User login
Osteoarthritis as a Chronic Disease: Maximizing Management in Primary Care
Osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability worldwide. In the United States alone, it is believed that approximately 27 million people are affected by this degenerative condition. Many factors are known to increase the risk of developing OA, including heredity, obesity, joint or nerve injury, repeated overuse of certain joints, lack of physical activity, and aging. Treatment for OA needs to be individualized according to the stage of the disease, patient tolerability, comorbidities involved, and response to therapies to meet the goals of reducing pain, improving joint mobility and quality of life, and limiting functional impairment while avoiding drug toxicity. This supplement will review the latest advances in the pathogenesis, underlying phenotypes, and current therapeutic options of OA.
Click here to read Supplement.
Osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability worldwide. In the United States alone, it is believed that approximately 27 million people are affected by this degenerative condition. Many factors are known to increase the risk of developing OA, including heredity, obesity, joint or nerve injury, repeated overuse of certain joints, lack of physical activity, and aging. Treatment for OA needs to be individualized according to the stage of the disease, patient tolerability, comorbidities involved, and response to therapies to meet the goals of reducing pain, improving joint mobility and quality of life, and limiting functional impairment while avoiding drug toxicity. This supplement will review the latest advances in the pathogenesis, underlying phenotypes, and current therapeutic options of OA.
Click here to read Supplement.
Osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability worldwide. In the United States alone, it is believed that approximately 27 million people are affected by this degenerative condition. Many factors are known to increase the risk of developing OA, including heredity, obesity, joint or nerve injury, repeated overuse of certain joints, lack of physical activity, and aging. Treatment for OA needs to be individualized according to the stage of the disease, patient tolerability, comorbidities involved, and response to therapies to meet the goals of reducing pain, improving joint mobility and quality of life, and limiting functional impairment while avoiding drug toxicity. This supplement will review the latest advances in the pathogenesis, underlying phenotypes, and current therapeutic options of OA.
Click here to read Supplement.
A new approach to heparin production
Researchers have developed a new process for manufacturing ultra-low molecular weight heparin, and they believe it’s superior to current methods.
In addition to reducing the potential for contamination, this process produced 2 structurally homogeneous ultra-low molecular weight heparins that were identical in performance and safety to fondaparinux. But these heparins were purer, faster, and less expensive to produce.
Robert Linhardt, PhD, of Rensselaer Polytechnic Institute in New York, and his colleagues reported their discovery in the October 28 edition of Science.
“With this discovery, we have successfully demonstrated that replacing the current model of drug production with a chemoenzymatic approach can greatly reduce the cost of drug development and manufacturing, while also increasing drug performance and safety . . . ,” Dr Linhardt said.
These heparins—like fondaparinux—were chemically synthesized from non-animal materials. So the risk of contamination is reduced, when compared to the risk with traditional heparin production.
In addition, the new production process is superior to that used with fondaparinux, the researchers said. The new process requires 10 to 12 steps, whereas fondaparinux production requires roughly 50.
The investigators were also able to increase the heparin yield 500-fold with the new process. They said it could decrease the cost of manufacture by a similar amount.
And the ultra-low molecular weight heparins proved to be as effective as fondaparinux in rabbit models.
“[W]e were able to quickly build multiple doses in a simple laboratory setting and feel that this is something than can be quickly and easy commercialized to reduce the cost of this drug and help to shift how pharmaceutical companies approach the synthesis of carbohydrate-containing drugs,” Dr Linhardt said.
These findings are part of a larger body of work in the Linhardt lab to completely replace all types of heparin-based or other glycoprotein-based drugs with safer, low-cost, synthetic versions that do not rely on foreign, potentially contaminated animal sources. ![]()

Researchers have developed a new process for manufacturing ultra-low molecular weight heparin, and they believe it’s superior to current methods.
In addition to reducing the potential for contamination, this process produced 2 structurally homogeneous ultra-low molecular weight heparins that were identical in performance and safety to fondaparinux. But these heparins were purer, faster, and less expensive to produce.
Robert Linhardt, PhD, of Rensselaer Polytechnic Institute in New York, and his colleagues reported their discovery in the October 28 edition of Science.
“With this discovery, we have successfully demonstrated that replacing the current model of drug production with a chemoenzymatic approach can greatly reduce the cost of drug development and manufacturing, while also increasing drug performance and safety . . . ,” Dr Linhardt said.
These heparins—like fondaparinux—were chemically synthesized from non-animal materials. So the risk of contamination is reduced, when compared to the risk with traditional heparin production.
In addition, the new production process is superior to that used with fondaparinux, the researchers said. The new process requires 10 to 12 steps, whereas fondaparinux production requires roughly 50.
The investigators were also able to increase the heparin yield 500-fold with the new process. They said it could decrease the cost of manufacture by a similar amount.
And the ultra-low molecular weight heparins proved to be as effective as fondaparinux in rabbit models.
“[W]e were able to quickly build multiple doses in a simple laboratory setting and feel that this is something than can be quickly and easy commercialized to reduce the cost of this drug and help to shift how pharmaceutical companies approach the synthesis of carbohydrate-containing drugs,” Dr Linhardt said.
These findings are part of a larger body of work in the Linhardt lab to completely replace all types of heparin-based or other glycoprotein-based drugs with safer, low-cost, synthetic versions that do not rely on foreign, potentially contaminated animal sources. ![]()

Researchers have developed a new process for manufacturing ultra-low molecular weight heparin, and they believe it’s superior to current methods.
In addition to reducing the potential for contamination, this process produced 2 structurally homogeneous ultra-low molecular weight heparins that were identical in performance and safety to fondaparinux. But these heparins were purer, faster, and less expensive to produce.
Robert Linhardt, PhD, of Rensselaer Polytechnic Institute in New York, and his colleagues reported their discovery in the October 28 edition of Science.
“With this discovery, we have successfully demonstrated that replacing the current model of drug production with a chemoenzymatic approach can greatly reduce the cost of drug development and manufacturing, while also increasing drug performance and safety . . . ,” Dr Linhardt said.
These heparins—like fondaparinux—were chemically synthesized from non-animal materials. So the risk of contamination is reduced, when compared to the risk with traditional heparin production.
In addition, the new production process is superior to that used with fondaparinux, the researchers said. The new process requires 10 to 12 steps, whereas fondaparinux production requires roughly 50.
The investigators were also able to increase the heparin yield 500-fold with the new process. They said it could decrease the cost of manufacture by a similar amount.
And the ultra-low molecular weight heparins proved to be as effective as fondaparinux in rabbit models.
“[W]e were able to quickly build multiple doses in a simple laboratory setting and feel that this is something than can be quickly and easy commercialized to reduce the cost of this drug and help to shift how pharmaceutical companies approach the synthesis of carbohydrate-containing drugs,” Dr Linhardt said.
These findings are part of a larger body of work in the Linhardt lab to completely replace all types of heparin-based or other glycoprotein-based drugs with safer, low-cost, synthetic versions that do not rely on foreign, potentially contaminated animal sources. ![]()
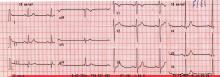
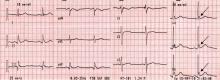
Atrial fibrillation management: Issues of concern
To the Editor: I read with interest the article by Drs. Callahan and Baranowski1 in your April 2011 issue about managing newly diagnosed atrial fibrillation. I believe several issues merit further discussion.
First of all, as mentioned in the article, pulmonary vein isolation, or radiofrequency catheter ablation of the left atrium, can cure paroxysmal atrial fibrillation. Callahan and Baranowski described the optimal indication for this procedure, but they failed to mention the potential adverse effects, that is, esophageal ulcer and atrio-esophageal fistula.2 Owing to the proximity of the esophagus and the accompanying vagus nerve to the posterior wall of the left atrium, it is estimated that 47% of patients develop thermal mucosal injury and 18% develop esophageal ulcer after ablation, while 0.5% develop atrio-esophageal fistula.3 Gastric hypomotility and pyloric spasm are reported as well. It would therefore be prudent to inform patients of such risks if a persistently symptomatic young patient demands this procedure, since the damage might be long-lasting.
