User login
Concern about Copper's Effectiveness in Preventing Hospital-Acquired Infections
Karen Appold’s cover story, “Copper,” in the September 2013 issue, offers an exciting and encouraging development in the struggle to prevent hospital-acquired infections, but I have two concerns. As copper tarnishes, it forms a surface patina of copper hydroxide and copper carbonate. Would this patina act as a physical barrier, preventing bacteria from coming into contact with elemental copper and inhibiting the antimicrobial effect? If so, the obvious solution is to polish the surface frequently enough to prevent tarnishing.
The second concern regards the use of copper-nickel alloys. Many people are sensitive to nickel, [with reactions that] usually manifest as contact dermatitis. A study by the North American Contact Dermatitis Group (NACDG), conducted between 1992-2004 and involving 25,626 patients who were patch-tested, showed a prevalence of nickel sensitivity of 18.8% in 2004, increased from 14.5% in 1992.1
With a current U.S. population of approximately 317 million, a prevalence of 18.8% would mean nearly 60 million people with nickel sensitivity. Extrapolating from the NACDG study, the rate is probably actually higher. Medical devices made with copper-nickel alloys that contact the patient’s skin would cause contact dermatitis, and implanted devices would have the potential for more severe allergic reactions.
I simply urge foresight and caution in the use of various copper alloys for medical applications.
Rod Duraski, MD, MBA, FACP, medical director, WGH Hospital Medicine, LaGrange, Ga.
Reference
Karen Appold’s cover story, “Copper,” in the September 2013 issue, offers an exciting and encouraging development in the struggle to prevent hospital-acquired infections, but I have two concerns. As copper tarnishes, it forms a surface patina of copper hydroxide and copper carbonate. Would this patina act as a physical barrier, preventing bacteria from coming into contact with elemental copper and inhibiting the antimicrobial effect? If so, the obvious solution is to polish the surface frequently enough to prevent tarnishing.
The second concern regards the use of copper-nickel alloys. Many people are sensitive to nickel, [with reactions that] usually manifest as contact dermatitis. A study by the North American Contact Dermatitis Group (NACDG), conducted between 1992-2004 and involving 25,626 patients who were patch-tested, showed a prevalence of nickel sensitivity of 18.8% in 2004, increased from 14.5% in 1992.1
With a current U.S. population of approximately 317 million, a prevalence of 18.8% would mean nearly 60 million people with nickel sensitivity. Extrapolating from the NACDG study, the rate is probably actually higher. Medical devices made with copper-nickel alloys that contact the patient’s skin would cause contact dermatitis, and implanted devices would have the potential for more severe allergic reactions.
I simply urge foresight and caution in the use of various copper alloys for medical applications.
Rod Duraski, MD, MBA, FACP, medical director, WGH Hospital Medicine, LaGrange, Ga.
Reference
Karen Appold’s cover story, “Copper,” in the September 2013 issue, offers an exciting and encouraging development in the struggle to prevent hospital-acquired infections, but I have two concerns. As copper tarnishes, it forms a surface patina of copper hydroxide and copper carbonate. Would this patina act as a physical barrier, preventing bacteria from coming into contact with elemental copper and inhibiting the antimicrobial effect? If so, the obvious solution is to polish the surface frequently enough to prevent tarnishing.
The second concern regards the use of copper-nickel alloys. Many people are sensitive to nickel, [with reactions that] usually manifest as contact dermatitis. A study by the North American Contact Dermatitis Group (NACDG), conducted between 1992-2004 and involving 25,626 patients who were patch-tested, showed a prevalence of nickel sensitivity of 18.8% in 2004, increased from 14.5% in 1992.1
With a current U.S. population of approximately 317 million, a prevalence of 18.8% would mean nearly 60 million people with nickel sensitivity. Extrapolating from the NACDG study, the rate is probably actually higher. Medical devices made with copper-nickel alloys that contact the patient’s skin would cause contact dermatitis, and implanted devices would have the potential for more severe allergic reactions.
I simply urge foresight and caution in the use of various copper alloys for medical applications.
Rod Duraski, MD, MBA, FACP, medical director, WGH Hospital Medicine, LaGrange, Ga.
Reference
Four Recommendations to Help Hospitalists Fight Antimicrobial Resistance
Prevent infections. This might be the most obvious way to fight antibiotic-resistance—if there’s no infection, there is no need to worry about one that can’t be treated. Hospitalists can help prevent infection by quickly and effectively treating those who are infected to prevent the spread, washing hands, and promoting effective cleaning habits.
Tracking. The CDC has programs to gather information on antibiotic-resistant infections, causes of infections, and risk factors for infections. With this information, hospitalists can stay aware of the threats. They can also help by remaining vigilant about signs of new resistance and helping to get that information to the CDC.
The CDC is now working on a new module that will collect antimicrobial-susceptibility data that’s generated in hospital labs, Dr. Patel says.
“This will be compiled in a national database and then made available to state and local public health departments that could track antimicrobial resistance trends in their own state,” she says. “We hope those data will then be used to identify new trends in anti-microbial resistance and used to strategize how to prevent resistance from being transmitted locally.”
Antibiotic stewardship. The CDC says prescribing antibiotics only when necessary and tailoring treatment as narrowly as possible might be the most important step in fighting antimicrobial resistance. The CDC estimates that up to half of antibiotic use in humans is unnecessary.
The CDC is working to capture data on antibiotic use in healthcare settings, which will be used for benchmarking antibiotic use among different institutions and regions.
“I think this additional information will really help healthcare institutions measure how well antibiotics are being used in their institutions and make appropriate adjustments,” Dr. Patel says.
New drugs and diagnostic tests. New antibiotics will be needed because, while resistance can be slowed, it cannot be stopped. However, the number of New Drug Application approvals for antibiotics has fallen drastically—nearly 20 from 1980 to 1984, but fewer than five from 2005 to 2012, according to the CDC report.
Prevent infections. This might be the most obvious way to fight antibiotic-resistance—if there’s no infection, there is no need to worry about one that can’t be treated. Hospitalists can help prevent infection by quickly and effectively treating those who are infected to prevent the spread, washing hands, and promoting effective cleaning habits.
Tracking. The CDC has programs to gather information on antibiotic-resistant infections, causes of infections, and risk factors for infections. With this information, hospitalists can stay aware of the threats. They can also help by remaining vigilant about signs of new resistance and helping to get that information to the CDC.
The CDC is now working on a new module that will collect antimicrobial-susceptibility data that’s generated in hospital labs, Dr. Patel says.
“This will be compiled in a national database and then made available to state and local public health departments that could track antimicrobial resistance trends in their own state,” she says. “We hope those data will then be used to identify new trends in anti-microbial resistance and used to strategize how to prevent resistance from being transmitted locally.”
Antibiotic stewardship. The CDC says prescribing antibiotics only when necessary and tailoring treatment as narrowly as possible might be the most important step in fighting antimicrobial resistance. The CDC estimates that up to half of antibiotic use in humans is unnecessary.
The CDC is working to capture data on antibiotic use in healthcare settings, which will be used for benchmarking antibiotic use among different institutions and regions.
“I think this additional information will really help healthcare institutions measure how well antibiotics are being used in their institutions and make appropriate adjustments,” Dr. Patel says.
New drugs and diagnostic tests. New antibiotics will be needed because, while resistance can be slowed, it cannot be stopped. However, the number of New Drug Application approvals for antibiotics has fallen drastically—nearly 20 from 1980 to 1984, but fewer than five from 2005 to 2012, according to the CDC report.
Prevent infections. This might be the most obvious way to fight antibiotic-resistance—if there’s no infection, there is no need to worry about one that can’t be treated. Hospitalists can help prevent infection by quickly and effectively treating those who are infected to prevent the spread, washing hands, and promoting effective cleaning habits.
Tracking. The CDC has programs to gather information on antibiotic-resistant infections, causes of infections, and risk factors for infections. With this information, hospitalists can stay aware of the threats. They can also help by remaining vigilant about signs of new resistance and helping to get that information to the CDC.
The CDC is now working on a new module that will collect antimicrobial-susceptibility data that’s generated in hospital labs, Dr. Patel says.
“This will be compiled in a national database and then made available to state and local public health departments that could track antimicrobial resistance trends in their own state,” she says. “We hope those data will then be used to identify new trends in anti-microbial resistance and used to strategize how to prevent resistance from being transmitted locally.”
Antibiotic stewardship. The CDC says prescribing antibiotics only when necessary and tailoring treatment as narrowly as possible might be the most important step in fighting antimicrobial resistance. The CDC estimates that up to half of antibiotic use in humans is unnecessary.
The CDC is working to capture data on antibiotic use in healthcare settings, which will be used for benchmarking antibiotic use among different institutions and regions.
“I think this additional information will really help healthcare institutions measure how well antibiotics are being used in their institutions and make appropriate adjustments,” Dr. Patel says.
New drugs and diagnostic tests. New antibiotics will be needed because, while resistance can be slowed, it cannot be stopped. However, the number of New Drug Application approvals for antibiotics has fallen drastically—nearly 20 from 1980 to 1984, but fewer than five from 2005 to 2012, according to the CDC report.
Mediterranean diet: Higher fat but lower risk
Counsel patients at high risk for cardiovascular disease and stroke to follow a Mediterranean diet, which is associated with a 30% risk reduction.1
Strength of recommendation
A: Based on one well-design randomized controlled trial (RCT).
Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279-1290.
Illustrative case
A 62-year-old patient with diabetes, obesity, and a family history of early onset coronary artery disease is motivated to make significant lifestyle changes. You recommend moderate aerobic exercise for 30 minutes 5 times a week, but wonder whether a low-fat diet or a Mediterranean diet would be more effective in lowering her risk.
