User login
The EHR Report Podcast: Meaningful Use, Part 1
Welcome to the debut of the EHR Report Podcast!
Starting this month, the authors of the EHR Report column, Dr. Neil Skolnik and Dr. Chris Notte, will deliver even more of their EHR know-how via their new podcast. Tune in regularly for candid analyses of EHR strategies, advanced tips on best practices, and one-on-one interviews with innovators in the field of EHRs.
In this, the first of a two-part series, Dr. Skolnik and Dr. Notte offer listeners an in-depth discussion of exactly what it takes for physicians to achieve meaningful use of their electronic health records software and earn federal incentives.
To download the podcast, click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
Welcome to the debut of the EHR Report Podcast!
Starting this month, the authors of the EHR Report column, Dr. Neil Skolnik and Dr. Chris Notte, will deliver even more of their EHR know-how via their new podcast. Tune in regularly for candid analyses of EHR strategies, advanced tips on best practices, and one-on-one interviews with innovators in the field of EHRs.
In this, the first of a two-part series, Dr. Skolnik and Dr. Notte offer listeners an in-depth discussion of exactly what it takes for physicians to achieve meaningful use of their electronic health records software and earn federal incentives.
To download the podcast, click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
Welcome to the debut of the EHR Report Podcast!
Starting this month, the authors of the EHR Report column, Dr. Neil Skolnik and Dr. Chris Notte, will deliver even more of their EHR know-how via their new podcast. Tune in regularly for candid analyses of EHR strategies, advanced tips on best practices, and one-on-one interviews with innovators in the field of EHRs.
In this, the first of a two-part series, Dr. Skolnik and Dr. Notte offer listeners an in-depth discussion of exactly what it takes for physicians to achieve meaningful use of their electronic health records software and earn federal incentives.
To download the podcast, click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
The EHR Report Podcast: Meaningful Use, Part 1
Welcome to the debut of the EHR Report Podcast!
Starting this month, the authors of the EHR Report column, Dr. Neil Skolnik and Dr. Chris Notte, will deliver even more of their EHR know-how via their new podcast. Tune in regularly for candid analyses of EHR strategies, advanced tips on best practices, and one-on-one interviews with innovators in the field of EHRs.
In this, the first of a two-part series, Dr. Skolnik and Dr. Notte offer listeners an in-depth discussion of exactly what it takes for physicians to achieve meaningful use of their electronic health records software and earn federal incentives.
To download the podcast, click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
Welcome to the debut of the EHR Report Podcast!
Starting this month, the authors of the EHR Report column, Dr. Neil Skolnik and Dr. Chris Notte, will deliver even more of their EHR know-how via their new podcast. Tune in regularly for candid analyses of EHR strategies, advanced tips on best practices, and one-on-one interviews with innovators in the field of EHRs.
In this, the first of a two-part series, Dr. Skolnik and Dr. Notte offer listeners an in-depth discussion of exactly what it takes for physicians to achieve meaningful use of their electronic health records software and earn federal incentives.
To download the podcast, click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
Welcome to the debut of the EHR Report Podcast!
Starting this month, the authors of the EHR Report column, Dr. Neil Skolnik and Dr. Chris Notte, will deliver even more of their EHR know-how via their new podcast. Tune in regularly for candid analyses of EHR strategies, advanced tips on best practices, and one-on-one interviews with innovators in the field of EHRs.
In this, the first of a two-part series, Dr. Skolnik and Dr. Notte offer listeners an in-depth discussion of exactly what it takes for physicians to achieve meaningful use of their electronic health records software and earn federal incentives.
To download the podcast, click here.
To read the related column, click here.
To listen via this Web page, click on the player below:
When Your Patient With Depression Has a Family
Julie Totten was 24 years old when her brother Mark took his life. Shortly after, she helped her father, who had been suffering from undiagnosed depression all his life, get treated for the illness. In dealing with the depression that afflicted her father and brother, she felt alone, lost, and responsible. She thought there must be a lot of other families like hers.
So, 10 years after Mark’s death, Julie founded Families for Depression Awareness, a nonprofit organization to help families, including family caregivers like her, recognize and cope with depressive disorders to get people well and prevent suicides. The organization’s website, helps families recognize and cope with depression, and focuses on getting people into treatment to prevent suicide.
Dr. Bill Beardslee, chairman of psychiatry at Children’s Hospital Boston, lost an older sister to suicide when he was in medical school. "The depression took her over and after a valiant struggle against it, she took her own life some years later.
"It has taken me many years to deal with that and many conversations with my father, my mother and my wife, my friends, and, more recently, my children. Above all, it has given me a sense of how awful this illness can be for families," he wrote on the Hachette Book Group website. In his book, Out of the Darkened Room (New York: Little, Brown & Co., 2002), he describes the experiences of families with depression and strategies that families find helpful. It is highly recommended to psychiatrists to share with their patients and families.
Parents with depression worry that their children are suffering. Dr. Beardslee, who also is the Gardner-Monks Professor of Child Psychiatry at Harvard Medical School, Boston, has developed interventions with his colleagues aimed at helping these families. A major goal is "breaking the silence and helping the family talk together about depression." He conceptualizes depression as a chronic medical illness but also a family calamity and has developed an intervention to help families talk together and make meaning together.
His website, Families Preventing and Overcoming Depression provides details on the Family Talk Preventive Intervention. This is a public health, strength-based, and family-centered intervention designed to support families in which one or both parents have depression. This evidence-based practice partners with families to improve relationships and functioning by educating families on depression risk factors and understanding the benefits of applying protective factors to promote resilience.
Dr. Beardslee has many international collaborators in many different countries: Australia, Finland, the Netherlands, Norway, Sweden, Columbia, Costa Rica, and Iceland. Family interventions in these countries are supported by government, and in the public health, and medical and mental health systems. Interventions in these countries are more widespread and systematically available than they are in the United States.
A symposium on this topic will be held at the American Psychiatric Association’s annual meeting in Philadelphia, moderated by Dr. Ellen Berman. It is called "When Your Patient Is a Parent: Supporting the Family and Addressing the Needs of Children." As part of that symposium, Dr. Beardslee will present "Clinical Implications of Evidence-Based Preventive Interventions for Families With Parental Depression." Finally, Ms. Totten will present "A Family Perspective," and I will present "Overview of Needs of the Children of Parents With Mental Illness." See you there!
Dr. Heru is an associate professor of psychiatry at the University of Colorado at Denver, Aurora. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic
Julie Totten was 24 years old when her brother Mark took his life. Shortly after, she helped her father, who had been suffering from undiagnosed depression all his life, get treated for the illness. In dealing with the depression that afflicted her father and brother, she felt alone, lost, and responsible. She thought there must be a lot of other families like hers.
So, 10 years after Mark’s death, Julie founded Families for Depression Awareness, a nonprofit organization to help families, including family caregivers like her, recognize and cope with depressive disorders to get people well and prevent suicides. The organization’s website, helps families recognize and cope with depression, and focuses on getting people into treatment to prevent suicide.
Dr. Bill Beardslee, chairman of psychiatry at Children’s Hospital Boston, lost an older sister to suicide when he was in medical school. "The depression took her over and after a valiant struggle against it, she took her own life some years later.
"It has taken me many years to deal with that and many conversations with my father, my mother and my wife, my friends, and, more recently, my children. Above all, it has given me a sense of how awful this illness can be for families," he wrote on the Hachette Book Group website. In his book, Out of the Darkened Room (New York: Little, Brown & Co., 2002), he describes the experiences of families with depression and strategies that families find helpful. It is highly recommended to psychiatrists to share with their patients and families.
Parents with depression worry that their children are suffering. Dr. Beardslee, who also is the Gardner-Monks Professor of Child Psychiatry at Harvard Medical School, Boston, has developed interventions with his colleagues aimed at helping these families. A major goal is "breaking the silence and helping the family talk together about depression." He conceptualizes depression as a chronic medical illness but also a family calamity and has developed an intervention to help families talk together and make meaning together.
His website, Families Preventing and Overcoming Depression provides details on the Family Talk Preventive Intervention. This is a public health, strength-based, and family-centered intervention designed to support families in which one or both parents have depression. This evidence-based practice partners with families to improve relationships and functioning by educating families on depression risk factors and understanding the benefits of applying protective factors to promote resilience.
Dr. Beardslee has many international collaborators in many different countries: Australia, Finland, the Netherlands, Norway, Sweden, Columbia, Costa Rica, and Iceland. Family interventions in these countries are supported by government, and in the public health, and medical and mental health systems. Interventions in these countries are more widespread and systematically available than they are in the United States.
A symposium on this topic will be held at the American Psychiatric Association’s annual meeting in Philadelphia, moderated by Dr. Ellen Berman. It is called "When Your Patient Is a Parent: Supporting the Family and Addressing the Needs of Children." As part of that symposium, Dr. Beardslee will present "Clinical Implications of Evidence-Based Preventive Interventions for Families With Parental Depression." Finally, Ms. Totten will present "A Family Perspective," and I will present "Overview of Needs of the Children of Parents With Mental Illness." See you there!
Dr. Heru is an associate professor of psychiatry at the University of Colorado at Denver, Aurora. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic
Julie Totten was 24 years old when her brother Mark took his life. Shortly after, she helped her father, who had been suffering from undiagnosed depression all his life, get treated for the illness. In dealing with the depression that afflicted her father and brother, she felt alone, lost, and responsible. She thought there must be a lot of other families like hers.
So, 10 years after Mark’s death, Julie founded Families for Depression Awareness, a nonprofit organization to help families, including family caregivers like her, recognize and cope with depressive disorders to get people well and prevent suicides. The organization’s website, helps families recognize and cope with depression, and focuses on getting people into treatment to prevent suicide.
Dr. Bill Beardslee, chairman of psychiatry at Children’s Hospital Boston, lost an older sister to suicide when he was in medical school. "The depression took her over and after a valiant struggle against it, she took her own life some years later.
"It has taken me many years to deal with that and many conversations with my father, my mother and my wife, my friends, and, more recently, my children. Above all, it has given me a sense of how awful this illness can be for families," he wrote on the Hachette Book Group website. In his book, Out of the Darkened Room (New York: Little, Brown & Co., 2002), he describes the experiences of families with depression and strategies that families find helpful. It is highly recommended to psychiatrists to share with their patients and families.
Parents with depression worry that their children are suffering. Dr. Beardslee, who also is the Gardner-Monks Professor of Child Psychiatry at Harvard Medical School, Boston, has developed interventions with his colleagues aimed at helping these families. A major goal is "breaking the silence and helping the family talk together about depression." He conceptualizes depression as a chronic medical illness but also a family calamity and has developed an intervention to help families talk together and make meaning together.
His website, Families Preventing and Overcoming Depression provides details on the Family Talk Preventive Intervention. This is a public health, strength-based, and family-centered intervention designed to support families in which one or both parents have depression. This evidence-based practice partners with families to improve relationships and functioning by educating families on depression risk factors and understanding the benefits of applying protective factors to promote resilience.
Dr. Beardslee has many international collaborators in many different countries: Australia, Finland, the Netherlands, Norway, Sweden, Columbia, Costa Rica, and Iceland. Family interventions in these countries are supported by government, and in the public health, and medical and mental health systems. Interventions in these countries are more widespread and systematically available than they are in the United States.
A symposium on this topic will be held at the American Psychiatric Association’s annual meeting in Philadelphia, moderated by Dr. Ellen Berman. It is called "When Your Patient Is a Parent: Supporting the Family and Addressing the Needs of Children." As part of that symposium, Dr. Beardslee will present "Clinical Implications of Evidence-Based Preventive Interventions for Families With Parental Depression." Finally, Ms. Totten will present "A Family Perspective," and I will present "Overview of Needs of the Children of Parents With Mental Illness." See you there!
Dr. Heru is an associate professor of psychiatry at the University of Colorado at Denver, Aurora. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic
FDA Investigates Major Bleeding Events in Dabigatran Patients
A little more than a year after the new anticoagulant dabigatran (Pradaxa) was approved for stroke prevention in nonvalvular atrial fibrillation (NVAF) patients, the FDA is evaluating post-marketing reports of serious bleeds in patients taking the drug.
The FDA is trying to determine if patients on Pradaxa are experiencing severe bleeding more than expected based on results of the clinical trial that led to Pradaxa’s approval, according to FDA spokeswoman Sandy Walsh.
“At this time, FDA continues to believe that Pradaxa provides an important health benefit when used as directed and recommends that healthcare professionals who prescribe Pradaxa follow the recommendations in the approved drug label,” Walsh tells The Hospitalist.

—Robert Pendleton, MD, director of the hospitalist program, University of Utah Healthcare; medical director, University Healthcare Thrombosis Service
Patients should not stop taking dabigatran without first talking to their doctors, the FDA announcement cautions. While “serious, even fatal events have been reported,” according to the FDA’s announcement, Walsh says the FDA isn’t prepared to say how many reports of serious bleeding events have been received because they’re still being reviewed.
“We often put out ‘early’ communications when we learn of drug safety issues,” she says. “We want to be transparent and make [the] public [aware of] what we do know, but our analysis is not final. At this point, the FDA is still evaluating this issue.”
Bleeding that leads to serious or fatal outcomes is a well-recognized complication of all anticoagulant therapies.
Dabigatran, a direct thrombin inhibitor, was approved in October 2010, becoming the first new oral anticoagulant approved in 50 years. It was the first approved among several new anti-coagulants that are poised to enter the market and are expected to challenge warfarin (Coumadin), the longtime standard of care.
The new drugs—including rivaroxaban (Xarelto), a Factor Xa-inhibitor that was approved in 2011—have been eagerly anticipated because they don’t require frequent blood draws for monitoring, as warfarin does. Hospitalists are especially interested in the new anticoagulant therapies because they treat numerous patients at an increased risk of clotting.
In the RE-LY trial, the 18,000-patient clinical trial comparing dabigatran and warfarin, major bleeding events occurred at similar rates with the two drugs.
Dabigatran manufacturer Boehringer Ingelheim is working with the FDA to evaluate the major bleeding reports, but spokeswoman Anna Moses says the drug has been performing according to expectations.
“Global data collected to date on major bleeding are consistent with our expectations based on the RE-LY trial and are in alignment with the U.S. Prescribing Information, which clearly state the benefits and risks associated with Pradaxa,” Moses says. “Overall, the positive-benefit-risk ratio of Pradaxa in NVAF remains unchanged.” (Visit the manufacturer website for prescribing information [PDF].)
Robert Pendleton, MD, director of the hospitalist program at the University of Utah Healthcare and Medical Director of the University Healthcare Thrombosis Service, expressed no surprise at the FDA’s statement.
“Although the data with new anticoagulants like Pradaxa is very favorable in a clinical trial setting, there’s great risk of enhanced demonstration of harm in the real-world setting if it’s not used optimally,” Dr. Pendleton says. “There will be more liberal sort of prescribing in a less-pure patient population.
So if people are not particularly cognizant of a patient’s renal function, their body weight, prior history of bleeding, etc., then you’re sort of applying new drugs in patients who are even more prone to bleed.”
Dr. Pendleton notes that in subgroup analyses, the slight benefits of the new drugs have come in patients with poor warfarin control, so if patients with good warfarin control are switched, outcomes could generally be expected not to be better, and could be worse.
“It won’t cause me to take people who I have prescribed Pradaxa and switch them back to warfarin,” he says, “but part of that is [that] here, in our healthcare system, we’re pretty cautious in who gets put on one of the new agents. And so those that do are patients who are most like those in the clinical trial.”
Tom Collins is a freelance writer in Florida.
A little more than a year after the new anticoagulant dabigatran (Pradaxa) was approved for stroke prevention in nonvalvular atrial fibrillation (NVAF) patients, the FDA is evaluating post-marketing reports of serious bleeds in patients taking the drug.
The FDA is trying to determine if patients on Pradaxa are experiencing severe bleeding more than expected based on results of the clinical trial that led to Pradaxa’s approval, according to FDA spokeswoman Sandy Walsh.
“At this time, FDA continues to believe that Pradaxa provides an important health benefit when used as directed and recommends that healthcare professionals who prescribe Pradaxa follow the recommendations in the approved drug label,” Walsh tells The Hospitalist.

—Robert Pendleton, MD, director of the hospitalist program, University of Utah Healthcare; medical director, University Healthcare Thrombosis Service
Patients should not stop taking dabigatran without first talking to their doctors, the FDA announcement cautions. While “serious, even fatal events have been reported,” according to the FDA’s announcement, Walsh says the FDA isn’t prepared to say how many reports of serious bleeding events have been received because they’re still being reviewed.
“We often put out ‘early’ communications when we learn of drug safety issues,” she says. “We want to be transparent and make [the] public [aware of] what we do know, but our analysis is not final. At this point, the FDA is still evaluating this issue.”
Bleeding that leads to serious or fatal outcomes is a well-recognized complication of all anticoagulant therapies.
Dabigatran, a direct thrombin inhibitor, was approved in October 2010, becoming the first new oral anticoagulant approved in 50 years. It was the first approved among several new anti-coagulants that are poised to enter the market and are expected to challenge warfarin (Coumadin), the longtime standard of care.
The new drugs—including rivaroxaban (Xarelto), a Factor Xa-inhibitor that was approved in 2011—have been eagerly anticipated because they don’t require frequent blood draws for monitoring, as warfarin does. Hospitalists are especially interested in the new anticoagulant therapies because they treat numerous patients at an increased risk of clotting.
In the RE-LY trial, the 18,000-patient clinical trial comparing dabigatran and warfarin, major bleeding events occurred at similar rates with the two drugs.
Dabigatran manufacturer Boehringer Ingelheim is working with the FDA to evaluate the major bleeding reports, but spokeswoman Anna Moses says the drug has been performing according to expectations.
“Global data collected to date on major bleeding are consistent with our expectations based on the RE-LY trial and are in alignment with the U.S. Prescribing Information, which clearly state the benefits and risks associated with Pradaxa,” Moses says. “Overall, the positive-benefit-risk ratio of Pradaxa in NVAF remains unchanged.” (Visit the manufacturer website for prescribing information [PDF].)
Robert Pendleton, MD, director of the hospitalist program at the University of Utah Healthcare and Medical Director of the University Healthcare Thrombosis Service, expressed no surprise at the FDA’s statement.
“Although the data with new anticoagulants like Pradaxa is very favorable in a clinical trial setting, there’s great risk of enhanced demonstration of harm in the real-world setting if it’s not used optimally,” Dr. Pendleton says. “There will be more liberal sort of prescribing in a less-pure patient population.
So if people are not particularly cognizant of a patient’s renal function, their body weight, prior history of bleeding, etc., then you’re sort of applying new drugs in patients who are even more prone to bleed.”
Dr. Pendleton notes that in subgroup analyses, the slight benefits of the new drugs have come in patients with poor warfarin control, so if patients with good warfarin control are switched, outcomes could generally be expected not to be better, and could be worse.
“It won’t cause me to take people who I have prescribed Pradaxa and switch them back to warfarin,” he says, “but part of that is [that] here, in our healthcare system, we’re pretty cautious in who gets put on one of the new agents. And so those that do are patients who are most like those in the clinical trial.”
Tom Collins is a freelance writer in Florida.
A little more than a year after the new anticoagulant dabigatran (Pradaxa) was approved for stroke prevention in nonvalvular atrial fibrillation (NVAF) patients, the FDA is evaluating post-marketing reports of serious bleeds in patients taking the drug.
The FDA is trying to determine if patients on Pradaxa are experiencing severe bleeding more than expected based on results of the clinical trial that led to Pradaxa’s approval, according to FDA spokeswoman Sandy Walsh.
“At this time, FDA continues to believe that Pradaxa provides an important health benefit when used as directed and recommends that healthcare professionals who prescribe Pradaxa follow the recommendations in the approved drug label,” Walsh tells The Hospitalist.

—Robert Pendleton, MD, director of the hospitalist program, University of Utah Healthcare; medical director, University Healthcare Thrombosis Service
Patients should not stop taking dabigatran without first talking to their doctors, the FDA announcement cautions. While “serious, even fatal events have been reported,” according to the FDA’s announcement, Walsh says the FDA isn’t prepared to say how many reports of serious bleeding events have been received because they’re still being reviewed.
“We often put out ‘early’ communications when we learn of drug safety issues,” she says. “We want to be transparent and make [the] public [aware of] what we do know, but our analysis is not final. At this point, the FDA is still evaluating this issue.”
Bleeding that leads to serious or fatal outcomes is a well-recognized complication of all anticoagulant therapies.
Dabigatran, a direct thrombin inhibitor, was approved in October 2010, becoming the first new oral anticoagulant approved in 50 years. It was the first approved among several new anti-coagulants that are poised to enter the market and are expected to challenge warfarin (Coumadin), the longtime standard of care.
The new drugs—including rivaroxaban (Xarelto), a Factor Xa-inhibitor that was approved in 2011—have been eagerly anticipated because they don’t require frequent blood draws for monitoring, as warfarin does. Hospitalists are especially interested in the new anticoagulant therapies because they treat numerous patients at an increased risk of clotting.
In the RE-LY trial, the 18,000-patient clinical trial comparing dabigatran and warfarin, major bleeding events occurred at similar rates with the two drugs.
Dabigatran manufacturer Boehringer Ingelheim is working with the FDA to evaluate the major bleeding reports, but spokeswoman Anna Moses says the drug has been performing according to expectations.
“Global data collected to date on major bleeding are consistent with our expectations based on the RE-LY trial and are in alignment with the U.S. Prescribing Information, which clearly state the benefits and risks associated with Pradaxa,” Moses says. “Overall, the positive-benefit-risk ratio of Pradaxa in NVAF remains unchanged.” (Visit the manufacturer website for prescribing information [PDF].)
Robert Pendleton, MD, director of the hospitalist program at the University of Utah Healthcare and Medical Director of the University Healthcare Thrombosis Service, expressed no surprise at the FDA’s statement.
“Although the data with new anticoagulants like Pradaxa is very favorable in a clinical trial setting, there’s great risk of enhanced demonstration of harm in the real-world setting if it’s not used optimally,” Dr. Pendleton says. “There will be more liberal sort of prescribing in a less-pure patient population.
So if people are not particularly cognizant of a patient’s renal function, their body weight, prior history of bleeding, etc., then you’re sort of applying new drugs in patients who are even more prone to bleed.”
Dr. Pendleton notes that in subgroup analyses, the slight benefits of the new drugs have come in patients with poor warfarin control, so if patients with good warfarin control are switched, outcomes could generally be expected not to be better, and could be worse.
“It won’t cause me to take people who I have prescribed Pradaxa and switch them back to warfarin,” he says, “but part of that is [that] here, in our healthcare system, we’re pretty cautious in who gets put on one of the new agents. And so those that do are patients who are most like those in the clinical trial.”
Tom Collins is a freelance writer in Florida.
VIDEO: Car Seat Safety
In this video, Dr. Beers explains why a rear facing car seat is safer for your child. Safety data show that the use of rear facing seats in the first 2 years of life provides better support for a child's back and neck muscles in addition to providing maximum protection in the event of an auto accident.
In this video, Dr. Beers explains why a rear facing car seat is safer for your child. Safety data show that the use of rear facing seats in the first 2 years of life provides better support for a child's back and neck muscles in addition to providing maximum protection in the event of an auto accident.
In this video, Dr. Beers explains why a rear facing car seat is safer for your child. Safety data show that the use of rear facing seats in the first 2 years of life provides better support for a child's back and neck muscles in addition to providing maximum protection in the event of an auto accident.
Blinatumomab Induces Complete Remissions in Acute Lymphoblastic Leukemia
SAN DIEGO – The novel antibody blinatumomab induced high complete remission rates in adults with relapsed B-precursor acute lymphoblastic leukemia in early clinical trials, according to Dr. Max S. Topp.
In a phase II study with a dose-finding phase, 9 of 12 patients who received blinatumomab 5 mcg/m2 per day for 1 week, followed by a 15-mcg dose on subsequent weeks, had either a complete remission (CR) or a CR with partial hematologic recovery (CRh), Dr. Topp of the University of Würzburg (Germany) said at the annual meeting of the American Society of Hematology.
"We have exceptionally high rates of hematological complete remissions in these patients, and it ought to be noted that every patient has achieved MRD [minimal residual disease] negativity," said Dr. Topp.
At a median follow-up of 9.7 months, the median overall survival had not been reached, he added.
Blinatumomab is a bispecific T-cell engager designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. It has shown good activity in a phase I clinical trial in patients with relapsed non-Hodgkin’s lymphoma, and in a study of patients with B-ALL who were positive for MRD (J. Clin. Oncol. 2011;29:2493-8).
The MT 103-206 trial was an open-label, multicenter phase II trial of blinatumomab in patients with relapsed/refractory B-precursor ALL, or Philadelphia chromosome–positive ALL (Ph+ALL) who were ineligible for tyrosine kinase inhibitors or who were in relapse following an allogeneic stem cell transplant.
The trial had a dose-finding run-in phase, with four patient cohorts. Dr. Topp focused on cohorts 2a and 3, in which patients received the selected dose schedule: an initial dose of 5 mcg/m2 IV daily for the 1st week of cycle 1, followed by 15 mcg/m2 per day for weeks 2-4 of every 4-week cycle, and every subsequent cycle. Patients had 2 weeks off between each cycle.
Patients who had a CR or CRh within the first two treatment cycles underwent consolidation with three additional cycles of blinatumonab and allogeneic stem cell transplant.
At the selected dose, the most common clinical adverse events were fever in 67%, headache in 33%, and tremor in 33%. Most of the events occurred during the first cycle, and no patients had to permanently discontinue therapy because of adverse events.
Among all cohorts (totaling 25 patients), there were 17 who had a CR or CRh: 5 of 7 patients who received a 15-mcg dose throughout treatment (cohort 1); 3 of 6 patients who received escalating doses of 5-, 15-, and 30-mcg doses (cohort 2b); and 9 of 12 patients in cohorts 2a and 3 combined. All patients with a CR or CRh were also MRD negative, defined as an MRD less than 104 measured by polymerase chain reaction evaluation of individual rearrangement of immunoglobulin or T-cell receptor genes by a central laboratory.
Dr. Topp explained that there were high response rates among all patient subgroups, including patients with Ph+ALL, and those with the t(4,11) translocation.
As of early November 2011, 6 of 17 patients with complete responses had relapses. One of four patients who had undergone allogeneic hematopoietic stem cell transplant had a medullary relapse; this patient was CD19 negative. A total of 5 of 13 patients had a relapse prior to transplant – 2 medullary relapses (1 CD19-negative and 1 positive) and 3 extramedullary relapses (1 CD19 negative and 2 positive).
One patient who had a medullary relapse but retained CD19 expression was retreated with blinatumomab and had a CRh of 7 months’ duration; the patient achieved a second, ongoing CRh after more blinatumomab.
The median duration of complete hematologic remission was 7.1 months (218 days) among 18 patients (12 responders) in cohorts 1, 2a, and 2b.
Asked in an interview whether an agent targeted against CD19 might work in combination with an anti-CD20 agent such as rituximab (Rituxan), Dr. Alan S. Wayne, a leukemia specialist and session comoderator who was not involved in the study, said that CD20 is not as attractive a target in ALL as it is in lymphoma or other hematologic malignancies.
"The question of CD20 in ALL is a little challenging, because the expression is less universal and even within individual cases across blasts," said Dr. Wayne, who is also head of the hematologic disease division of the pediatric oncology branch at the National Cancer Institute.
He noted, however, that there is evidence to suggest that pretreatment of patients with steroids may increase CD20 expression.
"This is an exciting new era for combining agents with a variety of different mechanisms of action, and also toxicity profiles. One could imagine, for example, [using] steroid to increase CD20 expression, rituximab, and then another CD19- or CD22-targeting agent," he said.
The MT 103-206 trial was supported by Micromet. Dr. Topp and coauthors Dr. Ralf Bargou and Dr. Nicola Goekbuget disclosed consulting for and/or receiving honoraria from the company. Three other coauthors are employees of the company. Dr. Wayne reported no relevant financial disclosures.
SAN DIEGO – The novel antibody blinatumomab induced high complete remission rates in adults with relapsed B-precursor acute lymphoblastic leukemia in early clinical trials, according to Dr. Max S. Topp.
In a phase II study with a dose-finding phase, 9 of 12 patients who received blinatumomab 5 mcg/m2 per day for 1 week, followed by a 15-mcg dose on subsequent weeks, had either a complete remission (CR) or a CR with partial hematologic recovery (CRh), Dr. Topp of the University of Würzburg (Germany) said at the annual meeting of the American Society of Hematology.
"We have exceptionally high rates of hematological complete remissions in these patients, and it ought to be noted that every patient has achieved MRD [minimal residual disease] negativity," said Dr. Topp.
At a median follow-up of 9.7 months, the median overall survival had not been reached, he added.
Blinatumomab is a bispecific T-cell engager designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. It has shown good activity in a phase I clinical trial in patients with relapsed non-Hodgkin’s lymphoma, and in a study of patients with B-ALL who were positive for MRD (J. Clin. Oncol. 2011;29:2493-8).
The MT 103-206 trial was an open-label, multicenter phase II trial of blinatumomab in patients with relapsed/refractory B-precursor ALL, or Philadelphia chromosome–positive ALL (Ph+ALL) who were ineligible for tyrosine kinase inhibitors or who were in relapse following an allogeneic stem cell transplant.
The trial had a dose-finding run-in phase, with four patient cohorts. Dr. Topp focused on cohorts 2a and 3, in which patients received the selected dose schedule: an initial dose of 5 mcg/m2 IV daily for the 1st week of cycle 1, followed by 15 mcg/m2 per day for weeks 2-4 of every 4-week cycle, and every subsequent cycle. Patients had 2 weeks off between each cycle.
Patients who had a CR or CRh within the first two treatment cycles underwent consolidation with three additional cycles of blinatumonab and allogeneic stem cell transplant.
At the selected dose, the most common clinical adverse events were fever in 67%, headache in 33%, and tremor in 33%. Most of the events occurred during the first cycle, and no patients had to permanently discontinue therapy because of adverse events.
Among all cohorts (totaling 25 patients), there were 17 who had a CR or CRh: 5 of 7 patients who received a 15-mcg dose throughout treatment (cohort 1); 3 of 6 patients who received escalating doses of 5-, 15-, and 30-mcg doses (cohort 2b); and 9 of 12 patients in cohorts 2a and 3 combined. All patients with a CR or CRh were also MRD negative, defined as an MRD less than 104 measured by polymerase chain reaction evaluation of individual rearrangement of immunoglobulin or T-cell receptor genes by a central laboratory.
Dr. Topp explained that there were high response rates among all patient subgroups, including patients with Ph+ALL, and those with the t(4,11) translocation.
As of early November 2011, 6 of 17 patients with complete responses had relapses. One of four patients who had undergone allogeneic hematopoietic stem cell transplant had a medullary relapse; this patient was CD19 negative. A total of 5 of 13 patients had a relapse prior to transplant – 2 medullary relapses (1 CD19-negative and 1 positive) and 3 extramedullary relapses (1 CD19 negative and 2 positive).
One patient who had a medullary relapse but retained CD19 expression was retreated with blinatumomab and had a CRh of 7 months’ duration; the patient achieved a second, ongoing CRh after more blinatumomab.
The median duration of complete hematologic remission was 7.1 months (218 days) among 18 patients (12 responders) in cohorts 1, 2a, and 2b.
Asked in an interview whether an agent targeted against CD19 might work in combination with an anti-CD20 agent such as rituximab (Rituxan), Dr. Alan S. Wayne, a leukemia specialist and session comoderator who was not involved in the study, said that CD20 is not as attractive a target in ALL as it is in lymphoma or other hematologic malignancies.
"The question of CD20 in ALL is a little challenging, because the expression is less universal and even within individual cases across blasts," said Dr. Wayne, who is also head of the hematologic disease division of the pediatric oncology branch at the National Cancer Institute.
He noted, however, that there is evidence to suggest that pretreatment of patients with steroids may increase CD20 expression.
"This is an exciting new era for combining agents with a variety of different mechanisms of action, and also toxicity profiles. One could imagine, for example, [using] steroid to increase CD20 expression, rituximab, and then another CD19- or CD22-targeting agent," he said.
The MT 103-206 trial was supported by Micromet. Dr. Topp and coauthors Dr. Ralf Bargou and Dr. Nicola Goekbuget disclosed consulting for and/or receiving honoraria from the company. Three other coauthors are employees of the company. Dr. Wayne reported no relevant financial disclosures.
SAN DIEGO – The novel antibody blinatumomab induced high complete remission rates in adults with relapsed B-precursor acute lymphoblastic leukemia in early clinical trials, according to Dr. Max S. Topp.
In a phase II study with a dose-finding phase, 9 of 12 patients who received blinatumomab 5 mcg/m2 per day for 1 week, followed by a 15-mcg dose on subsequent weeks, had either a complete remission (CR) or a CR with partial hematologic recovery (CRh), Dr. Topp of the University of Würzburg (Germany) said at the annual meeting of the American Society of Hematology.
"We have exceptionally high rates of hematological complete remissions in these patients, and it ought to be noted that every patient has achieved MRD [minimal residual disease] negativity," said Dr. Topp.
At a median follow-up of 9.7 months, the median overall survival had not been reached, he added.
Blinatumomab is a bispecific T-cell engager designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. It has shown good activity in a phase I clinical trial in patients with relapsed non-Hodgkin’s lymphoma, and in a study of patients with B-ALL who were positive for MRD (J. Clin. Oncol. 2011;29:2493-8).
The MT 103-206 trial was an open-label, multicenter phase II trial of blinatumomab in patients with relapsed/refractory B-precursor ALL, or Philadelphia chromosome–positive ALL (Ph+ALL) who were ineligible for tyrosine kinase inhibitors or who were in relapse following an allogeneic stem cell transplant.
The trial had a dose-finding run-in phase, with four patient cohorts. Dr. Topp focused on cohorts 2a and 3, in which patients received the selected dose schedule: an initial dose of 5 mcg/m2 IV daily for the 1st week of cycle 1, followed by 15 mcg/m2 per day for weeks 2-4 of every 4-week cycle, and every subsequent cycle. Patients had 2 weeks off between each cycle.
Patients who had a CR or CRh within the first two treatment cycles underwent consolidation with three additional cycles of blinatumonab and allogeneic stem cell transplant.
At the selected dose, the most common clinical adverse events were fever in 67%, headache in 33%, and tremor in 33%. Most of the events occurred during the first cycle, and no patients had to permanently discontinue therapy because of adverse events.
Among all cohorts (totaling 25 patients), there were 17 who had a CR or CRh: 5 of 7 patients who received a 15-mcg dose throughout treatment (cohort 1); 3 of 6 patients who received escalating doses of 5-, 15-, and 30-mcg doses (cohort 2b); and 9 of 12 patients in cohorts 2a and 3 combined. All patients with a CR or CRh were also MRD negative, defined as an MRD less than 104 measured by polymerase chain reaction evaluation of individual rearrangement of immunoglobulin or T-cell receptor genes by a central laboratory.
Dr. Topp explained that there were high response rates among all patient subgroups, including patients with Ph+ALL, and those with the t(4,11) translocation.
As of early November 2011, 6 of 17 patients with complete responses had relapses. One of four patients who had undergone allogeneic hematopoietic stem cell transplant had a medullary relapse; this patient was CD19 negative. A total of 5 of 13 patients had a relapse prior to transplant – 2 medullary relapses (1 CD19-negative and 1 positive) and 3 extramedullary relapses (1 CD19 negative and 2 positive).
One patient who had a medullary relapse but retained CD19 expression was retreated with blinatumomab and had a CRh of 7 months’ duration; the patient achieved a second, ongoing CRh after more blinatumomab.
The median duration of complete hematologic remission was 7.1 months (218 days) among 18 patients (12 responders) in cohorts 1, 2a, and 2b.
Asked in an interview whether an agent targeted against CD19 might work in combination with an anti-CD20 agent such as rituximab (Rituxan), Dr. Alan S. Wayne, a leukemia specialist and session comoderator who was not involved in the study, said that CD20 is not as attractive a target in ALL as it is in lymphoma or other hematologic malignancies.
"The question of CD20 in ALL is a little challenging, because the expression is less universal and even within individual cases across blasts," said Dr. Wayne, who is also head of the hematologic disease division of the pediatric oncology branch at the National Cancer Institute.
He noted, however, that there is evidence to suggest that pretreatment of patients with steroids may increase CD20 expression.
"This is an exciting new era for combining agents with a variety of different mechanisms of action, and also toxicity profiles. One could imagine, for example, [using] steroid to increase CD20 expression, rituximab, and then another CD19- or CD22-targeting agent," he said.
The MT 103-206 trial was supported by Micromet. Dr. Topp and coauthors Dr. Ralf Bargou and Dr. Nicola Goekbuget disclosed consulting for and/or receiving honoraria from the company. Three other coauthors are employees of the company. Dr. Wayne reported no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: A total of 9 of 12 patients with relapsed B-precursor acute lymphoblastic leukemia who received blinatumomab 5 mcg/m2 per day for 1 week, followed by a 15-mcg dose on subsequent weeks, had either a complete remission or a complete response with partial hematologic recovery,
Data Source: Open-label phase II trial with a dose-finding phase.
Disclosures: The MT 103-206 trial was supported by Micromet. Dr. Topp and coauthors Dr. Ralf Bargou and Dr. Nicola Goekbuget disclosed consulting for and/or receiving honoraria from the company. Three other coauthors are employees of the company. Dr. Wayne reported no relevant financial disclosures.
Intermediate Care: Role for Hospitalists
Hospitalized patients are becoming increasingly complex. The care of such patients may be impacted by the limited resources of the general ward and might benefit from more intensive monitoring in an intensive care unit (ICU)‐like setting. In light of this problem, the intermediate care units (ImCU) may provide a cost‐effective alternative by providing higher levels of staffing tailored to patient needs, without incurring the cost of an ICU admission. The ImCU can reduce costs and improves ICU utilization for sicker patients, decrease ICU readmissions, promote greater flexibility in patient triage, and decrease mortality rates in hospital wards.18
The characteristics of ImCUs depend on resource availability, institutional infrastructure, and the organization and funding of the parent healthcare system. The ImCU may function as a step‐up or step‐down unit, or may provide specialty care for cardiac, neurologic, respiratory, or surgical conditions.811 These units can expand opportunities for co‐management and, at the same time, offer the occasion for training residents to follow up patients through different levels of care (from the general ward to ImCU). In the same way, the multidisciplinary approach of the ImCU can improve the center's teaching potential.
Characterizing the ImCU population requires the assessment of their severity of illness, which is crucial for the evaluation of risk‐adjusted outcomes. The present study evaluated the impact of a hospitalist‐led ImCU on observed‐to‐expected mortality ratios, as well as its role in co‐management and teaching.
PATIENTS AND METHODS
We performed a retrospective observational study, with data collected from April 2006 to April 2010 in a single academic medical center in Pamplona, Spain. The ImCU is a 9‐bed unit adjacent to, but independent from, the mixed ICU. Each bed is equipped with continuous telemetry, pulse oximetry, noninvasive arterial blood pressure, central venous pressure monitoring, and noninvasive pressure support ventilation. The signals are relayed to a central monitoring station and the nurse‐to‐patient ratio is 1:3.
The ImCU rounding team is multidisciplinary, and involves the hospital pharmacist, a nurse, the ImCU resident, the specialist or surgeon, and the attending hospitalist. After the triage process, ImCU patients were admitted to the attending hospitalist, who was responsible for admission and discharge of all ImCU patients. The hospitalist ordered diagnostic or therapeutic interventions as needed, with the exception of orders for procedures or consultations related with specialist/surgeon's specific needs.
Admission and discharge criteria for the ImCU were set according to guidelines defined by The American College of Critical Care Medicine,10 and also served as inclusion criteria for the present study. Exclusion criteria included: age less than 18 years old, severe respiratory failure, status epilepticus, and catastrophic brain illness. Patients admitted for drug administration and desensitization, and also ImCU readmissions, were excluded from data analysis. Patients came from medical and surgical wards, ICU, the operating room, and the emergency room.
A total of 756 patients were admitted to our ImCU during the study period. Patient demographics, past medical history, physiologic parameters at the time of admission, and survival to hospital discharge were recorded for all patients. Patient demographics include: age, gender, location before ImCU admission, length of stay before ImCU admission, reason for ImCU admission, anatomic site of surgery (if applicable), planned or unplanned admission, and infection status (nosocomial). Past medical history includes: the presence of arterial hypertension, diabetes, cirrhosis, chronic renal failure, chronic heart failure, cancer, hematological malignancy, chronic obstructive pulmonary disease (COPD), human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), immunosuppression, radiotherapy, chemotherapy, steroid treatment, and alcoholism. Physiologic parameters abstracted are described in Table 1. We used the Simplified Acute Physiology Score II (SAPS II),12 as a prognostic and severity score. SAPS II is the only previously validated score in intermediate care.13 In‐hospital mortality was the clinical outcome measured.
|
| Vital signs |
| Glasgow Coma Scale |
| Serum bilirubin |
| Serum creatinine |
| Urea nitrogen |
| Leucocyte count |
| Serum sodium |
| Serum potassium |
| Bicarbonate levels |
| Urinary output in the first 24 hr |
| Oxygenation and ventilatory support |
Data were entered into a computer database by the authors. Statistical analysis was not blinded, and was performed using SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean standard deviation or median (25%‐75% interquartile range). For nonparametric measure of statistical dependence of quantitative variables, we used Spearman's correlation coefficient. Discrimination was evaluated by calculating the area under receiver operating characteristic curve (AUROC).
