User login
PsA: Brodalumab demonstrates favorable efficacy in phase 3 trials
Key clinical point: Brodalumab demonstrated significant and rapid improvements in signs and symptoms of psoriatic arthritis (PsA) vs. placebo in 2 phase 3 trials.
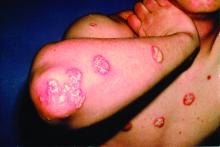
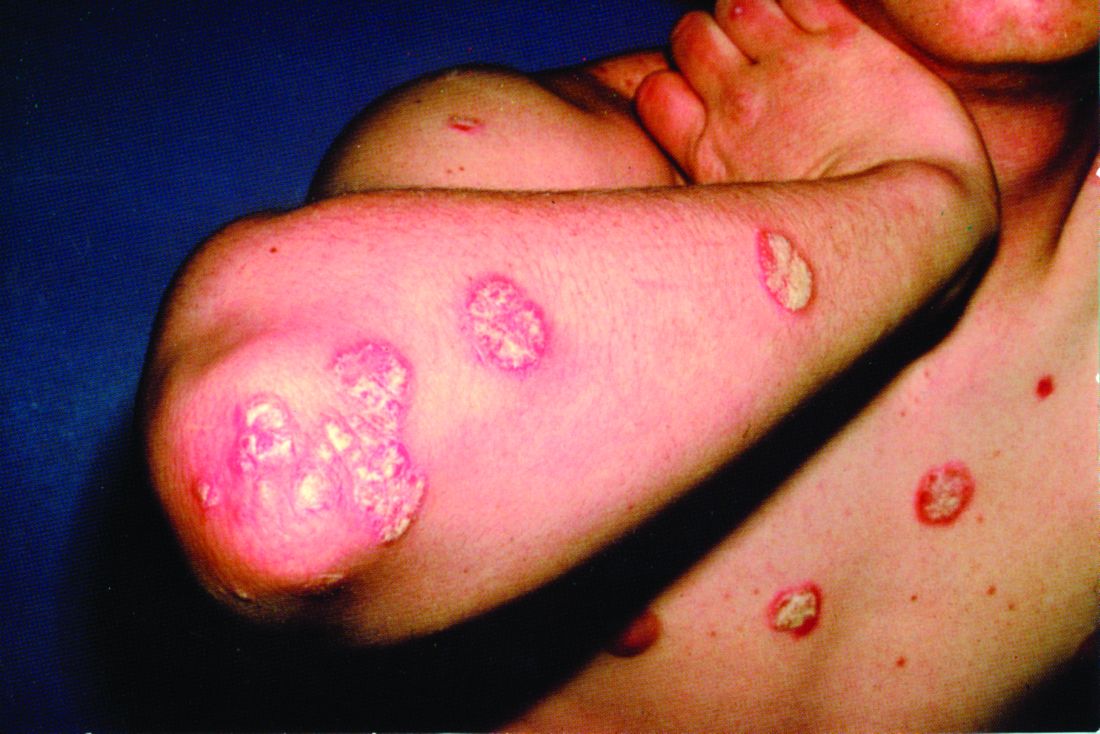
Major finding: The percentage of patients achieving American College of Rheumatology (ACR)20 response at week 16 was significantly higher in the 140 mg and 210 mg brodalumab treatment groups than in the placebo group (45.8% and 47.9%, respectively vs. 20.9%; P less than .0001). Results were similar at week 24. The proportion of brodalumab-treated patients achieving ACR50/70, Psoriasis Area and Severity Index 75/90/100 and resolution of dactylitis and enthesitis was significantly higher than placebo-treated patients (P less than .01). Brodalumab was well tolerated.
Study details: In the AMVISION-1 and AMVISION-2 trials, a total of 962 adult patients with active PsA refractory to conventional treatment were randomly assigned (1:1:1) to either subcutaneous brodalumab 140 mg or 210 mg or placebo at weeks 0, 1 and every 2 weeks up to 24 weeks.
Disclosures: The trials were funded by LEO Pharma. K Raymond is an employee of LEO Pharma. KF Hjuler was an employee of LEO Pharma at the time the study was conducted. PJ Mease, PS Helliwell, KF Hjuler and IB McInnes reported ties with various pharmaceutical companies.
Source: Mease PJ et al. Ann Rheum Dis. 2021 Feb. doi: 10.1136/annrheumdis-2019-216835.
Key clinical point: Brodalumab demonstrated significant and rapid improvements in signs and symptoms of psoriatic arthritis (PsA) vs. placebo in 2 phase 3 trials.