In addition, in deciding on long-term anticoagulation for patients with atrial fibrillation, the CHADS2 score is often utilized (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack). Although it is validated and widely applicable, the CHADS2 score carries the disadvantages of oversimplification and of overclassifying atrial fibrillation patients into the intermediate-risk category.4 Lip et al,5 in a seminal article surveying a large group of patients who had nonvalvular atrial fibrillation, proposed using a new and also simple risk stratification scheme, the 2009 Birmingham scheme. This scheme uses the acronym CHA2DS2-VASc and differs from the CHADS2 score in that patients age 75 or older get 2 points, those age 65 to 74 get 1 point, those with vascular disease get 1 point, and women get 1 point. They show that this new scheme fares marginally better than the original CHADS2 score, with fewer patients wrongly assigned to the intermediate-risk category. That means a lower percentage of patients will receive unnecessary anticoagulation and suffer from unneeded anguish. Subsequent studies also prove that this newer scoring index possesses higher sensitivity and predicts thromboembolic events more accurately than the CHADS2 score. Thus, I believe this should also be factored into the decision process when initiating warfarin in atrial fibrillation patients, especially in light of the fact that scanty evidence exists for the use of newer anticoagulants based on the CHADS2 score.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Ginzburg L. Esophageal ulceration: a complication of radiofrequency ablation treatment of atrial fibrillation. Gastrointest Endosc 2009; 70:551–552.
- Bahnson TD. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2009; 32:248–260.
- Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation—friend or foe? Thromb Haemost 2010; 104:45–48.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–172.
To the Editor: I read with interest the article by Drs. Callahan and Baranowski1 in your April 2011 issue about managing newly diagnosed atrial fibrillation. I believe several issues merit further discussion.
First of all, as mentioned in the article, pulmonary vein isolation, or radiofrequency catheter ablation of the left atrium, can cure paroxysmal atrial fibrillation. Callahan and Baranowski described the optimal indication for this procedure, but they failed to mention the potential adverse effects, that is, esophageal ulcer and atrio-esophageal fistula.2 Owing to the proximity of the esophagus and the accompanying vagus nerve to the posterior wall of the left atrium, it is estimated that 47% of patients develop thermal mucosal injury and 18% develop esophageal ulcer after ablation, while 0.5% develop atrio-esophageal fistula.3 Gastric hypomotility and pyloric spasm are reported as well. It would therefore be prudent to inform patients of such risks if a persistently symptomatic young patient demands this procedure, since the damage might be long-lasting.
In addition, in deciding on long-term anticoagulation for patients with atrial fibrillation, the CHADS2 score is often utilized (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack). Although it is validated and widely applicable, the CHADS2 score carries the disadvantages of oversimplification and of overclassifying atrial fibrillation patients into the intermediate-risk category.4 Lip et al,5 in a seminal article surveying a large group of patients who had nonvalvular atrial fibrillation, proposed using a new and also simple risk stratification scheme, the 2009 Birmingham scheme. This scheme uses the acronym CHA2DS2-VASc and differs from the CHADS2 score in that patients age 75 or older get 2 points, those age 65 to 74 get 1 point, those with vascular disease get 1 point, and women get 1 point. They show that this new scheme fares marginally better than the original CHADS2 score, with fewer patients wrongly assigned to the intermediate-risk category. That means a lower percentage of patients will receive unnecessary anticoagulation and suffer from unneeded anguish. Subsequent studies also prove that this newer scoring index possesses higher sensitivity and predicts thromboembolic events more accurately than the CHADS2 score. Thus, I believe this should also be factored into the decision process when initiating warfarin in atrial fibrillation patients, especially in light of the fact that scanty evidence exists for the use of newer anticoagulants based on the CHADS2 score.
To the Editor: I read with interest the article by Drs. Callahan and Baranowski1 in your April 2011 issue about managing newly diagnosed atrial fibrillation. I believe several issues merit further discussion.
First of all, as mentioned in the article, pulmonary vein isolation, or radiofrequency catheter ablation of the left atrium, can cure paroxysmal atrial fibrillation. Callahan and Baranowski described the optimal indication for this procedure, but they failed to mention the potential adverse effects, that is, esophageal ulcer and atrio-esophageal fistula.2 Owing to the proximity of the esophagus and the accompanying vagus nerve to the posterior wall of the left atrium, it is estimated that 47% of patients develop thermal mucosal injury and 18% develop esophageal ulcer after ablation, while 0.5% develop atrio-esophageal fistula.3 Gastric hypomotility and pyloric spasm are reported as well. It would therefore be prudent to inform patients of such risks if a persistently symptomatic young patient demands this procedure, since the damage might be long-lasting.
In addition, in deciding on long-term anticoagulation for patients with atrial fibrillation, the CHADS2 score is often utilized (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack). Although it is validated and widely applicable, the CHADS2 score carries the disadvantages of oversimplification and of overclassifying atrial fibrillation patients into the intermediate-risk category.4 Lip et al,5 in a seminal article surveying a large group of patients who had nonvalvular atrial fibrillation, proposed using a new and also simple risk stratification scheme, the 2009 Birmingham scheme. This scheme uses the acronym CHA2DS2-VASc and differs from the CHADS2 score in that patients age 75 or older get 2 points, those age 65 to 74 get 1 point, those with vascular disease get 1 point, and women get 1 point. They show that this new scheme fares marginally better than the original CHADS2 score, with fewer patients wrongly assigned to the intermediate-risk category. That means a lower percentage of patients will receive unnecessary anticoagulation and suffer from unneeded anguish. Subsequent studies also prove that this newer scoring index possesses higher sensitivity and predicts thromboembolic events more accurately than the CHADS2 score. Thus, I believe this should also be factored into the decision process when initiating warfarin in atrial fibrillation patients, especially in light of the fact that scanty evidence exists for the use of newer anticoagulants based on the CHADS2 score.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Ginzburg L. Esophageal ulceration: a complication of radiofrequency ablation treatment of atrial fibrillation. Gastrointest Endosc 2009; 70:551–552.
- Bahnson TD. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2009; 32:248–260.
- Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation—friend or foe? Thromb Haemost 2010; 104:45–48.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–172.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Ginzburg L. Esophageal ulceration: a complication of radiofrequency ablation treatment of atrial fibrillation. Gastrointest Endosc 2009; 70:551–552.
- Bahnson TD. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2009; 32:248–260.
- Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation—friend or foe? Thromb Haemost 2010; 104:45–48.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–172.
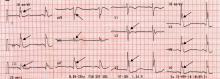
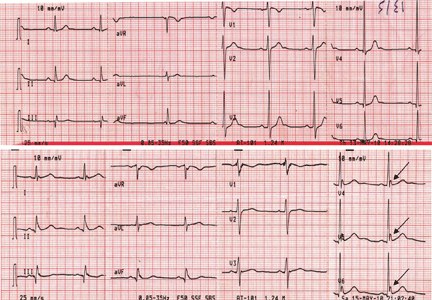
In reply: Atrial fibrillation management: Issues of concern
In Reply: Dr. Chao raises several important points regarding our manuscript on the management of newly diagnosed atrial fibrillation.1
Dr. Chao mentions some of the complications of pulmonary vein antrum isolation. A review of catheter ablation for atrial fibrillation was outside the scope of our manuscript, so the details of the procedure and potential complications were not covered. Dr. Chao does mention some of the important potential complications. However, the complication rates he cites are not generally supported by the available medical literature. Thermal mucosal injury of the esophagus was reported at rates as low as 4% in the same studies cited by Dr. Chao in patients undergoing pulmonary vein antrum isolation with conscious sedation. The rate of 47% was seen in patients undergoing the procedure with general anesthesia. The rate of atrio-esophageal fistula is not well known. As of 2010, about 49 cases were reported in the literature.2 Rates have been described ranging from 0.01% to 0.2%,3–9 far lower than the rate mentioned by Dr. Chao. A careful review with the patient of the risks, benefits, and alternatives is standard practice before any elective, invasive procedure.
