User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Will the Federal Non-Compete Ban Take Effect?
(with very limited exceptions). The final rule will not go into effect until 120 days after its publication in the Federal Register, which took place on May 7, and numerous legal challenges appear to be on the horizon.
The principal components of the rule are as follows:
- After the effective date, most non-compete agreements (which prevent departing employees from signing with a new employer for a defined period within a specific geographic area) are banned nationwide.
- The rule exempts certain “senior executives,” ie individuals who earn more than $151,164 annually and serve in policy-making positions.
- There is another major exception for non-competes connected with a sale of a business.
- While not explicitly stated, the rule arguably exempts non-profits, tax-exempt hospitals, and other tax-exempt entities.
- Employers must provide verbal and written notice to employees regarding existing agreements, which would be voided under the rule.
The final rule is the latest skirmish in an ongoing, years-long debate. Twelve states have already put non-compete bans in place, according to a recent paper, and they may serve as a harbinger of things to come should the federal ban go into effect. Each state rule varies in its specifics as states respond to local market conditions. While some states ban all non-compete agreements outright, others limit them based on variables, such as income and employment circumstances. Of course, should the federal ban take effect, it will supersede whatever rules the individual states have in place.
In drafting the rule, the FTC reasoned that non-compete clauses constitute restraint of trade, and eliminating them could potentially increase worker earnings as well as lower health care costs by billions of dollars. In its statements on the proposed ban, the FTC claimed that it could lower health spending across the board by almost $150 billion per year and return $300 million to workers each year in earnings. The agency cited a large body of research that non-competes make it harder for workers to move between jobs and can raise prices for goods and services, while suppressing wages for workers and inhibiting the creation of new businesses.
Most physicians affected by non-compete agreements heavily favor the new rule, because it would give them more control over their careers and expand their practice and income opportunities. It would allow them to get a new job with a competing organization, bucking a long-standing trend that hospitals and health care systems have heavily relied on to keep staff in place.
The rule would, however, keep in place “non-solicitation” rules that many health care organizations have put in place. That means that if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to join him or her at the new employment venue.
Within that clause, however, the FTC has specified that if such non-solicitation agreement has the “equivalent effect” of a non-compete, the agency would deem it such. That means, even if that rule stands, it could be contested and may be interpreted as violating the non-compete provision. So, there is value in reading all the fine print should the rule move forward.
Physicians in independent practices who employ physician assistants and nurse practitioners have expressed concerns that their expensively trained employees might be tempted to accept a nearby, higher-paying position. The “non-solicitation” clause would theoretically prevent them from taking patients and co-workers with them — unless it were successfully contested. Many questions remain.
Further complicating the non-compete ban issue is how it might impact nonprofit institutions. Most hospitals structured as nonprofits would theoretically be exempt from the rule, although it is not specifically stated in the rule itself, because the FTC Act gives the Commission jurisdiction over for-profit companies only. This would obviously create an unfair advantage for nonprofits, who could continue writing non-compete clauses with impunity.
All of these questions may be moot, of course, because a number of powerful entities with deep pockets have lined up in opposition to the rule. Some of them have even questioned the FTC’s authority to pass the rule at all, on the grounds that Section 5 of the FTC Act does not give it the authority to police labor markets. A lawsuit has already been filed by the US Chamber of Commerce. Other large groups in opposition are the American Medical Group Association, the American Hospital Association, and numerous large hospital and healthcare networks.
Only time will tell whether this issue will be regulated on a national level or remain the purview of each individual state.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
(with very limited exceptions). The final rule will not go into effect until 120 days after its publication in the Federal Register, which took place on May 7, and numerous legal challenges appear to be on the horizon.
The principal components of the rule are as follows:
- After the effective date, most non-compete agreements (which prevent departing employees from signing with a new employer for a defined period within a specific geographic area) are banned nationwide.
- The rule exempts certain “senior executives,” ie individuals who earn more than $151,164 annually and serve in policy-making positions.
- There is another major exception for non-competes connected with a sale of a business.
- While not explicitly stated, the rule arguably exempts non-profits, tax-exempt hospitals, and other tax-exempt entities.
- Employers must provide verbal and written notice to employees regarding existing agreements, which would be voided under the rule.
The final rule is the latest skirmish in an ongoing, years-long debate. Twelve states have already put non-compete bans in place, according to a recent paper, and they may serve as a harbinger of things to come should the federal ban go into effect. Each state rule varies in its specifics as states respond to local market conditions. While some states ban all non-compete agreements outright, others limit them based on variables, such as income and employment circumstances. Of course, should the federal ban take effect, it will supersede whatever rules the individual states have in place.
In drafting the rule, the FTC reasoned that non-compete clauses constitute restraint of trade, and eliminating them could potentially increase worker earnings as well as lower health care costs by billions of dollars. In its statements on the proposed ban, the FTC claimed that it could lower health spending across the board by almost $150 billion per year and return $300 million to workers each year in earnings. The agency cited a large body of research that non-competes make it harder for workers to move between jobs and can raise prices for goods and services, while suppressing wages for workers and inhibiting the creation of new businesses.
Most physicians affected by non-compete agreements heavily favor the new rule, because it would give them more control over their careers and expand their practice and income opportunities. It would allow them to get a new job with a competing organization, bucking a long-standing trend that hospitals and health care systems have heavily relied on to keep staff in place.
The rule would, however, keep in place “non-solicitation” rules that many health care organizations have put in place. That means that if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to join him or her at the new employment venue.
Within that clause, however, the FTC has specified that if such non-solicitation agreement has the “equivalent effect” of a non-compete, the agency would deem it such. That means, even if that rule stands, it could be contested and may be interpreted as violating the non-compete provision. So, there is value in reading all the fine print should the rule move forward.
Physicians in independent practices who employ physician assistants and nurse practitioners have expressed concerns that their expensively trained employees might be tempted to accept a nearby, higher-paying position. The “non-solicitation” clause would theoretically prevent them from taking patients and co-workers with them — unless it were successfully contested. Many questions remain.
Further complicating the non-compete ban issue is how it might impact nonprofit institutions. Most hospitals structured as nonprofits would theoretically be exempt from the rule, although it is not specifically stated in the rule itself, because the FTC Act gives the Commission jurisdiction over for-profit companies only. This would obviously create an unfair advantage for nonprofits, who could continue writing non-compete clauses with impunity.
All of these questions may be moot, of course, because a number of powerful entities with deep pockets have lined up in opposition to the rule. Some of them have even questioned the FTC’s authority to pass the rule at all, on the grounds that Section 5 of the FTC Act does not give it the authority to police labor markets. A lawsuit has already been filed by the US Chamber of Commerce. Other large groups in opposition are the American Medical Group Association, the American Hospital Association, and numerous large hospital and healthcare networks.
Only time will tell whether this issue will be regulated on a national level or remain the purview of each individual state.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
(with very limited exceptions). The final rule will not go into effect until 120 days after its publication in the Federal Register, which took place on May 7, and numerous legal challenges appear to be on the horizon.
The principal components of the rule are as follows:
- After the effective date, most non-compete agreements (which prevent departing employees from signing with a new employer for a defined period within a specific geographic area) are banned nationwide.
- The rule exempts certain “senior executives,” ie individuals who earn more than $151,164 annually and serve in policy-making positions.
- There is another major exception for non-competes connected with a sale of a business.
- While not explicitly stated, the rule arguably exempts non-profits, tax-exempt hospitals, and other tax-exempt entities.
- Employers must provide verbal and written notice to employees regarding existing agreements, which would be voided under the rule.
The final rule is the latest skirmish in an ongoing, years-long debate. Twelve states have already put non-compete bans in place, according to a recent paper, and they may serve as a harbinger of things to come should the federal ban go into effect. Each state rule varies in its specifics as states respond to local market conditions. While some states ban all non-compete agreements outright, others limit them based on variables, such as income and employment circumstances. Of course, should the federal ban take effect, it will supersede whatever rules the individual states have in place.
In drafting the rule, the FTC reasoned that non-compete clauses constitute restraint of trade, and eliminating them could potentially increase worker earnings as well as lower health care costs by billions of dollars. In its statements on the proposed ban, the FTC claimed that it could lower health spending across the board by almost $150 billion per year and return $300 million to workers each year in earnings. The agency cited a large body of research that non-competes make it harder for workers to move between jobs and can raise prices for goods and services, while suppressing wages for workers and inhibiting the creation of new businesses.
Most physicians affected by non-compete agreements heavily favor the new rule, because it would give them more control over their careers and expand their practice and income opportunities. It would allow them to get a new job with a competing organization, bucking a long-standing trend that hospitals and health care systems have heavily relied on to keep staff in place.
The rule would, however, keep in place “non-solicitation” rules that many health care organizations have put in place. That means that if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to join him or her at the new employment venue.
Within that clause, however, the FTC has specified that if such non-solicitation agreement has the “equivalent effect” of a non-compete, the agency would deem it such. That means, even if that rule stands, it could be contested and may be interpreted as violating the non-compete provision. So, there is value in reading all the fine print should the rule move forward.
Physicians in independent practices who employ physician assistants and nurse practitioners have expressed concerns that their expensively trained employees might be tempted to accept a nearby, higher-paying position. The “non-solicitation” clause would theoretically prevent them from taking patients and co-workers with them — unless it were successfully contested. Many questions remain.
Further complicating the non-compete ban issue is how it might impact nonprofit institutions. Most hospitals structured as nonprofits would theoretically be exempt from the rule, although it is not specifically stated in the rule itself, because the FTC Act gives the Commission jurisdiction over for-profit companies only. This would obviously create an unfair advantage for nonprofits, who could continue writing non-compete clauses with impunity.
All of these questions may be moot, of course, because a number of powerful entities with deep pockets have lined up in opposition to the rule. Some of them have even questioned the FTC’s authority to pass the rule at all, on the grounds that Section 5 of the FTC Act does not give it the authority to police labor markets. A lawsuit has already been filed by the US Chamber of Commerce. Other large groups in opposition are the American Medical Group Association, the American Hospital Association, and numerous large hospital and healthcare networks.
Only time will tell whether this issue will be regulated on a national level or remain the purview of each individual state.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Fluoride, Water, and Kids’ Brains: It’s Complicated
This transcript has been edited for clarity.
I recently looked back at my folder full of these medical study commentaries, this weekly video series we call Impact Factor, and realized that I’ve been doing this for a long time. More than 400 articles, believe it or not.
I’ve learned a lot in that time — about medicine, of course — but also about how people react to certain topics. If you’ve been with me this whole time, or even for just a chunk of it, you’ll know that I tend to take a measured approach to most topics. No one study is ever truly definitive, after all. But regardless of how even-keeled I may be, there are some topics that I just know in advance are going to be a bit divisive: studies about gun control; studies about vitamin D; and, of course, studies about fluoride.
Shall We Shake This Hornet’s Nest?
The fluoridation of the US water system began in 1945 with the goal of reducing cavities in the population. The CDC named water fluoridation one of the 10 great public health achievements of the 20th century, along with such inarguable achievements as the recognition of tobacco as a health hazard.
But fluoridation has never been without its detractors. One problem is that the spectrum of beliefs about the potential harm of fluoridation is huge. On one end, you have science-based concerns such as the recognition that excessive fluoride intake can cause fluorosis and stain tooth enamel. I’ll note that the EPA regulates fluoride levels — there is a fair amount of naturally occurring fluoride in water tables around the world — to prevent this. And, of course, on the other end of the spectrum, you have beliefs that are essentially conspiracy theories: “They” add fluoride to the water supply to control us.
The challenge for me is that when one “side” of a scientific debate includes the crazy theories, it can be hard to discuss that whole spectrum, since there are those who will see evidence of any adverse fluoride effect as confirmation that the conspiracy theory is true.
I can’t help this. So I’ll just say this up front: I am about to tell you about a study that shows some potential risk from fluoride exposure. I will tell you up front that there are some significant caveats to the study that call the results into question. And I will tell you up front that no one is controlling your mind, or my mind, with fluoride; they do it with social media.
Let’s Dive Into These Shark-Infested, Fluoridated Waters
We’re talking about the study, “Maternal Urinary Fluoride and Child Neurobehavior at Age 36 Months,” which appears in JAMA Network Open.
It’s a study of 229 mother-child pairs from the Los Angeles area. The moms had their urinary fluoride level measured once before 30 weeks of gestation. A neurobehavioral battery called the Preschool Child Behavior Checklist was administered to the children at age 36 months.
The main thing you’ll hear about this study — in headlines, Facebook posts, and manifestos locked in drawers somewhere — is the primary result: A 0.68-mg/L increase in urinary fluoride in the mothers, about 25 percentile points, was associated with a doubling of the risk for neurobehavioral problems in their kids when they were 3 years old.
Yikes.
But this is not a randomized trial. Researchers didn’t randomly assign some women to have high fluoride intake and some women to have low fluoride intake. They knew that other factors that might lead to neurobehavioral problems could also lead to higher fluoride intake. They represent these factors in what’s known as a directed acyclic graph, as seen here, and account for them statistically using a regression equation.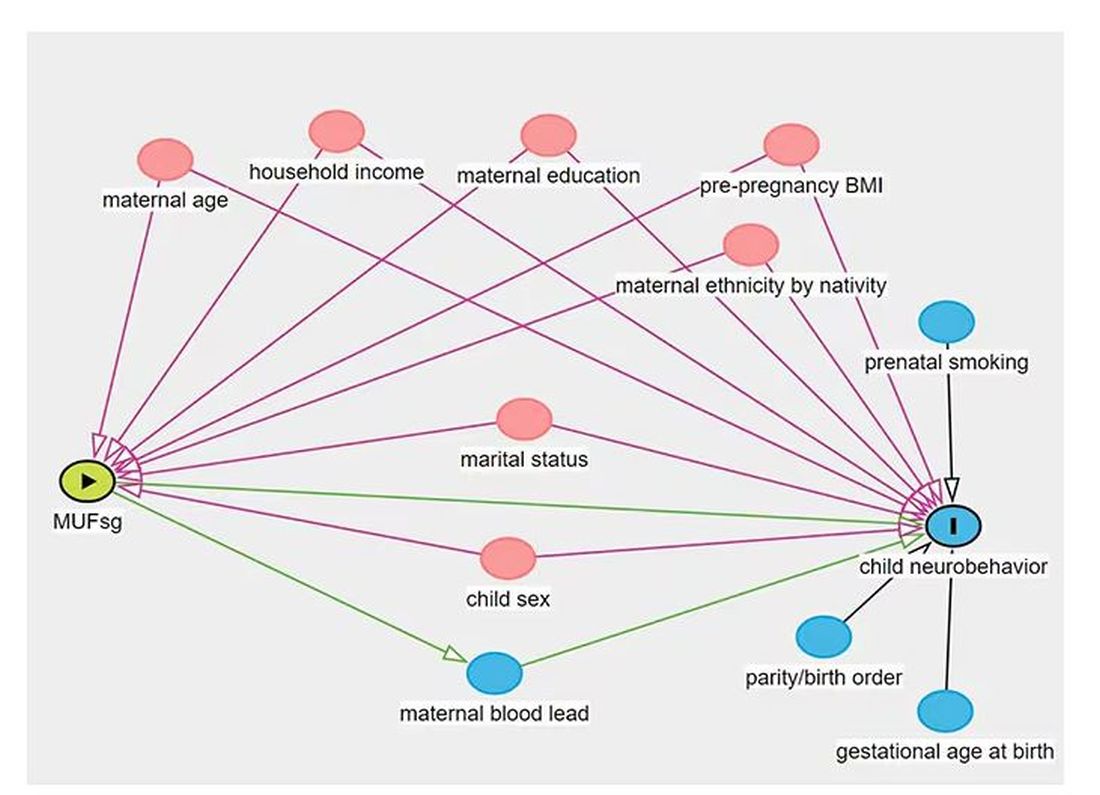
Not represented here are neighborhood characteristics. Los Angeles does not have uniformly fluoridated water, and neurobehavioral problems in kids are strongly linked to stressors in their environments. Fluoride level could be an innocent bystander.
I’m really just describing the classic issue of correlation versus causation here, the bane of all observational research and — let’s be honest — a bit of a crutch that allows us to disregard the results of studies we don’t like, provided the study wasn’t a randomized trial.
But I have a deeper issue with this study than the old “failure to adjust for relevant confounders” thing, as important as that is.
