User login
High rates of work-related trauma, PTSD in intern physicians
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”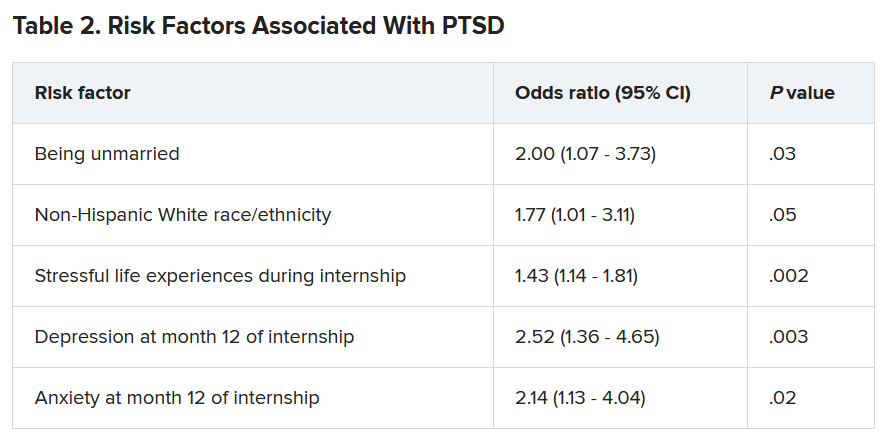
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Reflections on 10 years of hospitalist productivity
Successful programs will recruit lifelong learners
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
Successful programs will recruit lifelong learners
Successful programs will recruit lifelong learners
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
The workload of individual hospitalists has long been a hot-button issue. In a 2013 survey of hospitalists, 40% felt workloads were unsafe on a monthly basis, and 22% reported ordering unnecessary testing or procedures because of time pressure.1 In a 2014 analysis of over 20,000 admissions to an academic hospital medicine service, increasing workload led to increased length of stay and cost per case.2 Although these studies suggest a “sweet spot” for hospitalist workload, many groups face constant pressure to increase revenue.
Over the past decade there has been a significant change in how hospital medicine programs are financed. In the 2010 State of Hospital Medicine (SoHM), the median financial support per physician hospitalist in adult hospital medicine groups (HMGs) was $98,253. By the 2020 SoHM, the financial support was $198,750, an increase of $100,497 in just 10 years. When this is combined with the explosive growth in the number of hospitalists, there is one inescapable conclusion – hospital medicine is expensive.
Over this same 10 years, net collections per hospitalist grew from $194,440 in 2010 to $216,779 in 2020, an increase of $22,339. The increase was caused by higher collections per encounter, not more encounters. Additionally, median compensation for adult/internal medicine hospitalists increased over the same period from $215,000 to $307,336, an increase of $92,336, or 43%. That is an increase of 3.7% per year, more than twice the rate of inflation or wage growth in the general economy over the same period. About 75% of this increase was funded by hospital support. It is clear – health care systems continue to find value in investing in hospitalists and hospital medicine programs.
With mounting costs for hospitals, there is pressure for the hospitalist model of care to change or for yearly billable encounters per hospitalist full-time equivalent to increase. Yet, the productivity of hospitalists, as measured by median billable encounters per year has remained flat. The 2010 SoHM listed median number of billable encounters per year for an internal medicine hospitalist as 2,230. In 2020, the number is 2,246 – a trivial 0.7% increase per decade, what amounts to a rounding error. There has been wiggle up and down over the years, but I suspect these are not trends but noise.
So the question is why. I think it is partly because hospital medicine leaders together with the leaders of their health care systems seem to be reaching an equilibrium. Productivity will always remain an expectation. This expectation will vary based on local circumstances. But for many HMGs, the days when productivity is pushed as the primary objective seem to be disappearing. Most hospital leaders seem to now understand that high productivity can be detrimental to other program goals.
But if productivity is flat, do 40% of hospitalists still feel they are providing unsafe care on a monthly basis? Without another study we don’t know, but here are some reasons why I’m hopeful. First, the hospitalist workforce is more experienced than 10 years ago and may be more efficient. Second, hospital medicine groups are larger and are therefore enabled to schedule more flexibly or enact jeopardy systems to level out workload on busy days. And lastly, hospitalists who feel they are providing unsafe care find greener pastures. The 2010 SoHM reported adult hospital medicine programs had a median 14.3% turnover rate. The 2020 SoHM turnover was 10.9%. While this is up from 2018 (7.4%) and 2016 (6.9%), the general trend is down.
Additionally, we all need to consider the possibility that there will be a disruptive innovation that will allow greater productivity for individual hospitalists while maintaining value. It is apparent the EHR is not yet that breakthrough. We all need to keep our eyes open, stay flexible, and be prepared to meet evolving demands on our programs.
We will see constant demands on hospitalists. But I’m hopeful that going forward expectations will increasingly shift away from simply working harder and seeing more patients, toward goals related to improving performance. Training programs generally produce excellent clinicians, but they often do not equip physicians to be excellent hospitalists. Successful hospital medicine programs will recruit lifelong learners and career hospitalists who are flexible and willing to innovate and adapt. The best programs will have structures in place to help excellent clinicians mature into the role of excellent hospitalists, and leaders that create and foster an environment of excellence.
Discover more 2020 SoHM Report data at www.hospitalmedicine.org/sohm.
Dr. Frederickson is medical director, hospital medicine and palliative care, at CHI Health, Omaha, Neb., and assistant professor at Creighton University, Omaha.
References
1. Michtalik HJ et al. Impact of Attending Physician Workload on Patient Care: A Survey of Hospitalists. JAMA Intern Med. 2013;173(5):375-7. doi: 10.1001/jamainternmed.2013.1864.
2. Elliott DJ et al. Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786-93. doi: 10.1001/jamainternmed.2014.300.
Safety-net burden linked with poorer inpatient cirrhosis outcomes
Patients with cirrhosis treated at hospitals with the highest safety-net burden, defined by their proportion of Medicaid or uninsured patients, had a 5% higher mortality rate than patients who were treated at hospitals with the lowest burden, according to a study of over 300,000 patients.
The study, which was published in the Journal of Clinical Gastroenterology, analyzed inpatient data from the National Inpatient Sample (NIS) database focusing on a 4-year time span between 2012 and 2016. The hospitals were categorized by safety-net burden, which was defined as having either a high, medium, or low number of uninsured patients or patients with Medicaid.
This is the first-known study to evaluate the impact of a hospital’s safety-net burden on hospitalization outcomes in cirrhosis patients, wrote authors Robert J. Wong, MD, MS, of Stanford (Calif.) University and Grishma Hirode, MAS, of the University of Toronto. Previous studies have shown that safety-net hospitals, especially those with a high safety-net burden, have poorer patient outcomes. These hospitals also serve a patient population that is at high risk for chronic liver disease and cirrhosis.
The new analysis included 322,944 individual hospitalizations of patients with cirrhosis. Of these, 57.8% were male, 63.7% were White, 9.9% were Black, and 15.6% were Hispanic. In terms of safety-net burden, 107,446 hospitalizations were at high-burden hospitals, 103,508 were at medium-burden hospitals, and 111,990 hospitalizations were at low-burden hospitals.
Overall, cirrhosis-related hospitalizations in hospitals with the highest burden were found to have significantly greater odds of in-hospital mortality than the lowest tertile hospitals (odds ratio, 1.05, P = .044). The patients were also younger (mean age, 56.7 years vs. 59.8 years in low-burden hospitals). They also had a higher proportion of male patients, minority patients, Hispanic patients, and patients with Medicaid or no insurance.
The odds of hospitalization in the highest tertile hospitals were found to be significantly higher, compared with the middle and lowest tertiles for Blacks and Hispanics, compared with Whites (OR 1.26 and OR 1.63, respectively). Black patients (OR, 1.26; 95%CI, 1.17-1.35; P < .001) and Hispanic patients (OR, 1.63; 95% CI, 1.50-1.78; P< .001) were more likely to be admitted for care at high-burden hospitals (26% to 54%). In-hospital mortality rates among all hospitalizations were 5.95% and the rate did not significantly differ by hospital burden status.
