User login
Antibody Drug Conjugates: a growing field of targeted therapy for breast cancer
The landscape of breast cancer care and how we're working on pushing the targeted treatment movement forward is rapidly changing, especially with antibody drug conjugates (ADCs).
I like to think of ADCs as targeted missiles. They're essentially composed of antibodies against specific antigens, or targets of interest, and then they're combined with a linker to a chemotherapy payload. It's a way to deliver the chemotherapy in a more targeted manner than traditional chemotherapy, which is an exciting opportunity to allow us to target those patients who otherwise prefer agents that were more difficult to tolerate before this technology was invented.
This field has grown exponentially in the last 5 to 10 years and has presented multiple new opportunities for research. What is most exciting is that we have new targets for these treatments—new antigens that we can target with novel ADCs.
The NeoSTAR trial evaluated the ADC sacituzumab govitecan (SG), which is used for patients who have earlier stage triple negative breast cancer before surgery. The idea of this is to hopefully spare patients from many of the more toxic effects of traditional chemotherapy, while still providing them precision in terms of the treatment that we're targeting in the body.
I was involved in the ASCENT trial, which is also notable for precise treatment. It demonstrated the superiority of SG in metastatic triple negative breast cancer, and more recently the US Food and Drug Administration label has been expanded to the metastatic hormone-positive space as well. As we're developing these ADCs and broadening their use, we're able to reach larger patient populations. It's really exciting because we know there's such an appetite among our patients to use these agents, given how effective they can be and, in some situations, less toxic than the standard chemotherapy they would have otherwise gotten.
The other big category of trials involves another ADC called trastuzumab deruxtecan (TD). Trastuzumab is conjugated against HER2, a breast cancer specific agent, and is combined with the linker, deruxtecan—a very potent chemotherapy payload. TD was initially used in patients who had HER2-positive breast cancer. In fact, trastuzumab, the first half of the drug, was used as an antibody in and of itself in a lot of earlier stage and metastatic cancer for years. We've known about that for a long time. But more recently, with the series of DESTINY trials, we have seen the major impact that TD in HER2 can have compared to other chemotherapy agents and against other ADCs as well.
What was so exciting about the trials presented in 2022 is that they created a new category of patients called HER2-low. Before, we had always considered patients as HER2-positive or HER2-negative. We now know it's not that binary. We had already known by the way we do the pathology that people can have levels of HER2 expression. HER2-low patients are people who would have been considered HER2-negative before this—but have some HER2 expression. They have what we consider low on a scale of 0 to 3+, typically. Therefore, they're 1+ or 2+, not 0, and not 3+, because that would be considered HER2-positive. It's a little more complicated because when it's triple 2-positive, they could also do a back-up test, if a patient is positive on that, they are considered as HER2-positive.
There is now a new category of HER2-low patients who were also shown to have a tremendous improvement benefit with TD; this new category of patients could be candidates for this treatment, although they would never have been used for HER2 targeting before. These agents can be so effective that it's even causing us to rethink our classifications of disease.
Some of what we're working on right now is specifically looking at how patients are resistant to these agents; that's one of our major focuses as Mass General. We know these treatments are highly effective, but unfortunately, they don't last forever. Very few of these cancer treatments do, because as we know, cancer has this remarkable ability to evolve resistance to agents that we use. The amazing thing about ADCs is they've extended (in some cases) overall survival for patients, which is fantastic. But as I said, we know that they are not lasting forever. Part of what we're involved in with that clinical and research setting is to look at patients who've had success with these agents and ultimately progressed, and then figure out what changed before and after treatment (down to the single cell or genetic level) in order to continue expanding use of these treatments, extending use of the treatments, and making them more effective for more patients.
The landscape of breast cancer care and how we're working on pushing the targeted treatment movement forward is rapidly changing, especially with antibody drug conjugates (ADCs).
I like to think of ADCs as targeted missiles. They're essentially composed of antibodies against specific antigens, or targets of interest, and then they're combined with a linker to a chemotherapy payload. It's a way to deliver the chemotherapy in a more targeted manner than traditional chemotherapy, which is an exciting opportunity to allow us to target those patients who otherwise prefer agents that were more difficult to tolerate before this technology was invented.
This field has grown exponentially in the last 5 to 10 years and has presented multiple new opportunities for research. What is most exciting is that we have new targets for these treatments—new antigens that we can target with novel ADCs.
The NeoSTAR trial evaluated the ADC sacituzumab govitecan (SG), which is used for patients who have earlier stage triple negative breast cancer before surgery. The idea of this is to hopefully spare patients from many of the more toxic effects of traditional chemotherapy, while still providing them precision in terms of the treatment that we're targeting in the body.
I was involved in the ASCENT trial, which is also notable for precise treatment. It demonstrated the superiority of SG in metastatic triple negative breast cancer, and more recently the US Food and Drug Administration label has been expanded to the metastatic hormone-positive space as well. As we're developing these ADCs and broadening their use, we're able to reach larger patient populations. It's really exciting because we know there's such an appetite among our patients to use these agents, given how effective they can be and, in some situations, less toxic than the standard chemotherapy they would have otherwise gotten.
The other big category of trials involves another ADC called trastuzumab deruxtecan (TD). Trastuzumab is conjugated against HER2, a breast cancer specific agent, and is combined with the linker, deruxtecan—a very potent chemotherapy payload. TD was initially used in patients who had HER2-positive breast cancer. In fact, trastuzumab, the first half of the drug, was used as an antibody in and of itself in a lot of earlier stage and metastatic cancer for years. We've known about that for a long time. But more recently, with the series of DESTINY trials, we have seen the major impact that TD in HER2 can have compared to other chemotherapy agents and against other ADCs as well.
What was so exciting about the trials presented in 2022 is that they created a new category of patients called HER2-low. Before, we had always considered patients as HER2-positive or HER2-negative. We now know it's not that binary. We had already known by the way we do the pathology that people can have levels of HER2 expression. HER2-low patients are people who would have been considered HER2-negative before this—but have some HER2 expression. They have what we consider low on a scale of 0 to 3+, typically. Therefore, they're 1+ or 2+, not 0, and not 3+, because that would be considered HER2-positive. It's a little more complicated because when it's triple 2-positive, they could also do a back-up test, if a patient is positive on that, they are considered as HER2-positive.
There is now a new category of HER2-low patients who were also shown to have a tremendous improvement benefit with TD; this new category of patients could be candidates for this treatment, although they would never have been used for HER2 targeting before. These agents can be so effective that it's even causing us to rethink our classifications of disease.
Some of what we're working on right now is specifically looking at how patients are resistant to these agents; that's one of our major focuses as Mass General. We know these treatments are highly effective, but unfortunately, they don't last forever. Very few of these cancer treatments do, because as we know, cancer has this remarkable ability to evolve resistance to agents that we use. The amazing thing about ADCs is they've extended (in some cases) overall survival for patients, which is fantastic. But as I said, we know that they are not lasting forever. Part of what we're involved in with that clinical and research setting is to look at patients who've had success with these agents and ultimately progressed, and then figure out what changed before and after treatment (down to the single cell or genetic level) in order to continue expanding use of these treatments, extending use of the treatments, and making them more effective for more patients.
The landscape of breast cancer care and how we're working on pushing the targeted treatment movement forward is rapidly changing, especially with antibody drug conjugates (ADCs).
I like to think of ADCs as targeted missiles. They're essentially composed of antibodies against specific antigens, or targets of interest, and then they're combined with a linker to a chemotherapy payload. It's a way to deliver the chemotherapy in a more targeted manner than traditional chemotherapy, which is an exciting opportunity to allow us to target those patients who otherwise prefer agents that were more difficult to tolerate before this technology was invented.
This field has grown exponentially in the last 5 to 10 years and has presented multiple new opportunities for research. What is most exciting is that we have new targets for these treatments—new antigens that we can target with novel ADCs.
The NeoSTAR trial evaluated the ADC sacituzumab govitecan (SG), which is used for patients who have earlier stage triple negative breast cancer before surgery. The idea of this is to hopefully spare patients from many of the more toxic effects of traditional chemotherapy, while still providing them precision in terms of the treatment that we're targeting in the body.
I was involved in the ASCENT trial, which is also notable for precise treatment. It demonstrated the superiority of SG in metastatic triple negative breast cancer, and more recently the US Food and Drug Administration label has been expanded to the metastatic hormone-positive space as well. As we're developing these ADCs and broadening their use, we're able to reach larger patient populations. It's really exciting because we know there's such an appetite among our patients to use these agents, given how effective they can be and, in some situations, less toxic than the standard chemotherapy they would have otherwise gotten.
The other big category of trials involves another ADC called trastuzumab deruxtecan (TD). Trastuzumab is conjugated against HER2, a breast cancer specific agent, and is combined with the linker, deruxtecan—a very potent chemotherapy payload. TD was initially used in patients who had HER2-positive breast cancer. In fact, trastuzumab, the first half of the drug, was used as an antibody in and of itself in a lot of earlier stage and metastatic cancer for years. We've known about that for a long time. But more recently, with the series of DESTINY trials, we have seen the major impact that TD in HER2 can have compared to other chemotherapy agents and against other ADCs as well.
What was so exciting about the trials presented in 2022 is that they created a new category of patients called HER2-low. Before, we had always considered patients as HER2-positive or HER2-negative. We now know it's not that binary. We had already known by the way we do the pathology that people can have levels of HER2 expression. HER2-low patients are people who would have been considered HER2-negative before this—but have some HER2 expression. They have what we consider low on a scale of 0 to 3+, typically. Therefore, they're 1+ or 2+, not 0, and not 3+, because that would be considered HER2-positive. It's a little more complicated because when it's triple 2-positive, they could also do a back-up test, if a patient is positive on that, they are considered as HER2-positive.
