User login
Untreated COVID often involves relapse, clarifying antiviral rebound discussion
These findings offer a natural history of COVID-19 that will inform discussions and research concerning antiviral therapy, lead author Jonathan Z. Li, MD, associate professor of infectious disease at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues reported in Annals of Internal Medicine.
“There are increasing reports that high-risk patients are avoiding nirmatrelvir-ritonavir due to concerns about post-Paxlovid rebound, but there remains a gap in our knowledge of the frequency of symptom and viral relapse during untreated natural infection,” Dr. Li said in a written comment.
To address this gap, Dr. Li and colleagues analyzed data from 563 participants from the placebo group of the Adaptive Platform Treatment Trial for Outpatients with COVID-19 (ACTIV-2/A5401).
From days 0-28, patients recorded severity of 13 symptoms, with scores ranging from absent to severe (absent = 0, mild = 1, moderate = 2, severe = 3). RNA testing was performed on samples from nasal swabs on days 0–14, 21, and 28.
“The symptom rebound definition was determined by consensus of the study team, which comprises more than 10 infectious disease, pulmonary, and critical care physicians, as likely representing a clinically meaningful change in symptoms,” Dr. Li said.
Symptom scores needed to increase by at least 4 points to reach the threshold. For instance, a patient would qualify for relapse if they had worsening of four symptoms from mild to moderate, emergence of two new moderate symptoms, or emergence of one new moderate and two new mild symptoms.
The threshold for viral relapse was defined by an increase of at least 0.5 log10 RNA copies/mL from one nasal swab to the next, while high-level viral relapse was defined by an increase of at least 5.0 log10 RNA copies/mL. The former threshold was chosen based on previous analysis of viral rebound after nirmatrelvir treatment in the EPIC-HR phase 3 trial, whereas the high-level relapse point was based on Dr. Li and colleagues’ previous work linking this cutoff with the presence of infectious virus.
Their present analysis revealed that 26% of patients had symptom relapse at a median of 11 days after first symptom onset. Viral relapse occurred in 31% of patients, while high-level viral relapse occurred in 13% of participants. In about 9 out 10 cases, these relapses were detected at only one time point, suggesting they were transient. Of note, symptom relapse and high-level viral relapse occurred simultaneously in only 3% of patients.
This lack of correlation was “surprising” and “highlights that recovery from any infection is not always a linear process,” Dr. Li said.
This finding also suggests that untreated patients with recurring symptoms probably pose a low risk of contagion, according to David Wohl, MD, coauthor of the paper and professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill.
Paxlovid may not be to blame for COVID-19 rebound
“These results provide important context for the reports of Paxlovid rebound and show that baseline rates of symptom and viral relapse should be accounted for when studying the risk of rebound after antiviral therapy,” Dr. Li said.
Dr. Wohl suggested that these data can also play a role in conversations with patients who experience rebound after taking antiviral therapy.
“Many who have a return of their symptoms after taking Paxlovid blame the drug, and that may be justified, but this study suggests it happens in untreated people too,” Dr. Wohl said in a written comment.
Longer antiviral therapy deserves investigation
This is a “very important study” because it offers a baseline for comparing the natural history of COVID-19 with clinical course after antiviral therapy, said Timothy Henrich, MD, associate professor in the division of experimental medicine at University of California, San Francisco.
“Unlike this natural history, where it’s kind of sputtering up and down as it goes down, [after antiviral therapy,] it goes away for several days, and then it comes back up; and when it comes up, people have symptoms again,” Dr. Henrich said in an interview.
This suggests that each type of rebound is a unique phenomenon and, from a clinical perspective, that antiviral therapy may need to be extended.
“We treat for too short a period of time,” Dr. Henrich said. “We’re able to suppress [SARS-CoV-2] to the point where we’re not detecting it in the nasal pharynx, but it’s clearly still there. And it’s clearly still in a place that can replicate without the drug.”
That said, treating for longer may not be a sure-fire solution, especially if antiviral therapy is started early in the clinical course, as this could delay SARS-CoV-2-specific immune responses that are necessary for resolution, Dr. Henrich added,
“We need further study of longer-term therapies,” he said.
An array of research questions need to be addressed, according to Aditya Shah, MBBS, an infectious disease specialist at Mayo Clinic, Rochester, Minn. In a written comment, he probed the significance of rebound in various clinical scenarios.
“What [type of] rebound matters and what doesn’t?” Dr. Shah asked. “Does symptom rebound matter? How many untreated and treated ‘symptom rebounders’ need additional treatment or health care? If rebound does not really matter, but if Paxlovid helps in certain unvaccinated and high-risk patients, then does rebound matter? Future research should also focus on Paxlovid utility in vaccinated but high-risk patients. Is it as beneficial in them as it is in unvaccinated high-risk patients?”
While potentially regimen-altering questions like these remain unanswered, Dr. Henrich advised providers to keep patients focused on what we do know about the benefits of antiviral therapy given the current 5-day course, which is that it reduces the risk of severe disease and hospitalization.
The investigators disclosed relationships with Merck, Gilead, ViiV, and others. Dr. Henrich disclosed grant support from Merck and a consulting role with Roche. Dr. Shah disclosed no conflicts of interest.
These findings offer a natural history of COVID-19 that will inform discussions and research concerning antiviral therapy, lead author Jonathan Z. Li, MD, associate professor of infectious disease at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues reported in Annals of Internal Medicine.
“There are increasing reports that high-risk patients are avoiding nirmatrelvir-ritonavir due to concerns about post-Paxlovid rebound, but there remains a gap in our knowledge of the frequency of symptom and viral relapse during untreated natural infection,” Dr. Li said in a written comment.
To address this gap, Dr. Li and colleagues analyzed data from 563 participants from the placebo group of the Adaptive Platform Treatment Trial for Outpatients with COVID-19 (ACTIV-2/A5401).
From days 0-28, patients recorded severity of 13 symptoms, with scores ranging from absent to severe (absent = 0, mild = 1, moderate = 2, severe = 3). RNA testing was performed on samples from nasal swabs on days 0–14, 21, and 28.
“The symptom rebound definition was determined by consensus of the study team, which comprises more than 10 infectious disease, pulmonary, and critical care physicians, as likely representing a clinically meaningful change in symptoms,” Dr. Li said.
Symptom scores needed to increase by at least 4 points to reach the threshold. For instance, a patient would qualify for relapse if they had worsening of four symptoms from mild to moderate, emergence of two new moderate symptoms, or emergence of one new moderate and two new mild symptoms.
The threshold for viral relapse was defined by an increase of at least 0.5 log10 RNA copies/mL from one nasal swab to the next, while high-level viral relapse was defined by an increase of at least 5.0 log10 RNA copies/mL. The former threshold was chosen based on previous analysis of viral rebound after nirmatrelvir treatment in the EPIC-HR phase 3 trial, whereas the high-level relapse point was based on Dr. Li and colleagues’ previous work linking this cutoff with the presence of infectious virus.
Their present analysis revealed that 26% of patients had symptom relapse at a median of 11 days after first symptom onset. Viral relapse occurred in 31% of patients, while high-level viral relapse occurred in 13% of participants. In about 9 out 10 cases, these relapses were detected at only one time point, suggesting they were transient. Of note, symptom relapse and high-level viral relapse occurred simultaneously in only 3% of patients.
This lack of correlation was “surprising” and “highlights that recovery from any infection is not always a linear process,” Dr. Li said.
This finding also suggests that untreated patients with recurring symptoms probably pose a low risk of contagion, according to David Wohl, MD, coauthor of the paper and professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill.
Paxlovid may not be to blame for COVID-19 rebound
“These results provide important context for the reports of Paxlovid rebound and show that baseline rates of symptom and viral relapse should be accounted for when studying the risk of rebound after antiviral therapy,” Dr. Li said.
Dr. Wohl suggested that these data can also play a role in conversations with patients who experience rebound after taking antiviral therapy.
“Many who have a return of their symptoms after taking Paxlovid blame the drug, and that may be justified, but this study suggests it happens in untreated people too,” Dr. Wohl said in a written comment.
Longer antiviral therapy deserves investigation
This is a “very important study” because it offers a baseline for comparing the natural history of COVID-19 with clinical course after antiviral therapy, said Timothy Henrich, MD, associate professor in the division of experimental medicine at University of California, San Francisco.
“Unlike this natural history, where it’s kind of sputtering up and down as it goes down, [after antiviral therapy,] it goes away for several days, and then it comes back up; and when it comes up, people have symptoms again,” Dr. Henrich said in an interview.
This suggests that each type of rebound is a unique phenomenon and, from a clinical perspective, that antiviral therapy may need to be extended.
