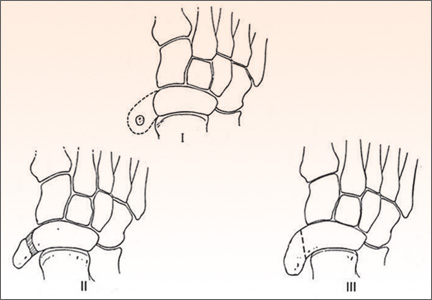
User login
New and Noteworthy Information—March 2014
Patients who are dementia-free but have two parents with late-onset Alzheimer’s disease may show signs of the disease during brain imaging decades before symptoms appear, researchers reported online ahead of print February 12 in Neurology. A total of 52 persons with normal cognition—including four demographically balanced groups with maternal, paternal, and maternal and paternal family history of late-onset Alzheimer’s disease, as well as those with a negative family history—underwent MRI, 11C-Pittsburgh compound B (PiB) PET, and 18F-fluoro-2-deoxyglucose PET. Subjects with both parents with a history of Alzheimer’s disease had more severe abnormalities in all three biomarkers, compared with the other groups, regarding the number of regions affected and magnitude of impairment. PiB retention and hypometabolism were most pronounced in participants with a maternal and paternal history of Alzheimer’s disease, according to the investigators.
Patients with nonvalvular atrial fibrillation are encouraged to take oral anticoagulants to prevent stroke, according to an updated guideline published in the February 25, 2014, issue of Neurology. Treatment with anticoagulants is especially important for people who have already had a stroke or a transient ischemic attack, according to the authors. The current guideline concludes that new anticoagulants such as dabigatran, rivaroxaban, and apixaban are at least as effective as, if not more effective than, warfarin and entail a lower risk of bleeding in the brain. An advantage of the new drugs is that they do not require frequent blood testing as warfarin does. The guideline also recommends new anticoagulants for the elderly, people with mild dementia, and people at moderate risk of falls.
Giving patients medications to lower blood pressure during the first 48 hours after a stroke may not reduce the likelihood of death or major disability, according to research published February 5 in JAMA. Within 48 hours of onset, 4,071 patients with nonthrombolysed ischemic stroke and elevated systolic blood pressure were randomized to receive antihypertensive treatment or to discontinuation of antihypertensive medications. Mean systolic blood pressure was reduced from 166.7 mm Hg to 144.7 mm Hg within 24 hours in the antihypertensive treatment group and from 165.6 mm Hg to 152.9 mm Hg in the control group within 24 hours after randomization. At 14 days or hospital discharge, researchers recorded 683 incidences of death or major disability in the antihypertensive treatment group and 681 incidences in the control group.
The FDA has granted accelerated approval for Northera (droxidopa) capsules for the treatment of neurogenic orthostatic hypotension (NOH) caused by primary autonomic failure (eg, Parkinson’s disease or multiple system atrophy). Accelerated approval is granted to medicines that fill a serious unmet medical need. The capsules have a boxed warning to alert health care professionals and patients about the risk of supine hypertension, which can cause stroke. Two clinical trials involving people with NOH demonstrated droxidopa’s effectiveness over a period of two weeks. The drug, which is manufactured by Chelsea Therapeutics in Charlotte, North Carolina, has not been demonstrated to provide improvement in patient symptoms beyond two weeks. The most common adverse events reported by clinical trial participants taking droxidopa were headache, dizziness, nausea, high blood pressure, and fatigue.
Earlier treatment with an antiepileptic drug (AED) results in a shorter total seizure duration among children with febrile status epilepticus, according to a study published online ahead of print February 6 in Epilepsia. A total of 199 children (ages 1 month to 6 years), were included in the prospective, multicenter study. The median time from seizure onset to first administration of an AED by EMS or emergency department personnel was 30 minutes. The mean seizure duration for children who were given medication before admission to the emergency department was 81 minutes, compared with 95 minutes for those who were not treated beforehand. The median time from first dose of an AED to the end of a seizure was 38 minutes. “Reducing the time from seizure onset to AED initiation was significantly related to shorter seizure duration,” the investigators concluded.
The FDA has granted 510(k) clearance to the Reveal LINQ Insertable Cardiac Monitor (ICM) System. The device is indicated for patients who have symptoms such as dizziness, palpitation, syncope, and chest pain that may suggest a cardiac arrhythmia, and for patients at increased risk for cardiac arrhythmias. The Reveal LINQ ICM is part of a system that allows physicians to monitor a patient’s heart continuously and wirelessly for as long as three years. The system also provides remote monitoring through the Carelink Network, which allows physicians to request notifications to alert them if their patients have had cardiac events. The Reveal LINQ ICM is approximately one-third the size of an AAA battery. The device is manufactured by Medtronic, which is headquartered in Minneapolis.
The final stage of the normal inflammatory process may be disrupted in patients with Alzheimer’s disease, according to research published online ahead of print February 14 in Alzheimer’s and Dementia. Researchers analyzed specialized proresolving mediators (SPMs), receptors, a biosynthetic enzyme, and downstream effectors involved in inflammation resolution in postmortem hippocampal tissue from patients with and without Alzheimer’s disease. SPMs were analyzed in CSF. Levels of the SPM lipoxin A4 (LXA4) were reduced in patients with Alzheimer’s disease in the CSF and the hippocampus. An enzyme involved in LXA4 synthesis and two SPM receptors were elevated in brains of patients with Alzheimer’s disease. LXA4 and RvD1 levels in CSF correlated with Mini-Mental State Examination scores. Stimulation of inflammation resolution may reduce neuronal death in the brain, said the investigators.
Toxic chemicals may be triggering the recent increases in neurodevelopmental disabilities among children, according to a study published in the March issue of Lancet Neurology. In 2006, researchers identified five industrial chemicals as developmental neurotoxicants. The current study offers updated findings about those chemicals and adds information on six newly recognized ones, including manganese, fluoride, chlorpyrifos and DDT (ie, pesticides), tetrachloroethylene (a solvent), and the polybrominated diphenyl ethers (flame retardants). The study found that manganese is associated with diminished intellectual function and impaired motor skills, solvents are linked to hyperactivity and aggressive behavior, and certain pesticides may cause cognitive delays. More neurotoxicants may remain undiscovered, according to the investigators, who propose a global prevention strategy to control what they call a pandemic of developmental neurotoxicity.
For relatives of patients with multiple sclerosis (MS), the risk of developing the disease may be lower than previously assumed, according to a study published in the March issue of Brain. Researchers from Karolinska Institutet assessed the familial risks for MS using population registers and health care registries. They identified 28,396 patients with MS, along with first- and second-degree relatives and cousins. The investigators used matched population-based controls to calculate relative risks and found lower estimates of familial MS risks than previously reported. Despite a well-established lower prevalence of MS among males, the relative risks were equal among maternal and paternal relations. Using 74,757 twin pairs, the researchers estimated the disease’s heritability to be 0.64 and its shared environmental component to be 0.01.
Football helmets differ in their ability to reduce the risk of concussion, researchers reported online ahead of print January 31 in the Journal of Neurosurgery. The investigators conducted a retrospective analysis of head impact data from 1,833 collegiate football players from 2005 to 2010 who wore helmet-mounted accelerometer arrays for games and practices. The researchers compared concussion rates between players who wore the Riddell VSR4 and Riddell Revolution helmets. A total of 1,281,444 head impacts were recorded, and 64 concussions were diagnosed. The investigators found that the relative risk of sustaining a concussion in a Revolution helmet versus a VSR4 helmet was 46.1%. “Although helmet design may never prevent all concussions from occurring in football, evidence illustrates that it can reduce the incidence of this injury,” the researchers concluded.
Women have a worse quality of life, compared with men, for as long as 12 months after a stroke, even after adjustment for key sociodemographic variables, stroke severity, and disability, according to a study published online ahead of print February 7 in Neurology. Researchers assessed the quality of life in 1,370 patients (53.7% male; median age, 65) with ischemic stroke or transient ischemic attack (TIA) at three and 12 months postdischarge. Women had a significantly lower quality of life at three and 12 months poststroke. After multivariable adjustment for sociodemographic, clinical, and stroke-related factors, the investigators found that women continued to have a lower quality of life at three and 12 months. Women also had a poorer outcome in the dimensions of mobility, pain or discomfort, and anxiety or depression at three and 12 months.
High levels of high-density lipoprotein (HDL) cholesterol and low levels of low-density lipoprotein (LDL) cholesterol may be correlated with lower levels of amyloid plaque deposition in the brain, according to a study published in the February issue of JAMA Neurology. Investigators examined 74 individuals age 70 or older, including three participants with mild dementia, 33 cognitively normal participants, and 38 people with mild cognitive impairment. Cerebral amyloid-beta was measured with carbon C11–labeled Pittsburgh Compound B (PiB) PET. Statistical models that controlled for age and APOE ɛ4 revealed independent associations among the levels of LDL cholesterol, HDL cholesterol, and PiB index. Higher LDL cholesterol and lower HDL cholesterol levels were associated with a higher PiB index. The finding suggests an important role for cholesterol in amyloid-beta processing, said the researchers.
Patients with acute ischemic stroke who receive prompt treatment with t-PA may avoid a lengthy stay in an ICU, according to a study published February 12 in PLOS One. In a retrospective chart review of 153 patients who received IV t-PA for stroke, those with an NIH Stroke Scale (NIHSS) score of 10 or higher had a 7.7-times higher risk of requiring ICU resources, compared with patients who presented with an NIHSS score lower than 10. Eighty-one percent of patients with ICU needs developed them before the end of t-PA infusion, while 7% of those without ICU needs at the end of the t-PA infusion required ICU care later on. “We propose that patients without ICU needs by the end of the t-PA infusion might be safely monitored in a non-ICU setting if NIHSS at presentation is low,” the researchers advised.
—Erik Greb and Colby Stong
Patients who are dementia-free but have two parents with late-onset Alzheimer’s disease may show signs of the disease during brain imaging decades before symptoms appear, researchers reported online ahead of print February 12 in Neurology. A total of 52 persons with normal cognition—including four demographically balanced groups with maternal, paternal, and maternal and paternal family history of late-onset Alzheimer’s disease, as well as those with a negative family history—underwent MRI, 11C-Pittsburgh compound B (PiB) PET, and 18F-fluoro-2-deoxyglucose PET. Subjects with both parents with a history of Alzheimer’s disease had more severe abnormalities in all three biomarkers, compared with the other groups, regarding the number of regions affected and magnitude of impairment. PiB retention and hypometabolism were most pronounced in participants with a maternal and paternal history of Alzheimer’s disease, according to the investigators.
Patients with nonvalvular atrial fibrillation are encouraged to take oral anticoagulants to prevent stroke, according to an updated guideline published in the February 25, 2014, issue of Neurology. Treatment with anticoagulants is especially important for people who have already had a stroke or a transient ischemic attack, according to the authors. The current guideline concludes that new anticoagulants such as dabigatran, rivaroxaban, and apixaban are at least as effective as, if not more effective than, warfarin and entail a lower risk of bleeding in the brain. An advantage of the new drugs is that they do not require frequent blood testing as warfarin does. The guideline also recommends new anticoagulants for the elderly, people with mild dementia, and people at moderate risk of falls.
Giving patients medications to lower blood pressure during the first 48 hours after a stroke may not reduce the likelihood of death or major disability, according to research published February 5 in JAMA. Within 48 hours of onset, 4,071 patients with nonthrombolysed ischemic stroke and elevated systolic blood pressure were randomized to receive antihypertensive treatment or to discontinuation of antihypertensive medications. Mean systolic blood pressure was reduced from 166.7 mm Hg to 144.7 mm Hg within 24 hours in the antihypertensive treatment group and from 165.6 mm Hg to 152.9 mm Hg in the control group within 24 hours after randomization. At 14 days or hospital discharge, researchers recorded 683 incidences of death or major disability in the antihypertensive treatment group and 681 incidences in the control group.
The FDA has granted accelerated approval for Northera (droxidopa) capsules for the treatment of neurogenic orthostatic hypotension (NOH) caused by primary autonomic failure (eg, Parkinson’s disease or multiple system atrophy). Accelerated approval is granted to medicines that fill a serious unmet medical need. The capsules have a boxed warning to alert health care professionals and patients about the risk of supine hypertension, which can cause stroke. Two clinical trials involving people with NOH demonstrated droxidopa’s effectiveness over a period of two weeks. The drug, which is manufactured by Chelsea Therapeutics in Charlotte, North Carolina, has not been demonstrated to provide improvement in patient symptoms beyond two weeks. The most common adverse events reported by clinical trial participants taking droxidopa were headache, dizziness, nausea, high blood pressure, and fatigue.
Earlier treatment with an antiepileptic drug (AED) results in a shorter total seizure duration among children with febrile status epilepticus, according to a study published online ahead of print February 6 in Epilepsia. A total of 199 children (ages 1 month to 6 years), were included in the prospective, multicenter study. The median time from seizure onset to first administration of an AED by EMS or emergency department personnel was 30 minutes. The mean seizure duration for children who were given medication before admission to the emergency department was 81 minutes, compared with 95 minutes for those who were not treated beforehand. The median time from first dose of an AED to the end of a seizure was 38 minutes. “Reducing the time from seizure onset to AED initiation was significantly related to shorter seizure duration,” the investigators concluded.
The FDA has granted 510(k) clearance to the Reveal LINQ Insertable Cardiac Monitor (ICM) System. The device is indicated for patients who have symptoms such as dizziness, palpitation, syncope, and chest pain that may suggest a cardiac arrhythmia, and for patients at increased risk for cardiac arrhythmias. The Reveal LINQ ICM is part of a system that allows physicians to monitor a patient’s heart continuously and wirelessly for as long as three years. The system also provides remote monitoring through the Carelink Network, which allows physicians to request notifications to alert them if their patients have had cardiac events. The Reveal LINQ ICM is approximately one-third the size of an AAA battery. The device is manufactured by Medtronic, which is headquartered in Minneapolis.
The final stage of the normal inflammatory process may be disrupted in patients with Alzheimer’s disease, according to research published online ahead of print February 14 in Alzheimer’s and Dementia. Researchers analyzed specialized proresolving mediators (SPMs), receptors, a biosynthetic enzyme, and downstream effectors involved in inflammation resolution in postmortem hippocampal tissue from patients with and without Alzheimer’s disease. SPMs were analyzed in CSF. Levels of the SPM lipoxin A4 (LXA4) were reduced in patients with Alzheimer’s disease in the CSF and the hippocampus. An enzyme involved in LXA4 synthesis and two SPM receptors were elevated in brains of patients with Alzheimer’s disease. LXA4 and RvD1 levels in CSF correlated with Mini-Mental State Examination scores. Stimulation of inflammation resolution may reduce neuronal death in the brain, said the investigators.
Toxic chemicals may be triggering the recent increases in neurodevelopmental disabilities among children, according to a study published in the March issue of Lancet Neurology. In 2006, researchers identified five industrial chemicals as developmental neurotoxicants. The current study offers updated findings about those chemicals and adds information on six newly recognized ones, including manganese, fluoride, chlorpyrifos and DDT (ie, pesticides), tetrachloroethylene (a solvent), and the polybrominated diphenyl ethers (flame retardants). The study found that manganese is associated with diminished intellectual function and impaired motor skills, solvents are linked to hyperactivity and aggressive behavior, and certain pesticides may cause cognitive delays. More neurotoxicants may remain undiscovered, according to the investigators, who propose a global prevention strategy to control what they call a pandemic of developmental neurotoxicity.
For relatives of patients with multiple sclerosis (MS), the risk of developing the disease may be lower than previously assumed, according to a study published in the March issue of Brain. Researchers from Karolinska Institutet assessed the familial risks for MS using population registers and health care registries. They identified 28,396 patients with MS, along with first- and second-degree relatives and cousins. The investigators used matched population-based controls to calculate relative risks and found lower estimates of familial MS risks than previously reported. Despite a well-established lower prevalence of MS among males, the relative risks were equal among maternal and paternal relations. Using 74,757 twin pairs, the researchers estimated the disease’s heritability to be 0.64 and its shared environmental component to be 0.01.
Football helmets differ in their ability to reduce the risk of concussion, researchers reported online ahead of print January 31 in the Journal of Neurosurgery. The investigators conducted a retrospective analysis of head impact data from 1,833 collegiate football players from 2005 to 2010 who wore helmet-mounted accelerometer arrays for games and practices. The researchers compared concussion rates between players who wore the Riddell VSR4 and Riddell Revolution helmets. A total of 1,281,444 head impacts were recorded, and 64 concussions were diagnosed. The investigators found that the relative risk of sustaining a concussion in a Revolution helmet versus a VSR4 helmet was 46.1%. “Although helmet design may never prevent all concussions from occurring in football, evidence illustrates that it can reduce the incidence of this injury,” the researchers concluded.
Women have a worse quality of life, compared with men, for as long as 12 months after a stroke, even after adjustment for key sociodemographic variables, stroke severity, and disability, according to a study published online ahead of print February 7 in Neurology. Researchers assessed the quality of life in 1,370 patients (53.7% male; median age, 65) with ischemic stroke or transient ischemic attack (TIA) at three and 12 months postdischarge. Women had a significantly lower quality of life at three and 12 months poststroke. After multivariable adjustment for sociodemographic, clinical, and stroke-related factors, the investigators found that women continued to have a lower quality of life at three and 12 months. Women also had a poorer outcome in the dimensions of mobility, pain or discomfort, and anxiety or depression at three and 12 months.
High levels of high-density lipoprotein (HDL) cholesterol and low levels of low-density lipoprotein (LDL) cholesterol may be correlated with lower levels of amyloid plaque deposition in the brain, according to a study published in the February issue of JAMA Neurology. Investigators examined 74 individuals age 70 or older, including three participants with mild dementia, 33 cognitively normal participants, and 38 people with mild cognitive impairment. Cerebral amyloid-beta was measured with carbon C11–labeled Pittsburgh Compound B (PiB) PET. Statistical models that controlled for age and APOE ɛ4 revealed independent associations among the levels of LDL cholesterol, HDL cholesterol, and PiB index. Higher LDL cholesterol and lower HDL cholesterol levels were associated with a higher PiB index. The finding suggests an important role for cholesterol in amyloid-beta processing, said the researchers.
Patients with acute ischemic stroke who receive prompt treatment with t-PA may avoid a lengthy stay in an ICU, according to a study published February 12 in PLOS One. In a retrospective chart review of 153 patients who received IV t-PA for stroke, those with an NIH Stroke Scale (NIHSS) score of 10 or higher had a 7.7-times higher risk of requiring ICU resources, compared with patients who presented with an NIHSS score lower than 10. Eighty-one percent of patients with ICU needs developed them before the end of t-PA infusion, while 7% of those without ICU needs at the end of the t-PA infusion required ICU care later on. “We propose that patients without ICU needs by the end of the t-PA infusion might be safely monitored in a non-ICU setting if NIHSS at presentation is low,” the researchers advised.
—Erik Greb and Colby Stong
Patients who are dementia-free but have two parents with late-onset Alzheimer’s disease may show signs of the disease during brain imaging decades before symptoms appear, researchers reported online ahead of print February 12 in Neurology. A total of 52 persons with normal cognition—including four demographically balanced groups with maternal, paternal, and maternal and paternal family history of late-onset Alzheimer’s disease, as well as those with a negative family history—underwent MRI, 11C-Pittsburgh compound B (PiB) PET, and 18F-fluoro-2-deoxyglucose PET. Subjects with both parents with a history of Alzheimer’s disease had more severe abnormalities in all three biomarkers, compared with the other groups, regarding the number of regions affected and magnitude of impairment. PiB retention and hypometabolism were most pronounced in participants with a maternal and paternal history of Alzheimer’s disease, according to the investigators.
Patients with nonvalvular atrial fibrillation are encouraged to take oral anticoagulants to prevent stroke, according to an updated guideline published in the February 25, 2014, issue of Neurology. Treatment with anticoagulants is especially important for people who have already had a stroke or a transient ischemic attack, according to the authors. The current guideline concludes that new anticoagulants such as dabigatran, rivaroxaban, and apixaban are at least as effective as, if not more effective than, warfarin and entail a lower risk of bleeding in the brain. An advantage of the new drugs is that they do not require frequent blood testing as warfarin does. The guideline also recommends new anticoagulants for the elderly, people with mild dementia, and people at moderate risk of falls.
Giving patients medications to lower blood pressure during the first 48 hours after a stroke may not reduce the likelihood of death or major disability, according to research published February 5 in JAMA. Within 48 hours of onset, 4,071 patients with nonthrombolysed ischemic stroke and elevated systolic blood pressure were randomized to receive antihypertensive treatment or to discontinuation of antihypertensive medications. Mean systolic blood pressure was reduced from 166.7 mm Hg to 144.7 mm Hg within 24 hours in the antihypertensive treatment group and from 165.6 mm Hg to 152.9 mm Hg in the control group within 24 hours after randomization. At 14 days or hospital discharge, researchers recorded 683 incidences of death or major disability in the antihypertensive treatment group and 681 incidences in the control group.
The FDA has granted accelerated approval for Northera (droxidopa) capsules for the treatment of neurogenic orthostatic hypotension (NOH) caused by primary autonomic failure (eg, Parkinson’s disease or multiple system atrophy). Accelerated approval is granted to medicines that fill a serious unmet medical need. The capsules have a boxed warning to alert health care professionals and patients about the risk of supine hypertension, which can cause stroke. Two clinical trials involving people with NOH demonstrated droxidopa’s effectiveness over a period of two weeks. The drug, which is manufactured by Chelsea Therapeutics in Charlotte, North Carolina, has not been demonstrated to provide improvement in patient symptoms beyond two weeks. The most common adverse events reported by clinical trial participants taking droxidopa were headache, dizziness, nausea, high blood pressure, and fatigue.
Earlier treatment with an antiepileptic drug (AED) results in a shorter total seizure duration among children with febrile status epilepticus, according to a study published online ahead of print February 6 in Epilepsia. A total of 199 children (ages 1 month to 6 years), were included in the prospective, multicenter study. The median time from seizure onset to first administration of an AED by EMS or emergency department personnel was 30 minutes. The mean seizure duration for children who were given medication before admission to the emergency department was 81 minutes, compared with 95 minutes for those who were not treated beforehand. The median time from first dose of an AED to the end of a seizure was 38 minutes. “Reducing the time from seizure onset to AED initiation was significantly related to shorter seizure duration,” the investigators concluded.
The FDA has granted 510(k) clearance to the Reveal LINQ Insertable Cardiac Monitor (ICM) System. The device is indicated for patients who have symptoms such as dizziness, palpitation, syncope, and chest pain that may suggest a cardiac arrhythmia, and for patients at increased risk for cardiac arrhythmias. The Reveal LINQ ICM is part of a system that allows physicians to monitor a patient’s heart continuously and wirelessly for as long as three years. The system also provides remote monitoring through the Carelink Network, which allows physicians to request notifications to alert them if their patients have had cardiac events. The Reveal LINQ ICM is approximately one-third the size of an AAA battery. The device is manufactured by Medtronic, which is headquartered in Minneapolis.