The study protocol was approved by the institutional review board at the Clnica Universidad de Navarra in Pamplona, Spain.
RESULTS
Four hundred fifty‐six patients were included in data analysis. Three hundred patients were excluded: 61 low‐risk patients (drug administration and desensitization), 147 readmissions, and 92 patients for missing variables. Patient characteristics, including probability of death following ImCU admission and discharge location, are summarized in Table 2. The mean age was 65.6 years, and about 35% of patients had a SAPS II‐based risk of death higher than 25% at the time of ImCU admission. The median length of stay was 4 (3‐7) days.
| |
| Age (yr) | 65.6 14.3 |
| Gender | |
| Male | 283 (62.1%) |
| Female | 173 (37.9%) |
| Location prior to admission | |
| General ward | 252 (55.3%) |
| Emergency room | 96 (21.1%) |
| ICU | 63 (13.8%) |
| Operating room | 28 (6.1%) |
| Other hospital | 17 (3.7%) |
| Probability of in‐hospital mortality based on SAPS II | |
| <10% | 128 (28.1%) |
| 11%‐25% | 176 (38.6%) |
| 26%‐50% | 107 (23.4%) |
| >50% | 45 (9.9%) |
| Global expected mortality (in‐hospital) | 23.2% |
| Global observed mortality (in‐hospital) | 20.6% (94/456) |
| O/E mortality ratio | 0.89 |
| Discharge location | |
| General ward | 352/456 (77.2%) |
| ICU | 65/456 (14.3%) |
| Home | 1/456 (0.2%) |
| Other hospital | 11/456 (2.4%) |
| Death location | |
| ImCU | 27/456 (5.9%) |
| ICU (transferred patients) | 32/65* (49.2%) |
| General ward | 35/352* (9.9%) |
Outcomes
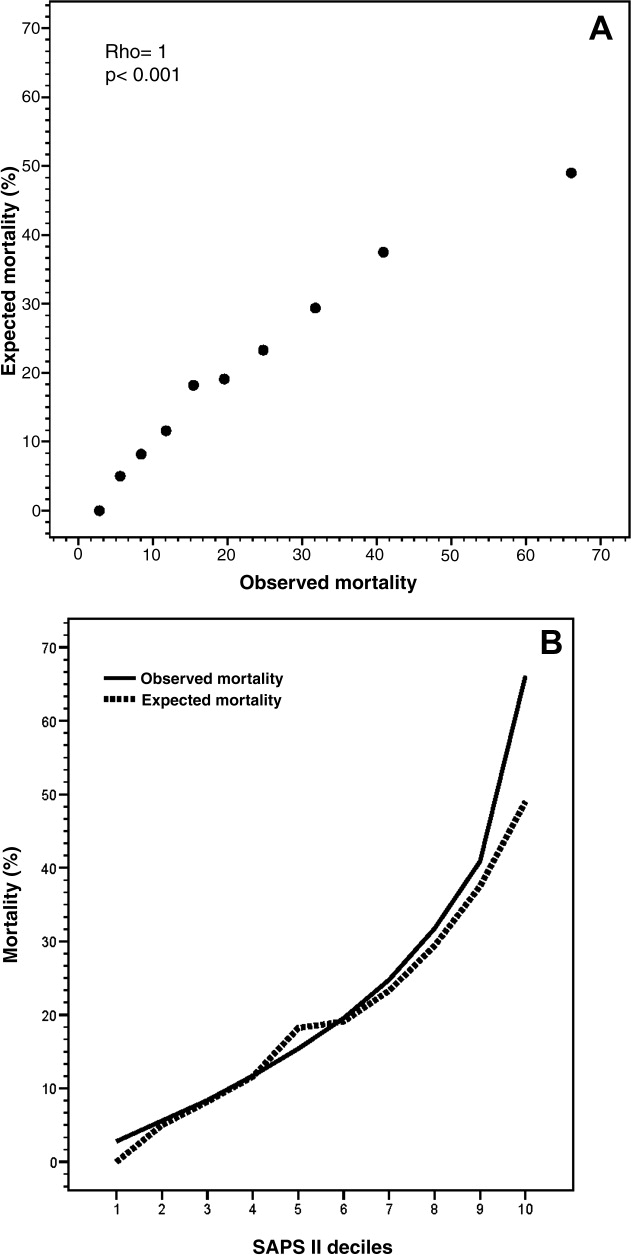
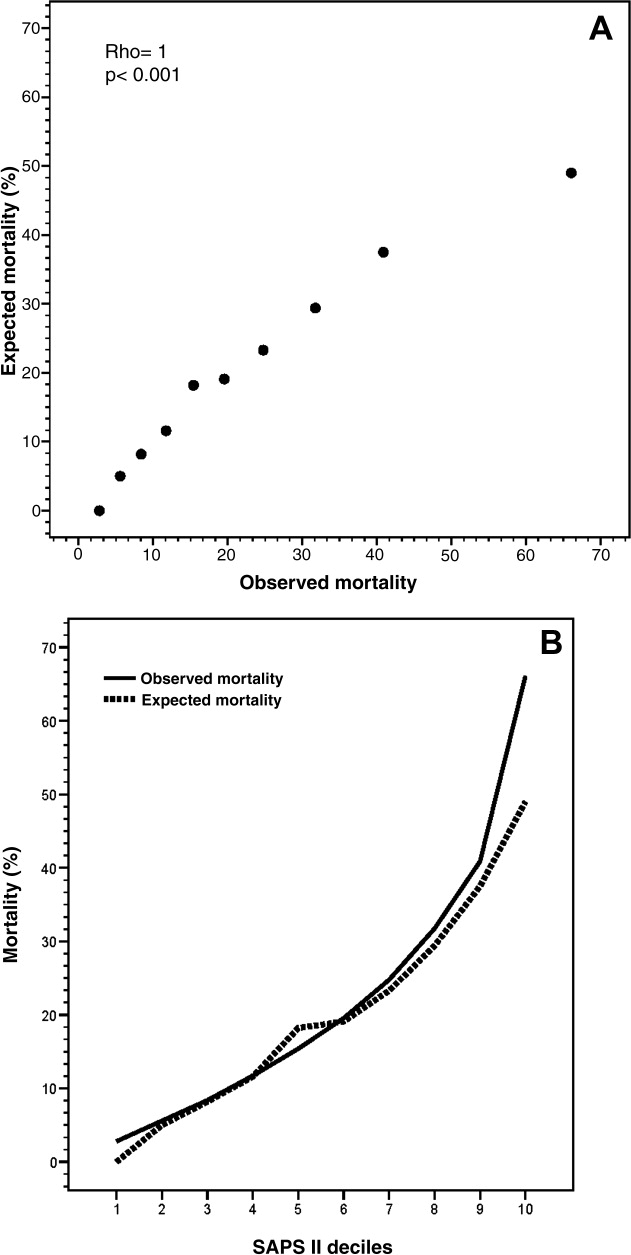
The mean SAPS II of the cohort was 37 12 points, and the expected mortality derived from this score was 23.2%. The observed in‐hospital mortality was 20.6% (94/456) resulting in an observed‐to‐expected mortality ratio of 0.89 (Table 2). Reasons for ImCU admission, as well as mortality ratios, are described in Table 3. The correlation between SAPS II predicted and observed death rates was accurate and statistically significant (Rho = 1.0, P < 0.001) (Figure 1). The AUROC for SAPS II predicting in‐hospital mortality was 0.75 (P < 0.001).
| Condition | Patients | SAPS II | Expected Mortality | Observed Mortality | O/E Ratio |
|---|---|---|---|---|---|
| |||||
| Respiratory failure | 153 (33.6%) | 36.1 9.7 | 21.5 15.3% | 25.5% (39) | 1.19 |
| Sepsis | 88 (19.3%) | 45.7 15.1 | 37.5 25.1% | 22.7% (20) | 0.61 |
| Cardiovascular | 72 (15.8%) | 35.7 11.0 | 21.3 16.6% | 23.6% (17) | 1.11 |
| Perioperative | 59 (12.9%) | 28.9 9.9 | 12.9 11.7% | 5.1% (3) | 0.40 |
| Complex monitoring | 34 (7.5%) | 33.2 12.1 | 19.1 16.3% | 14.7% (5) | 0.77 |
| GI complications | 33 (7.2%) | 32.1 8.3 | 15.6 10.7% | 12.1% (4) | 0.78 |
| Neurologic | 10 (2.2%) | 40.9 10.6 | 29.7 20.0% | 30.0% (3) | 1.01 |
| Liver failure | 7 (1.5%) | 42.1 17.2 | 30.9 29.4% | 42.9% (3) | 1.39 |
Co‐Management and Teaching
During the study period, 382/456 (83.8%) patients were co‐managed with 9 medical and 7 surgical teams (Table 4). From the period of 2006‐2008, a total of 37/106 (34.9%) patients were co‐managed with surgeons, and just 5/37 (13.5%) were co‐managed preoperatively before ImCU admission. In the next 2 years, the patient total increased to 69/106 (65.1%), and preoperative surgical co‐management significantly increased to 25/69 (36.2%) (P = 0.014).
| Medical | |||
|---|---|---|---|
| Surgical | |||
| |||
| Oncology | 100 (21.9%) | Neurology | 17 (3.7%) |
| Hepatology | 43 (9.4%) | Cardiology | 14 (3.1%) |
| Pulmonology | 36 (7.9%) | Nephrology | 14 (3.1%) |
| Hematology | 20 (4.4%) | Others | 13 (2.9%) |
| Gastroenterology | 19 (4.2%) | ||
| Total | 276 | ||
| General | 44 (9.6%) | Orthopedics | 6 (1.3%) |
| Vascular | 23 (5.0%) | Urology | 5 (1.1%) |
| Thoracic | 11 (2.4%) | Others | 10 (2.2%) |
| Neurosurgery | 7 (1.5%) | ||
| Total | 106 | ||
Our academic medical center enrolls 46 new residents every year. Since the creation of the ImCU in 2006, residents from different medical subspecialties and from general surgery received training in intermediate care and hospital medicine. All residents rotated into the ImCU for 1‐3 months working 8 hours a day. In 2006, when the unit was opened, 2 residents from internal medicine (4.3%) rotated in the ImCU. Thereafter, a significant increase in the number of training residents was observed, reaching 30.4% of the total resident pool (14/46) in 2010 (P = 0.002).
DISCUSSION
To the best of our knowledge, this is the first description of hospitalists in intermediate care. In Spain, where hospital medicine is early in development but expanding, critical and intermediate care units are usually staffed by intensivists or anesthesiologists. Staffing an ImCU with hospitalists, using a multidisciplinary co‐management model, is a novel staffing solution for acutely ill patients.
Approximately 35% of ICU patients are low risk, admitted mainly for monitoring purposes.9, 14 In contrast, some patients are treated on general wards when they should receive more intensive care and monitoring.15 Intermediate care units could improve cost containment and triage flexibility, while tailoring treatments according to patient needs. In general, ImCUs require lower nurse‐to‐patient ratios, and less expensive equipment and supplies than ICUs, while retaining the capability of responding appropriately to acute events.16 Moreover, patient and family satisfaction may be increased as a result of more liberal visitation policies and a less noisy environment.17
This study was not designed to measure the cost‐effectiveness of the ImCU. Surprisingly, there are few reports in the last 2 decades demonstrating the efficacy and cost containment of intermediate care. The majority of the studies were retrospective or uncontrolled observations.27 To our knowledge, only 1 randomized controlled trial1 and 1 multicenter prospective cost study exist.8 Further research is needed in this area, with larger, prospective randomized controlled trials, before the benefits and limitations of intermediate care can be fully determined.
Description of the ImCU patients depends on accurate severity scoring. The efficacy and reliability of these scores has been described only for ICU patients and their role for predicting mortality in the ImCU is uncertain. There is only 1 report using SAPS II in intermediate care, showing good discriminant power and calibration in a cohort of 433 patients.13 Auriant et al described, in that cohort, an observed mortality rate of 8.1% with an expected mortality rate of 8.7%.13 In contrast, our expected mortality rate was considerably higher (23.2%). Although ImCUs are generally created for low‐risk patients and monitoring purposes, our population was more similar to an ICU population, with very high risk for major complications and mortality.1823 The contribution of oncologic patients (22% of the total series; most of them with advanced disease, elevated SAPS II [42.2 13.6] and do‐not‐resuscitate orders), probably contributed to the higher acuity of our ImCU population. The correlation of our present data supports the value of SAPS II as a prognostic score in intermediate care, even for patients sicker than those reported by Auriant et al.13 Intermediate care is also a valuable setting to expand a co‐management model with different medical and surgical specialties.
Similarly, since the creation of the ImCU at our institution in 2006, there is a substantial increase in the number of residents rotating through our ImCU. Previous studies showed positive results of hospitalists as clinical educators in various settings.24, 25
In conclusion, intermediate care serves as an expansion of role for hospitalists at our institution; and clinicians, trainees, and patients may benefit from co‐management and teaching opportunities at this unique level of care. An ImCU led by hospitalists showed encouraging results in terms of observed‐to‐expected mortality ratios for acutely ill patients. SAPS II is a useful tool for prognostic evaluation of ImCU patients. However, results of this study should be confirmed with larger, prospective trials at multiple centers.
Acknowledgements
The authors thank Dr Efren Manjarrez for the final manuscript revision, and the ImCU Nursing Staff for their unconditional support in patient care.
- ,,,,,.The cost‐effectiveness of a special care unit to care for the chronically critically ill.J Nurs Adm.1995;25:47–53.
- ,.Noninvasive respiratory care unit. A cost‐effective solution for the future.Chest.1988;93:390–394.
- ,,,.The noninvasive respiratory care unit. Patterns of use and financial implications.Chest.1991;99:205–208.
- ,,,,,.Decreases in mortality on a large urban medical service by facilitating access to critical care. An alternative to rationing.Arch Intern Med.1988;148:1403–1405.
- ,,,.Impact of an intermediate care area on ICU utilization after cardiac surgery.Crit Care Med.1986;14:869–872.
- ,,.Closure of an intermediate care unit. Impact on critical care utilization.Chest.1993;104:876–881.
- ,.A case‐control study of patients readmitted to the intensive care unit.Crit Care Med.1993;21:1547–1553.
- ,,, et al.Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit.Respir Med.2005;99:894–900.
- ,,,,.A multicenter description of intermediate‐care patients. Comparison with ICU low‐risk monitor patients.Chest.2002;121:1253–1261.
- ,,, et al.Guidelines on admission and discharge for adult intermediate care units. American College of Critical Care Medicine of the Society of Critical Care Medicine.Crit Care Med.1998;26:607–610.
- ,.Do we need intermediate care units?Intensive Care Med.1999;25:1345–1349.
- ,,.A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study.JAMA.1993;270:2957–2963.
- ,,,,.Simplified acute physiology score II for measuring severity of illness in intermediate care units.Crit Care Med.1998;26:1368–1371.
- ,,,,,.The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost.Chest.1995;108:490–499.
- ,.Identifying patients with high risk of high cost.Chest1991;99:530–531.
- ,,.Structural models for intermediate care areas.Crit Care Med.1999;27:2266–2271.
- ,,,.Characteristics of pediatric intermediate care units in pediatric training programs.Crit Care Med.1991;19:1004–1007.
- ,,, et al.Prognostic performance and customization of the SAPS II: results of a multicenter Austrian study.Int Care Med.1999;25:192–197.
- ,,,,,.Comparison of Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II) scoring systems in a single Greek intensive care unit.Crit Care Med.2000;28:426–432.
- ,,,.External validation of the SAPS II, APACHE II and APACHE III prognostic models in South England: a multicentre study.Intensive Care Med.2003;29:249–256.
- ,,,,,.SAPS II revisited.Intensive Care Med.2005;31:416–423.
- ,,, et al.Mortality prediction using SAPS II: an update for French intensive care units.Crit Care.2005;9:R645–R652.
- ,,,.Predicting death and readmission after intensive care discharge.Br J Anaesth.2008;100:656–662.
- ,,, et al.Hospitalists as teachers.J Gen Intern Med.2004;19:8–15.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
Hospitalized patients are becoming increasingly complex. The care of such patients may be impacted by the limited resources of the general ward and might benefit from more intensive monitoring in an intensive care unit (ICU)‐like setting. In light of this problem, the intermediate care units (ImCU) may provide a cost‐effective alternative by providing higher levels of staffing tailored to patient needs, without incurring the cost of an ICU admission. The ImCU can reduce costs and improves ICU utilization for sicker patients, decrease ICU readmissions, promote greater flexibility in patient triage, and decrease mortality rates in hospital wards.18
The characteristics of ImCUs depend on resource availability, institutional infrastructure, and the organization and funding of the parent healthcare system. The ImCU may function as a step‐up or step‐down unit, or may provide specialty care for cardiac, neurologic, respiratory, or surgical conditions.811 These units can expand opportunities for co‐management and, at the same time, offer the occasion for training residents to follow up patients through different levels of care (from the general ward to ImCU). In the same way, the multidisciplinary approach of the ImCU can improve the center's teaching potential.
Characterizing the ImCU population requires the assessment of their severity of illness, which is crucial for the evaluation of risk‐adjusted outcomes. The present study evaluated the impact of a hospitalist‐led ImCU on observed‐to‐expected mortality ratios, as well as its role in co‐management and teaching.
PATIENTS AND METHODS
We performed a retrospective observational study, with data collected from April 2006 to April 2010 in a single academic medical center in Pamplona, Spain. The ImCU is a 9‐bed unit adjacent to, but independent from, the mixed ICU. Each bed is equipped with continuous telemetry, pulse oximetry, noninvasive arterial blood pressure, central venous pressure monitoring, and noninvasive pressure support ventilation. The signals are relayed to a central monitoring station and the nurse‐to‐patient ratio is 1:3.
The ImCU rounding team is multidisciplinary, and involves the hospital pharmacist, a nurse, the ImCU resident, the specialist or surgeon, and the attending hospitalist. After the triage process, ImCU patients were admitted to the attending hospitalist, who was responsible for admission and discharge of all ImCU patients. The hospitalist ordered diagnostic or therapeutic interventions as needed, with the exception of orders for procedures or consultations related with specialist/surgeon's specific needs.
Admission and discharge criteria for the ImCU were set according to guidelines defined by The American College of Critical Care Medicine,10 and also served as inclusion criteria for the present study. Exclusion criteria included: age less than 18 years old, severe respiratory failure, status epilepticus, and catastrophic brain illness. Patients admitted for drug administration and desensitization, and also ImCU readmissions, were excluded from data analysis. Patients came from medical and surgical wards, ICU, the operating room, and the emergency room.
A total of 756 patients were admitted to our ImCU during the study period. Patient demographics, past medical history, physiologic parameters at the time of admission, and survival to hospital discharge were recorded for all patients. Patient demographics include: age, gender, location before ImCU admission, length of stay before ImCU admission, reason for ImCU admission, anatomic site of surgery (if applicable), planned or unplanned admission, and infection status (nosocomial). Past medical history includes: the presence of arterial hypertension, diabetes, cirrhosis, chronic renal failure, chronic heart failure, cancer, hematological malignancy, chronic obstructive pulmonary disease (COPD), human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), immunosuppression, radiotherapy, chemotherapy, steroid treatment, and alcoholism. Physiologic parameters abstracted are described in Table 1. We used the Simplified Acute Physiology Score II (SAPS II),12 as a prognostic and severity score. SAPS II is the only previously validated score in intermediate care.13 In‐hospital mortality was the clinical outcome measured.
|
| Vital signs |
| Glasgow Coma Scale |
| Serum bilirubin |
| Serum creatinine |
| Urea nitrogen |
| Leucocyte count |
| Serum sodium |
| Serum potassium |
| Bicarbonate levels |
| Urinary output in the first 24 hr |
| Oxygenation and ventilatory support |
Data were entered into a computer database by the authors. Statistical analysis was not blinded, and was performed using SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean standard deviation or median (25%‐75% interquartile range). For nonparametric measure of statistical dependence of quantitative variables, we used Spearman's correlation coefficient. Discrimination was evaluated by calculating the area under receiver operating characteristic curve (AUROC).
The study protocol was approved by the institutional review board at the Clnica Universidad de Navarra in Pamplona, Spain.
RESULTS
Four hundred fifty‐six patients were included in data analysis. Three hundred patients were excluded: 61 low‐risk patients (drug administration and desensitization), 147 readmissions, and 92 patients for missing variables. Patient characteristics, including probability of death following ImCU admission and discharge location, are summarized in Table 2. The mean age was 65.6 years, and about 35% of patients had a SAPS II‐based risk of death higher than 25% at the time of ImCU admission. The median length of stay was 4 (3‐7) days.
| |
| Age (yr) | 65.6 14.3 |
| Gender | |
| Male | 283 (62.1%) |
| Female | 173 (37.9%) |
| Location prior to admission | |
| General ward | 252 (55.3%) |
| Emergency room | 96 (21.1%) |
| ICU | 63 (13.8%) |
| Operating room | 28 (6.1%) |
| Other hospital | 17 (3.7%) |
| Probability of in‐hospital mortality based on SAPS II | |
| <10% | 128 (28.1%) |
| 11%‐25% | 176 (38.6%) |
| 26%‐50% | 107 (23.4%) |
| >50% | 45 (9.9%) |
| Global expected mortality (in‐hospital) | 23.2% |
| Global observed mortality (in‐hospital) | 20.6% (94/456) |
| O/E mortality ratio | 0.89 |
| Discharge location | |
| General ward | 352/456 (77.2%) |
| ICU | 65/456 (14.3%) |
| Home | 1/456 (0.2%) |
| Other hospital | 11/456 (2.4%) |
| Death location | |
| ImCU | 27/456 (5.9%) |
| ICU (transferred patients) | 32/65* (49.2%) |
| General ward | 35/352* (9.9%) |
Outcomes
The mean SAPS II of the cohort was 37 12 points, and the expected mortality derived from this score was 23.2%. The observed in‐hospital mortality was 20.6% (94/456) resulting in an observed‐to‐expected mortality ratio of 0.89 (Table 2). Reasons for ImCU admission, as well as mortality ratios, are described in Table 3. The correlation between SAPS II predicted and observed death rates was accurate and statistically significant (Rho = 1.0, P < 0.001) (Figure 1). The AUROC for SAPS II predicting in‐hospital mortality was 0.75 (P < 0.001).

| Condition | Patients | SAPS II | Expected Mortality | Observed Mortality | O/E Ratio |
|---|---|---|---|---|---|
| |||||
| Respiratory failure | 153 (33.6%) | 36.1 9.7 | 21.5 15.3% | 25.5% (39) | 1.19 |
| Sepsis | 88 (19.3%) | 45.7 15.1 | 37.5 25.1% | 22.7% (20) | 0.61 |
| Cardiovascular | 72 (15.8%) | 35.7 11.0 | 21.3 16.6% | 23.6% (17) | 1.11 |
| Perioperative | 59 (12.9%) | 28.9 9.9 | 12.9 11.7% | 5.1% (3) | 0.40 |
| Complex monitoring | 34 (7.5%) | 33.2 12.1 | 19.1 16.3% | 14.7% (5) | 0.77 |
| GI complications | 33 (7.2%) | 32.1 8.3 | 15.6 10.7% | 12.1% (4) | 0.78 |
| Neurologic | 10 (2.2%) | 40.9 10.6 | 29.7 20.0% | 30.0% (3) | 1.01 |
| Liver failure | 7 (1.5%) | 42.1 17.2 | 30.9 29.4% | 42.9% (3) | 1.39 |
Co‐Management and Teaching
During the study period, 382/456 (83.8%) patients were co‐managed with 9 medical and 7 surgical teams (Table 4). From the period of 2006‐2008, a total of 37/106 (34.9%) patients were co‐managed with surgeons, and just 5/37 (13.5%) were co‐managed preoperatively before ImCU admission. In the next 2 years, the patient total increased to 69/106 (65.1%), and preoperative surgical co‐management significantly increased to 25/69 (36.2%) (P = 0.014).
| Medical | |||
|---|---|---|---|
| Surgical | |||
| |||
| Oncology | 100 (21.9%) | Neurology | 17 (3.7%) |
| Hepatology | 43 (9.4%) | Cardiology | 14 (3.1%) |
| Pulmonology | 36 (7.9%) | Nephrology | 14 (3.1%) |
| Hematology | 20 (4.4%) | Others | 13 (2.9%) |
| Gastroenterology | 19 (4.2%) | ||
| Total | 276 | ||
| General | 44 (9.6%) | Orthopedics | 6 (1.3%) |
| Vascular | 23 (5.0%) | Urology | 5 (1.1%) |
| Thoracic | 11 (2.4%) | Others | 10 (2.2%) |
| Neurosurgery | 7 (1.5%) | ||
| Total | 106 | ||
Our academic medical center enrolls 46 new residents every year. Since the creation of the ImCU in 2006, residents from different medical subspecialties and from general surgery received training in intermediate care and hospital medicine. All residents rotated into the ImCU for 1‐3 months working 8 hours a day. In 2006, when the unit was opened, 2 residents from internal medicine (4.3%) rotated in the ImCU. Thereafter, a significant increase in the number of training residents was observed, reaching 30.4% of the total resident pool (14/46) in 2010 (P = 0.002).
DISCUSSION
To the best of our knowledge, this is the first description of hospitalists in intermediate care. In Spain, where hospital medicine is early in development but expanding, critical and intermediate care units are usually staffed by intensivists or anesthesiologists. Staffing an ImCU with hospitalists, using a multidisciplinary co‐management model, is a novel staffing solution for acutely ill patients.
Approximately 35% of ICU patients are low risk, admitted mainly for monitoring purposes.9, 14 In contrast, some patients are treated on general wards when they should receive more intensive care and monitoring.15 Intermediate care units could improve cost containment and triage flexibility, while tailoring treatments according to patient needs. In general, ImCUs require lower nurse‐to‐patient ratios, and less expensive equipment and supplies than ICUs, while retaining the capability of responding appropriately to acute events.16 Moreover, patient and family satisfaction may be increased as a result of more liberal visitation policies and a less noisy environment.17
This study was not designed to measure the cost‐effectiveness of the ImCU. Surprisingly, there are few reports in the last 2 decades demonstrating the efficacy and cost containment of intermediate care. The majority of the studies were retrospective or uncontrolled observations.27 To our knowledge, only 1 randomized controlled trial1 and 1 multicenter prospective cost study exist.8 Further research is needed in this area, with larger, prospective randomized controlled trials, before the benefits and limitations of intermediate care can be fully determined.
Description of the ImCU patients depends on accurate severity scoring. The efficacy and reliability of these scores has been described only for ICU patients and their role for predicting mortality in the ImCU is uncertain. There is only 1 report using SAPS II in intermediate care, showing good discriminant power and calibration in a cohort of 433 patients.13 Auriant et al described, in that cohort, an observed mortality rate of 8.1% with an expected mortality rate of 8.7%.13 In contrast, our expected mortality rate was considerably higher (23.2%). Although ImCUs are generally created for low‐risk patients and monitoring purposes, our population was more similar to an ICU population, with very high risk for major complications and mortality.1823 The contribution of oncologic patients (22% of the total series; most of them with advanced disease, elevated SAPS II [42.2 13.6] and do‐not‐resuscitate orders), probably contributed to the higher acuity of our ImCU population. The correlation of our present data supports the value of SAPS II as a prognostic score in intermediate care, even for patients sicker than those reported by Auriant et al.13 Intermediate care is also a valuable setting to expand a co‐management model with different medical and surgical specialties.
Similarly, since the creation of the ImCU at our institution in 2006, there is a substantial increase in the number of residents rotating through our ImCU. Previous studies showed positive results of hospitalists as clinical educators in various settings.24, 25
In conclusion, intermediate care serves as an expansion of role for hospitalists at our institution; and clinicians, trainees, and patients may benefit from co‐management and teaching opportunities at this unique level of care. An ImCU led by hospitalists showed encouraging results in terms of observed‐to‐expected mortality ratios for acutely ill patients. SAPS II is a useful tool for prognostic evaluation of ImCU patients. However, results of this study should be confirmed with larger, prospective trials at multiple centers.
Acknowledgements
The authors thank Dr Efren Manjarrez for the final manuscript revision, and the ImCU Nursing Staff for their unconditional support in patient care.
Hospitalized patients are becoming increasingly complex. The care of such patients may be impacted by the limited resources of the general ward and might benefit from more intensive monitoring in an intensive care unit (ICU)‐like setting. In light of this problem, the intermediate care units (ImCU) may provide a cost‐effective alternative by providing higher levels of staffing tailored to patient needs, without incurring the cost of an ICU admission. The ImCU can reduce costs and improves ICU utilization for sicker patients, decrease ICU readmissions, promote greater flexibility in patient triage, and decrease mortality rates in hospital wards.18
The characteristics of ImCUs depend on resource availability, institutional infrastructure, and the organization and funding of the parent healthcare system. The ImCU may function as a step‐up or step‐down unit, or may provide specialty care for cardiac, neurologic, respiratory, or surgical conditions.811 These units can expand opportunities for co‐management and, at the same time, offer the occasion for training residents to follow up patients through different levels of care (from the general ward to ImCU). In the same way, the multidisciplinary approach of the ImCU can improve the center's teaching potential.
Characterizing the ImCU population requires the assessment of their severity of illness, which is crucial for the evaluation of risk‐adjusted outcomes. The present study evaluated the impact of a hospitalist‐led ImCU on observed‐to‐expected mortality ratios, as well as its role in co‐management and teaching.
PATIENTS AND METHODS
We performed a retrospective observational study, with data collected from April 2006 to April 2010 in a single academic medical center in Pamplona, Spain. The ImCU is a 9‐bed unit adjacent to, but independent from, the mixed ICU. Each bed is equipped with continuous telemetry, pulse oximetry, noninvasive arterial blood pressure, central venous pressure monitoring, and noninvasive pressure support ventilation. The signals are relayed to a central monitoring station and the nurse‐to‐patient ratio is 1:3.
The ImCU rounding team is multidisciplinary, and involves the hospital pharmacist, a nurse, the ImCU resident, the specialist or surgeon, and the attending hospitalist. After the triage process, ImCU patients were admitted to the attending hospitalist, who was responsible for admission and discharge of all ImCU patients. The hospitalist ordered diagnostic or therapeutic interventions as needed, with the exception of orders for procedures or consultations related with specialist/surgeon's specific needs.
Admission and discharge criteria for the ImCU were set according to guidelines defined by The American College of Critical Care Medicine,10 and also served as inclusion criteria for the present study. Exclusion criteria included: age less than 18 years old, severe respiratory failure, status epilepticus, and catastrophic brain illness. Patients admitted for drug administration and desensitization, and also ImCU readmissions, were excluded from data analysis. Patients came from medical and surgical wards, ICU, the operating room, and the emergency room.
A total of 756 patients were admitted to our ImCU during the study period. Patient demographics, past medical history, physiologic parameters at the time of admission, and survival to hospital discharge were recorded for all patients. Patient demographics include: age, gender, location before ImCU admission, length of stay before ImCU admission, reason for ImCU admission, anatomic site of surgery (if applicable), planned or unplanned admission, and infection status (nosocomial). Past medical history includes: the presence of arterial hypertension, diabetes, cirrhosis, chronic renal failure, chronic heart failure, cancer, hematological malignancy, chronic obstructive pulmonary disease (COPD), human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), immunosuppression, radiotherapy, chemotherapy, steroid treatment, and alcoholism. Physiologic parameters abstracted are described in Table 1. We used the Simplified Acute Physiology Score II (SAPS II),12 as a prognostic and severity score. SAPS II is the only previously validated score in intermediate care.13 In‐hospital mortality was the clinical outcome measured.
|
| Vital signs |
| Glasgow Coma Scale |
| Serum bilirubin |
| Serum creatinine |
| Urea nitrogen |
| Leucocyte count |
| Serum sodium |
| Serum potassium |
| Bicarbonate levels |
| Urinary output in the first 24 hr |
| Oxygenation and ventilatory support |
Data were entered into a computer database by the authors. Statistical analysis was not blinded, and was performed using SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL). Continuous variables were reported as mean standard deviation or median (25%‐75% interquartile range). For nonparametric measure of statistical dependence of quantitative variables, we used Spearman's correlation coefficient. Discrimination was evaluated by calculating the area under receiver operating characteristic curve (AUROC).
The study protocol was approved by the institutional review board at the Clnica Universidad de Navarra in Pamplona, Spain.
RESULTS
Four hundred fifty‐six patients were included in data analysis. Three hundred patients were excluded: 61 low‐risk patients (drug administration and desensitization), 147 readmissions, and 92 patients for missing variables. Patient characteristics, including probability of death following ImCU admission and discharge location, are summarized in Table 2. The mean age was 65.6 years, and about 35% of patients had a SAPS II‐based risk of death higher than 25% at the time of ImCU admission. The median length of stay was 4 (3‐7) days.
| |
| Age (yr) | 65.6 14.3 |
| Gender | |
| Male | 283 (62.1%) |
| Female | 173 (37.9%) |
| Location prior to admission | |
| General ward | 252 (55.3%) |
| Emergency room | 96 (21.1%) |
| ICU | 63 (13.8%) |
| Operating room | 28 (6.1%) |
| Other hospital | 17 (3.7%) |
| Probability of in‐hospital mortality based on SAPS II | |
| <10% | 128 (28.1%) |
| 11%‐25% | 176 (38.6%) |
| 26%‐50% | 107 (23.4%) |
| >50% | 45 (9.9%) |
| Global expected mortality (in‐hospital) | 23.2% |
| Global observed mortality (in‐hospital) | 20.6% (94/456) |
| O/E mortality ratio | 0.89 |
| Discharge location | |
| General ward | 352/456 (77.2%) |
| ICU | 65/456 (14.3%) |
| Home | 1/456 (0.2%) |
| Other hospital | 11/456 (2.4%) |
| Death location | |
| ImCU | 27/456 (5.9%) |
| ICU (transferred patients) | 32/65* (49.2%) |
| General ward | 35/352* (9.9%) |
Outcomes
The mean SAPS II of the cohort was 37 12 points, and the expected mortality derived from this score was 23.2%. The observed in‐hospital mortality was 20.6% (94/456) resulting in an observed‐to‐expected mortality ratio of 0.89 (Table 2). Reasons for ImCU admission, as well as mortality ratios, are described in Table 3. The correlation between SAPS II predicted and observed death rates was accurate and statistically significant (Rho = 1.0, P < 0.001) (Figure 1). The AUROC for SAPS II predicting in‐hospital mortality was 0.75 (P < 0.001).

| Condition | Patients | SAPS II | Expected Mortality | Observed Mortality | O/E Ratio |
|---|---|---|---|---|---|
| |||||
| Respiratory failure | 153 (33.6%) | 36.1 9.7 | 21.5 15.3% | 25.5% (39) | 1.19 |
| Sepsis | 88 (19.3%) | 45.7 15.1 | 37.5 25.1% | 22.7% (20) | 0.61 |
| Cardiovascular | 72 (15.8%) | 35.7 11.0 | 21.3 16.6% | 23.6% (17) | 1.11 |
| Perioperative | 59 (12.9%) | 28.9 9.9 | 12.9 11.7% | 5.1% (3) | 0.40 |
| Complex monitoring | 34 (7.5%) | 33.2 12.1 | 19.1 16.3% | 14.7% (5) | 0.77 |
| GI complications | 33 (7.2%) | 32.1 8.3 | 15.6 10.7% | 12.1% (4) | 0.78 |
| Neurologic | 10 (2.2%) | 40.9 10.6 | 29.7 20.0% | 30.0% (3) | 1.01 |
| Liver failure | 7 (1.5%) | 42.1 17.2 | 30.9 29.4% | 42.9% (3) | 1.39 |
Co‐Management and Teaching
During the study period, 382/456 (83.8%) patients were co‐managed with 9 medical and 7 surgical teams (Table 4). From the period of 2006‐2008, a total of 37/106 (34.9%) patients were co‐managed with surgeons, and just 5/37 (13.5%) were co‐managed preoperatively before ImCU admission. In the next 2 years, the patient total increased to 69/106 (65.1%), and preoperative surgical co‐management significantly increased to 25/69 (36.2%) (P = 0.014).
| Medical | |||
|---|---|---|---|
| Surgical | |||
| |||
| Oncology | 100 (21.9%) | Neurology | 17 (3.7%) |
| Hepatology | 43 (9.4%) | Cardiology | 14 (3.1%) |
| Pulmonology | 36 (7.9%) | Nephrology | 14 (3.1%) |
| Hematology | 20 (4.4%) | Others | 13 (2.9%) |
| Gastroenterology | 19 (4.2%) | ||
| Total | 276 | ||
| General | 44 (9.6%) | Orthopedics | 6 (1.3%) |
| Vascular | 23 (5.0%) | Urology | 5 (1.1%) |
| Thoracic | 11 (2.4%) | Others | 10 (2.2%) |
| Neurosurgery | 7 (1.5%) | ||
| Total | 106 | ||
Our academic medical center enrolls 46 new residents every year. Since the creation of the ImCU in 2006, residents from different medical subspecialties and from general surgery received training in intermediate care and hospital medicine. All residents rotated into the ImCU for 1‐3 months working 8 hours a day. In 2006, when the unit was opened, 2 residents from internal medicine (4.3%) rotated in the ImCU. Thereafter, a significant increase in the number of training residents was observed, reaching 30.4% of the total resident pool (14/46) in 2010 (P = 0.002).
DISCUSSION
To the best of our knowledge, this is the first description of hospitalists in intermediate care. In Spain, where hospital medicine is early in development but expanding, critical and intermediate care units are usually staffed by intensivists or anesthesiologists. Staffing an ImCU with hospitalists, using a multidisciplinary co‐management model, is a novel staffing solution for acutely ill patients.
Approximately 35% of ICU patients are low risk, admitted mainly for monitoring purposes.9, 14 In contrast, some patients are treated on general wards when they should receive more intensive care and monitoring.15 Intermediate care units could improve cost containment and triage flexibility, while tailoring treatments according to patient needs. In general, ImCUs require lower nurse‐to‐patient ratios, and less expensive equipment and supplies than ICUs, while retaining the capability of responding appropriately to acute events.16 Moreover, patient and family satisfaction may be increased as a result of more liberal visitation policies and a less noisy environment.17
This study was not designed to measure the cost‐effectiveness of the ImCU. Surprisingly, there are few reports in the last 2 decades demonstrating the efficacy and cost containment of intermediate care. The majority of the studies were retrospective or uncontrolled observations.27 To our knowledge, only 1 randomized controlled trial1 and 1 multicenter prospective cost study exist.8 Further research is needed in this area, with larger, prospective randomized controlled trials, before the benefits and limitations of intermediate care can be fully determined.
Description of the ImCU patients depends on accurate severity scoring. The efficacy and reliability of these scores has been described only for ICU patients and their role for predicting mortality in the ImCU is uncertain. There is only 1 report using SAPS II in intermediate care, showing good discriminant power and calibration in a cohort of 433 patients.13 Auriant et al described, in that cohort, an observed mortality rate of 8.1% with an expected mortality rate of 8.7%.13 In contrast, our expected mortality rate was considerably higher (23.2%). Although ImCUs are generally created for low‐risk patients and monitoring purposes, our population was more similar to an ICU population, with very high risk for major complications and mortality.1823 The contribution of oncologic patients (22% of the total series; most of them with advanced disease, elevated SAPS II [42.2 13.6] and do‐not‐resuscitate orders), probably contributed to the higher acuity of our ImCU population. The correlation of our present data supports the value of SAPS II as a prognostic score in intermediate care, even for patients sicker than those reported by Auriant et al.13 Intermediate care is also a valuable setting to expand a co‐management model with different medical and surgical specialties.
Similarly, since the creation of the ImCU at our institution in 2006, there is a substantial increase in the number of residents rotating through our ImCU. Previous studies showed positive results of hospitalists as clinical educators in various settings.24, 25
In conclusion, intermediate care serves as an expansion of role for hospitalists at our institution; and clinicians, trainees, and patients may benefit from co‐management and teaching opportunities at this unique level of care. An ImCU led by hospitalists showed encouraging results in terms of observed‐to‐expected mortality ratios for acutely ill patients. SAPS II is a useful tool for prognostic evaluation of ImCU patients. However, results of this study should be confirmed with larger, prospective trials at multiple centers.
Acknowledgements
The authors thank Dr Efren Manjarrez for the final manuscript revision, and the ImCU Nursing Staff for their unconditional support in patient care.
- ,,,,,.The cost‐effectiveness of a special care unit to care for the chronically critically ill.J Nurs Adm.1995;25:47–53.
- ,.Noninvasive respiratory care unit. A cost‐effective solution for the future.Chest.1988;93:390–394.
- ,,,.The noninvasive respiratory care unit. Patterns of use and financial implications.Chest.1991;99:205–208.
- ,,,,,.Decreases in mortality on a large urban medical service by facilitating access to critical care. An alternative to rationing.Arch Intern Med.1988;148:1403–1405.
- ,,,.Impact of an intermediate care area on ICU utilization after cardiac surgery.Crit Care Med.1986;14:869–872.
