User login
Be Careful What You Ask For
I pose here a list of questions to consider before embarking on the creation of a new specialty in hospital medicine
1) What distinguishes the body of knowledge of hospital medicine from internal medicine (or pediatrics, for our colleagues in that field)?
While there is a body of literature supporting operational aspects of hospital care, as far as I can tell there is no difference in the way a hospitalist or office-based internist should treat pneumonia. Hospitalists develop areas of expertise in case management, understanding of hospital-based quality-improvement systems, communication skills, etc, but these fall short of a body of knowledge for a medical specialty. Books on hospital medicine do not differ from standard medicine texts in terms of disease pathophysiology, clinical presentation, diagnosis, or management. What then is the new body of knowledge?
2) Does hospital medicine really want to exclude office-based primary care doctors from managing their own cases in the hospital if they so choose?
Creating a new specialty of hospital medicine certainly would tend to do that. Let’s look at emergency medicine, for example. It used to be common for internists and surgeons to work in emergency rooms. That no longer is the case in many parts of this country because of the emergence of a new specialty. Do we want the same to be true for office-based doctors who care for their own patients?
3) Creating a new specialty requires special training. What is that going to be? Who teaches it and who will do it?
New subspecialties require additional training. For instance, electrophysiology is now a subspecialty of cardiology and requires an additional one or two years of training after a three-year cardiology fellowship. Working for 2-3 years as Dr. Nelson has proposed in the field of hospital medicine is not additional training, it is just additional practice. What is the formal training that the Society of Hospital Medicine proposes to qualify someone as a Board-certified hospitalist? Is it likely that young doctors are going to want to add on an additional 2 or 3 years of training beyond their internal medicine residency before they can start paying off their medical school loans? What will this training actually entail, and how will it merge with the internal medicine training programs that already exist?
I would point out that residents in fact are hospitalists in training. Certainly the vast majority of their clinical experience occurs in the hospital. Except for primary care residencies, I would estimate that 2/3 of the clinical care that internal medicine residents experience is in the hospital.
4) What about the primary care doctor or hospitalist who wants to switch careers?
Is the Society of Hospital Medicine going to require that a physician who has been in practice for 5 or 10 years and decides to switch to hospital medicine go through further training? Is that likely to occur? Alternatively, what about the hospitalist who gets tired of that field and wishes to become a primary care doctor? Might not office-based internists move to create their own specialty and thereby exclude hospitalists from work in that setting?
5) What about the malpractice risks that a new specialty will create?
Let’s imagine a world in which there are internists certified as hospitalists or as primary care physicians. Imagine this malpractice scenario. An office-based doctor caring for his/her own patients in the hospital is sued for some issue or another. The plaintiff attorney standing near the jury faces the doctor and asks “Doctor [he sighs, looking gravely serious], I understand there is a subspecialty in hospital medicine. Are you [now facing the jury] certified in that specialty? The doctor responds “No.” The attorney [turning abruptly back towards the nervous doctor] asks “No? Why not?” Let’s imagine another scenario. A hospitalist working part-time in an office-based practice 1 or 2 days a week faces a similar malpractice situation where he or she is sued. Attorney: “Doctor, I understand there is a subspecialty in primary care medicine? Are you certified in that specialty? Doctor: “No.” Attorney: “No? Why not?”
6) Why create more tests and expenses?
Enough said!
7) Do you want to bite the hand that feeds you?
In our hospital the vast majority of hospitalist admissions are from primary care doctors. Try to eliminate their admitting privileges and see what happens. It will be like the Flu vaccine fiasco this year. There is little vaccine available, but now everyone who has never gotten it in the past is asking for it. My guess is that most primary care doctors will protect their privileges and start admitting and caring for their own patients.
Why don’t we consider a more modest proposal? Here are three ideas.
First, identify areas of expertise that hospitalists actually develop. For instance, can they become procedural experts? Certainly the performance of lumbar punctures, thoracenteses, paracenteses, and central lines is something that most office-based doctors are not comfortable in carrying out any longer. Can we help create credentialing for these important procedures? That would go a long way towards initiating a set of skills that differentiates a hospitalist from an office-based doctor. Why not become a credentialing society for performance of these and other procedures? Monitoring numbers of procedures might constitute one measure, for example, of how to initiate credentialing. For instance, most centers no longer allow a cardiologist who has not performed a certain number of cardiac catheterizations a year to maintain privileges for that procedure. This does not seem discriminatory. It seems wise. I do not think office-based doctors would view credentialing for procedures as discriminatory.
Secondly, what about working to modify existing internal medicine training to perhaps provide added qualifications within hospital medicine for residents committed to the field? The board exams might actually differ then for primary care residents and for those interested in hospital medicine.
Thirdly, what about concentrating efforts on recertification? My guess is that very few residents coming out of practice would not feel qualified to take the hospital medicine or the ambulatory portion of an internal medicine exam. On the other hand, 10 years later during recertification many office-based doctors will not feel qualified to take an exam that emphasizes the treatment of vancomycin-resistant enterococci or management of cardiac arrests. Perhaps the recertification exam is the time to ask doctors to differentiate themselves. Some may wish to maintain certification in both hospital-based and ambulatory care, while others may choose one path or the other.
SHM has become the great organization it is in part because it reached out to hospitalists working in both community and teaching hospitals. Can we not bridge the gap with our office-based colleagues as well? In the field of internal medicine are we going to set ourselves up to become blue and red states? How about a nice shade of violet?
I pose here a list of questions to consider before embarking on the creation of a new specialty in hospital medicine
1) What distinguishes the body of knowledge of hospital medicine from internal medicine (or pediatrics, for our colleagues in that field)?
While there is a body of literature supporting operational aspects of hospital care, as far as I can tell there is no difference in the way a hospitalist or office-based internist should treat pneumonia. Hospitalists develop areas of expertise in case management, understanding of hospital-based quality-improvement systems, communication skills, etc, but these fall short of a body of knowledge for a medical specialty. Books on hospital medicine do not differ from standard medicine texts in terms of disease pathophysiology, clinical presentation, diagnosis, or management. What then is the new body of knowledge?
2) Does hospital medicine really want to exclude office-based primary care doctors from managing their own cases in the hospital if they so choose?
Creating a new specialty of hospital medicine certainly would tend to do that. Let’s look at emergency medicine, for example. It used to be common for internists and surgeons to work in emergency rooms. That no longer is the case in many parts of this country because of the emergence of a new specialty. Do we want the same to be true for office-based doctors who care for their own patients?
3) Creating a new specialty requires special training. What is that going to be? Who teaches it and who will do it?
New subspecialties require additional training. For instance, electrophysiology is now a subspecialty of cardiology and requires an additional one or two years of training after a three-year cardiology fellowship. Working for 2-3 years as Dr. Nelson has proposed in the field of hospital medicine is not additional training, it is just additional practice. What is the formal training that the Society of Hospital Medicine proposes to qualify someone as a Board-certified hospitalist? Is it likely that young doctors are going to want to add on an additional 2 or 3 years of training beyond their internal medicine residency before they can start paying off their medical school loans? What will this training actually entail, and how will it merge with the internal medicine training programs that already exist?
I would point out that residents in fact are hospitalists in training. Certainly the vast majority of their clinical experience occurs in the hospital. Except for primary care residencies, I would estimate that 2/3 of the clinical care that internal medicine residents experience is in the hospital.
4) What about the primary care doctor or hospitalist who wants to switch careers?
Is the Society of Hospital Medicine going to require that a physician who has been in practice for 5 or 10 years and decides to switch to hospital medicine go through further training? Is that likely to occur? Alternatively, what about the hospitalist who gets tired of that field and wishes to become a primary care doctor? Might not office-based internists move to create their own specialty and thereby exclude hospitalists from work in that setting?
5) What about the malpractice risks that a new specialty will create?
Let’s imagine a world in which there are internists certified as hospitalists or as primary care physicians. Imagine this malpractice scenario. An office-based doctor caring for his/her own patients in the hospital is sued for some issue or another. The plaintiff attorney standing near the jury faces the doctor and asks “Doctor [he sighs, looking gravely serious], I understand there is a subspecialty in hospital medicine. Are you [now facing the jury] certified in that specialty? The doctor responds “No.” The attorney [turning abruptly back towards the nervous doctor] asks “No? Why not?” Let’s imagine another scenario. A hospitalist working part-time in an office-based practice 1 or 2 days a week faces a similar malpractice situation where he or she is sued. Attorney: “Doctor, I understand there is a subspecialty in primary care medicine? Are you certified in that specialty? Doctor: “No.” Attorney: “No? Why not?”
6) Why create more tests and expenses?
Enough said!
7) Do you want to bite the hand that feeds you?
In our hospital the vast majority of hospitalist admissions are from primary care doctors. Try to eliminate their admitting privileges and see what happens. It will be like the Flu vaccine fiasco this year. There is little vaccine available, but now everyone who has never gotten it in the past is asking for it. My guess is that most primary care doctors will protect their privileges and start admitting and caring for their own patients.
Why don’t we consider a more modest proposal? Here are three ideas.
First, identify areas of expertise that hospitalists actually develop. For instance, can they become procedural experts? Certainly the performance of lumbar punctures, thoracenteses, paracenteses, and central lines is something that most office-based doctors are not comfortable in carrying out any longer. Can we help create credentialing for these important procedures? That would go a long way towards initiating a set of skills that differentiates a hospitalist from an office-based doctor. Why not become a credentialing society for performance of these and other procedures? Monitoring numbers of procedures might constitute one measure, for example, of how to initiate credentialing. For instance, most centers no longer allow a cardiologist who has not performed a certain number of cardiac catheterizations a year to maintain privileges for that procedure. This does not seem discriminatory. It seems wise. I do not think office-based doctors would view credentialing for procedures as discriminatory.
Secondly, what about working to modify existing internal medicine training to perhaps provide added qualifications within hospital medicine for residents committed to the field? The board exams might actually differ then for primary care residents and for those interested in hospital medicine.
Thirdly, what about concentrating efforts on recertification? My guess is that very few residents coming out of practice would not feel qualified to take the hospital medicine or the ambulatory portion of an internal medicine exam. On the other hand, 10 years later during recertification many office-based doctors will not feel qualified to take an exam that emphasizes the treatment of vancomycin-resistant enterococci or management of cardiac arrests. Perhaps the recertification exam is the time to ask doctors to differentiate themselves. Some may wish to maintain certification in both hospital-based and ambulatory care, while others may choose one path or the other.
SHM has become the great organization it is in part because it reached out to hospitalists working in both community and teaching hospitals. Can we not bridge the gap with our office-based colleagues as well? In the field of internal medicine are we going to set ourselves up to become blue and red states? How about a nice shade of violet?
I pose here a list of questions to consider before embarking on the creation of a new specialty in hospital medicine
1) What distinguishes the body of knowledge of hospital medicine from internal medicine (or pediatrics, for our colleagues in that field)?
While there is a body of literature supporting operational aspects of hospital care, as far as I can tell there is no difference in the way a hospitalist or office-based internist should treat pneumonia. Hospitalists develop areas of expertise in case management, understanding of hospital-based quality-improvement systems, communication skills, etc, but these fall short of a body of knowledge for a medical specialty. Books on hospital medicine do not differ from standard medicine texts in terms of disease pathophysiology, clinical presentation, diagnosis, or management. What then is the new body of knowledge?
2) Does hospital medicine really want to exclude office-based primary care doctors from managing their own cases in the hospital if they so choose?
Creating a new specialty of hospital medicine certainly would tend to do that. Let’s look at emergency medicine, for example. It used to be common for internists and surgeons to work in emergency rooms. That no longer is the case in many parts of this country because of the emergence of a new specialty. Do we want the same to be true for office-based doctors who care for their own patients?
3) Creating a new specialty requires special training. What is that going to be? Who teaches it and who will do it?
New subspecialties require additional training. For instance, electrophysiology is now a subspecialty of cardiology and requires an additional one or two years of training after a three-year cardiology fellowship. Working for 2-3 years as Dr. Nelson has proposed in the field of hospital medicine is not additional training, it is just additional practice. What is the formal training that the Society of Hospital Medicine proposes to qualify someone as a Board-certified hospitalist? Is it likely that young doctors are going to want to add on an additional 2 or 3 years of training beyond their internal medicine residency before they can start paying off their medical school loans? What will this training actually entail, and how will it merge with the internal medicine training programs that already exist?
I would point out that residents in fact are hospitalists in training. Certainly the vast majority of their clinical experience occurs in the hospital. Except for primary care residencies, I would estimate that 2/3 of the clinical care that internal medicine residents experience is in the hospital.
4) What about the primary care doctor or hospitalist who wants to switch careers?
Is the Society of Hospital Medicine going to require that a physician who has been in practice for 5 or 10 years and decides to switch to hospital medicine go through further training? Is that likely to occur? Alternatively, what about the hospitalist who gets tired of that field and wishes to become a primary care doctor? Might not office-based internists move to create their own specialty and thereby exclude hospitalists from work in that setting?
5) What about the malpractice risks that a new specialty will create?
Let’s imagine a world in which there are internists certified as hospitalists or as primary care physicians. Imagine this malpractice scenario. An office-based doctor caring for his/her own patients in the hospital is sued for some issue or another. The plaintiff attorney standing near the jury faces the doctor and asks “Doctor [he sighs, looking gravely serious], I understand there is a subspecialty in hospital medicine. Are you [now facing the jury] certified in that specialty? The doctor responds “No.” The attorney [turning abruptly back towards the nervous doctor] asks “No? Why not?” Let’s imagine another scenario. A hospitalist working part-time in an office-based practice 1 or 2 days a week faces a similar malpractice situation where he or she is sued. Attorney: “Doctor, I understand there is a subspecialty in primary care medicine? Are you certified in that specialty? Doctor: “No.” Attorney: “No? Why not?”
6) Why create more tests and expenses?
Enough said!
7) Do you want to bite the hand that feeds you?
In our hospital the vast majority of hospitalist admissions are from primary care doctors. Try to eliminate their admitting privileges and see what happens. It will be like the Flu vaccine fiasco this year. There is little vaccine available, but now everyone who has never gotten it in the past is asking for it. My guess is that most primary care doctors will protect their privileges and start admitting and caring for their own patients.
Why don’t we consider a more modest proposal? Here are three ideas.
First, identify areas of expertise that hospitalists actually develop. For instance, can they become procedural experts? Certainly the performance of lumbar punctures, thoracenteses, paracenteses, and central lines is something that most office-based doctors are not comfortable in carrying out any longer. Can we help create credentialing for these important procedures? That would go a long way towards initiating a set of skills that differentiates a hospitalist from an office-based doctor. Why not become a credentialing society for performance of these and other procedures? Monitoring numbers of procedures might constitute one measure, for example, of how to initiate credentialing. For instance, most centers no longer allow a cardiologist who has not performed a certain number of cardiac catheterizations a year to maintain privileges for that procedure. This does not seem discriminatory. It seems wise. I do not think office-based doctors would view credentialing for procedures as discriminatory.
Secondly, what about working to modify existing internal medicine training to perhaps provide added qualifications within hospital medicine for residents committed to the field? The board exams might actually differ then for primary care residents and for those interested in hospital medicine.
Thirdly, what about concentrating efforts on recertification? My guess is that very few residents coming out of practice would not feel qualified to take the hospital medicine or the ambulatory portion of an internal medicine exam. On the other hand, 10 years later during recertification many office-based doctors will not feel qualified to take an exam that emphasizes the treatment of vancomycin-resistant enterococci or management of cardiac arrests. Perhaps the recertification exam is the time to ask doctors to differentiate themselves. Some may wish to maintain certification in both hospital-based and ambulatory care, while others may choose one path or the other.
SHM has become the great organization it is in part because it reached out to hospitalists working in both community and teaching hospitals. Can we not bridge the gap with our office-based colleagues as well? In the field of internal medicine are we going to set ourselves up to become blue and red states? How about a nice shade of violet?
An Ongoing Analysis of the 2003-04 SHM Productivity and Compensation Survey
The survey analysis of productivity breaks this performance measure into two categories:
- Inputs: The hours worked by hospitalists. Three categories of hours worked are analyzed in this chapter: inpatient hours worked, non-patient hours worked, and on-call hours worked. Please note, the analysis excludes outpatient hours worked because only 15% of the survey respondents reported any outpatient hours.
- Outputs: The work completed by hospitalists. This includes charges generated, collections generated, patient encounters, patient admissions and consults, and relative value units (RVUs) of work completed. These measures are analyzed in chapter 5 (to be published in the March/April Hospitalist issue.
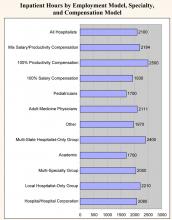
Overall, surveyed physician hospitalists worked a median of 2,100 inpatient hours per year. They had a median of 50 non-patient hours per year (about one per week) and worked a median of 600 on-call hours per year. The analyses below look at productivity inputs by region, employment model, specialty/provider type, and compensation model.
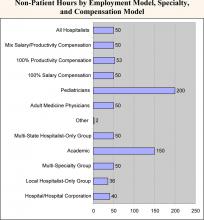
- Academic hospitalists work the least amount of inpatient hours (1,700 vs. an overall median of 2,100). However, they work significantly more non‑patient hours (150 vs. 50), probably because of their teaching responsibilities.
- Hospitalists that work for hospitalist-only groups work more inpatient hours than the overall median: multistate hospitalist only groups are 14% higher (2,400 vs. 2,100), while local hospitalist-only groups are 5% higher (2,210 vs. 2,100).
- Regarding on-call hours, hospitalists that work for hospital-based groups have a median of zero. This is probably because only 27% of hospital-based groups have call-based staffing, significantly less than other employment categories (see Chapter 1). This also is probably the explanation for the median of zero for eastern hospitalists, as that region has a high proportion of hospital-based groups.
- Adult medicine hospitalists work 24% more inpatient hours than pediatric hospitalists (2,111 vs. 1,700). Conversely, pediatric hospitalists have four times as many non-patient hours (200 vs. 50). This is likely explained by the fact that pediatricians are twice as likely to work in academia (see chapter 1).
- Non-physician hospitalists have a median of 1,900 inpatient hours and a median of only 10 non-patient hours
- There is a strong relationship between compensation model and hours worked. Hospitalists that work under a 100% productivity model have a median number of inpatient hours that is 30% more than those that work in a 100% salary model (2,500 vs. 1,930). Hospitalists that work in a mixed model fall in the middle (2,184).
- There is minimal difference in the non-patient hours worked among the three categories (approximately 50). However, 100% productivity-model hospitalists have a median number of on-call hours, which is almost 3 times greater than that of 100% salary-based hospitalists (1,250 vs. 416). Again, mixed-model hospitalists fall in the middle (700).
The survey analysis of productivity breaks this performance measure into two categories:
- Inputs: The hours worked by hospitalists. Three categories of hours worked are analyzed in this chapter: inpatient hours worked, non-patient hours worked, and on-call hours worked. Please note, the analysis excludes outpatient hours worked because only 15% of the survey respondents reported any outpatient hours.
- Outputs: The work completed by hospitalists. This includes charges generated, collections generated, patient encounters, patient admissions and consults, and relative value units (RVUs) of work completed. These measures are analyzed in chapter 5 (to be published in the March/April Hospitalist issue.
