User login
Decrease in cognitive functioning
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
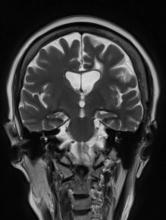
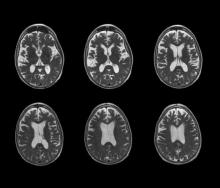
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of late-onset Alzheimer's disease (AD).
AD is a neurodegenerative disease associated with progressive impairment of behavioral and cognitive functions, including memory, comprehension, language, attention, reasoning, and judgment. At least two thirds of cases of dementia in people ≥ 65 years of age are due to AD, making it the most common type of dementia. At present, there is no cure for AD, which is associated with a long preclinical stage and a progressive disease course. In the United States, AD is the sixth leading cause of death.
Individuals with AD develop amyloid plaques in the hippocampus and in other areas of the cerebral cortex. The symptoms of AD vary depending on the stage of the disease; however, in most patients with late-onset AD (≥ 65 years of age), the most common presenting symptom is episodic short-term memory loss, with relative sparing of long-term memory. Subsequently, patients may experience impairments in problem-solving, judgment, executive functioning, motivation, and organization. It is not uncommon for individuals with AD to lack insight into the impairments they are experiences, or even to deny deficits.
Neuropsychiatric symptoms, such as apathy, social withdrawal, disinhibition, agitation, psychosis, and wandering are common in the mid- to late stages of the disease. Patients may also experience difficulty performing learned motor tasks (dyspraxia), olfactory dysfunction, and sleep disturbances; develop extrapyramidal motor signs (eg, dystonia, akathisia, and parkinsonian symptoms) followed by difficulties with primitive reflexes and incontinence, and may ultimately become totally dependent on caregivers.
A thorough history and physical examination are essential for the diagnosis of AD. Because some patients may lack insight into their disease, it is vital to elicit a history from the patient's family and caregivers as well. Onset and early symptoms are important to note to aid in differentiating AD from other types of dementia. In most patients with late-onset AD, comprehensive clinical assessment can provide reasonable diagnostic certainty. This should include a detailed neurologic examination to rule out other conditions; most patients with AD will have a normal neurologic exam.
A mental status examination to evaluate concentration, attention, recent and remote memory, language, visuospatial functioning, praxis, and executive functioning should also be conducted. Brief standard examinations, such the Mini-Mental State Examination, can be used for initial screening purposes, although they are less sensitive and specific than more comprehensive tests. Follow-up visits for patients diagnosed with AD should therefore include a full mental status examination to gauge disease progression as well as the development of neuropsychiatric symptoms.
Brain imaging can be beneficial both for diagnosing AD and monitoring the disease's clinical course. MRI or CT of the brain can help eliminate alternate causes of dementia, such as stroke or tumors, from consideration. Dilated lateral ventricles and widened cortical sulci, particularly in the temporal area, are typical findings in AD.
The standard medical treatment for AD includes cholinesterase inhibitors (ChEIs) and a partial N-methyl-D-aspartate (NMDA) antagonist. Both US and European guidelines list ChEIs (donepezil, rivastigmine, galantamine, tacrine) as first-line pharmacotherapies for mild to moderate AD; however, these agents only show modest efficacy on cognitive deficits and nonsignificant efficacy on functional capacity in mild to moderate AD. Memantine, a partial NMDA antagonist, shows very limited efficacy on cognitive symptoms, with no improvement in functional domains. Newly approved anti-amyloid therapies include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may help to mitigate the secondary symptoms of AD, such as depression, agitation, aggression, hallucinations, delusions, and sleep disorders. Behavioral interventions (eg, patient-centered approaches and caregiver training) may be beneficial for managing the cognitive and behavioral manifestations of AD and are often combined with pharmacologic interventions (eg, anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, antidepressants or mood stabilizers for mood disorders). Regular physical activity and exercise also be beneficial for brain health and delaying disease progression.
Numerous novel agents are under investigation for AD, including anti-tau therapy, anti-neuroinflammatory therapy, neuroprotective agents (such as NMDA receptor modulators), and brain stimulation.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 79-year-old man presents to his primary care provider (PCP) for an annual examination. The patient is accompanied by his oldest daughter, with whom he has lived since the death of his spouse approximately 9 months earlier. During the examination, the patient's daughter expresses concern about her father's cognitive functioning. Specifically, she has observed him becoming increasingly forgetful since he moved in with her. She states he has repeatedly forgotten the names of her dogs and has forgotten food in the microwave or on the stove on several occasions. Recently, after leaving a restaurant, her father was unable to remember where he had parked his car, and she suspects he has gotten lost while driving to and from familiar places several times. When questioned, the patient denies impairment and states occasional memory loss is "just part of the aging process."
Neither the patient nor his daughter reports any difficulties with his ability to groom and dress himself. His medical history is notable for high cholesterol, which is managed with a statin. The patient is a former smoker (24 pack-years) and occasionally drinks alcohol. His current height and weight are 5 ft 11 in and 177 lb, respectively.
The patient appears well nourished and oriented to time and place, although he appears to have moderate difficulty hearing and questions sometimes need to be repeated to him. His blood pressure, pulse oximetry, and heart rate are within normal ranges. Laboratory tests are all within normal ranges. The patient scores 16 on the Mini-Mental State Examination. His PCP orders MRI, which reveals atrophy on both hippocampi.
Cold snare polypectomy tops hot snare for small polyps
In the Taiwan Cold Polypectomy Study, CSP was not only safer than HSP, with a significantly lower risk for delayed bleeding, it was also more efficient, report Li-Chun Chang, MD, PhD, from the National Taiwan University Hospital, Taipei, and colleagues.
The study was published online in Annals of Internal Medicine.
This large study “strengthens the already significant evidence that CSP is as effective and safer than HSP for polyps 4-10 mm in size,” Rajesh N. Keswani, MD, Northwestern University, Chicago, told this news organization.
“This study evaluated all significant endpoints – safety (decreased bleeding risk with CSP), effectiveness (equivalent complete resection rates between CSP and HSP), and efficiency (CSP faster than HSP),” said Dr. Keswani, who wasn’t involved in the study.
Previous randomized controlled trials have shown that CSP is as effective as HSP but more efficient in removing small polyps. The reduction in delayed bleeding associated with CSP had been shown only in high-risk patients using antiplatelet agents or anticoagulants, however. Less was known about CSP’s effect on delayed bleeding in the general population.
To investigate, Dr. Chang and colleagues randomly assigned 4,270 adults aged 40 and older who were undergoing polypectomy to remove polyps measuring 4-10 mm to CSP or HSP.
Compared with HSP, CSP was associated with a significantly lower risk for all delayed bleeding (within 14 days after polypectomy) and severe delayed bleeding (defined as a decrease in hemoglobin of 20 g/L or more, requiring transfusion or hemostasis).
Eight of 2,137 patients (0.4%) in the CSP group had delayed bleeding versus 31 of 2,133 patients (1.5%) in the HSP group. Severe bleeding occurred in one patient who had CSP (0.05%) and eight who had HSP (0.4%).
The CSP group also had fewer emergency service visits than the HSP group – 4 visits (0.2%) versus 13 visits (0.6%).
CSP was more efficient, with mean polypectomy time reduced 26.9%, compared with HSP, with no difference between groups in successful tissue retrieval, en bloc resection, and complete histologic resection.
“CSP saves time setting up electrosurgical generators or conducting submucosal injection. Moreover, the lower rate of delayed bleeding means fewer emergency service visits or hospital stays, saving medical expenses,” Dr. Chang and colleagues write in their article.
“Given the benefit in safety and cost-effectiveness, CSP may replace HSP for removal of small polyps in the general population,” they add.