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of mortality in the United States. CVD accounts for one in every 3 deaths,2 and stroke is a leading cause of long-term disability.2 The direct cost of treating CVD is estimated at $312.6 billion annually.2
Many modifiable risk factors contribute to CVD, including smoking, sedentary lifestyle, obesity, alcohol consumption, and poorly controlled chronic disease, as well as an unhealthy diet. A recent report from the American Heart Association suggests that 13% of deaths from CVD can be attributed to poor diet.2
Focus counseling on patients at risk
Primary care providers (PCPs) often struggle to effectively counsel patients on behavior change strategies, but face many barriers. Chief among them are the lack of time, training, and confidence in their counseling techniques, as well as a lack of patient motivation and readiness to change.3 In recognition of these barriers, the US Preventive Services Task Force recently recommended that PCPs focus behavioral counseling efforts on patients at high risk for heart disease.4
Large observational studies have found an association between trans fat and an increased risk of CVD, as well as a decreased risk of CVD in patients adhering to a Mediterranean diet.5-11 This type of diet typically includes a high intake of olive oil, fruit, nuts, vegetables, and cereals; moderate intake of fish and poultry; and low intake of dairy products, red meat, processed meats, and sweets. It also includes wine in moderation, consumed with meals.
Data on the physiologic properties of olive oil, including its antioxidant, vasodilating, and antiplatelet effects—as well as its effects on low-density lipoprotein cholesterol (LDL-C) that may inhibit atherogenesis—support the link between a Mediterranean diet and a decreased risk of CVD found in the observational studies.12,13 Until recently, however, no RCT had compared the effect of a Mediterranean diet with that of a low-fat diet for primary prevention of CVD.
STUDY SUMMARY: Mediterranean diet significantly lowers risk
Prevencion con Dieta Mediterranea (PREDIMED) was a large RCT (N=7447) comparing 2 variations of a Mediterranean diet with a low-fat diet for primary prevention of CVD. This Spanish study enrolled men 55 to 80 years of age and women ages 60 to 80 at high risk for developing CVD. The risk was based on either a diagnosis of type 2 diabetes or the presence of ≥3 major risk factors, including smoking, hypertension, elevated LDL-C, low high-density lipoprotein cholesterol, overweight or obese, and a family history of early heart disease.
Participants were randomly assigned to one of 3 dietary groups: One group was assigned to a Mediterranean diet supplemented with ≥4 tablespoons per day of extra virgin olive oil; a second group was put on a Mediterranean diet supplemented by 30 grams (about 1/3 cup) of mixed nuts daily; a third group (the controls) was advised to follow a low-fat diet. The majority of baseline characteristics and medications taken throughout the study were similar among all 3 groups.
Those in both Mediterranean diet groups were followed for a median of 4.8 years, during which time they received quarterly dietary classes and individual and group counseling. The controls received baseline training, plus a leaflet about low-fat diets annually. In year 3, however, the researchers began giving the control group the same level of counseling as those in the Mediterranean diet groups to avoid confounding results.
Adherence to the diets was determined by a self-reported 14-item dietary screening questionnaire, plus urinary hydroxytyrosol and serum alpha-linoleic acid levels to assess for olive oil and mixed nut compliance. Self-reporting5 and biometric data indicated good compliance with the Mediterranean diets, and there was no difference found in levels of exercise among the groups.
After 5 years, those in the Mediterranean diet groups had consumed significantly more olive oil, nuts, vegetables, fruits, wine, legumes, fish, seafood, and sofrito sauce (a popular tomato-based sauce) than the control group. Participants in the low-fat diet group had decreased their fat intake by 2%, while those in the Mediterranean groups had increased fat intake (by 2.03% for the olive oil group and 2.1% for the nut group). Overall, 37% of energy intake by those in the low-fat diet group came from fat (exceeding the <30% of calories derived from fat intake that defines a low-fat diet) vs 39% fat intake for those in both Mediterranean diet groups.
The primary outcome was a composite of myocardial infarction (MI), stroke, and death from cardiovascular causes, and there were clinically meaningful and statistically significant differences between the Mediterranean diet groups and the controls. The primary outcome rate for the supplemental olive oil group was 3.8%; 3.4% for the extra nuts group; and 4.4% for the controls. This represents a 30% reduction in risk for combined stroke, MI, and death due to cardiovascular causes for the Mediterranean diet groups (hazard ratio [HR]=0.7; 95% confidence interval [CI], 0.53-0.91; P=.009; number needed to treat [NNT]=148 for the olive oil group and HR=0.7; 95% CI, 0.53-0.94; P=.02; NNT=100 for the group consuming extra nuts). Similar benefits were found in the multivariable adjusted analyses. The results correspond to 3 fewer events (stroke, MI, or cardiovascular death) per 1000 person-years for this high-risk population.
The only individual outcome that showed a significant decrease was stroke, with an NNT of 125 in both Mediterranean diet groups. Outcomes for the controls were similar before and after they began receiving quarterly counseling.
WHAT'S NEW?: Mediterranean diet is better than a lower-fat regimen
This study indicates that a Mediterranean diet, with increased intake of either olive oil or mixed nuts, is more protective against CVD than a recommended low-fat diet. It also shows that advising patients at high risk to follow a Mediterranean diet, providing dietary counseling, and monitoring them for adherence, rather than simply recommending a low-fat diet, can significantly decrease the risk of stroke.
Rates of CVD are higher in the United States than in Spain, so implementing a Mediterranean diet on a large scale in this country has the potential to produce a greater response than that seen in this study.
CAVEATS: Would a true low-fat diet be a better comparison?
Although the control group’s diet was meant to be low fat, the participants did not achieve this, possibly due to the relatively low level of dietary education and personalized counseling at the start of the study. Their inability to reach the <30% fat target could also reflect the difficulty patients have, in general, in decreasing fat content in their diet, which may mean the diet they maintained was a more realistic comparison.
This study used one brand of olive oil and a particular mixture of nuts (walnuts, hazelnuts, and almonds); it is possible that variations on either of these could affect the benefits of the diet.
CHALLENGES TO IMPLEMENTATION: Fitting a Mediterranean diet into an American lifestyle
The typical US diet is significantly different from that of most Spaniards. Americans may find it difficult to add either ≥4 tablespoons of olive oil or 30 g (1/3 cup) of nuts daily, for example, due to both cost and availability. Limited access to both individual and group counseling could be a barrier, as well.
On the other hand, this practice changer has the potential to simplify dietary counseling by allowing clinicians to focus on just one type of diet, for which there are many resources available both online and in print. We believe it makes sense to recommend a Mediterranean diet, while continuing to recommend increased exercise, smoking cessation, and improved control of chronic disease to lower patients’ risk of poor outcomes from CVD.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean Diet. N Engl J Med. 2013;368:1279-1290.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
3. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546-552.
4. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults, topic page. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/usp- sphys.htm. Accessed August 1, 2013.
5. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491-1499.
6. Oomen C, Ocké MC, Feskens JM, et al. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746-751.
7. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785.
8. Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433-1439.
9. Kris-Etherton P, Eckel RH, Howard BV, et al; Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Education Program/AHA Step 1 Dietary Pattern on cardiovascular disease. Circulation. 2001;103:1823-1825.
10. Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. The association of Mediterranean diet with lower risk of acute coronary syndromes, in hypertensive subjects. Int J Cardiol. 2002;82:141-147.
11. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. The role of traditional Mediterranean-type of diet and lifestyle, in the devel- opment of acute coronary syndromes: preliminary results from CARDIO 2000 study. Centr Eur J Public Health. 2002;10:11-15.
12. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
13. Vincent-Baudry S, Defoort C, Gerber M, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82:964-971.
Counsel patients at high risk for cardiovascular disease and stroke to follow a Mediterranean diet, which is associated with a 30% risk reduction.1
Strength of recommendation
A: Based on one well-design randomized controlled trial (RCT).
Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279-1290.
Illustrative case
A 62-year-old patient with diabetes, obesity, and a family history of early onset coronary artery disease is motivated to make significant lifestyle changes. You recommend moderate aerobic exercise for 30 minutes 5 times a week, but wonder whether a low-fat diet or a Mediterranean diet would be more effective in lowering her risk.
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of mortality in the United States. CVD accounts for one in every 3 deaths,2 and stroke is a leading cause of long-term disability.2 The direct cost of treating CVD is estimated at $312.6 billion annually.2
Many modifiable risk factors contribute to CVD, including smoking, sedentary lifestyle, obesity, alcohol consumption, and poorly controlled chronic disease, as well as an unhealthy diet. A recent report from the American Heart Association suggests that 13% of deaths from CVD can be attributed to poor diet.2
Focus counseling on patients at risk
Primary care providers (PCPs) often struggle to effectively counsel patients on behavior change strategies, but face many barriers. Chief among them are the lack of time, training, and confidence in their counseling techniques, as well as a lack of patient motivation and readiness to change.3 In recognition of these barriers, the US Preventive Services Task Force recently recommended that PCPs focus behavioral counseling efforts on patients at high risk for heart disease.4
Large observational studies have found an association between trans fat and an increased risk of CVD, as well as a decreased risk of CVD in patients adhering to a Mediterranean diet.5-11 This type of diet typically includes a high intake of olive oil, fruit, nuts, vegetables, and cereals; moderate intake of fish and poultry; and low intake of dairy products, red meat, processed meats, and sweets. It also includes wine in moderation, consumed with meals.
Data on the physiologic properties of olive oil, including its antioxidant, vasodilating, and antiplatelet effects—as well as its effects on low-density lipoprotein cholesterol (LDL-C) that may inhibit atherogenesis—support the link between a Mediterranean diet and a decreased risk of CVD found in the observational studies.12,13 Until recently, however, no RCT had compared the effect of a Mediterranean diet with that of a low-fat diet for primary prevention of CVD.
STUDY SUMMARY: Mediterranean diet significantly lowers risk
Prevencion con Dieta Mediterranea (PREDIMED) was a large RCT (N=7447) comparing 2 variations of a Mediterranean diet with a low-fat diet for primary prevention of CVD. This Spanish study enrolled men 55 to 80 years of age and women ages 60 to 80 at high risk for developing CVD. The risk was based on either a diagnosis of type 2 diabetes or the presence of ≥3 major risk factors, including smoking, hypertension, elevated LDL-C, low high-density lipoprotein cholesterol, overweight or obese, and a family history of early heart disease.