Multiple anticoagulation schemes have been proposed, including the Birmingham 2009 scheme.10 We included the CHADS2 score in our paper because it is widely accepted and well validated. The Birmingham 2009 scheme acknowledges other potential risk factors such as female sex, history of vascular disease, and age between 65 and 75 years. It will be interesting to see if it will ever supplant the CHADS2 score. However, no risk stratification scheme should replace sound clinical judgment. Individual patient factors must be considered when deciding whether anticoagulation is appropriate for an individual patient.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Seigel MO, Parenti DM, Simon GL. Atrial-esophageal fistula after atrial radiofrequency catheter ablation. Clin Infect Dis 2010; 51:73–76.
- Dagres N, Hindricks G, Kottkamp H, et al. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol 2009; 20:1014–1019.
- Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 2004; 109:2724–2726.
- Cappato R, Calkins H, Chen SA, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009; 53:1798-1803.
- Dagres N, Kottkamp H, Piorkowski C, et al. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol 2006; 17:1213–1215.
- Ghia KK, Chugh A, Good E, et al. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol 2009; 24:33–36.
- Mohr FW, Nikolaus D, Falk V, et al. Curative treatment of atrial fibrillation: acute and midterm results of intraoperative radiofrequency ablation of atrial fibrillation
in 150 patients. J Thorac Cardiovasc Surg 2002; 123:919–927. - Ren JF, Lin D, Marchlinski FE, Callans DJ, Patel V. Esophageal imaging and strategies for avoiding injury during left atrial ablation for atrial fibrillation. Heart Rhythm 2006; 3:1156–1161.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
In Reply: Dr. Chao raises several important points regarding our manuscript on the management of newly diagnosed atrial fibrillation.1
Dr. Chao mentions some of the complications of pulmonary vein antrum isolation. A review of catheter ablation for atrial fibrillation was outside the scope of our manuscript, so the details of the procedure and potential complications were not covered. Dr. Chao does mention some of the important potential complications. However, the complication rates he cites are not generally supported by the available medical literature. Thermal mucosal injury of the esophagus was reported at rates as low as 4% in the same studies cited by Dr. Chao in patients undergoing pulmonary vein antrum isolation with conscious sedation. The rate of 47% was seen in patients undergoing the procedure with general anesthesia. The rate of atrio-esophageal fistula is not well known. As of 2010, about 49 cases were reported in the literature.2 Rates have been described ranging from 0.01% to 0.2%,3–9 far lower than the rate mentioned by Dr. Chao. A careful review with the patient of the risks, benefits, and alternatives is standard practice before any elective, invasive procedure.
Multiple anticoagulation schemes have been proposed, including the Birmingham 2009 scheme.10 We included the CHADS2 score in our paper because it is widely accepted and well validated. The Birmingham 2009 scheme acknowledges other potential risk factors such as female sex, history of vascular disease, and age between 65 and 75 years. It will be interesting to see if it will ever supplant the CHADS2 score. However, no risk stratification scheme should replace sound clinical judgment. Individual patient factors must be considered when deciding whether anticoagulation is appropriate for an individual patient.
In Reply: Dr. Chao raises several important points regarding our manuscript on the management of newly diagnosed atrial fibrillation.1
Dr. Chao mentions some of the complications of pulmonary vein antrum isolation. A review of catheter ablation for atrial fibrillation was outside the scope of our manuscript, so the details of the procedure and potential complications were not covered. Dr. Chao does mention some of the important potential complications. However, the complication rates he cites are not generally supported by the available medical literature. Thermal mucosal injury of the esophagus was reported at rates as low as 4% in the same studies cited by Dr. Chao in patients undergoing pulmonary vein antrum isolation with conscious sedation. The rate of 47% was seen in patients undergoing the procedure with general anesthesia. The rate of atrio-esophageal fistula is not well known. As of 2010, about 49 cases were reported in the literature.2 Rates have been described ranging from 0.01% to 0.2%,3–9 far lower than the rate mentioned by Dr. Chao. A careful review with the patient of the risks, benefits, and alternatives is standard practice before any elective, invasive procedure.
Multiple anticoagulation schemes have been proposed, including the Birmingham 2009 scheme.10 We included the CHADS2 score in our paper because it is widely accepted and well validated. The Birmingham 2009 scheme acknowledges other potential risk factors such as female sex, history of vascular disease, and age between 65 and 75 years. It will be interesting to see if it will ever supplant the CHADS2 score. However, no risk stratification scheme should replace sound clinical judgment. Individual patient factors must be considered when deciding whether anticoagulation is appropriate for an individual patient.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Seigel MO, Parenti DM, Simon GL. Atrial-esophageal fistula after atrial radiofrequency catheter ablation. Clin Infect Dis 2010; 51:73–76.
- Dagres N, Hindricks G, Kottkamp H, et al. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol 2009; 20:1014–1019.
- Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 2004; 109:2724–2726.
- Cappato R, Calkins H, Chen SA, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009; 53:1798-1803.
- Dagres N, Kottkamp H, Piorkowski C, et al. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol 2006; 17:1213–1215.
- Ghia KK, Chugh A, Good E, et al. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol 2009; 24:33–36.
- Mohr FW, Nikolaus D, Falk V, et al. Curative treatment of atrial fibrillation: acute and midterm results of intraoperative radiofrequency ablation of atrial fibrillation
in 150 patients. J Thorac Cardiovasc Surg 2002; 123:919–927. - Ren JF, Lin D, Marchlinski FE, Callans DJ, Patel V. Esophageal imaging and strategies for avoiding injury during left atrial ablation for atrial fibrillation. Heart Rhythm 2006; 3:1156–1161.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Seigel MO, Parenti DM, Simon GL. Atrial-esophageal fistula after atrial radiofrequency catheter ablation. Clin Infect Dis 2010; 51:73–76.
- Dagres N, Hindricks G, Kottkamp H, et al. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol 2009; 20:1014–1019.
- Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 2004; 109:2724–2726.
- Cappato R, Calkins H, Chen SA, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009; 53:1798-1803.
- Dagres N, Kottkamp H, Piorkowski C, et al. Rapid detection and successful treatment of esophageal perforation after radiofrequency ablation of atrial fibrillation: lessons from five cases. J Cardiovasc Electrophysiol 2006; 17:1213–1215.
- Ghia KK, Chugh A, Good E, et al. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol 2009; 24:33–36.
- Mohr FW, Nikolaus D, Falk V, et al. Curative treatment of atrial fibrillation: acute and midterm results of intraoperative radiofrequency ablation of atrial fibrillation
in 150 patients. J Thorac Cardiovasc Surg 2002; 123:919–927. - Ren JF, Lin D, Marchlinski FE, Callans DJ, Patel V. Esophageal imaging and strategies for avoiding injury during left atrial ablation for atrial fibrillation. Heart Rhythm 2006; 3:1156–1161.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
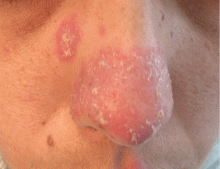
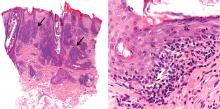
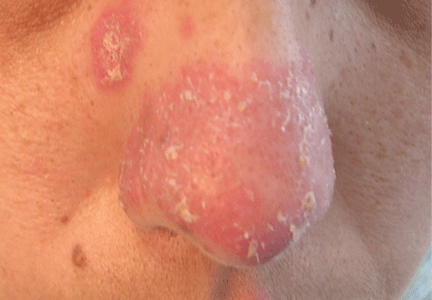
Angioedema due to the renin inhibitor aliskiren
To the Editor: The interesting report by Korniyenko and colleagues of the delayed diagnosis of visceral angioedema due to angiotensin-converting enzyme (ACE) inhibitor therapy1 should alert readers that a long duration of use of ACE inhibitors should not rule out the diagnosis of ACE inhibitor-induced angioedema, as symptoms can be delayed for up to a decade.
