User login
Caring for Asian immigrants: Tips on culture that can enhance patient care
› Ask Asian immigrants open-ended questions and encourage them to share their use of alternative remedies. C
› Consider providing an interpretation service for patients not proficient in English, as opposed to asking family members to help. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though often considered a “model minority,” Asian immigrants pose significant challenges for Western health care providers, including radically different ideas of disease causation, differing communication styles, and somatic presentations of mental illness. Asian diversity is tremendous, but several cultural trends are held in common: strong family structures, respect, adaptability, and, for first generation immigrants, widespread use of traditional therapies.1
While Asians and Pacific Islanders (APIs) represent only 5.6% of the US population, or 17.3 million people, that figure represents a 46% increase between 2000 and 2010, the most rapid for any ethnic group.2 A 79% increase is anticipated by 2050, bringing Asians to 9.3% of the US population. In order of population, API subpopulations include Chinese, Filipinos, Asian Indians, Vietnamese, Koreans, and Japanese.2 More than half of Asian Americans reside in the states of California, New York, and Hawaii, although enclaves exist in most major cities.3
Addressing the health needs of Asian immigrants in an increasingly diverse society mandates that US physicians develop the necessary skills to communicate, even when expectations for care may be very different. Fortunately, excellent resources are available (TABLE 1).
Barriers to good healthcare
The most formidable obstacle is limited English proficiency of patients, making them significantly less likely to seek care.4 They often struggle to arrange an appointment, although they arrive on time.5
Inadequate interpretation services. Frequently family members must interpret for patients, despite a federal mandate (Title VI of the 1964 Civil Rights Act) requiring professional services be provided at no charge if there is a federal payer (Medicare or Medicaid) involved.6 Unfortunately, these services are not currently reimbursable. Use of family or friends as interpreters, while convenient, results in far less accurate interpretation, frequent embarrassment, and loss of patient confidentiality. Trained medical interpreters or even telephone services are preferable, as they are much more accurate. Interviews involve a triad comprised of provider, patient, and interpreter, with the provider speaking directly to the patient using first-person address at all times. The interpreter should sit to the side or slightly behind the patient. All communication should be interpreted sentence by sentence so everyone is able to understand the entire conversation. It is well documented that proper interpretive services vastly improve the quality of care.7
Patient illiteracy. Health care illiteracy leads to medication errors due to the inability to understand instructions.8 Some immigrants have the added disadvantage of being illiterate both in English and in their native tongue.4 If not remedied, these situations easily lead to drug overdoses or missed allergies.9 Older immigrants neither understand the intricacies of the US health care system nor possess the language skills to master it.4
Stereotyping by caregivers must be surmounted if patients are to receive quality care. Many Asian patients report that physicians fail to understand them as unique individuals apart from their ethnic identity. Others feel excluded from the decision-making process or find culturally sensitive treatment options lacking.10
Subtleties of relational interaction. Asian culture has been defined as possessing a high Power Distance Index (PDI).11 The PDI refers to the distance or level of respect which an individual must afford to a superior, and this ideal is reflected in Asian conformance to a strict social hierarchy. Thus, physicians are viewed as authority figures and it is proper to nod or smile to indicate polite deference.12 However, showing respect and “buying in” to treatment recommendations are entirely different matters. Cultural factors make it difficult for patients to openly disagree with physician recommendations without feeling as though they have been disrespectful.12 Asian cultures are also “high context” cultures, having far more unwritten rules for conduct and communication that often prove baffling to westerners from “lower context” cultures.
Financial limitations. Socioeconomic influences also play a role. Although Asians have a higher income than other minority groups, 12.5% of Asians still live in poverty and 17.2% lack health insurance.2 Lack of coverage makes many Asians reluctant to seek regular medical care.13
Special medical concerns
Asian-Americans face a variety of challenging medical issues, including disproportionately high rates of tuberculosis (TB) and hepatitis B.
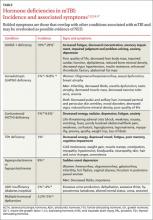
TB. Although rates of TB infection in the United States are low, rates in Asian immigrants are up to 100 times greater than that of the general population, more than any other immigrant group.14 Screening with interferon gamma release assays (IGRAs), such as T-SPOT TB, should be routine for Asian immigrants, since IGRAs do not cross-react with the bacillus Calmette-Guérin (BCG) vaccine. The Centers for Disease Control and Prevention now recommends IGRA blood testing in lieu of tuberculin skin testing (TST) for immigrants who received BCG in infancy, with the exception of children <5 years, for whom the TST is still preferable.15 Patients with positive IGRA tests are also more likely to be amenable to treatment.
Chronic hepatitis B infection in Asian immigrants is often due to perinatal transmission in their home countries. Rates of hepatitis B virus (HBV) infection in the United States have been steadily declining since vaccination began in 1981. However, chronic HBV infection in Asian immigrants approaches 10% of that population.16 An estimated 15% to 20% of patients with chronic HBV develop cirrhosis within 5 years and are at high risk for hepatocellular carcinoma (HCC).17,18
Evaluate HBV carriers annually with liver function testing (LFTs), hepatitis B surface Antigen (HBsAg), and hepatitis B e-Antigen (HBeAg). A positive HBsAg result indicates the virus is present; a positive HBeAg result indicates the virus is actively replicating. LFT elevation (AST >200 IU/L) or a positive HBeAg test should prompt referral to a gastroenterologist for liver biopsy and therapy. Screening for HCC with alpha-fetoprotein (AFP) levels and liver ultrasounds every 6 to 12 months has been recommended for all chronic HBV carriers,19 but this interval remains controversial. Screen men ≥40 years and women ≥50, or anyone who has had HBV infection >10 years, every 6 months.19,20 More recent recommendations favor ultrasound over AFP for screening, as the latter test lacks adequate sensitivity and specificity.20 Test partners and family members of HBV patients, and vaccinate them against HBV if not already immune.
Other medical issues. Asians from the Indian subcontinent have a significantly elevated risk of heart disease, in part due to low HDL-cholesterol levels.21 South Asian immigrant populations have a 3- to 5-fold increased risk of myocardial infarction and cardiovascular death compared with other ethnicities, and often exhibit coronary disease before the age of 40.22 The recent adoption of a Western diet and sedentary lifestyle has provoked an epidemic of diabetes throughout urban Asia and in Asians living abroad. This may be related to the “thrifty gene hypothesis,” which suggests that genes which evolved early in human history to facilitate storage of fat for periods of famine are detrimental in modern society where food is plentiful. A study of Asian Indian immigrants in Atlanta demonstrated an 18.3% prevalence rate for diabetes, higher than any other ethnic group.23 Tobacco and its causal relationship with lung cancer and heart disease only adds to this concern. Southeast Asians in particular demonstrate alarmingly high rates of tobacco consumption, and lung cancer is the leading cause of death among Asian Americans.12,24
Recently, a new acquired immune deficiency syndrome has been described in East Asia. In this syndrome, interferon–gamma (IFN–gamma) is blocked by an auto-antibody. Although not communicable like human immunodeficiency virus, this autoimmune syndrome may lead to similar opportunistic infections such as atypical mycobacterial infections.25
Mental health concerns
Perhaps no topic deserves more emphasis than that of mental health. In the aftermath of the war in Vietnam, Southeast Asian immigrants suffered a great deal from traumatic immigration experiences, with severe adjustment reactions.26 A high incidence of posttraumatic stress disorder (PTSD) among the Hmong in particular reflects their turbulent national history.27
While the incidence of mental illness among Asians is comparable to that of the general population, Asian Americans are less likely to report such problems or to use mental health services26 due to the stigmatization of mental illness in Asian culture.28 Consequently, these symptoms may be subconsciously converted into the more socially acceptable medium of physical illness, which “saves face” and preserves family honor.29 In many ways, Asian culture still perceives mental illness as personal weakness.30 In Hmong culture, inability to speak about being depressed stems not just from cultural bias but from linguistic constraints—the language simply lacks a word for depression.31 Even Asian cultures recognizing mental illness deem depression to be more dependent on circumstances than on the psyche.31 A first-generation immigrant with mental illness is therefore more likely to present with somatic symptoms than a mood disturbance, and is likely to be resistant to counseling or medication for depression. (See “Cultural influence on self-perception: A case ”).
Asian health care beliefs and illnesses
Asian culture substantially influences the ways in which an individual perceives disease, experiences illness, and copes with the phenomenon of sickness. The interplay between illness, disease, and sickness was first elucidated by the pioneering work of Arthur Kleinman, MD, in 1978.32 The term disease denotes a pathological process, while illness describes the subjective impact of disease in a patient’s life. Sickness is the sum of both, as it relates to the total picture of biological and social disruption. The culturally competent physician must understand not only the patient’s disease but also his experience of illness. Asking Dr. Kleinman’s questions will help physicians understand their patient’s perception of illness (TABLE 2).32
Traditional Chinese medicine
Many East Asians derive their conception of illness from traditional Chinese medicine (TCM), a broad range of therapies including herbs, acupuncture, massage (tuina), and diet that has been used for millennia. Similarly, South Asians are influenced by the Ayurvedic or Unani traditions. TCM views the body as an energy system, rather than a machine, through which the life force, or chi, flows. Health is not just the absence of disease, but a proper balance of the antithetic forces, yin and yang, maintained by herbs, diet, and acupuncture.33 TCM is often preferred for treating chronic conditions and viral syndromes, or as a substitute for Western medications with adverse effects.34 It is most popular among newly arrived immigrants, those with low literacy, and those with limited access to conventional medical treatment.34 Most Western physicians know little about TCM, feel uncomfortable when their patients use it, and fail to recognize its popularity.34,35 Likewise, most Asian patients are reluctant to discuss their use of TCM unless questioned about it in a nonjudgmental manner.
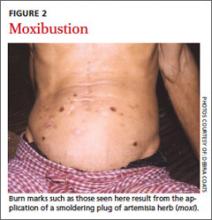
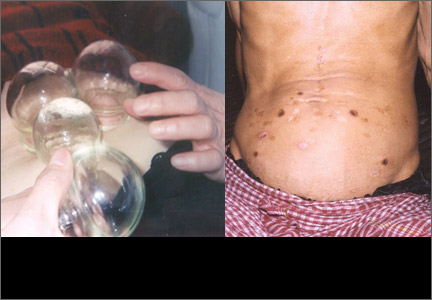
Many East Asian cultures practice a distinct form of folk healing known as “coining,” in which a coin dipped in cold oil or Tiger Balm is rubbed against the skin, enabling “wind illness” to escape the body (FIGURE 1). Linear bands of painless petechiae develop. The more extensive the bruising, the more illness is thought to be released. Failure to expel “wind-cold” from the body is believed to account for many ailments. Other traditions are moxibustion and cupping. In moxibustion, a smoldering plug of dried artemisia herb (moxi) is either impaled upon an acupuncture needle or placed directly on the skin to create a burn (FIGURE 2). In cupping, a flame is quickly passed through a glass bowl which is then placed against the skin. The resulting suction creates a circular bruise and draws blood to the area (FIGURE 3). Many folk remedies have been mistaken for child abuse by individuals unfamiliar with such practices.36 Occasionally TCM may result in harm from burns, unsterilized acupuncture needles, or (most commonly) adulterated herbal formulations.37
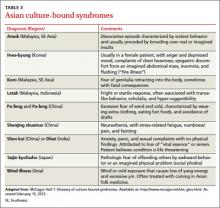
Culture-bound syndromes
Asian folk illnesses usually go unrecognized by western practitioners, and many of these are somatic presentations of mental illness or stress (TABLE 3). A classic example is the Korean folk condition Hwa-byung, which may include the sensation of an abdominal mass. US practitioners might pursue a fruitless abdominal workup before suspecting a psychiatric condition, even though a careful history would likely elicit other anxiety symptoms and loss of sleep and appetite.26
Asian social conventions
Asian cultural conventions often create considerable confusion. In India, head waggling (shaking the head back and forth) is equivalent to nodding in conversation, indicating an acknowledgement of communication. To western eyes, it appears that the patient is resisting advice rather than welcoming it. In East Asians, smiling expresses a variety of emotions, including polite disagreement. Acute embarrassment may provoke giggling. Eye contact is usually for social equals; avoiding it, especially between the sexes, is the norm. Only the right hand should be used when giving patients a prescription; the left hand is considered unclean. A patient’s head should only be touched with advance permission, as it is viewed as the seat of the soul and is therefore sacred. Under no circumstances should a patient ever see the bottom of the practitioner’s feet or be touched by them.38 Demonstrating respect (especially for older Asians) and preserving modesty are essential when examining patients.
Naming conventions can also be confusing. In China and much of Southeast Asia, it is customary for the surname to precede the given name, often with the 2 run together, rather than the other way round. It is best to ask how a patient would prefer to be addressed, regardless of how the name appears on the medical chart.38
Cultivating knowledge of Asian culture provides a framework from which practitioners can better understand and treat their patients. By asking respectful, open ended questions and encouraging patients to take an active role in their own treatment, physicians become therapeutic allies actively engaged in the healing process. Asking patients to share their use of alternative remedies allows the option of rationally integrating those most meaningful for the patient.
The cross-cultural interview
It is helpful to have a specific approach in mind when interviewing patients from other cultures. A number of mnemonic techniques exist.39-41 Perhaps the most useful of these is the LEARN model, which stands for Listen, Explain, Acknowledge, Recommend, and Negotiate.39 The physician first listens carefully to the patient’s perception of his illness before explaining any medical (disease) issues. This exchange is followed by acknowledging differences and similarities between the 2 viewpoints. Finally, the physician recommends a treatment plan and negotiates patient agreement.39 Negotiation implies flexibility and willingness to compromise with reasonable cultural demands, without compromising patient care. Use of the LEARN model aids in the identification and resolution of any cultural conflicts that might arise during the course of the clinical interview.
Teach back and patient activation
An extremely useful technique for all cultures is termed “teach back” or “show me,” which involves asking patients to repeat their care instructions at the end of the visit. This extra step provides an opportunity to correct errors that might have occurred during the transmission of instructions.42 Caregivers should also encourage or “activate” patients to become more involved in managing their own health care. Patient activation measures may be assessed on a one-to-4 point scale.43 Using both of these techniques combats passivity, promotes patient acceptance, and improves outcomes.
A caring environment
There are various strategies and approaches that can help make a medical practice more immigrant friendly (TABLE 4).44,45 Instructing office staff to assist patients in getting to the clinic is critical for those with limited mobility or who lack English proficiency. Adding evening hours that can also accommodate walk-ins helps working patients. For practices with larger immigrant populations, recognizing Asian holidays like Chinese New Year, Diwali, or Tet will be well received. These practices have been directly correlated with more positive health outcomes and better patient satisfaction.44
Conveying complex instructions to patients with little English takes effort for even the most unflappable providers. While written follow-up instructions in English could be interpreted by a more fluent family member, the ideal solution would be to have materials available in the native language. Fortunately, several Web sites, such as SPIRAL (Selective Patient Information in Asian Languages) provide downloadable Asian language instructions.46
Physicians should try to implement the Culturally & Linguistically Appropriate Services (CLAS) guidelines and mandates from the Office of Minority Health (http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15).6 They go far towards providing optimal care for patients of all cultures. Cultural competence does not imply being an expert in all cultures, let alone those of Asia. However, health care providers can develop the skills necessary for effective cross-cultural communication, which, to be most effective, must be accompanied by a caring attitude and respectful practice environment.
CORRESPONDENCE
Gregory Juckett, MD, MPH, West Virginia University School of Medicine, Box 9247, Robert C. Byrd Health Sciences Center, Morgantown, West Virginia, 26506; [email protected]
1. Min PG, ed. Asian Americans: Contemporary Trends and Issues. 2nd ed. Thousand Oaks, California: Pine Forge Press; 2006.
2. Ortman JM, Guarneri CE; National Census Bureau. United States population projections: 2000 to 2050. Available at: http://www.census.gov/population/projections/files/analytical-document09.pdf. Accessed February 20, 2012.
3. Barnes JS, Bennett CE; US Census Bureau Web site. The Asian population: 2000. Available at: http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed February 2, 2012.
4. Kim G, Worley CB, Allen RS, et al. Vulnerability of older Latino and Asian immigrants with limited English proficiency. J Am Geriatr Soc. 2011;59:1246-1252.
5. Silver D, Blustein J, Weitzman BC. Transportation to clinic: findings from a pilot clinic-based survey of low-income suburbanites. J Immigr Minor Health. 2012;14:350-355.
6. US Department of Health and Human Services Office of Minority Health Web site. The National CLAS Standards. Available at: http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Updated May 3, 2013. Accessed June 1, 2013.
7. Karliner LS, Jacobs EA, Chen AH, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42:727-754.
8. Wilson E, Chen AH, Grumbach K, et al. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800-806.
9. Ku L, Flores G. Pay now or pay later: providing interpreter services in health care. Health Aff (Millwood). 2005;24:435-444.
10. Ngo-Metzger Q, Massagali MP, Clarridge BR, et al. Linguistic and cultural barriers to care. J Gen Intern Med. 2003;18:44-52.
11. Basabe N, Ros M. Cultural dimensions and social behavior correlates: individualism-collectivism and power distance. Revue Internationale De Pscyhologie Sociale. 2005;17:189-225.
12. Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences. Results of a national survey. J Gen Intern Med. 2004;19:111-119.
13. Collins KS, Hughes DL, Doty MM, et al; The Commonwealth Fund. Diverse communities, common concerns: assessing health care quality for minority Americans. Available at: http://www.commonwealthfund.org/Publications/Fund-Reports/2002/Mar/Diverse-Communities--Common-Concerns--Assessing-Health-Care-Quality-for-Minority-Americans.aspx. Published March 2002. Accessed December 20, 2013.
14. Houston HR, Harada N, Makinodan T. Development of a culturally sensitive educational intervention program to reduce high incidence of tuberculosis among foreign-born Vietnamese. Ethn Health. 2002;7:255-265.
15. Mazurel GH, Jereb J, Vernon A, et al; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
16. Hutton DW, Tan D, So SK, et al. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460-469.
17. Fattovich G, Brollo L, Giustina G, et al. Natural history and prognostic factors in chronic hepatitis B. Gut. 1991;32:294-298.
18. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956.
19. Smith C. Managing Adult Patients with Chronic HBV. Hepatitis B Foundation. Accessed February 15, 2012, at http://www.hepb.org/professionals/management_guidelines.htm.
20. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
21. Hamaad A, Lip G. Assessing heart disease in your ethnic patients. Pulse. 2003;63:48-49.
22. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924-e929.
23. Venkataraman R, Nanda NC, Beweja G, et al. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol. 2004;94:977-980.
24. Nishtar S. Prevention of coronary heart disease in south Asia. Lancet. 2002;360:1015-1018.
25. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725-734.
26. Sorkin DH, Nguyen H, Ngo-Metzger Q. Assessing the mental health needs and barriers to care among a diverse sample of Asian American older adults. J Gen Intern Med. 2011;26:595-602.
27. PTSD, depression epidemic among Cambodian immigrants [press release]. Bethesda, MD: National Institutes of Health; August 2, 2005.
28. Sue S, Sue DW, Sue L, et al. Psychopathology among Asian Americans: a model minority? Cult Divers Ment Health. 1995;1:39-51.
29. Parker G, Cheah YC, Roy K. Do the Chinese somaticize depression? A cross-cultural study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:287-293.
30. Sribney W, Elliot K, Aguilar-Gaxiola S, et al. The role of nonmedical human services and alternative medicine. In: Ruiz P, Primm A, eds. Disparities in Psychiatric Care. Baltimore, MD: Lippincott, Williams & Wilkins; 2010:274-289.
31. Lee HY, Lytle K, Yang PN, et al. Mental health literacy in Hmong and Cambodian elderly refugees: a barrier to understanding, recognizing, and responding to depression. Int J Aging Hum Dev. 2010;71:323-344.
32. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthroplologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
33. Patwardhan B, Warude D, Pushpangadan P, et al. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465-473.
34. Wu AP, Burke A, LeBaron S. Use of traditional medicine by immigrant Chinese patients. Fam Med. 2007;39:195-200.
35. Nguyen G, Bowman M. Culture, language, and health literacy: communicating about health with Asians and Pacific Islanders. Fam Med. 2007;39:208-210.
36. Oates RK. Overturning the diagnosis of child abuse. Arch Dis Child. 1984;59:665-666.
37. Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr Drug Metab. 2011;12:989-996.
38. Galanti G. Communication and time orientation. In: Caring for Patients from Different Cultures. 4th ed. Philadelphia, PA: University of Pennsylvania Press; 2008:27-51.
39. Berlin E, Fowkes WC Jr. A teaching framework for cross-cultural health care: application in family practice. West J Med. 1983;139:934-938.
40. Stuart MR, Lieberman JA III, eds. The Fifteen Minute Hour: Applied Psychotherapy for the Primary Care Physician. 2nd ed. Westport, CT: Praeger; 1993:101-183.
41. Kobylarz FA, Heath JM, Like RC. The ETHNIC(S) mnemonic: a clinical tool for ethnogeriatric education. J Am Geriat Soc. 2002;50:1582-1589.
42. Kountz DS. Strategies for improving low health literacy. Postgrad Med. 2009;121:171-177.
43. Patient Activation Measure Assessment. Insignia Health Web site. Available at: http://www.insigniahealth.com/solutions/patientactivation-measure. Accessed February 20, 2012.
44. Glenn-Vega A. Achieving a more minority-friendly practice. Fam Pract Manag. 2002;9:39-43.
45. Galanti G. Making a Difference. In: Caring for Patients from Different Cultures. 3rd ed. Philadelphia, PA: University of Pennsylvania Press; 2003:1222-1229.
46. SPIRAL: Selected Patient Information in Asian Languages. Tufts University Hirsh Health Sciences Web site. Available at: http://spiral.tufts.edu/topic.shtml. Accessed February 10, 2012.
› Ask Asian immigrants open-ended questions and encourage them to share their use of alternative remedies. C
› Consider providing an interpretation service for patients not proficient in English, as opposed to asking family members to help. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though often considered a “model minority,” Asian immigrants pose significant challenges for Western health care providers, including radically different ideas of disease causation, differing communication styles, and somatic presentations of mental illness. Asian diversity is tremendous, but several cultural trends are held in common: strong family structures, respect, adaptability, and, for first generation immigrants, widespread use of traditional therapies.1
While Asians and Pacific Islanders (APIs) represent only 5.6% of the US population, or 17.3 million people, that figure represents a 46% increase between 2000 and 2010, the most rapid for any ethnic group.2 A 79% increase is anticipated by 2050, bringing Asians to 9.3% of the US population. In order of population, API subpopulations include Chinese, Filipinos, Asian Indians, Vietnamese, Koreans, and Japanese.2 More than half of Asian Americans reside in the states of California, New York, and Hawaii, although enclaves exist in most major cities.3
Addressing the health needs of Asian immigrants in an increasingly diverse society mandates that US physicians develop the necessary skills to communicate, even when expectations for care may be very different. Fortunately, excellent resources are available (TABLE 1).
Barriers to good healthcare
The most formidable obstacle is limited English proficiency of patients, making them significantly less likely to seek care.4 They often struggle to arrange an appointment, although they arrive on time.5
Inadequate interpretation services. Frequently family members must interpret for patients, despite a federal mandate (Title VI of the 1964 Civil Rights Act) requiring professional services be provided at no charge if there is a federal payer (Medicare or Medicaid) involved.6 Unfortunately, these services are not currently reimbursable. Use of family or friends as interpreters, while convenient, results in far less accurate interpretation, frequent embarrassment, and loss of patient confidentiality. Trained medical interpreters or even telephone services are preferable, as they are much more accurate. Interviews involve a triad comprised of provider, patient, and interpreter, with the provider speaking directly to the patient using first-person address at all times. The interpreter should sit to the side or slightly behind the patient. All communication should be interpreted sentence by sentence so everyone is able to understand the entire conversation. It is well documented that proper interpretive services vastly improve the quality of care.7
Patient illiteracy. Health care illiteracy leads to medication errors due to the inability to understand instructions.8 Some immigrants have the added disadvantage of being illiterate both in English and in their native tongue.4 If not remedied, these situations easily lead to drug overdoses or missed allergies.9 Older immigrants neither understand the intricacies of the US health care system nor possess the language skills to master it.4
Stereotyping by caregivers must be surmounted if patients are to receive quality care. Many Asian patients report that physicians fail to understand them as unique individuals apart from their ethnic identity. Others feel excluded from the decision-making process or find culturally sensitive treatment options lacking.10
Subtleties of relational interaction. Asian culture has been defined as possessing a high Power Distance Index (PDI).11 The PDI refers to the distance or level of respect which an individual must afford to a superior, and this ideal is reflected in Asian conformance to a strict social hierarchy. Thus, physicians are viewed as authority figures and it is proper to nod or smile to indicate polite deference.12 However, showing respect and “buying in” to treatment recommendations are entirely different matters. Cultural factors make it difficult for patients to openly disagree with physician recommendations without feeling as though they have been disrespectful.12 Asian cultures are also “high context” cultures, having far more unwritten rules for conduct and communication that often prove baffling to westerners from “lower context” cultures.
Financial limitations. Socioeconomic influences also play a role. Although Asians have a higher income than other minority groups, 12.5% of Asians still live in poverty and 17.2% lack health insurance.2 Lack of coverage makes many Asians reluctant to seek regular medical care.13
Special medical concerns
Asian-Americans face a variety of challenging medical issues, including disproportionately high rates of tuberculosis (TB) and hepatitis B.
TB. Although rates of TB infection in the United States are low, rates in Asian immigrants are up to 100 times greater than that of the general population, more than any other immigrant group.14 Screening with interferon gamma release assays (IGRAs), such as T-SPOT TB, should be routine for Asian immigrants, since IGRAs do not cross-react with the bacillus Calmette-Guérin (BCG) vaccine. The Centers for Disease Control and Prevention now recommends IGRA blood testing in lieu of tuberculin skin testing (TST) for immigrants who received BCG in infancy, with the exception of children <5 years, for whom the TST is still preferable.15 Patients with positive IGRA tests are also more likely to be amenable to treatment.
Chronic hepatitis B infection in Asian immigrants is often due to perinatal transmission in their home countries. Rates of hepatitis B virus (HBV) infection in the United States have been steadily declining since vaccination began in 1981. However, chronic HBV infection in Asian immigrants approaches 10% of that population.16 An estimated 15% to 20% of patients with chronic HBV develop cirrhosis within 5 years and are at high risk for hepatocellular carcinoma (HCC).17,18
Evaluate HBV carriers annually with liver function testing (LFTs), hepatitis B surface Antigen (HBsAg), and hepatitis B e-Antigen (HBeAg). A positive HBsAg result indicates the virus is present; a positive HBeAg result indicates the virus is actively replicating. LFT elevation (AST >200 IU/L) or a positive HBeAg test should prompt referral to a gastroenterologist for liver biopsy and therapy. Screening for HCC with alpha-fetoprotein (AFP) levels and liver ultrasounds every 6 to 12 months has been recommended for all chronic HBV carriers,19 but this interval remains controversial. Screen men ≥40 years and women ≥50, or anyone who has had HBV infection >10 years, every 6 months.19,20 More recent recommendations favor ultrasound over AFP for screening, as the latter test lacks adequate sensitivity and specificity.20 Test partners and family members of HBV patients, and vaccinate them against HBV if not already immune.
Other medical issues. Asians from the Indian subcontinent have a significantly elevated risk of heart disease, in part due to low HDL-cholesterol levels.21 South Asian immigrant populations have a 3- to 5-fold increased risk of myocardial infarction and cardiovascular death compared with other ethnicities, and often exhibit coronary disease before the age of 40.22 The recent adoption of a Western diet and sedentary lifestyle has provoked an epidemic of diabetes throughout urban Asia and in Asians living abroad. This may be related to the “thrifty gene hypothesis,” which suggests that genes which evolved early in human history to facilitate storage of fat for periods of famine are detrimental in modern society where food is plentiful. A study of Asian Indian immigrants in Atlanta demonstrated an 18.3% prevalence rate for diabetes, higher than any other ethnic group.23 Tobacco and its causal relationship with lung cancer and heart disease only adds to this concern. Southeast Asians in particular demonstrate alarmingly high rates of tobacco consumption, and lung cancer is the leading cause of death among Asian Americans.12,24
Recently, a new acquired immune deficiency syndrome has been described in East Asia. In this syndrome, interferon–gamma (IFN–gamma) is blocked by an auto-antibody. Although not communicable like human immunodeficiency virus, this autoimmune syndrome may lead to similar opportunistic infections such as atypical mycobacterial infections.25
Mental health concerns
Perhaps no topic deserves more emphasis than that of mental health. In the aftermath of the war in Vietnam, Southeast Asian immigrants suffered a great deal from traumatic immigration experiences, with severe adjustment reactions.26 A high incidence of posttraumatic stress disorder (PTSD) among the Hmong in particular reflects their turbulent national history.27
While the incidence of mental illness among Asians is comparable to that of the general population, Asian Americans are less likely to report such problems or to use mental health services26 due to the stigmatization of mental illness in Asian culture.28 Consequently, these symptoms may be subconsciously converted into the more socially acceptable medium of physical illness, which “saves face” and preserves family honor.29 In many ways, Asian culture still perceives mental illness as personal weakness.30 In Hmong culture, inability to speak about being depressed stems not just from cultural bias but from linguistic constraints—the language simply lacks a word for depression.31 Even Asian cultures recognizing mental illness deem depression to be more dependent on circumstances than on the psyche.31 A first-generation immigrant with mental illness is therefore more likely to present with somatic symptoms than a mood disturbance, and is likely to be resistant to counseling or medication for depression. (See “Cultural influence on self-perception: A case ”).
Asian health care beliefs and illnesses
Asian culture substantially influences the ways in which an individual perceives disease, experiences illness, and copes with the phenomenon of sickness. The interplay between illness, disease, and sickness was first elucidated by the pioneering work of Arthur Kleinman, MD, in 1978.32 The term disease denotes a pathological process, while illness describes the subjective impact of disease in a patient’s life. Sickness is the sum of both, as it relates to the total picture of biological and social disruption. The culturally competent physician must understand not only the patient’s disease but also his experience of illness. Asking Dr. Kleinman’s questions will help physicians understand their patient’s perception of illness (TABLE 2).32
Traditional Chinese medicine
Many East Asians derive their conception of illness from traditional Chinese medicine (TCM), a broad range of therapies including herbs, acupuncture, massage (tuina), and diet that has been used for millennia. Similarly, South Asians are influenced by the Ayurvedic or Unani traditions. TCM views the body as an energy system, rather than a machine, through which the life force, or chi, flows. Health is not just the absence of disease, but a proper balance of the antithetic forces, yin and yang, maintained by herbs, diet, and acupuncture.33 TCM is often preferred for treating chronic conditions and viral syndromes, or as a substitute for Western medications with adverse effects.34 It is most popular among newly arrived immigrants, those with low literacy, and those with limited access to conventional medical treatment.34 Most Western physicians know little about TCM, feel uncomfortable when their patients use it, and fail to recognize its popularity.34,35 Likewise, most Asian patients are reluctant to discuss their use of TCM unless questioned about it in a nonjudgmental manner.
Many East Asian cultures practice a distinct form of folk healing known as “coining,” in which a coin dipped in cold oil or Tiger Balm is rubbed against the skin, enabling “wind illness” to escape the body (FIGURE 1). Linear bands of painless petechiae develop. The more extensive the bruising, the more illness is thought to be released. Failure to expel “wind-cold” from the body is believed to account for many ailments. Other traditions are moxibustion and cupping. In moxibustion, a smoldering plug of dried artemisia herb (moxi) is either impaled upon an acupuncture needle or placed directly on the skin to create a burn (FIGURE 2). In cupping, a flame is quickly passed through a glass bowl which is then placed against the skin. The resulting suction creates a circular bruise and draws blood to the area (FIGURE 3). Many folk remedies have been mistaken for child abuse by individuals unfamiliar with such practices.36 Occasionally TCM may result in harm from burns, unsterilized acupuncture needles, or (most commonly) adulterated herbal formulations.37
Culture-bound syndromes
Asian folk illnesses usually go unrecognized by western practitioners, and many of these are somatic presentations of mental illness or stress (TABLE 3). A classic example is the Korean folk condition Hwa-byung, which may include the sensation of an abdominal mass. US practitioners might pursue a fruitless abdominal workup before suspecting a psychiatric condition, even though a careful history would likely elicit other anxiety symptoms and loss of sleep and appetite.26
Asian social conventions
Asian cultural conventions often create considerable confusion. In India, head waggling (shaking the head back and forth) is equivalent to nodding in conversation, indicating an acknowledgement of communication. To western eyes, it appears that the patient is resisting advice rather than welcoming it. In East Asians, smiling expresses a variety of emotions, including polite disagreement. Acute embarrassment may provoke giggling. Eye contact is usually for social equals; avoiding it, especially between the sexes, is the norm. Only the right hand should be used when giving patients a prescription; the left hand is considered unclean. A patient’s head should only be touched with advance permission, as it is viewed as the seat of the soul and is therefore sacred. Under no circumstances should a patient ever see the bottom of the practitioner’s feet or be touched by them.38 Demonstrating respect (especially for older Asians) and preserving modesty are essential when examining patients.
Naming conventions can also be confusing. In China and much of Southeast Asia, it is customary for the surname to precede the given name, often with the 2 run together, rather than the other way round. It is best to ask how a patient would prefer to be addressed, regardless of how the name appears on the medical chart.38
Cultivating knowledge of Asian culture provides a framework from which practitioners can better understand and treat their patients. By asking respectful, open ended questions and encouraging patients to take an active role in their own treatment, physicians become therapeutic allies actively engaged in the healing process. Asking patients to share their use of alternative remedies allows the option of rationally integrating those most meaningful for the patient.
The cross-cultural interview
It is helpful to have a specific approach in mind when interviewing patients from other cultures. A number of mnemonic techniques exist.39-41 Perhaps the most useful of these is the LEARN model, which stands for Listen, Explain, Acknowledge, Recommend, and Negotiate.39 The physician first listens carefully to the patient’s perception of his illness before explaining any medical (disease) issues. This exchange is followed by acknowledging differences and similarities between the 2 viewpoints. Finally, the physician recommends a treatment plan and negotiates patient agreement.39 Negotiation implies flexibility and willingness to compromise with reasonable cultural demands, without compromising patient care. Use of the LEARN model aids in the identification and resolution of any cultural conflicts that might arise during the course of the clinical interview.