The exposure of interest in this study is maternal urinary fluoride, as measured in a spot sample. It’s not often that I get to go deep on nephrology in this space, but let’s think about that for a second. Let’s assume for a moment that fluoride is toxic to the developing fetal brain, the main concern raised by the results of the study. How would that work? Presumably, mom would be ingesting fluoride from various sources (like the water supply), and that fluoride would get into her blood, and from her blood across the placenta to the baby’s blood, and into the baby’s brain.
Is Urinary Fluoride a Good Measure of Blood Fluoride?
It’s not great. Empirically, we have data that tell us that levels of urine fluoride are not all that similar to levels of serum fluoride. In 2014, a study investigated the correlation between urine and serum fluoride in a cohort of 60 schoolchildren and found a correlation coefficient of around 0.5.
Why isn’t urine fluoride a great proxy for serum fluoride? The most obvious reason is the urine concentration. Human urine concentration can range from about 50 mmol to 1200 mmol (a 24-fold difference) depending on hydration status. Over the course of 24 hours, for example, the amount of fluoride you put out in your urine may be fairly stable in relation to intake, but for a spot urine sample it would be wildly variable. The authors know this, of course, and so they divide the measured urine fluoride by the specific gravity of the urine to give a sort of “dilution adjusted” value. That’s what is actually used in this study. But specific gravity is, itself, an imperfect measure of how dilute the urine is.
This is something that comes up a lot in urinary biomarker research and it’s not that hard to get around. The best thing would be to just measure blood levels of fluoride. The second best option is 24-hour fluoride excretion. After that, the next best thing would be to adjust the spot concentration by other markers of urinary dilution — creatinine or osmolality — as sensitivity analyses. Any of these approaches would lend credence to the results of the study.
Urinary fluoride excretion is pH dependent. The more acidic the urine, the less fluoride is excreted. Many things — including, importantly, diet — affect urine pH. And it is not a stretch to think that diet may also affect the developing fetus. Neither urine pH nor dietary habits were accounted for in this study.
So, here we are. We have an observational study suggesting a harm that may be associated with fluoride. There may be a causal link here, in which case we need further studies to weigh the harm against the more well-established public health benefit. Or, this is all correlation — an illusion created by the limitations of observational data, and the unique challenges of estimating intake from a single urine sample. In other words, this study has something for everyone, fluoride boosters and skeptics alike. Let the arguments begin. But, if possible, leave me out of it.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently looked back at my folder full of these medical study commentaries, this weekly video series we call Impact Factor, and realized that I’ve been doing this for a long time. More than 400 articles, believe it or not.
I’ve learned a lot in that time — about medicine, of course — but also about how people react to certain topics. If you’ve been with me this whole time, or even for just a chunk of it, you’ll know that I tend to take a measured approach to most topics. No one study is ever truly definitive, after all. But regardless of how even-keeled I may be, there are some topics that I just know in advance are going to be a bit divisive: studies about gun control; studies about vitamin D; and, of course, studies about fluoride.
Shall We Shake This Hornet’s Nest?
The fluoridation of the US water system began in 1945 with the goal of reducing cavities in the population. The CDC named water fluoridation one of the 10 great public health achievements of the 20th century, along with such inarguable achievements as the recognition of tobacco as a health hazard.
But fluoridation has never been without its detractors. One problem is that the spectrum of beliefs about the potential harm of fluoridation is huge. On one end, you have science-based concerns such as the recognition that excessive fluoride intake can cause fluorosis and stain tooth enamel. I’ll note that the EPA regulates fluoride levels — there is a fair amount of naturally occurring fluoride in water tables around the world — to prevent this. And, of course, on the other end of the spectrum, you have beliefs that are essentially conspiracy theories: “They” add fluoride to the water supply to control us.
The challenge for me is that when one “side” of a scientific debate includes the crazy theories, it can be hard to discuss that whole spectrum, since there are those who will see evidence of any adverse fluoride effect as confirmation that the conspiracy theory is true.
I can’t help this. So I’ll just say this up front: I am about to tell you about a study that shows some potential risk from fluoride exposure. I will tell you up front that there are some significant caveats to the study that call the results into question. And I will tell you up front that no one is controlling your mind, or my mind, with fluoride; they do it with social media.
Let’s Dive Into These Shark-Infested, Fluoridated Waters
We’re talking about the study, “Maternal Urinary Fluoride and Child Neurobehavior at Age 36 Months,” which appears in JAMA Network Open.
It’s a study of 229 mother-child pairs from the Los Angeles area. The moms had their urinary fluoride level measured once before 30 weeks of gestation. A neurobehavioral battery called the Preschool Child Behavior Checklist was administered to the children at age 36 months.
The main thing you’ll hear about this study — in headlines, Facebook posts, and manifestos locked in drawers somewhere — is the primary result: A 0.68-mg/L increase in urinary fluoride in the mothers, about 25 percentile points, was associated with a doubling of the risk for neurobehavioral problems in their kids when they were 3 years old.
Yikes.
But this is not a randomized trial. Researchers didn’t randomly assign some women to have high fluoride intake and some women to have low fluoride intake. They knew that other factors that might lead to neurobehavioral problems could also lead to higher fluoride intake. They represent these factors in what’s known as a directed acyclic graph, as seen here, and account for them statistically using a regression equation.
Not represented here are neighborhood characteristics. Los Angeles does not have uniformly fluoridated water, and neurobehavioral problems in kids are strongly linked to stressors in their environments. Fluoride level could be an innocent bystander.
I’m really just describing the classic issue of correlation versus causation here, the bane of all observational research and — let’s be honest — a bit of a crutch that allows us to disregard the results of studies we don’t like, provided the study wasn’t a randomized trial.
But I have a deeper issue with this study than the old “failure to adjust for relevant confounders” thing, as important as that is.
The exposure of interest in this study is maternal urinary fluoride, as measured in a spot sample. It’s not often that I get to go deep on nephrology in this space, but let’s think about that for a second. Let’s assume for a moment that fluoride is toxic to the developing fetal brain, the main concern raised by the results of the study. How would that work? Presumably, mom would be ingesting fluoride from various sources (like the water supply), and that fluoride would get into her blood, and from her blood across the placenta to the baby’s blood, and into the baby’s brain.
Is Urinary Fluoride a Good Measure of Blood Fluoride?
It’s not great. Empirically, we have data that tell us that levels of urine fluoride are not all that similar to levels of serum fluoride. In 2014, a study investigated the correlation between urine and serum fluoride in a cohort of 60 schoolchildren and found a correlation coefficient of around 0.5.
Why isn’t urine fluoride a great proxy for serum fluoride? The most obvious reason is the urine concentration. Human urine concentration can range from about 50 mmol to 1200 mmol (a 24-fold difference) depending on hydration status. Over the course of 24 hours, for example, the amount of fluoride you put out in your urine may be fairly stable in relation to intake, but for a spot urine sample it would be wildly variable. The authors know this, of course, and so they divide the measured urine fluoride by the specific gravity of the urine to give a sort of “dilution adjusted” value. That’s what is actually used in this study. But specific gravity is, itself, an imperfect measure of how dilute the urine is.
This is something that comes up a lot in urinary biomarker research and it’s not that hard to get around. The best thing would be to just measure blood levels of fluoride. The second best option is 24-hour fluoride excretion. After that, the next best thing would be to adjust the spot concentration by other markers of urinary dilution — creatinine or osmolality — as sensitivity analyses. Any of these approaches would lend credence to the results of the study.
Urinary fluoride excretion is pH dependent. The more acidic the urine, the less fluoride is excreted. Many things — including, importantly, diet — affect urine pH. And it is not a stretch to think that diet may also affect the developing fetus. Neither urine pH nor dietary habits were accounted for in this study.
So, here we are. We have an observational study suggesting a harm that may be associated with fluoride. There may be a causal link here, in which case we need further studies to weigh the harm against the more well-established public health benefit. Or, this is all correlation — an illusion created by the limitations of observational data, and the unique challenges of estimating intake from a single urine sample. In other words, this study has something for everyone, fluoride boosters and skeptics alike. Let the arguments begin. But, if possible, leave me out of it.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently looked back at my folder full of these medical study commentaries, this weekly video series we call Impact Factor, and realized that I’ve been doing this for a long time. More than 400 articles, believe it or not.
I’ve learned a lot in that time — about medicine, of course — but also about how people react to certain topics. If you’ve been with me this whole time, or even for just a chunk of it, you’ll know that I tend to take a measured approach to most topics. No one study is ever truly definitive, after all. But regardless of how even-keeled I may be, there are some topics that I just know in advance are going to be a bit divisive: studies about gun control; studies about vitamin D; and, of course, studies about fluoride.
Shall We Shake This Hornet’s Nest?
The fluoridation of the US water system began in 1945 with the goal of reducing cavities in the population. The CDC named water fluoridation one of the 10 great public health achievements of the 20th century, along with such inarguable achievements as the recognition of tobacco as a health hazard.
But fluoridation has never been without its detractors. One problem is that the spectrum of beliefs about the potential harm of fluoridation is huge. On one end, you have science-based concerns such as the recognition that excessive fluoride intake can cause fluorosis and stain tooth enamel. I’ll note that the EPA regulates fluoride levels — there is a fair amount of naturally occurring fluoride in water tables around the world — to prevent this. And, of course, on the other end of the spectrum, you have beliefs that are essentially conspiracy theories: “They” add fluoride to the water supply to control us.
The challenge for me is that when one “side” of a scientific debate includes the crazy theories, it can be hard to discuss that whole spectrum, since there are those who will see evidence of any adverse fluoride effect as confirmation that the conspiracy theory is true.
I can’t help this. So I’ll just say this up front: I am about to tell you about a study that shows some potential risk from fluoride exposure. I will tell you up front that there are some significant caveats to the study that call the results into question. And I will tell you up front that no one is controlling your mind, or my mind, with fluoride; they do it with social media.
Let’s Dive Into These Shark-Infested, Fluoridated Waters
We’re talking about the study, “Maternal Urinary Fluoride and Child Neurobehavior at Age 36 Months,” which appears in JAMA Network Open.
It’s a study of 229 mother-child pairs from the Los Angeles area. The moms had their urinary fluoride level measured once before 30 weeks of gestation. A neurobehavioral battery called the Preschool Child Behavior Checklist was administered to the children at age 36 months.
The main thing you’ll hear about this study — in headlines, Facebook posts, and manifestos locked in drawers somewhere — is the primary result: A 0.68-mg/L increase in urinary fluoride in the mothers, about 25 percentile points, was associated with a doubling of the risk for neurobehavioral problems in their kids when they were 3 years old.
Yikes.
But this is not a randomized trial. Researchers didn’t randomly assign some women to have high fluoride intake and some women to have low fluoride intake. They knew that other factors that might lead to neurobehavioral problems could also lead to higher fluoride intake. They represent these factors in what’s known as a directed acyclic graph, as seen here, and account for them statistically using a regression equation.
Not represented here are neighborhood characteristics. Los Angeles does not have uniformly fluoridated water, and neurobehavioral problems in kids are strongly linked to stressors in their environments. Fluoride level could be an innocent bystander.
I’m really just describing the classic issue of correlation versus causation here, the bane of all observational research and — let’s be honest — a bit of a crutch that allows us to disregard the results of studies we don’t like, provided the study wasn’t a randomized trial.
But I have a deeper issue with this study than the old “failure to adjust for relevant confounders” thing, as important as that is.
The exposure of interest in this study is maternal urinary fluoride, as measured in a spot sample. It’s not often that I get to go deep on nephrology in this space, but let’s think about that for a second. Let’s assume for a moment that fluoride is toxic to the developing fetal brain, the main concern raised by the results of the study. How would that work? Presumably, mom would be ingesting fluoride from various sources (like the water supply), and that fluoride would get into her blood, and from her blood across the placenta to the baby’s blood, and into the baby’s brain.
Is Urinary Fluoride a Good Measure of Blood Fluoride?
It’s not great. Empirically, we have data that tell us that levels of urine fluoride are not all that similar to levels of serum fluoride. In 2014, a study investigated the correlation between urine and serum fluoride in a cohort of 60 schoolchildren and found a correlation coefficient of around 0.5.
Why isn’t urine fluoride a great proxy for serum fluoride? The most obvious reason is the urine concentration. Human urine concentration can range from about 50 mmol to 1200 mmol (a 24-fold difference) depending on hydration status. Over the course of 24 hours, for example, the amount of fluoride you put out in your urine may be fairly stable in relation to intake, but for a spot urine sample it would be wildly variable. The authors know this, of course, and so they divide the measured urine fluoride by the specific gravity of the urine to give a sort of “dilution adjusted” value. That’s what is actually used in this study. But specific gravity is, itself, an imperfect measure of how dilute the urine is.
This is something that comes up a lot in urinary biomarker research and it’s not that hard to get around. The best thing would be to just measure blood levels of fluoride. The second best option is 24-hour fluoride excretion. After that, the next best thing would be to adjust the spot concentration by other markers of urinary dilution — creatinine or osmolality — as sensitivity analyses. Any of these approaches would lend credence to the results of the study.
Urinary fluoride excretion is pH dependent. The more acidic the urine, the less fluoride is excreted. Many things — including, importantly, diet — affect urine pH. And it is not a stretch to think that diet may also affect the developing fetus. Neither urine pH nor dietary habits were accounted for in this study.
So, here we are. We have an observational study suggesting a harm that may be associated with fluoride. There may be a causal link here, in which case we need further studies to weigh the harm against the more well-established public health benefit. Or, this is all correlation — an illusion created by the limitations of observational data, and the unique challenges of estimating intake from a single urine sample. In other words, this study has something for everyone, fluoride boosters and skeptics alike. Let the arguments begin. But, if possible, leave me out of it.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ob.Gyns. Can Help Patients Manage Weight With Anti-Obesity Medications
SAN FRANCISCO — An estimated two out of five adult women in the United States have obesity, and given the potential challenges of losing pregnancy weight postpartum or staving off the weight gain associated with menopause, women are likely to be receptive toward weight management help from their ob.gyns. A whole new armamentarium of anti-obesity medications has become available in the past decade, providing physicians and patients with more treatment options.
Ob.gyns. are therefore well-poised to offer counseling and treatment for obesity management for their patients, Johanna G. Finkle, MD, clinical assistant professor of obstetrics and gynecology and a weight management specialist at the University of Kansas Heath System, told attendees at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. Dr. Finkle provided an extensive overview of what ob.gyns. need to know if they are interested in prescribing anti-obesity medications or simply providing their patients with information about the available drugs.
Kitila S. Heyward, MD, an ob.gyn. at Atrium Health in Monroe, North Carolina, who attended the talk, tries to prescribe anti-obesity medications but has run into roadblocks that Dr. Finkle’s talk helped her understand how to overcome.
“I thought it was very helpful because [I] and one of my midwives, in practice, have been trying to get things prescribed, and we can’t figure out the loopholes,” Dr. Heyward said. “Also, the failure rates are really helpful to us so that we know how to counsel people.”
Even for clinicians who aren’t prescribing these medications, Dr. Heyward said the talk was illuminating. “It offered a better understanding of the medications that your patients are on and how it can affect things like birth control, management of surgery, pregnancy, and things along those lines from a clinical day-by-day standpoint,” she said.
Starting With the Basics
Dr. Finkle began by emphasizing the importance of using patient-first language in discussing obesity, which means using terms such as “weight, excess weight, overweight, body mass index,” and “affected by obesity” instead of “obese, morbidly obese, heaviness, or large.” She also cited the Obesity Medicine Association’s definition of obesity: “a chronic, relapsing and treatable multifactorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.”
Though Dr. Finkle acknowledged the limitations of relying on BMI for defining obesity, it remains the standard tool in current practice, with a BMI of 25-29.9 defining overweight and a BMI of 30 or greater defining obesity. Other diagnostic criteria for obesity in women, however, include a percentage body fat over 32% or a waist circumference of more than 35 inches.
“Women are at risk for weight gain through their entire lifespan” Dr. Finkle said, and in women with polycystic ovarian syndrome, 60%-80% have pre-obesity or obesity. In menopause, the triple threat of decreased estrogen, decreased activity, and changes in diet all contribute to obesity risk and no evidence suggests that hormone therapy can prevent weight gain.
Healthy nutrition, physical activity, and behavioral modification remain key pillars of weight management, but interventions such as surgery or medications are also important tools, she said.