There is now a new category of HER2-low patients who were also shown to have a tremendous improvement benefit with TD; this new category of patients could be candidates for this treatment, although they would never have been used for HER2 targeting before. These agents can be so effective that it's even causing us to rethink our classifications of disease.
Some of what we're working on right now is specifically looking at how patients are resistant to these agents; that's one of our major focuses as Mass General. We know these treatments are highly effective, but unfortunately, they don't last forever. Very few of these cancer treatments do, because as we know, cancer has this remarkable ability to evolve resistance to agents that we use. The amazing thing about ADCs is they've extended (in some cases) overall survival for patients, which is fantastic. But as I said, we know that they are not lasting forever. Part of what we're involved in with that clinical and research setting is to look at patients who've had success with these agents and ultimately progressed, and then figure out what changed before and after treatment (down to the single cell or genetic level) in order to continue expanding use of these treatments, extending use of the treatments, and making them more effective for more patients.
Why is buprenorphine use flatlining?
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths are at a record high in the United States, and many of these deaths can be prevented with medications such as buprenorphine, said lead author Kao-Ping Chua, MD, of the University of Michigan, Ann Arbor, in an interview. “However, buprenorphine cannot prevent opioid overdose deaths if patients are never started on the medication or only stay on the medication for a short time. For that reason, rates of buprenorphine initiation and retention are critical metrics for measuring how well the U.S. health care system is responding to the opioid epidemic,” he said.
“At the time we started our study, several other research groups had evaluated U.S. rates of buprenorphine initiation and retention using data through 2020. However, more recent national data were lacking,” Dr. Chua told this news organization. “We felt that this was an important knowledge gap given the many changes in society that have occurred since 2020,” he noted. “For example, it was possible that the relaxation of social distancing measures during 2021 and 2022 might have reduced barriers to health care visits, thereby increasing opportunities to initiate treatment for opioid addiction with buprenorphine,” he said.
Dr. Chua and colleagues used data from the IQVIA Longitudinal Prescription Database, which reports 92% of prescriptions dispensed from retail pharmacies in the United States. “Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain,” they write.
Monthly buprenorphine initiation was defined as the number of patients initiating therapy per 100,000 individuals. For retention, the researchers used a National Quality Forum-endorsed quality measure that defined retention as continuous use of buprenorphine for at least 180 days.
A total of 3,006,629 patients began buprenorphine therapy during the study period; approximately 43% were female.
During the first years of the study period, from January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 per 100,000 to 15.9 per 100,000, with a statistically significant monthly percentage change of 0.62% (P < .001).
However, from October 2018 through October 2022, the monthly percentage remained essentially the same (P = .62) with a monthly percentage change of −0.03%.
From March 2020 through December 2020, the median monthly buprenorphine initiation rate was 14.4 per 100,000, only slightly lower than the rates from January 2019 through February 2020 and from January 2021 through October 2022 (15.5 per 100,000 and 15.0 per 100,000, respectively).
Over the entire study period from January 2016 through October 2022, the median monthly retention rate for buprenorphine use was 22.2%. This rate increased minimally, with no significant changes in slope and a monthly percentage change of 0.08% (P = .04).
The study findings were limited by several factors, including a lack of data on race and ethnicity, in-clinic administration of buprenorphine, and buprenorphine dispensing through methadone outpatient programs, the researchers note. Also, data did not indicate whether some patients began buprenorphine to treat pain, they say. The timing of the flattening of buprenorphine use also suggests the influence of factors beyond the COVID-19 pandemic, they write.
However, the results were strengthened by the large sample size and suggest that efforts to date to increase buprenorphine use have been unsuccessful, the researchers write. “A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers,” they conclude.
Study highlights underuse of buprenorphine option
“Our study shows that buprenorphine initiation rates have been flat since the end of 2018 and that rates of 180-day retention in buprenorphine therapy have remained low throughout 2016-2022,” Dr. Chua told this news organization. “Neither of these findings are particularly surprising, but they are disappointing,” he said. “There were a lot of policy and clinical efforts to maintain and expand access to buprenorphine during the COVID-19 pandemic, such as allowing buprenorphine to be prescribed via telehealth without an in-person visit and eliminating training requirements for the waiver that previously was required to prescribe buprenorphine.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that they were insufficient to meet the rising need for this medication,” he said.
The current study “adds to a growing body of research suggesting that clinicians are not maximizing opportunities to initiate buprenorphine treatment among patients with opioid addiction,” Dr. Chua said. He cited another of his recent studies in which 1 in 12 patients were prescribed buprenorphine within 30 days of an emergency department visit for opioid overdose from August 2019 to April 2021, but half of patients with emergency department visits with anaphylaxis were prescribed anepinephrine auto-injector.
“My hope is that our new study will further underscore to clinicians how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” he said.
The federal government’s recent elimination of the waiver needed to prescribe buprenorphine may move the needle, but to what degree remains to be seen, Dr. Chua added. “It is possible this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients, and pharmacists,” he said.
Lack of education remains a barrier to buprenorphine use
The current study is important to determine whether attempts to increase buprenorphine initiation and treatment retention are working, said Reuben J. Strayer, MD, director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York, in an interview.
Dr. Strayer was not involved in the current study, but said he was surprised that initiation of buprenorphine didn’t decrease more dramatically during the pandemic, given the significant barriers to accessing care during that time.
However, “efforts to increase buprenorphine initiation and retention have not been sufficiently effective,” Dr. Strayer said. “The rise of fentanyl as a primary street opioid, replacing heroin, has dissuaded both patients and providers from initiating buprenorphine for fear of precipitated withdrawal.”
The elimination of the DATA 2000 (X) waiver was the removal of a potential barrier to increased buprenorphine use, said Dr. Strayer. “Now that the DATA 2000 (X) waiver has been eliminated, the focus of buprenorphine access is educating primary care and inpatient providers on its use, so that patients with OUD [opioid use disorder] can be treated, regardless of the venue at which they seek care,” he said.
Looking ahead, “The priority in buprenorphine research is determining the most effective way to initiate buprenorphine without the risk of precipitated withdrawal,” Dr. Strayer added.
The study was supported in part by the Benter Foundation, the Michigan Department of Health and Human Services, and the Susan B. Meister Child Health Evaluation and Research Center in the department of pediatrics at the University of Michigan. Dr. Chua was supported by the National Institute on Drug Abuse. Dr. Strayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Obesity Management in Youth-Onset Type 2 Diabetes
Newly diagnosed type 2 diabetes has steadily risen by 4%-5% annually in the United States over the past 20 years; However, during the first year of the pandemic, the Journal of Pediatrics reported a possible 77% increase in youth-reported cases. Coupled with nearly 1 in 3 children being overweight, treatment for this patient population has recently evolved.
Dr Amy S. Shah of the Cincinnati Children's Hospital Medical Center reports on the current therapeutic and lifestyle modifications guidelines for this patient population, including the consideration of prescribing glucagon-like peptide 1 receptor agonists such as liraglutide, dulaglutide, and exenatide.
--
Amy S. Shah, MD, MS, Professor of Pediatrics, Division of Endocrinology, Director of the Adolescents Type 2 Diabetes Program, Cincinnati Children's Hospital Medical Center, The University of Cincinnati, Cincinnati, Ohio
Amy S. Shah, MD, MS, has disclosed the following relevant financial relationships:
Received research grant from: National Institutes of Health
Received income in an amount equal to or greater than $250 from: Journal of the American Medical Association (Associate Editor)
Newly diagnosed type 2 diabetes has steadily risen by 4%-5% annually in the United States over the past 20 years; However, during the first year of the pandemic, the Journal of Pediatrics reported a possible 77% increase in youth-reported cases. Coupled with nearly 1 in 3 children being overweight, treatment for this patient population has recently evolved.
Dr Amy S. Shah of the Cincinnati Children's Hospital Medical Center reports on the current therapeutic and lifestyle modifications guidelines for this patient population, including the consideration of prescribing glucagon-like peptide 1 receptor agonists such as liraglutide, dulaglutide, and exenatide.
--
Amy S. Shah, MD, MS, Professor of Pediatrics, Division of Endocrinology, Director of the Adolescents Type 2 Diabetes Program, Cincinnati Children's Hospital Medical Center, The University of Cincinnati, Cincinnati, Ohio
Amy S. Shah, MD, MS, has disclosed the following relevant financial relationships:
Received research grant from: National Institutes of Health
Received income in an amount equal to or greater than $250 from: Journal of the American Medical Association (Associate Editor)
Newly diagnosed type 2 diabetes has steadily risen by 4%-5% annually in the United States over the past 20 years; However, during the first year of the pandemic, the Journal of Pediatrics reported a possible 77% increase in youth-reported cases. Coupled with nearly 1 in 3 children being overweight, treatment for this patient population has recently evolved.
Dr Amy S. Shah of the Cincinnati Children's Hospital Medical Center reports on the current therapeutic and lifestyle modifications guidelines for this patient population, including the consideration of prescribing glucagon-like peptide 1 receptor agonists such as liraglutide, dulaglutide, and exenatide.
