User login
Profound brain changes found in patients who died of COVID-19
The most comprehensive molecular study to date of brain tissue from people who died of COVID-19 provides clear evidence that SARS-CoV-2 causes profound molecular changes in the brain, despite no molecular trace of the virus in brain tissue.
“The signature the virus leaves in the brain speaks of strong inflammation and disrupted brain circuits and resembles signatures the field has observed in Alzheimer’s or other neurodegenerative diseases,” senior author Tony Wyss-Coray, PhD, professor of neurology and neurological sciences, Stanford (Calif.) University, told this news organization.
The study was published online June 21 in Nature.
Signs of distress
“We know that up to a third of SARS-CoV-2-infected people show brain symptoms including brain fog, memory problems, and fatigue, and a growing number of people have such symptoms long after they [have] seemingly recovered from virus infection,” said Dr. Wyss-Coray.
“However, we have very little understanding of how the virus causes these symptoms and what its effects are on the brain at a molecular level,” he added.
Using single-cell RNA sequencing, the researchers profiled the transcriptomes of 65,309 nuclei isolated from frontal cortex and choroid plexus samples from eight patients who died of COVID-19 and 14 controls who died of other causes.
There was no molecular evidence of SARS-CoV-2 in brain tissue samples from the patients who died of COVID-19.
Yet, “we were very surprised to learn that no matter which type of cell we studied (different types of nerve cells, immune cells, or different support cells in the brain) there were prominent changes” compared with brain tissue samples from controls who died of other causes, said Dr. Wyss-Coray.
The changes in the COVID-19 brains showed signatures of inflammation, abnormal nerve cell communication, and chronic neurodegeneration.
“Across cell types, COVID-19 perturbations overlap with those in chronic brain disorders and reside in genetic variants associated with cognition, schizophrenia, and depression,” the researchers report.
“Viral infection appears to trigger inflammatory responses throughout the body that may cause inflammatory signaling across the blood–brain barrier, which in turn could ‘trip off’ neuroinflammation in the brain,” Dr. Wyss-Coray said.
The findings may help explain the brain fog, fatigue, and other neurological and psychiatric symptoms of long COVID.
“While we studied only brains from people who died of COVID-19, we believe it is likely that similar, but hopefully weaker, signs of inflammation and chronic neurodegeneration will be found in COVID-19 survivors, especially those with chronic brain symptoms,” Dr. Wyss-Coray said.
This research was funded by the Nomis Foundation, the National Institutes of Health, Nan Fung Life Sciences, the Wu Tsai Neurosciences Institute and the Stanford Alzheimer’s Disease Research Center. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The most comprehensive molecular study to date of brain tissue from people who died of COVID-19 provides clear evidence that SARS-CoV-2 causes profound molecular changes in the brain, despite no molecular trace of the virus in brain tissue.
“The signature the virus leaves in the brain speaks of strong inflammation and disrupted brain circuits and resembles signatures the field has observed in Alzheimer’s or other neurodegenerative diseases,” senior author Tony Wyss-Coray, PhD, professor of neurology and neurological sciences, Stanford (Calif.) University, told this news organization.
The study was published online June 21 in Nature.
Signs of distress
“We know that up to a third of SARS-CoV-2-infected people show brain symptoms including brain fog, memory problems, and fatigue, and a growing number of people have such symptoms long after they [have] seemingly recovered from virus infection,” said Dr. Wyss-Coray.
“However, we have very little understanding of how the virus causes these symptoms and what its effects are on the brain at a molecular level,” he added.
Using single-cell RNA sequencing, the researchers profiled the transcriptomes of 65,309 nuclei isolated from frontal cortex and choroid plexus samples from eight patients who died of COVID-19 and 14 controls who died of other causes.
There was no molecular evidence of SARS-CoV-2 in brain tissue samples from the patients who died of COVID-19.
Yet, “we were very surprised to learn that no matter which type of cell we studied (different types of nerve cells, immune cells, or different support cells in the brain) there were prominent changes” compared with brain tissue samples from controls who died of other causes, said Dr. Wyss-Coray.
The changes in the COVID-19 brains showed signatures of inflammation, abnormal nerve cell communication, and chronic neurodegeneration.
“Across cell types, COVID-19 perturbations overlap with those in chronic brain disorders and reside in genetic variants associated with cognition, schizophrenia, and depression,” the researchers report.
“Viral infection appears to trigger inflammatory responses throughout the body that may cause inflammatory signaling across the blood–brain barrier, which in turn could ‘trip off’ neuroinflammation in the brain,” Dr. Wyss-Coray said.
The findings may help explain the brain fog, fatigue, and other neurological and psychiatric symptoms of long COVID.
“While we studied only brains from people who died of COVID-19, we believe it is likely that similar, but hopefully weaker, signs of inflammation and chronic neurodegeneration will be found in COVID-19 survivors, especially those with chronic brain symptoms,” Dr. Wyss-Coray said.
This research was funded by the Nomis Foundation, the National Institutes of Health, Nan Fung Life Sciences, the Wu Tsai Neurosciences Institute and the Stanford Alzheimer’s Disease Research Center. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The most comprehensive molecular study to date of brain tissue from people who died of COVID-19 provides clear evidence that SARS-CoV-2 causes profound molecular changes in the brain, despite no molecular trace of the virus in brain tissue.
