User login
Adherence to Evidence-Based Outpatient Antimicrobial Prescribing Guidelines at a Tribal Health System
Tuba City Regional Health Care Corporation (TCRHCC) is located on the Navajo Reservation in northeastern Arizona and provides medical coverage to a 6000-square-mile area, serving more than 33,000 residents of the Navajo, Hopi, and San Juan Southern Paiute tribes.1,2 In 2021, there were 334,497 outpatient visits. TCRHCC departments involved in prescribing outpatient antibiotics include the emergency, internal medicine, family medicine, pediatrics, dentistry, surgery, podiatry, obstetrics and gynecology, and midwifery.
Antimicrobial resistance is one of the largest public health threats, causing an estimated 2 million infections and 23,000 deaths every year in the United States.3 This can lead to increased health care costs, morbidity, and mortality. A large, modifiable risk factor is the inappropriate prescribing of antibiotics: An estimated half of all outpatient antibiotics prescribed may be inappropriate in some manner, such as antibiotic choice, dosing, or duration. In addition, at least 30% of US antibiotic prescriptions are unnecessary, leading to significant overuse.3 As such, antimicrobial stewardship is a cornerstone of improving antibiotic use, patient care, and safety.
The goals of antimicrobial stewardship are to measure antimicrobial prescribing, improve clinician prescribing, minimize misdiagnosis or delayed diagnoses, and ensure the right drug, dose, and duration are selected when antimicrobial therapy is appropriate.3 The Centers for Disease Control and Prevention recommends 4 core elements of outpatient antimicrobial stewardship: commitment, action for policy and practice, tracking and reporting, and education and expertise.3 This study focuses on the pillars of action for policy and practice and tracking and reporting.
Methods
The study objectives were not designed to achieve statistical power. A retrospective chart review was performed for patients of any age who were seen in an ambulatory care setting at TCRHCC from August 1, 2020, to August 1, 2021, with a visit diagnosis included in the outpatient antimicrobial prescribing guidelines.4,5 A random sample of 10% of charts of each diagnosis code was used for analysis. An Excel spreadsheet with all patient charts, separated by diagnosis code, was created. Each chart was then assigned a number, and the Excel function RAND was used to select a random number from the pool. This was continued until 10% of each category, or at least 1 chart from diagnosis code categories with less than 10 total charts available, were selected.
Inclusion criteria were patients seen in ambulatory clinics or the emergency department, an infectious disease diagnosis addressed in the facility guidelines, diagnosis and treatment occurred between August 1, 2020, and August 1, 2021, and the patient was discharged home after the visit. Exclusion criteria were patients who required inpatient admission, patient visits to the clinic established solely for COVID-19 vaccination or testing as no other care was ever provided at this location, patients who refused treatment, patients who failed empiric therapy and required treatment adjustments, or patients who were initially treated and received an antibiotic prescription at a facility outside the TCRHCC system.
After chart review and analysis were completed, a prescriber survey and educational intervention were performed from March 2, 2022, to March 31, 2022. This consisted of an anonymous survey to gather demographic data and prescribing habits pre-education, a short educational brief on the existence, location, and recommended use of the outpatient antimicrobial prescribing guidelines, and a posteducation survey to assess knowledge of the guidelines and willingness to adhere to them after the educational intervention.
Results
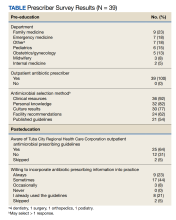
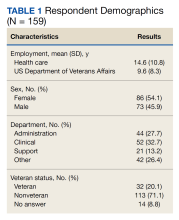
We reviewed 8779 patient records. A random sample of 10% of the records of each diagnosis code was taken and 876 charts were reviewed. Of the charts reviewed, 351 patients met the inclusion criteria and were included in the analysis. A goal of 90% was established as the target for prescriber adherence for the study based on author consensus for a reasonable goal. Of the 351 evaluated charts, 62 (16.1%) were pediatric patients (aged < 19 years) and 289 (83.9%) were adults (aged ≥ 19 years). Fifty-two (84%) of the pediatric charts and 249 (86%) of the adult charts demonstrated prescribers had appropriately followed guidelines for a combined total of 301 of the 351 charts and an overall adherence rate of 86%. This was 4 points below the established goal of 90%, warranting further investigation. An analysis of prescribers and locations revealed no trends or patterns of nonadherence. A prescriber survey and educational intervention were designed and disseminated to all prescribers at the facility with the approval and assistance of the chief of medicine.
Thirty-nine prescribers responded to the survey. In the pre-educational survey, clinical resources were the most common source of guidance with 36 prescribers (92%) indicating they used them to make an appropriate selection of an antimicrobial; 32 (82%) used personal knowledge, 30 (77%) used culture results, and 24 (62%) used facility guidelines. This was consistent with the posteducational questions: 12 (31%) indicated they were not aware of the facility guidelines before the educational intervention.
Discussion
This study’s objective was to evaluate prescriber adherence to the facility outpatient prescribing guidelines after they were implemented in 2019 and to plan for interventions if necessary. Overall prescriber adherence was high with 86% of the sampled charts adherent. This was below the goal of 90%, so evaluation of the nonadherent charts was warranted for the determination of any patterns to guide the planned interventions with the facility prescribers. However, no trends were identified, so the intervention was designed as a general survey and educational session for all prescribers. Overall prescriber response was positive, with a total of 34 responding prescribers (87%) indicating a willingness to use the guidelines.
Limitations
This is a retrospective observational study performed through chart review that allowed for frequency analysis but did not allow for statistical analysis, so the significance of results cannot be obtained. Additionally, this study was not able to compare rates of adherence before and after the educational intervention, so the effectiveness of the intervention cannot be assessed.
Conclusions
This retrospective observational study’s data demonstrate that prescribers are adhering at a high rate to recommended empiric antimicrobials for outpatient treatment with an 86% adherence rate. Response to educational intervention indicated a larger proportion of prescribers than previously will use the guidelines. However, the impact this will have on appropriate prescribing rates in the future could not be assessed during this study.
1. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2021. 2012. Accessed January 25, 2023. Accessed January 30, 2023. https://tchealth.org/pdfdownload/2021_TCRHCC_Annual_Report.pdf
2. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2013. 2013. Accessed January 25, 2023. Accessed January 30, 2023. https://www.tchealth.org/pdfdownload/2013_Annual_Report.pdf
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(No. RR-6):1–12. doi:10.15585/mmwr.rr6506a1
4. Tuba City Regional Health Care Corporation. Antimicrobial stewardship adult outpatient guidelines. 2019.
5. Tuba City Regional Health Care Corporation. Antimicrobial stewardship pediatric outpatient guidelines. 2019.
Tuba City Regional Health Care Corporation (TCRHCC) is located on the Navajo Reservation in northeastern Arizona and provides medical coverage to a 6000-square-mile area, serving more than 33,000 residents of the Navajo, Hopi, and San Juan Southern Paiute tribes.1,2 In 2021, there were 334,497 outpatient visits. TCRHCC departments involved in prescribing outpatient antibiotics include the emergency, internal medicine, family medicine, pediatrics, dentistry, surgery, podiatry, obstetrics and gynecology, and midwifery.
Antimicrobial resistance is one of the largest public health threats, causing an estimated 2 million infections and 23,000 deaths every year in the United States.3 This can lead to increased health care costs, morbidity, and mortality. A large, modifiable risk factor is the inappropriate prescribing of antibiotics: An estimated half of all outpatient antibiotics prescribed may be inappropriate in some manner, such as antibiotic choice, dosing, or duration. In addition, at least 30% of US antibiotic prescriptions are unnecessary, leading to significant overuse.3 As such, antimicrobial stewardship is a cornerstone of improving antibiotic use, patient care, and safety.
The goals of antimicrobial stewardship are to measure antimicrobial prescribing, improve clinician prescribing, minimize misdiagnosis or delayed diagnoses, and ensure the right drug, dose, and duration are selected when antimicrobial therapy is appropriate.3 The Centers for Disease Control and Prevention recommends 4 core elements of outpatient antimicrobial stewardship: commitment, action for policy and practice, tracking and reporting, and education and expertise.3 This study focuses on the pillars of action for policy and practice and tracking and reporting.
Methods
The study objectives were not designed to achieve statistical power. A retrospective chart review was performed for patients of any age who were seen in an ambulatory care setting at TCRHCC from August 1, 2020, to August 1, 2021, with a visit diagnosis included in the outpatient antimicrobial prescribing guidelines.4,5 A random sample of 10% of charts of each diagnosis code was used for analysis. An Excel spreadsheet with all patient charts, separated by diagnosis code, was created. Each chart was then assigned a number, and the Excel function RAND was used to select a random number from the pool. This was continued until 10% of each category, or at least 1 chart from diagnosis code categories with less than 10 total charts available, were selected.
Inclusion criteria were patients seen in ambulatory clinics or the emergency department, an infectious disease diagnosis addressed in the facility guidelines, diagnosis and treatment occurred between August 1, 2020, and August 1, 2021, and the patient was discharged home after the visit. Exclusion criteria were patients who required inpatient admission, patient visits to the clinic established solely for COVID-19 vaccination or testing as no other care was ever provided at this location, patients who refused treatment, patients who failed empiric therapy and required treatment adjustments, or patients who were initially treated and received an antibiotic prescription at a facility outside the TCRHCC system.
After chart review and analysis were completed, a prescriber survey and educational intervention were performed from March 2, 2022, to March 31, 2022. This consisted of an anonymous survey to gather demographic data and prescribing habits pre-education, a short educational brief on the existence, location, and recommended use of the outpatient antimicrobial prescribing guidelines, and a posteducation survey to assess knowledge of the guidelines and willingness to adhere to them after the educational intervention.
Results
We reviewed 8779 patient records. A random sample of 10% of the records of each diagnosis code was taken and 876 charts were reviewed. Of the charts reviewed, 351 patients met the inclusion criteria and were included in the analysis. A goal of 90% was established as the target for prescriber adherence for the study based on author consensus for a reasonable goal. Of the 351 evaluated charts, 62 (16.1%) were pediatric patients (aged < 19 years) and 289 (83.9%) were adults (aged ≥ 19 years). Fifty-two (84%) of the pediatric charts and 249 (86%) of the adult charts demonstrated prescribers had appropriately followed guidelines for a combined total of 301 of the 351 charts and an overall adherence rate of 86%. This was 4 points below the established goal of 90%, warranting further investigation. An analysis of prescribers and locations revealed no trends or patterns of nonadherence. A prescriber survey and educational intervention were designed and disseminated to all prescribers at the facility with the approval and assistance of the chief of medicine.
Thirty-nine prescribers responded to the survey. In the pre-educational survey, clinical resources were the most common source of guidance with 36 prescribers (92%) indicating they used them to make an appropriate selection of an antimicrobial; 32 (82%) used personal knowledge, 30 (77%) used culture results, and 24 (62%) used facility guidelines. This was consistent with the posteducational questions: 12 (31%) indicated they were not aware of the facility guidelines before the educational intervention.
Discussion
This study’s objective was to evaluate prescriber adherence to the facility outpatient prescribing guidelines after they were implemented in 2019 and to plan for interventions if necessary. Overall prescriber adherence was high with 86% of the sampled charts adherent. This was below the goal of 90%, so evaluation of the nonadherent charts was warranted for the determination of any patterns to guide the planned interventions with the facility prescribers. However, no trends were identified, so the intervention was designed as a general survey and educational session for all prescribers. Overall prescriber response was positive, with a total of 34 responding prescribers (87%) indicating a willingness to use the guidelines.
Limitations
This is a retrospective observational study performed through chart review that allowed for frequency analysis but did not allow for statistical analysis, so the significance of results cannot be obtained. Additionally, this study was not able to compare rates of adherence before and after the educational intervention, so the effectiveness of the intervention cannot be assessed.
Conclusions
This retrospective observational study’s data demonstrate that prescribers are adhering at a high rate to recommended empiric antimicrobials for outpatient treatment with an 86% adherence rate. Response to educational intervention indicated a larger proportion of prescribers than previously will use the guidelines. However, the impact this will have on appropriate prescribing rates in the future could not be assessed during this study.
Tuba City Regional Health Care Corporation (TCRHCC) is located on the Navajo Reservation in northeastern Arizona and provides medical coverage to a 6000-square-mile area, serving more than 33,000 residents of the Navajo, Hopi, and San Juan Southern Paiute tribes.1,2 In 2021, there were 334,497 outpatient visits. TCRHCC departments involved in prescribing outpatient antibiotics include the emergency, internal medicine, family medicine, pediatrics, dentistry, surgery, podiatry, obstetrics and gynecology, and midwifery.
Antimicrobial resistance is one of the largest public health threats, causing an estimated 2 million infections and 23,000 deaths every year in the United States.3 This can lead to increased health care costs, morbidity, and mortality. A large, modifiable risk factor is the inappropriate prescribing of antibiotics: An estimated half of all outpatient antibiotics prescribed may be inappropriate in some manner, such as antibiotic choice, dosing, or duration. In addition, at least 30% of US antibiotic prescriptions are unnecessary, leading to significant overuse.3 As such, antimicrobial stewardship is a cornerstone of improving antibiotic use, patient care, and safety.
The goals of antimicrobial stewardship are to measure antimicrobial prescribing, improve clinician prescribing, minimize misdiagnosis or delayed diagnoses, and ensure the right drug, dose, and duration are selected when antimicrobial therapy is appropriate.3 The Centers for Disease Control and Prevention recommends 4 core elements of outpatient antimicrobial stewardship: commitment, action for policy and practice, tracking and reporting, and education and expertise.3 This study focuses on the pillars of action for policy and practice and tracking and reporting.
Methods
The study objectives were not designed to achieve statistical power. A retrospective chart review was performed for patients of any age who were seen in an ambulatory care setting at TCRHCC from August 1, 2020, to August 1, 2021, with a visit diagnosis included in the outpatient antimicrobial prescribing guidelines.4,5 A random sample of 10% of charts of each diagnosis code was used for analysis. An Excel spreadsheet with all patient charts, separated by diagnosis code, was created. Each chart was then assigned a number, and the Excel function RAND was used to select a random number from the pool. This was continued until 10% of each category, or at least 1 chart from diagnosis code categories with less than 10 total charts available, were selected.
Inclusion criteria were patients seen in ambulatory clinics or the emergency department, an infectious disease diagnosis addressed in the facility guidelines, diagnosis and treatment occurred between August 1, 2020, and August 1, 2021, and the patient was discharged home after the visit. Exclusion criteria were patients who required inpatient admission, patient visits to the clinic established solely for COVID-19 vaccination or testing as no other care was ever provided at this location, patients who refused treatment, patients who failed empiric therapy and required treatment adjustments, or patients who were initially treated and received an antibiotic prescription at a facility outside the TCRHCC system.
After chart review and analysis were completed, a prescriber survey and educational intervention were performed from March 2, 2022, to March 31, 2022. This consisted of an anonymous survey to gather demographic data and prescribing habits pre-education, a short educational brief on the existence, location, and recommended use of the outpatient antimicrobial prescribing guidelines, and a posteducation survey to assess knowledge of the guidelines and willingness to adhere to them after the educational intervention.
Results
We reviewed 8779 patient records. A random sample of 10% of the records of each diagnosis code was taken and 876 charts were reviewed. Of the charts reviewed, 351 patients met the inclusion criteria and were included in the analysis. A goal of 90% was established as the target for prescriber adherence for the study based on author consensus for a reasonable goal. Of the 351 evaluated charts, 62 (16.1%) were pediatric patients (aged < 19 years) and 289 (83.9%) were adults (aged ≥ 19 years). Fifty-two (84%) of the pediatric charts and 249 (86%) of the adult charts demonstrated prescribers had appropriately followed guidelines for a combined total of 301 of the 351 charts and an overall adherence rate of 86%. This was 4 points below the established goal of 90%, warranting further investigation. An analysis of prescribers and locations revealed no trends or patterns of nonadherence. A prescriber survey and educational intervention were designed and disseminated to all prescribers at the facility with the approval and assistance of the chief of medicine.
Thirty-nine prescribers responded to the survey. In the pre-educational survey, clinical resources were the most common source of guidance with 36 prescribers (92%) indicating they used them to make an appropriate selection of an antimicrobial; 32 (82%) used personal knowledge, 30 (77%) used culture results, and 24 (62%) used facility guidelines. This was consistent with the posteducational questions: 12 (31%) indicated they were not aware of the facility guidelines before the educational intervention.
Discussion
This study’s objective was to evaluate prescriber adherence to the facility outpatient prescribing guidelines after they were implemented in 2019 and to plan for interventions if necessary. Overall prescriber adherence was high with 86% of the sampled charts adherent. This was below the goal of 90%, so evaluation of the nonadherent charts was warranted for the determination of any patterns to guide the planned interventions with the facility prescribers. However, no trends were identified, so the intervention was designed as a general survey and educational session for all prescribers. Overall prescriber response was positive, with a total of 34 responding prescribers (87%) indicating a willingness to use the guidelines.
Limitations
This is a retrospective observational study performed through chart review that allowed for frequency analysis but did not allow for statistical analysis, so the significance of results cannot be obtained. Additionally, this study was not able to compare rates of adherence before and after the educational intervention, so the effectiveness of the intervention cannot be assessed.
Conclusions
This retrospective observational study’s data demonstrate that prescribers are adhering at a high rate to recommended empiric antimicrobials for outpatient treatment with an 86% adherence rate. Response to educational intervention indicated a larger proportion of prescribers than previously will use the guidelines. However, the impact this will have on appropriate prescribing rates in the future could not be assessed during this study.
1. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2021. 2012. Accessed January 25, 2023. Accessed January 30, 2023. https://tchealth.org/pdfdownload/2021_TCRHCC_Annual_Report.pdf
2. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2013. 2013. Accessed January 25, 2023. Accessed January 30, 2023. https://www.tchealth.org/pdfdownload/2013_Annual_Report.pdf
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(No. RR-6):1–12. doi:10.15585/mmwr.rr6506a1
4. Tuba City Regional Health Care Corporation. Antimicrobial stewardship adult outpatient guidelines. 2019.
5. Tuba City Regional Health Care Corporation. Antimicrobial stewardship pediatric outpatient guidelines. 2019.
1. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2021. 2012. Accessed January 25, 2023. Accessed January 30, 2023. https://tchealth.org/pdfdownload/2021_TCRHCC_Annual_Report.pdf
2. Tuba City Regional Health Care Corporation. TCRHCC Annual Report 2013. 2013. Accessed January 25, 2023. Accessed January 30, 2023. https://www.tchealth.org/pdfdownload/2013_Annual_Report.pdf
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65(No. RR-6):1–12. doi:10.15585/mmwr.rr6506a1
4. Tuba City Regional Health Care Corporation. Antimicrobial stewardship adult outpatient guidelines. 2019.
5. Tuba City Regional Health Care Corporation. Antimicrobial stewardship pediatric outpatient guidelines. 2019.
Central Sleep Apnea in Adults: Diagnosis and Treatment
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring. If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring. If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring. If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
Augmented Reality Demonstration Survey Results From a Veteran Affairs Medical Center
Building the health care system of the future requires the thoughtful development and integration of innovative technologies to positively transform care.1-4 Extended reality (XR) represents a spectrum of emerging technologies that have the potential to enhance health care. This includes virtual reality (VR), where a computer-generated visual experience fills the screen; augmented reality (AR), which allows users to see computer-generated images superimposed into an otherwise normal real-world field of view; and mixed reality (MR), which allows users to interact and manipulate computer-generated AR images.
