User login
The U.S. Obesity Epidemic and Surging Liver Cancer
If there is one truism that trumps everything else these days about U.S. health, it’s that America is a chubby country that keeps getting fatter.
The consequences seep into every corner of the nation’s medical state, including the surprising fact that obesity and the type 2 diabetes it causes are likely pushing up the incidence of liver cancer – hepatocellular carcinoma – to unprecedented heights.
When I covered Digestive Disease Week in San Diego recently, one of the biggest stories I heard was that U.S. liver-cancer rates tripled from 1975-2007, and that the numbers continued to rise from the mid to the late 2000s. (My full report on this is here).
Granted, factors other than just obesity play into the liver cancer surge, notably the sizable number of Americans infected with either hepatitis B or C virus, and the fact that as they age their risk for developing hepatocellular carcinoma rises.
But new U.S. infections by hepatitis B and C are largely under control these days (although people infected elsewhere continue to emigrate to the United States). The part of the booming liver-cancer story that is by no means under control is the obesity part.
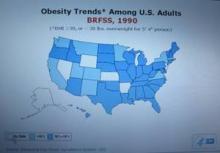
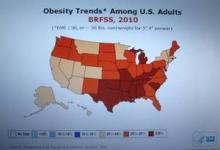
Every time I see a new CDC map for U.S. obesity prevalence, the colors on it keep getting redder and darker (the CDC’s code for higher prevalence rates).
Earlier this year, the CDC reported a 36% obesity prevalence rate for the entire U.S. population – and still on the rise – and just a few weeks ago we heard that obesity among children and adolescents had hit a new high of 17%. With obesity seemingly on an unchanging upward trajectory, one can only wonder what rates of liver cancer it might produce in the future. Obesity carries a special relationship with the liver, and it’s not pretty. Just consider any goose headed to a foie-gras future.
Until now, the evidence linking obesity and liver cancer, and type 2 diabetes and liver cancer has been epidemiologic. Compelling, but just an association. At DDW, a new study provided more observational data on the diabetes-liver cancer link, and while still circumstantial it further supports the notion and also carries an intriguing punchline.
The study, done in Taiwan, examined 97,000 hepatocellular carcinoma patients and 195,000 matched controls. The analysis showed that people with diabetes had a two-fold increased risk for liver cancer compared with those without diabetes. Even more striking, the analysis also showed that people with diabetes treated with the oral hypoglycemic drug metformin had their risk for liver cancer cut in half compared with those not on metformin, and those with diabetes treated with a glitazone drug (such as pioglitazone-Actos) had their risk cut nearly in half.
The best solution would be if people avoided obesity and type 2 diabetes all together. Both conditions cause a lot of medical problems, and this new evidence indicates more strongly than ever before that liver cancer is one of them.
— Mitchel Zoler (on Twitter @mitchelzoler)
If there is one truism that trumps everything else these days about U.S. health, it’s that America is a chubby country that keeps getting fatter.
The consequences seep into every corner of the nation’s medical state, including the surprising fact that obesity and the type 2 diabetes it causes are likely pushing up the incidence of liver cancer – hepatocellular carcinoma – to unprecedented heights.
When I covered Digestive Disease Week in San Diego recently, one of the biggest stories I heard was that U.S. liver-cancer rates tripled from 1975-2007, and that the numbers continued to rise from the mid to the late 2000s. (My full report on this is here).
Granted, factors other than just obesity play into the liver cancer surge, notably the sizable number of Americans infected with either hepatitis B or C virus, and the fact that as they age their risk for developing hepatocellular carcinoma rises.
But new U.S. infections by hepatitis B and C are largely under control these days (although people infected elsewhere continue to emigrate to the United States). The part of the booming liver-cancer story that is by no means under control is the obesity part.
Every time I see a new CDC map for U.S. obesity prevalence, the colors on it keep getting redder and darker (the CDC’s code for higher prevalence rates).
Earlier this year, the CDC reported a 36% obesity prevalence rate for the entire U.S. population – and still on the rise – and just a few weeks ago we heard that obesity among children and adolescents had hit a new high of 17%. With obesity seemingly on an unchanging upward trajectory, one can only wonder what rates of liver cancer it might produce in the future. Obesity carries a special relationship with the liver, and it’s not pretty. Just consider any goose headed to a foie-gras future.
Until now, the evidence linking obesity and liver cancer, and type 2 diabetes and liver cancer has been epidemiologic. Compelling, but just an association. At DDW, a new study provided more observational data on the diabetes-liver cancer link, and while still circumstantial it further supports the notion and also carries an intriguing punchline.
The study, done in Taiwan, examined 97,000 hepatocellular carcinoma patients and 195,000 matched controls. The analysis showed that people with diabetes had a two-fold increased risk for liver cancer compared with those without diabetes. Even more striking, the analysis also showed that people with diabetes treated with the oral hypoglycemic drug metformin had their risk for liver cancer cut in half compared with those not on metformin, and those with diabetes treated with a glitazone drug (such as pioglitazone-Actos) had their risk cut nearly in half.
The best solution would be if people avoided obesity and type 2 diabetes all together. Both conditions cause a lot of medical problems, and this new evidence indicates more strongly than ever before that liver cancer is one of them.
— Mitchel Zoler (on Twitter @mitchelzoler)
If there is one truism that trumps everything else these days about U.S. health, it’s that America is a chubby country that keeps getting fatter.
The consequences seep into every corner of the nation’s medical state, including the surprising fact that obesity and the type 2 diabetes it causes are likely pushing up the incidence of liver cancer – hepatocellular carcinoma – to unprecedented heights.
When I covered Digestive Disease Week in San Diego recently, one of the biggest stories I heard was that U.S. liver-cancer rates tripled from 1975-2007, and that the numbers continued to rise from the mid to the late 2000s. (My full report on this is here).
Granted, factors other than just obesity play into the liver cancer surge, notably the sizable number of Americans infected with either hepatitis B or C virus, and the fact that as they age their risk for developing hepatocellular carcinoma rises.
But new U.S. infections by hepatitis B and C are largely under control these days (although people infected elsewhere continue to emigrate to the United States). The part of the booming liver-cancer story that is by no means under control is the obesity part.
Every time I see a new CDC map for U.S. obesity prevalence, the colors on it keep getting redder and darker (the CDC’s code for higher prevalence rates).
Earlier this year, the CDC reported a 36% obesity prevalence rate for the entire U.S. population – and still on the rise – and just a few weeks ago we heard that obesity among children and adolescents had hit a new high of 17%. With obesity seemingly on an unchanging upward trajectory, one can only wonder what rates of liver cancer it might produce in the future. Obesity carries a special relationship with the liver, and it’s not pretty. Just consider any goose headed to a foie-gras future.
Until now, the evidence linking obesity and liver cancer, and type 2 diabetes and liver cancer has been epidemiologic. Compelling, but just an association. At DDW, a new study provided more observational data on the diabetes-liver cancer link, and while still circumstantial it further supports the notion and also carries an intriguing punchline.
The study, done in Taiwan, examined 97,000 hepatocellular carcinoma patients and 195,000 matched controls. The analysis showed that people with diabetes had a two-fold increased risk for liver cancer compared with those without diabetes. Even more striking, the analysis also showed that people with diabetes treated with the oral hypoglycemic drug metformin had their risk for liver cancer cut in half compared with those not on metformin, and those with diabetes treated with a glitazone drug (such as pioglitazone-Actos) had their risk cut nearly in half.
The best solution would be if people avoided obesity and type 2 diabetes all together. Both conditions cause a lot of medical problems, and this new evidence indicates more strongly than ever before that liver cancer is one of them.
— Mitchel Zoler (on Twitter @mitchelzoler)
Biosimilars and their use in hematology and oncology
The patent expiration of several biopharmaceuticals such as erythropoietin (erythropoiesis-stimulating agents, ESAs), granulocyte colony-stimulating factor (G-CSF, filgrastim) and others, has led to the emergence of biosimilar medicines. These are defined as copy versions of approved medicinal products with demonstrated similarity in physicochemical characteristics, efficacy, and safety based on a comprehensive comparability exercise. Strict guidelines for the development of biosimilars, ranging from preclinical to phase III trials and postmarketing studies, are already in place in Europe, and the United States Food and Drug Administration (FDA) recently issued draft guidance on biosimilars. A number of biosimilar ESAs and G-CSFs have been approved. Biosimilar monoclonal antibodies are an attractive target for development, with draft guidance from the European Medicines Agency currently under review. Biosimilar medicines may provide cost-effective alternatives to their branded counterparts, potentially benefitting public health by improving access to these medications. It is therefore important to raise awareness of these products among treating physicians. Furthermore, finalization of FDA guidance is important for the development of biosimilar medicines for the US market...
*For a PDF of the full article, click on the link to the left of this introduction.
The patent expiration of several biopharmaceuticals such as erythropoietin (erythropoiesis-stimulating agents, ESAs), granulocyte colony-stimulating factor (G-CSF, filgrastim) and others, has led to the emergence of biosimilar medicines. These are defined as copy versions of approved medicinal products with demonstrated similarity in physicochemical characteristics, efficacy, and safety based on a comprehensive comparability exercise. Strict guidelines for the development of biosimilars, ranging from preclinical to phase III trials and postmarketing studies, are already in place in Europe, and the United States Food and Drug Administration (FDA) recently issued draft guidance on biosimilars. A number of biosimilar ESAs and G-CSFs have been approved. Biosimilar monoclonal antibodies are an attractive target for development, with draft guidance from the European Medicines Agency currently under review. Biosimilar medicines may provide cost-effective alternatives to their branded counterparts, potentially benefitting public health by improving access to these medications. It is therefore important to raise awareness of these products among treating physicians. Furthermore, finalization of FDA guidance is important for the development of biosimilar medicines for the US market...
*For a PDF of the full article, click on the link to the left of this introduction.
The patent expiration of several biopharmaceuticals such as erythropoietin (erythropoiesis-stimulating agents, ESAs), granulocyte colony-stimulating factor (G-CSF, filgrastim) and others, has led to the emergence of biosimilar medicines. These are defined as copy versions of approved medicinal products with demonstrated similarity in physicochemical characteristics, efficacy, and safety based on a comprehensive comparability exercise. Strict guidelines for the development of biosimilars, ranging from preclinical to phase III trials and postmarketing studies, are already in place in Europe, and the United States Food and Drug Administration (FDA) recently issued draft guidance on biosimilars. A number of biosimilar ESAs and G-CSFs have been approved. Biosimilar monoclonal antibodies are an attractive target for development, with draft guidance from the European Medicines Agency currently under review. Biosimilar medicines may provide cost-effective alternatives to their branded counterparts, potentially benefitting public health by improving access to these medications. It is therefore important to raise awareness of these products among treating physicians. Furthermore, finalization of FDA guidance is important for the development of biosimilar medicines for the US market...
*For a PDF of the full article, click on the link to the left of this introduction.
Medullary thyroid cancer: advances in treatment and management of common adverse events associated with therapy
Thyroid cancer is the most common malignancy of the endocrine system. Medullary thyroid cancer (MTC), an intermediate differentiated histotype of thyroid cancer, accounts for approximately 4% of all thyroid cancer cases in the United States. MTC tumors are characterized by increased activation of the proto-oncogene RET, which encodes a receptor tyrosine kinase that promotes cell growth, differentiation, and survival. RET mutations are present in almost all patients with hereditary MTC and in up to 50% of patients with sporadic MTC. MTC tumors also are characterized by overexpression of vascular endothelial growth factor receptors. Until recently, systemic therapy options for MTC treatment were limited. However, based on promising efficacy demonstrated in other solid tumor types, many oral tyrosine kinase inhibitors are being investigated for the treatment of patients with MTC. Recently, vandetanib was approved in the United States for the treatment of patients with symptomatic or progressive MTC with locally advanced or metastatic disease. Common adverse events associated with tyrosine kinase inhibitors under investigation for MTC include diarrhea, rash, hypertension, and QTc prolongation.
*For a PDF of the full article, click on the link to the left of this introduction.
Thyroid cancer is the most common malignancy of the endocrine system. Medullary thyroid cancer (MTC), an intermediate differentiated histotype of thyroid cancer, accounts for approximately 4% of all thyroid cancer cases in the United States. MTC tumors are characterized by increased activation of the proto-oncogene RET, which encodes a receptor tyrosine kinase that promotes cell growth, differentiation, and survival. RET mutations are present in almost all patients with hereditary MTC and in up to 50% of patients with sporadic MTC. MTC tumors also are characterized by overexpression of vascular endothelial growth factor receptors. Until recently, systemic therapy options for MTC treatment were limited. However, based on promising efficacy demonstrated in other solid tumor types, many oral tyrosine kinase inhibitors are being investigated for the treatment of patients with MTC. Recently, vandetanib was approved in the United States for the treatment of patients with symptomatic or progressive MTC with locally advanced or metastatic disease. Common adverse events associated with tyrosine kinase inhibitors under investigation for MTC include diarrhea, rash, hypertension, and QTc prolongation.
*For a PDF of the full article, click on the link to the left of this introduction.