The final stage of the normal inflammatory process may be disrupted in patients with Alzheimer’s disease, according to research published online ahead of print February 14 in Alzheimer’s and Dementia. Researchers analyzed specialized proresolving mediators (SPMs), receptors, a biosynthetic enzyme, and downstream effectors involved in inflammation resolution in postmortem hippocampal tissue from patients with and without Alzheimer’s disease. SPMs were analyzed in CSF. Levels of the SPM lipoxin A4 (LXA4) were reduced in patients with Alzheimer’s disease in the CSF and the hippocampus. An enzyme involved in LXA4 synthesis and two SPM receptors were elevated in brains of patients with Alzheimer’s disease. LXA4 and RvD1 levels in CSF correlated with Mini-Mental State Examination scores. Stimulation of inflammation resolution may reduce neuronal death in the brain, said the investigators.
Toxic chemicals may be triggering the recent increases in neurodevelopmental disabilities among children, according to a study published in the March issue of Lancet Neurology. In 2006, researchers identified five industrial chemicals as developmental neurotoxicants. The current study offers updated findings about those chemicals and adds information on six newly recognized ones, including manganese, fluoride, chlorpyrifos and DDT (ie, pesticides), tetrachloroethylene (a solvent), and the polybrominated diphenyl ethers (flame retardants). The study found that manganese is associated with diminished intellectual function and impaired motor skills, solvents are linked to hyperactivity and aggressive behavior, and certain pesticides may cause cognitive delays. More neurotoxicants may remain undiscovered, according to the investigators, who propose a global prevention strategy to control what they call a pandemic of developmental neurotoxicity.
For relatives of patients with multiple sclerosis (MS), the risk of developing the disease may be lower than previously assumed, according to a study published in the March issue of Brain. Researchers from Karolinska Institutet assessed the familial risks for MS using population registers and health care registries. They identified 28,396 patients with MS, along with first- and second-degree relatives and cousins. The investigators used matched population-based controls to calculate relative risks and found lower estimates of familial MS risks than previously reported. Despite a well-established lower prevalence of MS among males, the relative risks were equal among maternal and paternal relations. Using 74,757 twin pairs, the researchers estimated the disease’s heritability to be 0.64 and its shared environmental component to be 0.01.
Football helmets differ in their ability to reduce the risk of concussion, researchers reported online ahead of print January 31 in the Journal of Neurosurgery. The investigators conducted a retrospective analysis of head impact data from 1,833 collegiate football players from 2005 to 2010 who wore helmet-mounted accelerometer arrays for games and practices. The researchers compared concussion rates between players who wore the Riddell VSR4 and Riddell Revolution helmets. A total of 1,281,444 head impacts were recorded, and 64 concussions were diagnosed. The investigators found that the relative risk of sustaining a concussion in a Revolution helmet versus a VSR4 helmet was 46.1%. “Although helmet design may never prevent all concussions from occurring in football, evidence illustrates that it can reduce the incidence of this injury,” the researchers concluded.
Women have a worse quality of life, compared with men, for as long as 12 months after a stroke, even after adjustment for key sociodemographic variables, stroke severity, and disability, according to a study published online ahead of print February 7 in Neurology. Researchers assessed the quality of life in 1,370 patients (53.7% male; median age, 65) with ischemic stroke or transient ischemic attack (TIA) at three and 12 months postdischarge. Women had a significantly lower quality of life at three and 12 months poststroke. After multivariable adjustment for sociodemographic, clinical, and stroke-related factors, the investigators found that women continued to have a lower quality of life at three and 12 months. Women also had a poorer outcome in the dimensions of mobility, pain or discomfort, and anxiety or depression at three and 12 months.
High levels of high-density lipoprotein (HDL) cholesterol and low levels of low-density lipoprotein (LDL) cholesterol may be correlated with lower levels of amyloid plaque deposition in the brain, according to a study published in the February issue of JAMA Neurology. Investigators examined 74 individuals age 70 or older, including three participants with mild dementia, 33 cognitively normal participants, and 38 people with mild cognitive impairment. Cerebral amyloid-beta was measured with carbon C11–labeled Pittsburgh Compound B (PiB) PET. Statistical models that controlled for age and APOE ɛ4 revealed independent associations among the levels of LDL cholesterol, HDL cholesterol, and PiB index. Higher LDL cholesterol and lower HDL cholesterol levels were associated with a higher PiB index. The finding suggests an important role for cholesterol in amyloid-beta processing, said the researchers.
Patients with acute ischemic stroke who receive prompt treatment with t-PA may avoid a lengthy stay in an ICU, according to a study published February 12 in PLOS One. In a retrospective chart review of 153 patients who received IV t-PA for stroke, those with an NIH Stroke Scale (NIHSS) score of 10 or higher had a 7.7-times higher risk of requiring ICU resources, compared with patients who presented with an NIHSS score lower than 10. Eighty-one percent of patients with ICU needs developed them before the end of t-PA infusion, while 7% of those without ICU needs at the end of the t-PA infusion required ICU care later on. “We propose that patients without ICU needs by the end of the t-PA infusion might be safely monitored in a non-ICU setting if NIHSS at presentation is low,” the researchers advised.
—Erik Greb and Colby Stong
Keeping ‘good health’ healthy
As providers we are often questioned about healthy habits, working out, and diets, but in the pediatric population, what works for adults may be very harmful to the growing body. Many of the food products that are advertised as "healthy" are nowhere close to healthy.
With childhood obesity on the rise, many parents and teens are looking for ways to lose weight or maintain a healthy lifestyle to avoid obesity. But, many resort to restricted diets that lack important nutrients for proper growth.
Adolescents also are notorious for skipping meals, snacking, and late night eating. Teens in particular resort to starvation to lose weight quickly. This is usually ineffective because most will binge on unhealthy food when they become hungry, negating the effects of the decreased intake (J. Pediatr. Nurs. 2005;20:258-67). In terms of skipping breakfast in particular, a study of schools that participated in a breakfast program showed that there was an increase in math grades and physical performance in children who ate breakfast. Generally, teens who consistently ate breakfast had better nutrition than those who did not.
The use of diet aids and stimulants is another quick weight loss trick that has detrimental side effects. Hydroxycut, diuretics, and amphetamines are just a few of the many substances used. Hydroxycut use has been linked to elevated liver enzymes, jaundice, and seizures. The Food and Drug Administration has urged consumers to stop using this product. Improper use of diuretics can cause electrolyte imbalances, and improper use of some stimulants has been indicated as a cause of sudden death.
Fad diets come out weekly and usually include low-carbohydrate/low-fat or meatless diets. Vegetarian diets also are becoming more popular. The problem with any diet in the adolescent age group is that their bodies are growing, and they actually have a higher demand for certain nutrients, in particular iron, zinc, and calcium.
A typical adolescent diet is low in iron, calcium, folic acid, fiber, and zinc. Low iron intake has been shown to impair cognitive function and physical performance. Low calcium intake increases the risk of fractures and osteoporosis later in life.
Vegetarian diets, depending on how restricted they are, can leave children with significant deficiencies. Children with rapid growth have increased iron needs. Iron from meat sources are more readily absorbed than from plant sources, and iron absorption from plant sources is greatly affected by dietary components. Therefore, there is less absorption of iron if consumed with legumes, nuts, and soy protein. Supplementing the diet with consumption of vitamin C during a meal significantly increases the absorption of iron (Hum. Nutr. Appl. Nutr. 1986;40:97-113). Careful consideration of what food is eliminated in a diet and ensuring its replacement by food substitution or vitamin supplements can prevent deficiencies.
Exercise and weight training are another avenue for weight loss but unfortunately are not used as often because they require discipline and time. But exercising and weight lifting aren’t without risk either. The growing adolescent has open growth plates. Therefore, with intense resistance exercises, there is a risk of injuring the growth plate, which could negatively impact the growth of the affected bone. Encouraging teens to train with supervision and to get accurate instructions are crucial to avoiding injury.
The use of protein shakes is popular among male teens, who are often looking to bulk up. Whey protein is commonly used and is safe when taken in proper amounts and with good hydration. The average teen needs approximately 50 g of protein per day. Excessive protein has been thought to cause kidney disease, but the research does not support this claim. Although lowering protein intake can be beneficial for a person with kidney disease, that does not extrapolate to excessive protein leading to kidney disease. But adequate hydration should be encouraged.
It is always prudent to monitor for eating disorders and the use of illicit drugs to improve physique. In adolescents who seem to be overly competitive or overly obsessed with their appearance, addressing concerns directly, informing parents of your observations, and making the appropriate referrals can prevent significant injury and health consequences.
Here are some general recommendations to help you guide your patients:
• Visit choosemyplate.gov, a comprehensive site that reviews dietary guidelines and individualizes the guidelines based on age, sex, and activity level.
• Educate families on reading food labels so they can make good choices.
• Warn against following restricted diets that could lead to nutritional deficiencies resulting in illness.
• Educate about the danger of weight loss drugs and their risks.
• Advise families that strength training can be effective but only when well designed and supervised to avoid injury.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Scan this QR code to view similar columns.
As providers we are often questioned about healthy habits, working out, and diets, but in the pediatric population, what works for adults may be very harmful to the growing body. Many of the food products that are advertised as "healthy" are nowhere close to healthy.
With childhood obesity on the rise, many parents and teens are looking for ways to lose weight or maintain a healthy lifestyle to avoid obesity. But, many resort to restricted diets that lack important nutrients for proper growth.
Adolescents also are notorious for skipping meals, snacking, and late night eating. Teens in particular resort to starvation to lose weight quickly. This is usually ineffective because most will binge on unhealthy food when they become hungry, negating the effects of the decreased intake (J. Pediatr. Nurs. 2005;20:258-67). In terms of skipping breakfast in particular, a study of schools that participated in a breakfast program showed that there was an increase in math grades and physical performance in children who ate breakfast. Generally, teens who consistently ate breakfast had better nutrition than those who did not.
The use of diet aids and stimulants is another quick weight loss trick that has detrimental side effects. Hydroxycut, diuretics, and amphetamines are just a few of the many substances used. Hydroxycut use has been linked to elevated liver enzymes, jaundice, and seizures. The Food and Drug Administration has urged consumers to stop using this product. Improper use of diuretics can cause electrolyte imbalances, and improper use of some stimulants has been indicated as a cause of sudden death.
Fad diets come out weekly and usually include low-carbohydrate/low-fat or meatless diets. Vegetarian diets also are becoming more popular. The problem with any diet in the adolescent age group is that their bodies are growing, and they actually have a higher demand for certain nutrients, in particular iron, zinc, and calcium.
A typical adolescent diet is low in iron, calcium, folic acid, fiber, and zinc. Low iron intake has been shown to impair cognitive function and physical performance. Low calcium intake increases the risk of fractures and osteoporosis later in life.
Vegetarian diets, depending on how restricted they are, can leave children with significant deficiencies. Children with rapid growth have increased iron needs. Iron from meat sources are more readily absorbed than from plant sources, and iron absorption from plant sources is greatly affected by dietary components. Therefore, there is less absorption of iron if consumed with legumes, nuts, and soy protein. Supplementing the diet with consumption of vitamin C during a meal significantly increases the absorption of iron (Hum. Nutr. Appl. Nutr. 1986;40:97-113). Careful consideration of what food is eliminated in a diet and ensuring its replacement by food substitution or vitamin supplements can prevent deficiencies.
Exercise and weight training are another avenue for weight loss but unfortunately are not used as often because they require discipline and time. But exercising and weight lifting aren’t without risk either. The growing adolescent has open growth plates. Therefore, with intense resistance exercises, there is a risk of injuring the growth plate, which could negatively impact the growth of the affected bone. Encouraging teens to train with supervision and to get accurate instructions are crucial to avoiding injury.
The use of protein shakes is popular among male teens, who are often looking to bulk up. Whey protein is commonly used and is safe when taken in proper amounts and with good hydration. The average teen needs approximately 50 g of protein per day. Excessive protein has been thought to cause kidney disease, but the research does not support this claim. Although lowering protein intake can be beneficial for a person with kidney disease, that does not extrapolate to excessive protein leading to kidney disease. But adequate hydration should be encouraged.
It is always prudent to monitor for eating disorders and the use of illicit drugs to improve physique. In adolescents who seem to be overly competitive or overly obsessed with their appearance, addressing concerns directly, informing parents of your observations, and making the appropriate referrals can prevent significant injury and health consequences.
Here are some general recommendations to help you guide your patients:
• Visit choosemyplate.gov, a comprehensive site that reviews dietary guidelines and individualizes the guidelines based on age, sex, and activity level.
• Educate families on reading food labels so they can make good choices.
• Warn against following restricted diets that could lead to nutritional deficiencies resulting in illness.
• Educate about the danger of weight loss drugs and their risks.
• Advise families that strength training can be effective but only when well designed and supervised to avoid injury.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Scan this QR code to view similar columns.
As providers we are often questioned about healthy habits, working out, and diets, but in the pediatric population, what works for adults may be very harmful to the growing body. Many of the food products that are advertised as "healthy" are nowhere close to healthy.
With childhood obesity on the rise, many parents and teens are looking for ways to lose weight or maintain a healthy lifestyle to avoid obesity. But, many resort to restricted diets that lack important nutrients for proper growth.
Adolescents also are notorious for skipping meals, snacking, and late night eating. Teens in particular resort to starvation to lose weight quickly. This is usually ineffective because most will binge on unhealthy food when they become hungry, negating the effects of the decreased intake (J. Pediatr. Nurs. 2005;20:258-67). In terms of skipping breakfast in particular, a study of schools that participated in a breakfast program showed that there was an increase in math grades and physical performance in children who ate breakfast. Generally, teens who consistently ate breakfast had better nutrition than those who did not.
The use of diet aids and stimulants is another quick weight loss trick that has detrimental side effects. Hydroxycut, diuretics, and amphetamines are just a few of the many substances used. Hydroxycut use has been linked to elevated liver enzymes, jaundice, and seizures. The Food and Drug Administration has urged consumers to stop using this product. Improper use of diuretics can cause electrolyte imbalances, and improper use of some stimulants has been indicated as a cause of sudden death.
Fad diets come out weekly and usually include low-carbohydrate/low-fat or meatless diets. Vegetarian diets also are becoming more popular. The problem with any diet in the adolescent age group is that their bodies are growing, and they actually have a higher demand for certain nutrients, in particular iron, zinc, and calcium.
A typical adolescent diet is low in iron, calcium, folic acid, fiber, and zinc. Low iron intake has been shown to impair cognitive function and physical performance. Low calcium intake increases the risk of fractures and osteoporosis later in life.
Vegetarian diets, depending on how restricted they are, can leave children with significant deficiencies. Children with rapid growth have increased iron needs. Iron from meat sources are more readily absorbed than from plant sources, and iron absorption from plant sources is greatly affected by dietary components. Therefore, there is less absorption of iron if consumed with legumes, nuts, and soy protein. Supplementing the diet with consumption of vitamin C during a meal significantly increases the absorption of iron (Hum. Nutr. Appl. Nutr. 1986;40:97-113). Careful consideration of what food is eliminated in a diet and ensuring its replacement by food substitution or vitamin supplements can prevent deficiencies.
Exercise and weight training are another avenue for weight loss but unfortunately are not used as often because they require discipline and time. But exercising and weight lifting aren’t without risk either. The growing adolescent has open growth plates. Therefore, with intense resistance exercises, there is a risk of injuring the growth plate, which could negatively impact the growth of the affected bone. Encouraging teens to train with supervision and to get accurate instructions are crucial to avoiding injury.
The use of protein shakes is popular among male teens, who are often looking to bulk up. Whey protein is commonly used and is safe when taken in proper amounts and with good hydration. The average teen needs approximately 50 g of protein per day. Excessive protein has been thought to cause kidney disease, but the research does not support this claim. Although lowering protein intake can be beneficial for a person with kidney disease, that does not extrapolate to excessive protein leading to kidney disease. But adequate hydration should be encouraged.
It is always prudent to monitor for eating disorders and the use of illicit drugs to improve physique. In adolescents who seem to be overly competitive or overly obsessed with their appearance, addressing concerns directly, informing parents of your observations, and making the appropriate referrals can prevent significant injury and health consequences.
Here are some general recommendations to help you guide your patients:
• Visit choosemyplate.gov, a comprehensive site that reviews dietary guidelines and individualizes the guidelines based on age, sex, and activity level.
• Educate families on reading food labels so they can make good choices.
• Warn against following restricted diets that could lead to nutritional deficiencies resulting in illness.
• Educate about the danger of weight loss drugs and their risks.
• Advise families that strength training can be effective but only when well designed and supervised to avoid injury.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Scan this QR code to view similar columns.
Drugs get orphan designation for AML, MM

Credit: FDA
The US Food and Drug Administration (FDA) has granted orphan designation for the histone deacetylase (HDAC) inhibitor pracinostat to treat acute myeloid leukemia (AML) and the proteasome inhibitor marizomib to treat multiple myeloma (MM).
Orphan designation is available for drugs that treat or prevent rare diseases affecting fewer than 200,000 people in the US.
The designation qualifies the sponsor of a drug for development incentives, including tax credits for qualified clinical testing, prescription drug user fee exemptions, and 7-year marketing exclusivity upon FDA approval.
About pracinostat
The oral HDAC inhibitor pracinostat has been tested in phase 1 and 2 trials of adult and pediatric patients with advanced hematologic disorders and solid tumors.
The drug has been generally well tolerated in more than 200 patients to date, according to the drug’s maker, MEI Pharma.
In a dose-escalation phase 1 trial, pracinostat demonstrated single-agent activity in elderly AML patients. Two of 14 patients (14%) achieved a complete remission, with responses persisting more than 206 days and 362 days.
Researchers are currently conducting a phase 2 trial of pracinostat in combination with azacitidine in elderly patients with newly diagnosed AML. Preliminary data from this trial are expected to be available by December 2014.
About marizomib
The proteasome inhibitor marizomib is under development for the treatment of MM and other malignancies.
Intravenous marizomib has been evaluated in more than 230 patients across 4 phase 1/2 studies, as a single agent or in combination with dexamethasone or an HDAC inhibitor.
Researchers are currently evaluating marizomib in combination with dexamethasone in an ongoing phase 2 trial of highly refractory MM patients, including those who are refractory to carfilzomib.
Marizomib is also being tested in combination with pomalidomide and dexamethasone in a phase 1/2 study of patients with relapsed and refractory MM.
The drug’s maker, Triphase Accelerator Corporation, is currently developing an oral formulation of marizomib. ![]()

Credit: FDA
The US Food and Drug Administration (FDA) has granted orphan designation for the histone deacetylase (HDAC) inhibitor pracinostat to treat acute myeloid leukemia (AML) and the proteasome inhibitor marizomib to treat multiple myeloma (MM).
Orphan designation is available for drugs that treat or prevent rare diseases affecting fewer than 200,000 people in the US.
The designation qualifies the sponsor of a drug for development incentives, including tax credits for qualified clinical testing, prescription drug user fee exemptions, and 7-year marketing exclusivity upon FDA approval.
About pracinostat
The oral HDAC inhibitor pracinostat has been tested in phase 1 and 2 trials of adult and pediatric patients with advanced hematologic disorders and solid tumors.
The drug has been generally well tolerated in more than 200 patients to date, according to the drug’s maker, MEI Pharma.
In a dose-escalation phase 1 trial, pracinostat demonstrated single-agent activity in elderly AML patients. Two of 14 patients (14%) achieved a complete remission, with responses persisting more than 206 days and 362 days.
Researchers are currently conducting a phase 2 trial of pracinostat in combination with azacitidine in elderly patients with newly diagnosed AML. Preliminary data from this trial are expected to be available by December 2014.
About marizomib
The proteasome inhibitor marizomib is under development for the treatment of MM and other malignancies.
Intravenous marizomib has been evaluated in more than 230 patients across 4 phase 1/2 studies, as a single agent or in combination with dexamethasone or an HDAC inhibitor.
Researchers are currently evaluating marizomib in combination with dexamethasone in an ongoing phase 2 trial of highly refractory MM patients, including those who are refractory to carfilzomib.
Marizomib is also being tested in combination with pomalidomide and dexamethasone in a phase 1/2 study of patients with relapsed and refractory MM.
The drug’s maker, Triphase Accelerator Corporation, is currently developing an oral formulation of marizomib. ![]()

Credit: FDA
The US Food and Drug Administration (FDA) has granted orphan designation for the histone deacetylase (HDAC) inhibitor pracinostat to treat acute myeloid leukemia (AML) and the proteasome inhibitor marizomib to treat multiple myeloma (MM).
Orphan designation is available for drugs that treat or prevent rare diseases affecting fewer than 200,000 people in the US.
The designation qualifies the sponsor of a drug for development incentives, including tax credits for qualified clinical testing, prescription drug user fee exemptions, and 7-year marketing exclusivity upon FDA approval.
About pracinostat
The oral HDAC inhibitor pracinostat has been tested in phase 1 and 2 trials of adult and pediatric patients with advanced hematologic disorders and solid tumors.
The drug has been generally well tolerated in more than 200 patients to date, according to the drug’s maker, MEI Pharma.
In a dose-escalation phase 1 trial, pracinostat demonstrated single-agent activity in elderly AML patients. Two of 14 patients (14%) achieved a complete remission, with responses persisting more than 206 days and 362 days.
Researchers are currently conducting a phase 2 trial of pracinostat in combination with azacitidine in elderly patients with newly diagnosed AML. Preliminary data from this trial are expected to be available by December 2014.
About marizomib
The proteasome inhibitor marizomib is under development for the treatment of MM and other malignancies.
Intravenous marizomib has been evaluated in more than 230 patients across 4 phase 1/2 studies, as a single agent or in combination with dexamethasone or an HDAC inhibitor.
Researchers are currently evaluating marizomib in combination with dexamethasone in an ongoing phase 2 trial of highly refractory MM patients, including those who are refractory to carfilzomib.
Marizomib is also being tested in combination with pomalidomide and dexamethasone in a phase 1/2 study of patients with relapsed and refractory MM.
The drug’s maker, Triphase Accelerator Corporation, is currently developing an oral formulation of marizomib. ![]()
Neutropenia prophylaxis, incidence increase with age

Credit: Volker Brinkmann
New research suggests that older non-Hodgkin lymphoma patients are more likely than their younger counterparts to receive prophylaxis for neutropenia, but the older patients still have a higher incidence of severe neutropenia.
Most of the patients studied had received granulocyte colony-stimulating factor (G-CSF) as neutropenia prophylaxis, but more than half of patients in each age group developed grade 3/4 neutropenia.
And although patients aged 65 and older were more likely to receive G-CSF, they had a higher incidence of grade 3/4 neutropenia than patients who were younger than 65.