“One size does not fit all in terms of treatment,” Dr. Finkle said. ”When I talk to a patient, I think about other medical complications that I can treat with these medications.”
Women for whom anti-obesity medications may be indicated are those with a BMI of 30 or greater, and those with a BMI of at least 27 along with at least one obesity-related comorbidity, such as hypertension, high cholesterol, diabetes, or sleep apnea. The goal of treating obesity with medication is at least a 5%-10% reduction of body weight.
Three Pharmacotherapy Categories
Dr. Finkle reviewed three basic categories of anti-obesity medications: Food and Drug Administration–approved short-term and long-term medications and then off-label drugs that can also aid in healthy weight loss. Short-term options include phentermine, diethylpropion, phendimetrazine, and benzphetamine. Long-term options include orlistat, phentermine/topiramate ER, naltrexone HCl/bupropion HCl ER, and the three GLP-1 receptor agonist drugs, liraglutide, semaglutide, and tirzepatide.
The short-term medications are stimulants that increase satiety, but adverse effects can include tachycardia, hypertension, insomnia, dry mouth, constipation, and diarrhea.
These medications are contraindicated for anyone with uncontrolled hypertension, hyperthyroidism, cardiovascular disease, MAOI use, glaucoma, or history of substance use. The goal is a 5% weight loss in 3 months, and 3 months is the maximum prescribing term.
Then Dr. Finkle reviewed the side effects and contraindications for the oral long-term medications. Orlistat, which can aid in up to 5% weight loss, can result in oily stools and fecal incontinence and is contraindicated for people with chronic malabsorption or cholestasis.
Phentermine/topiramate ER, which can aid in up to 10% weight loss, can result in hypertension, paresthesia, or constipation, and is contraindicated for those with glaucoma, hyperthyroidism, and kidney stones. After the starting dose of 3.75 mg/23 mg, Dr. Finkle increases patients’ dose every 2 weeks, ”but if they’re not tolerating it, if they’re having significant side effects, or they’re losing weight, you do not increase the medication.”
Side effects of naltrexone HCl/bupropion HCl ER, which can lead to 5%-6% weight loss, can include hypertension, suicidal ideation, and glaucoma, and it’s contraindicated in those taking opioids or with a history of seizures or anorexia.
The GLP-1 Receptor Agonists
Next Dr. Finkle discussed the newest but most effective medications, the GLP-1 agonists liraglutide, semaglutide, and tirzepatide. The main contraindications for these drugs are a personal or family history of medullary thyroid cancer, multiple endocrine neoplasia type II syndrome, or any hypersensitivity to this drug class. The two main serious risks are pancreatitis — a 1% risk — and gallstones. Though Dr. Finkle included suicidal ideation as a potential risk of these drugs, the most recent evidence suggests there is no link between suicidal ideation and GLP-1 agonists. The most common side effects are nausea, vomiting, diarrhea, constipation, dyspepsia, and an increased heart rate, though these eventually resolve.
“We always start low with these medications,” Dr. Finkle said, and then titrate the dose up each week, “but if they are having awful side effects, just stay on that dose longer.”
The mechanisms of all three drugs for treating obesity are similar; they work to curb central satiety and slow gastric emptying, though they also have additional mechanisms with benefits for blood glucose levels and for the liver and heart.
- Liraglutide, the first of these drugs approved, is a daily subcutaneous injection that starts at a dose of 0.6 mg and goes up to 3 mg. Patients should lose 4% of weight in 16 weeks or else they are non-responders, Dr. Finkle said.
- Semaglutide, a GLP-1 agonist given as a weekly subcutaneous injection, starts at a dose of 0.25 mg and goes up to 2.4 mg; patients should expect a 5% weight loss in 16 weeks if they are responders. Long term, however, patients lose up to an average 15% of body weight with semaglutide; a third of patients lost more than 20% of body weight in clinical trials, compared with 7%-8% body weight loss with liraglutide. An additional benefit of semaglutide is a 20% reduction in risk of cardiovascular disease.
- Tirzepatide is a combined GLP-1 and GIP agonist, also delivered as a weekly subcutaneous injection, that should result in an estimated 5% weight loss in 16 weeks for responders. But tirzepatide is the most effective of the three, with 91% of patients losing at least 5% body weight and more than half of patients (56%) losing at least 20%.
The big drawbacks to the GLP-1 agonists, however, are their high cost, common lack of insurance coverage, and continued shortages. Dr. Finkle recommended using manufacturer coupons, comparison shopping on Good Rx, and appealing prior authorization requirements to help patients pay for the GLP-1 agonists.
“Drug availability is my second problem. There’s not enough drug,” she said, and her patients often have to call around to different pharmacies to find out which ones are carrying the drug and at what doses. She will sometimes switch their doses as needed based on availability.
It’s also important for physicians to be aware of guidance from the American Society of Anesthesiologists regarding GLP-1 agonist use prior to surgery because of their slowed gastric-emptying mechanism. To reduce the risk of aspiration, patients undergoing general anesthesia should not take liraglutide on the day of surgery, and semaglutide and tirzepatide should be held for 1 week prior to the procedure. New research in JAMA Surgery, however, suggests holding these medications for longer than a week may be wiser.
Getting Patients Started
All the short-term and long-term medications are contraindicated during pregnancy and breastfeeding, Dr. Finkle said. Animal studies with GLP-1 agonists suggest adverse fetal effects when used during pregnancy, but the limited data in human studies so far have not shown a risk of major malformations. Dr. Finkle said the recommendations for now are to stop all GLP-1 receptor agonist drugs 2 months before patients attempt to become pregnant and not to begin them again until after they are no longer breastfeeding.
Finally, Dr. Finkle reviewed off-label medications that can result in modest weight loss, including topiramate, phentermine (not to be used for longer than 12 weeks), bupropion, naltrexone, and metformin. Metformin is likely to result in only 2% weight loss, but it may enhance the effects of GLP-1s, she said.
For ob.gyns. who want to get their patients started on one of these medications, Dr. Finkle first recommends asking patients if it’s okay to discuss their weight. ”Studies show that if you just ask permission to discuss someone’s weight, they go on to lose weight and lose more than someone who has never been asked,” Dr. Finkle said. Then she takes a history.
”When I see a patient, I ask, ‘Tell me why you’re here today,’ ” Dr. Finkle said.
This gives me a lot of insight as to why they’re coming in and it helps me understand where they’re at in terms of other things, such as depression or anxiety with weight, and it helps me to tailor my treatment.”
A full medical history is important for learning about potential contraindications or picking medications that might help with other conditions, such as topiramate for migraines. Finally, Dr. Finkle advises a lab screening with a comprehensive metabolic panel, lipid panel, HbA1c, and vitamin D.
“The [comprehensive metabolic panel] allows me to know about creatinine and liver function,” she said. If these are elevated, she will still prescribe GLP-1s but will monitor the values more closely. “Then I discuss options with the patient. They may be eligible for bariatric surgery or medications. We talk about lifestyle behavioral management, and then I go through the medications and we set goals.”
Goals include nutrition and exercise; start modest and have them work their way up by doing activities they enjoy. In addition, patients taking GLP-1s need to eat enough protein — 80 to 100 grams a day, though she starts them at 60 grams — and do regular muscle strengthening since they can lose muscle mass.
Indications for referral to an obesity medicine specialist are a history of gastric bypass/sleeve surgery, having type 2 diabetes, having an eating disorder, or having failed one of these anti-obesity medications.
Finally, Dr. Finkle reviewed medications that can cause weight gain: medroxyprogesterone acetate for birth control; beta blockers for hypertension or migraine; the antidepressants amitriptyline, paroxetine, venlafaxine, and trazodone; the mood stabilizers gabapentin, lithium, valproate, and carbamazepine; and diphenhydramine and zolpidem for sleep.
No external funding was used for the talk. Dr. Finkle and Dr. Heyward had no disclosures.
SAN FRANCISCO — An estimated two out of five adult women in the United States have obesity, and given the potential challenges of losing pregnancy weight postpartum or staving off the weight gain associated with menopause, women are likely to be receptive toward weight management help from their ob.gyns. A whole new armamentarium of anti-obesity medications has become available in the past decade, providing physicians and patients with more treatment options.
Ob.gyns. are therefore well-poised to offer counseling and treatment for obesity management for their patients, Johanna G. Finkle, MD, clinical assistant professor of obstetrics and gynecology and a weight management specialist at the University of Kansas Heath System, told attendees at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. Dr. Finkle provided an extensive overview of what ob.gyns. need to know if they are interested in prescribing anti-obesity medications or simply providing their patients with information about the available drugs.
Kitila S. Heyward, MD, an ob.gyn. at Atrium Health in Monroe, North Carolina, who attended the talk, tries to prescribe anti-obesity medications but has run into roadblocks that Dr. Finkle’s talk helped her understand how to overcome.
“I thought it was very helpful because [I] and one of my midwives, in practice, have been trying to get things prescribed, and we can’t figure out the loopholes,” Dr. Heyward said. “Also, the failure rates are really helpful to us so that we know how to counsel people.”
Even for clinicians who aren’t prescribing these medications, Dr. Heyward said the talk was illuminating. “It offered a better understanding of the medications that your patients are on and how it can affect things like birth control, management of surgery, pregnancy, and things along those lines from a clinical day-by-day standpoint,” she said.
Starting With the Basics
Dr. Finkle began by emphasizing the importance of using patient-first language in discussing obesity, which means using terms such as “weight, excess weight, overweight, body mass index,” and “affected by obesity” instead of “obese, morbidly obese, heaviness, or large.” She also cited the Obesity Medicine Association’s definition of obesity: “a chronic, relapsing and treatable multifactorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.”
Though Dr. Finkle acknowledged the limitations of relying on BMI for defining obesity, it remains the standard tool in current practice, with a BMI of 25-29.9 defining overweight and a BMI of 30 or greater defining obesity. Other diagnostic criteria for obesity in women, however, include a percentage body fat over 32% or a waist circumference of more than 35 inches.
“Women are at risk for weight gain through their entire lifespan” Dr. Finkle said, and in women with polycystic ovarian syndrome, 60%-80% have pre-obesity or obesity. In menopause, the triple threat of decreased estrogen, decreased activity, and changes in diet all contribute to obesity risk and no evidence suggests that hormone therapy can prevent weight gain.
Healthy nutrition, physical activity, and behavioral modification remain key pillars of weight management, but interventions such as surgery or medications are also important tools, she said.
“One size does not fit all in terms of treatment,” Dr. Finkle said. ”When I talk to a patient, I think about other medical complications that I can treat with these medications.”
Women for whom anti-obesity medications may be indicated are those with a BMI of 30 or greater, and those with a BMI of at least 27 along with at least one obesity-related comorbidity, such as hypertension, high cholesterol, diabetes, or sleep apnea. The goal of treating obesity with medication is at least a 5%-10% reduction of body weight.
Three Pharmacotherapy Categories
Dr. Finkle reviewed three basic categories of anti-obesity medications: Food and Drug Administration–approved short-term and long-term medications and then off-label drugs that can also aid in healthy weight loss. Short-term options include phentermine, diethylpropion, phendimetrazine, and benzphetamine. Long-term options include orlistat, phentermine/topiramate ER, naltrexone HCl/bupropion HCl ER, and the three GLP-1 receptor agonist drugs, liraglutide, semaglutide, and tirzepatide.
The short-term medications are stimulants that increase satiety, but adverse effects can include tachycardia, hypertension, insomnia, dry mouth, constipation, and diarrhea.
These medications are contraindicated for anyone with uncontrolled hypertension, hyperthyroidism, cardiovascular disease, MAOI use, glaucoma, or history of substance use. The goal is a 5% weight loss in 3 months, and 3 months is the maximum prescribing term.
Then Dr. Finkle reviewed the side effects and contraindications for the oral long-term medications. Orlistat, which can aid in up to 5% weight loss, can result in oily stools and fecal incontinence and is contraindicated for people with chronic malabsorption or cholestasis.
Phentermine/topiramate ER, which can aid in up to 10% weight loss, can result in hypertension, paresthesia, or constipation, and is contraindicated for those with glaucoma, hyperthyroidism, and kidney stones. After the starting dose of 3.75 mg/23 mg, Dr. Finkle increases patients’ dose every 2 weeks, ”but if they’re not tolerating it, if they’re having significant side effects, or they’re losing weight, you do not increase the medication.”
Side effects of naltrexone HCl/bupropion HCl ER, which can lead to 5%-6% weight loss, can include hypertension, suicidal ideation, and glaucoma, and it’s contraindicated in those taking opioids or with a history of seizures or anorexia.
The GLP-1 Receptor Agonists
Next Dr. Finkle discussed the newest but most effective medications, the GLP-1 agonists liraglutide, semaglutide, and tirzepatide. The main contraindications for these drugs are a personal or family history of medullary thyroid cancer, multiple endocrine neoplasia type II syndrome, or any hypersensitivity to this drug class. The two main serious risks are pancreatitis — a 1% risk — and gallstones. Though Dr. Finkle included suicidal ideation as a potential risk of these drugs, the most recent evidence suggests there is no link between suicidal ideation and GLP-1 agonists. The most common side effects are nausea, vomiting, diarrhea, constipation, dyspepsia, and an increased heart rate, though these eventually resolve.
“We always start low with these medications,” Dr. Finkle said, and then titrate the dose up each week, “but if they are having awful side effects, just stay on that dose longer.”
The mechanisms of all three drugs for treating obesity are similar; they work to curb central satiety and slow gastric emptying, though they also have additional mechanisms with benefits for blood glucose levels and for the liver and heart.
- Liraglutide, the first of these drugs approved, is a daily subcutaneous injection that starts at a dose of 0.6 mg and goes up to 3 mg. Patients should lose 4% of weight in 16 weeks or else they are non-responders, Dr. Finkle said.
- Semaglutide, a GLP-1 agonist given as a weekly subcutaneous injection, starts at a dose of 0.25 mg and goes up to 2.4 mg; patients should expect a 5% weight loss in 16 weeks if they are responders. Long term, however, patients lose up to an average 15% of body weight with semaglutide; a third of patients lost more than 20% of body weight in clinical trials, compared with 7%-8% body weight loss with liraglutide. An additional benefit of semaglutide is a 20% reduction in risk of cardiovascular disease.
- Tirzepatide is a combined GLP-1 and GIP agonist, also delivered as a weekly subcutaneous injection, that should result in an estimated 5% weight loss in 16 weeks for responders. But tirzepatide is the most effective of the three, with 91% of patients losing at least 5% body weight and more than half of patients (56%) losing at least 20%.
The big drawbacks to the GLP-1 agonists, however, are their high cost, common lack of insurance coverage, and continued shortages. Dr. Finkle recommended using manufacturer coupons, comparison shopping on Good Rx, and appealing prior authorization requirements to help patients pay for the GLP-1 agonists.
“Drug availability is my second problem. There’s not enough drug,” she said, and her patients often have to call around to different pharmacies to find out which ones are carrying the drug and at what doses. She will sometimes switch their doses as needed based on availability.
It’s also important for physicians to be aware of guidance from the American Society of Anesthesiologists regarding GLP-1 agonist use prior to surgery because of their slowed gastric-emptying mechanism. To reduce the risk of aspiration, patients undergoing general anesthesia should not take liraglutide on the day of surgery, and semaglutide and tirzepatide should be held for 1 week prior to the procedure. New research in JAMA Surgery, however, suggests holding these medications for longer than a week may be wiser.
Getting Patients Started
All the short-term and long-term medications are contraindicated during pregnancy and breastfeeding, Dr. Finkle said. Animal studies with GLP-1 agonists suggest adverse fetal effects when used during pregnancy, but the limited data in human studies so far have not shown a risk of major malformations. Dr. Finkle said the recommendations for now are to stop all GLP-1 receptor agonist drugs 2 months before patients attempt to become pregnant and not to begin them again until after they are no longer breastfeeding.
Finally, Dr. Finkle reviewed off-label medications that can result in modest weight loss, including topiramate, phentermine (not to be used for longer than 12 weeks), bupropion, naltrexone, and metformin. Metformin is likely to result in only 2% weight loss, but it may enhance the effects of GLP-1s, she said.
