User login
Is This Golfer's Pacemaker Malfunctioning?
ANSWER
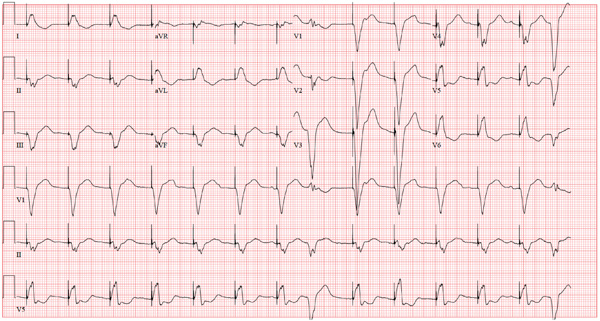
The ECG reveals an atrial-sensed and ventricular-paced rhythm of 83 beats/min. In this case, the pacemaker is functioning appropriately as programmed.
Pacemaker code consists of three letters: The first refers to the chamber(s) paced, the second to the chamber(s) sensed, and the third to the pacemaker’s response to a sensed beat. This patient has a pacing lead in the right atrium and one in the right ventricle and is programmed DDD. Each D in this case stands for dual: The first to indicate that both leads are programmed to pace, the second to indicate that both chambers may be sensed, and the third to indicate that the response to sensing can be either to inhibit or trigger a ventricular-paced beat in response to what happens in the atrium. Hence, there are four possible scenarios with a DDD pacemaker: AS-VS (atrial sensed-ventricle sensed; eg, intrinsic AV conduction requiring no pacing), AS-VP (atrial sensed-ventricle paced), AP-VS (atrial paced-ventricle sensed), and AP-VP (atrial paced-ventricle paced).
In this case, the atrial rate (83 beats/min) is faster than the pacemaker’s lower programmed rate. In order to see atrial pacing on the ECG, the intrinsic atrial rate would have to be less than the programmed rate of 60 beats/min. As soon as the pacemaker senses atrial conduction (either spontaneous or paced), it starts a timer (programmed at 130 ms in this case). If there is no spontaneous ventricular depolarization by the end of the timer, the pacemaker delivers an impulse to the ventricle, resulting in a paced ventricular beat. An often-made mistake (as this case illustrates) is the assumption that if one does not see pacing spikes, the pacemaker is not functioning properly.
ANSWER
The ECG reveals an atrial-sensed and ventricular-paced rhythm of 83 beats/min. In this case, the pacemaker is functioning appropriately as programmed.
Pacemaker code consists of three letters: The first refers to the chamber(s) paced, the second to the chamber(s) sensed, and the third to the pacemaker’s response to a sensed beat. This patient has a pacing lead in the right atrium and one in the right ventricle and is programmed DDD. Each D in this case stands for dual: The first to indicate that both leads are programmed to pace, the second to indicate that both chambers may be sensed, and the third to indicate that the response to sensing can be either to inhibit or trigger a ventricular-paced beat in response to what happens in the atrium. Hence, there are four possible scenarios with a DDD pacemaker: AS-VS (atrial sensed-ventricle sensed; eg, intrinsic AV conduction requiring no pacing), AS-VP (atrial sensed-ventricle paced), AP-VS (atrial paced-ventricle sensed), and AP-VP (atrial paced-ventricle paced).
In this case, the atrial rate (83 beats/min) is faster than the pacemaker’s lower programmed rate. In order to see atrial pacing on the ECG, the intrinsic atrial rate would have to be less than the programmed rate of 60 beats/min. As soon as the pacemaker senses atrial conduction (either spontaneous or paced), it starts a timer (programmed at 130 ms in this case). If there is no spontaneous ventricular depolarization by the end of the timer, the pacemaker delivers an impulse to the ventricle, resulting in a paced ventricular beat. An often-made mistake (as this case illustrates) is the assumption that if one does not see pacing spikes, the pacemaker is not functioning properly.
ANSWER
The ECG reveals an atrial-sensed and ventricular-paced rhythm of 83 beats/min. In this case, the pacemaker is functioning appropriately as programmed.
Pacemaker code consists of three letters: The first refers to the chamber(s) paced, the second to the chamber(s) sensed, and the third to the pacemaker’s response to a sensed beat. This patient has a pacing lead in the right atrium and one in the right ventricle and is programmed DDD. Each D in this case stands for dual: The first to indicate that both leads are programmed to pace, the second to indicate that both chambers may be sensed, and the third to indicate that the response to sensing can be either to inhibit or trigger a ventricular-paced beat in response to what happens in the atrium. Hence, there are four possible scenarios with a DDD pacemaker: AS-VS (atrial sensed-ventricle sensed; eg, intrinsic AV conduction requiring no pacing), AS-VP (atrial sensed-ventricle paced), AP-VS (atrial paced-ventricle sensed), and AP-VP (atrial paced-ventricle paced).
In this case, the atrial rate (83 beats/min) is faster than the pacemaker’s lower programmed rate. In order to see atrial pacing on the ECG, the intrinsic atrial rate would have to be less than the programmed rate of 60 beats/min. As soon as the pacemaker senses atrial conduction (either spontaneous or paced), it starts a timer (programmed at 130 ms in this case). If there is no spontaneous ventricular depolarization by the end of the timer, the pacemaker delivers an impulse to the ventricle, resulting in a paced ventricular beat. An often-made mistake (as this case illustrates) is the assumption that if one does not see pacing spikes, the pacemaker is not functioning properly.
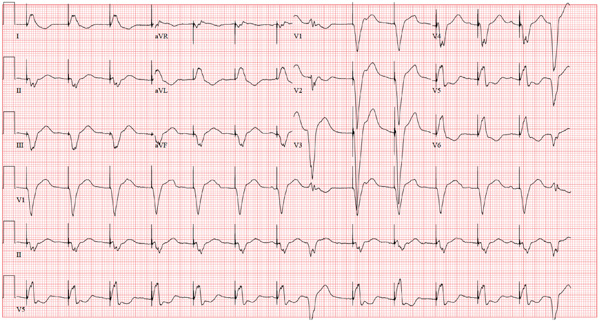
A 75-year-old man has a history of New York Heart Association Class II congestive heart failure (CHF), coronary artery disease (CAD) with coronary artery by-pass graft (CABG) surgery, and aortic stenosis with a bioprosthetic aortic valve replacement (AVR). He developed second-degree heart block (Mobitz II) following his four-vessel CABG and AVR four years ago, requiring placement of a dual-chamber pacemaker. He has been asymptomatic and plays golf two to three times per week. One week ago, he went to an urgent care center for treatment of a laceration on his leg and was told that part of his pacemaker wasn’t working. He presents to you now for follow-up on the pacemaker. Medical history is also remarkable for COPD, left inguinal hernia repair, hyperlipidemia, and bilateral cataracts. Family history is positive for CAD, diabetes, and stroke. He has a remote history of smoking and drinks one martini after each golf game. His medications include aspirin, lovastatin, and metoprolol. He has no drug allergies. The review of systems is negative except for a recent repair to a 3-cm laceration on the left leg. Physical examination reveals a well-developed, pleasant man in no distress. His height is 74” and weight, 179 lb. Blood pressure is 118/84 mm Hg; pulse, 80 beats/min and regular; respiratory rate, 14 breaths/min; temperature, 98.8°F; and O2 saturation, 97% on room air. Pertinent physical findings include a well-healed pacemaker site without signs of recent trauma. The lungs are clear in all fields, the cardiac exam is within normal limits, and there is no jugular venous distention. The abdomen is benign, and there is no peripheral edema. The laceration repair on his left leg is healing well (no erythema, induration, wound separation, or dehiscence). The pacemaker is programmed DDD at a lower rate of 60 beats/min and an upper rate of 120 beats/min, with paced and sensed atrioventricular (AV) delays programmed at 130 ms. An ECG reveals the following: a ventricular rate of 83 beats/min; PR interval, not measured; QRS duration, 162 ms; QT/QTc interval, 480/564 ms; P axis, unmeasurable; R axis, 254°; and T axis, 56°. What is your interpretation of this ECG? Is there any indication that the pacemaker is not functioning?
Is Leprosy the Cause of This Girl's Lesion?
ANSWER
The correct answer is postinflammatory hypopigmentation (choice “c”), in this case secondary to eczema in a classic antecubital location. Leprosy (choice “a”) is more common than one might imagine, but it does not appear overnight and does not involve overt inflammation. Vitiligo (choice “b”) does not appear suddenly and rarely involves the type of inflammation seen in this case. Lichen sclerosis et atrophicus (choice “d”) is an inflammatory condition that presents with hypopigmentation and epidermal atrophy; however, it is gradual in onset and would not exhibit papulosquamous inflammation.
DISCUSSION
The more color in the skin, the more the loss of that color stands out. Patients and families with darker skin are often understandably upset by the contrast. Providers need a differential for pigment loss, including the items mentioned—some of which have the potential to be dreadfully serious.
Two relevant facts stand out in this case: the rapidity of onset and the history of eczema, in which secondary pigment loss can occur. As mentioned, it is especially obvious in those with darker skin. Fortunately, once the eczema calms down, the hypopigmentation resolves and normal color returns.
Paradoxically, it’s not at all unusual to see postinflammatory hyperpigmentation, especially in those with skin of types IV and V (eg, African-Americans, some Hispanics, and those of Indian ancestry). Eczema is a common cause, but the inflammation can be from almost any source, including trauma, burns, or even acne.
Had this patient’s diagnosis not been obvious, a biopsy might have been indicated due to the serious nature of some of the items in the differential. Vitiligo, for example, can be very disfiguring, especially on a dark-skinned individual. It tends to become widespread and permanent, unless it’s caught and treated early on. Other conditions involving hypopigmentation include sarcoidosis, lupus, and morphea.
All of these conditions are unusual, if not rare, compared with atopic dermatitis (AD), which this patient has. AD is so common that almost 20% of newborns develop it. Eczema is one of the more typical manifestations, along with dry, sensitive skin, seasonal allergies, and reactive airway disease. Corroboration of the diagnosis is usually easily accomplished by taking a family history.
TREATMENT
Fortunately, this patient’s hypopigmentation will resolve quickly with treatment of her eczema, using a low-strength steroid cream (eg, hydrocortisone 2.5% cream or ointment). But a good portion of the “treatment” of AD is done by educating the family about the nature of the condition, as well as providing reassurance about the absence of the more serious items in the differential.
