User login
Albuminuria: When urine predicts kidney and cardiovascular disease
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
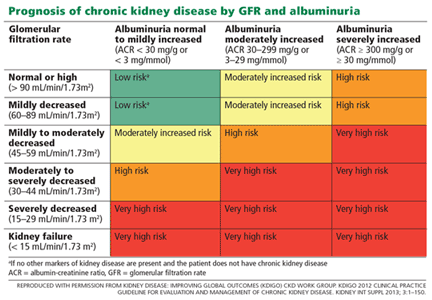
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
KEY POINTS
- Albuminuria is best measured by the albumin-to-creatinine ratio.
- In several studies, albuminuria has been independently associated with a higher risk of death, cardiovascular events, heart failure, stroke, and progression of chronic kidney disease.
- Despite strong evidence linking albuminuria to adverse outcomes, evidence is limited in favor of routinely screening for it in the general population.
- Evaluating and managing albuminuria require understanding the limits of its clinical measures, controlling other risk factors for progression of renal disease, managing it medically, and referring to a specialist in certain situations.
An 85-year-old with muscle pain
An 85-year-old man with hypertension, hyperlipidemia, and coronary artery disease presented to our clinic with diffuse muscle pain. The pain had been present for about 3 months, but it had become noticeably worse over the past few weeks.
He was not aware of any trauma. He described the muscle pain as dull and particularly severe in his lower extremities (his thighs and calves). The pain did not limit his daily activities, nor did physical exertion or the time of day have any effect on the level of the pain.
His medications at that time included metoprolol, aspirin, hydrochlorothiazide, simvastatin, and a daily multivitamin.
He was not in acute distress. On neurologic and musculoskeletal examinations, all deep-tendon reflexes were intact, with no tenderness to palpation of the upper and lower extremities. No abnormalities were noted on the joint examination. He had full range of motion, with 5/5 muscle strength in the upper and lower extremities bilaterally and normal muscle tone. He was able to walk with ease. Results of initial laboratory testing, including creatine kinase and erythrocyte sedimentation rate, were normal.
1. What should be the next best step in the evaluation of this patient’s muscle pain?
- Order tests for cyclic citrullinated peptide (CCP) antibody and rheumatoid factor
- Advise him to refrain from physical activity until his symptoms resolve
- Take a more detailed history, including a review of medications and supplements
- Recommend a trial of a nonsteroidal anti-inflammatory drug (NSAID)
- Send him for radiographic imaging
Since his muscle pain has persisted for several months without improvement, a more detailed history should be taken, including a review of current medications and supplements.
Testing CCP antibody and rheumatoid factor would be useful if rheumatoid arthritis were suspected, but in the absence of demonstrable arthritis on examination, these tests would have low specificity even if the results were positive.
An NSAID may temporarily alleviate his pain, but it will not help establish a diagnosis. And in elderly patients, NSAIDs are not without complications and so should be prescribed only in appropriate situations.
Imaging would be appropriate at this point only if there was clinical suspicion of a specific disease. However, our patient has no focal deficits, and the suspicion of fracture or malignancy is low.
The medical history should include asking about current drug regimens, recent medication changes, and the use of herbal supplements, since polypharmacy is common in elderly patients with multiple comorbidities.
On further questioning, our patient said that his dose of simvastatin had been increased from 40 mg daily to 80 mg daily about 1 month before his symptoms appeared. He was taking a daily multivitamin but was not using herbal supplements or other over-the-counter products. He did not recall any constitutional symptoms before the onset of his current symptoms, and he had never had similar muscle pain in the past.
2. Based on the additional information from the history, what is the most likely cause of his muscle pain?
- Limited myositis secondary to recent viral infection
- Rhabdomyolysis
- Hypothyroidism
- Drug-drug interaction
- Statin-induced myalgia
Our patient’s history provided nothing to suggest viral myositis. Hypothyroidism should always be considered in patients with myalgia, but this is not likely in our patient, as he does not display other characteristics, such as diminished reflexes, hypotonia, cold intolerance, and mood instability. Even though calcium channel blockers have been known to cause myalgia in patients on statins, a drug-drug reaction is not likely, as he had not started taking a calcium channel blocker before his symptoms began. This patient did not show signs or symptoms of rhabdomyolysis, a type of myopathy in which necrosis of the muscle tissue occurs, generally causing profound weakness and pain.1
Therefore, statin-induced myopathy is the most likely cause of his diffuse muscle pain, particularly since his simvastatin had been increased 1 month before the onset of symptoms.
3. What should be the next step in his management?
- Decrease the dose of simvastatin to the last known dose he was able to tolerate
- Continue simvastatin at the same dose and then monitor
- Switch to another statin
- Add coenzyme Q10
- Stop simvastatin
Decreasing the statin dosage to the last well-tolerated dose would not be appropriate in a patient with myopathy, as the symptoms would probably not improve.2–4 Also, one should not switch to a different statin while a patient is experiencing symptoms. Rather, the statin should be stopped for at least 6 weeks or until the symptoms have fully resolved.1
Adding coenzyme Q10 is another option, especially in a patient with previously diagnosed coronary artery disease,5 when continued statin therapy is thought necessary to reduce the likelihood of repeat coronary events.
We discontinued his simvastatin. Followup 3 weeks later in the outpatient clinic showed that his symptoms were slowly improving. The symptoms had resolved completely 4 months later.
4. How should we manage our patient’s hyperlipidemia once his symptoms have resolved?
- Restart simvastatin at the 80-mg dose
- Restart simvastatin at the 40-mg dose
- Start a hydrophilic statin at full dose
- Use a drug from another class of lipid-lowering drugs
- Wait another 3 months before prescribing any lipid-lowering drug
His treatment for hyperlipidemia should be continued, considering his comorbidities. However, restarting the same statin, even at a lower dose, will likely cause his symptoms to recur. Thus, a different statin should be tried once his muscle pain has resolved.
Other classes of lipid-lowering drugs are usually less efficacious than statins, particularly when trying to control low-density lipoprotein (LDL) cholesterol, so a drug from another class should not be used until other statin options have been attempted.2,6,7
Simvastatin is lipophilic. Trying a statin with hydrophilic properties (eg, pravastatin, rosuvastatin, fluvastatin) has been shown to convey similar cardioprotective effects with a lower propensity for myalgia, as lipophilic statins have a higher propensity to penetrate muscle tissue than do hydrophilic statins.3,4,8
Once his symptoms resolved, our patient was started on a hydrophilic statin, fluvastatin 20 mg daily. Unfortunately, his pain recurred 3 weeks later. The statin was stopped, and his symptoms again resolved.
5. Since our patient was unable to tolerate a second statin, what should be the next step in his management?
- Restart simvastatin
- Use a drug from another class to control the hyperlipidemia
- Wait at least 6 months after symptoms resolve before trying any lipid-lowering drug
- Initiate therapy with coenzyme Q10 and fish oil
- Wait for symptoms to resolve, then restart a hydrophilic statin at a lower dose and lower frequency
Restarting simvastatin will likely cause a recurrence of the myalgia. Other lipid-lowering drugs such as nicotinic acid, bile acid resins, and fibrates are not as efficacious as statins. Coenzyme Q10 and fish oil can reduce lipid levels, but they are not as efficacious as statins.
In view of our patient’s lipid profile—LDL cholesterol elevated at 167 mg/dL, high-density lipoprotein cholesterol 31 mg/dL, triglycerides 47 mg/dL—it is important to treat his hyperlipidemia. Therefore, another attempt at statin therapy should be made once his symptoms have resolved.
Studies have shown that restarting a statin at a low dose and low frequency is effective in patients who have experienced intolerance to a statin.3,4 Our patient was treated with low-dose pravastatin (20 mg), resulting in a moderate improvement in his LDL cholesterol to 123 mg/dL.
STATIN-INDUCED MYOPATHY: ADDRESSING THE DILEMMA
Treating hyperlipidemia is important to prevent vascular events in patients with or without coronary artery disease. Statins are the most effective agents available for controlling hypercholesterolemia, specifically LDL levels, as well as for preventing myocardial infarction.
Unfortunately, significant side effects have been reported, and myopathy is the most prevalent. Statin-induced myopathy includes a combination of muscle tenderness, myalgia, and weakness.2–11 In randomized controlled trials, the risk of myopathy was estimated to be between 1.5% and 5%.6 In unselected clinic patients on high-dose statins, the rate of muscle complaints may be as high as 20%.12
The cause of statin-induced myopathy is not known, although studies have linked it to genetic defects.7 Risk factors have been identified and include personal and family history of myalgia, Asian ethnicity, hypothyroidism, and type 1 diabetes. The incidence of statin-induced myalgia is two to three times higher in patients on corticosteroid therapy. Other risk factors include female sex, liver disease, and renal dysfunction.7,8
A less common etiology is anti-HMG coenzyme A reductase antibodies. Studies have shown that these antibody levels correlate well with the amount of myositis as measured by creatine kinase levels. However, there is no consensus yet on screening for these antibodies.13
Statin therapy poses a dilemma, as there is a thin line between the benefits and the risks of side effects, especially statin-induced myopathy.3,4 Current recommendations include discontinuing the statin until symptoms fully resolve. Creatine kinase levels may be useful in assessing for potential muscle breakdown, especially in patients with reduced renal function, as this predisposes them to statin-induced myopathy, yet normal values do not preclude the diagnosis of statin-induced myopathy.3,4,7,8
Once symptoms resolve and laboratory test results normalize, a trial of a different statin is recommended. If patients become symptomatic, a trial of a low-dose hydrophilic statin at a once- or twice-weekly interval has been recommended. Several studies have assessed the efficacy of a low-dose statin with decreased frequency of administration and have consistently shown significant improvement in lipid levels.3,4 For instance, once-weekly rosuvastatin at a dose between 5 mg and 20 mg resulted in a 29% reduction in LDL cholesterol levels, and 80% of patients did not experience a recurrence of myalgia.3 Furthermore, a study of patients treated with 5 mg to 10 mg of rosuvastatin twice a week resulted in a 26% decrease in LDL cholesterol levels.4 This study also showed that when an additional non-statin lipid-lowering drug was prescribed (eg, ezetimibe, bile acid resin, nicotinic acid), more than half of the patients reached their goal lipid level.4
The addition of coenzyme Q10 and fish oil has also been suggested. Although, the evidence to support this is inconclusive, the potential benefit outweighs the risk, since the side effects are minimal.1 However, no study yet has evaluated the risks vs the benefits in patients with elevated creatine kinase.
Statin-induced myopathy is a commonly encountered adverse effect. Currently, there are no guidelines on restarting statin therapy after statin-induced myopathy; however, data suggest that statin therapy should be restarted once symptoms resolve, and that variations in dose and frequency may be necessary.1–8,14
- Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011; 78:393–403.
- Foley KA, Simpson RJ, Crouse JR, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol 2008; 101:1747–1748.
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99:1409–1412.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Tomaszewski M, Stepien KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep 2011; 63:859–866.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Guyton JR. Benefit versus risk in statin treatment. Am J Cardiol 2006; 97:95C–97C.
- Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
An 85-year-old man with hypertension, hyperlipidemia, and coronary artery disease presented to our clinic with diffuse muscle pain. The pain had been present for about 3 months, but it had become noticeably worse over the past few weeks.
He was not aware of any trauma. He described the muscle pain as dull and particularly severe in his lower extremities (his thighs and calves). The pain did not limit his daily activities, nor did physical exertion or the time of day have any effect on the level of the pain.
His medications at that time included metoprolol, aspirin, hydrochlorothiazide, simvastatin, and a daily multivitamin.
He was not in acute distress. On neurologic and musculoskeletal examinations, all deep-tendon reflexes were intact, with no tenderness to palpation of the upper and lower extremities. No abnormalities were noted on the joint examination. He had full range of motion, with 5/5 muscle strength in the upper and lower extremities bilaterally and normal muscle tone. He was able to walk with ease. Results of initial laboratory testing, including creatine kinase and erythrocyte sedimentation rate, were normal.
1. What should be the next best step in the evaluation of this patient’s muscle pain?
- Order tests for cyclic citrullinated peptide (CCP) antibody and rheumatoid factor
- Advise him to refrain from physical activity until his symptoms resolve
- Take a more detailed history, including a review of medications and supplements
- Recommend a trial of a nonsteroidal anti-inflammatory drug (NSAID)
- Send him for radiographic imaging
Since his muscle pain has persisted for several months without improvement, a more detailed history should be taken, including a review of current medications and supplements.
Testing CCP antibody and rheumatoid factor would be useful if rheumatoid arthritis were suspected, but in the absence of demonstrable arthritis on examination, these tests would have low specificity even if the results were positive.
An NSAID may temporarily alleviate his pain, but it will not help establish a diagnosis. And in elderly patients, NSAIDs are not without complications and so should be prescribed only in appropriate situations.
Imaging would be appropriate at this point only if there was clinical suspicion of a specific disease. However, our patient has no focal deficits, and the suspicion of fracture or malignancy is low.
The medical history should include asking about current drug regimens, recent medication changes, and the use of herbal supplements, since polypharmacy is common in elderly patients with multiple comorbidities.
On further questioning, our patient said that his dose of simvastatin had been increased from 40 mg daily to 80 mg daily about 1 month before his symptoms appeared. He was taking a daily multivitamin but was not using herbal supplements or other over-the-counter products. He did not recall any constitutional symptoms before the onset of his current symptoms, and he had never had similar muscle pain in the past.
2. Based on the additional information from the history, what is the most likely cause of his muscle pain?
- Limited myositis secondary to recent viral infection
- Rhabdomyolysis
- Hypothyroidism
- Drug-drug interaction
- Statin-induced myalgia
Our patient’s history provided nothing to suggest viral myositis. Hypothyroidism should always be considered in patients with myalgia, but this is not likely in our patient, as he does not display other characteristics, such as diminished reflexes, hypotonia, cold intolerance, and mood instability. Even though calcium channel blockers have been known to cause myalgia in patients on statins, a drug-drug reaction is not likely, as he had not started taking a calcium channel blocker before his symptoms began. This patient did not show signs or symptoms of rhabdomyolysis, a type of myopathy in which necrosis of the muscle tissue occurs, generally causing profound weakness and pain.1
Therefore, statin-induced myopathy is the most likely cause of his diffuse muscle pain, particularly since his simvastatin had been increased 1 month before the onset of symptoms.
3. What should be the next step in his management?
- Decrease the dose of simvastatin to the last known dose he was able to tolerate
- Continue simvastatin at the same dose and then monitor
- Switch to another statin
- Add coenzyme Q10
- Stop simvastatin
Decreasing the statin dosage to the last well-tolerated dose would not be appropriate in a patient with myopathy, as the symptoms would probably not improve.2–4 Also, one should not switch to a different statin while a patient is experiencing symptoms. Rather, the statin should be stopped for at least 6 weeks or until the symptoms have fully resolved.1
Adding coenzyme Q10 is another option, especially in a patient with previously diagnosed coronary artery disease,5 when continued statin therapy is thought necessary to reduce the likelihood of repeat coronary events.
We discontinued his simvastatin. Followup 3 weeks later in the outpatient clinic showed that his symptoms were slowly improving. The symptoms had resolved completely 4 months later.
4. How should we manage our patient’s hyperlipidemia once his symptoms have resolved?
- Restart simvastatin at the 80-mg dose
- Restart simvastatin at the 40-mg dose
- Start a hydrophilic statin at full dose
- Use a drug from another class of lipid-lowering drugs
- Wait another 3 months before prescribing any lipid-lowering drug
His treatment for hyperlipidemia should be continued, considering his comorbidities. However, restarting the same statin, even at a lower dose, will likely cause his symptoms to recur. Thus, a different statin should be tried once his muscle pain has resolved.
Other classes of lipid-lowering drugs are usually less efficacious than statins, particularly when trying to control low-density lipoprotein (LDL) cholesterol, so a drug from another class should not be used until other statin options have been attempted.2,6,7
Simvastatin is lipophilic. Trying a statin with hydrophilic properties (eg, pravastatin, rosuvastatin, fluvastatin) has been shown to convey similar cardioprotective effects with a lower propensity for myalgia, as lipophilic statins have a higher propensity to penetrate muscle tissue than do hydrophilic statins.3,4,8
Once his symptoms resolved, our patient was started on a hydrophilic statin, fluvastatin 20 mg daily. Unfortunately, his pain recurred 3 weeks later. The statin was stopped, and his symptoms again resolved.
5. Since our patient was unable to tolerate a second statin, what should be the next step in his management?
- Restart simvastatin
- Use a drug from another class to control the hyperlipidemia
- Wait at least 6 months after symptoms resolve before trying any lipid-lowering drug
- Initiate therapy with coenzyme Q10 and fish oil
- Wait for symptoms to resolve, then restart a hydrophilic statin at a lower dose and lower frequency
Restarting simvastatin will likely cause a recurrence of the myalgia. Other lipid-lowering drugs such as nicotinic acid, bile acid resins, and fibrates are not as efficacious as statins. Coenzyme Q10 and fish oil can reduce lipid levels, but they are not as efficacious as statins.
In view of our patient’s lipid profile—LDL cholesterol elevated at 167 mg/dL, high-density lipoprotein cholesterol 31 mg/dL, triglycerides 47 mg/dL—it is important to treat his hyperlipidemia. Therefore, another attempt at statin therapy should be made once his symptoms have resolved.
Studies have shown that restarting a statin at a low dose and low frequency is effective in patients who have experienced intolerance to a statin.3,4 Our patient was treated with low-dose pravastatin (20 mg), resulting in a moderate improvement in his LDL cholesterol to 123 mg/dL.
STATIN-INDUCED MYOPATHY: ADDRESSING THE DILEMMA
Treating hyperlipidemia is important to prevent vascular events in patients with or without coronary artery disease. Statins are the most effective agents available for controlling hypercholesterolemia, specifically LDL levels, as well as for preventing myocardial infarction.
Unfortunately, significant side effects have been reported, and myopathy is the most prevalent. Statin-induced myopathy includes a combination of muscle tenderness, myalgia, and weakness.2–11 In randomized controlled trials, the risk of myopathy was estimated to be between 1.5% and 5%.6 In unselected clinic patients on high-dose statins, the rate of muscle complaints may be as high as 20%.12
The cause of statin-induced myopathy is not known, although studies have linked it to genetic defects.7 Risk factors have been identified and include personal and family history of myalgia, Asian ethnicity, hypothyroidism, and type 1 diabetes. The incidence of statin-induced myalgia is two to three times higher in patients on corticosteroid therapy. Other risk factors include female sex, liver disease, and renal dysfunction.7,8
A less common etiology is anti-HMG coenzyme A reductase antibodies. Studies have shown that these antibody levels correlate well with the amount of myositis as measured by creatine kinase levels. However, there is no consensus yet on screening for these antibodies.13
Statin therapy poses a dilemma, as there is a thin line between the benefits and the risks of side effects, especially statin-induced myopathy.3,4 Current recommendations include discontinuing the statin until symptoms fully resolve. Creatine kinase levels may be useful in assessing for potential muscle breakdown, especially in patients with reduced renal function, as this predisposes them to statin-induced myopathy, yet normal values do not preclude the diagnosis of statin-induced myopathy.3,4,7,8
Once symptoms resolve and laboratory test results normalize, a trial of a different statin is recommended. If patients become symptomatic, a trial of a low-dose hydrophilic statin at a once- or twice-weekly interval has been recommended. Several studies have assessed the efficacy of a low-dose statin with decreased frequency of administration and have consistently shown significant improvement in lipid levels.3,4 For instance, once-weekly rosuvastatin at a dose between 5 mg and 20 mg resulted in a 29% reduction in LDL cholesterol levels, and 80% of patients did not experience a recurrence of myalgia.3 Furthermore, a study of patients treated with 5 mg to 10 mg of rosuvastatin twice a week resulted in a 26% decrease in LDL cholesterol levels.4 This study also showed that when an additional non-statin lipid-lowering drug was prescribed (eg, ezetimibe, bile acid resin, nicotinic acid), more than half of the patients reached their goal lipid level.4
The addition of coenzyme Q10 and fish oil has also been suggested. Although, the evidence to support this is inconclusive, the potential benefit outweighs the risk, since the side effects are minimal.1 However, no study yet has evaluated the risks vs the benefits in patients with elevated creatine kinase.
Statin-induced myopathy is a commonly encountered adverse effect. Currently, there are no guidelines on restarting statin therapy after statin-induced myopathy; however, data suggest that statin therapy should be restarted once symptoms resolve, and that variations in dose and frequency may be necessary.1–8,14
An 85-year-old man with hypertension, hyperlipidemia, and coronary artery disease presented to our clinic with diffuse muscle pain. The pain had been present for about 3 months, but it had become noticeably worse over the past few weeks.
He was not aware of any trauma. He described the muscle pain as dull and particularly severe in his lower extremities (his thighs and calves). The pain did not limit his daily activities, nor did physical exertion or the time of day have any effect on the level of the pain.
His medications at that time included metoprolol, aspirin, hydrochlorothiazide, simvastatin, and a daily multivitamin.
He was not in acute distress. On neurologic and musculoskeletal examinations, all deep-tendon reflexes were intact, with no tenderness to palpation of the upper and lower extremities. No abnormalities were noted on the joint examination. He had full range of motion, with 5/5 muscle strength in the upper and lower extremities bilaterally and normal muscle tone. He was able to walk with ease. Results of initial laboratory testing, including creatine kinase and erythrocyte sedimentation rate, were normal.
1. What should be the next best step in the evaluation of this patient’s muscle pain?
- Order tests for cyclic citrullinated peptide (CCP) antibody and rheumatoid factor
- Advise him to refrain from physical activity until his symptoms resolve
- Take a more detailed history, including a review of medications and supplements
- Recommend a trial of a nonsteroidal anti-inflammatory drug (NSAID)
- Send him for radiographic imaging
Since his muscle pain has persisted for several months without improvement, a more detailed history should be taken, including a review of current medications and supplements.
Testing CCP antibody and rheumatoid factor would be useful if rheumatoid arthritis were suspected, but in the absence of demonstrable arthritis on examination, these tests would have low specificity even if the results were positive.
