User login
Progress in breast cancer screening over the past 50 years: A remarkable story, but still work to do
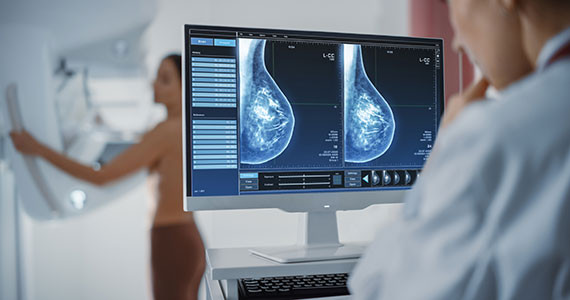
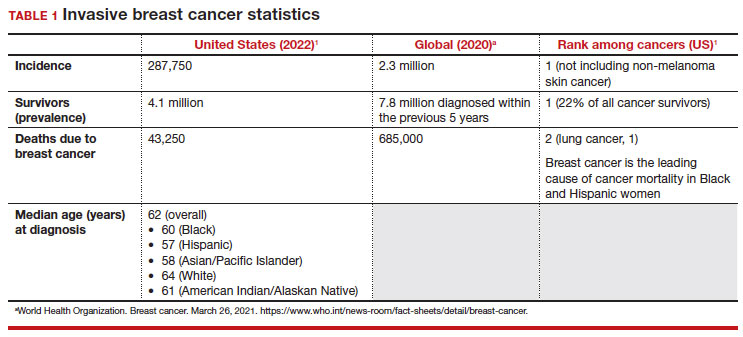
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3
While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
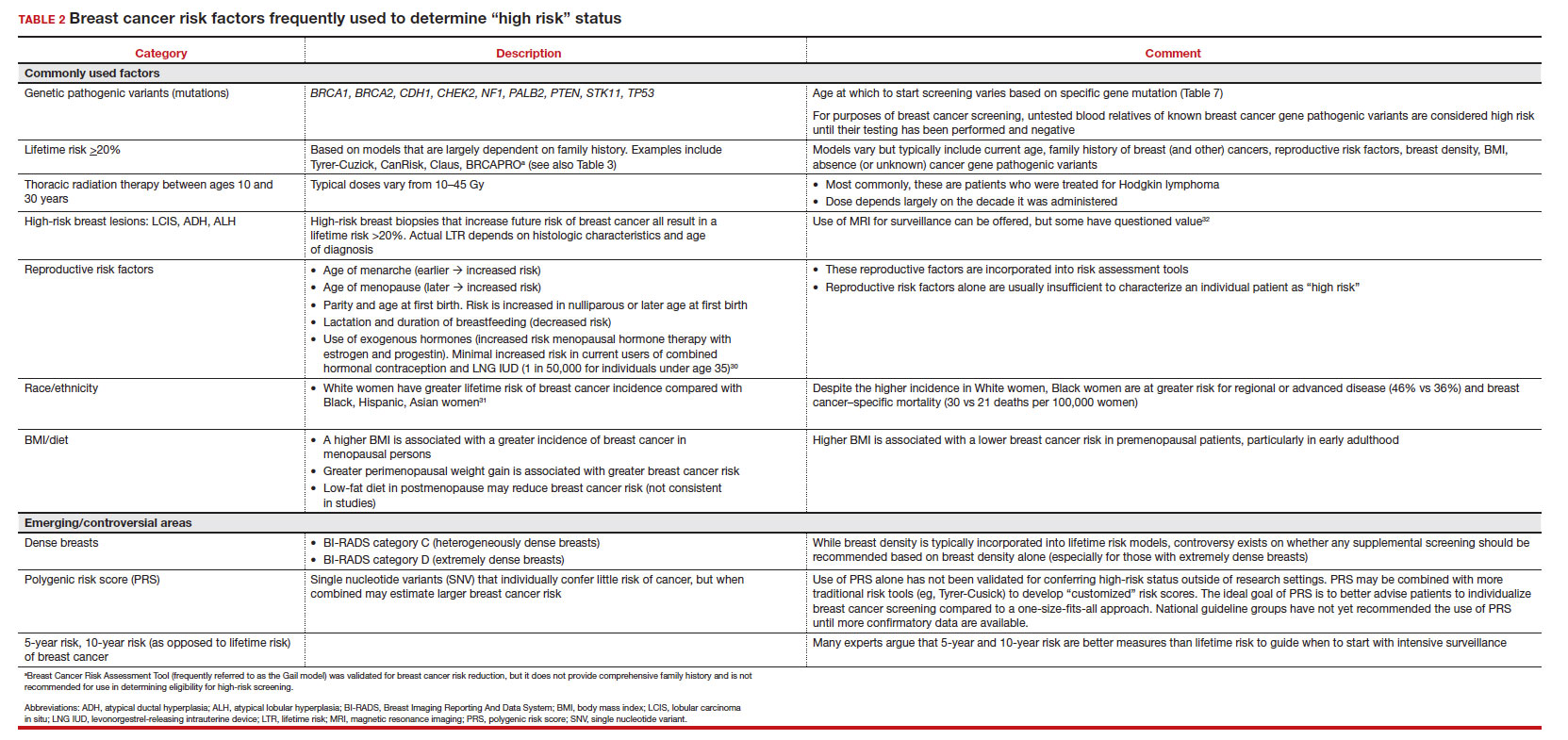
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.
Breast cancer risk assessment
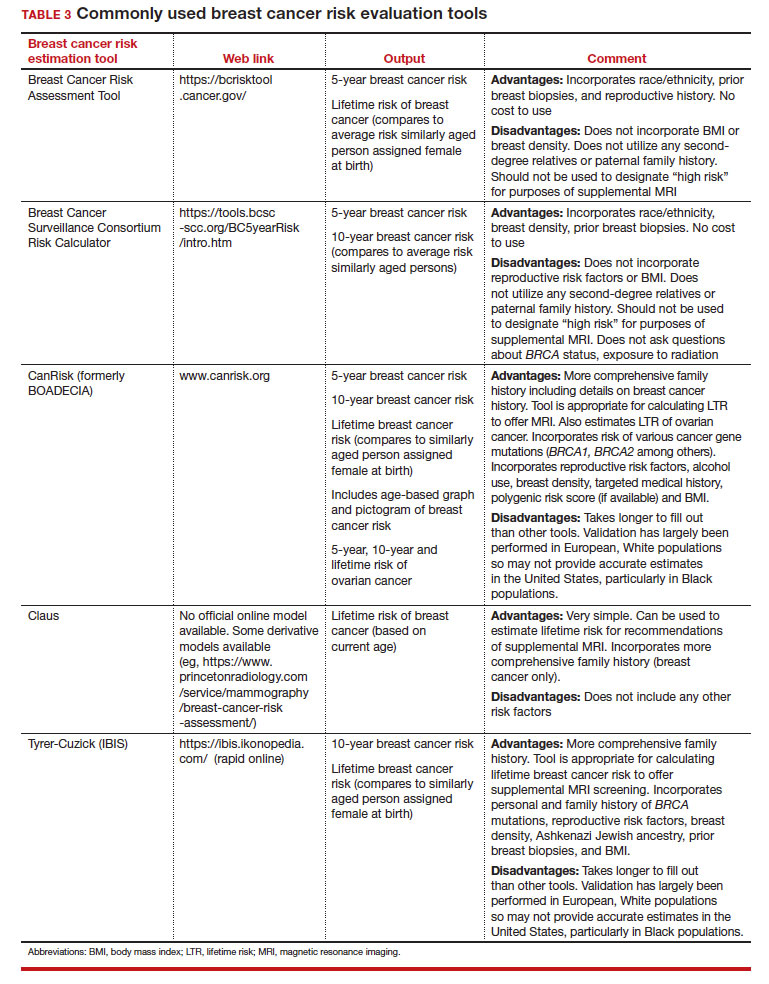
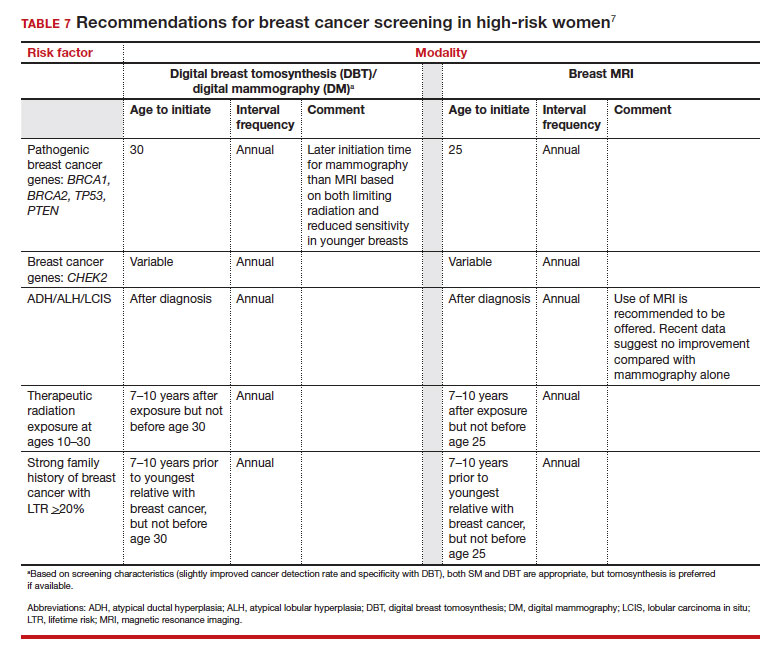
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7
Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10
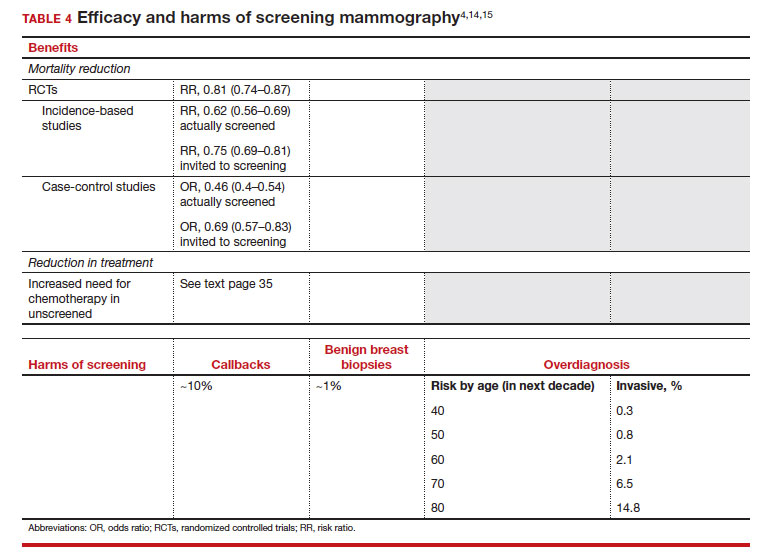
With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).
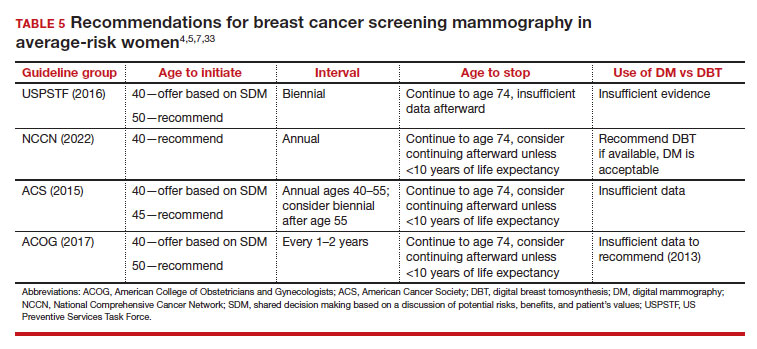
Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.
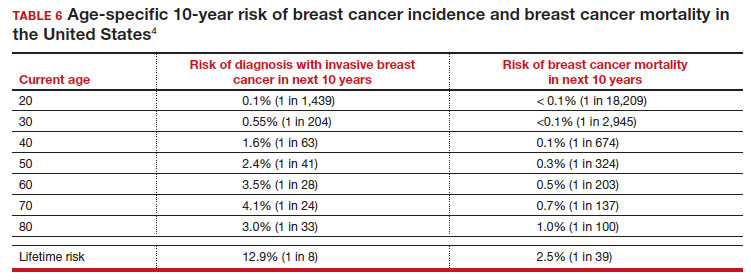
For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
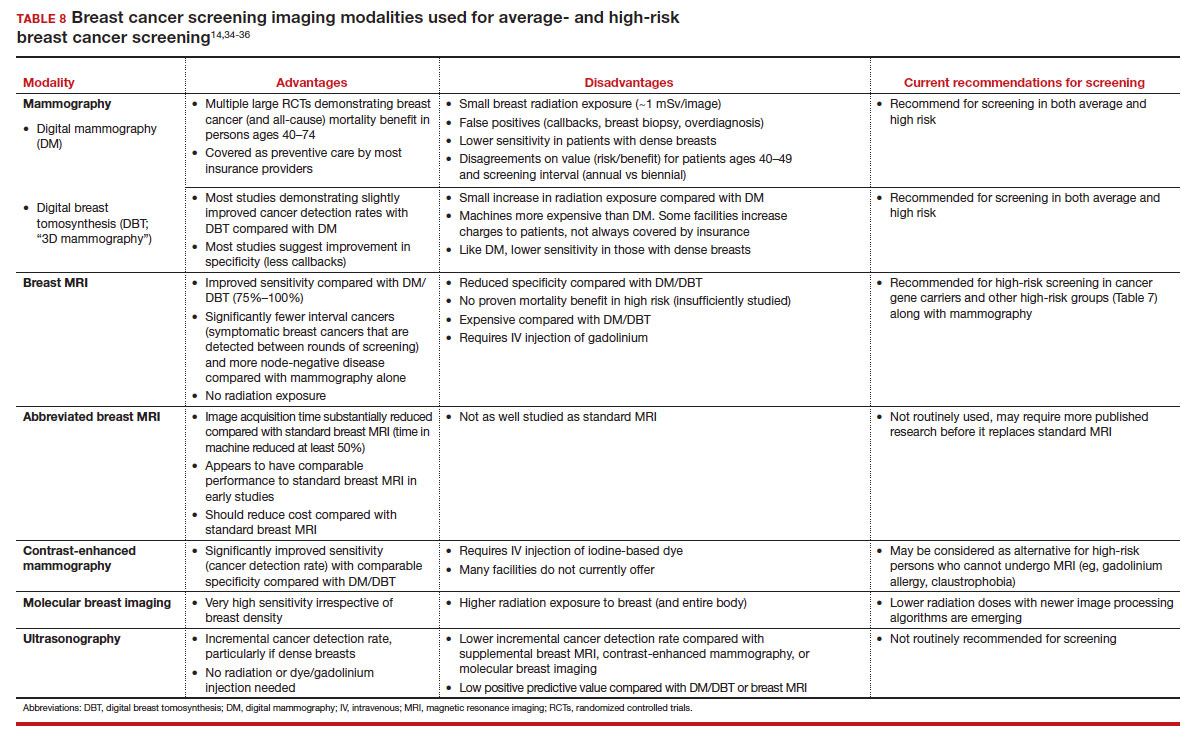
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.
Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).
Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
2023 Update on bone health
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
I recently heard a lecture where the speaker quoted this statistic: “A 50-year-old woman who does not currently have heart disease or cancer has a life expectancy of 91.” Hopefully, anyone reading this article already is aware of the fact that as our patients age, hip fracture results in greater morbidity and mortality than early breast cancer. It should be well known to clinicians (and, ultimately, to our patients) that localized breast cancer has a survival rate of 99%,1 whereas hip fracture carries a 21% mortality in the first year after the event.2 In addition, approximately one-third of women who fracture their hip do not have osteoporosis.3 Furthermore, the role of muscle mass, strength, and performance in bone health has become well established.4
With this in mind, a recent encounter with a patient in my clinical practice illustrates what I believe is an increasing problem today. The patient had been on long-term prednisone systemically for polymyalgia rheumatica. Her dual energy x-ray absorptiometry (DXA) bone mass measurements were among the worst osteoporotic numbers I have witnessed. She related to me the “argument” that occurred between her rheumatologist and endocrinologist. One wanted her to use injectable parathyroid hormone analog daily, while the other advised yearly infusion of zoledronic acid. She chose the yearly infusion. I inquired if either physician had mentioned anything to her about using nonskid rugs in the bathroom, grab bars, being careful of black ice, a calcium-rich diet, vitamin D supplementation, good eyesight, illumination so she does not miss a step, mindful walking, and maintaining optimal balance, muscle mass, strength, and performance-enhancing exercise? She replied, “No, just which drug I should take.”
Realize that the goal for our patients should be to avoid the morbidity and mortality associated especially with hip fracture. The goal is not to have a better bone mass measurement on your DXA scan as you age. This is exactly why the name of this column, years ago, was changed from “Update on osteoporosis” to “Update on bone health.” Similarly, in 2021, the NOF (National Osteoporosis Foundation) became the BHOF (Bone Health and Osteoporosis Foundation). Thus, our understanding and interest in bone health should and must go beyond simply bone mass measurement with DXA technology. The articles highlighted in this year’s Update reflect the importance of this concept.
Know SERMs’ effects on bone health for appropriate prescribing
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
Selective estrogen receptor modulators (SERMs) are synthetic molecules that bind to the estrogen receptor and can have agonistic activity in some tissues and antagonistic activity in others. In a recent article, I reviewed the known data regarding the effects of various SERMs on bone health.5
A rundown on 4 SERMs and their effects on bone
Tamoxifen is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of breast cancer in women with estrogen receptor–positive tumors. The only prospective study of tamoxifen versus placebo in which fracture risk was studied in women at risk for but not diagnosed with breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial. In this study, more than 13,000 women were randomly assigned to treatment with tamoxifen or placebo, with a primary objective of studying the incidence of invasive breast cancer in these high-risk women. With 7 years of follow-up, women receiving tamoxifen had significantly fewer fractures of the hip, radius, and spine (80 vs 116 in the placebo group), resulting in a combined relative risk (RR) of 0.68 (95% confidence interval [CI], 0.51–0.92).6
Raloxifene, another SERM, was extensively studied in the MORE (Multiple Outcomes of Raloxifene Evaluation) trial.7 This study involved more than 7,700 postmenopausal women with osteoporosis, average age 67. The incidence of first vertebral fracture was decreased from 4.3% with placebo to 1.9% with raloxifene (RR, 0.55; 95% CI, 0.29–0.71), and subsequent vertebral fractures were decreased from 20.2% with placebo to 14.1% with raloxifene (RR, 0.70; 95% CI, 0.60–0.90). In 2007, the FDA approved raloxifene for “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” as well as for “postmenopausal women at high risk for invasive breast cancer” based on the Study of Tamoxifen and Raloxifene (STAR) trial that involved almost 20,000 postmenopausal women deemed at high risk for breast cancer.8
The concept of combining an estrogen with a SERM, known as a TSEC (tissue selective estrogen complex) was studied and brought to market as conjugated equine estrogen (CEE) 0.45 mg and bazedoxifene (BZA) 20 mg. CEE and BZA individually have been shown to prevent vertebral fracture.9,10 The combination of BZA and CEE has been shown to improve bone density compared with placebo.11 There are, however, no fracture prevention data for this combination therapy. This was the basis on which the combination agent received regulatory approval for prevention of osteoporosis in postmenopausal women. This combination drug is also FDA approved for treating moderate to severe vasomotor symptoms of menopause.
Ospemifene is yet another SERM that is clinically available, at an oral dose of 60 mg, and is indicated for the treatment of moderate to severe dyspareunia secondary to vulvovaginal atrophy, or genitourinary syndrome of menopause (GSM). Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to estradiol and raloxifene.12 Clinical data from three phase 1 or phase 2 clinical trials revealed that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.13 While actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, there is a good correlation between biochemical markers for bone turnover and occurrence of fracture.14 Women who need treatment for osteoporosis should not be treated with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
SERMs, unlike estrogen, have no class labeling. In fact, in the endometrium and vagina, they have variable effects. To date, however, in postmenopausal women, all SERMs have shown estrogenic activity in bone as well as being antiestrogenic in breast. Tamoxifen, well known for its use in estrogen receptor–positive breast cancer patients, demonstrates positive effects on bone and fracture reduction compared with placebo. Raloxifene is approved for prevention and treatment of osteoporosis and for breast cancer chemoprevention in high-risk patients. The TSEC combination of CEE and the SERM bazedoxifene is approved for treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis. Finally, the SERM ospemifene, approved for treating moderate to severe dyspareunia or dryness due to vulvovaginal atrophy, or GSM, has demonstrated evidence of a positive effect on bone turnover and metabolism. Clinicians need to be aware of these effects when choosing medications for their patients.
Continue to: Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?...
Gut microbiome constituents may influence the development of osteoporosis: A potential treatment target?
Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
The role of the microbiome in many arenas is rapidly emerging. Apparently, its relationship in bone metabolism is still in its infancy. A review of PubMed articles showed that 1 paper was published in 2012, none until 2 more in 2015, with a total of 221 published through November 1, 2022. A recent review by Cronin and colleagues on the microbiome’s role in regulating bone metabolism came out of a workshop held by the Osteoporosis and Bone Research Academy of the Royal Osteoporosis Society in the United Kingdom.15
The gut microbiome’s relationship with bone health
The authors noted that the human microbiota functions at the interface between diet, medication use, lifestyle, host immune development, and health. Hence, it is closely aligned with many of the recognized modifiable factors that influence bone mass accrual in the young and bone maintenance and skeletal decline in older populations. Microbiome research and discovery supports a role of the human gut microbiome in the regulation of bone metabolism and the pathogenesis of osteoporosis as well as its prevention and treatment.
