It is important to note that while precision medicine holds promise in improving diabetes management, it is still an evolving field, and its widespread implementation is not yet fully realized. Collaboration between clinicians, researchers, and technological advancements will continue to drive the progress of precision medicine in T2DM management.
Metabolic syndrome, type 2 diabetes mellitus (T2DM), and the “diabetes syndrome,” are interrelated, serious health conditions that share common risk factors and mechanisms. While they are each distinct conditions, a significant association exists between them, with metabolic syndrome often being considered a precursor to the development of typical T2DM.
Metabolic syndrome is a cluster of individual metabolic abnormalities that includes a combination of risk factors such as abdominal obesity, high blood pressure, elevated insulin levels, high triglyceride levels, and low levels of high-density lipoprotein (HDL) cholesterol related to genes and epigenetic changes associated with insulin resistance. These risk factors increase the likelihood of developing cardiovascular diseases, such as heart disease and stroke, and, when combined with significant damage to β -cell function and the influence of concordant environmental precipitants, result in hyperglycemia/overt diabetes—classically defined as T2DM.
It is estimated that there will be a staggering 3.1 billion people living with T2DM by 2050, according to a recent article in The Lancet. This devastating number will place a heavy burden on the health care system.
However, this typical pathophysiologic definition of T2DM is imprecise. Twenty percent of patients with T2DM have islet-cell antibodies that are typical of the immune destruction of β-cells in patients with type 1 diabetes mellitus (T1DM). Furthermore, approximately 40% of patients with T1DM have insulin resistance.
Thus, to better understand and distinguish the disease processes unique to each individual, we have defined a new beta cell classification for all forms of diabetes mellitus (DM). In this classification, there are 4 common pathophysiologic causes of all DM (including classic T2DM), with resultant damage to the β-cells (ie, genetic and epigenetic changes, inflammation, an abnormal environment, and insulin resistance), which results in 11 mechanisms of hyperglycemia, represented as “the egregious eleven” in Figure 1.
Additionally, Figure 2 illustrates the association between overlapping genes/epigenetic changes responsible for DM and the increased susceptibility to developing various microvascular complications commonly observed in all forms of DM, including classic T2DM. These complications, now recognized as components of the diabetes syndrome, encompass a range of conditions with shared interrelated pathophysiologic mechanisms, such as arteriosclerotic vascular disease (ASVD), dementia, some cancers, nonalcoholic fatty liver disease or nonalcoholic steatohepatitis (NAFLD/NASH), or psoriasis.
The likelihood of developing a specific type of DM, with classic complications or associated conditions, is contingent on an individual’s genes, epigenetic factors, inflammation, insulin resistance, and environmental exposures over time. It has now been postulated that these factors can be identified in a particular individual by a set of genomics, metabolomics, proteomics, and markers of these processes.
This more precise approach has the added benefit of giving rise to a more accurate individualization of therapy—precision medicine.
Precision medicine is an approach to healthcare that considers an individual's specific characteristics, such as genetic makeup, lifestyle, and environmental factors, to tailor medical treatments and interventions. In the context of this discussion on T2DM, precision medicine’s goal is to provide targeted therapies and interventions based on an individual's unique -omic profile to improve treatment outcomes and minimize side effects. An additional benefit of precision medicine use in diabetes syndrome is giving the diabetes specialist the opportunity to treat the whole patient, looking for complications and associated conditions earlier via defining the presence or absence of various markers of their individual pathophysiology. Additionally, we have come to recognize that many of the medications for treating T2DM (eg, glucagon-like peptide 1 receptor agonists [GLP-1 RA], dipeptidyl peptidase 4 inhibitors [DPP-4 inhibitors], sodium-glucose cotransporter-2 inhibitors [SGLT-2 inhibitors], metformin, Cycloset [bromocriptine mesylate]) can offer other benefits for the patient—treating not only multiple mechanisms of hyperglycemia (the egregious eleven: use the fewest number of agents in combination to treat the most number of mechanisms of hyperglycemia) but also recognize that they can prevent and treat the complications and associated conditions of the diabetes syndrome: cardiovascular, renal, liver, some cancers, psoriasis, and dementia.
The classic link between metabolic syndrome and T2DM is important to consider when applying precision medicine approaches to the management of T2DM. Here are some examples of how precision medicine is being applied in the management of T2DM:
Genetic testing: Genetic testing can help identify specific genetic variants or mutations that may influence an individual's risk of developing T2DM or their response to certain medications. By understanding a person's genetic predisposition, clinicians can make more informed decisions about treatment options and develop personalized strategies for their patients.
Pharmacogenomics: Certain genetic variations can impact how a person metabolizes and responds to specific diabetes medications. By analyzing an individual's genetic profile, medications that are more likely to be effective and have fewer adverse effects for that patient may be selected.
Continuous glucose monitoring (CGM): CGM devices provide real-time information about an individual’s blood glucose levels, allowing for more precise management of diabetes. By continuously monitoring glucose levels, patterns can be identified, allowing for adjustments to medication dosages, dietary recommendations, and lifestyle modifications on an individualized basis.
Lifestyle interventions: Precision medicine also recognizes that lifestyle factors play a crucial role in the development and management of T2DM. Lifestyle interventions, such as diet and exercise plans, based on an individual's preferences, metabolic profile, and response to different interventions can be personalized (ie, some individuals may benefit more from a low-carbohydrate diet, while others may respond better to a Mediterranean-style diet).
Predictive modeling and risk stratification: Precision medicine leverages data analytics and predictive modeling to assess an individual's risk of developing complications associated with T2DM. By analyzing various factors such as medical history, genetics, lifestyle, and biomarkers, individuals who are at a higher risk of developing complications can be identified, and their treatment plans can be tailored accordingly. Precision medicine enables early identification of individuals who are at a higher risk of developing T2DM based on their metabolic syndrome status.
In summary, precision medicine for T2DM considers the link between metabolic syndrome and diabetes syndrome to develop personalized approaches for prevention, early intervention, and treatment. By understanding an individual's metabolic and genetic profile, targeted strategies to optimize management and improve outcomes for patients with metabolic syndrome and those at risk of developing diabetes can be implemented.
It is important to note that while precision medicine holds promise in improving diabetes management, it is still an evolving field, and its widespread implementation is not yet fully realized. Collaboration between clinicians, researchers, and technological advancements will continue to drive the progress of precision medicine in T2DM management.
Metabolic syndrome, type 2 diabetes mellitus (T2DM), and the “diabetes syndrome,” are interrelated, serious health conditions that share common risk factors and mechanisms. While they are each distinct conditions, a significant association exists between them, with metabolic syndrome often being considered a precursor to the development of typical T2DM.
Metabolic syndrome is a cluster of individual metabolic abnormalities that includes a combination of risk factors such as abdominal obesity, high blood pressure, elevated insulin levels, high triglyceride levels, and low levels of high-density lipoprotein (HDL) cholesterol related to genes and epigenetic changes associated with insulin resistance. These risk factors increase the likelihood of developing cardiovascular diseases, such as heart disease and stroke, and, when combined with significant damage to β -cell function and the influence of concordant environmental precipitants, result in hyperglycemia/overt diabetes—classically defined as T2DM.
It is estimated that there will be a staggering 3.1 billion people living with T2DM by 2050, according to a recent article in The Lancet. This devastating number will place a heavy burden on the health care system.
However, this typical pathophysiologic definition of T2DM is imprecise. Twenty percent of patients with T2DM have islet-cell antibodies that are typical of the immune destruction of β-cells in patients with type 1 diabetes mellitus (T1DM). Furthermore, approximately 40% of patients with T1DM have insulin resistance.