ANSWER
The correct answer is postinflammatory hypopigmentation (choice “c”), in this case secondary to eczema in a classic antecubital location. Leprosy (choice “a”) is more common than one might imagine, but it does not appear overnight and does not involve overt inflammation. Vitiligo (choice “b”) does not appear suddenly and rarely involves the type of inflammation seen in this case. Lichen sclerosis et atrophicus (choice “d”) is an inflammatory condition that presents with hypopigmentation and epidermal atrophy; however, it is gradual in onset and would not exhibit papulosquamous inflammation.
DISCUSSION
The more color in the skin, the more the loss of that color stands out. Patients and families with darker skin are often understandably upset by the contrast. Providers need a differential for pigment loss, including the items mentioned—some of which have the potential to be dreadfully serious.
Two relevant facts stand out in this case: the rapidity of onset and the history of eczema, in which secondary pigment loss can occur. As mentioned, it is especially obvious in those with darker skin. Fortunately, once the eczema calms down, the hypopigmentation resolves and normal color returns.
Paradoxically, it’s not at all unusual to see postinflammatory hyperpigmentation, especially in those with skin of types IV and V (eg, African-Americans, some Hispanics, and those of Indian ancestry). Eczema is a common cause, but the inflammation can be from almost any source, including trauma, burns, or even acne.
Had this patient’s diagnosis not been obvious, a biopsy might have been indicated due to the serious nature of some of the items in the differential. Vitiligo, for example, can be very disfiguring, especially on a dark-skinned individual. It tends to become widespread and permanent, unless it’s caught and treated early on. Other conditions involving hypopigmentation include sarcoidosis, lupus, and morphea.
All of these conditions are unusual, if not rare, compared with atopic dermatitis (AD), which this patient has. AD is so common that almost 20% of newborns develop it. Eczema is one of the more typical manifestations, along with dry, sensitive skin, seasonal allergies, and reactive airway disease. Corroboration of the diagnosis is usually easily accomplished by taking a family history.
TREATMENT
Fortunately, this patient’s hypopigmentation will resolve quickly with treatment of her eczema, using a low-strength steroid cream (eg, hydrocortisone 2.5% cream or ointment). But a good portion of the “treatment” of AD is done by educating the family about the nature of the condition, as well as providing reassurance about the absence of the more serious items in the differential.
ANSWER
The correct answer is postinflammatory hypopigmentation (choice “c”), in this case secondary to eczema in a classic antecubital location. Leprosy (choice “a”) is more common than one might imagine, but it does not appear overnight and does not involve overt inflammation. Vitiligo (choice “b”) does not appear suddenly and rarely involves the type of inflammation seen in this case. Lichen sclerosis et atrophicus (choice “d”) is an inflammatory condition that presents with hypopigmentation and epidermal atrophy; however, it is gradual in onset and would not exhibit papulosquamous inflammation.
DISCUSSION
The more color in the skin, the more the loss of that color stands out. Patients and families with darker skin are often understandably upset by the contrast. Providers need a differential for pigment loss, including the items mentioned—some of which have the potential to be dreadfully serious.
Two relevant facts stand out in this case: the rapidity of onset and the history of eczema, in which secondary pigment loss can occur. As mentioned, it is especially obvious in those with darker skin. Fortunately, once the eczema calms down, the hypopigmentation resolves and normal color returns.
Paradoxically, it’s not at all unusual to see postinflammatory hyperpigmentation, especially in those with skin of types IV and V (eg, African-Americans, some Hispanics, and those of Indian ancestry). Eczema is a common cause, but the inflammation can be from almost any source, including trauma, burns, or even acne.
Had this patient’s diagnosis not been obvious, a biopsy might have been indicated due to the serious nature of some of the items in the differential. Vitiligo, for example, can be very disfiguring, especially on a dark-skinned individual. It tends to become widespread and permanent, unless it’s caught and treated early on. Other conditions involving hypopigmentation include sarcoidosis, lupus, and morphea.
All of these conditions are unusual, if not rare, compared with atopic dermatitis (AD), which this patient has. AD is so common that almost 20% of newborns develop it. Eczema is one of the more typical manifestations, along with dry, sensitive skin, seasonal allergies, and reactive airway disease. Corroboration of the diagnosis is usually easily accomplished by taking a family history.
TREATMENT
Fortunately, this patient’s hypopigmentation will resolve quickly with treatment of her eczema, using a low-strength steroid cream (eg, hydrocortisone 2.5% cream or ointment). But a good portion of the “treatment” of AD is done by educating the family about the nature of the condition, as well as providing reassurance about the absence of the more serious items in the differential.
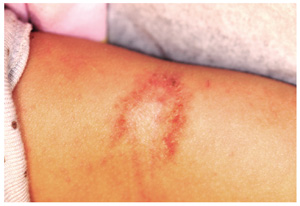
The parents of this 8-month-old infant are alarmed by skin changes that occurred practically overnight on the child’s arm—especially since the child’s grandparents suggested it might represent vitiligo or even leprosy. The child’s pediatrician thought “ringworm” was more likely, but the clotrimazole cream he recommended was no help. The child has an extensive history of atopy, including eczema affecting the trunk and face. The parents have used topical steroid cream on the affected areas with some good effect, but the loss of color in the antecubital site has made them reluctant to use the product on this new site. Examination shows a papulosquamous lesion, 3.5 cm in diameter, on the left lateral antecubital area, with marked central hypopigmentation. The child and her parents are Vietnamese, with type IV skin, making the pigment loss all the more obvious. The periphery of the lesion, in addition to being bumpy and scaly, is moderately inflamed. The rest of the child’s skin is dry but otherwise unremarkable.
Pregnancy Registries: Advantages and Disadvantages
Despite the fact that prescription medications are commonly used by pregnant women, for most products and for new drugs in particular, there is typically little to no human safety information available to aid clinicians and patients in managing risk. As randomized clinical trials are usually not considered ethical to perform in pregnancy, observational epidemiologic studies are often the next best option to address human pregnancy exposure. An increasingly common approach to gathering human safety data is postmarketing pregnancy registries.
These pregnancy registries are initiated many times by agreement between the manufacturer and a regulatory agency as a postmarketing commitment or requirement following shortly after drug approval. Furthermore, because use of a new drug in pregnant women might be relatively rare, a pregnancy registry may be the only feasible method for gathering preliminary safety information as quickly as possible so that potential signals might be detected and clinical decision making can be better informed.
Pregnancy registries vary in design, but all involve collection of data on exposure to the drug of interest in pregnant women, and collection of outcome data for those pregnancies. The primary outcome of interest is typically major congenital anomalies; some registries also collect outcome data on fetal/infant growth, preterm delivery, pregnancy loss, specific neonatal outcomes, and postnatal longer term growth and development. The rates of these outcomes can be compared with general population reference rates, or rates occurring in a specific comparison group that might be more similar to the exposed women, for example, in terms of the underlying maternal condition being treated by the drug.
In addition to early information on a new drug, some of the major advantages of many pregnancy registry designs are the ability to collect information on the exposure and other pregnancy details before the mother knows what the outcome of her pregnancy will be; direct collection of exposure information from the mother herself, so that important factors such as drug and alcohol use, dose, and exact timing of exposure to the drug of interest; information on important other factors such as tobacco, alcohol, and multivitamin use.
The most challenging aspect of pregnancy registries is recruitment, for which registries largely depend on obstetric providers and other specialty physicians. Although low numbers of recruited pregnancies may be caused by limited use of a new drug, clearly most pregnancy registries enroll a very small fraction of all exposed pregnancies that are in existence. A second, related issue is that there may be bias in the self-selection of women who do find out about the registry and agree to participate, thus raising questions about the generalizability of the findings. A third issue is that many registries experience high rates of "lost to follow-up," in which outcome information is unobtainable from the health care provider or the pregnant woman – in some cases as high as 40%. There is also a concern about bias involved with the timing in gestation when a pregnancy enters a registry, such as the later in gestation a pregnancy is enrolled, the more likely that prenatal diagnosis, pregnancy loss, or other adverse outcomes have already occurred – thus making the enrollment essentially retrospective.
Another concern is that few registries have a concurrently enrolled group of unexposed women for purposes of comparison. Thus, their findings are commonly compared to external reference statistics which may not be the most appropriate. Finally, in some registries, the absence of information on individual dose and specific timing in gestation of exposure may preclude evaluating the biological plausibility of any registry findings. All of these issues can lead to long delays in accumulation of sufficient information to draw meaningful inferences, and potential concerns about interpretation of results.
How can awareness of pregnancy registries and more representative enrollment of exposed women be improved? A variety of methods are used to inform physicians and their patients about existing pregnancy registries for the purpose of encouraging referrals, including the Food and Drug Administration website, information in product labeling and on product websites, direct to provider or direct to consumer advertising, and in commonly used resources for clinicians such as this column and Reprotox, an information system developed by the Reproductive Toxicology Center. However, with the rapidly increasing number of registries, it is challenging for physicians to remain current on which medications are being monitored through a registry, what the criteria for enrollment are, and how a physician or patient can find out more. Pregnancy registry designs that are disease based – such as encompassing all medications used to treat a specific disease in pregnancy – help simplify the referral process by broadening the criteria for enrollment. Particularly for specialty physicians, this can ease the burden of identifying eligible women for enrollment.
How can enrollment be accomplished as early as possible in pregnancy (after exposure, but before the outcome is known), and how can more complete ascertainment of exposure and outcome be improved? An approach that some registries have used to address this is by "direct to consumer" campaigns. Registries such as the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Registry require that the pregnant woman herself enroll in the study, and therefore, the study is marketed directly to those women, although physician referral is encouraged. At least in this case, this has led to low rates of lost to follow-up (less than 5%), recruitment timing that is typically before the seventh or eighth week of gestation, and collection of specific information on dose and timing in gestation of exposure.