- ,,.Closure of an intermediate care unit. Impact on critical care utilization.Chest.1993;104:876–881.
- ,.A case‐control study of patients readmitted to the intensive care unit.Crit Care Med.1993;21:1547–1553.
- ,,, et al.Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit.Respir Med.2005;99:894–900.
- ,,,,.A multicenter description of intermediate‐care patients. Comparison with ICU low‐risk monitor patients.Chest.2002;121:1253–1261.
- ,,, et al.Guidelines on admission and discharge for adult intermediate care units. American College of Critical Care Medicine of the Society of Critical Care Medicine.Crit Care Med.1998;26:607–610.
- ,.Do we need intermediate care units?Intensive Care Med.1999;25:1345–1349.
- ,,.A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study.JAMA.1993;270:2957–2963.
- ,,,,.Simplified acute physiology score II for measuring severity of illness in intermediate care units.Crit Care Med.1998;26:1368–1371.
- ,,,,,.The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost.Chest.1995;108:490–499.
- ,.Identifying patients with high risk of high cost.Chest1991;99:530–531.
- ,,.Structural models for intermediate care areas.Crit Care Med.1999;27:2266–2271.
- ,,,.Characteristics of pediatric intermediate care units in pediatric training programs.Crit Care Med.1991;19:1004–1007.
- ,,, et al.Prognostic performance and customization of the SAPS II: results of a multicenter Austrian study.Int Care Med.1999;25:192–197.
- ,,,,,.Comparison of Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II) scoring systems in a single Greek intensive care unit.Crit Care Med.2000;28:426–432.
- ,,,.External validation of the SAPS II, APACHE II and APACHE III prognostic models in South England: a multicentre study.Intensive Care Med.2003;29:249–256.
- ,,,,,.SAPS II revisited.Intensive Care Med.2005;31:416–423.
- ,,, et al.Mortality prediction using SAPS II: an update for French intensive care units.Crit Care.2005;9:R645–R652.
- ,,,.Predicting death and readmission after intensive care discharge.Br J Anaesth.2008;100:656–662.
- ,,, et al.Hospitalists as teachers.J Gen Intern Med.2004;19:8–15.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
- ,,,,,.The cost‐effectiveness of a special care unit to care for the chronically critically ill.J Nurs Adm.1995;25:47–53.
- ,.Noninvasive respiratory care unit. A cost‐effective solution for the future.Chest.1988;93:390–394.
- ,,,.The noninvasive respiratory care unit. Patterns of use and financial implications.Chest.1991;99:205–208.
- ,,,,,.Decreases in mortality on a large urban medical service by facilitating access to critical care. An alternative to rationing.Arch Intern Med.1988;148:1403–1405.
- ,,,.Impact of an intermediate care area on ICU utilization after cardiac surgery.Crit Care Med.1986;14:869–872.
- ,,.Closure of an intermediate care unit. Impact on critical care utilization.Chest.1993;104:876–881.
- ,.A case‐control study of patients readmitted to the intensive care unit.Crit Care Med.1993;21:1547–1553.
- ,,, et al.Costs of the COPD. Differences between intensive care unit and respiratory intermediate care unit.Respir Med.2005;99:894–900.
- ,,,,.A multicenter description of intermediate‐care patients. Comparison with ICU low‐risk monitor patients.Chest.2002;121:1253–1261.
- ,,, et al.Guidelines on admission and discharge for adult intermediate care units. American College of Critical Care Medicine of the Society of Critical Care Medicine.Crit Care Med.1998;26:607–610.
- ,.Do we need intermediate care units?Intensive Care Med.1999;25:1345–1349.
- ,,.A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study.JAMA.1993;270:2957–2963.
- ,,,,.Simplified acute physiology score II for measuring severity of illness in intermediate care units.Crit Care Med.1998;26:1368–1371.
- ,,,,,.The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost.Chest.1995;108:490–499.
- ,.Identifying patients with high risk of high cost.Chest1991;99:530–531.
- ,,.Structural models for intermediate care areas.Crit Care Med.1999;27:2266–2271.
- ,,,.Characteristics of pediatric intermediate care units in pediatric training programs.Crit Care Med.1991;19:1004–1007.
- ,,, et al.Prognostic performance and customization of the SAPS II: results of a multicenter Austrian study.Int Care Med.1999;25:192–197.
- ,,,,,.Comparison of Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Acute Physiology Score II (SAPS II) scoring systems in a single Greek intensive care unit.Crit Care Med.2000;28:426–432.
- ,,,.External validation of the SAPS II, APACHE II and APACHE III prognostic models in South England: a multicentre study.Intensive Care Med.2003;29:249–256.
- ,,,,,.SAPS II revisited.Intensive Care Med.2005;31:416–423.
- ,,, et al.Mortality prediction using SAPS II: an update for French intensive care units.Crit Care.2005;9:R645–R652.
- ,,,.Predicting death and readmission after intensive care discharge.Br J Anaesth.2008;100:656–662.
- ,,, et al.Hospitalists as teachers.J Gen Intern Med.2004;19:8–15.
- ,,, et al.The positive impact of initiation of hospitalist clinician educators.J Gen Intern Med.2004;19:293–301.
Copyright © 2012 Society of Hospital Medicine
Hospitalist Practice Models
Over the past 15 years, there has been dramatic growth in the number of hospitalist physicians in the United States and in the number of hospitals served by them.13 Hospitals are motivated to hire experienced hospitalists to staff their inpatient services,4 with goals that include obtaining cost‐savings and higher quality.59 The rapid growth of Hospital Medicine saw multiple types of hospital practice models emerge with differing job characteristics, clinical duties, workload, and compensation schemes.10 The extent of the variability of hospitalist jobs across practice models is not known.
Intensifying recruitment efforts and the concomitant increase in compensation for hospitalists over the last decade suggest that demand for hospitalists is strong and sustained.11 As a result, today's cohort of hospitalists has a wide range of choices of types of jobs, practice models, and locations. The diversity of available hospitalist jobs is characterized, for example, by setting (community hospital vs academic hospital), employer (hospital vs private practice), job duties (the amount and type of clinical work, and other administrative, teaching, or research duties), and intensity (work hours and duties to maximize income or lifestyle). How these choices relate to job satisfaction and burnout are also unknown.
The Society of Hospital Medicine (SHM) has administered surveys to hospitalist group leaders biennially since 2003.1215 These surveys, however, do not address issues related to individual hospitalist worklife, recruitment, and retention. In 2005, SHM convened a Career Satisfaction Task Force that designed and executed a national survey of hospitalists in 2009‐2010. The objective of this study is to evaluate how job characteristics vary by practice model, and the association of these characteristics and practice models with job satisfaction and burnout.
METHODS
Survey Instrument
A detailed description of the survey design, sampling strategy, data collection, and response rate calculations is described elsewhere.16 Portions of the 118‐item survey instrument assessed characteristics of the respondents' hospitalist group (12 items), details about their individual work patterns (12 items), and demographics (9 items). Work patterns were evaluated by the average number of clinical work days, consecutive days, hours per month, percentage of work assigned to night duty, and number of patient encounters. Average hours spent on nonclinical work, and the percentage of time allocated for clinical, administrative, teaching, and research activities were solicited. Additional items assessed specific clinical responsibilities, pretax earnings in FY2010, the availability of information technology capabilities, and the adequacy of available resources. Job and specialty satisfaction and 11 satisfaction domain measures were measured using validated scales.1726 Burnout symptoms were measured using a validated single‐item measure.26, 27
Sampling Strategy
We surveyed a national stratified sample of hospitalists in the US and Puerto Rico. We used the largest database of hospitalists (>24,000 names) currently available and maintained by the SHM as our sampling frame. We linked hospitalist employer information to hospital statistics from the American Hospital Association database28 to stratify the sample by number of hospital beds, geographic region, employment model, and specialty training, oversampling pediatric hospitalists due to small numbers. A respondent sample of about 700 hospitalists was calculated to be adequate to detect a 0.5 point difference in job satisfaction scores between subgroups assuming 90% power and alpha of 0.05. However, we sampled a total of 5389 addresses from the database to overcome the traditionally low physician response rates, duplicate sampling, bad addresses, and non‐hospitalists being included in the sampling frame. In addition, 2 multistate hospitalist companies (EmCare, In Compass Health) and 1 for‐profit hospital chain (HCA, Inc) financially sponsored this project with the stipulation that all of their hospitalist employees (n = 884) would be surveyed.
Data Collection
The healthcare consulting firm, Press Ganey, provided support with survey layout and administration following the modified Dillman method.29 Three rounds of coded surveys and solicitation letters from the investigators were mailed 2 weeks apart in November and December 2009. Because of low response rates to the mailed survey, an online survey was created using Survey Monkey and sent to 650 surveyees for whom e‐mail addresses were available, and administered at a kiosk for sample physicians during the SHM 2010 annual meeting.
Data Analysis
Nonresponse bias was measured by comparing characteristics between respondents of separate survey waves.30 We determined the validity of mailing addresses immediately following the survey period by mapping each address using Google, and if the address was a hospital, researching online whether or not the intended recipient was currently employed there. Practice characteristics were compared across 5 model categories distilled from the SHM & Medical Group Management Association survey: local hospitalist‐only group, multistate hospitalist group, multispecialty physician group, employer hospital, and university or medical school. Weighted proportions, means, and medians were calculated to account for oversampling of pediatric hospitalists. Differences in categorical measures were assessed using the chi‐square test and the design‐based F test for comparing weighted data. Weighted means (99% confidence intervals) and medians (interquartile ranges) were calculated. Because each parameter yielded a single outlier value across the 5 practice models, differences across weighted means were assessed using generalized linear models with the single outlier value chosen as the reference mean. Pair‐wise Wilcoxon rank sum test was used to compare median values. In these 4‐way comparisons of means and medians, significance was defined as P value of 0.0125 per Bonferroni correction. A single survey item solicited respondents to choose exactly 4 of 13 considerations most pertinent to job satisfaction. The proportion of respondents who scored 4 on a 5‐point Likert scale of the 11 satisfaction domains and 2 global measures of satisfaction, and burnout symptoms defined as 3 on a 5‐point single item measure were bar‐graphed. Chi‐square statistics were used to evaluate for differences across practice models. Statistical significance was defined by alpha less than 0.05, unless otherwise specified. All analyses were performed using STATA version 11.0 (College Station, TX). This study was approved by the Loyola University Institutional Review Board.
Survey data required cleaning prior to analysis. Missing gender information was imputed using the respondents' name. Responses to the item that asked to indicate the proportion of work dedicated to administrative responsibilities, clinical care, teaching, and research that did not add up to 100% were dropped. Two responses that indicated full‐time equivalent (FTE) of 0%, but whose respondents otherwise completed the survey implying they worked as clinical hospitalists, were replaced with values calculated from the given number of work hours relative to the median work hours in our sample. Out of range or implausible responses to the following items were dropped from analyses: the average number of billable encounters during a typical day or shift, number of shifts performing clinical activities during a typical month, pretax earnings, the year the respondent completed residency training, and the number of whole years practiced as a hospitalist. The proportion of selective item nonresponse was small and we did not, otherwise, impute missing data.
RESULTS
Response Rate
Of the 5389 originally sampled addresses, 1868 were undeliverable. Addresses were further excluded if they appeared in duplicate or were outdated. This yielded a total of 3105 eligible surveyees in the sample. As illustrated in Figure 1, 841 responded to the mailed survey and 5 responded to the Web‐based survey. After rejecting 67 non‐hospitalist respondents and 3 duplicate surveys, a total of 776 surveys were included in the final analysis. The adjusted response rate was 25.6% (776/3035). Members of SHM were more likely to return the survey than nonmembers. The adjusted response rate from hospitalists affiliated with the 3 sponsoring institutions was 6% (40/662). Because these respondents were more likely to be non‐members of SHM, we opted to analyze the responses from the sponsor hospitalists together with the sampled hospitalists. The demographics of the resulting pool of 816 respondents affiliated with over 650 unique hospitalist groups were representative of the original survey frame. We analyzed data from 794 of these who responded to the item indicating their hospitalist practice model. Demographic characteristics of responders and nonresponders to the practice model survey item were similar.
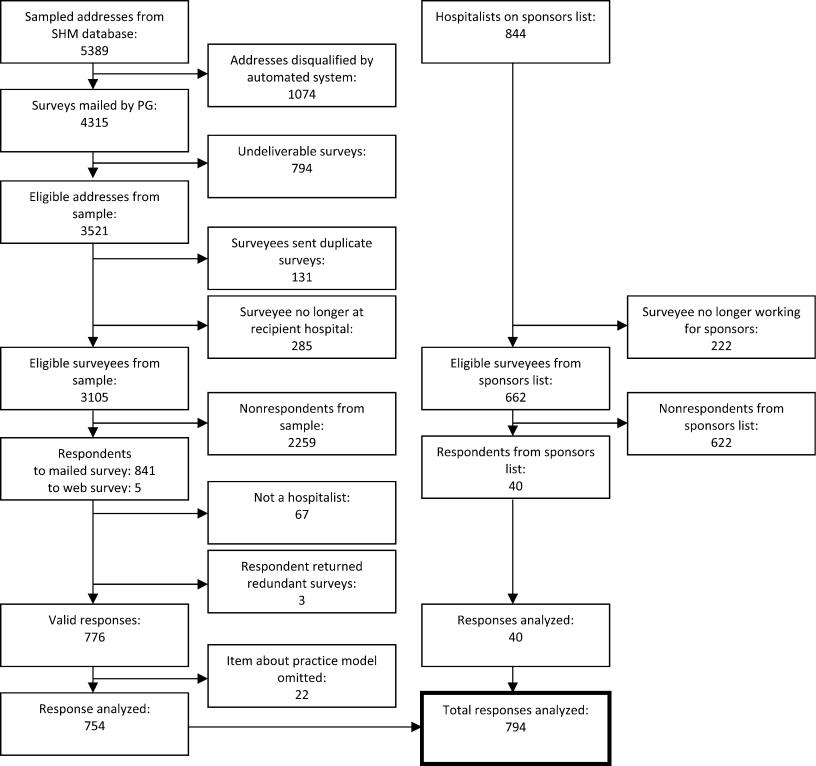
Characteristics of Hospitalists and Their Groups
Table 1 summarizes the characteristics of hospitalist respondents and their organizations by practice model. More (44%) respondents identified their practice model as directly employed by the hospital than other models, including multispecialty physician group (15%), multistate hospitalist group (14%), university or medical school (14%), local hospitalist group (12%), and other (2%). The median age of hospitalist respondents was 42 years, with 6.8 years of mean experience as a hospitalist. One third were women, 84% were married, and 46% had dependent children 6 years old or younger at home. Notably, hospitalists in multistate groups had fewer years of experience, and fewer hospitalists in local and multistate groups were married compared to hospitalists in other practice models.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| Hospitalist characteristics | ||||||
| Age, weighted mean (99% CI) | 45 (42, 48) | 44 (42, 47) | 45 (43, 47) | 45 (43, 46) | 43 (40, 46) | |
| Years hospitalist experience, weighted mean (99% CI) | 8 (6, 9)* | 5 (4, 6)* | 8 (7, 9) | 7 (6, 7) | 8 (6, 9) | <0.010* |
| Women, weighted % | 29 | 30 | 39 | 31 | 43 | 0.118 |
| Married, weighted % | 76 | 77 | 82 | 89 | 81 | 0.009 |
| At least 1 dependent child younger than age 6 living in home, weighted % | 47 | 48 | 43 | 47 | 45 | 0.905 |
| Pediatric specialty, n (%) | <10 | <10 | 11 (10%) | 57 (16%) | 36 (34%) | <0.001 |
| Hospitalist group characteristics | ||||||
| Region, weighted % | <0.001 | |||||
| Northeast (AHA 1 & 2) | 13 | 10 | 16 | 27 | 13 | |
| South (AHA 3 & 4) | 19 | 37 | 13 | 24 | 21 | |
| Midwest (AHA 5 & 6) | 23 | 24 | 25 | 22 | 26 | |
| Mountain (AHA 7 & 8) | 22 | 20 | 16 | 13 | 24 | |
| West (AHA 9) | 24 | 10 | 31 | 14 | 16 | |
| No. beds of primary hospital, weighted % | <0.001 | |||||
| Up to 149 | 17 | 26 | 12 | 24 | 14 | |
| 150299 | 30 | 36 | 36 | 33 | 21 | |
| 300449 | 26 | 24 | 29 | 20 | 19 | |
| 450599 | 13 | 8 | 17 | 11 | 21 | |
| 600 or more | 12 | 6 | 7 | 13 | 24 | |
| No. of hospital facilities served by current practice, weighted % | <0.001 | |||||
| 1 | 53 | 70 | 67 | 77 | 66 | |
| 2 | 20 | 22 | 20 | 16 | 24 | |
| 3 or more | 27 | 9 | 13 | 7 | 10 | |
| No. of physicians in current practice, median (IQR) | 10 (5, 18) | 8 (6, 12)* | 14 (8, 25)* | 12 (6, 18) | 12 (7, 20) | <0.001*, 0.001 |
| No. of non‐physician providers in current practice, median (IQR) | 0 (0, 2) | 0 (0, 2) | 0 (0, 3) | 1 (0, 2) | 0 (0, 2) | |
| Available information technology capabilities, weighted % | ||||||
| EHR to access physician notes | 57 | 57 | 75 | 58 | 79 | <0.001 |
| EHR to access nursing documentations | 68 | 67 | 74 | 75 | 76 | 0.357 |
| EHR to access laboratory or test results | 97 | 89 | 95 | 96 | 96 | 0.054 |
| Electronic order entry | 30 | 19 | 53 | 38 | 56 | <0.001 |
| Electronic billing | 38 | 31 | 36 | 36 | 38 | 0.818 |
| Access to EHR at home or off site | 78 | 73 | 78 | 82 | 84 | 0.235 |
| Access to Up‐to‐Date or other clinical guideline resources | 80 | 77 | 91 | 92 | 96 | <0.001 |
| Access to schedules, calendars, or other organizational resources | 56 | 57 | 66 | 67 | 75 | 0.024 |
| E‐mail, Web‐based paging, or other communication resources | 74 | 63 | 88 | 89 | 90 | <0.001 |
Several differences in respondent group characteristics by practice model were found. Respondents in multistate hospitalist groups were more likely from the South and Midwest, while respondents from multispecialty groups were likely from the West. More multistate group practices were based in smaller hospitals, while academic hospitalists tended to practice in hospitals with 600 or more beds. Respondents employed by hospitals were more likely to practice at 1 hospital facility only, while local group practices were more likely to practice at 3 or more facilities. The median number of physicians in a hospitalist group was 11 (interquartile range [IQR] 6, 19). Local and multistate groups had fewer hospitalists compared to other models. Nonphysician providers were employed by nearly half of all hospitalist practices. Although almost all groups had access to some information technology, more academic hospitalists had access to electronic order entry, electronic physician notes, electronic clinical guidelines resources and communication technology, while local and multistate groups were least likely to have access to these resources.
Work Pattern Variations
Table 2 further details hospitalist work hours by practice model. The majority of hospitalists (78%) reported their position was full‐time (FTE 1.0), while 13% reported working less than full‐time (FTE <1.0). Only 5% of local group hospitalists worked part‐time, while 20% of multispecialty group hospitalists did. An additional 9% reported FTE >1.0, indicating their work hours exceeded the definition of a full‐time physician in their practice. Among full‐time hospitalists, local group members worked a greater number of shifts per month than employees of multispecialty groups, hospitals, and academic medical centers. Academic hospitalists reported higher numbers of consecutive clinical days worked on average, but fewer night shifts compared to hospitalists employed by multistate groups, multispecialty groups, and hospitals; fewer billable encounters than hospitalists in local and multistate groups; and more nonclinical work hours than hospitalists of any other practice model. Academic hospitalists also spent more time on teaching and research than other practice models. Hospitalists spent 11%‐18% of their time on administrative and committee responsibilities, with the least amount spent by hospitalists in multistate groups and the most in academic practice.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| FTE, weighted % | 0.058 | |||||
| FTE < 1.0 | 6 | 13 | 20 | 12 | 14 | |
| FTE = 1.0 | 85 | 75 | 74 | 80 | 82 | |
| FTE > 1.0 | 10 | 13 | 6 | 8 | 5 | |
| Workload parameters, weighted mean (99% CI) | ||||||
| Clinical shifts per month for FTE 1.0 | 19 (17, 20)* | 17 (16, 19) | 15 (14, 17)* | 16 (15, 16) | 15 (13, 17) | <0.001* |
| Hours per clinical shift | 10 (9, 11) | 11 (10, 11)* | 10 (10, 11.0) | 11 (10, 11.0) | 10 (9, 10)* | 0.006*, 0.002 |
| Consecutive days on clinical shift | 8 (6, 9) | 7 (6, 7)* | 6 (6, 7) | 7 (6, 7) | 9 (7, 10)* | 0.002*, <0.001 |
| % Clinical shifts on nights | 20 (15, 25) | 23 (18, 28)* | 23 (17, 29) | 21 (17, 24) | 14 (9, 18)* | 0.001*, 0.002 |
| % Night shifts spent in hospital | 61 (49, 74)* | 63 (52, 75) | 72 (62, 83) | 73 (67, 80) | 43 (29, 57)* | 0.010*, 0.003, <0.001 |
| Billable encounters per clinical shift | 17 (14, 19)* | 17 (16, 18) | 14 (13, 15) | 15 (14, 16) | 13 (11, 14)* | <0.001*, 0.002 |
| Hours nonclinical work per month | 23 (12, 34)* | 19 (11, 27) | 31 (20, 42) | 30 (24, 36) | 71 (55, 86)* | <0.001* |
| Hours clinical and nonclinical work per month for FTE 1.0 | 202 (186, 219) | 211 (196, 226) | 184 (170, 198)* | 193 (186, 201) | 221 (203, 238)* | <0.001* |
| Professional activity, weighted mean % (99% CI) | ||||||
| Clinical | 84 (78, 89)* | 86 (81, 90) | 78 (72, 84) | 79 (76, 82) | 58 (51, 64)* | <0.001* |
| Teaching | 2.3 (1, 5)* | 3 (1, 4) | 6 (4, 9) | 6 (5, 8) | 17 (14, 20)* | <0.001* |
| Administration and Committee work | 13 (8, 19) | 11 (8, 15)* | 16 (10, 21) | 14 (12, 17) | 19 (14, 24)* | 0.001* |
| Research | 0 (0, 0)* | 1 (0, 2) | 0 (0, 1) | 1 (0, 1) | 7 (3, 11)* | <0.001* |
Table 3 tabulates other work pattern characteristics. Most hospitalists indicated that their current clinical work as hospitalists involved the general medical wards (100%), medical consultations (98%), and comanagement with specialists (92%). There were wide differences in participation in comanagement (100%, local groups vs 71%, academic), intensive care unit (ICU) responsibilities (94%, multistate groups vs 27%, academic), and nursing home care (30%, local groups vs 8%, academic). Among activities that are potentially not reimbursable, academic hospitalists were less likely to participate in coordination of patient transfers and code or rapid response teams, while multistate groups were least likely to participate in quality improvement activities. In total, 99% of hospitalists reported participating in at least 1 potentially nonreimbursable clinical activity.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| Reimbursable activities, overlapping weighted % | ||||||
| General medical ward | 100 | 99 | 100 | 99 | 99 | 0.809 |
| Medical consultations | 99 | 99 | 100 | 98 | 95 | 0.043 |
| Comanagement with specialists | 100 | 96 | 96 | 93 | 71 | <0.001 |
| Preoperative evaluations | 92 | 92 | 90 | 88 | 77 | 0.002 |
| Intensive care unit | 86 | 94 | 67 | 75 | 27 | <0.001 |
| Skilled nursing facility or long‐term acute care facility | 30 | 19 | 12 | 16 | 8 | <0.001 |
| Outpatient general medical practice | 4 | 4 | 5 | 5 | 10 | 0.241 |
| Potentially nonreimbursable activities, overlapping weighted % | ||||||
| Coordination of patient transfers | 92 | 94 | 95 | 93 | 82 | 0.005 |
| Quality improvement or patient safety initiatives | 81 | 78 | 83 | 89 | 89 | 0.029 |
| Code team or rapid response team | 56 | 57 | 53 | 62 | 37 | <0.001 |
| Information technology design or implementation | 42 | 39 | 47 | 51 | 51 | 0.154 |
| Admission triage for emergency department | 49 | 46 | 43 | 40 | 31 | 0.132 |
| Compensation scheme, weighted % | <0.001 | |||||
| Salary only | 18 | 21 | 30 | 29 | 47 | |
| Salary plus performance incentive | 54 | 72 | 59 | 67 | 53 | |
| Fee‐for‐service | 20 | 1 | 7 | 2 | 0 | |
| Capitation | 0 | 0 | 0 | 0 | 0 | |
| Other | 9 | 7 | 4 | 3 | 0 | |
| Compensation links to incentives, overlapping weighted % | ||||||
| No incentives | 40 | 28 | 29 | 29 | 48 | 0.003 |
| Patient satisfaction | 23 | 39 | 38 | 38 | 14 | <0.001 |
| Length of stay | 18 | 17 | 20 | 13 | 10 | 0.208 |
| Overall cost | 8 | 11 | 9 | 5 | 6 | 0.270 |
| Test utilization | 2 | 2 | 7 | 1 | 0 | <0.001 |
| Clinical processes and outcomes | 26 | 34 | 44 | 43 | 24 | <0.001 |
| Other | 17 | 29 | 26 | 31 | 25 | 0.087 |
| Earnings, weighted mean dollars (99% CI) | 226,065 (202,891, 249,240)* | 225,613 (210,772, 240,454) | 202,617 (186,036, 219,198) | 206,087 (198,413, 213,460) | 166,478 (151,135, 181,821)* | <0.001* |
Hospitalist compensation schemes were significantly different across the practice models. Salary‐only schemes were most common among academic hospitalists (47%), while 72% of multistate groups used performance incentives in addition to salary. More local groups used fee‐for‐service compensation than other models. Incentives differed by practice model, with more multistate groups having incentives based on patient satisfaction, while more multispecialty physician groups had incentives based on clinical processes and outcomes than other models. Finally, mean earnings for academic hospitalists were significantly lower than for hospitalists of other practice models. Local and multistate group hospitalists earned more than any other practice model (all P <0.001), and $60,000 more than the lowest compensated academic hospitalists.
Components of Job Satisfaction
Hospitalists' rankings of the most important factors for job satisfaction revealed differences across models (Figure 2). Overall, hospitalists were most likely to consider optimal workload and compensation as important factors for job satisfaction from a list of 13 considerations. Local groups and academics were least likely to rank optimal workload as a top factor, and local group hospitalists were more likely to rank optimal autonomy than those of other models. Academic hospitalists had less concern for substantial pay, and more concern for the variety of tasks they perform and recognition by leaders, than other hospitalists.
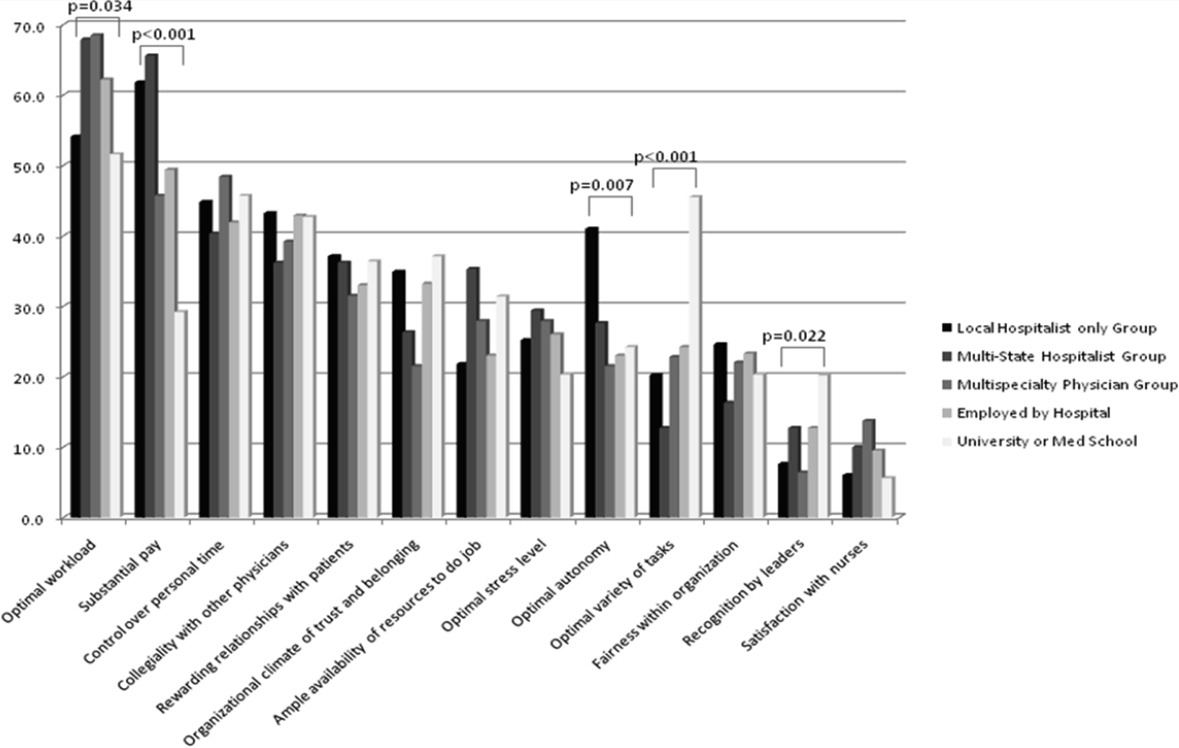
Job Satisfaction and Burnout Risk
Differences in the ratings of 4 of the 11 satisfaction and job characteristic domains were found across the practice models (Figure 3). Multispecialty group hospitalists were less satisfied with autonomy and their relationship with patients than other practice models, and along with multistate groups, reported the highest perceived workload. Organizational fairness was rated much higher by local group hospitalists than other practice models. Despite these differences in work patterns and satisfaction, there were no differences found in level of global job satisfaction, specialty satisfaction, or burnout across the practice models. Overall, 62% of respondents reported high job satisfaction (4 on a 1 to 5 scale), and 30% indicated burnout symptoms.
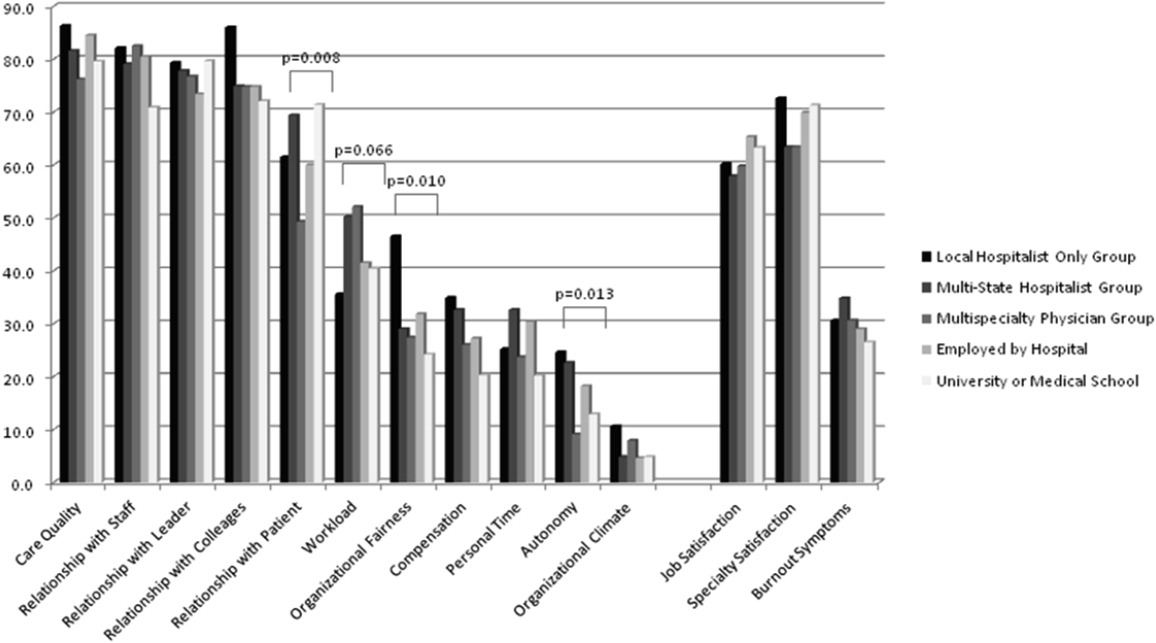
DISCUSSION
In our sample of US hospitalists, we found major differences in work patterns and compensation across hospitalist practice models, but no differences in job satisfaction, specialty satisfaction, and burnout. In particular, differences across these models included variations in hospitalist workload, hours, pay, and distribution of work activities. We found that hospitalists perform a variety of clinical and nonclinical tasks, for many of which there are not standard reimbursement mechanisms. We also found that features of a job that individual hospitalists considered most important vary by practice model.
Previous analysis of this data explored the overall state of hospitalist satisfaction.16 The present analysis offers a glimpse into hospitalists' systems‐orientation through a deeper look at their work patterns. The growth in the number of hospitalists who participate in intensive care medicine, specialty comanagement, and other work that involves close working relationships with specialist physicians confirms collaborative care as one of the dominant drivers of the hospitalist movement. At the level of indirect patient care, nearly all hospitalists contributed to work that facilitates coordination, quality, patient safety, or information technology. Understanding the integrative value of hospitalists outside of their clinical productivity may be of interest to hospital administrators.
Global satisfaction measures were similar across practice models. This finding is particularly interesting given the major differences in job characteristics seen among the practice models. This similarity in global satisfaction despite real differences in the nature of the job suggests that individuals find settings that allow them to address their individual professional goals. Our study demonstrates that, in 2010, Hospital Medicine has evolved enough to accommodate a wide variety of goals and needs.
While global satisfaction did not differ among practice types, hospitalists from various models did report differences in factors considered important to global satisfaction. While workload and pay were rated as influential across most models, the degree of importance was significantly different. In academic settings, substantial pay was not a top consideration for overall job satisfaction, whereas in local and multistate hospitalist groups, pay was a very close second in importance to optimal workload. These results may prove helpful for individual hospitalists trying to find their optimal job. For example, someone who is less concerned about workload, but wants to be paid well and have a high degree of autonomy, may find satisfaction in local hospitalist groups. However, for someone who is willing to sacrifice a higher salary for variety of activities, academic Hospital Medicine may be a better fit.
There is a concerning aspect of hospitalist job satisfaction that different practice models do not seem to solve. Control over personal time is a top consideration for many hospitalists across practice models, yet their satisfaction with personal time is low. As control over personal time is seen as a draw to the Hospital Medicine specialty, group leaders may need to evaluate their programs to ensure that schedules and workload support efforts for hospitalists to balance work and homelife commitments.
There are additional findings that are important for Hospital Medicine group leaders. Regardless of practice model, compensation and workload are often used as tools to recruit and retain hospitalists. While these tools may be effective, leaders may find more nuanced approaches to improving their hospitalists' overall satisfaction. Leaders of local hospitalist groups may find their hospitalists tolerant of heavier workloads as long as they are adequately rewarded and are given real autonomy over their work. However, leaders of academic programs may be missing the primary factor that can improve their hospitalists' satisfaction. Rather than asking for higher salaries to remain competitive, it may be more effective to advocate for time and training for their hospitalists to pursue important other activities beyond direct clinical care. Given that resources will always be limited, group leaders need to understand all of the elements that can contribute to hospitalist job satisfaction.
We point out several limitations to this study. First, our adjusted response rate of 25.6% is low for survey research, in general. As mentioned above, hospitalists are not easily identified in any available national physician database. Therefore, we deliberately designed our sampling strategy to error on the side of including ineligible surveyees to reduce systematic exclusion of practicing hospitalists. Using simple post hoc methods, we identified many nonhospitalists and bad addresses from our sample, but because these methods were exclusionary as opposed to confirmatory, we believe that a significant proportion of remaining nonrespondents may also have been ineligible for the survey. Although this does not fully address concerns about potential response bias, we believe that our sample representing a large number of hospitalist groups is adequate to make estimations about a nationally representative sample of practicing hospitalists. Second, in spite of our inclusive approach, we may still have excluded categories of practicing hospitalists. We were careful not to allow SHM members to represent all US hospitalists and included non‐members in the sampling frame, but the possibility of systematic exclusion that may alter our results remains a concern. Additionally, one of our goals was to characterize pediatric hospitalists independently from their adult‐patient counterparts. Despite oversampling of pediatricians, their sample was too small for a more detailed comparison across practice models. Also, self‐reported data about workload and compensation are subject to inaccuracies related to recall and cognitive biases. Last, this is a cross‐sectional study of hospitalist satisfaction at one point in time. Consequently, our sample may not be representative of very dissatisfied hospitalists who have already left their jobs.
The diversity found across existing practice models and the characteristics of the practices provide physicians with the opportunity to bring their unique skills and motivations to the hospitalist movement. As hospitals and other organizations seek to create, maintain, or grow hospitalist programs, the data provided here may prove useful to understand the relationship between practice characteristics and individual job satisfaction. Additionally, hospitalists looking for a job can consider these results as additional information to guide their choice of practice model and work patterns.
Acknowledgements
The authors thank Kenneth A. Rasinski for assistance with survey items refinement, and members of the SHM Career Satisfaction Task Force for their assistance in survey development.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1(2):75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92(2):265–273,vii.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20(2):101–107.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130(4 pt 2):350–354.
- ,.Effects of an HMO hospitalist program on inpatient utilization.Am J Manag Care.2001;7(11):1051–1057.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,,,.Trends in market demand for internal medicine 1999 to 2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21(10):1079–1085.
- SHM. 2003–2004 Survey by the Society of Hospital Medicine on Productivity and Compensation: Analysis of Results. 2004 [updated 2004]. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Practice_Resources Available at: http://cme.medscape.com/viewarticle/578134. Accessed October 21,2010.
- State of Hospital Medicine: 2010 Report Based on 2009 Data.Englewood, CO and Philadelphia, PA:Medical Group Management Association and Society of Hospital Medicine;2010.
- ,,,,.Worklife and satisfaction of hospitalists: toward flourishing careers.J Gen Intern Med.2011, Jul 20. PMID: 21773849.
- ,,, et al.Worklife and satisfaction of general internists.Arch Intern Med.2002;162(6):649–656.
- ,,, et al.Organizational climate, stress, and error in primary care: the MEMO study. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol 1: Research Findings.Rockville, MD:Agency for Healthcare Research and Quality;2005;1:65–77.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130(4 pt 2):343–349.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109(8):648–653.
- .Taking the Measure of Work: A Guide to Validated Scales for Organizational Research and Diagnosis.Thousand Oaks, CA:Sage Publications;2002.
- ,,,,.Job Demands and Worker Health.Ann Arbor, MI:University of Michigan, Institute for Social Research;1980.
- .On the dimensionality of organizational justice: a construct validation of a measure.J Appl Psychol.2001;86(3):386–400.
- ,.Effect of job demands and social support on worker stress—a study of VDT users.Behav Inform Technol.1995;14(1):32–40.
- ,,, et al.Measuring physician job satisfaction in a changing workplace and a challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine.Med Care.1999;37(11):1174–1182.
- ,,, et al.Working conditions in primary care: physician reactions and care quality.Ann Intern Med.2009;151(1):28–U48.
- ,,.Validation of a single‐item measure of burnout against the Maslach Burnout Inventory among physicians.Stress Health.2004;20(2):75–79.
- American Hospital Association. AHA Hospital Statistics. 2009 [updated 2009]. Available at: http://www.ahadata.com/ahadata/html/AHAStatistics.html. Accessed April 12,2011.
- ,,, et al.How to obtain excellent response rates when surveying physicians.Fam Pract.2009;26(1):65–68.
- ,.Estimating nonresponse bias in mail surveys.J Marketing Res.1977;14(3):396–402.
Over the past 15 years, there has been dramatic growth in the number of hospitalist physicians in the United States and in the number of hospitals served by them.13 Hospitals are motivated to hire experienced hospitalists to staff their inpatient services,4 with goals that include obtaining cost‐savings and higher quality.59 The rapid growth of Hospital Medicine saw multiple types of hospital practice models emerge with differing job characteristics, clinical duties, workload, and compensation schemes.10 The extent of the variability of hospitalist jobs across practice models is not known.
Intensifying recruitment efforts and the concomitant increase in compensation for hospitalists over the last decade suggest that demand for hospitalists is strong and sustained.11 As a result, today's cohort of hospitalists has a wide range of choices of types of jobs, practice models, and locations. The diversity of available hospitalist jobs is characterized, for example, by setting (community hospital vs academic hospital), employer (hospital vs private practice), job duties (the amount and type of clinical work, and other administrative, teaching, or research duties), and intensity (work hours and duties to maximize income or lifestyle). How these choices relate to job satisfaction and burnout are also unknown.
The Society of Hospital Medicine (SHM) has administered surveys to hospitalist group leaders biennially since 2003.1215 These surveys, however, do not address issues related to individual hospitalist worklife, recruitment, and retention. In 2005, SHM convened a Career Satisfaction Task Force that designed and executed a national survey of hospitalists in 2009‐2010. The objective of this study is to evaluate how job characteristics vary by practice model, and the association of these characteristics and practice models with job satisfaction and burnout.