Participants were randomly assigned to one of 3 dietary groups: One group was assigned to a Mediterranean diet supplemented with ≥4 tablespoons per day of extra virgin olive oil; a second group was put on a Mediterranean diet supplemented by 30 grams (about 1/3 cup) of mixed nuts daily; a third group (the controls) was advised to follow a low-fat diet. The majority of baseline characteristics and medications taken throughout the study were similar among all 3 groups.
Those in both Mediterranean diet groups were followed for a median of 4.8 years, during which time they received quarterly dietary classes and individual and group counseling. The controls received baseline training, plus a leaflet about low-fat diets annually. In year 3, however, the researchers began giving the control group the same level of counseling as those in the Mediterranean diet groups to avoid confounding results.
Adherence to the diets was determined by a self-reported 14-item dietary screening questionnaire, plus urinary hydroxytyrosol and serum alpha-linoleic acid levels to assess for olive oil and mixed nut compliance. Self-reporting5 and biometric data indicated good compliance with the Mediterranean diets, and there was no difference found in levels of exercise among the groups.
After 5 years, those in the Mediterranean diet groups had consumed significantly more olive oil, nuts, vegetables, fruits, wine, legumes, fish, seafood, and sofrito sauce (a popular tomato-based sauce) than the control group. Participants in the low-fat diet group had decreased their fat intake by 2%, while those in the Mediterranean groups had increased fat intake (by 2.03% for the olive oil group and 2.1% for the nut group). Overall, 37% of energy intake by those in the low-fat diet group came from fat (exceeding the <30% of calories derived from fat intake that defines a low-fat diet) vs 39% fat intake for those in both Mediterranean diet groups.
The primary outcome was a composite of myocardial infarction (MI), stroke, and death from cardiovascular causes, and there were clinically meaningful and statistically significant differences between the Mediterranean diet groups and the controls. The primary outcome rate for the supplemental olive oil group was 3.8%; 3.4% for the extra nuts group; and 4.4% for the controls. This represents a 30% reduction in risk for combined stroke, MI, and death due to cardiovascular causes for the Mediterranean diet groups (hazard ratio [HR]=0.7; 95% confidence interval [CI], 0.53-0.91; P=.009; number needed to treat [NNT]=148 for the olive oil group and HR=0.7; 95% CI, 0.53-0.94; P=.02; NNT=100 for the group consuming extra nuts). Similar benefits were found in the multivariable adjusted analyses. The results correspond to 3 fewer events (stroke, MI, or cardiovascular death) per 1000 person-years for this high-risk population.
The only individual outcome that showed a significant decrease was stroke, with an NNT of 125 in both Mediterranean diet groups. Outcomes for the controls were similar before and after they began receiving quarterly counseling.
WHAT'S NEW?: Mediterranean diet is better than a lower-fat regimen
This study indicates that a Mediterranean diet, with increased intake of either olive oil or mixed nuts, is more protective against CVD than a recommended low-fat diet. It also shows that advising patients at high risk to follow a Mediterranean diet, providing dietary counseling, and monitoring them for adherence, rather than simply recommending a low-fat diet, can significantly decrease the risk of stroke.
Rates of CVD are higher in the United States than in Spain, so implementing a Mediterranean diet on a large scale in this country has the potential to produce a greater response than that seen in this study.
CAVEATS: Would a true low-fat diet be a better comparison?
Although the control group’s diet was meant to be low fat, the participants did not achieve this, possibly due to the relatively low level of dietary education and personalized counseling at the start of the study. Their inability to reach the <30% fat target could also reflect the difficulty patients have, in general, in decreasing fat content in their diet, which may mean the diet they maintained was a more realistic comparison.
This study used one brand of olive oil and a particular mixture of nuts (walnuts, hazelnuts, and almonds); it is possible that variations on either of these could affect the benefits of the diet.
CHALLENGES TO IMPLEMENTATION: Fitting a Mediterranean diet into an American lifestyle
The typical US diet is significantly different from that of most Spaniards. Americans may find it difficult to add either ≥4 tablespoons of olive oil or 30 g (1/3 cup) of nuts daily, for example, due to both cost and availability. Limited access to both individual and group counseling could be a barrier, as well.
On the other hand, this practice changer has the potential to simplify dietary counseling by allowing clinicians to focus on just one type of diet, for which there are many resources available both online and in print. We believe it makes sense to recommend a Mediterranean diet, while continuing to recommend increased exercise, smoking cessation, and improved control of chronic disease to lower patients’ risk of poor outcomes from CVD.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Counsel patients at high risk for cardiovascular disease and stroke to follow a Mediterranean diet, which is associated with a 30% risk reduction.1
Strength of recommendation
A: Based on one well-design randomized controlled trial (RCT).
Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279-1290.
Illustrative case
A 62-year-old patient with diabetes, obesity, and a family history of early onset coronary artery disease is motivated to make significant lifestyle changes. You recommend moderate aerobic exercise for 30 minutes 5 times a week, but wonder whether a low-fat diet or a Mediterranean diet would be more effective in lowering her risk.
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of mortality in the United States. CVD accounts for one in every 3 deaths,2 and stroke is a leading cause of long-term disability.2 The direct cost of treating CVD is estimated at $312.6 billion annually.2
Many modifiable risk factors contribute to CVD, including smoking, sedentary lifestyle, obesity, alcohol consumption, and poorly controlled chronic disease, as well as an unhealthy diet. A recent report from the American Heart Association suggests that 13% of deaths from CVD can be attributed to poor diet.2
Focus counseling on patients at risk
Primary care providers (PCPs) often struggle to effectively counsel patients on behavior change strategies, but face many barriers. Chief among them are the lack of time, training, and confidence in their counseling techniques, as well as a lack of patient motivation and readiness to change.3 In recognition of these barriers, the US Preventive Services Task Force recently recommended that PCPs focus behavioral counseling efforts on patients at high risk for heart disease.4
Large observational studies have found an association between trans fat and an increased risk of CVD, as well as a decreased risk of CVD in patients adhering to a Mediterranean diet.5-11 This type of diet typically includes a high intake of olive oil, fruit, nuts, vegetables, and cereals; moderate intake of fish and poultry; and low intake of dairy products, red meat, processed meats, and sweets. It also includes wine in moderation, consumed with meals.
Data on the physiologic properties of olive oil, including its antioxidant, vasodilating, and antiplatelet effects—as well as its effects on low-density lipoprotein cholesterol (LDL-C) that may inhibit atherogenesis—support the link between a Mediterranean diet and a decreased risk of CVD found in the observational studies.12,13 Until recently, however, no RCT had compared the effect of a Mediterranean diet with that of a low-fat diet for primary prevention of CVD.
STUDY SUMMARY: Mediterranean diet significantly lowers risk
Prevencion con Dieta Mediterranea (PREDIMED) was a large RCT (N=7447) comparing 2 variations of a Mediterranean diet with a low-fat diet for primary prevention of CVD. This Spanish study enrolled men 55 to 80 years of age and women ages 60 to 80 at high risk for developing CVD. The risk was based on either a diagnosis of type 2 diabetes or the presence of ≥3 major risk factors, including smoking, hypertension, elevated LDL-C, low high-density lipoprotein cholesterol, overweight or obese, and a family history of early heart disease.
Participants were randomly assigned to one of 3 dietary groups: One group was assigned to a Mediterranean diet supplemented with ≥4 tablespoons per day of extra virgin olive oil; a second group was put on a Mediterranean diet supplemented by 30 grams (about 1/3 cup) of mixed nuts daily; a third group (the controls) was advised to follow a low-fat diet. The majority of baseline characteristics and medications taken throughout the study were similar among all 3 groups.
Those in both Mediterranean diet groups were followed for a median of 4.8 years, during which time they received quarterly dietary classes and individual and group counseling. The controls received baseline training, plus a leaflet about low-fat diets annually. In year 3, however, the researchers began giving the control group the same level of counseling as those in the Mediterranean diet groups to avoid confounding results.
Adherence to the diets was determined by a self-reported 14-item dietary screening questionnaire, plus urinary hydroxytyrosol and serum alpha-linoleic acid levels to assess for olive oil and mixed nut compliance. Self-reporting5 and biometric data indicated good compliance with the Mediterranean diets, and there was no difference found in levels of exercise among the groups.
After 5 years, those in the Mediterranean diet groups had consumed significantly more olive oil, nuts, vegetables, fruits, wine, legumes, fish, seafood, and sofrito sauce (a popular tomato-based sauce) than the control group. Participants in the low-fat diet group had decreased their fat intake by 2%, while those in the Mediterranean groups had increased fat intake (by 2.03% for the olive oil group and 2.1% for the nut group). Overall, 37% of energy intake by those in the low-fat diet group came from fat (exceeding the <30% of calories derived from fat intake that defines a low-fat diet) vs 39% fat intake for those in both Mediterranean diet groups.
The primary outcome was a composite of myocardial infarction (MI), stroke, and death from cardiovascular causes, and there were clinically meaningful and statistically significant differences between the Mediterranean diet groups and the controls. The primary outcome rate for the supplemental olive oil group was 3.8%; 3.4% for the extra nuts group; and 4.4% for the controls. This represents a 30% reduction in risk for combined stroke, MI, and death due to cardiovascular causes for the Mediterranean diet groups (hazard ratio [HR]=0.7; 95% confidence interval [CI], 0.53-0.91; P=.009; number needed to treat [NNT]=148 for the olive oil group and HR=0.7; 95% CI, 0.53-0.94; P=.02; NNT=100 for the group consuming extra nuts). Similar benefits were found in the multivariable adjusted analyses. The results correspond to 3 fewer events (stroke, MI, or cardiovascular death) per 1000 person-years for this high-risk population.
The only individual outcome that showed a significant decrease was stroke, with an NNT of 125 in both Mediterranean diet groups. Outcomes for the controls were similar before and after they began receiving quarterly counseling.
WHAT'S NEW?: Mediterranean diet is better than a lower-fat regimen
This study indicates that a Mediterranean diet, with increased intake of either olive oil or mixed nuts, is more protective against CVD than a recommended low-fat diet. It also shows that advising patients at high risk to follow a Mediterranean diet, providing dietary counseling, and monitoring them for adherence, rather than simply recommending a low-fat diet, can significantly decrease the risk of stroke.