Dr. Keswani agreed. “Based on the accumulated evidence over the past decade, CSP is the clear standard of care for polyps 4-10 mm in size,” he said in an interview.
“For polyps less than 4 mm, it remains reasonable to use either large capacity/jumbo forceps or CSP. Cautery should be reserved only for polyps greater than 10 mm, although there is ongoing work regarding cold versus hot EMR [endoscopic mucosal resection],” Dr. Keswani said.
The trial was principal investigator–initiated and partially funded by Boston Scientific, which had no role in the study design, data collection or analysis, data interpretation, manuscript preparation, or decision to submit the manuscript for publication. Dr. Keswani is a consultant for Boston Scientific and Neptune Medical and receives research support from Virgo.
A version of this article first appeared on Medscape.com.
In the Taiwan Cold Polypectomy Study, CSP was not only safer than HSP, with a significantly lower risk for delayed bleeding, it was also more efficient, report Li-Chun Chang, MD, PhD, from the National Taiwan University Hospital, Taipei, and colleagues.
The study was published online in Annals of Internal Medicine.
This large study “strengthens the already significant evidence that CSP is as effective and safer than HSP for polyps 4-10 mm in size,” Rajesh N. Keswani, MD, Northwestern University, Chicago, told this news organization.
“This study evaluated all significant endpoints – safety (decreased bleeding risk with CSP), effectiveness (equivalent complete resection rates between CSP and HSP), and efficiency (CSP faster than HSP),” said Dr. Keswani, who wasn’t involved in the study.
Previous randomized controlled trials have shown that CSP is as effective as HSP but more efficient in removing small polyps. The reduction in delayed bleeding associated with CSP had been shown only in high-risk patients using antiplatelet agents or anticoagulants, however. Less was known about CSP’s effect on delayed bleeding in the general population.
To investigate, Dr. Chang and colleagues randomly assigned 4,270 adults aged 40 and older who were undergoing polypectomy to remove polyps measuring 4-10 mm to CSP or HSP.
Compared with HSP, CSP was associated with a significantly lower risk for all delayed bleeding (within 14 days after polypectomy) and severe delayed bleeding (defined as a decrease in hemoglobin of 20 g/L or more, requiring transfusion or hemostasis).
Eight of 2,137 patients (0.4%) in the CSP group had delayed bleeding versus 31 of 2,133 patients (1.5%) in the HSP group. Severe bleeding occurred in one patient who had CSP (0.05%) and eight who had HSP (0.4%).
The CSP group also had fewer emergency service visits than the HSP group – 4 visits (0.2%) versus 13 visits (0.6%).
CSP was more efficient, with mean polypectomy time reduced 26.9%, compared with HSP, with no difference between groups in successful tissue retrieval, en bloc resection, and complete histologic resection.
“CSP saves time setting up electrosurgical generators or conducting submucosal injection. Moreover, the lower rate of delayed bleeding means fewer emergency service visits or hospital stays, saving medical expenses,” Dr. Chang and colleagues write in their article.
“Given the benefit in safety and cost-effectiveness, CSP may replace HSP for removal of small polyps in the general population,” they add.
Dr. Keswani agreed. “Based on the accumulated evidence over the past decade, CSP is the clear standard of care for polyps 4-10 mm in size,” he said in an interview.
“For polyps less than 4 mm, it remains reasonable to use either large capacity/jumbo forceps or CSP. Cautery should be reserved only for polyps greater than 10 mm, although there is ongoing work regarding cold versus hot EMR [endoscopic mucosal resection],” Dr. Keswani said.
The trial was principal investigator–initiated and partially funded by Boston Scientific, which had no role in the study design, data collection or analysis, data interpretation, manuscript preparation, or decision to submit the manuscript for publication. Dr. Keswani is a consultant for Boston Scientific and Neptune Medical and receives research support from Virgo.
A version of this article first appeared on Medscape.com.
In the Taiwan Cold Polypectomy Study, CSP was not only safer than HSP, with a significantly lower risk for delayed bleeding, it was also more efficient, report Li-Chun Chang, MD, PhD, from the National Taiwan University Hospital, Taipei, and colleagues.
The study was published online in Annals of Internal Medicine.
This large study “strengthens the already significant evidence that CSP is as effective and safer than HSP for polyps 4-10 mm in size,” Rajesh N. Keswani, MD, Northwestern University, Chicago, told this news organization.
“This study evaluated all significant endpoints – safety (decreased bleeding risk with CSP), effectiveness (equivalent complete resection rates between CSP and HSP), and efficiency (CSP faster than HSP),” said Dr. Keswani, who wasn’t involved in the study.
Previous randomized controlled trials have shown that CSP is as effective as HSP but more efficient in removing small polyps. The reduction in delayed bleeding associated with CSP had been shown only in high-risk patients using antiplatelet agents or anticoagulants, however. Less was known about CSP’s effect on delayed bleeding in the general population.
To investigate, Dr. Chang and colleagues randomly assigned 4,270 adults aged 40 and older who were undergoing polypectomy to remove polyps measuring 4-10 mm to CSP or HSP.
Compared with HSP, CSP was associated with a significantly lower risk for all delayed bleeding (within 14 days after polypectomy) and severe delayed bleeding (defined as a decrease in hemoglobin of 20 g/L or more, requiring transfusion or hemostasis).
Eight of 2,137 patients (0.4%) in the CSP group had delayed bleeding versus 31 of 2,133 patients (1.5%) in the HSP group. Severe bleeding occurred in one patient who had CSP (0.05%) and eight who had HSP (0.4%).
The CSP group also had fewer emergency service visits than the HSP group – 4 visits (0.2%) versus 13 visits (0.6%).
CSP was more efficient, with mean polypectomy time reduced 26.9%, compared with HSP, with no difference between groups in successful tissue retrieval, en bloc resection, and complete histologic resection.
“CSP saves time setting up electrosurgical generators or conducting submucosal injection. Moreover, the lower rate of delayed bleeding means fewer emergency service visits or hospital stays, saving medical expenses,” Dr. Chang and colleagues write in their article.
“Given the benefit in safety and cost-effectiveness, CSP may replace HSP for removal of small polyps in the general population,” they add.
Dr. Keswani agreed. “Based on the accumulated evidence over the past decade, CSP is the clear standard of care for polyps 4-10 mm in size,” he said in an interview.
“For polyps less than 4 mm, it remains reasonable to use either large capacity/jumbo forceps or CSP. Cautery should be reserved only for polyps greater than 10 mm, although there is ongoing work regarding cold versus hot EMR [endoscopic mucosal resection],” Dr. Keswani said.
The trial was principal investigator–initiated and partially funded by Boston Scientific, which had no role in the study design, data collection or analysis, data interpretation, manuscript preparation, or decision to submit the manuscript for publication. Dr. Keswani is a consultant for Boston Scientific and Neptune Medical and receives research support from Virgo.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Difficulty remembering words
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
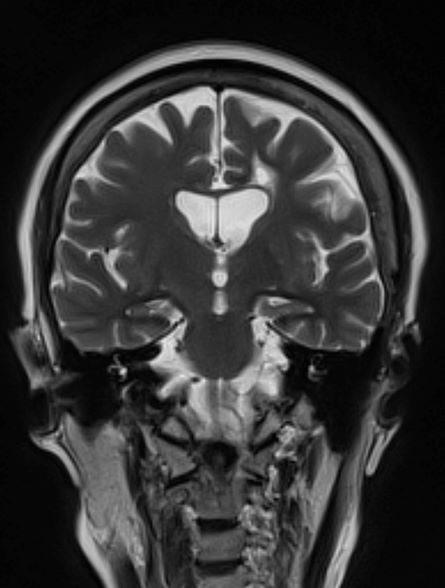
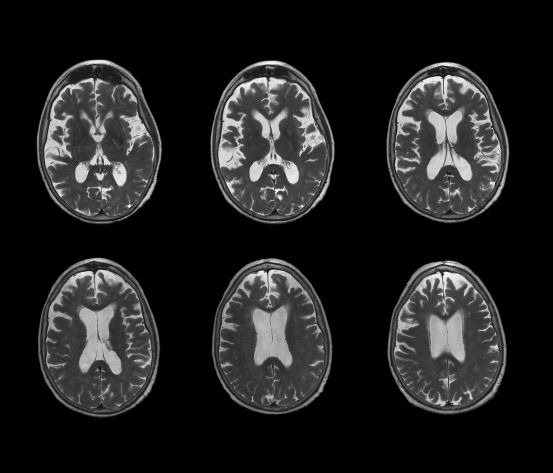
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of early-onset Alzheimer's disease (AD) with aphasia.
