User login
Time-Restricted Eating Is Not a Metabolic Magic Bullet
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.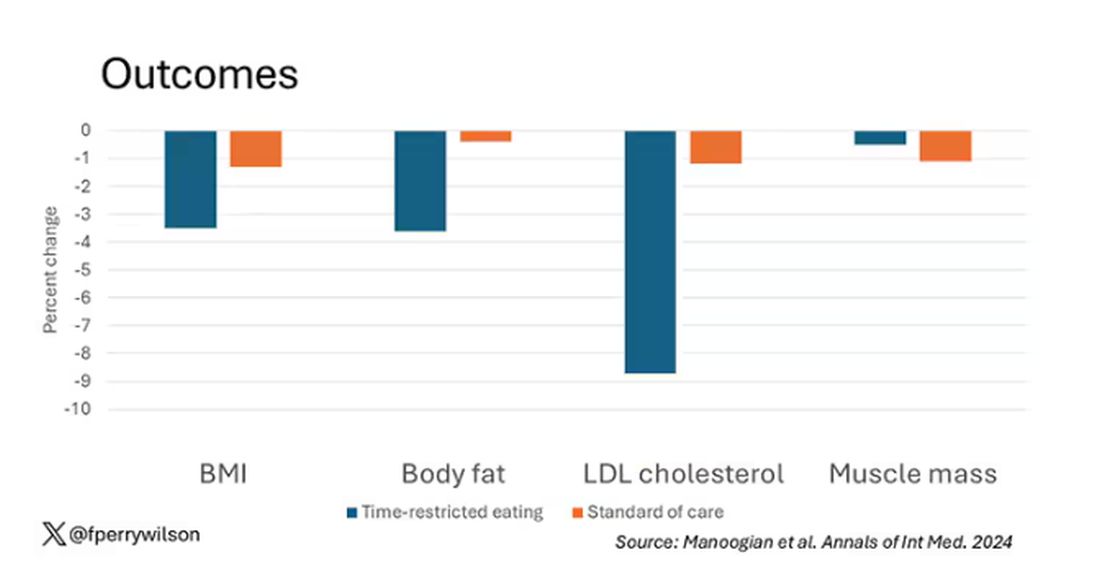
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.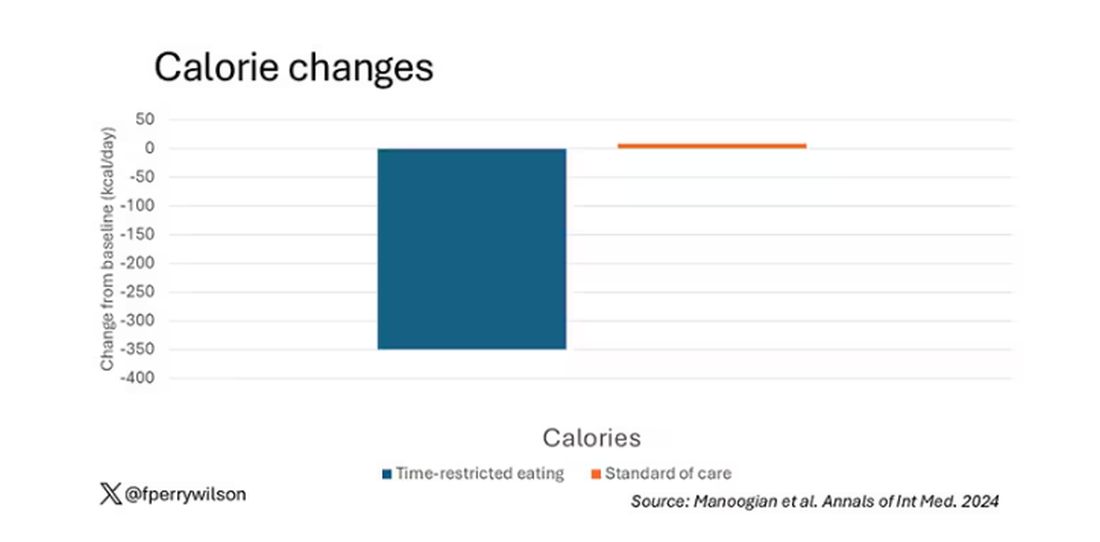
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 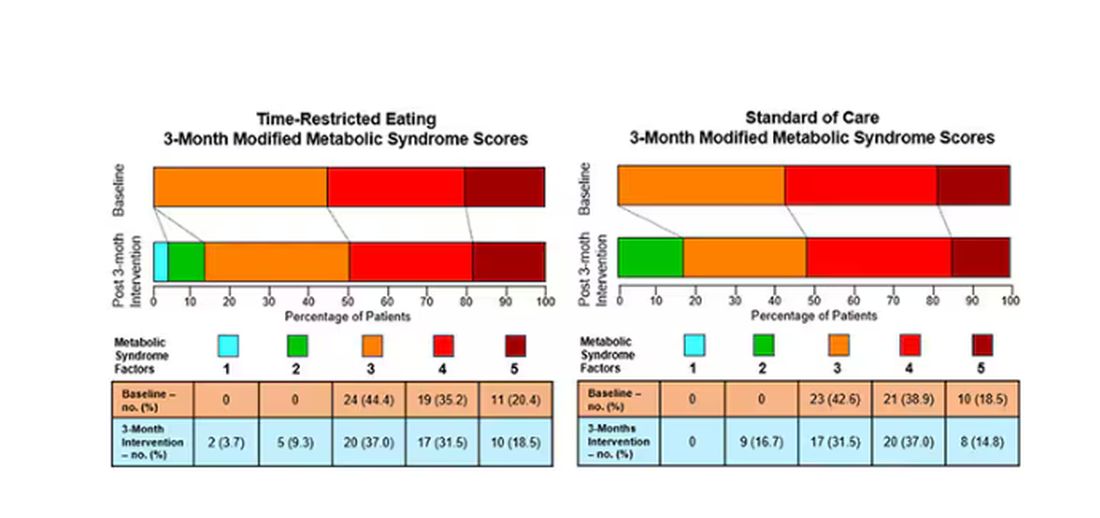
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Detecting Type 2 Diabetes Through Voice: How Does It Work?
An international study, Colive Voice, presented at the European Association for the Study of Diabetes (EASD) 2024 conference, shows that These results “open up possibilities for developing a first-line, noninvasive, and rapid screening tool for T2D, feasible with just a few seconds of voice recording on a smartphone or during consultations,” explained the study’s principal investigator Guy Fagherazzi, PhD, a diabetes epidemiologist at the Luxembourg Institute of Health, in an interview with this news organization.
How did the idea of detecting diabetes through voice come about?
During the COVID-19 pandemic, we began analyzing voice recordings from patients with chronic diseases. We wanted to find solutions to assess people’s health remotely, without physical contact. We quickly realized that this approach could be extended to other diseases. Because my main research focus has always been diabetes, I looked into how voice characteristics might correlate with diabetes. Previous studies had indicated that patients with diabetes have distinct voices compared with the general population, and this insight formed the starting point.
What mechanism could explain why patients with T2D have different voice characteristics?
It’s challenging to pinpoint a single factor that would explain why patients with T2D have different voices from those without diabetes. Several factors are involved.
Some biological mechanisms, especially those affecting the vascular system, influence symptoms in people with metabolic diseases such as diabetes. For example, people with T2D have more frequent cardiorespiratory fatigue. Obesity and overweight are also key factors, as these conditions can slightly alter vocal parameters compared with people of normal weight. Hypertension, common in patients with T2D, adds to the complexity.
Neurologic complications can affect the nerves and muscles involved in voice production, particularly the vocal cords.
Therefore, respiratory fatigue, neuropathies, and other conditions such as dehydration and gastric acid reflux, which are more common in patients with diabetes, can contribute to differences in voice.
These differences might not be noticeable to the human ear. That’s why we often don’t notice the link between voice and diabetes. However, technological advancements in signal processing and artificial intelligence allow us to extract a large amount of information from these subtle variations. By analyzing these small differences, we can detect diabetes with a reasonable degree of accuracy.
In your study, you mention that voice tone can indicate diabetic status. Could you elaborate?
Yes, voice tone can be affected, though it’s a complex, multidimensional phenomenon.
Patients who have had diabetes for 5-10 years, or longer, tend to have a rougher voice than those without diabetes of the same age and gender. In our study, we were able to extract many voice characteristics from the raw audio signal, which is why it’s difficult to isolate one specific factor that stands out.
Is there a difference in voice changes between patients with well-managed diabetes and those whose disease is uncontrolled?
The roughness of the voice tends to increase with the duration of diabetes. It’s more noticeable in people with poorly controlled diabetes. Our hypothesis, based on the results we presented at the EASD conference, is that fluctuations in blood sugar levels, both hypo- and hyperglycemia, may cause short-term changes in the voice. There are also many subtle, rapid changes that could potentially be detected, though we haven’t confirmed this yet. We’re currently conducting additional studies to explore this.
Why did you ask participants to read a passage from the Universal Declaration of Human Rights?
We used a highly standardized approach. Participants completed several recordings, including holding the sound “Aaaaaa” for as long as possible in one breath. They also read a passage, which helps us better distinguish between patients with and those without diabetes. This method works slightly better than other sounds typically used for analyzing diseases. We chose this particular text in the participant’s native language because it’s neutral and doesn’t trigger emotional fluctuations. Because Colive Voice is an international, multilingual study, we use official translations in various languages.
Your research focuses on T2D. Do you plan to study type 1 diabetes (T1D) as well?
We believe that individuals with T1D also exhibit voice changes over time. However, our current focus is on T2D because our goal is to develop large-scale screening methods. T1D, typically diagnosed in childhood, requires different screening approaches. For now, our research mainly involves adults.
Were there any gender differences in the accuracy of your voice analysis?
Yes, voice studies generally show that women have different vocal signatures from men, partly owing to hormonal fluctuations that affect pitch and tone. Detecting differences between healthy individuals and those with diabetes can sometimes be more challenging in women, depending on the condition. In our study, we achieved about 70% accuracy for women compared with 75% for men.
The EASD results focused on a US-based population. When can we expect data from France?
We started with the US because we could quickly gather a large number of patients. Now, we’re expanding to global and language-specific analyses. French data are certainly a priority, and we’re working on it. We encourage people to participate — it takes only 20 minutes and contributes to innovative research on noninvasive diabetes detection. Participants can sign up at www.colivevoice.org
Dr. Fagherazzi heads the Deep Digital Phenotyping laboratory and the Department of Precision Health at the Luxembourg Institute of Health. His research focuses on integrating new technologies and digital data into diabetes research. He has declared no relevant financial relationships.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
An international study, Colive Voice, presented at the European Association for the Study of Diabetes (EASD) 2024 conference, shows that These results “open up possibilities for developing a first-line, noninvasive, and rapid screening tool for T2D, feasible with just a few seconds of voice recording on a smartphone or during consultations,” explained the study’s principal investigator Guy Fagherazzi, PhD, a diabetes epidemiologist at the Luxembourg Institute of Health, in an interview with this news organization.
How did the idea of detecting diabetes through voice come about?
During the COVID-19 pandemic, we began analyzing voice recordings from patients with chronic diseases. We wanted to find solutions to assess people’s health remotely, without physical contact. We quickly realized that this approach could be extended to other diseases. Because my main research focus has always been diabetes, I looked into how voice characteristics might correlate with diabetes. Previous studies had indicated that patients with diabetes have distinct voices compared with the general population, and this insight formed the starting point.
What mechanism could explain why patients with T2D have different voice characteristics?
It’s challenging to pinpoint a single factor that would explain why patients with T2D have different voices from those without diabetes. Several factors are involved.
Some biological mechanisms, especially those affecting the vascular system, influence symptoms in people with metabolic diseases such as diabetes. For example, people with T2D have more frequent cardiorespiratory fatigue. Obesity and overweight are also key factors, as these conditions can slightly alter vocal parameters compared with people of normal weight. Hypertension, common in patients with T2D, adds to the complexity.
Neurologic complications can affect the nerves and muscles involved in voice production, particularly the vocal cords.
Therefore, respiratory fatigue, neuropathies, and other conditions such as dehydration and gastric acid reflux, which are more common in patients with diabetes, can contribute to differences in voice.
These differences might not be noticeable to the human ear. That’s why we often don’t notice the link between voice and diabetes. However, technological advancements in signal processing and artificial intelligence allow us to extract a large amount of information from these subtle variations. By analyzing these small differences, we can detect diabetes with a reasonable degree of accuracy.
In your study, you mention that voice tone can indicate diabetic status. Could you elaborate?
Yes, voice tone can be affected, though it’s a complex, multidimensional phenomenon.
Patients who have had diabetes for 5-10 years, or longer, tend to have a rougher voice than those without diabetes of the same age and gender. In our study, we were able to extract many voice characteristics from the raw audio signal, which is why it’s difficult to isolate one specific factor that stands out.
Is there a difference in voice changes between patients with well-managed diabetes and those whose disease is uncontrolled?
The roughness of the voice tends to increase with the duration of diabetes. It’s more noticeable in people with poorly controlled diabetes. Our hypothesis, based on the results we presented at the EASD conference, is that fluctuations in blood sugar levels, both hypo- and hyperglycemia, may cause short-term changes in the voice. There are also many subtle, rapid changes that could potentially be detected, though we haven’t confirmed this yet. We’re currently conducting additional studies to explore this.
Why did you ask participants to read a passage from the Universal Declaration of Human Rights?
We used a highly standardized approach. Participants completed several recordings, including holding the sound “Aaaaaa” for as long as possible in one breath. They also read a passage, which helps us better distinguish between patients with and those without diabetes. This method works slightly better than other sounds typically used for analyzing diseases. We chose this particular text in the participant’s native language because it’s neutral and doesn’t trigger emotional fluctuations. Because Colive Voice is an international, multilingual study, we use official translations in various languages.
Your research focuses on T2D. Do you plan to study type 1 diabetes (T1D) as well?
We believe that individuals with T1D also exhibit voice changes over time. However, our current focus is on T2D because our goal is to develop large-scale screening methods. T1D, typically diagnosed in childhood, requires different screening approaches. For now, our research mainly involves adults.
Were there any gender differences in the accuracy of your voice analysis?