“The signature the virus leaves in the brain speaks of strong inflammation and disrupted brain circuits and resembles signatures the field has observed in Alzheimer’s or other neurodegenerative diseases,” senior author Tony Wyss-Coray, PhD, professor of neurology and neurological sciences, Stanford (Calif.) University, told this news organization.
The study was published online June 21 in Nature.
Signs of distress
“We know that up to a third of SARS-CoV-2-infected people show brain symptoms including brain fog, memory problems, and fatigue, and a growing number of people have such symptoms long after they [have] seemingly recovered from virus infection,” said Dr. Wyss-Coray.
“However, we have very little understanding of how the virus causes these symptoms and what its effects are on the brain at a molecular level,” he added.
Using single-cell RNA sequencing, the researchers profiled the transcriptomes of 65,309 nuclei isolated from frontal cortex and choroid plexus samples from eight patients who died of COVID-19 and 14 controls who died of other causes.
There was no molecular evidence of SARS-CoV-2 in brain tissue samples from the patients who died of COVID-19.
Yet, “we were very surprised to learn that no matter which type of cell we studied (different types of nerve cells, immune cells, or different support cells in the brain) there were prominent changes” compared with brain tissue samples from controls who died of other causes, said Dr. Wyss-Coray.
The changes in the COVID-19 brains showed signatures of inflammation, abnormal nerve cell communication, and chronic neurodegeneration.
“Across cell types, COVID-19 perturbations overlap with those in chronic brain disorders and reside in genetic variants associated with cognition, schizophrenia, and depression,” the researchers report.
“Viral infection appears to trigger inflammatory responses throughout the body that may cause inflammatory signaling across the blood–brain barrier, which in turn could ‘trip off’ neuroinflammation in the brain,” Dr. Wyss-Coray said.
The findings may help explain the brain fog, fatigue, and other neurological and psychiatric symptoms of long COVID.
“While we studied only brains from people who died of COVID-19, we believe it is likely that similar, but hopefully weaker, signs of inflammation and chronic neurodegeneration will be found in COVID-19 survivors, especially those with chronic brain symptoms,” Dr. Wyss-Coray said.
This research was funded by the Nomis Foundation, the National Institutes of Health, Nan Fung Life Sciences, the Wu Tsai Neurosciences Institute and the Stanford Alzheimer’s Disease Research Center. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Post–acute kidney injury proteinuria predicts subsequent kidney disease progression
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Few clinical guidelines exist for treating post-COVID symptoms
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
C. difficile guidelines offer new possibilities
The American College of Gastroenterology has issued new guidelines on management of Clostridioides difficile infection that now include roles for fecal microbial transplant (FMT), combination testing, and bezlotoxumab.
The ACG’s previous guidelines on the diagnosis, management, and treatment of what was then still called Clostridium difficile were published in 2013. Since then, the organism’s name changed to Clostridioides difficile, and that’s just the beginning of the changes reflected in the scientific literature, wrote lead author Colleen R. Kelly, MD, of Brown University, Providence, R.I., and colleagues.
“Other developments include the increased recognition of diagnostic challenges in the era of nucleic acid amplification–based testing, new therapeutic options for treatment and prevention of recurrence, and increasing evidence to support fecal microbiota transplantation (FMT) in recurrent and severe infection,” the authors said.
The guidelines, published in the American Journal of Gastroenterology, include 23 graded recommendations addressing issues of prevention, diagnosis, treatment, prevention of recurrence, and guidance for special populations in the management of C. difficile infection (CDI).
New faces among familiar ones
In terms of diagnosis, the new guidelines recommend using both a highly sensitive testing modality and a highly specific one to help distinguish colonization from active infection. Specifically, the authors recommend that stool is first tested using a highly sensitive test, either nucleic acid amplification testing or glutamate dehydrogenase, followed by an enzyme immunoassays for its high specificity.
Changes to treatment recommendations include the initial use of oral vancomycin or oral fidaxomicin for cases of nonsevere CDI. Oral metronidazole may be considered for initial nonsevere CDI in low-risk patients, the authors noted. The evidence is strong for the continued recommendations of vancomycin (125 mg four times daily for 10 days) and fidaxomicin (200 mg twice daily for 10 days) for patients with severe CDI. For patients with fulminant CDI, the recommendations call for medical therapy including volume resuscitation and oral vancomycin, although combination therapy with parenteral metronidazole may be considered despite the very low quality of evidence.
A notable update to the guidelines is the recommendation of fecal microbiota transplant (FMT) for both severe and fulminant CDI cases that are resistant to antibiotics and to prevent recurrence in at-risk patients. Although the quality of evidence is ranked as low, the recommendation is strong, the authors wrote. “Beyond improved cure rates, FMT may result in decreased rates of CDI-related colectomy and sepsis and may offer survival benefit in this critically ill patient population.” However, most patients in studies of FMT required multiple treatments in combination with anti-CDI antibiotics.
Other recommendations to prevent recurrence include oral vancomycin prophylaxis during the subsequent use of systemic antibiotics in patients with a history of CDI. The guidelines also recommend bezlotoxumab for prevention of CDI recurrence in high-risk patients, and advise against discontinuing antisecretory therapy in CDI patients if there is an appropriate indication for use.