Lee S. Schwartzberg, MD, of The West Clinic in Memphis, Tennessee, and his colleagues reported these findings in Supportive Care in Cancer.
The researchers conducted a review of 1579 patients with non-Hodgkin lymphoma. Nearly 46% of patients were 65 years of age or older, and 54.1% were younger than 65.
Most patients had received treatment with R-CHOP every 3 weeks. And the dose levels were about the same in both groups of patients. The mean relative dose intensity was 80.4% among younger patients and 73.9% among the older patients.
The incidence of treatment delays was similar between the 2 groups—24.6% among the older patients and 26.5% among the younger patients.
But older patients were more likely to experience dose reductions—24.9% compared to 9.6% of younger patients.
A majority of all patients—86.9%—received G-CSF, but older patients were more likely to receive it upfront.
Among the older patients, 80.1% received G-CSF as primary prophylaxis, 11.6% received it as secondary prophylaxis, and 8.3% received it as treatment.
Among the younger patients, 71.9% received G-CSF as primary prophylaxis, 17.4% received it as secondary prophylaxis, and 10.7% received it as treatment.
The incidence of grade 3/4 neutropenia was 52.3% for the younger patients and 63.2% for the older patients. ![]()

Credit: Volker Brinkmann
New research suggests that older non-Hodgkin lymphoma patients are more likely than their younger counterparts to receive prophylaxis for neutropenia, but the older patients still have a higher incidence of severe neutropenia.
Most of the patients studied had received granulocyte colony-stimulating factor (G-CSF) as neutropenia prophylaxis, but more than half of patients in each age group developed grade 3/4 neutropenia.
And although patients aged 65 and older were more likely to receive G-CSF, they had a higher incidence of grade 3/4 neutropenia than patients who were younger than 65.
Lee S. Schwartzberg, MD, of The West Clinic in Memphis, Tennessee, and his colleagues reported these findings in Supportive Care in Cancer.
The researchers conducted a review of 1579 patients with non-Hodgkin lymphoma. Nearly 46% of patients were 65 years of age or older, and 54.1% were younger than 65.
Most patients had received treatment with R-CHOP every 3 weeks. And the dose levels were about the same in both groups of patients. The mean relative dose intensity was 80.4% among younger patients and 73.9% among the older patients.
The incidence of treatment delays was similar between the 2 groups—24.6% among the older patients and 26.5% among the younger patients.
But older patients were more likely to experience dose reductions—24.9% compared to 9.6% of younger patients.
A majority of all patients—86.9%—received G-CSF, but older patients were more likely to receive it upfront.
Among the older patients, 80.1% received G-CSF as primary prophylaxis, 11.6% received it as secondary prophylaxis, and 8.3% received it as treatment.
Among the younger patients, 71.9% received G-CSF as primary prophylaxis, 17.4% received it as secondary prophylaxis, and 10.7% received it as treatment.
The incidence of grade 3/4 neutropenia was 52.3% for the younger patients and 63.2% for the older patients. ![]()

Credit: Volker Brinkmann
New research suggests that older non-Hodgkin lymphoma patients are more likely than their younger counterparts to receive prophylaxis for neutropenia, but the older patients still have a higher incidence of severe neutropenia.
Most of the patients studied had received granulocyte colony-stimulating factor (G-CSF) as neutropenia prophylaxis, but more than half of patients in each age group developed grade 3/4 neutropenia.
And although patients aged 65 and older were more likely to receive G-CSF, they had a higher incidence of grade 3/4 neutropenia than patients who were younger than 65.
Lee S. Schwartzberg, MD, of The West Clinic in Memphis, Tennessee, and his colleagues reported these findings in Supportive Care in Cancer.
The researchers conducted a review of 1579 patients with non-Hodgkin lymphoma. Nearly 46% of patients were 65 years of age or older, and 54.1% were younger than 65.
Most patients had received treatment with R-CHOP every 3 weeks. And the dose levels were about the same in both groups of patients. The mean relative dose intensity was 80.4% among younger patients and 73.9% among the older patients.
The incidence of treatment delays was similar between the 2 groups—24.6% among the older patients and 26.5% among the younger patients.
But older patients were more likely to experience dose reductions—24.9% compared to 9.6% of younger patients.
A majority of all patients—86.9%—received G-CSF, but older patients were more likely to receive it upfront.
Among the older patients, 80.1% received G-CSF as primary prophylaxis, 11.6% received it as secondary prophylaxis, and 8.3% received it as treatment.
Among the younger patients, 71.9% received G-CSF as primary prophylaxis, 17.4% received it as secondary prophylaxis, and 10.7% received it as treatment.
The incidence of grade 3/4 neutropenia was 52.3% for the younger patients and 63.2% for the older patients. ![]()
Bacteria may protect against GVHD-related mortality
GRAPEVINE, TEXAS—Intestinal bacteria can offer protection from death related to graft-vs-host disease (GVHD), according to research presented at the 2014 BMT Tandem Meetings.
Experiments showed that Blautia, commensal bacteria found in the intestinal tract, can protect against GVHD-related mortality in mice and in humans.
So efforts to support Blautia survival—such as restricting the use of antibiotics and promoting better nutrition—may
prevent GVHD-related death, according to researchers.
Robert Jenq, MD, of Memorial Sloan-Kettering Cancer Center in New York, discussed this possibility when presenting this research, which was designated one of the “Best Abstracts” at the meeting (abstract 1*).
Dr Jenq noted that researchers have been trying for decades to determine whether the intestinal flora impact GVHD. Clinical studies have suggested that prophylaxis against anaerobes and gram-positive bacteria can reduce GVHD.
And murine studies have indicated that prophylaxis against gram-negative bacteria can reduce GVHD, that Lactobacillus can reduce GVHD, and that donor microbiota do not impact GVHD.
“If you’re confused, so are we,” Dr Jenq said. “It seems like it’s a mixed picture.”
So he and his colleagues conducted a series of experiments in an attempt to determine if any bacterial subgroups impact the risk of gut GVHD in mice and humans.
Bacteria seem to impact GVHD
The researchers first studied 76 adult transplant patients, analyzing stool samples taken at roughly 10 days after transplant (+/- 4 days). The team performed 16S gene sequencing using the Roche 454 platform.
This revealed the presence of several types of bacteria, including 6 gram-positive Firmicutes, 2 gram-negative Proteobacteria, and 2 gram-negative Bacteroidetes.
The researchers then used a computational assay to determine which of these bacteria might be associated with protection from GVHD. And they identified 2 possibilities—Lactobacillus and Blautia.
Additional analyses revealed that Blautia and Lactobacillus were significantly associated with GVHD-related mortality at 1500 days after transplant (P=0.03 and 0.01, respectively). But there was no significant association with Bacteroides (P=0.6), Enterobacteriales (P=0.2), or Enterococcus (P=0.3).
Blautia appears to affect GVHD-related mortality
To confirm their initial findings, Dr Jenq and his colleagues analyzed a second cohort of 50 adult transplant patients. The team analyzed stool samples for the abundance of bacterial subgroups using a different sequencing platform, Illumina miseq.
This time, they found that Blautia abundance predicted GVHD-related mortality at more than 500 days after transplant, but the abundance of Lactobacillus did not (P=0.01 and 1, respectively).
“Not enough Blautia in your gut seems to lead to an increase in GVHD-related mortality,” Dr Jenq said. “So what does this do to overall survival? In the first cohort, there’s a big difference in overall survival between the ‘haves’ and ‘have nots’ with Blautia [P=0.0008]. And this also holds up in the second cohort [P=0.04].”
Further analyses of data from both cohorts suggested that Blautia abundance was associated with GVHD-related mortality (P=0.004) and relapse-related mortality (P=0.01) but not non-relapse- and non-GVHD-related mortality (P=0.4).
“I don’t have a good explanation for [the relationship between Blautia and relapse-related death],” Dr Jenq said. “This was a surprise finding.”
The researchers also looked at Blautia’s ability to predict GVHD-related mortality. They found that, around day 10 after transplant, Blautia abundance predicts “very strongly” for GVHD-related death.
Another question was whether known GVHD risk factors—such as donor type, race, gender, and performance status—impact Blautia abundance. But an analysis revealed that Blautia is an independent risk factor for GVHD-related mortality.
A possible mechanism
To gain more insight into the association between Blautia and GVHD-related death, Dr Jenq and his colleagues decided to study it in mice.
The team killed off Blautia in mice using vancomycin and ampicillin, then introduced either murine Blautia or murine Enterococcus, transplanted the mice with MHC-disparate T cells, and monitored them for GVHD.
Mice that received Blautia had significantly better overall survival (at more than 80 days after transplant) than mice that received Enterococcus (P<0.001).
“So how is this happening?” Dr Jenq asked. “We think, potentially, it might be due to short-chain fatty acids . . . butyric acid, propionic acid, and acetic acid. These are metabolites that bacteria produce when they ferment glucose and other sugars.”
To test this theory, the researchers treated mice with antibiotics and introduced Blautia or Enterococcus.
Blautia increased the level of short-chain fatty acids (butyrate and propionate) when compared to Enterococcus, although levels were not as high as those observed in mice that did not receive antibiotics. Nevertheless, these results point to a possible mechanism, according to Dr Jenq.
Explaining Blautia reduction
Dr Jenq also noted that antibiotics may contribute to the decrease in Blautia observed in transplant patients. When patients come in for transplant, they often have more than 25% Blautia in their stool. But the bacteria decrease to negligible levels by day 2 after transplant.
To determine the role of antibiotics, the researchers treated mice with 4 different antibiotics and looked at the levels of different bacteria.
They found that aztreonam and cefepime increased the levels of Bacteroidales and Clostridiales (the family to which Blautia belongs), but imipenem and metronidazole decreased bacteria levels.
So antibiotics do affect Blautia levels, Dr Jenq said, but they’re only part of the problem. He noted that patients’ Blautia levels start to decrease before antibiotics are administered. So he and his colleagues believe nutrition might also play a part.
The team found a significant difference in Blautia abundance between patients who received total parenteral nutrition and those who did not (P<0.001).
The researchers also discovered that reduced caloric intake led to a loss of Blautia and other Clostridiales. They analyzed 50 samples from 5 patients and found that patients who consumed fewer than 500 calories had a marked reduction in Blautia (P<0.0001).
And experiments in mice confirmed this association. A week of calorie restriction significantly reduced the abundance of Blautia and other Clostridiales (P=0.0002).
“In GVHD, as we all know, patients and mice eat less because of the nausea,” Dr Jenq said. “And we found that GVHD itself can also lead to a reduction in Clostridiales, both in humans [P=0.02] and in mice [P=0.01].”
Protecting Blautia to prevent GVHD
Having confirmed the role of nutrition in Blautia reduction, the researchers set out to identify a nutrition-based intervention to support Blautia in transplant recipients.
They settled on a sugar called raffinose, which is found in beans, cruciferous vegetables, and whole grains. It passes undigested through the upper intestine but is fermented in the lower intestine and metabolized to produce short-chain fatty acids.
The team tested raffinose in mice by introducing it into their drinking water. At 100 days after transplant, mice that received raffinose had significantly better overall survival than controls (P<0.001).
Based on these results, Dr Jenq and his colleagues believe nutritional intervention can protect Blautia and, therefore, may prevent GVHD and related death. The team thinks encouraging eating, gastric nutritional supplementation, and flora-targeted nutritional supplements might all prove effective.
But other interventions might work as well, such as reintroducing endogenous flora (via autologous fecal microbiota transplant), reintroducing select bacteria with beneficial potential, selecting antibiotics that spare bacteria with beneficial potential, and identifying and introducing bacterial metabolites that mediate anti-inflammatory effects. ![]()
*Data in the abstract differ from data presented.
GRAPEVINE, TEXAS—Intestinal bacteria can offer protection from death related to graft-vs-host disease (GVHD), according to research presented at the 2014 BMT Tandem Meetings.
Experiments showed that Blautia, commensal bacteria found in the intestinal tract, can protect against GVHD-related mortality in mice and in humans.
So efforts to support Blautia survival—such as restricting the use of antibiotics and promoting better nutrition—may
prevent GVHD-related death, according to researchers.
Robert Jenq, MD, of Memorial Sloan-Kettering Cancer Center in New York, discussed this possibility when presenting this research, which was designated one of the “Best Abstracts” at the meeting (abstract 1*).
Dr Jenq noted that researchers have been trying for decades to determine whether the intestinal flora impact GVHD. Clinical studies have suggested that prophylaxis against anaerobes and gram-positive bacteria can reduce GVHD.
And murine studies have indicated that prophylaxis against gram-negative bacteria can reduce GVHD, that Lactobacillus can reduce GVHD, and that donor microbiota do not impact GVHD.
“If you’re confused, so are we,” Dr Jenq said. “It seems like it’s a mixed picture.”
So he and his colleagues conducted a series of experiments in an attempt to determine if any bacterial subgroups impact the risk of gut GVHD in mice and humans.
Bacteria seem to impact GVHD
The researchers first studied 76 adult transplant patients, analyzing stool samples taken at roughly 10 days after transplant (+/- 4 days). The team performed 16S gene sequencing using the Roche 454 platform.
This revealed the presence of several types of bacteria, including 6 gram-positive Firmicutes, 2 gram-negative Proteobacteria, and 2 gram-negative Bacteroidetes.
The researchers then used a computational assay to determine which of these bacteria might be associated with protection from GVHD. And they identified 2 possibilities—Lactobacillus and Blautia.
Additional analyses revealed that Blautia and Lactobacillus were significantly associated with GVHD-related mortality at 1500 days after transplant (P=0.03 and 0.01, respectively). But there was no significant association with Bacteroides (P=0.6), Enterobacteriales (P=0.2), or Enterococcus (P=0.3).
Blautia appears to affect GVHD-related mortality
To confirm their initial findings, Dr Jenq and his colleagues analyzed a second cohort of 50 adult transplant patients. The team analyzed stool samples for the abundance of bacterial subgroups using a different sequencing platform, Illumina miseq.
This time, they found that Blautia abundance predicted GVHD-related mortality at more than 500 days after transplant, but the abundance of Lactobacillus did not (P=0.01 and 1, respectively).
“Not enough Blautia in your gut seems to lead to an increase in GVHD-related mortality,” Dr Jenq said. “So what does this do to overall survival? In the first cohort, there’s a big difference in overall survival between the ‘haves’ and ‘have nots’ with Blautia [P=0.0008]. And this also holds up in the second cohort [P=0.04].”
Further analyses of data from both cohorts suggested that Blautia abundance was associated with GVHD-related mortality (P=0.004) and relapse-related mortality (P=0.01) but not non-relapse- and non-GVHD-related mortality (P=0.4).
“I don’t have a good explanation for [the relationship between Blautia and relapse-related death],” Dr Jenq said. “This was a surprise finding.”
The researchers also looked at Blautia’s ability to predict GVHD-related mortality. They found that, around day 10 after transplant, Blautia abundance predicts “very strongly” for GVHD-related death.
Another question was whether known GVHD risk factors—such as donor type, race, gender, and performance status—impact Blautia abundance. But an analysis revealed that Blautia is an independent risk factor for GVHD-related mortality.
A possible mechanism
To gain more insight into the association between Blautia and GVHD-related death, Dr Jenq and his colleagues decided to study it in mice.
The team killed off Blautia in mice using vancomycin and ampicillin, then introduced either murine Blautia or murine Enterococcus, transplanted the mice with MHC-disparate T cells, and monitored them for GVHD.
Mice that received Blautia had significantly better overall survival (at more than 80 days after transplant) than mice that received Enterococcus (P<0.001).
“So how is this happening?” Dr Jenq asked. “We think, potentially, it might be due to short-chain fatty acids . . . butyric acid, propionic acid, and acetic acid. These are metabolites that bacteria produce when they ferment glucose and other sugars.”
To test this theory, the researchers treated mice with antibiotics and introduced Blautia or Enterococcus.
Blautia increased the level of short-chain fatty acids (butyrate and propionate) when compared to Enterococcus, although levels were not as high as those observed in mice that did not receive antibiotics. Nevertheless, these results point to a possible mechanism, according to Dr Jenq.
Explaining Blautia reduction
Dr Jenq also noted that antibiotics may contribute to the decrease in Blautia observed in transplant patients. When patients come in for transplant, they often have more than 25% Blautia in their stool. But the bacteria decrease to negligible levels by day 2 after transplant.
To determine the role of antibiotics, the researchers treated mice with 4 different antibiotics and looked at the levels of different bacteria.
They found that aztreonam and cefepime increased the levels of Bacteroidales and Clostridiales (the family to which Blautia belongs), but imipenem and metronidazole decreased bacteria levels.
So antibiotics do affect Blautia levels, Dr Jenq said, but they’re only part of the problem. He noted that patients’ Blautia levels start to decrease before antibiotics are administered. So he and his colleagues believe nutrition might also play a part.
The team found a significant difference in Blautia abundance between patients who received total parenteral nutrition and those who did not (P<0.001).
The researchers also discovered that reduced caloric intake led to a loss of Blautia and other Clostridiales. They analyzed 50 samples from 5 patients and found that patients who consumed fewer than 500 calories had a marked reduction in Blautia (P<0.0001).
And experiments in mice confirmed this association. A week of calorie restriction significantly reduced the abundance of Blautia and other Clostridiales (P=0.0002).
“In GVHD, as we all know, patients and mice eat less because of the nausea,” Dr Jenq said. “And we found that GVHD itself can also lead to a reduction in Clostridiales, both in humans [P=0.02] and in mice [P=0.01].”
Protecting Blautia to prevent GVHD
Having confirmed the role of nutrition in Blautia reduction, the researchers set out to identify a nutrition-based intervention to support Blautia in transplant recipients.
They settled on a sugar called raffinose, which is found in beans, cruciferous vegetables, and whole grains. It passes undigested through the upper intestine but is fermented in the lower intestine and metabolized to produce short-chain fatty acids.
The team tested raffinose in mice by introducing it into their drinking water. At 100 days after transplant, mice that received raffinose had significantly better overall survival than controls (P<0.001).
Based on these results, Dr Jenq and his colleagues believe nutritional intervention can protect Blautia and, therefore, may prevent GVHD and related death. The team thinks encouraging eating, gastric nutritional supplementation, and flora-targeted nutritional supplements might all prove effective.
But other interventions might work as well, such as reintroducing endogenous flora (via autologous fecal microbiota transplant), reintroducing select bacteria with beneficial potential, selecting antibiotics that spare bacteria with beneficial potential, and identifying and introducing bacterial metabolites that mediate anti-inflammatory effects. ![]()
*Data in the abstract differ from data presented.
GRAPEVINE, TEXAS—Intestinal bacteria can offer protection from death related to graft-vs-host disease (GVHD), according to research presented at the 2014 BMT Tandem Meetings.
Experiments showed that Blautia, commensal bacteria found in the intestinal tract, can protect against GVHD-related mortality in mice and in humans.
So efforts to support Blautia survival—such as restricting the use of antibiotics and promoting better nutrition—may
prevent GVHD-related death, according to researchers.
Robert Jenq, MD, of Memorial Sloan-Kettering Cancer Center in New York, discussed this possibility when presenting this research, which was designated one of the “Best Abstracts” at the meeting (abstract 1*).
Dr Jenq noted that researchers have been trying for decades to determine whether the intestinal flora impact GVHD. Clinical studies have suggested that prophylaxis against anaerobes and gram-positive bacteria can reduce GVHD.
And murine studies have indicated that prophylaxis against gram-negative bacteria can reduce GVHD, that Lactobacillus can reduce GVHD, and that donor microbiota do not impact GVHD.
“If you’re confused, so are we,” Dr Jenq said. “It seems like it’s a mixed picture.”
So he and his colleagues conducted a series of experiments in an attempt to determine if any bacterial subgroups impact the risk of gut GVHD in mice and humans.
Bacteria seem to impact GVHD
The researchers first studied 76 adult transplant patients, analyzing stool samples taken at roughly 10 days after transplant (+/- 4 days). The team performed 16S gene sequencing using the Roche 454 platform.
This revealed the presence of several types of bacteria, including 6 gram-positive Firmicutes, 2 gram-negative Proteobacteria, and 2 gram-negative Bacteroidetes.
The researchers then used a computational assay to determine which of these bacteria might be associated with protection from GVHD. And they identified 2 possibilities—Lactobacillus and Blautia.
Additional analyses revealed that Blautia and Lactobacillus were significantly associated with GVHD-related mortality at 1500 days after transplant (P=0.03 and 0.01, respectively). But there was no significant association with Bacteroides (P=0.6), Enterobacteriales (P=0.2), or Enterococcus (P=0.3).
Blautia appears to affect GVHD-related mortality
To confirm their initial findings, Dr Jenq and his colleagues analyzed a second cohort of 50 adult transplant patients. The team analyzed stool samples for the abundance of bacterial subgroups using a different sequencing platform, Illumina miseq.
This time, they found that Blautia abundance predicted GVHD-related mortality at more than 500 days after transplant, but the abundance of Lactobacillus did not (P=0.01 and 1, respectively).
“Not enough Blautia in your gut seems to lead to an increase in GVHD-related mortality,” Dr Jenq said. “So what does this do to overall survival? In the first cohort, there’s a big difference in overall survival between the ‘haves’ and ‘have nots’ with Blautia [P=0.0008]. And this also holds up in the second cohort [P=0.04].”
Further analyses of data from both cohorts suggested that Blautia abundance was associated with GVHD-related mortality (P=0.004) and relapse-related mortality (P=0.01) but not non-relapse- and non-GVHD-related mortality (P=0.4).
“I don’t have a good explanation for [the relationship between Blautia and relapse-related death],” Dr Jenq said. “This was a surprise finding.”
The researchers also looked at Blautia’s ability to predict GVHD-related mortality. They found that, around day 10 after transplant, Blautia abundance predicts “very strongly” for GVHD-related death.
Another question was whether known GVHD risk factors—such as donor type, race, gender, and performance status—impact Blautia abundance. But an analysis revealed that Blautia is an independent risk factor for GVHD-related mortality.
A possible mechanism
To gain more insight into the association between Blautia and GVHD-related death, Dr Jenq and his colleagues decided to study it in mice.
The team killed off Blautia in mice using vancomycin and ampicillin, then introduced either murine Blautia or murine Enterococcus, transplanted the mice with MHC-disparate T cells, and monitored them for GVHD.
Mice that received Blautia had significantly better overall survival (at more than 80 days after transplant) than mice that received Enterococcus (P<0.001).
“So how is this happening?” Dr Jenq asked. “We think, potentially, it might be due to short-chain fatty acids . . . butyric acid, propionic acid, and acetic acid. These are metabolites that bacteria produce when they ferment glucose and other sugars.”
To test this theory, the researchers treated mice with antibiotics and introduced Blautia or Enterococcus.
Blautia increased the level of short-chain fatty acids (butyrate and propionate) when compared to Enterococcus, although levels were not as high as those observed in mice that did not receive antibiotics. Nevertheless, these results point to a possible mechanism, according to Dr Jenq.