For ob.gyns. who want to get their patients started on one of these medications, Dr. Finkle first recommends asking patients if it’s okay to discuss their weight. ”Studies show that if you just ask permission to discuss someone’s weight, they go on to lose weight and lose more than someone who has never been asked,” Dr. Finkle said. Then she takes a history.
”When I see a patient, I ask, ‘Tell me why you’re here today,’ ” Dr. Finkle said.
This gives me a lot of insight as to why they’re coming in and it helps me understand where they’re at in terms of other things, such as depression or anxiety with weight, and it helps me to tailor my treatment.”
A full medical history is important for learning about potential contraindications or picking medications that might help with other conditions, such as topiramate for migraines. Finally, Dr. Finkle advises a lab screening with a comprehensive metabolic panel, lipid panel, HbA1c, and vitamin D.
“The [comprehensive metabolic panel] allows me to know about creatinine and liver function,” she said. If these are elevated, she will still prescribe GLP-1s but will monitor the values more closely. “Then I discuss options with the patient. They may be eligible for bariatric surgery or medications. We talk about lifestyle behavioral management, and then I go through the medications and we set goals.”
Goals include nutrition and exercise; start modest and have them work their way up by doing activities they enjoy. In addition, patients taking GLP-1s need to eat enough protein — 80 to 100 grams a day, though she starts them at 60 grams — and do regular muscle strengthening since they can lose muscle mass.
Indications for referral to an obesity medicine specialist are a history of gastric bypass/sleeve surgery, having type 2 diabetes, having an eating disorder, or having failed one of these anti-obesity medications.
Finally, Dr. Finkle reviewed medications that can cause weight gain: medroxyprogesterone acetate for birth control; beta blockers for hypertension or migraine; the antidepressants amitriptyline, paroxetine, venlafaxine, and trazodone; the mood stabilizers gabapentin, lithium, valproate, and carbamazepine; and diphenhydramine and zolpidem for sleep.
No external funding was used for the talk. Dr. Finkle and Dr. Heyward had no disclosures.
SAN FRANCISCO — An estimated two out of five adult women in the United States have obesity, and given the potential challenges of losing pregnancy weight postpartum or staving off the weight gain associated with menopause, women are likely to be receptive toward weight management help from their ob.gyns. A whole new armamentarium of anti-obesity medications has become available in the past decade, providing physicians and patients with more treatment options.
Ob.gyns. are therefore well-poised to offer counseling and treatment for obesity management for their patients, Johanna G. Finkle, MD, clinical assistant professor of obstetrics and gynecology and a weight management specialist at the University of Kansas Heath System, told attendees at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. Dr. Finkle provided an extensive overview of what ob.gyns. need to know if they are interested in prescribing anti-obesity medications or simply providing their patients with information about the available drugs.
Kitila S. Heyward, MD, an ob.gyn. at Atrium Health in Monroe, North Carolina, who attended the talk, tries to prescribe anti-obesity medications but has run into roadblocks that Dr. Finkle’s talk helped her understand how to overcome.
“I thought it was very helpful because [I] and one of my midwives, in practice, have been trying to get things prescribed, and we can’t figure out the loopholes,” Dr. Heyward said. “Also, the failure rates are really helpful to us so that we know how to counsel people.”
Even for clinicians who aren’t prescribing these medications, Dr. Heyward said the talk was illuminating. “It offered a better understanding of the medications that your patients are on and how it can affect things like birth control, management of surgery, pregnancy, and things along those lines from a clinical day-by-day standpoint,” she said.
Starting With the Basics
Dr. Finkle began by emphasizing the importance of using patient-first language in discussing obesity, which means using terms such as “weight, excess weight, overweight, body mass index,” and “affected by obesity” instead of “obese, morbidly obese, heaviness, or large.” She also cited the Obesity Medicine Association’s definition of obesity: “a chronic, relapsing and treatable multifactorial, neurobehavioral disease, wherein an increase in body fat promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.”
Though Dr. Finkle acknowledged the limitations of relying on BMI for defining obesity, it remains the standard tool in current practice, with a BMI of 25-29.9 defining overweight and a BMI of 30 or greater defining obesity. Other diagnostic criteria for obesity in women, however, include a percentage body fat over 32% or a waist circumference of more than 35 inches.
“Women are at risk for weight gain through their entire lifespan” Dr. Finkle said, and in women with polycystic ovarian syndrome, 60%-80% have pre-obesity or obesity. In menopause, the triple threat of decreased estrogen, decreased activity, and changes in diet all contribute to obesity risk and no evidence suggests that hormone therapy can prevent weight gain.
Healthy nutrition, physical activity, and behavioral modification remain key pillars of weight management, but interventions such as surgery or medications are also important tools, she said.
“One size does not fit all in terms of treatment,” Dr. Finkle said. ”When I talk to a patient, I think about other medical complications that I can treat with these medications.”
Women for whom anti-obesity medications may be indicated are those with a BMI of 30 or greater, and those with a BMI of at least 27 along with at least one obesity-related comorbidity, such as hypertension, high cholesterol, diabetes, or sleep apnea. The goal of treating obesity with medication is at least a 5%-10% reduction of body weight.
Three Pharmacotherapy Categories
Dr. Finkle reviewed three basic categories of anti-obesity medications: Food and Drug Administration–approved short-term and long-term medications and then off-label drugs that can also aid in healthy weight loss. Short-term options include phentermine, diethylpropion, phendimetrazine, and benzphetamine. Long-term options include orlistat, phentermine/topiramate ER, naltrexone HCl/bupropion HCl ER, and the three GLP-1 receptor agonist drugs, liraglutide, semaglutide, and tirzepatide.
The short-term medications are stimulants that increase satiety, but adverse effects can include tachycardia, hypertension, insomnia, dry mouth, constipation, and diarrhea.
These medications are contraindicated for anyone with uncontrolled hypertension, hyperthyroidism, cardiovascular disease, MAOI use, glaucoma, or history of substance use. The goal is a 5% weight loss in 3 months, and 3 months is the maximum prescribing term.
Then Dr. Finkle reviewed the side effects and contraindications for the oral long-term medications. Orlistat, which can aid in up to 5% weight loss, can result in oily stools and fecal incontinence and is contraindicated for people with chronic malabsorption or cholestasis.
Phentermine/topiramate ER, which can aid in up to 10% weight loss, can result in hypertension, paresthesia, or constipation, and is contraindicated for those with glaucoma, hyperthyroidism, and kidney stones. After the starting dose of 3.75 mg/23 mg, Dr. Finkle increases patients’ dose every 2 weeks, ”but if they’re not tolerating it, if they’re having significant side effects, or they’re losing weight, you do not increase the medication.”
Side effects of naltrexone HCl/bupropion HCl ER, which can lead to 5%-6% weight loss, can include hypertension, suicidal ideation, and glaucoma, and it’s contraindicated in those taking opioids or with a history of seizures or anorexia.
The GLP-1 Receptor Agonists
Next Dr. Finkle discussed the newest but most effective medications, the GLP-1 agonists liraglutide, semaglutide, and tirzepatide. The main contraindications for these drugs are a personal or family history of medullary thyroid cancer, multiple endocrine neoplasia type II syndrome, or any hypersensitivity to this drug class. The two main serious risks are pancreatitis — a 1% risk — and gallstones. Though Dr. Finkle included suicidal ideation as a potential risk of these drugs, the most recent evidence suggests there is no link between suicidal ideation and GLP-1 agonists. The most common side effects are nausea, vomiting, diarrhea, constipation, dyspepsia, and an increased heart rate, though these eventually resolve.
“We always start low with these medications,” Dr. Finkle said, and then titrate the dose up each week, “but if they are having awful side effects, just stay on that dose longer.”
The mechanisms of all three drugs for treating obesity are similar; they work to curb central satiety and slow gastric emptying, though they also have additional mechanisms with benefits for blood glucose levels and for the liver and heart.
- Liraglutide, the first of these drugs approved, is a daily subcutaneous injection that starts at a dose of 0.6 mg and goes up to 3 mg. Patients should lose 4% of weight in 16 weeks or else they are non-responders, Dr. Finkle said.
- Semaglutide, a GLP-1 agonist given as a weekly subcutaneous injection, starts at a dose of 0.25 mg and goes up to 2.4 mg; patients should expect a 5% weight loss in 16 weeks if they are responders. Long term, however, patients lose up to an average 15% of body weight with semaglutide; a third of patients lost more than 20% of body weight in clinical trials, compared with 7%-8% body weight loss with liraglutide. An additional benefit of semaglutide is a 20% reduction in risk of cardiovascular disease.
- Tirzepatide is a combined GLP-1 and GIP agonist, also delivered as a weekly subcutaneous injection, that should result in an estimated 5% weight loss in 16 weeks for responders. But tirzepatide is the most effective of the three, with 91% of patients losing at least 5% body weight and more than half of patients (56%) losing at least 20%.
The big drawbacks to the GLP-1 agonists, however, are their high cost, common lack of insurance coverage, and continued shortages. Dr. Finkle recommended using manufacturer coupons, comparison shopping on Good Rx, and appealing prior authorization requirements to help patients pay for the GLP-1 agonists.
“Drug availability is my second problem. There’s not enough drug,” she said, and her patients often have to call around to different pharmacies to find out which ones are carrying the drug and at what doses. She will sometimes switch their doses as needed based on availability.
It’s also important for physicians to be aware of guidance from the American Society of Anesthesiologists regarding GLP-1 agonist use prior to surgery because of their slowed gastric-emptying mechanism. To reduce the risk of aspiration, patients undergoing general anesthesia should not take liraglutide on the day of surgery, and semaglutide and tirzepatide should be held for 1 week prior to the procedure. New research in JAMA Surgery, however, suggests holding these medications for longer than a week may be wiser.
Getting Patients Started
All the short-term and long-term medications are contraindicated during pregnancy and breastfeeding, Dr. Finkle said. Animal studies with GLP-1 agonists suggest adverse fetal effects when used during pregnancy, but the limited data in human studies so far have not shown a risk of major malformations. Dr. Finkle said the recommendations for now are to stop all GLP-1 receptor agonist drugs 2 months before patients attempt to become pregnant and not to begin them again until after they are no longer breastfeeding.
Finally, Dr. Finkle reviewed off-label medications that can result in modest weight loss, including topiramate, phentermine (not to be used for longer than 12 weeks), bupropion, naltrexone, and metformin. Metformin is likely to result in only 2% weight loss, but it may enhance the effects of GLP-1s, she said.
For ob.gyns. who want to get their patients started on one of these medications, Dr. Finkle first recommends asking patients if it’s okay to discuss their weight. ”Studies show that if you just ask permission to discuss someone’s weight, they go on to lose weight and lose more than someone who has never been asked,” Dr. Finkle said. Then she takes a history.
”When I see a patient, I ask, ‘Tell me why you’re here today,’ ” Dr. Finkle said.
This gives me a lot of insight as to why they’re coming in and it helps me understand where they’re at in terms of other things, such as depression or anxiety with weight, and it helps me to tailor my treatment.”
A full medical history is important for learning about potential contraindications or picking medications that might help with other conditions, such as topiramate for migraines. Finally, Dr. Finkle advises a lab screening with a comprehensive metabolic panel, lipid panel, HbA1c, and vitamin D.
“The [comprehensive metabolic panel] allows me to know about creatinine and liver function,” she said. If these are elevated, she will still prescribe GLP-1s but will monitor the values more closely. “Then I discuss options with the patient. They may be eligible for bariatric surgery or medications. We talk about lifestyle behavioral management, and then I go through the medications and we set goals.”
Goals include nutrition and exercise; start modest and have them work their way up by doing activities they enjoy. In addition, patients taking GLP-1s need to eat enough protein — 80 to 100 grams a day, though she starts them at 60 grams — and do regular muscle strengthening since they can lose muscle mass.
Indications for referral to an obesity medicine specialist are a history of gastric bypass/sleeve surgery, having type 2 diabetes, having an eating disorder, or having failed one of these anti-obesity medications.
Finally, Dr. Finkle reviewed medications that can cause weight gain: medroxyprogesterone acetate for birth control; beta blockers for hypertension or migraine; the antidepressants amitriptyline, paroxetine, venlafaxine, and trazodone; the mood stabilizers gabapentin, lithium, valproate, and carbamazepine; and diphenhydramine and zolpidem for sleep.
No external funding was used for the talk. Dr. Finkle and Dr. Heyward had no disclosures.
FROM ACOG 2024
Are Secondary Osteoporosis Causes Under-Investigated?
NEW ORLEANS — Postmenopausal women with osteoporosis may not be receiving all the recommended tests to rule out secondary causes of bone loss prior to treatment initiation, new research found.
In a single-center chart review of 150 postmenopausal women who had been diagnosed and treated for osteoporosis, most had received a complete blood cell count, basic metabolic panel, thyroid screening, and vitamin D testing. However, one in four had not been tested for a parathyroid hormone (PTH) level, and in nearly two thirds, a 24-hour urine calcium collection had not been ordered.
Overall, less than a third had received the complete workup for secondary osteoporosis causes as recommended by the American Association of Clinical Endocrinologists (AACE) and the Endocrine Society.
“An appropriate evaluation for secondary causes of osteoporosis is essential because it impacts different treatment options and modalities. We discovered low rates of complete testing for secondary causes of osteoporosis in our patient population prior to treatment initiation,” said Kajol Manglani, MD, an internal medicine resident at Georgetown University/MedStar Washington Hospital Center, Washington, DC, and colleagues, in a poster at the American Association of Clinical Endocrinology (AACE) annual meeting held on May 9-12, 2024.
First author Sheetal Bulchandani, MD, said in an interview, “It depends a lot on clinical judgment, but there are certain things that everybody with osteoporosis should be evaluated for. We looked for the things that all the guidelines recommend.”
Studies have suggested that up to 30% of postmenopausal women with osteoporosis have secondary causes, noted Dr. Bulchandani, who conducted the study as a postdoctoral fellow with colleagues at Georgetown University/MedStar Washington Hospital and is now in private endocrine practice in Petersburg, Virginia.
“It’s important not to assume that every woman who walks in with osteoporosis has postmenopausal osteoporosis. I think it would be appropriate to at least discuss with the patients what would warrant certain kinds of clinical workup. … If you don’t figure out if there is an underlying cause, you may end up using an unnecessary medication,” Dr. Bulchandani said.
Are You Missing Something Treatable?
For example, she said, if the patient has underlying hyperparathyroidism and is treated with osteoporosis medications, “you might not see the desired or expected outcome in their bone density.”
Asked to comment, Rachel Pessah-Pollack, MD, clinical associate professor at the Holman Division of Endocrinology, Diabetes, and Metabolism at New York University School of Medicine, New York City, told this news organization, “Certainly, if you have patients who have osteoporosis, it’s important to take a good history and consider secondary causes of bone loss because you may find a treatable etiology that actually can improve their bone density without even starting on a medication.”
Dr. Pessah-Pollack, who was an author of the 2020 AACE/American College of Endocrinology 2020 Clinical Practice Guidelines for the Diagnosis and Treatment of Osteoporosis, said a 24-hour urine calcium collection, not a spot calcium check, is “super important because you’re looking to see if there’s any evidence of hypercalciuria or malabsorption that may be associated with higher rates of bone loss. … These may be a little more cumbersome and harder to get patients to do and more logistics to arrange. But clearly, if you pick up hypercalciuria, that is a potentially treatable etiology and can improve bone density as well.”
Another example, Dr. Pessah-Pollack said, is “if they have a low serum calcium level and high PTH, that would be a real reason to look for celiac disease. By not getting that PTH level, you may be missing that potential diagnosis. There is a wide range of additional causes of osteoporosis ranging from common conditions such as hyperthyroidism to rare conditions such as Cushing disease.”
Differences in Ordering Found Across Specialties
The 150 postmenopausal women were all receiving treatment with either alendronate, denosumab, or zoledronic acid. Their average age was 64.7 years, and 63% were seeing an endocrinologist.
Complete workups as per AACE and Endocrine Society guidelines had been performed in just 28% of those who saw an endocrinologist and 12.5% of patients seen by a rheumatologist, in contrast to 84% of those who saw the head of the hospital’s fracture prevention program.
Overall, across all specialties, just 28.67% had the complete recommended workup for secondary osteoporosis causes.
The most missed test was a 24-hour urine calcium collection, ordered for just 38% of the patients, while PTH was ordered for 73% and phosphorus for 80%. The rest were more commonly ordered: Thyroid-stimulating hormone level for 92.7%, complete blood cell count for 91.3%, basic metabolic panel for 100%, and vitamin D level for 96%.