Based on the lack of quality evidence, the guidelines recommend against the use of probiotics for preventing CDI in patients being treated with antibiotics and for prevention of recurrent infection.
Special populations
For patients with inflammatory bowel disease, the guidelines recommend C. difficile testing when these individuals present with acute flares and diarrhea, and the use of vancomycin for treatment. In addition, the authors strongly recommended FMT for recurrent CDI in these patients. For pregnant, postpartum, and breastfeeding patients with CDI, the guidelines recommend vancomycin, and either vancomycin or fidaxomicin may be used for treating CDI in immunocompromised patients, the authors noted.
The updated guidelines are designed to complement those issued by the Infections Disease Society of America and Society of Healthcare Epidemiologists of America, the researchers noted.
Reflecting the research
The previous guidelines for C. difficile were issued in 2013, and much has changed since then in terms of epidemiology, diagnosis, treatment, and infection control, Sahil Khanna, MBBS, MS, of the Mayo Clinic, Rochester, Minn., said in an interview.
Notably, diagnostic testing has “made leaps and bounds” and new treatments have become available that were not included in earlier guidelines, said Dr. Khanna. In particular, the new guidelines are recommending a two-step diagnostic assay; “the diagnostic algorithm has changed, and hopefully that will help us change practice” to identify active infection more quickly and efficiently.
Another important update is the recommendation of fidaxomicin as an option for initial nonfulminant CDI as an alternative to vancomycin, Dr. Khanna said, noting that metronidazole remains an option for low-risk patients. An additional change is the advice to use a different treatment for a second recurrent infection rather than repeating the initial treatment.
The recommendation of bezlotoxumab for prevention of CDI recurrence in patients who are at high risk of recurrence is the first time this drug has appeared in major guidelines, Dr. Khanna observed.
The recommendation in support of fecal microbiota transplant is a key update to the management of CDI, including the guidance that the procedure can be repeated if necessary, he said.
Looking ahead, “Additional research is needed to fully understand the best testing algorithms for CDI,” Dr. Khanna explained. “More studies also are needed to show how FMT fully fits into the picture, and some current studies are looking at its potential earlier in the course of infection.”
The guidelines were developed in collaboration with the Practice Parameters Committee of the American College of Gastroenterology and received no outside funding. Dr. Kelly disclosed serving as a site investigator of a clinical trial for Finch Therapeutics and is an unpaid clinical advisory board member for OpenBiome. Dr. Khanna has coauthored previous guidelines on C. difficile. He disclosed consulting relationships with Finch, GlaxoSmithKline, Jetson, ProbioTech, and Shire/Takeda, as well as research support from Rebiotix, Seres, and Vedanta.
The American College of Gastroenterology has issued new guidelines on management of Clostridioides difficile infection that now include roles for fecal microbial transplant (FMT), combination testing, and bezlotoxumab.
The ACG’s previous guidelines on the diagnosis, management, and treatment of what was then still called Clostridium difficile were published in 2013. Since then, the organism’s name changed to Clostridioides difficile, and that’s just the beginning of the changes reflected in the scientific literature, wrote lead author Colleen R. Kelly, MD, of Brown University, Providence, R.I., and colleagues.
“Other developments include the increased recognition of diagnostic challenges in the era of nucleic acid amplification–based testing, new therapeutic options for treatment and prevention of recurrence, and increasing evidence to support fecal microbiota transplantation (FMT) in recurrent and severe infection,” the authors said.
The guidelines, published in the American Journal of Gastroenterology, include 23 graded recommendations addressing issues of prevention, diagnosis, treatment, prevention of recurrence, and guidance for special populations in the management of C. difficile infection (CDI).
New faces among familiar ones
In terms of diagnosis, the new guidelines recommend using both a highly sensitive testing modality and a highly specific one to help distinguish colonization from active infection. Specifically, the authors recommend that stool is first tested using a highly sensitive test, either nucleic acid amplification testing or glutamate dehydrogenase, followed by an enzyme immunoassays for its high specificity.
Changes to treatment recommendations include the initial use of oral vancomycin or oral fidaxomicin for cases of nonsevere CDI. Oral metronidazole may be considered for initial nonsevere CDI in low-risk patients, the authors noted. The evidence is strong for the continued recommendations of vancomycin (125 mg four times daily for 10 days) and fidaxomicin (200 mg twice daily for 10 days) for patients with severe CDI. For patients with fulminant CDI, the recommendations call for medical therapy including volume resuscitation and oral vancomycin, although combination therapy with parenteral metronidazole may be considered despite the very low quality of evidence.
A notable update to the guidelines is the recommendation of fecal microbiota transplant (FMT) for both severe and fulminant CDI cases that are resistant to antibiotics and to prevent recurrence in at-risk patients. Although the quality of evidence is ranked as low, the recommendation is strong, the authors wrote. “Beyond improved cure rates, FMT may result in decreased rates of CDI-related colectomy and sepsis and may offer survival benefit in this critically ill patient population.” However, most patients in studies of FMT required multiple treatments in combination with anti-CDI antibiotics.
Other recommendations to prevent recurrence include oral vancomycin prophylaxis during the subsequent use of systemic antibiotics in patients with a history of CDI. The guidelines also recommend bezlotoxumab for prevention of CDI recurrence in high-risk patients, and advise against discontinuing antisecretory therapy in CDI patients if there is an appropriate indication for use.