Multiple drug, disease-based, multiple sponsor registries such as the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drugs in Pregnancy Registry (patients call 888-233-2334), and the National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) offer distinct advantages but are not always feasible for a specific product. A national pregnancy registry for all new drugs has been suggested as another solution to many of the challenges facing single product registries and to streamline referral and follow-up. In addition, including pregnant women in selected preapproval studies has several advantages. Finally, creative new technologies for earlier and more complete ascertainment and referral, such as use of electronic medical records, should be fully explored. The need for safety information on new drugs is urgent.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. Dr. Cohen directs the perinatal psychiatry program at Massachusetts General Hospital, which provides information about pregnancy and mental health. Dr. Koren is professor of pediatrics, pharmacology, pharmacy, and medical genetics at the University of Toronto. He heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk program. Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; a clinical professor of pharmacy at the University of California, San Francisco; and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation." Dr. Cohen is the principal investigator on the National Pregnancy Registry for Atypical Antipsychotics, which is sponsored by multiple atypical antipsychotic manufacturers.
Dr. Chambers, Dr. Koren, and Mr. Briggs said they had no relevant financial disclosures.
Despite the fact that prescription medications are commonly used by pregnant women, for most products and for new drugs in particular, there is typically little to no human safety information available to aid clinicians and patients in managing risk. As randomized clinical trials are usually not considered ethical to perform in pregnancy, observational epidemiologic studies are often the next best option to address human pregnancy exposure. An increasingly common approach to gathering human safety data is postmarketing pregnancy registries.
These pregnancy registries are initiated many times by agreement between the manufacturer and a regulatory agency as a postmarketing commitment or requirement following shortly after drug approval. Furthermore, because use of a new drug in pregnant women might be relatively rare, a pregnancy registry may be the only feasible method for gathering preliminary safety information as quickly as possible so that potential signals might be detected and clinical decision making can be better informed.
Pregnancy registries vary in design, but all involve collection of data on exposure to the drug of interest in pregnant women, and collection of outcome data for those pregnancies. The primary outcome of interest is typically major congenital anomalies; some registries also collect outcome data on fetal/infant growth, preterm delivery, pregnancy loss, specific neonatal outcomes, and postnatal longer term growth and development. The rates of these outcomes can be compared with general population reference rates, or rates occurring in a specific comparison group that might be more similar to the exposed women, for example, in terms of the underlying maternal condition being treated by the drug.
In addition to early information on a new drug, some of the major advantages of many pregnancy registry designs are the ability to collect information on the exposure and other pregnancy details before the mother knows what the outcome of her pregnancy will be; direct collection of exposure information from the mother herself, so that important factors such as drug and alcohol use, dose, and exact timing of exposure to the drug of interest; information on important other factors such as tobacco, alcohol, and multivitamin use.
The most challenging aspect of pregnancy registries is recruitment, for which registries largely depend on obstetric providers and other specialty physicians. Although low numbers of recruited pregnancies may be caused by limited use of a new drug, clearly most pregnancy registries enroll a very small fraction of all exposed pregnancies that are in existence. A second, related issue is that there may be bias in the self-selection of women who do find out about the registry and agree to participate, thus raising questions about the generalizability of the findings. A third issue is that many registries experience high rates of "lost to follow-up," in which outcome information is unobtainable from the health care provider or the pregnant woman – in some cases as high as 40%. There is also a concern about bias involved with the timing in gestation when a pregnancy enters a registry, such as the later in gestation a pregnancy is enrolled, the more likely that prenatal diagnosis, pregnancy loss, or other adverse outcomes have already occurred – thus making the enrollment essentially retrospective.
Another concern is that few registries have a concurrently enrolled group of unexposed women for purposes of comparison. Thus, their findings are commonly compared to external reference statistics which may not be the most appropriate. Finally, in some registries, the absence of information on individual dose and specific timing in gestation of exposure may preclude evaluating the biological plausibility of any registry findings. All of these issues can lead to long delays in accumulation of sufficient information to draw meaningful inferences, and potential concerns about interpretation of results.
How can awareness of pregnancy registries and more representative enrollment of exposed women be improved? A variety of methods are used to inform physicians and their patients about existing pregnancy registries for the purpose of encouraging referrals, including the Food and Drug Administration website, information in product labeling and on product websites, direct to provider or direct to consumer advertising, and in commonly used resources for clinicians such as this column and Reprotox, an information system developed by the Reproductive Toxicology Center. However, with the rapidly increasing number of registries, it is challenging for physicians to remain current on which medications are being monitored through a registry, what the criteria for enrollment are, and how a physician or patient can find out more. Pregnancy registry designs that are disease based – such as encompassing all medications used to treat a specific disease in pregnancy – help simplify the referral process by broadening the criteria for enrollment. Particularly for specialty physicians, this can ease the burden of identifying eligible women for enrollment.
How can enrollment be accomplished as early as possible in pregnancy (after exposure, but before the outcome is known), and how can more complete ascertainment of exposure and outcome be improved? An approach that some registries have used to address this is by "direct to consumer" campaigns. Registries such as the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Registry require that the pregnant woman herself enroll in the study, and therefore, the study is marketed directly to those women, although physician referral is encouraged. At least in this case, this has led to low rates of lost to follow-up (less than 5%), recruitment timing that is typically before the seventh or eighth week of gestation, and collection of specific information on dose and timing in gestation of exposure.
Multiple drug, disease-based, multiple sponsor registries such as the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drugs in Pregnancy Registry (patients call 888-233-2334), and the National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) offer distinct advantages but are not always feasible for a specific product. A national pregnancy registry for all new drugs has been suggested as another solution to many of the challenges facing single product registries and to streamline referral and follow-up. In addition, including pregnant women in selected preapproval studies has several advantages. Finally, creative new technologies for earlier and more complete ascertainment and referral, such as use of electronic medical records, should be fully explored. The need for safety information on new drugs is urgent.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. Dr. Cohen directs the perinatal psychiatry program at Massachusetts General Hospital, which provides information about pregnancy and mental health. Dr. Koren is professor of pediatrics, pharmacology, pharmacy, and medical genetics at the University of Toronto. He heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk program. Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; a clinical professor of pharmacy at the University of California, San Francisco; and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation." Dr. Cohen is the principal investigator on the National Pregnancy Registry for Atypical Antipsychotics, which is sponsored by multiple atypical antipsychotic manufacturers.
Dr. Chambers, Dr. Koren, and Mr. Briggs said they had no relevant financial disclosures.
Despite the fact that prescription medications are commonly used by pregnant women, for most products and for new drugs in particular, there is typically little to no human safety information available to aid clinicians and patients in managing risk. As randomized clinical trials are usually not considered ethical to perform in pregnancy, observational epidemiologic studies are often the next best option to address human pregnancy exposure. An increasingly common approach to gathering human safety data is postmarketing pregnancy registries.
These pregnancy registries are initiated many times by agreement between the manufacturer and a regulatory agency as a postmarketing commitment or requirement following shortly after drug approval. Furthermore, because use of a new drug in pregnant women might be relatively rare, a pregnancy registry may be the only feasible method for gathering preliminary safety information as quickly as possible so that potential signals might be detected and clinical decision making can be better informed.
Pregnancy registries vary in design, but all involve collection of data on exposure to the drug of interest in pregnant women, and collection of outcome data for those pregnancies. The primary outcome of interest is typically major congenital anomalies; some registries also collect outcome data on fetal/infant growth, preterm delivery, pregnancy loss, specific neonatal outcomes, and postnatal longer term growth and development. The rates of these outcomes can be compared with general population reference rates, or rates occurring in a specific comparison group that might be more similar to the exposed women, for example, in terms of the underlying maternal condition being treated by the drug.
In addition to early information on a new drug, some of the major advantages of many pregnancy registry designs are the ability to collect information on the exposure and other pregnancy details before the mother knows what the outcome of her pregnancy will be; direct collection of exposure information from the mother herself, so that important factors such as drug and alcohol use, dose, and exact timing of exposure to the drug of interest; information on important other factors such as tobacco, alcohol, and multivitamin use.
The most challenging aspect of pregnancy registries is recruitment, for which registries largely depend on obstetric providers and other specialty physicians. Although low numbers of recruited pregnancies may be caused by limited use of a new drug, clearly most pregnancy registries enroll a very small fraction of all exposed pregnancies that are in existence. A second, related issue is that there may be bias in the self-selection of women who do find out about the registry and agree to participate, thus raising questions about the generalizability of the findings. A third issue is that many registries experience high rates of "lost to follow-up," in which outcome information is unobtainable from the health care provider or the pregnant woman – in some cases as high as 40%. There is also a concern about bias involved with the timing in gestation when a pregnancy enters a registry, such as the later in gestation a pregnancy is enrolled, the more likely that prenatal diagnosis, pregnancy loss, or other adverse outcomes have already occurred – thus making the enrollment essentially retrospective.
Another concern is that few registries have a concurrently enrolled group of unexposed women for purposes of comparison. Thus, their findings are commonly compared to external reference statistics which may not be the most appropriate. Finally, in some registries, the absence of information on individual dose and specific timing in gestation of exposure may preclude evaluating the biological plausibility of any registry findings. All of these issues can lead to long delays in accumulation of sufficient information to draw meaningful inferences, and potential concerns about interpretation of results.
How can awareness of pregnancy registries and more representative enrollment of exposed women be improved? A variety of methods are used to inform physicians and their patients about existing pregnancy registries for the purpose of encouraging referrals, including the Food and Drug Administration website, information in product labeling and on product websites, direct to provider or direct to consumer advertising, and in commonly used resources for clinicians such as this column and Reprotox, an information system developed by the Reproductive Toxicology Center. However, with the rapidly increasing number of registries, it is challenging for physicians to remain current on which medications are being monitored through a registry, what the criteria for enrollment are, and how a physician or patient can find out more. Pregnancy registry designs that are disease based – such as encompassing all medications used to treat a specific disease in pregnancy – help simplify the referral process by broadening the criteria for enrollment. Particularly for specialty physicians, this can ease the burden of identifying eligible women for enrollment.
How can enrollment be accomplished as early as possible in pregnancy (after exposure, but before the outcome is known), and how can more complete ascertainment of exposure and outcome be improved? An approach that some registries have used to address this is by "direct to consumer" campaigns. Registries such as the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Registry require that the pregnant woman herself enroll in the study, and therefore, the study is marketed directly to those women, although physician referral is encouraged. At least in this case, this has led to low rates of lost to follow-up (less than 5%), recruitment timing that is typically before the seventh or eighth week of gestation, and collection of specific information on dose and timing in gestation of exposure.