METHODS
Survey Instrument
A detailed description of the survey design, sampling strategy, data collection, and response rate calculations is described elsewhere.16 Portions of the 118‐item survey instrument assessed characteristics of the respondents' hospitalist group (12 items), details about their individual work patterns (12 items), and demographics (9 items). Work patterns were evaluated by the average number of clinical work days, consecutive days, hours per month, percentage of work assigned to night duty, and number of patient encounters. Average hours spent on nonclinical work, and the percentage of time allocated for clinical, administrative, teaching, and research activities were solicited. Additional items assessed specific clinical responsibilities, pretax earnings in FY2010, the availability of information technology capabilities, and the adequacy of available resources. Job and specialty satisfaction and 11 satisfaction domain measures were measured using validated scales.1726 Burnout symptoms were measured using a validated single‐item measure.26, 27
Sampling Strategy
We surveyed a national stratified sample of hospitalists in the US and Puerto Rico. We used the largest database of hospitalists (>24,000 names) currently available and maintained by the SHM as our sampling frame. We linked hospitalist employer information to hospital statistics from the American Hospital Association database28 to stratify the sample by number of hospital beds, geographic region, employment model, and specialty training, oversampling pediatric hospitalists due to small numbers. A respondent sample of about 700 hospitalists was calculated to be adequate to detect a 0.5 point difference in job satisfaction scores between subgroups assuming 90% power and alpha of 0.05. However, we sampled a total of 5389 addresses from the database to overcome the traditionally low physician response rates, duplicate sampling, bad addresses, and non‐hospitalists being included in the sampling frame. In addition, 2 multistate hospitalist companies (EmCare, In Compass Health) and 1 for‐profit hospital chain (HCA, Inc) financially sponsored this project with the stipulation that all of their hospitalist employees (n = 884) would be surveyed.
Data Collection
The healthcare consulting firm, Press Ganey, provided support with survey layout and administration following the modified Dillman method.29 Three rounds of coded surveys and solicitation letters from the investigators were mailed 2 weeks apart in November and December 2009. Because of low response rates to the mailed survey, an online survey was created using Survey Monkey and sent to 650 surveyees for whom e‐mail addresses were available, and administered at a kiosk for sample physicians during the SHM 2010 annual meeting.
Data Analysis
Nonresponse bias was measured by comparing characteristics between respondents of separate survey waves.30 We determined the validity of mailing addresses immediately following the survey period by mapping each address using Google, and if the address was a hospital, researching online whether or not the intended recipient was currently employed there. Practice characteristics were compared across 5 model categories distilled from the SHM & Medical Group Management Association survey: local hospitalist‐only group, multistate hospitalist group, multispecialty physician group, employer hospital, and university or medical school. Weighted proportions, means, and medians were calculated to account for oversampling of pediatric hospitalists. Differences in categorical measures were assessed using the chi‐square test and the design‐based F test for comparing weighted data. Weighted means (99% confidence intervals) and medians (interquartile ranges) were calculated. Because each parameter yielded a single outlier value across the 5 practice models, differences across weighted means were assessed using generalized linear models with the single outlier value chosen as the reference mean. Pair‐wise Wilcoxon rank sum test was used to compare median values. In these 4‐way comparisons of means and medians, significance was defined as P value of 0.0125 per Bonferroni correction. A single survey item solicited respondents to choose exactly 4 of 13 considerations most pertinent to job satisfaction. The proportion of respondents who scored 4 on a 5‐point Likert scale of the 11 satisfaction domains and 2 global measures of satisfaction, and burnout symptoms defined as 3 on a 5‐point single item measure were bar‐graphed. Chi‐square statistics were used to evaluate for differences across practice models. Statistical significance was defined by alpha less than 0.05, unless otherwise specified. All analyses were performed using STATA version 11.0 (College Station, TX). This study was approved by the Loyola University Institutional Review Board.
Survey data required cleaning prior to analysis. Missing gender information was imputed using the respondents' name. Responses to the item that asked to indicate the proportion of work dedicated to administrative responsibilities, clinical care, teaching, and research that did not add up to 100% were dropped. Two responses that indicated full‐time equivalent (FTE) of 0%, but whose respondents otherwise completed the survey implying they worked as clinical hospitalists, were replaced with values calculated from the given number of work hours relative to the median work hours in our sample. Out of range or implausible responses to the following items were dropped from analyses: the average number of billable encounters during a typical day or shift, number of shifts performing clinical activities during a typical month, pretax earnings, the year the respondent completed residency training, and the number of whole years practiced as a hospitalist. The proportion of selective item nonresponse was small and we did not, otherwise, impute missing data.
RESULTS
Response Rate
Of the 5389 originally sampled addresses, 1868 were undeliverable. Addresses were further excluded if they appeared in duplicate or were outdated. This yielded a total of 3105 eligible surveyees in the sample. As illustrated in Figure 1, 841 responded to the mailed survey and 5 responded to the Web‐based survey. After rejecting 67 non‐hospitalist respondents and 3 duplicate surveys, a total of 776 surveys were included in the final analysis. The adjusted response rate was 25.6% (776/3035). Members of SHM were more likely to return the survey than nonmembers. The adjusted response rate from hospitalists affiliated with the 3 sponsoring institutions was 6% (40/662). Because these respondents were more likely to be non‐members of SHM, we opted to analyze the responses from the sponsor hospitalists together with the sampled hospitalists. The demographics of the resulting pool of 816 respondents affiliated with over 650 unique hospitalist groups were representative of the original survey frame. We analyzed data from 794 of these who responded to the item indicating their hospitalist practice model. Demographic characteristics of responders and nonresponders to the practice model survey item were similar.

Characteristics of Hospitalists and Their Groups
Table 1 summarizes the characteristics of hospitalist respondents and their organizations by practice model. More (44%) respondents identified their practice model as directly employed by the hospital than other models, including multispecialty physician group (15%), multistate hospitalist group (14%), university or medical school (14%), local hospitalist group (12%), and other (2%). The median age of hospitalist respondents was 42 years, with 6.8 years of mean experience as a hospitalist. One third were women, 84% were married, and 46% had dependent children 6 years old or younger at home. Notably, hospitalists in multistate groups had fewer years of experience, and fewer hospitalists in local and multistate groups were married compared to hospitalists in other practice models.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| Hospitalist characteristics | ||||||
| Age, weighted mean (99% CI) | 45 (42, 48) | 44 (42, 47) | 45 (43, 47) | 45 (43, 46) | 43 (40, 46) | |
| Years hospitalist experience, weighted mean (99% CI) | 8 (6, 9)* | 5 (4, 6)* | 8 (7, 9) | 7 (6, 7) | 8 (6, 9) | <0.010* |
| Women, weighted % | 29 | 30 | 39 | 31 | 43 | 0.118 |
| Married, weighted % | 76 | 77 | 82 | 89 | 81 | 0.009 |
| At least 1 dependent child younger than age 6 living in home, weighted % | 47 | 48 | 43 | 47 | 45 | 0.905 |
| Pediatric specialty, n (%) | <10 | <10 | 11 (10%) | 57 (16%) | 36 (34%) | <0.001 |
| Hospitalist group characteristics | ||||||
| Region, weighted % | <0.001 | |||||
| Northeast (AHA 1 & 2) | 13 | 10 | 16 | 27 | 13 | |
| South (AHA 3 & 4) | 19 | 37 | 13 | 24 | 21 | |
| Midwest (AHA 5 & 6) | 23 | 24 | 25 | 22 | 26 | |
| Mountain (AHA 7 & 8) | 22 | 20 | 16 | 13 | 24 | |
| West (AHA 9) | 24 | 10 | 31 | 14 | 16 | |
| No. beds of primary hospital, weighted % | <0.001 | |||||
| Up to 149 | 17 | 26 | 12 | 24 | 14 | |
| 150299 | 30 | 36 | 36 | 33 | 21 | |
| 300449 | 26 | 24 | 29 | 20 | 19 | |
| 450599 | 13 | 8 | 17 | 11 | 21 | |
| 600 or more | 12 | 6 | 7 | 13 | 24 | |
| No. of hospital facilities served by current practice, weighted % | <0.001 | |||||
| 1 | 53 | 70 | 67 | 77 | 66 | |
| 2 | 20 | 22 | 20 | 16 | 24 | |
| 3 or more | 27 | 9 | 13 | 7 | 10 | |
| No. of physicians in current practice, median (IQR) | 10 (5, 18) | 8 (6, 12)* | 14 (8, 25)* | 12 (6, 18) | 12 (7, 20) | <0.001*, 0.001 |
| No. of non‐physician providers in current practice, median (IQR) | 0 (0, 2) | 0 (0, 2) | 0 (0, 3) | 1 (0, 2) | 0 (0, 2) | |
| Available information technology capabilities, weighted % | ||||||
| EHR to access physician notes | 57 | 57 | 75 | 58 | 79 | <0.001 |
| EHR to access nursing documentations | 68 | 67 | 74 | 75 | 76 | 0.357 |
| EHR to access laboratory or test results | 97 | 89 | 95 | 96 | 96 | 0.054 |
| Electronic order entry | 30 | 19 | 53 | 38 | 56 | <0.001 |
| Electronic billing | 38 | 31 | 36 | 36 | 38 | 0.818 |
| Access to EHR at home or off site | 78 | 73 | 78 | 82 | 84 | 0.235 |
| Access to Up‐to‐Date or other clinical guideline resources | 80 | 77 | 91 | 92 | 96 | <0.001 |
| Access to schedules, calendars, or other organizational resources | 56 | 57 | 66 | 67 | 75 | 0.024 |
| E‐mail, Web‐based paging, or other communication resources | 74 | 63 | 88 | 89 | 90 | <0.001 |
Several differences in respondent group characteristics by practice model were found. Respondents in multistate hospitalist groups were more likely from the South and Midwest, while respondents from multispecialty groups were likely from the West. More multistate group practices were based in smaller hospitals, while academic hospitalists tended to practice in hospitals with 600 or more beds. Respondents employed by hospitals were more likely to practice at 1 hospital facility only, while local group practices were more likely to practice at 3 or more facilities. The median number of physicians in a hospitalist group was 11 (interquartile range [IQR] 6, 19). Local and multistate groups had fewer hospitalists compared to other models. Nonphysician providers were employed by nearly half of all hospitalist practices. Although almost all groups had access to some information technology, more academic hospitalists had access to electronic order entry, electronic physician notes, electronic clinical guidelines resources and communication technology, while local and multistate groups were least likely to have access to these resources.
Work Pattern Variations
Table 2 further details hospitalist work hours by practice model. The majority of hospitalists (78%) reported their position was full‐time (FTE 1.0), while 13% reported working less than full‐time (FTE <1.0). Only 5% of local group hospitalists worked part‐time, while 20% of multispecialty group hospitalists did. An additional 9% reported FTE >1.0, indicating their work hours exceeded the definition of a full‐time physician in their practice. Among full‐time hospitalists, local group members worked a greater number of shifts per month than employees of multispecialty groups, hospitals, and academic medical centers. Academic hospitalists reported higher numbers of consecutive clinical days worked on average, but fewer night shifts compared to hospitalists employed by multistate groups, multispecialty groups, and hospitals; fewer billable encounters than hospitalists in local and multistate groups; and more nonclinical work hours than hospitalists of any other practice model. Academic hospitalists also spent more time on teaching and research than other practice models. Hospitalists spent 11%‐18% of their time on administrative and committee responsibilities, with the least amount spent by hospitalists in multistate groups and the most in academic practice.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| FTE, weighted % | 0.058 | |||||
| FTE < 1.0 | 6 | 13 | 20 | 12 | 14 | |
| FTE = 1.0 | 85 | 75 | 74 | 80 | 82 | |
| FTE > 1.0 | 10 | 13 | 6 | 8 | 5 | |
| Workload parameters, weighted mean (99% CI) | ||||||
| Clinical shifts per month for FTE 1.0 | 19 (17, 20)* | 17 (16, 19) | 15 (14, 17)* | 16 (15, 16) | 15 (13, 17) | <0.001* |
| Hours per clinical shift | 10 (9, 11) | 11 (10, 11)* | 10 (10, 11.0) | 11 (10, 11.0) | 10 (9, 10)* | 0.006*, 0.002 |
| Consecutive days on clinical shift | 8 (6, 9) | 7 (6, 7)* | 6 (6, 7) | 7 (6, 7) | 9 (7, 10)* | 0.002*, <0.001 |
| % Clinical shifts on nights | 20 (15, 25) | 23 (18, 28)* | 23 (17, 29) | 21 (17, 24) | 14 (9, 18)* | 0.001*, 0.002 |
| % Night shifts spent in hospital | 61 (49, 74)* | 63 (52, 75) | 72 (62, 83) | 73 (67, 80) | 43 (29, 57)* | 0.010*, 0.003, <0.001 |
| Billable encounters per clinical shift | 17 (14, 19)* | 17 (16, 18) | 14 (13, 15) | 15 (14, 16) | 13 (11, 14)* | <0.001*, 0.002 |
| Hours nonclinical work per month | 23 (12, 34)* | 19 (11, 27) | 31 (20, 42) | 30 (24, 36) | 71 (55, 86)* | <0.001* |
| Hours clinical and nonclinical work per month for FTE 1.0 | 202 (186, 219) | 211 (196, 226) | 184 (170, 198)* | 193 (186, 201) | 221 (203, 238)* | <0.001* |
| Professional activity, weighted mean % (99% CI) | ||||||
| Clinical | 84 (78, 89)* | 86 (81, 90) | 78 (72, 84) | 79 (76, 82) | 58 (51, 64)* | <0.001* |
| Teaching | 2.3 (1, 5)* | 3 (1, 4) | 6 (4, 9) | 6 (5, 8) | 17 (14, 20)* | <0.001* |
| Administration and Committee work | 13 (8, 19) | 11 (8, 15)* | 16 (10, 21) | 14 (12, 17) | 19 (14, 24)* | 0.001* |
| Research | 0 (0, 0)* | 1 (0, 2) | 0 (0, 1) | 1 (0, 1) | 7 (3, 11)* | <0.001* |
Table 3 tabulates other work pattern characteristics. Most hospitalists indicated that their current clinical work as hospitalists involved the general medical wards (100%), medical consultations (98%), and comanagement with specialists (92%). There were wide differences in participation in comanagement (100%, local groups vs 71%, academic), intensive care unit (ICU) responsibilities (94%, multistate groups vs 27%, academic), and nursing home care (30%, local groups vs 8%, academic). Among activities that are potentially not reimbursable, academic hospitalists were less likely to participate in coordination of patient transfers and code or rapid response teams, while multistate groups were least likely to participate in quality improvement activities. In total, 99% of hospitalists reported participating in at least 1 potentially nonreimbursable clinical activity.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| Reimbursable activities, overlapping weighted % | ||||||
| General medical ward | 100 | 99 | 100 | 99 | 99 | 0.809 |
| Medical consultations | 99 | 99 | 100 | 98 | 95 | 0.043 |
| Comanagement with specialists | 100 | 96 | 96 | 93 | 71 | <0.001 |
| Preoperative evaluations | 92 | 92 | 90 | 88 | 77 | 0.002 |
| Intensive care unit | 86 | 94 | 67 | 75 | 27 | <0.001 |
| Skilled nursing facility or long‐term acute care facility | 30 | 19 | 12 | 16 | 8 | <0.001 |
| Outpatient general medical practice | 4 | 4 | 5 | 5 | 10 | 0.241 |
| Potentially nonreimbursable activities, overlapping weighted % | ||||||
| Coordination of patient transfers | 92 | 94 | 95 | 93 | 82 | 0.005 |
| Quality improvement or patient safety initiatives | 81 | 78 | 83 | 89 | 89 | 0.029 |
| Code team or rapid response team | 56 | 57 | 53 | 62 | 37 | <0.001 |
| Information technology design or implementation | 42 | 39 | 47 | 51 | 51 | 0.154 |
| Admission triage for emergency department | 49 | 46 | 43 | 40 | 31 | 0.132 |
| Compensation scheme, weighted % | <0.001 | |||||
| Salary only | 18 | 21 | 30 | 29 | 47 | |
| Salary plus performance incentive | 54 | 72 | 59 | 67 | 53 | |
| Fee‐for‐service | 20 | 1 | 7 | 2 | 0 | |
| Capitation | 0 | 0 | 0 | 0 | 0 | |
| Other | 9 | 7 | 4 | 3 | 0 | |
| Compensation links to incentives, overlapping weighted % | ||||||
| No incentives | 40 | 28 | 29 | 29 | 48 | 0.003 |
| Patient satisfaction | 23 | 39 | 38 | 38 | 14 | <0.001 |
| Length of stay | 18 | 17 | 20 | 13 | 10 | 0.208 |
| Overall cost | 8 | 11 | 9 | 5 | 6 | 0.270 |
| Test utilization | 2 | 2 | 7 | 1 | 0 | <0.001 |
| Clinical processes and outcomes | 26 | 34 | 44 | 43 | 24 | <0.001 |
| Other | 17 | 29 | 26 | 31 | 25 | 0.087 |
| Earnings, weighted mean dollars (99% CI) | 226,065 (202,891, 249,240)* | 225,613 (210,772, 240,454) | 202,617 (186,036, 219,198) | 206,087 (198,413, 213,460) | 166,478 (151,135, 181,821)* | <0.001* |
Hospitalist compensation schemes were significantly different across the practice models. Salary‐only schemes were most common among academic hospitalists (47%), while 72% of multistate groups used performance incentives in addition to salary. More local groups used fee‐for‐service compensation than other models. Incentives differed by practice model, with more multistate groups having incentives based on patient satisfaction, while more multispecialty physician groups had incentives based on clinical processes and outcomes than other models. Finally, mean earnings for academic hospitalists were significantly lower than for hospitalists of other practice models. Local and multistate group hospitalists earned more than any other practice model (all P <0.001), and $60,000 more than the lowest compensated academic hospitalists.
Components of Job Satisfaction
Hospitalists' rankings of the most important factors for job satisfaction revealed differences across models (Figure 2). Overall, hospitalists were most likely to consider optimal workload and compensation as important factors for job satisfaction from a list of 13 considerations. Local groups and academics were least likely to rank optimal workload as a top factor, and local group hospitalists were more likely to rank optimal autonomy than those of other models. Academic hospitalists had less concern for substantial pay, and more concern for the variety of tasks they perform and recognition by leaders, than other hospitalists.

Job Satisfaction and Burnout Risk
Differences in the ratings of 4 of the 11 satisfaction and job characteristic domains were found across the practice models (Figure 3). Multispecialty group hospitalists were less satisfied with autonomy and their relationship with patients than other practice models, and along with multistate groups, reported the highest perceived workload. Organizational fairness was rated much higher by local group hospitalists than other practice models. Despite these differences in work patterns and satisfaction, there were no differences found in level of global job satisfaction, specialty satisfaction, or burnout across the practice models. Overall, 62% of respondents reported high job satisfaction (4 on a 1 to 5 scale), and 30% indicated burnout symptoms.

DISCUSSION
In our sample of US hospitalists, we found major differences in work patterns and compensation across hospitalist practice models, but no differences in job satisfaction, specialty satisfaction, and burnout. In particular, differences across these models included variations in hospitalist workload, hours, pay, and distribution of work activities. We found that hospitalists perform a variety of clinical and nonclinical tasks, for many of which there are not standard reimbursement mechanisms. We also found that features of a job that individual hospitalists considered most important vary by practice model.
Previous analysis of this data explored the overall state of hospitalist satisfaction.16 The present analysis offers a glimpse into hospitalists' systems‐orientation through a deeper look at their work patterns. The growth in the number of hospitalists who participate in intensive care medicine, specialty comanagement, and other work that involves close working relationships with specialist physicians confirms collaborative care as one of the dominant drivers of the hospitalist movement. At the level of indirect patient care, nearly all hospitalists contributed to work that facilitates coordination, quality, patient safety, or information technology. Understanding the integrative value of hospitalists outside of their clinical productivity may be of interest to hospital administrators.
Global satisfaction measures were similar across practice models. This finding is particularly interesting given the major differences in job characteristics seen among the practice models. This similarity in global satisfaction despite real differences in the nature of the job suggests that individuals find settings that allow them to address their individual professional goals. Our study demonstrates that, in 2010, Hospital Medicine has evolved enough to accommodate a wide variety of goals and needs.
While global satisfaction did not differ among practice types, hospitalists from various models did report differences in factors considered important to global satisfaction. While workload and pay were rated as influential across most models, the degree of importance was significantly different. In academic settings, substantial pay was not a top consideration for overall job satisfaction, whereas in local and multistate hospitalist groups, pay was a very close second in importance to optimal workload. These results may prove helpful for individual hospitalists trying to find their optimal job. For example, someone who is less concerned about workload, but wants to be paid well and have a high degree of autonomy, may find satisfaction in local hospitalist groups. However, for someone who is willing to sacrifice a higher salary for variety of activities, academic Hospital Medicine may be a better fit.
There is a concerning aspect of hospitalist job satisfaction that different practice models do not seem to solve. Control over personal time is a top consideration for many hospitalists across practice models, yet their satisfaction with personal time is low. As control over personal time is seen as a draw to the Hospital Medicine specialty, group leaders may need to evaluate their programs to ensure that schedules and workload support efforts for hospitalists to balance work and homelife commitments.
There are additional findings that are important for Hospital Medicine group leaders. Regardless of practice model, compensation and workload are often used as tools to recruit and retain hospitalists. While these tools may be effective, leaders may find more nuanced approaches to improving their hospitalists' overall satisfaction. Leaders of local hospitalist groups may find their hospitalists tolerant of heavier workloads as long as they are adequately rewarded and are given real autonomy over their work. However, leaders of academic programs may be missing the primary factor that can improve their hospitalists' satisfaction. Rather than asking for higher salaries to remain competitive, it may be more effective to advocate for time and training for their hospitalists to pursue important other activities beyond direct clinical care. Given that resources will always be limited, group leaders need to understand all of the elements that can contribute to hospitalist job satisfaction.
We point out several limitations to this study. First, our adjusted response rate of 25.6% is low for survey research, in general. As mentioned above, hospitalists are not easily identified in any available national physician database. Therefore, we deliberately designed our sampling strategy to error on the side of including ineligible surveyees to reduce systematic exclusion of practicing hospitalists. Using simple post hoc methods, we identified many nonhospitalists and bad addresses from our sample, but because these methods were exclusionary as opposed to confirmatory, we believe that a significant proportion of remaining nonrespondents may also have been ineligible for the survey. Although this does not fully address concerns about potential response bias, we believe that our sample representing a large number of hospitalist groups is adequate to make estimations about a nationally representative sample of practicing hospitalists. Second, in spite of our inclusive approach, we may still have excluded categories of practicing hospitalists. We were careful not to allow SHM members to represent all US hospitalists and included non‐members in the sampling frame, but the possibility of systematic exclusion that may alter our results remains a concern. Additionally, one of our goals was to characterize pediatric hospitalists independently from their adult‐patient counterparts. Despite oversampling of pediatricians, their sample was too small for a more detailed comparison across practice models. Also, self‐reported data about workload and compensation are subject to inaccuracies related to recall and cognitive biases. Last, this is a cross‐sectional study of hospitalist satisfaction at one point in time. Consequently, our sample may not be representative of very dissatisfied hospitalists who have already left their jobs.
The diversity found across existing practice models and the characteristics of the practices provide physicians with the opportunity to bring their unique skills and motivations to the hospitalist movement. As hospitals and other organizations seek to create, maintain, or grow hospitalist programs, the data provided here may prove useful to understand the relationship between practice characteristics and individual job satisfaction. Additionally, hospitalists looking for a job can consider these results as additional information to guide their choice of practice model and work patterns.
Acknowledgements
The authors thank Kenneth A. Rasinski for assistance with survey items refinement, and members of the SHM Career Satisfaction Task Force for their assistance in survey development.
Over the past 15 years, there has been dramatic growth in the number of hospitalist physicians in the United States and in the number of hospitals served by them.13 Hospitals are motivated to hire experienced hospitalists to staff their inpatient services,4 with goals that include obtaining cost‐savings and higher quality.59 The rapid growth of Hospital Medicine saw multiple types of hospital practice models emerge with differing job characteristics, clinical duties, workload, and compensation schemes.10 The extent of the variability of hospitalist jobs across practice models is not known.
Intensifying recruitment efforts and the concomitant increase in compensation for hospitalists over the last decade suggest that demand for hospitalists is strong and sustained.11 As a result, today's cohort of hospitalists has a wide range of choices of types of jobs, practice models, and locations. The diversity of available hospitalist jobs is characterized, for example, by setting (community hospital vs academic hospital), employer (hospital vs private practice), job duties (the amount and type of clinical work, and other administrative, teaching, or research duties), and intensity (work hours and duties to maximize income or lifestyle). How these choices relate to job satisfaction and burnout are also unknown.
The Society of Hospital Medicine (SHM) has administered surveys to hospitalist group leaders biennially since 2003.1215 These surveys, however, do not address issues related to individual hospitalist worklife, recruitment, and retention. In 2005, SHM convened a Career Satisfaction Task Force that designed and executed a national survey of hospitalists in 2009‐2010. The objective of this study is to evaluate how job characteristics vary by practice model, and the association of these characteristics and practice models with job satisfaction and burnout.
METHODS
Survey Instrument
A detailed description of the survey design, sampling strategy, data collection, and response rate calculations is described elsewhere.16 Portions of the 118‐item survey instrument assessed characteristics of the respondents' hospitalist group (12 items), details about their individual work patterns (12 items), and demographics (9 items). Work patterns were evaluated by the average number of clinical work days, consecutive days, hours per month, percentage of work assigned to night duty, and number of patient encounters. Average hours spent on nonclinical work, and the percentage of time allocated for clinical, administrative, teaching, and research activities were solicited. Additional items assessed specific clinical responsibilities, pretax earnings in FY2010, the availability of information technology capabilities, and the adequacy of available resources. Job and specialty satisfaction and 11 satisfaction domain measures were measured using validated scales.1726 Burnout symptoms were measured using a validated single‐item measure.26, 27
Sampling Strategy
We surveyed a national stratified sample of hospitalists in the US and Puerto Rico. We used the largest database of hospitalists (>24,000 names) currently available and maintained by the SHM as our sampling frame. We linked hospitalist employer information to hospital statistics from the American Hospital Association database28 to stratify the sample by number of hospital beds, geographic region, employment model, and specialty training, oversampling pediatric hospitalists due to small numbers. A respondent sample of about 700 hospitalists was calculated to be adequate to detect a 0.5 point difference in job satisfaction scores between subgroups assuming 90% power and alpha of 0.05. However, we sampled a total of 5389 addresses from the database to overcome the traditionally low physician response rates, duplicate sampling, bad addresses, and non‐hospitalists being included in the sampling frame. In addition, 2 multistate hospitalist companies (EmCare, In Compass Health) and 1 for‐profit hospital chain (HCA, Inc) financially sponsored this project with the stipulation that all of their hospitalist employees (n = 884) would be surveyed.
Data Collection
The healthcare consulting firm, Press Ganey, provided support with survey layout and administration following the modified Dillman method.29 Three rounds of coded surveys and solicitation letters from the investigators were mailed 2 weeks apart in November and December 2009. Because of low response rates to the mailed survey, an online survey was created using Survey Monkey and sent to 650 surveyees for whom e‐mail addresses were available, and administered at a kiosk for sample physicians during the SHM 2010 annual meeting.
Data Analysis
Nonresponse bias was measured by comparing characteristics between respondents of separate survey waves.30 We determined the validity of mailing addresses immediately following the survey period by mapping each address using Google, and if the address was a hospital, researching online whether or not the intended recipient was currently employed there. Practice characteristics were compared across 5 model categories distilled from the SHM & Medical Group Management Association survey: local hospitalist‐only group, multistate hospitalist group, multispecialty physician group, employer hospital, and university or medical school. Weighted proportions, means, and medians were calculated to account for oversampling of pediatric hospitalists. Differences in categorical measures were assessed using the chi‐square test and the design‐based F test for comparing weighted data. Weighted means (99% confidence intervals) and medians (interquartile ranges) were calculated. Because each parameter yielded a single outlier value across the 5 practice models, differences across weighted means were assessed using generalized linear models with the single outlier value chosen as the reference mean. Pair‐wise Wilcoxon rank sum test was used to compare median values. In these 4‐way comparisons of means and medians, significance was defined as P value of 0.0125 per Bonferroni correction. A single survey item solicited respondents to choose exactly 4 of 13 considerations most pertinent to job satisfaction. The proportion of respondents who scored 4 on a 5‐point Likert scale of the 11 satisfaction domains and 2 global measures of satisfaction, and burnout symptoms defined as 3 on a 5‐point single item measure were bar‐graphed. Chi‐square statistics were used to evaluate for differences across practice models. Statistical significance was defined by alpha less than 0.05, unless otherwise specified. All analyses were performed using STATA version 11.0 (College Station, TX). This study was approved by the Loyola University Institutional Review Board.
Survey data required cleaning prior to analysis. Missing gender information was imputed using the respondents' name. Responses to the item that asked to indicate the proportion of work dedicated to administrative responsibilities, clinical care, teaching, and research that did not add up to 100% were dropped. Two responses that indicated full‐time equivalent (FTE) of 0%, but whose respondents otherwise completed the survey implying they worked as clinical hospitalists, were replaced with values calculated from the given number of work hours relative to the median work hours in our sample. Out of range or implausible responses to the following items were dropped from analyses: the average number of billable encounters during a typical day or shift, number of shifts performing clinical activities during a typical month, pretax earnings, the year the respondent completed residency training, and the number of whole years practiced as a hospitalist. The proportion of selective item nonresponse was small and we did not, otherwise, impute missing data.
RESULTS
Response Rate
Of the 5389 originally sampled addresses, 1868 were undeliverable. Addresses were further excluded if they appeared in duplicate or were outdated. This yielded a total of 3105 eligible surveyees in the sample. As illustrated in Figure 1, 841 responded to the mailed survey and 5 responded to the Web‐based survey. After rejecting 67 non‐hospitalist respondents and 3 duplicate surveys, a total of 776 surveys were included in the final analysis. The adjusted response rate was 25.6% (776/3035). Members of SHM were more likely to return the survey than nonmembers. The adjusted response rate from hospitalists affiliated with the 3 sponsoring institutions was 6% (40/662). Because these respondents were more likely to be non‐members of SHM, we opted to analyze the responses from the sponsor hospitalists together with the sampled hospitalists. The demographics of the resulting pool of 816 respondents affiliated with over 650 unique hospitalist groups were representative of the original survey frame. We analyzed data from 794 of these who responded to the item indicating their hospitalist practice model. Demographic characteristics of responders and nonresponders to the practice model survey item were similar.

Characteristics of Hospitalists and Their Groups
Table 1 summarizes the characteristics of hospitalist respondents and their organizations by practice model. More (44%) respondents identified their practice model as directly employed by the hospital than other models, including multispecialty physician group (15%), multistate hospitalist group (14%), university or medical school (14%), local hospitalist group (12%), and other (2%). The median age of hospitalist respondents was 42 years, with 6.8 years of mean experience as a hospitalist. One third were women, 84% were married, and 46% had dependent children 6 years old or younger at home. Notably, hospitalists in multistate groups had fewer years of experience, and fewer hospitalists in local and multistate groups were married compared to hospitalists in other practice models.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| Hospitalist characteristics | ||||||
| Age, weighted mean (99% CI) | 45 (42, 48) | 44 (42, 47) | 45 (43, 47) | 45 (43, 46) | 43 (40, 46) | |
| Years hospitalist experience, weighted mean (99% CI) | 8 (6, 9)* | 5 (4, 6)* | 8 (7, 9) | 7 (6, 7) | 8 (6, 9) | <0.010* |
| Women, weighted % | 29 | 30 | 39 | 31 | 43 | 0.118 |
| Married, weighted % | 76 | 77 | 82 | 89 | 81 | 0.009 |
| At least 1 dependent child younger than age 6 living in home, weighted % | 47 | 48 | 43 | 47 | 45 | 0.905 |
| Pediatric specialty, n (%) | <10 | <10 | 11 (10%) | 57 (16%) | 36 (34%) | <0.001 |
| Hospitalist group characteristics | ||||||
| Region, weighted % | <0.001 | |||||
| Northeast (AHA 1 & 2) | 13 | 10 | 16 | 27 | 13 | |
| South (AHA 3 & 4) | 19 | 37 | 13 | 24 | 21 | |
| Midwest (AHA 5 & 6) | 23 | 24 | 25 | 22 | 26 | |
| Mountain (AHA 7 & 8) | 22 | 20 | 16 | 13 | 24 | |
| West (AHA 9) | 24 | 10 | 31 | 14 | 16 | |
| No. beds of primary hospital, weighted % | <0.001 | |||||
| Up to 149 | 17 | 26 | 12 | 24 | 14 | |
| 150299 | 30 | 36 | 36 | 33 | 21 | |
| 300449 | 26 | 24 | 29 | 20 | 19 | |
| 450599 | 13 | 8 | 17 | 11 | 21 | |
| 600 or more | 12 | 6 | 7 | 13 | 24 | |
| No. of hospital facilities served by current practice, weighted % | <0.001 | |||||
| 1 | 53 | 70 | 67 | 77 | 66 | |
| 2 | 20 | 22 | 20 | 16 | 24 | |
| 3 or more | 27 | 9 | 13 | 7 | 10 | |
| No. of physicians in current practice, median (IQR) | 10 (5, 18) | 8 (6, 12)* | 14 (8, 25)* | 12 (6, 18) | 12 (7, 20) | <0.001*, 0.001 |
| No. of non‐physician providers in current practice, median (IQR) | 0 (0, 2) | 0 (0, 2) | 0 (0, 3) | 1 (0, 2) | 0 (0, 2) | |
| Available information technology capabilities, weighted % | ||||||
| EHR to access physician notes | 57 | 57 | 75 | 58 | 79 | <0.001 |
| EHR to access nursing documentations | 68 | 67 | 74 | 75 | 76 | 0.357 |
| EHR to access laboratory or test results | 97 | 89 | 95 | 96 | 96 | 0.054 |
| Electronic order entry | 30 | 19 | 53 | 38 | 56 | <0.001 |
| Electronic billing | 38 | 31 | 36 | 36 | 38 | 0.818 |
| Access to EHR at home or off site | 78 | 73 | 78 | 82 | 84 | 0.235 |
| Access to Up‐to‐Date or other clinical guideline resources | 80 | 77 | 91 | 92 | 96 | <0.001 |
| Access to schedules, calendars, or other organizational resources | 56 | 57 | 66 | 67 | 75 | 0.024 |
| E‐mail, Web‐based paging, or other communication resources | 74 | 63 | 88 | 89 | 90 | <0.001 |
Several differences in respondent group characteristics by practice model were found. Respondents in multistate hospitalist groups were more likely from the South and Midwest, while respondents from multispecialty groups were likely from the West. More multistate group practices were based in smaller hospitals, while academic hospitalists tended to practice in hospitals with 600 or more beds. Respondents employed by hospitals were more likely to practice at 1 hospital facility only, while local group practices were more likely to practice at 3 or more facilities. The median number of physicians in a hospitalist group was 11 (interquartile range [IQR] 6, 19). Local and multistate groups had fewer hospitalists compared to other models. Nonphysician providers were employed by nearly half of all hospitalist practices. Although almost all groups had access to some information technology, more academic hospitalists had access to electronic order entry, electronic physician notes, electronic clinical guidelines resources and communication technology, while local and multistate groups were least likely to have access to these resources.
Work Pattern Variations
Table 2 further details hospitalist work hours by practice model. The majority of hospitalists (78%) reported their position was full‐time (FTE 1.0), while 13% reported working less than full‐time (FTE <1.0). Only 5% of local group hospitalists worked part‐time, while 20% of multispecialty group hospitalists did. An additional 9% reported FTE >1.0, indicating their work hours exceeded the definition of a full‐time physician in their practice. Among full‐time hospitalists, local group members worked a greater number of shifts per month than employees of multispecialty groups, hospitals, and academic medical centers. Academic hospitalists reported higher numbers of consecutive clinical days worked on average, but fewer night shifts compared to hospitalists employed by multistate groups, multispecialty groups, and hospitals; fewer billable encounters than hospitalists in local and multistate groups; and more nonclinical work hours than hospitalists of any other practice model. Academic hospitalists also spent more time on teaching and research than other practice models. Hospitalists spent 11%‐18% of their time on administrative and committee responsibilities, with the least amount spent by hospitalists in multistate groups and the most in academic practice.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| FTE, weighted % | 0.058 | |||||
| FTE < 1.0 | 6 | 13 | 20 | 12 | 14 | |
| FTE = 1.0 | 85 | 75 | 74 | 80 | 82 | |
| FTE > 1.0 | 10 | 13 | 6 | 8 | 5 | |
| Workload parameters, weighted mean (99% CI) | ||||||
| Clinical shifts per month for FTE 1.0 | 19 (17, 20)* | 17 (16, 19) | 15 (14, 17)* | 16 (15, 16) | 15 (13, 17) | <0.001* |
| Hours per clinical shift | 10 (9, 11) | 11 (10, 11)* | 10 (10, 11.0) | 11 (10, 11.0) | 10 (9, 10)* | 0.006*, 0.002 |
| Consecutive days on clinical shift | 8 (6, 9) | 7 (6, 7)* | 6 (6, 7) | 7 (6, 7) | 9 (7, 10)* | 0.002*, <0.001 |
| % Clinical shifts on nights | 20 (15, 25) | 23 (18, 28)* | 23 (17, 29) | 21 (17, 24) | 14 (9, 18)* | 0.001*, 0.002 |
| % Night shifts spent in hospital | 61 (49, 74)* | 63 (52, 75) | 72 (62, 83) | 73 (67, 80) | 43 (29, 57)* | 0.010*, 0.003, <0.001 |
| Billable encounters per clinical shift | 17 (14, 19)* | 17 (16, 18) | 14 (13, 15) | 15 (14, 16) | 13 (11, 14)* | <0.001*, 0.002 |
| Hours nonclinical work per month | 23 (12, 34)* | 19 (11, 27) | 31 (20, 42) | 30 (24, 36) | 71 (55, 86)* | <0.001* |
| Hours clinical and nonclinical work per month for FTE 1.0 | 202 (186, 219) | 211 (196, 226) | 184 (170, 198)* | 193 (186, 201) | 221 (203, 238)* | <0.001* |
| Professional activity, weighted mean % (99% CI) | ||||||
| Clinical | 84 (78, 89)* | 86 (81, 90) | 78 (72, 84) | 79 (76, 82) | 58 (51, 64)* | <0.001* |
| Teaching | 2.3 (1, 5)* | 3 (1, 4) | 6 (4, 9) | 6 (5, 8) | 17 (14, 20)* | <0.001* |
| Administration and Committee work | 13 (8, 19) | 11 (8, 15)* | 16 (10, 21) | 14 (12, 17) | 19 (14, 24)* | 0.001* |
| Research | 0 (0, 0)* | 1 (0, 2) | 0 (0, 1) | 1 (0, 1) | 7 (3, 11)* | <0.001* |
Table 3 tabulates other work pattern characteristics. Most hospitalists indicated that their current clinical work as hospitalists involved the general medical wards (100%), medical consultations (98%), and comanagement with specialists (92%). There were wide differences in participation in comanagement (100%, local groups vs 71%, academic), intensive care unit (ICU) responsibilities (94%, multistate groups vs 27%, academic), and nursing home care (30%, local groups vs 8%, academic). Among activities that are potentially not reimbursable, academic hospitalists were less likely to participate in coordination of patient transfers and code or rapid response teams, while multistate groups were least likely to participate in quality improvement activities. In total, 99% of hospitalists reported participating in at least 1 potentially nonreimbursable clinical activity.
| Local Hospitalist‐Only Group | Multi‐State Hospitalist Group | Multispecialty Physician Group | Employer Hospital | University or Medical School | ||
|---|---|---|---|---|---|---|
| n = 95 | n = 111 | n = 115 | n = 348 | n = 107 | P Value | |
| ||||||
| Reimbursable activities, overlapping weighted % | ||||||
| General medical ward | 100 | 99 | 100 | 99 | 99 | 0.809 |
| Medical consultations | 99 | 99 | 100 | 98 | 95 | 0.043 |
| Comanagement with specialists | 100 | 96 | 96 | 93 | 71 | <0.001 |
| Preoperative evaluations | 92 | 92 | 90 | 88 | 77 | 0.002 |
| Intensive care unit | 86 | 94 | 67 | 75 | 27 | <0.001 |
| Skilled nursing facility or long‐term acute care facility | 30 | 19 | 12 | 16 | 8 | <0.001 |
| Outpatient general medical practice | 4 | 4 | 5 | 5 | 10 | 0.241 |
| Potentially nonreimbursable activities, overlapping weighted % | ||||||
| Coordination of patient transfers | 92 | 94 | 95 | 93 | 82 | 0.005 |
| Quality improvement or patient safety initiatives | 81 | 78 | 83 | 89 | 89 | 0.029 |
| Code team or rapid response team | 56 | 57 | 53 | 62 | 37 | <0.001 |
| Information technology design or implementation | 42 | 39 | 47 | 51 | 51 | 0.154 |
| Admission triage for emergency department | 49 | 46 | 43 | 40 | 31 | 0.132 |
| Compensation scheme, weighted % | <0.001 | |||||
| Salary only | 18 | 21 | 30 | 29 | 47 | |
| Salary plus performance incentive | 54 | 72 | 59 | 67 | 53 | |
| Fee‐for‐service | 20 | 1 | 7 | 2 | 0 | |
| Capitation | 0 | 0 | 0 | 0 | 0 | |
| Other | 9 | 7 | 4 | 3 | 0 | |
| Compensation links to incentives, overlapping weighted % | ||||||
| No incentives | 40 | 28 | 29 | 29 | 48 | 0.003 |
| Patient satisfaction | 23 | 39 | 38 | 38 | 14 | <0.001 |
| Length of stay | 18 | 17 | 20 | 13 | 10 | 0.208 |
| Overall cost | 8 | 11 | 9 | 5 | 6 | 0.270 |
| Test utilization | 2 | 2 | 7 | 1 | 0 | <0.001 |
| Clinical processes and outcomes | 26 | 34 | 44 | 43 | 24 | <0.001 |
| Other | 17 | 29 | 26 | 31 | 25 | 0.087 |
| Earnings, weighted mean dollars (99% CI) | 226,065 (202,891, 249,240)* | 225,613 (210,772, 240,454) | 202,617 (186,036, 219,198) | 206,087 (198,413, 213,460) | 166,478 (151,135, 181,821)* | <0.001* |
Hospitalist compensation schemes were significantly different across the practice models. Salary‐only schemes were most common among academic hospitalists (47%), while 72% of multistate groups used performance incentives in addition to salary. More local groups used fee‐for‐service compensation than other models. Incentives differed by practice model, with more multistate groups having incentives based on patient satisfaction, while more multispecialty physician groups had incentives based on clinical processes and outcomes than other models. Finally, mean earnings for academic hospitalists were significantly lower than for hospitalists of other practice models. Local and multistate group hospitalists earned more than any other practice model (all P <0.001), and $60,000 more than the lowest compensated academic hospitalists.