Yes, voice studies generally show that women have different vocal signatures from men, partly owing to hormonal fluctuations that affect pitch and tone. Detecting differences between healthy individuals and those with diabetes can sometimes be more challenging in women, depending on the condition. In our study, we achieved about 70% accuracy for women compared with 75% for men.
The EASD results focused on a US-based population. When can we expect data from France?
We started with the US because we could quickly gather a large number of patients. Now, we’re expanding to global and language-specific analyses. French data are certainly a priority, and we’re working on it. We encourage people to participate — it takes only 20 minutes and contributes to innovative research on noninvasive diabetes detection. Participants can sign up at www.colivevoice.org
Dr. Fagherazzi heads the Deep Digital Phenotyping laboratory and the Department of Precision Health at the Luxembourg Institute of Health. His research focuses on integrating new technologies and digital data into diabetes research. He has declared no relevant financial relationships.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
An international study, Colive Voice, presented at the European Association for the Study of Diabetes (EASD) 2024 conference, shows that These results “open up possibilities for developing a first-line, noninvasive, and rapid screening tool for T2D, feasible with just a few seconds of voice recording on a smartphone or during consultations,” explained the study’s principal investigator Guy Fagherazzi, PhD, a diabetes epidemiologist at the Luxembourg Institute of Health, in an interview with this news organization.
How did the idea of detecting diabetes through voice come about?
During the COVID-19 pandemic, we began analyzing voice recordings from patients with chronic diseases. We wanted to find solutions to assess people’s health remotely, without physical contact. We quickly realized that this approach could be extended to other diseases. Because my main research focus has always been diabetes, I looked into how voice characteristics might correlate with diabetes. Previous studies had indicated that patients with diabetes have distinct voices compared with the general population, and this insight formed the starting point.
What mechanism could explain why patients with T2D have different voice characteristics?
It’s challenging to pinpoint a single factor that would explain why patients with T2D have different voices from those without diabetes. Several factors are involved.
Some biological mechanisms, especially those affecting the vascular system, influence symptoms in people with metabolic diseases such as diabetes. For example, people with T2D have more frequent cardiorespiratory fatigue. Obesity and overweight are also key factors, as these conditions can slightly alter vocal parameters compared with people of normal weight. Hypertension, common in patients with T2D, adds to the complexity.
Neurologic complications can affect the nerves and muscles involved in voice production, particularly the vocal cords.
Therefore, respiratory fatigue, neuropathies, and other conditions such as dehydration and gastric acid reflux, which are more common in patients with diabetes, can contribute to differences in voice.
These differences might not be noticeable to the human ear. That’s why we often don’t notice the link between voice and diabetes. However, technological advancements in signal processing and artificial intelligence allow us to extract a large amount of information from these subtle variations. By analyzing these small differences, we can detect diabetes with a reasonable degree of accuracy.
In your study, you mention that voice tone can indicate diabetic status. Could you elaborate?
Yes, voice tone can be affected, though it’s a complex, multidimensional phenomenon.
Patients who have had diabetes for 5-10 years, or longer, tend to have a rougher voice than those without diabetes of the same age and gender. In our study, we were able to extract many voice characteristics from the raw audio signal, which is why it’s difficult to isolate one specific factor that stands out.
Is there a difference in voice changes between patients with well-managed diabetes and those whose disease is uncontrolled?
The roughness of the voice tends to increase with the duration of diabetes. It’s more noticeable in people with poorly controlled diabetes. Our hypothesis, based on the results we presented at the EASD conference, is that fluctuations in blood sugar levels, both hypo- and hyperglycemia, may cause short-term changes in the voice. There are also many subtle, rapid changes that could potentially be detected, though we haven’t confirmed this yet. We’re currently conducting additional studies to explore this.
Why did you ask participants to read a passage from the Universal Declaration of Human Rights?
We used a highly standardized approach. Participants completed several recordings, including holding the sound “Aaaaaa” for as long as possible in one breath. They also read a passage, which helps us better distinguish between patients with and those without diabetes. This method works slightly better than other sounds typically used for analyzing diseases. We chose this particular text in the participant’s native language because it’s neutral and doesn’t trigger emotional fluctuations. Because Colive Voice is an international, multilingual study, we use official translations in various languages.
Your research focuses on T2D. Do you plan to study type 1 diabetes (T1D) as well?
We believe that individuals with T1D also exhibit voice changes over time. However, our current focus is on T2D because our goal is to develop large-scale screening methods. T1D, typically diagnosed in childhood, requires different screening approaches. For now, our research mainly involves adults.
Were there any gender differences in the accuracy of your voice analysis?
Yes, voice studies generally show that women have different vocal signatures from men, partly owing to hormonal fluctuations that affect pitch and tone. Detecting differences between healthy individuals and those with diabetes can sometimes be more challenging in women, depending on the condition. In our study, we achieved about 70% accuracy for women compared with 75% for men.
The EASD results focused on a US-based population. When can we expect data from France?
We started with the US because we could quickly gather a large number of patients. Now, we’re expanding to global and language-specific analyses. French data are certainly a priority, and we’re working on it. We encourage people to participate — it takes only 20 minutes and contributes to innovative research on noninvasive diabetes detection. Participants can sign up at www.colivevoice.org
Dr. Fagherazzi heads the Deep Digital Phenotyping laboratory and the Department of Precision Health at the Luxembourg Institute of Health. His research focuses on integrating new technologies and digital data into diabetes research. He has declared no relevant financial relationships.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM EASD 2024
Cannabis Use Rising in Diabetes: What Do Endos Need to Know?
A recent US prevalence study estimated that 9% adults with diabetes used cannabis in the last month, a 33.7% increase between 2021 and 2022. Nearly half (48.9%) of users were younger than 50 years.
Cannabis use is also increasing sharply among those aged 65 years or older, many of whom have diabetes and other chronic conditions. In this demographic, the perceived risk surrounding regular cannabis use has dropped significantly, even as the data tell another story — that they are particularly at risk from emergency department visits for cannabis poisoning.
As legalization continues and cannabis products proliferate, endocrinologists will likely face more patients of all ages seeking advice about its use. Yet with few evidence-based resources to turn to, endocrinologists advising patients in this area are mostly left fending for themselves.
Evidence ‘Limited’
“The evidence on cannabis is limited mainly because of its scheduling in the United States,” Jay Shubrook, DO, a professor and diabetologist at College of Osteopathic Medicine, Touro University California, in Vallejo, California, told this news organization.
“It was declared to be a schedule I drug in the 1970s, which meant it was ‘dangerous’ and ‘had no medical benefit.’ This made it hard to access and study in human trials.”
That will likely change soon. On May 16, 2024, the US Department of Justice submitted a proposal to move marijuana from a schedule I to a schedule III drug under the Controlled Substances Act, emphasizing its accepted medical use. If approved, the door will open to more investigators seeking to study the effects of cannabis.
Yet, even in Canada, where recreational use has been legal since 2018 and cannabis is sold widely with government support, there are little hard data to guide practice. In 2019, Diabetes Canada issued a position statement on recreational cannabis use in people with type 1 diabetes (T1D) and type 2 diabetes (T2D). It sought to evaluate the effects of cannabis on metabolic factors and diabetes complications, as well as self-management behaviors in those aged 13 years or older.
The authors noted that five of the six studies upon which the statement was based did not consider or report the routes of cannabis administration, which have differing risks. In addition, their recommendations were based on grade D evidence and consensus.
What Patients Are Taking
Cannabis — also known as marijuana, weed, pot, or bud — refers to the dried flowers, leaves, stems, and seeds of the cannabis plant. The plant contains more than 100 compounds, including tetrahydrocannabinol (THC), which is responsible for the euphoric “high,” and other active compounds, including cannabidiol (CBD), which by itself is not mind-altering.
Cannabis can be ingested in several ways. It can be smoked (ie, joints, blunts, pipes, and water pipes), ingested in edible form (mixed or infused into foods), and inhaled using electronic vaporizing devices (ie, e-cigarettes or vape pens).
Compounds in cannabis can also be extracted to make oils and concentrates that can be vaped or inhaled. Smoking oils, concentrates, and extracts from the cannabis plant, known as “dabbing,” are on the rise in the United States.
There are no validated or standard dosage recommendations for cannabis strains and formulations, THC/CBD ratios, or modes of administration. Therefore, the Canadian Pharmacists Association prepared a guide for finding a safe and effective dose for medical purposes. GoodRx, a website with information on prescription drug prices, says that larger doses of THC pose greater risks, noting that the potency of cannabis has increased from 4% in 1995 to about 14% in 2019.
Potential Risks and Benefits: Canadian and US Perspectives
Health and safety risks vary with each of the different ways of using cannabis for individuals with and without diabetes, depending on a host of patient- and product-specific factors.
In a recent article proposing a “THC unit” for Canada’s legal cannabis market, researchers reported that consumers lack familiarity with THC levels, don’t know what constitutes a “low” or “high” THC amount, have trouble dosing, overconsume, and commonly experience adverse health events from cannabis use.
A recent study suggested that most clinicians are similarly uninformed, with “a lack of knowledge of beneficial effects, adverse effects, and of how to advise patients,” even for medical cannabis.
Diabetes Canada takes a stab at summarizing what’s known with respect to cannabis and diabetes, stating that:
“Research on recreational cannabis use suggests it may negatively impact diabetes metabolic factors and self-management behaviors. The safety of recreational cannabis use has not been demonstrated, whereas regular cannabis use is associated with worsening glycemic control, more diabetes-related complications, and poorer self-care behaviors, such as adequate glucose monitoring, adherence to medications, and compliance with dietary and physical activity recommendations for people living with both type 1 and type 2 diabetes.”
The American Diabetes Association’s information on cannabis consists of a patient-oriented article on CBD oil. The article stated:
“There’s a lot of hype surrounding CBD oil and diabetes. There is no noticeable effect on blood glucose (blood sugar) or insulin levels in people with type 2 diabetes. Researchers continue to study the effects of CBD on diabetes in animal studies.”
It concludes that:
“Although many claims continue to be made about CBD oil, there is little evidence of any benefit. It’s certainly not an alternative to traditional diabetes management. The safety of CBD is also unknown — it may have dangerous side effects that we won’t know about unless further research is done.”
A Roundup of Recent Studies
A smattering of recent studies have touched on various aspects of cannabis consumption and diabetes.
Angela Bryan, PhD, professor and co-director of CUChange at the University of Colorado Boulder, has been evaluating cannabis use in young adults (ages 21-40 years) in the SONIC study. Dr. Bryan reported at the American Diabetes Association (ADA) 84th Scientific Sessions that cannabis users were more likely to have a lower body mass index and less likely to develop T2D. Furthermore, chronic cannabis users were less likely to have measures of inflammation and no loss of insulin sensitivity.
Another study by Dr. Bryan’s group found that CBD-dominant forms of cannabis were associated with acute tension reduction, which might lead to longer-term reductions in anxiety. Bryan said the findings could be relevant in the context of diabetes distress.
Similarly positive results were found in a 15-week, double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD spray for neuropathic pain among treatment-resistant patients. The investigators reported that “clinically important improvements” were seen in pain, sleep quality, and subjective impressions of pain. Another small study of inhaled cannabis in treatment-refractory patients found a dose-dependent reduction in diabetic peripheral neuropathy pain.
Findings from a 9-year longitudinal study of approximately 18,000 Swedish men and women suggested no association between cannabis and subsequent T2D development after controlling for age, although these authors also called for longer follow-up and more detailed information about cannabis use to make “more robust” conclusions.
On the other side of the spectrum, a “rapid” review of recreational cannabis use in people with T1D and T2D found that recreational cannabis use may negatively impact diabetes metabolic factors and self-management behaviors and may increase risks for peripheral arterial occlusion, myocardial infarction, and renal disease. However, the authors cautioned that more robust research is needed to confirm the potential impact of cannabis on diabetes.
How to Advise Patients
When Dr. Shubrook was working with patients with diabetes in his family medicine practice in Ohio, cannabis wasn’t legal.
“’Don’t ask, don’t tell’ was the way we handled it then,” he said.
By contrast, in California, where he’s currently located, “it’s pretty well accepted and legal, and patients volunteer information about use, even if it’s recreational,” he said. “Realizing this was something we could talk about was really eye-opening to me.”
Talking to patients about cannabis use is a “20-minute conversation that details what they’re doing,” he said. He proceeds by asking questions: Are you using for recreational or medicinal purposes? What do you take? What do you take it for? Does it work?
“People will tell you,” Dr. Shubrook said. “They know exactly what it works or doesn’t work for and how it affects their glucose control, which in most cases is only minimally.”
He tells patients he would prefer they don’t inhale cannabis, given the risks posed to the lungs.
“Edibles may have a slower onset of effect, but depending on what they’re adding it to, glucose might be affected,” he noted. “And I have seen that chronic use can lead to hyperemesis syndrome.”
Overall, he said, “Take the time to talk to your patients about cannabis — it will allow them to be honest with you, and you can improve the specificity and safety of its use. If cannabis is legal in your state, encourage people to go to legal dispensaries, which will reduce the risk of it being laced with another drug that could increase the danger of use.”
A recent US prevalence study found that people with diabetes who use cannabis likely engage in other substance and psychoactive substance use, including tobacco use, binge drinking, and misuse of opioids and stimulants.
“Use of these additional substances could further exacerbate the health risks associated with diabetes and also emphasizes the importance of addressing polysubstance use among adults with diabetes,” the study’s author Benjamin H. Han, MD, Division of Geriatrics, Gerontology and Palliative Care, Department of Medicine, US San Diego School of Medicine in La Jolla, California, told this news organization.
“We were surprised at how strong the associations were, especially with use of substances that can increase cardiovascular risk,” Dr. Han added. “And given the strong association we found between cannabis use and use of other psychoactive substances in diabetes, clinicians must screen all their patients for psychoactive substance use.”
Diabetes Canada’s position paper states that despite the limited evidence, “there were sufficient data to begin developing recommendations for type 1 and type 2 diabetes about education, counseling, and management related to recreational cannabis use.”
Their recommendations include the following:
- Healthcare professionals should engage their patients in discussions about substance use on a regular basis, with a nonjudgmental approach.
- The use of recreational cannabis is not recommended for adolescents and adults with diabetes.