An NSAID may temporarily alleviate his pain, but it will not help establish a diagnosis. And in elderly patients, NSAIDs are not without complications and so should be prescribed only in appropriate situations.
Imaging would be appropriate at this point only if there was clinical suspicion of a specific disease. However, our patient has no focal deficits, and the suspicion of fracture or malignancy is low.
The medical history should include asking about current drug regimens, recent medication changes, and the use of herbal supplements, since polypharmacy is common in elderly patients with multiple comorbidities.
On further questioning, our patient said that his dose of simvastatin had been increased from 40 mg daily to 80 mg daily about 1 month before his symptoms appeared. He was taking a daily multivitamin but was not using herbal supplements or other over-the-counter products. He did not recall any constitutional symptoms before the onset of his current symptoms, and he had never had similar muscle pain in the past.
2. Based on the additional information from the history, what is the most likely cause of his muscle pain?
- Limited myositis secondary to recent viral infection
- Rhabdomyolysis
- Hypothyroidism
- Drug-drug interaction
- Statin-induced myalgia
Our patient’s history provided nothing to suggest viral myositis. Hypothyroidism should always be considered in patients with myalgia, but this is not likely in our patient, as he does not display other characteristics, such as diminished reflexes, hypotonia, cold intolerance, and mood instability. Even though calcium channel blockers have been known to cause myalgia in patients on statins, a drug-drug reaction is not likely, as he had not started taking a calcium channel blocker before his symptoms began. This patient did not show signs or symptoms of rhabdomyolysis, a type of myopathy in which necrosis of the muscle tissue occurs, generally causing profound weakness and pain.1
Therefore, statin-induced myopathy is the most likely cause of his diffuse muscle pain, particularly since his simvastatin had been increased 1 month before the onset of symptoms.
3. What should be the next step in his management?
- Decrease the dose of simvastatin to the last known dose he was able to tolerate
- Continue simvastatin at the same dose and then monitor
- Switch to another statin
- Add coenzyme Q10
- Stop simvastatin
Decreasing the statin dosage to the last well-tolerated dose would not be appropriate in a patient with myopathy, as the symptoms would probably not improve.2–4 Also, one should not switch to a different statin while a patient is experiencing symptoms. Rather, the statin should be stopped for at least 6 weeks or until the symptoms have fully resolved.1
Adding coenzyme Q10 is another option, especially in a patient with previously diagnosed coronary artery disease,5 when continued statin therapy is thought necessary to reduce the likelihood of repeat coronary events.
We discontinued his simvastatin. Followup 3 weeks later in the outpatient clinic showed that his symptoms were slowly improving. The symptoms had resolved completely 4 months later.
4. How should we manage our patient’s hyperlipidemia once his symptoms have resolved?
- Restart simvastatin at the 80-mg dose
- Restart simvastatin at the 40-mg dose
- Start a hydrophilic statin at full dose
- Use a drug from another class of lipid-lowering drugs
- Wait another 3 months before prescribing any lipid-lowering drug
His treatment for hyperlipidemia should be continued, considering his comorbidities. However, restarting the same statin, even at a lower dose, will likely cause his symptoms to recur. Thus, a different statin should be tried once his muscle pain has resolved.
Other classes of lipid-lowering drugs are usually less efficacious than statins, particularly when trying to control low-density lipoprotein (LDL) cholesterol, so a drug from another class should not be used until other statin options have been attempted.2,6,7
Simvastatin is lipophilic. Trying a statin with hydrophilic properties (eg, pravastatin, rosuvastatin, fluvastatin) has been shown to convey similar cardioprotective effects with a lower propensity for myalgia, as lipophilic statins have a higher propensity to penetrate muscle tissue than do hydrophilic statins.3,4,8
Once his symptoms resolved, our patient was started on a hydrophilic statin, fluvastatin 20 mg daily. Unfortunately, his pain recurred 3 weeks later. The statin was stopped, and his symptoms again resolved.
5. Since our patient was unable to tolerate a second statin, what should be the next step in his management?
- Restart simvastatin
- Use a drug from another class to control the hyperlipidemia
- Wait at least 6 months after symptoms resolve before trying any lipid-lowering drug
- Initiate therapy with coenzyme Q10 and fish oil
- Wait for symptoms to resolve, then restart a hydrophilic statin at a lower dose and lower frequency
Restarting simvastatin will likely cause a recurrence of the myalgia. Other lipid-lowering drugs such as nicotinic acid, bile acid resins, and fibrates are not as efficacious as statins. Coenzyme Q10 and fish oil can reduce lipid levels, but they are not as efficacious as statins.
In view of our patient’s lipid profile—LDL cholesterol elevated at 167 mg/dL, high-density lipoprotein cholesterol 31 mg/dL, triglycerides 47 mg/dL—it is important to treat his hyperlipidemia. Therefore, another attempt at statin therapy should be made once his symptoms have resolved.
Studies have shown that restarting a statin at a low dose and low frequency is effective in patients who have experienced intolerance to a statin.3,4 Our patient was treated with low-dose pravastatin (20 mg), resulting in a moderate improvement in his LDL cholesterol to 123 mg/dL.
STATIN-INDUCED MYOPATHY: ADDRESSING THE DILEMMA
Treating hyperlipidemia is important to prevent vascular events in patients with or without coronary artery disease. Statins are the most effective agents available for controlling hypercholesterolemia, specifically LDL levels, as well as for preventing myocardial infarction.
Unfortunately, significant side effects have been reported, and myopathy is the most prevalent. Statin-induced myopathy includes a combination of muscle tenderness, myalgia, and weakness.2–11 In randomized controlled trials, the risk of myopathy was estimated to be between 1.5% and 5%.6 In unselected clinic patients on high-dose statins, the rate of muscle complaints may be as high as 20%.12
The cause of statin-induced myopathy is not known, although studies have linked it to genetic defects.7 Risk factors have been identified and include personal and family history of myalgia, Asian ethnicity, hypothyroidism, and type 1 diabetes. The incidence of statin-induced myalgia is two to three times higher in patients on corticosteroid therapy. Other risk factors include female sex, liver disease, and renal dysfunction.7,8
A less common etiology is anti-HMG coenzyme A reductase antibodies. Studies have shown that these antibody levels correlate well with the amount of myositis as measured by creatine kinase levels. However, there is no consensus yet on screening for these antibodies.13
Statin therapy poses a dilemma, as there is a thin line between the benefits and the risks of side effects, especially statin-induced myopathy.3,4 Current recommendations include discontinuing the statin until symptoms fully resolve. Creatine kinase levels may be useful in assessing for potential muscle breakdown, especially in patients with reduced renal function, as this predisposes them to statin-induced myopathy, yet normal values do not preclude the diagnosis of statin-induced myopathy.3,4,7,8
Once symptoms resolve and laboratory test results normalize, a trial of a different statin is recommended. If patients become symptomatic, a trial of a low-dose hydrophilic statin at a once- or twice-weekly interval has been recommended. Several studies have assessed the efficacy of a low-dose statin with decreased frequency of administration and have consistently shown significant improvement in lipid levels.3,4 For instance, once-weekly rosuvastatin at a dose between 5 mg and 20 mg resulted in a 29% reduction in LDL cholesterol levels, and 80% of patients did not experience a recurrence of myalgia.3 Furthermore, a study of patients treated with 5 mg to 10 mg of rosuvastatin twice a week resulted in a 26% decrease in LDL cholesterol levels.4 This study also showed that when an additional non-statin lipid-lowering drug was prescribed (eg, ezetimibe, bile acid resin, nicotinic acid), more than half of the patients reached their goal lipid level.4
The addition of coenzyme Q10 and fish oil has also been suggested. Although, the evidence to support this is inconclusive, the potential benefit outweighs the risk, since the side effects are minimal.1 However, no study yet has evaluated the risks vs the benefits in patients with elevated creatine kinase.
Statin-induced myopathy is a commonly encountered adverse effect. Currently, there are no guidelines on restarting statin therapy after statin-induced myopathy; however, data suggest that statin therapy should be restarted once symptoms resolve, and that variations in dose and frequency may be necessary.1–8,14
- Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011; 78:393–403.
- Foley KA, Simpson RJ, Crouse JR, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol 2008; 101:1747–1748.
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99:1409–1412.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Tomaszewski M, Stepien KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep 2011; 63:859–866.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Guyton JR. Benefit versus risk in statin treatment. Am J Cardiol 2006; 97:95C–97C.
- Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011; 78:393–403.
- Foley KA, Simpson RJ, Crouse JR, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol 2008; 101:1747–1748.
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99:1409–1412.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Tomaszewski M, Stepien KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep 2011; 63:859–866.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Guyton JR. Benefit versus risk in statin treatment. Am J Cardiol 2006; 97:95C–97C.
- Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
New cholesterol guidelines: Worth the wait?
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
On November 12, 2013, a joint task force for the American College of Cardiology and American Heart Association released new guidelines for treating high blood cholesterol to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) in adults.1
This document arrives after several years of intense deliberation, 12 years after the third Adult Treatment Panel (ATP III) guidelines,2 and 8 years after an ATP III update recommending that low-density lipoprotein cholesterol (LDL-C) levels be lowered aggressively (to less than 70 mg/dL) as an option in patients at high risk.3 It represents a major shift in the approach to and management of high blood cholesterol and has sparked considerable controversy.
In the following commentary, we summarize the new guidelines and the philosophy employed by the task force in generating them. We will also examine some advantages and what we believe to be several shortcomings of the new guidelines. These latter points are illustrated through case examples.
IN RANDOMIZED CONTROLLED TRIALS WE TRUST
In collaboration with the National Heart, Lung, and Blood Institute of the National Institutes of Health, the American College of Cardiology and American Heart Association formed an expert panel task force in 2008.
The task force elected to use only evidence from randomized controlled trials, systematic reviews, and meta-analyses of randomized controlled trials (and only predefined outcomes of the trials, not post hoc analyses) in formulating its recommendations, with the goal of providing the strongest possible evidence.
The authors state that “By using [randomized controlled trial] data to identify those most likely to benefit [emphasis in original] from cholesterol-lowering statin therapy, the recommendations will be of value to primary care clinicians as well as specialists concerned with ASCVD prevention. Importantly, the recommendations were designed to be easy to use in the clinical setting, facilitating the implementation of a strategy of risk assessment and treatment focused on the prevention of ASCVD.”3 They also state the guidelines are meant to “inform clinical judgment, not replace it” and that clinician judgment in addition to discussion with patients remains vital.
During the deliberations, the National Heart, Lung, and Blood Institute removed itself from participating, stating its mission no longer included drafting new guidelines. Additionally, other initial members of the task force removed themselves because of disagreement and concerns about the direction of the new guidelines.
These guidelines, and their accompanying new cardiovascular risk calculator,4 were released without a preliminary period to allow for open discussion, comment, and critique by physicians outside the panel. No attempt was made to harmonize the guidelines with previous versions (eg, ATP III) or with current international guidelines.
WHAT’S NEW IN THE GUIDELINES?
The following are the major changes in the new guidelines for treating high blood cholesterol:
- Treatment goals for LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) are no longer recommended.
- High-intensity and moderate-intensity statin treatment is emphasized, and low-intensity statin therapy is nearly eliminated.
- “ASCVD” now includes stroke in addition to coronary heart disease and peripheral arterial disease.
- Four groups are targeted for treatment (see below).
- Nonstatin therapies have been markedly de-emphasized.
- No guidelines are provided for treating high triglyceride levels.
The new guidelines emphasize lifestyle modification as the foundation for reducing risk, regardless of cholesterol therapy. No recommendations are given for patients with New York Heart Association class II, III, or IV heart failure or for hemodialysis patients, because there were insufficient data from randomized controlled trials to support recommendations. Similarly, the guidelines apply only to people between the ages of 40 and 75 (risk calculator ages 40–79), because the authors believed there was not enough evidence from randomized controlled trials to allow development of guidelines outside of this age range.
FOUR MAJOR STATIN TREATMENT GROUPS
The new guidelines specify four groups that merit intensive or moderately intensive statin therapy (Table 1)1:
- People with clinical ASCVD
- People with LDL-C levels of 190 mg/dL or higher
- People with diabetes, age 40 to 75
- People without diabetes, age 40 to 75, with LDL-C levels 70–189 mg/dL, and a 10-year ASCVD risk of 7.5% or higher as determined by the new risk calculator4 (which also calculates the lifetime risk of ASCVD).
Below, we will address each of these four groups and provide case scenarios to consider. In general, our major disagreements with the new recommendations pertain to the first and fourth categories.
GROUP 1: PEOPLE WITH CLINICAL ASCVD
Advantages of the new guidelines
- They appropriately recommend statins in the highest tolerated doses as first-line treatment for this group at high risk.
- They designate all patients with ASCVD, including those with coronary, peripheral, and cerebrovascular disease, as a high-risk group.
- Without target LDL-C levels, treatment is simpler than before, requiring less monitoring of lipid levels. (This can also be seen as a limitation, as we discuss below.)
Limitations of the new guidelines
- They make follow-up LDL-C levels irrelevant, seeming to assume that there is no gradation in residual risk and, thus, no need to tailor therapy to the individual.
- Patients no longer have a goal to strive for or a way to monitor their progress.
- The guidelines ignore the pathophysiology of coronary artery disease and evidence of residual risk in patients on both moderate-intensity and high-intensity statin therapy.
- They also ignore the potential benefits of treating to lower LDL-C or non-HDL-C goals, thus eliminating consideration of multidrug therapy. They do not address patients with recurrent cardiovascular events already on maximal tolerated statin doses.
- They undermine the potential development and use of new therapies for dysplipidemia in patients with ASCVD.
Case 1: Is LDL-C 110 mg/dL low enough?
A 52-year-old African American man presents with newly discovered moderate coronary artery disease that is not severe enough to warrant stenting. He has no history of hypertension, diabetes mellitus, or smoking. His systolic blood pressure is 130 mm Hg, and his body mass index is 26 kg/m2. He exercises regularly and follows a low-cholesterol diet. He has the following fasting lipid values:
- Total cholesterol 290 mg/dL
- HDL-C 50 mg/dL
- Triglycerides 250 mg/dL
- Calculated LDL-C 190 mg/dL.
Two months later, after beginning atorvastatin 80 mg daily, meeting with a nutritionist, and redoubling his dietary efforts, his fasting lipid concentrations are:
- Total cholesterol 180 mg/dL
- HDL-C 55 mg/dL
- Triglycerides 75 mg/dL
- Calculated LDL-C 110 mg/dL.
Comment: Lack of LDL-C goals is a flaw
The new guidelines call for patients with known ASCVD, such as this patient, to receive intensive statin therapy—which he got.
However, once a patient is on therapy, the new guidelines do not encourage repeating the lipid panel other than to assess compliance. With intensive therapy, we expect a reduction in LDL-C of at least 50% (Table 1), but patient-to-patient differences in response to medications are common, and without repeat testing we would have no way of gauging this patient’s residual risk.
Further, the new guidelines emphasize the lack of hard outcome data supporting the addition of another lipid-lowering drug to a statin, although they do indicate that one can consider it. In a patient at high risk, such as this one, would you be comfortable with an LDL-C value of 110 mg/dL on maximum statin therapy? Would you consider adding a nonstatin drug?
A preponderance of data shows that LDL plays a causal role in ASCVD development and adverse events. Genetic data show that the LDL particle and the LDL receptor pathway are mechanistically linked to ASCVD pathogenesis, with lifetime exposure as a critical determinant of risk.5,6 Moreover, randomized controlled trials of statins and other studies of cholesterol-lowering show a reproducible relationship between the LDL-C level achieved and absolute risk (Figure 1).7–24 We believe the totality of data constitutes a strong rationale for targeting LDL-C and establishing goals for lowering its levels. For these reasons, we believe that removing LDL-C goals is a fundamental flaw of the new guidelines.
The reason for the lack of data from randomized controlled trials demonstrating benefits of adding therapies to statins (when LDL-C is still high) or benefits of treating to specific goals is that no such trials have been performed. Even trials of nonpharmacologic means of lowering LDL-C, such as ileal bypass, which was used in the Program on the Surgical Control of the Hyperlipidemias trial,20 provide independent evidence that lowering LDL-C reduces the risk of ASCVD (Figure 1).
In addition, trials of nonstatin drugs, such as the Coronary Drug Project,25 which tested niacin, also showed outcome benefits. On the other hand, studies such as the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health26 and Treatment of HDL to Reduce the Incidence of Vascular Events27 trials did not show additional risk reduction when niacin was added to statin therapy. However, the study designs arguably had flaws, including requirement of aggressive LDL-lowering with statins, with LDL-C levels below 70 to 80 mg/dL before randomization.
Therefore, these trials do not tell us what to do for a patient on maximal intensive therapy who has recurrent ASCVD events or who, like our patient, has an LDL-C level higher than previous targets.
For this patient, we would recommend adding a second medication to further lower his LDL-C, but discussing with him the absence of proven benefit in clinical trials and the risks of side effects. At present, lacking LDL-C goals in the new guidelines, we are keeping with the ATP III goals to help guide therapeutic choices and individualize patient management.
GROUP 2: PEOPLE WITH LDL-C ≥ 190
Advantages of the new guidelines
- They state that these patients should receive statins in the highest tolerated doses, which is universally accepted.
Limitations of the new guidelines
- The new guidelines mention only that one “may consider” adding a second agent if LDL-C remains above 190 mg/dL after maximum-dose therapy. Patients with familial hypercholesterolemia or other severe forms of hypercholesterolemia typically end up on multidrug therapy to further reduce LDL-C. The absence of randomized controlled trial data in this setting to show an additive value of second and third lipid-lowering agents does not mean these agents do not provide benefit.
GROUP 3: DIABETES, AGE 40–75, LDL-C 70–189, NO CLINICAL ASCVD
Advantages of the new guidelines
- They call for aggressive treatment of people with diabetes, a group at high risk that derives significant benefit from statin therapy, as shown in randomized controlled trials.
Limitations of the new guidelines
- Although high-intensity statin therapy is indicated for this group, we believe that, using the new risk calculator, some patients may receive overly aggressive treatment, thus increasing the possibility of statin side effects.
- The guidelines do not address patients younger than 40 or older than 75.
- Diabetic patients have a high residual risk of ASCVD events, even on statin therapy. Yet the guidelines ignore the potential benefits of more aggressive LDL-lowering or non-LDL secondary targets for therapy.
Case 2: How low is too low?
A 63-year-old white woman, a nonsmoker with recently diagnosed diabetes, is seen by her primary care physician. She has hypertension, for which she takes lisinopril 5 mg daily. Her fasting lipid values are:
- Total cholesterol 160 mg/dL
- HDL-C 64 mg/dL
- Triglycerides 100 mg/dL
- Calculated LDL-C 76 mg/dL.
Her systolic blood pressure is 129 mm Hg, and based on the new risk calculator, her 10-year risk of cardiovascular disease is 10.2%. According to the new guidelines, she should be started on high-intensity statin treatment (Table 1).
Although this is an acceptable initial course of action, it necessitates close vigilance, since it may actually drive her LDL-C level too low. Randomized controlled trials have typically used an LDL-C concentration of less than or equal to 25 mg/dL as the safety cutoff. With a typical LDL-C reduction of at least 50% on high-intensity statins, our patient’s expected LDL-C level will likely be in the low 30s. We believe this would be a good outcome, provided that she tolerates the medication without adverse effects. However, responses to statins vary from patient to patient.
High-intensity statin therapy may not be necessary to reduce risk adequately in all patients who have diabetes without preexisting vascular disease. The Collaborative Atorvastatin Diabetes Study12 compared atorvastatin 10 mg vs placebo in people with type 2 diabetes, age 40 to 75, who had one or more cardiovascular risk factors but no signs or symptoms of preexisting ASCVD and who had only average or below-average cholesterol levels—precisely like this patient. The trial was terminated early because of a clear benefit (a 37% reduction in the composite end point of major adverse cardiovascular events) in the intervention group. For our patient, we believe an alternative and acceptable approach would be to begin moderate-intensity statin therapy (eg, with atorvastatin 10 mg) (Table 1).
Alternatively, in a patient with diabetes and previous atherosclerotic vascular disease or with a high 10-year risk and high LDL-C, limiting treatment to high-intensity statin therapy by itself may deny them the potential benefits of combination therapies and targeting to lower LDL-C levels or non-HDL-C secondary targets. Guidelines from the American Diabetes Association28 and the American Association of Clinical Endocrinologists29 continue to recommend an LDL-C goal of less than 70 mg/dL in patients at high risk, a non-HDL-C less than 100 mg/dL, an apolipoprotein B less than 80 mg/dL, and an LDL particle number less than 1,000 nmol/L.
GROUP 4: AGE 40–75, LDL-C 70–189, NO ASCVD, BUT 10-YEAR RISK ≥ 7.5%
Advantages of the new guidelines
- They may reduce ASCVD events for patients at higher risk.
- The risk calculator is easy to use and focuses on global risk, ie, all forms of ASCVD.
- The guidelines promote discussion of risks and benefits between patients and providers.
Limitations of the new guidelines
- The new risk calculator is controversial (see below).
- There is potential for overtreatment, particularly in older patients.
- There is potential for undertreatment, particularly in patients with an elevated LDL-C but whose 10-year risk is less than 7.5% because they are young.
- The guidelines do not address patients younger than 40 or older than 75.
- They do not take into account some traditional risk factors, such as family history, and nontraditional risk factors such as C-reactive protein as measured by ultrasensitive assays, lipoprotein(a), and apolipoprotein B.
Risk calculator controversy
The new risk calculator has aroused strong opinions on both sides of the aisle.
Shortly after the new guidelines were released, cardiologists Dr. Paul Ridker and Dr. Nancy Cook from Brigham and Women’s Hospital in Boston published analyses30 showing that the new risk calculator, which was based on older data from several large cohorts such as the Atherosclerosis Risk in Communities study,31 the Cardiovascular Health Study,32 the Coronary Artery Risk Development in Young Adults study,33 and the Framingham Heart Study,34,35 was inaccurate in other cohorts. Specifically, in more-recent cohorts (the Women’s Health Study,36 Physicians’ Health Study,37 and Women’s Health Initiative38), the new calculator overestimates the 10-year risk of ASCVD by 75% to 150%.30 Using the new calculator would make approximately 30 million more Americans eligible for statin treatment. The concern is that patients at lower risk would be treated and exposed to potential side effects of statin therapy.