Components of Job Satisfaction
Hospitalists' rankings of the most important factors for job satisfaction revealed differences across models (Figure 2). Overall, hospitalists were most likely to consider optimal workload and compensation as important factors for job satisfaction from a list of 13 considerations. Local groups and academics were least likely to rank optimal workload as a top factor, and local group hospitalists were more likely to rank optimal autonomy than those of other models. Academic hospitalists had less concern for substantial pay, and more concern for the variety of tasks they perform and recognition by leaders, than other hospitalists.

Job Satisfaction and Burnout Risk
Differences in the ratings of 4 of the 11 satisfaction and job characteristic domains were found across the practice models (Figure 3). Multispecialty group hospitalists were less satisfied with autonomy and their relationship with patients than other practice models, and along with multistate groups, reported the highest perceived workload. Organizational fairness was rated much higher by local group hospitalists than other practice models. Despite these differences in work patterns and satisfaction, there were no differences found in level of global job satisfaction, specialty satisfaction, or burnout across the practice models. Overall, 62% of respondents reported high job satisfaction (4 on a 1 to 5 scale), and 30% indicated burnout symptoms.

DISCUSSION
In our sample of US hospitalists, we found major differences in work patterns and compensation across hospitalist practice models, but no differences in job satisfaction, specialty satisfaction, and burnout. In particular, differences across these models included variations in hospitalist workload, hours, pay, and distribution of work activities. We found that hospitalists perform a variety of clinical and nonclinical tasks, for many of which there are not standard reimbursement mechanisms. We also found that features of a job that individual hospitalists considered most important vary by practice model.
Previous analysis of this data explored the overall state of hospitalist satisfaction.16 The present analysis offers a glimpse into hospitalists' systems‐orientation through a deeper look at their work patterns. The growth in the number of hospitalists who participate in intensive care medicine, specialty comanagement, and other work that involves close working relationships with specialist physicians confirms collaborative care as one of the dominant drivers of the hospitalist movement. At the level of indirect patient care, nearly all hospitalists contributed to work that facilitates coordination, quality, patient safety, or information technology. Understanding the integrative value of hospitalists outside of their clinical productivity may be of interest to hospital administrators.
Global satisfaction measures were similar across practice models. This finding is particularly interesting given the major differences in job characteristics seen among the practice models. This similarity in global satisfaction despite real differences in the nature of the job suggests that individuals find settings that allow them to address their individual professional goals. Our study demonstrates that, in 2010, Hospital Medicine has evolved enough to accommodate a wide variety of goals and needs.
While global satisfaction did not differ among practice types, hospitalists from various models did report differences in factors considered important to global satisfaction. While workload and pay were rated as influential across most models, the degree of importance was significantly different. In academic settings, substantial pay was not a top consideration for overall job satisfaction, whereas in local and multistate hospitalist groups, pay was a very close second in importance to optimal workload. These results may prove helpful for individual hospitalists trying to find their optimal job. For example, someone who is less concerned about workload, but wants to be paid well and have a high degree of autonomy, may find satisfaction in local hospitalist groups. However, for someone who is willing to sacrifice a higher salary for variety of activities, academic Hospital Medicine may be a better fit.
There is a concerning aspect of hospitalist job satisfaction that different practice models do not seem to solve. Control over personal time is a top consideration for many hospitalists across practice models, yet their satisfaction with personal time is low. As control over personal time is seen as a draw to the Hospital Medicine specialty, group leaders may need to evaluate their programs to ensure that schedules and workload support efforts for hospitalists to balance work and homelife commitments.
There are additional findings that are important for Hospital Medicine group leaders. Regardless of practice model, compensation and workload are often used as tools to recruit and retain hospitalists. While these tools may be effective, leaders may find more nuanced approaches to improving their hospitalists' overall satisfaction. Leaders of local hospitalist groups may find their hospitalists tolerant of heavier workloads as long as they are adequately rewarded and are given real autonomy over their work. However, leaders of academic programs may be missing the primary factor that can improve their hospitalists' satisfaction. Rather than asking for higher salaries to remain competitive, it may be more effective to advocate for time and training for their hospitalists to pursue important other activities beyond direct clinical care. Given that resources will always be limited, group leaders need to understand all of the elements that can contribute to hospitalist job satisfaction.
We point out several limitations to this study. First, our adjusted response rate of 25.6% is low for survey research, in general. As mentioned above, hospitalists are not easily identified in any available national physician database. Therefore, we deliberately designed our sampling strategy to error on the side of including ineligible surveyees to reduce systematic exclusion of practicing hospitalists. Using simple post hoc methods, we identified many nonhospitalists and bad addresses from our sample, but because these methods were exclusionary as opposed to confirmatory, we believe that a significant proportion of remaining nonrespondents may also have been ineligible for the survey. Although this does not fully address concerns about potential response bias, we believe that our sample representing a large number of hospitalist groups is adequate to make estimations about a nationally representative sample of practicing hospitalists. Second, in spite of our inclusive approach, we may still have excluded categories of practicing hospitalists. We were careful not to allow SHM members to represent all US hospitalists and included non‐members in the sampling frame, but the possibility of systematic exclusion that may alter our results remains a concern. Additionally, one of our goals was to characterize pediatric hospitalists independently from their adult‐patient counterparts. Despite oversampling of pediatricians, their sample was too small for a more detailed comparison across practice models. Also, self‐reported data about workload and compensation are subject to inaccuracies related to recall and cognitive biases. Last, this is a cross‐sectional study of hospitalist satisfaction at one point in time. Consequently, our sample may not be representative of very dissatisfied hospitalists who have already left their jobs.
The diversity found across existing practice models and the characteristics of the practices provide physicians with the opportunity to bring their unique skills and motivations to the hospitalist movement. As hospitals and other organizations seek to create, maintain, or grow hospitalist programs, the data provided here may prove useful to understand the relationship between practice characteristics and individual job satisfaction. Additionally, hospitalists looking for a job can consider these results as additional information to guide their choice of practice model and work patterns.
Acknowledgements
The authors thank Kenneth A. Rasinski for assistance with survey items refinement, and members of the SHM Career Satisfaction Task Force for their assistance in survey development.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1(2):75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92(2):265–273,vii.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20(2):101–107.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130(4 pt 2):350–354.
- ,.Effects of an HMO hospitalist program on inpatient utilization.Am J Manag Care.2001;7(11):1051–1057.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,,,.Trends in market demand for internal medicine 1999 to 2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21(10):1079–1085.
- SHM. 2003–2004 Survey by the Society of Hospital Medicine on Productivity and Compensation: Analysis of Results. 2004 [updated 2004]. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Practice_Resources Available at: http://cme.medscape.com/viewarticle/578134. Accessed October 21,2010.
- State of Hospital Medicine: 2010 Report Based on 2009 Data.Englewood, CO and Philadelphia, PA:Medical Group Management Association and Society of Hospital Medicine;2010.
- ,,,,.Worklife and satisfaction of hospitalists: toward flourishing careers.J Gen Intern Med.2011, Jul 20. PMID: 21773849.
- ,,, et al.Worklife and satisfaction of general internists.Arch Intern Med.2002;162(6):649–656.
- ,,, et al.Organizational climate, stress, and error in primary care: the MEMO study. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol 1: Research Findings.Rockville, MD:Agency for Healthcare Research and Quality;2005;1:65–77.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130(4 pt 2):343–349.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109(8):648–653.
- .Taking the Measure of Work: A Guide to Validated Scales for Organizational Research and Diagnosis.Thousand Oaks, CA:Sage Publications;2002.
- ,,,,.Job Demands and Worker Health.Ann Arbor, MI:University of Michigan, Institute for Social Research;1980.
- .On the dimensionality of organizational justice: a construct validation of a measure.J Appl Psychol.2001;86(3):386–400.
- ,.Effect of job demands and social support on worker stress—a study of VDT users.Behav Inform Technol.1995;14(1):32–40.
- ,,, et al.Measuring physician job satisfaction in a changing workplace and a challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine.Med Care.1999;37(11):1174–1182.
- ,,, et al.Working conditions in primary care: physician reactions and care quality.Ann Intern Med.2009;151(1):28–U48.
- ,,.Validation of a single‐item measure of burnout against the Maslach Burnout Inventory among physicians.Stress Health.2004;20(2):75–79.
- American Hospital Association. AHA Hospital Statistics. 2009 [updated 2009]. Available at: http://www.ahadata.com/ahadata/html/AHAStatistics.html. Accessed April 12,2011.
- ,,, et al.How to obtain excellent response rates when surveying physicians.Fam Pract.2009;26(1):65–68.
- ,.Estimating nonresponse bias in mail surveys.J Marketing Res.1977;14(3):396–402.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1(2):75–80.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92(2):265–273,vii.
- ,,,.Health care market trends and the evolution of hospitalist use and roles.J Gen Intern Med.2005;20(2):101–107.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137(11):866–874.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130(4 pt 2):350–354.
- ,.Effects of an HMO hospitalist program on inpatient utilization.Am J Manag Care.2001;7(11):1051–1057.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- ,,,.Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,,,.Trends in market demand for internal medicine 1999 to 2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21(10):1079–1085.
- SHM. 2003–2004 Survey by the Society of Hospital Medicine on Productivity and Compensation: Analysis of Results. 2004 [updated 2004]. Available at: http://www.hospitalmedicine.org/AM/Template. cfm?Section=Practice_Resources Available at: http://cme.medscape.com/viewarticle/578134. Accessed October 21,2010.
- State of Hospital Medicine: 2010 Report Based on 2009 Data.Englewood, CO and Philadelphia, PA:Medical Group Management Association and Society of Hospital Medicine;2010.
- ,,,,.Worklife and satisfaction of hospitalists: toward flourishing careers.J Gen Intern Med.2011, Jul 20. PMID: 21773849.
- ,,, et al.Worklife and satisfaction of general internists.Arch Intern Med.2002;162(6):649–656.
- ,,, et al.Organizational climate, stress, and error in primary care: the MEMO study. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol 1: Research Findings.Rockville, MD:Agency for Healthcare Research and Quality;2005;1:65–77.
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians.Ann Intern Med.1999;130(4 pt 2):343–349.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109(8):648–653.
- .Taking the Measure of Work: A Guide to Validated Scales for Organizational Research and Diagnosis.Thousand Oaks, CA:Sage Publications;2002.
- ,,,,.Job Demands and Worker Health.Ann Arbor, MI:University of Michigan, Institute for Social Research;1980.
- .On the dimensionality of organizational justice: a construct validation of a measure.J Appl Psychol.2001;86(3):386–400.
- ,.Effect of job demands and social support on worker stress—a study of VDT users.Behav Inform Technol.1995;14(1):32–40.
- ,,, et al.Measuring physician job satisfaction in a changing workplace and a challenging environment. SGIM Career Satisfaction Study Group. Society of General Internal Medicine.Med Care.1999;37(11):1174–1182.
- ,,, et al.Working conditions in primary care: physician reactions and care quality.Ann Intern Med.2009;151(1):28–U48.
- ,,.Validation of a single‐item measure of burnout against the Maslach Burnout Inventory among physicians.Stress Health.2004;20(2):75–79.
- American Hospital Association. AHA Hospital Statistics. 2009 [updated 2009]. Available at: http://www.ahadata.com/ahadata/html/AHAStatistics.html. Accessed April 12,2011.
- ,,, et al.How to obtain excellent response rates when surveying physicians.Fam Pract.2009;26(1):65–68.
- ,.Estimating nonresponse bias in mail surveys.J Marketing Res.1977;14(3):396–402.
Copyright © 2012 Society of Hospital Medicine
ACUTE Center for Eating Disorders
Anorexia nervosa occurs in 0.9% of women and 0.3% of men in the United States1 and is associated with a prolonged course,2 extensive medical complications that can affect almost every organ system,3, 4 and a 5% mean crude mortality rate9.6 times expected for age‐matched women in the United States.2, 5 Those with anorexia nervosa die as a complication of their illness more frequently than any other mental illness.3 Anorexia nervosa is commonly diagnosed during the adolescent years,2 with almost 25% going on to develop chronic anorexia nervosa.2, 6 Consequently, many patients with severe anorexia nervosa will receive treatment by adult medicine practitioners.
Patients with anorexia nervosa frequently require hospitalization. Published guidelines suggest that those who are 70% or less than ideal body weight, bradycardic, hypotensive, or those with severe electrolyte disturbances warrant admission for medical stabilization.79 Once admitted, however, there are no published guidelines for best practices to medically stabilize patients.7, 10 Although most experts advocate a multidisciplinary approach with weight restoration and medical stability as the goals of hospital admission,8, 9 controversy exists in the literature about how best to achieve these goals.7, 10
It is known, however, that for patients with complicated medical illnesses, such as human immunodeficiency virus (HIV) and sepsis, higher volumes of patient caseloads treated by physicians with disease‐specific expertise has been found to lead to improved outcomes in patients.11, 12 The adult patient with severe anorexia nervosa who requires inpatient medical stabilization may also benefit from a multidisciplinary trained staff familiar with the medical management of anorexia nervosa. Accordingly, we have developed the Acute Comprehensive Urgent Treatment for Eating Disorders (ACUTE) Center.
PROGRAM DESCRIPTION
The ACUTE Center at Denver Health is a 5‐bed unit dedicated to the medical stabilization of patients with severe malnutrition due to anorexia nervosa or severe electrolyte disorders due to bulimia nervosa. ACUTE accepts patients 17 years and older with medical complications related to chronic malnutrition and refeeding.
ACUTE uses a multidisciplinary approach to patient care. The physician team is composed of a hospital medicine attending physician, consultative expertise by an internal medicine specialist in the management of the medical complications of eating disorders, and a psychiatrist specializing in eating disorders. There is a dedicated team of nurses, two dieticians, physical therapists, certified nursing assistants, speech therapists, a psychotherapist, and a chaplain.
ACUTE patients are on continuous telemetry monitoring for the duration of their hospitalization to monitor for arrhythmias as well as signs of covert exercise. As part of the initial intake, a full set of vital signs is obtained, including height and weight. Patients are weighed daily with their back to the scale. There is no discussion of weight fluctuations. Patients may walk at a slow pace around the unit. No exercise is allowed.
Each patient at the ACUTE Center has an individualized meal plan and are started on an oral caloric intake 200 kcal below their basal energy expenditure (BEE). Indirect calorimetry is performed on the first hospital day. Each patient meets on a daily basis with the registered dietician to choose meals that meet their caloric goals.
All patients have a sitter continuously for their first week, and thereafter sitter time may be reduced to supervision surrounding each meal. Patients who fail to finish their prescribed meal are required to drink a liquid supplement to meet caloric goals. Calories are increased weekly until the patient's weight shows a clear pattern of weight increase. 0
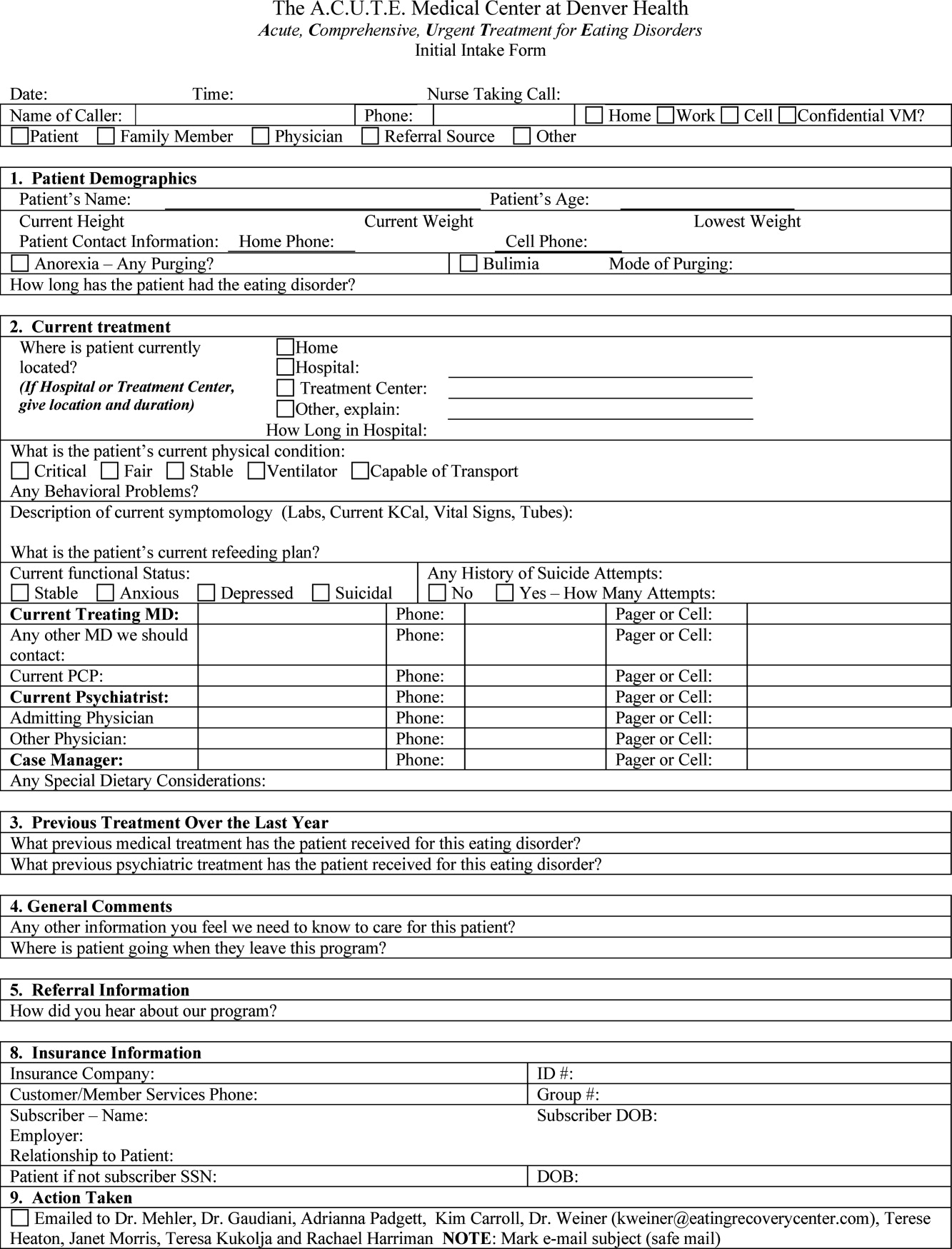
Patients are discharged from the ACUTE Center when they have achieved several basic goals: They are consuming greater than 2000 kcal per day, they are consistently gaining 23 pounds per week, their laboratory values have stabilized without electrolyte supplementation, and they are strong enough for an inpatient eating disorder program.
METHODS
Patients admitted to the ACUTE Center between October 2008 and December 2010 for medical stabilization and monitored refeeding were included. Patients with a diagnosis of bulimia nervosa were excluded. Demographic data and laboratory results were obtained electronically from our data repository, whereas weight, height, and other clinical characteristics were obtained by manual chart abstraction. The statistical analysis was conducted in SAS Enterprise Guide v4.1 (SAS Institute, Cary, NC).
RESULTS
In its first 27 months, the ACUTE Center had 76 total admissions, comprising 59 patients. Of the 76 admissions, the 62 admissions for medical stabilization and monitored refeeding of 54 patients with anorexia nervosa were included. Forty‐eight of the 54 (89%) included patients were female. Six patients were hospitalized twice, and 1 patient 3 times. There were 3 transfers to the intensive care unit, and no inpatient mortality. Of the 62 admissions, 11 (18%) discharges were to home, and 51 (82%) were to inpatient psychiatric eating disorder units.
The mean age at admission was 27 years (range 1765 years). The mean percent of ideal body weight (IBW) on admission was 62.2% 10.2%. The mean body mass index (BMI) was 12.9 2.0 kg/m2 on admission, and 13.1 1.9 kg/m2 upon discharge. The median length of stay was 16 days (interquartile range [IQR] 929 days). Median calculated BEE (1119 [10671184 IQR]) was higher than measured BEE by indirect calorimetry (792 [6341094]), (Table 1).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Age, yr | 27 (2135) | 1765 |
| Female | 56 | 90% |
| Length of hospitalization, days | 16 (929) | 570 |
| Calculated BEE | 1119 (10671184) | 9061491 |
| Measured BEE | 792 (6341094) | 5001742 |
| DEXA Z‐score | 2.2 1.1 | 4.40.7 |
| Height, in | 65 (6167) | 5774 |
| Weight on admission, lb | 76.1 14.4 | 50.8110.0 |
| % Ideal body weight on admission | 62.2 10.2 | 42.4101.0 |
| % Ideal body weight on discharge | 63.2 9.1 | 42.3 82.7 |
| BMI on admission | 12.9 2.0 | 8.719.7 |
| BMI nadir | 12.4 1.9 | 8.415.7 |
| BMI on discharge | 13.1 1.9 | 8.717.0 |
The majority of admission laboratory values, including serum albumin, blood urea nitrogen (BUN), creatinine, potassium, magnesium, and phosphate levels, were within normal limits. Fifty‐six percent were hyponatremic at admission, with a mean serum sodium level of 133 6 mmol/L (Table 2).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Sodium (135143 mmol/L) | 133 6 | 117145 |
| Potassium (3.65.1 mmol/L) | 3.8 (3.0 4.0) | 1.85.5 |
| Carbon dioxide (1827 mmol/L) | 28 (2531) | 1845 |
| Glucose (60199 mg/dL) | 85 (76105) | 41166 |
| BUN (622 mg/dL) | 16 (923) | 344 |
| Creatinine (0.61.2 mg/dL) | 0.7 (0.61.0) | 0.31.6 |
| Calcium (8.110.5 mg/dL) | 8.9 0.6 | 7.610.1 |
| Phosphorus (2.74.8 mg/dL) | 3.2 (2.83.7) | 2.15.7 |
| Magnesium (1.32.1 mEq/L) | 1.8 0.3 | 1.22.5 |
| AST (1040 U/L) | 38 (2391) | 122402 |
| ALT (745 U/L) | 45 (2498) | 152436 |
| Total bilirubin (0.01.2 mg/dL) | 0.5 (0.30.7) | 0.12.2 |
| Pre‐albumin (2052 mg/dL) | 21 7 | 842 |
| Albumin (3.05.3 g/dL) | 3.7 0.7 | 1.64.8 |
| WBC (4.510.0 k/L) | 4.0 (3.25.7) | 1.120.3 |
| Neutrophils (%) (48.069.0%) | 55.5 13.1 | 17.082.0 |
| Lymphocytes (%) (21.043.0%) | 34.9 13.0 | 10.864.0 |
| Platelet count (150450 k/L) | 266 (193371) | 40819 |
| Hematocrit (37.047.0%) | 36.1 5.4 | 19.145.7 |
| MCV (80100 fL) | 91 7 | 73105 |
| TSH (0.346.00 IU/mL) | 1.52 (0.962.84) | 0.1864.1 |
| INR (0.821.17) | 1.09 (1.001.22) | 0.812.05 |
| 1,25 Hydroxy vitamin D (3080 ng/mL) | 41 (3058) | 8171 |
DISCUSSION
Hospital Medicine is currently the fastest growing area of specialization in medicine.13 Palliative care, inpatient geriatrics, short stay units, and bedside procedures have evolved into hospitalist‐led services.1418 The management of the medical complications of severe eating disorders is another potential niche for hospitalists.
The ACUTE Center at Denver Health represents a center in which highly specialized, multidisciplinary care is provided for a rare and extremely ill population of patients. Prior to entering the ACUTE Center, the patients described in our program had each experienced prolonged and unsuccessful stays for medical stabilization in acute care hospitals across the country, after being denied treatment in eating disorder programs due to medical instability.
Patients transferred to ACUTE often received medical care reflecting a lack of specific expertise, training, and exposure. The most common management discrepancy we noted was over‐aggressive provision of intravenous fluids. Consequently, we often diurese 1020 pounds of edema weight, gained during a prior medical hospitalization, before beginning the process of weight restoration. This edema weight artificially increases admission weight and results in less than expected weight gain from admission to discharge.
Even without substantial weight gain, medical stabilization is evidenced by consistent caloric oral intake, and fluid and electrolyte stabilization after initial refeeding. Accordingly, patients who have been treated at the ACUTE Center often become eligible for admission to eating disorder programs at body weights below the typical 70% of ideal body weight that most programs use as a threshold for admission.
From a clinical research perspective, centers such as ACUTE allow for opportunities to better understand and investigate the nuances of patient care in the setting of severe malnutrition. From our cohort of patients to date, we have noted unique issues in albumin levels,19 coagulopathy,20 and liver function,21 among others. As an example, the cohort of patients with anorexia nervosa described here had profoundly low body weight, but relatively normal admission labs. Even the serum albumin, a parameter often used to reflect nutrition in an adult internal medicine setting, is usually normal, reflecting, in an otherwise generally healthy young population, the absence of a malignant, inflammatory, or infectious etiology of weight loss.19
Hospitalists also advocate for their patients by helping to maximize the benefits of their health care coverage. Many health care plans place limits on inpatient psychiatric care benefits. Patients who are severely malnourished from their eating disorder may waste valuable psychiatric care benefits undergoing medical stabilization in psychiatric units while physically unable to undergo psychotherapy. This has become increasingly important as health insurance plans continue to decrease coverage for residential care of patients with anorexia.22
In contrast, the medical benefits of most health plans are more robust. Accordingly, from the patient perspective, medical stabilization in an acute medical unit before admission to a psychiatry unit maximizes their ability to participate in the intensive psychiatric therapy which is still needed after medical stabilization. A recent study from a residential eating disorder program confirmed that a higher discharge BMI was the single best predictor of full recovery from anorexia nervosa.23
In the future, we believe that a continuing concentration of care and experience may also lend itself to the development of protocols and management guidelines which may benefit patients beyond our own unit. Severely malnourished patients with anorexia nervosa, or bulimic patients with complicated electrolyte disorders, are likely to benefit both medically and financially from centers of excellence. Inpatient or residential psychiatric eating disorder programs may act in synergy with medical eating disorders units, like ACUTE, to most efficiently care for the severely malnourished patient. Hospitalists, with the proper training and experience, are uniquely positioned to develop such centers of excellence.
- ,,,.The prevalence and correlates of eating disorders in the national comorbidity survey replication.Biol Psychiatry.2007;61:348–358.
- .The outcome of anorexia nervosa in the 20th century.Am J Psychiatry.2002;159:1284–1293.
- ,.Anorexia nervosa medical issues.J Womens Health.2003;12:331–340.
- .Diagnosis and care of patients with anorexia nervosa in primary care settings.Ann Intern Med.2001;134:1048–1059.
- ,,, et al.Mortality in eating disorders: a descriptive study.Int J Eat Disord.2000;28:20–26.
- ,,,,.Long‐term prognosis in anorexia nervosa: lessons from a 21‐year follow‐up study.Lancet.2000;355:721–722.
- ,,,,.Variations in admissions practices for adolescents with anorexia nervosa: a North American sample.J Adolesc Health.2008;43:425–431.
- American Psychiatric Association.Treatment of patients with eating disorders, third edition.Am J Psychiatry.2006;163(suppl 7):4–54.
- American Dietetic Association.Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders (ADA reports).J Am Diet Assoc.2006;106:2073–2082.
- ,.Clinical practice guidelines for treating restrictive eating disorder patients during medical hospitalization.Curr Opin Pediatr.2008;20:390–397.
- .Practice makes perfect: a volume‐outcome study of hospital patients with HIV disease.J Acquir Immune Defic Syndr.2008;47:226–233.
- ,,,.Association between physician caseload and patient outcome for sepsis treatment.Infect Control Hosp Epidemiol.2009;30:556–562.
- .Reflections: the hospitalist movement ten years later.J Hosp Med.2006;1:248–252.
- What will board certification be‐and mean‐for hospitalists?.Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2007;2:143–149.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,, et al.A hospitalist run short stay unit: features that predict length of stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- ,,.Serum albumin levels may not correlate with weight status in severe anorexia nervosa.Eat Disord.2009;17:322–326.
- ,,,,.The use of thrombelastography to determine coagulation status in severe anorexia nervosa: a case series.Int J Eat Disord.2010;43(4):382–385.
- ,,,.Liver function test abnormalities in anorexia nervosa—cause or effect.Int J Eat Disord.2010;43(4):378–381.
- .Eating disorders: a new front in insurance fight.New York Times. October 13, 2011. Available at: http://www.nytimes.com/2011/10/14/business/ruling‐offers‐hope‐to‐eating‐disorder‐sufferers. html?ref=business.
- ,.Long‐term outcome of residential treatment for anorexia nervosa and bulimia nervosa.Eat Disord.2011;19:132–144.
Anorexia nervosa occurs in 0.9% of women and 0.3% of men in the United States1 and is associated with a prolonged course,2 extensive medical complications that can affect almost every organ system,3, 4 and a 5% mean crude mortality rate9.6 times expected for age‐matched women in the United States.2, 5 Those with anorexia nervosa die as a complication of their illness more frequently than any other mental illness.3 Anorexia nervosa is commonly diagnosed during the adolescent years,2 with almost 25% going on to develop chronic anorexia nervosa.2, 6 Consequently, many patients with severe anorexia nervosa will receive treatment by adult medicine practitioners.
Patients with anorexia nervosa frequently require hospitalization. Published guidelines suggest that those who are 70% or less than ideal body weight, bradycardic, hypotensive, or those with severe electrolyte disturbances warrant admission for medical stabilization.79 Once admitted, however, there are no published guidelines for best practices to medically stabilize patients.7, 10 Although most experts advocate a multidisciplinary approach with weight restoration and medical stability as the goals of hospital admission,8, 9 controversy exists in the literature about how best to achieve these goals.7, 10
It is known, however, that for patients with complicated medical illnesses, such as human immunodeficiency virus (HIV) and sepsis, higher volumes of patient caseloads treated by physicians with disease‐specific expertise has been found to lead to improved outcomes in patients.11, 12 The adult patient with severe anorexia nervosa who requires inpatient medical stabilization may also benefit from a multidisciplinary trained staff familiar with the medical management of anorexia nervosa. Accordingly, we have developed the Acute Comprehensive Urgent Treatment for Eating Disorders (ACUTE) Center.
PROGRAM DESCRIPTION
The ACUTE Center at Denver Health is a 5‐bed unit dedicated to the medical stabilization of patients with severe malnutrition due to anorexia nervosa or severe electrolyte disorders due to bulimia nervosa. ACUTE accepts patients 17 years and older with medical complications related to chronic malnutrition and refeeding.
ACUTE uses a multidisciplinary approach to patient care. The physician team is composed of a hospital medicine attending physician, consultative expertise by an internal medicine specialist in the management of the medical complications of eating disorders, and a psychiatrist specializing in eating disorders. There is a dedicated team of nurses, two dieticians, physical therapists, certified nursing assistants, speech therapists, a psychotherapist, and a chaplain.
ACUTE patients are on continuous telemetry monitoring for the duration of their hospitalization to monitor for arrhythmias as well as signs of covert exercise. As part of the initial intake, a full set of vital signs is obtained, including height and weight. Patients are weighed daily with their back to the scale. There is no discussion of weight fluctuations. Patients may walk at a slow pace around the unit. No exercise is allowed.
Each patient at the ACUTE Center has an individualized meal plan and are started on an oral caloric intake 200 kcal below their basal energy expenditure (BEE). Indirect calorimetry is performed on the first hospital day. Each patient meets on a daily basis with the registered dietician to choose meals that meet their caloric goals.
All patients have a sitter continuously for their first week, and thereafter sitter time may be reduced to supervision surrounding each meal. Patients who fail to finish their prescribed meal are required to drink a liquid supplement to meet caloric goals. Calories are increased weekly until the patient's weight shows a clear pattern of weight increase. 0

Patients are discharged from the ACUTE Center when they have achieved several basic goals: They are consuming greater than 2000 kcal per day, they are consistently gaining 23 pounds per week, their laboratory values have stabilized without electrolyte supplementation, and they are strong enough for an inpatient eating disorder program.
METHODS
Patients admitted to the ACUTE Center between October 2008 and December 2010 for medical stabilization and monitored refeeding were included. Patients with a diagnosis of bulimia nervosa were excluded. Demographic data and laboratory results were obtained electronically from our data repository, whereas weight, height, and other clinical characteristics were obtained by manual chart abstraction. The statistical analysis was conducted in SAS Enterprise Guide v4.1 (SAS Institute, Cary, NC).
RESULTS
In its first 27 months, the ACUTE Center had 76 total admissions, comprising 59 patients. Of the 76 admissions, the 62 admissions for medical stabilization and monitored refeeding of 54 patients with anorexia nervosa were included. Forty‐eight of the 54 (89%) included patients were female. Six patients were hospitalized twice, and 1 patient 3 times. There were 3 transfers to the intensive care unit, and no inpatient mortality. Of the 62 admissions, 11 (18%) discharges were to home, and 51 (82%) were to inpatient psychiatric eating disorder units.
The mean age at admission was 27 years (range 1765 years). The mean percent of ideal body weight (IBW) on admission was 62.2% 10.2%. The mean body mass index (BMI) was 12.9 2.0 kg/m2 on admission, and 13.1 1.9 kg/m2 upon discharge. The median length of stay was 16 days (interquartile range [IQR] 929 days). Median calculated BEE (1119 [10671184 IQR]) was higher than measured BEE by indirect calorimetry (792 [6341094]), (Table 1).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Age, yr | 27 (2135) | 1765 |
| Female | 56 | 90% |
| Length of hospitalization, days | 16 (929) | 570 |
| Calculated BEE | 1119 (10671184) | 9061491 |
| Measured BEE | 792 (6341094) | 5001742 |
| DEXA Z‐score | 2.2 1.1 | 4.40.7 |
| Height, in | 65 (6167) | 5774 |
| Weight on admission, lb | 76.1 14.4 | 50.8110.0 |
| % Ideal body weight on admission | 62.2 10.2 | 42.4101.0 |
| % Ideal body weight on discharge | 63.2 9.1 | 42.3 82.7 |
| BMI on admission | 12.9 2.0 | 8.719.7 |
| BMI nadir | 12.4 1.9 | 8.415.7 |
| BMI on discharge | 13.1 1.9 | 8.717.0 |
The majority of admission laboratory values, including serum albumin, blood urea nitrogen (BUN), creatinine, potassium, magnesium, and phosphate levels, were within normal limits. Fifty‐six percent were hyponatremic at admission, with a mean serum sodium level of 133 6 mmol/L (Table 2).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Sodium (135143 mmol/L) | 133 6 | 117145 |
| Potassium (3.65.1 mmol/L) | 3.8 (3.0 4.0) | 1.85.5 |
| Carbon dioxide (1827 mmol/L) | 28 (2531) | 1845 |
| Glucose (60199 mg/dL) | 85 (76105) | 41166 |
| BUN (622 mg/dL) | 16 (923) | 344 |
| Creatinine (0.61.2 mg/dL) | 0.7 (0.61.0) | 0.31.6 |
| Calcium (8.110.5 mg/dL) | 8.9 0.6 | 7.610.1 |
| Phosphorus (2.74.8 mg/dL) | 3.2 (2.83.7) | 2.15.7 |
| Magnesium (1.32.1 mEq/L) | 1.8 0.3 | 1.22.5 |
| AST (1040 U/L) | 38 (2391) | 122402 |
| ALT (745 U/L) | 45 (2498) | 152436 |
| Total bilirubin (0.01.2 mg/dL) | 0.5 (0.30.7) | 0.12.2 |
| Pre‐albumin (2052 mg/dL) | 21 7 | 842 |
| Albumin (3.05.3 g/dL) | 3.7 0.7 | 1.64.8 |
| WBC (4.510.0 k/L) | 4.0 (3.25.7) | 1.120.3 |
| Neutrophils (%) (48.069.0%) | 55.5 13.1 | 17.082.0 |
| Lymphocytes (%) (21.043.0%) | 34.9 13.0 | 10.864.0 |
| Platelet count (150450 k/L) | 266 (193371) | 40819 |
| Hematocrit (37.047.0%) | 36.1 5.4 | 19.145.7 |
| MCV (80100 fL) | 91 7 | 73105 |
| TSH (0.346.00 IU/mL) | 1.52 (0.962.84) | 0.1864.1 |
| INR (0.821.17) | 1.09 (1.001.22) | 0.812.05 |
| 1,25 Hydroxy vitamin D (3080 ng/mL) | 41 (3058) | 8171 |
DISCUSSION
Hospital Medicine is currently the fastest growing area of specialization in medicine.13 Palliative care, inpatient geriatrics, short stay units, and bedside procedures have evolved into hospitalist‐led services.1418 The management of the medical complications of severe eating disorders is another potential niche for hospitalists.
The ACUTE Center at Denver Health represents a center in which highly specialized, multidisciplinary care is provided for a rare and extremely ill population of patients. Prior to entering the ACUTE Center, the patients described in our program had each experienced prolonged and unsuccessful stays for medical stabilization in acute care hospitals across the country, after being denied treatment in eating disorder programs due to medical instability.
Patients transferred to ACUTE often received medical care reflecting a lack of specific expertise, training, and exposure. The most common management discrepancy we noted was over‐aggressive provision of intravenous fluids. Consequently, we often diurese 1020 pounds of edema weight, gained during a prior medical hospitalization, before beginning the process of weight restoration. This edema weight artificially increases admission weight and results in less than expected weight gain from admission to discharge.
Even without substantial weight gain, medical stabilization is evidenced by consistent caloric oral intake, and fluid and electrolyte stabilization after initial refeeding. Accordingly, patients who have been treated at the ACUTE Center often become eligible for admission to eating disorder programs at body weights below the typical 70% of ideal body weight that most programs use as a threshold for admission.
From a clinical research perspective, centers such as ACUTE allow for opportunities to better understand and investigate the nuances of patient care in the setting of severe malnutrition. From our cohort of patients to date, we have noted unique issues in albumin levels,19 coagulopathy,20 and liver function,21 among others. As an example, the cohort of patients with anorexia nervosa described here had profoundly low body weight, but relatively normal admission labs. Even the serum albumin, a parameter often used to reflect nutrition in an adult internal medicine setting, is usually normal, reflecting, in an otherwise generally healthy young population, the absence of a malignant, inflammatory, or infectious etiology of weight loss.19
Hospitalists also advocate for their patients by helping to maximize the benefits of their health care coverage. Many health care plans place limits on inpatient psychiatric care benefits. Patients who are severely malnourished from their eating disorder may waste valuable psychiatric care benefits undergoing medical stabilization in psychiatric units while physically unable to undergo psychotherapy. This has become increasingly important as health insurance plans continue to decrease coverage for residential care of patients with anorexia.22
In contrast, the medical benefits of most health plans are more robust. Accordingly, from the patient perspective, medical stabilization in an acute medical unit before admission to a psychiatry unit maximizes their ability to participate in the intensive psychiatric therapy which is still needed after medical stabilization. A recent study from a residential eating disorder program confirmed that a higher discharge BMI was the single best predictor of full recovery from anorexia nervosa.23
In the future, we believe that a continuing concentration of care and experience may also lend itself to the development of protocols and management guidelines which may benefit patients beyond our own unit. Severely malnourished patients with anorexia nervosa, or bulimic patients with complicated electrolyte disorders, are likely to benefit both medically and financially from centers of excellence. Inpatient or residential psychiatric eating disorder programs may act in synergy with medical eating disorders units, like ACUTE, to most efficiently care for the severely malnourished patient. Hospitalists, with the proper training and experience, are uniquely positioned to develop such centers of excellence.