Explaining Blautia reduction
Dr Jenq also noted that antibiotics may contribute to the decrease in Blautia observed in transplant patients. When patients come in for transplant, they often have more than 25% Blautia in their stool. But the bacteria decrease to negligible levels by day 2 after transplant.
To determine the role of antibiotics, the researchers treated mice with 4 different antibiotics and looked at the levels of different bacteria.
They found that aztreonam and cefepime increased the levels of Bacteroidales and Clostridiales (the family to which Blautia belongs), but imipenem and metronidazole decreased bacteria levels.
So antibiotics do affect Blautia levels, Dr Jenq said, but they’re only part of the problem. He noted that patients’ Blautia levels start to decrease before antibiotics are administered. So he and his colleagues believe nutrition might also play a part.
The team found a significant difference in Blautia abundance between patients who received total parenteral nutrition and those who did not (P<0.001).
The researchers also discovered that reduced caloric intake led to a loss of Blautia and other Clostridiales. They analyzed 50 samples from 5 patients and found that patients who consumed fewer than 500 calories had a marked reduction in Blautia (P<0.0001).
And experiments in mice confirmed this association. A week of calorie restriction significantly reduced the abundance of Blautia and other Clostridiales (P=0.0002).
“In GVHD, as we all know, patients and mice eat less because of the nausea,” Dr Jenq said. “And we found that GVHD itself can also lead to a reduction in Clostridiales, both in humans [P=0.02] and in mice [P=0.01].”
Protecting Blautia to prevent GVHD
Having confirmed the role of nutrition in Blautia reduction, the researchers set out to identify a nutrition-based intervention to support Blautia in transplant recipients.
They settled on a sugar called raffinose, which is found in beans, cruciferous vegetables, and whole grains. It passes undigested through the upper intestine but is fermented in the lower intestine and metabolized to produce short-chain fatty acids.
The team tested raffinose in mice by introducing it into their drinking water. At 100 days after transplant, mice that received raffinose had significantly better overall survival than controls (P<0.001).
Based on these results, Dr Jenq and his colleagues believe nutritional intervention can protect Blautia and, therefore, may prevent GVHD and related death. The team thinks encouraging eating, gastric nutritional supplementation, and flora-targeted nutritional supplements might all prove effective.
But other interventions might work as well, such as reintroducing endogenous flora (via autologous fecal microbiota transplant), reintroducing select bacteria with beneficial potential, selecting antibiotics that spare bacteria with beneficial potential, and identifying and introducing bacterial metabolites that mediate anti-inflammatory effects. ![]()
*Data in the abstract differ from data presented.
Research and Publication Trends
In 1996, Wachter and Goldman heralded the arrival of hospitalists in the healthcare system. They recognized the need to link the clinical role of a hospitalist with other activities, both to provide a creative outlet and to assist in the creation of research and development arms.[1] The explosive growth of hospital medicine followed, and hospitalists rapidly entered the mainstream of the healthcare system.[2]
A consensus conference in 2009 identified the challenges faced by hospitalists in conducting research as a key obstacle in the evolution of the profession into an academic field.[3] Strategies for building and facilitating hospitalist research programs have been described.[4, 5, 6, 7] However, a survey of US academic hospitalist leaders found more than 40% feared their faculty was not developing sustainable nonclinical activities.[8]
Data describing research aspirations and support systems among hospitalists are sparse, and no previous study has described the trends in hospitalist publications. In this work we describe the current standing of hospital medicine research through a survey of both academic and non‐academic hospitalists and a review of hospitalist‐related publications.
METHODS
The Indiana University institutional review board approved this study.
Survey of Hospitalists
A 29‐item questionnaire that addressed research activities, barriers, and mentorship was designed and piloted with pediatrics trainees at Indiana University. The final version (see Supporting Survey in the online version of this article) was approved by the Society of Hospital Medicine (SHM) research committee and posted on Zoomerang (
Review of Publications
A PubMed search was conducted on October 8, 2013 for records with either hospital medicine or hospitalist in the affiliation field. This field provides the departmental name and address information for the first author, except for the not‐yet‐indexed publisher‐supplied records, which could include all author addresses.[10] Editorials and letters to the editor were excluded, and results were limited to English. All resulting articles were manually curated and retained only if the affiliation criteria of hospitalist or hospital medicine (as a relevant single phrase) were associated with the first author. All articles meeting the criteria were reviewed by 1 of the authors and categorized as a review, a case report, or as original research (when methodology was described in the abstract). Original research articles were assigned a category based on their methodology and research type, as defined in published literature. The categories included basic sciences, clinical, health information, health services, quality improvement (QI), education, and translational research.[11, 12, 13, 14, 15] If the article overlapped categories, a secondary category was also assigned. A second author independently evaluated a subset of articles. This subset was then used to calculate the overall concordance between the authors based on their agreement on either the primary or secondary category designations.
To capture data on research funding, each original research article was searched for statements directly linking the first author or the work to the funding source(s).
Publications in the Journal of Hospital Medicine (JHM) were reviewed to serve as a gauge of research interests in the field of hospital medicine that may not be reflected by the publications resulting from the PubMed search. JHM was selected as the journal best representing hospital medicine based on its stated mission of commitment to the advancement of the hospital medicine specialty.[16] All original research articles in JHM were assigned a category by 1 of the authors based on the methodology in the abstract.
Statistical Methods
The survey responses were summarized using descriptive statistics. Univariate tests of association between respondent characteristics and peer‐reviewed authorship were performed using the Fisher's exact test. P values of 0.05 were considered significant. Data from the publication searches were presented as descriptive statistics.
RESULTS
Survey
The survey link was emailed to 11,611 SHM members: 11,102 members received the link and 509 emails were returned as undeliverable. A total of 645 member responses were received (5.8% response rate).
The most common demographic characteristics identified included male gender, age 45 or younger, and white race. The locations of the current practices were distributed equally across the United States. Over half of the respondents were trained in internal medicine, and a quarter were trained in pediatrics. Eleven percent had undertaken fellowship training after residency. Thirty‐seven percent did not hold an academic rank, and among those who did, most were assistant professors. (Table 1)
| Characteristics | Responses, N (%) | |||
|---|---|---|---|---|
| All Responses | Responses With Funding | |||
| ||||
| Gender | 597 | 67 | ||
| Female | 248 (41) | 33 (49) | ||
| Male | 349 (58) | 34 (51) | ||
| Age, y | 599 | 67 | ||
| 2535 | 157 (26) | 17 (25) | ||
| 3645 | 274 (46) | 39 (58) | ||
| 4655 | 105 (17) | 6 (9) | ||
| 5665 | 56 (9) | 5 (7) | ||
| >65 | 7 (1) | 0 | ||
| Current practice location | 596 | 67 | ||
| Midwest | 147 (25) | 18 (27) | ||
| Northeast | 113 (19) | 12 (18) | ||
| South | 172 (29) | 14 (21) | ||
| West | 142 (4) | 16 (24) | ||
| Other | 22 (34) | 7 (10) | ||
| Race | 595 | 67 | ||
| White | 444 (75) | 58 (87) | ||
| Black | 18 (3) | 0 | ||
| Hispanic | 22 (4) | 1 (1) | ||
| Asian | 85 (14) | 8 (12) | ||
| Other | 26 (4) | |||
| Faculty appointment | 593 | 68 | ||
| Nonacademic | 221 (37) | 4 (6) | ||
| Instructor/lecturer | 60 (10) | 6 (9) | ||
| Assistant professor | 197 (33) | 32 (47) | ||
| Associate professor | 68 (11) | 19 (28) | ||
| Full professor | 14 (2) | 4 (6) | ||
| Other | 33 (6) | 3 (4) | ||
| Fellowship training | 68 | 14 | ||
| General IM/hospitalist | 15 (22) | 6 (43) | ||
| Pediatric hospital medicine | 7 (10) | 2 (14) | ||
| Other | 46 (68) | 6 (43) | ||
| Residency completed | 616 | 68 | ||
| IM | 340 (55) | 36 (53) | ||
| Pediatrics | 154 (25) | 27 (40) | ||
| Family medicine | 53 (9) | 1 (1) | ||
| IM/pediatrics | 48 (8) | 2 (3) | ||
| Other | 21 (3) | 2 (3) | ||
Overall availability of mentorship was low, but respondents with academic appointments were more likely to have a mentor than those without academic appointments (32% vs 2.7%, p<0.001). Hospitalists most likely identified their own mentors, and meetings between the hospitalist and mentor occurred more frequently than once every 3 months.
There were 213 (33%) respondents who identified themselves as currently conducting research, 96 (45%) of whom were trained in pediatrics. Ninety‐two (28%) of those with academic appointments and 157 (71%) of those without academic appointments had no current or future plans to engage in research. QI research, followed by clinical research, emerged as the most frequent type of research that hospitalists were either currently engaged in or planned to embark on. Most respondents identified factors other than age, family or financial issues, the grant process, or a lack of institutional support as the reason for not conducting research. (Table 2)
| Activity | Responses, N (%) | ||||
|---|---|---|---|---|---|
| Adult Medicine | Pediatric Medicine | ||||
| |||||
| No plan to conduct research | 245 | 26 | |||
| Reasons for not doing research | |||||
| Lack of institutional support | 42 (17) | 3 (12) | |||
| Family issues | 14 (6) | 1 (4) | |||
| Financial | 8 (3) | 0 | |||
| Grant process | 4 (2) | 2 (8) | |||
| Age | 5 (2) | 0 | |||
| Other | 171 (70) | 20 (77) | |||
| Currently doing research | 117 | 96 | |||
| Quality improvement | 79 (68) | 73 (76) | |||
| Clinical | 59 (50) | 62 (65) | |||
| Health services | 31 (26) | 30 (31) | |||
| Health informatics | 28 (24) | 11 (11) | |||
| Translational | 10 (8) | 7 (7) | |||
| Basic science | 3 (3) | 0 | |||
| Other | 17 (14) | 10 (10) | |||
| Plan on doing research | 183 | 30 | |||
| Quality improvement | 72 (39) | 25 (83) | |||
| Clinical | 65 (35) | 25 (83) | |||
| Health services | 20 (11) | 2 (7) | |||
| Health informatics | 25 (14) | 3 (10) | |||
| Translational | 8 (4) | 3 (10) | |||
| Basic science | 3 (2) | 0 | |||
| Other | 8 (4) | 0 | |||
| Peer‐review publications | 458 | 151 | |||
| No | 270 (59) | 62 (41) | |||
| Yes | 188 (41) | 89 (59) | |||
| Frequency | |||||
| Less than once/year | 111 (59) | 41 (46) | |||
| Once/year | 22 (12) | 20 (22) | |||
| Twice/year | 16 (8) | 16 (18) | |||
| More than twice/year | 23 (12) | 10 (11) | |||
| Other | 13 (7) | 1 (1) | |||
| Publication Type | |||||
| Original research | 97 (52) | 75 (84) | |||
| Case report/series | 80 (42) | 41 (46) | |||
| Reviews | 63 (34) | 25 (28) | |||
| Clinical trials | 36 (19) | 9 (10) | |||
| Practice guidelines | 18 (10) | 12 (13) | |||
| Meta‐analysis | 14 (7) | 8 (9) | |||
| Other | 23 (12) | 0 | |||
Sixty‐eight (10%) respondents held research funding, and 6 identified the grant process as an impediment to doing research. The most commonly reported funding source was from government and institutions, followed by support from foundations (see Supporting Figure 1A in the online version of this article). Responders with research funding were predominantly young, white, and assistant or associate professors. Fourteen hospitalists with funding reported completing a fellowship. (Table 1)
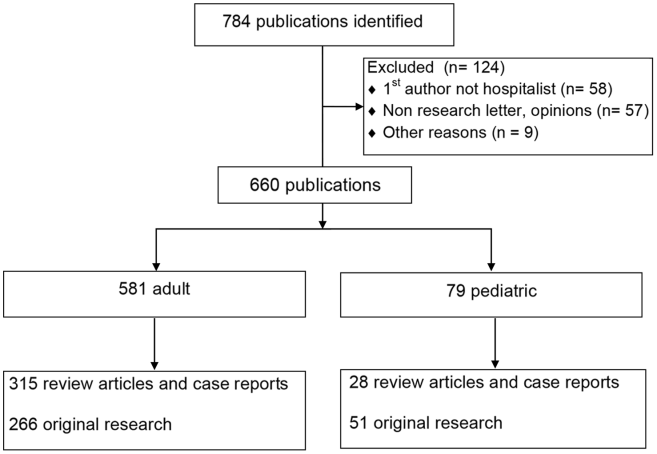
More than half of the respondents (n=332) had not authored peer‐reviewed publications. Of the 277 who had published successfully, 89 (31%) were trained in pediatrics. For those with publications, 152 (55%) reported publishing less than once per year. The type of article published most frequently was original research followed by case reports/series and reviews. (Table 2)
Variables individually associated with an increased likelihood of authoring peer‐reviewed publications included the completion of a fellowship, having an academic appointment, the availability of funding and mentorship, a background of pediatrics training, and more than 25% dedicated research time. (Table 3)
| Authored Peer‐Reviewed Publications, N (%) | |||||
|---|---|---|---|---|---|
| Characteristics | No | Yes | P | ||
| |||||
| Age, y | 327 | 272 | 0.437 | ||
| 2535 | 85 (26) | 72 (26) | |||
| 3645 | 146 (45) | 128 (47) | |||
| 4655 | 64 (20) | 41 (15) | |||
| 5665 | 30 (9) | 26 (10) | |||
| >65 | 2 (1) | 5 (2) | |||
| Gender | 327 | 270 | 0.067 | ||
| Female | 147 (45) | 101 (37) | |||
| Male | 180 (55) | 169 (63) | |||
| Faculty appointment | 301 | 247 | <0.001 | ||
| Nonacademic | 161 (53) | 63 (25) | |||
| Academic | 140 (46) | 184 (74) | |||
| Residency | 331 | 275 | <0.001 | ||
| Family | 39 (12) | 14 (5) | |||
| Internal medicine | 184 (56) | 151 (55) | |||
| Internal medicine (pediatrics) | 33 (10) | 15 (5) | |||
| Pediatrics | 62 (19) | 89 (32) | |||
| Other | 13 (4) | 6 (2) | |||
| Completed fellowship training | 332 | 19 (6) | 277 | 47 (17) | <0.001 |
| Current research/career mentor | 327 | 30 (9) | 272 | 96 (35) | <0.001 |
| Meet with mentor | 29 | 88 | 0.433 | ||
| More often than every 6 months | 21 (72) | 71 (81) | |||
| Every 6 months or less | 8 (28) | 17 (19) | |||
| Time for research | 54 | 153 | <0.001 | ||
| 25% or less | 53 (98) | 122 (80) | |||
| More than 25% | 1 (2) | 31 (20) | |||
| Has funding | 54 | 8 (15) | 156 | 60 (38) | <0.001 |
Publications Review
The PubMed search yielded 784 publications with hospital medicine or hospitalist in the affiliation field. After manual review, 660 articles were retained. (Figure 1)
The volume of hospitalist‐led publications has been increasing. Between 2006 and October 2013 there was a 5‐fold increase in hospitalist‐led publications (36 in 2006 to 179 in the first 10 months of 2013). Of the 660 articles culled from the PubMed search, 581 (88%) represented the work of authors affiliated with adult hospital medicine; 266 (46%) of these represented original research (the rest were reviews and case reports). Seventy‐nine (12%) of the 660 PubMed articles were related to pediatric hospital medicine; 51 (65%) of these represented original research. (Figure 1) In the period studied there was a variation from year to year in the proportion of publications representing original research, with a range of 37% to 71% comprising original research in adult hospital medicine publications and 50% to 81% in pediatric hospital medicine publications (Figure 2A).
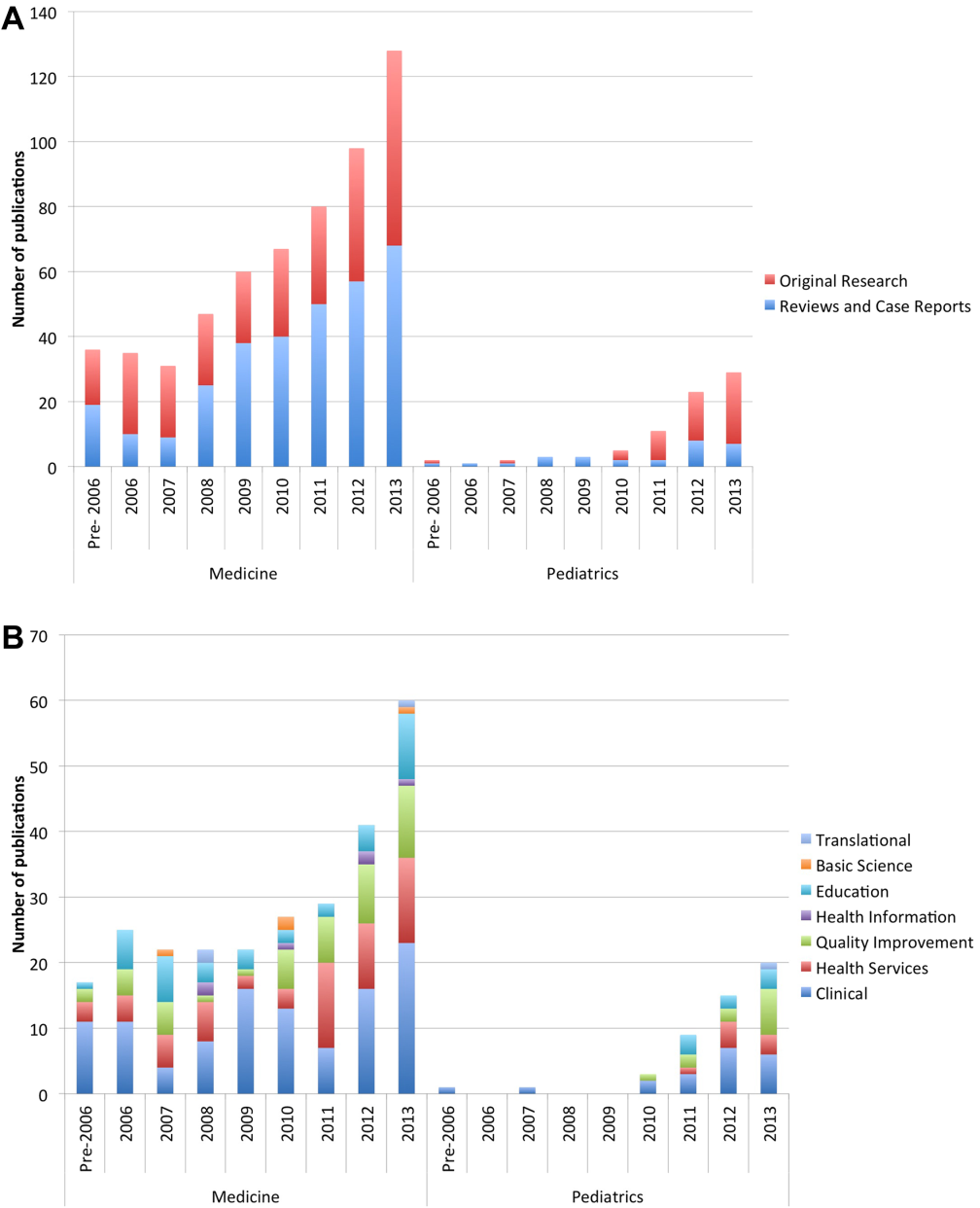
Nearly half (41%) of the original research in adult and pediatric medicine represented clinical research. Health services (21%) and QI (19%) were the next most frequent research categories published. Publications pertaining to research in education represented 15% of all original research. Health services and QI research are growing on a relatively stable base of clinical research. These trends were similar between adult and pediatric hospital medicine. (Figure 2B) The concordance rate on the assigned research categories was 82%, based on 67 publications that were independently reviewed by 2 authors.
There were 457 original research articles published in JHM between 2006 and early October 2013. JHM publications followed a trend similar to the publications of hospitalist‐affiliated first authors from PubMed, with the majority (47%) reflecting clinical research followed by health services (25%) and QI (25%). (see Supporting Figure 3 in the online version of this article)
In our review, adult medicine hospitalist authors were affiliated with 124 different universities or centers. However, 5 centers represented nearly half the publication volume. The Cleveland Clinic Foundation, University of California San Francisco (UCSF), Harvard, Northwestern, and the University of Chicago were the top producers. Fewer centers produce original research, with 66 counted in our search. Centers most prolific in producing original research are UCSF, Northwestern, University of Chicago, Harvard, and Johns Hopkins. Their combined output represented 56% of all published original published research. (see Supporting Figure 2A,B in the online version of this article)
In our review, publications attributed to pediatric hospitalists were the product of 34 different centers. Cincinnati Children's Hospital Medical Center, Children's National Medical Center (Georgetown University), and the Monroe Carell Children's Hospital (Vanderbilt) were the most productive in publishing. The same centers were also the most productive in publishing original research. (see Supporting Figure 2C,D in the online version of this article)
Funding data from the 317 original research articles found in PubMed showed that 52% had funding listed for the first author and/or the work. These publications were the work of 181 different first authors, of whom 39 (22%) had 1 or more funding sources specifically associated with them in the publications. The majority of these authors reported government funding (n=24), followed by support from foundations (n=12), institutions (n=8), and industries (n=6) (see Supporting Figure 1B in the online version of this article).
DISCUSSION
Using results from both the survey and our review of publications in PubMed provided complementary information that has enriched our evaluation and reporting of the current state of research and publications in hospital medicine.
The initial growth of the field of hospital medicine can be attributed to its clinical contributions.[17] However, hospital medicine faces numerous challenges in its evolution into an academic specialty.[3] Job satisfaction rates among hospitalists may be falling,[18, 19] and pursuing intellectual outlets such as research may improve both satisfaction and productivity.[20, 21] Therefore, it is important to study the predictors of success for the nonclinical intellectual endeavors of hospitalists.
Across the career spectrum in academic medicine, effective mentorship has been found to be beneficial in enhancing teaching skills, productivity, and satisfaction.[22] Similar to prior studies, we found that mentorship was not readily accessible, and its absence was associated with a decreased likelihood of peer‐reviewed publications.[23, 24] Hospital medicine remains a youthful specialty, with the mean age of clinicians in the 40s.[18] In our survey, hospitalists aged 36 to 45 years reported the highest rates of publications and funding. If these hospitalists can be retained in the field, they may eventually serve as mentors to those entering the specialty. Strategies to provide mentorship have been described,[25] and continued efforts to innovate are needed in the development of mentorship potential.