Clinicians and researchers have begun exploring the potential of XR to address a wide variety of health care challenges. A recent systematic review concluded that many clinical studies in this area have small sample sizes and are in the preclinical, proof-of-concept stage, but demonstrate the potential and impact of the underlying VR, AR, and MR technologies.5 Common emerging health care uses for XR include medical education, training, presurgical planning, surgical guidance, distraction therapy for pain and anxiety, and home health indications, including rehabilitation.5-39
Importantly, some researchers have raised concerns regarding the adaptability of the health care workforce with emerging technologies, and their interest in new methods of delivering care.7,39 Successful deployment of any novel health care technology depends on multiple factors, including alignment with staff needs, receptivity to those solutions, customization to specific preferences, and usability.1,3,40-42 Unfortunately, the implementation of some health care technologies, such as electronic health records that did not account for end-user requirements, resulted in employee fatigue, burnout, and negative staffing turnover.42-44 Conversely, elevated employee morale and operational performance have been directly linked to a climate of inclusion and innovation.45-47 In this assessment, we sought to understand US Department of Veterans Affairs (VA) employees’ perceptions and expert opinions related to the introduction of new AR/MR technology.
Methods
The VA Palo Alto Health Care System (VAPAHCS) consists of 3 inpatient hospitals and 7 outpatient clinics, provides a full range of care services to > 90,000 enrolled veterans with 800 hospital beds, 3 nursing homes, and a 100-bed domiciliary. The facility also runs data-driven care projects in research, innovation, and evidence-based practice group under nursing services.48 This project was performed by the VA National Center for Collaborative Healthcare Innovation at the VAPAHCS campus.
The combined technical system used for this assessment included a wireless communication network, AR/MR hardware, and software. Medivis AnatomyX software displayed an interactive human anatomy atlas segmented into about 6000 individual interactive parts. Medivis SurgicalAR received US Food and Drug Administration clearance for presurgical planning and was used to transform and display deidentified diagnostic images (eg, magnetic resonance images and computed tomography) in 3-dimensional (3D) interactive holograms (Figures 1 and 2).
The wireless Microsoft HoloLens 2 AR/MR headset was used for viewing and sensor-enabled collaborative interaction. Multiple participants in the same physical location simultaneously participated and interacted with 3D holograms. The interactive hologram data were enabled for 3D stereoscopic viewing and manipulation.
Setting and Participants
We reviewed published studies that used questionnaires to evaluate institutions’ level of innovation and new technology user acceptance to develop the questionnaire.49-56 Questions and methods were modified, with a focus on understanding the impact on hospital employees. The questionnaire consisted of 2 predemonstration and 3 postdemonstration sections. The first section included background questions. The second (predemonstration) and third (postdemonstration) sections provided matched questions on feelings about the VA. The fourth section included 2 unmatched questions about how the participant felt this technology would impact veterans and whether the VA should implement similar technologies. We used a 5-point Likert scale for sections 2, 3 and 4 (1 = not at all to 5 = extremely). Two unmatched free-text questions asked how the technology could be used in the participant’s hospital service, and another open-ended question asked for any additional comments. To reduce potential reporting bias, 2 VA employees that did not work at VAPAHCS assisted with the survey distribution and collection. VAPAHCS staff were informed by all employee email and facility intranet of the opportunity to participate; the voluntary demonstration and survey took place on February 10 and 11, 2020.
Data Analysis
All matching pre/post questions were analyzed together to determine statistically significant differences using the Wilcoxon signed rank matched pairs test and pooled t test. Survey respondents were also grouped by employment type to evaluate the impact on subgroups. Results were also grouped by VA tenure into 4 categorical 10-year increments (0-10, 11-20, 21-30, 31-40). Additionally, analysis of variance (ANOVA) was performed on employment types and VA tenure to understand whether there was a statistically significant difference in responses by these subgroups. Respondents’ optional free-text answers were manually reviewed by 2 authors (ZPV and DMA), classified, coded by the common themes, and analyzed for comparison.
Results
A total of 166 participants completed the predemonstration survey, which was a requirement for participating in the AR demonstration. Of those, 159 staff members (95.8%) also completed at least part of the postdemonstration paired structured questions, and their results were included in the analysis.
Paired Questions
For questions about how innovative the VA is, 108 of 152 participants (71.1%) provided higher scores after the demonstration, 42 (27.6%) had no change, and 2 (1.3%) provided decreased scores. The mean innovative score increased from 3.4 predemonstration to 4.5 postdemonstration on a Likert scale, which is a 1.1 point increase from predemonstration to postdemonstration (95% CI, 0.9- 1.2) or a 22% increase (95% CI, 18%-24%) (P < .001). Respondents level of excitement about VA also increased with 82 of 157 participants (52.2%) providing higher scores after the demonstration, 71 (45.2%) had no change, and 4 scores (2.5%) decreased. The predemonstration mean excitement score of 3.7 increased to 4.3 postdemonstration, which is a 0.6 point increase from before to after the demonstration (95% CI, 0.5-0.7) or a 12% increase (95% CI, 10%-14%) (P < .001). In the survey, 36 of 149 participants (24.2%) had higher scores for their expectation to continue working at VA postdemonstration, 109 (73.2%) had no change, and 4 scores (2.7%) decreased. The mean employee retention score increased from 4.2 predemonstration to 4.5 postdemonstration, which is a 0.3 point increase between pre/post (95% CI, 0.2-0.4) or a 6% increase (95% CI, 4%-8%) (P < .001)
The pre/post questions were analyzed using 1-way ANOVA by hospital department and VA tenure. The responses by department were not statistically significant. Of the 159 employees assessed, 101 respondents (63.5%) had 0 to 10 years VA tenure, 44 (27.7%) had 11 to 20 years, 10 (6.3%) had 21 to 30 years, and 4 (2.5%) had > 31 to 40 years. Length of VA tenure did not impact respondent excitement. Respondents opinions on innovation in the 0 to 10 year and the 11 to 20 year groups rose from 3.2 and 3.7 predemonstration to 4.3 and 4.6 postdemonstration, respectively (P < .001 for both statistical comparisons) (Table 2). Interestingly, the 0 to 10 group saw a 9% rise from a 4.0 score predemonstration to a 4.4 score postdemonstration (P < .001), indicating that the demonstration had a positive impact on their plans to continue employment at VA (Table 3).
Sex did not play a significant role in how respondents answered questions regarding VA excitement or innovation. However, there was a statistically significant difference in how male and female respondents answered the predemonstration question about their plans to continue VA employment, according to the Wilcoxon rank sum test. Predemonstration, female respondents had a mean score of 4.1, which was 6% lower than the 4.4 score of male colleagues (P = .04). Veteran status did have an impact on how respondents felt about VA innovation, and their plans to continue employment at VA. After the demonstration, veteran staff felt the VA was more innovative compared with nonveterans: 4.7 vs 4.4, respectively, a 6% difference (P = .02) Similarly, for the continued VA employment question, veterans had a mean score of 4.8 vs 4.4 for nonveterans, an 8% difference (P = .03) These results suggest that the demonstration had more of an impact on veteran employees vs nonveteran employees.
Unpaired Questions
There were 2 structured unpaired postdemonstration questions. Respondents agreed that similar technology would impact veteran health care with mean (SD) of 4.6 (0.6) and a median score of 5 on a 5-point Likert scale. Respondents also agreed on the importance of implementing similar innovations with mean (SD) of 4.7 (0.5), and a median score of 5.
The survey asked how this technology could benefit their hospital service department and had 64 responses. Forty-six respondents saw applications for education or patient care/surgery. Other responses shared excitement about the technology and its potential to positively impact patient education. There were 37 responses to the open-ended question: 21 respondents expressed excitement for the technology, and 10 respondents reiterated that the demonstration would be of benefit to patient care/surgery and training.
Discussion
Successful development, design, and deployment of any new health care tool depends on leveraging insights from the employees that will be using and supporting these systems. Correspondingly, understanding the impact that advanced technologies have on health care employees’ satisfaction, morale, and retention is critical to our overall institutional strategy. Our findings show that a one-time experience with AR/MR technology elicited positive employee reactions. Of note, the survey revealed statistically significant improvements in staff’s view of the VA, with the greatest positive impact for questions about innovation, followed by excitement to work at the VA, and likelihood to continue work at the VA. It is very disruptive and costly when health care employees leave, and improving employee satisfaction and morale is important for better patient care and patient satisfaction, which is priority for VAPAHCS leadership.57-62
The paired predemonstration and postdemonstration scores were similarly high, nearing the top threshold available for the Likert scale (4.3 to 4.5). Furthermore, the least incremental improvement for these responses was observed for topics that had the highest initial baseline score. Therefore, the improvements observed for the paired questions may have more to do with the high baseline values.
Of additional interest, the self-reported likelihood of continuing to work at the VA increased the most for female employees, veteran employees, and employees with the least number of years at the VA. These demographic differences have important implications for VA staff recruitment and retention strategies.62 The unpaired questions about the impact on veteran care and whether the VA should continue similar work demonstrated extremely high support with median scores of 5 for both questions. The free-text postdemonstration responses also demonstrate similar positive themes, with a disposition for excitement about both the training and patient care applications for this technology. In addition, respondents felt strongly that this and other similar technologies will positively impact the health care for veterans and that the VA should continue these efforts.
Strengths and Limitations
A strength of this assessment is the ability to evaluate survey responses that were systematically collected and matched from the same individual immediately before and after exposure to the new technology. The free-text responses provided additional important information that both confirmed the results and provided additional valued supplementary guidance for future implementation strategies, which is critical for our translational implementation goals. An additional strength is that the voluntary surveys were managed by non-VAPAHCS colleagues, limiting potential bias. Importantly, the number of respondents allowed a statistically significant assessment of important health care employee metrics. These results have emphasized how being part of an innovative organization, and the introduction of advanced AR/MR technology, improve employees’ satisfaction and morale about where they work as well as their intention to stay at their institution.
A limitation of this assessment was the lack of comparative data for employee acceptance of other technologies at VAPAHCS. This limits our ability to differentiate whether the strong positive results observed in this evaluation were a result of the specific technology assessed, or of new and advanced health care technology in general. Nonetheless, our unpaired questions, which received extremely high scores, also included participant questions about comparing the system with other similar technologies. This assessment was also focused on veteran care, which limits generalizability.
Conclusions
One-time exposure to advanced AR technology for health care significantly increased employee morale as measured by excitement about working at the VA as well as employee intention to continue employment at the VA. These collateral benefits of the technology are particularly important in health care because our employees are our most important asset and improving employee morale equates to better patient care. Positive impacts were most pronounced for women employees, newer VA employees, and employees who are also veterans. These more detailed insights are also positioned to have a direct impact on employee recruitment and retention strategies. Additional valuable insights regarding the most applicable use of the technology in the clinical setting were also obtained.
Acknowledgments
We thank Andrew Spiegelman, Hyewon Kim, Jonathan Sills, and Alexander Erickson for their assistance in developing the survey questions. We also thank Jason Rhodes and Mark Bulson for traveling to our facility to assist with managing the anonymous surveys during the demonstration event.
1. World Economic Forum. Health and healthcare in the fourth industrial revolution: Global Future Council on the future of health and healthcare 2016-2018. April 2019. Accessed January 27, 2023. https://www3.weforum.org/docs/WEF__Shaping_the_Future_of_Health_Council_Report.pdf
2. Iveroth E, Fryk P, Rapp B. Information technology strategy and alignment issues in health care organizations. Health Care Manage Rev. 2013;38(3):188-200. doi:10.1097/HMR.0b013e31826119d7
3. Thakur R, Hsu SH, Fontenot G. Innovation in healthcare: issues and future trends. J Bus Res. 2012;65(4):562-569. doi:10.1016/j.jbusres.2011.02.022
4. Thimbleby H. Technology and the future of healthcare. J Public Health Res. 2013;2(3):e28. Published 2013 Dec 1. doi:10.4081/jphr.2013.e28
5. Viglialoro RM, Condino S, Turini G, Carbone M, Ferrari V, Gesi M. augmented reality, mixed reality, and hybrid approach in healthcare simulation: a systematic review. Applied Sciences. 2021;11(5):2338. doi:10.3390/app11052338
6. Rawlins CR, Veigulis Z, Hebert C, Curtin C, Osborne T. Effect of immersive virtual reality on pain and anxiety at a Veterans Affairs health care facility. Front Virt Real. 2021;(2):136. doi:10.3389/frvir.2021.719681
7. Chawdhary G, Shoman N. Emerging artificial intelligence applications in otological imaging. Curr Opin Otolaryngol Head Neck Surg. 2021;29(5):357-364. doi:10.1097/MOO.0000000000000754
8. Asadzadeh A, Samad-Soltani T, Rezaei-Hachesu P. Applications of virtual and augmented reality in infectious disease epidemics with a focus on the COVID-19 outbreak. Inform Med Unlocked. 2021;24:100579. doi:10.1016/j.imu.2021.100579
9. Ashwini KB, Savitha R, Harish A. Application of augmented reality technology for home healthcare product visualization. ECS Transas. 2022;107(1):10921. doi:10.1149/10701.10921ecst
10. Brooks AL. VR/Technologies for Rehabilitation. In: Brooks AL, Brahman S, Kapralos B, Nakajima A, Tyerman J, Jain LC, eds. Recent Advances in Technologies for Inclusive Well-Being Virtual Patients, Gamification and Simulation. Intelligent Systems Reference Library. Springer; 2021:241-252. doi:10.1007/978-3-030-59608-8_13
11. Koulouris D, Menychtas A, Maglogiannis I. An IoT-enabled platform for the assessment of physical and mental activities utilizing augmented reality exergaming. Sensors (Basel). 2022;22(9):3181. Published 2022 Apr 21. doi:10.3390/s22093181
12. Deiss YR, Korkut S, Inglese T. Increase therapy understanding and medication adherence for patients with inflammatory skin diseases through augmented reality. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Health, Operations Management, and Design: 13th International Conference, DHM 2022, Held as Part of the 24th HCI International Conference, HCII 2022. 2022:21-40. doi:10.1007/978-3-031-06018-2_2
13. Bertino E, Gao W, Steffan B, et al, eds. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer; 2022:21-40.
14. Ruhaiyem NIR, Mazlan NA. Image Modeling Through Augmented Reality for Skin Allergies Recognition. Lecture Notes on Data Engineering and Communications Technologies. 2021:72-79. doi: 10.1007/978-3-030-70713-2_8
15. Park BJ, Perkons NR, Profka E, et al. Three-dimensional augmented reality visualization informs locoregional therapy in a translational model of hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(10):1612-1618.e1. doi:10.1016/j.jvir.2020.01.028
16. Leo J, Zhou Z, Yang H, et al, eds. Interactive cardiovascular surgical planning via augmented reality. 5th Asian CHI Symposium 2021; 2021. doi:10.1145/3429360.3468195
17. Zuo Y, Jiang T, Dou J, et al. A novel evaluation model for a mixed-reality surgical navigation system: where Microsoft Hololens meets the operating room. Surg Innov. 2020;27(2):193-202. doi:10.1177/1553350619893236
18. Ghaednia H, Fourman MS, Lans A, et al. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617-1625. doi:10.1016/j.spinee.2021.03.018
19. Liu Y, Lee MG, Kim JS. Spine surgery assisted by augmented reality: where have we been?. Yonsei Med J. 2022;63(4):305-316. doi:10.3349/ymj.2022.63.4.305
20. Kimmel S, Cobus V, Heuten W, eds. opticARe—augmented reality mobile patient monitoring in intensive care units. Proceedings of the ACM Symposium on Virtual Reality Software and Technology, VRST; 2021. doi:10.1145/3489849.3489852
21. Voštinár P, Horváthová D, Mitter M, Bako M. The look at the various uses of VR. Open Computer Sci. 2021;11(1):241-250. doi:10.1515/comp-2020-0123
22. Zhao J, Xu X, Jiang H, Ding Y. The effectiveness of virtual reality-based technology on anatomy teaching: a meta-analysis of randomized controlled studies. BMC Med Educ. 2020;20(1):127. Published 2020 Apr 25. doi:10.1186/s12909-020-1994-z
23. Ricci S, Calandrino A, Borgonovo G, Chirico M, Casadio M. Viewpoint: virtual and augmented reality in basic and advanced life support training. JMIR Serious Games. 2022;10(1):e28595. Published 2022 Mar 23. doi:10.2196/28595
24. Ricci S, Mobilio GA, Calandrino A, et al. RiNeo MR: A mixed-reality tool for newborn life support training. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:5043-5046. doi:10.1109/EMBC46164.2021.9629612
25. Dhar P, Rocks T, Samarasinghe RM, Stephenson G, Smith C. Augmented reality in medical education: students’ experiences and learning outcomes. Med Educ Online. 2021;26(1):1953953. doi:10.1080/10872981.2021.1953953
26. Pears M, Konstantinidis S. The future of immersive technology in global surgery education [published online ahead of print, 2021 Jul 1]. Indian J Surg. 2021;84(suppl 1):1-5. doi:10.1007/s12262-021-02998-6
27. Liang CJ, Start C, Boley H, Kamat VR, Menassa CC, Aebersold M. Enhancing stroke assessment simulation experience in clinical training using augmented reality. Virt Real. 2021;25(3):575-584. doi:10.1007/s10055-020-00475-1
28. Lacey G, Gozdzielewska L, McAloney-Kocaman K, Ruttle J, Cronin S, Price L. Psychomotor learning theory informing the design and evaluation of an interactive augmented reality hand hygiene training app for healthcare workers. Educ Inf Technol. 2022;27(3):3813-3832. doi:10.1007/s10639-021-10752-4
29. Ryan GV, Callaghan S, Rafferty A, Higgins MF, Mangina E, McAuliffe F. Learning outcomes of immersive technologies in health care student education: systematic review of the literature. J Med Internet Res. 2022;24(2):e30082. Published 2022 Feb 1. doi:10.2196/30082
30. Yu FU, Yan HU, Sundstedt V. A Systematic literature review of virtual, augmented, and mixed reality game applications in healthcare. ACM Trans Comput Healthcare. 2022;3(2);1-27. doi:10.1145/3472303
31. Weeks JK, Amiel JM. Enhancing neuroanatomy education with augmented reality. Med Educ. 2019;53(5):516-517. doi:10.1111/medu.13843
32. Williams MA, McVeigh J, Handa AI, Lee R. Augmented reality in surgical training: a systematic review. Postgrad Med J. 2020;96(1139):537-542. doi:10.1136/postgradmedj-2020-137600

33. Triepels CPR, Smeets CFA, Notten KJB, et al. Does three-dimensional anatomy improve student understanding? Clin Anat. 2020;33(1):25-33. doi:10.1002/ca.23405
34. Pietruski P, Majak M, S´wia¸tek-Najwer E, et al. Supporting fibula free flap harvest with augmented reality: A proof-of-concept study. Laryngoscope. 2020;130(5):1173-1179. doi:10.1002/lary.28090
35. Perkins SL, Krajancich B, Yang CJ, Hargreaves BA, Daniel BL, Berry MF. A patient-specific mixed-reality visualization tool for thoracic surgical planning. Ann Thorac Surg. 2020;110(1):290-295. doi:10.1016/j.athoracsur.2020.01.060
36. Müller F, Roner S, Liebmann F, Spirig JM, Fürnstahl P, Farshad M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020;20(4):621-628. doi:10.1016/j.spinee.2019.10.012
37. Kaplan AD, Cruit J, Endsley M, Beers SM, Sawyer BD, Hancock PA. The effects of virtual reality, augmented reality, and mixed reality as training enhancement methods: a meta-analysis. Hum Factors. 2021;63(4):706-726. doi:10.1177/0018720820904229
38. Jud L, Fotouhi J, Andronic O, et al. Applicability of augmented reality in orthopedic surgery - a systematic review. BMC Musculoskelet Disord. 2020;21(1):103. Published 2020 Feb 15. doi:10.1186/s12891-020-3110-2
39. Ara J, Karim FB, Alsubaie MSA, et al. Comprehensive analysis of augmented reality technology in modern healthcare system. Int J Adv Comput Sci Appl. 2021;12(6):845-854. doi:10.14569/IJACSA.2021.0120698
40. Webster A, Gardner J. Aligning technology and institutional readiness: the adoption of innovation. Technol Anal Strateg Manag. 2019;31(10):1229-1241. doi:10.1080/09537325.2019.1601694
41. Hastall MR, Dockweiler C, Mühlhaus J. achieving end user acceptance: building blocks for an evidence-based user-centered framework for health technology development and assessment. In: Antona, M, Stephanidis C, eds. Universal Access in Human–Computer Interaction. Human and Technological Environments. UAHCI 2017. Lecture Notes in Computer Science, vol 10279. Springer, Cham; 2017. doi:10.1007/978-3-319-58700-4_2
42. Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
43. Khairat S, Coleman C, Ottmar P, Jayachander DI, Bice T, Carson SS. Association of Electronic Health Record Use With Physician Fatigue and Efficiency. JAMA Netw Open. 2020;3(6):e207385. Published 2020 Jun 1. doi:10.1001/jamanetworkopen.2020.7385
44. Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clin Proc. 2020;95(3):476-487. doi:10.1016/j.mayocp.2019.09.024
45. Lee YJ. Comparison of job satisfaction between nonprofit and public employees. Nonprofit Volunt Sect Q. 2016;45(2):295-313. doi:10.1177/0899764015584061
46. Brimhall KC. Inclusion is important... but how do I include? Examining the effects of leader engagement on inclusion, innovation, job satisfaction, and perceived quality of care in a diverse nonprofit health care organization. Nonprofit Volunt Sect Q. 2019;48(4):716-737. doi:10.1177/0899764019829834
47. Moreira MR, Gherman M, Sousa PS. Does innovation influence the performance of healthcare organizations?. Innovation (North Syd). 2017;19(3):335-352. doi:10.1080/14479338.2017.1293489
48. US Department of Veterans Affairs. VA Palo Alto Healthcare System. Updated December 29, 2020. Accessed January 27, 2023. https://www.paloalto.va.gov/about/index.asp
49. Siegel SM, Kaemmerer WF. Measuring the perceived support for innovation in organizations. J Appl Psychol. 1978;63(5):553-562. doi:10.1037/0021-9010.63.5.553
50. Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav. 1998;19(3):235-258. doi:10.1002/(SICI)1099-1379(199805)19:3<235::AID-JOB837>3.0.CO;2-C
51. Aarons GA. Measuring provider attitudes toward evidence-based practice: consideration of organizational context and individual differences. Child Adolesc Psychiatr Clin N Am. 2005;14(2):255-viii. doi:10.1016/j.chc.2004.04.008
52. Van der Heijden H. User acceptance of hedonic information systems. MIS Q. 2004;28(4):695-704. doi:10.2307/25148660
53. Venkatesh V, Speier C, Morris MG. User acceptance enablers in individual decision making about technology: Toward an integrated model. Decis Sci. 2002;33(2):297-316. doi:10.1111/j.1540-5915.2002.tb01646.x
54. Puri A, Kim B, Nguyen O, Stolee P, Tung J, Lee J. User acceptance of wrist-worn activity trackers among community-dwelling older adults: mixed method study. JMIR Mhealth Uhealth. 2017;5(11):e173. Published 2017 Nov 15. doi:10.2196/mhealth.8211
55. Huang YC, Backman SJ, Backman KF, Moore D. Exploring user acceptance of 3D virtual worlds in travel and tourism marketing. Tourism Management. 2013;36:490-501. doi:10.1016/j.tourman.2012.09.009
56. Rasimah CM, Ahmad A, Zaman HB. Evaluation of user acceptance of mixed reality technology. AJET. 2011;27(8). doi:10.14742/ajet.899
57. Choi J, Boyle DK. RN workgroup job satisfaction and patient falls in acute care hospital units. J Nurs Adm. 2013;43(11):586-591. doi:10.1097/01.NNA.0000434509.66749.7c58. Tzeng HM, Ketefian S. The relationship between nurses’ job satisfaction and inpatient satisfaction: an exploratory study in a Taiwan teaching hospital. J Nurs Care Qual. 2002;16(2):39-49. doi:10.1097/00001786-200201000-00005
59. Williams ES, Skinner AC. Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119-139. doi:10.1097/00004010-200304000-00004
60. Waldman JD, Kelly F, Arora S, Smith HL. The shocking cost of turnover in health care. Health Care Manage Rev. 2004;29(1):2-7. doi:10.1097/00004010-200401000-00002
61. Hayes LJ, O’Brien-Pallas L, Duffield C, et al. Nurse turnover: a literature review - an update. Int J Nurs Stud. 2012;49(7):887-905. doi:10.1016/j.ijnurstu.2011.10.001
62. US Department of Veterans Affairs. FY 2021/FY 2019 Annual performance plan and report. February 2020. Accessed January 27, 2023. https://www.va.gov/oei/docs/VA2019-2021appr.pdf
Building the health care system of the future requires the thoughtful development and integration of innovative technologies to positively transform care.1-4 Extended reality (XR) represents a spectrum of emerging technologies that have the potential to enhance health care. This includes virtual reality (VR), where a computer-generated visual experience fills the screen; augmented reality (AR), which allows users to see computer-generated images superimposed into an otherwise normal real-world field of view; and mixed reality (MR), which allows users to interact and manipulate computer-generated AR images.