Numerous factors which influence the gut microbiome and the development of osteoporosis overlap. These include body mass index (BMI), vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Cronin and colleagues reviewed a number of clinical studies and concluded that “the available evidence suggests that probiotic supplements can attenuate bone loss in postmenopausal women, although the studies investigating this have been short term and individually have had small sample sizes. Moving forward, it will be important to conduct larger scale studies to evaluate if the skeletal response differs with different types of probiotic and also to determine if the effects are sustained in the longer term.”15
Composition of the microbiota
A recent study by Yang and colleagues focused on changes in gut and vaginal microbiota composition in patients with postmenopausal osteoporosis. They analyzed data from 132 postmenopausal women with osteoporosis (n = 34), osteopenia (n = 47), and controls (n = 51) based on their T-scores.16
Significant differences were observed in the microbial compositions of fecal samples between groups (P<.05), with some species enhanced in the control group whereas other species were higher in the osteoporosis group. Similar but less pronounced differences were seen in the vaginal microbiome but of different species.
The authors concluded that “The results show that changes in BMD in postmenopausal women are associated with the changes in gut microbiome and vaginal microbiome; however, changes in gut microbiome are more closely correlated with postmenopausal osteoporosis than vaginal microbiome.”16
While we are not yet ready to try to clinically alter the gut microbiome with various interventions, realizing that there is crosstalk between the gut microbiome and bone health is another factor to consider, and it begins with an appreciation of the various factors where the 2 overlap—BMI, vitamin D, alcohol intake, diet, corticosteroid use, physical activity, sex hormone deficiency, genetic variability, and chronic inflammatory disorders.
Continue to: Sarcopenia, osteoporosis, and frailty: A fracture risk triple play...
Sarcopenia, osteoporosis, and frailty: A fracture risk triple play
Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
Laskou and colleagues aimed to explore the relationship between sarcopenia, osteoporosis, and frailty in community-dwelling adults participating in a cohort study in the United Kingdom and to determine if the coexistence of osteoporosis and sarcopenia is associated with a significantly heavier health burden.17
Study details
The authors examined data from 206 women with an average age of 75.5 years. Sarcopenia was defined using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria, which includes low grip strength or slow chair rise and low muscle quantity. Osteoporosis was defined by standard measurements as a T-score of less than or equal to -2.5 standard deviations at the femoral neck or use of any osteoporosis medications. Frailty was defined using the Fried definition, which includes having 3 or more of the following 5 domains: weakness, slowness, exhaustion, low physical activity, and unintentional weight loss. Having 1 or 2 domains is “prefrailty” and no domains signifies nonfrail.
Frailty confers additional risk
The study results showed that among the 206 women, the prevalence of frailty and prefrailty was 9.2% and 60.7%, respectively. Of the 5 Fried frailty components, low walking speed and low physical activity followed by self-reported exhaustion were the most prevalent (96.6%, 87.5%, and 75.8%, respectively) among frail participants. Having sarcopenia only was strongly associated with frailty (odds ratio [OR], 8.28; 95% CI, 1.27–54.03; P=.027]). The likelihood of being frail was substantially higher with the presence of coexisting sarcopenia and osteoporosis (OR, 26.15; 95% CI, 3.31–218.76; P=.003).
Thus, both these conditions confer a high health burden for the individual as well as for health care systems. Osteosarcopenia is the term given when low bone mass and sarcopenia occur in consort. Previous data have shown that when osteoporosis or even osteopenia is combined with sarcopenia, it can result in a 3-fold increase in the risk of falls and a 4-fold increase in the risk of fracture compared with women who have osteopenia or osteoporosis alone.18
Sarcopenia, osteoporosis, and frailty are highly prevalent in older adults but are frequently underrecognized. Sarcopenia is characterized by progressive and generalized decline in muscle strength, function, and muscle mass with increasing age. Sarcopenia increases the likelihood of falls and adversely impacts functional independence and quality of life. Osteoporosis predisposes to low energy, fragility fractures, and is associated with chronic pain, impaired physical function, loss of independence, and higher risk of institutionalization. Clinicians need to be aware that when sarcopenia coexists with any degree of low bone mass, it will significantly increase the risk of falls and fracture compared with having osteopenia or osteoporosis alone.
Continue to: Denosumab effective in reducing falls, strengthening muscle...
Denosumab effective in reducing falls, strengthening muscle
Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Results of a previous study showed that denosumab treatment significantly decreased falls and resulted in significant improvement in all sarcopenic measures.19 Furthermore, 1 year after denosumab was discontinued, a significant worsening occurred in both falls and sarcopenic measures. In that study, the control group, treated with alendronate or zoledronate, also showed improvement on some tests of muscle performance but no improvement in the risk of falls.
Those results agreed with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis) trial.20 This study revealed that denosumab treatment not only reduced the risk of vertebral, nonvertebral, and hip fracture over 36 months but also that the denosumab-treated group had fewer falls compared with the placebo-treated group (4.5% vs 5.7%; P = .02).
Denosumab found to increase muscle strength
More recently, Rupp and colleagues conducted a retrospective cohort study that included women with osteoporosis or osteopenia who received vitamin D only (n = 52), alendronate 70 mg/week (n = 26), or denosumab (n = 52).21
After a mean follow-up period of 17.6 (SD, 9.0) months, the authors observed a significantly higher increase in grip force in both the denosumab (P<.001) and bisphosphonate groups (P = .001) compared with the vitamin D group. In addition, the denosumab group showed a significantly higher increase in chair rising test performance compared with the bisphosphonate group (denosumab vs bisphosphonate, P = 0.03). They concluded that denosumab resulted in increased muscle strength in the upper and lower limbs, indicating systemic rather than site-specific effects as compared with the bisphosphonate.
The authors concluded that based on these findings, denosumab might be favored over other osteoporosis treatments in patients with low BMD coexisting with poor muscle strength. ●
Osteoporosis and sarcopenia may share similar underlying risk factors. Muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. In previous studies, denosumab as well as various bisphosphonates improved measures of sarcopenia, although only denosumab was associated with a reduction in the risk of falls. The study by Rupp and colleagues suggests that denosumab treatment may result in increased muscle strength in upper and lower limbs, indicating some systemic effect and not simply site-specific activity. Thus, in choosing a bone-specific agent for patients with abnormal muscle strength, mass, or performance, clinicians may want to consider denosumab as a choice for these reasons.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Georgia: American Cancer Society; 2020. Accessed November 7, 2022. https://www.cancer.org/content /dam/cancer-org/research/cancer-facts-and-statistics /annual-cancer-facts-and-figures/2020/cancer-facts-and -figures-2020.pdf
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34:195-202.
- de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society White Paper 2021. Climacteric. 2021;24:498-504.
- Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric. 2022;25:56-59.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652-1662.
- Ettinger B, Black DM, Mitlak BH, et al; for the Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637645.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004:291:1701-1712.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Kangas L, Härkönen P, Väänänen K, et al. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Cronin O, Lanham-New SA, Corfe BM, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110:273-284.
- Yang X, Chang T, Yuan Q, et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front Immunol. 2022;13:930244.
- Laskou F, Fuggle NR, Patel HP, et al. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J Cachexia Sarcopenia Muscle. 2022;13:220-229.
- Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424-3431.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Rupp T, von Vopelius E, Strahl A, et al. Beneficial effects of denosumab on muscle performance in patients with low BMD: a retrospective, propensity score-matched study. Osteoporos Int. 2022;33:2177-2184.
Rucaparib benefit in BRCA+ prostate cancer confirmed
The finding, which comes from the TRITON3 clinical trial, provides evidence of clinical benefit for an indication for rucaparib that was granted an accelerated approval in May 2020.
“Rucaparib reduced the risk of progression or death by half in patients with BRCA alterations,” said lead author Alan H. Bryce, MD, medical director of the Genomic Oncology Clinic at Mayo Clinic Arizona, in Phoenix.
For the subgroup of patients with BRCA alterations, the median PFS was 11.2 months with rucaparib vs. 6.4 months (hazard ratio, 0.50; P < .001) among those who received physician’s choice of therapy, which included docetaxel or a second-generation ARPI, such as abiraterone or enzalutamide.
In another subgroup of patients whose disease had ATM alterations, the median PFS was 8.1 months with rucaparib vs. 6.8 months with physician’s choice of drug. The difference was not statistically significant.
However, the difference was significant in the intention-to-treat (ITT) population (comprising both subgroups), for whom the median PFS was 10.2 months with rucaparib vs. 6.4 months with physician’s choice of drug (HR, 0.61; P < .001 by log-rank test).
Dr. Bryce pointed out that three-quarters of the patients in the physician’s-choice arm who had progressive disease crossed over to rucaparib upon progression and that overall survival (OS) results are immature. At 62 months, median OS did not significantly differ in the BRCA subgroup (24.3 vs. 20.8 months favoring rucaparib; P = .21) or in the ITT group (23.6 vs. 20.9 months; P = .67).
Importantly, rucaparib was well tolerated. In all treatment groups, the most frequent adverse events were asthenia and fatigue, Bryce said. “There were no cases of myelodysplastic syndrome or acute myeloid leukemia reported.”
These results from the TRITON3 trial were presented at the 2023 ASCO Genitourinary Cancers Symposium and were published simultaneously in the New England Journal of Medicine.
Suggested benefit
Rucaparib is the first PARP inhibitor approved for use in patients with mCRPC that harbors deleterious BRCA mutations (germline and/or somatic) who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy. This prostate cancer indication was granted an accelerated approval in May 2020 by the U.S. Food and Drug Administration on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial, the forerunner of the current study.
The TRITON2 study was a single-arm clinical trial that involved three cohorts: 62 patients with a BRCA mutation (germline and/or somatic) and measurable disease; 115 patients with a BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency–positive mCRPC.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%. For the patients with measurable disease and a BRCA mutation, the objective response rate was 44%. The objective response rate was similar for those with a germline BRCA mutation.
Study details
The current phase 3 randomized TRITON3 clinical trial was conducted to confirm the earlier findings and to expand upon the data in mCRPC. The participants in this trial were patients with mCRPC who had specific gene alterations, including BRCA and ATM alterations, who had experienced disease progression after androgen receptor–directed therapy but who had not yet received chemotherapy.
A total of 270 men were assigned to receive rucaparib (600 mg twice daily); 135 patients received their physician’s choice of medication. Within the two study arms, 302 patients had a BRCA alteration, and 103 patients had an ATM alteration. The ITT population consisted of all the patients who had been randomly assigned to either of the two groups. A prespecified subgroup included patients with a BRCA alteration.
The primary outcome was the median duration of imaging-based PSF, as determined through independent review. Key secondary outcomes were overall survival and objective response rate.
The most common adverse events in the rucaparib group were fatigue, nausea, and anemia or decreased hemoglobin. In the control group, the most common adverse events were fatigue, diarrhea, and neuropathy. The most common events of grade 3 or higher were anemia or decreased hemoglobin, neutropenia or a decreased neutrophil count, and fatigue in the rucaparib group, and fatigue and neutropenia or a decreased neutrophil count among control patients.
No changes in standard of care
In a discussion of the study, Elena Castro, MD, PhD, of the Instituto de Investigación Biomédica de Málaga, Campanillas, Spain, emphasized that there is a clear benefit from the use of PARP inhibitors (such as rucaparib) for patients with BRCA alterations.
However, she highlighted the absence of convincing overall survival data and the absence of a clear benefit on PFS in the subgroup of patients with ATM alterations.
“These data raise several questions,” she noted, “such as, do patients with ATM alterations benefit at all? And should PARP inhibitors [such as rucaparib] precede or follow docetaxel therapy?”
Because of the high crossover rate, it may be possible to evaluate the directionality of docetaxel followed by PARP inhibitors and the other way around, she suggested.
Dr. Castro said that patients with BRCA alterations benefit from PARP inhibitors and are likely to derive more benefit from them than from taxanes.
“But those with ATM alterations are unlikely to benefit from rucaparib more than from taxanes,” she said.
In a comment, Hank Ng, MD, medical oncologist, NYU Langone Perlmutter Cancer Center, New York, said he is not convinced that the findings from TRITON 3 represent a new standard of care in BRCA 1/2 mutations or ATM.
“Currently, we know that, for patients with prostate cancer with BRCA1/2 or ATM, the standard of care is an androgen receptor pathway inhibitor (ARPI), such as abiraterone or enzalutamide, then docetaxel, and then a PARP inhibitor like rucaparib,” he said.
(Currently, rucaparib is indicated for use in patients with mCRPC with BRCA alterations after they have already received an ARPI and taxane-based chemotherapy.)
Dr. Ng also questioned the control arm of the TRITON 3 trial. All the participants in the trial had already experienced disease progression after treatment with a second-generation ARPI. But the physician’s choice of therapy allowed them to move on to another ARPI or to docetaxel.
Dr. NG commented that, “in almost all cases, after progression of one ARPI, switching to another ARPI does not provide much benefit – from what is visible from this abstract – and only 56% patients received docetaxel, and thus 44% received a not-beneficial treatment,” he said.
“I am not sure what the docetaxel subgroup showed, but potentially, if those numbers are convincing, we could move this [rucaparib] ahead of docetaxel,” he speculated.
However, he also pointed out that an overall survival benefit has not yet been shown; so far, the benefit that has been shown is with respect to imaging-based PFS.
Dr. Ng does agree that rucaparib is indicated in the second line after progression with one ARPI for patients who are not candidates for chemotherapy. “But this has not yet shown me that we should absolutely be offering rucaparib before docetaxel,” he said.
TRITON3 was supported by Clovis Oncology, manufacturer of rucaparib. Dr. Bryce has relationships with Bayer, Foundation Medicine, Janssen, Merck, Myovant Sciences, and Novartis and holds a patent for therapeutic targeting of cancer patients with NRG1 rearrangements. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis, Pfizer, and Roche.
A version of this article first appeared on Medscape.com.
The finding, which comes from the TRITON3 clinical trial, provides evidence of clinical benefit for an indication for rucaparib that was granted an accelerated approval in May 2020.
“Rucaparib reduced the risk of progression or death by half in patients with BRCA alterations,” said lead author Alan H. Bryce, MD, medical director of the Genomic Oncology Clinic at Mayo Clinic Arizona, in Phoenix.
For the subgroup of patients with BRCA alterations, the median PFS was 11.2 months with rucaparib vs. 6.4 months (hazard ratio, 0.50; P < .001) among those who received physician’s choice of therapy, which included docetaxel or a second-generation ARPI, such as abiraterone or enzalutamide.
In another subgroup of patients whose disease had ATM alterations, the median PFS was 8.1 months with rucaparib vs. 6.8 months with physician’s choice of drug. The difference was not statistically significant.
However, the difference was significant in the intention-to-treat (ITT) population (comprising both subgroups), for whom the median PFS was 10.2 months with rucaparib vs. 6.4 months with physician’s choice of drug (HR, 0.61; P < .001 by log-rank test).
Dr. Bryce pointed out that three-quarters of the patients in the physician’s-choice arm who had progressive disease crossed over to rucaparib upon progression and that overall survival (OS) results are immature. At 62 months, median OS did not significantly differ in the BRCA subgroup (24.3 vs. 20.8 months favoring rucaparib; P = .21) or in the ITT group (23.6 vs. 20.9 months; P = .67).
Importantly, rucaparib was well tolerated. In all treatment groups, the most frequent adverse events were asthenia and fatigue, Bryce said. “There were no cases of myelodysplastic syndrome or acute myeloid leukemia reported.”
These results from the TRITON3 trial were presented at the 2023 ASCO Genitourinary Cancers Symposium and were published simultaneously in the New England Journal of Medicine.
Suggested benefit
Rucaparib is the first PARP inhibitor approved for use in patients with mCRPC that harbors deleterious BRCA mutations (germline and/or somatic) who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy. This prostate cancer indication was granted an accelerated approval in May 2020 by the U.S. Food and Drug Administration on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial, the forerunner of the current study.
The TRITON2 study was a single-arm clinical trial that involved three cohorts: 62 patients with a BRCA mutation (germline and/or somatic) and measurable disease; 115 patients with a BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency–positive mCRPC.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%. For the patients with measurable disease and a BRCA mutation, the objective response rate was 44%. The objective response rate was similar for those with a germline BRCA mutation.
Study details
The current phase 3 randomized TRITON3 clinical trial was conducted to confirm the earlier findings and to expand upon the data in mCRPC. The participants in this trial were patients with mCRPC who had specific gene alterations, including BRCA and ATM alterations, who had experienced disease progression after androgen receptor–directed therapy but who had not yet received chemotherapy.
A total of 270 men were assigned to receive rucaparib (600 mg twice daily); 135 patients received their physician’s choice of medication. Within the two study arms, 302 patients had a BRCA alteration, and 103 patients had an ATM alteration. The ITT population consisted of all the patients who had been randomly assigned to either of the two groups. A prespecified subgroup included patients with a BRCA alteration.
The primary outcome was the median duration of imaging-based PSF, as determined through independent review. Key secondary outcomes were overall survival and objective response rate.
The most common adverse events in the rucaparib group were fatigue, nausea, and anemia or decreased hemoglobin. In the control group, the most common adverse events were fatigue, diarrhea, and neuropathy. The most common events of grade 3 or higher were anemia or decreased hemoglobin, neutropenia or a decreased neutrophil count, and fatigue in the rucaparib group, and fatigue and neutropenia or a decreased neutrophil count among control patients.
No changes in standard of care
In a discussion of the study, Elena Castro, MD, PhD, of the Instituto de Investigación Biomédica de Málaga, Campanillas, Spain, emphasized that there is a clear benefit from the use of PARP inhibitors (such as rucaparib) for patients with BRCA alterations.
However, she highlighted the absence of convincing overall survival data and the absence of a clear benefit on PFS in the subgroup of patients with ATM alterations.
“These data raise several questions,” she noted, “such as, do patients with ATM alterations benefit at all? And should PARP inhibitors [such as rucaparib] precede or follow docetaxel therapy?”
Because of the high crossover rate, it may be possible to evaluate the directionality of docetaxel followed by PARP inhibitors and the other way around, she suggested.
Dr. Castro said that patients with BRCA alterations benefit from PARP inhibitors and are likely to derive more benefit from them than from taxanes.
“But those with ATM alterations are unlikely to benefit from rucaparib more than from taxanes,” she said.
In a comment, Hank Ng, MD, medical oncologist, NYU Langone Perlmutter Cancer Center, New York, said he is not convinced that the findings from TRITON 3 represent a new standard of care in BRCA 1/2 mutations or ATM.
“Currently, we know that, for patients with prostate cancer with BRCA1/2 or ATM, the standard of care is an androgen receptor pathway inhibitor (ARPI), such as abiraterone or enzalutamide, then docetaxel, and then a PARP inhibitor like rucaparib,” he said.
(Currently, rucaparib is indicated for use in patients with mCRPC with BRCA alterations after they have already received an ARPI and taxane-based chemotherapy.)
Dr. Ng also questioned the control arm of the TRITON 3 trial. All the participants in the trial had already experienced disease progression after treatment with a second-generation ARPI. But the physician’s choice of therapy allowed them to move on to another ARPI or to docetaxel.
Dr. NG commented that, “in almost all cases, after progression of one ARPI, switching to another ARPI does not provide much benefit – from what is visible from this abstract – and only 56% patients received docetaxel, and thus 44% received a not-beneficial treatment,” he said.
“I am not sure what the docetaxel subgroup showed, but potentially, if those numbers are convincing, we could move this [rucaparib] ahead of docetaxel,” he speculated.
However, he also pointed out that an overall survival benefit has not yet been shown; so far, the benefit that has been shown is with respect to imaging-based PFS.
Dr. Ng does agree that rucaparib is indicated in the second line after progression with one ARPI for patients who are not candidates for chemotherapy. “But this has not yet shown me that we should absolutely be offering rucaparib before docetaxel,” he said.
TRITON3 was supported by Clovis Oncology, manufacturer of rucaparib. Dr. Bryce has relationships with Bayer, Foundation Medicine, Janssen, Merck, Myovant Sciences, and Novartis and holds a patent for therapeutic targeting of cancer patients with NRG1 rearrangements. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis, Pfizer, and Roche.
A version of this article first appeared on Medscape.com.
The finding, which comes from the TRITON3 clinical trial, provides evidence of clinical benefit for an indication for rucaparib that was granted an accelerated approval in May 2020.
“Rucaparib reduced the risk of progression or death by half in patients with BRCA alterations,” said lead author Alan H. Bryce, MD, medical director of the Genomic Oncology Clinic at Mayo Clinic Arizona, in Phoenix.
For the subgroup of patients with BRCA alterations, the median PFS was 11.2 months with rucaparib vs. 6.4 months (hazard ratio, 0.50; P < .001) among those who received physician’s choice of therapy, which included docetaxel or a second-generation ARPI, such as abiraterone or enzalutamide.
In another subgroup of patients whose disease had ATM alterations, the median PFS was 8.1 months with rucaparib vs. 6.8 months with physician’s choice of drug. The difference was not statistically significant.