Teach back and patient activation
An extremely useful technique for all cultures is termed “teach back” or “show me,” which involves asking patients to repeat their care instructions at the end of the visit. This extra step provides an opportunity to correct errors that might have occurred during the transmission of instructions.42 Caregivers should also encourage or “activate” patients to become more involved in managing their own health care. Patient activation measures may be assessed on a one-to-4 point scale.43 Using both of these techniques combats passivity, promotes patient acceptance, and improves outcomes.
A caring environment
There are various strategies and approaches that can help make a medical practice more immigrant friendly (TABLE 4).44,45 Instructing office staff to assist patients in getting to the clinic is critical for those with limited mobility or who lack English proficiency. Adding evening hours that can also accommodate walk-ins helps working patients. For practices with larger immigrant populations, recognizing Asian holidays like Chinese New Year, Diwali, or Tet will be well received. These practices have been directly correlated with more positive health outcomes and better patient satisfaction.44
Conveying complex instructions to patients with little English takes effort for even the most unflappable providers. While written follow-up instructions in English could be interpreted by a more fluent family member, the ideal solution would be to have materials available in the native language. Fortunately, several Web sites, such as SPIRAL (Selective Patient Information in Asian Languages) provide downloadable Asian language instructions.46
Physicians should try to implement the Culturally & Linguistically Appropriate Services (CLAS) guidelines and mandates from the Office of Minority Health (http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15).6 They go far towards providing optimal care for patients of all cultures. Cultural competence does not imply being an expert in all cultures, let alone those of Asia. However, health care providers can develop the skills necessary for effective cross-cultural communication, which, to be most effective, must be accompanied by a caring attitude and respectful practice environment.
CORRESPONDENCE
Gregory Juckett, MD, MPH, West Virginia University School of Medicine, Box 9247, Robert C. Byrd Health Sciences Center, Morgantown, West Virginia, 26506; [email protected]
› Ask Asian immigrants open-ended questions and encourage them to share their use of alternative remedies. C
› Consider providing an interpretation service for patients not proficient in English, as opposed to asking family members to help. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though often considered a “model minority,” Asian immigrants pose significant challenges for Western health care providers, including radically different ideas of disease causation, differing communication styles, and somatic presentations of mental illness. Asian diversity is tremendous, but several cultural trends are held in common: strong family structures, respect, adaptability, and, for first generation immigrants, widespread use of traditional therapies.1
While Asians and Pacific Islanders (APIs) represent only 5.6% of the US population, or 17.3 million people, that figure represents a 46% increase between 2000 and 2010, the most rapid for any ethnic group.2 A 79% increase is anticipated by 2050, bringing Asians to 9.3% of the US population. In order of population, API subpopulations include Chinese, Filipinos, Asian Indians, Vietnamese, Koreans, and Japanese.2 More than half of Asian Americans reside in the states of California, New York, and Hawaii, although enclaves exist in most major cities.3
Addressing the health needs of Asian immigrants in an increasingly diverse society mandates that US physicians develop the necessary skills to communicate, even when expectations for care may be very different. Fortunately, excellent resources are available (TABLE 1).
Barriers to good healthcare
The most formidable obstacle is limited English proficiency of patients, making them significantly less likely to seek care.4 They often struggle to arrange an appointment, although they arrive on time.5
Inadequate interpretation services. Frequently family members must interpret for patients, despite a federal mandate (Title VI of the 1964 Civil Rights Act) requiring professional services be provided at no charge if there is a federal payer (Medicare or Medicaid) involved.6 Unfortunately, these services are not currently reimbursable. Use of family or friends as interpreters, while convenient, results in far less accurate interpretation, frequent embarrassment, and loss of patient confidentiality. Trained medical interpreters or even telephone services are preferable, as they are much more accurate. Interviews involve a triad comprised of provider, patient, and interpreter, with the provider speaking directly to the patient using first-person address at all times. The interpreter should sit to the side or slightly behind the patient. All communication should be interpreted sentence by sentence so everyone is able to understand the entire conversation. It is well documented that proper interpretive services vastly improve the quality of care.7
Patient illiteracy. Health care illiteracy leads to medication errors due to the inability to understand instructions.8 Some immigrants have the added disadvantage of being illiterate both in English and in their native tongue.4 If not remedied, these situations easily lead to drug overdoses or missed allergies.9 Older immigrants neither understand the intricacies of the US health care system nor possess the language skills to master it.4
Stereotyping by caregivers must be surmounted if patients are to receive quality care. Many Asian patients report that physicians fail to understand them as unique individuals apart from their ethnic identity. Others feel excluded from the decision-making process or find culturally sensitive treatment options lacking.10
Subtleties of relational interaction. Asian culture has been defined as possessing a high Power Distance Index (PDI).11 The PDI refers to the distance or level of respect which an individual must afford to a superior, and this ideal is reflected in Asian conformance to a strict social hierarchy. Thus, physicians are viewed as authority figures and it is proper to nod or smile to indicate polite deference.12 However, showing respect and “buying in” to treatment recommendations are entirely different matters. Cultural factors make it difficult for patients to openly disagree with physician recommendations without feeling as though they have been disrespectful.12 Asian cultures are also “high context” cultures, having far more unwritten rules for conduct and communication that often prove baffling to westerners from “lower context” cultures.
Financial limitations. Socioeconomic influences also play a role. Although Asians have a higher income than other minority groups, 12.5% of Asians still live in poverty and 17.2% lack health insurance.2 Lack of coverage makes many Asians reluctant to seek regular medical care.13
Special medical concerns
Asian-Americans face a variety of challenging medical issues, including disproportionately high rates of tuberculosis (TB) and hepatitis B.
TB. Although rates of TB infection in the United States are low, rates in Asian immigrants are up to 100 times greater than that of the general population, more than any other immigrant group.14 Screening with interferon gamma release assays (IGRAs), such as T-SPOT TB, should be routine for Asian immigrants, since IGRAs do not cross-react with the bacillus Calmette-Guérin (BCG) vaccine. The Centers for Disease Control and Prevention now recommends IGRA blood testing in lieu of tuberculin skin testing (TST) for immigrants who received BCG in infancy, with the exception of children <5 years, for whom the TST is still preferable.15 Patients with positive IGRA tests are also more likely to be amenable to treatment.
Chronic hepatitis B infection in Asian immigrants is often due to perinatal transmission in their home countries. Rates of hepatitis B virus (HBV) infection in the United States have been steadily declining since vaccination began in 1981. However, chronic HBV infection in Asian immigrants approaches 10% of that population.16 An estimated 15% to 20% of patients with chronic HBV develop cirrhosis within 5 years and are at high risk for hepatocellular carcinoma (HCC).17,18
Evaluate HBV carriers annually with liver function testing (LFTs), hepatitis B surface Antigen (HBsAg), and hepatitis B e-Antigen (HBeAg). A positive HBsAg result indicates the virus is present; a positive HBeAg result indicates the virus is actively replicating. LFT elevation (AST >200 IU/L) or a positive HBeAg test should prompt referral to a gastroenterologist for liver biopsy and therapy. Screening for HCC with alpha-fetoprotein (AFP) levels and liver ultrasounds every 6 to 12 months has been recommended for all chronic HBV carriers,19 but this interval remains controversial. Screen men ≥40 years and women ≥50, or anyone who has had HBV infection >10 years, every 6 months.19,20 More recent recommendations favor ultrasound over AFP for screening, as the latter test lacks adequate sensitivity and specificity.20 Test partners and family members of HBV patients, and vaccinate them against HBV if not already immune.
Other medical issues. Asians from the Indian subcontinent have a significantly elevated risk of heart disease, in part due to low HDL-cholesterol levels.21 South Asian immigrant populations have a 3- to 5-fold increased risk of myocardial infarction and cardiovascular death compared with other ethnicities, and often exhibit coronary disease before the age of 40.22 The recent adoption of a Western diet and sedentary lifestyle has provoked an epidemic of diabetes throughout urban Asia and in Asians living abroad. This may be related to the “thrifty gene hypothesis,” which suggests that genes which evolved early in human history to facilitate storage of fat for periods of famine are detrimental in modern society where food is plentiful. A study of Asian Indian immigrants in Atlanta demonstrated an 18.3% prevalence rate for diabetes, higher than any other ethnic group.23 Tobacco and its causal relationship with lung cancer and heart disease only adds to this concern. Southeast Asians in particular demonstrate alarmingly high rates of tobacco consumption, and lung cancer is the leading cause of death among Asian Americans.12,24
Recently, a new acquired immune deficiency syndrome has been described in East Asia. In this syndrome, interferon–gamma (IFN–gamma) is blocked by an auto-antibody. Although not communicable like human immunodeficiency virus, this autoimmune syndrome may lead to similar opportunistic infections such as atypical mycobacterial infections.25
Mental health concerns
Perhaps no topic deserves more emphasis than that of mental health. In the aftermath of the war in Vietnam, Southeast Asian immigrants suffered a great deal from traumatic immigration experiences, with severe adjustment reactions.26 A high incidence of posttraumatic stress disorder (PTSD) among the Hmong in particular reflects their turbulent national history.27
While the incidence of mental illness among Asians is comparable to that of the general population, Asian Americans are less likely to report such problems or to use mental health services26 due to the stigmatization of mental illness in Asian culture.28 Consequently, these symptoms may be subconsciously converted into the more socially acceptable medium of physical illness, which “saves face” and preserves family honor.29 In many ways, Asian culture still perceives mental illness as personal weakness.30 In Hmong culture, inability to speak about being depressed stems not just from cultural bias but from linguistic constraints—the language simply lacks a word for depression.31 Even Asian cultures recognizing mental illness deem depression to be more dependent on circumstances than on the psyche.31 A first-generation immigrant with mental illness is therefore more likely to present with somatic symptoms than a mood disturbance, and is likely to be resistant to counseling or medication for depression. (See “Cultural influence on self-perception: A case ”).
Asian health care beliefs and illnesses
Asian culture substantially influences the ways in which an individual perceives disease, experiences illness, and copes with the phenomenon of sickness. The interplay between illness, disease, and sickness was first elucidated by the pioneering work of Arthur Kleinman, MD, in 1978.32 The term disease denotes a pathological process, while illness describes the subjective impact of disease in a patient’s life. Sickness is the sum of both, as it relates to the total picture of biological and social disruption. The culturally competent physician must understand not only the patient’s disease but also his experience of illness. Asking Dr. Kleinman’s questions will help physicians understand their patient’s perception of illness (TABLE 2).32
Traditional Chinese medicine
Many East Asians derive their conception of illness from traditional Chinese medicine (TCM), a broad range of therapies including herbs, acupuncture, massage (tuina), and diet that has been used for millennia. Similarly, South Asians are influenced by the Ayurvedic or Unani traditions. TCM views the body as an energy system, rather than a machine, through which the life force, or chi, flows. Health is not just the absence of disease, but a proper balance of the antithetic forces, yin and yang, maintained by herbs, diet, and acupuncture.33 TCM is often preferred for treating chronic conditions and viral syndromes, or as a substitute for Western medications with adverse effects.34 It is most popular among newly arrived immigrants, those with low literacy, and those with limited access to conventional medical treatment.34 Most Western physicians know little about TCM, feel uncomfortable when their patients use it, and fail to recognize its popularity.34,35 Likewise, most Asian patients are reluctant to discuss their use of TCM unless questioned about it in a nonjudgmental manner.
Many East Asian cultures practice a distinct form of folk healing known as “coining,” in which a coin dipped in cold oil or Tiger Balm is rubbed against the skin, enabling “wind illness” to escape the body (FIGURE 1). Linear bands of painless petechiae develop. The more extensive the bruising, the more illness is thought to be released. Failure to expel “wind-cold” from the body is believed to account for many ailments. Other traditions are moxibustion and cupping. In moxibustion, a smoldering plug of dried artemisia herb (moxi) is either impaled upon an acupuncture needle or placed directly on the skin to create a burn (FIGURE 2). In cupping, a flame is quickly passed through a glass bowl which is then placed against the skin. The resulting suction creates a circular bruise and draws blood to the area (FIGURE 3). Many folk remedies have been mistaken for child abuse by individuals unfamiliar with such practices.36 Occasionally TCM may result in harm from burns, unsterilized acupuncture needles, or (most commonly) adulterated herbal formulations.37
Culture-bound syndromes
Asian folk illnesses usually go unrecognized by western practitioners, and many of these are somatic presentations of mental illness or stress (TABLE 3). A classic example is the Korean folk condition Hwa-byung, which may include the sensation of an abdominal mass. US practitioners might pursue a fruitless abdominal workup before suspecting a psychiatric condition, even though a careful history would likely elicit other anxiety symptoms and loss of sleep and appetite.26
Asian social conventions
Asian cultural conventions often create considerable confusion. In India, head waggling (shaking the head back and forth) is equivalent to nodding in conversation, indicating an acknowledgement of communication. To western eyes, it appears that the patient is resisting advice rather than welcoming it. In East Asians, smiling expresses a variety of emotions, including polite disagreement. Acute embarrassment may provoke giggling. Eye contact is usually for social equals; avoiding it, especially between the sexes, is the norm. Only the right hand should be used when giving patients a prescription; the left hand is considered unclean. A patient’s head should only be touched with advance permission, as it is viewed as the seat of the soul and is therefore sacred. Under no circumstances should a patient ever see the bottom of the practitioner’s feet or be touched by them.38 Demonstrating respect (especially for older Asians) and preserving modesty are essential when examining patients.
Naming conventions can also be confusing. In China and much of Southeast Asia, it is customary for the surname to precede the given name, often with the 2 run together, rather than the other way round. It is best to ask how a patient would prefer to be addressed, regardless of how the name appears on the medical chart.38
Cultivating knowledge of Asian culture provides a framework from which practitioners can better understand and treat their patients. By asking respectful, open ended questions and encouraging patients to take an active role in their own treatment, physicians become therapeutic allies actively engaged in the healing process. Asking patients to share their use of alternative remedies allows the option of rationally integrating those most meaningful for the patient.
The cross-cultural interview
It is helpful to have a specific approach in mind when interviewing patients from other cultures. A number of mnemonic techniques exist.39-41 Perhaps the most useful of these is the LEARN model, which stands for Listen, Explain, Acknowledge, Recommend, and Negotiate.39 The physician first listens carefully to the patient’s perception of his illness before explaining any medical (disease) issues. This exchange is followed by acknowledging differences and similarities between the 2 viewpoints. Finally, the physician recommends a treatment plan and negotiates patient agreement.39 Negotiation implies flexibility and willingness to compromise with reasonable cultural demands, without compromising patient care. Use of the LEARN model aids in the identification and resolution of any cultural conflicts that might arise during the course of the clinical interview.
Teach back and patient activation
An extremely useful technique for all cultures is termed “teach back” or “show me,” which involves asking patients to repeat their care instructions at the end of the visit. This extra step provides an opportunity to correct errors that might have occurred during the transmission of instructions.42 Caregivers should also encourage or “activate” patients to become more involved in managing their own health care. Patient activation measures may be assessed on a one-to-4 point scale.43 Using both of these techniques combats passivity, promotes patient acceptance, and improves outcomes.
A caring environment
There are various strategies and approaches that can help make a medical practice more immigrant friendly (TABLE 4).44,45 Instructing office staff to assist patients in getting to the clinic is critical for those with limited mobility or who lack English proficiency. Adding evening hours that can also accommodate walk-ins helps working patients. For practices with larger immigrant populations, recognizing Asian holidays like Chinese New Year, Diwali, or Tet will be well received. These practices have been directly correlated with more positive health outcomes and better patient satisfaction.44
Conveying complex instructions to patients with little English takes effort for even the most unflappable providers. While written follow-up instructions in English could be interpreted by a more fluent family member, the ideal solution would be to have materials available in the native language. Fortunately, several Web sites, such as SPIRAL (Selective Patient Information in Asian Languages) provide downloadable Asian language instructions.46
Physicians should try to implement the Culturally & Linguistically Appropriate Services (CLAS) guidelines and mandates from the Office of Minority Health (http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15).6 They go far towards providing optimal care for patients of all cultures. Cultural competence does not imply being an expert in all cultures, let alone those of Asia. However, health care providers can develop the skills necessary for effective cross-cultural communication, which, to be most effective, must be accompanied by a caring attitude and respectful practice environment.
CORRESPONDENCE
Gregory Juckett, MD, MPH, West Virginia University School of Medicine, Box 9247, Robert C. Byrd Health Sciences Center, Morgantown, West Virginia, 26506; [email protected]
1. Min PG, ed. Asian Americans: Contemporary Trends and Issues. 2nd ed. Thousand Oaks, California: Pine Forge Press; 2006.
2. Ortman JM, Guarneri CE; National Census Bureau. United States population projections: 2000 to 2050. Available at: http://www.census.gov/population/projections/files/analytical-document09.pdf. Accessed February 20, 2012.
3. Barnes JS, Bennett CE; US Census Bureau Web site. The Asian population: 2000. Available at: http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed February 2, 2012.
4. Kim G, Worley CB, Allen RS, et al. Vulnerability of older Latino and Asian immigrants with limited English proficiency. J Am Geriatr Soc. 2011;59:1246-1252.
5. Silver D, Blustein J, Weitzman BC. Transportation to clinic: findings from a pilot clinic-based survey of low-income suburbanites. J Immigr Minor Health. 2012;14:350-355.
6. US Department of Health and Human Services Office of Minority Health Web site. The National CLAS Standards. Available at: http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Updated May 3, 2013. Accessed June 1, 2013.
7. Karliner LS, Jacobs EA, Chen AH, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42:727-754.
8. Wilson E, Chen AH, Grumbach K, et al. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800-806.
9. Ku L, Flores G. Pay now or pay later: providing interpreter services in health care. Health Aff (Millwood). 2005;24:435-444.
10. Ngo-Metzger Q, Massagali MP, Clarridge BR, et al. Linguistic and cultural barriers to care. J Gen Intern Med. 2003;18:44-52.
11. Basabe N, Ros M. Cultural dimensions and social behavior correlates: individualism-collectivism and power distance. Revue Internationale De Pscyhologie Sociale. 2005;17:189-225.
12. Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences. Results of a national survey. J Gen Intern Med. 2004;19:111-119.
13. Collins KS, Hughes DL, Doty MM, et al; The Commonwealth Fund. Diverse communities, common concerns: assessing health care quality for minority Americans. Available at: http://www.commonwealthfund.org/Publications/Fund-Reports/2002/Mar/Diverse-Communities--Common-Concerns--Assessing-Health-Care-Quality-for-Minority-Americans.aspx. Published March 2002. Accessed December 20, 2013.
14. Houston HR, Harada N, Makinodan T. Development of a culturally sensitive educational intervention program to reduce high incidence of tuberculosis among foreign-born Vietnamese. Ethn Health. 2002;7:255-265.
15. Mazurel GH, Jereb J, Vernon A, et al; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
16. Hutton DW, Tan D, So SK, et al. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460-469.
17. Fattovich G, Brollo L, Giustina G, et al. Natural history and prognostic factors in chronic hepatitis B. Gut. 1991;32:294-298.
18. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956.
19. Smith C. Managing Adult Patients with Chronic HBV. Hepatitis B Foundation. Accessed February 15, 2012, at http://www.hepb.org/professionals/management_guidelines.htm.
20. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
21. Hamaad A, Lip G. Assessing heart disease in your ethnic patients. Pulse. 2003;63:48-49.
22. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924-e929.
23. Venkataraman R, Nanda NC, Beweja G, et al. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol. 2004;94:977-980.
24. Nishtar S. Prevention of coronary heart disease in south Asia. Lancet. 2002;360:1015-1018.
25. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725-734.
26. Sorkin DH, Nguyen H, Ngo-Metzger Q. Assessing the mental health needs and barriers to care among a diverse sample of Asian American older adults. J Gen Intern Med. 2011;26:595-602.
27. PTSD, depression epidemic among Cambodian immigrants [press release]. Bethesda, MD: National Institutes of Health; August 2, 2005.
28. Sue S, Sue DW, Sue L, et al. Psychopathology among Asian Americans: a model minority? Cult Divers Ment Health. 1995;1:39-51.
29. Parker G, Cheah YC, Roy K. Do the Chinese somaticize depression? A cross-cultural study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:287-293.
30. Sribney W, Elliot K, Aguilar-Gaxiola S, et al. The role of nonmedical human services and alternative medicine. In: Ruiz P, Primm A, eds. Disparities in Psychiatric Care. Baltimore, MD: Lippincott, Williams & Wilkins; 2010:274-289.
31. Lee HY, Lytle K, Yang PN, et al. Mental health literacy in Hmong and Cambodian elderly refugees: a barrier to understanding, recognizing, and responding to depression. Int J Aging Hum Dev. 2010;71:323-344.
32. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthroplologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
33. Patwardhan B, Warude D, Pushpangadan P, et al. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465-473.
34. Wu AP, Burke A, LeBaron S. Use of traditional medicine by immigrant Chinese patients. Fam Med. 2007;39:195-200.
35. Nguyen G, Bowman M. Culture, language, and health literacy: communicating about health with Asians and Pacific Islanders. Fam Med. 2007;39:208-210.
36. Oates RK. Overturning the diagnosis of child abuse. Arch Dis Child. 1984;59:665-666.
37. Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr Drug Metab. 2011;12:989-996.
38. Galanti G. Communication and time orientation. In: Caring for Patients from Different Cultures. 4th ed. Philadelphia, PA: University of Pennsylvania Press; 2008:27-51.
39. Berlin E, Fowkes WC Jr. A teaching framework for cross-cultural health care: application in family practice. West J Med. 1983;139:934-938.
40. Stuart MR, Lieberman JA III, eds. The Fifteen Minute Hour: Applied Psychotherapy for the Primary Care Physician. 2nd ed. Westport, CT: Praeger; 1993:101-183.
41. Kobylarz FA, Heath JM, Like RC. The ETHNIC(S) mnemonic: a clinical tool for ethnogeriatric education. J Am Geriat Soc. 2002;50:1582-1589.
42. Kountz DS. Strategies for improving low health literacy. Postgrad Med. 2009;121:171-177.
43. Patient Activation Measure Assessment. Insignia Health Web site. Available at: http://www.insigniahealth.com/solutions/patientactivation-measure. Accessed February 20, 2012.
44. Glenn-Vega A. Achieving a more minority-friendly practice. Fam Pract Manag. 2002;9:39-43.
45. Galanti G. Making a Difference. In: Caring for Patients from Different Cultures. 3rd ed. Philadelphia, PA: University of Pennsylvania Press; 2003:1222-1229.
46. SPIRAL: Selected Patient Information in Asian Languages. Tufts University Hirsh Health Sciences Web site. Available at: http://spiral.tufts.edu/topic.shtml. Accessed February 10, 2012.
1. Min PG, ed. Asian Americans: Contemporary Trends and Issues. 2nd ed. Thousand Oaks, California: Pine Forge Press; 2006.
2. Ortman JM, Guarneri CE; National Census Bureau. United States population projections: 2000 to 2050. Available at: http://www.census.gov/population/projections/files/analytical-document09.pdf. Accessed February 20, 2012.
3. Barnes JS, Bennett CE; US Census Bureau Web site. The Asian population: 2000. Available at: http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed February 2, 2012.
4. Kim G, Worley CB, Allen RS, et al. Vulnerability of older Latino and Asian immigrants with limited English proficiency. J Am Geriatr Soc. 2011;59:1246-1252.
5. Silver D, Blustein J, Weitzman BC. Transportation to clinic: findings from a pilot clinic-based survey of low-income suburbanites. J Immigr Minor Health. 2012;14:350-355.
6. US Department of Health and Human Services Office of Minority Health Web site. The National CLAS Standards. Available at: http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=2&lvlID=15. Updated May 3, 2013. Accessed June 1, 2013.
7. Karliner LS, Jacobs EA, Chen AH, et al. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;42:727-754.
8. Wilson E, Chen AH, Grumbach K, et al. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800-806.
9. Ku L, Flores G. Pay now or pay later: providing interpreter services in health care. Health Aff (Millwood). 2005;24:435-444.
10. Ngo-Metzger Q, Massagali MP, Clarridge BR, et al. Linguistic and cultural barriers to care. J Gen Intern Med. 2003;18:44-52.
11. Basabe N, Ros M. Cultural dimensions and social behavior correlates: individualism-collectivism and power distance. Revue Internationale De Pscyhologie Sociale. 2005;17:189-225.
12. Ngo-Metzger Q, Legedza AT, Phillips RS. Asian Americans’ reports of their health care experiences. Results of a national survey. J Gen Intern Med. 2004;19:111-119.
13. Collins KS, Hughes DL, Doty MM, et al; The Commonwealth Fund. Diverse communities, common concerns: assessing health care quality for minority Americans. Available at: http://www.commonwealthfund.org/Publications/Fund-Reports/2002/Mar/Diverse-Communities--Common-Concerns--Assessing-Health-Care-Quality-for-Minority-Americans.aspx. Published March 2002. Accessed December 20, 2013.
14. Houston HR, Harada N, Makinodan T. Development of a culturally sensitive educational intervention program to reduce high incidence of tuberculosis among foreign-born Vietnamese. Ethn Health. 2002;7:255-265.
15. Mazurel GH, Jereb J, Vernon A, et al; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
16. Hutton DW, Tan D, So SK, et al. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147:460-469.
17. Fattovich G, Brollo L, Giustina G, et al. Natural history and prognostic factors in chronic hepatitis B. Gut. 1991;32:294-298.
18. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956.
19. Smith C. Managing Adult Patients with Chronic HBV. Hepatitis B Foundation. Accessed February 15, 2012, at http://www.hepb.org/professionals/management_guidelines.htm.
20. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
21. Hamaad A, Lip G. Assessing heart disease in your ethnic patients. Pulse. 2003;63:48-49.
22. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk: what clinicians should know. Circulation. 2006;113:e924-e929.
23. Venkataraman R, Nanda NC, Beweja G, et al. Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States. Am J Cardiol. 2004;94:977-980.
24. Nishtar S. Prevention of coronary heart disease in south Asia. Lancet. 2002;360:1015-1018.
25. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725-734.
26. Sorkin DH, Nguyen H, Ngo-Metzger Q. Assessing the mental health needs and barriers to care among a diverse sample of Asian American older adults. J Gen Intern Med. 2011;26:595-602.
27. PTSD, depression epidemic among Cambodian immigrants [press release]. Bethesda, MD: National Institutes of Health; August 2, 2005.
28. Sue S, Sue DW, Sue L, et al. Psychopathology among Asian Americans: a model minority? Cult Divers Ment Health. 1995;1:39-51.
29. Parker G, Cheah YC, Roy K. Do the Chinese somaticize depression? A cross-cultural study. Soc Psychiatry Psychiatr Epidemiol. 2001;36:287-293.
30. Sribney W, Elliot K, Aguilar-Gaxiola S, et al. The role of nonmedical human services and alternative medicine. In: Ruiz P, Primm A, eds. Disparities in Psychiatric Care. Baltimore, MD: Lippincott, Williams & Wilkins; 2010:274-289.
31. Lee HY, Lytle K, Yang PN, et al. Mental health literacy in Hmong and Cambodian elderly refugees: a barrier to understanding, recognizing, and responding to depression. Int J Aging Hum Dev. 2010;71:323-344.
32. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthroplologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
33. Patwardhan B, Warude D, Pushpangadan P, et al. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2:465-473.
34. Wu AP, Burke A, LeBaron S. Use of traditional medicine by immigrant Chinese patients. Fam Med. 2007;39:195-200.
35. Nguyen G, Bowman M. Culture, language, and health literacy: communicating about health with Asians and Pacific Islanders. Fam Med. 2007;39:208-210.
36. Oates RK. Overturning the diagnosis of child abuse. Arch Dis Child. 1984;59:665-666.
37. Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr Drug Metab. 2011;12:989-996.
38. Galanti G. Communication and time orientation. In: Caring for Patients from Different Cultures. 4th ed. Philadelphia, PA: University of Pennsylvania Press; 2008:27-51.
39. Berlin E, Fowkes WC Jr. A teaching framework for cross-cultural health care: application in family practice. West J Med. 1983;139:934-938.
40. Stuart MR, Lieberman JA III, eds. The Fifteen Minute Hour: Applied Psychotherapy for the Primary Care Physician. 2nd ed. Westport, CT: Praeger; 1993:101-183.
41. Kobylarz FA, Heath JM, Like RC. The ETHNIC(S) mnemonic: a clinical tool for ethnogeriatric education. J Am Geriat Soc. 2002;50:1582-1589.
42. Kountz DS. Strategies for improving low health literacy. Postgrad Med. 2009;121:171-177.
43. Patient Activation Measure Assessment. Insignia Health Web site. Available at: http://www.insigniahealth.com/solutions/patientactivation-measure. Accessed February 20, 2012.
44. Glenn-Vega A. Achieving a more minority-friendly practice. Fam Pract Manag. 2002;9:39-43.
45. Galanti G. Making a Difference. In: Caring for Patients from Different Cultures. 3rd ed. Philadelphia, PA: University of Pennsylvania Press; 2003:1222-1229.
46. SPIRAL: Selected Patient Information in Asian Languages. Tufts University Hirsh Health Sciences Web site. Available at: http://spiral.tufts.edu/topic.shtml. Accessed February 10, 2012.
What is the best approach to goiter in euthyroid patients?
IN THE ABSENCE OF OUTCOME STUDIES, experts recommend ultrasound evaluation of nontoxic mulinodular goiters (MNG) followed by fine-needle aspiration (FNA) of suspicious nodules (strength of recommendation [SOR]: C, consensus-based guidelines).
Thyroid hormone suppression therapy reduces the size of MNG (SOR: A, systematic review of randomized controlled trials [RCTs]), but it risks inducing hyperthyroidism (SOR: C, expert opinion).
Experts recommend thyroidectomy for compressive symptoms, progressive growth, or ultrasound or FNA results indicating thyroid cancer (SOR: C, consensus based guidelines).
Expert guidelines recommend repeat ultrasound at 6 to 18 months to follow up benign nodules or nonendemic MNG in patients at low risk of malignancy and subsequent follow-up of stable nodules every 3 to 5 years (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
This summary updates the 2007 Clinical Inquiry, “What is the best approach to goiter for euthyroid patients?”1
Initial evaluation of palpable goiter with a normal thyrotropin
In the United States, MNG is generally nonendemic and unrelated to iodine deficiency, as distinguished from endemic goiter caused by iodine deficiency in other parts of the world.
Our structured search of the literature found no randomized trials or prospective cohort studies comparing diagnostic approaches. The American Association of Clinical Endocrinologists’ (AACE) 2010 guidelines and American Thyroid Association (ATA) guidelines recommend ultrasound for all MNG.2,3 The AACE guidelines recommend thyroid scintigraphy when clinicians suspect retrosternal MNG.2
Ultrasound findings can change management, avoid biopsy
In a retrospective analysis of 223 patients with nodular thyroid disease, thyroid ultrasound altered clinical management of 63% of patients with abnormal thyroid exams.4 A single center retrospective cohort study of 650 FNA biopsies identified 4 morphologic patterns on ultrasound that predicted benign cytology with 100% specificity. The authors concluded that using ultrasound pattern to determine which patients require FNA could have obviated more than 60% of thyroid biopsies.5
Thyroid hormone suppression therapy risks hyperthyroidis
A systematic review of 9 RCTs of 18-month or shorter duration found that thyroid hormone suppression therapy reduced benign thyroid nodule volume (relative risk=1.88 compared with placebo or no treatment; 95% confidence interval [CI], 1.18-3.01; P=.008). The number needed to treat was 8 to reduce volume by >50% (risk difference=0.13; 95% CI, 0.06-0.19; P=.0003).6 However, thyroid hormone suppression therapy risks inducing hyperthyroidism and is not routinely recommended by the AACE or the ATA.2,3
Thyroidectomy: The treatment of choice
Thyroidectomy is the definitive therapy for MNG. A narrative review of 15 mostly retrospective cohort studies demonstrated MNG recurrence rates of 0% to 0.3% after total thyroidectomy, with follow-up intervals of 4.8 to 30 years.7
AACE consensus opinion recommends thyroidectomy for compressive symptoms, progressive growth, or when ultrasound or FNA results indicate thyroid cancer.2
A retrospective cohort study of 462 thyroidectomies for MNG found incidental thyroid carcinomas in 8.9% (41 patients). Risk factors included neck irradiation (odds ratio [OR]=21.64; 95% CI, 3.28-143), parenchymal calcifications on imaging (OR=2.30; 95% CI, 0.85-6.23), and family history of thyroid disease (OR=8.2; 95% CI, 2.15-29.87). Living in a goiter-endemic area was protective (OR=0.24; 95% CI, 0.07-0.83).8
Follow-up of patients with initial benign evaluation
Consensus opinion regarding follow-up of MNG is based on observational studies of the natural history of the condition. Benign MNG rarely progresses to malignancy. A review of 6 cohort studies, including 1265 patients with untreated nontoxic MNG who were followed for 60 to 130 months from 1990 to 2007, yielded an annual incidence range of 1.3 to 3.7 new cases of thyroid carcinoma per 1000 patients.9
Some goiters are more likely to enlarge. A retrospective cohort study of 488 patients treated surgically for MNG identified risk factors for enlargement: African American (OR=3.3; 95% CI, 2.0-5.4), age >40 years (OR=2.1; 95% CI, 1.2-3.8), and body mass index >30 (OR=2.5; 95% CI, 1.5-4.0).10
RECOMMENDATIONS
The AACE and the ATA recommend that patients with MNG with benign nodules have a repeat examination, TSH, and ultrasound in 6 to 18 months. Follow-up of stable nodules can then be done in 3 to 5 years.
An enlarging nodule requires repeat FNA.2 If palpation or ultrasound reveal evidence of nodule growth (more than a 50% change in volume or a 20% increase in at least 2 nodule dimensions, with a minimal increase of 2 mm in solid nodules or the solid portion of mixed cystic-solid nodules), the AACE and ATA recommend FNA, preferably with ultrasound guidance.3 Low TSH suggests autonomous nodules and the ATA recommends radionuclide scanning with FNA of hypofunctioning nodules with suspicious US features.3
1. Hoffman MR, Meadows SE, Langlois JP. Clinical inquiries. What is the best approach to goiter for euthyroid patients? J Fam Pract. 2007;56:479-480.
2. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract. 2010;16(suppl 1):S1-S43.
3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
4. Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696-700.
5. Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207-213.
6. Sdano MT, Falciglia M, Welge JA, et al. Efficacy of thyroid hormone suppression for benign thyroid nodules: metaanalysis of randomized trials. Otolaryngol Head Neck Surg. 2005;133:391-396.
7. Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32:1301-1312.
8. Botrugno I, Lovisetto F, Cobianchi L, et al. Incidental carcinoma in multinodular goiter: risk factors. Am Surg. 2011;77:1553-1558.
9. Winbladh A, Järhult J. Fate of the non-operated, non-toxic goiter in a defined population. Brit J Surg. 2008;95:338-343.
10. Phitayakorn R, Super DM, McHenry CR. An investigation of epidemiologic factors associated with large nodular goiter. J Surg Res. 2006;133:16-21.
IN THE ABSENCE OF OUTCOME STUDIES, experts recommend ultrasound evaluation of nontoxic mulinodular goiters (MNG) followed by fine-needle aspiration (FNA) of suspicious nodules (strength of recommendation [SOR]: C, consensus-based guidelines).
Thyroid hormone suppression therapy reduces the size of MNG (SOR: A, systematic review of randomized controlled trials [RCTs]), but it risks inducing hyperthyroidism (SOR: C, expert opinion).
Experts recommend thyroidectomy for compressive symptoms, progressive growth, or ultrasound or FNA results indicating thyroid cancer (SOR: C, consensus based guidelines).
Expert guidelines recommend repeat ultrasound at 6 to 18 months to follow up benign nodules or nonendemic MNG in patients at low risk of malignancy and subsequent follow-up of stable nodules every 3 to 5 years (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
This summary updates the 2007 Clinical Inquiry, “What is the best approach to goiter for euthyroid patients?”1
Initial evaluation of palpable goiter with a normal thyrotropin
In the United States, MNG is generally nonendemic and unrelated to iodine deficiency, as distinguished from endemic goiter caused by iodine deficiency in other parts of the world.
Our structured search of the literature found no randomized trials or prospective cohort studies comparing diagnostic approaches. The American Association of Clinical Endocrinologists’ (AACE) 2010 guidelines and American Thyroid Association (ATA) guidelines recommend ultrasound for all MNG.2,3 The AACE guidelines recommend thyroid scintigraphy when clinicians suspect retrosternal MNG.2
Ultrasound findings can change management, avoid biopsy
In a retrospective analysis of 223 patients with nodular thyroid disease, thyroid ultrasound altered clinical management of 63% of patients with abnormal thyroid exams.4 A single center retrospective cohort study of 650 FNA biopsies identified 4 morphologic patterns on ultrasound that predicted benign cytology with 100% specificity. The authors concluded that using ultrasound pattern to determine which patients require FNA could have obviated more than 60% of thyroid biopsies.5
Thyroid hormone suppression therapy risks hyperthyroidis
A systematic review of 9 RCTs of 18-month or shorter duration found that thyroid hormone suppression therapy reduced benign thyroid nodule volume (relative risk=1.88 compared with placebo or no treatment; 95% confidence interval [CI], 1.18-3.01; P=.008). The number needed to treat was 8 to reduce volume by >50% (risk difference=0.13; 95% CI, 0.06-0.19; P=.0003).6 However, thyroid hormone suppression therapy risks inducing hyperthyroidism and is not routinely recommended by the AACE or the ATA.2,3
Thyroidectomy: The treatment of choice
Thyroidectomy is the definitive therapy for MNG. A narrative review of 15 mostly retrospective cohort studies demonstrated MNG recurrence rates of 0% to 0.3% after total thyroidectomy, with follow-up intervals of 4.8 to 30 years.7
AACE consensus opinion recommends thyroidectomy for compressive symptoms, progressive growth, or when ultrasound or FNA results indicate thyroid cancer.2
A retrospective cohort study of 462 thyroidectomies for MNG found incidental thyroid carcinomas in 8.9% (41 patients). Risk factors included neck irradiation (odds ratio [OR]=21.64; 95% CI, 3.28-143), parenchymal calcifications on imaging (OR=2.30; 95% CI, 0.85-6.23), and family history of thyroid disease (OR=8.2; 95% CI, 2.15-29.87). Living in a goiter-endemic area was protective (OR=0.24; 95% CI, 0.07-0.83).8
Follow-up of patients with initial benign evaluation
Consensus opinion regarding follow-up of MNG is based on observational studies of the natural history of the condition. Benign MNG rarely progresses to malignancy. A review of 6 cohort studies, including 1265 patients with untreated nontoxic MNG who were followed for 60 to 130 months from 1990 to 2007, yielded an annual incidence range of 1.3 to 3.7 new cases of thyroid carcinoma per 1000 patients.9
Some goiters are more likely to enlarge. A retrospective cohort study of 488 patients treated surgically for MNG identified risk factors for enlargement: African American (OR=3.3; 95% CI, 2.0-5.4), age >40 years (OR=2.1; 95% CI, 1.2-3.8), and body mass index >30 (OR=2.5; 95% CI, 1.5-4.0).10
RECOMMENDATIONS
The AACE and the ATA recommend that patients with MNG with benign nodules have a repeat examination, TSH, and ultrasound in 6 to 18 months. Follow-up of stable nodules can then be done in 3 to 5 years.
An enlarging nodule requires repeat FNA.2 If palpation or ultrasound reveal evidence of nodule growth (more than a 50% change in volume or a 20% increase in at least 2 nodule dimensions, with a minimal increase of 2 mm in solid nodules or the solid portion of mixed cystic-solid nodules), the AACE and ATA recommend FNA, preferably with ultrasound guidance.3 Low TSH suggests autonomous nodules and the ATA recommends radionuclide scanning with FNA of hypofunctioning nodules with suspicious US features.3
IN THE ABSENCE OF OUTCOME STUDIES, experts recommend ultrasound evaluation of nontoxic mulinodular goiters (MNG) followed by fine-needle aspiration (FNA) of suspicious nodules (strength of recommendation [SOR]: C, consensus-based guidelines).
Thyroid hormone suppression therapy reduces the size of MNG (SOR: A, systematic review of randomized controlled trials [RCTs]), but it risks inducing hyperthyroidism (SOR: C, expert opinion).
Experts recommend thyroidectomy for compressive symptoms, progressive growth, or ultrasound or FNA results indicating thyroid cancer (SOR: C, consensus based guidelines).
Expert guidelines recommend repeat ultrasound at 6 to 18 months to follow up benign nodules or nonendemic MNG in patients at low risk of malignancy and subsequent follow-up of stable nodules every 3 to 5 years (SOR: C, consensus-based guidelines).
EVIDENCE SUMMARY
This summary updates the 2007 Clinical Inquiry, “What is the best approach to goiter for euthyroid patients?”1
Initial evaluation of palpable goiter with a normal thyrotropin
In the United States, MNG is generally nonendemic and unrelated to iodine deficiency, as distinguished from endemic goiter caused by iodine deficiency in other parts of the world.
Our structured search of the literature found no randomized trials or prospective cohort studies comparing diagnostic approaches. The American Association of Clinical Endocrinologists’ (AACE) 2010 guidelines and American Thyroid Association (ATA) guidelines recommend ultrasound for all MNG.2,3 The AACE guidelines recommend thyroid scintigraphy when clinicians suspect retrosternal MNG.2
Ultrasound findings can change management, avoid biopsy
In a retrospective analysis of 223 patients with nodular thyroid disease, thyroid ultrasound altered clinical management of 63% of patients with abnormal thyroid exams.4 A single center retrospective cohort study of 650 FNA biopsies identified 4 morphologic patterns on ultrasound that predicted benign cytology with 100% specificity. The authors concluded that using ultrasound pattern to determine which patients require FNA could have obviated more than 60% of thyroid biopsies.5
Thyroid hormone suppression therapy risks hyperthyroidis
A systematic review of 9 RCTs of 18-month or shorter duration found that thyroid hormone suppression therapy reduced benign thyroid nodule volume (relative risk=1.88 compared with placebo or no treatment; 95% confidence interval [CI], 1.18-3.01; P=.008). The number needed to treat was 8 to reduce volume by >50% (risk difference=0.13; 95% CI, 0.06-0.19; P=.0003).6 However, thyroid hormone suppression therapy risks inducing hyperthyroidism and is not routinely recommended by the AACE or the ATA.2,3
Thyroidectomy: The treatment of choice
Thyroidectomy is the definitive therapy for MNG. A narrative review of 15 mostly retrospective cohort studies demonstrated MNG recurrence rates of 0% to 0.3% after total thyroidectomy, with follow-up intervals of 4.8 to 30 years.7
AACE consensus opinion recommends thyroidectomy for compressive symptoms, progressive growth, or when ultrasound or FNA results indicate thyroid cancer.2
A retrospective cohort study of 462 thyroidectomies for MNG found incidental thyroid carcinomas in 8.9% (41 patients). Risk factors included neck irradiation (odds ratio [OR]=21.64; 95% CI, 3.28-143), parenchymal calcifications on imaging (OR=2.30; 95% CI, 0.85-6.23), and family history of thyroid disease (OR=8.2; 95% CI, 2.15-29.87). Living in a goiter-endemic area was protective (OR=0.24; 95% CI, 0.07-0.83).8
Follow-up of patients with initial benign evaluation
Consensus opinion regarding follow-up of MNG is based on observational studies of the natural history of the condition. Benign MNG rarely progresses to malignancy. A review of 6 cohort studies, including 1265 patients with untreated nontoxic MNG who were followed for 60 to 130 months from 1990 to 2007, yielded an annual incidence range of 1.3 to 3.7 new cases of thyroid carcinoma per 1000 patients.9
Some goiters are more likely to enlarge. A retrospective cohort study of 488 patients treated surgically for MNG identified risk factors for enlargement: African American (OR=3.3; 95% CI, 2.0-5.4), age >40 years (OR=2.1; 95% CI, 1.2-3.8), and body mass index >30 (OR=2.5; 95% CI, 1.5-4.0).10
RECOMMENDATIONS
The AACE and the ATA recommend that patients with MNG with benign nodules have a repeat examination, TSH, and ultrasound in 6 to 18 months. Follow-up of stable nodules can then be done in 3 to 5 years.
An enlarging nodule requires repeat FNA.2 If palpation or ultrasound reveal evidence of nodule growth (more than a 50% change in volume or a 20% increase in at least 2 nodule dimensions, with a minimal increase of 2 mm in solid nodules or the solid portion of mixed cystic-solid nodules), the AACE and ATA recommend FNA, preferably with ultrasound guidance.3 Low TSH suggests autonomous nodules and the ATA recommends radionuclide scanning with FNA of hypofunctioning nodules with suspicious US features.3
1. Hoffman MR, Meadows SE, Langlois JP. Clinical inquiries. What is the best approach to goiter for euthyroid patients? J Fam Pract. 2007;56:479-480.
2. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract. 2010;16(suppl 1):S1-S43.
3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
4. Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696-700.
5. Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207-213.
6. Sdano MT, Falciglia M, Welge JA, et al. Efficacy of thyroid hormone suppression for benign thyroid nodules: metaanalysis of randomized trials. Otolaryngol Head Neck Surg. 2005;133:391-396.
7. Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32:1301-1312.
8. Botrugno I, Lovisetto F, Cobianchi L, et al. Incidental carcinoma in multinodular goiter: risk factors. Am Surg. 2011;77:1553-1558.
9. Winbladh A, Järhult J. Fate of the non-operated, non-toxic goiter in a defined population. Brit J Surg. 2008;95:338-343.
10. Phitayakorn R, Super DM, McHenry CR. An investigation of epidemiologic factors associated with large nodular goiter. J Surg Res. 2006;133:16-21.
1. Hoffman MR, Meadows SE, Langlois JP. Clinical inquiries. What is the best approach to goiter for euthyroid patients? J Fam Pract. 2007;56:479-480.
2. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Pract. 2010;16(suppl 1):S1-S43.
3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
4. Marqusee E, Benson CB, Frates MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696-700.
5. Bonavita JA, Mayo J, Babb J, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207-213.
6. Sdano MT, Falciglia M, Welge JA, et al. Efficacy of thyroid hormone suppression for benign thyroid nodules: metaanalysis of randomized trials. Otolaryngol Head Neck Surg. 2005;133:391-396.
7. Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32:1301-1312.
8. Botrugno I, Lovisetto F, Cobianchi L, et al. Incidental carcinoma in multinodular goiter: risk factors. Am Surg. 2011;77:1553-1558.
9. Winbladh A, Järhult J. Fate of the non-operated, non-toxic goiter in a defined population. Brit J Surg. 2008;95:338-343.
10. Phitayakorn R, Super DM, McHenry CR. An investigation of epidemiologic factors associated with large nodular goiter. J Surg Res. 2006;133:16-21.
Evidence-based answers from the Family Physicians Inquiries Network
Neuroendocrine dysfunction following mild TBI: When to screen for it
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
› Consider neuroendocrine dysfunction (NED) following confirmed traumatic brain injury of any severity when symptoms suggestive of NED persist for >3 months after injury. A
› Order blood studies to detect deficiencies in pituitary and other key hormones when NED is suspected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Centers for Disease Control and Prevention (CDC) reports that >1.7 million cases of traumatic brain injury (TBI) occur annually in the United States.1 More than 266,000 military service members sustained at least one TBI from 2000 to 2012.2 Most TBIs (80%-85%), military and civilian, are classified as mild (mTBI), and most mTBI patients (80%-85%) experience a complete functional recovery within 3 months of injury.1,3 The remaining 15% to 20% of mTBI patients experience persistent symptoms and difficulty in rehabilitation, particularly if there are concomitant disorders, such as post-traumatic stress disorder (PTSD), sleep disorders, acute stress disorder, substance abuse disorder, and depression.4,5 Symptoms that mTBI and these other disorders have in common can make differential diagnosis difficult, requiring a high degree of clinical awareness by primary care providers. An additional concern following mTBI is neuroendocrine dysfunction (NED). This association has not been widely discussed and therefore may go largely undiagnosed.6 Consider NED in the setting of prolonged symptoms or in patients experiencing difficulty with rehabilitation following mTBI.7,8
NED following mTBI is more common than once thought
The term “neuroendocrine dysfunction,” as discussed in this article, refers to a variety of conditions caused by imbalances in the body’s hormone production directly related to the pituitary, hypothalamus, and their axes following TBI. Until the past decade, the incidence of TBI-associated pituitary dysfunction was thought to be an uncommon event, usually associated with catastrophic head injuries. Studies of NED in TBI patients focused primarily on moderate or severe TBI, usually from motor vehicle incidents, falls, and assaults.7 Other research has since shown that NED occurs more commonly than once believed.9 And while the risk of NED may be higher for patients who sustain more severe brain injuries, NED also occurs in mTBI.7,9,10,11 Interestingly, a recent literature review indicated that the incidence of NED in mTBI was 16.8%, while the incidence with moderate TBI was reported at 10.9%.7 Other research has noted that the incidence of NED in mTBI may be as high as 42%.9,12 No evidence suggests that the severity of NED is related to a specific hormonal dysfunction, nor is there evidence that NED may be associated with a specific mechanism of injury.
Pituitary anatomy is susceptible to injury and dysfunction
The anatomic and physiologic complexities of the hypothalamus and pituitary gland increase their susceptibility to injury from TBI. The pituitary gland is connected to the hypothalamus by a blood vessel-containing stalk, making the pituitary gland—particularly the anterior portion—susceptible to damage during a head injury.13 The hypothalamus secretes thyrotropin-releasing hormone (TRH) and luteinizing-releasing hormone (LRH) to stimulate or suppress the production of anterior pituitary gland hormones, which in turn stimulate the release of hormones and other substances from target organs. Anterior pituitary hormones are growth hormone (GH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), and prolactin (PRL). The posterior pituitary secretes oxytocin and vasopressin, also known as antidiuretic hormone (ADH).13
Impact from a direct blow with an object or from a concussive blast can cause focal trauma or rotational shearing of tissue internally. Resultant vascular injury, rupture, cerebral edema, vasospasm, pituitary swelling, or inflammation may then initiate an endocrine response that drives a cascade of complex hormonal processes.5,7,8 Anterior pituitary deficiencies account for the majority of chronic neuroendocrine disorders following mTBI. GH and gonadotropin deficiencies are the most common, but TSH deficiency (secondary hypothyroidism) and ACTH deficiency (adrenal insufficiency) may occur as well, although in <10% of cases with TBI associated NED.12
Clinical features of NED mimic those of other conditions
The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances (TABLE).7,12,14-17 Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, postconcussive syndrome (PCS), and memory and attention difficulties.18 The onset of NED may be immediate (eg, in diabetes insipidus [DI] or syndrome of inappropriate antidiuretic hormone [SIADH], which are very rare in mTBI) and potentially life-threatening (eg, in sodium and potassium imbalances), or may be nonspecific and take years to manifest.6,10,15,19 Additionally, symptoms of NED may spontaneously resolve or persist. Studies have demonstrated pituitary dysfunction in the acute postinjury phase as well as its development as late as 2 to 3 years after injury.7,8,11,20
Due to the range of symptoms related to the combinations of possible hormonal derangements, NED can be an elusive diagnosis and may have a deleterious effect on individuals who sustain TBI.12 For example, an undiagnosed GH deficiency—which can result in increased abdominal fat mass and decreased lean muscle mass as well as impaired cardiac function, dyslipidemia, and insulin resistance—makes it more difficult for an affected individual either to recover from additional injuries or to maintain fitness. Considering NED may avoid a delay in diagnosis and improve prognosis.7,8,20
Findings leading to recommendations on diagnosis
Primary care providers, military and civilian alike, can benefit from the findings and recommendations of an expert panel assembled by the Defense Centers of Excellence (DCoE) for Psychological Health and Traumatic Brain Injury to address NED in mTBI. The panel that convened in December 2010 included experts representing the military services, the Department of Veterans Affairs, DCoE, and civilian sectors. Based on the group’s recommendations combined with literature review findings, the DCoE developed a clinical recommendation to encourage primary care providers to consider screening for NED in patients with persistent symptoms following mTBI.21 Key findings and issues identified by the group included the following:
• The most frequent mechanism of injury in the military deployed population is blast-induced TBI. Such injury could occur in the civilian population at construction blast sites or in factories producing or using highly flammable substances.
• The prevalence of any anterior pituitary hormone deficiency is as high as 30% to hormone deficiency is as high as 30% to 80% at 24 to 36 months post injury.
• The prevalence of posterior pituitary hormone deficiency is as high as 4% to 7% at 12 months post injury.
• The anterior pituitary hormones most frequently affected in survivors of TBI are ACTH, gonadotropin, prolactin, and GH.
• In 2004 Agha et al,22 reported >28% of survivors of TBI had at least one anterior pituitary hormone deficiency.
• According to research by Agha et al23 in 2005, >20% of survivors of TBI developed DI; those who developed DI, either acutely or permanently, were more likely to have sustained a severe TBI.
• The development of pituitary dysfunction is independent of the severity of TBI.
• In 2005, civilian guidelines4 recommended screening for pituitary dysfunction in all patients who sustained a moderate to severe TBI.
• In 2010, civilian guidelines7 recommended screening for pituitary dysfunction in patients who sustained a mild TBI.
When to screen for NED after TBI
Given the complexities described—including the similarity of NED to other post-mTBI medical diagnoses and such concomitant disorders as a sleep disorder, memory difficulties, depression, PTSD, and PCS24—consider NED in the primary care setting following confirmed TBI of any severity level when symptoms suggestive of NED persist for >3 months following injury or appear up to 36 months later.7,8,12,20
Order a lab evaluation of blood levels for cortisol (drawn at 8 am), LH, FSH, PRL, insulin-like growth factor-1, TSH, free thyroxine-4, and testosterone for men (8 am) and estradiol for women (8 am). With frankly abnormal lab results or with borderline results and strong clinical suspicion for NED, refer for further endocrinology workup (FIGURE).21 Earlier diagnosis of NED results in more rapid improvement of symptoms and an improved prognosis.7,8,20 Postinjury screening for NED should be one component of a thorough clinical evaluation by a qualified provider, and not used in isolation for clinical decision making. NED screening should not be routinely ordered during the early stages of mTBI, defined as <3 months postinjury.
Provider awareness and willingness to include NED screening in a timely manner, and to refer to specialty services as indicated for symptoms that may be sleep related or psychiatric in nature, may increase the opportunities for early treatment, better rehabilitation outcomes, and better overall quality of life.
Looking ahead
While the DCoE expert group made recommendations on screening for NED in the military combat population, they also acknowledged that NED diagnosis and treatment would benefit from additional areas of research:
• the effect of GH replacement (for GH-deficient patients or as prophylaxis for all TBI patients) on rehabilitation response and quality of life
• the role of multiple TBIs on long-term cognition and possible premature aging
• the role of NED over time
• biomarkers for diagnosis
• factors affecting resiliency
• resiliency in the context of increased or decreased susceptibility to the development of an acute clinical syndrome, as well as susceptibility in developing the spectrum of consequences of TBI.
The research areas given the highest priority by the group were incidence and prevalence studies of pituitary dysfunction after TBI in the combat military population, including pre- and postdeployment rates of dysfunction and the incidence of comorbidities. Also of benefit would be a retrospective study of the consequences of pituitary dysfunction that additionally addresses the effects of comorbid conditions commonly associated with TBI. Considering the rapid expansion in the field of mTBI, additional research and provider awareness concerning early identification and treatments may improve the outcomes for those with persistent mTBI symptoms.
CORRESPONDENCE
Theres A. West, DNP, APN, BC, Defense and Veterans Brain Injury Center, 1335 East West Highway, 6th floor, Silver Spring, MD 20910; [email protected]
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
1. Injury prevention & control: Traumatic brain injury national TBI estimates. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/traumaticbraininjury/statistics.html. Accessed August 12, 2013.
2. Armed Forces Surveillance Center. DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Centers Web site. Available at: www.dvbic.org/dod-worldwide-Numbers-tbi. Accessed August 12, 2013.
3. Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury (mTBI). Available at: http://www.healthquality.va.gov/management_of_concussion_mtbi.asp. Published April 2009. Accessed August 12, 2013.
4. Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711-724.
5. Krahulik D, Zapletalova J, Frysak Z, et al. Dysfunction of hypothalamic-hyperphysical axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581-584.
6. Behan LA, Phillips J, Thompson CJ, et al. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79:753-759.
7. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary. 2010;13:146-153.
8. Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679-691.
9. Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-hormone abnormalities after blast related mild traumatic brain injury. Front Neurol. 2012;3:11.
10. Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687-1697.
11. Benvenga S, Campenmi A, Ruggeri R, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353-1361.
12. Schneider H, Kreitschman-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429-1438.
13. Amar AP, Weiss MH. Pituitary anatomy and physiology. Neurosurg Clin N Am. 2003;14:11-23.
14. Tanriverdi F, Unluhizarci K, Kocyigit I, et al. Brief communication: Pituitary volume and function in competing and retired male boxers. Ann Intern Med. 2008;148:827-831.
15. Bondanelli M, De Marinis L, Ambrosio MR, et al. Occurrence of pituitary dysfunction following traumatic brain injury. J Neurotrauma. 2004;21:685-696.
16. Klose M, Watt T, Brennum J, et al. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life in 12 months after injury. J Clin Endocrinol Metab. 2007;92:3861-3868.
17. Aimeretti G, Ambrosio MR, Di Somma C, et al. Residual pituitary function after brain injury-induced hypopituitaryism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085-6092.
18. Agha A, Phillips J, Thompson CJ. Hypopituitarism following traumatic brain injury. Br J Neurosurg. 2007;21:210-216.
19. Cohan P, Wang C, McArthur DL, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 2005;33:2358-2366.
20. Rothman MS, Arciniegas DS, Filley CM, et al. The neuroendocrine effects of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007;19:363-372.
21. Defense Centers of Excellence. Neuroendocrine screening post mild TBI clinical recommendation. Available at: http://www.dcoe.mil/Content/Navigation/Documents/DCoE_TBI_NED_Reference_Card.pdf. Published February 2012. Accessed August 12, 2013.
22. Agha A, Rogers B, Mylotte D, et al. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf). 2004;60:584-591.
23. Agha A, Phillips J, O’Kelly P, et al. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med. 2005;118:1416.
24. Guerrero AF, Alfonso A. Traumatic brain injury related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574-580.
Obesity in the elderly: More complicated than you think
Should older obese people try to lose weight? Such a simple question is more complicated than one would think.
At issue is whether obesity is harmful in older people, and whether treating it will reduce their health risks. True, obesity is an independent risk factor for cardiovascular disease and is associated with many comorbidities, including type 2 diabetes mellitus, hyperlipidemia, heart failure, and hypertension.1 An independent association also exists between obesity and all-cause mortality.2 However, there is also evidence suggesting that obesity in this age group is associated with a lower, not higher, risk of death—a finding termed the obesity paradox.3 And for that matter, what exactly constitutes obesity in elderly people, who naturally undergo changes in body composition as they age?
This article examines the literature on these controversial issues, including changes in body composition with age, the definition of obesity in older adults, the obesity paradox, and treatment of obesity in older adults.
AMERICANS ARE GETTING OLDER—AND BIGGER
Americans are living longer than ever before; life expectancy has reached a new high of 77.8 years.4,5 According to the US Census Bureau,6 about 27 million people in the United States are over age 70, and this number is expected to nearly double by 2030.
Meanwhile, the prevalence of obesity, defined as a body mass index (BMI) of 30 kg/m2 or higher, has increased in the last 25 years in all age groups in the United States, including those age 65 and older.7,8 These two trends add up to an increase in the number of obese older people. In 2000, 22.9% of people age 60 to 69 and 15.5% of those over age 70 and older were obese.9 This amounted to a 56% increase in the former group and a 36% increase in the latter group in the interval since 1991.5,9
BUT WHAT CONSTITUTES ‘OBESITY’?
Obesity is the excess accumulation of body fat, leading to a higher risk of medical illness and premature death. But measuring it is not as simple as one might think.
The body mass index can mislead
The BMI, ie, weight in kilograms divided by the square of the height in meters, correlates fairly well with body fat stores and is generally used to classify medical risk.
However, the BMI can classify some older people as overweight (BMI 30.0–34.9 kg/m2) or obese (BMI ≥ 35.0 kg/m2) who actually do not have an excess of body fat—and can fail to classify others as overweight or obese who do. For example, if a person loses height as a result of vertebral compression fractures, his or her BMI would become higher, even with no change in weight or body fat. Conversely, changes in body composition with age, including loss of muscle and an increase in fat, may not be reflected in the BMI, even if the person really does have too much body fat.10
This second limitation of the BMI is important when estimating risk in older adults, who have a particular fat distribution. Visceral, subcutaneous, intramuscular, and intrahepatic fat increase with age, and they are all risk factors for insulin resistance and type 2 diabetes mellitus.11 And in older people, having too much visceral fat is more prevalent than the BMI might predict.10
Percent body fat awaits investigation
Percent body fat is another way to assess body fat. Defined as the total weight of fat divided by total weight, it is measured in various ways.
Dual-energy x-ray absorptiometry, computed tomography, and magnetic resonance imaging can measure percent body fat, and they can differentiate visceral from subcutaneous fat (which is less metabolically active). Unfortunately, most of these tests are used for this purpose only in research, and they are relatively expensive.
Commercially available bioelectrical impedance devices send a weak electric current through the body and measure the resistance, and using this information and four other factors (height, weight, age, and sex), they calculate percent body fat. This method is fast, easy, painless, and cheap. A disadvantage is that the handheld devices measure body composition of the upper body only. Because the lower body is excluded, they do not give an accurate measurement of body fat of the abdomen and hips. Also, they cannot differentiate visceral from subcutaneous fat.
Bioelectrical impedance devices work well in healthy individuals with stable water balance. The values are only an estimate of fat-free mass, and therefore this method is not the gold standard for assessing body fat. Bioelectrical impedance is better at tracking body composition in an individual over time than at diagnosing obesity.
Percent body fat can vary by sex and race. Asians, for example, have higher percent body fats at lower BMIs, particularly when younger.12 Also, Gallagher et al12 found that percent body fat increased with age at every given BMI in both men and women (Table 1).
The traditional universal cutoffs for defining obesity by percent body fat are 25% in men and 35% in women. However, research has indicated that cutoffs of 20% to 25% in men and 30% to 38% in women may better identify those at risk of metabolic disease.13 Guidelines and evidence-based cutoffs for percent body fat must await further investigation.
Waist circumference is useful
In older adults, obesity can be diagnosed by a measurement such as waist circumference, which correlates highly with total fat and intra-abdominal fat.14 It is very cost-effective, simple, and useful for the office assessment of adiposity.
The measurement should be made halfway between the iliac crest and the lower anterior ribs, with the patient standing, and at the end of expiration.
The traditional standard for waist circumference is less than 89 cm (35 inches) for women and 102 cm (40 inches) for men. However, opinion differs, and different reference ranges exist depending on ethnicity. Additionally, because stature and body composition change with age, concerns have been raised about misclassification of the health risks related to obesity in older adults using the current standard.15,16
The waist circumference is as good as or even better than the BMI as a measure of excess adiposity in older adults.16–18 This is in part because of the age-dependent height decrease in older adults.15,19 (Recall that the BMI is calculated using the height squared as the denominator; as a result, the BMI would give a higher reading and thus an overestimate of total body fat.) Conversely, we can underestimate the amount of adiposity because of decreases in abdominal muscle tone.17
Cutoffs for waist circumference should be age-specific.16
Investigators in the Netherlands15,16 prospectively took 4,996 measurements in 2,232 people with a mean age of 70, from 1992 through 2006. They concluded that the best cutoffs for predicting the health risks of obesity in the elderly were 109 cm (43 inches) in men and 98 cm (39 inches) in women.
A group of researchers has proposed that the cutoffs be shifted upward in older adults, with new values for those age 70 and over.20 The Health Survey for England aimed to describe the patterns and trends in waist circumference and abdominal obesity and overweight in people age 70 through 89, comparing both the standard and the new cutoffs. Optimal cutoffs recommended for abdominal obesity for patients age 70 and older were 100 to 106 cm in men and 99 cm in women.20 Estimates of the prevalence of abdominal obesity are much lower using the new cutoffs.
SARCOPENIA: LOSS OF MUSCLE WITH AGE
With age comes sarcopenia—the progressive loss of muscle mass, primarily skeletal muscle, resulting in a decrease in strength and power.21 The process begins as early as the 20s or 30s.22 It is distinct from wasting (involuntary weight loss from inadequate intake), seen in starvation.21
Sarcopenia is defined as an appendicular skeletal muscle mass index (the appendicular skeletal mass divided by the square of the height in meters) of less than 2 standard deviations below a young adult reference, and a percentage of body fat over the 60th percentile for the individual’s sex and age.23,24 Estimates of its prevalence vary, but it is common and it increases with age.14,20
Sarcopenic obesity: Less muscle, more fat
Progressive loss of skeletal muscle with age, along with an increase and redistribution of body fat, is known as sarcopenic obesity.25 It is associated with higher morbidity and mortality rates as well as a decline in functional strength, which leads to frailty.23 This loss of muscle mass may go unnoticed in an older person until he or she begins to lose physical function.
As noted, in an older person with sarcopenic obesity, the BMI may mislead because of the high percentage of fat and the low lean mass.26
Why we change with age
This change in body composition with age is a result of several factors. Illness or inactivity can lead to loss of muscle, while body fat is preserved.17 The combination of reduced physical activity, a lower resting metabolic rate, and an unchanged intake of food can increase the likelihood of sarcopenia.27 Also possibly contributing are hormonal changes, including reduced production of growth hormone and testosterone and decreased responsiveness to thyroid hormone and leptin.28
Moreover, the interaction of several factors can lead to a vicious circle of progressive loss of muscle and increase in fat. As people age, their physical activity tends to decrease, resulting in muscle loss. As muscle mass decreases, the amount of available insulin-responsive tissue is reduced, resulting in insulin resistance, which in turn promotes the metabolic syndrome and an increase in fat. With more fat, people produce more of the adipokines tumor necrosis factor alpha and interleukin 6, which further promote insulin resistance.
Other changes contribute to a decrease in muscle quality and performance, including an increase in intramuscular and intrahepatic fat, which is associated with insulin resistance.11 The increases in adipose stores occur mostly in intra-abdominal fat rather than in subcutaneous fat.
ADVERSE EFFECTS OF OBESITY
A number of comorbidities arise with obesity, regardless of age.19
The diseases most strongly associated with obesity are the metabolic syndrome and type 2 diabetes mellitus.17 Studies have shown that in older adults, obesity as measured by waist circumference is associated with hyperglycemia and dyslipidemia.29,30
Metabolic abnormalities may ensue in obese older people through complex mechanisms involving an age-related decline in sex hormones. For example, late-onset hypogonadism in men, which is more common in those who are obese, is related to the metabolic syndrome.29
These mechanisms are also complex in women. Because estrogens can be produced in adipose tissue, obese postmenopausal women have higher concentrations of estrogens than their lean counterparts, and this may lead to metabolic abnormalities.31 (On the other hand, higher estrogen levels in obese menopausal women can protect against osteoporosis by increasing bone mass.)
Older people who weigh more and have more adipose tissue, especially those who became obese at a young age, have a greater risk of osteoarthritis of the knee,32,33 which when combined with obesity can cause disability and physical impairment.19 And cardiovascular risk factors,18,33 hypertension,34 and certain cancers35 are more common in older people with higher waist circumference.
THE OBESITY PARADOX
In general, obesity in younger adults has been shown to shorten life expectancy. This risk of death is often associated with obesity-related health problems.
In older people, the effect of obesity is much more complex.36 The optimal weight in terms of survival increases with age. More interesting is the finding that although the risk of cardiovascular disease is higher in overweight or obese older adults, studies also suggest that in this age group, being overweight or obese is paradoxically associated with lower mortality rates from these diseases.26 This phenomenon is called the obesity paradox.37
For those over age 75, the relative risk of death from all causes and from cardiovascular disease has been found to decrease with increasing BMI.25 The relationship between BMI and death from all causes in older adults may actually be a U-shaped curve, meaning that the risk of death rises at both extremes of BMI values.26
Possible explanations for the paradox
Several hypotheses have been proposed to explain the change in the relationship between BMI and the risk of death that occurs with aging.
The BMI is an imperfect measure of obesity. The obesity paradox may be an artifact of using the BMI to measure obesity in older adults.17 As described above, sarcopenic obesity must be considered in those over age 65 because the BMI does not differentiate between fat and muscle. Older adults tend to have a higher proportion of body and visceral fat that is distributed differently, making the waist circumference or waist-hip ratio a more appropriate measure of obesity in this group.38 Janssen et al39 found that in people age 65 and older, after controlling for waist circumference, higher BMI values were associated with lower death rates; after controlling for BMI, waist circumference was associated with a higher risk of death.
