User login
How best to manage treatment-resistant depression?
• While lithium produces significant remission rates in augmentation efforts for treatment-resistant depression, it is more likely to cause side effects than many other psychotropic agents. A
• Medication and cognitive therapy are equally effective when augmenting antidepressant therapy; cognitive therapy, however, takes longer to achieve remission. B
• Since the efficacy of many agents is similar when augmenting treatment, it’s important to factor the cost of the medication, side effects, and patient preference into the decision process. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The sobering truth about major depression is that too often it goes unrecognized or undertreated.1,2 And even when it is treated correctly, up to 34% of patients fail to respond to treatment.3 In the United States alone, the lifetime prevalence of the disease is 16.2%, and more than 6% of adults experience symptoms of major depression in any given year.2 Obviously, we need to do more for these patients.
Treatment-resistant depression has been defined as the failure to achieve remission after continuous therapy for about 6 to 12 weeks with an adequate dose of a single antidepressant.4 Remission is typically defined as a 50% reduction of scores on depression severity scales, with the 17-item Hamilton Rating Scale of Depression (HRSD17) and the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR16) being the most often used. An adequate dose is the lowest effective dose that doesn’t cause intolerable side effects.
What are our best options for treatment-resistant depression?
To answer this question, we reviewed all English language studies in PubMed or Medline that were performed among adults using the search terms “augmentation, antidepressants, major depression.” We excluded studies involving patients with comorbid anxiety, bipolar disorder, or other major mental illnesses.
Based on our review of the literature, we found support for several augmentative treatments for patients with treatment-resistant depression (TABLE). Of note, though: Most of these studies were randomized trials dating back nearly 2 decades and had limitations. Most were not blinded, nor did they have consistent placebo controls. The studies were typically small (albeit frequently still showing efficacy for the agent despite lower statistical power) and of relatively short duration (typically 6-14 weeks). There were few studies that looked at treatment approaches over longer periods or that considered indications and timelines for tapering of medications once remission had been achieved. A major exception to the rule was the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, which we’ll discuss below.5
The research points to several viable options: Clinicians can switch antidepressants, augment these agents by adding others—usually nonantidepressants—or completely change the therapeutic approach. Since most of the research discusses augmentation, we’ll focus on that here. Because the decision to use electroconvulsive therapy, vagus nerve stimulators, or other nonpharmaceutical approaches is made in consultation with a psychiatrist, we will not discuss these options.
TABLE
How these agents compare for treatment-resistant depression
| Agent/approach | Remission rate | Starting dose | Titrated to… | Frequency of side effects | SOR rating |
|---|---|---|---|---|---|
| Lithium5-9 | 53%-60% | 300 mg BID-TID | Doses of 800-1500 mg and lithium level of 0.5-1 mmol/L | >25% | A |
| Thyroid supplementation10-13 | 25%-59% | 20-50 mcg | Often not titrated | 11%-25% | A |
| Aripiprazole14-17 | 25%-37% | 2-5 mg | 15-20 mg | 16%-25% | A |
| Olanzapine/fluoxetine18 | 25% | Fixed dose | Fixed dose | 10%-40% | A |
| Risperidone19-21 | 25%-71% | 0.25-1 mg | 0.5-3 mg | 6%-10% | A |
| Quetiapine22,23 | 36%-49% | 25-200 mg | 100-600 mg | >25% | B |
| Mirtazapine24 | 45% | 15 mg | 30 mg | 6%-10% | B |
| Cognitive therapy34 | 23% | NA | NA | 0%-5% | B |
| Folic acid (Leucovorin)26 | 18% | 15 mg | 30 mg | 11%-25% | C |
| SAMe27 | 43% | 800 mg | 1600 mg | 50% | C |
| NA, not applicable; SAMe, S-adenosyl-L-methionine; SOR, strength of recommendation. | |||||
Lithium: Good results, but with side effects
Lithium is one of the oldest and most well studied agents.6-9 Most studies used 900 mg divided into 3 daily doses and titrated to lithium plasma levels of 0.5 to 1 mmol/L. Unfortunately, though, lithium is more likely to cause side effects than many other psychotropic agents (>25%).5-9 And while the research has found that lithium produces significant remission rates, it’s not very effective in patients who have failed multiple antidepressant trials, with only 12.5% to 15.9% of patients in that cohort achieving remission.10,11
Thyroid hormone has only mild side effects
Low-dose thyroid supplementation has been used for decades in euthyroid patients with treatment-resistant depression; remission rates range from 25% to 59%.10-13 Most studies used a low fixed-dose therapy between 25 and 50 mcg daily; side effects were mild and occurred at rates similar to placebo.13
Atypical antipsychotics: Many pluses, but weight gain is an issue
Atypical antipsychotics are an attractive alternative to typical antipsychotics for treatment-resistant depression. They are much less likely to cause extrapyramidal symptoms, tardive dyskinesia, and other motor symptoms, but as a trade-off they often cause weight gain, abnormal glucose metabolism, dyslipidemia, and hyperprolactinemia.
Aripiprazole (Abilify) is 1 of 2 medications approved by the Food and Drug Administration (FDA) for treatment-resistant depression. Studies have shown remission rates of 25% to 37%, with side effects in 16% to 25% of patients.14-16 Akathisia, the major side effect, can be reduced by lowering the starting dose to 2.5 mg.17
Olanzapine, combined with fluoxetine in a fixed-dose pill (Symbyax), is the other FDA-approved agent for treatment-resistant depression. Trivedi and colleagues showed a remission rate of 25.5% and side effects ranging from 10% to 40%.18
Risperidone (Risperdal) rivals the efficacy of the medications previously discussed, but starting doses and titration schedules vary widely, making it difficult to determine which treatment course would be most efficacious.19-21
Quetiapine (Seroquel) has produced mixed results in treatment-resistant patients. That may have been because some studies used lower daily doses—25 to 100 mg—vs 150 to 600 mg in other trials.22 Bauer found higher remission rates compared to placebo (36% vs 24%)23 while Garakani22 did not (49% for quetiapine vs. 63% for placebo when using an intention-to-treat analysis; a similar lack of efficacy was found if those who dropped out of the study were excluded; P<0.29). Garakani also found that dry mouth, sedation, and other side effects occurred in up to 76% of patients. (Of note: Most studies of atypical antipsychotics are industry funded.)
Mirtazapine has not been well studied
Unfortunately, there are very little data on mirtazapine (Remeron). When the drug was added to ongoing antidepressant therapy, a single double-blind, randomized controlled trial found significantly better response rates compared with placebo.24 One advantage of the drug was that it helped relieve the sexual side effects of ongoing selective serotonin reuptake inhibitor (SSRI) therapy.25
Folic acid and SAMe also haven’t been well studied
Up to 50% of Americans have low levels of central nervous system L-methylfolate, which is a key co-factor in monoamine neurotransmitter production. Although lower plasma folate has been linked to depression, folate supplementation as a primary treatment for major depression has not been well studied and its use in treatment-resistant depression is limited to 1 study by Alpert and colleagues.26 Using an open-label, nonplacebo-controlled design in which folinic acid—an activated form of folic acid—was compared with placebo, researchers found remission rates of 18%, which is not significantly higher than the placebo response seen in other studies.
Similarly, there’s limited research on S-adenosyl-L-methionine (SAMe). Using a similar open-label, nonplacebo-controlled design, Alpert found a 43% remission rate in patients with treatment-resistant depression. Side effects occurred in up to half of patients, prompting 6.6% of patients to leave the study.27
Omega-3 fatty acids: The news is mixed
Data are contradictory on the value of omega-3 fatty acid for major depression28,29 and the evidence to support its use in treatment-resistant depression is likewise limited and contradictory.30 Until the data are more consistent and robust, it’s unclear whether omega-3 fatty acid supplementation can benefit patients.
Exercise helps
Some studies suggest that exercise can have a dose-responsive effect on clinical depression.31 As a result, the Treatment with Exercise Augmentation for Depression (TREAD) trial is underway to examine whether it can augment drug therapy. Preliminary evidence suggests that 30 minutes of aerobic exercise most days of the week can be effective.32,33 (For more on exercise, see “Does exercise alleviate symptoms of depression?”)
Cognitive therapy helps patients, and there are no side effects
The STAR*D study showed equivalent efficacy in achieving remission (23% vs. 33%) when comparing augmentation with cognitive therapy and augmentation with medications, with a low rate of side effects seen for cognitive therapy.34 Notably, it took longer to achieve remission (55 vs 40 days) when cognitive therapy was added to antidepressants. (For more on the STAR*D trial, see “What we learned from the STAR*D trial”) It’s also worth mentioning that other researchers did not find similar results for augmentation when they compared cognitive behavioral and brief supportive psychotherapy with medication therapy only.35
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study is noteworthy because of its size, duration, design, and impact on the treatment of depression.5 This was a multicenter study of 3671 patients with major unipolar depression. Remission was determined by 2 rating scales—HRSD17 and QIDS-SR16*—and side effects were measured at each visit. There was no placebo control, no consideration for atypical antipsychotic medications or other therapies, and randomization was limited.
The FIGURE below shows how STAR*D was structured. In Level 1, patients were treated with citalopram (Celexa). If they didn’t go into remission, they were encouraged to proceed to Level 2, which involved 3 arms: switching agents, augmenting agents, or cognitive therapy. Patients chose which arm they wanted and were randomized to the specific medications used in that level. Switch agents included bupropion SR (Wellbutrin SR), sertraline (Zoloft), and venlafaxine XR (Effexor XR). Augmentation was with buspirone (BuSpar) and bupropion. Cognitive therapy was with or without continued citalopram with a second augmentation level of bupropion SR or venlafaxine XR. Level 3 also involved switch and augmentation arms using nortriptyline (Aventyl, Pamelor) or mirtazapine (Remeron) for switch agents and lithium or thyroxine for augmentation. Level 4 involved switching to tranylcypromine (Parnate) or venlafaxine XR plus mirtazapine.
Remission rates were 36.8% for those in Level 1, 30.6% in Level 2, 13.7% in Level 3, and 13% in Level 4. The cumulative remission rate was 67%. While remission rates for switch strategies in Levels 2 and 3 appeared to be lower for pharmacologic agents compared with augmentation strategies (27% vs 35% in Level 2 and 10.7% vs 20.5% in Level 3), the sample sizes were too small for the differences to be statistically significant, and the study was underpowered to establish the superiority of switch vs augmentation strategies.
Finally, it’s important to mention that patients of primary care physicians had similar remission rates, when compared with psychiatrists’ patients.42 Keep in mind, however, that the care provided in the STAR*D trial was structured and protocol-driven. After randomization into 1 medication group or another, dose adjustments were standardized at set intervals and based on inadequate response to validated depression severity assessment tools. Outcomes were patient-based and uniformly applied. This study structure may explain why remission rates were identical regardless of the specialty of the treating physician.
*The 17-item Hamilton Rating Scale of Depression and the 16-item Quick Inventory of Depressive Symptomatology–Self Report.
FIGURE
Structure of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D)
These agents don’t appear helpful in augmentation efforts
Several other agents have been studied with no effect on remission rates. These include pindolol,36 modafinil,37 buspirone, lamotrigine,38 stimulants, and estrogen replacement.
Consider side effects, cost, and patient preference
Because most studies showed that the efficacy of the tested drugs was similar, how do you decide which augmenting agent to prescribe? The usual standard is lithium, which offers rates of remission that are high, but not statistically significantly better when compared with thyroid supplementation and cognitive therapy. Quetiapine, while not more efficacious, may lower scores on depression rating scales more quickly than lithium.39 Additionally, the STAR*D trial suggests that many agents may be used in augmentation with similar results.
In the final analysis, the family physician has to consider factors other than efficacy. You also have to factor in the costs of medicines and lab testing, patient preference, and side effects. Lithium is most likely to cause side effects. Atypical antipsychotics seem to have lower short-term side effect profiles with efficacy similar to cognitive therapy. However, the potential drawbacks of antipsychotics, including aberrations in glucose metabolism, weight, and lipid profiles, are not typically seen in short-term studies. Obviously, more long-term studies are necessary before 1 agent can be deemed superior.
When should you stop therapy?
That question still doesn’t have a definitive answer. The STAR*D trial found that patients who achieved full remission were less likely to relapse/worsen than those who had only a partial response. The time to relapse ranged from 2.5 to 4.5 months and was shorter for those patients requiring 2 or more levels of treatment. As there was no control of the therapeutic interventions used during this time, we can’t be certain about what caused the relapse, but burden of disease, income levels, and ethnicity all played roles in symptom severity, decreasing remission rates, and increasing relapse rates.40,41
While we cannot identify the ideal or even preferred duration of augmentation in patients with treatment-resistant depression, it seems clear that relapse is extremely common and tends to occur relatively early after achieving remission.
CORRESPONDENCE Paul Hicks, MD, Department of Family and Community Medicine, 1450 North Cherry Avenue, Tucson, AZ 85719; [email protected]
1. Kocsis JH, Gelenberg AJ, Rothbaum B, et al. Chronic forms of major depression are still undertreated in the 21st century: systematic assessment of 801 patients presenting for treatment. J Affect Disord. 2008;110:55-61.
2. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095-3105.
3. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179-200.
4. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649-659.
5. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients who required one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917.
6. Cappiello A, McDougle CJ, Delgado PL, et al. Lithium and desipramine versus desipramine alone in the treatment of severe major depression: a preliminary study. Int Clin Psychopharmacol. 1998;13:191-198.
7. Katona CL, Abou-Saleh MT, Harrison DA, et al. Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Br J Psychiatry. 1995;166:80-86.
8. Bauer M, Bschor T, Kunz D, et al. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000;157:1429-1435.
9. Joffe RT, Singer W, Levitt AJ, et al. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry. 1993;50:387-393.
10. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530.
11. Nierenberg AA, Papakostas GI, Petersen T, et al. Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J Clin Psychopharmacol. 2003;23:92-95.
12. Aronson R, Offman HJ, Joffe RT, et al. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry. 1996;53:842-848.
13. Joffe RT, Sokolov ST, Levitt AJ. Lithium and triiodothyronine augmentation of antidepressants. Can J Psychiatry. 2006;51:791-793.
14. Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197-206.
15. Trivedi MH, Thase ME, Fava M, et al. Adjunctive aripiprazole in major depressive disorder: analysis of efficacy and safety in patients with anxious and atypical features. J Clin Psychiatry. 2008;69:1928-1936.
16. Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156-165.
17. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry. 2005;66:1216-1220.
18. Trivedi MH, Thase ME, Osuntokun O, et al. An integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depression. J Clin Psychiatry. 2009;70:387-396.
19. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16:21-30
20. Mahmoud RA, Pandina GJ, Turkoz I, et al. Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med. 2007;147:593-602
21. Keitner GI, Garlow SJ, Ryan CE, et al. A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J Psychiatr Res. 2009;43:205-214.
22. Garakani A, Martinez JM, Marcus S, et al. A randomized, double-blind, and placebo-controlled trial of quetiapine augmentation of fluoxetine in major depressive disorder. Int Clin Psychopharmacol. 2008;23:269-275.
23. Bauer M, Pretorius HW, Constant EL, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry. 2009;70:540-549.
24. Carpenter LL, Yasmin S, Price LH. Double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51:183-188.
25. Ozmenler NK, Karlidere T, Bozkurt A, et al. Mirtazapine augmentation in depressed patients with sexual dysfunction due to selective serotonin reuptake inhibitors. Hum Psychopharmacol. 2008;23:321-326.
26. Alpert JE, Mischoulon D, Rubenstein GE, et al. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14:33-38.
27. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-Lmethionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24:661-664.
28. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477-479.
29. Carney RM, Freedland KE, Rubin EH, et al. Depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;302:1651-1657.
30. Peet M, Horrobin DE. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913-919.
31. Mead GE, Morley W, Campbell P, et al. Exercise for depression. Cochrane Database Syst Rev. 2009;(3):CD004366.-
32. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12:205-213.
33. Trivedi MH, Greer TL, Grannemann BD, et al. TREAD: Treatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials. 2006;3:291-305.
34. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739-752.
35. Kocsis JH, Gelenberg AJ, Rothbaum BO, et al. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009;66:1178-1188.
36. Perry EB, Berman RM, Sanacora G, et al. Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitors: a double-blind, randomized, controlled trial. J Clin Psychiatry. 2004;65:238-243.
37. Rasmussen NA, Schrøder P, Olsen LR, et al. Modafi nil augmentation in depressed patients with partial response to antidepressants: a pilot study on self-reported symptoms covered by the Major Depression Inventory (MDI) and the Symptom Checklist (SCL-92). Nord J Psychiatry. 2005;59:173-178.
38. Santos MA, Rocha FL, Hara C. Efficacy and safety of antidepressant augmentation with lamotrigine in patients with treatment-resistant depression: a randomized, placebo-controlled, double-blind study. Prim Care Companion J Clin Psychiatry. 2008;10:187-190.
39. Dorée JP, Des Rosiers J, Lew V, et al. Quetiapine augmentation of treatment-resistant depression: a comparison with lithium. Curr Med Res Opin. 2007;23:333-341.
40. Lesser IM, Castro DB, Gaynes BN, et al. Ethnicity/race and outcome in the treatment of depression: results from STAR*D. Med Care. 2007;45:1043-1051.
41. Lesser IM, Leuchter AF, Trivedi MH, et al. Characteristics of insured and noninsured outpatients with depression in STAR(*) D. Psychiatr Serv. 2005;56:995-1004.
42. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23:551-560.
• While lithium produces significant remission rates in augmentation efforts for treatment-resistant depression, it is more likely to cause side effects than many other psychotropic agents. A
• Medication and cognitive therapy are equally effective when augmenting antidepressant therapy; cognitive therapy, however, takes longer to achieve remission. B
• Since the efficacy of many agents is similar when augmenting treatment, it’s important to factor the cost of the medication, side effects, and patient preference into the decision process. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The sobering truth about major depression is that too often it goes unrecognized or undertreated.1,2 And even when it is treated correctly, up to 34% of patients fail to respond to treatment.3 In the United States alone, the lifetime prevalence of the disease is 16.2%, and more than 6% of adults experience symptoms of major depression in any given year.2 Obviously, we need to do more for these patients.
Treatment-resistant depression has been defined as the failure to achieve remission after continuous therapy for about 6 to 12 weeks with an adequate dose of a single antidepressant.4 Remission is typically defined as a 50% reduction of scores on depression severity scales, with the 17-item Hamilton Rating Scale of Depression (HRSD17) and the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR16) being the most often used. An adequate dose is the lowest effective dose that doesn’t cause intolerable side effects.
What are our best options for treatment-resistant depression?
To answer this question, we reviewed all English language studies in PubMed or Medline that were performed among adults using the search terms “augmentation, antidepressants, major depression.” We excluded studies involving patients with comorbid anxiety, bipolar disorder, or other major mental illnesses.
Based on our review of the literature, we found support for several augmentative treatments for patients with treatment-resistant depression (TABLE). Of note, though: Most of these studies were randomized trials dating back nearly 2 decades and had limitations. Most were not blinded, nor did they have consistent placebo controls. The studies were typically small (albeit frequently still showing efficacy for the agent despite lower statistical power) and of relatively short duration (typically 6-14 weeks). There were few studies that looked at treatment approaches over longer periods or that considered indications and timelines for tapering of medications once remission had been achieved. A major exception to the rule was the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, which we’ll discuss below.5
The research points to several viable options: Clinicians can switch antidepressants, augment these agents by adding others—usually nonantidepressants—or completely change the therapeutic approach. Since most of the research discusses augmentation, we’ll focus on that here. Because the decision to use electroconvulsive therapy, vagus nerve stimulators, or other nonpharmaceutical approaches is made in consultation with a psychiatrist, we will not discuss these options.
TABLE
How these agents compare for treatment-resistant depression
| Agent/approach | Remission rate | Starting dose | Titrated to… | Frequency of side effects | SOR rating |
|---|---|---|---|---|---|
| Lithium5-9 | 53%-60% | 300 mg BID-TID | Doses of 800-1500 mg and lithium level of 0.5-1 mmol/L | >25% | A |
| Thyroid supplementation10-13 | 25%-59% | 20-50 mcg | Often not titrated | 11%-25% | A |
| Aripiprazole14-17 | 25%-37% | 2-5 mg | 15-20 mg | 16%-25% | A |
| Olanzapine/fluoxetine18 | 25% | Fixed dose | Fixed dose | 10%-40% | A |
| Risperidone19-21 | 25%-71% | 0.25-1 mg | 0.5-3 mg | 6%-10% | A |
| Quetiapine22,23 | 36%-49% | 25-200 mg | 100-600 mg | >25% | B |
| Mirtazapine24 | 45% | 15 mg | 30 mg | 6%-10% | B |
| Cognitive therapy34 | 23% | NA | NA | 0%-5% | B |
| Folic acid (Leucovorin)26 | 18% | 15 mg | 30 mg | 11%-25% | C |
| SAMe27 | 43% | 800 mg | 1600 mg | 50% | C |
| NA, not applicable; SAMe, S-adenosyl-L-methionine; SOR, strength of recommendation. | |||||
Lithium: Good results, but with side effects
Lithium is one of the oldest and most well studied agents.6-9 Most studies used 900 mg divided into 3 daily doses and titrated to lithium plasma levels of 0.5 to 1 mmol/L. Unfortunately, though, lithium is more likely to cause side effects than many other psychotropic agents (>25%).5-9 And while the research has found that lithium produces significant remission rates, it’s not very effective in patients who have failed multiple antidepressant trials, with only 12.5% to 15.9% of patients in that cohort achieving remission.10,11
Thyroid hormone has only mild side effects
Low-dose thyroid supplementation has been used for decades in euthyroid patients with treatment-resistant depression; remission rates range from 25% to 59%.10-13 Most studies used a low fixed-dose therapy between 25 and 50 mcg daily; side effects were mild and occurred at rates similar to placebo.13
Atypical antipsychotics: Many pluses, but weight gain is an issue
Atypical antipsychotics are an attractive alternative to typical antipsychotics for treatment-resistant depression. They are much less likely to cause extrapyramidal symptoms, tardive dyskinesia, and other motor symptoms, but as a trade-off they often cause weight gain, abnormal glucose metabolism, dyslipidemia, and hyperprolactinemia.
Aripiprazole (Abilify) is 1 of 2 medications approved by the Food and Drug Administration (FDA) for treatment-resistant depression. Studies have shown remission rates of 25% to 37%, with side effects in 16% to 25% of patients.14-16 Akathisia, the major side effect, can be reduced by lowering the starting dose to 2.5 mg.17
Olanzapine, combined with fluoxetine in a fixed-dose pill (Symbyax), is the other FDA-approved agent for treatment-resistant depression. Trivedi and colleagues showed a remission rate of 25.5% and side effects ranging from 10% to 40%.18
Risperidone (Risperdal) rivals the efficacy of the medications previously discussed, but starting doses and titration schedules vary widely, making it difficult to determine which treatment course would be most efficacious.19-21
Quetiapine (Seroquel) has produced mixed results in treatment-resistant patients. That may have been because some studies used lower daily doses—25 to 100 mg—vs 150 to 600 mg in other trials.22 Bauer found higher remission rates compared to placebo (36% vs 24%)23 while Garakani22 did not (49% for quetiapine vs. 63% for placebo when using an intention-to-treat analysis; a similar lack of efficacy was found if those who dropped out of the study were excluded; P<0.29). Garakani also found that dry mouth, sedation, and other side effects occurred in up to 76% of patients. (Of note: Most studies of atypical antipsychotics are industry funded.)
Mirtazapine has not been well studied
Unfortunately, there are very little data on mirtazapine (Remeron). When the drug was added to ongoing antidepressant therapy, a single double-blind, randomized controlled trial found significantly better response rates compared with placebo.24 One advantage of the drug was that it helped relieve the sexual side effects of ongoing selective serotonin reuptake inhibitor (SSRI) therapy.25
Folic acid and SAMe also haven’t been well studied
Up to 50% of Americans have low levels of central nervous system L-methylfolate, which is a key co-factor in monoamine neurotransmitter production. Although lower plasma folate has been linked to depression, folate supplementation as a primary treatment for major depression has not been well studied and its use in treatment-resistant depression is limited to 1 study by Alpert and colleagues.26 Using an open-label, nonplacebo-controlled design in which folinic acid—an activated form of folic acid—was compared with placebo, researchers found remission rates of 18%, which is not significantly higher than the placebo response seen in other studies.
Similarly, there’s limited research on S-adenosyl-L-methionine (SAMe). Using a similar open-label, nonplacebo-controlled design, Alpert found a 43% remission rate in patients with treatment-resistant depression. Side effects occurred in up to half of patients, prompting 6.6% of patients to leave the study.27
Omega-3 fatty acids: The news is mixed
Data are contradictory on the value of omega-3 fatty acid for major depression28,29 and the evidence to support its use in treatment-resistant depression is likewise limited and contradictory.30 Until the data are more consistent and robust, it’s unclear whether omega-3 fatty acid supplementation can benefit patients.
Exercise helps
Some studies suggest that exercise can have a dose-responsive effect on clinical depression.31 As a result, the Treatment with Exercise Augmentation for Depression (TREAD) trial is underway to examine whether it can augment drug therapy. Preliminary evidence suggests that 30 minutes of aerobic exercise most days of the week can be effective.32,33 (For more on exercise, see “Does exercise alleviate symptoms of depression?”)
Cognitive therapy helps patients, and there are no side effects
The STAR*D study showed equivalent efficacy in achieving remission (23% vs. 33%) when comparing augmentation with cognitive therapy and augmentation with medications, with a low rate of side effects seen for cognitive therapy.34 Notably, it took longer to achieve remission (55 vs 40 days) when cognitive therapy was added to antidepressants. (For more on the STAR*D trial, see “What we learned from the STAR*D trial”) It’s also worth mentioning that other researchers did not find similar results for augmentation when they compared cognitive behavioral and brief supportive psychotherapy with medication therapy only.35
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study is noteworthy because of its size, duration, design, and impact on the treatment of depression.5 This was a multicenter study of 3671 patients with major unipolar depression. Remission was determined by 2 rating scales—HRSD17 and QIDS-SR16*—and side effects were measured at each visit. There was no placebo control, no consideration for atypical antipsychotic medications or other therapies, and randomization was limited.
The FIGURE below shows how STAR*D was structured. In Level 1, patients were treated with citalopram (Celexa). If they didn’t go into remission, they were encouraged to proceed to Level 2, which involved 3 arms: switching agents, augmenting agents, or cognitive therapy. Patients chose which arm they wanted and were randomized to the specific medications used in that level. Switch agents included bupropion SR (Wellbutrin SR), sertraline (Zoloft), and venlafaxine XR (Effexor XR). Augmentation was with buspirone (BuSpar) and bupropion. Cognitive therapy was with or without continued citalopram with a second augmentation level of bupropion SR or venlafaxine XR. Level 3 also involved switch and augmentation arms using nortriptyline (Aventyl, Pamelor) or mirtazapine (Remeron) for switch agents and lithium or thyroxine for augmentation. Level 4 involved switching to tranylcypromine (Parnate) or venlafaxine XR plus mirtazapine.
Remission rates were 36.8% for those in Level 1, 30.6% in Level 2, 13.7% in Level 3, and 13% in Level 4. The cumulative remission rate was 67%. While remission rates for switch strategies in Levels 2 and 3 appeared to be lower for pharmacologic agents compared with augmentation strategies (27% vs 35% in Level 2 and 10.7% vs 20.5% in Level 3), the sample sizes were too small for the differences to be statistically significant, and the study was underpowered to establish the superiority of switch vs augmentation strategies.
Finally, it’s important to mention that patients of primary care physicians had similar remission rates, when compared with psychiatrists’ patients.42 Keep in mind, however, that the care provided in the STAR*D trial was structured and protocol-driven. After randomization into 1 medication group or another, dose adjustments were standardized at set intervals and based on inadequate response to validated depression severity assessment tools. Outcomes were patient-based and uniformly applied. This study structure may explain why remission rates were identical regardless of the specialty of the treating physician.
*The 17-item Hamilton Rating Scale of Depression and the 16-item Quick Inventory of Depressive Symptomatology–Self Report.
FIGURE
Structure of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D)
These agents don’t appear helpful in augmentation efforts
Several other agents have been studied with no effect on remission rates. These include pindolol,36 modafinil,37 buspirone, lamotrigine,38 stimulants, and estrogen replacement.
Consider side effects, cost, and patient preference
Because most studies showed that the efficacy of the tested drugs was similar, how do you decide which augmenting agent to prescribe? The usual standard is lithium, which offers rates of remission that are high, but not statistically significantly better when compared with thyroid supplementation and cognitive therapy. Quetiapine, while not more efficacious, may lower scores on depression rating scales more quickly than lithium.39 Additionally, the STAR*D trial suggests that many agents may be used in augmentation with similar results.
In the final analysis, the family physician has to consider factors other than efficacy. You also have to factor in the costs of medicines and lab testing, patient preference, and side effects. Lithium is most likely to cause side effects. Atypical antipsychotics seem to have lower short-term side effect profiles with efficacy similar to cognitive therapy. However, the potential drawbacks of antipsychotics, including aberrations in glucose metabolism, weight, and lipid profiles, are not typically seen in short-term studies. Obviously, more long-term studies are necessary before 1 agent can be deemed superior.
When should you stop therapy?
That question still doesn’t have a definitive answer. The STAR*D trial found that patients who achieved full remission were less likely to relapse/worsen than those who had only a partial response. The time to relapse ranged from 2.5 to 4.5 months and was shorter for those patients requiring 2 or more levels of treatment. As there was no control of the therapeutic interventions used during this time, we can’t be certain about what caused the relapse, but burden of disease, income levels, and ethnicity all played roles in symptom severity, decreasing remission rates, and increasing relapse rates.40,41
While we cannot identify the ideal or even preferred duration of augmentation in patients with treatment-resistant depression, it seems clear that relapse is extremely common and tends to occur relatively early after achieving remission.
CORRESPONDENCE Paul Hicks, MD, Department of Family and Community Medicine, 1450 North Cherry Avenue, Tucson, AZ 85719; [email protected]
• While lithium produces significant remission rates in augmentation efforts for treatment-resistant depression, it is more likely to cause side effects than many other psychotropic agents. A
• Medication and cognitive therapy are equally effective when augmenting antidepressant therapy; cognitive therapy, however, takes longer to achieve remission. B
• Since the efficacy of many agents is similar when augmenting treatment, it’s important to factor the cost of the medication, side effects, and patient preference into the decision process. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The sobering truth about major depression is that too often it goes unrecognized or undertreated.1,2 And even when it is treated correctly, up to 34% of patients fail to respond to treatment.3 In the United States alone, the lifetime prevalence of the disease is 16.2%, and more than 6% of adults experience symptoms of major depression in any given year.2 Obviously, we need to do more for these patients.
Treatment-resistant depression has been defined as the failure to achieve remission after continuous therapy for about 6 to 12 weeks with an adequate dose of a single antidepressant.4 Remission is typically defined as a 50% reduction of scores on depression severity scales, with the 17-item Hamilton Rating Scale of Depression (HRSD17) and the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR16) being the most often used. An adequate dose is the lowest effective dose that doesn’t cause intolerable side effects.
What are our best options for treatment-resistant depression?
To answer this question, we reviewed all English language studies in PubMed or Medline that were performed among adults using the search terms “augmentation, antidepressants, major depression.” We excluded studies involving patients with comorbid anxiety, bipolar disorder, or other major mental illnesses.
Based on our review of the literature, we found support for several augmentative treatments for patients with treatment-resistant depression (TABLE). Of note, though: Most of these studies were randomized trials dating back nearly 2 decades and had limitations. Most were not blinded, nor did they have consistent placebo controls. The studies were typically small (albeit frequently still showing efficacy for the agent despite lower statistical power) and of relatively short duration (typically 6-14 weeks). There were few studies that looked at treatment approaches over longer periods or that considered indications and timelines for tapering of medications once remission had been achieved. A major exception to the rule was the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, which we’ll discuss below.5
The research points to several viable options: Clinicians can switch antidepressants, augment these agents by adding others—usually nonantidepressants—or completely change the therapeutic approach. Since most of the research discusses augmentation, we’ll focus on that here. Because the decision to use electroconvulsive therapy, vagus nerve stimulators, or other nonpharmaceutical approaches is made in consultation with a psychiatrist, we will not discuss these options.
TABLE
How these agents compare for treatment-resistant depression
| Agent/approach | Remission rate | Starting dose | Titrated to… | Frequency of side effects | SOR rating |
|---|---|---|---|---|---|
| Lithium5-9 | 53%-60% | 300 mg BID-TID | Doses of 800-1500 mg and lithium level of 0.5-1 mmol/L | >25% | A |
| Thyroid supplementation10-13 | 25%-59% | 20-50 mcg | Often not titrated | 11%-25% | A |
| Aripiprazole14-17 | 25%-37% | 2-5 mg | 15-20 mg | 16%-25% | A |
| Olanzapine/fluoxetine18 | 25% | Fixed dose | Fixed dose | 10%-40% | A |
| Risperidone19-21 | 25%-71% | 0.25-1 mg | 0.5-3 mg | 6%-10% | A |
| Quetiapine22,23 | 36%-49% | 25-200 mg | 100-600 mg | >25% | B |
| Mirtazapine24 | 45% | 15 mg | 30 mg | 6%-10% | B |
| Cognitive therapy34 | 23% | NA | NA | 0%-5% | B |
| Folic acid (Leucovorin)26 | 18% | 15 mg | 30 mg | 11%-25% | C |
| SAMe27 | 43% | 800 mg | 1600 mg | 50% | C |
| NA, not applicable; SAMe, S-adenosyl-L-methionine; SOR, strength of recommendation. | |||||
Lithium: Good results, but with side effects
Lithium is one of the oldest and most well studied agents.6-9 Most studies used 900 mg divided into 3 daily doses and titrated to lithium plasma levels of 0.5 to 1 mmol/L. Unfortunately, though, lithium is more likely to cause side effects than many other psychotropic agents (>25%).5-9 And while the research has found that lithium produces significant remission rates, it’s not very effective in patients who have failed multiple antidepressant trials, with only 12.5% to 15.9% of patients in that cohort achieving remission.10,11
Thyroid hormone has only mild side effects
Low-dose thyroid supplementation has been used for decades in euthyroid patients with treatment-resistant depression; remission rates range from 25% to 59%.10-13 Most studies used a low fixed-dose therapy between 25 and 50 mcg daily; side effects were mild and occurred at rates similar to placebo.13
Atypical antipsychotics: Many pluses, but weight gain is an issue
Atypical antipsychotics are an attractive alternative to typical antipsychotics for treatment-resistant depression. They are much less likely to cause extrapyramidal symptoms, tardive dyskinesia, and other motor symptoms, but as a trade-off they often cause weight gain, abnormal glucose metabolism, dyslipidemia, and hyperprolactinemia.
Aripiprazole (Abilify) is 1 of 2 medications approved by the Food and Drug Administration (FDA) for treatment-resistant depression. Studies have shown remission rates of 25% to 37%, with side effects in 16% to 25% of patients.14-16 Akathisia, the major side effect, can be reduced by lowering the starting dose to 2.5 mg.17
Olanzapine, combined with fluoxetine in a fixed-dose pill (Symbyax), is the other FDA-approved agent for treatment-resistant depression. Trivedi and colleagues showed a remission rate of 25.5% and side effects ranging from 10% to 40%.18
Risperidone (Risperdal) rivals the efficacy of the medications previously discussed, but starting doses and titration schedules vary widely, making it difficult to determine which treatment course would be most efficacious.19-21
Quetiapine (Seroquel) has produced mixed results in treatment-resistant patients. That may have been because some studies used lower daily doses—25 to 100 mg—vs 150 to 600 mg in other trials.22 Bauer found higher remission rates compared to placebo (36% vs 24%)23 while Garakani22 did not (49% for quetiapine vs. 63% for placebo when using an intention-to-treat analysis; a similar lack of efficacy was found if those who dropped out of the study were excluded; P<0.29). Garakani also found that dry mouth, sedation, and other side effects occurred in up to 76% of patients. (Of note: Most studies of atypical antipsychotics are industry funded.)
Mirtazapine has not been well studied
Unfortunately, there are very little data on mirtazapine (Remeron). When the drug was added to ongoing antidepressant therapy, a single double-blind, randomized controlled trial found significantly better response rates compared with placebo.24 One advantage of the drug was that it helped relieve the sexual side effects of ongoing selective serotonin reuptake inhibitor (SSRI) therapy.25
Folic acid and SAMe also haven’t been well studied
Up to 50% of Americans have low levels of central nervous system L-methylfolate, which is a key co-factor in monoamine neurotransmitter production. Although lower plasma folate has been linked to depression, folate supplementation as a primary treatment for major depression has not been well studied and its use in treatment-resistant depression is limited to 1 study by Alpert and colleagues.26 Using an open-label, nonplacebo-controlled design in which folinic acid—an activated form of folic acid—was compared with placebo, researchers found remission rates of 18%, which is not significantly higher than the placebo response seen in other studies.
Similarly, there’s limited research on S-adenosyl-L-methionine (SAMe). Using a similar open-label, nonplacebo-controlled design, Alpert found a 43% remission rate in patients with treatment-resistant depression. Side effects occurred in up to half of patients, prompting 6.6% of patients to leave the study.27
Omega-3 fatty acids: The news is mixed
Data are contradictory on the value of omega-3 fatty acid for major depression28,29 and the evidence to support its use in treatment-resistant depression is likewise limited and contradictory.30 Until the data are more consistent and robust, it’s unclear whether omega-3 fatty acid supplementation can benefit patients.
Exercise helps
Some studies suggest that exercise can have a dose-responsive effect on clinical depression.31 As a result, the Treatment with Exercise Augmentation for Depression (TREAD) trial is underway to examine whether it can augment drug therapy. Preliminary evidence suggests that 30 minutes of aerobic exercise most days of the week can be effective.32,33 (For more on exercise, see “Does exercise alleviate symptoms of depression?”)
Cognitive therapy helps patients, and there are no side effects
The STAR*D study showed equivalent efficacy in achieving remission (23% vs. 33%) when comparing augmentation with cognitive therapy and augmentation with medications, with a low rate of side effects seen for cognitive therapy.34 Notably, it took longer to achieve remission (55 vs 40 days) when cognitive therapy was added to antidepressants. (For more on the STAR*D trial, see “What we learned from the STAR*D trial”) It’s also worth mentioning that other researchers did not find similar results for augmentation when they compared cognitive behavioral and brief supportive psychotherapy with medication therapy only.35
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study is noteworthy because of its size, duration, design, and impact on the treatment of depression.5 This was a multicenter study of 3671 patients with major unipolar depression. Remission was determined by 2 rating scales—HRSD17 and QIDS-SR16*—and side effects were measured at each visit. There was no placebo control, no consideration for atypical antipsychotic medications or other therapies, and randomization was limited.
The FIGURE below shows how STAR*D was structured. In Level 1, patients were treated with citalopram (Celexa). If they didn’t go into remission, they were encouraged to proceed to Level 2, which involved 3 arms: switching agents, augmenting agents, or cognitive therapy. Patients chose which arm they wanted and were randomized to the specific medications used in that level. Switch agents included bupropion SR (Wellbutrin SR), sertraline (Zoloft), and venlafaxine XR (Effexor XR). Augmentation was with buspirone (BuSpar) and bupropion. Cognitive therapy was with or without continued citalopram with a second augmentation level of bupropion SR or venlafaxine XR. Level 3 also involved switch and augmentation arms using nortriptyline (Aventyl, Pamelor) or mirtazapine (Remeron) for switch agents and lithium or thyroxine for augmentation. Level 4 involved switching to tranylcypromine (Parnate) or venlafaxine XR plus mirtazapine.
Remission rates were 36.8% for those in Level 1, 30.6% in Level 2, 13.7% in Level 3, and 13% in Level 4. The cumulative remission rate was 67%. While remission rates for switch strategies in Levels 2 and 3 appeared to be lower for pharmacologic agents compared with augmentation strategies (27% vs 35% in Level 2 and 10.7% vs 20.5% in Level 3), the sample sizes were too small for the differences to be statistically significant, and the study was underpowered to establish the superiority of switch vs augmentation strategies.
Finally, it’s important to mention that patients of primary care physicians had similar remission rates, when compared with psychiatrists’ patients.42 Keep in mind, however, that the care provided in the STAR*D trial was structured and protocol-driven. After randomization into 1 medication group or another, dose adjustments were standardized at set intervals and based on inadequate response to validated depression severity assessment tools. Outcomes were patient-based and uniformly applied. This study structure may explain why remission rates were identical regardless of the specialty of the treating physician.
*The 17-item Hamilton Rating Scale of Depression and the 16-item Quick Inventory of Depressive Symptomatology–Self Report.
FIGURE
Structure of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D)
These agents don’t appear helpful in augmentation efforts
Several other agents have been studied with no effect on remission rates. These include pindolol,36 modafinil,37 buspirone, lamotrigine,38 stimulants, and estrogen replacement.
Consider side effects, cost, and patient preference
Because most studies showed that the efficacy of the tested drugs was similar, how do you decide which augmenting agent to prescribe? The usual standard is lithium, which offers rates of remission that are high, but not statistically significantly better when compared with thyroid supplementation and cognitive therapy. Quetiapine, while not more efficacious, may lower scores on depression rating scales more quickly than lithium.39 Additionally, the STAR*D trial suggests that many agents may be used in augmentation with similar results.
In the final analysis, the family physician has to consider factors other than efficacy. You also have to factor in the costs of medicines and lab testing, patient preference, and side effects. Lithium is most likely to cause side effects. Atypical antipsychotics seem to have lower short-term side effect profiles with efficacy similar to cognitive therapy. However, the potential drawbacks of antipsychotics, including aberrations in glucose metabolism, weight, and lipid profiles, are not typically seen in short-term studies. Obviously, more long-term studies are necessary before 1 agent can be deemed superior.
When should you stop therapy?
That question still doesn’t have a definitive answer. The STAR*D trial found that patients who achieved full remission were less likely to relapse/worsen than those who had only a partial response. The time to relapse ranged from 2.5 to 4.5 months and was shorter for those patients requiring 2 or more levels of treatment. As there was no control of the therapeutic interventions used during this time, we can’t be certain about what caused the relapse, but burden of disease, income levels, and ethnicity all played roles in symptom severity, decreasing remission rates, and increasing relapse rates.40,41
While we cannot identify the ideal or even preferred duration of augmentation in patients with treatment-resistant depression, it seems clear that relapse is extremely common and tends to occur relatively early after achieving remission.
CORRESPONDENCE Paul Hicks, MD, Department of Family and Community Medicine, 1450 North Cherry Avenue, Tucson, AZ 85719; [email protected]
1. Kocsis JH, Gelenberg AJ, Rothbaum B, et al. Chronic forms of major depression are still undertreated in the 21st century: systematic assessment of 801 patients presenting for treatment. J Affect Disord. 2008;110:55-61.
2. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095-3105.
3. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179-200.
4. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649-659.
5. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients who required one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917.
6. Cappiello A, McDougle CJ, Delgado PL, et al. Lithium and desipramine versus desipramine alone in the treatment of severe major depression: a preliminary study. Int Clin Psychopharmacol. 1998;13:191-198.
7. Katona CL, Abou-Saleh MT, Harrison DA, et al. Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Br J Psychiatry. 1995;166:80-86.
8. Bauer M, Bschor T, Kunz D, et al. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000;157:1429-1435.
9. Joffe RT, Singer W, Levitt AJ, et al. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry. 1993;50:387-393.
10. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530.
11. Nierenberg AA, Papakostas GI, Petersen T, et al. Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J Clin Psychopharmacol. 2003;23:92-95.
12. Aronson R, Offman HJ, Joffe RT, et al. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry. 1996;53:842-848.
13. Joffe RT, Sokolov ST, Levitt AJ. Lithium and triiodothyronine augmentation of antidepressants. Can J Psychiatry. 2006;51:791-793.
14. Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197-206.
15. Trivedi MH, Thase ME, Fava M, et al. Adjunctive aripiprazole in major depressive disorder: analysis of efficacy and safety in patients with anxious and atypical features. J Clin Psychiatry. 2008;69:1928-1936.
16. Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156-165.
17. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry. 2005;66:1216-1220.
18. Trivedi MH, Thase ME, Osuntokun O, et al. An integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depression. J Clin Psychiatry. 2009;70:387-396.
19. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16:21-30
20. Mahmoud RA, Pandina GJ, Turkoz I, et al. Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med. 2007;147:593-602
21. Keitner GI, Garlow SJ, Ryan CE, et al. A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J Psychiatr Res. 2009;43:205-214.
22. Garakani A, Martinez JM, Marcus S, et al. A randomized, double-blind, and placebo-controlled trial of quetiapine augmentation of fluoxetine in major depressive disorder. Int Clin Psychopharmacol. 2008;23:269-275.
23. Bauer M, Pretorius HW, Constant EL, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry. 2009;70:540-549.
24. Carpenter LL, Yasmin S, Price LH. Double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51:183-188.
25. Ozmenler NK, Karlidere T, Bozkurt A, et al. Mirtazapine augmentation in depressed patients with sexual dysfunction due to selective serotonin reuptake inhibitors. Hum Psychopharmacol. 2008;23:321-326.
26. Alpert JE, Mischoulon D, Rubenstein GE, et al. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14:33-38.
27. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-Lmethionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24:661-664.
28. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477-479.
29. Carney RM, Freedland KE, Rubin EH, et al. Depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;302:1651-1657.
30. Peet M, Horrobin DE. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913-919.
31. Mead GE, Morley W, Campbell P, et al. Exercise for depression. Cochrane Database Syst Rev. 2009;(3):CD004366.-
32. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12:205-213.
33. Trivedi MH, Greer TL, Grannemann BD, et al. TREAD: Treatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials. 2006;3:291-305.
34. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739-752.
35. Kocsis JH, Gelenberg AJ, Rothbaum BO, et al. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009;66:1178-1188.
36. Perry EB, Berman RM, Sanacora G, et al. Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitors: a double-blind, randomized, controlled trial. J Clin Psychiatry. 2004;65:238-243.
37. Rasmussen NA, Schrøder P, Olsen LR, et al. Modafi nil augmentation in depressed patients with partial response to antidepressants: a pilot study on self-reported symptoms covered by the Major Depression Inventory (MDI) and the Symptom Checklist (SCL-92). Nord J Psychiatry. 2005;59:173-178.
38. Santos MA, Rocha FL, Hara C. Efficacy and safety of antidepressant augmentation with lamotrigine in patients with treatment-resistant depression: a randomized, placebo-controlled, double-blind study. Prim Care Companion J Clin Psychiatry. 2008;10:187-190.
39. Dorée JP, Des Rosiers J, Lew V, et al. Quetiapine augmentation of treatment-resistant depression: a comparison with lithium. Curr Med Res Opin. 2007;23:333-341.
40. Lesser IM, Castro DB, Gaynes BN, et al. Ethnicity/race and outcome in the treatment of depression: results from STAR*D. Med Care. 2007;45:1043-1051.
41. Lesser IM, Leuchter AF, Trivedi MH, et al. Characteristics of insured and noninsured outpatients with depression in STAR(*) D. Psychiatr Serv. 2005;56:995-1004.
42. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23:551-560.
1. Kocsis JH, Gelenberg AJ, Rothbaum B, et al. Chronic forms of major depression are still undertreated in the 21st century: systematic assessment of 801 patients presenting for treatment. J Affect Disord. 2008;110:55-61.
2. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095-3105.
3. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179-200.
4. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649-659.
5. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients who required one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917.
6. Cappiello A, McDougle CJ, Delgado PL, et al. Lithium and desipramine versus desipramine alone in the treatment of severe major depression: a preliminary study. Int Clin Psychopharmacol. 1998;13:191-198.
7. Katona CL, Abou-Saleh MT, Harrison DA, et al. Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Br J Psychiatry. 1995;166:80-86.
8. Bauer M, Bschor T, Kunz D, et al. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry. 2000;157:1429-1435.
9. Joffe RT, Singer W, Levitt AJ, et al. A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry. 1993;50:387-393.
10. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519-1530.
11. Nierenberg AA, Papakostas GI, Petersen T, et al. Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J Clin Psychopharmacol. 2003;23:92-95.
12. Aronson R, Offman HJ, Joffe RT, et al. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry. 1996;53:842-848.
13. Joffe RT, Sokolov ST, Levitt AJ. Lithium and triiodothyronine augmentation of antidepressants. Can J Psychiatry. 2006;51:791-793.
14. Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197-206.
15. Trivedi MH, Thase ME, Fava M, et al. Adjunctive aripiprazole in major depressive disorder: analysis of efficacy and safety in patients with anxious and atypical features. J Clin Psychiatry. 2008;69:1928-1936.
16. Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156-165.
17. Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry. 2005;66:1216-1220.
18. Trivedi MH, Thase ME, Osuntokun O, et al. An integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depression. J Clin Psychiatry. 2009;70:387-396.
19. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16:21-30
20. Mahmoud RA, Pandina GJ, Turkoz I, et al. Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med. 2007;147:593-602
21. Keitner GI, Garlow SJ, Ryan CE, et al. A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J Psychiatr Res. 2009;43:205-214.
22. Garakani A, Martinez JM, Marcus S, et al. A randomized, double-blind, and placebo-controlled trial of quetiapine augmentation of fluoxetine in major depressive disorder. Int Clin Psychopharmacol. 2008;23:269-275.
23. Bauer M, Pretorius HW, Constant EL, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry. 2009;70:540-549.
24. Carpenter LL, Yasmin S, Price LH. Double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51:183-188.
25. Ozmenler NK, Karlidere T, Bozkurt A, et al. Mirtazapine augmentation in depressed patients with sexual dysfunction due to selective serotonin reuptake inhibitors. Hum Psychopharmacol. 2008;23:321-326.
26. Alpert JE, Mischoulon D, Rubenstein GE, et al. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14:33-38.
27. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-Lmethionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24:661-664.
28. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477-479.
29. Carney RM, Freedland KE, Rubin EH, et al. Depression in patients with coronary heart disease: a randomized controlled trial. JAMA. 2009;302:1651-1657.
30. Peet M, Horrobin DE. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913-919.
31. Mead GE, Morley W, Campbell P, et al. Exercise for depression. Cochrane Database Syst Rev. 2009;(3):CD004366.-
32. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12:205-213.
33. Trivedi MH, Greer TL, Grannemann BD, et al. TREAD: Treatment with Exercise Augmentation for Depression: study rationale and design. Clin Trials. 2006;3:291-305.
34. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739-752.
35. Kocsis JH, Gelenberg AJ, Rothbaum BO, et al. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009;66:1178-1188.
36. Perry EB, Berman RM, Sanacora G, et al. Pindolol augmentation in depressed patients resistant to selective serotonin reuptake inhibitors: a double-blind, randomized, controlled trial. J Clin Psychiatry. 2004;65:238-243.
37. Rasmussen NA, Schrøder P, Olsen LR, et al. Modafi nil augmentation in depressed patients with partial response to antidepressants: a pilot study on self-reported symptoms covered by the Major Depression Inventory (MDI) and the Symptom Checklist (SCL-92). Nord J Psychiatry. 2005;59:173-178.
38. Santos MA, Rocha FL, Hara C. Efficacy and safety of antidepressant augmentation with lamotrigine in patients with treatment-resistant depression: a randomized, placebo-controlled, double-blind study. Prim Care Companion J Clin Psychiatry. 2008;10:187-190.
39. Dorée JP, Des Rosiers J, Lew V, et al. Quetiapine augmentation of treatment-resistant depression: a comparison with lithium. Curr Med Res Opin. 2007;23:333-341.
40. Lesser IM, Castro DB, Gaynes BN, et al. Ethnicity/race and outcome in the treatment of depression: results from STAR*D. Med Care. 2007;45:1043-1051.
41. Lesser IM, Leuchter AF, Trivedi MH, et al. Characteristics of insured and noninsured outpatients with depression in STAR(*) D. Psychiatr Serv. 2005;56:995-1004.
42. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23:551-560.
Glycemic variability: Too often overlooked in type 2 diabetes?
• Consider evaluating 24-hour variability in glucose levels with patients’ self-monitoring glucose meters (in addition to monitoring glycosylated hemoglobin [HbA1c] levels at regular intervals). C
• If glycemic goals are unmet 2 to 3 months after initiating treatment with exercise and diet or with oral agent monotherapy, consider starting insulin therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though we have the knowledge and the means to reduce complications of type 2 diabetes mellitus (T2DM), most patients may not be reaching all the glycemic goals necessary to achieve optimal risk reduction.1 Maintaining an acceptable level of glycosylated hemoglobin (HbA1c) is one of the important glycemic goals. But that measurement is an average of glucose levels occurring over the prior 3 months. Regardless of a given HbA1c measurement, an emerging body of evidence supports the presumption that glycemic variability over each 24-hour cycle is an independent risk factor for vascular complications.2-15
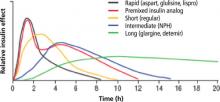
In this article, I review the literature pertaining to the risk associated with glycemic variability and to the benefit in correcting it. I also review the comparative outcomes achievable with normal human insulin and insulin analogs, as well as the advisability of starting insulin earlier in the management process.
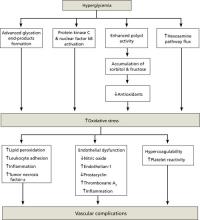
Glycemic variability increases vascular risk independently
HbA1c, considered the gold standard for monitoring glycemic control in patients with T2DM, is an average of the full range of glucose values in the preceding 3 months, including fasting plasma glucose (FPG) and 2-hour postprandial glucose (PPG) levels. Studies have linked lowering HbA1c to reducing the risk and progression of micro- and macrovascular complications associated with diabetes.16,17 But evidence shows that other glycemic values are also important.
The Diabetes Control and Complications Trial (DCCT) was a landmark study in which patients with type 1 diabetes mellitus who received targeted intensive insulin therapy experienced delayed onset and slowed progression of micro-vascular complications compared with those who received conventional insulin treatment.16 Interestingly, this study also reported that patients randomized to receive conventional insulin treatment did not exhibit a reduction in the risk of progression of microvascular disease despite having HbA1c values comparable to those in the intensive-treatment group. One hypothesis is that glucose excursions occurred more frequently in the conventionally treated group, which received fewer daily insulin injections.5
Acute glucose fluctuations during the postprandial period trigger oxidative stress and are more predictive of atherosclerosis development than are FPG or HbA1c6,7 (see “Implications of glycemic variability” below). This suggests that therapy for patients with T2DM should not only target HbA1c as a long-term goal, but also aim to avoid acute glucose fluctuations as an immediate goal. Several studies have shown that postprandial hyperglycemia is an independent risk factor for vascular complications in patients with T2DM.2,7-9,12,14,15
Evidence of increased vascular risk with glycemic variability. The Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe study (DECODE) followed more than 25,000 patients for more than 7 years and found that increased mortality was more closely associated with increased 2-hour PPG levels than with FPG.14 In the Framingham Offspring Study, Meigs et al9 reported that, in nondiabetic subjects, an elevated glucose level 2 hours after an oral challenge increased the relative risk for cardiovascular disease by up to 40%, independent of fasting hyperglycemia.