Rates of CVD are higher in the United States than in Spain, so implementing a Mediterranean diet on a large scale in this country has the potential to produce a greater response than that seen in this study.
CAVEATS: Would a true low-fat diet be a better comparison?
Although the control group’s diet was meant to be low fat, the participants did not achieve this, possibly due to the relatively low level of dietary education and personalized counseling at the start of the study. Their inability to reach the <30% fat target could also reflect the difficulty patients have, in general, in decreasing fat content in their diet, which may mean the diet they maintained was a more realistic comparison.
This study used one brand of olive oil and a particular mixture of nuts (walnuts, hazelnuts, and almonds); it is possible that variations on either of these could affect the benefits of the diet.
CHALLENGES TO IMPLEMENTATION: Fitting a Mediterranean diet into an American lifestyle
The typical US diet is significantly different from that of most Spaniards. Americans may find it difficult to add either ≥4 tablespoons of olive oil or 30 g (1/3 cup) of nuts daily, for example, due to both cost and availability. Limited access to both individual and group counseling could be a barrier, as well.
On the other hand, this practice changer has the potential to simplify dietary counseling by allowing clinicians to focus on just one type of diet, for which there are many resources available both online and in print. We believe it makes sense to recommend a Mediterranean diet, while continuing to recommend increased exercise, smoking cessation, and improved control of chronic disease to lower patients’ risk of poor outcomes from CVD.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean Diet. N Engl J Med. 2013;368:1279-1290.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
3. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546-552.
4. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults, topic page. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/usp- sphys.htm. Accessed August 1, 2013.
5. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491-1499.
6. Oomen C, Ocké MC, Feskens JM, et al. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746-751.
7. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785.
8. Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433-1439.
9. Kris-Etherton P, Eckel RH, Howard BV, et al; Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Education Program/AHA Step 1 Dietary Pattern on cardiovascular disease. Circulation. 2001;103:1823-1825.
10. Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. The association of Mediterranean diet with lower risk of acute coronary syndromes, in hypertensive subjects. Int J Cardiol. 2002;82:141-147.
11. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. The role of traditional Mediterranean-type of diet and lifestyle, in the devel- opment of acute coronary syndromes: preliminary results from CARDIO 2000 study. Centr Eur J Public Health. 2002;10:11-15.
12. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
13. Vincent-Baudry S, Defoort C, Gerber M, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82:964-971.
1. Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean Diet. N Engl J Med. 2013;368:1279-1290.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
3. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546-552.
4. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults, topic page. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/usp- sphys.htm. Accessed August 1, 2013.
5. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491-1499.
6. Oomen C, Ocké MC, Feskens JM, et al. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746-751.
7. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785.
8. Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433-1439.
9. Kris-Etherton P, Eckel RH, Howard BV, et al; Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Education Program/AHA Step 1 Dietary Pattern on cardiovascular disease. Circulation. 2001;103:1823-1825.
10. Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. The association of Mediterranean diet with lower risk of acute coronary syndromes, in hypertensive subjects. Int J Cardiol. 2002;82:141-147.
11. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. The role of traditional Mediterranean-type of diet and lifestyle, in the devel- opment of acute coronary syndromes: preliminary results from CARDIO 2000 study. Centr Eur J Public Health. 2002;10:11-15.
12. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
13. Vincent-Baudry S, Defoort C, Gerber M, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82:964-971.
Copyright © 2013 Family Physicians Inquiries Network. All rights reserved.
Hospitalists Poised to Prevent, Combat Antibiotic-Resistant Pathogens
Describing formally for the first time the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than two million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
And because those numbers are based only on the data available—and the agency assumes that many infections are not captured—the CDC says its estimate is a conservative one and the real number is probably higher.
The report is a call to action for hospitalists, who are in an almost ideal position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC.
“I think it’s a sobering number, and it indicates how far we have to go in combating this problem of antimicrobial resistance,” Dr. Patel says.
The medical community, she adds, cannot expect that new treatments will become available to fight all of these new infections.
“All of the drugs also are going to have some gaps in their range of activity, so there’s no drug coming that’s going to be effective against all the antimicrobial-resistant drugs that we face today,” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013,” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 drug-resistant infections a year and 600 deaths; and drug-resistant Neisseria gonorrhoeae, at 246,000 drug-resistant infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
There are 12 pathogens in the second category, described as “a serious concern” requiring “prompt and sustained action to ensure the problem does not grow.”
Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is actually decreasing, especially in healthcare institutions, and because there are antibiotics that still work on MRSA.
“If either of those things were to change—for example, if the rate of infections were to increase, or if these isolates were to become more resistant—then we would have to think about changing this from a serious threat to an urgent threat,” Dr. Patel says.
Another infection in the serious category that should be on hospitalists’ radar is drug-resistant Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
The report estimates as much as $20 billion in excess healthcare costs due to antimicrobial-resistant infections, with $35 billion in lost productivity in 2008 dollars.1
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the sheer numbers are sure to get people to take notice.
“Two million is lots of patients,” she says. “It’s eye-opening, really, for many doctors and patients and society.”
The silver lining, she says, is that the field is moving toward diagnostic tools that will provide quick feedback on the type of infection at work.
It may be that hospitalists have no choice but to give an antibiotic to a patient because of the risk involved in not giving one; however, providers should quickly tailor that treatment to target the specific pathogen when more information is available.
—Ketino Kobaidze, MD, assistant professor, Emory University School of Medicine, Atlanta, member, antimicrobial stewardship and infectious disease control committees, Emory University Hospital Midtown
“The most important thing, I think, for hospital medicine and medicine anywhere, is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care,” Dr. Kobaidze says. “Time is really an important issue here.
“As hospitalists, we need to be extremely cautious not to give them something they don’t need.”
Dr. Kobaidze was particularly struck by gonorrhea being listed in the “urgent” threat category.
“It was so easy to treat before,” she says. “It was nothing, piece of cake. This makes me a little bit concerned.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play.
“I think this has a clear impact on hospitalists, who are the primary caregivers of many of these ill patients,” he says. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use. Hospitalists are also the people who can help protect patients from the spread of these in the hospital by following appropriate infection prevention guidelines and educating their colleagues of the importance of this.”
He also stresses the importance of being aware of threats within your specific region.
“Many of these MDROs [multi-drug resistant organisms] have regional prevalence,” he says. “And it’s important to know which bugs are in your region so you can work with your institution and public health to tackle these.”
Tom Collins is a freelance writer in South Florida.
Reference
Describing formally for the first time the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than two million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
And because those numbers are based only on the data available—and the agency assumes that many infections are not captured—the CDC says its estimate is a conservative one and the real number is probably higher.
The report is a call to action for hospitalists, who are in an almost ideal position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC.
“I think it’s a sobering number, and it indicates how far we have to go in combating this problem of antimicrobial resistance,” Dr. Patel says.
The medical community, she adds, cannot expect that new treatments will become available to fight all of these new infections.
“All of the drugs also are going to have some gaps in their range of activity, so there’s no drug coming that’s going to be effective against all the antimicrobial-resistant drugs that we face today,” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013,” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 drug-resistant infections a year and 600 deaths; and drug-resistant Neisseria gonorrhoeae, at 246,000 drug-resistant infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
There are 12 pathogens in the second category, described as “a serious concern” requiring “prompt and sustained action to ensure the problem does not grow.”
Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is actually decreasing, especially in healthcare institutions, and because there are antibiotics that still work on MRSA.
“If either of those things were to change—for example, if the rate of infections were to increase, or if these isolates were to become more resistant—then we would have to think about changing this from a serious threat to an urgent threat,” Dr. Patel says.
Another infection in the serious category that should be on hospitalists’ radar is drug-resistant Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
The report estimates as much as $20 billion in excess healthcare costs due to antimicrobial-resistant infections, with $35 billion in lost productivity in 2008 dollars.1
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the sheer numbers are sure to get people to take notice.
“Two million is lots of patients,” she says. “It’s eye-opening, really, for many doctors and patients and society.”
The silver lining, she says, is that the field is moving toward diagnostic tools that will provide quick feedback on the type of infection at work.
It may be that hospitalists have no choice but to give an antibiotic to a patient because of the risk involved in not giving one; however, providers should quickly tailor that treatment to target the specific pathogen when more information is available.
—Ketino Kobaidze, MD, assistant professor, Emory University School of Medicine, Atlanta, member, antimicrobial stewardship and infectious disease control committees, Emory University Hospital Midtown
“The most important thing, I think, for hospital medicine and medicine anywhere, is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care,” Dr. Kobaidze says. “Time is really an important issue here.
“As hospitalists, we need to be extremely cautious not to give them something they don’t need.”
Dr. Kobaidze was particularly struck by gonorrhea being listed in the “urgent” threat category.
“It was so easy to treat before,” she says. “It was nothing, piece of cake. This makes me a little bit concerned.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play.
“I think this has a clear impact on hospitalists, who are the primary caregivers of many of these ill patients,” he says. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use. Hospitalists are also the people who can help protect patients from the spread of these in the hospital by following appropriate infection prevention guidelines and educating their colleagues of the importance of this.”
He also stresses the importance of being aware of threats within your specific region.
“Many of these MDROs [multi-drug resistant organisms] have regional prevalence,” he says. “And it’s important to know which bugs are in your region so you can work with your institution and public health to tackle these.”
Tom Collins is a freelance writer in South Florida.
Reference
Describing formally for the first time the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than two million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
And because those numbers are based only on the data available—and the agency assumes that many infections are not captured—the CDC says its estimate is a conservative one and the real number is probably higher.
The report is a call to action for hospitalists, who are in an almost ideal position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC.
“I think it’s a sobering number, and it indicates how far we have to go in combating this problem of antimicrobial resistance,” Dr. Patel says.
The medical community, she adds, cannot expect that new treatments will become available to fight all of these new infections.
“All of the drugs also are going to have some gaps in their range of activity, so there’s no drug coming that’s going to be effective against all the antimicrobial-resistant drugs that we face today,” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013,” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 drug-resistant infections a year and 600 deaths; and drug-resistant Neisseria gonorrhoeae, at 246,000 drug-resistant infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
There are 12 pathogens in the second category, described as “a serious concern” requiring “prompt and sustained action to ensure the problem does not grow.”
Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is actually decreasing, especially in healthcare institutions, and because there are antibiotics that still work on MRSA.
“If either of those things were to change—for example, if the rate of infections were to increase, or if these isolates were to become more resistant—then we would have to think about changing this from a serious threat to an urgent threat,” Dr. Patel says.
Another infection in the serious category that should be on hospitalists’ radar is drug-resistant Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
The report estimates as much as $20 billion in excess healthcare costs due to antimicrobial-resistant infections, with $35 billion in lost productivity in 2008 dollars.1
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the sheer numbers are sure to get people to take notice.
“Two million is lots of patients,” she says. “It’s eye-opening, really, for many doctors and patients and society.”
The silver lining, she says, is that the field is moving toward diagnostic tools that will provide quick feedback on the type of infection at work.
It may be that hospitalists have no choice but to give an antibiotic to a patient because of the risk involved in not giving one; however, providers should quickly tailor that treatment to target the specific pathogen when more information is available.
—Ketino Kobaidze, MD, assistant professor, Emory University School of Medicine, Atlanta, member, antimicrobial stewardship and infectious disease control committees, Emory University Hospital Midtown
“The most important thing, I think, for hospital medicine and medicine anywhere, is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care,” Dr. Kobaidze says. “Time is really an important issue here.
“As hospitalists, we need to be extremely cautious not to give them something they don’t need.”
Dr. Kobaidze was particularly struck by gonorrhea being listed in the “urgent” threat category.
“It was so easy to treat before,” she says. “It was nothing, piece of cake. This makes me a little bit concerned.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play.
“I think this has a clear impact on hospitalists, who are the primary caregivers of many of these ill patients,” he says. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use. Hospitalists are also the people who can help protect patients from the spread of these in the hospital by following appropriate infection prevention guidelines and educating their colleagues of the importance of this.”
He also stresses the importance of being aware of threats within your specific region.
“Many of these MDROs [multi-drug resistant organisms] have regional prevalence,” he says. “And it’s important to know which bugs are in your region so you can work with your institution and public health to tackle these.”
Tom Collins is a freelance writer in South Florida.
Reference
Culture Shift Required to Defeat Defensive Medicine
Hospitalist Allen Kachalia, MD, JD, of Brigham and Women’s Hospital in Boston, sees defensive medicine as a source of unnecessary costs—and a threat to patient safety.
In fact, he and his colleagues offered an oral presentation at HM13 earlier this year titled, “Overutilization and Defensive Medicine in U.S. Hospitals: A Randomized National Survey of Hospitalists.” In a survey of 1,020 hospitalists, it was reported that defensive medicine was practiced in 37% of pre-operative evaluations and 58% of syncope cases.
Dr. Kachalia says he understands the pressures that can lead physicians to order unnecessary tests, particularly when patients request them. So what does he say about those requests?
“The answer is a simple one but takes time and effort: If you don’t think that something is clinically indicated, you should talk with the patient, explaining to them why you don’t think it’s necessary,” he says. “And, hopefully, you can come to mutual agreement. Ordering things just for the sake of preventing legal liability is just not the right thing to do.”
Dr. Kachalia says he believes that a paradigm shift in how medical liability is handled in this country is needed to change those habits.
But culture change also takes time.
Bryan Weiss, MBA, managing director of the consulting services practice at Irving, Texas-based MedSynergies, says the first step of that change may be having physicians admit that few doctors know a lot about malpractice issues, because they are typically negotiated, arranged, and paid for by their employers, whether that’s a hospital or large management companies.
“It’s not me versus them,” says Weiss, a Team Hospitalist member. “As a specialty, we need to be in this together, to push the education and awareness that it’s OK not to know, so let’s work together to make it better. But it’s not going to happen overnight.”
Hospitalist Allen Kachalia, MD, JD, of Brigham and Women’s Hospital in Boston, sees defensive medicine as a source of unnecessary costs—and a threat to patient safety.
In fact, he and his colleagues offered an oral presentation at HM13 earlier this year titled, “Overutilization and Defensive Medicine in U.S. Hospitals: A Randomized National Survey of Hospitalists.” In a survey of 1,020 hospitalists, it was reported that defensive medicine was practiced in 37% of pre-operative evaluations and 58% of syncope cases.
Dr. Kachalia says he understands the pressures that can lead physicians to order unnecessary tests, particularly when patients request them. So what does he say about those requests?
“The answer is a simple one but takes time and effort: If you don’t think that something is clinically indicated, you should talk with the patient, explaining to them why you don’t think it’s necessary,” he says. “And, hopefully, you can come to mutual agreement. Ordering things just for the sake of preventing legal liability is just not the right thing to do.”
Dr. Kachalia says he believes that a paradigm shift in how medical liability is handled in this country is needed to change those habits.
But culture change also takes time.
Bryan Weiss, MBA, managing director of the consulting services practice at Irving, Texas-based MedSynergies, says the first step of that change may be having physicians admit that few doctors know a lot about malpractice issues, because they are typically negotiated, arranged, and paid for by their employers, whether that’s a hospital or large management companies.
“It’s not me versus them,” says Weiss, a Team Hospitalist member. “As a specialty, we need to be in this together, to push the education and awareness that it’s OK not to know, so let’s work together to make it better. But it’s not going to happen overnight.”
Hospitalist Allen Kachalia, MD, JD, of Brigham and Women’s Hospital in Boston, sees defensive medicine as a source of unnecessary costs—and a threat to patient safety.
In fact, he and his colleagues offered an oral presentation at HM13 earlier this year titled, “Overutilization and Defensive Medicine in U.S. Hospitals: A Randomized National Survey of Hospitalists.” In a survey of 1,020 hospitalists, it was reported that defensive medicine was practiced in 37% of pre-operative evaluations and 58% of syncope cases.
Dr. Kachalia says he understands the pressures that can lead physicians to order unnecessary tests, particularly when patients request them. So what does he say about those requests?
“The answer is a simple one but takes time and effort: If you don’t think that something is clinically indicated, you should talk with the patient, explaining to them why you don’t think it’s necessary,” he says. “And, hopefully, you can come to mutual agreement. Ordering things just for the sake of preventing legal liability is just not the right thing to do.”
Dr. Kachalia says he believes that a paradigm shift in how medical liability is handled in this country is needed to change those habits.
But culture change also takes time.
Bryan Weiss, MBA, managing director of the consulting services practice at Irving, Texas-based MedSynergies, says the first step of that change may be having physicians admit that few doctors know a lot about malpractice issues, because they are typically negotiated, arranged, and paid for by their employers, whether that’s a hospital or large management companies.
“It’s not me versus them,” says Weiss, a Team Hospitalist member. “As a specialty, we need to be in this together, to push the education and awareness that it’s OK not to know, so let’s work together to make it better. But it’s not going to happen overnight.”
Why Hospitalists Remain Outside Malpractice Insurers' High-Risk Categories, For Now
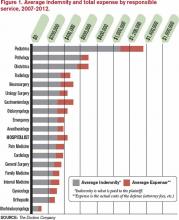
Source: The Doctors Company
Ten years ago, the national headlines on malpractice insurance were staggering. Media reports catalogued OB-GYNs who proclaimed they were shutting down their private practices in the face of runaway premiums. Surgeons and other proceduralists decried payments tied to lawsuits they’d argue were arbitrary and capricious. And the American Medical Association (AMA) made announcement after announcement about states being in a “malpractice crisis.”
In recent years, premiums have actually dropped and stabilized at levels that most physicians agree are manageable for bottom lines. But, in that time, there has been scant discussion about hospital medicine’s relationship with malpractice. It’s not because the issue isn’t omnipresent for all healthcare practitioners, including the relatively nascent specialty that is HM.
Practice management experts say anecdotally that delayed diagnosis of, or treatment for, a spinal epidural abscess (SEA) is likely to get more than a few hospitalists sued. And, the proliferation of co-management of other specialties—particularly those with higher risk of incidence and higher premiums than internal medicine—open up hospitalists to further liability.
The issue is that at less than 20 years as a specialty, HM is in its infancy when it comes to its interaction with malpractice premiums. Health insurance companies and trade groups that track the insurance industry are just beginning to have enough data on claims, premiums, and payouts to make recommendations on risk factors, risk mitigation, and potential trends.
Still, even in a landscape of limited information, there are a few rules of thumb hospitalist group leaders should live by when it comes to managing exposure to malpractice cases, according to interviews with a half dozen healthcare professionals:
- Know how your coverage works. Is there “tail coverage” that ensures you have protection for incidents that happened at an institution where you no longer practice? Even though hospital-employed physicians rarely have rate discussions directly (the hospital typically covers premiums as part of the compensation package), take the time to learn the basic details.
- Be diligent in documentation. Note concerns in charts when appropriate, and stand up for your point of view. There’s a fine line between picking fights with other physicians involved in a patient’s care and making your concerns known, but don’t be afraid to put your clinical view on the record.
- Avoid the practice of “defensive medicine.” Ordering tests and procedures that aren’t clinically necessary might seem like it can serve as a protection from later lawsuits, but it adds to healthcare costs and is just not the right thing to do, says hospitalist Allen Kachalia, MD, JD, of Brigham and Women’s Hospital in Boston, who has studied the phenomenon (see “Culture Shift Necessary to Defeat “Defensive” Medicine,” on p. 38).
- Recognize the risks associated with co-management. Caring for neurology, cardiology, and other subspecialty patients is a revenue boost for HM groups, but when some of those complex cases have adverse events, the hospitalist who interacted with the patient daily could be included in a lawsuit.
- Focus on communication skills. An analysis of claims data by The Doctors Company (TDC) (www.thedoctors.com), a medical malpractice insurance company exclusively endorsed by SHM, reports that the second most common factor contributing to patient injury by hospitalists is “communication breakdown among healthcare professionals.”
- Manage workloads to avoid burnout. Don’t take on too many patients at the expense of being involved in hospital committees or quality initiatives.