The survival effect suggests that people who are susceptible to the negative effects of obesity die sooner,40 and those who survive until old age may be resistant to the effects of obesity.41 If true, the survival effect would explain why the death rate seems to be unaffected by BMI in the older population.
Unhealthy weight loss. Smoking and diseases such as cancer that can cause early death may also induce weight loss, further complicating the relationship between BMI and death.19 After age 80, the association between BMI and the risk of death is weak because those with a low BMI include not only those who have always been lean and physically active, but also those who lost weight through chronic ill health or smoking.17
Further study needed. Thus, a number of confounding variables may muddy the association between obesity and death in older adults. Obesity should not be misinterpreted as being harmless or beneficial in older adults. Stevens et al36 found that a greater BMI was associated with a higher rate of death from all causes and from cardiovascular disease in men and women up to age 75, but that the relative risk of death associated with a greater BMI decreased with age.
Optimal BMI targets in older people have yet to be validated in a large prospective trial. However, multiple studies have examined the relationship between BMI and all-cause mortality in older adults and have identified a BMI of 24 to 35 as “ideal” and associated with the lowest risk of death, with a lower range for men and a higher range for women.42,43 The topic has been reviewed by Oreopoulos et al.26 More research is needed to evaluate this relationship.
THE BENEFIT OF WEIGHT LOSS IN OLDER ADULTS IS CONTROVERSIAL
In younger obese people, weight loss brings a multitude of benefits by reducing the risk of complications arising from obesity. However, in older adults, the effects of weight loss remain controversial, and evidence to guide treatment is limited.44,45 The few trials that have been published have typically focused on cardiovascular risk factors rather than physical function.45
In a 1-year trial, 107 people age 65 or older were randomized to a control group, to weight management, to exercise, or to weight management plus exercise. The combination of weight loss and exercise yielded the greatest improvement in physical function.46
Intentional vs unintentional weight loss
Intentional weight loss is altogether different from unintentional weight loss.
In most cases, weight loss in older adults is unintentional and may indicate underlying disease and impending death.17 For example, older men who lose weight unintentionally have significantly greater rates of smoking, disability, cancer, and respiratory disease and less obesity and physical activity than those who lose weight intentionally.47
Studies have shown an increase in life expectancy in older patients with type 2 diabetes mellitus who lost weight intentionally.48,49 In fact, moderate weight loss—just 5% to 10%—has been shown to improve cardiovascular risk factors,44 osteoarthritis, and type 2 diabetes.50
Bales and Buhr44 performed a systematic review of 16 studies that had lasted at least 6 months. Patients were age 60 or older with a minimum baseline BMI of 27 kg/m2 who intentionally lost at least 3% of body weight or 2 kg. Levels of the inflammatory markers C-reactive protein, tumor necrosis factor alpha, and interleukin 6 declined with weight, along with blood pressure, fasting glucose, waist circumference, and low-density lipoprotein cholesterol. On the downside, bone mineral density and lean body mass also declined slightly. The best way to avoid losing lean body mass and to preserve bone density during weight loss is to include a program of resistance-training exercises.
No clinical trial has evaluated the effects of intentional weight loss on death rates in older obese people.25 As a result, evidence-based recommendations cannot be made. Rather, advice on weight loss must be individualized, with special emphasis on the patient’s weight history and medical comorbidities.44
Oreopoulos et al26 summarized the possible effects of BMI, abdominal fat, lean body mass, and intentional weight loss on morbidity and mortality outcomes in older adults (Table 2).
TREATMENT GUIDELINES AND RECOMMENDATIONS
Many of the methods of weight management in older adults are the same as in young and middle-aged adults.51 Recommendations for all age groups include lifestyle changes, increased activity, dietary changes, drug therapy, and bariatric surgery.
Whether there should be separate guidelines for older adults is controversial. In view of the obesity paradox, physicians have been reluctant to recommend weight loss in elderly patients. Caution is advised in recommending weight loss solely on the basis of body weight, as studies have shown that the weight associated with maximal survival increases with age. Because of age-related changes in body composition and reduced energy requirements and expenditure, recommendations for the young and middle-aged should not be applied directly to older adults.
In this group, especially those who have survived into old age with good health and an intact functional status, one could argue that significant caloric restriction should not be recommended. In these people, the goal is often to maintain weight and incorporate a daily exercise program rather than to aggressively lose weight. Adding resistance training can improve physical function, which can improve quality of life. There is less emphasis on cardiovascular risk, but both outcomes apply for both age groups.52
Intentional weight loss should be recommended to high-risk older adults, including those with cardiovascular disease, type 2 diabetes mellitus, and metabolic syndrome, because the absolute risk of death and morbidity is higher in this group. Most health benefits can be achieved with modest weight loss.53 Potential benefits include prevention of cognitive impairment, protection from bone fractures, an increase in antioxidant defense, a reserve of fat and energy stores, and an increase in longevity.26
Treatment differs from that in the younger population primarily because of the importance of preventing loss of muscle with intentional weight loss. People of all ages who lose weight intentionally lose fat and, to a lesser extent, skeletal muscle. Older patients have already lost muscle mass, but further changes in body composition, especially a further reduction in muscle mass, can be limited by consuming about 1.0 g/kg of high-quality protein in the diet and by engaging in resistance training and weight training.52
Improving quality of life and physical function are important goals. Information is emerging about when obesity needs to be managed in older adults. There is also evidence to support dietary and exercise therapy.54 Weight-loss options include lifestyle interventions, pharmacotherapy, and bariatric surgery.
Lifestyle interventions: Diet and exercise
The goal is to induce an energy deficit by reducing energy intake, increasing energy expenditure, or both—by 500 to 1,000 calories a day. This generally leads to a loss of 1 to 2 lb per week, and possibly up to 10% of weight in 6 months. Loss of about 10 to 20 lb with diet and exercise can translate to a relatively large reduction in visceral fat, with subsequent improvement in metabolic abnormalities.
A regular exercise program is important for improving overall physical function, which can slow progression to frailty. Adding aerobic, endurance, and resistance training helps preserve fat-free mass, which otherwise tends to diminish during active weight loss.55–57
The exercise program should begin at the outset of the weight-loss effort to help maintain weight loss and to prevent weight regain.58 Exercise is not essential for reaching the targeted weight loss, but starting early is important to reduce the loss of lean muscle that is usually already seen in the older population.
Several studies indicate that diet and exercise are just as effective in middle-aged and older people (over age 60) as in the younger population.58–60 Older people in the Diabetes Prevention Program were more compliant with lifestyle interventions and lost more weight than younger participants49: 60% of the older group met the 7% weight-loss goal at the end of 24 weeks, compared with 43% of those under age 45. At 3 years, the numbers were 63% vs 27%.
In a small randomized controlled trial,61 fat mass decreased by 6.6 kg in 17 people assigned to a program of diet and exercise, compared with a gain of 1.7 kg in a control group of 10 patients. Fat-free mass decreased by about 1 kg in both groups. The authors concluded that diet plus exercise (resistance training and strength training in this trial) could ameliorate frailty in obese older adults.
If exercise is appropriate, a physician should write a prescription for it, especially for resistance training, strengthening, flexibility, and stretching. This is important for patients with sarcopenic obesity and for those at high risk of chronic bone loss. The 2007 American College of Sports Medicine guidelines recommend muscle-strengthening activity of 8 to 10 exercises involving the major muscle groups, 10 to 15 repetitions at least twice a week. Flexibility and balance exercises should be included for those at risk of falls.62
Pharmacotherapy
At present, there are two general classes of weight-loss drugs: appetite suppressants and drugs that interfere with nutrient absorption.
Appetite suppressants include the sympathomimetics, which stimulate the release of dopamine and norepinephrine, resulting in increased satiety. Data—and therefore, recommendations—on their use in the elderly are very scarce, as most randomized controlled trials included only a small number of older people. A meta-analysis of drug therapy to treat obesity noted that the study population ranged in age from 34 to 54.63
The only approved drug currently available for use in older adults is orlistat, which blocks absorption of dietary fat by binding to intestinal lipase. A randomized controlled trial found the weight loss with orlistat to be comparable in older and younger adults.64,65
Review medications than can cause weight gain
When assessing older adults, always review the drugs they are taking. Those known to cause weight gain include certain of the following:
- Antiepileptics (eg, gabapentin)
- Antipsychotics (eg, olanzapine)
- Antidepressants (eg, tricyclics)
- Antihyperglycemic drugs (eg, sulfonylureas, thiazolidinediones)
- Beta-blockers
- Steroids.
If medically appropriate, a weight-neutral drug should be substituted for one suspected of causing weight gain. If a different physician (eg, a specialist) prescribed the original drug, he or she should be notified or consulted about any change.
Bariatric surgery
Bariatric surgery is the most effective weight-loss option, and more older patients are undergoing it than in the past. Dorman et al66 showed that the number of patients age 65 or older undergoing bariatric surgery increased from the year 2005 (when they accounted for 2%) to 2009 (when they accounted for 4.8%).
However, very few studies have provided information on the safety and effectiveness of bariatric surgery in older people. Several reports concluded that rates of perioperative morbidity and mortality are higher in older patients.67–69 Surgery resulted in marked weight loss and improvement in obesity-related complications and physical disability in older patients, although by a lower rate than in younger patients.
Varela et al70 examined the outcomes of bariatric surgery in a database from the University Health System Consortium Centers between 1999 and 2005. Patients over age 60 accounted for 1,339 (2.7%) of all bariatric operations performed. Compared with young and middle-aged patients, older patients had more comorbidities, longer hospital stays, and more complications, in addition to a higher in-hospital mortality rate. When risk-adjusted, the observed-to-expected mortality ratio for the older group was 0.9, compared with 0.7 in the young and middle-aged cohort.
Willkomm et al71 found an apparently higher operative risk profile in those over age 65 (n = 100) than in younger patients (n = 1,374), with higher rates of sleep apnea, diabetes, and hypertension. However, the operative outcomes were similar in the two groups in terms of operative time, length of stay, and 30-day readmission rates. The authors concluded that patients over age 65 had excellent outcomes compared with younger patients, suggesting that older age is not a risk factor for complications or death with bariatric surgery.
The American College of Surgeons National Surgical Quality Improvement Program evaluated the outcomes of 48,378 adults with a BMI greater than or equal to 35 kg/m2 who underwent bariatric surgery between 2005 and 2009.66 During this time, the number of patients age 65 and older seeking bariatric surgery increased from 1.5% to 4%. A total of 1,449 patients were in this age range. Thirty-day mortality rates did not differ significantly by age group and were less than 1% for all age ranges. Being age 65 or older was a significant predictor of prolonged length of stay but not of major adverse events. Significant predictors of major adverse events were a BMI greater than or equal to 55 kg/m2, cardiac comorbidities, a severe American Society of Anesthesiologists score, albumin levels lower than 3 g/dL, and creatinine levels greater than 1.5 mg/dL.
The most up-to-date study of the outcomes of bariatric surgery in patients over age 70 was a retrospective review at a single institution from 2007 to 2008 of 42 patients who underwent bariatric surgery.72 Twenty-two patients had laparoscopic gastric banding, 12 had laparoscopic sleeve gastrectomy, and 8 underwent laparoscopic Roux-en-Y gastric bypass. No patient died, complications occurred in 9 patients, and the rates of postoperative use of medications for hypertension, hyperlipidemia, diabetes, and osteoarthritis were reduced by about half. With the increasing number of patients seeking bariatric surgery, especially those over age 70, further prospective studies will determine if the outcomes are statistically significant.
If bariatric surgery is considered
The outcomes, complications, and mortality rates associated with bariatric surgery have been shown to be acceptable for adults age 65 and older. Perioperative risk assessment in the older obese patient seeking bariatric surgery is paramount to ensure that the benefits of the procedure justify any associated risks to the patient. Consequently, patients over age 65 should not be excluded out of hand: the patient’s individual risk of major adverse events must be identified beforehand.
If the patient is at risk, efforts should be made to reduce the risk to an acceptable level, including cardiac risk stratification, optimization of drug therapy, and discussions with the bariatric surgeon to plan on a less-invasive laparoscopic procedure. Otherwise, older obese patients can safely proceed with conventional bariatric surgery, which will help them achieve durable weight loss, improve quality of life, and reduce associated comorbidities.
The aforementioned studies of bariatric surgery are retrospective, include small numbers of patients, and lack long-term follow-up. The issues of long-term safety and the risk of death and morbidity in the aging population will require randomized controlled trials to answer these important questions.
At our hospital, we have seen an increase in the number of patients referred for a possible additional procedure (revision) to correct a problem from a previous bariatric surgery. The problems arising from the previous surgery can lead to weight gain or to excessive weight loss and malnutrition. To date, our institution has no policy on when to consider a revisional procedure in an older patient. All patients, including older ones, are assessed for the procedure on a case-by-case basis.
- Bray GA, Macdiarmid J. The epidemic of obesity. West J Med 2000; 172:78–79.
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med 1999; 341:1097–1105.
- Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007; 10:433–442.
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004; 291:1238–1245.
- Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. National vital statistics reports; vol 58no 10. Hyattsville, MD: National Center for Health Statistics. 2010.
- US Census Bureau International Database (IDB). Population projections of the US by age, sex, race, Hispanic origin, population division. http://www.census.gov/ipc/www/idb/country.php. Accessed September 13, 2013.
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004; 291:2847–2850.
- Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA 1994; 272:205–211.
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999; 282:1519–1522.
- Horani MH, Mooradian AD. Management of obesity in the elderly: special considerations. Treat Endocrinol 2002; 1:387–398.
- Beaufrère B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr 2000; 54(suppl 3):S48–S53.
- Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000; 72:694–701.
- Snitker S. Use of body fatness cutoff points (author reply). Mayo Clin Proc 2010; 85:1057; author reply 1057–1058.
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147:755–763. Erratum in Am J Epidemiol 1999; 149:1161.
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord 2001; 25:1730–1735.
- Molarius A, Seidell JC, Visscher TL, Hofman A. Misclassification of high-risk older subjects using waist action levels established for young and middle-aged adults—results from the Rotterdam Study. J Am Geriatr Soc 2000; 48:1638–1645.
- Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull 2011; 97:169–196.
- Turcato E, Bosello O, Di Francesco V, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord 2000; 24:1005–1010.
- Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005; 29:1011–1029.
- Heim N, Snijder MB, Heymans MW, Deeg DJ, Seidell JC, Visser M. Optimal cutoff values for high-risk waist circumference in older adults based on related health outcomes. Am J Epidemiol 2011; 174:479–489.
- Roubenoff R, Castaneda C. Sarcopenia—understanding the dynamics of aging muscle. JAMA 2001; 286:1230–1231.
- Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int J Obes Relat Metab Disord 2002; 26:953–960.
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004; 12:1995–2004.
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001; 137:231–243.
- Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 2004; 12:887–888.
- Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med 2009; 25:643–659.
- Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr 2000; 54(suppl 3):S92–S103.
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37:1595–1607.
- Corona G, Mannucci E, Forti G, Maggi M. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int J Androl 2009; 32:587–598.
- Haarbo J, Hassager C, Riis BJ, Christiansen C. Relation of body fat distribution to serum lipids and lipoproteins in elderly women. Atherosclerosis 1989; 80:57–62.
- Cignarella A, Kratz M, Bolego C. Emerging role of estrogen in the control of cardiometabolic disease. Trends Pharmacol Sci 2010; 31:183–189.
- Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med 1988; 109:18–24.
- Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med 1999; 107:542–548.
- Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Effect of aging on the relationship between multiple risk factors and waist circumference. J Am Geriatr Soc 2000; 48:788–794.
- Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med 2000; 160:2117–2128.
- Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med 1998; 338:1–7.
- Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007; 10:433–442.
- Zamboni M, Armellini F, Harris T, et al. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr 1997; 66:111–115.
- Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc 2005; 53:2112–2118.
- Inelmen EM, Sergi G, Coin A, Miotto F, Peruzza S, Enzi G. Can obesity be a risk factor in elderly people? Obes Rev 2003; 4:147–155.
- Elia M. Obesity in the elderly. Obes Res 2001; 9(suppl 4):244S–248S.
- Losonczy KG, Harris TB, Cornoni-Huntley J, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol 1995; 141:312–321.
- Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol 2006; 163:938–949.
- Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc 2008; 9:302–312.
- Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing 2010; 39:176–184.
- Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011; 364:1218–1229.
- Wannamethee SG, Shaper AG, Whincup PH, Walker M. Characteristics of older men who lose weight intentionally or unintentionally. Am J Epidemiol 2000; 151:667–675.
- Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med 1990; 7:228–233.
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000; 23:1499–1504.
- Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29:2102-2107.
- National Heart, Lung, and Blood Institute in cooperation with The National Institute of Diabetes and Digestive and Kidney Diseases. Clinical guidelines on the identification, evaluation and treatment of the overweight and obesity in adults, the evidence report. NIH Publication number 98-4803 http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf. Accessed September 13, 2013.
- Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005; 82:923–934.
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40-64 years. Am J Epidemiol 1995; 141:1128–1141.
- McTigue KM, Hess R, Ziouras J. Obesity in older adults: a systematic review of the evidence for diagnosis and treatment. Obesity (Silver Spring). 2006; 14:1485–1497.
- Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol 1995; 79:818–823.
- Pavlou KN, Krey S, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am J Clin Nutr 1989; 49(suppl 5):1115–1123.
- Kraemer WJ, Volek JS, Clark KL, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc 1999; 31:1320–1329.
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341.
- Banks M, Klein S, Sinacore D, Siener C, Villareal DT. Effects of weight loss and exercise on frailty in obese elderly subjects. J Am Geriatr Soc 2005; 53:S16.
- Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004; 50:1501–1510.
- Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 2006; 166:860–866.
- Nelson ME, Rejeski WJ, Blair SN, et al; American College of Sports Medicine; American Heart Association. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116:1094–1105.
- Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 2005; 142:532–546.
- Segal KR, Lucas C, Boldrin M, Hauptman J. Weight loss efficacy of orlistat in obese elderly adults (abstract). Obes Res 1999; 7(suppl):26S.
- Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000; 9:160–167.
- Dorman RB, Abraham AA, Al-Refaie WB, Parsons HM, Ikramuddin S, Habermann EB. Bariatric surgery outcomes in the elderly: an ACS NSQIP study. J Gastrointest Surg 2012; 16:35–44.
- Sugerman HJ, DeMaria EJ, Kellum JM, Sugerman EL, Meador JG, Wolfe LG. Effects of bariatric surgery in older patients. Ann Surg 2004; 240:243–247.
- St. Peter SD, Craft RO, Tiede JL, Swain JM. Impact of advanced age on weight loss and health benefits after laparoscopic gastric bypass. Arch Surg 2005; 140:165–168.
- Sosa JL, Pombo H, Pallavicini H, Ruiz-Rodriguez M. Laparoscopic gastric bypass beyond age 60. Obes Surg 2004; 14:1398–1401.
- Varela JE, Wilson SE, Nguyen NT. Outcomes of bariatric surgery in the elderly. Am Surg 2006; 72:865–869.
- Willkomm CM, Fisher TL, Barnes GS, Kennedy CI, Kuhn JA. Surgical weight loss >65 years old: is it worth the risk? Surg Obes Relat Dis 2010; 6:491–496.
- Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med 2001; 161:1194–1203.
Should older obese people try to lose weight? Such a simple question is more complicated than one would think.
At issue is whether obesity is harmful in older people, and whether treating it will reduce their health risks. True, obesity is an independent risk factor for cardiovascular disease and is associated with many comorbidities, including type 2 diabetes mellitus, hyperlipidemia, heart failure, and hypertension.1 An independent association also exists between obesity and all-cause mortality.2 However, there is also evidence suggesting that obesity in this age group is associated with a lower, not higher, risk of death—a finding termed the obesity paradox.3 And for that matter, what exactly constitutes obesity in elderly people, who naturally undergo changes in body composition as they age?
This article examines the literature on these controversial issues, including changes in body composition with age, the definition of obesity in older adults, the obesity paradox, and treatment of obesity in older adults.
AMERICANS ARE GETTING OLDER—AND BIGGER
Americans are living longer than ever before; life expectancy has reached a new high of 77.8 years.4,5 According to the US Census Bureau,6 about 27 million people in the United States are over age 70, and this number is expected to nearly double by 2030.
Meanwhile, the prevalence of obesity, defined as a body mass index (BMI) of 30 kg/m2 or higher, has increased in the last 25 years in all age groups in the United States, including those age 65 and older.7,8 These two trends add up to an increase in the number of obese older people. In 2000, 22.9% of people age 60 to 69 and 15.5% of those over age 70 and older were obese.9 This amounted to a 56% increase in the former group and a 36% increase in the latter group in the interval since 1991.5,9
BUT WHAT CONSTITUTES ‘OBESITY’?
Obesity is the excess accumulation of body fat, leading to a higher risk of medical illness and premature death. But measuring it is not as simple as one might think.
The body mass index can mislead
The BMI, ie, weight in kilograms divided by the square of the height in meters, correlates fairly well with body fat stores and is generally used to classify medical risk.
However, the BMI can classify some older people as overweight (BMI 30.0–34.9 kg/m2) or obese (BMI ≥ 35.0 kg/m2) who actually do not have an excess of body fat—and can fail to classify others as overweight or obese who do. For example, if a person loses height as a result of vertebral compression fractures, his or her BMI would become higher, even with no change in weight or body fat. Conversely, changes in body composition with age, including loss of muscle and an increase in fat, may not be reflected in the BMI, even if the person really does have too much body fat.10
This second limitation of the BMI is important when estimating risk in older adults, who have a particular fat distribution. Visceral, subcutaneous, intramuscular, and intrahepatic fat increase with age, and they are all risk factors for insulin resistance and type 2 diabetes mellitus.11 And in older people, having too much visceral fat is more prevalent than the BMI might predict.10
Percent body fat awaits investigation
Percent body fat is another way to assess body fat. Defined as the total weight of fat divided by total weight, it is measured in various ways.
Dual-energy x-ray absorptiometry, computed tomography, and magnetic resonance imaging can measure percent body fat, and they can differentiate visceral from subcutaneous fat (which is less metabolically active). Unfortunately, most of these tests are used for this purpose only in research, and they are relatively expensive.
Commercially available bioelectrical impedance devices send a weak electric current through the body and measure the resistance, and using this information and four other factors (height, weight, age, and sex), they calculate percent body fat. This method is fast, easy, painless, and cheap. A disadvantage is that the handheld devices measure body composition of the upper body only. Because the lower body is excluded, they do not give an accurate measurement of body fat of the abdomen and hips. Also, they cannot differentiate visceral from subcutaneous fat.
Bioelectrical impedance devices work well in healthy individuals with stable water balance. The values are only an estimate of fat-free mass, and therefore this method is not the gold standard for assessing body fat. Bioelectrical impedance is better at tracking body composition in an individual over time than at diagnosing obesity.
Percent body fat can vary by sex and race. Asians, for example, have higher percent body fats at lower BMIs, particularly when younger.12 Also, Gallagher et al12 found that percent body fat increased with age at every given BMI in both men and women (Table 1).
The traditional universal cutoffs for defining obesity by percent body fat are 25% in men and 35% in women. However, research has indicated that cutoffs of 20% to 25% in men and 30% to 38% in women may better identify those at risk of metabolic disease.13 Guidelines and evidence-based cutoffs for percent body fat must await further investigation.
Waist circumference is useful
In older adults, obesity can be diagnosed by a measurement such as waist circumference, which correlates highly with total fat and intra-abdominal fat.14 It is very cost-effective, simple, and useful for the office assessment of adiposity.
The measurement should be made halfway between the iliac crest and the lower anterior ribs, with the patient standing, and at the end of expiration.
The traditional standard for waist circumference is less than 89 cm (35 inches) for women and 102 cm (40 inches) for men. However, opinion differs, and different reference ranges exist depending on ethnicity. Additionally, because stature and body composition change with age, concerns have been raised about misclassification of the health risks related to obesity in older adults using the current standard.15,16
The waist circumference is as good as or even better than the BMI as a measure of excess adiposity in older adults.16–18 This is in part because of the age-dependent height decrease in older adults.15,19 (Recall that the BMI is calculated using the height squared as the denominator; as a result, the BMI would give a higher reading and thus an overestimate of total body fat.) Conversely, we can underestimate the amount of adiposity because of decreases in abdominal muscle tone.17
Cutoffs for waist circumference should be age-specific.16
Investigators in the Netherlands15,16 prospectively took 4,996 measurements in 2,232 people with a mean age of 70, from 1992 through 2006. They concluded that the best cutoffs for predicting the health risks of obesity in the elderly were 109 cm (43 inches) in men and 98 cm (39 inches) in women.
A group of researchers has proposed that the cutoffs be shifted upward in older adults, with new values for those age 70 and over.20 The Health Survey for England aimed to describe the patterns and trends in waist circumference and abdominal obesity and overweight in people age 70 through 89, comparing both the standard and the new cutoffs. Optimal cutoffs recommended for abdominal obesity for patients age 70 and older were 100 to 106 cm in men and 99 cm in women.20 Estimates of the prevalence of abdominal obesity are much lower using the new cutoffs.
SARCOPENIA: LOSS OF MUSCLE WITH AGE
With age comes sarcopenia—the progressive loss of muscle mass, primarily skeletal muscle, resulting in a decrease in strength and power.21 The process begins as early as the 20s or 30s.22 It is distinct from wasting (involuntary weight loss from inadequate intake), seen in starvation.21
Sarcopenia is defined as an appendicular skeletal muscle mass index (the appendicular skeletal mass divided by the square of the height in meters) of less than 2 standard deviations below a young adult reference, and a percentage of body fat over the 60th percentile for the individual’s sex and age.23,24 Estimates of its prevalence vary, but it is common and it increases with age.14,20
Sarcopenic obesity: Less muscle, more fat
Progressive loss of skeletal muscle with age, along with an increase and redistribution of body fat, is known as sarcopenic obesity.25 It is associated with higher morbidity and mortality rates as well as a decline in functional strength, which leads to frailty.23 This loss of muscle mass may go unnoticed in an older person until he or she begins to lose physical function.
As noted, in an older person with sarcopenic obesity, the BMI may mislead because of the high percentage of fat and the low lean mass.26
Why we change with age
This change in body composition with age is a result of several factors. Illness or inactivity can lead to loss of muscle, while body fat is preserved.17 The combination of reduced physical activity, a lower resting metabolic rate, and an unchanged intake of food can increase the likelihood of sarcopenia.27 Also possibly contributing are hormonal changes, including reduced production of growth hormone and testosterone and decreased responsiveness to thyroid hormone and leptin.28
Moreover, the interaction of several factors can lead to a vicious circle of progressive loss of muscle and increase in fat. As people age, their physical activity tends to decrease, resulting in muscle loss. As muscle mass decreases, the amount of available insulin-responsive tissue is reduced, resulting in insulin resistance, which in turn promotes the metabolic syndrome and an increase in fat. With more fat, people produce more of the adipokines tumor necrosis factor alpha and interleukin 6, which further promote insulin resistance.
Other changes contribute to a decrease in muscle quality and performance, including an increase in intramuscular and intrahepatic fat, which is associated with insulin resistance.11 The increases in adipose stores occur mostly in intra-abdominal fat rather than in subcutaneous fat.
ADVERSE EFFECTS OF OBESITY
A number of comorbidities arise with obesity, regardless of age.19
The diseases most strongly associated with obesity are the metabolic syndrome and type 2 diabetes mellitus.17 Studies have shown that in older adults, obesity as measured by waist circumference is associated with hyperglycemia and dyslipidemia.29,30
Metabolic abnormalities may ensue in obese older people through complex mechanisms involving an age-related decline in sex hormones. For example, late-onset hypogonadism in men, which is more common in those who are obese, is related to the metabolic syndrome.29
These mechanisms are also complex in women. Because estrogens can be produced in adipose tissue, obese postmenopausal women have higher concentrations of estrogens than their lean counterparts, and this may lead to metabolic abnormalities.31 (On the other hand, higher estrogen levels in obese menopausal women can protect against osteoporosis by increasing bone mass.)
Older people who weigh more and have more adipose tissue, especially those who became obese at a young age, have a greater risk of osteoarthritis of the knee,32,33 which when combined with obesity can cause disability and physical impairment.19 And cardiovascular risk factors,18,33 hypertension,34 and certain cancers35 are more common in older people with higher waist circumference.
THE OBESITY PARADOX
In general, obesity in younger adults has been shown to shorten life expectancy. This risk of death is often associated with obesity-related health problems.
In older people, the effect of obesity is much more complex.36 The optimal weight in terms of survival increases with age. More interesting is the finding that although the risk of cardiovascular disease is higher in overweight or obese older adults, studies also suggest that in this age group, being overweight or obese is paradoxically associated with lower mortality rates from these diseases.26 This phenomenon is called the obesity paradox.37
For those over age 75, the relative risk of death from all causes and from cardiovascular disease has been found to decrease with increasing BMI.25 The relationship between BMI and death from all causes in older adults may actually be a U-shaped curve, meaning that the risk of death rises at both extremes of BMI values.26
Possible explanations for the paradox
Several hypotheses have been proposed to explain the change in the relationship between BMI and the risk of death that occurs with aging.
The BMI is an imperfect measure of obesity. The obesity paradox may be an artifact of using the BMI to measure obesity in older adults.17 As described above, sarcopenic obesity must be considered in those over age 65 because the BMI does not differentiate between fat and muscle. Older adults tend to have a higher proportion of body and visceral fat that is distributed differently, making the waist circumference or waist-hip ratio a more appropriate measure of obesity in this group.38 Janssen et al39 found that in people age 65 and older, after controlling for waist circumference, higher BMI values were associated with lower death rates; after controlling for BMI, waist circumference was associated with a higher risk of death.
The survival effect suggests that people who are susceptible to the negative effects of obesity die sooner,40 and those who survive until old age may be resistant to the effects of obesity.41 If true, the survival effect would explain why the death rate seems to be unaffected by BMI in the older population.
Unhealthy weight loss. Smoking and diseases such as cancer that can cause early death may also induce weight loss, further complicating the relationship between BMI and death.19 After age 80, the association between BMI and the risk of death is weak because those with a low BMI include not only those who have always been lean and physically active, but also those who lost weight through chronic ill health or smoking.17
Further study needed. Thus, a number of confounding variables may muddy the association between obesity and death in older adults. Obesity should not be misinterpreted as being harmless or beneficial in older adults. Stevens et al36 found that a greater BMI was associated with a higher rate of death from all causes and from cardiovascular disease in men and women up to age 75, but that the relative risk of death associated with a greater BMI decreased with age.
Optimal BMI targets in older people have yet to be validated in a large prospective trial. However, multiple studies have examined the relationship between BMI and all-cause mortality in older adults and have identified a BMI of 24 to 35 as “ideal” and associated with the lowest risk of death, with a lower range for men and a higher range for women.42,43 The topic has been reviewed by Oreopoulos et al.26 More research is needed to evaluate this relationship.
THE BENEFIT OF WEIGHT LOSS IN OLDER ADULTS IS CONTROVERSIAL
In younger obese people, weight loss brings a multitude of benefits by reducing the risk of complications arising from obesity. However, in older adults, the effects of weight loss remain controversial, and evidence to guide treatment is limited.44,45 The few trials that have been published have typically focused on cardiovascular risk factors rather than physical function.45
In a 1-year trial, 107 people age 65 or older were randomized to a control group, to weight management, to exercise, or to weight management plus exercise. The combination of weight loss and exercise yielded the greatest improvement in physical function.46
Intentional vs unintentional weight loss
Intentional weight loss is altogether different from unintentional weight loss.
In most cases, weight loss in older adults is unintentional and may indicate underlying disease and impending death.17 For example, older men who lose weight unintentionally have significantly greater rates of smoking, disability, cancer, and respiratory disease and less obesity and physical activity than those who lose weight intentionally.47
Studies have shown an increase in life expectancy in older patients with type 2 diabetes mellitus who lost weight intentionally.48,49 In fact, moderate weight loss—just 5% to 10%—has been shown to improve cardiovascular risk factors,44 osteoarthritis, and type 2 diabetes.50
Bales and Buhr44 performed a systematic review of 16 studies that had lasted at least 6 months. Patients were age 60 or older with a minimum baseline BMI of 27 kg/m2 who intentionally lost at least 3% of body weight or 2 kg. Levels of the inflammatory markers C-reactive protein, tumor necrosis factor alpha, and interleukin 6 declined with weight, along with blood pressure, fasting glucose, waist circumference, and low-density lipoprotein cholesterol. On the downside, bone mineral density and lean body mass also declined slightly. The best way to avoid losing lean body mass and to preserve bone density during weight loss is to include a program of resistance-training exercises.
No clinical trial has evaluated the effects of intentional weight loss on death rates in older obese people.25 As a result, evidence-based recommendations cannot be made. Rather, advice on weight loss must be individualized, with special emphasis on the patient’s weight history and medical comorbidities.44
Oreopoulos et al26 summarized the possible effects of BMI, abdominal fat, lean body mass, and intentional weight loss on morbidity and mortality outcomes in older adults (Table 2).
TREATMENT GUIDELINES AND RECOMMENDATIONS
Many of the methods of weight management in older adults are the same as in young and middle-aged adults.51 Recommendations for all age groups include lifestyle changes, increased activity, dietary changes, drug therapy, and bariatric surgery.
Whether there should be separate guidelines for older adults is controversial. In view of the obesity paradox, physicians have been reluctant to recommend weight loss in elderly patients. Caution is advised in recommending weight loss solely on the basis of body weight, as studies have shown that the weight associated with maximal survival increases with age. Because of age-related changes in body composition and reduced energy requirements and expenditure, recommendations for the young and middle-aged should not be applied directly to older adults.
In this group, especially those who have survived into old age with good health and an intact functional status, one could argue that significant caloric restriction should not be recommended. In these people, the goal is often to maintain weight and incorporate a daily exercise program rather than to aggressively lose weight. Adding resistance training can improve physical function, which can improve quality of life. There is less emphasis on cardiovascular risk, but both outcomes apply for both age groups.52
Intentional weight loss should be recommended to high-risk older adults, including those with cardiovascular disease, type 2 diabetes mellitus, and metabolic syndrome, because the absolute risk of death and morbidity is higher in this group. Most health benefits can be achieved with modest weight loss.53 Potential benefits include prevention of cognitive impairment, protection from bone fractures, an increase in antioxidant defense, a reserve of fat and energy stores, and an increase in longevity.26
Treatment differs from that in the younger population primarily because of the importance of preventing loss of muscle with intentional weight loss. People of all ages who lose weight intentionally lose fat and, to a lesser extent, skeletal muscle. Older patients have already lost muscle mass, but further changes in body composition, especially a further reduction in muscle mass, can be limited by consuming about 1.0 g/kg of high-quality protein in the diet and by engaging in resistance training and weight training.52
Improving quality of life and physical function are important goals. Information is emerging about when obesity needs to be managed in older adults. There is also evidence to support dietary and exercise therapy.54 Weight-loss options include lifestyle interventions, pharmacotherapy, and bariatric surgery.