Overall, surveyed physician hospitalists worked a median of 2,100 inpatient hours per year. They had a median of 50 non-patient hours per year (about one per week) and worked a median of 600 on-call hours per year. The analyses below look at productivity inputs by region, employment model, specialty/provider type, and compensation model.
- Academic hospitalists work the least amount of inpatient hours (1,700 vs. an overall median of 2,100). However, they work significantly more non‑patient hours (150 vs. 50), probably because of their teaching responsibilities.
- Hospitalists that work for hospitalist-only groups work more inpatient hours than the overall median: multistate hospitalist only groups are 14% higher (2,400 vs. 2,100), while local hospitalist-only groups are 5% higher (2,210 vs. 2,100).
- Regarding on-call hours, hospitalists that work for hospital-based groups have a median of zero. This is probably because only 27% of hospital-based groups have call-based staffing, significantly less than other employment categories (see Chapter 1). This also is probably the explanation for the median of zero for eastern hospitalists, as that region has a high proportion of hospital-based groups.
- Adult medicine hospitalists work 24% more inpatient hours than pediatric hospitalists (2,111 vs. 1,700). Conversely, pediatric hospitalists have four times as many non-patient hours (200 vs. 50). This is likely explained by the fact that pediatricians are twice as likely to work in academia (see chapter 1).
- Non-physician hospitalists have a median of 1,900 inpatient hours and a median of only 10 non-patient hours
- There is a strong relationship between compensation model and hours worked. Hospitalists that work under a 100% productivity model have a median number of inpatient hours that is 30% more than those that work in a 100% salary model (2,500 vs. 1,930). Hospitalists that work in a mixed model fall in the middle (2,184).
- There is minimal difference in the non-patient hours worked among the three categories (approximately 50). However, 100% productivity-model hospitalists have a median number of on-call hours, which is almost 3 times greater than that of 100% salary-based hospitalists (1,250 vs. 416). Again, mixed-model hospitalists fall in the middle (700).
The survey analysis of productivity breaks this performance measure into two categories:
- Inputs: The hours worked by hospitalists. Three categories of hours worked are analyzed in this chapter: inpatient hours worked, non-patient hours worked, and on-call hours worked. Please note, the analysis excludes outpatient hours worked because only 15% of the survey respondents reported any outpatient hours.
- Outputs: The work completed by hospitalists. This includes charges generated, collections generated, patient encounters, patient admissions and consults, and relative value units (RVUs) of work completed. These measures are analyzed in chapter 5 (to be published in the March/April Hospitalist issue.
Overall, surveyed physician hospitalists worked a median of 2,100 inpatient hours per year. They had a median of 50 non-patient hours per year (about one per week) and worked a median of 600 on-call hours per year. The analyses below look at productivity inputs by region, employment model, specialty/provider type, and compensation model.
- Academic hospitalists work the least amount of inpatient hours (1,700 vs. an overall median of 2,100). However, they work significantly more non‑patient hours (150 vs. 50), probably because of their teaching responsibilities.
- Hospitalists that work for hospitalist-only groups work more inpatient hours than the overall median: multistate hospitalist only groups are 14% higher (2,400 vs. 2,100), while local hospitalist-only groups are 5% higher (2,210 vs. 2,100).
- Regarding on-call hours, hospitalists that work for hospital-based groups have a median of zero. This is probably because only 27% of hospital-based groups have call-based staffing, significantly less than other employment categories (see Chapter 1). This also is probably the explanation for the median of zero for eastern hospitalists, as that region has a high proportion of hospital-based groups.
- Adult medicine hospitalists work 24% more inpatient hours than pediatric hospitalists (2,111 vs. 1,700). Conversely, pediatric hospitalists have four times as many non-patient hours (200 vs. 50). This is likely explained by the fact that pediatricians are twice as likely to work in academia (see chapter 1).
- Non-physician hospitalists have a median of 1,900 inpatient hours and a median of only 10 non-patient hours
- There is a strong relationship between compensation model and hours worked. Hospitalists that work under a 100% productivity model have a median number of inpatient hours that is 30% more than those that work in a 100% salary model (2,500 vs. 1,930). Hospitalists that work in a mixed model fall in the middle (2,184).
- There is minimal difference in the non-patient hours worked among the three categories (approximately 50). However, 100% productivity-model hospitalists have a median number of on-call hours, which is almost 3 times greater than that of 100% salary-based hospitalists (1,250 vs. 416). Again, mixed-model hospitalists fall in the middle (700).
Thanks for the Memories
This issue of The Hospitalist marks the beginning of my sixth year as the chief executive officer at SHM. Much has happened at SHM and in our specialty in the last 5 years, and
I thought I would use this space to share with everyone what we have accomplished together and to recognize the many individuals who have made all of this possible.
Past
When I first came to SHM in January of 2000, SHM had two employees, three or four committees, and about 500members. There were estimated to be 1000-2000 hospitalists in the country. SHM did stage an Annual Meeting with 300 attendees and published a newsletter of 16 pages with minimal ad revenue and a circulation of about 1000. SHM had no external grants and limited relationships with industry.
SHM was a fledgling national organization with no local presence. SHM had minimal assets or infrastructure and was very reliant on ACP for support and direction. Most of the innovation and direction fell to a few hospitalists around the country, who, while devoted to SHM (then NAIP) and our specialty, still had a very full plate just doing their day jobs, growing their hospital medicine groups. It was amazing what they had accomplished with minimal staff support or infrastructure.
At the start of the new millennium, SHM didn’t know how many hospitals had hospitalists. There was no data on how hard hospitalists should be expected to work or how much they should be paid. There was limited data on the background or training of those doctors who were going into hospital medicine, and there was no understanding of what the knowledge base was for this new specialty. There was a vague sense that the importance of hospitalists was more than just seeing their own patients, but there was little understanding of what value hospitalists could add to their health communities.
Present
Over the last 5 years, together we have made enormous progress. We have changed our name from the National Association of Inpatient Physicians to the Society of Hospital Medicine to better reflect all the stakeholders in our growing specialty. We have grown our Philadelphia staff to 13 and employ another five staff in Boston, Atlanta, and California. The Hospitalist newsletter is now the recognized publication in hospital medicine with 65-80 pages per issue, 2-3 supplements each year, and a circulation well over 10,000. There are more than $75,000 in recruitment ads in each issue, as much a testament to the growth of the specialty as anything else.
SHM’s Annual Meeting now attracts almost 1000 attendees and is the primary networking opportunity for the fastest-growing medical specialty. SHM has almost 5000 members, and there are an estimated 10,000-12,000 hospitalists now practicing in over 1500 hospitals. SHM currently has more than 40 local chapters meeting at least once a year throughout the country.
SHM has developed unique expertise in the management aspects of hospital medicine and holds practice management courses at least three times each year. In addition, SHM has realized that hospitalists will need to be the leaders of the hospitals of the future and has created Leadership Academies to train these future leaders. SHM has worked with grants from the Hartford Foundation to establish the hospitalist as the physician for the acutely ill elderly. SHM is working with the Robert Wood Johnson Foundation and others in helping to create the physical design of the hospital of the future.
SHM is just completing the Core Curriculum for Hospital Medicine, which will define the knowledge base for our specialty and serve as the basis for SHM’s growing educational enterprise. SHM is defining the value that hospitalists add beyond just direct patient care. This phenomenon has been the basis for hospitals looking for innovative ways to grow and support their hospital medicine groups. SHM will publish these white papers for hospitalists and hospital executives to use in designing their hospital medicine programs.
SHM has defined the productivity and compensation data for our specialty in our biannual surveys that are the best source for hospitalist data. SHM has developed a Washington presence and is defining the advocacy issues for hospital medicine, including substantial reform of payment to de-emphasize compensation based solely on the unit of the visit or the procedure.
SHM is now an organization with almost $3 million in assets, completely autonomous, and functioning on its own. We have a strong and growing relationship with ACP, and SHM has reached out to partner with many other organizations, including the AHA, ACCP, JCAHO, RWJ, Hartford Foundation, CDC, AACN, ASHP, ABIM, AAP, SGIM, AAIM and many others.
Future
And there is much to look forward to in the next 5 years. In the coming months, SHM will launch the first journal in hospital medicine in January 2006. SHM’s Web site will come into the 21st century with the ability for each member to have their own Web page. The Web site will be the one location that hospitalists can come to for CME and other educational information. SHM will be working with AACP, AACN, ASHP, and others to establish an Acute Care Collaborative, reorganizing hospital workflow to deliver measurable higher-quality health care using interdisciplinary teams of health professionals. This will help to define the hospital of the future.
There will be a certification for hospitalists in the near future. This will define how hospitalists add value and how we are different from other internists, pediatricians, and family practitioners. SHM will also be using the Core Curriculum to not only drive SHM post-graduate education, but to help redefine residency training to produce more and better-trained individuals for a future that includes 30,000 to 40,000 hospitalists.
This has been quite a ride in the last 5 years. I have been fortunate enough to have had a front row seat. And I am not going anywhere soon. This is way too much fun. I just wanted to share with you a few others who have been instrumental in growing SHM.
A Special Thank You to Those Who Did the Work
SHM Presidents
John Nelson
Win Whitcomb
Bob Wachter
Ron Angus
Mark Williams
Jeff Dichter
Jeanne Huddleston
SHM Board Members (in addition to all Presidents)
Bill Atchley
Brad Flansbaum
David Zipes
Diane Craig
Herb Rogove
Jan Merin
Lisa Kettering
Mark Aronson
Mary Jo Gorman
Mike Ruhlen
Mitch Wilson
Pat Cawley
Peter Lindenauer
Richard Slataper
Russ Holman
Steve Pantilat
Editors, The Hospitalist
Scott Flanders
Jim Pile
Committee & Council Chairs (in addition to Board members)
Alpesh Amin
Andy Auerbach
Don Krause
Jack Percelay
Joe Li
Lakshmi Halasyamani
Mike Pistoria
Natalie Correia
Neil Kripalani
Preetha Basaviah
Sanjay Saint
Shaun Frost
Stacy Goldsholl
Sylvia McKean
Teresa Jones
Tim Cornell
Vineet Arora
SHM Staff
Angela Musial
Erica Pearson
Jane Mihelic
Kevin Stevens
Marie Francois
Marilyn Rivera
Michelle D’Agostino
Vera Bensch
Vernita Jackson
Veronica BeUs
Joe Miller
Tina Budnitz
This issue of The Hospitalist marks the beginning of my sixth year as the chief executive officer at SHM. Much has happened at SHM and in our specialty in the last 5 years, and
I thought I would use this space to share with everyone what we have accomplished together and to recognize the many individuals who have made all of this possible.
Past
When I first came to SHM in January of 2000, SHM had two employees, three or four committees, and about 500members. There were estimated to be 1000-2000 hospitalists in the country. SHM did stage an Annual Meeting with 300 attendees and published a newsletter of 16 pages with minimal ad revenue and a circulation of about 1000. SHM had no external grants and limited relationships with industry.
SHM was a fledgling national organization with no local presence. SHM had minimal assets or infrastructure and was very reliant on ACP for support and direction. Most of the innovation and direction fell to a few hospitalists around the country, who, while devoted to SHM (then NAIP) and our specialty, still had a very full plate just doing their day jobs, growing their hospital medicine groups. It was amazing what they had accomplished with minimal staff support or infrastructure.
At the start of the new millennium, SHM didn’t know how many hospitals had hospitalists. There was no data on how hard hospitalists should be expected to work or how much they should be paid. There was limited data on the background or training of those doctors who were going into hospital medicine, and there was no understanding of what the knowledge base was for this new specialty. There was a vague sense that the importance of hospitalists was more than just seeing their own patients, but there was little understanding of what value hospitalists could add to their health communities.
Present
Over the last 5 years, together we have made enormous progress. We have changed our name from the National Association of Inpatient Physicians to the Society of Hospital Medicine to better reflect all the stakeholders in our growing specialty. We have grown our Philadelphia staff to 13 and employ another five staff in Boston, Atlanta, and California. The Hospitalist newsletter is now the recognized publication in hospital medicine with 65-80 pages per issue, 2-3 supplements each year, and a circulation well over 10,000. There are more than $75,000 in recruitment ads in each issue, as much a testament to the growth of the specialty as anything else.
SHM’s Annual Meeting now attracts almost 1000 attendees and is the primary networking opportunity for the fastest-growing medical specialty. SHM has almost 5000 members, and there are an estimated 10,000-12,000 hospitalists now practicing in over 1500 hospitals. SHM currently has more than 40 local chapters meeting at least once a year throughout the country.
SHM has developed unique expertise in the management aspects of hospital medicine and holds practice management courses at least three times each year. In addition, SHM has realized that hospitalists will need to be the leaders of the hospitals of the future and has created Leadership Academies to train these future leaders. SHM has worked with grants from the Hartford Foundation to establish the hospitalist as the physician for the acutely ill elderly. SHM is working with the Robert Wood Johnson Foundation and others in helping to create the physical design of the hospital of the future.
SHM is just completing the Core Curriculum for Hospital Medicine, which will define the knowledge base for our specialty and serve as the basis for SHM’s growing educational enterprise. SHM is defining the value that hospitalists add beyond just direct patient care. This phenomenon has been the basis for hospitals looking for innovative ways to grow and support their hospital medicine groups. SHM will publish these white papers for hospitalists and hospital executives to use in designing their hospital medicine programs.
SHM has defined the productivity and compensation data for our specialty in our biannual surveys that are the best source for hospitalist data. SHM has developed a Washington presence and is defining the advocacy issues for hospital medicine, including substantial reform of payment to de-emphasize compensation based solely on the unit of the visit or the procedure.
SHM is now an organization with almost $3 million in assets, completely autonomous, and functioning on its own. We have a strong and growing relationship with ACP, and SHM has reached out to partner with many other organizations, including the AHA, ACCP, JCAHO, RWJ, Hartford Foundation, CDC, AACN, ASHP, ABIM, AAP, SGIM, AAIM and many others.
Future
And there is much to look forward to in the next 5 years. In the coming months, SHM will launch the first journal in hospital medicine in January 2006. SHM’s Web site will come into the 21st century with the ability for each member to have their own Web page. The Web site will be the one location that hospitalists can come to for CME and other educational information. SHM will be working with AACP, AACN, ASHP, and others to establish an Acute Care Collaborative, reorganizing hospital workflow to deliver measurable higher-quality health care using interdisciplinary teams of health professionals. This will help to define the hospital of the future.
There will be a certification for hospitalists in the near future. This will define how hospitalists add value and how we are different from other internists, pediatricians, and family practitioners. SHM will also be using the Core Curriculum to not only drive SHM post-graduate education, but to help redefine residency training to produce more and better-trained individuals for a future that includes 30,000 to 40,000 hospitalists.
This has been quite a ride in the last 5 years. I have been fortunate enough to have had a front row seat. And I am not going anywhere soon. This is way too much fun. I just wanted to share with you a few others who have been instrumental in growing SHM.
A Special Thank You to Those Who Did the Work
SHM Presidents
John Nelson
Win Whitcomb
Bob Wachter
Ron Angus
Mark Williams
Jeff Dichter
Jeanne Huddleston
SHM Board Members (in addition to all Presidents)
Bill Atchley
Brad Flansbaum
David Zipes
Diane Craig
Herb Rogove
Jan Merin
Lisa Kettering
Mark Aronson
Mary Jo Gorman
Mike Ruhlen
Mitch Wilson
Pat Cawley
Peter Lindenauer
Richard Slataper
Russ Holman
Steve Pantilat
Editors, The Hospitalist
Scott Flanders
Jim Pile
Committee & Council Chairs (in addition to Board members)
Alpesh Amin
Andy Auerbach
Don Krause
Jack Percelay
Joe Li
Lakshmi Halasyamani
Mike Pistoria
Natalie Correia
Neil Kripalani
Preetha Basaviah
Sanjay Saint
Shaun Frost
Stacy Goldsholl
Sylvia McKean
Teresa Jones
Tim Cornell
Vineet Arora
SHM Staff
Angela Musial
Erica Pearson
Jane Mihelic
Kevin Stevens
Marie Francois
Marilyn Rivera
Michelle D’Agostino
Vera Bensch
Vernita Jackson
Veronica BeUs
Joe Miller
Tina Budnitz
This issue of The Hospitalist marks the beginning of my sixth year as the chief executive officer at SHM. Much has happened at SHM and in our specialty in the last 5 years, and
I thought I would use this space to share with everyone what we have accomplished together and to recognize the many individuals who have made all of this possible.
Past
When I first came to SHM in January of 2000, SHM had two employees, three or four committees, and about 500members. There were estimated to be 1000-2000 hospitalists in the country. SHM did stage an Annual Meeting with 300 attendees and published a newsletter of 16 pages with minimal ad revenue and a circulation of about 1000. SHM had no external grants and limited relationships with industry.
SHM was a fledgling national organization with no local presence. SHM had minimal assets or infrastructure and was very reliant on ACP for support and direction. Most of the innovation and direction fell to a few hospitalists around the country, who, while devoted to SHM (then NAIP) and our specialty, still had a very full plate just doing their day jobs, growing their hospital medicine groups. It was amazing what they had accomplished with minimal staff support or infrastructure.
At the start of the new millennium, SHM didn’t know how many hospitals had hospitalists. There was no data on how hard hospitalists should be expected to work or how much they should be paid. There was limited data on the background or training of those doctors who were going into hospital medicine, and there was no understanding of what the knowledge base was for this new specialty. There was a vague sense that the importance of hospitalists was more than just seeing their own patients, but there was little understanding of what value hospitalists could add to their health communities.
Present
Over the last 5 years, together we have made enormous progress. We have changed our name from the National Association of Inpatient Physicians to the Society of Hospital Medicine to better reflect all the stakeholders in our growing specialty. We have grown our Philadelphia staff to 13 and employ another five staff in Boston, Atlanta, and California. The Hospitalist newsletter is now the recognized publication in hospital medicine with 65-80 pages per issue, 2-3 supplements each year, and a circulation well over 10,000. There are more than $75,000 in recruitment ads in each issue, as much a testament to the growth of the specialty as anything else.
SHM’s Annual Meeting now attracts almost 1000 attendees and is the primary networking opportunity for the fastest-growing medical specialty. SHM has almost 5000 members, and there are an estimated 10,000-12,000 hospitalists now practicing in over 1500 hospitals. SHM currently has more than 40 local chapters meeting at least once a year throughout the country.
SHM has developed unique expertise in the management aspects of hospital medicine and holds practice management courses at least three times each year. In addition, SHM has realized that hospitalists will need to be the leaders of the hospitals of the future and has created Leadership Academies to train these future leaders. SHM has worked with grants from the Hartford Foundation to establish the hospitalist as the physician for the acutely ill elderly. SHM is working with the Robert Wood Johnson Foundation and others in helping to create the physical design of the hospital of the future.
SHM is just completing the Core Curriculum for Hospital Medicine, which will define the knowledge base for our specialty and serve as the basis for SHM’s growing educational enterprise. SHM is defining the value that hospitalists add beyond just direct patient care. This phenomenon has been the basis for hospitals looking for innovative ways to grow and support their hospital medicine groups. SHM will publish these white papers for hospitalists and hospital executives to use in designing their hospital medicine programs.
SHM has defined the productivity and compensation data for our specialty in our biannual surveys that are the best source for hospitalist data. SHM has developed a Washington presence and is defining the advocacy issues for hospital medicine, including substantial reform of payment to de-emphasize compensation based solely on the unit of the visit or the procedure.