The high rate of vitamin D testing is noteworthy, Dr. Pessah-Pollack said. “The fact that 96% of women are having vitamin D levels checked as part of an osteoporosis evaluation means that everybody’s aware about vitamin D deficiency, and people want to know what their vitamin D levels are. … That’s good because we want to identify vitamin D deficiency in our osteoporosis patients.”
But the low rate of complete secondary screening even by endocrinologists is concerning. “I look at this study as an opportunity for education that we can reinforce the importance of a secondary evaluation for our osteoporosis patients and really tailor which additional tests should be ordered for the individual patient,” Dr. Pessah-Pollack said.
In the poster, Dr. Bulchandani and colleagues wrote, “Further intervention will be aimed to ensure physicians undertake adequate evaluation before considering further treatment directions.” Possibilities that have been discussed include electronic health record alerts and educational materials for primary care providers, she told this news organization.
Dr. Manglani and Dr. Bulchandani had no disclosures. Dr. Pessah-Pollack is an advisor for Boehringer Ingelheim and Eli Lilly.
A version of this article appeared on Medscape.com.
NEW ORLEANS — Postmenopausal women with osteoporosis may not be receiving all the recommended tests to rule out secondary causes of bone loss prior to treatment initiation, new research found.
In a single-center chart review of 150 postmenopausal women who had been diagnosed and treated for osteoporosis, most had received a complete blood cell count, basic metabolic panel, thyroid screening, and vitamin D testing. However, one in four had not been tested for a parathyroid hormone (PTH) level, and in nearly two thirds, a 24-hour urine calcium collection had not been ordered.
Overall, less than a third had received the complete workup for secondary osteoporosis causes as recommended by the American Association of Clinical Endocrinologists (AACE) and the Endocrine Society.
“An appropriate evaluation for secondary causes of osteoporosis is essential because it impacts different treatment options and modalities. We discovered low rates of complete testing for secondary causes of osteoporosis in our patient population prior to treatment initiation,” said Kajol Manglani, MD, an internal medicine resident at Georgetown University/MedStar Washington Hospital Center, Washington, DC, and colleagues, in a poster at the American Association of Clinical Endocrinology (AACE) annual meeting held on May 9-12, 2024.
First author Sheetal Bulchandani, MD, said in an interview, “It depends a lot on clinical judgment, but there are certain things that everybody with osteoporosis should be evaluated for. We looked for the things that all the guidelines recommend.”
Studies have suggested that up to 30% of postmenopausal women with osteoporosis have secondary causes, noted Dr. Bulchandani, who conducted the study as a postdoctoral fellow with colleagues at Georgetown University/MedStar Washington Hospital and is now in private endocrine practice in Petersburg, Virginia.
“It’s important not to assume that every woman who walks in with osteoporosis has postmenopausal osteoporosis. I think it would be appropriate to at least discuss with the patients what would warrant certain kinds of clinical workup. … If you don’t figure out if there is an underlying cause, you may end up using an unnecessary medication,” Dr. Bulchandani said.
Are You Missing Something Treatable?
For example, she said, if the patient has underlying hyperparathyroidism and is treated with osteoporosis medications, “you might not see the desired or expected outcome in their bone density.”
Asked to comment, Rachel Pessah-Pollack, MD, clinical associate professor at the Holman Division of Endocrinology, Diabetes, and Metabolism at New York University School of Medicine, New York City, told this news organization, “Certainly, if you have patients who have osteoporosis, it’s important to take a good history and consider secondary causes of bone loss because you may find a treatable etiology that actually can improve their bone density without even starting on a medication.”
Dr. Pessah-Pollack, who was an author of the 2020 AACE/American College of Endocrinology 2020 Clinical Practice Guidelines for the Diagnosis and Treatment of Osteoporosis, said a 24-hour urine calcium collection, not a spot calcium check, is “super important because you’re looking to see if there’s any evidence of hypercalciuria or malabsorption that may be associated with higher rates of bone loss. … These may be a little more cumbersome and harder to get patients to do and more logistics to arrange. But clearly, if you pick up hypercalciuria, that is a potentially treatable etiology and can improve bone density as well.”
Another example, Dr. Pessah-Pollack said, is “if they have a low serum calcium level and high PTH, that would be a real reason to look for celiac disease. By not getting that PTH level, you may be missing that potential diagnosis. There is a wide range of additional causes of osteoporosis ranging from common conditions such as hyperthyroidism to rare conditions such as Cushing disease.”
Differences in Ordering Found Across Specialties
The 150 postmenopausal women were all receiving treatment with either alendronate, denosumab, or zoledronic acid. Their average age was 64.7 years, and 63% were seeing an endocrinologist.
Complete workups as per AACE and Endocrine Society guidelines had been performed in just 28% of those who saw an endocrinologist and 12.5% of patients seen by a rheumatologist, in contrast to 84% of those who saw the head of the hospital’s fracture prevention program.
Overall, across all specialties, just 28.67% had the complete recommended workup for secondary osteoporosis causes.
The most missed test was a 24-hour urine calcium collection, ordered for just 38% of the patients, while PTH was ordered for 73% and phosphorus for 80%. The rest were more commonly ordered: Thyroid-stimulating hormone level for 92.7%, complete blood cell count for 91.3%, basic metabolic panel for 100%, and vitamin D level for 96%.
The high rate of vitamin D testing is noteworthy, Dr. Pessah-Pollack said. “The fact that 96% of women are having vitamin D levels checked as part of an osteoporosis evaluation means that everybody’s aware about vitamin D deficiency, and people want to know what their vitamin D levels are. … That’s good because we want to identify vitamin D deficiency in our osteoporosis patients.”
But the low rate of complete secondary screening even by endocrinologists is concerning. “I look at this study as an opportunity for education that we can reinforce the importance of a secondary evaluation for our osteoporosis patients and really tailor which additional tests should be ordered for the individual patient,” Dr. Pessah-Pollack said.
In the poster, Dr. Bulchandani and colleagues wrote, “Further intervention will be aimed to ensure physicians undertake adequate evaluation before considering further treatment directions.” Possibilities that have been discussed include electronic health record alerts and educational materials for primary care providers, she told this news organization.
Dr. Manglani and Dr. Bulchandani had no disclosures. Dr. Pessah-Pollack is an advisor for Boehringer Ingelheim and Eli Lilly.
A version of this article appeared on Medscape.com.
NEW ORLEANS — Postmenopausal women with osteoporosis may not be receiving all the recommended tests to rule out secondary causes of bone loss prior to treatment initiation, new research found.
In a single-center chart review of 150 postmenopausal women who had been diagnosed and treated for osteoporosis, most had received a complete blood cell count, basic metabolic panel, thyroid screening, and vitamin D testing. However, one in four had not been tested for a parathyroid hormone (PTH) level, and in nearly two thirds, a 24-hour urine calcium collection had not been ordered.
Overall, less than a third had received the complete workup for secondary osteoporosis causes as recommended by the American Association of Clinical Endocrinologists (AACE) and the Endocrine Society.
“An appropriate evaluation for secondary causes of osteoporosis is essential because it impacts different treatment options and modalities. We discovered low rates of complete testing for secondary causes of osteoporosis in our patient population prior to treatment initiation,” said Kajol Manglani, MD, an internal medicine resident at Georgetown University/MedStar Washington Hospital Center, Washington, DC, and colleagues, in a poster at the American Association of Clinical Endocrinology (AACE) annual meeting held on May 9-12, 2024.
First author Sheetal Bulchandani, MD, said in an interview, “It depends a lot on clinical judgment, but there are certain things that everybody with osteoporosis should be evaluated for. We looked for the things that all the guidelines recommend.”
Studies have suggested that up to 30% of postmenopausal women with osteoporosis have secondary causes, noted Dr. Bulchandani, who conducted the study as a postdoctoral fellow with colleagues at Georgetown University/MedStar Washington Hospital and is now in private endocrine practice in Petersburg, Virginia.
“It’s important not to assume that every woman who walks in with osteoporosis has postmenopausal osteoporosis. I think it would be appropriate to at least discuss with the patients what would warrant certain kinds of clinical workup. … If you don’t figure out if there is an underlying cause, you may end up using an unnecessary medication,” Dr. Bulchandani said.
Are You Missing Something Treatable?
For example, she said, if the patient has underlying hyperparathyroidism and is treated with osteoporosis medications, “you might not see the desired or expected outcome in their bone density.”
Asked to comment, Rachel Pessah-Pollack, MD, clinical associate professor at the Holman Division of Endocrinology, Diabetes, and Metabolism at New York University School of Medicine, New York City, told this news organization, “Certainly, if you have patients who have osteoporosis, it’s important to take a good history and consider secondary causes of bone loss because you may find a treatable etiology that actually can improve their bone density without even starting on a medication.”
Dr. Pessah-Pollack, who was an author of the 2020 AACE/American College of Endocrinology 2020 Clinical Practice Guidelines for the Diagnosis and Treatment of Osteoporosis, said a 24-hour urine calcium collection, not a spot calcium check, is “super important because you’re looking to see if there’s any evidence of hypercalciuria or malabsorption that may be associated with higher rates of bone loss. … These may be a little more cumbersome and harder to get patients to do and more logistics to arrange. But clearly, if you pick up hypercalciuria, that is a potentially treatable etiology and can improve bone density as well.”
Another example, Dr. Pessah-Pollack said, is “if they have a low serum calcium level and high PTH, that would be a real reason to look for celiac disease. By not getting that PTH level, you may be missing that potential diagnosis. There is a wide range of additional causes of osteoporosis ranging from common conditions such as hyperthyroidism to rare conditions such as Cushing disease.”
Differences in Ordering Found Across Specialties
The 150 postmenopausal women were all receiving treatment with either alendronate, denosumab, or zoledronic acid. Their average age was 64.7 years, and 63% were seeing an endocrinologist.
Complete workups as per AACE and Endocrine Society guidelines had been performed in just 28% of those who saw an endocrinologist and 12.5% of patients seen by a rheumatologist, in contrast to 84% of those who saw the head of the hospital’s fracture prevention program.
Overall, across all specialties, just 28.67% had the complete recommended workup for secondary osteoporosis causes.
The most missed test was a 24-hour urine calcium collection, ordered for just 38% of the patients, while PTH was ordered for 73% and phosphorus for 80%. The rest were more commonly ordered: Thyroid-stimulating hormone level for 92.7%, complete blood cell count for 91.3%, basic metabolic panel for 100%, and vitamin D level for 96%.
The high rate of vitamin D testing is noteworthy, Dr. Pessah-Pollack said. “The fact that 96% of women are having vitamin D levels checked as part of an osteoporosis evaluation means that everybody’s aware about vitamin D deficiency, and people want to know what their vitamin D levels are. … That’s good because we want to identify vitamin D deficiency in our osteoporosis patients.”
But the low rate of complete secondary screening even by endocrinologists is concerning. “I look at this study as an opportunity for education that we can reinforce the importance of a secondary evaluation for our osteoporosis patients and really tailor which additional tests should be ordered for the individual patient,” Dr. Pessah-Pollack said.
In the poster, Dr. Bulchandani and colleagues wrote, “Further intervention will be aimed to ensure physicians undertake adequate evaluation before considering further treatment directions.” Possibilities that have been discussed include electronic health record alerts and educational materials for primary care providers, she told this news organization.
Dr. Manglani and Dr. Bulchandani had no disclosures. Dr. Pessah-Pollack is an advisor for Boehringer Ingelheim and Eli Lilly.
A version of this article appeared on Medscape.com.
Research Highlights From ESMO Breast Cancer
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
Among the topics the speakers addressed were breast cancer prevention, early breast cancer, advanced breast cancer, and supportive care.
In recent years, the way clinicians look at carcinogenesis in breast cancer has changed, and many new targets for potential early detection and prevention have emerged, said Suzette Delaloge, MD, of Gustave Roussy, Paris, France, in her presentation at the meeting.
Instant risk assessment at different time points could potentially intercept cancer among high-risk individuals, she said.
A study by Mikael Eriksson, PhD, and colleagues focused on external validation of the Profound AI tool to identify breast cancer risk in the general population. The researchers showed an area under the curve of 0.72 in their AI risk model, which has the potential to be clinically meaningful, although it must be prospectively validated, Dr. Delaloge said in her presentation.
She also reviewed two studies on the use of genes to further refine breast cancer risk among carriers. One of these, a prospective study presented in a session by Kelly-Anne Phillips, MD, of Peter MacCallum Cancer Center, Melbourne, Australia, used the CANRISK online risk assessment tool and validated increased breast cancer risk in BRCA1 and BRCA2 carriers, with AUCs of 0.79 and 0.78, respectively. The other study, which was by Maria Rezqallah Aron, MD, and colleagues examined polygenic scores as a way to refine breast cancer risk stratification among carriers of the ALM and PALB2 genes as well. These genes might be useful in identifying individuals who could benefit from early intervention, including surgery, Dr. Delaloge said.
Translational Research
“Preparing my talk, I felt like a kid in a candy store,” because of the amount of new translational research presented, including several studies of endocrine treatment–based approaches to therapy, said Marleen Kok, MD, of the Netherlands Cancer Institute, Amsterdam.
In her presentation, Dr. Kok highlighted findings from an analysis of patients in the monarchE study (a trial of high-risk patients) showing a consistent improvement in invasive disease-free survival for the subset of patients with germline BRCA1 and BRCA2 mutations who received abemaciclib plus endocrine therapy.
The value of tumor-infiltrating lymphocytes (TILs) on patients who are not receiving chemotherapy is important because of the focus on prognosis, and prospective trials are underway, she said.
A poster on the impact of chemotherapy and stromal tumor-infiltrating lymphocytes (sTILs) in stage I triple-negative breast cancer showed no association between chemotherapy and better outcomes regardless of sTILs in patients who did and did not receive chemotherapy, which has implications for potential treatment sparing in this population, Dr. Kok noted.
Artificial Intelligence (AI) was the subject of several posters at the meeting, and Dr. Kok identified a multisite European study of an automated HER2 scoring system as notable for its size and accuracy. In the study, the accuracy among pathologists was much higher with the assistance of AI, she said. Using AI for more complex analysis has shown success, she said.
Dr. Kok ended her talk with a poster that surveyed breast cancer patients about their understanding of their disease. The results showed that less than half (44%) of patients reported that their healthcare providers had given them enough information to learn about their breast cancer type, and less than one third could recall terminology about biomarkers; the study is important because it shows that clinicians need to do better in explaining these terms to patients, Dr. Kok said.
Early Breast Cancer
Right-sizing therapy, meaning identifying the right treatment for every patient, is a key element of new research in early breast cancer, said Erika Hamilton, MD, of the Sarah Cannon Research Institute, Nashville, Tenn.
She highlighted safety and treatment duration updates from the NATALEE study, which compared adjuvant ribociclib plus nonsteroidal aromatase inhibitor (NSAI) to NSAI alone for ER+/HER2- breast cancer. The current analysis presented at the meeting showed significant benefits with the addition of ribociclib and no evidence of new safety signals or adverse event exacerbations at 3 years, she said. Dose modifications had no significant impact on efficacy, she added.
The findings of no impact of dose reduction on efficacy in both the NATALEE and monarchE studies provide important information on whether dosage can be reduced in patients, which will increase the odds that patients will tolerate extended therapy with good outcomes and stay on their prescribed therapies, Dr. Hamilton emphasized.
The CARABELA study, a phase 2 trial of neoadjuvant letrozole plus abemaciclib vs adriamycin and cyclophosphamide (AC), showed clinically similar response rates but did not meet its endpoint for residual cancer burden (RCB) scores. These data add to results from other studies and show that it is too soon to universally replace neoadjuvant chemotherapy as first-line treatment for highly proliferative ER+ breast cancer, Dr. Hamilton said in her presentation.
Advanced Breast Cancer
Take-home messages about advanced breast cancer include growing evidence for the potential benefits of antibody drug conjugates (ADCs), said Eva Ciruelos, MD, of University Hospital, Madrid, Spain. The TROPION-BREAST01 study, a phase 3 randomized trial, showed significant and clinically meaningful improvement in progression-free survival in patients with previously treated, inoperable, or metastatic HR+/HER2- breast cancer who received datopotamab deruxtecan (Dato-DXd) compared with those who received chemotherapy.
