User login
Combined reperfusion strategies in ST-segment elevation MI: Rationale and current role
Effective and rapid reperfusion is crucial in patients with acute ST-segment elevation myocardial infarction (MI). The preferred strategy for reperfusion—when it can be performed in a timely fashion at an experienced facility—is primary percutaneous coronary intervention (PCI), which produces outcomes superior to those of pharmacologic thrombolysis.1
Unfortunately, in the United States about half of patients present to hospitals that do not have PCI capability,2 and in one analysis, 91% of transferred patients had a door-to-balloon time greater than the recommended 90 minutes, with a mean of 152 minutes.3 (In this case, the door-to-balloon time was the time that elapsed between entry into the first hospital and inflation of the PCI balloon at the second hospital.)
In situations such as these, a combined approach may be appropriate, with thrombolysis delivered by paramedics or at a local facility, followed by transfer to a PCI facility and performance of PCI within a few hours. However, this is feasible only if standardized community-based or regional protocols for prompt transfer and reperfusion are in place.
In this paper we discuss the rationale and the clinical data behind several approaches to combined reperfusion, as well as experiences with community-based care protocols.
WITHIN 3 HOURS OF SYMPTOM ONSET, THROMBOLYSIS IS AS GOOD AS PCI
The PRAGUE-2 Trial
In the randomized PRAGUE-2 trial,4 patients with ST-elevation MI who presented to a non-PCI facility had better outcomes if they were transferred promptly for PCI (median door-to-balloon time 97 minutes), as opposed to receiving local therapy with streptokinase. However, for patients presenting within 3 hours of symptom onset, the mortality rates were comparable with either strategy.4
See the glossary of clinical trial names below
The CAPTIM trial
In the CAPTIM trial,5 patients who presented within 2 hours of symptom onset and who were randomized to receive prehospital thrombolysis had outcomes similar to those of patients treated with primary PCI, despite a short door-to-balloon time (82 minutes).
The Vienna STEMI Registry
In the Vienna STEMI Registry,6 the mortality rates with primary PCI and with thrombolysis were similar when patients presented within 2 hours of symptom onset. However, as the time from symptom onset increased, primary PCI appeared to offer an increasing survival benefit compared with thrombolysis.
Comments: Thrombolysis is effective mostly in the first 2 to 3 hours, with some benefit up to 12 hours
Previous studies have shown that the sooner thrombolysis is given after symptom onset, the more effective it is. If it is given within an hour of symptom onset, the relative reduction in the mortality rate is 50% and the absolute reduction is 6.5% compared with no reperfusion therapy. If it is started in the second hour, the absolute reduction in the mortality rate drops to 4%, and a lesser benefit extends to patients presenting up to 12 hours after symptom onset.7 This time-dependent benefit is due to the fact that very early reperfusion of the occluded coronary artery may lead to full recovery of ischemic tissue and thus prevent necrosis. In addition, thrombolysis in the first 2 hours is highly efficacious in lysing a fresh thrombus.
These data support the current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA), which state no preference for either thrombolytic therapy or PCI in ST-elevation MI if the presentation is less than 3 hours after symptom onset.8
Of note, in the CAPTIM trial and in the Vienna STEMI Registry, rescue PCI was available and was in fact used after thrombolysis in about 25% of patients, which might have contributed to the benefit of early thrombolysis.

PRIMARY PCI MAY NOT BE SUPERIOR IF TRANSFER TIME IS LONG
Another time-related factor to consider is the PCI-related delay, ie, the theoretical difference between the expected time from first medical contact to balloon inflation (if the patient undergoes primary PCI) and the time from first medical contact to the start of thrombolytic therapy (if the patient undergoes primary thrombolysis).
A meta-analysis of 13 trials comparing PCI and thrombolysis showed that a PCI-related delay of more than 60 minutes might negate the potential advantage of primary PCI over immediate thrombolysis in terms of deaths.9
This observation has been further refined by data from the National Registry of Myocardial Infarction.10 In this analysis, patient factors, including age, duration of symptoms, and infarct location, significantly affected the point at which the PCI-related delay negated the survival advantage of primary PCI. The survival advantage of primary PCI was lost more rapidly—with a PCI-related delay as short as 40 minutes—in patients who presented sooner, were younger, or had anterior MI. Primary PCI maintained its survival advantage even with a PCI-related delay longer than 100 minutes in older patients or patients with nonanterior MI presenting more than 3 hours after symptom onset. Given that median door-to-balloon times in the United States may exceed 150 minutes when transfer is involved, 3 primary PCI may be no better than primary thrombolysis in transferred patients who present early or who have large infarcts.
Although these results were derived from a post hoc analysis of a registry and the delay times reported were sometimes inaccurate, they suggest that both the PCI-related delay time and patient characteristics should be considered when selecting a reperfusion strategy. Thrombolytic therapy before and in conjunction with primary PCI was considered a potential solution to these concerns.
In addition, while the benefit of any reperfusion strategy depends on the time of presentation, the loss in benefit by later presentation is less pronounced with primary PCI than with thrombolysis, making thrombolysis less attractive in later presentations (> 3 hours).11
Also, while thrombolytic therapy in patients older than 75 years was associated with a lower mortality rate compared with no therapy in a large Swedish registry,12 this benefit was less striking than in younger patients. A meta-analysis of thrombolysis trials failed to show a similar benefit in patients over age 75 vs younger patients,13 whereas primary PCI remained effective and superior to thrombolysis in the elderly, with more absolute reduction in mortality rates in the elderly subgroup than with younger patients. 14 This makes thrombolysis less attractive in the elderly, either as a stand-alone therapy or in conjunction with PCI. Studies of combined thrombolysis and PCI included very few patients over age 75.15–17
THREE COMBINATION REPERFUSION STRATEGIES
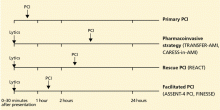
Facilitated PCI is a strategy of thrombolysis immediately followed by PCI, with a planned door-to-balloon time of 90 to 120 minutes.
Pharmacoinvasive therapy means giving thrombolysis at a non-PCI facility and then promptly and systematically transferring the patient to a PCI facility, where PCI is performed 2 to 24 hours after the start of thrombolytic therapy, regardless of whether thrombolysis results in successful reperfusion. 15 Thus, the time to PCI is longer than with facilitated PCI. Facilitated PCI addresses the value of pretreatment with thrombolytics or glycoprotein IIb/IIIa inhibitors in patients otherwise eligible for primary PCI, whereas pharmacoinvasive therapy addresses the value of routine early PCI after thrombolysis in patients who are not eligible for primary PCI.16
Rescue PCI refers to PCI that is performed urgently if thrombolysis fails, failure being defined as persistent hemodynamic or electrical instability, persistent ischemic symptoms, or failure to achieve at least a 50% to 70% resolution of the maximal ST-segment elevation 90 minutes after the infusion is started.
FACILITATED PCI: NEGATIVE RESULTS IN CLINICAL TRIALS
ASSENT-4 PCI trial
In the ASSENT-4 PCI trial,18 patients receiving full thrombolytic therapy before PCI had a higher rate of in-hospital death, bleeding, and cardiovascular events at 90 days than patients treated with primary PCI.
This trial recruited patients arriving at hospitals with or without PCI capability. The door-to-balloon time was about 110 minutes in both groups, which might not have been prolonged enough to show a benefit from a timely addition of thrombolysis. In addition, antiplatelet therapy was limited in these patients: glycoprotein IIb/IIIa inhibitors were not given, and clopidogrel (Plavix) was not appropriately preloaded, and this might have offset the potential benefit of early PCI. In fact, data suggest that platelet activation and aggregation are heightened after thrombolysis, 21–23 and that glycoprotein IIb/IIIa antagonists can inhibit these effects.23
The FINESSE trial
In the FINESSE trial,19 patients were randomized to undergo primary PCI, to undergo PCI facilitated (ie, preceded) by abciximab (Reo-Pro), or to undergo PCI facilitated by half-dose reteplase (Retavase) and full-dose abciximab. Despite a median door-to-balloon time of 132 minutes, the three strategies were associated with similar rates of death, heart failure, or ischemic outcome at 90 days. Even though the dosage of heparin was weight-adjusted, more major bleeding events occurred with the facilitated strategies.
Comments: Some subgroups may still benefit from facilitated PCI
The results of ASSENT-4 PCI and FINESSE led to the conclusion that PCI facilitated by full-dose thrombolysis should be avoided, and called into question the value of PCI facilitation using glycoprotein IIb/IIIa inhibitors with or without half-dose thrombolytic therapy.
However, subgroup analyses of these trials identified some subgroups that may benefit from a facilitated strategy. In ASSENT-4 PCI, 45% of patients were enrolled at PCI hospitals with a minimal PCI-related delay time. These patients had the worst outcome with the facilitated strategy. In contrast, patients who had a short time from pain onset to thrombolysis (2 to 3 hours) and who were given prehospital thrombolysis had a trend toward better outcomes with facilitated PCI.24 And in FINESSE, 60% of patients were enrolled at centers with PCI capability. Analysis of a small subgroup of patients with a Thrombolysis in Myocardial Infarction study (TIMI) risk score of 3 or greater presenting to non-PCI hospitals within 4 hours of symptom onset suggested a potential reduction of ischemic events with the facilitated strategy in these patients.25
Thus, for patients seen in the first 2 to 3 hours after symptom onset, immediate thrombolysis is recommended if PCI will likely be delayed, with or without plans for subsequent early PCI. “Time is muscle,” especially during the first 3 hours.
PHARMACOINVASIVE STRATEGY: GOOD RESULTS IN HIGH-RISK PATIENTS
A number of randomized studies during the last 10 years have examined the value of a pharmacoinvasive strategy.15,16,26–29
The TRANSFER-AMI trial
The TRANSFER-AMI trial15 randomized 1,059 patients with high-risk ST-elevation MI (ie, anterior or high-risk inferior) at non-PCI centers to undergo either pharmacoinvasive care, ie, full-dose tenecteplase (TNKase) with immediate transfer for PCI or standard care, ie, tenecteplase with transfer for rescue PCI if the patient had persistent ST-segment elevation, chest pain, or hemodynamic instability.15 The goal was to perform PCI within 6 hours of thrombolysis, and the median time to PCI was 3.9 hours (range 2–6 hours). In the standard-care group, 35% of patients needed to be transferred for rescue PCI. Unlike in the ASSENT-4 trial, over 80% of patients received aggressive antiplatelet therapy with both 300 mg of clopidogrel and glycoprotein IIb/IIIa inhibitors.
The rate of cardiovascular events at 30 days was significantly lower with pharmacoinvasive therapy than with standard care and rescue PCI (11% vs 17%, P = .004). This difference was driven by lower rates of recurrent ischemia, reinfarction, and heart failure.
The CARESS-in-AMI study
The CARESS-in-AMI study16 found a similar improvement in ischemic outcomes in 600 patients with high-risk ST-elevation MI arriving at non-PCI centers if they had received pharmacoinvasive therapy. Patients received half-dose reteplase and abciximab and were randomized either to be immediately transferred for PCI (median time to PCI 2.25 hours) or to be transferred only if they had persistent ST-segment elevation or clinical deterioration.16 The event rate was low with pharmacoinvasive therapy, comparable to that achieved in primary PCI trials.
Interestingly, no significant increase was seen in the risk of major and minor bleeding in these two trials despite the use of a femoral approach for PCI in over 80% of the cases; this is probably due to the delays between thrombolytic administration and PCI and to the use of a highly fibrin-specific thrombolytic agent and adjusted-dose heparin.
Meta-analysis of pharmacoinvasive trials
A meta-analysis29 of studies of systematic early PCI (mainly with stenting) within 24 hours of thrombolysis showed a reduction in the rates of mortality and reinfarction with this strategy, without an increase in the risk of major or intracranial bleeding.30 In contrast to the results of the trials of facilitated PCI, a pharmacoinvasive strategy improved outcomes in these trials because the delay between thrombolysis and PCI was more than 2 hours, ie, long enough to prevent bleeding complications, and because most patients randomized in these trials presented within 2 to 3 hours of symptom onset, when the time to reperfusion is critical. After 3 hours, the PCI-mediated myocardial salvage is less time-dependent. Moreover, trials of pharmacoinvasive strategy used aggressive antiplatelet therapy with clopidogrel and glycoprotein IIb/IIIa inhibitors.
Comment: Pharmacoinvasive strategy in the guidelines
These results and those of the subgroup analysis from the FINESSE trial suggest that patients with high-risk ST-elevation MI treated at non-PCI hospitals have better outcomes without an increase in major bleeding events when given thrombolysis and then immediately transferred for routine PCI, rather than being transferred only if reperfusion fails.
Hence, the 2009 update of the ACC/AHA guidelines31 gives a class IIa recommendation for transferring patients with anterior ST-elevation MI or high-risk inferior ST-elevation MI treated with thrombolysis to a PCI-capable facility where PCI is performed as part of a pharmacoinvasive or rescue strategy soon after thrombolysis.
This strategy has been particularly studied in patients younger than 75 years presenting with high-risk types of ST-elevation MI early (< 3 hours) after symptom onset. If not at high risk, the patient may be transferred to a PCI facility after receiving thrombolysis or observed in the initial facility (class IIb recommendation). Consideration should be given to starting anticoagulant and antiplatelet therapy before and during transfer—ie, 300 mg of clopidogrel before transfer for PCI and glycoprotein IIb/IIIa inhibitor therapy during PCI.
The European Society of Cardiology (ESC) guidelines32 recommend early routine angiography 3 to 24 hours after successful thrombolysis. This time window was selected to avoid PCI during the prothrombotic period in the first few hours after thrombolysis and to minimize the risk of reocclusion with PCI delays of more than 24 hours (class IIa recommendation).
Larger randomized trials are still needed to establish whether the pharmacoinvasive strategy confers a survival benefit, to determine its usefulness in low-risk inferior or lateral ST-elevation MI, and to further refine the time window when PCI is both safe and beneficial after thrombolysis.33
RESCUE PCI REDUCES MORTALITY RATES
Rescue PCI is the most accepted form of thrombolysis-PCI combination.
The REACT trial
The REACT trial20 showed that rescue PCI performed at a mean of 4.5 hours after failed thrombolysis reduces the rate of adverse cardiovascular events by more than 50% at 6 to 12 months and reduces the 5-year mortality rate by more than 50% compared with conservative management.20 As in the pharmacoinvasive strategy, aggressive antiplatelet regimens were used in the REACT trial.
A meta-analysis of rescue PCI trials
A meta-analysis of rescue PCI trials34 confirmed these results, showing a reduction in heart failure and reinfarction and a trend toward a lower mortality rate with rescue PCI.34 After thrombolysis, 40% of patients do not achieve grade 3 TIMI flow, which explains why in modern clinical trials 30% of patients treated with thrombolysis require rescue PCI.5,15,16,35
For patients with high-risk ST-elevation MI, current ACC/AHA guidelines assign a class IIa recommendation to rescue PCI.31
WHEN PATIENTS WITH ST-ELEVATION MI PRESENT TO A NON-PCI HOSPITAL
Transfer for primary PCI vs thrombolysis at the non-PCI hospital
The DANAMI-2 trial36 found that immediate transfer for PCI was superior to onsite thrombolytic therapy, as measured by a reduction in the rate of ischemic events (composite of death, myocardial infarction, or stroke at 30 days): 8.5% vs 14.2% (P < .001). There were no deaths during transfer.3
The PRAGUE-2 trial4 showed similar results for patients presenting 3 to 12 hours after symptom onset (30-day mortality rate 6% with immediate transfer vs 15.3% with on-site thrombolysis, P < .002), whereas patients presenting within 3 hours of symptom onset had a similar mortality rate with either therapy.4
Comment. These trials showed that transfer for primary PCI is superior to thrombolytic therapy when performed in a timely fashion. However, they were done in countries with established transfer networks and short distances between community hospitals and PCI centers, with a PCI-related delay of only 44 minutes and a door-to-balloon time of 90 minutes despite transfer. The large-scale application of this prompt transfer policy is not practical in most regions in the United States. Thus, a strategy of local thrombolysis followed by routine early transfer for routine or rescue PCI seems warranted when the door-to-balloon time or the PCI-related delay time is expected to be too long.
Experiences with community-based systems of care and prehospital thrombolysis
In Minnesota, Henry et al37 developed a PCI-based treatment system and an integrated transfer program for ST-elevation MI involving 30 hospitals within 210 miles of the Minneapolis Heart Institute. Participating hospitals were divided into two zones: zone 1 hospitals were within 60 miles, and zone 2 facilities were between 60 and 210 miles from the Heart Institute. Zone 2 patients received half-dose tenecteplase (if thrombolytic therapy was not contraindicated) in anticipation of a lengthy transfer time.
The median door-to-balloon time for zone 1 patients was 95 minutes (interquartile range 82 and 116 minutes) and for zone 2 patients 120 minutes (interquartile range 100 and 145 minutes). The diagnosis of ST-elevation MI was made by the emergency department physician, who activated the system with a phone call. The patient was then directly transferred to the catheterization laboratory, most often by helicopter.