In addition, the risk calculator relies heavily on age and sex and does not include other factors such as triglyceride level, family history, C-reactive protein, or lipoprotein(a). Importantly, and somewhat ironically given the otherwise absolute adherence to randomized controlled trial data for guideline development, the risk calculator has never been verified in prospective studies to adequately show that using it reduces ASCVD events.
Case 3: Overtreating a primary prevention patient
Based on the risk calculator, essentially any African American man in his early 60s with no other risk factors has a 10-year risk of ASCVD of 7.5% or higher and, according to the new guidelines, should receive at least moderate-intensity statin therapy.
For example, consider a 64-year-old African American man whose systolic blood pressure is 129 mm Hg, who does not smoke, does not have diabetes, and does not have hypertension, and whose total cholesterol level is 180 mg/dL, HDL-C 70 mg/dL, triglycerides 130 mg/dL, and calculated LDL-C 84 mg/dL. His calculated 10-year risk is, surprisingly, 7.5%.
Alternatively, his twin brother is a two-pack-per-day smoker with untreated hypertension and systolic blood pressure 150 mm Hg, with fasting total cholesterol 153 mg/dL, HDL-C 70 mg/dL, triglycerides 60 mg/dL, and LDL-C 71 mg/dL. His calculated 10-year risk is 10.5%, so according to the new guidelines, he too should receive high-intensity statin therapy. Yet this patient clearly needs better blood pressure control and smoking cessation as his primary risk-reduction efforts, not a statin. While assessing global risk is important, a shortcoming of the new guidelines is that they can inappropriately lead to treating the risk score, not individualizing the treatment to the patient. Because of the errors inherent in the risk calculator, some experts have called for a temporary halt on implementing the new guidelines until the risk calculator can be further validated. In November 2013, the American Heart Association and the American College of Cardiology reaffirmed their support of the new guidelines and recommended that they be implemented as planned. As of the time this manuscript goes to print, there are no plans to halt implementation of the new guidelines.
Case 4: Undertreating a primary prevention patient
A 25-year-old white man with no medical history has a total cholesterol level of 310 mg/dL, HDL-C 50 mg/dL, triglycerides 400 mg/dL, and calculated LDL-C 180 mg/dL. He does not smoke or have hypertension or diabetes but has a strong family history of premature coronary disease (his father died of myocardial infarction at age 42). His body mass index is 25 kg/m2. Because he is less than 40 years old, the risk calculator does not apply to him.
If we assume he remains untreated and returns at age 40 with the same clinical factors and laboratory values, his calculated 10-year risk of an ASCVD event according to the new risk calculator will still be only 3.1%. Assuming his medical history remains unchanged as he continues to age, his 10-year risk would not reach 7.5% until he is 58. Would you feel comfortable waiting 33 years before starting statin therapy in this patient?
Waiting for dyslipidemic patients to reach middle age before starting LDL-C-lowering therapy is a failure of prevention. For practical reasons, there are no data from randomized controlled trials with hard outcomes in younger people. Nevertheless, a tenet of preventive cardiology is that cumulative exposure accelerates the “vascular age” ahead of the chronological age. This case illustrates why individualized recommendations guided by LDL-C goals as a target for therapy are needed. For this 25-year-old patient, we would recommend starting an intermediate- or high-potency statin.
Case 5: Rheumatoid arthritis
A 60-year-old postmenopausal white woman with severe rheumatoid arthritis presents for cholesterol evaluation. Her total cholesterol level is 235 mg/dL, HDL-C 50 mg/dL, and LDL-C 165 mg/dL. She does not smoke or have hypertension or diabetes. Her systolic blood pressure is 110 mm Hg. She has elevated C-reactive protein on an ultrasensitive assay and elevated lipoprotein(a).
Her calculated 10-year risk of ASCVD is 3.0%. Assuming her medical history remains the same, she would not reach a calculated 10-year risk of at least 7.5% until age 70. We suggest starting moderate- or high-dose statin therapy in this case, based on data (not from randomized controlled trials) showing an increased risk of ASCVD events in patients with rheumatologic disease, increased lipoprotein(a), and inflammatory markers like C-reactive protein. However, the current guidelines do not address this scenario, other than to suggest that clinician consideration can be given to other risk markers such as these, and that these findings should be discussed in detail with the patient. The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial16 showed a dramatic ASCVD risk reduction in just such patients (Figure 1).
APPLAUSE—AND RESERVATIONS
The newest guidelines for treating high blood cholesterol represent a monumental shift away from using target levels of LDL-C and non-HDL-C and toward a focus on statin intensity for patients in the four highest-risk groups.
We applaud the expert panel for its idealistic approach of using only data from randomized controlled trials, for placing more emphasis on higher-intensity statin treatment, for including stroke in the new definition of ASCVD, and for focusing more attention on treating diabetic patients more aggressively. Simplifying the guidelines is a noble goal. Emphasizing moderate-to-high-intensity statin therapy in patients at moderate-to-high risk should have substantial long-term public health benefits.
However, as we have shown in the case examples, there are significant limitations, and some patients can end up being overtreated, while others may be undertreated.
Guidelines need to be crafted by looking at all the evidence, including the pathophysiology of the disease process, not just data from randomized controlled trials. It is difficult to implement a guideline that on one hand used randomized controlled trials exclusively for recommendations, but on the other hand used an untested risk calculator to guide therapy. Randomized controlled trials are not available for every scenario.
Further, absence of randomized controlled trial data in a given scenario should not be interpreted as evidence of lack of benefit. An example of this is a primary-prevention patient under age 40 with elevated LDL-C below the 190 mg/dL cutoff who otherwise is healthy and without risk factors (eg, Case 4). By disregarding all evidence that is not from randomized controlled trials, the expert panel fails to account for the extensive pathophysiology of ASCVD, which often begins at a young age and takes decades to develop.5,6,39 An entire generation of patients who have not reached the age of inclusion in most randomized controlled trials with hard outcomes is excluded (unless the LDL-C level is very high), potentially setting back decades of progress in the field of prevention. Prevention only works if started. With childhood and young adult obesity sharply rising, we should not fail to address the under-40-year-old patient population in our guidelines.
Guidelines are designed to be expert opinion, not to dictate practice. Focusing on the individual patient instead of the general population at risk, the expert panel appropriately emphasizes the “importance of clinician judgment, weighing potential benefits, adverse effects, drug-drug interactions and patient preferences.” However, by excluding all data that do not come from randomized controlled trials, the panel neglects a very large base of knowledge and leaves many clinicians without as much expert opinion as we had hoped for.
LDL-C goals are important: they provide a scorecard to help the patient with lifestyle and dietary changes. They provide the health care provider guidance in making treatment decisions and focusing on treatment of a single patient, not a population. Moreover, if a patient has difficulty taking standard doses of statins because of side effects, the absence of LDL-C goals makes decision-making nearly impossible. We hope physicians will rely on LDL-C goals in such situations, falling back on the ATP III recommendations, although many patients may simply go untreated until they present with ASCVD or until they “age in” to a higher risk category.
We suggest caution in strict adherence to the new guidelines and instead urge physicians to consider a hybrid of the old guidelines (using the ATP III LDL-C goals) and the new ones (emphasizing global risk assessment and high-intensity statin treatment).
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Heart Association. 2013 Prevention guidelines tools. CV risk calculator. http://my.americanheart.org/professional/StatementsGuidelines/PreventionGuidelines/Prevention-Guidelines_UCM_457698_SubHomePage.jsp. Accessed December 10, 2013.
- Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009; 29:431–438.
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50(suppl):S172–S177.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- de Lemos JA, Blazing MA, Wiviott SD, et al; for the A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Downs JR, Clearfield M, Weis S, et al; for the AFCAPS/TexCAPS Research Group. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/TexCAPS. JAMA 1998; 279:1615–1622.
- Koren MJ, Hunninghake DB, on behalf of the ALLIANCE investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics. J Am Coll Cardiol 2004; 44:1772–1779.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; on behalf of the CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Sacks FM, Pfeffer MA, Moye LA, et al; for the Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335:1001–1009.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Pedersen TR, Faegeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Ridker PM, Danielson E, Fonseca FAH, et al; for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Nakamura H, Arakawa K, Itakura H, et al; for the MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin Japan (MEGA Study): a prospective rabndomised controlled trial. Lancet 2006; 368:1155–1163.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Myocardial Ischemia Reduction with Aggreessive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia: report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990; 323:946–955.
- Cannon CP, Braunwald E, McCabe CH, et al; for the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Shepherd J, Cobbe SM, Ford I, et al; for the West of Scotland Coronary Prevention Study Group. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995; 333:1301–1308.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1989; 8:1245–1255.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013; 19:536–557.
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013doi: 10.1016/S0140-6736(13)62388-0. [Epub ahead of print]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989; 129:687–702.
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276.
- Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116.
- Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham study. Ann N Y Acad Sci 1963; 107:539–556.
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979; 110:281–290.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belancer C, Buring JE, Cook N, et al; The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321:129–135.
- Langer R, White E, Lewis C, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13:S107–S121.
- Strong JP, Malcom GT, Oalmann MC, Wissler RW. The PDAY study: natural history, risk factors, and pathobiology. Ann N Y Acad Sci 1997; 811:226–235.
(Re)turning the Pages of Residency
It's hard to imagine a busy urban hospital without its chorus of beepers.[1] This statement, the first sentence of an article published in 1988, rings (or beeps or buzzes) true to any resident physician today. At that time, pagers had replaced overhead paging, and provided a rapid method to contact physicians who were often scattered throughout the hospital. Still, it was an imperfect solution as the ubiquitous pager constantly interrupted patient care and other tasks, failed to prioritize information, and added to an already stressful working environment. Notably, interns were paged on average once per hour, and occasionally 5 or more times per hour, a frequency that was felt to be detrimental to patient care and to the working environment of resident physicians.[1]
Little has changed. Despite the instant, multidirectional communication platforms available today, alphanumeric paging remains a mainstay of communication between physicians and other members of the care team. Importantly, paging contributes to communication errors (eg, by failing to convey urgency, having incomplete information, or being missed entirely by coverage gaps),[2, 3] and interrupts resident workflow, thereby negatively affecting work efficiency and educational activities, and adding to perceived workload.[4, 5]
In this era of duty hour restrictions, there has been concern that residents experience increased workload due to having fewer hours to do the same amount of work.[6, 7] As such, the Accreditation Council of Graduate Medical Education emphasizes the quality of those hours, with a focus on several aspects of the resident working environment as key to improved educational and patient safety outcomes.[8, 9, 10]
Geographic localization of physicians to patient care units has been proposed as a means to improve communication and agreement on plans of care,[11, 12] and also to reduce resident workload by decreasing inefficiencies attributable to traveling throughout the hospital.[13] O'Leary, et al. (2009) found that when physicians were localized to 1 hospital unit, there was greater agreement between physicians and nurses on various aspects of care, such as planned tests and anticipated length of stay. In addition, members of the patient care team were better able to identify one another, and there was a perceived increase in face‐to‐face communication, and a perceived decrease in text paging.[11]
In consideration of these factors, in July 2011, at New YorkPresbyterian Hospital/Weill Cornell (NYPH/WC), an 800‐bed tertiary care teaching hospital in New York, New York, we geographically localized 2 internal medicine resident teams, and partially localized 2 additional teams. We investigated whether interns on teams that were geographically localized received fewer pages than interns on teams that were not localized. This study was reviewed by the institutional review board of Weill Cornell Medical College and met the requirements for exemption.
METHODS
We conducted a retrospective analysis of the number of pages received by interns during the day (7:00 am to 7:00 pm) on 5 general internal medicine teams during a 1‐month ward rotation between October 17, 2011 and November 13, 2011 at NYPH/WC. The general medicine teams were composed of 1 attending, 1 resident, and 2 interns each. Two teams were geographically localized to a 32‐bed unit (geographic localization model [GLM]). Two teams were partially localized to a 26‐bed unit, which included a respiratory care step‐down unit (partial localization model [PLM]). A fifth and final team admitted patients irrespective of their assigned bed location (standard model [SM]). Both the GLM and the PLM occasionally carried patients on other units to allow for overall census management and patient throughput. The total number of pages received by each intern over the study period was collected by retrospective analysis of electronic paging logs. Night pages (7 pm7 am) were excluded because of night float coverage. Weekend pages were excluded because data were inaccurate due to coverage for days off.
The daily number of admissions and daily census per team were recorded by physician assistants, who also assigned new patients to appropriate teams according to an admissions algorithm (see Supporting Figure 1 in the online version of this article). The percent of geographically localized patients on each team was estimated from the percentage of localized patients on the day of discharge averaged over the study period. For the SM team, percent localization was defined as the number of patients on the patient care unit that contained the team's work area.
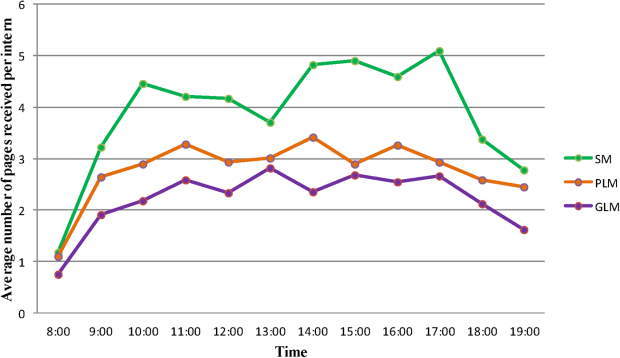
Standard multivariate linear regression techniques were used to analyze the relationship between the number of pages received per intern and the type of team, controlling for the potential effect of total census and number of admissions. The regression model was used to determine adjusted marginal point estimates and 95% confidence intervals (CIs) for the average number of pages per intern per hour for each type of team. All statistical analyses were conducted using Stata version 12 (StataCorp, College Station, TX).
RESULTS
Over the 28‐day study period, a total of 6652 pages were received by 10 interns on 5 general internal medicine teams from 7 am to 7 pm Monday through Friday. The average daily census, average daily admissions, and percent of patients localized to patient care units for the individual teams are shown in Table 1. In univariate analysis, the mean daily pages per intern were not significantly different between the 2 teams within the GLM, nor between the 2 teams in the PLM, allowing them to be combined in multivariate analysis (data not shown). The number of pages received per intern per hour, adjusted for team census and number of admissions, was 2.2 (95% CI: 2.02.4) in the GLM, 2.8 (95% CI: 2.6‐3.0) in the PLM, and 3.9 (95% CI: 3.6‐4.2) in the SM (Table 1). All of these differences were statistically significant (P0.001).
| Standard Model* | Partial Localization Model | Geographically Localized Model | |
|---|---|---|---|
| |||
| Percent of patients localized | 37% | 45% | 85% |
| Team census, mean (range per day) | 16.1 (1320) | 15.9 (1120) | 15.6 (1119) |
| Team admissions, mean (range per day) | 2.7 (15) | 2.9 (06) | 3.5 (07) |
| Pages per hour per intern, unadjusted, mean (95% CI) | 3.9 (3.6‐4.1) | 2.8 (2.6‐3.0) | 2.2 (2.02.4) |
| Pages per hour per intern, adjusted for census and admissions, mean (95% CI) | 3.9 (3.6‐4.2) | 2.8 (2.6‐3.0) | 2.2 (2.02.4) |
Figure 1 shows the pattern of daytime paging for each model. The GLM and PLM had a similar pattern, with an initial ramp up in the first 2 hours of the day, holding steady until approximately 4 pm, and then decrease until 7 pm. The SM had a steeper initial rise, and then continued to increase slowly until a peak at 4 pm.
DISCUSSION
This study corroborates that of Singh et al. (2012), who found that geographic localization led to significantly fewer pages.[14] Our results strengthen the evidence by demonstrating that even modest differences between the percent of patients localized to a care unit led to a significant decrease in the number of pages, indicating a dose‐response effect. The paging frequency we measured is higher than described in Singh et al. (1.4 pages per hour for localized teams), yet our average census appears to be 4 patients higher, which may account for some of that difference. We also show that interns on teams whose patients are more widely scattered throughout the hospital may experience upward of 5 pages per hour, an interruption by pager every 12 minutes, all day long.
A pager interruption is not solely limited to a disruption by noxious sound or vibration. The page recipient must then read the page and respond accordingly, which may involve a phone call, placing an order, walking to another location, or other work tasks. Although some of these interruptions must be handled immediately, such as a clinically deteriorating patient, many are not urgent, and could wait until the physician's current task or thought process is complete. There is also the potentially risky assumption on the part of the sender that the message has been received and will be acted upon. Furthermore, frequent paging is a common interruption to physician workflow; interruptions contribute to increased perceived physician workload[4, 5] and are likely detrimental to patient safety.[15, 16]
The most common metrics used to measure resident workload are patient census and number of admissions,[13] but these metrics have provided a mixed and likely incomplete picture. Recent research suggests that other factors, such as work efficiency (including interruptions, time spent obtaining test results, and time in transit) and work intensity (such as the acuity and complexity of patients), contribute significantly to actual and perceived resident workload.[13]
Our analysis was a single‐site, retrospective study, which occurred over 1 month and was limited to internal medicine teams. Additionally, geographic localization logically should lead to increased face‐to‐face interruptions, which we were unable to measure with this project, but direct communication is more efficient and less prone to error, which would likely lead to fewer overall interruptions. Although we anticipate that our findings are applicable to geographically localized patient care units in other hospitals, further investigation is warranted.
The paging chorus has only grown louder over the last 25 years, with likely downstream effects on patient safety and resident education. To mitigate these effects, it is incumbent upon us to approach our training and patient care environments with a critical and creative lens, and to explore opportunities to decrease interruptions and streamline our communication systems.
Acknowledgements
The authors acknowledge the assistance with data analysis of Arthur Evans, MD, MPH, and review of the manuscript by Brendan Reilly, MD.
Disclosures: Dr. Fanucchi and Ms. Unterbrink have no conflicts of interest to disclose. Dr. Logio reports receiving royalties from McGraw‐Hill for Core Concepts in Patient Safety online modules.
- , . The sounds of the hospital. Paging patterns in three teaching hospitals. N Engl J Med. 1988;319(24):1585–1589.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . Alphanumeric paging: a potential source of problems in patient care and communication. J Surg Educ. 2011;68(6):447–451.
- , , , , . Hospital doctors' workflow interruptions and activities: an observation study. BMJ Qual Saf. 2011;20(6):491–497.
- , , , , . The association of workflow interruptions and hospital doctors' workload: a prospective observational study. BMJ Qual Saf. 2012;21(5):399–407.
- , . Resident workload—let's treat the disease, not just the symptom. Comment on: Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff. JAMA Intern Med. 2013;173(8):655–656.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , . The ACGME 2011 Duty Hour Standards: Enhancing Quality of Care, Supervision, and Resident Professional Development. Chicago, IL: Accreditation Council for Graduate Medical Education; 2011.
- , , , . Institute of Medicine Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, DC: National Academies Press; 2009.
- , , , , , . Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883–888.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Service census caps and unit‐based admissions: resident workload, conference attendance, duty hour compliance, and patient safety. Mayo Clin Proc. 2012;87(4):320–327.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- . The science of interruption. BMJ Qual Saf. 2012;21(5):357–360.
- , , , et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284–289.
It's hard to imagine a busy urban hospital without its chorus of beepers.[1] This statement, the first sentence of an article published in 1988, rings (or beeps or buzzes) true to any resident physician today. At that time, pagers had replaced overhead paging, and provided a rapid method to contact physicians who were often scattered throughout the hospital. Still, it was an imperfect solution as the ubiquitous pager constantly interrupted patient care and other tasks, failed to prioritize information, and added to an already stressful working environment. Notably, interns were paged on average once per hour, and occasionally 5 or more times per hour, a frequency that was felt to be detrimental to patient care and to the working environment of resident physicians.[1]
Little has changed. Despite the instant, multidirectional communication platforms available today, alphanumeric paging remains a mainstay of communication between physicians and other members of the care team. Importantly, paging contributes to communication errors (eg, by failing to convey urgency, having incomplete information, or being missed entirely by coverage gaps),[2, 3] and interrupts resident workflow, thereby negatively affecting work efficiency and educational activities, and adding to perceived workload.[4, 5]
In this era of duty hour restrictions, there has been concern that residents experience increased workload due to having fewer hours to do the same amount of work.[6, 7] As such, the Accreditation Council of Graduate Medical Education emphasizes the quality of those hours, with a focus on several aspects of the resident working environment as key to improved educational and patient safety outcomes.[8, 9, 10]
Geographic localization of physicians to patient care units has been proposed as a means to improve communication and agreement on plans of care,[11, 12] and also to reduce resident workload by decreasing inefficiencies attributable to traveling throughout the hospital.[13] O'Leary, et al. (2009) found that when physicians were localized to 1 hospital unit, there was greater agreement between physicians and nurses on various aspects of care, such as planned tests and anticipated length of stay. In addition, members of the patient care team were better able to identify one another, and there was a perceived increase in face‐to‐face communication, and a perceived decrease in text paging.[11]
In consideration of these factors, in July 2011, at New YorkPresbyterian Hospital/Weill Cornell (NYPH/WC), an 800‐bed tertiary care teaching hospital in New York, New York, we geographically localized 2 internal medicine resident teams, and partially localized 2 additional teams. We investigated whether interns on teams that were geographically localized received fewer pages than interns on teams that were not localized. This study was reviewed by the institutional review board of Weill Cornell Medical College and met the requirements for exemption.
METHODS
We conducted a retrospective analysis of the number of pages received by interns during the day (7:00 am to 7:00 pm) on 5 general internal medicine teams during a 1‐month ward rotation between October 17, 2011 and November 13, 2011 at NYPH/WC. The general medicine teams were composed of 1 attending, 1 resident, and 2 interns each. Two teams were geographically localized to a 32‐bed unit (geographic localization model [GLM]). Two teams were partially localized to a 26‐bed unit, which included a respiratory care step‐down unit (partial localization model [PLM]). A fifth and final team admitted patients irrespective of their assigned bed location (standard model [SM]). Both the GLM and the PLM occasionally carried patients on other units to allow for overall census management and patient throughput. The total number of pages received by each intern over the study period was collected by retrospective analysis of electronic paging logs. Night pages (7 pm7 am) were excluded because of night float coverage. Weekend pages were excluded because data were inaccurate due to coverage for days off.