Risk factors for angioedema for patients on ACE inhibitor therapy that have been identified so far include black race, the XPNPEP2 C-2399A polymorphism in men (which leads to decreased aminopeptidase P activity),2 and concomitant use of the mTOR inhibitors sirolimus (Rapamune) or everolimus (Afinitor) in renal transplant recipients.3 All of these factors further decrease metabolism of the vasoactive peptide bradykinin. However, the effect of cofactors such as use of nonsteroidal anti-inflammatory drugs, aspirin, simvastatin, estrogen, or a tendency for angioedema (such as in patients with recurrent or episodic idiopathic angioedema) on lowering the threshold for angioedema or increasing the severity of the angioedema episode or episodes after starting ACE inhibitor therapy remains unknown.
Physicians should also be aware of angioedema as a significant side effect of the new renin inhibitor aliskiren (marketed by Novartis Pharmaceuticals as Rasilez in the United Kingdom and as Tekturna in the United States) for treatment of essential hypertension.4 A pooled analysis of 31 studies in 12,188 patients showed the incidence of angioedema associated with aliskiren monotherapy to be 0.4%, similar to that with ACE inhibitors: relative risk 0.31, 95% confidence interval 0.07–1.47 for 150 mg; relative risk 0.57, 95% confidence interval 0.17–1.89 for 300 mg).5 However, no patients were hospitalized with a serious angioedema event.
Although the mechanism of action of aliskiren via renin inhibition would suggest that bradykinin may not be the causative agent of angioedema, physicians should ensure that patients who report significant angioedema episodes or those who present with angioedema have their medication history thoroughly reviewed to prevent a serious untoward event.
- Korniyenko A, Alviar CL, Cordova JP, Messerli FH. Visceral angioedema due to angiotensin-converting enzyme inhibitor therapy. Cleve Clin J Med 2011; 78:297–304.
- Woodard-Grice AV, Lucisano AC, Byrd JB, Stone ER, Simmons WH, Brown NJ. Sex-dependent and race-dependent association of XPNPEP2 C-2399A polymorphism with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet Genomics 2010; 20:532–536.
- Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5:703–708.
- Aliskiren: risk of angioedema and renal dysfunction. Drug Safety Update. Medicines and Healthcare products Regulatory Agency. 2009; 10:2. http://www.mhra.gov.uk/home/groups/pl-p/documents/publication/con046452.pdf. Accessed September 6, 2011.
- White WB, Bresalier R, Kaplan AP, et al. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens (Greenwich) 2010; 12:765–775.
To the Editor: The interesting report by Korniyenko and colleagues of the delayed diagnosis of visceral angioedema due to angiotensin-converting enzyme (ACE) inhibitor therapy1 should alert readers that a long duration of use of ACE inhibitors should not rule out the diagnosis of ACE inhibitor-induced angioedema, as symptoms can be delayed for up to a decade.
Risk factors for angioedema for patients on ACE inhibitor therapy that have been identified so far include black race, the XPNPEP2 C-2399A polymorphism in men (which leads to decreased aminopeptidase P activity),2 and concomitant use of the mTOR inhibitors sirolimus (Rapamune) or everolimus (Afinitor) in renal transplant recipients.3 All of these factors further decrease metabolism of the vasoactive peptide bradykinin. However, the effect of cofactors such as use of nonsteroidal anti-inflammatory drugs, aspirin, simvastatin, estrogen, or a tendency for angioedema (such as in patients with recurrent or episodic idiopathic angioedema) on lowering the threshold for angioedema or increasing the severity of the angioedema episode or episodes after starting ACE inhibitor therapy remains unknown.
Physicians should also be aware of angioedema as a significant side effect of the new renin inhibitor aliskiren (marketed by Novartis Pharmaceuticals as Rasilez in the United Kingdom and as Tekturna in the United States) for treatment of essential hypertension.4 A pooled analysis of 31 studies in 12,188 patients showed the incidence of angioedema associated with aliskiren monotherapy to be 0.4%, similar to that with ACE inhibitors: relative risk 0.31, 95% confidence interval 0.07–1.47 for 150 mg; relative risk 0.57, 95% confidence interval 0.17–1.89 for 300 mg).5 However, no patients were hospitalized with a serious angioedema event.
Although the mechanism of action of aliskiren via renin inhibition would suggest that bradykinin may not be the causative agent of angioedema, physicians should ensure that patients who report significant angioedema episodes or those who present with angioedema have their medication history thoroughly reviewed to prevent a serious untoward event.
To the Editor: The interesting report by Korniyenko and colleagues of the delayed diagnosis of visceral angioedema due to angiotensin-converting enzyme (ACE) inhibitor therapy1 should alert readers that a long duration of use of ACE inhibitors should not rule out the diagnosis of ACE inhibitor-induced angioedema, as symptoms can be delayed for up to a decade.
Risk factors for angioedema for patients on ACE inhibitor therapy that have been identified so far include black race, the XPNPEP2 C-2399A polymorphism in men (which leads to decreased aminopeptidase P activity),2 and concomitant use of the mTOR inhibitors sirolimus (Rapamune) or everolimus (Afinitor) in renal transplant recipients.3 All of these factors further decrease metabolism of the vasoactive peptide bradykinin. However, the effect of cofactors such as use of nonsteroidal anti-inflammatory drugs, aspirin, simvastatin, estrogen, or a tendency for angioedema (such as in patients with recurrent or episodic idiopathic angioedema) on lowering the threshold for angioedema or increasing the severity of the angioedema episode or episodes after starting ACE inhibitor therapy remains unknown.
Physicians should also be aware of angioedema as a significant side effect of the new renin inhibitor aliskiren (marketed by Novartis Pharmaceuticals as Rasilez in the United Kingdom and as Tekturna in the United States) for treatment of essential hypertension.4 A pooled analysis of 31 studies in 12,188 patients showed the incidence of angioedema associated with aliskiren monotherapy to be 0.4%, similar to that with ACE inhibitors: relative risk 0.31, 95% confidence interval 0.07–1.47 for 150 mg; relative risk 0.57, 95% confidence interval 0.17–1.89 for 300 mg).5 However, no patients were hospitalized with a serious angioedema event.
Although the mechanism of action of aliskiren via renin inhibition would suggest that bradykinin may not be the causative agent of angioedema, physicians should ensure that patients who report significant angioedema episodes or those who present with angioedema have their medication history thoroughly reviewed to prevent a serious untoward event.
- Korniyenko A, Alviar CL, Cordova JP, Messerli FH. Visceral angioedema due to angiotensin-converting enzyme inhibitor therapy. Cleve Clin J Med 2011; 78:297–304.
- Woodard-Grice AV, Lucisano AC, Byrd JB, Stone ER, Simmons WH, Brown NJ. Sex-dependent and race-dependent association of XPNPEP2 C-2399A polymorphism with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet Genomics 2010; 20:532–536.
- Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5:703–708.
- Aliskiren: risk of angioedema and renal dysfunction. Drug Safety Update. Medicines and Healthcare products Regulatory Agency. 2009; 10:2. http://www.mhra.gov.uk/home/groups/pl-p/documents/publication/con046452.pdf. Accessed September 6, 2011.
- White WB, Bresalier R, Kaplan AP, et al. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens (Greenwich) 2010; 12:765–775.
- Korniyenko A, Alviar CL, Cordova JP, Messerli FH. Visceral angioedema due to angiotensin-converting enzyme inhibitor therapy. Cleve Clin J Med 2011; 78:297–304.
- Woodard-Grice AV, Lucisano AC, Byrd JB, Stone ER, Simmons WH, Brown NJ. Sex-dependent and race-dependent association of XPNPEP2 C-2399A polymorphism with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet Genomics 2010; 20:532–536.
- Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased incidence of angioedema with ACE inhibitors in combination with mTOR inhibitors in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5:703–708.
- Aliskiren: risk of angioedema and renal dysfunction. Drug Safety Update. Medicines and Healthcare products Regulatory Agency. 2009; 10:2. http://www.mhra.gov.uk/home/groups/pl-p/documents/publication/con046452.pdf. Accessed September 6, 2011.
- White WB, Bresalier R, Kaplan AP, et al. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens (Greenwich) 2010; 12:765–775.