“Despite adjusting for safety-net burden, our study continued to demonstrate ethnic disparities in in-hospital mortality among cirrhosis-related hospitalizations,” the researchers wrote. Overall, the odds of in-hospital mortality were 27% higher in Black patients as compared with White patients.
However, significantly lower mortality was observed in Hispanic patients as compared with White patients (4.9% vs. 6.0%, P < .001), but why this occurred was not entirely clear. “Hispanic patients may be more likely to have NASH [nonalcoholic steatohepatitis]-related cirrhosis, which generally has a slower disease progression, compared with [hepatitis C virus] or alcoholic cirrhosis. As such, it is likely that NASH-cirrhosis Hispanic patients had less severe disease at presentation,” the researchers wrote.
Study design has limitations, but shows concerning trends
The study findings were limited by several factors including the inability to show causality based on the observational study design and cross-sectional nature of the database, the researchers said. The NIS database records individual hospitalizations, not individual patient data which means that it may include repeat hospitalizations from the same patient. In addition, the study was limited by a lack of data on outpatient cirrhosis outcomes and non–liver-related comorbidities.
However, the finding that ethnic minorities with cirrhosis were significantly more likely to be hospitalized in high safety-net hospitals than White patients is concerning, and more research is needed, they said.
“These observations highlight that, while disparities in resources and health care delivery inherent to safety-net health systems may partly explain and provide opportunities to improve cirrhosis hospitalization care, they alone do not explain all of the ethnic disparities in cirrhosis outcomes observed,” they concluded.
The current study was important to conduct at this time because rates of cirrhosis are on the rise, Michael Volk, MD, of Loma Linda (Calif.) University Health, said in an interview. “Millions of patients receive care in safety-net hospitals across the country.”
Dr. Volk said that he was not surprised by the overall outcomes. “Unfortunately, I expected that patient outcomes would be worse at safety-net hospitals than wealthier hospitals. However, I was surprised that Blacks had higher in-hospital mortality than Whites, even after adjusting for the hospital.”
Dr. Volk echoed the study’s stated limitation of the lack of data to address disparities.
“Additional research is needed to determine whether the higher in-hospital mortality among Blacks is related to biological differences such as differential rates of disease progression, or social differences such as access to outpatient care,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Volk had no relevant financial conflicts to disclose.
Patients with cirrhosis treated at hospitals with the highest safety-net burden, defined by their proportion of Medicaid or uninsured patients, had a 5% higher mortality rate than patients who were treated at hospitals with the lowest burden, according to a study of over 300,000 patients.
The study, which was published in the Journal of Clinical Gastroenterology, analyzed inpatient data from the National Inpatient Sample (NIS) database focusing on a 4-year time span between 2012 and 2016. The hospitals were categorized by safety-net burden, which was defined as having either a high, medium, or low number of uninsured patients or patients with Medicaid.
This is the first-known study to evaluate the impact of a hospital’s safety-net burden on hospitalization outcomes in cirrhosis patients, wrote authors Robert J. Wong, MD, MS, of Stanford (Calif.) University and Grishma Hirode, MAS, of the University of Toronto. Previous studies have shown that safety-net hospitals, especially those with a high safety-net burden, have poorer patient outcomes. These hospitals also serve a patient population that is at high risk for chronic liver disease and cirrhosis.
The new analysis included 322,944 individual hospitalizations of patients with cirrhosis. Of these, 57.8% were male, 63.7% were White, 9.9% were Black, and 15.6% were Hispanic. In terms of safety-net burden, 107,446 hospitalizations were at high-burden hospitals, 103,508 were at medium-burden hospitals, and 111,990 hospitalizations were at low-burden hospitals.
Overall, cirrhosis-related hospitalizations in hospitals with the highest burden were found to have significantly greater odds of in-hospital mortality than the lowest tertile hospitals (odds ratio, 1.05, P = .044). The patients were also younger (mean age, 56.7 years vs. 59.8 years in low-burden hospitals). They also had a higher proportion of male patients, minority patients, Hispanic patients, and patients with Medicaid or no insurance.
The odds of hospitalization in the highest tertile hospitals were found to be significantly higher, compared with the middle and lowest tertiles for Blacks and Hispanics, compared with Whites (OR 1.26 and OR 1.63, respectively). Black patients (OR, 1.26; 95%CI, 1.17-1.35; P < .001) and Hispanic patients (OR, 1.63; 95% CI, 1.50-1.78; P< .001) were more likely to be admitted for care at high-burden hospitals (26% to 54%). In-hospital mortality rates among all hospitalizations were 5.95% and the rate did not significantly differ by hospital burden status.
“Despite adjusting for safety-net burden, our study continued to demonstrate ethnic disparities in in-hospital mortality among cirrhosis-related hospitalizations,” the researchers wrote. Overall, the odds of in-hospital mortality were 27% higher in Black patients as compared with White patients.
However, significantly lower mortality was observed in Hispanic patients as compared with White patients (4.9% vs. 6.0%, P < .001), but why this occurred was not entirely clear. “Hispanic patients may be more likely to have NASH [nonalcoholic steatohepatitis]-related cirrhosis, which generally has a slower disease progression, compared with [hepatitis C virus] or alcoholic cirrhosis. As such, it is likely that NASH-cirrhosis Hispanic patients had less severe disease at presentation,” the researchers wrote.
Study design has limitations, but shows concerning trends
The study findings were limited by several factors including the inability to show causality based on the observational study design and cross-sectional nature of the database, the researchers said. The NIS database records individual hospitalizations, not individual patient data which means that it may include repeat hospitalizations from the same patient. In addition, the study was limited by a lack of data on outpatient cirrhosis outcomes and non–liver-related comorbidities.
However, the finding that ethnic minorities with cirrhosis were significantly more likely to be hospitalized in high safety-net hospitals than White patients is concerning, and more research is needed, they said.
“These observations highlight that, while disparities in resources and health care delivery inherent to safety-net health systems may partly explain and provide opportunities to improve cirrhosis hospitalization care, they alone do not explain all of the ethnic disparities in cirrhosis outcomes observed,” they concluded.
The current study was important to conduct at this time because rates of cirrhosis are on the rise, Michael Volk, MD, of Loma Linda (Calif.) University Health, said in an interview. “Millions of patients receive care in safety-net hospitals across the country.”
Dr. Volk said that he was not surprised by the overall outcomes. “Unfortunately, I expected that patient outcomes would be worse at safety-net hospitals than wealthier hospitals. However, I was surprised that Blacks had higher in-hospital mortality than Whites, even after adjusting for the hospital.”
Dr. Volk echoed the study’s stated limitation of the lack of data to address disparities.
“Additional research is needed to determine whether the higher in-hospital mortality among Blacks is related to biological differences such as differential rates of disease progression, or social differences such as access to outpatient care,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Volk had no relevant financial conflicts to disclose.
Patients with cirrhosis treated at hospitals with the highest safety-net burden, defined by their proportion of Medicaid or uninsured patients, had a 5% higher mortality rate than patients who were treated at hospitals with the lowest burden, according to a study of over 300,000 patients.
The study, which was published in the Journal of Clinical Gastroenterology, analyzed inpatient data from the National Inpatient Sample (NIS) database focusing on a 4-year time span between 2012 and 2016. The hospitals were categorized by safety-net burden, which was defined as having either a high, medium, or low number of uninsured patients or patients with Medicaid.
This is the first-known study to evaluate the impact of a hospital’s safety-net burden on hospitalization outcomes in cirrhosis patients, wrote authors Robert J. Wong, MD, MS, of Stanford (Calif.) University and Grishma Hirode, MAS, of the University of Toronto. Previous studies have shown that safety-net hospitals, especially those with a high safety-net burden, have poorer patient outcomes. These hospitals also serve a patient population that is at high risk for chronic liver disease and cirrhosis.
The new analysis included 322,944 individual hospitalizations of patients with cirrhosis. Of these, 57.8% were male, 63.7% were White, 9.9% were Black, and 15.6% were Hispanic. In terms of safety-net burden, 107,446 hospitalizations were at high-burden hospitals, 103,508 were at medium-burden hospitals, and 111,990 hospitalizations were at low-burden hospitals.