AD is a neurodegenerative disorder characterized by cognitive and behavioral impairment that significantly interferes with a patient's social and occupational functioning. There is currently no cure for AD, which has a long preclinical period and a progressive course. Individuals with AD develop amyloid plaques in the hippocampus, a structure deep in the brain that helps to encode memories, and in other areas of the cerebral cortex that are involved in thinking and making decisions.
Patients with AD typically present with insidiously progressive memory loss; over the course of several years, other areas of cognition are impaired. Subsequent to memory loss, patients may also experience language disorders (eg, anomic aphasia or anomia) and impairment in visuospatial skills and executive functions. In many patients, slowly progressive behavioral changes are also observed.
AD is most prevalent in individuals older than 65 years; however, early‐onset AD (in individuals aged 60 years or older) can also occur. Early-onset AD shares the same essential neuropathological characteristics (ie, amyloid plaques and neurofibrillary tangles) as late-onset (65 years or older) AD, but it differs in several ways. For example, memory loss is an extremely common presenting symptom in late-onset AD, whereas nonamnestic presentation (ie, language, visuospatial, or executive impairment) is very rare, occurring in only about 5% of cases. Conversely, nonamnestic presentations may occur in 30%-40% of patients with early-onset AD. Frequent nonamnestic cognitive manifestations in patients with early-onset AD are those seen in mild to moderate AD, including visual agnosia (55.1%), aphasia (57.9%), and behavioral changes (61.7%). In addition, several studies have suggested that early-onset AD may have a more aggressive course than late-onset AD does, including faster cognitive and functional decline.
Presently, only symptomatic therapies are available for AD. The standard medical treatment for AD includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Newly approved antiamyloid therapies are also available for patients with mild cognitive impairment or mild dementia. These include aducanumab, a first-in-class amyloid beta–directed antibody that was approved in 2021, and lecanemab, another amyloid beta–directed antibody that was approved in 2023. Both aducanumab and lecanemab are recommended for the treatment of patients with mild cognitive impairment or mild dementia stage of disease, the population in which the safety and efficacy of these newer agents were demonstrated in clinical trials.
Psychotropic agents may be used to treat the secondary symptoms of AD (eg, depression, agitation, aggression, hallucinations, delusions, sleep disorders), which can be problematic. Behavioral interventions ranging from patient-centered approaches to caregiver training may also be used to help manage cognitive and behavioral manifestations of AD, often in combination with pharmacologic interventions, such as anxiolytics for anxiety and agitation, neuroleptics for delusions or hallucinations, and antidepressants or mood stabilizers for mood disorders and specific manifestations (eg, episodes of anger or rage). Routine physical activity and exercise may affect AD progression and may possibly exert a protective effect on brain health.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 53-year-old woman, who is a high school mathematics teacher, presents with reports of progressively increasing cognitive impairments. Specifically, she notes increasing difficulty with remembering words as well as challenges with her executive functioning. She was recently reprimanded by her principal for missing several mandatory staff meetings and deadlines for submitting student grades. The patient states her symptoms began approximately 2 years ago. She initially attributed them to hormonal changes because of menopause but is becoming concerned about the impact they are having on her ability to function. She recently began experiencing difficulties with spatial perception, which resulted in her falling down the stairs of her home and spraining an ankle. The patient lives alone and has no children. Her medical history is unremarkable except for a motor vehicle accident 5 years earlier that resulted in her sustaining a concussion and a fractured wrist. She does not currently take any medications. There is no history of tobacco use or excessive alcohol consumption. Her current height and weight are 5 ft 3 in and 147 lb, respectively.
No abnormalities are noted on physical exam; the patient's blood pressure, pulse oximetry, and heart rate are within normal ranges. Laboratory tests are all within normal ranges, including thyroid-stimulating hormone and vitamin B12 levels. The patient scores 16 on the Montreal Cognitive Assessment test. Her clinician orders an MRI, which reveals deep indentations around the front and sides of the brain.
Expert offers caveats to perioperative antirheumatic drug guideline
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
FROM RWCS 2023
Adaptations to education, training vital to alleviating rheumatologist shortage
To address the growing workforce shortage in rheumatology, medical educators will have to adapt and learn how to train a new generation of rheumatologists, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, told attendees she is “heavily invested in the training of our future rheumatologists” and strives to help them “operate at the top of the level across the spectrum.”
“They’re carrying forward our field,” Dr. Dua said. “We need to propagate our field and we need them to go out and serve and continue to make rheumatology awesome.”
The American College of Rheumatology’s 2015 workforce study estimates that by 2030, there will be a shortage of more than 4,000 rheumatologists in the United States.
Rheumatology may have inadvertently created the problem through rheumatologists diagnosing disease earlier and prescribing better treatments, with patients subsequently living longer with disease, she noted. Compounding the problem is an increasing number of rheumatologists looking to retire over the next decade and the continued need for care in rural areas where there are few practicing rheumatologists.
Interest in rheumatology is increasing
The good news is there is increasing interest in the field. “This has really shifted, I would say, from about 10 years ago when I was looking at fellowships,” Dr. Dua said. “It’s not really an interest problem. But the issue is that the training programs and slots don’t necessarily exist to fill the gap of the people who are leaving the field.”
The key to bringing more people into rheumatology is to understand how Millennials and Generation Z differ from generations that came before them. In general, members of Generation Z “tend to prefer an à la carte approach to education” with hands-on experiences, and they prefer customized feedback that is actionable, Dr. Dua explained.
“As a medical educator, there are different demands, and these are changing over time, so we have to figure out how we can best serve them and educate them,” she said.
This also means connecting with younger generations on social media. A research letter published in JAMA Network Open in 2021 found a minority of 650 physicians across 14 specialties had a presence on social media platforms, with 44.9% of physicians surveyed present on LinkedIn, 23.4% on Facebook, 18.6% of on Twitter, and 14.9% on ResearchGate. “There is a lot of room to grow, and this is where some of our future teaching is headed,” Dr. Dua said.
Future of rheumatology education
Does this mean rheumatologists should start dancing in TikTok videos? Maybe not, but Dr. Dua noted there are ways to bring understanding, recall, comprehension, and behavioral change through active learning, spaced learning, case-based modules, podcasts, videos, and other educational strategies.
“We need to find ways to engage our learners and connect with them and teach them,” she said.
Rheumatologists are already bringing innovation to the education space with initiatives like educational podcasts, remote learning developed during the COVID-19 pandemic, development of rheumatology Objective Structured Clinical Examinations using challenging patient scenarios, and other virtual learning opportunities. “We really have been forced to push the envelope,” Dr. Dua said.
“The future of medical education is here. It’s exciting. Embrace it,” she said.
Training nurse practitioners and physician assistants?