The daily number of admissions and daily census per team were recorded by physician assistants, who also assigned new patients to appropriate teams according to an admissions algorithm (see Supporting Figure 1 in the online version of this article). The percent of geographically localized patients on each team was estimated from the percentage of localized patients on the day of discharge averaged over the study period. For the SM team, percent localization was defined as the number of patients on the patient care unit that contained the team's work area.

Standard multivariate linear regression techniques were used to analyze the relationship between the number of pages received per intern and the type of team, controlling for the potential effect of total census and number of admissions. The regression model was used to determine adjusted marginal point estimates and 95% confidence intervals (CIs) for the average number of pages per intern per hour for each type of team. All statistical analyses were conducted using Stata version 12 (StataCorp, College Station, TX).
RESULTS
Over the 28‐day study period, a total of 6652 pages were received by 10 interns on 5 general internal medicine teams from 7 am to 7 pm Monday through Friday. The average daily census, average daily admissions, and percent of patients localized to patient care units for the individual teams are shown in Table 1. In univariate analysis, the mean daily pages per intern were not significantly different between the 2 teams within the GLM, nor between the 2 teams in the PLM, allowing them to be combined in multivariate analysis (data not shown). The number of pages received per intern per hour, adjusted for team census and number of admissions, was 2.2 (95% CI: 2.02.4) in the GLM, 2.8 (95% CI: 2.6‐3.0) in the PLM, and 3.9 (95% CI: 3.6‐4.2) in the SM (Table 1). All of these differences were statistically significant (P0.001).
| Standard Model* | Partial Localization Model | Geographically Localized Model | |
|---|---|---|---|
| |||
| Percent of patients localized | 37% | 45% | 85% |
| Team census, mean (range per day) | 16.1 (1320) | 15.9 (1120) | 15.6 (1119) |
| Team admissions, mean (range per day) | 2.7 (15) | 2.9 (06) | 3.5 (07) |
| Pages per hour per intern, unadjusted, mean (95% CI) | 3.9 (3.6‐4.1) | 2.8 (2.6‐3.0) | 2.2 (2.02.4) |
| Pages per hour per intern, adjusted for census and admissions, mean (95% CI) | 3.9 (3.6‐4.2) | 2.8 (2.6‐3.0) | 2.2 (2.02.4) |
Figure 1 shows the pattern of daytime paging for each model. The GLM and PLM had a similar pattern, with an initial ramp up in the first 2 hours of the day, holding steady until approximately 4 pm, and then decrease until 7 pm. The SM had a steeper initial rise, and then continued to increase slowly until a peak at 4 pm.
DISCUSSION
This study corroborates that of Singh et al. (2012), who found that geographic localization led to significantly fewer pages.[14] Our results strengthen the evidence by demonstrating that even modest differences between the percent of patients localized to a care unit led to a significant decrease in the number of pages, indicating a dose‐response effect. The paging frequency we measured is higher than described in Singh et al. (1.4 pages per hour for localized teams), yet our average census appears to be 4 patients higher, which may account for some of that difference. We also show that interns on teams whose patients are more widely scattered throughout the hospital may experience upward of 5 pages per hour, an interruption by pager every 12 minutes, all day long.
A pager interruption is not solely limited to a disruption by noxious sound or vibration. The page recipient must then read the page and respond accordingly, which may involve a phone call, placing an order, walking to another location, or other work tasks. Although some of these interruptions must be handled immediately, such as a clinically deteriorating patient, many are not urgent, and could wait until the physician's current task or thought process is complete. There is also the potentially risky assumption on the part of the sender that the message has been received and will be acted upon. Furthermore, frequent paging is a common interruption to physician workflow; interruptions contribute to increased perceived physician workload[4, 5] and are likely detrimental to patient safety.[15, 16]
The most common metrics used to measure resident workload are patient census and number of admissions,[13] but these metrics have provided a mixed and likely incomplete picture. Recent research suggests that other factors, such as work efficiency (including interruptions, time spent obtaining test results, and time in transit) and work intensity (such as the acuity and complexity of patients), contribute significantly to actual and perceived resident workload.[13]
Our analysis was a single‐site, retrospective study, which occurred over 1 month and was limited to internal medicine teams. Additionally, geographic localization logically should lead to increased face‐to‐face interruptions, which we were unable to measure with this project, but direct communication is more efficient and less prone to error, which would likely lead to fewer overall interruptions. Although we anticipate that our findings are applicable to geographically localized patient care units in other hospitals, further investigation is warranted.
The paging chorus has only grown louder over the last 25 years, with likely downstream effects on patient safety and resident education. To mitigate these effects, it is incumbent upon us to approach our training and patient care environments with a critical and creative lens, and to explore opportunities to decrease interruptions and streamline our communication systems.
Acknowledgements
The authors acknowledge the assistance with data analysis of Arthur Evans, MD, MPH, and review of the manuscript by Brendan Reilly, MD.
Disclosures: Dr. Fanucchi and Ms. Unterbrink have no conflicts of interest to disclose. Dr. Logio reports receiving royalties from McGraw‐Hill for Core Concepts in Patient Safety online modules.
It's hard to imagine a busy urban hospital without its chorus of beepers.[1] This statement, the first sentence of an article published in 1988, rings (or beeps or buzzes) true to any resident physician today. At that time, pagers had replaced overhead paging, and provided a rapid method to contact physicians who were often scattered throughout the hospital. Still, it was an imperfect solution as the ubiquitous pager constantly interrupted patient care and other tasks, failed to prioritize information, and added to an already stressful working environment. Notably, interns were paged on average once per hour, and occasionally 5 or more times per hour, a frequency that was felt to be detrimental to patient care and to the working environment of resident physicians.[1]
Little has changed. Despite the instant, multidirectional communication platforms available today, alphanumeric paging remains a mainstay of communication between physicians and other members of the care team. Importantly, paging contributes to communication errors (eg, by failing to convey urgency, having incomplete information, or being missed entirely by coverage gaps),[2, 3] and interrupts resident workflow, thereby negatively affecting work efficiency and educational activities, and adding to perceived workload.[4, 5]
In this era of duty hour restrictions, there has been concern that residents experience increased workload due to having fewer hours to do the same amount of work.[6, 7] As such, the Accreditation Council of Graduate Medical Education emphasizes the quality of those hours, with a focus on several aspects of the resident working environment as key to improved educational and patient safety outcomes.[8, 9, 10]
Geographic localization of physicians to patient care units has been proposed as a means to improve communication and agreement on plans of care,[11, 12] and also to reduce resident workload by decreasing inefficiencies attributable to traveling throughout the hospital.[13] O'Leary, et al. (2009) found that when physicians were localized to 1 hospital unit, there was greater agreement between physicians and nurses on various aspects of care, such as planned tests and anticipated length of stay. In addition, members of the patient care team were better able to identify one another, and there was a perceived increase in face‐to‐face communication, and a perceived decrease in text paging.[11]
In consideration of these factors, in July 2011, at New YorkPresbyterian Hospital/Weill Cornell (NYPH/WC), an 800‐bed tertiary care teaching hospital in New York, New York, we geographically localized 2 internal medicine resident teams, and partially localized 2 additional teams. We investigated whether interns on teams that were geographically localized received fewer pages than interns on teams that were not localized. This study was reviewed by the institutional review board of Weill Cornell Medical College and met the requirements for exemption.
METHODS
We conducted a retrospective analysis of the number of pages received by interns during the day (7:00 am to 7:00 pm) on 5 general internal medicine teams during a 1‐month ward rotation between October 17, 2011 and November 13, 2011 at NYPH/WC. The general medicine teams were composed of 1 attending, 1 resident, and 2 interns each. Two teams were geographically localized to a 32‐bed unit (geographic localization model [GLM]). Two teams were partially localized to a 26‐bed unit, which included a respiratory care step‐down unit (partial localization model [PLM]). A fifth and final team admitted patients irrespective of their assigned bed location (standard model [SM]). Both the GLM and the PLM occasionally carried patients on other units to allow for overall census management and patient throughput. The total number of pages received by each intern over the study period was collected by retrospective analysis of electronic paging logs. Night pages (7 pm7 am) were excluded because of night float coverage. Weekend pages were excluded because data were inaccurate due to coverage for days off.
The daily number of admissions and daily census per team were recorded by physician assistants, who also assigned new patients to appropriate teams according to an admissions algorithm (see Supporting Figure 1 in the online version of this article). The percent of geographically localized patients on each team was estimated from the percentage of localized patients on the day of discharge averaged over the study period. For the SM team, percent localization was defined as the number of patients on the patient care unit that contained the team's work area.

Standard multivariate linear regression techniques were used to analyze the relationship between the number of pages received per intern and the type of team, controlling for the potential effect of total census and number of admissions. The regression model was used to determine adjusted marginal point estimates and 95% confidence intervals (CIs) for the average number of pages per intern per hour for each type of team. All statistical analyses were conducted using Stata version 12 (StataCorp, College Station, TX).
RESULTS
Over the 28‐day study period, a total of 6652 pages were received by 10 interns on 5 general internal medicine teams from 7 am to 7 pm Monday through Friday. The average daily census, average daily admissions, and percent of patients localized to patient care units for the individual teams are shown in Table 1. In univariate analysis, the mean daily pages per intern were not significantly different between the 2 teams within the GLM, nor between the 2 teams in the PLM, allowing them to be combined in multivariate analysis (data not shown). The number of pages received per intern per hour, adjusted for team census and number of admissions, was 2.2 (95% CI: 2.02.4) in the GLM, 2.8 (95% CI: 2.6‐3.0) in the PLM, and 3.9 (95% CI: 3.6‐4.2) in the SM (Table 1). All of these differences were statistically significant (P0.001).
| Standard Model* | Partial Localization Model | Geographically Localized Model | |
|---|---|---|---|
| |||
| Percent of patients localized | 37% | 45% | 85% |
| Team census, mean (range per day) | 16.1 (1320) | 15.9 (1120) | 15.6 (1119) |
| Team admissions, mean (range per day) | 2.7 (15) | 2.9 (06) | 3.5 (07) |
| Pages per hour per intern, unadjusted, mean (95% CI) | 3.9 (3.6‐4.1) | 2.8 (2.6‐3.0) | 2.2 (2.02.4) |
| Pages per hour per intern, adjusted for census and admissions, mean (95% CI) | 3.9 (3.6‐4.2) | 2.8 (2.6‐3.0) | 2.2 (2.02.4) |
Figure 1 shows the pattern of daytime paging for each model. The GLM and PLM had a similar pattern, with an initial ramp up in the first 2 hours of the day, holding steady until approximately 4 pm, and then decrease until 7 pm. The SM had a steeper initial rise, and then continued to increase slowly until a peak at 4 pm.
DISCUSSION
This study corroborates that of Singh et al. (2012), who found that geographic localization led to significantly fewer pages.[14] Our results strengthen the evidence by demonstrating that even modest differences between the percent of patients localized to a care unit led to a significant decrease in the number of pages, indicating a dose‐response effect. The paging frequency we measured is higher than described in Singh et al. (1.4 pages per hour for localized teams), yet our average census appears to be 4 patients higher, which may account for some of that difference. We also show that interns on teams whose patients are more widely scattered throughout the hospital may experience upward of 5 pages per hour, an interruption by pager every 12 minutes, all day long.
A pager interruption is not solely limited to a disruption by noxious sound or vibration. The page recipient must then read the page and respond accordingly, which may involve a phone call, placing an order, walking to another location, or other work tasks. Although some of these interruptions must be handled immediately, such as a clinically deteriorating patient, many are not urgent, and could wait until the physician's current task or thought process is complete. There is also the potentially risky assumption on the part of the sender that the message has been received and will be acted upon. Furthermore, frequent paging is a common interruption to physician workflow; interruptions contribute to increased perceived physician workload[4, 5] and are likely detrimental to patient safety.[15, 16]
The most common metrics used to measure resident workload are patient census and number of admissions,[13] but these metrics have provided a mixed and likely incomplete picture. Recent research suggests that other factors, such as work efficiency (including interruptions, time spent obtaining test results, and time in transit) and work intensity (such as the acuity and complexity of patients), contribute significantly to actual and perceived resident workload.[13]
Our analysis was a single‐site, retrospective study, which occurred over 1 month and was limited to internal medicine teams. Additionally, geographic localization logically should lead to increased face‐to‐face interruptions, which we were unable to measure with this project, but direct communication is more efficient and less prone to error, which would likely lead to fewer overall interruptions. Although we anticipate that our findings are applicable to geographically localized patient care units in other hospitals, further investigation is warranted.
The paging chorus has only grown louder over the last 25 years, with likely downstream effects on patient safety and resident education. To mitigate these effects, it is incumbent upon us to approach our training and patient care environments with a critical and creative lens, and to explore opportunities to decrease interruptions and streamline our communication systems.
Acknowledgements
The authors acknowledge the assistance with data analysis of Arthur Evans, MD, MPH, and review of the manuscript by Brendan Reilly, MD.
Disclosures: Dr. Fanucchi and Ms. Unterbrink have no conflicts of interest to disclose. Dr. Logio reports receiving royalties from McGraw‐Hill for Core Concepts in Patient Safety online modules.
- , . The sounds of the hospital. Paging patterns in three teaching hospitals. N Engl J Med. 1988;319(24):1585–1589.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . Alphanumeric paging: a potential source of problems in patient care and communication. J Surg Educ. 2011;68(6):447–451.
- , , , , . Hospital doctors' workflow interruptions and activities: an observation study. BMJ Qual Saf. 2011;20(6):491–497.
- , , , , . The association of workflow interruptions and hospital doctors' workload: a prospective observational study. BMJ Qual Saf. 2012;21(5):399–407.
- , . Resident workload—let's treat the disease, not just the symptom. Comment on: Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff. JAMA Intern Med. 2013;173(8):655–656.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , . The ACGME 2011 Duty Hour Standards: Enhancing Quality of Care, Supervision, and Resident Professional Development. Chicago, IL: Accreditation Council for Graduate Medical Education; 2011.
- , , , . Institute of Medicine Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, DC: National Academies Press; 2009.
- , , , , , . Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883–888.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Service census caps and unit‐based admissions: resident workload, conference attendance, duty hour compliance, and patient safety. Mayo Clin Proc. 2012;87(4):320–327.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- . The science of interruption. BMJ Qual Saf. 2012;21(5):357–360.
- , , , et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284–289.
- , . The sounds of the hospital. Paging patterns in three teaching hospitals. N Engl J Med. 1988;319(24):1585–1589.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . Alphanumeric paging: a potential source of problems in patient care and communication. J Surg Educ. 2011;68(6):447–451.
- , , , , . Hospital doctors' workflow interruptions and activities: an observation study. BMJ Qual Saf. 2011;20(6):491–497.
- , , , , . The association of workflow interruptions and hospital doctors' workload: a prospective observational study. BMJ Qual Saf. 2012;21(5):399–407.
- , . Resident workload—let's treat the disease, not just the symptom. Comment on: Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff. JAMA Intern Med. 2013;173(8):655–656.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–655.
- , . The ACGME 2011 Duty Hour Standards: Enhancing Quality of Care, Supervision, and Resident Professional Development. Chicago, IL: Accreditation Council for Graduate Medical Education; 2011.
- , , , . Institute of Medicine Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, DC: National Academies Press; 2009.
- , , , , , . Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883–888.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Service census caps and unit‐based admissions: resident workload, conference attendance, duty hour compliance, and patient safety. Mayo Clin Proc. 2012;87(4):320–327.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- . The science of interruption. BMJ Qual Saf. 2012;21(5):357–360.
- , , , et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284–289.
Improving Admission Process Efficiency
Maintaining high‐quality patient care, optimizing patient safety, and providing adequate trainee supervision has been an area of debate in medical education recently, and many physicians remain concerned that excessive regulation and duty hour restrictions may prevent residents from obtaining sufficient experience and developing an appropriate sense of autonomy.[1, 2, 3, 4] However, pediatric hospital medicine (PHM) has seen dramatic increases in evening and nighttime in‐house attending coverage, and the trend is expected to continue.[5, 6] Whether it be for financial, educational, or patient‐centered reasons, increased in‐house attending coverage at an academic medical setting, almost by definition, increases direct resident supervision.[7]
Increased supervision may result in better educational outcomes,[8] but many forces, such as night float systems and electronic medical records (EMRs), pull residents away from the bedside, leaving them with fewer opportunities to make decisions and a reduced sense of personal responsibility and patient ownership. Experiential learning is of great value in medical training, and without this, residents may exit their training with less confidence and competence, only rarely having been able to make important medical decisions on their own.[9, 10]
Counter to the shift toward increased supervision, we recently amended our process for pediatric admissions to the PHM service by transitioning from mandatory to on‐demand attending input during the admissions process. We hypothesized that this would improve its efficiency by encouraging residents to develop an increased sense of patient ownership and would not significantly impact patient care.
METHODS
Setting
This cohort study was conducted at the Golisano Children's Hospital (GCH) at the University of Rochester in Rochester, New York. The pediatric residency program at this tertiary care center includes 48 pediatric residents and 21 medicinepediatric residents. The PHM division, comprised of 8 pediatric hospitalists, provides care to approximately one‐third of the children with medical illnesses admitted to GCH. During the daytime, PHM attendings provide in‐house supervision for 2 resident teams, each consisting of a senior resident and 2 interns. At night, PHM attendings take calls from home. Residents are encouraged to contact attendings, available by cell phone and pager, with questions or concerns regarding patient care. The institutional review board of the University of Rochester Medical Center approved this study and informed consent was waived.
Process Change
Prior to the change, a pediatric emergency department (ED) provider at GCH directly contacted the PHM attending for all admissions to the PHM service (Figure 1). If the PHM attending accepted the admission, the ED provider then notified the pediatric admitting officer (PAO), a third‐year pediatric or fourth‐year medicinepediatric resident, who either performed or delegated the admission duties (eg, history and physical exam, admission orders).
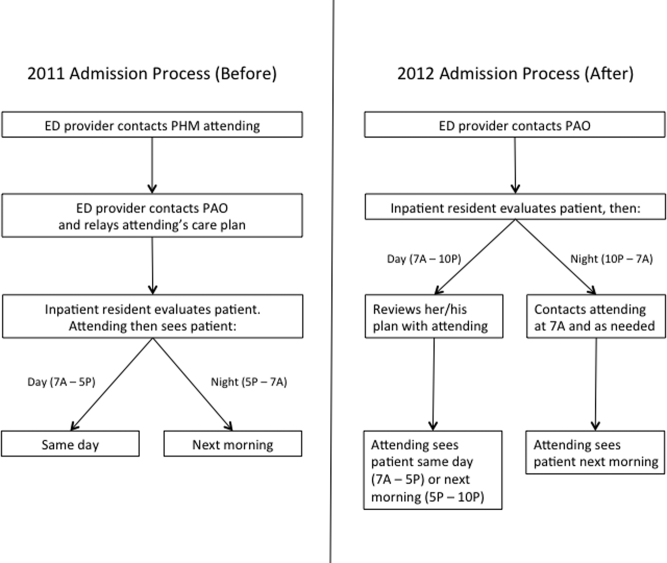
On June 18, 2012, a new process for pediatric admissions was implemented (Figure 1). The ED provider now called the PAO, and not the attending, to discuss an admission to the PHM service. The PAO was empowered to accept the patient on behalf of the PHM attending, and perform or delegate the admission duties. During daytime hours (7:00 am5:00 pm), the PAO was expected to alert the PHM attending of the admission to allow the attending to see the patient on the day of admission. The PHM attending discussed the case with the admitting resident after the resident had an opportunity to assess the patient and formulate a management plan. During evening hours (5:00 pm10:00 pm), the admitting resident was expected to contact the PHM attending on call after evaluating the patient and developing a plan. Overnight (10:00 pm7:00 am), the PAO was given discretion as to whether she/he needed to contact the PHM attending on call; the PHM service attending then saw the patient in the morning. Residents were strongly encouraged to call the PHM attending with any questions or concerns or if they did not feel an admission was appropriate to the PHM service.
Study Population
The study population included all patients <19 years of age admitted to the PHM service from the ED. The pre‐ and post‐intervention cohorts included patients admitted from July 1, 2011 to September 30, 2011 and July 1, 2012 to September 30, 2012, respectively. These dates were chosen because residents are least experienced in the summer months, and hence we would predict the greatest disparity during this time. Patients who were directly admitted via transport from an outside facility, office or from home, or who were transferred from another service within GCH were excluded. Patients were identified from administrative databases.
Data Collection
Date and time of admission, severity of illness (SOI) scores, and risk of mortality (ROM) scores were obtained from the administrative dataset. The EMR was then used to extract the following variables: gender; date and time of the ED provider's admission request and first inpatient resident order; date and time of patient discharge, defined as the time the after‐visit summary was finalized by an inpatient provider; and the number of rapid response team (RRT) activations within 24 hours of the first inpatient resident order. The order time difference was calculated by subtracting the date and time of the ED provider admission request from the first inpatient order. Cases in which the order time difference was negative were excluded from the order time analysis due to the possibility that some extenuating circumstance for these patients, not related to the admission process, caused the early inpatient order. Length of stay (LOS) was calculated as the difference between the date and time of ED admission request and date and time of patient discharge.
The first 24 hours of each admission were reviewed independently by 3 PHM attending investigators. Neither reviewer evaluated a chart for which he had cosigned the admission note. Charts were assessed to determine whether a reasonable standard of care (SOC) was provided by the inpatient resident during admission. For instances in which SOC was not felt to have been provided by the resident, the chart was reviewed by the second investigator. If there was disagreement between the 2 investigators, a third PHM attending was used to determine the majority opinion. Due to the nature of data collected, it was not possible to blind reviewers.