Overall, cirrhosis-related hospitalizations in hospitals with the highest burden were found to have significantly greater odds of in-hospital mortality than the lowest tertile hospitals (odds ratio, 1.05, P = .044). The patients were also younger (mean age, 56.7 years vs. 59.8 years in low-burden hospitals). They also had a higher proportion of male patients, minority patients, Hispanic patients, and patients with Medicaid or no insurance.
The odds of hospitalization in the highest tertile hospitals were found to be significantly higher, compared with the middle and lowest tertiles for Blacks and Hispanics, compared with Whites (OR 1.26 and OR 1.63, respectively). Black patients (OR, 1.26; 95%CI, 1.17-1.35; P < .001) and Hispanic patients (OR, 1.63; 95% CI, 1.50-1.78; P< .001) were more likely to be admitted for care at high-burden hospitals (26% to 54%). In-hospital mortality rates among all hospitalizations were 5.95% and the rate did not significantly differ by hospital burden status.
“Despite adjusting for safety-net burden, our study continued to demonstrate ethnic disparities in in-hospital mortality among cirrhosis-related hospitalizations,” the researchers wrote. Overall, the odds of in-hospital mortality were 27% higher in Black patients as compared with White patients.
However, significantly lower mortality was observed in Hispanic patients as compared with White patients (4.9% vs. 6.0%, P < .001), but why this occurred was not entirely clear. “Hispanic patients may be more likely to have NASH [nonalcoholic steatohepatitis]-related cirrhosis, which generally has a slower disease progression, compared with [hepatitis C virus] or alcoholic cirrhosis. As such, it is likely that NASH-cirrhosis Hispanic patients had less severe disease at presentation,” the researchers wrote.
Study design has limitations, but shows concerning trends
The study findings were limited by several factors including the inability to show causality based on the observational study design and cross-sectional nature of the database, the researchers said. The NIS database records individual hospitalizations, not individual patient data which means that it may include repeat hospitalizations from the same patient. In addition, the study was limited by a lack of data on outpatient cirrhosis outcomes and non–liver-related comorbidities.
However, the finding that ethnic minorities with cirrhosis were significantly more likely to be hospitalized in high safety-net hospitals than White patients is concerning, and more research is needed, they said.
“These observations highlight that, while disparities in resources and health care delivery inherent to safety-net health systems may partly explain and provide opportunities to improve cirrhosis hospitalization care, they alone do not explain all of the ethnic disparities in cirrhosis outcomes observed,” they concluded.
The current study was important to conduct at this time because rates of cirrhosis are on the rise, Michael Volk, MD, of Loma Linda (Calif.) University Health, said in an interview. “Millions of patients receive care in safety-net hospitals across the country.”
Dr. Volk said that he was not surprised by the overall outcomes. “Unfortunately, I expected that patient outcomes would be worse at safety-net hospitals than wealthier hospitals. However, I was surprised that Blacks had higher in-hospital mortality than Whites, even after adjusting for the hospital.”
Dr. Volk echoed the study’s stated limitation of the lack of data to address disparities.
“Additional research is needed to determine whether the higher in-hospital mortality among Blacks is related to biological differences such as differential rates of disease progression, or social differences such as access to outpatient care,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Volk had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Preparing pediatric hospital medicine fellows for leadership
Reflecting on a longitudinal leadership elective experience
The practice of pediatric hospital medicine (PHM) has been evolving and rapidly expanding over the last several decades. Not only has the scope of clinical practice matured and become more defined, but hospitalists now also have the responsibility to advance the performance of hospitals and health care systems. Pediatric hospitalists are increasingly incorporating medical education, research, high-value care, patient quality and safety initiatives, and process improvement into their careers.1 As a result, pediatric hospitalists are occupying a wider range of administrative and leadership positions within the health care system.
The field of PHM has highlighted the importance of leadership in the practice of hospital medicine by dedicating a chapter to “Leadership in Healthcare” in the PHM Core Competencies.1 The competencies define the expertise required of hospitalists and serve as guidance for the development of education, training, and career development series. Hospitalists may seek out opportunities for leadership training at an institutional or national level. Options may include advanced degrees, national conferences, division training seminars, or self-directed learning through reading or observational experiences. Unfortunately, all of these take time and motivation. As a result, hospitalists tend to pursue these opportunities only after they have already been appointed to leadership positions.
PHM fellowship is the optimal time to build a foundation of leadership skills. Over the course of a 2-year fellowship, fellows have a combined 16 weeks dedicated to educational activities beyond direct patient care.2 The Accreditation Council for Graduate Medical Education (ACGME) encourages educational innovation during this time, allowing programs to create unique opportunities for their fellows that will promote progress towards their ultimate career goals.3 This curricular framework provides the flexibility to integrate leadership training into fellowship training.
Many fellows are eager for leadership experiences and mentorship, myself included. As a pediatric chief resident, I was immersed in a diverse range of clinical, educational, research, and administrative responsibilities. I found myself in a leadership position with no prior education on how to manage people or team dynamics, make high-stress decisions on behalf of a group of people, or handle conflict. Although I learned new strategies on a daily basis, the experience showed me how much more I still had to learn in order to be a successful leader. This was one of the reasons I decided to pursue fellowship training. I think many PHM fellowship applicants feel similarly. They may have served in a leadership position in the past but feel underprepared to fulfill leadership positions in the next phase of their careers.
But despite this eagerness, evidence suggests that fellows do not feel that they receive as much management training as they need to start their careers. In a 2014 survey of PHM fellowship graduates, many held formal leadership positions within their institution (23/51) and within national organizations (6/51), despite having only five years of hospitalist experience on average (including time spent in fellowship). When asked about training needs, respondents identified “hospital program management” as an area where they wished they received more training during fellowship.4
Anyone who has gone through the PHM fellowship interview process can tell you that a common refrain of program directors is, “One of the goals of our program is to create future leaders in PHM.” This led me to wonder: how do fellowship programs prepare their fellows for future leadership positions?
I began my fellowship training at Nationwide Children’s Hospital in the summer of 2020. The program had just designed a longitudinal leadership elective, which the second-year fellow and I decided to pilot together. As I reflected on the first half of this academic year, I realized that it is unique experiences like this elective that make me thankful I pursued fellowship. I want to share with the hospitalist community the structure of the elective and why it has been particularly valuable with the hope that it will inspire similar opportunities for other fellows.
The program is semi-structured but allows the fellow and preceptors the flexibility to decide what activities would benefit that particular fellow. We attend a variety of administrative and committee meetings with each preceptor that expose us to the responsibilities of their positions, their leadership style in action, their approach to crisis management, and differences in divisional operations. On a monthly basis we meet with a preceptor to discuss a topic related to leadership. Examples of topics include how to run a more effective meeting, barriers to organizational change, leading in crisis, and the importance of mission, vision, values, and goals of organizations. The preceptor sends us articles or other learning materials they have found useful on the topic, and these serve as a starting point for our discussions. These discussions provide a point of reflection as we apply the day’s concept to our own prior experiences or to our observations during the elective.
The combination of learning experiences, discussions, and dedicated preceptorship has prepared me far better for future leadership than my past personal and observational experiences. I have summarized my top three reasons why this structure of leadership development is particularly valuable to me as a fellow.
First, the longitudinal structure of the elective allows us to learn from multiple preceptors over the course of the academic year. The preceptors include the current chief of hospital pediatrics at Nationwide Children’s Hospital; the division director of hospital medicine at the Ohio State University Wexner Medical Center; and the physician lead for hospital medicine at one of the satellite hospitals in the region. With faculty from the Department of Pediatrics and the Department of Internal Medicine-Pediatrics in these leadership positions, we have the unique ability to compare and contrast operational systems between the two different hospital systems.
Recently, we also had the opportunity to meet with both the chairman of the department of pediatrics and chief medical officer. All of these physician leaders hold a variety of administrative roles and have differing leadership philosophies, each providing useful insights. For instance, one leader ensures his team holds him accountable as the leader by always asking for honest feedback. He recommends telling those you work with to “never let me fail.” Another leader acknowledges that creating five-year plans can be daunting but encouraged us to still be intentional with our direction on a smaller scale by writing down goals for the year and sharing with a mentor. Ultimately, I came away with a wide variety of perspectives to reference as I go forward.