Thus, to better understand and distinguish the disease processes unique to each individual, we have defined a new beta cell classification for all forms of diabetes mellitus (DM). In this classification, there are 4 common pathophysiologic causes of all DM (including classic T2DM), with resultant damage to the β-cells (ie, genetic and epigenetic changes, inflammation, an abnormal environment, and insulin resistance), which results in 11 mechanisms of hyperglycemia, represented as “the egregious eleven” in Figure 1.
Additionally, Figure 2 illustrates the association between overlapping genes/epigenetic changes responsible for DM and the increased susceptibility to developing various microvascular complications commonly observed in all forms of DM, including classic T2DM. These complications, now recognized as components of the diabetes syndrome, encompass a range of conditions with shared interrelated pathophysiologic mechanisms, such as arteriosclerotic vascular disease (ASVD), dementia, some cancers, nonalcoholic fatty liver disease or nonalcoholic steatohepatitis (NAFLD/NASH), or psoriasis.
The likelihood of developing a specific type of DM, with classic complications or associated conditions, is contingent on an individual’s genes, epigenetic factors, inflammation, insulin resistance, and environmental exposures over time. It has now been postulated that these factors can be identified in a particular individual by a set of genomics, metabolomics, proteomics, and markers of these processes.
This more precise approach has the added benefit of giving rise to a more accurate individualization of therapy—precision medicine.
Precision medicine is an approach to healthcare that considers an individual's specific characteristics, such as genetic makeup, lifestyle, and environmental factors, to tailor medical treatments and interventions. In the context of this discussion on T2DM, precision medicine’s goal is to provide targeted therapies and interventions based on an individual's unique -omic profile to improve treatment outcomes and minimize side effects. An additional benefit of precision medicine use in diabetes syndrome is giving the diabetes specialist the opportunity to treat the whole patient, looking for complications and associated conditions earlier via defining the presence or absence of various markers of their individual pathophysiology. Additionally, we have come to recognize that many of the medications for treating T2DM (eg, glucagon-like peptide 1 receptor agonists [GLP-1 RA], dipeptidyl peptidase 4 inhibitors [DPP-4 inhibitors], sodium-glucose cotransporter-2 inhibitors [SGLT-2 inhibitors], metformin, Cycloset [bromocriptine mesylate]) can offer other benefits for the patient—treating not only multiple mechanisms of hyperglycemia (the egregious eleven: use the fewest number of agents in combination to treat the most number of mechanisms of hyperglycemia) but also recognize that they can prevent and treat the complications and associated conditions of the diabetes syndrome: cardiovascular, renal, liver, some cancers, psoriasis, and dementia.
The classic link between metabolic syndrome and T2DM is important to consider when applying precision medicine approaches to the management of T2DM. Here are some examples of how precision medicine is being applied in the management of T2DM:
Genetic testing: Genetic testing can help identify specific genetic variants or mutations that may influence an individual's risk of developing T2DM or their response to certain medications. By understanding a person's genetic predisposition, clinicians can make more informed decisions about treatment options and develop personalized strategies for their patients.
Pharmacogenomics: Certain genetic variations can impact how a person metabolizes and responds to specific diabetes medications. By analyzing an individual's genetic profile, medications that are more likely to be effective and have fewer adverse effects for that patient may be selected.
Continuous glucose monitoring (CGM): CGM devices provide real-time information about an individual’s blood glucose levels, allowing for more precise management of diabetes. By continuously monitoring glucose levels, patterns can be identified, allowing for adjustments to medication dosages, dietary recommendations, and lifestyle modifications on an individualized basis.
Lifestyle interventions: Precision medicine also recognizes that lifestyle factors play a crucial role in the development and management of T2DM. Lifestyle interventions, such as diet and exercise plans, based on an individual's preferences, metabolic profile, and response to different interventions can be personalized (ie, some individuals may benefit more from a low-carbohydrate diet, while others may respond better to a Mediterranean-style diet).
Predictive modeling and risk stratification: Precision medicine leverages data analytics and predictive modeling to assess an individual's risk of developing complications associated with T2DM. By analyzing various factors such as medical history, genetics, lifestyle, and biomarkers, individuals who are at a higher risk of developing complications can be identified, and their treatment plans can be tailored accordingly. Precision medicine enables early identification of individuals who are at a higher risk of developing T2DM based on their metabolic syndrome status.
In summary, precision medicine for T2DM considers the link between metabolic syndrome and diabetes syndrome to develop personalized approaches for prevention, early intervention, and treatment. By understanding an individual's metabolic and genetic profile, targeted strategies to optimize management and improve outcomes for patients with metabolic syndrome and those at risk of developing diabetes can be implemented.
It is important to note that while precision medicine holds promise in improving diabetes management, it is still an evolving field, and its widespread implementation is not yet fully realized. Collaboration between clinicians, researchers, and technological advancements will continue to drive the progress of precision medicine in T2DM management.
Most Americans in favor of regulated therapeutic psychedelics
It is a surprisingly large percentage, said officials at the University of California, Berkeley, Center for the Science of Psychedelics, which conducted the online survey of 1,500 registered voters in early June.
“That is a stunning number,” said Michael Pollan, cofounder of the center, and author of “How to Change Your Mind,” a book that explored potential uses of psychedelics.
In a briefing with reporters, Mr. Pollan said that he believes the large support base, in part, reflects campaigns that have “been successful by highlighting the effectiveness of psychedelics as therapy for mental illness.”
However, the poll also showed that 61% of voters said that they do not perceive psychedelics as “good for society,” and 69% do not perceive them as “something for people like me.”
These negative sentiments “suggest a fragile kind of support – the kind of support where you’re only hearing one side of the story,” said Mr. Pollan.
Still, poll respondents supported other potential policy changes, including 56% in support of the U.S. Food and Drug Administration vetting and approving psychedelics so they could be available by prescription.
50% have tried psychedelics
Almost 80% said that it should be easier for researchers to study psychedelics, and just under one-half said that they backed removing criminal penalties for personal use and possession.
The poll results also show that almost half of respondents had heard about psychedelics recently, with 48% saying they had heard about the drugs’ use in treating mental illness.
Respondents who were most familiar with and positive about psychedelics tended to be White, male, aged 30-50 years, liberal, highly educated, living in a Western state, and have little to no religious or spiritual practice.
Overall, 52% of survey respondents said that they or someone close to them had used a psychedelic, with almost half of that use coming in the past 5 years. Some 40% said that the use had been more than a decade ago.
Almost three-quarters of psychedelic use was reported as recreational, but the second-biggest category was therapeutic use at 39%. About one-third of respondents said that they or someone close to them had microdosed.
Conservative voters had lower levels of awareness and first-degree connection use as well as the least amount of support for regulated therapeutic use, with only 45% saying they would back such a policy, compared with 80% of liberal voters and 66% of moderate voters.
Black individuals were the least likely to be familiar with psychedelics: Just 29% said that they had heard a little or a lot about the drugs, compared with 39% of Latinx individuals and 51% of White individuals. And just one-quarter reported first-degree use, compared with half of Latinx individuals and 56% of White individuals.
Who should be eligible?
When asked who should be eligible for treatment with psychedelics, 80% said that they were comfortable with its use for those with terminal illnesses. More than two-thirds expressed comfort with the drugs being used to help veterans and people with treatment-resistant depression and anxiety.