Based on the lack of quality evidence, the guidelines recommend against the use of probiotics for preventing CDI in patients being treated with antibiotics and for prevention of recurrent infection.
Special populations
For patients with inflammatory bowel disease, the guidelines recommend C. difficile testing when these individuals present with acute flares and diarrhea, and the use of vancomycin for treatment. In addition, the authors strongly recommended FMT for recurrent CDI in these patients. For pregnant, postpartum, and breastfeeding patients with CDI, the guidelines recommend vancomycin, and either vancomycin or fidaxomicin may be used for treating CDI in immunocompromised patients, the authors noted.
The updated guidelines are designed to complement those issued by the Infections Disease Society of America and Society of Healthcare Epidemiologists of America, the researchers noted.
Reflecting the research
The previous guidelines for C. difficile were issued in 2013, and much has changed since then in terms of epidemiology, diagnosis, treatment, and infection control, Sahil Khanna, MBBS, MS, of the Mayo Clinic, Rochester, Minn., said in an interview.
Notably, diagnostic testing has “made leaps and bounds” and new treatments have become available that were not included in earlier guidelines, said Dr. Khanna. In particular, the new guidelines are recommending a two-step diagnostic assay; “the diagnostic algorithm has changed, and hopefully that will help us change practice” to identify active infection more quickly and efficiently.
Another important update is the recommendation of fidaxomicin as an option for initial nonfulminant CDI as an alternative to vancomycin, Dr. Khanna said, noting that metronidazole remains an option for low-risk patients. An additional change is the advice to use a different treatment for a second recurrent infection rather than repeating the initial treatment.
The recommendation of bezlotoxumab for prevention of CDI recurrence in patients who are at high risk of recurrence is the first time this drug has appeared in major guidelines, Dr. Khanna observed.
The recommendation in support of fecal microbiota transplant is a key update to the management of CDI, including the guidance that the procedure can be repeated if necessary, he said.
Looking ahead, “Additional research is needed to fully understand the best testing algorithms for CDI,” Dr. Khanna explained. “More studies also are needed to show how FMT fully fits into the picture, and some current studies are looking at its potential earlier in the course of infection.”
The guidelines were developed in collaboration with the Practice Parameters Committee of the American College of Gastroenterology and received no outside funding. Dr. Kelly disclosed serving as a site investigator of a clinical trial for Finch Therapeutics and is an unpaid clinical advisory board member for OpenBiome. Dr. Khanna has coauthored previous guidelines on C. difficile. He disclosed consulting relationships with Finch, GlaxoSmithKline, Jetson, ProbioTech, and Shire/Takeda, as well as research support from Rebiotix, Seres, and Vedanta.
The American College of Gastroenterology has issued new guidelines on management of Clostridioides difficile infection that now include roles for fecal microbial transplant (FMT), combination testing, and bezlotoxumab.
The ACG’s previous guidelines on the diagnosis, management, and treatment of what was then still called Clostridium difficile were published in 2013. Since then, the organism’s name changed to Clostridioides difficile, and that’s just the beginning of the changes reflected in the scientific literature, wrote lead author Colleen R. Kelly, MD, of Brown University, Providence, R.I., and colleagues.
“Other developments include the increased recognition of diagnostic challenges in the era of nucleic acid amplification–based testing, new therapeutic options for treatment and prevention of recurrence, and increasing evidence to support fecal microbiota transplantation (FMT) in recurrent and severe infection,” the authors said.
The guidelines, published in the American Journal of Gastroenterology, include 23 graded recommendations addressing issues of prevention, diagnosis, treatment, prevention of recurrence, and guidance for special populations in the management of C. difficile infection (CDI).
New faces among familiar ones
In terms of diagnosis, the new guidelines recommend using both a highly sensitive testing modality and a highly specific one to help distinguish colonization from active infection. Specifically, the authors recommend that stool is first tested using a highly sensitive test, either nucleic acid amplification testing or glutamate dehydrogenase, followed by an enzyme immunoassays for its high specificity.
Changes to treatment recommendations include the initial use of oral vancomycin or oral fidaxomicin for cases of nonsevere CDI. Oral metronidazole may be considered for initial nonsevere CDI in low-risk patients, the authors noted. The evidence is strong for the continued recommendations of vancomycin (125 mg four times daily for 10 days) and fidaxomicin (200 mg twice daily for 10 days) for patients with severe CDI. For patients with fulminant CDI, the recommendations call for medical therapy including volume resuscitation and oral vancomycin, although combination therapy with parenteral metronidazole may be considered despite the very low quality of evidence.
A notable update to the guidelines is the recommendation of fecal microbiota transplant (FMT) for both severe and fulminant CDI cases that are resistant to antibiotics and to prevent recurrence in at-risk patients. Although the quality of evidence is ranked as low, the recommendation is strong, the authors wrote. “Beyond improved cure rates, FMT may result in decreased rates of CDI-related colectomy and sepsis and may offer survival benefit in this critically ill patient population.” However, most patients in studies of FMT required multiple treatments in combination with anti-CDI antibiotics.