Second, the learning is contextualized. I can take concepts that I learn through reading and discussions and construct meaning based on observations from meetings or other encounters with different leaders. For example, after reviewing several articles on strategies to make meetings more effective, I started noticing what went well and what didn’t go well in every meeting I attended. I observed preceptors employing many of the strategies successfully with positive feedback. This included not only simple practices, such as setting an agenda to provide a compass for the conversation, but also more nuanced practices like controlling the meeting but not the conversation.
After reading about leadership styles I also found myself analyzing the qualities and strategies of leaders I encountered and reflecting on their approach, noticing what I could possibly interlace in my own practice. Several of the leaders I spoke with during the elective recommended paying attention to the actions of the ineffective bosses or mentors because they can teach you something too: how not to act. I even started applying this strategy to the popular television series The Office – Michael Scott, the regional manager of a fictional paper company, demonstrates some of the best and worst leadership skills in every episode. I am developing a repertoire of strategies to lead and motivate people.
Finally, the design allows for real-time application of new methods to my current practice. One particularly useful tool I have learned is Leader Standard Work, a systematic method to get leaders to maintain stability, problem solve, and drive continuous improvement within their organization.5 I have used elements of Leader Standard Work on a personal level to improve my time management skills and increase my productivity. For example, I reconceptualized my calendar as a standardized checklist and I organized it to allot more time to critical activities, such as my research and scholarly output, and less on administrative tasks. I am also implementing changes to how I prepare and run meetings, collaborate, and communicate with members of my research team.
Mastery requires practice and feedback, so applying concepts even on a small, personal scale shortly after learning them has been very valuable. Over the last several months I have often wished I had this type of structured leadership education during my year as a chief resident. I think I could have been more intentional in my decision-making, possibly being a stronger leader for the program. Now that I am transferring skills into practice right away, I am setting the stage for lasting changes in behavior that will hopefully benefit all those that I work with in the future.
Leadership development through a customizable longitudinal elective may be an effective way to prepare PHM fellow graduates for future leadership positions. Fellows can emerge with the skills and real-world practice to allow them to feel confident in future positions. However, leadership doesn’t end when we get the position. We must remember to continuously ask for feedback and build upon our experiences to evolve as leaders in PHM.
Dr. Westphal is a first-year pediatric hospital medicine fellow at Nationwide Children’s Hospital in Columbus, Ohio with an interest in improving the delivery of quality care for hospitalized infants.
References
1. Maniscalco, J, et al. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C). J Hosp Med. 2020;S1;E12-E17. doi: 10.12788/jhm.3391.
2. Jerardi KE, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017 Jul;140(1):e20170698. doi: 10.1542/peds.2017-0698.
3. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. 2020 Edition. Accessed 2021 Jan 14.
4. Oshimura, JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7. doi: 10.1542/hpeds.2016-0031.
5. Murli, J. Standard Work for Lean Leaders: One of the Keys to Sustaining Performance Gains. Lean Institute Enterprise, Lean Institute Enterprise Inc. 4 Dec 2013. www.lean.org/common/display/?o=2493
Reflecting on a longitudinal leadership elective experience
Reflecting on a longitudinal leadership elective experience
The practice of pediatric hospital medicine (PHM) has been evolving and rapidly expanding over the last several decades. Not only has the scope of clinical practice matured and become more defined, but hospitalists now also have the responsibility to advance the performance of hospitals and health care systems. Pediatric hospitalists are increasingly incorporating medical education, research, high-value care, patient quality and safety initiatives, and process improvement into their careers.1 As a result, pediatric hospitalists are occupying a wider range of administrative and leadership positions within the health care system.
The field of PHM has highlighted the importance of leadership in the practice of hospital medicine by dedicating a chapter to “Leadership in Healthcare” in the PHM Core Competencies.1 The competencies define the expertise required of hospitalists and serve as guidance for the development of education, training, and career development series. Hospitalists may seek out opportunities for leadership training at an institutional or national level. Options may include advanced degrees, national conferences, division training seminars, or self-directed learning through reading or observational experiences. Unfortunately, all of these take time and motivation. As a result, hospitalists tend to pursue these opportunities only after they have already been appointed to leadership positions.
PHM fellowship is the optimal time to build a foundation of leadership skills. Over the course of a 2-year fellowship, fellows have a combined 16 weeks dedicated to educational activities beyond direct patient care.2 The Accreditation Council for Graduate Medical Education (ACGME) encourages educational innovation during this time, allowing programs to create unique opportunities for their fellows that will promote progress towards their ultimate career goals.3 This curricular framework provides the flexibility to integrate leadership training into fellowship training.
Many fellows are eager for leadership experiences and mentorship, myself included. As a pediatric chief resident, I was immersed in a diverse range of clinical, educational, research, and administrative responsibilities. I found myself in a leadership position with no prior education on how to manage people or team dynamics, make high-stress decisions on behalf of a group of people, or handle conflict. Although I learned new strategies on a daily basis, the experience showed me how much more I still had to learn in order to be a successful leader. This was one of the reasons I decided to pursue fellowship training. I think many PHM fellowship applicants feel similarly. They may have served in a leadership position in the past but feel underprepared to fulfill leadership positions in the next phase of their careers.
But despite this eagerness, evidence suggests that fellows do not feel that they receive as much management training as they need to start their careers. In a 2014 survey of PHM fellowship graduates, many held formal leadership positions within their institution (23/51) and within national organizations (6/51), despite having only five years of hospitalist experience on average (including time spent in fellowship). When asked about training needs, respondents identified “hospital program management” as an area where they wished they received more training during fellowship.4
Anyone who has gone through the PHM fellowship interview process can tell you that a common refrain of program directors is, “One of the goals of our program is to create future leaders in PHM.” This led me to wonder: how do fellowship programs prepare their fellows for future leadership positions?
I began my fellowship training at Nationwide Children’s Hospital in the summer of 2020. The program had just designed a longitudinal leadership elective, which the second-year fellow and I decided to pilot together. As I reflected on the first half of this academic year, I realized that it is unique experiences like this elective that make me thankful I pursued fellowship. I want to share with the hospitalist community the structure of the elective and why it has been particularly valuable with the hope that it will inspire similar opportunities for other fellows.
The program is semi-structured but allows the fellow and preceptors the flexibility to decide what activities would benefit that particular fellow. We attend a variety of administrative and committee meetings with each preceptor that expose us to the responsibilities of their positions, their leadership style in action, their approach to crisis management, and differences in divisional operations. On a monthly basis we meet with a preceptor to discuss a topic related to leadership. Examples of topics include how to run a more effective meeting, barriers to organizational change, leading in crisis, and the importance of mission, vision, values, and goals of organizations. The preceptor sends us articles or other learning materials they have found useful on the topic, and these serve as a starting point for our discussions. These discussions provide a point of reflection as we apply the day’s concept to our own prior experiences or to our observations during the elective.
The combination of learning experiences, discussions, and dedicated preceptorship has prepared me far better for future leadership than my past personal and observational experiences. I have summarized my top three reasons why this structure of leadership development is particularly valuable to me as a fellow.
First, the longitudinal structure of the elective allows us to learn from multiple preceptors over the course of the academic year. The preceptors include the current chief of hospital pediatrics at Nationwide Children’s Hospital; the division director of hospital medicine at the Ohio State University Wexner Medical Center; and the physician lead for hospital medicine at one of the satellite hospitals in the region. With faculty from the Department of Pediatrics and the Department of Internal Medicine-Pediatrics in these leadership positions, we have the unique ability to compare and contrast operational systems between the two different hospital systems.
Recently, we also had the opportunity to meet with both the chairman of the department of pediatrics and chief medical officer. All of these physician leaders hold a variety of administrative roles and have differing leadership philosophies, each providing useful insights. For instance, one leader ensures his team holds him accountable as the leader by always asking for honest feedback. He recommends telling those you work with to “never let me fail.” Another leader acknowledges that creating five-year plans can be daunting but encouraged us to still be intentional with our direction on a smaller scale by writing down goals for the year and sharing with a mentor. Ultimately, I came away with a wide variety of perspectives to reference as I go forward.