PHM attending investigators also assessed how often the inpatient resident's antibiotic choice was changed by the admitting PHM attending. This evaluation excluded topical antibiotics and antibiotics not related to the admitting diagnosis (eg, continuation of outpatient antibiotics for otitis media). A change in antibiotics was defined as a change in class or a change within classes, initiation, or discontinuation of an antibiotic by the attending. Switching the route of administration was considered a change if it was not done as part of the transition to discharge. Antibiotic choice was considered in agreement if a change was made by the PHM attending based on new patient information that was not available to the admitting inpatient resident if it could be reasonably concluded that the attending would have otherwise agreed with the original choice. If this determination could not be made, the antibiotic agreement was classified as unknown. Data regarding antibiotic agreement were analyzed in 2 ways. The first included all patients for which agreement could be determined. For this analysis, if a patient was not prescribed an antibiotic by the resident or attending, there was considered to have been antibiotic agreement. The second analysis included only the patients for whom an antibiotic was started by the inpatient resident or admitting attending.
Finally, RRT activations within the first 24 hours of admission in the 2012 cohort were evaluated to determine whether the RRT could have been prevented by the original admission process. This determination was made via majority opinion of 3 PHM attendings who each independently reviewed the cases.
Statistical Analysis
The distributions of continuous variables (eg, order time difference, LOS) and the ordinal variables (ROM and SOI) were compared using Wilcoxon rank sum tests. 2 tests or Fisher exact tests were used to assess the differences in categorical variables (eg, SOC, gender). All tests were 2‐sided, and the significance level was set at 0.05. Analyses were conducted using the SAS statistical package version 9.3 (SAS Institute Inc., Cary, NC) and SPSS version 21 (IBM/SPSS, Armonk, NY).
RESULTS
The initial search identified 532 admissions. Of these, 140 were excluded (72 were via route other than the ED, 44 were not admitted to PHM, 14 were outside the study period, and 10 did not meet age criteria). Therefore, 182 admissions in the 2011 cohort and 210 admissions in the 2012 cohort were included. For all patients in the 2012 cohort, the correct admission process was followed.
Demographic characteristics between cohorts were similar (Table 1). Data for ROM and SOI were available for 141 (78%) 2011 patients and for 169 (81%) 2012 patients. The distribution of patients over the study months differed between cohorts. Age, gender, ROM, and SOI were not significantly different.
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Male gender, n (%) | 107 (59) | 105 (50) | 0.082 |
| Median age, y (IQR) | 2 (010) | 2 (07) | 0.689 |
| Month admitted, n (%) | 0.002 | ||
| July | 60 (33) | 87 (41) | |
| August | 57 (31) | 81 (39) | |
| September | 65 (36) | 42 (20) | |
| Nighttime admission, n (%)* | 71 (39) | 90 (43) | 0.440 |
| Risk of mortality, n (%) | 0.910 | ||
| 1, lowest risk | 114 (81) | 138 (82) | |
| 2 | 22 (16) | 23 (14) | |
| 3 | 5 (4) | 6 (4) | |
| 4, highest risk | 0 (0) | 2 (1) | |
| Severity of illness, n (%) | 0.095 | ||
| 1, lowest severity | 60 (43) | 86 (51) | |
| 2 | 54 (38) | 62 (37) | |
| 3 | 25 (18) | 15 (9) | |
| 4, highest severity | 2 (1) | 6 (4) | |
The median difference in time from the ED provider admission request to the first inpatient resident order was roughly half as long in 2012 than in 2011 (123 vs 62 minutes, P<0.001) (Table 2). There were 12 cases in which the inpatient order came prior to the ED admission request in 2012 and 2 cases in 2011, and these were excluded from the order time difference analysis. LOS was not significantly different between groups (P=0.348). There were no differences in the frequency of antibiotic changes when all patients were considered or in the subgroup in whom antibiotics were prescribed by either the resident or attending. The number of cases for which the admitting resident's plan was deemed not to have met standard of care were few and not significantly different (P=1). None of these patients experienced harm as a result, and in all cases, SOC was determined to have been provided by the admitting PHM attending. The frequency of RRT calls within the first 24 hours of admission on PHM patients was not significantly different (P=0.114).
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Time from admission decision to first inpatient order, min, median (IQR)a | 123 (70188) | 62 (30105) | <0.001 |
| Length of stay, h, median (IQR)b | 44 (3167) | 41 (2271) | 0.348 |
| Change by attending to resident's antibiotic choice in all patients, n (%) | 13/182 (7) | 18/210 (9) | 0.617 |
| Change by attending to resident's antibiotic choice in patients who received antibiotics, n (%) | 13/97 (13) | 18/96 (19) | 0.312 |
| Resident met standard of care, n (%) | 180/182 (99) | 207/210 (99) | 1 |
| RRT called within first 24 hours, n (%) | 2/182 (1) | 8/210 (4) | 0.114 |
When only patients admitted during the night in 2011 and 2012 were compared, results were consistent with the overall finding that there was a shorter time to inpatient admission order without a difference in other studied variables (Table 3).
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Time from admission decision to first inpatient order, min, median (IQR)ab | 90 (40151) | 42 (1767) | 0.002 |
| Length of stay, h, median (IQR)b | 53 (3461) | 36 (1769) | 0.307 |
| Change by attending to resident's antibiotic choice in all patients, n (%) | 7/70 (10) | 7/88 (8) | 1 |
| Resident met standard of care, n (%) | 70/71 (99) | 88/90 (98) | 1 |
| RRT called within first 24 hours, n (%) | 2/71 (3) | 6/90 (7) | 0.468 |
DISCUSSION
The purpose of this study was to evaluate an admission process that removed an ineffective method of attending oversight and allowed residents an opportunity to develop patient care plans prior to attending input. The key change from the original process was removing the step in which the ED provider contacted the PHM attending for new admissions, thus eliminating mandatory inpatient attending input, removing an impediment to workflow, and empowering inpatient pediatric residents to assess new patients and develop management plans. Our data show a reduction in the time difference between the ED admission request and the inpatient resident's first order by more than an hour, indicating a more efficient admission process. Although one might expect that eliminating the act of a phone call would shorten this time by a few minutes, it cannot account for the extent of the difference we found. We postulate that an increased sense of accountability motivated inpatient residents to evaluate and begin management sooner, a topic that requires further exploration.
A more efficient admission process benefits emergency medicine residents and other ED providers as well. It is well documented that ED crowding is associated with decreased quality of care,[11, 12] and ED efficiency is receiving increased attention with newly reportable quality metrics such as Admit Decision Time to Emergency Department Departure Time for Admitted Patients.[13]
Our data do not attenuate the importance of hospitalists in patient care, as evidenced by the fact that PHM attendings continued to frequently amend the residents' antibiotic choicethe only variable we evaluated in terms of change in planand recognized several cases in which the residents' plan did not meet standard of care. Furthermore, attendings continued to be available by phone and pager for guidance and education when needed or requested by the residents. Instead, our data show that removing mandated attending input at the time of admission did not significantly impact major patient outcomes, which may partly be attributable to the general safety of the inpatient pediatric wards.[14, 15] In our study, a comprehensive analysis of patient harm was not possible given the variable list and infrequency with which SOC was not met or RRTs were called. Furthermore, our residency program continues to comply with national pediatric residency requirements for nighttime supervision.[7]
Our PHM division, which had previously allocated 2 hours of attending clinical time per call night, now averages <15 minutes. These data conflict with the current trend in PHM toward more, rather than less, direct attending oversight. Many PHM divisions have moved toward 24/7 in‐house coverage,[5] a situation that often results in shiftwork and multiple handoffs. Removing the in‐house attending overnight would allow for the rapidly growing PHM subspecialty to allocate hospitalists elsewhere depending on their scholarly needs, particularly as divisions seek to become increasingly involved in medical education, research, and hospital leadership.[16, 17] Although one might posit a financial benefit to having in‐house attendings determine the appropriateness of an admission overnight, we identified no case in which the insurance denied an admission.
Safety equivalence of an in‐house to on‐call attending is poorly studied in PHM. However, even in intensive care units, where the majority of morbidity and mortality occur, it is unclear that the presence of an attending, let alone mandating phone calls, positively impacts survival. One prospective trial failed to demonstrate a difference in patient outcomes in the critical care setting when comparing mandated attending in‐house involvement to optional attending availability by phone.[18] Furthermore, several studies have found no association with time of admission and mortality, implying there is no criticality specifically requiring nighttime coverage.[19, 20]
One adult study of nocturnists showed that residents felt they had more contact with attendings who were in‐house than attendings taking home calls.[21] However, when the residents were asked why they did not contact the attending, the only difference between at‐home and in‐house attendings was that for attendings available by phone, residents were less likely to know who to call and were hesitant to wake the attending.
This study had several limitations. First, we could not effectively blind reviewers; a salient point given that the reviewers benefited from the new system with a reduced nighttime workload. We attempted to minimize this bias by employing multiple independent evaluations followed by group consensus whenever possible. Second, even though we had 3 hospitalists independently review each 2012 RRT to determine whether it was preventable by the prior system, this task was prone to retrospective bias. Third, there was a significant difference in the month of admission between cohorts. Rather than biasing toward our observed time difference, the fact that more patients were admitted in July 2012the beginning of the academic yearmay have decreased our observed difference given that residents were less experienced. Forth, this study used certain measurable outcomes as proxies for quality of care and patient harm and was likely underpowered to truly detect a difference in some of the more infrequent variables. Furthermore, we did not evaluate other potential harms, such as cost. Fifth, we did not evaluate whether or not the new process changed ED provider behavior (ie, an ED provider may wait longer to request admission overnight given that the PHM attending is not mandated to provide input until the morning). Finally, although LOS was used as a balancing measure, it would likely have taken major events or omissions during the admission process to cause it to change significantly, and therefore the lack of statistical difference in this metric does not necessarily imply that more subtle aspects of care were the same between groups. We also chose not to include readmission rate for this reason, as any change could not conclusively be attributed to the new admission process.
CONCLUSION
Increasing resident autonomy by removing mandated input during PHM admissions makes the process more efficient and results in no significant changes to major patient outcomes. These data may be used by rapidly growing PHM divisions to redefine faculty clinical responsibilities, particularly at night.
ACKNOWLEDGMENTS
Disclosures: This project was supported by the University of Rochester CTSA award number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
- Accreditation Council for Graduate Medical Education Task Force on Quality Care and Professionalism. The ACGME 2011 duty hour standards: enhancing quality of care, supervision, and resident professional development. Accreditation Council for Graduate Medical Education, Chicago, IL; 2011. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/jgme‐monograph[1].pdf. Last accessed on December 18, 2013.
- , , , , . Impact of reduction in working hours for doctors in training on postgraduate medical education and patients' outcomes: systemic review. BMJ. 2011;342:d1580.
- , . ACGME 2011 duty‐hour guidelines: consequences expected by radiology residency directors and chief residents. J Am Coll Radiol. 2012;9(11):820–827.
- . Justifying patient risks associated with medical education. JAMA. 2007;298(9):1046–1048.
- , , , , , . Survey of academic pediatric hospitalist programs in the U.S.: organizational, administrative and financial factors. J Hosp Med. 2013;8(6):285–291.
- , , , . Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299–303.
- ACGME Program Requirements for Graduate Medical Education in Pediatrics. ACGME Approved: September 30, 2012; Effective: July 1, 2013. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013‐PR‐FAQ‐PIF/320_pediatrics_07012013.pdf. Accessed September 17, 2013.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , . Twenty‐four‐hour intensivist staffing in teaching hospitals: tension between safety today and safety tomorrow. Chest. 2012;141(5):1315–1320.
- . Medical education on the brink: 62 years of front‐line observations and opinions. Tex Heart Inst J. 2012;39(3):322–329.
- , . Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008;51:6–7.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- The Specifications Manual for National Hospital Inpatient Quality Measures. A Collaboration of the Centers for Medicare 128(1):72–78.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- Section on Hospital Medicine. Guiding principles for Pediatric Hospital Medicine programs. Pediatrics. 2013;132(4):782–786. SHM fact sheet: about hospital medicine. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Media_Kit42(5):120–126.
- , , , et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368(23):2201–2209.
- , , , , , . Association between time of admission to the ICU and mortality: a systematic review and meta‐analysis. Chest. 2010;138(1):68–75.
- , , , . After‐hours admissions are not associated with increased risk‐adjusted mortality in pediatric intensive care. Intensive Care Med. 2008;34(1):148–151.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
Maintaining high‐quality patient care, optimizing patient safety, and providing adequate trainee supervision has been an area of debate in medical education recently, and many physicians remain concerned that excessive regulation and duty hour restrictions may prevent residents from obtaining sufficient experience and developing an appropriate sense of autonomy.[1, 2, 3, 4] However, pediatric hospital medicine (PHM) has seen dramatic increases in evening and nighttime in‐house attending coverage, and the trend is expected to continue.[5, 6] Whether it be for financial, educational, or patient‐centered reasons, increased in‐house attending coverage at an academic medical setting, almost by definition, increases direct resident supervision.[7]
Increased supervision may result in better educational outcomes,[8] but many forces, such as night float systems and electronic medical records (EMRs), pull residents away from the bedside, leaving them with fewer opportunities to make decisions and a reduced sense of personal responsibility and patient ownership. Experiential learning is of great value in medical training, and without this, residents may exit their training with less confidence and competence, only rarely having been able to make important medical decisions on their own.[9, 10]
Counter to the shift toward increased supervision, we recently amended our process for pediatric admissions to the PHM service by transitioning from mandatory to on‐demand attending input during the admissions process. We hypothesized that this would improve its efficiency by encouraging residents to develop an increased sense of patient ownership and would not significantly impact patient care.
METHODS
Setting
This cohort study was conducted at the Golisano Children's Hospital (GCH) at the University of Rochester in Rochester, New York. The pediatric residency program at this tertiary care center includes 48 pediatric residents and 21 medicinepediatric residents. The PHM division, comprised of 8 pediatric hospitalists, provides care to approximately one‐third of the children with medical illnesses admitted to GCH. During the daytime, PHM attendings provide in‐house supervision for 2 resident teams, each consisting of a senior resident and 2 interns. At night, PHM attendings take calls from home. Residents are encouraged to contact attendings, available by cell phone and pager, with questions or concerns regarding patient care. The institutional review board of the University of Rochester Medical Center approved this study and informed consent was waived.
Process Change
Prior to the change, a pediatric emergency department (ED) provider at GCH directly contacted the PHM attending for all admissions to the PHM service (Figure 1). If the PHM attending accepted the admission, the ED provider then notified the pediatric admitting officer (PAO), a third‐year pediatric or fourth‐year medicinepediatric resident, who either performed or delegated the admission duties (eg, history and physical exam, admission orders).

On June 18, 2012, a new process for pediatric admissions was implemented (Figure 1). The ED provider now called the PAO, and not the attending, to discuss an admission to the PHM service. The PAO was empowered to accept the patient on behalf of the PHM attending, and perform or delegate the admission duties. During daytime hours (7:00 am5:00 pm), the PAO was expected to alert the PHM attending of the admission to allow the attending to see the patient on the day of admission. The PHM attending discussed the case with the admitting resident after the resident had an opportunity to assess the patient and formulate a management plan. During evening hours (5:00 pm10:00 pm), the admitting resident was expected to contact the PHM attending on call after evaluating the patient and developing a plan. Overnight (10:00 pm7:00 am), the PAO was given discretion as to whether she/he needed to contact the PHM attending on call; the PHM service attending then saw the patient in the morning. Residents were strongly encouraged to call the PHM attending with any questions or concerns or if they did not feel an admission was appropriate to the PHM service.
Study Population
The study population included all patients <19 years of age admitted to the PHM service from the ED. The pre‐ and post‐intervention cohorts included patients admitted from July 1, 2011 to September 30, 2011 and July 1, 2012 to September 30, 2012, respectively. These dates were chosen because residents are least experienced in the summer months, and hence we would predict the greatest disparity during this time. Patients who were directly admitted via transport from an outside facility, office or from home, or who were transferred from another service within GCH were excluded. Patients were identified from administrative databases.
Data Collection
Date and time of admission, severity of illness (SOI) scores, and risk of mortality (ROM) scores were obtained from the administrative dataset. The EMR was then used to extract the following variables: gender; date and time of the ED provider's admission request and first inpatient resident order; date and time of patient discharge, defined as the time the after‐visit summary was finalized by an inpatient provider; and the number of rapid response team (RRT) activations within 24 hours of the first inpatient resident order. The order time difference was calculated by subtracting the date and time of the ED provider admission request from the first inpatient order. Cases in which the order time difference was negative were excluded from the order time analysis due to the possibility that some extenuating circumstance for these patients, not related to the admission process, caused the early inpatient order. Length of stay (LOS) was calculated as the difference between the date and time of ED admission request and date and time of patient discharge.
The first 24 hours of each admission were reviewed independently by 3 PHM attending investigators. Neither reviewer evaluated a chart for which he had cosigned the admission note. Charts were assessed to determine whether a reasonable standard of care (SOC) was provided by the inpatient resident during admission. For instances in which SOC was not felt to have been provided by the resident, the chart was reviewed by the second investigator. If there was disagreement between the 2 investigators, a third PHM attending was used to determine the majority opinion. Due to the nature of data collected, it was not possible to blind reviewers.
PHM attending investigators also assessed how often the inpatient resident's antibiotic choice was changed by the admitting PHM attending. This evaluation excluded topical antibiotics and antibiotics not related to the admitting diagnosis (eg, continuation of outpatient antibiotics for otitis media). A change in antibiotics was defined as a change in class or a change within classes, initiation, or discontinuation of an antibiotic by the attending. Switching the route of administration was considered a change if it was not done as part of the transition to discharge. Antibiotic choice was considered in agreement if a change was made by the PHM attending based on new patient information that was not available to the admitting inpatient resident if it could be reasonably concluded that the attending would have otherwise agreed with the original choice. If this determination could not be made, the antibiotic agreement was classified as unknown. Data regarding antibiotic agreement were analyzed in 2 ways. The first included all patients for which agreement could be determined. For this analysis, if a patient was not prescribed an antibiotic by the resident or attending, there was considered to have been antibiotic agreement. The second analysis included only the patients for whom an antibiotic was started by the inpatient resident or admitting attending.
Finally, RRT activations within the first 24 hours of admission in the 2012 cohort were evaluated to determine whether the RRT could have been prevented by the original admission process. This determination was made via majority opinion of 3 PHM attendings who each independently reviewed the cases.
Statistical Analysis
The distributions of continuous variables (eg, order time difference, LOS) and the ordinal variables (ROM and SOI) were compared using Wilcoxon rank sum tests. 2 tests or Fisher exact tests were used to assess the differences in categorical variables (eg, SOC, gender). All tests were 2‐sided, and the significance level was set at 0.05. Analyses were conducted using the SAS statistical package version 9.3 (SAS Institute Inc., Cary, NC) and SPSS version 21 (IBM/SPSS, Armonk, NY).
RESULTS
The initial search identified 532 admissions. Of these, 140 were excluded (72 were via route other than the ED, 44 were not admitted to PHM, 14 were outside the study period, and 10 did not meet age criteria). Therefore, 182 admissions in the 2011 cohort and 210 admissions in the 2012 cohort were included. For all patients in the 2012 cohort, the correct admission process was followed.
Demographic characteristics between cohorts were similar (Table 1). Data for ROM and SOI were available for 141 (78%) 2011 patients and for 169 (81%) 2012 patients. The distribution of patients over the study months differed between cohorts. Age, gender, ROM, and SOI were not significantly different.
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Male gender, n (%) | 107 (59) | 105 (50) | 0.082 |
| Median age, y (IQR) | 2 (010) | 2 (07) | 0.689 |
| Month admitted, n (%) | 0.002 | ||
| July | 60 (33) | 87 (41) | |
| August | 57 (31) | 81 (39) | |
| September | 65 (36) | 42 (20) | |
| Nighttime admission, n (%)* | 71 (39) | 90 (43) | 0.440 |
| Risk of mortality, n (%) | 0.910 | ||
| 1, lowest risk | 114 (81) | 138 (82) | |
| 2 | 22 (16) | 23 (14) | |
| 3 | 5 (4) | 6 (4) | |
| 4, highest risk | 0 (0) | 2 (1) | |
| Severity of illness, n (%) | 0.095 | ||
| 1, lowest severity | 60 (43) | 86 (51) | |
| 2 | 54 (38) | 62 (37) | |
| 3 | 25 (18) | 15 (9) | |
| 4, highest severity | 2 (1) | 6 (4) | |
The median difference in time from the ED provider admission request to the first inpatient resident order was roughly half as long in 2012 than in 2011 (123 vs 62 minutes, P<0.001) (Table 2). There were 12 cases in which the inpatient order came prior to the ED admission request in 2012 and 2 cases in 2011, and these were excluded from the order time difference analysis. LOS was not significantly different between groups (P=0.348). There were no differences in the frequency of antibiotic changes when all patients were considered or in the subgroup in whom antibiotics were prescribed by either the resident or attending. The number of cases for which the admitting resident's plan was deemed not to have met standard of care were few and not significantly different (P=1). None of these patients experienced harm as a result, and in all cases, SOC was determined to have been provided by the admitting PHM attending. The frequency of RRT calls within the first 24 hours of admission on PHM patients was not significantly different (P=0.114).
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Time from admission decision to first inpatient order, min, median (IQR)a | 123 (70188) | 62 (30105) | <0.001 |
| Length of stay, h, median (IQR)b | 44 (3167) | 41 (2271) | 0.348 |
| Change by attending to resident's antibiotic choice in all patients, n (%) | 13/182 (7) | 18/210 (9) | 0.617 |
| Change by attending to resident's antibiotic choice in patients who received antibiotics, n (%) | 13/97 (13) | 18/96 (19) | 0.312 |
| Resident met standard of care, n (%) | 180/182 (99) | 207/210 (99) | 1 |
| RRT called within first 24 hours, n (%) | 2/182 (1) | 8/210 (4) | 0.114 |
When only patients admitted during the night in 2011 and 2012 were compared, results were consistent with the overall finding that there was a shorter time to inpatient admission order without a difference in other studied variables (Table 3).