“We treat for too short a period of time,” Dr. Henrich said. “We’re able to suppress [SARS-CoV-2] to the point where we’re not detecting it in the nasal pharynx, but it’s clearly still there. And it’s clearly still in a place that can replicate without the drug.”
That said, treating for longer may not be a sure-fire solution, especially if antiviral therapy is started early in the clinical course, as this could delay SARS-CoV-2-specific immune responses that are necessary for resolution, Dr. Henrich added,
“We need further study of longer-term therapies,” he said.
An array of research questions need to be addressed, according to Aditya Shah, MBBS, an infectious disease specialist at Mayo Clinic, Rochester, Minn. In a written comment, he probed the significance of rebound in various clinical scenarios.
“What [type of] rebound matters and what doesn’t?” Dr. Shah asked. “Does symptom rebound matter? How many untreated and treated ‘symptom rebounders’ need additional treatment or health care? If rebound does not really matter, but if Paxlovid helps in certain unvaccinated and high-risk patients, then does rebound matter? Future research should also focus on Paxlovid utility in vaccinated but high-risk patients. Is it as beneficial in them as it is in unvaccinated high-risk patients?”
While potentially regimen-altering questions like these remain unanswered, Dr. Henrich advised providers to keep patients focused on what we do know about the benefits of antiviral therapy given the current 5-day course, which is that it reduces the risk of severe disease and hospitalization.
The investigators disclosed relationships with Merck, Gilead, ViiV, and others. Dr. Henrich disclosed grant support from Merck and a consulting role with Roche. Dr. Shah disclosed no conflicts of interest.
These findings offer a natural history of COVID-19 that will inform discussions and research concerning antiviral therapy, lead author Jonathan Z. Li, MD, associate professor of infectious disease at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues reported in Annals of Internal Medicine.
“There are increasing reports that high-risk patients are avoiding nirmatrelvir-ritonavir due to concerns about post-Paxlovid rebound, but there remains a gap in our knowledge of the frequency of symptom and viral relapse during untreated natural infection,” Dr. Li said in a written comment.
To address this gap, Dr. Li and colleagues analyzed data from 563 participants from the placebo group of the Adaptive Platform Treatment Trial for Outpatients with COVID-19 (ACTIV-2/A5401).
From days 0-28, patients recorded severity of 13 symptoms, with scores ranging from absent to severe (absent = 0, mild = 1, moderate = 2, severe = 3). RNA testing was performed on samples from nasal swabs on days 0–14, 21, and 28.
“The symptom rebound definition was determined by consensus of the study team, which comprises more than 10 infectious disease, pulmonary, and critical care physicians, as likely representing a clinically meaningful change in symptoms,” Dr. Li said.
Symptom scores needed to increase by at least 4 points to reach the threshold. For instance, a patient would qualify for relapse if they had worsening of four symptoms from mild to moderate, emergence of two new moderate symptoms, or emergence of one new moderate and two new mild symptoms.
The threshold for viral relapse was defined by an increase of at least 0.5 log10 RNA copies/mL from one nasal swab to the next, while high-level viral relapse was defined by an increase of at least 5.0 log10 RNA copies/mL. The former threshold was chosen based on previous analysis of viral rebound after nirmatrelvir treatment in the EPIC-HR phase 3 trial, whereas the high-level relapse point was based on Dr. Li and colleagues’ previous work linking this cutoff with the presence of infectious virus.
Their present analysis revealed that 26% of patients had symptom relapse at a median of 11 days after first symptom onset. Viral relapse occurred in 31% of patients, while high-level viral relapse occurred in 13% of participants. In about 9 out 10 cases, these relapses were detected at only one time point, suggesting they were transient. Of note, symptom relapse and high-level viral relapse occurred simultaneously in only 3% of patients.
This lack of correlation was “surprising” and “highlights that recovery from any infection is not always a linear process,” Dr. Li said.
This finding also suggests that untreated patients with recurring symptoms probably pose a low risk of contagion, according to David Wohl, MD, coauthor of the paper and professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill.
Paxlovid may not be to blame for COVID-19 rebound
“These results provide important context for the reports of Paxlovid rebound and show that baseline rates of symptom and viral relapse should be accounted for when studying the risk of rebound after antiviral therapy,” Dr. Li said.
Dr. Wohl suggested that these data can also play a role in conversations with patients who experience rebound after taking antiviral therapy.
“Many who have a return of their symptoms after taking Paxlovid blame the drug, and that may be justified, but this study suggests it happens in untreated people too,” Dr. Wohl said in a written comment.
Longer antiviral therapy deserves investigation
This is a “very important study” because it offers a baseline for comparing the natural history of COVID-19 with clinical course after antiviral therapy, said Timothy Henrich, MD, associate professor in the division of experimental medicine at University of California, San Francisco.
“Unlike this natural history, where it’s kind of sputtering up and down as it goes down, [after antiviral therapy,] it goes away for several days, and then it comes back up; and when it comes up, people have symptoms again,” Dr. Henrich said in an interview.
This suggests that each type of rebound is a unique phenomenon and, from a clinical perspective, that antiviral therapy may need to be extended.
“We treat for too short a period of time,” Dr. Henrich said. “We’re able to suppress [SARS-CoV-2] to the point where we’re not detecting it in the nasal pharynx, but it’s clearly still there. And it’s clearly still in a place that can replicate without the drug.”
That said, treating for longer may not be a sure-fire solution, especially if antiviral therapy is started early in the clinical course, as this could delay SARS-CoV-2-specific immune responses that are necessary for resolution, Dr. Henrich added,
“We need further study of longer-term therapies,” he said.
An array of research questions need to be addressed, according to Aditya Shah, MBBS, an infectious disease specialist at Mayo Clinic, Rochester, Minn. In a written comment, he probed the significance of rebound in various clinical scenarios.
“What [type of] rebound matters and what doesn’t?” Dr. Shah asked. “Does symptom rebound matter? How many untreated and treated ‘symptom rebounders’ need additional treatment or health care? If rebound does not really matter, but if Paxlovid helps in certain unvaccinated and high-risk patients, then does rebound matter? Future research should also focus on Paxlovid utility in vaccinated but high-risk patients. Is it as beneficial in them as it is in unvaccinated high-risk patients?”
While potentially regimen-altering questions like these remain unanswered, Dr. Henrich advised providers to keep patients focused on what we do know about the benefits of antiviral therapy given the current 5-day course, which is that it reduces the risk of severe disease and hospitalization.
The investigators disclosed relationships with Merck, Gilead, ViiV, and others. Dr. Henrich disclosed grant support from Merck and a consulting role with Roche. Dr. Shah disclosed no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
The way I see it
I’ve worn glasses since I was 8, when a routine school vision test showed I was nearsighted. Except for an ill-fated 3-month attempt at contact lenses when I was 16, glasses have been just another part of my daily routine.
The last time I got new ones was in 2018, and my vision always seemed “off” after that. I took them back to the store a few times and was told I’d adjust to them and that things would be fine, So after a few weeks of doggedly wearing them I adjusted to them. I still felt like something was slightly off, but then I was busy, and then came the pandemic, and then my eye doctor retired and I had to find a new one ... so going to get my glasses prescription rechecked kept getting pushed back.
As so many of us do over time, I’ve gotten used to taking my glasses off to read things up close, like a book, or to do a detailed jigsaw puzzle. This has gotten worse over time, and so finally I made an appointment with a new eye doctor.
I handed him my previous prescription. He did a reading off the lenses, looked at the prescription again, gave me a perplexed look, and started the usual eye exam, asking me to read different lines as he switched lenses around. This went on for 10-15 minutes.
“The right lens wasn’t made correctly,” he told me. “You’ve been working off your left eye for the last 5 years.”
He returned my glasses and I put them on. He covered my left eye and showed me how, without realizing it, I was tilting my head back to bring distant items into focus on the right – the opposite of what I should be doing – and with both eyes would adjust my position to use the left eye.
The next morning, while working at my desk, I realized for the first time that I had my head turned slightly right to bring the left eye a tad closer to the screen. In a job where we’re trained to look for such minutiae in patients I’d missed it on myself. A friend even suggested I submit my story as a case report – “An unusual cause of a head-tilt in a middle-aged male” – to a journal.
It’s an interesting commentary on how adaptable the brain is at handling vision changes. It was several hundred million years ago when the brain figured out how to invert images that were seen upside down, and it continues to find ways to compensate for field cuts, cranial nerve palsies, and other lesions. Including flawed spectacles.
When my new eyeglasses arrive, my brain will have to readjust. This time, though, I’m curious and will try to pay better attention to my own reactions. If I can.
so we don’t notice an issue.
