User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Iatrogenic hyponatremia in a patient with bipolar disorder
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
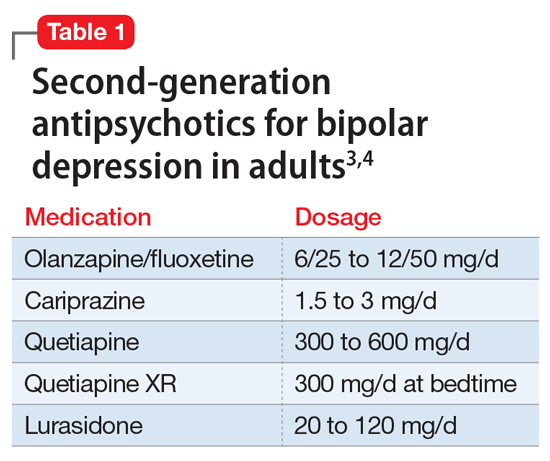
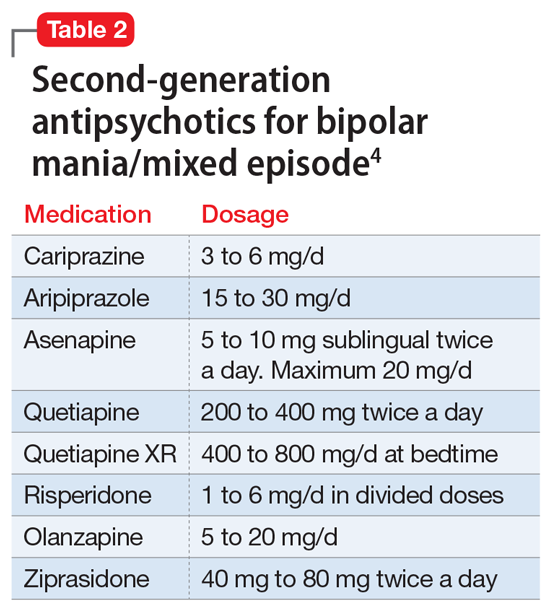
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.
The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
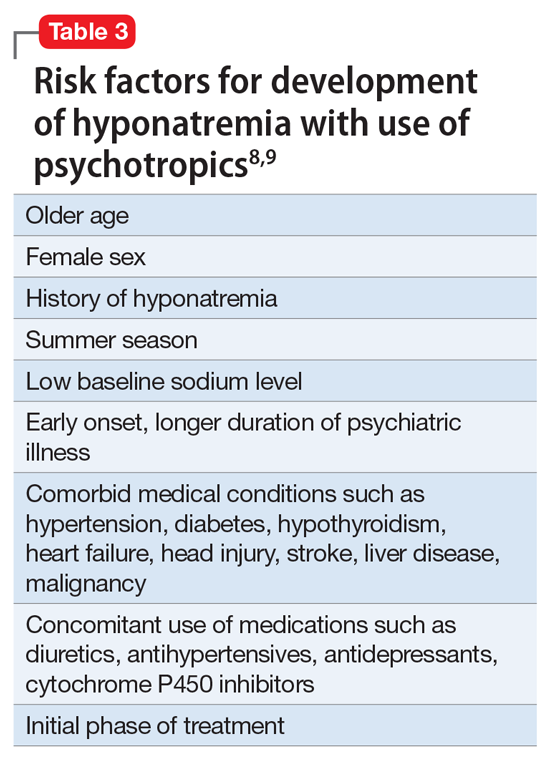
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.
Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Antidepressant may cut COVID-19–related hospitalization, mortality: TOGETHER
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Clinicians underprescribe behavior therapy for preschool ADHD
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Borderline personality disorder: 6 studies of biological interventions
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.
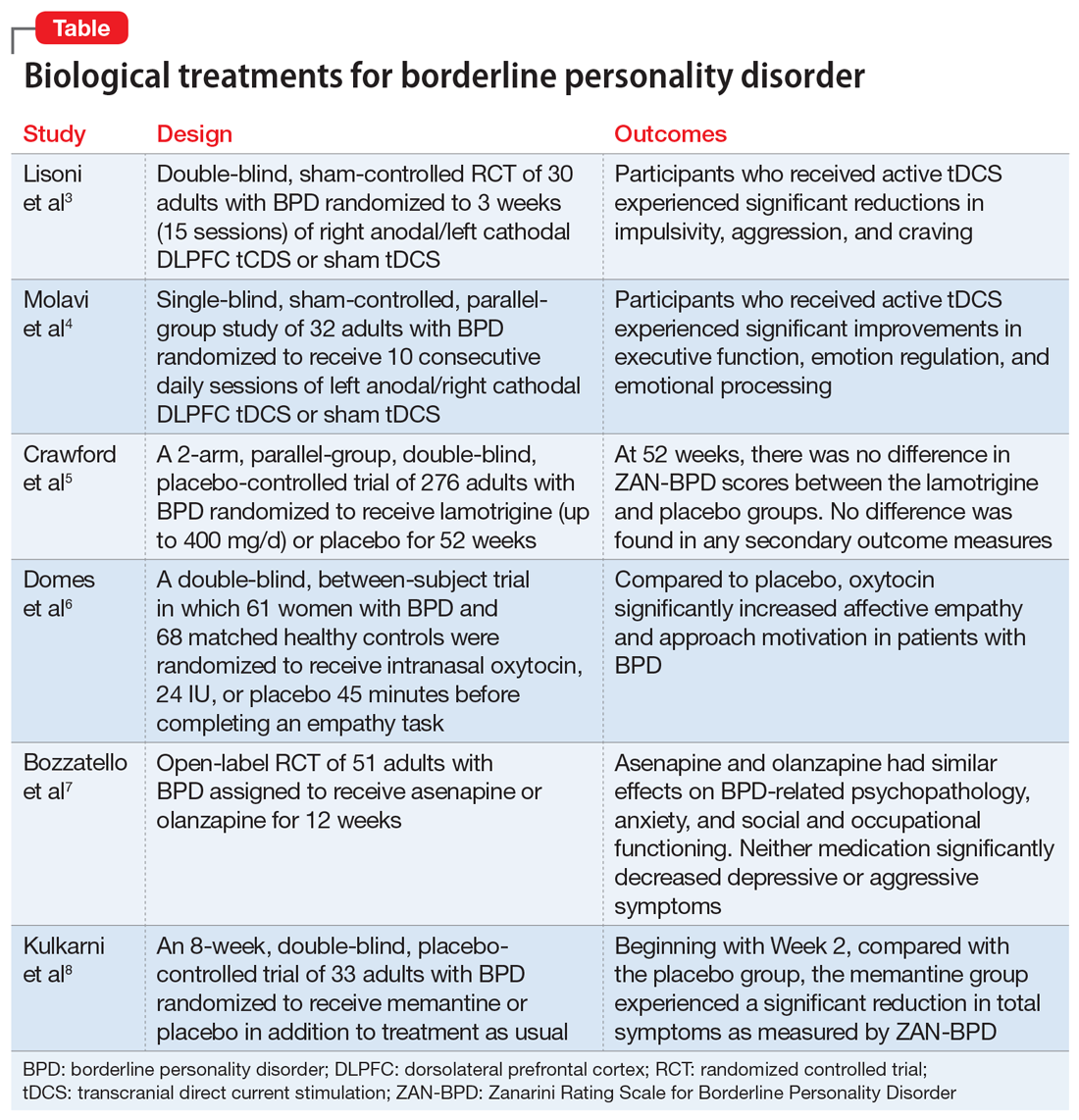
1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.

1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
FIRST OF 2 PARTS
Borderline personality disorder (BPD) is marked by an ongoing pattern of mood instability, cognitive distortions, problems with self-image, and impulsive behavior, often resulting in problems in relationships. BPD is associated with serious impairment in psychosocial functioning.1 Patients with BPD tend to use more mental health services than patients with other personality disorders or those with major depressive disorder (MDD).2 However, there has been little consensus on the best treatment(s) for this serious and debilitating disorder, and some clinicians view BPD as difficult to treat.