To be sure, many of the same tenets of being a productive hospitalist with high patient satisfaction scores—maintain manageable censuses; focus on patient centeredness; and use checklists, technology, and regimented protocols to reduce adverse events—translate very well to being a lower-risk hospitalist in relation to malpractice cases.
When you’re “thinking of patient satisfaction strategies, also think of them as risk mitigation strategies,” says John Nelson, MD, MHM, FACP, medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash., an SHM co-founder and practice management columnist for The Hospitalist. “They overlap tremendously.”
A History Lesson
Medical malpractice has been around for centuries and has two prevailing goals: 1) to provide monetary remuneration to patients who have been injured via substandard care and 2) to deter that poor treatment through fiscal punishment.
Malpractice lawsuits were not prevalent enough to be a major medical concern until the early 1800s. By the middle of the 19th century, the country hit its first periods of crisis.1 Cycles ebbed and flowed from there, with malpractice premiums causing crises in the 1980s and again in the early 2000s.
continued below...
Now, rates for medical professional liability insurance have been dropping for seven years, and an eighth straight annual decline is expected this year, according to Mike Matray, the editor of trade publication Medical Liability Monitor and the chief content officer of its associated website, www.mymedicalmalpracticeinsurance.com.
“We are in the longest, deepest soft market that the malpractice insurance industry has ever been in,” he says. “Right now, things are really good for the doctors, as far as rates coming down.”
Matray says he understands that declining rates may seem immaterial to a physician who receives an insurance bill that eats into the bottom line. For some specialties, that premium can be as high as $200,000 per physician, per year—or more.
“I’m not saying it isn’t expensive,” he adds. “It’s expensive to run a medical practice. At the same time, medical malpractice insurance is less expensive in today’s dollars than it was in 2005.”
The reduction in rates is multi-faceted. Prominently, state-level tort reforms like non-economic damage caps, health courts, and arbitration hearings are making it harder to bring cases to trial, particularly for lawyers who take cases on contingency. Second, frivolous lawsuits “are making an impression on jury pools,” Matray says, which means fewer filed claims and fewer cases that make it to trial. Third, this soft cycle has outlasted the typical pattern of rates falling for three to four years before rebounding.
“A lot of smart actuaries keep saying this has to change soon, because in a soft market there is a lot of competition,” he says, noting that in order to compete for low rates, insurance companies offer credits to clients and use their own reserve cash piles. “So things are really going to change in the next couple of years.”

—Robin Diamond, senior vice president and chief patient safety officer, The Doctors Company
In Need of Data, Patience
So what does it all mean for hospitalists and HM group leaders looking to be proactive about medical malpractice liability insurance? Patience is required.
For starters, there is no designated premium category for hospitalists. Much like the situation that exists for coding issues, the closest proxy for HM is internal medicine. According to Medical Liability Monitor, the premium paid by internal medicine physicians as of July 1, 2012, varied widely across the country. In South Florida, internal medicine insurance premiums in Miami, Dade, and Broward counties were between $42,000 and $46,000 per year. In South Dakota, one insurer reported rates of just under $4,000 per year. There is no average or median figure available, and Matray notes that actual rates paid can vary from county to county.
Moreover, it is difficult for group leaders or hospital executives to use past history to negotiate rates with insurers because of a shortage of reliable data. In its spring 2013 newsletter, the PIAA (formerly known as the Physician Insurers Association of America) published its first report on hospitalist claims reported to its Data Sharing Project. Of the 92,868 closed claims reported from 2002-2011, just 312, or 0.3%, named hospitalists as the defendant.
The data also showed that, of those claims, 20% were settled through insurance company payments. Those payments totaled $17.1 million, with an average payout to a claimant (known as the indemnity) of $272,553 per claim. Overall, hospitalists had a 20% paid-to-closed ratio, totaling more than $17.1 million. By comparison, the percent of paid-to-closed claims for all physicians was 29.3%, according to PIAA.
In a separate data set compiled this year by TDC, 34% of allegations against hospitalists were related to missed or failed diagnoses, with 28% tied to “improper management of treatment.” Twelve percent of allegations were the result of either improper medication management or ordering errors.
Robin Diamond, TDC’s senior vice president and chief patient safety officer, says that teasing out trends from the initial data can be challenging. Hospitalists, she says, can deal with so many different patients, diseases, and severity levels that it is difficult to draw conclusions.
“Hospital medicine is different than other specialties, because the hospitalist treats a broad range of patients in an acute care setting—from a pediatric patient to an adult patient with many chronic illnesses,” she says.
Divya Parikh, PIAA’s director of research and loss prevention, says HM group leaders should avoid reading too much into the first batch of data, because it’s a small sample size.
“A big part of that is we feel that a lot of hospitalists are intermingled into the other medical specialties,” she says. “So this becomes a very small subset where they are distinctly identified as hospitalists. And that’s the challenge.”
In particular, Parikh is curious to see whether HM’s rate of claims paid through insurance payments drops from 20% (already below the overall healthcare industry average). “It will be interesting as we proceed...to see if they begin to mitigate areas of risk where we used to see a lot of claims,” she adds. “If you look at a hospital setting, there has been some shift change in what the errors are. And, what you’d hope with hospitalists within these environments who are really owning this specialty, is that you’d see a decrease in that. There would be that connective care. There would be the patient that felt that they had an individual who was their go-to individual throughout their care at a hospital.”
A Peek at the Future
Insurers have begun compiling claims data on hospitalists and are taking a longer-term view of the specialty. TDC, for example, has analyzed its data and identified characteristics it says make a low-risk hospitalist, an analysis the company says is the first of its kind (see Figure 1). The insurer adds that it sees its responsibility as making sure everyone understands the hospitalist’s role within the acute care setting so that its pricing is commensurate with the liability risk.
Source: The Doctors Company
“We’re looking at the systems within the hospitalist group, as well as how well that group is integrating with the hospital where they’re practicing,” Diamond says. “What kind of patient mix is this particular hospitalist group seeing in that particular hospital, because it can be different in a large healthcare corporation in Manhattan, New York, from a community hospital in rural Texas.”
The growing popularity of hospitalists taking on co-management responsibilities for other specialties is another trend to keep an eye on, as it creates what insurers call “vicarious liability.” Working together in teams with other specialties can improve communication, reduce errors during transitions of care, and create better outcomes. However, in instances where there are problems, being on a care team means hospitalists can open themselves to liability. To mitigate that risk, hospitalists can look to other groups that have dealt with shared liability issues in the past, Parikh says.
“Historically, you would have seen it with anesthesiology,” she explains. “And one huge improvement anesthesiologists have made when a patient comes in for a surgery now is they come out, introduce themselves, say hello, and tell you what’s going on. They put a face to the name, so that it’s not just a no-name anesthesiologist who gets included in the lawsuit as well because they’re naming everybody in the group.”
But, holistically, the best long-term mitigation strategy appears to be tort reform and new ways of looking at the way in which healthcare liability issues are handled in the U.S., says Anupam Jena, MD, PhD, assistant professor of healthcare policy and medicine at Harvard Medical School, and an internist at Massachusetts General Hospital, both in Boston. Dr. Jena says that there is limited evidence that enacted malpractice reforms have produced more than a 2% to 5% reduction in healthcare spending compared to states that have not.2 Instead, healthcare leaders should push for the elimination of defensive medicine, which he says contributes the lion’s share of the estimated $50 billion annual cost of malpractice liability across the country.
“Do I think the country is in a malpractice crisis? No,” he says. “Do I think that defensive medicine is larger than we think it is? Yes.
“If physicians practice as they felt they should practice without ordering extra tests and procedures, my guess would be you could reduce healthcare spending by substantially more than $50 billion.”
Richard Quinn is a freelance writer in New Jersey.
References
- Spiegel AD, Kavaler F. America’s first medical malpractice crisis, 1835-1865. J Community Health. 1997;22:283-308.
- Chandra A, Jena A, Seabury, S. Defensive medicine may be costlier than it seems. The Wall Street Journal website. http://online.wsj.com/article/SB10001424127887323701904578280112638373302.html. Accessed September 21, 2013.
Source: The Doctors Company
Ten years ago, the national headlines on malpractice insurance were staggering. Media reports catalogued OB-GYNs who proclaimed they were shutting down their private practices in the face of runaway premiums. Surgeons and other proceduralists decried payments tied to lawsuits they’d argue were arbitrary and capricious. And the American Medical Association (AMA) made announcement after announcement about states being in a “malpractice crisis.”
In recent years, premiums have actually dropped and stabilized at levels that most physicians agree are manageable for bottom lines. But, in that time, there has been scant discussion about hospital medicine’s relationship with malpractice. It’s not because the issue isn’t omnipresent for all healthcare practitioners, including the relatively nascent specialty that is HM.
Practice management experts say anecdotally that delayed diagnosis of, or treatment for, a spinal epidural abscess (SEA) is likely to get more than a few hospitalists sued. And, the proliferation of co-management of other specialties—particularly those with higher risk of incidence and higher premiums than internal medicine—open up hospitalists to further liability.
The issue is that at less than 20 years as a specialty, HM is in its infancy when it comes to its interaction with malpractice premiums. Health insurance companies and trade groups that track the insurance industry are just beginning to have enough data on claims, premiums, and payouts to make recommendations on risk factors, risk mitigation, and potential trends.
Still, even in a landscape of limited information, there are a few rules of thumb hospitalist group leaders should live by when it comes to managing exposure to malpractice cases, according to interviews with a half dozen healthcare professionals:
- Know how your coverage works. Is there “tail coverage” that ensures you have protection for incidents that happened at an institution where you no longer practice? Even though hospital-employed physicians rarely have rate discussions directly (the hospital typically covers premiums as part of the compensation package), take the time to learn the basic details.
- Be diligent in documentation. Note concerns in charts when appropriate, and stand up for your point of view. There’s a fine line between picking fights with other physicians involved in a patient’s care and making your concerns known, but don’t be afraid to put your clinical view on the record.
- Avoid the practice of “defensive medicine.” Ordering tests and procedures that aren’t clinically necessary might seem like it can serve as a protection from later lawsuits, but it adds to healthcare costs and is just not the right thing to do, says hospitalist Allen Kachalia, MD, JD, of Brigham and Women’s Hospital in Boston, who has studied the phenomenon (see “Culture Shift Necessary to Defeat “Defensive” Medicine,” on p. 38).