SHM is now an organization with almost $3 million in assets, completely autonomous, and functioning on its own. We have a strong and growing relationship with ACP, and SHM has reached out to partner with many other organizations, including the AHA, ACCP, JCAHO, RWJ, Hartford Foundation, CDC, AACN, ASHP, ABIM, AAP, SGIM, AAIM and many others.
Future
And there is much to look forward to in the next 5 years. In the coming months, SHM will launch the first journal in hospital medicine in January 2006. SHM’s Web site will come into the 21st century with the ability for each member to have their own Web page. The Web site will be the one location that hospitalists can come to for CME and other educational information. SHM will be working with AACP, AACN, ASHP, and others to establish an Acute Care Collaborative, reorganizing hospital workflow to deliver measurable higher-quality health care using interdisciplinary teams of health professionals. This will help to define the hospital of the future.
There will be a certification for hospitalists in the near future. This will define how hospitalists add value and how we are different from other internists, pediatricians, and family practitioners. SHM will also be using the Core Curriculum to not only drive SHM post-graduate education, but to help redefine residency training to produce more and better-trained individuals for a future that includes 30,000 to 40,000 hospitalists.
This has been quite a ride in the last 5 years. I have been fortunate enough to have had a front row seat. And I am not going anywhere soon. This is way too much fun. I just wanted to share with you a few others who have been instrumental in growing SHM.
A Special Thank You to Those Who Did the Work
SHM Presidents
John Nelson
Win Whitcomb
Bob Wachter
Ron Angus
Mark Williams
Jeff Dichter
Jeanne Huddleston
SHM Board Members (in addition to all Presidents)
Bill Atchley
Brad Flansbaum
David Zipes
Diane Craig
Herb Rogove
Jan Merin
Lisa Kettering
Mark Aronson
Mary Jo Gorman
Mike Ruhlen
Mitch Wilson
Pat Cawley
Peter Lindenauer
Richard Slataper
Russ Holman
Steve Pantilat
Editors, The Hospitalist
Scott Flanders
Jim Pile
Committee & Council Chairs (in addition to Board members)
Alpesh Amin
Andy Auerbach
Don Krause
Jack Percelay
Joe Li
Lakshmi Halasyamani
Mike Pistoria
Natalie Correia
Neil Kripalani
Preetha Basaviah
Sanjay Saint
Shaun Frost
Stacy Goldsholl
Sylvia McKean
Teresa Jones
Tim Cornell
Vineet Arora
SHM Staff
Angela Musial
Erica Pearson
Jane Mihelic
Kevin Stevens
Marie Francois
Marilyn Rivera
Michelle D’Agostino
Vera Bensch
Vernita Jackson
Veronica BeUs
Joe Miller
Tina Budnitz
The Campaign to Save 100,000 Lives
I am on a plane on my way back to Minnesota after being professionally rejuvenated by the content of the Institute of Healthcare Improvement’s 16th Annual Forum, in Orlando, FL. The theme of the meeting called on all hospitals, and hence I believe all hospitalists, to save lives. Dr. Donald Berwick, President and CEO of the Institute of Healthcare Improvement (IHI) kicked off this years’ Annual Forum with his plenary speech “Some is not a number, Soon is not a time.” Saving some lives, some time in the future is not a clear goal. “Some is not a number and soon is not a time.” So, he put the challenge forth for hospitals to join IHI in a campaign to save 100K lives by June 14, 2006 at 9:00 a.m. EDT.
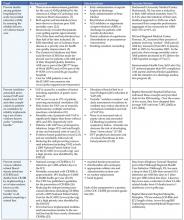
“Some is not a number. Soon is not a time.” We all get “why” this is important, at least in so much as what we have been told by the Institute of Medicine Reports “To Err is Human” and “Crossing the Quality Chasm”. But “how” can this be done? By doing things that we already know impact mortality in a hospital setting. By engaging in the reliable care delivery of six changes that save lives. These include recommendations in each of the following areas: rapid response or emergency medical teams, reliable care for acute myocardial infarctions, reliable use of the ventilator pneumonia and central venous line “bundles”, surgical site infection prophylaxis, and prevention of adverse drug events with reconciliation. Each is described in more detail below.
- Rapid Response Teams (also known as Medical Emergency or Pre-Code Teams): This is a team of healthcare providers that may be summoned at any time by anyone in the hospital to assist in the care of a patient who appears acutely ill, before the patient has respiratory failure, a cardiac arrest or other adverse event. The aim is to prevent situations of “failure to rescue”, to recognize the early signs and symptoms of clinical deterioration prior to requiring transfer to the intensive care unit.
- Reliable Care for Acute Myocardial Infarction (AMI): For appropriate AMI patients, reliable use of all of the following treatments: early administration of aspirin, aspirin at discharge, early administration of a beta-blocker, beta-blocker at discharge, ACE‑inhibitor or angiotensin receptor blocker (ARB) at discharge (if systolic dysfunction), timely reperfusion, and smoking cessation counseling.
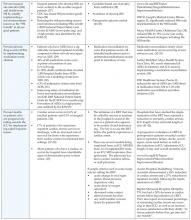
- Reliable use of the Ventilator Bundle: A number of hospitals have initiated the use of the ventilator bundle to prevent ventilator associated pneumonia (VAP). VAP carries a high mortality rate. The “bundle” is a grouping of 5 treatments/preventions measured as a composite (% of patients that get all 5).
- Elevate head of bed to 30 degrees
- Peptic ulcer prophylaxis
- Deep venous thrombosis prophylaxis
- Daily “sedation vacation”
- Daily assessment of readiness to extubate
Not all of the items have a specific relationship to VAP (e.g., DVT prophylaxis), but when reliably performed in concert with the other items, leads to a decrease in VAP.
- Reliable use of Central Venous Line Bundles: This is a grouping of 5 preventative measures that when done in concert and measured as a composite have had maximal effectiveness for the reduction of central line associated blood stream infections (CLABs) in some hospitals.
- Hand hygiene
- Maximal barrier precautions
- Chlorhexidine skin antisepsis
- Appropriate catheter site and administration system care
- No routine line replacement
- Surgical site infection (SSI) prophylaxis with a “SSI bundle”: Hospitals participating with the IHI in a variety of different formats have found the most substantial reduction/prevention of SSIs when 3 preventative measures are done in concert with each other for every surgical patient. These preventative measures include:
- Guideline-based use of prophylactic perioperative antibiotics (including both choice and timing of administration of antibiotic)
- Appropriate hair removal (avoiding shaving)
- Perioperative glucose control
- Prevention of adverse drug events with medication reconciliation: This refers to the procedures that can be put in place at the time of any transition of care to mitigate the increased risk of wrong dose of medication or even wrong drug being administered immediately following that transition. Each time we have to transfer information from one sheet of paper to another or from a sheet of paper to a computer, there is chance for human error. Medication reconciliation can virtually eliminate errors occurring at transitions in care.
I have placed in Table 1, the information (goals, background, proposed interventions and success stories) handed out during Dr. Donald Berwick’s opening plenary session, the kick-off for the campaign to save 100,000 lives.
Two key components of the descriptions above deserve further explanation. One of the key components is the concept of reliability. Reliability is how often something in health care does what it is supposed to do, in the time frame it is supposed to do it in. The formula is the number of times that something (delivery of a medication or service) is done correctly divided by the number of times that same something is attempted. In work published by Karl Weick, one common principle within high reliability organizations is that of a preoccupation with failure. As such, the notation of reliability is a measure of defects. Currently much of healthcare (including use of beta-blockers after AMI) functions at a 10-1 level of performance (one defect in 10 tries) or less than a 90% success rate. Organizations that have actively embraced this concept of reliability in their quality improvement work have rejected the usual satisfaction with 10-1 performance. Shouldn’t 99 out of 100 (or 999 out of a 1,000) patients with an AMI get what they are supposed to get?
The other key component embedded within some of the six items that save lives is the concept of bundles. Rather than considering individual measures for each of the items within a bundle, a composite or aggregate measure is reported. Bottom line is that doing any one or two of the items in a bundle is not good enough. It will not achieve the same reduction in hospital acquired infection rates or mortality, as doing all of the items in concert for every appropriate patient.
How can hospitalists help achieve this national goal, to participate in this campaign with the IHI? As individuals, we can be a hospital “precinct captain” or champion, speak to our hospital boards, convene colleagues to standardize to science, start medication reconciliation, and seek composite reliability in our own individual practices.
The IHI will measure this campaign in four ways.
Level 1. Number of hospitals “signing up”
Level 2. Changes in process of care reported
Level 3. Actual changes in deaths and death rates (sample amongst volunteer hospitals)
Level 4. Hospital Standardized Mortality Rates (work of Brian Jarman)
More detailed and specific information about the campaign (and how to participate) can be found on IHI’s Web site (www.ihi.org/ihi/programs/campaign).
“Some is not a number. Soon is not a time.”
The number: 100,000 lives.
The time: June 14, 2006 – 9 a.m. EDT.
References
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). J Am Coll Cardiol. 2004;44:671-719.
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized controlled trials and recommendations of clinical experts: treatments for myocardial infarction. JAMA. 1992;268:240-8.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction – evidence from clinical trials. N Engl J Med. 1996;335:1660-7.
- McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635-45.
- Adams K, Corrigan JM, eds. Priority areas for national action: transforming health care quality. Washington, DC: The National Academics Press, 2003.
- Lappe JM, Muhlstein JB, Lappe DL, et al. Improvements in 1-year cardiovascular clinical outcomes associated with a hospital-based discharge medication program. Ann Intern Med. 2004;141:446-53.
- Hackensack University Medical Center AMI Report, Sept 10, 2004.
- McLeod Regional Medical Center Storyboard for the 2004 IHI National Forum.
- Craven DE, Steger KA. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect. 1996;11:32-53.
- Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120:555-61.
- Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator associated pneumonia in a large U.S. database. Chest. 2002;122:2115-21.
- Guidelines for Preventing Health-Care-Associated Pneumonia, 2003. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Commi Tee. MMWR. 2004;53(No.RR‑3):1-36.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
- Rello J, Lorente C, Bodi M, Diaz E, Ricart M, Kollef MH. Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia? A survey based on the opinions of an international panel of intensivists. Chest. 2002;122:656-61.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomized trial. Lancet. 1999;354:1851-58.
- Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-77.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330:377-81.
- Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA. 1996;275:308-314.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med. 1998;338:791-97.
- Cook D, Heyland, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med. 1999;27:2812-17.
- Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161:1268-79.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:338S-400S.
- Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014-20.
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and aTributable mortality. JAMA. 1994;271:1598-1601.
- Saint S. Chapter 16. Prevention of intravascular catheter-related infection. Making health care safer: a critical analysis of patient safety practices. AHRQ evidence report, number 43, July 20, 2001. www.ncbi.nlm.nih.gov/vooks.
- O’Grady NP; Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. MMWR Morb Mort Wkly Rep. 2002;51(RR-10):1-29.
- Kirkland KB, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- Mangram AJ, Horan TC, Pearson ML, et al. Guidelines for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20:247-78.
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301-06.
- Phillips DP, Christenfeld N, Glynn LM. Increase in U.S. medication-error deaths between 1983 and 1993. Lancet. 1998;351:643-4.
- Rothschild JM, Federic FA, Gandhi TK, Kaushal R, Williams DH, Bates DW. Analysis of medication-related malpractice claims. Causes, preventability, and costs. Arch Intern Med. 2002;162:2414-20.
- Pronovost P, Weast B, Schwarz M, et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18:201-205.
- Rozich JD, Resar RK. Medication safety: one organization’s approach to the challenge. JCOM. 2001;8(10):27-34.
- Rozich JD, Howard RJ, Justeson JM, Macken PD, Lindsay ME, Resar RK. Standardization as a mechanism to improve safety in healthcare. Jt Comm J Qual Saf. 2004;30:5-14.
- Whittington J, Cohen H. OSF Healthcare’s journey in patient safety. Qual Manag Health Care. 2004;13:53-59.
- Needleman J, Buerhaus P, Mattke S, et al. Nursing-staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715-22.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,270 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297-308.
- Sandroni C, Ferro G, Santangelo S, et al. In-hospital cardiac arrest: survival depends mainly on the emergency response. Resuscitation. 2004;62:291-7.
- Schein RM, Hazday N, Pena M, et al. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98:1388-92.
- Hillman K, Parr M, Flabouris A, Bishop G, Stewart A. Redefining in-hospital resuscitation: the concept of the medical emergency team. Resuscitation. 2001;48:105-10.
- Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387-90.
- Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. MJA. 2003;179:283-7.
- Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trail of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Car Med. 2004;32:916-21.
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr AL. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67: 352-62.
- Van de Berghe G, Wouters P, Weekers F, et all. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-67.
I am on a plane on my way back to Minnesota after being professionally rejuvenated by the content of the Institute of Healthcare Improvement’s 16th Annual Forum, in Orlando, FL. The theme of the meeting called on all hospitals, and hence I believe all hospitalists, to save lives. Dr. Donald Berwick, President and CEO of the Institute of Healthcare Improvement (IHI) kicked off this years’ Annual Forum with his plenary speech “Some is not a number, Soon is not a time.” Saving some lives, some time in the future is not a clear goal. “Some is not a number and soon is not a time.” So, he put the challenge forth for hospitals to join IHI in a campaign to save 100K lives by June 14, 2006 at 9:00 a.m. EDT.
“Some is not a number. Soon is not a time.” We all get “why” this is important, at least in so much as what we have been told by the Institute of Medicine Reports “To Err is Human” and “Crossing the Quality Chasm”. But “how” can this be done? By doing things that we already know impact mortality in a hospital setting. By engaging in the reliable care delivery of six changes that save lives. These include recommendations in each of the following areas: rapid response or emergency medical teams, reliable care for acute myocardial infarctions, reliable use of the ventilator pneumonia and central venous line “bundles”, surgical site infection prophylaxis, and prevention of adverse drug events with reconciliation. Each is described in more detail below.
- Rapid Response Teams (also known as Medical Emergency or Pre-Code Teams): This is a team of healthcare providers that may be summoned at any time by anyone in the hospital to assist in the care of a patient who appears acutely ill, before the patient has respiratory failure, a cardiac arrest or other adverse event. The aim is to prevent situations of “failure to rescue”, to recognize the early signs and symptoms of clinical deterioration prior to requiring transfer to the intensive care unit.
- Reliable Care for Acute Myocardial Infarction (AMI): For appropriate AMI patients, reliable use of all of the following treatments: early administration of aspirin, aspirin at discharge, early administration of a beta-blocker, beta-blocker at discharge, ACE‑inhibitor or angiotensin receptor blocker (ARB) at discharge (if systolic dysfunction), timely reperfusion, and smoking cessation counseling.
- Reliable use of the Ventilator Bundle: A number of hospitals have initiated the use of the ventilator bundle to prevent ventilator associated pneumonia (VAP). VAP carries a high mortality rate. The “bundle” is a grouping of 5 treatments/preventions measured as a composite (% of patients that get all 5).
- Elevate head of bed to 30 degrees
- Peptic ulcer prophylaxis
- Deep venous thrombosis prophylaxis
- Daily “sedation vacation”
- Daily assessment of readiness to extubate
Not all of the items have a specific relationship to VAP (e.g., DVT prophylaxis), but when reliably performed in concert with the other items, leads to a decrease in VAP.
- Reliable use of Central Venous Line Bundles: This is a grouping of 5 preventative measures that when done in concert and measured as a composite have had maximal effectiveness for the reduction of central line associated blood stream infections (CLABs) in some hospitals.
- Hand hygiene
- Maximal barrier precautions
- Chlorhexidine skin antisepsis
- Appropriate catheter site and administration system care
- No routine line replacement
- Surgical site infection (SSI) prophylaxis with a “SSI bundle”: Hospitals participating with the IHI in a variety of different formats have found the most substantial reduction/prevention of SSIs when 3 preventative measures are done in concert with each other for every surgical patient. These preventative measures include:
- Guideline-based use of prophylactic perioperative antibiotics (including both choice and timing of administration of antibiotic)
- Appropriate hair removal (avoiding shaving)
- Perioperative glucose control
- Prevention of adverse drug events with medication reconciliation: This refers to the procedures that can be put in place at the time of any transition of care to mitigate the increased risk of wrong dose of medication or even wrong drug being administered immediately following that transition. Each time we have to transfer information from one sheet of paper to another or from a sheet of paper to a computer, there is chance for human error. Medication reconciliation can virtually eliminate errors occurring at transitions in care.
I have placed in Table 1, the information (goals, background, proposed interventions and success stories) handed out during Dr. Donald Berwick’s opening plenary session, the kick-off for the campaign to save 100,000 lives.
Two key components of the descriptions above deserve further explanation. One of the key components is the concept of reliability. Reliability is how often something in health care does what it is supposed to do, in the time frame it is supposed to do it in. The formula is the number of times that something (delivery of a medication or service) is done correctly divided by the number of times that same something is attempted. In work published by Karl Weick, one common principle within high reliability organizations is that of a preoccupation with failure. As such, the notation of reliability is a measure of defects. Currently much of healthcare (including use of beta-blockers after AMI) functions at a 10-1 level of performance (one defect in 10 tries) or less than a 90% success rate. Organizations that have actively embraced this concept of reliability in their quality improvement work have rejected the usual satisfaction with 10-1 performance. Shouldn’t 99 out of 100 (or 999 out of a 1,000) patients with an AMI get what they are supposed to get?
The other key component embedded within some of the six items that save lives is the concept of bundles. Rather than considering individual measures for each of the items within a bundle, a composite or aggregate measure is reported. Bottom line is that doing any one or two of the items in a bundle is not good enough. It will not achieve the same reduction in hospital acquired infection rates or mortality, as doing all of the items in concert for every appropriate patient.
How can hospitalists help achieve this national goal, to participate in this campaign with the IHI? As individuals, we can be a hospital “precinct captain” or champion, speak to our hospital boards, convene colleagues to standardize to science, start medication reconciliation, and seek composite reliability in our own individual practices.
The IHI will measure this campaign in four ways.
Level 1. Number of hospitals “signing up”
Level 2. Changes in process of care reported
Level 3. Actual changes in deaths and death rates (sample amongst volunteer hospitals)
Level 4. Hospital Standardized Mortality Rates (work of Brian Jarman)
More detailed and specific information about the campaign (and how to participate) can be found on IHI’s Web site (www.ihi.org/ihi/programs/campaign).
“Some is not a number. Soon is not a time.”
The number: 100,000 lives.
The time: June 14, 2006 – 9 a.m. EDT.
References
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). J Am Coll Cardiol. 2004;44:671-719.
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized controlled trials and recommendations of clinical experts: treatments for myocardial infarction. JAMA. 1992;268:240-8.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction – evidence from clinical trials. N Engl J Med. 1996;335:1660-7.
- McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635-45.
- Adams K, Corrigan JM, eds. Priority areas for national action: transforming health care quality. Washington, DC: The National Academics Press, 2003.
- Lappe JM, Muhlstein JB, Lappe DL, et al. Improvements in 1-year cardiovascular clinical outcomes associated with a hospital-based discharge medication program. Ann Intern Med. 2004;141:446-53.
- Hackensack University Medical Center AMI Report, Sept 10, 2004.