However, the difference was significant in the intention-to-treat (ITT) population (comprising both subgroups), for whom the median PFS was 10.2 months with rucaparib vs. 6.4 months with physician’s choice of drug (HR, 0.61; P < .001 by log-rank test).
Dr. Bryce pointed out that three-quarters of the patients in the physician’s-choice arm who had progressive disease crossed over to rucaparib upon progression and that overall survival (OS) results are immature. At 62 months, median OS did not significantly differ in the BRCA subgroup (24.3 vs. 20.8 months favoring rucaparib; P = .21) or in the ITT group (23.6 vs. 20.9 months; P = .67).
Importantly, rucaparib was well tolerated. In all treatment groups, the most frequent adverse events were asthenia and fatigue, Bryce said. “There were no cases of myelodysplastic syndrome or acute myeloid leukemia reported.”
These results from the TRITON3 trial were presented at the 2023 ASCO Genitourinary Cancers Symposium and were published simultaneously in the New England Journal of Medicine.
Suggested benefit
Rucaparib is the first PARP inhibitor approved for use in patients with mCRPC that harbors deleterious BRCA mutations (germline and/or somatic) who have already been treated with androgen receptor–directed therapy and a taxane-based chemotherapy. This prostate cancer indication was granted an accelerated approval in May 2020 by the U.S. Food and Drug Administration on the basis of response rates and effect on levels of prostate-specific antigen (PSA) from the TRITON2 clinical trial, the forerunner of the current study.
The TRITON2 study was a single-arm clinical trial that involved three cohorts: 62 patients with a BRCA mutation (germline and/or somatic) and measurable disease; 115 patients with a BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease; and 209 patients with homologous recombination deficiency–positive mCRPC.
In an analysis of 115 patients with a deleterious BRCA mutation (germline and/or somatic) and measurable or nonmeasurable disease, the confirmed PSA response rate was 55%. For the patients with measurable disease and a BRCA mutation, the objective response rate was 44%. The objective response rate was similar for those with a germline BRCA mutation.
Study details
The current phase 3 randomized TRITON3 clinical trial was conducted to confirm the earlier findings and to expand upon the data in mCRPC. The participants in this trial were patients with mCRPC who had specific gene alterations, including BRCA and ATM alterations, who had experienced disease progression after androgen receptor–directed therapy but who had not yet received chemotherapy.
A total of 270 men were assigned to receive rucaparib (600 mg twice daily); 135 patients received their physician’s choice of medication. Within the two study arms, 302 patients had a BRCA alteration, and 103 patients had an ATM alteration. The ITT population consisted of all the patients who had been randomly assigned to either of the two groups. A prespecified subgroup included patients with a BRCA alteration.
The primary outcome was the median duration of imaging-based PSF, as determined through independent review. Key secondary outcomes were overall survival and objective response rate.
The most common adverse events in the rucaparib group were fatigue, nausea, and anemia or decreased hemoglobin. In the control group, the most common adverse events were fatigue, diarrhea, and neuropathy. The most common events of grade 3 or higher were anemia or decreased hemoglobin, neutropenia or a decreased neutrophil count, and fatigue in the rucaparib group, and fatigue and neutropenia or a decreased neutrophil count among control patients.
No changes in standard of care
In a discussion of the study, Elena Castro, MD, PhD, of the Instituto de Investigación Biomédica de Málaga, Campanillas, Spain, emphasized that there is a clear benefit from the use of PARP inhibitors (such as rucaparib) for patients with BRCA alterations.
However, she highlighted the absence of convincing overall survival data and the absence of a clear benefit on PFS in the subgroup of patients with ATM alterations.
“These data raise several questions,” she noted, “such as, do patients with ATM alterations benefit at all? And should PARP inhibitors [such as rucaparib] precede or follow docetaxel therapy?”
Because of the high crossover rate, it may be possible to evaluate the directionality of docetaxel followed by PARP inhibitors and the other way around, she suggested.
Dr. Castro said that patients with BRCA alterations benefit from PARP inhibitors and are likely to derive more benefit from them than from taxanes.
“But those with ATM alterations are unlikely to benefit from rucaparib more than from taxanes,” she said.
In a comment, Hank Ng, MD, medical oncologist, NYU Langone Perlmutter Cancer Center, New York, said he is not convinced that the findings from TRITON 3 represent a new standard of care in BRCA 1/2 mutations or ATM.
“Currently, we know that, for patients with prostate cancer with BRCA1/2 or ATM, the standard of care is an androgen receptor pathway inhibitor (ARPI), such as abiraterone or enzalutamide, then docetaxel, and then a PARP inhibitor like rucaparib,” he said.
(Currently, rucaparib is indicated for use in patients with mCRPC with BRCA alterations after they have already received an ARPI and taxane-based chemotherapy.)
Dr. Ng also questioned the control arm of the TRITON 3 trial. All the participants in the trial had already experienced disease progression after treatment with a second-generation ARPI. But the physician’s choice of therapy allowed them to move on to another ARPI or to docetaxel.
Dr. NG commented that, “in almost all cases, after progression of one ARPI, switching to another ARPI does not provide much benefit – from what is visible from this abstract – and only 56% patients received docetaxel, and thus 44% received a not-beneficial treatment,” he said.
“I am not sure what the docetaxel subgroup showed, but potentially, if those numbers are convincing, we could move this [rucaparib] ahead of docetaxel,” he speculated.
However, he also pointed out that an overall survival benefit has not yet been shown; so far, the benefit that has been shown is with respect to imaging-based PFS.
Dr. Ng does agree that rucaparib is indicated in the second line after progression with one ARPI for patients who are not candidates for chemotherapy. “But this has not yet shown me that we should absolutely be offering rucaparib before docetaxel,” he said.
TRITON3 was supported by Clovis Oncology, manufacturer of rucaparib. Dr. Bryce has relationships with Bayer, Foundation Medicine, Janssen, Merck, Myovant Sciences, and Novartis and holds a patent for therapeutic targeting of cancer patients with NRG1 rearrangements. Dr. Castro has relationships with Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Janssen-Cilag, Merck, MSD Oncology, Novartis, Pfizer, and Roche.
A version of this article first appeared on Medscape.com.
AT ASCO GU 2023
Transplant vs. chemo: Similar AML survival rates
Notably, all patients who relapsed after consolidation chemotherapy were able to receive allogeneic HCT, suggesting that transplantation may be safely delayed in some patients until their first relapse.
“The results of this randomized clinical trial indicate that the probability of survival after [allogeneic] HCT is not superior to that of conventional consolidation chemotherapy” among patients 60 years or younger with intermediate-risk AML, the authors concluded.
However, two experts highlighted several caveats to the study, which suggest the results may not translate to current clinical practice.
The study was published online in JAMA Oncology.
Approximately 50%-70% of patients with AML who receive intensive induction chemotherapy for AML and achieve a first complete remission are referred for post-remission therapy.
While consolidation chemotherapy with high-dose cytarabine has shown a benefit for those with a favorable risk profile, patients considered high-risk with adequate performance status may be candidates for allogeneic HCT.
However, determining the optimal post-remission treatment option for patients who fall into the intermediate-risk category can be more challenging.
To compare outcomes among intermediate-risk patients, researchers from Germany conducted a multicenter trial, enrolling 143 adults aged 60 or younger with intermediate-risk AML who had achieved first complete remission or complete remission with incomplete blood cell count recovery following conventional induction therapy.
The patients, who had a mean age of 48.2 years, were randomly assigned to consolidation treatment with allogeneic HCT (n = 76) or chemotherapy with high-dose cytarabine (n = 67), with the option for salvage HCT in the case of relapse. Overall, 12 patients in the HCT group received one consolidation course of high-dose cytarabine after achieving complete remission to bridge until allogeneic HCT, while all other patients in this group received allogeneic HCT directly after induction therapy.
Overall, disease-free survival at 2 years was significantly higher in the allogeneic HCT group (69%), compared with the consolidation therapy group (40%; P = .001). And the cumulative incidence of relapse at 2 years in the allogeneic HCT group was also lower, at 20%, compared with 58% in the consolidation therapy group (P < .001).
The overall survival data, however, painted a slightly more complex picture. In the intention-to-treat analysis, the probability of survival at 2 years was similar between the allogeneic HCT group (74%, or 56 of 76 patients), compared with consolidation chemotherapy (84%, or 56 of 67 patients; P = .22).
In addition, the rates of nonrelapse mortality at 2 years were higher in the allogeneic HCT group (9%) versus chemotherapy (2%; P = .005).
Although the rate of nonrelapse mortality was higher with allogeneic HCT, the relatively low rate with each treatment strategies was “an important and rewarding finding,” the authors noted. “This achievement is clearly due to the availability of less toxic but still effective conditioning therapies and modern antiviral and antifungal prophylaxis.”
In addition, among the 41 patients who relapsed after consolidation chemotherapy, all received allogeneic HCT, and the authors observed no significant differences between the groups in terms of health-related quality of life measures.
Results ‘may not translate to real-life clinical practice’
An important caveat is that the findings do not reflect some key updated strategies currently used in clinical practice, said Diego Adrianzen Herrera, MD, from the University of Vermont’s Larner College of Medicine, Burlington, who was not involved in the study.
“A charitable interpretation of the results is that a clear, large survival benefit of transplant in first complete remission is not apparent, which in turn can inform decision-making in certain circumstances for patients meeting the trial criteria, [including] younger patients with a readily available donor,” he told this news organization.
“However, risk stratification strategies currently used were not followed,” he said.
For instance, molecular risk stratification was not universally used, which may have led the researchers to overrepresent the number of patients considered to have favorable risk disease and “could have skewed the results in favor of the chemotherapy arm,” he explained.
In addition, minimal residual disease surveillance by flow cytometry was not used. Plus, Dr. Herrera added, in practice, not all patients can be salvaged and taken to HCT when in their second complete remission, or even achieve complete remission again.
“Unfortunately, these issues make the clinical significance of these results limited,” he concluded.
Margaret Kasner, MD, who was not associated with the research, agreed that aspects of the study design may not translate to real-life clinical practice, particularly in terms of quality-of-life outcomes.
“Although the [study] showed no difference in quality of life in the patient groups, this is likely due to the patient selection,” Dr. Kasner, of the Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, said in an interview. “Most patients do not allow themselves to be randomized between these two very different strategies, so those who are willing to be randomized may be a different population in terms how their quality of life is affected by relapse.”
The authors acknowledged some of these limitations, adding that the routine use of minimal residual disease monitoring in some patients was only established once the trial was underway, and the number of patients with complete minimal residual disease was therefore limited.
In addition, Dr. Herrera explained that because HCT involves significant disruptions to daily life and extensive follow-up and monitoring, decisions to use the strategy are not taken lightly by clinicians or patients.
“This is a major issue,” he said. “HCT remains a therapeutic option which causes significant apprehension to patients.”
Nevertheless, “in my experience most patients would prefer an upfront strategy if there is a definitive need for transplant,” he added. “I think the main question patients have is whether they absolutely need an HCT and how can we better identify up front who will be in the relapse-free group at 2 years.”
The study received grant funding from the Deutsche Forschungsgemeinschaft. The authors’ disclosures are detailed in the original article. Dr. Herrera and Dr. Kasner report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Notably, all patients who relapsed after consolidation chemotherapy were able to receive allogeneic HCT, suggesting that transplantation may be safely delayed in some patients until their first relapse.
“The results of this randomized clinical trial indicate that the probability of survival after [allogeneic] HCT is not superior to that of conventional consolidation chemotherapy” among patients 60 years or younger with intermediate-risk AML, the authors concluded.
However, two experts highlighted several caveats to the study, which suggest the results may not translate to current clinical practice.
The study was published online in JAMA Oncology.
Approximately 50%-70% of patients with AML who receive intensive induction chemotherapy for AML and achieve a first complete remission are referred for post-remission therapy.
While consolidation chemotherapy with high-dose cytarabine has shown a benefit for those with a favorable risk profile, patients considered high-risk with adequate performance status may be candidates for allogeneic HCT.
However, determining the optimal post-remission treatment option for patients who fall into the intermediate-risk category can be more challenging.
To compare outcomes among intermediate-risk patients, researchers from Germany conducted a multicenter trial, enrolling 143 adults aged 60 or younger with intermediate-risk AML who had achieved first complete remission or complete remission with incomplete blood cell count recovery following conventional induction therapy.
The patients, who had a mean age of 48.2 years, were randomly assigned to consolidation treatment with allogeneic HCT (n = 76) or chemotherapy with high-dose cytarabine (n = 67), with the option for salvage HCT in the case of relapse. Overall, 12 patients in the HCT group received one consolidation course of high-dose cytarabine after achieving complete remission to bridge until allogeneic HCT, while all other patients in this group received allogeneic HCT directly after induction therapy.
Overall, disease-free survival at 2 years was significantly higher in the allogeneic HCT group (69%), compared with the consolidation therapy group (40%; P = .001). And the cumulative incidence of relapse at 2 years in the allogeneic HCT group was also lower, at 20%, compared with 58% in the consolidation therapy group (P < .001).
The overall survival data, however, painted a slightly more complex picture. In the intention-to-treat analysis, the probability of survival at 2 years was similar between the allogeneic HCT group (74%, or 56 of 76 patients), compared with consolidation chemotherapy (84%, or 56 of 67 patients; P = .22).
In addition, the rates of nonrelapse mortality at 2 years were higher in the allogeneic HCT group (9%) versus chemotherapy (2%; P = .005).
Although the rate of nonrelapse mortality was higher with allogeneic HCT, the relatively low rate with each treatment strategies was “an important and rewarding finding,” the authors noted. “This achievement is clearly due to the availability of less toxic but still effective conditioning therapies and modern antiviral and antifungal prophylaxis.”
In addition, among the 41 patients who relapsed after consolidation chemotherapy, all received allogeneic HCT, and the authors observed no significant differences between the groups in terms of health-related quality of life measures.
Results ‘may not translate to real-life clinical practice’
An important caveat is that the findings do not reflect some key updated strategies currently used in clinical practice, said Diego Adrianzen Herrera, MD, from the University of Vermont’s Larner College of Medicine, Burlington, who was not involved in the study.
“A charitable interpretation of the results is that a clear, large survival benefit of transplant in first complete remission is not apparent, which in turn can inform decision-making in certain circumstances for patients meeting the trial criteria, [including] younger patients with a readily available donor,” he told this news organization.
“However, risk stratification strategies currently used were not followed,” he said.
For instance, molecular risk stratification was not universally used, which may have led the researchers to overrepresent the number of patients considered to have favorable risk disease and “could have skewed the results in favor of the chemotherapy arm,” he explained.
In addition, minimal residual disease surveillance by flow cytometry was not used. Plus, Dr. Herrera added, in practice, not all patients can be salvaged and taken to HCT when in their second complete remission, or even achieve complete remission again.
“Unfortunately, these issues make the clinical significance of these results limited,” he concluded.
Margaret Kasner, MD, who was not associated with the research, agreed that aspects of the study design may not translate to real-life clinical practice, particularly in terms of quality-of-life outcomes.
“Although the [study] showed no difference in quality of life in the patient groups, this is likely due to the patient selection,” Dr. Kasner, of the Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, said in an interview. “Most patients do not allow themselves to be randomized between these two very different strategies, so those who are willing to be randomized may be a different population in terms how their quality of life is affected by relapse.”
The authors acknowledged some of these limitations, adding that the routine use of minimal residual disease monitoring in some patients was only established once the trial was underway, and the number of patients with complete minimal residual disease was therefore limited.
In addition, Dr. Herrera explained that because HCT involves significant disruptions to daily life and extensive follow-up and monitoring, decisions to use the strategy are not taken lightly by clinicians or patients.
“This is a major issue,” he said. “HCT remains a therapeutic option which causes significant apprehension to patients.”
Nevertheless, “in my experience most patients would prefer an upfront strategy if there is a definitive need for transplant,” he added. “I think the main question patients have is whether they absolutely need an HCT and how can we better identify up front who will be in the relapse-free group at 2 years.”
The study received grant funding from the Deutsche Forschungsgemeinschaft. The authors’ disclosures are detailed in the original article. Dr. Herrera and Dr. Kasner report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Notably, all patients who relapsed after consolidation chemotherapy were able to receive allogeneic HCT, suggesting that transplantation may be safely delayed in some patients until their first relapse.
“The results of this randomized clinical trial indicate that the probability of survival after [allogeneic] HCT is not superior to that of conventional consolidation chemotherapy” among patients 60 years or younger with intermediate-risk AML, the authors concluded.
However, two experts highlighted several caveats to the study, which suggest the results may not translate to current clinical practice.
The study was published online in JAMA Oncology.
Approximately 50%-70% of patients with AML who receive intensive induction chemotherapy for AML and achieve a first complete remission are referred for post-remission therapy.
While consolidation chemotherapy with high-dose cytarabine has shown a benefit for those with a favorable risk profile, patients considered high-risk with adequate performance status may be candidates for allogeneic HCT.
However, determining the optimal post-remission treatment option for patients who fall into the intermediate-risk category can be more challenging.
To compare outcomes among intermediate-risk patients, researchers from Germany conducted a multicenter trial, enrolling 143 adults aged 60 or younger with intermediate-risk AML who had achieved first complete remission or complete remission with incomplete blood cell count recovery following conventional induction therapy.
The patients, who had a mean age of 48.2 years, were randomly assigned to consolidation treatment with allogeneic HCT (n = 76) or chemotherapy with high-dose cytarabine (n = 67), with the option for salvage HCT in the case of relapse. Overall, 12 patients in the HCT group received one consolidation course of high-dose cytarabine after achieving complete remission to bridge until allogeneic HCT, while all other patients in this group received allogeneic HCT directly after induction therapy.
Overall, disease-free survival at 2 years was significantly higher in the allogeneic HCT group (69%), compared with the consolidation therapy group (40%; P = .001). And the cumulative incidence of relapse at 2 years in the allogeneic HCT group was also lower, at 20%, compared with 58% in the consolidation therapy group (P < .001).
The overall survival data, however, painted a slightly more complex picture. In the intention-to-treat analysis, the probability of survival at 2 years was similar between the allogeneic HCT group (74%, or 56 of 76 patients), compared with consolidation chemotherapy (84%, or 56 of 67 patients; P = .22).
In addition, the rates of nonrelapse mortality at 2 years were higher in the allogeneic HCT group (9%) versus chemotherapy (2%; P = .005).
Although the rate of nonrelapse mortality was higher with allogeneic HCT, the relatively low rate with each treatment strategies was “an important and rewarding finding,” the authors noted. “This achievement is clearly due to the availability of less toxic but still effective conditioning therapies and modern antiviral and antifungal prophylaxis.”
In addition, among the 41 patients who relapsed after consolidation chemotherapy, all received allogeneic HCT, and the authors observed no significant differences between the groups in terms of health-related quality of life measures.
Results ‘may not translate to real-life clinical practice’
An important caveat is that the findings do not reflect some key updated strategies currently used in clinical practice, said Diego Adrianzen Herrera, MD, from the University of Vermont’s Larner College of Medicine, Burlington, who was not involved in the study.
“A charitable interpretation of the results is that a clear, large survival benefit of transplant in first complete remission is not apparent, which in turn can inform decision-making in certain circumstances for patients meeting the trial criteria, [including] younger patients with a readily available donor,” he told this news organization.
“However, risk stratification strategies currently used were not followed,” he said.
For instance, molecular risk stratification was not universally used, which may have led the researchers to overrepresent the number of patients considered to have favorable risk disease and “could have skewed the results in favor of the chemotherapy arm,” he explained.
In addition, minimal residual disease surveillance by flow cytometry was not used. Plus, Dr. Herrera added, in practice, not all patients can be salvaged and taken to HCT when in their second complete remission, or even achieve complete remission again.
“Unfortunately, these issues make the clinical significance of these results limited,” he concluded.
Margaret Kasner, MD, who was not associated with the research, agreed that aspects of the study design may not translate to real-life clinical practice, particularly in terms of quality-of-life outcomes.
“Although the [study] showed no difference in quality of life in the patient groups, this is likely due to the patient selection,” Dr. Kasner, of the Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, said in an interview. “Most patients do not allow themselves to be randomized between these two very different strategies, so those who are willing to be randomized may be a different population in terms how their quality of life is affected by relapse.”
The authors acknowledged some of these limitations, adding that the routine use of minimal residual disease monitoring in some patients was only established once the trial was underway, and the number of patients with complete minimal residual disease was therefore limited.
In addition, Dr. Herrera explained that because HCT involves significant disruptions to daily life and extensive follow-up and monitoring, decisions to use the strategy are not taken lightly by clinicians or patients.
“This is a major issue,” he said. “HCT remains a therapeutic option which causes significant apprehension to patients.”
Nevertheless, “in my experience most patients would prefer an upfront strategy if there is a definitive need for transplant,” he added. “I think the main question patients have is whether they absolutely need an HCT and how can we better identify up front who will be in the relapse-free group at 2 years.”