Mixed outcomes with Hba1c reduction only. Macrovascular risk reduction with intensive HbA1c management was not apparent in 3 recent studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD),18 Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE),19 and Veterans Affairs Diabetes Trial (VADT).20 The ACCORD study, in fact, showed an increase in cardiovascular events in the intensively managed group (HbA1c target <6.0%). Indeed, previous studies had suggested an association between fasting hypoglycemia and poor cardiovascular outcomes.3,4 Retrospective subanalysis of the ACCORD study suggested that patients with poorer glycemic control had a greater risk of hypoglycemia independent of HbA1c values, and that patients who had difficulty reaching lower HbA1c levels may have had poorer cardiovascular outcomes.21
The apparent absence of a reduction in macrovascular events in the ACCORD, ADVANCE, and VADT studies also suggests an additive effect of nonglycemic risk factors that frequently accompany diabetes—ie, hypertension, hyperlipidemia, and hypercoagulability/pro-inflammatory states.
Long-term follow-up in the United Kingdom Prospective Diabetes Study (UKPDS) showed ongoing risk reduction for both microvascular and macrovascular complications.22 A separate meta-analysis showed a significant 10% reduction in cardiovascular events with intensive glycemic control when data were combined from the ACCORD trial, ADVANCE trial, VADT, and the UKPDS.23
An improvement in long-term outcomes for patients with T2DM might be expected when initiating a targeted, intensified, multi-factorial interventional regimen to reduce not only HbA1c, but also glucose variability. The STENO-2 trial showed that a targeted multifactorial treatment regimen in patients with T2DM could decrease long-term vascular complications.24
Consider assessing true variability in your patients. Because postprandial glucose levels alone may not equate to overall glycemic variability, you may want to ask select patients to take readings with their glucose meters at various times of the day across several days to get a more accurate picture.5
Normal physiologic insulin secretion prevents glucose fluctuations in healthy adults. in patients with diabetes, abnormalities in insulin secretion are part of the pathophysiologic process, resulting in chronic sustained hyperglycemia and acute daily fluctuations in glucose levels. These glycemic disorders are associated with a state of increased oxidative stress and possible subsequent development of vascular complications.
Cellular response to hyperglycemia. oxidative stress, the imbalance between production of reactive oxygen species and the ability to eliminate them, is central to the pathogenesis of cardiovascular complications of diabetes, including accelerated atherosclerotic macrovascular disease (FIGURE 1). Both insulin resistance and hyperglycemia are implicated in the pathogenesis of these complications.65,66 hyperglycemia is hypothesized to induce vascular injury via at least 4 biochemical pathways: enhanced polyol activity leading to sorbitol and fructose accumulation; increased formation of advanced glycation end products; activation of protein kinase c and nuclear factor kB; and increased hexosamine pathway flux.67 endothelium activation is a pro-inflammatory, proliferative, and pro-coagulatory setting, ultimately leading to arterial narrowing and susceptibility to atheroma deposition. hyperglycemia can also induce alterations in the coagulation system, resulting in increased thrombosis.68
Association of glycemic variability with oxidative stress. macrovascular complications, particularly cardiovascular disease, contribute significantly to the increased morbidity and mortality with diabetes.24 oxidative stress has been implicated as a major factor in the development of these complications.66-68 other cell-culture evidence suggests that normal protective mechanisms of oxidative stress are impaired by chronic hyperglycemia. When exposed to intermittent glycemic variability, cells have exhibited more pronounced toxicity.69,70 risso et al71 further established that variability in glycemic control resulted in more endothelial cell damage than did chronic sustained hyperglycemia.
Despite the experimental evidence that suggests glycemic variability is associated with increased risk of vascular complications, there are limited clinical data establishing glycemic variability as an independent predictor of these complications. monnier et al72 provided data in patients with type 2 diabetes mellitus (T2Dm) to support the concept of acute glucose fluctuations as a more important trigger of oxidative stress than chronic hyperglycemia. if these data are confirmed in larger clinical trials, a monitoring paradigm for patients with T2Dm could include increased focus on preventing glucose excursions in addition to reducing HbA1c.
FIGURE 1
How oxidative stress secondary to hyperglycemia leads to vascular complications in diabetes66-68
Following through with targeted, intensified management
Consider the following treatment goals for patients with T2DM: (1) lowering HbA1c levels; (2) lowering fasting blood glucose levels; (3) minimizing glycemic variability, including postprandial glucose excursions. TABLE 1 lists the values that the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have assigned to these glycemic-control goals.
In addition to managing glycemic levels, reducing risk of cardiovascular disease in T2DM involves aggressive interventions, as needed, to correct blood pressure and lipid levels.24,25
TABLE 1
Aim to reach 3 glycemic goals in treating type 2 diabetes mellitus
| ADA | AACE | |
|---|---|---|
| Fasting blood glucose (mg/dL) | 90-130 | <110 |
| Postprandial plasma glucose (mg/dL) | <180 | <140 |
| HbA1c (%) | <7* | ≤6.5 |
| *Recommended “in general”; however, the guideline indicates that for “the individual patient,” HbA1c should be as close to normal (<6%) as possible without causing hypoglycemia. | ||
| AACE, American Association of clinical endocrinologists; ADA, American Diabetes Association; HbA1c, glycosylated hemoglobin. | ||
| Sources: ADA, http://care.diabetesjournals.org/content/33/Supplement_1/S11/T11.expansion.htm; AACE, http://www.metcare.com/files/physician-resources/clinical-guidelines/dm-guidelines.pdf | ||
Challenges to achieving glycemic control
Despite current recommendations for more aggressive management of patients with T2DM,25 estimates are that as many as 60% of patients with T2DM do not achieve glycemic targets, and, as the disease progresses, many of the available treatment options fail to sustain levels previously reached.1,26,27
A shortcoming of older treatment strategies still in use is the slow transition to more effective therapy, resulting in long periods of inadequate glycemic control.1 Brown et al27 found that patients receiving monotherapy with either a sulfonylurea or metformin had HbA1c levels >8% for a mean of 20 months and 14 months, respectively, before treatment was changed. Current recommendations call for treatment changes within 2 to 3 months of initiation of therapy if the HbA1c goal is not reached.27-29
Turning to insulin earlier. Insulin is most effective for lowering HbA1c and delaying subsequent complications related to diabetes; however, there is often reluctance to using it early in diabetes management. Consequently, by the time insulin therapy is started, many patients will have had unacceptable glycemic levels for 10 years or more and may already be developing complications.27 And, as noted, the HbA1c level is an average measurement that does not detect glycemic variability. Continuous glucose monitoring will likely lead to more responsive adjustments in treatment regimens and to improved quality of care for patients with T2DM.
Insulin has many beneficial effects
Insulin exerts an anti-inflammatory effect by reducing the increase in C-reactive protein and serum amyloid A.30 It also partially restores insulin-stimulated endothelial function,31 facilitates vasodilation by increasing nitric oxide production,32 and improves fibrinolytic profiles.33 Early initiation of insulin therapy can increase peripheral insulin sensitivity and preserve beta cell function.34-36
When oral agents have failed, insulin can significantly improve patients’ beta cell function,34,35,37 and short periods of insulin therapy in patients newly diagnosed with T2DM may even set the foundation for better long-term control.38,39
But not all insulin is alike
Ideally, insulin therapy should mimic physiologic insulin secretion. However, conventional human insulin products fail to do so because of their suboptimal pharmacodynamic profiles. With recombinant DNA technology, molecular modifications of the human insulin molecule have overcome some of the limitations of conventional human insulin products.
Unfortunately, many practitioners still hold insulin in reserve until combination therapy with oral agents has failed, possibly resulting in years of suboptimal glycemic control. Newer strategies recommend earlier initiation of insulin—ie, once diet and exercise fail, or when treatment with 1 oral agent fails. The development of insulin analogs is a significant milestone on the road to achieving improved outcomes for patients with T2DM.
Rapid-acting agents
Compared with regular human insulin, newer rapid-acting insulin analogs may improve glycemic control when used at mealtimes. However, due to their shorter half-lives, these insulin analogs require augmentation with basal insulin to control hyperglycemia between meals and during the night.
Insulin lispro was the first commercially available rapid-acting insulin analog, introduced in 1996. This agent differs from human insulin by an inversion of amino acid residues in positions 28 and 29 of the insulin B-chain. Inversion prevents the formation of hexamers and dimers that tend to diffuse more slowly, thereby facilitating a rapid uptake of the insulin analog into blood and tissues.40,41 The second such agent, marketed in 2000, was insulin aspart, in which aspartic acid replaces proline at position 28 of the B-chain of human insulin.41,42 The most recent rapid-acting analog is insulin glulisine, in which lysine replaces asparagine near the N-terminus of the B-chain, and glutamic acid replaces lysine near the C-terminus of human insulin.
The molecular changes made in creating these analogs allows them to dissociate quickly into monomers that are absorbed rapidly and achieve faster peak levels compared with regular human insulin.41,42 These changes do not, however, interfere with the analogs’ ability to bind to the insulin receptor.43,44
Dosing considerations. Absorption of regular human insulin is not sufficiently rapid at mealtimes to control prandial glucose levels.45 Therefore, it is essential to give regular insulin 30 to 60 minutes before meals. For patients who have erratic daily schedules, adhering to this sort of routine can be difficult. But even if scheduling is not a problem, the prolonged duration of action of human insulin can predispose patients to hypoglycemia. Moreover, absorption of regular insulin can vary dramatically from day to day.46,47
The insulin analogs correct the pharmacokinetic and pharmacodynamic deficiencies of regular insulin, producing plasma profiles that more closely simulate normal, physiologic meal-stimulated insulin release.48-50 The 3 rapid-acting agents (aspart, glulisine, lispro) have very similar onset and duration of action, with peak effect occurring close to injection time (TABLE 2 and FIGURE 2).48-50
Advantages of rapid-acting agents. These agents can be administered closer to meals, giving patients more flexibility and likely tighter postprandial glucose control, with reductions in glycemic excursions. Another advantage is the ability to better match insulin dose to anticipated carbohydrate in-take, affording better postprandial control.51-53 Rapid-acting analogs also result in fewer episodes of hypoglycemia. In a meta-analysis of 2576 patients, hypoglycemic events occurred 25% less often with insulin lispro compared with regular human insulin in patients with type 1 diabetes mellitus (T1DM).52 In clinical trials, insulin aspart and insulin glulisine have also caused fewer hypoglycemic events compared with regular human insulin.51-53
Long-acting agents
Basal, or long-acting, insulins are important for maintaining normoglycemia over 24 hours. Neutral protamine Hagedorn (NPH) insulin reaches its peak effect 4 to 10 hours after injection, and its total effect lasts only 12 to 18 hours. NPH is therefore often dosed twice daily. Absorption of NPH can vary significantly, causing day-to-day blood glucose fluctuations.46,47 Therefore, this agent’s activity does not closely resemble normal physiologic basal insulin secretion.
The newer long-acting insulin analogs—insulin detemir and insulin glargine—were designed to more closely replicate normal physiologic basal insulin secretion. Insulin glargine was first to reach the market, in 2001. It contains glycine instead of asparagine in the alpha-chain and 2 arginine residues at the C-terminus, and the addition of zinc enhances the aggregation and slow release at a neutral pH. Insulin glargine precipitates in the subcutaneous tissue, which slows its absorption and results in a relatively flat insulin plasma profile and extended action.54,55 Insulin detemir is a combination of the original insulin molecule and a saturated fatty acid (myristic acid). Insulin detemir is designed to bind albumin (98% albumin-bound in circulation) through this fatty acid chain in the plasma after injection, resulting in an extended plasma profile.54,56 Insulin glargine and NPH form crystalline depots, but detemir is soluble and the subcutaneous depot remains in a liquid state; this may account for differences in absorption variability.56
Advantages of the long-acting insulin analogs. Compared with conventional basal insulin such as NPH, the analogs have a prolonged duration of action (up to 24 hours) without pronounced peaks, permitting once-daily dosing in many patients (TABLE 2 and FIGURE 2).46,47,50,55 The pharmacodynamic and pharmacokinetic properties of the long- acting agents make them less likely than NPH to cause nocturnal and overall hypoglycemia, a benefit that has been observed in several clinical trials.57-61
Comparative clinical trials evaluating glycemic variability. Both of the long-acting analogs have shown lower within-subject variability in blood and plasma glucose measurements when compared with NPH.62-64 In head-to-head comparisons of the analogs in glucose clamp studies, insulin detemir has demonstrated less within-subject variability of blood glucose levels than insulin glargine, in patients with T1DM or T2DM.56,64 In clinical practice, different patients may have better results with one of these basal insulins as opposed to the other, and treatment choices will need to be tailored to the individual patient.
TABLE 2
Pharmacokinetic properties giving insulin analogs an advantage over regular insulin46-50
| Insulin preparation | Onset of action | Peak action | Duration of action |
|---|---|---|---|
| Short-acting | |||
| Regular | 30-60 minutes | 2-3 hours | 8-10 hours |
| Lispro | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Aspart | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Glulisine | 20 minutes | 90 minutes | 5.3 hours |
| Long-acting | |||
| NPH | 2-4 hours | 4-10 hours | 12-18 hours |
| Glargine | 2-4 hours | Relatively flat | Up to 24 hours |
| Detemir | 1-2 hours | Relatively flat | Up to 24 hours |
| NPH, neutral protamine Hagedorn. | |||
FIGURE 2
Pharmacokinetic profiles of human insulin and insulin analogs
Adapted from: Burton S. J Fam Pract. 2006;55(12 suppl):S10-S17.
CORRESPONDENCE Eric L. Johnson, MD, University of North Dakota School of Medicine and Health Sciences, Department of Family & Community Medicine, 501 North Columbia Road, Grand Forks, ND 58202-9037; [email protected]
1. Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17-20.
2. Kadowaki S, Okamura T, Hozawa A, et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia. 2008;51:575-582.
3. Wändell PE, Theobald H. The association between low fasting glucose value and mortality. Curr Diabetes Rev. 2007;3:274-279.
4. Wei M, Gibbons LW, Mitchell TL, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047-2052.
5. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178-181.
6. Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830-1834.
7. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107-2114.
8. Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494.
9. Meigs JB, Nathan DM, D’Agostino RB, Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845-1850.
10. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360-367.
11. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
12. Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36:689-692.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617-621.
14. Balkau B, Hu G, Qiao Q, et al. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia. 2004;47:2118-2128.
15. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
16. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
17. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412.
18. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
19. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
20. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
21. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.-
22. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
23. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;11:2288-2298.
24. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
25. Standards of medical care in diabetes—2010 Diabetes Care. 2010;33(suppl 1):S11-S61.
26. Barnett AH. Treating to goal: challenges of current management. Eur J Endocrinol. 2004;151(suppl 2):T3-T7.
27. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
28. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
29. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193-203.
30. Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849-854.
31. Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611-2618.
32. Chaudhuri A, Kanjwal Y, Mohanty P, et al. Insulin-induced vasodilatation of internal carotid artery. Metabolism. 1999;48:1470-1473.
33. Melidonis A, Stefanidis A, Tournis S, et al. The role of strict metabolic control by insulin infusion on fibrinolytic profile during an acute coronary event in diabetic patients. Clin Cardiol. 2000;23:160-164.
34. Garvey WT, Olefsky JM, Griffin J, et al. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222-234.
35. Rolla A. The pathophysiological basis for intensive insulin replacement. Int J Obes Relat Metab Disord. 2004;28(suppl 2):S3-S7.
36. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231-2237.
37. Glaser B, Leibovich G, Nesher R, et al. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh). 1988;118:365-373.
38. Andrews WJ, Vasquez B, Nagulesparan M, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33:634-642.
39. Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028-1032.
40. Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7:1303-1325.
41. Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527-533.
42. Garber AJ. Pharmacologic modifications of hormones to improve their therapeutic potential for diabetes management. Diabetes Obes Metab. 2005;7:666-674.
43. Hansen BF, Danielsen GM, Drejer K, et al. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. 1996;315(pt 1):271-279.
44. Slieker LJ, Brooke GS, DiMarchi RD, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997;40(suppl 2):S54-S61.
45. Dimitriadis GD, Gerich JE. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6:374-377.
46. Roy B, Chou MC, Field JB. Time-action characteristics of regular and NPH insulin in insulin-treated diabetics. J Clin Endocrinol Metab. 1980;50:475-479.
47. Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care. 1984;7:188-199.
48. Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal-related plasma glucose excursions in type 1 diabetic subjects. Diabetes Care. 1991;14:571-577.
49. Becker RH, Frick AD, Burger F, et al. A comparison of the steady-state pharmacokinetics and pharmacodynamics of a novel rapid-acting insulin analog, insulin glulisine, and regular human insulin in healthy volunteers using the euglycemic clamp technique. Exp Clin Endocrinol Diabetes. 2005;113:292-297.
50. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
51. Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care. 1999;22:801-805.
52. Brunelle BL, Llewelyn J, Anderson JH, Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726-1731.
53. Home PD, Lindholm A, Hylleberg B, et al. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998;21:1904-1909.
54. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;5:648-659.
55. Bolli GB, Di Marchi RD, Park GD, et al. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42:1151-1167.
56. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290-299.
57. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
58. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
59. Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
60. Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269-1274.
61. Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569-1581.
62. Haak T, Tiengo A, Draeger E, et al. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56-64.
63. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
64. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
65. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453-458.
66. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625.
67. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
68. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993;36:1119-1125.
69. Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795-2804.
70. Jones SC, Saunders HJ, Qi W, et al. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999;42:1113-1119.
71. Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924-E930.
72. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
• Consider evaluating 24-hour variability in glucose levels with patients’ self-monitoring glucose meters (in addition to monitoring glycosylated hemoglobin [HbA1c] levels at regular intervals). C
• If glycemic goals are unmet 2 to 3 months after initiating treatment with exercise and diet or with oral agent monotherapy, consider starting insulin therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though we have the knowledge and the means to reduce complications of type 2 diabetes mellitus (T2DM), most patients may not be reaching all the glycemic goals necessary to achieve optimal risk reduction.1 Maintaining an acceptable level of glycosylated hemoglobin (HbA1c) is one of the important glycemic goals. But that measurement is an average of glucose levels occurring over the prior 3 months. Regardless of a given HbA1c measurement, an emerging body of evidence supports the presumption that glycemic variability over each 24-hour cycle is an independent risk factor for vascular complications.2-15
In this article, I review the literature pertaining to the risk associated with glycemic variability and to the benefit in correcting it. I also review the comparative outcomes achievable with normal human insulin and insulin analogs, as well as the advisability of starting insulin earlier in the management process.
Glycemic variability increases vascular risk independently
HbA1c, considered the gold standard for monitoring glycemic control in patients with T2DM, is an average of the full range of glucose values in the preceding 3 months, including fasting plasma glucose (FPG) and 2-hour postprandial glucose (PPG) levels. Studies have linked lowering HbA1c to reducing the risk and progression of micro- and macrovascular complications associated with diabetes.16,17 But evidence shows that other glycemic values are also important.
The Diabetes Control and Complications Trial (DCCT) was a landmark study in which patients with type 1 diabetes mellitus who received targeted intensive insulin therapy experienced delayed onset and slowed progression of micro-vascular complications compared with those who received conventional insulin treatment.16 Interestingly, this study also reported that patients randomized to receive conventional insulin treatment did not exhibit a reduction in the risk of progression of microvascular disease despite having HbA1c values comparable to those in the intensive-treatment group. One hypothesis is that glucose excursions occurred more frequently in the conventionally treated group, which received fewer daily insulin injections.5
Acute glucose fluctuations during the postprandial period trigger oxidative stress and are more predictive of atherosclerosis development than are FPG or HbA1c6,7 (see “Implications of glycemic variability” below). This suggests that therapy for patients with T2DM should not only target HbA1c as a long-term goal, but also aim to avoid acute glucose fluctuations as an immediate goal. Several studies have shown that postprandial hyperglycemia is an independent risk factor for vascular complications in patients with T2DM.2,7-9,12,14,15
Evidence of increased vascular risk with glycemic variability. The Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe study (DECODE) followed more than 25,000 patients for more than 7 years and found that increased mortality was more closely associated with increased 2-hour PPG levels than with FPG.14 In the Framingham Offspring Study, Meigs et al9 reported that, in nondiabetic subjects, an elevated glucose level 2 hours after an oral challenge increased the relative risk for cardiovascular disease by up to 40%, independent of fasting hyperglycemia.
Mixed outcomes with Hba1c reduction only. Macrovascular risk reduction with intensive HbA1c management was not apparent in 3 recent studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD),18 Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE),19 and Veterans Affairs Diabetes Trial (VADT).20 The ACCORD study, in fact, showed an increase in cardiovascular events in the intensively managed group (HbA1c target <6.0%). Indeed, previous studies had suggested an association between fasting hypoglycemia and poor cardiovascular outcomes.3,4 Retrospective subanalysis of the ACCORD study suggested that patients with poorer glycemic control had a greater risk of hypoglycemia independent of HbA1c values, and that patients who had difficulty reaching lower HbA1c levels may have had poorer cardiovascular outcomes.21
The apparent absence of a reduction in macrovascular events in the ACCORD, ADVANCE, and VADT studies also suggests an additive effect of nonglycemic risk factors that frequently accompany diabetes—ie, hypertension, hyperlipidemia, and hypercoagulability/pro-inflammatory states.
Long-term follow-up in the United Kingdom Prospective Diabetes Study (UKPDS) showed ongoing risk reduction for both microvascular and macrovascular complications.22 A separate meta-analysis showed a significant 10% reduction in cardiovascular events with intensive glycemic control when data were combined from the ACCORD trial, ADVANCE trial, VADT, and the UKPDS.23
An improvement in long-term outcomes for patients with T2DM might be expected when initiating a targeted, intensified, multi-factorial interventional regimen to reduce not only HbA1c, but also glucose variability. The STENO-2 trial showed that a targeted multifactorial treatment regimen in patients with T2DM could decrease long-term vascular complications.24
Consider assessing true variability in your patients. Because postprandial glucose levels alone may not equate to overall glycemic variability, you may want to ask select patients to take readings with their glucose meters at various times of the day across several days to get a more accurate picture.5
Normal physiologic insulin secretion prevents glucose fluctuations in healthy adults. in patients with diabetes, abnormalities in insulin secretion are part of the pathophysiologic process, resulting in chronic sustained hyperglycemia and acute daily fluctuations in glucose levels. These glycemic disorders are associated with a state of increased oxidative stress and possible subsequent development of vascular complications.
Cellular response to hyperglycemia. oxidative stress, the imbalance between production of reactive oxygen species and the ability to eliminate them, is central to the pathogenesis of cardiovascular complications of diabetes, including accelerated atherosclerotic macrovascular disease (FIGURE 1). Both insulin resistance and hyperglycemia are implicated in the pathogenesis of these complications.65,66 hyperglycemia is hypothesized to induce vascular injury via at least 4 biochemical pathways: enhanced polyol activity leading to sorbitol and fructose accumulation; increased formation of advanced glycation end products; activation of protein kinase c and nuclear factor kB; and increased hexosamine pathway flux.67 endothelium activation is a pro-inflammatory, proliferative, and pro-coagulatory setting, ultimately leading to arterial narrowing and susceptibility to atheroma deposition. hyperglycemia can also induce alterations in the coagulation system, resulting in increased thrombosis.68
Association of glycemic variability with oxidative stress. macrovascular complications, particularly cardiovascular disease, contribute significantly to the increased morbidity and mortality with diabetes.24 oxidative stress has been implicated as a major factor in the development of these complications.66-68 other cell-culture evidence suggests that normal protective mechanisms of oxidative stress are impaired by chronic hyperglycemia. When exposed to intermittent glycemic variability, cells have exhibited more pronounced toxicity.69,70 risso et al71 further established that variability in glycemic control resulted in more endothelial cell damage than did chronic sustained hyperglycemia.
Despite the experimental evidence that suggests glycemic variability is associated with increased risk of vascular complications, there are limited clinical data establishing glycemic variability as an independent predictor of these complications. monnier et al72 provided data in patients with type 2 diabetes mellitus (T2Dm) to support the concept of acute glucose fluctuations as a more important trigger of oxidative stress than chronic hyperglycemia. if these data are confirmed in larger clinical trials, a monitoring paradigm for patients with T2Dm could include increased focus on preventing glucose excursions in addition to reducing HbA1c.
FIGURE 1
How oxidative stress secondary to hyperglycemia leads to vascular complications in diabetes66-68
Following through with targeted, intensified management
Consider the following treatment goals for patients with T2DM: (1) lowering HbA1c levels; (2) lowering fasting blood glucose levels; (3) minimizing glycemic variability, including postprandial glucose excursions. TABLE 1 lists the values that the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have assigned to these glycemic-control goals.
In addition to managing glycemic levels, reducing risk of cardiovascular disease in T2DM involves aggressive interventions, as needed, to correct blood pressure and lipid levels.24,25
TABLE 1
Aim to reach 3 glycemic goals in treating type 2 diabetes mellitus
| ADA | AACE | |
|---|---|---|
| Fasting blood glucose (mg/dL) | 90-130 | <110 |
| Postprandial plasma glucose (mg/dL) | <180 | <140 |
| HbA1c (%) | <7* | ≤6.5 |
| *Recommended “in general”; however, the guideline indicates that for “the individual patient,” HbA1c should be as close to normal (<6%) as possible without causing hypoglycemia. | ||
| AACE, American Association of clinical endocrinologists; ADA, American Diabetes Association; HbA1c, glycosylated hemoglobin. | ||
| Sources: ADA, http://care.diabetesjournals.org/content/33/Supplement_1/S11/T11.expansion.htm; AACE, http://www.metcare.com/files/physician-resources/clinical-guidelines/dm-guidelines.pdf | ||
Challenges to achieving glycemic control
Despite current recommendations for more aggressive management of patients with T2DM,25 estimates are that as many as 60% of patients with T2DM do not achieve glycemic targets, and, as the disease progresses, many of the available treatment options fail to sustain levels previously reached.1,26,27
A shortcoming of older treatment strategies still in use is the slow transition to more effective therapy, resulting in long periods of inadequate glycemic control.1 Brown et al27 found that patients receiving monotherapy with either a sulfonylurea or metformin had HbA1c levels >8% for a mean of 20 months and 14 months, respectively, before treatment was changed. Current recommendations call for treatment changes within 2 to 3 months of initiation of therapy if the HbA1c goal is not reached.27-29
Turning to insulin earlier. Insulin is most effective for lowering HbA1c and delaying subsequent complications related to diabetes; however, there is often reluctance to using it early in diabetes management. Consequently, by the time insulin therapy is started, many patients will have had unacceptable glycemic levels for 10 years or more and may already be developing complications.27 And, as noted, the HbA1c level is an average measurement that does not detect glycemic variability. Continuous glucose monitoring will likely lead to more responsive adjustments in treatment regimens and to improved quality of care for patients with T2DM.
Insulin has many beneficial effects
Insulin exerts an anti-inflammatory effect by reducing the increase in C-reactive protein and serum amyloid A.30 It also partially restores insulin-stimulated endothelial function,31 facilitates vasodilation by increasing nitric oxide production,32 and improves fibrinolytic profiles.33 Early initiation of insulin therapy can increase peripheral insulin sensitivity and preserve beta cell function.34-36
When oral agents have failed, insulin can significantly improve patients’ beta cell function,34,35,37 and short periods of insulin therapy in patients newly diagnosed with T2DM may even set the foundation for better long-term control.38,39
But not all insulin is alike
Ideally, insulin therapy should mimic physiologic insulin secretion. However, conventional human insulin products fail to do so because of their suboptimal pharmacodynamic profiles. With recombinant DNA technology, molecular modifications of the human insulin molecule have overcome some of the limitations of conventional human insulin products.
Unfortunately, many practitioners still hold insulin in reserve until combination therapy with oral agents has failed, possibly resulting in years of suboptimal glycemic control. Newer strategies recommend earlier initiation of insulin—ie, once diet and exercise fail, or when treatment with 1 oral agent fails. The development of insulin analogs is a significant milestone on the road to achieving improved outcomes for patients with T2DM.
Rapid-acting agents
Compared with regular human insulin, newer rapid-acting insulin analogs may improve glycemic control when used at mealtimes. However, due to their shorter half-lives, these insulin analogs require augmentation with basal insulin to control hyperglycemia between meals and during the night.
Insulin lispro was the first commercially available rapid-acting insulin analog, introduced in 1996. This agent differs from human insulin by an inversion of amino acid residues in positions 28 and 29 of the insulin B-chain. Inversion prevents the formation of hexamers and dimers that tend to diffuse more slowly, thereby facilitating a rapid uptake of the insulin analog into blood and tissues.40,41 The second such agent, marketed in 2000, was insulin aspart, in which aspartic acid replaces proline at position 28 of the B-chain of human insulin.41,42 The most recent rapid-acting analog is insulin glulisine, in which lysine replaces asparagine near the N-terminus of the B-chain, and glutamic acid replaces lysine near the C-terminus of human insulin.
The molecular changes made in creating these analogs allows them to dissociate quickly into monomers that are absorbed rapidly and achieve faster peak levels compared with regular human insulin.41,42 These changes do not, however, interfere with the analogs’ ability to bind to the insulin receptor.43,44
Dosing considerations. Absorption of regular human insulin is not sufficiently rapid at mealtimes to control prandial glucose levels.45 Therefore, it is essential to give regular insulin 30 to 60 minutes before meals. For patients who have erratic daily schedules, adhering to this sort of routine can be difficult. But even if scheduling is not a problem, the prolonged duration of action of human insulin can predispose patients to hypoglycemia. Moreover, absorption of regular insulin can vary dramatically from day to day.46,47
The insulin analogs correct the pharmacokinetic and pharmacodynamic deficiencies of regular insulin, producing plasma profiles that more closely simulate normal, physiologic meal-stimulated insulin release.48-50 The 3 rapid-acting agents (aspart, glulisine, lispro) have very similar onset and duration of action, with peak effect occurring close to injection time (TABLE 2 and FIGURE 2).48-50
Advantages of rapid-acting agents. These agents can be administered closer to meals, giving patients more flexibility and likely tighter postprandial glucose control, with reductions in glycemic excursions. Another advantage is the ability to better match insulin dose to anticipated carbohydrate in-take, affording better postprandial control.51-53 Rapid-acting analogs also result in fewer episodes of hypoglycemia. In a meta-analysis of 2576 patients, hypoglycemic events occurred 25% less often with insulin lispro compared with regular human insulin in patients with type 1 diabetes mellitus (T1DM).52 In clinical trials, insulin aspart and insulin glulisine have also caused fewer hypoglycemic events compared with regular human insulin.51-53
Long-acting agents
Basal, or long-acting, insulins are important for maintaining normoglycemia over 24 hours. Neutral protamine Hagedorn (NPH) insulin reaches its peak effect 4 to 10 hours after injection, and its total effect lasts only 12 to 18 hours. NPH is therefore often dosed twice daily. Absorption of NPH can vary significantly, causing day-to-day blood glucose fluctuations.46,47 Therefore, this agent’s activity does not closely resemble normal physiologic basal insulin secretion.
The newer long-acting insulin analogs—insulin detemir and insulin glargine—were designed to more closely replicate normal physiologic basal insulin secretion. Insulin glargine was first to reach the market, in 2001. It contains glycine instead of asparagine in the alpha-chain and 2 arginine residues at the C-terminus, and the addition of zinc enhances the aggregation and slow release at a neutral pH. Insulin glargine precipitates in the subcutaneous tissue, which slows its absorption and results in a relatively flat insulin plasma profile and extended action.54,55 Insulin detemir is a combination of the original insulin molecule and a saturated fatty acid (myristic acid). Insulin detemir is designed to bind albumin (98% albumin-bound in circulation) through this fatty acid chain in the plasma after injection, resulting in an extended plasma profile.54,56 Insulin glargine and NPH form crystalline depots, but detemir is soluble and the subcutaneous depot remains in a liquid state; this may account for differences in absorption variability.56
Advantages of the long-acting insulin analogs. Compared with conventional basal insulin such as NPH, the analogs have a prolonged duration of action (up to 24 hours) without pronounced peaks, permitting once-daily dosing in many patients (TABLE 2 and FIGURE 2).46,47,50,55 The pharmacodynamic and pharmacokinetic properties of the long- acting agents make them less likely than NPH to cause nocturnal and overall hypoglycemia, a benefit that has been observed in several clinical trials.57-61
Comparative clinical trials evaluating glycemic variability. Both of the long-acting analogs have shown lower within-subject variability in blood and plasma glucose measurements when compared with NPH.62-64 In head-to-head comparisons of the analogs in glucose clamp studies, insulin detemir has demonstrated less within-subject variability of blood glucose levels than insulin glargine, in patients with T1DM or T2DM.56,64 In clinical practice, different patients may have better results with one of these basal insulins as opposed to the other, and treatment choices will need to be tailored to the individual patient.
TABLE 2
Pharmacokinetic properties giving insulin analogs an advantage over regular insulin46-50
| Insulin preparation | Onset of action | Peak action | Duration of action |
|---|---|---|---|
| Short-acting | |||
| Regular | 30-60 minutes | 2-3 hours | 8-10 hours |
| Lispro | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Aspart | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Glulisine | 20 minutes | 90 minutes | 5.3 hours |
| Long-acting | |||
| NPH | 2-4 hours | 4-10 hours | 12-18 hours |
| Glargine | 2-4 hours | Relatively flat | Up to 24 hours |
| Detemir | 1-2 hours | Relatively flat | Up to 24 hours |
| NPH, neutral protamine Hagedorn. | |||
FIGURE 2
Pharmacokinetic profiles of human insulin and insulin analogs
Adapted from: Burton S. J Fam Pract. 2006;55(12 suppl):S10-S17.
CORRESPONDENCE Eric L. Johnson, MD, University of North Dakota School of Medicine and Health Sciences, Department of Family & Community Medicine, 501 North Columbia Road, Grand Forks, ND 58202-9037; [email protected]
• Consider evaluating 24-hour variability in glucose levels with patients’ self-monitoring glucose meters (in addition to monitoring glycosylated hemoglobin [HbA1c] levels at regular intervals). C
• If glycemic goals are unmet 2 to 3 months after initiating treatment with exercise and diet or with oral agent monotherapy, consider starting insulin therapy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Though we have the knowledge and the means to reduce complications of type 2 diabetes mellitus (T2DM), most patients may not be reaching all the glycemic goals necessary to achieve optimal risk reduction.1 Maintaining an acceptable level of glycosylated hemoglobin (HbA1c) is one of the important glycemic goals. But that measurement is an average of glucose levels occurring over the prior 3 months. Regardless of a given HbA1c measurement, an emerging body of evidence supports the presumption that glycemic variability over each 24-hour cycle is an independent risk factor for vascular complications.2-15
In this article, I review the literature pertaining to the risk associated with glycemic variability and to the benefit in correcting it. I also review the comparative outcomes achievable with normal human insulin and insulin analogs, as well as the advisability of starting insulin earlier in the management process.
Glycemic variability increases vascular risk independently
HbA1c, considered the gold standard for monitoring glycemic control in patients with T2DM, is an average of the full range of glucose values in the preceding 3 months, including fasting plasma glucose (FPG) and 2-hour postprandial glucose (PPG) levels. Studies have linked lowering HbA1c to reducing the risk and progression of micro- and macrovascular complications associated with diabetes.16,17 But evidence shows that other glycemic values are also important.
The Diabetes Control and Complications Trial (DCCT) was a landmark study in which patients with type 1 diabetes mellitus who received targeted intensive insulin therapy experienced delayed onset and slowed progression of micro-vascular complications compared with those who received conventional insulin treatment.16 Interestingly, this study also reported that patients randomized to receive conventional insulin treatment did not exhibit a reduction in the risk of progression of microvascular disease despite having HbA1c values comparable to those in the intensive-treatment group. One hypothesis is that glucose excursions occurred more frequently in the conventionally treated group, which received fewer daily insulin injections.5
Acute glucose fluctuations during the postprandial period trigger oxidative stress and are more predictive of atherosclerosis development than are FPG or HbA1c6,7 (see “Implications of glycemic variability” below). This suggests that therapy for patients with T2DM should not only target HbA1c as a long-term goal, but also aim to avoid acute glucose fluctuations as an immediate goal. Several studies have shown that postprandial hyperglycemia is an independent risk factor for vascular complications in patients with T2DM.2,7-9,12,14,15
Evidence of increased vascular risk with glycemic variability. The Diabetes Epidemiology: COllaborative analysis of Diagnostic criteria in Europe study (DECODE) followed more than 25,000 patients for more than 7 years and found that increased mortality was more closely associated with increased 2-hour PPG levels than with FPG.14 In the Framingham Offspring Study, Meigs et al9 reported that, in nondiabetic subjects, an elevated glucose level 2 hours after an oral challenge increased the relative risk for cardiovascular disease by up to 40%, independent of fasting hyperglycemia.
Mixed outcomes with Hba1c reduction only. Macrovascular risk reduction with intensive HbA1c management was not apparent in 3 recent studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD),18 Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE),19 and Veterans Affairs Diabetes Trial (VADT).20 The ACCORD study, in fact, showed an increase in cardiovascular events in the intensively managed group (HbA1c target <6.0%). Indeed, previous studies had suggested an association between fasting hypoglycemia and poor cardiovascular outcomes.3,4 Retrospective subanalysis of the ACCORD study suggested that patients with poorer glycemic control had a greater risk of hypoglycemia independent of HbA1c values, and that patients who had difficulty reaching lower HbA1c levels may have had poorer cardiovascular outcomes.21
The apparent absence of a reduction in macrovascular events in the ACCORD, ADVANCE, and VADT studies also suggests an additive effect of nonglycemic risk factors that frequently accompany diabetes—ie, hypertension, hyperlipidemia, and hypercoagulability/pro-inflammatory states.
Long-term follow-up in the United Kingdom Prospective Diabetes Study (UKPDS) showed ongoing risk reduction for both microvascular and macrovascular complications.22 A separate meta-analysis showed a significant 10% reduction in cardiovascular events with intensive glycemic control when data were combined from the ACCORD trial, ADVANCE trial, VADT, and the UKPDS.23
An improvement in long-term outcomes for patients with T2DM might be expected when initiating a targeted, intensified, multi-factorial interventional regimen to reduce not only HbA1c, but also glucose variability. The STENO-2 trial showed that a targeted multifactorial treatment regimen in patients with T2DM could decrease long-term vascular complications.24
Consider assessing true variability in your patients. Because postprandial glucose levels alone may not equate to overall glycemic variability, you may want to ask select patients to take readings with their glucose meters at various times of the day across several days to get a more accurate picture.5
Normal physiologic insulin secretion prevents glucose fluctuations in healthy adults. in patients with diabetes, abnormalities in insulin secretion are part of the pathophysiologic process, resulting in chronic sustained hyperglycemia and acute daily fluctuations in glucose levels. These glycemic disorders are associated with a state of increased oxidative stress and possible subsequent development of vascular complications.
Cellular response to hyperglycemia. oxidative stress, the imbalance between production of reactive oxygen species and the ability to eliminate them, is central to the pathogenesis of cardiovascular complications of diabetes, including accelerated atherosclerotic macrovascular disease (FIGURE 1). Both insulin resistance and hyperglycemia are implicated in the pathogenesis of these complications.65,66 hyperglycemia is hypothesized to induce vascular injury via at least 4 biochemical pathways: enhanced polyol activity leading to sorbitol and fructose accumulation; increased formation of advanced glycation end products; activation of protein kinase c and nuclear factor kB; and increased hexosamine pathway flux.67 endothelium activation is a pro-inflammatory, proliferative, and pro-coagulatory setting, ultimately leading to arterial narrowing and susceptibility to atheroma deposition. hyperglycemia can also induce alterations in the coagulation system, resulting in increased thrombosis.68
Association of glycemic variability with oxidative stress. macrovascular complications, particularly cardiovascular disease, contribute significantly to the increased morbidity and mortality with diabetes.24 oxidative stress has been implicated as a major factor in the development of these complications.66-68 other cell-culture evidence suggests that normal protective mechanisms of oxidative stress are impaired by chronic hyperglycemia. When exposed to intermittent glycemic variability, cells have exhibited more pronounced toxicity.69,70 risso et al71 further established that variability in glycemic control resulted in more endothelial cell damage than did chronic sustained hyperglycemia.
Despite the experimental evidence that suggests glycemic variability is associated with increased risk of vascular complications, there are limited clinical data establishing glycemic variability as an independent predictor of these complications. monnier et al72 provided data in patients with type 2 diabetes mellitus (T2Dm) to support the concept of acute glucose fluctuations as a more important trigger of oxidative stress than chronic hyperglycemia. if these data are confirmed in larger clinical trials, a monitoring paradigm for patients with T2Dm could include increased focus on preventing glucose excursions in addition to reducing HbA1c.
FIGURE 1
How oxidative stress secondary to hyperglycemia leads to vascular complications in diabetes66-68
Following through with targeted, intensified management
Consider the following treatment goals for patients with T2DM: (1) lowering HbA1c levels; (2) lowering fasting blood glucose levels; (3) minimizing glycemic variability, including postprandial glucose excursions. TABLE 1 lists the values that the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have assigned to these glycemic-control goals.
In addition to managing glycemic levels, reducing risk of cardiovascular disease in T2DM involves aggressive interventions, as needed, to correct blood pressure and lipid levels.24,25
TABLE 1
Aim to reach 3 glycemic goals in treating type 2 diabetes mellitus
| ADA | AACE | |
|---|---|---|
| Fasting blood glucose (mg/dL) | 90-130 | <110 |
| Postprandial plasma glucose (mg/dL) | <180 | <140 |
| HbA1c (%) | <7* | ≤6.5 |
| *Recommended “in general”; however, the guideline indicates that for “the individual patient,” HbA1c should be as close to normal (<6%) as possible without causing hypoglycemia. | ||
| AACE, American Association of clinical endocrinologists; ADA, American Diabetes Association; HbA1c, glycosylated hemoglobin. | ||
| Sources: ADA, http://care.diabetesjournals.org/content/33/Supplement_1/S11/T11.expansion.htm; AACE, http://www.metcare.com/files/physician-resources/clinical-guidelines/dm-guidelines.pdf | ||
Challenges to achieving glycemic control
Despite current recommendations for more aggressive management of patients with T2DM,25 estimates are that as many as 60% of patients with T2DM do not achieve glycemic targets, and, as the disease progresses, many of the available treatment options fail to sustain levels previously reached.1,26,27
A shortcoming of older treatment strategies still in use is the slow transition to more effective therapy, resulting in long periods of inadequate glycemic control.1 Brown et al27 found that patients receiving monotherapy with either a sulfonylurea or metformin had HbA1c levels >8% for a mean of 20 months and 14 months, respectively, before treatment was changed. Current recommendations call for treatment changes within 2 to 3 months of initiation of therapy if the HbA1c goal is not reached.27-29
Turning to insulin earlier. Insulin is most effective for lowering HbA1c and delaying subsequent complications related to diabetes; however, there is often reluctance to using it early in diabetes management. Consequently, by the time insulin therapy is started, many patients will have had unacceptable glycemic levels for 10 years or more and may already be developing complications.27 And, as noted, the HbA1c level is an average measurement that does not detect glycemic variability. Continuous glucose monitoring will likely lead to more responsive adjustments in treatment regimens and to improved quality of care for patients with T2DM.
Insulin has many beneficial effects
Insulin exerts an anti-inflammatory effect by reducing the increase in C-reactive protein and serum amyloid A.30 It also partially restores insulin-stimulated endothelial function,31 facilitates vasodilation by increasing nitric oxide production,32 and improves fibrinolytic profiles.33 Early initiation of insulin therapy can increase peripheral insulin sensitivity and preserve beta cell function.34-36
When oral agents have failed, insulin can significantly improve patients’ beta cell function,34,35,37 and short periods of insulin therapy in patients newly diagnosed with T2DM may even set the foundation for better long-term control.38,39
But not all insulin is alike
Ideally, insulin therapy should mimic physiologic insulin secretion. However, conventional human insulin products fail to do so because of their suboptimal pharmacodynamic profiles. With recombinant DNA technology, molecular modifications of the human insulin molecule have overcome some of the limitations of conventional human insulin products.
Unfortunately, many practitioners still hold insulin in reserve until combination therapy with oral agents has failed, possibly resulting in years of suboptimal glycemic control. Newer strategies recommend earlier initiation of insulin—ie, once diet and exercise fail, or when treatment with 1 oral agent fails. The development of insulin analogs is a significant milestone on the road to achieving improved outcomes for patients with T2DM.
Rapid-acting agents
Compared with regular human insulin, newer rapid-acting insulin analogs may improve glycemic control when used at mealtimes. However, due to their shorter half-lives, these insulin analogs require augmentation with basal insulin to control hyperglycemia between meals and during the night.
Insulin lispro was the first commercially available rapid-acting insulin analog, introduced in 1996. This agent differs from human insulin by an inversion of amino acid residues in positions 28 and 29 of the insulin B-chain. Inversion prevents the formation of hexamers and dimers that tend to diffuse more slowly, thereby facilitating a rapid uptake of the insulin analog into blood and tissues.40,41 The second such agent, marketed in 2000, was insulin aspart, in which aspartic acid replaces proline at position 28 of the B-chain of human insulin.41,42 The most recent rapid-acting analog is insulin glulisine, in which lysine replaces asparagine near the N-terminus of the B-chain, and glutamic acid replaces lysine near the C-terminus of human insulin.
The molecular changes made in creating these analogs allows them to dissociate quickly into monomers that are absorbed rapidly and achieve faster peak levels compared with regular human insulin.41,42 These changes do not, however, interfere with the analogs’ ability to bind to the insulin receptor.43,44
Dosing considerations. Absorption of regular human insulin is not sufficiently rapid at mealtimes to control prandial glucose levels.45 Therefore, it is essential to give regular insulin 30 to 60 minutes before meals. For patients who have erratic daily schedules, adhering to this sort of routine can be difficult. But even if scheduling is not a problem, the prolonged duration of action of human insulin can predispose patients to hypoglycemia. Moreover, absorption of regular insulin can vary dramatically from day to day.46,47
The insulin analogs correct the pharmacokinetic and pharmacodynamic deficiencies of regular insulin, producing plasma profiles that more closely simulate normal, physiologic meal-stimulated insulin release.48-50 The 3 rapid-acting agents (aspart, glulisine, lispro) have very similar onset and duration of action, with peak effect occurring close to injection time (TABLE 2 and FIGURE 2).48-50
Advantages of rapid-acting agents. These agents can be administered closer to meals, giving patients more flexibility and likely tighter postprandial glucose control, with reductions in glycemic excursions. Another advantage is the ability to better match insulin dose to anticipated carbohydrate in-take, affording better postprandial control.51-53 Rapid-acting analogs also result in fewer episodes of hypoglycemia. In a meta-analysis of 2576 patients, hypoglycemic events occurred 25% less often with insulin lispro compared with regular human insulin in patients with type 1 diabetes mellitus (T1DM).52 In clinical trials, insulin aspart and insulin glulisine have also caused fewer hypoglycemic events compared with regular human insulin.51-53
Long-acting agents
Basal, or long-acting, insulins are important for maintaining normoglycemia over 24 hours. Neutral protamine Hagedorn (NPH) insulin reaches its peak effect 4 to 10 hours after injection, and its total effect lasts only 12 to 18 hours. NPH is therefore often dosed twice daily. Absorption of NPH can vary significantly, causing day-to-day blood glucose fluctuations.46,47 Therefore, this agent’s activity does not closely resemble normal physiologic basal insulin secretion.
The newer long-acting insulin analogs—insulin detemir and insulin glargine—were designed to more closely replicate normal physiologic basal insulin secretion. Insulin glargine was first to reach the market, in 2001. It contains glycine instead of asparagine in the alpha-chain and 2 arginine residues at the C-terminus, and the addition of zinc enhances the aggregation and slow release at a neutral pH. Insulin glargine precipitates in the subcutaneous tissue, which slows its absorption and results in a relatively flat insulin plasma profile and extended action.54,55 Insulin detemir is a combination of the original insulin molecule and a saturated fatty acid (myristic acid). Insulin detemir is designed to bind albumin (98% albumin-bound in circulation) through this fatty acid chain in the plasma after injection, resulting in an extended plasma profile.54,56 Insulin glargine and NPH form crystalline depots, but detemir is soluble and the subcutaneous depot remains in a liquid state; this may account for differences in absorption variability.56
Advantages of the long-acting insulin analogs. Compared with conventional basal insulin such as NPH, the analogs have a prolonged duration of action (up to 24 hours) without pronounced peaks, permitting once-daily dosing in many patients (TABLE 2 and FIGURE 2).46,47,50,55 The pharmacodynamic and pharmacokinetic properties of the long- acting agents make them less likely than NPH to cause nocturnal and overall hypoglycemia, a benefit that has been observed in several clinical trials.57-61
Comparative clinical trials evaluating glycemic variability. Both of the long-acting analogs have shown lower within-subject variability in blood and plasma glucose measurements when compared with NPH.62-64 In head-to-head comparisons of the analogs in glucose clamp studies, insulin detemir has demonstrated less within-subject variability of blood glucose levels than insulin glargine, in patients with T1DM or T2DM.56,64 In clinical practice, different patients may have better results with one of these basal insulins as opposed to the other, and treatment choices will need to be tailored to the individual patient.
TABLE 2
Pharmacokinetic properties giving insulin analogs an advantage over regular insulin46-50
| Insulin preparation | Onset of action | Peak action | Duration of action |
|---|---|---|---|
| Short-acting | |||
| Regular | 30-60 minutes | 2-3 hours | 8-10 hours |
| Lispro | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Aspart | 5-15 minutes | 30-90 minutes | 4-6 hours |
| Glulisine | 20 minutes | 90 minutes | 5.3 hours |
| Long-acting | |||
| NPH | 2-4 hours | 4-10 hours | 12-18 hours |
| Glargine | 2-4 hours | Relatively flat | Up to 24 hours |
| Detemir | 1-2 hours | Relatively flat | Up to 24 hours |
| NPH, neutral protamine Hagedorn. | |||
FIGURE 2
Pharmacokinetic profiles of human insulin and insulin analogs
Adapted from: Burton S. J Fam Pract. 2006;55(12 suppl):S10-S17.
CORRESPONDENCE Eric L. Johnson, MD, University of North Dakota School of Medicine and Health Sciences, Department of Family & Community Medicine, 501 North Columbia Road, Grand Forks, ND 58202-9037; [email protected]
1. Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17-20.
2. Kadowaki S, Okamura T, Hozawa A, et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia. 2008;51:575-582.
3. Wändell PE, Theobald H. The association between low fasting glucose value and mortality. Curr Diabetes Rev. 2007;3:274-279.
4. Wei M, Gibbons LW, Mitchell TL, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047-2052.
5. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178-181.
6. Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830-1834.
7. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107-2114.
8. Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494.
9. Meigs JB, Nathan DM, D’Agostino RB, Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845-1850.
10. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360-367.
11. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
12. Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36:689-692.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617-621.
14. Balkau B, Hu G, Qiao Q, et al. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia. 2004;47:2118-2128.
15. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
16. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
17. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412.
18. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
19. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
20. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
21. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.-
22. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
23. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;11:2288-2298.
24. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
25. Standards of medical care in diabetes—2010 Diabetes Care. 2010;33(suppl 1):S11-S61.
26. Barnett AH. Treating to goal: challenges of current management. Eur J Endocrinol. 2004;151(suppl 2):T3-T7.
27. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
28. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
29. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193-203.
30. Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849-854.
31. Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611-2618.
32. Chaudhuri A, Kanjwal Y, Mohanty P, et al. Insulin-induced vasodilatation of internal carotid artery. Metabolism. 1999;48:1470-1473.
33. Melidonis A, Stefanidis A, Tournis S, et al. The role of strict metabolic control by insulin infusion on fibrinolytic profile during an acute coronary event in diabetic patients. Clin Cardiol. 2000;23:160-164.
34. Garvey WT, Olefsky JM, Griffin J, et al. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222-234.
35. Rolla A. The pathophysiological basis for intensive insulin replacement. Int J Obes Relat Metab Disord. 2004;28(suppl 2):S3-S7.
36. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231-2237.
37. Glaser B, Leibovich G, Nesher R, et al. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh). 1988;118:365-373.
38. Andrews WJ, Vasquez B, Nagulesparan M, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33:634-642.
39. Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028-1032.
40. Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7:1303-1325.
41. Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527-533.
42. Garber AJ. Pharmacologic modifications of hormones to improve their therapeutic potential for diabetes management. Diabetes Obes Metab. 2005;7:666-674.
43. Hansen BF, Danielsen GM, Drejer K, et al. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. 1996;315(pt 1):271-279.
44. Slieker LJ, Brooke GS, DiMarchi RD, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997;40(suppl 2):S54-S61.
45. Dimitriadis GD, Gerich JE. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6:374-377.
46. Roy B, Chou MC, Field JB. Time-action characteristics of regular and NPH insulin in insulin-treated diabetics. J Clin Endocrinol Metab. 1980;50:475-479.
47. Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care. 1984;7:188-199.
48. Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal-related plasma glucose excursions in type 1 diabetic subjects. Diabetes Care. 1991;14:571-577.
49. Becker RH, Frick AD, Burger F, et al. A comparison of the steady-state pharmacokinetics and pharmacodynamics of a novel rapid-acting insulin analog, insulin glulisine, and regular human insulin in healthy volunteers using the euglycemic clamp technique. Exp Clin Endocrinol Diabetes. 2005;113:292-297.
50. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
51. Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care. 1999;22:801-805.
52. Brunelle BL, Llewelyn J, Anderson JH, Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726-1731.
53. Home PD, Lindholm A, Hylleberg B, et al. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998;21:1904-1909.
54. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;5:648-659.
55. Bolli GB, Di Marchi RD, Park GD, et al. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42:1151-1167.
56. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290-299.
57. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
58. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
59. Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
60. Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269-1274.
61. Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569-1581.
62. Haak T, Tiengo A, Draeger E, et al. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56-64.
63. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
64. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
65. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453-458.
66. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625.
67. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
68. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993;36:1119-1125.
69. Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795-2804.
70. Jones SC, Saunders HJ, Qi W, et al. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999;42:1113-1119.
71. Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924-E930.
72. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
1. Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17-20.
2. Kadowaki S, Okamura T, Hozawa A, et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia. 2008;51:575-582.
3. Wändell PE, Theobald H. The association between low fasting glucose value and mortality. Curr Diabetes Rev. 2007;3:274-279.
4. Wei M, Gibbons LW, Mitchell TL, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047-2052.
5. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178-181.
6. Temelkova-Kurktschiev TS, Koehler C, Henkel E, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830-1834.
7. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107-2114.
8. Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494.
9. Meigs JB, Nathan DM, D’Agostino RB, Sr, et al. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845-1850.
10. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360-367.
11. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
12. Donahue RP, Abbott RD, Reed DM, et al. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry. Honolulu Heart Program. Diabetes. 1987;36:689-692.
13. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617-621.
14. Balkau B, Hu G, Qiao Q, et al. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia. 2004;47:2118-2128.
15. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
16. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
17. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412.
18. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
19. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
20. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
21. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.-
22. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
23. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;11:2288-2298.
24. Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
25. Standards of medical care in diabetes—2010 Diabetes Care. 2010;33(suppl 1):S11-S61.
26. Barnett AH. Treating to goal: challenges of current management. Eur J Endocrinol. 2004;151(suppl 2):T3-T7.
27. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540.
28. Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342-1349.
29. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193-203.
30. Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109:849-854.
31. Rask-Madsen C, Ihlemann N, Krarup T, et al. Insulin therapy improves insulin-stimulated endothelial function in patients with type 2 diabetes and ischemic heart disease. Diabetes. 2001;50:2611-2618.
32. Chaudhuri A, Kanjwal Y, Mohanty P, et al. Insulin-induced vasodilatation of internal carotid artery. Metabolism. 1999;48:1470-1473.
33. Melidonis A, Stefanidis A, Tournis S, et al. The role of strict metabolic control by insulin infusion on fibrinolytic profile during an acute coronary event in diabetic patients. Clin Cardiol. 2000;23:160-164.
34. Garvey WT, Olefsky JM, Griffin J, et al. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222-234.
35. Rolla A. The pathophysiological basis for intensive insulin replacement. Int J Obes Relat Metab Disord. 2004;28(suppl 2):S3-S7.
36. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231-2237.
37. Glaser B, Leibovich G, Nesher R, et al. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh). 1988;118:365-373.
38. Andrews WJ, Vasquez B, Nagulesparan M, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984;33:634-642.
39. Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028-1032.
40. Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7:1303-1325.
41. Brems DN, Alter LA, Beckage MJ, et al. Altering the association properties of insulin by amino acid replacement. Protein Eng. 1992;5:527-533.
42. Garber AJ. Pharmacologic modifications of hormones to improve their therapeutic potential for diabetes management. Diabetes Obes Metab. 2005;7:666-674.
43. Hansen BF, Danielsen GM, Drejer K, et al. Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. 1996;315(pt 1):271-279.
44. Slieker LJ, Brooke GS, DiMarchi RD, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997;40(suppl 2):S54-S61.
45. Dimitriadis GD, Gerich JE. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6:374-377.
46. Roy B, Chou MC, Field JB. Time-action characteristics of regular and NPH insulin in insulin-treated diabetics. J Clin Endocrinol Metab. 1980;50:475-479.
47. Binder C, Lauritzen T, Faber O, et al. Insulin pharmacokinetics. Diabetes Care. 1984;7:188-199.
48. Kang S, Creagh FM, Peters JR, et al. Comparison of subcutaneous soluble human insulin and insulin analogues (AspB9, GluB27; AspB10; AspB28) on meal-related plasma glucose excursions in type 1 diabetic subjects. Diabetes Care. 1991;14:571-577.
49. Becker RH, Frick AD, Burger F, et al. A comparison of the steady-state pharmacokinetics and pharmacodynamics of a novel rapid-acting insulin analog, insulin glulisine, and regular human insulin in healthy volunteers using the euglycemic clamp technique. Exp Clin Endocrinol Diabetes. 2005;113:292-297.
50. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
51. Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care. 1999;22:801-805.
52. Brunelle BL, Llewelyn J, Anderson JH, Jr, et al. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726-1731.
53. Home PD, Lindholm A, Hylleberg B, et al. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998;21:1904-1909.
54. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;5:648-659.
55. Bolli GB, Di Marchi RD, Park GD, et al. Insulin analogues and their potential in the management of diabetes mellitus. Diabetologia. 1999;42:1151-1167.
56. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290-299.
57. Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
58. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
59. Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
60. Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269-1274.
61. Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569-1581.
62. Haak T, Tiengo A, Draeger E, et al. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2005;7:56-64.
63. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
64. Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614-1620.
65. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453-458.
66. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625.
67. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820.
68. Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia. 1993;36:1119-1125.
69. Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795-2804.
70. Jones SC, Saunders HJ, Qi W, et al. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999;42:1113-1119.
71. Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924-E930.
72. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687.
How best to manage dysfunctional uterine bleeding
• Assess postmenopausal women for cancer by endometrial biopsy, transvaginal ultrasound, or saline infusion sonohysterogram. A
• Treat mild dysfunctional uterine bleeding (DUB) with nonsteroidal anti-inflammatory drugs, levonorgestrel intrauterine device (IUD), or danazol. A
• Treat moderate DUB with oral contraceptive pills C, levonorgestrel IUD, danazol, or tranexamic acid. A
• Treat severe DUB with the same agents used for moderate DUB, or with IV estrogen followed by oral contraceptive pills. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Test your skills: How would you treat these 3 patients?
CASE 1: Casey is a 14-year-old with a normal body mass index who has had heavy vaginal bleeding for 10 days. For the last 3 days, the bleeding has been so heavy she has been soaking more than 15 pads a day. She feels tired and is light-headed and dizzy when she stands up. Casey had her first period 13 months ago. Since then, her periods have varied in length from 18 to 40 days, with heavy bleeding for 7 to 14 days. She tells you she is not taking any prescription or herbal medications or over-the-counter supplements, and does not have any other medical problems. She is not sexually active. Her physical examination was remarkable only for pale skin and a positive tilt test. She feels frustrated and wants something done immediately.
CASE 2: Sarah is a 35-year-old obese woman whose chief complaint is irregular periods. For the past 3 years, she has had only 3 to 6 periods per year, each period lasting for 3 to 10 days. Her most recent period was 4 months ago. Sarah has a moderate amount of acne and facial hair.
CASE 3: Joan is a 53-year-old postmenopausal woman who has never been pregnant. She has a history of type 2 diabetes mellitus, hypertension, and obesity. She has come to your office for a routine physical examination. She tells you that her periods were regular until she stopped menstruating 4 years ago. But for the past 6 to 8 months she says she’s had irregular bleeding every 30 to 45 days, each period of bleeding lasting for 3 to 7 days. Her previous Pap smears and mammograms were normal. She has no family history of breast, gastrointestinal, or genital tract cancer. Her physical examination, including her pelvic examination, is negative.
These 3 women are fairly typical patients in a family medicine practice. Most women experience episodes of abnormal uterine bleeding (AUB) at some point in their reproductive lives. The condition occurs in approximately 1 in every 3 women of reproductive age and 1 in 10 postmenopausal women, and the impact on quality of life is often substantial.1,2 Abnormal bleeding can be divided into 4 major categories: genital tract pathology, systemic disease, exposure to medication or radiation, and dysfunctional uterine bleeding (DUB). Specific conditions within each category are listed in TABLE 1. The focus of this article will be on DUB, the category that remains after the other possibilities are excluded.
First, find out what your patient means by “abnormal”
A normal menstrual cycle varies in length between 24 and 35 days, with menstrual flow lasting 2 to 7 days. Blood loss of 30 to 80 cc per cycle is considered normal.3-5 To quantify blood loss, ask the patient how many pads or tampons she uses each period (<21 would be normal), how often she has to change pads (every 3 hours is usual), the size of clots (less than 1 cm is normal), and whether she has to get up at night to change pads.6 If blood loss is sufficient to cause anemia, the condition is always considered abnormal and requires further evaluation. When your patient’s description leaves you in doubt about whether her bleeding is abnormal, base your evaluation and treatment on her perception of a change in her menstrual cycle.
Stages of the reproductive life cycle
The meaning of abnormal bleeding varies with your patient’s stage in her reproductive life cycle. Uterine bleeding in a premenarchal child or a postmenopausal woman is always abnormal and must be evaluated.7-9
Premenarchal children with vaginal bleeding should be evaluated for trauma, sexual or physical abuse, foreign bodies, signs of precocious puberty, and possible infectious etiologies.7 If the cause of the bleeding is not obvious, these patients should be immediately referred to a pediatric gynecologist.
Postmenopausal bleeding is defined as any bleeding that occurs more than 1 year after the last menstrual period.8 Cancer is the primary concern in these women and must always be excluded. (More on that, in a bit.)
History may reveal underlying pathology
The initial approach to evaluating abnormal bleeding is a thorough history and physical. Ask about stress, dietary habits, exercise, medications, radiation exposure, visual disturbances, headache, weight loss or gain, galactorrhea, palpitations, abdominal symptoms, jaundice, or excessive hair growth. Your patient’s answers to these questions may point to pathologies that underlie the abnormal bleeding, as listed in TABLE 1.
TABLE 1
Abnormal uterine bleeding: A typology3,5,17,18,22-24,30
| Genital tract pathology |
| Vulva Cancer Lichen sclerosis Sexually transmitted diseases (STDs) Vagina STDs Trauma Foreign body Cancer Cervix STDs Cervicitis Cancer Uterus Endometritis Hyperplasia Cancer Polyps Leiomyomas |
| Systemic disease |
| Crohn’s disease Von Willebrand’s disease Thrombocytopenia Acute leukemia Advanced liver disease Hyper/hypothyroidism Chronic renal disease Pituitary disease Emotional or physical stress |
| Medication/iatrogenic cause |
| Tamoxifen Corticosteroids Chemotherapy Anticoagulants Warfarin Aspirin Clopidogrel Antipsychotics Hormonal therapy Oral contraceptives Medroxyprogesterone acetate Intrauterine devices Herbal supplement Black cohosh Soy supplements Radiation |
| Dysfunctional uterine bleeding |
| Anovulatory (90%) Hypothalamic suppression Pituitary adenoma Eating disorders Thyroid disorders Adrenal disorders Primary ovarian disorders (such as polycystic ovarian syndrome) Ovulatory (10%) Structural anomalies |
In postmenopausal women, rule out cancer
The initial work-up for a postmenopausal patient should begin with a pelvic examination, followed by an assessment of her endometrial cavity by transvaginal ultrasound (TVUS), saline infusion sonohysterogram (SIS), or biopsy. An SIS, in particular, is often superior to TVUS in screening for anatomic anomalies.10 If a sonogram shows an endometrial thickness greater than 5 mm or the patient has risk factors for endometrial neoplasia, an endometrial biopsy for histologic diagnosis will be needed.11 Risk factors for endometrial cancer include age older than 40, infertility, diabetes mellitus, hypertension, obesity, and estrogen medication. Repeat the sampling if the biopsy is inadequate. If the patient continues to have uterine bleeding, further evaluation with hysteroscopy by a gynecologist should be considered.12-14
Evaluating bleeding in women of child-bearing age
Bleeding in this age group is most often related to pregnancy, so the diagnostic work-up should begin with a urine pregnancy test.3,15 If pregnancy is ruled out, most etiologies in these women are benign, respond to conservative therapy, and can often be managed exclusively by family physicians.
After excluding pregnancy, look for genital tract pathology, including infection, polyps, uterine fibroids, and signs of cancer; iatrogenic causes such as medications or radiation; and systemic illnesses. In teenagers, look for inherited clotting disorders such as Von Willebrand’s disorder. If cervical cancer screening is not up to date, do a Pap smear.16 Cervical dysplasia generally does not cause heavy vaginal bleeding, but can cause postcoital bleeding.17 A complete blood count and a thyroid-stimulating hormone (TSH) level will allow you to rule out anemia, leukemia, thrombocytopenia, and thyroid disorders.11
Dysfunctional uterine bleeding (DUB): A diagnosis of exclusion
Once you have ruled out genital tract pathology, systemic disease, and iatrogenic causes, you are left with a diagnosis of dysfunctional uterine bleeding. DUB occurs most commonly at the onset of regular menstrual cycles or when menstruation is coming to an end during menopause.
The menstrual cycle in a woman with DUB may be ovulatory or anovulatory. Women who have ovulatory cycles usually know the characteristics of their menses and are often aware of minor variations in the timing or flow. A patient with an anatomic problem who has ovulatory cycles will usually present with complaints of menorrhagia.
Anovulatory cycles are more typical, occurring in 90% of patients with DUB.18 In anovulatory cycles, the corpus luteum is not produced and the ovaries do not secrete progesterone. In the absence of progesterone, constant estrogen stimulation produces a proliferative endometrium that is not sustainable. As 1 area of bleeding heals, another site begins to slough, and the result is an irregular and prolonged bleeding pattern that is unpredictable. The clinical result in this scenario is varying cycle lengths and differing amounts of menstrual blood loss.
Treatment depends on the etiology
Cervical and endometrial cancer should be ruled out early, because early diagnosis and treatment may improve survival. If the source of abnormal bleeding is an anatomic abnormality such as an endometrial polyp, removing the polyp under hysteroscopic guidance should alleviate the problem. If the bleeding is due to medication exposure or a systemic disease such as hypothyroidism, withdrawing the off ending agent or treating the systemic disorder will generally alleviate the problem.
For patients with DUB, hormonal (medroxyprogesterone acetate, oral contraceptive pills [OCPs], levonorgestrel intrauterine device [IUD]) and nonhormonal treatment (nonsteroidal anti-inflammatory drugs, tranexamic acid, danazol) decisions are based on the age of the patient, severity of bleeding and symptoms, and the patient’s hematocrit.19 Patients with persistent bleeding despite medical treatment require a complete reevaluation and referral to a gynecologist if an explanation is not found or if surgical treatment is required.
CASE 1: Casey
Where does Casey fit in this typology? She is in the perimenarchal stage of her reproductive life cycle, when anovulatory bleeding is common. For the first 18 to 24 months after menarche, the immature hypothalamic-pituitary-ovarian axis may fail to respond to estrogen and progesterone stimulation, resulting in anovulation and irregular, often heavy bleeding. Her urine test rules out pregnancy. Blood tests confirm the anemia her pallor and fatigue suggest. Her initial, empiric treatment would be iron supplementation for anemia and cyclic medroxyprogesterone acetate or OCPs (TABLE 2) to regulate her periods. If these conservative measures are not sufficient, further evaluation would be indicated.
Blood dyscrasias (5%-20% incidence in teenagers) and systemic disorders, including Von Willebrand’s disease, idiopathic thrombocytopenic purpura, and leukemia, are the major diseases to consider.20-23 An endometrial biopsy is not indicated, because the incidence of endometrial cancer in Casey’s age group is less than 1 in 100,000. 24
CASE 2: Sarah
How can you explain Sarah’s irregular periods? A negative urine test rules out pregnancy, and her responses to questions about diet, exercise, and stress rule out hypothalamic suppression. She doesn’t complain of headaches, visual field changes, or galactorrhea, which would exclude a pituitary microadenoma. She does not exhibit symptoms of a thyroid disorder and her TSH is normal. Complaints of frequent urination, thirst, or weight loss could be indications of diabetes mellitus, but Sarah does not present with these symptoms. Her facial hair and acne suggest androgen excess originating from the adrenal glands or ovaries.
Sarah’s history of infrequent and heavy menses, as well as an absence of breast tenderness, bloating, or mittelschmerz, indicate she is not ovulating. The most likely explanation for her failure to ovulate is polycystic ovarian syndrome (PCOS), and you can initiate treatment immediately. The major treatment options for this disorder are observation, medroxyprogesterone acetate, and OCPs (TABLE 2).
If Sarah does not respond to hormonal therapy, a thorough reevaluation is indicated, including additional laboratory tests and a pelvic sonogram to evaluate the uterus and ovaries. Other tests to consider include prolactin, fasting blood sugar, early morning 17-hydroxy-progesterone, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S), testosterone, and cortisol. For an extensive review of PCOS and its relationship with endocrine, metabolic, and reproductive disorders, as well as cardiovascular disease and obstructive sleep apnea, see the excellent review by Ehrmann.25 If hormonal therapy is unsuccessful, a hysteroscopy with endometrial ablation could then be offered. In refractory cases, a hysterectomy can be performed.
Although Sarah is only 35, her prolonged exposure to unopposed estrogen (>3 years, according to her history) warrants an endometrial biopsy. The presence of other endometrial cancer risk factors (obesity, chronic anovulation, nulliparity) supports this decision. The incidence of endometrial cancer is 2.3 cases per 100,000 patients in 30- to 34-year-old women, 6.1 cases per 100,000 patients in 35- to 39-year-old women, increasing to 36.5 cases per 100,000 in women ages 40 to 49 years. 24
If Sarah is troubled by her infertility, consider referring her to a specialist. Treatment options for her infertility would include weight loss, insulin-lowering medications, and clomiphene citrate to induce ovulation.
CASE 3: Joan
Uterine bleeding in a postmenopausal patient like Joan is always abnormal. In 5% to 10% of cases, such bleeding indicates endometrial cancer. 26,27 An endometrial biopsy to rule out cancer is the first order of business. If the biopsy is nondiagnostic or reveals endometrial polyps or submucosal fibroids, the next step would be a diagnostic hysteroscopy. Alternatively, Joan’s endometrium could first be evaluated with a TVUS. If the sonogram showed an endometrium 5 mm in thickness or more, an endometrial biopsy could be performed then.26-29
If these tests rule out a cancer diagnosis, your next step would be to try low-dose cyclic OCPs or medroxyprogesterone acetate (TABLE 2) to control the bleeding. If hormonal therapy is not effective or Joan doesn’t want to try it, an endometrial ablation in conjunction with a hysteroscopy performed by a gynecologist is another option. But if Joan’s bleeding is light, it may be due simply to her postmenopausal hypoestrogenic state, and can be left untreated as long as Joan is comfortable with this option.
TABLE 2
Medical treatment for dysfunctional uterine bleeding
| Mild (bleeding is minimal and symptoms limited) |
| Moderate (moderate amounts of bleeding, mild anemia, and mild orthostatic symptoms or fatigue) |
|
| Severe (heavy bleeding, moderate to severe anemia, significant orthostatic symptoms) |
|
| IUD, intrauterine device; NSAIDs, nonsteroidal anti-inflammatory drugs; OCPs, oral contraceptive pills. |
Lessons learned
Patients like Casey, Sarah, and Joan can be successfully managed by the family physician. A thorough history, physical examination, and basic laboratory tests will usually suffice to rule out anatomic, systemic, or iatrogenic explanations. Pregnancy, the most common explanation for abnormal uterine bleeding, can be ruled out with a urine pregnancy test. Patients like Sarah and Joan, who have some of the risk factors for endometrial cancer, require an evaluation of the endometrium to rule out that possibility. When none of these etiologies is the culprit, your working diagnosis is DUB, and medical treatment for it is well within your competence.
CORRESPONDENCE David L. Maness, DO, MSS, Department of Family Medicine, University of Tennessee Health Science Center, College of Medicine, 1301 Primacy Parkway, Memphis, TN 38119; [email protected]
1. Wren BG. Dysfunctional uterine bleeding. Aust Fam Physician. 1998;27:371-377.
2. Astrup K, Olivarius Nde F. Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand. 2004;83:203-207.
3. Albers JR, Hull SJ, Wesley RM. Abnormal uterine bleeding. Am Fam Physician. 2004;69:1916-1926.
4. Munster K, Schmidt L, Helm P. Length and variation in the menstrual cycle—a cross-sectional study from a Danish country. Br J Obstet Gynaecol. 1992;99:422-429.
5. Halberg L, Hogdahl AM, Nilsson L, et al. Menstrual blood loss-a population study. Acta Obstet Gynecol Scand. 1966;45:320-351.
6. Warner PE, Critchley HO, Lumsden MA, et al. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J Obstet Gynecol. 2004;190:1216-1223.
7. Hill NC, Oppenheimer LW, Morton KE. The aetiology of vaginal bleeding in children. A 20-year review. Br J Obstet Gynaecol. 1989;96:467-470.
8. Amman M, Anguino H, Bauman RA, et al. Postmenopausal uterine bleeding. Pasadena, Calif: Kaiser Permanente Southern California; December 2006. NGC 005688. Available at: www.guideline.gov. Accessed July 19, 2010.
9. Hataska H. The evaluation of abnormal uterine bleeding. Clin Obstet Gynecol. 2005;48:258-273.
10. Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2006;(2):CD003855.-
11. Amman M, Anguino H, Bauman RA, et al. Chronic abnormal uterine bleeding in nongravid women. Pasadena, Calif: Kaiser Permanente Southern California; December 2006. NGC 005687. Available at: www.guideline.gov. Accessed July 19, 2010.
12. Bradley LD, Widrich T. State-of-the-art flexible hysteroscopy for office gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
13. Nagele F, O’Connor H, Davies A, et al. 2500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87-92.
14. Serden SP. Diagnostic hysteroscopy to evaluate the cause of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:277-286.
15. Shwayder JM. Pathophysiology of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:219-234.
16. Vilos GA, Lefebvre G, Graves GR. Guidelines for the management of abnormal uterine bleeding. SOGC Clinical Practice Guidelines; August 2001. Available at: www.sogc.org/guidelines/public/106E-CPG-August2001.pdf. Accessed July 19, 2010.
17. Rosenthal AN, Panoskaltsis T, Smith T, et al. The frequency of significant pathology in women attending a general gynaecological service for postcoital bleeding. BJOG. 2001;108:103-106.
18. Beers MH, Berkow R. eds. Dysfunctional uterine bleeding, In: The Merck Manual. 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999:1941-1942.
19. Singh RH, Blumenthal P. Hormonal management of abnormal uterine bleeding. Clin Obstet Gynecol. 2005;48:337-352.
20. Edlund M, Blomback M, von Schoultz B, et al. On the value of menorrhagia as a predictor for coagulation disorders. Am J Hematol. 1996;53:234-238.
21. Kouides PA. Evaluation of abnormal bleeding in women. Curr Hematol Rep. 2002;1:11-18.
22. Kadir RA, Economides DL, Sabin CA, et al. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351:485-489.
23. Dilley A, Drews C, Miller C, et al. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol. 2001;97:630-636.
24. Ries LAG, Melbert D, Krapcho M, et al. eds SEER Cancer Statistics Review, 1975-2005.Bethesda, Md: National Cancer Institute. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008. Accessed July 19, 2010.
25. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1226.
26. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding. A Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
27. Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal bleeding. Obstet Gynecol. 2002;99:663-670.
28. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510-1517.
29. Medverd JR, Dubinsky TJ. Cost analysis model: US versus endometrial biopsy in evaluation of peri- and post-menopausal abnormal vaginal bleeding. Radiology. 2002;222:619-627.
30. Scott S. Abnormal bleeding in the pediatric patient. Postgrad Obstet Gynecol. 2006;26:1-5.
31. Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD001016.-
32. Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2008;(1):CD001016.-
33. Stewart A, Cummins C, Gold L, et al. The effectiveness of levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. Br J Obstet Gynaecol. 2001;108:74-86.
34. Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(4):CD000249.-
35. Beaumont H, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(2):CD001017.-
36. Wellington K, Wagstaff AJ. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs. 2003;63:1417-1433.
• Assess postmenopausal women for cancer by endometrial biopsy, transvaginal ultrasound, or saline infusion sonohysterogram. A
• Treat mild dysfunctional uterine bleeding (DUB) with nonsteroidal anti-inflammatory drugs, levonorgestrel intrauterine device (IUD), or danazol. A
• Treat moderate DUB with oral contraceptive pills C, levonorgestrel IUD, danazol, or tranexamic acid. A
• Treat severe DUB with the same agents used for moderate DUB, or with IV estrogen followed by oral contraceptive pills. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Test your skills: How would you treat these 3 patients?
CASE 1: Casey is a 14-year-old with a normal body mass index who has had heavy vaginal bleeding for 10 days. For the last 3 days, the bleeding has been so heavy she has been soaking more than 15 pads a day. She feels tired and is light-headed and dizzy when she stands up. Casey had her first period 13 months ago. Since then, her periods have varied in length from 18 to 40 days, with heavy bleeding for 7 to 14 days. She tells you she is not taking any prescription or herbal medications or over-the-counter supplements, and does not have any other medical problems. She is not sexually active. Her physical examination was remarkable only for pale skin and a positive tilt test. She feels frustrated and wants something done immediately.
CASE 2: Sarah is a 35-year-old obese woman whose chief complaint is irregular periods. For the past 3 years, she has had only 3 to 6 periods per year, each period lasting for 3 to 10 days. Her most recent period was 4 months ago. Sarah has a moderate amount of acne and facial hair.
CASE 3: Joan is a 53-year-old postmenopausal woman who has never been pregnant. She has a history of type 2 diabetes mellitus, hypertension, and obesity. She has come to your office for a routine physical examination. She tells you that her periods were regular until she stopped menstruating 4 years ago. But for the past 6 to 8 months she says she’s had irregular bleeding every 30 to 45 days, each period of bleeding lasting for 3 to 7 days. Her previous Pap smears and mammograms were normal. She has no family history of breast, gastrointestinal, or genital tract cancer. Her physical examination, including her pelvic examination, is negative.
These 3 women are fairly typical patients in a family medicine practice. Most women experience episodes of abnormal uterine bleeding (AUB) at some point in their reproductive lives. The condition occurs in approximately 1 in every 3 women of reproductive age and 1 in 10 postmenopausal women, and the impact on quality of life is often substantial.1,2 Abnormal bleeding can be divided into 4 major categories: genital tract pathology, systemic disease, exposure to medication or radiation, and dysfunctional uterine bleeding (DUB). Specific conditions within each category are listed in TABLE 1. The focus of this article will be on DUB, the category that remains after the other possibilities are excluded.
First, find out what your patient means by “abnormal”
A normal menstrual cycle varies in length between 24 and 35 days, with menstrual flow lasting 2 to 7 days. Blood loss of 30 to 80 cc per cycle is considered normal.3-5 To quantify blood loss, ask the patient how many pads or tampons she uses each period (<21 would be normal), how often she has to change pads (every 3 hours is usual), the size of clots (less than 1 cm is normal), and whether she has to get up at night to change pads.6 If blood loss is sufficient to cause anemia, the condition is always considered abnormal and requires further evaluation. When your patient’s description leaves you in doubt about whether her bleeding is abnormal, base your evaluation and treatment on her perception of a change in her menstrual cycle.
Stages of the reproductive life cycle
The meaning of abnormal bleeding varies with your patient’s stage in her reproductive life cycle. Uterine bleeding in a premenarchal child or a postmenopausal woman is always abnormal and must be evaluated.7-9
Premenarchal children with vaginal bleeding should be evaluated for trauma, sexual or physical abuse, foreign bodies, signs of precocious puberty, and possible infectious etiologies.7 If the cause of the bleeding is not obvious, these patients should be immediately referred to a pediatric gynecologist.
Postmenopausal bleeding is defined as any bleeding that occurs more than 1 year after the last menstrual period.8 Cancer is the primary concern in these women and must always be excluded. (More on that, in a bit.)
History may reveal underlying pathology
The initial approach to evaluating abnormal bleeding is a thorough history and physical. Ask about stress, dietary habits, exercise, medications, radiation exposure, visual disturbances, headache, weight loss or gain, galactorrhea, palpitations, abdominal symptoms, jaundice, or excessive hair growth. Your patient’s answers to these questions may point to pathologies that underlie the abnormal bleeding, as listed in TABLE 1.
TABLE 1
Abnormal uterine bleeding: A typology3,5,17,18,22-24,30
| Genital tract pathology |
| Vulva Cancer Lichen sclerosis Sexually transmitted diseases (STDs) Vagina STDs Trauma Foreign body Cancer Cervix STDs Cervicitis Cancer Uterus Endometritis Hyperplasia Cancer Polyps Leiomyomas |
| Systemic disease |
| Crohn’s disease Von Willebrand’s disease Thrombocytopenia Acute leukemia Advanced liver disease Hyper/hypothyroidism Chronic renal disease Pituitary disease Emotional or physical stress |
| Medication/iatrogenic cause |
| Tamoxifen Corticosteroids Chemotherapy Anticoagulants Warfarin Aspirin Clopidogrel Antipsychotics Hormonal therapy Oral contraceptives Medroxyprogesterone acetate Intrauterine devices Herbal supplement Black cohosh Soy supplements Radiation |
| Dysfunctional uterine bleeding |
| Anovulatory (90%) Hypothalamic suppression Pituitary adenoma Eating disorders Thyroid disorders Adrenal disorders Primary ovarian disorders (such as polycystic ovarian syndrome) Ovulatory (10%) Structural anomalies |
In postmenopausal women, rule out cancer
The initial work-up for a postmenopausal patient should begin with a pelvic examination, followed by an assessment of her endometrial cavity by transvaginal ultrasound (TVUS), saline infusion sonohysterogram (SIS), or biopsy. An SIS, in particular, is often superior to TVUS in screening for anatomic anomalies.10 If a sonogram shows an endometrial thickness greater than 5 mm or the patient has risk factors for endometrial neoplasia, an endometrial biopsy for histologic diagnosis will be needed.11 Risk factors for endometrial cancer include age older than 40, infertility, diabetes mellitus, hypertension, obesity, and estrogen medication. Repeat the sampling if the biopsy is inadequate. If the patient continues to have uterine bleeding, further evaluation with hysteroscopy by a gynecologist should be considered.12-14
Evaluating bleeding in women of child-bearing age
Bleeding in this age group is most often related to pregnancy, so the diagnostic work-up should begin with a urine pregnancy test.3,15 If pregnancy is ruled out, most etiologies in these women are benign, respond to conservative therapy, and can often be managed exclusively by family physicians.
After excluding pregnancy, look for genital tract pathology, including infection, polyps, uterine fibroids, and signs of cancer; iatrogenic causes such as medications or radiation; and systemic illnesses. In teenagers, look for inherited clotting disorders such as Von Willebrand’s disorder. If cervical cancer screening is not up to date, do a Pap smear.16 Cervical dysplasia generally does not cause heavy vaginal bleeding, but can cause postcoital bleeding.17 A complete blood count and a thyroid-stimulating hormone (TSH) level will allow you to rule out anemia, leukemia, thrombocytopenia, and thyroid disorders.11
Dysfunctional uterine bleeding (DUB): A diagnosis of exclusion
Once you have ruled out genital tract pathology, systemic disease, and iatrogenic causes, you are left with a diagnosis of dysfunctional uterine bleeding. DUB occurs most commonly at the onset of regular menstrual cycles or when menstruation is coming to an end during menopause.
The menstrual cycle in a woman with DUB may be ovulatory or anovulatory. Women who have ovulatory cycles usually know the characteristics of their menses and are often aware of minor variations in the timing or flow. A patient with an anatomic problem who has ovulatory cycles will usually present with complaints of menorrhagia.
Anovulatory cycles are more typical, occurring in 90% of patients with DUB.18 In anovulatory cycles, the corpus luteum is not produced and the ovaries do not secrete progesterone. In the absence of progesterone, constant estrogen stimulation produces a proliferative endometrium that is not sustainable. As 1 area of bleeding heals, another site begins to slough, and the result is an irregular and prolonged bleeding pattern that is unpredictable. The clinical result in this scenario is varying cycle lengths and differing amounts of menstrual blood loss.
Treatment depends on the etiology
Cervical and endometrial cancer should be ruled out early, because early diagnosis and treatment may improve survival. If the source of abnormal bleeding is an anatomic abnormality such as an endometrial polyp, removing the polyp under hysteroscopic guidance should alleviate the problem. If the bleeding is due to medication exposure or a systemic disease such as hypothyroidism, withdrawing the off ending agent or treating the systemic disorder will generally alleviate the problem.
For patients with DUB, hormonal (medroxyprogesterone acetate, oral contraceptive pills [OCPs], levonorgestrel intrauterine device [IUD]) and nonhormonal treatment (nonsteroidal anti-inflammatory drugs, tranexamic acid, danazol) decisions are based on the age of the patient, severity of bleeding and symptoms, and the patient’s hematocrit.19 Patients with persistent bleeding despite medical treatment require a complete reevaluation and referral to a gynecologist if an explanation is not found or if surgical treatment is required.
CASE 1: Casey
Where does Casey fit in this typology? She is in the perimenarchal stage of her reproductive life cycle, when anovulatory bleeding is common. For the first 18 to 24 months after menarche, the immature hypothalamic-pituitary-ovarian axis may fail to respond to estrogen and progesterone stimulation, resulting in anovulation and irregular, often heavy bleeding. Her urine test rules out pregnancy. Blood tests confirm the anemia her pallor and fatigue suggest. Her initial, empiric treatment would be iron supplementation for anemia and cyclic medroxyprogesterone acetate or OCPs (TABLE 2) to regulate her periods. If these conservative measures are not sufficient, further evaluation would be indicated.
Blood dyscrasias (5%-20% incidence in teenagers) and systemic disorders, including Von Willebrand’s disease, idiopathic thrombocytopenic purpura, and leukemia, are the major diseases to consider.20-23 An endometrial biopsy is not indicated, because the incidence of endometrial cancer in Casey’s age group is less than 1 in 100,000. 24
CASE 2: Sarah
How can you explain Sarah’s irregular periods? A negative urine test rules out pregnancy, and her responses to questions about diet, exercise, and stress rule out hypothalamic suppression. She doesn’t complain of headaches, visual field changes, or galactorrhea, which would exclude a pituitary microadenoma. She does not exhibit symptoms of a thyroid disorder and her TSH is normal. Complaints of frequent urination, thirst, or weight loss could be indications of diabetes mellitus, but Sarah does not present with these symptoms. Her facial hair and acne suggest androgen excess originating from the adrenal glands or ovaries.
Sarah’s history of infrequent and heavy menses, as well as an absence of breast tenderness, bloating, or mittelschmerz, indicate she is not ovulating. The most likely explanation for her failure to ovulate is polycystic ovarian syndrome (PCOS), and you can initiate treatment immediately. The major treatment options for this disorder are observation, medroxyprogesterone acetate, and OCPs (TABLE 2).
If Sarah does not respond to hormonal therapy, a thorough reevaluation is indicated, including additional laboratory tests and a pelvic sonogram to evaluate the uterus and ovaries. Other tests to consider include prolactin, fasting blood sugar, early morning 17-hydroxy-progesterone, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S), testosterone, and cortisol. For an extensive review of PCOS and its relationship with endocrine, metabolic, and reproductive disorders, as well as cardiovascular disease and obstructive sleep apnea, see the excellent review by Ehrmann.25 If hormonal therapy is unsuccessful, a hysteroscopy with endometrial ablation could then be offered. In refractory cases, a hysterectomy can be performed.
Although Sarah is only 35, her prolonged exposure to unopposed estrogen (>3 years, according to her history) warrants an endometrial biopsy. The presence of other endometrial cancer risk factors (obesity, chronic anovulation, nulliparity) supports this decision. The incidence of endometrial cancer is 2.3 cases per 100,000 patients in 30- to 34-year-old women, 6.1 cases per 100,000 patients in 35- to 39-year-old women, increasing to 36.5 cases per 100,000 in women ages 40 to 49 years. 24
If Sarah is troubled by her infertility, consider referring her to a specialist. Treatment options for her infertility would include weight loss, insulin-lowering medications, and clomiphene citrate to induce ovulation.
CASE 3: Joan
Uterine bleeding in a postmenopausal patient like Joan is always abnormal. In 5% to 10% of cases, such bleeding indicates endometrial cancer. 26,27 An endometrial biopsy to rule out cancer is the first order of business. If the biopsy is nondiagnostic or reveals endometrial polyps or submucosal fibroids, the next step would be a diagnostic hysteroscopy. Alternatively, Joan’s endometrium could first be evaluated with a TVUS. If the sonogram showed an endometrium 5 mm in thickness or more, an endometrial biopsy could be performed then.26-29
If these tests rule out a cancer diagnosis, your next step would be to try low-dose cyclic OCPs or medroxyprogesterone acetate (TABLE 2) to control the bleeding. If hormonal therapy is not effective or Joan doesn’t want to try it, an endometrial ablation in conjunction with a hysteroscopy performed by a gynecologist is another option. But if Joan’s bleeding is light, it may be due simply to her postmenopausal hypoestrogenic state, and can be left untreated as long as Joan is comfortable with this option.
TABLE 2
Medical treatment for dysfunctional uterine bleeding
| Mild (bleeding is minimal and symptoms limited) |
| Moderate (moderate amounts of bleeding, mild anemia, and mild orthostatic symptoms or fatigue) |
|
| Severe (heavy bleeding, moderate to severe anemia, significant orthostatic symptoms) |
|
| IUD, intrauterine device; NSAIDs, nonsteroidal anti-inflammatory drugs; OCPs, oral contraceptive pills. |
Lessons learned
Patients like Casey, Sarah, and Joan can be successfully managed by the family physician. A thorough history, physical examination, and basic laboratory tests will usually suffice to rule out anatomic, systemic, or iatrogenic explanations. Pregnancy, the most common explanation for abnormal uterine bleeding, can be ruled out with a urine pregnancy test. Patients like Sarah and Joan, who have some of the risk factors for endometrial cancer, require an evaluation of the endometrium to rule out that possibility. When none of these etiologies is the culprit, your working diagnosis is DUB, and medical treatment for it is well within your competence.
CORRESPONDENCE David L. Maness, DO, MSS, Department of Family Medicine, University of Tennessee Health Science Center, College of Medicine, 1301 Primacy Parkway, Memphis, TN 38119; [email protected]
• Assess postmenopausal women for cancer by endometrial biopsy, transvaginal ultrasound, or saline infusion sonohysterogram. A
• Treat mild dysfunctional uterine bleeding (DUB) with nonsteroidal anti-inflammatory drugs, levonorgestrel intrauterine device (IUD), or danazol. A
• Treat moderate DUB with oral contraceptive pills C, levonorgestrel IUD, danazol, or tranexamic acid. A
• Treat severe DUB with the same agents used for moderate DUB, or with IV estrogen followed by oral contraceptive pills. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Test your skills: How would you treat these 3 patients?
CASE 1: Casey is a 14-year-old with a normal body mass index who has had heavy vaginal bleeding for 10 days. For the last 3 days, the bleeding has been so heavy she has been soaking more than 15 pads a day. She feels tired and is light-headed and dizzy when she stands up. Casey had her first period 13 months ago. Since then, her periods have varied in length from 18 to 40 days, with heavy bleeding for 7 to 14 days. She tells you she is not taking any prescription or herbal medications or over-the-counter supplements, and does not have any other medical problems. She is not sexually active. Her physical examination was remarkable only for pale skin and a positive tilt test. She feels frustrated and wants something done immediately.
CASE 2: Sarah is a 35-year-old obese woman whose chief complaint is irregular periods. For the past 3 years, she has had only 3 to 6 periods per year, each period lasting for 3 to 10 days. Her most recent period was 4 months ago. Sarah has a moderate amount of acne and facial hair.
CASE 3: Joan is a 53-year-old postmenopausal woman who has never been pregnant. She has a history of type 2 diabetes mellitus, hypertension, and obesity. She has come to your office for a routine physical examination. She tells you that her periods were regular until she stopped menstruating 4 years ago. But for the past 6 to 8 months she says she’s had irregular bleeding every 30 to 45 days, each period of bleeding lasting for 3 to 7 days. Her previous Pap smears and mammograms were normal. She has no family history of breast, gastrointestinal, or genital tract cancer. Her physical examination, including her pelvic examination, is negative.
These 3 women are fairly typical patients in a family medicine practice. Most women experience episodes of abnormal uterine bleeding (AUB) at some point in their reproductive lives. The condition occurs in approximately 1 in every 3 women of reproductive age and 1 in 10 postmenopausal women, and the impact on quality of life is often substantial.1,2 Abnormal bleeding can be divided into 4 major categories: genital tract pathology, systemic disease, exposure to medication or radiation, and dysfunctional uterine bleeding (DUB). Specific conditions within each category are listed in TABLE 1. The focus of this article will be on DUB, the category that remains after the other possibilities are excluded.
First, find out what your patient means by “abnormal”
A normal menstrual cycle varies in length between 24 and 35 days, with menstrual flow lasting 2 to 7 days. Blood loss of 30 to 80 cc per cycle is considered normal.3-5 To quantify blood loss, ask the patient how many pads or tampons she uses each period (<21 would be normal), how often she has to change pads (every 3 hours is usual), the size of clots (less than 1 cm is normal), and whether she has to get up at night to change pads.6 If blood loss is sufficient to cause anemia, the condition is always considered abnormal and requires further evaluation. When your patient’s description leaves you in doubt about whether her bleeding is abnormal, base your evaluation and treatment on her perception of a change in her menstrual cycle.
Stages of the reproductive life cycle
The meaning of abnormal bleeding varies with your patient’s stage in her reproductive life cycle. Uterine bleeding in a premenarchal child or a postmenopausal woman is always abnormal and must be evaluated.7-9
Premenarchal children with vaginal bleeding should be evaluated for trauma, sexual or physical abuse, foreign bodies, signs of precocious puberty, and possible infectious etiologies.7 If the cause of the bleeding is not obvious, these patients should be immediately referred to a pediatric gynecologist.
Postmenopausal bleeding is defined as any bleeding that occurs more than 1 year after the last menstrual period.8 Cancer is the primary concern in these women and must always be excluded. (More on that, in a bit.)
History may reveal underlying pathology
The initial approach to evaluating abnormal bleeding is a thorough history and physical. Ask about stress, dietary habits, exercise, medications, radiation exposure, visual disturbances, headache, weight loss or gain, galactorrhea, palpitations, abdominal symptoms, jaundice, or excessive hair growth. Your patient’s answers to these questions may point to pathologies that underlie the abnormal bleeding, as listed in TABLE 1.
TABLE 1
Abnormal uterine bleeding: A typology3,5,17,18,22-24,30
| Genital tract pathology |
| Vulva Cancer Lichen sclerosis Sexually transmitted diseases (STDs) Vagina STDs Trauma Foreign body Cancer Cervix STDs Cervicitis Cancer Uterus Endometritis Hyperplasia Cancer Polyps Leiomyomas |
| Systemic disease |
| Crohn’s disease Von Willebrand’s disease Thrombocytopenia Acute leukemia Advanced liver disease Hyper/hypothyroidism Chronic renal disease Pituitary disease Emotional or physical stress |
| Medication/iatrogenic cause |
| Tamoxifen Corticosteroids Chemotherapy Anticoagulants Warfarin Aspirin Clopidogrel Antipsychotics Hormonal therapy Oral contraceptives Medroxyprogesterone acetate Intrauterine devices Herbal supplement Black cohosh Soy supplements Radiation |
| Dysfunctional uterine bleeding |
| Anovulatory (90%) Hypothalamic suppression Pituitary adenoma Eating disorders Thyroid disorders Adrenal disorders Primary ovarian disorders (such as polycystic ovarian syndrome) Ovulatory (10%) Structural anomalies |
In postmenopausal women, rule out cancer
The initial work-up for a postmenopausal patient should begin with a pelvic examination, followed by an assessment of her endometrial cavity by transvaginal ultrasound (TVUS), saline infusion sonohysterogram (SIS), or biopsy. An SIS, in particular, is often superior to TVUS in screening for anatomic anomalies.10 If a sonogram shows an endometrial thickness greater than 5 mm or the patient has risk factors for endometrial neoplasia, an endometrial biopsy for histologic diagnosis will be needed.11 Risk factors for endometrial cancer include age older than 40, infertility, diabetes mellitus, hypertension, obesity, and estrogen medication. Repeat the sampling if the biopsy is inadequate. If the patient continues to have uterine bleeding, further evaluation with hysteroscopy by a gynecologist should be considered.12-14
Evaluating bleeding in women of child-bearing age
Bleeding in this age group is most often related to pregnancy, so the diagnostic work-up should begin with a urine pregnancy test.3,15 If pregnancy is ruled out, most etiologies in these women are benign, respond to conservative therapy, and can often be managed exclusively by family physicians.
After excluding pregnancy, look for genital tract pathology, including infection, polyps, uterine fibroids, and signs of cancer; iatrogenic causes such as medications or radiation; and systemic illnesses. In teenagers, look for inherited clotting disorders such as Von Willebrand’s disorder. If cervical cancer screening is not up to date, do a Pap smear.16 Cervical dysplasia generally does not cause heavy vaginal bleeding, but can cause postcoital bleeding.17 A complete blood count and a thyroid-stimulating hormone (TSH) level will allow you to rule out anemia, leukemia, thrombocytopenia, and thyroid disorders.11
Dysfunctional uterine bleeding (DUB): A diagnosis of exclusion
Once you have ruled out genital tract pathology, systemic disease, and iatrogenic causes, you are left with a diagnosis of dysfunctional uterine bleeding. DUB occurs most commonly at the onset of regular menstrual cycles or when menstruation is coming to an end during menopause.
The menstrual cycle in a woman with DUB may be ovulatory or anovulatory. Women who have ovulatory cycles usually know the characteristics of their menses and are often aware of minor variations in the timing or flow. A patient with an anatomic problem who has ovulatory cycles will usually present with complaints of menorrhagia.
Anovulatory cycles are more typical, occurring in 90% of patients with DUB.18 In anovulatory cycles, the corpus luteum is not produced and the ovaries do not secrete progesterone. In the absence of progesterone, constant estrogen stimulation produces a proliferative endometrium that is not sustainable. As 1 area of bleeding heals, another site begins to slough, and the result is an irregular and prolonged bleeding pattern that is unpredictable. The clinical result in this scenario is varying cycle lengths and differing amounts of menstrual blood loss.
Treatment depends on the etiology
Cervical and endometrial cancer should be ruled out early, because early diagnosis and treatment may improve survival. If the source of abnormal bleeding is an anatomic abnormality such as an endometrial polyp, removing the polyp under hysteroscopic guidance should alleviate the problem. If the bleeding is due to medication exposure or a systemic disease such as hypothyroidism, withdrawing the off ending agent or treating the systemic disorder will generally alleviate the problem.
For patients with DUB, hormonal (medroxyprogesterone acetate, oral contraceptive pills [OCPs], levonorgestrel intrauterine device [IUD]) and nonhormonal treatment (nonsteroidal anti-inflammatory drugs, tranexamic acid, danazol) decisions are based on the age of the patient, severity of bleeding and symptoms, and the patient’s hematocrit.19 Patients with persistent bleeding despite medical treatment require a complete reevaluation and referral to a gynecologist if an explanation is not found or if surgical treatment is required.
CASE 1: Casey
Where does Casey fit in this typology? She is in the perimenarchal stage of her reproductive life cycle, when anovulatory bleeding is common. For the first 18 to 24 months after menarche, the immature hypothalamic-pituitary-ovarian axis may fail to respond to estrogen and progesterone stimulation, resulting in anovulation and irregular, often heavy bleeding. Her urine test rules out pregnancy. Blood tests confirm the anemia her pallor and fatigue suggest. Her initial, empiric treatment would be iron supplementation for anemia and cyclic medroxyprogesterone acetate or OCPs (TABLE 2) to regulate her periods. If these conservative measures are not sufficient, further evaluation would be indicated.
Blood dyscrasias (5%-20% incidence in teenagers) and systemic disorders, including Von Willebrand’s disease, idiopathic thrombocytopenic purpura, and leukemia, are the major diseases to consider.20-23 An endometrial biopsy is not indicated, because the incidence of endometrial cancer in Casey’s age group is less than 1 in 100,000. 24
CASE 2: Sarah
How can you explain Sarah’s irregular periods? A negative urine test rules out pregnancy, and her responses to questions about diet, exercise, and stress rule out hypothalamic suppression. She doesn’t complain of headaches, visual field changes, or galactorrhea, which would exclude a pituitary microadenoma. She does not exhibit symptoms of a thyroid disorder and her TSH is normal. Complaints of frequent urination, thirst, or weight loss could be indications of diabetes mellitus, but Sarah does not present with these symptoms. Her facial hair and acne suggest androgen excess originating from the adrenal glands or ovaries.
Sarah’s history of infrequent and heavy menses, as well as an absence of breast tenderness, bloating, or mittelschmerz, indicate she is not ovulating. The most likely explanation for her failure to ovulate is polycystic ovarian syndrome (PCOS), and you can initiate treatment immediately. The major treatment options for this disorder are observation, medroxyprogesterone acetate, and OCPs (TABLE 2).
If Sarah does not respond to hormonal therapy, a thorough reevaluation is indicated, including additional laboratory tests and a pelvic sonogram to evaluate the uterus and ovaries. Other tests to consider include prolactin, fasting blood sugar, early morning 17-hydroxy-progesterone, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S), testosterone, and cortisol. For an extensive review of PCOS and its relationship with endocrine, metabolic, and reproductive disorders, as well as cardiovascular disease and obstructive sleep apnea, see the excellent review by Ehrmann.25 If hormonal therapy is unsuccessful, a hysteroscopy with endometrial ablation could then be offered. In refractory cases, a hysterectomy can be performed.
Although Sarah is only 35, her prolonged exposure to unopposed estrogen (>3 years, according to her history) warrants an endometrial biopsy. The presence of other endometrial cancer risk factors (obesity, chronic anovulation, nulliparity) supports this decision. The incidence of endometrial cancer is 2.3 cases per 100,000 patients in 30- to 34-year-old women, 6.1 cases per 100,000 patients in 35- to 39-year-old women, increasing to 36.5 cases per 100,000 in women ages 40 to 49 years. 24
If Sarah is troubled by her infertility, consider referring her to a specialist. Treatment options for her infertility would include weight loss, insulin-lowering medications, and clomiphene citrate to induce ovulation.
CASE 3: Joan
Uterine bleeding in a postmenopausal patient like Joan is always abnormal. In 5% to 10% of cases, such bleeding indicates endometrial cancer. 26,27 An endometrial biopsy to rule out cancer is the first order of business. If the biopsy is nondiagnostic or reveals endometrial polyps or submucosal fibroids, the next step would be a diagnostic hysteroscopy. Alternatively, Joan’s endometrium could first be evaluated with a TVUS. If the sonogram showed an endometrium 5 mm in thickness or more, an endometrial biopsy could be performed then.26-29
If these tests rule out a cancer diagnosis, your next step would be to try low-dose cyclic OCPs or medroxyprogesterone acetate (TABLE 2) to control the bleeding. If hormonal therapy is not effective or Joan doesn’t want to try it, an endometrial ablation in conjunction with a hysteroscopy performed by a gynecologist is another option. But if Joan’s bleeding is light, it may be due simply to her postmenopausal hypoestrogenic state, and can be left untreated as long as Joan is comfortable with this option.
TABLE 2
Medical treatment for dysfunctional uterine bleeding
| Mild (bleeding is minimal and symptoms limited) |
| Moderate (moderate amounts of bleeding, mild anemia, and mild orthostatic symptoms or fatigue) |
|
| Severe (heavy bleeding, moderate to severe anemia, significant orthostatic symptoms) |
|
| IUD, intrauterine device; NSAIDs, nonsteroidal anti-inflammatory drugs; OCPs, oral contraceptive pills. |
Lessons learned
Patients like Casey, Sarah, and Joan can be successfully managed by the family physician. A thorough history, physical examination, and basic laboratory tests will usually suffice to rule out anatomic, systemic, or iatrogenic explanations. Pregnancy, the most common explanation for abnormal uterine bleeding, can be ruled out with a urine pregnancy test. Patients like Sarah and Joan, who have some of the risk factors for endometrial cancer, require an evaluation of the endometrium to rule out that possibility. When none of these etiologies is the culprit, your working diagnosis is DUB, and medical treatment for it is well within your competence.
CORRESPONDENCE David L. Maness, DO, MSS, Department of Family Medicine, University of Tennessee Health Science Center, College of Medicine, 1301 Primacy Parkway, Memphis, TN 38119; [email protected]
1. Wren BG. Dysfunctional uterine bleeding. Aust Fam Physician. 1998;27:371-377.
2. Astrup K, Olivarius Nde F. Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand. 2004;83:203-207.
3. Albers JR, Hull SJ, Wesley RM. Abnormal uterine bleeding. Am Fam Physician. 2004;69:1916-1926.
4. Munster K, Schmidt L, Helm P. Length and variation in the menstrual cycle—a cross-sectional study from a Danish country. Br J Obstet Gynaecol. 1992;99:422-429.
5. Halberg L, Hogdahl AM, Nilsson L, et al. Menstrual blood loss-a population study. Acta Obstet Gynecol Scand. 1966;45:320-351.
6. Warner PE, Critchley HO, Lumsden MA, et al. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J Obstet Gynecol. 2004;190:1216-1223.
7. Hill NC, Oppenheimer LW, Morton KE. The aetiology of vaginal bleeding in children. A 20-year review. Br J Obstet Gynaecol. 1989;96:467-470.
8. Amman M, Anguino H, Bauman RA, et al. Postmenopausal uterine bleeding. Pasadena, Calif: Kaiser Permanente Southern California; December 2006. NGC 005688. Available at: www.guideline.gov. Accessed July 19, 2010.
9. Hataska H. The evaluation of abnormal uterine bleeding. Clin Obstet Gynecol. 2005;48:258-273.
10. Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2006;(2):CD003855.-
11. Amman M, Anguino H, Bauman RA, et al. Chronic abnormal uterine bleeding in nongravid women. Pasadena, Calif: Kaiser Permanente Southern California; December 2006. NGC 005687. Available at: www.guideline.gov. Accessed July 19, 2010.
12. Bradley LD, Widrich T. State-of-the-art flexible hysteroscopy for office gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
13. Nagele F, O’Connor H, Davies A, et al. 2500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87-92.
14. Serden SP. Diagnostic hysteroscopy to evaluate the cause of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:277-286.
15. Shwayder JM. Pathophysiology of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:219-234.
16. Vilos GA, Lefebvre G, Graves GR. Guidelines for the management of abnormal uterine bleeding. SOGC Clinical Practice Guidelines; August 2001. Available at: www.sogc.org/guidelines/public/106E-CPG-August2001.pdf. Accessed July 19, 2010.
17. Rosenthal AN, Panoskaltsis T, Smith T, et al. The frequency of significant pathology in women attending a general gynaecological service for postcoital bleeding. BJOG. 2001;108:103-106.
18. Beers MH, Berkow R. eds. Dysfunctional uterine bleeding, In: The Merck Manual. 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999:1941-1942.
19. Singh RH, Blumenthal P. Hormonal management of abnormal uterine bleeding. Clin Obstet Gynecol. 2005;48:337-352.
20. Edlund M, Blomback M, von Schoultz B, et al. On the value of menorrhagia as a predictor for coagulation disorders. Am J Hematol. 1996;53:234-238.
21. Kouides PA. Evaluation of abnormal bleeding in women. Curr Hematol Rep. 2002;1:11-18.
22. Kadir RA, Economides DL, Sabin CA, et al. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351:485-489.
23. Dilley A, Drews C, Miller C, et al. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol. 2001;97:630-636.
24. Ries LAG, Melbert D, Krapcho M, et al. eds SEER Cancer Statistics Review, 1975-2005.Bethesda, Md: National Cancer Institute. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008. Accessed July 19, 2010.
25. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1226.
26. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding. A Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
27. Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal bleeding. Obstet Gynecol. 2002;99:663-670.
28. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510-1517.