Commenting on the shortage of rheumatologists, Philip J. Mease, MD, clinical professor at the University of Washington and director of rheumatology research at Swedish Medical Center, both in Seattle, said one answer to the problem may be training more nurse practitioners (NPs) and physician assistants (PAs) to bridge the gap.
“Some are suggesting that part of the answer to the deficiency of rheumatologists will be having two NPs or PAs to every single rheumatologist that there is out there,” he said. “I work with three, and the issue of ... getting access to them when they are in school to demonstrate how sexy rheumatology is, is something that is deficient, way deficient.” Rheumatologists should be putting themselves out there with preceptorships and lectures to recruit more NPs and PAs to rheumatology, he explained. “That’s a 24/7 process.”
Dr. Dua, who is cochair of the E-Learning Subcommittee within the ACR Workforce Solutions Steering Committee, said the subcomittee’s focus has been connecting with primary care doctors, pediatricians, NPs, and PAs to “expand who can provide some rheumatologic care.”
Lindsay Orme, MD, a family medicine doctor from Caldwell, Idaho, shared her experience serving as faculty for a family medicine residency program, training family medicine doctors in rural areas.
“Our curriculum hasn’t had a section for what trainees are expected to learn in rheumatology. When I did the same program years ago in Idaho, it was very well defined: What I should know how to do without consulting a cardiologist, what I should know how to do without consulting an obstetrician, what I should be able to manage in terms of [chronic kidney disease] before referring to nephrology,” she explained. “No one ever taught me what I could manage in rheumatology.
“I do think we need to find some defined areas that we’re more comfortable teaching primary care doctors to manage because there is no one – there are no rheumatologists in Boise or any of the surrounding towns that accept Medicaid patients now. They are all expected to go 250 miles away,” she said.
“That’s a major, major problem,” Dr. Dua acknowledged. “Really, for me, the goal is to develop resources that you can tap into to be able to at least figure out where things stand, and at least bide time until they can get in with that rheumatologist 250 miles away and make sure that you’re getting the training, or feel comfortable with whatever it is you’re forced to manage from a rheumatologic sense.”
More engagement, more adaptation
Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said one thing he’s noticed over the years is that, as time spent in the hospital has decreased, the time residents and fellows spend with practitioners in front of patients has also decreased. “It just isn’t there, and that’s where you really learn,” he said.
“You are 100% correct the two generations are different. What I think is important in life is very different than what the fellows think is different in life at this point, and how much work I’m willing to put in or how much work they’re willing to put in, in the same way, is very different,” he explained. “What they want to spend their time on, I don’t, and vice versa. We do have to adapt, but I do think that they need more time in front of patients with very experienced physicians.”
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said if education is to move forward, “it’s got to change dramatically.”
“The competencies aren’t always knowledge,” he said. “Knowledge has now been replaced by everything at your fingertips. I don’t need to know all the formulas and everything right now.”
Engagement should be the “main statistic that we need to be striving for,” Dr. Cush explained. “Engagement as the measure of ... education’s value, I think, is where it has to go.”
Dr. Dua reported being a consultant and serving on an advisory board for Sanofi, Novartis, AbbVie, and Chemocentryx/Amgen.
To address the growing workforce shortage in rheumatology, medical educators will have to adapt and learn how to train a new generation of rheumatologists, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, told attendees she is “heavily invested in the training of our future rheumatologists” and strives to help them “operate at the top of the level across the spectrum.”
“They’re carrying forward our field,” Dr. Dua said. “We need to propagate our field and we need them to go out and serve and continue to make rheumatology awesome.”
The American College of Rheumatology’s 2015 workforce study estimates that by 2030, there will be a shortage of more than 4,000 rheumatologists in the United States.
Rheumatology may have inadvertently created the problem through rheumatologists diagnosing disease earlier and prescribing better treatments, with patients subsequently living longer with disease, she noted. Compounding the problem is an increasing number of rheumatologists looking to retire over the next decade and the continued need for care in rural areas where there are few practicing rheumatologists.
Interest in rheumatology is increasing
The good news is there is increasing interest in the field. “This has really shifted, I would say, from about 10 years ago when I was looking at fellowships,” Dr. Dua said. “It’s not really an interest problem. But the issue is that the training programs and slots don’t necessarily exist to fill the gap of the people who are leaving the field.”
The key to bringing more people into rheumatology is to understand how Millennials and Generation Z differ from generations that came before them. In general, members of Generation Z “tend to prefer an à la carte approach to education” with hands-on experiences, and they prefer customized feedback that is actionable, Dr. Dua explained.
“As a medical educator, there are different demands, and these are changing over time, so we have to figure out how we can best serve them and educate them,” she said.
This also means connecting with younger generations on social media. A research letter published in JAMA Network Open in 2021 found a minority of 650 physicians across 14 specialties had a presence on social media platforms, with 44.9% of physicians surveyed present on LinkedIn, 23.4% on Facebook, 18.6% of on Twitter, and 14.9% on ResearchGate. “There is a lot of room to grow, and this is where some of our future teaching is headed,” Dr. Dua said.
Future of rheumatology education
Does this mean rheumatologists should start dancing in TikTok videos? Maybe not, but Dr. Dua noted there are ways to bring understanding, recall, comprehension, and behavioral change through active learning, spaced learning, case-based modules, podcasts, videos, and other educational strategies.
“We need to find ways to engage our learners and connect with them and teach them,” she said.
Rheumatologists are already bringing innovation to the education space with initiatives like educational podcasts, remote learning developed during the COVID-19 pandemic, development of rheumatology Objective Structured Clinical Examinations using challenging patient scenarios, and other virtual learning opportunities. “We really have been forced to push the envelope,” Dr. Dua said.
“The future of medical education is here. It’s exciting. Embrace it,” she said.
Training nurse practitioners and physician assistants?
Commenting on the shortage of rheumatologists, Philip J. Mease, MD, clinical professor at the University of Washington and director of rheumatology research at Swedish Medical Center, both in Seattle, said one answer to the problem may be training more nurse practitioners (NPs) and physician assistants (PAs) to bridge the gap.
“Some are suggesting that part of the answer to the deficiency of rheumatologists will be having two NPs or PAs to every single rheumatologist that there is out there,” he said. “I work with three, and the issue of ... getting access to them when they are in school to demonstrate how sexy rheumatology is, is something that is deficient, way deficient.” Rheumatologists should be putting themselves out there with preceptorships and lectures to recruit more NPs and PAs to rheumatology, he explained. “That’s a 24/7 process.”
Dr. Dua, who is cochair of the E-Learning Subcommittee within the ACR Workforce Solutions Steering Committee, said the subcomittee’s focus has been connecting with primary care doctors, pediatricians, NPs, and PAs to “expand who can provide some rheumatologic care.”
Lindsay Orme, MD, a family medicine doctor from Caldwell, Idaho, shared her experience serving as faculty for a family medicine residency program, training family medicine doctors in rural areas.
“Our curriculum hasn’t had a section for what trainees are expected to learn in rheumatology. When I did the same program years ago in Idaho, it was very well defined: What I should know how to do without consulting a cardiologist, what I should know how to do without consulting an obstetrician, what I should be able to manage in terms of [chronic kidney disease] before referring to nephrology,” she explained. “No one ever taught me what I could manage in rheumatology.
“I do think we need to find some defined areas that we’re more comfortable teaching primary care doctors to manage because there is no one – there are no rheumatologists in Boise or any of the surrounding towns that accept Medicaid patients now. They are all expected to go 250 miles away,” she said.
“That’s a major, major problem,” Dr. Dua acknowledged. “Really, for me, the goal is to develop resources that you can tap into to be able to at least figure out where things stand, and at least bide time until they can get in with that rheumatologist 250 miles away and make sure that you’re getting the training, or feel comfortable with whatever it is you’re forced to manage from a rheumatologic sense.”