--
Amy S. Shah, MD, MS, Professor of Pediatrics, Division of Endocrinology, Director of the Adolescents Type 2 Diabetes Program, Cincinnati Children's Hospital Medical Center, The University of Cincinnati, Cincinnati, Ohio
Amy S. Shah, MD, MS, has disclosed the following relevant financial relationships:
Received research grant from: National Institutes of Health
Received income in an amount equal to or greater than $250 from: Journal of the American Medical Association (Associate Editor)

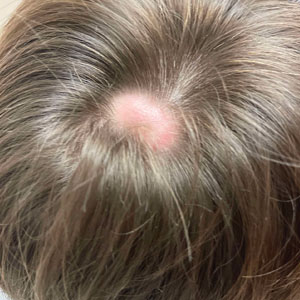
Scalp Nodule Associated With Hair Loss
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
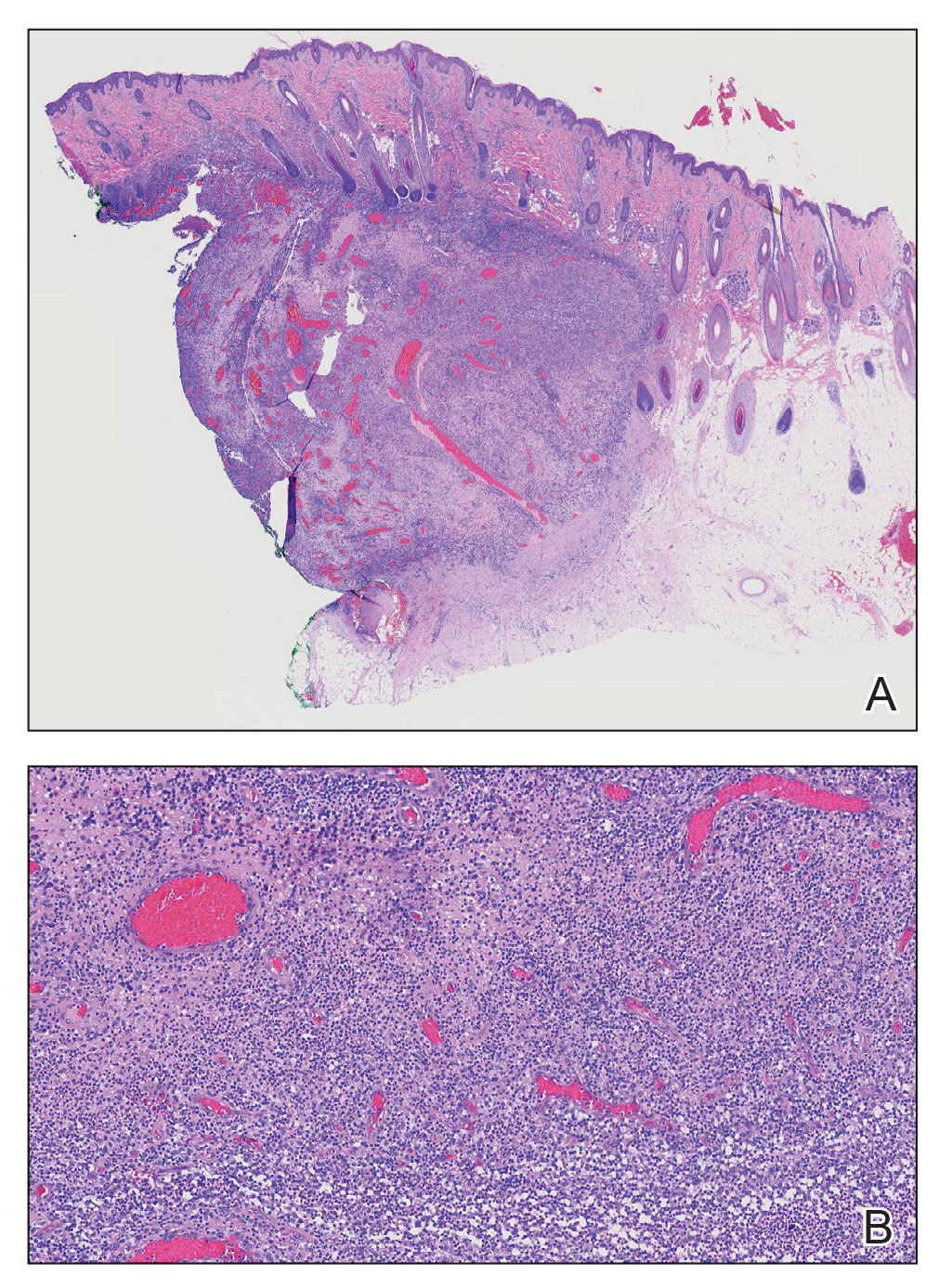
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1
Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
The Diagnosis: Alopecic and Aseptic Nodule of the Scalp
Alopecic and aseptic nodule of the scalp (AANS) is an underdiagnosed condition presenting with one or few inflammatory nodules on the scalp with overlying nonscarring alopecia. The nodules can be soft, fluctuant, or firm and are characterized by negative fungal and bacterial stains as well as cultures.1 Trichoscopic features such as black or yellow dots, fine vellus hairs, and broken hairs have been reported.1-3 Dilated follicular openings may be seen and are termed the Eastern pancake sign, as they resemble the bubble cavities formed during the cooking of atayef.2 The histologic features of AANS often are nonspecific but show a nodular or pseudocystic, lymphohistiocytic to acute inflammatory component centered in the dermis.1 Granulomatous inflammation or isolated giant cells have been reported within the deep dermis.1,4 In our patient, histopathology revealed admixed acute and granulomatous inflammation within the deep dermis (Figure). Treatment of AANS includes oral antibiotics such as doxycycline, intralesional corticosteroids, or excision.1

Although the etiology of AANS currently is unclear, a process of follicular plugging or a deep folliculitis sparing the bulge stem cells has been theorized. Young males are disproportionately affected.1 It is uncertain how much overlap there is, if any, between AANS and pseudocyst of the scalp, the latter of which primarily is reported in the Japanese literature and demonstrates alopecic nodules between the forehead and vertex of the scalp with pseudocystic architecture and granulomatous infiltration on histopathology.4-7
There are several clinical and histologic differences between AANS and other diagnoses in the differential. Dermoid cysts tend to present at birth, with 70% of cases presenting before the age of 6 years, and without overlying skin changes.8 They represent a benign entrapment of ectoderm along embryonic closure lines during development.9 Histologic examination typically will show a squamous-lined cyst within the dermis with associated adnexal structures.10 Cylindromas are benign neoplasms of eccrine sweat glands named after the histologic presentation of cylinder-shaped basaloid cell populations when cross-sectioned.11,12 When cylindromas coalesce on the scalp, they form a distinctive morphology sometimes loosely resembling a turban, giving them the previously more common name turban tumors.11,13 Cylindromas appear as slow-growing protuberant tumors that are erythematous or flesh colored. Cylindromas are 9 times more common in females.13 Pilar cysts have a stratified squamous epithelium lining with a palisaded outer layer and are derived from the outer root sheath of hair follicles.14 Clinically, pilar cysts are smooth mobile cysts that favor skin with a dense concentration of hair follicles.14,15 On palpation, pilar cysts are firm due to their keratinous contents and typically are nontender unless inflamed.15 Lipomas are benign mesenchymal tumors with mature adipocytes that often appear as subcutaneous nodules without overlying skin changes, though they can involve deep fascia. On palpation, lipomas generally are soft, mobile, and nontender.16
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
- Bellinato F, Maurelli M, Colato C, et al. Alopecic and aseptic nodules of the scalp: a new case with a systematic review of the literature [published online May 1, 2021]. Clin Case Rep. 2021;9:E04153. doi:10.1002/ccr3.4153
- Lázaro-Simó AI, Sancho MI, Quintana-Codina M, et al. Alopecic and aseptic nodules of the scalp with trichoscopic and ultrasonographic findings. Indian J Dermatol. 2017;62:515-518.
- Garrido-Colmenero C, Arias-Santiago S, Aneiros Fernández J, et al. Trichoscopy and ultrasonography features of aseptic and alopecic nodules of the scalp. J Eur Acad Dermatol Venereol. 2016;30:507-509. doi:10.1111/jdv.12903
- Seol JE, Park IH, Kim DH, et al. Alopecic and aseptic nodules of the scalp/pseudocyst of the scalp: clinicopathological and therapeutic analyses in 11 Korean patients. Dermatology. 2016;232:165-170.
- Lee SS, Kim SY, Im M, et al. Pseudocyst of the scalp. Ann Dermatol. 2011;23(suppl 2):S267-S269.
- Eisenberg EL. Alopecia-associated pseudocyst of the scalp. J Am Acad Dermatol. 2012;67:E114-E116.
- Tsuruta D, Hayashi A, Kobayashi H, et al. Pseudocyst of the scalp. Dermatology. 2005;210:333-335.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Julapalli MR, Cohen BA, Hollier LH, et al. Congenital, ill-defined, yellowish plaque: the nasal dermoid. Pediatr Dermatol. 2006;23:556-559.
- Reissis D, Pfaff MJ, Patel A, et al. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. 2014;87:349-357.
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61.
- Albores-Saavedra J, Heard SC, McLaren B, et al. Cylindroma (dermal analog tumor) of the breast: a comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. Am J Clin Pathol. 2005;123:866-873.
- Myers DJ, Fillman EP. Cylindroma. StatPearls. StatPearls Publishing; 2022.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128. doi:10.4103/1947-2714.107532
- Al Aboud DM, Yarrarapu SNS, Patel BC. Pilar cyst. StatPearls. StatPearls Publishing; 2022. 16. Kolb L, Yarrarapu SNS, Ameer MA, et al. Lipoma. StatPearls. StatPearls Publishing; 2022.
A 9-year-old boy presented with a soft subcutaneous nodule with overlying alopecia on the right parietal scalp of 5 months’ duration that had grown in size, became increasingly alopecic, and was complicated by intermittent pain. An excisional biopsy of the nodule revealed deep dermal mixed inflammation with scattered granulomas. No foreign material, definitive cystic spaces, or cyst wall lining was identified. Special stains including periodic acid– Schiff, Fite acid-fast, and Twort Gram were negative for infectious organisms. His postoperative course was uneventful, and no recurrence of the nodule was reported.

Lose weight, gain huge debt: N.Y. provider has sued more than 300 patients who had bariatric surgery
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Seven months after Lahavah Wallace’s weight-loss operation, a New York bariatric surgery practice sued her, accusing her of “intentionally” failing to pay nearly $18,000 of her bill.