Current treatments for BPD include psychological and pharmacological interventions. Neuromodulation techniques, such as repetitive transcranial magnetic stimulation, may also positively affect BPD symptomatology. In recent years, there have been some promising findings in the treatment of BPD. In this 2-part article, we focus on current (within the last 5 years) findings from randomized controlled trials (RCTs) of BPD treatments. Here in Part 1, we focus on 6 studies that evaluated biological interventions (Table,3-8). In Part 2, we will focus on RCTs that investigated psychological interventions.

1. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
Impulsivity has been described as the core feature of BPD that best explains its behavioral, cognitive, and clinical manifestations. Studies have repeatedly demonstrated the role of the prefrontal cortex in modulating impulsivity. Dysfunction of the
Continue to: Study design...
Study design
- In a double-blind, sham-controlled trial, adults who met DSM-IV-TR criteria for BPD were randomized to 3 weeks (15 sessions) of right anodal/left cathodal DLPFC tCDS (n = 15) or sham tDCS (n = 15). This study included patients with comorbid psychiatric disorders, including substance use disorders. Discontinuation or alteration of existing medications was not allowed.
- The presence, severity, and change over time of BPD core symptoms was assessed at baseline and after 3 weeks using several clinical scales, self-questionnaires, and neuropsychological tests, including the Barratt Impulsiveness Scale-11 (BIS-11), Buss-Perry Aggression Questionnaire (BP-AQ), Difficulties in Emotion Regulation Scale (DERS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Hamilton Anxiety Rating Scale (HAM-A), Irritability-Depression Anxiety Scale (IDA), Visual Analog Scales (VAS), and Iowa Gambling Task.
Outcomes
- Participants in the active tDCS group experienced significant reductions in impulsivity, aggression, and craving as measured by the BIS-11, BP-AQ, and VAS.
- Compared to the sham group, the active tDCS group had greater reductions in HAM-D and BDI scores.
- HAM-A and IDA scores were improved in both groups, although the active tDCS group showed greater reductions in IDA scores compared with the sham group.
- As measured by DERS, active tDCS did not improve affective dysregulation more than sham tDCS.
Conclusions/limitations
- Bilateral tDCS targeting the right DLPFC with anodal stimulation is a safe, well-tolerated technique that may modulate core dimensions of BPD, including impulsivity, aggression, and craving.
- Excitatory anodal stimulation of the right DLFPC coupled with inhibitory cathodal stimulation on the left DLPFC may be an effective montage for targeting impulsivity in patients with BPD.
- Study limitations include a small sample size, use of targeted questionnaires only, inclusion of patients with BPD who also had certain comorbid psychiatric disorders, lack of analysis of the contributions of medications, lack of functional neuroimaging, and lack of a follow-up phase.
2. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
Emotional dysregulation is considered a core feature of BPD psychopathology and is closely associated with executive dysfunction and cognitive control. Manifestations of executive dysfunction include aggressiveness, impulsive decision-making, disinhibition, and self-destructive behaviors. Neuroimaging of patients with BPD has shown enhanced activity in the insula, posterior cingulate cortex, and amygdala, with reduced activity in the medial PFC, subgenual anterior cingulate cortex, and DLPFC. Molavi et al4 postulated that increasing DLPFC activation with left anodal tDCS would result in improved executive functioning and emotion dysregulation in patients with BPD.
Study design
- In this single-blind, sham-controlled, parallel-group study, adults who met DSM-5 criteria for BPD were randomized to receive 10 consecutive daily sessions of left anodal/right cathodal DLPFC tDCS (n = 16) or sham tDCS (n = 16).
- The effect of tDCS on executive dysfunction, emotion dysregulation, and emotional processing was measured using the Executive Skills Questionnaire for Adults (ESQ), Emotion Regulation Questionnaire (ERQ), and Emotional Processing Scale (EPS). Measurements occurred at baseline and after 10 sessions of active or sham tDCS.
Outcomes
- Participants who received active tDCS experienced significant improvements in ESQ overall score and most of the executive function domains measured by the ESQ.
- Those in the active tDCS group also experienced significant improvement in emotion regulation as measured by the cognitive reappraisal subscale (but not the expressive suppression subscale) of the ERQ after the intervention.
- Overall emotional processing as measured by the EPS was significantly improved in the active tDCS group following the intervention.
Conclusions/limitations
- Repeated bilateral left anodal/right cathodal tDCS stimulation of the DLPFC significantly improved executive functioning and aspects of emotion regulation and emotional processing in patients with BPD. This improvement was presumed to be the result of increased activity of left DLPFC.
- Study limitations include a single-blind design, lack of follow-up to assess durability and stability of response over time, reliance on self-report measures, lack of functional neuroimaging, and limited focality of tDCS.
3. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
One of the hallmark symptoms of BPD is mood dysregulation. Current treatment guidelines recommend the use of mood stabilizers for BPD despite limited quality evidence of effectiveness and a lack of FDA-approved medications with this indication. In this RCT, Crawford et al5 examined whether lamotrigine is a clinically effective and cost-effective treatment for people with BPD.
Continue to: Study design...
Study design
- In this 2-arm, parallel-group, double-blind, placebo-controlled trial, 276 adults who met DSM-IV criteria for BPD were randomized to receive lamotrigine (up to 400 mg/d) or placebo for 52 weeks.
- The primary outcome was the score on the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) at 52 weeks. Secondary outcomes included depressive symptoms, deliberate self-harm, social functioning, health-related quality of life, resource use and costs, treatment adverse effects, and adverse events. These were assessed using the BDI; Acts of Deliberate Self-Harm Inventory; Social Functioning Questionnaire; Alcohol, Smoking, and Substance Involvement Screening Test; and the EQ-5D-3L.
Outcomes
- Mean ZAN-BPD score decreased at 12 weeks in both groups, after which time the score remained stable.
- There was no difference in ZAN-BPD scores at 52 weeks between treatment arms. No difference was found in any secondary outcome measures.
- Difference in costs between groups was not significant.
Conclusions/limitations
- There was no evidence that lamotrigine led to clinical improvements in BPD symptomatology, social functioning, health-related quality of life, or substance use.
- Lamotrigine is neither clinically effective nor a cost-effective use of resources in the treatment of BPD.
- Limitations include a low level of adherence.
4. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
A core feature of BPD is impairment in empathy; adequate empathy is required for intact social functioning. Oxytocin is a neuropeptide that helps regulate complex social cognition and behavior. Prior research has found that oxytocin administration enhances emotion regulation and empathy. Women with BPD have been observed to have lower levels of oxytocin. Domes et al6 conducted an RCT to see if oxytocin could have a beneficial effect on social approach and social cognition in women with BPD.
Study design
- In a double-blind, placebo-controlled, between-subject trial, 61 women who met DSM-IV criteria for BPD and 68 matched healthy controls were randomized to receive intranasal oxytocin, 24 IU, or placebo 45 minutes before completing an empathy task.
- An extended version of the Multifaceted Empathy Test was used to assess empathy and approach motivation.
Outcomes
- For cognitive empathy, patients with BPD exhibited significantly lower overall performance compared to controls. There was no effect of oxytocin on this performance in either group.
- Patients with BPD had significantly lower affective empathy compared with controls. After oxytocin administration, patients with BPD had significantly higher affective empathy than those with BPD who received placebo, reaching the level of healthy controls who received placebo.
- For positive stimuli, patients with BPD showed lower affective empathy than controls. Oxytocin treatment increased affective empathy in both groups.
- For negative stimuli, oxytocin increased affective empathy more in patients with BPD than in controls.
- Patients with BPD demonstrated less approach motivation than controls. Oxytocin increased approach motivation more in patients with BPD than in controls. For approach motivation toward positive stimuli, oxytocin had a significant effect on patients with BPD.
Continue to: Conclusions/limitations...
Conclusions/limitations
- Patients with BPD showed reduced cognitive and affective empathy and less approach behavior motivation than healthy controls.
- Patients with BPD who received oxytocin attained a level of affective empathy and approach motivation similar to that of healthy controls who received placebo. For positive stimuli, both groups exhibited comparable improvements from oxytocin. For negative stimuli, patients with BPD patients showed significant improvement with oxytocin, whereas healthy controls received no such benefit.
- Limitations include the use of self-report scales, lack of a control group, and inclusion of patients using psychotherapeutic medications. The study lacks generalizability because only women were included; the effect of exogenous oxytocin on men may differ.
5. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
The last decade has seen a noticeable shift in clinical practice from the use of antidepressants to mood stabilizers and second-generation antipsychotics (SGAs) in the treatment of BPD. Studies have demonstrated therapeutic effects of antipsychotic drugs across a wide range of BPD symptoms. Among SGAs, olanzapine is the most extensively studied across case reports, open-label studies, and RCTs of patients with BPD. In an RCT, Bozzatello et al7 compared the efficacy and tolerability of asenapine to olanzapine.
Study design
- In this open-label RCT, adults who met DSM-5 criteria for BPD were assigned to receive asenapine (n = 25) or olanzapine (n = 26) for 12 weeks.
- Study measurements included the Clinical Global Impression Scale, Severity item, HAM-D, HAM-A, Social and Occupational Functioning Assessment Scale, Borderline Personality Disorder Severity Index (BPDSI), BIS-11, Modified Overt Aggression Scale, and Dosage Record Treatment Emergent Symptom Scale.
Outcomes
- Asenapine and olanzapine had similar effects on BPD-related psychopathology, anxiety, and social and occupational functioning.
- Neither medication significantly decreased depressive or aggressive symptoms.
- Asenapine was superior to olanzapine in reducing the affective instability score of the BPDSI.
- Akathisia and restlessness/anxiety were more common with asenapine, and somnolence and fatigue were more common with olanzapine.
Conclusions/limitations
- The overall efficacy of asenapine was not different from olanzapine, and both medications were well-tolerated.
- Neither medication led to an improvement in depression or aggression, but asenapine was superior to olanzapine in reducing the severity of affective instability.
- Limitations include an open-label design, lack of placebo group, small sample size, high drop-out rate, exclusion of participants with co-occurring MDD and substance abuse/dependence, lack of data on prior pharmacotherapies and psychotherapies, and lack of power to detect a difference on the dissociation/paranoid ideation item of BPDSI.
6. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
It has been hypothesized that glutamate dysregulation and excitotoxicity are crucial to the development of the cognitive disturbances that underlie BPD. As such, glutamate modulators such as memantine hold promise for the treatment of BPD. In this RCT, Kulkarni et al8 examined the efficacy and tolerability of memantine compared with treatment as usual in patients with BPD.
Continue to: Study design...
Study design
- In an 8-week, double-blind, placebo-controlled trial, adults diagnosed with BPD according to the Diagnostic Interview for Borderline Patients were randomized to receive memantine (n = 17) or placebo (n = 16) in addition to treatment as usual. Treatment as usual included the use of antidepressants, mood stabilizers, and antipsychotics as well as psychotherapy and other psychosocial interventions.
- Patients were initiated on placebo or memantine, 10 mg/d. Memantine was increased to 20 mg/d after 7 days.
- ZAN-BPD score was the primary outcome and was measured at baseline and 2, 4, 6, and 8 weeks. An adverse effects questionnaire was administered every 2 weeks to assess tolerability.
Outcomes
- During the first 2 weeks of treatment, there were no significant improvements in ZAN-BPD score in the memantine group compared with the placebo group.
- Beginning with Week 2, compared with the placebo group, the memantine group experienced a significant reduction in total symptoms as measured by ZAN-BPD.
- There were no statistically significant differences in adverse events between groups.
Conclusions/limitations
- Memantine appears to be a well-tolerated treatment option for patients with BPD and merits further study.
- Limitations include a small sample size, and an inability to reach plateau of ZAN-BPD total score in either group. Also, there is considerable individual variability in memantine steady-state plasma concentrations, but plasma levels were not measured in this study.
Bottom Line
Findings from small randomized controlled trials suggest that transcranial direct current stimulation, oxytocin, asenapine, olanzapine, and memantine may have beneficial effects on some core symptoms of borderline personality disorder. These findings need to be replicated in larger studies.
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
1. Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002; 159:276-283.
2. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
3. Lisoni J, Miotto P, Barlati S, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261
4. Molavi P, Aziziaram S, Basharpoor S, et al. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93-102. doi: 10.1016/j.jad.2020.05.007
5. Crawford MJ, Sanatinia R, Barrett B, et al; LABILE study team. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial. Am J Psychiatry. 2018;175(8):756-764. doi: 10.1176/appi.ajp.2018.17091006
6. Domes G, Ower N, von Dawans B, et al. Effects of intranasal oxytocin administration on empathy and approach motivation in women with borderline personality disorder: a randomized controlled trial. Transl Psychiatry. 2019;9(1):328. doi: 10.1038/s41398-019-0658-4
7. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819. doi: 10.1007/s40263-017-0458-4
8. Kulkarni J, Thomas N, Hudaib AR, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. CNS Drugs. 2018;32(2):179-187. doi: 10.1007/s40263-018-0506-8
Service animals and emotional support animals: Should you write that letter?
For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals

For centuries, animals, especially dogs, have assisted humans in a variety of ways in their daily lives. Animals that assist people with disabilities fall into 2 broad categories: disability service animals, and emotional support animals (ESAs). Often there is confusion in how these categories differ because of the animal’s role and the laws related to them.
This article describes the differences between disability service animals and ESAs, and outlines the forensic and ethical concerns you should consider before agreeing to write a letter for a patient outlining their need for a disability service animal or ESA. A letter may protect a patient and their service animal or ESA in situations where laws and regulations typically prohibit animals, such as on a flight or when renting an apartment or house. Note that a description of how to conduct the formal patient evaluation before writing a verification letter is beyond the scope of this article.
The differences between disability service animals and ESAs
Purpose and training. Disability service animals, or service animals, are dogs of any breed (and in some cases miniature horses) that are specially trained to perform tasks for an individual with a disability (physical, sensory, psychiatric, intellectual, or other mental disability).1-3 These tasks must be directly related to the individual’s disability.1,2 On the other hand, ESAs, which can be any species of animal, provide support and minimize the impact of an individual’s emotional or psychological disability based on their presence alone. Unlike disability service animals, ESAs are not trained to perform a specific task or duty.2,3
There is no legal requirement for service animals to know specific commands, and professional training is not required—individuals can train the animals themselves.1 Service animals, mainly dogs, can be trained to perform numerous tasks, including4:
- attending to an individual’s mobility and activities of daily living
- guiding an individual who is deaf or hearing impaired
- helping to remind an individual to take their medications
- assisting an individual during and/or after a seizure
- alerting individuals with diabetes in advance of low or high blood sugar episodes
- supporting an individual with autism
- assisting an individual with a psychiatric or mental disability
- applying sensory commands such as lying on the person or resting their head on the individual’s lap to help the individual regain behavioral control.
Service dog verification works via an honor system, which can be problematic, especially in the case of psychiatric service dogs, whose handlers may not have a visible disability (Box 11,5).
Box 1
In the United States, there is no national service dog certification program—meaning there is no official test that a dog has to pass in order to obtain formal recognition as a service animal—nor is there a central and mandatory service dog registry.5 Instead, service dog verification works through an honor system, which can be problematic.5 In many states, misrepresenting one’s dog as a service dog is considered a misdemeanor.5 Unfortunately, other than the guidance set forth by the Americans with Disabilities Act, there are no criteria by which one can recognize a genuine service dog vs one being passed off as a service dog.5
In situations in public settings where it is not obvious or there’s doubt that the dog is a service animal (such as when a person visits a restaurant or store), employees are not allowed to request documentation for the dog, require the dog demonstrate its task, or inquire about the nature of the person’s disability.1
However, they can ask 2 questions1:
1. Is the animal required because of a disability?
2. What work or task has the animal been trained to perform?
Legal protections. Under the Americans with Disabilities Act (ADA), individuals with disabilities can bring their service animals into buildings or facilities where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs, religious organizations, or places of worship that are not open to the public.6,7 ESAs do not qualify as service animals under the ADA and are not given the same legal accommodations as service animals.1,3 Although ESAs were initially covered by the Air Carrier Access Act, they are no longer allowed in aircraft cabins after the US Department of Transportation revised this Act’s regulations in December 2020. ESAs are covered under the Fair Housing Act. Box 21-3,6-15 further discusses these laws and protections.
Evidence.