Other recommendations to prevent recurrence include oral vancomycin prophylaxis during the subsequent use of systemic antibiotics in patients with a history of CDI. The guidelines also recommend bezlotoxumab for prevention of CDI recurrence in high-risk patients, and advise against discontinuing antisecretory therapy in CDI patients if there is an appropriate indication for use.
Based on the lack of quality evidence, the guidelines recommend against the use of probiotics for preventing CDI in patients being treated with antibiotics and for prevention of recurrent infection.
Special populations
For patients with inflammatory bowel disease, the guidelines recommend C. difficile testing when these individuals present with acute flares and diarrhea, and the use of vancomycin for treatment. In addition, the authors strongly recommended FMT for recurrent CDI in these patients. For pregnant, postpartum, and breastfeeding patients with CDI, the guidelines recommend vancomycin, and either vancomycin or fidaxomicin may be used for treating CDI in immunocompromised patients, the authors noted.
The updated guidelines are designed to complement those issued by the Infections Disease Society of America and Society of Healthcare Epidemiologists of America, the researchers noted.
Reflecting the research
The previous guidelines for C. difficile were issued in 2013, and much has changed since then in terms of epidemiology, diagnosis, treatment, and infection control, Sahil Khanna, MBBS, MS, of the Mayo Clinic, Rochester, Minn., said in an interview.
Notably, diagnostic testing has “made leaps and bounds” and new treatments have become available that were not included in earlier guidelines, said Dr. Khanna. In particular, the new guidelines are recommending a two-step diagnostic assay; “the diagnostic algorithm has changed, and hopefully that will help us change practice” to identify active infection more quickly and efficiently.
Another important update is the recommendation of fidaxomicin as an option for initial nonfulminant CDI as an alternative to vancomycin, Dr. Khanna said, noting that metronidazole remains an option for low-risk patients. An additional change is the advice to use a different treatment for a second recurrent infection rather than repeating the initial treatment.
The recommendation of bezlotoxumab for prevention of CDI recurrence in patients who are at high risk of recurrence is the first time this drug has appeared in major guidelines, Dr. Khanna observed.
The recommendation in support of fecal microbiota transplant is a key update to the management of CDI, including the guidance that the procedure can be repeated if necessary, he said.
Looking ahead, “Additional research is needed to fully understand the best testing algorithms for CDI,” Dr. Khanna explained. “More studies also are needed to show how FMT fully fits into the picture, and some current studies are looking at its potential earlier in the course of infection.”
The guidelines were developed in collaboration with the Practice Parameters Committee of the American College of Gastroenterology and received no outside funding. Dr. Kelly disclosed serving as a site investigator of a clinical trial for Finch Therapeutics and is an unpaid clinical advisory board member for OpenBiome. Dr. Khanna has coauthored previous guidelines on C. difficile. He disclosed consulting relationships with Finch, GlaxoSmithKline, Jetson, ProbioTech, and Shire/Takeda, as well as research support from Rebiotix, Seres, and Vedanta.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
FIND: A framework for success as a first-year hospitalist
Congratulations! You’re about to start your first year as a hospitalist, and in many cases your first real job. Hospital medicine is an incredibly rewarding subspecialty, but the progression from resident to attending physician can be daunting. To facilitate this transition, we present FIND (Familiarity, Identity, Network, and Direction) – a novel, sequential framework for success as a first-year hospitalist. For each component, we provide a narrative overview and a summary bullet point for quick reference.
Familiarity
- Lay the foundation: Learn the ins and outs of your job, EMR, and team.
Familiarize yourself with your surroundings. Know where your patients are located, where you can document, where to find equipment for procedures, and how to reach information technology. Proactively set up the electronic medical record on your home computer and phone. Make sure to review your responsibilities, including your call schedule, your shifts, your assigned patient panel, when you can leave campus, and how people should contact you. Also, others should know your expectations of them, especially if you are working with trainees.
Maintain a file with all of your orientation materials, including phone numbers and emails of key personnel. Know who your people are – who can access your calendar, who you can call with a clinical question or to escalate care, who can assist you with billing, and who helps with the throughput of your patients in the hospital. Take time to review your benefits, including parental leave, insurance coverage, retirement planning, vacation time, and ancillary services like laundry for your white coat. Familiarizing yourself with these basics will provide comfort and lay the foundation for your first year.
Identity
- Perform self-reflection: Overcome imposter syndrome and invest in hobbies.
One of the fundamental realizations that will occur with your first hospitalist job is that you are the attending. You walk in with a vision of your first job; be prepared to be surprised. You have earned the privilege of deciding on patient plans, and you are no longer obligated to staff with a senior physician. This is both empowering and terrifying. In a way, it may oddly remind you of intern year. A new hospital, new EMR, new colleagues, and imposter syndrome will trick you into doubting your decisions.
How to battle it? Positive thinking. You do know the basics of inpatient medicine and you do have a support system to cheer you on. As part of imposter syndrome, you may feel pressured to focus solely on work. Yet, your first job as a hospitalist is finally an amazing opportunity to focus on you. What hobbies have you been neglecting: cooking, photography, reading, more time with family, a new pet? You have the power to schedule your off-weeks. Are you interested in academics? Reserve a portion of your time off to explore scholarship opportunities at your institution. Your first job as a hospitalist is a chance to develop your identity, both as a physician and as an individual.