Amazing stuff if you think about it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve worn glasses since I was 8, when a routine school vision test showed I was nearsighted. Except for an ill-fated 3-month attempt at contact lenses when I was 16, glasses have been just another part of my daily routine.
The last time I got new ones was in 2018, and my vision always seemed “off” after that. I took them back to the store a few times and was told I’d adjust to them and that things would be fine, So after a few weeks of doggedly wearing them I adjusted to them. I still felt like something was slightly off, but then I was busy, and then came the pandemic, and then my eye doctor retired and I had to find a new one ... so going to get my glasses prescription rechecked kept getting pushed back.
As so many of us do over time, I’ve gotten used to taking my glasses off to read things up close, like a book, or to do a detailed jigsaw puzzle. This has gotten worse over time, and so finally I made an appointment with a new eye doctor.
I handed him my previous prescription. He did a reading off the lenses, looked at the prescription again, gave me a perplexed look, and started the usual eye exam, asking me to read different lines as he switched lenses around. This went on for 10-15 minutes.
“The right lens wasn’t made correctly,” he told me. “You’ve been working off your left eye for the last 5 years.”
He returned my glasses and I put them on. He covered my left eye and showed me how, without realizing it, I was tilting my head back to bring distant items into focus on the right – the opposite of what I should be doing – and with both eyes would adjust my position to use the left eye.
The next morning, while working at my desk, I realized for the first time that I had my head turned slightly right to bring the left eye a tad closer to the screen. In a job where we’re trained to look for such minutiae in patients I’d missed it on myself. A friend even suggested I submit my story as a case report – “An unusual cause of a head-tilt in a middle-aged male” – to a journal.
It’s an interesting commentary on how adaptable the brain is at handling vision changes. It was several hundred million years ago when the brain figured out how to invert images that were seen upside down, and it continues to find ways to compensate for field cuts, cranial nerve palsies, and other lesions. Including flawed spectacles.
When my new eyeglasses arrive, my brain will have to readjust. This time, though, I’m curious and will try to pay better attention to my own reactions. If I can.
so we don’t notice an issue.
Amazing stuff if you think about it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve worn glasses since I was 8, when a routine school vision test showed I was nearsighted. Except for an ill-fated 3-month attempt at contact lenses when I was 16, glasses have been just another part of my daily routine.
The last time I got new ones was in 2018, and my vision always seemed “off” after that. I took them back to the store a few times and was told I’d adjust to them and that things would be fine, So after a few weeks of doggedly wearing them I adjusted to them. I still felt like something was slightly off, but then I was busy, and then came the pandemic, and then my eye doctor retired and I had to find a new one ... so going to get my glasses prescription rechecked kept getting pushed back.
As so many of us do over time, I’ve gotten used to taking my glasses off to read things up close, like a book, or to do a detailed jigsaw puzzle. This has gotten worse over time, and so finally I made an appointment with a new eye doctor.
I handed him my previous prescription. He did a reading off the lenses, looked at the prescription again, gave me a perplexed look, and started the usual eye exam, asking me to read different lines as he switched lenses around. This went on for 10-15 minutes.
“The right lens wasn’t made correctly,” he told me. “You’ve been working off your left eye for the last 5 years.”
He returned my glasses and I put them on. He covered my left eye and showed me how, without realizing it, I was tilting my head back to bring distant items into focus on the right – the opposite of what I should be doing – and with both eyes would adjust my position to use the left eye.
The next morning, while working at my desk, I realized for the first time that I had my head turned slightly right to bring the left eye a tad closer to the screen. In a job where we’re trained to look for such minutiae in patients I’d missed it on myself. A friend even suggested I submit my story as a case report – “An unusual cause of a head-tilt in a middle-aged male” – to a journal.
It’s an interesting commentary on how adaptable the brain is at handling vision changes. It was several hundred million years ago when the brain figured out how to invert images that were seen upside down, and it continues to find ways to compensate for field cuts, cranial nerve palsies, and other lesions. Including flawed spectacles.
When my new eyeglasses arrive, my brain will have to readjust. This time, though, I’m curious and will try to pay better attention to my own reactions. If I can.
so we don’t notice an issue.
Amazing stuff if you think about it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Alzheimer’s Disease Overview
Expert discusses pros, cons of molecular tests for melanoma
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
SAN DIEGO – , according to Gregory A. Hosler, MD, PhD.
At the annual Cutaneous Malignancy Update, Dr. Hosler, director of dermatopathology for ProPath, highlighted the following molecular tests currently used for the diagnosis of challenging melanocytic lesions:
Comparative genomic hybridization (CGH). This technique allows for the detection of chromosomal copy number changes throughout the tumor genome. “With CGH, test (tumor) DNA and normal DNA are differentially labeled and compared to a reference library. Gains and losses of portions of the tumor genome are determined by comparing the relative signals from these two groups,” said Dr. Hosler, clinical professor of pathology and dermatology at the University of Texas Southwestern Medical Center, Dallas.
“In the past, your library was a metaphase of spread of chromosomes, which introduced technical challenges and made performance of the assay labor intensive. Because of this, CGH is not routinely performed by clinical laboratories and is used more as an exploratory/research technique.”
Array CGH (also known as SNP array). Newer versions of CGH use short DNA sequences that are tiled onto a chip. “The interesting thing about these chips is that you can purchase them or design them on your own,” Dr. Hosler said. “The chips may cover the entire genome or cover specific areas of the genome at higher resolution.” One upside of array CGH, he continued, is that it allows one to detect essentially all gains or losses of chromosomal material in a single reaction. “It is not subject to the artifacts associated with cutting thin sections like with fluorescence in situ hybridization (FISH); it can detect copy number neutral loss of heterozygosity, and it is more scalable,” Dr. Hosler said at the meeting, which was hosted by Scripps MD Anderson Cancer Center.
One downside of array CGH is that does not allow one to analyze specific cells, “so if you have a tumor that’s heterogeneous, the assay is agnostic to this and spits out a result based on all the material provided,” he said. “You can’t parse out different areas of the lesion. It also does not track balanced translocations.” In addition, he said, “there are also questions about reimbursement and these are lab-developed tests, so each lab’s assay is different. Finally, it requires specialized equipment and expertise for interpretation.”
FISH. First-generation melanoma FISH assays, which became available in 2009, used six probes and four colors and had a sensitivity of about 87% and specificity of about 95%, Dr. Hosler said, but there were problems with those assays, particularly related to Spitz nevi. Spitz nevi often duplicate their chromosomes, “so instead of being diploid they’re tetraploid,” he said.
“The second-generation melanoma FISH assays addressed this by adding centromeres to the assay, and targeted probes could be compared to the centromeres on the same chromosome to determine if these were true copy number gains, due to genetic instability, or gains or losses of entire arms or whole chromosomes. This modification and the addition of new targets really improved upon the sensitivity and specificity (94% and 98%, respectively),” he said, noting that this assay is widely used.
Upsides of melanoma FISH assays are that they are a “fairly routine methodology” in large clinical laboratories, he said, and that many labs are familiar with interpretation. “I would say the biggest advantage to FISH is its ability to analyze specific cells, which is useful with small or heterogeneous tumors,” Dr. Hosler said. “Also, there is a genetic reimbursement code for it, and it yields diagnostic and potentially prognostic information.” For example, certain copy number changes have shown to portend a worse prognosis if they’re present in a melanocytic tumor, including alterations in CDKN2A, CCND1, MYC, topoisomerase, and BAP1.
Downsides of melanoma FISH assays are that they are expensive, labor-intensive, and require experts to interpret the results. “The stacking and truncation of cell nuclei innate to paraffin-embedded FISH make interpretation difficult,” he said. “Also, all colors cannot be viewed simultaneously, and each lab’s assay potentially is different, requiring validation. These are not [Food and Drug Administration]-approved tests.”
Next generation sequencing (NGS). Also known as high-throughput sequencing, this technique allows for the generation of millions of sequencing reads that are aligned to a standard human genome, and likely represents the wave of the future. “With NGS you can increase breadth, so you can sequence the entire genome if you want, but you can also increase depth, meaning increasing the number of reads over a single target of the genome,” Dr. Hosler said. “That’s useful if you’re looking for a low frequency mutation.”