More engagement, more adaptation
Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said one thing he’s noticed over the years is that, as time spent in the hospital has decreased, the time residents and fellows spend with practitioners in front of patients has also decreased. “It just isn’t there, and that’s where you really learn,” he said.
“You are 100% correct the two generations are different. What I think is important in life is very different than what the fellows think is different in life at this point, and how much work I’m willing to put in or how much work they’re willing to put in, in the same way, is very different,” he explained. “What they want to spend their time on, I don’t, and vice versa. We do have to adapt, but I do think that they need more time in front of patients with very experienced physicians.”
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said if education is to move forward, “it’s got to change dramatically.”
“The competencies aren’t always knowledge,” he said. “Knowledge has now been replaced by everything at your fingertips. I don’t need to know all the formulas and everything right now.”
Engagement should be the “main statistic that we need to be striving for,” Dr. Cush explained. “Engagement as the measure of ... education’s value, I think, is where it has to go.”
Dr. Dua reported being a consultant and serving on an advisory board for Sanofi, Novartis, AbbVie, and Chemocentryx/Amgen.
To address the growing workforce shortage in rheumatology, medical educators will have to adapt and learn how to train a new generation of rheumatologists, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, told attendees she is “heavily invested in the training of our future rheumatologists” and strives to help them “operate at the top of the level across the spectrum.”
“They’re carrying forward our field,” Dr. Dua said. “We need to propagate our field and we need them to go out and serve and continue to make rheumatology awesome.”
The American College of Rheumatology’s 2015 workforce study estimates that by 2030, there will be a shortage of more than 4,000 rheumatologists in the United States.
Rheumatology may have inadvertently created the problem through rheumatologists diagnosing disease earlier and prescribing better treatments, with patients subsequently living longer with disease, she noted. Compounding the problem is an increasing number of rheumatologists looking to retire over the next decade and the continued need for care in rural areas where there are few practicing rheumatologists.
Interest in rheumatology is increasing
The good news is there is increasing interest in the field. “This has really shifted, I would say, from about 10 years ago when I was looking at fellowships,” Dr. Dua said. “It’s not really an interest problem. But the issue is that the training programs and slots don’t necessarily exist to fill the gap of the people who are leaving the field.”
The key to bringing more people into rheumatology is to understand how Millennials and Generation Z differ from generations that came before them. In general, members of Generation Z “tend to prefer an à la carte approach to education” with hands-on experiences, and they prefer customized feedback that is actionable, Dr. Dua explained.
“As a medical educator, there are different demands, and these are changing over time, so we have to figure out how we can best serve them and educate them,” she said.
This also means connecting with younger generations on social media. A research letter published in JAMA Network Open in 2021 found a minority of 650 physicians across 14 specialties had a presence on social media platforms, with 44.9% of physicians surveyed present on LinkedIn, 23.4% on Facebook, 18.6% of on Twitter, and 14.9% on ResearchGate. “There is a lot of room to grow, and this is where some of our future teaching is headed,” Dr. Dua said.
Future of rheumatology education
Does this mean rheumatologists should start dancing in TikTok videos? Maybe not, but Dr. Dua noted there are ways to bring understanding, recall, comprehension, and behavioral change through active learning, spaced learning, case-based modules, podcasts, videos, and other educational strategies.
“We need to find ways to engage our learners and connect with them and teach them,” she said.
Rheumatologists are already bringing innovation to the education space with initiatives like educational podcasts, remote learning developed during the COVID-19 pandemic, development of rheumatology Objective Structured Clinical Examinations using challenging patient scenarios, and other virtual learning opportunities. “We really have been forced to push the envelope,” Dr. Dua said.
“The future of medical education is here. It’s exciting. Embrace it,” she said.
Training nurse practitioners and physician assistants?
Commenting on the shortage of rheumatologists, Philip J. Mease, MD, clinical professor at the University of Washington and director of rheumatology research at Swedish Medical Center, both in Seattle, said one answer to the problem may be training more nurse practitioners (NPs) and physician assistants (PAs) to bridge the gap.
“Some are suggesting that part of the answer to the deficiency of rheumatologists will be having two NPs or PAs to every single rheumatologist that there is out there,” he said. “I work with three, and the issue of ... getting access to them when they are in school to demonstrate how sexy rheumatology is, is something that is deficient, way deficient.” Rheumatologists should be putting themselves out there with preceptorships and lectures to recruit more NPs and PAs to rheumatology, he explained. “That’s a 24/7 process.”
Dr. Dua, who is cochair of the E-Learning Subcommittee within the ACR Workforce Solutions Steering Committee, said the subcomittee’s focus has been connecting with primary care doctors, pediatricians, NPs, and PAs to “expand who can provide some rheumatologic care.”
Lindsay Orme, MD, a family medicine doctor from Caldwell, Idaho, shared her experience serving as faculty for a family medicine residency program, training family medicine doctors in rural areas.
“Our curriculum hasn’t had a section for what trainees are expected to learn in rheumatology. When I did the same program years ago in Idaho, it was very well defined: What I should know how to do without consulting a cardiologist, what I should know how to do without consulting an obstetrician, what I should be able to manage in terms of [chronic kidney disease] before referring to nephrology,” she explained. “No one ever taught me what I could manage in rheumatology.
“I do think we need to find some defined areas that we’re more comfortable teaching primary care doctors to manage because there is no one – there are no rheumatologists in Boise or any of the surrounding towns that accept Medicaid patients now. They are all expected to go 250 miles away,” she said.
“That’s a major, major problem,” Dr. Dua acknowledged. “Really, for me, the goal is to develop resources that you can tap into to be able to at least figure out where things stand, and at least bide time until they can get in with that rheumatologist 250 miles away and make sure that you’re getting the training, or feel comfortable with whatever it is you’re forced to manage from a rheumatologic sense.”
More engagement, more adaptation
Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said one thing he’s noticed over the years is that, as time spent in the hospital has decreased, the time residents and fellows spend with practitioners in front of patients has also decreased. “It just isn’t there, and that’s where you really learn,” he said.
“You are 100% correct the two generations are different. What I think is important in life is very different than what the fellows think is different in life at this point, and how much work I’m willing to put in or how much work they’re willing to put in, in the same way, is very different,” he explained. “What they want to spend their time on, I don’t, and vice versa. We do have to adapt, but I do think that they need more time in front of patients with very experienced physicians.”
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said if education is to move forward, “it’s got to change dramatically.”
“The competencies aren’t always knowledge,” he said. “Knowledge has now been replaced by everything at your fingertips. I don’t need to know all the formulas and everything right now.”
Engagement should be the “main statistic that we need to be striving for,” Dr. Cush explained. “Engagement as the measure of ... education’s value, I think, is where it has to go.”
Dr. Dua reported being a consultant and serving on an advisory board for Sanofi, Novartis, AbbVie, and Chemocentryx/Amgen.
FROM RWCS 2023
A purple warrior rises in the battle against diabetes
One-eyed, one-horned, flying purple veggie eater
Big Fruits and Vegetables is at it again. You notice how they’re always like “Oh, vegetables are good for your health,” and “Eating fruits every day makes you live longer,” but come on. It’s a marketing ploy, leading us astray from our personal savior, McDonald’s.
Just look at this latest bit of research: According to researchers from Finland, eating purple vegetables can protect against diabetes. Considering nearly 40 million Americans have diabetes (and nearly 100 million have prediabetes), anything to reduce the incidence of diabetes (people with diabetes account for one-fourth of every dollar spent in U.S. health care) would be beneficial. So, let’s humor the fruits and veggies people this time and hear them out.