Multiple drug, disease-based, multiple sponsor registries such as the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drugs in Pregnancy Registry (patients call 888-233-2334), and the National Pregnancy Registry for Atypical Antipsychotics (866-961-2388) offer distinct advantages but are not always feasible for a specific product. A national pregnancy registry for all new drugs has been suggested as another solution to many of the challenges facing single product registries and to streamline referral and follow-up. In addition, including pregnant women in selected preapproval studies has several advantages. Finally, creative new technologies for earlier and more complete ascertainment and referral, such as use of electronic medical records, should be fully explored. The need for safety information on new drugs is urgent.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. Dr. Cohen directs the perinatal psychiatry program at Massachusetts General Hospital, which provides information about pregnancy and mental health. Dr. Koren is professor of pediatrics, pharmacology, pharmacy, and medical genetics at the University of Toronto. He heads the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation at the Hospital for Sick Children, Toronto, where he is director of the Motherisk program. Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; a clinical professor of pharmacy at the University of California, San Francisco; and an adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of "Drugs in Pregnancy and Lactation." Dr. Cohen is the principal investigator on the National Pregnancy Registry for Atypical Antipsychotics, which is sponsored by multiple atypical antipsychotic manufacturers.
Dr. Chambers, Dr. Koren, and Mr. Briggs said they had no relevant financial disclosures.
Radiation Therapy With or Without Temozolomide in Treating Patients With Low-Grade Glioma
Objectives: This phase III trial asks whether the addition of temozolomide (Temodar) to radiation therapy can improve progression-free survival and/or overall survival of patients with grade 2 glioma at the most recent pathological diagnosis.
Key entry or exclusion criteria: Patients with a previous pathological diagnosis of grade 3 or 4 are excluded, as are patients with prior radiotherapy, cytotoxic chemotherapy, radiosurgery, or investigational therapy for the brain tumor. Prior surgery is permitted.
Locations: 173 sites
Goal: 540 patients
Study sponsor: Eastern Cooperative Oncology Group in collaboration with the National Cancer Institute.
Link for more information: clinicaltrials.gov/ct2/show/study/NCT00978458
NIH clinical trials identifier: NCT00978458
Objectives: This phase III trial asks whether the addition of temozolomide (Temodar) to radiation therapy can improve progression-free survival and/or overall survival of patients with grade 2 glioma at the most recent pathological diagnosis.
Key entry or exclusion criteria: Patients with a previous pathological diagnosis of grade 3 or 4 are excluded, as are patients with prior radiotherapy, cytotoxic chemotherapy, radiosurgery, or investigational therapy for the brain tumor. Prior surgery is permitted.
Locations: 173 sites
Goal: 540 patients
Study sponsor: Eastern Cooperative Oncology Group in collaboration with the National Cancer Institute.
Link for more information: clinicaltrials.gov/ct2/show/study/NCT00978458
NIH clinical trials identifier: NCT00978458
Objectives: This phase III trial asks whether the addition of temozolomide (Temodar) to radiation therapy can improve progression-free survival and/or overall survival of patients with grade 2 glioma at the most recent pathological diagnosis.
Key entry or exclusion criteria: Patients with a previous pathological diagnosis of grade 3 or 4 are excluded, as are patients with prior radiotherapy, cytotoxic chemotherapy, radiosurgery, or investigational therapy for the brain tumor. Prior surgery is permitted.
Locations: 173 sites
Goal: 540 patients
Study sponsor: Eastern Cooperative Oncology Group in collaboration with the National Cancer Institute.
Link for more information: clinicaltrials.gov/ct2/show/study/NCT00978458
NIH clinical trials identifier: NCT00978458
Less Liver Cancer in HCV Patients Given Antiviral Therapy
Antiviral therapy may reduce the risk of hepatocellular carcinoma in patients with hepatitis C infections, Dr. Nina Kimer reported in the Oct. 22 issue of BMJ Open, published online.
Moreover, the effect seems to persist regardless of whether sustained virologic response is achieved.
Dr. Kimer, of Copenhagen University Hospital, Hvidovre, Denmark, and colleagues conducted a meta-analysis of trials looking at hepatitis C-related cirrhosis or fibrosis (BMJ Open 2012;2:e001313 [doi:10.1136/bmjopen-2012-001313]).
The primary analysis focused on randomized controlled trials, although prospective cohort studies with control groups were included in sensitivity analyses.
The authors searched the Cochrane Library, PubMed, EMBASE, and Web of Science databases, as well as reference lists from relevant papers, conference proceedings, and the World Health Organization Trial Search Portal.
Studies looking at HIV and chronic hepatitis B were excluded, for a total of eight randomized trials and five prospective cohort studies.
The duration of therapy (which included pegylated interferon in two trials, interferon plus ribavirin in one trial, and interferon monotherapy in the remaining trials) varied from 1 to 5 years. Follow-up ranged from 2 to 8.7 years.
Overall, the authors found that 81 of 1,156 patients who received antiviral therapy and 129 of 1,074 control patients who received no therapy developed hepatocellular carcinoma.
The relative risk for antiviral therapy, compared with no treatment, was 0.53 (95% confidence interval, 0.34-0.81).
"The corresponding number needed to treat to prevent one case of [hepatocellular carcinoma] was eight patients," added the researchers.
And while the effect of antivirals was more pronounced among patients with a virological response (RR 0.15; 95% CI, 0.05-0.45), there was nevertheless a clear reduction in risk for nonresponders to antivirals, with a relative risk of 0.57, compared with controls (95% CI 0.37-0.85).
"Although the intervention was more beneficial among sustained virological responders than nonresponders, there was a clear effect in both patient groups. ... Antiviral therapy may have beneficial effects on the risk of developing HCC that are unrelated to the virological response."
On the other hand, there was no reported effect of antiviral therapy on all-cause mortality, liver-related mortality, or liver-related morbidity in this population.
Dr. Kimer conceded several limitations to this analysis. "Only two of the included trials evaluated pegylated interferon, which is the current standard treatment for chronic hepatitis C."
Moreover, the duration of treatment in several included studies was "relatively long, which may increase the proportion of responders."
Finally, Dr. Kimer added that the researchers were unable to perform subgroup analysis to determine which treatment duration or dose is best.
"Based on the duration of follow-up and the lack of clear evidence concerning morbidity or mortality, we cannot exclude that interferon delays rather than prevents carcinogenesis," concluded the investigators.
Nevertheless, the "protection from HCC might be even better among patients in current antiviral therapy since the proportion of virological responders continues to increase with ongoing improvements in therapy," they wrote.
"Additional randomized trials with longer follow-up are still warranted to determine whether this is the case," they noted.
The authors disclosed having no outside funding and no competing interests related to this study.
sustained virologic response, hepatitis C-related cirrhosis, fibrosis,
Antiviral therapy may reduce the risk of hepatocellular carcinoma in patients with hepatitis C infections, Dr. Nina Kimer reported in the Oct. 22 issue of BMJ Open, published online.
Moreover, the effect seems to persist regardless of whether sustained virologic response is achieved.
Dr. Kimer, of Copenhagen University Hospital, Hvidovre, Denmark, and colleagues conducted a meta-analysis of trials looking at hepatitis C-related cirrhosis or fibrosis (BMJ Open 2012;2:e001313 [doi:10.1136/bmjopen-2012-001313]).
The primary analysis focused on randomized controlled trials, although prospective cohort studies with control groups were included in sensitivity analyses.
The authors searched the Cochrane Library, PubMed, EMBASE, and Web of Science databases, as well as reference lists from relevant papers, conference proceedings, and the World Health Organization Trial Search Portal.
Studies looking at HIV and chronic hepatitis B were excluded, for a total of eight randomized trials and five prospective cohort studies.
The duration of therapy (which included pegylated interferon in two trials, interferon plus ribavirin in one trial, and interferon monotherapy in the remaining trials) varied from 1 to 5 years. Follow-up ranged from 2 to 8.7 years.
Overall, the authors found that 81 of 1,156 patients who received antiviral therapy and 129 of 1,074 control patients who received no therapy developed hepatocellular carcinoma.
The relative risk for antiviral therapy, compared with no treatment, was 0.53 (95% confidence interval, 0.34-0.81).
"The corresponding number needed to treat to prevent one case of [hepatocellular carcinoma] was eight patients," added the researchers.
And while the effect of antivirals was more pronounced among patients with a virological response (RR 0.15; 95% CI, 0.05-0.45), there was nevertheless a clear reduction in risk for nonresponders to antivirals, with a relative risk of 0.57, compared with controls (95% CI 0.37-0.85).
"Although the intervention was more beneficial among sustained virological responders than nonresponders, there was a clear effect in both patient groups. ... Antiviral therapy may have beneficial effects on the risk of developing HCC that are unrelated to the virological response."
On the other hand, there was no reported effect of antiviral therapy on all-cause mortality, liver-related mortality, or liver-related morbidity in this population.
Dr. Kimer conceded several limitations to this analysis. "Only two of the included trials evaluated pegylated interferon, which is the current standard treatment for chronic hepatitis C."
Moreover, the duration of treatment in several included studies was "relatively long, which may increase the proportion of responders."
Finally, Dr. Kimer added that the researchers were unable to perform subgroup analysis to determine which treatment duration or dose is best.
"Based on the duration of follow-up and the lack of clear evidence concerning morbidity or mortality, we cannot exclude that interferon delays rather than prevents carcinogenesis," concluded the investigators.
Nevertheless, the "protection from HCC might be even better among patients in current antiviral therapy since the proportion of virological responders continues to increase with ongoing improvements in therapy," they wrote.
"Additional randomized trials with longer follow-up are still warranted to determine whether this is the case," they noted.
The authors disclosed having no outside funding and no competing interests related to this study.
Antiviral therapy may reduce the risk of hepatocellular carcinoma in patients with hepatitis C infections, Dr. Nina Kimer reported in the Oct. 22 issue of BMJ Open, published online.
Moreover, the effect seems to persist regardless of whether sustained virologic response is achieved.
Dr. Kimer, of Copenhagen University Hospital, Hvidovre, Denmark, and colleagues conducted a meta-analysis of trials looking at hepatitis C-related cirrhosis or fibrosis (BMJ Open 2012;2:e001313 [doi:10.1136/bmjopen-2012-001313]).
The primary analysis focused on randomized controlled trials, although prospective cohort studies with control groups were included in sensitivity analyses.
The authors searched the Cochrane Library, PubMed, EMBASE, and Web of Science databases, as well as reference lists from relevant papers, conference proceedings, and the World Health Organization Trial Search Portal.
Studies looking at HIV and chronic hepatitis B were excluded, for a total of eight randomized trials and five prospective cohort studies.