- People with T1D should avoid recreational cannabis use because of the increased risk for diabetic ketoacidosis.
- For adults with T1D or T2D who intend to use cannabis recreationally, individualized assessment and counseling should be offered to inform them of the general risks of cannabis, with a focus on harm reduction and reduction of the risk for potential adverse effects on diabetes management and complications.
- People with T1D or T2D should be offered education on and encouraged to read public information available through resources from various Canadian health authorities about the general risks of cannabis use to reduce the risk for nondiabetes-related adverse effects of cannabis consumption.
Of note, in 2018, the Canadian government produced an exhaustive compendium of information on cannabis for healthcare professionals that includes information relevant to managing patients with diabetes.
Dr. Shubrook and Dr. Han reported no competing interests.
A version of this article appeared on Medscape.com.
A recent US prevalence study estimated that 9% adults with diabetes used cannabis in the last month, a 33.7% increase between 2021 and 2022. Nearly half (48.9%) of users were younger than 50 years.
Cannabis use is also increasing sharply among those aged 65 years or older, many of whom have diabetes and other chronic conditions. In this demographic, the perceived risk surrounding regular cannabis use has dropped significantly, even as the data tell another story — that they are particularly at risk from emergency department visits for cannabis poisoning.
As legalization continues and cannabis products proliferate, endocrinologists will likely face more patients of all ages seeking advice about its use. Yet with few evidence-based resources to turn to, endocrinologists advising patients in this area are mostly left fending for themselves.
Evidence ‘Limited’
“The evidence on cannabis is limited mainly because of its scheduling in the United States,” Jay Shubrook, DO, a professor and diabetologist at College of Osteopathic Medicine, Touro University California, in Vallejo, California, told this news organization.
“It was declared to be a schedule I drug in the 1970s, which meant it was ‘dangerous’ and ‘had no medical benefit.’ This made it hard to access and study in human trials.”
That will likely change soon. On May 16, 2024, the US Department of Justice submitted a proposal to move marijuana from a schedule I to a schedule III drug under the Controlled Substances Act, emphasizing its accepted medical use. If approved, the door will open to more investigators seeking to study the effects of cannabis.
Yet, even in Canada, where recreational use has been legal since 2018 and cannabis is sold widely with government support, there are little hard data to guide practice. In 2019, Diabetes Canada issued a position statement on recreational cannabis use in people with type 1 diabetes (T1D) and type 2 diabetes (T2D). It sought to evaluate the effects of cannabis on metabolic factors and diabetes complications, as well as self-management behaviors in those aged 13 years or older.
The authors noted that five of the six studies upon which the statement was based did not consider or report the routes of cannabis administration, which have differing risks. In addition, their recommendations were based on grade D evidence and consensus.
What Patients Are Taking
Cannabis — also known as marijuana, weed, pot, or bud — refers to the dried flowers, leaves, stems, and seeds of the cannabis plant. The plant contains more than 100 compounds, including tetrahydrocannabinol (THC), which is responsible for the euphoric “high,” and other active compounds, including cannabidiol (CBD), which by itself is not mind-altering.
Cannabis can be ingested in several ways. It can be smoked (ie, joints, blunts, pipes, and water pipes), ingested in edible form (mixed or infused into foods), and inhaled using electronic vaporizing devices (ie, e-cigarettes or vape pens).
Compounds in cannabis can also be extracted to make oils and concentrates that can be vaped or inhaled. Smoking oils, concentrates, and extracts from the cannabis plant, known as “dabbing,” are on the rise in the United States.
There are no validated or standard dosage recommendations for cannabis strains and formulations, THC/CBD ratios, or modes of administration. Therefore, the Canadian Pharmacists Association prepared a guide for finding a safe and effective dose for medical purposes. GoodRx, a website with information on prescription drug prices, says that larger doses of THC pose greater risks, noting that the potency of cannabis has increased from 4% in 1995 to about 14% in 2019.
Potential Risks and Benefits: Canadian and US Perspectives
Health and safety risks vary with each of the different ways of using cannabis for individuals with and without diabetes, depending on a host of patient- and product-specific factors.
In a recent article proposing a “THC unit” for Canada’s legal cannabis market, researchers reported that consumers lack familiarity with THC levels, don’t know what constitutes a “low” or “high” THC amount, have trouble dosing, overconsume, and commonly experience adverse health events from cannabis use.
A recent study suggested that most clinicians are similarly uninformed, with “a lack of knowledge of beneficial effects, adverse effects, and of how to advise patients,” even for medical cannabis.
Diabetes Canada takes a stab at summarizing what’s known with respect to cannabis and diabetes, stating that:
“Research on recreational cannabis use suggests it may negatively impact diabetes metabolic factors and self-management behaviors. The safety of recreational cannabis use has not been demonstrated, whereas regular cannabis use is associated with worsening glycemic control, more diabetes-related complications, and poorer self-care behaviors, such as adequate glucose monitoring, adherence to medications, and compliance with dietary and physical activity recommendations for people living with both type 1 and type 2 diabetes.”
The American Diabetes Association’s information on cannabis consists of a patient-oriented article on CBD oil. The article stated:
“There’s a lot of hype surrounding CBD oil and diabetes. There is no noticeable effect on blood glucose (blood sugar) or insulin levels in people with type 2 diabetes. Researchers continue to study the effects of CBD on diabetes in animal studies.”
It concludes that:
“Although many claims continue to be made about CBD oil, there is little evidence of any benefit. It’s certainly not an alternative to traditional diabetes management. The safety of CBD is also unknown — it may have dangerous side effects that we won’t know about unless further research is done.”
A Roundup of Recent Studies
A smattering of recent studies have touched on various aspects of cannabis consumption and diabetes.
Angela Bryan, PhD, professor and co-director of CUChange at the University of Colorado Boulder, has been evaluating cannabis use in young adults (ages 21-40 years) in the SONIC study. Dr. Bryan reported at the American Diabetes Association (ADA) 84th Scientific Sessions that cannabis users were more likely to have a lower body mass index and less likely to develop T2D. Furthermore, chronic cannabis users were less likely to have measures of inflammation and no loss of insulin sensitivity.
Another study by Dr. Bryan’s group found that CBD-dominant forms of cannabis were associated with acute tension reduction, which might lead to longer-term reductions in anxiety. Bryan said the findings could be relevant in the context of diabetes distress.
Similarly positive results were found in a 15-week, double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD spray for neuropathic pain among treatment-resistant patients. The investigators reported that “clinically important improvements” were seen in pain, sleep quality, and subjective impressions of pain. Another small study of inhaled cannabis in treatment-refractory patients found a dose-dependent reduction in diabetic peripheral neuropathy pain.
Findings from a 9-year longitudinal study of approximately 18,000 Swedish men and women suggested no association between cannabis and subsequent T2D development after controlling for age, although these authors also called for longer follow-up and more detailed information about cannabis use to make “more robust” conclusions.
On the other side of the spectrum, a “rapid” review of recreational cannabis use in people with T1D and T2D found that recreational cannabis use may negatively impact diabetes metabolic factors and self-management behaviors and may increase risks for peripheral arterial occlusion, myocardial infarction, and renal disease. However, the authors cautioned that more robust research is needed to confirm the potential impact of cannabis on diabetes.
How to Advise Patients
When Dr. Shubrook was working with patients with diabetes in his family medicine practice in Ohio, cannabis wasn’t legal.
“’Don’t ask, don’t tell’ was the way we handled it then,” he said.
By contrast, in California, where he’s currently located, “it’s pretty well accepted and legal, and patients volunteer information about use, even if it’s recreational,” he said. “Realizing this was something we could talk about was really eye-opening to me.”
Talking to patients about cannabis use is a “20-minute conversation that details what they’re doing,” he said. He proceeds by asking questions: Are you using for recreational or medicinal purposes? What do you take? What do you take it for? Does it work?
“People will tell you,” Dr. Shubrook said. “They know exactly what it works or doesn’t work for and how it affects their glucose control, which in most cases is only minimally.”
He tells patients he would prefer they don’t inhale cannabis, given the risks posed to the lungs.
“Edibles may have a slower onset of effect, but depending on what they’re adding it to, glucose might be affected,” he noted. “And I have seen that chronic use can lead to hyperemesis syndrome.”
Overall, he said, “Take the time to talk to your patients about cannabis — it will allow them to be honest with you, and you can improve the specificity and safety of its use. If cannabis is legal in your state, encourage people to go to legal dispensaries, which will reduce the risk of it being laced with another drug that could increase the danger of use.”
A recent US prevalence study found that people with diabetes who use cannabis likely engage in other substance and psychoactive substance use, including tobacco use, binge drinking, and misuse of opioids and stimulants.
“Use of these additional substances could further exacerbate the health risks associated with diabetes and also emphasizes the importance of addressing polysubstance use among adults with diabetes,” the study’s author Benjamin H. Han, MD, Division of Geriatrics, Gerontology and Palliative Care, Department of Medicine, US San Diego School of Medicine in La Jolla, California, told this news organization.
“We were surprised at how strong the associations were, especially with use of substances that can increase cardiovascular risk,” Dr. Han added. “And given the strong association we found between cannabis use and use of other psychoactive substances in diabetes, clinicians must screen all their patients for psychoactive substance use.”
Diabetes Canada’s position paper states that despite the limited evidence, “there were sufficient data to begin developing recommendations for type 1 and type 2 diabetes about education, counseling, and management related to recreational cannabis use.”
Their recommendations include the following:
- Healthcare professionals should engage their patients in discussions about substance use on a regular basis, with a nonjudgmental approach.
- The use of recreational cannabis is not recommended for adolescents and adults with diabetes.
- People with T1D should avoid recreational cannabis use because of the increased risk for diabetic ketoacidosis.
- For adults with T1D or T2D who intend to use cannabis recreationally, individualized assessment and counseling should be offered to inform them of the general risks of cannabis, with a focus on harm reduction and reduction of the risk for potential adverse effects on diabetes management and complications.
- People with T1D or T2D should be offered education on and encouraged to read public information available through resources from various Canadian health authorities about the general risks of cannabis use to reduce the risk for nondiabetes-related adverse effects of cannabis consumption.
Of note, in 2018, the Canadian government produced an exhaustive compendium of information on cannabis for healthcare professionals that includes information relevant to managing patients with diabetes.
Dr. Shubrook and Dr. Han reported no competing interests.
A version of this article appeared on Medscape.com.
A recent US prevalence study estimated that 9% adults with diabetes used cannabis in the last month, a 33.7% increase between 2021 and 2022. Nearly half (48.9%) of users were younger than 50 years.
Cannabis use is also increasing sharply among those aged 65 years or older, many of whom have diabetes and other chronic conditions. In this demographic, the perceived risk surrounding regular cannabis use has dropped significantly, even as the data tell another story — that they are particularly at risk from emergency department visits for cannabis poisoning.
As legalization continues and cannabis products proliferate, endocrinologists will likely face more patients of all ages seeking advice about its use. Yet with few evidence-based resources to turn to, endocrinologists advising patients in this area are mostly left fending for themselves.
Evidence ‘Limited’
“The evidence on cannabis is limited mainly because of its scheduling in the United States,” Jay Shubrook, DO, a professor and diabetologist at College of Osteopathic Medicine, Touro University California, in Vallejo, California, told this news organization.
“It was declared to be a schedule I drug in the 1970s, which meant it was ‘dangerous’ and ‘had no medical benefit.’ This made it hard to access and study in human trials.”
That will likely change soon. On May 16, 2024, the US Department of Justice submitted a proposal to move marijuana from a schedule I to a schedule III drug under the Controlled Substances Act, emphasizing its accepted medical use. If approved, the door will open to more investigators seeking to study the effects of cannabis.
Yet, even in Canada, where recreational use has been legal since 2018 and cannabis is sold widely with government support, there are little hard data to guide practice. In 2019, Diabetes Canada issued a position statement on recreational cannabis use in people with type 1 diabetes (T1D) and type 2 diabetes (T2D). It sought to evaluate the effects of cannabis on metabolic factors and diabetes complications, as well as self-management behaviors in those aged 13 years or older.
The authors noted that five of the six studies upon which the statement was based did not consider or report the routes of cannabis administration, which have differing risks. In addition, their recommendations were based on grade D evidence and consensus.
What Patients Are Taking
Cannabis — also known as marijuana, weed, pot, or bud — refers to the dried flowers, leaves, stems, and seeds of the cannabis plant. The plant contains more than 100 compounds, including tetrahydrocannabinol (THC), which is responsible for the euphoric “high,” and other active compounds, including cannabidiol (CBD), which by itself is not mind-altering.
Cannabis can be ingested in several ways. It can be smoked (ie, joints, blunts, pipes, and water pipes), ingested in edible form (mixed or infused into foods), and inhaled using electronic vaporizing devices (ie, e-cigarettes or vape pens).
Compounds in cannabis can also be extracted to make oils and concentrates that can be vaped or inhaled. Smoking oils, concentrates, and extracts from the cannabis plant, known as “dabbing,” are on the rise in the United States.
There are no validated or standard dosage recommendations for cannabis strains and formulations, THC/CBD ratios, or modes of administration. Therefore, the Canadian Pharmacists Association prepared a guide for finding a safe and effective dose for medical purposes. GoodRx, a website with information on prescription drug prices, says that larger doses of THC pose greater risks, noting that the potency of cannabis has increased from 4% in 1995 to about 14% in 2019.
Potential Risks and Benefits: Canadian and US Perspectives
Health and safety risks vary with each of the different ways of using cannabis for individuals with and without diabetes, depending on a host of patient- and product-specific factors.
In a recent article proposing a “THC unit” for Canada’s legal cannabis market, researchers reported that consumers lack familiarity with THC levels, don’t know what constitutes a “low” or “high” THC amount, have trouble dosing, overconsume, and commonly experience adverse health events from cannabis use.
A recent study suggested that most clinicians are similarly uninformed, with “a lack of knowledge of beneficial effects, adverse effects, and of how to advise patients,” even for medical cannabis.
Diabetes Canada takes a stab at summarizing what’s known with respect to cannabis and diabetes, stating that:
“Research on recreational cannabis use suggests it may negatively impact diabetes metabolic factors and self-management behaviors. The safety of recreational cannabis use has not been demonstrated, whereas regular cannabis use is associated with worsening glycemic control, more diabetes-related complications, and poorer self-care behaviors, such as adequate glucose monitoring, adherence to medications, and compliance with dietary and physical activity recommendations for people living with both type 1 and type 2 diabetes.”