In reply: Angioedema due to the renin inhibitor aliskiren
In Reply: We agree with Dr. Khan that the duration of ACE inhibitor therapy should never be used to rule out ACE inhibitor-associated angioedema. In an Italian study of 85 cases of angioedema with ACE inhibitor therapy, the mean ACE inhibitor exposure was a full 12 months before angioedema was diagnosed.1 More disturbing was the fact that another 12 months elapsed before the ACE inhibitor actually was discontinued. This would indicate that neither the patient nor the physician related the angioedema to ACE inhibitor therapy. In patients with visceral angioedema, since the diagnosis is unusually challenging, even a further delay can be expected.
Angioedema has been reported with aliskiren, but the 0.04% incidence reported by White et al2 may reflect very simply that physicians are more alert and on the lookout now more than they ever were when ACE inhibitors were first available. Obviously, greater awareness will lead to more frequent diagnosis. As Dr. Khan points out, there is no known mechanism by which aliskiren should cause angioedema, whereas there is fairly solid evidence that ACE inhibitor-associated angioedema is mediated by bradykinin.3,4
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ 2006; 175:1065–1070.
- White WB, Bresalier R, Kaplan AP, et al. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens (Greenwich) 2010; 12:765–775.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Cunnion KM Wagner E, Frank MM. Complement and kinin. In: Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology, 10th Ed. New York, NY: Lange Medical Books; 2001:186-888.
In Reply: We agree with Dr. Khan that the duration of ACE inhibitor therapy should never be used to rule out ACE inhibitor-associated angioedema. In an Italian study of 85 cases of angioedema with ACE inhibitor therapy, the mean ACE inhibitor exposure was a full 12 months before angioedema was diagnosed.1 More disturbing was the fact that another 12 months elapsed before the ACE inhibitor actually was discontinued. This would indicate that neither the patient nor the physician related the angioedema to ACE inhibitor therapy. In patients with visceral angioedema, since the diagnosis is unusually challenging, even a further delay can be expected.
Angioedema has been reported with aliskiren, but the 0.04% incidence reported by White et al2 may reflect very simply that physicians are more alert and on the lookout now more than they ever were when ACE inhibitors were first available. Obviously, greater awareness will lead to more frequent diagnosis. As Dr. Khan points out, there is no known mechanism by which aliskiren should cause angioedema, whereas there is fairly solid evidence that ACE inhibitor-associated angioedema is mediated by bradykinin.3,4
In Reply: We agree with Dr. Khan that the duration of ACE inhibitor therapy should never be used to rule out ACE inhibitor-associated angioedema. In an Italian study of 85 cases of angioedema with ACE inhibitor therapy, the mean ACE inhibitor exposure was a full 12 months before angioedema was diagnosed.1 More disturbing was the fact that another 12 months elapsed before the ACE inhibitor actually was discontinued. This would indicate that neither the patient nor the physician related the angioedema to ACE inhibitor therapy. In patients with visceral angioedema, since the diagnosis is unusually challenging, even a further delay can be expected.
Angioedema has been reported with aliskiren, but the 0.04% incidence reported by White et al2 may reflect very simply that physicians are more alert and on the lookout now more than they ever were when ACE inhibitors were first available. Obviously, greater awareness will lead to more frequent diagnosis. As Dr. Khan points out, there is no known mechanism by which aliskiren should cause angioedema, whereas there is fairly solid evidence that ACE inhibitor-associated angioedema is mediated by bradykinin.3,4
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ 2006; 175:1065–1070.
- White WB, Bresalier R, Kaplan AP, et al. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens (Greenwich) 2010; 12:765–775.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Cunnion KM Wagner E, Frank MM. Complement and kinin. In: Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology, 10th Ed. New York, NY: Lange Medical Books; 2001:186-888.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ 2006; 175:1065–1070.
- White WB, Bresalier R, Kaplan AP, et al. Safety and tolerability of the direct renin inhibitor aliskiren: a pooled analysis of clinical experience in more than 12,000 patients with hypertension. J Clin Hypertens (Greenwich) 2010; 12:765–775.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Cunnion KM Wagner E, Frank MM. Complement and kinin. In: Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology, 10th Ed. New York, NY: Lange Medical Books; 2001:186-888.
Do you get up a lot at night to go to the bathroom?
If you get up more than once every night to empty your bladder, you should tell your doctor. He or she may be able to do something about it.
Many people get this problem as they get older. It even has a medical name: nocturia (noct = night, uria = urination). However, it is not something you just have to put up with.
What causes nocturia?
A large urine output can be a sign of a number of diseases. Diabetes, for example, is high on the list and not something you should ignore.
Less room in the bladder, overactive bladder, or an enlarged prostate gland can also make you have to go to the bathroom more often. There are drugs that can help with this. Another common cause is urinary tract infection.
Poor sleep. If you keep waking up for other reasons, you may be getting up just because you are awake. Your doctor may be able to suggest treatments to help you sleep better.
Dear Diary…
To help figure out what is causing the problem, your doctor may ask you to keep a “voiding diary” (a urination diary) for a few days to a week. This means you’ll have to measure everything that comes out—every time—and write down the amount and time. Some people use a special plastic measuring container that fits in the toilet.
Some common-sense advice
- Don’t drink a lot before you go to bed, especially alcoholic or caffeinated drinks.
- If you take a diuretic (water pill) for your blood pressure or heart, don’t take it right before you go to bed.
- If your feet and ankles swell, try wearing compression stockings during the day, and sitting with your legs up in the afternoon.
- Get some moderate exercise every day, such as walking.
- Bed is for sleeping. Avoid reading or watching TV when you’re in bed. Keep the bedroom dark and quiet, if possible. And if it’s cold, put another blanket on the bed.
Be safe
To avoid the risk of tripping and falling down on the way to and from the bathroom, make sure that the path is clear before you go to bed. Clean up the clutter. Get rid of throw rugs. Keep a nightlight on so you can see where you are going. Try to discourage your dog or cat from sleeping in the path you’re going to take. Wear slippers that don’t slip.
It may not be possible to make the problem go away completely, but you may be able to improve it. And if you can sleep better, you’ll probably feel better in the daytime too.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health.
If you get up more than once every night to empty your bladder, you should tell your doctor. He or she may be able to do something about it.
Many people get this problem as they get older. It even has a medical name: nocturia (noct = night, uria = urination). However, it is not something you just have to put up with.
What causes nocturia?
A large urine output can be a sign of a number of diseases. Diabetes, for example, is high on the list and not something you should ignore.
Less room in the bladder, overactive bladder, or an enlarged prostate gland can also make you have to go to the bathroom more often. There are drugs that can help with this. Another common cause is urinary tract infection.
Poor sleep. If you keep waking up for other reasons, you may be getting up just because you are awake. Your doctor may be able to suggest treatments to help you sleep better.
Dear Diary…
To help figure out what is causing the problem, your doctor may ask you to keep a “voiding diary” (a urination diary) for a few days to a week. This means you’ll have to measure everything that comes out—every time—and write down the amount and time. Some people use a special plastic measuring container that fits in the toilet.
Some common-sense advice
- Don’t drink a lot before you go to bed, especially alcoholic or caffeinated drinks.
- If you take a diuretic (water pill) for your blood pressure or heart, don’t take it right before you go to bed.
- If your feet and ankles swell, try wearing compression stockings during the day, and sitting with your legs up in the afternoon.
- Get some moderate exercise every day, such as walking.
- Bed is for sleeping. Avoid reading or watching TV when you’re in bed. Keep the bedroom dark and quiet, if possible. And if it’s cold, put another blanket on the bed.
Be safe
To avoid the risk of tripping and falling down on the way to and from the bathroom, make sure that the path is clear before you go to bed. Clean up the clutter. Get rid of throw rugs. Keep a nightlight on so you can see where you are going. Try to discourage your dog or cat from sleeping in the path you’re going to take. Wear slippers that don’t slip.
It may not be possible to make the problem go away completely, but you may be able to improve it. And if you can sleep better, you’ll probably feel better in the daytime too.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health.
If you get up more than once every night to empty your bladder, you should tell your doctor. He or she may be able to do something about it.
Many people get this problem as they get older. It even has a medical name: nocturia (noct = night, uria = urination). However, it is not something you just have to put up with.
What causes nocturia?