Long Island Minimally Invasive Surgery, which does business as the New York Bariatric Group, went on to accuse Ms. Wallace of “embezzlement,” alleging she kept insurance payments that should have been turned over to the practice.
Ms. Wallace denies the allegations, which the bariatric practice has leveled against patients in hundreds of debt-collection lawsuits filed over the past 4 years, court records in New York state show.
In about 60 cases, the lawsuits demanded $100,000 or more from patients. Some patients were found liable for tens of thousands of dollars in interest charges or wound up shackled with debt that could take a decade or more to shake. Others are facing the likely prospect of six-figure financial penalties, court records show.
Backed by a major private equity firm, the bariatric practice spends millions each year on advertisements featuring patients who have dropped 100 pounds or more after bariatric procedures, sometimes having had a portion of their stomachs removed. The ads have run on TV, online, and on New York City subway posters.
The online ads, often showcasing the slogan “Stop obesity for life,” appealed to Ms. Wallace, who lives in Brooklyn and works as a legal assistant for the state of New York. She said she turned over checks from her insurer to the bariatric group and was stunned when the medical practice hauled her into court citing an “out-of-network payment agreement” she had signed before her surgery.
“I really didn’t know what I was signing,” Ms. Wallace told KFF Health News. “I didn’t pay enough attention.”
Shawn Garber, MD, a bariatric surgeon who founded the practice in 2000 on Long Island and serves as its CEO, said that “prior to rendering services” his office staff advises patients of the costs and their responsibility to pay the bill.
The bariatric group has cited these out-of-network payment agreements in at least 300 lawsuits filed against patients from January 2019 to 2022 demanding nearly $19 million to cover medical bills, interest charges, and attorney’s fees, a KFF Health News review of New York state court records found.
Danny De Voe, a partner at Sahn Ward Braff Koblenz law firm in Uniondale, N.Y., who filed many of those suits, declined to comment, citing attorney-client privilege.
In most cases, the medical practice had agreed to accept an insurance company’s out-of-network rate as full payment for its services – with caveats, according to court filings.
In the agreements they signed, patients promised to pay any coinsurance, meeting any deductible, and pass on to the medical practice any reimbursement checks they received from their health plans within 7 days.
KFF Health News found – while legal fees and other costs can layer on thousands more.
Elisabeth Benjamin, a lawyer with the Community Service Society of New York, said conflicts can arise when insurers send checks to pay for out-of-network medical services to patients rather than reimbursing a medical provider directly.
“We would prefer to see regulators step in and stop that practice,” she said, adding it “causes tension between providers and patients.”
That’s certainly true for Ms. Wallace. The surgery practice sued her in August 2022demanding $17,981 in fees it said remained unpaid after her January 2022 laparoscopic sleeve gastrectomy, an operation in which much of the stomach is removed to assist weight loss.
The lawsuit also tacked on a demand for $5,993 in attorney’s fees, court records show.
The suit alleges Ms. Wallace signed the contract even though she “had no intention” of paying her bills. The complaint goes on to accuse her of “committing embezzlement” by “willfully, intentionally, deliberately and maliciously” depositing checks from her health plan into her personal account.
The suit doesn’t include details to substantiate these claims, and Ms. Wallace said in her court response they are not true. Ms. Wallace said she turned over checks for the charges.
“They billed the insurance for everything they possibly could,” Ms. Wallace said.
In September, Ms. Wallace filed for bankruptcy, hoping to discharge the bariatric care debt along with about $4,700 in unrelated credit card charges.
The medical practice fired back in November by filing an “adversary complaint” in her Brooklyn bankruptcy court proceeding that argues her medical debt should not be forgiven because Ms. Wallace committed fraud.
The adversary complaint, which is pending in the bankruptcy case, accuses Ms. Wallace of “fraudulently” inducing the surgery center to perform “elective medical procedures” without requiring payment up front.
Both the harsh wording and claims of wrongdoing have infuriated Ms. Wallace and her attorney, Jacob Silver, of Brooklyn.
Mr. Silver wants the medical practice to turn over records of the payments received from Ms. Wallace. “There is no fraud here,” he said. “This is frivolous. We are taking a no-settlement position.”
Gaining debt
Few patients sued by the bariatric practice mount a defense in court and those who do fight often lose, court records show.
The medical practice won default judgments totaling nearly $6 million in about 90 of the 300 cases in the sample reviewed by KFF Health News. Default judgments are entered when the defendant fails to respond.
Many cases either are pending, or it is not clear from court filings how they were resolved.
Some patients tried to argue that the fees were too high or that they didn’t understand going in how much they could owe. One woman, trying to push back against a demand for more than $100,000, said in a legal filing that she “was given numerous papers to sign without anyone of the staff members explaining to me what it actually meant.” Another patient, who was sued for more than $40,000, wrote: “I don’t have the means to pay this bill.”
Among the cases described in court records:
- A Westchester County, N.Y., woman was sued for $102,556 and settled for $72,000 in May 2021. She agreed to pay $7,500 upon signing the settlement and $500 a month from September 2021 to May 2032.
- A Peekskill, N.Y., woman in a December 2019 judgment was held liable for $384,092, which included $94,047 in interest.
- A Newburgh, N.Y., man was sued in 2021 for $252,309 in medical bills, 12% interest, and $84,103 in attorneys’ fees. The case is pending.
Robert Cohen, a longtime attorney for the bariatric practice, testified in a November 2021 hearing that the lawyers take “a contingency fee of one-third of our recovery” in these cases. In that case, Mr. Cohen had requested $13,578 based on his contingency fee arrangement. He testified that he spent 7.3 hours on the case and that his customary billing rate was $475 per hour, which came to $3,467.50. The judge awarded the lower amount, according to a transcript of the hearing.
Teresa LaMasters, MD, president of the American Society for Metabolic and Bariatric Surgery, said suing patients for large sums “is not a common practice” among bariatric surgeons.
“This is not what the vast majority in the field would espouse,” she said.
But Dr. Garber, the NYBG’s chief executive, suggested patients deserve blame.
“These lawsuits stem from these patients stealing the insurance money rather than forwarding it onto NYBG as they are morally and contractually obligated to do,” Dr. Garber wrote in an email to KFF Health News.
Dr. Garber added: “The issue is not with what we bill, but rather with the fact that the insurance companies refuse to send payment directly to us.”
‘A kooky system’
Defense attorneys argue that many patients don’t fully comprehend the perils of failing to pay on time – for whatever reason.
In a few cases, patients admitted pocketing checks they were obligated to turn over to the medical practice. But for the most part, court records don’t specify how many such checks were issued and for what amounts – or whether the patient improperly cashed them.
“It’s a kooky system,” said Paul Brite, an attorney who has faced off against the bariatric practice in court.
“You sign these documents that could cost you tons of money. It shouldn’t be that way,” he said. “This can ruin their financial life.”
New York lawmakers have acted to limit the damage from medical debt, including “surprise bills.”
In November, Democratic Gov. Kathy Hochul signed legislation that prohibits health care providers from slapping liens on a primary residence or garnishing wages.
But contracts with onerous repayment terms represent an “evolving area of law” and an alarming “new twist” on concerns over medical debt, said Ms. Benjamin, the community service society lawyer.
She said contract “accelerator clauses” that trigger severe penalties if patients miss payments should not be permitted for medical debt.
“If you default, the full amount is due,” she said. “This is really a bummer.”
‘Fair market value’
The debt collection lawsuits argue that weight-loss patients had agreed to pay “fair market value” for services – and the doctors are only trying to secure money they are due.
But some prices far exceed typical insurance payments for obesity treatments across the country, according to a medical billing data registry. Surgeons performed about 200,000 bariatric operations in 2020, according to the bariatric surgery society.
Ms. Wallace, the Brooklyn legal assistant, was billed $60,500 for her lap sleeve gastrectomy, though how much her insurance actually paid remains to be hashed out in court.
Michael Arrigo, a California medical billing expert at No World Borders, called the prices “outrageous” and “unreasonable and, in fact, likely unconscionable.”
“I disagree that these are fair market charges,” he said.
Dr. LaMasters called the gastrectomy price billed to Ms. Wallace “really expensive” and “a severe outlier.” While charges vary by region, she quoted a typical price of around $22,000.
Dr. Garber said NYBG “bills at usual and customary rates” determined by Fair Health, a New York City-based repository of insurance claims data. Fair Health “sets these rates based upon the acceptable price for our geographic location,” he said.
But Rachel Kent, Fair Health’s senior director of marketing, told KFF Health News that the group “does not set rates, nor determine or take any position on what constitutes ‘usual and customary rates.’ ” Instead, it reports the prices providers are charging in a given area.
Overall, Fair Health data shows huge price variations even in adjacent ZIP codes in the metro area. In Long Island’s Roslyn Heights neighborhood, where NYBG is based, Fair Health lists the out-of-network price charged by providers in the area as $60,500, the figure Ms. Wallace was billed.
But in several other New York City–area ZIP codes the price charged for the gastrectomy procedure hovers around $20,000, according to the data bank. The price in Manhattan is $17,500, for instance, according to Fair Health.
Nationwide, the average cost in 2021 for bariatric surgery done in a hospital was $32,868, according to a KFF analysis of health insurance claims.
Private equity arrives
Dr. Garber said in a court affidavit in May 2022 that he founded the bariatric practice “with a singular focus: providing safe, effective care to patients suffering from obesity and its resulting complications.”
Under his leadership, the practice has “developed into New York’s elite institution for obesity treatment,” Dr. Garber said. He said the group’s surgeons are “highly sought after to train other bariatric surgeons throughout the country and are active in the development of new, cutting-edge bariatric surgery techniques.”
In 2017, Dr. Garber and partners agreed on a business plan to help spur growth and “attract private equity investment,” according to the affidavit.