29. Medverd JR, Dubinsky TJ. Cost analysis model: US versus endometrial biopsy in evaluation of peri- and post-menopausal abnormal vaginal bleeding. Radiology. 2002;222:619-627.
30. Scott S. Abnormal bleeding in the pediatric patient. Postgrad Obstet Gynecol. 2006;26:1-5.
31. Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD001016.-
32. Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2008;(1):CD001016.-
33. Stewart A, Cummins C, Gold L, et al. The effectiveness of levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. Br J Obstet Gynaecol. 2001;108:74-86.
34. Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(4):CD000249.-
35. Beaumont H, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(2):CD001017.-
36. Wellington K, Wagstaff AJ. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs. 2003;63:1417-1433.
1. Wren BG. Dysfunctional uterine bleeding. Aust Fam Physician. 1998;27:371-377.
2. Astrup K, Olivarius Nde F. Frequency of spontaneously occurring postmenopausal bleeding in the general population. Acta Obstet Gynecol Scand. 2004;83:203-207.
3. Albers JR, Hull SJ, Wesley RM. Abnormal uterine bleeding. Am Fam Physician. 2004;69:1916-1926.
4. Munster K, Schmidt L, Helm P. Length and variation in the menstrual cycle—a cross-sectional study from a Danish country. Br J Obstet Gynaecol. 1992;99:422-429.
5. Halberg L, Hogdahl AM, Nilsson L, et al. Menstrual blood loss-a population study. Acta Obstet Gynecol Scand. 1966;45:320-351.
6. Warner PE, Critchley HO, Lumsden MA, et al. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J Obstet Gynecol. 2004;190:1216-1223.
7. Hill NC, Oppenheimer LW, Morton KE. The aetiology of vaginal bleeding in children. A 20-year review. Br J Obstet Gynaecol. 1989;96:467-470.
8. Amman M, Anguino H, Bauman RA, et al. Postmenopausal uterine bleeding. Pasadena, Calif: Kaiser Permanente Southern California; December 2006. NGC 005688. Available at: www.guideline.gov. Accessed July 19, 2010.
9. Hataska H. The evaluation of abnormal uterine bleeding. Clin Obstet Gynecol. 2005;48:258-273.
10. Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2006;(2):CD003855.-
11. Amman M, Anguino H, Bauman RA, et al. Chronic abnormal uterine bleeding in nongravid women. Pasadena, Calif: Kaiser Permanente Southern California; December 2006. NGC 005687. Available at: www.guideline.gov. Accessed July 19, 2010.
12. Bradley LD, Widrich T. State-of-the-art flexible hysteroscopy for office gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
13. Nagele F, O’Connor H, Davies A, et al. 2500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87-92.
14. Serden SP. Diagnostic hysteroscopy to evaluate the cause of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:277-286.
15. Shwayder JM. Pathophysiology of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:219-234.
16. Vilos GA, Lefebvre G, Graves GR. Guidelines for the management of abnormal uterine bleeding. SOGC Clinical Practice Guidelines; August 2001. Available at: www.sogc.org/guidelines/public/106E-CPG-August2001.pdf. Accessed July 19, 2010.
17. Rosenthal AN, Panoskaltsis T, Smith T, et al. The frequency of significant pathology in women attending a general gynaecological service for postcoital bleeding. BJOG. 2001;108:103-106.
18. Beers MH, Berkow R. eds. Dysfunctional uterine bleeding, In: The Merck Manual. 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999:1941-1942.
19. Singh RH, Blumenthal P. Hormonal management of abnormal uterine bleeding. Clin Obstet Gynecol. 2005;48:337-352.
20. Edlund M, Blomback M, von Schoultz B, et al. On the value of menorrhagia as a predictor for coagulation disorders. Am J Hematol. 1996;53:234-238.
21. Kouides PA. Evaluation of abnormal bleeding in women. Curr Hematol Rep. 2002;1:11-18.
22. Kadir RA, Economides DL, Sabin CA, et al. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351:485-489.
23. Dilley A, Drews C, Miller C, et al. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol. 2001;97:630-636.
24. Ries LAG, Melbert D, Krapcho M, et al. eds SEER Cancer Statistics Review, 1975-2005.Bethesda, Md: National Cancer Institute. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008. Accessed July 19, 2010.
25. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1226.
26. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding. A Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488-1494.
27. Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal bleeding. Obstet Gynecol. 2002;99:663-670.
28. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510-1517.
29. Medverd JR, Dubinsky TJ. Cost analysis model: US versus endometrial biopsy in evaluation of peri- and post-menopausal abnormal vaginal bleeding. Radiology. 2002;222:619-627.
30. Scott S. Abnormal bleeding in the pediatric patient. Postgrad Obstet Gynecol. 2006;26:1-5.
31. Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD001016.-
32. Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2008;(1):CD001016.-
33. Stewart A, Cummins C, Gold L, et al. The effectiveness of levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. Br J Obstet Gynaecol. 2001;108:74-86.
34. Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(4):CD000249.-
35. Beaumont H, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2002;(2):CD001017.-
36. Wellington K, Wagstaff AJ. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs. 2003;63:1417-1433.
Nonspecific low back pain: Evaluation and treatment tips
• Avoid imaging in cases of uncomplicated low back pain (unless there are specific clinical indications). A
• Use acetaminophen, nonsteroidal anti-inflammatory drugs, or muscle relaxants for short-term relief of acute nonspecific low back pain. A
• Consider matching specific physical therapy options to the patient’s history and exam findings. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 50-year-old construction worker comes in for an office visit because he’s been experiencing intermittent low back pain that’s been occurring more frequently. He says he has not been injured and that he always takes care on the job to minimize physical risk. He reports no symptoms other than the back pain.
How would you proceed with this patient’s care? Would you order a plain radiograph to be sure nothing dire is causing the patient’s pain—or would you skip it? And would you know how to match your patient’s history and exam findings with specific physical therapy interventions?
The following review brings the latest guidelines and research to bear on these questions—and others—as you care for patients with nonspecific low back pain.
Categorizing low back pain to direct your investigation
The 2007 Joint Clinical Practice Guideline issued by the American College of Physicians and the American Pain Society encourages clinicians to perform a focused history and physical exam to classify patients into 1 of 3 broad categories: nonspecific low back pain (LBP), LBP potentially associated with radiculopathy or spinal stenosis, or LBP potentially associated with another specific spinal cause.1
Patients in the last category often exhibit findings in the history and physical examination suggestive of severe or progressive neurologic defi cits. Refer these patients for further diagnostic testing.
Patients presenting with persistent (>4 weeks) LBP and signs and symptoms of radiculopathy or spinal stenosis are best referred for, preferably, magnetic resonance imaging (MRI) or for computed tomography (CT)—but only if the patient is a candidate for surgery or epidural steroid injection.
For patients with nonspecific LBP, which accounts for most cases, practice guidelines recommend against routine use of imaging or other diagnostic procedures.1
Unfortunately, however, some clinicians still use routine imaging in the absence of significant findings or without clear indication.2 One argument used to justify this action—particularly by some who consider nonspecific LBP to be a diagnosis of exclusion —is the desire to rule out a serious underlying spinal condition.
What the research tells us about routine imaging
A recently published systematic review and meta-analysis compiled data relevant to more than 1800 subjects from 6 randomized controlled trials (RCTs). The authors examined early, routine use of lumbar imaging (radiography, MRI, or CT) in patients with acute or subacute LBP. They found that, without clear indication from findings in the history and physical examination, immediate imaging does not improve clinical outcomes (ie, diminish pain or improve daily function).3
Another study took a closer look at advanced imaging for LBP. In an RCT including more than 300 patients with a mean age of 53 years, investigators compared outcomes for patients receiving either plain radiographs or rapid MRI. No differences were noted in outcomes for back-related disability, pain, health survey results, preference scores, satisfaction, or costs at 12 months.
Furthermore, patients receiving rapid MRI were more likely to undergo surgery, which also failed to improve outcomes. As a result, the authors cautioned against unnecessary use of advanced imaging, as it could increase costs of care and possibly increase surgical intervention without improved outcomes.4 These studies substantiate practice guidelines regarding the use of imaging for patients with nonspecific LBP.
Not helpful, and perhaps harmful?
When imaging is unwarranted, it unnecessarily exposes patients to ionizing radiation, especially objectionable for younger women.1 Imaging can also lead to the identifi cation of pathology unrelated to a patient’s LBP.1,5
As mentioned above, patients receiving rapid MRI were more likely to receive surgical intervention that did not improve outcomes.4 This observation may reflect, in part, findings of pathoanatomical abnormalities that have little or no correlation with patient symptoms. In a random sample of 148 subjects ages 36 to 71 years—nearly half of whom had never experienced back pain—Jarvik and colleagues5 found MRI evidence of annular tears, disc bulges, disc protrusions, facet joint degeneration, end plate changes, and mild spondylolisthesis. The authors concluded that such MRI findings are therefore of limited diagnostic value.5
Labeling can be harmful, too. Identification of pathology that could well be unrelated to LBP can result in a specific, presumed diagnosis, possibly inducing a phenomenon known as the labeling effect. The search for a specific diagnosis or label for patients with nonspecific LBP could cause them to perceive their low back condition as being more serious than it actually is. Patients may then develop distorted beliefs regarding the true nature of their health status. The labeling effect could even alter the natural course of an otherwise benign condition.6,7
It’s time to treat: Tell patients to remain active
Practice guidelines for nonspecific LBP recommend providing patients with evidence-based education that emphasizes the favorable course of this condition and that encourages them to remain active.1 This recommendation was assessed retrospectively in a study of nearly 1200 patients receiving physical therapy for acute LBP. The authors found that adherence to the recommendation for activity and exercise yielded significant reductions in disability and pain, and resulted in significantly fewer visits and lower charges.8
For acute cases of nonspecific LBP, good evidence supports the short-term effectiveness of acetaminophen and nonsteroidal anti-inflammatory drugs, as well as skeletal muscle relaxants.1,9 For chronic cases, good evidence exists for prescribing tricyclic antidepressants.9
Nonpharmacologic interventions include, in acute cases, active care, spinal manipulation, and superficial heat (eg, hot packs). For subacute and chronic cases, think about intensive interdisciplinary rehabilitation interventions—therapeutic exercise, soft-tissue manual techniques, acupuncture, movement re-education techniques, spinal manipulation, cognitive-behavioral therapy, or progressive relaxation.1,10
Further customize your Tx approach
While recent data suggest that some front-line physicians who treat patients with LBP may have insufficient knowledge to do so11-13 (see “Are we out of step?” ), there are promising developments, as well. Many primary care clinicians and researchers believe that nonspecific LBP resembles a heterogeneous condition and that intervention is more effective when treatment is matched to the patient’s history and examination findings.14,15 In a survey of more than 600 primary care clinicians from multiple disciplines, including physical therapy, chiropractic, and medicine, 93% of the participants reported that they treat nonspecific LBP cases differently, depending on signs and symptoms, with 74% believing there are recognizable subgroups to guide management.14
An example of subgrouping patients with nonspecific LBP is the idea of treatment-based classification, which evidence has found to be reliable, effective, and cost-efficient for patients with LBP (TABLE).11 In an RCT, Brennan and colleagues15 examined 123 patients with acute LBP (ie, back pain lasting <90 days) referred to a physical therapist for treatment. Patients were examined and then classified into 1 of 3 subgroups according to the type of treatment expected to work best for them: manipulation, stabilization, or specific exercise. Each patient was then randomly assigned to receive 1 of the 3 treatments. Post-treatment analysis compared outcomes between patients who received treatment matched to their classification and those whose treatment did not match their classification.
At 4 weeks, the matched-treatment group had significantly greater reductions in disability compared with the unmatched-treatment group; this difference was still evident at 1 year. The authors agreed that LBP should not be thought of as a homogenous condition, and found that outcomes can be improved with subgrouping to guide intervention selection.15
Recent data suggest that frontline clinicians who typically treat patients with low back pain (LBP) may have insufficient knowledge to do so.11,12 Using an observational design, Buchbinder et al12 surveyed nearly 4000 general practitioners, with and without special interest in LBP, to assess their knowledge in managing acute LBP and their attitudes toward patients with LBP.
Using a 5-point Likert scale ranging from “strongly agree” to “strongly disagree,” the investigators asked physicians questions related to such topics as bed rest, work, imaging, physical activity, interventions by physicians or other healthcare providers, patient expectations, chronic LBP, patient motivation, and usefulness and utilization of practice guidelines. Interestingly, physicians with a special interest in treating LBP actually had poorer management beliefs (ie, not in accord with best available evidence) than those who did not have such an interest.1
A similar study found that both family practitioners and orthopedists were deficient in knowledge for treating patients with nonspecific LBP; orthopedists were less informed than family practitioners.13
TABLE
Matching physical therapy to low back pain attributes can improve outcomes
| Treatment recommendation | History and examination findings |
|---|---|
| Manipulation |
|
| Stabilization/motor control |
|
| Specific exercise | |
|
|
|
|
|
|
| Traction* |
|
| ROM, range of motion. | |
| *This fourth category was not included in the original study; patient selection criteria were developed at a later date. | |
| Source: Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290-302.11 Adapted with permission from the Orthopaedic and Sports Physical Therapy Sections of the American Physical Therapy Association and The Journal of Orthopaedic and Sports Physical Therapy. | |
CORRESPONDENCE William R. VanWye, PT, DPT, 1600 Albany Street, Beech Grove, IN 46107; [email protected]
1. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
2. Di Iorio D, Henley E, Doughty A. A survey of primary care physician practice patterns and adherence to acute low back problem guidelines. Arch Fam Med. 2000;9:1015-1021.
3. Chou R, Fu R, Carrino JA, et al. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463-472.
4. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289:2810-2818.
5. Jarvik JG, Hollingworth W, Heagerty P, et al. The longitudinal assessment of imaging and disability of the back (LAIDBack) study. Spine. 2001;26:1158-1166.
6. Abenhaim L, Rossignol M, Gobeille D, et al. The prognostic consequences in the making of the initial medical diagnosis of work-related back injuries. Spine. 1995;20:791-795.
7. Waddell G, Burton AK. Occupational health guidelines for the management of low back pain at work: evidence review. Occup Med. 2001;51:124-135.
8. Fritz JM, Cleland JA, Brennan GP. Does adherence to the guideline recommendation for active treatments improve the quality of care for patients with acute low back pain delivered by physical therapists? Med Care. 2007;45:973-980.
9. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
10. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
11. Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290-302.
12. Buchbinder R, Staples M, Jolley D. Doctors with a special interest in back pain have poorer knowledge about how to treat back pain. Spine. 2009;34:1218-1226.
13. Finestone AS, Raveh A, Mirovsky Y, et al. Orthopaedists’ and family practitioners’ knowledge of simple low back pain management. Spine. 2009;34:1600-1603.
14. Kent P, Keating J. Do primary-care clinicians think that nonspecific low back pain is one condition? Spine. 2004;29:1022-1031.
15. Brennan GP, Fritz JM, Hunter SJ, et al. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: results of a randomized clinical trial. Spine. 2006;31:623-631.
• Avoid imaging in cases of uncomplicated low back pain (unless there are specific clinical indications). A
• Use acetaminophen, nonsteroidal anti-inflammatory drugs, or muscle relaxants for short-term relief of acute nonspecific low back pain. A
• Consider matching specific physical therapy options to the patient’s history and exam findings. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 50-year-old construction worker comes in for an office visit because he’s been experiencing intermittent low back pain that’s been occurring more frequently. He says he has not been injured and that he always takes care on the job to minimize physical risk. He reports no symptoms other than the back pain.
How would you proceed with this patient’s care? Would you order a plain radiograph to be sure nothing dire is causing the patient’s pain—or would you skip it? And would you know how to match your patient’s history and exam findings with specific physical therapy interventions?
The following review brings the latest guidelines and research to bear on these questions—and others—as you care for patients with nonspecific low back pain.
Categorizing low back pain to direct your investigation
The 2007 Joint Clinical Practice Guideline issued by the American College of Physicians and the American Pain Society encourages clinicians to perform a focused history and physical exam to classify patients into 1 of 3 broad categories: nonspecific low back pain (LBP), LBP potentially associated with radiculopathy or spinal stenosis, or LBP potentially associated with another specific spinal cause.1
Patients in the last category often exhibit findings in the history and physical examination suggestive of severe or progressive neurologic defi cits. Refer these patients for further diagnostic testing.
Patients presenting with persistent (>4 weeks) LBP and signs and symptoms of radiculopathy or spinal stenosis are best referred for, preferably, magnetic resonance imaging (MRI) or for computed tomography (CT)—but only if the patient is a candidate for surgery or epidural steroid injection.
For patients with nonspecific LBP, which accounts for most cases, practice guidelines recommend against routine use of imaging or other diagnostic procedures.1
Unfortunately, however, some clinicians still use routine imaging in the absence of significant findings or without clear indication.2 One argument used to justify this action—particularly by some who consider nonspecific LBP to be a diagnosis of exclusion —is the desire to rule out a serious underlying spinal condition.
What the research tells us about routine imaging
A recently published systematic review and meta-analysis compiled data relevant to more than 1800 subjects from 6 randomized controlled trials (RCTs). The authors examined early, routine use of lumbar imaging (radiography, MRI, or CT) in patients with acute or subacute LBP. They found that, without clear indication from findings in the history and physical examination, immediate imaging does not improve clinical outcomes (ie, diminish pain or improve daily function).3
Another study took a closer look at advanced imaging for LBP. In an RCT including more than 300 patients with a mean age of 53 years, investigators compared outcomes for patients receiving either plain radiographs or rapid MRI. No differences were noted in outcomes for back-related disability, pain, health survey results, preference scores, satisfaction, or costs at 12 months.
Furthermore, patients receiving rapid MRI were more likely to undergo surgery, which also failed to improve outcomes. As a result, the authors cautioned against unnecessary use of advanced imaging, as it could increase costs of care and possibly increase surgical intervention without improved outcomes.4 These studies substantiate practice guidelines regarding the use of imaging for patients with nonspecific LBP.
Not helpful, and perhaps harmful?
When imaging is unwarranted, it unnecessarily exposes patients to ionizing radiation, especially objectionable for younger women.1 Imaging can also lead to the identifi cation of pathology unrelated to a patient’s LBP.1,5
As mentioned above, patients receiving rapid MRI were more likely to receive surgical intervention that did not improve outcomes.4 This observation may reflect, in part, findings of pathoanatomical abnormalities that have little or no correlation with patient symptoms. In a random sample of 148 subjects ages 36 to 71 years—nearly half of whom had never experienced back pain—Jarvik and colleagues5 found MRI evidence of annular tears, disc bulges, disc protrusions, facet joint degeneration, end plate changes, and mild spondylolisthesis. The authors concluded that such MRI findings are therefore of limited diagnostic value.5
Labeling can be harmful, too. Identification of pathology that could well be unrelated to LBP can result in a specific, presumed diagnosis, possibly inducing a phenomenon known as the labeling effect. The search for a specific diagnosis or label for patients with nonspecific LBP could cause them to perceive their low back condition as being more serious than it actually is. Patients may then develop distorted beliefs regarding the true nature of their health status. The labeling effect could even alter the natural course of an otherwise benign condition.6,7
It’s time to treat: Tell patients to remain active
Practice guidelines for nonspecific LBP recommend providing patients with evidence-based education that emphasizes the favorable course of this condition and that encourages them to remain active.1 This recommendation was assessed retrospectively in a study of nearly 1200 patients receiving physical therapy for acute LBP. The authors found that adherence to the recommendation for activity and exercise yielded significant reductions in disability and pain, and resulted in significantly fewer visits and lower charges.8
For acute cases of nonspecific LBP, good evidence supports the short-term effectiveness of acetaminophen and nonsteroidal anti-inflammatory drugs, as well as skeletal muscle relaxants.1,9 For chronic cases, good evidence exists for prescribing tricyclic antidepressants.9
Nonpharmacologic interventions include, in acute cases, active care, spinal manipulation, and superficial heat (eg, hot packs). For subacute and chronic cases, think about intensive interdisciplinary rehabilitation interventions—therapeutic exercise, soft-tissue manual techniques, acupuncture, movement re-education techniques, spinal manipulation, cognitive-behavioral therapy, or progressive relaxation.1,10
Further customize your Tx approach
While recent data suggest that some front-line physicians who treat patients with LBP may have insufficient knowledge to do so11-13 (see “Are we out of step?” ), there are promising developments, as well. Many primary care clinicians and researchers believe that nonspecific LBP resembles a heterogeneous condition and that intervention is more effective when treatment is matched to the patient’s history and examination findings.14,15 In a survey of more than 600 primary care clinicians from multiple disciplines, including physical therapy, chiropractic, and medicine, 93% of the participants reported that they treat nonspecific LBP cases differently, depending on signs and symptoms, with 74% believing there are recognizable subgroups to guide management.14
An example of subgrouping patients with nonspecific LBP is the idea of treatment-based classification, which evidence has found to be reliable, effective, and cost-efficient for patients with LBP (TABLE).11 In an RCT, Brennan and colleagues15 examined 123 patients with acute LBP (ie, back pain lasting <90 days) referred to a physical therapist for treatment. Patients were examined and then classified into 1 of 3 subgroups according to the type of treatment expected to work best for them: manipulation, stabilization, or specific exercise. Each patient was then randomly assigned to receive 1 of the 3 treatments. Post-treatment analysis compared outcomes between patients who received treatment matched to their classification and those whose treatment did not match their classification.
At 4 weeks, the matched-treatment group had significantly greater reductions in disability compared with the unmatched-treatment group; this difference was still evident at 1 year. The authors agreed that LBP should not be thought of as a homogenous condition, and found that outcomes can be improved with subgrouping to guide intervention selection.15
Recent data suggest that frontline clinicians who typically treat patients with low back pain (LBP) may have insufficient knowledge to do so.11,12 Using an observational design, Buchbinder et al12 surveyed nearly 4000 general practitioners, with and without special interest in LBP, to assess their knowledge in managing acute LBP and their attitudes toward patients with LBP.
Using a 5-point Likert scale ranging from “strongly agree” to “strongly disagree,” the investigators asked physicians questions related to such topics as bed rest, work, imaging, physical activity, interventions by physicians or other healthcare providers, patient expectations, chronic LBP, patient motivation, and usefulness and utilization of practice guidelines. Interestingly, physicians with a special interest in treating LBP actually had poorer management beliefs (ie, not in accord with best available evidence) than those who did not have such an interest.1
A similar study found that both family practitioners and orthopedists were deficient in knowledge for treating patients with nonspecific LBP; orthopedists were less informed than family practitioners.13
TABLE
Matching physical therapy to low back pain attributes can improve outcomes
| Treatment recommendation | History and examination findings |
|---|---|
| Manipulation |
|
| Stabilization/motor control |
|
| Specific exercise | |
|
|
|
|
|
|
| Traction* |
|
| ROM, range of motion. | |
| *This fourth category was not included in the original study; patient selection criteria were developed at a later date. | |
| Source: Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290-302.11 Adapted with permission from the Orthopaedic and Sports Physical Therapy Sections of the American Physical Therapy Association and The Journal of Orthopaedic and Sports Physical Therapy. | |
CORRESPONDENCE William R. VanWye, PT, DPT, 1600 Albany Street, Beech Grove, IN 46107; [email protected]
• Avoid imaging in cases of uncomplicated low back pain (unless there are specific clinical indications). A
• Use acetaminophen, nonsteroidal anti-inflammatory drugs, or muscle relaxants for short-term relief of acute nonspecific low back pain. A
• Consider matching specific physical therapy options to the patient’s history and exam findings. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 50-year-old construction worker comes in for an office visit because he’s been experiencing intermittent low back pain that’s been occurring more frequently. He says he has not been injured and that he always takes care on the job to minimize physical risk. He reports no symptoms other than the back pain.
How would you proceed with this patient’s care? Would you order a plain radiograph to be sure nothing dire is causing the patient’s pain—or would you skip it? And would you know how to match your patient’s history and exam findings with specific physical therapy interventions?
The following review brings the latest guidelines and research to bear on these questions—and others—as you care for patients with nonspecific low back pain.
Categorizing low back pain to direct your investigation
The 2007 Joint Clinical Practice Guideline issued by the American College of Physicians and the American Pain Society encourages clinicians to perform a focused history and physical exam to classify patients into 1 of 3 broad categories: nonspecific low back pain (LBP), LBP potentially associated with radiculopathy or spinal stenosis, or LBP potentially associated with another specific spinal cause.1
Patients in the last category often exhibit findings in the history and physical examination suggestive of severe or progressive neurologic defi cits. Refer these patients for further diagnostic testing.
Patients presenting with persistent (>4 weeks) LBP and signs and symptoms of radiculopathy or spinal stenosis are best referred for, preferably, magnetic resonance imaging (MRI) or for computed tomography (CT)—but only if the patient is a candidate for surgery or epidural steroid injection.
For patients with nonspecific LBP, which accounts for most cases, practice guidelines recommend against routine use of imaging or other diagnostic procedures.1
Unfortunately, however, some clinicians still use routine imaging in the absence of significant findings or without clear indication.2 One argument used to justify this action—particularly by some who consider nonspecific LBP to be a diagnosis of exclusion —is the desire to rule out a serious underlying spinal condition.
What the research tells us about routine imaging
A recently published systematic review and meta-analysis compiled data relevant to more than 1800 subjects from 6 randomized controlled trials (RCTs). The authors examined early, routine use of lumbar imaging (radiography, MRI, or CT) in patients with acute or subacute LBP. They found that, without clear indication from findings in the history and physical examination, immediate imaging does not improve clinical outcomes (ie, diminish pain or improve daily function).3
Another study took a closer look at advanced imaging for LBP. In an RCT including more than 300 patients with a mean age of 53 years, investigators compared outcomes for patients receiving either plain radiographs or rapid MRI. No differences were noted in outcomes for back-related disability, pain, health survey results, preference scores, satisfaction, or costs at 12 months.
Furthermore, patients receiving rapid MRI were more likely to undergo surgery, which also failed to improve outcomes. As a result, the authors cautioned against unnecessary use of advanced imaging, as it could increase costs of care and possibly increase surgical intervention without improved outcomes.4 These studies substantiate practice guidelines regarding the use of imaging for patients with nonspecific LBP.
Not helpful, and perhaps harmful?
When imaging is unwarranted, it unnecessarily exposes patients to ionizing radiation, especially objectionable for younger women.1 Imaging can also lead to the identifi cation of pathology unrelated to a patient’s LBP.1,5
As mentioned above, patients receiving rapid MRI were more likely to receive surgical intervention that did not improve outcomes.4 This observation may reflect, in part, findings of pathoanatomical abnormalities that have little or no correlation with patient symptoms. In a random sample of 148 subjects ages 36 to 71 years—nearly half of whom had never experienced back pain—Jarvik and colleagues5 found MRI evidence of annular tears, disc bulges, disc protrusions, facet joint degeneration, end plate changes, and mild spondylolisthesis. The authors concluded that such MRI findings are therefore of limited diagnostic value.5
Labeling can be harmful, too. Identification of pathology that could well be unrelated to LBP can result in a specific, presumed diagnosis, possibly inducing a phenomenon known as the labeling effect. The search for a specific diagnosis or label for patients with nonspecific LBP could cause them to perceive their low back condition as being more serious than it actually is. Patients may then develop distorted beliefs regarding the true nature of their health status. The labeling effect could even alter the natural course of an otherwise benign condition.6,7
It’s time to treat: Tell patients to remain active
Practice guidelines for nonspecific LBP recommend providing patients with evidence-based education that emphasizes the favorable course of this condition and that encourages them to remain active.1 This recommendation was assessed retrospectively in a study of nearly 1200 patients receiving physical therapy for acute LBP. The authors found that adherence to the recommendation for activity and exercise yielded significant reductions in disability and pain, and resulted in significantly fewer visits and lower charges.8
For acute cases of nonspecific LBP, good evidence supports the short-term effectiveness of acetaminophen and nonsteroidal anti-inflammatory drugs, as well as skeletal muscle relaxants.1,9 For chronic cases, good evidence exists for prescribing tricyclic antidepressants.9
Nonpharmacologic interventions include, in acute cases, active care, spinal manipulation, and superficial heat (eg, hot packs). For subacute and chronic cases, think about intensive interdisciplinary rehabilitation interventions—therapeutic exercise, soft-tissue manual techniques, acupuncture, movement re-education techniques, spinal manipulation, cognitive-behavioral therapy, or progressive relaxation.1,10
Further customize your Tx approach
While recent data suggest that some front-line physicians who treat patients with LBP may have insufficient knowledge to do so11-13 (see “Are we out of step?” ), there are promising developments, as well. Many primary care clinicians and researchers believe that nonspecific LBP resembles a heterogeneous condition and that intervention is more effective when treatment is matched to the patient’s history and examination findings.14,15 In a survey of more than 600 primary care clinicians from multiple disciplines, including physical therapy, chiropractic, and medicine, 93% of the participants reported that they treat nonspecific LBP cases differently, depending on signs and symptoms, with 74% believing there are recognizable subgroups to guide management.14
An example of subgrouping patients with nonspecific LBP is the idea of treatment-based classification, which evidence has found to be reliable, effective, and cost-efficient for patients with LBP (TABLE).11 In an RCT, Brennan and colleagues15 examined 123 patients with acute LBP (ie, back pain lasting <90 days) referred to a physical therapist for treatment. Patients were examined and then classified into 1 of 3 subgroups according to the type of treatment expected to work best for them: manipulation, stabilization, or specific exercise. Each patient was then randomly assigned to receive 1 of the 3 treatments. Post-treatment analysis compared outcomes between patients who received treatment matched to their classification and those whose treatment did not match their classification.
At 4 weeks, the matched-treatment group had significantly greater reductions in disability compared with the unmatched-treatment group; this difference was still evident at 1 year. The authors agreed that LBP should not be thought of as a homogenous condition, and found that outcomes can be improved with subgrouping to guide intervention selection.15
Recent data suggest that frontline clinicians who typically treat patients with low back pain (LBP) may have insufficient knowledge to do so.11,12 Using an observational design, Buchbinder et al12 surveyed nearly 4000 general practitioners, with and without special interest in LBP, to assess their knowledge in managing acute LBP and their attitudes toward patients with LBP.
Using a 5-point Likert scale ranging from “strongly agree” to “strongly disagree,” the investigators asked physicians questions related to such topics as bed rest, work, imaging, physical activity, interventions by physicians or other healthcare providers, patient expectations, chronic LBP, patient motivation, and usefulness and utilization of practice guidelines. Interestingly, physicians with a special interest in treating LBP actually had poorer management beliefs (ie, not in accord with best available evidence) than those who did not have such an interest.1
A similar study found that both family practitioners and orthopedists were deficient in knowledge for treating patients with nonspecific LBP; orthopedists were less informed than family practitioners.13
TABLE
Matching physical therapy to low back pain attributes can improve outcomes
| Treatment recommendation | History and examination findings |
|---|---|
| Manipulation |
|
| Stabilization/motor control |
|
| Specific exercise | |
|
|
|
|
|
|
| Traction* |
|
| ROM, range of motion. | |
| *This fourth category was not included in the original study; patient selection criteria were developed at a later date. | |
| Source: Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290-302.11 Adapted with permission from the Orthopaedic and Sports Physical Therapy Sections of the American Physical Therapy Association and The Journal of Orthopaedic and Sports Physical Therapy. | |
CORRESPONDENCE William R. VanWye, PT, DPT, 1600 Albany Street, Beech Grove, IN 46107; [email protected]
1. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
2. Di Iorio D, Henley E, Doughty A. A survey of primary care physician practice patterns and adherence to acute low back problem guidelines. Arch Fam Med. 2000;9:1015-1021.
3. Chou R, Fu R, Carrino JA, et al. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463-472.
4. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289:2810-2818.
5. Jarvik JG, Hollingworth W, Heagerty P, et al. The longitudinal assessment of imaging and disability of the back (LAIDBack) study. Spine. 2001;26:1158-1166.
6. Abenhaim L, Rossignol M, Gobeille D, et al. The prognostic consequences in the making of the initial medical diagnosis of work-related back injuries. Spine. 1995;20:791-795.
7. Waddell G, Burton AK. Occupational health guidelines for the management of low back pain at work: evidence review. Occup Med. 2001;51:124-135.
8. Fritz JM, Cleland JA, Brennan GP. Does adherence to the guideline recommendation for active treatments improve the quality of care for patients with acute low back pain delivered by physical therapists? Med Care. 2007;45:973-980.
9. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
10. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
11. Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290-302.
12. Buchbinder R, Staples M, Jolley D. Doctors with a special interest in back pain have poorer knowledge about how to treat back pain. Spine. 2009;34:1218-1226.
13. Finestone AS, Raveh A, Mirovsky Y, et al. Orthopaedists’ and family practitioners’ knowledge of simple low back pain management. Spine. 2009;34:1600-1603.
14. Kent P, Keating J. Do primary-care clinicians think that nonspecific low back pain is one condition? Spine. 2004;29:1022-1031.
15. Brennan GP, Fritz JM, Hunter SJ, et al. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: results of a randomized clinical trial. Spine. 2006;31:623-631.
1. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
2. Di Iorio D, Henley E, Doughty A. A survey of primary care physician practice patterns and adherence to acute low back problem guidelines. Arch Fam Med. 2000;9:1015-1021.
3. Chou R, Fu R, Carrino JA, et al. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463-472.
4. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289:2810-2818.
5. Jarvik JG, Hollingworth W, Heagerty P, et al. The longitudinal assessment of imaging and disability of the back (LAIDBack) study. Spine. 2001;26:1158-1166.
6. Abenhaim L, Rossignol M, Gobeille D, et al. The prognostic consequences in the making of the initial medical diagnosis of work-related back injuries. Spine. 1995;20:791-795.
7. Waddell G, Burton AK. Occupational health guidelines for the management of low back pain at work: evidence review. Occup Med. 2001;51:124-135.
8. Fritz JM, Cleland JA, Brennan GP. Does adherence to the guideline recommendation for active treatments improve the quality of care for patients with acute low back pain delivered by physical therapists? Med Care. 2007;45:973-980.
9. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
10. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
11. Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther. 2007;37:290-302.
12. Buchbinder R, Staples M, Jolley D. Doctors with a special interest in back pain have poorer knowledge about how to treat back pain. Spine. 2009;34:1218-1226.
13. Finestone AS, Raveh A, Mirovsky Y, et al. Orthopaedists’ and family practitioners’ knowledge of simple low back pain management. Spine. 2009;34:1600-1603.
14. Kent P, Keating J. Do primary-care clinicians think that nonspecific low back pain is one condition? Spine. 2004;29:1022-1031.
15. Brennan GP, Fritz JM, Hunter SJ, et al. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: results of a randomized clinical trial. Spine. 2006;31:623-631.
Give your sports physicals a performance boost
• Cover the 12 components of the preparticipation physical evaluation (PPE) recommended by the American Heart Association to screen young athletes for potentially life-threatening cardiovascular disease. B
• Perform a genitourinary exam as part of the PPE for young men; assess young women for the criteria associated with the female athlete triad. C
• Perform auscultation while the patient is squatting and while doing the Valsalva maneuver to determine whether any murmurs you detected on examination are associated with hypertrophic cardiomyopathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With preparticipation physical evaluations (PPEs) required for competitive athletic activities at colleges nationwide1 and at high schools and middle schools in the vast majority of states,2 the start of a new school year often brings a barrage of student visits. Yet despite this almost universal requirement, there is no universal standard for the PPE.
There is, however, a new evidence-based guideline. Preparticipation Physical Evaluation, 4th edition,3 released earlier this year, was created by the American Academy of Family Physicians, American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopaedic Society for Sports Medicine, and American Osteopathic Academy of Sports Medicine.
This guideline describes how to conduct a thorough medical history and a targeted physical exam, with a focus on activity-related risks to various organ systems. Regardless of the specific activity the student is interested in pursuing, however, emphasis on cardiac, neurological, and musculoskeletal systems is crucial because of the frequency and gravity of complications.
Help with the medical history: A downloadable form
The history is the most important part of the PPE, which should be scheduled at least 6 weeks before the sports season starts to allow time for follow-up testing or consultation. A form (which can be downloaded along with a physical exam form from the American College of Sports Medicine’s [ACSM] Web site at http://www.ppesportsevaluation.org/body.html) features 54 questions, which cover a range of organ systems and highlight areas of common sports injury and disability. The answers to these questions alone can identify 75% of problems affecting athletes,3 including chronic conditions and medications that may require adjustment or closer monitoring.
Start with the cardiovascular system
As many as 85% of sudden deaths in young athletes are related to underlying cardiac abnormalities, according to a 10-year study of 150 such cases.4 Indeed, sudden cardiac death occurs in approximately 1 in 200,000 high school athletes.5,6 Not surprisingly, those engaged in high-intensity activity are at highest risk.7
Thus, screening school-aged athletes for potential causes of sudden cardiac death is a primary objective of the PPE.3 The American Heart Association (AHA) recommends a 12-part evaluation (TABLE 1) of the heart, with 8 history questions and 4 physical exam components, to properly screen for cardiac disease in the young athlete.8 Syncope, chest pain, and dyspnea, particularly if associated with exertion, may be signs of underlying cardiac disorders that warrant confirmatory testing;5 a family history of premature death or disability from cardiac disease indicate a need for additional testing, as well. Further evaluation normally entails a combination of cardiac testing and consultation with a cardiologist.
Ask about asthma, including exercise-induced asthma (EIA). Identifying any respiratory conditions is vital to ensure adequate treatment and optimal performance. Patients with EIA are normally asymptomatic at rest, with no disturbance of peak expiratory flow. The presence of cough, chest tightness, wheezing, dyspnea, or loss of endurance during exercise suggests an EIA diagnosis.9 If a patient reports any such symptoms, order pulmonary function tests for confirmation.
EIA affects between 10% and 50% of athletes, depending on the sport.10 In up to 80% of athletes with EIA, use of inhaled short-acting beta-agonists prior to participation can help to prevent symptoms.9 Any athlete who reports symptoms of asthma, whether or not it is exercise-induced, requires treatment to prevent serious respiratory sequelae.
TABLE 1
Ask these 12 questions during cardiovascular screening5
| Personal history |
|---|
Has the patient had: 1. exertional chest pain/discomfort? |
| Family history |
| Has a family member: 6. died prematurely (sudden or unexpected) before age 50 due to heart disease? 7. suffered a disability from heart disease before age 50? 8. been diagnosed with a cardiac condition, such as hypertrophic or dilated cardiomyopathy, long QT syndrome, ion channelopathies, or Marfan syndrome? |
| Physical exam |
| Does the patient have: 9. a heart murmur? 10. femoral pulses? 11. physical stigmata of Marfan syndrome? 12. brachial artery blood pressure? |
Take a neurologic history
The neurologic system is highly susceptible to injury during sports activity. Identifying a history of concussion, nerve injury, or neurologic deficit is important both to prevent future injury and to avoid worsening of a current disability.
Always ask about transient neuropraxia, also known as a “stinger” or “burner”—a traction or compression injury to the nerves of the brachial plexus or cervical nerve roots that is sustained by 50% to 65% of college football players.11 The injury causes numbness, weakness, or both, in an upper extremity. Although symptoms are commonly transient, the injury may be severe enough to cause more prolonged symptoms; about 5% to 10% of the time, symptoms related to transient neuropraxia persist for hours to weeks.11
A history of numbness or weakness that occurs simultaneously in more than 1 extremity is suggestive of underlying cervical cord neuropraxia.12 Patients with such symptoms require further evaluation for the presence of spinal stenosis, cervical ligamentous injury, and spinal cord injury before being cleared to participate in sports.
Concussion is a common neurologic insult among competitive athletes (See “Update on concussion: Here’s what the experts have to say”), accounting for anywhere from 6.5% to 18.5% of injuries in collegiate contact sports.1,13 Identifying students who have had head injuries and are therefore at increased risk for future concussion is a key function of the PPE.13,14
An individual who suffers even a mild head injury prior to full resolution of an initial concussion is at risk for second impact syndrome—a devastating brain injury that causes a loss of autoregulation of cerebral blood flow, leading to rapid swelling, herniation, and death. Thus, any athlete with a recent history of concussion requires further evaluation—and disqualification from play until symptoms fully resolve. In fact, anyone with a history of concussion—recent or not—may need neuropsychologic testing to assess baseline level of cognitive function. Thorough documentation is critical in such cases. In the event of a repeat concussion, having this information may assist with treatment and return-to-play decisions.
Review other significant findings noted on the history form. Follow up, as needed, with questions about any missing or dysfunctional organ or bodily system (The ACSM Web site includes a supplemental history form for athletes with special needs to document additional details, if necessary).
In some cases, protective equipment may be sufficient (a student with defective vision in 1 eye can wear protective eyewear to prevent injury to the other eye, for instance). In other cases, a conversation with the patient and his or her family regarding the risk of serious injury may be in order. Parents of a child with only 1 kidney, for example, should be advised that contact sports pose a small, but real risk of damage to that kidney, potentially resulting in the need for dialysis.
Take the opportunity to discuss body image, mental health
For many adolescents—up to 50%, by some counts15—the PPE is their only interaction with a clinician. Thus, it provides a good opportunity for you to talk to young athletes about such sensitive topics as high-risk behaviors, mental health issues, body image, and personal safety. To help ensure that students feel free to talk openly, the new PPE forms have removed key questions about high-risk behaviors from the patient history (which requires a parent’s signature). Instead, a series of questions is listed under the heading “physician reminders” at the top of the form for the physical exam,3 which is typically conducted in a private setting.
Overweight? Underweight? Height and weight, a standard part of the physical along with blood pressure and pulse rate, may clue you in to the existence of an eating disorder. Calculate body mass index (BMI), looking for students who are underweight (BMI <19 kg/m2) or overweight (BMI >25 kg/m2). Overweight athletes are at increased risk for heat illness and may need a preseason conditioning program.16 Underweight athletes—particularly young women—may be at additional risk.
Female athlete triad. When examining young women, be alert to signs and symptoms of the female athlete triad, a syndrome of disordered eating, amenorrhea, and osteopenia or osteoporosis. This related spectrum of medical problems can pose a significant health risk, as it involves a cycle of low energy intake that “turns off” the reproductive cycle and creates a hypoestrogenic state. The lack of estrogen has a devastating effect on bone mineral resorption and can lead to osteopenia.17 While the prevalence of the female athlete triad is low in the general population, 1 study noted that nearly 6% of young female athletes met the criteria for 2 of the 3 components of the triad, and as many as 20% had at least 1.18
Is eye protection needed? Ear plugs?
Check vision in each eye, both with and without corrective lenses. A student whose corrected vision is 20/50 or worse in 1 eye qualifies as functionally one-eyed—and must wear full eye protection during athletic activities.19 Document pupil size for all patients to ensure that anisocoria is not confused with a neurological insult in the event of a sports injury.
Water sports? Examine the nares, inspecting the septum for signs of deviation or perforation. Th is portion of the exam, however, can be tailored to the type of sport in which the patient plans to participate. Students who expect to be on a swim team or participate in other water sports require an evaluation of the ears, including the tympanic membranes and external auditory canals. Swimming and humid environments are risk factors for otitis externa, and repeated cold water exposure is a risk for the development of external auditory exostoses.20,21 A swimmer with perforated tympanic membranes should be advised to wear ear plugs to protect the middle ear.
Is the patient a wrestler? Pay close attention to the ears of any student who plans to join a wrestling team, documenting the absence—or presence—of cauliflower ear. A notation about the presence of this ear-deforming condition in the medical record ensures that any new auricular hematoma can be evaluated and treated with knowledge of the prior injury.
Cardiac findings that warrant further work-up
Elevated blood pressure, the most common cardiovascular abnormality in people participating in competitive sports,22 is categorized in stages as defined by the Second Task Force on Blood Pressure Control in Children23 and the Joint National Commission VII for adults ages 18 and older.24 Children with significant hypertension (TABLE 2) and adults with stage I hypertension (140-159/90-99 mm Hg) may participate as long as there is no indication of end organ disease. A child with severe hypertension or an adult with stage II hypertension (>159/99 mm Hg), however, should not be cleared for participation until he or she has had further evaluation and treatment.22,25
Similarly, any patient with an elevated heart rate needs a medical work-up prior to participation to determine the underlying cause of tachycardia.22 This may be a sign of an underlying cardiac arrhythmia or another medical condition that must be treated prior to athletic competition.
Auscultate the heart to screen for underlying cardiac disease. Listen to all 4 standard regions, with the patient in both a supine and a standing position. If you detect a murmur, perform auscultation while the patient squats and while performing the Valsalva maneuver. The murmur of hypertrophic cardiomyopathy (HCM)—the key cause of sudden cardiac death—is systolic, increasing with standing and the Valsalva maneuver and decreasing with squatting and a supine position. Any murmur that is 3/6 or greater in sound or has characteristics of HCM needs further evaluation before the patient can be cleared for sports activities. (See “Hypertrophic cardiomyopathy: Ask athletes these 9 questions,” J Fam Pract. 2009;58:576-584).
Palpate the femoral pulses. Absence of, or decreased, femoral pulses compared with brachial pulses may suggest coarctation of the aorta.
TABLE 2
Hypertension in children: Is it significant or severe?24
| Age | Significant HTN (mm Hg) | Severe HTN (mm Hg) |
|---|---|---|
| 6-9 y Systolic Diastolic | 122-129 78-85 | >129 >85 |
| 10-12 y Systolic Diastolic | 126-133 82-89 | >133 >89 |
| 13-15 y Systolic Diastolic | 136-143 86-91 | >143 >91 |
| 16-18 y Systolic Diastolic | 142-149 92-97 | >149 >97 |
| HTN, hypertension. | ||
Check lungs, abdomen, and skin
Auscultate the lungs, which should be clear and absent of adventitious or diminished breath sounds. Any patient with abnormal breath sounds requires further evaluation and/or treatment before being cleared for sports participation.
Examine the abdomen to assess the presence of organomegaly. Enlargement of the liver and spleen may be a sign of an underlying disease process. Mononucleosis, which causes splenomegaly, is associated with a 0.1% to 0.2% rate of rupture that may be related to trauma or the Valsalva maneuver.26 Athletes with organomegaly should not be cleared for athletic activity without further evaluation.3
Perform a genitourinary examination on young men. Check for the presence of both testicles and palpate for masses and inguinal hernias. A patient with a solitary testicle will require protective gear during certain sporting events to prevent injury.27
Assess the condition of the skin. In this case, the activity the student plans to pursue will determine the extent of the examination. Skin condition is particularly important for wrestlers, as the close contact involved predisposes the athletes to infectious skin conditions. Molluscum contagiosum, tinea corporis, and herpes gladiatorum, in particular, should be treated before the student is cleared to participate in wrestling. Regardless of the sport involved, however, identify chronic skin problems and institute prophylaxis, as needed.
Conduct a general musculoskeletal exam
A brief evaluation of strength and mobility of each joint and muscle group is sufficient to determine if a student has adequate baseline musculoskeletal function to compete.3 A joint-specific exam will not only help to assess the particular function and stability of each joint, but may reveal subtle deficits of particular joints or muscle groups that may be amenable to rehabilitation or other treatments prior to the start of the sport season.
The emphasis here, too, may vary based on the sport the patient plans to participate in. If you examine the ankles of a volleyball or soccer player and find ligamentous laxity from a prior injury, for example, consider prescribing a brace or physical therapy.
Follow up with a neurologic exam, which is often paired with the musculoskeletal exam and is considered adequate if the patient possesses full strength in all muscle groups. A patient with a history of multiple stingers may warrant a more detailed examination of strength, reflex, and sensation in the upper extremities to screen for signs of residual nerve injury. Similarly, a patient with a history of multiple concussions may need a more detailed exam that includes the cranial nerves, evaluation of balance, and possibly baseline neuropsychological testing.
Screen for Marfan syndrome
This genetic disorder involves mutations of the fibrillin gene that lead to a diverse presentation of abnormalities in multiple organ systems. Primarily because of the effects of Marfan syndrome on the cardiovascular system, it is important to identify it as part of the PPE. Individuals with this disorder have an increased risk for valvular disorders and for aortic dilation that can lead to dissection or rupture. If the history reveals that the patient has a family member with this disorder or has a history of spontaneous pneumothorax or mitral valve prolapse, look closely for the following skeletal and cardiac abnormalities:
- pectus carinatum or excavatum
- arm span-to-height ratio >1.05
- arachnodactyly
- pes planus
- scoliosis
- reduced elbow extension
- highly arched palate
- murmur of mitral valve prolapse or regurgitation.
If you find any of these abnormalities, postpone sports clearance until the patient undergoes further evaluation.28 To establish a diagnosis of Marfan syndrome, the patient must have major criteria in 2 organ systems, with involvement of a third system.
Make a determination: Should the patient be cleared?
The final part of the PPE, of course, is your decision as to whether the patient should be cleared to engage in competitive activities. Clearance falls into 4 categories: (1) Clearance without restriction; (2) clearance with recommendations for further evaluation or treatment; (3) not cleared; restricted until completion of further testing/consultation; (4) complete restriction from certain or all sports.
The goal of the PPE is to provide safe participation in sports for all athletes, not to disqualify anyone. Fortunately, the vast majority of student athletes qualify for clearance without restriction. About 3% to 10% of those who undergo preparticipation screening require further evaluation prior to sports clearance, and less than 1% are disqualified.3
Being prevented from participating in sports can be a major stressor that interferes with the student’s sense of well-being. The inability to exercise or participate in organized sports poses a risk to the patient’s overall health, and may contribute to social isolation. In fact, being restricted from playing organized sports has been reported to be as stressful as the death of a close friend.29
Help in making the determination. Nonetheless, there are circumstances when restriction is necessary. In addition to the new PPE, there are 2 sources you can turn to for help in making that determination. The Bethesda guidelines—Eligibility Recommendations for Competitive Athletes with Cardiovascular Abnormalities, published by the American College of Cardiology and available at http://content.onlinejacc.org/cgi/content/full/45/8/1318, call for disqualifying students only when the activity would pose a clear danger to their health.23
The American Academy of Pediatrics (AAP) has published a set of sports participation guidelines of its own (available at http://www.guideline.gov/summary/summary.aspx?doc_id=13439) for children with conditions that range from diabetes mellitus and human immunodeficiency virus to mitral valve prolapse, rheumatoid arthritis, and bleeding disorders. There are, of course, some disorders for which the AAP would disqualify students from participation, but in many cases it recommends a “qualified Yes.”27,30
CORRESPONDENCE Jason Womack, MD, UMDNJ - Robert Wood Johnson Medical School, 1 Robert Wood Johnson Place, Department of Family Medicine, MEB 2nd floor, New Brunswick, NJ 08903; [email protected]
1. Klossner D. ed. NCAA Sports Medicine Handbook. 20th ed. Indianapolis, Ind: National College Athletic Association; 2009.
2. Schultz SJ, Zinder SM, Valovich TC. NFHS Sports Medicine Handbook. Indianapolis, Ind: National Federation of State High School Associations; July 2001.
3. Bernhard DT, Roberts WO. eds. Preparticipation Physical Evaluation. 4th ed. Indianapolis, Ind: American American College of Sports Medicine; 2010. Available at: http://www.ppe-sportsevaluation.org/body.html. Accessed June 25, 2010.
4. Maron BJ, Shirani J, Poliac LC, et al. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199-204.
5. Luckstead EF, Sr. Cardiac risk factors and participation guidelines for youth sports. Pediatr Clin North Am. 2002;49:681-707.
6. Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32:1881-1884.
7. Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064-1075.
8. Maron BJ, Thompson PD, Ackerman MJ, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643-1655.
9. National Institutes of Health. National Asthma Education and Prevention Program, Expert panel report: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Institutes of Health; 2003. NIH publication 03-5074.
10. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
11. Shannon B, Klimkiewicz JJ. Cervical burners in the athlete. Clin Sports Med. 2002;21:29-35, vi.
12. Torg JS. Cervical spinal stenosis with cord neurapraxia: evaluations and decisions regarding participation in athletics. Curr Sports Med Rep. 2002;1:43-46.
13. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport—the Third International Conference on Concussion in Sport held in Zurich, November 2008. J Clin Neurosci. 2009;16:755-763.
14. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549-2555.
15. Metzl JD. The adolescent preparticipation physical examination. Is it helpful? Clin Sports Med. 2000;19:577-592.
16. Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness. Pediatrics. 2000;106(1 pt 1):158-159.
17. Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
18. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
19. Napier SM, Baker RS, Sanford DG, et al. Eye injuries in athletics and recreation. Surv Ophthalmol. 1996;41:229-244.
20. Osguthorpe JD, Nielsen DR. Otitis externa: review and clinical update. Am Fam Physician. 2006;74:1510-1516.
21. House JW, Wilkinson EP. External auditory exostoses: evaluation and treatment. Otolaryngol Head Neck Surg. 2008;138:672-678.
22. Maron BJ, Zipes DP, et al. 36th Bethesda Conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005;45:1318-1375.
23. Report of the Second Task Force on Blood Pressure Control in Children—1987. Task Force on Blood Pressure Control in Children. Pediatrics. 1987;79:1-25.
24. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
25. American Academy of Pediatrics Committee on Sports Medicine and Fitness Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 1997;99:637-638.
26. Putukian M, O’Connor FG, Stricker P, et al. Mononucleosis and athletic participation: an evidence-based subject review. Clin J Sport Med. 2008;18:309-315.
27. Rice SG and the American Academy of Pediatrics Council on Sports Medicine and Fitness. Medical conditions affecting sports participation. Pediatrics. 2008;121:841-848.
28. De Paepe A, Devereux RB, Dietz HC, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417-426.
29. Stevens MB, Smith GN. The preparticipation sports assessment. Fam Pract Recert. 1986;8:68-88.
30. National Guideline Clearinghouse. Medical conditions affecting sports participation. 2008. Available at: http://guideline.gov/summary/summary.Aspx?doc_id=13439. Accessed June 30, 2010.
• Cover the 12 components of the preparticipation physical evaluation (PPE) recommended by the American Heart Association to screen young athletes for potentially life-threatening cardiovascular disease. B
• Perform a genitourinary exam as part of the PPE for young men; assess young women for the criteria associated with the female athlete triad. C
• Perform auscultation while the patient is squatting and while doing the Valsalva maneuver to determine whether any murmurs you detected on examination are associated with hypertrophic cardiomyopathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With preparticipation physical evaluations (PPEs) required for competitive athletic activities at colleges nationwide1 and at high schools and middle schools in the vast majority of states,2 the start of a new school year often brings a barrage of student visits. Yet despite this almost universal requirement, there is no universal standard for the PPE.
There is, however, a new evidence-based guideline. Preparticipation Physical Evaluation, 4th edition,3 released earlier this year, was created by the American Academy of Family Physicians, American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopaedic Society for Sports Medicine, and American Osteopathic Academy of Sports Medicine.