Second, the learning is contextualized. I can take concepts that I learn through reading and discussions and construct meaning based on observations from meetings or other encounters with different leaders. For example, after reviewing several articles on strategies to make meetings more effective, I started noticing what went well and what didn’t go well in every meeting I attended. I observed preceptors employing many of the strategies successfully with positive feedback. This included not only simple practices, such as setting an agenda to provide a compass for the conversation, but also more nuanced practices like controlling the meeting but not the conversation.
After reading about leadership styles I also found myself analyzing the qualities and strategies of leaders I encountered and reflecting on their approach, noticing what I could possibly interlace in my own practice. Several of the leaders I spoke with during the elective recommended paying attention to the actions of the ineffective bosses or mentors because they can teach you something too: how not to act. I even started applying this strategy to the popular television series The Office – Michael Scott, the regional manager of a fictional paper company, demonstrates some of the best and worst leadership skills in every episode. I am developing a repertoire of strategies to lead and motivate people.
Finally, the design allows for real-time application of new methods to my current practice. One particularly useful tool I have learned is Leader Standard Work, a systematic method to get leaders to maintain stability, problem solve, and drive continuous improvement within their organization.5 I have used elements of Leader Standard Work on a personal level to improve my time management skills and increase my productivity. For example, I reconceptualized my calendar as a standardized checklist and I organized it to allot more time to critical activities, such as my research and scholarly output, and less on administrative tasks. I am also implementing changes to how I prepare and run meetings, collaborate, and communicate with members of my research team.
Mastery requires practice and feedback, so applying concepts even on a small, personal scale shortly after learning them has been very valuable. Over the last several months I have often wished I had this type of structured leadership education during my year as a chief resident. I think I could have been more intentional in my decision-making, possibly being a stronger leader for the program. Now that I am transferring skills into practice right away, I am setting the stage for lasting changes in behavior that will hopefully benefit all those that I work with in the future.
Leadership development through a customizable longitudinal elective may be an effective way to prepare PHM fellow graduates for future leadership positions. Fellows can emerge with the skills and real-world practice to allow them to feel confident in future positions. However, leadership doesn’t end when we get the position. We must remember to continuously ask for feedback and build upon our experiences to evolve as leaders in PHM.
Dr. Westphal is a first-year pediatric hospital medicine fellow at Nationwide Children’s Hospital in Columbus, Ohio with an interest in improving the delivery of quality care for hospitalized infants.
References
1. Maniscalco, J, et al. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C). J Hosp Med. 2020;S1;E12-E17. doi: 10.12788/jhm.3391.
2. Jerardi KE, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017 Jul;140(1):e20170698. doi: 10.1542/peds.2017-0698.
3. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. 2020 Edition. Accessed 2021 Jan 14.
4. Oshimura, JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7. doi: 10.1542/hpeds.2016-0031.
5. Murli, J. Standard Work for Lean Leaders: One of the Keys to Sustaining Performance Gains. Lean Institute Enterprise, Lean Institute Enterprise Inc. 4 Dec 2013. www.lean.org/common/display/?o=2493
The practice of pediatric hospital medicine (PHM) has been evolving and rapidly expanding over the last several decades. Not only has the scope of clinical practice matured and become more defined, but hospitalists now also have the responsibility to advance the performance of hospitals and health care systems. Pediatric hospitalists are increasingly incorporating medical education, research, high-value care, patient quality and safety initiatives, and process improvement into their careers.1 As a result, pediatric hospitalists are occupying a wider range of administrative and leadership positions within the health care system.
The field of PHM has highlighted the importance of leadership in the practice of hospital medicine by dedicating a chapter to “Leadership in Healthcare” in the PHM Core Competencies.1 The competencies define the expertise required of hospitalists and serve as guidance for the development of education, training, and career development series. Hospitalists may seek out opportunities for leadership training at an institutional or national level. Options may include advanced degrees, national conferences, division training seminars, or self-directed learning through reading or observational experiences. Unfortunately, all of these take time and motivation. As a result, hospitalists tend to pursue these opportunities only after they have already been appointed to leadership positions.
PHM fellowship is the optimal time to build a foundation of leadership skills. Over the course of a 2-year fellowship, fellows have a combined 16 weeks dedicated to educational activities beyond direct patient care.2 The Accreditation Council for Graduate Medical Education (ACGME) encourages educational innovation during this time, allowing programs to create unique opportunities for their fellows that will promote progress towards their ultimate career goals.3 This curricular framework provides the flexibility to integrate leadership training into fellowship training.
Many fellows are eager for leadership experiences and mentorship, myself included. As a pediatric chief resident, I was immersed in a diverse range of clinical, educational, research, and administrative responsibilities. I found myself in a leadership position with no prior education on how to manage people or team dynamics, make high-stress decisions on behalf of a group of people, or handle conflict. Although I learned new strategies on a daily basis, the experience showed me how much more I still had to learn in order to be a successful leader. This was one of the reasons I decided to pursue fellowship training. I think many PHM fellowship applicants feel similarly. They may have served in a leadership position in the past but feel underprepared to fulfill leadership positions in the next phase of their careers.
But despite this eagerness, evidence suggests that fellows do not feel that they receive as much management training as they need to start their careers. In a 2014 survey of PHM fellowship graduates, many held formal leadership positions within their institution (23/51) and within national organizations (6/51), despite having only five years of hospitalist experience on average (including time spent in fellowship). When asked about training needs, respondents identified “hospital program management” as an area where they wished they received more training during fellowship.4
Anyone who has gone through the PHM fellowship interview process can tell you that a common refrain of program directors is, “One of the goals of our program is to create future leaders in PHM.” This led me to wonder: how do fellowship programs prepare their fellows for future leadership positions?
I began my fellowship training at Nationwide Children’s Hospital in the summer of 2020. The program had just designed a longitudinal leadership elective, which the second-year fellow and I decided to pilot together. As I reflected on the first half of this academic year, I realized that it is unique experiences like this elective that make me thankful I pursued fellowship. I want to share with the hospitalist community the structure of the elective and why it has been particularly valuable with the hope that it will inspire similar opportunities for other fellows.
The program is semi-structured but allows the fellow and preceptors the flexibility to decide what activities would benefit that particular fellow. We attend a variety of administrative and committee meetings with each preceptor that expose us to the responsibilities of their positions, their leadership style in action, their approach to crisis management, and differences in divisional operations. On a monthly basis we meet with a preceptor to discuss a topic related to leadership. Examples of topics include how to run a more effective meeting, barriers to organizational change, leading in crisis, and the importance of mission, vision, values, and goals of organizations. The preceptor sends us articles or other learning materials they have found useful on the topic, and these serve as a starting point for our discussions. These discussions provide a point of reflection as we apply the day’s concept to our own prior experiences or to our observations during the elective.
The combination of learning experiences, discussions, and dedicated preceptorship has prepared me far better for future leadership than my past personal and observational experiences. I have summarized my top three reasons why this structure of leadership development is particularly valuable to me as a fellow.
First, the longitudinal structure of the elective allows us to learn from multiple preceptors over the course of the academic year. The preceptors include the current chief of hospital pediatrics at Nationwide Children’s Hospital; the division director of hospital medicine at the Ohio State University Wexner Medical Center; and the physician lead for hospital medicine at one of the satellite hospitals in the region. With faculty from the Department of Pediatrics and the Department of Internal Medicine-Pediatrics in these leadership positions, we have the unique ability to compare and contrast operational systems between the two different hospital systems.
Recently, we also had the opportunity to meet with both the chairman of the department of pediatrics and chief medical officer. All of these physician leaders hold a variety of administrative roles and have differing leadership philosophies, each providing useful insights. For instance, one leader ensures his team holds him accountable as the leader by always asking for honest feedback. He recommends telling those you work with to “never let me fail.” Another leader acknowledges that creating five-year plans can be daunting but encouraged us to still be intentional with our direction on a smaller scale by writing down goals for the year and sharing with a mentor. Ultimately, I came away with a wide variety of perspectives to reference as I go forward.