Less than one-half of respondents said that psychedelics should be available to everyone older than 21 years. And voters seemed to be less inclined to say psychedelics should be used to treat people with addiction, with just 45% indicating that they were very or somewhat comfortable with that use.
Mr. Pollan said that reflects perhaps some lack of knowledge or education.
“The story about addiction and psychedelics hasn’t gotten out,” he said. “I kind of get that intuitively the idea of using a drug to treat a drug doesn’t sound right to a lot of people. But in fact, there’s good evidence it works,” Mr. Pollan said.
Respondents said that doctors, nurses, and scientists were the most trusted source of information about psychedelics, whereas the FDA received lower confidence. Law enforcement was least trusted by liberals and most trusted by conservatives.
Mr. Pollan noted the reversal in attitudes, with Americans mostly now looking to the scientific and medical establishment for guidance on psychedelics.
“We went from a counterculture drug to something that is being taken seriously by scientists as a potential therapy,” he said.
The poll’s margin of error was ± 2.5%.
A version of this article first appeared on Medscape.com.
It is a surprisingly large percentage, said officials at the University of California, Berkeley, Center for the Science of Psychedelics, which conducted the online survey of 1,500 registered voters in early June.
“That is a stunning number,” said Michael Pollan, cofounder of the center, and author of “How to Change Your Mind,” a book that explored potential uses of psychedelics.
In a briefing with reporters, Mr. Pollan said that he believes the large support base, in part, reflects campaigns that have “been successful by highlighting the effectiveness of psychedelics as therapy for mental illness.”
However, the poll also showed that 61% of voters said that they do not perceive psychedelics as “good for society,” and 69% do not perceive them as “something for people like me.”
These negative sentiments “suggest a fragile kind of support – the kind of support where you’re only hearing one side of the story,” said Mr. Pollan.
Still, poll respondents supported other potential policy changes, including 56% in support of the U.S. Food and Drug Administration vetting and approving psychedelics so they could be available by prescription.
50% have tried psychedelics
Almost 80% said that it should be easier for researchers to study psychedelics, and just under one-half said that they backed removing criminal penalties for personal use and possession.
The poll results also show that almost half of respondents had heard about psychedelics recently, with 48% saying they had heard about the drugs’ use in treating mental illness.
Respondents who were most familiar with and positive about psychedelics tended to be White, male, aged 30-50 years, liberal, highly educated, living in a Western state, and have little to no religious or spiritual practice.
Overall, 52% of survey respondents said that they or someone close to them had used a psychedelic, with almost half of that use coming in the past 5 years. Some 40% said that the use had been more than a decade ago.
Almost three-quarters of psychedelic use was reported as recreational, but the second-biggest category was therapeutic use at 39%. About one-third of respondents said that they or someone close to them had microdosed.
Conservative voters had lower levels of awareness and first-degree connection use as well as the least amount of support for regulated therapeutic use, with only 45% saying they would back such a policy, compared with 80% of liberal voters and 66% of moderate voters.
Black individuals were the least likely to be familiar with psychedelics: Just 29% said that they had heard a little or a lot about the drugs, compared with 39% of Latinx individuals and 51% of White individuals. And just one-quarter reported first-degree use, compared with half of Latinx individuals and 56% of White individuals.
Who should be eligible?
When asked who should be eligible for treatment with psychedelics, 80% said that they were comfortable with its use for those with terminal illnesses. More than two-thirds expressed comfort with the drugs being used to help veterans and people with treatment-resistant depression and anxiety.
Less than one-half of respondents said that psychedelics should be available to everyone older than 21 years. And voters seemed to be less inclined to say psychedelics should be used to treat people with addiction, with just 45% indicating that they were very or somewhat comfortable with that use.
Mr. Pollan said that reflects perhaps some lack of knowledge or education.
“The story about addiction and psychedelics hasn’t gotten out,” he said. “I kind of get that intuitively the idea of using a drug to treat a drug doesn’t sound right to a lot of people. But in fact, there’s good evidence it works,” Mr. Pollan said.
Respondents said that doctors, nurses, and scientists were the most trusted source of information about psychedelics, whereas the FDA received lower confidence. Law enforcement was least trusted by liberals and most trusted by conservatives.
Mr. Pollan noted the reversal in attitudes, with Americans mostly now looking to the scientific and medical establishment for guidance on psychedelics.
“We went from a counterculture drug to something that is being taken seriously by scientists as a potential therapy,” he said.
The poll’s margin of error was ± 2.5%.
A version of this article first appeared on Medscape.com.
It is a surprisingly large percentage, said officials at the University of California, Berkeley, Center for the Science of Psychedelics, which conducted the online survey of 1,500 registered voters in early June.
“That is a stunning number,” said Michael Pollan, cofounder of the center, and author of “How to Change Your Mind,” a book that explored potential uses of psychedelics.
In a briefing with reporters, Mr. Pollan said that he believes the large support base, in part, reflects campaigns that have “been successful by highlighting the effectiveness of psychedelics as therapy for mental illness.”
However, the poll also showed that 61% of voters said that they do not perceive psychedelics as “good for society,” and 69% do not perceive them as “something for people like me.”
These negative sentiments “suggest a fragile kind of support – the kind of support where you’re only hearing one side of the story,” said Mr. Pollan.
Still, poll respondents supported other potential policy changes, including 56% in support of the U.S. Food and Drug Administration vetting and approving psychedelics so they could be available by prescription.
50% have tried psychedelics
Almost 80% said that it should be easier for researchers to study psychedelics, and just under one-half said that they backed removing criminal penalties for personal use and possession.
The poll results also show that almost half of respondents had heard about psychedelics recently, with 48% saying they had heard about the drugs’ use in treating mental illness.
Respondents who were most familiar with and positive about psychedelics tended to be White, male, aged 30-50 years, liberal, highly educated, living in a Western state, and have little to no religious or spiritual practice.
Overall, 52% of survey respondents said that they or someone close to them had used a psychedelic, with almost half of that use coming in the past 5 years. Some 40% said that the use had been more than a decade ago.
Almost three-quarters of psychedelic use was reported as recreational, but the second-biggest category was therapeutic use at 39%. About one-third of respondents said that they or someone close to them had microdosed.
Conservative voters had lower levels of awareness and first-degree connection use as well as the least amount of support for regulated therapeutic use, with only 45% saying they would back such a policy, compared with 80% of liberal voters and 66% of moderate voters.
Black individuals were the least likely to be familiar with psychedelics: Just 29% said that they had heard a little or a lot about the drugs, compared with 39% of Latinx individuals and 51% of White individuals. And just one-quarter reported first-degree use, compared with half of Latinx individuals and 56% of White individuals.
Who should be eligible?
When asked who should be eligible for treatment with psychedelics, 80% said that they were comfortable with its use for those with terminal illnesses. More than two-thirds expressed comfort with the drugs being used to help veterans and people with treatment-resistant depression and anxiety.
Less than one-half of respondents said that psychedelics should be available to everyone older than 21 years. And voters seemed to be less inclined to say psychedelics should be used to treat people with addiction, with just 45% indicating that they were very or somewhat comfortable with that use.
Mr. Pollan said that reflects perhaps some lack of knowledge or education.
“The story about addiction and psychedelics hasn’t gotten out,” he said. “I kind of get that intuitively the idea of using a drug to treat a drug doesn’t sound right to a lot of people. But in fact, there’s good evidence it works,” Mr. Pollan said.
Respondents said that doctors, nurses, and scientists were the most trusted source of information about psychedelics, whereas the FDA received lower confidence. Law enforcement was least trusted by liberals and most trusted by conservatives.