Anorexia nervosa occurs in 0.9% of women and 0.3% of men in the United States1 and is associated with a prolonged course,2 extensive medical complications that can affect almost every organ system,3, 4 and a 5% mean crude mortality rate9.6 times expected for age‐matched women in the United States.2, 5 Those with anorexia nervosa die as a complication of their illness more frequently than any other mental illness.3 Anorexia nervosa is commonly diagnosed during the adolescent years,2 with almost 25% going on to develop chronic anorexia nervosa.2, 6 Consequently, many patients with severe anorexia nervosa will receive treatment by adult medicine practitioners.
Patients with anorexia nervosa frequently require hospitalization. Published guidelines suggest that those who are 70% or less than ideal body weight, bradycardic, hypotensive, or those with severe electrolyte disturbances warrant admission for medical stabilization.79 Once admitted, however, there are no published guidelines for best practices to medically stabilize patients.7, 10 Although most experts advocate a multidisciplinary approach with weight restoration and medical stability as the goals of hospital admission,8, 9 controversy exists in the literature about how best to achieve these goals.7, 10
It is known, however, that for patients with complicated medical illnesses, such as human immunodeficiency virus (HIV) and sepsis, higher volumes of patient caseloads treated by physicians with disease‐specific expertise has been found to lead to improved outcomes in patients.11, 12 The adult patient with severe anorexia nervosa who requires inpatient medical stabilization may also benefit from a multidisciplinary trained staff familiar with the medical management of anorexia nervosa. Accordingly, we have developed the Acute Comprehensive Urgent Treatment for Eating Disorders (ACUTE) Center.
PROGRAM DESCRIPTION
The ACUTE Center at Denver Health is a 5‐bed unit dedicated to the medical stabilization of patients with severe malnutrition due to anorexia nervosa or severe electrolyte disorders due to bulimia nervosa. ACUTE accepts patients 17 years and older with medical complications related to chronic malnutrition and refeeding.
ACUTE uses a multidisciplinary approach to patient care. The physician team is composed of a hospital medicine attending physician, consultative expertise by an internal medicine specialist in the management of the medical complications of eating disorders, and a psychiatrist specializing in eating disorders. There is a dedicated team of nurses, two dieticians, physical therapists, certified nursing assistants, speech therapists, a psychotherapist, and a chaplain.
ACUTE patients are on continuous telemetry monitoring for the duration of their hospitalization to monitor for arrhythmias as well as signs of covert exercise. As part of the initial intake, a full set of vital signs is obtained, including height and weight. Patients are weighed daily with their back to the scale. There is no discussion of weight fluctuations. Patients may walk at a slow pace around the unit. No exercise is allowed.
Each patient at the ACUTE Center has an individualized meal plan and are started on an oral caloric intake 200 kcal below their basal energy expenditure (BEE). Indirect calorimetry is performed on the first hospital day. Each patient meets on a daily basis with the registered dietician to choose meals that meet their caloric goals.
All patients have a sitter continuously for their first week, and thereafter sitter time may be reduced to supervision surrounding each meal. Patients who fail to finish their prescribed meal are required to drink a liquid supplement to meet caloric goals. Calories are increased weekly until the patient's weight shows a clear pattern of weight increase. 0

Patients are discharged from the ACUTE Center when they have achieved several basic goals: They are consuming greater than 2000 kcal per day, they are consistently gaining 23 pounds per week, their laboratory values have stabilized without electrolyte supplementation, and they are strong enough for an inpatient eating disorder program.
METHODS
Patients admitted to the ACUTE Center between October 2008 and December 2010 for medical stabilization and monitored refeeding were included. Patients with a diagnosis of bulimia nervosa were excluded. Demographic data and laboratory results were obtained electronically from our data repository, whereas weight, height, and other clinical characteristics were obtained by manual chart abstraction. The statistical analysis was conducted in SAS Enterprise Guide v4.1 (SAS Institute, Cary, NC).
RESULTS
In its first 27 months, the ACUTE Center had 76 total admissions, comprising 59 patients. Of the 76 admissions, the 62 admissions for medical stabilization and monitored refeeding of 54 patients with anorexia nervosa were included. Forty‐eight of the 54 (89%) included patients were female. Six patients were hospitalized twice, and 1 patient 3 times. There were 3 transfers to the intensive care unit, and no inpatient mortality. Of the 62 admissions, 11 (18%) discharges were to home, and 51 (82%) were to inpatient psychiatric eating disorder units.
The mean age at admission was 27 years (range 1765 years). The mean percent of ideal body weight (IBW) on admission was 62.2% 10.2%. The mean body mass index (BMI) was 12.9 2.0 kg/m2 on admission, and 13.1 1.9 kg/m2 upon discharge. The median length of stay was 16 days (interquartile range [IQR] 929 days). Median calculated BEE (1119 [10671184 IQR]) was higher than measured BEE by indirect calorimetry (792 [6341094]), (Table 1).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Age, yr | 27 (2135) | 1765 |
| Female | 56 | 90% |
| Length of hospitalization, days | 16 (929) | 570 |
| Calculated BEE | 1119 (10671184) | 9061491 |
| Measured BEE | 792 (6341094) | 5001742 |
| DEXA Z‐score | 2.2 1.1 | 4.40.7 |
| Height, in | 65 (6167) | 5774 |
| Weight on admission, lb | 76.1 14.4 | 50.8110.0 |
| % Ideal body weight on admission | 62.2 10.2 | 42.4101.0 |
| % Ideal body weight on discharge | 63.2 9.1 | 42.3 82.7 |
| BMI on admission | 12.9 2.0 | 8.719.7 |
| BMI nadir | 12.4 1.9 | 8.415.7 |
| BMI on discharge | 13.1 1.9 | 8.717.0 |
The majority of admission laboratory values, including serum albumin, blood urea nitrogen (BUN), creatinine, potassium, magnesium, and phosphate levels, were within normal limits. Fifty‐six percent were hyponatremic at admission, with a mean serum sodium level of 133 6 mmol/L (Table 2).
| Median (Interquartile Range)* | Range | |
|---|---|---|
| ||
| Sodium (135143 mmol/L) | 133 6 | 117145 |
| Potassium (3.65.1 mmol/L) | 3.8 (3.0 4.0) | 1.85.5 |
| Carbon dioxide (1827 mmol/L) | 28 (2531) | 1845 |
| Glucose (60199 mg/dL) | 85 (76105) | 41166 |
| BUN (622 mg/dL) | 16 (923) | 344 |
| Creatinine (0.61.2 mg/dL) | 0.7 (0.61.0) | 0.31.6 |
| Calcium (8.110.5 mg/dL) | 8.9 0.6 | 7.610.1 |
| Phosphorus (2.74.8 mg/dL) | 3.2 (2.83.7) | 2.15.7 |
| Magnesium (1.32.1 mEq/L) | 1.8 0.3 | 1.22.5 |
| AST (1040 U/L) | 38 (2391) | 122402 |
| ALT (745 U/L) | 45 (2498) | 152436 |
| Total bilirubin (0.01.2 mg/dL) | 0.5 (0.30.7) | 0.12.2 |
| Pre‐albumin (2052 mg/dL) | 21 7 | 842 |
| Albumin (3.05.3 g/dL) | 3.7 0.7 | 1.64.8 |
| WBC (4.510.0 k/L) | 4.0 (3.25.7) | 1.120.3 |
| Neutrophils (%) (48.069.0%) | 55.5 13.1 | 17.082.0 |
| Lymphocytes (%) (21.043.0%) | 34.9 13.0 | 10.864.0 |
| Platelet count (150450 k/L) | 266 (193371) | 40819 |
| Hematocrit (37.047.0%) | 36.1 5.4 | 19.145.7 |
| MCV (80100 fL) | 91 7 | 73105 |
| TSH (0.346.00 IU/mL) | 1.52 (0.962.84) | 0.1864.1 |
| INR (0.821.17) | 1.09 (1.001.22) | 0.812.05 |
| 1,25 Hydroxy vitamin D (3080 ng/mL) | 41 (3058) | 8171 |
DISCUSSION
Hospital Medicine is currently the fastest growing area of specialization in medicine.13 Palliative care, inpatient geriatrics, short stay units, and bedside procedures have evolved into hospitalist‐led services.1418 The management of the medical complications of severe eating disorders is another potential niche for hospitalists.
The ACUTE Center at Denver Health represents a center in which highly specialized, multidisciplinary care is provided for a rare and extremely ill population of patients. Prior to entering the ACUTE Center, the patients described in our program had each experienced prolonged and unsuccessful stays for medical stabilization in acute care hospitals across the country, after being denied treatment in eating disorder programs due to medical instability.
Patients transferred to ACUTE often received medical care reflecting a lack of specific expertise, training, and exposure. The most common management discrepancy we noted was over‐aggressive provision of intravenous fluids. Consequently, we often diurese 1020 pounds of edema weight, gained during a prior medical hospitalization, before beginning the process of weight restoration. This edema weight artificially increases admission weight and results in less than expected weight gain from admission to discharge.
Even without substantial weight gain, medical stabilization is evidenced by consistent caloric oral intake, and fluid and electrolyte stabilization after initial refeeding. Accordingly, patients who have been treated at the ACUTE Center often become eligible for admission to eating disorder programs at body weights below the typical 70% of ideal body weight that most programs use as a threshold for admission.
From a clinical research perspective, centers such as ACUTE allow for opportunities to better understand and investigate the nuances of patient care in the setting of severe malnutrition. From our cohort of patients to date, we have noted unique issues in albumin levels,19 coagulopathy,20 and liver function,21 among others. As an example, the cohort of patients with anorexia nervosa described here had profoundly low body weight, but relatively normal admission labs. Even the serum albumin, a parameter often used to reflect nutrition in an adult internal medicine setting, is usually normal, reflecting, in an otherwise generally healthy young population, the absence of a malignant, inflammatory, or infectious etiology of weight loss.19
Hospitalists also advocate for their patients by helping to maximize the benefits of their health care coverage. Many health care plans place limits on inpatient psychiatric care benefits. Patients who are severely malnourished from their eating disorder may waste valuable psychiatric care benefits undergoing medical stabilization in psychiatric units while physically unable to undergo psychotherapy. This has become increasingly important as health insurance plans continue to decrease coverage for residential care of patients with anorexia.22
In contrast, the medical benefits of most health plans are more robust. Accordingly, from the patient perspective, medical stabilization in an acute medical unit before admission to a psychiatry unit maximizes their ability to participate in the intensive psychiatric therapy which is still needed after medical stabilization. A recent study from a residential eating disorder program confirmed that a higher discharge BMI was the single best predictor of full recovery from anorexia nervosa.23
In the future, we believe that a continuing concentration of care and experience may also lend itself to the development of protocols and management guidelines which may benefit patients beyond our own unit. Severely malnourished patients with anorexia nervosa, or bulimic patients with complicated electrolyte disorders, are likely to benefit both medically and financially from centers of excellence. Inpatient or residential psychiatric eating disorder programs may act in synergy with medical eating disorders units, like ACUTE, to most efficiently care for the severely malnourished patient. Hospitalists, with the proper training and experience, are uniquely positioned to develop such centers of excellence.
- ,,,.The prevalence and correlates of eating disorders in the national comorbidity survey replication.Biol Psychiatry.2007;61:348–358.
- .The outcome of anorexia nervosa in the 20th century.Am J Psychiatry.2002;159:1284–1293.
- ,.Anorexia nervosa medical issues.J Womens Health.2003;12:331–340.
- .Diagnosis and care of patients with anorexia nervosa in primary care settings.Ann Intern Med.2001;134:1048–1059.
- ,,, et al.Mortality in eating disorders: a descriptive study.Int J Eat Disord.2000;28:20–26.
- ,,,,.Long‐term prognosis in anorexia nervosa: lessons from a 21‐year follow‐up study.Lancet.2000;355:721–722.
- ,,,,.Variations in admissions practices for adolescents with anorexia nervosa: a North American sample.J Adolesc Health.2008;43:425–431.
- American Psychiatric Association.Treatment of patients with eating disorders, third edition.Am J Psychiatry.2006;163(suppl 7):4–54.
- American Dietetic Association.Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders (ADA reports).J Am Diet Assoc.2006;106:2073–2082.
- ,.Clinical practice guidelines for treating restrictive eating disorder patients during medical hospitalization.Curr Opin Pediatr.2008;20:390–397.
- .Practice makes perfect: a volume‐outcome study of hospital patients with HIV disease.J Acquir Immune Defic Syndr.2008;47:226–233.
- ,,,.Association between physician caseload and patient outcome for sepsis treatment.Infect Control Hosp Epidemiol.2009;30:556–562.
- .Reflections: the hospitalist movement ten years later.J Hosp Med.2006;1:248–252.
- What will board certification be‐and mean‐for hospitalists?.Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2007;2:143–149.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,, et al.A hospitalist run short stay unit: features that predict length of stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- ,,.Serum albumin levels may not correlate with weight status in severe anorexia nervosa.Eat Disord.2009;17:322–326.
- ,,,,.The use of thrombelastography to determine coagulation status in severe anorexia nervosa: a case series.Int J Eat Disord.2010;43(4):382–385.
- ,,,.Liver function test abnormalities in anorexia nervosa—cause or effect.Int J Eat Disord.2010;43(4):378–381.
- .Eating disorders: a new front in insurance fight.New York Times. October 13, 2011. Available at: http://www.nytimes.com/2011/10/14/business/ruling‐offers‐hope‐to‐eating‐disorder‐sufferers. html?ref=business.
- ,.Long‐term outcome of residential treatment for anorexia nervosa and bulimia nervosa.Eat Disord.2011;19:132–144.
- ,,,.The prevalence and correlates of eating disorders in the national comorbidity survey replication.Biol Psychiatry.2007;61:348–358.
- .The outcome of anorexia nervosa in the 20th century.Am J Psychiatry.2002;159:1284–1293.
- ,.Anorexia nervosa medical issues.J Womens Health.2003;12:331–340.
- .Diagnosis and care of patients with anorexia nervosa in primary care settings.Ann Intern Med.2001;134:1048–1059.
- ,,, et al.Mortality in eating disorders: a descriptive study.Int J Eat Disord.2000;28:20–26.
- ,,,,.Long‐term prognosis in anorexia nervosa: lessons from a 21‐year follow‐up study.Lancet.2000;355:721–722.
- ,,,,.Variations in admissions practices for adolescents with anorexia nervosa: a North American sample.J Adolesc Health.2008;43:425–431.
- American Psychiatric Association.Treatment of patients with eating disorders, third edition.Am J Psychiatry.2006;163(suppl 7):4–54.
- American Dietetic Association.Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders (ADA reports).J Am Diet Assoc.2006;106:2073–2082.
- ,.Clinical practice guidelines for treating restrictive eating disorder patients during medical hospitalization.Curr Opin Pediatr.2008;20:390–397.
- .Practice makes perfect: a volume‐outcome study of hospital patients with HIV disease.J Acquir Immune Defic Syndr.2008;47:226–233.
- ,,,.Association between physician caseload and patient outcome for sepsis treatment.Infect Control Hosp Epidemiol.2009;30:556–562.
- .Reflections: the hospitalist movement ten years later.J Hosp Med.2006;1:248–252.
- What will board certification be‐and mean‐for hospitalists?.Palliative care in hospitals.J Hosp Med.2006;1:21–28.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1:5–6.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2007;2:143–149.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360:1102–1112.
- ,,, et al.A hospitalist run short stay unit: features that predict length of stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- ,,.Serum albumin levels may not correlate with weight status in severe anorexia nervosa.Eat Disord.2009;17:322–326.
- ,,,,.The use of thrombelastography to determine coagulation status in severe anorexia nervosa: a case series.Int J Eat Disord.2010;43(4):382–385.
- ,,,.Liver function test abnormalities in anorexia nervosa—cause or effect.Int J Eat Disord.2010;43(4):378–381.
- .Eating disorders: a new front in insurance fight.New York Times. October 13, 2011. Available at: http://www.nytimes.com/2011/10/14/business/ruling‐offers‐hope‐to‐eating‐disorder‐sufferers. html?ref=business.
- ,.Long‐term outcome of residential treatment for anorexia nervosa and bulimia nervosa.Eat Disord.2011;19:132–144.
Macrolides for Mycoplasmal Pneumonia
Mycoplasma pneumoniae is a common cause of community‐acquired pneumonia (CAP), among school‐age children and adolescents.14 Though pneumonia caused by M. pneumoniae is typically self‐limited, severe illness may occur.5 M. pneumoniae has also been implicated in airway inflammation, which may lead to the onset and development of chronic pulmonary disease.610 Few studies have directly addressed appropriate treatment strategies for M. pneumoniae pneumonia,11 and, despite its high prevalence and potential for causing severe complications, treatment recommendations remain inconsistent.
The efficacy of macrolide therapy in particular for M. pneumoniae remains unclear. In vitro susceptibility studies have shown bacteriostatic activity of erythromycin, clarithromycin, and azithromycin against M. pneumoniae.1218 Additionally, several small retrospective studies have shown that among children with atypical CAP (including M. pneumoniae pneumonia), those treated with macrolides were less likely to have persistence or progression of signs and symptoms after 3 days of therapy.19, 20 Lu et al21 found a shorter duration of fever among macrolide recipients compared with non‐recipients. In adults, Shames et al22 found a shorter duration of fever and hospitalization among erythromycin recipients compared with controls. Other randomized controlled trials have also addressed the use of macrolides in treatment of M. pneumoniae, but the ability to draw meaningful conclusions is limited by small samples sizes and by lack of details about the number of patients with M. pneumoniae.11
In addition to their antimicrobial effect, macrolides also have anti‐inflammatory properties.2327 The importance of these anti‐inflammatory properties is supported by studies showing clinical cure in patients treated with macrolides despite persistence of M. pneumoniae organisms,2831 clinical improvement despite the administration of doses that provide tissue levels below the minimum inhibitory concentration of the organism,3234 and clinical cure in patients with macrolide‐resistant M. pneumoniae.18, 35
The objectives of the current study were to examine the impact of macrolide therapy on the length of stay (LOS) and short‐ and longer‐term readmissions, including longer‐term asthma‐related readmissions, in children hospitalized with M. pneumoniae pneumonia.
METHODS
Data Source
Data for this retrospective cohort study were obtained from the Pediatric Health Information System (PHIS), which contains administrative data from 38 freestanding children's hospitals. Data quality and reliability are assured through a joint effort by the Child Health Corporation of America (Shawnee Mission, KS) and PHIS‐participating hospitals as described previously.36, 37 Encrypted medical record numbers allow for tracking of individual patients across hospitalizations. This study was reviewed and approved by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia (Philadelphia, PA).
Patients
Children 6‐18 years of age with CAP were eligible if they were discharged from a participating hospital between January 1, 2006 and December 31, 2008. Subjects were included if they received antibiotic therapy on the first day of hospitalization and if they satisfied one of the following International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code criteria: 1) Principal diagnosis of M. pneumoniae pneumonia (483.0); 2) Principal diagnosis of a pneumonia‐related symptom (eg, fever, cough) (780.6 or 786.00‐786.52 [except 786.1]) and a secondary diagnosis of M. pneumoniae pneumonia; or 3) Principal diagnosis of pneumonia (481‐483.8 [except 483.0], 485‐486) and a secondary diagnosis of Mycoplasma (041.81).
Children younger than 6 years of age were excluded due to the low prevalence of M. pneumoniae infection.2, 38 Patients with comorbid conditions predisposing to severe or recurrent pneumonia (eg, cystic fibrosis, malignancy) were excluded using a previously reported classification scheme.39 In addition, we excluded patient data from 2 hospitals due to incomplete reporting of discharge information; thus data from 36 hospitals were included in this study.
Validation of Discharge Diagnosis Codes for Mycoplasma pneumoniae
To assess for misclassification of the diagnosis of M. pneumoniae, we reviewed records of a randomly selected subset of subjects from The Children's Hospital of Philadelphia; 14 of 15 patients had signs of lower respiratory tract infection in conjunction with a positive M. pneumoniae polymerase chain reaction test from nasopharyngeal washings to confirm the diagnosis of M. pneumoniae pneumonia. Hence, the positive predictive value of our algorithm for diagnosing M. pneumoniae pneumonia was 93.3%.
Study Definitions
We identified children with asthma in 2 ways. Asthma‐related hospitalizations were identified by an ICD‐9 code for asthma (493.0‐493.92) in any discharge diagnosis field during any hospitalization in the 24 months prior to the current hospitalization. Baseline controller medications were identified by receipt of inhaled corticosteroids (eg, fluticasone) or leukotriene receptor antagonists on the first day of hospitalization.
Systemic corticosteroids (either oral or intravenous) included dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Measures of disease severity included admission to the intensive care unit within 48 hours of hospitalization, and administration of vancomycin or clindamycin, vasoactive infusions (epinephrine, norepinephrine, dopamine, and dobutamine), and invasive (endotracheal intubation) and noninvasive (continuous positive airway pressure) mechanical ventilation within 24 hours of hospitalization, as previously described.40, 41 Viral respiratory season was defined as October through March.
Measured Outcomes
The primary outcomes of interest were hospital LOS and all‐cause readmission within 28 days and 15 months after index discharge. We examined readmissions for asthma 15 months after index discharge as a secondary outcome measure because of the potential role for M. pneumoniae infection in long‐term lung dysfunction, including asthma.42 The 15‐month time frame was selected based on longitudinal data available in PHIS for the entire study cohort.
Measured Exposures
The main exposure was early initiation of macrolide therapy, defined as receipt of erythromycin, clarithromycin, or azithromycin on the first day of hospitalization.
Data Analysis
Continuous variables were described using median and interquartile range (IQR) or range values, and compared using the Wilcoxon rank‐sum test. Categorical variables were described using counts and frequencies, and compared using the chi‐square test. Multivariable linear (for LOS) and logistic (for readmission) regression analyses were performed to assess the independent association of macrolide therapy with the primary outcomes. Because the LOS data had a skewed distribution, our analyses were performed using logarithmically transformed LOS values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent difference in LOS between subjects receiving and not receiving macrolide therapy.
Building of the multivariable models began with the inclusion of macrolide therapy. Variables associated with primary outcomes on univariate analysis (P < 0.20) were also considered for inclusion as potential confounders.43 These variables were included in the final multivariable model if they remained significant after adjusting for other factors, or if their inclusion in the model resulted in a 15% or greater change in the effect size of the primary association of interest (ie, macrolide therapy).44 Because corticosteroids also have anti‐inflammatory properties, we assessed for interactions with macrolide therapy. There was no interaction between macrolide and systemic corticosteroid therapy (P = 0.26, Likelihood ratio test), therefore our primary model adjusted for systemic corticosteroids.
Despite adjusting for systemic corticosteroid therapy in our primary analysis, residual confounding by indication for corticosteroid therapy might exist. We therefore repeated the analysis after stratifying by receipt or non‐receipt of systemic corticosteroid therapy. Because the benefit of macrolides in preventing long‐term dysfunction may be limited to those without a prior diagnosis of asthma, we repeated the analysis of readmissions within 15 months of index discharge (any readmission and asthma‐related readmissions) while limiting the cohort to those without evidence of asthma (ie, no prior asthma‐related hospitalizations and no chronic asthma medications). Because children with underlying conditions or circumstances that would predispose to prolonged hospitalizations may have been included, despite our restriction of the cohort to those without an identified chronic complex condition, we also repeated the analysis while limiting the cohort to those with a LOS 7 days. Finally, all analyses were clustered on hospital using the robust standard errors of Huber and White to account for the correlation of exposures and outcomes among children within centers.
Data were analyzed using Stata version 11 (Stata Corporation, College Station, TX). Statistical significance was determined a priori as a two‐tailed P value <0.05.
RESULTS
Patient Characteristics
During the study, 690 children ages 6 to 18 years met inclusion criteria. Characteristics of these patients are shown in Table 1. The median age was 10 years (IQR, 7‐13 years). Ten patients (1.4%) also had a concomitant discharge diagnosis of pneumococcal pneumonia, while 19 patients (2.7%) had a concomitant discharge diagnosis of viral pneumonia; 1 of these patients had discharge diagnoses of both viral and pneumococcal pneumonia.
| Empiric Macrolide Therapy | ||||
|---|---|---|---|---|
| Variable | All Subjects | Yes | No | P |
| ||||
| Demographics | ||||
| Male sex | 356 (51.6) | 200 (49.4) | 156 (54.7) | 0.166 |
| Race | ||||
| Black | 135 (19.6) | 81 (20.0) | 54 (19.0) | 0.506 |
| White | 484 (70.1) | 287 (70.9) | 197 (69.1) | |
| Other | 62 (9.0) | 31 (7.7) | 31 (10.9) | |
| Missing | 9 (1.3) | 6 (1.5) | 3 (1.1) | |
| Presentation during viral respiratory season | 420 (60.9) | 242 (59.8) | 178 (62.5) | |
| Prior asthma hospitalization | 41 (5.9) | 31 (7.7) | 10 (3.5) | 0.023 |
| Intensive care unit admission | 127 (18.4) | 74 (18.3) | 53 (18.6) | 0.914 |
| Laboratory tests and procedures | ||||
| Additional radiologic imaging* | 24 (3.5) | 13 (3.2) | 11 (3.9) | 0.646 |
| Arterial blood gas | 116 (17.3) | 72 (18.5) | 44 (15.6) | 0.316 |
| Complete blood count | 433 (64.4) | 249 (64.0) | 184 (65.0) | 0.788 |
| Blood culture | 280 (41.7) | 167 (42.9) | 113 (39.9) | 0.436 |
| Mechanical ventilation | 16 (2.3) | 5 (1.2) | 11 (3.86) | 0.024 |
| Medications | ||||
| Chronic asthma medication | 116 (16.8) | 72 (17.8) | 44 (15.4) | 0.419 |
| Beta‐agonist therapy | 328 (47.5) | 215 (53.1) | 113 (39.7) | 0.001 |
| Vasoactive infusions | 22 (3.2) | 13 (3.2) | 9 (3.2) | 0.969 |
| Systemic corticosteroids | 252 (36.5) | 191 (47.2) | 61 (21.4) | <0.001 |
| Clindamycin or vancomycin | 86 (12.5) | 24 (5.9) | 62 (21.8) | <0.001 |
Macrolide therapy was administered to 405 (58.7%) patients. Systemic corticosteroid therapy was administered to 252 (36.5%) patients. Overall, 191 (27.7%) of the 690 patients received both macrolides and systemic corticosteroids empirically, while 224 (32.5%) received neither; 61 (8.8%) received corticosteroids but not macrolides, while 214 (31.0%) received macrolides but not corticosteroids. Asthma hospitalization within the 24 months prior to admission was more common among those receiving macrolides (N = 60/405, 14.8%) than among those not receiving macrolides (N = 30/285, 10.5%) (P = 0.023). Macrolide recipients also more commonly received concomitant systemic corticosteroids (N = 191/405, 47.2%) than macrolide non‐recipients (N = 61/285, 21.4%) (P < 0.001) and more commonly received beta‐agonist therapy (N = 215/405, 53.1%) than macrolide non‐recipients (N = 113/285, 39.7%) (P = 0.001).
Length of Stay
The overall median LOS was 3 days (IQR, 2‐6 days); the median LOS was 3 days (IQR, 2‐5 days) for empiric macrolide recipients and 4 days (IQR, 2‐9 days) for non‐recipients (P < 0.001). Overall, 22.9% (N = 158) of children had an LOS 7 days and 8.8% (N = 61) of children had an LOS 14 days. The LOS was 7 days for 15.3% (N = 62) of macrolide recipients and 33.7% (N = 96) of non‐recipients. LOS was 7 days for 17.5% (N = 44) of systemic steroid recipients and 26% (N = 114) of non‐recipients. In unadjusted analysis, macrolide therapy (beta‐coefficient, 0.49; 95% confidence interval [CI]: 0.72 to 0.25; P < 0.001) and systemic corticosteroid administration (beta‐coefficient, 0.26; CI: 0.37 to 0.14; P < 0.001) were associated with shorter hospital LOS (Appendix 1).
In multivariable analysis, macrolide therapy remained associated with a shorter LOS (Table 2; Appendix 2). Systemic corticosteroid administration was associated with a 23% shorter LOS (adjusted beta‐coefficient, 0.26; 95% CI: 0.39 to 0.14; P < 0.001). In contrast, previous hospitalization for asthma was associated with a 31% longer LOS (adjusted beta‐coefficient, 0.27; 95% CI: 0.09‐0.045; P = 0.004). Receipt of beta‐agonist therapy or chronic asthma medications were not associated with significant differences in LOS. In analysis stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy, empiric macrolide therapy remained associated with a significantly shorter LOS in both systemic corticosteroid recipients and non‐recipient (Table 4). When the cohort was restricted to subjects with a LOS 7 days, macrolide therapy remained significantly associated with a shorter LOS (adjusted percent change, 20%; 95% CI: 32% to 5%; P = 0.015).
| Association of Empiric Macrolide Therapy With Outcomes* | |
|---|---|
| |
| Length of stay (days) | |
| Adjusted beta‐coefficient (95 % CI) | 0.38 (0.59 to 0.17) |
| Adjusted percent change (95% CI) | 32% (45% to 15%) |
| P value | 0.001 |
| Any readmission within 28 days | |
| Adjusted odds ratio (95% CI) | 1.12 (0.22 to 5.78) |
| P value | 0.890 |
| Any readmission within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.00 (0.59 to 1.70) |
| P value | 0.991 |
| Asthma hospitalization within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.09 (0.54 to 2.17) |
| P value | 0.820 |
Readmission
Overall, 8 children (1.2%) were readmitted for pneumonia‐associated conditions within 28 days of index discharge. Readmission occurred in 1.2% of macrolide recipients and 1.1% of non‐recipients (P = 0.83) (Table 4). In unadjusted analysis, neither macrolide therapy (odds ratio [OR], 1.18; 95% CI: 0.25‐5.45; P = 0.84) nor systemic corticosteroid administration (OR, 1.04; 95% CI: 0.27‐4.10; P = 0.95) was associated with 28‐day readmission (Appendix 3). In multivariable analysis, empiric macrolide therapy was not associated with 28‐day readmission in the overall cohort (Table 2; Appendix 4)), or when the analysis was stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
| Concomitant Systemic Corticosteroid Therapy* | ||
|---|---|---|
| Yes | No | |
| ||
| Length of stay | ||
| Adjusted beta‐coefficient (95% CI) | 0.40 (0.74 to 0.07) | 0.37 (0.58 to 0.16) |
| Adjusted percent change (95% CI) | 33% (52% to 7%) | 31% (44% to 15%) |
| P value | 0.020 | 0.001 |
| Readmission within 28 days | ||
| Adjusted odds ratio (95% CI) | 1.09 (0.05 to 26.7) | 1.50 (0.21 to 10.8) |
| P value | 0.960 | 0.687 |
| Readmission within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.57 (0.65 to 3.82) | 0.81 (0.45 to 1.46) |
| P value | 0.32 | 0.49 |
| Asthma hospitalization within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.51 (0.58 to 3.93) | 0.85 (0.36 to 1.97) |
| P value | 0.395 | 0.700 |
| Empiric Macrolide Therapy | ||
|---|---|---|
| N/Total (%) | ||
| Readmission | Yes | No |
| Any readmission within 28 days | ||
| Overall | 5/405 (1.2) | 3/285 (1.1) |
| Systemic corticosteroid therapy | 2/186 (1.1) | 1/66 (1.5) |
| No systemic corticosteroid therapy | 3/177 (1.7) | 2/261 (0.8) |
| Any readmission within 15 mo | ||
| Overall | 96/405 (23.7) | 64/285 (22.5) |
| Systemic corticosteroid therapy | 52/186 (28.0) | 17/66 (25.8) |
| No systemic corticosteroid therapy | 32/177 (18.1) | 59/261 (22.6) |
| Asthma hospitalization within 15 mo | ||
| Overall | 61/405 (15.1) | 34/285 (11.9) |
| Systemic corticosteroid therapy | 39/186 (21.0) | 13/66 (19.7) |
| No systemic corticosteroid therapy | 14/177 (7.9) | 29/261 (11.1) |
Overall, 160 children (23.2%) were readmitted within 15 months of index discharge; 95 were readmitted for asthma during this time (Table 3). Overall readmission occurred in 23.7% of macrolide recipients and 22.5% of macrolide non‐recipients (P = 0.702). Asthma readmission occurred in 15.1% of macrolide recipients and 11.9% of macrolide non‐recipients (P = 0.240). In unadjusted analysis, empiric macrolide therapy was not significantly associated with any readmission within 15 months (OR, 1.07; 95% CI: 0.69‐1.68; P = 0.759) or with asthma‐related readmission within 15 months (OR, 1.31; 95% CI: 0.73‐ 2.36; P = 0.369). In multivariable analysis, neither any readmission nor asthma readmission within 15 months was associated with empiric macrolide therapy overall (Table 2) or when stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
The analyses for readmissions within 15 months of index discharge were repeated while limiting the cohort to those without prior asthma hospitalizations or chronic asthma medications. In this subset of patients, readmissions for any reason occurred in 55 (18.6%) of 295 macrolide recipients and 50 (22.0%) of 227 non‐recipients. The difference was not statistically significant in multivariable analysis (adjusted odds ratio, 0.79; 95% CI: 0.41‐1.51; P = 0.47). Readmissions for asthma occurred in 30 (10.2%) of 295 macrolide recipients and 26 (11.5%) of 227 non‐recipients; this difference was also not significant in multivariable analysis (adjusted odds ratio, 0.83; 95% CI: 0.36‐1.93; P = 0.83). The magnitude of the estimate of effect for 28‐day and 15‐month readmissions, and 15‐month asthma hospitalizations, was similar to the primary analysis when the cohort was restricted to subjects with a LOS 7 days.
DISCUSSION
This multicenter study examined the role of macrolide therapy in children hospitalized with M. pneumoniae pneumonia. Empiric macrolide therapy was associated with an approximately 30% shorter hospital LOS and, in stratified analysis, remained associated with a significantly shorter hospital LOS in both systemic corticosteroid recipients and non‐recipients. Empiric macrolide therapy was not associated with short‐ or longer‐term hospital readmission.
Previous small randomized trials have been inconclusive regarding the potential benefit of macrolide therapy in M. pneumoniae pneumonia.11 Our study, which demonstrated a shorter LOS among macrolides recipients compared with non‐recipients, has several advantages over prior studies including a substantively larger sample size and multicenter design. Animal models support our observations regarding the potential beneficial antimicrobial role of macrolides. M. pneumoniae concentrations in bronchoalveolar lavage specimens were significantly lower among experimentally infected mice treated with clarithromycin, a macolide‐class antibiotic, compared with either placebo or dexamethasone.45 Combination therapy with clarithromycin and dexamethasone reduced histopathologic inflammation to a greater degree than dexamethasone alone.45
While the relative importance of the antimicrobial and anti‐inflammatory properties of macrolides is not known, observational studies of children infected with macrolide‐resistant M. pneumoniae suggest that the antimicrobial properties of macrolides may provide disproportionate clinical benefit. The duration of fever in macrolide recipients with macrolide‐resistant M. pneumoniae (median duration, 9 days) reported by Suzuki et al46 was significantly longer than those with macrolide‐susceptible infections (median duration, 5 days), and similar to the duration of fever in patients with M. pneumoniae infection treated with placebo (median duration, 8 days) reported by Kingston et al.47 Additionally, macrolide therapy was associated with significant improvements in lung function in patients with asthma and concomitant M. pneumoniae infection, but not in patients with asthma without documented M. pneumoniae infection.9 As corticosteroids also have anti‐inflammatory properties, we expect that any anti‐inflammatory benefit of macrolide therapy would be mitigated by the concomitant administration of corticosteroids. The shorter LOS associated with empiric macrolide therapy in our study was comparable among corticosteroid recipients and non‐recipients.
Atypical bacterial pathogens, including M. pneumoniae, are associated with diffuse lower airway inflammation6, 48 and airway hyperresponsiveness,6 and have been implicated as a cause of acute asthma exacerbations.7, 4954 Among patients with previously diagnosed asthma, acute M. pneumoniae infection was identified in up to 20% of those having acute exacerbations.7, 54 Macrolide therapy has a beneficial effect on lung function and airway hyperresponsiveness in adults with asthma.9, 55 Among mice infected with M. pneumoniae, 3 days of macrolide therapy resulted in a significant reduction in airway hyperresponsiveness compared with placebo or dexamethasone; however, after 6 days of therapy, there was no significant difference in airway hyperresponsiveness between those receiving macrolides, dexamethasone, or placebo, suggesting that the benefit of macrolides on airway hyperresponsiveness may be brief. Our findings of a shorter LOS but no difference in readmissions at 28 days or longer, for macrolide recipients compared with non‐recipients, support the limited benefit of macrolide therapy beyond the initial reduction in bacterial load seen in the first few days of therapy.
M. pneumoniae infection has also been implicated as a cause of chronic pulmonary disease, including asthma.610 In the mouse model, peribronchial and perivascular mononuclear infiltrates, increased airway methacholine reactivity, and increased airway obstruction were observed 530 days after M. pneumoniae inoculation.6 M. pneumoniae has been identified in 26 (50%) of 51 children experiencing their first asthma attack,7 and 23 (42%) of 55 adults with chronic, stable asthma.9 Nevertheless, results of other studies addressing the issue are inconsistent, and the role of M. pneumoniae in the development of asthma remains unclear.56 In order to investigate the impact of macrolide therapy on the development of chronic pulmonary disease requiring hospitalization, we examined the readmission rates in the 15 months following index discharge. The proportion of children hospitalized with asthma following the hospitalization for M. pneumoniae pneumonia was higher for both macrolide recipients and non‐recipients compared with the 24‐months prior to infection. These results support a possible role for M. pneumoniae in chronic pulmonary disease. However, macrolide therapy was not associated with long‐term overall hospital readmission or long‐term asthma readmission, either in the entire cohort or in the subset of patients without prior asthma hospitalizations or medications.
This study had several limitations. First, because the identification of children with M. pneumoniae pneumonia relied on ICD‐9 discharge diagnosis codes, it is possible that there was misclassification of disease. We minimized the inclusion of children without M. pneumoniae by including only children who received antibiotic therapy on the first day of hospitalization and by excluding patients younger than 6 years of age, a group at relatively low‐risk for M. pneumoniae infection. Further, our algorithm for identification of M. pneumoniae pneumonia was validated through review of the medical records at 1 institution and was found to have a high positive predictive value. However, the positive predictive value of these ICD‐9 codes may vary across institutions. Additionally, the sensitivity of ICD‐9 codes for identifying children with M. pneumoniae pneumonia is not known. Also, not all children with pneumonia undergo testing for M. pneumoniae, and different tests have varying sensitivity and specificity.57, 58 Thus, some children with M. pneumoniae pneumonia were not diagnosed and so were not included in our study. It is not known how inclusion of these children would affect our results.
Second, the antibiotic information used in this study was limited to empiric antibiotic therapy. It is possible that some patients received macrolide therapy before admission. It is also likely that identification of M. pneumoniae during the hospitalization prompted the addition or substitution of macrolide therapy for some patients. If this therapy was initiated beyond the first day of hospitalization, these children would be classified as macrolide non‐recipients. Since macrolide administration was associated with a shorter hospital LOS, such misclassification would bias our results towards finding no difference in LOS between macrolide recipients and non‐recipients. It is therefore possible that the benefit of macrolide therapy is even greater than found in our study.
Third, there may be unmeasured confounding or residual confounding by indication for adjunct corticosteroid therapy related to clinical presentation. We expect that corticosteroid recipients would be sicker than non‐recipients. We included variables associated with a greater severity of illness (such as intensive care unit admission) in the multivariable analysis. Additionally, the shorter LOS among macrolide recipients remained when the analysis was stratified by receipt or non‐receipt of systemic corticosteroid therapy.
Fourth, we were only able to record readmissions occurring at the same hospital as the index admission; any readmission presenting to a different hospital following their index admission did not appear in our records, and was therefore not counted. It is thus possible that the true number of readmissions is higher than that represented here. Finally, despite the large number of patients included in this study, the number of short‐term readmissions was relatively small. Thus, we may have been underpowered to detect small but significant differences in short‐term readmission rates.
In conclusion, macrolide therapy was associated with shorter hospital LOS, but not with short‐term or longer‐term readmission in children presenting with M. pneumoniae pneumonia.