Data from an additional safety analysis were presented at the meeting; although Dato-DXd, a trophoblast cell-surface antigen 2 (TROP2)–directed antibody-drug conjugate, was well-tolerated, it is important to remain aware of toxicities, notably oral mucositis, which occurred in 55.6% of the patients in the study across all grades, and ocular surface toxicity, which occurred in 40% of patients across all grades, Dr. Ciruelos emphasized.
Key research in the area of advanced triple-negative breast cancer included data from the IMPASSION 132 study. This study is “specifically centered on early relapsers,” a population often excluded from other trials, Dr. Ciruelos said. In this study, patients with advanced triple-negative breast cancer were randomized to chemotherapy with or without atezolizumab, and the study showed no benefits with atezolizumab for overall survival, progression-free survival, or overall response rate, she said. “This is something to work with, because this is a very refractory population,” Dr. Ciruelos noted.
New immunotherapy combinations are needed to improve survival in advanced breast cancer patients, Dr. Ciruelos said. At the meeting, researchers presented interim data from a subset of patients in the MORPHEUS-pan breast cancer trial, a phase 1B/2 study involving multiple treatment combinations in locally advanced/metastatic breast cancer patients.
The interim analysis included 18-week data from triple-negative breast cancer patients and compared outcomes for patients randomized to atezolizumab with or without sacituzumab govitecan (SG).
The study was small, with only 31 patients in the combination arm and 11 controls, but the results were promising, with an overall response rate of 76.7% in the combination arm vs 66.7% in the control arm, Dr. Ciruelos said.
Supportive Care
Key supportive care takeaways included data on pregnancy in young breast cancer survivors and the safety of vaginal estrogen therapy in breast cancer patients with genitourinary symptoms, said Anne May, MD, of the University Medical Center Utrecht, Utrecht, Netherlands.
A study previously published in JAMA including nearly 5000 BRCA carriers who were diagnosed with invasive breast cancer at age 40 years or younger showed no association between pregnancy after breast cancer and adverse maternal or fetal outcomes, and pregnancy had no significant impact on overall survival. The authors presented new data on the safety of assisted reproductive techniques (ART) based on the 543 pregnancies in the original study, at the meeting. Of these, 436 conceived naturally, and 107 used ART. After a median of 9.1 years, ART had no effect on disease-free survival compared to natural conception (hazard ratio [HR], 0.64). Based on these findings, fertility preservation should be offered to all women who receive a breast cancer diagnosis and are interested in future fertility, Dr. May said.
Conceiving after breast cancer treatment and follow-up should not be contraindicated for young BRCA carriers, she added.No trial data are available for the effects of vaginal estrogen therapy (VET) on disease-free survival in breast cancer survivors with genitourinary symptoms caused by declining estrogen levels, Dr. May said. However, researchers in France and Switzerland conducted an emulation of a hypothetical target trial using data from the French National social security system for more than 130,000 individuals. Although VET therapy had no impact on disease-free survival in most breast cancer survivors overall, it did have a negative impact in a subset of patients with HR-positive and HR-negative tumors who were treated with aromatase inhibitors. The study was hypothetical, but important because the results suggest that clinicians can safely propose VTE to patients who report genitourinary symptoms after treatment for early-stage breast cancer with tamoxifen, but VTE should be avoided in patients treated with aromatase inhibitors, Dr. May said.
Dr. Delaloge disclosed research support to her institution from AstraZeneca, MSD, Bristol Myers Squibb, Sanofi, Taiho, Novartis, European Commission, INCa, Banque des Territoires, and Fondation Philanthropia. She also disclosed honoraria to her institution from AstraZeneca, Gilead, Novartis, Elsan, Besins, Sanofi, Exact Sciences, and Lilly, as well as travel support from Novartis.
Dr. Kok disclosed research funding from AstraZeneca, Bristol Myers Squibb, Daichi, and Roche, and advisory board membership/speaker’s fees from Alderaan Biotechnology, BIONTECH, Domain Therapeutics, AstraZeneca, Daichi, Bristol Myers Squibb, Gilead, Medscape, MSD, and Roche.
Dr. Hamilton disclosed a consulting advisory role (to her institution) for Accutar Biotechology, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Entos, Forsum Pharma, Gilead Sciences, Greenwich LifeSciences, Jazz Pharmaceuticals, Lilly, Medical Pharma Services, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Roche/Genentech, Stemline Therapeutics, ands others. She also disclosed contracted research/grant support to her institution only from Abbvie, Acerta Pharma, Accutar Biotechnology , ADC Therapeutics, AKESOBIO Australia , Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, BeiGene, Black Diamond and others.
Dr. Ciruelos disclosed serving as an external advisor for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, and Lilly, as well as serving as a speaker for Roche, MSD, Gilead, AstraZeneca, Daichii Sankyo, Reveal Genomics, Pfizer, Novartis, Lilly, and Pierre Fabre. She also disclosed travel grants from Roche, Pfizer, and AstraZeneca, and research grants from Seagen and Roche.
Dr. May had no financial conflicts to disclose.
FROM ESMO BREAST CANCER 2024
Does More Systemic Treatment for Advanced Cancer Improve Survival?
This conclusion of a new study published online May 16 in JAMA Oncology may help reassure oncologists that giving systemic anticancer therapy (SACT) at the most advanced stages of cancer will not improve the patient’s life, the authors wrote. It also may encourage them to instead focus more on honest communication with patients about their choices, Maureen E. Canavan, PhD, at the Cancer and Outcomes, Public Policy and Effectiveness Research (COPPER) Center at the Yale School of Medicine in New Haven, Connecticut, and colleagues, wrote in their paper.
How Was the Study Conducted?
Researchers used Flatiron Health, a nationwide electronic health records database of academic and community practices throughout the United State. They identified 78,446 adults with advanced or metastatic stages of one of six common cancers (breast, colorectal, urothelial, non–small cell lung cancer [NSCLC], pancreatic and renal cell carcinoma) who were treated at healthcare practices from 2015 to 2019. They then stratified practices into quintiles based on how often the practices treated patients with any systemic therapy, including chemotherapy and immunotherapy, in their last 14 days of life. They compared whether patients in practices with greater use of systemic treatment at very advanced stages had longer overall survival.
What Were the Main Findings?
“We saw that there were absolutely no survival differences between the practices that used more systemic therapy for very advanced cancer than the practices that use less,” said senior author Kerin Adelson, MD, chief quality and value officer at MD Anderson Cancer Center in Houston, Texas. In some cancers, those in the lowest quintile (those with the lowest rates of systemic end-of-life care) lived fewer years compared with those in the highest quintiles. In other cancers, those in the lowest quintiles lived more years than those in the highest quintiles.
“What’s important is that none of those differences, after you control for other factors, was statistically significant,” Dr. Adelson said. “That was the same in every cancer type we looked at.”
An example is seen in advanced urothelial cancer. Those in the first quintile (lowest rates of systemic care at end of life) had an SACT rate range of 4.0-9.1. The SACT rate range in the highest quintile was 19.8-42.6. But the median overall survival (OS) rate for those in the lowest quintile was 12.7 months, not statistically different from the median OS in the highest quintile (11 months.)
How Does This Study Add to the Literature?
The American Society of Clinical Oncology (ASCO) and the National Quality Forum (NQF) developed a cancer quality metric to reduce SACT at the end of life. The NQF 0210 is a ratio of patients who get systemic treatment within 14 days of death over all patients who die of cancer. The quality metric has been widely adopted and used in value-based care reporting.
But the metric has been criticized because it focuses only on people who died and not people who lived longer because they benefited from the systemic therapy, the authors wrote.
Dr. Canavan’s team focused on all patients treated in the practice, not just those who died, Dr. Adelson said. This may put that criticism to rest, Dr. Adelson said.
“I personally believed the ASCO and NQF metric was appropriate and the criticisms were off base,” said Otis Brawley, MD, associate director of community outreach and engagement at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University School of Medicine in Baltimore. “Canavan’s study is evidence suggesting the metrics were appropriate.”
This study included not just chemotherapy, as some other studies have, but targeted therapies and immunotherapies as well. Dr. Adelson said some think that the newer drugs might change the prognosis at end of life. But this study shows “even those drugs are not helping patients to survive with very advanced cancer,” she said.
Could This Change Practice?
The authors noted that end-of life SACT has been linked with more acute care use, delays in conversations about care goals, late enrollment in hospice, higher costs, and potentially shorter and poorer quality life.
Dr. Adelson said she’s hoping that the knowledge that there’s no survival benefit for use of SACT for patients with advanced solid tumors who are nearing the end of life will lead instead to more conversations about prognosis with patients and transitions to palliative care.
“Palliative care has actually been shown to improve quality of life and, in some studies, even survival,” she said.
“I doubt it will change practice, but it should,” Dr. Brawley said. “The study suggests that doctors and patients have too much hope for chemotherapy as patients’ disease progresses. In the US especially, there is a tendency to believe we have better therapies than we truly do and we have difficulty accepting that the patient is dying. Many patients get third- and fourth-line chemotherapy that is highly likely to increase suffering without realistic hope of prolonging life and especially no hope of prolonging life with good quality.”
Dr. Adelson disclosed ties with AbbVie, Quantum Health, Gilead, ParetoHealth, and Carrum Health. Various coauthors disclosed ties with Roche, AbbVie, Johnson & Johnson, Genentech, the National Comprehensive Cancer Network, and AstraZeneca. The study was funded by Flatiron Health, an independent member of the Roche group. Dr. Brawley reports no relevant financial disclosures.
This conclusion of a new study published online May 16 in JAMA Oncology may help reassure oncologists that giving systemic anticancer therapy (SACT) at the most advanced stages of cancer will not improve the patient’s life, the authors wrote. It also may encourage them to instead focus more on honest communication with patients about their choices, Maureen E. Canavan, PhD, at the Cancer and Outcomes, Public Policy and Effectiveness Research (COPPER) Center at the Yale School of Medicine in New Haven, Connecticut, and colleagues, wrote in their paper.
How Was the Study Conducted?
Researchers used Flatiron Health, a nationwide electronic health records database of academic and community practices throughout the United State. They identified 78,446 adults with advanced or metastatic stages of one of six common cancers (breast, colorectal, urothelial, non–small cell lung cancer [NSCLC], pancreatic and renal cell carcinoma) who were treated at healthcare practices from 2015 to 2019. They then stratified practices into quintiles based on how often the practices treated patients with any systemic therapy, including chemotherapy and immunotherapy, in their last 14 days of life. They compared whether patients in practices with greater use of systemic treatment at very advanced stages had longer overall survival.
What Were the Main Findings?
“We saw that there were absolutely no survival differences between the practices that used more systemic therapy for very advanced cancer than the practices that use less,” said senior author Kerin Adelson, MD, chief quality and value officer at MD Anderson Cancer Center in Houston, Texas. In some cancers, those in the lowest quintile (those with the lowest rates of systemic end-of-life care) lived fewer years compared with those in the highest quintiles. In other cancers, those in the lowest quintiles lived more years than those in the highest quintiles.
“What’s important is that none of those differences, after you control for other factors, was statistically significant,” Dr. Adelson said. “That was the same in every cancer type we looked at.”
An example is seen in advanced urothelial cancer. Those in the first quintile (lowest rates of systemic care at end of life) had an SACT rate range of 4.0-9.1. The SACT rate range in the highest quintile was 19.8-42.6. But the median overall survival (OS) rate for those in the lowest quintile was 12.7 months, not statistically different from the median OS in the highest quintile (11 months.)
How Does This Study Add to the Literature?
The American Society of Clinical Oncology (ASCO) and the National Quality Forum (NQF) developed a cancer quality metric to reduce SACT at the end of life. The NQF 0210 is a ratio of patients who get systemic treatment within 14 days of death over all patients who die of cancer. The quality metric has been widely adopted and used in value-based care reporting.
But the metric has been criticized because it focuses only on people who died and not people who lived longer because they benefited from the systemic therapy, the authors wrote.
Dr. Canavan’s team focused on all patients treated in the practice, not just those who died, Dr. Adelson said. This may put that criticism to rest, Dr. Adelson said.
“I personally believed the ASCO and NQF metric was appropriate and the criticisms were off base,” said Otis Brawley, MD, associate director of community outreach and engagement at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University School of Medicine in Baltimore. “Canavan’s study is evidence suggesting the metrics were appropriate.”
This study included not just chemotherapy, as some other studies have, but targeted therapies and immunotherapies as well. Dr. Adelson said some think that the newer drugs might change the prognosis at end of life. But this study shows “even those drugs are not helping patients to survive with very advanced cancer,” she said.
Could This Change Practice?
The authors noted that end-of life SACT has been linked with more acute care use, delays in conversations about care goals, late enrollment in hospice, higher costs, and potentially shorter and poorer quality life.
Dr. Adelson said she’s hoping that the knowledge that there’s no survival benefit for use of SACT for patients with advanced solid tumors who are nearing the end of life will lead instead to more conversations about prognosis with patients and transitions to palliative care.
“Palliative care has actually been shown to improve quality of life and, in some studies, even survival,” she said.
“I doubt it will change practice, but it should,” Dr. Brawley said. “The study suggests that doctors and patients have too much hope for chemotherapy as patients’ disease progresses. In the US especially, there is a tendency to believe we have better therapies than we truly do and we have difficulty accepting that the patient is dying. Many patients get third- and fourth-line chemotherapy that is highly likely to increase suffering without realistic hope of prolonging life and especially no hope of prolonging life with good quality.”
Dr. Adelson disclosed ties with AbbVie, Quantum Health, Gilead, ParetoHealth, and Carrum Health. Various coauthors disclosed ties with Roche, AbbVie, Johnson & Johnson, Genentech, the National Comprehensive Cancer Network, and AstraZeneca. The study was funded by Flatiron Health, an independent member of the Roche group. Dr. Brawley reports no relevant financial disclosures.
This conclusion of a new study published online May 16 in JAMA Oncology may help reassure oncologists that giving systemic anticancer therapy (SACT) at the most advanced stages of cancer will not improve the patient’s life, the authors wrote. It also may encourage them to instead focus more on honest communication with patients about their choices, Maureen E. Canavan, PhD, at the Cancer and Outcomes, Public Policy and Effectiveness Research (COPPER) Center at the Yale School of Medicine in New Haven, Connecticut, and colleagues, wrote in their paper.
How Was the Study Conducted?
Researchers used Flatiron Health, a nationwide electronic health records database of academic and community practices throughout the United State. They identified 78,446 adults with advanced or metastatic stages of one of six common cancers (breast, colorectal, urothelial, non–small cell lung cancer [NSCLC], pancreatic and renal cell carcinoma) who were treated at healthcare practices from 2015 to 2019. They then stratified practices into quintiles based on how often the practices treated patients with any systemic therapy, including chemotherapy and immunotherapy, in their last 14 days of life. They compared whether patients in practices with greater use of systemic treatment at very advanced stages had longer overall survival.
What Were the Main Findings?
“We saw that there were absolutely no survival differences between the practices that used more systemic therapy for very advanced cancer than the practices that use less,” said senior author Kerin Adelson, MD, chief quality and value officer at MD Anderson Cancer Center in Houston, Texas. In some cancers, those in the lowest quintile (those with the lowest rates of systemic end-of-life care) lived fewer years compared with those in the highest quintiles. In other cancers, those in the lowest quintiles lived more years than those in the highest quintiles.
“What’s important is that none of those differences, after you control for other factors, was statistically significant,” Dr. Adelson said. “That was the same in every cancer type we looked at.”
An example is seen in advanced urothelial cancer. Those in the first quintile (lowest rates of systemic care at end of life) had an SACT rate range of 4.0-9.1. The SACT rate range in the highest quintile was 19.8-42.6. But the median overall survival (OS) rate for those in the lowest quintile was 12.7 months, not statistically different from the median OS in the highest quintile (11 months.)
How Does This Study Add to the Literature?
The American Society of Clinical Oncology (ASCO) and the National Quality Forum (NQF) developed a cancer quality metric to reduce SACT at the end of life. The NQF 0210 is a ratio of patients who get systemic treatment within 14 days of death over all patients who die of cancer. The quality metric has been widely adopted and used in value-based care reporting.
But the metric has been criticized because it focuses only on people who died and not people who lived longer because they benefited from the systemic therapy, the authors wrote.