The duration of therapy (which included pegylated interferon in two trials, interferon plus ribavirin in one trial, and interferon monotherapy in the remaining trials) varied from 1 to 5 years. Follow-up ranged from 2 to 8.7 years.
Overall, the authors found that 81 of 1,156 patients who received antiviral therapy and 129 of 1,074 control patients who received no therapy developed hepatocellular carcinoma.
The relative risk for antiviral therapy, compared with no treatment, was 0.53 (95% confidence interval, 0.34-0.81).
"The corresponding number needed to treat to prevent one case of [hepatocellular carcinoma] was eight patients," added the researchers.
And while the effect of antivirals was more pronounced among patients with a virological response (RR 0.15; 95% CI, 0.05-0.45), there was nevertheless a clear reduction in risk for nonresponders to antivirals, with a relative risk of 0.57, compared with controls (95% CI 0.37-0.85).
"Although the intervention was more beneficial among sustained virological responders than nonresponders, there was a clear effect in both patient groups. ... Antiviral therapy may have beneficial effects on the risk of developing HCC that are unrelated to the virological response."
On the other hand, there was no reported effect of antiviral therapy on all-cause mortality, liver-related mortality, or liver-related morbidity in this population.
Dr. Kimer conceded several limitations to this analysis. "Only two of the included trials evaluated pegylated interferon, which is the current standard treatment for chronic hepatitis C."
Moreover, the duration of treatment in several included studies was "relatively long, which may increase the proportion of responders."
Finally, Dr. Kimer added that the researchers were unable to perform subgroup analysis to determine which treatment duration or dose is best.
"Based on the duration of follow-up and the lack of clear evidence concerning morbidity or mortality, we cannot exclude that interferon delays rather than prevents carcinogenesis," concluded the investigators.
Nevertheless, the "protection from HCC might be even better among patients in current antiviral therapy since the proportion of virological responders continues to increase with ongoing improvements in therapy," they wrote.
"Additional randomized trials with longer follow-up are still warranted to determine whether this is the case," they noted.
The authors disclosed having no outside funding and no competing interests related to this study.
sustained virologic response, hepatitis C-related cirrhosis, fibrosis,
sustained virologic response, hepatitis C-related cirrhosis, fibrosis,
FROM BMJ OPEN
Major Finding: Antiviral therapy for hepatitis C carried a relative risk of 0.53 for developing hepatocellular carcinoma, compared with no treatment (95% confidence interval, 0.34-0.81).
Data Source: A meta-analysis of randomized controlled trials.
Disclosures: The authors disclosed having no outside funding and no competing interests related to this study.
Hospitalists Can Be Prime Partners in QI, Patient Safety Efforts
NEW YORK—Hospitalists are poised to become key allies with hospital quality and safety officers nationwide, according to veteran hospitalist Jennifer Myers, MD, FHM, director of quality and safety education for Penn Medicine in Philadelphia.
Addressing hospitalists at the seventh annual Mid-Atlantic Hospital Medicine Symposium at Mount Sinai School of Medicine in New York, Dr. Myers said that while the challenges associated with quality improvement (QI) are many, HM leaders have the in-house relationships and respect to push the issue.
"There's really no other specialty more perfectly poised to lead this work," she told more than 180 symposium attendees Friday.
Dr. Myers, in an address titled "Enhancing Patient Safety," told The Hospitalist that HM leaders pursue three broad goals: to participate in QI programs already in place, to help create or foster a culture focused on addressing mistakes, and to teach those lessons to young physicians.
She urged physicians to actively report on mistakes and near misses, and earnestly address the processes that led to them. If a vehicle to discuss the mistakes doesn't exist at an institution, hospitalists can push to start one, she said. If a hospital doesn't have an electronic incident reporting system, a hospitalist can push to get one. "This is the goal," Dr. Myers added. "People coming to work and feeling they can be safe and report errors in the spirit of improvement."
She noted that many hospitalists already oversee quality and safety programs without any formal training. She recommended some of those physicians consider the Quality and Safety Educators Academy (QSEA), a three-day academy designed as a faculty development program and sponsored by SHM and the Alliance for Academic Internal Medicine (AAIM). The academy is March 7-9, 2013, in Tempe, Ariz.
NEW YORK—Hospitalists are poised to become key allies with hospital quality and safety officers nationwide, according to veteran hospitalist Jennifer Myers, MD, FHM, director of quality and safety education for Penn Medicine in Philadelphia.
Addressing hospitalists at the seventh annual Mid-Atlantic Hospital Medicine Symposium at Mount Sinai School of Medicine in New York, Dr. Myers said that while the challenges associated with quality improvement (QI) are many, HM leaders have the in-house relationships and respect to push the issue.
"There's really no other specialty more perfectly poised to lead this work," she told more than 180 symposium attendees Friday.
Dr. Myers, in an address titled "Enhancing Patient Safety," told The Hospitalist that HM leaders pursue three broad goals: to participate in QI programs already in place, to help create or foster a culture focused on addressing mistakes, and to teach those lessons to young physicians.
She urged physicians to actively report on mistakes and near misses, and earnestly address the processes that led to them. If a vehicle to discuss the mistakes doesn't exist at an institution, hospitalists can push to start one, she said. If a hospital doesn't have an electronic incident reporting system, a hospitalist can push to get one. "This is the goal," Dr. Myers added. "People coming to work and feeling they can be safe and report errors in the spirit of improvement."
She noted that many hospitalists already oversee quality and safety programs without any formal training. She recommended some of those physicians consider the Quality and Safety Educators Academy (QSEA), a three-day academy designed as a faculty development program and sponsored by SHM and the Alliance for Academic Internal Medicine (AAIM). The academy is March 7-9, 2013, in Tempe, Ariz.
NEW YORK—Hospitalists are poised to become key allies with hospital quality and safety officers nationwide, according to veteran hospitalist Jennifer Myers, MD, FHM, director of quality and safety education for Penn Medicine in Philadelphia.
Addressing hospitalists at the seventh annual Mid-Atlantic Hospital Medicine Symposium at Mount Sinai School of Medicine in New York, Dr. Myers said that while the challenges associated with quality improvement (QI) are many, HM leaders have the in-house relationships and respect to push the issue.
"There's really no other specialty more perfectly poised to lead this work," she told more than 180 symposium attendees Friday.
Dr. Myers, in an address titled "Enhancing Patient Safety," told The Hospitalist that HM leaders pursue three broad goals: to participate in QI programs already in place, to help create or foster a culture focused on addressing mistakes, and to teach those lessons to young physicians.
She urged physicians to actively report on mistakes and near misses, and earnestly address the processes that led to them. If a vehicle to discuss the mistakes doesn't exist at an institution, hospitalists can push to start one, she said. If a hospital doesn't have an electronic incident reporting system, a hospitalist can push to get one. "This is the goal," Dr. Myers added. "People coming to work and feeling they can be safe and report errors in the spirit of improvement."
She noted that many hospitalists already oversee quality and safety programs without any formal training. She recommended some of those physicians consider the Quality and Safety Educators Academy (QSEA), a three-day academy designed as a faculty development program and sponsored by SHM and the Alliance for Academic Internal Medicine (AAIM). The academy is March 7-9, 2013, in Tempe, Ariz.
Localized Hospitalist Teams Enhance Workflow Productivity
Using localized inpatient teams of hospitalists and physician assistants in single nursing units can boost physicians' productivity, hospital efficiency, and patient outcomes, according to a study in the Journal of Hospital Medicine.
The study, "Impact of Localizing General Medical Teams to a Single Nursing Unit," compared the effectiveness of using localized medical teams with nonlocalized teams in caring for patients in the nursing unit of an academic medical center from April to mid-July 2010. The localized team members received 51% fewer paged messages, logged more encounters with patients, and generated more relative value units (RVUs) during the workday compared with the nonlocalized teams, researchers reported.
These findings point to an overall significant increase in team productivity. The risk of 30-day readmissions and the patient charges incurred remained the same.
Lead author Siddhartha Singh, MD, MS, associate chief medical officer at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee, says the study's most surprising finding was that patients averaged longer length of stay (LOS) under localized team care. However, Dr. Singh says, "if somebody wants to try out localization, the big message from our study is that it's a good thing as far as workflow is concerned."
Dr. Singh hopes the research will spark future studies about localized hospitalist teams and the optimal amount of localization needed to improve productivity and efficiency.
"When others try to localize patients, they need to be careful of 100% localization," he says. "My sense is, without having studied this any further, there's a sweet spot that optimizes the care provided to the patients [and maximizes] hospital efficiency and physician assistant productivity. I'm hoping that the next set of research on this topic tries to investigate that."
Using localized inpatient teams of hospitalists and physician assistants in single nursing units can boost physicians' productivity, hospital efficiency, and patient outcomes, according to a study in the Journal of Hospital Medicine.
The study, "Impact of Localizing General Medical Teams to a Single Nursing Unit," compared the effectiveness of using localized medical teams with nonlocalized teams in caring for patients in the nursing unit of an academic medical center from April to mid-July 2010. The localized team members received 51% fewer paged messages, logged more encounters with patients, and generated more relative value units (RVUs) during the workday compared with the nonlocalized teams, researchers reported.
These findings point to an overall significant increase in team productivity. The risk of 30-day readmissions and the patient charges incurred remained the same.
Lead author Siddhartha Singh, MD, MS, associate chief medical officer at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee, says the study's most surprising finding was that patients averaged longer length of stay (LOS) under localized team care. However, Dr. Singh says, "if somebody wants to try out localization, the big message from our study is that it's a good thing as far as workflow is concerned."
Dr. Singh hopes the research will spark future studies about localized hospitalist teams and the optimal amount of localization needed to improve productivity and efficiency.
"When others try to localize patients, they need to be careful of 100% localization," he says. "My sense is, without having studied this any further, there's a sweet spot that optimizes the care provided to the patients [and maximizes] hospital efficiency and physician assistant productivity. I'm hoping that the next set of research on this topic tries to investigate that."
Using localized inpatient teams of hospitalists and physician assistants in single nursing units can boost physicians' productivity, hospital efficiency, and patient outcomes, according to a study in the Journal of Hospital Medicine.