A large urine output can be a sign of a number of diseases. Diabetes, for example, is high on the list and not something you should ignore.
Less room in the bladder, overactive bladder, or an enlarged prostate gland can also make you have to go to the bathroom more often. There are drugs that can help with this. Another common cause is urinary tract infection.
Poor sleep. If you keep waking up for other reasons, you may be getting up just because you are awake. Your doctor may be able to suggest treatments to help you sleep better.
Dear Diary…
To help figure out what is causing the problem, your doctor may ask you to keep a “voiding diary” (a urination diary) for a few days to a week. This means you’ll have to measure everything that comes out—every time—and write down the amount and time. Some people use a special plastic measuring container that fits in the toilet.
Some common-sense advice
- Don’t drink a lot before you go to bed, especially alcoholic or caffeinated drinks.
- If you take a diuretic (water pill) for your blood pressure or heart, don’t take it right before you go to bed.
- If your feet and ankles swell, try wearing compression stockings during the day, and sitting with your legs up in the afternoon.
- Get some moderate exercise every day, such as walking.
- Bed is for sleeping. Avoid reading or watching TV when you’re in bed. Keep the bedroom dark and quiet, if possible. And if it’s cold, put another blanket on the bed.
Be safe
To avoid the risk of tripping and falling down on the way to and from the bathroom, make sure that the path is clear before you go to bed. Clean up the clutter. Get rid of throw rugs. Keep a nightlight on so you can see where you are going. Try to discourage your dog or cat from sleeping in the path you’re going to take. Wear slippers that don’t slip.
It may not be possible to make the problem go away completely, but you may be able to improve it. And if you can sleep better, you’ll probably feel better in the daytime too.
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health.
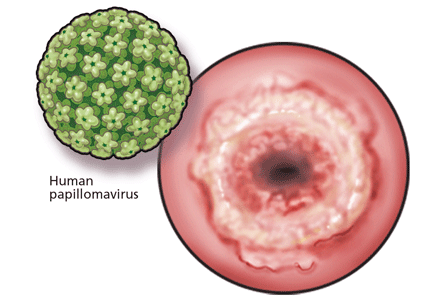
Cervical cancer screening: Less testing, smarter testing
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
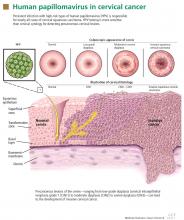
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
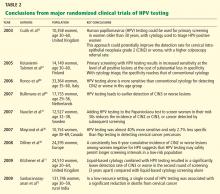
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
KEY POINTS
- Persistent infection with one of the 18 high-risk types of HPV is associated with the development of nearly all cases of cervical cancer.
- The 2009 ACOG guidelines recommend starting to screen with the Pap test at an older age (21 years) than in the past, and they recommend a longer screening interval for women in their 20s, ie, every 2 years instead of yearly.
- Women age 30 and older should undergo both Pap and HPV testing. If both tests are negative, screening should be done again no sooner than 3 years. Alternatively, women age 30 or older who have had three consecutive negative Pap tests can be screened by Pap testing every 3 years.
- Although vaccination can prevent most primary infections with high-risk HPV, it does not eliminate the need for continuing cervical cancer screening, as it does not protect against all high-risk HPV subtypes.
- Screening can stop at age 65 to 70 in women who have had three negative Pap tests in a row and no abnormal tests within the past 10 years.
Osborn waves: An inverse correlation with core body temperature
Although Osborn waves are a marker of hypothermia, they also occur in nonhypothermic conditions. Brainstem death is a precursor of the J wave, and this is explained by impaired thermoregulatory ability resulting from hypothalamic dysfunction and subsequent hypothermia.
The three electrocardiograms presented here illustrate several points:
- Classic findings in hypothermia include J waves, sinus bradycardia, prolongation of the PR interval, widening of the QRS complex, and prolongation of the QT interval.
- The lower the core body temperature, the higher the amplitude of the J wave.
- The J wave in brain death (unlike hypothermic causes of the J wave) is not associated with the characteristic signs of shivering in the surface electrocardiogram.
- As hypothermia becomes more profound, the J wave becomes evident in all leads, not only the inferolateral leads.
- Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol 1953; 175:389–398.
Although Osborn waves are a marker of hypothermia, they also occur in nonhypothermic conditions. Brainstem death is a precursor of the J wave, and this is explained by impaired thermoregulatory ability resulting from hypothalamic dysfunction and subsequent hypothermia.
The three electrocardiograms presented here illustrate several points:
- Classic findings in hypothermia include J waves, sinus bradycardia, prolongation of the PR interval, widening of the QRS complex, and prolongation of the QT interval.
- The lower the core body temperature, the higher the amplitude of the J wave.
- The J wave in brain death (unlike hypothermic causes of the J wave) is not associated with the characteristic signs of shivering in the surface electrocardiogram.
- As hypothermia becomes more profound, the J wave becomes evident in all leads, not only the inferolateral leads.
Although Osborn waves are a marker of hypothermia, they also occur in nonhypothermic conditions. Brainstem death is a precursor of the J wave, and this is explained by impaired thermoregulatory ability resulting from hypothalamic dysfunction and subsequent hypothermia.
The three electrocardiograms presented here illustrate several points:
- Classic findings in hypothermia include J waves, sinus bradycardia, prolongation of the PR interval, widening of the QRS complex, and prolongation of the QT interval.
- The lower the core body temperature, the higher the amplitude of the J wave.
- The J wave in brain death (unlike hypothermic causes of the J wave) is not associated with the characteristic signs of shivering in the surface electrocardiogram.
- As hypothermia becomes more profound, the J wave becomes evident in all leads, not only the inferolateral leads.
- Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol 1953; 175:389–398.
- Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol 1953; 175:389–398.
An erythematous plaque on the nose
A 38-year-old woman presented with a pruriginous and erythematous lesion on her nose that appeared during periods of cold weather. She said she is completely asymptomatic during the summer months.
Q: What is the most likely diagnosis?
- Lupus pernio
- Rosacea
- Seborrheic dermatitis
- Chilblain lupus erythematosus
- Lupus vulgaris
A: The diagnosis is chilblain lupus erythematosus.
The differential diagnosis of an erythematous lesion on the nose of a middle-aged woman also includes rosacea, lupus pernio, lupus vulgaris, and seborrheic dermatitis. Some of these lesions are exacerbated by cold. Usually, the diagnosis is based on clinical findings, but in some cases histologic features on biopsy study confirm the diagnosis.
Lesions of lupus pernio (sarcoidosis) remain unaltered with changes in temperature, and biopsy study usually shows granulomas without caseous necrosis with little inflammatory infiltrate at the periphery.
Rosacea usually gets worse with heat and with alcohol consumption, although it can be exacerbated by cold. Biopsy study shows a nonspecific perivascular and perifollicular lymphohistiocytic infiltrate accompanied occasionally by multinucleated cells.
Seborrheic dermatitis is a papulosquamous disorder characterized by greasy scaling over inflamed skin on the scalp, face, and trunk. Disease activity is increased in winter and spring, with remissions commonly occurring in summer. The histologic features of seborrheic dermatitis are nonspecific; in this case, the histologic features were compatible with chilblain lupus without changes of seborrheic dermatitis.
Lupus vulgaris is a chronic form of cutaneous tuberculosis characterized by redbrown papules with central atrophy. The nose and ears are usually affected. Histologically, granulomatous tubercles with epithelioid cells and caseation necrosis are usually found.
CHILBLAIN LUPUS ERYTHEMATOSUS
Pernio, or chilblain, is a localized inflammatory lesion of the skin resulting from an abnormal response to cold.1 The cutaneous lesions of chilblain may be classified as idiopathic, autoimmune-related (as in systemic lupus erythematosus, subacute cutaneous lupus), and induced by drugs such as terbinafine (Lamisil)2 or infliximab (Remicade).,3
Chilblain lupus is a rare form of cutaneous lupus erythematosus and should not be confused with lupus pernio, which is a misleading name used for a type of cutaneous sarcoidosis.4
Chilblain lupus is characterized by reddish-purple plaques in acral areas (more often the hands and feet, but also the nose and ears) that are induced by exposure to cold—unlike other lesions of lupus erythematosus, which worsen with exposure to sunlight. The main difference from the cutaneous variety of sarcoidosis (lupus pernio) is the histopathologic appearance. In patients with chilblain lupus, epidermal atrophy, perivascular and periadnexal inflammatory infiltrates, and degeneration of the basal layer are found, whereas in lupus pernio (sarcoidosis), we observe granulomas without caseous necrosis, but with few inflammatory infiltrates on the periphery.