For example, NGS allows one to detect alterations of BRAF and KIT and other potentially actionable alterations. It can also be used to detect mutations in benign and malignant melanocytic lesions, including historically diagnostically challenging Spitz and desmoplastic subgroups. Several different NGS technologies exist, and there are different strategies behind each assay, including whole genome sequencing, whole exome sequencing, transcriptome sequencing, and targeted panels. “I’ve seen panels of 10 and I’ve seen panels of 1,500; there’s a wide range,” Dr. Hosler said. “The biggest challenge with NGS, currently, is that it’s difficult to interpret. Trying to figure out what’s important and what’s not important can be challenging. Often you need a team of people who are experts in bioinformatics to interpret these results.”
Slow turnaround time is another downside. “It can take a month to get results, and sometimes clinicians don’t want to wait that long, especially if they think a lesion is melanoma, so that’s an area of focus for NGS laboratories,” he said. “And there are questions on reimbursement. If you run NGS on every unusual melanocytic lesion, that’s not a good use of health care dollars. Who’s paying for it? I don’t have an answer for you. It’s all over the map right now. Each lab’s test and billing practice is different.”
Dr. Hosler reported having no relevant financial disclosures. ProPath is a nationwide pathology practice.
AT MELANOMA 2023
Antibiotics and SJS/TEN: Study provides global prevalence
of SJS/TEN in connection with antibiotics.
“SJS/TEN is considered the most severe form of drug hypersensitivity reaction, and antibiotics are an important risk,” Erika Yue Lee, MD, and associates wrote in JAMA Dermatology.
Their analysis, which involved 38 studies published since 1987 with 2,917 patients from more than 20 countries, showed that 86% of all SJS/TEN cases were associated with a single drug, with the rest involving multiple drug triggers, infections, or other causes. More than a quarter (28%) of those patients had used an antibiotic, and the sulfonamides were the class most often triggering SJS/TEN, said Dr. Lee of the University of Toronto and associates.
Sulfonamides were responsible for 32% of the antibiotic-associated cases, which works out to 11% of all SJS/TEN cases included in the analysis. Penicillins were next with 22% of all antibiotic-associated cases, followed by the cephalosporins (11%), fluoroquinolones (4%), and macrolides (2%), the investigators reported.
A subgroup analysis conducted by age indicated that “there was no difference in the proportion of antibiotics associated with SJS/TEN between adult and pediatric groups,” they noted.
There were differences, however, among the various antibiotic classes. Sulfonamides represented 54% of antibiotic-triggered reactions in children, compared with 25% in adults, but adults were significantly more likely to have cephalosporin (23%) and fluoroquinolone (5%) involvement than were children (2% and 0, respectively). Macrolide-induced SJS/TEN was more common in children (18% vs. 1%), while the penicillin rate was 18% for both age groups, Dr. Lee and associates said.
A second subgroup analysis establishing the proportion of antibiotic-induced SJS/TEN by continent ranked Australia highest with 43%, but that was based on only one study of 42 patients. North America was slightly lower at 37%, but the analysis included 14 studies and 932 patients. Asia’s 16 studies and 1,298 patients were divided into three regions, with the lowest being the southeast at 16%, according to the researchers.
“Global sulfonamide antibiotic use has been decreasing since 2000 despite an ongoing upward trend of use in other antibiotic classes,” they wrote, but “antibiotics remain one of the most common culprit drugs for SJS/TEN in both adults and children worldwide.”
One of Dr. Lee’s associates has received personal fees from Janssen, AstraZeneca, UpToDate, Verve, BioCryst, Regeneron Pharmaceuticals, and Novavax and has served as codirector of IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing and has a patent pending for detection of HLA-A*32:01 in connection with diagnosing drug reaction without any financial remuneration outside this study.
of SJS/TEN in connection with antibiotics.
“SJS/TEN is considered the most severe form of drug hypersensitivity reaction, and antibiotics are an important risk,” Erika Yue Lee, MD, and associates wrote in JAMA Dermatology.
Their analysis, which involved 38 studies published since 1987 with 2,917 patients from more than 20 countries, showed that 86% of all SJS/TEN cases were associated with a single drug, with the rest involving multiple drug triggers, infections, or other causes. More than a quarter (28%) of those patients had used an antibiotic, and the sulfonamides were the class most often triggering SJS/TEN, said Dr. Lee of the University of Toronto and associates.
Sulfonamides were responsible for 32% of the antibiotic-associated cases, which works out to 11% of all SJS/TEN cases included in the analysis. Penicillins were next with 22% of all antibiotic-associated cases, followed by the cephalosporins (11%), fluoroquinolones (4%), and macrolides (2%), the investigators reported.
A subgroup analysis conducted by age indicated that “there was no difference in the proportion of antibiotics associated with SJS/TEN between adult and pediatric groups,” they noted.
There were differences, however, among the various antibiotic classes. Sulfonamides represented 54% of antibiotic-triggered reactions in children, compared with 25% in adults, but adults were significantly more likely to have cephalosporin (23%) and fluoroquinolone (5%) involvement than were children (2% and 0, respectively). Macrolide-induced SJS/TEN was more common in children (18% vs. 1%), while the penicillin rate was 18% for both age groups, Dr. Lee and associates said.
A second subgroup analysis establishing the proportion of antibiotic-induced SJS/TEN by continent ranked Australia highest with 43%, but that was based on only one study of 42 patients. North America was slightly lower at 37%, but the analysis included 14 studies and 932 patients. Asia’s 16 studies and 1,298 patients were divided into three regions, with the lowest being the southeast at 16%, according to the researchers.
“Global sulfonamide antibiotic use has been decreasing since 2000 despite an ongoing upward trend of use in other antibiotic classes,” they wrote, but “antibiotics remain one of the most common culprit drugs for SJS/TEN in both adults and children worldwide.”
One of Dr. Lee’s associates has received personal fees from Janssen, AstraZeneca, UpToDate, Verve, BioCryst, Regeneron Pharmaceuticals, and Novavax and has served as codirector of IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing and has a patent pending for detection of HLA-A*32:01 in connection with diagnosing drug reaction without any financial remuneration outside this study.
of SJS/TEN in connection with antibiotics.
“SJS/TEN is considered the most severe form of drug hypersensitivity reaction, and antibiotics are an important risk,” Erika Yue Lee, MD, and associates wrote in JAMA Dermatology.
Their analysis, which involved 38 studies published since 1987 with 2,917 patients from more than 20 countries, showed that 86% of all SJS/TEN cases were associated with a single drug, with the rest involving multiple drug triggers, infections, or other causes. More than a quarter (28%) of those patients had used an antibiotic, and the sulfonamides were the class most often triggering SJS/TEN, said Dr. Lee of the University of Toronto and associates.
Sulfonamides were responsible for 32% of the antibiotic-associated cases, which works out to 11% of all SJS/TEN cases included in the analysis. Penicillins were next with 22% of all antibiotic-associated cases, followed by the cephalosporins (11%), fluoroquinolones (4%), and macrolides (2%), the investigators reported.
A subgroup analysis conducted by age indicated that “there was no difference in the proportion of antibiotics associated with SJS/TEN between adult and pediatric groups,” they noted.
There were differences, however, among the various antibiotic classes. Sulfonamides represented 54% of antibiotic-triggered reactions in children, compared with 25% in adults, but adults were significantly more likely to have cephalosporin (23%) and fluoroquinolone (5%) involvement than were children (2% and 0, respectively). Macrolide-induced SJS/TEN was more common in children (18% vs. 1%), while the penicillin rate was 18% for both age groups, Dr. Lee and associates said.
A second subgroup analysis establishing the proportion of antibiotic-induced SJS/TEN by continent ranked Australia highest with 43%, but that was based on only one study of 42 patients. North America was slightly lower at 37%, but the analysis included 14 studies and 932 patients. Asia’s 16 studies and 1,298 patients were divided into three regions, with the lowest being the southeast at 16%, according to the researchers.
“Global sulfonamide antibiotic use has been decreasing since 2000 despite an ongoing upward trend of use in other antibiotic classes,” they wrote, but “antibiotics remain one of the most common culprit drugs for SJS/TEN in both adults and children worldwide.”
One of Dr. Lee’s associates has received personal fees from Janssen, AstraZeneca, UpToDate, Verve, BioCryst, Regeneron Pharmaceuticals, and Novavax and has served as codirector of IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing and has a patent pending for detection of HLA-A*32:01 in connection with diagnosing drug reaction without any financial remuneration outside this study.
FROM JAMA DERMATOLOGY
MCL Workup
Telemedicine usage still high among rheumatologists as interest wanes in other specialties
There was an explosion in the use of telemedicine during the COVID-19 pandemic, but usage has stabilized and varies between specialties. However, telemedicine use is still somewhat high among rheumatologists, according to speakers at the 2023 Rheumatology Winter Clinical Symposium.
Speaking in general about the future of rheumatology, Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said it is up to rheumatologists to adapt to the changing winds in the specialty.