The in-hospital death rate for patients who presented to the PCI center and for patients in zones 1 and 2 was similarly low (about 5%).37
In France, the FAST-MI registry,17 which collected outcome data for different reperfusion strategies, found that thrombolysis yielded in-hospital and midterm results that were comparable to those of primary PCI. Of note, thrombolysis was started early after symptom onset (about 2 hours), and was started in the ambulance in two-thirds of cases. Nearly all patients underwent a pharmacoinvasive strategy that combined thrombolysis with coronary angiography and PCI within 24 hours of symptom onset. These findings suggest that timely thrombolysis followed by semiurgent transfer for PCI is an alternative to primary PCI for patients presenting to hospitals with no PCI capability, and that this alternative offers similar benefit to that of primary PCI.
Five centers in the United States have reported their experience with half-dose thrombolysis in the prehospital setting (in the field or during transfer) or at a non-PCI hospital, followed by prompt transfer to a PCI facility. In this registry of almost 3,000 patients,38 patients treated with thrombolysis had better outcomes than patients directly transferred for primary PCI, with a significantly lower 30-day mortality rate (3.8% vs from 6.4%), and no increase in bleeding.38,39 The mean door-to-balloon time was long (168 minutes in the primary PCI group and 196 minutes in the thrombolysis-PCI group), which might explain the benefit achieved with prompt thrombolysis.
CARDIOGENIC SHOCK
Patients presenting with left ventricular cardiogenic shock derive a large mortality benefit from revascularization, whether they are transferred or directly admitted to a PCI center. 40 Moreover, in the SHOCK registry, patients with predominant right ventricular cardiogenic shock had an in-hospital mortality rate similar to that of patients with predominant left ventricular cardiogenic shock, and revascularization (PCI or surgical revascularization) was associated with a strikingly lower mortality rate in both groups.41
Thus, all patients with left or right cardiogenic shock should be revascularized on an emergency basis, either surgically or percutaneously.
While trials of pharmacoinvasive therapy excluded patients with cardiogenic shock,15,16 thrombolytic therapy was associated with improved outcomes in the drug-therapy group of the SHOCK trial and in hypotensive patients randomized in the early thrombolysis trials.13 Thus, the ACC/AHA guidelines recommend thrombolytic therapy before transfer if a patient presents in shock within 3 to 6 hours of onset of the MI and delays in transport and intervention are anticipated.8
PUTTING IT ALL TOGETHER: MANAGEMENT STRATEGIES
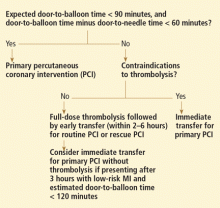
If an effective transfer system is in place, primary PCI not preceded by thrombolytic therapy or glycoprotein IIb/IIIa inhibitor therapy is the preferred approach, according to ACC/AHA and ESC guidelines.31,32 Giving thrombolytics immediately before PCI is harmful and thus should be avoided when the expected door-to-balloon time is 90 minutes or less.
All hospitals (whether or not they offer PCI) and regional emergency medical services should participate in a community-based system of care for ST-elevation MI, with protocols for expeditious transfer as defined and coordinated by the American Heart Association initiative “Mission: Lifeline.” In addition, a system of field triage and direct transport to the catheterization laboratory of a PCI facility after field activation significantly reduces door-to-balloon times and improves outcomes.42
If such a system is not in place, then a pharmacoinvasive strategy seems best: ie, local full-dose thrombolysis (if not contraindicated) followed by transfer to a PCI facility and routine performance of PCI 2 to 6 hours after thrombolysis—in conjunction with aggressive early dual oral antiplatelet therapy and “downstream” glycoprotein IIb/IIIa inhibition. This approach is associated with outcomes similar to those of primary PCI.15–17,37
Prehospital thrombolysis delivered by paramedics and followed by early transfer to a PCI facility has been associated with further reduction in mortality rates compared with in-hospital thrombolysis (as in the Swedish registry43), and a reduction in death rate comparable to that of primary PCI in patients presenting early. This is an adequate strategy in regions where such a system can be established.5,17,38,43,44
Patients presenting more than 3 to 4 hours after symptom onset, older patients, and patients with lower-risk MI or a higher risk of bleeding may still be suited for primary PCI even when the door-to-balloon time is 90 to 120 minutes, as stated by the European guidelines,32 or when the PCI-related delay time is as long as 100 minutes. 10 On the other hand, while the ACC/AHA guidelines recognize that in these patients the mortality advantage of primary PCI vs thrombolytic therapy is maintained with more prolonged door-to-balloon times, they nevertheless state that the focus should be on developing systems of care to increase the number of patients with access to primary PCI in less than 90 minutes rather than extending the acceptable window for door-to-balloon time.
In conclusion, for patients presenting with ST-elevation MI who cannot undergo timely primary PCI, the best approach seems to be prehospital thrombolysis delivered by paramedics or local thrombolysis at the non-PCI hospital followed by transferring the patient and performing PCI within a few hours. This is especially important in patients with high-risk ST-elevation MI who present early after symptom onset, when the extent of myocardial necrosis associated with delayed primary PCI is largest.
In addition, every community should develop a coordinated transfer strategy between non-PCI and PCI hospitals.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Waters RE, Singh KP, Roe MT, et al. Rationale and strategies for implementing community-based transfer protocols for primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004; 43:2153–2159.
- Chakrabarti A, Krumholz HM, Wang Y, Rumsfeld JS, Nallamothu BK; National Cardiovascular Data Registry. Time-to-reperfusion in patients undergoing interhospital transfer for primary percutaneous coronary intervention in the U.S: an analysis of 2005 and 2006 data from the National Cardiovascular Data Registry. J Am Coll Cardiol 2008; 51:2442–2443.
- Widimský P, Budesínský T, Vorác D, et al; ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J 2003; 24:94–104.
- Steg PG, Bonnefoy E, Chabaud S, et al; Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial infarction (CAPTIM) Investigators. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003; 108:2851–2856.
- Kalla K, Christ G, Karnik R, et al; Vienna STEMI Registry Group. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry). Circulation 2006; 113:2398–2405.
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996; 348:771–775.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 2003; 92:824–826.
- Pinto DS, Kirtane AJ, Nallamothu BK, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006; 114:2019–2025.
- Boersma E; Primary Coronary Angioplasty vs Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006; 27:779–788.
- Stenestrand U, Wallentin L; Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med 2003; 163:965–971.
- Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343:311–322.
- Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993; 328:673–679.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab RE-teplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FAST-MI). Circulation 2008; 118:268–276.
- Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006; 367:569–578.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Carver A, Rafelt S, Gershlick AH, Fairbrother KL, Hughes S, Wilcox R; REACT Investigators. Longer-term follow-up of patients recruited to the REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial. J Am Coll Cardiol 2009; 54:118–126.
- Rasmanis G, Vesterqvist O, Gréen K, Edhag O, Henriksson P. Evidence of increased platelet activation after thrombolysis in patients with acute myocardial infarction. Br Heart J 1992; 68:374–376.
- Gurbel PA, Serebruany VL, Shustov AR, et al. Effects of reteplase and alteplase on platelet aggregation and major receptor expression during the first 24 hours of acute myocardial infarction treatment. GUSTO-III Investigators. Global Use of Strategies to Open Occluded Coronary Arteries. J Am Coll Cardiol 1998; 31:1466–1473.
- Coulter SA, Cannon CP, Ault KA, et al. High levels of platelet inhibition with abciximab despite heightened platelet activation and aggregation during thrombolysis for acute myocardial infarction: results from TIMI (thrombolysis in myocardial infarction) 14. Circulation 2000; 101:2690–2695.
- Ross AM, Huber K, Zeymer U, et al. The impact of place of enrollment and delay to reperfusion on 90-day post-infarction mortality in the ASSENT-4 PCI trial: assessment of the safety and efficacy of a new treatment strategy with percutaneous coronary intervention. JACC Cardiovasc Interv 2009; 2:925–930.
- Herrmann HC, Lu J, Brodie BR, et al; FINESSE Investigators. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv 2009; 2:917–924.
- Scheller B, Hennen B, Hammer B, et al; SIAM III Study Group. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol 2003; 42:634–641.
- Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al; GRACIA (Grupo de Análisis de la Cardiopatía Isquémica Aguda) Group. Routine invasive strategy within 24 hours of thrombolysis versus ischaemiaguided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet 2004; 364:1045–1053.
- Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study). J Am Coll Cardiol 2005; 46:417–424.
- Bøhmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010; 55:102–110.
- Wijeysundera HC, You JJ, Nallamothu BK, Krumholz HM, Cantor WJ, Ko DT. An early invasive strategy versus ischemia-guided management after fibrinolytic therapy for ST-segment elevation myocardial infarction: a meta-analysis of contemporary randomized controlled trials. Am Heart J 2008; 156:564–572,572.e1–e2.
- Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009; 54:2205–2241.
- Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008; 29:2909–2945.
- Mukherjee D, Moliterno DJ. The timely coupling of mechanical revascularization following thrombolysis for myocardial infarction. Cardiology 2007; 107:337–339.
- Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 2007; 49:422–430.
- The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329:1615–1622.
- Andersen HR, Nielsen TT, Rasmussen K, et al; DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349:733–742.
- Henry TD, Sharkey SW, Burke MN, et al. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007; 116:721–728.
- Denktas AE, Athar H, Henry TD, et al. Reduced-dose fibrinolytic acceleration of ST-segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv 2008; 1:504–510.
- Smalling RW. Ischemic time: the new gold standard for ST-segment elevation myocardial infarction care. J Am Coll Cardiol 2009; 54:2154–2156.
- Hochman JS, Sleeper LA, White HD, et al; SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. One-year survival following early revascularization for cardiogenic shock. JAMA 2001; 285:190–192.
- Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 2003; 41:1273–1279.
- Pedersen SH, Galatius S, Hansen PR, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009; 54:2296–2302.
- Björklund E, Stenestrand U, Lindbäck J, Svensson L, Wallentin L, Lindahl B. Pre-hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance-transported real-life patients with ST-elevation myocardial infarction. Eur Heart J 2006; 27:1146–1152.
- The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993; 329:383–389.
Effective and rapid reperfusion is crucial in patients with acute ST-segment elevation myocardial infarction (MI). The preferred strategy for reperfusion—when it can be performed in a timely fashion at an experienced facility—is primary percutaneous coronary intervention (PCI), which produces outcomes superior to those of pharmacologic thrombolysis.1
Unfortunately, in the United States about half of patients present to hospitals that do not have PCI capability,2 and in one analysis, 91% of transferred patients had a door-to-balloon time greater than the recommended 90 minutes, with a mean of 152 minutes.3 (In this case, the door-to-balloon time was the time that elapsed between entry into the first hospital and inflation of the PCI balloon at the second hospital.)
In situations such as these, a combined approach may be appropriate, with thrombolysis delivered by paramedics or at a local facility, followed by transfer to a PCI facility and performance of PCI within a few hours. However, this is feasible only if standardized community-based or regional protocols for prompt transfer and reperfusion are in place.
In this paper we discuss the rationale and the clinical data behind several approaches to combined reperfusion, as well as experiences with community-based care protocols.
WITHIN 3 HOURS OF SYMPTOM ONSET, THROMBOLYSIS IS AS GOOD AS PCI
The PRAGUE-2 Trial
In the randomized PRAGUE-2 trial,4 patients with ST-elevation MI who presented to a non-PCI facility had better outcomes if they were transferred promptly for PCI (median door-to-balloon time 97 minutes), as opposed to receiving local therapy with streptokinase. However, for patients presenting within 3 hours of symptom onset, the mortality rates were comparable with either strategy.4
See the glossary of clinical trial names below
The CAPTIM trial
In the CAPTIM trial,5 patients who presented within 2 hours of symptom onset and who were randomized to receive prehospital thrombolysis had outcomes similar to those of patients treated with primary PCI, despite a short door-to-balloon time (82 minutes).
The Vienna STEMI Registry
In the Vienna STEMI Registry,6 the mortality rates with primary PCI and with thrombolysis were similar when patients presented within 2 hours of symptom onset. However, as the time from symptom onset increased, primary PCI appeared to offer an increasing survival benefit compared with thrombolysis.
Comments: Thrombolysis is effective mostly in the first 2 to 3 hours, with some benefit up to 12 hours
Previous studies have shown that the sooner thrombolysis is given after symptom onset, the more effective it is. If it is given within an hour of symptom onset, the relative reduction in the mortality rate is 50% and the absolute reduction is 6.5% compared with no reperfusion therapy. If it is started in the second hour, the absolute reduction in the mortality rate drops to 4%, and a lesser benefit extends to patients presenting up to 12 hours after symptom onset.7 This time-dependent benefit is due to the fact that very early reperfusion of the occluded coronary artery may lead to full recovery of ischemic tissue and thus prevent necrosis. In addition, thrombolysis in the first 2 hours is highly efficacious in lysing a fresh thrombus.
These data support the current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA), which state no preference for either thrombolytic therapy or PCI in ST-elevation MI if the presentation is less than 3 hours after symptom onset.8
Of note, in the CAPTIM trial and in the Vienna STEMI Registry, rescue PCI was available and was in fact used after thrombolysis in about 25% of patients, which might have contributed to the benefit of early thrombolysis.

PRIMARY PCI MAY NOT BE SUPERIOR IF TRANSFER TIME IS LONG
Another time-related factor to consider is the PCI-related delay, ie, the theoretical difference between the expected time from first medical contact to balloon inflation (if the patient undergoes primary PCI) and the time from first medical contact to the start of thrombolytic therapy (if the patient undergoes primary thrombolysis).
A meta-analysis of 13 trials comparing PCI and thrombolysis showed that a PCI-related delay of more than 60 minutes might negate the potential advantage of primary PCI over immediate thrombolysis in terms of deaths.9
This observation has been further refined by data from the National Registry of Myocardial Infarction.10 In this analysis, patient factors, including age, duration of symptoms, and infarct location, significantly affected the point at which the PCI-related delay negated the survival advantage of primary PCI. The survival advantage of primary PCI was lost more rapidly—with a PCI-related delay as short as 40 minutes—in patients who presented sooner, were younger, or had anterior MI. Primary PCI maintained its survival advantage even with a PCI-related delay longer than 100 minutes in older patients or patients with nonanterior MI presenting more than 3 hours after symptom onset. Given that median door-to-balloon times in the United States may exceed 150 minutes when transfer is involved, 3 primary PCI may be no better than primary thrombolysis in transferred patients who present early or who have large infarcts.
Although these results were derived from a post hoc analysis of a registry and the delay times reported were sometimes inaccurate, they suggest that both the PCI-related delay time and patient characteristics should be considered when selecting a reperfusion strategy. Thrombolytic therapy before and in conjunction with primary PCI was considered a potential solution to these concerns.
In addition, while the benefit of any reperfusion strategy depends on the time of presentation, the loss in benefit by later presentation is less pronounced with primary PCI than with thrombolysis, making thrombolysis less attractive in later presentations (> 3 hours).11
Also, while thrombolytic therapy in patients older than 75 years was associated with a lower mortality rate compared with no therapy in a large Swedish registry,12 this benefit was less striking than in younger patients. A meta-analysis of thrombolysis trials failed to show a similar benefit in patients over age 75 vs younger patients,13 whereas primary PCI remained effective and superior to thrombolysis in the elderly, with more absolute reduction in mortality rates in the elderly subgroup than with younger patients. 14 This makes thrombolysis less attractive in the elderly, either as a stand-alone therapy or in conjunction with PCI. Studies of combined thrombolysis and PCI included very few patients over age 75.15–17
THREE COMBINATION REPERFUSION STRATEGIES
Facilitated PCI is a strategy of thrombolysis immediately followed by PCI, with a planned door-to-balloon time of 90 to 120 minutes.
Pharmacoinvasive therapy means giving thrombolysis at a non-PCI facility and then promptly and systematically transferring the patient to a PCI facility, where PCI is performed 2 to 24 hours after the start of thrombolytic therapy, regardless of whether thrombolysis results in successful reperfusion. 15 Thus, the time to PCI is longer than with facilitated PCI. Facilitated PCI addresses the value of pretreatment with thrombolytics or glycoprotein IIb/IIIa inhibitors in patients otherwise eligible for primary PCI, whereas pharmacoinvasive therapy addresses the value of routine early PCI after thrombolysis in patients who are not eligible for primary PCI.16
Rescue PCI refers to PCI that is performed urgently if thrombolysis fails, failure being defined as persistent hemodynamic or electrical instability, persistent ischemic symptoms, or failure to achieve at least a 50% to 70% resolution of the maximal ST-segment elevation 90 minutes after the infusion is started.
FACILITATED PCI: NEGATIVE RESULTS IN CLINICAL TRIALS
ASSENT-4 PCI trial
In the ASSENT-4 PCI trial,18 patients receiving full thrombolytic therapy before PCI had a higher rate of in-hospital death, bleeding, and cardiovascular events at 90 days than patients treated with primary PCI.