Thyroid cancer is the most common malignancy of the endocrine system. Medullary thyroid cancer (MTC), an intermediate differentiated histotype of thyroid cancer, accounts for approximately 4% of all thyroid cancer cases in the United States. MTC tumors are characterized by increased activation of the proto-oncogene RET, which encodes a receptor tyrosine kinase that promotes cell growth, differentiation, and survival. RET mutations are present in almost all patients with hereditary MTC and in up to 50% of patients with sporadic MTC. MTC tumors also are characterized by overexpression of vascular endothelial growth factor receptors. Until recently, systemic therapy options for MTC treatment were limited. However, based on promising efficacy demonstrated in other solid tumor types, many oral tyrosine kinase inhibitors are being investigated for the treatment of patients with MTC. Recently, vandetanib was approved in the United States for the treatment of patients with symptomatic or progressive MTC with locally advanced or metastatic disease. Common adverse events associated with tyrosine kinase inhibitors under investigation for MTC include diarrhea, rash, hypertension, and QTc prolongation.
*For a PDF of the full article, click on the link to the left of this introduction.
Cancer recurrence and survival in patients with early-stage triple-negative breast cancer
Background: Triple-negative breast cancer (TNBC) has fewer treatment options and is associated with a poor prognosis in the metastatic and adjuvant setting.
Objective: To evaluate the impact of triple-negative (TN) status on disease recurrence and survival among stage I-III patients who were treated with adjuvant chemotherapy in a community-based clinical practice setting.
Methods: Data were extracted from the 2003-2008 Georgia Cancer Specialist Database. Stage I-III breast cancer patients who received adjuvant chemotherapy were followed from initial diagnosis until death, recurrence, or loss to follow-up. The influence of TN status on disease-free survival (DFS) and recurrence was assessed.
Results: The study included 1,572 patients, of whom 26.3% had TNBC. The 5-year DFS was 76.8% for TNBC patients and 89.0% for non-TNBC patients (P less than .001); 5-year recurrence rates were 18.8% for TNBC and 11.2% for non-TNBC (P less than .001). The adjusted likelihood for DFS was lower for TNBC patients (hazard ratio [HR], 0.37; P less than .001), and risk for recurrence was higher (HR, 2.85; P less than .001) compared with non-TNBC patients. In the subpopulation with confirmed race, the comparable adjusted HRs were 0.27 and 4.70 (P less than .001, for both), respectively. African American race was an independent risk factor for worse outcome.
Limitations: Some potential confounding factors are not accounted for in this study, including accessibility to health care, differences in chemotherapy type, dose intensity, and socioeconomic status.
Conclusions: Patients with stage I-III TNBC had shorter DFS and higher recurrence risk, despite having received chemotherapy. The results emphasize the need for more effective treatments.
*To read the full article, click on the PDF icon at the top of this introduction.
Background: Triple-negative breast cancer (TNBC) has fewer treatment options and is associated with a poor prognosis in the metastatic and adjuvant setting.
Objective: To evaluate the impact of triple-negative (TN) status on disease recurrence and survival among stage I-III patients who were treated with adjuvant chemotherapy in a community-based clinical practice setting.
Methods: Data were extracted from the 2003-2008 Georgia Cancer Specialist Database. Stage I-III breast cancer patients who received adjuvant chemotherapy were followed from initial diagnosis until death, recurrence, or loss to follow-up. The influence of TN status on disease-free survival (DFS) and recurrence was assessed.
Results: The study included 1,572 patients, of whom 26.3% had TNBC. The 5-year DFS was 76.8% for TNBC patients and 89.0% for non-TNBC patients (P less than .001); 5-year recurrence rates were 18.8% for TNBC and 11.2% for non-TNBC (P less than .001). The adjusted likelihood for DFS was lower for TNBC patients (hazard ratio [HR], 0.37; P less than .001), and risk for recurrence was higher (HR, 2.85; P less than .001) compared with non-TNBC patients. In the subpopulation with confirmed race, the comparable adjusted HRs were 0.27 and 4.70 (P less than .001, for both), respectively. African American race was an independent risk factor for worse outcome.
Limitations: Some potential confounding factors are not accounted for in this study, including accessibility to health care, differences in chemotherapy type, dose intensity, and socioeconomic status.
Conclusions: Patients with stage I-III TNBC had shorter DFS and higher recurrence risk, despite having received chemotherapy. The results emphasize the need for more effective treatments.
*To read the full article, click on the PDF icon at the top of this introduction.
Background: Triple-negative breast cancer (TNBC) has fewer treatment options and is associated with a poor prognosis in the metastatic and adjuvant setting.
Objective: To evaluate the impact of triple-negative (TN) status on disease recurrence and survival among stage I-III patients who were treated with adjuvant chemotherapy in a community-based clinical practice setting.
Methods: Data were extracted from the 2003-2008 Georgia Cancer Specialist Database. Stage I-III breast cancer patients who received adjuvant chemotherapy were followed from initial diagnosis until death, recurrence, or loss to follow-up. The influence of TN status on disease-free survival (DFS) and recurrence was assessed.
Results: The study included 1,572 patients, of whom 26.3% had TNBC. The 5-year DFS was 76.8% for TNBC patients and 89.0% for non-TNBC patients (P less than .001); 5-year recurrence rates were 18.8% for TNBC and 11.2% for non-TNBC (P less than .001). The adjusted likelihood for DFS was lower for TNBC patients (hazard ratio [HR], 0.37; P less than .001), and risk for recurrence was higher (HR, 2.85; P less than .001) compared with non-TNBC patients. In the subpopulation with confirmed race, the comparable adjusted HRs were 0.27 and 4.70 (P less than .001, for both), respectively. African American race was an independent risk factor for worse outcome.
Limitations: Some potential confounding factors are not accounted for in this study, including accessibility to health care, differences in chemotherapy type, dose intensity, and socioeconomic status.
Conclusions: Patients with stage I-III TNBC had shorter DFS and higher recurrence risk, despite having received chemotherapy. The results emphasize the need for more effective treatments.
*To read the full article, click on the PDF icon at the top of this introduction.
Quality of Life Undiminished by Telaprevir in Chronic Hepatitis C
SAN DIEGO – Although the addition of telaprevir to peginterferon/ribavirin therapy for treatment of chronic hepatitis C exacerbates treatment-related side effects, the triple combination does not diminish patient quality of life relative to treatment with the peginterferon/ribavirin regimen alone, a study has shown.
In other words, adding the protease inhibitor "does not further diminish patient quality of life," lead investigator Dr. Zobair Younossi explained at the annual Digestive Disease Week. "The most important contributor to the quality of life measurement in interferon therapy is interferon itself, which is so overwhelming in terms of side effects, especially grade 4 and 5 effects, that it probably overshadows everything else," he said.
Studies have shown that the addition of telaprevir to standard peginterferon alfa-2a/ribavirin (PR) significantly improves treatment efficacy in treatment-naive patients with genotype 1 hepatitis C virus (HCV), but there is a perception that the additional side effect burden from adding telaprevir is prohibitive in some patients, said Dr. Younossi, chairman of the department of medicine at Inova Health System in Falls Church, Va.
Dr. Younossi and colleagues conducted post hoc analyses of data from the ADVANCE trial, in which adding telaprevir to the treatment mix significantly improved patients’ sustained virologic response compared with standard PR therapy.
In the ADVANCE study, 1,088 treatment naive HCV genotype 1 patients were assigned to one of three treatment arms: 48 weeks of standard PR therapy; 12 weeks of telaprevir plus 24 weeks PR; or 12 weeks of telaprevir plus 48 weeks of PR. Nearly 80% of patients in both telaprevir groups achieved sustained virologic response, compared with 46% of patients in the standard PR treatment group (N. Engl. J. Med. 2011;364:2405-16).
In terms of side effects, "across all phase III studies, the incidence of rash and anemia (which are the effects we’re talking about with the protease inhibitors) was 56% and 34%, respectively, among telaprevir-treated patients, and 36% and 17% in patients receiving standard treatment," Dr. Younossi said.
To assess whether and to what degree these increases played a role in patient quality of life, Dr. Younossi and colleagues analyzed the results of EQ-5D quality of life questionnaires completed at baseline and at weeks 4, 12, 24, 36, 48, and 72 by 722 patients. They derived a summary index by calculating the percentages of patients reporting problems for each of the five health-related quality of life dimensions measured (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression).
After adjustment for age and sex, the baseline mean index values for the EQ-5D were 0.92 for the telaprevir plus 24-week PR group, 0.90 for the telaprevir plus 48-week PR group, and 0.91 for the 48-week PR-only group. The percentages of patients reporting any problems in each of the five qualitative dimensions at baseline were 8.2% for mobility, 2.0% for self-care, 12.9% for usual activities, 25.7% for pain/discomfort, and 25.6% for anxiety/depression, he said.
Across all the treatment groups, the EQ-5D index scores worsened during the first 12 weeks of treatment initiation. Specifically, mean values were 0.80 for the pooled-telaprevir groups and 0.83 for the PR-only group, according to Dr. Younossi.
Also, the respective percentages of patients in the pooled-telaprevir and PR-only groups reporting any problems at week 12 were 56% and 50% for usual activities, 51% and 42% for anxiety/depression, and 60% and 63% for pain/discomfort, he said. Change from baseline in terms of reported impact on mobility and self-care were small and not reported.
At week 48, the corresponding mean EQ-5D values were 0.93 for the telaprevir plus 24-week PR group, 0.83 for the telaprevir plus 48-week PR group, and 0.84 for the PR-only group.
By week 72 the EQ-5D index values returned to baseline levels, Dr. Younossi said.
Adjusted for age and sex, the mean EQ-5D index at week 72 was higher among the patients achieving sustained virologic response (SVR) compared with those who did not, with respective values of 0.90 and 0.86. "The 4% difference is within the range of published values for the minimal clinically important difference for the EQ-5D," he said.
Furthermore, at week 72, there were fewer patients among those who experienced SVR and reported problems in each dimension, compared with those who did not experience SVR.
At week 72, after adjustment for the index at baseline, patient age, sex, race, advanced liver disease, self-reported comorbidities, and the number of adverse events during treatment, only SVR was a positive predictor of the EQ-5D index. "We saw that [SVR] was a statistically significant and meaningful predictor of health-related quality of life," he said.
The study findings are consistent with the published research on the impacts of interferon-based regimens on health-related quality of life in this patient population, "and support the value of shorter treatment duration and [SVR] from a patient-reported outcomes perspective," said Dr. Younossi.
"We certainly cannot say that adding telaprevir causes fewer side effects. It’s clear there are more side effects, but it appears that the most troublesome side effects are related to the interferon therapy," he explained. When considered in the context of the improved SVR, "the burden of the increased incidence of anemia and rash associated with telaprevir, of which few cases are severe, appears to be outweighed by the overall treatment response."
This study was sponsored by Vertex. Dr. Younossi disclosed relationships with Biolex, Vertex, Salix, GlaxoSmithKline, and Tibotec.
SAN DIEGO – Although the addition of telaprevir to peginterferon/ribavirin therapy for treatment of chronic hepatitis C exacerbates treatment-related side effects, the triple combination does not diminish patient quality of life relative to treatment with the peginterferon/ribavirin regimen alone, a study has shown.
In other words, adding the protease inhibitor "does not further diminish patient quality of life," lead investigator Dr. Zobair Younossi explained at the annual Digestive Disease Week. "The most important contributor to the quality of life measurement in interferon therapy is interferon itself, which is so overwhelming in terms of side effects, especially grade 4 and 5 effects, that it probably overshadows everything else," he said.
Studies have shown that the addition of telaprevir to standard peginterferon alfa-2a/ribavirin (PR) significantly improves treatment efficacy in treatment-naive patients with genotype 1 hepatitis C virus (HCV), but there is a perception that the additional side effect burden from adding telaprevir is prohibitive in some patients, said Dr. Younossi, chairman of the department of medicine at Inova Health System in Falls Church, Va.
Dr. Younossi and colleagues conducted post hoc analyses of data from the ADVANCE trial, in which adding telaprevir to the treatment mix significantly improved patients’ sustained virologic response compared with standard PR therapy.
In the ADVANCE study, 1,088 treatment naive HCV genotype 1 patients were assigned to one of three treatment arms: 48 weeks of standard PR therapy; 12 weeks of telaprevir plus 24 weeks PR; or 12 weeks of telaprevir plus 48 weeks of PR. Nearly 80% of patients in both telaprevir groups achieved sustained virologic response, compared with 46% of patients in the standard PR treatment group (N. Engl. J. Med. 2011;364:2405-16).
In terms of side effects, "across all phase III studies, the incidence of rash and anemia (which are the effects we’re talking about with the protease inhibitors) was 56% and 34%, respectively, among telaprevir-treated patients, and 36% and 17% in patients receiving standard treatment," Dr. Younossi said.
To assess whether and to what degree these increases played a role in patient quality of life, Dr. Younossi and colleagues analyzed the results of EQ-5D quality of life questionnaires completed at baseline and at weeks 4, 12, 24, 36, 48, and 72 by 722 patients. They derived a summary index by calculating the percentages of patients reporting problems for each of the five health-related quality of life dimensions measured (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression).
After adjustment for age and sex, the baseline mean index values for the EQ-5D were 0.92 for the telaprevir plus 24-week PR group, 0.90 for the telaprevir plus 48-week PR group, and 0.91 for the 48-week PR-only group. The percentages of patients reporting any problems in each of the five qualitative dimensions at baseline were 8.2% for mobility, 2.0% for self-care, 12.9% for usual activities, 25.7% for pain/discomfort, and 25.6% for anxiety/depression, he said.