PROPOSED DIAGNOSTIC CRITERIA
Su et al5 have proposed diagnostic criteria for chilblain lupus. Their two major criteria are skin lesions in acral locations induced by exposure to cold or a drop in temperature, and evidence of lupus erythematosus in the skin lesions by histopathologic examination or immunofluorescence study. Both of these criteria must be met, plus one of three minor criteria: the coexistence of systemic lupus erythematosus or of skin lesions of discoid lupus erythematosus; response to lupus therapy; and negative results of testing for cryoglobulin and cold agglutinins.
CHILBLAIN LUPUS VS SYSTEMIC LUPUS
Chilblain lupus is an uncommon manifestation of systemic lupus erythematosus, and it is reported to occur in about 20% of patients with that condition.6 Often, the onset of chilblain lupus precedes the systemic disease. Patients with systemic lupus erythematosus and chilblain lupus do not usually present with renal disease, mucosal lesions, or central nervous system involvement. However, Raynaud phenomenon and photosensitivity have been reported to be more frequently associated with chilblain lupus.7
A disorder of peripheral circulation could be involved in the pathogenesis of chilblain lupus, and the association with Raynaud phenomenon, livedo reticularis, antiphospholipid syndrome, and changes in nailfold capillaries supports this hypothesis. Antinuclear antibody and anti-Ro/SS-A antibody are commonly detected in the serum of patients with chilblain lupus, and anti-Ro/SS-A antibody seems to be a major serologic marker of chilblain lupus in patients with systemic lupus erythematosus.7
TREATMENT
Protection from cold by physical measures is very important, as well as the use of topical or oral antibiotics if the lesions are infected. In severe cases unresponsive to topical corticosteroids, a calcium channel blocker is a good therapeutic option; antimalarials, commonly used in the treatment of lupus erythematosus, can also have a positive effect in patients with chilblain lupus.
CASE CONCLUDED
Our patient was advised to protect herself from the cold. Topical corticosteroids and oral hydroxychloroquine (200 mg/day) were prescribed, and they produced a good response. In severe cases, oral corticosteroids, etretinate (Tegison), mycophenolate (CellCept), or thalidomide (Thalomid) may be used.8
- Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics 2005; 116:e472–e475.
- Bonsmann G, Schiller M, Luger TA, Ständer S. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol 2001; 44:925–931.
- Richez C, Dumoulin C, Schaeverbeke T. Infliximab induced chilblain lupus in a patient with rheumatoid arthritis. J Rheumatol 2005; 32:760–761.
- Arias-Santiago SA, Girón-Prieto MS, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Lupus pernio or chilblain lupus?: two different entities. Chest 2009; 136:946–947.
- Su WP, Perniciaro C, Rogers RS, White JW. Chilblain lupus erythematosus (lupus pernio): clinical review of the Mayo Clinic experience and proposal of diagnostic criteria. Cutis 1994; 54:395–399.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol 1996; 135:355–362.
- Franceschini F, Calzavara-Pinton P, Quinzanini M, et al. Chilblain lupus erythematosus is associated with antibodies to SSA/Ro. Lupus 1999; 8:215–219.
- Bouaziz JD, Barete S, Le Pelletier F, Amoura Z, Piette JC, Francès C. Cutaneous lesions of the digits in systemic lupus erythematosus: 50 cases. Lupus 2007; 16:163–167.
A 38-year-old woman presented with a pruriginous and erythematous lesion on her nose that appeared during periods of cold weather. She said she is completely asymptomatic during the summer months.
Q: What is the most likely diagnosis?
- Lupus pernio
- Rosacea
- Seborrheic dermatitis
- Chilblain lupus erythematosus
- Lupus vulgaris
A: The diagnosis is chilblain lupus erythematosus.
The differential diagnosis of an erythematous lesion on the nose of a middle-aged woman also includes rosacea, lupus pernio, lupus vulgaris, and seborrheic dermatitis. Some of these lesions are exacerbated by cold. Usually, the diagnosis is based on clinical findings, but in some cases histologic features on biopsy study confirm the diagnosis.
Lesions of lupus pernio (sarcoidosis) remain unaltered with changes in temperature, and biopsy study usually shows granulomas without caseous necrosis with little inflammatory infiltrate at the periphery.
Rosacea usually gets worse with heat and with alcohol consumption, although it can be exacerbated by cold. Biopsy study shows a nonspecific perivascular and perifollicular lymphohistiocytic infiltrate accompanied occasionally by multinucleated cells.
Seborrheic dermatitis is a papulosquamous disorder characterized by greasy scaling over inflamed skin on the scalp, face, and trunk. Disease activity is increased in winter and spring, with remissions commonly occurring in summer. The histologic features of seborrheic dermatitis are nonspecific; in this case, the histologic features were compatible with chilblain lupus without changes of seborrheic dermatitis.
Lupus vulgaris is a chronic form of cutaneous tuberculosis characterized by redbrown papules with central atrophy. The nose and ears are usually affected. Histologically, granulomatous tubercles with epithelioid cells and caseation necrosis are usually found.
CHILBLAIN LUPUS ERYTHEMATOSUS
Pernio, or chilblain, is a localized inflammatory lesion of the skin resulting from an abnormal response to cold.1 The cutaneous lesions of chilblain may be classified as idiopathic, autoimmune-related (as in systemic lupus erythematosus, subacute cutaneous lupus), and induced by drugs such as terbinafine (Lamisil)2 or infliximab (Remicade).,3
Chilblain lupus is a rare form of cutaneous lupus erythematosus and should not be confused with lupus pernio, which is a misleading name used for a type of cutaneous sarcoidosis.4
Chilblain lupus is characterized by reddish-purple plaques in acral areas (more often the hands and feet, but also the nose and ears) that are induced by exposure to cold—unlike other lesions of lupus erythematosus, which worsen with exposure to sunlight. The main difference from the cutaneous variety of sarcoidosis (lupus pernio) is the histopathologic appearance. In patients with chilblain lupus, epidermal atrophy, perivascular and periadnexal inflammatory infiltrates, and degeneration of the basal layer are found, whereas in lupus pernio (sarcoidosis), we observe granulomas without caseous necrosis, but with few inflammatory infiltrates on the periphery.
PROPOSED DIAGNOSTIC CRITERIA
Su et al5 have proposed diagnostic criteria for chilblain lupus. Their two major criteria are skin lesions in acral locations induced by exposure to cold or a drop in temperature, and evidence of lupus erythematosus in the skin lesions by histopathologic examination or immunofluorescence study. Both of these criteria must be met, plus one of three minor criteria: the coexistence of systemic lupus erythematosus or of skin lesions of discoid lupus erythematosus; response to lupus therapy; and negative results of testing for cryoglobulin and cold agglutinins.
CHILBLAIN LUPUS VS SYSTEMIC LUPUS
Chilblain lupus is an uncommon manifestation of systemic lupus erythematosus, and it is reported to occur in about 20% of patients with that condition.6 Often, the onset of chilblain lupus precedes the systemic disease. Patients with systemic lupus erythematosus and chilblain lupus do not usually present with renal disease, mucosal lesions, or central nervous system involvement. However, Raynaud phenomenon and photosensitivity have been reported to be more frequently associated with chilblain lupus.7
A disorder of peripheral circulation could be involved in the pathogenesis of chilblain lupus, and the association with Raynaud phenomenon, livedo reticularis, antiphospholipid syndrome, and changes in nailfold capillaries supports this hypothesis. Antinuclear antibody and anti-Ro/SS-A antibody are commonly detected in the serum of patients with chilblain lupus, and anti-Ro/SS-A antibody seems to be a major serologic marker of chilblain lupus in patients with systemic lupus erythematosus.7
TREATMENT
Protection from cold by physical measures is very important, as well as the use of topical or oral antibiotics if the lesions are infected. In severe cases unresponsive to topical corticosteroids, a calcium channel blocker is a good therapeutic option; antimalarials, commonly used in the treatment of lupus erythematosus, can also have a positive effect in patients with chilblain lupus.