Clinicians and researchers have begun exploring the potential of XR to address a wide variety of health care challenges. A recent systematic review concluded that many clinical studies in this area have small sample sizes and are in the preclinical, proof-of-concept stage, but demonstrate the potential and impact of the underlying VR, AR, and MR technologies.5 Common emerging health care uses for XR include medical education, training, presurgical planning, surgical guidance, distraction therapy for pain and anxiety, and home health indications, including rehabilitation.5-39
Importantly, some researchers have raised concerns regarding the adaptability of the health care workforce with emerging technologies, and their interest in new methods of delivering care.7,39 Successful deployment of any novel health care technology depends on multiple factors, including alignment with staff needs, receptivity to those solutions, customization to specific preferences, and usability.1,3,40-42 Unfortunately, the implementation of some health care technologies, such as electronic health records that did not account for end-user requirements, resulted in employee fatigue, burnout, and negative staffing turnover.42-44 Conversely, elevated employee morale and operational performance have been directly linked to a climate of inclusion and innovation.45-47 In this assessment, we sought to understand US Department of Veterans Affairs (VA) employees’ perceptions and expert opinions related to the introduction of new AR/MR technology.
Methods
The VA Palo Alto Health Care System (VAPAHCS) consists of 3 inpatient hospitals and 7 outpatient clinics, provides a full range of care services to > 90,000 enrolled veterans with 800 hospital beds, 3 nursing homes, and a 100-bed domiciliary. The facility also runs data-driven care projects in research, innovation, and evidence-based practice group under nursing services.48 This project was performed by the VA National Center for Collaborative Healthcare Innovation at the VAPAHCS campus.
The combined technical system used for this assessment included a wireless communication network, AR/MR hardware, and software. Medivis AnatomyX software displayed an interactive human anatomy atlas segmented into about 6000 individual interactive parts. Medivis SurgicalAR received US Food and Drug Administration clearance for presurgical planning and was used to transform and display deidentified diagnostic images (eg, magnetic resonance images and computed tomography) in 3-dimensional (3D) interactive holograms (Figures 1 and 2).
The wireless Microsoft HoloLens 2 AR/MR headset was used for viewing and sensor-enabled collaborative interaction. Multiple participants in the same physical location simultaneously participated and interacted with 3D holograms. The interactive hologram data were enabled for 3D stereoscopic viewing and manipulation.
Setting and Participants
We reviewed published studies that used questionnaires to evaluate institutions’ level of innovation and new technology user acceptance to develop the questionnaire.49-56 Questions and methods were modified, with a focus on understanding the impact on hospital employees. The questionnaire consisted of 2 predemonstration and 3 postdemonstration sections. The first section included background questions. The second (predemonstration) and third (postdemonstration) sections provided matched questions on feelings about the VA. The fourth section included 2 unmatched questions about how the participant felt this technology would impact veterans and whether the VA should implement similar technologies. We used a 5-point Likert scale for sections 2, 3 and 4 (1 = not at all to 5 = extremely). Two unmatched free-text questions asked how the technology could be used in the participant’s hospital service, and another open-ended question asked for any additional comments. To reduce potential reporting bias, 2 VA employees that did not work at VAPAHCS assisted with the survey distribution and collection. VAPAHCS staff were informed by all employee email and facility intranet of the opportunity to participate; the voluntary demonstration and survey took place on February 10 and 11, 2020.
Data Analysis
All matching pre/post questions were analyzed together to determine statistically significant differences using the Wilcoxon signed rank matched pairs test and pooled t test. Survey respondents were also grouped by employment type to evaluate the impact on subgroups. Results were also grouped by VA tenure into 4 categorical 10-year increments (0-10, 11-20, 21-30, 31-40). Additionally, analysis of variance (ANOVA) was performed on employment types and VA tenure to understand whether there was a statistically significant difference in responses by these subgroups. Respondents’ optional free-text answers were manually reviewed by 2 authors (ZPV and DMA), classified, coded by the common themes, and analyzed for comparison.
Results
A total of 166 participants completed the predemonstration survey, which was a requirement for participating in the AR demonstration. Of those, 159 staff members (95.8%) also completed at least part of the postdemonstration paired structured questions, and their results were included in the analysis.
Paired Questions
For questions about how innovative the VA is, 108 of 152 participants (71.1%) provided higher scores after the demonstration, 42 (27.6%) had no change, and 2 (1.3%) provided decreased scores. The mean innovative score increased from 3.4 predemonstration to 4.5 postdemonstration on a Likert scale, which is a 1.1 point increase from predemonstration to postdemonstration (95% CI, 0.9- 1.2) or a 22% increase (95% CI, 18%-24%) (P < .001). Respondents level of excitement about VA also increased with 82 of 157 participants (52.2%) providing higher scores after the demonstration, 71 (45.2%) had no change, and 4 scores (2.5%) decreased. The predemonstration mean excitement score of 3.7 increased to 4.3 postdemonstration, which is a 0.6 point increase from before to after the demonstration (95% CI, 0.5-0.7) or a 12% increase (95% CI, 10%-14%) (P < .001). In the survey, 36 of 149 participants (24.2%) had higher scores for their expectation to continue working at VA postdemonstration, 109 (73.2%) had no change, and 4 scores (2.7%) decreased. The mean employee retention score increased from 4.2 predemonstration to 4.5 postdemonstration, which is a 0.3 point increase between pre/post (95% CI, 0.2-0.4) or a 6% increase (95% CI, 4%-8%) (P < .001)
The pre/post questions were analyzed using 1-way ANOVA by hospital department and VA tenure. The responses by department were not statistically significant. Of the 159 employees assessed, 101 respondents (63.5%) had 0 to 10 years VA tenure, 44 (27.7%) had 11 to 20 years, 10 (6.3%) had 21 to 30 years, and 4 (2.5%) had > 31 to 40 years. Length of VA tenure did not impact respondent excitement. Respondents opinions on innovation in the 0 to 10 year and the 11 to 20 year groups rose from 3.2 and 3.7 predemonstration to 4.3 and 4.6 postdemonstration, respectively (P < .001 for both statistical comparisons) (Table 2). Interestingly, the 0 to 10 group saw a 9% rise from a 4.0 score predemonstration to a 4.4 score postdemonstration (P < .001), indicating that the demonstration had a positive impact on their plans to continue employment at VA (Table 3).
Sex did not play a significant role in how respondents answered questions regarding VA excitement or innovation. However, there was a statistically significant difference in how male and female respondents answered the predemonstration question about their plans to continue VA employment, according to the Wilcoxon rank sum test. Predemonstration, female respondents had a mean score of 4.1, which was 6% lower than the 4.4 score of male colleagues (P = .04). Veteran status did have an impact on how respondents felt about VA innovation, and their plans to continue employment at VA. After the demonstration, veteran staff felt the VA was more innovative compared with nonveterans: 4.7 vs 4.4, respectively, a 6% difference (P = .02) Similarly, for the continued VA employment question, veterans had a mean score of 4.8 vs 4.4 for nonveterans, an 8% difference (P = .03) These results suggest that the demonstration had more of an impact on veteran employees vs nonveteran employees.
Unpaired Questions
There were 2 structured unpaired postdemonstration questions. Respondents agreed that similar technology would impact veteran health care with mean (SD) of 4.6 (0.6) and a median score of 5 on a 5-point Likert scale. Respondents also agreed on the importance of implementing similar innovations with mean (SD) of 4.7 (0.5), and a median score of 5.
The survey asked how this technology could benefit their hospital service department and had 64 responses. Forty-six respondents saw applications for education or patient care/surgery. Other responses shared excitement about the technology and its potential to positively impact patient education. There were 37 responses to the open-ended question: 21 respondents expressed excitement for the technology, and 10 respondents reiterated that the demonstration would be of benefit to patient care/surgery and training.
Discussion
Successful development, design, and deployment of any new health care tool depends on leveraging insights from the employees that will be using and supporting these systems. Correspondingly, understanding the impact that advanced technologies have on health care employees’ satisfaction, morale, and retention is critical to our overall institutional strategy. Our findings show that a one-time experience with AR/MR technology elicited positive employee reactions. Of note, the survey revealed statistically significant improvements in staff’s view of the VA, with the greatest positive impact for questions about innovation, followed by excitement to work at the VA, and likelihood to continue work at the VA. It is very disruptive and costly when health care employees leave, and improving employee satisfaction and morale is important for better patient care and patient satisfaction, which is priority for VAPAHCS leadership.57-62
The paired predemonstration and postdemonstration scores were similarly high, nearing the top threshold available for the Likert scale (4.3 to 4.5). Furthermore, the least incremental improvement for these responses was observed for topics that had the highest initial baseline score. Therefore, the improvements observed for the paired questions may have more to do with the high baseline values.
Of additional interest, the self-reported likelihood of continuing to work at the VA increased the most for female employees, veteran employees, and employees with the least number of years at the VA. These demographic differences have important implications for VA staff recruitment and retention strategies.62 The unpaired questions about the impact on veteran care and whether the VA should continue similar work demonstrated extremely high support with median scores of 5 for both questions. The free-text postdemonstration responses also demonstrate similar positive themes, with a disposition for excitement about both the training and patient care applications for this technology. In addition, respondents felt strongly that this and other similar technologies will positively impact the health care for veterans and that the VA should continue these efforts.
Strengths and Limitations
A strength of this assessment is the ability to evaluate survey responses that were systematically collected and matched from the same individual immediately before and after exposure to the new technology. The free-text responses provided additional important information that both confirmed the results and provided additional valued supplementary guidance for future implementation strategies, which is critical for our translational implementation goals. An additional strength is that the voluntary surveys were managed by non-VAPAHCS colleagues, limiting potential bias. Importantly, the number of respondents allowed a statistically significant assessment of important health care employee metrics. These results have emphasized how being part of an innovative organization, and the introduction of advanced AR/MR technology, improve employees’ satisfaction and morale about where they work as well as their intention to stay at their institution.
A limitation of this assessment was the lack of comparative data for employee acceptance of other technologies at VAPAHCS. This limits our ability to differentiate whether the strong positive results observed in this evaluation were a result of the specific technology assessed, or of new and advanced health care technology in general. Nonetheless, our unpaired questions, which received extremely high scores, also included participant questions about comparing the system with other similar technologies. This assessment was also focused on veteran care, which limits generalizability.
Conclusions
One-time exposure to advanced AR technology for health care significantly increased employee morale as measured by excitement about working at the VA as well as employee intention to continue employment at the VA. These collateral benefits of the technology are particularly important in health care because our employees are our most important asset and improving employee morale equates to better patient care. Positive impacts were most pronounced for women employees, newer VA employees, and employees who are also veterans. These more detailed insights are also positioned to have a direct impact on employee recruitment and retention strategies. Additional valuable insights regarding the most applicable use of the technology in the clinical setting were also obtained.
Acknowledgments
We thank Andrew Spiegelman, Hyewon Kim, Jonathan Sills, and Alexander Erickson for their assistance in developing the survey questions. We also thank Jason Rhodes and Mark Bulson for traveling to our facility to assist with managing the anonymous surveys during the demonstration event.
Building the health care system of the future requires the thoughtful development and integration of innovative technologies to positively transform care.1-4 Extended reality (XR) represents a spectrum of emerging technologies that have the potential to enhance health care. This includes virtual reality (VR), where a computer-generated visual experience fills the screen; augmented reality (AR), which allows users to see computer-generated images superimposed into an otherwise normal real-world field of view; and mixed reality (MR), which allows users to interact and manipulate computer-generated AR images.
Clinicians and researchers have begun exploring the potential of XR to address a wide variety of health care challenges. A recent systematic review concluded that many clinical studies in this area have small sample sizes and are in the preclinical, proof-of-concept stage, but demonstrate the potential and impact of the underlying VR, AR, and MR technologies.5 Common emerging health care uses for XR include medical education, training, presurgical planning, surgical guidance, distraction therapy for pain and anxiety, and home health indications, including rehabilitation.5-39
Importantly, some researchers have raised concerns regarding the adaptability of the health care workforce with emerging technologies, and their interest in new methods of delivering care.7,39 Successful deployment of any novel health care technology depends on multiple factors, including alignment with staff needs, receptivity to those solutions, customization to specific preferences, and usability.1,3,40-42 Unfortunately, the implementation of some health care technologies, such as electronic health records that did not account for end-user requirements, resulted in employee fatigue, burnout, and negative staffing turnover.42-44 Conversely, elevated employee morale and operational performance have been directly linked to a climate of inclusion and innovation.45-47 In this assessment, we sought to understand US Department of Veterans Affairs (VA) employees’ perceptions and expert opinions related to the introduction of new AR/MR technology.
Methods
The VA Palo Alto Health Care System (VAPAHCS) consists of 3 inpatient hospitals and 7 outpatient clinics, provides a full range of care services to > 90,000 enrolled veterans with 800 hospital beds, 3 nursing homes, and a 100-bed domiciliary. The facility also runs data-driven care projects in research, innovation, and evidence-based practice group under nursing services.48 This project was performed by the VA National Center for Collaborative Healthcare Innovation at the VAPAHCS campus.
The combined technical system used for this assessment included a wireless communication network, AR/MR hardware, and software. Medivis AnatomyX software displayed an interactive human anatomy atlas segmented into about 6000 individual interactive parts. Medivis SurgicalAR received US Food and Drug Administration clearance for presurgical planning and was used to transform and display deidentified diagnostic images (eg, magnetic resonance images and computed tomography) in 3-dimensional (3D) interactive holograms (Figures 1 and 2).
The wireless Microsoft HoloLens 2 AR/MR headset was used for viewing and sensor-enabled collaborative interaction. Multiple participants in the same physical location simultaneously participated and interacted with 3D holograms. The interactive hologram data were enabled for 3D stereoscopic viewing and manipulation.
Setting and Participants
We reviewed published studies that used questionnaires to evaluate institutions’ level of innovation and new technology user acceptance to develop the questionnaire.49-56 Questions and methods were modified, with a focus on understanding the impact on hospital employees. The questionnaire consisted of 2 predemonstration and 3 postdemonstration sections. The first section included background questions. The second (predemonstration) and third (postdemonstration) sections provided matched questions on feelings about the VA. The fourth section included 2 unmatched questions about how the participant felt this technology would impact veterans and whether the VA should implement similar technologies. We used a 5-point Likert scale for sections 2, 3 and 4 (1 = not at all to 5 = extremely). Two unmatched free-text questions asked how the technology could be used in the participant’s hospital service, and another open-ended question asked for any additional comments. To reduce potential reporting bias, 2 VA employees that did not work at VAPAHCS assisted with the survey distribution and collection. VAPAHCS staff were informed by all employee email and facility intranet of the opportunity to participate; the voluntary demonstration and survey took place on February 10 and 11, 2020.
Data Analysis
All matching pre/post questions were analyzed together to determine statistically significant differences using the Wilcoxon signed rank matched pairs test and pooled t test. Survey respondents were also grouped by employment type to evaluate the impact on subgroups. Results were also grouped by VA tenure into 4 categorical 10-year increments (0-10, 11-20, 21-30, 31-40). Additionally, analysis of variance (ANOVA) was performed on employment types and VA tenure to understand whether there was a statistically significant difference in responses by these subgroups. Respondents’ optional free-text answers were manually reviewed by 2 authors (ZPV and DMA), classified, coded by the common themes, and analyzed for comparison.