The American Diabetes Association’s information on cannabis consists of a patient-oriented article on CBD oil. The article stated:
“There’s a lot of hype surrounding CBD oil and diabetes. There is no noticeable effect on blood glucose (blood sugar) or insulin levels in people with type 2 diabetes. Researchers continue to study the effects of CBD on diabetes in animal studies.”
It concludes that:
“Although many claims continue to be made about CBD oil, there is little evidence of any benefit. It’s certainly not an alternative to traditional diabetes management. The safety of CBD is also unknown — it may have dangerous side effects that we won’t know about unless further research is done.”
A Roundup of Recent Studies
A smattering of recent studies have touched on various aspects of cannabis consumption and diabetes.
Angela Bryan, PhD, professor and co-director of CUChange at the University of Colorado Boulder, has been evaluating cannabis use in young adults (ages 21-40 years) in the SONIC study. Dr. Bryan reported at the American Diabetes Association (ADA) 84th Scientific Sessions that cannabis users were more likely to have a lower body mass index and less likely to develop T2D. Furthermore, chronic cannabis users were less likely to have measures of inflammation and no loss of insulin sensitivity.
Another study by Dr. Bryan’s group found that CBD-dominant forms of cannabis were associated with acute tension reduction, which might lead to longer-term reductions in anxiety. Bryan said the findings could be relevant in the context of diabetes distress.
Similarly positive results were found in a 15-week, double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD spray for neuropathic pain among treatment-resistant patients. The investigators reported that “clinically important improvements” were seen in pain, sleep quality, and subjective impressions of pain. Another small study of inhaled cannabis in treatment-refractory patients found a dose-dependent reduction in diabetic peripheral neuropathy pain.
Findings from a 9-year longitudinal study of approximately 18,000 Swedish men and women suggested no association between cannabis and subsequent T2D development after controlling for age, although these authors also called for longer follow-up and more detailed information about cannabis use to make “more robust” conclusions.
On the other side of the spectrum, a “rapid” review of recreational cannabis use in people with T1D and T2D found that recreational cannabis use may negatively impact diabetes metabolic factors and self-management behaviors and may increase risks for peripheral arterial occlusion, myocardial infarction, and renal disease. However, the authors cautioned that more robust research is needed to confirm the potential impact of cannabis on diabetes.
How to Advise Patients
When Dr. Shubrook was working with patients with diabetes in his family medicine practice in Ohio, cannabis wasn’t legal.
“’Don’t ask, don’t tell’ was the way we handled it then,” he said.
By contrast, in California, where he’s currently located, “it’s pretty well accepted and legal, and patients volunteer information about use, even if it’s recreational,” he said. “Realizing this was something we could talk about was really eye-opening to me.”
Talking to patients about cannabis use is a “20-minute conversation that details what they’re doing,” he said. He proceeds by asking questions: Are you using for recreational or medicinal purposes? What do you take? What do you take it for? Does it work?
“People will tell you,” Dr. Shubrook said. “They know exactly what it works or doesn’t work for and how it affects their glucose control, which in most cases is only minimally.”
He tells patients he would prefer they don’t inhale cannabis, given the risks posed to the lungs.
“Edibles may have a slower onset of effect, but depending on what they’re adding it to, glucose might be affected,” he noted. “And I have seen that chronic use can lead to hyperemesis syndrome.”
Overall, he said, “Take the time to talk to your patients about cannabis — it will allow them to be honest with you, and you can improve the specificity and safety of its use. If cannabis is legal in your state, encourage people to go to legal dispensaries, which will reduce the risk of it being laced with another drug that could increase the danger of use.”
A recent US prevalence study found that people with diabetes who use cannabis likely engage in other substance and psychoactive substance use, including tobacco use, binge drinking, and misuse of opioids and stimulants.
“Use of these additional substances could further exacerbate the health risks associated with diabetes and also emphasizes the importance of addressing polysubstance use among adults with diabetes,” the study’s author Benjamin H. Han, MD, Division of Geriatrics, Gerontology and Palliative Care, Department of Medicine, US San Diego School of Medicine in La Jolla, California, told this news organization.
“We were surprised at how strong the associations were, especially with use of substances that can increase cardiovascular risk,” Dr. Han added. “And given the strong association we found between cannabis use and use of other psychoactive substances in diabetes, clinicians must screen all their patients for psychoactive substance use.”
Diabetes Canada’s position paper states that despite the limited evidence, “there were sufficient data to begin developing recommendations for type 1 and type 2 diabetes about education, counseling, and management related to recreational cannabis use.”
Their recommendations include the following:
- Healthcare professionals should engage their patients in discussions about substance use on a regular basis, with a nonjudgmental approach.
- The use of recreational cannabis is not recommended for adolescents and adults with diabetes.
- People with T1D should avoid recreational cannabis use because of the increased risk for diabetic ketoacidosis.
- For adults with T1D or T2D who intend to use cannabis recreationally, individualized assessment and counseling should be offered to inform them of the general risks of cannabis, with a focus on harm reduction and reduction of the risk for potential adverse effects on diabetes management and complications.
- People with T1D or T2D should be offered education on and encouraged to read public information available through resources from various Canadian health authorities about the general risks of cannabis use to reduce the risk for nondiabetes-related adverse effects of cannabis consumption.
Of note, in 2018, the Canadian government produced an exhaustive compendium of information on cannabis for healthcare professionals that includes information relevant to managing patients with diabetes.
Dr. Shubrook and Dr. Han reported no competing interests.
A version of this article appeared on Medscape.com.
Higher Daily Buprenorphine Doses Help Manage OUD: AMA Recommends Policy Change
Higher daily buprenorphine doses may help patients better manage opioid use disorder (OUD), data from a National Institutes of Health (NIH) study suggested.
The new data highlight that the dose size currently recommended by the US Food and Drug Administration (FDA) and insurance caps on doses are outdated and harmful in the age of fentanyl overdoses, according to the American Medical Association (AMA) and physicians who have studied the issue.
Findings of the study, led by Sarah Axeen, PhD, with the Schaeffer Center for Health Policy & Economics, University of Southern California, Los Angeles, were published in JAMA Network Open.
The researchers reviewed insurance claims data from more than 35,000 people diagnosed with OUD who started on buprenorphine treatment between 2016 and 2021. They found that 12.5% had an emergency department (ED) or inpatient visit related to behavioral health within the study period.
They analyzed whether a patient’s buprenorphine dose was linked with the length of time between treatment start and an ED or inpatient visit.
Higher Doses, Better Outcomes
The FDA’s recommended target dose for buprenorphine is 16 mg/d. Dr. Axeen’s team found that those taking higher daily doses (> 16 to 24 mg) took 20% longer to have an ED or inpatient visit related to behavioral health within the first year after receiving treatment than those who took > 8 to 16 mg/d.
“Those taking daily doses of more than 24 mg of buprenorphine went 50% longer before having a subsequent emergency or inpatient healthcare visit related to behavioral health within the first year after receiving treatment, compared to those receiving > 8 to 16 mg a day,” the researchers said in a press release.
AMA Says the Findings Should Change Policies
Bobby Mukkamala, MD, president-elect of the AMA and Chair of the AMA Substance Use and Pain Care Task Force, said the association welcomed the study findings and urged policymakers and insurance providers to act on them with updated policies.
“The findings support AMA policy calling for flexibility in buprenorphine dosing, allowing patients to receive doses exceeding FDA-approved limits when clinically recommended by their prescriber,” he said in a statement. “Policymakers must take note of these findings and the growing body of evidence that further affirm buprenorphine as a safe, effective, and lifesaving tool in the fight against the illicit fentanyl overdose epidemic. It is also critically important for health insurance companies, Medicaid, and Medicare to remove dosage caps for buprenorphine.”
‘Tangible Economic Impact’
Lucinda Grande, MD, a family physician and addiction specialist with Pioneer Family Practice in Lacey, Washington, said in an interview that she was happy to see this study because “it is the first buprenorphine dose study that addresses an outcome with a tangible economic impact that would affect the bottom line of payers and healthcare systems” and may capture the attention of policymakers in changing what she says are outdated recommendations.
“This study is also unusual because it looked specifically at the dose range above 24 mg. Even though that top tier included only a tiny proportion (1.8%) of patients, it was the group that had the greatest long-term benefit from buprenorphine,” Dr. Grande said, adding that other studies have not included that high a dose.
Dr. Grande, who published on a related topic in 2023, noted that Medicaid patients were excluded from the current study, and they make up a substantial portion of those using buprenorphine for OUD. Had they been included, she said, she suspects the evidence would have been even stronger in favor of higher doses.
Physicians can prescribe higher doses off-label, but buprenorphine is expensive, and some insurers have caps based on the FDA recommendations. Dr. Grande says she rarely prescribes > 32 mg/d, and the patients who need the higher doses often have chronic pain. “In Washington State,” she said, “we have had the luxury of prescribing up to 32 mg daily to Medicaid patients for years. I have had a lot of opportunity to work in that dose rage for people who really need it, and I can really see a difference.”
As fentanyl has grown into the primary illicit opioid, she says, the FDA recommendations for buprenorphine have become progressively weaker.
“Fentanyl is 50 times more potent than heroin, the opioid prevalent when the FDA guidelines were written,” she said. “It’s like a popgun that you’re using against a cannon.”
This manuscript was prepared with support from the National Institute on Drug Abuse. Dr. Axeen reported no relevant financial disclosures. Coauthor Jessica S. Merlin, MD, reported grants from Cambia Health Foundation outside the submitted work. Adam J. Gordon, MD, reported grants from NIH and the Veterans Affairs (institution) during the conduct of the study; he reported service as editor-in-chief with the Association for Multidisciplinary Education and Research in Substance use and Addiction. Bradley D. Stein, MD, reported grants from the NIH during the conduct of the study. Dr. Mukkamala and Dr. Grande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher daily buprenorphine doses may help patients better manage opioid use disorder (OUD), data from a National Institutes of Health (NIH) study suggested.
The new data highlight that the dose size currently recommended by the US Food and Drug Administration (FDA) and insurance caps on doses are outdated and harmful in the age of fentanyl overdoses, according to the American Medical Association (AMA) and physicians who have studied the issue.
Findings of the study, led by Sarah Axeen, PhD, with the Schaeffer Center for Health Policy & Economics, University of Southern California, Los Angeles, were published in JAMA Network Open.
The researchers reviewed insurance claims data from more than 35,000 people diagnosed with OUD who started on buprenorphine treatment between 2016 and 2021. They found that 12.5% had an emergency department (ED) or inpatient visit related to behavioral health within the study period.
They analyzed whether a patient’s buprenorphine dose was linked with the length of time between treatment start and an ED or inpatient visit.
Higher Doses, Better Outcomes
The FDA’s recommended target dose for buprenorphine is 16 mg/d. Dr. Axeen’s team found that those taking higher daily doses (> 16 to 24 mg) took 20% longer to have an ED or inpatient visit related to behavioral health within the first year after receiving treatment than those who took > 8 to 16 mg/d.
“Those taking daily doses of more than 24 mg of buprenorphine went 50% longer before having a subsequent emergency or inpatient healthcare visit related to behavioral health within the first year after receiving treatment, compared to those receiving > 8 to 16 mg a day,” the researchers said in a press release.
AMA Says the Findings Should Change Policies
Bobby Mukkamala, MD, president-elect of the AMA and Chair of the AMA Substance Use and Pain Care Task Force, said the association welcomed the study findings and urged policymakers and insurance providers to act on them with updated policies.
“The findings support AMA policy calling for flexibility in buprenorphine dosing, allowing patients to receive doses exceeding FDA-approved limits when clinically recommended by their prescriber,” he said in a statement. “Policymakers must take note of these findings and the growing body of evidence that further affirm buprenorphine as a safe, effective, and lifesaving tool in the fight against the illicit fentanyl overdose epidemic. It is also critically important for health insurance companies, Medicaid, and Medicare to remove dosage caps for buprenorphine.”
‘Tangible Economic Impact’
Lucinda Grande, MD, a family physician and addiction specialist with Pioneer Family Practice in Lacey, Washington, said in an interview that she was happy to see this study because “it is the first buprenorphine dose study that addresses an outcome with a tangible economic impact that would affect the bottom line of payers and healthcare systems” and may capture the attention of policymakers in changing what she says are outdated recommendations.
“This study is also unusual because it looked specifically at the dose range above 24 mg. Even though that top tier included only a tiny proportion (1.8%) of patients, it was the group that had the greatest long-term benefit from buprenorphine,” Dr. Grande said, adding that other studies have not included that high a dose.
Dr. Grande, who published on a related topic in 2023, noted that Medicaid patients were excluded from the current study, and they make up a substantial portion of those using buprenorphine for OUD. Had they been included, she said, she suspects the evidence would have been even stronger in favor of higher doses.
Physicians can prescribe higher doses off-label, but buprenorphine is expensive, and some insurers have caps based on the FDA recommendations. Dr. Grande says she rarely prescribes > 32 mg/d, and the patients who need the higher doses often have chronic pain. “In Washington State,” she said, “we have had the luxury of prescribing up to 32 mg daily to Medicaid patients for years. I have had a lot of opportunity to work in that dose rage for people who really need it, and I can really see a difference.”
As fentanyl has grown into the primary illicit opioid, she says, the FDA recommendations for buprenorphine have become progressively weaker.
“Fentanyl is 50 times more potent than heroin, the opioid prevalent when the FDA guidelines were written,” she said. “It’s like a popgun that you’re using against a cannon.”
This manuscript was prepared with support from the National Institute on Drug Abuse. Dr. Axeen reported no relevant financial disclosures. Coauthor Jessica S. Merlin, MD, reported grants from Cambia Health Foundation outside the submitted work. Adam J. Gordon, MD, reported grants from NIH and the Veterans Affairs (institution) during the conduct of the study; he reported service as editor-in-chief with the Association for Multidisciplinary Education and Research in Substance use and Addiction. Bradley D. Stein, MD, reported grants from the NIH during the conduct of the study. Dr. Mukkamala and Dr. Grande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher daily buprenorphine doses may help patients better manage opioid use disorder (OUD), data from a National Institutes of Health (NIH) study suggested.