Across all the treatment groups, the EQ-5D index scores worsened during the first 12 weeks of treatment initiation. Specifically, mean values were 0.80 for the pooled-telaprevir groups and 0.83 for the PR-only group, according to Dr. Younossi.
Also, the respective percentages of patients in the pooled-telaprevir and PR-only groups reporting any problems at week 12 were 56% and 50% for usual activities, 51% and 42% for anxiety/depression, and 60% and 63% for pain/discomfort, he said. Change from baseline in terms of reported impact on mobility and self-care were small and not reported.
At week 48, the corresponding mean EQ-5D values were 0.93 for the telaprevir plus 24-week PR group, 0.83 for the telaprevir plus 48-week PR group, and 0.84 for the PR-only group.
By week 72 the EQ-5D index values returned to baseline levels, Dr. Younossi said.
Adjusted for age and sex, the mean EQ-5D index at week 72 was higher among the patients achieving sustained virologic response (SVR) compared with those who did not, with respective values of 0.90 and 0.86. "The 4% difference is within the range of published values for the minimal clinically important difference for the EQ-5D," he said.
Furthermore, at week 72, there were fewer patients among those who experienced SVR and reported problems in each dimension, compared with those who did not experience SVR.
At week 72, after adjustment for the index at baseline, patient age, sex, race, advanced liver disease, self-reported comorbidities, and the number of adverse events during treatment, only SVR was a positive predictor of the EQ-5D index. "We saw that [SVR] was a statistically significant and meaningful predictor of health-related quality of life," he said.
The study findings are consistent with the published research on the impacts of interferon-based regimens on health-related quality of life in this patient population, "and support the value of shorter treatment duration and [SVR] from a patient-reported outcomes perspective," said Dr. Younossi.
"We certainly cannot say that adding telaprevir causes fewer side effects. It’s clear there are more side effects, but it appears that the most troublesome side effects are related to the interferon therapy," he explained. When considered in the context of the improved SVR, "the burden of the increased incidence of anemia and rash associated with telaprevir, of which few cases are severe, appears to be outweighed by the overall treatment response."
This study was sponsored by Vertex. Dr. Younossi disclosed relationships with Biolex, Vertex, Salix, GlaxoSmithKline, and Tibotec.
SAN DIEGO – Although the addition of telaprevir to peginterferon/ribavirin therapy for treatment of chronic hepatitis C exacerbates treatment-related side effects, the triple combination does not diminish patient quality of life relative to treatment with the peginterferon/ribavirin regimen alone, a study has shown.
In other words, adding the protease inhibitor "does not further diminish patient quality of life," lead investigator Dr. Zobair Younossi explained at the annual Digestive Disease Week. "The most important contributor to the quality of life measurement in interferon therapy is interferon itself, which is so overwhelming in terms of side effects, especially grade 4 and 5 effects, that it probably overshadows everything else," he said.
Studies have shown that the addition of telaprevir to standard peginterferon alfa-2a/ribavirin (PR) significantly improves treatment efficacy in treatment-naive patients with genotype 1 hepatitis C virus (HCV), but there is a perception that the additional side effect burden from adding telaprevir is prohibitive in some patients, said Dr. Younossi, chairman of the department of medicine at Inova Health System in Falls Church, Va.
Dr. Younossi and colleagues conducted post hoc analyses of data from the ADVANCE trial, in which adding telaprevir to the treatment mix significantly improved patients’ sustained virologic response compared with standard PR therapy.
In the ADVANCE study, 1,088 treatment naive HCV genotype 1 patients were assigned to one of three treatment arms: 48 weeks of standard PR therapy; 12 weeks of telaprevir plus 24 weeks PR; or 12 weeks of telaprevir plus 48 weeks of PR. Nearly 80% of patients in both telaprevir groups achieved sustained virologic response, compared with 46% of patients in the standard PR treatment group (N. Engl. J. Med. 2011;364:2405-16).
In terms of side effects, "across all phase III studies, the incidence of rash and anemia (which are the effects we’re talking about with the protease inhibitors) was 56% and 34%, respectively, among telaprevir-treated patients, and 36% and 17% in patients receiving standard treatment," Dr. Younossi said.
To assess whether and to what degree these increases played a role in patient quality of life, Dr. Younossi and colleagues analyzed the results of EQ-5D quality of life questionnaires completed at baseline and at weeks 4, 12, 24, 36, 48, and 72 by 722 patients. They derived a summary index by calculating the percentages of patients reporting problems for each of the five health-related quality of life dimensions measured (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression).
After adjustment for age and sex, the baseline mean index values for the EQ-5D were 0.92 for the telaprevir plus 24-week PR group, 0.90 for the telaprevir plus 48-week PR group, and 0.91 for the 48-week PR-only group. The percentages of patients reporting any problems in each of the five qualitative dimensions at baseline were 8.2% for mobility, 2.0% for self-care, 12.9% for usual activities, 25.7% for pain/discomfort, and 25.6% for anxiety/depression, he said.
Across all the treatment groups, the EQ-5D index scores worsened during the first 12 weeks of treatment initiation. Specifically, mean values were 0.80 for the pooled-telaprevir groups and 0.83 for the PR-only group, according to Dr. Younossi.
Also, the respective percentages of patients in the pooled-telaprevir and PR-only groups reporting any problems at week 12 were 56% and 50% for usual activities, 51% and 42% for anxiety/depression, and 60% and 63% for pain/discomfort, he said. Change from baseline in terms of reported impact on mobility and self-care were small and not reported.
At week 48, the corresponding mean EQ-5D values were 0.93 for the telaprevir plus 24-week PR group, 0.83 for the telaprevir plus 48-week PR group, and 0.84 for the PR-only group.
By week 72 the EQ-5D index values returned to baseline levels, Dr. Younossi said.
Adjusted for age and sex, the mean EQ-5D index at week 72 was higher among the patients achieving sustained virologic response (SVR) compared with those who did not, with respective values of 0.90 and 0.86. "The 4% difference is within the range of published values for the minimal clinically important difference for the EQ-5D," he said.
Furthermore, at week 72, there were fewer patients among those who experienced SVR and reported problems in each dimension, compared with those who did not experience SVR.
At week 72, after adjustment for the index at baseline, patient age, sex, race, advanced liver disease, self-reported comorbidities, and the number of adverse events during treatment, only SVR was a positive predictor of the EQ-5D index. "We saw that [SVR] was a statistically significant and meaningful predictor of health-related quality of life," he said.
The study findings are consistent with the published research on the impacts of interferon-based regimens on health-related quality of life in this patient population, "and support the value of shorter treatment duration and [SVR] from a patient-reported outcomes perspective," said Dr. Younossi.
"We certainly cannot say that adding telaprevir causes fewer side effects. It’s clear there are more side effects, but it appears that the most troublesome side effects are related to the interferon therapy," he explained. When considered in the context of the improved SVR, "the burden of the increased incidence of anemia and rash associated with telaprevir, of which few cases are severe, appears to be outweighed by the overall treatment response."
This study was sponsored by Vertex. Dr. Younossi disclosed relationships with Biolex, Vertex, Salix, GlaxoSmithKline, and Tibotec.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: After adjustment for age and sex in treatment-naive chronic HCV patients, the baseline mean index values for health-related quality of life on the EQ-5D were 0.92 for the telaprevir plus 24-week PR group, 0.90 for the telaprevir plus 48-week PR group, and 0.91 for the 48-week PR-only group. At treatment week 12, mean values dropped to 0.80 for the pooled telaprevir groups and to 0.83 for the PR-only group, and then rebounded to baseline levels at 72 weeks, indicating that the addition of telaprevir to PR did not further impair quality of life.
Data Source: Findings are based on post hoc analyses of the ADVANCE randomized controlled trial comparing telaprevir plus PR vs. PR alone in chronic HCV.
Disclosures: This study was sponsored by Vertex. Dr. Younossi disclosed relationships with Biolex, Vertex, Salix, GlaxoSmithKline, and Tibotec.
Liver Cancer Rates Continue to Rise, Vigilance Warranted
SAN DIEGO – The U.S. incidence of hepatocellular carcinoma continues to soar, and will likely remain on that trajectory for at least a couple of decades, fed in large part by the obesity and type 2 diabetes epidemics, as well as by infections with hepatitis virus types C and B.
"I think rates will increase for another 10-20 years," predicted Dr. Alita Mishra, one of two researchers who reported results at the meeting from independent studies that documented increased rates of U.S. hepatocellular carcinoma (HCC) cases during the 2000s.
Greater vigilance is therefore needed to spot incident cases early, she said in an interview. While patients infected with hepatitis C virus who develop cirrhosis usually undergo routine, serial ultrasound screening for liver lesions, regular surveillance occurs less often in patients with cirrhosis who are infected with hepatitis B virus, or those with cirrhosis due to non-alcoholic fatty liver disease (NAFLD) secondary to obesity or type two diabetes. "Patients with cirrhosis should undergo regular HCC screening regardless of the underlying cause," Dr. Mishra said at the annual Digestive Disease Week.
One analysis, based on data collected by the Surveillance Epidemiology and End Results (SEER) registry of the National Cancer Institute, showed that U.S. HCC rates rose three-fold from 1975-2007, including a 33% rise during 1998-2007, Jessica A. Davila, Ph.D. reported at the meeting.
The second analysis, using data collected by the Nationwide Inpatient Sample (NIS), showed that the number of patients hospitalized with HCC per 100,000 hospital discharges jumped from 148 in 2005 to 213, said Dr. Mishra, a hospitalist at Inova Farifax (Va.) Hospital.
"HCC is rising because of hepatitis C viral infection, especially in people born during 1945-1965," Dr. Mishra said in an interview. Many of these people don’t know they are infected, and it usually takes decades for them to develop HCC.
The second big factor is the rising prevalence of obesity and type 2 diabetes. "Hepatitis C infections are now falling, so perhaps the rise in new HCC cases will eventually peak, but not if other factors like obesity and type 2 diabetes continue to push it up," she said.
"What is driving a lot of the increase is hepatitis C virus, and the high prevalence of hepatitis B virus in foreign-born Asians," said Dr. Davila, a clinical epidemiologist at the Houston VA Medical Center and Baylor College of Medicine in Houston.
"A lot also has to do with obesity and type 2 diabetes and their association with non-alcoholic fatty liver disease, especially in middle-aged, Hispanic women. I think we’ll see the greatest increase in HCC in women during the next 2 decades," Dr. Davila said. She also predicted increasing numbers of hepatitis C virus-driven HCC cases in the short term "as the [infected] cohort ages, increasing numbers will develop advanced fibrosis and eventually HCC," she said.
Dr. Davila’s study used data from SEER, which the National Cancer Institute began in 1973 to collect data on cancer cases from about 14% of the U.S. population in selected states and metropolitan areas. During 1975-2007, SEER tallied a total of 21,472 HCC cases, about 80% of which occurred in people aged 50-79 years, and about three-quarters of cases in men.
HCC incidence rose from 1.6 cases per 100,000 people during 1975-1977 to 4.8 per 100,000 in 2005-2007. Roughly a tripling of cases during the three decades occurred in both men and in women. The greatest increase occurred among people aged 50-59 years, which jumped nearly fivefold, from 2.6 per 100,000 in 1975-1977 to 12.6 per 100,000 in 2005-2007. The smallest rise was 2.4-fold among people aged 70-79 years.
By 2005-2007, the highest rate was among Asians, 10.3 per 100,000, followed by 8.2 per 100,000 in Hispanics, 7.5 per 100,000 in blacks, and 3.7/100,000 in whites (see table).
Dr. Mishra’s study used data collected in NIS by the federal Agency for Healthcare Research and Quality from about 1,000 hospitals in 44 states. The number of patients hospitalized with HCC (not confined to incident cases) rose from 9,537 in 2005 to 13,689 in 2009. During the 5-year period, in-hospital mortality of HCC cases dropped from 120 per 1,000 cases in 2005 to 95 per 1,000 cases in 2009, and the median length of stay fell by about 0.5 days.
Despite reduced hospitalized time, median hospital charges for each HCC hospitalized case rose from about $21,000 in 2005 to nearly $29,000 in 2009. Paralleling this increase was an uptick in the percent of cases having "major" or "extreme" illness, from 52% in 2005 to 63% in 2009, and the average number of comorbidities also rose steadily during the 5 years studied.
Hospitalized patients with HCC "are getting sicker, more complicated, and have more comorbidities," Dr. Mishra said. She also noted that the rate of liver transplants remained "very low" during the period studied.
Dr. Davila and Dr. Mishra reported having no conflicts of interest.
SAN DIEGO – The U.S. incidence of hepatocellular carcinoma continues to soar, and will likely remain on that trajectory for at least a couple of decades, fed in large part by the obesity and type 2 diabetes epidemics, as well as by infections with hepatitis virus types C and B.
"I think rates will increase for another 10-20 years," predicted Dr. Alita Mishra, one of two researchers who reported results at the meeting from independent studies that documented increased rates of U.S. hepatocellular carcinoma (HCC) cases during the 2000s.
Greater vigilance is therefore needed to spot incident cases early, she said in an interview. While patients infected with hepatitis C virus who develop cirrhosis usually undergo routine, serial ultrasound screening for liver lesions, regular surveillance occurs less often in patients with cirrhosis who are infected with hepatitis B virus, or those with cirrhosis due to non-alcoholic fatty liver disease (NAFLD) secondary to obesity or type two diabetes. "Patients with cirrhosis should undergo regular HCC screening regardless of the underlying cause," Dr. Mishra said at the annual Digestive Disease Week.