Second, the learning is contextualized. I can take concepts that I learn through reading and discussions and construct meaning based on observations from meetings or other encounters with different leaders. For example, after reviewing several articles on strategies to make meetings more effective, I started noticing what went well and what didn’t go well in every meeting I attended. I observed preceptors employing many of the strategies successfully with positive feedback. This included not only simple practices, such as setting an agenda to provide a compass for the conversation, but also more nuanced practices like controlling the meeting but not the conversation.
After reading about leadership styles I also found myself analyzing the qualities and strategies of leaders I encountered and reflecting on their approach, noticing what I could possibly interlace in my own practice. Several of the leaders I spoke with during the elective recommended paying attention to the actions of the ineffective bosses or mentors because they can teach you something too: how not to act. I even started applying this strategy to the popular television series The Office – Michael Scott, the regional manager of a fictional paper company, demonstrates some of the best and worst leadership skills in every episode. I am developing a repertoire of strategies to lead and motivate people.
Finally, the design allows for real-time application of new methods to my current practice. One particularly useful tool I have learned is Leader Standard Work, a systematic method to get leaders to maintain stability, problem solve, and drive continuous improvement within their organization.5 I have used elements of Leader Standard Work on a personal level to improve my time management skills and increase my productivity. For example, I reconceptualized my calendar as a standardized checklist and I organized it to allot more time to critical activities, such as my research and scholarly output, and less on administrative tasks. I am also implementing changes to how I prepare and run meetings, collaborate, and communicate with members of my research team.
Mastery requires practice and feedback, so applying concepts even on a small, personal scale shortly after learning them has been very valuable. Over the last several months I have often wished I had this type of structured leadership education during my year as a chief resident. I think I could have been more intentional in my decision-making, possibly being a stronger leader for the program. Now that I am transferring skills into practice right away, I am setting the stage for lasting changes in behavior that will hopefully benefit all those that I work with in the future.
Leadership development through a customizable longitudinal elective may be an effective way to prepare PHM fellow graduates for future leadership positions. Fellows can emerge with the skills and real-world practice to allow them to feel confident in future positions. However, leadership doesn’t end when we get the position. We must remember to continuously ask for feedback and build upon our experiences to evolve as leaders in PHM.
Dr. Westphal is a first-year pediatric hospital medicine fellow at Nationwide Children’s Hospital in Columbus, Ohio with an interest in improving the delivery of quality care for hospitalized infants.
References
1. Maniscalco, J, et al. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology (C). J Hosp Med. 2020;S1;E12-E17. doi: 10.12788/jhm.3391.
2. Jerardi KE, et al; Council of Pediatric Hospital Medicine Fellowship Directors. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017 Jul;140(1):e20170698. doi: 10.1542/peds.2017-0698.
3. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. 2020 Edition. Accessed 2021 Jan 14.
4. Oshimura, JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7. doi: 10.1542/hpeds.2016-0031.
5. Murli, J. Standard Work for Lean Leaders: One of the Keys to Sustaining Performance Gains. Lean Institute Enterprise, Lean Institute Enterprise Inc. 4 Dec 2013. www.lean.org/common/display/?o=2493
Prophylactic anticoagulation tied to lower death rate in COVID
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S., international MIS-C studies yield disparate results
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
That requires rapid pragmatic evaluation of therapies. Two real-world observational studies published online June 16 in The New England Journal of Medicine do that, with differing results.
In the Overcoming COVID-19 study, investigators assessed initial therapy and outcomes for patients with MIS-C using surveillance data from 58 pediatric hospitals nationwide.
The results suggest that patients with MIS-C who were younger than 21 years of age and who were initially treated with intravenous immunoglobulin (IVIG) plus glucocorticoids fared better in terms of cardiovascular function.
The study included 518 children (median age, 8.7 years) who were admitted to the hospital between March and October 2020 and who received at least one immunomodulatory therapy. In a propensity score–matched analysis, those given IVIG plus glucocorticoids (n = 103) had a lower risk for the primary outcome of cardiovascular dysfunction on or after day 2 than those given IVIG alone (n = 103), at 17% versus 31% (risk ratio, 0.56; 95% confidence interval, 0.34-0.94).
Risks for individual aspects of the study’s composite outcome were also lower with IVIG plus glucocorticoids. Left ventricular dysfunction occurred in 8% and 17%, respectively (RR, 0.46; 95% CI, 0.19-1.15). Shock requiring vasopressor use emerged in 13% and 24%, respectively (RR, 0.54; 95% CI, 0.29-1.00).
In addition, there were fewer cases in which adjunctive therapy was given on day one among those who received combination therapy than among those who received IVIG alone, at 34% versus 70% (RR, 0.49; 95% CI, 0.36-0.65), but the risk for fever was not lower on or after day two (31% and 40%, respectively; RR, 0.78; 95% CI, 0.53-1.13).
Lead author Mary Beth F. Son, MD, director of the rheumatology program at Boston Children’s Hospital, who is also associate professor of pediatrics at Harvard Medical School, stressed that the study did not assess which MIS-C patients should receive treatment. “Rather, we studied children who had been treated with one of two initial regimens and then assessed short-term outcomes,” she told this news organization.
Going forward, it will be important to study which children should receive immunomodulatory treatment, Dr. Son said. “Specifically, can the less ill children receive IVIG alone or no treatment? This is an unanswered question at the moment, which could be addressed with a randomized controlled trial.”
Future directions, she added, will include assessing long-term cardiac outcomes for patients with MIS-C as well as studying outpatient regimens, especially those that involve steroids.
Earlier this year, French investigators found better outcomes with combined corticosteroids and IVIG than with IVIG alone. They suggested that combination therapy should be the standard of care, given the present state of therapeutic knowledge.
Maybe not so standard
Different results emerged, however, from an international study of MIS-C that compared three, rather than two, treatment approaches. Collaborators from the Best Available Treatment Study for MIS-C (BATS) evaluated data for 614 children with suspected MIS-C between June 2020 and February 2021 in 32 countries and found no substantial differences in recovery among children whose primary treatment was IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone.
The study by Andrew J. McArdle, MB BChir, MSC, a clinical research fellow at Imperial College London, and colleagues was published June 16 in The New England Journal of Medicine.
In the BATS cohort, 246 received IVIG alone, 208 received IVIG plus glucocorticoids, and 99 received glucocorticoids alone. Twenty-two patients received other combinations, including biologics, and 39 received no immunomodulatory therapy.
Among patients who were included in the primary analysis, death occurred or inotropic or ventilatory support was employed in 56 of 180 of the patients who received IVIG plus glucocorticoids, compared with 44 of 211 patients treated with IVIG alone, for an adjusted odds ratio (aOR) of 0.77 (95% CI, 0.33-1.82). Among those who received glucocorticoids alone, 17 of 83 met the primary endpoint of death or inotropic or ventilatory support, for an aOR relative to IVIG alone of 0.54 (95% CI, 0.22-1.33).
After adjustments, the likelihood for reduced disease severity was similar in the two groups relative to IVIG alone, at 0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone. Time to reduction in disease severity was also comparable across all groups.
Some of the differences between the U.S. study and the global studies could be the result of the larger size of the international cohort and possibly a difference in the strains of virus in the United States and abroad, according to S. Sexson Tejtel, MD, PhD, MPH, a pediatric cardiologist at Texas Children’s Hospital and an assistant professor at Baylor College of Medicine, Houston, Texas. “Some strains make children sicker than others, and they’re going to need more treatment,” said Dr. Sexson Tejtel, who was not involved in either study.
Dr. Sexson Tejtel also noted that the U.S. researchers did not assess outcomes among children treated with steroids alone. “It would be interesting to know what steroids alone look like in the U.S. MIS-C population,” she said in an interview.
BATS corresponding author Michael Levin, MBE, PhD, FRCPCH, an Imperial College professor of pediatrics and international child health, told this news organization that the differing results may have arisen because of the international study’s three-treatment focus, its wider spectrum of patients, and its different endpoints: Death and inotropic support on or after day 2, versus echocardiographic left ventricular dysfunction or inotropic usage.