“The future is going to happen no matter what, so the question is, are you going to go along with it? Are you going to be a part of it? Are you going to be resistant to it?” Dr. Cush asked attendees. “Your recent experience with COVID would tell you maybe what your path is going to be if you’re dying to get back to the way it once was.”
Rheumatologists can expect changes in where they work, how they’re paid, increases in their workload, and new innovations in connecting with patients, he said.
“You’re going to be integrating a new style of medicine, you’re going to be digitally connected,” he explained. “All these networks are going to be working together to make you supposedly better at what you do, or maybe they’re working together to make you obsolete – and I think you better start protecting your space.”
One major area of change, telemedicine, already occurred as a result of the COVID-19 pandemic and will “begin to dominate” over the next decade, Dr. Cush said. An analysis conducted by consulting firm McKinsey & Company found telehealth usage increased 78-fold between February and April 2020 before leveling off at a 38-fold higher rate, compared with prepandemic levels. In the same analysis, rheumatology ranked third in terms of telehealth usage claims behind psychiatry and substance use disorder treatment, Dr. Cush observed, as other specialties have “fallen off quite a bit.”
“The common denominators are chronic care, cognitive care, nonprocedural care, pattern recognition, and monitoring, and this is what you do,” he said. “This is why, in many ways, for you to abandon telemedicine I think is a gigantic mistake.”
Changes to telemedicine
The most immediate change to telemedicine will come when the Biden administration officially ends the COVID-19 public health emergency in May 2023, and temporary telehealth services will be extended for approximately 5 months after the end of the public health emergency. Legislation passed by Congress will ensure some of the flexibilities in telemedicine will be extended until the end of December 2024.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said he sees telemedicine as persisting even after the official COVID-19 public health emergency ends. “There’s a lot of push from the American Medical Association, from the American College of Physicians. You’re going to see people – this will not go away because [there’s] also going to be that demand.”
Despite decreased usage since April 2020, telehealth was estimated to be a $60 billion industry in 2022 and will likely increase over the next decade, Dr. Cush noted. “I question [the decline] because I think it still is a major part of your [future in] 2033.”
The number of physicians who have at least three licenses to practice in other U.S. states increased from 50,454 in 2010 to 72,752 in 2020, and that trend will continue, Dr. Wells explained. It is now becoming easier for physicians to become licensed in other states with companies like CompHealth that offer services to simplify obtaining medical licenses with states that participate in the Interstate Medical Licensure Compact.
“It’s a telemedicine easy pass,” Dr. Cush said.
Concerns in telemedicine
Commenting on the presentation, Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at the Hospital for Special Surgery (HSS), both in New York, pointed out that because telemedicine is governed by U.S. states, rather than the federal government, a physician needs to be licensed in the state where the patient is located. While many states relaxed their restrictions during COVID-19, as states began tightening their restrictions later, “many physicians didn’t want to have three licenses,” he said.
“There’s an expense in getting three licenses. There’s an expense in obtaining it and maintaining it, and the reimbursement for the telemedicine visit did not reach that expectation,” Dr. Gibofsky explained. With the exception of the orthopedic surgeons at HSS who practice in New York and a satellite office in Florida, none of the surgeons at his center have obtained more than one license to practice telemedicine in other states.
“Our volume of telemedicine at HSS has remained about the same at 30%, but fewer physicians are doing it because they don’t want to maintain multiple licensures,” he said. “So don’t overlook the role of legal concerns in terms of who’s going to be allowed to do what where. Your talk was great in terms of an exuberance of what’s going to be available, but it’s not going to relieve the physician from the burden of being responsible for their use.”
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, asked the presenters about the balance between seeing patients for virtual and in-person visits. “The question is what’s the sweet spot? Are there people you’re willing to see virtually forever?” he asked, noting that he has patients scheduling telemedicine visits that he hasn’t seen since before the COVID-19 pandemic.
“That’s not going to work for me. At some point, you have to lay hands on people,” he said.
Dr. Wells said his current practice is 40% virtual, and his staff converts potential no-shows into a telemedicine consultation over the phone. “My no-show rate has gone down to zero. Somebody’s scheduled for a visit, they don’t show up, my [medical assistants] get them on the phone, they put them on hold, tee up the refills. I turn them into a telephone call,” he said. “We don’t accept the no-show at all because we can do a telephone [consultation].”
In Dr. Cush’s practice, he alternates telemedicine visits with in-person visits. “If you come back for two videos in a row, you’re catching hell from me for that,” he said. Responding to how Dr. Wells incorporates telemedicine into his practice, Dr. Cush said many rheumatologists “don’t have the setups to support the care, and that’s why it’s hard to do and that’s why we’re not as great as we could be.”
“This is the way we were trained. We’re used to seeing these patients in the clinic that often. Not every single patient needs to be seen that frequently if they’re stable and doing fine,” Dr. Wells countered.
Dr. Cush and Dr. Wells reported having financial relationships with numerous pharmaceutical companies.
There was an explosion in the use of telemedicine during the COVID-19 pandemic, but usage has stabilized and varies between specialties. However, telemedicine use is still somewhat high among rheumatologists, according to speakers at the 2023 Rheumatology Winter Clinical Symposium.
Speaking in general about the future of rheumatology, Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said it is up to rheumatologists to adapt to the changing winds in the specialty.
“The future is going to happen no matter what, so the question is, are you going to go along with it? Are you going to be a part of it? Are you going to be resistant to it?” Dr. Cush asked attendees. “Your recent experience with COVID would tell you maybe what your path is going to be if you’re dying to get back to the way it once was.”
Rheumatologists can expect changes in where they work, how they’re paid, increases in their workload, and new innovations in connecting with patients, he said.
“You’re going to be integrating a new style of medicine, you’re going to be digitally connected,” he explained. “All these networks are going to be working together to make you supposedly better at what you do, or maybe they’re working together to make you obsolete – and I think you better start protecting your space.”
One major area of change, telemedicine, already occurred as a result of the COVID-19 pandemic and will “begin to dominate” over the next decade, Dr. Cush said. An analysis conducted by consulting firm McKinsey & Company found telehealth usage increased 78-fold between February and April 2020 before leveling off at a 38-fold higher rate, compared with prepandemic levels. In the same analysis, rheumatology ranked third in terms of telehealth usage claims behind psychiatry and substance use disorder treatment, Dr. Cush observed, as other specialties have “fallen off quite a bit.”
“The common denominators are chronic care, cognitive care, nonprocedural care, pattern recognition, and monitoring, and this is what you do,” he said. “This is why, in many ways, for you to abandon telemedicine I think is a gigantic mistake.”
Changes to telemedicine
The most immediate change to telemedicine will come when the Biden administration officially ends the COVID-19 public health emergency in May 2023, and temporary telehealth services will be extended for approximately 5 months after the end of the public health emergency. Legislation passed by Congress will ensure some of the flexibilities in telemedicine will be extended until the end of December 2024.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said he sees telemedicine as persisting even after the official COVID-19 public health emergency ends. “There’s a lot of push from the American Medical Association, from the American College of Physicians. You’re going to see people – this will not go away because [there’s] also going to be that demand.”
Despite decreased usage since April 2020, telehealth was estimated to be a $60 billion industry in 2022 and will likely increase over the next decade, Dr. Cush noted. “I question [the decline] because I think it still is a major part of your [future in] 2033.”
The number of physicians who have at least three licenses to practice in other U.S. states increased from 50,454 in 2010 to 72,752 in 2020, and that trend will continue, Dr. Wells explained. It is now becoming easier for physicians to become licensed in other states with companies like CompHealth that offer services to simplify obtaining medical licenses with states that participate in the Interstate Medical Licensure Compact.
“It’s a telemedicine easy pass,” Dr. Cush said.
Concerns in telemedicine
Commenting on the presentation, Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at the Hospital for Special Surgery (HSS), both in New York, pointed out that because telemedicine is governed by U.S. states, rather than the federal government, a physician needs to be licensed in the state where the patient is located. While many states relaxed their restrictions during COVID-19, as states began tightening their restrictions later, “many physicians didn’t want to have three licenses,” he said.
“There’s an expense in getting three licenses. There’s an expense in obtaining it and maintaining it, and the reimbursement for the telemedicine visit did not reach that expectation,” Dr. Gibofsky explained. With the exception of the orthopedic surgeons at HSS who practice in New York and a satellite office in Florida, none of the surgeons at his center have obtained more than one license to practice telemedicine in other states.