One analysis, based on data collected by the Surveillance Epidemiology and End Results (SEER) registry of the National Cancer Institute, showed that U.S. HCC rates rose three-fold from 1975-2007, including a 33% rise during 1998-2007, Jessica A. Davila, Ph.D. reported at the meeting.
The second analysis, using data collected by the Nationwide Inpatient Sample (NIS), showed that the number of patients hospitalized with HCC per 100,000 hospital discharges jumped from 148 in 2005 to 213, said Dr. Mishra, a hospitalist at Inova Farifax (Va.) Hospital.
"HCC is rising because of hepatitis C viral infection, especially in people born during 1945-1965," Dr. Mishra said in an interview. Many of these people don’t know they are infected, and it usually takes decades for them to develop HCC.
The second big factor is the rising prevalence of obesity and type 2 diabetes. "Hepatitis C infections are now falling, so perhaps the rise in new HCC cases will eventually peak, but not if other factors like obesity and type 2 diabetes continue to push it up," she said.
"What is driving a lot of the increase is hepatitis C virus, and the high prevalence of hepatitis B virus in foreign-born Asians," said Dr. Davila, a clinical epidemiologist at the Houston VA Medical Center and Baylor College of Medicine in Houston.
"A lot also has to do with obesity and type 2 diabetes and their association with non-alcoholic fatty liver disease, especially in middle-aged, Hispanic women. I think we’ll see the greatest increase in HCC in women during the next 2 decades," Dr. Davila said. She also predicted increasing numbers of hepatitis C virus-driven HCC cases in the short term "as the [infected] cohort ages, increasing numbers will develop advanced fibrosis and eventually HCC," she said.
Dr. Davila’s study used data from SEER, which the National Cancer Institute began in 1973 to collect data on cancer cases from about 14% of the U.S. population in selected states and metropolitan areas. During 1975-2007, SEER tallied a total of 21,472 HCC cases, about 80% of which occurred in people aged 50-79 years, and about three-quarters of cases in men.
HCC incidence rose from 1.6 cases per 100,000 people during 1975-1977 to 4.8 per 100,000 in 2005-2007. Roughly a tripling of cases during the three decades occurred in both men and in women. The greatest increase occurred among people aged 50-59 years, which jumped nearly fivefold, from 2.6 per 100,000 in 1975-1977 to 12.6 per 100,000 in 2005-2007. The smallest rise was 2.4-fold among people aged 70-79 years.
By 2005-2007, the highest rate was among Asians, 10.3 per 100,000, followed by 8.2 per 100,000 in Hispanics, 7.5 per 100,000 in blacks, and 3.7/100,000 in whites (see table).
Dr. Mishra’s study used data collected in NIS by the federal Agency for Healthcare Research and Quality from about 1,000 hospitals in 44 states. The number of patients hospitalized with HCC (not confined to incident cases) rose from 9,537 in 2005 to 13,689 in 2009. During the 5-year period, in-hospital mortality of HCC cases dropped from 120 per 1,000 cases in 2005 to 95 per 1,000 cases in 2009, and the median length of stay fell by about 0.5 days.
Despite reduced hospitalized time, median hospital charges for each HCC hospitalized case rose from about $21,000 in 2005 to nearly $29,000 in 2009. Paralleling this increase was an uptick in the percent of cases having "major" or "extreme" illness, from 52% in 2005 to 63% in 2009, and the average number of comorbidities also rose steadily during the 5 years studied.
Hospitalized patients with HCC "are getting sicker, more complicated, and have more comorbidities," Dr. Mishra said. She also noted that the rate of liver transplants remained "very low" during the period studied.
Dr. Davila and Dr. Mishra reported having no conflicts of interest.
SAN DIEGO – The U.S. incidence of hepatocellular carcinoma continues to soar, and will likely remain on that trajectory for at least a couple of decades, fed in large part by the obesity and type 2 diabetes epidemics, as well as by infections with hepatitis virus types C and B.
"I think rates will increase for another 10-20 years," predicted Dr. Alita Mishra, one of two researchers who reported results at the meeting from independent studies that documented increased rates of U.S. hepatocellular carcinoma (HCC) cases during the 2000s.
Greater vigilance is therefore needed to spot incident cases early, she said in an interview. While patients infected with hepatitis C virus who develop cirrhosis usually undergo routine, serial ultrasound screening for liver lesions, regular surveillance occurs less often in patients with cirrhosis who are infected with hepatitis B virus, or those with cirrhosis due to non-alcoholic fatty liver disease (NAFLD) secondary to obesity or type two diabetes. "Patients with cirrhosis should undergo regular HCC screening regardless of the underlying cause," Dr. Mishra said at the annual Digestive Disease Week.
One analysis, based on data collected by the Surveillance Epidemiology and End Results (SEER) registry of the National Cancer Institute, showed that U.S. HCC rates rose three-fold from 1975-2007, including a 33% rise during 1998-2007, Jessica A. Davila, Ph.D. reported at the meeting.
The second analysis, using data collected by the Nationwide Inpatient Sample (NIS), showed that the number of patients hospitalized with HCC per 100,000 hospital discharges jumped from 148 in 2005 to 213, said Dr. Mishra, a hospitalist at Inova Farifax (Va.) Hospital.
"HCC is rising because of hepatitis C viral infection, especially in people born during 1945-1965," Dr. Mishra said in an interview. Many of these people don’t know they are infected, and it usually takes decades for them to develop HCC.
The second big factor is the rising prevalence of obesity and type 2 diabetes. "Hepatitis C infections are now falling, so perhaps the rise in new HCC cases will eventually peak, but not if other factors like obesity and type 2 diabetes continue to push it up," she said.
"What is driving a lot of the increase is hepatitis C virus, and the high prevalence of hepatitis B virus in foreign-born Asians," said Dr. Davila, a clinical epidemiologist at the Houston VA Medical Center and Baylor College of Medicine in Houston.
"A lot also has to do with obesity and type 2 diabetes and their association with non-alcoholic fatty liver disease, especially in middle-aged, Hispanic women. I think we’ll see the greatest increase in HCC in women during the next 2 decades," Dr. Davila said. She also predicted increasing numbers of hepatitis C virus-driven HCC cases in the short term "as the [infected] cohort ages, increasing numbers will develop advanced fibrosis and eventually HCC," she said.
Dr. Davila’s study used data from SEER, which the National Cancer Institute began in 1973 to collect data on cancer cases from about 14% of the U.S. population in selected states and metropolitan areas. During 1975-2007, SEER tallied a total of 21,472 HCC cases, about 80% of which occurred in people aged 50-79 years, and about three-quarters of cases in men.
HCC incidence rose from 1.6 cases per 100,000 people during 1975-1977 to 4.8 per 100,000 in 2005-2007. Roughly a tripling of cases during the three decades occurred in both men and in women. The greatest increase occurred among people aged 50-59 years, which jumped nearly fivefold, from 2.6 per 100,000 in 1975-1977 to 12.6 per 100,000 in 2005-2007. The smallest rise was 2.4-fold among people aged 70-79 years.
By 2005-2007, the highest rate was among Asians, 10.3 per 100,000, followed by 8.2 per 100,000 in Hispanics, 7.5 per 100,000 in blacks, and 3.7/100,000 in whites (see table).
Dr. Mishra’s study used data collected in NIS by the federal Agency for Healthcare Research and Quality from about 1,000 hospitals in 44 states. The number of patients hospitalized with HCC (not confined to incident cases) rose from 9,537 in 2005 to 13,689 in 2009. During the 5-year period, in-hospital mortality of HCC cases dropped from 120 per 1,000 cases in 2005 to 95 per 1,000 cases in 2009, and the median length of stay fell by about 0.5 days.
Despite reduced hospitalized time, median hospital charges for each HCC hospitalized case rose from about $21,000 in 2005 to nearly $29,000 in 2009. Paralleling this increase was an uptick in the percent of cases having "major" or "extreme" illness, from 52% in 2005 to 63% in 2009, and the average number of comorbidities also rose steadily during the 5 years studied.
Hospitalized patients with HCC "are getting sicker, more complicated, and have more comorbidities," Dr. Mishra said. She also noted that the rate of liver transplants remained "very low" during the period studied.
Dr. Davila and Dr. Mishra reported having no conflicts of interest.
FROM THE ANNUAL DIGESTIVE DISEASE WEEK
Major Finding: U.S. hepatocellular carcinoma cases rose threefold during 1975-2007, while hospitalized cases rose by more than a third during 2005-2009.
Data Source: Data came from a review of U.S. HCC cases collected by the SEER registry during 1975-2007, and from a review of hospitalized U.S. HCC cases collected in the NIS registry during 2005-2009.
Disclosures: Dr. Davila and Dr. Mishra reported having no conflicts of interest.
A Special Supplement on Men's Health
Since chronic diseases are largely self-managed, the family physician must work collaboratively with each patient to individualize therapy. One key consideration in individualizing therapy is patient gender, since men and women often manifest, deal with, and manage diseases differently. This supplement highlights these gender-related differences by focusing on the management of 6 diseases in men, including benign prostatic hyperplasia, gout, diabetes, acute coronary syndrome, coronary heart disease, and dyslipidemia.
Since chronic diseases are largely self-managed, the family physician must work collaboratively with each patient to individualize therapy. One key consideration in individualizing therapy is patient gender, since men and women often manifest, deal with, and manage diseases differently. This supplement highlights these gender-related differences by focusing on the management of 6 diseases in men, including benign prostatic hyperplasia, gout, diabetes, acute coronary syndrome, coronary heart disease, and dyslipidemia.
Since chronic diseases are largely self-managed, the family physician must work collaboratively with each patient to individualize therapy. One key consideration in individualizing therapy is patient gender, since men and women often manifest, deal with, and manage diseases differently. This supplement highlights these gender-related differences by focusing on the management of 6 diseases in men, including benign prostatic hyperplasia, gout, diabetes, acute coronary syndrome, coronary heart disease, and dyslipidemia.
The Treatment of Gout
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Ruoff has disclosed that he is on the speakers’ bureau for and has received research grants from Takeda Pharmaceuticals.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from URL Pharma, Inc.
DB is a 50-year-old obese male visiting the clinic for symptoms suggestive of allergic rhinitis. The nurse has informed the family physician that DB was limping from the waiting room to the examination room. DB reports that he has been experiencing pain in his left big toe and ankle over the past few days. The last time this happened, the pain resolved within 7 to 10 days.
DB reports that he has experienced 4 or 5 similar episodes over the past 3 years. The first attacks affected his left big toe, but he now also experiences some pain in his left ankle. The pain is moderate, peaks in 1 to 2 days, and resolves within 7 to 10 days. Acetaminophen provided little pain relief so DB now takes ibuprofen 400 mg 3 times daily, as it “helps take the edge off.” Other medications include aspirin 81 mg per day and an oral antihistamine as needed for hay fever. DB reports that he eats seafood 2 to 3 times per week and red meat 1 to 2 times per week; he drinks 2 six-packs of beer per week.
Physical examination: weight, 186 lb (body mass index [BMI], 27 kg/m2); blood pressure, 126/76 mm Hg; and temperature, 98.8°F. His left big toe and ankle are red, slightly swollen, and warm with a small subcutaneous nodule noted on the first metatarsophalangeal joint. There is no sign of skin or joint infection.
The impression from his history and physical exam is that DB is suffering from an acute attack of gout, but the family physician also considers other diagnoses.