Network
- Engage your support system: Communicate with nursing, administration, colleagues.
Networking, or building a web of mutually beneficial professional relationships, is imperative for long-term career success. Hospitalists should focus on developing their network across multiple departments, such as nursing, subspecialties, medical education, and hospital administration. Curating a broad network will increase your visibility within your organization, showcase your unique services, and demonstrate your value.
To make networking encounters impactful, express interest, actively listen, ask relevant questions, and seek areas of mutual benefit. It’s equally important to cultivate these new relationships after the initial encounter and to demonstrate how your skill set will aid colleagues in achieving their professional goals. Over time, as you establish your niche, deliberate networking with those who share similar interests can lead to a wealth of new experiences and opportunities. Intentionally mastering networking early in your career provides insight into different aspects of the hospital system, new perspectives on ideas, and access to valuable guidance from other professionals. Engaging in networking to establish your support system is an essential step towards success as a first-year hospitalist.
Direction
- Visualize your path: Find a mentor and develop a mission statement and career plan.
Once you’re familiar with your work environment, confident in your identity, and acquainted with your support network, you’re ready for the final step – direction. Hospital medicine offers many professional avenues and clarifying your career path is challenging when attempted alone. A mentor is the necessary catalyst to find direction and purpose.
Selecting and engaging with a mentor will bolster your professional advancement, academic productivity, and most importantly, career satisfaction.1 At its best, mentorship is a symbiotic relationship. Your mentor should inspire you, challenge you, and support your growth and emotional well-being. In turn, as the mentee, you should be proactive, establish expectations, and take responsibility for maintaining communication to ensure a successful relationship. As your career takes shape over time, you may require a mentorship team to fulfill your unique needs.
When you’ve established a relationship with your mentor, take time to develop 1-year and 5-year plans. Your 1-year plan should focus on a few “quick wins,” often projects or opportunities at your home institution. Small victories in your first year will boost your confidence, motivation, and sense of control. Your 5-year plan should delineate the steps necessary to make your first major career transition, such as from instructor to assistant professor. Working with your mentor to draft a career mission statement is a useful first step in this process. Beginning with the end in mind, will help you visualize your direction.2
We hope that the FIND framework will help you find your path to success as a first-year hospitalist.
Dr. Nelson is a hospitalist and instructor of medicine at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Dr. Ashford is assistant professor and program director, department of internal medicine/pediatrics, at the University of Nebraska Medical Center, Omaha. Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Crecelius is assistant professor of clinical medicine at Indiana University, Indianapolis. This article is sponsored by the SHM Physicians in Training committee, which submits quarterly content to the Hospitalist on topics relevant to trainees and early -career hospitalists.
References
1. Zerzan JT et al. Making the most of mentors: a guide for mentees. Acad Med. 2009;84:140-4. doi: 10.1097/ACM.0b013e3181906e8f.
2. Covey F. The seven habits of highly effective people. 25th anniversary edition. New York: Simon and Schuster, 2013.
Congratulations! You’re about to start your first year as a hospitalist, and in many cases your first real job. Hospital medicine is an incredibly rewarding subspecialty, but the progression from resident to attending physician can be daunting. To facilitate this transition, we present FIND (Familiarity, Identity, Network, and Direction) – a novel, sequential framework for success as a first-year hospitalist. For each component, we provide a narrative overview and a summary bullet point for quick reference.
Familiarity
- Lay the foundation: Learn the ins and outs of your job, EMR, and team.
Familiarize yourself with your surroundings. Know where your patients are located, where you can document, where to find equipment for procedures, and how to reach information technology. Proactively set up the electronic medical record on your home computer and phone. Make sure to review your responsibilities, including your call schedule, your shifts, your assigned patient panel, when you can leave campus, and how people should contact you. Also, others should know your expectations of them, especially if you are working with trainees.
Maintain a file with all of your orientation materials, including phone numbers and emails of key personnel. Know who your people are – who can access your calendar, who you can call with a clinical question or to escalate care, who can assist you with billing, and who helps with the throughput of your patients in the hospital. Take time to review your benefits, including parental leave, insurance coverage, retirement planning, vacation time, and ancillary services like laundry for your white coat. Familiarizing yourself with these basics will provide comfort and lay the foundation for your first year.
Identity
- Perform self-reflection: Overcome imposter syndrome and invest in hobbies.
One of the fundamental realizations that will occur with your first hospitalist job is that you are the attending. You walk in with a vision of your first job; be prepared to be surprised. You have earned the privilege of deciding on patient plans, and you are no longer obligated to staff with a senior physician. This is both empowering and terrifying. In a way, it may oddly remind you of intern year. A new hospital, new EMR, new colleagues, and imposter syndrome will trick you into doubting your decisions.
How to battle it? Positive thinking. You do know the basics of inpatient medicine and you do have a support system to cheer you on. As part of imposter syndrome, you may feel pressured to focus solely on work. Yet, your first job as a hospitalist is finally an amazing opportunity to focus on you. What hobbies have you been neglecting: cooking, photography, reading, more time with family, a new pet? You have the power to schedule your off-weeks. Are you interested in academics? Reserve a portion of your time off to explore scholarship opportunities at your institution. Your first job as a hospitalist is a chance to develop your identity, both as a physician and as an individual.