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Time from admission decision to first inpatient order, min, median (IQR)ab | 90 (40151) | 42 (1767) | 0.002 |
| Length of stay, h, median (IQR)b | 53 (3461) | 36 (1769) | 0.307 |
| Change by attending to resident's antibiotic choice in all patients, n (%) | 7/70 (10) | 7/88 (8) | 1 |
| Resident met standard of care, n (%) | 70/71 (99) | 88/90 (98) | 1 |
| RRT called within first 24 hours, n (%) | 2/71 (3) | 6/90 (7) | 0.468 |
DISCUSSION
The purpose of this study was to evaluate an admission process that removed an ineffective method of attending oversight and allowed residents an opportunity to develop patient care plans prior to attending input. The key change from the original process was removing the step in which the ED provider contacted the PHM attending for new admissions, thus eliminating mandatory inpatient attending input, removing an impediment to workflow, and empowering inpatient pediatric residents to assess new patients and develop management plans. Our data show a reduction in the time difference between the ED admission request and the inpatient resident's first order by more than an hour, indicating a more efficient admission process. Although one might expect that eliminating the act of a phone call would shorten this time by a few minutes, it cannot account for the extent of the difference we found. We postulate that an increased sense of accountability motivated inpatient residents to evaluate and begin management sooner, a topic that requires further exploration.
A more efficient admission process benefits emergency medicine residents and other ED providers as well. It is well documented that ED crowding is associated with decreased quality of care,[11, 12] and ED efficiency is receiving increased attention with newly reportable quality metrics such as Admit Decision Time to Emergency Department Departure Time for Admitted Patients.[13]
Our data do not attenuate the importance of hospitalists in patient care, as evidenced by the fact that PHM attendings continued to frequently amend the residents' antibiotic choicethe only variable we evaluated in terms of change in planand recognized several cases in which the residents' plan did not meet standard of care. Furthermore, attendings continued to be available by phone and pager for guidance and education when needed or requested by the residents. Instead, our data show that removing mandated attending input at the time of admission did not significantly impact major patient outcomes, which may partly be attributable to the general safety of the inpatient pediatric wards.[14, 15] In our study, a comprehensive analysis of patient harm was not possible given the variable list and infrequency with which SOC was not met or RRTs were called. Furthermore, our residency program continues to comply with national pediatric residency requirements for nighttime supervision.[7]
Our PHM division, which had previously allocated 2 hours of attending clinical time per call night, now averages <15 minutes. These data conflict with the current trend in PHM toward more, rather than less, direct attending oversight. Many PHM divisions have moved toward 24/7 in‐house coverage,[5] a situation that often results in shiftwork and multiple handoffs. Removing the in‐house attending overnight would allow for the rapidly growing PHM subspecialty to allocate hospitalists elsewhere depending on their scholarly needs, particularly as divisions seek to become increasingly involved in medical education, research, and hospital leadership.[16, 17] Although one might posit a financial benefit to having in‐house attendings determine the appropriateness of an admission overnight, we identified no case in which the insurance denied an admission.
Safety equivalence of an in‐house to on‐call attending is poorly studied in PHM. However, even in intensive care units, where the majority of morbidity and mortality occur, it is unclear that the presence of an attending, let alone mandating phone calls, positively impacts survival. One prospective trial failed to demonstrate a difference in patient outcomes in the critical care setting when comparing mandated attending in‐house involvement to optional attending availability by phone.[18] Furthermore, several studies have found no association with time of admission and mortality, implying there is no criticality specifically requiring nighttime coverage.[19, 20]
One adult study of nocturnists showed that residents felt they had more contact with attendings who were in‐house than attendings taking home calls.[21] However, when the residents were asked why they did not contact the attending, the only difference between at‐home and in‐house attendings was that for attendings available by phone, residents were less likely to know who to call and were hesitant to wake the attending.
This study had several limitations. First, we could not effectively blind reviewers; a salient point given that the reviewers benefited from the new system with a reduced nighttime workload. We attempted to minimize this bias by employing multiple independent evaluations followed by group consensus whenever possible. Second, even though we had 3 hospitalists independently review each 2012 RRT to determine whether it was preventable by the prior system, this task was prone to retrospective bias. Third, there was a significant difference in the month of admission between cohorts. Rather than biasing toward our observed time difference, the fact that more patients were admitted in July 2012the beginning of the academic yearmay have decreased our observed difference given that residents were less experienced. Forth, this study used certain measurable outcomes as proxies for quality of care and patient harm and was likely underpowered to truly detect a difference in some of the more infrequent variables. Furthermore, we did not evaluate other potential harms, such as cost. Fifth, we did not evaluate whether or not the new process changed ED provider behavior (ie, an ED provider may wait longer to request admission overnight given that the PHM attending is not mandated to provide input until the morning). Finally, although LOS was used as a balancing measure, it would likely have taken major events or omissions during the admission process to cause it to change significantly, and therefore the lack of statistical difference in this metric does not necessarily imply that more subtle aspects of care were the same between groups. We also chose not to include readmission rate for this reason, as any change could not conclusively be attributed to the new admission process.
CONCLUSION
Increasing resident autonomy by removing mandated input during PHM admissions makes the process more efficient and results in no significant changes to major patient outcomes. These data may be used by rapidly growing PHM divisions to redefine faculty clinical responsibilities, particularly at night.
ACKNOWLEDGMENTS
Disclosures: This project was supported by the University of Rochester CTSA award number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
Maintaining high‐quality patient care, optimizing patient safety, and providing adequate trainee supervision has been an area of debate in medical education recently, and many physicians remain concerned that excessive regulation and duty hour restrictions may prevent residents from obtaining sufficient experience and developing an appropriate sense of autonomy.[1, 2, 3, 4] However, pediatric hospital medicine (PHM) has seen dramatic increases in evening and nighttime in‐house attending coverage, and the trend is expected to continue.[5, 6] Whether it be for financial, educational, or patient‐centered reasons, increased in‐house attending coverage at an academic medical setting, almost by definition, increases direct resident supervision.[7]
Increased supervision may result in better educational outcomes,[8] but many forces, such as night float systems and electronic medical records (EMRs), pull residents away from the bedside, leaving them with fewer opportunities to make decisions and a reduced sense of personal responsibility and patient ownership. Experiential learning is of great value in medical training, and without this, residents may exit their training with less confidence and competence, only rarely having been able to make important medical decisions on their own.[9, 10]
Counter to the shift toward increased supervision, we recently amended our process for pediatric admissions to the PHM service by transitioning from mandatory to on‐demand attending input during the admissions process. We hypothesized that this would improve its efficiency by encouraging residents to develop an increased sense of patient ownership and would not significantly impact patient care.
METHODS
Setting
This cohort study was conducted at the Golisano Children's Hospital (GCH) at the University of Rochester in Rochester, New York. The pediatric residency program at this tertiary care center includes 48 pediatric residents and 21 medicinepediatric residents. The PHM division, comprised of 8 pediatric hospitalists, provides care to approximately one‐third of the children with medical illnesses admitted to GCH. During the daytime, PHM attendings provide in‐house supervision for 2 resident teams, each consisting of a senior resident and 2 interns. At night, PHM attendings take calls from home. Residents are encouraged to contact attendings, available by cell phone and pager, with questions or concerns regarding patient care. The institutional review board of the University of Rochester Medical Center approved this study and informed consent was waived.
Process Change
Prior to the change, a pediatric emergency department (ED) provider at GCH directly contacted the PHM attending for all admissions to the PHM service (Figure 1). If the PHM attending accepted the admission, the ED provider then notified the pediatric admitting officer (PAO), a third‐year pediatric or fourth‐year medicinepediatric resident, who either performed or delegated the admission duties (eg, history and physical exam, admission orders).

On June 18, 2012, a new process for pediatric admissions was implemented (Figure 1). The ED provider now called the PAO, and not the attending, to discuss an admission to the PHM service. The PAO was empowered to accept the patient on behalf of the PHM attending, and perform or delegate the admission duties. During daytime hours (7:00 am5:00 pm), the PAO was expected to alert the PHM attending of the admission to allow the attending to see the patient on the day of admission. The PHM attending discussed the case with the admitting resident after the resident had an opportunity to assess the patient and formulate a management plan. During evening hours (5:00 pm10:00 pm), the admitting resident was expected to contact the PHM attending on call after evaluating the patient and developing a plan. Overnight (10:00 pm7:00 am), the PAO was given discretion as to whether she/he needed to contact the PHM attending on call; the PHM service attending then saw the patient in the morning. Residents were strongly encouraged to call the PHM attending with any questions or concerns or if they did not feel an admission was appropriate to the PHM service.
Study Population
The study population included all patients <19 years of age admitted to the PHM service from the ED. The pre‐ and post‐intervention cohorts included patients admitted from July 1, 2011 to September 30, 2011 and July 1, 2012 to September 30, 2012, respectively. These dates were chosen because residents are least experienced in the summer months, and hence we would predict the greatest disparity during this time. Patients who were directly admitted via transport from an outside facility, office or from home, or who were transferred from another service within GCH were excluded. Patients were identified from administrative databases.
Data Collection
Date and time of admission, severity of illness (SOI) scores, and risk of mortality (ROM) scores were obtained from the administrative dataset. The EMR was then used to extract the following variables: gender; date and time of the ED provider's admission request and first inpatient resident order; date and time of patient discharge, defined as the time the after‐visit summary was finalized by an inpatient provider; and the number of rapid response team (RRT) activations within 24 hours of the first inpatient resident order. The order time difference was calculated by subtracting the date and time of the ED provider admission request from the first inpatient order. Cases in which the order time difference was negative were excluded from the order time analysis due to the possibility that some extenuating circumstance for these patients, not related to the admission process, caused the early inpatient order. Length of stay (LOS) was calculated as the difference between the date and time of ED admission request and date and time of patient discharge.
The first 24 hours of each admission were reviewed independently by 3 PHM attending investigators. Neither reviewer evaluated a chart for which he had cosigned the admission note. Charts were assessed to determine whether a reasonable standard of care (SOC) was provided by the inpatient resident during admission. For instances in which SOC was not felt to have been provided by the resident, the chart was reviewed by the second investigator. If there was disagreement between the 2 investigators, a third PHM attending was used to determine the majority opinion. Due to the nature of data collected, it was not possible to blind reviewers.
PHM attending investigators also assessed how often the inpatient resident's antibiotic choice was changed by the admitting PHM attending. This evaluation excluded topical antibiotics and antibiotics not related to the admitting diagnosis (eg, continuation of outpatient antibiotics for otitis media). A change in antibiotics was defined as a change in class or a change within classes, initiation, or discontinuation of an antibiotic by the attending. Switching the route of administration was considered a change if it was not done as part of the transition to discharge. Antibiotic choice was considered in agreement if a change was made by the PHM attending based on new patient information that was not available to the admitting inpatient resident if it could be reasonably concluded that the attending would have otherwise agreed with the original choice. If this determination could not be made, the antibiotic agreement was classified as unknown. Data regarding antibiotic agreement were analyzed in 2 ways. The first included all patients for which agreement could be determined. For this analysis, if a patient was not prescribed an antibiotic by the resident or attending, there was considered to have been antibiotic agreement. The second analysis included only the patients for whom an antibiotic was started by the inpatient resident or admitting attending.
Finally, RRT activations within the first 24 hours of admission in the 2012 cohort were evaluated to determine whether the RRT could have been prevented by the original admission process. This determination was made via majority opinion of 3 PHM attendings who each independently reviewed the cases.
Statistical Analysis
The distributions of continuous variables (eg, order time difference, LOS) and the ordinal variables (ROM and SOI) were compared using Wilcoxon rank sum tests. 2 tests or Fisher exact tests were used to assess the differences in categorical variables (eg, SOC, gender). All tests were 2‐sided, and the significance level was set at 0.05. Analyses were conducted using the SAS statistical package version 9.3 (SAS Institute Inc., Cary, NC) and SPSS version 21 (IBM/SPSS, Armonk, NY).
RESULTS
The initial search identified 532 admissions. Of these, 140 were excluded (72 were via route other than the ED, 44 were not admitted to PHM, 14 were outside the study period, and 10 did not meet age criteria). Therefore, 182 admissions in the 2011 cohort and 210 admissions in the 2012 cohort were included. For all patients in the 2012 cohort, the correct admission process was followed.
Demographic characteristics between cohorts were similar (Table 1). Data for ROM and SOI were available for 141 (78%) 2011 patients and for 169 (81%) 2012 patients. The distribution of patients over the study months differed between cohorts. Age, gender, ROM, and SOI were not significantly different.
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Male gender, n (%) | 107 (59) | 105 (50) | 0.082 |
| Median age, y (IQR) | 2 (010) | 2 (07) | 0.689 |
| Month admitted, n (%) | 0.002 | ||
| July | 60 (33) | 87 (41) | |
| August | 57 (31) | 81 (39) | |
| September | 65 (36) | 42 (20) | |
| Nighttime admission, n (%)* | 71 (39) | 90 (43) | 0.440 |
| Risk of mortality, n (%) | 0.910 | ||
| 1, lowest risk | 114 (81) | 138 (82) | |
| 2 | 22 (16) | 23 (14) | |
| 3 | 5 (4) | 6 (4) | |
| 4, highest risk | 0 (0) | 2 (1) | |
| Severity of illness, n (%) | 0.095 | ||
| 1, lowest severity | 60 (43) | 86 (51) | |
| 2 | 54 (38) | 62 (37) | |
| 3 | 25 (18) | 15 (9) | |
| 4, highest severity | 2 (1) | 6 (4) | |
The median difference in time from the ED provider admission request to the first inpatient resident order was roughly half as long in 2012 than in 2011 (123 vs 62 minutes, P<0.001) (Table 2). There were 12 cases in which the inpatient order came prior to the ED admission request in 2012 and 2 cases in 2011, and these were excluded from the order time difference analysis. LOS was not significantly different between groups (P=0.348). There were no differences in the frequency of antibiotic changes when all patients were considered or in the subgroup in whom antibiotics were prescribed by either the resident or attending. The number of cases for which the admitting resident's plan was deemed not to have met standard of care were few and not significantly different (P=1). None of these patients experienced harm as a result, and in all cases, SOC was determined to have been provided by the admitting PHM attending. The frequency of RRT calls within the first 24 hours of admission on PHM patients was not significantly different (P=0.114).
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Time from admission decision to first inpatient order, min, median (IQR)a | 123 (70188) | 62 (30105) | <0.001 |
| Length of stay, h, median (IQR)b | 44 (3167) | 41 (2271) | 0.348 |
| Change by attending to resident's antibiotic choice in all patients, n (%) | 13/182 (7) | 18/210 (9) | 0.617 |
| Change by attending to resident's antibiotic choice in patients who received antibiotics, n (%) | 13/97 (13) | 18/96 (19) | 0.312 |
| Resident met standard of care, n (%) | 180/182 (99) | 207/210 (99) | 1 |
| RRT called within first 24 hours, n (%) | 2/182 (1) | 8/210 (4) | 0.114 |
When only patients admitted during the night in 2011 and 2012 were compared, results were consistent with the overall finding that there was a shorter time to inpatient admission order without a difference in other studied variables (Table 3).
| Variable | 2011 | 2012 | P Value |
|---|---|---|---|
| |||
| Time from admission decision to first inpatient order, min, median (IQR)ab | 90 (40151) | 42 (1767) | 0.002 |
| Length of stay, h, median (IQR)b | 53 (3461) | 36 (1769) | 0.307 |
| Change by attending to resident's antibiotic choice in all patients, n (%) | 7/70 (10) | 7/88 (8) | 1 |
| Resident met standard of care, n (%) | 70/71 (99) | 88/90 (98) | 1 |
| RRT called within first 24 hours, n (%) | 2/71 (3) | 6/90 (7) | 0.468 |
DISCUSSION
The purpose of this study was to evaluate an admission process that removed an ineffective method of attending oversight and allowed residents an opportunity to develop patient care plans prior to attending input. The key change from the original process was removing the step in which the ED provider contacted the PHM attending for new admissions, thus eliminating mandatory inpatient attending input, removing an impediment to workflow, and empowering inpatient pediatric residents to assess new patients and develop management plans. Our data show a reduction in the time difference between the ED admission request and the inpatient resident's first order by more than an hour, indicating a more efficient admission process. Although one might expect that eliminating the act of a phone call would shorten this time by a few minutes, it cannot account for the extent of the difference we found. We postulate that an increased sense of accountability motivated inpatient residents to evaluate and begin management sooner, a topic that requires further exploration.
A more efficient admission process benefits emergency medicine residents and other ED providers as well. It is well documented that ED crowding is associated with decreased quality of care,[11, 12] and ED efficiency is receiving increased attention with newly reportable quality metrics such as Admit Decision Time to Emergency Department Departure Time for Admitted Patients.[13]
Our data do not attenuate the importance of hospitalists in patient care, as evidenced by the fact that PHM attendings continued to frequently amend the residents' antibiotic choicethe only variable we evaluated in terms of change in planand recognized several cases in which the residents' plan did not meet standard of care. Furthermore, attendings continued to be available by phone and pager for guidance and education when needed or requested by the residents. Instead, our data show that removing mandated attending input at the time of admission did not significantly impact major patient outcomes, which may partly be attributable to the general safety of the inpatient pediatric wards.[14, 15] In our study, a comprehensive analysis of patient harm was not possible given the variable list and infrequency with which SOC was not met or RRTs were called. Furthermore, our residency program continues to comply with national pediatric residency requirements for nighttime supervision.[7]
Our PHM division, which had previously allocated 2 hours of attending clinical time per call night, now averages <15 minutes. These data conflict with the current trend in PHM toward more, rather than less, direct attending oversight. Many PHM divisions have moved toward 24/7 in‐house coverage,[5] a situation that often results in shiftwork and multiple handoffs. Removing the in‐house attending overnight would allow for the rapidly growing PHM subspecialty to allocate hospitalists elsewhere depending on their scholarly needs, particularly as divisions seek to become increasingly involved in medical education, research, and hospital leadership.[16, 17] Although one might posit a financial benefit to having in‐house attendings determine the appropriateness of an admission overnight, we identified no case in which the insurance denied an admission.
Safety equivalence of an in‐house to on‐call attending is poorly studied in PHM. However, even in intensive care units, where the majority of morbidity and mortality occur, it is unclear that the presence of an attending, let alone mandating phone calls, positively impacts survival. One prospective trial failed to demonstrate a difference in patient outcomes in the critical care setting when comparing mandated attending in‐house involvement to optional attending availability by phone.[18] Furthermore, several studies have found no association with time of admission and mortality, implying there is no criticality specifically requiring nighttime coverage.[19, 20]
One adult study of nocturnists showed that residents felt they had more contact with attendings who were in‐house than attendings taking home calls.[21] However, when the residents were asked why they did not contact the attending, the only difference between at‐home and in‐house attendings was that for attendings available by phone, residents were less likely to know who to call and were hesitant to wake the attending.
This study had several limitations. First, we could not effectively blind reviewers; a salient point given that the reviewers benefited from the new system with a reduced nighttime workload. We attempted to minimize this bias by employing multiple independent evaluations followed by group consensus whenever possible. Second, even though we had 3 hospitalists independently review each 2012 RRT to determine whether it was preventable by the prior system, this task was prone to retrospective bias. Third, there was a significant difference in the month of admission between cohorts. Rather than biasing toward our observed time difference, the fact that more patients were admitted in July 2012the beginning of the academic yearmay have decreased our observed difference given that residents were less experienced. Forth, this study used certain measurable outcomes as proxies for quality of care and patient harm and was likely underpowered to truly detect a difference in some of the more infrequent variables. Furthermore, we did not evaluate other potential harms, such as cost. Fifth, we did not evaluate whether or not the new process changed ED provider behavior (ie, an ED provider may wait longer to request admission overnight given that the PHM attending is not mandated to provide input until the morning). Finally, although LOS was used as a balancing measure, it would likely have taken major events or omissions during the admission process to cause it to change significantly, and therefore the lack of statistical difference in this metric does not necessarily imply that more subtle aspects of care were the same between groups. We also chose not to include readmission rate for this reason, as any change could not conclusively be attributed to the new admission process.
CONCLUSION
Increasing resident autonomy by removing mandated input during PHM admissions makes the process more efficient and results in no significant changes to major patient outcomes. These data may be used by rapidly growing PHM divisions to redefine faculty clinical responsibilities, particularly at night.
ACKNOWLEDGMENTS
Disclosures: This project was supported by the University of Rochester CTSA award number UL1 TR000042 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
- Accreditation Council for Graduate Medical Education Task Force on Quality Care and Professionalism. The ACGME 2011 duty hour standards: enhancing quality of care, supervision, and resident professional development. Accreditation Council for Graduate Medical Education, Chicago, IL; 2011. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/jgme‐monograph[1].pdf. Last accessed on December 18, 2013.
- , , , , . Impact of reduction in working hours for doctors in training on postgraduate medical education and patients' outcomes: systemic review. BMJ. 2011;342:d1580.
- , . ACGME 2011 duty‐hour guidelines: consequences expected by radiology residency directors and chief residents. J Am Coll Radiol. 2012;9(11):820–827.
- . Justifying patient risks associated with medical education. JAMA. 2007;298(9):1046–1048.
- , , , , , . Survey of academic pediatric hospitalist programs in the U.S.: organizational, administrative and financial factors. J Hosp Med. 2013;8(6):285–291.
- , , , . Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299–303.
- ACGME Program Requirements for Graduate Medical Education in Pediatrics. ACGME Approved: September 30, 2012; Effective: July 1, 2013. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013‐PR‐FAQ‐PIF/320_pediatrics_07012013.pdf. Accessed September 17, 2013.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , . Twenty‐four‐hour intensivist staffing in teaching hospitals: tension between safety today and safety tomorrow. Chest. 2012;141(5):1315–1320.
- . Medical education on the brink: 62 years of front‐line observations and opinions. Tex Heart Inst J. 2012;39(3):322–329.
- , . Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008;51:6–7.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- The Specifications Manual for National Hospital Inpatient Quality Measures. A Collaboration of the Centers for Medicare 128(1):72–78.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- Section on Hospital Medicine. Guiding principles for Pediatric Hospital Medicine programs. Pediatrics. 2013;132(4):782–786. SHM fact sheet: about hospital medicine. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Media_Kit42(5):120–126.
- , , , et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368(23):2201–2209.
- , , , , , . Association between time of admission to the ICU and mortality: a systematic review and meta‐analysis. Chest. 2010;138(1):68–75.