CASE CONCLUDED
Our patient was advised to protect herself from the cold. Topical corticosteroids and oral hydroxychloroquine (200 mg/day) were prescribed, and they produced a good response. In severe cases, oral corticosteroids, etretinate (Tegison), mycophenolate (CellCept), or thalidomide (Thalomid) may be used.8
A 38-year-old woman presented with a pruriginous and erythematous lesion on her nose that appeared during periods of cold weather. She said she is completely asymptomatic during the summer months.
Q: What is the most likely diagnosis?
- Lupus pernio
- Rosacea
- Seborrheic dermatitis
- Chilblain lupus erythematosus
- Lupus vulgaris
A: The diagnosis is chilblain lupus erythematosus.
The differential diagnosis of an erythematous lesion on the nose of a middle-aged woman also includes rosacea, lupus pernio, lupus vulgaris, and seborrheic dermatitis. Some of these lesions are exacerbated by cold. Usually, the diagnosis is based on clinical findings, but in some cases histologic features on biopsy study confirm the diagnosis.
Lesions of lupus pernio (sarcoidosis) remain unaltered with changes in temperature, and biopsy study usually shows granulomas without caseous necrosis with little inflammatory infiltrate at the periphery.
Rosacea usually gets worse with heat and with alcohol consumption, although it can be exacerbated by cold. Biopsy study shows a nonspecific perivascular and perifollicular lymphohistiocytic infiltrate accompanied occasionally by multinucleated cells.
Seborrheic dermatitis is a papulosquamous disorder characterized by greasy scaling over inflamed skin on the scalp, face, and trunk. Disease activity is increased in winter and spring, with remissions commonly occurring in summer. The histologic features of seborrheic dermatitis are nonspecific; in this case, the histologic features were compatible with chilblain lupus without changes of seborrheic dermatitis.
Lupus vulgaris is a chronic form of cutaneous tuberculosis characterized by redbrown papules with central atrophy. The nose and ears are usually affected. Histologically, granulomatous tubercles with epithelioid cells and caseation necrosis are usually found.
CHILBLAIN LUPUS ERYTHEMATOSUS
Pernio, or chilblain, is a localized inflammatory lesion of the skin resulting from an abnormal response to cold.1 The cutaneous lesions of chilblain may be classified as idiopathic, autoimmune-related (as in systemic lupus erythematosus, subacute cutaneous lupus), and induced by drugs such as terbinafine (Lamisil)2 or infliximab (Remicade).,3
Chilblain lupus is a rare form of cutaneous lupus erythematosus and should not be confused with lupus pernio, which is a misleading name used for a type of cutaneous sarcoidosis.4
Chilblain lupus is characterized by reddish-purple plaques in acral areas (more often the hands and feet, but also the nose and ears) that are induced by exposure to cold—unlike other lesions of lupus erythematosus, which worsen with exposure to sunlight. The main difference from the cutaneous variety of sarcoidosis (lupus pernio) is the histopathologic appearance. In patients with chilblain lupus, epidermal atrophy, perivascular and periadnexal inflammatory infiltrates, and degeneration of the basal layer are found, whereas in lupus pernio (sarcoidosis), we observe granulomas without caseous necrosis, but with few inflammatory infiltrates on the periphery.
PROPOSED DIAGNOSTIC CRITERIA
Su et al5 have proposed diagnostic criteria for chilblain lupus. Their two major criteria are skin lesions in acral locations induced by exposure to cold or a drop in temperature, and evidence of lupus erythematosus in the skin lesions by histopathologic examination or immunofluorescence study. Both of these criteria must be met, plus one of three minor criteria: the coexistence of systemic lupus erythematosus or of skin lesions of discoid lupus erythematosus; response to lupus therapy; and negative results of testing for cryoglobulin and cold agglutinins.
CHILBLAIN LUPUS VS SYSTEMIC LUPUS
Chilblain lupus is an uncommon manifestation of systemic lupus erythematosus, and it is reported to occur in about 20% of patients with that condition.6 Often, the onset of chilblain lupus precedes the systemic disease. Patients with systemic lupus erythematosus and chilblain lupus do not usually present with renal disease, mucosal lesions, or central nervous system involvement. However, Raynaud phenomenon and photosensitivity have been reported to be more frequently associated with chilblain lupus.7
A disorder of peripheral circulation could be involved in the pathogenesis of chilblain lupus, and the association with Raynaud phenomenon, livedo reticularis, antiphospholipid syndrome, and changes in nailfold capillaries supports this hypothesis. Antinuclear antibody and anti-Ro/SS-A antibody are commonly detected in the serum of patients with chilblain lupus, and anti-Ro/SS-A antibody seems to be a major serologic marker of chilblain lupus in patients with systemic lupus erythematosus.7
TREATMENT
Protection from cold by physical measures is very important, as well as the use of topical or oral antibiotics if the lesions are infected. In severe cases unresponsive to topical corticosteroids, a calcium channel blocker is a good therapeutic option; antimalarials, commonly used in the treatment of lupus erythematosus, can also have a positive effect in patients with chilblain lupus.
CASE CONCLUDED
Our patient was advised to protect herself from the cold. Topical corticosteroids and oral hydroxychloroquine (200 mg/day) were prescribed, and they produced a good response. In severe cases, oral corticosteroids, etretinate (Tegison), mycophenolate (CellCept), or thalidomide (Thalomid) may be used.8
- Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics 2005; 116:e472–e475.
- Bonsmann G, Schiller M, Luger TA, Ständer S. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol 2001; 44:925–931.
- Richez C, Dumoulin C, Schaeverbeke T. Infliximab induced chilblain lupus in a patient with rheumatoid arthritis. J Rheumatol 2005; 32:760–761.
- Arias-Santiago SA, Girón-Prieto MS, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Lupus pernio or chilblain lupus?: two different entities. Chest 2009; 136:946–947.
- Su WP, Perniciaro C, Rogers RS, White JW. Chilblain lupus erythematosus (lupus pernio): clinical review of the Mayo Clinic experience and proposal of diagnostic criteria. Cutis 1994; 54:395–399.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol 1996; 135:355–362.
- Franceschini F, Calzavara-Pinton P, Quinzanini M, et al. Chilblain lupus erythematosus is associated with antibodies to SSA/Ro. Lupus 1999; 8:215–219.
- Bouaziz JD, Barete S, Le Pelletier F, Amoura Z, Piette JC, Francès C. Cutaneous lesions of the digits in systemic lupus erythematosus: 50 cases. Lupus 2007; 16:163–167.
- Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics 2005; 116:e472–e475.
- Bonsmann G, Schiller M, Luger TA, Ständer S. Terbinafine-induced subacute cutaneous lupus erythematosus. J Am Acad Dermatol 2001; 44:925–931.
- Richez C, Dumoulin C, Schaeverbeke T. Infliximab induced chilblain lupus in a patient with rheumatoid arthritis. J Rheumatol 2005; 32:760–761.
- Arias-Santiago SA, Girón-Prieto MS, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Lupus pernio or chilblain lupus?: two different entities. Chest 2009; 136:946–947.
- Su WP, Perniciaro C, Rogers RS, White JW. Chilblain lupus erythematosus (lupus pernio): clinical review of the Mayo Clinic experience and proposal of diagnostic criteria. Cutis 1994; 54:395–399.
- Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol 1996; 135:355–362.
- Franceschini F, Calzavara-Pinton P, Quinzanini M, et al. Chilblain lupus erythematosus is associated with antibodies to SSA/Ro. Lupus 1999; 8:215–219.
- Bouaziz JD, Barete S, Le Pelletier F, Amoura Z, Piette JC, Francès C. Cutaneous lesions of the digits in systemic lupus erythematosus: 50 cases. Lupus 2007; 16:163–167.