Dr. Canavan’s team focused on all patients treated in the practice, not just those who died, Dr. Adelson said. This may put that criticism to rest, Dr. Adelson said.
“I personally believed the ASCO and NQF metric was appropriate and the criticisms were off base,” said Otis Brawley, MD, associate director of community outreach and engagement at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University School of Medicine in Baltimore. “Canavan’s study is evidence suggesting the metrics were appropriate.”
This study included not just chemotherapy, as some other studies have, but targeted therapies and immunotherapies as well. Dr. Adelson said some think that the newer drugs might change the prognosis at end of life. But this study shows “even those drugs are not helping patients to survive with very advanced cancer,” she said.
Could This Change Practice?
The authors noted that end-of life SACT has been linked with more acute care use, delays in conversations about care goals, late enrollment in hospice, higher costs, and potentially shorter and poorer quality life.
Dr. Adelson said she’s hoping that the knowledge that there’s no survival benefit for use of SACT for patients with advanced solid tumors who are nearing the end of life will lead instead to more conversations about prognosis with patients and transitions to palliative care.
“Palliative care has actually been shown to improve quality of life and, in some studies, even survival,” she said.
“I doubt it will change practice, but it should,” Dr. Brawley said. “The study suggests that doctors and patients have too much hope for chemotherapy as patients’ disease progresses. In the US especially, there is a tendency to believe we have better therapies than we truly do and we have difficulty accepting that the patient is dying. Many patients get third- and fourth-line chemotherapy that is highly likely to increase suffering without realistic hope of prolonging life and especially no hope of prolonging life with good quality.”
Dr. Adelson disclosed ties with AbbVie, Quantum Health, Gilead, ParetoHealth, and Carrum Health. Various coauthors disclosed ties with Roche, AbbVie, Johnson & Johnson, Genentech, the National Comprehensive Cancer Network, and AstraZeneca. The study was funded by Flatiron Health, an independent member of the Roche group. Dr. Brawley reports no relevant financial disclosures.
FROM JAMA ONCOLOGY
Urine Tests Could Be ‘Enormous Step’ in Diagnosing Cancer
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Emerging science suggests that the body’s “liquid gold” could be particularly useful for liquid biopsies, offering a convenient, pain-free, and cost-effective way to spot otherwise hard-to-detect cancers.
“The search for cancer biomarkers that can be detected in urine could provide an enormous step forward to decrease cancer patient mortality,” said Kenneth R. Shroyer, MD, PhD, a pathologist at Stony Brook University, Stony Brook, New York, who studies cancer biomarkers.
Physicians have long known that urine can reveal a lot about our health — that’s why urinalysis has been part of medicine for 6000 years. Urine tests can detect diabetes, pregnancy, drug use, and urinary or kidney conditions.
But other conditions leave clues in urine, too, and cancer may be one of the most promising. “Urine testing could detect biomarkers of early-stage cancers, not only from local but also distant sites,” Dr. Shroyer said. It could also help flag recurrence in cancer survivors who have undergone treatment.
Granted, cancer biomarkers in urine are not nearly as widely studied as those in the blood, Dr. Shroyer noted. But a new wave of urine tests suggests research is gaining pace.
“The recent availability of high-throughput screening technologies has enabled researchers to investigate cancer from a top-down, comprehensive approach,” said Pak Kin Wong, PhD, professor of mechanical engineering, biomedical engineering, and surgery at The Pennsylvania State University. “We are starting to understand the rich information that can be obtained from urine.”
Urine is mostly water (about 95%) and urea, a metabolic byproduct that imparts that signature yellow color (about 2%). The other 3% is a mix of waste products, minerals, and other compounds the kidneys removed from the blood. Even in trace amounts, these substances say a lot.
Among them are “exfoliated cancer cells, cell-free DNA, hormones, and the urine microbiota — the collection of microbes in our urinary tract system,” Dr. Wong said.
“It is highly promising to be one of the major biological fluids used for screening, diagnosis, prognosis, and monitoring treatment efficiency in the era of precision medicine,” Dr. Wong said.
How Urine Testing Could Reveal Cancer
Still, as exciting as the prospect is, there’s a lot to consider in the hunt for cancer biomarkers in urine. These biomarkers must be able to pass through the renal nephrons (filtering units), remain stable in urine, and have high-level sensitivity, Dr. Shroyer said. They should also have high specificity for cancer vs benign conditions and be expressed at early stages, before the primary tumor has spread.
“At this stage, few circulating biomarkers have been found that are both sensitive and specific for early-stage disease,” said Dr. Shroyer.
But there are a few promising examples under investigation in humans:
Prostate cancer. Researchers at the University of Michigan have developed a urine test that detects high-grade prostate cancer more accurately than existing tests, including PHI, SelectMDx, 4Kscore, EPI, MPS, and IsoPSA.
The MyProstateScore 2.0 (MPS2) test, which looks for 18 genes associated with high-grade tumors, could reduce unnecessary biopsies in men with elevated prostate-specific antigen levels, according to a paper published in JAMA Oncology.
It makes sense. The prostate gland secretes fluid that becomes part of the semen, traces of which enter urine. After a digital rectal exam, even more prostate fluid enters the urine. If a patient has prostate cancer, genetic material from the cancer cells will infiltrate the urine.
In the MPS2 test, researchers used polymerase chain reaction (PCR) testing in urine. “The technology used for COVID PCR is essentially the same as the PCR used to detect transcripts associated with high-grade prostate cancer in urine,” said study author Arul Chinnaiyan, MD, PhD, director of the Michigan Center for Translational Pathology at the University of Michigan, Ann Arbor. “In the case of the MPS2 test, we are doing PCR on 18 genes simultaneously on urine samples.”
A statistical model uses levels of that genetic material to predict the risk for high-grade disease, helping doctors decide what to do next. At 95% sensitivity, the MPS2 model could eliminate 35%-45% of unnecessary biopsies, compared with 15%-30% for the other tests, and reduce repeat biopsies by 46%-51%, compared with 9%-21% for the other tests.
Head and neck cancer. In a paper published in JCI Insight, researchers described a test that finds ultra-short fragments of DNA in urine to enable early detection of head and neck cancers caused by human papillomavirus.
“Our data show that a relatively small volume of urine (30-60 mL) gives overall detection results comparable to a tube of blood,” said study author Muneesh Tewari, MD, PhD, professor of hematology and oncology at the University of Michigan .
A larger volume of urine could potentially “make cancer detection even more sensitive than blood,” Dr. Tewari said, “allowing cancers to be detected at the earliest stages when they are more curable.”
The team used a technique called droplet digital PCR to detect DNA fragments that are “ultra-short” (less than 50 base pairs long) and usually missed by conventional PCR testing. This transrenal cell-free tumor DNA, which travels from the tumor into the bloodstream, is broken down small enough to pass through the kidneys and into the urine. But the fragments are still long enough to carry information about the tumor’s genetic signature.
This test could spot cancer before a tumor grows big enough — about a centimeter wide and carrying a billion cells — to spot on a CT scan or other imaging test. “When we are instead detecting fragments of DNA released from a tumor,” said Dr. Tewari, “our testing methods are very sensitive and can detect DNA in urine that came from just 5-10 cells in a tumor that died and released their DNA into the blood, which then made its way into the urine.”
Pancreatic cancer. Pancreatic ductal adenocarcinoma is one of the deadliest cancers, largely because it is diagnosed so late. A urine panel now in clinical trials could help doctors diagnose the cancer before it has spread so more people can have the tumor surgically removed, improving prognosis.
Using enzyme-linked immunosorbent assay test, a common lab method that detects antibodies and other proteins, the team measured expression levels for three genes (LYVE1, REG1B, and TFF1) in urine samples collected from people up to 5 years before they were diagnosed with pancreatic cancer. The researchers combined this result with patients’ urinary creatinine levels, a common component of existing urinalysis, and their age to develop a risk score.
This score performed similarly to an existing blood test, CA19-9, in predicting patients’ risk for pancreatic cancer up to 1 year before diagnosis. When combined with CA19-9, the urinary panel helped spot cancer up to 2 years before diagnosis.
According to a paper in the International Journal of Cancer, “the urine panel and affiliated PancRISK are currently being validated in a prospective clinical study (UroPanc).” If all goes well, they could be implemented in clinical practice in a few years as a “noninvasive stratification tool” to identify patients for further testing, speeding up diagnosis, and saving lives.
Limitations and Promises
Each cancer type is different, and more research is needed to map out which substances in urine predict which cancers and to develop tests for mass adoption. “There are medical and technological hurdles to the large-scale implementation of urine analysis for complex diseases such as cancer,” said Dr. Wong.
One possibility: Scientists and clinicians could collaborate and use artificial intelligence techniques to combine urine test results with other data.
“It is likely that future diagnostics may combine urine with other biological samples such as feces and saliva, among others,” said Dr. Wong. “This is especially true when novel data science and machine learning techniques can integrate comprehensive data from patients that span genetic, proteomic, metabolic, microbiomic, and even behavioral data to evaluate a patient’s condition.”
One thing that excites Dr. Tewari about urine-based cancer testing: “We think it could be especially impactful for patients living in rural areas or other areas with less access to healthcare services,” he said.
A version of this article appeared on Medscape.com.
Maternal Complication Risk Higher For Cesarean Deliveries With Low-Lying Placenta
SAN FRANCISCO — Patients with a low-lying placenta who underwent cesarean deliveries were at higher risk for multiple complications even if they did not have placenta previa, according to data presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Rates of preterm delivery, postpartum hemorrhage, placenta accreta, and need for hysterectomy and transfusion were all significantly higher in patients with low-lying placenta than in patients without, Jacob Thomas, MD, of Advocate Aurora Health in Chicago, Illinois, and Ascension Illinois St. Alexius Medical Center in Hoffman Estates, reported at the meeting.
A low-lying placenta is defined as a placental edge less than 20 mm from the internal os but not covering it. Most studies looking at low-lying placentas, however, group them with placenta previa, making it difficult to know if there are differences in risk of adverse outcomes for those who don’t have placenta previa.
“These are not necessarily shocking findings, but it shows that even low-lying placentas have significant morbidity in and of themselves, not just when they’re lumped with placenta previas,” Dr. Thomas said in an interview. “This means, if you’re doing a C-section for a low-lying placenta, you probably want to treat it a lot like you would treat a placenta previa. You may have blood ready, whether or not you’re going to give it, and you’re going to be more prepared for those complications.”
Noting that approximately 30% of patients with low-lying placenta had preterm deliveries, Dr. Thomas added that these patients might need to be counseled differently as well. The researchers did not have data on how preterm the deliveries were — many could have been 35-37 weeks, for example — but “how you prepare those patients is different,” he said.
Breanna Bolivar, MD, MPH, an obgyn hospitalist at MAHEC Ob/Gyn Specialists in Asheville, North Carolina, said the findings confirm her experience in practice.
“Low-lying placentas are treated very similarly to placenta previas and the results seem similar to patients that have placenta previas,” Dr. Bolivar said in an interview. “In my practice, I treat patients with low-lying placenta the same as I do with placenta previa. I have the same risk factors in mind, and I prepare in the same way.”
The researchers conducted a retrospective analysis of all patients who underwent a cesarean delivery in the National Inpatient Sample from 2017 to 2019 through the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality. After excluding patients with placenta previa, the researchers compared outcomes among patients with ICD-10 codes for low-lying placenta to those of patients without low-lying placenta. The researchers specifically looked at preterm delivery, hemorrhage, hysterectomy, placenta accreta spectrum (PAS), sepsis, shock, disseminated intravascular coagulation, and blood transfusion.
Among 700,635 patients with cesarean deliveries in the database, 0.4% had low-lying placenta. These patients were more likely to be older, to be anemic, and to deliver at a large or urban teaching hospital. They were less likely to have public insurance or a previous cesarean.
After controlling for confounders that differed between the two populations, the researchers found a higher likelihood of all adverse maternal outcomes studied in patients with low-lying placenta (P < .05). These patients had three times greater risk for preterm delivery (adjusted odds ratio [aOR], 3.07; 95% CI, 2.81-3.35) and nearly three times greater risk for shock (aOR 2.55; 95% CI, 1.44-4.52), and transfusion (aOR, 2.56; 95% CI, 2.14-3.06).
Compared to those without low-lying placenta, risk for patients with low-lying placenta was even higher for hemorrhage (aOR, 8.87; 95% CI, 8.10-9.73), hysterectomy (aOR, 9.42; 95% CI, 7.11-12.47), and PAS (aOR, 13.41; 95% CI, 10.34-17.39).
Within the group with low-lying placenta, older patients were modestly, but significantly, more likely to have hemorrhage, hysterectomy, and PAS (aOR, 1.06 for all). The risk was more elevated and significant in patients with tobacco use for hemorrhage (aOR, 1.43), hysterectomy (aOR, 1.40), and PAS (aOR, 1.40). Patients with anemia were also significantly more likely to experience PAS (aOR, 1.34).
“Interestingly, in this population, prior cesarean was not associated with increased rates of hemorrhage or hysterectomy,” the researchers reported. The findings can also “help guide research in terms of questions for the future,” Dr. Thomas said, such as looking at complication rates for vaginal deliveries in people with low-lying placenta.
No external funding was noted, and the authors all had no disclosures. Dr. Bolivar had no disclosures.
SAN FRANCISCO — Patients with a low-lying placenta who underwent cesarean deliveries were at higher risk for multiple complications even if they did not have placenta previa, according to data presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Rates of preterm delivery, postpartum hemorrhage, placenta accreta, and need for hysterectomy and transfusion were all significantly higher in patients with low-lying placenta than in patients without, Jacob Thomas, MD, of Advocate Aurora Health in Chicago, Illinois, and Ascension Illinois St. Alexius Medical Center in Hoffman Estates, reported at the meeting.
A low-lying placenta is defined as a placental edge less than 20 mm from the internal os but not covering it. Most studies looking at low-lying placentas, however, group them with placenta previa, making it difficult to know if there are differences in risk of adverse outcomes for those who don’t have placenta previa.
“These are not necessarily shocking findings, but it shows that even low-lying placentas have significant morbidity in and of themselves, not just when they’re lumped with placenta previas,” Dr. Thomas said in an interview. “This means, if you’re doing a C-section for a low-lying placenta, you probably want to treat it a lot like you would treat a placenta previa. You may have blood ready, whether or not you’re going to give it, and you’re going to be more prepared for those complications.”
Noting that approximately 30% of patients with low-lying placenta had preterm deliveries, Dr. Thomas added that these patients might need to be counseled differently as well. The researchers did not have data on how preterm the deliveries were — many could have been 35-37 weeks, for example — but “how you prepare those patients is different,” he said.
Breanna Bolivar, MD, MPH, an obgyn hospitalist at MAHEC Ob/Gyn Specialists in Asheville, North Carolina, said the findings confirm her experience in practice.
“Low-lying placentas are treated very similarly to placenta previas and the results seem similar to patients that have placenta previas,” Dr. Bolivar said in an interview. “In my practice, I treat patients with low-lying placenta the same as I do with placenta previa. I have the same risk factors in mind, and I prepare in the same way.”
The researchers conducted a retrospective analysis of all patients who underwent a cesarean delivery in the National Inpatient Sample from 2017 to 2019 through the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality. After excluding patients with placenta previa, the researchers compared outcomes among patients with ICD-10 codes for low-lying placenta to those of patients without low-lying placenta. The researchers specifically looked at preterm delivery, hemorrhage, hysterectomy, placenta accreta spectrum (PAS), sepsis, shock, disseminated intravascular coagulation, and blood transfusion.
Among 700,635 patients with cesarean deliveries in the database, 0.4% had low-lying placenta. These patients were more likely to be older, to be anemic, and to deliver at a large or urban teaching hospital. They were less likely to have public insurance or a previous cesarean.
After controlling for confounders that differed between the two populations, the researchers found a higher likelihood of all adverse maternal outcomes studied in patients with low-lying placenta (P < .05). These patients had three times greater risk for preterm delivery (adjusted odds ratio [aOR], 3.07; 95% CI, 2.81-3.35) and nearly three times greater risk for shock (aOR 2.55; 95% CI, 1.44-4.52), and transfusion (aOR, 2.56; 95% CI, 2.14-3.06).