Background
Gout is a heterogeneous disorder that peaks in incidence in the fifth decade. Gout is caused by hyperuricemia, generally as a result of reduced excretion of uric acid by the kidneys; hyperuricemia may also result from overproduction of uric acid. Data from the National Health and Nutrition Examination Survey (NHANES) 2007-2008 indicate that the prevalence of gout continues to rise in the United States, likely related to the increasing frequency of adiposity and hypertension. Overall, about 75% of the 8.3 million people with gout are men.1
Risk Factors
Clinically defined hyperuricemia—a serum urate (sUA) level greater than 6.8 mg/dL, the concentration at which urate exceeds its solubility in most biological fluids—is the major risk factor for gout. However, not all persons with hyperuricemia have gout. Data from NHANES 2007-2008, in which the definition of hyperuricemia was an sUA level greater than 7.0 mg/dL for men and greater than 5.7 mg/dL for women, showed the mean sUA level to be 6.1 mg/dL in men and 4.9 mg/dL in women, corresponding to hyperuricemia prevalences of 21.2% and 21.6%, respectively.1
There are several other risk factors for gout, including hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease (CVD), and metabolic syndrome.2 For a man with hypertension, the relative risk (RR) of gout is 2.3 compared with a normotensive man.3 Furthermore, it is well established that the use of diuretics increases the risk of gout (RR, 1.8).3 Several other medications increase sUA level as well: aspirin (including low-dose), cyclosporine, pyrazinamide, ethambutol, and niacin.2
Lifestyle and diet also pose a risk for gout. The risk of gout increases with BMI such that, compared with a man with a BMI of 21 to 22.9 kg/m2, the RR of gout is doubled for a man with a BMI of 25 to 29.9 kg/m2; for a man with a BMI of 35 kg/m2 or more, the RR is tripled.3 Sugar-sweetened soft drinks (but not diet soft drinks) and fructose-rich fruits and fruit juices also increase the risk of gout, as do a high alcohol intake, particularly beer, and a diet rich in meat (especially organ meat, turkey, or wild game) or seafood.4 A moderate intake of purine-rich vegetables (eg, peas, beans, lentils, spinach, mushrooms, oatmeal, and cauliflower) or protein is not associated with an increased risk of gout, while a high consumption of dairy products is associated with a decreased risk.5,6
Untreated or poorly treated gout usually leads to further acute attacks and progressive joint and tissue damage. In addition, gout and hyperuricemia serve as risk factors for other diseases. Adults with gout are 3 times as likely to develop metabolic syndrome as adults without gout.7 An elevated sUA level is also an independent risk factor for the development of hypertension (RR, 1.1), as well as myocardial infarction (MI; RR, 1.9), and stroke (RR, 1.6).8,9 An increasing sUA level also increases the risk of renal failure.10,11 In a study of 49,413 men followed for a mean of 5.4 years, the age-adjusted RR of renal failure was 1.5 in men with an sUA level of 6.5 to 8.4 mg/dL and 8.5 in men with an sUA level of 8.5 to 13.0 mg/dL compared with men with an sUA level of 5.0 to 6.4 mg/dL.11
Clinical Presentation
The deposition of monosodium urate (MSU) crystals in joints and tissues is very common and typically causes no signs or symptoms in the majority of persons. Even in men with an sUA level of 9 mg/dL or greater, the cumulative incidence of gouty arthritis has been found to be 22% over 5 years.12 However, as crystal deposition progresses, acute, painful attacks occur more frequently, with the development of chronic tophaceous gout after several years.13
Laboratory results for DB:
- Serum uric acid, 7.9 mg/dL
- White blood cell count, 15,800/mm3
- Serum creatinine, 1.2 mg/dL (estimated creatinine clearance, 90 mL/min)
- Erythrocyte sedimentation rate, 23 mm/h
- Low-density lipoprotein cholesterol (nonfasting), 127 mg/dL
Laboratory confirmation of hyperuricemia together with the pain, swelling, and tenderness of DB’s toe and ankle, other findings from his medical history and physical exam (eg, the use of aspirin daily), and exclusion of alternative diagnoses, such as septic arthritis, enable the family physician to arrive at a presumptive diagnosis of gouty arthritis. Aspiration of MSU crystals from DB’s toe or ankle is the gold standard and would allow for a definitive diagnosis. Although the sUA level was found to be high, it should be noted that a normal sUA level is often found during an acute attack; should this occur, the sUA level should be checked again 1 to 2 weeks after the acute attack has resolved.
Goals of Treatment
The cornerstone of gout management is daily, long-term treatment with urate-lowering therapy (ULT) combined with as-needed treatment for an acute attack. In addition, since initiation of ULT mobilizes MSU crystals, which often leads to a short-term increase in acute attacks, prophylaxis with an appropriate anti-inflammatory therapy is recommended at the time ULT is initiated.14
The therapeutic goals of gout treatment are 2-pronged: treatment of an acute gout attack and management of chronic gout. For an acute attack, the goals are to exclude a diagnosis of septic arthritis; reduce inflammation and terminate the attack; and seek, assess, and control associated diseases, such as diabetes mellitus, hypertension, hyperlipidemia, and CVD. If this latter goal is not possible during the acute attack, plans should be made to assess associated diseases once the acute attack has resolved.14 Lowering the sUA level is not a goal of therapy for an acute attack, but it is the primary goal of ULT for chronic gout. Lowering the sUA level to less than 6.0 mg/dL, which is well below the saturation point of urate in most biological fluids, is intended to prevent further acute attacks, tophus formation, and tissue damage.14
Treatment of an Acute Attack
The mainstay of treatment for an acute attack is anti-inflammatory therapy to reduce pain and inflammation.14 Therapy should be initiated at the onset of the attack and continued until the attack is terminated, which is typically 1 to 2 weeks. Anti-inflammatory therapy traditionally has in-cluded colchicine, a nonsteroidal anti-inflammatory drug (NSAID), or a corticosteroid.14
Nonsteroidal Anti-inflammatory Drugs
The NSAIDs are all thought to provide similar efficacy when used in maximum doses.15,16 Since gastrointestinal toxicity is a concern with NSAIDs, coadministration of a proton pump inhibitor, H2 antagonist, or misoprostol is advised for patients with an increased risk of peptic ulcers, bleeds, or perforations.17 The risk of MI, stroke, cardiovascular death, and atrial fibrillation/flutter with NSAID therapy should be considered, especially because gout often coexists with cardiovascular disorders.15,18,19 Furthermore, NSAIDs are contraindicated in patients with heart failure or renal insufficiency.20,21
Corticosteroids. A systematic review of clinical trials involving systemic corticosteroids that found a few prospective trials of low to moderate quality concluded that there was inconclusive evidence for the efficacy and effectiveness of corticosteroids in the treatment of acute gout.22 No serious adverse events (AEs) were reported. A more recent prospective trial found comparable pain reduction and incidence of AEs with naproxen 500 mg twice daily and prednisolone 35 mg once daily for 5 days in patients with monoarticular gout.23 Furthermore, clinical experience indicates that intra-articular aspiration and injection of a long-acting corticosteroid is an effective and safe treatment for an acute attack.14,15 Corticosteroids may be useful in patients who have an inadequate response to, are intolerant of, or have a contraindication to NSAIDs and colchicine.14,15
Colchicine. Much of the recent clinical investigation regarding pharmacologic treatment of an acute gout attack has involved colchicine. To overcome the limitations of the standard dose-to-toxicity regimen of colchicine, a low-dose regimen of colchicine (1.2 mg followed by 0.6 mg 1 hour later) was investigated and subsequently approved by the US Food and Drug Administration (FDA).24
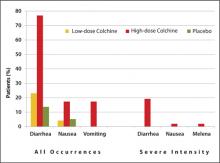
Approval was based on a randomized, double-blind comparison with high-dose colchicine (1.2 mg followed by 0.6 mg every hour for 6 hours) and placebo in 184 patients with an acute gout attack.25 The primary endpoint, a 50% or greater reduction in pain at 24 hours without the use of rescue medication, was reached in 28 of 74 patients (38%) in the low-dose group, 17 of 52 patients (33%) in the high-dose group, and 9 of 58 patients (16%) in the placebo group (P = .005 and P = .034, respectively, versus placebo). An AE occurred in 36.5% and 76.9% of study participants in the low-dose and high-dose colchicine groups, respectively, and in 27.1% of participants in the placebo group. Gastrointestinal AEs (eg, diarrhea, nausea, and vomiting) were less common in the low-dose colchicine group ( FIGURE ). All AEs in the low-dose group were mild to moderate in intensity, while 10 of 52 patients (19.2%) in the high-dose group had an AE of severe intensity. Concomitant use of numerous drugs can increase the concentration of colchicine. Examples include atorvastatin, fluvastatin, pravastatin, simvastatin, fibrates, gemfibrozil, digoxin, clarithromycin, erythromycin, fluconazole, itraconazole, ketoconazole, protease inhibitors, diltiazem, verapamil, and cyclosporine, as well as grapefruit juice.26
FIGURE
Frequency of selected adverse events occurring over 24 hours with low-dose vs high-dose colchicine25
Treatment plan:
TABLE
Care plan for a patient with gout27
| Acute flare | Chronic gout | |
|---|---|---|
| Goals |
|
|
| Educational points |
|
|
| sUA, serum uric acid; ULT, urate-lowering therapy. Source: Reproduced with permission. Becker MA, et al. J Fam Pract. 2010;59(6):S1-S8. Quadrant HealthCom Inc. Copyright 2010. | ||
Urate-Lowering Therapy
Urate lowering therapy is indicated for most, but not all, patients with gout. ULT is generally not recommended for those who have suffered a single attack of gout and have no complications, since 40% of these patients will not experience another attack within a year. However, should a second attack occur within a year of the first attack, ULT is recommended. Some patients who have experienced a single attack may elect to initiate ULT after being educated about the risks of the disease and the risks and benefits of ULT.14 Patients who have had an attack of gout and also have a comorbidity (eg, visible gouty tophi, renal insufficiency, uric acid stones, or use of a diuretic for hypertension) should begin ULT, since the risk of further attacks is higher in these patients, and kidney or joint damage is more likely.17
Initiation of ULT should not occur until 1 to 2 weeks after an acute attack has resolved, since beginning ULT during an acute attack is thought to prolong the attack.17 Because gout is a chronic, largely self-managed disease, patient education is a cornerstone of successful long-term treatment. Implementation of a care plan for both an acute flare and chronic gout is recommended ( TABLE ).27
Anti-inflammatory prophylaxis should begin at the same time that ULT is initiated, since an acute attack is likely due to a transient rise in the sUA level resulting from mobilization of MSU crystals. Colchicine, which is the only drug approved by the FDA for prophylaxis of an acute gout attack, can be used daily in a low-dose regimen (0.6 mg once or twice daily) for up to 6 months.17,26 Alternatively, an NSAID can be used.17
One recent investigation pooled the results of 3 phase III clinical trials of ULT in 4101 patients with gout.28 Patients received prophylaxis for 8 weeks or 6 months with low-dose colchicine 0.6 mg once daily or the combination of naproxen 250 mg twice daily with lansoprazole 15 mg once daily. The incidence of acute gout attacks increased sharply (up to 40%) at the end of 8 weeks of prophylaxis with either colchicine or naproxen and then declined steadily, whereas the rates of acute attacks were consistently low (3% to 5%) at the end of 6 months of prophylaxis with either colchicine or naproxen/lansoprazole. With the 8-week prophylaxis regimen, diarrhea was more common in the colchicine group (n = 993) than in the naproxen group (n = 829) (8.4% vs 2.7%, respectively; P < .001). With the 6-month prophylaxis regimen, liver function abnormalities (7.7% vs 4.3%; P = .023) and headache (2.8% vs 0.9%; P = .037) were more common with colchicine (n = 1807) than naproxen, while gastrointestinal/abdominal pains (3.2% vs 1.2%; P = .012) and dental/oral soft tissue infections (2.3% vs 0.6%; P = .006) were more common with naproxen (n = 346) than colchicine.
Uricostatic Agents
Uricostatic therapy with a xanthine oxidase inhibitor (ie, allopurinol or febuxostat) is the most commonly used ULT. Allopurinol is effective in lowering the sUA level and has been shown to lower the rates of all-cause mortality and cardiovascular events, and, in patients with chronic kidney disease, slow the progression of renal disease.29,30 One key point that must be kept in mind is that the efficacy of allopurinol to lower the sUA level is dose-dependent, although limited safety data are available for doses >300 mg per day.14,31,32 One recent prospective clinical trial showed that 26% of patients achieved an sUA level of 5 mg/dL or less following 2 months of treatment with allopurinol 300 mg per day compared with 78% of those who subsequently doubled the dose to 300 mg twice daily.31 Two patients discontinued treatment with allopurinol because of an AE. Finally, the dose of allopurinol must be adjusted based on renal function to minimize the risk of AEs, particularly skin rashes.33
Febuxostat is also effective in lowering the sUA level. In patients with an sUA level of 8.0 mg/dL or higher and a creatinine clearance of 50 mL/min or higher at baseline, an sUA level of less than 6.0 mg/dL was achieved in 53% of patients treated with febuxostat 80 mg (n = 256) versus 21% of patients treated with allopurinol 300 mg once daily (n = 253) after 1 year (P < .001).34 The most frequent treatment-related AE was liver function abnormality, which occurred in 4% of patients in each group. Results of a 6-month trial showed that achievement of an sUA level of less than 6.0 mg/dL was achieved in 45% and 67% of patients treated with febuxostat 40 mg or 80 mg daily, respectively, and 42% of those treated with allopurinol 300 mg (200 mg in moderate renal impairment) daily.35 Febuxostat also has been shown to slow the progression of, or even stabilize, renal function.36
Treatment plan (continued):
- For an acute gout attack: Continue colchicine as needed
- ULT: Initiate allopurinol 100 mg once daily; increase to 200 mg once daily in 1 week, and 300 mg once daily in another week
- -Alternatively, initiate febuxostat 40 mg once daily; increase to 80 mg once daily if an sUA level of less than 6.0 mg/dL is not achieved within 2 weeks
- For prophylaxis of an acute attack when initiating ULT: Initiate colchicine 0.6 mg once daily; may increase to 0.6 mg twice daily if needed
- -Alternatively, initiate naproxen 250 mg twice daily with a proton pump inhibitor
- Measure sUA in 1 month; if the sUA level is greater than 6.0 mg/dL, increase allopurinol to 200 mg twice daily
- -Measure sUA in 1 month; if the sUA level is still greater than 6.0 mg/dL, increase allopurinol to 300 mg twice daily
- Implement the care plan ( TABLE )27
- -Inquire about and address issues to promote adherence and self-management
- -Discuss the most common AEs with allopurinol and colchicine and the actions the patient should take if an AE occurs
- Once the sUA level is 6.0 mg/dL or less, monitor sUA annually (including serum creatinine)14
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(suppl 5):S9-S12.
3. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742-748.
4. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309-312.
5. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
6. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277-1281.
7. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109-115.
8. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031-1036.
9. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503-1507.
10. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642-650.
11. Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10(6):403-409.
12. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421-426.
13. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(Suppl 5):S5-S8.
14. Hamburger M, Baraf HS, Adamson TC III, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123 (6 suppl 1):3-36.
15. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312-1324.
16. Schumacher HR Jr, Boice JA, Daikh DI, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324(7352):1488-1492.
17. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46(8):1372-1374.
18. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.-
19. Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.-
20. NSAIDS and chronic kidney disease. US Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/news/docs/nsaid_video.htm. Published 2012. Accessed April 22, 2012.
21. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
22. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
23. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854-1860.
24. Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
25. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
26. Colcrys [package insert]. Philadelphia, PA: AR Scientific, Inc.; 2011.
27. Becker MA, Ruoff GE. What do I need to know about gout? J Fam Pract. 2010;59(6 suppl):S1-S8.
28. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386-2397.
29. Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009;48(7):804-806.
30. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388-1393.
31. Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892-897.
32. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421.
33. Zyloprim [package insert]. San Diego, CA: Prometheus Laboratories Inc.; 2003.
34. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450-2461.
35. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:doi:10.1186/ar2978.
36. Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7-13.
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Ruoff has disclosed that he is on the speakers’ bureau for and has received research grants from Takeda Pharmaceuticals.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from URL Pharma, Inc.
DB is a 50-year-old obese male visiting the clinic for symptoms suggestive of allergic rhinitis. The nurse has informed the family physician that DB was limping from the waiting room to the examination room. DB reports that he has been experiencing pain in his left big toe and ankle over the past few days. The last time this happened, the pain resolved within 7 to 10 days.
DB reports that he has experienced 4 or 5 similar episodes over the past 3 years. The first attacks affected his left big toe, but he now also experiences some pain in his left ankle. The pain is moderate, peaks in 1 to 2 days, and resolves within 7 to 10 days. Acetaminophen provided little pain relief so DB now takes ibuprofen 400 mg 3 times daily, as it “helps take the edge off.” Other medications include aspirin 81 mg per day and an oral antihistamine as needed for hay fever. DB reports that he eats seafood 2 to 3 times per week and red meat 1 to 2 times per week; he drinks 2 six-packs of beer per week.
Physical examination: weight, 186 lb (body mass index [BMI], 27 kg/m2); blood pressure, 126/76 mm Hg; and temperature, 98.8°F. His left big toe and ankle are red, slightly swollen, and warm with a small subcutaneous nodule noted on the first metatarsophalangeal joint. There is no sign of skin or joint infection.
The impression from his history and physical exam is that DB is suffering from an acute attack of gout, but the family physician also considers other diagnoses.
Background
Gout is a heterogeneous disorder that peaks in incidence in the fifth decade. Gout is caused by hyperuricemia, generally as a result of reduced excretion of uric acid by the kidneys; hyperuricemia may also result from overproduction of uric acid. Data from the National Health and Nutrition Examination Survey (NHANES) 2007-2008 indicate that the prevalence of gout continues to rise in the United States, likely related to the increasing frequency of adiposity and hypertension. Overall, about 75% of the 8.3 million people with gout are men.1
Risk Factors
Clinically defined hyperuricemia—a serum urate (sUA) level greater than 6.8 mg/dL, the concentration at which urate exceeds its solubility in most biological fluids—is the major risk factor for gout. However, not all persons with hyperuricemia have gout. Data from NHANES 2007-2008, in which the definition of hyperuricemia was an sUA level greater than 7.0 mg/dL for men and greater than 5.7 mg/dL for women, showed the mean sUA level to be 6.1 mg/dL in men and 4.9 mg/dL in women, corresponding to hyperuricemia prevalences of 21.2% and 21.6%, respectively.1
There are several other risk factors for gout, including hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease (CVD), and metabolic syndrome.2 For a man with hypertension, the relative risk (RR) of gout is 2.3 compared with a normotensive man.3 Furthermore, it is well established that the use of diuretics increases the risk of gout (RR, 1.8).3 Several other medications increase sUA level as well: aspirin (including low-dose), cyclosporine, pyrazinamide, ethambutol, and niacin.2
Lifestyle and diet also pose a risk for gout. The risk of gout increases with BMI such that, compared with a man with a BMI of 21 to 22.9 kg/m2, the RR of gout is doubled for a man with a BMI of 25 to 29.9 kg/m2; for a man with a BMI of 35 kg/m2 or more, the RR is tripled.3 Sugar-sweetened soft drinks (but not diet soft drinks) and fructose-rich fruits and fruit juices also increase the risk of gout, as do a high alcohol intake, particularly beer, and a diet rich in meat (especially organ meat, turkey, or wild game) or seafood.4 A moderate intake of purine-rich vegetables (eg, peas, beans, lentils, spinach, mushrooms, oatmeal, and cauliflower) or protein is not associated with an increased risk of gout, while a high consumption of dairy products is associated with a decreased risk.5,6
Untreated or poorly treated gout usually leads to further acute attacks and progressive joint and tissue damage. In addition, gout and hyperuricemia serve as risk factors for other diseases. Adults with gout are 3 times as likely to develop metabolic syndrome as adults without gout.7 An elevated sUA level is also an independent risk factor for the development of hypertension (RR, 1.1), as well as myocardial infarction (MI; RR, 1.9), and stroke (RR, 1.6).8,9 An increasing sUA level also increases the risk of renal failure.10,11 In a study of 49,413 men followed for a mean of 5.4 years, the age-adjusted RR of renal failure was 1.5 in men with an sUA level of 6.5 to 8.4 mg/dL and 8.5 in men with an sUA level of 8.5 to 13.0 mg/dL compared with men with an sUA level of 5.0 to 6.4 mg/dL.11
Clinical Presentation
The deposition of monosodium urate (MSU) crystals in joints and tissues is very common and typically causes no signs or symptoms in the majority of persons. Even in men with an sUA level of 9 mg/dL or greater, the cumulative incidence of gouty arthritis has been found to be 22% over 5 years.12 However, as crystal deposition progresses, acute, painful attacks occur more frequently, with the development of chronic tophaceous gout after several years.13
Laboratory results for DB:
- Serum uric acid, 7.9 mg/dL
- White blood cell count, 15,800/mm3
- Serum creatinine, 1.2 mg/dL (estimated creatinine clearance, 90 mL/min)
- Erythrocyte sedimentation rate, 23 mm/h
- Low-density lipoprotein cholesterol (nonfasting), 127 mg/dL
Laboratory confirmation of hyperuricemia together with the pain, swelling, and tenderness of DB’s toe and ankle, other findings from his medical history and physical exam (eg, the use of aspirin daily), and exclusion of alternative diagnoses, such as septic arthritis, enable the family physician to arrive at a presumptive diagnosis of gouty arthritis. Aspiration of MSU crystals from DB’s toe or ankle is the gold standard and would allow for a definitive diagnosis. Although the sUA level was found to be high, it should be noted that a normal sUA level is often found during an acute attack; should this occur, the sUA level should be checked again 1 to 2 weeks after the acute attack has resolved.
Goals of Treatment
The cornerstone of gout management is daily, long-term treatment with urate-lowering therapy (ULT) combined with as-needed treatment for an acute attack. In addition, since initiation of ULT mobilizes MSU crystals, which often leads to a short-term increase in acute attacks, prophylaxis with an appropriate anti-inflammatory therapy is recommended at the time ULT is initiated.14
The therapeutic goals of gout treatment are 2-pronged: treatment of an acute gout attack and management of chronic gout. For an acute attack, the goals are to exclude a diagnosis of septic arthritis; reduce inflammation and terminate the attack; and seek, assess, and control associated diseases, such as diabetes mellitus, hypertension, hyperlipidemia, and CVD. If this latter goal is not possible during the acute attack, plans should be made to assess associated diseases once the acute attack has resolved.14 Lowering the sUA level is not a goal of therapy for an acute attack, but it is the primary goal of ULT for chronic gout. Lowering the sUA level to less than 6.0 mg/dL, which is well below the saturation point of urate in most biological fluids, is intended to prevent further acute attacks, tophus formation, and tissue damage.14
Treatment of an Acute Attack
The mainstay of treatment for an acute attack is anti-inflammatory therapy to reduce pain and inflammation.14 Therapy should be initiated at the onset of the attack and continued until the attack is terminated, which is typically 1 to 2 weeks. Anti-inflammatory therapy traditionally has in-cluded colchicine, a nonsteroidal anti-inflammatory drug (NSAID), or a corticosteroid.14
Nonsteroidal Anti-inflammatory Drugs
The NSAIDs are all thought to provide similar efficacy when used in maximum doses.15,16 Since gastrointestinal toxicity is a concern with NSAIDs, coadministration of a proton pump inhibitor, H2 antagonist, or misoprostol is advised for patients with an increased risk of peptic ulcers, bleeds, or perforations.17 The risk of MI, stroke, cardiovascular death, and atrial fibrillation/flutter with NSAID therapy should be considered, especially because gout often coexists with cardiovascular disorders.15,18,19 Furthermore, NSAIDs are contraindicated in patients with heart failure or renal insufficiency.20,21
Corticosteroids. A systematic review of clinical trials involving systemic corticosteroids that found a few prospective trials of low to moderate quality concluded that there was inconclusive evidence for the efficacy and effectiveness of corticosteroids in the treatment of acute gout.22 No serious adverse events (AEs) were reported. A more recent prospective trial found comparable pain reduction and incidence of AEs with naproxen 500 mg twice daily and prednisolone 35 mg once daily for 5 days in patients with monoarticular gout.23 Furthermore, clinical experience indicates that intra-articular aspiration and injection of a long-acting corticosteroid is an effective and safe treatment for an acute attack.14,15 Corticosteroids may be useful in patients who have an inadequate response to, are intolerant of, or have a contraindication to NSAIDs and colchicine.14,15
Colchicine. Much of the recent clinical investigation regarding pharmacologic treatment of an acute gout attack has involved colchicine. To overcome the limitations of the standard dose-to-toxicity regimen of colchicine, a low-dose regimen of colchicine (1.2 mg followed by 0.6 mg 1 hour later) was investigated and subsequently approved by the US Food and Drug Administration (FDA).24
Approval was based on a randomized, double-blind comparison with high-dose colchicine (1.2 mg followed by 0.6 mg every hour for 6 hours) and placebo in 184 patients with an acute gout attack.25 The primary endpoint, a 50% or greater reduction in pain at 24 hours without the use of rescue medication, was reached in 28 of 74 patients (38%) in the low-dose group, 17 of 52 patients (33%) in the high-dose group, and 9 of 58 patients (16%) in the placebo group (P = .005 and P = .034, respectively, versus placebo). An AE occurred in 36.5% and 76.9% of study participants in the low-dose and high-dose colchicine groups, respectively, and in 27.1% of participants in the placebo group. Gastrointestinal AEs (eg, diarrhea, nausea, and vomiting) were less common in the low-dose colchicine group ( FIGURE ). All AEs in the low-dose group were mild to moderate in intensity, while 10 of 52 patients (19.2%) in the high-dose group had an AE of severe intensity. Concomitant use of numerous drugs can increase the concentration of colchicine. Examples include atorvastatin, fluvastatin, pravastatin, simvastatin, fibrates, gemfibrozil, digoxin, clarithromycin, erythromycin, fluconazole, itraconazole, ketoconazole, protease inhibitors, diltiazem, verapamil, and cyclosporine, as well as grapefruit juice.26
FIGURE
Frequency of selected adverse events occurring over 24 hours with low-dose vs high-dose colchicine25
Treatment plan:
TABLE
Care plan for a patient with gout27
| Acute flare | Chronic gout | |
|---|---|---|
| Goals |
|
|
| Educational points |
|
|
| sUA, serum uric acid; ULT, urate-lowering therapy. Source: Reproduced with permission. Becker MA, et al. J Fam Pract. 2010;59(6):S1-S8. Quadrant HealthCom Inc. Copyright 2010. | ||
Urate-Lowering Therapy
Urate lowering therapy is indicated for most, but not all, patients with gout. ULT is generally not recommended for those who have suffered a single attack of gout and have no complications, since 40% of these patients will not experience another attack within a year. However, should a second attack occur within a year of the first attack, ULT is recommended. Some patients who have experienced a single attack may elect to initiate ULT after being educated about the risks of the disease and the risks and benefits of ULT.14 Patients who have had an attack of gout and also have a comorbidity (eg, visible gouty tophi, renal insufficiency, uric acid stones, or use of a diuretic for hypertension) should begin ULT, since the risk of further attacks is higher in these patients, and kidney or joint damage is more likely.17
Initiation of ULT should not occur until 1 to 2 weeks after an acute attack has resolved, since beginning ULT during an acute attack is thought to prolong the attack.17 Because gout is a chronic, largely self-managed disease, patient education is a cornerstone of successful long-term treatment. Implementation of a care plan for both an acute flare and chronic gout is recommended ( TABLE ).27
Anti-inflammatory prophylaxis should begin at the same time that ULT is initiated, since an acute attack is likely due to a transient rise in the sUA level resulting from mobilization of MSU crystals. Colchicine, which is the only drug approved by the FDA for prophylaxis of an acute gout attack, can be used daily in a low-dose regimen (0.6 mg once or twice daily) for up to 6 months.17,26 Alternatively, an NSAID can be used.17
One recent investigation pooled the results of 3 phase III clinical trials of ULT in 4101 patients with gout.28 Patients received prophylaxis for 8 weeks or 6 months with low-dose colchicine 0.6 mg once daily or the combination of naproxen 250 mg twice daily with lansoprazole 15 mg once daily. The incidence of acute gout attacks increased sharply (up to 40%) at the end of 8 weeks of prophylaxis with either colchicine or naproxen and then declined steadily, whereas the rates of acute attacks were consistently low (3% to 5%) at the end of 6 months of prophylaxis with either colchicine or naproxen/lansoprazole. With the 8-week prophylaxis regimen, diarrhea was more common in the colchicine group (n = 993) than in the naproxen group (n = 829) (8.4% vs 2.7%, respectively; P < .001). With the 6-month prophylaxis regimen, liver function abnormalities (7.7% vs 4.3%; P = .023) and headache (2.8% vs 0.9%; P = .037) were more common with colchicine (n = 1807) than naproxen, while gastrointestinal/abdominal pains (3.2% vs 1.2%; P = .012) and dental/oral soft tissue infections (2.3% vs 0.6%; P = .006) were more common with naproxen (n = 346) than colchicine.