Results
A total of 166 participants completed the predemonstration survey, which was a requirement for participating in the AR demonstration. Of those, 159 staff members (95.8%) also completed at least part of the postdemonstration paired structured questions, and their results were included in the analysis.
Paired Questions
For questions about how innovative the VA is, 108 of 152 participants (71.1%) provided higher scores after the demonstration, 42 (27.6%) had no change, and 2 (1.3%) provided decreased scores. The mean innovative score increased from 3.4 predemonstration to 4.5 postdemonstration on a Likert scale, which is a 1.1 point increase from predemonstration to postdemonstration (95% CI, 0.9- 1.2) or a 22% increase (95% CI, 18%-24%) (P < .001). Respondents level of excitement about VA also increased with 82 of 157 participants (52.2%) providing higher scores after the demonstration, 71 (45.2%) had no change, and 4 scores (2.5%) decreased. The predemonstration mean excitement score of 3.7 increased to 4.3 postdemonstration, which is a 0.6 point increase from before to after the demonstration (95% CI, 0.5-0.7) or a 12% increase (95% CI, 10%-14%) (P < .001). In the survey, 36 of 149 participants (24.2%) had higher scores for their expectation to continue working at VA postdemonstration, 109 (73.2%) had no change, and 4 scores (2.7%) decreased. The mean employee retention score increased from 4.2 predemonstration to 4.5 postdemonstration, which is a 0.3 point increase between pre/post (95% CI, 0.2-0.4) or a 6% increase (95% CI, 4%-8%) (P < .001)
The pre/post questions were analyzed using 1-way ANOVA by hospital department and VA tenure. The responses by department were not statistically significant. Of the 159 employees assessed, 101 respondents (63.5%) had 0 to 10 years VA tenure, 44 (27.7%) had 11 to 20 years, 10 (6.3%) had 21 to 30 years, and 4 (2.5%) had > 31 to 40 years. Length of VA tenure did not impact respondent excitement. Respondents opinions on innovation in the 0 to 10 year and the 11 to 20 year groups rose from 3.2 and 3.7 predemonstration to 4.3 and 4.6 postdemonstration, respectively (P < .001 for both statistical comparisons) (Table 2). Interestingly, the 0 to 10 group saw a 9% rise from a 4.0 score predemonstration to a 4.4 score postdemonstration (P < .001), indicating that the demonstration had a positive impact on their plans to continue employment at VA (Table 3).
Sex did not play a significant role in how respondents answered questions regarding VA excitement or innovation. However, there was a statistically significant difference in how male and female respondents answered the predemonstration question about their plans to continue VA employment, according to the Wilcoxon rank sum test. Predemonstration, female respondents had a mean score of 4.1, which was 6% lower than the 4.4 score of male colleagues (P = .04). Veteran status did have an impact on how respondents felt about VA innovation, and their plans to continue employment at VA. After the demonstration, veteran staff felt the VA was more innovative compared with nonveterans: 4.7 vs 4.4, respectively, a 6% difference (P = .02) Similarly, for the continued VA employment question, veterans had a mean score of 4.8 vs 4.4 for nonveterans, an 8% difference (P = .03) These results suggest that the demonstration had more of an impact on veteran employees vs nonveteran employees.
Unpaired Questions
There were 2 structured unpaired postdemonstration questions. Respondents agreed that similar technology would impact veteran health care with mean (SD) of 4.6 (0.6) and a median score of 5 on a 5-point Likert scale. Respondents also agreed on the importance of implementing similar innovations with mean (SD) of 4.7 (0.5), and a median score of 5.
The survey asked how this technology could benefit their hospital service department and had 64 responses. Forty-six respondents saw applications for education or patient care/surgery. Other responses shared excitement about the technology and its potential to positively impact patient education. There were 37 responses to the open-ended question: 21 respondents expressed excitement for the technology, and 10 respondents reiterated that the demonstration would be of benefit to patient care/surgery and training.
Discussion
Successful development, design, and deployment of any new health care tool depends on leveraging insights from the employees that will be using and supporting these systems. Correspondingly, understanding the impact that advanced technologies have on health care employees’ satisfaction, morale, and retention is critical to our overall institutional strategy. Our findings show that a one-time experience with AR/MR technology elicited positive employee reactions. Of note, the survey revealed statistically significant improvements in staff’s view of the VA, with the greatest positive impact for questions about innovation, followed by excitement to work at the VA, and likelihood to continue work at the VA. It is very disruptive and costly when health care employees leave, and improving employee satisfaction and morale is important for better patient care and patient satisfaction, which is priority for VAPAHCS leadership.57-62
The paired predemonstration and postdemonstration scores were similarly high, nearing the top threshold available for the Likert scale (4.3 to 4.5). Furthermore, the least incremental improvement for these responses was observed for topics that had the highest initial baseline score. Therefore, the improvements observed for the paired questions may have more to do with the high baseline values.
Of additional interest, the self-reported likelihood of continuing to work at the VA increased the most for female employees, veteran employees, and employees with the least number of years at the VA. These demographic differences have important implications for VA staff recruitment and retention strategies.62 The unpaired questions about the impact on veteran care and whether the VA should continue similar work demonstrated extremely high support with median scores of 5 for both questions. The free-text postdemonstration responses also demonstrate similar positive themes, with a disposition for excitement about both the training and patient care applications for this technology. In addition, respondents felt strongly that this and other similar technologies will positively impact the health care for veterans and that the VA should continue these efforts.
Strengths and Limitations
A strength of this assessment is the ability to evaluate survey responses that were systematically collected and matched from the same individual immediately before and after exposure to the new technology. The free-text responses provided additional important information that both confirmed the results and provided additional valued supplementary guidance for future implementation strategies, which is critical for our translational implementation goals. An additional strength is that the voluntary surveys were managed by non-VAPAHCS colleagues, limiting potential bias. Importantly, the number of respondents allowed a statistically significant assessment of important health care employee metrics. These results have emphasized how being part of an innovative organization, and the introduction of advanced AR/MR technology, improve employees’ satisfaction and morale about where they work as well as their intention to stay at their institution.
A limitation of this assessment was the lack of comparative data for employee acceptance of other technologies at VAPAHCS. This limits our ability to differentiate whether the strong positive results observed in this evaluation were a result of the specific technology assessed, or of new and advanced health care technology in general. Nonetheless, our unpaired questions, which received extremely high scores, also included participant questions about comparing the system with other similar technologies. This assessment was also focused on veteran care, which limits generalizability.
Conclusions
One-time exposure to advanced AR technology for health care significantly increased employee morale as measured by excitement about working at the VA as well as employee intention to continue employment at the VA. These collateral benefits of the technology are particularly important in health care because our employees are our most important asset and improving employee morale equates to better patient care. Positive impacts were most pronounced for women employees, newer VA employees, and employees who are also veterans. These more detailed insights are also positioned to have a direct impact on employee recruitment and retention strategies. Additional valuable insights regarding the most applicable use of the technology in the clinical setting were also obtained.
Acknowledgments
We thank Andrew Spiegelman, Hyewon Kim, Jonathan Sills, and Alexander Erickson for their assistance in developing the survey questions. We also thank Jason Rhodes and Mark Bulson for traveling to our facility to assist with managing the anonymous surveys during the demonstration event.
1. World Economic Forum. Health and healthcare in the fourth industrial revolution: Global Future Council on the future of health and healthcare 2016-2018. April 2019. Accessed January 27, 2023. https://www3.weforum.org/docs/WEF__Shaping_the_Future_of_Health_Council_Report.pdf
2. Iveroth E, Fryk P, Rapp B. Information technology strategy and alignment issues in health care organizations. Health Care Manage Rev. 2013;38(3):188-200. doi:10.1097/HMR.0b013e31826119d7
3. Thakur R, Hsu SH, Fontenot G. Innovation in healthcare: issues and future trends. J Bus Res. 2012;65(4):562-569. doi:10.1016/j.jbusres.2011.02.022
4. Thimbleby H. Technology and the future of healthcare. J Public Health Res. 2013;2(3):e28. Published 2013 Dec 1. doi:10.4081/jphr.2013.e28
5. Viglialoro RM, Condino S, Turini G, Carbone M, Ferrari V, Gesi M. augmented reality, mixed reality, and hybrid approach in healthcare simulation: a systematic review. Applied Sciences. 2021;11(5):2338. doi:10.3390/app11052338
6. Rawlins CR, Veigulis Z, Hebert C, Curtin C, Osborne T. Effect of immersive virtual reality on pain and anxiety at a Veterans Affairs health care facility. Front Virt Real. 2021;(2):136. doi:10.3389/frvir.2021.719681
7. Chawdhary G, Shoman N. Emerging artificial intelligence applications in otological imaging. Curr Opin Otolaryngol Head Neck Surg. 2021;29(5):357-364. doi:10.1097/MOO.0000000000000754
8. Asadzadeh A, Samad-Soltani T, Rezaei-Hachesu P. Applications of virtual and augmented reality in infectious disease epidemics with a focus on the COVID-19 outbreak. Inform Med Unlocked. 2021;24:100579. doi:10.1016/j.imu.2021.100579
9. Ashwini KB, Savitha R, Harish A. Application of augmented reality technology for home healthcare product visualization. ECS Transas. 2022;107(1):10921. doi:10.1149/10701.10921ecst
10. Brooks AL. VR/Technologies for Rehabilitation. In: Brooks AL, Brahman S, Kapralos B, Nakajima A, Tyerman J, Jain LC, eds. Recent Advances in Technologies for Inclusive Well-Being Virtual Patients, Gamification and Simulation. Intelligent Systems Reference Library. Springer; 2021:241-252. doi:10.1007/978-3-030-59608-8_13
11. Koulouris D, Menychtas A, Maglogiannis I. An IoT-enabled platform for the assessment of physical and mental activities utilizing augmented reality exergaming. Sensors (Basel). 2022;22(9):3181. Published 2022 Apr 21. doi:10.3390/s22093181
12. Deiss YR, Korkut S, Inglese T. Increase therapy understanding and medication adherence for patients with inflammatory skin diseases through augmented reality. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Health, Operations Management, and Design: 13th International Conference, DHM 2022, Held as Part of the 24th HCI International Conference, HCII 2022. 2022:21-40. doi:10.1007/978-3-031-06018-2_2
13. Bertino E, Gao W, Steffan B, et al, eds. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer; 2022:21-40.
14. Ruhaiyem NIR, Mazlan NA. Image Modeling Through Augmented Reality for Skin Allergies Recognition. Lecture Notes on Data Engineering and Communications Technologies. 2021:72-79. doi: 10.1007/978-3-030-70713-2_8
15. Park BJ, Perkons NR, Profka E, et al. Three-dimensional augmented reality visualization informs locoregional therapy in a translational model of hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(10):1612-1618.e1. doi:10.1016/j.jvir.2020.01.028
16. Leo J, Zhou Z, Yang H, et al, eds. Interactive cardiovascular surgical planning via augmented reality. 5th Asian CHI Symposium 2021; 2021. doi:10.1145/3429360.3468195
17. Zuo Y, Jiang T, Dou J, et al. A novel evaluation model for a mixed-reality surgical navigation system: where Microsoft Hololens meets the operating room. Surg Innov. 2020;27(2):193-202. doi:10.1177/1553350619893236
18. Ghaednia H, Fourman MS, Lans A, et al. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617-1625. doi:10.1016/j.spinee.2021.03.018
19. Liu Y, Lee MG, Kim JS. Spine surgery assisted by augmented reality: where have we been?. Yonsei Med J. 2022;63(4):305-316. doi:10.3349/ymj.2022.63.4.305
20. Kimmel S, Cobus V, Heuten W, eds. opticARe—augmented reality mobile patient monitoring in intensive care units. Proceedings of the ACM Symposium on Virtual Reality Software and Technology, VRST; 2021. doi:10.1145/3489849.3489852
21. Voštinár P, Horváthová D, Mitter M, Bako M. The look at the various uses of VR. Open Computer Sci. 2021;11(1):241-250. doi:10.1515/comp-2020-0123
22. Zhao J, Xu X, Jiang H, Ding Y. The effectiveness of virtual reality-based technology on anatomy teaching: a meta-analysis of randomized controlled studies. BMC Med Educ. 2020;20(1):127. Published 2020 Apr 25. doi:10.1186/s12909-020-1994-z
23. Ricci S, Calandrino A, Borgonovo G, Chirico M, Casadio M. Viewpoint: virtual and augmented reality in basic and advanced life support training. JMIR Serious Games. 2022;10(1):e28595. Published 2022 Mar 23. doi:10.2196/28595
24. Ricci S, Mobilio GA, Calandrino A, et al. RiNeo MR: A mixed-reality tool for newborn life support training. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:5043-5046. doi:10.1109/EMBC46164.2021.9629612
25. Dhar P, Rocks T, Samarasinghe RM, Stephenson G, Smith C. Augmented reality in medical education: students’ experiences and learning outcomes. Med Educ Online. 2021;26(1):1953953. doi:10.1080/10872981.2021.1953953
26. Pears M, Konstantinidis S. The future of immersive technology in global surgery education [published online ahead of print, 2021 Jul 1]. Indian J Surg. 2021;84(suppl 1):1-5. doi:10.1007/s12262-021-02998-6
27. Liang CJ, Start C, Boley H, Kamat VR, Menassa CC, Aebersold M. Enhancing stroke assessment simulation experience in clinical training using augmented reality. Virt Real. 2021;25(3):575-584. doi:10.1007/s10055-020-00475-1
28. Lacey G, Gozdzielewska L, McAloney-Kocaman K, Ruttle J, Cronin S, Price L. Psychomotor learning theory informing the design and evaluation of an interactive augmented reality hand hygiene training app for healthcare workers. Educ Inf Technol. 2022;27(3):3813-3832. doi:10.1007/s10639-021-10752-4
29. Ryan GV, Callaghan S, Rafferty A, Higgins MF, Mangina E, McAuliffe F. Learning outcomes of immersive technologies in health care student education: systematic review of the literature. J Med Internet Res. 2022;24(2):e30082. Published 2022 Feb 1. doi:10.2196/30082
30. Yu FU, Yan HU, Sundstedt V. A Systematic literature review of virtual, augmented, and mixed reality game applications in healthcare. ACM Trans Comput Healthcare. 2022;3(2);1-27. doi:10.1145/3472303
31. Weeks JK, Amiel JM. Enhancing neuroanatomy education with augmented reality. Med Educ. 2019;53(5):516-517. doi:10.1111/medu.13843
32. Williams MA, McVeigh J, Handa AI, Lee R. Augmented reality in surgical training: a systematic review. Postgrad Med J. 2020;96(1139):537-542. doi:10.1136/postgradmedj-2020-137600

33. Triepels CPR, Smeets CFA, Notten KJB, et al. Does three-dimensional anatomy improve student understanding? Clin Anat. 2020;33(1):25-33. doi:10.1002/ca.23405
34. Pietruski P, Majak M, S´wia¸tek-Najwer E, et al. Supporting fibula free flap harvest with augmented reality: A proof-of-concept study. Laryngoscope. 2020;130(5):1173-1179. doi:10.1002/lary.28090
35. Perkins SL, Krajancich B, Yang CJ, Hargreaves BA, Daniel BL, Berry MF. A patient-specific mixed-reality visualization tool for thoracic surgical planning. Ann Thorac Surg. 2020;110(1):290-295. doi:10.1016/j.athoracsur.2020.01.060
36. Müller F, Roner S, Liebmann F, Spirig JM, Fürnstahl P, Farshad M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020;20(4):621-628. doi:10.1016/j.spinee.2019.10.012
37. Kaplan AD, Cruit J, Endsley M, Beers SM, Sawyer BD, Hancock PA. The effects of virtual reality, augmented reality, and mixed reality as training enhancement methods: a meta-analysis. Hum Factors. 2021;63(4):706-726. doi:10.1177/0018720820904229
38. Jud L, Fotouhi J, Andronic O, et al. Applicability of augmented reality in orthopedic surgery - a systematic review. BMC Musculoskelet Disord. 2020;21(1):103. Published 2020 Feb 15. doi:10.1186/s12891-020-3110-2
39. Ara J, Karim FB, Alsubaie MSA, et al. Comprehensive analysis of augmented reality technology in modern healthcare system. Int J Adv Comput Sci Appl. 2021;12(6):845-854. doi:10.14569/IJACSA.2021.0120698
40. Webster A, Gardner J. Aligning technology and institutional readiness: the adoption of innovation. Technol Anal Strateg Manag. 2019;31(10):1229-1241. doi:10.1080/09537325.2019.1601694
41. Hastall MR, Dockweiler C, Mühlhaus J. achieving end user acceptance: building blocks for an evidence-based user-centered framework for health technology development and assessment. In: Antona, M, Stephanidis C, eds. Universal Access in Human–Computer Interaction. Human and Technological Environments. UAHCI 2017. Lecture Notes in Computer Science, vol 10279. Springer, Cham; 2017. doi:10.1007/978-3-319-58700-4_2
42. Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
43. Khairat S, Coleman C, Ottmar P, Jayachander DI, Bice T, Carson SS. Association of Electronic Health Record Use With Physician Fatigue and Efficiency. JAMA Netw Open. 2020;3(6):e207385. Published 2020 Jun 1. doi:10.1001/jamanetworkopen.2020.7385
44. Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clin Proc. 2020;95(3):476-487. doi:10.1016/j.mayocp.2019.09.024
45. Lee YJ. Comparison of job satisfaction between nonprofit and public employees. Nonprofit Volunt Sect Q. 2016;45(2):295-313. doi:10.1177/0899764015584061
46. Brimhall KC. Inclusion is important... but how do I include? Examining the effects of leader engagement on inclusion, innovation, job satisfaction, and perceived quality of care in a diverse nonprofit health care organization. Nonprofit Volunt Sect Q. 2019;48(4):716-737. doi:10.1177/0899764019829834
47. Moreira MR, Gherman M, Sousa PS. Does innovation influence the performance of healthcare organizations?. Innovation (North Syd). 2017;19(3):335-352. doi:10.1080/14479338.2017.1293489
48. US Department of Veterans Affairs. VA Palo Alto Healthcare System. Updated December 29, 2020. Accessed January 27, 2023. https://www.paloalto.va.gov/about/index.asp
49. Siegel SM, Kaemmerer WF. Measuring the perceived support for innovation in organizations. J Appl Psychol. 1978;63(5):553-562. doi:10.1037/0021-9010.63.5.553
50. Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav. 1998;19(3):235-258. doi:10.1002/(SICI)1099-1379(199805)19:3<235::AID-JOB837>3.0.CO;2-C
51. Aarons GA. Measuring provider attitudes toward evidence-based practice: consideration of organizational context and individual differences. Child Adolesc Psychiatr Clin N Am. 2005;14(2):255-viii. doi:10.1016/j.chc.2004.04.008
52. Van der Heijden H. User acceptance of hedonic information systems. MIS Q. 2004;28(4):695-704. doi:10.2307/25148660
53. Venkatesh V, Speier C, Morris MG. User acceptance enablers in individual decision making about technology: Toward an integrated model. Decis Sci. 2002;33(2):297-316. doi:10.1111/j.1540-5915.2002.tb01646.x
54. Puri A, Kim B, Nguyen O, Stolee P, Tung J, Lee J. User acceptance of wrist-worn activity trackers among community-dwelling older adults: mixed method study. JMIR Mhealth Uhealth. 2017;5(11):e173. Published 2017 Nov 15. doi:10.2196/mhealth.8211
55. Huang YC, Backman SJ, Backman KF, Moore D. Exploring user acceptance of 3D virtual worlds in travel and tourism marketing. Tourism Management. 2013;36:490-501. doi:10.1016/j.tourman.2012.09.009
56. Rasimah CM, Ahmad A, Zaman HB. Evaluation of user acceptance of mixed reality technology. AJET. 2011;27(8). doi:10.14742/ajet.899
57. Choi J, Boyle DK. RN workgroup job satisfaction and patient falls in acute care hospital units. J Nurs Adm. 2013;43(11):586-591. doi:10.1097/01.NNA.0000434509.66749.7c58. Tzeng HM, Ketefian S. The relationship between nurses’ job satisfaction and inpatient satisfaction: an exploratory study in a Taiwan teaching hospital. J Nurs Care Qual. 2002;16(2):39-49. doi:10.1097/00001786-200201000-00005
59. Williams ES, Skinner AC. Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119-139. doi:10.1097/00004010-200304000-00004
60. Waldman JD, Kelly F, Arora S, Smith HL. The shocking cost of turnover in health care. Health Care Manage Rev. 2004;29(1):2-7. doi:10.1097/00004010-200401000-00002
61. Hayes LJ, O’Brien-Pallas L, Duffield C, et al. Nurse turnover: a literature review - an update. Int J Nurs Stud. 2012;49(7):887-905. doi:10.1016/j.ijnurstu.2011.10.001
62. US Department of Veterans Affairs. FY 2021/FY 2019 Annual performance plan and report. February 2020. Accessed January 27, 2023. https://www.va.gov/oei/docs/VA2019-2021appr.pdf
1. World Economic Forum. Health and healthcare in the fourth industrial revolution: Global Future Council on the future of health and healthcare 2016-2018. April 2019. Accessed January 27, 2023. https://www3.weforum.org/docs/WEF__Shaping_the_Future_of_Health_Council_Report.pdf
2. Iveroth E, Fryk P, Rapp B. Information technology strategy and alignment issues in health care organizations. Health Care Manage Rev. 2013;38(3):188-200. doi:10.1097/HMR.0b013e31826119d7
3. Thakur R, Hsu SH, Fontenot G. Innovation in healthcare: issues and future trends. J Bus Res. 2012;65(4):562-569. doi:10.1016/j.jbusres.2011.02.022
4. Thimbleby H. Technology and the future of healthcare. J Public Health Res. 2013;2(3):e28. Published 2013 Dec 1. doi:10.4081/jphr.2013.e28
5. Viglialoro RM, Condino S, Turini G, Carbone M, Ferrari V, Gesi M. augmented reality, mixed reality, and hybrid approach in healthcare simulation: a systematic review. Applied Sciences. 2021;11(5):2338. doi:10.3390/app11052338
6. Rawlins CR, Veigulis Z, Hebert C, Curtin C, Osborne T. Effect of immersive virtual reality on pain and anxiety at a Veterans Affairs health care facility. Front Virt Real. 2021;(2):136. doi:10.3389/frvir.2021.719681
7. Chawdhary G, Shoman N. Emerging artificial intelligence applications in otological imaging. Curr Opin Otolaryngol Head Neck Surg. 2021;29(5):357-364. doi:10.1097/MOO.0000000000000754
8. Asadzadeh A, Samad-Soltani T, Rezaei-Hachesu P. Applications of virtual and augmented reality in infectious disease epidemics with a focus on the COVID-19 outbreak. Inform Med Unlocked. 2021;24:100579. doi:10.1016/j.imu.2021.100579
9. Ashwini KB, Savitha R, Harish A. Application of augmented reality technology for home healthcare product visualization. ECS Transas. 2022;107(1):10921. doi:10.1149/10701.10921ecst
10. Brooks AL. VR/Technologies for Rehabilitation. In: Brooks AL, Brahman S, Kapralos B, Nakajima A, Tyerman J, Jain LC, eds. Recent Advances in Technologies for Inclusive Well-Being Virtual Patients, Gamification and Simulation. Intelligent Systems Reference Library. Springer; 2021:241-252. doi:10.1007/978-3-030-59608-8_13
11. Koulouris D, Menychtas A, Maglogiannis I. An IoT-enabled platform for the assessment of physical and mental activities utilizing augmented reality exergaming. Sensors (Basel). 2022;22(9):3181. Published 2022 Apr 21. doi:10.3390/s22093181
12. Deiss YR, Korkut S, Inglese T. Increase therapy understanding and medication adherence for patients with inflammatory skin diseases through augmented reality. Digital Human Modeling and Applications in Health, Safety, Ergonomics and Risk Management. Health, Operations Management, and Design: 13th International Conference, DHM 2022, Held as Part of the 24th HCI International Conference, HCII 2022. 2022:21-40. doi:10.1007/978-3-031-06018-2_2