- McLeod Regional Medical Center Storyboard for the 2004 IHI National Forum.
- Craven DE, Steger KA. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect. 1996;11:32-53.
- Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120:555-61.
- Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator associated pneumonia in a large U.S. database. Chest. 2002;122:2115-21.
- Guidelines for Preventing Health-Care-Associated Pneumonia, 2003. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Commi Tee. MMWR. 2004;53(No.RR‑3):1-36.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
- Rello J, Lorente C, Bodi M, Diaz E, Ricart M, Kollef MH. Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia? A survey based on the opinions of an international panel of intensivists. Chest. 2002;122:656-61.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomized trial. Lancet. 1999;354:1851-58.
- Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-77.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330:377-81.
- Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA. 1996;275:308-314.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med. 1998;338:791-97.
- Cook D, Heyland, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med. 1999;27:2812-17.
- Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161:1268-79.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:338S-400S.
- Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014-20.
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and aTributable mortality. JAMA. 1994;271:1598-1601.
- Saint S. Chapter 16. Prevention of intravascular catheter-related infection. Making health care safer: a critical analysis of patient safety practices. AHRQ evidence report, number 43, July 20, 2001. www.ncbi.nlm.nih.gov/vooks.
- O’Grady NP; Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. MMWR Morb Mort Wkly Rep. 2002;51(RR-10):1-29.
- Kirkland KB, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- Mangram AJ, Horan TC, Pearson ML, et al. Guidelines for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20:247-78.
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301-06.
- Phillips DP, Christenfeld N, Glynn LM. Increase in U.S. medication-error deaths between 1983 and 1993. Lancet. 1998;351:643-4.
- Rothschild JM, Federic FA, Gandhi TK, Kaushal R, Williams DH, Bates DW. Analysis of medication-related malpractice claims. Causes, preventability, and costs. Arch Intern Med. 2002;162:2414-20.
- Pronovost P, Weast B, Schwarz M, et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18:201-205.
- Rozich JD, Resar RK. Medication safety: one organization’s approach to the challenge. JCOM. 2001;8(10):27-34.
- Rozich JD, Howard RJ, Justeson JM, Macken PD, Lindsay ME, Resar RK. Standardization as a mechanism to improve safety in healthcare. Jt Comm J Qual Saf. 2004;30:5-14.
- Whittington J, Cohen H. OSF Healthcare’s journey in patient safety. Qual Manag Health Care. 2004;13:53-59.
- Needleman J, Buerhaus P, Mattke S, et al. Nursing-staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715-22.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,270 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297-308.
- Sandroni C, Ferro G, Santangelo S, et al. In-hospital cardiac arrest: survival depends mainly on the emergency response. Resuscitation. 2004;62:291-7.
- Schein RM, Hazday N, Pena M, et al. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98:1388-92.
- Hillman K, Parr M, Flabouris A, Bishop G, Stewart A. Redefining in-hospital resuscitation: the concept of the medical emergency team. Resuscitation. 2001;48:105-10.
- Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387-90.
- Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. MJA. 2003;179:283-7.
- Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trail of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Car Med. 2004;32:916-21.
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr AL. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67: 352-62.
- Van de Berghe G, Wouters P, Weekers F, et all. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-67.
I am on a plane on my way back to Minnesota after being professionally rejuvenated by the content of the Institute of Healthcare Improvement’s 16th Annual Forum, in Orlando, FL. The theme of the meeting called on all hospitals, and hence I believe all hospitalists, to save lives. Dr. Donald Berwick, President and CEO of the Institute of Healthcare Improvement (IHI) kicked off this years’ Annual Forum with his plenary speech “Some is not a number, Soon is not a time.” Saving some lives, some time in the future is not a clear goal. “Some is not a number and soon is not a time.” So, he put the challenge forth for hospitals to join IHI in a campaign to save 100K lives by June 14, 2006 at 9:00 a.m. EDT.
“Some is not a number. Soon is not a time.” We all get “why” this is important, at least in so much as what we have been told by the Institute of Medicine Reports “To Err is Human” and “Crossing the Quality Chasm”. But “how” can this be done? By doing things that we already know impact mortality in a hospital setting. By engaging in the reliable care delivery of six changes that save lives. These include recommendations in each of the following areas: rapid response or emergency medical teams, reliable care for acute myocardial infarctions, reliable use of the ventilator pneumonia and central venous line “bundles”, surgical site infection prophylaxis, and prevention of adverse drug events with reconciliation. Each is described in more detail below.
- Rapid Response Teams (also known as Medical Emergency or Pre-Code Teams): This is a team of healthcare providers that may be summoned at any time by anyone in the hospital to assist in the care of a patient who appears acutely ill, before the patient has respiratory failure, a cardiac arrest or other adverse event. The aim is to prevent situations of “failure to rescue”, to recognize the early signs and symptoms of clinical deterioration prior to requiring transfer to the intensive care unit.
- Reliable Care for Acute Myocardial Infarction (AMI): For appropriate AMI patients, reliable use of all of the following treatments: early administration of aspirin, aspirin at discharge, early administration of a beta-blocker, beta-blocker at discharge, ACE‑inhibitor or angiotensin receptor blocker (ARB) at discharge (if systolic dysfunction), timely reperfusion, and smoking cessation counseling.
- Reliable use of the Ventilator Bundle: A number of hospitals have initiated the use of the ventilator bundle to prevent ventilator associated pneumonia (VAP). VAP carries a high mortality rate. The “bundle” is a grouping of 5 treatments/preventions measured as a composite (% of patients that get all 5).
- Elevate head of bed to 30 degrees
- Peptic ulcer prophylaxis
- Deep venous thrombosis prophylaxis
- Daily “sedation vacation”
- Daily assessment of readiness to extubate
Not all of the items have a specific relationship to VAP (e.g., DVT prophylaxis), but when reliably performed in concert with the other items, leads to a decrease in VAP.
- Reliable use of Central Venous Line Bundles: This is a grouping of 5 preventative measures that when done in concert and measured as a composite have had maximal effectiveness for the reduction of central line associated blood stream infections (CLABs) in some hospitals.
- Hand hygiene
- Maximal barrier precautions
- Chlorhexidine skin antisepsis
- Appropriate catheter site and administration system care
- No routine line replacement
- Surgical site infection (SSI) prophylaxis with a “SSI bundle”: Hospitals participating with the IHI in a variety of different formats have found the most substantial reduction/prevention of SSIs when 3 preventative measures are done in concert with each other for every surgical patient. These preventative measures include:
- Guideline-based use of prophylactic perioperative antibiotics (including both choice and timing of administration of antibiotic)
- Appropriate hair removal (avoiding shaving)
- Perioperative glucose control
- Prevention of adverse drug events with medication reconciliation: This refers to the procedures that can be put in place at the time of any transition of care to mitigate the increased risk of wrong dose of medication or even wrong drug being administered immediately following that transition. Each time we have to transfer information from one sheet of paper to another or from a sheet of paper to a computer, there is chance for human error. Medication reconciliation can virtually eliminate errors occurring at transitions in care.
I have placed in Table 1, the information (goals, background, proposed interventions and success stories) handed out during Dr. Donald Berwick’s opening plenary session, the kick-off for the campaign to save 100,000 lives.
Two key components of the descriptions above deserve further explanation. One of the key components is the concept of reliability. Reliability is how often something in health care does what it is supposed to do, in the time frame it is supposed to do it in. The formula is the number of times that something (delivery of a medication or service) is done correctly divided by the number of times that same something is attempted. In work published by Karl Weick, one common principle within high reliability organizations is that of a preoccupation with failure. As such, the notation of reliability is a measure of defects. Currently much of healthcare (including use of beta-blockers after AMI) functions at a 10-1 level of performance (one defect in 10 tries) or less than a 90% success rate. Organizations that have actively embraced this concept of reliability in their quality improvement work have rejected the usual satisfaction with 10-1 performance. Shouldn’t 99 out of 100 (or 999 out of a 1,000) patients with an AMI get what they are supposed to get?
The other key component embedded within some of the six items that save lives is the concept of bundles. Rather than considering individual measures for each of the items within a bundle, a composite or aggregate measure is reported. Bottom line is that doing any one or two of the items in a bundle is not good enough. It will not achieve the same reduction in hospital acquired infection rates or mortality, as doing all of the items in concert for every appropriate patient.
How can hospitalists help achieve this national goal, to participate in this campaign with the IHI? As individuals, we can be a hospital “precinct captain” or champion, speak to our hospital boards, convene colleagues to standardize to science, start medication reconciliation, and seek composite reliability in our own individual practices.
The IHI will measure this campaign in four ways.
Level 1. Number of hospitals “signing up”
Level 2. Changes in process of care reported
Level 3. Actual changes in deaths and death rates (sample amongst volunteer hospitals)
Level 4. Hospital Standardized Mortality Rates (work of Brian Jarman)
More detailed and specific information about the campaign (and how to participate) can be found on IHI’s Web site (www.ihi.org/ihi/programs/campaign).
“Some is not a number. Soon is not a time.”
The number: 100,000 lives.
The time: June 14, 2006 – 9 a.m. EDT.
References
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). J Am Coll Cardiol. 2004;44:671-719.
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized controlled trials and recommendations of clinical experts: treatments for myocardial infarction. JAMA. 1992;268:240-8.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction – evidence from clinical trials. N Engl J Med. 1996;335:1660-7.
- McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635-45.
- Adams K, Corrigan JM, eds. Priority areas for national action: transforming health care quality. Washington, DC: The National Academics Press, 2003.
- Lappe JM, Muhlstein JB, Lappe DL, et al. Improvements in 1-year cardiovascular clinical outcomes associated with a hospital-based discharge medication program. Ann Intern Med. 2004;141:446-53.
- Hackensack University Medical Center AMI Report, Sept 10, 2004.
- McLeod Regional Medical Center Storyboard for the 2004 IHI National Forum.
- Craven DE, Steger KA. Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect. 1996;11:32-53.
- Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120:555-61.
- Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator associated pneumonia in a large U.S. database. Chest. 2002;122:2115-21.
- Guidelines for Preventing Health-Care-Associated Pneumonia, 2003. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Commi Tee. MMWR. 2004;53(No.RR‑3):1-36.
- Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-13.
- Rello J, Lorente C, Bodi M, Diaz E, Ricart M, Kollef MH. Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia? A survey based on the opinions of an international panel of intensivists. Chest. 2002;122:656-61.
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomized trial. Lancet. 1999;354:1851-58.
- Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-77.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330:377-81.
- Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: resolving discordant meta-analyses. JAMA. 1996;275:308-314.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med. 1998;338:791-97.
- Cook D, Heyland, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. Crit Care Med. 1999;27:2812-17.
- Attia J, Ray JG, Cook DJ, Douketis J, Ginsberg JS, Geerts WH. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med. 2001;161:1268-79.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:338S-400S.
- Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014-20.
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and aTributable mortality. JAMA. 1994;271:1598-1601.
- Saint S. Chapter 16. Prevention of intravascular catheter-related infection. Making health care safer: a critical analysis of patient safety practices. AHRQ evidence report, number 43, July 20, 2001. www.ncbi.nlm.nih.gov/vooks.
- O’Grady NP; Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. MMWR Morb Mort Wkly Rep. 2002;51(RR-10):1-29.
- Kirkland KB, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- Mangram AJ, Horan TC, Pearson ML, et al. Guidelines for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20:247-78.
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301-06.
- Phillips DP, Christenfeld N, Glynn LM. Increase in U.S. medication-error deaths between 1983 and 1993. Lancet. 1998;351:643-4.
- Rothschild JM, Federic FA, Gandhi TK, Kaushal R, Williams DH, Bates DW. Analysis of medication-related malpractice claims. Causes, preventability, and costs. Arch Intern Med. 2002;162:2414-20.
- Pronovost P, Weast B, Schwarz M, et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18:201-205.
- Rozich JD, Resar RK. Medication safety: one organization’s approach to the challenge. JCOM. 2001;8(10):27-34.
- Rozich JD, Howard RJ, Justeson JM, Macken PD, Lindsay ME, Resar RK. Standardization as a mechanism to improve safety in healthcare. Jt Comm J Qual Saf. 2004;30:5-14.
- Whittington J, Cohen H. OSF Healthcare’s journey in patient safety. Qual Manag Health Care. 2004;13:53-59.
- Needleman J, Buerhaus P, Mattke S, et al. Nursing-staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715-22.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,270 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297-308.
- Sandroni C, Ferro G, Santangelo S, et al. In-hospital cardiac arrest: survival depends mainly on the emergency response. Resuscitation. 2004;62:291-7.
- Schein RM, Hazday N, Pena M, et al. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98:1388-92.
- Hillman K, Parr M, Flabouris A, Bishop G, Stewart A. Redefining in-hospital resuscitation: the concept of the medical emergency team. Resuscitation. 2001;48:105-10.
- Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387-90.
- Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. MJA. 2003;179:283-7.
- Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trail of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Car Med. 2004;32:916-21.
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr AL. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67: 352-62.
- Van de Berghe G, Wouters P, Weekers F, et all. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-67.
Chemotherapy for Advanced Head and Neck Cancer
Caution with Concurrent Nucleotide Analogs
Squamous Cell Carcinoma of the Anus in a Patient with Chronic Lymphocytic Leukemia
Involuntary commitment, ‘false’ memories
Self-proclaimed ‘exorcist’ claims he was improperly committed
Court of claims (NY)
In response to a 911 call, police arrested a man who, the caller said, was trying to choke and stab an individual. Upon his arrest, the man claimed that he was an exorcist. He justified his attack by alleging that his victim was a medium for demons and spirits.
The suspect was taken to a psychiatric hospital; staff decided that he endangered himself and others and should be committed. During his commitment, he was restrained and forcibly given medication.
The man later sued the hospital for intentional infliction of emotional distress and malpractice. He charged that the hospital denied him the right to a court hearing after he was admitted.
The court dismissed the case. The court noted that the plaintiff did not present expert testimony to support his emotional distress claim.
Dr. Grant’s observations
Involuntary commitment. Although the standards for involuntary commitment vary from state to state, some general principles apply.
A patient who endangers himself or others may be held for varying periods until a court hearing can be arranged. State law determines how long someone can be held before a court-ordered commitment. Although the patient has a right to a court hearing, the state is not obligated to conduct that hearing sooner than is determined by state law. Clinicians need to learn the laws governing involuntary commitment in the states in which they practice.
Patients who are involuntarily committed are not required to accept treatment, however. Competent adults generally must give informed consent to treatment, but this rule is usually suspended in an emergency. When a patient is a danger to self or others, that person can be restrained and medicated against his or her will for as long as the emergency lasts. In such cases, the clinician should clearly document:
- indications for using restraint and forced medication (include a detailed assessment of the patient’s dangerous behaviors)
- the patient’s response to previous behavioral approaches or treatments
- grounds for believing that the patient’s refusal of other interventions is clearly a product of the illness.
Patient: psychiatrists planted false memories, gave wrong diagnosis
Green County (WI) circuit court
A 55-year-old woman was seen in a hospital clinic’s weight-loss program and developed anxiety symptoms as she reached normal weight. Her psychologist assigned her to read a book about surviving incest, which focused on repressed memories that surface during recovery. The woman then received hypnosis from a psychiatrist who was not trained as a hypnotist.
During the hypnosis sessions, the patient reported “remembering” past instances of abuse that she had not previously recalled. The psychiatrist guided her to relive or reenact one event, in which she reported remembering being anally raped. The patient became more depressed and required hospitalization. Another psychiatrist, who took over the case when the first psychiatrist left the clinic, diagnosed the patient as having multiple personality disorder.
The patient later questioned the diagnosis and came to believe that her treatment had been inappropriate and that the memories had been planted. The patient, once a registered nurse, is now disabled.
In court, the jury heard:
- charges of negligence against the treating physicians on behalf of the woman and her son, who was briefly treated by the original psychiatrist
- charges that neither the psychiatrists nor the clinic obtained informed consent before treating the woman or her son.
- The jury decided for the physician, clinic, and hospital on all charges.
Dr. Grant’s observations
This case involves several complex and controversial areas in psychiatry: recovered memory, multiple personality disorder, and use of hypnosis. Although the jury found for the physician, clinic, and hospital, these areas provide fertile ground for lawsuits, many of which are successful.
The case involved two distinct legal causes of action:
- negligent care
- lack of informed consent.
Recovered memory. The veracity of recovered memory has been vigorously debated.1 Because the credibility of recovered memory cannot be established, the clinician should clearly state in the chart that the past incident the patient reports during therapy may not have happened. The clinician also must avoid imposing his or her beliefs on the patient (such as assuming that patients with eating disorders have been sexually abused) or advocating for action on the patient’s part.
Hypnosis used to recover memories of abuse may be particularly complex legally.2 A clinician using hypnosis may jeopardize therapeutic disinterest by interjecting suggestions—often without realizing that he or she is doing so.
To avoid negligence claims, clinicians should stay within their areas of competence when treating patients. If hypnosis is deemed clinically necessary, a clinician not trained in hypnosis should refer the patient to a certified clinical hypnotist.
Multiple personality disorder is included in DSM-IV-TR as dissociative identity disorder, but approximately one-third of psychiatrists question whether this is a legitimate diagnosis.3 Clearly documenting the basis for this—or any—diagnosis may help the clinician avoid a lawsuit or defend against a negligence charge.
Informed consent. Failure to inform patients about the risks associated with recovered memories is one of the most common allegations against clinicians in recovered memory cases.
Canterbury v. Spence, the landmark case of informed consent, offers some guidance. The court found that the clinician must provide reasonable disclosure of:
- therapy alternatives open to the patient
- goals expected to be achieved
- the risks involved with recovering memories.4
Some have proposed that clinicians should disclose the risk of recovering false memories of sexual and physical abuse before starting treatment.5 The clinician should then clearly document this disclosure.
1. Pope HG, Jr. Psychology astray: Fallacies in studies of repressed memory and childhood trauma. Boca Raton, FL: Upton Books, 1997.
2. Borawick v. Shay. 68 F3d 597 (2d Cir. 1995).
3. Pope HG, Jr, Oliva PS, Hudson JI, et al. Attitudes toward DSM-IV dissociative disorders diagnoses among board-certified American psychiatrists. Am J Psychiatry 1999;156:321-3.
4. Canterbury v. Spence. 464 F2d 775 (DC Cir 1972).
5. Cannell J, Hudson JI, Pope HG, Jr. Standards for informed consent in recovered memory therapy. J Am Acad Psychiatry Law 2001;29:138-47.
Self-proclaimed ‘exorcist’ claims he was improperly committed
Court of claims (NY)
In response to a 911 call, police arrested a man who, the caller said, was trying to choke and stab an individual. Upon his arrest, the man claimed that he was an exorcist. He justified his attack by alleging that his victim was a medium for demons and spirits.
The suspect was taken to a psychiatric hospital; staff decided that he endangered himself and others and should be committed. During his commitment, he was restrained and forcibly given medication.
The man later sued the hospital for intentional infliction of emotional distress and malpractice. He charged that the hospital denied him the right to a court hearing after he was admitted.
The court dismissed the case. The court noted that the plaintiff did not present expert testimony to support his emotional distress claim.
Dr. Grant’s observations
Involuntary commitment. Although the standards for involuntary commitment vary from state to state, some general principles apply.