This guideline describes how to conduct a thorough medical history and a targeted physical exam, with a focus on activity-related risks to various organ systems. Regardless of the specific activity the student is interested in pursuing, however, emphasis on cardiac, neurological, and musculoskeletal systems is crucial because of the frequency and gravity of complications.
Help with the medical history: A downloadable form
The history is the most important part of the PPE, which should be scheduled at least 6 weeks before the sports season starts to allow time for follow-up testing or consultation. A form (which can be downloaded along with a physical exam form from the American College of Sports Medicine’s [ACSM] Web site at http://www.ppesportsevaluation.org/body.html) features 54 questions, which cover a range of organ systems and highlight areas of common sports injury and disability. The answers to these questions alone can identify 75% of problems affecting athletes,3 including chronic conditions and medications that may require adjustment or closer monitoring.
Start with the cardiovascular system
As many as 85% of sudden deaths in young athletes are related to underlying cardiac abnormalities, according to a 10-year study of 150 such cases.4 Indeed, sudden cardiac death occurs in approximately 1 in 200,000 high school athletes.5,6 Not surprisingly, those engaged in high-intensity activity are at highest risk.7
Thus, screening school-aged athletes for potential causes of sudden cardiac death is a primary objective of the PPE.3 The American Heart Association (AHA) recommends a 12-part evaluation (TABLE 1) of the heart, with 8 history questions and 4 physical exam components, to properly screen for cardiac disease in the young athlete.8 Syncope, chest pain, and dyspnea, particularly if associated with exertion, may be signs of underlying cardiac disorders that warrant confirmatory testing;5 a family history of premature death or disability from cardiac disease indicate a need for additional testing, as well. Further evaluation normally entails a combination of cardiac testing and consultation with a cardiologist.
Ask about asthma, including exercise-induced asthma (EIA). Identifying any respiratory conditions is vital to ensure adequate treatment and optimal performance. Patients with EIA are normally asymptomatic at rest, with no disturbance of peak expiratory flow. The presence of cough, chest tightness, wheezing, dyspnea, or loss of endurance during exercise suggests an EIA diagnosis.9 If a patient reports any such symptoms, order pulmonary function tests for confirmation.
EIA affects between 10% and 50% of athletes, depending on the sport.10 In up to 80% of athletes with EIA, use of inhaled short-acting beta-agonists prior to participation can help to prevent symptoms.9 Any athlete who reports symptoms of asthma, whether or not it is exercise-induced, requires treatment to prevent serious respiratory sequelae.
TABLE 1
Ask these 12 questions during cardiovascular screening5
| Personal history |
|---|
Has the patient had: 1. exertional chest pain/discomfort? |
| Family history |
| Has a family member: 6. died prematurely (sudden or unexpected) before age 50 due to heart disease? 7. suffered a disability from heart disease before age 50? 8. been diagnosed with a cardiac condition, such as hypertrophic or dilated cardiomyopathy, long QT syndrome, ion channelopathies, or Marfan syndrome? |
| Physical exam |
| Does the patient have: 9. a heart murmur? 10. femoral pulses? 11. physical stigmata of Marfan syndrome? 12. brachial artery blood pressure? |
Take a neurologic history
The neurologic system is highly susceptible to injury during sports activity. Identifying a history of concussion, nerve injury, or neurologic deficit is important both to prevent future injury and to avoid worsening of a current disability.
Always ask about transient neuropraxia, also known as a “stinger” or “burner”—a traction or compression injury to the nerves of the brachial plexus or cervical nerve roots that is sustained by 50% to 65% of college football players.11 The injury causes numbness, weakness, or both, in an upper extremity. Although symptoms are commonly transient, the injury may be severe enough to cause more prolonged symptoms; about 5% to 10% of the time, symptoms related to transient neuropraxia persist for hours to weeks.11
A history of numbness or weakness that occurs simultaneously in more than 1 extremity is suggestive of underlying cervical cord neuropraxia.12 Patients with such symptoms require further evaluation for the presence of spinal stenosis, cervical ligamentous injury, and spinal cord injury before being cleared to participate in sports.
Concussion is a common neurologic insult among competitive athletes (See “Update on concussion: Here’s what the experts have to say”), accounting for anywhere from 6.5% to 18.5% of injuries in collegiate contact sports.1,13 Identifying students who have had head injuries and are therefore at increased risk for future concussion is a key function of the PPE.13,14
An individual who suffers even a mild head injury prior to full resolution of an initial concussion is at risk for second impact syndrome—a devastating brain injury that causes a loss of autoregulation of cerebral blood flow, leading to rapid swelling, herniation, and death. Thus, any athlete with a recent history of concussion requires further evaluation—and disqualification from play until symptoms fully resolve. In fact, anyone with a history of concussion—recent or not—may need neuropsychologic testing to assess baseline level of cognitive function. Thorough documentation is critical in such cases. In the event of a repeat concussion, having this information may assist with treatment and return-to-play decisions.
Review other significant findings noted on the history form. Follow up, as needed, with questions about any missing or dysfunctional organ or bodily system (The ACSM Web site includes a supplemental history form for athletes with special needs to document additional details, if necessary).
In some cases, protective equipment may be sufficient (a student with defective vision in 1 eye can wear protective eyewear to prevent injury to the other eye, for instance). In other cases, a conversation with the patient and his or her family regarding the risk of serious injury may be in order. Parents of a child with only 1 kidney, for example, should be advised that contact sports pose a small, but real risk of damage to that kidney, potentially resulting in the need for dialysis.
Take the opportunity to discuss body image, mental health
For many adolescents—up to 50%, by some counts15—the PPE is their only interaction with a clinician. Thus, it provides a good opportunity for you to talk to young athletes about such sensitive topics as high-risk behaviors, mental health issues, body image, and personal safety. To help ensure that students feel free to talk openly, the new PPE forms have removed key questions about high-risk behaviors from the patient history (which requires a parent’s signature). Instead, a series of questions is listed under the heading “physician reminders” at the top of the form for the physical exam,3 which is typically conducted in a private setting.
Overweight? Underweight? Height and weight, a standard part of the physical along with blood pressure and pulse rate, may clue you in to the existence of an eating disorder. Calculate body mass index (BMI), looking for students who are underweight (BMI <19 kg/m2) or overweight (BMI >25 kg/m2). Overweight athletes are at increased risk for heat illness and may need a preseason conditioning program.16 Underweight athletes—particularly young women—may be at additional risk.
Female athlete triad. When examining young women, be alert to signs and symptoms of the female athlete triad, a syndrome of disordered eating, amenorrhea, and osteopenia or osteoporosis. This related spectrum of medical problems can pose a significant health risk, as it involves a cycle of low energy intake that “turns off” the reproductive cycle and creates a hypoestrogenic state. The lack of estrogen has a devastating effect on bone mineral resorption and can lead to osteopenia.17 While the prevalence of the female athlete triad is low in the general population, 1 study noted that nearly 6% of young female athletes met the criteria for 2 of the 3 components of the triad, and as many as 20% had at least 1.18
Is eye protection needed? Ear plugs?
Check vision in each eye, both with and without corrective lenses. A student whose corrected vision is 20/50 or worse in 1 eye qualifies as functionally one-eyed—and must wear full eye protection during athletic activities.19 Document pupil size for all patients to ensure that anisocoria is not confused with a neurological insult in the event of a sports injury.
Water sports? Examine the nares, inspecting the septum for signs of deviation or perforation. Th is portion of the exam, however, can be tailored to the type of sport in which the patient plans to participate. Students who expect to be on a swim team or participate in other water sports require an evaluation of the ears, including the tympanic membranes and external auditory canals. Swimming and humid environments are risk factors for otitis externa, and repeated cold water exposure is a risk for the development of external auditory exostoses.20,21 A swimmer with perforated tympanic membranes should be advised to wear ear plugs to protect the middle ear.
Is the patient a wrestler? Pay close attention to the ears of any student who plans to join a wrestling team, documenting the absence—or presence—of cauliflower ear. A notation about the presence of this ear-deforming condition in the medical record ensures that any new auricular hematoma can be evaluated and treated with knowledge of the prior injury.
Cardiac findings that warrant further work-up
Elevated blood pressure, the most common cardiovascular abnormality in people participating in competitive sports,22 is categorized in stages as defined by the Second Task Force on Blood Pressure Control in Children23 and the Joint National Commission VII for adults ages 18 and older.24 Children with significant hypertension (TABLE 2) and adults with stage I hypertension (140-159/90-99 mm Hg) may participate as long as there is no indication of end organ disease. A child with severe hypertension or an adult with stage II hypertension (>159/99 mm Hg), however, should not be cleared for participation until he or she has had further evaluation and treatment.22,25
Similarly, any patient with an elevated heart rate needs a medical work-up prior to participation to determine the underlying cause of tachycardia.22 This may be a sign of an underlying cardiac arrhythmia or another medical condition that must be treated prior to athletic competition.
Auscultate the heart to screen for underlying cardiac disease. Listen to all 4 standard regions, with the patient in both a supine and a standing position. If you detect a murmur, perform auscultation while the patient squats and while performing the Valsalva maneuver. The murmur of hypertrophic cardiomyopathy (HCM)—the key cause of sudden cardiac death—is systolic, increasing with standing and the Valsalva maneuver and decreasing with squatting and a supine position. Any murmur that is 3/6 or greater in sound or has characteristics of HCM needs further evaluation before the patient can be cleared for sports activities. (See “Hypertrophic cardiomyopathy: Ask athletes these 9 questions,” J Fam Pract. 2009;58:576-584).
Palpate the femoral pulses. Absence of, or decreased, femoral pulses compared with brachial pulses may suggest coarctation of the aorta.
TABLE 2
Hypertension in children: Is it significant or severe?24
| Age | Significant HTN (mm Hg) | Severe HTN (mm Hg) |
|---|---|---|
| 6-9 y Systolic Diastolic | 122-129 78-85 | >129 >85 |
| 10-12 y Systolic Diastolic | 126-133 82-89 | >133 >89 |
| 13-15 y Systolic Diastolic | 136-143 86-91 | >143 >91 |
| 16-18 y Systolic Diastolic | 142-149 92-97 | >149 >97 |
| HTN, hypertension. | ||
Check lungs, abdomen, and skin
Auscultate the lungs, which should be clear and absent of adventitious or diminished breath sounds. Any patient with abnormal breath sounds requires further evaluation and/or treatment before being cleared for sports participation.
Examine the abdomen to assess the presence of organomegaly. Enlargement of the liver and spleen may be a sign of an underlying disease process. Mononucleosis, which causes splenomegaly, is associated with a 0.1% to 0.2% rate of rupture that may be related to trauma or the Valsalva maneuver.26 Athletes with organomegaly should not be cleared for athletic activity without further evaluation.3
Perform a genitourinary examination on young men. Check for the presence of both testicles and palpate for masses and inguinal hernias. A patient with a solitary testicle will require protective gear during certain sporting events to prevent injury.27
Assess the condition of the skin. In this case, the activity the student plans to pursue will determine the extent of the examination. Skin condition is particularly important for wrestlers, as the close contact involved predisposes the athletes to infectious skin conditions. Molluscum contagiosum, tinea corporis, and herpes gladiatorum, in particular, should be treated before the student is cleared to participate in wrestling. Regardless of the sport involved, however, identify chronic skin problems and institute prophylaxis, as needed.
Conduct a general musculoskeletal exam
A brief evaluation of strength and mobility of each joint and muscle group is sufficient to determine if a student has adequate baseline musculoskeletal function to compete.3 A joint-specific exam will not only help to assess the particular function and stability of each joint, but may reveal subtle deficits of particular joints or muscle groups that may be amenable to rehabilitation or other treatments prior to the start of the sport season.
The emphasis here, too, may vary based on the sport the patient plans to participate in. If you examine the ankles of a volleyball or soccer player and find ligamentous laxity from a prior injury, for example, consider prescribing a brace or physical therapy.
Follow up with a neurologic exam, which is often paired with the musculoskeletal exam and is considered adequate if the patient possesses full strength in all muscle groups. A patient with a history of multiple stingers may warrant a more detailed examination of strength, reflex, and sensation in the upper extremities to screen for signs of residual nerve injury. Similarly, a patient with a history of multiple concussions may need a more detailed exam that includes the cranial nerves, evaluation of balance, and possibly baseline neuropsychological testing.
Screen for Marfan syndrome
This genetic disorder involves mutations of the fibrillin gene that lead to a diverse presentation of abnormalities in multiple organ systems. Primarily because of the effects of Marfan syndrome on the cardiovascular system, it is important to identify it as part of the PPE. Individuals with this disorder have an increased risk for valvular disorders and for aortic dilation that can lead to dissection or rupture. If the history reveals that the patient has a family member with this disorder or has a history of spontaneous pneumothorax or mitral valve prolapse, look closely for the following skeletal and cardiac abnormalities:
- pectus carinatum or excavatum
- arm span-to-height ratio >1.05
- arachnodactyly
- pes planus
- scoliosis
- reduced elbow extension
- highly arched palate
- murmur of mitral valve prolapse or regurgitation.
If you find any of these abnormalities, postpone sports clearance until the patient undergoes further evaluation.28 To establish a diagnosis of Marfan syndrome, the patient must have major criteria in 2 organ systems, with involvement of a third system.
Make a determination: Should the patient be cleared?
The final part of the PPE, of course, is your decision as to whether the patient should be cleared to engage in competitive activities. Clearance falls into 4 categories: (1) Clearance without restriction; (2) clearance with recommendations for further evaluation or treatment; (3) not cleared; restricted until completion of further testing/consultation; (4) complete restriction from certain or all sports.
The goal of the PPE is to provide safe participation in sports for all athletes, not to disqualify anyone. Fortunately, the vast majority of student athletes qualify for clearance without restriction. About 3% to 10% of those who undergo preparticipation screening require further evaluation prior to sports clearance, and less than 1% are disqualified.3
Being prevented from participating in sports can be a major stressor that interferes with the student’s sense of well-being. The inability to exercise or participate in organized sports poses a risk to the patient’s overall health, and may contribute to social isolation. In fact, being restricted from playing organized sports has been reported to be as stressful as the death of a close friend.29
Help in making the determination. Nonetheless, there are circumstances when restriction is necessary. In addition to the new PPE, there are 2 sources you can turn to for help in making that determination. The Bethesda guidelines—Eligibility Recommendations for Competitive Athletes with Cardiovascular Abnormalities, published by the American College of Cardiology and available at http://content.onlinejacc.org/cgi/content/full/45/8/1318, call for disqualifying students only when the activity would pose a clear danger to their health.23
The American Academy of Pediatrics (AAP) has published a set of sports participation guidelines of its own (available at http://www.guideline.gov/summary/summary.aspx?doc_id=13439) for children with conditions that range from diabetes mellitus and human immunodeficiency virus to mitral valve prolapse, rheumatoid arthritis, and bleeding disorders. There are, of course, some disorders for which the AAP would disqualify students from participation, but in many cases it recommends a “qualified Yes.”27,30
CORRESPONDENCE Jason Womack, MD, UMDNJ - Robert Wood Johnson Medical School, 1 Robert Wood Johnson Place, Department of Family Medicine, MEB 2nd floor, New Brunswick, NJ 08903; [email protected]
• Cover the 12 components of the preparticipation physical evaluation (PPE) recommended by the American Heart Association to screen young athletes for potentially life-threatening cardiovascular disease. B
• Perform a genitourinary exam as part of the PPE for young men; assess young women for the criteria associated with the female athlete triad. C
• Perform auscultation while the patient is squatting and while doing the Valsalva maneuver to determine whether any murmurs you detected on examination are associated with hypertrophic cardiomyopathy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With preparticipation physical evaluations (PPEs) required for competitive athletic activities at colleges nationwide1 and at high schools and middle schools in the vast majority of states,2 the start of a new school year often brings a barrage of student visits. Yet despite this almost universal requirement, there is no universal standard for the PPE.
There is, however, a new evidence-based guideline. Preparticipation Physical Evaluation, 4th edition,3 released earlier this year, was created by the American Academy of Family Physicians, American Academy of Pediatrics, American College of Sports Medicine, American Medical Society for Sports Medicine, American Orthopaedic Society for Sports Medicine, and American Osteopathic Academy of Sports Medicine.
This guideline describes how to conduct a thorough medical history and a targeted physical exam, with a focus on activity-related risks to various organ systems. Regardless of the specific activity the student is interested in pursuing, however, emphasis on cardiac, neurological, and musculoskeletal systems is crucial because of the frequency and gravity of complications.
Help with the medical history: A downloadable form
The history is the most important part of the PPE, which should be scheduled at least 6 weeks before the sports season starts to allow time for follow-up testing or consultation. A form (which can be downloaded along with a physical exam form from the American College of Sports Medicine’s [ACSM] Web site at http://www.ppesportsevaluation.org/body.html) features 54 questions, which cover a range of organ systems and highlight areas of common sports injury and disability. The answers to these questions alone can identify 75% of problems affecting athletes,3 including chronic conditions and medications that may require adjustment or closer monitoring.
Start with the cardiovascular system
As many as 85% of sudden deaths in young athletes are related to underlying cardiac abnormalities, according to a 10-year study of 150 such cases.4 Indeed, sudden cardiac death occurs in approximately 1 in 200,000 high school athletes.5,6 Not surprisingly, those engaged in high-intensity activity are at highest risk.7
Thus, screening school-aged athletes for potential causes of sudden cardiac death is a primary objective of the PPE.3 The American Heart Association (AHA) recommends a 12-part evaluation (TABLE 1) of the heart, with 8 history questions and 4 physical exam components, to properly screen for cardiac disease in the young athlete.8 Syncope, chest pain, and dyspnea, particularly if associated with exertion, may be signs of underlying cardiac disorders that warrant confirmatory testing;5 a family history of premature death or disability from cardiac disease indicate a need for additional testing, as well. Further evaluation normally entails a combination of cardiac testing and consultation with a cardiologist.
Ask about asthma, including exercise-induced asthma (EIA). Identifying any respiratory conditions is vital to ensure adequate treatment and optimal performance. Patients with EIA are normally asymptomatic at rest, with no disturbance of peak expiratory flow. The presence of cough, chest tightness, wheezing, dyspnea, or loss of endurance during exercise suggests an EIA diagnosis.9 If a patient reports any such symptoms, order pulmonary function tests for confirmation.
EIA affects between 10% and 50% of athletes, depending on the sport.10 In up to 80% of athletes with EIA, use of inhaled short-acting beta-agonists prior to participation can help to prevent symptoms.9 Any athlete who reports symptoms of asthma, whether or not it is exercise-induced, requires treatment to prevent serious respiratory sequelae.
TABLE 1
Ask these 12 questions during cardiovascular screening5
| Personal history |
|---|
Has the patient had: 1. exertional chest pain/discomfort? |
| Family history |
| Has a family member: 6. died prematurely (sudden or unexpected) before age 50 due to heart disease? 7. suffered a disability from heart disease before age 50? 8. been diagnosed with a cardiac condition, such as hypertrophic or dilated cardiomyopathy, long QT syndrome, ion channelopathies, or Marfan syndrome? |
| Physical exam |
| Does the patient have: 9. a heart murmur? 10. femoral pulses? 11. physical stigmata of Marfan syndrome? 12. brachial artery blood pressure? |
Take a neurologic history
The neurologic system is highly susceptible to injury during sports activity. Identifying a history of concussion, nerve injury, or neurologic deficit is important both to prevent future injury and to avoid worsening of a current disability.
Always ask about transient neuropraxia, also known as a “stinger” or “burner”—a traction or compression injury to the nerves of the brachial plexus or cervical nerve roots that is sustained by 50% to 65% of college football players.11 The injury causes numbness, weakness, or both, in an upper extremity. Although symptoms are commonly transient, the injury may be severe enough to cause more prolonged symptoms; about 5% to 10% of the time, symptoms related to transient neuropraxia persist for hours to weeks.11
A history of numbness or weakness that occurs simultaneously in more than 1 extremity is suggestive of underlying cervical cord neuropraxia.12 Patients with such symptoms require further evaluation for the presence of spinal stenosis, cervical ligamentous injury, and spinal cord injury before being cleared to participate in sports.
Concussion is a common neurologic insult among competitive athletes (See “Update on concussion: Here’s what the experts have to say”), accounting for anywhere from 6.5% to 18.5% of injuries in collegiate contact sports.1,13 Identifying students who have had head injuries and are therefore at increased risk for future concussion is a key function of the PPE.13,14
An individual who suffers even a mild head injury prior to full resolution of an initial concussion is at risk for second impact syndrome—a devastating brain injury that causes a loss of autoregulation of cerebral blood flow, leading to rapid swelling, herniation, and death. Thus, any athlete with a recent history of concussion requires further evaluation—and disqualification from play until symptoms fully resolve. In fact, anyone with a history of concussion—recent or not—may need neuropsychologic testing to assess baseline level of cognitive function. Thorough documentation is critical in such cases. In the event of a repeat concussion, having this information may assist with treatment and return-to-play decisions.
Review other significant findings noted on the history form. Follow up, as needed, with questions about any missing or dysfunctional organ or bodily system (The ACSM Web site includes a supplemental history form for athletes with special needs to document additional details, if necessary).
In some cases, protective equipment may be sufficient (a student with defective vision in 1 eye can wear protective eyewear to prevent injury to the other eye, for instance). In other cases, a conversation with the patient and his or her family regarding the risk of serious injury may be in order. Parents of a child with only 1 kidney, for example, should be advised that contact sports pose a small, but real risk of damage to that kidney, potentially resulting in the need for dialysis.
Take the opportunity to discuss body image, mental health
For many adolescents—up to 50%, by some counts15—the PPE is their only interaction with a clinician. Thus, it provides a good opportunity for you to talk to young athletes about such sensitive topics as high-risk behaviors, mental health issues, body image, and personal safety. To help ensure that students feel free to talk openly, the new PPE forms have removed key questions about high-risk behaviors from the patient history (which requires a parent’s signature). Instead, a series of questions is listed under the heading “physician reminders” at the top of the form for the physical exam,3 which is typically conducted in a private setting.
Overweight? Underweight? Height and weight, a standard part of the physical along with blood pressure and pulse rate, may clue you in to the existence of an eating disorder. Calculate body mass index (BMI), looking for students who are underweight (BMI <19 kg/m2) or overweight (BMI >25 kg/m2). Overweight athletes are at increased risk for heat illness and may need a preseason conditioning program.16 Underweight athletes—particularly young women—may be at additional risk.
Female athlete triad. When examining young women, be alert to signs and symptoms of the female athlete triad, a syndrome of disordered eating, amenorrhea, and osteopenia or osteoporosis. This related spectrum of medical problems can pose a significant health risk, as it involves a cycle of low energy intake that “turns off” the reproductive cycle and creates a hypoestrogenic state. The lack of estrogen has a devastating effect on bone mineral resorption and can lead to osteopenia.17 While the prevalence of the female athlete triad is low in the general population, 1 study noted that nearly 6% of young female athletes met the criteria for 2 of the 3 components of the triad, and as many as 20% had at least 1.18
Is eye protection needed? Ear plugs?
Check vision in each eye, both with and without corrective lenses. A student whose corrected vision is 20/50 or worse in 1 eye qualifies as functionally one-eyed—and must wear full eye protection during athletic activities.19 Document pupil size for all patients to ensure that anisocoria is not confused with a neurological insult in the event of a sports injury.
Water sports? Examine the nares, inspecting the septum for signs of deviation or perforation. Th is portion of the exam, however, can be tailored to the type of sport in which the patient plans to participate. Students who expect to be on a swim team or participate in other water sports require an evaluation of the ears, including the tympanic membranes and external auditory canals. Swimming and humid environments are risk factors for otitis externa, and repeated cold water exposure is a risk for the development of external auditory exostoses.20,21 A swimmer with perforated tympanic membranes should be advised to wear ear plugs to protect the middle ear.
Is the patient a wrestler? Pay close attention to the ears of any student who plans to join a wrestling team, documenting the absence—or presence—of cauliflower ear. A notation about the presence of this ear-deforming condition in the medical record ensures that any new auricular hematoma can be evaluated and treated with knowledge of the prior injury.
Cardiac findings that warrant further work-up
Elevated blood pressure, the most common cardiovascular abnormality in people participating in competitive sports,22 is categorized in stages as defined by the Second Task Force on Blood Pressure Control in Children23 and the Joint National Commission VII for adults ages 18 and older.24 Children with significant hypertension (TABLE 2) and adults with stage I hypertension (140-159/90-99 mm Hg) may participate as long as there is no indication of end organ disease. A child with severe hypertension or an adult with stage II hypertension (>159/99 mm Hg), however, should not be cleared for participation until he or she has had further evaluation and treatment.22,25
Similarly, any patient with an elevated heart rate needs a medical work-up prior to participation to determine the underlying cause of tachycardia.22 This may be a sign of an underlying cardiac arrhythmia or another medical condition that must be treated prior to athletic competition.
Auscultate the heart to screen for underlying cardiac disease. Listen to all 4 standard regions, with the patient in both a supine and a standing position. If you detect a murmur, perform auscultation while the patient squats and while performing the Valsalva maneuver. The murmur of hypertrophic cardiomyopathy (HCM)—the key cause of sudden cardiac death—is systolic, increasing with standing and the Valsalva maneuver and decreasing with squatting and a supine position. Any murmur that is 3/6 or greater in sound or has characteristics of HCM needs further evaluation before the patient can be cleared for sports activities. (See “Hypertrophic cardiomyopathy: Ask athletes these 9 questions,” J Fam Pract. 2009;58:576-584).
Palpate the femoral pulses. Absence of, or decreased, femoral pulses compared with brachial pulses may suggest coarctation of the aorta.
TABLE 2
Hypertension in children: Is it significant or severe?24
| Age | Significant HTN (mm Hg) | Severe HTN (mm Hg) |
|---|---|---|
| 6-9 y Systolic Diastolic | 122-129 78-85 | >129 >85 |
| 10-12 y Systolic Diastolic | 126-133 82-89 | >133 >89 |
| 13-15 y Systolic Diastolic | 136-143 86-91 | >143 >91 |
| 16-18 y Systolic Diastolic | 142-149 92-97 | >149 >97 |
| HTN, hypertension. | ||
Check lungs, abdomen, and skin
Auscultate the lungs, which should be clear and absent of adventitious or diminished breath sounds. Any patient with abnormal breath sounds requires further evaluation and/or treatment before being cleared for sports participation.
Examine the abdomen to assess the presence of organomegaly. Enlargement of the liver and spleen may be a sign of an underlying disease process. Mononucleosis, which causes splenomegaly, is associated with a 0.1% to 0.2% rate of rupture that may be related to trauma or the Valsalva maneuver.26 Athletes with organomegaly should not be cleared for athletic activity without further evaluation.3
Perform a genitourinary examination on young men. Check for the presence of both testicles and palpate for masses and inguinal hernias. A patient with a solitary testicle will require protective gear during certain sporting events to prevent injury.27
Assess the condition of the skin. In this case, the activity the student plans to pursue will determine the extent of the examination. Skin condition is particularly important for wrestlers, as the close contact involved predisposes the athletes to infectious skin conditions. Molluscum contagiosum, tinea corporis, and herpes gladiatorum, in particular, should be treated before the student is cleared to participate in wrestling. Regardless of the sport involved, however, identify chronic skin problems and institute prophylaxis, as needed.
Conduct a general musculoskeletal exam
A brief evaluation of strength and mobility of each joint and muscle group is sufficient to determine if a student has adequate baseline musculoskeletal function to compete.3 A joint-specific exam will not only help to assess the particular function and stability of each joint, but may reveal subtle deficits of particular joints or muscle groups that may be amenable to rehabilitation or other treatments prior to the start of the sport season.
The emphasis here, too, may vary based on the sport the patient plans to participate in. If you examine the ankles of a volleyball or soccer player and find ligamentous laxity from a prior injury, for example, consider prescribing a brace or physical therapy.
Follow up with a neurologic exam, which is often paired with the musculoskeletal exam and is considered adequate if the patient possesses full strength in all muscle groups. A patient with a history of multiple stingers may warrant a more detailed examination of strength, reflex, and sensation in the upper extremities to screen for signs of residual nerve injury. Similarly, a patient with a history of multiple concussions may need a more detailed exam that includes the cranial nerves, evaluation of balance, and possibly baseline neuropsychological testing.
Screen for Marfan syndrome
This genetic disorder involves mutations of the fibrillin gene that lead to a diverse presentation of abnormalities in multiple organ systems. Primarily because of the effects of Marfan syndrome on the cardiovascular system, it is important to identify it as part of the PPE. Individuals with this disorder have an increased risk for valvular disorders and for aortic dilation that can lead to dissection or rupture. If the history reveals that the patient has a family member with this disorder or has a history of spontaneous pneumothorax or mitral valve prolapse, look closely for the following skeletal and cardiac abnormalities:
- pectus carinatum or excavatum
- arm span-to-height ratio >1.05
- arachnodactyly
- pes planus
- scoliosis
- reduced elbow extension
- highly arched palate
- murmur of mitral valve prolapse or regurgitation.
If you find any of these abnormalities, postpone sports clearance until the patient undergoes further evaluation.28 To establish a diagnosis of Marfan syndrome, the patient must have major criteria in 2 organ systems, with involvement of a third system.
Make a determination: Should the patient be cleared?
The final part of the PPE, of course, is your decision as to whether the patient should be cleared to engage in competitive activities. Clearance falls into 4 categories: (1) Clearance without restriction; (2) clearance with recommendations for further evaluation or treatment; (3) not cleared; restricted until completion of further testing/consultation; (4) complete restriction from certain or all sports.
The goal of the PPE is to provide safe participation in sports for all athletes, not to disqualify anyone. Fortunately, the vast majority of student athletes qualify for clearance without restriction. About 3% to 10% of those who undergo preparticipation screening require further evaluation prior to sports clearance, and less than 1% are disqualified.3
Being prevented from participating in sports can be a major stressor that interferes with the student’s sense of well-being. The inability to exercise or participate in organized sports poses a risk to the patient’s overall health, and may contribute to social isolation. In fact, being restricted from playing organized sports has been reported to be as stressful as the death of a close friend.29
Help in making the determination. Nonetheless, there are circumstances when restriction is necessary. In addition to the new PPE, there are 2 sources you can turn to for help in making that determination. The Bethesda guidelines—Eligibility Recommendations for Competitive Athletes with Cardiovascular Abnormalities, published by the American College of Cardiology and available at http://content.onlinejacc.org/cgi/content/full/45/8/1318, call for disqualifying students only when the activity would pose a clear danger to their health.23
The American Academy of Pediatrics (AAP) has published a set of sports participation guidelines of its own (available at http://www.guideline.gov/summary/summary.aspx?doc_id=13439) for children with conditions that range from diabetes mellitus and human immunodeficiency virus to mitral valve prolapse, rheumatoid arthritis, and bleeding disorders. There are, of course, some disorders for which the AAP would disqualify students from participation, but in many cases it recommends a “qualified Yes.”27,30
CORRESPONDENCE Jason Womack, MD, UMDNJ - Robert Wood Johnson Medical School, 1 Robert Wood Johnson Place, Department of Family Medicine, MEB 2nd floor, New Brunswick, NJ 08903; [email protected]
1. Klossner D. ed. NCAA Sports Medicine Handbook. 20th ed. Indianapolis, Ind: National College Athletic Association; 2009.
2. Schultz SJ, Zinder SM, Valovich TC. NFHS Sports Medicine Handbook. Indianapolis, Ind: National Federation of State High School Associations; July 2001.
3. Bernhard DT, Roberts WO. eds. Preparticipation Physical Evaluation. 4th ed. Indianapolis, Ind: American American College of Sports Medicine; 2010. Available at: http://www.ppe-sportsevaluation.org/body.html. Accessed June 25, 2010.
4. Maron BJ, Shirani J, Poliac LC, et al. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199-204.
5. Luckstead EF, Sr. Cardiac risk factors and participation guidelines for youth sports. Pediatr Clin North Am. 2002;49:681-707.
6. Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32:1881-1884.
7. Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064-1075.
8. Maron BJ, Thompson PD, Ackerman MJ, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643-1655.
9. National Institutes of Health. National Asthma Education and Prevention Program, Expert panel report: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Institutes of Health; 2003. NIH publication 03-5074.
10. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
11. Shannon B, Klimkiewicz JJ. Cervical burners in the athlete. Clin Sports Med. 2002;21:29-35, vi.
12. Torg JS. Cervical spinal stenosis with cord neurapraxia: evaluations and decisions regarding participation in athletics. Curr Sports Med Rep. 2002;1:43-46.
13. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport—the Third International Conference on Concussion in Sport held in Zurich, November 2008. J Clin Neurosci. 2009;16:755-763.
14. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549-2555.
15. Metzl JD. The adolescent preparticipation physical examination. Is it helpful? Clin Sports Med. 2000;19:577-592.
16. Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness. Pediatrics. 2000;106(1 pt 1):158-159.
17. Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
18. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
19. Napier SM, Baker RS, Sanford DG, et al. Eye injuries in athletics and recreation. Surv Ophthalmol. 1996;41:229-244.
20. Osguthorpe JD, Nielsen DR. Otitis externa: review and clinical update. Am Fam Physician. 2006;74:1510-1516.
21. House JW, Wilkinson EP. External auditory exostoses: evaluation and treatment. Otolaryngol Head Neck Surg. 2008;138:672-678.
22. Maron BJ, Zipes DP, et al. 36th Bethesda Conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005;45:1318-1375.
23. Report of the Second Task Force on Blood Pressure Control in Children—1987. Task Force on Blood Pressure Control in Children. Pediatrics. 1987;79:1-25.
24. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
25. American Academy of Pediatrics Committee on Sports Medicine and Fitness Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 1997;99:637-638.
26. Putukian M, O’Connor FG, Stricker P, et al. Mononucleosis and athletic participation: an evidence-based subject review. Clin J Sport Med. 2008;18:309-315.
27. Rice SG and the American Academy of Pediatrics Council on Sports Medicine and Fitness. Medical conditions affecting sports participation. Pediatrics. 2008;121:841-848.
28. De Paepe A, Devereux RB, Dietz HC, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417-426.
29. Stevens MB, Smith GN. The preparticipation sports assessment. Fam Pract Recert. 1986;8:68-88.
30. National Guideline Clearinghouse. Medical conditions affecting sports participation. 2008. Available at: http://guideline.gov/summary/summary.Aspx?doc_id=13439. Accessed June 30, 2010.
1. Klossner D. ed. NCAA Sports Medicine Handbook. 20th ed. Indianapolis, Ind: National College Athletic Association; 2009.
2. Schultz SJ, Zinder SM, Valovich TC. NFHS Sports Medicine Handbook. Indianapolis, Ind: National Federation of State High School Associations; July 2001.
3. Bernhard DT, Roberts WO. eds. Preparticipation Physical Evaluation. 4th ed. Indianapolis, Ind: American American College of Sports Medicine; 2010. Available at: http://www.ppe-sportsevaluation.org/body.html. Accessed June 25, 2010.
4. Maron BJ, Shirani J, Poliac LC, et al. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199-204.
5. Luckstead EF, Sr. Cardiac risk factors and participation guidelines for youth sports. Pediatr Clin North Am. 2002;49:681-707.
6. Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32:1881-1884.
7. Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064-1075.
8. Maron BJ, Thompson PD, Ackerman MJ, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115:1643-1655.
9. National Institutes of Health. National Asthma Education and Prevention Program, Expert panel report: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Institutes of Health; 2003. NIH publication 03-5074.
10. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
11. Shannon B, Klimkiewicz JJ. Cervical burners in the athlete. Clin Sports Med. 2002;21:29-35, vi.
12. Torg JS. Cervical spinal stenosis with cord neurapraxia: evaluations and decisions regarding participation in athletics. Curr Sports Med Rep. 2002;1:43-46.
13. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport—the Third International Conference on Concussion in Sport held in Zurich, November 2008. J Clin Neurosci. 2009;16:755-763.
14. Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549-2555.
15. Metzl JD. The adolescent preparticipation physical examination. Is it helpful? Clin Sports Med. 2000;19:577-592.
16. Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness. Pediatrics. 2000;106(1 pt 1):158-159.
17. Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
18. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
19. Napier SM, Baker RS, Sanford DG, et al. Eye injuries in athletics and recreation. Surv Ophthalmol. 1996;41:229-244.
20. Osguthorpe JD, Nielsen DR. Otitis externa: review and clinical update. Am Fam Physician. 2006;74:1510-1516.
21. House JW, Wilkinson EP. External auditory exostoses: evaluation and treatment. Otolaryngol Head Neck Surg. 2008;138:672-678.
22. Maron BJ, Zipes DP, et al. 36th Bethesda Conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2005;45:1318-1375.
23. Report of the Second Task Force on Blood Pressure Control in Children—1987. Task Force on Blood Pressure Control in Children. Pediatrics. 1987;79:1-25.
24. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
25. American Academy of Pediatrics Committee on Sports Medicine and Fitness Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 1997;99:637-638.
26. Putukian M, O’Connor FG, Stricker P, et al. Mononucleosis and athletic participation: an evidence-based subject review. Clin J Sport Med. 2008;18:309-315.
27. Rice SG and the American Academy of Pediatrics Council on Sports Medicine and Fitness. Medical conditions affecting sports participation. Pediatrics. 2008;121:841-848.
28. De Paepe A, Devereux RB, Dietz HC, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417-426.
29. Stevens MB, Smith GN. The preparticipation sports assessment. Fam Pract Recert. 1986;8:68-88.
30. National Guideline Clearinghouse. Medical conditions affecting sports participation. 2008. Available at: http://guideline.gov/summary/summary.Aspx?doc_id=13439. Accessed June 30, 2010.
Update on concussion: Here’s what the experts say
• Don’t allow an athlete who has symptoms at rest or with exertion to return to play. C
• Consider neuropsychological testing in conjunction with continued clinical assessment for objective measurements to assist in managing concussion. B
• Recommend up-to-date protective equipment for athletes. Recent improvements, especially in football, have been shown to help decrease the incidence of concussion. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE: Jeff, a 15-year-old high school ice hockey player, asks you to write a note for his coach, stating that he has recovered from his concussion and can return to play. He says that 2 days ago he collided with another player and was knocked unconscious for roughly 10 seconds. He had a headache for the rest of that evening, and complained that the light was hurting his eyes. Now he has no symptoms at rest, but activity gives him a slight headache.
How would you evaluate this patient to determine whether he can return to play?
Concussions like Jeff’s are common in sports-related activities, and family physicians are frequently asked to manage the condition and decide when the injured athlete can safely return to play.
Concussions occur in both helmeted and nonhelmeted sports, and are most common in collision sports.1 A 2007 estimate from the Centers for Disease Control and Prevention (CDC) suggests that 1.1 million people are seen in emergency departments in the United States each year for concussion-related injuries, while nearly another 235,000 people are hospitalized.2 As astounding as these numbers are, many experts believe they underestimate the true incidence of concussion, given the propensity for athletes not to report symptoms for fear of being held out of sporting events.3,4
Further complicating matters: There has historically been a lack of agreement over what, exactly, constitutes a concussion and how to manage these injuries.
Refining concussion terminology
Concussion has often been referred to as mild traumatic brain injury (MTBI), although more recent expert opinion suggests the terms refer to different injury constructs and should not be used interchangeably.5 Over the years there has been little agreement on the definition, grading, and treatment of these injuries.6-8 On 3 occasions in the last decade, the sports medicine community has held symposia designed to refine an expert consensus on these issues: in 2001 in Vienna, in 2004 in Prague, and in 2008 in Zurich.5,9,10 These recommendations provide a useful framework for caring for patients like Jeff.
A definition. According to the consensus statement that emerged from the most recent Zurich conference, sports concussion can be defined as a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.” Common features may include:5
- A direct blow to the head or elsewhere in the body with an impulsive force transmitted to the head.
- Rapid onset of neurological impairments that resolve spontaneously over time.
- Possible neuropathological changes, although the clinical symptoms reflect a functional disturbance rather than structural injury.
- A graded set of clinical symptoms that may or may not involve loss of consciousness.
- No abnormality on standard structural neuroimaging studies.
Athletes with concussion show a range of signs and symptoms
Athletes who suffer from a concussion may show signs of being dazed or disoriented, or experience retrograde amnesia (where they can’t remember things that happened before the traumatic event) or anterograde amnesia (where they can’t remember things that happened after the event). They may also suffer from poor coordination, decreased attention span, emotional lability, or loss of consciousness. After the traumatic event, they may complain of headache, dizziness, nausea or vomiting, photophobia, phonophobia, inability to concentrate, sleep disturbances, fatigue, and memory disturbances. Academic performance can also be severely impaired during the postconcussive period.
Symptoms of concussion may be short-lived or persist for many weeks. Postconcussive syndrome is a term used to describe the condition of prolonged and persistent concussive symptoms. Recent studies in military personal have also shown a strong association between post-traumatic stress disorder (PTSD) and clinical depression in soldiers who have suffered from a traumatic brain injury.11
Start with the ABCs, then check the spine
If you are a team physician on the spot when the injury occurs, you can do the initial assessment on the field of play. A certified athletic trainer can also do this first assessment. Start by checking the basics: airway, breathing, and circulation. Once the ABCs have been completed, palpate the head and neck to rule out a head or cervical spine injury. If the player complains of neck pain or you can palpate bony tenderness or step-off over the spinous processes, suspect a possible cervical spine injury. Make sure the player is put onto a spine board with cervical spine precautions and transported to the nearest medical facility.
Look for neurologic deficits
If the cervical spine is cleared, you can do the rest of the assessment in a quiet location either on the sideline or in your office. Your history should include a narrative of how the injury occurred, an estimate of the force involved, the duration of any symptoms, and any previous concussions. The physical examination comes next, and should include a neurologic assessment and a full cognitive evaluation. Reassess frequently after the traumatic event to monitor for any signs of neurologic decline. If any neurologic deficits are found, the patient should be transported to the nearest medical facility for neuroimaging studies to rule out a structural brain injury.
The SCAT2: A convenient assessment tool
Standardized tools now exist to help you evaluate patients with concussion. The Pocket Sport Concussion Assessment Tool (SCAT2) on page 430 has been endorsed by the Zurich conference.5 It is a condensed version of the conference recommendations, suitable for use on the field of play. The SCAT2 includes a symptom scale, mental status tests, instructions on neurologic screening, and guidelines for return to play.
Pocket Sports Concussion Assessment Tool (SCAT2)
Adapted from: Pocket SCAT2. Available at: http://bjsm.bmj.com/content/43/Suppl_1/i89.full.pdf. Accessed July 7, 2010.
No system for grading severity is recommended
Many different classification systems for grading the severity of concussion have been proposed, but none of them is endorsed by the Zurich conference.5,7,12-15 The classification schemes that have been proposed are complex, not evidence-based, and unable to encompass the full range of concussion symptoms. Thus, the 3rd International Conference on Concussion in Sport abandoned all attempts to use or create classification systems, but recommended that each case be treated clinically on the basis of the symptoms displayed and the duration of the impairment.5 Athletes with severe impairment or prolonged symptoms may require referral to a sport medicine specialist with expertise in the management of concussion.
The third conference did agree on a range of “modifying factors” that may influence management and possibly predict the potential for prolonged or persistent symptoms (TABLE). The conference participants endorsed that any athlete displaying these features should be managed in a multidisciplinary manner coordinated by a physician with specific expertise in the management of concussive injuries.
TABLE
"Modifying factors” that may influence concussion management
| Symptoms | How many? |
| How long did symptoms last? (>10 days?) | |
| How severe? | |
| Signs | Loss of consciousness lasting >1 minute, amnesia |
| Sequelae | Concussive convulsions |
| Timing | Repeated concussions, concussions occurring close together in time, or recent concussion |
| Threshold | Repeated concussions with progressively less impact force or slower recovery after each |
| Age | Child or adolescent <18 years |
| Comorbidities | Migraine, depression, other mental health disorders, attention deficit hyperactivity disorder, learning disabilities, sleep disorders |
| Medications | Psychoactive drugs, anticoagulants |
| Behavior | Dangerous style of play |
| Sport in which injury occurred | High-risk, contact, and collision sports, “high sporting level” |
| Source: McCrory P, et al. Br J Sports Med. 2009.5 | |
Return to play is the crucial decision
Just as multiple systems for classifying severity have been proposed, so have guidelines for return to play.13-15 Again, each of the proposed guidelines has been based on expert opinion and no single set of guidelines has ever been proven to be accurate.10 There is, therefore, no universally accepted guide for making the decision of when an athlete can safely return to play. It is universally accepted, however, that no athlete should return to play if he or she is still symptomatic at rest or with any exertional maneuvers.3,7,16 Additionally, the athlete should not be taking any medication that could minimize any of the signs or symptoms of concussion when the physician is determining whether he or she can return to activity.
Once you are assured that the player has no symptoms at rest, you can start him or her on a graded, step-by-step regimen for returning to play. Athletes should spend 24 to 48 hours at each level before progressing to the next. If symptoms return at any point, instruct the athlete to drop back down a step for 24 hours and then proceed with the progression as tolerated.9,10 The stages of activity are: 3,10
- Light aerobic exercise
- Moderate to intense aerobic exercise
- Sport-specific activities/noncontact training drills
- Full contact activities
- Game play.
Neuropsychological testing can help you decide
In 1989, Barth and colleagues evaluated 2300 college football players, 200 of whom had suspected concussion.17 Neuropsychological testing at 24 hours, 5 days, and 10 days showed a decline from baseline following a concussion, with the majority of the athletes returning to baseline by 10 days postconcussion. This finding led researchers to believe that testing could help identify concussions, and several computer-based testing products were developed.11,18
Neuropsychological testing should include measures of concentration, motor dexterity, information processing, visual and verbal memory, executive function, and brain stem function.19 Testing can be performed in the athletic setting with a Web-based computer program, by a sports medicine specialist with an interest in concussion, or by a neuro-psychologist with expertise in concussion.
Improvement in cognitive function as a concussion resolves may come prior to, or follow, the resolution of clinical symptoms. Therefore, it is important to properly assess cognition and symptoms before you make a recommendation about returning to play.3,10 Baseline performance parameters must be established before the season starts.
Neuropsychological testing can provide both an objective measure of the neuro-cognitive effects of concussion and the ability to track recovery. It may also assist in making return-to-play recommendations in complicated cases, but bear in mind that no data are available to suggest that return to play is safe once neuropsychological testing has returned to normal.3,9 Test results can aid clinical decision making, but cannot substitute for it. Testing may be most helpful in athletes with repeated concussions or those with persistent symptoms.10
Educating athletes, parents, and coaches in prevention
No foolproof method exists for preventing concussion in sports. Sports medicine research has focused on designing and testing safer equipment and on devising new rules to make play safer.20-22 At present, there is no evidence that protective equipment will prevent concussions, but recent studies by Collins and Viano suggest that newer football helmets may assist in decreasing the incidence of concussions.20,22,23
The Zurich consensus statement warns that protective equipment can have a paradoxical effect, influencing athletes to take risks that they might otherwise avoid, thus increasing injury rates.5 Trials of rule changes in different sports have been and continue to be conducted, such as barring spearing in football and restricting helmet-to-helmet hits. Given the frequency of concussion, further research is clearly needed. In the meantime, family physicians can play a major role in educating players, parents, and coaches about the seriousness of concussive injury and the need for identifying concussion promptly and allowing adequate time for recovery.
What do you tell Jeff?
Your answer for Jeff is, “You’re not ready to go back to practice or play. You feel OK when you’re resting, but when you get up, your headache returns. Come back to the office in a day or 2, and I’ll re-evaluate you. If you don’t have any symptoms then, you can start a program of graduated activity, beginning with some light aerobic exercise. If you feel all right with that, you can go on to a moderate and then an intense aerobic workout. If you still feel good, you can go on to sports-specific activities with no contact training, and then full contact training.
“At each stage, you will need to be re-evaluated by me or by your team trainer. Once you’ve finished the program without any reactivation of symptoms, I’ll clear you for play.”
CORRESPONDENCE Shawn M. Ferullo, MD, One Boston Medical Center Place, Boston, MA 02118; [email protected]
1. Cantu R. Cerebral concussion in sport: management and prevention. Sports Med. 1992;14:64-74.
2. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreational activities. MMWR Morb Mortal Wkly Rep. 2007;56:733-737.
3. American College of Sports Medicine. Special communications: concussion (mild traumatic brain injury) and the team physician: a consensus statement. Med Sci Sports Exerc. 2006;38:395-399.
4. Van Kampen D, Lovell M, Pardini J, et al. The “value added” of neurocognitive testing after sports-related concussion. Am J Sports Med. 2006;34:1630-1636.
5. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43(suppl I):i76-i84.
6. Powell J. Cerebral concussion: causes, effects and risks in sports. J Athl Training. 2001;36:307-311.
7. Cantu R. Posttraumatic retrograde and anterograde amnesia: pathophysiology and implications in grading and safe return to play. J Athl Training. 2001;36:244-248.
8. Oliaro S, Anderson S, Hooker D. Management of cerebral concussion in sports: the athletic trainer’s perspective. J Athl Training. 2001;36:257-262.
9. Concussion in sport group: Aubry M, Cantu R, Dvorak J, et al. Summary and Agreement Statement of the 1st International Symposium on Concussion in Sport. Vienna 2001. Clin J Sports Med. 2002;12:6-11.
10. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport. Prague 2004. Br J Sports Med. 2005;39:196-204.
11. Hoge C, McGurk D, Thomas J, et al. Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med. 2008;358:453-463.
12. Harmon KG. Assessment and management of concussion in sports. Am Fam Physician. 1999;60:887-892.
13. American Academy of Neurology. Practice parameter: the management of concussion in sport (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997;48:581-585.
14. Cantu R. Guidelines for return to contact sports after cerebral concussion. Phys Sports Med. 1986;14:75-83.
15. Colorado Medical Society School and Sports Medicine Committee. Guidelines for the management of concussion in sports. Colo Med. 1990;87:4.-
16. Guskiewicz KM, Bruce SL, Cantu R, et al. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association Position Statement. Br J Sports Med. 2006;40:6-10.
17. Barth JT, Alves WA, Ryan TV, et al. Mild head injury in sports; neuropsychological sequelae and recovery of function. In: Levin HS, Eisenberg HM, Benton AL, eds. Mild Head Injury. New York: Oxford University Press; 1989:257–275.
18. Randolph C. Implementation of neuropsychological testing models for the high school, collegiate and professional sport settings. J Athl Training. 2001;36:288-296.
19. Maroon J, Lovell M, Norwig J, et al. Cerebral concussion in athletes: evaluation and neuropsychological testing. Neurosurgery. 2000;47:659-672.
20. Collins M, Lovell M, Iverson G, et al. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three year prospective cohort study. Neurosurgery. 2006;58:275-286.
21. Delaney J, Al-Kashmiri A, Drummond R, et al. The effect of protective headgear on head injuries and concussions in adolescent football (soccer) players. Br J Sports Med. 2008;42:110-115.
22. Viano D, Pellman E, Whitnall C, et al. Concussion in professional football: performance of newer helmets in reconstructed game impacts – Part 13. Neurosurgery. 2006;59:591-606.
23. Pellman E, Lovell M, Viano D, et al. Concussion in professional football: recovery of NFL and high school athletes assessed by neuropsychological testing – Part 12. Neurosurgery. 2006;58:236-274.
• Don’t allow an athlete who has symptoms at rest or with exertion to return to play. C
• Consider neuropsychological testing in conjunction with continued clinical assessment for objective measurements to assist in managing concussion. B
• Recommend up-to-date protective equipment for athletes. Recent improvements, especially in football, have been shown to help decrease the incidence of concussion. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE: Jeff, a 15-year-old high school ice hockey player, asks you to write a note for his coach, stating that he has recovered from his concussion and can return to play. He says that 2 days ago he collided with another player and was knocked unconscious for roughly 10 seconds. He had a headache for the rest of that evening, and complained that the light was hurting his eyes. Now he has no symptoms at rest, but activity gives him a slight headache.
How would you evaluate this patient to determine whether he can return to play?
Concussions like Jeff’s are common in sports-related activities, and family physicians are frequently asked to manage the condition and decide when the injured athlete can safely return to play.
Concussions occur in both helmeted and nonhelmeted sports, and are most common in collision sports.1 A 2007 estimate from the Centers for Disease Control and Prevention (CDC) suggests that 1.1 million people are seen in emergency departments in the United States each year for concussion-related injuries, while nearly another 235,000 people are hospitalized.2 As astounding as these numbers are, many experts believe they underestimate the true incidence of concussion, given the propensity for athletes not to report symptoms for fear of being held out of sporting events.3,4
Further complicating matters: There has historically been a lack of agreement over what, exactly, constitutes a concussion and how to manage these injuries.
Refining concussion terminology
Concussion has often been referred to as mild traumatic brain injury (MTBI), although more recent expert opinion suggests the terms refer to different injury constructs and should not be used interchangeably.5 Over the years there has been little agreement on the definition, grading, and treatment of these injuries.6-8 On 3 occasions in the last decade, the sports medicine community has held symposia designed to refine an expert consensus on these issues: in 2001 in Vienna, in 2004 in Prague, and in 2008 in Zurich.5,9,10 These recommendations provide a useful framework for caring for patients like Jeff.
A definition. According to the consensus statement that emerged from the most recent Zurich conference, sports concussion can be defined as a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.” Common features may include:5
- A direct blow to the head or elsewhere in the body with an impulsive force transmitted to the head.
- Rapid onset of neurological impairments that resolve spontaneously over time.
- Possible neuropathological changes, although the clinical symptoms reflect a functional disturbance rather than structural injury.
- A graded set of clinical symptoms that may or may not involve loss of consciousness.
- No abnormality on standard structural neuroimaging studies.
Athletes with concussion show a range of signs and symptoms
Athletes who suffer from a concussion may show signs of being dazed or disoriented, or experience retrograde amnesia (where they can’t remember things that happened before the traumatic event) or anterograde amnesia (where they can’t remember things that happened after the event). They may also suffer from poor coordination, decreased attention span, emotional lability, or loss of consciousness. After the traumatic event, they may complain of headache, dizziness, nausea or vomiting, photophobia, phonophobia, inability to concentrate, sleep disturbances, fatigue, and memory disturbances. Academic performance can also be severely impaired during the postconcussive period.
Symptoms of concussion may be short-lived or persist for many weeks. Postconcussive syndrome is a term used to describe the condition of prolonged and persistent concussive symptoms. Recent studies in military personal have also shown a strong association between post-traumatic stress disorder (PTSD) and clinical depression in soldiers who have suffered from a traumatic brain injury.11
Start with the ABCs, then check the spine
If you are a team physician on the spot when the injury occurs, you can do the initial assessment on the field of play. A certified athletic trainer can also do this first assessment. Start by checking the basics: airway, breathing, and circulation. Once the ABCs have been completed, palpate the head and neck to rule out a head or cervical spine injury. If the player complains of neck pain or you can palpate bony tenderness or step-off over the spinous processes, suspect a possible cervical spine injury. Make sure the player is put onto a spine board with cervical spine precautions and transported to the nearest medical facility.