Due to the difficulty in reconciling inconsistent definitions for ESAs, there is limited high-quality data pertaining to the potential benefits and risks of ESAs.9 Currently, ESAs are not an evidence-based treatment for psychiatric disorders. To date, a handful of small studies have focused on ESAs. However, data from actual tests of the clinical risks and benefits of ESAs do not exist.9 In practice, ESAs are equivalent to pets. It stands to reason that similar to pets, ESAs could reduce loneliness, improve life satisfaction, and provide a sense of well-being.9 A systematic review suggested that pets provide benefits to patients with mental health conditions “through the intensity of connectivity with their owners and the contribution they make to emotional support in times of crises together with their ability to help manage symptoms when they arise.”18 In response to a congressional mandate, the US Department of Veterans Affairs launched a multi-site study from December 2014 to June 2019 to examine how limitations on activity and quality of life in veterans with posttraumatic stress disorder are impacted by the provision of a service dog vs an emotional support dog.19 As of October 14, 2021, results had not been published.19
Continue to: What’s in a disability service animal/ESA letter?
What’s in a disability service animal/ESA letter?
If you decide to write a letter advocating for your patient to have a service animal or ESA, the letter should appear on letterhead, be written by a licensed mental health professional, and include the following2,20:
- statement that the letter is being written at the patient’s request and is being given directly to the patient for use as the patient sees fit
- confirmation of the patient’s DSM-5 mental health diagnosis
- explanation of how the animal helps alleviate symptoms of the patient’s condition, briefly describing any interaction(s) between the animal and patient that you may have observed, and if applicable, a mention of any training the animal may have received from a qualified trainer if applicable
- explanation of the possible negative effects of the patient not having the animal with him or her
- statement that you are not vouching for the animal’s behavior
- verification of your involvement in your patient’s treatment and your assessment of the patient as their licensed mental health professional (including details such as date and type of license you have and the state/other jurisdiction where it was issued).
In a letter for a service animal, also indicate that your patient is psychiatrically disabled to the extent that your patient is not able to perform at least one major life task without the daily assistance of a service animal.2Should you write your patient a letter?
Writing a letter advocating for a patient to have a service animal or ESA may appear innocuous, but doing so may have serious ramifications. Writing a letter certifying a dog as a service animal does not make that animal a service animal; the dog must be specifically trained for a task or tasks directly related to that individual’s disability. There are no current standards for conducting evaluations to determine the need a patient has for a service animal or ESA. How to conduct such evaluations is beyond the scope of this article. There are meager opportunities for formal education and training on how to conduct these evaluations.9 Online resources may be incomplete or inaccurate, and this information is often produced by lay animal enthusiasts and organizations, which can lead to a biased depiction of these animals.9
If you decide to write a letter for your patient, consider the following forensic and ethical concerns.
Remain objective. As an advocate for your patient, you may find it difficult to remain neutral and objective when asked to determine if your patient has a disability, the severity of the disability, the impact of the disability on your patient’s life, and the need for a service animal or ESA. Ensure that your advocacy for your patient does not impair your objectivity; if that is difficult, consider referring your patient to a third party who can conduct an objective evaluation.
Understand the risks. If you make written recommendations for special accommodations in a letter and those recommendations are disputed by an agency, that agency could initiate legal action and you may be called to justify your recommendations in a deposition or open court.9,21 Before writing the letter, ask yourself, “Can I defend my determination that my patient is disabled by a DSM-5 disorder and that this disability requires the presence of an animal in exception to existing policy?”21 Be prepared to state in a legal proceeding that the presence of a service animal or ESA is necessary. If you are unwilling to risk exposure to a legal action, then you should likely refrain from writing the letter. It is a crime to fraudulently certify an animal as a service animal in some jurisdictions, and such conduct could result in disciplinary action by your licensing board.21
Conduct a systematic examination. When you write a letter for your patient, you are explicitly declaring your patient has a disability or condition. Comprehensive disability determinations are complex and are best conducted by assessing for objective evidence of psychiatric disorders and impairment through the use of standard, systematic examination methods.22 Unstandardized measures (eg, asking patients open-ended questions and then relying on your clinical judgement and interpretation in arriving at conclusions) are not as effective.22 In addition, consider the possibility that your patient may malinger their symptoms in an effort to obtain a letter supporting a service animal or ESA. Assessing for malingering is essential to making a disability determination, especially if a disability claim is based primarily on self-report.22
Anticipate pushback. Problems can arise when a patient wants a letter that you cannot or will not provide due to your scope of practice. Consider how you would resolve the situation when you do not believe your patient has a disability that requires the presence of a service animal or ESA—or you believe that your patient no longer needs a service animal or ESA—and the patient disagrees.21 Disagreeing with your patient’s assessment could result in a conflict of interest that could damage the therapeutic relationship.21
Box 2
The Americans with Disabilities Act (ADA) of 1990, as amended by the ADA Amendments Act of 2008, prohibits discrimination on the basis of disability in several areas, including state and local governments (under Title II of the ADA) and places of public accommodations, commercial facilities, and private entities (under Title III of the ADA).6,7 Thus, individuals with disabilities can bring their service animals into the building or facility where members of the public, program participants, clients, customers, patrons, or invitees are allowed.2 This does not include private clubs not open to the public, religious organizations, or places of worship.6,7
Service animals. Although the ADA recognizes miniature horses as service animals, only dogs are recognized as service animals in regards to Title II and Title III protections under the ADA as of March 15, 2011.2 Federal agencies do not have to comply with the ADA1; however, Section 504 of the Rehabilitation Act of 1973 is the federal law that protects the rights of people with disabilities to participate in federal programs and services.1,8 It states that no qualified individual with a disability shall be excluded from, denied the benefits of, or be subjected to discrimination under any program or activity that receives federal funding or is conducted by federal agencies.8 Courts have strived to interpret the Rehabilitation Act and the ADA in a consistent manner, specifically applying the ADA regulations regarding service animals (including its narrow definition regarding specifically trained tasks and emotional support) to the Rehabilitation Act.9-11
Similarly, commercial airlines do not have to comply with the ADA1 ; however, the Air Carrier Access Act (ACAA) of 1986 is the federal law that protects the rights of people with disabilities in air travel.1,12 On December 2, 2020, the US Department of Transportation announced that it was revising its ACAA regulation regarding service animals on aircraft (this final rule will be effective 30 days after date of publication in the Federal Register).13 Among the many revisions, the US Department of Transportation narrowed the definition of service animals to only dogs that were individually trained to work or perform tasks for the benefits of a person with a disability.13 It requires airlines to treat psychiatric service animals the same as other service animals.13 Although the US Department of Transportation has chosen to closely align its ACAA service animal definition with US Department of Justice service animal definition under the ADA, the substantive requirements in this final rule differ from US Department of Justice’s requirements for service animals under the ADA in various areas (for example, by allowing airlines to require service animal documentation and prohibiting the use of voice control over a service animal).13
Emotional support animals. Regulations regarding ESAs are primarily set by individual states1,3; however, ESAs may qualify for a waiver of a no-pet rule or a pet deposit under the Fair Housing Amendments Act (FHAA) of 1988.2,14 Under the FHAA, if an individual has a disability, as defined by the ADA, that requires the presence of an ESA, or if they have symptoms that are ameliorated by the presence of an ESA, the landlord must comply with this request and allow the animal into the facility without charging pet fees.15
Bottom Line
Disability service animals and emotional support animals (ESAs) differ in their roles and legal protections. Before writing a letter in support of a patient’s request for a service animal or ESA, take into account the forensic and ethical implications of doing so.
Related Resources
- US Department of Justice. Civil Rights Division. Disability Rights Section. ADA requirements. Service animals. Updated February 24, 2020. https://www.ada.gov/service_ animals_2010.htm
American Veterinary Medical Association. Service, emotional support and therapy animals. https://www. avma.org/resources-tools/animal-health-welfare/ service-emotional-support-and-therapy-animals
US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. https://www.transportation.gov/briefingroom/us-department-transportation-announces-finalrule-traveling-air-service-animals
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf
1. US Department of Justice. Frequently asked questions about service animals and the ADA. Published July 20, 2015. Accessed on July 28, 2021. https://www.ada.gov/regs2010/service_animal_qa.pdf
2. ADA National Network. Service animals and emotional support animals: where are they allowed and under what conditions? Published 2014. Accessed July 28, 2021. https://adata.org/sites/adata.org/files/files/Service_Animal_Booklet_2014(2).pdf
3. Huben-Kearney A. What to do if patients want service or emotional support animals. Psychiatric News. Published September 28, 2020. Accessed July 28, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.10a24
4. Fine AH. The role of therapy and service animals in the lives of persons with disabilities. Rev Sci Tech. 2018;37(1):141-149.