A patient who endangers himself or others may be held for varying periods until a court hearing can be arranged. State law determines how long someone can be held before a court-ordered commitment. Although the patient has a right to a court hearing, the state is not obligated to conduct that hearing sooner than is determined by state law. Clinicians need to learn the laws governing involuntary commitment in the states in which they practice.
Patients who are involuntarily committed are not required to accept treatment, however. Competent adults generally must give informed consent to treatment, but this rule is usually suspended in an emergency. When a patient is a danger to self or others, that person can be restrained and medicated against his or her will for as long as the emergency lasts. In such cases, the clinician should clearly document:
- indications for using restraint and forced medication (include a detailed assessment of the patient’s dangerous behaviors)
- the patient’s response to previous behavioral approaches or treatments
- grounds for believing that the patient’s refusal of other interventions is clearly a product of the illness.
Patient: psychiatrists planted false memories, gave wrong diagnosis
Green County (WI) circuit court
A 55-year-old woman was seen in a hospital clinic’s weight-loss program and developed anxiety symptoms as she reached normal weight. Her psychologist assigned her to read a book about surviving incest, which focused on repressed memories that surface during recovery. The woman then received hypnosis from a psychiatrist who was not trained as a hypnotist.
During the hypnosis sessions, the patient reported “remembering” past instances of abuse that she had not previously recalled. The psychiatrist guided her to relive or reenact one event, in which she reported remembering being anally raped. The patient became more depressed and required hospitalization. Another psychiatrist, who took over the case when the first psychiatrist left the clinic, diagnosed the patient as having multiple personality disorder.
The patient later questioned the diagnosis and came to believe that her treatment had been inappropriate and that the memories had been planted. The patient, once a registered nurse, is now disabled.
In court, the jury heard:
- charges of negligence against the treating physicians on behalf of the woman and her son, who was briefly treated by the original psychiatrist
- charges that neither the psychiatrists nor the clinic obtained informed consent before treating the woman or her son.
- The jury decided for the physician, clinic, and hospital on all charges.
Dr. Grant’s observations
This case involves several complex and controversial areas in psychiatry: recovered memory, multiple personality disorder, and use of hypnosis. Although the jury found for the physician, clinic, and hospital, these areas provide fertile ground for lawsuits, many of which are successful.
The case involved two distinct legal causes of action:
- negligent care
- lack of informed consent.
Recovered memory. The veracity of recovered memory has been vigorously debated.1 Because the credibility of recovered memory cannot be established, the clinician should clearly state in the chart that the past incident the patient reports during therapy may not have happened. The clinician also must avoid imposing his or her beliefs on the patient (such as assuming that patients with eating disorders have been sexually abused) or advocating for action on the patient’s part.
Hypnosis used to recover memories of abuse may be particularly complex legally.2 A clinician using hypnosis may jeopardize therapeutic disinterest by interjecting suggestions—often without realizing that he or she is doing so.
To avoid negligence claims, clinicians should stay within their areas of competence when treating patients. If hypnosis is deemed clinically necessary, a clinician not trained in hypnosis should refer the patient to a certified clinical hypnotist.
Multiple personality disorder is included in DSM-IV-TR as dissociative identity disorder, but approximately one-third of psychiatrists question whether this is a legitimate diagnosis.3 Clearly documenting the basis for this—or any—diagnosis may help the clinician avoid a lawsuit or defend against a negligence charge.
Informed consent. Failure to inform patients about the risks associated with recovered memories is one of the most common allegations against clinicians in recovered memory cases.
Canterbury v. Spence, the landmark case of informed consent, offers some guidance. The court found that the clinician must provide reasonable disclosure of:
- therapy alternatives open to the patient
- goals expected to be achieved
- the risks involved with recovering memories.4
Some have proposed that clinicians should disclose the risk of recovering false memories of sexual and physical abuse before starting treatment.5 The clinician should then clearly document this disclosure.
Self-proclaimed ‘exorcist’ claims he was improperly committed
Court of claims (NY)
In response to a 911 call, police arrested a man who, the caller said, was trying to choke and stab an individual. Upon his arrest, the man claimed that he was an exorcist. He justified his attack by alleging that his victim was a medium for demons and spirits.
The suspect was taken to a psychiatric hospital; staff decided that he endangered himself and others and should be committed. During his commitment, he was restrained and forcibly given medication.
The man later sued the hospital for intentional infliction of emotional distress and malpractice. He charged that the hospital denied him the right to a court hearing after he was admitted.
The court dismissed the case. The court noted that the plaintiff did not present expert testimony to support his emotional distress claim.
Dr. Grant’s observations
Involuntary commitment. Although the standards for involuntary commitment vary from state to state, some general principles apply.
A patient who endangers himself or others may be held for varying periods until a court hearing can be arranged. State law determines how long someone can be held before a court-ordered commitment. Although the patient has a right to a court hearing, the state is not obligated to conduct that hearing sooner than is determined by state law. Clinicians need to learn the laws governing involuntary commitment in the states in which they practice.
Patients who are involuntarily committed are not required to accept treatment, however. Competent adults generally must give informed consent to treatment, but this rule is usually suspended in an emergency. When a patient is a danger to self or others, that person can be restrained and medicated against his or her will for as long as the emergency lasts. In such cases, the clinician should clearly document:
- indications for using restraint and forced medication (include a detailed assessment of the patient’s dangerous behaviors)
- the patient’s response to previous behavioral approaches or treatments
- grounds for believing that the patient’s refusal of other interventions is clearly a product of the illness.
Patient: psychiatrists planted false memories, gave wrong diagnosis
Green County (WI) circuit court
A 55-year-old woman was seen in a hospital clinic’s weight-loss program and developed anxiety symptoms as she reached normal weight. Her psychologist assigned her to read a book about surviving incest, which focused on repressed memories that surface during recovery. The woman then received hypnosis from a psychiatrist who was not trained as a hypnotist.
During the hypnosis sessions, the patient reported “remembering” past instances of abuse that she had not previously recalled. The psychiatrist guided her to relive or reenact one event, in which she reported remembering being anally raped. The patient became more depressed and required hospitalization. Another psychiatrist, who took over the case when the first psychiatrist left the clinic, diagnosed the patient as having multiple personality disorder.
The patient later questioned the diagnosis and came to believe that her treatment had been inappropriate and that the memories had been planted. The patient, once a registered nurse, is now disabled.
In court, the jury heard:
- charges of negligence against the treating physicians on behalf of the woman and her son, who was briefly treated by the original psychiatrist
- charges that neither the psychiatrists nor the clinic obtained informed consent before treating the woman or her son.
- The jury decided for the physician, clinic, and hospital on all charges.
Dr. Grant’s observations
This case involves several complex and controversial areas in psychiatry: recovered memory, multiple personality disorder, and use of hypnosis. Although the jury found for the physician, clinic, and hospital, these areas provide fertile ground for lawsuits, many of which are successful.
The case involved two distinct legal causes of action:
- negligent care
- lack of informed consent.
Recovered memory. The veracity of recovered memory has been vigorously debated.1 Because the credibility of recovered memory cannot be established, the clinician should clearly state in the chart that the past incident the patient reports during therapy may not have happened. The clinician also must avoid imposing his or her beliefs on the patient (such as assuming that patients with eating disorders have been sexually abused) or advocating for action on the patient’s part.
Hypnosis used to recover memories of abuse may be particularly complex legally.2 A clinician using hypnosis may jeopardize therapeutic disinterest by interjecting suggestions—often without realizing that he or she is doing so.
To avoid negligence claims, clinicians should stay within their areas of competence when treating patients. If hypnosis is deemed clinically necessary, a clinician not trained in hypnosis should refer the patient to a certified clinical hypnotist.
Multiple personality disorder is included in DSM-IV-TR as dissociative identity disorder, but approximately one-third of psychiatrists question whether this is a legitimate diagnosis.3 Clearly documenting the basis for this—or any—diagnosis may help the clinician avoid a lawsuit or defend against a negligence charge.
Informed consent. Failure to inform patients about the risks associated with recovered memories is one of the most common allegations against clinicians in recovered memory cases.
Canterbury v. Spence, the landmark case of informed consent, offers some guidance. The court found that the clinician must provide reasonable disclosure of:
- therapy alternatives open to the patient
- goals expected to be achieved
- the risks involved with recovering memories.4
Some have proposed that clinicians should disclose the risk of recovering false memories of sexual and physical abuse before starting treatment.5 The clinician should then clearly document this disclosure.
1. Pope HG, Jr. Psychology astray: Fallacies in studies of repressed memory and childhood trauma. Boca Raton, FL: Upton Books, 1997.
2. Borawick v. Shay. 68 F3d 597 (2d Cir. 1995).
3. Pope HG, Jr, Oliva PS, Hudson JI, et al. Attitudes toward DSM-IV dissociative disorders diagnoses among board-certified American psychiatrists. Am J Psychiatry 1999;156:321-3.
4. Canterbury v. Spence. 464 F2d 775 (DC Cir 1972).
5. Cannell J, Hudson JI, Pope HG, Jr. Standards for informed consent in recovered memory therapy. J Am Acad Psychiatry Law 2001;29:138-47.
1. Pope HG, Jr. Psychology astray: Fallacies in studies of repressed memory and childhood trauma. Boca Raton, FL: Upton Books, 1997.
2. Borawick v. Shay. 68 F3d 597 (2d Cir. 1995).
3. Pope HG, Jr, Oliva PS, Hudson JI, et al. Attitudes toward DSM-IV dissociative disorders diagnoses among board-certified American psychiatrists. Am J Psychiatry 1999;156:321-3.
4. Canterbury v. Spence. 464 F2d 775 (DC Cir 1972).
5. Cannell J, Hudson JI, Pope HG, Jr. Standards for informed consent in recovered memory therapy. J Am Acad Psychiatry Law 2001;29:138-47.
Delirium: Apply the ‘4 Ps’ for comprehensive treatment
Four principles of treating delirium can help protect medical/surgical patients at risk for morbidity and functional decline. These principals—which I call the “four Ps”—are prompt identification, protection, pragmatic intervention, and pharmacotherapy.
This article describes an up-to-date, “four-Ps” approach to treating delirium—including use of antipsychotics and supportive care—and offers evidence and case reports to address these clinical questions:
- What causes delirium?
- Does delirium worsen prognosis?
- Can delirium be prevented?
FOUR ‘Ps’ FOR TREATING DELIRIUM
When a patient’s mental status changes dramatically (Box 1),1 identifying potential delirium causes requires careful medical, psychiatric, and neurologic assessment. Assimilating this information is as essential to positive outcomes as are intensive nursing care and appropriate interventions.
- Disturbance of consciousness (i.e. reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention
- A change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia
- The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of a 24-hour period
- There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiologic consequences of a general medical condition
Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders (4th ed., text rev). Copyright 2000. American Psychiatric Publishing.
Prompt identification. Delirium often goes unrecognized, delaying treatment. Easily administered rating scales—such as the Delirium Rating Scale (DRS)2 and the Confusion Assessment Method (CAM)3 —can help detect emerging symptoms.
Patient protection. Provide intensive nursing care—often one-to-one observation and containment—and, where possible, enlist the family in reassuring and calming the patient. Restraints may be needed to safeguard against injury and to prevent the patient from removing or dislocating monitoring equipment and IV access.
Pragmatic intervention. With medical colleagues, begin treating biochemical and physiologic abnormalities that are the most likely and most remediable contributors (Box 2).2,4-6 Review the patient’s medications and discontinue or replace any that may be causing delirium.
Pharmacotherapy. Based on clinical studies, antipsychotics appear to possess antidelirium properties and may be considered as one part of a patient’s treatment plan. Interpreting these studies is complicated, however, by delirium’s complexity, numerous causes, and presumed mechanisms, as well as the transience of some forms. For ethical reasons, no placebo-controlled studies of delirium treatment have been done.
EVIDENCE ON ANTIPSYCHOTICS
Haloperidol has been the drug of choice for managing delirium because it is less likely to cause hypotension and sedation than other neuroleptics. Optimum haloperidol dosing in delirium has not been established, but the usual range is 2 to 6 mg every 4 to 6 hours, depending on the patient’s age and delirium severity.
Instances of QTc interval prolongation have been reported with high-dose IV haloperidol (> 100 mg). This life-threatening effect—which can induce torsades de pointes dysrhythmia, ventricular tachycardias, and fibrillation—is very rare, quite variable, and unpredictable. It probably is a function of total dose and neuroleptic administration rate.
Atypical antipsychotics share haloperidol’s advantages over first-generation neuroleptics, with lower potential for dystonic reactions, parkinsonian side effects, and tardive dyskinesia. Preliminary evidence suggests that atypicals may be safe and effective in treating delirium, although no randomized controlled trials have been done and accurate dose-response curves have not been established. Low to modest dosages have been used in case series.
Risperidone. Two prospective, open-label trials—each with 10 patients—suggest that low-dose risperidone is effective for treating delirium:
- In one trial, risperidone given at an average dosage of 1.7 mg/d was effective in 80% of patients with delirium, and one patient responded to 0.5 mg/d. Some patients experienced sleepiness or mild drug-induced parkinsonism.7
- In the other trial, risperidone was started at 0.5 mg twice daily, with additional doses allowed on day 1 for cognitive and behavioral symptoms. This dosage was maintained until DRS scores declined to ≤12, then was reduced by 50% and continued until day 6. Mean maintenance dosage was 0.75 mg/d. Two patients discontinued risperidone because of sedation or hypotension.8
At least 10% to 30% of hospitalized medically ill patients develop delirium, and rates approach 40% after age 65.4 Especially in older patients, delirium is a risk factor for:
- prolonged hospital stays
- increased morbidity and mortality
- increased functional decline and need for custodial care after hospital discharge.2
Risk factors. Prospectively identified risk factors for delirium include pre-existing dementia; age >65 years; serious medical illness; alcohol/sedative withdrawal; abnormal serum sodium, potassium, or blood glucose levels; vision or hearing impairment; hypoxia; malnutrition; and fever. Medication—particularly anticholinergic drugs—is one of the most common delirium triggers in susceptible patients.5
The most common underlying disorders that increase delirium risk in older patients are hip fracture, dementia, infections, and cerebrovascular events.6
In a larger prospective study, 64 patients (mean age 67) with delirium were treated with risperidone, given at a mean dose of 2.6 +/- 1.7 mg/d at day 3. This dosage was effective in 90% of patients and significantly improved all symptoms, as measured with scales including the DRS. Two patients (3%) experienced adverse effects.9
No significant differences in response frequency were seen in a 7-day, double-blind comparison of flexibly-dosed risperidone (starting at 0.5 mg bid) and haloperidol (starting at 0.75 mg bid) in 28 patients with delirium. Symptom severity decreased for each group, as measured with the Memorial Delirium Assessment Scale. One patient receiving haloperidol experienced mild akathisia, but no others reported clinically significant side effects.10
Quetiapine. In a retrospective review, the charts of 11 patients who received quetiapine for delirium were compared with those of 11 similar patients treated with haloperidol. DRS scores improved by >50% in 10 of 11 patients in both groups, with similar onset of effect, treatment duration, and overall clinical improvement.11 Small prospective trials with flexible dosing schedules have reported similar results.12,13
In a study of 12 older hospitalized patients with delirium, quetiapine at a mean dosage of 93.75 +/-23.31 mg/d was associated with significant DRS score improvements. Interestingly, patients’ Mini-Mental State Examination and Clock-Drawing Test scores continued to improve 3 months after their delirium symptoms stabilized.14
Olanzapine. In a prospective trial, hospitalized patients with delirium were randomly assigned to receive enteral olanzapine or haloperidol. Delirium symptoms decreased across 5 days in both groups, and clinical improvement was similar. Some patients receiving haloperidol reported extrapyramidal symptoms, whereas those receiving olanzapine reported no adverse effects.15
Parenteral forms of some atypicals (aripiprazole, olanzapine, and ziprasidone) have become available and may increase this class’ usefulness in treating delirium.
Other drugs. Benzodiazepines appear ineffective and generally play only an adjunctive role in treating delirium. An exception may be delirium induced by acute alcohol or benzodiazepine withdrawal. Sedating antidepressants have been used as hypnotics in patients with delirium, but supporting evidence is lacking.
Other drug classes—general anesthetics, narcotics, cholinomimetics—may help manage the dangerously hyperactive delirious patient, but the literature contains no systematic analyses.
WHAT CAUSES DELIRIUM?
Delirium’s pathophysiology is not completely understood, although most authors believe several mechanisms are involved.
The brain’s exclusively oxidative metabolism and its systems’ hierarchical vulnerability to substrate deficiency—as might occur in even transient hypoxia or hypotension—appear to play important roles. Factors such as fever and stress that increase metabolic demand on the brain intensify the effects of oxygen deficiency or circulatory compromise.
At least three molecular mechanisms have been proposed for delirium, including cholinergic transmission disruption, monoaminergic dysfunction, and cytokine release ( Box 3).16-19 These mechanisms may interact, cascading into a common final pathway that results in delirium.
Features not considered essential to delirium’s diagnosis—such as visual hallucinations or aggressive behaviors—indicate that additional cortical and subcortical systems are involved.
CASE REPORT: DRUG-DRUG INTERACTION
Three days after hip replacement surgery, Mr. S, age 64, becomes confused, distractible, and combative. He is alert one minute and somnolent the next. His arms and legs jerk involuntarily, and his muscle tone is diffusely increased. He talks with absent friends and family as though they are present in his hospital room. His body temperature and blood pressure fluctuate widely, despite no evidence of infection.
Cholinergic transmission disruption
The greater a medication’s anticholinergic activity, the greater its risk of causing delirium. Combining drugs with anticholinergic effects—such as theophylline, warfarin, or codeine (Table19)—compounds the delirium risk.
Acetylcholine-secreting neurons—widely if sparsely distributed throughout the brain—affect arousal, attention, memory, and sleep regulation. Acetylcholine is produced by oxidative metabolism and thus is vulnerable to physiologic disturbances that increase oxygen demand or disrupt oxygen supply.
Anticholinergic poisoning and abuse of anticholinergic substances are known to cause acute delirium—a finding that supports the key role of acetylcholine in maintaining alertness and concentration. Agents that enhance cholinergic transmission—such as the cholinesterase inhibitor physostigmine—can effectively treat drug-induced delirium.
Monoaminergic dysfunction
The principal monoamines of dopamine, serotonin, and norepinephrine help sustain attention, regulate the sleep-wake cycle, inhibit affective responses, and modulate aggressive and impulsive behaviors. Treating patients with dopamine and serotonin agonists can cause psychotic symptoms.
Glutamate—a monoamine neurotransmitter with excitatory properties—is released during metabolic stress and likely contributes to the psychotic features sometimes seen in delirium.
Cytokine release
Infection in a distant organ, such as gallbladder or kidney, is known to cause delirium. Cytokines such as interleukins and interferon-alpha are polypeptides secreted by macrophagesin response to tissue injury. They easily cross the blood-brain barrier and stimulate glial cells to release more cytokines, which interfere with neurotransmitter synthesis and transmission.