“Our volume of telemedicine at HSS has remained about the same at 30%, but fewer physicians are doing it because they don’t want to maintain multiple licensures,” he said. “So don’t overlook the role of legal concerns in terms of who’s going to be allowed to do what where. Your talk was great in terms of an exuberance of what’s going to be available, but it’s not going to relieve the physician from the burden of being responsible for their use.”
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, asked the presenters about the balance between seeing patients for virtual and in-person visits. “The question is what’s the sweet spot? Are there people you’re willing to see virtually forever?” he asked, noting that he has patients scheduling telemedicine visits that he hasn’t seen since before the COVID-19 pandemic.
“That’s not going to work for me. At some point, you have to lay hands on people,” he said.
Dr. Wells said his current practice is 40% virtual, and his staff converts potential no-shows into a telemedicine consultation over the phone. “My no-show rate has gone down to zero. Somebody’s scheduled for a visit, they don’t show up, my [medical assistants] get them on the phone, they put them on hold, tee up the refills. I turn them into a telephone call,” he said. “We don’t accept the no-show at all because we can do a telephone [consultation].”
In Dr. Cush’s practice, he alternates telemedicine visits with in-person visits. “If you come back for two videos in a row, you’re catching hell from me for that,” he said. Responding to how Dr. Wells incorporates telemedicine into his practice, Dr. Cush said many rheumatologists “don’t have the setups to support the care, and that’s why it’s hard to do and that’s why we’re not as great as we could be.”
“This is the way we were trained. We’re used to seeing these patients in the clinic that often. Not every single patient needs to be seen that frequently if they’re stable and doing fine,” Dr. Wells countered.
Dr. Cush and Dr. Wells reported having financial relationships with numerous pharmaceutical companies.
There was an explosion in the use of telemedicine during the COVID-19 pandemic, but usage has stabilized and varies between specialties. However, telemedicine use is still somewhat high among rheumatologists, according to speakers at the 2023 Rheumatology Winter Clinical Symposium.
Speaking in general about the future of rheumatology, Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said it is up to rheumatologists to adapt to the changing winds in the specialty.
“The future is going to happen no matter what, so the question is, are you going to go along with it? Are you going to be a part of it? Are you going to be resistant to it?” Dr. Cush asked attendees. “Your recent experience with COVID would tell you maybe what your path is going to be if you’re dying to get back to the way it once was.”
Rheumatologists can expect changes in where they work, how they’re paid, increases in their workload, and new innovations in connecting with patients, he said.
“You’re going to be integrating a new style of medicine, you’re going to be digitally connected,” he explained. “All these networks are going to be working together to make you supposedly better at what you do, or maybe they’re working together to make you obsolete – and I think you better start protecting your space.”
One major area of change, telemedicine, already occurred as a result of the COVID-19 pandemic and will “begin to dominate” over the next decade, Dr. Cush said. An analysis conducted by consulting firm McKinsey & Company found telehealth usage increased 78-fold between February and April 2020 before leveling off at a 38-fold higher rate, compared with prepandemic levels. In the same analysis, rheumatology ranked third in terms of telehealth usage claims behind psychiatry and substance use disorder treatment, Dr. Cush observed, as other specialties have “fallen off quite a bit.”
“The common denominators are chronic care, cognitive care, nonprocedural care, pattern recognition, and monitoring, and this is what you do,” he said. “This is why, in many ways, for you to abandon telemedicine I think is a gigantic mistake.”
Changes to telemedicine
The most immediate change to telemedicine will come when the Biden administration officially ends the COVID-19 public health emergency in May 2023, and temporary telehealth services will be extended for approximately 5 months after the end of the public health emergency. Legislation passed by Congress will ensure some of the flexibilities in telemedicine will be extended until the end of December 2024.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said he sees telemedicine as persisting even after the official COVID-19 public health emergency ends. “There’s a lot of push from the American Medical Association, from the American College of Physicians. You’re going to see people – this will not go away because [there’s] also going to be that demand.”
Despite decreased usage since April 2020, telehealth was estimated to be a $60 billion industry in 2022 and will likely increase over the next decade, Dr. Cush noted. “I question [the decline] because I think it still is a major part of your [future in] 2033.”
The number of physicians who have at least three licenses to practice in other U.S. states increased from 50,454 in 2010 to 72,752 in 2020, and that trend will continue, Dr. Wells explained. It is now becoming easier for physicians to become licensed in other states with companies like CompHealth that offer services to simplify obtaining medical licenses with states that participate in the Interstate Medical Licensure Compact.
“It’s a telemedicine easy pass,” Dr. Cush said.
Concerns in telemedicine
Commenting on the presentation, Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at the Hospital for Special Surgery (HSS), both in New York, pointed out that because telemedicine is governed by U.S. states, rather than the federal government, a physician needs to be licensed in the state where the patient is located. While many states relaxed their restrictions during COVID-19, as states began tightening their restrictions later, “many physicians didn’t want to have three licenses,” he said.
“There’s an expense in getting three licenses. There’s an expense in obtaining it and maintaining it, and the reimbursement for the telemedicine visit did not reach that expectation,” Dr. Gibofsky explained. With the exception of the orthopedic surgeons at HSS who practice in New York and a satellite office in Florida, none of the surgeons at his center have obtained more than one license to practice telemedicine in other states.
“Our volume of telemedicine at HSS has remained about the same at 30%, but fewer physicians are doing it because they don’t want to maintain multiple licensures,” he said. “So don’t overlook the role of legal concerns in terms of who’s going to be allowed to do what where. Your talk was great in terms of an exuberance of what’s going to be available, but it’s not going to relieve the physician from the burden of being responsible for their use.”
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, asked the presenters about the balance between seeing patients for virtual and in-person visits. “The question is what’s the sweet spot? Are there people you’re willing to see virtually forever?” he asked, noting that he has patients scheduling telemedicine visits that he hasn’t seen since before the COVID-19 pandemic.
“That’s not going to work for me. At some point, you have to lay hands on people,” he said.
Dr. Wells said his current practice is 40% virtual, and his staff converts potential no-shows into a telemedicine consultation over the phone. “My no-show rate has gone down to zero. Somebody’s scheduled for a visit, they don’t show up, my [medical assistants] get them on the phone, they put them on hold, tee up the refills. I turn them into a telephone call,” he said. “We don’t accept the no-show at all because we can do a telephone [consultation].”
In Dr. Cush’s practice, he alternates telemedicine visits with in-person visits. “If you come back for two videos in a row, you’re catching hell from me for that,” he said. Responding to how Dr. Wells incorporates telemedicine into his practice, Dr. Cush said many rheumatologists “don’t have the setups to support the care, and that’s why it’s hard to do and that’s why we’re not as great as we could be.”
“This is the way we were trained. We’re used to seeing these patients in the clinic that often. Not every single patient needs to be seen that frequently if they’re stable and doing fine,” Dr. Wells countered.
Dr. Cush and Dr. Wells reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2023
Pulmonary embolism workup needed for any sudden onset of exertional dyspnea
A diagnostic workup for pulmonary embolism (PE) should be performed in all patients with recent onset of exertional dyspnea, according to the authors of an article published in the Journal of Thrombosis and Haemostasis. That conclusion emerged from an analysis of PE prevalence in 417 patients with recent marked exertional dyspnea performing previously well-tolerated physical activities.
Exertional dyspnea is a frequently encountered complaint in clinical practice. Missteps in both diagnosis and early management, however, have been found to be prevalent in emergency department practices. with a complicated clinical course or mortality as a consequence, stated the researchers. Also, failure to diagnose PE is a common malpractice allegation.
Noting that the prevalence of PE among patients with dyspnea on exertion has not been reported, the authors hypothesized: “PE might be a frequent underlying condition in patients presenting for care complaining of marked dyspnea on exertion of recent onset.”
In a multicenter prospective, cross-sectional study among 14 university or hospital centers in Italy, patients who were referred for outpatient evaluation with recent (< 1 month) dyspnea on exertion with a severity of 3 or 4 on the modified Medical Research Council dyspnea scale were potentially eligible for the study. Prior deep-vein thrombosis (DVT), PE, and use of therapeutic anticoagulation were among exclusion criteria. All patients aged 75 years or younger with recent (< 1 month) marked exertional dyspnea had a systematic workup for PE, irrespective of concomitant signs or symptoms of venous thromboembolism and alternative explanations for dyspnea. The main study outcome was prevalence of PE in the entire cohort of patients with recent marked dyspnea on exertion.
When about 400 patients had been enrolled after an interim analysis in which the preestablished stopping rule (if the lower limit of the 95% confidence interval of the prevalence of PE exceeds 20%) was met, the study was prematurely terminated. PE was found, after exclusion of 134 patients based on low PE clinical probability and normal D-dimer, in 134 (47.3%) of the remaining 283 patients. The overall PE prevalence was 32.1% (95% confidence interval, 27.8-36.8).