5. Wlodarczyk J. When pigs fly: emotional support animals, service dogs and the politics of legitimacy across species boundaries. Med Humanit. 2019;45(1):82-91.
6. Americans with Disabilities Act of 1990. Pub L. 101-336, 104 Stat. 327.
7. ADA Amendments Act of 2008. Pub L. 110-325.
8. Rehabilitation Act of 1973. Pub L. 93-112, 87 Stat 355.
9. Carroll JD, Mohlenhoff BS, Kersten CM, et al. Laws and ethics related to emotional support animals. J Am Acad Psychiatry Law. 2020;48(4):509-518.
10. Sanchez v US Dept of Energy. 870 F3d 1185 (10th Circuit 2017).
11. Berardelli v Allied Services Inst. of Rehab. Med., 900 F3d 104 (3rd Circuit 2018).
12. Air Carrier Access Act of 1986. 49 USC §41705.
13. US Department of Transportation. US Department of Transportation announces final rule on traveling by air with service animals. Published December 2, 2020. Accessed July 28, 2021. https://www.transportation.gov/briefing-room/us-department-transportation-announces-final-rule-traveling-air-service-animals
14. Fair Housing Amendments Act of 1988. Pub. L. 100-430. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg1619.pdf
15. Boness CL, Younggren JN, Frumkin IB. The certification of emotional support animals: difference between clinical and forensic mental health practitioners. Professional Psychology: Research and Practice. 2017;48(3):216-223.
16. Lane DR, McNicholas J, Collis GM. Dogs for the disabled: benefits to recipients and welfare of the dog. Applied Animal Behaviour Science. 1998;59(1-3):49-60.
17. Hall SS, MacMichael J, Turner A, et al. A survey of the impact of owning a service dog on quality of life for individuals with physical and hearing disability: a pilot study. Health Qual Life Outcomes. 2017;15(1):59. doi:10.1186/s12955-017-0640-x
18. Brooks HL, Rushton K, Lovell K, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):31. doi: 10.1186/s12888-018-1613-2
19. US National Library of Medicine: ClinicalTrials.gov. Can service dogs improve activity and quality of life in veterans with PTSD? (SDPTSD). Updated August 15, 2019. Accessed October 14, 2021. https://clinicaltrials.gov/ct2/show/study/NCT02039843
20. Clay RA. Is that a pet or therapeutic aid? American Psychological Association. 2016;47(8):38. https://www.apa.org/monitor/2016/09/pet-aid
21. Younggren JN, Boisvert JA, Boness CL. Examining emotional support animals and role conflicts in professional psychology. Prof Psychol Res Pr. 2016;47(4):255-260.
22. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(4 Suppl):S3-S50. https://www.aapl.org/docs/pdf/Evaluation%20of%20Psychiatric%20Disability.pdf
I have a dream … for psychiatry
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
One of the most inspiring speeches ever made is Rev. Martin Luther King’s “I have a dream” about ending discrimination and achieving social justice. Many of the tenets of that classic speech are relevant to psychiatric patients who have been subjected to discrimination and bias instead of the compassion and support that they deserve, as do patients with other medical disorders.
Like Rev. King, we all have dreams, spoken and unspoken. They may be related to our various goals or objectives as individuals, spouses, parents, professionals, friends, or citizens of the world. Here, I will elaborate on my dream as a psychiatric physician, educator, and researcher, with decades of experience treating thousands of patients, many of whom I followed for a long time. I have come to see the world through the eyes and painful journeys of suffering psychiatric patients.
Vision of a better world for our patients
So, here is my dream, comprised of multiple parts that many clinician-readers may have incorporated in their own dreams about psychiatry. I have a dream:
- that the ugly, stubborn stigma of mental illness evaporates and is replaced with empathy and compassion
- that genuine full parity be implemented for all psychiatric patients
- that the public becomes far more educated about their own mental health, and cognizant of psychiatric symptoms in their family members and friends, so they can urge them to promptly seek medical help. The public should be aware that the success rate of treating psychiatric disorders is similar to that of many general medical conditions, such as heart, lung, kidney, and liver diseases
- that psychiatry continues to evolve into a clinical neuroscience, respected and appreciated like its sister neurology, and emphasizing that all mental illnesses are biologically rooted in various brain circuits
- that neuroscience literacy among psychiatrists increases dramatically, while maintaining our biopsychosocial clinical framework
- that federal funding for research into the causes and treatments of psychiatric disorders increases by an order of magnitude, to help accelerate the discovery of cures for disabling psychiatric disorders, which have a serious personal, societal, and financial toll
- that some of the many fabulously wealthy billionaires in this country (and around the world) adopt psychiatry as their favorite charity, and establish powerful and very well-funded research foundations to explore the brain and solve its mysteries in health and disease
- that effective treatments for and interventions to prevent alcohol and substance use disorders are discovered, including vaccines for alcoholism and other drugs of abuse. This would save countless lives lost to addiction
- that Medicare opens its huge wallet and supports thousands of additional residency training positions to address the serious shortage of psychiatrists
- that pharmaceutical companies, admittedly the only entities with the requisite infrastructure to develop new drugs for psychiatry, be creatively incentivized to discover drugs with new mechanisms of action to effectively treat psychiatric conditions for which there are no FDA-approved medications, such as the negative symptoms and cognitive deficits of schizophrenia, personality disorders (such as borderline personality), autism, and Alzheimer’s disease
- that the jailing, incarceration, and criminalization of patients with serious mental illness ceases immediately and is replaced with hospitalization and dignified medical treatment instead of prison sentences with murders and rapists. Building more hospitals instead of more prisons is the civilized and ethical approach to psychiatric brain disorders
- that the public recognizes that persons suffering from schizophrenia are more likely to be victims of crime rather than perpetrators. Tell that to the misguided media
- that clinicians in primary care specialties, where up to 50% of patients have a diagnosable and treatable psychiatric illness, be much better trained in psychiatry during their residency. Currently, residents in family medicine, general internal medicine, pediatrics, and obstetrics/gynecology receive 0 months to 1 month of psychiatry in their 4 years of training. Many are unable to handle the large number of psychiatric disorders in their patients. In addition, psychiatrists and primary care physicians should be colocalized so psychiatric and primary care patients can both benefit from true collaborative care, because many are dually afflicted
- that the syndemic1 (ie, multiple epidemics) that often is effectively addressed for the sake of our patients and society at large. The ongoing syndemic includes poverty, child abuse, human trafficking, domestic violence, racism, suicide, gun violence, broken families, and social media addiction across all ages
- that psychiatric practitioners embrace and adopt validated rating scales in their practice to quantify the severity of the patient’s illness and adverse effects at each visit, and to assess the degree of improvement in both. Measurement is at the foundation of science. Psychiatry will be a stronger medical specialty with measurement-based practice
- that licensing boards stop discriminating against physicians who have recovered from a psychiatric disorder or addiction. This form of stigma is destructive to the functioning of highly trained medical professionals who recover with treatment and can return to work
- that the number of psychiatric hospital beds in the country is significantly expanded to accommodate the high demand, and that psychiatric wards in general hospitals not be repurposed for more lucrative, procedure-oriented programs
- that insurance companies stop the absurdity of authorizing only 3 to 4 days for the inpatient treatment of patients who are acutely psychotic, manic, or suicidally depressed. It is impossible for such serious brain disorders to improve rapidly. This leads to discharging patients who are still unstable and who might relapse quickly after discharge, risking harm to themselves, or ending up in jail
- that HIPAA laws are revised to allow psychiatrists to collect or exchange information about ailing adult members of the family. Collateral information is a vital component of psychiatric evaluation, and its prohibition can be harmful to the patient. The family often is the most likely support system for the mentally ill individual, and must be informed about what their family member needs after discharge
- that long-acting antipsychotics are used very early and widely to prevent the tragic consequences of psychotic relapses,2 and long-lasting antidepressants are developed to prevent the relapse and risk of suicide in many patients who stop their antidepressant medication once they feel better, and do not recognize that like hypertension or diabetes, depression requires ongoing pharmacotherapy to prevent relapse
- that the time to get a court order for involuntary administration of antipsychotic medication to acutely psychotic patients is reduced to 1 day because a large body of published evidence shows that a longer duration of untreated psychosis has a deleterious neurotoxic effect on the brain, worsening outcomes and prognosis.3 The legal system should catch up with scientific findings.