Regardless of the differences between the two studies, neither establishes the most effective single or combination treatment, writes Roberta L. DeBiasi, MD, of the Division of Pediatric Infectious Diseases at Children’s National Hospital and Research Institute and George Washington University, Washington, in an accompanying editorial. “Specifically, neither study was powered to include an evaluation of approaches that steer away from broad immunosuppression with glucocorticoids and that focus on more targeted and titratable treatments with biologic agents, such as anakinra and infliximab,” she writes.
Dr. DeBiasi adds that long-term follow-up studies of cardiac and noncardiac outcomes in these patients will launch soon. “Meanwhile, continued collaboration across centers is essential to decreasing the short-term incidence of death and complications,” she writes.
“It will be interesting as we apply results from these studies as they come out to see how they change our practice,” Dr. Sexson Tejtel said. “And it would be good to have some randomized clinical trials.”
For Dr. Levin, the bottom line is that all three treatments are associated with recovery for a majority of children. “This is good news for clinicians who have been guessing which treatment to use,” he said. “Both studies are attempts to provide doctors with some evidence on which to base treatment decisions and are not the final answer. Our study is ongoing, and with larger numbers of patients it may give clearer answers.”
The Overcoming COVID-19 study was funded by the U.S. Centers for Disease Control and Prevention. Several coauthors have reported support from industry outside of the submitted work. BATS was funded by the European Union’s Horizons 2020 Program. The study authors have disclosed no relevant financial relationships. One coauthor’s spouse is employed by GlaxoSmithKline. Dr. DeBiasi and Dr. Sexson Tejtel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Understanding the grieving process
Loss is inevitable – and understanding essential
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
Loss is inevitable – and understanding essential
Loss is inevitable – and understanding essential
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
I arrived on the 6th floor nursing unit one day last fall to find halls abuzz with people. Something didn’t feel right, and then I a saw a nursing colleague with tears streaming down her face. My heart dropped. She looked up at me and said, “Dr Hass, K died last night.” She started to sob. I stood dumbfounded for a moment. We had lost a beloved coworker to COVID.
There has been a collective sense of grief in our country since the beginning of the COVID-19 pandemic as we have all been suffering losses: smiles, touch, in-person relationships, a “normal life.” But it went to another level for us at Alta Bates Summit Medical Center in Oakland, Calif., with the passing of a couple of our beloved teammates in the fall. Strong emotions triggered by these events caused me to pause and think: “What is grief? Is it another word for sadness? How do we work through it?”
What is the difference between sadness and grief? While related, they are temporally and functionally quite different. Sadness is an emotion, and like all emotions, we feel it in brief episodes. Those moments of profound sadness only last minutes at a time. Sadness leads to decreased physiological arousal, especially after crying. When less intense, the physiological slowing is thought to allow for some mental clarity that lets the loss sink in and moves us toward a recalibration process. These episodes of sadness occur more frequently and with greater intensity the closer we are to the triggering event.
While emotions last minutes, mood, another affective state, lasts hours to days and is less intense and specific in content. A sad mood can be present much of the time after a significant loss. Emotions predispose to moods and vice versa.
Grief, on the other hand, is a complex and lengthy process that moves us from a place of loss to a new place with a new equilibrium without the lost object. While sadness is about fully acknowledging the loss, the grieving process is about getting beyond it. The bigger the loss, the bigger the hole in your life and the longer the grieving process. Grief is a multi-emotional process with people often experiencing a range of emotions, such as shock, anger, and fear in addition to sadness.
As I grappled with my sense of loss, I realized that understanding the grieving process was going to help me as I navigate this world now full of loss. Here are a few things we should all keep in mind.
A sense of mindful self-awareness
As we work through our grief, a mindful self-awareness can help us identify our emotions and see them as part of the grieving process. Simply anticipating emotions can lessen the impact of them when they come. As they come on, try to name the emotion, e.g., “I am so sad,” and feel the experience in the body. The sadness can be cathartic, and by focusing on the body and not the head, we can also drop the sometimes healthy, sometimes unhealthy rants and ruminations that can accompany these events. If we experience the emotions with mindful self-awareness, we can see our emotions as part of a healing, grieving process, and we will likely be able to handle them more gracefully.
In the days after the death of my nursing colleague, my sad mood would be interrupted with flares of anger triggered by thoughts of those not wearing masks or spreading misinformation. Moving my thoughts to the emotions, I would say to myself, “I am really angry, and I am angry because of these deaths.” I felt the recognition of the emotions helped me better ride the big waves on the grieving journey.
Counter to the thinking of the 20th century, research by George Bonanno at Columbia University found that the majority of bereavement is met with resilience. We will be sad, we might have moments of anger or denial or fear, but for most of us, despite the gravity of the loss, our innate resilience will lead about 50-80% of us to recover to near our baseline in months. It is nice to know we are not repressing things if we don’t pass through all the stages postulated by Elizabeth Kubler-Ross, the dominate paradigm in the field.1
For those grieving, this idea of resilience being the norm can provide reassurance during tough moments. While our degree of resilience will depend on our loss and our circumstance, the work of Lucy Hone, PhD, suggests that resilience can be fostered. Many of the negative feelings we experience have a flip side we can seek out. We can be grateful for what remains and what the departed has left us with. We can aid in our grieving journey by using many of the resources available from UC Berkeley’s Greater Good in Action (https://ggia.berkeley.edu/).
While most grief is met with resilience, complicated grieving with persistent negative moods and emotions is common. We should consider seeking professional help if our emotions and pattern of thought continue to feel unhealthy.
Meaning and wisdom, not acceptance
Another change in our understanding of grief is this: Instead of “acceptance” being seen as the end result of grieving, meaning and wisdom are now recognized as the outcomes. Research has found that efforts to find meaning in loss facilitates the grieving process. As time passes and our sadness lessens, the loved one doesn’t leave us but stays with us as a better understanding of the beauty and complexity of life. The loss, through grieving, is transformed to wisdom that will guide us through future challenges and help us make sense of the world.
Last week, masked and robed and with an iPad in hand so the family could join the conversation, I was talking to Ms. B who is hospitalized with COVID-19. She said, “I just keep thinking, ‘Why is this happening to me? To all of us?’ And then I realized that it is a message from God that we need to do a better job of taking care of each other, and I suddenly felt a little better. What do you think, Dr. Hass?”
“Wow,” I said. “Thank you for sharing that. There is definitely some truth there. There is a lot to learn from the pandemic about how we care for each other. I need to keep that in mind when I start feeling down.”
So much is going on now: climate change, racial violence, frightening political dysfunction, and a global pandemic that has upended our daily routines and the economy. It is hard to keep track of all the loss and uncertainty. We might not know why feelings of sadness, anger and anxiety come on, but if we can meet these emotions with mindful equanimity, see them as part of our intrinsic healing process and keep in mind that our path will likely be towards one of wisdom and sense-making, we can better navigate these profoundly unsettling times.
Just as sadness is not grief, joy alone does not lead to happiness. A happy life comes as much from meaning as joy. While unbridled joy might be in short supply, our grief, our work as hospitalists with the suffering, and confronting the many problems our world faces gives us the opportunity to lead a meaningful life. If we couple this search for meaning with healthy habits that promote wellbeing, such as hugs, investing in relationships, and moving our body in the natural world, we can survive these crazy times and be wiser beings as a result of our experiences.
Dr. Hass is a hospitalist at Sutter East Bay Medical Group in Oakland, Calif. He is a member of the clinical faculty at the University of California, Berkeley-UC San Francisco joint medical program, and an adviser on health and health care at the Greater Good Science Center at UC Berkeley.
Reference
1. Bonanno GA, and Boerner K. The stage theory of grief. JAMA. 2007;297(24):2692-2694. doi:10.1001/jama.297.24.2693-a.
Reversal agents curb DOAC-related bleeding but deaths still high
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
Agents that reverse the effect of direct oral anticoagulants (DOACs) are highly effective in patients with severe bleeding, but mortality rates remain high despite their use, a meta-analysis shows.
Effective hemostasis was achieved in 78.5% of patients treated with a reversal agent, whereas failure to achieve hemostasis was associated with more than a threefold higher relative risk for death (relative risk, 3.63; 95% confidence interval, 2.56-5.16).