The study, "Impact of Localizing General Medical Teams to a Single Nursing Unit," compared the effectiveness of using localized medical teams with nonlocalized teams in caring for patients in the nursing unit of an academic medical center from April to mid-July 2010. The localized team members received 51% fewer paged messages, logged more encounters with patients, and generated more relative value units (RVUs) during the workday compared with the nonlocalized teams, researchers reported.
These findings point to an overall significant increase in team productivity. The risk of 30-day readmissions and the patient charges incurred remained the same.
Lead author Siddhartha Singh, MD, MS, associate chief medical officer at Froedtert Hospital and the Medical College of Wisconsin in Milwaukee, says the study's most surprising finding was that patients averaged longer length of stay (LOS) under localized team care. However, Dr. Singh says, "if somebody wants to try out localization, the big message from our study is that it's a good thing as far as workflow is concerned."
Dr. Singh hopes the research will spark future studies about localized hospitalist teams and the optimal amount of localization needed to improve productivity and efficiency.
"When others try to localize patients, they need to be careful of 100% localization," he says. "My sense is, without having studied this any further, there's a sweet spot that optimizes the care provided to the patients [and maximizes] hospital efficiency and physician assistant productivity. I'm hoping that the next set of research on this topic tries to investigate that."
Second TNF Blocker Approved for Refractory UC
Adalimumab, a subcutaneously administered tumor necrosis factor blocker, has been approved for treating adults with moderately to severely active ulcerative colitis who have not had an adequate response with conventional treatments, the Food and Drug Administration announced.
The safety and effectiveness of adalimumab for this patient population was established in two clinical studies of 908 patients with moderately to severely active ulcerative colitis (UC).
Adalimumab, marketed as Humira by Abbott Laboratories, was first approved for treating rheumatoid arthritis in 2002, followed by psoriatic arthritis in 2005, ankylosing spondylitis in 2006, Crohn’s disease in 2007, and plaque psoriasis and juvenile idiopathic arthritis in 2008.
Adalimumab is the second TNF blocker to be approved for ulcerative colitis; infliximab (Remicade), an intravenous TNF blocker, was previously approved for treating ulcerative colitis.
Clinical remission rates in the two studies were significantly greater among patients treated with infliximab than among those who received placebo: In an 8-week study, which did not include patients who had previously been treated with a TNF blocker, the clinical remission rate at 8 weeks was 18.5% among those on adalimumab vs. 9.2% in those on placebo, a 9.3% difference.
In the second study, which followed patients for 1 year and included some who had been treated with infliximab, the clinical remission rate at 8 weeks was 16.5% among those on adalimumab, vs. 9.3% among those on placebo, a 7.2% difference.
At a meeting on Aug. 28 held to review these data, the majority of the FDA’s Gastrointestinal Drugs Advisory Committee agreed that these differences represented clinically meaningful benefits and supported approval of adalimumab for this indication – adults with moderately to severely active ulcerative colitis who have not had an adequate response to conventional treatment.
Panelists cited the need for more treatments for ulcerative colitis and for a subcutaneous TNF blocker for these patients, as well as its potential steroid-sparing effects.
In the studies, no new side effects were identified, the agency said.
The FDA statement points out that the effectiveness of adalimumab "has not been established in patients with ulcerative colitis who have lost response to or were intolerant to TNF blockers."
The approved dosing regimen for adalimumab is a starting dose of 160 mg, followed by a second dose of 80 mg 2 weeks later and then a maintenance dose of 40 mg every other week.
"The drug should only continue to be used in patients who have shown evidence of clinical remission by 8 weeks of therapy," according to the FDA statement.
Adalimumab is the first self-administered biologic treatment for ulcerative colitis to be approved.
It is good news for patients who have ulcerative colitis and the health care providers who take care of them that a second anti-TNF agent is now available. The FDA’s approval of Humira (adalimumab) for moderate to severe ulcer-ative colitis provides us with an additional option that we definitely need because there are many patients who suffer from ulcerative colitis and fail to respond to the "conventional" treatments with aminosalicylates or steroids and thiopurines, or lose response to infliximab. Adalimumab is an option for many of these types of patients and may be the first choice anti–tumor necrosis factor agent for some patients or providers, due to the injectable delivery method for this therapy.
Despite this good news, however, we need to acknowledge that 8-week remission rates of 18.5% and 16.5% leave a lot of room for improvement. Future studies with adalimumab in ulcerative colitis will focus on how to optimize this therapy and will explore adjustable dosing schedules, combination therapies, and other important longer-term outcomes such as sustained remission and mucosal healing.
David T. Rubin, M.D., AGAF, is a professor of medicine and co-director of the Inflammatory Bowel Disease Center at the University of Chicago. He has served as a consultant for Janssen and for Abbott.
It is good news for patients who have ulcerative colitis and the health care providers who take care of them that a second anti-TNF agent is now available. The FDA’s approval of Humira (adalimumab) for moderate to severe ulcer-ative colitis provides us with an additional option that we definitely need because there are many patients who suffer from ulcerative colitis and fail to respond to the "conventional" treatments with aminosalicylates or steroids and thiopurines, or lose response to infliximab. Adalimumab is an option for many of these types of patients and may be the first choice anti–tumor necrosis factor agent for some patients or providers, due to the injectable delivery method for this therapy.
Despite this good news, however, we need to acknowledge that 8-week remission rates of 18.5% and 16.5% leave a lot of room for improvement. Future studies with adalimumab in ulcerative colitis will focus on how to optimize this therapy and will explore adjustable dosing schedules, combination therapies, and other important longer-term outcomes such as sustained remission and mucosal healing.
David T. Rubin, M.D., AGAF, is a professor of medicine and co-director of the Inflammatory Bowel Disease Center at the University of Chicago. He has served as a consultant for Janssen and for Abbott.
It is good news for patients who have ulcerative colitis and the health care providers who take care of them that a second anti-TNF agent is now available. The FDA’s approval of Humira (adalimumab) for moderate to severe ulcer-ative colitis provides us with an additional option that we definitely need because there are many patients who suffer from ulcerative colitis and fail to respond to the "conventional" treatments with aminosalicylates or steroids and thiopurines, or lose response to infliximab. Adalimumab is an option for many of these types of patients and may be the first choice anti–tumor necrosis factor agent for some patients or providers, due to the injectable delivery method for this therapy.
Despite this good news, however, we need to acknowledge that 8-week remission rates of 18.5% and 16.5% leave a lot of room for improvement. Future studies with adalimumab in ulcerative colitis will focus on how to optimize this therapy and will explore adjustable dosing schedules, combination therapies, and other important longer-term outcomes such as sustained remission and mucosal healing.
David T. Rubin, M.D., AGAF, is a professor of medicine and co-director of the Inflammatory Bowel Disease Center at the University of Chicago. He has served as a consultant for Janssen and for Abbott.
Adalimumab, a subcutaneously administered tumor necrosis factor blocker, has been approved for treating adults with moderately to severely active ulcerative colitis who have not had an adequate response with conventional treatments, the Food and Drug Administration announced.
The safety and effectiveness of adalimumab for this patient population was established in two clinical studies of 908 patients with moderately to severely active ulcerative colitis (UC).
Adalimumab, marketed as Humira by Abbott Laboratories, was first approved for treating rheumatoid arthritis in 2002, followed by psoriatic arthritis in 2005, ankylosing spondylitis in 2006, Crohn’s disease in 2007, and plaque psoriasis and juvenile idiopathic arthritis in 2008.
Adalimumab is the second TNF blocker to be approved for ulcerative colitis; infliximab (Remicade), an intravenous TNF blocker, was previously approved for treating ulcerative colitis.
Clinical remission rates in the two studies were significantly greater among patients treated with infliximab than among those who received placebo: In an 8-week study, which did not include patients who had previously been treated with a TNF blocker, the clinical remission rate at 8 weeks was 18.5% among those on adalimumab vs. 9.2% in those on placebo, a 9.3% difference.
In the second study, which followed patients for 1 year and included some who had been treated with infliximab, the clinical remission rate at 8 weeks was 16.5% among those on adalimumab, vs. 9.3% among those on placebo, a 7.2% difference.
At a meeting on Aug. 28 held to review these data, the majority of the FDA’s Gastrointestinal Drugs Advisory Committee agreed that these differences represented clinically meaningful benefits and supported approval of adalimumab for this indication – adults with moderately to severely active ulcerative colitis who have not had an adequate response to conventional treatment.
Panelists cited the need for more treatments for ulcerative colitis and for a subcutaneous TNF blocker for these patients, as well as its potential steroid-sparing effects.
In the studies, no new side effects were identified, the agency said.
The FDA statement points out that the effectiveness of adalimumab "has not been established in patients with ulcerative colitis who have lost response to or were intolerant to TNF blockers."
The approved dosing regimen for adalimumab is a starting dose of 160 mg, followed by a second dose of 80 mg 2 weeks later and then a maintenance dose of 40 mg every other week.
"The drug should only continue to be used in patients who have shown evidence of clinical remission by 8 weeks of therapy," according to the FDA statement.
Adalimumab is the first self-administered biologic treatment for ulcerative colitis to be approved.
Adalimumab, a subcutaneously administered tumor necrosis factor blocker, has been approved for treating adults with moderately to severely active ulcerative colitis who have not had an adequate response with conventional treatments, the Food and Drug Administration announced.
The safety and effectiveness of adalimumab for this patient population was established in two clinical studies of 908 patients with moderately to severely active ulcerative colitis (UC).
Adalimumab, marketed as Humira by Abbott Laboratories, was first approved for treating rheumatoid arthritis in 2002, followed by psoriatic arthritis in 2005, ankylosing spondylitis in 2006, Crohn’s disease in 2007, and plaque psoriasis and juvenile idiopathic arthritis in 2008.
Adalimumab is the second TNF blocker to be approved for ulcerative colitis; infliximab (Remicade), an intravenous TNF blocker, was previously approved for treating ulcerative colitis.
Clinical remission rates in the two studies were significantly greater among patients treated with infliximab than among those who received placebo: In an 8-week study, which did not include patients who had previously been treated with a TNF blocker, the clinical remission rate at 8 weeks was 18.5% among those on adalimumab vs. 9.2% in those on placebo, a 9.3% difference.
In the second study, which followed patients for 1 year and included some who had been treated with infliximab, the clinical remission rate at 8 weeks was 16.5% among those on adalimumab, vs. 9.3% among those on placebo, a 7.2% difference.