Uricostatic Agents
Uricostatic therapy with a xanthine oxidase inhibitor (ie, allopurinol or febuxostat) is the most commonly used ULT. Allopurinol is effective in lowering the sUA level and has been shown to lower the rates of all-cause mortality and cardiovascular events, and, in patients with chronic kidney disease, slow the progression of renal disease.29,30 One key point that must be kept in mind is that the efficacy of allopurinol to lower the sUA level is dose-dependent, although limited safety data are available for doses >300 mg per day.14,31,32 One recent prospective clinical trial showed that 26% of patients achieved an sUA level of 5 mg/dL or less following 2 months of treatment with allopurinol 300 mg per day compared with 78% of those who subsequently doubled the dose to 300 mg twice daily.31 Two patients discontinued treatment with allopurinol because of an AE. Finally, the dose of allopurinol must be adjusted based on renal function to minimize the risk of AEs, particularly skin rashes.33
Febuxostat is also effective in lowering the sUA level. In patients with an sUA level of 8.0 mg/dL or higher and a creatinine clearance of 50 mL/min or higher at baseline, an sUA level of less than 6.0 mg/dL was achieved in 53% of patients treated with febuxostat 80 mg (n = 256) versus 21% of patients treated with allopurinol 300 mg once daily (n = 253) after 1 year (P < .001).34 The most frequent treatment-related AE was liver function abnormality, which occurred in 4% of patients in each group. Results of a 6-month trial showed that achievement of an sUA level of less than 6.0 mg/dL was achieved in 45% and 67% of patients treated with febuxostat 40 mg or 80 mg daily, respectively, and 42% of those treated with allopurinol 300 mg (200 mg in moderate renal impairment) daily.35 Febuxostat also has been shown to slow the progression of, or even stabilize, renal function.36
Treatment plan (continued):
- For an acute gout attack: Continue colchicine as needed
- ULT: Initiate allopurinol 100 mg once daily; increase to 200 mg once daily in 1 week, and 300 mg once daily in another week
- -Alternatively, initiate febuxostat 40 mg once daily; increase to 80 mg once daily if an sUA level of less than 6.0 mg/dL is not achieved within 2 weeks
- For prophylaxis of an acute attack when initiating ULT: Initiate colchicine 0.6 mg once daily; may increase to 0.6 mg twice daily if needed
- -Alternatively, initiate naproxen 250 mg twice daily with a proton pump inhibitor
- Measure sUA in 1 month; if the sUA level is greater than 6.0 mg/dL, increase allopurinol to 200 mg twice daily
- -Measure sUA in 1 month; if the sUA level is still greater than 6.0 mg/dL, increase allopurinol to 300 mg twice daily
- Implement the care plan ( TABLE )27
- -Inquire about and address issues to promote adherence and self-management
- -Discuss the most common AEs with allopurinol and colchicine and the actions the patient should take if an AE occurs
- Once the sUA level is 6.0 mg/dL or less, monitor sUA annually (including serum creatinine)14
Managing the Multiple Symptoms of Benign Prostatic Hyperplasia — CME
Managing Type 2 Diabetes in Men
Meeting New Challenges with Antiplatelet Therapy in Primary Care
Dr. Ruoff has disclosed that he is on the speakers’ bureau for and has received research grants from Takeda Pharmaceuticals.
SUPPORT
This program is sponsored by the PCEC and is supported by funding from URL Pharma, Inc.
DB is a 50-year-old obese male visiting the clinic for symptoms suggestive of allergic rhinitis. The nurse has informed the family physician that DB was limping from the waiting room to the examination room. DB reports that he has been experiencing pain in his left big toe and ankle over the past few days. The last time this happened, the pain resolved within 7 to 10 days.
DB reports that he has experienced 4 or 5 similar episodes over the past 3 years. The first attacks affected his left big toe, but he now also experiences some pain in his left ankle. The pain is moderate, peaks in 1 to 2 days, and resolves within 7 to 10 days. Acetaminophen provided little pain relief so DB now takes ibuprofen 400 mg 3 times daily, as it “helps take the edge off.” Other medications include aspirin 81 mg per day and an oral antihistamine as needed for hay fever. DB reports that he eats seafood 2 to 3 times per week and red meat 1 to 2 times per week; he drinks 2 six-packs of beer per week.
Physical examination: weight, 186 lb (body mass index [BMI], 27 kg/m2); blood pressure, 126/76 mm Hg; and temperature, 98.8°F. His left big toe and ankle are red, slightly swollen, and warm with a small subcutaneous nodule noted on the first metatarsophalangeal joint. There is no sign of skin or joint infection.
The impression from his history and physical exam is that DB is suffering from an acute attack of gout, but the family physician also considers other diagnoses.
Background
Gout is a heterogeneous disorder that peaks in incidence in the fifth decade. Gout is caused by hyperuricemia, generally as a result of reduced excretion of uric acid by the kidneys; hyperuricemia may also result from overproduction of uric acid. Data from the National Health and Nutrition Examination Survey (NHANES) 2007-2008 indicate that the prevalence of gout continues to rise in the United States, likely related to the increasing frequency of adiposity and hypertension. Overall, about 75% of the 8.3 million people with gout are men.1
Risk Factors
Clinically defined hyperuricemia—a serum urate (sUA) level greater than 6.8 mg/dL, the concentration at which urate exceeds its solubility in most biological fluids—is the major risk factor for gout. However, not all persons with hyperuricemia have gout. Data from NHANES 2007-2008, in which the definition of hyperuricemia was an sUA level greater than 7.0 mg/dL for men and greater than 5.7 mg/dL for women, showed the mean sUA level to be 6.1 mg/dL in men and 4.9 mg/dL in women, corresponding to hyperuricemia prevalences of 21.2% and 21.6%, respectively.1
There are several other risk factors for gout, including hypertension, diabetes, hyperlipidemia, chronic kidney disease, cardiovascular disease (CVD), and metabolic syndrome.2 For a man with hypertension, the relative risk (RR) of gout is 2.3 compared with a normotensive man.3 Furthermore, it is well established that the use of diuretics increases the risk of gout (RR, 1.8).3 Several other medications increase sUA level as well: aspirin (including low-dose), cyclosporine, pyrazinamide, ethambutol, and niacin.2
Lifestyle and diet also pose a risk for gout. The risk of gout increases with BMI such that, compared with a man with a BMI of 21 to 22.9 kg/m2, the RR of gout is doubled for a man with a BMI of 25 to 29.9 kg/m2; for a man with a BMI of 35 kg/m2 or more, the RR is tripled.3 Sugar-sweetened soft drinks (but not diet soft drinks) and fructose-rich fruits and fruit juices also increase the risk of gout, as do a high alcohol intake, particularly beer, and a diet rich in meat (especially organ meat, turkey, or wild game) or seafood.4 A moderate intake of purine-rich vegetables (eg, peas, beans, lentils, spinach, mushrooms, oatmeal, and cauliflower) or protein is not associated with an increased risk of gout, while a high consumption of dairy products is associated with a decreased risk.5,6
Untreated or poorly treated gout usually leads to further acute attacks and progressive joint and tissue damage. In addition, gout and hyperuricemia serve as risk factors for other diseases. Adults with gout are 3 times as likely to develop metabolic syndrome as adults without gout.7 An elevated sUA level is also an independent risk factor for the development of hypertension (RR, 1.1), as well as myocardial infarction (MI; RR, 1.9), and stroke (RR, 1.6).8,9 An increasing sUA level also increases the risk of renal failure.10,11 In a study of 49,413 men followed for a mean of 5.4 years, the age-adjusted RR of renal failure was 1.5 in men with an sUA level of 6.5 to 8.4 mg/dL and 8.5 in men with an sUA level of 8.5 to 13.0 mg/dL compared with men with an sUA level of 5.0 to 6.4 mg/dL.11
Clinical Presentation
The deposition of monosodium urate (MSU) crystals in joints and tissues is very common and typically causes no signs or symptoms in the majority of persons. Even in men with an sUA level of 9 mg/dL or greater, the cumulative incidence of gouty arthritis has been found to be 22% over 5 years.12 However, as crystal deposition progresses, acute, painful attacks occur more frequently, with the development of chronic tophaceous gout after several years.13
Laboratory results for DB:
- Serum uric acid, 7.9 mg/dL
- White blood cell count, 15,800/mm3
- Serum creatinine, 1.2 mg/dL (estimated creatinine clearance, 90 mL/min)
- Erythrocyte sedimentation rate, 23 mm/h
- Low-density lipoprotein cholesterol (nonfasting), 127 mg/dL
Laboratory confirmation of hyperuricemia together with the pain, swelling, and tenderness of DB’s toe and ankle, other findings from his medical history and physical exam (eg, the use of aspirin daily), and exclusion of alternative diagnoses, such as septic arthritis, enable the family physician to arrive at a presumptive diagnosis of gouty arthritis. Aspiration of MSU crystals from DB’s toe or ankle is the gold standard and would allow for a definitive diagnosis. Although the sUA level was found to be high, it should be noted that a normal sUA level is often found during an acute attack; should this occur, the sUA level should be checked again 1 to 2 weeks after the acute attack has resolved.