They formed a separate company to handle the bariatric practice’s business side. Known as management services organizations, such companies provide a way for private equity investors to circumvent laws in some states that prohibit nonphysicians from owning a stake in a medical practice.
In August 2019, the private equity firm Sentinel Capital Partners bought 65% of the MSO for $156.5 million, according to Dr. Garber’s affidavit. The management company is now known as New You Bariatric Group. The private equity firm did not respond to requests for comment.
Dr. Garber, in a September 2021 American Society for Metabolic and Bariatric Surgery webinar viewable online, said the weight-loss practice spends $6 million a year on media and marketing directly to patients – and is on a roll. Nationally, bariatric surgery is growing 6% annually, he said. NYBG boasts two dozen offices in the tri-state area of New York, New Jersey, and Connecticut and is poised to expand into more states.
“Since private equity, we’ve been growing at 30%-40% year over year,” Dr. Garber said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
UTI imaging falls short in some primary care settings
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
WASHINGTON –
“Timely imaging is recommended after febrile UTI (fUTI) in young children to identify treatable urologic conditions,” wrote Jonathan Hatoun, MD, of Boston Children’s Hospital, and colleagues in a poster presented at the Pediatric Academic Societies annual meeting.
The American Academy of Pediatrics (AAP) currently recommends renal-bladder ultrasound (RBUS) after fUTI with voiding cystourethrogram (VCUG) after abnormal RBUS or second fUTI, but data on clinician adherence to these recommendations are limited, the researchers said.
To characterize practice patterns regarding fUTI, the researchers reviewed data from children younger than 24 months of age with fUTI who were treated at a primary care network in Massachusetts in 2019. The definition of fUTI was temperature of 38° C or higher, positive urinalysis, and more than 50,000 CFU on urine culture. The median age of the patients was 9 months; 84% were female.
In a multivariate analysis, post-UTI imaging followed the AAP guidelines in 82 cases (69.5%). The main reasons for nonadherence were lack of RBUS in 21 patients, VCUG despite normal RBUS in 9 patients, no VCUG after abnormal RBUS in 4 patients, and no VCUG after a second fUTI in 2 patients.
Overall, nonadherence was a result of not ordering a recommended study in 23% of cases (errors of omission) and ordering an unnecessary study in 8% of cases (errors of commission).
Commercial insurance, larger number of providers in practice, and younger provider age were significant independent predictors of adherence (odds ratios 2.82, 1.38, and 0.96, respectively).
The findings were limited by the use of data from a single center; however, the results suggest that targeted training may improve guideline adherence, the researchers wrote. Additional research and quality improvement studies are needed to understand and address the impact of insurance on guideline adherence for imaging after febrile UTIs, they noted.
Provider education is essential to continued quality of care
When it comes to febrile UTIs, “it is important to stay focused on the quality of care being provided, as opposed to the usual benchmark of quantity of care,” Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, said in an interview.
“This is a very simple but interesting study on provider compliance with practice guidelines,” said Dr. Joos, who was not involved in the study. “I was surprised that the providers did so well in ordering the correct imaging in 70% of the cases,” he said.
Of particular interest, Dr. Joos noted, was that “the authors also showed that older providers and those working in smaller practices are less likely to comply with this particular imaging guideline. This can be summed up as the ‘I didn’t know the guideline’ effect.”
To improve quality of care, “more research and effort should be directed at updating providers when strong new evidence changes previous practices and guidelines,” Dr. Joos told this news organization.
The study received no outside funding. The researchers and Dr. Joos had no financial conflicts to disclose.
AT PAS 2023
Two Canadian provinces lift licensing barriers for U.S. doctors
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
Some decisions aren’t right or wrong; they’re just devastating
There is one situation, while not common, that is often among the most difficult for me: the person who must be told at diagnosis that they are already dying. I am still reminded of a patient I saw early in my career.
A woman in her 40s was admitted to the hospital complaining of severe shortness of breath. In retrospect, she had been sick for months. She had not sought help because she was young and thought it would pass – the results of a “bad bug” that she just couldn’t shake.
But in the past few weeks, the persistence of symptoms became associated with weight loss, profound fatigue, loss of appetite, and nausea.
By the time she was hospitalized she was emaciated, though she appeared pregnant – a sign of the fluid that had built up in her abdomen. Imaging showed that her abdomen was filled with disease (carcinomatosis) and her liver and lungs were nearly replaced with metastatic disease.
A biopsy revealed an aggressive cancer that had no identifying histologic marker: carcinoma, not otherwise specified, or cancer of unknown primary.
I still remember seeing her. She had a deer-in-headlights stare that held me as I approached. I introduced myself and sat down so we were eye to eye.
“Tell me what you know,” I said.
“I know I have cancer and they don’t know where it started. I know surgery is not an option and that’s why they’ve asked you to come. Whatever. I’m ready. I want to fight this because I know I can beat it,” she said.
I remember that she looked very sick; her thin face and arms contrasted with her large, distended abdomen. Her breathing was labored, her skin almost gray. For a moment I didn’t know what to say.
As doctors, we like to believe that our decisions are guided by data: the randomized trials and meta-analyses that set standards of care; phase 2 trials that establish evidence (or lack thereof) of activity; case-control studies that suggest the impacts of treatment; and at the very least, case studies that document that “N of 1” experience. We have expert panels and pathways that lay out what treatments we should be using to help ensure access to quality care in every clinic on every corner of every cancer center in the United States.
These data and pathways tell us objectively what we can expect from therapy, who is at most risk for toxicities, and profiles of patients for whom treatment is not likely to be of benefit. In an ideal world, this objectivity would help us help people decide on an approach. But life is not objective, and sometimes individualizing care is as important as data.
In this scenario, I knew only one thing: She was dying. She had an overwhelming tumor burden. But I still asked myself a question that many in, and outside of, oncology ask themselves: Could she be saved?
This question was made even more difficult because she was young. She had her whole life ahead of her. It seemed incongruous that she would be here now, facing the gravity of her situation.
Looking at her, I saw the person, not a data point in a trial or a statistic in a textbook. She was terrified. And she was not ready to die.
I sat down and reviewed what I knew about her cancer and what I did not know. I went through potential treatments we could try and the toxicities associated with each. I made clear that these treatments, based on how sick she was, could kill her.
“Whatever we do,” I said, “you do not have disease that I can cure.”
She cried then, realizing what a horrible situation she was in and that she would no longer go back to her normal life. Indeed, she seemed to grasp that she was probably facing the end of her life and that it could be short.
“My concern is,” I continued, “that treatment could do the exact opposite of what I hope it would do. It could kill you sooner than this cancer will.”
Instead of making a treatment plan, I decided that it would be best to come back another day, so I said my goodbyes and left. Still, I could not stop thinking about her and what I should suggest as her next steps.
I asked colleagues what they would suggest. Some recommended hospice care, others recommended treatment. Clearly, there was no one way to proceed.
One might wonder: Why is it so hard to do the right thing?
Ask any clinician and I think you will hear the same answer: Because we do not have the luxury of certainty.
Am I certain that this person will not benefit from intubation? Am I certain that she has only weeks to live? Am I sure that there are no treatments that will work?
The answer to these questions is no – I am not certain. It is that uncertainty that always makes me pause because it reminds me of my own humanity.
I stopped by the next day to see her surrounded by family. After some pleasantries I took the opportunity to reiterate much of our conversation from the other day. After some questions, I looked at her and asked if she wanted to talk more about her options. I was prepared to suggest treatment, anticipating that she would want it. Instead, she told me she didn’t want to proceed.
“I feel like I’m dying, and if what you have to give me isn’t going to cure me, then I’d prefer not to suffer while it happens. You said it’s up to me. I don’t want it.”
First, do no harm. It’s one of the tenets of medicine – to provide care that will benefit the people who have trusted us with their lives, whether that be longevity, relief of symptoms, or helping them achieve their last wishes. Throughout one’s life, goals might change but that edict remains the same.
But that can be difficult, especially in oncology and especially when one is not prepared for their own end of life. It can be hard for doctors to discuss the end of life; it’s easier to focus on the next treatment, instilling hope that there’s more that can be done. And there are people with end-stage cancer who insist on continuing treatment in the same circumstances, preferring to “die fighting” than to “give up.” Involving supportive and palliative care specialists early has helped in both situations, which is certainly a good thing.
We talked a while more and then arranged for our palliative care team to see her. I wish I could say I was at peace with her decision, but I wasn’t. The truth is, whatever she decided would probably have the same impact: I wouldn’t be able to stop thinking about it.
Dr. Dizon is professor of medicine, department of medicine, at Brown University and director of medical oncology at Rhode Island Hospital, both in Providence, R.I. He disclosed conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
There is one situation, while not common, that is often among the most difficult for me: the person who must be told at diagnosis that they are already dying. I am still reminded of a patient I saw early in my career.
A woman in her 40s was admitted to the hospital complaining of severe shortness of breath. In retrospect, she had been sick for months. She had not sought help because she was young and thought it would pass – the results of a “bad bug” that she just couldn’t shake.
But in the past few weeks, the persistence of symptoms became associated with weight loss, profound fatigue, loss of appetite, and nausea.
By the time she was hospitalized she was emaciated, though she appeared pregnant – a sign of the fluid that had built up in her abdomen. Imaging showed that her abdomen was filled with disease (carcinomatosis) and her liver and lungs were nearly replaced with metastatic disease.
A biopsy revealed an aggressive cancer that had no identifying histologic marker: carcinoma, not otherwise specified, or cancer of unknown primary.
I still remember seeing her. She had a deer-in-headlights stare that held me as I approached. I introduced myself and sat down so we were eye to eye.
“Tell me what you know,” I said.
“I know I have cancer and they don’t know where it started. I know surgery is not an option and that’s why they’ve asked you to come. Whatever. I’m ready. I want to fight this because I know I can beat it,” she said.