- , , , . After‐hours admissions are not associated with increased risk‐adjusted mortality in pediatric intensive care. Intensive Care Med. 2008;34(1):148–151.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
- Accreditation Council for Graduate Medical Education Task Force on Quality Care and Professionalism. The ACGME 2011 duty hour standards: enhancing quality of care, supervision, and resident professional development. Accreditation Council for Graduate Medical Education, Chicago, IL; 2011. Available at: http://www.acgme.org/acgmeweb/Portals/0/PDFs/jgme‐monograph[1].pdf. Last accessed on December 18, 2013.
- , , , , . Impact of reduction in working hours for doctors in training on postgraduate medical education and patients' outcomes: systemic review. BMJ. 2011;342:d1580.
- , . ACGME 2011 duty‐hour guidelines: consequences expected by radiology residency directors and chief residents. J Am Coll Radiol. 2012;9(11):820–827.
- . Justifying patient risks associated with medical education. JAMA. 2007;298(9):1046–1048.
- , , , , , . Survey of academic pediatric hospitalist programs in the U.S.: organizational, administrative and financial factors. J Hosp Med. 2013;8(6):285–291.
- , , , . Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299–303.
- ACGME Program Requirements for Graduate Medical Education in Pediatrics. ACGME Approved: September 30, 2012; Effective: July 1, 2013. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013‐PR‐FAQ‐PIF/320_pediatrics_07012013.pdf. Accessed September 17, 2013.
- , , , et al. A systematic review: the effect of clinical supervision on patient and residency education outcomes. Acad Med. 2012;87(4):428–442.
- , . Twenty‐four‐hour intensivist staffing in teaching hospitals: tension between safety today and safety tomorrow. Chest. 2012;141(5):1315–1320.
- . Medical education on the brink: 62 years of front‐line observations and opinions. Tex Heart Inst J. 2012;39(3):322–329.
- , . Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008;51:6–7.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1–10.
- The Specifications Manual for National Hospital Inpatient Quality Measures. A Collaboration of the Centers for Medicare 128(1):72–78.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- Section on Hospital Medicine. Guiding principles for Pediatric Hospital Medicine programs. Pediatrics. 2013;132(4):782–786. SHM fact sheet: about hospital medicine. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Media_Kit42(5):120–126.
- , , , et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368(23):2201–2209.
- , , , , , . Association between time of admission to the ICU and mortality: a systematic review and meta‐analysis. Chest. 2010;138(1):68–75.
- , , , . After‐hours admissions are not associated with increased risk‐adjusted mortality in pediatric intensive care. Intensive Care Med. 2008;34(1):148–151.
- , , , , , . Effects of increased overnight supervision on resident education, decision‐making, and autonomy. J Hosp Med. 2012;7(8):606–610.
© 2013 Society of Hospital Medicine
Bringing CME to the Bedside
Hospitalists, and physicians in general, recognize the need for continuing medical education (CME) to update their knowledge and skills to provide the best possible care for patients. Interactive and personalized learning activities provide the most effective approaches for maintaining or improving physician competency.[1, 2] Despite guidelines that recommend a shift of CME from the traditional large lecture format to case‐based and highly interactive learning techniques,[3] this has been challenging to achieve in practice.
In this issue of the Journal of Hospital Medicine, Sehgal and collaborators at the University of California, San Francisco (UCSF) report innovative and highly appealing CME activity that provides a short, focused experience for the practicing hospitalist seeking to update his or her skills.[4] The UCSF Hospitalist Mini‐College (UHMC) embraced the principles for creation of effective CME by conducting needs assessment from community hospitalists and constructing a program that provides focused, interactive, small‐group, intensive experiences and then evaluating the experience to improve subsequent iterations of the Mini‐College. The UHMC immerses participants in a relatively intense experience that includes close interaction with prominent faculty, hands‐on bedside experiences, practical skills, and attendance at sessions (resident report, morbidity and mortality conferences) that are part of every resident trainee's experience. Participants would be linked to their previous learning activities. As the authors point out, there may be a powerful stimulus to learning when practicing physicians return to the milieu of training environments. This observation deserves further investigation.
The report does not provide evidence that participation in the Mini‐College improved patient outcomes or physician performance in practice; these outcome measures remain elusive and an aspirational goal in medical education research. However, experienced clinician educators have come to recognize and adopt effective interventions that simply make sense in the same fashion that it makes sense to use a parachute when jumping out of an airplane in flight.[5] The UHMC makes sense. The medical education literature is replete with articles describing educational innovations and their 1‐ to 2‐year outcomes, leaving the reader wondering about sustainability. It is reassuring that Sehgal et al. report 5 years of experience with the UHMC, and that the program has consistently had a waiting list of hospitalists who want to participate despite the expense. Although it requires patience on the part of educational innovators, this report helps set a standard for reporting enduring innovation in the education arena.
The article provides a description that is sufficiently detailed for other academic medical centers to replicate the intervention or to effectively adapt the principles of the intervention for the needs of their local hospitalist community. The authors should be congratulated for sharing the details of their program and for sharing powerful comments by participants. For hospitalist medical educators interested in sharing details of effective and sustained innovations, publication of this article emphasizes the Journal of Hospital Medicine's interest in disseminating these important projects.
In summary, the report on the UHMC model challenges all of us in academic hospital medicine to think creatively about how to provide effective, engaging, and exciting learning opportunities beyond the years of medical school and residency training.
- . Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380–385.
- , . Continuing medical education: the link between physician learning and health care outcomes. Acad Med. 2011;86:1339.
- , , . Continuing medical education: AMEE Education Guide No. 35. Med Teach. 2008;30:652–666.
- , , . Bringing continuing medical education to the bedside: The University of California, San Francisco hospitalist mini‐college. J HospMed. 2014;9:129–134.
- , . Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327:1459–1461.
Hospitalists, and physicians in general, recognize the need for continuing medical education (CME) to update their knowledge and skills to provide the best possible care for patients. Interactive and personalized learning activities provide the most effective approaches for maintaining or improving physician competency.[1, 2] Despite guidelines that recommend a shift of CME from the traditional large lecture format to case‐based and highly interactive learning techniques,[3] this has been challenging to achieve in practice.
In this issue of the Journal of Hospital Medicine, Sehgal and collaborators at the University of California, San Francisco (UCSF) report innovative and highly appealing CME activity that provides a short, focused experience for the practicing hospitalist seeking to update his or her skills.[4] The UCSF Hospitalist Mini‐College (UHMC) embraced the principles for creation of effective CME by conducting needs assessment from community hospitalists and constructing a program that provides focused, interactive, small‐group, intensive experiences and then evaluating the experience to improve subsequent iterations of the Mini‐College. The UHMC immerses participants in a relatively intense experience that includes close interaction with prominent faculty, hands‐on bedside experiences, practical skills, and attendance at sessions (resident report, morbidity and mortality conferences) that are part of every resident trainee's experience. Participants would be linked to their previous learning activities. As the authors point out, there may be a powerful stimulus to learning when practicing physicians return to the milieu of training environments. This observation deserves further investigation.
The report does not provide evidence that participation in the Mini‐College improved patient outcomes or physician performance in practice; these outcome measures remain elusive and an aspirational goal in medical education research. However, experienced clinician educators have come to recognize and adopt effective interventions that simply make sense in the same fashion that it makes sense to use a parachute when jumping out of an airplane in flight.[5] The UHMC makes sense. The medical education literature is replete with articles describing educational innovations and their 1‐ to 2‐year outcomes, leaving the reader wondering about sustainability. It is reassuring that Sehgal et al. report 5 years of experience with the UHMC, and that the program has consistently had a waiting list of hospitalists who want to participate despite the expense. Although it requires patience on the part of educational innovators, this report helps set a standard for reporting enduring innovation in the education arena.
The article provides a description that is sufficiently detailed for other academic medical centers to replicate the intervention or to effectively adapt the principles of the intervention for the needs of their local hospitalist community. The authors should be congratulated for sharing the details of their program and for sharing powerful comments by participants. For hospitalist medical educators interested in sharing details of effective and sustained innovations, publication of this article emphasizes the Journal of Hospital Medicine's interest in disseminating these important projects.
In summary, the report on the UHMC model challenges all of us in academic hospital medicine to think creatively about how to provide effective, engaging, and exciting learning opportunities beyond the years of medical school and residency training.
Hospitalists, and physicians in general, recognize the need for continuing medical education (CME) to update their knowledge and skills to provide the best possible care for patients. Interactive and personalized learning activities provide the most effective approaches for maintaining or improving physician competency.[1, 2] Despite guidelines that recommend a shift of CME from the traditional large lecture format to case‐based and highly interactive learning techniques,[3] this has been challenging to achieve in practice.
In this issue of the Journal of Hospital Medicine, Sehgal and collaborators at the University of California, San Francisco (UCSF) report innovative and highly appealing CME activity that provides a short, focused experience for the practicing hospitalist seeking to update his or her skills.[4] The UCSF Hospitalist Mini‐College (UHMC) embraced the principles for creation of effective CME by conducting needs assessment from community hospitalists and constructing a program that provides focused, interactive, small‐group, intensive experiences and then evaluating the experience to improve subsequent iterations of the Mini‐College. The UHMC immerses participants in a relatively intense experience that includes close interaction with prominent faculty, hands‐on bedside experiences, practical skills, and attendance at sessions (resident report, morbidity and mortality conferences) that are part of every resident trainee's experience. Participants would be linked to their previous learning activities. As the authors point out, there may be a powerful stimulus to learning when practicing physicians return to the milieu of training environments. This observation deserves further investigation.
The report does not provide evidence that participation in the Mini‐College improved patient outcomes or physician performance in practice; these outcome measures remain elusive and an aspirational goal in medical education research. However, experienced clinician educators have come to recognize and adopt effective interventions that simply make sense in the same fashion that it makes sense to use a parachute when jumping out of an airplane in flight.[5] The UHMC makes sense. The medical education literature is replete with articles describing educational innovations and their 1‐ to 2‐year outcomes, leaving the reader wondering about sustainability. It is reassuring that Sehgal et al. report 5 years of experience with the UHMC, and that the program has consistently had a waiting list of hospitalists who want to participate despite the expense. Although it requires patience on the part of educational innovators, this report helps set a standard for reporting enduring innovation in the education arena.
The article provides a description that is sufficiently detailed for other academic medical centers to replicate the intervention or to effectively adapt the principles of the intervention for the needs of their local hospitalist community. The authors should be congratulated for sharing the details of their program and for sharing powerful comments by participants. For hospitalist medical educators interested in sharing details of effective and sustained innovations, publication of this article emphasizes the Journal of Hospital Medicine's interest in disseminating these important projects.
In summary, the report on the UHMC model challenges all of us in academic hospital medicine to think creatively about how to provide effective, engaging, and exciting learning opportunities beyond the years of medical school and residency training.
- . Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380–385.
- , . Continuing medical education: the link between physician learning and health care outcomes. Acad Med. 2011;86:1339.
- , , . Continuing medical education: AMEE Education Guide No. 35. Med Teach. 2008;30:652–666.
- , , . Bringing continuing medical education to the bedside: The University of California, San Francisco hospitalist mini‐college. J HospMed. 2014;9:129–134.
- , . Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327:1459–1461.
- . Effects of continuing medical education on improving physician clinical care and patient health: a review of systematic reviews. Int J Technol Assess Health Care. 2005;21:380–385.
- , . Continuing medical education: the link between physician learning and health care outcomes. Acad Med. 2011;86:1339.
- , , . Continuing medical education: AMEE Education Guide No. 35. Med Teach. 2008;30:652–666.
- , , . Bringing continuing medical education to the bedside: The University of California, San Francisco hospitalist mini‐college. J HospMed. 2014;9:129–134.
- , . Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327:1459–1461.
Perioral dermatitis and diet
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
Could it be the carbs?
In my practice, I have observed consistent improvements in recalcitrant perioral dermatitis when patients switch to low-carbohydrate diets. Several of my patients with perioral dermatitis that responded poorly to oral doxycycline, topical metronidazole, and topical tacrolimus – or recurred upon cessation of therapy – have proven to have gluten sensitivity or intolerance. Their skin condition improves when they go on a gluten-free diet. But I have also seen considerable improvements after patients undertake low-carbohydrate, high-protein diets, even if those patients have no diagnosed gluten sensitivity. These improvements have occurred with minimal oral and topical treatments, and these patients have not experienced recurrences.
There have been no well-controlled studies, or even case reports to my knowledge, linking carbohydrate or gluten intake to perioral dermatitis. Could the improvement be serendipitous, or is there some basis for carbohydrates contributing to inflammatory status in the oral and gastrointestinal mucosa?
Alcohol, spicy foods, and chocolate have been linked to exacerbation of erythemogenic and papulopustular rosacea. However, the precipitating ingredients in these foods have not been identified. Could the common link simply be an abundance of carbohydrates?
More studies are needed to better define the role of diet in perioral dermatitis. In the meantime, I am seeing good results with low-carb/carb-free diets and will continue to suggest them to prevent recurrences in my patients with perioral dermatitis.
Dr. Talakoub is in private practice in McLean, Va.
First-in-man bioengineered graft proves enduring for vascular access
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: The 6-month enduring patency rate of an investigational tissue-engineered vascular graft for hemodialysis access was 100%, markedly better than rates achievable with synthetic PTFE grafts, the current benchmark technology.
Data source: An initial report from an ongoing prospective first-in-man study in which, to date, 28 patients with end-stage renal disease have been implanted with a novel tissue-engineered vascular graft for use as a hemodialysis access.
Disclosures: The study was funded by the Department of Defense and Humacyte. The presenter is a consultant to the company.
Is Spreading Pain Due to Injury?
Answer
The radiograph shows a right apical mass. This clinical and radiographic presentation is strongly suggestive of a Pancoast tumor. Such lung masses (typically non–small cell carcinomas) can cause brachial plexus compression when they progress, which results in thoracic outlet obstruction and symptoms similar to those seen in this patient.
The patient was admitted by a hospitalist service, and further imaging did confirm the presence of a lung mass, as well as extension to the chest wall and cervicothoracic portion of the spinal canal. CT-guided biopsy of the mass is pending.
Answer
The radiograph shows a right apical mass. This clinical and radiographic presentation is strongly suggestive of a Pancoast tumor. Such lung masses (typically non–small cell carcinomas) can cause brachial plexus compression when they progress, which results in thoracic outlet obstruction and symptoms similar to those seen in this patient.
The patient was admitted by a hospitalist service, and further imaging did confirm the presence of a lung mass, as well as extension to the chest wall and cervicothoracic portion of the spinal canal. CT-guided biopsy of the mass is pending.
Answer
The radiograph shows a right apical mass. This clinical and radiographic presentation is strongly suggestive of a Pancoast tumor. Such lung masses (typically non–small cell carcinomas) can cause brachial plexus compression when they progress, which results in thoracic outlet obstruction and symptoms similar to those seen in this patient.
The patient was admitted by a hospitalist service, and further imaging did confirm the presence of a lung mass, as well as extension to the chest wall and cervicothoracic portion of the spinal canal. CT-guided biopsy of the mass is pending.
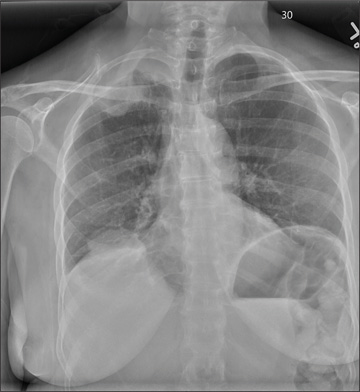
A 53-year-old woman presents with complaints of right-side chest wall, neck, and shoulder pain. Her symptoms started two months ago, when she says she injured herself while doing yard work. She initially self-treated but subsequently went to various emergency departments and walk-in clinics on several occasions; no definitive diagnosis was established. Recently, she has noticed increasing weakness in her right arm and hand as well. Medical history is significant for hypertension. Family history is remarkable for non-Hodgkin’s lymphoma (mother). Social history reveals that the patient is a smoker, with a pack-a-day habit for at least 40 years. On physical exam, you note normal vital signs. The patient has good range of motion in her extremities; however, the strength in her right upper extremity is significantly diminished. Her deltoid, biceps, triceps, and hand grip are all about 2/5. She also notes a paresthesia along her right anterior chest wall, although sensation is intact. Chest radiograph is ordered (shown). What is your impression?
Man, 45, With Greasy Rash and Deformed Nails
A 45-year-old man presented to the dermatology office complaining of a pruritic rash on his neck, chest, abdomen, and upper back. The rash had been present since the patient was 20, intermittently flaring and causing severe pruritus. For the past two weeks, it had become increasingly bothersome.
The patient described the rash as “greasy” brown plaques diffusely scattered on his body. The rash on his neck was the most bothersome, and the patient felt an uncontrollable need to scratch that area.
Since it first developed 25 years ago, he had used OTC hydrocortisone cream as needed to treat the rash. Although effective for past flares, the cream provided only minimal relief during the current episode.
The patient’s medical history included brittle nails with a worsening of nail quality in recent years. The family history revealed that the patient’s father and sister were affected by the same type of rash, which developed in adolescence for each of them, as well as brittle nails.
On physical examination, the skin was warm and moist to the touch. Flat, slightly elevated, greasy brown papules were scattered on the chest, abdomen, and upper back, with mild surrounding erythema (see Figure 1). Excoriated lesions were noted on the anterior surface of the neck, with pinpoint bleeding resulting from constant irritation. The patient’s fingernails were deformed, with longitudinal ridges and v-shaped notching of the free margin. The remainder of the physical exam was unremarkable, and review of systems was negative.
This patient’s symptoms could result from a variety of causes. Seborrheic dermatitis is a common skin condition that presents with brown plaques similar to those on the patient’s trunk. Another possible diagnosis is Grover’s disease, a rare disorder also known as transient acantholytic dermatosis, in which keratotic plaques appear on the torso and are thought to occur from trauma to sun-damaged skin. An additional consideration is Hailey-Hailey disease, a rare genetic disorder also known as benign familial pemphigus, which is characterized by red-brown plaques located predominantly on flexure surfaces.1 Skin biopsy should be performed for a definitive diagnosis.
Given the family history of a similar rash occurring in first-degree relatives and the distinct physical exam findings, the most likely diagnosis for this patient is keratosis follicularis, also known as Darier disease (DD) or Darier-White disease.
DISCUSSION
Named after Ferdinand-Jean Darier, who discovered this rare genodermatosis, DD is a rare genetic skin disorder caused by mutations of the ATP2A2 gene, located on the long arm of chromosome 12 at position 24,11.1,2 The mutation disrupts the encoding of the enzyme sarco/endoplasmic reticulum calcium-ATPase 2 (SERCA2). This enzyme is important in the transport of calcium ions across the cell membrane, and insufficient amounts lead to a defect in intracellular calcium signaling.2,3
This genetic mutation is inherited as an autosomal dominant trait with complete penetrance. DD affects men and women equally, with progressive skin signs of interfamilial and intrafamilial variability.4 Skin manifestations occur from late childhood to early adulthood and are typical during adolescence.4 Acute flare-ups can be triggered by heat, perspiration, sunlight, ultraviolet B exposure, stress, or certain medications (in particular, lithium).2 DD is not contagious.2
CLINICAL PRESENTATION
The characteristics of DD include yellow or brown, rough, firm papules that are frequently crusted. The papules often appear in seborrheic areas of the body, such as the chest, back, ears, nasolabial fold, forehead, scalp, and groin.4 The severity of expression varies from mild, with few lesions, to severe, in which the entire body is covered with disfiguring, macerated plaques emitting a strong odor. On biopsy, the histopathologic findings are typical of dyskeratosis and acantholysis.4
Fingernails (and occasionally toenails) display broad, white or red, somewhat translucent, longitudinal bands accompanied by v-shaped notching1,4,5 (see Figure 2). Such nail changes are diagnostic and occur in 92% to 95% of patients with DD.6 They may, in fact, occur in the absence of cutaneous disease. All nails may be affected, but usually only two to three are involved.6
Although uncommon in DD, white, umbilicated, or cobblestone plaques may be found on intraoral mucous membranes (ie, tongue, buccal mucosa, palate, epiglottis, pharyngeal wall, and esophagus); due to confluence, papules may mimic leukoplakia.7 Lesions may also appear on the vulva or rectum.1,5 In severe cases, the salivary glands can become blocked, and the gums can hypertrophy.5
Since epidermal and brain tissue both derive from ectoderm, pathologic processes that affect one organ system may also affect the other.8 Indeed, among patients with DD, neuropsychiatric problems—including epilepsy, learning difficulties, and schizoaffective disorder—are commonly reported.1 To confirm an association between DD and ATP2A2 mutations, Jacobsen and colleagues performed an analysis of 19 unrelated DD patients with neuropsychiatric phenotypes. They discovered evidence to support the gene’s pleiotropic effects in the brain and hypothesized that mutations in the enzyme SERCA2 correlate with these phenotypes, most specifically for mood disorders.9
TREATMENT AND MANAGEMENT
Although no cure is currently available for DD, both short- and long-term treatment options are available; the choice should be based on the severity of an individual patient’s signs and symptoms. For mild cases, topical therapy, such as general emollients, corticosteroid ointments, and high sun protection factor sunscreen, is sufficient.1
For moderate cases, topical retinoids, including tretinoin cream, adapalene gel or cream, and tazarotene gel, may be necessary.4 Keratolytics, including salicylic acid in propylene glycol gel, may be used to regulate hyperkeratosis.4 Celecoxib, a COX-2 inhibitor, is another option that may restore the down regulation of SERCA2. This can prevent progression of the disease.10
Long-term management includes use of oral retinoid therapy (eg, acitretin), which might reduce the frequency of inflammatory flares.1 Systemic adverse effects from long-term use of oral retinoids are cause for concern, however. Close monitoring along with patient education can limit the occurrence of complications.11
If DD is uncontrolled with medication, dermabrasion and erbium:YAG laser ablation have been used to successfully treat chronic cases.12 Although these treatment options may remove existing lesions, it is important to inform patients that the disease has not been cured, that remission is difficult to attain, and that lesions may recur.