The study received grant funding from the Deutsche Forschungsgemeinschaft. The authors’ disclosures are detailed in the original article. Dr. Herrera and Dr. Kasner report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Teaching the Teacher: Novel Faculty Development for VA Hospitalists
Educating the next generation of health professionals is 1 of 4 congressionally mandated statutory missions of the US Department of Veterans Affairs (VA).1 Even before the COVID-19 pandemic, the number of veterans accessing VA health care was increasing, and those veterans are older and more medically complex than those who seek care outside the VA.2 Almost half of medical residents reported a decline in the quality of their clinical education since the institution of the 2011 duty hours regulations, and in the past decade, more attention has been paid to the need for structured faculty development programs that focus on clinicians’ roles as medical educators.3-6 Hospitalists in particular shoulder a large portion of inpatient medicine education.7 As a result, hospitalists have adapted known frameworks for medical education to their unique clinical setting and developed novel frameworks to meet the needs of their learners.8,9
Access to technology and social media have shaped the educational experience of young learners who are accustomed to quick answers and the rapidity of change.10 The clinical teaching landscape changed again with COVID-19, requiring at least temporary abandonment of traditional in-person teaching methods, which upended well-established educational norms.11,12 In this evolving field, even seasoned preceptors may feel ill-equipped to manage the nuances of modern clinical education and may struggle to recognize which teaching skills are most critical.13,14 Baseline core teaching competencies for medical educators have been previously described and are separate from clinical competencies; however, to our knowledge, no needs assessment has previously been performed specifically for VA hospitalist clinician educators.15
Between May and June of 2020, we distributed an online needs assessment to academic VA hospitalists to identify perceived barriers to effective clinical education and preferred strategies to overcome them. We received 71 responses from 140 hospitalists (50% response rate) on the Veterans Health Administration (VHA) academic hospitalist listserv. Of respondents, 59 (83%) reported teaching health professions trainees every year. VA hospitalists reported educating a diverse group of interprofessional learners, including medical residents and students, physician assistant students, nursing students, pharmacy residents and students, and podiatry students.
Only 14 respondents (20%) were aware of faculty development training available to them through their VA facility, while 53 (75%) were aware of similar resources through academic affiliates or other outside sources. More than 95% of respondents (n = 68) reported interest in receiving VA-specific faculty development to improve skills as clinician educators. The most preferred forms of delivery were in-person or virtual real-time workshops. VA hospitalists reported the least confidence in their ability to support struggling learners, balance supervision and autonomy, and develop individualized learning plans (Table 1).
With a better understanding of the needs of academic VA hospitalists, we sought to develop, implement, and measure the impact of a faculty development program that meets the specific needs of inpatient clinicians in the VA. Here we introduce the program, its content, and the experiences of initial participants.
Teaching the Teacher
Teaching the Teacher began at a single VA institution as a series of in-person, discussion-based faculty development workshops. The series met a local need for collaborative professional development in clinical education for hospitalists and specialists who round with health professions learners on the inpatient wards. Both novice and experienced clinicians participated in the series with positive feedback. Based on the results of the national needs assessment, the program has since expanded to other sites with support from the VHA Hospital Medicine Program Office. The project’s overarching goal was to facilitate sharing of best practices across VA sites and create a network of local and national VA educators that participants could continue to access even after course completion.
Teaching the Teacher is structured into 5 facilitated hour-long sessions that can be completed either daily for 1 week or weekly for 1 month at the discretion of each institution. Each session is dedicated to a subject identified on the needs assessment as being highest yield. The hospitalist needs assessment also identified the preference for targeted faculty development that is relevant specifically to VA clinicians. To meet this need, Teaching the Teacher delivers its content through the unique lens of VA medicine. The educational mission of the VA is threaded throughout all presentations, and tips to maximize teaching in the VA’s unique clinical environments are embedded into each hour. Examples include discussions on how to incorporate veteran patients into bedside teaching, handling challenging patient-practitioner interactions as they pertain to patients, and the use of VA resources to find and teach evidence-based medicine.Each session includes a set of learning objectives; within that framework, facilitators allow participants to guide the nuances of content based on their individual and institutional priorities. The pandemic continues to shape much of the course content, as both hospitalists and their trainees grapple with mental health challenges, decreased bedside teaching, and wide variations in baseline trainee competence due to different institutional responses to teaching during a pandemic.12,16 Content is regularly updated to incorporate new literature and feedback from participants and prioritize active participation. Continuing medical education/continuing educational units credit is available through the VA for course completion.
In the first session on modern learners, participants discuss the current generation of health professions trainees, including how personality characteristics and COVID-19 have impacted their learning experiences, and strategies to improve our ability to teach them successfully (Table 2).
The course was originally designed to be in person, but the COVID-19 pandemic forced a shift to online format. To achieve a high-quality learning environment, the course implemented best practices in virtual synchronous instruction, including setting expectations for participation and screen use at the beginning of the series and optimizing audiovisual technology.17 During each seminar, the use of breakout rooms, polling, and the chat function fostered and sustained engagement.17 After each seminar, participants received a recording of the session, a copy of the materials reviewed, and links to referenced readings.17 The course preserved the interactive aspect of the curriculum through both these previously described techniques and our novel approaches, such as facilitated live interactions with online VA resources.
The pandemic also had an impact on curriculum content, as facilitation of online learning was a new and necessary skill set for instructors and participants. To meet this evolving need, additions in content addressed best practices in synchronous and asynchronous online learning, and augmented discussions on navigating asynchronous learning modalities such as social media. A virtual format allowed for dissemination of this course across the country and for recruitment of new course facilitators from remote sites. The team of instructors included academic hospitalist faculty from 3 VA institutions.
Program Impact
Ten academically affiliated VA hospital medicine sections across 6 states have participated in Teaching the Teacher and several more are scheduled at other sites. Of the 10, 5 completed the course in collaboration with another VA site. Ninety-seven clinicians completed < 1 session synchronously but given the asynchronous option, this number likely underestimates the total audience. Participants included physicians, nurse practitioners, and physician assistants.
Surveys were conducted before and after the program, with 58 participants completing the presurvey, 32 the postsurvey, and 27 completing both. Of the 32 postsurvey respondents, 31 (97%) would recommend the seminar to colleagues. The live, discussion-based format was the most valued aspect of the course structure, with engaging facilitators and course content also ranking highly. Just over half (n = 17) indicated specific behavioral changes they plan to enact after completing the series, such as connecting with and better understanding learners, prioritizing high-quality feedback more deliberately, and bringing medicine to the bedside. The most common critiques of the course were requests for more time for feedback skills.
Discussion
Teaching the Teacher is a VA-specific faculty development seminar for hospitalists. Participants who responded to a survey reported that it met their needs as VA clinician educators. This is the first published needs assessment of academic VA hospitalists in their roles as clinician educators and the first faculty development initiative to address those specific needs using a collaborative, multisite approach. Although this program is a pilot, the positive response it has received has set a precedent for increased development and growth.
Teaching the Teacher presents a novel approach with a condensed curriculum that is more convenient and accessible to VA clinicians than previous programs with similar goals. Hospitalists have busy and variable work schedules, and it can be difficult to find time to participate in a traditional faculty development program. While these programs are becoming more commonplace, they are often longitudinal and require a significant time and/or financial commitment from participants.18 In contrast, Teaching the Teacher is only 5 hours long, can be viewed either synchronously or asynchronously, and is no cost to participants. In the future, other specialties may similarly value an efficient faculty development curriculum, and participation from clinicians outside of hospital medicine could augment the richness of content.
Teaching the Teacher’s curriculum is not meant to be exhaustive, but rather to spark conversation among colleagues. According to survey respondents, the most lauded aspect of this program was the facilitated, discussion-based structure, wherein participants are presented with common challenges and encouraged to share their experiences and solutions with colleagues. Of particular interest to the program’s mission of greater community building are the VA facilities that chose to complete the seminar with another hospitalist section from a different institution. Within this structure lies an opportunity for seasoned educators to informally mentor junior colleagues both within and across institutions, and foster connections among educators that continue beyond the completion of the series. We envision this program growing into an enduring professional development course that begins at onboarding and is revisited at regular intervals thereafter.
Another compelling aspect of this project is the interprofessional design, bringing physicians, nurse practitioners, and physician assistants together. Health education, like clinical care, is shifting to a team approach.19 The curriculum addresses topics previously described as high priority for interprofessional faculty development, such as fostering healthy team leadership, motivating learners, and appraising evidence and online resources.20 A pilot project in VA primary care facilities found that deliberate interprofessional education improved collaboration among health care professionals.21 Prior to Teaching the Teacher, no similar faculty development program provided interprofessional learning and collaboration for VA hospitalists.
Limitations and Future Directions
There are several limitations to this preliminary study. Participation at each site was voluntary and did not always reach the full potential audience of hospitalist clinician educators. As one participant stated, future directions include doing “more to involve teachers who need to learn [these skills]. The ones who attended [from our institution] were already the best teachers.” In addition, despite the asynchronous option, lack of protected time for faculty development may be a limiting factor in participation. Support from institutional and national leadership would likely improve participation.
Measured endpoints to date consist primarily of participant satisfaction and do not yet capture objective changes in teaching. Data collection is ongoing to assess immediate and longitudinal changes in confidence and behaviors of attendees and how this might affect their health professions learners.
Last, our initial needs assessment only targeted academic hospitalists, and the needs of VA hospitalists in rural areas or at facilities without academic affiliation may be different. More research is needed to understand the diverse faculty that comprises both urban and rural VA sites, what their professional development needs are, and how those needs can be met.
Conclusions
Teaching the Teacher is a faculty development pilot, tailored to meet the needs of VA hospitalist clinician educators, that has been voluntarily adopted at multiple VA sites. The facilitated discussion format allows participants to guide the conversation and personalize content, thereby promoting a culture of discussing challenges and best practices among colleagues that we hope endures beyond the bounds of the curriculum. The program focuses on elevating the specific teaching mission of the VA and could be incorporated into onboarding and regular VA-sponsored faculty development updates. While Teaching the Teacher was originally developed for VA hospitalists, most of the content is applicable to clinicians outside hospital medicine. This project serves as a model for training clinical educators and has opportunities to expand across VA as a customizable didactic platform.
Acknowledgments
We thank Brian Schneider, MD, for his tireless support of this program, as well as all the VA clinicians who have shared their time, talents, and wisdom with us since this program’s inception.
1. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Updated September 24, 2019. Accessed November 29, 2022. https://www.va.gov/oaa/oaa_mission.asp
2. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
3. Drolet BC, Christopher DA, Fischer SA. Residents’ response to duty-hour regulations--a follow-up national survey. N Engl J Med. 2012;366(24):e35. doi:10.1056/NEJMp1202848
4. Hatem CJ, Lown BA, Newman LR. The academic health center coming of age: helping faculty become better teachers and agents of educational change. Acad Med. 2006;81(11):941-944. doi:10.1097/01.ACM.0000242490.56586.64
5. Harvey MM, Berkley HH, O’Malley PG, Durning SJ. Preparing future medical educators: development and pilot evaluation of a student-led medical education elective. Mil Med. 2020;185(1-2):e131-e137. doi:10.1093/milmed/usz175
6. Jason H. Future medical education: Preparing, priorities, possibilities. Med Teach. 2018;40(10):996-1003. doi:10.1080/0142159X.2018.1503412
7. Natarajan P, Ranji SR, Auerbach AD, Hauer KE. Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490-498. doi:10.1002/jhm.537
8. Pascoe JM, Nixon J, Lang VJ. Maximizing teaching on the wards: review and application of the One-Minute Preceptor and SNAPPS models. J Hosp Med. 2015;10(2):125-130. doi:10.1002/jhm.2302
9. Martin SK, Farnan JM, Arora VM. Future: new strategies for hospitalists to overcome challenges in teaching on today’s wards. J Hosp Med. 2013;8(7):409-413. doi:10.1002/jhm.2057
10. Waljee JF, Chopra V, Saint S. Mentoring Millennials. JAMA. 2020;323(17):1716-1717. doi:10.1001/jama.2020.3085
11. Papapanou M, Routsi E, Tsamakis K, et al. Medical education challenges and innovations during COVID-19 pandemic. Postgrad Med J. 2022;98(1159):321-327. doi:10.1136/postgradmedj-2021-140032
12. Hilburg R, Patel N, Ambruso S, Biewald MA, Farouk SS. Medical education during the Coronavirus Disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27(5):412-417. doi:10.1053/j.ackd.2020.05.017
13. Simpson D, Marcdante K, Souza KH, Anderson A, Holmboe E. Job roles of the 2025 medical educator. J Grad Med Educ. 2018;10(3):243-246. doi:10.4300/JGME-D-18-00253.1
14. Armstrong EG, Mackey M, Spear SJ. Medical education as a process management problem. Acad Med. 2004;79(8):721-728. doi:10.1097/00001888-200408000-00002
15. Srinivasan M, Li ST, Meyers FJ, et al. “Teaching as a Competency”: competencies for medical educators. Acad Med. 2011;86(10):1211-1220. doi:10.1097/ACM.0b013e31822c5b9a
16. Clark E, Freytag J, Hysong SJ, Dang B, Giordano TP, Kulkarni PA. 964. Impact of the COVID-19 pandemic on bedside medical education: a mixed-methods study. Open Forum Infect Dis. 2021;8(Suppl 1):S574. Published 2021 Dec 4. doi:10.1093/ofid/ofab466.1159
17. Ohnigian S, Richards JB, Monette DL, Roberts DH. optimizing remote learning: leveraging zoom to develop and implement successful education sessions. J Med Educ Curric Dev. 2021;8:23821205211020760. Published 2021 Jun 28. doi:10.1177/23821205211020760
18. Burgess A, Matar E, Neuen B, Fox GJ. A longitudinal faculty development program: supporting a culture of teaching. BMC Med Educ. 2019;19(1):400. Published 2019 Nov 1. doi:10.1186/s12909-019-1832-3
19. Stoddard HA, Brownfield ED. Clinician-educators as dual professionals: a contemporary reappraisal. Acad Med. 2016;91(7):921-924. doi:10.1097/ACM.0000000000001210
20. Schönwetter DJ, Hamilton J, Sawatzky JA. Exploring professional development needs of educators in the health sciences professions. J Dent Educ. 2015;79(2):113-123.
21. Meyer EM, Zapatka S, Brienza RS. The development of professional identity and the formation of teams in the Veterans Affairs Connecticut Healthcare System’s Center of Excellence in Primary Care Education Program (CoEPCE). Acad Med. 2015;90(6):802-809. doi:10.1097/ACM.0000000000000594
Educating the next generation of health professionals is 1 of 4 congressionally mandated statutory missions of the US Department of Veterans Affairs (VA).1 Even before the COVID-19 pandemic, the number of veterans accessing VA health care was increasing, and those veterans are older and more medically complex than those who seek care outside the VA.2 Almost half of medical residents reported a decline in the quality of their clinical education since the institution of the 2011 duty hours regulations, and in the past decade, more attention has been paid to the need for structured faculty development programs that focus on clinicians’ roles as medical educators.3-6 Hospitalists in particular shoulder a large portion of inpatient medicine education.7 As a result, hospitalists have adapted known frameworks for medical education to their unique clinical setting and developed novel frameworks to meet the needs of their learners.8,9
Access to technology and social media have shaped the educational experience of young learners who are accustomed to quick answers and the rapidity of change.10 The clinical teaching landscape changed again with COVID-19, requiring at least temporary abandonment of traditional in-person teaching methods, which upended well-established educational norms.11,12 In this evolving field, even seasoned preceptors may feel ill-equipped to manage the nuances of modern clinical education and may struggle to recognize which teaching skills are most critical.13,14 Baseline core teaching competencies for medical educators have been previously described and are separate from clinical competencies; however, to our knowledge, no needs assessment has previously been performed specifically for VA hospitalist clinician educators.15
Between May and June of 2020, we distributed an online needs assessment to academic VA hospitalists to identify perceived barriers to effective clinical education and preferred strategies to overcome them. We received 71 responses from 140 hospitalists (50% response rate) on the Veterans Health Administration (VHA) academic hospitalist listserv. Of respondents, 59 (83%) reported teaching health professions trainees every year. VA hospitalists reported educating a diverse group of interprofessional learners, including medical residents and students, physician assistant students, nursing students, pharmacy residents and students, and podiatry students.
Only 14 respondents (20%) were aware of faculty development training available to them through their VA facility, while 53 (75%) were aware of similar resources through academic affiliates or other outside sources. More than 95% of respondents (n = 68) reported interest in receiving VA-specific faculty development to improve skills as clinician educators. The most preferred forms of delivery were in-person or virtual real-time workshops. VA hospitalists reported the least confidence in their ability to support struggling learners, balance supervision and autonomy, and develop individualized learning plans (Table 1).
With a better understanding of the needs of academic VA hospitalists, we sought to develop, implement, and measure the impact of a faculty development program that meets the specific needs of inpatient clinicians in the VA. Here we introduce the program, its content, and the experiences of initial participants.
Teaching the Teacher
Teaching the Teacher began at a single VA institution as a series of in-person, discussion-based faculty development workshops. The series met a local need for collaborative professional development in clinical education for hospitalists and specialists who round with health professions learners on the inpatient wards. Both novice and experienced clinicians participated in the series with positive feedback. Based on the results of the national needs assessment, the program has since expanded to other sites with support from the VHA Hospital Medicine Program Office. The project’s overarching goal was to facilitate sharing of best practices across VA sites and create a network of local and national VA educators that participants could continue to access even after course completion.
Teaching the Teacher is structured into 5 facilitated hour-long sessions that can be completed either daily for 1 week or weekly for 1 month at the discretion of each institution. Each session is dedicated to a subject identified on the needs assessment as being highest yield. The hospitalist needs assessment also identified the preference for targeted faculty development that is relevant specifically to VA clinicians. To meet this need, Teaching the Teacher delivers its content through the unique lens of VA medicine. The educational mission of the VA is threaded throughout all presentations, and tips to maximize teaching in the VA’s unique clinical environments are embedded into each hour. Examples include discussions on how to incorporate veteran patients into bedside teaching, handling challenging patient-practitioner interactions as they pertain to patients, and the use of VA resources to find and teach evidence-based medicine.Each session includes a set of learning objectives; within that framework, facilitators allow participants to guide the nuances of content based on their individual and institutional priorities. The pandemic continues to shape much of the course content, as both hospitalists and their trainees grapple with mental health challenges, decreased bedside teaching, and wide variations in baseline trainee competence due to different institutional responses to teaching during a pandemic.12,16 Content is regularly updated to incorporate new literature and feedback from participants and prioritize active participation. Continuing medical education/continuing educational units credit is available through the VA for course completion.
In the first session on modern learners, participants discuss the current generation of health professions trainees, including how personality characteristics and COVID-19 have impacted their learning experiences, and strategies to improve our ability to teach them successfully (Table 2).
The course was originally designed to be in person, but the COVID-19 pandemic forced a shift to online format. To achieve a high-quality learning environment, the course implemented best practices in virtual synchronous instruction, including setting expectations for participation and screen use at the beginning of the series and optimizing audiovisual technology.17 During each seminar, the use of breakout rooms, polling, and the chat function fostered and sustained engagement.17 After each seminar, participants received a recording of the session, a copy of the materials reviewed, and links to referenced readings.17 The course preserved the interactive aspect of the curriculum through both these previously described techniques and our novel approaches, such as facilitated live interactions with online VA resources.
The pandemic also had an impact on curriculum content, as facilitation of online learning was a new and necessary skill set for instructors and participants. To meet this evolving need, additions in content addressed best practices in synchronous and asynchronous online learning, and augmented discussions on navigating asynchronous learning modalities such as social media. A virtual format allowed for dissemination of this course across the country and for recruitment of new course facilitators from remote sites. The team of instructors included academic hospitalist faculty from 3 VA institutions.
Program Impact
Ten academically affiliated VA hospital medicine sections across 6 states have participated in Teaching the Teacher and several more are scheduled at other sites. Of the 10, 5 completed the course in collaboration with another VA site. Ninety-seven clinicians completed < 1 session synchronously but given the asynchronous option, this number likely underestimates the total audience. Participants included physicians, nurse practitioners, and physician assistants.
Surveys were conducted before and after the program, with 58 participants completing the presurvey, 32 the postsurvey, and 27 completing both. Of the 32 postsurvey respondents, 31 (97%) would recommend the seminar to colleagues. The live, discussion-based format was the most valued aspect of the course structure, with engaging facilitators and course content also ranking highly. Just over half (n = 17) indicated specific behavioral changes they plan to enact after completing the series, such as connecting with and better understanding learners, prioritizing high-quality feedback more deliberately, and bringing medicine to the bedside. The most common critiques of the course were requests for more time for feedback skills.
Discussion
Teaching the Teacher is a VA-specific faculty development seminar for hospitalists. Participants who responded to a survey reported that it met their needs as VA clinician educators. This is the first published needs assessment of academic VA hospitalists in their roles as clinician educators and the first faculty development initiative to address those specific needs using a collaborative, multisite approach. Although this program is a pilot, the positive response it has received has set a precedent for increased development and growth.
Teaching the Teacher presents a novel approach with a condensed curriculum that is more convenient and accessible to VA clinicians than previous programs with similar goals. Hospitalists have busy and variable work schedules, and it can be difficult to find time to participate in a traditional faculty development program. While these programs are becoming more commonplace, they are often longitudinal and require a significant time and/or financial commitment from participants.18 In contrast, Teaching the Teacher is only 5 hours long, can be viewed either synchronously or asynchronously, and is no cost to participants. In the future, other specialties may similarly value an efficient faculty development curriculum, and participation from clinicians outside of hospital medicine could augment the richness of content.