Major finding: The percentage of patients achieving American College of Rheumatology (ACR)20 response at week 16 was significantly higher in the 140 mg and 210 mg brodalumab treatment groups than in the placebo group (45.8% and 47.9%, respectively vs. 20.9%; P less than .0001). Results were similar at week 24. The proportion of brodalumab-treated patients achieving ACR50/70, Psoriasis Area and Severity Index 75/90/100 and resolution of dactylitis and enthesitis was significantly higher than placebo-treated patients (P less than .01). Brodalumab was well tolerated.
Study details: In the AMVISION-1 and AMVISION-2 trials, a total of 962 adult patients with active PsA refractory to conventional treatment were randomly assigned (1:1:1) to either subcutaneous brodalumab 140 mg or 210 mg or placebo at weeks 0, 1 and every 2 weeks up to 24 weeks.
Disclosures: The trials were funded by LEO Pharma. K Raymond is an employee of LEO Pharma. KF Hjuler was an employee of LEO Pharma at the time the study was conducted. PJ Mease, PS Helliwell, KF Hjuler and IB McInnes reported ties with various pharmaceutical companies.
Source: Mease PJ et al. Ann Rheum Dis. 2021 Feb. doi: 10.1136/annrheumdis-2019-216835.
Key clinical point: Brodalumab demonstrated significant and rapid improvements in signs and symptoms of psoriatic arthritis (PsA) vs. placebo in 2 phase 3 trials.
Major finding: The percentage of patients achieving American College of Rheumatology (ACR)20 response at week 16 was significantly higher in the 140 mg and 210 mg brodalumab treatment groups than in the placebo group (45.8% and 47.9%, respectively vs. 20.9%; P less than .0001). Results were similar at week 24. The proportion of brodalumab-treated patients achieving ACR50/70, Psoriasis Area and Severity Index 75/90/100 and resolution of dactylitis and enthesitis was significantly higher than placebo-treated patients (P less than .01). Brodalumab was well tolerated.
Study details: In the AMVISION-1 and AMVISION-2 trials, a total of 962 adult patients with active PsA refractory to conventional treatment were randomly assigned (1:1:1) to either subcutaneous brodalumab 140 mg or 210 mg or placebo at weeks 0, 1 and every 2 weeks up to 24 weeks.
Disclosures: The trials were funded by LEO Pharma. K Raymond is an employee of LEO Pharma. KF Hjuler was an employee of LEO Pharma at the time the study was conducted. PJ Mease, PS Helliwell, KF Hjuler and IB McInnes reported ties with various pharmaceutical companies.
Source: Mease PJ et al. Ann Rheum Dis. 2021 Feb. doi: 10.1136/annrheumdis-2019-216835.
Is pain linked to mortality risk in patients with psoriatic arthritis?
Key clinical point: Pain intensity has limited predictive value for preterm or excess mortality, whereas recent glucocorticoid use and comorbidities were associated with an increased risk of early mortality in patients with psoriatic arthritis.
Major finding: Higher mean pain intensity was associated with an increased risk of mortality (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02-1.10). However, this association attenuated after adjusting for additional confounders. Recent glucocorticoid use (OR, 5.60; 95% CI, 3.71-8.45), concurrent chronic obstructive pulmonary disease (OR, 1.72; 95% CI, 1.06-2.80), diabetes mellitus (OR, 1.86; 95% CI, 1.19-2.90), cancer (OR, 7.17; 95% CI, 4.70-10.93), and cardiovascular disease (OR, 3.04; 95% CI, 2.06-4.49) were all associated with early mortality.
Study details: This nested case-control study included 276 patients with psoriatic arthritis who died (cases) and 1,187 matched controls using data from the nationwide DANBIO register and Danish healthcare registers.
Disclosures: This study was supported by the Danish Psoriasis Foundation Grant, the Danish Rheumatism Foundation Grant, and a grant from Aalborg University and Aalborg University hospital. The authors declared no conflicts of interest.
Source: Vela J et al. Rheumatology (Oxford). 2021 Mar 1. doi: 10.1093/rheumatology/keab192.
Key clinical point: Pain intensity has limited predictive value for preterm or excess mortality, whereas recent glucocorticoid use and comorbidities were associated with an increased risk of early mortality in patients with psoriatic arthritis.