Appendix
| Variable | Beta Coefficient | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Sex | 0.12 | (0.22, 0.02) | 0.022 |
| Race | |||
| Blackreference category | |||
| White | 0.01 | (0.21, 0.23) | 0.933 |
| Other | 0.13 | (0.39, 0.13) | 0.323 |
| Missing | 0.46 | (0.81, 0.11) | 0.012 |
| Presentation during viral respiratory season | 0.05 | (0.19, 0.09) | 0.462 |
| Prior asthma hospitalization | 0.36 | (0.64, 0.08) | 0.015 |
| Intensive care unit admission | 1.05 | (0.87, 1.23) | <0.001 |
| Labs and procedures performed | |||
| Additional radiologic imaging | 0.23 | (0.20, 0.67) | 0.287 |
| Arterial blood gas | 0.69 | (0.50, 0.87) | <0.001 |
| Complete blood count | 0.34 | (0.24, 0.45) | <0.001 |
| Blood culture | 0.17 | (0.98, 0.44) | 0.204 |
| Mechanical ventilation | 1.15 | (0.68, 1.63) | <0.001 |
| Therapies received | |||
| Empiric macrolide therapy | 0.49 | (0.72, 0.25) | <0.001 |
| Systemic steroids | 0.26 | (0.38, 0.14) | <0.001 |
| Chronic asthma medications | 0.20 | (0.38, 0.013) | 0.037 |
| Beta‐agonist therapy | 0.07 | (0.21, 0.08) | 0.357 |
| Vasoactive infusion | 1.08 | (0.727, 1.45) | <0.001 |
| Clindamycin or vancomycin | 0.55 | (0.34, 0.75) | <0.001 |
| Variable | Odds Ratio* | Confidence Interval | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Sex | 0.56 | (0.23, 1.33) | 0.190 |
| Race | |||
| Blackreference category | |||
| White | 0.46 | (0.19, 1.14) | 0.093 |
| Other | |||
| Missing | |||
| Presentation during viral respiratory season | 0.64 | (0.09, 4.75) | 0.662 |
| Prior asthma hospitalization | |||
| Intensive care unit admission | 4.54 | (1.21, 17.03) | 0.025 |
| Laboratory tests and procedures | |||
| Additional radiologic imaging | 10.00 | (2.25, 44.47) | 0.002 |
| Arterial blood gas | |||
| Complete blood count | 0.92 | (0.24, 3.48) | 0.901 |
| Blood culture | 0.85 | (0.30, 2.36) | 0.738 |
| Mechanical ventilation | |||
| Medications | |||
| Macrolide therapy | 1.18 | (0.25, 5.45) | 0.837 |
| Systemic corticosteroids | 1.04 | (0.276, 4.09) | 0.951 |
| Chronic asthma medication | 1.66 | (0.71, 3.88) | 0.242 |
| Beta‐agonist therapy | 0.66 | (0.16, 2.65) | 0.557 |
| Vasoactive infusions | |||
| Clindamycin or vancomycin | 1.00 | (0.10, 9.90) | 0.998 |
| Variable | Coefficient | Confidence Interval | P Value | % Change | Confidence Interval for % Change |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 0.287 | (0.012, 0.045) | 0.001 | 2.9 | (1.2, 4.6) |
| Prior asthma hospitalization | 0.272 | (0.094, 0.45) | 0.004 | 31.3 | (9.9, 56.8) |
| Intensive care unit admission | 1.015 | (0.802, 1.23) | <0.001 | 175.9 | (123.0, 241.3) |
| Therapies received | |||||
| Macrolide therapy | 0.379 | (0.59, 0.166) | 0.001 | 31.6 | (44.6, 15.3) |
| Systemic corticosteroids | 0.264 | (0.391, 0.138) | <0.001 | 23.2 | (32.3, 12.9) |
| Chronic asthma medications | 0.056 | (0.255, 0.142) | 0.568 | 5.5 | (22.5, 15.2) |
| Albuterol | 0.07 | (0.059, 0.199) | 0.281 | 7.2 | (5.8, 22.0) |
| Clindamycin or vancomycin | 0.311 | (0.063, 0.559) | 0.015 | 36.5 | (6.5, 74.9) |
| Variable | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Age | 0.91 | 0.72, 1.15 | 0.423 |
| Prior asthma hospitalization | 1.94 | 0.42, 8.90 | 0.394 |
| Intensive care unit admission | 5.73 | 2.03, 16.20 | 0.001 |
| Therapies received | |||
| Macrolide therapy | 1.12 | 0.22, 5.78 | 0.890 |
| Systemic corticosteroids | 0.696 | 0.10, 4.70 | 0.710 |
| Chronic asthma medications | 1.98 | 0.32, 12.20 | 0.460 |
| Albuterol | 0.519 | 0.081, 3.31 | 0.488 |
| Clindamycin or vancomycin | 0.904 | 0.07, 11.13 | 0.937 |
- ,,, et al.Prospective surveillance for atypical pathogens in children with community‐acquired pneumonia in Japan.J Infect Chemother.2006;12:36–41.
- ,,.Incidence of community‐acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population‐based study in primary health care.Respirology.2004;9:109–114.
- ,,.Mycoplasma pneumoniae infections in University of Wisconsin students.Am Rev Respir Dis.1967;96:237–244.
- .Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients.Clin Infect Dis.1993;17(suppl 1):S37–S46.
- .Mycoplasma pneumoniae. In: Long SS, Pickering LK, Prober CG, eds.Principles and Practice of Pediatric Infectious Diseases.3rd ed.Philadelphia, PA:Churchill Livingstone;2008:979–985.
- ,,, et al.Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection‐associated chronic reactive airway disease.Infect Immun.2002;70:649–654.
- ,,, et al.Mycoplasma pneumoniae and asthma in children.Clin Infect Dis.2004;38:1341–1346.
- ,,,.Isolation of Mycoplasma pneumoniae from asthmatic patients.Ann Allergy.1993;70:23–25.
- ,,,.Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin.Chest.2002;121:1782–1788.
- ,,,,.A link between chronic asthma and chronic infection.J Allergy Clin Immunol.2001;107:595–601.
- ,,.Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children.Cochrane Database Syst Rev.2010;7:CD004875.
- ,,,.In vitro susceptibilities of mycoplasmas and ureaplasmas to new macrolides and aryl‐fluoroquinolones.Antimicrob Agents Chemother.1988;32:1500–1502.
- ,,.Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae.Int J Antimicrob Agents.2003;21:574–577.
- ,,,,.The in vitro activity of some 14‐, 15‐ and 16‐ membered macrolides against Staphylococcus spp., Legionella spp., Mycoplasma spp. and Ureaplasma urealyticum.Drugs Exp Clin Res.1991;17:91–99.
- ,,, et al.In vitro and in vivo activities of macrolides against Mycoplasma pneumoniae.Antimicrob Agents Chemother.1994;38:790–798.
- ,.Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum.Eur J Clin Microbiol Infect Dis.1990;9:838–841.
- ,,, et al.Characteristics of macrolide‐resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro.Microbiol Immunol.2001;45:617–620.
- ,,, et al.Characterization and molecular analysis of macrolide‐resistant Mycoplasma pneumoniae clinical isolates obtained in Japan.Antimicrob Agents Chemother.2004;48:4624–4630.
- ,,,.Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired lower respiratory tract infections.Clin Infect Dis.2001;32:1281–1289.
- ,,, et al.Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2–5 years of age with community‐acquired pneumonia.Clin Infect Dis.2002;35:1345–1352.
- ,,,,.Macrolide use shortens fever duration in Mycoplasma pneumoniae infection in children: a 2‐year experience.J Microbiol Immunol Infect.2008;41:307–310.
- ,,,,.Comparison of antibiotics in the treatment of mycoplasmal pneumonia.Arch Intern Med.1970;125:680–684.
- ,,, et al.Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae‐induced pneumonia.Antimicrob Agents Chemother.2003;47:1614–1620.
- ,.Antibiotics in asthma.Curr Allergy Asthma Rep.2004;4:132–138.
- ,.Immunomodulatory activity and effectiveness of macrolides in chronic airway disease.Chest.2004;125:70S–78S.
- ,,, et al.Interleukin‐8 gene repression by clarithromycin is mediated by the activator protein‐1 binding site in human bronchial epithelial cells.Am J Respir Cell Mol Biol.2000;22:51–60.
- ,,, et al.Clarithromycin inhibits NF‐kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells.Antimicrob Agents Chemother.2001;45:44–47.
- ,,,,.Epidemiology of Mycoplasma pneumoniae infection in families.JAMA.1966;197:859–866.
- ,,.Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy.N Engl J Med.1967;276:1172–1175.
- ,,.Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control.J Infect Dis.1971;123:74–92.
- .Is there a role for antibiotics in the treatment of asthma? Involvement of atypical organisms.BioDrugs.2000;14:349–354.
- ,.Diffuse panbronchiolitis: role of macrolides in therapy.Am J Respir Med.2002;1:119–131.
- ,,,,,.Long‐term low‐dose administration of erythromycin to patients with diffuse panbronchiolitis.Respiration.1991;58:145–149.
- ,,,,,.[Long‐term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis].Nihon Kyobu Shikkan Gakkai Zasshi.1990;28:1305–1313.
- ,,, et al.A comparative clinical study of macrolide‐sensitive and macrolide‐resistant Mycoplasma pneumoniae infections in pediatric patients.J Infect Chemother.2009;15:380–383.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Etiology of childhood pneumonia: serologic results of a prospective, population‐based study.Pediatr Infect Dis J.1998;17:986–991.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107:E99.
- ,,,,,.Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia.Pediatrics.2011;127:e255–e263.
- ,,, et al.Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood.J Hosp Med.2011;6:256–263.
- ,,, et al.Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma.Am J Respir Crit Care Med.1998;158:998–1001.
- ,.The impact of confounder selection criteria on effect estimation.Am J Epidemiol.1989;129:125–137.
- ,,.The risk of determining risk with multivariable models.Ann Intern Med.1993;118:201–210.
- ,,, et al.The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection.J Infect Dis.2008;198:1180–1188.
- ,,, et al.Clinical evaluation of macrolide‐resistant Mycoplasma pneumoniae.Antimicrob Agents Chemother.2006;50:709–712.
- ,,, et al.Eaton agent pneumonia.JAMA.1961;176:118–123.
- .The role of viral and atypical bacterial pathogens in asthma pathogenesis.Pediatr Pulmonol Suppl.1999;18:141–143.
- ,,.The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child.Ann Allergy.1970;28:43–49.
- ,,,.Association of viral and mycoplasma infections with exacerbations of asthma.Ann Allergy.1974;33:145–149.
- ,,, et al.Acute Chlamydia pneumoniae and Mycoplasma pneumoniae infections in community‐acquired pneumonia and exacerbations of COPD or asthma: therapeutic considerations.J Chemother.2004;16:70–76.
- ,,, et al.Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults.Ann Allergy.1986;57:263–265.
- ,,, et al.Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection.Eur Respir J.1994;7:2165–2168.
- ,,, et al.Atypical pathogen infection in adults with acute exacerbation of bronchial asthma.Am J Respir Crit Care Med.2003;167:406–410.
- ,,,,,.Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma.Chest.1991;99:670–673.
- ,,,,.Atypical bacteria and macrolides in asthma.Allergy Asthma Clin Immunol.2008;4:111–116.
- ,,.Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods.Eur J Clin Microbiol Infect Dis.2010;29:1055–1069.
- ,,,,,.A multicenter pilot external quality assessment programme to assess the quality of molecular detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae.J Microbiol Methods.2010;82:131–135.
Mycoplasma pneumoniae is a common cause of community‐acquired pneumonia (CAP), among school‐age children and adolescents.14 Though pneumonia caused by M. pneumoniae is typically self‐limited, severe illness may occur.5 M. pneumoniae has also been implicated in airway inflammation, which may lead to the onset and development of chronic pulmonary disease.610 Few studies have directly addressed appropriate treatment strategies for M. pneumoniae pneumonia,11 and, despite its high prevalence and potential for causing severe complications, treatment recommendations remain inconsistent.
The efficacy of macrolide therapy in particular for M. pneumoniae remains unclear. In vitro susceptibility studies have shown bacteriostatic activity of erythromycin, clarithromycin, and azithromycin against M. pneumoniae.1218 Additionally, several small retrospective studies have shown that among children with atypical CAP (including M. pneumoniae pneumonia), those treated with macrolides were less likely to have persistence or progression of signs and symptoms after 3 days of therapy.19, 20 Lu et al21 found a shorter duration of fever among macrolide recipients compared with non‐recipients. In adults, Shames et al22 found a shorter duration of fever and hospitalization among erythromycin recipients compared with controls. Other randomized controlled trials have also addressed the use of macrolides in treatment of M. pneumoniae, but the ability to draw meaningful conclusions is limited by small samples sizes and by lack of details about the number of patients with M. pneumoniae.11
In addition to their antimicrobial effect, macrolides also have anti‐inflammatory properties.2327 The importance of these anti‐inflammatory properties is supported by studies showing clinical cure in patients treated with macrolides despite persistence of M. pneumoniae organisms,2831 clinical improvement despite the administration of doses that provide tissue levels below the minimum inhibitory concentration of the organism,3234 and clinical cure in patients with macrolide‐resistant M. pneumoniae.18, 35
The objectives of the current study were to examine the impact of macrolide therapy on the length of stay (LOS) and short‐ and longer‐term readmissions, including longer‐term asthma‐related readmissions, in children hospitalized with M. pneumoniae pneumonia.
METHODS
Data Source
Data for this retrospective cohort study were obtained from the Pediatric Health Information System (PHIS), which contains administrative data from 38 freestanding children's hospitals. Data quality and reliability are assured through a joint effort by the Child Health Corporation of America (Shawnee Mission, KS) and PHIS‐participating hospitals as described previously.36, 37 Encrypted medical record numbers allow for tracking of individual patients across hospitalizations. This study was reviewed and approved by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia (Philadelphia, PA).
Patients
Children 6‐18 years of age with CAP were eligible if they were discharged from a participating hospital between January 1, 2006 and December 31, 2008. Subjects were included if they received antibiotic therapy on the first day of hospitalization and if they satisfied one of the following International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code criteria: 1) Principal diagnosis of M. pneumoniae pneumonia (483.0); 2) Principal diagnosis of a pneumonia‐related symptom (eg, fever, cough) (780.6 or 786.00‐786.52 [except 786.1]) and a secondary diagnosis of M. pneumoniae pneumonia; or 3) Principal diagnosis of pneumonia (481‐483.8 [except 483.0], 485‐486) and a secondary diagnosis of Mycoplasma (041.81).
Children younger than 6 years of age were excluded due to the low prevalence of M. pneumoniae infection.2, 38 Patients with comorbid conditions predisposing to severe or recurrent pneumonia (eg, cystic fibrosis, malignancy) were excluded using a previously reported classification scheme.39 In addition, we excluded patient data from 2 hospitals due to incomplete reporting of discharge information; thus data from 36 hospitals were included in this study.
Validation of Discharge Diagnosis Codes for Mycoplasma pneumoniae
To assess for misclassification of the diagnosis of M. pneumoniae, we reviewed records of a randomly selected subset of subjects from The Children's Hospital of Philadelphia; 14 of 15 patients had signs of lower respiratory tract infection in conjunction with a positive M. pneumoniae polymerase chain reaction test from nasopharyngeal washings to confirm the diagnosis of M. pneumoniae pneumonia. Hence, the positive predictive value of our algorithm for diagnosing M. pneumoniae pneumonia was 93.3%.
Study Definitions
We identified children with asthma in 2 ways. Asthma‐related hospitalizations were identified by an ICD‐9 code for asthma (493.0‐493.92) in any discharge diagnosis field during any hospitalization in the 24 months prior to the current hospitalization. Baseline controller medications were identified by receipt of inhaled corticosteroids (eg, fluticasone) or leukotriene receptor antagonists on the first day of hospitalization.
Systemic corticosteroids (either oral or intravenous) included dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Measures of disease severity included admission to the intensive care unit within 48 hours of hospitalization, and administration of vancomycin or clindamycin, vasoactive infusions (epinephrine, norepinephrine, dopamine, and dobutamine), and invasive (endotracheal intubation) and noninvasive (continuous positive airway pressure) mechanical ventilation within 24 hours of hospitalization, as previously described.40, 41 Viral respiratory season was defined as October through March.
Measured Outcomes
The primary outcomes of interest were hospital LOS and all‐cause readmission within 28 days and 15 months after index discharge. We examined readmissions for asthma 15 months after index discharge as a secondary outcome measure because of the potential role for M. pneumoniae infection in long‐term lung dysfunction, including asthma.42 The 15‐month time frame was selected based on longitudinal data available in PHIS for the entire study cohort.
Measured Exposures
The main exposure was early initiation of macrolide therapy, defined as receipt of erythromycin, clarithromycin, or azithromycin on the first day of hospitalization.
Data Analysis
Continuous variables were described using median and interquartile range (IQR) or range values, and compared using the Wilcoxon rank‐sum test. Categorical variables were described using counts and frequencies, and compared using the chi‐square test. Multivariable linear (for LOS) and logistic (for readmission) regression analyses were performed to assess the independent association of macrolide therapy with the primary outcomes. Because the LOS data had a skewed distribution, our analyses were performed using logarithmically transformed LOS values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent difference in LOS between subjects receiving and not receiving macrolide therapy.
Building of the multivariable models began with the inclusion of macrolide therapy. Variables associated with primary outcomes on univariate analysis (P < 0.20) were also considered for inclusion as potential confounders.43 These variables were included in the final multivariable model if they remained significant after adjusting for other factors, or if their inclusion in the model resulted in a 15% or greater change in the effect size of the primary association of interest (ie, macrolide therapy).44 Because corticosteroids also have anti‐inflammatory properties, we assessed for interactions with macrolide therapy. There was no interaction between macrolide and systemic corticosteroid therapy (P = 0.26, Likelihood ratio test), therefore our primary model adjusted for systemic corticosteroids.
Despite adjusting for systemic corticosteroid therapy in our primary analysis, residual confounding by indication for corticosteroid therapy might exist. We therefore repeated the analysis after stratifying by receipt or non‐receipt of systemic corticosteroid therapy. Because the benefit of macrolides in preventing long‐term dysfunction may be limited to those without a prior diagnosis of asthma, we repeated the analysis of readmissions within 15 months of index discharge (any readmission and asthma‐related readmissions) while limiting the cohort to those without evidence of asthma (ie, no prior asthma‐related hospitalizations and no chronic asthma medications). Because children with underlying conditions or circumstances that would predispose to prolonged hospitalizations may have been included, despite our restriction of the cohort to those without an identified chronic complex condition, we also repeated the analysis while limiting the cohort to those with a LOS 7 days. Finally, all analyses were clustered on hospital using the robust standard errors of Huber and White to account for the correlation of exposures and outcomes among children within centers.
Data were analyzed using Stata version 11 (Stata Corporation, College Station, TX). Statistical significance was determined a priori as a two‐tailed P value <0.05.
RESULTS
Patient Characteristics
During the study, 690 children ages 6 to 18 years met inclusion criteria. Characteristics of these patients are shown in Table 1. The median age was 10 years (IQR, 7‐13 years). Ten patients (1.4%) also had a concomitant discharge diagnosis of pneumococcal pneumonia, while 19 patients (2.7%) had a concomitant discharge diagnosis of viral pneumonia; 1 of these patients had discharge diagnoses of both viral and pneumococcal pneumonia.
| Empiric Macrolide Therapy | ||||
|---|---|---|---|---|
| Variable | All Subjects | Yes | No | P |
| ||||
| Demographics | ||||
| Male sex | 356 (51.6) | 200 (49.4) | 156 (54.7) | 0.166 |
| Race | ||||
| Black | 135 (19.6) | 81 (20.0) | 54 (19.0) | 0.506 |
| White | 484 (70.1) | 287 (70.9) | 197 (69.1) | |
| Other | 62 (9.0) | 31 (7.7) | 31 (10.9) | |
| Missing | 9 (1.3) | 6 (1.5) | 3 (1.1) | |
| Presentation during viral respiratory season | 420 (60.9) | 242 (59.8) | 178 (62.5) | |
| Prior asthma hospitalization | 41 (5.9) | 31 (7.7) | 10 (3.5) | 0.023 |
| Intensive care unit admission | 127 (18.4) | 74 (18.3) | 53 (18.6) | 0.914 |
| Laboratory tests and procedures | ||||
| Additional radiologic imaging* | 24 (3.5) | 13 (3.2) | 11 (3.9) | 0.646 |
| Arterial blood gas | 116 (17.3) | 72 (18.5) | 44 (15.6) | 0.316 |
| Complete blood count | 433 (64.4) | 249 (64.0) | 184 (65.0) | 0.788 |
| Blood culture | 280 (41.7) | 167 (42.9) | 113 (39.9) | 0.436 |
| Mechanical ventilation | 16 (2.3) | 5 (1.2) | 11 (3.86) | 0.024 |
| Medications | ||||
| Chronic asthma medication | 116 (16.8) | 72 (17.8) | 44 (15.4) | 0.419 |
| Beta‐agonist therapy | 328 (47.5) | 215 (53.1) | 113 (39.7) | 0.001 |
| Vasoactive infusions | 22 (3.2) | 13 (3.2) | 9 (3.2) | 0.969 |
| Systemic corticosteroids | 252 (36.5) | 191 (47.2) | 61 (21.4) | <0.001 |
| Clindamycin or vancomycin | 86 (12.5) | 24 (5.9) | 62 (21.8) | <0.001 |
Macrolide therapy was administered to 405 (58.7%) patients. Systemic corticosteroid therapy was administered to 252 (36.5%) patients. Overall, 191 (27.7%) of the 690 patients received both macrolides and systemic corticosteroids empirically, while 224 (32.5%) received neither; 61 (8.8%) received corticosteroids but not macrolides, while 214 (31.0%) received macrolides but not corticosteroids. Asthma hospitalization within the 24 months prior to admission was more common among those receiving macrolides (N = 60/405, 14.8%) than among those not receiving macrolides (N = 30/285, 10.5%) (P = 0.023). Macrolide recipients also more commonly received concomitant systemic corticosteroids (N = 191/405, 47.2%) than macrolide non‐recipients (N = 61/285, 21.4%) (P < 0.001) and more commonly received beta‐agonist therapy (N = 215/405, 53.1%) than macrolide non‐recipients (N = 113/285, 39.7%) (P = 0.001).
Length of Stay
The overall median LOS was 3 days (IQR, 2‐6 days); the median LOS was 3 days (IQR, 2‐5 days) for empiric macrolide recipients and 4 days (IQR, 2‐9 days) for non‐recipients (P < 0.001). Overall, 22.9% (N = 158) of children had an LOS 7 days and 8.8% (N = 61) of children had an LOS 14 days. The LOS was 7 days for 15.3% (N = 62) of macrolide recipients and 33.7% (N = 96) of non‐recipients. LOS was 7 days for 17.5% (N = 44) of systemic steroid recipients and 26% (N = 114) of non‐recipients. In unadjusted analysis, macrolide therapy (beta‐coefficient, 0.49; 95% confidence interval [CI]: 0.72 to 0.25; P < 0.001) and systemic corticosteroid administration (beta‐coefficient, 0.26; CI: 0.37 to 0.14; P < 0.001) were associated with shorter hospital LOS (Appendix 1).
In multivariable analysis, macrolide therapy remained associated with a shorter LOS (Table 2; Appendix 2). Systemic corticosteroid administration was associated with a 23% shorter LOS (adjusted beta‐coefficient, 0.26; 95% CI: 0.39 to 0.14; P < 0.001). In contrast, previous hospitalization for asthma was associated with a 31% longer LOS (adjusted beta‐coefficient, 0.27; 95% CI: 0.09‐0.045; P = 0.004). Receipt of beta‐agonist therapy or chronic asthma medications were not associated with significant differences in LOS. In analysis stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy, empiric macrolide therapy remained associated with a significantly shorter LOS in both systemic corticosteroid recipients and non‐recipient (Table 4). When the cohort was restricted to subjects with a LOS 7 days, macrolide therapy remained significantly associated with a shorter LOS (adjusted percent change, 20%; 95% CI: 32% to 5%; P = 0.015).
| Association of Empiric Macrolide Therapy With Outcomes* | |
|---|---|
| |
| Length of stay (days) | |
| Adjusted beta‐coefficient (95 % CI) | 0.38 (0.59 to 0.17) |
| Adjusted percent change (95% CI) | 32% (45% to 15%) |
| P value | 0.001 |
| Any readmission within 28 days | |
| Adjusted odds ratio (95% CI) | 1.12 (0.22 to 5.78) |
| P value | 0.890 |
| Any readmission within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.00 (0.59 to 1.70) |
| P value | 0.991 |
| Asthma hospitalization within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.09 (0.54 to 2.17) |
| P value | 0.820 |
Readmission
Overall, 8 children (1.2%) were readmitted for pneumonia‐associated conditions within 28 days of index discharge. Readmission occurred in 1.2% of macrolide recipients and 1.1% of non‐recipients (P = 0.83) (Table 4). In unadjusted analysis, neither macrolide therapy (odds ratio [OR], 1.18; 95% CI: 0.25‐5.45; P = 0.84) nor systemic corticosteroid administration (OR, 1.04; 95% CI: 0.27‐4.10; P = 0.95) was associated with 28‐day readmission (Appendix 3). In multivariable analysis, empiric macrolide therapy was not associated with 28‐day readmission in the overall cohort (Table 2; Appendix 4)), or when the analysis was stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
| Concomitant Systemic Corticosteroid Therapy* | ||
|---|---|---|
| Yes | No | |
| ||
| Length of stay | ||
| Adjusted beta‐coefficient (95% CI) | 0.40 (0.74 to 0.07) | 0.37 (0.58 to 0.16) |
| Adjusted percent change (95% CI) | 33% (52% to 7%) | 31% (44% to 15%) |
| P value | 0.020 | 0.001 |
| Readmission within 28 days | ||
| Adjusted odds ratio (95% CI) | 1.09 (0.05 to 26.7) | 1.50 (0.21 to 10.8) |
| P value | 0.960 | 0.687 |
| Readmission within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.57 (0.65 to 3.82) | 0.81 (0.45 to 1.46) |
| P value | 0.32 | 0.49 |
| Asthma hospitalization within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.51 (0.58 to 3.93) | 0.85 (0.36 to 1.97) |
| P value | 0.395 | 0.700 |
| Empiric Macrolide Therapy | ||
|---|---|---|
| N/Total (%) | ||
| Readmission | Yes | No |
| Any readmission within 28 days | ||
| Overall | 5/405 (1.2) | 3/285 (1.1) |
| Systemic corticosteroid therapy | 2/186 (1.1) | 1/66 (1.5) |
| No systemic corticosteroid therapy | 3/177 (1.7) | 2/261 (0.8) |
| Any readmission within 15 mo | ||
| Overall | 96/405 (23.7) | 64/285 (22.5) |
| Systemic corticosteroid therapy | 52/186 (28.0) | 17/66 (25.8) |
| No systemic corticosteroid therapy | 32/177 (18.1) | 59/261 (22.6) |
| Asthma hospitalization within 15 mo | ||
| Overall | 61/405 (15.1) | 34/285 (11.9) |
| Systemic corticosteroid therapy | 39/186 (21.0) | 13/66 (19.7) |
| No systemic corticosteroid therapy | 14/177 (7.9) | 29/261 (11.1) |
Overall, 160 children (23.2%) were readmitted within 15 months of index discharge; 95 were readmitted for asthma during this time (Table 3). Overall readmission occurred in 23.7% of macrolide recipients and 22.5% of macrolide non‐recipients (P = 0.702). Asthma readmission occurred in 15.1% of macrolide recipients and 11.9% of macrolide non‐recipients (P = 0.240). In unadjusted analysis, empiric macrolide therapy was not significantly associated with any readmission within 15 months (OR, 1.07; 95% CI: 0.69‐1.68; P = 0.759) or with asthma‐related readmission within 15 months (OR, 1.31; 95% CI: 0.73‐ 2.36; P = 0.369). In multivariable analysis, neither any readmission nor asthma readmission within 15 months was associated with empiric macrolide therapy overall (Table 2) or when stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
The analyses for readmissions within 15 months of index discharge were repeated while limiting the cohort to those without prior asthma hospitalizations or chronic asthma medications. In this subset of patients, readmissions for any reason occurred in 55 (18.6%) of 295 macrolide recipients and 50 (22.0%) of 227 non‐recipients. The difference was not statistically significant in multivariable analysis (adjusted odds ratio, 0.79; 95% CI: 0.41‐1.51; P = 0.47). Readmissions for asthma occurred in 30 (10.2%) of 295 macrolide recipients and 26 (11.5%) of 227 non‐recipients; this difference was also not significant in multivariable analysis (adjusted odds ratio, 0.83; 95% CI: 0.36‐1.93; P = 0.83). The magnitude of the estimate of effect for 28‐day and 15‐month readmissions, and 15‐month asthma hospitalizations, was similar to the primary analysis when the cohort was restricted to subjects with a LOS 7 days.
DISCUSSION
This multicenter study examined the role of macrolide therapy in children hospitalized with M. pneumoniae pneumonia. Empiric macrolide therapy was associated with an approximately 30% shorter hospital LOS and, in stratified analysis, remained associated with a significantly shorter hospital LOS in both systemic corticosteroid recipients and non‐recipients. Empiric macrolide therapy was not associated with short‐ or longer‐term hospital readmission.
Previous small randomized trials have been inconclusive regarding the potential benefit of macrolide therapy in M. pneumoniae pneumonia.11 Our study, which demonstrated a shorter LOS among macrolides recipients compared with non‐recipients, has several advantages over prior studies including a substantively larger sample size and multicenter design. Animal models support our observations regarding the potential beneficial antimicrobial role of macrolides. M. pneumoniae concentrations in bronchoalveolar lavage specimens were significantly lower among experimentally infected mice treated with clarithromycin, a macolide‐class antibiotic, compared with either placebo or dexamethasone.45 Combination therapy with clarithromycin and dexamethasone reduced histopathologic inflammation to a greater degree than dexamethasone alone.45
While the relative importance of the antimicrobial and anti‐inflammatory properties of macrolides is not known, observational studies of children infected with macrolide‐resistant M. pneumoniae suggest that the antimicrobial properties of macrolides may provide disproportionate clinical benefit. The duration of fever in macrolide recipients with macrolide‐resistant M. pneumoniae (median duration, 9 days) reported by Suzuki et al46 was significantly longer than those with macrolide‐susceptible infections (median duration, 5 days), and similar to the duration of fever in patients with M. pneumoniae infection treated with placebo (median duration, 8 days) reported by Kingston et al.47 Additionally, macrolide therapy was associated with significant improvements in lung function in patients with asthma and concomitant M. pneumoniae infection, but not in patients with asthma without documented M. pneumoniae infection.9 As corticosteroids also have anti‐inflammatory properties, we expect that any anti‐inflammatory benefit of macrolide therapy would be mitigated by the concomitant administration of corticosteroids. The shorter LOS associated with empiric macrolide therapy in our study was comparable among corticosteroid recipients and non‐recipients.
Atypical bacterial pathogens, including M. pneumoniae, are associated with diffuse lower airway inflammation6, 48 and airway hyperresponsiveness,6 and have been implicated as a cause of acute asthma exacerbations.7, 4954 Among patients with previously diagnosed asthma, acute M. pneumoniae infection was identified in up to 20% of those having acute exacerbations.7, 54 Macrolide therapy has a beneficial effect on lung function and airway hyperresponsiveness in adults with asthma.9, 55 Among mice infected with M. pneumoniae, 3 days of macrolide therapy resulted in a significant reduction in airway hyperresponsiveness compared with placebo or dexamethasone; however, after 6 days of therapy, there was no significant difference in airway hyperresponsiveness between those receiving macrolides, dexamethasone, or placebo, suggesting that the benefit of macrolides on airway hyperresponsiveness may be brief. Our findings of a shorter LOS but no difference in readmissions at 28 days or longer, for macrolide recipients compared with non‐recipients, support the limited benefit of macrolide therapy beyond the initial reduction in bacterial load seen in the first few days of therapy.
M. pneumoniae infection has also been implicated as a cause of chronic pulmonary disease, including asthma.610 In the mouse model, peribronchial and perivascular mononuclear infiltrates, increased airway methacholine reactivity, and increased airway obstruction were observed 530 days after M. pneumoniae inoculation.6 M. pneumoniae has been identified in 26 (50%) of 51 children experiencing their first asthma attack,7 and 23 (42%) of 55 adults with chronic, stable asthma.9 Nevertheless, results of other studies addressing the issue are inconsistent, and the role of M. pneumoniae in the development of asthma remains unclear.56 In order to investigate the impact of macrolide therapy on the development of chronic pulmonary disease requiring hospitalization, we examined the readmission rates in the 15 months following index discharge. The proportion of children hospitalized with asthma following the hospitalization for M. pneumoniae pneumonia was higher for both macrolide recipients and non‐recipients compared with the 24‐months prior to infection. These results support a possible role for M. pneumoniae in chronic pulmonary disease. However, macrolide therapy was not associated with long‐term overall hospital readmission or long‐term asthma readmission, either in the entire cohort or in the subset of patients without prior asthma hospitalizations or medications.
This study had several limitations. First, because the identification of children with M. pneumoniae pneumonia relied on ICD‐9 discharge diagnosis codes, it is possible that there was misclassification of disease. We minimized the inclusion of children without M. pneumoniae by including only children who received antibiotic therapy on the first day of hospitalization and by excluding patients younger than 6 years of age, a group at relatively low‐risk for M. pneumoniae infection. Further, our algorithm for identification of M. pneumoniae pneumonia was validated through review of the medical records at 1 institution and was found to have a high positive predictive value. However, the positive predictive value of these ICD‐9 codes may vary across institutions. Additionally, the sensitivity of ICD‐9 codes for identifying children with M. pneumoniae pneumonia is not known. Also, not all children with pneumonia undergo testing for M. pneumoniae, and different tests have varying sensitivity and specificity.57, 58 Thus, some children with M. pneumoniae pneumonia were not diagnosed and so were not included in our study. It is not known how inclusion of these children would affect our results.
Second, the antibiotic information used in this study was limited to empiric antibiotic therapy. It is possible that some patients received macrolide therapy before admission. It is also likely that identification of M. pneumoniae during the hospitalization prompted the addition or substitution of macrolide therapy for some patients. If this therapy was initiated beyond the first day of hospitalization, these children would be classified as macrolide non‐recipients. Since macrolide administration was associated with a shorter hospital LOS, such misclassification would bias our results towards finding no difference in LOS between macrolide recipients and non‐recipients. It is therefore possible that the benefit of macrolide therapy is even greater than found in our study.
Third, there may be unmeasured confounding or residual confounding by indication for adjunct corticosteroid therapy related to clinical presentation. We expect that corticosteroid recipients would be sicker than non‐recipients. We included variables associated with a greater severity of illness (such as intensive care unit admission) in the multivariable analysis. Additionally, the shorter LOS among macrolide recipients remained when the analysis was stratified by receipt or non‐receipt of systemic corticosteroid therapy.
Fourth, we were only able to record readmissions occurring at the same hospital as the index admission; any readmission presenting to a different hospital following their index admission did not appear in our records, and was therefore not counted. It is thus possible that the true number of readmissions is higher than that represented here. Finally, despite the large number of patients included in this study, the number of short‐term readmissions was relatively small. Thus, we may have been underpowered to detect small but significant differences in short‐term readmission rates.
In conclusion, macrolide therapy was associated with shorter hospital LOS, but not with short‐term or longer‐term readmission in children presenting with M. pneumoniae pneumonia.
Appendix
| Variable | Beta Coefficient | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Sex | 0.12 | (0.22, 0.02) | 0.022 |
| Race | |||
| Blackreference category | |||
| White | 0.01 | (0.21, 0.23) | 0.933 |
| Other | 0.13 | (0.39, 0.13) | 0.323 |
| Missing | 0.46 | (0.81, 0.11) | 0.012 |
| Presentation during viral respiratory season | 0.05 | (0.19, 0.09) | 0.462 |
| Prior asthma hospitalization | 0.36 | (0.64, 0.08) | 0.015 |
| Intensive care unit admission | 1.05 | (0.87, 1.23) | <0.001 |
| Labs and procedures performed | |||
| Additional radiologic imaging | 0.23 | (0.20, 0.67) | 0.287 |
| Arterial blood gas | 0.69 | (0.50, 0.87) | <0.001 |
| Complete blood count | 0.34 | (0.24, 0.45) | <0.001 |
| Blood culture | 0.17 | (0.98, 0.44) | 0.204 |
| Mechanical ventilation | 1.15 | (0.68, 1.63) | <0.001 |
| Therapies received | |||
| Empiric macrolide therapy | 0.49 | (0.72, 0.25) | <0.001 |
| Systemic steroids | 0.26 | (0.38, 0.14) | <0.001 |
| Chronic asthma medications | 0.20 | (0.38, 0.013) | 0.037 |
| Beta‐agonist therapy | 0.07 | (0.21, 0.08) | 0.357 |
| Vasoactive infusion | 1.08 | (0.727, 1.45) | <0.001 |
| Clindamycin or vancomycin | 0.55 | (0.34, 0.75) | <0.001 |
| Variable | Odds Ratio* | Confidence Interval | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Sex | 0.56 | (0.23, 1.33) | 0.190 |
| Race | |||
| Blackreference category | |||
| White | 0.46 | (0.19, 1.14) | 0.093 |
| Other | |||
| Missing | |||
| Presentation during viral respiratory season | 0.64 | (0.09, 4.75) | 0.662 |
| Prior asthma hospitalization | |||
| Intensive care unit admission | 4.54 | (1.21, 17.03) | 0.025 |
| Laboratory tests and procedures | |||
| Additional radiologic imaging | 10.00 | (2.25, 44.47) | 0.002 |
| Arterial blood gas | |||
| Complete blood count | 0.92 | (0.24, 3.48) | 0.901 |
| Blood culture | 0.85 | (0.30, 2.36) | 0.738 |
| Mechanical ventilation | |||
| Medications | |||
| Macrolide therapy | 1.18 | (0.25, 5.45) | 0.837 |
| Systemic corticosteroids | 1.04 | (0.276, 4.09) | 0.951 |
| Chronic asthma medication | 1.66 | (0.71, 3.88) | 0.242 |
| Beta‐agonist therapy | 0.66 | (0.16, 2.65) | 0.557 |
| Vasoactive infusions | |||
| Clindamycin or vancomycin | 1.00 | (0.10, 9.90) | 0.998 |
| Variable | Coefficient | Confidence Interval | P Value | % Change | Confidence Interval for % Change |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 0.287 | (0.012, 0.045) | 0.001 | 2.9 | (1.2, 4.6) |
| Prior asthma hospitalization | 0.272 | (0.094, 0.45) | 0.004 | 31.3 | (9.9, 56.8) |
| Intensive care unit admission | 1.015 | (0.802, 1.23) | <0.001 | 175.9 | (123.0, 241.3) |
| Therapies received | |||||
| Macrolide therapy | 0.379 | (0.59, 0.166) | 0.001 | 31.6 | (44.6, 15.3) |
| Systemic corticosteroids | 0.264 | (0.391, 0.138) | <0.001 | 23.2 | (32.3, 12.9) |
| Chronic asthma medications | 0.056 | (0.255, 0.142) | 0.568 | 5.5 | (22.5, 15.2) |
| Albuterol | 0.07 | (0.059, 0.199) | 0.281 | 7.2 | (5.8, 22.0) |
| Clindamycin or vancomycin | 0.311 | (0.063, 0.559) | 0.015 | 36.5 | (6.5, 74.9) |
| Variable | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Age | 0.91 | 0.72, 1.15 | 0.423 |
| Prior asthma hospitalization | 1.94 | 0.42, 8.90 | 0.394 |
| Intensive care unit admission | 5.73 | 2.03, 16.20 | 0.001 |
| Therapies received | |||
| Macrolide therapy | 1.12 | 0.22, 5.78 | 0.890 |
| Systemic corticosteroids | 0.696 | 0.10, 4.70 | 0.710 |
| Chronic asthma medications | 1.98 | 0.32, 12.20 | 0.460 |
| Albuterol | 0.519 | 0.081, 3.31 | 0.488 |
| Clindamycin or vancomycin | 0.904 | 0.07, 11.13 | 0.937 |
Mycoplasma pneumoniae is a common cause of community‐acquired pneumonia (CAP), among school‐age children and adolescents.14 Though pneumonia caused by M. pneumoniae is typically self‐limited, severe illness may occur.5 M. pneumoniae has also been implicated in airway inflammation, which may lead to the onset and development of chronic pulmonary disease.610 Few studies have directly addressed appropriate treatment strategies for M. pneumoniae pneumonia,11 and, despite its high prevalence and potential for causing severe complications, treatment recommendations remain inconsistent.
The efficacy of macrolide therapy in particular for M. pneumoniae remains unclear. In vitro susceptibility studies have shown bacteriostatic activity of erythromycin, clarithromycin, and azithromycin against M. pneumoniae.1218 Additionally, several small retrospective studies have shown that among children with atypical CAP (including M. pneumoniae pneumonia), those treated with macrolides were less likely to have persistence or progression of signs and symptoms after 3 days of therapy.19, 20 Lu et al21 found a shorter duration of fever among macrolide recipients compared with non‐recipients. In adults, Shames et al22 found a shorter duration of fever and hospitalization among erythromycin recipients compared with controls. Other randomized controlled trials have also addressed the use of macrolides in treatment of M. pneumoniae, but the ability to draw meaningful conclusions is limited by small samples sizes and by lack of details about the number of patients with M. pneumoniae.11
In addition to their antimicrobial effect, macrolides also have anti‐inflammatory properties.2327 The importance of these anti‐inflammatory properties is supported by studies showing clinical cure in patients treated with macrolides despite persistence of M. pneumoniae organisms,2831 clinical improvement despite the administration of doses that provide tissue levels below the minimum inhibitory concentration of the organism,3234 and clinical cure in patients with macrolide‐resistant M. pneumoniae.18, 35
The objectives of the current study were to examine the impact of macrolide therapy on the length of stay (LOS) and short‐ and longer‐term readmissions, including longer‐term asthma‐related readmissions, in children hospitalized with M. pneumoniae pneumonia.