Teaching the Teacher’s curriculum is not meant to be exhaustive, but rather to spark conversation among colleagues. According to survey respondents, the most lauded aspect of this program was the facilitated, discussion-based structure, wherein participants are presented with common challenges and encouraged to share their experiences and solutions with colleagues. Of particular interest to the program’s mission of greater community building are the VA facilities that chose to complete the seminar with another hospitalist section from a different institution. Within this structure lies an opportunity for seasoned educators to informally mentor junior colleagues both within and across institutions, and foster connections among educators that continue beyond the completion of the series. We envision this program growing into an enduring professional development course that begins at onboarding and is revisited at regular intervals thereafter.
Another compelling aspect of this project is the interprofessional design, bringing physicians, nurse practitioners, and physician assistants together. Health education, like clinical care, is shifting to a team approach.19 The curriculum addresses topics previously described as high priority for interprofessional faculty development, such as fostering healthy team leadership, motivating learners, and appraising evidence and online resources.20 A pilot project in VA primary care facilities found that deliberate interprofessional education improved collaboration among health care professionals.21 Prior to Teaching the Teacher, no similar faculty development program provided interprofessional learning and collaboration for VA hospitalists.
Limitations and Future Directions
There are several limitations to this preliminary study. Participation at each site was voluntary and did not always reach the full potential audience of hospitalist clinician educators. As one participant stated, future directions include doing “more to involve teachers who need to learn [these skills]. The ones who attended [from our institution] were already the best teachers.” In addition, despite the asynchronous option, lack of protected time for faculty development may be a limiting factor in participation. Support from institutional and national leadership would likely improve participation.
Measured endpoints to date consist primarily of participant satisfaction and do not yet capture objective changes in teaching. Data collection is ongoing to assess immediate and longitudinal changes in confidence and behaviors of attendees and how this might affect their health professions learners.
Last, our initial needs assessment only targeted academic hospitalists, and the needs of VA hospitalists in rural areas or at facilities without academic affiliation may be different. More research is needed to understand the diverse faculty that comprises both urban and rural VA sites, what their professional development needs are, and how those needs can be met.
Conclusions
Teaching the Teacher is a faculty development pilot, tailored to meet the needs of VA hospitalist clinician educators, that has been voluntarily adopted at multiple VA sites. The facilitated discussion format allows participants to guide the conversation and personalize content, thereby promoting a culture of discussing challenges and best practices among colleagues that we hope endures beyond the bounds of the curriculum. The program focuses on elevating the specific teaching mission of the VA and could be incorporated into onboarding and regular VA-sponsored faculty development updates. While Teaching the Teacher was originally developed for VA hospitalists, most of the content is applicable to clinicians outside hospital medicine. This project serves as a model for training clinical educators and has opportunities to expand across VA as a customizable didactic platform.
Acknowledgments
We thank Brian Schneider, MD, for his tireless support of this program, as well as all the VA clinicians who have shared their time, talents, and wisdom with us since this program’s inception.
Educating the next generation of health professionals is 1 of 4 congressionally mandated statutory missions of the US Department of Veterans Affairs (VA).1 Even before the COVID-19 pandemic, the number of veterans accessing VA health care was increasing, and those veterans are older and more medically complex than those who seek care outside the VA.2 Almost half of medical residents reported a decline in the quality of their clinical education since the institution of the 2011 duty hours regulations, and in the past decade, more attention has been paid to the need for structured faculty development programs that focus on clinicians’ roles as medical educators.3-6 Hospitalists in particular shoulder a large portion of inpatient medicine education.7 As a result, hospitalists have adapted known frameworks for medical education to their unique clinical setting and developed novel frameworks to meet the needs of their learners.8,9
Access to technology and social media have shaped the educational experience of young learners who are accustomed to quick answers and the rapidity of change.10 The clinical teaching landscape changed again with COVID-19, requiring at least temporary abandonment of traditional in-person teaching methods, which upended well-established educational norms.11,12 In this evolving field, even seasoned preceptors may feel ill-equipped to manage the nuances of modern clinical education and may struggle to recognize which teaching skills are most critical.13,14 Baseline core teaching competencies for medical educators have been previously described and are separate from clinical competencies; however, to our knowledge, no needs assessment has previously been performed specifically for VA hospitalist clinician educators.15
Between May and June of 2020, we distributed an online needs assessment to academic VA hospitalists to identify perceived barriers to effective clinical education and preferred strategies to overcome them. We received 71 responses from 140 hospitalists (50% response rate) on the Veterans Health Administration (VHA) academic hospitalist listserv. Of respondents, 59 (83%) reported teaching health professions trainees every year. VA hospitalists reported educating a diverse group of interprofessional learners, including medical residents and students, physician assistant students, nursing students, pharmacy residents and students, and podiatry students.
Only 14 respondents (20%) were aware of faculty development training available to them through their VA facility, while 53 (75%) were aware of similar resources through academic affiliates or other outside sources. More than 95% of respondents (n = 68) reported interest in receiving VA-specific faculty development to improve skills as clinician educators. The most preferred forms of delivery were in-person or virtual real-time workshops. VA hospitalists reported the least confidence in their ability to support struggling learners, balance supervision and autonomy, and develop individualized learning plans (Table 1).
With a better understanding of the needs of academic VA hospitalists, we sought to develop, implement, and measure the impact of a faculty development program that meets the specific needs of inpatient clinicians in the VA. Here we introduce the program, its content, and the experiences of initial participants.
Teaching the Teacher
Teaching the Teacher began at a single VA institution as a series of in-person, discussion-based faculty development workshops. The series met a local need for collaborative professional development in clinical education for hospitalists and specialists who round with health professions learners on the inpatient wards. Both novice and experienced clinicians participated in the series with positive feedback. Based on the results of the national needs assessment, the program has since expanded to other sites with support from the VHA Hospital Medicine Program Office. The project’s overarching goal was to facilitate sharing of best practices across VA sites and create a network of local and national VA educators that participants could continue to access even after course completion.
Teaching the Teacher is structured into 5 facilitated hour-long sessions that can be completed either daily for 1 week or weekly for 1 month at the discretion of each institution. Each session is dedicated to a subject identified on the needs assessment as being highest yield. The hospitalist needs assessment also identified the preference for targeted faculty development that is relevant specifically to VA clinicians. To meet this need, Teaching the Teacher delivers its content through the unique lens of VA medicine. The educational mission of the VA is threaded throughout all presentations, and tips to maximize teaching in the VA’s unique clinical environments are embedded into each hour. Examples include discussions on how to incorporate veteran patients into bedside teaching, handling challenging patient-practitioner interactions as they pertain to patients, and the use of VA resources to find and teach evidence-based medicine.Each session includes a set of learning objectives; within that framework, facilitators allow participants to guide the nuances of content based on their individual and institutional priorities. The pandemic continues to shape much of the course content, as both hospitalists and their trainees grapple with mental health challenges, decreased bedside teaching, and wide variations in baseline trainee competence due to different institutional responses to teaching during a pandemic.12,16 Content is regularly updated to incorporate new literature and feedback from participants and prioritize active participation. Continuing medical education/continuing educational units credit is available through the VA for course completion.
In the first session on modern learners, participants discuss the current generation of health professions trainees, including how personality characteristics and COVID-19 have impacted their learning experiences, and strategies to improve our ability to teach them successfully (Table 2).
The course was originally designed to be in person, but the COVID-19 pandemic forced a shift to online format. To achieve a high-quality learning environment, the course implemented best practices in virtual synchronous instruction, including setting expectations for participation and screen use at the beginning of the series and optimizing audiovisual technology.17 During each seminar, the use of breakout rooms, polling, and the chat function fostered and sustained engagement.17 After each seminar, participants received a recording of the session, a copy of the materials reviewed, and links to referenced readings.17 The course preserved the interactive aspect of the curriculum through both these previously described techniques and our novel approaches, such as facilitated live interactions with online VA resources.
The pandemic also had an impact on curriculum content, as facilitation of online learning was a new and necessary skill set for instructors and participants. To meet this evolving need, additions in content addressed best practices in synchronous and asynchronous online learning, and augmented discussions on navigating asynchronous learning modalities such as social media. A virtual format allowed for dissemination of this course across the country and for recruitment of new course facilitators from remote sites. The team of instructors included academic hospitalist faculty from 3 VA institutions.
Program Impact
Ten academically affiliated VA hospital medicine sections across 6 states have participated in Teaching the Teacher and several more are scheduled at other sites. Of the 10, 5 completed the course in collaboration with another VA site. Ninety-seven clinicians completed < 1 session synchronously but given the asynchronous option, this number likely underestimates the total audience. Participants included physicians, nurse practitioners, and physician assistants.
Surveys were conducted before and after the program, with 58 participants completing the presurvey, 32 the postsurvey, and 27 completing both. Of the 32 postsurvey respondents, 31 (97%) would recommend the seminar to colleagues. The live, discussion-based format was the most valued aspect of the course structure, with engaging facilitators and course content also ranking highly. Just over half (n = 17) indicated specific behavioral changes they plan to enact after completing the series, such as connecting with and better understanding learners, prioritizing high-quality feedback more deliberately, and bringing medicine to the bedside. The most common critiques of the course were requests for more time for feedback skills.
Discussion
Teaching the Teacher is a VA-specific faculty development seminar for hospitalists. Participants who responded to a survey reported that it met their needs as VA clinician educators. This is the first published needs assessment of academic VA hospitalists in their roles as clinician educators and the first faculty development initiative to address those specific needs using a collaborative, multisite approach. Although this program is a pilot, the positive response it has received has set a precedent for increased development and growth.
Teaching the Teacher presents a novel approach with a condensed curriculum that is more convenient and accessible to VA clinicians than previous programs with similar goals. Hospitalists have busy and variable work schedules, and it can be difficult to find time to participate in a traditional faculty development program. While these programs are becoming more commonplace, they are often longitudinal and require a significant time and/or financial commitment from participants.18 In contrast, Teaching the Teacher is only 5 hours long, can be viewed either synchronously or asynchronously, and is no cost to participants. In the future, other specialties may similarly value an efficient faculty development curriculum, and participation from clinicians outside of hospital medicine could augment the richness of content.
Teaching the Teacher’s curriculum is not meant to be exhaustive, but rather to spark conversation among colleagues. According to survey respondents, the most lauded aspect of this program was the facilitated, discussion-based structure, wherein participants are presented with common challenges and encouraged to share their experiences and solutions with colleagues. Of particular interest to the program’s mission of greater community building are the VA facilities that chose to complete the seminar with another hospitalist section from a different institution. Within this structure lies an opportunity for seasoned educators to informally mentor junior colleagues both within and across institutions, and foster connections among educators that continue beyond the completion of the series. We envision this program growing into an enduring professional development course that begins at onboarding and is revisited at regular intervals thereafter.
Another compelling aspect of this project is the interprofessional design, bringing physicians, nurse practitioners, and physician assistants together. Health education, like clinical care, is shifting to a team approach.19 The curriculum addresses topics previously described as high priority for interprofessional faculty development, such as fostering healthy team leadership, motivating learners, and appraising evidence and online resources.20 A pilot project in VA primary care facilities found that deliberate interprofessional education improved collaboration among health care professionals.21 Prior to Teaching the Teacher, no similar faculty development program provided interprofessional learning and collaboration for VA hospitalists.
Limitations and Future Directions
There are several limitations to this preliminary study. Participation at each site was voluntary and did not always reach the full potential audience of hospitalist clinician educators. As one participant stated, future directions include doing “more to involve teachers who need to learn [these skills]. The ones who attended [from our institution] were already the best teachers.” In addition, despite the asynchronous option, lack of protected time for faculty development may be a limiting factor in participation. Support from institutional and national leadership would likely improve participation.
Measured endpoints to date consist primarily of participant satisfaction and do not yet capture objective changes in teaching. Data collection is ongoing to assess immediate and longitudinal changes in confidence and behaviors of attendees and how this might affect their health professions learners.
Last, our initial needs assessment only targeted academic hospitalists, and the needs of VA hospitalists in rural areas or at facilities without academic affiliation may be different. More research is needed to understand the diverse faculty that comprises both urban and rural VA sites, what their professional development needs are, and how those needs can be met.
Conclusions
Teaching the Teacher is a faculty development pilot, tailored to meet the needs of VA hospitalist clinician educators, that has been voluntarily adopted at multiple VA sites. The facilitated discussion format allows participants to guide the conversation and personalize content, thereby promoting a culture of discussing challenges and best practices among colleagues that we hope endures beyond the bounds of the curriculum. The program focuses on elevating the specific teaching mission of the VA and could be incorporated into onboarding and regular VA-sponsored faculty development updates. While Teaching the Teacher was originally developed for VA hospitalists, most of the content is applicable to clinicians outside hospital medicine. This project serves as a model for training clinical educators and has opportunities to expand across VA as a customizable didactic platform.
Acknowledgments
We thank Brian Schneider, MD, for his tireless support of this program, as well as all the VA clinicians who have shared their time, talents, and wisdom with us since this program’s inception.
1. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Updated September 24, 2019. Accessed November 29, 2022. https://www.va.gov/oaa/oaa_mission.asp
2. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
3. Drolet BC, Christopher DA, Fischer SA. Residents’ response to duty-hour regulations--a follow-up national survey. N Engl J Med. 2012;366(24):e35. doi:10.1056/NEJMp1202848
4. Hatem CJ, Lown BA, Newman LR. The academic health center coming of age: helping faculty become better teachers and agents of educational change. Acad Med. 2006;81(11):941-944. doi:10.1097/01.ACM.0000242490.56586.64
5. Harvey MM, Berkley HH, O’Malley PG, Durning SJ. Preparing future medical educators: development and pilot evaluation of a student-led medical education elective. Mil Med. 2020;185(1-2):e131-e137. doi:10.1093/milmed/usz175
6. Jason H. Future medical education: Preparing, priorities, possibilities. Med Teach. 2018;40(10):996-1003. doi:10.1080/0142159X.2018.1503412
7. Natarajan P, Ranji SR, Auerbach AD, Hauer KE. Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490-498. doi:10.1002/jhm.537
8. Pascoe JM, Nixon J, Lang VJ. Maximizing teaching on the wards: review and application of the One-Minute Preceptor and SNAPPS models. J Hosp Med. 2015;10(2):125-130. doi:10.1002/jhm.2302
9. Martin SK, Farnan JM, Arora VM. Future: new strategies for hospitalists to overcome challenges in teaching on today’s wards. J Hosp Med. 2013;8(7):409-413. doi:10.1002/jhm.2057
10. Waljee JF, Chopra V, Saint S. Mentoring Millennials. JAMA. 2020;323(17):1716-1717. doi:10.1001/jama.2020.3085
11. Papapanou M, Routsi E, Tsamakis K, et al. Medical education challenges and innovations during COVID-19 pandemic. Postgrad Med J. 2022;98(1159):321-327. doi:10.1136/postgradmedj-2021-140032
12. Hilburg R, Patel N, Ambruso S, Biewald MA, Farouk SS. Medical education during the Coronavirus Disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27(5):412-417. doi:10.1053/j.ackd.2020.05.017
13. Simpson D, Marcdante K, Souza KH, Anderson A, Holmboe E. Job roles of the 2025 medical educator. J Grad Med Educ. 2018;10(3):243-246. doi:10.4300/JGME-D-18-00253.1
14. Armstrong EG, Mackey M, Spear SJ. Medical education as a process management problem. Acad Med. 2004;79(8):721-728. doi:10.1097/00001888-200408000-00002
15. Srinivasan M, Li ST, Meyers FJ, et al. “Teaching as a Competency”: competencies for medical educators. Acad Med. 2011;86(10):1211-1220. doi:10.1097/ACM.0b013e31822c5b9a
16. Clark E, Freytag J, Hysong SJ, Dang B, Giordano TP, Kulkarni PA. 964. Impact of the COVID-19 pandemic on bedside medical education: a mixed-methods study. Open Forum Infect Dis. 2021;8(Suppl 1):S574. Published 2021 Dec 4. doi:10.1093/ofid/ofab466.1159
17. Ohnigian S, Richards JB, Monette DL, Roberts DH. optimizing remote learning: leveraging zoom to develop and implement successful education sessions. J Med Educ Curric Dev. 2021;8:23821205211020760. Published 2021 Jun 28. doi:10.1177/23821205211020760
18. Burgess A, Matar E, Neuen B, Fox GJ. A longitudinal faculty development program: supporting a culture of teaching. BMC Med Educ. 2019;19(1):400. Published 2019 Nov 1. doi:10.1186/s12909-019-1832-3
19. Stoddard HA, Brownfield ED. Clinician-educators as dual professionals: a contemporary reappraisal. Acad Med. 2016;91(7):921-924. doi:10.1097/ACM.0000000000001210
20. Schönwetter DJ, Hamilton J, Sawatzky JA. Exploring professional development needs of educators in the health sciences professions. J Dent Educ. 2015;79(2):113-123.
21. Meyer EM, Zapatka S, Brienza RS. The development of professional identity and the formation of teams in the Veterans Affairs Connecticut Healthcare System’s Center of Excellence in Primary Care Education Program (CoEPCE). Acad Med. 2015;90(6):802-809. doi:10.1097/ACM.0000000000000594
1. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Updated September 24, 2019. Accessed November 29, 2022. https://www.va.gov/oaa/oaa_mission.asp
2. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
3. Drolet BC, Christopher DA, Fischer SA. Residents’ response to duty-hour regulations--a follow-up national survey. N Engl J Med. 2012;366(24):e35. doi:10.1056/NEJMp1202848
4. Hatem CJ, Lown BA, Newman LR. The academic health center coming of age: helping faculty become better teachers and agents of educational change. Acad Med. 2006;81(11):941-944. doi:10.1097/01.ACM.0000242490.56586.64
5. Harvey MM, Berkley HH, O’Malley PG, Durning SJ. Preparing future medical educators: development and pilot evaluation of a student-led medical education elective. Mil Med. 2020;185(1-2):e131-e137. doi:10.1093/milmed/usz175
6. Jason H. Future medical education: Preparing, priorities, possibilities. Med Teach. 2018;40(10):996-1003. doi:10.1080/0142159X.2018.1503412
7. Natarajan P, Ranji SR, Auerbach AD, Hauer KE. Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490-498. doi:10.1002/jhm.537
8. Pascoe JM, Nixon J, Lang VJ. Maximizing teaching on the wards: review and application of the One-Minute Preceptor and SNAPPS models. J Hosp Med. 2015;10(2):125-130. doi:10.1002/jhm.2302
9. Martin SK, Farnan JM, Arora VM. Future: new strategies for hospitalists to overcome challenges in teaching on today’s wards. J Hosp Med. 2013;8(7):409-413. doi:10.1002/jhm.2057
10. Waljee JF, Chopra V, Saint S. Mentoring Millennials. JAMA. 2020;323(17):1716-1717. doi:10.1001/jama.2020.3085
11. Papapanou M, Routsi E, Tsamakis K, et al. Medical education challenges and innovations during COVID-19 pandemic. Postgrad Med J. 2022;98(1159):321-327. doi:10.1136/postgradmedj-2021-140032
12. Hilburg R, Patel N, Ambruso S, Biewald MA, Farouk SS. Medical education during the Coronavirus Disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27(5):412-417. doi:10.1053/j.ackd.2020.05.017
13. Simpson D, Marcdante K, Souza KH, Anderson A, Holmboe E. Job roles of the 2025 medical educator. J Grad Med Educ. 2018;10(3):243-246. doi:10.4300/JGME-D-18-00253.1
14. Armstrong EG, Mackey M, Spear SJ. Medical education as a process management problem. Acad Med. 2004;79(8):721-728. doi:10.1097/00001888-200408000-00002
15. Srinivasan M, Li ST, Meyers FJ, et al. “Teaching as a Competency”: competencies for medical educators. Acad Med. 2011;86(10):1211-1220. doi:10.1097/ACM.0b013e31822c5b9a
16. Clark E, Freytag J, Hysong SJ, Dang B, Giordano TP, Kulkarni PA. 964. Impact of the COVID-19 pandemic on bedside medical education: a mixed-methods study. Open Forum Infect Dis. 2021;8(Suppl 1):S574. Published 2021 Dec 4. doi:10.1093/ofid/ofab466.1159
17. Ohnigian S, Richards JB, Monette DL, Roberts DH. optimizing remote learning: leveraging zoom to develop and implement successful education sessions. J Med Educ Curric Dev. 2021;8:23821205211020760. Published 2021 Jun 28. doi:10.1177/23821205211020760
18. Burgess A, Matar E, Neuen B, Fox GJ. A longitudinal faculty development program: supporting a culture of teaching. BMC Med Educ. 2019;19(1):400. Published 2019 Nov 1. doi:10.1186/s12909-019-1832-3
19. Stoddard HA, Brownfield ED. Clinician-educators as dual professionals: a contemporary reappraisal. Acad Med. 2016;91(7):921-924. doi:10.1097/ACM.0000000000001210
20. Schönwetter DJ, Hamilton J, Sawatzky JA. Exploring professional development needs of educators in the health sciences professions. J Dent Educ. 2015;79(2):113-123.
21. Meyer EM, Zapatka S, Brienza RS. The development of professional identity and the formation of teams in the Veterans Affairs Connecticut Healthcare System’s Center of Excellence in Primary Care Education Program (CoEPCE). Acad Med. 2015;90(6):802-809. doi:10.1097/ACM.0000000000000594
Surviving CLL: Higher risk of other cancer DXs
The report, which appeared in January in Blood Cancer Journal, found that patients diagnosed with CLL between 1989 and 2019 were 63% more likely to were diagnosed with SPM than a matched population: standardized incidence ratio = 1.63, 95% confidence interval (CI), 1.59-1.68.