It all comes down to a chemical called anthocyanin, which is a pigment that gives fruits and vegetables such as blueberries, radishes, and red cabbages their purplish color. Anthocyanin also has probiotic and anti-inflammatory effects, meaning it can help improve intestinal lining health and regulate glucose and lipid metabolic pathways. Obviously, good things if you want to avoid diabetes.
The investigators also found that, while standard anthocyanin was beneficial, acylated anthocyanin (which has an acyl group added to the sugar molecules of anthocyanin) is really what you want to go for. The acylated version, found in abundance in purple potatoes, purple carrots, radishes, and red cabbages, is tougher to digest, but the positive effects it has in the body are enhanced over the standard version.
Now, this all a compelling bit of research, but at the end of the day, you’re still eating fruits and vegetables, and we are red-blooded Americans here. We don’t do healthy foods. Although, if you were to dye our burgers with anthocyanin and make them purple, you’d have our attention. Purple is our favorite color.
Manuka honey better as building material than antibiotic
Milk, according to the old saying, builds strong bones, but when it comes to patients with bone loss caused by various medical reasons, researchers found that manuka honey, produced only in New Zealand and some parts of Australia, may also do the job. They soaked collagen scaffolds used for bone implants in various concentrations of the honey and found that 5% led to higher mineral formation and osteoprotegerin production, which suggests increased bone production.
But, and this is a pretty big one, the other half of the study – testing manuka honey’s ability to ward off bacteria – wasn’t so successful. Bone implants, apparently, count for almost half of all hospital-acquired infections, which obviously can put a damper on the healing process. The hope was that a biomaterial would be more effective than something like metal in lessening bacteria formation. Nope.
When the researchers soaked paper disks in honey and added them to cultures of Pseudomonas aeruginosa and Staphylococcus aureus, none of the various concentrations stopped bacterial growth in the scaffolding, even when they added antibiotics.
The sticky conclusion, you could say, is more bitter than sweet.
It may sound like Korn, but can it play ‘Freak on a Leash’?
Like all right-thinking Americans, we love corn, corn-based products, and almost corn. Corn on the cob grilled in the husk? Mmm. Plus, we’re big fans of the band Korn. Also, we once had a reporter here named Tim Kirn. And don’t even get us started with Karn. Best Family Feud host ever.
But what about Quorn? Oh sure, the fungi-based meat alternative is full of yummy mycoprotein, but can it prevent colorectal cancer? Can we add Quorn to our favorites list? Let’s see what Science has to say.
Researchers at Northumbria University in Newcastle upon Tyne, England, fed a group of 20 men some meat (240 g/day) for 2 weeks – hopefully, they were allowed to eat some other food as well – and then gave them the same amount of Quorn, excuse us, fungi-derived mycoprotein equivalents, for 2 more weeks, with a 4-week washout period in between.
Levels of cancer-causing chemicals known as genotoxins fell significantly in the mycoprotein phase but rose during the meat phase. The mycoprotein diet also improved gut health “by increasing the abundance of protective bacteria such as Lactobacilli, Roseburia, and Akkermansia, which are associated with offering protection against chemically induced tumours, inflammation and bowel cancer,” they said in a statement from the university.
The meat phase, on the other hand, resulted in an increase in “gut bacteria linked with issues such as cancer, cardiovascular diseases, weight gain and other negative health outcomes,” they noted.
Science, then, seems to approve of Quorn, and that’s good enough for us. We’re adding Quorn to our diet, starting with a fungi-derived mycoproteinburger tonight while we’re watching the Cornell Big Red take the court against their archrivals, the Big Green of Dartmouth College. GO RED!
One-eyed, one-horned, flying purple veggie eater
Big Fruits and Vegetables is at it again. You notice how they’re always like “Oh, vegetables are good for your health,” and “Eating fruits every day makes you live longer,” but come on. It’s a marketing ploy, leading us astray from our personal savior, McDonald’s.
Just look at this latest bit of research: According to researchers from Finland, eating purple vegetables can protect against diabetes. Considering nearly 40 million Americans have diabetes (and nearly 100 million have prediabetes), anything to reduce the incidence of diabetes (people with diabetes account for one-fourth of every dollar spent in U.S. health care) would be beneficial. So, let’s humor the fruits and veggies people this time and hear them out.
It all comes down to a chemical called anthocyanin, which is a pigment that gives fruits and vegetables such as blueberries, radishes, and red cabbages their purplish color. Anthocyanin also has probiotic and anti-inflammatory effects, meaning it can help improve intestinal lining health and regulate glucose and lipid metabolic pathways. Obviously, good things if you want to avoid diabetes.
The investigators also found that, while standard anthocyanin was beneficial, acylated anthocyanin (which has an acyl group added to the sugar molecules of anthocyanin) is really what you want to go for. The acylated version, found in abundance in purple potatoes, purple carrots, radishes, and red cabbages, is tougher to digest, but the positive effects it has in the body are enhanced over the standard version.
Now, this all a compelling bit of research, but at the end of the day, you’re still eating fruits and vegetables, and we are red-blooded Americans here. We don’t do healthy foods. Although, if you were to dye our burgers with anthocyanin and make them purple, you’d have our attention. Purple is our favorite color.
Manuka honey better as building material than antibiotic
Milk, according to the old saying, builds strong bones, but when it comes to patients with bone loss caused by various medical reasons, researchers found that manuka honey, produced only in New Zealand and some parts of Australia, may also do the job. They soaked collagen scaffolds used for bone implants in various concentrations of the honey and found that 5% led to higher mineral formation and osteoprotegerin production, which suggests increased bone production.
But, and this is a pretty big one, the other half of the study – testing manuka honey’s ability to ward off bacteria – wasn’t so successful. Bone implants, apparently, count for almost half of all hospital-acquired infections, which obviously can put a damper on the healing process. The hope was that a biomaterial would be more effective than something like metal in lessening bacteria formation. Nope.
When the researchers soaked paper disks in honey and added them to cultures of Pseudomonas aeruginosa and Staphylococcus aureus, none of the various concentrations stopped bacterial growth in the scaffolding, even when they added antibiotics.
The sticky conclusion, you could say, is more bitter than sweet.
It may sound like Korn, but can it play ‘Freak on a Leash’?
Like all right-thinking Americans, we love corn, corn-based products, and almost corn. Corn on the cob grilled in the husk? Mmm. Plus, we’re big fans of the band Korn. Also, we once had a reporter here named Tim Kirn. And don’t even get us started with Karn. Best Family Feud host ever.
But what about Quorn? Oh sure, the fungi-based meat alternative is full of yummy mycoprotein, but can it prevent colorectal cancer? Can we add Quorn to our favorites list? Let’s see what Science has to say.
Researchers at Northumbria University in Newcastle upon Tyne, England, fed a group of 20 men some meat (240 g/day) for 2 weeks – hopefully, they were allowed to eat some other food as well – and then gave them the same amount of Quorn, excuse us, fungi-derived mycoprotein equivalents, for 2 more weeks, with a 4-week washout period in between.
Levels of cancer-causing chemicals known as genotoxins fell significantly in the mycoprotein phase but rose during the meat phase. The mycoprotein diet also improved gut health “by increasing the abundance of protective bacteria such as Lactobacilli, Roseburia, and Akkermansia, which are associated with offering protection against chemically induced tumours, inflammation and bowel cancer,” they said in a statement from the university.
The meat phase, on the other hand, resulted in an increase in “gut bacteria linked with issues such as cancer, cardiovascular diseases, weight gain and other negative health outcomes,” they noted.