Look for neurologic deficits
If the cervical spine is cleared, you can do the rest of the assessment in a quiet location either on the sideline or in your office. Your history should include a narrative of how the injury occurred, an estimate of the force involved, the duration of any symptoms, and any previous concussions. The physical examination comes next, and should include a neurologic assessment and a full cognitive evaluation. Reassess frequently after the traumatic event to monitor for any signs of neurologic decline. If any neurologic deficits are found, the patient should be transported to the nearest medical facility for neuroimaging studies to rule out a structural brain injury.
The SCAT2: A convenient assessment tool
Standardized tools now exist to help you evaluate patients with concussion. The Pocket Sport Concussion Assessment Tool (SCAT2) on page 430 has been endorsed by the Zurich conference.5 It is a condensed version of the conference recommendations, suitable for use on the field of play. The SCAT2 includes a symptom scale, mental status tests, instructions on neurologic screening, and guidelines for return to play.
Pocket Sports Concussion Assessment Tool (SCAT2)
Adapted from: Pocket SCAT2. Available at: http://bjsm.bmj.com/content/43/Suppl_1/i89.full.pdf. Accessed July 7, 2010.
No system for grading severity is recommended
Many different classification systems for grading the severity of concussion have been proposed, but none of them is endorsed by the Zurich conference.5,7,12-15 The classification schemes that have been proposed are complex, not evidence-based, and unable to encompass the full range of concussion symptoms. Thus, the 3rd International Conference on Concussion in Sport abandoned all attempts to use or create classification systems, but recommended that each case be treated clinically on the basis of the symptoms displayed and the duration of the impairment.5 Athletes with severe impairment or prolonged symptoms may require referral to a sport medicine specialist with expertise in the management of concussion.
The third conference did agree on a range of “modifying factors” that may influence management and possibly predict the potential for prolonged or persistent symptoms (TABLE). The conference participants endorsed that any athlete displaying these features should be managed in a multidisciplinary manner coordinated by a physician with specific expertise in the management of concussive injuries.
TABLE
"Modifying factors” that may influence concussion management
| Symptoms | How many? |
| How long did symptoms last? (>10 days?) | |
| How severe? | |
| Signs | Loss of consciousness lasting >1 minute, amnesia |
| Sequelae | Concussive convulsions |
| Timing | Repeated concussions, concussions occurring close together in time, or recent concussion |
| Threshold | Repeated concussions with progressively less impact force or slower recovery after each |
| Age | Child or adolescent <18 years |
| Comorbidities | Migraine, depression, other mental health disorders, attention deficit hyperactivity disorder, learning disabilities, sleep disorders |
| Medications | Psychoactive drugs, anticoagulants |
| Behavior | Dangerous style of play |
| Sport in which injury occurred | High-risk, contact, and collision sports, “high sporting level” |
| Source: McCrory P, et al. Br J Sports Med. 2009.5 | |
Return to play is the crucial decision
Just as multiple systems for classifying severity have been proposed, so have guidelines for return to play.13-15 Again, each of the proposed guidelines has been based on expert opinion and no single set of guidelines has ever been proven to be accurate.10 There is, therefore, no universally accepted guide for making the decision of when an athlete can safely return to play. It is universally accepted, however, that no athlete should return to play if he or she is still symptomatic at rest or with any exertional maneuvers.3,7,16 Additionally, the athlete should not be taking any medication that could minimize any of the signs or symptoms of concussion when the physician is determining whether he or she can return to activity.
Once you are assured that the player has no symptoms at rest, you can start him or her on a graded, step-by-step regimen for returning to play. Athletes should spend 24 to 48 hours at each level before progressing to the next. If symptoms return at any point, instruct the athlete to drop back down a step for 24 hours and then proceed with the progression as tolerated.9,10 The stages of activity are: 3,10
- Light aerobic exercise
- Moderate to intense aerobic exercise
- Sport-specific activities/noncontact training drills
- Full contact activities
- Game play.
Neuropsychological testing can help you decide
In 1989, Barth and colleagues evaluated 2300 college football players, 200 of whom had suspected concussion.17 Neuropsychological testing at 24 hours, 5 days, and 10 days showed a decline from baseline following a concussion, with the majority of the athletes returning to baseline by 10 days postconcussion. This finding led researchers to believe that testing could help identify concussions, and several computer-based testing products were developed.11,18
Neuropsychological testing should include measures of concentration, motor dexterity, information processing, visual and verbal memory, executive function, and brain stem function.19 Testing can be performed in the athletic setting with a Web-based computer program, by a sports medicine specialist with an interest in concussion, or by a neuro-psychologist with expertise in concussion.
Improvement in cognitive function as a concussion resolves may come prior to, or follow, the resolution of clinical symptoms. Therefore, it is important to properly assess cognition and symptoms before you make a recommendation about returning to play.3,10 Baseline performance parameters must be established before the season starts.
Neuropsychological testing can provide both an objective measure of the neuro-cognitive effects of concussion and the ability to track recovery. It may also assist in making return-to-play recommendations in complicated cases, but bear in mind that no data are available to suggest that return to play is safe once neuropsychological testing has returned to normal.3,9 Test results can aid clinical decision making, but cannot substitute for it. Testing may be most helpful in athletes with repeated concussions or those with persistent symptoms.10
Educating athletes, parents, and coaches in prevention
No foolproof method exists for preventing concussion in sports. Sports medicine research has focused on designing and testing safer equipment and on devising new rules to make play safer.20-22 At present, there is no evidence that protective equipment will prevent concussions, but recent studies by Collins and Viano suggest that newer football helmets may assist in decreasing the incidence of concussions.20,22,23
The Zurich consensus statement warns that protective equipment can have a paradoxical effect, influencing athletes to take risks that they might otherwise avoid, thus increasing injury rates.5 Trials of rule changes in different sports have been and continue to be conducted, such as barring spearing in football and restricting helmet-to-helmet hits. Given the frequency of concussion, further research is clearly needed. In the meantime, family physicians can play a major role in educating players, parents, and coaches about the seriousness of concussive injury and the need for identifying concussion promptly and allowing adequate time for recovery.
What do you tell Jeff?
Your answer for Jeff is, “You’re not ready to go back to practice or play. You feel OK when you’re resting, but when you get up, your headache returns. Come back to the office in a day or 2, and I’ll re-evaluate you. If you don’t have any symptoms then, you can start a program of graduated activity, beginning with some light aerobic exercise. If you feel all right with that, you can go on to a moderate and then an intense aerobic workout. If you still feel good, you can go on to sports-specific activities with no contact training, and then full contact training.
“At each stage, you will need to be re-evaluated by me or by your team trainer. Once you’ve finished the program without any reactivation of symptoms, I’ll clear you for play.”
CORRESPONDENCE Shawn M. Ferullo, MD, One Boston Medical Center Place, Boston, MA 02118; [email protected]
• Don’t allow an athlete who has symptoms at rest or with exertion to return to play. C
• Consider neuropsychological testing in conjunction with continued clinical assessment for objective measurements to assist in managing concussion. B
• Recommend up-to-date protective equipment for athletes. Recent improvements, especially in football, have been shown to help decrease the incidence of concussion. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE: Jeff, a 15-year-old high school ice hockey player, asks you to write a note for his coach, stating that he has recovered from his concussion and can return to play. He says that 2 days ago he collided with another player and was knocked unconscious for roughly 10 seconds. He had a headache for the rest of that evening, and complained that the light was hurting his eyes. Now he has no symptoms at rest, but activity gives him a slight headache.
How would you evaluate this patient to determine whether he can return to play?
Concussions like Jeff’s are common in sports-related activities, and family physicians are frequently asked to manage the condition and decide when the injured athlete can safely return to play.
Concussions occur in both helmeted and nonhelmeted sports, and are most common in collision sports.1 A 2007 estimate from the Centers for Disease Control and Prevention (CDC) suggests that 1.1 million people are seen in emergency departments in the United States each year for concussion-related injuries, while nearly another 235,000 people are hospitalized.2 As astounding as these numbers are, many experts believe they underestimate the true incidence of concussion, given the propensity for athletes not to report symptoms for fear of being held out of sporting events.3,4
Further complicating matters: There has historically been a lack of agreement over what, exactly, constitutes a concussion and how to manage these injuries.
Refining concussion terminology
Concussion has often been referred to as mild traumatic brain injury (MTBI), although more recent expert opinion suggests the terms refer to different injury constructs and should not be used interchangeably.5 Over the years there has been little agreement on the definition, grading, and treatment of these injuries.6-8 On 3 occasions in the last decade, the sports medicine community has held symposia designed to refine an expert consensus on these issues: in 2001 in Vienna, in 2004 in Prague, and in 2008 in Zurich.5,9,10 These recommendations provide a useful framework for caring for patients like Jeff.
A definition. According to the consensus statement that emerged from the most recent Zurich conference, sports concussion can be defined as a “complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces.” Common features may include:5
- A direct blow to the head or elsewhere in the body with an impulsive force transmitted to the head.
- Rapid onset of neurological impairments that resolve spontaneously over time.
- Possible neuropathological changes, although the clinical symptoms reflect a functional disturbance rather than structural injury.
- A graded set of clinical symptoms that may or may not involve loss of consciousness.
- No abnormality on standard structural neuroimaging studies.
Athletes with concussion show a range of signs and symptoms
Athletes who suffer from a concussion may show signs of being dazed or disoriented, or experience retrograde amnesia (where they can’t remember things that happened before the traumatic event) or anterograde amnesia (where they can’t remember things that happened after the event). They may also suffer from poor coordination, decreased attention span, emotional lability, or loss of consciousness. After the traumatic event, they may complain of headache, dizziness, nausea or vomiting, photophobia, phonophobia, inability to concentrate, sleep disturbances, fatigue, and memory disturbances. Academic performance can also be severely impaired during the postconcussive period.
Symptoms of concussion may be short-lived or persist for many weeks. Postconcussive syndrome is a term used to describe the condition of prolonged and persistent concussive symptoms. Recent studies in military personal have also shown a strong association between post-traumatic stress disorder (PTSD) and clinical depression in soldiers who have suffered from a traumatic brain injury.11
Start with the ABCs, then check the spine
If you are a team physician on the spot when the injury occurs, you can do the initial assessment on the field of play. A certified athletic trainer can also do this first assessment. Start by checking the basics: airway, breathing, and circulation. Once the ABCs have been completed, palpate the head and neck to rule out a head or cervical spine injury. If the player complains of neck pain or you can palpate bony tenderness or step-off over the spinous processes, suspect a possible cervical spine injury. Make sure the player is put onto a spine board with cervical spine precautions and transported to the nearest medical facility.
Look for neurologic deficits
If the cervical spine is cleared, you can do the rest of the assessment in a quiet location either on the sideline or in your office. Your history should include a narrative of how the injury occurred, an estimate of the force involved, the duration of any symptoms, and any previous concussions. The physical examination comes next, and should include a neurologic assessment and a full cognitive evaluation. Reassess frequently after the traumatic event to monitor for any signs of neurologic decline. If any neurologic deficits are found, the patient should be transported to the nearest medical facility for neuroimaging studies to rule out a structural brain injury.
The SCAT2: A convenient assessment tool
Standardized tools now exist to help you evaluate patients with concussion. The Pocket Sport Concussion Assessment Tool (SCAT2) on page 430 has been endorsed by the Zurich conference.5 It is a condensed version of the conference recommendations, suitable for use on the field of play. The SCAT2 includes a symptom scale, mental status tests, instructions on neurologic screening, and guidelines for return to play.
Pocket Sports Concussion Assessment Tool (SCAT2)
Adapted from: Pocket SCAT2. Available at: http://bjsm.bmj.com/content/43/Suppl_1/i89.full.pdf. Accessed July 7, 2010.
No system for grading severity is recommended
Many different classification systems for grading the severity of concussion have been proposed, but none of them is endorsed by the Zurich conference.5,7,12-15 The classification schemes that have been proposed are complex, not evidence-based, and unable to encompass the full range of concussion symptoms. Thus, the 3rd International Conference on Concussion in Sport abandoned all attempts to use or create classification systems, but recommended that each case be treated clinically on the basis of the symptoms displayed and the duration of the impairment.5 Athletes with severe impairment or prolonged symptoms may require referral to a sport medicine specialist with expertise in the management of concussion.
The third conference did agree on a range of “modifying factors” that may influence management and possibly predict the potential for prolonged or persistent symptoms (TABLE). The conference participants endorsed that any athlete displaying these features should be managed in a multidisciplinary manner coordinated by a physician with specific expertise in the management of concussive injuries.
TABLE
"Modifying factors” that may influence concussion management
| Symptoms | How many? |
| How long did symptoms last? (>10 days?) | |
| How severe? | |
| Signs | Loss of consciousness lasting >1 minute, amnesia |
| Sequelae | Concussive convulsions |
| Timing | Repeated concussions, concussions occurring close together in time, or recent concussion |
| Threshold | Repeated concussions with progressively less impact force or slower recovery after each |
| Age | Child or adolescent <18 years |
| Comorbidities | Migraine, depression, other mental health disorders, attention deficit hyperactivity disorder, learning disabilities, sleep disorders |
| Medications | Psychoactive drugs, anticoagulants |
| Behavior | Dangerous style of play |
| Sport in which injury occurred | High-risk, contact, and collision sports, “high sporting level” |
| Source: McCrory P, et al. Br J Sports Med. 2009.5 | |
Return to play is the crucial decision
Just as multiple systems for classifying severity have been proposed, so have guidelines for return to play.13-15 Again, each of the proposed guidelines has been based on expert opinion and no single set of guidelines has ever been proven to be accurate.10 There is, therefore, no universally accepted guide for making the decision of when an athlete can safely return to play. It is universally accepted, however, that no athlete should return to play if he or she is still symptomatic at rest or with any exertional maneuvers.3,7,16 Additionally, the athlete should not be taking any medication that could minimize any of the signs or symptoms of concussion when the physician is determining whether he or she can return to activity.
Once you are assured that the player has no symptoms at rest, you can start him or her on a graded, step-by-step regimen for returning to play. Athletes should spend 24 to 48 hours at each level before progressing to the next. If symptoms return at any point, instruct the athlete to drop back down a step for 24 hours and then proceed with the progression as tolerated.9,10 The stages of activity are: 3,10
- Light aerobic exercise
- Moderate to intense aerobic exercise
- Sport-specific activities/noncontact training drills
- Full contact activities
- Game play.
Neuropsychological testing can help you decide
In 1989, Barth and colleagues evaluated 2300 college football players, 200 of whom had suspected concussion.17 Neuropsychological testing at 24 hours, 5 days, and 10 days showed a decline from baseline following a concussion, with the majority of the athletes returning to baseline by 10 days postconcussion. This finding led researchers to believe that testing could help identify concussions, and several computer-based testing products were developed.11,18
Neuropsychological testing should include measures of concentration, motor dexterity, information processing, visual and verbal memory, executive function, and brain stem function.19 Testing can be performed in the athletic setting with a Web-based computer program, by a sports medicine specialist with an interest in concussion, or by a neuro-psychologist with expertise in concussion.
Improvement in cognitive function as a concussion resolves may come prior to, or follow, the resolution of clinical symptoms. Therefore, it is important to properly assess cognition and symptoms before you make a recommendation about returning to play.3,10 Baseline performance parameters must be established before the season starts.
Neuropsychological testing can provide both an objective measure of the neuro-cognitive effects of concussion and the ability to track recovery. It may also assist in making return-to-play recommendations in complicated cases, but bear in mind that no data are available to suggest that return to play is safe once neuropsychological testing has returned to normal.3,9 Test results can aid clinical decision making, but cannot substitute for it. Testing may be most helpful in athletes with repeated concussions or those with persistent symptoms.10
Educating athletes, parents, and coaches in prevention
No foolproof method exists for preventing concussion in sports. Sports medicine research has focused on designing and testing safer equipment and on devising new rules to make play safer.20-22 At present, there is no evidence that protective equipment will prevent concussions, but recent studies by Collins and Viano suggest that newer football helmets may assist in decreasing the incidence of concussions.20,22,23
The Zurich consensus statement warns that protective equipment can have a paradoxical effect, influencing athletes to take risks that they might otherwise avoid, thus increasing injury rates.5 Trials of rule changes in different sports have been and continue to be conducted, such as barring spearing in football and restricting helmet-to-helmet hits. Given the frequency of concussion, further research is clearly needed. In the meantime, family physicians can play a major role in educating players, parents, and coaches about the seriousness of concussive injury and the need for identifying concussion promptly and allowing adequate time for recovery.
What do you tell Jeff?
Your answer for Jeff is, “You’re not ready to go back to practice or play. You feel OK when you’re resting, but when you get up, your headache returns. Come back to the office in a day or 2, and I’ll re-evaluate you. If you don’t have any symptoms then, you can start a program of graduated activity, beginning with some light aerobic exercise. If you feel all right with that, you can go on to a moderate and then an intense aerobic workout. If you still feel good, you can go on to sports-specific activities with no contact training, and then full contact training.
“At each stage, you will need to be re-evaluated by me or by your team trainer. Once you’ve finished the program without any reactivation of symptoms, I’ll clear you for play.”
CORRESPONDENCE Shawn M. Ferullo, MD, One Boston Medical Center Place, Boston, MA 02118; [email protected]
1. Cantu R. Cerebral concussion in sport: management and prevention. Sports Med. 1992;14:64-74.
2. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreational activities. MMWR Morb Mortal Wkly Rep. 2007;56:733-737.
3. American College of Sports Medicine. Special communications: concussion (mild traumatic brain injury) and the team physician: a consensus statement. Med Sci Sports Exerc. 2006;38:395-399.
4. Van Kampen D, Lovell M, Pardini J, et al. The “value added” of neurocognitive testing after sports-related concussion. Am J Sports Med. 2006;34:1630-1636.
5. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43(suppl I):i76-i84.
6. Powell J. Cerebral concussion: causes, effects and risks in sports. J Athl Training. 2001;36:307-311.
7. Cantu R. Posttraumatic retrograde and anterograde amnesia: pathophysiology and implications in grading and safe return to play. J Athl Training. 2001;36:244-248.
8. Oliaro S, Anderson S, Hooker D. Management of cerebral concussion in sports: the athletic trainer’s perspective. J Athl Training. 2001;36:257-262.
9. Concussion in sport group: Aubry M, Cantu R, Dvorak J, et al. Summary and Agreement Statement of the 1st International Symposium on Concussion in Sport. Vienna 2001. Clin J Sports Med. 2002;12:6-11.
10. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport. Prague 2004. Br J Sports Med. 2005;39:196-204.
11. Hoge C, McGurk D, Thomas J, et al. Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med. 2008;358:453-463.
12. Harmon KG. Assessment and management of concussion in sports. Am Fam Physician. 1999;60:887-892.
13. American Academy of Neurology. Practice parameter: the management of concussion in sport (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997;48:581-585.
14. Cantu R. Guidelines for return to contact sports after cerebral concussion. Phys Sports Med. 1986;14:75-83.
15. Colorado Medical Society School and Sports Medicine Committee. Guidelines for the management of concussion in sports. Colo Med. 1990;87:4.-
16. Guskiewicz KM, Bruce SL, Cantu R, et al. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association Position Statement. Br J Sports Med. 2006;40:6-10.
17. Barth JT, Alves WA, Ryan TV, et al. Mild head injury in sports; neuropsychological sequelae and recovery of function. In: Levin HS, Eisenberg HM, Benton AL, eds. Mild Head Injury. New York: Oxford University Press; 1989:257–275.
18. Randolph C. Implementation of neuropsychological testing models for the high school, collegiate and professional sport settings. J Athl Training. 2001;36:288-296.
19. Maroon J, Lovell M, Norwig J, et al. Cerebral concussion in athletes: evaluation and neuropsychological testing. Neurosurgery. 2000;47:659-672.
20. Collins M, Lovell M, Iverson G, et al. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three year prospective cohort study. Neurosurgery. 2006;58:275-286.
21. Delaney J, Al-Kashmiri A, Drummond R, et al. The effect of protective headgear on head injuries and concussions in adolescent football (soccer) players. Br J Sports Med. 2008;42:110-115.
22. Viano D, Pellman E, Whitnall C, et al. Concussion in professional football: performance of newer helmets in reconstructed game impacts – Part 13. Neurosurgery. 2006;59:591-606.
23. Pellman E, Lovell M, Viano D, et al. Concussion in professional football: recovery of NFL and high school athletes assessed by neuropsychological testing – Part 12. Neurosurgery. 2006;58:236-274.
1. Cantu R. Cerebral concussion in sport: management and prevention. Sports Med. 1992;14:64-74.
2. Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries from sports and recreational activities. MMWR Morb Mortal Wkly Rep. 2007;56:733-737.
3. American College of Sports Medicine. Special communications: concussion (mild traumatic brain injury) and the team physician: a consensus statement. Med Sci Sports Exerc. 2006;38:395-399.
4. Van Kampen D, Lovell M, Pardini J, et al. The “value added” of neurocognitive testing after sports-related concussion. Am J Sports Med. 2006;34:1630-1636.
5. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43(suppl I):i76-i84.
6. Powell J. Cerebral concussion: causes, effects and risks in sports. J Athl Training. 2001;36:307-311.
7. Cantu R. Posttraumatic retrograde and anterograde amnesia: pathophysiology and implications in grading and safe return to play. J Athl Training. 2001;36:244-248.
8. Oliaro S, Anderson S, Hooker D. Management of cerebral concussion in sports: the athletic trainer’s perspective. J Athl Training. 2001;36:257-262.
9. Concussion in sport group: Aubry M, Cantu R, Dvorak J, et al. Summary and Agreement Statement of the 1st International Symposium on Concussion in Sport. Vienna 2001. Clin J Sports Med. 2002;12:6-11.
10. McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport. Prague 2004. Br J Sports Med. 2005;39:196-204.
11. Hoge C, McGurk D, Thomas J, et al. Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med. 2008;358:453-463.
12. Harmon KG. Assessment and management of concussion in sports. Am Fam Physician. 1999;60:887-892.
13. American Academy of Neurology. Practice parameter: the management of concussion in sport (summary statement). Report of the Quality Standards Subcommittee. Neurology. 1997;48:581-585.
14. Cantu R. Guidelines for return to contact sports after cerebral concussion. Phys Sports Med. 1986;14:75-83.
15. Colorado Medical Society School and Sports Medicine Committee. Guidelines for the management of concussion in sports. Colo Med. 1990;87:4.-
16. Guskiewicz KM, Bruce SL, Cantu R, et al. Research based recommendations on management of sport related concussion: summary of the National Athletic Trainers’ Association Position Statement. Br J Sports Med. 2006;40:6-10.
17. Barth JT, Alves WA, Ryan TV, et al. Mild head injury in sports; neuropsychological sequelae and recovery of function. In: Levin HS, Eisenberg HM, Benton AL, eds. Mild Head Injury. New York: Oxford University Press; 1989:257–275.
18. Randolph C. Implementation of neuropsychological testing models for the high school, collegiate and professional sport settings. J Athl Training. 2001;36:288-296.
19. Maroon J, Lovell M, Norwig J, et al. Cerebral concussion in athletes: evaluation and neuropsychological testing. Neurosurgery. 2000;47:659-672.
20. Collins M, Lovell M, Iverson G, et al. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three year prospective cohort study. Neurosurgery. 2006;58:275-286.
21. Delaney J, Al-Kashmiri A, Drummond R, et al. The effect of protective headgear on head injuries and concussions in adolescent football (soccer) players. Br J Sports Med. 2008;42:110-115.
22. Viano D, Pellman E, Whitnall C, et al. Concussion in professional football: performance of newer helmets in reconstructed game impacts – Part 13. Neurosurgery. 2006;59:591-606.
23. Pellman E, Lovell M, Viano D, et al. Concussion in professional football: recovery of NFL and high school athletes assessed by neuropsychological testing – Part 12. Neurosurgery. 2006;58:236-274.
A triage guide for tinnitus
• Let patients know that they can learn to manage their reactions to tinnitus with methods that include stress reduction, therapeutic sound, and coping skills. A
• Refer patients with tinnitus to an audiologist for a hearing evaluation, assessment of the tinnitus, and, if indicated, support in learning to manage reactions to tinnitus. A
• Give patients with suicidal ideation or extreme anxiety or depression in response to tinnitus a same-day referral to a mental health professional. A
• Provide an urgent referral to an otolaryngologist or emergency care if you suspect sudden sensorineural hearing loss or another urgent medical condition. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, I have this ringing in my ears.”
With an estimated 10% to 15% of adults experiencing chronic tinnitus,1 most primary care physicians are familiar with this complaint. The prevalence of tinnitus increases with age and with exposure to high levels of noise—the most commonly reported cause.1 With people living longer and such “toxic” noise levels on the rise, tinnitus is a condition you can expect to encounter even more frequently.
Despite the prevalence of tinnitus, however, there are no clinical standards or best practice guidelines for managing it. Thus, many physicians are uncertain about what to tell patients with this distressing disorder, and when (or whether) to refer them to specialists. So patients are sometimes told that “nothing can be done” and that they simply must “learn to live with” tinnitus.
Such negative messages from a trusted physician can have a detrimental effect, causing some patients to stop seeking help and to become increasingly disturbed by tinnitus.2 What’s more, these messages are untrue. Some conditions that result in tinnitus can be treated. And, although tinnitus itself cannot normally be cured, there are numerous interventions and educational strategies that can help patients change their reactions to—and learn to cope with—the ringing in their ears. We developed this evidence-based review and tinnitus triage guide (TABLE 1) to help family physicians respond appropriately to this distressing, but common, condition.
TABLE 1
Tinnitus triage guide27
| If the patient | Refer to | Status/considerations |
|---|---|---|
| Has neural deficits such as facial weakness, head trauma, or other urgent medical condition | Otolaryngology or ED | Emergency |
| Has unexplained sudden hearing loss | Audiology and otolaryngology | Emergency; must see audiologist prior to otolaryngologist on same day |
| Expresses suicidal ideation or manifests obvious mental illness | Mental health or ED | May be emergency; report suicide ideation; provide escort, if necessary |
Has any of the following:
| Otolaryngology and audiology | Urgent; schedule otolaryngology exam as soon as possible |
Has symptoms that suggest a neurophysiologic origin of tinnitus without:
| Audiology and otolaryngology | Nonurgent; schedule audiology exam before patient sees otolaryngologist |
| ED, emergency department. | ||
Is it transient noise, or tinnitus?
Virtually everyone experiences “transient ear noise,” which is usually described as a whistling sound accompanied by a sensation of sudden temporary hearing loss.3,4 These idiopathic episodes are usually unilateral, and often accompanied by a feeling of ear blockage.
To distinguish between tinnitus—the perception of sound that is produced internally, rather than by an external stimulus—and transient ear noise, consider the duration and frequency. Transient ear noise generally disappears within seconds (and does not require diagnostic testing or treatment). Tinnitus, which can have a variety of underlying pathologies, is defined as ear or head noise that lasts at least 5 minutes and occurs at least twice a week.5
Neurophysiologic tinnitus is most common
Neurophysiologic (sensorineural) tinnitus, which originates within the auditory nervous system, accounts for the vast majority of cases. The pathology exists anywhere between the cochlea and the auditory cortex, and excludes any sounds generated by mechanical (somatic) processes.6
The ringing may be relatively soft; in some cases, it can be heard only in quiet environments or while the patient is trying to sleep. In others, the tinnitus may be constant, interfering with concentration and daily activities, as well as sleep. In the most severe cases, tinnitus may be associated with severe depression and anxiety, even to the point of suicidal ideation.7
Notably, however, the loudness or other perceptual characteristics of tinnitus do not necessarily indicate the degree to which it is a problem for the patient.7 Although patients often report that tinnitus interferes with their hearing, they usually also have hearing loss, which an audiologic evaluation will reveal.7-9
Certain medications can trigger or exacerbate tinnitus, including aspirin, nonsteroidal anti-inflammatory drugs, loop diuretics, and quinine.2 Fairly high doses are usually required to cause tinnitus, however, and the effects are typically temporary. Patients have also reported exacerbation of tinnitus due to alcohol, salt, and caffeine intake. Ototoxicity from aminoglycosides and platinum-containing chemotherapeutic drugs is a well-known cause of hearing loss and tinnitus, but these effects are often irreversible.10,11
Neurophysiologic tinnitus is generally not serious from a medical standpoint. While all patients with this condition should undergo an audiologic exam and hearing evaluation, only about 20% of adults who experience tinnitus require intervention.12-14 Although there is no cure, patients with clinically significant tinnitus can be taught stress management and therapeutic use of sound techniques, as well as lifestyle modifications (TABLE 2) to minimize its detrimental effects.
TABLE 2
Managing neurophysiologic tinnitus: A range of options2,5,25-27
| Cognitive-behavioral therapy |
| Elimination of tinnitus-inducing medications (eg, NSAIDs, loop diuretics, and quinine) |
| Hearing aids, sound generators, or other sound devices |
| Lifestyle modifications (eg, improve sleep hygiene, exercise regularly, limit salt intake) |
| Medication (antidepressants or anxiolytics) |
| Patient education that stresses that there are numerous techniques that can be used to manage reactions to tinnitus |
| Stress reduction techniques (eg, imagery, meditation, and deep breathing techniques) |
| Therapeutic sound (eg, using interesting sound to direct attention away from tinnitus, low-level background sound to reduce auditory contrast, and soothing sound for relief) |
| NSAIDs, nonsteroidal anti-inflammatory drugs. |
Somatic tinnitus may be serious
Somatic tinnitus, also known as somato-sound, refers to the perception of sound that originates within the body—in vascular, muscular, skeletal, or respiratory structures, or in the temporomandibular joint.4 These “body sounds” have an internal acoustic source.9
Pulsatile tinnitus, which pulses in synchrony with the heartbeat, is the most common somatosound.15,16 Most patients with pulsatile tinnitus have benign venous “hums,” but serious conditions such as arteriovenous malformations, glomus tumors, and carotid stenosis must be considered. Auscultation over the neck and temporal bone may reveal bruits that can help localize the lesion. We recommend either magnetic resonance imaging (MRI) of the head or computed tomography (CT) angiography, accompanied by timely referral to an otolaryngologist for a focused evaluation.15,17,18
Somatosounds can also be nonpulsatile, indicating a nonvascular source. Examples of nonvascular somatosounds include middle-ear muscle spasms and eustachian tube dysfunction. Nonpulsatile somatic tinnitus is rarely progressive or dangerous. It is reasonable to offer reassurance to patients with nonpulsatile tinnitus, followed by a referral to an otolaryngologist if the symptoms interfere with daily activities.
Unilateral tinnitus is a red flag
In most cases, tinnitus is bilateral. Unilateral tinnitus may indicate a more serious medical condition. It is a common presenting sign of both vestibular schwannoma (also known as acoustic neuroma) and Meniere’s disease.
Patients with unilateral tinnitus should receive a hearing test as soon as possible; if asymmetric hearing loss is found, MRI is indicated, both with and without contrast of the internal auditory canal, to rule out vestibular schwannoma.
Idiopathic sudden sensorineural hearing loss (ISSNHL), which may be associated with new onset unilateral tinnitus, should be considered an otologic emergency. When you suspect ISSNHL, you’ll need to make a same-day referral for an otologic examination.
If left untreated, the ISSNHL and associated tinnitus will resolve partially or completely at least 50% of the time. This recovery rate may be improved with glucocorticoid treatments.19 Prompt initiation of corticosteroid therapy can be a factor in the chances of recovery—the more rapidly such patients are seen and treated, the better their prognosis.20
Tinnitus triage: Key points
Following our triage guide (TABLE 1) should result in appropriate care in most cases. Here are some considerations to keep in mind:
Urgent medical referral. Any patient with tinnitus and symptoms suggestive of serious underlying treatable pathology requires an urgent otolaryngology referral. That includes ISSNHL, which you should suspect whenever a patient reports an unexplained decrease in hearing, as well as pulsatile tinnitus, vestibular symptoms, and long-standing ear pain, drainage, or malodor that does not resolve with routine treatment. If possible, such patients should undergo an audiologic assessment prior to the otolaryngology visit; however, the otolaryngology exam is the primary concern.
Facial paralysis, severe vertigo, or sudden onset pulsatile tinnitus can indicate a serious intracranial condition. These symptoms may point to cerebrovascular disease or neoplasm, and should be treated as an otologic emergency.
Mental health referral. Some tinnitus patients require a mental health assessment, either because of obvious manifestations of a mental illness or because of expressed suicidal ideation. If there’s a question about the patient’s mental health, consider consulting with a mental health provider or using basic screening tools for anxiety and depression to help determine the need for referral, as well as the urgency.12
Some patients experience extreme anxiety or depression in response to tinnitus and should be referred to a mental health professional on the day they present with symptoms. Suicidal ideation warrants special attention, of course—possibly including the need to escort the patient to the emergency department or to a behavioral specialist.21-23
Nonurgent medical referral. Ideally, all patients who present with tinnitus should see an audiologist and an otolaryngologist, but those who have no serious symptoms should be referred on a nonurgent basis. Such patients need to have a comprehensive hearing evaluation—ideally, before they see the otolaryngologist so the test results are available at the time of the exam. The audiologist should also assess the severity of the tinnitus, using a validated questionnaire such as the Tinnitus Handicap Inventory, for the initial assessment and to monitor changes in the severity of the tinnitus as an outcome measure of therapy.24
Enlist an interdisciplinary team
For patients with somatic tinnitus, the treatment—and the specialist who provides it—depends on the underlying cause. A patient who has unilateral tinnitus may be referred by an audiologist or otolaryngologist to a neurologist, for example, if he or she is found to have Meniere’s disease; a patient with pulsatile tinnitus may be sent back to his or her primary care physician after diagnostic testing has ruled out serious causes.
For patients with neurophysiologic tinnitus (and any patient with untreatable somatic tinnitus), a well-organized interdisciplinary team that includes the family physician, an audiologist, and a psychologist is the best approach. The variety of available management options (TABLE 2) incorporate medical approaches, complementary and alternative treatments, psychological interventions, and sound-based methods. Lifestyle modifications, such as improved sleep hygiene, regular exercise, and dietary modifications, may help, as well.25-27 First-line strategies include:
Adjusting medications. Eliminating tinnitus-inducing medications, if medically safe, is a common starting point. No prescription drug has been developed specifically for tinnitus. But some antidepressants or anxiolytics (eg, amitriptyline or lorazepam) are commonly used to address coexisting sleep and mental health disorders—primarily depression and anxiety—that may be associated with, or exacerbated by, tinnitus.28-30
Addressing hearing problems. Patients should undergo a hearing evaluation and receive help in managing a hearing problem, if necessary. Hearing aids improve hearing and reduce the perception of tinnitus.31
Using therapeutic sound. Some audiologists are trained to implement various forms of sound-based therapy. Tinnitus retraining therapy involves the use of background sound to facilitate habituation to tinnitus; tinnitus masking involves the use of soothing sound to provide a sense of relief. Progressive tinnitus management is a more recent method that educates patients in the use of all types of therapeutic sound.32 These sound-based methods often include the use of hearing aids, sound generators, and other devices.
Circling in a mental health professional. It is essential to involve psychologists or other mental health specialists in the care of patients with clinically significant tinnitus to ensure that psychological and other barriers to successful management of the condition are identified and addressed. Cognitive-behavioral therapy (CBT) has been shown to be helpful for patients with tinnitus.33 In fact, we have been successful in teaching patients to manage their reactions to tinnitus—resulting in a better quality of life—using a combination of educational counseling, therapeutic sound, and CBT. JFP
Acknowledgments
Funding for this work was provided by Veterans Health Administration, and Veterans Affairs Rehabilitation Research and Development (RR&D) Service (C4488R). Thanks to Robert Folmer, PhD, William Martin, PhD, Dennis Trune, PhD, and Baker Shi, MD, PhD, for advice that contributed to this manuscript. Special thanks to Martin Schechter, PhD, for his significant contributions to our research. The authors also wish to thank Stephen Fausti, PhD, and Sara Ruth Oliver, AuD, for their consistent support of our research.
CORRESPONDENCE James A. Henry, PhD, VA Medical Center (NCRAR), Post Office Box 1034, Portland, OR 97207; [email protected]
1. Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:16-41.
2. Jastreboff PJ, Hazell JWP. Tinnitus Retraining Therapy: Implementing the Neurophysiological Model. New York: Cambridge University Press; 2004.
3. Kiang NYS, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Wolstenholme GEW, Knight J, eds. Sensorineural Hearing Loss. London: J. & A. Churchill; 1970:241-273.
4. Henry JA, Dennis K, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005;48:1204-1235.
5. Dauman R, Tyler RS. Some considerations on the classification of tinnitus. In: Aran J-M, Dauman R, eds. Proceedings of the Fourth International Tinnitus Seminar. Amsterdam/New York: Kugler Publications; 1992:225-229.
6. Hazell J. Incidence, classification, and models of tinnitus. In: Ludman H, Wright T, eds. Diseases of the Ear. London: Arnold; 1998:185-195.
7. Dobie RA. Overview: suffering from tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:1-7.
8. Zaugg TL, et al. Difficulties caused by patients’ misconceptions that hearing problems are due to tinnitus. In: Patuzzi R, ed. Proceedings of the Seventh International Tinnitus Seminar. Perth: University of Western Australia; 2002:226-228.
9. Coles RRA. Classification of causes, mechanisms of patient disturbance, and associated counseling. In: Vernon JA, Moller AR, eds. Mechanisms of Tinnitus. Needham Heights, Mass: Allyn & Bacon; 1995:11-19.
10. Fausti SA, et al. Ototoxicity. In: Northern JL, ed. Hearing Disorders. Needham Heights, Mass: Allyn & Bacon; 1995:149-164.
11. Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear Res. 2002;164:206-214.
12. Henry JA, Zaugg TL, Myers PJ, et al. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12:170-187.
13. Jastreboff PJ, Hazell JWP. Treatment of tinnitus based on a neurophysiological model. In: Vernon JA, ed. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998:201-217.
14. Davis A, Refaie AE. Epidemiology of tinnitus. In: Tyler R, ed. Tinnitus Handbook. San Diego: Singular Publishing Group; 2000:1-23.
15. Lockwood AH, Burkard RF, Salvi RJ. Imaging tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:253-264.
16. Sismanis A. Pulsatile tinnitus. Otolaryngol Clin North Am. 2003;36:389-402.
17. Sismanis A. Pulsatile tinnitus. In: Vernon JA, ed. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998:28-33.
18. Wackym PA, Friedland DR. Otologic evaluation. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:205-219.
19. Hamid M, Trune D. Issues, indications, and controversies regarding intratympanic steroid perfusion. Curr Opin Otolaryngol Head Neck Surg. 2008;16:434-440.
20. Jeyakumar A, et al. Treatment of idiopathic sudden sensorineural hearing loss. Acta Otolaryngol. 2006;126:708-713.
21. Brown GK, et al. Suicide intent and accurate expectations of lethality: predictors of medical lethality of suicide attempts. J Consult Clin Psychol. 2004;72:1170-1174.
22. Hawton K. Studying survivors of nearly lethal suicide attempts: an important strategy in suicide research. Suicide Life Threat Behav. 2001;32(1 suppl):76-84.
23. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617-626.
24. Newman CW, Sandridge SA, Jacobson GP. Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J Am Acad Audiol. 1998;9:153-160.
25. Tyler RS, ed. Tinnitus Treatment: Clinical Protocols. New York: Thieme Medical Publishers, Inc; 2005.
26. Vernon JA. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998.
27. Henry JA, Zaugg TL, Myers PM, et al. Progressive Tinnitus Management: Clinical Handbook for Audiologists. San Diego, Calif: Plural Publishing; 2010.
28. Robinson SK, Viirre ES, Stein MB. Antidepressant therapy for tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:278-293.
29. Dobie RA. Clinical trials and drug therapy for tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:266-277.
30. Henry JA, Zaugg TL, Schechter MA. Clinical guide for audiologic tinnitus management I: assessment. Am J Audiol. 2005;14:21-48.
31. Surr RK, Montgomery AA, Mueller HG. Effect of amplification on tinnitus among new hearing aid users. Ear Hear. 1985;6:71-75.
32. Henry JA, et al. Using therapeutic sound with progressive audiologic tinnitus management. Trends Amplif. 2008;12:185-206.
33. Martinez Devesa P, Waddell A, Perera R, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2007;(1):CD005233.-
• Let patients know that they can learn to manage their reactions to tinnitus with methods that include stress reduction, therapeutic sound, and coping skills. A
• Refer patients with tinnitus to an audiologist for a hearing evaluation, assessment of the tinnitus, and, if indicated, support in learning to manage reactions to tinnitus. A
• Give patients with suicidal ideation or extreme anxiety or depression in response to tinnitus a same-day referral to a mental health professional. A
• Provide an urgent referral to an otolaryngologist or emergency care if you suspect sudden sensorineural hearing loss or another urgent medical condition. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, I have this ringing in my ears.”
With an estimated 10% to 15% of adults experiencing chronic tinnitus,1 most primary care physicians are familiar with this complaint. The prevalence of tinnitus increases with age and with exposure to high levels of noise—the most commonly reported cause.1 With people living longer and such “toxic” noise levels on the rise, tinnitus is a condition you can expect to encounter even more frequently.
Despite the prevalence of tinnitus, however, there are no clinical standards or best practice guidelines for managing it. Thus, many physicians are uncertain about what to tell patients with this distressing disorder, and when (or whether) to refer them to specialists. So patients are sometimes told that “nothing can be done” and that they simply must “learn to live with” tinnitus.
Such negative messages from a trusted physician can have a detrimental effect, causing some patients to stop seeking help and to become increasingly disturbed by tinnitus.2 What’s more, these messages are untrue. Some conditions that result in tinnitus can be treated. And, although tinnitus itself cannot normally be cured, there are numerous interventions and educational strategies that can help patients change their reactions to—and learn to cope with—the ringing in their ears. We developed this evidence-based review and tinnitus triage guide (TABLE 1) to help family physicians respond appropriately to this distressing, but common, condition.
TABLE 1
Tinnitus triage guide27
| If the patient | Refer to | Status/considerations |
|---|---|---|
| Has neural deficits such as facial weakness, head trauma, or other urgent medical condition | Otolaryngology or ED | Emergency |
| Has unexplained sudden hearing loss | Audiology and otolaryngology | Emergency; must see audiologist prior to otolaryngologist on same day |
| Expresses suicidal ideation or manifests obvious mental illness | Mental health or ED | May be emergency; report suicide ideation; provide escort, if necessary |
Has any of the following:
| Otolaryngology and audiology | Urgent; schedule otolaryngology exam as soon as possible |
Has symptoms that suggest a neurophysiologic origin of tinnitus without:
| Audiology and otolaryngology | Nonurgent; schedule audiology exam before patient sees otolaryngologist |
| ED, emergency department. | ||
Is it transient noise, or tinnitus?
Virtually everyone experiences “transient ear noise,” which is usually described as a whistling sound accompanied by a sensation of sudden temporary hearing loss.3,4 These idiopathic episodes are usually unilateral, and often accompanied by a feeling of ear blockage.
To distinguish between tinnitus—the perception of sound that is produced internally, rather than by an external stimulus—and transient ear noise, consider the duration and frequency. Transient ear noise generally disappears within seconds (and does not require diagnostic testing or treatment). Tinnitus, which can have a variety of underlying pathologies, is defined as ear or head noise that lasts at least 5 minutes and occurs at least twice a week.5
Neurophysiologic tinnitus is most common
Neurophysiologic (sensorineural) tinnitus, which originates within the auditory nervous system, accounts for the vast majority of cases. The pathology exists anywhere between the cochlea and the auditory cortex, and excludes any sounds generated by mechanical (somatic) processes.6
The ringing may be relatively soft; in some cases, it can be heard only in quiet environments or while the patient is trying to sleep. In others, the tinnitus may be constant, interfering with concentration and daily activities, as well as sleep. In the most severe cases, tinnitus may be associated with severe depression and anxiety, even to the point of suicidal ideation.7
Notably, however, the loudness or other perceptual characteristics of tinnitus do not necessarily indicate the degree to which it is a problem for the patient.7 Although patients often report that tinnitus interferes with their hearing, they usually also have hearing loss, which an audiologic evaluation will reveal.7-9
Certain medications can trigger or exacerbate tinnitus, including aspirin, nonsteroidal anti-inflammatory drugs, loop diuretics, and quinine.2 Fairly high doses are usually required to cause tinnitus, however, and the effects are typically temporary. Patients have also reported exacerbation of tinnitus due to alcohol, salt, and caffeine intake. Ototoxicity from aminoglycosides and platinum-containing chemotherapeutic drugs is a well-known cause of hearing loss and tinnitus, but these effects are often irreversible.10,11
Neurophysiologic tinnitus is generally not serious from a medical standpoint. While all patients with this condition should undergo an audiologic exam and hearing evaluation, only about 20% of adults who experience tinnitus require intervention.12-14 Although there is no cure, patients with clinically significant tinnitus can be taught stress management and therapeutic use of sound techniques, as well as lifestyle modifications (TABLE 2) to minimize its detrimental effects.
TABLE 2
Managing neurophysiologic tinnitus: A range of options2,5,25-27
| Cognitive-behavioral therapy |
| Elimination of tinnitus-inducing medications (eg, NSAIDs, loop diuretics, and quinine) |
| Hearing aids, sound generators, or other sound devices |
| Lifestyle modifications (eg, improve sleep hygiene, exercise regularly, limit salt intake) |
| Medication (antidepressants or anxiolytics) |
| Patient education that stresses that there are numerous techniques that can be used to manage reactions to tinnitus |
| Stress reduction techniques (eg, imagery, meditation, and deep breathing techniques) |
| Therapeutic sound (eg, using interesting sound to direct attention away from tinnitus, low-level background sound to reduce auditory contrast, and soothing sound for relief) |
| NSAIDs, nonsteroidal anti-inflammatory drugs. |
Somatic tinnitus may be serious
Somatic tinnitus, also known as somato-sound, refers to the perception of sound that originates within the body—in vascular, muscular, skeletal, or respiratory structures, or in the temporomandibular joint.4 These “body sounds” have an internal acoustic source.9
Pulsatile tinnitus, which pulses in synchrony with the heartbeat, is the most common somatosound.15,16 Most patients with pulsatile tinnitus have benign venous “hums,” but serious conditions such as arteriovenous malformations, glomus tumors, and carotid stenosis must be considered. Auscultation over the neck and temporal bone may reveal bruits that can help localize the lesion. We recommend either magnetic resonance imaging (MRI) of the head or computed tomography (CT) angiography, accompanied by timely referral to an otolaryngologist for a focused evaluation.15,17,18
Somatosounds can also be nonpulsatile, indicating a nonvascular source. Examples of nonvascular somatosounds include middle-ear muscle spasms and eustachian tube dysfunction. Nonpulsatile somatic tinnitus is rarely progressive or dangerous. It is reasonable to offer reassurance to patients with nonpulsatile tinnitus, followed by a referral to an otolaryngologist if the symptoms interfere with daily activities.
Unilateral tinnitus is a red flag
In most cases, tinnitus is bilateral. Unilateral tinnitus may indicate a more serious medical condition. It is a common presenting sign of both vestibular schwannoma (also known as acoustic neuroma) and Meniere’s disease.
Patients with unilateral tinnitus should receive a hearing test as soon as possible; if asymmetric hearing loss is found, MRI is indicated, both with and without contrast of the internal auditory canal, to rule out vestibular schwannoma.
Idiopathic sudden sensorineural hearing loss (ISSNHL), which may be associated with new onset unilateral tinnitus, should be considered an otologic emergency. When you suspect ISSNHL, you’ll need to make a same-day referral for an otologic examination.
If left untreated, the ISSNHL and associated tinnitus will resolve partially or completely at least 50% of the time. This recovery rate may be improved with glucocorticoid treatments.19 Prompt initiation of corticosteroid therapy can be a factor in the chances of recovery—the more rapidly such patients are seen and treated, the better their prognosis.20
Tinnitus triage: Key points
Following our triage guide (TABLE 1) should result in appropriate care in most cases. Here are some considerations to keep in mind:
Urgent medical referral. Any patient with tinnitus and symptoms suggestive of serious underlying treatable pathology requires an urgent otolaryngology referral. That includes ISSNHL, which you should suspect whenever a patient reports an unexplained decrease in hearing, as well as pulsatile tinnitus, vestibular symptoms, and long-standing ear pain, drainage, or malodor that does not resolve with routine treatment. If possible, such patients should undergo an audiologic assessment prior to the otolaryngology visit; however, the otolaryngology exam is the primary concern.
Facial paralysis, severe vertigo, or sudden onset pulsatile tinnitus can indicate a serious intracranial condition. These symptoms may point to cerebrovascular disease or neoplasm, and should be treated as an otologic emergency.
Mental health referral. Some tinnitus patients require a mental health assessment, either because of obvious manifestations of a mental illness or because of expressed suicidal ideation. If there’s a question about the patient’s mental health, consider consulting with a mental health provider or using basic screening tools for anxiety and depression to help determine the need for referral, as well as the urgency.12
Some patients experience extreme anxiety or depression in response to tinnitus and should be referred to a mental health professional on the day they present with symptoms. Suicidal ideation warrants special attention, of course—possibly including the need to escort the patient to the emergency department or to a behavioral specialist.21-23
Nonurgent medical referral. Ideally, all patients who present with tinnitus should see an audiologist and an otolaryngologist, but those who have no serious symptoms should be referred on a nonurgent basis. Such patients need to have a comprehensive hearing evaluation—ideally, before they see the otolaryngologist so the test results are available at the time of the exam. The audiologist should also assess the severity of the tinnitus, using a validated questionnaire such as the Tinnitus Handicap Inventory, for the initial assessment and to monitor changes in the severity of the tinnitus as an outcome measure of therapy.24
Enlist an interdisciplinary team
For patients with somatic tinnitus, the treatment—and the specialist who provides it—depends on the underlying cause. A patient who has unilateral tinnitus may be referred by an audiologist or otolaryngologist to a neurologist, for example, if he or she is found to have Meniere’s disease; a patient with pulsatile tinnitus may be sent back to his or her primary care physician after diagnostic testing has ruled out serious causes.
For patients with neurophysiologic tinnitus (and any patient with untreatable somatic tinnitus), a well-organized interdisciplinary team that includes the family physician, an audiologist, and a psychologist is the best approach. The variety of available management options (TABLE 2) incorporate medical approaches, complementary and alternative treatments, psychological interventions, and sound-based methods. Lifestyle modifications, such as improved sleep hygiene, regular exercise, and dietary modifications, may help, as well.25-27 First-line strategies include:
Adjusting medications. Eliminating tinnitus-inducing medications, if medically safe, is a common starting point. No prescription drug has been developed specifically for tinnitus. But some antidepressants or anxiolytics (eg, amitriptyline or lorazepam) are commonly used to address coexisting sleep and mental health disorders—primarily depression and anxiety—that may be associated with, or exacerbated by, tinnitus.28-30
Addressing hearing problems. Patients should undergo a hearing evaluation and receive help in managing a hearing problem, if necessary. Hearing aids improve hearing and reduce the perception of tinnitus.31
Using therapeutic sound. Some audiologists are trained to implement various forms of sound-based therapy. Tinnitus retraining therapy involves the use of background sound to facilitate habituation to tinnitus; tinnitus masking involves the use of soothing sound to provide a sense of relief. Progressive tinnitus management is a more recent method that educates patients in the use of all types of therapeutic sound.32 These sound-based methods often include the use of hearing aids, sound generators, and other devices.
Circling in a mental health professional. It is essential to involve psychologists or other mental health specialists in the care of patients with clinically significant tinnitus to ensure that psychological and other barriers to successful management of the condition are identified and addressed. Cognitive-behavioral therapy (CBT) has been shown to be helpful for patients with tinnitus.33 In fact, we have been successful in teaching patients to manage their reactions to tinnitus—resulting in a better quality of life—using a combination of educational counseling, therapeutic sound, and CBT. JFP
Acknowledgments
Funding for this work was provided by Veterans Health Administration, and Veterans Affairs Rehabilitation Research and Development (RR&D) Service (C4488R). Thanks to Robert Folmer, PhD, William Martin, PhD, Dennis Trune, PhD, and Baker Shi, MD, PhD, for advice that contributed to this manuscript. Special thanks to Martin Schechter, PhD, for his significant contributions to our research. The authors also wish to thank Stephen Fausti, PhD, and Sara Ruth Oliver, AuD, for their consistent support of our research.
CORRESPONDENCE James A. Henry, PhD, VA Medical Center (NCRAR), Post Office Box 1034, Portland, OR 97207; [email protected]
• Let patients know that they can learn to manage their reactions to tinnitus with methods that include stress reduction, therapeutic sound, and coping skills. A
• Refer patients with tinnitus to an audiologist for a hearing evaluation, assessment of the tinnitus, and, if indicated, support in learning to manage reactions to tinnitus. A
• Give patients with suicidal ideation or extreme anxiety or depression in response to tinnitus a same-day referral to a mental health professional. A
• Provide an urgent referral to an otolaryngologist or emergency care if you suspect sudden sensorineural hearing loss or another urgent medical condition. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, I have this ringing in my ears.”
With an estimated 10% to 15% of adults experiencing chronic tinnitus,1 most primary care physicians are familiar with this complaint. The prevalence of tinnitus increases with age and with exposure to high levels of noise—the most commonly reported cause.1 With people living longer and such “toxic” noise levels on the rise, tinnitus is a condition you can expect to encounter even more frequently.
Despite the prevalence of tinnitus, however, there are no clinical standards or best practice guidelines for managing it. Thus, many physicians are uncertain about what to tell patients with this distressing disorder, and when (or whether) to refer them to specialists. So patients are sometimes told that “nothing can be done” and that they simply must “learn to live with” tinnitus.
Such negative messages from a trusted physician can have a detrimental effect, causing some patients to stop seeking help and to become increasingly disturbed by tinnitus.2 What’s more, these messages are untrue. Some conditions that result in tinnitus can be treated. And, although tinnitus itself cannot normally be cured, there are numerous interventions and educational strategies that can help patients change their reactions to—and learn to cope with—the ringing in their ears. We developed this evidence-based review and tinnitus triage guide (TABLE 1) to help family physicians respond appropriately to this distressing, but common, condition.
TABLE 1
Tinnitus triage guide27
| If the patient | Refer to | Status/considerations |
|---|---|---|
| Has neural deficits such as facial weakness, head trauma, or other urgent medical condition | Otolaryngology or ED | Emergency |
| Has unexplained sudden hearing loss | Audiology and otolaryngology | Emergency; must see audiologist prior to otolaryngologist on same day |
| Expresses suicidal ideation or manifests obvious mental illness | Mental health or ED | May be emergency; report suicide ideation; provide escort, if necessary |
Has any of the following:
| Otolaryngology and audiology | Urgent; schedule otolaryngology exam as soon as possible |
Has symptoms that suggest a neurophysiologic origin of tinnitus without:
| Audiology and otolaryngology | Nonurgent; schedule audiology exam before patient sees otolaryngologist |
| ED, emergency department. | ||
Is it transient noise, or tinnitus?