Just as Martin Luther King’s dream resonated loudly for decades and led to salutary legal and societal changes, I hope that what I dream about will eventually become reality. My dream is shared by all my fellow psychiatrists, and it will come true if we unite, lobby continuously, and advocate vigorously for our patients and our noble profession. I am sure we shall overcome our challenges someday.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Namer Y, Razum O. Surviving syndemics. Lancet. 2021;398(10295):118-119.
2. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
3. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
Maria A. Oquendo, MD, PhD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Maria A. Oquendo, MD, PhD. Dr. Oquendo is the Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania and Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania. Until 2016, she was Professor of Psychiatry and Vice Chairman for Education at Columbia University. In 2017, she was elected to the National Academy of Medicine, one of the highest honors in medicine. Dr. Oquendo has used positron emission tomography and magnetic resonance imaging to map brain abnormalities in mood disorders and suicidal behavior. Dr. Oquendo is Past President of the American Psychiatric Association (APA), the International Academy of Suicide Research, and the American College of Neuropsychopharmacology (ACNP). She is President of the American Foundation for Suicide Prevention Board of Directors, Vice President of the College of International Neuropsychopharmacology, and has served on the National Institute of Mental Health’s Advisory Council. A recipient of multiple awards in the US, Europe, and South America, most recently, she received the Virginia Kneeland Award for Distinguished Women in Medicine (Columbia University 2016), the Award for Mood Disorders Research (ACP 2017), the Alexandra Symonds Award (APA 2017), the APA’s Research Award (2018), the Dolores Shockley Award (ACNP 2018), the Alexander Glassman Award (Columbia University 2021), and the Senior Investigator Klerman Award (Depression and Bipolar Support Alliance 2021).
Dr. Aftab: A major focus of your presidential year at APA was on prevention in psychiatry (especially suicide prevention), and working toward prevention through collaboration with colleagues in other medical specialties. What is your perspective on where our field presently stands in this regard?
Dr. Oquendo: There are more and more studies that focus on early childhood or pre-adolescence and the utility of intervening at the first sign of a potential issue. This is quite different from what was the case when I was training. Back then, the idea was that in many cases it was best to wait because kids might “grow out of it.” The implication was that care or intervention were “stigmatizing,” or that it could affect the child’s self-esteem and we wanted to “spare” the child. What we are learning now is that there are advantages of intervening early even if the issues are subtle, potentially preventing development of more serious problems down the line. Still, much work remains to be done. Because so many of the disorders we treat are in fact neurodevelopmental, we desperately need more investigators focused on childhood and adolescent mental health. We also need scientists to identify biomarkers that will permit identification of individuals at risk before the emergence of symptoms. Developing that workforce should be front and center if we are to make a dent in the rising rates of psychiatric disorders.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Oquendo: Our profession’s recognition that the doctor-patient relationship remains a powerful element of healing is one of its greatest strengths. Psychiatry is the only area of medicine in which practitioners are students of the doctor-patient relationship. That provides an unparalleled ability to leverage it for good. Another strength is that many who enter psychiatry are humanists, so as a field, we are collectively engaged in working towards improving conditions for our patients and our community.
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Oquendo: The most challenging issue that plagues psychiatry is not of our making, but we do have to address it. The ongoing lack of parity in the US and the insurance industry’s approach of using carve-outs and other strategies to keep psychiatry reimbursement low has led many, if not most, to practice on a cash basis only. This hurts our patients, but also our reputation among our medical colleagues. We need to use creative solutions and engage in advocacy to bring about change.
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Oquendo: An ongoing threat relates to the low reimbursement for psychiatric services, which tends to drive clinicians towards cash-based practices. Advocacy at the state and federal level as well as with large employers may be one strategy to remedy this inequity.
Dr. Aftab: What do you envision for the future of psychiatry? What sort of opportunities lie ahead for us?
Dr. Oquendo: The adoption of the advances in psychiatry that permit greater reach, such as the adoption of integrated mental health services, utilization of physician extenders, etc., has been slow in psychiatry, but I think the pace is accelerating. This is important because of an upcoming opportunity: the burgeoning need for our help. With stigma quickly decreasing and the younger generations being open about their needs and prioritization of mental health and wellness, it will be a new era, one in which we can make a huge difference in the health and quality of life of the population.
Calcineurin inhibitor–induced psychosis
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.
Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
Mrs. C, age 68, is experiencing worsening paranoid delusions. She believes that because she lied about her income when she was younger, the FBI is tracking her and wants to poison her food and spray dangerous fumes in her house. Her paranoia has made it hard for her to sleep, eat, or feel safe in her home.
Mrs. C’s daughter reports that her mother’s delusions started 3 years ago and have worsened in recent months. Mrs. C has no psychiatric history. Her medical history is significant for renal transplant in 2015, type 2 diabetes, hyperlipidemia, hypertension, and hypothyroidism. She is currently normotensive. Mrs. C’s home medications include cyclosporine, which is a calcineurin inhibitor, 125 mg twice daily (trough level = 138 mcg/L); mycophenolate mofetil, 500 mg twice daily; cinacalcet, 30 mg 3 times a week; metformin, 500 mg twice daily; amlodipine, 5 mg twice daily; levothyroxine, 112 mcg/d; and atorvastatin, 40 mg at bedtime.
As you review her medications, you wonder if the cyclosporine she began receiving after her kidney transplant could be causing or contributing to her worsening paranoid delusions.
Kidney transplantation has become an ideal treatment for patients with end-stage renal disease. In 2020, 22,817 kidney transplants were performed in the United States.1 Compared with dialysis, kidney transplant allows patients the chance to return to a satisfactory quality of life.2 However, to ensure a successful and long-lasting transplant, patients must be started and maintained on lifelong immunosuppressant agents that have potential adverse effects, including nephrotoxicity and hypertension. Further, immunosuppressant agents—particularly calcineurin inhibitors—are associated with potential adverse effects on mental health. The most commonly documented mental health-related adverse effects include insomnia, anxiety, depression, and confusion.3 In this article, we discuss the risk of severe psychosis associated with the use of calcineurin inhibitors.

Calcineurin inhibitors and psychiatric symptoms
Cyclosporine and tacrolimus are calcineurin inhibitors that are commonly used as immunosuppressant agents after kidney transplantation. They primarily work by specifically and competitively binding to and inhibiting the calcineurin protein to reduce the transcriptional activation of cytokine genes for interleukin-2, tumor necrosis factor-alpha, interleukin-3, interleukin-4, CD40L (CD40 ligand), granulocyte-macrophage colony-stimulating factor, and interferon-gamma.4,5 The ultimate downstream effect is reduced proliferation of T lymphocytes and thereby an immunosuppressed state that will protect against organ rejection. However, this is not the only downstream effect that can occur from inhibiting calcineurin. Cyclosporine and tacrolimus may modulate the activity of dopamine and N-methyl-
An increased effect of dopamine in the mesocortical dopaminergic pathway has long been a suspected cause for psychotic symptoms. A study conducted in rodents suggested that tacrolimus selectively modifies the responsivity and sensitivity of postsynaptic dopamine-2 (D2) and dopamine-3 (D3) receptors.9 These receptors are important when discussing psychosis because antipsychotic medications work primarily by blocking dopamine D2, while many also block the D3 receptor. We hypothesize that modifying the responsivity and sensitivity of these 2 receptors could increase the risk of a person developing psychosis. It may also provide insight into how to best treat a psychotic episode.