“Our results provide patients and their treating physicians with an overview of the risk of SPM development. This information can be used in treatment decision-making and for planning appropriate surveillance activities and interventions,” study lead author Lina van der Straten, MD, PhD, of the Albert Schweitzer Hospital and Erasmus University Medical Center in the Netherlands, said in an interview.
Ohio State University hematologist David Bond, MD, who’s familiar with the findings, said in an interview that “it’s been well-established that patients with CLL are at increased risk for second primary malignancies. This is thought to be due to impaired immune surveillance and possibly carcinogenic effects of CLL treatments.” It’s not clear, he said, “whether the rate of second cancers differs between chemoimmunotherapy-treated patients and those receiving newer oral kinase inhibitors.”
Previous research into CLL and SPM has been sparse, Dr. van der Straten said, and most studies haven’t looked at SPM over time and taken into account the widespread use of chemoimmunotherapy and agents such as ibrutinib and venetoclax.
It’s important to study this topic, she said, since “cancers diagnosed after the CLL diagnosis can outweigh the improved longevity and contribute to excess morbidity and mortality in long-term CLL survivors.”
With the help of the Netherlands Cancer Registry, researchers tracked 24,815 patients with CLL who were diagnosed over the 20-year period; 4,369 developed SPM. “We demonstrated that the risk of SPM development was higher than in the general population with an excess of 125 malignancies per 10,000 person-years in the CLL cohort,” Dr. van der Straten said. “The risk of SPM development was found to be heightened in solid and hematological cancers. Patients with CLL had an increased risk of developing cancers at the following sites or types: skin, acute myeloid leukemia, soft-tissue sarcomas, thyroid, kidney, unknown primary localization, non-Hodgkin lymphomas, lung and bronchus, and colon and rectum.”
Specifically, the study reports that “elevated risk was observed for solid (SIR = 1.67; 95% CI, 1.65-1.75) and hematological SPMs (SIR = 1.42; 95% CI, 1.24-1.62). The highest risk for SPMs was noted beyond 5 years post diagnosis (SIR = 1.70; 95% CI, 1.62-1.77), for male individuals (SIR = 1.70; 95% CI, 1.64-1.77), and patients aged 18-69 years (SR = 1.92; 95% CI, 1.79-2.05).
“Patients with CLL exposed to treatment have a higher risk of SPM development than patients who will never receive therapy,” Dr. van der Straten said. Research has shown that “treatment with fludarabine, cyclophosphamide, and rituximab has been associated with a 2.38 increased risk for SPM development, particularly acute myeloid leukemia. Indeed, we found an increased risk for hematological malignancies in patients diagnosed between 2003-2009 and 2010-2019, which might be explained by the broader administration of fludarabine-based strategies in these calendar periods.”
Multiple factors could explain the higher risk of SPM in patients with CLL, including “a dysregulated immune system, treatment-related effects, and surveillance bias,” Dr. van der Straten said. “In addition, it is proposed that the immune dysfunctional nature of CLL might enhance the effect of common carcinogens, such as UV exposure and smoking, in increasing the probability of skin and respiratory cancers.”
She added that “the risk and the spectrum of SPMs were comparable for the 2003-2009 and 2010-2019 periods, suggesting that both the introduction of chemoimmunotherapy and, in part, targeted therapies did not dramatically alter the SPM landscape. However, due to the short follow-up period for the small cohort of patients receiving targeted therapies, further research is warranted.”
Dr. Bond said the findings “are largely in line with prior studies and strengthen their conclusions. Immune surveillance appears to be critical to reducing the risk for some but not all malignancies including lung cancer and melanoma, and the treatments given for CLL can cause immune suppression and thus may increase the risk.”
Moving forward, he said, “this research highlights the importance of second cancers to patients with CLL. It also highlights the need for secondary cancer screening for CLL patients, patient education to avoid known cancer risk factors including smoking and excess UV light exposure, and the need as a field to continue to invest in research into characteristics of second cancers and mitigation strategies.”
Study funding was not reported. The authors and Dr. Bond report no disclosures.
The report, which appeared in January in Blood Cancer Journal, found that patients diagnosed with CLL between 1989 and 2019 were 63% more likely to were diagnosed with SPM than a matched population: standardized incidence ratio = 1.63, 95% confidence interval (CI), 1.59-1.68.
“Our results provide patients and their treating physicians with an overview of the risk of SPM development. This information can be used in treatment decision-making and for planning appropriate surveillance activities and interventions,” study lead author Lina van der Straten, MD, PhD, of the Albert Schweitzer Hospital and Erasmus University Medical Center in the Netherlands, said in an interview.
Ohio State University hematologist David Bond, MD, who’s familiar with the findings, said in an interview that “it’s been well-established that patients with CLL are at increased risk for second primary malignancies. This is thought to be due to impaired immune surveillance and possibly carcinogenic effects of CLL treatments.” It’s not clear, he said, “whether the rate of second cancers differs between chemoimmunotherapy-treated patients and those receiving newer oral kinase inhibitors.”
Previous research into CLL and SPM has been sparse, Dr. van der Straten said, and most studies haven’t looked at SPM over time and taken into account the widespread use of chemoimmunotherapy and agents such as ibrutinib and venetoclax.
It’s important to study this topic, she said, since “cancers diagnosed after the CLL diagnosis can outweigh the improved longevity and contribute to excess morbidity and mortality in long-term CLL survivors.”
With the help of the Netherlands Cancer Registry, researchers tracked 24,815 patients with CLL who were diagnosed over the 20-year period; 4,369 developed SPM. “We demonstrated that the risk of SPM development was higher than in the general population with an excess of 125 malignancies per 10,000 person-years in the CLL cohort,” Dr. van der Straten said. “The risk of SPM development was found to be heightened in solid and hematological cancers. Patients with CLL had an increased risk of developing cancers at the following sites or types: skin, acute myeloid leukemia, soft-tissue sarcomas, thyroid, kidney, unknown primary localization, non-Hodgkin lymphomas, lung and bronchus, and colon and rectum.”
Specifically, the study reports that “elevated risk was observed for solid (SIR = 1.67; 95% CI, 1.65-1.75) and hematological SPMs (SIR = 1.42; 95% CI, 1.24-1.62). The highest risk for SPMs was noted beyond 5 years post diagnosis (SIR = 1.70; 95% CI, 1.62-1.77), for male individuals (SIR = 1.70; 95% CI, 1.64-1.77), and patients aged 18-69 years (SR = 1.92; 95% CI, 1.79-2.05).
“Patients with CLL exposed to treatment have a higher risk of SPM development than patients who will never receive therapy,” Dr. van der Straten said. Research has shown that “treatment with fludarabine, cyclophosphamide, and rituximab has been associated with a 2.38 increased risk for SPM development, particularly acute myeloid leukemia. Indeed, we found an increased risk for hematological malignancies in patients diagnosed between 2003-2009 and 2010-2019, which might be explained by the broader administration of fludarabine-based strategies in these calendar periods.”
Multiple factors could explain the higher risk of SPM in patients with CLL, including “a dysregulated immune system, treatment-related effects, and surveillance bias,” Dr. van der Straten said. “In addition, it is proposed that the immune dysfunctional nature of CLL might enhance the effect of common carcinogens, such as UV exposure and smoking, in increasing the probability of skin and respiratory cancers.”
She added that “the risk and the spectrum of SPMs were comparable for the 2003-2009 and 2010-2019 periods, suggesting that both the introduction of chemoimmunotherapy and, in part, targeted therapies did not dramatically alter the SPM landscape. However, due to the short follow-up period for the small cohort of patients receiving targeted therapies, further research is warranted.”
Dr. Bond said the findings “are largely in line with prior studies and strengthen their conclusions. Immune surveillance appears to be critical to reducing the risk for some but not all malignancies including lung cancer and melanoma, and the treatments given for CLL can cause immune suppression and thus may increase the risk.”
Moving forward, he said, “this research highlights the importance of second cancers to patients with CLL. It also highlights the need for secondary cancer screening for CLL patients, patient education to avoid known cancer risk factors including smoking and excess UV light exposure, and the need as a field to continue to invest in research into characteristics of second cancers and mitigation strategies.”
Study funding was not reported. The authors and Dr. Bond report no disclosures.
The report, which appeared in January in Blood Cancer Journal, found that patients diagnosed with CLL between 1989 and 2019 were 63% more likely to were diagnosed with SPM than a matched population: standardized incidence ratio = 1.63, 95% confidence interval (CI), 1.59-1.68.
“Our results provide patients and their treating physicians with an overview of the risk of SPM development. This information can be used in treatment decision-making and for planning appropriate surveillance activities and interventions,” study lead author Lina van der Straten, MD, PhD, of the Albert Schweitzer Hospital and Erasmus University Medical Center in the Netherlands, said in an interview.
Ohio State University hematologist David Bond, MD, who’s familiar with the findings, said in an interview that “it’s been well-established that patients with CLL are at increased risk for second primary malignancies. This is thought to be due to impaired immune surveillance and possibly carcinogenic effects of CLL treatments.” It’s not clear, he said, “whether the rate of second cancers differs between chemoimmunotherapy-treated patients and those receiving newer oral kinase inhibitors.”
Previous research into CLL and SPM has been sparse, Dr. van der Straten said, and most studies haven’t looked at SPM over time and taken into account the widespread use of chemoimmunotherapy and agents such as ibrutinib and venetoclax.
It’s important to study this topic, she said, since “cancers diagnosed after the CLL diagnosis can outweigh the improved longevity and contribute to excess morbidity and mortality in long-term CLL survivors.”
With the help of the Netherlands Cancer Registry, researchers tracked 24,815 patients with CLL who were diagnosed over the 20-year period; 4,369 developed SPM. “We demonstrated that the risk of SPM development was higher than in the general population with an excess of 125 malignancies per 10,000 person-years in the CLL cohort,” Dr. van der Straten said. “The risk of SPM development was found to be heightened in solid and hematological cancers. Patients with CLL had an increased risk of developing cancers at the following sites or types: skin, acute myeloid leukemia, soft-tissue sarcomas, thyroid, kidney, unknown primary localization, non-Hodgkin lymphomas, lung and bronchus, and colon and rectum.”
Specifically, the study reports that “elevated risk was observed for solid (SIR = 1.67; 95% CI, 1.65-1.75) and hematological SPMs (SIR = 1.42; 95% CI, 1.24-1.62). The highest risk for SPMs was noted beyond 5 years post diagnosis (SIR = 1.70; 95% CI, 1.62-1.77), for male individuals (SIR = 1.70; 95% CI, 1.64-1.77), and patients aged 18-69 years (SR = 1.92; 95% CI, 1.79-2.05).
“Patients with CLL exposed to treatment have a higher risk of SPM development than patients who will never receive therapy,” Dr. van der Straten said. Research has shown that “treatment with fludarabine, cyclophosphamide, and rituximab has been associated with a 2.38 increased risk for SPM development, particularly acute myeloid leukemia. Indeed, we found an increased risk for hematological malignancies in patients diagnosed between 2003-2009 and 2010-2019, which might be explained by the broader administration of fludarabine-based strategies in these calendar periods.”
Multiple factors could explain the higher risk of SPM in patients with CLL, including “a dysregulated immune system, treatment-related effects, and surveillance bias,” Dr. van der Straten said. “In addition, it is proposed that the immune dysfunctional nature of CLL might enhance the effect of common carcinogens, such as UV exposure and smoking, in increasing the probability of skin and respiratory cancers.”
She added that “the risk and the spectrum of SPMs were comparable for the 2003-2009 and 2010-2019 periods, suggesting that both the introduction of chemoimmunotherapy and, in part, targeted therapies did not dramatically alter the SPM landscape. However, due to the short follow-up period for the small cohort of patients receiving targeted therapies, further research is warranted.”
Dr. Bond said the findings “are largely in line with prior studies and strengthen their conclusions. Immune surveillance appears to be critical to reducing the risk for some but not all malignancies including lung cancer and melanoma, and the treatments given for CLL can cause immune suppression and thus may increase the risk.”
Moving forward, he said, “this research highlights the importance of second cancers to patients with CLL. It also highlights the need for secondary cancer screening for CLL patients, patient education to avoid known cancer risk factors including smoking and excess UV light exposure, and the need as a field to continue to invest in research into characteristics of second cancers and mitigation strategies.”
Study funding was not reported. The authors and Dr. Bond report no disclosures.
FROM BLOOD CANCER JOURNAL
Strategy to reduce peritoneal metastases in gastric cancer
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- Surgery and postoperative chemotherapy are standard of care for advanced gastric cancer, but up to half of patients develop peritoneal metastases with poor prognosis.
- There is no consensus on how to prevent peritoneal metastases.
- With hyperthermic intraperitoneal chemotherapy, the abdominal cavity is bathed in chemotherapy that has been heated, directly killing free cancer cells and micrometastases.
- The findings suggest that adding hyperthermic intraperitoneal chemotherapy to standard treatment greatly reduces the risk of peritoneal metastases.
Study design
- The investigators randomly assigned 134 patients with advanced gastric cancer evenly to receive either systemic chemotherapy alone or systemic chemotherapy plus hyperthermic intraperitoneal chemotherapy after radical gastrectomy.
- The hyperthermic intraperitoneal chemotherapy group had 3 L of heated saline containing 40 mg/m2 of cisplatin circulated in their peritoneal cavities for an hour. The procedure was performed twice within 72 hours of surgery.
- Systemic chemotherapy consisted of six to eight cycles of S-1 combined with oxaliplatin (SOX regimen) starting 4-6 weeks after surgery.
- Most patients (90%) had stage III disease, and the rest stage II.
- Median follow-up was 44 months.
Key results
- Overall, the 3-year DFS rate was 73.8% with hyperthermic intraperitoneal chemotherapy versus 61.2% without it (P = .031).
- In addition, 21% of patients in the hyperthermic intraperitoneal chemotherapy group developed peritoneal metastases versus 40.3% with standard care (P = .015)
- The 3-year overall survival was 73.9% in the hyperthermic intraperitoneal chemotherapy group versus 77.6% in the standard care arm, but the difference was not significant (P = .737).
- There were no serious adverse events related to hyperthermic intraperitoneal chemotherapy, and postoperative complications were similar between the groups.
- Grade 3 or 4 adverse events occurred in 14.2% of patients; there were no statistically significant between-group differences.
- Metastases to other sites, such as the liver and distant lymph nodes, were also similar between the two arms.
Limitations
- Follow-up might have been too short to detect a difference in overall survival.
- The trial was conducted at a single-center and was relatively small.
Disclosures
- The study received no external funding, and the investigators did not report any financial relationships.
This is a summary of a preprint research study, “Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Plus Systemic Chemotherapy Versus Systemic Chemotherapy Alone in Locally Advanced Gastric Cancer After D2 Radical Resection: A Randomized Controlled Study,” led by Pengfei Yu of the Zhejiang Cancer Hospital, Hangzhou, China. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- Surgery and postoperative chemotherapy are standard of care for advanced gastric cancer, but up to half of patients develop peritoneal metastases with poor prognosis.
- There is no consensus on how to prevent peritoneal metastases.
- With hyperthermic intraperitoneal chemotherapy, the abdominal cavity is bathed in chemotherapy that has been heated, directly killing free cancer cells and micrometastases.
- The findings suggest that adding hyperthermic intraperitoneal chemotherapy to standard treatment greatly reduces the risk of peritoneal metastases.
Study design
- The investigators randomly assigned 134 patients with advanced gastric cancer evenly to receive either systemic chemotherapy alone or systemic chemotherapy plus hyperthermic intraperitoneal chemotherapy after radical gastrectomy.
- The hyperthermic intraperitoneal chemotherapy group had 3 L of heated saline containing 40 mg/m2 of cisplatin circulated in their peritoneal cavities for an hour. The procedure was performed twice within 72 hours of surgery.
- Systemic chemotherapy consisted of six to eight cycles of S-1 combined with oxaliplatin (SOX regimen) starting 4-6 weeks after surgery.
- Most patients (90%) had stage III disease, and the rest stage II.
- Median follow-up was 44 months.
Key results
- Overall, the 3-year DFS rate was 73.8% with hyperthermic intraperitoneal chemotherapy versus 61.2% without it (P = .031).
- In addition, 21% of patients in the hyperthermic intraperitoneal chemotherapy group developed peritoneal metastases versus 40.3% with standard care (P = .015)
- The 3-year overall survival was 73.9% in the hyperthermic intraperitoneal chemotherapy group versus 77.6% in the standard care arm, but the difference was not significant (P = .737).
- There were no serious adverse events related to hyperthermic intraperitoneal chemotherapy, and postoperative complications were similar between the groups.
- Grade 3 or 4 adverse events occurred in 14.2% of patients; there were no statistically significant between-group differences.
- Metastases to other sites, such as the liver and distant lymph nodes, were also similar between the two arms.
Limitations
- Follow-up might have been too short to detect a difference in overall survival.
- The trial was conducted at a single-center and was relatively small.
Disclosures
- The study received no external funding, and the investigators did not report any financial relationships.
This is a summary of a preprint research study, “Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Plus Systemic Chemotherapy Versus Systemic Chemotherapy Alone in Locally Advanced Gastric Cancer After D2 Radical Resection: A Randomized Controlled Study,” led by Pengfei Yu of the Zhejiang Cancer Hospital, Hangzhou, China. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
The study covered in this summary was published on researchsquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
Why this matters
- Surgery and postoperative chemotherapy are standard of care for advanced gastric cancer, but up to half of patients develop peritoneal metastases with poor prognosis.
- There is no consensus on how to prevent peritoneal metastases.
- With hyperthermic intraperitoneal chemotherapy, the abdominal cavity is bathed in chemotherapy that has been heated, directly killing free cancer cells and micrometastases.
- The findings suggest that adding hyperthermic intraperitoneal chemotherapy to standard treatment greatly reduces the risk of peritoneal metastases.
Study design
- The investigators randomly assigned 134 patients with advanced gastric cancer evenly to receive either systemic chemotherapy alone or systemic chemotherapy plus hyperthermic intraperitoneal chemotherapy after radical gastrectomy.
- The hyperthermic intraperitoneal chemotherapy group had 3 L of heated saline containing 40 mg/m2 of cisplatin circulated in their peritoneal cavities for an hour. The procedure was performed twice within 72 hours of surgery.
- Systemic chemotherapy consisted of six to eight cycles of S-1 combined with oxaliplatin (SOX regimen) starting 4-6 weeks after surgery.
- Most patients (90%) had stage III disease, and the rest stage II.
- Median follow-up was 44 months.
Key results
- Overall, the 3-year DFS rate was 73.8% with hyperthermic intraperitoneal chemotherapy versus 61.2% without it (P = .031).
- In addition, 21% of patients in the hyperthermic intraperitoneal chemotherapy group developed peritoneal metastases versus 40.3% with standard care (P = .015)
- The 3-year overall survival was 73.9% in the hyperthermic intraperitoneal chemotherapy group versus 77.6% in the standard care arm, but the difference was not significant (P = .737).
- There were no serious adverse events related to hyperthermic intraperitoneal chemotherapy, and postoperative complications were similar between the groups.
- Grade 3 or 4 adverse events occurred in 14.2% of patients; there were no statistically significant between-group differences.
- Metastases to other sites, such as the liver and distant lymph nodes, were also similar between the two arms.
Limitations
- Follow-up might have been too short to detect a difference in overall survival.
- The trial was conducted at a single-center and was relatively small.
Disclosures
- The study received no external funding, and the investigators did not report any financial relationships.
This is a summary of a preprint research study, “Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Plus Systemic Chemotherapy Versus Systemic Chemotherapy Alone in Locally Advanced Gastric Cancer After D2 Radical Resection: A Randomized Controlled Study,” led by Pengfei Yu of the Zhejiang Cancer Hospital, Hangzhou, China. The study has not been peer reviewed. The full text can be found at researchsquare.com.
A version of this article first appeared on Medscape.com.
Factors linked to higher risk for death in young cancer survivors
according to new data from the St. Jude Lifetime Cohort.
Survivors with a greater number and severity of modifiable chronic health conditions as well as those living in the most versus least resource-deprived areas had a significantly higher risk of all-cause and health-related late death.
Finding ways to mitigate these factors “will be important to improving health outcomes and developing risk-stratification strategies to optimize care delivery to survivors at varying risk of adverse health events,” the researchers wrote.
The study indicates that treating chronic health conditions alone may not be enough to increase a cancer survivor’s lifespan; improving local environments matters too.
“It is important for clinicians to ask patients about their specific situation,” first author Matthew J. Ehrhardt, MD, department of oncology, St. Jude Children’s Research Hospital, Memphis, said in a news release. “It’s easy to prescribe medications or to tell people to exercise. It takes more time and more thoughtfulness to sit and understand environments in which they are residing.”
“As clinicians, we may have limited ability to modify some of those factors. But we can work closely with the rest of the health care team, such as social workers, for example, to help survivors to identify and access local resources,” Dr. Ehrhardt added.
The study was published online in JAMA Network Open.
A growing population of childhood cancer survivors faces an increased risk for premature death in the years following their diagnosis. However, associations between social determinants of health, modifiable health conditions, and late mortality in childhood cancer survivors remain unclear.