Successfully promoted hospitalists identify peer‐reviewed publications as a key activity that supports promotion.[26] However, similar to Reid et al.,[23] our survey found that hospitalists reported low rates of peer‐reviewed publications. Hospitalists have unique access to the inpatient population, and setting up collaborative efforts between specialists and hospitalists, or participating in multi‐institutional projects that require patient recruitment,[27] may facilitate research and publication productivity. A specific emerging opportunity for this expertise is the need for collecting and identifying disease presentations to correlate with the exploding genetic data now available.[28]
QI research was identified from our survey results as the most frequent type of research that hospitalists were either engaged in or planned to pursue. However, based on our review of published research, the volume of QI research is surpassed by that of clinical research. Many factors contribute to this. First, an overlap between the categories of clinical and QI research may have led to lower numbers in QI. Second, there may be a lag between the interest in QI translating into publications. This may be related both to the dearth of QI mentorship and to the barriers in publishing QI. These barriers include increasing competition in target journals, the lack of generalizability of QI efforts, and the compressed time frames of rapid improvement cycles that differ from the slower pace of clinical research and its measurements.[29] Hospitalists may also perform QI that results in scholarly output other than publications (eg, grand rounds, posters, or presentations) that we did not address. In the absence of QI publications, the systematic documentation of QI efforts in a portfolio may assist career advancement.[30]
The review of publications in the PubMed database through early October 2013 showed a consistent increase in the number of publications produced by hospitalist first authors. Clinical research was represented most frequently followed by health services and QI research. The predominance of clinical research parallels the large clinical role of hospitalists; however, the diversity of research categories represented reflects the growing penetration and involvement of hospitalists in the arenas of QI, health services, and education. Although our search identified fewer pediatric hospitalist articles, pediatric hospitalist literature is also on the rise. There are other indicators of the enthusiasm for research among pediatric hospitalists, as nearly half the respondents in our survey who are currently engaged in research and nearly a third who had successfully published or had funding support were trained in pediatrics.
Publications by first authors who were hospitalists or affiliated with hospital medicine represented the effort of more than 100 institutions, implying a widespread engagement in hospital medicine‐related scholarship. However, fewer centers produce original research, and over half the original research output is the product of 8 centers. Strategies to select and support person‐job fit,[31] availability of mentorship, the presence of existing infrastructure, funding, and departmental priorities are all likely to affect an institution's publication productivity. To emulate the success of these centers, a closer study of the strategies they employ[5] would be instructive for the broader hospitalist community.
Although our survey data showed that the presence of funding is associated with success in publishing, the percentage of hospitalists who report funding both from the survey and PubMed publication reports is <25%. This underscores the need for innovations that help hospitalists obtain support and incentives for their work.
This study has limitations. A survey is a cross‐sectional snap shot, and associations do not imply causation. Survey response rates have been falling,[32] and our convenience sampling without incentives engendered a low response rate. This response rate is similar to that of other surveys administered through SHM (SHM membership and marketing data, October 2013). Although statistical significance is presented, the differences may not be generalizable given the low response rate. We cannot quantify all responder biases or comment on how the membership fee to SHM may affect the sample cohort. The demographics of our respondents parallel that of the SHM membership base in age and gender. However, 25% of our respondents were trained in pediatrics, whereas only 4.3% of the SHM membership base is pediatrics trained (SHM membership and marketing data, October 2013). We did not inquire about contributions from job dissatisfaction to the lack of participation in research activities, and this may represent an area for further research.
The search methodology used in this study is likely to under‐report hospitalist‐related research, because collaborative publications in which the lead author is not a hospitalist were not included. Furthermore, many hospitalists are associated with centers that do not have a hospitalist or hospital medicine title or department, and our search terms would have missed the publications stemming from these centers. Pediatric hospitalist literature is likely to be further under‐represented, as centers may not have separate pediatric hospitalist departments.
The assignment of each publication into a research category was based on definitions found in the literature. However, this designation ultimately remains a subjective process that may introduce bias.
Although the initial growth spurt of hospital medicine can be attributed to its clinical success, the increase in hospital medicine‐led peer‐reviewed publications in increasingly diverse domains provides evidence that supports the field's concomitant academic and scholarly maturation. Research into factors that impede or inspire hospitalists to participate in research, innovations that provide mentorship and funding for the specific interests of hospitalists, and the emulation of strategies employed by centers productive in publications are required to successfully foster the multidimensional growth of the field.
Acknowledgements
The authors thank Dr. Antoinette Laskey for her mentorship in survey development, Elaine Bammerlin for copyediting assistance, and the Society of Hospital Medicine members for taking the survey.
Disclosures: An Dang Do, MD, PhD, completed the major part of this work as a Morris Green Scholar at Indiana University School of Medicine. An N. Dang Do, MD, PhD, and Amy M. Munchhof, MD, PhD, contributed equally to this work. Areeba Kara, MD, is supported by a grant from the Methodist Health Foundation and by award number T15OC000047 from the Office of the National Coordinator for Health Information Technology, Office of the Secretary, US Department of Health & Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the National Coordinator For Health Information Technology, Office of the Secretary, US Department of Health & Human Services, or the National Institutes of Health.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- , , , . The status of hospital medicine groups in the United States. J Hosp Med. 2006;1(2):75–80.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308–313.
- , , , . An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314–318.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 25, 2013.
- US National Library of Medicine website. MEDLINE/PubMed data element (field) descriptions. Available at: http://www.nlm.nih.gov/bsd/mms/medlineelements.html. Accessed October 30, 2013.
- American Educational Research Association website. Available at: http://www.aera.net/EducationResearch/WhatisEducationResearch/tab id/13453/Default.aspx. Accessed October 30, 2013.
- National Institutes of Health website. Glossary of NIH terms. Available at: http://grants.nih.gov/grants/glossary.htm. Accessed February 26, 2013.
- US National Science Foundation website. National Center for Science and Engineering Statistics. Definitions of research and development: an annotated compilation of official sources. Available at: http://www.nsf.gov/statistics/randdef/fedgov.cfm. Accessed February 26, 2013.
- , . Health services research: an evolving definition of the field. US National Library of Medicine website. National Institutes of Health. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430351. Accessed February 26, 2013.
- Centers for Medicare and Medicaid Services website. Outcome measures. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/OutcomeMeasu res.html. Accessed August 22, 2013.
- Journal of Hospital Medicine. Available at: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1553–5606/homepage/ProductInformation.html. Accessed October 30, 2013.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , , . Preparing for “diastole”: Advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103–1115.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2011;27(1):23–27.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1–2.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , . The APA and the rise of pediatric generalist network research. Acad Pediatr. 2011;11(3):195–204.
- , , , et al. Phenotype harmonization and cross‐study collaboration in GWAS consortia: the GENEVA experience. Genet Epidemiol. 2011;35(3):159–173.
- , . Clinicians in quality improvement: a new career pathway in academic medicine. JAMA. 2009;301(7):766–768.
- , , , , Documenting quality improvement and patient safety efforts: the quality portfolio. A statement from the Academic Hospitalist Taskforce [published online ahead of print June 27, 2013]. J Gen Intern Med. doi: 10.1007/s11606‐013‐2532‐z.
- , , , , . Person‐job fit: an exploratory cross‐sectional analysis of hospitalists. J Hosp Med. 2012;8(2):96–101.
- , , , . Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40(1):213–226.
In 1996, Wachter and Goldman heralded the arrival of hospitalists in the healthcare system. They recognized the need to link the clinical role of a hospitalist with other activities, both to provide a creative outlet and to assist in the creation of research and development arms.[1] The explosive growth of hospital medicine followed, and hospitalists rapidly entered the mainstream of the healthcare system.[2]
A consensus conference in 2009 identified the challenges faced by hospitalists in conducting research as a key obstacle in the evolution of the profession into an academic field.[3] Strategies for building and facilitating hospitalist research programs have been described.[4, 5, 6, 7] However, a survey of US academic hospitalist leaders found more than 40% feared their faculty was not developing sustainable nonclinical activities.[8]
Data describing research aspirations and support systems among hospitalists are sparse, and no previous study has described the trends in hospitalist publications. In this work we describe the current standing of hospital medicine research through a survey of both academic and non‐academic hospitalists and a review of hospitalist‐related publications.
METHODS
The Indiana University institutional review board approved this study.
Survey of Hospitalists
A 29‐item questionnaire that addressed research activities, barriers, and mentorship was designed and piloted with pediatrics trainees at Indiana University. The final version (see Supporting Survey in the online version of this article) was approved by the Society of Hospital Medicine (SHM) research committee and posted on Zoomerang (
Review of Publications
A PubMed search was conducted on October 8, 2013 for records with either hospital medicine or hospitalist in the affiliation field. This field provides the departmental name and address information for the first author, except for the not‐yet‐indexed publisher‐supplied records, which could include all author addresses.[10] Editorials and letters to the editor were excluded, and results were limited to English. All resulting articles were manually curated and retained only if the affiliation criteria of hospitalist or hospital medicine (as a relevant single phrase) were associated with the first author. All articles meeting the criteria were reviewed by 1 of the authors and categorized as a review, a case report, or as original research (when methodology was described in the abstract). Original research articles were assigned a category based on their methodology and research type, as defined in published literature. The categories included basic sciences, clinical, health information, health services, quality improvement (QI), education, and translational research.[11, 12, 13, 14, 15] If the article overlapped categories, a secondary category was also assigned. A second author independently evaluated a subset of articles. This subset was then used to calculate the overall concordance between the authors based on their agreement on either the primary or secondary category designations.
To capture data on research funding, each original research article was searched for statements directly linking the first author or the work to the funding source(s).
Publications in the Journal of Hospital Medicine (JHM) were reviewed to serve as a gauge of research interests in the field of hospital medicine that may not be reflected by the publications resulting from the PubMed search. JHM was selected as the journal best representing hospital medicine based on its stated mission of commitment to the advancement of the hospital medicine specialty.[16] All original research articles in JHM were assigned a category by 1 of the authors based on the methodology in the abstract.
Statistical Methods
The survey responses were summarized using descriptive statistics. Univariate tests of association between respondent characteristics and peer‐reviewed authorship were performed using the Fisher's exact test. P values of 0.05 were considered significant. Data from the publication searches were presented as descriptive statistics.
RESULTS
Survey
The survey link was emailed to 11,611 SHM members: 11,102 members received the link and 509 emails were returned as undeliverable. A total of 645 member responses were received (5.8% response rate).
The most common demographic characteristics identified included male gender, age 45 or younger, and white race. The locations of the current practices were distributed equally across the United States. Over half of the respondents were trained in internal medicine, and a quarter were trained in pediatrics. Eleven percent had undertaken fellowship training after residency. Thirty‐seven percent did not hold an academic rank, and among those who did, most were assistant professors. (Table 1)
| Characteristics | Responses, N (%) | |||
|---|---|---|---|---|
| All Responses | Responses With Funding | |||
| ||||
| Gender | 597 | 67 | ||
| Female | 248 (41) | 33 (49) | ||
| Male | 349 (58) | 34 (51) | ||
| Age, y | 599 | 67 | ||
| 2535 | 157 (26) | 17 (25) | ||
| 3645 | 274 (46) | 39 (58) | ||
| 4655 | 105 (17) | 6 (9) | ||
| 5665 | 56 (9) | 5 (7) | ||
| >65 | 7 (1) | 0 | ||
| Current practice location | 596 | 67 | ||
| Midwest | 147 (25) | 18 (27) | ||
| Northeast | 113 (19) | 12 (18) | ||
| South | 172 (29) | 14 (21) | ||
| West | 142 (4) | 16 (24) | ||
| Other | 22 (34) | 7 (10) | ||
| Race | 595 | 67 | ||
| White | 444 (75) | 58 (87) | ||
| Black | 18 (3) | 0 | ||
| Hispanic | 22 (4) | 1 (1) | ||
| Asian | 85 (14) | 8 (12) | ||
| Other | 26 (4) | |||
| Faculty appointment | 593 | 68 | ||
| Nonacademic | 221 (37) | 4 (6) | ||
| Instructor/lecturer | 60 (10) | 6 (9) | ||
| Assistant professor | 197 (33) | 32 (47) | ||
| Associate professor | 68 (11) | 19 (28) | ||
| Full professor | 14 (2) | 4 (6) | ||
| Other | 33 (6) | 3 (4) | ||
| Fellowship training | 68 | 14 | ||
| General IM/hospitalist | 15 (22) | 6 (43) | ||
| Pediatric hospital medicine | 7 (10) | 2 (14) | ||
| Other | 46 (68) | 6 (43) | ||
| Residency completed | 616 | 68 | ||
| IM | 340 (55) | 36 (53) | ||
| Pediatrics | 154 (25) | 27 (40) | ||
| Family medicine | 53 (9) | 1 (1) | ||
| IM/pediatrics | 48 (8) | 2 (3) | ||
| Other | 21 (3) | 2 (3) | ||
Overall availability of mentorship was low, but respondents with academic appointments were more likely to have a mentor than those without academic appointments (32% vs 2.7%, p<0.001). Hospitalists most likely identified their own mentors, and meetings between the hospitalist and mentor occurred more frequently than once every 3 months.
There were 213 (33%) respondents who identified themselves as currently conducting research, 96 (45%) of whom were trained in pediatrics. Ninety‐two (28%) of those with academic appointments and 157 (71%) of those without academic appointments had no current or future plans to engage in research. QI research, followed by clinical research, emerged as the most frequent type of research that hospitalists were either currently engaged in or planned to embark on. Most respondents identified factors other than age, family or financial issues, the grant process, or a lack of institutional support as the reason for not conducting research. (Table 2)
| Activity | Responses, N (%) | ||||
|---|---|---|---|---|---|
| Adult Medicine | Pediatric Medicine | ||||
| |||||
| No plan to conduct research | 245 | 26 | |||
| Reasons for not doing research | |||||
| Lack of institutional support | 42 (17) | 3 (12) | |||
| Family issues | 14 (6) | 1 (4) | |||
| Financial | 8 (3) | 0 | |||
| Grant process | 4 (2) | 2 (8) | |||
| Age | 5 (2) | 0 | |||
| Other | 171 (70) | 20 (77) | |||
| Currently doing research | 117 | 96 | |||
| Quality improvement | 79 (68) | 73 (76) | |||
| Clinical | 59 (50) | 62 (65) | |||
| Health services | 31 (26) | 30 (31) | |||
| Health informatics | 28 (24) | 11 (11) | |||
| Translational | 10 (8) | 7 (7) | |||
| Basic science | 3 (3) | 0 | |||
| Other | 17 (14) | 10 (10) | |||
| Plan on doing research | 183 | 30 | |||
| Quality improvement | 72 (39) | 25 (83) | |||
| Clinical | 65 (35) | 25 (83) | |||
| Health services | 20 (11) | 2 (7) | |||
| Health informatics | 25 (14) | 3 (10) | |||
| Translational | 8 (4) | 3 (10) | |||
| Basic science | 3 (2) | 0 | |||
| Other | 8 (4) | 0 | |||
| Peer‐review publications | 458 | 151 | |||
| No | 270 (59) | 62 (41) | |||
| Yes | 188 (41) | 89 (59) | |||
| Frequency | |||||
| Less than once/year | 111 (59) | 41 (46) | |||
| Once/year | 22 (12) | 20 (22) | |||
| Twice/year | 16 (8) | 16 (18) | |||
| More than twice/year | 23 (12) | 10 (11) | |||
| Other | 13 (7) | 1 (1) | |||
| Publication Type | |||||
| Original research | 97 (52) | 75 (84) | |||
| Case report/series | 80 (42) | 41 (46) | |||
| Reviews | 63 (34) | 25 (28) | |||
| Clinical trials | 36 (19) | 9 (10) | |||
| Practice guidelines | 18 (10) | 12 (13) | |||
| Meta‐analysis | 14 (7) | 8 (9) | |||
| Other | 23 (12) | 0 | |||
Sixty‐eight (10%) respondents held research funding, and 6 identified the grant process as an impediment to doing research. The most commonly reported funding source was from government and institutions, followed by support from foundations (see Supporting Figure 1A in the online version of this article). Responders with research funding were predominantly young, white, and assistant or associate professors. Fourteen hospitalists with funding reported completing a fellowship. (Table 1)

More than half of the respondents (n=332) had not authored peer‐reviewed publications. Of the 277 who had published successfully, 89 (31%) were trained in pediatrics. For those with publications, 152 (55%) reported publishing less than once per year. The type of article published most frequently was original research followed by case reports/series and reviews. (Table 2)
Variables individually associated with an increased likelihood of authoring peer‐reviewed publications included the completion of a fellowship, having an academic appointment, the availability of funding and mentorship, a background of pediatrics training, and more than 25% dedicated research time. (Table 3)
| Authored Peer‐Reviewed Publications, N (%) | |||||
|---|---|---|---|---|---|
| Characteristics | No | Yes | P | ||
| |||||
| Age, y | 327 | 272 | 0.437 | ||
| 2535 | 85 (26) | 72 (26) | |||
| 3645 | 146 (45) | 128 (47) | |||
| 4655 | 64 (20) | 41 (15) | |||
| 5665 | 30 (9) | 26 (10) | |||
| >65 | 2 (1) | 5 (2) | |||
| Gender | 327 | 270 | 0.067 | ||
| Female | 147 (45) | 101 (37) | |||
| Male | 180 (55) | 169 (63) | |||
| Faculty appointment | 301 | 247 | <0.001 | ||
| Nonacademic | 161 (53) | 63 (25) | |||
| Academic | 140 (46) | 184 (74) | |||
| Residency | 331 | 275 | <0.001 | ||
| Family | 39 (12) | 14 (5) | |||
| Internal medicine | 184 (56) | 151 (55) | |||
| Internal medicine (pediatrics) | 33 (10) | 15 (5) | |||
| Pediatrics | 62 (19) | 89 (32) | |||
| Other | 13 (4) | 6 (2) | |||
| Completed fellowship training | 332 | 19 (6) | 277 | 47 (17) | <0.001 |
| Current research/career mentor | 327 | 30 (9) | 272 | 96 (35) | <0.001 |
| Meet with mentor | 29 | 88 | 0.433 | ||
| More often than every 6 months | 21 (72) | 71 (81) | |||
| Every 6 months or less | 8 (28) | 17 (19) | |||
| Time for research | 54 | 153 | <0.001 | ||
| 25% or less | 53 (98) | 122 (80) | |||
| More than 25% | 1 (2) | 31 (20) | |||
| Has funding | 54 | 8 (15) | 156 | 60 (38) | <0.001 |
Publications Review
The PubMed search yielded 784 publications with hospital medicine or hospitalist in the affiliation field. After manual review, 660 articles were retained. (Figure 1)
The volume of hospitalist‐led publications has been increasing. Between 2006 and October 2013 there was a 5‐fold increase in hospitalist‐led publications (36 in 2006 to 179 in the first 10 months of 2013). Of the 660 articles culled from the PubMed search, 581 (88%) represented the work of authors affiliated with adult hospital medicine; 266 (46%) of these represented original research (the rest were reviews and case reports). Seventy‐nine (12%) of the 660 PubMed articles were related to pediatric hospital medicine; 51 (65%) of these represented original research. (Figure 1) In the period studied there was a variation from year to year in the proportion of publications representing original research, with a range of 37% to 71% comprising original research in adult hospital medicine publications and 50% to 81% in pediatric hospital medicine publications (Figure 2A).

Nearly half (41%) of the original research in adult and pediatric medicine represented clinical research. Health services (21%) and QI (19%) were the next most frequent research categories published. Publications pertaining to research in education represented 15% of all original research. Health services and QI research are growing on a relatively stable base of clinical research. These trends were similar between adult and pediatric hospital medicine. (Figure 2B) The concordance rate on the assigned research categories was 82%, based on 67 publications that were independently reviewed by 2 authors.
There were 457 original research articles published in JHM between 2006 and early October 2013. JHM publications followed a trend similar to the publications of hospitalist‐affiliated first authors from PubMed, with the majority (47%) reflecting clinical research followed by health services (25%) and QI (25%). (see Supporting Figure 3 in the online version of this article)
In our review, adult medicine hospitalist authors were affiliated with 124 different universities or centers. However, 5 centers represented nearly half the publication volume. The Cleveland Clinic Foundation, University of California San Francisco (UCSF), Harvard, Northwestern, and the University of Chicago were the top producers. Fewer centers produce original research, with 66 counted in our search. Centers most prolific in producing original research are UCSF, Northwestern, University of Chicago, Harvard, and Johns Hopkins. Their combined output represented 56% of all published original published research. (see Supporting Figure 2A,B in the online version of this article)
In our review, publications attributed to pediatric hospitalists were the product of 34 different centers. Cincinnati Children's Hospital Medical Center, Children's National Medical Center (Georgetown University), and the Monroe Carell Children's Hospital (Vanderbilt) were the most productive in publishing. The same centers were also the most productive in publishing original research. (see Supporting Figure 2C,D in the online version of this article)
Funding data from the 317 original research articles found in PubMed showed that 52% had funding listed for the first author and/or the work. These publications were the work of 181 different first authors, of whom 39 (22%) had 1 or more funding sources specifically associated with them in the publications. The majority of these authors reported government funding (n=24), followed by support from foundations (n=12), institutions (n=8), and industries (n=6) (see Supporting Figure 1B in the online version of this article).
DISCUSSION
Using results from both the survey and our review of publications in PubMed provided complementary information that has enriched our evaluation and reporting of the current state of research and publications in hospital medicine.
The initial growth of the field of hospital medicine can be attributed to its clinical contributions.[17] However, hospital medicine faces numerous challenges in its evolution into an academic specialty.[3] Job satisfaction rates among hospitalists may be falling,[18, 19] and pursuing intellectual outlets such as research may improve both satisfaction and productivity.[20, 21] Therefore, it is important to study the predictors of success for the nonclinical intellectual endeavors of hospitalists.
Across the career spectrum in academic medicine, effective mentorship has been found to be beneficial in enhancing teaching skills, productivity, and satisfaction.[22] Similar to prior studies, we found that mentorship was not readily accessible, and its absence was associated with a decreased likelihood of peer‐reviewed publications.[23, 24] Hospital medicine remains a youthful specialty, with the mean age of clinicians in the 40s.[18] In our survey, hospitalists aged 36 to 45 years reported the highest rates of publications and funding. If these hospitalists can be retained in the field, they may eventually serve as mentors to those entering the specialty. Strategies to provide mentorship have been described,[25] and continued efforts to innovate are needed in the development of mentorship potential.