At a meeting on Aug. 28 held to review these data, the majority of the FDA’s Gastrointestinal Drugs Advisory Committee agreed that these differences represented clinically meaningful benefits and supported approval of adalimumab for this indication – adults with moderately to severely active ulcerative colitis who have not had an adequate response to conventional treatment.
Panelists cited the need for more treatments for ulcerative colitis and for a subcutaneous TNF blocker for these patients, as well as its potential steroid-sparing effects.
In the studies, no new side effects were identified, the agency said.
The FDA statement points out that the effectiveness of adalimumab "has not been established in patients with ulcerative colitis who have lost response to or were intolerant to TNF blockers."
The approved dosing regimen for adalimumab is a starting dose of 160 mg, followed by a second dose of 80 mg 2 weeks later and then a maintenance dose of 40 mg every other week.
"The drug should only continue to be used in patients who have shown evidence of clinical remission by 8 weeks of therapy," according to the FDA statement.
Adalimumab is the first self-administered biologic treatment for ulcerative colitis to be approved.
Crohn's Responded to Ustekinumab
Ustekinumab induced a clinical response in patients with moderate to severe Crohn’s disease that was resistant to tumor necrosis factor antagonists, in a phase IIb clinical trial published online Oct. 17 in the New England Journal of Medicine.
However, the agent did not improve remission rates, compared with placebo, said Dr. William J. Sandborn, AGAF, who is professor of medicine and chief of the division of gastroenterology at the University of California San Diego, La Jolla, and his associates.
"A sizable proportion" of patients with moderate to severe Crohn’s disease do not respond to TNF antagonists, have an unsustained response, or must discontinue the medications because of adverse effects. After ustekinumab showed efficacy in such patients in a phase IIa clinical study, Dr. Sandborn and his colleagues performed a 36-week double-blind phase IIb trial in 526 adults at 153 medical centers in 12 countries.
Ustekinumab, a human IgG monoclonal antibody that inhibits the receptors for interleukin-12 and interleukin-23 on T cells, natural killer cells, and antigen-presenting cells, has Food and Drug Administration approval for use in plaque psoriasis. This clinical trial was sponsored by an affiliate of the manufacturer, Janssen Biotech.
During an 8-week induction phase, the study subjects were randomly assigned to receive intravenous placebo (132 patients) or ustekinumab in 1-mg/kg (131 patients), 3-mg/kg (132 patients), or 6-mg/kg (131 patients) doses. Then, during weeks 8-36, the study subjects who showed a response to induction therapy and those who did not show a response were separately randomized to receive either subcutaneous ustekinumab (90 mg) or placebo at week 8 and week 16, as maintenance therapy.
Treatment efficacy was assessed at week 22, and patients were followed through week 36 for a safety analysis. A total of 36.1% of the subjects discontinued the study before week 36.
The primary end point was a clinical response, defined as a decrease of 100 points or more on the Crohn’s Disease Activity Index (CDAI) score.
A total of 39.7% of patients receiving the 6-mg induction dose showed a clinical response, which was significantly greater than the 23.5% of patients receiving placebo, the investigators said (N. Engl. J. Med. 2012 [doi:10.1056/NEJMoa1203572]).
A greater number of patients receiving the lower doses of ustekinumab than receiving placebo showed a clinical response, but the differences between these low-dose groups and the placebo group did not reach statistical significance.
The 6-mg/kg dose was effective across most demographic and disease characteristics, judging from the findings of a subgroup analysis. It was consistently effective in patients who had failed on their first attempt at therapy with TNF antagonists, patients who had failed on two or more TNF antagonists, and patients who had only a transient response to TNF antagonists.
However, rates of clinical remission did not differ significantly between patients receiving ustekinumab and those receiving placebo, Dr. Sandborn and his associates said.
At all follow-up visits, the proportion of patients who had a 70-point clinical response was significantly higher, the reductions in mean CDAI scores were significantly greater, and the reductions in C-reactive protein levels were significantly greater in patients receiving 6 mg per kg of ustekinumab than in the placebo group.
As a maintenance therapy, 90 mg of subcutaneous ustekinumab appeared to be effective in patients who responded to the induction dose of the agent. The proportion of patients who showed a clinical response at week 22 was 69.4% in those receiving maintenance ustekinumab, significantly greater than the 42.5% response rate among those receiving maintenance placebo.
Among patients who responded to induction-phase ustekinumab, 41.7% of those who also received maintenance ustekinumab achieved clinical remission at week 22, compared with only 27.4% of those who received maintenance placebo.
Similarly, among patients who showed a response to induction ustekinumab, reductions in both CDAI scores and CRP levels were sustained if they continued on maintenance ustekinumab but were not sustained if they continued on placebo for the maintenance period.
However, patients who did not show a response to induction ustekinumab also did not benefit from additional ustekinumab in the maintenance phase of the study.
The results of the safety analysis were "somewhat limited" by the small sample size and the short duration of treatment. No deaths, serious opportunistic infections, or major adverse cardiovascular events were reported, "but large studies of longer duration are needed to assess uncommon adverse events," the investigators said.
Of note, one patient receiving ustekinumab as both induction and maintenance therapy developed a basal cell carcinoma. Among patients taking ustekinumab in the induction phase of the study, six developed serious infections: Clostridium difficile, viral gastroenteritis, UTI, anal abscess, vaginal abscess, and a staph infection of a central catheter.
Ustekinumab induced a clinical response in patients with moderate to severe Crohn’s disease that was resistant to tumor necrosis factor antagonists, in a phase IIb clinical trial published online Oct. 17 in the New England Journal of Medicine.
However, the agent did not improve remission rates, compared with placebo, said Dr. William J. Sandborn, AGAF, who is professor of medicine and chief of the division of gastroenterology at the University of California San Diego, La Jolla, and his associates.
"A sizable proportion" of patients with moderate to severe Crohn’s disease do not respond to TNF antagonists, have an unsustained response, or must discontinue the medications because of adverse effects. After ustekinumab showed efficacy in such patients in a phase IIa clinical study, Dr. Sandborn and his colleagues performed a 36-week double-blind phase IIb trial in 526 adults at 153 medical centers in 12 countries.
Ustekinumab, a human IgG monoclonal antibody that inhibits the receptors for interleukin-12 and interleukin-23 on T cells, natural killer cells, and antigen-presenting cells, has Food and Drug Administration approval for use in plaque psoriasis. This clinical trial was sponsored by an affiliate of the manufacturer, Janssen Biotech.
During an 8-week induction phase, the study subjects were randomly assigned to receive intravenous placebo (132 patients) or ustekinumab in 1-mg/kg (131 patients), 3-mg/kg (132 patients), or 6-mg/kg (131 patients) doses. Then, during weeks 8-36, the study subjects who showed a response to induction therapy and those who did not show a response were separately randomized to receive either subcutaneous ustekinumab (90 mg) or placebo at week 8 and week 16, as maintenance therapy.
Treatment efficacy was assessed at week 22, and patients were followed through week 36 for a safety analysis. A total of 36.1% of the subjects discontinued the study before week 36.
The primary end point was a clinical response, defined as a decrease of 100 points or more on the Crohn’s Disease Activity Index (CDAI) score.
A total of 39.7% of patients receiving the 6-mg induction dose showed a clinical response, which was significantly greater than the 23.5% of patients receiving placebo, the investigators said (N. Engl. J. Med. 2012 [doi:10.1056/NEJMoa1203572]).
A greater number of patients receiving the lower doses of ustekinumab than receiving placebo showed a clinical response, but the differences between these low-dose groups and the placebo group did not reach statistical significance.
The 6-mg/kg dose was effective across most demographic and disease characteristics, judging from the findings of a subgroup analysis. It was consistently effective in patients who had failed on their first attempt at therapy with TNF antagonists, patients who had failed on two or more TNF antagonists, and patients who had only a transient response to TNF antagonists.
However, rates of clinical remission did not differ significantly between patients receiving ustekinumab and those receiving placebo, Dr. Sandborn and his associates said.
At all follow-up visits, the proportion of patients who had a 70-point clinical response was significantly higher, the reductions in mean CDAI scores were significantly greater, and the reductions in C-reactive protein levels were significantly greater in patients receiving 6 mg per kg of ustekinumab than in the placebo group.
As a maintenance therapy, 90 mg of subcutaneous ustekinumab appeared to be effective in patients who responded to the induction dose of the agent. The proportion of patients who showed a clinical response at week 22 was 69.4% in those receiving maintenance ustekinumab, significantly greater than the 42.5% response rate among those receiving maintenance placebo.
Among patients who responded to induction-phase ustekinumab, 41.7% of those who also received maintenance ustekinumab achieved clinical remission at week 22, compared with only 27.4% of those who received maintenance placebo.
Similarly, among patients who showed a response to induction ustekinumab, reductions in both CDAI scores and CRP levels were sustained if they continued on maintenance ustekinumab but were not sustained if they continued on placebo for the maintenance period.
However, patients who did not show a response to induction ustekinumab also did not benefit from additional ustekinumab in the maintenance phase of the study.
The results of the safety analysis were "somewhat limited" by the small sample size and the short duration of treatment. No deaths, serious opportunistic infections, or major adverse cardiovascular events were reported, "but large studies of longer duration are needed to assess uncommon adverse events," the investigators said.
Of note, one patient receiving ustekinumab as both induction and maintenance therapy developed a basal cell carcinoma. Among patients taking ustekinumab in the induction phase of the study, six developed serious infections: Clostridium difficile, viral gastroenteritis, UTI, anal abscess, vaginal abscess, and a staph infection of a central catheter.
Ustekinumab induced a clinical response in patients with moderate to severe Crohn’s disease that was resistant to tumor necrosis factor antagonists, in a phase IIb clinical trial published online Oct. 17 in the New England Journal of Medicine.
However, the agent did not improve remission rates, compared with placebo, said Dr. William J. Sandborn, AGAF, who is professor of medicine and chief of the division of gastroenterology at the University of California San Diego, La Jolla, and his associates.
"A sizable proportion" of patients with moderate to severe Crohn’s disease do not respond to TNF antagonists, have an unsustained response, or must discontinue the medications because of adverse effects. After ustekinumab showed efficacy in such patients in a phase IIa clinical study, Dr. Sandborn and his colleagues performed a 36-week double-blind phase IIb trial in 526 adults at 153 medical centers in 12 countries.