This trial recruited patients arriving at hospitals with or without PCI capability. The door-to-balloon time was about 110 minutes in both groups, which might not have been prolonged enough to show a benefit from a timely addition of thrombolysis. In addition, antiplatelet therapy was limited in these patients: glycoprotein IIb/IIIa inhibitors were not given, and clopidogrel (Plavix) was not appropriately preloaded, and this might have offset the potential benefit of early PCI. In fact, data suggest that platelet activation and aggregation are heightened after thrombolysis, 21–23 and that glycoprotein IIb/IIIa antagonists can inhibit these effects.23
The FINESSE trial
In the FINESSE trial,19 patients were randomized to undergo primary PCI, to undergo PCI facilitated (ie, preceded) by abciximab (Reo-Pro), or to undergo PCI facilitated by half-dose reteplase (Retavase) and full-dose abciximab. Despite a median door-to-balloon time of 132 minutes, the three strategies were associated with similar rates of death, heart failure, or ischemic outcome at 90 days. Even though the dosage of heparin was weight-adjusted, more major bleeding events occurred with the facilitated strategies.
Comments: Some subgroups may still benefit from facilitated PCI
The results of ASSENT-4 PCI and FINESSE led to the conclusion that PCI facilitated by full-dose thrombolysis should be avoided, and called into question the value of PCI facilitation using glycoprotein IIb/IIIa inhibitors with or without half-dose thrombolytic therapy.
However, subgroup analyses of these trials identified some subgroups that may benefit from a facilitated strategy. In ASSENT-4 PCI, 45% of patients were enrolled at PCI hospitals with a minimal PCI-related delay time. These patients had the worst outcome with the facilitated strategy. In contrast, patients who had a short time from pain onset to thrombolysis (2 to 3 hours) and who were given prehospital thrombolysis had a trend toward better outcomes with facilitated PCI.24 And in FINESSE, 60% of patients were enrolled at centers with PCI capability. Analysis of a small subgroup of patients with a Thrombolysis in Myocardial Infarction study (TIMI) risk score of 3 or greater presenting to non-PCI hospitals within 4 hours of symptom onset suggested a potential reduction of ischemic events with the facilitated strategy in these patients.25
Thus, for patients seen in the first 2 to 3 hours after symptom onset, immediate thrombolysis is recommended if PCI will likely be delayed, with or without plans for subsequent early PCI. “Time is muscle,” especially during the first 3 hours.
PHARMACOINVASIVE STRATEGY: GOOD RESULTS IN HIGH-RISK PATIENTS
A number of randomized studies during the last 10 years have examined the value of a pharmacoinvasive strategy.15,16,26–29
The TRANSFER-AMI trial
The TRANSFER-AMI trial15 randomized 1,059 patients with high-risk ST-elevation MI (ie, anterior or high-risk inferior) at non-PCI centers to undergo either pharmacoinvasive care, ie, full-dose tenecteplase (TNKase) with immediate transfer for PCI or standard care, ie, tenecteplase with transfer for rescue PCI if the patient had persistent ST-segment elevation, chest pain, or hemodynamic instability.15 The goal was to perform PCI within 6 hours of thrombolysis, and the median time to PCI was 3.9 hours (range 2–6 hours). In the standard-care group, 35% of patients needed to be transferred for rescue PCI. Unlike in the ASSENT-4 trial, over 80% of patients received aggressive antiplatelet therapy with both 300 mg of clopidogrel and glycoprotein IIb/IIIa inhibitors.
The rate of cardiovascular events at 30 days was significantly lower with pharmacoinvasive therapy than with standard care and rescue PCI (11% vs 17%, P = .004). This difference was driven by lower rates of recurrent ischemia, reinfarction, and heart failure.
The CARESS-in-AMI study
The CARESS-in-AMI study16 found a similar improvement in ischemic outcomes in 600 patients with high-risk ST-elevation MI arriving at non-PCI centers if they had received pharmacoinvasive therapy. Patients received half-dose reteplase and abciximab and were randomized either to be immediately transferred for PCI (median time to PCI 2.25 hours) or to be transferred only if they had persistent ST-segment elevation or clinical deterioration.16 The event rate was low with pharmacoinvasive therapy, comparable to that achieved in primary PCI trials.
Interestingly, no significant increase was seen in the risk of major and minor bleeding in these two trials despite the use of a femoral approach for PCI in over 80% of the cases; this is probably due to the delays between thrombolytic administration and PCI and to the use of a highly fibrin-specific thrombolytic agent and adjusted-dose heparin.
Meta-analysis of pharmacoinvasive trials
A meta-analysis29 of studies of systematic early PCI (mainly with stenting) within 24 hours of thrombolysis showed a reduction in the rates of mortality and reinfarction with this strategy, without an increase in the risk of major or intracranial bleeding.30 In contrast to the results of the trials of facilitated PCI, a pharmacoinvasive strategy improved outcomes in these trials because the delay between thrombolysis and PCI was more than 2 hours, ie, long enough to prevent bleeding complications, and because most patients randomized in these trials presented within 2 to 3 hours of symptom onset, when the time to reperfusion is critical. After 3 hours, the PCI-mediated myocardial salvage is less time-dependent. Moreover, trials of pharmacoinvasive strategy used aggressive antiplatelet therapy with clopidogrel and glycoprotein IIb/IIIa inhibitors.
Comment: Pharmacoinvasive strategy in the guidelines
These results and those of the subgroup analysis from the FINESSE trial suggest that patients with high-risk ST-elevation MI treated at non-PCI hospitals have better outcomes without an increase in major bleeding events when given thrombolysis and then immediately transferred for routine PCI, rather than being transferred only if reperfusion fails.
Hence, the 2009 update of the ACC/AHA guidelines31 gives a class IIa recommendation for transferring patients with anterior ST-elevation MI or high-risk inferior ST-elevation MI treated with thrombolysis to a PCI-capable facility where PCI is performed as part of a pharmacoinvasive or rescue strategy soon after thrombolysis.
This strategy has been particularly studied in patients younger than 75 years presenting with high-risk types of ST-elevation MI early (< 3 hours) after symptom onset. If not at high risk, the patient may be transferred to a PCI facility after receiving thrombolysis or observed in the initial facility (class IIb recommendation). Consideration should be given to starting anticoagulant and antiplatelet therapy before and during transfer—ie, 300 mg of clopidogrel before transfer for PCI and glycoprotein IIb/IIIa inhibitor therapy during PCI.
The European Society of Cardiology (ESC) guidelines32 recommend early routine angiography 3 to 24 hours after successful thrombolysis. This time window was selected to avoid PCI during the prothrombotic period in the first few hours after thrombolysis and to minimize the risk of reocclusion with PCI delays of more than 24 hours (class IIa recommendation).
Larger randomized trials are still needed to establish whether the pharmacoinvasive strategy confers a survival benefit, to determine its usefulness in low-risk inferior or lateral ST-elevation MI, and to further refine the time window when PCI is both safe and beneficial after thrombolysis.33
RESCUE PCI REDUCES MORTALITY RATES
Rescue PCI is the most accepted form of thrombolysis-PCI combination.
The REACT trial
The REACT trial20 showed that rescue PCI performed at a mean of 4.5 hours after failed thrombolysis reduces the rate of adverse cardiovascular events by more than 50% at 6 to 12 months and reduces the 5-year mortality rate by more than 50% compared with conservative management.20 As in the pharmacoinvasive strategy, aggressive antiplatelet regimens were used in the REACT trial.
A meta-analysis of rescue PCI trials
A meta-analysis of rescue PCI trials34 confirmed these results, showing a reduction in heart failure and reinfarction and a trend toward a lower mortality rate with rescue PCI.34 After thrombolysis, 40% of patients do not achieve grade 3 TIMI flow, which explains why in modern clinical trials 30% of patients treated with thrombolysis require rescue PCI.5,15,16,35
For patients with high-risk ST-elevation MI, current ACC/AHA guidelines assign a class IIa recommendation to rescue PCI.31
WHEN PATIENTS WITH ST-ELEVATION MI PRESENT TO A NON-PCI HOSPITAL
Transfer for primary PCI vs thrombolysis at the non-PCI hospital
The DANAMI-2 trial36 found that immediate transfer for PCI was superior to onsite thrombolytic therapy, as measured by a reduction in the rate of ischemic events (composite of death, myocardial infarction, or stroke at 30 days): 8.5% vs 14.2% (P < .001). There were no deaths during transfer.3
The PRAGUE-2 trial4 showed similar results for patients presenting 3 to 12 hours after symptom onset (30-day mortality rate 6% with immediate transfer vs 15.3% with on-site thrombolysis, P < .002), whereas patients presenting within 3 hours of symptom onset had a similar mortality rate with either therapy.4
Comment. These trials showed that transfer for primary PCI is superior to thrombolytic therapy when performed in a timely fashion. However, they were done in countries with established transfer networks and short distances between community hospitals and PCI centers, with a PCI-related delay of only 44 minutes and a door-to-balloon time of 90 minutes despite transfer. The large-scale application of this prompt transfer policy is not practical in most regions in the United States. Thus, a strategy of local thrombolysis followed by routine early transfer for routine or rescue PCI seems warranted when the door-to-balloon time or the PCI-related delay time is expected to be too long.
Experiences with community-based systems of care and prehospital thrombolysis
In Minnesota, Henry et al37 developed a PCI-based treatment system and an integrated transfer program for ST-elevation MI involving 30 hospitals within 210 miles of the Minneapolis Heart Institute. Participating hospitals were divided into two zones: zone 1 hospitals were within 60 miles, and zone 2 facilities were between 60 and 210 miles from the Heart Institute. Zone 2 patients received half-dose tenecteplase (if thrombolytic therapy was not contraindicated) in anticipation of a lengthy transfer time.
The median door-to-balloon time for zone 1 patients was 95 minutes (interquartile range 82 and 116 minutes) and for zone 2 patients 120 minutes (interquartile range 100 and 145 minutes). The diagnosis of ST-elevation MI was made by the emergency department physician, who activated the system with a phone call. The patient was then directly transferred to the catheterization laboratory, most often by helicopter.
The in-hospital death rate for patients who presented to the PCI center and for patients in zones 1 and 2 was similarly low (about 5%).37
In France, the FAST-MI registry,17 which collected outcome data for different reperfusion strategies, found that thrombolysis yielded in-hospital and midterm results that were comparable to those of primary PCI. Of note, thrombolysis was started early after symptom onset (about 2 hours), and was started in the ambulance in two-thirds of cases. Nearly all patients underwent a pharmacoinvasive strategy that combined thrombolysis with coronary angiography and PCI within 24 hours of symptom onset. These findings suggest that timely thrombolysis followed by semiurgent transfer for PCI is an alternative to primary PCI for patients presenting to hospitals with no PCI capability, and that this alternative offers similar benefit to that of primary PCI.
Five centers in the United States have reported their experience with half-dose thrombolysis in the prehospital setting (in the field or during transfer) or at a non-PCI hospital, followed by prompt transfer to a PCI facility. In this registry of almost 3,000 patients,38 patients treated with thrombolysis had better outcomes than patients directly transferred for primary PCI, with a significantly lower 30-day mortality rate (3.8% vs from 6.4%), and no increase in bleeding.38,39 The mean door-to-balloon time was long (168 minutes in the primary PCI group and 196 minutes in the thrombolysis-PCI group), which might explain the benefit achieved with prompt thrombolysis.
CARDIOGENIC SHOCK
Patients presenting with left ventricular cardiogenic shock derive a large mortality benefit from revascularization, whether they are transferred or directly admitted to a PCI center. 40 Moreover, in the SHOCK registry, patients with predominant right ventricular cardiogenic shock had an in-hospital mortality rate similar to that of patients with predominant left ventricular cardiogenic shock, and revascularization (PCI or surgical revascularization) was associated with a strikingly lower mortality rate in both groups.41
Thus, all patients with left or right cardiogenic shock should be revascularized on an emergency basis, either surgically or percutaneously.
While trials of pharmacoinvasive therapy excluded patients with cardiogenic shock,15,16 thrombolytic therapy was associated with improved outcomes in the drug-therapy group of the SHOCK trial and in hypotensive patients randomized in the early thrombolysis trials.13 Thus, the ACC/AHA guidelines recommend thrombolytic therapy before transfer if a patient presents in shock within 3 to 6 hours of onset of the MI and delays in transport and intervention are anticipated.8
PUTTING IT ALL TOGETHER: MANAGEMENT STRATEGIES
If an effective transfer system is in place, primary PCI not preceded by thrombolytic therapy or glycoprotein IIb/IIIa inhibitor therapy is the preferred approach, according to ACC/AHA and ESC guidelines.31,32 Giving thrombolytics immediately before PCI is harmful and thus should be avoided when the expected door-to-balloon time is 90 minutes or less.
All hospitals (whether or not they offer PCI) and regional emergency medical services should participate in a community-based system of care for ST-elevation MI, with protocols for expeditious transfer as defined and coordinated by the American Heart Association initiative “Mission: Lifeline.” In addition, a system of field triage and direct transport to the catheterization laboratory of a PCI facility after field activation significantly reduces door-to-balloon times and improves outcomes.42
If such a system is not in place, then a pharmacoinvasive strategy seems best: ie, local full-dose thrombolysis (if not contraindicated) followed by transfer to a PCI facility and routine performance of PCI 2 to 6 hours after thrombolysis—in conjunction with aggressive early dual oral antiplatelet therapy and “downstream” glycoprotein IIb/IIIa inhibition. This approach is associated with outcomes similar to those of primary PCI.15–17,37
Prehospital thrombolysis delivered by paramedics and followed by early transfer to a PCI facility has been associated with further reduction in mortality rates compared with in-hospital thrombolysis (as in the Swedish registry43), and a reduction in death rate comparable to that of primary PCI in patients presenting early. This is an adequate strategy in regions where such a system can be established.5,17,38,43,44
Patients presenting more than 3 to 4 hours after symptom onset, older patients, and patients with lower-risk MI or a higher risk of bleeding may still be suited for primary PCI even when the door-to-balloon time is 90 to 120 minutes, as stated by the European guidelines,32 or when the PCI-related delay time is as long as 100 minutes. 10 On the other hand, while the ACC/AHA guidelines recognize that in these patients the mortality advantage of primary PCI vs thrombolytic therapy is maintained with more prolonged door-to-balloon times, they nevertheless state that the focus should be on developing systems of care to increase the number of patients with access to primary PCI in less than 90 minutes rather than extending the acceptable window for door-to-balloon time.
In conclusion, for patients presenting with ST-elevation MI who cannot undergo timely primary PCI, the best approach seems to be prehospital thrombolysis delivered by paramedics or local thrombolysis at the non-PCI hospital followed by transferring the patient and performing PCI within a few hours. This is especially important in patients with high-risk ST-elevation MI who present early after symptom onset, when the extent of myocardial necrosis associated with delayed primary PCI is largest.
In addition, every community should develop a coordinated transfer strategy between non-PCI and PCI hospitals.
Effective and rapid reperfusion is crucial in patients with acute ST-segment elevation myocardial infarction (MI). The preferred strategy for reperfusion—when it can be performed in a timely fashion at an experienced facility—is primary percutaneous coronary intervention (PCI), which produces outcomes superior to those of pharmacologic thrombolysis.1
Unfortunately, in the United States about half of patients present to hospitals that do not have PCI capability,2 and in one analysis, 91% of transferred patients had a door-to-balloon time greater than the recommended 90 minutes, with a mean of 152 minutes.3 (In this case, the door-to-balloon time was the time that elapsed between entry into the first hospital and inflation of the PCI balloon at the second hospital.)
In situations such as these, a combined approach may be appropriate, with thrombolysis delivered by paramedics or at a local facility, followed by transfer to a PCI facility and performance of PCI within a few hours. However, this is feasible only if standardized community-based or regional protocols for prompt transfer and reperfusion are in place.
In this paper we discuss the rationale and the clinical data behind several approaches to combined reperfusion, as well as experiences with community-based care protocols.
WITHIN 3 HOURS OF SYMPTOM ONSET, THROMBOLYSIS IS AS GOOD AS PCI
The PRAGUE-2 Trial
In the randomized PRAGUE-2 trial,4 patients with ST-elevation MI who presented to a non-PCI facility had better outcomes if they were transferred promptly for PCI (median door-to-balloon time 97 minutes), as opposed to receiving local therapy with streptokinase. However, for patients presenting within 3 hours of symptom onset, the mortality rates were comparable with either strategy.4
See the glossary of clinical trial names below
The CAPTIM trial
In the CAPTIM trial,5 patients who presented within 2 hours of symptom onset and who were randomized to receive prehospital thrombolysis had outcomes similar to those of patients treated with primary PCI, despite a short door-to-balloon time (82 minutes).
The Vienna STEMI Registry
In the Vienna STEMI Registry,6 the mortality rates with primary PCI and with thrombolysis were similar when patients presented within 2 hours of symptom onset. However, as the time from symptom onset increased, primary PCI appeared to offer an increasing survival benefit compared with thrombolysis.