Successfully promoted hospitalists identify peer‐reviewed publications as a key activity that supports promotion.[26] However, similar to Reid et al.,[23] our survey found that hospitalists reported low rates of peer‐reviewed publications. Hospitalists have unique access to the inpatient population, and setting up collaborative efforts between specialists and hospitalists, or participating in multi‐institutional projects that require patient recruitment,[27] may facilitate research and publication productivity. A specific emerging opportunity for this expertise is the need for collecting and identifying disease presentations to correlate with the exploding genetic data now available.[28]
QI research was identified from our survey results as the most frequent type of research that hospitalists were either engaged in or planned to pursue. However, based on our review of published research, the volume of QI research is surpassed by that of clinical research. Many factors contribute to this. First, an overlap between the categories of clinical and QI research may have led to lower numbers in QI. Second, there may be a lag between the interest in QI translating into publications. This may be related both to the dearth of QI mentorship and to the barriers in publishing QI. These barriers include increasing competition in target journals, the lack of generalizability of QI efforts, and the compressed time frames of rapid improvement cycles that differ from the slower pace of clinical research and its measurements.[29] Hospitalists may also perform QI that results in scholarly output other than publications (eg, grand rounds, posters, or presentations) that we did not address. In the absence of QI publications, the systematic documentation of QI efforts in a portfolio may assist career advancement.[30]
The review of publications in the PubMed database through early October 2013 showed a consistent increase in the number of publications produced by hospitalist first authors. Clinical research was represented most frequently followed by health services and QI research. The predominance of clinical research parallels the large clinical role of hospitalists; however, the diversity of research categories represented reflects the growing penetration and involvement of hospitalists in the arenas of QI, health services, and education. Although our search identified fewer pediatric hospitalist articles, pediatric hospitalist literature is also on the rise. There are other indicators of the enthusiasm for research among pediatric hospitalists, as nearly half the respondents in our survey who are currently engaged in research and nearly a third who had successfully published or had funding support were trained in pediatrics.
Publications by first authors who were hospitalists or affiliated with hospital medicine represented the effort of more than 100 institutions, implying a widespread engagement in hospital medicine‐related scholarship. However, fewer centers produce original research, and over half the original research output is the product of 8 centers. Strategies to select and support person‐job fit,[31] availability of mentorship, the presence of existing infrastructure, funding, and departmental priorities are all likely to affect an institution's publication productivity. To emulate the success of these centers, a closer study of the strategies they employ[5] would be instructive for the broader hospitalist community.
Although our survey data showed that the presence of funding is associated with success in publishing, the percentage of hospitalists who report funding both from the survey and PubMed publication reports is <25%. This underscores the need for innovations that help hospitalists obtain support and incentives for their work.
This study has limitations. A survey is a cross‐sectional snap shot, and associations do not imply causation. Survey response rates have been falling,[32] and our convenience sampling without incentives engendered a low response rate. This response rate is similar to that of other surveys administered through SHM (SHM membership and marketing data, October 2013). Although statistical significance is presented, the differences may not be generalizable given the low response rate. We cannot quantify all responder biases or comment on how the membership fee to SHM may affect the sample cohort. The demographics of our respondents parallel that of the SHM membership base in age and gender. However, 25% of our respondents were trained in pediatrics, whereas only 4.3% of the SHM membership base is pediatrics trained (SHM membership and marketing data, October 2013). We did not inquire about contributions from job dissatisfaction to the lack of participation in research activities, and this may represent an area for further research.
The search methodology used in this study is likely to under‐report hospitalist‐related research, because collaborative publications in which the lead author is not a hospitalist were not included. Furthermore, many hospitalists are associated with centers that do not have a hospitalist or hospital medicine title or department, and our search terms would have missed the publications stemming from these centers. Pediatric hospitalist literature is likely to be further under‐represented, as centers may not have separate pediatric hospitalist departments.
The assignment of each publication into a research category was based on definitions found in the literature. However, this designation ultimately remains a subjective process that may introduce bias.
Although the initial growth spurt of hospital medicine can be attributed to its clinical success, the increase in hospital medicine‐led peer‐reviewed publications in increasingly diverse domains provides evidence that supports the field's concomitant academic and scholarly maturation. Research into factors that impede or inspire hospitalists to participate in research, innovations that provide mentorship and funding for the specific interests of hospitalists, and the emulation of strategies employed by centers productive in publications are required to successfully foster the multidimensional growth of the field.
Acknowledgements
The authors thank Dr. Antoinette Laskey for her mentorship in survey development, Elaine Bammerlin for copyediting assistance, and the Society of Hospital Medicine members for taking the survey.
Disclosures: An Dang Do, MD, PhD, completed the major part of this work as a Morris Green Scholar at Indiana University School of Medicine. An N. Dang Do, MD, PhD, and Amy M. Munchhof, MD, PhD, contributed equally to this work. Areeba Kara, MD, is supported by a grant from the Methodist Health Foundation and by award number T15OC000047 from the Office of the National Coordinator for Health Information Technology, Office of the Secretary, US Department of Health & Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the National Coordinator For Health Information Technology, Office of the Secretary, US Department of Health & Human Services, or the National Institutes of Health.
In 1996, Wachter and Goldman heralded the arrival of hospitalists in the healthcare system. They recognized the need to link the clinical role of a hospitalist with other activities, both to provide a creative outlet and to assist in the creation of research and development arms.[1] The explosive growth of hospital medicine followed, and hospitalists rapidly entered the mainstream of the healthcare system.[2]
A consensus conference in 2009 identified the challenges faced by hospitalists in conducting research as a key obstacle in the evolution of the profession into an academic field.[3] Strategies for building and facilitating hospitalist research programs have been described.[4, 5, 6, 7] However, a survey of US academic hospitalist leaders found more than 40% feared their faculty was not developing sustainable nonclinical activities.[8]
Data describing research aspirations and support systems among hospitalists are sparse, and no previous study has described the trends in hospitalist publications. In this work we describe the current standing of hospital medicine research through a survey of both academic and non‐academic hospitalists and a review of hospitalist‐related publications.
METHODS
The Indiana University institutional review board approved this study.
Survey of Hospitalists
A 29‐item questionnaire that addressed research activities, barriers, and mentorship was designed and piloted with pediatrics trainees at Indiana University. The final version (see Supporting Survey in the online version of this article) was approved by the Society of Hospital Medicine (SHM) research committee and posted on Zoomerang (
Review of Publications
A PubMed search was conducted on October 8, 2013 for records with either hospital medicine or hospitalist in the affiliation field. This field provides the departmental name and address information for the first author, except for the not‐yet‐indexed publisher‐supplied records, which could include all author addresses.[10] Editorials and letters to the editor were excluded, and results were limited to English. All resulting articles were manually curated and retained only if the affiliation criteria of hospitalist or hospital medicine (as a relevant single phrase) were associated with the first author. All articles meeting the criteria were reviewed by 1 of the authors and categorized as a review, a case report, or as original research (when methodology was described in the abstract). Original research articles were assigned a category based on their methodology and research type, as defined in published literature. The categories included basic sciences, clinical, health information, health services, quality improvement (QI), education, and translational research.[11, 12, 13, 14, 15] If the article overlapped categories, a secondary category was also assigned. A second author independently evaluated a subset of articles. This subset was then used to calculate the overall concordance between the authors based on their agreement on either the primary or secondary category designations.
To capture data on research funding, each original research article was searched for statements directly linking the first author or the work to the funding source(s).
Publications in the Journal of Hospital Medicine (JHM) were reviewed to serve as a gauge of research interests in the field of hospital medicine that may not be reflected by the publications resulting from the PubMed search. JHM was selected as the journal best representing hospital medicine based on its stated mission of commitment to the advancement of the hospital medicine specialty.[16] All original research articles in JHM were assigned a category by 1 of the authors based on the methodology in the abstract.
Statistical Methods
The survey responses were summarized using descriptive statistics. Univariate tests of association between respondent characteristics and peer‐reviewed authorship were performed using the Fisher's exact test. P values of 0.05 were considered significant. Data from the publication searches were presented as descriptive statistics.
RESULTS
Survey
The survey link was emailed to 11,611 SHM members: 11,102 members received the link and 509 emails were returned as undeliverable. A total of 645 member responses were received (5.8% response rate).
The most common demographic characteristics identified included male gender, age 45 or younger, and white race. The locations of the current practices were distributed equally across the United States. Over half of the respondents were trained in internal medicine, and a quarter were trained in pediatrics. Eleven percent had undertaken fellowship training after residency. Thirty‐seven percent did not hold an academic rank, and among those who did, most were assistant professors. (Table 1)
| Characteristics | Responses, N (%) | |||
|---|---|---|---|---|
| All Responses | Responses With Funding | |||
| ||||
| Gender | 597 | 67 | ||
| Female | 248 (41) | 33 (49) | ||
| Male | 349 (58) | 34 (51) | ||
| Age, y | 599 | 67 | ||
| 2535 | 157 (26) | 17 (25) | ||
| 3645 | 274 (46) | 39 (58) | ||
| 4655 | 105 (17) | 6 (9) | ||
| 5665 | 56 (9) | 5 (7) | ||
| >65 | 7 (1) | 0 | ||
| Current practice location | 596 | 67 | ||
| Midwest | 147 (25) | 18 (27) | ||
| Northeast | 113 (19) | 12 (18) | ||
| South | 172 (29) | 14 (21) | ||
| West | 142 (4) | 16 (24) | ||
| Other | 22 (34) | 7 (10) | ||
| Race | 595 | 67 | ||
| White | 444 (75) | 58 (87) | ||
| Black | 18 (3) | 0 | ||
| Hispanic | 22 (4) | 1 (1) | ||
| Asian | 85 (14) | 8 (12) | ||
| Other | 26 (4) | |||
| Faculty appointment | 593 | 68 | ||
| Nonacademic | 221 (37) | 4 (6) | ||
| Instructor/lecturer | 60 (10) | 6 (9) | ||
| Assistant professor | 197 (33) | 32 (47) | ||
| Associate professor | 68 (11) | 19 (28) | ||
| Full professor | 14 (2) | 4 (6) | ||
| Other | 33 (6) | 3 (4) | ||
| Fellowship training | 68 | 14 | ||
| General IM/hospitalist | 15 (22) | 6 (43) | ||
| Pediatric hospital medicine | 7 (10) | 2 (14) | ||
| Other | 46 (68) | 6 (43) | ||
| Residency completed | 616 | 68 | ||
| IM | 340 (55) | 36 (53) | ||
| Pediatrics | 154 (25) | 27 (40) | ||
| Family medicine | 53 (9) | 1 (1) | ||
| IM/pediatrics | 48 (8) | 2 (3) | ||
| Other | 21 (3) | 2 (3) | ||
Overall availability of mentorship was low, but respondents with academic appointments were more likely to have a mentor than those without academic appointments (32% vs 2.7%, p<0.001). Hospitalists most likely identified their own mentors, and meetings between the hospitalist and mentor occurred more frequently than once every 3 months.
There were 213 (33%) respondents who identified themselves as currently conducting research, 96 (45%) of whom were trained in pediatrics. Ninety‐two (28%) of those with academic appointments and 157 (71%) of those without academic appointments had no current or future plans to engage in research. QI research, followed by clinical research, emerged as the most frequent type of research that hospitalists were either currently engaged in or planned to embark on. Most respondents identified factors other than age, family or financial issues, the grant process, or a lack of institutional support as the reason for not conducting research. (Table 2)
| Activity | Responses, N (%) | ||||
|---|---|---|---|---|---|
| Adult Medicine | Pediatric Medicine | ||||
| |||||
| No plan to conduct research | 245 | 26 | |||
| Reasons for not doing research | |||||
| Lack of institutional support | 42 (17) | 3 (12) | |||
| Family issues | 14 (6) | 1 (4) | |||
| Financial | 8 (3) | 0 | |||
| Grant process | 4 (2) | 2 (8) | |||
| Age | 5 (2) | 0 | |||
| Other | 171 (70) | 20 (77) | |||
| Currently doing research | 117 | 96 | |||
| Quality improvement | 79 (68) | 73 (76) | |||
| Clinical | 59 (50) | 62 (65) | |||
| Health services | 31 (26) | 30 (31) | |||
| Health informatics | 28 (24) | 11 (11) | |||
| Translational | 10 (8) | 7 (7) | |||
| Basic science | 3 (3) | 0 | |||
| Other | 17 (14) | 10 (10) | |||
| Plan on doing research | 183 | 30 | |||
| Quality improvement | 72 (39) | 25 (83) | |||
| Clinical | 65 (35) | 25 (83) | |||
| Health services | 20 (11) | 2 (7) | |||
| Health informatics | 25 (14) | 3 (10) | |||
| Translational | 8 (4) | 3 (10) | |||
| Basic science | 3 (2) | 0 | |||
| Other | 8 (4) | 0 | |||
| Peer‐review publications | 458 | 151 | |||
| No | 270 (59) | 62 (41) | |||
| Yes | 188 (41) | 89 (59) | |||
| Frequency | |||||
| Less than once/year | 111 (59) | 41 (46) | |||
| Once/year | 22 (12) | 20 (22) | |||
| Twice/year | 16 (8) | 16 (18) | |||
| More than twice/year | 23 (12) | 10 (11) | |||
| Other | 13 (7) | 1 (1) | |||
| Publication Type | |||||
| Original research | 97 (52) | 75 (84) | |||
| Case report/series | 80 (42) | 41 (46) | |||
| Reviews | 63 (34) | 25 (28) | |||
| Clinical trials | 36 (19) | 9 (10) | |||
| Practice guidelines | 18 (10) | 12 (13) | |||
| Meta‐analysis | 14 (7) | 8 (9) | |||
| Other | 23 (12) | 0 | |||
Sixty‐eight (10%) respondents held research funding, and 6 identified the grant process as an impediment to doing research. The most commonly reported funding source was from government and institutions, followed by support from foundations (see Supporting Figure 1A in the online version of this article). Responders with research funding were predominantly young, white, and assistant or associate professors. Fourteen hospitalists with funding reported completing a fellowship. (Table 1)

More than half of the respondents (n=332) had not authored peer‐reviewed publications. Of the 277 who had published successfully, 89 (31%) were trained in pediatrics. For those with publications, 152 (55%) reported publishing less than once per year. The type of article published most frequently was original research followed by case reports/series and reviews. (Table 2)
Variables individually associated with an increased likelihood of authoring peer‐reviewed publications included the completion of a fellowship, having an academic appointment, the availability of funding and mentorship, a background of pediatrics training, and more than 25% dedicated research time. (Table 3)
| Authored Peer‐Reviewed Publications, N (%) | |||||
|---|---|---|---|---|---|
| Characteristics | No | Yes | P | ||
| |||||
| Age, y | 327 | 272 | 0.437 | ||
| 2535 | 85 (26) | 72 (26) | |||
| 3645 | 146 (45) | 128 (47) | |||
| 4655 | 64 (20) | 41 (15) | |||
| 5665 | 30 (9) | 26 (10) | |||
| >65 | 2 (1) | 5 (2) | |||
| Gender | 327 | 270 | 0.067 | ||
| Female | 147 (45) | 101 (37) | |||
| Male | 180 (55) | 169 (63) | |||
| Faculty appointment | 301 | 247 | <0.001 | ||
| Nonacademic | 161 (53) | 63 (25) | |||
| Academic | 140 (46) | 184 (74) | |||
| Residency | 331 | 275 | <0.001 | ||
| Family | 39 (12) | 14 (5) | |||
| Internal medicine | 184 (56) | 151 (55) | |||
| Internal medicine (pediatrics) | 33 (10) | 15 (5) | |||
| Pediatrics | 62 (19) | 89 (32) | |||
| Other | 13 (4) | 6 (2) | |||
| Completed fellowship training | 332 | 19 (6) | 277 | 47 (17) | <0.001 |
| Current research/career mentor | 327 | 30 (9) | 272 | 96 (35) | <0.001 |
| Meet with mentor | 29 | 88 | 0.433 | ||
| More often than every 6 months | 21 (72) | 71 (81) | |||
| Every 6 months or less | 8 (28) | 17 (19) | |||
| Time for research | 54 | 153 | <0.001 | ||
| 25% or less | 53 (98) | 122 (80) | |||
| More than 25% | 1 (2) | 31 (20) | |||
| Has funding | 54 | 8 (15) | 156 | 60 (38) | <0.001 |
Publications Review
The PubMed search yielded 784 publications with hospital medicine or hospitalist in the affiliation field. After manual review, 660 articles were retained. (Figure 1)
The volume of hospitalist‐led publications has been increasing. Between 2006 and October 2013 there was a 5‐fold increase in hospitalist‐led publications (36 in 2006 to 179 in the first 10 months of 2013). Of the 660 articles culled from the PubMed search, 581 (88%) represented the work of authors affiliated with adult hospital medicine; 266 (46%) of these represented original research (the rest were reviews and case reports). Seventy‐nine (12%) of the 660 PubMed articles were related to pediatric hospital medicine; 51 (65%) of these represented original research. (Figure 1) In the period studied there was a variation from year to year in the proportion of publications representing original research, with a range of 37% to 71% comprising original research in adult hospital medicine publications and 50% to 81% in pediatric hospital medicine publications (Figure 2A).

Nearly half (41%) of the original research in adult and pediatric medicine represented clinical research. Health services (21%) and QI (19%) were the next most frequent research categories published. Publications pertaining to research in education represented 15% of all original research. Health services and QI research are growing on a relatively stable base of clinical research. These trends were similar between adult and pediatric hospital medicine. (Figure 2B) The concordance rate on the assigned research categories was 82%, based on 67 publications that were independently reviewed by 2 authors.
There were 457 original research articles published in JHM between 2006 and early October 2013. JHM publications followed a trend similar to the publications of hospitalist‐affiliated first authors from PubMed, with the majority (47%) reflecting clinical research followed by health services (25%) and QI (25%). (see Supporting Figure 3 in the online version of this article)
In our review, adult medicine hospitalist authors were affiliated with 124 different universities or centers. However, 5 centers represented nearly half the publication volume. The Cleveland Clinic Foundation, University of California San Francisco (UCSF), Harvard, Northwestern, and the University of Chicago were the top producers. Fewer centers produce original research, with 66 counted in our search. Centers most prolific in producing original research are UCSF, Northwestern, University of Chicago, Harvard, and Johns Hopkins. Their combined output represented 56% of all published original published research. (see Supporting Figure 2A,B in the online version of this article)
In our review, publications attributed to pediatric hospitalists were the product of 34 different centers. Cincinnati Children's Hospital Medical Center, Children's National Medical Center (Georgetown University), and the Monroe Carell Children's Hospital (Vanderbilt) were the most productive in publishing. The same centers were also the most productive in publishing original research. (see Supporting Figure 2C,D in the online version of this article)
Funding data from the 317 original research articles found in PubMed showed that 52% had funding listed for the first author and/or the work. These publications were the work of 181 different first authors, of whom 39 (22%) had 1 or more funding sources specifically associated with them in the publications. The majority of these authors reported government funding (n=24), followed by support from foundations (n=12), institutions (n=8), and industries (n=6) (see Supporting Figure 1B in the online version of this article).
DISCUSSION
Using results from both the survey and our review of publications in PubMed provided complementary information that has enriched our evaluation and reporting of the current state of research and publications in hospital medicine.
The initial growth of the field of hospital medicine can be attributed to its clinical contributions.[17] However, hospital medicine faces numerous challenges in its evolution into an academic specialty.[3] Job satisfaction rates among hospitalists may be falling,[18, 19] and pursuing intellectual outlets such as research may improve both satisfaction and productivity.[20, 21] Therefore, it is important to study the predictors of success for the nonclinical intellectual endeavors of hospitalists.
Across the career spectrum in academic medicine, effective mentorship has been found to be beneficial in enhancing teaching skills, productivity, and satisfaction.[22] Similar to prior studies, we found that mentorship was not readily accessible, and its absence was associated with a decreased likelihood of peer‐reviewed publications.[23, 24] Hospital medicine remains a youthful specialty, with the mean age of clinicians in the 40s.[18] In our survey, hospitalists aged 36 to 45 years reported the highest rates of publications and funding. If these hospitalists can be retained in the field, they may eventually serve as mentors to those entering the specialty. Strategies to provide mentorship have been described,[25] and continued efforts to innovate are needed in the development of mentorship potential.
Successfully promoted hospitalists identify peer‐reviewed publications as a key activity that supports promotion.[26] However, similar to Reid et al.,[23] our survey found that hospitalists reported low rates of peer‐reviewed publications. Hospitalists have unique access to the inpatient population, and setting up collaborative efforts between specialists and hospitalists, or participating in multi‐institutional projects that require patient recruitment,[27] may facilitate research and publication productivity. A specific emerging opportunity for this expertise is the need for collecting and identifying disease presentations to correlate with the exploding genetic data now available.[28]
QI research was identified from our survey results as the most frequent type of research that hospitalists were either engaged in or planned to pursue. However, based on our review of published research, the volume of QI research is surpassed by that of clinical research. Many factors contribute to this. First, an overlap between the categories of clinical and QI research may have led to lower numbers in QI. Second, there may be a lag between the interest in QI translating into publications. This may be related both to the dearth of QI mentorship and to the barriers in publishing QI. These barriers include increasing competition in target journals, the lack of generalizability of QI efforts, and the compressed time frames of rapid improvement cycles that differ from the slower pace of clinical research and its measurements.[29] Hospitalists may also perform QI that results in scholarly output other than publications (eg, grand rounds, posters, or presentations) that we did not address. In the absence of QI publications, the systematic documentation of QI efforts in a portfolio may assist career advancement.[30]
The review of publications in the PubMed database through early October 2013 showed a consistent increase in the number of publications produced by hospitalist first authors. Clinical research was represented most frequently followed by health services and QI research. The predominance of clinical research parallels the large clinical role of hospitalists; however, the diversity of research categories represented reflects the growing penetration and involvement of hospitalists in the arenas of QI, health services, and education. Although our search identified fewer pediatric hospitalist articles, pediatric hospitalist literature is also on the rise. There are other indicators of the enthusiasm for research among pediatric hospitalists, as nearly half the respondents in our survey who are currently engaged in research and nearly a third who had successfully published or had funding support were trained in pediatrics.
Publications by first authors who were hospitalists or affiliated with hospital medicine represented the effort of more than 100 institutions, implying a widespread engagement in hospital medicine‐related scholarship. However, fewer centers produce original research, and over half the original research output is the product of 8 centers. Strategies to select and support person‐job fit,[31] availability of mentorship, the presence of existing infrastructure, funding, and departmental priorities are all likely to affect an institution's publication productivity. To emulate the success of these centers, a closer study of the strategies they employ[5] would be instructive for the broader hospitalist community.
Although our survey data showed that the presence of funding is associated with success in publishing, the percentage of hospitalists who report funding both from the survey and PubMed publication reports is <25%. This underscores the need for innovations that help hospitalists obtain support and incentives for their work.
This study has limitations. A survey is a cross‐sectional snap shot, and associations do not imply causation. Survey response rates have been falling,[32] and our convenience sampling without incentives engendered a low response rate. This response rate is similar to that of other surveys administered through SHM (SHM membership and marketing data, October 2013). Although statistical significance is presented, the differences may not be generalizable given the low response rate. We cannot quantify all responder biases or comment on how the membership fee to SHM may affect the sample cohort. The demographics of our respondents parallel that of the SHM membership base in age and gender. However, 25% of our respondents were trained in pediatrics, whereas only 4.3% of the SHM membership base is pediatrics trained (SHM membership and marketing data, October 2013). We did not inquire about contributions from job dissatisfaction to the lack of participation in research activities, and this may represent an area for further research.