13. Bertino E, Gao W, Steffan B, et al, eds. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Springer; 2022:21-40.
14. Ruhaiyem NIR, Mazlan NA. Image Modeling Through Augmented Reality for Skin Allergies Recognition. Lecture Notes on Data Engineering and Communications Technologies. 2021:72-79. doi: 10.1007/978-3-030-70713-2_8
15. Park BJ, Perkons NR, Profka E, et al. Three-dimensional augmented reality visualization informs locoregional therapy in a translational model of hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(10):1612-1618.e1. doi:10.1016/j.jvir.2020.01.028
16. Leo J, Zhou Z, Yang H, et al, eds. Interactive cardiovascular surgical planning via augmented reality. 5th Asian CHI Symposium 2021; 2021. doi:10.1145/3429360.3468195
17. Zuo Y, Jiang T, Dou J, et al. A novel evaluation model for a mixed-reality surgical navigation system: where Microsoft Hololens meets the operating room. Surg Innov. 2020;27(2):193-202. doi:10.1177/1553350619893236
18. Ghaednia H, Fourman MS, Lans A, et al. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021;21(10):1617-1625. doi:10.1016/j.spinee.2021.03.018
19. Liu Y, Lee MG, Kim JS. Spine surgery assisted by augmented reality: where have we been?. Yonsei Med J. 2022;63(4):305-316. doi:10.3349/ymj.2022.63.4.305
20. Kimmel S, Cobus V, Heuten W, eds. opticARe—augmented reality mobile patient monitoring in intensive care units. Proceedings of the ACM Symposium on Virtual Reality Software and Technology, VRST; 2021. doi:10.1145/3489849.3489852
21. Voštinár P, Horváthová D, Mitter M, Bako M. The look at the various uses of VR. Open Computer Sci. 2021;11(1):241-250. doi:10.1515/comp-2020-0123
22. Zhao J, Xu X, Jiang H, Ding Y. The effectiveness of virtual reality-based technology on anatomy teaching: a meta-analysis of randomized controlled studies. BMC Med Educ. 2020;20(1):127. Published 2020 Apr 25. doi:10.1186/s12909-020-1994-z
23. Ricci S, Calandrino A, Borgonovo G, Chirico M, Casadio M. Viewpoint: virtual and augmented reality in basic and advanced life support training. JMIR Serious Games. 2022;10(1):e28595. Published 2022 Mar 23. doi:10.2196/28595
24. Ricci S, Mobilio GA, Calandrino A, et al. RiNeo MR: A mixed-reality tool for newborn life support training. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:5043-5046. doi:10.1109/EMBC46164.2021.9629612
25. Dhar P, Rocks T, Samarasinghe RM, Stephenson G, Smith C. Augmented reality in medical education: students’ experiences and learning outcomes. Med Educ Online. 2021;26(1):1953953. doi:10.1080/10872981.2021.1953953
26. Pears M, Konstantinidis S. The future of immersive technology in global surgery education [published online ahead of print, 2021 Jul 1]. Indian J Surg. 2021;84(suppl 1):1-5. doi:10.1007/s12262-021-02998-6
27. Liang CJ, Start C, Boley H, Kamat VR, Menassa CC, Aebersold M. Enhancing stroke assessment simulation experience in clinical training using augmented reality. Virt Real. 2021;25(3):575-584. doi:10.1007/s10055-020-00475-1
28. Lacey G, Gozdzielewska L, McAloney-Kocaman K, Ruttle J, Cronin S, Price L. Psychomotor learning theory informing the design and evaluation of an interactive augmented reality hand hygiene training app for healthcare workers. Educ Inf Technol. 2022;27(3):3813-3832. doi:10.1007/s10639-021-10752-4
29. Ryan GV, Callaghan S, Rafferty A, Higgins MF, Mangina E, McAuliffe F. Learning outcomes of immersive technologies in health care student education: systematic review of the literature. J Med Internet Res. 2022;24(2):e30082. Published 2022 Feb 1. doi:10.2196/30082
30. Yu FU, Yan HU, Sundstedt V. A Systematic literature review of virtual, augmented, and mixed reality game applications in healthcare. ACM Trans Comput Healthcare. 2022;3(2);1-27. doi:10.1145/3472303
31. Weeks JK, Amiel JM. Enhancing neuroanatomy education with augmented reality. Med Educ. 2019;53(5):516-517. doi:10.1111/medu.13843
32. Williams MA, McVeigh J, Handa AI, Lee R. Augmented reality in surgical training: a systematic review. Postgrad Med J. 2020;96(1139):537-542. doi:10.1136/postgradmedj-2020-137600

33. Triepels CPR, Smeets CFA, Notten KJB, et al. Does three-dimensional anatomy improve student understanding? Clin Anat. 2020;33(1):25-33. doi:10.1002/ca.23405
34. Pietruski P, Majak M, S´wia¸tek-Najwer E, et al. Supporting fibula free flap harvest with augmented reality: A proof-of-concept study. Laryngoscope. 2020;130(5):1173-1179. doi:10.1002/lary.28090
35. Perkins SL, Krajancich B, Yang CJ, Hargreaves BA, Daniel BL, Berry MF. A patient-specific mixed-reality visualization tool for thoracic surgical planning. Ann Thorac Surg. 2020;110(1):290-295. doi:10.1016/j.athoracsur.2020.01.060
36. Müller F, Roner S, Liebmann F, Spirig JM, Fürnstahl P, Farshad M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020;20(4):621-628. doi:10.1016/j.spinee.2019.10.012
37. Kaplan AD, Cruit J, Endsley M, Beers SM, Sawyer BD, Hancock PA. The effects of virtual reality, augmented reality, and mixed reality as training enhancement methods: a meta-analysis. Hum Factors. 2021;63(4):706-726. doi:10.1177/0018720820904229
38. Jud L, Fotouhi J, Andronic O, et al. Applicability of augmented reality in orthopedic surgery - a systematic review. BMC Musculoskelet Disord. 2020;21(1):103. Published 2020 Feb 15. doi:10.1186/s12891-020-3110-2
39. Ara J, Karim FB, Alsubaie MSA, et al. Comprehensive analysis of augmented reality technology in modern healthcare system. Int J Adv Comput Sci Appl. 2021;12(6):845-854. doi:10.14569/IJACSA.2021.0120698
40. Webster A, Gardner J. Aligning technology and institutional readiness: the adoption of innovation. Technol Anal Strateg Manag. 2019;31(10):1229-1241. doi:10.1080/09537325.2019.1601694
41. Hastall MR, Dockweiler C, Mühlhaus J. achieving end user acceptance: building blocks for an evidence-based user-centered framework for health technology development and assessment. In: Antona, M, Stephanidis C, eds. Universal Access in Human–Computer Interaction. Human and Technological Environments. UAHCI 2017. Lecture Notes in Computer Science, vol 10279. Springer, Cham; 2017. doi:10.1007/978-3-319-58700-4_2
42. Ratwani RM, Fairbanks RJ, Hettinger AZ, Benda NC. Electronic health record usability: analysis of the user-centered design processes of eleven electronic health record vendors. J Am Med Inform Assoc. 2015;22(6):1179-1182. doi:10.1093/jamia/ocv050
43. Khairat S, Coleman C, Ottmar P, Jayachander DI, Bice T, Carson SS. Association of Electronic Health Record Use With Physician Fatigue and Efficiency. JAMA Netw Open. 2020;3(6):e207385. Published 2020 Jun 1. doi:10.1001/jamanetworkopen.2020.7385
44. Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clin Proc. 2020;95(3):476-487. doi:10.1016/j.mayocp.2019.09.024
45. Lee YJ. Comparison of job satisfaction between nonprofit and public employees. Nonprofit Volunt Sect Q. 2016;45(2):295-313. doi:10.1177/0899764015584061
46. Brimhall KC. Inclusion is important... but how do I include? Examining the effects of leader engagement on inclusion, innovation, job satisfaction, and perceived quality of care in a diverse nonprofit health care organization. Nonprofit Volunt Sect Q. 2019;48(4):716-737. doi:10.1177/0899764019829834
47. Moreira MR, Gherman M, Sousa PS. Does innovation influence the performance of healthcare organizations?. Innovation (North Syd). 2017;19(3):335-352. doi:10.1080/14479338.2017.1293489
48. US Department of Veterans Affairs. VA Palo Alto Healthcare System. Updated December 29, 2020. Accessed January 27, 2023. https://www.paloalto.va.gov/about/index.asp
49. Siegel SM, Kaemmerer WF. Measuring the perceived support for innovation in organizations. J Appl Psychol. 1978;63(5):553-562. doi:10.1037/0021-9010.63.5.553
50. Anderson NR, West MA. Measuring climate for work group innovation: development and validation of the team climate inventory. J Organ Behav. 1998;19(3):235-258. doi:10.1002/(SICI)1099-1379(199805)19:3<235::AID-JOB837>3.0.CO;2-C
51. Aarons GA. Measuring provider attitudes toward evidence-based practice: consideration of organizational context and individual differences. Child Adolesc Psychiatr Clin N Am. 2005;14(2):255-viii. doi:10.1016/j.chc.2004.04.008
52. Van der Heijden H. User acceptance of hedonic information systems. MIS Q. 2004;28(4):695-704. doi:10.2307/25148660
53. Venkatesh V, Speier C, Morris MG. User acceptance enablers in individual decision making about technology: Toward an integrated model. Decis Sci. 2002;33(2):297-316. doi:10.1111/j.1540-5915.2002.tb01646.x
54. Puri A, Kim B, Nguyen O, Stolee P, Tung J, Lee J. User acceptance of wrist-worn activity trackers among community-dwelling older adults: mixed method study. JMIR Mhealth Uhealth. 2017;5(11):e173. Published 2017 Nov 15. doi:10.2196/mhealth.8211
55. Huang YC, Backman SJ, Backman KF, Moore D. Exploring user acceptance of 3D virtual worlds in travel and tourism marketing. Tourism Management. 2013;36:490-501. doi:10.1016/j.tourman.2012.09.009
56. Rasimah CM, Ahmad A, Zaman HB. Evaluation of user acceptance of mixed reality technology. AJET. 2011;27(8). doi:10.14742/ajet.899
57. Choi J, Boyle DK. RN workgroup job satisfaction and patient falls in acute care hospital units. J Nurs Adm. 2013;43(11):586-591. doi:10.1097/01.NNA.0000434509.66749.7c58. Tzeng HM, Ketefian S. The relationship between nurses’ job satisfaction and inpatient satisfaction: an exploratory study in a Taiwan teaching hospital. J Nurs Care Qual. 2002;16(2):39-49. doi:10.1097/00001786-200201000-00005
59. Williams ES, Skinner AC. Outcomes of physician job satisfaction: a narrative review, implications, and directions for future research. Health Care Manage Rev. 2003;28(2):119-139. doi:10.1097/00004010-200304000-00004
60. Waldman JD, Kelly F, Arora S, Smith HL. The shocking cost of turnover in health care. Health Care Manage Rev. 2004;29(1):2-7. doi:10.1097/00004010-200401000-00002
61. Hayes LJ, O’Brien-Pallas L, Duffield C, et al. Nurse turnover: a literature review - an update. Int J Nurs Stud. 2012;49(7):887-905. doi:10.1016/j.ijnurstu.2011.10.001
62. US Department of Veterans Affairs. FY 2021/FY 2019 Annual performance plan and report. February 2020. Accessed January 27, 2023. https://www.va.gov/oei/docs/VA2019-2021appr.pdf
Patient-based mouse MVID model hints at mechanism
Researchers have identified a novel mutation in a patient with microvillus inclusion disease (MVID) and rapidly developed a specific mouse model to find insights into the disease process.
MVID is characterized by severe diarrhea, generally beginning within a few hours of birth. The condition is caused by inactivating mutations in the gene myosin VB (MYO5B). Affected individuals usually require lifetime use of total parenteral nutrition or small-bowel transplantation.
More than 100 MYO5B mutations have been identified in MVID patients, most of whom inherit two unique mutant alleles. This can lead to variability in phenotypes, which single-mutation animal models have been unable to mimic. Generally, patients have atrophy of microvilli on enterocytes as well as inclusion bodies within enterocytes that contain microvilli.
In the study, published in Cellular and Molecular Gastroenterology and Hepatology, researchers describe genetic sequencing of MYO5B mutations in a patient and both parents. One mutation is predicted to lead to protein truncation (c.1821delG), and the other (c.1555G>A) appeared to be inherited from the patient’s mother. The patient suffered from severe diarrhea after birth and was intermittently feeding intolerant.
The researchers conducted a range of diagnostic tests and biopsies. One particularly interesting finding was expression of the A-kinase anchoring protein 350 in the vicinity of the inclusion, wrote Andreanna Burman and her coauthors at Vanderbilt University, Nashville, Tenn. This protein takes part in a protein-mediating scaffolding pathway, suggesting that it could play a role in the development of the inclusions.
The researchers used multiplexed immunofluorescence (MxIF) staining of a biopsy of the duodenum to look for expression of a range of proteins. They found a striking decrease in enterocytes that express glucose transporter 2, implying that malabsorption might be due at least in part to enterocytes that fail to mature.
The patient also had internalization of apical nutrient transporters, which has been reported in other MVID patients. The researchers also developed a mouse model that incorporated the patient’s novel compound heterozygous genotype, which they cross-bred to produce a tamoxifen-inducible mouse model.
After the tamoxifen injection, the patient-mimicking animals exhibited a severe watery diarrhea, losing 19% of their body weight by day 4. The animals’ intestines showed a similar phenotype to that of the patient.
The apical sodium transporters sodium-glucose cotransporter 1 (SGLT1), apical sodium-dependent bile transporter, and NHE3 were internalized away from the apical membrane of the enterocytes in the patient-mimicking model animals. This structural difference may explain the limited absorption of sodium and water and the resultant watery diarrhea. The researchers also noted disruption of the actinin-4+ terminal web structure, as well as increased SGLT1 localization with lysosome-associated membrane protein 1+ lysosomes within the mouse model enterocytes. This association may indicate degradation of mislocalized proteins, the authors noted, which has also been seen in expanded RAB7+ vesicles within MVID patient tissues.
Other signs pointed to the expansion of immature cells in the upper crypt and lower villus, as well as faster shedding of enterocytes in the villus than in control animals. Scanning electron microscope images also showed immature and disorganized microvilli in the enterocytes of the patient-mimicking mice.
Taken together, “these findings are consistent with a deficit in enterocyte maturation in Myo5b(G519R) mice and in the patient with the MYO5B(G519R) mutation,” the authors wrote.
The authors suggest that their approach could be used more generally to quickly create mouse models of patient-specific monogenic congenital disorders.
The authors disclosed no conflicts of interest. The research was funded by the National Foundation of Science, National Institutes of Health, Vanderbilt Digestive Diseases Research Center Pilot and Feasibility grant, an American Physiological Society John F. Perkins, Jr. Research Career Enhancement Award, and a gift from the Christine Volpe Fund.
Genetically engineered mouse models (GEMMs) have offered tremendous insight into the genetics, biology, and pathobiology of the gastrointestinal tract. Yet, in the past, the time necessary to generate these GEMMs has presented challenges for modeling human diseases. With the ongoing identification of disease-associated polymorphisms or mutations, including through approaches like genomewide association studies (GWAS), defining the function of these specific genetic alterations in disease pathogenesis is of great importance.
Overall, this study demonstrates the value of both patient-mimicking mouse models and multiplexed staining to define the molecular mechanisms of congenital diseases in vivo.
Dr. Jonathan P. Katz is associate professor of medicine, department of medicine, gastroenterology division, University of Pennsylvania Perelman School of Medicine, Philadelphia. He has no relevant conflicts of interest.