To assess late mortality, the study team analyzed data on 9,440 participants (median age at assessment, 27.5 years; range, 5.3-71.9 years) who lived at least 5 years after being diagnosed with a childhood cancer between 1962 and 2012.
During a median follow-up of about 18 years, childhood cancer survivors had an increased rate of both all-cause and health-related late mortality (standardized mortality rate, 7.6 for both). Among specific health-related causes of death, SMRs were 16.0 for subsequent neoplasms, 9.0 for pulmonary causes, 4.2 for cardiac causes, and 4.3 for other health-related causes.
To evaluate ties between modifiable chronic health conditions, social determinants, and late mortality, the researchers restricted their analysis to 3,407 adult study participants for whom relevant data were available. Modifiable chronic health conditions included dyslipidemia, hypertension, diabetes, underweight or obesity, bone mineral deficiency, and hypothyroidism.
After adjusting for individual factors, including age at diagnosis and treatment, as well as neighborhood-level factors, the researchers observed a significantly increased risk for death among survivors with one or more modifiable chronic health conditions of grade 2 or higher (relative risk, 2.2), two chronic health conditions of grade 2 or higher (RR, 2.6) or three chronic health conditions of grade 2 or higher (RR, 3.6).
These findings suggest that “increased late mortality experienced by childhood cancer survivors in adulthood may not be predetermined by treatment-related risk factors alone,” the researchers said.
In addition, survivors living in the most disadvantaged areas, as measured by the area deprivation index (ADI), had a five- to eightfold increased risk of late death from any cause compared with those living in the least disadvantaged areas, even after adjusting for modifiable chronic health conditions, cancer treatment, demographics, and individual socioeconomic factors.
The findings have important public health implications, Dr. Ehrhardt and colleagues said. The results can, for instance, help identify and stratify cancer survivors at higher lifetime risk for specific chronic conditions and late death.
This risk-stratified approach to care, however, is “relatively static” and does not account for risk factors acquired after cancer diagnosis and treatment, such as social determinants of health.
That is why also focusing on socioeconomic factors is important, and transitional care services following cancer treatment should consider that survivors in disadvantaged neighborhoods may lack supportive resources to address health issues, potentially leading to increased risk for death, the researchers said.
The knowledge that living in a resource-poor neighborhood may raise the risk for late death in childhood cancer survivors “strengthens support for public health policies that will direct resources to such regions and facilitate a multipronged approach to risk mitigation,” the authors concluded.
This study was supported by grants from the National Institutes of Health and the American Lebanese Syrian Associated Charities. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new data from the St. Jude Lifetime Cohort.
Survivors with a greater number and severity of modifiable chronic health conditions as well as those living in the most versus least resource-deprived areas had a significantly higher risk of all-cause and health-related late death.
Finding ways to mitigate these factors “will be important to improving health outcomes and developing risk-stratification strategies to optimize care delivery to survivors at varying risk of adverse health events,” the researchers wrote.
The study indicates that treating chronic health conditions alone may not be enough to increase a cancer survivor’s lifespan; improving local environments matters too.
“It is important for clinicians to ask patients about their specific situation,” first author Matthew J. Ehrhardt, MD, department of oncology, St. Jude Children’s Research Hospital, Memphis, said in a news release. “It’s easy to prescribe medications or to tell people to exercise. It takes more time and more thoughtfulness to sit and understand environments in which they are residing.”
“As clinicians, we may have limited ability to modify some of those factors. But we can work closely with the rest of the health care team, such as social workers, for example, to help survivors to identify and access local resources,” Dr. Ehrhardt added.
The study was published online in JAMA Network Open.
A growing population of childhood cancer survivors faces an increased risk for premature death in the years following their diagnosis. However, associations between social determinants of health, modifiable health conditions, and late mortality in childhood cancer survivors remain unclear.
To assess late mortality, the study team analyzed data on 9,440 participants (median age at assessment, 27.5 years; range, 5.3-71.9 years) who lived at least 5 years after being diagnosed with a childhood cancer between 1962 and 2012.
During a median follow-up of about 18 years, childhood cancer survivors had an increased rate of both all-cause and health-related late mortality (standardized mortality rate, 7.6 for both). Among specific health-related causes of death, SMRs were 16.0 for subsequent neoplasms, 9.0 for pulmonary causes, 4.2 for cardiac causes, and 4.3 for other health-related causes.
To evaluate ties between modifiable chronic health conditions, social determinants, and late mortality, the researchers restricted their analysis to 3,407 adult study participants for whom relevant data were available. Modifiable chronic health conditions included dyslipidemia, hypertension, diabetes, underweight or obesity, bone mineral deficiency, and hypothyroidism.
After adjusting for individual factors, including age at diagnosis and treatment, as well as neighborhood-level factors, the researchers observed a significantly increased risk for death among survivors with one or more modifiable chronic health conditions of grade 2 or higher (relative risk, 2.2), two chronic health conditions of grade 2 or higher (RR, 2.6) or three chronic health conditions of grade 2 or higher (RR, 3.6).
These findings suggest that “increased late mortality experienced by childhood cancer survivors in adulthood may not be predetermined by treatment-related risk factors alone,” the researchers said.
In addition, survivors living in the most disadvantaged areas, as measured by the area deprivation index (ADI), had a five- to eightfold increased risk of late death from any cause compared with those living in the least disadvantaged areas, even after adjusting for modifiable chronic health conditions, cancer treatment, demographics, and individual socioeconomic factors.
The findings have important public health implications, Dr. Ehrhardt and colleagues said. The results can, for instance, help identify and stratify cancer survivors at higher lifetime risk for specific chronic conditions and late death.
This risk-stratified approach to care, however, is “relatively static” and does not account for risk factors acquired after cancer diagnosis and treatment, such as social determinants of health.
That is why also focusing on socioeconomic factors is important, and transitional care services following cancer treatment should consider that survivors in disadvantaged neighborhoods may lack supportive resources to address health issues, potentially leading to increased risk for death, the researchers said.
The knowledge that living in a resource-poor neighborhood may raise the risk for late death in childhood cancer survivors “strengthens support for public health policies that will direct resources to such regions and facilitate a multipronged approach to risk mitigation,” the authors concluded.
This study was supported by grants from the National Institutes of Health and the American Lebanese Syrian Associated Charities. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new data from the St. Jude Lifetime Cohort.
Survivors with a greater number and severity of modifiable chronic health conditions as well as those living in the most versus least resource-deprived areas had a significantly higher risk of all-cause and health-related late death.
Finding ways to mitigate these factors “will be important to improving health outcomes and developing risk-stratification strategies to optimize care delivery to survivors at varying risk of adverse health events,” the researchers wrote.
The study indicates that treating chronic health conditions alone may not be enough to increase a cancer survivor’s lifespan; improving local environments matters too.
“It is important for clinicians to ask patients about their specific situation,” first author Matthew J. Ehrhardt, MD, department of oncology, St. Jude Children’s Research Hospital, Memphis, said in a news release. “It’s easy to prescribe medications or to tell people to exercise. It takes more time and more thoughtfulness to sit and understand environments in which they are residing.”
“As clinicians, we may have limited ability to modify some of those factors. But we can work closely with the rest of the health care team, such as social workers, for example, to help survivors to identify and access local resources,” Dr. Ehrhardt added.
The study was published online in JAMA Network Open.
A growing population of childhood cancer survivors faces an increased risk for premature death in the years following their diagnosis. However, associations between social determinants of health, modifiable health conditions, and late mortality in childhood cancer survivors remain unclear.
To assess late mortality, the study team analyzed data on 9,440 participants (median age at assessment, 27.5 years; range, 5.3-71.9 years) who lived at least 5 years after being diagnosed with a childhood cancer between 1962 and 2012.
During a median follow-up of about 18 years, childhood cancer survivors had an increased rate of both all-cause and health-related late mortality (standardized mortality rate, 7.6 for both). Among specific health-related causes of death, SMRs were 16.0 for subsequent neoplasms, 9.0 for pulmonary causes, 4.2 for cardiac causes, and 4.3 for other health-related causes.
To evaluate ties between modifiable chronic health conditions, social determinants, and late mortality, the researchers restricted their analysis to 3,407 adult study participants for whom relevant data were available. Modifiable chronic health conditions included dyslipidemia, hypertension, diabetes, underweight or obesity, bone mineral deficiency, and hypothyroidism.
After adjusting for individual factors, including age at diagnosis and treatment, as well as neighborhood-level factors, the researchers observed a significantly increased risk for death among survivors with one or more modifiable chronic health conditions of grade 2 or higher (relative risk, 2.2), two chronic health conditions of grade 2 or higher (RR, 2.6) or three chronic health conditions of grade 2 or higher (RR, 3.6).
These findings suggest that “increased late mortality experienced by childhood cancer survivors in adulthood may not be predetermined by treatment-related risk factors alone,” the researchers said.
In addition, survivors living in the most disadvantaged areas, as measured by the area deprivation index (ADI), had a five- to eightfold increased risk of late death from any cause compared with those living in the least disadvantaged areas, even after adjusting for modifiable chronic health conditions, cancer treatment, demographics, and individual socioeconomic factors.
The findings have important public health implications, Dr. Ehrhardt and colleagues said. The results can, for instance, help identify and stratify cancer survivors at higher lifetime risk for specific chronic conditions and late death.
This risk-stratified approach to care, however, is “relatively static” and does not account for risk factors acquired after cancer diagnosis and treatment, such as social determinants of health.
That is why also focusing on socioeconomic factors is important, and transitional care services following cancer treatment should consider that survivors in disadvantaged neighborhoods may lack supportive resources to address health issues, potentially leading to increased risk for death, the researchers said.
The knowledge that living in a resource-poor neighborhood may raise the risk for late death in childhood cancer survivors “strengthens support for public health policies that will direct resources to such regions and facilitate a multipronged approach to risk mitigation,” the authors concluded.
This study was supported by grants from the National Institutes of Health and the American Lebanese Syrian Associated Charities. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Endovascular therapy benefits large infarction: ANGEL-ASPECT
The trial was stopped early because a planned interim analysis showed efficacy of endovascular therapy in this patient population.
Among patients in China with acute ischemic stroke and a large cerebral infarction, treatment with endovascular therapy within 24 hours after stroke onset “resulted in a better functional outcome at 3 months than medical management alone,” lead author Xiaochuan Huo, MD, PhD, associate chief physician, interventional neurology department, Beijing Tiantan Hospital, Capital Medical University, told this news organization.
“This trial added important evidence for the benefits of endovascular therapy,” Dr. Huo added.
The findings were presented at the International Stroke Conference and were published online in The New England Journal of Medicine. The conference was presented by the American Stroke Association, a division of the American Heart Association.
Will change practice
Commenting on the results, Tudor G. Jovin, MD, professor and chair, department of neurology, Cooper Medical School of Rowan University, Camden, N.J., said he has “little doubt” this study will change practice.
Despite previous studies showing signals of benefit from thrombectomy for patients with large-core infarcts, and some even finding a large treatment effect, “somehow the world didn’t register this,” said Dr. Jovin.
“The stroke community was perhaps reluctant to accept these signals that were there in plain sight because we have been primed for such a long time that reperfusing large infarcts was, if not detrimental, not beneficial.”
But this study, along with another study showing similar results, SELECT 2, which was also presented at this meeting and was published in the same issue of NEJM, provide “overwhelming proof” and “have finally made the community aware,” said Dr. Jovin. “This is sort of a wake-up call to say, ‘Hey, this is real; patients with large infarcts also benefit from thrombectomy.’ “
This new research suggests it’s not necessary to learn the infarct size, at least in the early time window, and doing so just wastes precious time, added Dr. Jovin.
The impact of thrombectomy on patients with “super large infarcts” is still not clear, although these are “extremely rare” in the early time window, perhaps representing only about 1% of patients, said Dr. Jovin.
The increased rate of hemorrhages in study patients receiving thrombectomy “is the price you pay” for the benefits, he said. He noted that this is not any different from the situation with tissue plasminogen activator (tPA), which is routinely used because the benefits far outweigh the risks.
ANGEL-ASPECT
As patients with large infarctions are generally excluded from studies of thrombectomy, it’s been unclear whether they benefit from this therapy, the researchers noted.
The multicenter Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core (ANGEL-ASPECT) trial included 455 adult patients (median age, 68 years; 38.7% women) who had a large infarct core caused by acute large-vessel occlusion in the anterior circulation (Alberta Stroke Program Early CT Score [ASPECTS] 3-5 without core volume limitations or ASPECTS 0–2 with core volume between 70 and 100 mL).
Study participants had to have a score of 6-30 on the National Institutes of Health Stroke Scale (NIHSS) and a retrospectively determined prestroke score of 0 or 1 on the Modified Rankin Scale (mRS).
The median baseline NIHSS score of study patients was 16, the median ASPECTS was 3, and the median infarct-core volume was 62 mL.
Researchers randomly assigned patients to undergo either medical management alone or medical management as well as endovascular therapy. Medical management included intravenous (IV) thrombolysis for those who were eligible.
IV thrombolysis was administered before thrombectomy for about 28% of patients in each group. Some 78.7% of all patients arrived at the hospital outside the typical 4.5-hour window and were ineligible for thrombolysis.
A greater percentage of patients in the endovascular therapy group was receiving antihypertensive medications (83.0%) than in the medical management alone group (54.0%). About 20% of patients in each group were taking an anticoagulant medication.
When the trial was halted, outcome data were available for 336 patients. An additional 120 patients had undergone randomization, and 455 had completed 90 days of follow-up.
Better functional outcome
The primary outcome was the score on the mRS at 90 days. Results showed a shift in the distribution of scores on the mRS at 90 days toward better outcomes favoring endovascular therapy over medical management alone (generalized odds ratio, 1.37; 95% confidence interval [CI], 1.11-1.69; P = .004).
The efficacy of endovascular therapy with respect to the primary outcome was similar across predefined subgroups and across all trial sites. However, the trial was not powered to allow definite conclusions based on the results of subgroup analyses.
Although patients with an ASPECT score of 0-2 (indicating very large infarct cores) are considered unlikely to benefit from endovascular treatment, the researchers did find some signals of gain for these patients.
“Although no conclusions can be drawn because the trial was not powered for this analysis and the confidence interval for the odds ratio between the trial groups included 1, there may have been a benefit with endovascular therapy in this subgroup,” the authors wrote. “More trials are warranted to determine if this benefit is valid.”
As for secondary outcomes, the percentage of patients with a score of 0-2 on the mRS at 90 days was 30.0% in the endovascular therapy group and 11.6% in the medical management group (relative risk [RR], 2.62; 95% CI, 1.69-4.06).
The percentage of patients with a score of 0-3 on the mRS at 90 days was 47.0% in the endovascular therapy group and 33.3% in the medical management group (RR, 1.50; 95% CI, 1.17-1.91).
The primary safety outcome was symptomatic intracranial hemorrhage within 48 hours, which occurred in 6.1% of the endovascular therapy group, compared to 2.7% in the medical management group (RR, 2.07; 95% CI, 0.79-5.41; P = .12)
Mortality within 90 days was 21.7% in the endovascular therapy group and 20.0% in the medical management group. Other serious adverse events occurred in 40.0% in the endovascular therapy group and 38.2% in the medical management group (P = .70).
The percentage of patients receiving IV thrombolysis was relatively low, which may have affected outcomes in the medical management group. Another potential limitation was that urokinase rather than alteplase, which is probably more effective, was used for thrombolysis in a small percentage of patients.
Further, the study did not include patients older than 80 years or those with an ASPECT value greater than 5 and infarct core volume of 70-100 mL, and it included only Chinese patients, so the results may not be generalizable, the researchers noted.
These findings will likely change clinical practice, said Dr. Huo, who noted that the current guideline doesn’t provide “a high-level recommendation” for [endovascular therapy] in patients with a low ASPECT score.
“These new results will change the guideline” to suggest endovascular therapy for large-core patients, he said.
Welcome news
An accompanying editorial by Pierre Fayad, MD, department of neurological sciences, division of vascular neurology and stroke, University of Nebraska Medical Center, Omaha, welcomed results from this and other recent related studies.
From these new results, “it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes” if they arrive in a timely fashion at a center capable of performing the procedure and have an ASPECT value of 3-5 or an ischemic-core volume of 50 mL or greater, he wrote.
“The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment.”
The study received funding from Covidien Healthcare International Trading (Shanghai), Johnson & Johnson MedTech, Genesis MedTech (Shanghai), and Shanghai HeartCare Medical Technology. Dr. Huo and Dr. Jovin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial was stopped early because a planned interim analysis showed efficacy of endovascular therapy in this patient population.
Among patients in China with acute ischemic stroke and a large cerebral infarction, treatment with endovascular therapy within 24 hours after stroke onset “resulted in a better functional outcome at 3 months than medical management alone,” lead author Xiaochuan Huo, MD, PhD, associate chief physician, interventional neurology department, Beijing Tiantan Hospital, Capital Medical University, told this news organization.
“This trial added important evidence for the benefits of endovascular therapy,” Dr. Huo added.
The findings were presented at the International Stroke Conference and were published online in The New England Journal of Medicine. The conference was presented by the American Stroke Association, a division of the American Heart Association.
Will change practice
Commenting on the results, Tudor G. Jovin, MD, professor and chair, department of neurology, Cooper Medical School of Rowan University, Camden, N.J., said he has “little doubt” this study will change practice.
Despite previous studies showing signals of benefit from thrombectomy for patients with large-core infarcts, and some even finding a large treatment effect, “somehow the world didn’t register this,” said Dr. Jovin.
“The stroke community was perhaps reluctant to accept these signals that were there in plain sight because we have been primed for such a long time that reperfusing large infarcts was, if not detrimental, not beneficial.”
But this study, along with another study showing similar results, SELECT 2, which was also presented at this meeting and was published in the same issue of NEJM, provide “overwhelming proof” and “have finally made the community aware,” said Dr. Jovin. “This is sort of a wake-up call to say, ‘Hey, this is real; patients with large infarcts also benefit from thrombectomy.’ “
This new research suggests it’s not necessary to learn the infarct size, at least in the early time window, and doing so just wastes precious time, added Dr. Jovin.
The impact of thrombectomy on patients with “super large infarcts” is still not clear, although these are “extremely rare” in the early time window, perhaps representing only about 1% of patients, said Dr. Jovin.
The increased rate of hemorrhages in study patients receiving thrombectomy “is the price you pay” for the benefits, he said. He noted that this is not any different from the situation with tissue plasminogen activator (tPA), which is routinely used because the benefits far outweigh the risks.
ANGEL-ASPECT
As patients with large infarctions are generally excluded from studies of thrombectomy, it’s been unclear whether they benefit from this therapy, the researchers noted.
The multicenter Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core (ANGEL-ASPECT) trial included 455 adult patients (median age, 68 years; 38.7% women) who had a large infarct core caused by acute large-vessel occlusion in the anterior circulation (Alberta Stroke Program Early CT Score [ASPECTS] 3-5 without core volume limitations or ASPECTS 0–2 with core volume between 70 and 100 mL).
Study participants had to have a score of 6-30 on the National Institutes of Health Stroke Scale (NIHSS) and a retrospectively determined prestroke score of 0 or 1 on the Modified Rankin Scale (mRS).
The median baseline NIHSS score of study patients was 16, the median ASPECTS was 3, and the median infarct-core volume was 62 mL.
Researchers randomly assigned patients to undergo either medical management alone or medical management as well as endovascular therapy. Medical management included intravenous (IV) thrombolysis for those who were eligible.
IV thrombolysis was administered before thrombectomy for about 28% of patients in each group. Some 78.7% of all patients arrived at the hospital outside the typical 4.5-hour window and were ineligible for thrombolysis.
A greater percentage of patients in the endovascular therapy group was receiving antihypertensive medications (83.0%) than in the medical management alone group (54.0%). About 20% of patients in each group were taking an anticoagulant medication.
When the trial was halted, outcome data were available for 336 patients. An additional 120 patients had undergone randomization, and 455 had completed 90 days of follow-up.
Better functional outcome
The primary outcome was the score on the mRS at 90 days. Results showed a shift in the distribution of scores on the mRS at 90 days toward better outcomes favoring endovascular therapy over medical management alone (generalized odds ratio, 1.37; 95% confidence interval [CI], 1.11-1.69; P = .004).
The efficacy of endovascular therapy with respect to the primary outcome was similar across predefined subgroups and across all trial sites. However, the trial was not powered to allow definite conclusions based on the results of subgroup analyses.
Although patients with an ASPECT score of 0-2 (indicating very large infarct cores) are considered unlikely to benefit from endovascular treatment, the researchers did find some signals of gain for these patients.
“Although no conclusions can be drawn because the trial was not powered for this analysis and the confidence interval for the odds ratio between the trial groups included 1, there may have been a benefit with endovascular therapy in this subgroup,” the authors wrote. “More trials are warranted to determine if this benefit is valid.”
As for secondary outcomes, the percentage of patients with a score of 0-2 on the mRS at 90 days was 30.0% in the endovascular therapy group and 11.6% in the medical management group (relative risk [RR], 2.62; 95% CI, 1.69-4.06).
The percentage of patients with a score of 0-3 on the mRS at 90 days was 47.0% in the endovascular therapy group and 33.3% in the medical management group (RR, 1.50; 95% CI, 1.17-1.91).