I remember that she looked very sick; her thin face and arms contrasted with her large, distended abdomen. Her breathing was labored, her skin almost gray. For a moment I didn’t know what to say.
As doctors, we like to believe that our decisions are guided by data: the randomized trials and meta-analyses that set standards of care; phase 2 trials that establish evidence (or lack thereof) of activity; case-control studies that suggest the impacts of treatment; and at the very least, case studies that document that “N of 1” experience. We have expert panels and pathways that lay out what treatments we should be using to help ensure access to quality care in every clinic on every corner of every cancer center in the United States.
These data and pathways tell us objectively what we can expect from therapy, who is at most risk for toxicities, and profiles of patients for whom treatment is not likely to be of benefit. In an ideal world, this objectivity would help us help people decide on an approach. But life is not objective, and sometimes individualizing care is as important as data.
In this scenario, I knew only one thing: She was dying. She had an overwhelming tumor burden. But I still asked myself a question that many in, and outside of, oncology ask themselves: Could she be saved?
This question was made even more difficult because she was young. She had her whole life ahead of her. It seemed incongruous that she would be here now, facing the gravity of her situation.
Looking at her, I saw the person, not a data point in a trial or a statistic in a textbook. She was terrified. And she was not ready to die.
I sat down and reviewed what I knew about her cancer and what I did not know. I went through potential treatments we could try and the toxicities associated with each. I made clear that these treatments, based on how sick she was, could kill her.
“Whatever we do,” I said, “you do not have disease that I can cure.”
She cried then, realizing what a horrible situation she was in and that she would no longer go back to her normal life. Indeed, she seemed to grasp that she was probably facing the end of her life and that it could be short.
“My concern is,” I continued, “that treatment could do the exact opposite of what I hope it would do. It could kill you sooner than this cancer will.”
Instead of making a treatment plan, I decided that it would be best to come back another day, so I said my goodbyes and left. Still, I could not stop thinking about her and what I should suggest as her next steps.
I asked colleagues what they would suggest. Some recommended hospice care, others recommended treatment. Clearly, there was no one way to proceed.
One might wonder: Why is it so hard to do the right thing?
Ask any clinician and I think you will hear the same answer: Because we do not have the luxury of certainty.
Am I certain that this person will not benefit from intubation? Am I certain that she has only weeks to live? Am I sure that there are no treatments that will work?
The answer to these questions is no – I am not certain. It is that uncertainty that always makes me pause because it reminds me of my own humanity.
I stopped by the next day to see her surrounded by family. After some pleasantries I took the opportunity to reiterate much of our conversation from the other day. After some questions, I looked at her and asked if she wanted to talk more about her options. I was prepared to suggest treatment, anticipating that she would want it. Instead, she told me she didn’t want to proceed.
“I feel like I’m dying, and if what you have to give me isn’t going to cure me, then I’d prefer not to suffer while it happens. You said it’s up to me. I don’t want it.”
First, do no harm. It’s one of the tenets of medicine – to provide care that will benefit the people who have trusted us with their lives, whether that be longevity, relief of symptoms, or helping them achieve their last wishes. Throughout one’s life, goals might change but that edict remains the same.
But that can be difficult, especially in oncology and especially when one is not prepared for their own end of life. It can be hard for doctors to discuss the end of life; it’s easier to focus on the next treatment, instilling hope that there’s more that can be done. And there are people with end-stage cancer who insist on continuing treatment in the same circumstances, preferring to “die fighting” than to “give up.” Involving supportive and palliative care specialists early has helped in both situations, which is certainly a good thing.
We talked a while more and then arranged for our palliative care team to see her. I wish I could say I was at peace with her decision, but I wasn’t. The truth is, whatever she decided would probably have the same impact: I wouldn’t be able to stop thinking about it.
Dr. Dizon is professor of medicine, department of medicine, at Brown University and director of medical oncology at Rhode Island Hospital, both in Providence, R.I. He disclosed conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
There is one situation, while not common, that is often among the most difficult for me: the person who must be told at diagnosis that they are already dying. I am still reminded of a patient I saw early in my career.
A woman in her 40s was admitted to the hospital complaining of severe shortness of breath. In retrospect, she had been sick for months. She had not sought help because she was young and thought it would pass – the results of a “bad bug” that she just couldn’t shake.
But in the past few weeks, the persistence of symptoms became associated with weight loss, profound fatigue, loss of appetite, and nausea.
By the time she was hospitalized she was emaciated, though she appeared pregnant – a sign of the fluid that had built up in her abdomen. Imaging showed that her abdomen was filled with disease (carcinomatosis) and her liver and lungs were nearly replaced with metastatic disease.
A biopsy revealed an aggressive cancer that had no identifying histologic marker: carcinoma, not otherwise specified, or cancer of unknown primary.
I still remember seeing her. She had a deer-in-headlights stare that held me as I approached. I introduced myself and sat down so we were eye to eye.
“Tell me what you know,” I said.
“I know I have cancer and they don’t know where it started. I know surgery is not an option and that’s why they’ve asked you to come. Whatever. I’m ready. I want to fight this because I know I can beat it,” she said.
I remember that she looked very sick; her thin face and arms contrasted with her large, distended abdomen. Her breathing was labored, her skin almost gray. For a moment I didn’t know what to say.
As doctors, we like to believe that our decisions are guided by data: the randomized trials and meta-analyses that set standards of care; phase 2 trials that establish evidence (or lack thereof) of activity; case-control studies that suggest the impacts of treatment; and at the very least, case studies that document that “N of 1” experience. We have expert panels and pathways that lay out what treatments we should be using to help ensure access to quality care in every clinic on every corner of every cancer center in the United States.
These data and pathways tell us objectively what we can expect from therapy, who is at most risk for toxicities, and profiles of patients for whom treatment is not likely to be of benefit. In an ideal world, this objectivity would help us help people decide on an approach. But life is not objective, and sometimes individualizing care is as important as data.
In this scenario, I knew only one thing: She was dying. She had an overwhelming tumor burden. But I still asked myself a question that many in, and outside of, oncology ask themselves: Could she be saved?
This question was made even more difficult because she was young. She had her whole life ahead of her. It seemed incongruous that she would be here now, facing the gravity of her situation.
Looking at her, I saw the person, not a data point in a trial or a statistic in a textbook. She was terrified. And she was not ready to die.
I sat down and reviewed what I knew about her cancer and what I did not know. I went through potential treatments we could try and the toxicities associated with each. I made clear that these treatments, based on how sick she was, could kill her.
“Whatever we do,” I said, “you do not have disease that I can cure.”
She cried then, realizing what a horrible situation she was in and that she would no longer go back to her normal life. Indeed, she seemed to grasp that she was probably facing the end of her life and that it could be short.
“My concern is,” I continued, “that treatment could do the exact opposite of what I hope it would do. It could kill you sooner than this cancer will.”
Instead of making a treatment plan, I decided that it would be best to come back another day, so I said my goodbyes and left. Still, I could not stop thinking about her and what I should suggest as her next steps.
I asked colleagues what they would suggest. Some recommended hospice care, others recommended treatment. Clearly, there was no one way to proceed.
One might wonder: Why is it so hard to do the right thing?
Ask any clinician and I think you will hear the same answer: Because we do not have the luxury of certainty.
Am I certain that this person will not benefit from intubation? Am I certain that she has only weeks to live? Am I sure that there are no treatments that will work?
The answer to these questions is no – I am not certain. It is that uncertainty that always makes me pause because it reminds me of my own humanity.
I stopped by the next day to see her surrounded by family. After some pleasantries I took the opportunity to reiterate much of our conversation from the other day. After some questions, I looked at her and asked if she wanted to talk more about her options. I was prepared to suggest treatment, anticipating that she would want it. Instead, she told me she didn’t want to proceed.
“I feel like I’m dying, and if what you have to give me isn’t going to cure me, then I’d prefer not to suffer while it happens. You said it’s up to me. I don’t want it.”
First, do no harm. It’s one of the tenets of medicine – to provide care that will benefit the people who have trusted us with their lives, whether that be longevity, relief of symptoms, or helping them achieve their last wishes. Throughout one’s life, goals might change but that edict remains the same.
But that can be difficult, especially in oncology and especially when one is not prepared for their own end of life. It can be hard for doctors to discuss the end of life; it’s easier to focus on the next treatment, instilling hope that there’s more that can be done. And there are people with end-stage cancer who insist on continuing treatment in the same circumstances, preferring to “die fighting” than to “give up.” Involving supportive and palliative care specialists early has helped in both situations, which is certainly a good thing.
We talked a while more and then arranged for our palliative care team to see her. I wish I could say I was at peace with her decision, but I wasn’t. The truth is, whatever she decided would probably have the same impact: I wouldn’t be able to stop thinking about it.
Dr. Dizon is professor of medicine, department of medicine, at Brown University and director of medical oncology at Rhode Island Hospital, both in Providence, R.I. He disclosed conflicts of interest with Regeneron, AstraZeneca, Clovis, Bristol Myers Squibb, and Kazia.
A version of this article first appeared on Medscape.com.
Four profiles help identify kids at risk for suicide
The profiles were developed from their study of children and adolescents aged 5-18 years who had been admitted with a neuropsychiatric event to two children’s hospitals.
The researchers used Bayesian regression to identify the profiles developed from 32 covariates: age, sex, and 30 mental health diagnostic groups from April 2016 to March 2020. The profiles include low-, moderate-, high- and very-high-risk categories.
The study, led by Mert Sekmen with the division of hospital medicine at Monroe Carell Jr. Children’s Hospital, and a student at Vanderbilt University Medical Center in Nashville, Tenn., included 1,098 children, average age 14. Of those, 406 (37%) were diagnosed with a self-harm event.