Science, then, seems to approve of Quorn, and that’s good enough for us. We’re adding Quorn to our diet, starting with a fungi-derived mycoproteinburger tonight while we’re watching the Cornell Big Red take the court against their archrivals, the Big Green of Dartmouth College. GO RED!
One-eyed, one-horned, flying purple veggie eater
Big Fruits and Vegetables is at it again. You notice how they’re always like “Oh, vegetables are good for your health,” and “Eating fruits every day makes you live longer,” but come on. It’s a marketing ploy, leading us astray from our personal savior, McDonald’s.
Just look at this latest bit of research: According to researchers from Finland, eating purple vegetables can protect against diabetes. Considering nearly 40 million Americans have diabetes (and nearly 100 million have prediabetes), anything to reduce the incidence of diabetes (people with diabetes account for one-fourth of every dollar spent in U.S. health care) would be beneficial. So, let’s humor the fruits and veggies people this time and hear them out.
It all comes down to a chemical called anthocyanin, which is a pigment that gives fruits and vegetables such as blueberries, radishes, and red cabbages their purplish color. Anthocyanin also has probiotic and anti-inflammatory effects, meaning it can help improve intestinal lining health and regulate glucose and lipid metabolic pathways. Obviously, good things if you want to avoid diabetes.
The investigators also found that, while standard anthocyanin was beneficial, acylated anthocyanin (which has an acyl group added to the sugar molecules of anthocyanin) is really what you want to go for. The acylated version, found in abundance in purple potatoes, purple carrots, radishes, and red cabbages, is tougher to digest, but the positive effects it has in the body are enhanced over the standard version.
Now, this all a compelling bit of research, but at the end of the day, you’re still eating fruits and vegetables, and we are red-blooded Americans here. We don’t do healthy foods. Although, if you were to dye our burgers with anthocyanin and make them purple, you’d have our attention. Purple is our favorite color.
Manuka honey better as building material than antibiotic
Milk, according to the old saying, builds strong bones, but when it comes to patients with bone loss caused by various medical reasons, researchers found that manuka honey, produced only in New Zealand and some parts of Australia, may also do the job. They soaked collagen scaffolds used for bone implants in various concentrations of the honey and found that 5% led to higher mineral formation and osteoprotegerin production, which suggests increased bone production.
But, and this is a pretty big one, the other half of the study – testing manuka honey’s ability to ward off bacteria – wasn’t so successful. Bone implants, apparently, count for almost half of all hospital-acquired infections, which obviously can put a damper on the healing process. The hope was that a biomaterial would be more effective than something like metal in lessening bacteria formation. Nope.
When the researchers soaked paper disks in honey and added them to cultures of Pseudomonas aeruginosa and Staphylococcus aureus, none of the various concentrations stopped bacterial growth in the scaffolding, even when they added antibiotics.
The sticky conclusion, you could say, is more bitter than sweet.
It may sound like Korn, but can it play ‘Freak on a Leash’?
Like all right-thinking Americans, we love corn, corn-based products, and almost corn. Corn on the cob grilled in the husk? Mmm. Plus, we’re big fans of the band Korn. Also, we once had a reporter here named Tim Kirn. And don’t even get us started with Karn. Best Family Feud host ever.
But what about Quorn? Oh sure, the fungi-based meat alternative is full of yummy mycoprotein, but can it prevent colorectal cancer? Can we add Quorn to our favorites list? Let’s see what Science has to say.
Researchers at Northumbria University in Newcastle upon Tyne, England, fed a group of 20 men some meat (240 g/day) for 2 weeks – hopefully, they were allowed to eat some other food as well – and then gave them the same amount of Quorn, excuse us, fungi-derived mycoprotein equivalents, for 2 more weeks, with a 4-week washout period in between.
Levels of cancer-causing chemicals known as genotoxins fell significantly in the mycoprotein phase but rose during the meat phase. The mycoprotein diet also improved gut health “by increasing the abundance of protective bacteria such as Lactobacilli, Roseburia, and Akkermansia, which are associated with offering protection against chemically induced tumours, inflammation and bowel cancer,” they said in a statement from the university.
The meat phase, on the other hand, resulted in an increase in “gut bacteria linked with issues such as cancer, cardiovascular diseases, weight gain and other negative health outcomes,” they noted.
Science, then, seems to approve of Quorn, and that’s good enough for us. We’re adding Quorn to our diet, starting with a fungi-derived mycoproteinburger tonight while we’re watching the Cornell Big Red take the court against their archrivals, the Big Green of Dartmouth College. GO RED!
Trends in HPV vaccination among adults aged 27 to 45 years
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?
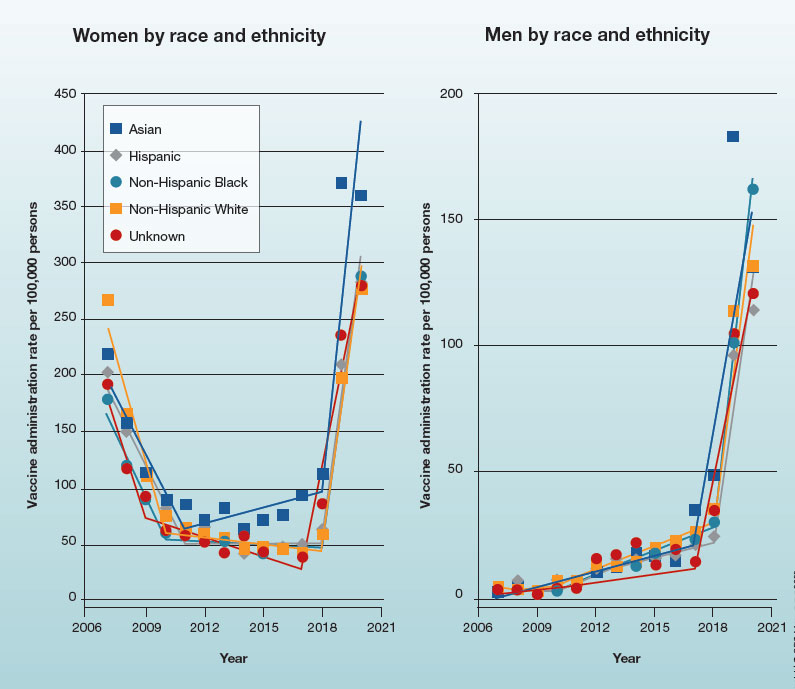
In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?

In 2019, the Advisory Committee on Immunization Practices recommended patient-clinician shared decision-making for human papillomavirus vaccination in adults aged 27 to 45 years. Has the recommendation increased vaccine uptake in this age group?

How to place an IUD with minimal patient discomfort
CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8
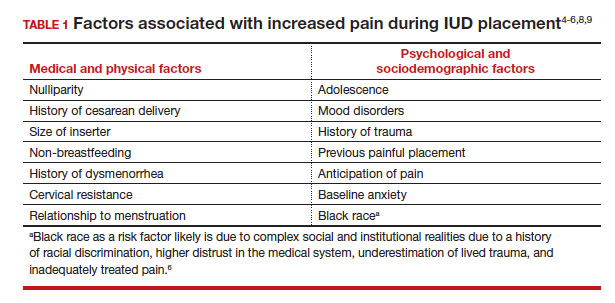
It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10
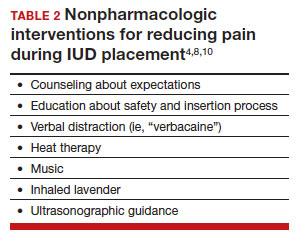
Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).
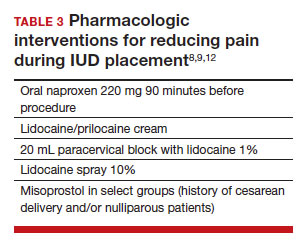
Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
Atopic dermatitis increases the risk for food allergy, food sensitivity, challenge-proven food allergy, and vice versa
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk of developing food allergy (FA), food sensitivity (FS), and challenge-proven food allergy (CPFA), and vice versa.