The new data highlight that the dose size currently recommended by the US Food and Drug Administration (FDA) and insurance caps on doses are outdated and harmful in the age of fentanyl overdoses, according to the American Medical Association (AMA) and physicians who have studied the issue.
Findings of the study, led by Sarah Axeen, PhD, with the Schaeffer Center for Health Policy & Economics, University of Southern California, Los Angeles, were published in JAMA Network Open.
The researchers reviewed insurance claims data from more than 35,000 people diagnosed with OUD who started on buprenorphine treatment between 2016 and 2021. They found that 12.5% had an emergency department (ED) or inpatient visit related to behavioral health within the study period.
They analyzed whether a patient’s buprenorphine dose was linked with the length of time between treatment start and an ED or inpatient visit.
Higher Doses, Better Outcomes
The FDA’s recommended target dose for buprenorphine is 16 mg/d. Dr. Axeen’s team found that those taking higher daily doses (> 16 to 24 mg) took 20% longer to have an ED or inpatient visit related to behavioral health within the first year after receiving treatment than those who took > 8 to 16 mg/d.
“Those taking daily doses of more than 24 mg of buprenorphine went 50% longer before having a subsequent emergency or inpatient healthcare visit related to behavioral health within the first year after receiving treatment, compared to those receiving > 8 to 16 mg a day,” the researchers said in a press release.
AMA Says the Findings Should Change Policies
Bobby Mukkamala, MD, president-elect of the AMA and Chair of the AMA Substance Use and Pain Care Task Force, said the association welcomed the study findings and urged policymakers and insurance providers to act on them with updated policies.
“The findings support AMA policy calling for flexibility in buprenorphine dosing, allowing patients to receive doses exceeding FDA-approved limits when clinically recommended by their prescriber,” he said in a statement. “Policymakers must take note of these findings and the growing body of evidence that further affirm buprenorphine as a safe, effective, and lifesaving tool in the fight against the illicit fentanyl overdose epidemic. It is also critically important for health insurance companies, Medicaid, and Medicare to remove dosage caps for buprenorphine.”
‘Tangible Economic Impact’
Lucinda Grande, MD, a family physician and addiction specialist with Pioneer Family Practice in Lacey, Washington, said in an interview that she was happy to see this study because “it is the first buprenorphine dose study that addresses an outcome with a tangible economic impact that would affect the bottom line of payers and healthcare systems” and may capture the attention of policymakers in changing what she says are outdated recommendations.
“This study is also unusual because it looked specifically at the dose range above 24 mg. Even though that top tier included only a tiny proportion (1.8%) of patients, it was the group that had the greatest long-term benefit from buprenorphine,” Dr. Grande said, adding that other studies have not included that high a dose.
Dr. Grande, who published on a related topic in 2023, noted that Medicaid patients were excluded from the current study, and they make up a substantial portion of those using buprenorphine for OUD. Had they been included, she said, she suspects the evidence would have been even stronger in favor of higher doses.
Physicians can prescribe higher doses off-label, but buprenorphine is expensive, and some insurers have caps based on the FDA recommendations. Dr. Grande says she rarely prescribes > 32 mg/d, and the patients who need the higher doses often have chronic pain. “In Washington State,” she said, “we have had the luxury of prescribing up to 32 mg daily to Medicaid patients for years. I have had a lot of opportunity to work in that dose rage for people who really need it, and I can really see a difference.”
As fentanyl has grown into the primary illicit opioid, she says, the FDA recommendations for buprenorphine have become progressively weaker.
“Fentanyl is 50 times more potent than heroin, the opioid prevalent when the FDA guidelines were written,” she said. “It’s like a popgun that you’re using against a cannon.”
This manuscript was prepared with support from the National Institute on Drug Abuse. Dr. Axeen reported no relevant financial disclosures. Coauthor Jessica S. Merlin, MD, reported grants from Cambia Health Foundation outside the submitted work. Adam J. Gordon, MD, reported grants from NIH and the Veterans Affairs (institution) during the conduct of the study; he reported service as editor-in-chief with the Association for Multidisciplinary Education and Research in Substance use and Addiction. Bradley D. Stein, MD, reported grants from the NIH during the conduct of the study. Dr. Mukkamala and Dr. Grande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medicare Will Speed Coverage of Some ‘Breakthrough’ Medical Devices
The Centers for Medicare & Medicaid Services (CMS) in August finalized a long-awaited plan to accelerate coverage decisions on medical devices that have impressed regulators.
The intent is to create a smoother pathway for some devices that earned a “breakthrough” designation from the US Food and Drug Administration (FDA), a label intended for innovative products that significantly advance treatment for serious illness.
“We’ll see how many they actually take on, but it could make a really big difference on how quickly devices make it through the Medicare coverage gauntlet and get to patients,” Emily P. Zeitler, MD, MHS, a cardiologist and assistant professor of medicine at Dartmouth’s Geisel School of Medicine in Hanover, New Hampshire, told this news organization.
Companies selling “breakthrough” devices face tougher challenges in securing Medicare payment than device makers whose products fit into already established niches. Previous Medicare decisions can serve as a path to coverage for those products.
The new CMS Transitional Coverage for Emerging Technologies (TCET) pathway addresses two causes of delay in securing Medicare coverage for newcomer devices:
- CMS delegates many decisions on new device coverage to Medicare Administrative Contractors (MACs). MACs are businesses owned by, or affiliated with, insurers such as Blue Cross plans. Companies sometimes need to work their way through several regional MACs to gain nationwide coverage of their devices.
- Congress sets different mandates for the FDA and CMS regarding medical products. The FDA needs to know a product is safe and effective enough for US sales. Companies sometimes produce enough evidence to show products can meet the FDA’s standard without generating sufficient data to compel Medicare coverage. Medicare covers about 66.7 million people: 59.1 million aged 65 years or older and 7.6 million with disabilities.
“Medicare beneficiaries are often older, have multiple comorbidities, and are often underrepresented or not represented in many clinical studies,” CMS said in its August notice on the TCET pathway.
“Consequently, a device’s potential benefits and harms for older patients with more comorbidities may not be well understood at the time of FDA market authorization,” the agency added.
Coverage With Evidence Development (CED) Experience
TCET is meant to help CMS officials clear coverage of breakthrough devices sooner, and on a nationwide basis, while sometimes continuing to study how well these products work for people enrolled in Medicare.
The TCET pathway builds on Medicare’s experience over two decades with its “CED” program. That program allows coverage of well-regarded new devices or treatments whose effects on older patients were not yet well understood.
In the TCET notice, CMS provided details of its plans for using contractors for evidence reviews and evidence development plans as part of its new coverage pathway.
Securing help from these experts outside of CMS, along with perhaps some new arrangements for in-house staff, would help the agency reach its goals for TCET, Dr. Zeitler said. She has published research on the CED process and has participated in cardiac registries associated with CED as both a researcher and a physician who implants devices.
The poster child for the benefits of the CED approach is the transcatheter aortic valve replacement (TAVR) devices, said Ralph Brindis, MD, MPH, a past president of the American College of Cardiology (ACC) and the senior medical officer for external affairs of the ACC’s National Cardiovascular Data Registry (NCDR).
Through the NCDR, ACC members worked with CMS for years in trying to find ways to gather evidence about the best uses of medical devices.
Brindis said this approach to evidence generation involved more tracking of off-label use and analysis of how TAVR devices performed in routine use through the data obtained through a registry, rather than relying solely on clinical trials requiring a much longer time frame for completion and at an increased cost.
This approach allowed cardiovascular surgeons and cardiologists to steer the course of real-world evidence gathering, he said.
“We were able to expand indications for TAVR without relying on industry to fund randomized clinical trials that they may not have had interest or bandwidth or finances” to do, Dr. Brindis said. “And we already had the data in hand.”
The TCET policy is welcomed, although there remain questions about how well CMS will be able to carry out this program due to funding limitations, Dr. Brindis said. Groups such as the ACC may be able to help CMS by encouraging Congress to provide the agency with more money to carry out the TCET plans, Dr. Brindis said.
“The professional societies will work hand in hand with CMS in trying to achieve these goals” with TCET, he said.
AdvaMed, the trade group for makers of medical devices, also called for beefing up the administrative budget for CMS to speed reviews of innovative devices.
“The limited number of devices CMS can handle demonstrates clearly to Congress the need for greater resources,” AdvaMed CEO Scott Whitaker said in a statement.
Mr. Whitaker also described CMS’ decision to exclude medical tests from the TCET pathway as a disappointment.
The agency said the majority of coverage decisions on diagnostic tests should stay with MACs. In some cases, there may be a need for CMS to use its long-standing processes for considering a national coverage decision for certain tests, the agency said.
“The final TCET notice is a step toward a stronger, more robust policy, but doesn’t go far enough to help the Medicare seniors depending on breakthrough diagnostics and treatments to alleviate their suffering,” AdvaMed’s Mr. Whitaker said.
Dr. Brindis said he had no relevant financial disclosures. Dr. Zeitler reported having received consulting and speaking fees, travel payments, and research support from Medtronic, Abbott, Biosense Webster, Sanofi, NIH/NIGMS, Element Science, Edwards, Boston Scientific, Philips, and V-Wave.
A version of this article appeared on Medscape.com.
The Centers for Medicare & Medicaid Services (CMS) in August finalized a long-awaited plan to accelerate coverage decisions on medical devices that have impressed regulators.
The intent is to create a smoother pathway for some devices that earned a “breakthrough” designation from the US Food and Drug Administration (FDA), a label intended for innovative products that significantly advance treatment for serious illness.
“We’ll see how many they actually take on, but it could make a really big difference on how quickly devices make it through the Medicare coverage gauntlet and get to patients,” Emily P. Zeitler, MD, MHS, a cardiologist and assistant professor of medicine at Dartmouth’s Geisel School of Medicine in Hanover, New Hampshire, told this news organization.
Companies selling “breakthrough” devices face tougher challenges in securing Medicare payment than device makers whose products fit into already established niches. Previous Medicare decisions can serve as a path to coverage for those products.
The new CMS Transitional Coverage for Emerging Technologies (TCET) pathway addresses two causes of delay in securing Medicare coverage for newcomer devices:
- CMS delegates many decisions on new device coverage to Medicare Administrative Contractors (MACs). MACs are businesses owned by, or affiliated with, insurers such as Blue Cross plans. Companies sometimes need to work their way through several regional MACs to gain nationwide coverage of their devices.
- Congress sets different mandates for the FDA and CMS regarding medical products. The FDA needs to know a product is safe and effective enough for US sales. Companies sometimes produce enough evidence to show products can meet the FDA’s standard without generating sufficient data to compel Medicare coverage. Medicare covers about 66.7 million people: 59.1 million aged 65 years or older and 7.6 million with disabilities.
“Medicare beneficiaries are often older, have multiple comorbidities, and are often underrepresented or not represented in many clinical studies,” CMS said in its August notice on the TCET pathway.
“Consequently, a device’s potential benefits and harms for older patients with more comorbidities may not be well understood at the time of FDA market authorization,” the agency added.
Coverage With Evidence Development (CED) Experience
TCET is meant to help CMS officials clear coverage of breakthrough devices sooner, and on a nationwide basis, while sometimes continuing to study how well these products work for people enrolled in Medicare.
The TCET pathway builds on Medicare’s experience over two decades with its “CED” program. That program allows coverage of well-regarded new devices or treatments whose effects on older patients were not yet well understood.
In the TCET notice, CMS provided details of its plans for using contractors for evidence reviews and evidence development plans as part of its new coverage pathway.
Securing help from these experts outside of CMS, along with perhaps some new arrangements for in-house staff, would help the agency reach its goals for TCET, Dr. Zeitler said. She has published research on the CED process and has participated in cardiac registries associated with CED as both a researcher and a physician who implants devices.
The poster child for the benefits of the CED approach is the transcatheter aortic valve replacement (TAVR) devices, said Ralph Brindis, MD, MPH, a past president of the American College of Cardiology (ACC) and the senior medical officer for external affairs of the ACC’s National Cardiovascular Data Registry (NCDR).
Through the NCDR, ACC members worked with CMS for years in trying to find ways to gather evidence about the best uses of medical devices.
Brindis said this approach to evidence generation involved more tracking of off-label use and analysis of how TAVR devices performed in routine use through the data obtained through a registry, rather than relying solely on clinical trials requiring a much longer time frame for completion and at an increased cost.
This approach allowed cardiovascular surgeons and cardiologists to steer the course of real-world evidence gathering, he said.
“We were able to expand indications for TAVR without relying on industry to fund randomized clinical trials that they may not have had interest or bandwidth or finances” to do, Dr. Brindis said. “And we already had the data in hand.”
The TCET policy is welcomed, although there remain questions about how well CMS will be able to carry out this program due to funding limitations, Dr. Brindis said. Groups such as the ACC may be able to help CMS by encouraging Congress to provide the agency with more money to carry out the TCET plans, Dr. Brindis said.
“The professional societies will work hand in hand with CMS in trying to achieve these goals” with TCET, he said.
AdvaMed, the trade group for makers of medical devices, also called for beefing up the administrative budget for CMS to speed reviews of innovative devices.
“The limited number of devices CMS can handle demonstrates clearly to Congress the need for greater resources,” AdvaMed CEO Scott Whitaker said in a statement.
Mr. Whitaker also described CMS’ decision to exclude medical tests from the TCET pathway as a disappointment.
The agency said the majority of coverage decisions on diagnostic tests should stay with MACs. In some cases, there may be a need for CMS to use its long-standing processes for considering a national coverage decision for certain tests, the agency said.
“The final TCET notice is a step toward a stronger, more robust policy, but doesn’t go far enough to help the Medicare seniors depending on breakthrough diagnostics and treatments to alleviate their suffering,” AdvaMed’s Mr. Whitaker said.
Dr. Brindis said he had no relevant financial disclosures. Dr. Zeitler reported having received consulting and speaking fees, travel payments, and research support from Medtronic, Abbott, Biosense Webster, Sanofi, NIH/NIGMS, Element Science, Edwards, Boston Scientific, Philips, and V-Wave.
A version of this article appeared on Medscape.com.