Mr. Pollan noted the reversal in attitudes, with Americans mostly now looking to the scientific and medical establishment for guidance on psychedelics.
“We went from a counterculture drug to something that is being taken seriously by scientists as a potential therapy,” he said.
The poll’s margin of error was ± 2.5%.
A version of this article first appeared on Medscape.com.
High-intensity interval training before major surgery may boost postoperative outcomes
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Cognitive benefit of highly touted MIND diet questioned
in healthy adults at risk for dementia, results of a new randomized trial show.
Given the strong base of evidence from observational studies that demonstrate the benefits of the MIND diet on cognitive decline, Alzheimer’s disease (AD), and neuropathologic changes such as reduced beta amyloid and tau associated with AD, the study’s results were “unexpected,” study investigator Lisa L. Barnes, PhD, with the Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“One possibility is the trial may not have been long enough to see an effect. It’s also possible that participants in the control diet group benefited just as much as those in the MIND diet group because they also improved their diets to focus on weight loss,” Dr. Barnes said.
“Although we did not see a specific effect of the MIND diet, people in both groups improved their cognitive function, suggesting that a healthy diet in general is good for cognitive function,” she added.
The findings were presented at the annual Alzheimer’s Association International Conference and simultaneously published online in the New England Journal of Medicine.
Randomized trial
A hybrid of the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diet, the MIND diet includes foods and nutrients that have been putatively associated with a decreased risk of dementia.
To further investigate, the researchers conducted a randomized trial that included 604 older adults without cognitive impairment who had a family history of dementia, a body mass index greater than 25, and a suboptimal diet determined via a 14-item questionnaire.
For 3 years, 301 were randomly assigned to follow the MIND-diet with mild calorie restriction and 303 to follow a control diet with mild calorie restriction only. All participants received counseling to help them adhere to their assigned diet, plus support to promote weight loss of 3%-5% by year 3.
The primary endpoint was the change from baseline in global cognition and in specific cognitive domains through year 3. Cognition was assessed with an established battery of 12 publicly available cognitive function tests.
The secondary endpoint was the change from baseline in MRI-derived measures of brain characteristics in a nonrandom sample of participants.
“We had good adherence to the assigned diets and both groups lost weight, on average about 5 kilograms in both groups,” Dr. Barnes noted in her presentation.
From baseline through 3 years, small improvements in global cognition scores were observed in both groups, with increases of 0.205 standardized units in the MIND-diet group versus 0.170 standardized units in the control-diet group.
However, in intention-to-treat analysis, the mean change in score did not differ significantly between groups, with an estimated mean difference at the end of the trial of 0.035 standardized units (P = .23).
At the trial’s conclusion, there were also no between-group differences in change in white-matter hyperintensities, hippocampal volumes, and total gray- and white-matter volumes on MRI.
Dr. Barnes noted that the trial was limited to well-educated, older adults, mostly of European descent. Other limitations include the small sample size of those who received MRI and follow-up that was shorter than a typical observational study.
Dr. Barnes noted that this is a single study and that there needs to be more randomized trials of the MIND diet that, as with the observational research, follow participants for a longer period of time.
More to brain health than diet
Reached for comment, Majid Fotuhi, MD, PhD, adjunct professor of neuroscience at George Washington University, Washington, noted that participants who enroll in clinical trials that focus on diet become more aware of their eating habits and shift toward a healthier diet.
“This may explain the reason why both groups of participants in this study improved,” said Dr. Fotuhi, medical director of NeuroGrow Brain Fitness Center, McLean, Va.
However, he believes that better brain health requires a multipronged approach.
“In order to see significant results, people need to improve their diet, become physically fit, sleep well, reduce their stress, engage in cognitively challenging activities, and develop a positive mind set,” said Dr. Fotuhi.
“Interventions that target only one of these goals may not produce results that are as remarkable as multimodal programs, which target all of these goals,” Dr. Fotuhi said.
Dr. Fotuhi developed a multidimensional “brain fitness program” that has shown to provide multiple benefits for individuals with memory loss, attention deficit hyperactivity disorder, and post-concussion syndrome.
“Having provided our 12-week program for thousands of patients in the past 10 years, I have noticed a synergistic effect in patients who incorporate all of these changes in their day-to-day life and maintain it over time. They often become sharper and feel better overall,” Dr. Fotuhi told this news organization.
The study was supported by the National Institute on Aging. Disclosures for study authors are listed with the original article. Dr. Fotuhi has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in healthy adults at risk for dementia, results of a new randomized trial show.
Given the strong base of evidence from observational studies that demonstrate the benefits of the MIND diet on cognitive decline, Alzheimer’s disease (AD), and neuropathologic changes such as reduced beta amyloid and tau associated with AD, the study’s results were “unexpected,” study investigator Lisa L. Barnes, PhD, with the Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“One possibility is the trial may not have been long enough to see an effect. It’s also possible that participants in the control diet group benefited just as much as those in the MIND diet group because they also improved their diets to focus on weight loss,” Dr. Barnes said.
“Although we did not see a specific effect of the MIND diet, people in both groups improved their cognitive function, suggesting that a healthy diet in general is good for cognitive function,” she added.
The findings were presented at the annual Alzheimer’s Association International Conference and simultaneously published online in the New England Journal of Medicine.
Randomized trial
A hybrid of the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diet, the MIND diet includes foods and nutrients that have been putatively associated with a decreased risk of dementia.
To further investigate, the researchers conducted a randomized trial that included 604 older adults without cognitive impairment who had a family history of dementia, a body mass index greater than 25, and a suboptimal diet determined via a 14-item questionnaire.
For 3 years, 301 were randomly assigned to follow the MIND-diet with mild calorie restriction and 303 to follow a control diet with mild calorie restriction only. All participants received counseling to help them adhere to their assigned diet, plus support to promote weight loss of 3%-5% by year 3.
The primary endpoint was the change from baseline in global cognition and in specific cognitive domains through year 3. Cognition was assessed with an established battery of 12 publicly available cognitive function tests.
The secondary endpoint was the change from baseline in MRI-derived measures of brain characteristics in a nonrandom sample of participants.
“We had good adherence to the assigned diets and both groups lost weight, on average about 5 kilograms in both groups,” Dr. Barnes noted in her presentation.
From baseline through 3 years, small improvements in global cognition scores were observed in both groups, with increases of 0.205 standardized units in the MIND-diet group versus 0.170 standardized units in the control-diet group.
However, in intention-to-treat analysis, the mean change in score did not differ significantly between groups, with an estimated mean difference at the end of the trial of 0.035 standardized units (P = .23).
At the trial’s conclusion, there were also no between-group differences in change in white-matter hyperintensities, hippocampal volumes, and total gray- and white-matter volumes on MRI.
Dr. Barnes noted that the trial was limited to well-educated, older adults, mostly of European descent. Other limitations include the small sample size of those who received MRI and follow-up that was shorter than a typical observational study.
Dr. Barnes noted that this is a single study and that there needs to be more randomized trials of the MIND diet that, as with the observational research, follow participants for a longer period of time.
More to brain health than diet
Reached for comment, Majid Fotuhi, MD, PhD, adjunct professor of neuroscience at George Washington University, Washington, noted that participants who enroll in clinical trials that focus on diet become more aware of their eating habits and shift toward a healthier diet.
“This may explain the reason why both groups of participants in this study improved,” said Dr. Fotuhi, medical director of NeuroGrow Brain Fitness Center, McLean, Va.
However, he believes that better brain health requires a multipronged approach.