Virtually everyone experiences “transient ear noise,” which is usually described as a whistling sound accompanied by a sensation of sudden temporary hearing loss.3,4 These idiopathic episodes are usually unilateral, and often accompanied by a feeling of ear blockage.
To distinguish between tinnitus—the perception of sound that is produced internally, rather than by an external stimulus—and transient ear noise, consider the duration and frequency. Transient ear noise generally disappears within seconds (and does not require diagnostic testing or treatment). Tinnitus, which can have a variety of underlying pathologies, is defined as ear or head noise that lasts at least 5 minutes and occurs at least twice a week.5
Neurophysiologic tinnitus is most common
Neurophysiologic (sensorineural) tinnitus, which originates within the auditory nervous system, accounts for the vast majority of cases. The pathology exists anywhere between the cochlea and the auditory cortex, and excludes any sounds generated by mechanical (somatic) processes.6
The ringing may be relatively soft; in some cases, it can be heard only in quiet environments or while the patient is trying to sleep. In others, the tinnitus may be constant, interfering with concentration and daily activities, as well as sleep. In the most severe cases, tinnitus may be associated with severe depression and anxiety, even to the point of suicidal ideation.7
Notably, however, the loudness or other perceptual characteristics of tinnitus do not necessarily indicate the degree to which it is a problem for the patient.7 Although patients often report that tinnitus interferes with their hearing, they usually also have hearing loss, which an audiologic evaluation will reveal.7-9
Certain medications can trigger or exacerbate tinnitus, including aspirin, nonsteroidal anti-inflammatory drugs, loop diuretics, and quinine.2 Fairly high doses are usually required to cause tinnitus, however, and the effects are typically temporary. Patients have also reported exacerbation of tinnitus due to alcohol, salt, and caffeine intake. Ototoxicity from aminoglycosides and platinum-containing chemotherapeutic drugs is a well-known cause of hearing loss and tinnitus, but these effects are often irreversible.10,11
Neurophysiologic tinnitus is generally not serious from a medical standpoint. While all patients with this condition should undergo an audiologic exam and hearing evaluation, only about 20% of adults who experience tinnitus require intervention.12-14 Although there is no cure, patients with clinically significant tinnitus can be taught stress management and therapeutic use of sound techniques, as well as lifestyle modifications (TABLE 2) to minimize its detrimental effects.
TABLE 2
Managing neurophysiologic tinnitus: A range of options2,5,25-27
| Cognitive-behavioral therapy |
| Elimination of tinnitus-inducing medications (eg, NSAIDs, loop diuretics, and quinine) |
| Hearing aids, sound generators, or other sound devices |
| Lifestyle modifications (eg, improve sleep hygiene, exercise regularly, limit salt intake) |
| Medication (antidepressants or anxiolytics) |
| Patient education that stresses that there are numerous techniques that can be used to manage reactions to tinnitus |
| Stress reduction techniques (eg, imagery, meditation, and deep breathing techniques) |
| Therapeutic sound (eg, using interesting sound to direct attention away from tinnitus, low-level background sound to reduce auditory contrast, and soothing sound for relief) |
| NSAIDs, nonsteroidal anti-inflammatory drugs. |
Somatic tinnitus may be serious
Somatic tinnitus, also known as somato-sound, refers to the perception of sound that originates within the body—in vascular, muscular, skeletal, or respiratory structures, or in the temporomandibular joint.4 These “body sounds” have an internal acoustic source.9
Pulsatile tinnitus, which pulses in synchrony with the heartbeat, is the most common somatosound.15,16 Most patients with pulsatile tinnitus have benign venous “hums,” but serious conditions such as arteriovenous malformations, glomus tumors, and carotid stenosis must be considered. Auscultation over the neck and temporal bone may reveal bruits that can help localize the lesion. We recommend either magnetic resonance imaging (MRI) of the head or computed tomography (CT) angiography, accompanied by timely referral to an otolaryngologist for a focused evaluation.15,17,18
Somatosounds can also be nonpulsatile, indicating a nonvascular source. Examples of nonvascular somatosounds include middle-ear muscle spasms and eustachian tube dysfunction. Nonpulsatile somatic tinnitus is rarely progressive or dangerous. It is reasonable to offer reassurance to patients with nonpulsatile tinnitus, followed by a referral to an otolaryngologist if the symptoms interfere with daily activities.
Unilateral tinnitus is a red flag
In most cases, tinnitus is bilateral. Unilateral tinnitus may indicate a more serious medical condition. It is a common presenting sign of both vestibular schwannoma (also known as acoustic neuroma) and Meniere’s disease.
Patients with unilateral tinnitus should receive a hearing test as soon as possible; if asymmetric hearing loss is found, MRI is indicated, both with and without contrast of the internal auditory canal, to rule out vestibular schwannoma.
Idiopathic sudden sensorineural hearing loss (ISSNHL), which may be associated with new onset unilateral tinnitus, should be considered an otologic emergency. When you suspect ISSNHL, you’ll need to make a same-day referral for an otologic examination.
If left untreated, the ISSNHL and associated tinnitus will resolve partially or completely at least 50% of the time. This recovery rate may be improved with glucocorticoid treatments.19 Prompt initiation of corticosteroid therapy can be a factor in the chances of recovery—the more rapidly such patients are seen and treated, the better their prognosis.20
Tinnitus triage: Key points
Following our triage guide (TABLE 1) should result in appropriate care in most cases. Here are some considerations to keep in mind:
Urgent medical referral. Any patient with tinnitus and symptoms suggestive of serious underlying treatable pathology requires an urgent otolaryngology referral. That includes ISSNHL, which you should suspect whenever a patient reports an unexplained decrease in hearing, as well as pulsatile tinnitus, vestibular symptoms, and long-standing ear pain, drainage, or malodor that does not resolve with routine treatment. If possible, such patients should undergo an audiologic assessment prior to the otolaryngology visit; however, the otolaryngology exam is the primary concern.
Facial paralysis, severe vertigo, or sudden onset pulsatile tinnitus can indicate a serious intracranial condition. These symptoms may point to cerebrovascular disease or neoplasm, and should be treated as an otologic emergency.
Mental health referral. Some tinnitus patients require a mental health assessment, either because of obvious manifestations of a mental illness or because of expressed suicidal ideation. If there’s a question about the patient’s mental health, consider consulting with a mental health provider or using basic screening tools for anxiety and depression to help determine the need for referral, as well as the urgency.12
Some patients experience extreme anxiety or depression in response to tinnitus and should be referred to a mental health professional on the day they present with symptoms. Suicidal ideation warrants special attention, of course—possibly including the need to escort the patient to the emergency department or to a behavioral specialist.21-23
Nonurgent medical referral. Ideally, all patients who present with tinnitus should see an audiologist and an otolaryngologist, but those who have no serious symptoms should be referred on a nonurgent basis. Such patients need to have a comprehensive hearing evaluation—ideally, before they see the otolaryngologist so the test results are available at the time of the exam. The audiologist should also assess the severity of the tinnitus, using a validated questionnaire such as the Tinnitus Handicap Inventory, for the initial assessment and to monitor changes in the severity of the tinnitus as an outcome measure of therapy.24
Enlist an interdisciplinary team
For patients with somatic tinnitus, the treatment—and the specialist who provides it—depends on the underlying cause. A patient who has unilateral tinnitus may be referred by an audiologist or otolaryngologist to a neurologist, for example, if he or she is found to have Meniere’s disease; a patient with pulsatile tinnitus may be sent back to his or her primary care physician after diagnostic testing has ruled out serious causes.
For patients with neurophysiologic tinnitus (and any patient with untreatable somatic tinnitus), a well-organized interdisciplinary team that includes the family physician, an audiologist, and a psychologist is the best approach. The variety of available management options (TABLE 2) incorporate medical approaches, complementary and alternative treatments, psychological interventions, and sound-based methods. Lifestyle modifications, such as improved sleep hygiene, regular exercise, and dietary modifications, may help, as well.25-27 First-line strategies include:
Adjusting medications. Eliminating tinnitus-inducing medications, if medically safe, is a common starting point. No prescription drug has been developed specifically for tinnitus. But some antidepressants or anxiolytics (eg, amitriptyline or lorazepam) are commonly used to address coexisting sleep and mental health disorders—primarily depression and anxiety—that may be associated with, or exacerbated by, tinnitus.28-30
Addressing hearing problems. Patients should undergo a hearing evaluation and receive help in managing a hearing problem, if necessary. Hearing aids improve hearing and reduce the perception of tinnitus.31
Using therapeutic sound. Some audiologists are trained to implement various forms of sound-based therapy. Tinnitus retraining therapy involves the use of background sound to facilitate habituation to tinnitus; tinnitus masking involves the use of soothing sound to provide a sense of relief. Progressive tinnitus management is a more recent method that educates patients in the use of all types of therapeutic sound.32 These sound-based methods often include the use of hearing aids, sound generators, and other devices.
Circling in a mental health professional. It is essential to involve psychologists or other mental health specialists in the care of patients with clinically significant tinnitus to ensure that psychological and other barriers to successful management of the condition are identified and addressed. Cognitive-behavioral therapy (CBT) has been shown to be helpful for patients with tinnitus.33 In fact, we have been successful in teaching patients to manage their reactions to tinnitus—resulting in a better quality of life—using a combination of educational counseling, therapeutic sound, and CBT. JFP
Acknowledgments
Funding for this work was provided by Veterans Health Administration, and Veterans Affairs Rehabilitation Research and Development (RR&D) Service (C4488R). Thanks to Robert Folmer, PhD, William Martin, PhD, Dennis Trune, PhD, and Baker Shi, MD, PhD, for advice that contributed to this manuscript. Special thanks to Martin Schechter, PhD, for his significant contributions to our research. The authors also wish to thank Stephen Fausti, PhD, and Sara Ruth Oliver, AuD, for their consistent support of our research.
CORRESPONDENCE James A. Henry, PhD, VA Medical Center (NCRAR), Post Office Box 1034, Portland, OR 97207; [email protected]
1. Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:16-41.
2. Jastreboff PJ, Hazell JWP. Tinnitus Retraining Therapy: Implementing the Neurophysiological Model. New York: Cambridge University Press; 2004.
3. Kiang NYS, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Wolstenholme GEW, Knight J, eds. Sensorineural Hearing Loss. London: J. & A. Churchill; 1970:241-273.
4. Henry JA, Dennis K, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005;48:1204-1235.
5. Dauman R, Tyler RS. Some considerations on the classification of tinnitus. In: Aran J-M, Dauman R, eds. Proceedings of the Fourth International Tinnitus Seminar. Amsterdam/New York: Kugler Publications; 1992:225-229.
6. Hazell J. Incidence, classification, and models of tinnitus. In: Ludman H, Wright T, eds. Diseases of the Ear. London: Arnold; 1998:185-195.
7. Dobie RA. Overview: suffering from tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:1-7.
8. Zaugg TL, et al. Difficulties caused by patients’ misconceptions that hearing problems are due to tinnitus. In: Patuzzi R, ed. Proceedings of the Seventh International Tinnitus Seminar. Perth: University of Western Australia; 2002:226-228.
9. Coles RRA. Classification of causes, mechanisms of patient disturbance, and associated counseling. In: Vernon JA, Moller AR, eds. Mechanisms of Tinnitus. Needham Heights, Mass: Allyn & Bacon; 1995:11-19.
10. Fausti SA, et al. Ototoxicity. In: Northern JL, ed. Hearing Disorders. Needham Heights, Mass: Allyn & Bacon; 1995:149-164.
11. Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear Res. 2002;164:206-214.
12. Henry JA, Zaugg TL, Myers PJ, et al. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12:170-187.
13. Jastreboff PJ, Hazell JWP. Treatment of tinnitus based on a neurophysiological model. In: Vernon JA, ed. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998:201-217.
14. Davis A, Refaie AE. Epidemiology of tinnitus. In: Tyler R, ed. Tinnitus Handbook. San Diego: Singular Publishing Group; 2000:1-23.
15. Lockwood AH, Burkard RF, Salvi RJ. Imaging tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:253-264.
16. Sismanis A. Pulsatile tinnitus. Otolaryngol Clin North Am. 2003;36:389-402.
17. Sismanis A. Pulsatile tinnitus. In: Vernon JA, ed. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998:28-33.
18. Wackym PA, Friedland DR. Otologic evaluation. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:205-219.
19. Hamid M, Trune D. Issues, indications, and controversies regarding intratympanic steroid perfusion. Curr Opin Otolaryngol Head Neck Surg. 2008;16:434-440.
20. Jeyakumar A, et al. Treatment of idiopathic sudden sensorineural hearing loss. Acta Otolaryngol. 2006;126:708-713.
21. Brown GK, et al. Suicide intent and accurate expectations of lethality: predictors of medical lethality of suicide attempts. J Consult Clin Psychol. 2004;72:1170-1174.
22. Hawton K. Studying survivors of nearly lethal suicide attempts: an important strategy in suicide research. Suicide Life Threat Behav. 2001;32(1 suppl):76-84.
23. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617-626.
24. Newman CW, Sandridge SA, Jacobson GP. Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J Am Acad Audiol. 1998;9:153-160.
25. Tyler RS, ed. Tinnitus Treatment: Clinical Protocols. New York: Thieme Medical Publishers, Inc; 2005.
26. Vernon JA. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998.
27. Henry JA, Zaugg TL, Myers PM, et al. Progressive Tinnitus Management: Clinical Handbook for Audiologists. San Diego, Calif: Plural Publishing; 2010.
28. Robinson SK, Viirre ES, Stein MB. Antidepressant therapy for tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:278-293.
29. Dobie RA. Clinical trials and drug therapy for tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:266-277.
30. Henry JA, Zaugg TL, Schechter MA. Clinical guide for audiologic tinnitus management I: assessment. Am J Audiol. 2005;14:21-48.
31. Surr RK, Montgomery AA, Mueller HG. Effect of amplification on tinnitus among new hearing aid users. Ear Hear. 1985;6:71-75.
32. Henry JA, et al. Using therapeutic sound with progressive audiologic tinnitus management. Trends Amplif. 2008;12:185-206.
33. Martinez Devesa P, Waddell A, Perera R, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2007;(1):CD005233.-
1. Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:16-41.
2. Jastreboff PJ, Hazell JWP. Tinnitus Retraining Therapy: Implementing the Neurophysiological Model. New York: Cambridge University Press; 2004.
3. Kiang NYS, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Wolstenholme GEW, Knight J, eds. Sensorineural Hearing Loss. London: J. & A. Churchill; 1970:241-273.
4. Henry JA, Dennis K, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005;48:1204-1235.
5. Dauman R, Tyler RS. Some considerations on the classification of tinnitus. In: Aran J-M, Dauman R, eds. Proceedings of the Fourth International Tinnitus Seminar. Amsterdam/New York: Kugler Publications; 1992:225-229.
6. Hazell J. Incidence, classification, and models of tinnitus. In: Ludman H, Wright T, eds. Diseases of the Ear. London: Arnold; 1998:185-195.
7. Dobie RA. Overview: suffering from tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:1-7.
8. Zaugg TL, et al. Difficulties caused by patients’ misconceptions that hearing problems are due to tinnitus. In: Patuzzi R, ed. Proceedings of the Seventh International Tinnitus Seminar. Perth: University of Western Australia; 2002:226-228.
9. Coles RRA. Classification of causes, mechanisms of patient disturbance, and associated counseling. In: Vernon JA, Moller AR, eds. Mechanisms of Tinnitus. Needham Heights, Mass: Allyn & Bacon; 1995:11-19.
10. Fausti SA, et al. Ototoxicity. In: Northern JL, ed. Hearing Disorders. Needham Heights, Mass: Allyn & Bacon; 1995:149-164.
11. Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear Res. 2002;164:206-214.
12. Henry JA, Zaugg TL, Myers PJ, et al. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12:170-187.
13. Jastreboff PJ, Hazell JWP. Treatment of tinnitus based on a neurophysiological model. In: Vernon JA, ed. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998:201-217.
14. Davis A, Refaie AE. Epidemiology of tinnitus. In: Tyler R, ed. Tinnitus Handbook. San Diego: Singular Publishing Group; 2000:1-23.
15. Lockwood AH, Burkard RF, Salvi RJ. Imaging tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:253-264.
16. Sismanis A. Pulsatile tinnitus. Otolaryngol Clin North Am. 2003;36:389-402.
17. Sismanis A. Pulsatile tinnitus. In: Vernon JA, ed. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998:28-33.
18. Wackym PA, Friedland DR. Otologic evaluation. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:205-219.
19. Hamid M, Trune D. Issues, indications, and controversies regarding intratympanic steroid perfusion. Curr Opin Otolaryngol Head Neck Surg. 2008;16:434-440.
20. Jeyakumar A, et al. Treatment of idiopathic sudden sensorineural hearing loss. Acta Otolaryngol. 2006;126:708-713.
21. Brown GK, et al. Suicide intent and accurate expectations of lethality: predictors of medical lethality of suicide attempts. J Consult Clin Psychol. 2004;72:1170-1174.
22. Hawton K. Studying survivors of nearly lethal suicide attempts: an important strategy in suicide research. Suicide Life Threat Behav. 2001;32(1 suppl):76-84.
23. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617-626.
24. Newman CW, Sandridge SA, Jacobson GP. Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J Am Acad Audiol. 1998;9:153-160.
25. Tyler RS, ed. Tinnitus Treatment: Clinical Protocols. New York: Thieme Medical Publishers, Inc; 2005.
26. Vernon JA. Tinnitus Treatment and Relief. Needham Heights, Mass: Allyn & Bacon; 1998.
27. Henry JA, Zaugg TL, Myers PM, et al. Progressive Tinnitus Management: Clinical Handbook for Audiologists. San Diego, Calif: Plural Publishing; 2010.
28. Robinson SK, Viirre ES, Stein MB. Antidepressant therapy for tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:278-293.
29. Dobie RA. Clinical trials and drug therapy for tinnitus. In: Snow JB, ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker Inc; 2004:266-277.
30. Henry JA, Zaugg TL, Schechter MA. Clinical guide for audiologic tinnitus management I: assessment. Am J Audiol. 2005;14:21-48.
31. Surr RK, Montgomery AA, Mueller HG. Effect of amplification on tinnitus among new hearing aid users. Ear Hear. 1985;6:71-75.
32. Henry JA, et al. Using therapeutic sound with progressive audiologic tinnitus management. Trends Amplif. 2008;12:185-206.
33. Martinez Devesa P, Waddell A, Perera R, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2007;(1):CD005233.-
Weight loss strategies that really work
• Calculate body mass index and diagnose obesity to increase the likelihood that obese patients will take steps to lose weight. B
• Prescribe a low-calorie diet for at least 6 months to help patients achieve a weight loss of at least 5% to 10%; prescribe physical activity for weight loss and weight maintenance. A
• Review the benefits and risks of bariatric surgery with patients who are severely obese, and provide a referral, when appropriate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s not only obese patients who are resistant to weight loss strategies. Many physicians contribute to the gap between current practice and optimal management of adult obesity, as well. There are a number of reasons for this—a dearth of knowledge, time, and reimbursement among them.1,2
What’s more, physicians are often pessimistic about how much headway patients can make in their weight loss efforts. That’s not surprising, given that the average weight loss achieved in well-controlled clinical trials tends to be modest and the recidivism rate is extremely high.3 Yet these same trials are cause for optimism, with substantial subsets of patients often achieving clinically meaningful long-term weight loss. The National Weight Control Registry, a long-term prospective study of “successful losers,” is another hopeful indicator: The registry includes approximately 6000 individuals who have lost, on average, more than 70 lb, and kept it off for an average of 6 years.4 (Listen to the audiocast at jfponline.com to find out how.)
Weight loss does not have to be huge to be clinically significant. Even a modest loss (5%-10% of total body weight) can have major health benefits. There’s much you can do to help.
Evidence suggests that patients are considerably more likely to lose weight when they are advised to do so and supported by their primary care physician.5-9 Because there is no way to predict which approach will be most effective for which patient, family physicians (FPs) should offer a variety of evidence-based treatments, including dietary change, increased physical activity, medication for selected patients, and surgery for severely obese adults (TABLE 1).
In 2009, the National Committee on Quality Assurance added body mass index (BMI) to the list of effectiveness-of-care measures that health plans and physicians are rated on.10 That addition, coupled with a recent study indicating that obesity accounts for more than 9% of annual health care expenditures,11 highlights the growing recognition that obesity should be considered a medical condition—not just a risk factor. While patients are increasingly likely to have a weight management insurance benefit that reimburses physicians and dietitians for multiple visits, many health plans still do not cover weight-related treatment. FPs can help by advocating for such coverage—and by taking steps to help patients win the battle against obesity.
TABLE 1
BMI, health risks, and weight loss: Which intervention for which patients?15
| Disease risk | Intervention | ||||
|---|---|---|---|---|---|
| BMI, kg/m2 (weight status) | Normal waist measurement | Elevated waist measurement | Diet and physical activity | Medication | Surgery |
| 25-29.9 (overweight) | Increased | High | 25-26.9: Yes, for patients with comorbidities 27-29.9: Yes | 25-26.9: NA 27-29.9: Yes, for patients with comorbidities | NA |
| 30-39.9 (obese) | 30-34.9: High 35-39.9: Very high | Very high | Yes | Yes | 30-34.9: Yes, for patients with comorbidities 35-39.9: Yes |
| ≥40 (extremely obese) | Extremely high | Extremely high | Yes | Yes | Yes |
| BMI, body mass index; NA, not appropriate. | |||||
2 weight loss tools that can jump-start your efforts
Physicians often cite time as a key reason for not providing weight loss counseling. But physician knowledge—actually, lack of knowledge—may be a bigger barrier. In 1 recent study, 44% of physicians said they did not feel qualified to treat obesity.12 In another, 72% of primary care physicians surveyed said that no one in their practice was trained to deal with weight-related issues.13
As the focus on obesity grows, clinical weight management tools are increasingly available. Two excellent examples are the California Medical Association Foundation’s Obesity Provider Toolkit (www.thecmafoundation.org/projects/obesityProject.aspx) and the clinical tools from North Carolina’s Eat Smart, Move More program (www.eatsmartmovemorenc.com/ESMMPlan/ESMMPlan.html). You can download a toolkit, review the strategies described, and adopt those you think would be most effective in your practice. Doing so needn’t be especially time-consuming; evidence suggests you can provide basic counseling about healthy behaviors in fewer than 5 minutes.14
Review guidelines. The National Heart, Lung, and Blood Institute issued the first evidence-based guidelines for the treatment of adult obesity in 1998.15 Many other groups—the US Preventive Services Task Force (USPSTF),16 the American Dietetic Association,17 and the American College of Physicians,18 among them—have followed suit, with guidelines addressing practical weight loss interventions and treatment related to specific comorbidities. The organizations all recommend using BMI to diagnose and classify obesity, assessing readiness to change, and setting realistic goals (TABLE 2).
TABLE 2
Obesity: Key components of evaluation and treatment
| Assess |
|
| Advise |
|
| Agree |
|
| Assist |
|
| Arrange |
|
| BMI, body mass index. | |
Diagnose obesity without delay
While most patients can report their height and weight with reasonable accuracy, few obese adults consider themselves to be obese.19 And, although the USPSTF 2009 recommendations call for screening all adults for obesity and introducing behavioral interventions to promote sustained weight loss as needed,20 most obese men and women receive neither a diagnosis nor an obesity management plan.21 Yet both are key components of long-term weight control. Physicians should calculate and document the BMI of all adult patients, and routinely diagnose obesity in patients with a BMI ≥30 kg/m2 (TABLE 1).
Get the patient’s perspective
In addition to using BMI and waist circumference measures and identifying comorbidities such as diabetes and hypertension, it is important to determine whether the patient is motivated to change. You can start by asking whether he or she has previously been told to lose weight; adults who have received weight control counseling in the past are more likely to be in a greater state of readiness.7
Review the patient’s medications. Once you have identified obesity, look for iatrogenic causes—most notably, current use of 1 or more medications that are associated with weight gain or known to affect satiety. If you identify any such drugs, it may be possible to find a suitable alternative (TABLE 3).
Factor in literacy. Patients with low literacy are less likely to fully comprehend the health benefits of weight loss or to report that they are ready to lose weight, as compared with those with higher literacy levels.22 Talking to such patients to determine what will spark their interest and motivation—ie, looking and feeling better, being able to play with children or grandchildren—can help.
Assess the patient’s dietary patterns. This can be done with the Rapid Eating Assessment for Participants (REAP), an office-based tool of adequate reliability and validity for nutrition assessment and counseling that can be administered in a few minutes.23
This office-based survey, available at http://bms.brown.edu/nutrition/acrobat/REAP%206.pdf, provides information that can be the basis for an action plan. While there is no direct evidence that having a plan leads to weight loss, receiving advice from a physician is strongly associated with efforts to lose weight.6 Be aware that obese individuals may not be as responsive to weight management counseling from a physician as those who are overweight, so they may require more assistance.21
TABLE 3
Drugs that promote weight gain—and alternatives to consider46,52,53
| Indication/drug class/medication | Alternatives* |
|---|---|
Anticonvulsants/psychotropics Anticonvulsants | Topiramate‡ |
| Antimanic (bipolar) Lithium | Valproic acid,† carbamazepine† |
Antipsychotics Typical: Chlorpromazine, thiothixene, haloperidol | Ziprasidone
|
Antidepressants Tricyclics: Amitriptyline, imipramine |
Nortriptyline, protriptyline, desipramine |
Antidiabetics Sulfonylureas | Gliclazide, metformin, acarbose |
Antihypertensives Beta-blockers Calcium channel blockers | Guanfacine, doxazosin Acebutolol, atenolol, betaxolol, bisoprolol, labetalol, metoprolol, nadolol, pindolol Amlodipine, diltiazem, felodipine, nicardipine, nifedipine, verapamil |
Anti-inflammatories Corticosteroids | NSAIDs, COX inhibitors |
| COX, cyclooxygenase; MAOI, monoamine oxidase inhibitor; NSAIDs, nonsteroidal anti-inflammatory drugs. | |
| * Table is not meant to imply equal efficacy for all choices for a given indication. | |
| † Valproic acid and carbamazepine are associated with weight gain, but less than that associated with lithium. | |
| ‡ Topiramate may not be adequate as a single agent. | |
Set a goal, prescribe a food plan
After discussing possible interventions based on the patient’s BMI and weight status (TABLE 1), set a safe and achievable goal to reduce body weight at a rate of 1 to 2 lb per week for 6 months to achieve an initial weight loss goal of up to 10%. Not only does such a target have proven health benefits,15,24 but defining success in realistic and achievable terms helps maintain patient motivation.
Although no single dietary approach has been found to have a metabolic advantage or be most likely to support long-term weight maintenance,15,25-28 energy balance is central. It is critical to induce a negative energy balance—with a deficit of 250 to 500 calories per day to achieve a weight loss of 0.5 to 2 lb per week.
Which diet is best?
Diets emphasizing varying contents of carbohydrate, fat, and protein have generally been found to be equally successful in promoting clinically meaningful weight loss and maintenance over the course of 2 years, although some studies have found specific approaches to encourage initial weight loss.26 Because different strategies have proven helpful to various patients, your best bet is to analyze the patient’s eating pattern and prescribe the diet he or she is most likely to adhere to for at least 6 months.
If the patient has comorbidities, choose the diet expected to have the greatest impact—a low-glycemic diet for an obese patient with diabetes, for example, and the Dietary Approaches to Stop Hypertension (DASH) food plan for a patient with hypertension.27 For premenopausal women, diets low in carbohydrates have been found to facilitate weight loss without negative health consequences.25
Offer specific—and actionable—strategies
Most clinically obese patients (63%, according to 1 study) do not receive weight loss counseling from their primary care physician. Often, they are told to lose weight, but not given advice on how to do so. Providing specific strategies can help patients stay motivated.
One set of specific weight-loss messages comes from the Centers for Disease Control and Prevention (CDC)’s Research to Practice Series.29 Patients are advised to:
- limit eating away from home
- select healthy options when eating out
- eat more fruits and vegetables
- avoid large portion sizes
- eat low energy-dense foods (ie, foods that are high in micronutrients but low in calories per gram).
The CDC also encourages new moms to breastfeed. In addition to helping the women themselves control their weight, breastfed infants appear to be more likely to maintain a normal body weight—an important consideration as overweight children are more likely than children of normal weight to become overweight adults.30 (To learn more about pediatric obesity, see “How best to help kids lose weight”.)
The “5-3-2-1-almost none” plan offers additional advice. Every day, patients should:
- Eat ≥5 servings of fruits and vegetables
- Have 3 structured meals (including breakfast)
- Limit TV/video game use to ≤2 hours
- Engage in ≥1 hour of moderate to vigorous physical activity
- Limit sugar-sweetened drinks to almost none.31
Encourage patients to keep a diary. Suggesting that patients track their food and beverage intake, as well as their physical activity, is another helpful strategy, as self-monitoring creates a sense of accountability and awareness. Patients are likely to need ongoing encouragement to do so, however, because record keeping typically declines with time.32,33
Prescribe physical activity, and sleep
Physical activity guidelines vary for active adults, older adults, and those with disabilities. To attain health benefits, however, the general recommendation is for ≥150 minutes of moderate-intensity physical activity per week, plus muscle strengthening activities at least twice a week.33,34 Help patients identify strategies that will improve adherence, such as wearing a pedometer to gauge miles walked per day or working out with a buddy.34
Sleep duration, too, may affect weight. Although there is insufficient evidence to support the idea that sleep is an independent risk factor for obesity,35 it appears that those who sleep too much (9-10 hours per night) or too little (5-6 hours) have a 3- to 5-lb weight gain compared with those who sleep for 8 hours. One possible explanation is that there is a disruption in the production of hormones that affects appetite.4 Advise patients that getting enough sleep can help them control their weight.
Consider meal replacement, pharmacotherapy
For select patients, adherence to a low-calorie diet can be facilitated by the use of meal replacement products, as well as by pharmacotherapy. Each approach yields about a 5% to 7% weight loss.36 High-protein, high-fiber calorie-controlled shakes or bars can be particularly helpful for patients who have difficulty with food selection or portion control and can be effectively monitored by a physician or dietitian.37,38 Dietary fiber is also helpful in decreasing food intake and hunger.
Discuss the risks of supplements. Patients are bombarded by advertisements for dietary supplements. You can help by initiating a discussion of the lack of evidence of the efficacy and/or safety of most such products.
The most widely used herbal supplement, ephedrine, was taken off the market in 2006 for safety reasons. The US Food and Drug Administration recommends avoiding many over-the-counter dietary products because a significant number of them have been found to contain undeclared active prescription ingredients.39
In general, published trials of dietary supplements are of suboptimal quality. There is limited evidence that caffeine may have a positive effect on thermogenesis and fat oxidation.4 A review of 1 promising dietary supplement, chitosan, found that the effect was minimal and unlikely to be of clinical significance.40 The evidence is weak for meaningful changes in weight or body composition for green tea catechins, conjugated linoleic acid, and chromium picolinate. Dietary calcium appears to aid in weight management, but the magnitude is controversial.41 Preliminary data about amino acids and neurotransmitter modulation are promising, but too little is currently known about these approaches.42 Fiber supplements, unlike most dietary supplements, are recommended, as dietary fiber decreases food intake and hunger.
Pharmacotherapy may boost weight loss. Two types of weight loss drugs—a lipase inhibitor (orlistat [Xenical]) and an appetite suppressant (sibutramine [Meridia])—are on the market. Research shows that pharmacotherapy, when combined with diet or physical activity, may enhance weight loss (usually <11 lb/year) in some adults, although the optimal duration of drug use has not been determined.16,17,43,44 The choice of medication should be based on the expected response to the medication. There is no evidence that either medication promotes more sustained weight loss than the other. In clinical trials, however, sibutramine produced a weight loss of 4.9 lb more than orlistat.44
While patients with hypertension have been found to achieve modest weight loss using either agent, orlistat reduced both diastolic and systolic pressure, while diastolic blood pressure increased with sibutramine.45 Metformin may help prevent excess weight gain associated with short-term use of atypical antipsychotics, although its use for this purpose is off-label.46
When to consider bariatric surgery
Bariatric surgery continues to provide greater sustained weight loss and metabolic improvements than other conventional treatments.47 Identify patients who have failed at comprehensive weight loss programs and who are at high risk for obesity-associated morbidity and mortality (TABLE 1), and discuss the benefits (eg, reduction in comorbidities and at least a short-term improvement in health-related quality of life) and risks (eg, pulmonary embolism, anastomosis leakage, procedure-specific problems such as band slippage and erosion [after gastric banding], and possibly even death) of bariatric surgery. Refer potential candidates to an appropriate surgeon and facility.
Be aware, however, that the field of bariatric surgery is rapidly changing in terms of types of procedures, standards for perioperative care, patient selection, and reimbursement policies,48,49 and patients need to check with their insurance company before making any treatment decisions.
Common bariatric procedures include Roux-en Y gastric bypass (the gold standard); adjustable gastric banding; biliopancreatic diversion; and sleeve gastrectomy. Most are done laparoscopically. The sleeve gastrectomy, in which a vertical sleeve is created while much of the stomach is removed, may be useful for patients who are high-volume eaters, or to prepare extremely obese patients for gastric bypass. Patient selection is important, and multidisciplinary care is generally considered essential. After the surgery, the FP will play a key role, monitoring the patient’s medications, nutrition, physical activity, chronic conditions, and overall quality of life.
Adopt a team approach
Encourage your office staff to review the Surgeon General’s Vision for a Healthy and Fit Nation 2010 (www.surgeongeneral.gov/library/obesityvision/obesityvision2010.pdf), which focuses on promoting, modeling, and working with patients to achieve a healthy lifestyle. The Surgeon General advocates a team approach to weight management, and recommends that patients have referrals to dietitians, psychologists, and community services.17,50
Interventions that include not only lifestyle modifications, but also behavioral modifications, such as hypnosis, can also be helpful. Computer-based strategies, such as Internet-based weight management programs and automated messaging, may be useful, as well, to break down barriers resulting from factors such as cost, time constraints, and lack of transportation or child care.51 Even when such programs are in use, however, it is important to remember that patients value—and benefit from—the support of their primary care physician.
Acknowledgement
This work has been presented in various Continuing Medical Education programs to audiences in North Carolina.
CORRESPONDENCE Kathryn M. Kolasa, PhD, RD, LDN, Department of Family Medicine, Mailstop 626, Brody School of Medicine at East Carolina University, Greenville, NC 27834; [email protected]
1. Manson JE, Skerrett PJ, Greenland P, et al. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch Intern Med. 2004;164:249-258.
2. Murray J. What others take for granted. Am Fam Physician. 2008;77:1677.-
3. Martens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8:270-278.
4. Hill JO, Wyatt H, Phelan S, et al. The National Weight Control Registry: is it useful in helping deal with our obesity epidemic? J Nutr Educ Behav. 2005;37:206-210.
5. Calfas KJ, Sallis JF, Zabinski MF, et al. Preliminary evaluation of a multicomponent program for nutrition and physical activity change in primary care: PACE+ for adults. Prev Med. 2002;34:153-161.
6. Ockene IS, Herbert JR, Ockene JK, et al. Effect of physician delivered nutrition counseling training and an office support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population (WATCH). Arch Intern Med. 1999;159:725-731.
7. Simkin-Silverman LR, Gleason KA, King WC, et al. Predictors of weight control advice in primary care practices: patient health and psychosocial characteristics. Prev Med. 2005;40:71-82.
8. Flocke SA, Clark A, Schlessman K, et al. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med. 2005;37:415-421.
9. Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170:146-154.
10. National Committee for Quality Assurance. Obesity screening measures highlight additions to HEDIS 2009. February 14, 2008. Available at: http://www.ncqa.org/tabid/662/Default.aspx. Accessed June 7, 2010.
11. Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood). 2009;28:w822-w831.
12. Jay M, Kalet A, Ark T, et al. Physicians’ attitudes about obesity and their associations with competency and specialty: a cross-sectional study. BMC Health Serv Res. 2009;9:106.-
13. STOP Obesity Alliance. STOP Obesity Alliance surveys show doctors, patients share role in weight loss, but ask, now what? March 16, 2010. Available at: http://www.eurekalert.org/pub_releases/2010-03/cca-soa031510.php. Accessed June 7, 2010.
14. Ferguson C, Langwith C, Muldoon A, et al. STOP Obesity Alliance Research Team, George Washington University School of Public Health and Health Services. Improving obesity management in adult primary care. White paper. March 16, 2010.
15. National Institutes of Health, National Heart, Lung and Blood Institute. North American Association for the Study of Obesity. The Practical Guide: Identification, Evaluation , and Treatment of Overweight and Obesity in Adults. 2000. NIH Publication No. 00-4084.
16. US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930-932.
17. American Dietetic Association. Adult weight management evidence based nutrition practice guidelines. Available at: http://www.adaevidencelibrary.com. Accessed September 9, 2009.
18. Snow V, Barry P, Fitterman N, et al. Clinical efficacy assessment subcommittee of the American College of Physicians. Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;142:525-531.
19. Truesdale KP, Stevens J. Do the obese know they are obese? N C Med J. 2008;69:188-194.
20. Agency for Healthcare Research and Quality. Guide to clinical preventive services, 2009. Available at: http://www.ahrq.gov/clinic/pocketgd09/. Accessed March 30, 2010.
21. Bardia A, Holtan SG, Slezak JM, et al. Diagnosis of obesity by primary care physicians and impact on obesity management. Mayo Clin Proc. 2007;82:927-932.
22. Kennen EM, Davis TC, Huang J, et al. Tipping the scales: the effect of literacy on obese patients’ knowledge and readiness to lose weight. South Med J. 2005;98:15-18.
23. Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. J Nutr Educ Behav. 2006;38:286-292.
24. Dansinger ML, Tatsioni A, Wong JB, et al. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41-50.
25. Atkins, Zone, Ornish, or LEARN—which diet kept weight off? J Fam Pract 2007;56:434.-
26. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859-873.
27. Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139-1148.
28. Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007;(3):CD005105.-
29. Centers for Disease Control and Prevention. Obesity and overweight for professionals: Resources/DNPAO/CDC. Updated 2009. Available at: http://www.cdc.gov/obesity/resources.html#R2P. Accessed December 10, 2009.
30. Rowland K, Coffey J. Are overweight children more likely to be overweight adults? J Fam Pract. 2009;58:431-432.
31. Eat Smart, Move More. North Carolina. Prescription for health. Available at: PrescriptionPadColor.pdf. Accessed June 11, 2010.
32. Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118-126.
33. Burke LE, Swigart V, Turk MW, et al. Experiences of self-monitoring: successes and struggles during treatment for weight loss. Qual Health Res. 2009;19:815.-
34. Cudjoe S, Moss S, Nguyen L, et al. Clinical inquiries. How do exercise and diet compare for weight loss? J Fam Pract. 2007;56:841-844.
35. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16:643-653.
36. Early JL, Apovian CM, Aronne LJ, et al. Sibutramine plus meal replacement therapy for body weight loss and maintenance in obese patients. Obesity (Silver Spring). 2007;15:1464-1472.
37. Ashley JM, St Jeor ST, Schrage JP, et al. Weight control in the physician’s office. Arch Intern Med. 2001;161:1599-1604.
38. Bowerman S, Bellman M, Saltsman P, et al. Implementation of a primary care physician network obesity management program. Obes Res. 2001;9(suppl 4):321S-325S.
39. Kuehn B. Tainted diet drugs. JAMA. 2009;301:817.-
40. Jull AB, Ni Mhurchu C, Bennett DA, et al. Chitosan for overweight or obesity. Cochrane Database Syst Rev. 2008;(3):CD003892.-
41. Teegarden D, Gunther CW. Can the controversial relationship between dietary calcium and body weight be mechanistically explained by alterations in appetite and food intake? Nutr Rev. 2008;66:601-605.
42. Greenway R, Heber D. Herbal and alternative approaches to obesity. In: Bray GA, Bouchard C, eds. Handbook of Obesity: Clinical Applications. 3rd ed. New York: Informa Healthcare; 2008: 425-443.
43. Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;144:532-546.
44. Neovius M, Johansson K, Rossner S. Head-to-head studies evaluating efficacy of pharmacotherapy for obesity: a systematic review and meta-analysis. Obes Rev. 2008;9:420-427.
45. Siebenhofer A, Korvath K, Jeitler K, et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database Syst Rev. 2009;(3):CD007654.-
46. Miller LJ. Management of atypical antipsychotic drug-induced weight gain: focus on metformin. Pharmacotherapy. 2009;29:725-735.
47. Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009;(2):CD003641.-
48. Blackburn GL, Hutter MM, Harvey AM, et al. Expert panel on weight loss surgery: executive report update. Obesity (Silver Spring). 2009;17:842-862.
49. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14(suppl 1):S1-S83.
50. Helman T. Weight management by family physicians. Arbor Clin Nutrition Updates. 2002 Feb;116:1-2. Available at: www.arborcom.com/free/116.pdf. Accessed June 16, 2010.
51. Holt J, Warren L, Wallace R, et al. Clinical inquiries. What behavioral interventions are safe and effective for treating obesity? J Fam Pract. 2006;55:536-538.
52. Cheskin LJ, Bartlett SJ, Zayas R, et al. Prescription medications: a modifiable contributor to obesity. South Med J. 1999;92:898-904.
53. Parsons B, Allison DB, Loebel A, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103-110.
• Calculate body mass index and diagnose obesity to increase the likelihood that obese patients will take steps to lose weight. B
• Prescribe a low-calorie diet for at least 6 months to help patients achieve a weight loss of at least 5% to 10%; prescribe physical activity for weight loss and weight maintenance. A
• Review the benefits and risks of bariatric surgery with patients who are severely obese, and provide a referral, when appropriate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s not only obese patients who are resistant to weight loss strategies. Many physicians contribute to the gap between current practice and optimal management of adult obesity, as well. There are a number of reasons for this—a dearth of knowledge, time, and reimbursement among them.1,2
What’s more, physicians are often pessimistic about how much headway patients can make in their weight loss efforts. That’s not surprising, given that the average weight loss achieved in well-controlled clinical trials tends to be modest and the recidivism rate is extremely high.3 Yet these same trials are cause for optimism, with substantial subsets of patients often achieving clinically meaningful long-term weight loss. The National Weight Control Registry, a long-term prospective study of “successful losers,” is another hopeful indicator: The registry includes approximately 6000 individuals who have lost, on average, more than 70 lb, and kept it off for an average of 6 years.4 (Listen to the audiocast at jfponline.com to find out how.)
Weight loss does not have to be huge to be clinically significant. Even a modest loss (5%-10% of total body weight) can have major health benefits. There’s much you can do to help.
Evidence suggests that patients are considerably more likely to lose weight when they are advised to do so and supported by their primary care physician.5-9 Because there is no way to predict which approach will be most effective for which patient, family physicians (FPs) should offer a variety of evidence-based treatments, including dietary change, increased physical activity, medication for selected patients, and surgery for severely obese adults (TABLE 1).
In 2009, the National Committee on Quality Assurance added body mass index (BMI) to the list of effectiveness-of-care measures that health plans and physicians are rated on.10 That addition, coupled with a recent study indicating that obesity accounts for more than 9% of annual health care expenditures,11 highlights the growing recognition that obesity should be considered a medical condition—not just a risk factor. While patients are increasingly likely to have a weight management insurance benefit that reimburses physicians and dietitians for multiple visits, many health plans still do not cover weight-related treatment. FPs can help by advocating for such coverage—and by taking steps to help patients win the battle against obesity.
TABLE 1
BMI, health risks, and weight loss: Which intervention for which patients?15
| Disease risk | Intervention | ||||
|---|---|---|---|---|---|
| BMI, kg/m2 (weight status) | Normal waist measurement | Elevated waist measurement | Diet and physical activity | Medication | Surgery |
| 25-29.9 (overweight) | Increased | High | 25-26.9: Yes, for patients with comorbidities 27-29.9: Yes | 25-26.9: NA 27-29.9: Yes, for patients with comorbidities | NA |
| 30-39.9 (obese) | 30-34.9: High 35-39.9: Very high | Very high | Yes | Yes | 30-34.9: Yes, for patients with comorbidities 35-39.9: Yes |
| ≥40 (extremely obese) | Extremely high | Extremely high | Yes | Yes | Yes |
| BMI, body mass index; NA, not appropriate. | |||||
2 weight loss tools that can jump-start your efforts
Physicians often cite time as a key reason for not providing weight loss counseling. But physician knowledge—actually, lack of knowledge—may be a bigger barrier. In 1 recent study, 44% of physicians said they did not feel qualified to treat obesity.12 In another, 72% of primary care physicians surveyed said that no one in their practice was trained to deal with weight-related issues.13
As the focus on obesity grows, clinical weight management tools are increasingly available. Two excellent examples are the California Medical Association Foundation’s Obesity Provider Toolkit (www.thecmafoundation.org/projects/obesityProject.aspx) and the clinical tools from North Carolina’s Eat Smart, Move More program (www.eatsmartmovemorenc.com/ESMMPlan/ESMMPlan.html). You can download a toolkit, review the strategies described, and adopt those you think would be most effective in your practice. Doing so needn’t be especially time-consuming; evidence suggests you can provide basic counseling about healthy behaviors in fewer than 5 minutes.14
Review guidelines. The National Heart, Lung, and Blood Institute issued the first evidence-based guidelines for the treatment of adult obesity in 1998.15 Many other groups—the US Preventive Services Task Force (USPSTF),16 the American Dietetic Association,17 and the American College of Physicians,18 among them—have followed suit, with guidelines addressing practical weight loss interventions and treatment related to specific comorbidities. The organizations all recommend using BMI to diagnose and classify obesity, assessing readiness to change, and setting realistic goals (TABLE 2).
TABLE 2
Obesity: Key components of evaluation and treatment
| Assess |
|
| Advise |
|
| Agree |
|
| Assist |
|
| Arrange |
|
| BMI, body mass index. | |
Diagnose obesity without delay
While most patients can report their height and weight with reasonable accuracy, few obese adults consider themselves to be obese.19 And, although the USPSTF 2009 recommendations call for screening all adults for obesity and introducing behavioral interventions to promote sustained weight loss as needed,20 most obese men and women receive neither a diagnosis nor an obesity management plan.21 Yet both are key components of long-term weight control. Physicians should calculate and document the BMI of all adult patients, and routinely diagnose obesity in patients with a BMI ≥30 kg/m2 (TABLE 1).
Get the patient’s perspective
In addition to using BMI and waist circumference measures and identifying comorbidities such as diabetes and hypertension, it is important to determine whether the patient is motivated to change. You can start by asking whether he or she has previously been told to lose weight; adults who have received weight control counseling in the past are more likely to be in a greater state of readiness.7
Review the patient’s medications. Once you have identified obesity, look for iatrogenic causes—most notably, current use of 1 or more medications that are associated with weight gain or known to affect satiety. If you identify any such drugs, it may be possible to find a suitable alternative (TABLE 3).
Factor in literacy. Patients with low literacy are less likely to fully comprehend the health benefits of weight loss or to report that they are ready to lose weight, as compared with those with higher literacy levels.22 Talking to such patients to determine what will spark their interest and motivation—ie, looking and feeling better, being able to play with children or grandchildren—can help.
Assess the patient’s dietary patterns. This can be done with the Rapid Eating Assessment for Participants (REAP), an office-based tool of adequate reliability and validity for nutrition assessment and counseling that can be administered in a few minutes.23
This office-based survey, available at http://bms.brown.edu/nutrition/acrobat/REAP%206.pdf, provides information that can be the basis for an action plan. While there is no direct evidence that having a plan leads to weight loss, receiving advice from a physician is strongly associated with efforts to lose weight.6 Be aware that obese individuals may not be as responsive to weight management counseling from a physician as those who are overweight, so they may require more assistance.21
TABLE 3
Drugs that promote weight gain—and alternatives to consider46,52,53
| Indication/drug class/medication | Alternatives* |
|---|---|
Anticonvulsants/psychotropics Anticonvulsants | Topiramate‡ |
| Antimanic (bipolar) Lithium | Valproic acid,† carbamazepine† |
Antipsychotics Typical: Chlorpromazine, thiothixene, haloperidol | Ziprasidone
|
Antidepressants Tricyclics: Amitriptyline, imipramine |
Nortriptyline, protriptyline, desipramine |
Antidiabetics Sulfonylureas | Gliclazide, metformin, acarbose |
Antihypertensives Beta-blockers Calcium channel blockers | Guanfacine, doxazosin Acebutolol, atenolol, betaxolol, bisoprolol, labetalol, metoprolol, nadolol, pindolol Amlodipine, diltiazem, felodipine, nicardipine, nifedipine, verapamil |
Anti-inflammatories Corticosteroids | NSAIDs, COX inhibitors |
| COX, cyclooxygenase; MAOI, monoamine oxidase inhibitor; NSAIDs, nonsteroidal anti-inflammatory drugs. | |
| * Table is not meant to imply equal efficacy for all choices for a given indication. | |
| † Valproic acid and carbamazepine are associated with weight gain, but less than that associated with lithium. | |
| ‡ Topiramate may not be adequate as a single agent. | |
Set a goal, prescribe a food plan
After discussing possible interventions based on the patient’s BMI and weight status (TABLE 1), set a safe and achievable goal to reduce body weight at a rate of 1 to 2 lb per week for 6 months to achieve an initial weight loss goal of up to 10%. Not only does such a target have proven health benefits,15,24 but defining success in realistic and achievable terms helps maintain patient motivation.
Although no single dietary approach has been found to have a metabolic advantage or be most likely to support long-term weight maintenance,15,25-28 energy balance is central. It is critical to induce a negative energy balance—with a deficit of 250 to 500 calories per day to achieve a weight loss of 0.5 to 2 lb per week.
Which diet is best?
Diets emphasizing varying contents of carbohydrate, fat, and protein have generally been found to be equally successful in promoting clinically meaningful weight loss and maintenance over the course of 2 years, although some studies have found specific approaches to encourage initial weight loss.26 Because different strategies have proven helpful to various patients, your best bet is to analyze the patient’s eating pattern and prescribe the diet he or she is most likely to adhere to for at least 6 months.
If the patient has comorbidities, choose the diet expected to have the greatest impact—a low-glycemic diet for an obese patient with diabetes, for example, and the Dietary Approaches to Stop Hypertension (DASH) food plan for a patient with hypertension.27 For premenopausal women, diets low in carbohydrates have been found to facilitate weight loss without negative health consequences.25
Offer specific—and actionable—strategies
Most clinically obese patients (63%, according to 1 study) do not receive weight loss counseling from their primary care physician. Often, they are told to lose weight, but not given advice on how to do so. Providing specific strategies can help patients stay motivated.
One set of specific weight-loss messages comes from the Centers for Disease Control and Prevention (CDC)’s Research to Practice Series.29 Patients are advised to:
- limit eating away from home
- select healthy options when eating out
- eat more fruits and vegetables
- avoid large portion sizes
- eat low energy-dense foods (ie, foods that are high in micronutrients but low in calories per gram).
The CDC also encourages new moms to breastfeed. In addition to helping the women themselves control their weight, breastfed infants appear to be more likely to maintain a normal body weight—an important consideration as overweight children are more likely than children of normal weight to become overweight adults.30 (To learn more about pediatric obesity, see “How best to help kids lose weight”.)
The “5-3-2-1-almost none” plan offers additional advice. Every day, patients should:
- Eat ≥5 servings of fruits and vegetables
- Have 3 structured meals (including breakfast)
- Limit TV/video game use to ≤2 hours
- Engage in ≥1 hour of moderate to vigorous physical activity
- Limit sugar-sweetened drinks to almost none.31
Encourage patients to keep a diary. Suggesting that patients track their food and beverage intake, as well as their physical activity, is another helpful strategy, as self-monitoring creates a sense of accountability and awareness. Patients are likely to need ongoing encouragement to do so, however, because record keeping typically declines with time.32,33
Prescribe physical activity, and sleep
Physical activity guidelines vary for active adults, older adults, and those with disabilities. To attain health benefits, however, the general recommendation is for ≥150 minutes of moderate-intensity physical activity per week, plus muscle strengthening activities at least twice a week.33,34 Help patients identify strategies that will improve adherence, such as wearing a pedometer to gauge miles walked per day or working out with a buddy.34
Sleep duration, too, may affect weight. Although there is insufficient evidence to support the idea that sleep is an independent risk factor for obesity,35 it appears that those who sleep too much (9-10 hours per night) or too little (5-6 hours) have a 3- to 5-lb weight gain compared with those who sleep for 8 hours. One possible explanation is that there is a disruption in the production of hormones that affects appetite.4 Advise patients that getting enough sleep can help them control their weight.
Consider meal replacement, pharmacotherapy
For select patients, adherence to a low-calorie diet can be facilitated by the use of meal replacement products, as well as by pharmacotherapy. Each approach yields about a 5% to 7% weight loss.36 High-protein, high-fiber calorie-controlled shakes or bars can be particularly helpful for patients who have difficulty with food selection or portion control and can be effectively monitored by a physician or dietitian.37,38 Dietary fiber is also helpful in decreasing food intake and hunger.
Discuss the risks of supplements. Patients are bombarded by advertisements for dietary supplements. You can help by initiating a discussion of the lack of evidence of the efficacy and/or safety of most such products.
The most widely used herbal supplement, ephedrine, was taken off the market in 2006 for safety reasons. The US Food and Drug Administration recommends avoiding many over-the-counter dietary products because a significant number of them have been found to contain undeclared active prescription ingredients.39
In general, published trials of dietary supplements are of suboptimal quality. There is limited evidence that caffeine may have a positive effect on thermogenesis and fat oxidation.4 A review of 1 promising dietary supplement, chitosan, found that the effect was minimal and unlikely to be of clinical significance.40 The evidence is weak for meaningful changes in weight or body composition for green tea catechins, conjugated linoleic acid, and chromium picolinate. Dietary calcium appears to aid in weight management, but the magnitude is controversial.41 Preliminary data about amino acids and neurotransmitter modulation are promising, but too little is currently known about these approaches.42 Fiber supplements, unlike most dietary supplements, are recommended, as dietary fiber decreases food intake and hunger.