Genetically engineered mouse models (GEMMs) have offered tremendous insight into the genetics, biology, and pathobiology of the gastrointestinal tract. Yet, in the past, the time necessary to generate these GEMMs has presented challenges for modeling human diseases. With the ongoing identification of disease-associated polymorphisms or mutations, including through approaches like genomewide association studies (GWAS), defining the function of these specific genetic alterations in disease pathogenesis is of great importance.
Overall, this study demonstrates the value of both patient-mimicking mouse models and multiplexed staining to define the molecular mechanisms of congenital diseases in vivo.
Dr. Jonathan P. Katz is associate professor of medicine, department of medicine, gastroenterology division, University of Pennsylvania Perelman School of Medicine, Philadelphia. He has no relevant conflicts of interest.
Genetically engineered mouse models (GEMMs) have offered tremendous insight into the genetics, biology, and pathobiology of the gastrointestinal tract. Yet, in the past, the time necessary to generate these GEMMs has presented challenges for modeling human diseases. With the ongoing identification of disease-associated polymorphisms or mutations, including through approaches like genomewide association studies (GWAS), defining the function of these specific genetic alterations in disease pathogenesis is of great importance.
Overall, this study demonstrates the value of both patient-mimicking mouse models and multiplexed staining to define the molecular mechanisms of congenital diseases in vivo.
Dr. Jonathan P. Katz is associate professor of medicine, department of medicine, gastroenterology division, University of Pennsylvania Perelman School of Medicine, Philadelphia. He has no relevant conflicts of interest.
Researchers have identified a novel mutation in a patient with microvillus inclusion disease (MVID) and rapidly developed a specific mouse model to find insights into the disease process.
MVID is characterized by severe diarrhea, generally beginning within a few hours of birth. The condition is caused by inactivating mutations in the gene myosin VB (MYO5B). Affected individuals usually require lifetime use of total parenteral nutrition or small-bowel transplantation.
More than 100 MYO5B mutations have been identified in MVID patients, most of whom inherit two unique mutant alleles. This can lead to variability in phenotypes, which single-mutation animal models have been unable to mimic. Generally, patients have atrophy of microvilli on enterocytes as well as inclusion bodies within enterocytes that contain microvilli.
In the study, published in Cellular and Molecular Gastroenterology and Hepatology, researchers describe genetic sequencing of MYO5B mutations in a patient and both parents. One mutation is predicted to lead to protein truncation (c.1821delG), and the other (c.1555G>A) appeared to be inherited from the patient’s mother. The patient suffered from severe diarrhea after birth and was intermittently feeding intolerant.
The researchers conducted a range of diagnostic tests and biopsies. One particularly interesting finding was expression of the A-kinase anchoring protein 350 in the vicinity of the inclusion, wrote Andreanna Burman and her coauthors at Vanderbilt University, Nashville, Tenn. This protein takes part in a protein-mediating scaffolding pathway, suggesting that it could play a role in the development of the inclusions.
The researchers used multiplexed immunofluorescence (MxIF) staining of a biopsy of the duodenum to look for expression of a range of proteins. They found a striking decrease in enterocytes that express glucose transporter 2, implying that malabsorption might be due at least in part to enterocytes that fail to mature.
The patient also had internalization of apical nutrient transporters, which has been reported in other MVID patients. The researchers also developed a mouse model that incorporated the patient’s novel compound heterozygous genotype, which they cross-bred to produce a tamoxifen-inducible mouse model.
After the tamoxifen injection, the patient-mimicking animals exhibited a severe watery diarrhea, losing 19% of their body weight by day 4. The animals’ intestines showed a similar phenotype to that of the patient.
The apical sodium transporters sodium-glucose cotransporter 1 (SGLT1), apical sodium-dependent bile transporter, and NHE3 were internalized away from the apical membrane of the enterocytes in the patient-mimicking model animals. This structural difference may explain the limited absorption of sodium and water and the resultant watery diarrhea. The researchers also noted disruption of the actinin-4+ terminal web structure, as well as increased SGLT1 localization with lysosome-associated membrane protein 1+ lysosomes within the mouse model enterocytes. This association may indicate degradation of mislocalized proteins, the authors noted, which has also been seen in expanded RAB7+ vesicles within MVID patient tissues.
Other signs pointed to the expansion of immature cells in the upper crypt and lower villus, as well as faster shedding of enterocytes in the villus than in control animals. Scanning electron microscope images also showed immature and disorganized microvilli in the enterocytes of the patient-mimicking mice.
Taken together, “these findings are consistent with a deficit in enterocyte maturation in Myo5b(G519R) mice and in the patient with the MYO5B(G519R) mutation,” the authors wrote.
The authors suggest that their approach could be used more generally to quickly create mouse models of patient-specific monogenic congenital disorders.
The authors disclosed no conflicts of interest. The research was funded by the National Foundation of Science, National Institutes of Health, Vanderbilt Digestive Diseases Research Center Pilot and Feasibility grant, an American Physiological Society John F. Perkins, Jr. Research Career Enhancement Award, and a gift from the Christine Volpe Fund.
Researchers have identified a novel mutation in a patient with microvillus inclusion disease (MVID) and rapidly developed a specific mouse model to find insights into the disease process.
MVID is characterized by severe diarrhea, generally beginning within a few hours of birth. The condition is caused by inactivating mutations in the gene myosin VB (MYO5B). Affected individuals usually require lifetime use of total parenteral nutrition or small-bowel transplantation.
More than 100 MYO5B mutations have been identified in MVID patients, most of whom inherit two unique mutant alleles. This can lead to variability in phenotypes, which single-mutation animal models have been unable to mimic. Generally, patients have atrophy of microvilli on enterocytes as well as inclusion bodies within enterocytes that contain microvilli.
In the study, published in Cellular and Molecular Gastroenterology and Hepatology, researchers describe genetic sequencing of MYO5B mutations in a patient and both parents. One mutation is predicted to lead to protein truncation (c.1821delG), and the other (c.1555G>A) appeared to be inherited from the patient’s mother. The patient suffered from severe diarrhea after birth and was intermittently feeding intolerant.
The researchers conducted a range of diagnostic tests and biopsies. One particularly interesting finding was expression of the A-kinase anchoring protein 350 in the vicinity of the inclusion, wrote Andreanna Burman and her coauthors at Vanderbilt University, Nashville, Tenn. This protein takes part in a protein-mediating scaffolding pathway, suggesting that it could play a role in the development of the inclusions.
The researchers used multiplexed immunofluorescence (MxIF) staining of a biopsy of the duodenum to look for expression of a range of proteins. They found a striking decrease in enterocytes that express glucose transporter 2, implying that malabsorption might be due at least in part to enterocytes that fail to mature.
The patient also had internalization of apical nutrient transporters, which has been reported in other MVID patients. The researchers also developed a mouse model that incorporated the patient’s novel compound heterozygous genotype, which they cross-bred to produce a tamoxifen-inducible mouse model.
After the tamoxifen injection, the patient-mimicking animals exhibited a severe watery diarrhea, losing 19% of their body weight by day 4. The animals’ intestines showed a similar phenotype to that of the patient.
The apical sodium transporters sodium-glucose cotransporter 1 (SGLT1), apical sodium-dependent bile transporter, and NHE3 were internalized away from the apical membrane of the enterocytes in the patient-mimicking model animals. This structural difference may explain the limited absorption of sodium and water and the resultant watery diarrhea. The researchers also noted disruption of the actinin-4+ terminal web structure, as well as increased SGLT1 localization with lysosome-associated membrane protein 1+ lysosomes within the mouse model enterocytes. This association may indicate degradation of mislocalized proteins, the authors noted, which has also been seen in expanded RAB7+ vesicles within MVID patient tissues.
Other signs pointed to the expansion of immature cells in the upper crypt and lower villus, as well as faster shedding of enterocytes in the villus than in control animals. Scanning electron microscope images also showed immature and disorganized microvilli in the enterocytes of the patient-mimicking mice.
Taken together, “these findings are consistent with a deficit in enterocyte maturation in Myo5b(G519R) mice and in the patient with the MYO5B(G519R) mutation,” the authors wrote.
The authors suggest that their approach could be used more generally to quickly create mouse models of patient-specific monogenic congenital disorders.
The authors disclosed no conflicts of interest. The research was funded by the National Foundation of Science, National Institutes of Health, Vanderbilt Digestive Diseases Research Center Pilot and Feasibility grant, an American Physiological Society John F. Perkins, Jr. Research Career Enhancement Award, and a gift from the Christine Volpe Fund.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Yoga linked with improved gait speed, lower extremity strength in older adults
“Up to 50% of adults aged 80 years or older are estimated to be frail, and the global prevalence is expected to rise given the aging of our population,” therefore more interventions are needed to help with frailty, corresponding author Julia Loewenthal, MD, said in an interview.
Yoga integrates across multiple body systems including the musculoskeletal system, nervous system, and others, said Dr. Loewenthal of Brigham and Women’s Hospital, Boston. Previous research has shown that yoga has a positive effect on cardiovascular risk factors, mood, and quality of life, but the effects of yoga on frailty have not been well studied.
“We wanted to evaluate whether [yoga] might help with frailty, since it touches on so many systems, as frailty does,” she noted.
In a systematic review published in Annals of Internal Medicine, the researchers identified 33 randomized, controlled trials of yoga-based interventions including 2,384 adults aged 65 years and older. The studies mainly involved Iyengar or chair-based yoga methods. The study population included community-dwelling seniors, nursing home residents, and individuals with chronic diseases.
The studies assessed the effect of a range of yoga practices on frailty markers including gait speed, handgrip strength, balance, lower extremity strength and endurance, and multicomponent measures of physical performance.
Overall, individuals who were randomized to engage in a yoga practice showed improved gait speed and lower extremity strength, compared with controls who were inactive or received an education intervention, with moderate-certainty evidence. The researchers also found low-certainty evidence in favor of yoga for improved balance and for a composite measure of physical function, and low-certainty evidence in favor of yoga for improved handgrip strength.
The findings were limited by several factors, mainly the heterogenous study designs, populations, and yoga styles, the researchers noted. Other limitations included the small sample sizes, variation in descriptions of the studies’ randomizations, and a lack of data on race and ethnicity of the participants.
Yoga’s role in healthy aging
“Overall, we were not surprised by the results since we have seen similar findings from other mind-body practices such as tai chi,” Dr. Loewenthal said in an interview. “We were surprised by the degree of improvement many of the participants had with gait speed.
“Yoga practices usually include a mix of poses in the standing, seated, and lying-down positions,” Dr. Loewenthal said. Some of the studies in the review also involved chair-based methods with few standing poses, and some involved gentle or slow-paced practices. “We know that many of the practices helped with leg strength, and perhaps they are also helping with coordination between the brain and body for walking.”
The findings suggest that clinicians can view yoga practice in general as part of a strategy to support healthy aging, Dr. Loewenthal said. “While our work looked at frailty markers and not overall frailty, I think it would be reasonable to offer yoga as a strategy along with already-established interventions such as resistance training and the Mediterranean diet, and if older adults are already practicing yoga, this could help them understand how the practice is impacting the aging process.”
There are many styles of yoga that overlap and are related to one another, so it is hard to make recommendations about a single type, said Dr. Loewenthal. Many of the studies in the review involved Iyengar yoga, named for yoga master B.K.S. Iyengar, which focuses on precise alignment and breath and seems to be conducive for older populations. Iyengar yoga also involves use of props such as blocks, bolsters, straps, and chairs, which makes it well suited for older individuals who may have a chronic condition or other limitations.
“Some styles of yoga are very physical and may reach energy expenditure and cardiovascular effects similar to aerobic exercise, but this is generally not the case for most styles of yoga,” she added.
As for additional research, “I think it is important that trials use a validated definition of frailty as an outcome; all of the trials in our study used markers of frailty but did not look at overall frailty,” said Dr. Loewenthal. In addition, it is important to understand how yoga affects people who have different levels of frailty, since previous research shows that those who are the most frail benefit most from physical activity interventions.
Yoga as an entry point for physical activity
With the increasing population of older adults in the United States and around the world, frailty is a major health concern because its association with significant declines in health and potential loss of independence, Amanda Paluch, PhD, said in an interview.
“Therefore, it is important to identify programs that can prevent frailty to support longevity and living independently for older adults. Yoga can be a feasible solution to promote movement and prevent frailty,” said Dr. Paluch, of the University of Massachusetts, Amherst, an assistant professor in the department of kinesiology and Institute for Applied Life Sciences.
“Other studies have demonstrated that light-intensity movement, as in yoga, may be particularly beneficial for older adults,” Dr. Paluch said. “Additionally, research has demonstrated that balance training activities are important to maintain physical function, prevent falls, and maintain their independence for older adults, so it makes sense that yoga was associated with lower likelihood of frailty.”
Although there may be additional benefits with higher intensity activity, “yoga could be a great place to start for older adults who are starting at low activity levels,” she said.
The takeaway for clinicians is to consider encouraging more physical activity for their patients to support healthy aging, including reducing the risk factors for frailty, said Dr. Paluch. “Particularly for older adults, physical activity may not need to be of high intensity for benefits. Activities such as yoga that focus on flexibility, balance, and movement at lower intensities can support healthy aging, and yoga may be a particularly good option for older adults who are least active.”
The study received no outside funding. The researchers and Dr. Paluch had no financial conflicts to disclose.
“Up to 50% of adults aged 80 years or older are estimated to be frail, and the global prevalence is expected to rise given the aging of our population,” therefore more interventions are needed to help with frailty, corresponding author Julia Loewenthal, MD, said in an interview.
Yoga integrates across multiple body systems including the musculoskeletal system, nervous system, and others, said Dr. Loewenthal of Brigham and Women’s Hospital, Boston. Previous research has shown that yoga has a positive effect on cardiovascular risk factors, mood, and quality of life, but the effects of yoga on frailty have not been well studied.
“We wanted to evaluate whether [yoga] might help with frailty, since it touches on so many systems, as frailty does,” she noted.
In a systematic review published in Annals of Internal Medicine, the researchers identified 33 randomized, controlled trials of yoga-based interventions including 2,384 adults aged 65 years and older. The studies mainly involved Iyengar or chair-based yoga methods. The study population included community-dwelling seniors, nursing home residents, and individuals with chronic diseases.
The studies assessed the effect of a range of yoga practices on frailty markers including gait speed, handgrip strength, balance, lower extremity strength and endurance, and multicomponent measures of physical performance.
Overall, individuals who were randomized to engage in a yoga practice showed improved gait speed and lower extremity strength, compared with controls who were inactive or received an education intervention, with moderate-certainty evidence. The researchers also found low-certainty evidence in favor of yoga for improved balance and for a composite measure of physical function, and low-certainty evidence in favor of yoga for improved handgrip strength.
The findings were limited by several factors, mainly the heterogenous study designs, populations, and yoga styles, the researchers noted. Other limitations included the small sample sizes, variation in descriptions of the studies’ randomizations, and a lack of data on race and ethnicity of the participants.
Yoga’s role in healthy aging
“Overall, we were not surprised by the results since we have seen similar findings from other mind-body practices such as tai chi,” Dr. Loewenthal said in an interview. “We were surprised by the degree of improvement many of the participants had with gait speed.
“Yoga practices usually include a mix of poses in the standing, seated, and lying-down positions,” Dr. Loewenthal said. Some of the studies in the review also involved chair-based methods with few standing poses, and some involved gentle or slow-paced practices. “We know that many of the practices helped with leg strength, and perhaps they are also helping with coordination between the brain and body for walking.”
The findings suggest that clinicians can view yoga practice in general as part of a strategy to support healthy aging, Dr. Loewenthal said. “While our work looked at frailty markers and not overall frailty, I think it would be reasonable to offer yoga as a strategy along with already-established interventions such as resistance training and the Mediterranean diet, and if older adults are already practicing yoga, this could help them understand how the practice is impacting the aging process.”
There are many styles of yoga that overlap and are related to one another, so it is hard to make recommendations about a single type, said Dr. Loewenthal. Many of the studies in the review involved Iyengar yoga, named for yoga master B.K.S. Iyengar, which focuses on precise alignment and breath and seems to be conducive for older populations. Iyengar yoga also involves use of props such as blocks, bolsters, straps, and chairs, which makes it well suited for older individuals who may have a chronic condition or other limitations.
“Some styles of yoga are very physical and may reach energy expenditure and cardiovascular effects similar to aerobic exercise, but this is generally not the case for most styles of yoga,” she added.
As for additional research, “I think it is important that trials use a validated definition of frailty as an outcome; all of the trials in our study used markers of frailty but did not look at overall frailty,” said Dr. Loewenthal. In addition, it is important to understand how yoga affects people who have different levels of frailty, since previous research shows that those who are the most frail benefit most from physical activity interventions.
Yoga as an entry point for physical activity
With the increasing population of older adults in the United States and around the world, frailty is a major health concern because its association with significant declines in health and potential loss of independence, Amanda Paluch, PhD, said in an interview.
“Therefore, it is important to identify programs that can prevent frailty to support longevity and living independently for older adults. Yoga can be a feasible solution to promote movement and prevent frailty,” said Dr. Paluch, of the University of Massachusetts, Amherst, an assistant professor in the department of kinesiology and Institute for Applied Life Sciences.
“Other studies have demonstrated that light-intensity movement, as in yoga, may be particularly beneficial for older adults,” Dr. Paluch said. “Additionally, research has demonstrated that balance training activities are important to maintain physical function, prevent falls, and maintain their independence for older adults, so it makes sense that yoga was associated with lower likelihood of frailty.”
Although there may be additional benefits with higher intensity activity, “yoga could be a great place to start for older adults who are starting at low activity levels,” she said.
The takeaway for clinicians is to consider encouraging more physical activity for their patients to support healthy aging, including reducing the risk factors for frailty, said Dr. Paluch. “Particularly for older adults, physical activity may not need to be of high intensity for benefits. Activities such as yoga that focus on flexibility, balance, and movement at lower intensities can support healthy aging, and yoga may be a particularly good option for older adults who are least active.”
The study received no outside funding. The researchers and Dr. Paluch had no financial conflicts to disclose.
“Up to 50% of adults aged 80 years or older are estimated to be frail, and the global prevalence is expected to rise given the aging of our population,” therefore more interventions are needed to help with frailty, corresponding author Julia Loewenthal, MD, said in an interview.
Yoga integrates across multiple body systems including the musculoskeletal system, nervous system, and others, said Dr. Loewenthal of Brigham and Women’s Hospital, Boston. Previous research has shown that yoga has a positive effect on cardiovascular risk factors, mood, and quality of life, but the effects of yoga on frailty have not been well studied.
“We wanted to evaluate whether [yoga] might help with frailty, since it touches on so many systems, as frailty does,” she noted.
In a systematic review published in Annals of Internal Medicine, the researchers identified 33 randomized, controlled trials of yoga-based interventions including 2,384 adults aged 65 years and older. The studies mainly involved Iyengar or chair-based yoga methods. The study population included community-dwelling seniors, nursing home residents, and individuals with chronic diseases.
The studies assessed the effect of a range of yoga practices on frailty markers including gait speed, handgrip strength, balance, lower extremity strength and endurance, and multicomponent measures of physical performance.
Overall, individuals who were randomized to engage in a yoga practice showed improved gait speed and lower extremity strength, compared with controls who were inactive or received an education intervention, with moderate-certainty evidence. The researchers also found low-certainty evidence in favor of yoga for improved balance and for a composite measure of physical function, and low-certainty evidence in favor of yoga for improved handgrip strength.
The findings were limited by several factors, mainly the heterogenous study designs, populations, and yoga styles, the researchers noted. Other limitations included the small sample sizes, variation in descriptions of the studies’ randomizations, and a lack of data on race and ethnicity of the participants.
Yoga’s role in healthy aging
“Overall, we were not surprised by the results since we have seen similar findings from other mind-body practices such as tai chi,” Dr. Loewenthal said in an interview. “We were surprised by the degree of improvement many of the participants had with gait speed.
“Yoga practices usually include a mix of poses in the standing, seated, and lying-down positions,” Dr. Loewenthal said. Some of the studies in the review also involved chair-based methods with few standing poses, and some involved gentle or slow-paced practices. “We know that many of the practices helped with leg strength, and perhaps they are also helping with coordination between the brain and body for walking.”