Comments: Thrombolysis is effective mostly in the first 2 to 3 hours, with some benefit up to 12 hours
Previous studies have shown that the sooner thrombolysis is given after symptom onset, the more effective it is. If it is given within an hour of symptom onset, the relative reduction in the mortality rate is 50% and the absolute reduction is 6.5% compared with no reperfusion therapy. If it is started in the second hour, the absolute reduction in the mortality rate drops to 4%, and a lesser benefit extends to patients presenting up to 12 hours after symptom onset.7 This time-dependent benefit is due to the fact that very early reperfusion of the occluded coronary artery may lead to full recovery of ischemic tissue and thus prevent necrosis. In addition, thrombolysis in the first 2 hours is highly efficacious in lysing a fresh thrombus.
These data support the current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA), which state no preference for either thrombolytic therapy or PCI in ST-elevation MI if the presentation is less than 3 hours after symptom onset.8
Of note, in the CAPTIM trial and in the Vienna STEMI Registry, rescue PCI was available and was in fact used after thrombolysis in about 25% of patients, which might have contributed to the benefit of early thrombolysis.

PRIMARY PCI MAY NOT BE SUPERIOR IF TRANSFER TIME IS LONG
Another time-related factor to consider is the PCI-related delay, ie, the theoretical difference between the expected time from first medical contact to balloon inflation (if the patient undergoes primary PCI) and the time from first medical contact to the start of thrombolytic therapy (if the patient undergoes primary thrombolysis).
A meta-analysis of 13 trials comparing PCI and thrombolysis showed that a PCI-related delay of more than 60 minutes might negate the potential advantage of primary PCI over immediate thrombolysis in terms of deaths.9
This observation has been further refined by data from the National Registry of Myocardial Infarction.10 In this analysis, patient factors, including age, duration of symptoms, and infarct location, significantly affected the point at which the PCI-related delay negated the survival advantage of primary PCI. The survival advantage of primary PCI was lost more rapidly—with a PCI-related delay as short as 40 minutes—in patients who presented sooner, were younger, or had anterior MI. Primary PCI maintained its survival advantage even with a PCI-related delay longer than 100 minutes in older patients or patients with nonanterior MI presenting more than 3 hours after symptom onset. Given that median door-to-balloon times in the United States may exceed 150 minutes when transfer is involved, 3 primary PCI may be no better than primary thrombolysis in transferred patients who present early or who have large infarcts.
Although these results were derived from a post hoc analysis of a registry and the delay times reported were sometimes inaccurate, they suggest that both the PCI-related delay time and patient characteristics should be considered when selecting a reperfusion strategy. Thrombolytic therapy before and in conjunction with primary PCI was considered a potential solution to these concerns.
In addition, while the benefit of any reperfusion strategy depends on the time of presentation, the loss in benefit by later presentation is less pronounced with primary PCI than with thrombolysis, making thrombolysis less attractive in later presentations (> 3 hours).11
Also, while thrombolytic therapy in patients older than 75 years was associated with a lower mortality rate compared with no therapy in a large Swedish registry,12 this benefit was less striking than in younger patients. A meta-analysis of thrombolysis trials failed to show a similar benefit in patients over age 75 vs younger patients,13 whereas primary PCI remained effective and superior to thrombolysis in the elderly, with more absolute reduction in mortality rates in the elderly subgroup than with younger patients. 14 This makes thrombolysis less attractive in the elderly, either as a stand-alone therapy or in conjunction with PCI. Studies of combined thrombolysis and PCI included very few patients over age 75.15–17
THREE COMBINATION REPERFUSION STRATEGIES
Facilitated PCI is a strategy of thrombolysis immediately followed by PCI, with a planned door-to-balloon time of 90 to 120 minutes.
Pharmacoinvasive therapy means giving thrombolysis at a non-PCI facility and then promptly and systematically transferring the patient to a PCI facility, where PCI is performed 2 to 24 hours after the start of thrombolytic therapy, regardless of whether thrombolysis results in successful reperfusion. 15 Thus, the time to PCI is longer than with facilitated PCI. Facilitated PCI addresses the value of pretreatment with thrombolytics or glycoprotein IIb/IIIa inhibitors in patients otherwise eligible for primary PCI, whereas pharmacoinvasive therapy addresses the value of routine early PCI after thrombolysis in patients who are not eligible for primary PCI.16
Rescue PCI refers to PCI that is performed urgently if thrombolysis fails, failure being defined as persistent hemodynamic or electrical instability, persistent ischemic symptoms, or failure to achieve at least a 50% to 70% resolution of the maximal ST-segment elevation 90 minutes after the infusion is started.
FACILITATED PCI: NEGATIVE RESULTS IN CLINICAL TRIALS
ASSENT-4 PCI trial
In the ASSENT-4 PCI trial,18 patients receiving full thrombolytic therapy before PCI had a higher rate of in-hospital death, bleeding, and cardiovascular events at 90 days than patients treated with primary PCI.
This trial recruited patients arriving at hospitals with or without PCI capability. The door-to-balloon time was about 110 minutes in both groups, which might not have been prolonged enough to show a benefit from a timely addition of thrombolysis. In addition, antiplatelet therapy was limited in these patients: glycoprotein IIb/IIIa inhibitors were not given, and clopidogrel (Plavix) was not appropriately preloaded, and this might have offset the potential benefit of early PCI. In fact, data suggest that platelet activation and aggregation are heightened after thrombolysis, 21–23 and that glycoprotein IIb/IIIa antagonists can inhibit these effects.23
The FINESSE trial
In the FINESSE trial,19 patients were randomized to undergo primary PCI, to undergo PCI facilitated (ie, preceded) by abciximab (Reo-Pro), or to undergo PCI facilitated by half-dose reteplase (Retavase) and full-dose abciximab. Despite a median door-to-balloon time of 132 minutes, the three strategies were associated with similar rates of death, heart failure, or ischemic outcome at 90 days. Even though the dosage of heparin was weight-adjusted, more major bleeding events occurred with the facilitated strategies.
Comments: Some subgroups may still benefit from facilitated PCI
The results of ASSENT-4 PCI and FINESSE led to the conclusion that PCI facilitated by full-dose thrombolysis should be avoided, and called into question the value of PCI facilitation using glycoprotein IIb/IIIa inhibitors with or without half-dose thrombolytic therapy.
However, subgroup analyses of these trials identified some subgroups that may benefit from a facilitated strategy. In ASSENT-4 PCI, 45% of patients were enrolled at PCI hospitals with a minimal PCI-related delay time. These patients had the worst outcome with the facilitated strategy. In contrast, patients who had a short time from pain onset to thrombolysis (2 to 3 hours) and who were given prehospital thrombolysis had a trend toward better outcomes with facilitated PCI.24 And in FINESSE, 60% of patients were enrolled at centers with PCI capability. Analysis of a small subgroup of patients with a Thrombolysis in Myocardial Infarction study (TIMI) risk score of 3 or greater presenting to non-PCI hospitals within 4 hours of symptom onset suggested a potential reduction of ischemic events with the facilitated strategy in these patients.25
Thus, for patients seen in the first 2 to 3 hours after symptom onset, immediate thrombolysis is recommended if PCI will likely be delayed, with or without plans for subsequent early PCI. “Time is muscle,” especially during the first 3 hours.
PHARMACOINVASIVE STRATEGY: GOOD RESULTS IN HIGH-RISK PATIENTS
A number of randomized studies during the last 10 years have examined the value of a pharmacoinvasive strategy.15,16,26–29
The TRANSFER-AMI trial
The TRANSFER-AMI trial15 randomized 1,059 patients with high-risk ST-elevation MI (ie, anterior or high-risk inferior) at non-PCI centers to undergo either pharmacoinvasive care, ie, full-dose tenecteplase (TNKase) with immediate transfer for PCI or standard care, ie, tenecteplase with transfer for rescue PCI if the patient had persistent ST-segment elevation, chest pain, or hemodynamic instability.15 The goal was to perform PCI within 6 hours of thrombolysis, and the median time to PCI was 3.9 hours (range 2–6 hours). In the standard-care group, 35% of patients needed to be transferred for rescue PCI. Unlike in the ASSENT-4 trial, over 80% of patients received aggressive antiplatelet therapy with both 300 mg of clopidogrel and glycoprotein IIb/IIIa inhibitors.
The rate of cardiovascular events at 30 days was significantly lower with pharmacoinvasive therapy than with standard care and rescue PCI (11% vs 17%, P = .004). This difference was driven by lower rates of recurrent ischemia, reinfarction, and heart failure.
The CARESS-in-AMI study
The CARESS-in-AMI study16 found a similar improvement in ischemic outcomes in 600 patients with high-risk ST-elevation MI arriving at non-PCI centers if they had received pharmacoinvasive therapy. Patients received half-dose reteplase and abciximab and were randomized either to be immediately transferred for PCI (median time to PCI 2.25 hours) or to be transferred only if they had persistent ST-segment elevation or clinical deterioration.16 The event rate was low with pharmacoinvasive therapy, comparable to that achieved in primary PCI trials.
Interestingly, no significant increase was seen in the risk of major and minor bleeding in these two trials despite the use of a femoral approach for PCI in over 80% of the cases; this is probably due to the delays between thrombolytic administration and PCI and to the use of a highly fibrin-specific thrombolytic agent and adjusted-dose heparin.
Meta-analysis of pharmacoinvasive trials
A meta-analysis29 of studies of systematic early PCI (mainly with stenting) within 24 hours of thrombolysis showed a reduction in the rates of mortality and reinfarction with this strategy, without an increase in the risk of major or intracranial bleeding.30 In contrast to the results of the trials of facilitated PCI, a pharmacoinvasive strategy improved outcomes in these trials because the delay between thrombolysis and PCI was more than 2 hours, ie, long enough to prevent bleeding complications, and because most patients randomized in these trials presented within 2 to 3 hours of symptom onset, when the time to reperfusion is critical. After 3 hours, the PCI-mediated myocardial salvage is less time-dependent. Moreover, trials of pharmacoinvasive strategy used aggressive antiplatelet therapy with clopidogrel and glycoprotein IIb/IIIa inhibitors.
Comment: Pharmacoinvasive strategy in the guidelines
These results and those of the subgroup analysis from the FINESSE trial suggest that patients with high-risk ST-elevation MI treated at non-PCI hospitals have better outcomes without an increase in major bleeding events when given thrombolysis and then immediately transferred for routine PCI, rather than being transferred only if reperfusion fails.
Hence, the 2009 update of the ACC/AHA guidelines31 gives a class IIa recommendation for transferring patients with anterior ST-elevation MI or high-risk inferior ST-elevation MI treated with thrombolysis to a PCI-capable facility where PCI is performed as part of a pharmacoinvasive or rescue strategy soon after thrombolysis.
This strategy has been particularly studied in patients younger than 75 years presenting with high-risk types of ST-elevation MI early (< 3 hours) after symptom onset. If not at high risk, the patient may be transferred to a PCI facility after receiving thrombolysis or observed in the initial facility (class IIb recommendation). Consideration should be given to starting anticoagulant and antiplatelet therapy before and during transfer—ie, 300 mg of clopidogrel before transfer for PCI and glycoprotein IIb/IIIa inhibitor therapy during PCI.
The European Society of Cardiology (ESC) guidelines32 recommend early routine angiography 3 to 24 hours after successful thrombolysis. This time window was selected to avoid PCI during the prothrombotic period in the first few hours after thrombolysis and to minimize the risk of reocclusion with PCI delays of more than 24 hours (class IIa recommendation).
Larger randomized trials are still needed to establish whether the pharmacoinvasive strategy confers a survival benefit, to determine its usefulness in low-risk inferior or lateral ST-elevation MI, and to further refine the time window when PCI is both safe and beneficial after thrombolysis.33
RESCUE PCI REDUCES MORTALITY RATES
Rescue PCI is the most accepted form of thrombolysis-PCI combination.
The REACT trial
The REACT trial20 showed that rescue PCI performed at a mean of 4.5 hours after failed thrombolysis reduces the rate of adverse cardiovascular events by more than 50% at 6 to 12 months and reduces the 5-year mortality rate by more than 50% compared with conservative management.20 As in the pharmacoinvasive strategy, aggressive antiplatelet regimens were used in the REACT trial.
A meta-analysis of rescue PCI trials
A meta-analysis of rescue PCI trials34 confirmed these results, showing a reduction in heart failure and reinfarction and a trend toward a lower mortality rate with rescue PCI.34 After thrombolysis, 40% of patients do not achieve grade 3 TIMI flow, which explains why in modern clinical trials 30% of patients treated with thrombolysis require rescue PCI.5,15,16,35
For patients with high-risk ST-elevation MI, current ACC/AHA guidelines assign a class IIa recommendation to rescue PCI.31
WHEN PATIENTS WITH ST-ELEVATION MI PRESENT TO A NON-PCI HOSPITAL
Transfer for primary PCI vs thrombolysis at the non-PCI hospital
The DANAMI-2 trial36 found that immediate transfer for PCI was superior to onsite thrombolytic therapy, as measured by a reduction in the rate of ischemic events (composite of death, myocardial infarction, or stroke at 30 days): 8.5% vs 14.2% (P < .001). There were no deaths during transfer.3
The PRAGUE-2 trial4 showed similar results for patients presenting 3 to 12 hours after symptom onset (30-day mortality rate 6% with immediate transfer vs 15.3% with on-site thrombolysis, P < .002), whereas patients presenting within 3 hours of symptom onset had a similar mortality rate with either therapy.4
Comment. These trials showed that transfer for primary PCI is superior to thrombolytic therapy when performed in a timely fashion. However, they were done in countries with established transfer networks and short distances between community hospitals and PCI centers, with a PCI-related delay of only 44 minutes and a door-to-balloon time of 90 minutes despite transfer. The large-scale application of this prompt transfer policy is not practical in most regions in the United States. Thus, a strategy of local thrombolysis followed by routine early transfer for routine or rescue PCI seems warranted when the door-to-balloon time or the PCI-related delay time is expected to be too long.
Experiences with community-based systems of care and prehospital thrombolysis
In Minnesota, Henry et al37 developed a PCI-based treatment system and an integrated transfer program for ST-elevation MI involving 30 hospitals within 210 miles of the Minneapolis Heart Institute. Participating hospitals were divided into two zones: zone 1 hospitals were within 60 miles, and zone 2 facilities were between 60 and 210 miles from the Heart Institute. Zone 2 patients received half-dose tenecteplase (if thrombolytic therapy was not contraindicated) in anticipation of a lengthy transfer time.
The median door-to-balloon time for zone 1 patients was 95 minutes (interquartile range 82 and 116 minutes) and for zone 2 patients 120 minutes (interquartile range 100 and 145 minutes). The diagnosis of ST-elevation MI was made by the emergency department physician, who activated the system with a phone call. The patient was then directly transferred to the catheterization laboratory, most often by helicopter.
The in-hospital death rate for patients who presented to the PCI center and for patients in zones 1 and 2 was similarly low (about 5%).37
In France, the FAST-MI registry,17 which collected outcome data for different reperfusion strategies, found that thrombolysis yielded in-hospital and midterm results that were comparable to those of primary PCI. Of note, thrombolysis was started early after symptom onset (about 2 hours), and was started in the ambulance in two-thirds of cases. Nearly all patients underwent a pharmacoinvasive strategy that combined thrombolysis with coronary angiography and PCI within 24 hours of symptom onset. These findings suggest that timely thrombolysis followed by semiurgent transfer for PCI is an alternative to primary PCI for patients presenting to hospitals with no PCI capability, and that this alternative offers similar benefit to that of primary PCI.
Five centers in the United States have reported their experience with half-dose thrombolysis in the prehospital setting (in the field or during transfer) or at a non-PCI hospital, followed by prompt transfer to a PCI facility. In this registry of almost 3,000 patients,38 patients treated with thrombolysis had better outcomes than patients directly transferred for primary PCI, with a significantly lower 30-day mortality rate (3.8% vs from 6.4%), and no increase in bleeding.38,39 The mean door-to-balloon time was long (168 minutes in the primary PCI group and 196 minutes in the thrombolysis-PCI group), which might explain the benefit achieved with prompt thrombolysis.
CARDIOGENIC SHOCK
Patients presenting with left ventricular cardiogenic shock derive a large mortality benefit from revascularization, whether they are transferred or directly admitted to a PCI center. 40 Moreover, in the SHOCK registry, patients with predominant right ventricular cardiogenic shock had an in-hospital mortality rate similar to that of patients with predominant left ventricular cardiogenic shock, and revascularization (PCI or surgical revascularization) was associated with a strikingly lower mortality rate in both groups.41
Thus, all patients with left or right cardiogenic shock should be revascularized on an emergency basis, either surgically or percutaneously.