The search methodology used in this study is likely to under‐report hospitalist‐related research, because collaborative publications in which the lead author is not a hospitalist were not included. Furthermore, many hospitalists are associated with centers that do not have a hospitalist or hospital medicine title or department, and our search terms would have missed the publications stemming from these centers. Pediatric hospitalist literature is likely to be further under‐represented, as centers may not have separate pediatric hospitalist departments.
The assignment of each publication into a research category was based on definitions found in the literature. However, this designation ultimately remains a subjective process that may introduce bias.
Although the initial growth spurt of hospital medicine can be attributed to its clinical success, the increase in hospital medicine‐led peer‐reviewed publications in increasingly diverse domains provides evidence that supports the field's concomitant academic and scholarly maturation. Research into factors that impede or inspire hospitalists to participate in research, innovations that provide mentorship and funding for the specific interests of hospitalists, and the emulation of strategies employed by centers productive in publications are required to successfully foster the multidimensional growth of the field.
Acknowledgements
The authors thank Dr. Antoinette Laskey for her mentorship in survey development, Elaine Bammerlin for copyediting assistance, and the Society of Hospital Medicine members for taking the survey.
Disclosures: An Dang Do, MD, PhD, completed the major part of this work as a Morris Green Scholar at Indiana University School of Medicine. An N. Dang Do, MD, PhD, and Amy M. Munchhof, MD, PhD, contributed equally to this work. Areeba Kara, MD, is supported by a grant from the Methodist Health Foundation and by award number T15OC000047 from the Office of the National Coordinator for Health Information Technology, Office of the Secretary, US Department of Health & Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the National Coordinator For Health Information Technology, Office of the Secretary, US Department of Health & Human Services, or the National Institutes of Health.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- , , , . The status of hospital medicine groups in the United States. J Hosp Med. 2006;1(2):75–80.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308–313.
- , , , . An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314–318.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 25, 2013.
- US National Library of Medicine website. MEDLINE/PubMed data element (field) descriptions. Available at: http://www.nlm.nih.gov/bsd/mms/medlineelements.html. Accessed October 30, 2013.
- American Educational Research Association website. Available at: http://www.aera.net/EducationResearch/WhatisEducationResearch/tab id/13453/Default.aspx. Accessed October 30, 2013.
- National Institutes of Health website. Glossary of NIH terms. Available at: http://grants.nih.gov/grants/glossary.htm. Accessed February 26, 2013.
- US National Science Foundation website. National Center for Science and Engineering Statistics. Definitions of research and development: an annotated compilation of official sources. Available at: http://www.nsf.gov/statistics/randdef/fedgov.cfm. Accessed February 26, 2013.
- , . Health services research: an evolving definition of the field. US National Library of Medicine website. National Institutes of Health. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430351. Accessed February 26, 2013.
- Centers for Medicare and Medicaid Services website. Outcome measures. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/OutcomeMeasu res.html. Accessed August 22, 2013.
- Journal of Hospital Medicine. Available at: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1553–5606/homepage/ProductInformation.html. Accessed October 30, 2013.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , , . Preparing for “diastole”: Advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103–1115.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2011;27(1):23–27.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1–2.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , . The APA and the rise of pediatric generalist network research. Acad Pediatr. 2011;11(3):195–204.
- , , , et al. Phenotype harmonization and cross‐study collaboration in GWAS consortia: the GENEVA experience. Genet Epidemiol. 2011;35(3):159–173.
- , . Clinicians in quality improvement: a new career pathway in academic medicine. JAMA. 2009;301(7):766–768.
- , , , , Documenting quality improvement and patient safety efforts: the quality portfolio. A statement from the Academic Hospitalist Taskforce [published online ahead of print June 27, 2013]. J Gen Intern Med. doi: 10.1007/s11606‐013‐2532‐z.
- , , , , . Person‐job fit: an exploratory cross‐sectional analysis of hospitalists. J Hosp Med. 2012;8(2):96–101.
- , , , . Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40(1):213–226.
- , . The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514–517.
- , , , . The status of hospital medicine groups in the United States. J Hosp Med. 2006;1(2):75–80.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . The University of Michigan Specialist‐Hospitalist Allied Research Program: jumpstarting hospital medicine research. J Hosp Med. 2008;3(4):308–313.
- , , , . An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314–318.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed February 25, 2013.
- US National Library of Medicine website. MEDLINE/PubMed data element (field) descriptions. Available at: http://www.nlm.nih.gov/bsd/mms/medlineelements.html. Accessed October 30, 2013.
- American Educational Research Association website. Available at: http://www.aera.net/EducationResearch/WhatisEducationResearch/tab id/13453/Default.aspx. Accessed October 30, 2013.
- National Institutes of Health website. Glossary of NIH terms. Available at: http://grants.nih.gov/grants/glossary.htm. Accessed February 26, 2013.
- US National Science Foundation website. National Center for Science and Engineering Statistics. Definitions of research and development: an annotated compilation of official sources. Available at: http://www.nsf.gov/statistics/randdef/fedgov.cfm. Accessed February 26, 2013.
- , . Health services research: an evolving definition of the field. US National Library of Medicine website. National Institutes of Health. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1430351. Accessed February 26, 2013.
- Centers for Medicare and Medicaid Services website. Outcome measures. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/HospitalQualityInits/OutcomeMeasu res.html. Accessed August 22, 2013.
- Journal of Hospital Medicine. Available at: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1553–5606/homepage/ProductInformation.html. Accessed October 30, 2013.
- . A systematic review of outcomes and quality measures in adult patients cared for by hospitalists vs nonhospitalists. Mayo Clin Proc. 2009;84(3):248–254.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , , , , , . Career satisfaction and burnout in academic hospital medicine. Arch Intern Med. 2011;171(8):782–785.
- , , , . Preparing for “diastole”: Advanced training opportunities for academic hospitalists. J Hosp Med. 2006;1(6):368–377.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , . Mentoring in academic medicine: a systematic review. JAMA. 2006;296(9):1103–1115.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2011;27(1):23–27.
- , . The need for mentors in the odyssey of the academic hospitalist. J Hosp Med. 2011;6(1):1–2.
- , , , . Research in progress conference for hospitalists provides valuable peer mentoring. J Hosp Med. 2011;6(1):43–46.
- , , , , . Tried and true: a survey of successfully promoted academic hospitalists. J Hosp Med. 2011;6(7):411–415.
- , , , , . The APA and the rise of pediatric generalist network research. Acad Pediatr. 2011;11(3):195–204.
- , , , et al. Phenotype harmonization and cross‐study collaboration in GWAS consortia: the GENEVA experience. Genet Epidemiol. 2011;35(3):159–173.
- , . Clinicians in quality improvement: a new career pathway in academic medicine. JAMA. 2009;301(7):766–768.
- , , , , Documenting quality improvement and patient safety efforts: the quality portfolio. A statement from the Academic Hospitalist Taskforce [published online ahead of print June 27, 2013]. J Gen Intern Med. doi: 10.1007/s11606‐013‐2532‐z.
- , , , , . Person‐job fit: an exploratory cross‐sectional analysis of hospitalists. J Hosp Med. 2012;8(2):96–101.
- , , , . Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40(1):213–226.
© 2014 Society of Hospital Medicine
Surgical Treatment of Symptomatic Accessory Navicular in Children and Adolescents
The Orthopedic Stepchild
Throughout antiquity, physicians and surgeons have concerned themselves with maladies of the foot and ankle. The literature is rife with articles describing management of clubfoot deformities and traumatic amputations of feet and legs. Authors have described tenotomies and manipulation for clubfeet as well as optimal techniques and levels for amputations to promote healing and functional outcomes. In progressive and aggressive surgical centers in Austria and Germany, techniques for correction of the deformities created by disease and trauma formed the basis for today’s reconstructive methodologies.
During my orthopedic residency in the 1960s, we managed pediatric versions of clubfoot, vertical talus, and neuromuscular conditions of the lower extremity (myelomeningocele, muscular dystrophy, cerebral palsy); adolescent bunions and pathologic flat feet; and, in adults, residual polio, arthritis, bunions, lesser toe deformities, ankle disorders, and trauma. Then along came the excitement of total joint arthroplasty, with its spectacular results, and the thrill in devoting careers to athletes and their myriad problems. Other interesting subspecialties emerged, and the orthopedic focus on a significant part of our heritage, the issues of foot and ankle, was lost for decades. Care for these problems was left to a small cadre of pediatric doctors, and soon-to-retire orthopedists who tended to view the field as
less demanding. Dynamic young practitioners showed little or no interest in caring for foot and ankle patients, and no progress was made in clinical care, research, and development of orthopedic technology and devices.
In the late 1960s, a small group of middle-aged and senior devotees of the specialty met in New York to form the American Orthopaedic Foot Society (AOFS), later to become the American Orthopaedic Foot and Ankle Society (AOFAS). The group’s goal was to renew interest in the foot and ankle specialty among orthopedic surgeons. As everyone knows, AOFAS has flourished and become one of the most progressive, innovative, and dynamic of all the orthopedic subspecialty
groups. In 1985, John Gartland, president of the American Academy of Orthopaedic Surgeons, called together the leaders in the foot and ankle field to formulate a long-range plan to reclaim foot and ankle from the morass of substandard care
and to advance the subspecialty in every quarter. As AOFS (and later AOFAS) president, I was part of Gartland’s team. I recall we aimed to convince orthopedic chairs, the Residency Review Committee for Orthopaedic Surgery, and the Board
of Orthopaedic Surgery to increase training requirements, to develop foot and ankle educators, and to promote the area in training programs. Orthopedic educators developed fellowships (essentially nonexistent up until then) and organized and taught beginner and advanced continuing education courses at annual meetings and throughout the year.
There were other needs to be addressed. One was to educate nonorthopedic doctors to appreciate that foot and ankle problems had good nonoperative and surgical solutions, that there was an orthopedic subspecialty for these conditions, and that foot and ankle patients should be referred to its practitioners. Second, these patients’ public advocacy groups needed to know what knowledgeable orthopedists could provide and needed to be encouraged to seek care from these physicians rather than from less qualified providers and nonspecialists.
To an extraordinary degree, the goal of educating orthopedists has been achieved, and the field is now populated with young, energetic leaders, teachers, and practitioners. We have been less successful in educating potential referring physicians, the public, public advocacy groups, and third-party payers, including the US government. Progress has been made with private insurers and, as advisors, with the Centers for Medicare and Medicaid Services and state government health committees.
Driven by emerging market opportunities, the orthopedic device industry has made unanticipated and enormous advances in the distal lower extremity realm. Small
companies have been founded, and larger companies have dedicated entire divisions to making fixation devices and prosthetic implants for every procedure involving the foot or the ankle. Biomedical engineers, metallurgists, orthopedic
consultant researchers, and well-funded projects have led the surge to develop the best foot and ankle technology. In addition, industry courses and scholarships for residents, fellows, teaching programs, and young physicians have been generating interest in these advances. Although it may be argued that entrepreneurship brings enormous bias, it must be conceded that interest in the foot and ankle field has increased tremendously. Outreach programs for foot and ankle care in the Third World have emerged as an additional humanitarian benefit of the expansion of the field.
From its strong start as a medical specialty to its fall into ignorance and neglect, the foot and ankle field, the unwanted stepchild of medicine and orthopedics, has made a dramatic recovery and has become a premier example of what medicine can achieve through focused effort. As leaders in orthopedic medicine in North America, we must also acknowledge the huge contributions made by a sterling array of international researchers, educators, and practitioners.
Some journals in the United States and other countries now concentrate solely on foot and ankle. Nevertheless, it is appropriate that The American Journal of Orthopedics and other general orthopedic surgery publications focus on foot and
ankle (and other specialties) in an annual issue. As each orthopedist tends mainly to his or her own area of interest, it is essential that we all stay current on the field as a whole. The basic science, innovations, and concepts of one specialty are often applicable to the entire field, and a casual notation of an idea from such a focused issue may have unimagined benefits for the readership and their patients. ◾
Throughout antiquity, physicians and surgeons have concerned themselves with maladies of the foot and ankle. The literature is rife with articles describing management of clubfoot deformities and traumatic amputations of feet and legs. Authors have described tenotomies and manipulation for clubfeet as well as optimal techniques and levels for amputations to promote healing and functional outcomes. In progressive and aggressive surgical centers in Austria and Germany, techniques for correction of the deformities created by disease and trauma formed the basis for today’s reconstructive methodologies.
During my orthopedic residency in the 1960s, we managed pediatric versions of clubfoot, vertical talus, and neuromuscular conditions of the lower extremity (myelomeningocele, muscular dystrophy, cerebral palsy); adolescent bunions and pathologic flat feet; and, in adults, residual polio, arthritis, bunions, lesser toe deformities, ankle disorders, and trauma. Then along came the excitement of total joint arthroplasty, with its spectacular results, and the thrill in devoting careers to athletes and their myriad problems. Other interesting subspecialties emerged, and the orthopedic focus on a significant part of our heritage, the issues of foot and ankle, was lost for decades. Care for these problems was left to a small cadre of pediatric doctors, and soon-to-retire orthopedists who tended to view the field as
less demanding. Dynamic young practitioners showed little or no interest in caring for foot and ankle patients, and no progress was made in clinical care, research, and development of orthopedic technology and devices.
In the late 1960s, a small group of middle-aged and senior devotees of the specialty met in New York to form the American Orthopaedic Foot Society (AOFS), later to become the American Orthopaedic Foot and Ankle Society (AOFAS). The group’s goal was to renew interest in the foot and ankle specialty among orthopedic surgeons. As everyone knows, AOFAS has flourished and become one of the most progressive, innovative, and dynamic of all the orthopedic subspecialty
groups. In 1985, John Gartland, president of the American Academy of Orthopaedic Surgeons, called together the leaders in the foot and ankle field to formulate a long-range plan to reclaim foot and ankle from the morass of substandard care
and to advance the subspecialty in every quarter. As AOFS (and later AOFAS) president, I was part of Gartland’s team. I recall we aimed to convince orthopedic chairs, the Residency Review Committee for Orthopaedic Surgery, and the Board
of Orthopaedic Surgery to increase training requirements, to develop foot and ankle educators, and to promote the area in training programs. Orthopedic educators developed fellowships (essentially nonexistent up until then) and organized and taught beginner and advanced continuing education courses at annual meetings and throughout the year.
There were other needs to be addressed. One was to educate nonorthopedic doctors to appreciate that foot and ankle problems had good nonoperative and surgical solutions, that there was an orthopedic subspecialty for these conditions, and that foot and ankle patients should be referred to its practitioners. Second, these patients’ public advocacy groups needed to know what knowledgeable orthopedists could provide and needed to be encouraged to seek care from these physicians rather than from less qualified providers and nonspecialists.
To an extraordinary degree, the goal of educating orthopedists has been achieved, and the field is now populated with young, energetic leaders, teachers, and practitioners. We have been less successful in educating potential referring physicians, the public, public advocacy groups, and third-party payers, including the US government. Progress has been made with private insurers and, as advisors, with the Centers for Medicare and Medicaid Services and state government health committees.
Driven by emerging market opportunities, the orthopedic device industry has made unanticipated and enormous advances in the distal lower extremity realm. Small
companies have been founded, and larger companies have dedicated entire divisions to making fixation devices and prosthetic implants for every procedure involving the foot or the ankle. Biomedical engineers, metallurgists, orthopedic
consultant researchers, and well-funded projects have led the surge to develop the best foot and ankle technology. In addition, industry courses and scholarships for residents, fellows, teaching programs, and young physicians have been generating interest in these advances. Although it may be argued that entrepreneurship brings enormous bias, it must be conceded that interest in the foot and ankle field has increased tremendously. Outreach programs for foot and ankle care in the Third World have emerged as an additional humanitarian benefit of the expansion of the field.
From its strong start as a medical specialty to its fall into ignorance and neglect, the foot and ankle field, the unwanted stepchild of medicine and orthopedics, has made a dramatic recovery and has become a premier example of what medicine can achieve through focused effort. As leaders in orthopedic medicine in North America, we must also acknowledge the huge contributions made by a sterling array of international researchers, educators, and practitioners.
Some journals in the United States and other countries now concentrate solely on foot and ankle. Nevertheless, it is appropriate that The American Journal of Orthopedics and other general orthopedic surgery publications focus on foot and
ankle (and other specialties) in an annual issue. As each orthopedist tends mainly to his or her own area of interest, it is essential that we all stay current on the field as a whole. The basic science, innovations, and concepts of one specialty are often applicable to the entire field, and a casual notation of an idea from such a focused issue may have unimagined benefits for the readership and their patients. ◾
Throughout antiquity, physicians and surgeons have concerned themselves with maladies of the foot and ankle. The literature is rife with articles describing management of clubfoot deformities and traumatic amputations of feet and legs. Authors have described tenotomies and manipulation for clubfeet as well as optimal techniques and levels for amputations to promote healing and functional outcomes. In progressive and aggressive surgical centers in Austria and Germany, techniques for correction of the deformities created by disease and trauma formed the basis for today’s reconstructive methodologies.
During my orthopedic residency in the 1960s, we managed pediatric versions of clubfoot, vertical talus, and neuromuscular conditions of the lower extremity (myelomeningocele, muscular dystrophy, cerebral palsy); adolescent bunions and pathologic flat feet; and, in adults, residual polio, arthritis, bunions, lesser toe deformities, ankle disorders, and trauma. Then along came the excitement of total joint arthroplasty, with its spectacular results, and the thrill in devoting careers to athletes and their myriad problems. Other interesting subspecialties emerged, and the orthopedic focus on a significant part of our heritage, the issues of foot and ankle, was lost for decades. Care for these problems was left to a small cadre of pediatric doctors, and soon-to-retire orthopedists who tended to view the field as
less demanding. Dynamic young practitioners showed little or no interest in caring for foot and ankle patients, and no progress was made in clinical care, research, and development of orthopedic technology and devices.
In the late 1960s, a small group of middle-aged and senior devotees of the specialty met in New York to form the American Orthopaedic Foot Society (AOFS), later to become the American Orthopaedic Foot and Ankle Society (AOFAS). The group’s goal was to renew interest in the foot and ankle specialty among orthopedic surgeons. As everyone knows, AOFAS has flourished and become one of the most progressive, innovative, and dynamic of all the orthopedic subspecialty
groups. In 1985, John Gartland, president of the American Academy of Orthopaedic Surgeons, called together the leaders in the foot and ankle field to formulate a long-range plan to reclaim foot and ankle from the morass of substandard care
and to advance the subspecialty in every quarter. As AOFS (and later AOFAS) president, I was part of Gartland’s team. I recall we aimed to convince orthopedic chairs, the Residency Review Committee for Orthopaedic Surgery, and the Board
of Orthopaedic Surgery to increase training requirements, to develop foot and ankle educators, and to promote the area in training programs. Orthopedic educators developed fellowships (essentially nonexistent up until then) and organized and taught beginner and advanced continuing education courses at annual meetings and throughout the year.
There were other needs to be addressed. One was to educate nonorthopedic doctors to appreciate that foot and ankle problems had good nonoperative and surgical solutions, that there was an orthopedic subspecialty for these conditions, and that foot and ankle patients should be referred to its practitioners. Second, these patients’ public advocacy groups needed to know what knowledgeable orthopedists could provide and needed to be encouraged to seek care from these physicians rather than from less qualified providers and nonspecialists.
To an extraordinary degree, the goal of educating orthopedists has been achieved, and the field is now populated with young, energetic leaders, teachers, and practitioners. We have been less successful in educating potential referring physicians, the public, public advocacy groups, and third-party payers, including the US government. Progress has been made with private insurers and, as advisors, with the Centers for Medicare and Medicaid Services and state government health committees.
Driven by emerging market opportunities, the orthopedic device industry has made unanticipated and enormous advances in the distal lower extremity realm. Small
companies have been founded, and larger companies have dedicated entire divisions to making fixation devices and prosthetic implants for every procedure involving the foot or the ankle. Biomedical engineers, metallurgists, orthopedic
consultant researchers, and well-funded projects have led the surge to develop the best foot and ankle technology. In addition, industry courses and scholarships for residents, fellows, teaching programs, and young physicians have been generating interest in these advances. Although it may be argued that entrepreneurship brings enormous bias, it must be conceded that interest in the foot and ankle field has increased tremendously. Outreach programs for foot and ankle care in the Third World have emerged as an additional humanitarian benefit of the expansion of the field.
From its strong start as a medical specialty to its fall into ignorance and neglect, the foot and ankle field, the unwanted stepchild of medicine and orthopedics, has made a dramatic recovery and has become a premier example of what medicine can achieve through focused effort. As leaders in orthopedic medicine in North America, we must also acknowledge the huge contributions made by a sterling array of international researchers, educators, and practitioners.
Some journals in the United States and other countries now concentrate solely on foot and ankle. Nevertheless, it is appropriate that The American Journal of Orthopedics and other general orthopedic surgery publications focus on foot and
ankle (and other specialties) in an annual issue. As each orthopedist tends mainly to his or her own area of interest, it is essential that we all stay current on the field as a whole. The basic science, innovations, and concepts of one specialty are often applicable to the entire field, and a casual notation of an idea from such a focused issue may have unimagined benefits for the readership and their patients. ◾
Role for GIST genotyping stirs controversy
MILAN – Many U.S. patients with gastrointestinal stromal tumors today start on the wrong adjuvant treatment because their physicians don’t order genetic assessment of the cancer, said two American oncologists.
"Initial treatment for GIST [gastrointestinal stromal tumors] is enhanced by molecular decision making," Dr. Jonathan C. Trent said at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology.
"I’ve had patients referred to me from academic centers where the patient was treated with imatinib [Gleevec], which didn’t work, sunitinib [Sutent], which didn’t work, and regorafenib [Stivarga], which didn’t work. We did genetic testing, and it was a D842V mutation [in the PDGFRA gene]. This patient should never have been treated with these drugs," because GIST that carry this PDGFRA mutation are resistant to all three of these tyrosine kinase inhibitor [TKI] drugs, said Dr. Trent, professor of medicine and director of the bone and soft tissue program at the University of Miami.
"If you’re thinking of giving adjuvant therapy, you absolutely need molecular profiling because in the primary, resected-disease setting, a full 20% of patients will have a mutation that makes imatinib useless, the D842V mutation," said Dr. George D. Demetri, professor of medicine at Harvard University and director of the Center for Sarcoma and Bone Oncology at Dana Farber Cancer Institute, Boston. The potential consequence of not performing a genetic analysis is "you could overtreat 20% of patients with what is a still moderately expensive drug that will give them side effects for 3 years when they don’t need it. That is bad medicine," Dr. Demetri said in an interview. "I’m flabbergasted that more people are not getting molecular profiling for GIST patients being considered for adjuvant therapy. We have not communicated this well. Genetic testing is easily accessible at reference labs."
Dr. Demetri highlighted that identifying a D842V mutation in the PDGFRA gene of a GIST is good news for patients, because when this mutation appears in a primary tumor it flags a very indolent form of GIST. "The physician can tell patients that they don’t need to take this drug because it won’t do them any good, plus most patients with your mutation don’t have their tumor recur for many years and sometimes never."