Network
- Engage your support system: Communicate with nursing, administration, colleagues.
Networking, or building a web of mutually beneficial professional relationships, is imperative for long-term career success. Hospitalists should focus on developing their network across multiple departments, such as nursing, subspecialties, medical education, and hospital administration. Curating a broad network will increase your visibility within your organization, showcase your unique services, and demonstrate your value.
To make networking encounters impactful, express interest, actively listen, ask relevant questions, and seek areas of mutual benefit. It’s equally important to cultivate these new relationships after the initial encounter and to demonstrate how your skill set will aid colleagues in achieving their professional goals. Over time, as you establish your niche, deliberate networking with those who share similar interests can lead to a wealth of new experiences and opportunities. Intentionally mastering networking early in your career provides insight into different aspects of the hospital system, new perspectives on ideas, and access to valuable guidance from other professionals. Engaging in networking to establish your support system is an essential step towards success as a first-year hospitalist.
Direction
- Visualize your path: Find a mentor and develop a mission statement and career plan.
Once you’re familiar with your work environment, confident in your identity, and acquainted with your support network, you’re ready for the final step – direction. Hospital medicine offers many professional avenues and clarifying your career path is challenging when attempted alone. A mentor is the necessary catalyst to find direction and purpose.
Selecting and engaging with a mentor will bolster your professional advancement, academic productivity, and most importantly, career satisfaction.1 At its best, mentorship is a symbiotic relationship. Your mentor should inspire you, challenge you, and support your growth and emotional well-being. In turn, as the mentee, you should be proactive, establish expectations, and take responsibility for maintaining communication to ensure a successful relationship. As your career takes shape over time, you may require a mentorship team to fulfill your unique needs.
When you’ve established a relationship with your mentor, take time to develop 1-year and 5-year plans. Your 1-year plan should focus on a few “quick wins,” often projects or opportunities at your home institution. Small victories in your first year will boost your confidence, motivation, and sense of control. Your 5-year plan should delineate the steps necessary to make your first major career transition, such as from instructor to assistant professor. Working with your mentor to draft a career mission statement is a useful first step in this process. Beginning with the end in mind, will help you visualize your direction.2
We hope that the FIND framework will help you find your path to success as a first-year hospitalist.
Dr. Nelson is a hospitalist and instructor of medicine at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Dr. Ashford is assistant professor and program director, department of internal medicine/pediatrics, at the University of Nebraska Medical Center, Omaha. Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Crecelius is assistant professor of clinical medicine at Indiana University, Indianapolis. This article is sponsored by the SHM Physicians in Training committee, which submits quarterly content to the Hospitalist on topics relevant to trainees and early -career hospitalists.
References
1. Zerzan JT et al. Making the most of mentors: a guide for mentees. Acad Med. 2009;84:140-4. doi: 10.1097/ACM.0b013e3181906e8f.
2. Covey F. The seven habits of highly effective people. 25th anniversary edition. New York: Simon and Schuster, 2013.
Congratulations! You’re about to start your first year as a hospitalist, and in many cases your first real job. Hospital medicine is an incredibly rewarding subspecialty, but the progression from resident to attending physician can be daunting. To facilitate this transition, we present FIND (Familiarity, Identity, Network, and Direction) – a novel, sequential framework for success as a first-year hospitalist. For each component, we provide a narrative overview and a summary bullet point for quick reference.
Familiarity
- Lay the foundation: Learn the ins and outs of your job, EMR, and team.
Familiarize yourself with your surroundings. Know where your patients are located, where you can document, where to find equipment for procedures, and how to reach information technology. Proactively set up the electronic medical record on your home computer and phone. Make sure to review your responsibilities, including your call schedule, your shifts, your assigned patient panel, when you can leave campus, and how people should contact you. Also, others should know your expectations of them, especially if you are working with trainees.
Maintain a file with all of your orientation materials, including phone numbers and emails of key personnel. Know who your people are – who can access your calendar, who you can call with a clinical question or to escalate care, who can assist you with billing, and who helps with the throughput of your patients in the hospital. Take time to review your benefits, including parental leave, insurance coverage, retirement planning, vacation time, and ancillary services like laundry for your white coat. Familiarizing yourself with these basics will provide comfort and lay the foundation for your first year.
Identity
- Perform self-reflection: Overcome imposter syndrome and invest in hobbies.
One of the fundamental realizations that will occur with your first hospitalist job is that you are the attending. You walk in with a vision of your first job; be prepared to be surprised. You have earned the privilege of deciding on patient plans, and you are no longer obligated to staff with a senior physician. This is both empowering and terrifying. In a way, it may oddly remind you of intern year. A new hospital, new EMR, new colleagues, and imposter syndrome will trick you into doubting your decisions.
How to battle it? Positive thinking. You do know the basics of inpatient medicine and you do have a support system to cheer you on. As part of imposter syndrome, you may feel pressured to focus solely on work. Yet, your first job as a hospitalist is finally an amazing opportunity to focus on you. What hobbies have you been neglecting: cooking, photography, reading, more time with family, a new pet? You have the power to schedule your off-weeks. Are you interested in academics? Reserve a portion of your time off to explore scholarship opportunities at your institution. Your first job as a hospitalist is a chance to develop your identity, both as a physician and as an individual.