Ustekinumab, a human IgG monoclonal antibody that inhibits the receptors for interleukin-12 and interleukin-23 on T cells, natural killer cells, and antigen-presenting cells, has Food and Drug Administration approval for use in plaque psoriasis. This clinical trial was sponsored by an affiliate of the manufacturer, Janssen Biotech.
During an 8-week induction phase, the study subjects were randomly assigned to receive intravenous placebo (132 patients) or ustekinumab in 1-mg/kg (131 patients), 3-mg/kg (132 patients), or 6-mg/kg (131 patients) doses. Then, during weeks 8-36, the study subjects who showed a response to induction therapy and those who did not show a response were separately randomized to receive either subcutaneous ustekinumab (90 mg) or placebo at week 8 and week 16, as maintenance therapy.
Treatment efficacy was assessed at week 22, and patients were followed through week 36 for a safety analysis. A total of 36.1% of the subjects discontinued the study before week 36.
The primary end point was a clinical response, defined as a decrease of 100 points or more on the Crohn’s Disease Activity Index (CDAI) score.
A total of 39.7% of patients receiving the 6-mg induction dose showed a clinical response, which was significantly greater than the 23.5% of patients receiving placebo, the investigators said (N. Engl. J. Med. 2012 [doi:10.1056/NEJMoa1203572]).
A greater number of patients receiving the lower doses of ustekinumab than receiving placebo showed a clinical response, but the differences between these low-dose groups and the placebo group did not reach statistical significance.
The 6-mg/kg dose was effective across most demographic and disease characteristics, judging from the findings of a subgroup analysis. It was consistently effective in patients who had failed on their first attempt at therapy with TNF antagonists, patients who had failed on two or more TNF antagonists, and patients who had only a transient response to TNF antagonists.
However, rates of clinical remission did not differ significantly between patients receiving ustekinumab and those receiving placebo, Dr. Sandborn and his associates said.
At all follow-up visits, the proportion of patients who had a 70-point clinical response was significantly higher, the reductions in mean CDAI scores were significantly greater, and the reductions in C-reactive protein levels were significantly greater in patients receiving 6 mg per kg of ustekinumab than in the placebo group.
As a maintenance therapy, 90 mg of subcutaneous ustekinumab appeared to be effective in patients who responded to the induction dose of the agent. The proportion of patients who showed a clinical response at week 22 was 69.4% in those receiving maintenance ustekinumab, significantly greater than the 42.5% response rate among those receiving maintenance placebo.
Among patients who responded to induction-phase ustekinumab, 41.7% of those who also received maintenance ustekinumab achieved clinical remission at week 22, compared with only 27.4% of those who received maintenance placebo.
Similarly, among patients who showed a response to induction ustekinumab, reductions in both CDAI scores and CRP levels were sustained if they continued on maintenance ustekinumab but were not sustained if they continued on placebo for the maintenance period.
However, patients who did not show a response to induction ustekinumab also did not benefit from additional ustekinumab in the maintenance phase of the study.
The results of the safety analysis were "somewhat limited" by the small sample size and the short duration of treatment. No deaths, serious opportunistic infections, or major adverse cardiovascular events were reported, "but large studies of longer duration are needed to assess uncommon adverse events," the investigators said.
Of note, one patient receiving ustekinumab as both induction and maintenance therapy developed a basal cell carcinoma. Among patients taking ustekinumab in the induction phase of the study, six developed serious infections: Clostridium difficile, viral gastroenteritis, UTI, anal abscess, vaginal abscess, and a staph infection of a central catheter.
Major Finding: Of patients with moderate to severe Crohn's disease who received ustekinumab (6 mg/kg), 39.7% showed a decrease of 100 points or more in CDAI score, compared with 23.5% of those who received placebo.
Data Source: The data come from a 36-week international phase IIb randomized clinical trial comparing 3 doses of ustekinumab with placebo in 526 adults who had refractory Crohn’ disease.
Disclosures: This study was sponsored by Janssen Research and Development; Janssen Biotech makes ustekinumab. Dr. Sandborn and his associates reported numerous ties to industry sources.
HCV Rapid Tests Accurate in First-Line Screening
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-66).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5%-99.8% for whole blood and 96.8%-99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9%-99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3%-99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5%-99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9%-99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7%-98.4%) and specificity of 98.2% (95% CI, 92.2%-99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs), and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates, and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk factor–based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Steven L. Flamm, M.D., is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs), and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates, and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk factor–based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Steven L. Flamm, M.D., is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Chronic hepatitis C virus (HCV) infection is a common worldwide problem. It is estimated that 170 million people are afflicted. Unfortunately, the majority of infected people remain undiagnosed. In many parts of the world, patients do not have access to medical personnel or diagnostic testing so that the diagnosis of HCV can be made. For patients that do have access, standard testing for HCV is cumbersome and expensive, adding to the diagnostic burden and diminishing the opportunity for diagnosis. Even in the United States, it is estimated that the majority of people infected with HCV have not been diagnosed. These factors have provided the impetus to develop less cumbersome and inexpensive tests for HCV, with the hope that they could be implemented throughout the world to increase the number of patients diagnosed with HCV.
Although there have been numerous studies examining individual diagnostic tests for HCV, there has not been an exhaustive review of both rapid and point-of-care screening tests for HCV. Ms. Shivkumar and her associates performed a literature review and meta-analysis of 18 studies worldwide that use the tests to screen for HCV in adults. They assessed the studies for diagnostic accuracy variables including sensitivity, specificity, likelihood rates (LRs), and diagnostic odds ratios (DORs) for available rapid and point-of-care diagnostic tests.
The assessment indicates that point-of-care tests of blood (serum, plasma, or whole blood) have the highest accuracy, followed by rapid diagnostic tests of serum or plasma and then by point-of-care diagnostic tests of oral fluids. However, all tests showed excellent sensitivity, specificity, likelihood rates, and diagnostic odds ratios.
Strategies to improve the identification of patients infected with HCV are changing. In fact, the Centers for Disease Control and Prevention recently updated screening guidelines, recommending that screening change from ineffective risk factor–based screening to age-based screening. Testing strategies are also a source of potential improvement. This comprehensive global review suggests that rapid diagnostic and point-of-care tests are useful as initial screening tests for HCV. Although potential biases inherent in retrospective meta-analysis reviews are discussed, the quality of the analysis is excellent and the conclusions are valid.
These diagnostic test results are available rapidly in the field, and thus are available at the point of care. The tests are also relatively inexpensive and are easy to perform. With more effective therapies for HCV in development, broadening the ability to diagnose HCV worldwide is increasingly important. Integration of these tests into the diagnostic algorithm for HCV offers a more effective screening strategy with subsequent diagnosis of more people infected with HCV worldwide.
Steven L. Flamm, M.D., is a professor of gastroenterology, hepatology, and surgery at Northwestern University, Chicago. He has no disclosures.
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-66).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5%-99.8% for whole blood and 96.8%-99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9%-99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3%-99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5%-99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9%-99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7%-98.4%) and specificity of 98.2% (95% CI, 92.2%-99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Rapid diagnostic and point-of-care tests for hepatitis C virus are accurate enough to be used in first-line HCV screening worldwide, according to results from the first systematic review of evidence on such tests.
Blood-based point-of-care tests (POCTs) had better sensitivity and specificity overall than did blood-based rapid diagnostic tests (RDTs), Sushmita Shivkumar of McGill University, Montreal, Quebec, and her colleagues reported.
POCTs do not require sample processing or refrigeration, and have a shelf life of 6 months or more. Rapid diagnostic tests, or RDTs, require refrigeration and sample processing. Manufactured by a variety of firms under various marketing names, all the tests can detect HCV infection in under 30 minutes, with many taking less than 5 minutes, according to the investigators, who conducted a meta-analysis of 18 studies evaluating the accuracy of one or more RDT or POCT, compared with standard assays (Ann. Intern. Med. 2012;157:558-66).
Ms. Shivkumar and her colleagues found blood-based POCTs (using serum, plasma, or whole blood) to be the most accurate of the tests evaluated in the included studies, showing 98.9% sensitivity with a 95% confidence interval [CI] of 94.5%-99.8% for whole blood and 96.8%-99.6% for serum or plasma). RDTs of serum or plasma had 98.4% sensitivity (95% CI 88.9%-99.8%). Specificity was highest in POCTs of serum or plasma at 99.7% (95% CI, 99.3%-99.9%), followed by POCTs of whole blood at 99.5% (95% CI, 97.5%-99.9%), RDTs of serum or plasma at 98.6% (95% CI, 94.9%-99.6%). POCTs for oral fluids had a sensitivity of 97.1% (95% CI, 94.7%-98.4%) and specificity of 98.2% (95% CI, 92.2%-99.6%).
POCTs and RDTs cannot distinguish between acute and chronic HCV infections, which is one of the reasons that public health organizations continue to favor conventional assays. The Food and Drug Administration currently approves the use of a single POCT for HCV, and for use only in nontraditional settings; in the United Kingdom and Canada such tests have not yet been approved.
However, by being fast, accurate, and cheaper than conventional tests, the rapid diagnostic and point-of-care tests offer "great potential for expanded first-line screening for hepatitis C infection and demonstrate the utility of blood-based singleton POCTs and of multiplex POCTs designed to provide integrated HIV and HCV screening of at-risk populations," Ms. Shivkumar and her associates wrote in their analysis. "Their rapid turnaround time limits loss to follow-up and facilitates early linkages."
The authors also concluded that their findings showed that the rapid and point-of-care tests could play a "substantial role" in expanded global screening initiatives for HCV.
The blood-based POCTs, besides being the most accurate, have the advantage of not requiring refrigeration, the investigators wrote. This feature is key in developing countries in Africa and Asia, where prevalence of HCV infection is highest.
Ms. Shivkumar and her colleagues noted several weaknesses in their meta-analysis, mostly related to the quality of studies included. Only three of the studies they evaluated were blinded, while four received industry funding; the conventional assays used as reference varied in the studies, and HCV genotype information was not collected in most.
The study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.
Major Finding: Blood-based point-of-care tests for hepatitis C virus have the highest sensitivity for screening at 98.9%, with specificity ranging from 99.5% for whole blood and 99.7% for serum or plasma.
Data Source: A meta-analysis of 18 studies that evaluated the accuracy of one or more tests.
Disclosures: Ms. Shivkumar and colleagues’ study was supported by grants from the Canadian Institutes of Health Research. None of the authors declared conflicts of interest.