Compared to those without low-lying placenta, risk for patients with low-lying placenta was even higher for hemorrhage (aOR, 8.87; 95% CI, 8.10-9.73), hysterectomy (aOR, 9.42; 95% CI, 7.11-12.47), and PAS (aOR, 13.41; 95% CI, 10.34-17.39).
Within the group with low-lying placenta, older patients were modestly, but significantly, more likely to have hemorrhage, hysterectomy, and PAS (aOR, 1.06 for all). The risk was more elevated and significant in patients with tobacco use for hemorrhage (aOR, 1.43), hysterectomy (aOR, 1.40), and PAS (aOR, 1.40). Patients with anemia were also significantly more likely to experience PAS (aOR, 1.34).
“Interestingly, in this population, prior cesarean was not associated with increased rates of hemorrhage or hysterectomy,” the researchers reported. The findings can also “help guide research in terms of questions for the future,” Dr. Thomas said, such as looking at complication rates for vaginal deliveries in people with low-lying placenta.
No external funding was noted, and the authors all had no disclosures. Dr. Bolivar had no disclosures.
SAN FRANCISCO — Patients with a low-lying placenta who underwent cesarean deliveries were at higher risk for multiple complications even if they did not have placenta previa, according to data presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Rates of preterm delivery, postpartum hemorrhage, placenta accreta, and need for hysterectomy and transfusion were all significantly higher in patients with low-lying placenta than in patients without, Jacob Thomas, MD, of Advocate Aurora Health in Chicago, Illinois, and Ascension Illinois St. Alexius Medical Center in Hoffman Estates, reported at the meeting.
A low-lying placenta is defined as a placental edge less than 20 mm from the internal os but not covering it. Most studies looking at low-lying placentas, however, group them with placenta previa, making it difficult to know if there are differences in risk of adverse outcomes for those who don’t have placenta previa.
“These are not necessarily shocking findings, but it shows that even low-lying placentas have significant morbidity in and of themselves, not just when they’re lumped with placenta previas,” Dr. Thomas said in an interview. “This means, if you’re doing a C-section for a low-lying placenta, you probably want to treat it a lot like you would treat a placenta previa. You may have blood ready, whether or not you’re going to give it, and you’re going to be more prepared for those complications.”
Noting that approximately 30% of patients with low-lying placenta had preterm deliveries, Dr. Thomas added that these patients might need to be counseled differently as well. The researchers did not have data on how preterm the deliveries were — many could have been 35-37 weeks, for example — but “how you prepare those patients is different,” he said.
Breanna Bolivar, MD, MPH, an obgyn hospitalist at MAHEC Ob/Gyn Specialists in Asheville, North Carolina, said the findings confirm her experience in practice.
“Low-lying placentas are treated very similarly to placenta previas and the results seem similar to patients that have placenta previas,” Dr. Bolivar said in an interview. “In my practice, I treat patients with low-lying placenta the same as I do with placenta previa. I have the same risk factors in mind, and I prepare in the same way.”
The researchers conducted a retrospective analysis of all patients who underwent a cesarean delivery in the National Inpatient Sample from 2017 to 2019 through the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality. After excluding patients with placenta previa, the researchers compared outcomes among patients with ICD-10 codes for low-lying placenta to those of patients without low-lying placenta. The researchers specifically looked at preterm delivery, hemorrhage, hysterectomy, placenta accreta spectrum (PAS), sepsis, shock, disseminated intravascular coagulation, and blood transfusion.
Among 700,635 patients with cesarean deliveries in the database, 0.4% had low-lying placenta. These patients were more likely to be older, to be anemic, and to deliver at a large or urban teaching hospital. They were less likely to have public insurance or a previous cesarean.
After controlling for confounders that differed between the two populations, the researchers found a higher likelihood of all adverse maternal outcomes studied in patients with low-lying placenta (P < .05). These patients had three times greater risk for preterm delivery (adjusted odds ratio [aOR], 3.07; 95% CI, 2.81-3.35) and nearly three times greater risk for shock (aOR 2.55; 95% CI, 1.44-4.52), and transfusion (aOR, 2.56; 95% CI, 2.14-3.06).
Compared to those without low-lying placenta, risk for patients with low-lying placenta was even higher for hemorrhage (aOR, 8.87; 95% CI, 8.10-9.73), hysterectomy (aOR, 9.42; 95% CI, 7.11-12.47), and PAS (aOR, 13.41; 95% CI, 10.34-17.39).
Within the group with low-lying placenta, older patients were modestly, but significantly, more likely to have hemorrhage, hysterectomy, and PAS (aOR, 1.06 for all). The risk was more elevated and significant in patients with tobacco use for hemorrhage (aOR, 1.43), hysterectomy (aOR, 1.40), and PAS (aOR, 1.40). Patients with anemia were also significantly more likely to experience PAS (aOR, 1.34).
“Interestingly, in this population, prior cesarean was not associated with increased rates of hemorrhage or hysterectomy,” the researchers reported. The findings can also “help guide research in terms of questions for the future,” Dr. Thomas said, such as looking at complication rates for vaginal deliveries in people with low-lying placenta.
No external funding was noted, and the authors all had no disclosures. Dr. Bolivar had no disclosures.
FROM ACOG 2024
The Lure of Specialty Medicine Pulls Nurse Practitioners From Primary Care
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
For many patients, seeing a nurse practitioner has become a routine part of primary care, in which these “NPs” often perform the same tasks that patients have relied on doctors for.
But NPs in specialty care? That’s not routine, at least not yet. Increasingly, though, nurse practitioners and physician assistants are joining cardiology, dermatology, and other specialty practices, broadening their skills and increasing their income.
This development worries some people who track the health workforce, because current trends suggest primary care, which has counted on nurse practitioners to backstop physician shortages, soon might not be able to rely on them to the same extent.
“They’re succumbing to the same challenges that we have with physicians,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute at the Association of American Medical Colleges. The rates NPs can command in a specialty practice “are quite a bit higher” than practice salaries in primary care, he said.
When nurse practitioner programs began to proliferate in the 1970s, “at first it looked great, producing all these nurse practitioners that go to work with primary care physicians,” said Yalda Jabbarpour, MD, director of the American Academy of Family Physicians’ Robert Graham Center for Policy Studies. “But now only 30% are going into primary care.”
Dr. Jabbarpour was referring to the 2024 primary care scorecard by the Milbank Memorial Fund, which found that from 2016 to 2021 the proportion of nurse practitioners who worked in primary care practices hovered between 32% and 34%, even though their numbers grew rapidly. The proportion of physician assistants, also known as physician associates, in primary care ranged from 27% to 30%, the study found.
Both nurse practitioners and physician assistants are advanced practice clinicians who, in addition to graduate degrees, must complete distinct education, training, and certification steps. NPs can practice without a doctor’s supervision in more than two dozen states, while PAs have similar independence in only a handful of states.
About 88% of nurse practitioners are certified in an area of primary care, according to the American Association of Nurse Practitioners. But it is difficult to track exactly how many work in primary care or in specialty practices. Unlike physicians, they’re generally not required to be endorsed by a national standard-setting body to practice in specialties like oncology or cardiology, for example. The AANP declined to answer questions about its annual workforce survey or the extent to which primary care NPs are moving toward specialties.
Though data tracking the change is sparse, specialty practices are adding these advanced practice clinicians at almost the same rate as primary care practices, according to frequently cited research published in 2018.
The clearest evidence of the shift: From 2008 to 2016, there was a 22% increase in the number of specialty practices that employed nurse practitioners and physician assistants, according to that study. The increase in the number of primary care practices that employed these professionals was 24%.
Once more, the most recent projections by the Association of American Medical Colleges predict a dearth of at least 20,200 primary care physicians by 2036. There will also be a shortfall of non-primary care specialists, including a deficiency of at least 10,100 surgical physicians and up to 25,000 physicians in other specialties.
When it comes to the actual work performed, the lines between primary and specialty care are often blurred, said Candice Chen, MD, MPH, associate professor of health policy and management at George Washington University.
“You might be a nurse practitioner working in a gastroenterology clinic or cardiology clinic, but the scope of what you do is starting to overlap with primary care,” she said.
Nurse practitioners’ salaries vary widely by location, type of facility, and experience. Still, according to data from health care recruiter AMN Healthcare Physician Solutions, formerly known as Merritt Hawkins, the total annual average starting compensation, including signing bonus, for nurse practitioners and physician assistants in specialty practice was $172,544 in the year that ended March 31, slightly higher than the $166,544 for those in primary care.
According to forecasts from the federal Bureau of Labor Statistics, nurse practitioner jobs will increase faster than jobs in almost any other occupation in the decade leading up to 2032, growing by 123,600 jobs or 45%. (Wind turbine service technician is the only other occupation projected to grow as fast.) The growth rate for physician assistants is also much faster than average, at 27%. There are more than twice as many nurse practitioners as physician assistants, however: 323,900 versus 148,000, in 2022.
To Dr. Grover of the AAMC, numbers like this signal that there will probably be enough NPs, PAs, and physicians to meet primary care needs. At the same time, “expect more NPs and PAs to also flow out into other specialties,” he said.
When Pamela Ograbisz started working as a registered nurse 27 years ago, she worked in a cardiothoracic intensive care unit. After she became a family nurse practitioner a few years later, she found a job with a similar specialty practice, which trained her to take on a bigger role, first running their outpatient clinic, then working on the floor, and later in the intensive care unit.
If nurse practitioners want to specialize, often “the doctors mentor them just like they would with a physician residency,” said Ms. Ograbisz, now vice president of clinical operations at temporary placement recruiter LocumTenens.com.
If physician assistants want to specialize, they also can do so through mentoring, or they can receive “certificates of added qualifications” in 10 specialties to demonstrate their expertise. Most employers don’t “encourage or require” these certificates, however, said Jennifer Orozco, DMSc, PA-C, DFAAPA, chief medical officer at the American Academy of Physician Associates.
There are a number of training programs for family nurse practitioners who want to develop skills in other areas.
Raina Hoebelheinrich, 40, a family nurse practitioner at a regional medical center in Yankton, South Dakota, recently enrolled in a three-semester post-master’s endocrinology training program at Mount Marty University. She lives on a farm in nearby northeastern Nebraska with her husband and five sons.
Ms. Hoebelheinrich’s new skills could be helpful in her current hospital job, in which she sees a lot of patients with acute diabetes, or in a clinic setting like the one in Sioux Falls, South Dakota, where she is doing her clinical endocrinology training.
Lack of access to endocrinology care in rural areas is a real problem, and many people may travel hundreds of miles to see a specialist.
“There aren’t a lot of options,” she said.
A version of this article first appeared on Medscape.com.
Severe Maternal Morbidity Can Adversely Affect Mental Health
TOPLINE:
Individuals with severe maternal morbidity (SMM) are at an increased risk for mental health condition–related hospitalization or emergency department (ED) visits up to 13 years after delivery.
METHODOLOGY:
- This retrospective cohort study compared mental health hospitalizations and ED visits in postpartum individuals with and without SMM over 13 years after delivery from April 2008 to March 2021.
- The study analyzed 1,579,392 individuals aged 18-55 years with a first recorded liveborn or stillborn delivery from a pregnancy lasting 20-43 weeks, of which 35,825 (2.3%) had exposure to SMM.
- The SMM exposure was analyzed for events occurring after 20 weeks’ gestation and up to 42 days after delivery hospital discharge in the first recorded birth; those without SMM were considered unexposed.
- The main outcome was a combination of mental health hospitalizations or ED visits occurring at least 43 days after the index birth hospitalization.
TAKEAWAY:
- Individuals with SMM had a 1.3-fold increased risk of mental health hospitalizations or ED visits.
- The hospital or ED visits per 10,000 person-years were 59.2 for mood and anxiety disorders, 17.1 for substance abuse and related disorders, 4.8 for suicidality or self-harm, and 4.1 for schizophrenia spectrum or other psychotic disorders.
- Following SMM, an elevated risk was observed for all mental health outcomes except one (schizophrenia spectrum and other psychotic disorders), with the highest risk seen for suicidality and self-harm (aHR, 1.54).
IN PRACTICE:
“Knowledge of the short- and long-term risks of serious mental health conditions after SMM and its subtypes could inform the need for enhanced postpartum supportive resources,” the authors wrote.
SOURCE:
This study was led by Asia Blackman, MSc, Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Québec, Canada. It was published online in JAMA Network Open.
LIMITATIONS:
The study is limited by its observational design, missing data, and misclassification bias.
DISCLOSURES:
This study was supported by funding from the Canadian Institutes of Health Research. Three authors reported receiving personal fees or grants outside the submitted work. No other conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Individuals with severe maternal morbidity (SMM) are at an increased risk for mental health condition–related hospitalization or emergency department (ED) visits up to 13 years after delivery.
METHODOLOGY:
- This retrospective cohort study compared mental health hospitalizations and ED visits in postpartum individuals with and without SMM over 13 years after delivery from April 2008 to March 2021.
- The study analyzed 1,579,392 individuals aged 18-55 years with a first recorded liveborn or stillborn delivery from a pregnancy lasting 20-43 weeks, of which 35,825 (2.3%) had exposure to SMM.
- The SMM exposure was analyzed for events occurring after 20 weeks’ gestation and up to 42 days after delivery hospital discharge in the first recorded birth; those without SMM were considered unexposed.
- The main outcome was a combination of mental health hospitalizations or ED visits occurring at least 43 days after the index birth hospitalization.
TAKEAWAY:
- Individuals with SMM had a 1.3-fold increased risk of mental health hospitalizations or ED visits.
- The hospital or ED visits per 10,000 person-years were 59.2 for mood and anxiety disorders, 17.1 for substance abuse and related disorders, 4.8 for suicidality or self-harm, and 4.1 for schizophrenia spectrum or other psychotic disorders.
- Following SMM, an elevated risk was observed for all mental health outcomes except one (schizophrenia spectrum and other psychotic disorders), with the highest risk seen for suicidality and self-harm (aHR, 1.54).
IN PRACTICE:
“Knowledge of the short- and long-term risks of serious mental health conditions after SMM and its subtypes could inform the need for enhanced postpartum supportive resources,” the authors wrote.
SOURCE:
This study was led by Asia Blackman, MSc, Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Québec, Canada. It was published online in JAMA Network Open.
LIMITATIONS:
The study is limited by its observational design, missing data, and misclassification bias.
DISCLOSURES:
This study was supported by funding from the Canadian Institutes of Health Research. Three authors reported receiving personal fees or grants outside the submitted work. No other conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Individuals with severe maternal morbidity (SMM) are at an increased risk for mental health condition–related hospitalization or emergency department (ED) visits up to 13 years after delivery.
METHODOLOGY:
- This retrospective cohort study compared mental health hospitalizations and ED visits in postpartum individuals with and without SMM over 13 years after delivery from April 2008 to March 2021.
- The study analyzed 1,579,392 individuals aged 18-55 years with a first recorded liveborn or stillborn delivery from a pregnancy lasting 20-43 weeks, of which 35,825 (2.3%) had exposure to SMM.
- The SMM exposure was analyzed for events occurring after 20 weeks’ gestation and up to 42 days after delivery hospital discharge in the first recorded birth; those without SMM were considered unexposed.
- The main outcome was a combination of mental health hospitalizations or ED visits occurring at least 43 days after the index birth hospitalization.
TAKEAWAY:
- Individuals with SMM had a 1.3-fold increased risk of mental health hospitalizations or ED visits.
- The hospital or ED visits per 10,000 person-years were 59.2 for mood and anxiety disorders, 17.1 for substance abuse and related disorders, 4.8 for suicidality or self-harm, and 4.1 for schizophrenia spectrum or other psychotic disorders.
- Following SMM, an elevated risk was observed for all mental health outcomes except one (schizophrenia spectrum and other psychotic disorders), with the highest risk seen for suicidality and self-harm (aHR, 1.54).
IN PRACTICE:
“Knowledge of the short- and long-term risks of serious mental health conditions after SMM and its subtypes could inform the need for enhanced postpartum supportive resources,” the authors wrote.
SOURCE:
This study was led by Asia Blackman, MSc, Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Québec, Canada. It was published online in JAMA Network Open.
LIMITATIONS:
The study is limited by its observational design, missing data, and misclassification bias.
DISCLOSURES:
This study was supported by funding from the Canadian Institutes of Health Research. Three authors reported receiving personal fees or grants outside the submitted work. No other conflicts of interest were reported.
A version of this article first appeared on Medscape.com.