“In order to see significant results, people need to improve their diet, become physically fit, sleep well, reduce their stress, engage in cognitively challenging activities, and develop a positive mind set,” said Dr. Fotuhi.
“Interventions that target only one of these goals may not produce results that are as remarkable as multimodal programs, which target all of these goals,” Dr. Fotuhi said.
Dr. Fotuhi developed a multidimensional “brain fitness program” that has shown to provide multiple benefits for individuals with memory loss, attention deficit hyperactivity disorder, and post-concussion syndrome.
“Having provided our 12-week program for thousands of patients in the past 10 years, I have noticed a synergistic effect in patients who incorporate all of these changes in their day-to-day life and maintain it over time. They often become sharper and feel better overall,” Dr. Fotuhi told this news organization.
The study was supported by the National Institute on Aging. Disclosures for study authors are listed with the original article. Dr. Fotuhi has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in healthy adults at risk for dementia, results of a new randomized trial show.
Given the strong base of evidence from observational studies that demonstrate the benefits of the MIND diet on cognitive decline, Alzheimer’s disease (AD), and neuropathologic changes such as reduced beta amyloid and tau associated with AD, the study’s results were “unexpected,” study investigator Lisa L. Barnes, PhD, with the Rush Alzheimer’s Disease Center, Chicago, said in an interview.
“One possibility is the trial may not have been long enough to see an effect. It’s also possible that participants in the control diet group benefited just as much as those in the MIND diet group because they also improved their diets to focus on weight loss,” Dr. Barnes said.
“Although we did not see a specific effect of the MIND diet, people in both groups improved their cognitive function, suggesting that a healthy diet in general is good for cognitive function,” she added.
The findings were presented at the annual Alzheimer’s Association International Conference and simultaneously published online in the New England Journal of Medicine.
Randomized trial
A hybrid of the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diet, the MIND diet includes foods and nutrients that have been putatively associated with a decreased risk of dementia.
To further investigate, the researchers conducted a randomized trial that included 604 older adults without cognitive impairment who had a family history of dementia, a body mass index greater than 25, and a suboptimal diet determined via a 14-item questionnaire.
For 3 years, 301 were randomly assigned to follow the MIND-diet with mild calorie restriction and 303 to follow a control diet with mild calorie restriction only. All participants received counseling to help them adhere to their assigned diet, plus support to promote weight loss of 3%-5% by year 3.
The primary endpoint was the change from baseline in global cognition and in specific cognitive domains through year 3. Cognition was assessed with an established battery of 12 publicly available cognitive function tests.
The secondary endpoint was the change from baseline in MRI-derived measures of brain characteristics in a nonrandom sample of participants.
“We had good adherence to the assigned diets and both groups lost weight, on average about 5 kilograms in both groups,” Dr. Barnes noted in her presentation.
From baseline through 3 years, small improvements in global cognition scores were observed in both groups, with increases of 0.205 standardized units in the MIND-diet group versus 0.170 standardized units in the control-diet group.
However, in intention-to-treat analysis, the mean change in score did not differ significantly between groups, with an estimated mean difference at the end of the trial of 0.035 standardized units (P = .23).
At the trial’s conclusion, there were also no between-group differences in change in white-matter hyperintensities, hippocampal volumes, and total gray- and white-matter volumes on MRI.
Dr. Barnes noted that the trial was limited to well-educated, older adults, mostly of European descent. Other limitations include the small sample size of those who received MRI and follow-up that was shorter than a typical observational study.
Dr. Barnes noted that this is a single study and that there needs to be more randomized trials of the MIND diet that, as with the observational research, follow participants for a longer period of time.
More to brain health than diet
Reached for comment, Majid Fotuhi, MD, PhD, adjunct professor of neuroscience at George Washington University, Washington, noted that participants who enroll in clinical trials that focus on diet become more aware of their eating habits and shift toward a healthier diet.
“This may explain the reason why both groups of participants in this study improved,” said Dr. Fotuhi, medical director of NeuroGrow Brain Fitness Center, McLean, Va.
However, he believes that better brain health requires a multipronged approach.
“In order to see significant results, people need to improve their diet, become physically fit, sleep well, reduce their stress, engage in cognitively challenging activities, and develop a positive mind set,” said Dr. Fotuhi.
“Interventions that target only one of these goals may not produce results that are as remarkable as multimodal programs, which target all of these goals,” Dr. Fotuhi said.
Dr. Fotuhi developed a multidimensional “brain fitness program” that has shown to provide multiple benefits for individuals with memory loss, attention deficit hyperactivity disorder, and post-concussion syndrome.
“Having provided our 12-week program for thousands of patients in the past 10 years, I have noticed a synergistic effect in patients who incorporate all of these changes in their day-to-day life and maintain it over time. They often become sharper and feel better overall,” Dr. Fotuhi told this news organization.
The study was supported by the National Institute on Aging. Disclosures for study authors are listed with the original article. Dr. Fotuhi has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAIC 2023
Case report describes pediatric RIME triggered by norovirus
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
How staging laparoscopy informs pancreatic cancer care
TOPLINE:
and prompted a change in management in about one in five patients.
METHODOLOGY:
- The study included 1,004 patients who underwent staging laparoscopy at the Mayo Clinic, Rochester, Minn., from January 2017 to December 2021.
- Patients’ median age was 66 years; 48% of the cohort were female.
- Tumor location was proximal in 644 patients (64%) and distal in 360 patients (36%); median tumor size was 29 mm.
- Upfront resectable disease was present in 351 patients (35%), and borderline resectable or locally advanced anatomy was present in 653 (65%).
TAKEAWAY:
- Overall, 180 patients had a positive staging laparoscopy because of gross metastatic disease (n = 140) and/or positive peritoneal cytology (n = 96); patients who underwent neoadjuvant chemotherapy before staging laparoscopy had lower rates of positive laparoscopy (14% vs. 22%; P = .002).
- When the analysis was restricted to chemo-naive patients who had concurrent peritoneal lavage performed, 95 of 419 patients (23%) had positive laparoscopy.
- Among 721 patients who had a staged procedure with peritoneal washings, 151 (21%) had confirmed metastatic disease; cytology was positive in 96 (13%).
- Among patients with positive staging laparoscopy, median overall survival was 11 months in those with gross metastatic disease and 13 months in those with positive peritoneal cytology only (P = .40).
IN PRACTICE:
“Staging laparoscopy should be considered in the majority of patients prior to resection and/or initiation of neoadjuvant therapy, specifically in patients with high-risk features such as indeterminate extrapancreatic lesions on imaging, young age, large tumor size, distal tumor location, or elevated serum tumor markers,” the authors concluded.
SOURCE:
The study, led by Hallbera Gudmundsdottir, MD, of the Mayo Clinic was published in the Journal of the American College of Surgeons in June.
LIMITATIONS:
Staging laparoscopy may have been performed in higher-risk patients in earlier years of the study. The Mayo Clinic is a high-volume pancreatic surgery center that sees high-risk patients with advances lesions. This study population may not be generalizable to the those at other centers.
DISCLOSURES:
The authors did not disclose any financial interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
and prompted a change in management in about one in five patients.
METHODOLOGY:
- The study included 1,004 patients who underwent staging laparoscopy at the Mayo Clinic, Rochester, Minn., from January 2017 to December 2021.
- Patients’ median age was 66 years; 48% of the cohort were female.
- Tumor location was proximal in 644 patients (64%) and distal in 360 patients (36%); median tumor size was 29 mm.