Lifestyle interventions: Diet and exercise
The goal is to induce an energy deficit by reducing energy intake, increasing energy expenditure, or both—by 500 to 1,000 calories a day. This generally leads to a loss of 1 to 2 lb per week, and possibly up to 10% of weight in 6 months. Loss of about 10 to 20 lb with diet and exercise can translate to a relatively large reduction in visceral fat, with subsequent improvement in metabolic abnormalities.
A regular exercise program is important for improving overall physical function, which can slow progression to frailty. Adding aerobic, endurance, and resistance training helps preserve fat-free mass, which otherwise tends to diminish during active weight loss.55–57
The exercise program should begin at the outset of the weight-loss effort to help maintain weight loss and to prevent weight regain.58 Exercise is not essential for reaching the targeted weight loss, but starting early is important to reduce the loss of lean muscle that is usually already seen in the older population.
Several studies indicate that diet and exercise are just as effective in middle-aged and older people (over age 60) as in the younger population.58–60 Older people in the Diabetes Prevention Program were more compliant with lifestyle interventions and lost more weight than younger participants49: 60% of the older group met the 7% weight-loss goal at the end of 24 weeks, compared with 43% of those under age 45. At 3 years, the numbers were 63% vs 27%.
In a small randomized controlled trial,61 fat mass decreased by 6.6 kg in 17 people assigned to a program of diet and exercise, compared with a gain of 1.7 kg in a control group of 10 patients. Fat-free mass decreased by about 1 kg in both groups. The authors concluded that diet plus exercise (resistance training and strength training in this trial) could ameliorate frailty in obese older adults.
If exercise is appropriate, a physician should write a prescription for it, especially for resistance training, strengthening, flexibility, and stretching. This is important for patients with sarcopenic obesity and for those at high risk of chronic bone loss. The 2007 American College of Sports Medicine guidelines recommend muscle-strengthening activity of 8 to 10 exercises involving the major muscle groups, 10 to 15 repetitions at least twice a week. Flexibility and balance exercises should be included for those at risk of falls.62
Pharmacotherapy
At present, there are two general classes of weight-loss drugs: appetite suppressants and drugs that interfere with nutrient absorption.
Appetite suppressants include the sympathomimetics, which stimulate the release of dopamine and norepinephrine, resulting in increased satiety. Data—and therefore, recommendations—on their use in the elderly are very scarce, as most randomized controlled trials included only a small number of older people. A meta-analysis of drug therapy to treat obesity noted that the study population ranged in age from 34 to 54.63
The only approved drug currently available for use in older adults is orlistat, which blocks absorption of dietary fat by binding to intestinal lipase. A randomized controlled trial found the weight loss with orlistat to be comparable in older and younger adults.64,65
Review medications than can cause weight gain
When assessing older adults, always review the drugs they are taking. Those known to cause weight gain include certain of the following:
- Antiepileptics (eg, gabapentin)
- Antipsychotics (eg, olanzapine)
- Antidepressants (eg, tricyclics)
- Antihyperglycemic drugs (eg, sulfonylureas, thiazolidinediones)
- Beta-blockers
- Steroids.
If medically appropriate, a weight-neutral drug should be substituted for one suspected of causing weight gain. If a different physician (eg, a specialist) prescribed the original drug, he or she should be notified or consulted about any change.
Bariatric surgery
Bariatric surgery is the most effective weight-loss option, and more older patients are undergoing it than in the past. Dorman et al66 showed that the number of patients age 65 or older undergoing bariatric surgery increased from the year 2005 (when they accounted for 2%) to 2009 (when they accounted for 4.8%).
However, very few studies have provided information on the safety and effectiveness of bariatric surgery in older people. Several reports concluded that rates of perioperative morbidity and mortality are higher in older patients.67–69 Surgery resulted in marked weight loss and improvement in obesity-related complications and physical disability in older patients, although by a lower rate than in younger patients.
Varela et al70 examined the outcomes of bariatric surgery in a database from the University Health System Consortium Centers between 1999 and 2005. Patients over age 60 accounted for 1,339 (2.7%) of all bariatric operations performed. Compared with young and middle-aged patients, older patients had more comorbidities, longer hospital stays, and more complications, in addition to a higher in-hospital mortality rate. When risk-adjusted, the observed-to-expected mortality ratio for the older group was 0.9, compared with 0.7 in the young and middle-aged cohort.
Willkomm et al71 found an apparently higher operative risk profile in those over age 65 (n = 100) than in younger patients (n = 1,374), with higher rates of sleep apnea, diabetes, and hypertension. However, the operative outcomes were similar in the two groups in terms of operative time, length of stay, and 30-day readmission rates. The authors concluded that patients over age 65 had excellent outcomes compared with younger patients, suggesting that older age is not a risk factor for complications or death with bariatric surgery.
The American College of Surgeons National Surgical Quality Improvement Program evaluated the outcomes of 48,378 adults with a BMI greater than or equal to 35 kg/m2 who underwent bariatric surgery between 2005 and 2009.66 During this time, the number of patients age 65 and older seeking bariatric surgery increased from 1.5% to 4%. A total of 1,449 patients were in this age range. Thirty-day mortality rates did not differ significantly by age group and were less than 1% for all age ranges. Being age 65 or older was a significant predictor of prolonged length of stay but not of major adverse events. Significant predictors of major adverse events were a BMI greater than or equal to 55 kg/m2, cardiac comorbidities, a severe American Society of Anesthesiologists score, albumin levels lower than 3 g/dL, and creatinine levels greater than 1.5 mg/dL.
The most up-to-date study of the outcomes of bariatric surgery in patients over age 70 was a retrospective review at a single institution from 2007 to 2008 of 42 patients who underwent bariatric surgery.72 Twenty-two patients had laparoscopic gastric banding, 12 had laparoscopic sleeve gastrectomy, and 8 underwent laparoscopic Roux-en-Y gastric bypass. No patient died, complications occurred in 9 patients, and the rates of postoperative use of medications for hypertension, hyperlipidemia, diabetes, and osteoarthritis were reduced by about half. With the increasing number of patients seeking bariatric surgery, especially those over age 70, further prospective studies will determine if the outcomes are statistically significant.
If bariatric surgery is considered
The outcomes, complications, and mortality rates associated with bariatric surgery have been shown to be acceptable for adults age 65 and older. Perioperative risk assessment in the older obese patient seeking bariatric surgery is paramount to ensure that the benefits of the procedure justify any associated risks to the patient. Consequently, patients over age 65 should not be excluded out of hand: the patient’s individual risk of major adverse events must be identified beforehand.
If the patient is at risk, efforts should be made to reduce the risk to an acceptable level, including cardiac risk stratification, optimization of drug therapy, and discussions with the bariatric surgeon to plan on a less-invasive laparoscopic procedure. Otherwise, older obese patients can safely proceed with conventional bariatric surgery, which will help them achieve durable weight loss, improve quality of life, and reduce associated comorbidities.
The aforementioned studies of bariatric surgery are retrospective, include small numbers of patients, and lack long-term follow-up. The issues of long-term safety and the risk of death and morbidity in the aging population will require randomized controlled trials to answer these important questions.
At our hospital, we have seen an increase in the number of patients referred for a possible additional procedure (revision) to correct a problem from a previous bariatric surgery. The problems arising from the previous surgery can lead to weight gain or to excessive weight loss and malnutrition. To date, our institution has no policy on when to consider a revisional procedure in an older patient. All patients, including older ones, are assessed for the procedure on a case-by-case basis.
Should older obese people try to lose weight? Such a simple question is more complicated than one would think.
At issue is whether obesity is harmful in older people, and whether treating it will reduce their health risks. True, obesity is an independent risk factor for cardiovascular disease and is associated with many comorbidities, including type 2 diabetes mellitus, hyperlipidemia, heart failure, and hypertension.1 An independent association also exists between obesity and all-cause mortality.2 However, there is also evidence suggesting that obesity in this age group is associated with a lower, not higher, risk of death—a finding termed the obesity paradox.3 And for that matter, what exactly constitutes obesity in elderly people, who naturally undergo changes in body composition as they age?
This article examines the literature on these controversial issues, including changes in body composition with age, the definition of obesity in older adults, the obesity paradox, and treatment of obesity in older adults.
AMERICANS ARE GETTING OLDER—AND BIGGER
Americans are living longer than ever before; life expectancy has reached a new high of 77.8 years.4,5 According to the US Census Bureau,6 about 27 million people in the United States are over age 70, and this number is expected to nearly double by 2030.
Meanwhile, the prevalence of obesity, defined as a body mass index (BMI) of 30 kg/m2 or higher, has increased in the last 25 years in all age groups in the United States, including those age 65 and older.7,8 These two trends add up to an increase in the number of obese older people. In 2000, 22.9% of people age 60 to 69 and 15.5% of those over age 70 and older were obese.9 This amounted to a 56% increase in the former group and a 36% increase in the latter group in the interval since 1991.5,9
BUT WHAT CONSTITUTES ‘OBESITY’?
Obesity is the excess accumulation of body fat, leading to a higher risk of medical illness and premature death. But measuring it is not as simple as one might think.
The body mass index can mislead
The BMI, ie, weight in kilograms divided by the square of the height in meters, correlates fairly well with body fat stores and is generally used to classify medical risk.
However, the BMI can classify some older people as overweight (BMI 30.0–34.9 kg/m2) or obese (BMI ≥ 35.0 kg/m2) who actually do not have an excess of body fat—and can fail to classify others as overweight or obese who do. For example, if a person loses height as a result of vertebral compression fractures, his or her BMI would become higher, even with no change in weight or body fat. Conversely, changes in body composition with age, including loss of muscle and an increase in fat, may not be reflected in the BMI, even if the person really does have too much body fat.10
This second limitation of the BMI is important when estimating risk in older adults, who have a particular fat distribution. Visceral, subcutaneous, intramuscular, and intrahepatic fat increase with age, and they are all risk factors for insulin resistance and type 2 diabetes mellitus.11 And in older people, having too much visceral fat is more prevalent than the BMI might predict.10
Percent body fat awaits investigation
Percent body fat is another way to assess body fat. Defined as the total weight of fat divided by total weight, it is measured in various ways.
Dual-energy x-ray absorptiometry, computed tomography, and magnetic resonance imaging can measure percent body fat, and they can differentiate visceral from subcutaneous fat (which is less metabolically active). Unfortunately, most of these tests are used for this purpose only in research, and they are relatively expensive.
Commercially available bioelectrical impedance devices send a weak electric current through the body and measure the resistance, and using this information and four other factors (height, weight, age, and sex), they calculate percent body fat. This method is fast, easy, painless, and cheap. A disadvantage is that the handheld devices measure body composition of the upper body only. Because the lower body is excluded, they do not give an accurate measurement of body fat of the abdomen and hips. Also, they cannot differentiate visceral from subcutaneous fat.
Bioelectrical impedance devices work well in healthy individuals with stable water balance. The values are only an estimate of fat-free mass, and therefore this method is not the gold standard for assessing body fat. Bioelectrical impedance is better at tracking body composition in an individual over time than at diagnosing obesity.
Percent body fat can vary by sex and race. Asians, for example, have higher percent body fats at lower BMIs, particularly when younger.12 Also, Gallagher et al12 found that percent body fat increased with age at every given BMI in both men and women (Table 1).
The traditional universal cutoffs for defining obesity by percent body fat are 25% in men and 35% in women. However, research has indicated that cutoffs of 20% to 25% in men and 30% to 38% in women may better identify those at risk of metabolic disease.13 Guidelines and evidence-based cutoffs for percent body fat must await further investigation.
Waist circumference is useful
In older adults, obesity can be diagnosed by a measurement such as waist circumference, which correlates highly with total fat and intra-abdominal fat.14 It is very cost-effective, simple, and useful for the office assessment of adiposity.
The measurement should be made halfway between the iliac crest and the lower anterior ribs, with the patient standing, and at the end of expiration.
The traditional standard for waist circumference is less than 89 cm (35 inches) for women and 102 cm (40 inches) for men. However, opinion differs, and different reference ranges exist depending on ethnicity. Additionally, because stature and body composition change with age, concerns have been raised about misclassification of the health risks related to obesity in older adults using the current standard.15,16
The waist circumference is as good as or even better than the BMI as a measure of excess adiposity in older adults.16–18 This is in part because of the age-dependent height decrease in older adults.15,19 (Recall that the BMI is calculated using the height squared as the denominator; as a result, the BMI would give a higher reading and thus an overestimate of total body fat.) Conversely, we can underestimate the amount of adiposity because of decreases in abdominal muscle tone.17
Cutoffs for waist circumference should be age-specific.16
Investigators in the Netherlands15,16 prospectively took 4,996 measurements in 2,232 people with a mean age of 70, from 1992 through 2006. They concluded that the best cutoffs for predicting the health risks of obesity in the elderly were 109 cm (43 inches) in men and 98 cm (39 inches) in women.
A group of researchers has proposed that the cutoffs be shifted upward in older adults, with new values for those age 70 and over.20 The Health Survey for England aimed to describe the patterns and trends in waist circumference and abdominal obesity and overweight in people age 70 through 89, comparing both the standard and the new cutoffs. Optimal cutoffs recommended for abdominal obesity for patients age 70 and older were 100 to 106 cm in men and 99 cm in women.20 Estimates of the prevalence of abdominal obesity are much lower using the new cutoffs.
SARCOPENIA: LOSS OF MUSCLE WITH AGE
With age comes sarcopenia—the progressive loss of muscle mass, primarily skeletal muscle, resulting in a decrease in strength and power.21 The process begins as early as the 20s or 30s.22 It is distinct from wasting (involuntary weight loss from inadequate intake), seen in starvation.21
Sarcopenia is defined as an appendicular skeletal muscle mass index (the appendicular skeletal mass divided by the square of the height in meters) of less than 2 standard deviations below a young adult reference, and a percentage of body fat over the 60th percentile for the individual’s sex and age.23,24 Estimates of its prevalence vary, but it is common and it increases with age.14,20
Sarcopenic obesity: Less muscle, more fat
Progressive loss of skeletal muscle with age, along with an increase and redistribution of body fat, is known as sarcopenic obesity.25 It is associated with higher morbidity and mortality rates as well as a decline in functional strength, which leads to frailty.23 This loss of muscle mass may go unnoticed in an older person until he or she begins to lose physical function.
As noted, in an older person with sarcopenic obesity, the BMI may mislead because of the high percentage of fat and the low lean mass.26
Why we change with age
This change in body composition with age is a result of several factors. Illness or inactivity can lead to loss of muscle, while body fat is preserved.17 The combination of reduced physical activity, a lower resting metabolic rate, and an unchanged intake of food can increase the likelihood of sarcopenia.27 Also possibly contributing are hormonal changes, including reduced production of growth hormone and testosterone and decreased responsiveness to thyroid hormone and leptin.28
Moreover, the interaction of several factors can lead to a vicious circle of progressive loss of muscle and increase in fat. As people age, their physical activity tends to decrease, resulting in muscle loss. As muscle mass decreases, the amount of available insulin-responsive tissue is reduced, resulting in insulin resistance, which in turn promotes the metabolic syndrome and an increase in fat. With more fat, people produce more of the adipokines tumor necrosis factor alpha and interleukin 6, which further promote insulin resistance.
Other changes contribute to a decrease in muscle quality and performance, including an increase in intramuscular and intrahepatic fat, which is associated with insulin resistance.11 The increases in adipose stores occur mostly in intra-abdominal fat rather than in subcutaneous fat.
ADVERSE EFFECTS OF OBESITY
A number of comorbidities arise with obesity, regardless of age.19
The diseases most strongly associated with obesity are the metabolic syndrome and type 2 diabetes mellitus.17 Studies have shown that in older adults, obesity as measured by waist circumference is associated with hyperglycemia and dyslipidemia.29,30
Metabolic abnormalities may ensue in obese older people through complex mechanisms involving an age-related decline in sex hormones. For example, late-onset hypogonadism in men, which is more common in those who are obese, is related to the metabolic syndrome.29
These mechanisms are also complex in women. Because estrogens can be produced in adipose tissue, obese postmenopausal women have higher concentrations of estrogens than their lean counterparts, and this may lead to metabolic abnormalities.31 (On the other hand, higher estrogen levels in obese menopausal women can protect against osteoporosis by increasing bone mass.)
Older people who weigh more and have more adipose tissue, especially those who became obese at a young age, have a greater risk of osteoarthritis of the knee,32,33 which when combined with obesity can cause disability and physical impairment.19 And cardiovascular risk factors,18,33 hypertension,34 and certain cancers35 are more common in older people with higher waist circumference.
THE OBESITY PARADOX
In general, obesity in younger adults has been shown to shorten life expectancy. This risk of death is often associated with obesity-related health problems.
In older people, the effect of obesity is much more complex.36 The optimal weight in terms of survival increases with age. More interesting is the finding that although the risk of cardiovascular disease is higher in overweight or obese older adults, studies also suggest that in this age group, being overweight or obese is paradoxically associated with lower mortality rates from these diseases.26 This phenomenon is called the obesity paradox.37
For those over age 75, the relative risk of death from all causes and from cardiovascular disease has been found to decrease with increasing BMI.25 The relationship between BMI and death from all causes in older adults may actually be a U-shaped curve, meaning that the risk of death rises at both extremes of BMI values.26
Possible explanations for the paradox
Several hypotheses have been proposed to explain the change in the relationship between BMI and the risk of death that occurs with aging.
The BMI is an imperfect measure of obesity. The obesity paradox may be an artifact of using the BMI to measure obesity in older adults.17 As described above, sarcopenic obesity must be considered in those over age 65 because the BMI does not differentiate between fat and muscle. Older adults tend to have a higher proportion of body and visceral fat that is distributed differently, making the waist circumference or waist-hip ratio a more appropriate measure of obesity in this group.38 Janssen et al39 found that in people age 65 and older, after controlling for waist circumference, higher BMI values were associated with lower death rates; after controlling for BMI, waist circumference was associated with a higher risk of death.
The survival effect suggests that people who are susceptible to the negative effects of obesity die sooner,40 and those who survive until old age may be resistant to the effects of obesity.41 If true, the survival effect would explain why the death rate seems to be unaffected by BMI in the older population.
Unhealthy weight loss. Smoking and diseases such as cancer that can cause early death may also induce weight loss, further complicating the relationship between BMI and death.19 After age 80, the association between BMI and the risk of death is weak because those with a low BMI include not only those who have always been lean and physically active, but also those who lost weight through chronic ill health or smoking.17
Further study needed. Thus, a number of confounding variables may muddy the association between obesity and death in older adults. Obesity should not be misinterpreted as being harmless or beneficial in older adults. Stevens et al36 found that a greater BMI was associated with a higher rate of death from all causes and from cardiovascular disease in men and women up to age 75, but that the relative risk of death associated with a greater BMI decreased with age.
Optimal BMI targets in older people have yet to be validated in a large prospective trial. However, multiple studies have examined the relationship between BMI and all-cause mortality in older adults and have identified a BMI of 24 to 35 as “ideal” and associated with the lowest risk of death, with a lower range for men and a higher range for women.42,43 The topic has been reviewed by Oreopoulos et al.26 More research is needed to evaluate this relationship.
THE BENEFIT OF WEIGHT LOSS IN OLDER ADULTS IS CONTROVERSIAL
In younger obese people, weight loss brings a multitude of benefits by reducing the risk of complications arising from obesity. However, in older adults, the effects of weight loss remain controversial, and evidence to guide treatment is limited.44,45 The few trials that have been published have typically focused on cardiovascular risk factors rather than physical function.45
In a 1-year trial, 107 people age 65 or older were randomized to a control group, to weight management, to exercise, or to weight management plus exercise. The combination of weight loss and exercise yielded the greatest improvement in physical function.46
Intentional vs unintentional weight loss
Intentional weight loss is altogether different from unintentional weight loss.
In most cases, weight loss in older adults is unintentional and may indicate underlying disease and impending death.17 For example, older men who lose weight unintentionally have significantly greater rates of smoking, disability, cancer, and respiratory disease and less obesity and physical activity than those who lose weight intentionally.47
Studies have shown an increase in life expectancy in older patients with type 2 diabetes mellitus who lost weight intentionally.48,49 In fact, moderate weight loss—just 5% to 10%—has been shown to improve cardiovascular risk factors,44 osteoarthritis, and type 2 diabetes.50
Bales and Buhr44 performed a systematic review of 16 studies that had lasted at least 6 months. Patients were age 60 or older with a minimum baseline BMI of 27 kg/m2 who intentionally lost at least 3% of body weight or 2 kg. Levels of the inflammatory markers C-reactive protein, tumor necrosis factor alpha, and interleukin 6 declined with weight, along with blood pressure, fasting glucose, waist circumference, and low-density lipoprotein cholesterol. On the downside, bone mineral density and lean body mass also declined slightly. The best way to avoid losing lean body mass and to preserve bone density during weight loss is to include a program of resistance-training exercises.
No clinical trial has evaluated the effects of intentional weight loss on death rates in older obese people.25 As a result, evidence-based recommendations cannot be made. Rather, advice on weight loss must be individualized, with special emphasis on the patient’s weight history and medical comorbidities.44
Oreopoulos et al26 summarized the possible effects of BMI, abdominal fat, lean body mass, and intentional weight loss on morbidity and mortality outcomes in older adults (Table 2).
TREATMENT GUIDELINES AND RECOMMENDATIONS
Many of the methods of weight management in older adults are the same as in young and middle-aged adults.51 Recommendations for all age groups include lifestyle changes, increased activity, dietary changes, drug therapy, and bariatric surgery.
Whether there should be separate guidelines for older adults is controversial. In view of the obesity paradox, physicians have been reluctant to recommend weight loss in elderly patients. Caution is advised in recommending weight loss solely on the basis of body weight, as studies have shown that the weight associated with maximal survival increases with age. Because of age-related changes in body composition and reduced energy requirements and expenditure, recommendations for the young and middle-aged should not be applied directly to older adults.
In this group, especially those who have survived into old age with good health and an intact functional status, one could argue that significant caloric restriction should not be recommended. In these people, the goal is often to maintain weight and incorporate a daily exercise program rather than to aggressively lose weight. Adding resistance training can improve physical function, which can improve quality of life. There is less emphasis on cardiovascular risk, but both outcomes apply for both age groups.52
Intentional weight loss should be recommended to high-risk older adults, including those with cardiovascular disease, type 2 diabetes mellitus, and metabolic syndrome, because the absolute risk of death and morbidity is higher in this group. Most health benefits can be achieved with modest weight loss.53 Potential benefits include prevention of cognitive impairment, protection from bone fractures, an increase in antioxidant defense, a reserve of fat and energy stores, and an increase in longevity.26
Treatment differs from that in the younger population primarily because of the importance of preventing loss of muscle with intentional weight loss. People of all ages who lose weight intentionally lose fat and, to a lesser extent, skeletal muscle. Older patients have already lost muscle mass, but further changes in body composition, especially a further reduction in muscle mass, can be limited by consuming about 1.0 g/kg of high-quality protein in the diet and by engaging in resistance training and weight training.52
Improving quality of life and physical function are important goals. Information is emerging about when obesity needs to be managed in older adults. There is also evidence to support dietary and exercise therapy.54 Weight-loss options include lifestyle interventions, pharmacotherapy, and bariatric surgery.
Lifestyle interventions: Diet and exercise
The goal is to induce an energy deficit by reducing energy intake, increasing energy expenditure, or both—by 500 to 1,000 calories a day. This generally leads to a loss of 1 to 2 lb per week, and possibly up to 10% of weight in 6 months. Loss of about 10 to 20 lb with diet and exercise can translate to a relatively large reduction in visceral fat, with subsequent improvement in metabolic abnormalities.
A regular exercise program is important for improving overall physical function, which can slow progression to frailty. Adding aerobic, endurance, and resistance training helps preserve fat-free mass, which otherwise tends to diminish during active weight loss.55–57
The exercise program should begin at the outset of the weight-loss effort to help maintain weight loss and to prevent weight regain.58 Exercise is not essential for reaching the targeted weight loss, but starting early is important to reduce the loss of lean muscle that is usually already seen in the older population.
Several studies indicate that diet and exercise are just as effective in middle-aged and older people (over age 60) as in the younger population.58–60 Older people in the Diabetes Prevention Program were more compliant with lifestyle interventions and lost more weight than younger participants49: 60% of the older group met the 7% weight-loss goal at the end of 24 weeks, compared with 43% of those under age 45. At 3 years, the numbers were 63% vs 27%.
In a small randomized controlled trial,61 fat mass decreased by 6.6 kg in 17 people assigned to a program of diet and exercise, compared with a gain of 1.7 kg in a control group of 10 patients. Fat-free mass decreased by about 1 kg in both groups. The authors concluded that diet plus exercise (resistance training and strength training in this trial) could ameliorate frailty in obese older adults.
If exercise is appropriate, a physician should write a prescription for it, especially for resistance training, strengthening, flexibility, and stretching. This is important for patients with sarcopenic obesity and for those at high risk of chronic bone loss. The 2007 American College of Sports Medicine guidelines recommend muscle-strengthening activity of 8 to 10 exercises involving the major muscle groups, 10 to 15 repetitions at least twice a week. Flexibility and balance exercises should be included for those at risk of falls.62
Pharmacotherapy
At present, there are two general classes of weight-loss drugs: appetite suppressants and drugs that interfere with nutrient absorption.
Appetite suppressants include the sympathomimetics, which stimulate the release of dopamine and norepinephrine, resulting in increased satiety. Data—and therefore, recommendations—on their use in the elderly are very scarce, as most randomized controlled trials included only a small number of older people. A meta-analysis of drug therapy to treat obesity noted that the study population ranged in age from 34 to 54.63
The only approved drug currently available for use in older adults is orlistat, which blocks absorption of dietary fat by binding to intestinal lipase. A randomized controlled trial found the weight loss with orlistat to be comparable in older and younger adults.64,65
Review medications than can cause weight gain
When assessing older adults, always review the drugs they are taking. Those known to cause weight gain include certain of the following:
- Antiepileptics (eg, gabapentin)
- Antipsychotics (eg, olanzapine)
- Antidepressants (eg, tricyclics)
- Antihyperglycemic drugs (eg, sulfonylureas, thiazolidinediones)
- Beta-blockers
- Steroids.
If medically appropriate, a weight-neutral drug should be substituted for one suspected of causing weight gain. If a different physician (eg, a specialist) prescribed the original drug, he or she should be notified or consulted about any change.
Bariatric surgery
Bariatric surgery is the most effective weight-loss option, and more older patients are undergoing it than in the past. Dorman et al66 showed that the number of patients age 65 or older undergoing bariatric surgery increased from the year 2005 (when they accounted for 2%) to 2009 (when they accounted for 4.8%).
However, very few studies have provided information on the safety and effectiveness of bariatric surgery in older people. Several reports concluded that rates of perioperative morbidity and mortality are higher in older patients.67–69 Surgery resulted in marked weight loss and improvement in obesity-related complications and physical disability in older patients, although by a lower rate than in younger patients.
Varela et al70 examined the outcomes of bariatric surgery in a database from the University Health System Consortium Centers between 1999 and 2005. Patients over age 60 accounted for 1,339 (2.7%) of all bariatric operations performed. Compared with young and middle-aged patients, older patients had more comorbidities, longer hospital stays, and more complications, in addition to a higher in-hospital mortality rate. When risk-adjusted, the observed-to-expected mortality ratio for the older group was 0.9, compared with 0.7 in the young and middle-aged cohort.
Willkomm et al71 found an apparently higher operative risk profile in those over age 65 (n = 100) than in younger patients (n = 1,374), with higher rates of sleep apnea, diabetes, and hypertension. However, the operative outcomes were similar in the two groups in terms of operative time, length of stay, and 30-day readmission rates. The authors concluded that patients over age 65 had excellent outcomes compared with younger patients, suggesting that older age is not a risk factor for complications or death with bariatric surgery.
The American College of Surgeons National Surgical Quality Improvement Program evaluated the outcomes of 48,378 adults with a BMI greater than or equal to 35 kg/m2 who underwent bariatric surgery between 2005 and 2009.66 During this time, the number of patients age 65 and older seeking bariatric surgery increased from 1.5% to 4%. A total of 1,449 patients were in this age range. Thirty-day mortality rates did not differ significantly by age group and were less than 1% for all age ranges. Being age 65 or older was a significant predictor of prolonged length of stay but not of major adverse events. Significant predictors of major adverse events were a BMI greater than or equal to 55 kg/m2, cardiac comorbidities, a severe American Society of Anesthesiologists score, albumin levels lower than 3 g/dL, and creatinine levels greater than 1.5 mg/dL.
The most up-to-date study of the outcomes of bariatric surgery in patients over age 70 was a retrospective review at a single institution from 2007 to 2008 of 42 patients who underwent bariatric surgery.72 Twenty-two patients had laparoscopic gastric banding, 12 had laparoscopic sleeve gastrectomy, and 8 underwent laparoscopic Roux-en-Y gastric bypass. No patient died, complications occurred in 9 patients, and the rates of postoperative use of medications for hypertension, hyperlipidemia, diabetes, and osteoarthritis were reduced by about half. With the increasing number of patients seeking bariatric surgery, especially those over age 70, further prospective studies will determine if the outcomes are statistically significant.
If bariatric surgery is considered
The outcomes, complications, and mortality rates associated with bariatric surgery have been shown to be acceptable for adults age 65 and older. Perioperative risk assessment in the older obese patient seeking bariatric surgery is paramount to ensure that the benefits of the procedure justify any associated risks to the patient. Consequently, patients over age 65 should not be excluded out of hand: the patient’s individual risk of major adverse events must be identified beforehand.
If the patient is at risk, efforts should be made to reduce the risk to an acceptable level, including cardiac risk stratification, optimization of drug therapy, and discussions with the bariatric surgeon to plan on a less-invasive laparoscopic procedure. Otherwise, older obese patients can safely proceed with conventional bariatric surgery, which will help them achieve durable weight loss, improve quality of life, and reduce associated comorbidities.
The aforementioned studies of bariatric surgery are retrospective, include small numbers of patients, and lack long-term follow-up. The issues of long-term safety and the risk of death and morbidity in the aging population will require randomized controlled trials to answer these important questions.
At our hospital, we have seen an increase in the number of patients referred for a possible additional procedure (revision) to correct a problem from a previous bariatric surgery. The problems arising from the previous surgery can lead to weight gain or to excessive weight loss and malnutrition. To date, our institution has no policy on when to consider a revisional procedure in an older patient. All patients, including older ones, are assessed for the procedure on a case-by-case basis.
- Bray GA, Macdiarmid J. The epidemic of obesity. West J Med 2000; 172:78–79.
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med 1999; 341:1097–1105.
- Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007; 10:433–442.
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004; 291:1238–1245.
- Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. National vital statistics reports; vol 58no 10. Hyattsville, MD: National Center for Health Statistics. 2010.
- US Census Bureau International Database (IDB). Population projections of the US by age, sex, race, Hispanic origin, population division. http://www.census.gov/ipc/www/idb/country.php. Accessed September 13, 2013.
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004; 291:2847–2850.
- Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA 1994; 272:205–211.
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999; 282:1519–1522.
- Horani MH, Mooradian AD. Management of obesity in the elderly: special considerations. Treat Endocrinol 2002; 1:387–398.
- Beaufrère B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr 2000; 54(suppl 3):S48–S53.
- Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000; 72:694–701.
- Snitker S. Use of body fatness cutoff points (author reply). Mayo Clin Proc 2010; 85:1057; author reply 1057–1058.
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147:755–763. Erratum in Am J Epidemiol 1999; 149:1161.
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord 2001; 25:1730–1735.
- Molarius A, Seidell JC, Visscher TL, Hofman A. Misclassification of high-risk older subjects using waist action levels established for young and middle-aged adults—results from the Rotterdam Study. J Am Geriatr Soc 2000; 48:1638–1645.
- Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull 2011; 97:169–196.
- Turcato E, Bosello O, Di Francesco V, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord 2000; 24:1005–1010.
- Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005; 29:1011–1029.
- Heim N, Snijder MB, Heymans MW, Deeg DJ, Seidell JC, Visser M. Optimal cutoff values for high-risk waist circumference in older adults based on related health outcomes. Am J Epidemiol 2011; 174:479–489.
- Roubenoff R, Castaneda C. Sarcopenia—understanding the dynamics of aging muscle. JAMA 2001; 286:1230–1231.
- Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int J Obes Relat Metab Disord 2002; 26:953–960.
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004; 12:1995–2004.
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001; 137:231–243.
- Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 2004; 12:887–888.
- Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med 2009; 25:643–659.
- Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr 2000; 54(suppl 3):S92–S103.
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37:1595–1607.
- Corona G, Mannucci E, Forti G, Maggi M. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int J Androl 2009; 32:587–598.
- Haarbo J, Hassager C, Riis BJ, Christiansen C. Relation of body fat distribution to serum lipids and lipoproteins in elderly women. Atherosclerosis 1989; 80:57–62.
- Cignarella A, Kratz M, Bolego C. Emerging role of estrogen in the control of cardiometabolic disease. Trends Pharmacol Sci 2010; 31:183–189.
- Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med 1988; 109:18–24.
- Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med 1999; 107:542–548.
- Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Effect of aging on the relationship between multiple risk factors and waist circumference. J Am Geriatr Soc 2000; 48:788–794.
- Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med 2000; 160:2117–2128.
- Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med 1998; 338:1–7.
- Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007; 10:433–442.
- Zamboni M, Armellini F, Harris T, et al. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr 1997; 66:111–115.
- Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc 2005; 53:2112–2118.
- Inelmen EM, Sergi G, Coin A, Miotto F, Peruzza S, Enzi G. Can obesity be a risk factor in elderly people? Obes Rev 2003; 4:147–155.
- Elia M. Obesity in the elderly. Obes Res 2001; 9(suppl 4):244S–248S.
- Losonczy KG, Harris TB, Cornoni-Huntley J, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol 1995; 141:312–321.
- Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol 2006; 163:938–949.
- Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc 2008; 9:302–312.
- Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing 2010; 39:176–184.
- Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011; 364:1218–1229.
- Wannamethee SG, Shaper AG, Whincup PH, Walker M. Characteristics of older men who lose weight intentionally or unintentionally. Am J Epidemiol 2000; 151:667–675.
- Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med 1990; 7:228–233.
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000; 23:1499–1504.
- Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29:2102-2107.
- National Heart, Lung, and Blood Institute in cooperation with The National Institute of Diabetes and Digestive and Kidney Diseases. Clinical guidelines on the identification, evaluation and treatment of the overweight and obesity in adults, the evidence report. NIH Publication number 98-4803 http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf. Accessed September 13, 2013.
- Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005; 82:923–934.
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40-64 years. Am J Epidemiol 1995; 141:1128–1141.
- McTigue KM, Hess R, Ziouras J. Obesity in older adults: a systematic review of the evidence for diagnosis and treatment. Obesity (Silver Spring). 2006; 14:1485–1497.
- Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol 1995; 79:818–823.
- Pavlou KN, Krey S, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am J Clin Nutr 1989; 49(suppl 5):1115–1123.
- Kraemer WJ, Volek JS, Clark KL, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc 1999; 31:1320–1329.
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341.
- Banks M, Klein S, Sinacore D, Siener C, Villareal DT. Effects of weight loss and exercise on frailty in obese elderly subjects. J Am Geriatr Soc 2005; 53:S16.
- Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004; 50:1501–1510.
- Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 2006; 166:860–866.
- Nelson ME, Rejeski WJ, Blair SN, et al; American College of Sports Medicine; American Heart Association. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116:1094–1105.
- Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 2005; 142:532–546.
- Segal KR, Lucas C, Boldrin M, Hauptman J. Weight loss efficacy of orlistat in obese elderly adults (abstract). Obes Res 1999; 7(suppl):26S.
- Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000; 9:160–167.
- Dorman RB, Abraham AA, Al-Refaie WB, Parsons HM, Ikramuddin S, Habermann EB. Bariatric surgery outcomes in the elderly: an ACS NSQIP study. J Gastrointest Surg 2012; 16:35–44.
- Sugerman HJ, DeMaria EJ, Kellum JM, Sugerman EL, Meador JG, Wolfe LG. Effects of bariatric surgery in older patients. Ann Surg 2004; 240:243–247.
- St. Peter SD, Craft RO, Tiede JL, Swain JM. Impact of advanced age on weight loss and health benefits after laparoscopic gastric bypass. Arch Surg 2005; 140:165–168.
- Sosa JL, Pombo H, Pallavicini H, Ruiz-Rodriguez M. Laparoscopic gastric bypass beyond age 60. Obes Surg 2004; 14:1398–1401.
- Varela JE, Wilson SE, Nguyen NT. Outcomes of bariatric surgery in the elderly. Am Surg 2006; 72:865–869.
- Willkomm CM, Fisher TL, Barnes GS, Kennedy CI, Kuhn JA. Surgical weight loss >65 years old: is it worth the risk? Surg Obes Relat Dis 2010; 6:491–496.
- Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med 2001; 161:1194–1203.
- Bray GA, Macdiarmid J. The epidemic of obesity. West J Med 2000; 172:78–79.
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med 1999; 341:1097–1105.
- Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007; 10:433–442.
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004; 291:1238–1245.
- Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. National vital statistics reports; vol 58no 10. Hyattsville, MD: National Center for Health Statistics. 2010.
- US Census Bureau International Database (IDB). Population projections of the US by age, sex, race, Hispanic origin, population division. http://www.census.gov/ipc/www/idb/country.php. Accessed September 13, 2013.
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004; 291:2847–2850.
- Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA 1994; 272:205–211.
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999; 282:1519–1522.
- Horani MH, Mooradian AD. Management of obesity in the elderly: special considerations. Treat Endocrinol 2002; 1:387–398.
- Beaufrère B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr 2000; 54(suppl 3):S48–S53.
- Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000; 72:694–701.
- Snitker S. Use of body fatness cutoff points (author reply). Mayo Clin Proc 2010; 85:1057; author reply 1057–1058.
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147:755–763. Erratum in Am J Epidemiol 1999; 149:1161.
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord 2001; 25:1730–1735.
- Molarius A, Seidell JC, Visscher TL, Hofman A. Misclassification of high-risk older subjects using waist action levels established for young and middle-aged adults—results from the Rotterdam Study. J Am Geriatr Soc 2000; 48:1638–1645.
- Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull 2011; 97:169–196.
- Turcato E, Bosello O, Di Francesco V, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord 2000; 24:1005–1010.
- Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005; 29:1011–1029.
- Heim N, Snijder MB, Heymans MW, Deeg DJ, Seidell JC, Visser M. Optimal cutoff values for high-risk waist circumference in older adults based on related health outcomes. Am J Epidemiol 2011; 174:479–489.
- Roubenoff R, Castaneda C. Sarcopenia—understanding the dynamics of aging muscle. JAMA 2001; 286:1230–1231.
- Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int J Obes Relat Metab Disord 2002; 26:953–960.
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004; 12:1995–2004.
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001; 137:231–243.
- Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 2004; 12:887–888.
- Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med 2009; 25:643–659.
- Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr 2000; 54(suppl 3):S92–S103.
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37:1595–1607.
- Corona G, Mannucci E, Forti G, Maggi M. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int J Androl 2009; 32:587–598.
- Haarbo J, Hassager C, Riis BJ, Christiansen C. Relation of body fat distribution to serum lipids and lipoproteins in elderly women. Atherosclerosis 1989; 80:57–62.
- Cignarella A, Kratz M, Bolego C. Emerging role of estrogen in the control of cardiometabolic disease. Trends Pharmacol Sci 2010; 31:183–189.
- Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med 1988; 109:18–24.
- Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med 1999; 107:542–548.
- Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Effect of aging on the relationship between multiple risk factors and waist circumference. J Am Geriatr Soc 2000; 48:788–794.
- Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med 2000; 160:2117–2128.
- Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med 1998; 338:1–7.
- Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007; 10:433–442.
- Zamboni M, Armellini F, Harris T, et al. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr 1997; 66:111–115.
- Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc 2005; 53:2112–2118.
- Inelmen EM, Sergi G, Coin A, Miotto F, Peruzza S, Enzi G. Can obesity be a risk factor in elderly people? Obes Rev 2003; 4:147–155.
- Elia M. Obesity in the elderly. Obes Res 2001; 9(suppl 4):244S–248S.
- Losonczy KG, Harris TB, Cornoni-Huntley J, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol 1995; 141:312–321.
- Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol 2006; 163:938–949.
- Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc 2008; 9:302–312.
- Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing 2010; 39:176–184.
- Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011; 364:1218–1229.
- Wannamethee SG, Shaper AG, Whincup PH, Walker M. Characteristics of older men who lose weight intentionally or unintentionally. Am J Epidemiol 2000; 151:667–675.
- Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med 1990; 7:228–233.
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000; 23:1499–1504.
- Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29:2102-2107.
- National Heart, Lung, and Blood Institute in cooperation with The National Institute of Diabetes and Digestive and Kidney Diseases. Clinical guidelines on the identification, evaluation and treatment of the overweight and obesity in adults, the evidence report. NIH Publication number 98-4803 http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf. Accessed September 13, 2013.
- Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005; 82:923–934.
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40-64 years. Am J Epidemiol 1995; 141:1128–1141.
- McTigue KM, Hess R, Ziouras J. Obesity in older adults: a systematic review of the evidence for diagnosis and treatment. Obesity (Silver Spring). 2006; 14:1485–1497.
- Ryan AS, Pratley RE, Elahi D, Goldberg AP. Resistive training increases fat-free mass and maintains RMR despite weight loss in postmenopausal women. J Appl Physiol 1995; 79:818–823.
- Pavlou KN, Krey S, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am J Clin Nutr 1989; 49(suppl 5):1115–1123.
- Kraemer WJ, Volek JS, Clark KL, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc 1999; 31:1320–1329.
- Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001; 21:323–341.
- Banks M, Klein S, Sinacore D, Siener C, Villareal DT. Effects of weight loss and exercise on frailty in obese elderly subjects. J Am Geriatr Soc 2005; 53:S16.
- Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004; 50:1501–1510.
- Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 2006; 166:860–866.
- Nelson ME, Rejeski WJ, Blair SN, et al; American College of Sports Medicine; American Heart Association. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116:1094–1105.
- Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 2005; 142:532–546.
- Segal KR, Lucas C, Boldrin M, Hauptman J. Weight loss efficacy of orlistat in obese elderly adults (abstract). Obes Res 1999; 7(suppl):26S.
- Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000; 9:160–167.
- Dorman RB, Abraham AA, Al-Refaie WB, Parsons HM, Ikramuddin S, Habermann EB. Bariatric surgery outcomes in the elderly: an ACS NSQIP study. J Gastrointest Surg 2012; 16:35–44.
- Sugerman HJ, DeMaria EJ, Kellum JM, Sugerman EL, Meador JG, Wolfe LG. Effects of bariatric surgery in older patients. Ann Surg 2004; 240:243–247.
- St. Peter SD, Craft RO, Tiede JL, Swain JM. Impact of advanced age on weight loss and health benefits after laparoscopic gastric bypass. Arch Surg 2005; 140:165–168.
- Sosa JL, Pombo H, Pallavicini H, Ruiz-Rodriguez M. Laparoscopic gastric bypass beyond age 60. Obes Surg 2004; 14:1398–1401.
- Varela JE, Wilson SE, Nguyen NT. Outcomes of bariatric surgery in the elderly. Am Surg 2006; 72:865–869.
- Willkomm CM, Fisher TL, Barnes GS, Kennedy CI, Kuhn JA. Surgical weight loss >65 years old: is it worth the risk? Surg Obes Relat Dis 2010; 6:491–496.
- Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med 2001; 161:1194–1203.
KEY POINTS
- In older patients, the waist circumference may be more appropriate than the body mass index as a measure of adiposity.
- Data suggest that being moderately overweight may offer a survival advantage in older people, but a body mass index of 30 kg/m2 or higher continues to be associated with many health risks in this age group.
- In obese patients, intensive lifestyle interventions with an emphasis on exercise and strength training can optimize their overall health and quality of life.
- Weight-loss recommendations in older obese patients should take into account the benefits and risks of lifestyle interventions, drug therapy, and bariatric surgery.
How to spot heritable breast cancer: A primary care physician’s guide
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
PATIENT 1: A PERSONAL AND FAMILY HISTORY OF BREAST CANCER
A 55-year-old Ashkenazi Jewish woman presents to your clinic for her annual physical. She reports that she had been diagnosed with breast cancer 10 years ago and that it had been treated with lumpectomy. You recall that Ashkenazi Jewish ethnicity and a diagnosis of breast cancer before age 50 are red flags for a hereditary cancer syndrome, and you ask about her family history of cancer. She reports that her mother was diagnosed with breast cancer in her 60s. The patient wants to know if her daughter should start breast cancer screening.
What do you do next?
Facing increasing demands and a plethora of information to be discussed in a short time, primary care physicians may find it challenging to inform patients about the possibility of a hereditary cancer syndrome, to assess the risk, to organize genetic testing if appropriate, and to counsel patients about their management options. As our knowledge of the genetics of breast cancer continues to expand, this information will become more detailed and complex.
Nevertheless, primary care physicians can help identify patients who may have a syndrome of inherited cancer predisposition or whose family history raises concern for familial breast cancer. Patients in both groups may be candidates for genetic risk assessment, for special management options for women at high risk, or for both.
This article provides an overview of inherited conditions associated with higher breast cancer risk, and guidelines to help physicians recognize patients in their own practice for whom a genetics referral may be appropriate.
BREAST CANCER IS COMPLEX AND HETEROGENEOUS
Breast cancer is the second-leading cause of cancer deaths in women. According to the American Cancer Society, an estimated 234,340 new cases of breast cancer are expected to be diagnosed in women in the United States in 2013, and about 2,240 new cases are expected in men; 39,620 women and 410 men are expected to die of it.1
Breast cancer is a complex and heterogeneous disease, influenced by many factors, of which female sex and increasing age are the most significant. Modifiable risk factors include obesity, use of combined hormone replacement therapy, and physical inactivity. Other risk factors include dense breast tissue, having had a breast biopsy in the past, the finding of atypical hyperplasia on biopsy, a history of high-dose chest radiation, and reproductive factors that include early menarche, late menopause, nulliparity, and birth of first child after age 30.
After female sex and age, family history of the disease is the most significant risk factor for breast cancer.2 If a woman has a first-degree relative (mother, sister, daughter) with breast cancer, her risk is 1.8 times higher, and if she has a second-degree relative (aunt, grandmother) with breast cancer, her risk is 1.3 times higher.3
Hereditary cancer predisposition syndromes account for 5% to 10% of cases of breast cancer. These are caused by a germline mutation in a highly penetrant gene that considerably increases the risk of malignancies of the breast and other tissues. These conditions are inherited in an autosomal-dominant fashion, with age of onset tending to be significantly—several decades—younger than the median age of onset in the general population. The most common of these is hereditary breast and ovarian cancer syndrome, caused by germline mutations of the BRCA1 or BRCA2 gene.
Familial breast cancers account for 15% to 20% of cases. Here, the women who develop breast cancer have multiple family members who are also affected but without an obvious inheritance pattern, and the age of onset is similar to that in the general population.4
Sporadic forms of breast cancer account for the remaining 70% to 80% of cases. Their development can be attributed mainly to nonhereditary causes, such as the environmental and personal risk factors listed above. In general, sporadic forms of breast cancer occur at older ages, with no particular inheritance pattern and with frequency of occurrence in a family comparable to that in the general population.
IS A GENETICS CONSULTATION NEEDED?
In the case described above, the primary care physician gathered basic information about the patient’s cancer-related personal and family history. Asking a few key questions (Table 1)5,6 can help physicians understand two important things: whether a more detailed assessment of genetic risk and counseling by a genetics professional are indicated, and whether the patient would benefit from additional cancer screening and prevention.
Table 2 summarizes the National Comprehensive Cancer Network’s recommendations for cancer genetics consultation.5 These red flags for a hereditary breast cancer syndrome can help primary care providers identify patients for whom a cancer genetics referral is appropriate. Of note: the maternal and paternal family histories are equally important.
Because our patient was diagnosed with breast cancer before age 50 and is of Ashkenazi Jewish ethnicity, she meets these criteria and warrants a cancer genetics consultation.
What is a cancer-focused genetic counseling session?
The tenets of genetic counseling, described previously in this series,7 are relevant to hereditary cancer syndromes. Cancer risk assessment and genetic counseling constitute the process of identifying and counseling individuals at risk of familial or hereditary cancer.8
As in other genetic counseling scenarios, a detailed pedigree (family tree) is taken, and this information, along with the patient’s personal medical history, allows a genetics specialist to determine if the presentation is most suggestive of sporadic, familial, or hereditary cancer.
A common misconception among patients is that there is a single genetic test for hereditary breast cancer, when in fact many highly penetrant predisposition genes have been linked to heightened risk (see below). The syndromes summarized in Table 35,9–18 are part of the differential diagnosis for every patient presenting with a personal or family history of breast cancer, and the detailed information from the personal and family history, ascertained during the assessment, ensures the right syndrome is explored within a family.
Cancer-focused genetic counseling may also help a patient or family process the psychological and emotional responses that can occur when cancer risk is discussed: eg, fear of cancer and death; guilt a parent may feel for passing on a genetic predisposition; and survivor guilt experienced by family members who test negative.
Genetic counselors are trained to recognize patients who may benefit from additional counseling. Not all patients pursuing cancer-focused genetic testing need a thorough evaluation by a psychologist, unlike those with adult-onset neurodegenerative conditions such as Huntington disease. Rather, the genetic counselor discusses the psychological implications of cancer-focused genetic testing and can refer the patient to a psychologist, therapist, social worker, or others if he or she feels the patient may benefit.8
Some patients come to a genetic counseling session with concerns about whether their insurance will pay for testing, and about whether they will face discrimination because of the testing results. In most situations, genetic testing is deemed medically necessary and is covered by the patient’s insurance. When testing is necessary, genetic counselors are skilled at preauthorizing it and writing letters of medical necessity. They are also familiar with laws and regulations that protect patients, such as the Genetic Information Nondiscrimination Act, which protects patients from insurance and employment discrimination.
Because a cancer-focused genetic counseling session typically lasts 1 hour, the counselor has enough time to address these and any other concerns that might prevent a patient who is otherwise interested in genetic testing from pursuing it.
HOW CAN GENETIC TESTING HELP?
Genetic testing for hereditary cancer syndromes can have personal benefit for the patient and at-risk family members.
Note that the syndromes in Table 3 all increase the risk of more than one type of cancer. Patients with these syndromes frequently receive care from multiple subspecialists to mitigate those risks. Guidelines exist for each of these syndromes and, if followed, may prevent the morbidity and possibly death from the genotype-specific cancers that would otherwise be in the patient’s future. For patients found to have a hereditary cancer syndrome, medical management options include more-frequent cancer screening or surveillance, prophylactic surgery, and preventive medical treatment, which will be reviewed in a future article in this series.
Identifying the specific mutation in one family member allows at-risk relatives, both female and male, to then take advantage of predictive testing, with genetic counseling. If they test positive for the risk-increasing mutation, they too can take advantage of the management options for people at high risk. If they test negative, they can continue to undergo the same screening as recommended for the general population. Also, they may be relieved to know that their cancer risk is no greater than that in the general population.
The American Society of Clinical Oncology9 recommends genetic counseling and testing when all of the following are true:
- There is a personal or family history suggesting genetic cancer susceptibility
- The test can be adequately interpreted
- The results will aid in the diagnosis or influence the medical or surgical management of the patient or family at hereditary risk of cancer.
Professional society guidelines also recommend that genetic testing be done only with genetic counseling before and after.5,6,8 The National Society of Genetic Counselors provides a list of clinical genetic counselors, organized by geographical area, at www.nsgc.org.
PATIENT 1 RECEIVES GENETIC TESTING AND COUNSELING
Let’s return to the Ashkenazi Jewish patient who has a personal and family history of breast cancer, whom you referred for cancer genetics consultation and who attends this appointment. A detailed personal and family history is gathered, and a brief physical examination is done, which reveals that the patient has macrocephaly and a history of multiple uterine fibroids.
The genetic differential diagnosis for your patient includes hereditary breast and ovarian cancer syndrome (resulting from mutations in the BRCA1 and BRCA2 genes) and Cowden syndrome (from mutations in the PTEN gene) (TABLE 3). The counselor uses BRCAPRO, a statistical risk-assessment tool that estimates a patient’s risk of harboring a BRCA1 or BRCA2 mutation based on ethnicity and personal and family history of cancer, and finds her risk to be 31%. In view of this risk, genetic testing for BRCA1 and BRCA2 is offered after a detailed discussion of the genetic differential diagnosis, the implications of a positive vs a negative test result, the possibility of finding gene changes (variants) of unknown significance, and the implications of the test results for family members.
Your patient elects to pursue BRCA1 and BRCA2 genetic testing and the results are negative—no mutations in either gene are found. PTEN testing is recommended next, which your patient elects to undergo. A mutation in the PTEN gene is found, indicating that she has Cowden syndrome. This result and its implications are discussed in a posttest genetic counseling session.
Cowden syndrome is an autosomal-dominant condition that carries a heightened risk of benign and malignant neoplasms, including a lifetime risk of breast cancer of up to 85%, with the average age at diagnosis in the 40s. Mutations in the PTEN gene also predispose to other cancer types, including nonmedullary thyroid, uterine, renal, and colorectal cancers, as well as melanoma.9 Multiple benign skin lesions and gastrointestinal polyposis are common.20
During the appointment, medical management options for patients with PTEN mutations are presented (Table 4).9 Given that your patient’s breast cancer was initially treated with lumpectomy, her remaining breast tissue is at risk of a second malignancy. She has never undergone thyroid imaging, colonoscopy, or kidney imaging. She reports that lately she has had occasional abnormal uterine bleeding and pain, which she believes are caused by her uterine fibroids. Given these symptoms and in light of her PTEN mutation, hysterectomy may be presented to her as an option. The genetics team sends a detailed clinical note directly to the primary care physician so they can coordinate and “quarterback” the patient’s care.
Like many patients, your patient is very concerned about how this information may affect her daughter. She first expresses some guilt at having to tell her daughter that she may have “given” her a risk of cancer. However, during the course of the genetic counseling session, she accepts that she could not have prevented her daughter from possibly inheriting this mutation, and understands that sharing this information will enable her daughter to pursue testing to help her understand her own risks.
When a known mutation exists in the family, as is the case with your patient, predictive testing only for that mutation gives a 100% accurate result. During a separate genetic counseling appointment, the patient’s daughter opts to proceed with testing and is found to be negative for her mother’s PTEN mutation.
WHAT HAPPENS WHEN GENETIC TESTING IS NOT INDICATED?
Cancer genetic risk assessment and counseling provides benefits even when genetic testing is not indicated. In some situations genetic testing is not warranted, but referral for heightened surveillance for breast cancer is deemed necessary. Patients who have a personal or family history of cancer can still gain from a detailed assessment of their personal and family history and may come away relieved after learning that they or their family members are not at high risk of developing cancer. Such patients or families may be classified as demonstrating either familial or sporadic breast cancer diagnoses.
Familial breast cancer
Familial breast cancers, believed to account for 15% to 20% of all cases of breast cancer, share features with hereditary breast cancer syndromes.4 In affected families, the frequency of breast cancer is higher than in the general population (multiple family members may be affected), and the age of onset tends to be close to that in the general population.
Members of a family with familial breast cancer who have not yet developed the disease may be at increased risk of it. Several risk-assessment tools (the Gail, Tyrer-Cuzick, Claus, and other models)21–25 use personal and family history to estimate breast cancer risk.
Depending on the assessed risk, additional options for screening and surveillance are available. The American Cancer Society recommends magnetic resonance imaging (MRI) in addition to annual mammography for women whose lifetime risk of breast cancer is greater than 20%. They also recommend that women at moderately increased risk (ie, 15%–20% lifetime risk) talk to their doctor about the benefits and limitations of adding MRI screening to yearly mammography.1
Sporadic breast cancer
Sporadic forms of breast cancer account for 70% to 80% of cases of breast cancer. Sporadic breast cancers are thought to have mainly nonhereditary causes, with environment and personal risk factors playing a large role.
Women with apparently sporadic breast cancers are diagnosed at or beyond the average age at diagnosis in the general population and do not have a family history that suggests either a hereditary cancer syndrome or familial breast cancer. If they undergo a cancer risk assessment, they may be relieved to learn that other women in their family do not have a high probability of being affected, and that they themselves do not appear to be at increased risk of other malignancies.
PATIENT 2: NEGATIVE TEST RESULTS ARE SOMETIMES ‘UNINFORMATIVE’
A healthy 35-year-old woman is referred for a genetics consultation by her gynecologist because her mother developed breast cancer at age 40 and died of the disease. A detailed personal and family history and risk assessment are done. After pretest genetic counseling, testing for BRCA1 and BRCA2 mutations (hereditary breast and ovarian cancer syndrome) is ordered, and the patient’s test results are negative. Risk assessment determines that no other hereditary cancer syndrome is likely. Therefore, no other genetic testing is offered at this time.
Genetic testing is most informative when performed first on the family member at highest risk of having a mutation. For families with breast cancer, this is typically the person with cancer diagnosed at the earliest age.
Unfortunately, sometimes these family members cannot be tested because they are deceased or otherwise unavailable. In such situations, it is acceptable to offer testing to a close, unaffected relative, such as your patient. Pretest genetic counseling in these circumstances is key, highlighting the fact that negative (normal) results would be uninformative. In your case, we cannot know whether the patient’s mother would have tested positive for a BRCA1 or BRCA2 mutation and your patient is a “true negative,” or whether her mother would have tested negative as well.
In unaffected patients with uninformative genetic testing results, medical management is based on the patient’s personal risk factors and family history of cancer. For your patient, statistical risk modeling tools (the Gail, Claus, Couch, and Tyrer-Cuzick models) determine that her risk of developing breast cancer is 22% to 28.5%, qualifying her for MRI along with yearly mammography per the American Cancer Society guidelines previously discussed.
KNOWLEDGE CONTINUES TO EXPAND
Major advances in the understanding of breast cancer susceptibility were made in the last decade through genetic linkage mapping in families that have an overabundance of members with breast cancer.26–28 Additionally, as more information is acquired, other genes predisposing to cancer or modifying cancer risk may be identified and additional knowledge gained.
With the advent of gene-panel-based testing and exome sequencing, we will incidentally discover mutations that predispose to cancer in patients in whom we were not looking for these mutations. With improving technology and value-based health care delivery, providers must continue to embrace multidisciplinary care, and genetics will become central in guiding medical management. In the event of an incidental finding suggesting susceptibility to heritable cancer, a consult to genetic counseling is recommended.
Many studies of the genetics of breast cancer are now focusing on known hereditary breast cancer syndromes and on possibilities for risk reduction, lifestyle modification, and identification of genetic variations that may increase or decrease cancer risk for an individual patient. The Center for Personalized Genetic Healthcare at Cleveland Clinic is collaborating in one such study. Titled “Risk Factor Analysis of Hereditary Breast and Ovarian Cancer Syndrome,” it is an international study led by a leading breast cancer researcher, Dr. Steven Narod from the Women’s College Research Institute in Toronto, ON. This study is focusing on women with a BRCA1 or BRCA2 mutation and their personal cancer risk factors, lifestyle choices, and overall development of cancer. This research group and others are also focusing on identifying genetic “modifiers” of cancer risk in these high-risk women.29
For patients who do not have a hereditary cancer syndrome, research is further exploring novel genes and their relation to breast cancer risk. One such study in our laboratory has found that several genes once thought only to cause an increased risk of hereditary paraganglioma may also predispose to breast and thyroid cancer.29,31 Additional research in this area is under way to clarify these risks.
GOOD SCIENCE, BAD MEDICINE?
Other research studies have identified a number of genes currently thought to be “moderately penetrant” for breast cancer risk, meaning that they may confer a risk of breast cancer slightly greater than that in the general population, but in some instances the risk has not been proven to be high enough to alter a patient’s management.32,33
Although a few clinical laboratories currently offer testing for these kinds of genes, the clinical utility of this testing is questionable. Before offering testing on a clinical basis, we need clear, consistent data on the types of cancers associated with these genes and on the lifetime percentage risk of acquiring these cancers. Currently, it is difficult to understand whether a variant in a moderately penetrant gene is the true explanation behind a patient’s breast cancer diagnosis. If such a variant is identified and family members pursue testing for it, should those family members who test negative be considered to have the same risk of cancer as the general population? And should family members testing positive be offered prophylactic surgical options?
Without more data these questions cannot be answered, and until such data are gathered, we believe that testing for moderately penetrant genes should not be performed outside of a research study. The Center for Personalized Genetic Healthcare in Cleveland Clinic’s Genomic Medicine Institute can assist in educating and coordinating patients’ enrollment in such research studies.
PUTTING IT ALL TOGETHER
Primary care physicians are the first-line providers to individuals and families, many of whom have a personal or family history of breast cancer. Identifying patients at risk of breast cancer and hereditary cancer syndromes can be challenging in this era of shortened appointment times and patients with complex medical histories.
Reviewing an individual’s personal and cancer family history is a necessary first step in considering appropriate medical management recommendations for cancer screening and prevention, the cornerstone of personalized health care. Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from high-risk breast cancer surveillance.
Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history. Genetic counselors empower patients to make educated and informed decisions about genetic testing, cancer screening, and prevention.
As health care continues to focus more on prevention in this new era of genomic medicine and value-based delivery of health care, genetic counselors will serve as powerful allies to physicians.34
Acknowledgments: We would like to thank Dr. Colleen Clayton and Dr. Lynn Pattimakiel of the Medicine Institute, Cleveland Clinic, for their critical review of and thoughtful feedback on this manuscript.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
- American Cancer Society. Breast cancer: detailed guide( 2013). http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/index. Accessed November 12, 2013.
- McTiernan A, Gilligan MA, Redmond C. Assessing individual risk for breast cancer: risky business. J Clin Epidemiol 1997; 50:547–556.
- Teerlink CC, Albright FS, Lins L, Cannon-Albright LA. A comprehensive survey of cancer risks in extended families. Genet Med 2012; 14:107–114.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer risk reduction (version 1.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Genetic/familial high risk assessment: breast and ovarian (version 4.2013). http://www.nccn.org. Accessed November 21, 2013.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis (version 2.2013). http://www.nccn.org. Accessed November 21, 2013.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med 2012; 79:560–568.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the National Society of Genetic Counselors. J Genet Couns 2004; 13:83–114.
- Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012; 18:400–407.
- Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62:676–689.
- Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol 2004; 22:735–742.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336:1401–1408.
- Birch JM, Hartley AL, Tricker KJ, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res 1994; 54:1298–1304.
- Chompret A, Brugières L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer 2000; 82:1932–1937.
- Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol 2009; 27:1250–1256.
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 2003; 21:313–320.
- Fitzgerald RC, Hardwick R, Huntsman D, et al; International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010; 47:436–444.
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet 2013; 163:114–121.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 1994; 73:643–651.
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med 1997; 336:1409–1415.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 2004; 23:1111–1130.
- Gail MH, Anderson WF, Garcia-Closas M, Sherman ME. Absolute risk models for subtypes of breast cancer. J Natl Cancer Inst 2007; 99:1657–1659.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Kent P, O’Donoghue JM, O’Hanlon DM, Kerin MJ, Maher DJ, Given HF. Linkage analysis and the susceptibility gene (BRCA-1) in familial breast cancer. Eur J Surg Oncol 1995; 21:240–241.
- Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1993; 52:678–701.
- Ormiston W. Hereditary breast cancer. Eur J Cancer Care (Engl) 1996; 5:13–20.
- Couch FJ, Wang X, McGuffog L, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet 2013; 9:e1003212.
- Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA 2010; 304:2724–2731.
- Ni Y, He X, Chen J, et al. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependent destabilization of p53. Hum Mol Genet 2012; 21:300–310.
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011; 71:2222–2229.
- Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A 2010; 107:12629–12633.
- Eng C. Molecular genetics to genomic medicine: at the heart of value-based delivery of healthcare. Mol Genet Genom Med 2013; 1:4–6.
KEY POINTS
- Primary care physicians play a critical role in identifying patients at risk of inherited health problems.
- Hereditary cancers are important to detect because the age of onset is early, multiple primary cancers can develop, and cancer predisposition may be inherited.
- Hereditary syndromes account for only a minority of cases of breast cancer, but women who bear the responsible mutations have an extremely high risk.
- Patients with hereditary breast cancer syndromes and those with familial breast cancer can benefit from heightened surveillance for breast cancer.
- Cancer genetics risk assessment ensures that the correct genetic testing is offered to the most appropriate patients, with personalized interpretation of results and provision of future management recommendations based on the individual patient’s personal and family history.
Do all hospitalized patients need stress ulcer prophylaxis?
No. Based on current evidence and guidelines, routine acid-suppressive therapy to prevent stress ulcers has no benefit in hospitalized patients outside the critical-care setting. Only critically ill patients who meet specific criteria, as described in the guidelines of the American Society of Health System Pharmacists, should receive acid-suppressive therapy.
Unfortunately, routine stress ulcer prophylaxis is common in US hospitals, unnecessarily putting patients at risk of complications and adding costs.
STRESS ULCER AND CRITICAL ILLNESS
Stress ulcers—ulcerations of the upper part of the gastrointestinal (GI) mucosa in the setting of acute disease—usually involve the fundus and body of the stomach. The stomach is lined with a glycoprotein mucous layer rich in bicarbonates, forming a physiologic barrier to protect the gastric wall from acid insult by neutralizing hydrogen ions. Disruption of this protective layer can occur in critically ill patients (eg, those with shock or sepsis) through overproduction of uremic toxins, increased reflux of bile salts, compromised blood flow, and increased stomach acidity through gastrin stimulation of parietal cells.
More than 75% of patients with major burns or cranial trauma develop endoscopic mucosal abnormalities within 72 hours of injury.1 In critically ill patients, the risk of ulcer-related overt bleeding is estimated to be 5% to 25%. Furthermore, 1% to 5% of stress ulcers can be deep enough to erode into the submucosa, causing clinically significant GI bleeding, defined as bleeding complicated by hemodynamic compromise or a drop in hemoglobin that requires a blood transfusion.2 In contrast, in inpatients who are not critically ill, the risk of overt bleeding from stress ulcers is less than 1%.3
ADDRESSING RISK
A multicenter prospective cohort study of 2,252 intensive care patients2 reported two main risk factors for significant bleeding caused by stress ulcers: mechanical ventilation for more than 48 hours and coagulopathy, defined as a platelet count below 50 × 109/L, an international normalized ratio greater than 1.5, or a partial thromboplastin time more than twice the control value.4 In hemodynamically stable patients receiving anticoagulation in a general medical or surgical ward, the risk of GI bleeding was low, and acid suppression failed to lower the rate of stress ulcer occurrence.3
Other risk factors include severe sepsis, shock, liver failure, kidney failure, burns over 35% of the total body surface, organ transplantation, cranial trauma, spinal cord trauma, history of peptic ulcer disease, and history of upper GI bleeding.3,5,6 Steroid therapy is not considered a risk factor for stress ulcers unless it is used in the presence of another risk factor such as use of aspirin or nonsteroidal antiinflammatory drugs (NSAIDs).2
INDICATIONS FOR PROPHYLAXIS
Prophylaxis with a proton pump inhibitor (PPI) is indicated in specific conditions—ie, peptic ulcer disease, gastroesophageal reflux disease, chronic NSAID therapy, and Zollinger-Ellison syndrome—and to eradicate Helicobacter pylori infection.7 But in the United States, stress ulcer prophylaxis is overused in general-care floors despite the lack of supporting evidence.
The American Society of Health System Pharmacists guidelines recommend it in the intensive care unit for patients with any of the following: coagulopathy, prolonged mechanical ventilation (more than 48 hours), GI ulcer or bleeding within the past year, sepsis, a stay longer than 1 week in the intensive care unit, occult GI bleeding for 6 or more days, and steroid therapy with more than 250 mg of hydrocortisone daily.8 Hemodynamically stable patients admitted to general-care floors should not receive stress ulcer prophylaxis, as it only negligibly decreases the rate of GI bleeding, from 0.33% to 0.22%.9
WHY ROUTINE ULCER PROPHYLAXIS IS NOT FOR ALL HOSPITALIZED PATIENTS
Although stress ulcer prophylaxis is often considered benign, its lack of proven benefit, additional cost, and risk of adverse effects, including interactions with foods and other drugs, preclude using it routinely for all hospitalized patients.10,11 Chronic use of PPIs has been associated with complications, as discussed below.