Tacrolimus has been shown to elicit inhibition of NMDA-induced neurotransmitter release and augmentation of depolarization-induced neurotransmitter release.10 In theory, this potential inhibition at the NMDA receptors may lead to a compensatory and excessive release of glutamate. Elevated glutamate levels in the brain could lead to psychiatric symptoms, including psychosis. This is supported by the psychosis caused by many NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine. Furthermore, a study examining calcineurin in knockout mice showed that the spectrum of behavioral abnormalities was strikingly similar to those in schizophrenia models.11 These mice displayed impaired working memory, impaired attentional function, social withdrawal, and psychomotor agitation. This further supports the idea that calcineurin inhibition can play a significant role in causing psychiatric symptoms by affecting both dopamine and NMDA receptors.
Continue to: How to address calcineurin inhibitor–induced psychosis...
How to address calcineurin inhibitor–induced psychosis
Here we outline a potential treatment strategy to combat psychosis secondary to calcineurin inhibitors. First, evaluate the patient’s calcineurin inhibitor level (either cyclosporine or tacrolimus). Levels should be drawn as a true trough and doses adjusted if necessary via appropriate consultation with a transplant specialist. Because many of the adverse effects associated with these agents are dose-dependent, we suspect that the risk of psychosis and other mental health–related adverse effects may also follow this trend.
Assuming that the calcineurin inhibitor cannot be stopped, changed to a different agent, or subject to a dose decrease, we recommend initiating an antipsychotic medication to control psychotic symptoms. Given the potential effect of calcineurin inhibitors on dopamine, we suggest trialing a second-generation antipsychotic with relatively high affinity for dopamine D2 receptors, such as risperidone or paliperidone. However, compared with patients with schizophrenia, patients receiving a calcineurin inhibitor may be more likely to develop extrapyramidal symptoms (EPS). Therefore, to avoid potential adverse effects, consider using a lower starting dose or an antipsychotic medication with less dopamine D2 affinity, such as quetiapine, olanzapine, or aripiprazole. Furthermore, because post-transplant patients may be at a higher risk for depression, which may be secondary to medication adverse effects, we suggest avoiding first-generation antipsychotics (FGAs) such as haloperidol because FGAs may worsen depressive symptoms.
We recommend initiating risperidone, 1 mg twice a day, for patients with psychosis secondary to a calcineurin inhibitor. If the patient develops EPS, consider switching to an antipsychotic medication with a less potent dopamine D2 blockade, such as quetiapine, olanzapine, or aripiprazole. We recommend an antipsychotic switch rather than adding benztropine or diphenhydramine to the regimen because many transplant recipients may be older patients, and adding anticholinergic medications can be problematic for this population. However, if the patient is younger or has responded particularly well to risperidone, the benefit of adding an anticholinergic medication may outweigh the risks. This decision should be made on an individual basis and may include other options, such as a switch to quetiapine, olanzapine, or aripiprazole. While these agents may not block the D2 receptor as strongly as risperidone, they all are effective and approved for adjunct therapy in major depressive disorder. We recommend quetiapine and olanzapine as second-line agents because of their potential for sedation and significant weight gain. While aripiprazole has a great metabolic adverse effect profile, its mechanism of action as a partial D2 agonist may make it difficult to control psychotic symptoms in this patient population compared with true D2 antagonists.
Continue to: CASE CONTINUED...
CASE CONTINUED
Mrs. C is admitted to the inpatient psychiatric unit and started on risperidone, 1 mg twice daily. Initially, she complains of lightheadedness at night due to the risperidone, so her dose is changed to 2 mg at bedtime. Gradually, she begins to show mild improvement. Previously, she reported feeling frightened of staff members, but after a few days she reports that she feels safe at the hospital. However, her delusions of being monitored by the FBI persist.
After 9 days of hospitalization, Mrs. C is discharged home to the care of her daughter. At first, she does well, but unfortunately she begins to refuse to take her medication and returns to her baseline.
Related Resources
- Gok F, Eroglu MZ. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;3:314-316.
- Bersani G, Marino P, Valerani G, et al. Manic-like psychosis associated with elevated trough tacrolimus blood concentrations 17 years after kidney transplant. Case Rep Psychiatry. 2013;2013:926395. doi: 10.1155/2013/926395
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Atorvastatin • Lipitor
Benztropine • Cogentin
Cinacalcet • Sensipar
Cyclosporine • Gengraf
Haloperidol • Haldol
Ketamine • Ketalar
Levothyroxine • Synthroid
Metformin • Glucophage
Mycophenolate mofetil • CellCept
Olanzapine • Zyprexa
Quetiapine • Seroquel
Paliperidone • Invega
Risperidone • Risperdal
Tacrolimus • Prograf
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
1. Health Resources & Services Administration. US Government Information on Organ Donor Transplantation. Organ Donation Statistics. Updated October 1, 2020. Accessed October 8, 2021. https://www.organdonor.gov/learn/organ-donation-statistics/detailed-description#fig1
2. De Pasquale C, Veroux M, Indelicato L, et al. Psychopathological aspects of kidney transplantation: efficacy of a multidisciplinary team. World J Transplant. 2014;4(4):267-275.
3. Gengraf capsules [package insert]. North Chicago, IL: AbbVie Inc; 2017.
4. Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci. 1993;696:9-19.
5. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136-142.
6. Scherrer U, Vissing SF, Morgan BJ, et al. Cyclosporine-induced sympathetic activation and hypertension after heart transplantation. N Engl J Med. 1990;323(11):693-699.
7. Fulya G, Meliha ZE. Acute psychotic disorder associated with immunosuppressive agent use after renal transplantation: a case report. Psychiatry and Clinical Psychopharmacology. 2017;27(3):314-316.
8. Tan TC, Robinson PJ. Mechanisms of calcineurin inhibitor-induced neurotoxicity. Transplant Rev. 2006;20(1):49-60.
9. Masatsuna S, Norio M, Nori Takei, et al. Tacrolimus, a specific inhibitor of calcineurin, modifies the locomotor activity of quinpirole, but not that of SKF82958, in male rats. Eur J Pharmacol. 2002;438(1-2):93-97.
10. Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285-306.
11. Miyakawa T, Leiter LM, Gerber DJ. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100(15): 8987-8992.
Catching the runner’s high: Anxiety and the endocannabinoid system
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
"Effortless.” “Weightless.” “Limitless.” “Carefree.” In most cases, these are words one would not commonly associate with running. In fact, many people might experience an uptick in anxiety when fathoming the idea of going out for a run. Believe it or not, these words are direct quotes from runners describing the feeling of a “runner’s high”—a well-documented organic euphoria that cannot be purchased or abused. But how is the innately basic and monotonous act of running able to reliably transform something as complex as human emotion? This answer lies in the endocannabinoid system.
For decades, scientists and the public believed the runner’s high was associated with an exercise-induced increase in levels of opioid peptides called beta-endorphins. The problem with this theory is that beta-endorphin, released into the blood by the pituitary gland in response to exercise and stress, has difficulty passing through the blood-brain barrier, rendering central effects of this peripheral opioid unlikely.1 Only recently have researchers been examining the effects of exercise as it pertains to the endocannabinoid system.2 Interest in the study of this system is peaking as use of cannabis and hemp-based products reaches an all-time high.
Exploring the endocannabinoid system
Within the last 25 years, the endocannabinoid system has emerged as a highly relevant and unique neuromodulatory system. As with other neurotransmitter systems, the endocannabinoid system is comprised of the endogenous cannabinoids, their receptors, and an array of enzymes responsible for both synthesis and degradation. Endocannabinoid receptors are ubiquitous in the brain and take effect primarily in the cortex, amygdala, basal ganglia, hippocampus, hypothalamus, and cerebellum. One fascinating feature of endocannabinoids is that their precursors lie embedded within lipid membranes. Nearly on demand, endocannabinoids can be rapidly synthesized and released. This grants them almost immediate availability to get into the action at the synapse.2
One study of mice found that those who were exercised experienced improvements in anxiety behaviors and better tolerance to pain. Further, when the exercised mice were treated with endocannabinoid receptor antagonists, they remained anxious and were more sensitive to pain. Endorphin antagonists had no effect on the outcome of these tests.1 A similar study conducted in humans found improvement in subjective anxiety scores after 45 minutes of moderate-intensity exercise. This study also showed no change with naltrexone administration vs placebo, again supporting the hypothesis that endorphins play little role in this phenomenon. Interestingly, levels of endogenous cannabinoids were elevated following exercise.3 These findings suggest a possible link between activation of the endogenous endocannabinoid system and the anxiolytic properties of exercise.