- Upfront resectable disease was present in 351 patients (35%), and borderline resectable or locally advanced anatomy was present in 653 (65%).
TAKEAWAY:
- Overall, 180 patients had a positive staging laparoscopy because of gross metastatic disease (n = 140) and/or positive peritoneal cytology (n = 96); patients who underwent neoadjuvant chemotherapy before staging laparoscopy had lower rates of positive laparoscopy (14% vs. 22%; P = .002).
- When the analysis was restricted to chemo-naive patients who had concurrent peritoneal lavage performed, 95 of 419 patients (23%) had positive laparoscopy.
- Among 721 patients who had a staged procedure with peritoneal washings, 151 (21%) had confirmed metastatic disease; cytology was positive in 96 (13%).
- Among patients with positive staging laparoscopy, median overall survival was 11 months in those with gross metastatic disease and 13 months in those with positive peritoneal cytology only (P = .40).
IN PRACTICE:
“Staging laparoscopy should be considered in the majority of patients prior to resection and/or initiation of neoadjuvant therapy, specifically in patients with high-risk features such as indeterminate extrapancreatic lesions on imaging, young age, large tumor size, distal tumor location, or elevated serum tumor markers,” the authors concluded.
SOURCE:
The study, led by Hallbera Gudmundsdottir, MD, of the Mayo Clinic was published in the Journal of the American College of Surgeons in June.
LIMITATIONS:
Staging laparoscopy may have been performed in higher-risk patients in earlier years of the study. The Mayo Clinic is a high-volume pancreatic surgery center that sees high-risk patients with advances lesions. This study population may not be generalizable to the those at other centers.
DISCLOSURES:
The authors did not disclose any financial interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
and prompted a change in management in about one in five patients.
METHODOLOGY:
- The study included 1,004 patients who underwent staging laparoscopy at the Mayo Clinic, Rochester, Minn., from January 2017 to December 2021.
- Patients’ median age was 66 years; 48% of the cohort were female.
- Tumor location was proximal in 644 patients (64%) and distal in 360 patients (36%); median tumor size was 29 mm.
- Upfront resectable disease was present in 351 patients (35%), and borderline resectable or locally advanced anatomy was present in 653 (65%).
TAKEAWAY:
- Overall, 180 patients had a positive staging laparoscopy because of gross metastatic disease (n = 140) and/or positive peritoneal cytology (n = 96); patients who underwent neoadjuvant chemotherapy before staging laparoscopy had lower rates of positive laparoscopy (14% vs. 22%; P = .002).
- When the analysis was restricted to chemo-naive patients who had concurrent peritoneal lavage performed, 95 of 419 patients (23%) had positive laparoscopy.
- Among 721 patients who had a staged procedure with peritoneal washings, 151 (21%) had confirmed metastatic disease; cytology was positive in 96 (13%).
- Among patients with positive staging laparoscopy, median overall survival was 11 months in those with gross metastatic disease and 13 months in those with positive peritoneal cytology only (P = .40).
IN PRACTICE:
“Staging laparoscopy should be considered in the majority of patients prior to resection and/or initiation of neoadjuvant therapy, specifically in patients with high-risk features such as indeterminate extrapancreatic lesions on imaging, young age, large tumor size, distal tumor location, or elevated serum tumor markers,” the authors concluded.
SOURCE:
The study, led by Hallbera Gudmundsdottir, MD, of the Mayo Clinic was published in the Journal of the American College of Surgeons in June.
LIMITATIONS:
Staging laparoscopy may have been performed in higher-risk patients in earlier years of the study. The Mayo Clinic is a high-volume pancreatic surgery center that sees high-risk patients with advances lesions. This study population may not be generalizable to the those at other centers.
DISCLOSURES:
The authors did not disclose any financial interests.
A version of this article first appeared on Medscape.com.
Clinical index predicts common postpartum mental health disorders
Developed by Canadian researchers, the easily implementable PMH CAREPLAN index “creates a framework for clinically actionable risk stratification that could assist patients and providers in determining an individual’s level of risk for common postpartum mental health disorders and direct them to appropriate intervention,” wrote a group led by Simone N. Vigod, MD, MSc, head of the department of psychiatry at Women’s College Hospital, Toronto, in the British Journal of Psychiatry.
After giving birth, women are especially vulnerable to major depression, anxiety, PTSD, and obsessive-compulsive disorder, which have a general postpartum prevalence of 7%-20%.
Common PMH disorders are to be distinguished from the more rare but severe PMH disorders such as postpartum psychosis and bipolar disorder, the researchers stressed.
“We know there are interventions that can prevent these disorders, but these seem to work best in people who are at high risk for developing the illnesses, “ Dr. Vigod said. “So, we wanted to be able to determine the level of risk that a person might actually experience them.”
In an ideal world, she continued, physicians might be able to say to a patient: “You have a 50% chance of developing postpartum depression and anxiety, so it may be worth investing your time and resources in a course of preventive psychotherapy.” Or: “You have a 90% chance of developing these disorders, so it might be worth going back on your medications even though you are breastfeeding.” Or: “You have only a 1% chance of developing them, so probably it’s not worthwhile to go back on your medication prophylactically.”
A need for a new assessment tool, akin to the Framingham Risk Score for 10-year cardiovascular events and the FRAX scoring system for 10-year fracture risk, was evident since previous indices based largely on patient self-reporting have had moderate predictive capacity, and have not been adopted in clinical practice, Dr. Vigod and associates noted.
Split-cohort design
Using population-based health administrative data and hospital birth records from Ontario during 2012-2015, Dr. Vigod’s group created and internally validated a predictive model for common PMH disorders in a cohort of 152,362 mothers. They then converted it to a risk index after validation in an additional cohort of 75,772 mothers. The women had delivered live infants during 2012-2014.
A common PMH disorder occurred in 13,608 mothers, while 214,526 were unaffected.
Independently associated PMH variables were many: prenatal care provider, mental health diagnosis history and medications during pregnancy, psychiatric hospital admissions or ED visits, conception type and complications, and apprehension of newborn by child services. Other factors were region of maternal origin, extremes of gestational age at birth, primary maternal language, lactation intention, maternal age, and number of prenatal visits.
Based on a broad span of scores from 0 to 39, 1-year common PMH disorder risk ranged from 1.5% to 40.5%, with an overall 1-year prevalence of 6%, consistent with previous studies. That included 11,262 (5%) mothers with an anxiety or related disorder, 3,392 (1.5%) with a depressive episode, and 1,046 (0.5%) with both. The best trade-off of sensitivity/specificity for risk appeared to be at a screening threshold score of 17 or above.
Risk drivers
PMH-affected mothers were slightly younger than unaffected women (mean age, 29.9 years vs. 30.6 years), more likely to be primiparous (45.2% vs. 42%), and less likely to be recent immigrants (16.7% vs. 27.2%).
They were also more likely to have previously experienced postpartum depression (4.4% vs. 1.4%), any depression (15.3% vs. 4.4%), and any anxiety disorder (13.8% vs. 4.3%).
As to lifestyle, smoking was more common in women with PMH (15.0% vs. 10.2%), as were the use of nonprescribed substances (3% vs. 1.4%) and intimate partner violence in pregnancy (2.7% vs. 1.5%).
In addition, the affected group experienced more pregnancy complications than their unaffected peers (16% vs. 13.9%), preterm birth (8.2% vs. 6.8%), and Apgar scores below 7 at 1 or 5 minutes (10.5% vs. 7.6%).
Low income did not appear to have an impact since just over 20% in either group fell into the lowest neighborhood income quintile.