While trials of pharmacoinvasive therapy excluded patients with cardiogenic shock,15,16 thrombolytic therapy was associated with improved outcomes in the drug-therapy group of the SHOCK trial and in hypotensive patients randomized in the early thrombolysis trials.13 Thus, the ACC/AHA guidelines recommend thrombolytic therapy before transfer if a patient presents in shock within 3 to 6 hours of onset of the MI and delays in transport and intervention are anticipated.8
PUTTING IT ALL TOGETHER: MANAGEMENT STRATEGIES
If an effective transfer system is in place, primary PCI not preceded by thrombolytic therapy or glycoprotein IIb/IIIa inhibitor therapy is the preferred approach, according to ACC/AHA and ESC guidelines.31,32 Giving thrombolytics immediately before PCI is harmful and thus should be avoided when the expected door-to-balloon time is 90 minutes or less.
All hospitals (whether or not they offer PCI) and regional emergency medical services should participate in a community-based system of care for ST-elevation MI, with protocols for expeditious transfer as defined and coordinated by the American Heart Association initiative “Mission: Lifeline.” In addition, a system of field triage and direct transport to the catheterization laboratory of a PCI facility after field activation significantly reduces door-to-balloon times and improves outcomes.42
If such a system is not in place, then a pharmacoinvasive strategy seems best: ie, local full-dose thrombolysis (if not contraindicated) followed by transfer to a PCI facility and routine performance of PCI 2 to 6 hours after thrombolysis—in conjunction with aggressive early dual oral antiplatelet therapy and “downstream” glycoprotein IIb/IIIa inhibition. This approach is associated with outcomes similar to those of primary PCI.15–17,37
Prehospital thrombolysis delivered by paramedics and followed by early transfer to a PCI facility has been associated with further reduction in mortality rates compared with in-hospital thrombolysis (as in the Swedish registry43), and a reduction in death rate comparable to that of primary PCI in patients presenting early. This is an adequate strategy in regions where such a system can be established.5,17,38,43,44
Patients presenting more than 3 to 4 hours after symptom onset, older patients, and patients with lower-risk MI or a higher risk of bleeding may still be suited for primary PCI even when the door-to-balloon time is 90 to 120 minutes, as stated by the European guidelines,32 or when the PCI-related delay time is as long as 100 minutes. 10 On the other hand, while the ACC/AHA guidelines recognize that in these patients the mortality advantage of primary PCI vs thrombolytic therapy is maintained with more prolonged door-to-balloon times, they nevertheless state that the focus should be on developing systems of care to increase the number of patients with access to primary PCI in less than 90 minutes rather than extending the acceptable window for door-to-balloon time.
In conclusion, for patients presenting with ST-elevation MI who cannot undergo timely primary PCI, the best approach seems to be prehospital thrombolysis delivered by paramedics or local thrombolysis at the non-PCI hospital followed by transferring the patient and performing PCI within a few hours. This is especially important in patients with high-risk ST-elevation MI who present early after symptom onset, when the extent of myocardial necrosis associated with delayed primary PCI is largest.
In addition, every community should develop a coordinated transfer strategy between non-PCI and PCI hospitals.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Waters RE, Singh KP, Roe MT, et al. Rationale and strategies for implementing community-based transfer protocols for primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004; 43:2153–2159.
- Chakrabarti A, Krumholz HM, Wang Y, Rumsfeld JS, Nallamothu BK; National Cardiovascular Data Registry. Time-to-reperfusion in patients undergoing interhospital transfer for primary percutaneous coronary intervention in the U.S: an analysis of 2005 and 2006 data from the National Cardiovascular Data Registry. J Am Coll Cardiol 2008; 51:2442–2443.
- Widimský P, Budesínský T, Vorác D, et al; ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J 2003; 24:94–104.
- Steg PG, Bonnefoy E, Chabaud S, et al; Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial infarction (CAPTIM) Investigators. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003; 108:2851–2856.
- Kalla K, Christ G, Karnik R, et al; Vienna STEMI Registry Group. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry). Circulation 2006; 113:2398–2405.
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996; 348:771–775.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 2003; 92:824–826.
- Pinto DS, Kirtane AJ, Nallamothu BK, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006; 114:2019–2025.
- Boersma E; Primary Coronary Angioplasty vs Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006; 27:779–788.
- Stenestrand U, Wallentin L; Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med 2003; 163:965–971.
- Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343:311–322.
- Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993; 328:673–679.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab RE-teplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FAST-MI). Circulation 2008; 118:268–276.
- Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006; 367:569–578.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Carver A, Rafelt S, Gershlick AH, Fairbrother KL, Hughes S, Wilcox R; REACT Investigators. Longer-term follow-up of patients recruited to the REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial. J Am Coll Cardiol 2009; 54:118–126.
- Rasmanis G, Vesterqvist O, Gréen K, Edhag O, Henriksson P. Evidence of increased platelet activation after thrombolysis in patients with acute myocardial infarction. Br Heart J 1992; 68:374–376.
- Gurbel PA, Serebruany VL, Shustov AR, et al. Effects of reteplase and alteplase on platelet aggregation and major receptor expression during the first 24 hours of acute myocardial infarction treatment. GUSTO-III Investigators. Global Use of Strategies to Open Occluded Coronary Arteries. J Am Coll Cardiol 1998; 31:1466–1473.
- Coulter SA, Cannon CP, Ault KA, et al. High levels of platelet inhibition with abciximab despite heightened platelet activation and aggregation during thrombolysis for acute myocardial infarction: results from TIMI (thrombolysis in myocardial infarction) 14. Circulation 2000; 101:2690–2695.
- Ross AM, Huber K, Zeymer U, et al. The impact of place of enrollment and delay to reperfusion on 90-day post-infarction mortality in the ASSENT-4 PCI trial: assessment of the safety and efficacy of a new treatment strategy with percutaneous coronary intervention. JACC Cardiovasc Interv 2009; 2:925–930.
- Herrmann HC, Lu J, Brodie BR, et al; FINESSE Investigators. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv 2009; 2:917–924.
- Scheller B, Hennen B, Hammer B, et al; SIAM III Study Group. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol 2003; 42:634–641.
- Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al; GRACIA (Grupo de Análisis de la Cardiopatía Isquémica Aguda) Group. Routine invasive strategy within 24 hours of thrombolysis versus ischaemiaguided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet 2004; 364:1045–1053.
- Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study). J Am Coll Cardiol 2005; 46:417–424.
- Bøhmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010; 55:102–110.
- Wijeysundera HC, You JJ, Nallamothu BK, Krumholz HM, Cantor WJ, Ko DT. An early invasive strategy versus ischemia-guided management after fibrinolytic therapy for ST-segment elevation myocardial infarction: a meta-analysis of contemporary randomized controlled trials. Am Heart J 2008; 156:564–572,572.e1–e2.
- Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009; 54:2205–2241.
- Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008; 29:2909–2945.
- Mukherjee D, Moliterno DJ. The timely coupling of mechanical revascularization following thrombolysis for myocardial infarction. Cardiology 2007; 107:337–339.
- Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 2007; 49:422–430.
- The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329:1615–1622.
- Andersen HR, Nielsen TT, Rasmussen K, et al; DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349:733–742.
- Henry TD, Sharkey SW, Burke MN, et al. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007; 116:721–728.
- Denktas AE, Athar H, Henry TD, et al. Reduced-dose fibrinolytic acceleration of ST-segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv 2008; 1:504–510.
- Smalling RW. Ischemic time: the new gold standard for ST-segment elevation myocardial infarction care. J Am Coll Cardiol 2009; 54:2154–2156.
- Hochman JS, Sleeper LA, White HD, et al; SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. One-year survival following early revascularization for cardiogenic shock. JAMA 2001; 285:190–192.
- Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 2003; 41:1273–1279.
- Pedersen SH, Galatius S, Hansen PR, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009; 54:2296–2302.
- Björklund E, Stenestrand U, Lindbäck J, Svensson L, Wallentin L, Lindahl B. Pre-hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance-transported real-life patients with ST-elevation myocardial infarction. Eur Heart J 2006; 27:1146–1152.
- The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993; 329:383–389.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Waters RE, Singh KP, Roe MT, et al. Rationale and strategies for implementing community-based transfer protocols for primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004; 43:2153–2159.
- Chakrabarti A, Krumholz HM, Wang Y, Rumsfeld JS, Nallamothu BK; National Cardiovascular Data Registry. Time-to-reperfusion in patients undergoing interhospital transfer for primary percutaneous coronary intervention in the U.S: an analysis of 2005 and 2006 data from the National Cardiovascular Data Registry. J Am Coll Cardiol 2008; 51:2442–2443.
- Widimský P, Budesínský T, Vorác D, et al; ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J 2003; 24:94–104.
- Steg PG, Bonnefoy E, Chabaud S, et al; Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial infarction (CAPTIM) Investigators. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003; 108:2851–2856.
- Kalla K, Christ G, Karnik R, et al; Vienna STEMI Registry Group. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry). Circulation 2006; 113:2398–2405.
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996; 348:771–775.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 2003; 92:824–826.
- Pinto DS, Kirtane AJ, Nallamothu BK, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006; 114:2019–2025.
- Boersma E; Primary Coronary Angioplasty vs Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006; 27:779–788.
- Stenestrand U, Wallentin L; Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med 2003; 163:965–971.
- Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343:311–322.
- Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993; 328:673–679.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab RE-teplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FAST-MI). Circulation 2008; 118:268–276.
- Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006; 367:569–578.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Carver A, Rafelt S, Gershlick AH, Fairbrother KL, Hughes S, Wilcox R; REACT Investigators. Longer-term follow-up of patients recruited to the REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial. J Am Coll Cardiol 2009; 54:118–126.
- Rasmanis G, Vesterqvist O, Gréen K, Edhag O, Henriksson P. Evidence of increased platelet activation after thrombolysis in patients with acute myocardial infarction. Br Heart J 1992; 68:374–376.
- Gurbel PA, Serebruany VL, Shustov AR, et al. Effects of reteplase and alteplase on platelet aggregation and major receptor expression during the first 24 hours of acute myocardial infarction treatment. GUSTO-III Investigators. Global Use of Strategies to Open Occluded Coronary Arteries. J Am Coll Cardiol 1998; 31:1466–1473.
- Coulter SA, Cannon CP, Ault KA, et al. High levels of platelet inhibition with abciximab despite heightened platelet activation and aggregation during thrombolysis for acute myocardial infarction: results from TIMI (thrombolysis in myocardial infarction) 14. Circulation 2000; 101:2690–2695.
- Ross AM, Huber K, Zeymer U, et al. The impact of place of enrollment and delay to reperfusion on 90-day post-infarction mortality in the ASSENT-4 PCI trial: assessment of the safety and efficacy of a new treatment strategy with percutaneous coronary intervention. JACC Cardiovasc Interv 2009; 2:925–930.
- Herrmann HC, Lu J, Brodie BR, et al; FINESSE Investigators. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv 2009; 2:917–924.
- Scheller B, Hennen B, Hammer B, et al; SIAM III Study Group. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol 2003; 42:634–641.
- Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al; GRACIA (Grupo de Análisis de la Cardiopatía Isquémica Aguda) Group. Routine invasive strategy within 24 hours of thrombolysis versus ischaemiaguided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet 2004; 364:1045–1053.
- Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study). J Am Coll Cardiol 2005; 46:417–424.
- Bøhmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010; 55:102–110.
- Wijeysundera HC, You JJ, Nallamothu BK, Krumholz HM, Cantor WJ, Ko DT. An early invasive strategy versus ischemia-guided management after fibrinolytic therapy for ST-segment elevation myocardial infarction: a meta-analysis of contemporary randomized controlled trials. Am Heart J 2008; 156:564–572,572.e1–e2.
- Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009; 54:2205–2241.
- Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008; 29:2909–2945.
- Mukherjee D, Moliterno DJ. The timely coupling of mechanical revascularization following thrombolysis for myocardial infarction. Cardiology 2007; 107:337–339.
- Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 2007; 49:422–430.
- The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329:1615–1622.
- Andersen HR, Nielsen TT, Rasmussen K, et al; DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349:733–742.
- Henry TD, Sharkey SW, Burke MN, et al. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007; 116:721–728.
- Denktas AE, Athar H, Henry TD, et al. Reduced-dose fibrinolytic acceleration of ST-segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv 2008; 1:504–510.
- Smalling RW. Ischemic time: the new gold standard for ST-segment elevation myocardial infarction care. J Am Coll Cardiol 2009; 54:2154–2156.
- Hochman JS, Sleeper LA, White HD, et al; SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. One-year survival following early revascularization for cardiogenic shock. JAMA 2001; 285:190–192.
- Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 2003; 41:1273–1279.
- Pedersen SH, Galatius S, Hansen PR, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009; 54:2296–2302.
- Björklund E, Stenestrand U, Lindbäck J, Svensson L, Wallentin L, Lindahl B. Pre-hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance-transported real-life patients with ST-elevation myocardial infarction. Eur Heart J 2006; 27:1146–1152.
- The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993; 329:383–389.
KEY POINTS
- When the expected door-to-balloon time is less than 90 minutes and the door-to-balloon time minus the door-to-needle time is less than 60 minutes, the preferred approach is PCI not preceded by thrombolysis.
- Evidence suggests that routine early (but not immediate) PCI—ie, 2 to 6 hours after thrombolysis—is beneficial, particularly in patients with high-risk ST-elevation MI.
- Hospitals and emergency services should participate in community-based and regional systems of care, with standardized protocols to ensure expeditious transfer and prompt reperfusion.
- Prehospital thrombolysis followed by early transfer to a PCI facility as part of a community-based system of care may further improve outcomes of patients with very long transfer times.
Exchanging the skin bleb for the test tube
Detecting latent tuberculosis is important for public health reasons, since reactivated tuberculosis can lead to infection of vulnerable populations, including those with some degree of immunosuppression due to aging, comorbid disease, or immunosuppressive therapy such as corticosteroids or anti-tumor necrosis factor-alpha agents.
The widely used tuberculin skin test is safe and relatively inexpensive, although it is unwieldy because it must be done by a person skilled in its technique, and the patient must make a second trip to a health care provider 48 to 72 hours later to have it interpreted. A previous effort to make testing more user-friendly, the tine test, proved to be less reliable.
In this issue of the Journal, Drs. Cyndee Miranda, J. Walton Tomford, and Steven M. Gordon describe the relatively new ex vivo interferon-gamma-release assays, which have received the full support of the US Centers for Disease Control and Prevention and are beginning to supplant the tuberculin skin test.
Besides solving some of the logistic problems, these newer tests have additional benefits. The skin tests detect prior exposure to several nontuberculous mycobacterial species, including Mycobacterium bovis, the strain used in the bacille Calmette-Guérin (BCG) vaccine given in many countries around the world. Because many areas where BCG is given also have a high prevalence of tuberculosis and nontuberculous mycobacterial infection, this limited specificity can cause confusion when immigrants from these areas enter the United States and undergo skin testing. Many people unnecessarily receive antibiotic therapy for assumed latent tuberculosis, due to a false-positive tuberculin skin test.
The interferon-gamma-release assays utilize more limited mycobacterial material obtained from M tuberculosis and thus have a greater specificity but a similar sensitivity.
These tests are not perfect. There are challenges with the interpretation of some results, the assay kits are relatively costly, and laboratory technicians must handle the samples and assay kits with great care. Nonetheless, I believe that these tests are a positive step towards accurate recognition and treatment of patients with latent tuberculosis.
Detecting latent tuberculosis is important for public health reasons, since reactivated tuberculosis can lead to infection of vulnerable populations, including those with some degree of immunosuppression due to aging, comorbid disease, or immunosuppressive therapy such as corticosteroids or anti-tumor necrosis factor-alpha agents.
The widely used tuberculin skin test is safe and relatively inexpensive, although it is unwieldy because it must be done by a person skilled in its technique, and the patient must make a second trip to a health care provider 48 to 72 hours later to have it interpreted. A previous effort to make testing more user-friendly, the tine test, proved to be less reliable.
In this issue of the Journal, Drs. Cyndee Miranda, J. Walton Tomford, and Steven M. Gordon describe the relatively new ex vivo interferon-gamma-release assays, which have received the full support of the US Centers for Disease Control and Prevention and are beginning to supplant the tuberculin skin test.
Besides solving some of the logistic problems, these newer tests have additional benefits. The skin tests detect prior exposure to several nontuberculous mycobacterial species, including Mycobacterium bovis, the strain used in the bacille Calmette-Guérin (BCG) vaccine given in many countries around the world. Because many areas where BCG is given also have a high prevalence of tuberculosis and nontuberculous mycobacterial infection, this limited specificity can cause confusion when immigrants from these areas enter the United States and undergo skin testing. Many people unnecessarily receive antibiotic therapy for assumed latent tuberculosis, due to a false-positive tuberculin skin test.
The interferon-gamma-release assays utilize more limited mycobacterial material obtained from M tuberculosis and thus have a greater specificity but a similar sensitivity.