Major finding: AD was significantly associated with an increased risk for FS (pooled odds ratio [pOR] 4.17; 95% CI 3.03-5.75), FA (pOR 3.91; 95% CI 3.44-4.45), and CPFA (pOR 4.99; 95% CI 2.20-11.35). A significantly increased risk for AD was observed in individuals with FS (pOR 3.92; 95% CI,2.88-5.33), FA (pOR 4.06; 95% CI 3.54-4.65), and CPFA (pOR 4.93; 95% CI 2.74-8.84).
Study details: Findings are from a meta-analysis of 465 studies involving patients with AD (n = 225,568); reference individuals without AD (n = 1,128,322); individuals with FS, FA, and CPFA (n = 1,357,793); and reference individuals without FS, FA, and CPFA (n = 1,244,596).
Disclosures: This study did not receive any funding. Some authors declared serving as advisors, speakers, or consultants for or receiving speaking or consulting fees from various sources.
Source: Christensen MO et al. Prevalence of and association between atopic dermatitis and food sensitivity, food allergy and challenge-proven food allergy: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023 (Jan 25). Doi: 10.1111/jdv.18919
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk of developing food allergy (FA), food sensitivity (FS), and challenge-proven food allergy (CPFA), and vice versa.
Major finding: AD was significantly associated with an increased risk for FS (pooled odds ratio [pOR] 4.17; 95% CI 3.03-5.75), FA (pOR 3.91; 95% CI 3.44-4.45), and CPFA (pOR 4.99; 95% CI 2.20-11.35). A significantly increased risk for AD was observed in individuals with FS (pOR 3.92; 95% CI,2.88-5.33), FA (pOR 4.06; 95% CI 3.54-4.65), and CPFA (pOR 4.93; 95% CI 2.74-8.84).
Study details: Findings are from a meta-analysis of 465 studies involving patients with AD (n = 225,568); reference individuals without AD (n = 1,128,322); individuals with FS, FA, and CPFA (n = 1,357,793); and reference individuals without FS, FA, and CPFA (n = 1,244,596).
Disclosures: This study did not receive any funding. Some authors declared serving as advisors, speakers, or consultants for or receiving speaking or consulting fees from various sources.
Source: Christensen MO et al. Prevalence of and association between atopic dermatitis and food sensitivity, food allergy and challenge-proven food allergy: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023 (Jan 25). Doi: 10.1111/jdv.18919
Key clinical point: Patients with atopic dermatitis (AD) have an increased risk of developing food allergy (FA), food sensitivity (FS), and challenge-proven food allergy (CPFA), and vice versa.
Major finding: AD was significantly associated with an increased risk for FS (pooled odds ratio [pOR] 4.17; 95% CI 3.03-5.75), FA (pOR 3.91; 95% CI 3.44-4.45), and CPFA (pOR 4.99; 95% CI 2.20-11.35). A significantly increased risk for AD was observed in individuals with FS (pOR 3.92; 95% CI,2.88-5.33), FA (pOR 4.06; 95% CI 3.54-4.65), and CPFA (pOR 4.93; 95% CI 2.74-8.84).
Study details: Findings are from a meta-analysis of 465 studies involving patients with AD (n = 225,568); reference individuals without AD (n = 1,128,322); individuals with FS, FA, and CPFA (n = 1,357,793); and reference individuals without FS, FA, and CPFA (n = 1,244,596).
Disclosures: This study did not receive any funding. Some authors declared serving as advisors, speakers, or consultants for or receiving speaking or consulting fees from various sources.
Source: Christensen MO et al. Prevalence of and association between atopic dermatitis and food sensitivity, food allergy and challenge-proven food allergy: A systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2023 (Jan 25). Doi: 10.1111/jdv.18919
Atopic dermatitis: Association of itch with patient- vs physician-reported outcomes
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), itch was more closely associated with patient-reported outcome measures than with objective assessments by the physician.
Major finding: Itch severity had a weak positive correlation with objective Scoring Atopic Dermatitis (rho (ρ) 0.44; 95% CI 0.39-0.48), Eczema Area and Severity Index (ρ 0.41; 95% CI 0.36-0.46), and Investigator Global Assessment (ρ 0.46; 95% CI 0.42-0.51) scores, but a strong positive correlation with Patient Global Assessment score (ρ 0.68; 95% CI 0.65-0.71), Patient-Oriented Eczema Measure sum score (ρ 0.66; 95% CI 0.63-0.69), and Dermatology Life Quality Index score (ρ 0.61; 95% CI 0.57-0.65).
Study details: This study analyzed the data of 1134 adult patients with moderate-to-severe AD from the multicenter prospective TREATgermany registry, of which 1121 had provided data on their itch.
Disclosures: TREATgermany is sponsored by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., and others. Some authors reported ties with various organizations, including TREATgermany sponsors.
Source: Weisshaar E et al. Itching in atopic dermatitis: Patient- and physician-reported outcomes in the German atopic dermatitis registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854 (Jan 23). Doi: 10.2340/actadv.v103.4426
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), itch was more closely associated with patient-reported outcome measures than with objective assessments by the physician.
Major finding: Itch severity had a weak positive correlation with objective Scoring Atopic Dermatitis (rho (ρ) 0.44; 95% CI 0.39-0.48), Eczema Area and Severity Index (ρ 0.41; 95% CI 0.36-0.46), and Investigator Global Assessment (ρ 0.46; 95% CI 0.42-0.51) scores, but a strong positive correlation with Patient Global Assessment score (ρ 0.68; 95% CI 0.65-0.71), Patient-Oriented Eczema Measure sum score (ρ 0.66; 95% CI 0.63-0.69), and Dermatology Life Quality Index score (ρ 0.61; 95% CI 0.57-0.65).
Study details: This study analyzed the data of 1134 adult patients with moderate-to-severe AD from the multicenter prospective TREATgermany registry, of which 1121 had provided data on their itch.
Disclosures: TREATgermany is sponsored by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., and others. Some authors reported ties with various organizations, including TREATgermany sponsors.
Source: Weisshaar E et al. Itching in atopic dermatitis: Patient- and physician-reported outcomes in the German atopic dermatitis registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854 (Jan 23). Doi: 10.2340/actadv.v103.4426
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), itch was more closely associated with patient-reported outcome measures than with objective assessments by the physician.
Major finding: Itch severity had a weak positive correlation with objective Scoring Atopic Dermatitis (rho (ρ) 0.44; 95% CI 0.39-0.48), Eczema Area and Severity Index (ρ 0.41; 95% CI 0.36-0.46), and Investigator Global Assessment (ρ 0.46; 95% CI 0.42-0.51) scores, but a strong positive correlation with Patient Global Assessment score (ρ 0.68; 95% CI 0.65-0.71), Patient-Oriented Eczema Measure sum score (ρ 0.66; 95% CI 0.63-0.69), and Dermatology Life Quality Index score (ρ 0.61; 95% CI 0.57-0.65).
Study details: This study analyzed the data of 1134 adult patients with moderate-to-severe AD from the multicenter prospective TREATgermany registry, of which 1121 had provided data on their itch.
Disclosures: TREATgermany is sponsored by AbbVie Deutschland GmbH & Co. KG, Galderma S.A., and others. Some authors reported ties with various organizations, including TREATgermany sponsors.
Source: Weisshaar E et al. Itching in atopic dermatitis: Patient- and physician-reported outcomes in the German atopic dermatitis registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854 (Jan 23). Doi: 10.2340/actadv.v103.4426