PE was present in 40 of 204 (19.6%) patients without other findings suspicious for PE and in 94 of 213 patients (44.1%) with PE-suspicious findings. PE involved a main pulmonary artery in 37% and multiple lobes in 87% of the patients.
The researchers pointed out that, while the prevalence of PE was highest (44%) in patients who had concomitant signs or symptoms suspicious of PE or underlying DVT, PE was detected in almost 20% of patients without concomitant PE signs and symptoms. Also, the detected pulmonary emboli were deemed significant.
“Our findings suggest that, regardless of the diagnostic algorithm in use, physicians should rule in or out PE in patients who solely report recent onset of marked dyspnea on exertion,” they concluded.
Agreeing with the authors’ conclusions, Mary Jo S. Farmer, MD, PhD, of the department of medicine at University of Massachusetts, Worcester, stated in an interview, “The results of the current study support a diagnostic workup for pulmonary embolus in all patients with recent onset of exertional dyspnea.” She added, “Pulmonary emboli detected were significant as almost all were segmental or more proximal emboli involving multiple lobes. The observed overall prevalence of pulmonary embolus of 32% may seem high when compared with the low prevalence of 7%-13% reported in other studies of patients with suspected pulmonary embolus. However, the prevalence of pulmonary embolus among emergency department cohorts in European countries is generally higher, as is the diagnostic yield from [CT pulmonary angiogram] compared to North American countries. This could be explained by differences in applied thresholds for suspicion of pulmonary embolus. The incidence of COVID-19 and association with thrombosis was not reported.
“It has been reported that nonspecific clinical manifestations and absence of typical signs and symptoms can result in delay in diagnosis of pulmonary embolus or result in pulmonary embolus being missed, an unfortunate situation that could result in malpractice allegation.” Dr. Farmer concluded.
Among limitations of the study, the authors noted that their results are not applicable to patients older than 75 years or patients with chronic (more than 1 month) symptoms of dyspnea or less severe dyspnea (modified Medical Research Council dyspnea score of 2 or lower). Also, no attempt to stratify the clinical relevance of PE was made.
The study was funded by the Arianna Foundation on Anticoagulation, Bologna, Italy. The authors reported that they had no potential conflicts. Dr. Farmer also declared she had no relevant conflicts.
A diagnostic workup for pulmonary embolism (PE) should be performed in all patients with recent onset of exertional dyspnea, according to the authors of an article published in the Journal of Thrombosis and Haemostasis. That conclusion emerged from an analysis of PE prevalence in 417 patients with recent marked exertional dyspnea performing previously well-tolerated physical activities.
Exertional dyspnea is a frequently encountered complaint in clinical practice. Missteps in both diagnosis and early management, however, have been found to be prevalent in emergency department practices. with a complicated clinical course or mortality as a consequence, stated the researchers. Also, failure to diagnose PE is a common malpractice allegation.
Noting that the prevalence of PE among patients with dyspnea on exertion has not been reported, the authors hypothesized: “PE might be a frequent underlying condition in patients presenting for care complaining of marked dyspnea on exertion of recent onset.”
In a multicenter prospective, cross-sectional study among 14 university or hospital centers in Italy, patients who were referred for outpatient evaluation with recent (< 1 month) dyspnea on exertion with a severity of 3 or 4 on the modified Medical Research Council dyspnea scale were potentially eligible for the study. Prior deep-vein thrombosis (DVT), PE, and use of therapeutic anticoagulation were among exclusion criteria. All patients aged 75 years or younger with recent (< 1 month) marked exertional dyspnea had a systematic workup for PE, irrespective of concomitant signs or symptoms of venous thromboembolism and alternative explanations for dyspnea. The main study outcome was prevalence of PE in the entire cohort of patients with recent marked dyspnea on exertion.
When about 400 patients had been enrolled after an interim analysis in which the preestablished stopping rule (if the lower limit of the 95% confidence interval of the prevalence of PE exceeds 20%) was met, the study was prematurely terminated. PE was found, after exclusion of 134 patients based on low PE clinical probability and normal D-dimer, in 134 (47.3%) of the remaining 283 patients. The overall PE prevalence was 32.1% (95% confidence interval, 27.8-36.8).
PE was present in 40 of 204 (19.6%) patients without other findings suspicious for PE and in 94 of 213 patients (44.1%) with PE-suspicious findings. PE involved a main pulmonary artery in 37% and multiple lobes in 87% of the patients.
The researchers pointed out that, while the prevalence of PE was highest (44%) in patients who had concomitant signs or symptoms suspicious of PE or underlying DVT, PE was detected in almost 20% of patients without concomitant PE signs and symptoms. Also, the detected pulmonary emboli were deemed significant.
“Our findings suggest that, regardless of the diagnostic algorithm in use, physicians should rule in or out PE in patients who solely report recent onset of marked dyspnea on exertion,” they concluded.
Agreeing with the authors’ conclusions, Mary Jo S. Farmer, MD, PhD, of the department of medicine at University of Massachusetts, Worcester, stated in an interview, “The results of the current study support a diagnostic workup for pulmonary embolus in all patients with recent onset of exertional dyspnea.” She added, “Pulmonary emboli detected were significant as almost all were segmental or more proximal emboli involving multiple lobes. The observed overall prevalence of pulmonary embolus of 32% may seem high when compared with the low prevalence of 7%-13% reported in other studies of patients with suspected pulmonary embolus. However, the prevalence of pulmonary embolus among emergency department cohorts in European countries is generally higher, as is the diagnostic yield from [CT pulmonary angiogram] compared to North American countries. This could be explained by differences in applied thresholds for suspicion of pulmonary embolus. The incidence of COVID-19 and association with thrombosis was not reported.
“It has been reported that nonspecific clinical manifestations and absence of typical signs and symptoms can result in delay in diagnosis of pulmonary embolus or result in pulmonary embolus being missed, an unfortunate situation that could result in malpractice allegation.” Dr. Farmer concluded.
Among limitations of the study, the authors noted that their results are not applicable to patients older than 75 years or patients with chronic (more than 1 month) symptoms of dyspnea or less severe dyspnea (modified Medical Research Council dyspnea score of 2 or lower). Also, no attempt to stratify the clinical relevance of PE was made.
The study was funded by the Arianna Foundation on Anticoagulation, Bologna, Italy. The authors reported that they had no potential conflicts. Dr. Farmer also declared she had no relevant conflicts.
A diagnostic workup for pulmonary embolism (PE) should be performed in all patients with recent onset of exertional dyspnea, according to the authors of an article published in the Journal of Thrombosis and Haemostasis. That conclusion emerged from an analysis of PE prevalence in 417 patients with recent marked exertional dyspnea performing previously well-tolerated physical activities.
Exertional dyspnea is a frequently encountered complaint in clinical practice. Missteps in both diagnosis and early management, however, have been found to be prevalent in emergency department practices. with a complicated clinical course or mortality as a consequence, stated the researchers. Also, failure to diagnose PE is a common malpractice allegation.
Noting that the prevalence of PE among patients with dyspnea on exertion has not been reported, the authors hypothesized: “PE might be a frequent underlying condition in patients presenting for care complaining of marked dyspnea on exertion of recent onset.”
In a multicenter prospective, cross-sectional study among 14 university or hospital centers in Italy, patients who were referred for outpatient evaluation with recent (< 1 month) dyspnea on exertion with a severity of 3 or 4 on the modified Medical Research Council dyspnea scale were potentially eligible for the study. Prior deep-vein thrombosis (DVT), PE, and use of therapeutic anticoagulation were among exclusion criteria. All patients aged 75 years or younger with recent (< 1 month) marked exertional dyspnea had a systematic workup for PE, irrespective of concomitant signs or symptoms of venous thromboembolism and alternative explanations for dyspnea. The main study outcome was prevalence of PE in the entire cohort of patients with recent marked dyspnea on exertion.
When about 400 patients had been enrolled after an interim analysis in which the preestablished stopping rule (if the lower limit of the 95% confidence interval of the prevalence of PE exceeds 20%) was met, the study was prematurely terminated. PE was found, after exclusion of 134 patients based on low PE clinical probability and normal D-dimer, in 134 (47.3%) of the remaining 283 patients. The overall PE prevalence was 32.1% (95% confidence interval, 27.8-36.8).
PE was present in 40 of 204 (19.6%) patients without other findings suspicious for PE and in 94 of 213 patients (44.1%) with PE-suspicious findings. PE involved a main pulmonary artery in 37% and multiple lobes in 87% of the patients.