The findings suggest that clinicians can view yoga practice in general as part of a strategy to support healthy aging, Dr. Loewenthal said. “While our work looked at frailty markers and not overall frailty, I think it would be reasonable to offer yoga as a strategy along with already-established interventions such as resistance training and the Mediterranean diet, and if older adults are already practicing yoga, this could help them understand how the practice is impacting the aging process.”
There are many styles of yoga that overlap and are related to one another, so it is hard to make recommendations about a single type, said Dr. Loewenthal. Many of the studies in the review involved Iyengar yoga, named for yoga master B.K.S. Iyengar, which focuses on precise alignment and breath and seems to be conducive for older populations. Iyengar yoga also involves use of props such as blocks, bolsters, straps, and chairs, which makes it well suited for older individuals who may have a chronic condition or other limitations.
“Some styles of yoga are very physical and may reach energy expenditure and cardiovascular effects similar to aerobic exercise, but this is generally not the case for most styles of yoga,” she added.
As for additional research, “I think it is important that trials use a validated definition of frailty as an outcome; all of the trials in our study used markers of frailty but did not look at overall frailty,” said Dr. Loewenthal. In addition, it is important to understand how yoga affects people who have different levels of frailty, since previous research shows that those who are the most frail benefit most from physical activity interventions.
Yoga as an entry point for physical activity
With the increasing population of older adults in the United States and around the world, frailty is a major health concern because its association with significant declines in health and potential loss of independence, Amanda Paluch, PhD, said in an interview.
“Therefore, it is important to identify programs that can prevent frailty to support longevity and living independently for older adults. Yoga can be a feasible solution to promote movement and prevent frailty,” said Dr. Paluch, of the University of Massachusetts, Amherst, an assistant professor in the department of kinesiology and Institute for Applied Life Sciences.
“Other studies have demonstrated that light-intensity movement, as in yoga, may be particularly beneficial for older adults,” Dr. Paluch said. “Additionally, research has demonstrated that balance training activities are important to maintain physical function, prevent falls, and maintain their independence for older adults, so it makes sense that yoga was associated with lower likelihood of frailty.”
Although there may be additional benefits with higher intensity activity, “yoga could be a great place to start for older adults who are starting at low activity levels,” she said.
The takeaway for clinicians is to consider encouraging more physical activity for their patients to support healthy aging, including reducing the risk factors for frailty, said Dr. Paluch. “Particularly for older adults, physical activity may not need to be of high intensity for benefits. Activities such as yoga that focus on flexibility, balance, and movement at lower intensities can support healthy aging, and yoga may be a particularly good option for older adults who are least active.”
The study received no outside funding. The researchers and Dr. Paluch had no financial conflicts to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Dodging PE
I was (probably) the bane of my elementary school nurse.
I hated PE (I know, who didn’t?). But I also had childhood asthma. So it was an easy out to go to the school nurse, Mrs. Reed, because I was having an asthma attack, or at least claiming to have one.
She’d put me in a chair to “keep an eye” on me, occasionally have me take the prescription drug my pediatrician had ordered (Marax – anyone else remember that?), and knew to send me back to class about 5 minutes before PE was over.
Maybe Mrs. Reed liked me. Maybe it was just the path of least resistance to let me dodge PE. Maybe she’d hated PE, too, and was sympathetic. Who knows?
So twice a week through years of elementary school she and I went through the same routine of my showing up in her office. No matter how busy she was, she always told me to take a seat and do a therapeutic application of her stethoscope. She often told others who noticed my frequent visits that I was “a sickly child” even though I knew she saw through me and said it with a sense of sarcasm and humor.
Of course, life goes on, and one day 20 years ago Mrs. Reed showed up on my hospital census as a new consult after she’d had a minor stroke.
She remembered me very well. Her first comment, said with the same tone I recalled, was that she was amazed I’d lived to adulthood after having been such “a sickly child.” We both laughed.
Now, in her late 80s, she still comes to see me for this and that. Sometimes we reminisce about the intertwined journey our lives have taken us on. Sometimes she asks if I’ve been to PE recently.
Like any patient, she occasionally shows up on the wrong day, or at the wrong time. I always do my best to see her, though.
After all, I owe her big time for letting me dodge PE.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I was (probably) the bane of my elementary school nurse.
I hated PE (I know, who didn’t?). But I also had childhood asthma. So it was an easy out to go to the school nurse, Mrs. Reed, because I was having an asthma attack, or at least claiming to have one.
She’d put me in a chair to “keep an eye” on me, occasionally have me take the prescription drug my pediatrician had ordered (Marax – anyone else remember that?), and knew to send me back to class about 5 minutes before PE was over.
Maybe Mrs. Reed liked me. Maybe it was just the path of least resistance to let me dodge PE. Maybe she’d hated PE, too, and was sympathetic. Who knows?
So twice a week through years of elementary school she and I went through the same routine of my showing up in her office. No matter how busy she was, she always told me to take a seat and do a therapeutic application of her stethoscope. She often told others who noticed my frequent visits that I was “a sickly child” even though I knew she saw through me and said it with a sense of sarcasm and humor.
Of course, life goes on, and one day 20 years ago Mrs. Reed showed up on my hospital census as a new consult after she’d had a minor stroke.
She remembered me very well. Her first comment, said with the same tone I recalled, was that she was amazed I’d lived to adulthood after having been such “a sickly child.” We both laughed.
Now, in her late 80s, she still comes to see me for this and that. Sometimes we reminisce about the intertwined journey our lives have taken us on. Sometimes she asks if I’ve been to PE recently.
Like any patient, she occasionally shows up on the wrong day, or at the wrong time. I always do my best to see her, though.
After all, I owe her big time for letting me dodge PE.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I was (probably) the bane of my elementary school nurse.
I hated PE (I know, who didn’t?). But I also had childhood asthma. So it was an easy out to go to the school nurse, Mrs. Reed, because I was having an asthma attack, or at least claiming to have one.
She’d put me in a chair to “keep an eye” on me, occasionally have me take the prescription drug my pediatrician had ordered (Marax – anyone else remember that?), and knew to send me back to class about 5 minutes before PE was over.
Maybe Mrs. Reed liked me. Maybe it was just the path of least resistance to let me dodge PE. Maybe she’d hated PE, too, and was sympathetic. Who knows?
So twice a week through years of elementary school she and I went through the same routine of my showing up in her office. No matter how busy she was, she always told me to take a seat and do a therapeutic application of her stethoscope. She often told others who noticed my frequent visits that I was “a sickly child” even though I knew she saw through me and said it with a sense of sarcasm and humor.
Of course, life goes on, and one day 20 years ago Mrs. Reed showed up on my hospital census as a new consult after she’d had a minor stroke.
She remembered me very well. Her first comment, said with the same tone I recalled, was that she was amazed I’d lived to adulthood after having been such “a sickly child.” We both laughed.
Now, in her late 80s, she still comes to see me for this and that. Sometimes we reminisce about the intertwined journey our lives have taken us on. Sometimes she asks if I’ve been to PE recently.
Like any patient, she occasionally shows up on the wrong day, or at the wrong time. I always do my best to see her, though.
After all, I owe her big time for letting me dodge PE.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
TMEM16A, TMEM16F play crucial role in Paneth cell secretion
To defend the gut from microbes and pathogens, Paneth cells rely on TMEM16A, a calcium-activated chloride channel, and TMEM16F, a phospholipid scramblase, according to a new study published in Gastro Hep Advances.
The Paneth cells in mice missing TMEM16A or TMEM16F showed defects in signaling and release of secretary factors, researchers reported.
Inhibiting or activating TMEM16A and TMEM16F is likely to affect microbial content and immune functions in the small intestine, concluded Rainer Schreiber, Dr. rer. nat., of the Institute of Physiology at Universität Regensburg, Germany, and colleagues.
“Many small molecules and numerous natural or herbal compounds have been identified that either inhibit or activate TMEM16A or TMEM16F,” they wrote. “Some of these compounds may turn out to be useful therapeutics in inflammatory bowel disease, intestinal allergies, or abnormal colonization of the gut.”
Paneth cells play a central role in intestinal innate immune response, the authors wrote. Located at the base of small intestinal crypts and occasionally found in the proximal colon, these cells have defensive functions, such as protecting stem cells in response to invading microbes and eradicating ingested pathogens from intestinal crypts. Through secretion, they also regulate the composition and number of commensal intestinal bacteria. In inflammatory bowel disease, the Paneth cell zone expands due to an increase in cell size and cell number.
In previous studies, cholinergic stimulation provided enhanced protection in animals orally infected with virulent Salmonella enterica. However, the mechanisms of luminal stimulation of Paneth cell secretion in response to bacteria or lipopolysaccharide are unclear. Recent reports show that TMEM16A (also known as anoctamin 1, or ANO1) and TMEM16F (anoctamin 6, or ANO6) control intracellular calcium (Ca2+) signaling and that high local Ca2+ levels support exocytosis in intestinal cells.
The researchers analyzed the roles of the two molecules in Paneth cell secretion using mice with intestinal epithelial-specific knockout of TMEM16A or TMEM16F. They examined tissue structures and Paneth cells in the mice, as well as Paneth cell exocytosis in small intestinal organoids in vitro. They also compared Ca2+ signals between wild-type and knockout mice and analyzed bacterial colonization and intestinal apoptosis.
In wild-type mice, TMEM16A was detected at the apical pole of crypt epithelial cells, while TMEM16F was located predominantly at the basolateral side. Notably, TMEM16A was also located in intestinal smooth muscle cells.
Compared with wild-type mice, the TMEM16 knockout mice had pronounced accumulation of lysozyme in jejunal Paneth cells. This suggests a defect in Paneth cell secretion in the absence of TMEM16A and TMEM16F, the authors wrote.
Previous studies had found an accumulation of mucus in intestinal goblet cells in mice with tissue-specific knockout of TMEM16A and TMEM16F. In this study, a more detailed analysis of mucus using periodic acid-Schiff staining of duodenum, jejunum, and ileum confirmed those results and demonstrated enhanced mucus in the small intestine of knockout mice. This suggests that a lack of TMEM16A or TMEM16F causes a broad secretion defect in secretory cells, including Paneth cells, the authors wrote.
Because granules of Paneth cells contain antimicrobial peptides, cytokines, and other factors that control proliferation or epithelial cell death, the researchers analyzed the presence of Gram-positive and Gram-negative bacteria in the jejunum and ileum. Compared to wild-type mice, the number of bacteria was higher in the ileum of both TMEM16A and TMEM16F knockout mice and in the jejunum of TMEM16F knockout mice, suggesting reduced antimicrobial activity in the absence of TMEM16 proteins.
The researchers also compared regulated cell death of intestinal epithelial cells in jejuna of wild-type and knockout mice. They found largely reduced cell death in both TMEM16A and TMEM16F knockout mice.
“Intestinal inflammatory diseases such as Crohn’s disease, necrotizing enterocolitis, and intestinal microbiota dysbiosis have been related to abnormal Paneth cell physiology,” the authors wrote. “The present findings may therefore provide the basis for a novel anti-inflammatory therapy for intestinal diseases and may improve our understanding of the molecular mechanism of some of the currently available drugs.”
The study was supported by the Deutsche Forschungsgemeinschaft funding program. The authors disclosed no conflicts of interest.
To defend the gut from microbes and pathogens, Paneth cells rely on TMEM16A, a calcium-activated chloride channel, and TMEM16F, a phospholipid scramblase, according to a new study published in Gastro Hep Advances.
The Paneth cells in mice missing TMEM16A or TMEM16F showed defects in signaling and release of secretary factors, researchers reported.
Inhibiting or activating TMEM16A and TMEM16F is likely to affect microbial content and immune functions in the small intestine, concluded Rainer Schreiber, Dr. rer. nat., of the Institute of Physiology at Universität Regensburg, Germany, and colleagues.
“Many small molecules and numerous natural or herbal compounds have been identified that either inhibit or activate TMEM16A or TMEM16F,” they wrote. “Some of these compounds may turn out to be useful therapeutics in inflammatory bowel disease, intestinal allergies, or abnormal colonization of the gut.”
Paneth cells play a central role in intestinal innate immune response, the authors wrote. Located at the base of small intestinal crypts and occasionally found in the proximal colon, these cells have defensive functions, such as protecting stem cells in response to invading microbes and eradicating ingested pathogens from intestinal crypts. Through secretion, they also regulate the composition and number of commensal intestinal bacteria. In inflammatory bowel disease, the Paneth cell zone expands due to an increase in cell size and cell number.
In previous studies, cholinergic stimulation provided enhanced protection in animals orally infected with virulent Salmonella enterica. However, the mechanisms of luminal stimulation of Paneth cell secretion in response to bacteria or lipopolysaccharide are unclear. Recent reports show that TMEM16A (also known as anoctamin 1, or ANO1) and TMEM16F (anoctamin 6, or ANO6) control intracellular calcium (Ca2+) signaling and that high local Ca2+ levels support exocytosis in intestinal cells.
The researchers analyzed the roles of the two molecules in Paneth cell secretion using mice with intestinal epithelial-specific knockout of TMEM16A or TMEM16F. They examined tissue structures and Paneth cells in the mice, as well as Paneth cell exocytosis in small intestinal organoids in vitro. They also compared Ca2+ signals between wild-type and knockout mice and analyzed bacterial colonization and intestinal apoptosis.
In wild-type mice, TMEM16A was detected at the apical pole of crypt epithelial cells, while TMEM16F was located predominantly at the basolateral side. Notably, TMEM16A was also located in intestinal smooth muscle cells.
Compared with wild-type mice, the TMEM16 knockout mice had pronounced accumulation of lysozyme in jejunal Paneth cells. This suggests a defect in Paneth cell secretion in the absence of TMEM16A and TMEM16F, the authors wrote.
Previous studies had found an accumulation of mucus in intestinal goblet cells in mice with tissue-specific knockout of TMEM16A and TMEM16F. In this study, a more detailed analysis of mucus using periodic acid-Schiff staining of duodenum, jejunum, and ileum confirmed those results and demonstrated enhanced mucus in the small intestine of knockout mice. This suggests that a lack of TMEM16A or TMEM16F causes a broad secretion defect in secretory cells, including Paneth cells, the authors wrote.
Because granules of Paneth cells contain antimicrobial peptides, cytokines, and other factors that control proliferation or epithelial cell death, the researchers analyzed the presence of Gram-positive and Gram-negative bacteria in the jejunum and ileum. Compared to wild-type mice, the number of bacteria was higher in the ileum of both TMEM16A and TMEM16F knockout mice and in the jejunum of TMEM16F knockout mice, suggesting reduced antimicrobial activity in the absence of TMEM16 proteins.
The researchers also compared regulated cell death of intestinal epithelial cells in jejuna of wild-type and knockout mice. They found largely reduced cell death in both TMEM16A and TMEM16F knockout mice.
“Intestinal inflammatory diseases such as Crohn’s disease, necrotizing enterocolitis, and intestinal microbiota dysbiosis have been related to abnormal Paneth cell physiology,” the authors wrote. “The present findings may therefore provide the basis for a novel anti-inflammatory therapy for intestinal diseases and may improve our understanding of the molecular mechanism of some of the currently available drugs.”
The study was supported by the Deutsche Forschungsgemeinschaft funding program. The authors disclosed no conflicts of interest.
To defend the gut from microbes and pathogens, Paneth cells rely on TMEM16A, a calcium-activated chloride channel, and TMEM16F, a phospholipid scramblase, according to a new study published in Gastro Hep Advances.
The Paneth cells in mice missing TMEM16A or TMEM16F showed defects in signaling and release of secretary factors, researchers reported.
Inhibiting or activating TMEM16A and TMEM16F is likely to affect microbial content and immune functions in the small intestine, concluded Rainer Schreiber, Dr. rer. nat., of the Institute of Physiology at Universität Regensburg, Germany, and colleagues.
“Many small molecules and numerous natural or herbal compounds have been identified that either inhibit or activate TMEM16A or TMEM16F,” they wrote. “Some of these compounds may turn out to be useful therapeutics in inflammatory bowel disease, intestinal allergies, or abnormal colonization of the gut.”
Paneth cells play a central role in intestinal innate immune response, the authors wrote. Located at the base of small intestinal crypts and occasionally found in the proximal colon, these cells have defensive functions, such as protecting stem cells in response to invading microbes and eradicating ingested pathogens from intestinal crypts. Through secretion, they also regulate the composition and number of commensal intestinal bacteria. In inflammatory bowel disease, the Paneth cell zone expands due to an increase in cell size and cell number.
In previous studies, cholinergic stimulation provided enhanced protection in animals orally infected with virulent Salmonella enterica. However, the mechanisms of luminal stimulation of Paneth cell secretion in response to bacteria or lipopolysaccharide are unclear. Recent reports show that TMEM16A (also known as anoctamin 1, or ANO1) and TMEM16F (anoctamin 6, or ANO6) control intracellular calcium (Ca2+) signaling and that high local Ca2+ levels support exocytosis in intestinal cells.
The researchers analyzed the roles of the two molecules in Paneth cell secretion using mice with intestinal epithelial-specific knockout of TMEM16A or TMEM16F. They examined tissue structures and Paneth cells in the mice, as well as Paneth cell exocytosis in small intestinal organoids in vitro. They also compared Ca2+ signals between wild-type and knockout mice and analyzed bacterial colonization and intestinal apoptosis.
In wild-type mice, TMEM16A was detected at the apical pole of crypt epithelial cells, while TMEM16F was located predominantly at the basolateral side. Notably, TMEM16A was also located in intestinal smooth muscle cells.
Compared with wild-type mice, the TMEM16 knockout mice had pronounced accumulation of lysozyme in jejunal Paneth cells. This suggests a defect in Paneth cell secretion in the absence of TMEM16A and TMEM16F, the authors wrote.
Previous studies had found an accumulation of mucus in intestinal goblet cells in mice with tissue-specific knockout of TMEM16A and TMEM16F. In this study, a more detailed analysis of mucus using periodic acid-Schiff staining of duodenum, jejunum, and ileum confirmed those results and demonstrated enhanced mucus in the small intestine of knockout mice. This suggests that a lack of TMEM16A or TMEM16F causes a broad secretion defect in secretory cells, including Paneth cells, the authors wrote.
Because granules of Paneth cells contain antimicrobial peptides, cytokines, and other factors that control proliferation or epithelial cell death, the researchers analyzed the presence of Gram-positive and Gram-negative bacteria in the jejunum and ileum. Compared to wild-type mice, the number of bacteria was higher in the ileum of both TMEM16A and TMEM16F knockout mice and in the jejunum of TMEM16F knockout mice, suggesting reduced antimicrobial activity in the absence of TMEM16 proteins.
The researchers also compared regulated cell death of intestinal epithelial cells in jejuna of wild-type and knockout mice. They found largely reduced cell death in both TMEM16A and TMEM16F knockout mice.
“Intestinal inflammatory diseases such as Crohn’s disease, necrotizing enterocolitis, and intestinal microbiota dysbiosis have been related to abnormal Paneth cell physiology,” the authors wrote. “The present findings may therefore provide the basis for a novel anti-inflammatory therapy for intestinal diseases and may improve our understanding of the molecular mechanism of some of the currently available drugs.”
The study was supported by the Deutsche Forschungsgemeinschaft funding program. The authors disclosed no conflicts of interest.
FROM GASTRO HEP ADVANCES
Review explores the boundaries of endoscopic resection for esophageal adenocarcinoma
A growing body of evidence shows that deeper and larger tumors can be safely removed with endoscopy instead of surgery when individual patient risk is taken into account, according to a review by Eva P.D. Verheij, a doctoral candidate at Amsterdam University Medical Center, and colleagues.
“Management of patients with superficial esophageal adenocarcinoma (EAC) is becoming less invasive and more patient-tailored,” the researchers wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “In the future, watchful waiting may be a valid alternative to surgery in selected cases.”
The investigators examined new advances that have been made in the management of superficial esophageal adenocarcinomas by endoscopy, and they address how guidelines may be falling short in light of newly published evidence.