METHODS
Data Source
Data for this retrospective cohort study were obtained from the Pediatric Health Information System (PHIS), which contains administrative data from 38 freestanding children's hospitals. Data quality and reliability are assured through a joint effort by the Child Health Corporation of America (Shawnee Mission, KS) and PHIS‐participating hospitals as described previously.36, 37 Encrypted medical record numbers allow for tracking of individual patients across hospitalizations. This study was reviewed and approved by the Committees for the Protection of Human Subjects at The Children's Hospital of Philadelphia (Philadelphia, PA).
Patients
Children 6‐18 years of age with CAP were eligible if they were discharged from a participating hospital between January 1, 2006 and December 31, 2008. Subjects were included if they received antibiotic therapy on the first day of hospitalization and if they satisfied one of the following International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code criteria: 1) Principal diagnosis of M. pneumoniae pneumonia (483.0); 2) Principal diagnosis of a pneumonia‐related symptom (eg, fever, cough) (780.6 or 786.00‐786.52 [except 786.1]) and a secondary diagnosis of M. pneumoniae pneumonia; or 3) Principal diagnosis of pneumonia (481‐483.8 [except 483.0], 485‐486) and a secondary diagnosis of Mycoplasma (041.81).
Children younger than 6 years of age were excluded due to the low prevalence of M. pneumoniae infection.2, 38 Patients with comorbid conditions predisposing to severe or recurrent pneumonia (eg, cystic fibrosis, malignancy) were excluded using a previously reported classification scheme.39 In addition, we excluded patient data from 2 hospitals due to incomplete reporting of discharge information; thus data from 36 hospitals were included in this study.
Validation of Discharge Diagnosis Codes for Mycoplasma pneumoniae
To assess for misclassification of the diagnosis of M. pneumoniae, we reviewed records of a randomly selected subset of subjects from The Children's Hospital of Philadelphia; 14 of 15 patients had signs of lower respiratory tract infection in conjunction with a positive M. pneumoniae polymerase chain reaction test from nasopharyngeal washings to confirm the diagnosis of M. pneumoniae pneumonia. Hence, the positive predictive value of our algorithm for diagnosing M. pneumoniae pneumonia was 93.3%.
Study Definitions
We identified children with asthma in 2 ways. Asthma‐related hospitalizations were identified by an ICD‐9 code for asthma (493.0‐493.92) in any discharge diagnosis field during any hospitalization in the 24 months prior to the current hospitalization. Baseline controller medications were identified by receipt of inhaled corticosteroids (eg, fluticasone) or leukotriene receptor antagonists on the first day of hospitalization.
Systemic corticosteroids (either oral or intravenous) included dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Measures of disease severity included admission to the intensive care unit within 48 hours of hospitalization, and administration of vancomycin or clindamycin, vasoactive infusions (epinephrine, norepinephrine, dopamine, and dobutamine), and invasive (endotracheal intubation) and noninvasive (continuous positive airway pressure) mechanical ventilation within 24 hours of hospitalization, as previously described.40, 41 Viral respiratory season was defined as October through March.
Measured Outcomes
The primary outcomes of interest were hospital LOS and all‐cause readmission within 28 days and 15 months after index discharge. We examined readmissions for asthma 15 months after index discharge as a secondary outcome measure because of the potential role for M. pneumoniae infection in long‐term lung dysfunction, including asthma.42 The 15‐month time frame was selected based on longitudinal data available in PHIS for the entire study cohort.
Measured Exposures
The main exposure was early initiation of macrolide therapy, defined as receipt of erythromycin, clarithromycin, or azithromycin on the first day of hospitalization.
Data Analysis
Continuous variables were described using median and interquartile range (IQR) or range values, and compared using the Wilcoxon rank‐sum test. Categorical variables were described using counts and frequencies, and compared using the chi‐square test. Multivariable linear (for LOS) and logistic (for readmission) regression analyses were performed to assess the independent association of macrolide therapy with the primary outcomes. Because the LOS data had a skewed distribution, our analyses were performed using logarithmically transformed LOS values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent difference in LOS between subjects receiving and not receiving macrolide therapy.
Building of the multivariable models began with the inclusion of macrolide therapy. Variables associated with primary outcomes on univariate analysis (P < 0.20) were also considered for inclusion as potential confounders.43 These variables were included in the final multivariable model if they remained significant after adjusting for other factors, or if their inclusion in the model resulted in a 15% or greater change in the effect size of the primary association of interest (ie, macrolide therapy).44 Because corticosteroids also have anti‐inflammatory properties, we assessed for interactions with macrolide therapy. There was no interaction between macrolide and systemic corticosteroid therapy (P = 0.26, Likelihood ratio test), therefore our primary model adjusted for systemic corticosteroids.
Despite adjusting for systemic corticosteroid therapy in our primary analysis, residual confounding by indication for corticosteroid therapy might exist. We therefore repeated the analysis after stratifying by receipt or non‐receipt of systemic corticosteroid therapy. Because the benefit of macrolides in preventing long‐term dysfunction may be limited to those without a prior diagnosis of asthma, we repeated the analysis of readmissions within 15 months of index discharge (any readmission and asthma‐related readmissions) while limiting the cohort to those without evidence of asthma (ie, no prior asthma‐related hospitalizations and no chronic asthma medications). Because children with underlying conditions or circumstances that would predispose to prolonged hospitalizations may have been included, despite our restriction of the cohort to those without an identified chronic complex condition, we also repeated the analysis while limiting the cohort to those with a LOS 7 days. Finally, all analyses were clustered on hospital using the robust standard errors of Huber and White to account for the correlation of exposures and outcomes among children within centers.
Data were analyzed using Stata version 11 (Stata Corporation, College Station, TX). Statistical significance was determined a priori as a two‐tailed P value <0.05.
RESULTS
Patient Characteristics
During the study, 690 children ages 6 to 18 years met inclusion criteria. Characteristics of these patients are shown in Table 1. The median age was 10 years (IQR, 7‐13 years). Ten patients (1.4%) also had a concomitant discharge diagnosis of pneumococcal pneumonia, while 19 patients (2.7%) had a concomitant discharge diagnosis of viral pneumonia; 1 of these patients had discharge diagnoses of both viral and pneumococcal pneumonia.
| Empiric Macrolide Therapy | ||||
|---|---|---|---|---|
| Variable | All Subjects | Yes | No | P |
| ||||
| Demographics | ||||
| Male sex | 356 (51.6) | 200 (49.4) | 156 (54.7) | 0.166 |
| Race | ||||
| Black | 135 (19.6) | 81 (20.0) | 54 (19.0) | 0.506 |
| White | 484 (70.1) | 287 (70.9) | 197 (69.1) | |
| Other | 62 (9.0) | 31 (7.7) | 31 (10.9) | |
| Missing | 9 (1.3) | 6 (1.5) | 3 (1.1) | |
| Presentation during viral respiratory season | 420 (60.9) | 242 (59.8) | 178 (62.5) | |
| Prior asthma hospitalization | 41 (5.9) | 31 (7.7) | 10 (3.5) | 0.023 |
| Intensive care unit admission | 127 (18.4) | 74 (18.3) | 53 (18.6) | 0.914 |
| Laboratory tests and procedures | ||||
| Additional radiologic imaging* | 24 (3.5) | 13 (3.2) | 11 (3.9) | 0.646 |
| Arterial blood gas | 116 (17.3) | 72 (18.5) | 44 (15.6) | 0.316 |
| Complete blood count | 433 (64.4) | 249 (64.0) | 184 (65.0) | 0.788 |
| Blood culture | 280 (41.7) | 167 (42.9) | 113 (39.9) | 0.436 |
| Mechanical ventilation | 16 (2.3) | 5 (1.2) | 11 (3.86) | 0.024 |
| Medications | ||||
| Chronic asthma medication | 116 (16.8) | 72 (17.8) | 44 (15.4) | 0.419 |
| Beta‐agonist therapy | 328 (47.5) | 215 (53.1) | 113 (39.7) | 0.001 |
| Vasoactive infusions | 22 (3.2) | 13 (3.2) | 9 (3.2) | 0.969 |
| Systemic corticosteroids | 252 (36.5) | 191 (47.2) | 61 (21.4) | <0.001 |
| Clindamycin or vancomycin | 86 (12.5) | 24 (5.9) | 62 (21.8) | <0.001 |
Macrolide therapy was administered to 405 (58.7%) patients. Systemic corticosteroid therapy was administered to 252 (36.5%) patients. Overall, 191 (27.7%) of the 690 patients received both macrolides and systemic corticosteroids empirically, while 224 (32.5%) received neither; 61 (8.8%) received corticosteroids but not macrolides, while 214 (31.0%) received macrolides but not corticosteroids. Asthma hospitalization within the 24 months prior to admission was more common among those receiving macrolides (N = 60/405, 14.8%) than among those not receiving macrolides (N = 30/285, 10.5%) (P = 0.023). Macrolide recipients also more commonly received concomitant systemic corticosteroids (N = 191/405, 47.2%) than macrolide non‐recipients (N = 61/285, 21.4%) (P < 0.001) and more commonly received beta‐agonist therapy (N = 215/405, 53.1%) than macrolide non‐recipients (N = 113/285, 39.7%) (P = 0.001).
Length of Stay
The overall median LOS was 3 days (IQR, 2‐6 days); the median LOS was 3 days (IQR, 2‐5 days) for empiric macrolide recipients and 4 days (IQR, 2‐9 days) for non‐recipients (P < 0.001). Overall, 22.9% (N = 158) of children had an LOS 7 days and 8.8% (N = 61) of children had an LOS 14 days. The LOS was 7 days for 15.3% (N = 62) of macrolide recipients and 33.7% (N = 96) of non‐recipients. LOS was 7 days for 17.5% (N = 44) of systemic steroid recipients and 26% (N = 114) of non‐recipients. In unadjusted analysis, macrolide therapy (beta‐coefficient, 0.49; 95% confidence interval [CI]: 0.72 to 0.25; P < 0.001) and systemic corticosteroid administration (beta‐coefficient, 0.26; CI: 0.37 to 0.14; P < 0.001) were associated with shorter hospital LOS (Appendix 1).
In multivariable analysis, macrolide therapy remained associated with a shorter LOS (Table 2; Appendix 2). Systemic corticosteroid administration was associated with a 23% shorter LOS (adjusted beta‐coefficient, 0.26; 95% CI: 0.39 to 0.14; P < 0.001). In contrast, previous hospitalization for asthma was associated with a 31% longer LOS (adjusted beta‐coefficient, 0.27; 95% CI: 0.09‐0.045; P = 0.004). Receipt of beta‐agonist therapy or chronic asthma medications were not associated with significant differences in LOS. In analysis stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy, empiric macrolide therapy remained associated with a significantly shorter LOS in both systemic corticosteroid recipients and non‐recipient (Table 4). When the cohort was restricted to subjects with a LOS 7 days, macrolide therapy remained significantly associated with a shorter LOS (adjusted percent change, 20%; 95% CI: 32% to 5%; P = 0.015).
| Association of Empiric Macrolide Therapy With Outcomes* | |
|---|---|
| |
| Length of stay (days) | |
| Adjusted beta‐coefficient (95 % CI) | 0.38 (0.59 to 0.17) |
| Adjusted percent change (95% CI) | 32% (45% to 15%) |
| P value | 0.001 |
| Any readmission within 28 days | |
| Adjusted odds ratio (95% CI) | 1.12 (0.22 to 5.78) |
| P value | 0.890 |
| Any readmission within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.00 (0.59 to 1.70) |
| P value | 0.991 |
| Asthma hospitalization within 15 mo | |
| Adjusted odds ratio (95% CI) | 1.09 (0.54 to 2.17) |
| P value | 0.820 |
Readmission
Overall, 8 children (1.2%) were readmitted for pneumonia‐associated conditions within 28 days of index discharge. Readmission occurred in 1.2% of macrolide recipients and 1.1% of non‐recipients (P = 0.83) (Table 4). In unadjusted analysis, neither macrolide therapy (odds ratio [OR], 1.18; 95% CI: 0.25‐5.45; P = 0.84) nor systemic corticosteroid administration (OR, 1.04; 95% CI: 0.27‐4.10; P = 0.95) was associated with 28‐day readmission (Appendix 3). In multivariable analysis, empiric macrolide therapy was not associated with 28‐day readmission in the overall cohort (Table 2; Appendix 4)), or when the analysis was stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
| Concomitant Systemic Corticosteroid Therapy* | ||
|---|---|---|
| Yes | No | |
| ||
| Length of stay | ||
| Adjusted beta‐coefficient (95% CI) | 0.40 (0.74 to 0.07) | 0.37 (0.58 to 0.16) |
| Adjusted percent change (95% CI) | 33% (52% to 7%) | 31% (44% to 15%) |
| P value | 0.020 | 0.001 |
| Readmission within 28 days | ||
| Adjusted odds ratio (95% CI) | 1.09 (0.05 to 26.7) | 1.50 (0.21 to 10.8) |
| P value | 0.960 | 0.687 |
| Readmission within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.57 (0.65 to 3.82) | 0.81 (0.45 to 1.46) |
| P value | 0.32 | 0.49 |
| Asthma hospitalization within 15 mo | ||
| Adjusted odds ratio (95% CI) | 1.51 (0.58 to 3.93) | 0.85 (0.36 to 1.97) |
| P value | 0.395 | 0.700 |
| Empiric Macrolide Therapy | ||
|---|---|---|
| N/Total (%) | ||
| Readmission | Yes | No |
| Any readmission within 28 days | ||
| Overall | 5/405 (1.2) | 3/285 (1.1) |
| Systemic corticosteroid therapy | 2/186 (1.1) | 1/66 (1.5) |
| No systemic corticosteroid therapy | 3/177 (1.7) | 2/261 (0.8) |
| Any readmission within 15 mo | ||
| Overall | 96/405 (23.7) | 64/285 (22.5) |
| Systemic corticosteroid therapy | 52/186 (28.0) | 17/66 (25.8) |
| No systemic corticosteroid therapy | 32/177 (18.1) | 59/261 (22.6) |
| Asthma hospitalization within 15 mo | ||
| Overall | 61/405 (15.1) | 34/285 (11.9) |
| Systemic corticosteroid therapy | 39/186 (21.0) | 13/66 (19.7) |
| No systemic corticosteroid therapy | 14/177 (7.9) | 29/261 (11.1) |
Overall, 160 children (23.2%) were readmitted within 15 months of index discharge; 95 were readmitted for asthma during this time (Table 3). Overall readmission occurred in 23.7% of macrolide recipients and 22.5% of macrolide non‐recipients (P = 0.702). Asthma readmission occurred in 15.1% of macrolide recipients and 11.9% of macrolide non‐recipients (P = 0.240). In unadjusted analysis, empiric macrolide therapy was not significantly associated with any readmission within 15 months (OR, 1.07; 95% CI: 0.69‐1.68; P = 0.759) or with asthma‐related readmission within 15 months (OR, 1.31; 95% CI: 0.73‐ 2.36; P = 0.369). In multivariable analysis, neither any readmission nor asthma readmission within 15 months was associated with empiric macrolide therapy overall (Table 2) or when stratified by receipt or non‐receipt of concomitant systemic corticosteroid therapy (Table 3).
The analyses for readmissions within 15 months of index discharge were repeated while limiting the cohort to those without prior asthma hospitalizations or chronic asthma medications. In this subset of patients, readmissions for any reason occurred in 55 (18.6%) of 295 macrolide recipients and 50 (22.0%) of 227 non‐recipients. The difference was not statistically significant in multivariable analysis (adjusted odds ratio, 0.79; 95% CI: 0.41‐1.51; P = 0.47). Readmissions for asthma occurred in 30 (10.2%) of 295 macrolide recipients and 26 (11.5%) of 227 non‐recipients; this difference was also not significant in multivariable analysis (adjusted odds ratio, 0.83; 95% CI: 0.36‐1.93; P = 0.83). The magnitude of the estimate of effect for 28‐day and 15‐month readmissions, and 15‐month asthma hospitalizations, was similar to the primary analysis when the cohort was restricted to subjects with a LOS 7 days.
DISCUSSION
This multicenter study examined the role of macrolide therapy in children hospitalized with M. pneumoniae pneumonia. Empiric macrolide therapy was associated with an approximately 30% shorter hospital LOS and, in stratified analysis, remained associated with a significantly shorter hospital LOS in both systemic corticosteroid recipients and non‐recipients. Empiric macrolide therapy was not associated with short‐ or longer‐term hospital readmission.
Previous small randomized trials have been inconclusive regarding the potential benefit of macrolide therapy in M. pneumoniae pneumonia.11 Our study, which demonstrated a shorter LOS among macrolides recipients compared with non‐recipients, has several advantages over prior studies including a substantively larger sample size and multicenter design. Animal models support our observations regarding the potential beneficial antimicrobial role of macrolides. M. pneumoniae concentrations in bronchoalveolar lavage specimens were significantly lower among experimentally infected mice treated with clarithromycin, a macolide‐class antibiotic, compared with either placebo or dexamethasone.45 Combination therapy with clarithromycin and dexamethasone reduced histopathologic inflammation to a greater degree than dexamethasone alone.45
While the relative importance of the antimicrobial and anti‐inflammatory properties of macrolides is not known, observational studies of children infected with macrolide‐resistant M. pneumoniae suggest that the antimicrobial properties of macrolides may provide disproportionate clinical benefit. The duration of fever in macrolide recipients with macrolide‐resistant M. pneumoniae (median duration, 9 days) reported by Suzuki et al46 was significantly longer than those with macrolide‐susceptible infections (median duration, 5 days), and similar to the duration of fever in patients with M. pneumoniae infection treated with placebo (median duration, 8 days) reported by Kingston et al.47 Additionally, macrolide therapy was associated with significant improvements in lung function in patients with asthma and concomitant M. pneumoniae infection, but not in patients with asthma without documented M. pneumoniae infection.9 As corticosteroids also have anti‐inflammatory properties, we expect that any anti‐inflammatory benefit of macrolide therapy would be mitigated by the concomitant administration of corticosteroids. The shorter LOS associated with empiric macrolide therapy in our study was comparable among corticosteroid recipients and non‐recipients.
Atypical bacterial pathogens, including M. pneumoniae, are associated with diffuse lower airway inflammation6, 48 and airway hyperresponsiveness,6 and have been implicated as a cause of acute asthma exacerbations.7, 4954 Among patients with previously diagnosed asthma, acute M. pneumoniae infection was identified in up to 20% of those having acute exacerbations.7, 54 Macrolide therapy has a beneficial effect on lung function and airway hyperresponsiveness in adults with asthma.9, 55 Among mice infected with M. pneumoniae, 3 days of macrolide therapy resulted in a significant reduction in airway hyperresponsiveness compared with placebo or dexamethasone; however, after 6 days of therapy, there was no significant difference in airway hyperresponsiveness between those receiving macrolides, dexamethasone, or placebo, suggesting that the benefit of macrolides on airway hyperresponsiveness may be brief. Our findings of a shorter LOS but no difference in readmissions at 28 days or longer, for macrolide recipients compared with non‐recipients, support the limited benefit of macrolide therapy beyond the initial reduction in bacterial load seen in the first few days of therapy.
M. pneumoniae infection has also been implicated as a cause of chronic pulmonary disease, including asthma.610 In the mouse model, peribronchial and perivascular mononuclear infiltrates, increased airway methacholine reactivity, and increased airway obstruction were observed 530 days after M. pneumoniae inoculation.6 M. pneumoniae has been identified in 26 (50%) of 51 children experiencing their first asthma attack,7 and 23 (42%) of 55 adults with chronic, stable asthma.9 Nevertheless, results of other studies addressing the issue are inconsistent, and the role of M. pneumoniae in the development of asthma remains unclear.56 In order to investigate the impact of macrolide therapy on the development of chronic pulmonary disease requiring hospitalization, we examined the readmission rates in the 15 months following index discharge. The proportion of children hospitalized with asthma following the hospitalization for M. pneumoniae pneumonia was higher for both macrolide recipients and non‐recipients compared with the 24‐months prior to infection. These results support a possible role for M. pneumoniae in chronic pulmonary disease. However, macrolide therapy was not associated with long‐term overall hospital readmission or long‐term asthma readmission, either in the entire cohort or in the subset of patients without prior asthma hospitalizations or medications.
This study had several limitations. First, because the identification of children with M. pneumoniae pneumonia relied on ICD‐9 discharge diagnosis codes, it is possible that there was misclassification of disease. We minimized the inclusion of children without M. pneumoniae by including only children who received antibiotic therapy on the first day of hospitalization and by excluding patients younger than 6 years of age, a group at relatively low‐risk for M. pneumoniae infection. Further, our algorithm for identification of M. pneumoniae pneumonia was validated through review of the medical records at 1 institution and was found to have a high positive predictive value. However, the positive predictive value of these ICD‐9 codes may vary across institutions. Additionally, the sensitivity of ICD‐9 codes for identifying children with M. pneumoniae pneumonia is not known. Also, not all children with pneumonia undergo testing for M. pneumoniae, and different tests have varying sensitivity and specificity.57, 58 Thus, some children with M. pneumoniae pneumonia were not diagnosed and so were not included in our study. It is not known how inclusion of these children would affect our results.
Second, the antibiotic information used in this study was limited to empiric antibiotic therapy. It is possible that some patients received macrolide therapy before admission. It is also likely that identification of M. pneumoniae during the hospitalization prompted the addition or substitution of macrolide therapy for some patients. If this therapy was initiated beyond the first day of hospitalization, these children would be classified as macrolide non‐recipients. Since macrolide administration was associated with a shorter hospital LOS, such misclassification would bias our results towards finding no difference in LOS between macrolide recipients and non‐recipients. It is therefore possible that the benefit of macrolide therapy is even greater than found in our study.
Third, there may be unmeasured confounding or residual confounding by indication for adjunct corticosteroid therapy related to clinical presentation. We expect that corticosteroid recipients would be sicker than non‐recipients. We included variables associated with a greater severity of illness (such as intensive care unit admission) in the multivariable analysis. Additionally, the shorter LOS among macrolide recipients remained when the analysis was stratified by receipt or non‐receipt of systemic corticosteroid therapy.
Fourth, we were only able to record readmissions occurring at the same hospital as the index admission; any readmission presenting to a different hospital following their index admission did not appear in our records, and was therefore not counted. It is thus possible that the true number of readmissions is higher than that represented here. Finally, despite the large number of patients included in this study, the number of short‐term readmissions was relatively small. Thus, we may have been underpowered to detect small but significant differences in short‐term readmission rates.
In conclusion, macrolide therapy was associated with shorter hospital LOS, but not with short‐term or longer‐term readmission in children presenting with M. pneumoniae pneumonia.
Appendix
| Variable | Beta Coefficient | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Sex | 0.12 | (0.22, 0.02) | 0.022 |
| Race | |||
| Blackreference category | |||
| White | 0.01 | (0.21, 0.23) | 0.933 |
| Other | 0.13 | (0.39, 0.13) | 0.323 |
| Missing | 0.46 | (0.81, 0.11) | 0.012 |
| Presentation during viral respiratory season | 0.05 | (0.19, 0.09) | 0.462 |
| Prior asthma hospitalization | 0.36 | (0.64, 0.08) | 0.015 |
| Intensive care unit admission | 1.05 | (0.87, 1.23) | <0.001 |
| Labs and procedures performed | |||
| Additional radiologic imaging | 0.23 | (0.20, 0.67) | 0.287 |
| Arterial blood gas | 0.69 | (0.50, 0.87) | <0.001 |
| Complete blood count | 0.34 | (0.24, 0.45) | <0.001 |
| Blood culture | 0.17 | (0.98, 0.44) | 0.204 |
| Mechanical ventilation | 1.15 | (0.68, 1.63) | <0.001 |
| Therapies received | |||
| Empiric macrolide therapy | 0.49 | (0.72, 0.25) | <0.001 |
| Systemic steroids | 0.26 | (0.38, 0.14) | <0.001 |
| Chronic asthma medications | 0.20 | (0.38, 0.013) | 0.037 |
| Beta‐agonist therapy | 0.07 | (0.21, 0.08) | 0.357 |
| Vasoactive infusion | 1.08 | (0.727, 1.45) | <0.001 |
| Clindamycin or vancomycin | 0.55 | (0.34, 0.75) | <0.001 |
| Variable | Odds Ratio* | Confidence Interval | P Value |
|---|---|---|---|
| |||
| Demographics | |||
| Sex | 0.56 | (0.23, 1.33) | 0.190 |
| Race | |||
| Blackreference category | |||
| White | 0.46 | (0.19, 1.14) | 0.093 |
| Other | |||
| Missing | |||
| Presentation during viral respiratory season | 0.64 | (0.09, 4.75) | 0.662 |
| Prior asthma hospitalization | |||
| Intensive care unit admission | 4.54 | (1.21, 17.03) | 0.025 |
| Laboratory tests and procedures | |||
| Additional radiologic imaging | 10.00 | (2.25, 44.47) | 0.002 |
| Arterial blood gas | |||
| Complete blood count | 0.92 | (0.24, 3.48) | 0.901 |
| Blood culture | 0.85 | (0.30, 2.36) | 0.738 |
| Mechanical ventilation | |||
| Medications | |||
| Macrolide therapy | 1.18 | (0.25, 5.45) | 0.837 |
| Systemic corticosteroids | 1.04 | (0.276, 4.09) | 0.951 |
| Chronic asthma medication | 1.66 | (0.71, 3.88) | 0.242 |
| Beta‐agonist therapy | 0.66 | (0.16, 2.65) | 0.557 |
| Vasoactive infusions | |||
| Clindamycin or vancomycin | 1.00 | (0.10, 9.90) | 0.998 |
| Variable | Coefficient | Confidence Interval | P Value | % Change | Confidence Interval for % Change |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 0.287 | (0.012, 0.045) | 0.001 | 2.9 | (1.2, 4.6) |
| Prior asthma hospitalization | 0.272 | (0.094, 0.45) | 0.004 | 31.3 | (9.9, 56.8) |
| Intensive care unit admission | 1.015 | (0.802, 1.23) | <0.001 | 175.9 | (123.0, 241.3) |
| Therapies received | |||||
| Macrolide therapy | 0.379 | (0.59, 0.166) | 0.001 | 31.6 | (44.6, 15.3) |
| Systemic corticosteroids | 0.264 | (0.391, 0.138) | <0.001 | 23.2 | (32.3, 12.9) |
| Chronic asthma medications | 0.056 | (0.255, 0.142) | 0.568 | 5.5 | (22.5, 15.2) |
| Albuterol | 0.07 | (0.059, 0.199) | 0.281 | 7.2 | (5.8, 22.0) |
| Clindamycin or vancomycin | 0.311 | (0.063, 0.559) | 0.015 | 36.5 | (6.5, 74.9) |
| Variable | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Demographics | |||
| Age | 0.91 | 0.72, 1.15 | 0.423 |
| Prior asthma hospitalization | 1.94 | 0.42, 8.90 | 0.394 |
| Intensive care unit admission | 5.73 | 2.03, 16.20 | 0.001 |
| Therapies received | |||
| Macrolide therapy | 1.12 | 0.22, 5.78 | 0.890 |
| Systemic corticosteroids | 0.696 | 0.10, 4.70 | 0.710 |
| Chronic asthma medications | 1.98 | 0.32, 12.20 | 0.460 |
| Albuterol | 0.519 | 0.081, 3.31 | 0.488 |
| Clindamycin or vancomycin | 0.904 | 0.07, 11.13 | 0.937 |
- ,,, et al.Prospective surveillance for atypical pathogens in children with community‐acquired pneumonia in Japan.J Infect Chemother.2006;12:36–41.
- ,,.Incidence of community‐acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population‐based study in primary health care.Respirology.2004;9:109–114.
- ,,.Mycoplasma pneumoniae infections in University of Wisconsin students.Am Rev Respir Dis.1967;96:237–244.
- .Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients.Clin Infect Dis.1993;17(suppl 1):S37–S46.
- .Mycoplasma pneumoniae. In: Long SS, Pickering LK, Prober CG, eds.Principles and Practice of Pediatric Infectious Diseases.3rd ed.Philadelphia, PA:Churchill Livingstone;2008:979–985.
- ,,, et al.Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection‐associated chronic reactive airway disease.Infect Immun.2002;70:649–654.
- ,,, et al.Mycoplasma pneumoniae and asthma in children.Clin Infect Dis.2004;38:1341–1346.
- ,,,.Isolation of Mycoplasma pneumoniae from asthmatic patients.Ann Allergy.1993;70:23–25.
- ,,,.Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin.Chest.2002;121:1782–1788.
- ,,,,.A link between chronic asthma and chronic infection.J Allergy Clin Immunol.2001;107:595–601.
- ,,.Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children.Cochrane Database Syst Rev.2010;7:CD004875.
- ,,,.In vitro susceptibilities of mycoplasmas and ureaplasmas to new macrolides and aryl‐fluoroquinolones.Antimicrob Agents Chemother.1988;32:1500–1502.
- ,,.Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae.Int J Antimicrob Agents.2003;21:574–577.
- ,,,,.The in vitro activity of some 14‐, 15‐ and 16‐ membered macrolides against Staphylococcus spp., Legionella spp., Mycoplasma spp. and Ureaplasma urealyticum.Drugs Exp Clin Res.1991;17:91–99.
- ,,, et al.In vitro and in vivo activities of macrolides against Mycoplasma pneumoniae.Antimicrob Agents Chemother.1994;38:790–798.
- ,.Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum.Eur J Clin Microbiol Infect Dis.1990;9:838–841.
- ,,, et al.Characteristics of macrolide‐resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro.Microbiol Immunol.2001;45:617–620.
- ,,, et al.Characterization and molecular analysis of macrolide‐resistant Mycoplasma pneumoniae clinical isolates obtained in Japan.Antimicrob Agents Chemother.2004;48:4624–4630.
- ,,,.Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired lower respiratory tract infections.Clin Infect Dis.2001;32:1281–1289.
- ,,, et al.Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2–5 years of age with community‐acquired pneumonia.Clin Infect Dis.2002;35:1345–1352.
- ,,,,.Macrolide use shortens fever duration in Mycoplasma pneumoniae infection in children: a 2‐year experience.J Microbiol Immunol Infect.2008;41:307–310.
- ,,,,.Comparison of antibiotics in the treatment of mycoplasmal pneumonia.Arch Intern Med.1970;125:680–684.
- ,,, et al.Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae‐induced pneumonia.Antimicrob Agents Chemother.2003;47:1614–1620.
- ,.Antibiotics in asthma.Curr Allergy Asthma Rep.2004;4:132–138.
- ,.Immunomodulatory activity and effectiveness of macrolides in chronic airway disease.Chest.2004;125:70S–78S.
- ,,, et al.Interleukin‐8 gene repression by clarithromycin is mediated by the activator protein‐1 binding site in human bronchial epithelial cells.Am J Respir Cell Mol Biol.2000;22:51–60.
- ,,, et al.Clarithromycin inhibits NF‐kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells.Antimicrob Agents Chemother.2001;45:44–47.
- ,,,,.Epidemiology of Mycoplasma pneumoniae infection in families.JAMA.1966;197:859–866.
- ,,.Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy.N Engl J Med.1967;276:1172–1175.
- ,,.Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control.J Infect Dis.1971;123:74–92.
- .Is there a role for antibiotics in the treatment of asthma? Involvement of atypical organisms.BioDrugs.2000;14:349–354.
- ,.Diffuse panbronchiolitis: role of macrolides in therapy.Am J Respir Med.2002;1:119–131.
- ,,,,,.Long‐term low‐dose administration of erythromycin to patients with diffuse panbronchiolitis.Respiration.1991;58:145–149.
- ,,,,,.[Long‐term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis].Nihon Kyobu Shikkan Gakkai Zasshi.1990;28:1305–1313.
- ,,, et al.A comparative clinical study of macrolide‐sensitive and macrolide‐resistant Mycoplasma pneumoniae infections in pediatric patients.J Infect Chemother.2009;15:380–383.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Etiology of childhood pneumonia: serologic results of a prospective, population‐based study.Pediatr Infect Dis J.1998;17:986–991.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107:E99.
- ,,,,,.Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia.Pediatrics.2011;127:e255–e263.
- ,,, et al.Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood.J Hosp Med.2011;6:256–263.
- ,,, et al.Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma.Am J Respir Crit Care Med.1998;158:998–1001.
- ,.The impact of confounder selection criteria on effect estimation.Am J Epidemiol.1989;129:125–137.
- ,,.The risk of determining risk with multivariable models.Ann Intern Med.1993;118:201–210.
- ,,, et al.The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection.J Infect Dis.2008;198:1180–1188.
- ,,, et al.Clinical evaluation of macrolide‐resistant Mycoplasma pneumoniae.Antimicrob Agents Chemother.2006;50:709–712.
- ,,, et al.Eaton agent pneumonia.JAMA.1961;176:118–123.
- .The role of viral and atypical bacterial pathogens in asthma pathogenesis.Pediatr Pulmonol Suppl.1999;18:141–143.
- ,,.The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child.Ann Allergy.1970;28:43–49.
- ,,,.Association of viral and mycoplasma infections with exacerbations of asthma.Ann Allergy.1974;33:145–149.
- ,,, et al.Acute Chlamydia pneumoniae and Mycoplasma pneumoniae infections in community‐acquired pneumonia and exacerbations of COPD or asthma: therapeutic considerations.J Chemother.2004;16:70–76.
- ,,, et al.Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults.Ann Allergy.1986;57:263–265.
- ,,, et al.Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection.Eur Respir J.1994;7:2165–2168.
- ,,, et al.Atypical pathogen infection in adults with acute exacerbation of bronchial asthma.Am J Respir Crit Care Med.2003;167:406–410.
- ,,,,,.Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma.Chest.1991;99:670–673.
- ,,,,.Atypical bacteria and macrolides in asthma.Allergy Asthma Clin Immunol.2008;4:111–116.
- ,,.Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods.Eur J Clin Microbiol Infect Dis.2010;29:1055–1069.
- ,,,,,.A multicenter pilot external quality assessment programme to assess the quality of molecular detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae.J Microbiol Methods.2010;82:131–135.
- ,,, et al.Prospective surveillance for atypical pathogens in children with community‐acquired pneumonia in Japan.J Infect Chemother.2006;12:36–41.
- ,,.Incidence of community‐acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population‐based study in primary health care.Respirology.2004;9:109–114.
- ,,.Mycoplasma pneumoniae infections in University of Wisconsin students.Am Rev Respir Dis.1967;96:237–244.
- .Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients.Clin Infect Dis.1993;17(suppl 1):S37–S46.
- .Mycoplasma pneumoniae. In: Long SS, Pickering LK, Prober CG, eds.Principles and Practice of Pediatric Infectious Diseases.3rd ed.Philadelphia, PA:Churchill Livingstone;2008:979–985.
- ,,, et al.Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection‐associated chronic reactive airway disease.Infect Immun.2002;70:649–654.
- ,,, et al.Mycoplasma pneumoniae and asthma in children.Clin Infect Dis.2004;38:1341–1346.
- ,,,.Isolation of Mycoplasma pneumoniae from asthmatic patients.Ann Allergy.1993;70:23–25.
- ,,,.Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin.Chest.2002;121:1782–1788.
- ,,,,.A link between chronic asthma and chronic infection.J Allergy Clin Immunol.2001;107:595–601.
- ,,.Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children.Cochrane Database Syst Rev.2010;7:CD004875.
- ,,,.In vitro susceptibilities of mycoplasmas and ureaplasmas to new macrolides and aryl‐fluoroquinolones.Antimicrob Agents Chemother.1988;32:1500–1502.
- ,,.Inhibitory and bactericidal activities of gemifloxacin and other antimicrobials against Mycoplasma pneumoniae.Int J Antimicrob Agents.2003;21:574–577.
- ,,,,.The in vitro activity of some 14‐, 15‐ and 16‐ membered macrolides against Staphylococcus spp., Legionella spp., Mycoplasma spp. and Ureaplasma urealyticum.Drugs Exp Clin Res.1991;17:91–99.
- ,,, et al.In vitro and in vivo activities of macrolides against Mycoplasma pneumoniae.Antimicrob Agents Chemother.1994;38:790–798.
- ,.Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum.Eur J Clin Microbiol Infect Dis.1990;9:838–841.
- ,,, et al.Characteristics of macrolide‐resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro.Microbiol Immunol.2001;45:617–620.
- ,,, et al.Characterization and molecular analysis of macrolide‐resistant Mycoplasma pneumoniae clinical isolates obtained in Japan.Antimicrob Agents Chemother.2004;48:4624–4630.
- ,,,.Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired lower respiratory tract infections.Clin Infect Dis.2001;32:1281–1289.
- ,,, et al.Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2–5 years of age with community‐acquired pneumonia.Clin Infect Dis.2002;35:1345–1352.
- ,,,,.Macrolide use shortens fever duration in Mycoplasma pneumoniae infection in children: a 2‐year experience.J Microbiol Immunol Infect.2008;41:307–310.
- ,,,,.Comparison of antibiotics in the treatment of mycoplasmal pneumonia.Arch Intern Med.1970;125:680–684.
- ,,, et al.Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae‐induced pneumonia.Antimicrob Agents Chemother.2003;47:1614–1620.
- ,.Antibiotics in asthma.Curr Allergy Asthma Rep.2004;4:132–138.
- ,.Immunomodulatory activity and effectiveness of macrolides in chronic airway disease.Chest.2004;125:70S–78S.
- ,,, et al.Interleukin‐8 gene repression by clarithromycin is mediated by the activator protein‐1 binding site in human bronchial epithelial cells.Am J Respir Cell Mol Biol.2000;22:51–60.
- ,,, et al.Clarithromycin inhibits NF‐kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells.Antimicrob Agents Chemother.2001;45:44–47.
- ,,,,.Epidemiology of Mycoplasma pneumoniae infection in families.JAMA.1966;197:859–866.
- ,,.Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy.N Engl J Med.1967;276:1172–1175.
- ,,.Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control.J Infect Dis.1971;123:74–92.
- .Is there a role for antibiotics in the treatment of asthma? Involvement of atypical organisms.BioDrugs.2000;14:349–354.
- ,.Diffuse panbronchiolitis: role of macrolides in therapy.Am J Respir Med.2002;1:119–131.
- ,,,,,.Long‐term low‐dose administration of erythromycin to patients with diffuse panbronchiolitis.Respiration.1991;58:145–149.
- ,,,,,.[Long‐term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis].Nihon Kyobu Shikkan Gakkai Zasshi.1990;28:1305–1313.
- ,,, et al.A comparative clinical study of macrolide‐sensitive and macrolide‐resistant Mycoplasma pneumoniae infections in pediatric patients.J Infect Chemother.2009;15:380–383.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Etiology of childhood pneumonia: serologic results of a prospective, population‐based study.Pediatr Infect Dis J.1998;17:986–991.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107:E99.
- ,,,,,.Adjunct corticosteroids in children hospitalized with community‐acquired pneumonia.Pediatrics.2011;127:e255–e263.
- ,,, et al.Comparative effectiveness of pleural drainage procedures for the treatment of complicated pneumonia in childhood.J Hosp Med.2011;6:256–263.
- ,,, et al.Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma.Am J Respir Crit Care Med.1998;158:998–1001.
- ,.The impact of confounder selection criteria on effect estimation.Am J Epidemiol.1989;129:125–137.
- ,,.The risk of determining risk with multivariable models.Ann Intern Med.1993;118:201–210.
- ,,, et al.The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection.J Infect Dis.2008;198:1180–1188.
- ,,, et al.Clinical evaluation of macrolide‐resistant Mycoplasma pneumoniae.Antimicrob Agents Chemother.2006;50:709–712.
- ,,, et al.Eaton agent pneumonia.JAMA.1961;176:118–123.
- .The role of viral and atypical bacterial pathogens in asthma pathogenesis.Pediatr Pulmonol Suppl.1999;18:141–143.
- ,,.The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child.Ann Allergy.1970;28:43–49.
- ,,,.Association of viral and mycoplasma infections with exacerbations of asthma.Ann Allergy.1974;33:145–149.
- ,,, et al.Acute Chlamydia pneumoniae and Mycoplasma pneumoniae infections in community‐acquired pneumonia and exacerbations of COPD or asthma: therapeutic considerations.J Chemother.2004;16:70–76.
- ,,, et al.Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults.Ann Allergy.1986;57:263–265.
- ,,, et al.Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection.Eur Respir J.1994;7:2165–2168.
- ,,, et al.Atypical pathogen infection in adults with acute exacerbation of bronchial asthma.Am J Respir Crit Care Med.2003;167:406–410.
- ,,,,,.Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma.Chest.1991;99:670–673.
- ,,,,.Atypical bacteria and macrolides in asthma.Allergy Asthma Clin Immunol.2008;4:111–116.
- ,,.Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods.Eur J Clin Microbiol Infect Dis.2010;29:1055–1069.
- ,,,,,.A multicenter pilot external quality assessment programme to assess the quality of molecular detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae.J Microbiol Methods.2010;82:131–135.
Copyright © 2012 Society of Hospital Medicine