These tests are not perfect. There are challenges with the interpretation of some results, the assay kits are relatively costly, and laboratory technicians must handle the samples and assay kits with great care. Nonetheless, I believe that these tests are a positive step towards accurate recognition and treatment of patients with latent tuberculosis.
Detecting latent tuberculosis is important for public health reasons, since reactivated tuberculosis can lead to infection of vulnerable populations, including those with some degree of immunosuppression due to aging, comorbid disease, or immunosuppressive therapy such as corticosteroids or anti-tumor necrosis factor-alpha agents.
The widely used tuberculin skin test is safe and relatively inexpensive, although it is unwieldy because it must be done by a person skilled in its technique, and the patient must make a second trip to a health care provider 48 to 72 hours later to have it interpreted. A previous effort to make testing more user-friendly, the tine test, proved to be less reliable.
In this issue of the Journal, Drs. Cyndee Miranda, J. Walton Tomford, and Steven M. Gordon describe the relatively new ex vivo interferon-gamma-release assays, which have received the full support of the US Centers for Disease Control and Prevention and are beginning to supplant the tuberculin skin test.
Besides solving some of the logistic problems, these newer tests have additional benefits. The skin tests detect prior exposure to several nontuberculous mycobacterial species, including Mycobacterium bovis, the strain used in the bacille Calmette-Guérin (BCG) vaccine given in many countries around the world. Because many areas where BCG is given also have a high prevalence of tuberculosis and nontuberculous mycobacterial infection, this limited specificity can cause confusion when immigrants from these areas enter the United States and undergo skin testing. Many people unnecessarily receive antibiotic therapy for assumed latent tuberculosis, due to a false-positive tuberculin skin test.
The interferon-gamma-release assays utilize more limited mycobacterial material obtained from M tuberculosis and thus have a greater specificity but a similar sensitivity.
These tests are not perfect. There are challenges with the interpretation of some results, the assay kits are relatively costly, and laboratory technicians must handle the samples and assay kits with great care. Nonetheless, I believe that these tests are a positive step towards accurate recognition and treatment of patients with latent tuberculosis.
Interferon-gamma-release assays: Better than tuberculin skin testing?
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
KEY POINTS
- Prior vaccination with bacille Calmette-Guérin can cause the results of skin testing to be falsely positive, but it does not affect interferon-gamma-release assays.
- In 2005, the US Centers for Disease Control and Prevention recommended that interferon-gamma-release assays be used in all situations in which skin testing is currently used. Updated guidelines were published on June 25, 2010.
- Successful implementation of interferon-gamma-release assay testing requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians.
Open Mesenteric Approach Still Useful
SCOTTSDALE, ARIZ. -- Despite the fact that open surgery for patients with chronic mesenteric ischemia tends to be reserved for sicker, more complicated patients who aren't good candidates for endovascular revascularization, outcomes have not declined over time.
A single-center review of 116 patients with 203 obstructed mesenteric arteries who underwent open repairs since 1998 found no significant differences in outcomes in 58 patients treated before endovascular treatment became the norm and 58 treated with open surgery during the endovascular era. "We believe that open revascularization still plays an important role in the treatment of this disease," Dr. Evan Ryer and his associates reported at the annual meeting of the Society for Clinical Vascular Surgery.
All patients were symptomatic and had open surgery at the Mayo Clinic, Rochester, Minn., which adopted endovascular treatment in 2002 for most cases of chronic mesenteric ischemia. Starting in that year, approximately 70% of patients with the disease were treated using endovascular revascularization.
"Since 2002, open mesenteric revascularization has been used in only 58 of 176 patients (33%) treated for chronic mesenteric ischemia at the Mayo Clinic," Dr. Ryer said in an interview. "Endovascular revascularization, which is our primary modality of treatment in most patients with suitable lesions, was not performed in these cases because it had failed previously or the anatomy was considered unfavorable because of chronic occlusion, severe calcification, or long-segment stenosis," he explained.
Patients in the pre-endovascular era (1998-2001) and post-endovascular era (2002-2009) who underwent open surgery reported similar durations of symptoms and degrees of weight loss. Compared with the pre-endovascular era patients, however, the post-endovascular era group had significantly higher rates of hypertension (86% vs. 66%), hyperlipidemia (76% vs. 36%), coronary artery disease requiring intervention (29% vs. 14%), cardiac dysrhythmias (28% vs. 7%), postprandial pain (88% vs. 72%), food fear (71% vs. 45%), and need for total parenteral nutrition (10% vs. 2%), as well as higher Society for Vascular Surgery comorbidity severity scores (7 vs. 5).
The extent of disease was greater in post-endovascular era patients, who were more likely to have three-vessel disease (79% vs. 59%) and occluded superior mesenteric arteries (67% vs. 45%) compared with pre-endovascular era patients, Dr. Ryer said. Two-vessel disease accounted for 81% of cases in the pre-endovascular era and 69% of cases in the post-endovascular era. Only 1% of patients in the post-endovascular era and none in the earlier time period had single-vessel disease. The differences between eras in two- and single-vessel disease rates were not significant.
The two time periods did not differ significantly in the technical details of the open procedures or in any outcomes, he added.
In the pre- and post-endovascular eras, patients averaged 4 and 5 days in the intensive care unit, respectively, and 13 and 12 days in the hospital. Among short-term outcomes, symptoms improved in 56% and 54% of patients treated in the pre- and post-endovascular eras, respectively. Two patients in the earlier era and three patients in the more recent era died, and major complications developed in 17 and 21 patients, respectively. These differences between groups were not significant.
After 5 years of follow-up, survival rates were 84% for pre-endovascular era patients and 78% for those in the post-endovascular era. Recurrence-free survival rates were 84% and 76%, respectively, primary patency rates were 82% and 81%, and secondary patency rates were 86% and 82%. None of the differences in outcomes were significant between groups.
The investigators declared they had no conflicts.
SCOTTSDALE, ARIZ. -- Despite the fact that open surgery for patients with chronic mesenteric ischemia tends to be reserved for sicker, more complicated patients who aren't good candidates for endovascular revascularization, outcomes have not declined over time.
A single-center review of 116 patients with 203 obstructed mesenteric arteries who underwent open repairs since 1998 found no significant differences in outcomes in 58 patients treated before endovascular treatment became the norm and 58 treated with open surgery during the endovascular era. "We believe that open revascularization still plays an important role in the treatment of this disease," Dr. Evan Ryer and his associates reported at the annual meeting of the Society for Clinical Vascular Surgery.
All patients were symptomatic and had open surgery at the Mayo Clinic, Rochester, Minn., which adopted endovascular treatment in 2002 for most cases of chronic mesenteric ischemia. Starting in that year, approximately 70% of patients with the disease were treated using endovascular revascularization.
"Since 2002, open mesenteric revascularization has been used in only 58 of 176 patients (33%) treated for chronic mesenteric ischemia at the Mayo Clinic," Dr. Ryer said in an interview. "Endovascular revascularization, which is our primary modality of treatment in most patients with suitable lesions, was not performed in these cases because it had failed previously or the anatomy was considered unfavorable because of chronic occlusion, severe calcification, or long-segment stenosis," he explained.
Patients in the pre-endovascular era (1998-2001) and post-endovascular era (2002-2009) who underwent open surgery reported similar durations of symptoms and degrees of weight loss. Compared with the pre-endovascular era patients, however, the post-endovascular era group had significantly higher rates of hypertension (86% vs. 66%), hyperlipidemia (76% vs. 36%), coronary artery disease requiring intervention (29% vs. 14%), cardiac dysrhythmias (28% vs. 7%), postprandial pain (88% vs. 72%), food fear (71% vs. 45%), and need for total parenteral nutrition (10% vs. 2%), as well as higher Society for Vascular Surgery comorbidity severity scores (7 vs. 5).
The extent of disease was greater in post-endovascular era patients, who were more likely to have three-vessel disease (79% vs. 59%) and occluded superior mesenteric arteries (67% vs. 45%) compared with pre-endovascular era patients, Dr. Ryer said. Two-vessel disease accounted for 81% of cases in the pre-endovascular era and 69% of cases in the post-endovascular era. Only 1% of patients in the post-endovascular era and none in the earlier time period had single-vessel disease. The differences between eras in two- and single-vessel disease rates were not significant.
The two time periods did not differ significantly in the technical details of the open procedures or in any outcomes, he added.
In the pre- and post-endovascular eras, patients averaged 4 and 5 days in the intensive care unit, respectively, and 13 and 12 days in the hospital. Among short-term outcomes, symptoms improved in 56% and 54% of patients treated in the pre- and post-endovascular eras, respectively. Two patients in the earlier era and three patients in the more recent era died, and major complications developed in 17 and 21 patients, respectively. These differences between groups were not significant.
After 5 years of follow-up, survival rates were 84% for pre-endovascular era patients and 78% for those in the post-endovascular era. Recurrence-free survival rates were 84% and 76%, respectively, primary patency rates were 82% and 81%, and secondary patency rates were 86% and 82%. None of the differences in outcomes were significant between groups.
The investigators declared they had no conflicts.
SCOTTSDALE, ARIZ. -- Despite the fact that open surgery for patients with chronic mesenteric ischemia tends to be reserved for sicker, more complicated patients who aren't good candidates for endovascular revascularization, outcomes have not declined over time.
A single-center review of 116 patients with 203 obstructed mesenteric arteries who underwent open repairs since 1998 found no significant differences in outcomes in 58 patients treated before endovascular treatment became the norm and 58 treated with open surgery during the endovascular era. "We believe that open revascularization still plays an important role in the treatment of this disease," Dr. Evan Ryer and his associates reported at the annual meeting of the Society for Clinical Vascular Surgery.
All patients were symptomatic and had open surgery at the Mayo Clinic, Rochester, Minn., which adopted endovascular treatment in 2002 for most cases of chronic mesenteric ischemia. Starting in that year, approximately 70% of patients with the disease were treated using endovascular revascularization.
"Since 2002, open mesenteric revascularization has been used in only 58 of 176 patients (33%) treated for chronic mesenteric ischemia at the Mayo Clinic," Dr. Ryer said in an interview. "Endovascular revascularization, which is our primary modality of treatment in most patients with suitable lesions, was not performed in these cases because it had failed previously or the anatomy was considered unfavorable because of chronic occlusion, severe calcification, or long-segment stenosis," he explained.
Patients in the pre-endovascular era (1998-2001) and post-endovascular era (2002-2009) who underwent open surgery reported similar durations of symptoms and degrees of weight loss. Compared with the pre-endovascular era patients, however, the post-endovascular era group had significantly higher rates of hypertension (86% vs. 66%), hyperlipidemia (76% vs. 36%), coronary artery disease requiring intervention (29% vs. 14%), cardiac dysrhythmias (28% vs. 7%), postprandial pain (88% vs. 72%), food fear (71% vs. 45%), and need for total parenteral nutrition (10% vs. 2%), as well as higher Society for Vascular Surgery comorbidity severity scores (7 vs. 5).
The extent of disease was greater in post-endovascular era patients, who were more likely to have three-vessel disease (79% vs. 59%) and occluded superior mesenteric arteries (67% vs. 45%) compared with pre-endovascular era patients, Dr. Ryer said. Two-vessel disease accounted for 81% of cases in the pre-endovascular era and 69% of cases in the post-endovascular era. Only 1% of patients in the post-endovascular era and none in the earlier time period had single-vessel disease. The differences between eras in two- and single-vessel disease rates were not significant.
The two time periods did not differ significantly in the technical details of the open procedures or in any outcomes, he added.
In the pre- and post-endovascular eras, patients averaged 4 and 5 days in the intensive care unit, respectively, and 13 and 12 days in the hospital. Among short-term outcomes, symptoms improved in 56% and 54% of patients treated in the pre- and post-endovascular eras, respectively. Two patients in the earlier era and three patients in the more recent era died, and major complications developed in 17 and 21 patients, respectively. These differences between groups were not significant.
After 5 years of follow-up, survival rates were 84% for pre-endovascular era patients and 78% for those in the post-endovascular era. Recurrence-free survival rates were 84% and 76%, respectively, primary patency rates were 82% and 81%, and secondary patency rates were 86% and 82%. None of the differences in outcomes were significant between groups.
The investigators declared they had no conflicts.
Update on Pediatric Psoriasis, Part 1: Clinical Features and Demographics
Excessive opioids blamed for respiratory arrest…A rising PSA, but no evaluation…A hemorrhoid…or something else?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Another option for patients with liver disease
Consider prescribing rifaximin for patients with hepatic encephalopathy, not only as a treatment for acute episodes but also to prevent a recurrence.1
STRENGTH OF RECOMMENDATION:
A: Based on a high-quality randomized controlled trial (RCT)
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
ILLUSTRATIVE CASE
A 64-year-old patient with chronic liver disease has been hospitalized on 3 occasions for hepatic encephalopathy, all while he was taking lactulose. He is still taking it, but wonders if there are other ways to prevent future episodes of hepatic encephalopathy. What can you tell him?
Characterized by periods of impaired cognition of varying severity, hepatic encephalopathy is a common complication of chronic liver disease—and a frequent cause of hospitalization, morbidity, and mortality in this patient population. Up to 70% of patients with cirrhosis may have some degree of hepatic encephalopathy,2 which can occur without provocation or be triggered by gastrointestinal (GI) bleeding, infection, kidney disease, electrolyte abnormalities, shunt placement, respiratory disease, or anemia. Hepatic encephalopathy is thought to be caused by elevated ammonia levels.
Current first-line treatment is not problem-free
Patients with chronic liver disease and hepatic encephalopathy are often placed on nonabsorbable disaccharides, such as lactulose, to prevent recurrent hepatic encephalopathy. However, disaccharides’ effectiveness as prophylaxis is unproven.3 In addition, many patients have difficulty tolerating lactulose because of its taste and GI side effects.
A 2004 Cochrane review examined the effectiveness of lactulose in preventing hepatic encephalopathy.3 The reviewers also compared the effectiveness of an oral antibiotic, rifaximin, with lactulose for this purpose. Rifaximin, like lactu-lose, is believed to work by reducing ammonia in the gut. The antibiotic is a well-established treatment for acute hepatic encephalopathy, but not widely used for preventive purposes.
The reviewers found rifaximin to be more effective compared with lactulose at preventing recurrent episodes of hepatic encephalopathy (number needed to treat [NNT]=11).3 Other studies have also suggested that the antibiotic, which has minimal systemic absorption, may be as effective as, or more effective than, lactu-lose in preventing recurrences.4,5 The new RCT detailed in this PURL took another look at rifaximin’s usefulness as prophylaxis.
STUDY SUMMARY: Patients on rifaximin had better outcomes
The study by Bass et al was a double-blinded RCT enrolling 299 patients with chronic liver disease.1 Criteria for inclusion were age ≥18 years, a minimum of 2 prior episodes of hepatic encephalopathy, remission from hepatic encephalopathy at the time of enrollment, and mild to moderate liver disease severity, defined as a score ≤25 on the Model for End-Stage Liver Disease (MELD) scale.6 (The scale ranges from 6 to 40, with higher numbers indicating more severe disease.) The researchers excluded patients for whom liver transplant was imminent and those with conditions that precipitate hepatic encephalopathy, as described earlier.
Patients were assigned to either rifaximin 550 mg twice a day (140 patients) or placebo (159 patients) for 6 months. Both groups had similar baseline characteristics, including a high percentage of subjects (>90%) with concomitant lactulose use. The researchers assessed the patients at clinic visits every 2 weeks, both by their Conn score (the scale commonly used to grade hepatic encephalopathy) and grade of asterixis, and during telephone calls on alternate weeks. Analysis was by intention-to-treat.
The primary endpoint was the mean time to the first episode of hepatic encephalopathy, which was 130.0 (±56.5) days in the rifaximin group and 105.7 (±62.7) days in the control group. During the 6-month study period, 22% of patients in the rifaximin group experienced a breakthrough hepatic encephalopathy event, vs 45.9% of the placebo group (95% confidence interval, 0.28-0.64; P<0.001; hazard ratio=0.42; NNT=9). Both groups had high rates of compliance (~84%) and high rates of adverse events (80%). Two patients receiving rifaximin experienced Clostridium difficile infections, from which they recovered. Death rates were similar in both groups, and were attributed to liver disease progression.
WHAT’S NEW?: FDA approves rifaximin to prevent recurrence
This trial adds further support for the use of rifaximin in the prevention of recurrent episodes of hepatic encephalopathy. In addition, the US Food and Drug Administration approved the antibiotic for that purpose in March of this year.7 Given the lack of proven, well-tolerated treatments to prevent hepatic encephalopathy in patients with liver disease and the significant morbidity and mortality associated with this complication, family physicians should consider prescribing rifaximin for patients with prior episodes of hepatic encephalopathy. Rifaximin resistance is not common and, because its activity is concentrated in the gut, resistance is unlikely to become a significant issue.
CAVEATS: Long-term safety has not been established
Because of this study’s short duration (6 months) and relatively small sample size, we cannot be certain of its long-term effects or safety. However, patients with advanced liver disease and recurrent hepatic encephalopathy have a poor prognosis, and a treatment that is effective, even if just for 6 months, is meaningful.