Surgery is usually the first choice for the management of advanced esophageal adenocarcinoma. “Endoscopic treatment has become the cornerstone for early cancer confined to the mucosa,” the authors wrote.
“For low-risk submucosal EAC, which only invades the superficial submucosa (sm1, i.e. less than 500 mcm) without any other risk factors, endoscopic treatment as an alternative to surgery is gaining acceptance because multiple studies have demonstrated a very low risk of lymph node metastases (less than 2% for these lesions),” the investigators wrote. Although surgical resection with lymphadenectomy is currently the recommended treatment for cases with deep submucosal invasion, poor differentiation, or lymphovascular invasion, the investigators suggested that even these tumors may be within an endoscopist’s reach.
While the rate of lymph node metastasis for such patients has been reported to be as high as 46%, more recent endoscopic studies show a metastasis rate range of up to 20% after 23-63 months of follow-up.
“One possible explanation for the discrepancy in lymph node metastases rates between surgical and endoscopic studies could be the different preparation of slides for histopathological assessment,” the investigators wrote. “In general, the cuts in surgical specimen are made with wider intervals (±5 mm) than the cuts in endoscopic resection specimens (2-3 mm), with additional cuts in case of submucosal invasion. The hypothesis is that this wider interval may result in missing the area with the deepest tumor infiltration. This could result in an underdiagnosis of the actual invasion depth, and therefore an overestimation of the associated lymph node metastases risk.” A study published in August 2022 in Gastrointestinal Endoscopy found an annual metastases risk of 6.9% in patients with high-risk T1a EAC.
“Given its invasiveness and associated morbidity and mortality, esophagectomy may be overtreatment in those patients who will not develop lymph node metastases,” the investigators wrote. “Given the technical advances in endoscopy that enable us to radically remove large EACs, and to perform more meticulous follow-up, it might be time to swing the pendulum and only send those patients for surgery who have an indisputable indication for surgery, instead of performing esophagectomy as a prophylactic treatment.”
To truly find the limits of endoscopic resection for EAC, however, more research is needed.
“Ongoing studies are necessary to evaluate the lymph node metastases risk on an individual basis, using presence of histological risk factors. By predicting the risk of lymph node metastases, and considering patients’ wishes and condition, one might decide to perform esophagectomy or watchful waiting with strict endoscopic follow-up. In high-risk cases, we may use sentinel node navigated surgery in the future as an extra safety check before deciding on optimal management,” the authors wrote.
The investigators disclosed relationships Medtronic, C2 Therapeutics/Pentax Medical, MicroTech, and Aqua Medical.
Barrett’s esophagus (BE) is the only known precursor lesion to esophageal adenocarcinoma, a cancer with rising incidence and stage-dependent survival. Early detection of BE-related neoplasia provides the opportunity to intervene through endoscopic eradication therapy and avoid the morbidity associated with esophagectomy. Verheji and colleagues, a group from a robust BE expert center in the Netherlands, provide a comprehensive and detailed overview of the role of endoscopic therapy for superficial esophageal adenocarcinoma (EAC), which is gaining popularity. In this review, they nicely highlight the benefits of this approach as a minimally invasive, organ-preserving, safe, and effective treatment option.
Jennifer M. Kolb, MD, MS, is assistant professor of medicine, Vatche and Tamar Manoukian Division of Digestive Diseases University of California, Los Angeles. She also is affiliated with VA Greater Los Angeles Health Care System. She has no relevant conflicts of interest.
Barrett’s esophagus (BE) is the only known precursor lesion to esophageal adenocarcinoma, a cancer with rising incidence and stage-dependent survival. Early detection of BE-related neoplasia provides the opportunity to intervene through endoscopic eradication therapy and avoid the morbidity associated with esophagectomy. Verheji and colleagues, a group from a robust BE expert center in the Netherlands, provide a comprehensive and detailed overview of the role of endoscopic therapy for superficial esophageal adenocarcinoma (EAC), which is gaining popularity. In this review, they nicely highlight the benefits of this approach as a minimally invasive, organ-preserving, safe, and effective treatment option.
Jennifer M. Kolb, MD, MS, is assistant professor of medicine, Vatche and Tamar Manoukian Division of Digestive Diseases University of California, Los Angeles. She also is affiliated with VA Greater Los Angeles Health Care System. She has no relevant conflicts of interest.
Barrett’s esophagus (BE) is the only known precursor lesion to esophageal adenocarcinoma, a cancer with rising incidence and stage-dependent survival. Early detection of BE-related neoplasia provides the opportunity to intervene through endoscopic eradication therapy and avoid the morbidity associated with esophagectomy. Verheji and colleagues, a group from a robust BE expert center in the Netherlands, provide a comprehensive and detailed overview of the role of endoscopic therapy for superficial esophageal adenocarcinoma (EAC), which is gaining popularity. In this review, they nicely highlight the benefits of this approach as a minimally invasive, organ-preserving, safe, and effective treatment option.
Jennifer M. Kolb, MD, MS, is assistant professor of medicine, Vatche and Tamar Manoukian Division of Digestive Diseases University of California, Los Angeles. She also is affiliated with VA Greater Los Angeles Health Care System. She has no relevant conflicts of interest.
A growing body of evidence shows that deeper and larger tumors can be safely removed with endoscopy instead of surgery when individual patient risk is taken into account, according to a review by Eva P.D. Verheij, a doctoral candidate at Amsterdam University Medical Center, and colleagues.
“Management of patients with superficial esophageal adenocarcinoma (EAC) is becoming less invasive and more patient-tailored,” the researchers wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “In the future, watchful waiting may be a valid alternative to surgery in selected cases.”
The investigators examined new advances that have been made in the management of superficial esophageal adenocarcinomas by endoscopy, and they address how guidelines may be falling short in light of newly published evidence.
Surgery is usually the first choice for the management of advanced esophageal adenocarcinoma. “Endoscopic treatment has become the cornerstone for early cancer confined to the mucosa,” the authors wrote.
“For low-risk submucosal EAC, which only invades the superficial submucosa (sm1, i.e. less than 500 mcm) without any other risk factors, endoscopic treatment as an alternative to surgery is gaining acceptance because multiple studies have demonstrated a very low risk of lymph node metastases (less than 2% for these lesions),” the investigators wrote. Although surgical resection with lymphadenectomy is currently the recommended treatment for cases with deep submucosal invasion, poor differentiation, or lymphovascular invasion, the investigators suggested that even these tumors may be within an endoscopist’s reach.
While the rate of lymph node metastasis for such patients has been reported to be as high as 46%, more recent endoscopic studies show a metastasis rate range of up to 20% after 23-63 months of follow-up.
“One possible explanation for the discrepancy in lymph node metastases rates between surgical and endoscopic studies could be the different preparation of slides for histopathological assessment,” the investigators wrote. “In general, the cuts in surgical specimen are made with wider intervals (±5 mm) than the cuts in endoscopic resection specimens (2-3 mm), with additional cuts in case of submucosal invasion. The hypothesis is that this wider interval may result in missing the area with the deepest tumor infiltration. This could result in an underdiagnosis of the actual invasion depth, and therefore an overestimation of the associated lymph node metastases risk.” A study published in August 2022 in Gastrointestinal Endoscopy found an annual metastases risk of 6.9% in patients with high-risk T1a EAC.
“Given its invasiveness and associated morbidity and mortality, esophagectomy may be overtreatment in those patients who will not develop lymph node metastases,” the investigators wrote. “Given the technical advances in endoscopy that enable us to radically remove large EACs, and to perform more meticulous follow-up, it might be time to swing the pendulum and only send those patients for surgery who have an indisputable indication for surgery, instead of performing esophagectomy as a prophylactic treatment.”
To truly find the limits of endoscopic resection for EAC, however, more research is needed.
“Ongoing studies are necessary to evaluate the lymph node metastases risk on an individual basis, using presence of histological risk factors. By predicting the risk of lymph node metastases, and considering patients’ wishes and condition, one might decide to perform esophagectomy or watchful waiting with strict endoscopic follow-up. In high-risk cases, we may use sentinel node navigated surgery in the future as an extra safety check before deciding on optimal management,” the authors wrote.
The investigators disclosed relationships Medtronic, C2 Therapeutics/Pentax Medical, MicroTech, and Aqua Medical.
A growing body of evidence shows that deeper and larger tumors can be safely removed with endoscopy instead of surgery when individual patient risk is taken into account, according to a review by Eva P.D. Verheij, a doctoral candidate at Amsterdam University Medical Center, and colleagues.
“Management of patients with superficial esophageal adenocarcinoma (EAC) is becoming less invasive and more patient-tailored,” the researchers wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “In the future, watchful waiting may be a valid alternative to surgery in selected cases.”
The investigators examined new advances that have been made in the management of superficial esophageal adenocarcinomas by endoscopy, and they address how guidelines may be falling short in light of newly published evidence.
Surgery is usually the first choice for the management of advanced esophageal adenocarcinoma. “Endoscopic treatment has become the cornerstone for early cancer confined to the mucosa,” the authors wrote.
“For low-risk submucosal EAC, which only invades the superficial submucosa (sm1, i.e. less than 500 mcm) without any other risk factors, endoscopic treatment as an alternative to surgery is gaining acceptance because multiple studies have demonstrated a very low risk of lymph node metastases (less than 2% for these lesions),” the investigators wrote. Although surgical resection with lymphadenectomy is currently the recommended treatment for cases with deep submucosal invasion, poor differentiation, or lymphovascular invasion, the investigators suggested that even these tumors may be within an endoscopist’s reach.
While the rate of lymph node metastasis for such patients has been reported to be as high as 46%, more recent endoscopic studies show a metastasis rate range of up to 20% after 23-63 months of follow-up.
“One possible explanation for the discrepancy in lymph node metastases rates between surgical and endoscopic studies could be the different preparation of slides for histopathological assessment,” the investigators wrote. “In general, the cuts in surgical specimen are made with wider intervals (±5 mm) than the cuts in endoscopic resection specimens (2-3 mm), with additional cuts in case of submucosal invasion. The hypothesis is that this wider interval may result in missing the area with the deepest tumor infiltration. This could result in an underdiagnosis of the actual invasion depth, and therefore an overestimation of the associated lymph node metastases risk.” A study published in August 2022 in Gastrointestinal Endoscopy found an annual metastases risk of 6.9% in patients with high-risk T1a EAC.
“Given its invasiveness and associated morbidity and mortality, esophagectomy may be overtreatment in those patients who will not develop lymph node metastases,” the investigators wrote. “Given the technical advances in endoscopy that enable us to radically remove large EACs, and to perform more meticulous follow-up, it might be time to swing the pendulum and only send those patients for surgery who have an indisputable indication for surgery, instead of performing esophagectomy as a prophylactic treatment.”
To truly find the limits of endoscopic resection for EAC, however, more research is needed.
“Ongoing studies are necessary to evaluate the lymph node metastases risk on an individual basis, using presence of histological risk factors. By predicting the risk of lymph node metastases, and considering patients’ wishes and condition, one might decide to perform esophagectomy or watchful waiting with strict endoscopic follow-up. In high-risk cases, we may use sentinel node navigated surgery in the future as an extra safety check before deciding on optimal management,” the authors wrote.
The investigators disclosed relationships Medtronic, C2 Therapeutics/Pentax Medical, MicroTech, and Aqua Medical.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
New AGA guideline recommends blood and stool tests for monitoring ulcerative colitis
These guidelines were published in Gastroenterology.
The AGA guidelines outline use cases for three biomarkers that provide accurate insights into UC disease activity: serum C-reactive protein (CRP) (blood), fecal calprotectin (stool), and fecal lactoferrin (stool). AGA recommends a monitoring strategy that integrates noninvasive biomarkers for patients with UC in remission (no current symptoms) as well as those with current symptoms.
Patients with UC in symptomatic remission
- Perform interval biomarker monitoring every 6-12 months.
- AGA recommends stool-based biomarkers over blood testing.
- If biomarkers are normal, AGA suggests continuing biomarker monitoring and avoiding routine endoscopic assessment.
- If biomarkers are elevated, AGA suggests endoscopic assessment by a gastroenterologist.
- Listen to your body! Talk to your doctor about any new symptoms.
Patients with symptomatically active UC
- Biomarker testing should be the first step to determine the need for endoscopic assessment.
- For patients with mild symptoms who have normal or elevated biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms who have normal biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms and elevated biomarkers, AGA suggests treatment adjustment and avoiding endoscopic assessment.
With AGA guidelines guiding the use of noninvasive biomarkers, physicians can confidently offer a more convenient and closer monitoring option for their patients.
AGA will advocate for all insurers to cover the cost of biomarker testing in UC.
These guidelines were published in Gastroenterology.
The AGA guidelines outline use cases for three biomarkers that provide accurate insights into UC disease activity: serum C-reactive protein (CRP) (blood), fecal calprotectin (stool), and fecal lactoferrin (stool). AGA recommends a monitoring strategy that integrates noninvasive biomarkers for patients with UC in remission (no current symptoms) as well as those with current symptoms.
Patients with UC in symptomatic remission
- Perform interval biomarker monitoring every 6-12 months.
- AGA recommends stool-based biomarkers over blood testing.
- If biomarkers are normal, AGA suggests continuing biomarker monitoring and avoiding routine endoscopic assessment.
- If biomarkers are elevated, AGA suggests endoscopic assessment by a gastroenterologist.
- Listen to your body! Talk to your doctor about any new symptoms.
Patients with symptomatically active UC
- Biomarker testing should be the first step to determine the need for endoscopic assessment.
- For patients with mild symptoms who have normal or elevated biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms who have normal biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms and elevated biomarkers, AGA suggests treatment adjustment and avoiding endoscopic assessment.
With AGA guidelines guiding the use of noninvasive biomarkers, physicians can confidently offer a more convenient and closer monitoring option for their patients.
AGA will advocate for all insurers to cover the cost of biomarker testing in UC.
These guidelines were published in Gastroenterology.
The AGA guidelines outline use cases for three biomarkers that provide accurate insights into UC disease activity: serum C-reactive protein (CRP) (blood), fecal calprotectin (stool), and fecal lactoferrin (stool). AGA recommends a monitoring strategy that integrates noninvasive biomarkers for patients with UC in remission (no current symptoms) as well as those with current symptoms.
Patients with UC in symptomatic remission
- Perform interval biomarker monitoring every 6-12 months.
- AGA recommends stool-based biomarkers over blood testing.
- If biomarkers are normal, AGA suggests continuing biomarker monitoring and avoiding routine endoscopic assessment.
- If biomarkers are elevated, AGA suggests endoscopic assessment by a gastroenterologist.
- Listen to your body! Talk to your doctor about any new symptoms.
Patients with symptomatically active UC
- Biomarker testing should be the first step to determine the need for endoscopic assessment.
- For patients with mild symptoms who have normal or elevated biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms who have normal biomarkers, AGA suggests endoscopic assessment by a gastroenterologist.
- For patients with moderate to severe symptoms and elevated biomarkers, AGA suggests treatment adjustment and avoiding endoscopic assessment.
With AGA guidelines guiding the use of noninvasive biomarkers, physicians can confidently offer a more convenient and closer monitoring option for their patients.
AGA will advocate for all insurers to cover the cost of biomarker testing in UC.
Medicare requires new modifier for CRC follow-on colonoscopy claims
To unlock this free benefit, providers must properly apply modifier KX.
What codes does this apply to?
Providers must append modifier KX (“requirements specified in the medical policy have been met”) to HCPCS codes G0105 and G0121 when the screening colonoscopy follows a positive result from one of the following noninvasive stool-based CRC screening tests:
- Screening Guaiac-based Fecal Occult Blood Test (gFOBT) (CPT 82270).
- Screening Immunoassay-based Fecal Occult Blood Test (iFOBT) (HCPCS G0328).
- Cologuard™ – Multi-target Stool DNA (sDNA) Test (CPT 81528).
What happens if I don’t use the KX modifier?
Medicare will return the screening colonoscopy claim as “unprocessable” and you will receive one of following messages:
CARC 16: “Claim/service lacks information or has submission billing error(s)” and RARC N822: “Missing Procedure Modifier(s)”
or
RARC N823: “Incomplete/Invalid Procedure Modifier”
Attach modifier KX and resubmit the claim to Medicare.
Should I use modifier KX if I remove polyps?
No. If you remove polyps during a screening colonoscopy following a positive noninvasive stool-based test, report the appropriate CPT code (for example, 45380, 45384, 45385, or 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare.
Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet when polypectomy is needed during a screening colonoscopy. Medicare beneficiary coinsurance responsibility is 15% of the cost of the procedure from 2023 to 2026. The coinsurance responsibility falls to 10% from 2027 to 2029 and by 2030 it will be covered 100% by Medicare.
Where can I find more information?
See the MLN Matters notice and the CMS Manual System.
To unlock this free benefit, providers must properly apply modifier KX.
What codes does this apply to?
Providers must append modifier KX (“requirements specified in the medical policy have been met”) to HCPCS codes G0105 and G0121 when the screening colonoscopy follows a positive result from one of the following noninvasive stool-based CRC screening tests:
- Screening Guaiac-based Fecal Occult Blood Test (gFOBT) (CPT 82270).
- Screening Immunoassay-based Fecal Occult Blood Test (iFOBT) (HCPCS G0328).
- Cologuard™ – Multi-target Stool DNA (sDNA) Test (CPT 81528).
What happens if I don’t use the KX modifier?
Medicare will return the screening colonoscopy claim as “unprocessable” and you will receive one of following messages:
CARC 16: “Claim/service lacks information or has submission billing error(s)” and RARC N822: “Missing Procedure Modifier(s)”
or
RARC N823: “Incomplete/Invalid Procedure Modifier”
Attach modifier KX and resubmit the claim to Medicare.
Should I use modifier KX if I remove polyps?
No. If you remove polyps during a screening colonoscopy following a positive noninvasive stool-based test, report the appropriate CPT code (for example, 45380, 45384, 45385, or 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare.
Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet when polypectomy is needed during a screening colonoscopy. Medicare beneficiary coinsurance responsibility is 15% of the cost of the procedure from 2023 to 2026. The coinsurance responsibility falls to 10% from 2027 to 2029 and by 2030 it will be covered 100% by Medicare.
Where can I find more information?
See the MLN Matters notice and the CMS Manual System.
To unlock this free benefit, providers must properly apply modifier KX.
What codes does this apply to?
Providers must append modifier KX (“requirements specified in the medical policy have been met”) to HCPCS codes G0105 and G0121 when the screening colonoscopy follows a positive result from one of the following noninvasive stool-based CRC screening tests:
- Screening Guaiac-based Fecal Occult Blood Test (gFOBT) (CPT 82270).
- Screening Immunoassay-based Fecal Occult Blood Test (iFOBT) (HCPCS G0328).
- Cologuard™ – Multi-target Stool DNA (sDNA) Test (CPT 81528).
What happens if I don’t use the KX modifier?
Medicare will return the screening colonoscopy claim as “unprocessable” and you will receive one of following messages:
CARC 16: “Claim/service lacks information or has submission billing error(s)” and RARC N822: “Missing Procedure Modifier(s)”
or
RARC N823: “Incomplete/Invalid Procedure Modifier”
Attach modifier KX and resubmit the claim to Medicare.
Should I use modifier KX if I remove polyps?
No. If you remove polyps during a screening colonoscopy following a positive noninvasive stool-based test, report the appropriate CPT code (for example, 45380, 45384, 45385, or 45388) and add modifier PT (colorectal cancer screening test; converted to diagnostic test or other procedure) to each CPT code for Medicare.
Some Medicare beneficiaries are not aware that Medicare has not fully eliminated the coinsurance responsibility yet when polypectomy is needed during a screening colonoscopy. Medicare beneficiary coinsurance responsibility is 15% of the cost of the procedure from 2023 to 2026. The coinsurance responsibility falls to 10% from 2027 to 2029 and by 2030 it will be covered 100% by Medicare.
Where can I find more information?
See the MLN Matters notice and the CMS Manual System.