- Recognize the risks associated with co-management. Caring for neurology, cardiology, and other subspecialty patients is a revenue boost for HM groups, but when some of those complex cases have adverse events, the hospitalist who interacted with the patient daily could be included in a lawsuit.
- Focus on communication skills. An analysis of claims data by The Doctors Company (TDC) (www.thedoctors.com), a medical malpractice insurance company exclusively endorsed by SHM, reports that the second most common factor contributing to patient injury by hospitalists is “communication breakdown among healthcare professionals.”
- Manage workloads to avoid burnout. Don’t take on too many patients at the expense of being involved in hospital committees or quality initiatives.
To be sure, many of the same tenets of being a productive hospitalist with high patient satisfaction scores—maintain manageable censuses; focus on patient centeredness; and use checklists, technology, and regimented protocols to reduce adverse events—translate very well to being a lower-risk hospitalist in relation to malpractice cases.
When you’re “thinking of patient satisfaction strategies, also think of them as risk mitigation strategies,” says John Nelson, MD, MHM, FACP, medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash., an SHM co-founder and practice management columnist for The Hospitalist. “They overlap tremendously.”
A History Lesson
Medical malpractice has been around for centuries and has two prevailing goals: 1) to provide monetary remuneration to patients who have been injured via substandard care and 2) to deter that poor treatment through fiscal punishment.
Malpractice lawsuits were not prevalent enough to be a major medical concern until the early 1800s. By the middle of the 19th century, the country hit its first periods of crisis.1 Cycles ebbed and flowed from there, with malpractice premiums causing crises in the 1980s and again in the early 2000s.
continued below...
Now, rates for medical professional liability insurance have been dropping for seven years, and an eighth straight annual decline is expected this year, according to Mike Matray, the editor of trade publication Medical Liability Monitor and the chief content officer of its associated website, www.mymedicalmalpracticeinsurance.com.
“We are in the longest, deepest soft market that the malpractice insurance industry has ever been in,” he says. “Right now, things are really good for the doctors, as far as rates coming down.”
Matray says he understands that declining rates may seem immaterial to a physician who receives an insurance bill that eats into the bottom line. For some specialties, that premium can be as high as $200,000 per physician, per year—or more.
“I’m not saying it isn’t expensive,” he adds. “It’s expensive to run a medical practice. At the same time, medical malpractice insurance is less expensive in today’s dollars than it was in 2005.”
The reduction in rates is multi-faceted. Prominently, state-level tort reforms like non-economic damage caps, health courts, and arbitration hearings are making it harder to bring cases to trial, particularly for lawyers who take cases on contingency. Second, frivolous lawsuits “are making an impression on jury pools,” Matray says, which means fewer filed claims and fewer cases that make it to trial. Third, this soft cycle has outlasted the typical pattern of rates falling for three to four years before rebounding.
“A lot of smart actuaries keep saying this has to change soon, because in a soft market there is a lot of competition,” he says, noting that in order to compete for low rates, insurance companies offer credits to clients and use their own reserve cash piles. “So things are really going to change in the next couple of years.”

—Robin Diamond, senior vice president and chief patient safety officer, The Doctors Company
In Need of Data, Patience
So what does it all mean for hospitalists and HM group leaders looking to be proactive about medical malpractice liability insurance? Patience is required.
For starters, there is no designated premium category for hospitalists. Much like the situation that exists for coding issues, the closest proxy for HM is internal medicine. According to Medical Liability Monitor, the premium paid by internal medicine physicians as of July 1, 2012, varied widely across the country. In South Florida, internal medicine insurance premiums in Miami, Dade, and Broward counties were between $42,000 and $46,000 per year. In South Dakota, one insurer reported rates of just under $4,000 per year. There is no average or median figure available, and Matray notes that actual rates paid can vary from county to county.
Moreover, it is difficult for group leaders or hospital executives to use past history to negotiate rates with insurers because of a shortage of reliable data. In its spring 2013 newsletter, the PIAA (formerly known as the Physician Insurers Association of America) published its first report on hospitalist claims reported to its Data Sharing Project. Of the 92,868 closed claims reported from 2002-2011, just 312, or 0.3%, named hospitalists as the defendant.
The data also showed that, of those claims, 20% were settled through insurance company payments. Those payments totaled $17.1 million, with an average payout to a claimant (known as the indemnity) of $272,553 per claim. Overall, hospitalists had a 20% paid-to-closed ratio, totaling more than $17.1 million. By comparison, the percent of paid-to-closed claims for all physicians was 29.3%, according to PIAA.
In a separate data set compiled this year by TDC, 34% of allegations against hospitalists were related to missed or failed diagnoses, with 28% tied to “improper management of treatment.” Twelve percent of allegations were the result of either improper medication management or ordering errors.
Robin Diamond, TDC’s senior vice president and chief patient safety officer, says that teasing out trends from the initial data can be challenging. Hospitalists, she says, can deal with so many different patients, diseases, and severity levels that it is difficult to draw conclusions.
“Hospital medicine is different than other specialties, because the hospitalist treats a broad range of patients in an acute care setting—from a pediatric patient to an adult patient with many chronic illnesses,” she says.
Divya Parikh, PIAA’s director of research and loss prevention, says HM group leaders should avoid reading too much into the first batch of data, because it’s a small sample size.
“A big part of that is we feel that a lot of hospitalists are intermingled into the other medical specialties,” she says. “So this becomes a very small subset where they are distinctly identified as hospitalists. And that’s the challenge.”
In particular, Parikh is curious to see whether HM’s rate of claims paid through insurance payments drops from 20% (already below the overall healthcare industry average). “It will be interesting as we proceed...to see if they begin to mitigate areas of risk where we used to see a lot of claims,” she adds. “If you look at a hospital setting, there has been some shift change in what the errors are. And, what you’d hope with hospitalists within these environments who are really owning this specialty, is that you’d see a decrease in that. There would be that connective care. There would be the patient that felt that they had an individual who was their go-to individual throughout their care at a hospital.”
A Peek at the Future
Insurers have begun compiling claims data on hospitalists and are taking a longer-term view of the specialty. TDC, for example, has analyzed its data and identified characteristics it says make a low-risk hospitalist, an analysis the company says is the first of its kind (see Figure 1). The insurer adds that it sees its responsibility as making sure everyone understands the hospitalist’s role within the acute care setting so that its pricing is commensurate with the liability risk.
Source: The Doctors Company
“We’re looking at the systems within the hospitalist group, as well as how well that group is integrating with the hospital where they’re practicing,” Diamond says. “What kind of patient mix is this particular hospitalist group seeing in that particular hospital, because it can be different in a large healthcare corporation in Manhattan, New York, from a community hospital in rural Texas.”
The growing popularity of hospitalists taking on co-management responsibilities for other specialties is another trend to keep an eye on, as it creates what insurers call “vicarious liability.” Working together in teams with other specialties can improve communication, reduce errors during transitions of care, and create better outcomes. However, in instances where there are problems, being on a care team means hospitalists can open themselves to liability. To mitigate that risk, hospitalists can look to other groups that have dealt with shared liability issues in the past, Parikh says.
“Historically, you would have seen it with anesthesiology,” she explains. “And one huge improvement anesthesiologists have made when a patient comes in for a surgery now is they come out, introduce themselves, say hello, and tell you what’s going on. They put a face to the name, so that it’s not just a no-name anesthesiologist who gets included in the lawsuit as well because they’re naming everybody in the group.”
But, holistically, the best long-term mitigation strategy appears to be tort reform and new ways of looking at the way in which healthcare liability issues are handled in the U.S., says Anupam Jena, MD, PhD, assistant professor of healthcare policy and medicine at Harvard Medical School, and an internist at Massachusetts General Hospital, both in Boston. Dr. Jena says that there is limited evidence that enacted malpractice reforms have produced more than a 2% to 5% reduction in healthcare spending compared to states that have not.2 Instead, healthcare leaders should push for the elimination of defensive medicine, which he says contributes the lion’s share of the estimated $50 billion annual cost of malpractice liability across the country.
“Do I think the country is in a malpractice crisis? No,” he says. “Do I think that defensive medicine is larger than we think it is? Yes.
“If physicians practice as they felt they should practice without ordering extra tests and procedures, my guess would be you could reduce healthcare spending by substantially more than $50 billion.”
Richard Quinn is a freelance writer in New Jersey.
References
- Spiegel AD, Kavaler F. America’s first medical malpractice crisis, 1835-1865. J Community Health. 1997;22:283-308.
- Chandra A, Jena A, Seabury, S. Defensive medicine may be costlier than it seems. The Wall Street Journal website. http://online.wsj.com/article/SB10001424127887323701904578280112638373302.html. Accessed September 21, 2013.
Source: The Doctors Company
Ten years ago, the national headlines on malpractice insurance were staggering. Media reports catalogued OB-GYNs who proclaimed they were shutting down their private practices in the face of runaway premiums. Surgeons and other proceduralists decried payments tied to lawsuits they’d argue were arbitrary and capricious. And the American Medical Association (AMA) made announcement after announcement about states being in a “malpractice crisis.”
In recent years, premiums have actually dropped and stabilized at levels that most physicians agree are manageable for bottom lines. But, in that time, there has been scant discussion about hospital medicine’s relationship with malpractice. It’s not because the issue isn’t omnipresent for all healthcare practitioners, including the relatively nascent specialty that is HM.
Practice management experts say anecdotally that delayed diagnosis of, or treatment for, a spinal epidural abscess (SEA) is likely to get more than a few hospitalists sued. And, the proliferation of co-management of other specialties—particularly those with higher risk of incidence and higher premiums than internal medicine—open up hospitalists to further liability.
The issue is that at less than 20 years as a specialty, HM is in its infancy when it comes to its interaction with malpractice premiums. Health insurance companies and trade groups that track the insurance industry are just beginning to have enough data on claims, premiums, and payouts to make recommendations on risk factors, risk mitigation, and potential trends.