In its most recent guidelines for GIST management, the National Comprehensive Cancer Network (NCCN) said: "If tyrosine kinase inhibitors are considered as part of the treatment plan, genetic analysis of the tumor should be considered since the presence of mutations (or absence of mutations) in specific regions of the KIT and PDGFRA tyrosine kinase genes are correlated with response (or lack of a response) to specific tyrosine kinase inhibitors."
Similar language exists in the posted GIST management recommendations of the National Cancer Institute (NCI), which date from 2012: "KIT- and PDGFRA-mutational analysis may be of help in predicting responses to kinase inhibitors for patients with unresectable, metastatic, or recurrent GIST who are undergoing therapy with selective TKIs. However, the data are preliminary and mutational analysis for treatment decisions is not routine. There is currently no evidence that basing treatment decisions on mutational analysis improves OS [overall survival]."
Dr. Trent took issue with these positions and said that the NCCN and NCI need to call genetic assessment of primary GIST necessary, especially for patients considered for adjuvant treatment. But others saw reason for equivocation.
"Since the mutational status of GIST can impact your use of adjuvant therapy or even therapy for metastatic disease, most sarcoma physicians prefer to see it done," noted Dr. Robert G. Maki, professor of medicine and director of the sarcoma program at Mount Sinai Medical Center in New York. "That said, we often do not have clinical trial data to support" this approach to management. For example, no trial results clearly show that GIST that carry a PDGFRA D842V mutation do not respond well to imatinib and have better outcomes when treated with some other drug. "I would like to have these data to discuss the options" with patients, Dr. Maki said in an interview. The relatively well-described patterns of genetic mutation and drug sensitivity seen in GIST make this tumor different from other adult sarcomas, he added.
"We had a patient with the PDGFRA D842V mutation who clearly responded to imatinib," said Dr. Robert S. Benjamin, professor and chairman of sarcoma medical oncology at M.D. Anderson Cancer Center in Houston.
"You use imatinib empirically because it is the most benign drug, especially at the 400-mg/day level," Dr. Benjamin said in an interview. "It’s well tolerated, and it usually works." But Dr. Benjamin acknowledged the added value of learning a tumor’s genetic profile. "It’s analogous to infectious disease," where you might start a patient on an empiric antibiotic but then reconsider once you receive antibiotic-sensitivity results. Genotyping complements the clinical findings made after starting a patient on imatinib, he said.
Based on current data for GIST sensitivity to TKIs, Dr. Trent and Dr. Demetri summarized the current GIST mutation and treatment landscape this way:
• About 60% of GIST have the most common tyrosine kinase mutation, in exon 11 of the KIT gene, and are sensitive to 400 mg/day of imatinib.
• About 7% of GIST have the exon 9 mutation of KIT and are sensitive to a higher dosage of imatinib, ideally 800 mg/day if that is tolerated.
• About 20% of GIST have the D842V mutation in the PDGFRA tyrosine kinase gene, and these patients are candidates for enrollment in a trial, as no regimens are known effective for these tumors.
• About 12% of GIST have a mutation in the SDF gene, which appears to make them resistant to imatinib and sunitinib but which may be sensitive to another TKI, regorafenib.
• The remaining GIST have other, rare mutations.
Dr. Trent said that he had no disclosures. Dr. Demetri said that he has been a consultant to Bayer, Novartis, and other companies. Dr. Maki said that he has been a consultant to Eisai/Morphotek, Bayer, and other companies, and has received research support from Eisai/Morphotek, Tracon, and Bayer. Dr. Benjamin said that he has been a consultant to Johnson & Johnson, Merck, and Pfizer.
On Twitter @mitchelzoler
MILAN – Many U.S. patients with gastrointestinal stromal tumors today start on the wrong adjuvant treatment because their physicians don’t order genetic assessment of the cancer, said two American oncologists.
"Initial treatment for GIST [gastrointestinal stromal tumors] is enhanced by molecular decision making," Dr. Jonathan C. Trent said at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology.
"I’ve had patients referred to me from academic centers where the patient was treated with imatinib [Gleevec], which didn’t work, sunitinib [Sutent], which didn’t work, and regorafenib [Stivarga], which didn’t work. We did genetic testing, and it was a D842V mutation [in the PDGFRA gene]. This patient should never have been treated with these drugs," because GIST that carry this PDGFRA mutation are resistant to all three of these tyrosine kinase inhibitor [TKI] drugs, said Dr. Trent, professor of medicine and director of the bone and soft tissue program at the University of Miami.
"If you’re thinking of giving adjuvant therapy, you absolutely need molecular profiling because in the primary, resected-disease setting, a full 20% of patients will have a mutation that makes imatinib useless, the D842V mutation," said Dr. George D. Demetri, professor of medicine at Harvard University and director of the Center for Sarcoma and Bone Oncology at Dana Farber Cancer Institute, Boston. The potential consequence of not performing a genetic analysis is "you could overtreat 20% of patients with what is a still moderately expensive drug that will give them side effects for 3 years when they don’t need it. That is bad medicine," Dr. Demetri said in an interview. "I’m flabbergasted that more people are not getting molecular profiling for GIST patients being considered for adjuvant therapy. We have not communicated this well. Genetic testing is easily accessible at reference labs."
Dr. Demetri highlighted that identifying a D842V mutation in the PDGFRA gene of a GIST is good news for patients, because when this mutation appears in a primary tumor it flags a very indolent form of GIST. "The physician can tell patients that they don’t need to take this drug because it won’t do them any good, plus most patients with your mutation don’t have their tumor recur for many years and sometimes never."
In its most recent guidelines for GIST management, the National Comprehensive Cancer Network (NCCN) said: "If tyrosine kinase inhibitors are considered as part of the treatment plan, genetic analysis of the tumor should be considered since the presence of mutations (or absence of mutations) in specific regions of the KIT and PDGFRA tyrosine kinase genes are correlated with response (or lack of a response) to specific tyrosine kinase inhibitors."
Similar language exists in the posted GIST management recommendations of the National Cancer Institute (NCI), which date from 2012: "KIT- and PDGFRA-mutational analysis may be of help in predicting responses to kinase inhibitors for patients with unresectable, metastatic, or recurrent GIST who are undergoing therapy with selective TKIs. However, the data are preliminary and mutational analysis for treatment decisions is not routine. There is currently no evidence that basing treatment decisions on mutational analysis improves OS [overall survival]."
Dr. Trent took issue with these positions and said that the NCCN and NCI need to call genetic assessment of primary GIST necessary, especially for patients considered for adjuvant treatment. But others saw reason for equivocation.
"Since the mutational status of GIST can impact your use of adjuvant therapy or even therapy for metastatic disease, most sarcoma physicians prefer to see it done," noted Dr. Robert G. Maki, professor of medicine and director of the sarcoma program at Mount Sinai Medical Center in New York. "That said, we often do not have clinical trial data to support" this approach to management. For example, no trial results clearly show that GIST that carry a PDGFRA D842V mutation do not respond well to imatinib and have better outcomes when treated with some other drug. "I would like to have these data to discuss the options" with patients, Dr. Maki said in an interview. The relatively well-described patterns of genetic mutation and drug sensitivity seen in GIST make this tumor different from other adult sarcomas, he added.
"We had a patient with the PDGFRA D842V mutation who clearly responded to imatinib," said Dr. Robert S. Benjamin, professor and chairman of sarcoma medical oncology at M.D. Anderson Cancer Center in Houston.
"You use imatinib empirically because it is the most benign drug, especially at the 400-mg/day level," Dr. Benjamin said in an interview. "It’s well tolerated, and it usually works." But Dr. Benjamin acknowledged the added value of learning a tumor’s genetic profile. "It’s analogous to infectious disease," where you might start a patient on an empiric antibiotic but then reconsider once you receive antibiotic-sensitivity results. Genotyping complements the clinical findings made after starting a patient on imatinib, he said.
Based on current data for GIST sensitivity to TKIs, Dr. Trent and Dr. Demetri summarized the current GIST mutation and treatment landscape this way:
• About 60% of GIST have the most common tyrosine kinase mutation, in exon 11 of the KIT gene, and are sensitive to 400 mg/day of imatinib.
• About 7% of GIST have the exon 9 mutation of KIT and are sensitive to a higher dosage of imatinib, ideally 800 mg/day if that is tolerated.
• About 20% of GIST have the D842V mutation in the PDGFRA tyrosine kinase gene, and these patients are candidates for enrollment in a trial, as no regimens are known effective for these tumors.
• About 12% of GIST have a mutation in the SDF gene, which appears to make them resistant to imatinib and sunitinib but which may be sensitive to another TKI, regorafenib.
• The remaining GIST have other, rare mutations.
Dr. Trent said that he had no disclosures. Dr. Demetri said that he has been a consultant to Bayer, Novartis, and other companies. Dr. Maki said that he has been a consultant to Eisai/Morphotek, Bayer, and other companies, and has received research support from Eisai/Morphotek, Tracon, and Bayer. Dr. Benjamin said that he has been a consultant to Johnson & Johnson, Merck, and Pfizer.
On Twitter @mitchelzoler
MILAN – Many U.S. patients with gastrointestinal stromal tumors today start on the wrong adjuvant treatment because their physicians don’t order genetic assessment of the cancer, said two American oncologists.
"Initial treatment for GIST [gastrointestinal stromal tumors] is enhanced by molecular decision making," Dr. Jonathan C. Trent said at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology.
"I’ve had patients referred to me from academic centers where the patient was treated with imatinib [Gleevec], which didn’t work, sunitinib [Sutent], which didn’t work, and regorafenib [Stivarga], which didn’t work. We did genetic testing, and it was a D842V mutation [in the PDGFRA gene]. This patient should never have been treated with these drugs," because GIST that carry this PDGFRA mutation are resistant to all three of these tyrosine kinase inhibitor [TKI] drugs, said Dr. Trent, professor of medicine and director of the bone and soft tissue program at the University of Miami.
"If you’re thinking of giving adjuvant therapy, you absolutely need molecular profiling because in the primary, resected-disease setting, a full 20% of patients will have a mutation that makes imatinib useless, the D842V mutation," said Dr. George D. Demetri, professor of medicine at Harvard University and director of the Center for Sarcoma and Bone Oncology at Dana Farber Cancer Institute, Boston. The potential consequence of not performing a genetic analysis is "you could overtreat 20% of patients with what is a still moderately expensive drug that will give them side effects for 3 years when they don’t need it. That is bad medicine," Dr. Demetri said in an interview. "I’m flabbergasted that more people are not getting molecular profiling for GIST patients being considered for adjuvant therapy. We have not communicated this well. Genetic testing is easily accessible at reference labs."
Dr. Demetri highlighted that identifying a D842V mutation in the PDGFRA gene of a GIST is good news for patients, because when this mutation appears in a primary tumor it flags a very indolent form of GIST. "The physician can tell patients that they don’t need to take this drug because it won’t do them any good, plus most patients with your mutation don’t have their tumor recur for many years and sometimes never."
In its most recent guidelines for GIST management, the National Comprehensive Cancer Network (NCCN) said: "If tyrosine kinase inhibitors are considered as part of the treatment plan, genetic analysis of the tumor should be considered since the presence of mutations (or absence of mutations) in specific regions of the KIT and PDGFRA tyrosine kinase genes are correlated with response (or lack of a response) to specific tyrosine kinase inhibitors."
Similar language exists in the posted GIST management recommendations of the National Cancer Institute (NCI), which date from 2012: "KIT- and PDGFRA-mutational analysis may be of help in predicting responses to kinase inhibitors for patients with unresectable, metastatic, or recurrent GIST who are undergoing therapy with selective TKIs. However, the data are preliminary and mutational analysis for treatment decisions is not routine. There is currently no evidence that basing treatment decisions on mutational analysis improves OS [overall survival]."
Dr. Trent took issue with these positions and said that the NCCN and NCI need to call genetic assessment of primary GIST necessary, especially for patients considered for adjuvant treatment. But others saw reason for equivocation.
"Since the mutational status of GIST can impact your use of adjuvant therapy or even therapy for metastatic disease, most sarcoma physicians prefer to see it done," noted Dr. Robert G. Maki, professor of medicine and director of the sarcoma program at Mount Sinai Medical Center in New York. "That said, we often do not have clinical trial data to support" this approach to management. For example, no trial results clearly show that GIST that carry a PDGFRA D842V mutation do not respond well to imatinib and have better outcomes when treated with some other drug. "I would like to have these data to discuss the options" with patients, Dr. Maki said in an interview. The relatively well-described patterns of genetic mutation and drug sensitivity seen in GIST make this tumor different from other adult sarcomas, he added.
"We had a patient with the PDGFRA D842V mutation who clearly responded to imatinib," said Dr. Robert S. Benjamin, professor and chairman of sarcoma medical oncology at M.D. Anderson Cancer Center in Houston.
"You use imatinib empirically because it is the most benign drug, especially at the 400-mg/day level," Dr. Benjamin said in an interview. "It’s well tolerated, and it usually works." But Dr. Benjamin acknowledged the added value of learning a tumor’s genetic profile. "It’s analogous to infectious disease," where you might start a patient on an empiric antibiotic but then reconsider once you receive antibiotic-sensitivity results. Genotyping complements the clinical findings made after starting a patient on imatinib, he said.
Based on current data for GIST sensitivity to TKIs, Dr. Trent and Dr. Demetri summarized the current GIST mutation and treatment landscape this way:
• About 60% of GIST have the most common tyrosine kinase mutation, in exon 11 of the KIT gene, and are sensitive to 400 mg/day of imatinib.
• About 7% of GIST have the exon 9 mutation of KIT and are sensitive to a higher dosage of imatinib, ideally 800 mg/day if that is tolerated.
• About 20% of GIST have the D842V mutation in the PDGFRA tyrosine kinase gene, and these patients are candidates for enrollment in a trial, as no regimens are known effective for these tumors.
• About 12% of GIST have a mutation in the SDF gene, which appears to make them resistant to imatinib and sunitinib but which may be sensitive to another TKI, regorafenib.
• The remaining GIST have other, rare mutations.
Dr. Trent said that he had no disclosures. Dr. Demetri said that he has been a consultant to Bayer, Novartis, and other companies. Dr. Maki said that he has been a consultant to Eisai/Morphotek, Bayer, and other companies, and has received research support from Eisai/Morphotek, Tracon, and Bayer. Dr. Benjamin said that he has been a consultant to Johnson & Johnson, Merck, and Pfizer.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM SARCOMA AND GIST 2014
Liquid biopsies may solve GIST biopsy problem
MILAN – Although genetic analysis has become a key part of gastrointestinal-stromal tumor assessment before treating a primary tumor, its use at the time of recurrence remains problematic because of the heterogeneity of recurrent clones at the time of relapse, according to Dr. George D. Demetri.
"Liquid biopsy" is a new genetic assessment method with the potential to address the heterogeneity by sampling the complete spectrum of a patient’s tumor using free circulating tumor DNA in the patient’s plasma, rather than biopsying specific pieces of the tumor.
"The challenge from multiple, progressing tumors in a patient with GIST who is failing tyrosine-kinase inhibitor treatment is how to get around the limitation of tumor biopsy. How useful are biopsies when each corner of the tumor tells you something different" when a patient has recurrent GIST? said Dr. Demetri at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology. "Do you think you get a comprehensive look by biopsying the tumor? How many biopsies do you take?" asked Dr. Demetri, professor of medicine at Harvard Medical School and director of the center for sarcoma and bone oncology at Dana Farber Cancer Institute, both in Boston.
Dr. Demetri called for continued research to prove the efficacy and utility of liquid biopsies. "We need to develop liquid biopsies to get around the issue of tumor biopsies," he said.
The approach relies on the concept that tumor cells are constantly dying and releasing their DNA into a patient’s blood, and hence the free DNA circulating reflects the genetic profile, including all mutations and clones the patient’s tumor has at the time; this approach was pioneered by researchers at Johns Hopkins University (Sci. Transl. Med. 2012;4:162ra154).
Last year, researchers in Germany reported good correlations when assessing mutations in free circulating DNA in multiple plasma samples drawn from 38 patients with recurrent GIST and matching the results with the patients’ clinical state (Clin. Cancer Res. 2013;19:4854-67).
Dr. Demetri said his own laboratory recently compared mutational analyses in 32 patients with primary GIST and found that in 29 of 32 cases (91%), the mutational profile seen in the free circulating DNA matched that seen in biopsy specimens from each patient.
Currently, genetic assessment of free circulating DNA relies on looking for known mutations using specific amplification primers for those mutations, but next-generation sequencing could be used instead to search for any type of mutation, Dr. Demetri said.
Genetic analysis of GIST at the time of relapse is important, given today’s treatment options and the need to match the right treatment to the right genetic profile, but the challenge is how to perform this analysis in a meaningful way, commented Dr. Robert S. Benjamin, professor and chairman of sarcoma medical oncology at M.D. Anderson Cancer Center in Houston. "The idea of using free DNA in blood is very exciting," he said in an interview.
Dr. Demetri said he has been a consultant to Bayer, Novartis, Pfizer, Sanofi Oncology, Merck, GlaxoSmithKline, and Ariad. Dr. Benjamin said he has been a consultant to Johnson & Johnson, Merck, and Pfizer.
On Twitter @mitchelzoler
MILAN – Although genetic analysis has become a key part of gastrointestinal-stromal tumor assessment before treating a primary tumor, its use at the time of recurrence remains problematic because of the heterogeneity of recurrent clones at the time of relapse, according to Dr. George D. Demetri.
"Liquid biopsy" is a new genetic assessment method with the potential to address the heterogeneity by sampling the complete spectrum of a patient’s tumor using free circulating tumor DNA in the patient’s plasma, rather than biopsying specific pieces of the tumor.
"The challenge from multiple, progressing tumors in a patient with GIST who is failing tyrosine-kinase inhibitor treatment is how to get around the limitation of tumor biopsy. How useful are biopsies when each corner of the tumor tells you something different" when a patient has recurrent GIST? said Dr. Demetri at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology. "Do you think you get a comprehensive look by biopsying the tumor? How many biopsies do you take?" asked Dr. Demetri, professor of medicine at Harvard Medical School and director of the center for sarcoma and bone oncology at Dana Farber Cancer Institute, both in Boston.
Dr. Demetri called for continued research to prove the efficacy and utility of liquid biopsies. "We need to develop liquid biopsies to get around the issue of tumor biopsies," he said.
The approach relies on the concept that tumor cells are constantly dying and releasing their DNA into a patient’s blood, and hence the free DNA circulating reflects the genetic profile, including all mutations and clones the patient’s tumor has at the time; this approach was pioneered by researchers at Johns Hopkins University (Sci. Transl. Med. 2012;4:162ra154).
Last year, researchers in Germany reported good correlations when assessing mutations in free circulating DNA in multiple plasma samples drawn from 38 patients with recurrent GIST and matching the results with the patients’ clinical state (Clin. Cancer Res. 2013;19:4854-67).
Dr. Demetri said his own laboratory recently compared mutational analyses in 32 patients with primary GIST and found that in 29 of 32 cases (91%), the mutational profile seen in the free circulating DNA matched that seen in biopsy specimens from each patient.
Currently, genetic assessment of free circulating DNA relies on looking for known mutations using specific amplification primers for those mutations, but next-generation sequencing could be used instead to search for any type of mutation, Dr. Demetri said.
Genetic analysis of GIST at the time of relapse is important, given today’s treatment options and the need to match the right treatment to the right genetic profile, but the challenge is how to perform this analysis in a meaningful way, commented Dr. Robert S. Benjamin, professor and chairman of sarcoma medical oncology at M.D. Anderson Cancer Center in Houston. "The idea of using free DNA in blood is very exciting," he said in an interview.
Dr. Demetri said he has been a consultant to Bayer, Novartis, Pfizer, Sanofi Oncology, Merck, GlaxoSmithKline, and Ariad. Dr. Benjamin said he has been a consultant to Johnson & Johnson, Merck, and Pfizer.
On Twitter @mitchelzoler
MILAN – Although genetic analysis has become a key part of gastrointestinal-stromal tumor assessment before treating a primary tumor, its use at the time of recurrence remains problematic because of the heterogeneity of recurrent clones at the time of relapse, according to Dr. George D. Demetri.
"Liquid biopsy" is a new genetic assessment method with the potential to address the heterogeneity by sampling the complete spectrum of a patient’s tumor using free circulating tumor DNA in the patient’s plasma, rather than biopsying specific pieces of the tumor.
"The challenge from multiple, progressing tumors in a patient with GIST who is failing tyrosine-kinase inhibitor treatment is how to get around the limitation of tumor biopsy. How useful are biopsies when each corner of the tumor tells you something different" when a patient has recurrent GIST? said Dr. Demetri at Sarcoma and GIST 2014, hosted by the European Society for Medical Oncology. "Do you think you get a comprehensive look by biopsying the tumor? How many biopsies do you take?" asked Dr. Demetri, professor of medicine at Harvard Medical School and director of the center for sarcoma and bone oncology at Dana Farber Cancer Institute, both in Boston.
Dr. Demetri called for continued research to prove the efficacy and utility of liquid biopsies. "We need to develop liquid biopsies to get around the issue of tumor biopsies," he said.
The approach relies on the concept that tumor cells are constantly dying and releasing their DNA into a patient’s blood, and hence the free DNA circulating reflects the genetic profile, including all mutations and clones the patient’s tumor has at the time; this approach was pioneered by researchers at Johns Hopkins University (Sci. Transl. Med. 2012;4:162ra154).
Last year, researchers in Germany reported good correlations when assessing mutations in free circulating DNA in multiple plasma samples drawn from 38 patients with recurrent GIST and matching the results with the patients’ clinical state (Clin. Cancer Res. 2013;19:4854-67).
Dr. Demetri said his own laboratory recently compared mutational analyses in 32 patients with primary GIST and found that in 29 of 32 cases (91%), the mutational profile seen in the free circulating DNA matched that seen in biopsy specimens from each patient.
Currently, genetic assessment of free circulating DNA relies on looking for known mutations using specific amplification primers for those mutations, but next-generation sequencing could be used instead to search for any type of mutation, Dr. Demetri said.
Genetic analysis of GIST at the time of relapse is important, given today’s treatment options and the need to match the right treatment to the right genetic profile, but the challenge is how to perform this analysis in a meaningful way, commented Dr. Robert S. Benjamin, professor and chairman of sarcoma medical oncology at M.D. Anderson Cancer Center in Houston. "The idea of using free DNA in blood is very exciting," he said in an interview.
Dr. Demetri said he has been a consultant to Bayer, Novartis, Pfizer, Sanofi Oncology, Merck, GlaxoSmithKline, and Ariad. Dr. Benjamin said he has been a consultant to Johnson & Johnson, Merck, and Pfizer.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM SARCOMA AND GIST 2014