Traditionally, single diagnoses have been linked with risk of self-harm, independent of other comorbidities, but this study gauges risk for a set of diagnoses.
Findings were published online in Pediatrics.
The risk groups were described as follows:
- Low risk. (45% of the study population; median risk of 0.04 (interquartile range, 0.03-0.04; odds ratio, 0.08). The group included children aged 5-9 years with a non–mental health diagnosis, and without mood, behavioral, psychotic, developmental, trauma, or substance-related disorders.
- Moderate risk. (8% of the study group). This group had the same risk as the baseline risk for the entire cohort (37%) and served as the reference group, with a median risk of 0.30 (IQR, 0.27-0.33). This profile was characterized by several mood disorders and behavioral disorders but without depressive disorders.
- High risk. (36%) This group had an average risk of 0.69 (IQR, 0.67-0.71; OR, 5.09). This profile included female adolescents ages 14-17 with depression and anxiety in conjunction with substance- and trauma-related disorders. Personality and eating disorders were significant in this group. Importantly, the authors wrote, the high-risk group did not include behavioral and developmental disorders.
- Very high risk. (11%) The very-high-risk profile had the highest average risk of 0.79 (IQR, 0.73-0.79; OR, 7.21) and included male children aged 10-13. This profile, like the high-risk profile, included anxiety and depressive disorders. The very-high-risk profile differed from the high-risk with its inclusion of bipolar disorder; attention-deficit/hyperactivity disorder; and trauma-related and developmental disorders such as autism spectrum disorder or intellectual disability, along with conduct disorders. Neither the high- nor the very-high-risk profiles included a concurrent non–mental health diagnosis.
Differences by sex
The authors explained some of the differences by sex. They noted that in a study of children aged 5-11, deaths by suicide were more prevalent among boys. A mental health diagnosis was identified in 31%, the most common being ADHD, depression, and other unspecified co-occurring disorders.
“The very-high-risk group also reflects a concerning rise in death by suicide among (males) aged 10-13, who have seen rates nearly triple from 2007 to 2017,” the authors wrote.
The authors pointed out that, although incidence of anxiety and depressive disorders between male and female children is much the same before adolescence, “female adolescents are twice as likely to be diagnosed with either disorder during adolescence. Girls also have higher rates of suicidal ideation and attempts after puberty.”
Eating disorders were also included in the high-risk profile. A study showed that emergency department visits for adolescent girls attempting suicide were 51% higher from February to March 2021, compared with the same period in the pre-COVID-19 year 2019.
Jason Lewis, PhD, psychologist and section director of mood, anxiety and trauma disorders in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, who was not part of the research team, said the “constellations of risk factors put into acuity levels” helps to better project risk than knowing the risk associated with a particular diagnosis.
Gap closing between young children, adolescents
Dr. Lewis said he was surprised by the young age of 10-13 among the boys in the highest-risk category. That speaks to the differences from standard thinking this paper points out, he said. “Generally, we think about adolescents as being at the highest risk of suicide death and suicidal behavior,” he said.
Dr. Lewis said it’s important to note that the authors acknowledge these profiles are not static. He gave an example that the rate of suicide deaths among females is rising.
“As things like that change, some of these risk profiles will change as well.”
Dr. Lewis said the profiles may be especially helpful to medical providers in emergency departments or those making discharge decisions who don’t have an ongoing relationship with a patient.
The information could also help educators and lay people, “think about suicide in the youth population in ways we don’t normally think about it,” Dr. Lewis said.
Covariates considered for profiles were determined through expert consensus between pediatric psychiatrists, general pediatricians, pediatric hospitalists, pediatric complex care physicians, and pediatric pharmacoepidemiologists.
Age was broken into three groups: 5-9 years, 10-13 years, and 14-17 years based on Centers for Disease Control and Prevention reporting and previous studies that showed significant increases in suicide rates in these age-based subgroups.
Results are preliminary
The authors note that the profiles were developed using data from 1,000 children with neuropsychiatric complaints at two academic children’s hospitals and are thus preliminary.
“Future studies should focus on validating these risk profiles in a larger, more heterogeneous population of children and adolescents,” the authors write.
They also acknowledge that they were not able to include factors such as medication use, previous suicidal behavior, and family and social support, which also factor into risk.
The study authors and Dr. Lewis report no relevant financial relationships.
The profiles were developed from their study of children and adolescents aged 5-18 years who had been admitted with a neuropsychiatric event to two children’s hospitals.
The researchers used Bayesian regression to identify the profiles developed from 32 covariates: age, sex, and 30 mental health diagnostic groups from April 2016 to March 2020. The profiles include low-, moderate-, high- and very-high-risk categories.
The study, led by Mert Sekmen with the division of hospital medicine at Monroe Carell Jr. Children’s Hospital, and a student at Vanderbilt University Medical Center in Nashville, Tenn., included 1,098 children, average age 14. Of those, 406 (37%) were diagnosed with a self-harm event.
Traditionally, single diagnoses have been linked with risk of self-harm, independent of other comorbidities, but this study gauges risk for a set of diagnoses.
Findings were published online in Pediatrics.
The risk groups were described as follows:
- Low risk. (45% of the study population; median risk of 0.04 (interquartile range, 0.03-0.04; odds ratio, 0.08). The group included children aged 5-9 years with a non–mental health diagnosis, and without mood, behavioral, psychotic, developmental, trauma, or substance-related disorders.
- Moderate risk. (8% of the study group). This group had the same risk as the baseline risk for the entire cohort (37%) and served as the reference group, with a median risk of 0.30 (IQR, 0.27-0.33). This profile was characterized by several mood disorders and behavioral disorders but without depressive disorders.
- High risk. (36%) This group had an average risk of 0.69 (IQR, 0.67-0.71; OR, 5.09). This profile included female adolescents ages 14-17 with depression and anxiety in conjunction with substance- and trauma-related disorders. Personality and eating disorders were significant in this group. Importantly, the authors wrote, the high-risk group did not include behavioral and developmental disorders.
- Very high risk. (11%) The very-high-risk profile had the highest average risk of 0.79 (IQR, 0.73-0.79; OR, 7.21) and included male children aged 10-13. This profile, like the high-risk profile, included anxiety and depressive disorders. The very-high-risk profile differed from the high-risk with its inclusion of bipolar disorder; attention-deficit/hyperactivity disorder; and trauma-related and developmental disorders such as autism spectrum disorder or intellectual disability, along with conduct disorders. Neither the high- nor the very-high-risk profiles included a concurrent non–mental health diagnosis.
Differences by sex
The authors explained some of the differences by sex. They noted that in a study of children aged 5-11, deaths by suicide were more prevalent among boys. A mental health diagnosis was identified in 31%, the most common being ADHD, depression, and other unspecified co-occurring disorders.
“The very-high-risk group also reflects a concerning rise in death by suicide among (males) aged 10-13, who have seen rates nearly triple from 2007 to 2017,” the authors wrote.
The authors pointed out that, although incidence of anxiety and depressive disorders between male and female children is much the same before adolescence, “female adolescents are twice as likely to be diagnosed with either disorder during adolescence. Girls also have higher rates of suicidal ideation and attempts after puberty.”
Eating disorders were also included in the high-risk profile. A study showed that emergency department visits for adolescent girls attempting suicide were 51% higher from February to March 2021, compared with the same period in the pre-COVID-19 year 2019.
Jason Lewis, PhD, psychologist and section director of mood, anxiety and trauma disorders in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, who was not part of the research team, said the “constellations of risk factors put into acuity levels” helps to better project risk than knowing the risk associated with a particular diagnosis.
Gap closing between young children, adolescents
Dr. Lewis said he was surprised by the young age of 10-13 among the boys in the highest-risk category. That speaks to the differences from standard thinking this paper points out, he said. “Generally, we think about adolescents as being at the highest risk of suicide death and suicidal behavior,” he said.
Dr. Lewis said it’s important to note that the authors acknowledge these profiles are not static. He gave an example that the rate of suicide deaths among females is rising.
“As things like that change, some of these risk profiles will change as well.”
Dr. Lewis said the profiles may be especially helpful to medical providers in emergency departments or those making discharge decisions who don’t have an ongoing relationship with a patient.
The information could also help educators and lay people, “think about suicide in the youth population in ways we don’t normally think about it,” Dr. Lewis said.
Covariates considered for profiles were determined through expert consensus between pediatric psychiatrists, general pediatricians, pediatric hospitalists, pediatric complex care physicians, and pediatric pharmacoepidemiologists.
Age was broken into three groups: 5-9 years, 10-13 years, and 14-17 years based on Centers for Disease Control and Prevention reporting and previous studies that showed significant increases in suicide rates in these age-based subgroups.
Results are preliminary
The authors note that the profiles were developed using data from 1,000 children with neuropsychiatric complaints at two academic children’s hospitals and are thus preliminary.
“Future studies should focus on validating these risk profiles in a larger, more heterogeneous population of children and adolescents,” the authors write.
They also acknowledge that they were not able to include factors such as medication use, previous suicidal behavior, and family and social support, which also factor into risk.
The study authors and Dr. Lewis report no relevant financial relationships.
The profiles were developed from their study of children and adolescents aged 5-18 years who had been admitted with a neuropsychiatric event to two children’s hospitals.
The researchers used Bayesian regression to identify the profiles developed from 32 covariates: age, sex, and 30 mental health diagnostic groups from April 2016 to March 2020. The profiles include low-, moderate-, high- and very-high-risk categories.
The study, led by Mert Sekmen with the division of hospital medicine at Monroe Carell Jr. Children’s Hospital, and a student at Vanderbilt University Medical Center in Nashville, Tenn., included 1,098 children, average age 14. Of those, 406 (37%) were diagnosed with a self-harm event.
Traditionally, single diagnoses have been linked with risk of self-harm, independent of other comorbidities, but this study gauges risk for a set of diagnoses.