Also, because this study excluded patients with more severe liver disease (MELD score >25), we have no data to guide the use of rifaximin in this patient population. However, the mechanism of action and risk of adverse effects are likely to be similar.
Finally, the manufacturer of the drug was involved in the study design, data collection, data analysis, and manuscript preparation.
CHALLENGES TO IMPLEMENTATION: Drug cost and coverage are potential barriers
Rifaximin is available in the United States in 200- and 550-mg tablets, so it can be dosed at 1100 or 1200 mg per day in divided doses. The drug is not generic, however, and is costly: A month’s supply of the 550-mg tablets is about $1300 (a supply of the 200-mg tablets is even more expensive),8 and the drug may not be covered by insurance.
Acknowledgement
The PURls Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479.
3. Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044.-
4. Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407.
5. Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy. 2008;28:1019-1032.
6. Mayo Clinic. The MELD model, UNOS modification. Available at: http://www.mayoclinic.org/meld/mayomodel6.html. Accessed August 16, 2010.
7. US Food and Drug Administration. FDA approves new use of Xifaxan for patients with liver disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm206104.htm. Updated March 26, 2010. Accessed July 7, 2010.
8. Drugstore.com. Available at: http://www.drugstore.com/. Accessed August 20, 2010.
Consider prescribing rifaximin for patients with hepatic encephalopathy, not only as a treatment for acute episodes but also to prevent a recurrence.1
STRENGTH OF RECOMMENDATION:
A: Based on a high-quality randomized controlled trial (RCT)
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
ILLUSTRATIVE CASE
A 64-year-old patient with chronic liver disease has been hospitalized on 3 occasions for hepatic encephalopathy, all while he was taking lactulose. He is still taking it, but wonders if there are other ways to prevent future episodes of hepatic encephalopathy. What can you tell him?
Characterized by periods of impaired cognition of varying severity, hepatic encephalopathy is a common complication of chronic liver disease—and a frequent cause of hospitalization, morbidity, and mortality in this patient population. Up to 70% of patients with cirrhosis may have some degree of hepatic encephalopathy,2 which can occur without provocation or be triggered by gastrointestinal (GI) bleeding, infection, kidney disease, electrolyte abnormalities, shunt placement, respiratory disease, or anemia. Hepatic encephalopathy is thought to be caused by elevated ammonia levels.
Current first-line treatment is not problem-free
Patients with chronic liver disease and hepatic encephalopathy are often placed on nonabsorbable disaccharides, such as lactulose, to prevent recurrent hepatic encephalopathy. However, disaccharides’ effectiveness as prophylaxis is unproven.3 In addition, many patients have difficulty tolerating lactulose because of its taste and GI side effects.
A 2004 Cochrane review examined the effectiveness of lactulose in preventing hepatic encephalopathy.3 The reviewers also compared the effectiveness of an oral antibiotic, rifaximin, with lactulose for this purpose. Rifaximin, like lactu-lose, is believed to work by reducing ammonia in the gut. The antibiotic is a well-established treatment for acute hepatic encephalopathy, but not widely used for preventive purposes.
The reviewers found rifaximin to be more effective compared with lactulose at preventing recurrent episodes of hepatic encephalopathy (number needed to treat [NNT]=11).3 Other studies have also suggested that the antibiotic, which has minimal systemic absorption, may be as effective as, or more effective than, lactu-lose in preventing recurrences.4,5 The new RCT detailed in this PURL took another look at rifaximin’s usefulness as prophylaxis.
STUDY SUMMARY: Patients on rifaximin had better outcomes
The study by Bass et al was a double-blinded RCT enrolling 299 patients with chronic liver disease.1 Criteria for inclusion were age ≥18 years, a minimum of 2 prior episodes of hepatic encephalopathy, remission from hepatic encephalopathy at the time of enrollment, and mild to moderate liver disease severity, defined as a score ≤25 on the Model for End-Stage Liver Disease (MELD) scale.6 (The scale ranges from 6 to 40, with higher numbers indicating more severe disease.) The researchers excluded patients for whom liver transplant was imminent and those with conditions that precipitate hepatic encephalopathy, as described earlier.
Patients were assigned to either rifaximin 550 mg twice a day (140 patients) or placebo (159 patients) for 6 months. Both groups had similar baseline characteristics, including a high percentage of subjects (>90%) with concomitant lactulose use. The researchers assessed the patients at clinic visits every 2 weeks, both by their Conn score (the scale commonly used to grade hepatic encephalopathy) and grade of asterixis, and during telephone calls on alternate weeks. Analysis was by intention-to-treat.
The primary endpoint was the mean time to the first episode of hepatic encephalopathy, which was 130.0 (±56.5) days in the rifaximin group and 105.7 (±62.7) days in the control group. During the 6-month study period, 22% of patients in the rifaximin group experienced a breakthrough hepatic encephalopathy event, vs 45.9% of the placebo group (95% confidence interval, 0.28-0.64; P<0.001; hazard ratio=0.42; NNT=9). Both groups had high rates of compliance (~84%) and high rates of adverse events (80%). Two patients receiving rifaximin experienced Clostridium difficile infections, from which they recovered. Death rates were similar in both groups, and were attributed to liver disease progression.
WHAT’S NEW?: FDA approves rifaximin to prevent recurrence
This trial adds further support for the use of rifaximin in the prevention of recurrent episodes of hepatic encephalopathy. In addition, the US Food and Drug Administration approved the antibiotic for that purpose in March of this year.7 Given the lack of proven, well-tolerated treatments to prevent hepatic encephalopathy in patients with liver disease and the significant morbidity and mortality associated with this complication, family physicians should consider prescribing rifaximin for patients with prior episodes of hepatic encephalopathy. Rifaximin resistance is not common and, because its activity is concentrated in the gut, resistance is unlikely to become a significant issue.
CAVEATS: Long-term safety has not been established
Because of this study’s short duration (6 months) and relatively small sample size, we cannot be certain of its long-term effects or safety. However, patients with advanced liver disease and recurrent hepatic encephalopathy have a poor prognosis, and a treatment that is effective, even if just for 6 months, is meaningful.
Also, because this study excluded patients with more severe liver disease (MELD score >25), we have no data to guide the use of rifaximin in this patient population. However, the mechanism of action and risk of adverse effects are likely to be similar.
Finally, the manufacturer of the drug was involved in the study design, data collection, data analysis, and manuscript preparation.
CHALLENGES TO IMPLEMENTATION: Drug cost and coverage are potential barriers
Rifaximin is available in the United States in 200- and 550-mg tablets, so it can be dosed at 1100 or 1200 mg per day in divided doses. The drug is not generic, however, and is costly: A month’s supply of the 550-mg tablets is about $1300 (a supply of the 200-mg tablets is even more expensive),8 and the drug may not be covered by insurance.
Acknowledgement
The PURls Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
Consider prescribing rifaximin for patients with hepatic encephalopathy, not only as a treatment for acute episodes but also to prevent a recurrence.1
STRENGTH OF RECOMMENDATION:
A: Based on a high-quality randomized controlled trial (RCT)
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
ILLUSTRATIVE CASE
A 64-year-old patient with chronic liver disease has been hospitalized on 3 occasions for hepatic encephalopathy, all while he was taking lactulose. He is still taking it, but wonders if there are other ways to prevent future episodes of hepatic encephalopathy. What can you tell him?
Characterized by periods of impaired cognition of varying severity, hepatic encephalopathy is a common complication of chronic liver disease—and a frequent cause of hospitalization, morbidity, and mortality in this patient population. Up to 70% of patients with cirrhosis may have some degree of hepatic encephalopathy,2 which can occur without provocation or be triggered by gastrointestinal (GI) bleeding, infection, kidney disease, electrolyte abnormalities, shunt placement, respiratory disease, or anemia. Hepatic encephalopathy is thought to be caused by elevated ammonia levels.
Current first-line treatment is not problem-free
Patients with chronic liver disease and hepatic encephalopathy are often placed on nonabsorbable disaccharides, such as lactulose, to prevent recurrent hepatic encephalopathy. However, disaccharides’ effectiveness as prophylaxis is unproven.3 In addition, many patients have difficulty tolerating lactulose because of its taste and GI side effects.
A 2004 Cochrane review examined the effectiveness of lactulose in preventing hepatic encephalopathy.3 The reviewers also compared the effectiveness of an oral antibiotic, rifaximin, with lactulose for this purpose. Rifaximin, like lactu-lose, is believed to work by reducing ammonia in the gut. The antibiotic is a well-established treatment for acute hepatic encephalopathy, but not widely used for preventive purposes.
The reviewers found rifaximin to be more effective compared with lactulose at preventing recurrent episodes of hepatic encephalopathy (number needed to treat [NNT]=11).3 Other studies have also suggested that the antibiotic, which has minimal systemic absorption, may be as effective as, or more effective than, lactu-lose in preventing recurrences.4,5 The new RCT detailed in this PURL took another look at rifaximin’s usefulness as prophylaxis.
STUDY SUMMARY: Patients on rifaximin had better outcomes
The study by Bass et al was a double-blinded RCT enrolling 299 patients with chronic liver disease.1 Criteria for inclusion were age ≥18 years, a minimum of 2 prior episodes of hepatic encephalopathy, remission from hepatic encephalopathy at the time of enrollment, and mild to moderate liver disease severity, defined as a score ≤25 on the Model for End-Stage Liver Disease (MELD) scale.6 (The scale ranges from 6 to 40, with higher numbers indicating more severe disease.) The researchers excluded patients for whom liver transplant was imminent and those with conditions that precipitate hepatic encephalopathy, as described earlier.
Patients were assigned to either rifaximin 550 mg twice a day (140 patients) or placebo (159 patients) for 6 months. Both groups had similar baseline characteristics, including a high percentage of subjects (>90%) with concomitant lactulose use. The researchers assessed the patients at clinic visits every 2 weeks, both by their Conn score (the scale commonly used to grade hepatic encephalopathy) and grade of asterixis, and during telephone calls on alternate weeks. Analysis was by intention-to-treat.
The primary endpoint was the mean time to the first episode of hepatic encephalopathy, which was 130.0 (±56.5) days in the rifaximin group and 105.7 (±62.7) days in the control group. During the 6-month study period, 22% of patients in the rifaximin group experienced a breakthrough hepatic encephalopathy event, vs 45.9% of the placebo group (95% confidence interval, 0.28-0.64; P<0.001; hazard ratio=0.42; NNT=9). Both groups had high rates of compliance (~84%) and high rates of adverse events (80%). Two patients receiving rifaximin experienced Clostridium difficile infections, from which they recovered. Death rates were similar in both groups, and were attributed to liver disease progression.
WHAT’S NEW?: FDA approves rifaximin to prevent recurrence
This trial adds further support for the use of rifaximin in the prevention of recurrent episodes of hepatic encephalopathy. In addition, the US Food and Drug Administration approved the antibiotic for that purpose in March of this year.7 Given the lack of proven, well-tolerated treatments to prevent hepatic encephalopathy in patients with liver disease and the significant morbidity and mortality associated with this complication, family physicians should consider prescribing rifaximin for patients with prior episodes of hepatic encephalopathy. Rifaximin resistance is not common and, because its activity is concentrated in the gut, resistance is unlikely to become a significant issue.
CAVEATS: Long-term safety has not been established
Because of this study’s short duration (6 months) and relatively small sample size, we cannot be certain of its long-term effects or safety. However, patients with advanced liver disease and recurrent hepatic encephalopathy have a poor prognosis, and a treatment that is effective, even if just for 6 months, is meaningful.
Also, because this study excluded patients with more severe liver disease (MELD score >25), we have no data to guide the use of rifaximin in this patient population. However, the mechanism of action and risk of adverse effects are likely to be similar.
Finally, the manufacturer of the drug was involved in the study design, data collection, data analysis, and manuscript preparation.
CHALLENGES TO IMPLEMENTATION: Drug cost and coverage are potential barriers
Rifaximin is available in the United States in 200- and 550-mg tablets, so it can be dosed at 1100 or 1200 mg per day in divided doses. The drug is not generic, however, and is costly: A month’s supply of the 550-mg tablets is about $1300 (a supply of the 200-mg tablets is even more expensive),8 and the drug may not be covered by insurance.
Acknowledgement
The PURls Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479.
3. Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044.-
4. Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407.
5. Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy. 2008;28:1019-1032.
6. Mayo Clinic. The MELD model, UNOS modification. Available at: http://www.mayoclinic.org/meld/mayomodel6.html. Accessed August 16, 2010.
7. US Food and Drug Administration. FDA approves new use of Xifaxan for patients with liver disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm206104.htm. Updated March 26, 2010. Accessed July 7, 2010.
8. Drugstore.com. Available at: http://www.drugstore.com/. Accessed August 20, 2010.
1. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479.
3. Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044.-
4. Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407.
5. Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy. 2008;28:1019-1032.
6. Mayo Clinic. The MELD model, UNOS modification. Available at: http://www.mayoclinic.org/meld/mayomodel6.html. Accessed August 16, 2010.
7. US Food and Drug Administration. FDA approves new use of Xifaxan for patients with liver disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm206104.htm. Updated March 26, 2010. Accessed July 7, 2010.
8. Drugstore.com. Available at: http://www.drugstore.com/. Accessed August 20, 2010.
Copyright © 2010 The Family Physicians Inquiries Network.
All rights reserved.
Flu season’s almost here: Are you ready?
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Should you restrain yourself from ordering restraints?
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
Dear Dr. Mossman:
We often have to administer sedating medications to aggressive patients who pose an immediate threat of harm to themselves or others. But I am unsure about whether these “chemical restraints” create more liability problems than “physical restraints”—or vice versa. Does one type of restraint carry more legal risk than the other?—Submitted by “Dr. L”
Mental health professionals view “mechanical” or “physical” restraints in a way that really differs from how they felt 2 decades ago. In the 1980s, physical restraint use was a common response when patients seemed to be immediately dangerous to themselves or others. But recent practice guidelines say physical restraints are a “last resort,” to be used only when other treatment measures to prevent aggression fail to work.
What should psychiatrists do? Is use of physical restraints malpractice? Are “chemical” restraints better?
This article looks at:
- definitions of restraint
- medical risks of restraint
- evolution and status of restraint policy
- what you can do about legal risks of restraint.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
Definitions
In medical contexts, restraint typically refers to “any device or medication used to restrict a patient’s movement.”1 The longer, official US regulatory definitions of physical and chemical restraints appear in Table 1.2 Two important notes:
- Neither regulatory definition of restraint is limited to psychiatric patients; both definitions and the accompanying regulations on restraint apply to any patient in a hospital eligible for federal reimbursement.
- The definition of physical restraint would include holding a patient still while administering an injection.
The detailed interpretive rules (“Conditions of Participation for Hospitals”)3 for these regulations require hospitals to document conditions surrounding and reasons related to restraint incidents and to make this documentation available to federal surveyors.
Table 1
Federal regulatory definitions of ‘restraint’
| Physical restraint | Any manual method, physical or mechanical device, material, or equipment that immobilizes or reduces the ability of a patient to move his or her arms, legs, body, or head freely |
| Chemical restraint | A drug or medication when it is used as a restriction to manage the patient’s behavior or restrict the patient’s freedom of movement and is not a standard treatment or dosage for the patient’s condition |
| Source: Reference 2 | |
Medical risks of restraint
In 1998, the Hartford Courant investigative series “Deadly restraint”4 reported on 142 deaths of psychiatric patients and alerted the public to the potentially fatal consequences of physical restraint. Often, restraint deaths result from asphyxia when patients try to free themselves and get caught in positions that restrict breathing.5 Other injuries—particularly those produced by falls—can result from well-intentioned efforts to protect confused patients by restraining them.6
Evolution of restraint policy
Although restraining patients might inadvertently cause harm, isn’t it better to restrain someone, which prevents harm from aggression and accidents? Mental health professionals once thought the answer to this question was, “Of course!” But scientific data say, “Often not.”