Commenting on the index but not involved in developing it, LaTasha D. Nelson, MD, an associate professor or medicine and a maternal-fetal medicine specialist at Northwestern Medicine in Chicago, doubted the Canadian model would work as well in the more fragmented U.S. health care system, compared with Canada’s universal model with its large provincial health databases.
She also found the large number of variables and broad score range potentially problematic, especially if the risk threshold is set at less than half the maximum score at 17, at which some low-risk mothers might get screening and perhaps intervention. “Are we going to use up the resources we have for those who might not need help, or are we going to treat someone who really needs it?” she asked.
Another concern is the postpartum timing of assessment. At Dr. Nelson’s center, mothers are checked for mental health at two points during pregnancy and those with higher scores are triaged for further care.
Dr. Nelson was also puzzled by the score-lowering impact of prenatal care given by a nurse practitioner and “other” provider : –5 and –2, respectively, versus +3 for a midwife and +1 for a family doctor. “This may capture more relaxed, easy-going multiparous mothers who felt comfortable turning to an NP,” she said.
It may indeed reflect that the risk level of a person who sees those providers is overall lower, Dr. Vigod agreed. “This is one reason why we would want to see replication of these results in other jurisdictions and by other ways of diagnosis before putting it out into clinical practice.”
As to the score-lowering effect of not speaking English as the primary tongue, Dr. Nelson wondered, “is that because we’re taking better care of mothers who speak the main language and missing those who speak other languages? Are they not getting the same level of interrogation?”
It may be that individuals in these groups were less likely to access mental health care, Dr. Vigod agreed, or it might reflect the so-called healthy immigrant effect or culturally different levels of postpartum support. “It might mean that there are more people who benefit from community-level protective factors in these groups. We know that social support is an important protective factor.”
Despite her reservations about the index, Dr. Nelson said that increasing attention to the pre- and postnatal mental health of mothers is an important part of maternal care. “This is an issue that needs to be recognized.”
The next step, Dr. Vigod said, is to determine whether the index holds up in other populations. “Then, we would want to test it out to see if recommending interventions based on a certain level of risk improves outcomes. At what percentage risk would starting an antidepressant medication result in a reduced risk for postpartum depression or anxiety – 90%, 80%, 70%, or less?”
The study received funding from the Canadian Institutes of Health Research. Data were analyzed by ICES, an independent nonprofit research organization that holds population-based data. Dr. Vigod reported royalties from UpToDate for materials related to depression and pregnancy. Dr. Nelson disclosed no relevant competing interests.
Developed by Canadian researchers, the easily implementable PMH CAREPLAN index “creates a framework for clinically actionable risk stratification that could assist patients and providers in determining an individual’s level of risk for common postpartum mental health disorders and direct them to appropriate intervention,” wrote a group led by Simone N. Vigod, MD, MSc, head of the department of psychiatry at Women’s College Hospital, Toronto, in the British Journal of Psychiatry.
After giving birth, women are especially vulnerable to major depression, anxiety, PTSD, and obsessive-compulsive disorder, which have a general postpartum prevalence of 7%-20%.
Common PMH disorders are to be distinguished from the more rare but severe PMH disorders such as postpartum psychosis and bipolar disorder, the researchers stressed.
“We know there are interventions that can prevent these disorders, but these seem to work best in people who are at high risk for developing the illnesses, “ Dr. Vigod said. “So, we wanted to be able to determine the level of risk that a person might actually experience them.”
In an ideal world, she continued, physicians might be able to say to a patient: “You have a 50% chance of developing postpartum depression and anxiety, so it may be worth investing your time and resources in a course of preventive psychotherapy.” Or: “You have a 90% chance of developing these disorders, so it might be worth going back on your medications even though you are breastfeeding.” Or: “You have only a 1% chance of developing them, so probably it’s not worthwhile to go back on your medication prophylactically.”
A need for a new assessment tool, akin to the Framingham Risk Score for 10-year cardiovascular events and the FRAX scoring system for 10-year fracture risk, was evident since previous indices based largely on patient self-reporting have had moderate predictive capacity, and have not been adopted in clinical practice, Dr. Vigod and associates noted.
Split-cohort design
Using population-based health administrative data and hospital birth records from Ontario during 2012-2015, Dr. Vigod’s group created and internally validated a predictive model for common PMH disorders in a cohort of 152,362 mothers. They then converted it to a risk index after validation in an additional cohort of 75,772 mothers. The women had delivered live infants during 2012-2014.
A common PMH disorder occurred in 13,608 mothers, while 214,526 were unaffected.
Independently associated PMH variables were many: prenatal care provider, mental health diagnosis history and medications during pregnancy, psychiatric hospital admissions or ED visits, conception type and complications, and apprehension of newborn by child services. Other factors were region of maternal origin, extremes of gestational age at birth, primary maternal language, lactation intention, maternal age, and number of prenatal visits.
Based on a broad span of scores from 0 to 39, 1-year common PMH disorder risk ranged from 1.5% to 40.5%, with an overall 1-year prevalence of 6%, consistent with previous studies. That included 11,262 (5%) mothers with an anxiety or related disorder, 3,392 (1.5%) with a depressive episode, and 1,046 (0.5%) with both. The best trade-off of sensitivity/specificity for risk appeared to be at a screening threshold score of 17 or above.
Risk drivers
PMH-affected mothers were slightly younger than unaffected women (mean age, 29.9 years vs. 30.6 years), more likely to be primiparous (45.2% vs. 42%), and less likely to be recent immigrants (16.7% vs. 27.2%).
They were also more likely to have previously experienced postpartum depression (4.4% vs. 1.4%), any depression (15.3% vs. 4.4%), and any anxiety disorder (13.8% vs. 4.3%).
As to lifestyle, smoking was more common in women with PMH (15.0% vs. 10.2%), as were the use of nonprescribed substances (3% vs. 1.4%) and intimate partner violence in pregnancy (2.7% vs. 1.5%).
In addition, the affected group experienced more pregnancy complications than their unaffected peers (16% vs. 13.9%), preterm birth (8.2% vs. 6.8%), and Apgar scores below 7 at 1 or 5 minutes (10.5% vs. 7.6%).
Low income did not appear to have an impact since just over 20% in either group fell into the lowest neighborhood income quintile.
Commenting on the index but not involved in developing it, LaTasha D. Nelson, MD, an associate professor or medicine and a maternal-fetal medicine specialist at Northwestern Medicine in Chicago, doubted the Canadian model would work as well in the more fragmented U.S. health care system, compared with Canada’s universal model with its large provincial health databases.
She also found the large number of variables and broad score range potentially problematic, especially if the risk threshold is set at less than half the maximum score at 17, at which some low-risk mothers might get screening and perhaps intervention. “Are we going to use up the resources we have for those who might not need help, or are we going to treat someone who really needs it?” she asked.
Another concern is the postpartum timing of assessment. At Dr. Nelson’s center, mothers are checked for mental health at two points during pregnancy and those with higher scores are triaged for further care.
Dr. Nelson was also puzzled by the score-lowering impact of prenatal care given by a nurse practitioner and “other” provider : –5 and –2, respectively, versus +3 for a midwife and +1 for a family doctor. “This may capture more relaxed, easy-going multiparous mothers who felt comfortable turning to an NP,” she said.
It may indeed reflect that the risk level of a person who sees those providers is overall lower, Dr. Vigod agreed. “This is one reason why we would want to see replication of these results in other jurisdictions and by other ways of diagnosis before putting it out into clinical practice.”