The primary safety outcome was symptomatic intracranial hemorrhage within 48 hours, which occurred in 6.1% of the endovascular therapy group, compared to 2.7% in the medical management group (RR, 2.07; 95% CI, 0.79-5.41; P = .12)
Mortality within 90 days was 21.7% in the endovascular therapy group and 20.0% in the medical management group. Other serious adverse events occurred in 40.0% in the endovascular therapy group and 38.2% in the medical management group (P = .70).
The percentage of patients receiving IV thrombolysis was relatively low, which may have affected outcomes in the medical management group. Another potential limitation was that urokinase rather than alteplase, which is probably more effective, was used for thrombolysis in a small percentage of patients.
Further, the study did not include patients older than 80 years or those with an ASPECT value greater than 5 and infarct core volume of 70-100 mL, and it included only Chinese patients, so the results may not be generalizable, the researchers noted.
These findings will likely change clinical practice, said Dr. Huo, who noted that the current guideline doesn’t provide “a high-level recommendation” for [endovascular therapy] in patients with a low ASPECT score.
“These new results will change the guideline” to suggest endovascular therapy for large-core patients, he said.
Welcome news
An accompanying editorial by Pierre Fayad, MD, department of neurological sciences, division of vascular neurology and stroke, University of Nebraska Medical Center, Omaha, welcomed results from this and other recent related studies.
From these new results, “it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes” if they arrive in a timely fashion at a center capable of performing the procedure and have an ASPECT value of 3-5 or an ischemic-core volume of 50 mL or greater, he wrote.
“The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment.”
The study received funding from Covidien Healthcare International Trading (Shanghai), Johnson & Johnson MedTech, Genesis MedTech (Shanghai), and Shanghai HeartCare Medical Technology. Dr. Huo and Dr. Jovin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial was stopped early because a planned interim analysis showed efficacy of endovascular therapy in this patient population.
Among patients in China with acute ischemic stroke and a large cerebral infarction, treatment with endovascular therapy within 24 hours after stroke onset “resulted in a better functional outcome at 3 months than medical management alone,” lead author Xiaochuan Huo, MD, PhD, associate chief physician, interventional neurology department, Beijing Tiantan Hospital, Capital Medical University, told this news organization.
“This trial added important evidence for the benefits of endovascular therapy,” Dr. Huo added.
The findings were presented at the International Stroke Conference and were published online in The New England Journal of Medicine. The conference was presented by the American Stroke Association, a division of the American Heart Association.
Will change practice
Commenting on the results, Tudor G. Jovin, MD, professor and chair, department of neurology, Cooper Medical School of Rowan University, Camden, N.J., said he has “little doubt” this study will change practice.
Despite previous studies showing signals of benefit from thrombectomy for patients with large-core infarcts, and some even finding a large treatment effect, “somehow the world didn’t register this,” said Dr. Jovin.
“The stroke community was perhaps reluctant to accept these signals that were there in plain sight because we have been primed for such a long time that reperfusing large infarcts was, if not detrimental, not beneficial.”
But this study, along with another study showing similar results, SELECT 2, which was also presented at this meeting and was published in the same issue of NEJM, provide “overwhelming proof” and “have finally made the community aware,” said Dr. Jovin. “This is sort of a wake-up call to say, ‘Hey, this is real; patients with large infarcts also benefit from thrombectomy.’ “
This new research suggests it’s not necessary to learn the infarct size, at least in the early time window, and doing so just wastes precious time, added Dr. Jovin.
The impact of thrombectomy on patients with “super large infarcts” is still not clear, although these are “extremely rare” in the early time window, perhaps representing only about 1% of patients, said Dr. Jovin.
The increased rate of hemorrhages in study patients receiving thrombectomy “is the price you pay” for the benefits, he said. He noted that this is not any different from the situation with tissue plasminogen activator (tPA), which is routinely used because the benefits far outweigh the risks.
ANGEL-ASPECT
As patients with large infarctions are generally excluded from studies of thrombectomy, it’s been unclear whether they benefit from this therapy, the researchers noted.
The multicenter Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core (ANGEL-ASPECT) trial included 455 adult patients (median age, 68 years; 38.7% women) who had a large infarct core caused by acute large-vessel occlusion in the anterior circulation (Alberta Stroke Program Early CT Score [ASPECTS] 3-5 without core volume limitations or ASPECTS 0–2 with core volume between 70 and 100 mL).
Study participants had to have a score of 6-30 on the National Institutes of Health Stroke Scale (NIHSS) and a retrospectively determined prestroke score of 0 or 1 on the Modified Rankin Scale (mRS).
The median baseline NIHSS score of study patients was 16, the median ASPECTS was 3, and the median infarct-core volume was 62 mL.
Researchers randomly assigned patients to undergo either medical management alone or medical management as well as endovascular therapy. Medical management included intravenous (IV) thrombolysis for those who were eligible.
IV thrombolysis was administered before thrombectomy for about 28% of patients in each group. Some 78.7% of all patients arrived at the hospital outside the typical 4.5-hour window and were ineligible for thrombolysis.
A greater percentage of patients in the endovascular therapy group was receiving antihypertensive medications (83.0%) than in the medical management alone group (54.0%). About 20% of patients in each group were taking an anticoagulant medication.
When the trial was halted, outcome data were available for 336 patients. An additional 120 patients had undergone randomization, and 455 had completed 90 days of follow-up.
Better functional outcome
The primary outcome was the score on the mRS at 90 days. Results showed a shift in the distribution of scores on the mRS at 90 days toward better outcomes favoring endovascular therapy over medical management alone (generalized odds ratio, 1.37; 95% confidence interval [CI], 1.11-1.69; P = .004).
The efficacy of endovascular therapy with respect to the primary outcome was similar across predefined subgroups and across all trial sites. However, the trial was not powered to allow definite conclusions based on the results of subgroup analyses.
Although patients with an ASPECT score of 0-2 (indicating very large infarct cores) are considered unlikely to benefit from endovascular treatment, the researchers did find some signals of gain for these patients.
“Although no conclusions can be drawn because the trial was not powered for this analysis and the confidence interval for the odds ratio between the trial groups included 1, there may have been a benefit with endovascular therapy in this subgroup,” the authors wrote. “More trials are warranted to determine if this benefit is valid.”
As for secondary outcomes, the percentage of patients with a score of 0-2 on the mRS at 90 days was 30.0% in the endovascular therapy group and 11.6% in the medical management group (relative risk [RR], 2.62; 95% CI, 1.69-4.06).
The percentage of patients with a score of 0-3 on the mRS at 90 days was 47.0% in the endovascular therapy group and 33.3% in the medical management group (RR, 1.50; 95% CI, 1.17-1.91).
The primary safety outcome was symptomatic intracranial hemorrhage within 48 hours, which occurred in 6.1% of the endovascular therapy group, compared to 2.7% in the medical management group (RR, 2.07; 95% CI, 0.79-5.41; P = .12)
Mortality within 90 days was 21.7% in the endovascular therapy group and 20.0% in the medical management group. Other serious adverse events occurred in 40.0% in the endovascular therapy group and 38.2% in the medical management group (P = .70).
The percentage of patients receiving IV thrombolysis was relatively low, which may have affected outcomes in the medical management group. Another potential limitation was that urokinase rather than alteplase, which is probably more effective, was used for thrombolysis in a small percentage of patients.
Further, the study did not include patients older than 80 years or those with an ASPECT value greater than 5 and infarct core volume of 70-100 mL, and it included only Chinese patients, so the results may not be generalizable, the researchers noted.
These findings will likely change clinical practice, said Dr. Huo, who noted that the current guideline doesn’t provide “a high-level recommendation” for [endovascular therapy] in patients with a low ASPECT score.
“These new results will change the guideline” to suggest endovascular therapy for large-core patients, he said.
Welcome news
An accompanying editorial by Pierre Fayad, MD, department of neurological sciences, division of vascular neurology and stroke, University of Nebraska Medical Center, Omaha, welcomed results from this and other recent related studies.
From these new results, “it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes” if they arrive in a timely fashion at a center capable of performing the procedure and have an ASPECT value of 3-5 or an ischemic-core volume of 50 mL or greater, he wrote.
“The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment.”
The study received funding from Covidien Healthcare International Trading (Shanghai), Johnson & Johnson MedTech, Genesis MedTech (Shanghai), and Shanghai HeartCare Medical Technology. Dr. Huo and Dr. Jovin report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Similar effect of early, late BP reduction in stroke: CATIS-2
in the CATIS-2 trial.
The trial was presented by Liping Liu, MD, Beijing Tiantan Hospital, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
“Antihypertensive treatment can be delayed for at least 7 days following ischemic stroke onset, unless there are severe acute comorbidities that demand emergency blood pressure reduction to prevent serious complications,” Dr. Liu concluded.
But he acknowledged that the optimal BP management strategy in these patients remains uncertain and should be the focus of future research.
Discussing the trial at an ISC 2023 Highlights session, Lauren Sansing, MD, Yale University, New Haven, Conn., and ISC program vice chair, said: “These results seem to support waiting for a week or so before treating blood pressure in these patients.”
But Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and ISC program chair, countered: “To me, it’s kind of a neutral result, so what I take home from this is that you don’t necessarily have to wait.”
Dr. Jovin continued: “We used to think that it was mandatory not to treat blood pressure early because of the risk of deceasing the perfusion pressure, but this trial suggests the effects are neutral and there is probably as much benefit from lowering blood pressure for other reasons that offsets the potential harm.
“I think these are good data to rely on when we make these kinds of treatment decisions. Personally, I am a bit more aggressive with early blood pressure management and it’s good to see that you don’t get punished for that,” he added.
In his presentation, Dr. Liu explained that increased BP is common in acute stroke and is strongly associated with poor functional outcome and recurrence of ischemic stroke, but the optimal blood pressure management strategy in acute ischemic stroke remains controversial.
In the first CATIS trial (China Antihypertensive Trial in Acute Ischemic Stroke), which compared antihypertensive treatment within 48 hours of stroke onset with no antihypertensive treatment in ischemic stroke patients not receiving thrombolysis, the main results suggested that BP reduction with antihypertensive medications did not reduce the likelihood of death and major disability at 14 days or hospital discharge. But a subgroup analysis found that initiating antihypertensive treatment between 24 and 48 hours of stroke onset showed a beneficial effect on reducing death or major disability.
Current AHA/ASA guidelines suggest that, in patients with BP greater than 220/120 mm Hg who have not received thrombolysis or thrombectomy and have no comorbid conditions requiring urgent antihypertensive treatment, the benefit of initiating or reinitiating antihypertensive treatment within the first 48-72 hours is uncertain, although the guidelines say it might be reasonable to lower BP by around 15% during the first 24 hours after stroke onset, Dr. Liu noted.
The CATIS-2 trial was a multicenter, randomized, open-label, blinded-endpoints trial conducted at 106 centers in China that enrolled 4810 patients within 24-48 hours of onset of acute ischemic stroke who had elevated BP. Patients had not received thrombolytic therapy or mechanical thrombectomy.
Patients were randomly assigned to early antihypertensive therapy (initiated after randomization and aiming for a 10%-20% reduction in systolic BP) or delayed antihypertensive therapy (restarted antihypertensive therapy on day 8 of randomization, aiming for a BP of < 140/90 mm Hg).
The median age of the patients was 64 years, 65% were male, 80% had a history of hypertension, and the median National Institutes of Health Stroke Scale score was 3. Baseline BP averaged 163/92 mm Hg in both groups. The median time from stroke onset to antihypertensive treatment was 1.5 days in the early group and 8.5 days in the delayed group.
BP results showed that, at 24 hours after randomization, mean systolic pressure was reduced by 16.4 mm Hg (9.7%) in the early-treatment group and by 8.6 mm Hg (4.9%) in the delayed-treatment group (difference, –7.8 mm Hg; P < .0001).
At day 7, mean systolic pressure was 139.1 mm Hg in the early-treatment group, compared with 150.9 mm Hg in the delayed-treatment group, with a net difference in systolic BP of –11.9 mm Hg (P < .0001).
The primary outcome was the composite of death and major disability (modified Rankin Scale ≥ 3) at 3 months. This did not differ between the groups, occurring in 12.1% in the early antihypertensive treatment group versus 10.5% in the delayed antihypertensive treatment group (risk ratio, 1.15; P = .08).
There was also no difference in the major secondary outcome of shift in scores of mRS at 3 months, with a common odds ratio of 1.05 (95% confidence interval, 0.95-1.17).
There was no interaction with the composite outcome of death or major disability at 90 days in the prespecified subgroups.
Dr. Liu pointed out several limitations of the study. These included an observed primary outcome rate substantially lower than expected; the BP reduction seen within the first 7 days in the early-treatment group was moderate; and the results of the study cannot be applied to patients treated with thrombolysis or thrombectomy.
Dr. Liu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in the CATIS-2 trial.
The trial was presented by Liping Liu, MD, Beijing Tiantan Hospital, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
“Antihypertensive treatment can be delayed for at least 7 days following ischemic stroke onset, unless there are severe acute comorbidities that demand emergency blood pressure reduction to prevent serious complications,” Dr. Liu concluded.
But he acknowledged that the optimal BP management strategy in these patients remains uncertain and should be the focus of future research.
Discussing the trial at an ISC 2023 Highlights session, Lauren Sansing, MD, Yale University, New Haven, Conn., and ISC program vice chair, said: “These results seem to support waiting for a week or so before treating blood pressure in these patients.”
But Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and ISC program chair, countered: “To me, it’s kind of a neutral result, so what I take home from this is that you don’t necessarily have to wait.”
Dr. Jovin continued: “We used to think that it was mandatory not to treat blood pressure early because of the risk of deceasing the perfusion pressure, but this trial suggests the effects are neutral and there is probably as much benefit from lowering blood pressure for other reasons that offsets the potential harm.
“I think these are good data to rely on when we make these kinds of treatment decisions. Personally, I am a bit more aggressive with early blood pressure management and it’s good to see that you don’t get punished for that,” he added.
In his presentation, Dr. Liu explained that increased BP is common in acute stroke and is strongly associated with poor functional outcome and recurrence of ischemic stroke, but the optimal blood pressure management strategy in acute ischemic stroke remains controversial.
In the first CATIS trial (China Antihypertensive Trial in Acute Ischemic Stroke), which compared antihypertensive treatment within 48 hours of stroke onset with no antihypertensive treatment in ischemic stroke patients not receiving thrombolysis, the main results suggested that BP reduction with antihypertensive medications did not reduce the likelihood of death and major disability at 14 days or hospital discharge. But a subgroup analysis found that initiating antihypertensive treatment between 24 and 48 hours of stroke onset showed a beneficial effect on reducing death or major disability.
Current AHA/ASA guidelines suggest that, in patients with BP greater than 220/120 mm Hg who have not received thrombolysis or thrombectomy and have no comorbid conditions requiring urgent antihypertensive treatment, the benefit of initiating or reinitiating antihypertensive treatment within the first 48-72 hours is uncertain, although the guidelines say it might be reasonable to lower BP by around 15% during the first 24 hours after stroke onset, Dr. Liu noted.
The CATIS-2 trial was a multicenter, randomized, open-label, blinded-endpoints trial conducted at 106 centers in China that enrolled 4810 patients within 24-48 hours of onset of acute ischemic stroke who had elevated BP. Patients had not received thrombolytic therapy or mechanical thrombectomy.
Patients were randomly assigned to early antihypertensive therapy (initiated after randomization and aiming for a 10%-20% reduction in systolic BP) or delayed antihypertensive therapy (restarted antihypertensive therapy on day 8 of randomization, aiming for a BP of < 140/90 mm Hg).
The median age of the patients was 64 years, 65% were male, 80% had a history of hypertension, and the median National Institutes of Health Stroke Scale score was 3. Baseline BP averaged 163/92 mm Hg in both groups. The median time from stroke onset to antihypertensive treatment was 1.5 days in the early group and 8.5 days in the delayed group.
BP results showed that, at 24 hours after randomization, mean systolic pressure was reduced by 16.4 mm Hg (9.7%) in the early-treatment group and by 8.6 mm Hg (4.9%) in the delayed-treatment group (difference, –7.8 mm Hg; P < .0001).
At day 7, mean systolic pressure was 139.1 mm Hg in the early-treatment group, compared with 150.9 mm Hg in the delayed-treatment group, with a net difference in systolic BP of –11.9 mm Hg (P < .0001).
The primary outcome was the composite of death and major disability (modified Rankin Scale ≥ 3) at 3 months. This did not differ between the groups, occurring in 12.1% in the early antihypertensive treatment group versus 10.5% in the delayed antihypertensive treatment group (risk ratio, 1.15; P = .08).
There was also no difference in the major secondary outcome of shift in scores of mRS at 3 months, with a common odds ratio of 1.05 (95% confidence interval, 0.95-1.17).
There was no interaction with the composite outcome of death or major disability at 90 days in the prespecified subgroups.
Dr. Liu pointed out several limitations of the study. These included an observed primary outcome rate substantially lower than expected; the BP reduction seen within the first 7 days in the early-treatment group was moderate; and the results of the study cannot be applied to patients treated with thrombolysis or thrombectomy.
Dr. Liu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in the CATIS-2 trial.
The trial was presented by Liping Liu, MD, Beijing Tiantan Hospital, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
“Antihypertensive treatment can be delayed for at least 7 days following ischemic stroke onset, unless there are severe acute comorbidities that demand emergency blood pressure reduction to prevent serious complications,” Dr. Liu concluded.
But he acknowledged that the optimal BP management strategy in these patients remains uncertain and should be the focus of future research.
Discussing the trial at an ISC 2023 Highlights session, Lauren Sansing, MD, Yale University, New Haven, Conn., and ISC program vice chair, said: “These results seem to support waiting for a week or so before treating blood pressure in these patients.”
But Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and ISC program chair, countered: “To me, it’s kind of a neutral result, so what I take home from this is that you don’t necessarily have to wait.”
Dr. Jovin continued: “We used to think that it was mandatory not to treat blood pressure early because of the risk of deceasing the perfusion pressure, but this trial suggests the effects are neutral and there is probably as much benefit from lowering blood pressure for other reasons that offsets the potential harm.
“I think these are good data to rely on when we make these kinds of treatment decisions. Personally, I am a bit more aggressive with early blood pressure management and it’s good to see that you don’t get punished for that,” he added.
In his presentation, Dr. Liu explained that increased BP is common in acute stroke and is strongly associated with poor functional outcome and recurrence of ischemic stroke, but the optimal blood pressure management strategy in acute ischemic stroke remains controversial.
In the first CATIS trial (China Antihypertensive Trial in Acute Ischemic Stroke), which compared antihypertensive treatment within 48 hours of stroke onset with no antihypertensive treatment in ischemic stroke patients not receiving thrombolysis, the main results suggested that BP reduction with antihypertensive medications did not reduce the likelihood of death and major disability at 14 days or hospital discharge. But a subgroup analysis found that initiating antihypertensive treatment between 24 and 48 hours of stroke onset showed a beneficial effect on reducing death or major disability.
Current AHA/ASA guidelines suggest that, in patients with BP greater than 220/120 mm Hg who have not received thrombolysis or thrombectomy and have no comorbid conditions requiring urgent antihypertensive treatment, the benefit of initiating or reinitiating antihypertensive treatment within the first 48-72 hours is uncertain, although the guidelines say it might be reasonable to lower BP by around 15% during the first 24 hours after stroke onset, Dr. Liu noted.
The CATIS-2 trial was a multicenter, randomized, open-label, blinded-endpoints trial conducted at 106 centers in China that enrolled 4810 patients within 24-48 hours of onset of acute ischemic stroke who had elevated BP. Patients had not received thrombolytic therapy or mechanical thrombectomy.
Patients were randomly assigned to early antihypertensive therapy (initiated after randomization and aiming for a 10%-20% reduction in systolic BP) or delayed antihypertensive therapy (restarted antihypertensive therapy on day 8 of randomization, aiming for a BP of < 140/90 mm Hg).
The median age of the patients was 64 years, 65% were male, 80% had a history of hypertension, and the median National Institutes of Health Stroke Scale score was 3. Baseline BP averaged 163/92 mm Hg in both groups. The median time from stroke onset to antihypertensive treatment was 1.5 days in the early group and 8.5 days in the delayed group.
BP results showed that, at 24 hours after randomization, mean systolic pressure was reduced by 16.4 mm Hg (9.7%) in the early-treatment group and by 8.6 mm Hg (4.9%) in the delayed-treatment group (difference, –7.8 mm Hg; P < .0001).
At day 7, mean systolic pressure was 139.1 mm Hg in the early-treatment group, compared with 150.9 mm Hg in the delayed-treatment group, with a net difference in systolic BP of –11.9 mm Hg (P < .0001).
The primary outcome was the composite of death and major disability (modified Rankin Scale ≥ 3) at 3 months. This did not differ between the groups, occurring in 12.1% in the early antihypertensive treatment group versus 10.5% in the delayed antihypertensive treatment group (risk ratio, 1.15; P = .08).
There was also no difference in the major secondary outcome of shift in scores of mRS at 3 months, with a common odds ratio of 1.05 (95% confidence interval, 0.95-1.17).
There was no interaction with the composite outcome of death or major disability at 90 days in the prespecified subgroups.
Dr. Liu pointed out several limitations of the study. These included an observed primary outcome rate substantially lower than expected; the BP reduction seen within the first 7 days in the early-treatment group was moderate; and the results of the study cannot be applied to patients treated with thrombolysis or thrombectomy.
Dr. Liu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023