Studies conducted when physical restraint was more common found order-of-magnitude disparities in restraint rates at sites with similar patient populations. This suggested that institutional norms and practice styles—not patients’ problems or dangerousness—explained why much restraint occurred.7-9
Reacting to these kinds of findings, psychiatric hospitals in the United States and abroad implemented various methods and policy changes to reduce restraint. Follow-up studies typically showed that episodes of restraint and total time spent in restraints could decrease markedly without any increase in events that harmed patients or staff members.10 In addition, mental health professionals now recognize that being restrained is psychologically traumatic for patients, even when restraint causes no physical injury.11
Patients in psychiatric settings represent a minority of persons who get restrained. On inpatient medical/surgical units, patient confusion and wandering, fall prevention, and perceived medical necessity can lead to physical restraint use.12 Yet physical restraints as innocent-seeming as bed rails can lead to deaths and injuries.13
Nursing homes are another environment where restraints may be common but sometimes detrimental. A recent study found that in all aspects of nursing home patients’ health and functioning—behavior, cognitive performance, falls, walking, activities of daily living, pressure sores, and contractures—physical restraints lead to worse outcomes than leaving patients unrestrained.14
For all these reasons, restraining patients is often viewed as “poor practice”14 and a response of last resort for behavioral problems.15-17
Federal regulations
Publication of the Courant article spurred Congress to develop standards18 that, a decade later, permit restraint or seclusion only when less restrictive interventions will not prevent harm, only for limited periods, and only with careful medical monitoring. Restraint is permissible when no alternative exists, but facilities that use restraint must train staff members to recognize and avert situations that might lead to physical interventions and must generate proper documentation each time restraint is used.2
Federal regulations also apply to “chemical restraints” and aim to restrict their use. This doesn’t mean you can’t use drugs to treat patients, however. Regulations explicitly allow you to prescribe “standard treatment” (Table 2)3 to help your patients function or sleep better, to alleviate pain, or to reduce agitation—and such uses of medication are not “chemical restraint.” Rather, you’re using “chemical restraint” if you prescribe a drug to control bothersome behavior—for example, to “knock out” a patient with dementia whose “sundowning” bothers staff members.19 Psychiatrists should be familiar with the risks of medications used for behavioral control, particularly in elderly patients.20
Table 2
Federal criteria for ‘standard treatment‘
| Medication is used within FDA-approved pharmaceutical parameters and manufacturer indications |
| Medication use follows standards recognized by the medical community |
| Choice of medication is based on patient’s symptoms, overall clinical situation, and prescriber’s knowledge of the patient’s treatment response |
| Source: Reference 3 |
Avoiding legal risks
No study or systematic data will ever tell us whether physical or chemical restraints create a greater liability risk. Obviously, the best way to avoid legal liability for restraints is to minimize use of physical restraints and to avoid using medications as chemical restraints. Psychiatrists who work in hospitals or other institutional settings can politely but firmly decline to prescribe medications or to order physical restraints when staff members request these measures for non-therapeutic reasons—ie, for a patient who has calmed down but whom staff members believe “needs to learn a lesson” or “get some consequences” for throwing a chair. When restraints are necessary, psychiatrists (along with other staff members) should document the reasons why, including what other interventions were tried first.
Many psychiatric facilities and care systems have reduced incidence of restraint and time spent by patients in restraint through programs that broadly address institutional practices. Such programs usually involve a multi-disciplinary, multi-strategy commitment to alternatives—to helping staff members see that restraints represent a failure in treatment rather than a form of treatment, and to developing other mechanisms for averting or responding to patients’ aggression before restraint becomes the only option.10,21 Individual psychiatrists can play an important role in advocating and supporting institutional policies, practices, and training that help staff members minimize restraint use.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
1. Agens JE. Chemical and physical restraint use in the older person. BJMP. 2010;3:302.-
2. Code of Federal Regulations. Conditions of participation for hospitals: Condition of participation: Patient’s rights. Title 42, Part 482, § 482.13. Available at: http://edocket.access.gpo.gov/cfr_2004/octqtr/pdf/42cfr482.13.pdf. Accessed July 21, 2010.
3. Department of Health and Human Services, Centers for Medicare and Medicaid Services Pub. 100-07 State Operations (Provider Certification, Transmittal 37). Available at: https://146.123.140.205/transmittals/downloads/R37SOMA.pdf. Accessed July 20, 2010.
4. Weiss EM. Deadly restraint: a Hartford Courant investigative report. Hartford Courant. October 11-15, 1998.
5. Karger B, Fracasso T, Pfeiffer H. Fatalities related to medical restraint devices—asphyxia is a common finding. Forensic Sci Int. 2008;178:178-184.
6. Inouye SK, Brown CJ, Tinetti ME. Medicare nonpayment, hospital falls, and unintended consequences. N Engl J Med. 2009;360:2390-2393.
7. Betemps EJ, Somoza E, Buncher CR. Hospital characteristics, diagnoses, and staff reasons associated with use of seclusion and restraint. Hosp Community Psychiatry. 1993;44:367-371.
8. Crenshaw WB, Francis PS. A national survey on seclusion and restraint in state psychiatric hospitals. Psychiatr Serv. 1995;46:1026-1031.
9. Ray NK, Rappaport ME. Use of restraint and seclusion in psychiatric settings in New York State. Psychiatr Serv. 1995;46:1032-1037.
10. Smith GM, Davis RH, Bixler EO, et al. Pennsylvania State Hospital system’s seclusion and restraint reduction program. Psychiatr Serv. 2005;56:1115-1122.
11. Frueh BC, Knapp RG, Cusack KJ, et al. Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatr Serv. 2005;56:1123-1133.
12. Forrester DA, McCabe-Bender J, Walsh N, et al. Physical restraint management of hospitalized adults and follow-up study. J Nurses Staff Dev. 2000;16:267-276.
13. The Joint Commission. Bed rail-related entrapment deaths. Available at: http://www.jointcommission.org/ sentinelevents/alert/sea_27.htm. Accessed July 20, 2010.
14. Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Med Care. 2009;47:1164-1173.
15. Marder SR. A review of agitation in mental illness: treatment guidelines and current therapies. J Clin Psychiatry. 2006;67(suppl 10):13-21.
16. Borckardt JJ, Grubaugh AL, Pelic CG, et al. Enhancing patient safety in psychiatric settings. J Psychiatr Pract. 2007;13:355-361.
17. National Association of State Mental Health Program Directors. Position Statement on Seclusion and Restraint. Available at: http://www.nasmhpd.org/general_files/position_statement/posses1.htm. Accessed July 18, 2010.
18. Appelbaum P. Seclusion and restraint: Congress reacts to reports of abuse. Psychiatr Serv. 1999;50:881-882, 885.
19. Centers for Medicare and Medicaid Services. State operations manual: appendix A—survey protocol, regulations and interpretive guidelines for hospitals. Available at: http://www.cms.gov/manuals/downloads/som107ap_a_hospitals.pdf. Accessed July 20, 2010.
20. Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
21. Gaskin CJ, Elsom SJ, Happell B. Interventions for reducing the use of seclusion in psychiatric facilities: review of the literature. Br J Psychiatry. 2007;191:298-303.
Hallucinogen sequelae
I appreciated “The woman who saw the light” (Current Psychiatry, July 2010, p. 44-48) in which Dr. R. Andrew Sewell et al describe a 30-year-old woman with schizoaffective disorder and a 7-year history of visual disturbances, including “flashing lights.” The authors’ differential diagnosis did not include the possibility of visual disturbance secondary to atypical anti-psychotic serotonergic antagonism. Photopsia and similar phenomena are not uncommon with 5HT antagonist antidepressants, such as nefazodone.1 They also are well-known sequelae of lysergic acid diethylamide (LSD), a complex serotonin antagonist/agonist, and would be included under the DSM-IV-TR diagnosis hallucinogen persisting perceptual disorder (HPPD).2 Risperidone, a 5HT2-blocking atypical, and selective serotonin reuptake inhibitors may worsen HPPD effects.3,4 Visual disturbance with risperidone also has been reported in a patient with no LSD exposure.5 Dr. Sewell’s patient was treated sequentially with aripiprazole and olanzapine. Both have 5HT blocking properties.
I wonder if the patient has a history of hallucinogen or LSD exposure, or whether her visual symptoms might be related to the use of atypical anti-psychotics combined with sertraline. It would be interesting to see if her symptoms abated with use of a first-generation antipsychotic.
Charles Krasnow, MD
Adjunct clinical assistant professor of psychiatry
University of Michigan Medical School
Ann Arbor, MI
The authors respond
We agree with Dr. Krasnow that HPPD belongs within our differential diagnosis for photopsia and regret omitting it from our article. We consider this to be unlikely, however, because she had no prior LSD use, a history of well-formed visual hallucinations not characteristic of HPPD, and no other characteristic symptoms of HPPD (palinopsia, afterimages, illusory movement, etc.).
In addition, she tolerated olanzapine well, and there is anecdotal evidence and 1 case report to suggest that olanzapine exacerbates HPPD.1
HPPD typically is considered a rare sequela of LSD use, although even more rarely it may be caused by other drugs. Common visual disturbances attributed to HPPD are recurrent geometric hallucinations, perception of peripheral movement, colored flashes, intensified colors, palinopsia, positive afterimages, haloes around objects, macropsia, and micropsia occurring spontaneously in individuals with no prior psychopathology. These disturbances can be intermittent or continuous, slowly reversible or irreversible, but are severe, intrusive, and cause functional debility. Sufferers retain insight that these phenomena are the consequence of LSD use and usually seek psychiatric help.
HPPD may be diagnosed by the presence of an identifiable trigger, prodromal symptoms, and presentation onset; by the characteristics of the perceptual disturbances, their frequency, duration, intensity, and course; and by the accompanying negative affect and preserved insight.2
This LSD-induced persistence of visual imagery after the image is removed from the visual field is thought to result from dysfunction of serotonergic cortical inhibitory interneurons with GABAergic outputs that normally suppress visual processors.3 Clonazepam often is helpful.2
R. Andrew Sewell, MD
VA Connecticut Healthcare/Yale University
School of Medicine
New Haven, CT
David Kozin
McLean Hospital/Harvard Medical School
Belmont, MA
Miles G. Cunningham, MD, PhD
McLean Hospital/Harvard Medical School
Belmont, MA
1. Espiard ML, Lecardeur L, Abadie P, et al. Hallucinogen persisting perception disorder after psilocybin consumption: a case study. Eur Psychiatry. 2005;20:458-460.
2. Lerner AG, Gelkopf M, Skladman I, et al. Clonazepam treatment of lysergic acid diethylamide-induced hallucinogen persisting perception disorder with anxiety features. Int Clin Psychopharmacol. 2003;18:101-105.
3. Abraham HD, Aldridge AM. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.
I appreciated “The woman who saw the light” (Current Psychiatry, July 2010, p. 44-48) in which Dr. R. Andrew Sewell et al describe a 30-year-old woman with schizoaffective disorder and a 7-year history of visual disturbances, including “flashing lights.” The authors’ differential diagnosis did not include the possibility of visual disturbance secondary to atypical anti-psychotic serotonergic antagonism. Photopsia and similar phenomena are not uncommon with 5HT antagonist antidepressants, such as nefazodone.1 They also are well-known sequelae of lysergic acid diethylamide (LSD), a complex serotonin antagonist/agonist, and would be included under the DSM-IV-TR diagnosis hallucinogen persisting perceptual disorder (HPPD).2 Risperidone, a 5HT2-blocking atypical, and selective serotonin reuptake inhibitors may worsen HPPD effects.3,4 Visual disturbance with risperidone also has been reported in a patient with no LSD exposure.5 Dr. Sewell’s patient was treated sequentially with aripiprazole and olanzapine. Both have 5HT blocking properties.
I wonder if the patient has a history of hallucinogen or LSD exposure, or whether her visual symptoms might be related to the use of atypical anti-psychotics combined with sertraline. It would be interesting to see if her symptoms abated with use of a first-generation antipsychotic.
Charles Krasnow, MD
Adjunct clinical assistant professor of psychiatry
University of Michigan Medical School
Ann Arbor, MI
The authors respond
We agree with Dr. Krasnow that HPPD belongs within our differential diagnosis for photopsia and regret omitting it from our article. We consider this to be unlikely, however, because she had no prior LSD use, a history of well-formed visual hallucinations not characteristic of HPPD, and no other characteristic symptoms of HPPD (palinopsia, afterimages, illusory movement, etc.).
In addition, she tolerated olanzapine well, and there is anecdotal evidence and 1 case report to suggest that olanzapine exacerbates HPPD.1
HPPD typically is considered a rare sequela of LSD use, although even more rarely it may be caused by other drugs. Common visual disturbances attributed to HPPD are recurrent geometric hallucinations, perception of peripheral movement, colored flashes, intensified colors, palinopsia, positive afterimages, haloes around objects, macropsia, and micropsia occurring spontaneously in individuals with no prior psychopathology. These disturbances can be intermittent or continuous, slowly reversible or irreversible, but are severe, intrusive, and cause functional debility. Sufferers retain insight that these phenomena are the consequence of LSD use and usually seek psychiatric help.
HPPD may be diagnosed by the presence of an identifiable trigger, prodromal symptoms, and presentation onset; by the characteristics of the perceptual disturbances, their frequency, duration, intensity, and course; and by the accompanying negative affect and preserved insight.2
This LSD-induced persistence of visual imagery after the image is removed from the visual field is thought to result from dysfunction of serotonergic cortical inhibitory interneurons with GABAergic outputs that normally suppress visual processors.3 Clonazepam often is helpful.2
R. Andrew Sewell, MD
VA Connecticut Healthcare/Yale University
School of Medicine
New Haven, CT
David Kozin
McLean Hospital/Harvard Medical School
Belmont, MA
Miles G. Cunningham, MD, PhD
McLean Hospital/Harvard Medical School
Belmont, MA
I appreciated “The woman who saw the light” (Current Psychiatry, July 2010, p. 44-48) in which Dr. R. Andrew Sewell et al describe a 30-year-old woman with schizoaffective disorder and a 7-year history of visual disturbances, including “flashing lights.” The authors’ differential diagnosis did not include the possibility of visual disturbance secondary to atypical anti-psychotic serotonergic antagonism. Photopsia and similar phenomena are not uncommon with 5HT antagonist antidepressants, such as nefazodone.1 They also are well-known sequelae of lysergic acid diethylamide (LSD), a complex serotonin antagonist/agonist, and would be included under the DSM-IV-TR diagnosis hallucinogen persisting perceptual disorder (HPPD).2 Risperidone, a 5HT2-blocking atypical, and selective serotonin reuptake inhibitors may worsen HPPD effects.3,4 Visual disturbance with risperidone also has been reported in a patient with no LSD exposure.5 Dr. Sewell’s patient was treated sequentially with aripiprazole and olanzapine. Both have 5HT blocking properties.
I wonder if the patient has a history of hallucinogen or LSD exposure, or whether her visual symptoms might be related to the use of atypical anti-psychotics combined with sertraline. It would be interesting to see if her symptoms abated with use of a first-generation antipsychotic.
Charles Krasnow, MD
Adjunct clinical assistant professor of psychiatry
University of Michigan Medical School
Ann Arbor, MI
The authors respond
We agree with Dr. Krasnow that HPPD belongs within our differential diagnosis for photopsia and regret omitting it from our article. We consider this to be unlikely, however, because she had no prior LSD use, a history of well-formed visual hallucinations not characteristic of HPPD, and no other characteristic symptoms of HPPD (palinopsia, afterimages, illusory movement, etc.).
In addition, she tolerated olanzapine well, and there is anecdotal evidence and 1 case report to suggest that olanzapine exacerbates HPPD.1
HPPD typically is considered a rare sequela of LSD use, although even more rarely it may be caused by other drugs. Common visual disturbances attributed to HPPD are recurrent geometric hallucinations, perception of peripheral movement, colored flashes, intensified colors, palinopsia, positive afterimages, haloes around objects, macropsia, and micropsia occurring spontaneously in individuals with no prior psychopathology. These disturbances can be intermittent or continuous, slowly reversible or irreversible, but are severe, intrusive, and cause functional debility. Sufferers retain insight that these phenomena are the consequence of LSD use and usually seek psychiatric help.
HPPD may be diagnosed by the presence of an identifiable trigger, prodromal symptoms, and presentation onset; by the characteristics of the perceptual disturbances, their frequency, duration, intensity, and course; and by the accompanying negative affect and preserved insight.2
This LSD-induced persistence of visual imagery after the image is removed from the visual field is thought to result from dysfunction of serotonergic cortical inhibitory interneurons with GABAergic outputs that normally suppress visual processors.3 Clonazepam often is helpful.2
R. Andrew Sewell, MD
VA Connecticut Healthcare/Yale University
School of Medicine
New Haven, CT
David Kozin
McLean Hospital/Harvard Medical School
Belmont, MA
Miles G. Cunningham, MD, PhD
McLean Hospital/Harvard Medical School
Belmont, MA
1. Espiard ML, Lecardeur L, Abadie P, et al. Hallucinogen persisting perception disorder after psilocybin consumption: a case study. Eur Psychiatry. 2005;20:458-460.
2. Lerner AG, Gelkopf M, Skladman I, et al. Clonazepam treatment of lysergic acid diethylamide-induced hallucinogen persisting perception disorder with anxiety features. Int Clin Psychopharmacol. 2003;18:101-105.
3. Abraham HD, Aldridge AM. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.
1. Espiard ML, Lecardeur L, Abadie P, et al. Hallucinogen persisting perception disorder after psilocybin consumption: a case study. Eur Psychiatry. 2005;20:458-460.
2. Lerner AG, Gelkopf M, Skladman I, et al. Clonazepam treatment of lysergic acid diethylamide-induced hallucinogen persisting perception disorder with anxiety features. Int Clin Psychopharmacol. 2003;18:101-105.
3. Abraham HD, Aldridge AM. Adverse consequences of lysergic acid diethylamide. Addiction. 1993;88:1327-1334.