Because viral, bacterial, and fungal superinfections are common and may exacerbate the disease, be sure to check for signs of infection while examining the patient.4 Patients should be advised to avoid hot environments, and if that is not possible, to dress in cool cotton clothing to allow for proper ventilation and avoid the build-up of perspiration. Excessive perspiration along with poor hygiene can contribute to the formation of infections as well as trigger a flare-up. If an infection develops, patients should consult a health care provider.
Keeping the skin well moisturized can alleviate the constant pruritus that many patients experience. Daily sunscreen use is essential to avoid skin irritation caused by the sun, which can trigger an acute flare-up. Patients should be advised to avoid the long-term use of corticosteroid ointment. They should also contact their health care provider before using OTC treatments such as Burow’s solution.
CONCLUSION
A thorough history and physical exam are crucial in the diagnosis of DD. In this particular case, inquiry into family history was the key to proper diagnosis. That information, paired with a thorough physical exam, led to the correct diagnosis of this rare genetic skin disorder. A skin biopsy provided definitive confirmation.
This patient had a mild-to-moderate manifestation of DD. He was prescribed retinoid therapy, and routine follow-up visits were recommended to monitor the efficacy of medical therapy and to screen for secondary infections or neuropsychiatric disorders.
This case illustrates the importance of taking a full history and performing an in-depth physical exam when a patient presents with an unfamiliar complaint. Being thorough reduces the risk of missing a crucial element that can guide the diagnostic process.
REFERENCES
1. Creamer D, Barker J, Kerdel FA. Papular and papulosquamous dermatoses. In: Acute Adult Dermatology: Diagnosis and Management (A Colour Handbook). London, UK: Manson Publishing Ltd; 2011:48.
2. Kelly EB. Darier disease (DAR). In: Encyclopedia of Human Genetics and Disease. Santa Barbara, CA: ABC-CLIO; 2013:186-187.
3. Klausegger A, Laimer M, Bauer JW. Darier disease. [In German.] Hautarzt. 2013;64:22-25.
4. Ringpfeil F. Dermatologic disorders. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:101.
5. Disorders of keratinization. In: Ostler HB, Maibach HI, Hoke AW, Schwab IR, eds. Diseases of the Eye and Skin: A Color Atlas. Philadelphia, PA: Lippincott Williams & Wilkins; 2004:23-34.
6. Baran R, de Berker D, Holzberg M, Thomas L, eds. Baran & Dawber’s Diseases of the Nails and their Management. 4th ed. West Sussex, UK: John Wiley & Sons, Ltd; 2012:295-296.
7. Thiagarajan MK, Narasimhan M, Sankarasubramanian A. Darier disease with oral and esophageal involvement: a case report. Indian J Dent Res. 2011;22:843-846.
8. Medansky RS, Woloshin AA. Darier’s disease: an evaluation of its neuropsychiatric component. Arch Dermatol. 1961;84:482-484.
9. Jacobsen NJ, Lyons I, Hoogendoorn B, et al. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Hum Mol Genet. 1999;8:1631-1636.
10. Kamijo M, Nishiyama C, Takagi A, et al. Cyclooxygenase-2 inhibition restores ultraviolet B-induced downregulation of ATP2A2/SERCA2 in keratinocytes: possible therapeutic approach of cyclooxygenase-2 inhibition for treatment of Darier disease. Br J Dermatol. 2012;166: 1017-1022.
11. Brecher AR, Orlow SJ. Oral retinoid therapy for dermatologic conditions in children and adolescents. J Am Acad Dermatol. 2003;49:171-182.
12. Beier C, Kaufmann R. Efficacy of erbium:YAG laser ablation in Darier disease and Hailey-Hailey disease. Arch Dermatol. 1999;35:423-427.
A 45-year-old man presented to the dermatology office complaining of a pruritic rash on his neck, chest, abdomen, and upper back. The rash had been present since the patient was 20, intermittently flaring and causing severe pruritus. For the past two weeks, it had become increasingly bothersome.
The patient described the rash as “greasy” brown plaques diffusely scattered on his body. The rash on his neck was the most bothersome, and the patient felt an uncontrollable need to scratch that area.
Since it first developed 25 years ago, he had used OTC hydrocortisone cream as needed to treat the rash. Although effective for past flares, the cream provided only minimal relief during the current episode.
The patient’s medical history included brittle nails with a worsening of nail quality in recent years. The family history revealed that the patient’s father and sister were affected by the same type of rash, which developed in adolescence for each of them, as well as brittle nails.
On physical examination, the skin was warm and moist to the touch. Flat, slightly elevated, greasy brown papules were scattered on the chest, abdomen, and upper back, with mild surrounding erythema (see Figure 1). Excoriated lesions were noted on the anterior surface of the neck, with pinpoint bleeding resulting from constant irritation. The patient’s fingernails were deformed, with longitudinal ridges and v-shaped notching of the free margin. The remainder of the physical exam was unremarkable, and review of systems was negative.
This patient’s symptoms could result from a variety of causes. Seborrheic dermatitis is a common skin condition that presents with brown plaques similar to those on the patient’s trunk. Another possible diagnosis is Grover’s disease, a rare disorder also known as transient acantholytic dermatosis, in which keratotic plaques appear on the torso and are thought to occur from trauma to sun-damaged skin. An additional consideration is Hailey-Hailey disease, a rare genetic disorder also known as benign familial pemphigus, which is characterized by red-brown plaques located predominantly on flexure surfaces.1 Skin biopsy should be performed for a definitive diagnosis.
Given the family history of a similar rash occurring in first-degree relatives and the distinct physical exam findings, the most likely diagnosis for this patient is keratosis follicularis, also known as Darier disease (DD) or Darier-White disease.
DISCUSSION
Named after Ferdinand-Jean Darier, who discovered this rare genodermatosis, DD is a rare genetic skin disorder caused by mutations of the ATP2A2 gene, located on the long arm of chromosome 12 at position 24,11.1,2 The mutation disrupts the encoding of the enzyme sarco/endoplasmic reticulum calcium-ATPase 2 (SERCA2). This enzyme is important in the transport of calcium ions across the cell membrane, and insufficient amounts lead to a defect in intracellular calcium signaling.2,3
This genetic mutation is inherited as an autosomal dominant trait with complete penetrance. DD affects men and women equally, with progressive skin signs of interfamilial and intrafamilial variability.4 Skin manifestations occur from late childhood to early adulthood and are typical during adolescence.4 Acute flare-ups can be triggered by heat, perspiration, sunlight, ultraviolet B exposure, stress, or certain medications (in particular, lithium).2 DD is not contagious.2
CLINICAL PRESENTATION
The characteristics of DD include yellow or brown, rough, firm papules that are frequently crusted. The papules often appear in seborrheic areas of the body, such as the chest, back, ears, nasolabial fold, forehead, scalp, and groin.4 The severity of expression varies from mild, with few lesions, to severe, in which the entire body is covered with disfiguring, macerated plaques emitting a strong odor. On biopsy, the histopathologic findings are typical of dyskeratosis and acantholysis.4
Fingernails (and occasionally toenails) display broad, white or red, somewhat translucent, longitudinal bands accompanied by v-shaped notching1,4,5 (see Figure 2). Such nail changes are diagnostic and occur in 92% to 95% of patients with DD.6 They may, in fact, occur in the absence of cutaneous disease. All nails may be affected, but usually only two to three are involved.6
Although uncommon in DD, white, umbilicated, or cobblestone plaques may be found on intraoral mucous membranes (ie, tongue, buccal mucosa, palate, epiglottis, pharyngeal wall, and esophagus); due to confluence, papules may mimic leukoplakia.7 Lesions may also appear on the vulva or rectum.1,5 In severe cases, the salivary glands can become blocked, and the gums can hypertrophy.5
Since epidermal and brain tissue both derive from ectoderm, pathologic processes that affect one organ system may also affect the other.8 Indeed, among patients with DD, neuropsychiatric problems—including epilepsy, learning difficulties, and schizoaffective disorder—are commonly reported.1 To confirm an association between DD and ATP2A2 mutations, Jacobsen and colleagues performed an analysis of 19 unrelated DD patients with neuropsychiatric phenotypes. They discovered evidence to support the gene’s pleiotropic effects in the brain and hypothesized that mutations in the enzyme SERCA2 correlate with these phenotypes, most specifically for mood disorders.9
TREATMENT AND MANAGEMENT
Although no cure is currently available for DD, both short- and long-term treatment options are available; the choice should be based on the severity of an individual patient’s signs and symptoms. For mild cases, topical therapy, such as general emollients, corticosteroid ointments, and high sun protection factor sunscreen, is sufficient.1
For moderate cases, topical retinoids, including tretinoin cream, adapalene gel or cream, and tazarotene gel, may be necessary.4 Keratolytics, including salicylic acid in propylene glycol gel, may be used to regulate hyperkeratosis.4 Celecoxib, a COX-2 inhibitor, is another option that may restore the down regulation of SERCA2. This can prevent progression of the disease.10
Long-term management includes use of oral retinoid therapy (eg, acitretin), which might reduce the frequency of inflammatory flares.1 Systemic adverse effects from long-term use of oral retinoids are cause for concern, however. Close monitoring along with patient education can limit the occurrence of complications.11
If DD is uncontrolled with medication, dermabrasion and erbium:YAG laser ablation have been used to successfully treat chronic cases.12 Although these treatment options may remove existing lesions, it is important to inform patients that the disease has not been cured, that remission is difficult to attain, and that lesions may recur.
Because viral, bacterial, and fungal superinfections are common and may exacerbate the disease, be sure to check for signs of infection while examining the patient.4 Patients should be advised to avoid hot environments, and if that is not possible, to dress in cool cotton clothing to allow for proper ventilation and avoid the build-up of perspiration. Excessive perspiration along with poor hygiene can contribute to the formation of infections as well as trigger a flare-up. If an infection develops, patients should consult a health care provider.
Keeping the skin well moisturized can alleviate the constant pruritus that many patients experience. Daily sunscreen use is essential to avoid skin irritation caused by the sun, which can trigger an acute flare-up. Patients should be advised to avoid the long-term use of corticosteroid ointment. They should also contact their health care provider before using OTC treatments such as Burow’s solution.
CONCLUSION
A thorough history and physical exam are crucial in the diagnosis of DD. In this particular case, inquiry into family history was the key to proper diagnosis. That information, paired with a thorough physical exam, led to the correct diagnosis of this rare genetic skin disorder. A skin biopsy provided definitive confirmation.
This patient had a mild-to-moderate manifestation of DD. He was prescribed retinoid therapy, and routine follow-up visits were recommended to monitor the efficacy of medical therapy and to screen for secondary infections or neuropsychiatric disorders.
This case illustrates the importance of taking a full history and performing an in-depth physical exam when a patient presents with an unfamiliar complaint. Being thorough reduces the risk of missing a crucial element that can guide the diagnostic process.
REFERENCES
1. Creamer D, Barker J, Kerdel FA. Papular and papulosquamous dermatoses. In: Acute Adult Dermatology: Diagnosis and Management (A Colour Handbook). London, UK: Manson Publishing Ltd; 2011:48.
2. Kelly EB. Darier disease (DAR). In: Encyclopedia of Human Genetics and Disease. Santa Barbara, CA: ABC-CLIO; 2013:186-187.
3. Klausegger A, Laimer M, Bauer JW. Darier disease. [In German.] Hautarzt. 2013;64:22-25.
4. Ringpfeil F. Dermatologic disorders. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:101.
5. Disorders of keratinization. In: Ostler HB, Maibach HI, Hoke AW, Schwab IR, eds. Diseases of the Eye and Skin: A Color Atlas. Philadelphia, PA: Lippincott Williams & Wilkins; 2004:23-34.
6. Baran R, de Berker D, Holzberg M, Thomas L, eds. Baran & Dawber’s Diseases of the Nails and their Management. 4th ed. West Sussex, UK: John Wiley & Sons, Ltd; 2012:295-296.
7. Thiagarajan MK, Narasimhan M, Sankarasubramanian A. Darier disease with oral and esophageal involvement: a case report. Indian J Dent Res. 2011;22:843-846.
8. Medansky RS, Woloshin AA. Darier’s disease: an evaluation of its neuropsychiatric component. Arch Dermatol. 1961;84:482-484.
9. Jacobsen NJ, Lyons I, Hoogendoorn B, et al. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Hum Mol Genet. 1999;8:1631-1636.
10. Kamijo M, Nishiyama C, Takagi A, et al. Cyclooxygenase-2 inhibition restores ultraviolet B-induced downregulation of ATP2A2/SERCA2 in keratinocytes: possible therapeutic approach of cyclooxygenase-2 inhibition for treatment of Darier disease. Br J Dermatol. 2012;166: 1017-1022.
11. Brecher AR, Orlow SJ. Oral retinoid therapy for dermatologic conditions in children and adolescents. J Am Acad Dermatol. 2003;49:171-182.
12. Beier C, Kaufmann R. Efficacy of erbium:YAG laser ablation in Darier disease and Hailey-Hailey disease. Arch Dermatol. 1999;35:423-427.
A 45-year-old man presented to the dermatology office complaining of a pruritic rash on his neck, chest, abdomen, and upper back. The rash had been present since the patient was 20, intermittently flaring and causing severe pruritus. For the past two weeks, it had become increasingly bothersome.
The patient described the rash as “greasy” brown plaques diffusely scattered on his body. The rash on his neck was the most bothersome, and the patient felt an uncontrollable need to scratch that area.
Since it first developed 25 years ago, he had used OTC hydrocortisone cream as needed to treat the rash. Although effective for past flares, the cream provided only minimal relief during the current episode.
The patient’s medical history included brittle nails with a worsening of nail quality in recent years. The family history revealed that the patient’s father and sister were affected by the same type of rash, which developed in adolescence for each of them, as well as brittle nails.
On physical examination, the skin was warm and moist to the touch. Flat, slightly elevated, greasy brown papules were scattered on the chest, abdomen, and upper back, with mild surrounding erythema (see Figure 1). Excoriated lesions were noted on the anterior surface of the neck, with pinpoint bleeding resulting from constant irritation. The patient’s fingernails were deformed, with longitudinal ridges and v-shaped notching of the free margin. The remainder of the physical exam was unremarkable, and review of systems was negative.
This patient’s symptoms could result from a variety of causes. Seborrheic dermatitis is a common skin condition that presents with brown plaques similar to those on the patient’s trunk. Another possible diagnosis is Grover’s disease, a rare disorder also known as transient acantholytic dermatosis, in which keratotic plaques appear on the torso and are thought to occur from trauma to sun-damaged skin. An additional consideration is Hailey-Hailey disease, a rare genetic disorder also known as benign familial pemphigus, which is characterized by red-brown plaques located predominantly on flexure surfaces.1 Skin biopsy should be performed for a definitive diagnosis.
Given the family history of a similar rash occurring in first-degree relatives and the distinct physical exam findings, the most likely diagnosis for this patient is keratosis follicularis, also known as Darier disease (DD) or Darier-White disease.
DISCUSSION
Named after Ferdinand-Jean Darier, who discovered this rare genodermatosis, DD is a rare genetic skin disorder caused by mutations of the ATP2A2 gene, located on the long arm of chromosome 12 at position 24,11.1,2 The mutation disrupts the encoding of the enzyme sarco/endoplasmic reticulum calcium-ATPase 2 (SERCA2). This enzyme is important in the transport of calcium ions across the cell membrane, and insufficient amounts lead to a defect in intracellular calcium signaling.2,3
This genetic mutation is inherited as an autosomal dominant trait with complete penetrance. DD affects men and women equally, with progressive skin signs of interfamilial and intrafamilial variability.4 Skin manifestations occur from late childhood to early adulthood and are typical during adolescence.4 Acute flare-ups can be triggered by heat, perspiration, sunlight, ultraviolet B exposure, stress, or certain medications (in particular, lithium).2 DD is not contagious.2
CLINICAL PRESENTATION
The characteristics of DD include yellow or brown, rough, firm papules that are frequently crusted. The papules often appear in seborrheic areas of the body, such as the chest, back, ears, nasolabial fold, forehead, scalp, and groin.4 The severity of expression varies from mild, with few lesions, to severe, in which the entire body is covered with disfiguring, macerated plaques emitting a strong odor. On biopsy, the histopathologic findings are typical of dyskeratosis and acantholysis.4
Fingernails (and occasionally toenails) display broad, white or red, somewhat translucent, longitudinal bands accompanied by v-shaped notching1,4,5 (see Figure 2). Such nail changes are diagnostic and occur in 92% to 95% of patients with DD.6 They may, in fact, occur in the absence of cutaneous disease. All nails may be affected, but usually only two to three are involved.6
Although uncommon in DD, white, umbilicated, or cobblestone plaques may be found on intraoral mucous membranes (ie, tongue, buccal mucosa, palate, epiglottis, pharyngeal wall, and esophagus); due to confluence, papules may mimic leukoplakia.7 Lesions may also appear on the vulva or rectum.1,5 In severe cases, the salivary glands can become blocked, and the gums can hypertrophy.5
Since epidermal and brain tissue both derive from ectoderm, pathologic processes that affect one organ system may also affect the other.8 Indeed, among patients with DD, neuropsychiatric problems—including epilepsy, learning difficulties, and schizoaffective disorder—are commonly reported.1 To confirm an association between DD and ATP2A2 mutations, Jacobsen and colleagues performed an analysis of 19 unrelated DD patients with neuropsychiatric phenotypes. They discovered evidence to support the gene’s pleiotropic effects in the brain and hypothesized that mutations in the enzyme SERCA2 correlate with these phenotypes, most specifically for mood disorders.9
TREATMENT AND MANAGEMENT
Although no cure is currently available for DD, both short- and long-term treatment options are available; the choice should be based on the severity of an individual patient’s signs and symptoms. For mild cases, topical therapy, such as general emollients, corticosteroid ointments, and high sun protection factor sunscreen, is sufficient.1
For moderate cases, topical retinoids, including tretinoin cream, adapalene gel or cream, and tazarotene gel, may be necessary.4 Keratolytics, including salicylic acid in propylene glycol gel, may be used to regulate hyperkeratosis.4 Celecoxib, a COX-2 inhibitor, is another option that may restore the down regulation of SERCA2. This can prevent progression of the disease.10
Long-term management includes use of oral retinoid therapy (eg, acitretin), which might reduce the frequency of inflammatory flares.1 Systemic adverse effects from long-term use of oral retinoids are cause for concern, however. Close monitoring along with patient education can limit the occurrence of complications.11
If DD is uncontrolled with medication, dermabrasion and erbium:YAG laser ablation have been used to successfully treat chronic cases.12 Although these treatment options may remove existing lesions, it is important to inform patients that the disease has not been cured, that remission is difficult to attain, and that lesions may recur.
Because viral, bacterial, and fungal superinfections are common and may exacerbate the disease, be sure to check for signs of infection while examining the patient.4 Patients should be advised to avoid hot environments, and if that is not possible, to dress in cool cotton clothing to allow for proper ventilation and avoid the build-up of perspiration. Excessive perspiration along with poor hygiene can contribute to the formation of infections as well as trigger a flare-up. If an infection develops, patients should consult a health care provider.
Keeping the skin well moisturized can alleviate the constant pruritus that many patients experience. Daily sunscreen use is essential to avoid skin irritation caused by the sun, which can trigger an acute flare-up. Patients should be advised to avoid the long-term use of corticosteroid ointment. They should also contact their health care provider before using OTC treatments such as Burow’s solution.
CONCLUSION
A thorough history and physical exam are crucial in the diagnosis of DD. In this particular case, inquiry into family history was the key to proper diagnosis. That information, paired with a thorough physical exam, led to the correct diagnosis of this rare genetic skin disorder. A skin biopsy provided definitive confirmation.
This patient had a mild-to-moderate manifestation of DD. He was prescribed retinoid therapy, and routine follow-up visits were recommended to monitor the efficacy of medical therapy and to screen for secondary infections or neuropsychiatric disorders.
This case illustrates the importance of taking a full history and performing an in-depth physical exam when a patient presents with an unfamiliar complaint. Being thorough reduces the risk of missing a crucial element that can guide the diagnostic process.
REFERENCES
1. Creamer D, Barker J, Kerdel FA. Papular and papulosquamous dermatoses. In: Acute Adult Dermatology: Diagnosis and Management (A Colour Handbook). London, UK: Manson Publishing Ltd; 2011:48.
2. Kelly EB. Darier disease (DAR). In: Encyclopedia of Human Genetics and Disease. Santa Barbara, CA: ABC-CLIO; 2013:186-187.
3. Klausegger A, Laimer M, Bauer JW. Darier disease. [In German.] Hautarzt. 2013;64:22-25.
4. Ringpfeil F. Dermatologic disorders. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:101.
5. Disorders of keratinization. In: Ostler HB, Maibach HI, Hoke AW, Schwab IR, eds. Diseases of the Eye and Skin: A Color Atlas. Philadelphia, PA: Lippincott Williams & Wilkins; 2004:23-34.
6. Baran R, de Berker D, Holzberg M, Thomas L, eds. Baran & Dawber’s Diseases of the Nails and their Management. 4th ed. West Sussex, UK: John Wiley & Sons, Ltd; 2012:295-296.
7. Thiagarajan MK, Narasimhan M, Sankarasubramanian A. Darier disease with oral and esophageal involvement: a case report. Indian J Dent Res. 2011;22:843-846.
8. Medansky RS, Woloshin AA. Darier’s disease: an evaluation of its neuropsychiatric component. Arch Dermatol. 1961;84:482-484.
9. Jacobsen NJ, Lyons I, Hoogendoorn B, et al. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Hum Mol Genet. 1999;8:1631-1636.
10. Kamijo M, Nishiyama C, Takagi A, et al. Cyclooxygenase-2 inhibition restores ultraviolet B-induced downregulation of ATP2A2/SERCA2 in keratinocytes: possible therapeutic approach of cyclooxygenase-2 inhibition for treatment of Darier disease. Br J Dermatol. 2012;166: 1017-1022.
11. Brecher AR, Orlow SJ. Oral retinoid therapy for dermatologic conditions in children and adolescents. J Am Acad Dermatol. 2003;49:171-182.
12. Beier C, Kaufmann R. Efficacy of erbium:YAG laser ablation in Darier disease and Hailey-Hailey disease. Arch Dermatol. 1999;35:423-427.