“This has implications in practice because it emphasizes the need for achieving effective hemostasis, if not with only one agent, trying other agents or treatment modalities, because it is a strong predictor of survival,” lead author Antonio Gómez-Outes, MD, PhD, said in an interview.
The bad news, he said, is that the mortality rate was still significant, at 17.7%, and approximately half of patients with DOAC-related severe intracranial bleeding survived with long-term moderate/severe disability.
“The lesson is to prevent these bleeding events because once they appear, even if you give an antidote, the outcome is poor, particularly for intracranial bleeding,” said Dr. Gómez-Outes, division of pharmacology and clinical drug evaluation, Spanish Agency for Medicines and Medical Devices, Madrid.
To put this in context, mortality rates were close to 50% after intracranial bleeding a decade ago when there were no antidotes or reversal agents, he observed. “So to some extent, patient care has improved, and the outcome has improved, but there is a long road to improve regarding disability.”
More than 100,000 DOAC-related major bleeding cases occur each year in the United States and European Union, Dr. Gómez-Outes said, and about half are severe enough to require hospitalization and potentially the use of a reversal agent. These include idarucizumab (Praxbind) for dabigatran reversal and prothombin complex concentrates (4CCC) or andexanet alpha (Andexxa) for reversal of direct factor Xa inhibitors like rivaroxaban, apixaban, and edoxaban.
As reported in the June 22 issue of the Journal of the American College of Cardiology, the meta-analysis comprised 4,735 patients (mean age, 77 years; 57% male) with severe DOAC-related bleeding who received 4PCC (n = 2,688), idarucizumab (n = 1,111), or andexanet (n = 936) in 60 studies between January 2010 and December 2020.
Atrial fibrillation (AFib) was the most common reason for use of a DOAC (82%), followed by venous thromboembolism (14%). Rivaroxaban was used in 36%, apixaban in 32%, dabigatran in 31%, and edoxaban in 1%.
The index bleeding event was intracranial hemorrhage (ICH) in 55%. Anticoagulation was restarted in 57% of patients an average of 11 days after admission.
Mortality rates were 20.2% in patients with ICH and 15.4% in those with extracranial bleeding. There were no differences in death rates by reversal agent used, type of study, risk for bias, or study sponsorship in meta-regression analysis.
Rebleeding occurred in 13.2% of patients; 82.0% of these events were described as an ICH, and 78.0% occurred after anticoagulation was restarted.
The overall rate of thromboembolism was 4.6%. The risk was particularly high with andexanet, at 10.7%, and relatively low with idarucizumab (3.8%) and 4PCC (4.3%), the authors note.
“Our meta-analysis suggests specific reversal with andexanet is not superior to unspecific reversal with 4PCC, and that’s good news because many centers, in many countries, have no access to specific antidotes that are more costly,” Dr. Gómez-Outes said. “4PCC is an effective and relatively safe drug, so it’s still a good option for these patients.”
Labeling for andexanet includes a warning for thromboembolic events, but in the absence of direct comparisons, the findings should be interpreted with caution, he added. Further insights are expected from an ongoing randomized trial of andexanet and standard of care in 900 patients who present with acute ICH less than 15 hours after taking an oral factor Xa inhibitor. The preliminary completion date is set for 2023.
“The meta-analysis raises awareness about the rates of mortality and thromboembolism after reversal agent administration, although understanding the implications of these data is challenging,” Christopher Granger, MD, and Sean P. Pokomey, MD, MBA, Duke University Medical Center, Durham, N.C., say in an accompanying editorial.
The fact that failure to achieve hemostasis was associated with death is expected and might be related to the way hemostasis was defined, rather than the actual failure of the hemostatic treatments, they suggest. “The prothrombotic effects of each agent, including andexanet, need to be better understood, as clinicians work toward including reversal agents into algorithms for bleeding management.”
Effective hemostasis was defined in the studies through various methods as: “Excellent/good” using the Sarode and ANNEXA-4 scales; “yes” in the International Society on Thrombosis and Hemostasis Scale; and with other scales and through clinical judgment.
Although the size of the meta-analysis dwarfs previous reviews, the editorialists and authors point out that 47 of the 60 studies were retrospective, only two had control groups, and 45 had a high risk for bias.
In general, there was also poor reporting of key clinical data, such as postbleeding anticoagulation management, and a limitation of the mortality analysis is that it was based in selected patients with effective hemostasis assessed within 48 hours, which may not capture early deaths, the authors note.
“The morbidity and mortality from ischemic strokes as a result of undertreatment of stroke prevention in patients with AFib continue to dwarf the bleeding related mortality among patients with AFib and on DOACs, and thus the number one priority is to treat nearly all patients with AFib with a DOAC,” Dr. Granger and Dr. Pokomey conclude. “The availability of reversal agents for DOACs should provide reassurance, with another tool in our armamentarium, to providers to prescribe OACs for stroke prevention.”
No funding/grant support was received to conduct the study. Coauthor Ramón Lecumberri has received personal fees from Boehringer Ingelheim and Bristol Myers Squibb outside the submitted work. All other authors report no relevant financial relationships. Dr. Granger has received research and consulting fees from Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Bayer, Janssen, Boston Scientific, Apple, AstraZeneca, Novartis, AbbVie, Biomed, CeleCor, GSK, Novartis, Medtronic, Merck, Novo Nordisk, Philips, Rho, and the U.S. Food and Drug Administration. Dr. Pokomey has received modest consulting support from Bristol Myers Squibb, Pfizer, Boston Scientific, Medtronic, Janssen, and Zoll; modest research support from Gilead, Boston Scientific, Bristol Myers Squibb, Pfizer, and Janssen; and significant research support from the FDA.
A version of this article first appeared on Medscape.com.
Supreme Court upholds Affordable Care Act
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
The challengers were comprised of 18 GOP-dominated states, led by Texas, that took issue with the ACA’s individual mandate – which required most Americans to have health insurance or pay a tax penalty.
But Congress reduced the penalty to zero in 2017. Challengers argued that without the mandate, the rest of the law should be scrapped, too. The court ruled that eliminated the harm the states were claiming.
“To have standing, a plaintiff must ‘allege personal injury fairly traceable to the defendant’s allegedly unlawful conduct and likely to be redressed by the requested relief,’” the majority wrote. “No plaintiff has shown such an injury ‘fairly traceable’ to the ‘allegedly unlawful conduct’ challenged here.”
Justice Stephen Breyer authored the opinion. Justices Samuel Alito and Neil Gorsuch dissented.
The decision said that the mandate in question did not require the 18 states that brought the complaint to pay anything, and therefore they had no standing.
President Joe Biden has said he plans to build on the ACA – which was enacted while he was vice president – to offer coverage to more Americans.
This marks the third time the Supreme Court spared the Obama-era law from GOP attacks. The mandate was also upheld in 2012 in a 5 to 4 ruling.
American Medical Association president Gerald Harmon, MD, also called for building on the ruling to expand the law.
“With yet another court decision upholding the ACA now behind us, we remain committed to strengthening the current law and look forward to policymakers advancing solutions to improve the ACA,” Dr. Harmon said in a statement. “The AMA will continue working to expand access to health care and ensure that all Americans have meaningful, comprehensive, and affordable health coverage to improve the health of the nation.”
House Speaker Nancy Pelosi (D-Calif.), a longtime advocate for the ACA, called the decision a “landmark victory for Democrats.”
“Thanks to the tireless advocacy of Americans across the country and Democrats in Congress, the Affordable Care Act endures as a pillar of American health and economic security alongside Medicare, Medicaid and Social Security,” she said in a statement.
Senate Majority Leader Chuck Schumer (D-N.Y.) also celebrated the ruling.
“The Affordable Care Act has won. The Supreme Court has just ruled: the ACA is here to stay and now we’re going to try to make it bigger and better,” he said, according to CNN. “For more than a decade, the assault on our health care law was relentless from Republicans in Congress, from the executive branch itself and from Republican attorneys general in the courts. Each time in each arena, the ACA has prevailed.”
This article was updated June 17, 2021.
A version of this article first appeared on WebMD.com.
AHA: Don’t delay COVID shot while CDC reviews myocarditis cases
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.