Network
- Engage your support system: Communicate with nursing, administration, colleagues.
Networking, or building a web of mutually beneficial professional relationships, is imperative for long-term career success. Hospitalists should focus on developing their network across multiple departments, such as nursing, subspecialties, medical education, and hospital administration. Curating a broad network will increase your visibility within your organization, showcase your unique services, and demonstrate your value.
To make networking encounters impactful, express interest, actively listen, ask relevant questions, and seek areas of mutual benefit. It’s equally important to cultivate these new relationships after the initial encounter and to demonstrate how your skill set will aid colleagues in achieving their professional goals. Over time, as you establish your niche, deliberate networking with those who share similar interests can lead to a wealth of new experiences and opportunities. Intentionally mastering networking early in your career provides insight into different aspects of the hospital system, new perspectives on ideas, and access to valuable guidance from other professionals. Engaging in networking to establish your support system is an essential step towards success as a first-year hospitalist.
Direction
- Visualize your path: Find a mentor and develop a mission statement and career plan.
Once you’re familiar with your work environment, confident in your identity, and acquainted with your support network, you’re ready for the final step – direction. Hospital medicine offers many professional avenues and clarifying your career path is challenging when attempted alone. A mentor is the necessary catalyst to find direction and purpose.
Selecting and engaging with a mentor will bolster your professional advancement, academic productivity, and most importantly, career satisfaction.1 At its best, mentorship is a symbiotic relationship. Your mentor should inspire you, challenge you, and support your growth and emotional well-being. In turn, as the mentee, you should be proactive, establish expectations, and take responsibility for maintaining communication to ensure a successful relationship. As your career takes shape over time, you may require a mentorship team to fulfill your unique needs.
When you’ve established a relationship with your mentor, take time to develop 1-year and 5-year plans. Your 1-year plan should focus on a few “quick wins,” often projects or opportunities at your home institution. Small victories in your first year will boost your confidence, motivation, and sense of control. Your 5-year plan should delineate the steps necessary to make your first major career transition, such as from instructor to assistant professor. Working with your mentor to draft a career mission statement is a useful first step in this process. Beginning with the end in mind, will help you visualize your direction.2
We hope that the FIND framework will help you find your path to success as a first-year hospitalist.
Dr. Nelson is a hospitalist and instructor of medicine at Harvard Medical School and Beth Israel Deaconess Medical Center, both in Boston. Dr. Ashford is assistant professor and program director, department of internal medicine/pediatrics, at the University of Nebraska Medical Center, Omaha. Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Crecelius is assistant professor of clinical medicine at Indiana University, Indianapolis. This article is sponsored by the SHM Physicians in Training committee, which submits quarterly content to the Hospitalist on topics relevant to trainees and early -career hospitalists.
References
1. Zerzan JT et al. Making the most of mentors: a guide for mentees. Acad Med. 2009;84:140-4. doi: 10.1097/ACM.0b013e3181906e8f.
2. Covey F. The seven habits of highly effective people. 25th anniversary edition. New York: Simon and Schuster, 2013.
FDA to add myocarditis warning to mRNA COVID-19 vaccines
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
Tofacitinib shows mortality benefit in patients with COVID-19 pneumonia
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Sotagliflozin use in T2D patients linked with posthospitalization benefits in analysis
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
FROM ANNALS OF INTERNAL MEDICINE
New data on COVID-19’s cognitive fallout
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Fact or fiction? Intravascular contrast and acute kidney injury
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.
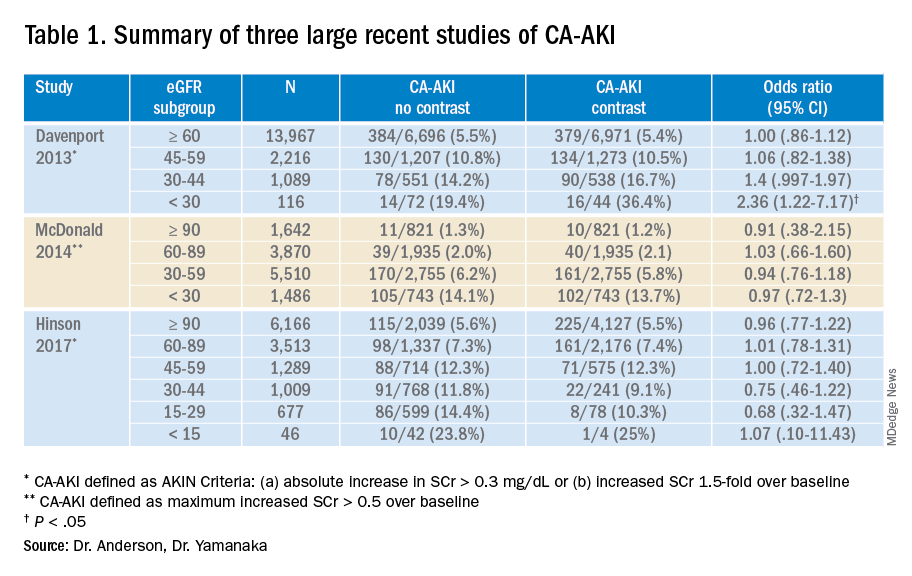
Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Withholding contrast may be the greater risk
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.


