For good reason, the endocannabinoid system has attracted substantial interest as a focus for a new class of drugs to treat anxiety and stress-related disorders. It remains unknown how we can best harness its many beneficial effects in a safe and effective manner. With that said, activities such as physical exercise, mindfulness meditation, yoga, and other forms of complementary medicine are immediately available and cost-effective methods that have at least preliminary data revealing their multiple health benefits, including improvement in the symptoms of anxiety and activation of endogenous cannabinoids.1,3-6
As we are all aware, medical training and practice is full of a variety of stresses and demands. Given these demands, finding a balance between mind, body, and spirit can seem like an impossible task. Besides the obvious physical benefits of regular exercise, running can serve to employ the stress-modifying effects of our endogenous cannabinoid system to reduce perceived anxiety and improve wellness. Indeed, at the start of any bout of exercise—especially running—the transition from rest can be startling. Rest assured that there is good news waiting beyond the first few miles. Your reward for patience and perseverance is a beautiful freedom experienced only by those who have earned it. The best part of it all? It is waiting for you right outside your door.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
1. Fuss J, Steinle J, Bindila L, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105-13108.
2. Patel S, Hill MN, Cheer JF, et al. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev. 2017;76(Pt A):56-66.
3. Siebers M, Biedermann SV, Bindila L, et al. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology. 2021;126:105173.
4. Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med. 2004;38(5):536-541.
5. Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169-183.
6. Watkins BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Aspects Med. 2018;64:68-78.
Warn patients about illicit drugs doctored with fentanyl
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
Fentanyl is now threatening overdoses in patients exposed to essentially any of the full array of recreational drugs – not just opioids – that are being sold illicitly, according to an overview of the problem presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Fentanyl can now be found in cocaine and methamphetamine. At this point, there is really no way to predict what is in a [street] drug,” Edwin A. Salsitz, MD, said at the meeting, sponsored by Medscape Live. He is associate clinical professor of medicine who works in the division of chemical dependency at Mount Sinai Beth Israel Medical Center in New York.
As proof of the frequency with which fentanyl is now being used as an additive, most patients with a drug use disorder, regardless of their drug of choice, are testing positive for fentanyl at Dr. Salsitz’s center. Many of those with positive fentanyl tests are unaware that their drugs had been doctored with this agent.
Relative to drugs sold as an opioid, such as heroin or oxycodone, the fentanyl dose in nonopioid drugs is typically more modest, but Dr. Salsitz pointed out that those expecting cocaine or methamphetamine often “have no heroin tolerance, so they are more vulnerable” to the adverse effects of fentanyl, including an overdose.
Although opioid tolerance might improve the chances for surviving a fentanyl overdose, the toxicology of fentanyl is not the same as other opioids. Death from heroin is typically a result of respiratory depression, but the onset is relatively slow, providing a greater opportunity to administer a reversal agent, such as naloxone.
Fentanyl not only produces respiratory depression but skeletal muscle rigidity. The rapid onset of “wooden chest syndrome” can occur within minutes, making the opportunity for intervention much smaller, Dr. Salsitz said.
To illustrate the phenomenon, Dr. Salsitz recounted a case.
After an argument with his mother, a 26-year-old male with a long history of intravenous drug use went to his bedroom. His mother, responding to the sound of a loud thud, rushed to the bedroom to find her son on the floor with a needle still in his arm. Resuscitation efforts by the mother and by the emergency responders, who arrived quickly, failed.
“The speed of his death made it clear that it was fentanyl related, and the postmortem toxicology confirmed that the exposure involved both heroin and fentanyl,” Dr. Salsitz said.
After the first wave of deaths in the opioid epidemic, which was attributed to inappropriate use of prescription opioids, the second wave was driven by heroin. In that wave, patients who became addicted to prescription opioids but were having more difficulty gaining access to them, turned to far cheaper and readily available street heroin. The third wave, driven by fentanyl, began several years ago when sellers of heroin began adding this synthetic opioid, which is relatively cheap, to intensify the high.
It is not expected to end quickly. The fentanyl added to heroin was never a prescription version. Rather, Dr. Salsitz said, it is synthesized in laboratories in China, Mexico, and the United States. It is relatively easy to produce and compact, which makes it easy to transport.
Exacerbating the risks that fentanyl poses when added to street drugs, even more potent versions, such as carfentanil, are also being added to cocaine, methamphetamines, and other nonopioid illicit drugs. When compared on a per-milligram basis, fentanyl is about 100 times more potent than heroin, but carfentanil is about 100 times more potent than fentanyl, according to Dr. Salsitz.
When the third wave of deaths in the opioid epidemic began around 2013, prescriptions of fentanyl, like many other opioid-type therapies were declining. The “perfect storm” that initiated the opioid epidemic was a product of intense focus on pain control and a misperception that prescription opioids posed a low risk of abuse potential, Dr. Salsitz said. By the time fentanyl was driving opioid deaths, the risks of opioids were widely appreciated and their use for prescription analgesia was declining.
Citing several cases, Dr. Salsitz noted that only 20 years after clinicians were being successfully sued for not offering enough analgesia, they were now going to jail for prescribing these drugs too liberally.
According to Dr. Salsitz, While psychiatrists might not have a role in this issue, Dr. Salsitz did see a role for these specialists in protecting patients from the adverse consequences of using illicit drugs doctored with fentanyl.
Noting that individuals with psychiatric disorders are more likely than the general population to self-medicate with drugs purchased illegally, Dr. Salsitz encouraged psychiatrists “to get involved” in asking about drug use and counseling patients on the risks of fentanyl substitution or additives.
“The message is that no one knows what are in these drugs, anymore,” he said.
In addition to making patients aware that many street drugs are now contaminated with fentanyl, Dr. Salsitz provided some safety tips. He suggested instructing patients to take a low dose of any newly acquired drug to gauge its effect, to avoid taking drugs alone, and to avoid mixing drugs. He also recommended using rapid fentanyl test strips in order to detect fentanyl contamination.
Even for the many psychiatrists who do not feel comfortable managing addiction, Dr. Salsitz recommended a proactive approach to address the current threat.
Test strips as an intervention
The seriousness of fentanyl contamination of illicit drugs, including cocaine and methamphetamine, was corroborated by two investigators at the School of Public Health and the Albert Einstein Medical School of Brown University, Providence, R.I. Brandon D.L. Marshall, PhD, associate professor of epidemiology in the School of Public Health, called fentanyl-contaminated cannabis “extremely rare,” but he said that it is being found in counterfeit prescription pills as well as in crystal methamphetamine and in both crack and powder cocaine.
He also advocated the use of fentanyl test strips.
“Test strips are an efficient, inexpensive, and effective way to determine whether fentanyl or related analogs are present in illicit drugs,” he said, noting that he is involved in a trial designed to determine whether fentanyl test strips can reduce the risk of fatal and nonfatal overdoses.
In a pilot study conducted in Baltimore, 69% of the 103 participants engaged in harm reduction behavior after using a fentanyl test strip and receiving a positive result (Addict Behav. 2020;110:106529). It is notable that 86% of the participants had a least one positive result when using the strips. More than half were surprised by the result.
One of the findings from this study was “that the lasting benefit of fentanyl test strip distribution is the opportunity to engage in discussions around safety and relationship building with historically underserved communities,” said the lead author, Ju Nyeong Park, PhD, assistant professor of medicine and epidemiology at Brown University. She moved to Brown after performing this work at Johns Hopkins University, Baltimore.
Dr. Park noted that “many patients in the community already know that they are using drugs containing fentanyl,” but for those who are concerned and wish to avoid contaminated drugs, fentanyl test strips “are a quick screening tool.” However, while the strips are helpful, she cautioned that they cannot be considered a definitive tool for detecting harm in illicit drugs.
“There may also be other chemicals present in tested drugs that confer risk,” she said.
Medscape Live and this news organization are owned by the same parent company. Dr. Salsitz, Dr. Marshall, and Dr. Park reported no potential conflicts of interest.
FROM PSYCHOPHARMACOLOGY UPDATE