Table
Drugs whose anticholinergic effects may increase the risk of delirium
| Drug | Anticholinergic level* |
|---|---|
| Cimetidine | 0.86 |
| Prednisolone | 0.55 |
| Theophylline | 0.44 |
| Digoxin | 0.25 |
| Lanoxin | 0.25 |
| Nifedipine | 0.22 |
| Ranitidine | 0.22 |
| Furosemide | 0.22 |
| Isosorbide | 0.15 |
| Warfarin | 0.12 |
| Dipyridamole | 0.11 |
| Codeine | 0.11 |
| * ng/mL in atropine equivalents | |
| Source: Adapted from reference 19. | |
For several years, Mr. S has been taking the monoamine oxidase inhibitor (MAOI) phenelzine, 30 mg/d, for depression maintenance treatment. On admission, he insisted that the MAOI be continued during hospitalization because it had relieved his severe depressions.
Within 24 hours of surgery, he was given the skeletal muscle relaxant cyclobenzaprine, 5 mg tid, for painful muscle spasms in the operated hip. When this brought little relief, the dosage was increased to 10 mg tid. Delirium and autonomic instability developed approximately 4 hours after the first 10-mg dose and gradually worsened.
The two drugs are discontinued, and Mr S. gradually recovers after several days of physiologic support, protection, and sedation in the intensive-care unit.
Discussion. Mr. S developed serotonin syndrome from a drug-drug interaction. Phenelzine inhibited serotonin metabolism, and cyclobenzaprine—a drug chemically similar to tricyclic antidepressants—inhibited serotonin reuptake, resulting in substantially increased CNS serotonergic activity.20 Serotonin syndrome symptoms include delirium, autonomic dysfunction, and neurologic signs such as myoclonus and rigidity when patients are taking drugs that enhance serotonergic transmission.
DOES DELIRIUM WORSEN PROGNOSIS?
In the largest study of delirium in older patients, Inouye et al21 examined outcomes of 727 consecutive patients age 65 and older with various medical diagnoses who were admitted to three teaching hospitals. Delirium was diagnosed in 88 patients (12%) at admission.
Within 3 months of hospital discharge, 165 (25%) of 663 patients had died or been newly admitted to a nursing home. After the authors controlled the data for age, gender, dementia, illness severity, and functional status, they found that delirium:
- tripled the likelihood of nursing home placement at hospital discharge and after 3 months (adjusted odds ratio [OR] for delirium 3.0)
- more than doubled the likelihood of death or new nursing home placement at discharge (OR for delirium 2.1) and after 3 months (OR for delirium 2.6).
They concluded that delirium was a significant predictor of functional decline at hospital discharge and also at follow-up in older patients.
Interestingly, although these authors did not find a statistically significant association between delirium and death alone, the risk of death was particularly strong for patients who were not demented (OR for delirium, 3.77). Similarly, Rabins and Folstein22 found higher mortality rates in medically ill patients diagnosed with delirium on hospital admission than in demented, cognitively intact, or depressed patients. After 1 year, the death rate remained higher in those who had been delirious than in those with dementia.
In a 12-month observational study comparing 243 older medical inpatients with delirium and 118 controls without delirium, McCusker et al23 found that:
- patients with delirium were twice as likely to die within 12 months as those without delirium
- the greater severity of delirium symptoms, the higher the risk of death in patients with delirium but without dementia.
In a recent study, some of the same investigators found that delirium symptoms—especially inattention, disorientation, and impaired memory—persisted for 12 months after hospital discharge in medical inpatients age 65 and older with or without dementia. Mean numbers of delirium symptoms at diagnosis and 12-month follow-up, respectively, were:
- 4.5 and 3.5 in patients with dementia
- 3.4 and 2.2 in patients without dementia.24
CASE REPORT: DELIRIUM AS PROGNOSTIC SIGN
Mrs. W, age 70, is hospitalized for treatment of anemia and dehydration after falling at home. She has metastatic adenocarcinoma of the colon and is hypernatremic and hypotensive on admission.
Within 24 hours, she becomes floridly delirious, despite transfusion of two units of packed red cells and IV fluid replacement. She receives IM haloperidol to reduce the agitation and counteract delirium. Head CT reveals mild, diffuse cerebral atrophy but no metastasis or subdural hematoma.
Although aggressive treatment corrects her electrolyte disturbance and dehydration and restores normal vital signs, the delirium does not resolve. She is discharged to a nursing home, where she is discovered dead in bed 1 week later.
Discussion. Delirium independently increases the risk of death during hospitalization and thereafter, particularly in older patients. As in the case of Mrs. W, delirium is a common preterminal event in cancer patients.25
Evidence suggests that delirium is a marker for declining functional status and of relatively poor outcomes in older patients. In patients who are hospitalized, however, the relative effects of comorbid medical and neurologic conditions on prognosis are difficult to differentiate from the effects of delirium.
CAN DELIRIUM BE PREVENTED?
Researchers at Yale University examined whether a multicomponent, nonpharmacologic intervention could reduce delirium incidence and episode duration in 852 at-risk hospitalized medical patients age 70 and older.26 Patients were randomly assigned to intervention or usual care and then observed daily until discharge. Interventions included protocols for orientation, mobilization, sleep hygiene, and sensory enhancement, as well as prompt treatment of dehydration.
Delirium occurred in 10% of the intervention group and in 15% of the usual-care group (matched odds ratio 0.6). Total days with delirium (105 vs. 161; P = 0.02) and total episodes (62 vs. 90; P = 0.03) were significantly lower in the intervention group. A potential source of bias in this study was a lack of randomization in assigning patients to intervention or usual care. Follow-up studies found that:
- The intervention increased health care costs for patients at high risk for delirium but had no significant effect on overall costs for patients at intermediate risk.27
- Delirium risk decreased the most (89%) in older patients who were most adherent to the intervention protocols during hospitalization.28
- Among the 705 patients who survived at least 6 months after discharge, those who had been in the intervention and usual-care groups showed similar functional and cognitive status and rates of depression, delirium, nursing home placement, and rehospitalization.29
CASE REPORT: A SUCCESSFUL INTERVENTION
Mr. A, age 66, who has moderate-to-severe chronic obstructive pulmonary disease, is hospitalized for surgery to remove a suspicious lung nodule. Two years ago, he experienced delirium following a transurethral prostatectomy. His hemoglobin is 9.1 g/dL (normal, 11.5 to 14 g/dL), defined as anemia related to chronic disease.
Because of his history of postoperative delirium, the hospital staff initiates preventive measures. Before surgery, he is given two units of blood for anemia. To assist with orientation, he and his family receive information about delirium, and his hearing aid—which has been malfunctioning—is readjusted to improve his auditory acuity. During surgery, his oxygen saturation and blood pressure are monitored scrupulously.
Afterward, no mental status changes are observed, and Mr. A recovers uneventfully. The surgery revealed a benign granuloma.
Discussion. Surgical patients such as Mr. A—particularly those with hemoglobin <10 g/dL—face a higher risk for delirium than medical patients do. The reason, although undetermined, may be related to unavoidable tissue injury and hemorrhage associated with surgery.30
Nonpharmacologic intervention shows promise in preventing delirium, but more evidence is needed to develop simpler, less-costly strategies for at-risk hospitalized patients and to preserve their functional status after discharge.
Related resources
- Cook IA. Guideline Watch. Practice guideline for the treatment of patients with delirium. American Psychiatric Association, August 2004. www.psych.org/psych_pract/treatg/pg/prac_guide.cfm (scroll down to “Delirium” under topic list). Accessed Dec. 14, 2004.
Drug brand names
- Aripiprazole • Abilify
- Cyclobenzapine • Flexeril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Phenylzine • Nardil
- Physostigmine • Antilirium
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosures
Dr. O’Connor reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th edition, text rev. Washington, DC: American Psychiatric Association, 2000.
2. Trzepacz PT, Mulsant BH, Amanda Dew M, et al. Is delirium different when it occurs with dementia? A study using the delirium rating scale. J Neuropsychiatry Clin Neurosci 1998;10:199-204.
3. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7.
4. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB (eds). Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;581-96.
5. Bourgeois JA, Seaman JS, Servis M. Delirium, dementia, and amnestic disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003;270.-
6. Rahkonen T, Makela H, Paanila S, et al. Delirium in elderly people without severe predisposing disorders: etiology and 1-year prognosis after discharge. Int Psychogeriatr 2000;12(4):473-81.
7. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment of delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry 2003;25(4):289-92.
8. Mittal D, Jimerson NA, Neely EP, et al. Risperidone in the treatment of delirium: results from a prospective open-label trial. J Clin Psychiatry 2004;65(5):662-7.
9. Parellada E, Baeza I, de Pablo J, Martinez G. Risperidone in the treatment of patients with delirium. J Clin Psychiatry 2004;65(3):348-53.
10. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004;45:297-301.
11. Schwartz TL, Masand PS. Treatment of delirium with quetiapine. Prim Care Companion J Clin Psychiatry 2000;2(1):10-12.
12. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry 2003;64(11):1316-21.
13. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol 2004;19(2):125-7.
14. Kim KY, Bader GM, Kotlyar V, Gropper D. Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol 2003;16(1):29-31.
15. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 2004;30(3):444-9.
16. Van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998;11:138-45.
17. Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999;10:330-4.
18. Mussi C, Ferrari R, Ascari S, et al. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol 1999;12:82-6.
19. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium.[see comment]. Am J Psychiatry 1992;149:1393-4.
20. Keck PE, Jr, Arnold LM. The serotonin syndrome. Psychiatr Ann 2000;30:333-43.
21. Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234-42.
22. Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982;140:149-53.
23. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med 2002;162(4):457-63.
24. McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18(9):696-704.
25. Greenberg DB. Preventing delirium at the end of life: lessons from recent research. Primary Care Companion J Clin Psychiatry 2003;5:62-7.
26. Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76.
27. Rizzo JA, Bogardus ST, Jr, Leo-Summers L, et al. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care 2001;39(7):740-52.
28. Inouye SK, Bogardus ST, Jr, Williams CS, et al. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med 2003;163(8):958-64.
29. Bogardus ST, Jr, Desai MM, Williams CS, et al. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114(5):383-90.
30. Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998;105(5):380-4.
Four principles of treating delirium can help protect medical/surgical patients at risk for morbidity and functional decline. These principals—which I call the “four Ps”—are prompt identification, protection, pragmatic intervention, and pharmacotherapy.
This article describes an up-to-date, “four-Ps” approach to treating delirium—including use of antipsychotics and supportive care—and offers evidence and case reports to address these clinical questions:
- What causes delirium?
- Does delirium worsen prognosis?
- Can delirium be prevented?
FOUR ‘Ps’ FOR TREATING DELIRIUM
When a patient’s mental status changes dramatically (Box 1),1 identifying potential delirium causes requires careful medical, psychiatric, and neurologic assessment. Assimilating this information is as essential to positive outcomes as are intensive nursing care and appropriate interventions.
- Disturbance of consciousness (i.e. reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention
- A change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia
- The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of a 24-hour period
- There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiologic consequences of a general medical condition
Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders (4th ed., text rev). Copyright 2000. American Psychiatric Publishing.
Prompt identification. Delirium often goes unrecognized, delaying treatment. Easily administered rating scales—such as the Delirium Rating Scale (DRS)2 and the Confusion Assessment Method (CAM)3 —can help detect emerging symptoms.
Patient protection. Provide intensive nursing care—often one-to-one observation and containment—and, where possible, enlist the family in reassuring and calming the patient. Restraints may be needed to safeguard against injury and to prevent the patient from removing or dislocating monitoring equipment and IV access.
Pragmatic intervention. With medical colleagues, begin treating biochemical and physiologic abnormalities that are the most likely and most remediable contributors (Box 2).2,4-6 Review the patient’s medications and discontinue or replace any that may be causing delirium.
Pharmacotherapy. Based on clinical studies, antipsychotics appear to possess antidelirium properties and may be considered as one part of a patient’s treatment plan. Interpreting these studies is complicated, however, by delirium’s complexity, numerous causes, and presumed mechanisms, as well as the transience of some forms. For ethical reasons, no placebo-controlled studies of delirium treatment have been done.
EVIDENCE ON ANTIPSYCHOTICS
Haloperidol has been the drug of choice for managing delirium because it is less likely to cause hypotension and sedation than other neuroleptics. Optimum haloperidol dosing in delirium has not been established, but the usual range is 2 to 6 mg every 4 to 6 hours, depending on the patient’s age and delirium severity.
Instances of QTc interval prolongation have been reported with high-dose IV haloperidol (> 100 mg). This life-threatening effect—which can induce torsades de pointes dysrhythmia, ventricular tachycardias, and fibrillation—is very rare, quite variable, and unpredictable. It probably is a function of total dose and neuroleptic administration rate.
Atypical antipsychotics share haloperidol’s advantages over first-generation neuroleptics, with lower potential for dystonic reactions, parkinsonian side effects, and tardive dyskinesia. Preliminary evidence suggests that atypicals may be safe and effective in treating delirium, although no randomized controlled trials have been done and accurate dose-response curves have not been established. Low to modest dosages have been used in case series.
Risperidone. Two prospective, open-label trials—each with 10 patients—suggest that low-dose risperidone is effective for treating delirium:
- In one trial, risperidone given at an average dosage of 1.7 mg/d was effective in 80% of patients with delirium, and one patient responded to 0.5 mg/d. Some patients experienced sleepiness or mild drug-induced parkinsonism.7
- In the other trial, risperidone was started at 0.5 mg twice daily, with additional doses allowed on day 1 for cognitive and behavioral symptoms. This dosage was maintained until DRS scores declined to ≤12, then was reduced by 50% and continued until day 6. Mean maintenance dosage was 0.75 mg/d. Two patients discontinued risperidone because of sedation or hypotension.8
At least 10% to 30% of hospitalized medically ill patients develop delirium, and rates approach 40% after age 65.4 Especially in older patients, delirium is a risk factor for:
- prolonged hospital stays
- increased morbidity and mortality
- increased functional decline and need for custodial care after hospital discharge.2
Risk factors. Prospectively identified risk factors for delirium include pre-existing dementia; age >65 years; serious medical illness; alcohol/sedative withdrawal; abnormal serum sodium, potassium, or blood glucose levels; vision or hearing impairment; hypoxia; malnutrition; and fever. Medication—particularly anticholinergic drugs—is one of the most common delirium triggers in susceptible patients.5
The most common underlying disorders that increase delirium risk in older patients are hip fracture, dementia, infections, and cerebrovascular events.6
In a larger prospective study, 64 patients (mean age 67) with delirium were treated with risperidone, given at a mean dose of 2.6 +/- 1.7 mg/d at day 3. This dosage was effective in 90% of patients and significantly improved all symptoms, as measured with scales including the DRS. Two patients (3%) experienced adverse effects.9
No significant differences in response frequency were seen in a 7-day, double-blind comparison of flexibly-dosed risperidone (starting at 0.5 mg bid) and haloperidol (starting at 0.75 mg bid) in 28 patients with delirium. Symptom severity decreased for each group, as measured with the Memorial Delirium Assessment Scale. One patient receiving haloperidol experienced mild akathisia, but no others reported clinically significant side effects.10
Quetiapine. In a retrospective review, the charts of 11 patients who received quetiapine for delirium were compared with those of 11 similar patients treated with haloperidol. DRS scores improved by >50% in 10 of 11 patients in both groups, with similar onset of effect, treatment duration, and overall clinical improvement.11 Small prospective trials with flexible dosing schedules have reported similar results.12,13
In a study of 12 older hospitalized patients with delirium, quetiapine at a mean dosage of 93.75 +/-23.31 mg/d was associated with significant DRS score improvements. Interestingly, patients’ Mini-Mental State Examination and Clock-Drawing Test scores continued to improve 3 months after their delirium symptoms stabilized.14
Olanzapine. In a prospective trial, hospitalized patients with delirium were randomly assigned to receive enteral olanzapine or haloperidol. Delirium symptoms decreased across 5 days in both groups, and clinical improvement was similar. Some patients receiving haloperidol reported extrapyramidal symptoms, whereas those receiving olanzapine reported no adverse effects.15
Parenteral forms of some atypicals (aripiprazole, olanzapine, and ziprasidone) have become available and may increase this class’ usefulness in treating delirium.
Other drugs. Benzodiazepines appear ineffective and generally play only an adjunctive role in treating delirium. An exception may be delirium induced by acute alcohol or benzodiazepine withdrawal. Sedating antidepressants have been used as hypnotics in patients with delirium, but supporting evidence is lacking.
Other drug classes—general anesthetics, narcotics, cholinomimetics—may help manage the dangerously hyperactive delirious patient, but the literature contains no systematic analyses.
WHAT CAUSES DELIRIUM?
Delirium’s pathophysiology is not completely understood, although most authors believe several mechanisms are involved.
The brain’s exclusively oxidative metabolism and its systems’ hierarchical vulnerability to substrate deficiency—as might occur in even transient hypoxia or hypotension—appear to play important roles. Factors such as fever and stress that increase metabolic demand on the brain intensify the effects of oxygen deficiency or circulatory compromise.
At least three molecular mechanisms have been proposed for delirium, including cholinergic transmission disruption, monoaminergic dysfunction, and cytokine release ( Box 3).16-19 These mechanisms may interact, cascading into a common final pathway that results in delirium.
Features not considered essential to delirium’s diagnosis—such as visual hallucinations or aggressive behaviors—indicate that additional cortical and subcortical systems are involved.
CASE REPORT: DRUG-DRUG INTERACTION
Three days after hip replacement surgery, Mr. S, age 64, becomes confused, distractible, and combative. He is alert one minute and somnolent the next. His arms and legs jerk involuntarily, and his muscle tone is diffusely increased. He talks with absent friends and family as though they are present in his hospital room. His body temperature and blood pressure fluctuate widely, despite no evidence of infection.
Cholinergic transmission disruption
The greater a medication’s anticholinergic activity, the greater its risk of causing delirium. Combining drugs with anticholinergic effects—such as theophylline, warfarin, or codeine (Table19)—compounds the delirium risk.
Acetylcholine-secreting neurons—widely if sparsely distributed throughout the brain—affect arousal, attention, memory, and sleep regulation. Acetylcholine is produced by oxidative metabolism and thus is vulnerable to physiologic disturbances that increase oxygen demand or disrupt oxygen supply.
Anticholinergic poisoning and abuse of anticholinergic substances are known to cause acute delirium—a finding that supports the key role of acetylcholine in maintaining alertness and concentration. Agents that enhance cholinergic transmission—such as the cholinesterase inhibitor physostigmine—can effectively treat drug-induced delirium.
Monoaminergic dysfunction
The principal monoamines of dopamine, serotonin, and norepinephrine help sustain attention, regulate the sleep-wake cycle, inhibit affective responses, and modulate aggressive and impulsive behaviors. Treating patients with dopamine and serotonin agonists can cause psychotic symptoms.
Glutamate—a monoamine neurotransmitter with excitatory properties—is released during metabolic stress and likely contributes to the psychotic features sometimes seen in delirium.
Cytokine release
Infection in a distant organ, such as gallbladder or kidney, is known to cause delirium. Cytokines such as interleukins and interferon-alpha are polypeptides secreted by macrophagesin response to tissue injury. They easily cross the blood-brain barrier and stimulate glial cells to release more cytokines, which interfere with neurotransmitter synthesis and transmission.