The Centers for Medicare & Medicaid Services (CMS) in August finalized a long-awaited plan to accelerate coverage decisions on medical devices that have impressed regulators.
The intent is to create a smoother pathway for some devices that earned a “breakthrough” designation from the US Food and Drug Administration (FDA), a label intended for innovative products that significantly advance treatment for serious illness.
“We’ll see how many they actually take on, but it could make a really big difference on how quickly devices make it through the Medicare coverage gauntlet and get to patients,” Emily P. Zeitler, MD, MHS, a cardiologist and assistant professor of medicine at Dartmouth’s Geisel School of Medicine in Hanover, New Hampshire, told this news organization.
Companies selling “breakthrough” devices face tougher challenges in securing Medicare payment than device makers whose products fit into already established niches. Previous Medicare decisions can serve as a path to coverage for those products.
The new CMS Transitional Coverage for Emerging Technologies (TCET) pathway addresses two causes of delay in securing Medicare coverage for newcomer devices:
- CMS delegates many decisions on new device coverage to Medicare Administrative Contractors (MACs). MACs are businesses owned by, or affiliated with, insurers such as Blue Cross plans. Companies sometimes need to work their way through several regional MACs to gain nationwide coverage of their devices.
- Congress sets different mandates for the FDA and CMS regarding medical products. The FDA needs to know a product is safe and effective enough for US sales. Companies sometimes produce enough evidence to show products can meet the FDA’s standard without generating sufficient data to compel Medicare coverage. Medicare covers about 66.7 million people: 59.1 million aged 65 years or older and 7.6 million with disabilities.
“Medicare beneficiaries are often older, have multiple comorbidities, and are often underrepresented or not represented in many clinical studies,” CMS said in its August notice on the TCET pathway.
“Consequently, a device’s potential benefits and harms for older patients with more comorbidities may not be well understood at the time of FDA market authorization,” the agency added.
Coverage With Evidence Development (CED) Experience
TCET is meant to help CMS officials clear coverage of breakthrough devices sooner, and on a nationwide basis, while sometimes continuing to study how well these products work for people enrolled in Medicare.
The TCET pathway builds on Medicare’s experience over two decades with its “CED” program. That program allows coverage of well-regarded new devices or treatments whose effects on older patients were not yet well understood.
In the TCET notice, CMS provided details of its plans for using contractors for evidence reviews and evidence development plans as part of its new coverage pathway.
Securing help from these experts outside of CMS, along with perhaps some new arrangements for in-house staff, would help the agency reach its goals for TCET, Dr. Zeitler said. She has published research on the CED process and has participated in cardiac registries associated with CED as both a researcher and a physician who implants devices.
The poster child for the benefits of the CED approach is the transcatheter aortic valve replacement (TAVR) devices, said Ralph Brindis, MD, MPH, a past president of the American College of Cardiology (ACC) and the senior medical officer for external affairs of the ACC’s National Cardiovascular Data Registry (NCDR).
Through the NCDR, ACC members worked with CMS for years in trying to find ways to gather evidence about the best uses of medical devices.
Brindis said this approach to evidence generation involved more tracking of off-label use and analysis of how TAVR devices performed in routine use through the data obtained through a registry, rather than relying solely on clinical trials requiring a much longer time frame for completion and at an increased cost.
This approach allowed cardiovascular surgeons and cardiologists to steer the course of real-world evidence gathering, he said.
“We were able to expand indications for TAVR without relying on industry to fund randomized clinical trials that they may not have had interest or bandwidth or finances” to do, Dr. Brindis said. “And we already had the data in hand.”
The TCET policy is welcomed, although there remain questions about how well CMS will be able to carry out this program due to funding limitations, Dr. Brindis said. Groups such as the ACC may be able to help CMS by encouraging Congress to provide the agency with more money to carry out the TCET plans, Dr. Brindis said.
“The professional societies will work hand in hand with CMS in trying to achieve these goals” with TCET, he said.
AdvaMed, the trade group for makers of medical devices, also called for beefing up the administrative budget for CMS to speed reviews of innovative devices.
“The limited number of devices CMS can handle demonstrates clearly to Congress the need for greater resources,” AdvaMed CEO Scott Whitaker said in a statement.
Mr. Whitaker also described CMS’ decision to exclude medical tests from the TCET pathway as a disappointment.
The agency said the majority of coverage decisions on diagnostic tests should stay with MACs. In some cases, there may be a need for CMS to use its long-standing processes for considering a national coverage decision for certain tests, the agency said.
“The final TCET notice is a step toward a stronger, more robust policy, but doesn’t go far enough to help the Medicare seniors depending on breakthrough diagnostics and treatments to alleviate their suffering,” AdvaMed’s Mr. Whitaker said.
Dr. Brindis said he had no relevant financial disclosures. Dr. Zeitler reported having received consulting and speaking fees, travel payments, and research support from Medtronic, Abbott, Biosense Webster, Sanofi, NIH/NIGMS, Element Science, Edwards, Boston Scientific, Philips, and V-Wave.
A version of this article appeared on Medscape.com.
Public Health, Not Politics, Should Drive Mask Policies, Says Ethicist
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Six Tips on Coronavirus Testing for Doctors and Patients
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
COVID Levels Start to Dip, New Variant Emerges
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
How Psychedelic Drugs Can Aid Patients at the End of Life
Palliative care has proven to be one of the most promising fields for research on interventions with psychedelic substances. One of the most prominent researchers in this area was the American psychopharmacologist Roland Griffiths, PhD.
In 2016, Dr. Griffiths and his team at Johns Hopkins University in Baltimore, Maryland, published one of the most relevant contributions to the field by demonstrating in a placebo-controlled study that psilocybin can reduce depressive and anxiety symptoms in patients with cancer. The study, conducted with 51 patients diagnosed with advanced-stage cancer, compared the effects of a low dose and a high dose of psilocybin, showing that the high dose resulted in improvements in mood, quality of life, and sense of life, reducing death-related anxiety.
In 2021, after a routine examination, Dr. Griffiths himself was diagnosed with advanced colon cancer. Unexpectedly, the researcher found himself in the position of his research subjects. In an interview with The New York Times in April 2023, he stated that, after some resistance, he agreed to undergo an LSD session.
In the conversation, he revealed that he had a 50% chance of being alive by Halloween. Despite the diagnosis, he showed no discouragement. “As a scientist, I feel like a kid in a candy store, considering all the research and questions that need to be answered about psychedelics and the theme of human flourishing,” he said.
In his last months of life, in the various appearances and interviews he gave, Dr. Griffiths demonstrated a perception of life uncommon in people facing death. “I’m excited to communicate, to shake off the dust and tell people: ‘Come on, wake up!’ ”
He passed away on October 16, 2023, at age 77 years, opening new horizons for clinical research with psychedelics and becoming an example of the therapeutic potential of these substances.
Innovative Treatments
“I believe this will be one of the next conditions, if not the next condition, to be considered for the designation of innovative treatment in future psilocybin regulation in the United States, where the field is more advanced,” said Lucas Maia, PhD, a psychopharmacologist and researcher affiliated with the Advanced Center for Psychedelic Medicine (CAMP) at the Federal University of Rio Grande do Norte (UFRN) and the Interdisciplinary Cooperation for Ayahuasca Research and Outreach (ICARO) at the State University of Campinas in São Paulo, Brazil.
Currently, MDMA (for the treatment of posttraumatic stress disorder), psilocybin (for depressive disorder), and MM120 (an LSD analogue used to treat generalized anxiety disorder) are the only psychedelic substances that have received the designation of innovative treatment by the Food and Drug Administration (FDA).
In 2022, Dr. Maia and a colleague from ICARO, Ana Cláudia Mesquita Garcia, PhD, a professor at the School of Nursing at the Federal University of Alfenas in Brazil and leader of the Interdisciplinary Center for Studies in Palliative Care, published a systematic review in the Journal of Pain and Symptom Management that evaluated the use of psychedelic-assisted treatments for symptom control in patients with serious or terminal illnesses.
Of the 20 articles reviewed, 9 (45%) used LSD, 5 (25%) psilocybin, 2 (10%) dipropyltryptamine (DPT), 1 (5%) used ketamine, and 1 (5%) used MDMA. In 10% of the studies, LSD and DPT were combined. Altogether, 347 participants (54%) received LSD, 116 (18%) psilocybin, 81 (13%) LSD and DPT, 64 (10%) DPT, 18 (3%) MDMA, and 14 (2%) ketamine.
The conclusion of the study is that psychedelics provide therapeutic effects on physical, psychological, social, and existential outcomes. They are associated with a reduction in pain and improvement in sleep. A decrease in depressive and anxiety symptoms is also observed; such symptoms are common in patients with serious diseases. In addition, interpersonal relationships become closer and more empathetic. Finally, there is a reduction in the fear of death and suffering, an increase in acceptance, and a redefinition of the disease.
In 55% of the studies, the adverse effects were mild to moderate and transient. They included nausea, vomiting, dry mouth, and fatigue, as well as anxiety, panic, and hallucinations. The researchers concluded that the scarcity and difficulty of access to professional training in psychedelic-assisted treatments represent a significant challenge for the advancement of these interventions, especially in countries in the Global South.
Another systematic review and meta-analysis published in July by researchers at the University of Michigan in Ann Arbor, Michigan, included seven studies with 132 participants and showed significant improvements in quality of life, pain control, and anxiety relief after psychedelic-assisted psychotherapy with psilocybin. The combined effects indicated statistically significant reductions in anxiety symptoms after 4.0-4.5 months and after 6.0-6.5 months post administration, compared with the initial evaluations.
One of the most advanced research studies currently being conducted is led by Stephen Ross, MD, a psychiatrist affiliated with New York University’s Langone Medical Center, New York City. The phase 2b clinical study is randomized, double blind, and placebo controlled, and involves 300 participants. The study aims to evaluate the effects of psilocybin-assisted psychotherapy on psychiatric and existential distress in patients with advanced cancer. Its expected completion date is in 2027.
“We still lack effective interventions in minimizing psychological, spiritual, and existential suffering,” said Dr. Garcia. “In this sense, respecting the contraindications of a physical nature (including pre-existing illnesses at study initiation, disease staging, patient functionality level, comorbidities, concurrent pharmacological treatments, etc) and of a psychiatric nature for the use of psychedelics, depending on the clinical picture, end-of-life patients facing existential crises and psychological suffering will likely benefit more from psychedelic-assisted psychotherapy, which highlights the need for more research and the integration of this treatment into clinical practice.”
Changing Perceptions
Since 2021, the Cancer Institute of the State of São Paulo (Icesp) has been providing palliative treatment with ketamine — an atypical psychedelic — following a rigorous and carefully monitored clinical protocol. The substance is already used off label to treat refractory depressive disorder. In addition, in 2020, Brazil’s National Health Surveillance Agency approved the use of Spravato, an intranasal antidepressant based on the ketamine derivative esketamine.
Icesp has hospice beds for clinical oncology patients, and a pain management team evaluates which patients meet the inclusion criteria for ketamine use. In addition to difficult-to-control pain, it is important that the patient present emotional, existential, or spiritual symptoms that amplify that pain.
After this evaluation, a psychoeducation process takes place, in which the patient receives clear information about the treatment, its potential benefits and risks, and understands how ketamine can be a viable option for managing their symptoms. Finally, it is essential that the patient accept the referral and demonstrate a willingness to participate in the treatment, agreeing to the proposed terms.
The treatment takes place in a hospital environment, with an ambiance that aims to provide comfort and safety. Clinicians consider not only the substance dose (such as 0.5 mg/kg) but also the emotional state (“set”) and the treatment environment (“setting”). The experience is facilitated through psychological support for the patient during and after treatment.
According to Alessandro Campolina, MD, PhD, a researcher at the Center for Translational Oncology Research at Icesp, it is important to highlight that quality of life is intrinsically linked to the patient’s self-perception, including how they see themselves in terms of health and in the context in which they live.
The doctor explains that psychedelic interventions can provide a “window of opportunity,” allowing a qualified clinician to help the patient explore new perspectives based on their experiences.
“Often, although the intensity of pain remains the same, the way the patient perceives it can change significantly. For example, a patient may report that, despite the pain, they now feel less concerned about it because they were able to contemplate more significant aspects of their life,” said Dr. Campolina.
“This observation shows that treatment is not limited to addressing the pain or primary symptoms, but also addresses the associated suffering. While some patients have profound insights, many others experience more subtle changes that, under the guidance of a competent therapist, can turn into valuable clinical insights, thus improving quality of life and how they deal with their pathologies.”
Dr. Griffiths exemplified this in the interview with the Times when he reflected on his own cancer. He came to believe, as if guided an external observer, that “there is a meaning and a purpose in this [disease] that go beyond your understanding, and the way you are dealing with it is exactly how you should.”
Toshio Chiba, MD, chief physician of the Palliative Care Service at Icesp, emphasized that ketamine is already in use. “It is not feasible to wait years for the approval of psilocybin or for the FDA’s decision on MDMA, especially if the patient needs immediate care,” he said.
Furthermore, recreational and therapeutic uses are distinct. “It is essential to note that responsibilities are shared between the professional and the patient,” said Dr. Chiba. “In the therapeutic setting, there is an ethical and civil responsibility of the medical professional, as well as the patient actively engaging in treatment.”
Early palliative care can also facilitate the establishment of care goals. “I prefer to avoid terms like ‘coping’ or ‘fighting the disease,’” said Dr. Chiba. “Nowadays, dealing with cancer is more about coexisting with the disease properly, as treatments can last for years.
“Of course, there are still highly lethal tumors. However, for neoplasms like breast, colorectal, and prostate cancers, we often talk about 5, 10, or even 15 years of coexistence [with the condition]. The lack of this information [about the disease, treatments, and existential issues] can generate distress in some patients, who end up excessively worrying about the future,” he added.
But palliative treatment with psychedelics as a panacea, he said.
In addition, Marcelo Falchi, MD, medical director of CAMP at UFRN, also emphasized that psychedelics are not a risk-free intervention. Substances like LSD and psilocybin, for example, can cause increases in blood pressure and tachycardia, which, may limit their use for patients at high cardiovascular risk. Crises of anxiety or dissociative symptoms also may occur, and they require mitigation strategies such as psychological support and attention to set and setting.