Major finding: Higher mean pain intensity was associated with an increased risk of mortality (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02-1.10). However, this association attenuated after adjusting for additional confounders. Recent glucocorticoid use (OR, 5.60; 95% CI, 3.71-8.45), concurrent chronic obstructive pulmonary disease (OR, 1.72; 95% CI, 1.06-2.80), diabetes mellitus (OR, 1.86; 95% CI, 1.19-2.90), cancer (OR, 7.17; 95% CI, 4.70-10.93), and cardiovascular disease (OR, 3.04; 95% CI, 2.06-4.49) were all associated with early mortality.
Study details: This nested case-control study included 276 patients with psoriatic arthritis who died (cases) and 1,187 matched controls using data from the nationwide DANBIO register and Danish healthcare registers.
Disclosures: This study was supported by the Danish Psoriasis Foundation Grant, the Danish Rheumatism Foundation Grant, and a grant from Aalborg University and Aalborg University hospital. The authors declared no conflicts of interest.
Source: Vela J et al. Rheumatology (Oxford). 2021 Mar 1. doi: 10.1093/rheumatology/keab192.
Key clinical point: Pain intensity has limited predictive value for preterm or excess mortality, whereas recent glucocorticoid use and comorbidities were associated with an increased risk of early mortality in patients with psoriatic arthritis.
Major finding: Higher mean pain intensity was associated with an increased risk of mortality (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02-1.10). However, this association attenuated after adjusting for additional confounders. Recent glucocorticoid use (OR, 5.60; 95% CI, 3.71-8.45), concurrent chronic obstructive pulmonary disease (OR, 1.72; 95% CI, 1.06-2.80), diabetes mellitus (OR, 1.86; 95% CI, 1.19-2.90), cancer (OR, 7.17; 95% CI, 4.70-10.93), and cardiovascular disease (OR, 3.04; 95% CI, 2.06-4.49) were all associated with early mortality.
Study details: This nested case-control study included 276 patients with psoriatic arthritis who died (cases) and 1,187 matched controls using data from the nationwide DANBIO register and Danish healthcare registers.
Disclosures: This study was supported by the Danish Psoriasis Foundation Grant, the Danish Rheumatism Foundation Grant, and a grant from Aalborg University and Aalborg University hospital. The authors declared no conflicts of interest.
Source: Vela J et al. Rheumatology (Oxford). 2021 Mar 1. doi: 10.1093/rheumatology/keab192.
Experts highlight recent breakthroughs in psoriatic arthritis
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2021
COVID-19 vaccination in RMD patients: Safety data “reassuring”
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES
FDA warning letters target OTC cannabidiol product claims for pain relief
The Food and Drug Administration has warned two manufacturers about illegal marketing of drugs containing cannabidiol (CBD) for over-the-counter use without an approved new drug application, for using substandard manufacturing processes, and for failure to comply with current good manufacturing practices. These warnings add to 51 previous warning letters issued by the FDA since 2015 to other manufacturers of products containing CBD who were violating the Federal Food, Drug, and Cosmetic Act.
In a news release, the agency explained that its two most recent letters, sent to Honest Globe Inc. on March 15 and BioLyte Laboratories LLC on March 18, were issued because CBD has “known pharmacologic effects on humans, with demonstrated risks, it cannot be legally marketed as an inactive ingredient in OTC drug products that are not reviewed and approved by the FDA.” They also describe the companies’ failures to comply with current good manufacturing practices.
“The FDA continues to alert the public to potential safety and efficacy concerns with unapproved CBD products sold online and in stores across the country,” FDA Principal Deputy Commissioner Amy P. Abernethy, MD, PhD, said in the release. “It’s important that consumers understand that the FDA has only approved one drug containing CBD as an ingredient [Epidiolex]. These other, unapproved, CBD products may have dangerous health impacts and side effects. We remain focused on exploring potential pathways for CBD products to be lawfully marketed while also educating the public about these outstanding questions of CBD’s safety. Meanwhile, we will continue to monitor and take action, as needed, against companies that unlawfully market their products – prioritizing those that pose a risk to public health.”
The specific products from Santa Ana, Calif.–based Honest Globe that the FDA called unapproved new drugs and misbranded under the Federal Food, Drug, and Cosmetic Act included Elixicure Original Pain Relief and Elixicure Lavender Pain Relief, both of which were described as containing CBD. Products from Grand Rapids, Mich.–based BioLyte Laboratories LLC that the FDA similarly cited for violations included Silver Gel, Silver Gel with Aloe, Silver Liquid Supplement, Therapeutic Pain Gel, Pain Relief Cream, and Magnesium Oil Spray.
The agency has asked the two companies to respond to its letters within 15 working days, “stating how they will address these violations or providing their reasoning and supporting information as to why they believe these products are not in violation of the law. Failure to adequately address the violations promptly may result in legal action, including product seizure and/or injunction.”