Still, even in a landscape of limited information, there are a few rules of thumb hospitalist group leaders should live by when it comes to managing exposure to malpractice cases, according to interviews with a half dozen healthcare professionals:
- Know how your coverage works. Is there “tail coverage” that ensures you have protection for incidents that happened at an institution where you no longer practice? Even though hospital-employed physicians rarely have rate discussions directly (the hospital typically covers premiums as part of the compensation package), take the time to learn the basic details.
- Be diligent in documentation. Note concerns in charts when appropriate, and stand up for your point of view. There’s a fine line between picking fights with other physicians involved in a patient’s care and making your concerns known, but don’t be afraid to put your clinical view on the record.
- Avoid the practice of “defensive medicine.” Ordering tests and procedures that aren’t clinically necessary might seem like it can serve as a protection from later lawsuits, but it adds to healthcare costs and is just not the right thing to do, says hospitalist Allen Kachalia, MD, JD, of Brigham and Women’s Hospital in Boston, who has studied the phenomenon (see “Culture Shift Necessary to Defeat “Defensive” Medicine,” on p. 38).
- Recognize the risks associated with co-management. Caring for neurology, cardiology, and other subspecialty patients is a revenue boost for HM groups, but when some of those complex cases have adverse events, the hospitalist who interacted with the patient daily could be included in a lawsuit.
- Focus on communication skills. An analysis of claims data by The Doctors Company (TDC) (www.thedoctors.com), a medical malpractice insurance company exclusively endorsed by SHM, reports that the second most common factor contributing to patient injury by hospitalists is “communication breakdown among healthcare professionals.”
- Manage workloads to avoid burnout. Don’t take on too many patients at the expense of being involved in hospital committees or quality initiatives.
To be sure, many of the same tenets of being a productive hospitalist with high patient satisfaction scores—maintain manageable censuses; focus on patient centeredness; and use checklists, technology, and regimented protocols to reduce adverse events—translate very well to being a lower-risk hospitalist in relation to malpractice cases.
When you’re “thinking of patient satisfaction strategies, also think of them as risk mitigation strategies,” says John Nelson, MD, MHM, FACP, medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash., an SHM co-founder and practice management columnist for The Hospitalist. “They overlap tremendously.”
A History Lesson
Medical malpractice has been around for centuries and has two prevailing goals: 1) to provide monetary remuneration to patients who have been injured via substandard care and 2) to deter that poor treatment through fiscal punishment.
Malpractice lawsuits were not prevalent enough to be a major medical concern until the early 1800s. By the middle of the 19th century, the country hit its first periods of crisis.1 Cycles ebbed and flowed from there, with malpractice premiums causing crises in the 1980s and again in the early 2000s.
continued below...
Now, rates for medical professional liability insurance have been dropping for seven years, and an eighth straight annual decline is expected this year, according to Mike Matray, the editor of trade publication Medical Liability Monitor and the chief content officer of its associated website, www.mymedicalmalpracticeinsurance.com.
“We are in the longest, deepest soft market that the malpractice insurance industry has ever been in,” he says. “Right now, things are really good for the doctors, as far as rates coming down.”
Matray says he understands that declining rates may seem immaterial to a physician who receives an insurance bill that eats into the bottom line. For some specialties, that premium can be as high as $200,000 per physician, per year—or more.
“I’m not saying it isn’t expensive,” he adds. “It’s expensive to run a medical practice. At the same time, medical malpractice insurance is less expensive in today’s dollars than it was in 2005.”
The reduction in rates is multi-faceted. Prominently, state-level tort reforms like non-economic damage caps, health courts, and arbitration hearings are making it harder to bring cases to trial, particularly for lawyers who take cases on contingency. Second, frivolous lawsuits “are making an impression on jury pools,” Matray says, which means fewer filed claims and fewer cases that make it to trial. Third, this soft cycle has outlasted the typical pattern of rates falling for three to four years before rebounding.
“A lot of smart actuaries keep saying this has to change soon, because in a soft market there is a lot of competition,” he says, noting that in order to compete for low rates, insurance companies offer credits to clients and use their own reserve cash piles. “So things are really going to change in the next couple of years.”

—Robin Diamond, senior vice president and chief patient safety officer, The Doctors Company
In Need of Data, Patience
So what does it all mean for hospitalists and HM group leaders looking to be proactive about medical malpractice liability insurance? Patience is required.
For starters, there is no designated premium category for hospitalists. Much like the situation that exists for coding issues, the closest proxy for HM is internal medicine. According to Medical Liability Monitor, the premium paid by internal medicine physicians as of July 1, 2012, varied widely across the country. In South Florida, internal medicine insurance premiums in Miami, Dade, and Broward counties were between $42,000 and $46,000 per year. In South Dakota, one insurer reported rates of just under $4,000 per year. There is no average or median figure available, and Matray notes that actual rates paid can vary from county to county.
Moreover, it is difficult for group leaders or hospital executives to use past history to negotiate rates with insurers because of a shortage of reliable data. In its spring 2013 newsletter, the PIAA (formerly known as the Physician Insurers Association of America) published its first report on hospitalist claims reported to its Data Sharing Project. Of the 92,868 closed claims reported from 2002-2011, just 312, or 0.3%, named hospitalists as the defendant.
The data also showed that, of those claims, 20% were settled through insurance company payments. Those payments totaled $17.1 million, with an average payout to a claimant (known as the indemnity) of $272,553 per claim. Overall, hospitalists had a 20% paid-to-closed ratio, totaling more than $17.1 million. By comparison, the percent of paid-to-closed claims for all physicians was 29.3%, according to PIAA.
In a separate data set compiled this year by TDC, 34% of allegations against hospitalists were related to missed or failed diagnoses, with 28% tied to “improper management of treatment.” Twelve percent of allegations were the result of either improper medication management or ordering errors.
Robin Diamond, TDC’s senior vice president and chief patient safety officer, says that teasing out trends from the initial data can be challenging. Hospitalists, she says, can deal with so many different patients, diseases, and severity levels that it is difficult to draw conclusions.
“Hospital medicine is different than other specialties, because the hospitalist treats a broad range of patients in an acute care setting—from a pediatric patient to an adult patient with many chronic illnesses,” she says.
Divya Parikh, PIAA’s director of research and loss prevention, says HM group leaders should avoid reading too much into the first batch of data, because it’s a small sample size.
“A big part of that is we feel that a lot of hospitalists are intermingled into the other medical specialties,” she says. “So this becomes a very small subset where they are distinctly identified as hospitalists. And that’s the challenge.”
In particular, Parikh is curious to see whether HM’s rate of claims paid through insurance payments drops from 20% (already below the overall healthcare industry average). “It will be interesting as we proceed...to see if they begin to mitigate areas of risk where we used to see a lot of claims,” she adds. “If you look at a hospital setting, there has been some shift change in what the errors are. And, what you’d hope with hospitalists within these environments who are really owning this specialty, is that you’d see a decrease in that. There would be that connective care. There would be the patient that felt that they had an individual who was their go-to individual throughout their care at a hospital.”
A Peek at the Future
Insurers have begun compiling claims data on hospitalists and are taking a longer-term view of the specialty. TDC, for example, has analyzed its data and identified characteristics it says make a low-risk hospitalist, an analysis the company says is the first of its kind (see Figure 1). The insurer adds that it sees its responsibility as making sure everyone understands the hospitalist’s role within the acute care setting so that its pricing is commensurate with the liability risk.
Source: The Doctors Company
“We’re looking at the systems within the hospitalist group, as well as how well that group is integrating with the hospital where they’re practicing,” Diamond says. “What kind of patient mix is this particular hospitalist group seeing in that particular hospital, because it can be different in a large healthcare corporation in Manhattan, New York, from a community hospital in rural Texas.”
The growing popularity of hospitalists taking on co-management responsibilities for other specialties is another trend to keep an eye on, as it creates what insurers call “vicarious liability.” Working together in teams with other specialties can improve communication, reduce errors during transitions of care, and create better outcomes. However, in instances where there are problems, being on a care team means hospitalists can open themselves to liability. To mitigate that risk, hospitalists can look to other groups that have dealt with shared liability issues in the past, Parikh says.
“Historically, you would have seen it with anesthesiology,” she explains. “And one huge improvement anesthesiologists have made when a patient comes in for a surgery now is they come out, introduce themselves, say hello, and tell you what’s going on. They put a face to the name, so that it’s not just a no-name anesthesiologist who gets included in the lawsuit as well because they’re naming everybody in the group.”
But, holistically, the best long-term mitigation strategy appears to be tort reform and new ways of looking at the way in which healthcare liability issues are handled in the U.S., says Anupam Jena, MD, PhD, assistant professor of healthcare policy and medicine at Harvard Medical School, and an internist at Massachusetts General Hospital, both in Boston. Dr. Jena says that there is limited evidence that enacted malpractice reforms have produced more than a 2% to 5% reduction in healthcare spending compared to states that have not.2 Instead, healthcare leaders should push for the elimination of defensive medicine, which he says contributes the lion’s share of the estimated $50 billion annual cost of malpractice liability across the country.
“Do I think the country is in a malpractice crisis? No,” he says. “Do I think that defensive medicine is larger than we think it is? Yes.
“If physicians practice as they felt they should practice without ordering extra tests and procedures, my guess would be you could reduce healthcare spending by substantially more than $50 billion.”
Richard Quinn is a freelance writer in New Jersey.
References
- Spiegel AD, Kavaler F. America’s first medical malpractice crisis, 1835-1865. J Community Health. 1997;22:283-308.
- Chandra A, Jena A, Seabury, S. Defensive medicine may be costlier than it seems. The Wall Street Journal website. http://online.wsj.com/article/SB10001424127887323701904578280112638373302.html. Accessed September 21, 2013.
Surgeons' Perception of Fluoroscopic Radiation Hazards to Vision
Strategies for Treating Scoliosis in Children With Spinal Muscular Atrophy
Consensus Recommendations From the American Acne & Rosacea Society on the Management of Rosacea, Part 1: A Status Report on the Disease State, General Measures, and Adjunctive Skin Care
Test your knowledge on consensus recommendations for rosacea with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.