Infection
Acid suppression may impair the destruction of ingested microorganisms, resulting in overgrowth of bacteria.12 Overuse of PPIs may increase the risk of several infections:
- Diarrhea due to Clostridium difficile12
- Community-acquired pneumonia, from increased microaspiration of overgrown microorganisms into the lung.12
- Spontaneous bacterial peritonitis in patients with cirrhosis,13 although the mechanism is not clear. (Small-bowel bacterial overgrowth is the hypothesized cause.)
Bone fracture
PPIs lower gastric acidity, and this can inhibit intestinal calcium absorption. Furthermore, PPIs may directly inhibit bone resorption by osteoclasts.14
Reduction in clopidogrel efficacy
PPIs may reduce the efficacy of clopidogrel as a result of competitive inhibition of cytochrome CYP2C19, which is necessary to metabolize clopidogrel to its active forms. Therefore, concomitant use of clopidogrel with omeprazole, esomeprazole, or other CYP2C19 inhibitors is not recommended.15
Nutritional deficiencies
The overgrown microorganisms consume cobalamin in the stomach, resulting in vitamin B12 deficiency. Acid-suppressive therapy can also reduce the absorption of magnesium and iron.12
Unnecessary cost
Heidelbaugh and Inadomi16 reviewed the non-evidence-based use of stress ulcer prophylaxis in patients admitted to a large university hospital and estimated that it entailed a cost to the hospital of $111,791 over the course of a year.
WHICH ULCER PROPHYLAXIS SHOULD BE USED IN CRITICALLY ILL PATIENTS?
Studies have shown histamine-2 blockers to be superior to antacids and sucralfate in preventing stress ulcer and GI bleeding,8,15 but no study has compared PPIs with sucralfate and antacids.
When indicated, an oral PPI is preferred over an oral histamine-2 blocker for GI prophylaxis.17 This practice is considered cost-effective and is associated with lower rates of stress ulcer and GI bleeding. In intubated patients, however, an intravenous histamine-2 blocker is preferable to an intravenous PPI.3,8,11 Interestingly, no difference was reported between PPIs and histamine-2 blockers in terms of mortality rate or reduction in the incidence of nosocomial pneumonia.17
OUR RECOMMENDATION
Only critically ill patients who meet the specific criteria described here should receive stress ulcer prophylaxis. More effort is needed to educate residents, medical staff, and pharmacists about current guidelines. Computerized ordering templates and reminders to discontinue prophylaxis at discharge or step-down may decrease overall use, reduce costs, and limit potential side effects.18
- DePriest JL. Stress ulcer prophylaxis. Do critically ill patients need it? Postgrad Med 1995; 98:159–168.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Qadeer MA, Richter JE, Brotman DJ. Hospital-acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings. J Hosp Med 2006; 1:13–20.
- Shuman RB, Schuster DP, Zuckerman GR. Prophylactic therapy for stress ulcer bleeding: a reappraisal. Ann Intern Med 1987; 106:562–567.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA 1996; 275:308–314.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–1391.e5.
- Barkun AN, Bardou M, Pham CQ, Martel M. Proton pump inhibitors vs histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol 2012; 107:507–520.
- Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med 2011; 171:991–997.
- Cook DJ. Stress ulcer prophylaxis: gastrointestinal bleeding and nosocomial pneumonia. Best evidence synthesis. Scand J Gastroenterol Suppl 1995; 210:48–52.
- Messori A, Trippoli S, Vaiani M, Gorini M, Corrado A. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: meta-analysis of randomised controlled trials. BMJ 2000; 321:1103–1106.
- Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol 2012; 5:219–232.
- Deshpande A, Pasupuleti V, Thota P, et al. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol 2013; 28:235–242.
- Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H(+)- ATPase. Curr Pharm Des 2002; 8:2033–2048.
- ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis. ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm 1999; 56:347–379.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2013; 41:693–705.
- Liberman JD, Whelan CT. Brief report: reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med 2006; 21:498–500.
No. Based on current evidence and guidelines, routine acid-suppressive therapy to prevent stress ulcers has no benefit in hospitalized patients outside the critical-care setting. Only critically ill patients who meet specific criteria, as described in the guidelines of the American Society of Health System Pharmacists, should receive acid-suppressive therapy.
Unfortunately, routine stress ulcer prophylaxis is common in US hospitals, unnecessarily putting patients at risk of complications and adding costs.
STRESS ULCER AND CRITICAL ILLNESS
Stress ulcers—ulcerations of the upper part of the gastrointestinal (GI) mucosa in the setting of acute disease—usually involve the fundus and body of the stomach. The stomach is lined with a glycoprotein mucous layer rich in bicarbonates, forming a physiologic barrier to protect the gastric wall from acid insult by neutralizing hydrogen ions. Disruption of this protective layer can occur in critically ill patients (eg, those with shock or sepsis) through overproduction of uremic toxins, increased reflux of bile salts, compromised blood flow, and increased stomach acidity through gastrin stimulation of parietal cells.
More than 75% of patients with major burns or cranial trauma develop endoscopic mucosal abnormalities within 72 hours of injury.1 In critically ill patients, the risk of ulcer-related overt bleeding is estimated to be 5% to 25%. Furthermore, 1% to 5% of stress ulcers can be deep enough to erode into the submucosa, causing clinically significant GI bleeding, defined as bleeding complicated by hemodynamic compromise or a drop in hemoglobin that requires a blood transfusion.2 In contrast, in inpatients who are not critically ill, the risk of overt bleeding from stress ulcers is less than 1%.3
ADDRESSING RISK
A multicenter prospective cohort study of 2,252 intensive care patients2 reported two main risk factors for significant bleeding caused by stress ulcers: mechanical ventilation for more than 48 hours and coagulopathy, defined as a platelet count below 50 × 109/L, an international normalized ratio greater than 1.5, or a partial thromboplastin time more than twice the control value.4 In hemodynamically stable patients receiving anticoagulation in a general medical or surgical ward, the risk of GI bleeding was low, and acid suppression failed to lower the rate of stress ulcer occurrence.3
Other risk factors include severe sepsis, shock, liver failure, kidney failure, burns over 35% of the total body surface, organ transplantation, cranial trauma, spinal cord trauma, history of peptic ulcer disease, and history of upper GI bleeding.3,5,6 Steroid therapy is not considered a risk factor for stress ulcers unless it is used in the presence of another risk factor such as use of aspirin or nonsteroidal antiinflammatory drugs (NSAIDs).2
INDICATIONS FOR PROPHYLAXIS
Prophylaxis with a proton pump inhibitor (PPI) is indicated in specific conditions—ie, peptic ulcer disease, gastroesophageal reflux disease, chronic NSAID therapy, and Zollinger-Ellison syndrome—and to eradicate Helicobacter pylori infection.7 But in the United States, stress ulcer prophylaxis is overused in general-care floors despite the lack of supporting evidence.
The American Society of Health System Pharmacists guidelines recommend it in the intensive care unit for patients with any of the following: coagulopathy, prolonged mechanical ventilation (more than 48 hours), GI ulcer or bleeding within the past year, sepsis, a stay longer than 1 week in the intensive care unit, occult GI bleeding for 6 or more days, and steroid therapy with more than 250 mg of hydrocortisone daily.8 Hemodynamically stable patients admitted to general-care floors should not receive stress ulcer prophylaxis, as it only negligibly decreases the rate of GI bleeding, from 0.33% to 0.22%.9
WHY ROUTINE ULCER PROPHYLAXIS IS NOT FOR ALL HOSPITALIZED PATIENTS
Although stress ulcer prophylaxis is often considered benign, its lack of proven benefit, additional cost, and risk of adverse effects, including interactions with foods and other drugs, preclude using it routinely for all hospitalized patients.10,11 Chronic use of PPIs has been associated with complications, as discussed below.
Infection
Acid suppression may impair the destruction of ingested microorganisms, resulting in overgrowth of bacteria.12 Overuse of PPIs may increase the risk of several infections:
- Diarrhea due to Clostridium difficile12
- Community-acquired pneumonia, from increased microaspiration of overgrown microorganisms into the lung.12
- Spontaneous bacterial peritonitis in patients with cirrhosis,13 although the mechanism is not clear. (Small-bowel bacterial overgrowth is the hypothesized cause.)
Bone fracture
PPIs lower gastric acidity, and this can inhibit intestinal calcium absorption. Furthermore, PPIs may directly inhibit bone resorption by osteoclasts.14
Reduction in clopidogrel efficacy
PPIs may reduce the efficacy of clopidogrel as a result of competitive inhibition of cytochrome CYP2C19, which is necessary to metabolize clopidogrel to its active forms. Therefore, concomitant use of clopidogrel with omeprazole, esomeprazole, or other CYP2C19 inhibitors is not recommended.15
Nutritional deficiencies
The overgrown microorganisms consume cobalamin in the stomach, resulting in vitamin B12 deficiency. Acid-suppressive therapy can also reduce the absorption of magnesium and iron.12
Unnecessary cost
Heidelbaugh and Inadomi16 reviewed the non-evidence-based use of stress ulcer prophylaxis in patients admitted to a large university hospital and estimated that it entailed a cost to the hospital of $111,791 over the course of a year.
WHICH ULCER PROPHYLAXIS SHOULD BE USED IN CRITICALLY ILL PATIENTS?
Studies have shown histamine-2 blockers to be superior to antacids and sucralfate in preventing stress ulcer and GI bleeding,8,15 but no study has compared PPIs with sucralfate and antacids.
When indicated, an oral PPI is preferred over an oral histamine-2 blocker for GI prophylaxis.17 This practice is considered cost-effective and is associated with lower rates of stress ulcer and GI bleeding. In intubated patients, however, an intravenous histamine-2 blocker is preferable to an intravenous PPI.3,8,11 Interestingly, no difference was reported between PPIs and histamine-2 blockers in terms of mortality rate or reduction in the incidence of nosocomial pneumonia.17
OUR RECOMMENDATION
Only critically ill patients who meet the specific criteria described here should receive stress ulcer prophylaxis. More effort is needed to educate residents, medical staff, and pharmacists about current guidelines. Computerized ordering templates and reminders to discontinue prophylaxis at discharge or step-down may decrease overall use, reduce costs, and limit potential side effects.18
No. Based on current evidence and guidelines, routine acid-suppressive therapy to prevent stress ulcers has no benefit in hospitalized patients outside the critical-care setting. Only critically ill patients who meet specific criteria, as described in the guidelines of the American Society of Health System Pharmacists, should receive acid-suppressive therapy.
Unfortunately, routine stress ulcer prophylaxis is common in US hospitals, unnecessarily putting patients at risk of complications and adding costs.
STRESS ULCER AND CRITICAL ILLNESS
Stress ulcers—ulcerations of the upper part of the gastrointestinal (GI) mucosa in the setting of acute disease—usually involve the fundus and body of the stomach. The stomach is lined with a glycoprotein mucous layer rich in bicarbonates, forming a physiologic barrier to protect the gastric wall from acid insult by neutralizing hydrogen ions. Disruption of this protective layer can occur in critically ill patients (eg, those with shock or sepsis) through overproduction of uremic toxins, increased reflux of bile salts, compromised blood flow, and increased stomach acidity through gastrin stimulation of parietal cells.
More than 75% of patients with major burns or cranial trauma develop endoscopic mucosal abnormalities within 72 hours of injury.1 In critically ill patients, the risk of ulcer-related overt bleeding is estimated to be 5% to 25%. Furthermore, 1% to 5% of stress ulcers can be deep enough to erode into the submucosa, causing clinically significant GI bleeding, defined as bleeding complicated by hemodynamic compromise or a drop in hemoglobin that requires a blood transfusion.2 In contrast, in inpatients who are not critically ill, the risk of overt bleeding from stress ulcers is less than 1%.3
ADDRESSING RISK
A multicenter prospective cohort study of 2,252 intensive care patients2 reported two main risk factors for significant bleeding caused by stress ulcers: mechanical ventilation for more than 48 hours and coagulopathy, defined as a platelet count below 50 × 109/L, an international normalized ratio greater than 1.5, or a partial thromboplastin time more than twice the control value.4 In hemodynamically stable patients receiving anticoagulation in a general medical or surgical ward, the risk of GI bleeding was low, and acid suppression failed to lower the rate of stress ulcer occurrence.3
Other risk factors include severe sepsis, shock, liver failure, kidney failure, burns over 35% of the total body surface, organ transplantation, cranial trauma, spinal cord trauma, history of peptic ulcer disease, and history of upper GI bleeding.3,5,6 Steroid therapy is not considered a risk factor for stress ulcers unless it is used in the presence of another risk factor such as use of aspirin or nonsteroidal antiinflammatory drugs (NSAIDs).2
INDICATIONS FOR PROPHYLAXIS
Prophylaxis with a proton pump inhibitor (PPI) is indicated in specific conditions—ie, peptic ulcer disease, gastroesophageal reflux disease, chronic NSAID therapy, and Zollinger-Ellison syndrome—and to eradicate Helicobacter pylori infection.7 But in the United States, stress ulcer prophylaxis is overused in general-care floors despite the lack of supporting evidence.
The American Society of Health System Pharmacists guidelines recommend it in the intensive care unit for patients with any of the following: coagulopathy, prolonged mechanical ventilation (more than 48 hours), GI ulcer or bleeding within the past year, sepsis, a stay longer than 1 week in the intensive care unit, occult GI bleeding for 6 or more days, and steroid therapy with more than 250 mg of hydrocortisone daily.8 Hemodynamically stable patients admitted to general-care floors should not receive stress ulcer prophylaxis, as it only negligibly decreases the rate of GI bleeding, from 0.33% to 0.22%.9
WHY ROUTINE ULCER PROPHYLAXIS IS NOT FOR ALL HOSPITALIZED PATIENTS
Although stress ulcer prophylaxis is often considered benign, its lack of proven benefit, additional cost, and risk of adverse effects, including interactions with foods and other drugs, preclude using it routinely for all hospitalized patients.10,11 Chronic use of PPIs has been associated with complications, as discussed below.
Infection
Acid suppression may impair the destruction of ingested microorganisms, resulting in overgrowth of bacteria.12 Overuse of PPIs may increase the risk of several infections:
- Diarrhea due to Clostridium difficile12
- Community-acquired pneumonia, from increased microaspiration of overgrown microorganisms into the lung.12
- Spontaneous bacterial peritonitis in patients with cirrhosis,13 although the mechanism is not clear. (Small-bowel bacterial overgrowth is the hypothesized cause.)
Bone fracture
PPIs lower gastric acidity, and this can inhibit intestinal calcium absorption. Furthermore, PPIs may directly inhibit bone resorption by osteoclasts.14
Reduction in clopidogrel efficacy
PPIs may reduce the efficacy of clopidogrel as a result of competitive inhibition of cytochrome CYP2C19, which is necessary to metabolize clopidogrel to its active forms. Therefore, concomitant use of clopidogrel with omeprazole, esomeprazole, or other CYP2C19 inhibitors is not recommended.15
Nutritional deficiencies
The overgrown microorganisms consume cobalamin in the stomach, resulting in vitamin B12 deficiency. Acid-suppressive therapy can also reduce the absorption of magnesium and iron.12
Unnecessary cost
Heidelbaugh and Inadomi16 reviewed the non-evidence-based use of stress ulcer prophylaxis in patients admitted to a large university hospital and estimated that it entailed a cost to the hospital of $111,791 over the course of a year.
WHICH ULCER PROPHYLAXIS SHOULD BE USED IN CRITICALLY ILL PATIENTS?
Studies have shown histamine-2 blockers to be superior to antacids and sucralfate in preventing stress ulcer and GI bleeding,8,15 but no study has compared PPIs with sucralfate and antacids.
When indicated, an oral PPI is preferred over an oral histamine-2 blocker for GI prophylaxis.17 This practice is considered cost-effective and is associated with lower rates of stress ulcer and GI bleeding. In intubated patients, however, an intravenous histamine-2 blocker is preferable to an intravenous PPI.3,8,11 Interestingly, no difference was reported between PPIs and histamine-2 blockers in terms of mortality rate or reduction in the incidence of nosocomial pneumonia.17
OUR RECOMMENDATION
Only critically ill patients who meet the specific criteria described here should receive stress ulcer prophylaxis. More effort is needed to educate residents, medical staff, and pharmacists about current guidelines. Computerized ordering templates and reminders to discontinue prophylaxis at discharge or step-down may decrease overall use, reduce costs, and limit potential side effects.18
- DePriest JL. Stress ulcer prophylaxis. Do critically ill patients need it? Postgrad Med 1995; 98:159–168.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Qadeer MA, Richter JE, Brotman DJ. Hospital-acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings. J Hosp Med 2006; 1:13–20.
- Shuman RB, Schuster DP, Zuckerman GR. Prophylactic therapy for stress ulcer bleeding: a reappraisal. Ann Intern Med 1987; 106:562–567.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA 1996; 275:308–314.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–1391.e5.
- Barkun AN, Bardou M, Pham CQ, Martel M. Proton pump inhibitors vs histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol 2012; 107:507–520.
- Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med 2011; 171:991–997.
- Cook DJ. Stress ulcer prophylaxis: gastrointestinal bleeding and nosocomial pneumonia. Best evidence synthesis. Scand J Gastroenterol Suppl 1995; 210:48–52.
- Messori A, Trippoli S, Vaiani M, Gorini M, Corrado A. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: meta-analysis of randomised controlled trials. BMJ 2000; 321:1103–1106.
- Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol 2012; 5:219–232.
- Deshpande A, Pasupuleti V, Thota P, et al. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol 2013; 28:235–242.
- Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H(+)- ATPase. Curr Pharm Des 2002; 8:2033–2048.
- ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis. ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm 1999; 56:347–379.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2013; 41:693–705.
- Liberman JD, Whelan CT. Brief report: reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med 2006; 21:498–500.
- DePriest JL. Stress ulcer prophylaxis. Do critically ill patients need it? Postgrad Med 1995; 98:159–168.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Qadeer MA, Richter JE, Brotman DJ. Hospital-acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings. J Hosp Med 2006; 1:13–20.
- Shuman RB, Schuster DP, Zuckerman GR. Prophylactic therapy for stress ulcer bleeding: a reappraisal. Ann Intern Med 1987; 106:562–567.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA 1996; 275:308–314.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–1391.e5.
- Barkun AN, Bardou M, Pham CQ, Martel M. Proton pump inhibitors vs histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol 2012; 107:507–520.
- Herzig SJ, Vaughn BP, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med 2011; 171:991–997.
- Cook DJ. Stress ulcer prophylaxis: gastrointestinal bleeding and nosocomial pneumonia. Best evidence synthesis. Scand J Gastroenterol Suppl 1995; 210:48–52.
- Messori A, Trippoli S, Vaiani M, Gorini M, Corrado A. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: meta-analysis of randomised controlled trials. BMJ 2000; 321:1103–1106.
- Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol 2012; 5:219–232.
- Deshpande A, Pasupuleti V, Thota P, et al. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol 2013; 28:235–242.
- Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H(+)- ATPase. Curr Pharm Des 2002; 8:2033–2048.
- ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis. ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm 1999; 56:347–379.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2013; 41:693–705.
- Liberman JD, Whelan CT. Brief report: reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med 2006; 21:498–500.
Deep T waves and chest pain
A 67-year-old man with a history of hypertension and hyperlipidemia presented to the emergency department after 3 hours of what he described as a burning sensation in his chest that woke him from sleep. He attributed it at first to a late-night meal and treated himself with some milk and yogurt, which seemed to relieve the symptoms. However, the pain recurred and was associated with difficulty breathing. At that point, he drove himself to the emergency department.
On arrival, his temperature was 36.5°C (97.7°F), blood pressure 134/67 mm Hg, heart rate 89 bpm, respirations 18/min, and oxygen saturation 98% on room air. His cardiovascular, lung, and neurologic examinations were normal. His cardiac enzyme levels (creatine kinase, creatine kinase MB fraction, and troponin T) were within normal limits.
Figure 1 depicts his initial electrocardiogram. It showed deep, symmetric T-wave inversions in the precordial leads especially in V2 and V3, changes known as Wellens syndrome. The ST-T changes in lead V1 suggested a very proximal lesion in the left anterior descending artery (LAD), before the first septal perforator. Also, lateral and high lateral (V5 and V6) findings indicated stenoses of the branching diagonals and left circumflex myocardial territory. Furthermore, the inferior ST-T changes indicated that his LAD may have wrapped around the cardiac apex. All of these findings were prognostically significant.
The patient was given aspirin and was started on intravenous unfractionated heparin and nitroglycerin. He was sent for urgent left-heart catheterization, which showed a 50% to 60% stenosis in the left main coronary artery, with involvement of the left circumflex artery proximally, in addition to a “tight” first-diagonal stenosis, a 90% stenosis in a large (> 3.0-mm) proximal segment of the LAD, an 80% stenosis in a large (> 3.0-mm) mid-LAD segment, and a 40% stenosis in a large (> 3.0-mm) second diagonal artery (Figure 2).
He was referred for cardiac surgery and underwent triple coronary artery bypass grafting: the left internal thoracic artery was grafted to the LAD, a reverse saphenous vein graft was performed to the diagonal artery, and a reverse saphenous vein graft was performed to the obtuse marginal artery.
A PRECURSOR TO INFARCTION
Wellens et al described specific precordial T-wave changes in patients with unstable angina who subsequently developed anterior wall myocardial infarction.1
The importance of Wellens syndrome is that it occurs in the pain-free interval when no other evidence of ischemia or angina may be present.1 Cardiac enzyme levels are typically normal or only minimally elevated; only 12% of patients with this syndrome have elevated cardiac biomarker levels.2
Given the extent of myocardial injury, urgent echocardiography can show a wall-motion abnormality even if cardiac enzyme levels are normal. This gives important insight into electrocardiographic changes and should prompt consideration of revascularization.
Even with extensive medical management, Wellens syndrome progresses to acute anterior wall ischemia. About 75% of patients with Wellens syndrome who receive medical management but do not undergo revascularization (eg, coronary artery bypass grafting, percutaneous coronary intervention) develop extensive anterior wall infarction within days.1,3 Despite negative cardiac biomarkers, Wellens syndrome is considered an acute coronary syndrome requiring urgent cardiac intervention.
- Movahed MR. Wellens’ syndrome or inverted U-waves? Clin Cardiol 2008; 31:133–134.
- de Zwaan C, Bär FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J 1989; 117:657–665.
- de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103:730–736.
A 67-year-old man with a history of hypertension and hyperlipidemia presented to the emergency department after 3 hours of what he described as a burning sensation in his chest that woke him from sleep. He attributed it at first to a late-night meal and treated himself with some milk and yogurt, which seemed to relieve the symptoms. However, the pain recurred and was associated with difficulty breathing. At that point, he drove himself to the emergency department.
On arrival, his temperature was 36.5°C (97.7°F), blood pressure 134/67 mm Hg, heart rate 89 bpm, respirations 18/min, and oxygen saturation 98% on room air. His cardiovascular, lung, and neurologic examinations were normal. His cardiac enzyme levels (creatine kinase, creatine kinase MB fraction, and troponin T) were within normal limits.
Figure 1 depicts his initial electrocardiogram. It showed deep, symmetric T-wave inversions in the precordial leads especially in V2 and V3, changes known as Wellens syndrome. The ST-T changes in lead V1 suggested a very proximal lesion in the left anterior descending artery (LAD), before the first septal perforator. Also, lateral and high lateral (V5 and V6) findings indicated stenoses of the branching diagonals and left circumflex myocardial territory. Furthermore, the inferior ST-T changes indicated that his LAD may have wrapped around the cardiac apex. All of these findings were prognostically significant.
The patient was given aspirin and was started on intravenous unfractionated heparin and nitroglycerin. He was sent for urgent left-heart catheterization, which showed a 50% to 60% stenosis in the left main coronary artery, with involvement of the left circumflex artery proximally, in addition to a “tight” first-diagonal stenosis, a 90% stenosis in a large (> 3.0-mm) proximal segment of the LAD, an 80% stenosis in a large (> 3.0-mm) mid-LAD segment, and a 40% stenosis in a large (> 3.0-mm) second diagonal artery (Figure 2).
He was referred for cardiac surgery and underwent triple coronary artery bypass grafting: the left internal thoracic artery was grafted to the LAD, a reverse saphenous vein graft was performed to the diagonal artery, and a reverse saphenous vein graft was performed to the obtuse marginal artery.
A PRECURSOR TO INFARCTION
Wellens et al described specific precordial T-wave changes in patients with unstable angina who subsequently developed anterior wall myocardial infarction.1
The importance of Wellens syndrome is that it occurs in the pain-free interval when no other evidence of ischemia or angina may be present.1 Cardiac enzyme levels are typically normal or only minimally elevated; only 12% of patients with this syndrome have elevated cardiac biomarker levels.2
Given the extent of myocardial injury, urgent echocardiography can show a wall-motion abnormality even if cardiac enzyme levels are normal. This gives important insight into electrocardiographic changes and should prompt consideration of revascularization.
Even with extensive medical management, Wellens syndrome progresses to acute anterior wall ischemia. About 75% of patients with Wellens syndrome who receive medical management but do not undergo revascularization (eg, coronary artery bypass grafting, percutaneous coronary intervention) develop extensive anterior wall infarction within days.1,3 Despite negative cardiac biomarkers, Wellens syndrome is considered an acute coronary syndrome requiring urgent cardiac intervention.
A 67-year-old man with a history of hypertension and hyperlipidemia presented to the emergency department after 3 hours of what he described as a burning sensation in his chest that woke him from sleep. He attributed it at first to a late-night meal and treated himself with some milk and yogurt, which seemed to relieve the symptoms. However, the pain recurred and was associated with difficulty breathing. At that point, he drove himself to the emergency department.
On arrival, his temperature was 36.5°C (97.7°F), blood pressure 134/67 mm Hg, heart rate 89 bpm, respirations 18/min, and oxygen saturation 98% on room air. His cardiovascular, lung, and neurologic examinations were normal. His cardiac enzyme levels (creatine kinase, creatine kinase MB fraction, and troponin T) were within normal limits.
Figure 1 depicts his initial electrocardiogram. It showed deep, symmetric T-wave inversions in the precordial leads especially in V2 and V3, changes known as Wellens syndrome. The ST-T changes in lead V1 suggested a very proximal lesion in the left anterior descending artery (LAD), before the first septal perforator. Also, lateral and high lateral (V5 and V6) findings indicated stenoses of the branching diagonals and left circumflex myocardial territory. Furthermore, the inferior ST-T changes indicated that his LAD may have wrapped around the cardiac apex. All of these findings were prognostically significant.
The patient was given aspirin and was started on intravenous unfractionated heparin and nitroglycerin. He was sent for urgent left-heart catheterization, which showed a 50% to 60% stenosis in the left main coronary artery, with involvement of the left circumflex artery proximally, in addition to a “tight” first-diagonal stenosis, a 90% stenosis in a large (> 3.0-mm) proximal segment of the LAD, an 80% stenosis in a large (> 3.0-mm) mid-LAD segment, and a 40% stenosis in a large (> 3.0-mm) second diagonal artery (Figure 2).
He was referred for cardiac surgery and underwent triple coronary artery bypass grafting: the left internal thoracic artery was grafted to the LAD, a reverse saphenous vein graft was performed to the diagonal artery, and a reverse saphenous vein graft was performed to the obtuse marginal artery.
A PRECURSOR TO INFARCTION
Wellens et al described specific precordial T-wave changes in patients with unstable angina who subsequently developed anterior wall myocardial infarction.1
The importance of Wellens syndrome is that it occurs in the pain-free interval when no other evidence of ischemia or angina may be present.1 Cardiac enzyme levels are typically normal or only minimally elevated; only 12% of patients with this syndrome have elevated cardiac biomarker levels.2
Given the extent of myocardial injury, urgent echocardiography can show a wall-motion abnormality even if cardiac enzyme levels are normal. This gives important insight into electrocardiographic changes and should prompt consideration of revascularization.
Even with extensive medical management, Wellens syndrome progresses to acute anterior wall ischemia. About 75% of patients with Wellens syndrome who receive medical management but do not undergo revascularization (eg, coronary artery bypass grafting, percutaneous coronary intervention) develop extensive anterior wall infarction within days.1,3 Despite negative cardiac biomarkers, Wellens syndrome is considered an acute coronary syndrome requiring urgent cardiac intervention.
- Movahed MR. Wellens’ syndrome or inverted U-waves? Clin Cardiol 2008; 31:133–134.
- de Zwaan C, Bär FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J 1989; 117:657–665.
- de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103:730–736.
- Movahed MR. Wellens’ syndrome or inverted U-waves? Clin Cardiol 2008; 31:133–134.
- de Zwaan C, Bär FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J 1989; 117:657–665.
- de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103:730–736.
Return of the 'pisse-mongers,' this time with data
Great effort has been spent on identifying easily measured biomarkers to predict the progression of coronary disease and chronic kidney disease (CKD). Interestingly, these two disease processes seem to share some biomarkers and perhaps some pathogenic mechanisms. An ultimate hope is that some of these markers will be found to also contribute directly to organ dysfunction and be amenable to therapy. Blood pressure and (in many people’s minds) low-density lipoprotein cholesterol fulfill this hope. The jury remains out on C-reactive protein and serum urate. There are others.
In this issue of the Journal, Stephen et al review the data indicating that albuminuria helps predict the progression of CKD, coronary disease, ventricular remodeling, and, in some studies, all-cause mortality. Proteinuria has generally been assumed to be a marker of renal injury, but, the authors point out, albumin can under some circumstances initiate inflammatory mechanisms and stimulate mediators of fibrosis.
Although not mentioned by Stephen et al, albumin (like hemoglobin) is susceptible to nonenzymatic glycosylation in patients with diabetes. There is a hint in the literature that glycosylated albumin may be preferentially excreted. Its effects on various tissues are incompletely studied, but it strikes me that perhaps this molecule plays a unique pathogenic role in diabetic renal and vascular disease, even more than native albumin. Further evaluation of this specific marker may lead to even stronger associations (although in a select population of patients with poorly controlled diabetes).
The focus on urine as a fluid with diagnostic and predictive characteristics is certainly not new. Both Hippocrates and Galen recognized the value of inspecting urine. Uroscopy (now urinalysis) may be the oldest surviving laboratory test. Recently, my friend Joe Nally, a coauthor with Stephen et al, shared with me a paper detailing the romantic yet checkered history of urinalysis.1
Gilles de Corbeil in the 12th century wrote a poem on judging urine, intending it as an aid for remembering the supposed 20 different diagnostic colors of urine and describing in detail the use of the urine flask, a bladder-shaped container for studying the partitioning of the urine colors and substance as representative of the diseased parts of the body. A urine flask was even illustrated in a version of Chaucer’s Canterbury Tales as a recognized accoutrement of the stylish physician (Figure 1). The “art” of uroscopy grew so successful over the centuries as a component of rampant medical charlatanry (casting no aspersions, of course, on current nephrologists) that the Royal College of Physicians in 1601 felt pressed to attack the “pisse-mongers” by stating, “It is ridiculous and foolish to divine the…course of disease…from the inspection of urine.”1 This dictate was apparently ignored then, but seemingly is too frequently followed by clinicians today, contributing to the oft-delayed diagnosis of glomerulonephritis and other renal diseases.
In 1637, Thomas Brian published The Pisse-Prophet or Certaine Pisse Pot Lectures, in which he railed against the witchcraft of uroscopy, which he said should only be performed by university-trained physicians. Jump forward to 1827, when Richard Bright elegantly described acute glomerulonephritis, although not the microscopic findings that would be illustrated in accurate detail by Golding Bird in his 1844 treatise, Urinary Deposits. Sitting on the bookshelf behind my desk is a copy of Richard W. Lippman’s Urine and Urinary Sediment: A Practical Manual and Atlas (1957). I have no urine flask—rheumatologists know their limitations.
As we enter 2014, all of us at the Journal offer you our sincere wishes for a personally healthy and universally peaceful new year.
- Haber MH. Pisse prophecy: a brief history of urinalysis. Clin Lab Med 1988; 8:415–430.
Great effort has been spent on identifying easily measured biomarkers to predict the progression of coronary disease and chronic kidney disease (CKD). Interestingly, these two disease processes seem to share some biomarkers and perhaps some pathogenic mechanisms. An ultimate hope is that some of these markers will be found to also contribute directly to organ dysfunction and be amenable to therapy. Blood pressure and (in many people’s minds) low-density lipoprotein cholesterol fulfill this hope. The jury remains out on C-reactive protein and serum urate. There are others.
In this issue of the Journal, Stephen et al review the data indicating that albuminuria helps predict the progression of CKD, coronary disease, ventricular remodeling, and, in some studies, all-cause mortality. Proteinuria has generally been assumed to be a marker of renal injury, but, the authors point out, albumin can under some circumstances initiate inflammatory mechanisms and stimulate mediators of fibrosis.
Although not mentioned by Stephen et al, albumin (like hemoglobin) is susceptible to nonenzymatic glycosylation in patients with diabetes. There is a hint in the literature that glycosylated albumin may be preferentially excreted. Its effects on various tissues are incompletely studied, but it strikes me that perhaps this molecule plays a unique pathogenic role in diabetic renal and vascular disease, even more than native albumin. Further evaluation of this specific marker may lead to even stronger associations (although in a select population of patients with poorly controlled diabetes).
The focus on urine as a fluid with diagnostic and predictive characteristics is certainly not new. Both Hippocrates and Galen recognized the value of inspecting urine. Uroscopy (now urinalysis) may be the oldest surviving laboratory test. Recently, my friend Joe Nally, a coauthor with Stephen et al, shared with me a paper detailing the romantic yet checkered history of urinalysis.1
Gilles de Corbeil in the 12th century wrote a poem on judging urine, intending it as an aid for remembering the supposed 20 different diagnostic colors of urine and describing in detail the use of the urine flask, a bladder-shaped container for studying the partitioning of the urine colors and substance as representative of the diseased parts of the body. A urine flask was even illustrated in a version of Chaucer’s Canterbury Tales as a recognized accoutrement of the stylish physician (Figure 1). The “art” of uroscopy grew so successful over the centuries as a component of rampant medical charlatanry (casting no aspersions, of course, on current nephrologists) that the Royal College of Physicians in 1601 felt pressed to attack the “pisse-mongers” by stating, “It is ridiculous and foolish to divine the…course of disease…from the inspection of urine.”1 This dictate was apparently ignored then, but seemingly is too frequently followed by clinicians today, contributing to the oft-delayed diagnosis of glomerulonephritis and other renal diseases.