The researchers pointed out that, while the prevalence of PE was highest (44%) in patients who had concomitant signs or symptoms suspicious of PE or underlying DVT, PE was detected in almost 20% of patients without concomitant PE signs and symptoms. Also, the detected pulmonary emboli were deemed significant.
“Our findings suggest that, regardless of the diagnostic algorithm in use, physicians should rule in or out PE in patients who solely report recent onset of marked dyspnea on exertion,” they concluded.
Agreeing with the authors’ conclusions, Mary Jo S. Farmer, MD, PhD, of the department of medicine at University of Massachusetts, Worcester, stated in an interview, “The results of the current study support a diagnostic workup for pulmonary embolus in all patients with recent onset of exertional dyspnea.” She added, “Pulmonary emboli detected were significant as almost all were segmental or more proximal emboli involving multiple lobes. The observed overall prevalence of pulmonary embolus of 32% may seem high when compared with the low prevalence of 7%-13% reported in other studies of patients with suspected pulmonary embolus. However, the prevalence of pulmonary embolus among emergency department cohorts in European countries is generally higher, as is the diagnostic yield from [CT pulmonary angiogram] compared to North American countries. This could be explained by differences in applied thresholds for suspicion of pulmonary embolus. The incidence of COVID-19 and association with thrombosis was not reported.
“It has been reported that nonspecific clinical manifestations and absence of typical signs and symptoms can result in delay in diagnosis of pulmonary embolus or result in pulmonary embolus being missed, an unfortunate situation that could result in malpractice allegation.” Dr. Farmer concluded.
Among limitations of the study, the authors noted that their results are not applicable to patients older than 75 years or patients with chronic (more than 1 month) symptoms of dyspnea or less severe dyspnea (modified Medical Research Council dyspnea score of 2 or lower). Also, no attempt to stratify the clinical relevance of PE was made.
The study was funded by the Arianna Foundation on Anticoagulation, Bologna, Italy. The authors reported that they had no potential conflicts. Dr. Farmer also declared she had no relevant conflicts.
FROM THE JOURNAL OF THROMBOSIS AND HAEMOSTASIS
Hormonal contraception and lactation: Reset your practices based on the evidence
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.
Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8
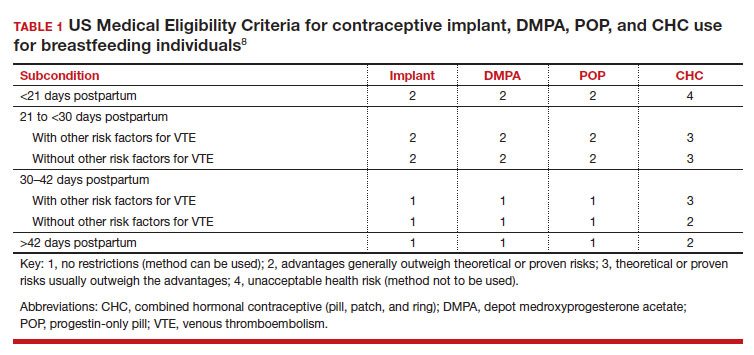
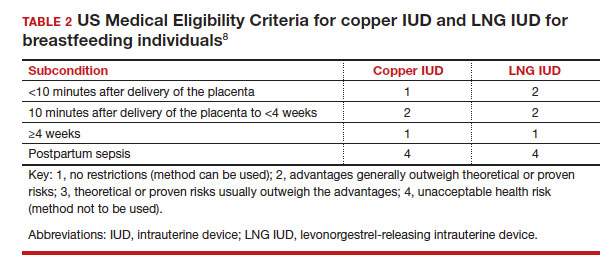
Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.

Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8


Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.

Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8


Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...
How can clinicians integrate evidence into contraception counseling?
The American College of Obstetricians and Gynecologists and the Academy of Breastfeeding Medicine offer guidance for how clinicians can address the use of hormonal contraception in breastfeeding patients. Both organizations recommend discussing the risks and benefits of hormonal contraception within the context of each person’s desire to breastfeed, potential for breastfeeding difficulty, and risk of pregnancy so that individuals can make their own informed decisions.17,18
Obstetric care clinicians have an important role in helping patients make informed infant feeding decisions without coercion or pressure. To start these discussions, clinicians can begin by assessing a patient’s breastfeeding goals by asking open-ended questions, such as:
- What have you heard about breastfeeding?
- What are your plans for returning to work or school after delivery?
- How did breastfeeding go with older children?
- What are your plans for feeding this baby?
In addition to gathering information about the patient’s priorities and goals, clinicians should identify any risk factors for breastfeeding challenges in the medical, surgical, or previous breastfeeding history. Clinicians can engage in a patient-centered approach to infant feeding decisions by anticipating any challenges and working together to develop strategies to address these challenges with the patient’s goals in mind.17
When counseling about contraception, a spectrum of approaches exists, from a nondirective information-sharing only model to directive counseling by the clinician. The shared decision-making model lies between these 2 approaches and recognizes the expertise of both the clinician and patient.19 To start these interactions, clinicians can ask about a patient’s reproductive goals by assessing the patient’s needs, values, and preferences for contraception. Potential questions include:
- What kinds of contraceptive methods have you used in the past?
- What is important to you in a contraceptive method?
- How important is it to you to avoid another pregnancy right now?
Clinicians can then share information about different contraceptive methods based on the desired qualities that the patient has identified and how each method fits or does not fit into the patient’s goals and preferences. This collaborative approach facilitates an open dialogue and supports patient autonomy in contraceptive decision-making.
Lastly, clinicians should be cognizant of their own potential biases that could affect their counseling, such as encouraging contraceptive use because of a patient’s young age, parity, or premature delivery, as in our case presentation. Similarly, clinicians also should recognize that breastfeeding and contraceptive decisions are personal and are made with cultural, historical, and social contexts in mind.20 Ultimately, counseling should be patient centered and individualized for each person’s priorities related to infant feeding and pregnancy prevention. ●
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
- Meek JY, Noble L; Section on Breastfeeding. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150:e2022057988.
- Centers for Disease Control and Prevention. Breastfeeding report card, United States 2022. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/pdf/2022-Breast feeding-Report-Card-H.pdf
- Centers for Disease Control and Prevention. Rates of any and exclusive breastfeeding by sociodemographic characteristic among children born in 2019. Accessed November 8, 2022. https://www.cdc.gov/breastfeeding/data/nis_data/data-files/2019/rates-any-exclusive-bf-socio-dem-2019.html
- American College of Obstetricians and Gynecologists. Committee opinion no. 821: barriers to breastfeeding: supporting initiation and continuation of breastfeeding. Obstet Gynecol. 2021;137:e54-e62.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12:211-221.
- Brownell E, Howard CR, Lawrence RA, et al. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. 2012;161:608-614.
- American College of Obstetricians and Gynecologists. Committee opinion no. 820: breastfeeding challenges. Obstet Gynecol. 2021;137:e42-e53.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(RR-3):1-104.
- Turok DK, Leeman L, Sanders JN, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol. 2017;217:665.e1-665.e8.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674-679.
- Carmo LSMP, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Averbach S, Kakaire O, McDiehl R, et al. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019;99:87-93.
- Krashin JW, Lemani C, Nkambule J, et al. A comparison of breastfeeding exclusivity and duration rates between immediate postpartum levonorgestrel versus etonogestrel implant users: a prospective cohort study. Breastfeed Med. 2019;14:69-76.
- Henkel A, Lerma K, Reyes G, et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am J Obstet Gynecol. 2023; 228:55.e1-55.e9.
- Parker LA, Sullivan S, Cacho N, et al. Effect of postpartum depo medroxyprogesterone acetate on lactation in mothers of very low-birth-weight infants. Breastfeed Med. 2021;16:835-842.
- Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13:334-342.
- American College of Obstetricians and Gynecologists. Committee opinion no. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
- Berens P, Labbok M; Academy of Breastfeeding Medicine. ABM Clinical Protocol #13: contraception during breastfeeding, revised 2015. Breastfeed Med. 2015;10:3-12.
- American College of Obstetricians and Gynecologists, Committee on Health Care for Underserved Women, Contraceptive Equity Expert Work Group, and Committee on Ethics. Committee statement no. 1: patient-centered contraceptive counseling. Obstet Gynecol. 2022;139:350-353.
- Bryant AG, Lyerly AD, DeVane-Johnson S, et al. Hormonal contraception, breastfeeding and bedside advocacy: the case for patient-centered care. Contraception. 2019;99:73-76.
Is it time to reconsider Rh testing and Rh D immune globulin treatment for miscarriage and abortion care in early pregnancy?
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.