“But research seems to agree that the risks can be managed effectively through a diligent process, allowing for the responsible exploration of the therapeutic potential of psychedelics,” said Dr. Falchi, who is responsible for CAMP’s postgraduate course in psychedelic therapies. The program provides training in substances used in Brazil, such as ketamine and ibogaine.
The use of psychedelics in palliative care requires a significant shift in how professionals relate to patients.
Unlike in traditional practice, where the prescription is followed by quick consultations, palliative care with psychedelics requires deep and continuous involvement, as Dr. Campolina pointed out. “We joke that it’s not a high-tech specialty, but ‘high touch,’ because it demands the constant presence of the doctor or therapist with the patient. This can involve sessions of several hours, with frequent monitoring and regular contact after sessions. This dynamic emphasizes the importance of human touch and connection during the process, reflecting a new way of practicing medicine.”
In his last months of life, Dr. Griffiths sought to emphasize this point, suggesting that, from a broader perspective, doctors and patients face the same fundamental questions. “We all know we are terminal,” he said. “Essentially, we shouldn’t need a stage 4 cancer diagnosis to awaken to this reality.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Palliative care has proven to be one of the most promising fields for research on interventions with psychedelic substances. One of the most prominent researchers in this area was the American psychopharmacologist Roland Griffiths, PhD.
In 2016, Dr. Griffiths and his team at Johns Hopkins University in Baltimore, Maryland, published one of the most relevant contributions to the field by demonstrating in a placebo-controlled study that psilocybin can reduce depressive and anxiety symptoms in patients with cancer. The study, conducted with 51 patients diagnosed with advanced-stage cancer, compared the effects of a low dose and a high dose of psilocybin, showing that the high dose resulted in improvements in mood, quality of life, and sense of life, reducing death-related anxiety.
In 2021, after a routine examination, Dr. Griffiths himself was diagnosed with advanced colon cancer. Unexpectedly, the researcher found himself in the position of his research subjects. In an interview with The New York Times in April 2023, he stated that, after some resistance, he agreed to undergo an LSD session.
In the conversation, he revealed that he had a 50% chance of being alive by Halloween. Despite the diagnosis, he showed no discouragement. “As a scientist, I feel like a kid in a candy store, considering all the research and questions that need to be answered about psychedelics and the theme of human flourishing,” he said.
In his last months of life, in the various appearances and interviews he gave, Dr. Griffiths demonstrated a perception of life uncommon in people facing death. “I’m excited to communicate, to shake off the dust and tell people: ‘Come on, wake up!’ ”
He passed away on October 16, 2023, at age 77 years, opening new horizons for clinical research with psychedelics and becoming an example of the therapeutic potential of these substances.
Innovative Treatments
“I believe this will be one of the next conditions, if not the next condition, to be considered for the designation of innovative treatment in future psilocybin regulation in the United States, where the field is more advanced,” said Lucas Maia, PhD, a psychopharmacologist and researcher affiliated with the Advanced Center for Psychedelic Medicine (CAMP) at the Federal University of Rio Grande do Norte (UFRN) and the Interdisciplinary Cooperation for Ayahuasca Research and Outreach (ICARO) at the State University of Campinas in São Paulo, Brazil.
Currently, MDMA (for the treatment of posttraumatic stress disorder), psilocybin (for depressive disorder), and MM120 (an LSD analogue used to treat generalized anxiety disorder) are the only psychedelic substances that have received the designation of innovative treatment by the Food and Drug Administration (FDA).
In 2022, Dr. Maia and a colleague from ICARO, Ana Cláudia Mesquita Garcia, PhD, a professor at the School of Nursing at the Federal University of Alfenas in Brazil and leader of the Interdisciplinary Center for Studies in Palliative Care, published a systematic review in the Journal of Pain and Symptom Management that evaluated the use of psychedelic-assisted treatments for symptom control in patients with serious or terminal illnesses.
Of the 20 articles reviewed, 9 (45%) used LSD, 5 (25%) psilocybin, 2 (10%) dipropyltryptamine (DPT), 1 (5%) used ketamine, and 1 (5%) used MDMA. In 10% of the studies, LSD and DPT were combined. Altogether, 347 participants (54%) received LSD, 116 (18%) psilocybin, 81 (13%) LSD and DPT, 64 (10%) DPT, 18 (3%) MDMA, and 14 (2%) ketamine.
The conclusion of the study is that psychedelics provide therapeutic effects on physical, psychological, social, and existential outcomes. They are associated with a reduction in pain and improvement in sleep. A decrease in depressive and anxiety symptoms is also observed; such symptoms are common in patients with serious diseases. In addition, interpersonal relationships become closer and more empathetic. Finally, there is a reduction in the fear of death and suffering, an increase in acceptance, and a redefinition of the disease.
In 55% of the studies, the adverse effects were mild to moderate and transient. They included nausea, vomiting, dry mouth, and fatigue, as well as anxiety, panic, and hallucinations. The researchers concluded that the scarcity and difficulty of access to professional training in psychedelic-assisted treatments represent a significant challenge for the advancement of these interventions, especially in countries in the Global South.
Another systematic review and meta-analysis published in July by researchers at the University of Michigan in Ann Arbor, Michigan, included seven studies with 132 participants and showed significant improvements in quality of life, pain control, and anxiety relief after psychedelic-assisted psychotherapy with psilocybin. The combined effects indicated statistically significant reductions in anxiety symptoms after 4.0-4.5 months and after 6.0-6.5 months post administration, compared with the initial evaluations.
One of the most advanced research studies currently being conducted is led by Stephen Ross, MD, a psychiatrist affiliated with New York University’s Langone Medical Center, New York City. The phase 2b clinical study is randomized, double blind, and placebo controlled, and involves 300 participants. The study aims to evaluate the effects of psilocybin-assisted psychotherapy on psychiatric and existential distress in patients with advanced cancer. Its expected completion date is in 2027.
“We still lack effective interventions in minimizing psychological, spiritual, and existential suffering,” said Dr. Garcia. “In this sense, respecting the contraindications of a physical nature (including pre-existing illnesses at study initiation, disease staging, patient functionality level, comorbidities, concurrent pharmacological treatments, etc) and of a psychiatric nature for the use of psychedelics, depending on the clinical picture, end-of-life patients facing existential crises and psychological suffering will likely benefit more from psychedelic-assisted psychotherapy, which highlights the need for more research and the integration of this treatment into clinical practice.”
Changing Perceptions
Since 2021, the Cancer Institute of the State of São Paulo (Icesp) has been providing palliative treatment with ketamine — an atypical psychedelic — following a rigorous and carefully monitored clinical protocol. The substance is already used off label to treat refractory depressive disorder. In addition, in 2020, Brazil’s National Health Surveillance Agency approved the use of Spravato, an intranasal antidepressant based on the ketamine derivative esketamine.
Icesp has hospice beds for clinical oncology patients, and a pain management team evaluates which patients meet the inclusion criteria for ketamine use. In addition to difficult-to-control pain, it is important that the patient present emotional, existential, or spiritual symptoms that amplify that pain.
After this evaluation, a psychoeducation process takes place, in which the patient receives clear information about the treatment, its potential benefits and risks, and understands how ketamine can be a viable option for managing their symptoms. Finally, it is essential that the patient accept the referral and demonstrate a willingness to participate in the treatment, agreeing to the proposed terms.
The treatment takes place in a hospital environment, with an ambiance that aims to provide comfort and safety. Clinicians consider not only the substance dose (such as 0.5 mg/kg) but also the emotional state (“set”) and the treatment environment (“setting”). The experience is facilitated through psychological support for the patient during and after treatment.
According to Alessandro Campolina, MD, PhD, a researcher at the Center for Translational Oncology Research at Icesp, it is important to highlight that quality of life is intrinsically linked to the patient’s self-perception, including how they see themselves in terms of health and in the context in which they live.
The doctor explains that psychedelic interventions can provide a “window of opportunity,” allowing a qualified clinician to help the patient explore new perspectives based on their experiences.
“Often, although the intensity of pain remains the same, the way the patient perceives it can change significantly. For example, a patient may report that, despite the pain, they now feel less concerned about it because they were able to contemplate more significant aspects of their life,” said Dr. Campolina.
“This observation shows that treatment is not limited to addressing the pain or primary symptoms, but also addresses the associated suffering. While some patients have profound insights, many others experience more subtle changes that, under the guidance of a competent therapist, can turn into valuable clinical insights, thus improving quality of life and how they deal with their pathologies.”
Dr. Griffiths exemplified this in the interview with the Times when he reflected on his own cancer. He came to believe, as if guided an external observer, that “there is a meaning and a purpose in this [disease] that go beyond your understanding, and the way you are dealing with it is exactly how you should.”
Toshio Chiba, MD, chief physician of the Palliative Care Service at Icesp, emphasized that ketamine is already in use. “It is not feasible to wait years for the approval of psilocybin or for the FDA’s decision on MDMA, especially if the patient needs immediate care,” he said.
Furthermore, recreational and therapeutic uses are distinct. “It is essential to note that responsibilities are shared between the professional and the patient,” said Dr. Chiba. “In the therapeutic setting, there is an ethical and civil responsibility of the medical professional, as well as the patient actively engaging in treatment.”
Early palliative care can also facilitate the establishment of care goals. “I prefer to avoid terms like ‘coping’ or ‘fighting the disease,’” said Dr. Chiba. “Nowadays, dealing with cancer is more about coexisting with the disease properly, as treatments can last for years.
“Of course, there are still highly lethal tumors. However, for neoplasms like breast, colorectal, and prostate cancers, we often talk about 5, 10, or even 15 years of coexistence [with the condition]. The lack of this information [about the disease, treatments, and existential issues] can generate distress in some patients, who end up excessively worrying about the future,” he added.
But palliative treatment with psychedelics as a panacea, he said.
In addition, Marcelo Falchi, MD, medical director of CAMP at UFRN, also emphasized that psychedelics are not a risk-free intervention. Substances like LSD and psilocybin, for example, can cause increases in blood pressure and tachycardia, which, may limit their use for patients at high cardiovascular risk. Crises of anxiety or dissociative symptoms also may occur, and they require mitigation strategies such as psychological support and attention to set and setting.
“But research seems to agree that the risks can be managed effectively through a diligent process, allowing for the responsible exploration of the therapeutic potential of psychedelics,” said Dr. Falchi, who is responsible for CAMP’s postgraduate course in psychedelic therapies. The program provides training in substances used in Brazil, such as ketamine and ibogaine.
The use of psychedelics in palliative care requires a significant shift in how professionals relate to patients.
Unlike in traditional practice, where the prescription is followed by quick consultations, palliative care with psychedelics requires deep and continuous involvement, as Dr. Campolina pointed out. “We joke that it’s not a high-tech specialty, but ‘high touch,’ because it demands the constant presence of the doctor or therapist with the patient. This can involve sessions of several hours, with frequent monitoring and regular contact after sessions. This dynamic emphasizes the importance of human touch and connection during the process, reflecting a new way of practicing medicine.”
In his last months of life, Dr. Griffiths sought to emphasize this point, suggesting that, from a broader perspective, doctors and patients face the same fundamental questions. “We all know we are terminal,” he said. “Essentially, we shouldn’t need a stage 4 cancer diagnosis to awaken to this reality.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Palliative care has proven to be one of the most promising fields for research on interventions with psychedelic substances. One of the most prominent researchers in this area was the American psychopharmacologist Roland Griffiths, PhD.
In 2016, Dr. Griffiths and his team at Johns Hopkins University in Baltimore, Maryland, published one of the most relevant contributions to the field by demonstrating in a placebo-controlled study that psilocybin can reduce depressive and anxiety symptoms in patients with cancer. The study, conducted with 51 patients diagnosed with advanced-stage cancer, compared the effects of a low dose and a high dose of psilocybin, showing that the high dose resulted in improvements in mood, quality of life, and sense of life, reducing death-related anxiety.
In 2021, after a routine examination, Dr. Griffiths himself was diagnosed with advanced colon cancer. Unexpectedly, the researcher found himself in the position of his research subjects. In an interview with The New York Times in April 2023, he stated that, after some resistance, he agreed to undergo an LSD session.
In the conversation, he revealed that he had a 50% chance of being alive by Halloween. Despite the diagnosis, he showed no discouragement. “As a scientist, I feel like a kid in a candy store, considering all the research and questions that need to be answered about psychedelics and the theme of human flourishing,” he said.
In his last months of life, in the various appearances and interviews he gave, Dr. Griffiths demonstrated a perception of life uncommon in people facing death. “I’m excited to communicate, to shake off the dust and tell people: ‘Come on, wake up!’ ”
He passed away on October 16, 2023, at age 77 years, opening new horizons for clinical research with psychedelics and becoming an example of the therapeutic potential of these substances.
Innovative Treatments
“I believe this will be one of the next conditions, if not the next condition, to be considered for the designation of innovative treatment in future psilocybin regulation in the United States, where the field is more advanced,” said Lucas Maia, PhD, a psychopharmacologist and researcher affiliated with the Advanced Center for Psychedelic Medicine (CAMP) at the Federal University of Rio Grande do Norte (UFRN) and the Interdisciplinary Cooperation for Ayahuasca Research and Outreach (ICARO) at the State University of Campinas in São Paulo, Brazil.
Currently, MDMA (for the treatment of posttraumatic stress disorder), psilocybin (for depressive disorder), and MM120 (an LSD analogue used to treat generalized anxiety disorder) are the only psychedelic substances that have received the designation of innovative treatment by the Food and Drug Administration (FDA).
In 2022, Dr. Maia and a colleague from ICARO, Ana Cláudia Mesquita Garcia, PhD, a professor at the School of Nursing at the Federal University of Alfenas in Brazil and leader of the Interdisciplinary Center for Studies in Palliative Care, published a systematic review in the Journal of Pain and Symptom Management that evaluated the use of psychedelic-assisted treatments for symptom control in patients with serious or terminal illnesses.
Of the 20 articles reviewed, 9 (45%) used LSD, 5 (25%) psilocybin, 2 (10%) dipropyltryptamine (DPT), 1 (5%) used ketamine, and 1 (5%) used MDMA. In 10% of the studies, LSD and DPT were combined. Altogether, 347 participants (54%) received LSD, 116 (18%) psilocybin, 81 (13%) LSD and DPT, 64 (10%) DPT, 18 (3%) MDMA, and 14 (2%) ketamine.