In 1637, Thomas Brian published The Pisse-Prophet or Certaine Pisse Pot Lectures, in which he railed against the witchcraft of uroscopy, which he said should only be performed by university-trained physicians. Jump forward to 1827, when Richard Bright elegantly described acute glomerulonephritis, although not the microscopic findings that would be illustrated in accurate detail by Golding Bird in his 1844 treatise, Urinary Deposits. Sitting on the bookshelf behind my desk is a copy of Richard W. Lippman’s Urine and Urinary Sediment: A Practical Manual and Atlas (1957). I have no urine flask—rheumatologists know their limitations.
As we enter 2014, all of us at the Journal offer you our sincere wishes for a personally healthy and universally peaceful new year.
Great effort has been spent on identifying easily measured biomarkers to predict the progression of coronary disease and chronic kidney disease (CKD). Interestingly, these two disease processes seem to share some biomarkers and perhaps some pathogenic mechanisms. An ultimate hope is that some of these markers will be found to also contribute directly to organ dysfunction and be amenable to therapy. Blood pressure and (in many people’s minds) low-density lipoprotein cholesterol fulfill this hope. The jury remains out on C-reactive protein and serum urate. There are others.
In this issue of the Journal, Stephen et al review the data indicating that albuminuria helps predict the progression of CKD, coronary disease, ventricular remodeling, and, in some studies, all-cause mortality. Proteinuria has generally been assumed to be a marker of renal injury, but, the authors point out, albumin can under some circumstances initiate inflammatory mechanisms and stimulate mediators of fibrosis.
Although not mentioned by Stephen et al, albumin (like hemoglobin) is susceptible to nonenzymatic glycosylation in patients with diabetes. There is a hint in the literature that glycosylated albumin may be preferentially excreted. Its effects on various tissues are incompletely studied, but it strikes me that perhaps this molecule plays a unique pathogenic role in diabetic renal and vascular disease, even more than native albumin. Further evaluation of this specific marker may lead to even stronger associations (although in a select population of patients with poorly controlled diabetes).
The focus on urine as a fluid with diagnostic and predictive characteristics is certainly not new. Both Hippocrates and Galen recognized the value of inspecting urine. Uroscopy (now urinalysis) may be the oldest surviving laboratory test. Recently, my friend Joe Nally, a coauthor with Stephen et al, shared with me a paper detailing the romantic yet checkered history of urinalysis.1
Gilles de Corbeil in the 12th century wrote a poem on judging urine, intending it as an aid for remembering the supposed 20 different diagnostic colors of urine and describing in detail the use of the urine flask, a bladder-shaped container for studying the partitioning of the urine colors and substance as representative of the diseased parts of the body. A urine flask was even illustrated in a version of Chaucer’s Canterbury Tales as a recognized accoutrement of the stylish physician (Figure 1). The “art” of uroscopy grew so successful over the centuries as a component of rampant medical charlatanry (casting no aspersions, of course, on current nephrologists) that the Royal College of Physicians in 1601 felt pressed to attack the “pisse-mongers” by stating, “It is ridiculous and foolish to divine the…course of disease…from the inspection of urine.”1 This dictate was apparently ignored then, but seemingly is too frequently followed by clinicians today, contributing to the oft-delayed diagnosis of glomerulonephritis and other renal diseases.
In 1637, Thomas Brian published The Pisse-Prophet or Certaine Pisse Pot Lectures, in which he railed against the witchcraft of uroscopy, which he said should only be performed by university-trained physicians. Jump forward to 1827, when Richard Bright elegantly described acute glomerulonephritis, although not the microscopic findings that would be illustrated in accurate detail by Golding Bird in his 1844 treatise, Urinary Deposits. Sitting on the bookshelf behind my desk is a copy of Richard W. Lippman’s Urine and Urinary Sediment: A Practical Manual and Atlas (1957). I have no urine flask—rheumatologists know their limitations.
As we enter 2014, all of us at the Journal offer you our sincere wishes for a personally healthy and universally peaceful new year.
- Haber MH. Pisse prophecy: a brief history of urinalysis. Clin Lab Med 1988; 8:415–430.
- Haber MH. Pisse prophecy: a brief history of urinalysis. Clin Lab Med 1988; 8:415–430.
Albuminuria: When urine predicts kidney and cardiovascular disease
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
KEY POINTS
- Albuminuria is best measured by the albumin-to-creatinine ratio.
- In several studies, albuminuria has been independently associated with a higher risk of death, cardiovascular events, heart failure, stroke, and progression of chronic kidney disease.
- Despite strong evidence linking albuminuria to adverse outcomes, evidence is limited in favor of routinely screening for it in the general population.
- Evaluating and managing albuminuria require understanding the limits of its clinical measures, controlling other risk factors for progression of renal disease, managing it medically, and referring to a specialist in certain situations.
An 85-year-old with muscle pain
An 85-year-old man with hypertension, hyperlipidemia, and coronary artery disease presented to our clinic with diffuse muscle pain. The pain had been present for about 3 months, but it had become noticeably worse over the past few weeks.
He was not aware of any trauma. He described the muscle pain as dull and particularly severe in his lower extremities (his thighs and calves). The pain did not limit his daily activities, nor did physical exertion or the time of day have any effect on the level of the pain.
His medications at that time included metoprolol, aspirin, hydrochlorothiazide, simvastatin, and a daily multivitamin.
He was not in acute distress. On neurologic and musculoskeletal examinations, all deep-tendon reflexes were intact, with no tenderness to palpation of the upper and lower extremities. No abnormalities were noted on the joint examination. He had full range of motion, with 5/5 muscle strength in the upper and lower extremities bilaterally and normal muscle tone. He was able to walk with ease. Results of initial laboratory testing, including creatine kinase and erythrocyte sedimentation rate, were normal.
1. What should be the next best step in the evaluation of this patient’s muscle pain?
- Order tests for cyclic citrullinated peptide (CCP) antibody and rheumatoid factor
- Advise him to refrain from physical activity until his symptoms resolve
- Take a more detailed history, including a review of medications and supplements
- Recommend a trial of a nonsteroidal anti-inflammatory drug (NSAID)
- Send him for radiographic imaging
Since his muscle pain has persisted for several months without improvement, a more detailed history should be taken, including a review of current medications and supplements.
Testing CCP antibody and rheumatoid factor would be useful if rheumatoid arthritis were suspected, but in the absence of demonstrable arthritis on examination, these tests would have low specificity even if the results were positive.
An NSAID may temporarily alleviate his pain, but it will not help establish a diagnosis. And in elderly patients, NSAIDs are not without complications and so should be prescribed only in appropriate situations.
Imaging would be appropriate at this point only if there was clinical suspicion of a specific disease. However, our patient has no focal deficits, and the suspicion of fracture or malignancy is low.
The medical history should include asking about current drug regimens, recent medication changes, and the use of herbal supplements, since polypharmacy is common in elderly patients with multiple comorbidities.
On further questioning, our patient said that his dose of simvastatin had been increased from 40 mg daily to 80 mg daily about 1 month before his symptoms appeared. He was taking a daily multivitamin but was not using herbal supplements or other over-the-counter products. He did not recall any constitutional symptoms before the onset of his current symptoms, and he had never had similar muscle pain in the past.
2. Based on the additional information from the history, what is the most likely cause of his muscle pain?
- Limited myositis secondary to recent viral infection
- Rhabdomyolysis
- Hypothyroidism
- Drug-drug interaction
- Statin-induced myalgia
Our patient’s history provided nothing to suggest viral myositis. Hypothyroidism should always be considered in patients with myalgia, but this is not likely in our patient, as he does not display other characteristics, such as diminished reflexes, hypotonia, cold intolerance, and mood instability. Even though calcium channel blockers have been known to cause myalgia in patients on statins, a drug-drug reaction is not likely, as he had not started taking a calcium channel blocker before his symptoms began. This patient did not show signs or symptoms of rhabdomyolysis, a type of myopathy in which necrosis of the muscle tissue occurs, generally causing profound weakness and pain.1
Therefore, statin-induced myopathy is the most likely cause of his diffuse muscle pain, particularly since his simvastatin had been increased 1 month before the onset of symptoms.
3. What should be the next step in his management?
- Decrease the dose of simvastatin to the last known dose he was able to tolerate
- Continue simvastatin at the same dose and then monitor
- Switch to another statin
- Add coenzyme Q10
- Stop simvastatin
Decreasing the statin dosage to the last well-tolerated dose would not be appropriate in a patient with myopathy, as the symptoms would probably not improve.2–4 Also, one should not switch to a different statin while a patient is experiencing symptoms. Rather, the statin should be stopped for at least 6 weeks or until the symptoms have fully resolved.1
Adding coenzyme Q10 is another option, especially in a patient with previously diagnosed coronary artery disease,5 when continued statin therapy is thought necessary to reduce the likelihood of repeat coronary events.
We discontinued his simvastatin. Followup 3 weeks later in the outpatient clinic showed that his symptoms were slowly improving. The symptoms had resolved completely 4 months later.
4. How should we manage our patient’s hyperlipidemia once his symptoms have resolved?
- Restart simvastatin at the 80-mg dose
- Restart simvastatin at the 40-mg dose
- Start a hydrophilic statin at full dose
- Use a drug from another class of lipid-lowering drugs
- Wait another 3 months before prescribing any lipid-lowering drug
His treatment for hyperlipidemia should be continued, considering his comorbidities. However, restarting the same statin, even at a lower dose, will likely cause his symptoms to recur. Thus, a different statin should be tried once his muscle pain has resolved.
Other classes of lipid-lowering drugs are usually less efficacious than statins, particularly when trying to control low-density lipoprotein (LDL) cholesterol, so a drug from another class should not be used until other statin options have been attempted.2,6,7
Simvastatin is lipophilic. Trying a statin with hydrophilic properties (eg, pravastatin, rosuvastatin, fluvastatin) has been shown to convey similar cardioprotective effects with a lower propensity for myalgia, as lipophilic statins have a higher propensity to penetrate muscle tissue than do hydrophilic statins.3,4,8
Once his symptoms resolved, our patient was started on a hydrophilic statin, fluvastatin 20 mg daily. Unfortunately, his pain recurred 3 weeks later. The statin was stopped, and his symptoms again resolved.
5. Since our patient was unable to tolerate a second statin, what should be the next step in his management?
- Restart simvastatin
- Use a drug from another class to control the hyperlipidemia
- Wait at least 6 months after symptoms resolve before trying any lipid-lowering drug
- Initiate therapy with coenzyme Q10 and fish oil
- Wait for symptoms to resolve, then restart a hydrophilic statin at a lower dose and lower frequency
Restarting simvastatin will likely cause a recurrence of the myalgia. Other lipid-lowering drugs such as nicotinic acid, bile acid resins, and fibrates are not as efficacious as statins. Coenzyme Q10 and fish oil can reduce lipid levels, but they are not as efficacious as statins.
In view of our patient’s lipid profile—LDL cholesterol elevated at 167 mg/dL, high-density lipoprotein cholesterol 31 mg/dL, triglycerides 47 mg/dL—it is important to treat his hyperlipidemia. Therefore, another attempt at statin therapy should be made once his symptoms have resolved.
Studies have shown that restarting a statin at a low dose and low frequency is effective in patients who have experienced intolerance to a statin.3,4 Our patient was treated with low-dose pravastatin (20 mg), resulting in a moderate improvement in his LDL cholesterol to 123 mg/dL.
STATIN-INDUCED MYOPATHY: ADDRESSING THE DILEMMA
Treating hyperlipidemia is important to prevent vascular events in patients with or without coronary artery disease. Statins are the most effective agents available for controlling hypercholesterolemia, specifically LDL levels, as well as for preventing myocardial infarction.
Unfortunately, significant side effects have been reported, and myopathy is the most prevalent. Statin-induced myopathy includes a combination of muscle tenderness, myalgia, and weakness.2–11 In randomized controlled trials, the risk of myopathy was estimated to be between 1.5% and 5%.6 In unselected clinic patients on high-dose statins, the rate of muscle complaints may be as high as 20%.12
The cause of statin-induced myopathy is not known, although studies have linked it to genetic defects.7 Risk factors have been identified and include personal and family history of myalgia, Asian ethnicity, hypothyroidism, and type 1 diabetes. The incidence of statin-induced myalgia is two to three times higher in patients on corticosteroid therapy. Other risk factors include female sex, liver disease, and renal dysfunction.7,8
A less common etiology is anti-HMG coenzyme A reductase antibodies. Studies have shown that these antibody levels correlate well with the amount of myositis as measured by creatine kinase levels. However, there is no consensus yet on screening for these antibodies.13
Statin therapy poses a dilemma, as there is a thin line between the benefits and the risks of side effects, especially statin-induced myopathy.3,4 Current recommendations include discontinuing the statin until symptoms fully resolve. Creatine kinase levels may be useful in assessing for potential muscle breakdown, especially in patients with reduced renal function, as this predisposes them to statin-induced myopathy, yet normal values do not preclude the diagnosis of statin-induced myopathy.3,4,7,8
Once symptoms resolve and laboratory test results normalize, a trial of a different statin is recommended. If patients become symptomatic, a trial of a low-dose hydrophilic statin at a once- or twice-weekly interval has been recommended. Several studies have assessed the efficacy of a low-dose statin with decreased frequency of administration and have consistently shown significant improvement in lipid levels.3,4 For instance, once-weekly rosuvastatin at a dose between 5 mg and 20 mg resulted in a 29% reduction in LDL cholesterol levels, and 80% of patients did not experience a recurrence of myalgia.3 Furthermore, a study of patients treated with 5 mg to 10 mg of rosuvastatin twice a week resulted in a 26% decrease in LDL cholesterol levels.4 This study also showed that when an additional non-statin lipid-lowering drug was prescribed (eg, ezetimibe, bile acid resin, nicotinic acid), more than half of the patients reached their goal lipid level.4
The addition of coenzyme Q10 and fish oil has also been suggested. Although, the evidence to support this is inconclusive, the potential benefit outweighs the risk, since the side effects are minimal.1 However, no study yet has evaluated the risks vs the benefits in patients with elevated creatine kinase.
Statin-induced myopathy is a commonly encountered adverse effect. Currently, there are no guidelines on restarting statin therapy after statin-induced myopathy; however, data suggest that statin therapy should be restarted once symptoms resolve, and that variations in dose and frequency may be necessary.1–8,14
- Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011; 78:393–403.
- Foley KA, Simpson RJ, Crouse JR, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol 2008; 101:1747–1748.
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99:1409–1412.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Tomaszewski M, Stepien KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep 2011; 63:859–866.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Guyton JR. Benefit versus risk in statin treatment. Am J Cardiol 2006; 97:95C–97C.
- Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
An 85-year-old man with hypertension, hyperlipidemia, and coronary artery disease presented to our clinic with diffuse muscle pain. The pain had been present for about 3 months, but it had become noticeably worse over the past few weeks.
He was not aware of any trauma. He described the muscle pain as dull and particularly severe in his lower extremities (his thighs and calves). The pain did not limit his daily activities, nor did physical exertion or the time of day have any effect on the level of the pain.
His medications at that time included metoprolol, aspirin, hydrochlorothiazide, simvastatin, and a daily multivitamin.
He was not in acute distress. On neurologic and musculoskeletal examinations, all deep-tendon reflexes were intact, with no tenderness to palpation of the upper and lower extremities. No abnormalities were noted on the joint examination. He had full range of motion, with 5/5 muscle strength in the upper and lower extremities bilaterally and normal muscle tone. He was able to walk with ease. Results of initial laboratory testing, including creatine kinase and erythrocyte sedimentation rate, were normal.
1. What should be the next best step in the evaluation of this patient’s muscle pain?
- Order tests for cyclic citrullinated peptide (CCP) antibody and rheumatoid factor
- Advise him to refrain from physical activity until his symptoms resolve
- Take a more detailed history, including a review of medications and supplements
- Recommend a trial of a nonsteroidal anti-inflammatory drug (NSAID)
- Send him for radiographic imaging
Since his muscle pain has persisted for several months without improvement, a more detailed history should be taken, including a review of current medications and supplements.
Testing CCP antibody and rheumatoid factor would be useful if rheumatoid arthritis were suspected, but in the absence of demonstrable arthritis on examination, these tests would have low specificity even if the results were positive.
An NSAID may temporarily alleviate his pain, but it will not help establish a diagnosis. And in elderly patients, NSAIDs are not without complications and so should be prescribed only in appropriate situations.
Imaging would be appropriate at this point only if there was clinical suspicion of a specific disease. However, our patient has no focal deficits, and the suspicion of fracture or malignancy is low.
The medical history should include asking about current drug regimens, recent medication changes, and the use of herbal supplements, since polypharmacy is common in elderly patients with multiple comorbidities.
On further questioning, our patient said that his dose of simvastatin had been increased from 40 mg daily to 80 mg daily about 1 month before his symptoms appeared. He was taking a daily multivitamin but was not using herbal supplements or other over-the-counter products. He did not recall any constitutional symptoms before the onset of his current symptoms, and he had never had similar muscle pain in the past.
2. Based on the additional information from the history, what is the most likely cause of his muscle pain?
- Limited myositis secondary to recent viral infection
- Rhabdomyolysis
- Hypothyroidism
- Drug-drug interaction
- Statin-induced myalgia
Our patient’s history provided nothing to suggest viral myositis. Hypothyroidism should always be considered in patients with myalgia, but this is not likely in our patient, as he does not display other characteristics, such as diminished reflexes, hypotonia, cold intolerance, and mood instability. Even though calcium channel blockers have been known to cause myalgia in patients on statins, a drug-drug reaction is not likely, as he had not started taking a calcium channel blocker before his symptoms began. This patient did not show signs or symptoms of rhabdomyolysis, a type of myopathy in which necrosis of the muscle tissue occurs, generally causing profound weakness and pain.1
Therefore, statin-induced myopathy is the most likely cause of his diffuse muscle pain, particularly since his simvastatin had been increased 1 month before the onset of symptoms.
3. What should be the next step in his management?
- Decrease the dose of simvastatin to the last known dose he was able to tolerate
- Continue simvastatin at the same dose and then monitor
- Switch to another statin
- Add coenzyme Q10
- Stop simvastatin
Decreasing the statin dosage to the last well-tolerated dose would not be appropriate in a patient with myopathy, as the symptoms would probably not improve.2–4 Also, one should not switch to a different statin while a patient is experiencing symptoms. Rather, the statin should be stopped for at least 6 weeks or until the symptoms have fully resolved.1
Adding coenzyme Q10 is another option, especially in a patient with previously diagnosed coronary artery disease,5 when continued statin therapy is thought necessary to reduce the likelihood of repeat coronary events.
We discontinued his simvastatin. Followup 3 weeks later in the outpatient clinic showed that his symptoms were slowly improving. The symptoms had resolved completely 4 months later.
4. How should we manage our patient’s hyperlipidemia once his symptoms have resolved?
- Restart simvastatin at the 80-mg dose
- Restart simvastatin at the 40-mg dose
- Start a hydrophilic statin at full dose
- Use a drug from another class of lipid-lowering drugs
- Wait another 3 months before prescribing any lipid-lowering drug
His treatment for hyperlipidemia should be continued, considering his comorbidities. However, restarting the same statin, even at a lower dose, will likely cause his symptoms to recur. Thus, a different statin should be tried once his muscle pain has resolved.
Other classes of lipid-lowering drugs are usually less efficacious than statins, particularly when trying to control low-density lipoprotein (LDL) cholesterol, so a drug from another class should not be used until other statin options have been attempted.2,6,7
Simvastatin is lipophilic. Trying a statin with hydrophilic properties (eg, pravastatin, rosuvastatin, fluvastatin) has been shown to convey similar cardioprotective effects with a lower propensity for myalgia, as lipophilic statins have a higher propensity to penetrate muscle tissue than do hydrophilic statins.3,4,8
Once his symptoms resolved, our patient was started on a hydrophilic statin, fluvastatin 20 mg daily. Unfortunately, his pain recurred 3 weeks later. The statin was stopped, and his symptoms again resolved.
5. Since our patient was unable to tolerate a second statin, what should be the next step in his management?
- Restart simvastatin
- Use a drug from another class to control the hyperlipidemia
- Wait at least 6 months after symptoms resolve before trying any lipid-lowering drug
- Initiate therapy with coenzyme Q10 and fish oil
- Wait for symptoms to resolve, then restart a hydrophilic statin at a lower dose and lower frequency
Restarting simvastatin will likely cause a recurrence of the myalgia. Other lipid-lowering drugs such as nicotinic acid, bile acid resins, and fibrates are not as efficacious as statins. Coenzyme Q10 and fish oil can reduce lipid levels, but they are not as efficacious as statins.
In view of our patient’s lipid profile—LDL cholesterol elevated at 167 mg/dL, high-density lipoprotein cholesterol 31 mg/dL, triglycerides 47 mg/dL—it is important to treat his hyperlipidemia. Therefore, another attempt at statin therapy should be made once his symptoms have resolved.
Studies have shown that restarting a statin at a low dose and low frequency is effective in patients who have experienced intolerance to a statin.3,4 Our patient was treated with low-dose pravastatin (20 mg), resulting in a moderate improvement in his LDL cholesterol to 123 mg/dL.
STATIN-INDUCED MYOPATHY: ADDRESSING THE DILEMMA
Treating hyperlipidemia is important to prevent vascular events in patients with or without coronary artery disease. Statins are the most effective agents available for controlling hypercholesterolemia, specifically LDL levels, as well as for preventing myocardial infarction.
Unfortunately, significant side effects have been reported, and myopathy is the most prevalent. Statin-induced myopathy includes a combination of muscle tenderness, myalgia, and weakness.2–11 In randomized controlled trials, the risk of myopathy was estimated to be between 1.5% and 5%.6 In unselected clinic patients on high-dose statins, the rate of muscle complaints may be as high as 20%.12
The cause of statin-induced myopathy is not known, although studies have linked it to genetic defects.7 Risk factors have been identified and include personal and family history of myalgia, Asian ethnicity, hypothyroidism, and type 1 diabetes. The incidence of statin-induced myalgia is two to three times higher in patients on corticosteroid therapy. Other risk factors include female sex, liver disease, and renal dysfunction.7,8
A less common etiology is anti-HMG coenzyme A reductase antibodies. Studies have shown that these antibody levels correlate well with the amount of myositis as measured by creatine kinase levels. However, there is no consensus yet on screening for these antibodies.13
Statin therapy poses a dilemma, as there is a thin line between the benefits and the risks of side effects, especially statin-induced myopathy.3,4 Current recommendations include discontinuing the statin until symptoms fully resolve. Creatine kinase levels may be useful in assessing for potential muscle breakdown, especially in patients with reduced renal function, as this predisposes them to statin-induced myopathy, yet normal values do not preclude the diagnosis of statin-induced myopathy.3,4,7,8
Once symptoms resolve and laboratory test results normalize, a trial of a different statin is recommended. If patients become symptomatic, a trial of a low-dose hydrophilic statin at a once- or twice-weekly interval has been recommended. Several studies have assessed the efficacy of a low-dose statin with decreased frequency of administration and have consistently shown significant improvement in lipid levels.3,4 For instance, once-weekly rosuvastatin at a dose between 5 mg and 20 mg resulted in a 29% reduction in LDL cholesterol levels, and 80% of patients did not experience a recurrence of myalgia.3 Furthermore, a study of patients treated with 5 mg to 10 mg of rosuvastatin twice a week resulted in a 26% decrease in LDL cholesterol levels.4 This study also showed that when an additional non-statin lipid-lowering drug was prescribed (eg, ezetimibe, bile acid resin, nicotinic acid), more than half of the patients reached their goal lipid level.4
The addition of coenzyme Q10 and fish oil has also been suggested. Although, the evidence to support this is inconclusive, the potential benefit outweighs the risk, since the side effects are minimal.1 However, no study yet has evaluated the risks vs the benefits in patients with elevated creatine kinase.
Statin-induced myopathy is a commonly encountered adverse effect. Currently, there are no guidelines on restarting statin therapy after statin-induced myopathy; however, data suggest that statin therapy should be restarted once symptoms resolve, and that variations in dose and frequency may be necessary.1–8,14
An 85-year-old man with hypertension, hyperlipidemia, and coronary artery disease presented to our clinic with diffuse muscle pain. The pain had been present for about 3 months, but it had become noticeably worse over the past few weeks.
He was not aware of any trauma. He described the muscle pain as dull and particularly severe in his lower extremities (his thighs and calves). The pain did not limit his daily activities, nor did physical exertion or the time of day have any effect on the level of the pain.
His medications at that time included metoprolol, aspirin, hydrochlorothiazide, simvastatin, and a daily multivitamin.
He was not in acute distress. On neurologic and musculoskeletal examinations, all deep-tendon reflexes were intact, with no tenderness to palpation of the upper and lower extremities. No abnormalities were noted on the joint examination. He had full range of motion, with 5/5 muscle strength in the upper and lower extremities bilaterally and normal muscle tone. He was able to walk with ease. Results of initial laboratory testing, including creatine kinase and erythrocyte sedimentation rate, were normal.
1. What should be the next best step in the evaluation of this patient’s muscle pain?
- Order tests for cyclic citrullinated peptide (CCP) antibody and rheumatoid factor
- Advise him to refrain from physical activity until his symptoms resolve
- Take a more detailed history, including a review of medications and supplements
- Recommend a trial of a nonsteroidal anti-inflammatory drug (NSAID)
- Send him for radiographic imaging
Since his muscle pain has persisted for several months without improvement, a more detailed history should be taken, including a review of current medications and supplements.
Testing CCP antibody and rheumatoid factor would be useful if rheumatoid arthritis were suspected, but in the absence of demonstrable arthritis on examination, these tests would have low specificity even if the results were positive.
An NSAID may temporarily alleviate his pain, but it will not help establish a diagnosis. And in elderly patients, NSAIDs are not without complications and so should be prescribed only in appropriate situations.
Imaging would be appropriate at this point only if there was clinical suspicion of a specific disease. However, our patient has no focal deficits, and the suspicion of fracture or malignancy is low.
The medical history should include asking about current drug regimens, recent medication changes, and the use of herbal supplements, since polypharmacy is common in elderly patients with multiple comorbidities.
On further questioning, our patient said that his dose of simvastatin had been increased from 40 mg daily to 80 mg daily about 1 month before his symptoms appeared. He was taking a daily multivitamin but was not using herbal supplements or other over-the-counter products. He did not recall any constitutional symptoms before the onset of his current symptoms, and he had never had similar muscle pain in the past.
2. Based on the additional information from the history, what is the most likely cause of his muscle pain?
- Limited myositis secondary to recent viral infection
- Rhabdomyolysis
- Hypothyroidism
- Drug-drug interaction
- Statin-induced myalgia
Our patient’s history provided nothing to suggest viral myositis. Hypothyroidism should always be considered in patients with myalgia, but this is not likely in our patient, as he does not display other characteristics, such as diminished reflexes, hypotonia, cold intolerance, and mood instability. Even though calcium channel blockers have been known to cause myalgia in patients on statins, a drug-drug reaction is not likely, as he had not started taking a calcium channel blocker before his symptoms began. This patient did not show signs or symptoms of rhabdomyolysis, a type of myopathy in which necrosis of the muscle tissue occurs, generally causing profound weakness and pain.1
Therefore, statin-induced myopathy is the most likely cause of his diffuse muscle pain, particularly since his simvastatin had been increased 1 month before the onset of symptoms.
3. What should be the next step in his management?
- Decrease the dose of simvastatin to the last known dose he was able to tolerate
- Continue simvastatin at the same dose and then monitor
- Switch to another statin
- Add coenzyme Q10
- Stop simvastatin
Decreasing the statin dosage to the last well-tolerated dose would not be appropriate in a patient with myopathy, as the symptoms would probably not improve.2–4 Also, one should not switch to a different statin while a patient is experiencing symptoms. Rather, the statin should be stopped for at least 6 weeks or until the symptoms have fully resolved.1
Adding coenzyme Q10 is another option, especially in a patient with previously diagnosed coronary artery disease,5 when continued statin therapy is thought necessary to reduce the likelihood of repeat coronary events.
We discontinued his simvastatin. Followup 3 weeks later in the outpatient clinic showed that his symptoms were slowly improving. The symptoms had resolved completely 4 months later.
4. How should we manage our patient’s hyperlipidemia once his symptoms have resolved?
- Restart simvastatin at the 80-mg dose
- Restart simvastatin at the 40-mg dose
- Start a hydrophilic statin at full dose
- Use a drug from another class of lipid-lowering drugs
- Wait another 3 months before prescribing any lipid-lowering drug
His treatment for hyperlipidemia should be continued, considering his comorbidities. However, restarting the same statin, even at a lower dose, will likely cause his symptoms to recur. Thus, a different statin should be tried once his muscle pain has resolved.
Other classes of lipid-lowering drugs are usually less efficacious than statins, particularly when trying to control low-density lipoprotein (LDL) cholesterol, so a drug from another class should not be used until other statin options have been attempted.2,6,7
Simvastatin is lipophilic. Trying a statin with hydrophilic properties (eg, pravastatin, rosuvastatin, fluvastatin) has been shown to convey similar cardioprotective effects with a lower propensity for myalgia, as lipophilic statins have a higher propensity to penetrate muscle tissue than do hydrophilic statins.3,4,8
Once his symptoms resolved, our patient was started on a hydrophilic statin, fluvastatin 20 mg daily. Unfortunately, his pain recurred 3 weeks later. The statin was stopped, and his symptoms again resolved.
5. Since our patient was unable to tolerate a second statin, what should be the next step in his management?
- Restart simvastatin
- Use a drug from another class to control the hyperlipidemia
- Wait at least 6 months after symptoms resolve before trying any lipid-lowering drug
- Initiate therapy with coenzyme Q10 and fish oil
- Wait for symptoms to resolve, then restart a hydrophilic statin at a lower dose and lower frequency
Restarting simvastatin will likely cause a recurrence of the myalgia. Other lipid-lowering drugs such as nicotinic acid, bile acid resins, and fibrates are not as efficacious as statins. Coenzyme Q10 and fish oil can reduce lipid levels, but they are not as efficacious as statins.
In view of our patient’s lipid profile—LDL cholesterol elevated at 167 mg/dL, high-density lipoprotein cholesterol 31 mg/dL, triglycerides 47 mg/dL—it is important to treat his hyperlipidemia. Therefore, another attempt at statin therapy should be made once his symptoms have resolved.
Studies have shown that restarting a statin at a low dose and low frequency is effective in patients who have experienced intolerance to a statin.3,4 Our patient was treated with low-dose pravastatin (20 mg), resulting in a moderate improvement in his LDL cholesterol to 123 mg/dL.
STATIN-INDUCED MYOPATHY: ADDRESSING THE DILEMMA
Treating hyperlipidemia is important to prevent vascular events in patients with or without coronary artery disease. Statins are the most effective agents available for controlling hypercholesterolemia, specifically LDL levels, as well as for preventing myocardial infarction.
Unfortunately, significant side effects have been reported, and myopathy is the most prevalent. Statin-induced myopathy includes a combination of muscle tenderness, myalgia, and weakness.2–11 In randomized controlled trials, the risk of myopathy was estimated to be between 1.5% and 5%.6 In unselected clinic patients on high-dose statins, the rate of muscle complaints may be as high as 20%.12
The cause of statin-induced myopathy is not known, although studies have linked it to genetic defects.7 Risk factors have been identified and include personal and family history of myalgia, Asian ethnicity, hypothyroidism, and type 1 diabetes. The incidence of statin-induced myalgia is two to three times higher in patients on corticosteroid therapy. Other risk factors include female sex, liver disease, and renal dysfunction.7,8
A less common etiology is anti-HMG coenzyme A reductase antibodies. Studies have shown that these antibody levels correlate well with the amount of myositis as measured by creatine kinase levels. However, there is no consensus yet on screening for these antibodies.13
Statin therapy poses a dilemma, as there is a thin line between the benefits and the risks of side effects, especially statin-induced myopathy.3,4 Current recommendations include discontinuing the statin until symptoms fully resolve. Creatine kinase levels may be useful in assessing for potential muscle breakdown, especially in patients with reduced renal function, as this predisposes them to statin-induced myopathy, yet normal values do not preclude the diagnosis of statin-induced myopathy.3,4,7,8
Once symptoms resolve and laboratory test results normalize, a trial of a different statin is recommended. If patients become symptomatic, a trial of a low-dose hydrophilic statin at a once- or twice-weekly interval has been recommended. Several studies have assessed the efficacy of a low-dose statin with decreased frequency of administration and have consistently shown significant improvement in lipid levels.3,4 For instance, once-weekly rosuvastatin at a dose between 5 mg and 20 mg resulted in a 29% reduction in LDL cholesterol levels, and 80% of patients did not experience a recurrence of myalgia.3 Furthermore, a study of patients treated with 5 mg to 10 mg of rosuvastatin twice a week resulted in a 26% decrease in LDL cholesterol levels.4 This study also showed that when an additional non-statin lipid-lowering drug was prescribed (eg, ezetimibe, bile acid resin, nicotinic acid), more than half of the patients reached their goal lipid level.4
The addition of coenzyme Q10 and fish oil has also been suggested. Although, the evidence to support this is inconclusive, the potential benefit outweighs the risk, since the side effects are minimal.1 However, no study yet has evaluated the risks vs the benefits in patients with elevated creatine kinase.
Statin-induced myopathy is a commonly encountered adverse effect. Currently, there are no guidelines on restarting statin therapy after statin-induced myopathy; however, data suggest that statin therapy should be restarted once symptoms resolve, and that variations in dose and frequency may be necessary.1–8,14
- Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011; 78:393–403.
- Foley KA, Simpson RJ, Crouse JR, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol 2008; 101:1747–1748.
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99:1409–1412.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Tomaszewski M, Stepien KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep 2011; 63:859–866.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Guyton JR. Benefit versus risk in statin treatment. Am J Cardiol 2006; 97:95C–97C.
- Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med 2011; 78:393–403.
- Foley KA, Simpson RJ, Crouse JR, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol 2008; 101:1747–1748.
- Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99:1409–1412.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Tomaszewski M, Stepien KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep 2011; 63:859–866.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Guyton JR. Benefit versus risk in statin treatment. Am J Cardiol 2006; 97:95C–97C.
- Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Werner JL, Christopher-Stine L, Ghazarian SR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum 2012; 64:4087–4093.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.