Goals of Treatment
The cornerstone of gout management is daily, long-term treatment with urate-lowering therapy (ULT) combined with as-needed treatment for an acute attack. In addition, since initiation of ULT mobilizes MSU crystals, which often leads to a short-term increase in acute attacks, prophylaxis with an appropriate anti-inflammatory therapy is recommended at the time ULT is initiated.14
The therapeutic goals of gout treatment are 2-pronged: treatment of an acute gout attack and management of chronic gout. For an acute attack, the goals are to exclude a diagnosis of septic arthritis; reduce inflammation and terminate the attack; and seek, assess, and control associated diseases, such as diabetes mellitus, hypertension, hyperlipidemia, and CVD. If this latter goal is not possible during the acute attack, plans should be made to assess associated diseases once the acute attack has resolved.14 Lowering the sUA level is not a goal of therapy for an acute attack, but it is the primary goal of ULT for chronic gout. Lowering the sUA level to less than 6.0 mg/dL, which is well below the saturation point of urate in most biological fluids, is intended to prevent further acute attacks, tophus formation, and tissue damage.14
Treatment of an Acute Attack
The mainstay of treatment for an acute attack is anti-inflammatory therapy to reduce pain and inflammation.14 Therapy should be initiated at the onset of the attack and continued until the attack is terminated, which is typically 1 to 2 weeks. Anti-inflammatory therapy traditionally has in-cluded colchicine, a nonsteroidal anti-inflammatory drug (NSAID), or a corticosteroid.14
Nonsteroidal Anti-inflammatory Drugs
The NSAIDs are all thought to provide similar efficacy when used in maximum doses.15,16 Since gastrointestinal toxicity is a concern with NSAIDs, coadministration of a proton pump inhibitor, H2 antagonist, or misoprostol is advised for patients with an increased risk of peptic ulcers, bleeds, or perforations.17 The risk of MI, stroke, cardiovascular death, and atrial fibrillation/flutter with NSAID therapy should be considered, especially because gout often coexists with cardiovascular disorders.15,18,19 Furthermore, NSAIDs are contraindicated in patients with heart failure or renal insufficiency.20,21
Corticosteroids. A systematic review of clinical trials involving systemic corticosteroids that found a few prospective trials of low to moderate quality concluded that there was inconclusive evidence for the efficacy and effectiveness of corticosteroids in the treatment of acute gout.22 No serious adverse events (AEs) were reported. A more recent prospective trial found comparable pain reduction and incidence of AEs with naproxen 500 mg twice daily and prednisolone 35 mg once daily for 5 days in patients with monoarticular gout.23 Furthermore, clinical experience indicates that intra-articular aspiration and injection of a long-acting corticosteroid is an effective and safe treatment for an acute attack.14,15 Corticosteroids may be useful in patients who have an inadequate response to, are intolerant of, or have a contraindication to NSAIDs and colchicine.14,15
Colchicine. Much of the recent clinical investigation regarding pharmacologic treatment of an acute gout attack has involved colchicine. To overcome the limitations of the standard dose-to-toxicity regimen of colchicine, a low-dose regimen of colchicine (1.2 mg followed by 0.6 mg 1 hour later) was investigated and subsequently approved by the US Food and Drug Administration (FDA).24
Approval was based on a randomized, double-blind comparison with high-dose colchicine (1.2 mg followed by 0.6 mg every hour for 6 hours) and placebo in 184 patients with an acute gout attack.25 The primary endpoint, a 50% or greater reduction in pain at 24 hours without the use of rescue medication, was reached in 28 of 74 patients (38%) in the low-dose group, 17 of 52 patients (33%) in the high-dose group, and 9 of 58 patients (16%) in the placebo group (P = .005 and P = .034, respectively, versus placebo). An AE occurred in 36.5% and 76.9% of study participants in the low-dose and high-dose colchicine groups, respectively, and in 27.1% of participants in the placebo group. Gastrointestinal AEs (eg, diarrhea, nausea, and vomiting) were less common in the low-dose colchicine group ( FIGURE ). All AEs in the low-dose group were mild to moderate in intensity, while 10 of 52 patients (19.2%) in the high-dose group had an AE of severe intensity. Concomitant use of numerous drugs can increase the concentration of colchicine. Examples include atorvastatin, fluvastatin, pravastatin, simvastatin, fibrates, gemfibrozil, digoxin, clarithromycin, erythromycin, fluconazole, itraconazole, ketoconazole, protease inhibitors, diltiazem, verapamil, and cyclosporine, as well as grapefruit juice.26
FIGURE
Frequency of selected adverse events occurring over 24 hours with low-dose vs high-dose colchicine25
Treatment plan:
TABLE
Care plan for a patient with gout27
| Acute flare | Chronic gout | |
|---|---|---|
| Goals |
|
|
| Educational points |
|
|
| sUA, serum uric acid; ULT, urate-lowering therapy. Source: Reproduced with permission. Becker MA, et al. J Fam Pract. 2010;59(6):S1-S8. Quadrant HealthCom Inc. Copyright 2010. | ||
Urate-Lowering Therapy
Urate lowering therapy is indicated for most, but not all, patients with gout. ULT is generally not recommended for those who have suffered a single attack of gout and have no complications, since 40% of these patients will not experience another attack within a year. However, should a second attack occur within a year of the first attack, ULT is recommended. Some patients who have experienced a single attack may elect to initiate ULT after being educated about the risks of the disease and the risks and benefits of ULT.14 Patients who have had an attack of gout and also have a comorbidity (eg, visible gouty tophi, renal insufficiency, uric acid stones, or use of a diuretic for hypertension) should begin ULT, since the risk of further attacks is higher in these patients, and kidney or joint damage is more likely.17
Initiation of ULT should not occur until 1 to 2 weeks after an acute attack has resolved, since beginning ULT during an acute attack is thought to prolong the attack.17 Because gout is a chronic, largely self-managed disease, patient education is a cornerstone of successful long-term treatment. Implementation of a care plan for both an acute flare and chronic gout is recommended ( TABLE ).27
Anti-inflammatory prophylaxis should begin at the same time that ULT is initiated, since an acute attack is likely due to a transient rise in the sUA level resulting from mobilization of MSU crystals. Colchicine, which is the only drug approved by the FDA for prophylaxis of an acute gout attack, can be used daily in a low-dose regimen (0.6 mg once or twice daily) for up to 6 months.17,26 Alternatively, an NSAID can be used.17
One recent investigation pooled the results of 3 phase III clinical trials of ULT in 4101 patients with gout.28 Patients received prophylaxis for 8 weeks or 6 months with low-dose colchicine 0.6 mg once daily or the combination of naproxen 250 mg twice daily with lansoprazole 15 mg once daily. The incidence of acute gout attacks increased sharply (up to 40%) at the end of 8 weeks of prophylaxis with either colchicine or naproxen and then declined steadily, whereas the rates of acute attacks were consistently low (3% to 5%) at the end of 6 months of prophylaxis with either colchicine or naproxen/lansoprazole. With the 8-week prophylaxis regimen, diarrhea was more common in the colchicine group (n = 993) than in the naproxen group (n = 829) (8.4% vs 2.7%, respectively; P < .001). With the 6-month prophylaxis regimen, liver function abnormalities (7.7% vs 4.3%; P = .023) and headache (2.8% vs 0.9%; P = .037) were more common with colchicine (n = 1807) than naproxen, while gastrointestinal/abdominal pains (3.2% vs 1.2%; P = .012) and dental/oral soft tissue infections (2.3% vs 0.6%; P = .006) were more common with naproxen (n = 346) than colchicine.
Uricostatic Agents
Uricostatic therapy with a xanthine oxidase inhibitor (ie, allopurinol or febuxostat) is the most commonly used ULT. Allopurinol is effective in lowering the sUA level and has been shown to lower the rates of all-cause mortality and cardiovascular events, and, in patients with chronic kidney disease, slow the progression of renal disease.29,30 One key point that must be kept in mind is that the efficacy of allopurinol to lower the sUA level is dose-dependent, although limited safety data are available for doses >300 mg per day.14,31,32 One recent prospective clinical trial showed that 26% of patients achieved an sUA level of 5 mg/dL or less following 2 months of treatment with allopurinol 300 mg per day compared with 78% of those who subsequently doubled the dose to 300 mg twice daily.31 Two patients discontinued treatment with allopurinol because of an AE. Finally, the dose of allopurinol must be adjusted based on renal function to minimize the risk of AEs, particularly skin rashes.33
Febuxostat is also effective in lowering the sUA level. In patients with an sUA level of 8.0 mg/dL or higher and a creatinine clearance of 50 mL/min or higher at baseline, an sUA level of less than 6.0 mg/dL was achieved in 53% of patients treated with febuxostat 80 mg (n = 256) versus 21% of patients treated with allopurinol 300 mg once daily (n = 253) after 1 year (P < .001).34 The most frequent treatment-related AE was liver function abnormality, which occurred in 4% of patients in each group. Results of a 6-month trial showed that achievement of an sUA level of less than 6.0 mg/dL was achieved in 45% and 67% of patients treated with febuxostat 40 mg or 80 mg daily, respectively, and 42% of those treated with allopurinol 300 mg (200 mg in moderate renal impairment) daily.35 Febuxostat also has been shown to slow the progression of, or even stabilize, renal function.36
Treatment plan (continued):
- For an acute gout attack: Continue colchicine as needed
- ULT: Initiate allopurinol 100 mg once daily; increase to 200 mg once daily in 1 week, and 300 mg once daily in another week
- -Alternatively, initiate febuxostat 40 mg once daily; increase to 80 mg once daily if an sUA level of less than 6.0 mg/dL is not achieved within 2 weeks
- For prophylaxis of an acute attack when initiating ULT: Initiate colchicine 0.6 mg once daily; may increase to 0.6 mg twice daily if needed
- -Alternatively, initiate naproxen 250 mg twice daily with a proton pump inhibitor
- Measure sUA in 1 month; if the sUA level is greater than 6.0 mg/dL, increase allopurinol to 200 mg twice daily
- -Measure sUA in 1 month; if the sUA level is still greater than 6.0 mg/dL, increase allopurinol to 300 mg twice daily
- Implement the care plan ( TABLE )27
- -Inquire about and address issues to promote adherence and self-management
- -Discuss the most common AEs with allopurinol and colchicine and the actions the patient should take if an AE occurs
- Once the sUA level is 6.0 mg/dL or less, monitor sUA annually (including serum creatinine)14
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(suppl 5):S9-S12.
3. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742-748.
4. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309-312.
5. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
6. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277-1281.
7. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109-115.
8. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031-1036.
9. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503-1507.
10. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642-650.
11. Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10(6):403-409.
12. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421-426.
13. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(Suppl 5):S5-S8.
14. Hamburger M, Baraf HS, Adamson TC III, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123 (6 suppl 1):3-36.
15. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312-1324.
16. Schumacher HR Jr, Boice JA, Daikh DI, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324(7352):1488-1492.
17. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46(8):1372-1374.
18. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.-
19. Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.-
20. NSAIDS and chronic kidney disease. US Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/news/docs/nsaid_video.htm. Published 2012. Accessed April 22, 2012.
21. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
22. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
23. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854-1860.
24. Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
25. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
26. Colcrys [package insert]. Philadelphia, PA: AR Scientific, Inc.; 2011.
27. Becker MA, Ruoff GE. What do I need to know about gout? J Fam Pract. 2010;59(6 suppl):S1-S8.
28. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386-2397.
29. Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009;48(7):804-806.
30. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388-1393.
31. Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892-897.
32. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421.
33. Zyloprim [package insert]. San Diego, CA: Prometheus Laboratories Inc.; 2003.
34. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450-2461.
35. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:doi:10.1186/ar2978.
36. Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7-13.
1. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141.
2. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(suppl 5):S9-S12.
3. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742-748.
4. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309-312.
5. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
6. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363(9417):1277-1281.
7. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109-115.
8. Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031-1036.
9. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503-1507.
10. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642-650.
11. Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol. 2000;10(6):403-409.
12. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82(3):421-426.
13. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75(Suppl 5):S5-S8.
14. Hamburger M, Baraf HS, Adamson TC III, et al. 2011 Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med. 2011;123 (6 suppl 1):3-36.
15. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312-1324.
16. Schumacher HR Jr, Boice JA, Daikh DI, et al. Randomised double blind trial of etoricoxib and indometacin in treatment of acute gouty arthritis. BMJ. 2002;324(7352):1488-1492.
17. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007;46(8):1372-1374.
18. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086.-
19. Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.-
20. NSAIDS and chronic kidney disease. US Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/news/docs/nsaid_video.htm. Published 2012. Accessed April 22, 2012.
21. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
22. Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
23. Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371(9627):1854-1860.
24. Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
25. Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060-1068.
26. Colcrys [package insert]. Philadelphia, PA: AR Scientific, Inc.; 2011.
27. Becker MA, Ruoff GE. What do I need to know about gout? J Fam Pract. 2010;59(6 suppl):S1-S8.
28. Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. Clin Ther. 2010;32(14):2386-2397.
29. Luk AJ, Levin GP, Moore EE, Zhou XH, Kestenbaum BR, Choi HK. Allopurinol and mortality in hyperuricaemic patients. Rheumatology (Oxford). 2009;48(7):804-806.
30. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388-1393.
31. Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300-600 mg/day versus benzbromarone 100-200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892-897.
32. Stamp LK, O’Donnell JL, Zhang M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63(2):412-421.
33. Zyloprim [package insert]. San Diego, CA: Prometheus Laboratories Inc.; 2003.
34. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450-2461.
35. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:doi:10.1186/ar2978.
36. Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17(1):7-13.
UV protection and sunscreens: What to tell patients
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
KEY POINTS
- Despite the known risks, nearly 28 million Americans use a sunbed or a sunlamp every year, and 70% of those are white women ages 16 to 29.
- Sunscreens have been a source of confusion in their labeling and their sun protection factor ratings. Revised FDA labeling requirements may help clinicians provide useful guidance to patients.
- The American Academy of Dermatology supports a ban on the nonmedical production and sale of indoor tanning devices.
- Recommendations to prevent UV damage include minimizing sun exposure during peak daylight hours, wearing clothing such as long-sleeve shirts, wide-brimmed hats, and sunglasses, and application of a broad-spectrum sunscreen with UV-A protection. Infants less than 6 months of age require additional protective measures.
How to avoid damaging ultraviolet light
- Seek shade or minimize your sun exposure between 10 am and 4 pm, when the sun’s rays are the most intense. Wear protective clothing such as a wide-brimmed hat and long sleeves. Wear UV-blocking sunglasses. Whenever possible, keep children under 6 months old out of direct sunlight.
- Apply sunscreen to exposed dry skin 10–15 minutes before going out into the sun. The amount to use for an adult is about 2 tablespoons. Wear sunscreen even when the sky is overcast, as UV rays penetrate clouds and windows (unless the windows are treated to block UV light). Also, take cover and use sunscreen around water, sand, and snow: they reflect UV radiation.
- Do not use tanning beds. They expose the skin to much larger amounts of damaging UV light than normal sun exposure. The American Dermatologic Association supports a ban on tanning beds, and the World Health Organization classifies them as carcinogenic.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Patient Education and Health Information web site, www.clevelandclinic.org/health
- Seek shade or minimize your sun exposure between 10 am and 4 pm, when the sun’s rays are the most intense. Wear protective clothing such as a wide-brimmed hat and long sleeves. Wear UV-blocking sunglasses. Whenever possible, keep children under 6 months old out of direct sunlight.
- Apply sunscreen to exposed dry skin 10–15 minutes before going out into the sun. The amount to use for an adult is about 2 tablespoons. Wear sunscreen even when the sky is overcast, as UV rays penetrate clouds and windows (unless the windows are treated to block UV light). Also, take cover and use sunscreen around water, sand, and snow: they reflect UV radiation.
- Do not use tanning beds. They expose the skin to much larger amounts of damaging UV light than normal sun exposure. The American Dermatologic Association supports a ban on tanning beds, and the World Health Organization classifies them as carcinogenic.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Patient Education and Health Information web site, www.clevelandclinic.org/health
- Seek shade or minimize your sun exposure between 10 am and 4 pm, when the sun’s rays are the most intense. Wear protective clothing such as a wide-brimmed hat and long sleeves. Wear UV-blocking sunglasses. Whenever possible, keep children under 6 months old out of direct sunlight.
- Apply sunscreen to exposed dry skin 10–15 minutes before going out into the sun. The amount to use for an adult is about 2 tablespoons. Wear sunscreen even when the sky is overcast, as UV rays penetrate clouds and windows (unless the windows are treated to block UV light). Also, take cover and use sunscreen around water, sand, and snow: they reflect UV radiation.
- Do not use tanning beds. They expose the skin to much larger amounts of damaging UV light than normal sun exposure. The American Dermatologic Association supports a ban on tanning beds, and the World Health Organization classifies them as carcinogenic.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Patient Education and Health Information web site, www.clevelandclinic.org/health