The conclusion of the study is that psychedelics provide therapeutic effects on physical, psychological, social, and existential outcomes. They are associated with a reduction in pain and improvement in sleep. A decrease in depressive and anxiety symptoms is also observed; such symptoms are common in patients with serious diseases. In addition, interpersonal relationships become closer and more empathetic. Finally, there is a reduction in the fear of death and suffering, an increase in acceptance, and a redefinition of the disease.
In 55% of the studies, the adverse effects were mild to moderate and transient. They included nausea, vomiting, dry mouth, and fatigue, as well as anxiety, panic, and hallucinations. The researchers concluded that the scarcity and difficulty of access to professional training in psychedelic-assisted treatments represent a significant challenge for the advancement of these interventions, especially in countries in the Global South.
Another systematic review and meta-analysis published in July by researchers at the University of Michigan in Ann Arbor, Michigan, included seven studies with 132 participants and showed significant improvements in quality of life, pain control, and anxiety relief after psychedelic-assisted psychotherapy with psilocybin. The combined effects indicated statistically significant reductions in anxiety symptoms after 4.0-4.5 months and after 6.0-6.5 months post administration, compared with the initial evaluations.
One of the most advanced research studies currently being conducted is led by Stephen Ross, MD, a psychiatrist affiliated with New York University’s Langone Medical Center, New York City. The phase 2b clinical study is randomized, double blind, and placebo controlled, and involves 300 participants. The study aims to evaluate the effects of psilocybin-assisted psychotherapy on psychiatric and existential distress in patients with advanced cancer. Its expected completion date is in 2027.
“We still lack effective interventions in minimizing psychological, spiritual, and existential suffering,” said Dr. Garcia. “In this sense, respecting the contraindications of a physical nature (including pre-existing illnesses at study initiation, disease staging, patient functionality level, comorbidities, concurrent pharmacological treatments, etc) and of a psychiatric nature for the use of psychedelics, depending on the clinical picture, end-of-life patients facing existential crises and psychological suffering will likely benefit more from psychedelic-assisted psychotherapy, which highlights the need for more research and the integration of this treatment into clinical practice.”
Changing Perceptions
Since 2021, the Cancer Institute of the State of São Paulo (Icesp) has been providing palliative treatment with ketamine — an atypical psychedelic — following a rigorous and carefully monitored clinical protocol. The substance is already used off label to treat refractory depressive disorder. In addition, in 2020, Brazil’s National Health Surveillance Agency approved the use of Spravato, an intranasal antidepressant based on the ketamine derivative esketamine.
Icesp has hospice beds for clinical oncology patients, and a pain management team evaluates which patients meet the inclusion criteria for ketamine use. In addition to difficult-to-control pain, it is important that the patient present emotional, existential, or spiritual symptoms that amplify that pain.
After this evaluation, a psychoeducation process takes place, in which the patient receives clear information about the treatment, its potential benefits and risks, and understands how ketamine can be a viable option for managing their symptoms. Finally, it is essential that the patient accept the referral and demonstrate a willingness to participate in the treatment, agreeing to the proposed terms.
The treatment takes place in a hospital environment, with an ambiance that aims to provide comfort and safety. Clinicians consider not only the substance dose (such as 0.5 mg/kg) but also the emotional state (“set”) and the treatment environment (“setting”). The experience is facilitated through psychological support for the patient during and after treatment.
According to Alessandro Campolina, MD, PhD, a researcher at the Center for Translational Oncology Research at Icesp, it is important to highlight that quality of life is intrinsically linked to the patient’s self-perception, including how they see themselves in terms of health and in the context in which they live.
The doctor explains that psychedelic interventions can provide a “window of opportunity,” allowing a qualified clinician to help the patient explore new perspectives based on their experiences.
“Often, although the intensity of pain remains the same, the way the patient perceives it can change significantly. For example, a patient may report that, despite the pain, they now feel less concerned about it because they were able to contemplate more significant aspects of their life,” said Dr. Campolina.
“This observation shows that treatment is not limited to addressing the pain or primary symptoms, but also addresses the associated suffering. While some patients have profound insights, many others experience more subtle changes that, under the guidance of a competent therapist, can turn into valuable clinical insights, thus improving quality of life and how they deal with their pathologies.”
Dr. Griffiths exemplified this in the interview with the Times when he reflected on his own cancer. He came to believe, as if guided an external observer, that “there is a meaning and a purpose in this [disease] that go beyond your understanding, and the way you are dealing with it is exactly how you should.”
Toshio Chiba, MD, chief physician of the Palliative Care Service at Icesp, emphasized that ketamine is already in use. “It is not feasible to wait years for the approval of psilocybin or for the FDA’s decision on MDMA, especially if the patient needs immediate care,” he said.
Furthermore, recreational and therapeutic uses are distinct. “It is essential to note that responsibilities are shared between the professional and the patient,” said Dr. Chiba. “In the therapeutic setting, there is an ethical and civil responsibility of the medical professional, as well as the patient actively engaging in treatment.”
Early palliative care can also facilitate the establishment of care goals. “I prefer to avoid terms like ‘coping’ or ‘fighting the disease,’” said Dr. Chiba. “Nowadays, dealing with cancer is more about coexisting with the disease properly, as treatments can last for years.
“Of course, there are still highly lethal tumors. However, for neoplasms like breast, colorectal, and prostate cancers, we often talk about 5, 10, or even 15 years of coexistence [with the condition]. The lack of this information [about the disease, treatments, and existential issues] can generate distress in some patients, who end up excessively worrying about the future,” he added.
But palliative treatment with psychedelics as a panacea, he said.
In addition, Marcelo Falchi, MD, medical director of CAMP at UFRN, also emphasized that psychedelics are not a risk-free intervention. Substances like LSD and psilocybin, for example, can cause increases in blood pressure and tachycardia, which, may limit their use for patients at high cardiovascular risk. Crises of anxiety or dissociative symptoms also may occur, and they require mitigation strategies such as psychological support and attention to set and setting.
“But research seems to agree that the risks can be managed effectively through a diligent process, allowing for the responsible exploration of the therapeutic potential of psychedelics,” said Dr. Falchi, who is responsible for CAMP’s postgraduate course in psychedelic therapies. The program provides training in substances used in Brazil, such as ketamine and ibogaine.
The use of psychedelics in palliative care requires a significant shift in how professionals relate to patients.
Unlike in traditional practice, where the prescription is followed by quick consultations, palliative care with psychedelics requires deep and continuous involvement, as Dr. Campolina pointed out. “We joke that it’s not a high-tech specialty, but ‘high touch,’ because it demands the constant presence of the doctor or therapist with the patient. This can involve sessions of several hours, with frequent monitoring and regular contact after sessions. This dynamic emphasizes the importance of human touch and connection during the process, reflecting a new way of practicing medicine.”
In his last months of life, Dr. Griffiths sought to emphasize this point, suggesting that, from a broader perspective, doctors and patients face the same fundamental questions. “We all know we are terminal,” he said. “Essentially, we shouldn’t need a stage 4 cancer diagnosis to awaken to this reality.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
CRC Screening After 75: Is Shared Decision-Making Helpful?
TOPLINE:
Physician training in shared decision-making does not increase the proportion of older adults who receive their preferred colorectal cancer (CRC) screening approach, new research suggests.
METHODOLOGY:
- Recent guidelines recommend that shared decision-making be employed when considering whether to stop or continue with CRC screening in adults older than 75 years of age.
- The impact of shared decision-making training on CRC decisions was assessed in 59 physicians and 449 patients (mean age, 80 years) across 36 primary care clinics in Massachusetts and Maine.
- Physicians received shared decision-making training plus pre-visit electronic reminders to discuss CRC screening (intervention) or only the reminders (comparator).
- Shared decision-making training focused on three options: stopping screening, switching to less invasive stool-based testing, and continuing colonoscopy.
- The primary outcome was concordance between patients’ preferred screening method and the screening they actually received, assessed over 12 months through surveys and electronic health records.
TAKEAWAY:
- Stool-based tests were preferred by 35% of patients, colonoscopy by 25%, and no further screening by 21%, whereas 16% were unsure and 4% did not provide a clear preference and were excluded.
- One year after the index visit, 39% of intervention patients and 29% of comparator patients completed CRC screening, a nonsignificant difference.
- Approximately 51% of patients in the intervention group received their preferred screening approach, as did 46% in the comparator group, a difference that was not statistically significant (P = .47).
- Two subgroups in the intervention group were significantly more likely to receive their desired screening: patients with a strong intention to follow through with their preferred approach and those who had longer discussions (5+ minutes) with their physicians about CRC screening.
IN PRACTICE:
“Although the [shared decision-making] training intervention did not make a statistically significant improvement in concordance in this sample, future work to refine and evaluate clinical decision support (in the form of an electronic advisory or reminder), as well as focused [shared decision-making] skills training for [primary care physicians], may promote high-quality, preference-concordant decisions about CRC testing for older adults,” the authors concluded.
SOURCE:
The study, with first author Karen R. Sepucha, PhD, Massachusetts General Hospital, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The study may have been underpowered to detect small differences in concordance rates. The limited racial and ethnic diversity and the high education level of the population restrict the generalizability of these results. The COVID-19 pandemic may have affected the ability of patients to follow through with CRC screening, potentially biasing the results.
DISCLOSURES:
The study was funded by the Patient-Centered Outcomes Research Institute (PCORI). Several authors reported receiving grants from PCORI and other organizations.
A version of this article first appeared on Medscape.com.
TOPLINE:
Physician training in shared decision-making does not increase the proportion of older adults who receive their preferred colorectal cancer (CRC) screening approach, new research suggests.
METHODOLOGY:
- Recent guidelines recommend that shared decision-making be employed when considering whether to stop or continue with CRC screening in adults older than 75 years of age.
- The impact of shared decision-making training on CRC decisions was assessed in 59 physicians and 449 patients (mean age, 80 years) across 36 primary care clinics in Massachusetts and Maine.
- Physicians received shared decision-making training plus pre-visit electronic reminders to discuss CRC screening (intervention) or only the reminders (comparator).
- Shared decision-making training focused on three options: stopping screening, switching to less invasive stool-based testing, and continuing colonoscopy.
- The primary outcome was concordance between patients’ preferred screening method and the screening they actually received, assessed over 12 months through surveys and electronic health records.
TAKEAWAY:
- Stool-based tests were preferred by 35% of patients, colonoscopy by 25%, and no further screening by 21%, whereas 16% were unsure and 4% did not provide a clear preference and were excluded.
- One year after the index visit, 39% of intervention patients and 29% of comparator patients completed CRC screening, a nonsignificant difference.
- Approximately 51% of patients in the intervention group received their preferred screening approach, as did 46% in the comparator group, a difference that was not statistically significant (P = .47).
- Two subgroups in the intervention group were significantly more likely to receive their desired screening: patients with a strong intention to follow through with their preferred approach and those who had longer discussions (5+ minutes) with their physicians about CRC screening.
IN PRACTICE:
“Although the [shared decision-making] training intervention did not make a statistically significant improvement in concordance in this sample, future work to refine and evaluate clinical decision support (in the form of an electronic advisory or reminder), as well as focused [shared decision-making] skills training for [primary care physicians], may promote high-quality, preference-concordant decisions about CRC testing for older adults,” the authors concluded.
SOURCE:
The study, with first author Karen R. Sepucha, PhD, Massachusetts General Hospital, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The study may have been underpowered to detect small differences in concordance rates. The limited racial and ethnic diversity and the high education level of the population restrict the generalizability of these results. The COVID-19 pandemic may have affected the ability of patients to follow through with CRC screening, potentially biasing the results.
DISCLOSURES:
The study was funded by the Patient-Centered Outcomes Research Institute (PCORI). Several authors reported receiving grants from PCORI and other organizations.
A version of this article first appeared on Medscape.com.
TOPLINE:
Physician training in shared decision-making does not increase the proportion of older adults who receive their preferred colorectal cancer (CRC) screening approach, new research suggests.
METHODOLOGY:
- Recent guidelines recommend that shared decision-making be employed when considering whether to stop or continue with CRC screening in adults older than 75 years of age.
- The impact of shared decision-making training on CRC decisions was assessed in 59 physicians and 449 patients (mean age, 80 years) across 36 primary care clinics in Massachusetts and Maine.
- Physicians received shared decision-making training plus pre-visit electronic reminders to discuss CRC screening (intervention) or only the reminders (comparator).
- Shared decision-making training focused on three options: stopping screening, switching to less invasive stool-based testing, and continuing colonoscopy.
- The primary outcome was concordance between patients’ preferred screening method and the screening they actually received, assessed over 12 months through surveys and electronic health records.
TAKEAWAY:
- Stool-based tests were preferred by 35% of patients, colonoscopy by 25%, and no further screening by 21%, whereas 16% were unsure and 4% did not provide a clear preference and were excluded.
- One year after the index visit, 39% of intervention patients and 29% of comparator patients completed CRC screening, a nonsignificant difference.
- Approximately 51% of patients in the intervention group received their preferred screening approach, as did 46% in the comparator group, a difference that was not statistically significant (P = .47).
- Two subgroups in the intervention group were significantly more likely to receive their desired screening: patients with a strong intention to follow through with their preferred approach and those who had longer discussions (5+ minutes) with their physicians about CRC screening.
IN PRACTICE:
“Although the [shared decision-making] training intervention did not make a statistically significant improvement in concordance in this sample, future work to refine and evaluate clinical decision support (in the form of an electronic advisory or reminder), as well as focused [shared decision-making] skills training for [primary care physicians], may promote high-quality, preference-concordant decisions about CRC testing for older adults,” the authors concluded.
SOURCE:
The study, with first author Karen R. Sepucha, PhD, Massachusetts General Hospital, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The study may have been underpowered to detect small differences in concordance rates. The limited racial and ethnic diversity and the high education level of the population restrict the generalizability of these results. The COVID-19 pandemic may have affected the ability of patients to follow through with CRC screening, potentially biasing the results.
DISCLOSURES:
The study was funded by the Patient-Centered Outcomes Research Institute (PCORI). Several authors reported receiving grants from PCORI and other organizations.
A version of this article first appeared on Medscape.com.