As to the score-lowering effect of not speaking English as the primary tongue, Dr. Nelson wondered, “is that because we’re taking better care of mothers who speak the main language and missing those who speak other languages? Are they not getting the same level of interrogation?”
It may be that individuals in these groups were less likely to access mental health care, Dr. Vigod agreed, or it might reflect the so-called healthy immigrant effect or culturally different levels of postpartum support. “It might mean that there are more people who benefit from community-level protective factors in these groups. We know that social support is an important protective factor.”
Despite her reservations about the index, Dr. Nelson said that increasing attention to the pre- and postnatal mental health of mothers is an important part of maternal care. “This is an issue that needs to be recognized.”
The next step, Dr. Vigod said, is to determine whether the index holds up in other populations. “Then, we would want to test it out to see if recommending interventions based on a certain level of risk improves outcomes. At what percentage risk would starting an antidepressant medication result in a reduced risk for postpartum depression or anxiety – 90%, 80%, 70%, or less?”
The study received funding from the Canadian Institutes of Health Research. Data were analyzed by ICES, an independent nonprofit research organization that holds population-based data. Dr. Vigod reported royalties from UpToDate for materials related to depression and pregnancy. Dr. Nelson disclosed no relevant competing interests.
Developed by Canadian researchers, the easily implementable PMH CAREPLAN index “creates a framework for clinically actionable risk stratification that could assist patients and providers in determining an individual’s level of risk for common postpartum mental health disorders and direct them to appropriate intervention,” wrote a group led by Simone N. Vigod, MD, MSc, head of the department of psychiatry at Women’s College Hospital, Toronto, in the British Journal of Psychiatry.
After giving birth, women are especially vulnerable to major depression, anxiety, PTSD, and obsessive-compulsive disorder, which have a general postpartum prevalence of 7%-20%.
Common PMH disorders are to be distinguished from the more rare but severe PMH disorders such as postpartum psychosis and bipolar disorder, the researchers stressed.
“We know there are interventions that can prevent these disorders, but these seem to work best in people who are at high risk for developing the illnesses, “ Dr. Vigod said. “So, we wanted to be able to determine the level of risk that a person might actually experience them.”
In an ideal world, she continued, physicians might be able to say to a patient: “You have a 50% chance of developing postpartum depression and anxiety, so it may be worth investing your time and resources in a course of preventive psychotherapy.” Or: “You have a 90% chance of developing these disorders, so it might be worth going back on your medications even though you are breastfeeding.” Or: “You have only a 1% chance of developing them, so probably it’s not worthwhile to go back on your medication prophylactically.”
A need for a new assessment tool, akin to the Framingham Risk Score for 10-year cardiovascular events and the FRAX scoring system for 10-year fracture risk, was evident since previous indices based largely on patient self-reporting have had moderate predictive capacity, and have not been adopted in clinical practice, Dr. Vigod and associates noted.
Split-cohort design
Using population-based health administrative data and hospital birth records from Ontario during 2012-2015, Dr. Vigod’s group created and internally validated a predictive model for common PMH disorders in a cohort of 152,362 mothers. They then converted it to a risk index after validation in an additional cohort of 75,772 mothers. The women had delivered live infants during 2012-2014.
A common PMH disorder occurred in 13,608 mothers, while 214,526 were unaffected.
Independently associated PMH variables were many: prenatal care provider, mental health diagnosis history and medications during pregnancy, psychiatric hospital admissions or ED visits, conception type and complications, and apprehension of newborn by child services. Other factors were region of maternal origin, extremes of gestational age at birth, primary maternal language, lactation intention, maternal age, and number of prenatal visits.
Based on a broad span of scores from 0 to 39, 1-year common PMH disorder risk ranged from 1.5% to 40.5%, with an overall 1-year prevalence of 6%, consistent with previous studies. That included 11,262 (5%) mothers with an anxiety or related disorder, 3,392 (1.5%) with a depressive episode, and 1,046 (0.5%) with both. The best trade-off of sensitivity/specificity for risk appeared to be at a screening threshold score of 17 or above.
Risk drivers
PMH-affected mothers were slightly younger than unaffected women (mean age, 29.9 years vs. 30.6 years), more likely to be primiparous (45.2% vs. 42%), and less likely to be recent immigrants (16.7% vs. 27.2%).
They were also more likely to have previously experienced postpartum depression (4.4% vs. 1.4%), any depression (15.3% vs. 4.4%), and any anxiety disorder (13.8% vs. 4.3%).
As to lifestyle, smoking was more common in women with PMH (15.0% vs. 10.2%), as were the use of nonprescribed substances (3% vs. 1.4%) and intimate partner violence in pregnancy (2.7% vs. 1.5%).
In addition, the affected group experienced more pregnancy complications than their unaffected peers (16% vs. 13.9%), preterm birth (8.2% vs. 6.8%), and Apgar scores below 7 at 1 or 5 minutes (10.5% vs. 7.6%).
Low income did not appear to have an impact since just over 20% in either group fell into the lowest neighborhood income quintile.
Commenting on the index but not involved in developing it, LaTasha D. Nelson, MD, an associate professor or medicine and a maternal-fetal medicine specialist at Northwestern Medicine in Chicago, doubted the Canadian model would work as well in the more fragmented U.S. health care system, compared with Canada’s universal model with its large provincial health databases.
She also found the large number of variables and broad score range potentially problematic, especially if the risk threshold is set at less than half the maximum score at 17, at which some low-risk mothers might get screening and perhaps intervention. “Are we going to use up the resources we have for those who might not need help, or are we going to treat someone who really needs it?” she asked.
Another concern is the postpartum timing of assessment. At Dr. Nelson’s center, mothers are checked for mental health at two points during pregnancy and those with higher scores are triaged for further care.
Dr. Nelson was also puzzled by the score-lowering impact of prenatal care given by a nurse practitioner and “other” provider : –5 and –2, respectively, versus +3 for a midwife and +1 for a family doctor. “This may capture more relaxed, easy-going multiparous mothers who felt comfortable turning to an NP,” she said.
It may indeed reflect that the risk level of a person who sees those providers is overall lower, Dr. Vigod agreed. “This is one reason why we would want to see replication of these results in other jurisdictions and by other ways of diagnosis before putting it out into clinical practice.”
As to the score-lowering effect of not speaking English as the primary tongue, Dr. Nelson wondered, “is that because we’re taking better care of mothers who speak the main language and missing those who speak other languages? Are they not getting the same level of interrogation?”
It may be that individuals in these groups were less likely to access mental health care, Dr. Vigod agreed, or it might reflect the so-called healthy immigrant effect or culturally different levels of postpartum support. “It might mean that there are more people who benefit from community-level protective factors in these groups. We know that social support is an important protective factor.”
Despite her reservations about the index, Dr. Nelson said that increasing attention to the pre- and postnatal mental health of mothers is an important part of maternal care. “This is an issue that needs to be recognized.”
The next step, Dr. Vigod said, is to determine whether the index holds up in other populations. “Then, we would want to test it out to see if recommending interventions based on a certain level of risk improves outcomes. At what percentage risk would starting an antidepressant medication result in a reduced risk for postpartum depression or anxiety – 90%, 80%, 70%, or less?”
The study received funding from the Canadian Institutes of Health Research. Data were analyzed by ICES, an independent nonprofit research organization that holds population-based data. Dr. Vigod reported royalties from UpToDate for materials related to depression and pregnancy. Dr. Nelson disclosed no relevant competing interests.
FROM THE BRITISH JOURNAL OF PSYCHIATRY