Table
Drugs whose anticholinergic effects may increase the risk of delirium
| Drug | Anticholinergic level* |
|---|---|
| Cimetidine | 0.86 |
| Prednisolone | 0.55 |
| Theophylline | 0.44 |
| Digoxin | 0.25 |
| Lanoxin | 0.25 |
| Nifedipine | 0.22 |
| Ranitidine | 0.22 |
| Furosemide | 0.22 |
| Isosorbide | 0.15 |
| Warfarin | 0.12 |
| Dipyridamole | 0.11 |
| Codeine | 0.11 |
| * ng/mL in atropine equivalents | |
| Source: Adapted from reference 19. | |
For several years, Mr. S has been taking the monoamine oxidase inhibitor (MAOI) phenelzine, 30 mg/d, for depression maintenance treatment. On admission, he insisted that the MAOI be continued during hospitalization because it had relieved his severe depressions.
Within 24 hours of surgery, he was given the skeletal muscle relaxant cyclobenzaprine, 5 mg tid, for painful muscle spasms in the operated hip. When this brought little relief, the dosage was increased to 10 mg tid. Delirium and autonomic instability developed approximately 4 hours after the first 10-mg dose and gradually worsened.
The two drugs are discontinued, and Mr S. gradually recovers after several days of physiologic support, protection, and sedation in the intensive-care unit.
Discussion. Mr. S developed serotonin syndrome from a drug-drug interaction. Phenelzine inhibited serotonin metabolism, and cyclobenzaprine—a drug chemically similar to tricyclic antidepressants—inhibited serotonin reuptake, resulting in substantially increased CNS serotonergic activity.20 Serotonin syndrome symptoms include delirium, autonomic dysfunction, and neurologic signs such as myoclonus and rigidity when patients are taking drugs that enhance serotonergic transmission.
DOES DELIRIUM WORSEN PROGNOSIS?
In the largest study of delirium in older patients, Inouye et al21 examined outcomes of 727 consecutive patients age 65 and older with various medical diagnoses who were admitted to three teaching hospitals. Delirium was diagnosed in 88 patients (12%) at admission.
Within 3 months of hospital discharge, 165 (25%) of 663 patients had died or been newly admitted to a nursing home. After the authors controlled the data for age, gender, dementia, illness severity, and functional status, they found that delirium:
- tripled the likelihood of nursing home placement at hospital discharge and after 3 months (adjusted odds ratio [OR] for delirium 3.0)
- more than doubled the likelihood of death or new nursing home placement at discharge (OR for delirium 2.1) and after 3 months (OR for delirium 2.6).
They concluded that delirium was a significant predictor of functional decline at hospital discharge and also at follow-up in older patients.
Interestingly, although these authors did not find a statistically significant association between delirium and death alone, the risk of death was particularly strong for patients who were not demented (OR for delirium, 3.77). Similarly, Rabins and Folstein22 found higher mortality rates in medically ill patients diagnosed with delirium on hospital admission than in demented, cognitively intact, or depressed patients. After 1 year, the death rate remained higher in those who had been delirious than in those with dementia.
In a 12-month observational study comparing 243 older medical inpatients with delirium and 118 controls without delirium, McCusker et al23 found that:
- patients with delirium were twice as likely to die within 12 months as those without delirium
- the greater severity of delirium symptoms, the higher the risk of death in patients with delirium but without dementia.
In a recent study, some of the same investigators found that delirium symptoms—especially inattention, disorientation, and impaired memory—persisted for 12 months after hospital discharge in medical inpatients age 65 and older with or without dementia. Mean numbers of delirium symptoms at diagnosis and 12-month follow-up, respectively, were:
- 4.5 and 3.5 in patients with dementia
- 3.4 and 2.2 in patients without dementia.24
CASE REPORT: DELIRIUM AS PROGNOSTIC SIGN
Mrs. W, age 70, is hospitalized for treatment of anemia and dehydration after falling at home. She has metastatic adenocarcinoma of the colon and is hypernatremic and hypotensive on admission.
Within 24 hours, she becomes floridly delirious, despite transfusion of two units of packed red cells and IV fluid replacement. She receives IM haloperidol to reduce the agitation and counteract delirium. Head CT reveals mild, diffuse cerebral atrophy but no metastasis or subdural hematoma.
Although aggressive treatment corrects her electrolyte disturbance and dehydration and restores normal vital signs, the delirium does not resolve. She is discharged to a nursing home, where she is discovered dead in bed 1 week later.
Discussion. Delirium independently increases the risk of death during hospitalization and thereafter, particularly in older patients. As in the case of Mrs. W, delirium is a common preterminal event in cancer patients.25
Evidence suggests that delirium is a marker for declining functional status and of relatively poor outcomes in older patients. In patients who are hospitalized, however, the relative effects of comorbid medical and neurologic conditions on prognosis are difficult to differentiate from the effects of delirium.
CAN DELIRIUM BE PREVENTED?
Researchers at Yale University examined whether a multicomponent, nonpharmacologic intervention could reduce delirium incidence and episode duration in 852 at-risk hospitalized medical patients age 70 and older.26 Patients were randomly assigned to intervention or usual care and then observed daily until discharge. Interventions included protocols for orientation, mobilization, sleep hygiene, and sensory enhancement, as well as prompt treatment of dehydration.
Delirium occurred in 10% of the intervention group and in 15% of the usual-care group (matched odds ratio 0.6). Total days with delirium (105 vs. 161; P = 0.02) and total episodes (62 vs. 90; P = 0.03) were significantly lower in the intervention group. A potential source of bias in this study was a lack of randomization in assigning patients to intervention or usual care. Follow-up studies found that:
- The intervention increased health care costs for patients at high risk for delirium but had no significant effect on overall costs for patients at intermediate risk.27
- Delirium risk decreased the most (89%) in older patients who were most adherent to the intervention protocols during hospitalization.28
- Among the 705 patients who survived at least 6 months after discharge, those who had been in the intervention and usual-care groups showed similar functional and cognitive status and rates of depression, delirium, nursing home placement, and rehospitalization.29
CASE REPORT: A SUCCESSFUL INTERVENTION
Mr. A, age 66, who has moderate-to-severe chronic obstructive pulmonary disease, is hospitalized for surgery to remove a suspicious lung nodule. Two years ago, he experienced delirium following a transurethral prostatectomy. His hemoglobin is 9.1 g/dL (normal, 11.5 to 14 g/dL), defined as anemia related to chronic disease.
Because of his history of postoperative delirium, the hospital staff initiates preventive measures. Before surgery, he is given two units of blood for anemia. To assist with orientation, he and his family receive information about delirium, and his hearing aid—which has been malfunctioning—is readjusted to improve his auditory acuity. During surgery, his oxygen saturation and blood pressure are monitored scrupulously.
Afterward, no mental status changes are observed, and Mr. A recovers uneventfully. The surgery revealed a benign granuloma.
Discussion. Surgical patients such as Mr. A—particularly those with hemoglobin <10 g/dL—face a higher risk for delirium than medical patients do. The reason, although undetermined, may be related to unavoidable tissue injury and hemorrhage associated with surgery.30
Nonpharmacologic intervention shows promise in preventing delirium, but more evidence is needed to develop simpler, less-costly strategies for at-risk hospitalized patients and to preserve their functional status after discharge.
Related resources
- Cook IA. Guideline Watch. Practice guideline for the treatment of patients with delirium. American Psychiatric Association, August 2004. www.psych.org/psych_pract/treatg/pg/prac_guide.cfm (scroll down to “Delirium” under topic list). Accessed Dec. 14, 2004.
Drug brand names
- Aripiprazole • Abilify
- Cyclobenzapine • Flexeril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Phenylzine • Nardil
- Physostigmine • Antilirium
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosures
Dr. O’Connor reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Four principles of treating delirium can help protect medical/surgical patients at risk for morbidity and functional decline. These principals—which I call the “four Ps”—are prompt identification, protection, pragmatic intervention, and pharmacotherapy.
This article describes an up-to-date, “four-Ps” approach to treating delirium—including use of antipsychotics and supportive care—and offers evidence and case reports to address these clinical questions:
- What causes delirium?
- Does delirium worsen prognosis?
- Can delirium be prevented?
FOUR ‘Ps’ FOR TREATING DELIRIUM
When a patient’s mental status changes dramatically (Box 1),1 identifying potential delirium causes requires careful medical, psychiatric, and neurologic assessment. Assimilating this information is as essential to positive outcomes as are intensive nursing care and appropriate interventions.
- Disturbance of consciousness (i.e. reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention
- A change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia
- The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of a 24-hour period
- There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiologic consequences of a general medical condition
Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders (4th ed., text rev). Copyright 2000. American Psychiatric Publishing.
Prompt identification. Delirium often goes unrecognized, delaying treatment. Easily administered rating scales—such as the Delirium Rating Scale (DRS)2 and the Confusion Assessment Method (CAM)3 —can help detect emerging symptoms.
Patient protection. Provide intensive nursing care—often one-to-one observation and containment—and, where possible, enlist the family in reassuring and calming the patient. Restraints may be needed to safeguard against injury and to prevent the patient from removing or dislocating monitoring equipment and IV access.
Pragmatic intervention. With medical colleagues, begin treating biochemical and physiologic abnormalities that are the most likely and most remediable contributors (Box 2).2,4-6 Review the patient’s medications and discontinue or replace any that may be causing delirium.
Pharmacotherapy. Based on clinical studies, antipsychotics appear to possess antidelirium properties and may be considered as one part of a patient’s treatment plan. Interpreting these studies is complicated, however, by delirium’s complexity, numerous causes, and presumed mechanisms, as well as the transience of some forms. For ethical reasons, no placebo-controlled studies of delirium treatment have been done.
EVIDENCE ON ANTIPSYCHOTICS
Haloperidol has been the drug of choice for managing delirium because it is less likely to cause hypotension and sedation than other neuroleptics. Optimum haloperidol dosing in delirium has not been established, but the usual range is 2 to 6 mg every 4 to 6 hours, depending on the patient’s age and delirium severity.
Instances of QTc interval prolongation have been reported with high-dose IV haloperidol (> 100 mg). This life-threatening effect—which can induce torsades de pointes dysrhythmia, ventricular tachycardias, and fibrillation—is very rare, quite variable, and unpredictable. It probably is a function of total dose and neuroleptic administration rate.
Atypical antipsychotics share haloperidol’s advantages over first-generation neuroleptics, with lower potential for dystonic reactions, parkinsonian side effects, and tardive dyskinesia. Preliminary evidence suggests that atypicals may be safe and effective in treating delirium, although no randomized controlled trials have been done and accurate dose-response curves have not been established. Low to modest dosages have been used in case series.
Risperidone. Two prospective, open-label trials—each with 10 patients—suggest that low-dose risperidone is effective for treating delirium:
- In one trial, risperidone given at an average dosage of 1.7 mg/d was effective in 80% of patients with delirium, and one patient responded to 0.5 mg/d. Some patients experienced sleepiness or mild drug-induced parkinsonism.7
- In the other trial, risperidone was started at 0.5 mg twice daily, with additional doses allowed on day 1 for cognitive and behavioral symptoms. This dosage was maintained until DRS scores declined to ≤12, then was reduced by 50% and continued until day 6. Mean maintenance dosage was 0.75 mg/d. Two patients discontinued risperidone because of sedation or hypotension.8
At least 10% to 30% of hospitalized medically ill patients develop delirium, and rates approach 40% after age 65.4 Especially in older patients, delirium is a risk factor for:
- prolonged hospital stays
- increased morbidity and mortality
- increased functional decline and need for custodial care after hospital discharge.2
Risk factors. Prospectively identified risk factors for delirium include pre-existing dementia; age >65 years; serious medical illness; alcohol/sedative withdrawal; abnormal serum sodium, potassium, or blood glucose levels; vision or hearing impairment; hypoxia; malnutrition; and fever. Medication—particularly anticholinergic drugs—is one of the most common delirium triggers in susceptible patients.5
The most common underlying disorders that increase delirium risk in older patients are hip fracture, dementia, infections, and cerebrovascular events.6
In a larger prospective study, 64 patients (mean age 67) with delirium were treated with risperidone, given at a mean dose of 2.6 +/- 1.7 mg/d at day 3. This dosage was effective in 90% of patients and significantly improved all symptoms, as measured with scales including the DRS. Two patients (3%) experienced adverse effects.9
No significant differences in response frequency were seen in a 7-day, double-blind comparison of flexibly-dosed risperidone (starting at 0.5 mg bid) and haloperidol (starting at 0.75 mg bid) in 28 patients with delirium. Symptom severity decreased for each group, as measured with the Memorial Delirium Assessment Scale. One patient receiving haloperidol experienced mild akathisia, but no others reported clinically significant side effects.10
Quetiapine. In a retrospective review, the charts of 11 patients who received quetiapine for delirium were compared with those of 11 similar patients treated with haloperidol. DRS scores improved by >50% in 10 of 11 patients in both groups, with similar onset of effect, treatment duration, and overall clinical improvement.11 Small prospective trials with flexible dosing schedules have reported similar results.12,13
In a study of 12 older hospitalized patients with delirium, quetiapine at a mean dosage of 93.75 +/-23.31 mg/d was associated with significant DRS score improvements. Interestingly, patients’ Mini-Mental State Examination and Clock-Drawing Test scores continued to improve 3 months after their delirium symptoms stabilized.14
Olanzapine. In a prospective trial, hospitalized patients with delirium were randomly assigned to receive enteral olanzapine or haloperidol. Delirium symptoms decreased across 5 days in both groups, and clinical improvement was similar. Some patients receiving haloperidol reported extrapyramidal symptoms, whereas those receiving olanzapine reported no adverse effects.15
Parenteral forms of some atypicals (aripiprazole, olanzapine, and ziprasidone) have become available and may increase this class’ usefulness in treating delirium.
Other drugs. Benzodiazepines appear ineffective and generally play only an adjunctive role in treating delirium. An exception may be delirium induced by acute alcohol or benzodiazepine withdrawal. Sedating antidepressants have been used as hypnotics in patients with delirium, but supporting evidence is lacking.
Other drug classes—general anesthetics, narcotics, cholinomimetics—may help manage the dangerously hyperactive delirious patient, but the literature contains no systematic analyses.
WHAT CAUSES DELIRIUM?
Delirium’s pathophysiology is not completely understood, although most authors believe several mechanisms are involved.
The brain’s exclusively oxidative metabolism and its systems’ hierarchical vulnerability to substrate deficiency—as might occur in even transient hypoxia or hypotension—appear to play important roles. Factors such as fever and stress that increase metabolic demand on the brain intensify the effects of oxygen deficiency or circulatory compromise.
At least three molecular mechanisms have been proposed for delirium, including cholinergic transmission disruption, monoaminergic dysfunction, and cytokine release ( Box 3).16-19 These mechanisms may interact, cascading into a common final pathway that results in delirium.
Features not considered essential to delirium’s diagnosis—such as visual hallucinations or aggressive behaviors—indicate that additional cortical and subcortical systems are involved.
CASE REPORT: DRUG-DRUG INTERACTION
Three days after hip replacement surgery, Mr. S, age 64, becomes confused, distractible, and combative. He is alert one minute and somnolent the next. His arms and legs jerk involuntarily, and his muscle tone is diffusely increased. He talks with absent friends and family as though they are present in his hospital room. His body temperature and blood pressure fluctuate widely, despite no evidence of infection.
Cholinergic transmission disruption
The greater a medication’s anticholinergic activity, the greater its risk of causing delirium. Combining drugs with anticholinergic effects—such as theophylline, warfarin, or codeine (Table19)—compounds the delirium risk.
Acetylcholine-secreting neurons—widely if sparsely distributed throughout the brain—affect arousal, attention, memory, and sleep regulation. Acetylcholine is produced by oxidative metabolism and thus is vulnerable to physiologic disturbances that increase oxygen demand or disrupt oxygen supply.
Anticholinergic poisoning and abuse of anticholinergic substances are known to cause acute delirium—a finding that supports the key role of acetylcholine in maintaining alertness and concentration. Agents that enhance cholinergic transmission—such as the cholinesterase inhibitor physostigmine—can effectively treat drug-induced delirium.
Monoaminergic dysfunction
The principal monoamines of dopamine, serotonin, and norepinephrine help sustain attention, regulate the sleep-wake cycle, inhibit affective responses, and modulate aggressive and impulsive behaviors. Treating patients with dopamine and serotonin agonists can cause psychotic symptoms.
Glutamate—a monoamine neurotransmitter with excitatory properties—is released during metabolic stress and likely contributes to the psychotic features sometimes seen in delirium.
Cytokine release
Infection in a distant organ, such as gallbladder or kidney, is known to cause delirium. Cytokines such as interleukins and interferon-alpha are polypeptides secreted by macrophagesin response to tissue injury. They easily cross the blood-brain barrier and stimulate glial cells to release more cytokines, which interfere with neurotransmitter synthesis and transmission.
Table
Drugs whose anticholinergic effects may increase the risk of delirium
| Drug | Anticholinergic level* |
|---|---|
| Cimetidine | 0.86 |
| Prednisolone | 0.55 |
| Theophylline | 0.44 |
| Digoxin | 0.25 |
| Lanoxin | 0.25 |
| Nifedipine | 0.22 |
| Ranitidine | 0.22 |
| Furosemide | 0.22 |
| Isosorbide | 0.15 |
| Warfarin | 0.12 |
| Dipyridamole | 0.11 |
| Codeine | 0.11 |
| * ng/mL in atropine equivalents | |
| Source: Adapted from reference 19. | |
For several years, Mr. S has been taking the monoamine oxidase inhibitor (MAOI) phenelzine, 30 mg/d, for depression maintenance treatment. On admission, he insisted that the MAOI be continued during hospitalization because it had relieved his severe depressions.
Within 24 hours of surgery, he was given the skeletal muscle relaxant cyclobenzaprine, 5 mg tid, for painful muscle spasms in the operated hip. When this brought little relief, the dosage was increased to 10 mg tid. Delirium and autonomic instability developed approximately 4 hours after the first 10-mg dose and gradually worsened.
The two drugs are discontinued, and Mr S. gradually recovers after several days of physiologic support, protection, and sedation in the intensive-care unit.
Discussion. Mr. S developed serotonin syndrome from a drug-drug interaction. Phenelzine inhibited serotonin metabolism, and cyclobenzaprine—a drug chemically similar to tricyclic antidepressants—inhibited serotonin reuptake, resulting in substantially increased CNS serotonergic activity.20 Serotonin syndrome symptoms include delirium, autonomic dysfunction, and neurologic signs such as myoclonus and rigidity when patients are taking drugs that enhance serotonergic transmission.
DOES DELIRIUM WORSEN PROGNOSIS?
In the largest study of delirium in older patients, Inouye et al21 examined outcomes of 727 consecutive patients age 65 and older with various medical diagnoses who were admitted to three teaching hospitals. Delirium was diagnosed in 88 patients (12%) at admission.
Within 3 months of hospital discharge, 165 (25%) of 663 patients had died or been newly admitted to a nursing home. After the authors controlled the data for age, gender, dementia, illness severity, and functional status, they found that delirium:
- tripled the likelihood of nursing home placement at hospital discharge and after 3 months (adjusted odds ratio [OR] for delirium 3.0)
- more than doubled the likelihood of death or new nursing home placement at discharge (OR for delirium 2.1) and after 3 months (OR for delirium 2.6).
They concluded that delirium was a significant predictor of functional decline at hospital discharge and also at follow-up in older patients.