Findings were published online in Pediatrics.
The risk groups were described as follows:
- Low risk. (45% of the study population; median risk of 0.04 (interquartile range, 0.03-0.04; odds ratio, 0.08). The group included children aged 5-9 years with a non–mental health diagnosis, and without mood, behavioral, psychotic, developmental, trauma, or substance-related disorders.
- Moderate risk. (8% of the study group). This group had the same risk as the baseline risk for the entire cohort (37%) and served as the reference group, with a median risk of 0.30 (IQR, 0.27-0.33). This profile was characterized by several mood disorders and behavioral disorders but without depressive disorders.
- High risk. (36%) This group had an average risk of 0.69 (IQR, 0.67-0.71; OR, 5.09). This profile included female adolescents ages 14-17 with depression and anxiety in conjunction with substance- and trauma-related disorders. Personality and eating disorders were significant in this group. Importantly, the authors wrote, the high-risk group did not include behavioral and developmental disorders.
- Very high risk. (11%) The very-high-risk profile had the highest average risk of 0.79 (IQR, 0.73-0.79; OR, 7.21) and included male children aged 10-13. This profile, like the high-risk profile, included anxiety and depressive disorders. The very-high-risk profile differed from the high-risk with its inclusion of bipolar disorder; attention-deficit/hyperactivity disorder; and trauma-related and developmental disorders such as autism spectrum disorder or intellectual disability, along with conduct disorders. Neither the high- nor the very-high-risk profiles included a concurrent non–mental health diagnosis.
Differences by sex
The authors explained some of the differences by sex. They noted that in a study of children aged 5-11, deaths by suicide were more prevalent among boys. A mental health diagnosis was identified in 31%, the most common being ADHD, depression, and other unspecified co-occurring disorders.
“The very-high-risk group also reflects a concerning rise in death by suicide among (males) aged 10-13, who have seen rates nearly triple from 2007 to 2017,” the authors wrote.
The authors pointed out that, although incidence of anxiety and depressive disorders between male and female children is much the same before adolescence, “female adolescents are twice as likely to be diagnosed with either disorder during adolescence. Girls also have higher rates of suicidal ideation and attempts after puberty.”
Eating disorders were also included in the high-risk profile. A study showed that emergency department visits for adolescent girls attempting suicide were 51% higher from February to March 2021, compared with the same period in the pre-COVID-19 year 2019.
Jason Lewis, PhD, psychologist and section director of mood, anxiety and trauma disorders in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, who was not part of the research team, said the “constellations of risk factors put into acuity levels” helps to better project risk than knowing the risk associated with a particular diagnosis.
Gap closing between young children, adolescents
Dr. Lewis said he was surprised by the young age of 10-13 among the boys in the highest-risk category. That speaks to the differences from standard thinking this paper points out, he said. “Generally, we think about adolescents as being at the highest risk of suicide death and suicidal behavior,” he said.
Dr. Lewis said it’s important to note that the authors acknowledge these profiles are not static. He gave an example that the rate of suicide deaths among females is rising.
“As things like that change, some of these risk profiles will change as well.”
Dr. Lewis said the profiles may be especially helpful to medical providers in emergency departments or those making discharge decisions who don’t have an ongoing relationship with a patient.
The information could also help educators and lay people, “think about suicide in the youth population in ways we don’t normally think about it,” Dr. Lewis said.
Covariates considered for profiles were determined through expert consensus between pediatric psychiatrists, general pediatricians, pediatric hospitalists, pediatric complex care physicians, and pediatric pharmacoepidemiologists.
Age was broken into three groups: 5-9 years, 10-13 years, and 14-17 years based on Centers for Disease Control and Prevention reporting and previous studies that showed significant increases in suicide rates in these age-based subgroups.
Results are preliminary
The authors note that the profiles were developed using data from 1,000 children with neuropsychiatric complaints at two academic children’s hospitals and are thus preliminary.
“Future studies should focus on validating these risk profiles in a larger, more heterogeneous population of children and adolescents,” the authors write.
They also acknowledge that they were not able to include factors such as medication use, previous suicidal behavior, and family and social support, which also factor into risk.
The study authors and Dr. Lewis report no relevant financial relationships.
FROM PEDIATRICS
Researchers seek to understand post-COVID autoimmune disease risk
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the COVID-19 pandemic started more than 3 years ago, the longer-lasting effects of SARS-CoV-2 infection have continued to reveal themselves. Approximately 28% of Americans report having ever experienced post-COVID conditions, such as brain fog, postexertional malaise, and joint pain, and 11% say they are still experiencing these long-term effects. Now, new research is showing that people who have had COVID are more likely to newly develop an autoimmune disease. Exactly why this is happening is less clear, experts say.
Two preprint studies and one study published in a peer-reviewed journal provide strong evidence that patients who have been infected with SARS-CoV-2 are at elevated risk of developing an autoimmune disease. The studies retrospectively reviewed medical records from three countries and compared the incidence of new-onset autoimmune disease among patients who had polymerase chain reaction–confirmed COVID-19 and those who had never been diagnosed with the virus.
A study analyzing the health records of 3.8 million U.S. patients – more than 888,460 with confirmed COVID-19 – found that the COVID-19 group was two to three times as likely to develop various autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and systemic sclerosis. A U.K. preprint study that included more than 458,000 people with confirmed COVID found that those who had previously been infected with SARS-CoV-2 were 22% more likely to develop an autoimmune disease compared with the control group. In this cohort, the diseases most strongly associated with COVID-19 were type 1 diabetes, inflammatory bowel disease, and psoriasis. A preprint study from German researchers found that COVID-19 patients were almost 43% more likely to develop an autoimmune disease, compared with those who had never been infected. COVID-19 was most strongly linked to vasculitis.
These large studies are telling us, “Yes, this link is there, so we have to accept it,” Sonia Sharma, PhD, of the Center for Autoimmunity and Inflammation at the La Jolla (Calif.) Institute for Immunology, told this news organization. But this is not the first time that autoimmune diseases have been linked to previous infections.
Researchers have known for decades that Epstein-Barr virus infection is linked to several autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. More recent research suggests the virus may activate certain genes associated with these immune disorders. Hepatitis C virus can induce cryoglobulinemia, and infection with cytomegalovirus has been implicated in several autoimmune diseases. Bacterial infections have also been linked to autoimmunity, such as group A streptococcus and rheumatic fever, as well as salmonella and reactive arthritis, to name only a few.
“In a way, this isn’t necessarily a new concept to physicians, particularly rheumatologists,” said Jeffrey A. Sparks, MD, a rheumatologist at Brigham and Women’s Hospital in Boston. “There’s a fine line between appropriately clearing an infection and the body overreacting and setting off a cascade where the immune system is chronically overactive that can manifest as an autoimmune disease,” he told this news organization.
A dysregulated response to infection
It takes the immune system a week or two to develop antigen-specific antibodies to a new pathogen. But for patients with serious infections – in this instance, COVID-19 – that’s time they don’t have. Therefore, the immune system has an alternative pathway, called extrafollicular activation, that creates fast-acting antibodies, explained Matthew Woodruff, PhD, an instructor of immunology and rheumatology at Emory University, Atlanta.
The trade-off is that these antibodies are not as specific and can target the body’s own tissues. This dysregulation of antibody selection is generally short lived and fades when more targeted antibodies are produced and take over, but in some cases, this process can lead to high levels of self-targeting antibodies that can harm the body’s organs and tissues. Research also suggests that for patients who experience long COVID, the same autoantibodies that drive the initial immune response are detectable in the body months after infection, though it is not known whether these lingering immune cells cause these longer-lasting symptoms.
“If you have a virus that causes hyperinflammation plus organ damage, that is a recipe for disaster,” Dr. Sharma said. “It’s a recipe for autoantibodies and autoreactive T cells that down the road can attack the body’s own tissues, especially in people whose immune system is trained in such a way to cause self-reactivity,” she added.
This hyperinflammation can result in rare but serious complications, such as multisystem inflammatory syndrome in children and adults, which can occur 2-6 weeks after SARS-CoV-2 infection. But even in these patients with severe illness, organ-specific complications tend to resolve in 6 months with “no significant sequelae 1 year after diagnosis,” according to the Centers for Disease Control and Prevention. And while long COVID can last for a year or longer, data suggest that symptoms do eventually resolve for most people. What is not clear is why acute autoimmunity triggered by COVID-19 can become a chronic condition in certain patients.
Predisposition to autoimmunity
P. J. Utz, MD, PhD, professor of immunology and rheumatology at Stanford (Calif.) University, said that people who develop autoimmune disease after SARS-CoV-2 infection may have already been predisposed toward autoimmunity. Especially for autoimmune diseases such as type 1 diabetes and lupus, autoantibodies can appear and circulate in the body for more than a decade in some people before they present with any clinical symptoms. “Their immune system is primed such that if they get infected with something – or they have some other environmental trigger that maybe we don’t know about yet – that is enough to then push them over the edge so that they get full-blown autoimmunity,” he said. What is not known is whether these patients’ conditions would have advanced to true clinical disease had they not been infected, he said.
He also noted that the presence of autoantibodies does not necessarily mean someone has autoimmune disease; healthy people can also have autoantibodies, and everyone develops them with age. “My advice would be, ‘Don’t lose sleep over this,’ “ he said.
Dr. Sparks agreed that while these retrospective studies did show an elevated risk of autoimmune disease after COVID-19, that risk appears to be relatively small. “As a practicing rheumatologist, we aren’t seeing a stampede of patients with new-onset rheumatic diseases,” he said. “It’s not like we’re overwhelmed with autoimmune patients, even though almost everyone’s had COVID. So, if there is a risk, it’s very modest.”
Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Utz receives research funding from Pfizer. Dr. Sharma and Dr. Woodruff have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.