The Food and Drug Administration has warned two manufacturers about illegal marketing of drugs containing cannabidiol (CBD) for over-the-counter use without an approved new drug application, for using substandard manufacturing processes, and for failure to comply with current good manufacturing practices. These warnings add to 51 previous warning letters issued by the FDA since 2015 to other manufacturers of products containing CBD who were violating the Federal Food, Drug, and Cosmetic Act.
In a news release, the agency explained that its two most recent letters, sent to Honest Globe Inc. on March 15 and BioLyte Laboratories LLC on March 18, were issued because CBD has “known pharmacologic effects on humans, with demonstrated risks, it cannot be legally marketed as an inactive ingredient in OTC drug products that are not reviewed and approved by the FDA.” They also describe the companies’ failures to comply with current good manufacturing practices.
“The FDA continues to alert the public to potential safety and efficacy concerns with unapproved CBD products sold online and in stores across the country,” FDA Principal Deputy Commissioner Amy P. Abernethy, MD, PhD, said in the release. “It’s important that consumers understand that the FDA has only approved one drug containing CBD as an ingredient [Epidiolex]. These other, unapproved, CBD products may have dangerous health impacts and side effects. We remain focused on exploring potential pathways for CBD products to be lawfully marketed while also educating the public about these outstanding questions of CBD’s safety. Meanwhile, we will continue to monitor and take action, as needed, against companies that unlawfully market their products – prioritizing those that pose a risk to public health.”
The specific products from Santa Ana, Calif.–based Honest Globe that the FDA called unapproved new drugs and misbranded under the Federal Food, Drug, and Cosmetic Act included Elixicure Original Pain Relief and Elixicure Lavender Pain Relief, both of which were described as containing CBD. Products from Grand Rapids, Mich.–based BioLyte Laboratories LLC that the FDA similarly cited for violations included Silver Gel, Silver Gel with Aloe, Silver Liquid Supplement, Therapeutic Pain Gel, Pain Relief Cream, and Magnesium Oil Spray.
The agency has asked the two companies to respond to its letters within 15 working days, “stating how they will address these violations or providing their reasoning and supporting information as to why they believe these products are not in violation of the law. Failure to adequately address the violations promptly may result in legal action, including product seizure and/or injunction.”
The Food and Drug Administration has warned two manufacturers about illegal marketing of drugs containing cannabidiol (CBD) for over-the-counter use without an approved new drug application, for using substandard manufacturing processes, and for failure to comply with current good manufacturing practices. These warnings add to 51 previous warning letters issued by the FDA since 2015 to other manufacturers of products containing CBD who were violating the Federal Food, Drug, and Cosmetic Act.
In a news release, the agency explained that its two most recent letters, sent to Honest Globe Inc. on March 15 and BioLyte Laboratories LLC on March 18, were issued because CBD has “known pharmacologic effects on humans, with demonstrated risks, it cannot be legally marketed as an inactive ingredient in OTC drug products that are not reviewed and approved by the FDA.” They also describe the companies’ failures to comply with current good manufacturing practices.
“The FDA continues to alert the public to potential safety and efficacy concerns with unapproved CBD products sold online and in stores across the country,” FDA Principal Deputy Commissioner Amy P. Abernethy, MD, PhD, said in the release. “It’s important that consumers understand that the FDA has only approved one drug containing CBD as an ingredient [Epidiolex]. These other, unapproved, CBD products may have dangerous health impacts and side effects. We remain focused on exploring potential pathways for CBD products to be lawfully marketed while also educating the public about these outstanding questions of CBD’s safety. Meanwhile, we will continue to monitor and take action, as needed, against companies that unlawfully market their products – prioritizing those that pose a risk to public health.”
The specific products from Santa Ana, Calif.–based Honest Globe that the FDA called unapproved new drugs and misbranded under the Federal Food, Drug, and Cosmetic Act included Elixicure Original Pain Relief and Elixicure Lavender Pain Relief, both of which were described as containing CBD. Products from Grand Rapids, Mich.–based BioLyte Laboratories LLC that the FDA similarly cited for violations included Silver Gel, Silver Gel with Aloe, Silver Liquid Supplement, Therapeutic Pain Gel, Pain Relief Cream, and Magnesium Oil Spray.
The agency has asked the two companies to respond to its letters within 15 working days, “stating how they will address these violations or providing their reasoning and supporting information as to why they believe these products are not in violation of the law. Failure to adequately address the violations promptly may result in legal action, including product seizure and/or injunction.”