Interestingly, although these authors did not find a statistically significant association between delirium and death alone, the risk of death was particularly strong for patients who were not demented (OR for delirium, 3.77). Similarly, Rabins and Folstein22 found higher mortality rates in medically ill patients diagnosed with delirium on hospital admission than in demented, cognitively intact, or depressed patients. After 1 year, the death rate remained higher in those who had been delirious than in those with dementia.
In a 12-month observational study comparing 243 older medical inpatients with delirium and 118 controls without delirium, McCusker et al23 found that:
- patients with delirium were twice as likely to die within 12 months as those without delirium
- the greater severity of delirium symptoms, the higher the risk of death in patients with delirium but without dementia.
In a recent study, some of the same investigators found that delirium symptoms—especially inattention, disorientation, and impaired memory—persisted for 12 months after hospital discharge in medical inpatients age 65 and older with or without dementia. Mean numbers of delirium symptoms at diagnosis and 12-month follow-up, respectively, were:
- 4.5 and 3.5 in patients with dementia
- 3.4 and 2.2 in patients without dementia.24
CASE REPORT: DELIRIUM AS PROGNOSTIC SIGN
Mrs. W, age 70, is hospitalized for treatment of anemia and dehydration after falling at home. She has metastatic adenocarcinoma of the colon and is hypernatremic and hypotensive on admission.
Within 24 hours, she becomes floridly delirious, despite transfusion of two units of packed red cells and IV fluid replacement. She receives IM haloperidol to reduce the agitation and counteract delirium. Head CT reveals mild, diffuse cerebral atrophy but no metastasis or subdural hematoma.
Although aggressive treatment corrects her electrolyte disturbance and dehydration and restores normal vital signs, the delirium does not resolve. She is discharged to a nursing home, where she is discovered dead in bed 1 week later.
Discussion. Delirium independently increases the risk of death during hospitalization and thereafter, particularly in older patients. As in the case of Mrs. W, delirium is a common preterminal event in cancer patients.25
Evidence suggests that delirium is a marker for declining functional status and of relatively poor outcomes in older patients. In patients who are hospitalized, however, the relative effects of comorbid medical and neurologic conditions on prognosis are difficult to differentiate from the effects of delirium.
CAN DELIRIUM BE PREVENTED?
Researchers at Yale University examined whether a multicomponent, nonpharmacologic intervention could reduce delirium incidence and episode duration in 852 at-risk hospitalized medical patients age 70 and older.26 Patients were randomly assigned to intervention or usual care and then observed daily until discharge. Interventions included protocols for orientation, mobilization, sleep hygiene, and sensory enhancement, as well as prompt treatment of dehydration.
Delirium occurred in 10% of the intervention group and in 15% of the usual-care group (matched odds ratio 0.6). Total days with delirium (105 vs. 161; P = 0.02) and total episodes (62 vs. 90; P = 0.03) were significantly lower in the intervention group. A potential source of bias in this study was a lack of randomization in assigning patients to intervention or usual care. Follow-up studies found that:
- The intervention increased health care costs for patients at high risk for delirium but had no significant effect on overall costs for patients at intermediate risk.27
- Delirium risk decreased the most (89%) in older patients who were most adherent to the intervention protocols during hospitalization.28
- Among the 705 patients who survived at least 6 months after discharge, those who had been in the intervention and usual-care groups showed similar functional and cognitive status and rates of depression, delirium, nursing home placement, and rehospitalization.29
CASE REPORT: A SUCCESSFUL INTERVENTION
Mr. A, age 66, who has moderate-to-severe chronic obstructive pulmonary disease, is hospitalized for surgery to remove a suspicious lung nodule. Two years ago, he experienced delirium following a transurethral prostatectomy. His hemoglobin is 9.1 g/dL (normal, 11.5 to 14 g/dL), defined as anemia related to chronic disease.
Because of his history of postoperative delirium, the hospital staff initiates preventive measures. Before surgery, he is given two units of blood for anemia. To assist with orientation, he and his family receive information about delirium, and his hearing aid—which has been malfunctioning—is readjusted to improve his auditory acuity. During surgery, his oxygen saturation and blood pressure are monitored scrupulously.
Afterward, no mental status changes are observed, and Mr. A recovers uneventfully. The surgery revealed a benign granuloma.
Discussion. Surgical patients such as Mr. A—particularly those with hemoglobin <10 g/dL—face a higher risk for delirium than medical patients do. The reason, although undetermined, may be related to unavoidable tissue injury and hemorrhage associated with surgery.30
Nonpharmacologic intervention shows promise in preventing delirium, but more evidence is needed to develop simpler, less-costly strategies for at-risk hospitalized patients and to preserve their functional status after discharge.
Related resources
- Cook IA. Guideline Watch. Practice guideline for the treatment of patients with delirium. American Psychiatric Association, August 2004. www.psych.org/psych_pract/treatg/pg/prac_guide.cfm (scroll down to “Delirium” under topic list). Accessed Dec. 14, 2004.
Drug brand names
- Aripiprazole • Abilify
- Cyclobenzapine • Flexeril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Phenylzine • Nardil
- Physostigmine • Antilirium
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Warfarin • Coumadin
- Ziprasidone • Geodon
Disclosures
Dr. O’Connor reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th edition, text rev. Washington, DC: American Psychiatric Association, 2000.
2. Trzepacz PT, Mulsant BH, Amanda Dew M, et al. Is delirium different when it occurs with dementia? A study using the delirium rating scale. J Neuropsychiatry Clin Neurosci 1998;10:199-204.
3. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7.
4. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB (eds). Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;581-96.
5. Bourgeois JA, Seaman JS, Servis M. Delirium, dementia, and amnestic disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003;270.-
6. Rahkonen T, Makela H, Paanila S, et al. Delirium in elderly people without severe predisposing disorders: etiology and 1-year prognosis after discharge. Int Psychogeriatr 2000;12(4):473-81.
7. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment of delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry 2003;25(4):289-92.
8. Mittal D, Jimerson NA, Neely EP, et al. Risperidone in the treatment of delirium: results from a prospective open-label trial. J Clin Psychiatry 2004;65(5):662-7.
9. Parellada E, Baeza I, de Pablo J, Martinez G. Risperidone in the treatment of patients with delirium. J Clin Psychiatry 2004;65(3):348-53.
10. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004;45:297-301.
11. Schwartz TL, Masand PS. Treatment of delirium with quetiapine. Prim Care Companion J Clin Psychiatry 2000;2(1):10-12.
12. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry 2003;64(11):1316-21.
13. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol 2004;19(2):125-7.
14. Kim KY, Bader GM, Kotlyar V, Gropper D. Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol 2003;16(1):29-31.
15. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 2004;30(3):444-9.
16. Van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998;11:138-45.
17. Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999;10:330-4.
18. Mussi C, Ferrari R, Ascari S, et al. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol 1999;12:82-6.
19. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium.[see comment]. Am J Psychiatry 1992;149:1393-4.
20. Keck PE, Jr, Arnold LM. The serotonin syndrome. Psychiatr Ann 2000;30:333-43.
21. Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234-42.
22. Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982;140:149-53.
23. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med 2002;162(4):457-63.
24. McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18(9):696-704.
25. Greenberg DB. Preventing delirium at the end of life: lessons from recent research. Primary Care Companion J Clin Psychiatry 2003;5:62-7.
26. Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76.
27. Rizzo JA, Bogardus ST, Jr, Leo-Summers L, et al. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care 2001;39(7):740-52.
28. Inouye SK, Bogardus ST, Jr, Williams CS, et al. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med 2003;163(8):958-64.
29. Bogardus ST, Jr, Desai MM, Williams CS, et al. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114(5):383-90.
30. Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998;105(5):380-4.
1. Diagnostic and statistical manual of mental disorders, 4th edition, text rev. Washington, DC: American Psychiatric Association, 2000.
2. Trzepacz PT, Mulsant BH, Amanda Dew M, et al. Is delirium different when it occurs with dementia? A study using the delirium rating scale. J Neuropsychiatry Clin Neurosci 1998;10:199-204.
3. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852-7.
4. Liptzin B. Clinical diagnosis and management of delirium. In: Stoudemire A, Fogel BS, Greenberg DB (eds). Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;581-96.
5. Bourgeois JA, Seaman JS, Servis M. Delirium, dementia, and amnestic disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry (4th ed). Washington, DC: American Psychiatric Publishing, 2003;270.-
6. Rahkonen T, Makela H, Paanila S, et al. Delirium in elderly people without severe predisposing disorders: etiology and 1-year prognosis after discharge. Int Psychogeriatr 2000;12(4):473-81.
7. Horikawa N, Yamazaki T, Miyamoto K, et al. Treatment of delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry 2003;25(4):289-92.
8. Mittal D, Jimerson NA, Neely EP, et al. Risperidone in the treatment of delirium: results from a prospective open-label trial. J Clin Psychiatry 2004;65(5):662-7.
9. Parellada E, Baeza I, de Pablo J, Martinez G. Risperidone in the treatment of patients with delirium. J Clin Psychiatry 2004;65(3):348-53.
10. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics 2004;45:297-301.
11. Schwartz TL, Masand PS. Treatment of delirium with quetiapine. Prim Care Companion J Clin Psychiatry 2000;2(1):10-12.
12. Sasaki Y, Matsuyama T, Inoue S, et al. A prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. J Clin Psychiatry 2003;64(11):1316-21.
13. Pae CU, Lee SJ, Lee CU, et al. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol 2004;19(2):125-7.
14. Kim KY, Bader GM, Kotlyar V, Gropper D. Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol 2003;16(1):29-31.
15. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med 2004;30(3):444-9.
16. Van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998;11:138-45.
17. Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999;10:330-4.
18. Mussi C, Ferrari R, Ascari S, et al. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J Geriatr Psychiatry Neurol 1999;12:82-6.
19. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium.[see comment]. Am J Psychiatry 1992;149:1393-4.
20. Keck PE, Jr, Arnold LM. The serotonin syndrome. Psychiatr Ann 2000;30:333-43.
21. Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234-42.
22. Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982;140:149-53.
23. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med 2002;162(4):457-63.
24. McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med 2003;18(9):696-704.
25. Greenberg DB. Preventing delirium at the end of life: lessons from recent research. Primary Care Companion J Clin Psychiatry 2003;5:62-7.
26. Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76.
27. Rizzo JA, Bogardus ST, Jr, Leo-Summers L, et al. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care 2001;39(7):740-52.
28. Inouye SK, Bogardus ST, Jr, Williams CS, et al. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch Intern Med 2003;163(8):958-64.
29. Bogardus ST, Jr, Desai MM, Williams CS, et al. The effects of a targeted multicomponent delirium intervention on postdischarge outcomes for hospitalized older adults. Am J Med 2003;114(5):383-90.
30. Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med 1998;105(5):380-4.
Scleroderma Responds to Stem Cell Transplant
SAN ANTONIO — A small group of patients with severe systemic sclerosis have shown a durable response to autologous hematopoietic stem cell transplantation, with 8 of 13 transplanted patients remaining alive after a mean follow-up of 44 months, Zora Marjanovic, M.D., reported at the annual meeting of the American College of Rheumatology.
Stem cell transplantation has in recent years been investigated for use in diseases such as scleroderma following observations that some patients with autoimmune disease who undergo transplantation for hematopoietic or other malignancies also may experience a remission of the autoimmune disease after the procedure.
In the first sequential open phase I-II study assessing the feasibility of autologous stem cell transplantation for systemic sclerosis with early visceral involvement, patients were eligible if they had rapidly progressing disease with heart, lung, or kidney involvement, Dr. Marjanovic said in a poster session.
The transplant protocol involved mobilization with cyclophosphamide plus recombinant human granulocyte colony-stimulating factor (GCSF) or GCSF alone if the left ventricular ejection fraction (LVEF) was less than 40%.
Subsequent conditioning, which took place at least 4 weeks after mobilization, used cyclophosphamide, 200 mg/kg, or melphalan, 140 mg/m
Outcomes following reinjection of CD34+ and hematopoietic stem cells were classified as major response, partial response, no response, disease progression, or relapse. Patients were assessed every 3 months.
Of the 14 patients enrolled in the nonrandomized trial, 13 were transplanted; 1 withdrew after mobilization.
One procedure-related death occurred, she said.
Six months following transplantation, nine patients responded to treatment—six had major responses and three had partial responses.
After a mean follow-up of 44 months, 8 of the responding patients were alive, 4 have died from disease progression. One nonresponding patient remains alive.
During the follow-up period, five patients relapsed but eventually responded to reintroduction of immunosuppression by mycophenolate mofetil. Four of these were partial responses, and one was a major response, said Dr. Marjanovic of University Hospital Center Saint-Louis, Paris, France.
This trial demonstrated that autologous hematopoietic stem cell transplantation is feasible in severe scleroderma, with low toxicity and significant clinical benefits, she said.
In a report published earlier and based on 12 of the patients, toxicity associated with the procedure included infections occurring during the neutropenic period of mobilization; these were managed with antibiotics (Br. J. Haematol. 2002;119:726-39).
There were also two episodes of mucositis and three cases of mild hepatic toxicity during intensification.
Stem cell transplantation is now being compared with monthly cyclophosphamide in an ongoing phase III trial, Dr. Marjanovic said.
In the Autologous Stem Cell International Scleroderma (ASTIS) trial, patients with diffuse systemic sclerosis and visceral involvement who are at risk for severe organ dysfunction and premature mortality are being prospectively randomized to the experimental transplant procedure or standard monthly intravenous pulse therapy with cyclophosphamide.
As of October 2004, 41 patients from 16 centers in eight European countries have been enrolled. The primary end point is event-free survival during 2 years of follow-up. Information is available at www.astistrial.com
SAN ANTONIO — A small group of patients with severe systemic sclerosis have shown a durable response to autologous hematopoietic stem cell transplantation, with 8 of 13 transplanted patients remaining alive after a mean follow-up of 44 months, Zora Marjanovic, M.D., reported at the annual meeting of the American College of Rheumatology.
Stem cell transplantation has in recent years been investigated for use in diseases such as scleroderma following observations that some patients with autoimmune disease who undergo transplantation for hematopoietic or other malignancies also may experience a remission of the autoimmune disease after the procedure.
In the first sequential open phase I-II study assessing the feasibility of autologous stem cell transplantation for systemic sclerosis with early visceral involvement, patients were eligible if they had rapidly progressing disease with heart, lung, or kidney involvement, Dr. Marjanovic said in a poster session.
The transplant protocol involved mobilization with cyclophosphamide plus recombinant human granulocyte colony-stimulating factor (GCSF) or GCSF alone if the left ventricular ejection fraction (LVEF) was less than 40%.
Subsequent conditioning, which took place at least 4 weeks after mobilization, used cyclophosphamide, 200 mg/kg, or melphalan, 140 mg/m
Outcomes following reinjection of CD34+ and hematopoietic stem cells were classified as major response, partial response, no response, disease progression, or relapse. Patients were assessed every 3 months.
Of the 14 patients enrolled in the nonrandomized trial, 13 were transplanted; 1 withdrew after mobilization.
One procedure-related death occurred, she said.
Six months following transplantation, nine patients responded to treatment—six had major responses and three had partial responses.
After a mean follow-up of 44 months, 8 of the responding patients were alive, 4 have died from disease progression. One nonresponding patient remains alive.
During the follow-up period, five patients relapsed but eventually responded to reintroduction of immunosuppression by mycophenolate mofetil. Four of these were partial responses, and one was a major response, said Dr. Marjanovic of University Hospital Center Saint-Louis, Paris, France.
This trial demonstrated that autologous hematopoietic stem cell transplantation is feasible in severe scleroderma, with low toxicity and significant clinical benefits, she said.
In a report published earlier and based on 12 of the patients, toxicity associated with the procedure included infections occurring during the neutropenic period of mobilization; these were managed with antibiotics (Br. J. Haematol. 2002;119:726-39).
There were also two episodes of mucositis and three cases of mild hepatic toxicity during intensification.
Stem cell transplantation is now being compared with monthly cyclophosphamide in an ongoing phase III trial, Dr. Marjanovic said.
In the Autologous Stem Cell International Scleroderma (ASTIS) trial, patients with diffuse systemic sclerosis and visceral involvement who are at risk for severe organ dysfunction and premature mortality are being prospectively randomized to the experimental transplant procedure or standard monthly intravenous pulse therapy with cyclophosphamide.
As of October 2004, 41 patients from 16 centers in eight European countries have been enrolled. The primary end point is event-free survival during 2 years of follow-up. Information is available at www.astistrial.com
SAN ANTONIO — A small group of patients with severe systemic sclerosis have shown a durable response to autologous hematopoietic stem cell transplantation, with 8 of 13 transplanted patients remaining alive after a mean follow-up of 44 months, Zora Marjanovic, M.D., reported at the annual meeting of the American College of Rheumatology.
Stem cell transplantation has in recent years been investigated for use in diseases such as scleroderma following observations that some patients with autoimmune disease who undergo transplantation for hematopoietic or other malignancies also may experience a remission of the autoimmune disease after the procedure.
In the first sequential open phase I-II study assessing the feasibility of autologous stem cell transplantation for systemic sclerosis with early visceral involvement, patients were eligible if they had rapidly progressing disease with heart, lung, or kidney involvement, Dr. Marjanovic said in a poster session.
The transplant protocol involved mobilization with cyclophosphamide plus recombinant human granulocyte colony-stimulating factor (GCSF) or GCSF alone if the left ventricular ejection fraction (LVEF) was less than 40%.
Subsequent conditioning, which took place at least 4 weeks after mobilization, used cyclophosphamide, 200 mg/kg, or melphalan, 140 mg/m
Outcomes following reinjection of CD34+ and hematopoietic stem cells were classified as major response, partial response, no response, disease progression, or relapse. Patients were assessed every 3 months.
Of the 14 patients enrolled in the nonrandomized trial, 13 were transplanted; 1 withdrew after mobilization.
One procedure-related death occurred, she said.
Six months following transplantation, nine patients responded to treatment—six had major responses and three had partial responses.
After a mean follow-up of 44 months, 8 of the responding patients were alive, 4 have died from disease progression. One nonresponding patient remains alive.
During the follow-up period, five patients relapsed but eventually responded to reintroduction of immunosuppression by mycophenolate mofetil. Four of these were partial responses, and one was a major response, said Dr. Marjanovic of University Hospital Center Saint-Louis, Paris, France.
This trial demonstrated that autologous hematopoietic stem cell transplantation is feasible in severe scleroderma, with low toxicity and significant clinical benefits, she said.
In a report published earlier and based on 12 of the patients, toxicity associated with the procedure included infections occurring during the neutropenic period of mobilization; these were managed with antibiotics (Br. J. Haematol. 2002;119:726-39).
There were also two episodes of mucositis and three cases of mild hepatic toxicity during intensification.
Stem cell transplantation is now being compared with monthly cyclophosphamide in an ongoing phase III trial, Dr. Marjanovic said.
In the Autologous Stem Cell International Scleroderma (ASTIS) trial, patients with diffuse systemic sclerosis and visceral involvement who are at risk for severe organ dysfunction and premature mortality are being prospectively randomized to the experimental transplant procedure or standard monthly intravenous pulse therapy with cyclophosphamide.
As of October 2004, 41 patients from 16 centers in eight European countries have been enrolled. The primary end point is event-free survival during 2 years of follow-up. Information is available at www.astistrial.com