Checkpoint inhibitor–induced rheumatic complications often arise late
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
FROM RWCS 2021
To improve psoriatic arthritis outcomes, address common comorbidities
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
FROM RWCS 2021
Recent psoriasis pathophysiology insights carry treatment implications
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Methotrexate-associated hepatotoxicity risk differs between psoriasis, PsA, and RA patients
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
COVID-19 vaccination recommended for rheumatology patients
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.
People with rheumatic diseases should get vaccinated against SARS-CoV-2 as soon as possible, the American College of Rheumatology (ACR) recommends.
“It may be that people with rheumatic diseases are at increased risk of developing COVID or serious COVID-related complications,” Jonathan Hausmann, MD, assistant professor of medicine at Harvard Medical School, Boston, said in an ACR podcast. “So the need to prevent COVID-19 is incredibly important in this group of patients.”
The guidelines recommend a delay in vaccination only in rare circumstances, such as for patients with very severe illness or who have recently been administered rituximab, Jeffrey R. Curtis, MD, MPH, lead author of the guidelines, said in the podcast.
“Our members have been inundated with questions and concerns from their patients on whether they should receive the vaccine,” ACR President David Karp, MD, PhD, said in a press release.
So the ACR convened a panel of nine rheumatologists, two infectious disease specialists, and two public health experts. Over the course of 8 weeks, the task force reviewed the literature and agreed on recommendations. The organization posted a summary of the guidelines on its website after its board of directors approved it Feb. 8. The paper is pending journal peer review.
Some risks are real
The task force confined its research to the COVID-19 vaccines being offered by Pfizer and Moderna because they are currently the only ones approved by the Food and Drug Administration. It found no reason to distinguish between the two vaccines in its recommendations.
Because little research has directly addressed the question concerning COVID-19 vaccination for patients with rheumatic diseases, the task force extrapolated from data on other vaccinations in people with rheumatic disease and on the COVID-19 vaccinations in other populations.
It analyzed reports that other types of vaccination, such as for influenza, triggered flares of rheumatic conditions. “It is really individual case reports or small cohorts where there may be a somewhat higher incidence of flare, but it’s usually not very large in its magnitude nor duration,” said Dr. Curtis of the University of Alabama at Birmingham.
The task force also considered the possibility that vaccinations could lead to a new autoimmune disorder, such as Guillain-Barré syndrome or Bell palsy. The risk is real, the task force decided, but not significant enough to influence their recommendations.
Likewise, in immunocompromised people, vaccinations with live virus, such as those for shingles, might trigger the infection the vaccination is meant to prevent. But this can’t happen with the Pfizer and Moderna COVID-19 vaccines because they contain messenger RNA instead of live viruses, Dr. Curtis said.
Although it might be optimal to administer the vaccines when rheumatic diseases are quiescent, the urgency of getting vaccinated overrides that consideration, Dr. Curtis said. “By and large, there was a general consensus to not want to delay vaccination until somebody was stable and doing great, because you don’t know how long that’s going to be,” he said.
How well does it work?
One unanswered question is whether the COVID-19 vaccines work as well for patients with rheumatic diseases. The task force was reassured by data showing efficacy across a range of subgroups, including some with immunosenescence, Dr. Curtis said. “But until we have data in rheumatology patients, we’re just not going to know,” he said.
The guidelines specify that some drug regimens be modified when patients are vaccinated.
For patients taking rituximab, vaccination should be delayed, but only for those who are able to maintain safe social distancing to reduce the risk for COVID-19 exposure, Dr. Curtis said. “If somebody has just gotten rituximab recently, it might be more ideal to complete the vaccine series about 2-4 weeks before the next rituximab dose,” he said. “So if you are giving that therapy, say, at 6-month intervals, if you could vaccinate them at around month 5 from the most recent rituximab cycle, that might be more ideal.”
The guidance calls for withholding JAK inhibitors for a week after each vaccine dose is administered.
It calls for holding SQ abatacept 1 week prior and 1 week after the first COVID-19 vaccine dose, with no interruption after the second dose.
For abatacept IV, clinicians should “time vaccine administration so that the first vaccination will occur 4 weeks after abatacept infusion (i.e., the entire dosing interval), and postpone the subsequent abatacept infusion by 1 week (i.e., a 5-week gap in total).” It recommends no medication adjustment for the second vaccine dose.
For cyclophosphamide, the guidance recommends timing administration to occur about a week after each vaccine dose, when feasible.
None of this advice should supersede clinical judgment, Dr. Curtis said.
A version of this article first appeared on Medscape.com.