Pharmacotherapy may boost weight loss. Two types of weight loss drugs—a lipase inhibitor (orlistat [Xenical]) and an appetite suppressant (sibutramine [Meridia])—are on the market. Research shows that pharmacotherapy, when combined with diet or physical activity, may enhance weight loss (usually <11 lb/year) in some adults, although the optimal duration of drug use has not been determined.16,17,43,44 The choice of medication should be based on the expected response to the medication. There is no evidence that either medication promotes more sustained weight loss than the other. In clinical trials, however, sibutramine produced a weight loss of 4.9 lb more than orlistat.44
While patients with hypertension have been found to achieve modest weight loss using either agent, orlistat reduced both diastolic and systolic pressure, while diastolic blood pressure increased with sibutramine.45 Metformin may help prevent excess weight gain associated with short-term use of atypical antipsychotics, although its use for this purpose is off-label.46
When to consider bariatric surgery
Bariatric surgery continues to provide greater sustained weight loss and metabolic improvements than other conventional treatments.47 Identify patients who have failed at comprehensive weight loss programs and who are at high risk for obesity-associated morbidity and mortality (TABLE 1), and discuss the benefits (eg, reduction in comorbidities and at least a short-term improvement in health-related quality of life) and risks (eg, pulmonary embolism, anastomosis leakage, procedure-specific problems such as band slippage and erosion [after gastric banding], and possibly even death) of bariatric surgery. Refer potential candidates to an appropriate surgeon and facility.
Be aware, however, that the field of bariatric surgery is rapidly changing in terms of types of procedures, standards for perioperative care, patient selection, and reimbursement policies,48,49 and patients need to check with their insurance company before making any treatment decisions.
Common bariatric procedures include Roux-en Y gastric bypass (the gold standard); adjustable gastric banding; biliopancreatic diversion; and sleeve gastrectomy. Most are done laparoscopically. The sleeve gastrectomy, in which a vertical sleeve is created while much of the stomach is removed, may be useful for patients who are high-volume eaters, or to prepare extremely obese patients for gastric bypass. Patient selection is important, and multidisciplinary care is generally considered essential. After the surgery, the FP will play a key role, monitoring the patient’s medications, nutrition, physical activity, chronic conditions, and overall quality of life.
Adopt a team approach
Encourage your office staff to review the Surgeon General’s Vision for a Healthy and Fit Nation 2010 (www.surgeongeneral.gov/library/obesityvision/obesityvision2010.pdf), which focuses on promoting, modeling, and working with patients to achieve a healthy lifestyle. The Surgeon General advocates a team approach to weight management, and recommends that patients have referrals to dietitians, psychologists, and community services.17,50
Interventions that include not only lifestyle modifications, but also behavioral modifications, such as hypnosis, can also be helpful. Computer-based strategies, such as Internet-based weight management programs and automated messaging, may be useful, as well, to break down barriers resulting from factors such as cost, time constraints, and lack of transportation or child care.51 Even when such programs are in use, however, it is important to remember that patients value—and benefit from—the support of their primary care physician.
Acknowledgement
This work has been presented in various Continuing Medical Education programs to audiences in North Carolina.
CORRESPONDENCE Kathryn M. Kolasa, PhD, RD, LDN, Department of Family Medicine, Mailstop 626, Brody School of Medicine at East Carolina University, Greenville, NC 27834; [email protected]
• Calculate body mass index and diagnose obesity to increase the likelihood that obese patients will take steps to lose weight. B
• Prescribe a low-calorie diet for at least 6 months to help patients achieve a weight loss of at least 5% to 10%; prescribe physical activity for weight loss and weight maintenance. A
• Review the benefits and risks of bariatric surgery with patients who are severely obese, and provide a referral, when appropriate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s not only obese patients who are resistant to weight loss strategies. Many physicians contribute to the gap between current practice and optimal management of adult obesity, as well. There are a number of reasons for this—a dearth of knowledge, time, and reimbursement among them.1,2
What’s more, physicians are often pessimistic about how much headway patients can make in their weight loss efforts. That’s not surprising, given that the average weight loss achieved in well-controlled clinical trials tends to be modest and the recidivism rate is extremely high.3 Yet these same trials are cause for optimism, with substantial subsets of patients often achieving clinically meaningful long-term weight loss. The National Weight Control Registry, a long-term prospective study of “successful losers,” is another hopeful indicator: The registry includes approximately 6000 individuals who have lost, on average, more than 70 lb, and kept it off for an average of 6 years.4 (Listen to the audiocast at jfponline.com to find out how.)
Weight loss does not have to be huge to be clinically significant. Even a modest loss (5%-10% of total body weight) can have major health benefits. There’s much you can do to help.
Evidence suggests that patients are considerably more likely to lose weight when they are advised to do so and supported by their primary care physician.5-9 Because there is no way to predict which approach will be most effective for which patient, family physicians (FPs) should offer a variety of evidence-based treatments, including dietary change, increased physical activity, medication for selected patients, and surgery for severely obese adults (TABLE 1).
In 2009, the National Committee on Quality Assurance added body mass index (BMI) to the list of effectiveness-of-care measures that health plans and physicians are rated on.10 That addition, coupled with a recent study indicating that obesity accounts for more than 9% of annual health care expenditures,11 highlights the growing recognition that obesity should be considered a medical condition—not just a risk factor. While patients are increasingly likely to have a weight management insurance benefit that reimburses physicians and dietitians for multiple visits, many health plans still do not cover weight-related treatment. FPs can help by advocating for such coverage—and by taking steps to help patients win the battle against obesity.
TABLE 1
BMI, health risks, and weight loss: Which intervention for which patients?15
| Disease risk | Intervention | ||||
|---|---|---|---|---|---|
| BMI, kg/m2 (weight status) | Normal waist measurement | Elevated waist measurement | Diet and physical activity | Medication | Surgery |
| 25-29.9 (overweight) | Increased | High | 25-26.9: Yes, for patients with comorbidities 27-29.9: Yes | 25-26.9: NA 27-29.9: Yes, for patients with comorbidities | NA |
| 30-39.9 (obese) | 30-34.9: High 35-39.9: Very high | Very high | Yes | Yes | 30-34.9: Yes, for patients with comorbidities 35-39.9: Yes |
| ≥40 (extremely obese) | Extremely high | Extremely high | Yes | Yes | Yes |
| BMI, body mass index; NA, not appropriate. | |||||
2 weight loss tools that can jump-start your efforts
Physicians often cite time as a key reason for not providing weight loss counseling. But physician knowledge—actually, lack of knowledge—may be a bigger barrier. In 1 recent study, 44% of physicians said they did not feel qualified to treat obesity.12 In another, 72% of primary care physicians surveyed said that no one in their practice was trained to deal with weight-related issues.13
As the focus on obesity grows, clinical weight management tools are increasingly available. Two excellent examples are the California Medical Association Foundation’s Obesity Provider Toolkit (www.thecmafoundation.org/projects/obesityProject.aspx) and the clinical tools from North Carolina’s Eat Smart, Move More program (www.eatsmartmovemorenc.com/ESMMPlan/ESMMPlan.html). You can download a toolkit, review the strategies described, and adopt those you think would be most effective in your practice. Doing so needn’t be especially time-consuming; evidence suggests you can provide basic counseling about healthy behaviors in fewer than 5 minutes.14
Review guidelines. The National Heart, Lung, and Blood Institute issued the first evidence-based guidelines for the treatment of adult obesity in 1998.15 Many other groups—the US Preventive Services Task Force (USPSTF),16 the American Dietetic Association,17 and the American College of Physicians,18 among them—have followed suit, with guidelines addressing practical weight loss interventions and treatment related to specific comorbidities. The organizations all recommend using BMI to diagnose and classify obesity, assessing readiness to change, and setting realistic goals (TABLE 2).
TABLE 2
Obesity: Key components of evaluation and treatment
| Assess |
|
| Advise |
|
| Agree |
|
| Assist |
|
| Arrange |
|
| BMI, body mass index. | |
Diagnose obesity without delay
While most patients can report their height and weight with reasonable accuracy, few obese adults consider themselves to be obese.19 And, although the USPSTF 2009 recommendations call for screening all adults for obesity and introducing behavioral interventions to promote sustained weight loss as needed,20 most obese men and women receive neither a diagnosis nor an obesity management plan.21 Yet both are key components of long-term weight control. Physicians should calculate and document the BMI of all adult patients, and routinely diagnose obesity in patients with a BMI ≥30 kg/m2 (TABLE 1).
Get the patient’s perspective
In addition to using BMI and waist circumference measures and identifying comorbidities such as diabetes and hypertension, it is important to determine whether the patient is motivated to change. You can start by asking whether he or she has previously been told to lose weight; adults who have received weight control counseling in the past are more likely to be in a greater state of readiness.7
Review the patient’s medications. Once you have identified obesity, look for iatrogenic causes—most notably, current use of 1 or more medications that are associated with weight gain or known to affect satiety. If you identify any such drugs, it may be possible to find a suitable alternative (TABLE 3).
Factor in literacy. Patients with low literacy are less likely to fully comprehend the health benefits of weight loss or to report that they are ready to lose weight, as compared with those with higher literacy levels.22 Talking to such patients to determine what will spark their interest and motivation—ie, looking and feeling better, being able to play with children or grandchildren—can help.
Assess the patient’s dietary patterns. This can be done with the Rapid Eating Assessment for Participants (REAP), an office-based tool of adequate reliability and validity for nutrition assessment and counseling that can be administered in a few minutes.23
This office-based survey, available at http://bms.brown.edu/nutrition/acrobat/REAP%206.pdf, provides information that can be the basis for an action plan. While there is no direct evidence that having a plan leads to weight loss, receiving advice from a physician is strongly associated with efforts to lose weight.6 Be aware that obese individuals may not be as responsive to weight management counseling from a physician as those who are overweight, so they may require more assistance.21
TABLE 3
Drugs that promote weight gain—and alternatives to consider46,52,53
| Indication/drug class/medication | Alternatives* |
|---|---|
Anticonvulsants/psychotropics Anticonvulsants | Topiramate‡ |
| Antimanic (bipolar) Lithium | Valproic acid,† carbamazepine† |
Antipsychotics Typical: Chlorpromazine, thiothixene, haloperidol | Ziprasidone
|
Antidepressants Tricyclics: Amitriptyline, imipramine |
Nortriptyline, protriptyline, desipramine |
Antidiabetics Sulfonylureas | Gliclazide, metformin, acarbose |
Antihypertensives Beta-blockers Calcium channel blockers | Guanfacine, doxazosin Acebutolol, atenolol, betaxolol, bisoprolol, labetalol, metoprolol, nadolol, pindolol Amlodipine, diltiazem, felodipine, nicardipine, nifedipine, verapamil |
Anti-inflammatories Corticosteroids | NSAIDs, COX inhibitors |
| COX, cyclooxygenase; MAOI, monoamine oxidase inhibitor; NSAIDs, nonsteroidal anti-inflammatory drugs. | |
| * Table is not meant to imply equal efficacy for all choices for a given indication. | |
| † Valproic acid and carbamazepine are associated with weight gain, but less than that associated with lithium. | |
| ‡ Topiramate may not be adequate as a single agent. | |
Set a goal, prescribe a food plan
After discussing possible interventions based on the patient’s BMI and weight status (TABLE 1), set a safe and achievable goal to reduce body weight at a rate of 1 to 2 lb per week for 6 months to achieve an initial weight loss goal of up to 10%. Not only does such a target have proven health benefits,15,24 but defining success in realistic and achievable terms helps maintain patient motivation.
Although no single dietary approach has been found to have a metabolic advantage or be most likely to support long-term weight maintenance,15,25-28 energy balance is central. It is critical to induce a negative energy balance—with a deficit of 250 to 500 calories per day to achieve a weight loss of 0.5 to 2 lb per week.
Which diet is best?
Diets emphasizing varying contents of carbohydrate, fat, and protein have generally been found to be equally successful in promoting clinically meaningful weight loss and maintenance over the course of 2 years, although some studies have found specific approaches to encourage initial weight loss.26 Because different strategies have proven helpful to various patients, your best bet is to analyze the patient’s eating pattern and prescribe the diet he or she is most likely to adhere to for at least 6 months.
If the patient has comorbidities, choose the diet expected to have the greatest impact—a low-glycemic diet for an obese patient with diabetes, for example, and the Dietary Approaches to Stop Hypertension (DASH) food plan for a patient with hypertension.27 For premenopausal women, diets low in carbohydrates have been found to facilitate weight loss without negative health consequences.25
Offer specific—and actionable—strategies
Most clinically obese patients (63%, according to 1 study) do not receive weight loss counseling from their primary care physician. Often, they are told to lose weight, but not given advice on how to do so. Providing specific strategies can help patients stay motivated.
One set of specific weight-loss messages comes from the Centers for Disease Control and Prevention (CDC)’s Research to Practice Series.29 Patients are advised to:
- limit eating away from home
- select healthy options when eating out
- eat more fruits and vegetables
- avoid large portion sizes
- eat low energy-dense foods (ie, foods that are high in micronutrients but low in calories per gram).
The CDC also encourages new moms to breastfeed. In addition to helping the women themselves control their weight, breastfed infants appear to be more likely to maintain a normal body weight—an important consideration as overweight children are more likely than children of normal weight to become overweight adults.30 (To learn more about pediatric obesity, see “How best to help kids lose weight”.)
The “5-3-2-1-almost none” plan offers additional advice. Every day, patients should:
- Eat ≥5 servings of fruits and vegetables
- Have 3 structured meals (including breakfast)
- Limit TV/video game use to ≤2 hours
- Engage in ≥1 hour of moderate to vigorous physical activity
- Limit sugar-sweetened drinks to almost none.31
Encourage patients to keep a diary. Suggesting that patients track their food and beverage intake, as well as their physical activity, is another helpful strategy, as self-monitoring creates a sense of accountability and awareness. Patients are likely to need ongoing encouragement to do so, however, because record keeping typically declines with time.32,33
Prescribe physical activity, and sleep
Physical activity guidelines vary for active adults, older adults, and those with disabilities. To attain health benefits, however, the general recommendation is for ≥150 minutes of moderate-intensity physical activity per week, plus muscle strengthening activities at least twice a week.33,34 Help patients identify strategies that will improve adherence, such as wearing a pedometer to gauge miles walked per day or working out with a buddy.34
Sleep duration, too, may affect weight. Although there is insufficient evidence to support the idea that sleep is an independent risk factor for obesity,35 it appears that those who sleep too much (9-10 hours per night) or too little (5-6 hours) have a 3- to 5-lb weight gain compared with those who sleep for 8 hours. One possible explanation is that there is a disruption in the production of hormones that affects appetite.4 Advise patients that getting enough sleep can help them control their weight.
Consider meal replacement, pharmacotherapy
For select patients, adherence to a low-calorie diet can be facilitated by the use of meal replacement products, as well as by pharmacotherapy. Each approach yields about a 5% to 7% weight loss.36 High-protein, high-fiber calorie-controlled shakes or bars can be particularly helpful for patients who have difficulty with food selection or portion control and can be effectively monitored by a physician or dietitian.37,38 Dietary fiber is also helpful in decreasing food intake and hunger.
Discuss the risks of supplements. Patients are bombarded by advertisements for dietary supplements. You can help by initiating a discussion of the lack of evidence of the efficacy and/or safety of most such products.
The most widely used herbal supplement, ephedrine, was taken off the market in 2006 for safety reasons. The US Food and Drug Administration recommends avoiding many over-the-counter dietary products because a significant number of them have been found to contain undeclared active prescription ingredients.39
In general, published trials of dietary supplements are of suboptimal quality. There is limited evidence that caffeine may have a positive effect on thermogenesis and fat oxidation.4 A review of 1 promising dietary supplement, chitosan, found that the effect was minimal and unlikely to be of clinical significance.40 The evidence is weak for meaningful changes in weight or body composition for green tea catechins, conjugated linoleic acid, and chromium picolinate. Dietary calcium appears to aid in weight management, but the magnitude is controversial.41 Preliminary data about amino acids and neurotransmitter modulation are promising, but too little is currently known about these approaches.42 Fiber supplements, unlike most dietary supplements, are recommended, as dietary fiber decreases food intake and hunger.
Pharmacotherapy may boost weight loss. Two types of weight loss drugs—a lipase inhibitor (orlistat [Xenical]) and an appetite suppressant (sibutramine [Meridia])—are on the market. Research shows that pharmacotherapy, when combined with diet or physical activity, may enhance weight loss (usually <11 lb/year) in some adults, although the optimal duration of drug use has not been determined.16,17,43,44 The choice of medication should be based on the expected response to the medication. There is no evidence that either medication promotes more sustained weight loss than the other. In clinical trials, however, sibutramine produced a weight loss of 4.9 lb more than orlistat.44
While patients with hypertension have been found to achieve modest weight loss using either agent, orlistat reduced both diastolic and systolic pressure, while diastolic blood pressure increased with sibutramine.45 Metformin may help prevent excess weight gain associated with short-term use of atypical antipsychotics, although its use for this purpose is off-label.46
When to consider bariatric surgery
Bariatric surgery continues to provide greater sustained weight loss and metabolic improvements than other conventional treatments.47 Identify patients who have failed at comprehensive weight loss programs and who are at high risk for obesity-associated morbidity and mortality (TABLE 1), and discuss the benefits (eg, reduction in comorbidities and at least a short-term improvement in health-related quality of life) and risks (eg, pulmonary embolism, anastomosis leakage, procedure-specific problems such as band slippage and erosion [after gastric banding], and possibly even death) of bariatric surgery. Refer potential candidates to an appropriate surgeon and facility.
Be aware, however, that the field of bariatric surgery is rapidly changing in terms of types of procedures, standards for perioperative care, patient selection, and reimbursement policies,48,49 and patients need to check with their insurance company before making any treatment decisions.
Common bariatric procedures include Roux-en Y gastric bypass (the gold standard); adjustable gastric banding; biliopancreatic diversion; and sleeve gastrectomy. Most are done laparoscopically. The sleeve gastrectomy, in which a vertical sleeve is created while much of the stomach is removed, may be useful for patients who are high-volume eaters, or to prepare extremely obese patients for gastric bypass. Patient selection is important, and multidisciplinary care is generally considered essential. After the surgery, the FP will play a key role, monitoring the patient’s medications, nutrition, physical activity, chronic conditions, and overall quality of life.
Adopt a team approach
Encourage your office staff to review the Surgeon General’s Vision for a Healthy and Fit Nation 2010 (www.surgeongeneral.gov/library/obesityvision/obesityvision2010.pdf), which focuses on promoting, modeling, and working with patients to achieve a healthy lifestyle. The Surgeon General advocates a team approach to weight management, and recommends that patients have referrals to dietitians, psychologists, and community services.17,50
Interventions that include not only lifestyle modifications, but also behavioral modifications, such as hypnosis, can also be helpful. Computer-based strategies, such as Internet-based weight management programs and automated messaging, may be useful, as well, to break down barriers resulting from factors such as cost, time constraints, and lack of transportation or child care.51 Even when such programs are in use, however, it is important to remember that patients value—and benefit from—the support of their primary care physician.
Acknowledgement
This work has been presented in various Continuing Medical Education programs to audiences in North Carolina.
CORRESPONDENCE Kathryn M. Kolasa, PhD, RD, LDN, Department of Family Medicine, Mailstop 626, Brody School of Medicine at East Carolina University, Greenville, NC 27834; [email protected]
1. Manson JE, Skerrett PJ, Greenland P, et al. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch Intern Med. 2004;164:249-258.
2. Murray J. What others take for granted. Am Fam Physician. 2008;77:1677.-
3. Martens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8:270-278.
4. Hill JO, Wyatt H, Phelan S, et al. The National Weight Control Registry: is it useful in helping deal with our obesity epidemic? J Nutr Educ Behav. 2005;37:206-210.
5. Calfas KJ, Sallis JF, Zabinski MF, et al. Preliminary evaluation of a multicomponent program for nutrition and physical activity change in primary care: PACE+ for adults. Prev Med. 2002;34:153-161.
6. Ockene IS, Herbert JR, Ockene JK, et al. Effect of physician delivered nutrition counseling training and an office support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population (WATCH). Arch Intern Med. 1999;159:725-731.
7. Simkin-Silverman LR, Gleason KA, King WC, et al. Predictors of weight control advice in primary care practices: patient health and psychosocial characteristics. Prev Med. 2005;40:71-82.
8. Flocke SA, Clark A, Schlessman K, et al. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med. 2005;37:415-421.
9. Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170:146-154.
10. National Committee for Quality Assurance. Obesity screening measures highlight additions to HEDIS 2009. February 14, 2008. Available at: http://www.ncqa.org/tabid/662/Default.aspx. Accessed June 7, 2010.
11. Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood). 2009;28:w822-w831.
12. Jay M, Kalet A, Ark T, et al. Physicians’ attitudes about obesity and their associations with competency and specialty: a cross-sectional study. BMC Health Serv Res. 2009;9:106.-
13. STOP Obesity Alliance. STOP Obesity Alliance surveys show doctors, patients share role in weight loss, but ask, now what? March 16, 2010. Available at: http://www.eurekalert.org/pub_releases/2010-03/cca-soa031510.php. Accessed June 7, 2010.
14. Ferguson C, Langwith C, Muldoon A, et al. STOP Obesity Alliance Research Team, George Washington University School of Public Health and Health Services. Improving obesity management in adult primary care. White paper. March 16, 2010.
15. National Institutes of Health, National Heart, Lung and Blood Institute. North American Association for the Study of Obesity. The Practical Guide: Identification, Evaluation , and Treatment of Overweight and Obesity in Adults. 2000. NIH Publication No. 00-4084.
16. US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930-932.
17. American Dietetic Association. Adult weight management evidence based nutrition practice guidelines. Available at: http://www.adaevidencelibrary.com. Accessed September 9, 2009.
18. Snow V, Barry P, Fitterman N, et al. Clinical efficacy assessment subcommittee of the American College of Physicians. Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;142:525-531.
19. Truesdale KP, Stevens J. Do the obese know they are obese? N C Med J. 2008;69:188-194.
20. Agency for Healthcare Research and Quality. Guide to clinical preventive services, 2009. Available at: http://www.ahrq.gov/clinic/pocketgd09/. Accessed March 30, 2010.
21. Bardia A, Holtan SG, Slezak JM, et al. Diagnosis of obesity by primary care physicians and impact on obesity management. Mayo Clin Proc. 2007;82:927-932.
22. Kennen EM, Davis TC, Huang J, et al. Tipping the scales: the effect of literacy on obese patients’ knowledge and readiness to lose weight. South Med J. 2005;98:15-18.
23. Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. J Nutr Educ Behav. 2006;38:286-292.
24. Dansinger ML, Tatsioni A, Wong JB, et al. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41-50.
25. Atkins, Zone, Ornish, or LEARN—which diet kept weight off? J Fam Pract 2007;56:434.-
26. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859-873.
27. Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139-1148.
28. Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007;(3):CD005105.-
29. Centers for Disease Control and Prevention. Obesity and overweight for professionals: Resources/DNPAO/CDC. Updated 2009. Available at: http://www.cdc.gov/obesity/resources.html#R2P. Accessed December 10, 2009.
30. Rowland K, Coffey J. Are overweight children more likely to be overweight adults? J Fam Pract. 2009;58:431-432.
31. Eat Smart, Move More. North Carolina. Prescription for health. Available at: PrescriptionPadColor.pdf. Accessed June 11, 2010.
32. Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118-126.
33. Burke LE, Swigart V, Turk MW, et al. Experiences of self-monitoring: successes and struggles during treatment for weight loss. Qual Health Res. 2009;19:815.-
34. Cudjoe S, Moss S, Nguyen L, et al. Clinical inquiries. How do exercise and diet compare for weight loss? J Fam Pract. 2007;56:841-844.
35. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16:643-653.
36. Early JL, Apovian CM, Aronne LJ, et al. Sibutramine plus meal replacement therapy for body weight loss and maintenance in obese patients. Obesity (Silver Spring). 2007;15:1464-1472.
37. Ashley JM, St Jeor ST, Schrage JP, et al. Weight control in the physician’s office. Arch Intern Med. 2001;161:1599-1604.
38. Bowerman S, Bellman M, Saltsman P, et al. Implementation of a primary care physician network obesity management program. Obes Res. 2001;9(suppl 4):321S-325S.
39. Kuehn B. Tainted diet drugs. JAMA. 2009;301:817.-
40. Jull AB, Ni Mhurchu C, Bennett DA, et al. Chitosan for overweight or obesity. Cochrane Database Syst Rev. 2008;(3):CD003892.-
41. Teegarden D, Gunther CW. Can the controversial relationship between dietary calcium and body weight be mechanistically explained by alterations in appetite and food intake? Nutr Rev. 2008;66:601-605.
42. Greenway R, Heber D. Herbal and alternative approaches to obesity. In: Bray GA, Bouchard C, eds. Handbook of Obesity: Clinical Applications. 3rd ed. New York: Informa Healthcare; 2008: 425-443.
43. Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;144:532-546.
44. Neovius M, Johansson K, Rossner S. Head-to-head studies evaluating efficacy of pharmacotherapy for obesity: a systematic review and meta-analysis. Obes Rev. 2008;9:420-427.
45. Siebenhofer A, Korvath K, Jeitler K, et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database Syst Rev. 2009;(3):CD007654.-
46. Miller LJ. Management of atypical antipsychotic drug-induced weight gain: focus on metformin. Pharmacotherapy. 2009;29:725-735.
47. Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009;(2):CD003641.-
48. Blackburn GL, Hutter MM, Harvey AM, et al. Expert panel on weight loss surgery: executive report update. Obesity (Silver Spring). 2009;17:842-862.
49. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14(suppl 1):S1-S83.
50. Helman T. Weight management by family physicians. Arbor Clin Nutrition Updates. 2002 Feb;116:1-2. Available at: www.arborcom.com/free/116.pdf. Accessed June 16, 2010.
51. Holt J, Warren L, Wallace R, et al. Clinical inquiries. What behavioral interventions are safe and effective for treating obesity? J Fam Pract. 2006;55:536-538.
52. Cheskin LJ, Bartlett SJ, Zayas R, et al. Prescription medications: a modifiable contributor to obesity. South Med J. 1999;92:898-904.
53. Parsons B, Allison DB, Loebel A, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103-110.
1. Manson JE, Skerrett PJ, Greenland P, et al. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch Intern Med. 2004;164:249-258.
2. Murray J. What others take for granted. Am Fam Physician. 2008;77:1677.-
3. Martens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8:270-278.
4. Hill JO, Wyatt H, Phelan S, et al. The National Weight Control Registry: is it useful in helping deal with our obesity epidemic? J Nutr Educ Behav. 2005;37:206-210.
5. Calfas KJ, Sallis JF, Zabinski MF, et al. Preliminary evaluation of a multicomponent program for nutrition and physical activity change in primary care: PACE+ for adults. Prev Med. 2002;34:153-161.
6. Ockene IS, Herbert JR, Ockene JK, et al. Effect of physician delivered nutrition counseling training and an office support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population (WATCH). Arch Intern Med. 1999;159:725-731.
7. Simkin-Silverman LR, Gleason KA, King WC, et al. Predictors of weight control advice in primary care practices: patient health and psychosocial characteristics. Prev Med. 2005;40:71-82.
8. Flocke SA, Clark A, Schlessman K, et al. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med. 2005;37:415-421.
9. Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170:146-154.
10. National Committee for Quality Assurance. Obesity screening measures highlight additions to HEDIS 2009. February 14, 2008. Available at: http://www.ncqa.org/tabid/662/Default.aspx. Accessed June 7, 2010.
11. Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood). 2009;28:w822-w831.
12. Jay M, Kalet A, Ark T, et al. Physicians’ attitudes about obesity and their associations with competency and specialty: a cross-sectional study. BMC Health Serv Res. 2009;9:106.-
13. STOP Obesity Alliance. STOP Obesity Alliance surveys show doctors, patients share role in weight loss, but ask, now what? March 16, 2010. Available at: http://www.eurekalert.org/pub_releases/2010-03/cca-soa031510.php. Accessed June 7, 2010.
14. Ferguson C, Langwith C, Muldoon A, et al. STOP Obesity Alliance Research Team, George Washington University School of Public Health and Health Services. Improving obesity management in adult primary care. White paper. March 16, 2010.
15. National Institutes of Health, National Heart, Lung and Blood Institute. North American Association for the Study of Obesity. The Practical Guide: Identification, Evaluation , and Treatment of Overweight and Obesity in Adults. 2000. NIH Publication No. 00-4084.
16. US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930-932.
17. American Dietetic Association. Adult weight management evidence based nutrition practice guidelines. Available at: http://www.adaevidencelibrary.com. Accessed September 9, 2009.
18. Snow V, Barry P, Fitterman N, et al. Clinical efficacy assessment subcommittee of the American College of Physicians. Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;142:525-531.
19. Truesdale KP, Stevens J. Do the obese know they are obese? N C Med J. 2008;69:188-194.
20. Agency for Healthcare Research and Quality. Guide to clinical preventive services, 2009. Available at: http://www.ahrq.gov/clinic/pocketgd09/. Accessed March 30, 2010.
21. Bardia A, Holtan SG, Slezak JM, et al. Diagnosis of obesity by primary care physicians and impact on obesity management. Mayo Clin Proc. 2007;82:927-932.
22. Kennen EM, Davis TC, Huang J, et al. Tipping the scales: the effect of literacy on obese patients’ knowledge and readiness to lose weight. South Med J. 2005;98:15-18.
23. Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the Rapid Eating and Activity Assessment for Patients (REAP): a new tool for primary care providers. J Nutr Educ Behav. 2006;38:286-292.
24. Dansinger ML, Tatsioni A, Wong JB, et al. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41-50.
25. Atkins, Zone, Ornish, or LEARN—which diet kept weight off? J Fam Pract 2007;56:434.-
26. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859-873.
27. Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139-1148.
28. Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007;(3):CD005105.-
29. Centers for Disease Control and Prevention. Obesity and overweight for professionals: Resources/DNPAO/CDC. Updated 2009. Available at: http://www.cdc.gov/obesity/resources.html#R2P. Accessed December 10, 2009.
30. Rowland K, Coffey J. Are overweight children more likely to be overweight adults? J Fam Pract. 2009;58:431-432.
31. Eat Smart, Move More. North Carolina. Prescription for health. Available at: PrescriptionPadColor.pdf. Accessed June 11, 2010.
32. Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118-126.
33. Burke LE, Swigart V, Turk MW, et al. Experiences of self-monitoring: successes and struggles during treatment for weight loss. Qual Health Res. 2009;19:815.-
34. Cudjoe S, Moss S, Nguyen L, et al. Clinical inquiries. How do exercise and diet compare for weight loss? J Fam Pract. 2007;56:841-844.
35. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16:643-653.
36. Early JL, Apovian CM, Aronne LJ, et al. Sibutramine plus meal replacement therapy for body weight loss and maintenance in obese patients. Obesity (Silver Spring). 2007;15:1464-1472.
37. Ashley JM, St Jeor ST, Schrage JP, et al. Weight control in the physician’s office. Arch Intern Med. 2001;161:1599-1604.
38. Bowerman S, Bellman M, Saltsman P, et al. Implementation of a primary care physician network obesity management program. Obes Res. 2001;9(suppl 4):321S-325S.
39. Kuehn B. Tainted diet drugs. JAMA. 2009;301:817.-
40. Jull AB, Ni Mhurchu C, Bennett DA, et al. Chitosan for overweight or obesity. Cochrane Database Syst Rev. 2008;(3):CD003892.-
41. Teegarden D, Gunther CW. Can the controversial relationship between dietary calcium and body weight be mechanistically explained by alterations in appetite and food intake? Nutr Rev. 2008;66:601-605.
42. Greenway R, Heber D. Herbal and alternative approaches to obesity. In: Bray GA, Bouchard C, eds. Handbook of Obesity: Clinical Applications. 3rd ed. New York: Informa Healthcare; 2008: 425-443.
43. Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;144:532-546.
44. Neovius M, Johansson K, Rossner S. Head-to-head studies evaluating efficacy of pharmacotherapy for obesity: a systematic review and meta-analysis. Obes Rev. 2008;9:420-427.
45. Siebenhofer A, Korvath K, Jeitler K, et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database Syst Rev. 2009;(3):CD007654.-
46. Miller LJ. Management of atypical antipsychotic drug-induced weight gain: focus on metformin. Pharmacotherapy. 2009;29:725-735.
47. Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009;(2):CD003641.-
48. Blackburn GL, Hutter MM, Harvey AM, et al. Expert panel on weight loss surgery: executive report update. Obesity (Silver Spring). 2009;17:842-862.
49. Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14(suppl 1):S1-S83.
50. Helman T. Weight management by family physicians. Arbor Clin Nutrition Updates. 2002 Feb;116:1-2. Available at: www.arborcom.com/free/116.pdf. Accessed June 16, 2010.
51. Holt J, Warren L, Wallace R, et al. Clinical inquiries. What behavioral interventions are safe and effective for treating obesity? J Fam Pract. 2006;55:536-538.
52. Cheskin LJ, Bartlett SJ, Zayas R, et al. Prescription medications: a modifiable contributor to obesity. South Med J. 1999;92:898-904.
53. Parsons B, Allison DB, Loebel A, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103-110.
8 ways to improve the informed consent process
• Let patients know that informed consent is an interactive process leading to mutual agreement, rather than a formality and forced choice. C
• Present all treatment options even if a patient’s insurance does not cover them all. C
• Discuss the advantages, disadvantages, and limitations of the tests you are ordering or recommending—particularly nonroutine lab work. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Once viewed as simply a legal preamble to treatment, informed consent today encompasses much more. An essential part of the ethical practice of medicine,1 it is also an opportunity to strengthen the doctor-patient relationship. Effective informed consent embodies the shift in primary care medicine to guide rather than dictate an individual’s health care decisions, often termed shared decision making.2 Furthermore, informed consent is increasingly relevant in today’s evolving legislative expectations3 and health care initiatives.4 For risk management, the physician has more direct control over the process of informed consent than most other areas pertaining to medical negligence. Thus, incorporating improvements to the process of informed consent is time well invested.
In this article I will discuss the components of informed consent and recommend practical steps to its effective delivery for mentally competent adult patients.
What informed consent requires
In the office setting, obtain informed consent before you start treatments or procedures, prescribe medications, or order most diagnostic tests. Informed consent requires that the patient understands the following:
- the material risks and benefits of a proposed treatment
- the reasonable alternative treatments
- the consequences of no treatment.
Physicians are not expected to disclose every risk to a patient. However, both common risks and uncommon but serious risks are considered material.5 In the extreme, a mentally competent patient may refuse lifesaving therapy if she or he understands the risk of doing so.6
The legal criteria that determine whether a physician satisfied the standard of care for informed consent vary from state to state. Some states test the physician’s conduct against what a “reasonable physician” should have disclosed. Other states apply what a “reasonable patient” would want to know, and some apply a combination of both. As such, legal cases provide little direction to physicians on how to decide what is material.7
Patients generally take 1 of 2 approaches when pursuing action against a physician that is related to informed consent.8
The first, and most common, cause of action is negligence. This occurs when a patient claims that the physician’s disclosures in the consent process were inadequate. The patient is then required to prove the elements of negligence: breach of standard of care, causation, and damages.
The second potential cause of action is battery, which is an unlawful touching. Proving battery is simpler because there are fewer elements to the claim. If a procedure or examination took place without the patient’s consent or was beyond the scope of the consent given, a battery action is possible, whether or not the outcome of a treatment was beneficial to the patient.
Now, on to the steps that can help improve the informed consent process.
1. Work on your rapport
The importance of good rapport between the patient and physician cannot be overemphasized. The level of rapport is a better predictor of the risk of litigation than the actual content of any particular discussion.9
A few tips to improve rapport. If you approach informed consent merely as a legal technicality, the tone you take in the discussion may reflect that attitude. Instead, enter into a consent discussion in such a manner that the patient understands it is an interactive process leading to mutual agreement rather than a formality. Should an adverse outcome occur, a patient who recalls feeling pressured may claim that not all the key information was presented. Don’t be dogmatic in making recommendations; scientific evidence and medical opinion can change with time. Hormone replacement therapy and cyclooxygenase-2 inhibitors are current examples that demonstrate the importance of allowing the patient to make a decision about therapy.
Effective communication reduces the likelihood of litigation.7 One model of more effective communication is the “teach back” approach,4 in which you identify the principal messages of the discussion and ask the patient to paraphrase them. This approach emphasizes the use of simple, clear language in layman’s terms, relying on your ability to explain rather than the patient’s ability to comprehend. Questions such as “Do you have any questions?” or “Do you understand?” are less effective than saying “I want to be sure we have the same understanding” or asking “Can you tell me in your own words?”4 (See “Putting informed consent principles into practice” by going to jfponline.com and scrolling to the end of this article.)
2. Discuss all treatment options—regardless of insurance coverage
Determining what should be disclosed as a material risk in the consent process can be challenging. It’s imperative to be familiar with the medical literature as well as the important risks and benefits of treatments. However, use statistics judiciously and meaningfully. Overusing statistical data can confuse and even alienate some patients. The goal is to achieve an understanding about whether a risk is relatively common or relatively rare, but serious.
Present all treatment options regardless of whether the patient’s insurance covers all of them.1 Consider a patient’s unique financial situation in the shared decision-making process.
Exhaustive lists of potential risks are impractical and, more important, are ineffective, as the risks have not been put into context for the patient. A list of routine risks is a good starting point and provides structure to the discussion. Then, by taking the patient’s point of view, identify important, patient-specific risks. Customizing the discussion for each individual is the key principle in the duty to inform.10 Common issues include how a side effect or adverse outcome might affect a person’s occupation, fertility, sexual function, appearance, etc. Other issues include the pain incurred, degree of rehabilitation, restrictions on lifestyle, etc.
3. Use the ABCDEF mnemonic
The following mnemonic is useful for guiding and documenting your discussion with the patient:
- Alternative therapies available
- Benefits of the therapy proposed
- Common but not devastating risks
- Devastating but not common risks
- Extra considerations specific to this patient
- Facial expressions, body language, and questions.
4. Decide how much medication information the patient needs
The learned intermediary doctrine is a legal concept whereby a pharmaceutical company is deemed to have discharged its responsibility to patients (in whole or in part) for side effects they have disclosed to physicians, commonly through the product monograph.11 To limit risks, it is prudent to use a limited number of first-line drugs in each class, rather than a lot of samples and new drugs, until you review monographs and the literature. As a final check on your duty to inform, encourage a patient to discuss with his or her pharmacist the drug you have prescribed, to further reduce inadvertent errors and side effects.
Decide how much information the patient needs. A recital of every risk in taking an antibiotic is untenable. However, certain drugs require more detailed discussion. Oral contraceptives, analgesics, and cardiovascular drugs are a few classes of medications that have infrequent, but serious, side effects. Generally, these risks are so devastating (eg, stroke associated with oral contraceptives) that lawsuits are common. Important information that is not directly health related includes occupational or driving limitations while a patient is taking a drug that alters mental status.
Ask patients to tell you about any supplements or alternative therapies they use. Many complementary treatments can have an effect on medical therapy.
If a patient asks about alternative medicine, disclose your level of training in the area and discuss candidly any known related medical issues. For example, a patient with neck pain may ask for an opinion about, or a referral for, chiropractic care. Discuss known risks, such as vertebral artery dissection, and be frank when you cannot endorse the therapy for lack of training or scientific evidence.
Discussing enrollment in a medication research trial increases a physician’s duties of disclosure before a patient decides to participate.12 You must convey that the therapy has unknown risks and may turn out to be harmful. Also, the sponsor of the study, the institutional review board, and government agencies (eg, the US Food and Drug Administration and the Department of Health and Human Services) may require discussion and documentation of specific risks.
5. Discuss how test results will be communicated
Laboratory or radiology investigations and their results introduce a unique set of issues. Particularly for nonroutine lab work, it’s prudent to discuss the advantages, disadvantages, and limitations of the test being ordered or recommended. These discussions can become the subject of suits when a patient receives a diagnosis and wonders in hindsight if his or her doctor missed the true diagnosis or should have been more aggressive in the investigation. Consider inviting your local laboratory or radiology group to make a presentation to keep you up-to-date on available options.
Obtaining informed consent provides a helpful segue to discussing how test results can best be shared with the patient. An all-too-common problem is that test results can become lost or misfiled. Describe your office policy on calling patients with results, and think about when it might be advisable for the patient to follow up with the office, to reduce error and liability—eg, cancer screenings, Pap tests, and biopsy results.
6. Keep a record of referrals
A patient generally has the right to refuse specialty treatment13 or referral to a specialist,14 once informed of the risks of delay or lack of treatment after making such a decision. If a patient still refuses referral, document the decision in case it results in a delayed diagnosis or an adverse outcome.
Generally, the specialist has the duty to inform the patient of the risks and benefits of the specialty treatment. However, it has been held that a primary care physician still bears some responsibility to assure the welfare of the patient in all phases of treatment.15 Thus, it is prudent to ensure that patients have not been lost to specialty follow-up. In a busy practice it is often difficult, of course, to keep track of the status of all referrals, and specialty offices differ in efforts to keep primary care physicians informed. Use the informed consent process to raise and discuss such issues. Encourage your patients to notify you or your staff if they have experienced a delay in care with a specialist you referred them to.
7. Avoid making guarantees about procedures
All procedures, including associated anesthesia, require a discussion of risks and benefits. If appropriate, also discuss available alternative procedures and your reasons for not recommending them. For example, a breast lump can be imaged, aspirated in the office, or surgically excised. All options need to be discussed and the course of action mutually agreed upon. A patient may not necessarily want the least invasive option.
Avoid assurances or guarantees regarding a specific outcome. Such guarantees can be the impetus for legal action (breach of warranty claim) should the promised outcome fail to occur. Exercise caution, for example, in explaining outcomes and risks for cosmetic procedures. If a patient has a complicated problem or unrealistically high expectations, consider a referral for a second opinion or for management by a specialist.
8. Document, document, document
Documentation is a necessary, final step. It records the process that is vital to good patient care and it may be the only proof that a discussion took place. Legal case opinions shed little light on what represents adequate documentation. Implement a record-keeping strategy that suits your practice setting and style. Products or guides for this purpose are available commercially or through medical societies, malpractice carriers, legislative initiatives, and special interest groups.16 If you use a preset consent form, make sure it is not intimidating or confusing. Initiatives to improve health literacy suggest that literature, to be effective, should be written at the fifth grade level.17
Forms with boilerplate language that simply require a signature are inferior to documents that give details of the meaningful discussion that took place. Drawings or notes stating which family members were present or what questions were asked can demonstrate the particulars of the discussion for a specific patient. It is also useful to document secondary resources you used, suggested, or gave to the patient, such as models, diagrams, pamphlets, CDs, and DVDs.
Dr. B holds a busy walk-in clinic after-hours in her office. She sees Harry, a 27-year-old man for the first time. Harry is uninsured and presents with fatigue and a 3-month cough. He is a nonsmoker and believes he is tired because he has been working 2 jobs and has had 2 severe viral illnesses in the last few months. Many of his symptoms suggest reactive airways and post-viral cough. However, Dr. B is concerned about other diseases based on Harry’s general appearance, such as Hodgkin’s lymphoma. Dr. B is also anxious, because one of the clinic doctors has recently been sued for missing lung cancer in a young man. She wonders how to best proceed with treatment to suit Harry’s needs and avoid unnecessary liability.
Dr. B does not have the benefit of a long-term relationship with Harry and may feel inclined to suggest an aggressive investigation to avoid missing disease and subsequent litigation. However, establishing good rapport and communicating effectively would better serve them both. Dr. B’s challenge is to ensure that Harry understands that he most likely has a simple disease process, but that they need to consider more serious possibilities. Harry must also understand the consequences of poor follow-up and delayed investigation. Dr. B should explain that deciding how to proceed will be a shared process that leads to a medically sound and financially practical option.
As an example, Dr. B could suggest a course of therapy for reactive airways, such as a beta-2 agonist and inhaled corticosteroid. She should describe both the common and the serious side effects for each. She should also counsel Harry to go over his medications with the pharmacist.
Dr. B may advise a screening chest x-ray now or offer close follow-up and a trial of inhalers for a reasonable period. She should explain that a chest x-ray has diagnostic limitations and that Harry may need further investigations, such as a chest cT or referral. at this point, Harry is ready to share in the decision on how to proceed.
At the end of this discussion, Harry should be asked to “teach back” his understanding of the treatment plan. Dr. B should then document the salient points (at minimum: “cost concerns,” “follow-up necessary to rule out serious pathology,” “risks and benefits of medications discussed and advised to also discuss with pharmacist,” “chest x-ray advised and discussed other investigation options,” and “teach-back method used to confirm plan”).
CORRESPONDENCE Preethy D. Kaibara, MD, Esq, Shufeldt Law, 2550 N. Thunderbird Circle, Suite 303, Mesa, AZ 85215; [email protected]
1. American Medical Association (AMA). Health and Ethics Policies of the AMA House of Delegates (Policy H-140.989 and H-160.998). Available at: http://www.ama-assn.org/ad-com/polfind/Hlth-Ethics.pdf. Accessed June 16, 1010.
2. Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285-294.
3. For example, The Patient Self-Determination Act 42 U.S.C.§§1395cc(a) et seq., as amended (2003).
4. National Quality Forum. Implementing a National Voluntary Consensus Standard for Informed Consent: A Users Guide for Healthcare Professionals. Washington, DC: National Quality Forum; 2005:13, 15, 27.
5. Salgo v Stanford University Board of Trustees, 154 Cal. App. 2d 560, 317 P. 2d 170 (1957).
6. Cruzan v Director, Missouri Department of Health, 497 U.S. 261 (1990).
7. Berg JW, Appelbaum PS, Lidz CW, et al. Informed Consent Legal Theory and Clinical Practice. 2nd ed. New York, NY: Oxford University Press; 2001:46-55.
8. 61 Am Jur 2d Physicians and Surgeons §151-152 pp 274-275.
9. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
10. Rozovsky FA. Consent to Treatment: A Practical Guide. 4th ed. Frederick, Md: Aspen Publishers; 2007:8-11.
11. Martin v Hacker, 83 NY 2d 1, N.E. 2d 1308 (1993).
12. Moore v The Regents of the University of California, 793 P.2d 479 (Cal 1990).
13. Moore v Preventative Medicine Group Inc., 178 Cal. App 3d 728, 223 Cal Rptr. 859 (Cal Ct. App. 1986).
14. Truman v Thomas, 27 Cal. 3d 285, 611 P. 2d 902, 165 Cal. Rptr. 308 (1980).
15. Prooth v Walsh, 432 N.Y.S. 2d 663 (Sup Ct. 1980).
16. Landro L. Consent forms that patients can understand. The Wall Street Journal. February 8, 2008. Available at: http://online.wsj.com/article/SB120224055435844931.html. Accessed February 10, 2008.
17. Safeer RS, Keenan ES. Health literacy: the gap between physicians and patients. Am Fam Phys. 2005;72:463-468.
• Let patients know that informed consent is an interactive process leading to mutual agreement, rather than a formality and forced choice. C
• Present all treatment options even if a patient’s insurance does not cover them all. C
• Discuss the advantages, disadvantages, and limitations of the tests you are ordering or recommending—particularly nonroutine lab work. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Once viewed as simply a legal preamble to treatment, informed consent today encompasses much more. An essential part of the ethical practice of medicine,1 it is also an opportunity to strengthen the doctor-patient relationship. Effective informed consent embodies the shift in primary care medicine to guide rather than dictate an individual’s health care decisions, often termed shared decision making.2 Furthermore, informed consent is increasingly relevant in today’s evolving legislative expectations3 and health care initiatives.4 For risk management, the physician has more direct control over the process of informed consent than most other areas pertaining to medical negligence. Thus, incorporating improvements to the process of informed consent is time well invested.
In this article I will discuss the components of informed consent and recommend practical steps to its effective delivery for mentally competent adult patients.
What informed consent requires
In the office setting, obtain informed consent before you start treatments or procedures, prescribe medications, or order most diagnostic tests. Informed consent requires that the patient understands the following:
- the material risks and benefits of a proposed treatment
- the reasonable alternative treatments
- the consequences of no treatment.
Physicians are not expected to disclose every risk to a patient. However, both common risks and uncommon but serious risks are considered material.5 In the extreme, a mentally competent patient may refuse lifesaving therapy if she or he understands the risk of doing so.6
The legal criteria that determine whether a physician satisfied the standard of care for informed consent vary from state to state. Some states test the physician’s conduct against what a “reasonable physician” should have disclosed. Other states apply what a “reasonable patient” would want to know, and some apply a combination of both. As such, legal cases provide little direction to physicians on how to decide what is material.7
Patients generally take 1 of 2 approaches when pursuing action against a physician that is related to informed consent.8
The first, and most common, cause of action is negligence. This occurs when a patient claims that the physician’s disclosures in the consent process were inadequate. The patient is then required to prove the elements of negligence: breach of standard of care, causation, and damages.
The second potential cause of action is battery, which is an unlawful touching. Proving battery is simpler because there are fewer elements to the claim. If a procedure or examination took place without the patient’s consent or was beyond the scope of the consent given, a battery action is possible, whether or not the outcome of a treatment was beneficial to the patient.
Now, on to the steps that can help improve the informed consent process.
1. Work on your rapport
The importance of good rapport between the patient and physician cannot be overemphasized. The level of rapport is a better predictor of the risk of litigation than the actual content of any particular discussion.9
A few tips to improve rapport. If you approach informed consent merely as a legal technicality, the tone you take in the discussion may reflect that attitude. Instead, enter into a consent discussion in such a manner that the patient understands it is an interactive process leading to mutual agreement rather than a formality. Should an adverse outcome occur, a patient who recalls feeling pressured may claim that not all the key information was presented. Don’t be dogmatic in making recommendations; scientific evidence and medical opinion can change with time. Hormone replacement therapy and cyclooxygenase-2 inhibitors are current examples that demonstrate the importance of allowing the patient to make a decision about therapy.
Effective communication reduces the likelihood of litigation.7 One model of more effective communication is the “teach back” approach,4 in which you identify the principal messages of the discussion and ask the patient to paraphrase them. This approach emphasizes the use of simple, clear language in layman’s terms, relying on your ability to explain rather than the patient’s ability to comprehend. Questions such as “Do you have any questions?” or “Do you understand?” are less effective than saying “I want to be sure we have the same understanding” or asking “Can you tell me in your own words?”4 (See “Putting informed consent principles into practice” by going to jfponline.com and scrolling to the end of this article.)
2. Discuss all treatment options—regardless of insurance coverage
Determining what should be disclosed as a material risk in the consent process can be challenging. It’s imperative to be familiar with the medical literature as well as the important risks and benefits of treatments. However, use statistics judiciously and meaningfully. Overusing statistical data can confuse and even alienate some patients. The goal is to achieve an understanding about whether a risk is relatively common or relatively rare, but serious.
Present all treatment options regardless of whether the patient’s insurance covers all of them.1 Consider a patient’s unique financial situation in the shared decision-making process.
Exhaustive lists of potential risks are impractical and, more important, are ineffective, as the risks have not been put into context for the patient. A list of routine risks is a good starting point and provides structure to the discussion. Then, by taking the patient’s point of view, identify important, patient-specific risks. Customizing the discussion for each individual is the key principle in the duty to inform.10 Common issues include how a side effect or adverse outcome might affect a person’s occupation, fertility, sexual function, appearance, etc. Other issues include the pain incurred, degree of rehabilitation, restrictions on lifestyle, etc.
3. Use the ABCDEF mnemonic
The following mnemonic is useful for guiding and documenting your discussion with the patient:
- Alternative therapies available
- Benefits of the therapy proposed
- Common but not devastating risks
- Devastating but not common risks
- Extra considerations specific to this patient
- Facial expressions, body language, and questions.
4. Decide how much medication information the patient needs
The learned intermediary doctrine is a legal concept whereby a pharmaceutical company is deemed to have discharged its responsibility to patients (in whole or in part) for side effects they have disclosed to physicians, commonly through the product monograph.11 To limit risks, it is prudent to use a limited number of first-line drugs in each class, rather than a lot of samples and new drugs, until you review monographs and the literature. As a final check on your duty to inform, encourage a patient to discuss with his or her pharmacist the drug you have prescribed, to further reduce inadvertent errors and side effects.
Decide how much information the patient needs. A recital of every risk in taking an antibiotic is untenable. However, certain drugs require more detailed discussion. Oral contraceptives, analgesics, and cardiovascular drugs are a few classes of medications that have infrequent, but serious, side effects. Generally, these risks are so devastating (eg, stroke associated with oral contraceptives) that lawsuits are common. Important information that is not directly health related includes occupational or driving limitations while a patient is taking a drug that alters mental status.
Ask patients to tell you about any supplements or alternative therapies they use. Many complementary treatments can have an effect on medical therapy.
If a patient asks about alternative medicine, disclose your level of training in the area and discuss candidly any known related medical issues. For example, a patient with neck pain may ask for an opinion about, or a referral for, chiropractic care. Discuss known risks, such as vertebral artery dissection, and be frank when you cannot endorse the therapy for lack of training or scientific evidence.
Discussing enrollment in a medication research trial increases a physician’s duties of disclosure before a patient decides to participate.12 You must convey that the therapy has unknown risks and may turn out to be harmful. Also, the sponsor of the study, the institutional review board, and government agencies (eg, the US Food and Drug Administration and the Department of Health and Human Services) may require discussion and documentation of specific risks.
5. Discuss how test results will be communicated
Laboratory or radiology investigations and their results introduce a unique set of issues. Particularly for nonroutine lab work, it’s prudent to discuss the advantages, disadvantages, and limitations of the test being ordered or recommended. These discussions can become the subject of suits when a patient receives a diagnosis and wonders in hindsight if his or her doctor missed the true diagnosis or should have been more aggressive in the investigation. Consider inviting your local laboratory or radiology group to make a presentation to keep you up-to-date on available options.
Obtaining informed consent provides a helpful segue to discussing how test results can best be shared with the patient. An all-too-common problem is that test results can become lost or misfiled. Describe your office policy on calling patients with results, and think about when it might be advisable for the patient to follow up with the office, to reduce error and liability—eg, cancer screenings, Pap tests, and biopsy results.
6. Keep a record of referrals
A patient generally has the right to refuse specialty treatment13 or referral to a specialist,14 once informed of the risks of delay or lack of treatment after making such a decision. If a patient still refuses referral, document the decision in case it results in a delayed diagnosis or an adverse outcome.
Generally, the specialist has the duty to inform the patient of the risks and benefits of the specialty treatment. However, it has been held that a primary care physician still bears some responsibility to assure the welfare of the patient in all phases of treatment.15 Thus, it is prudent to ensure that patients have not been lost to specialty follow-up. In a busy practice it is often difficult, of course, to keep track of the status of all referrals, and specialty offices differ in efforts to keep primary care physicians informed. Use the informed consent process to raise and discuss such issues. Encourage your patients to notify you or your staff if they have experienced a delay in care with a specialist you referred them to.
7. Avoid making guarantees about procedures
All procedures, including associated anesthesia, require a discussion of risks and benefits. If appropriate, also discuss available alternative procedures and your reasons for not recommending them. For example, a breast lump can be imaged, aspirated in the office, or surgically excised. All options need to be discussed and the course of action mutually agreed upon. A patient may not necessarily want the least invasive option.
Avoid assurances or guarantees regarding a specific outcome. Such guarantees can be the impetus for legal action (breach of warranty claim) should the promised outcome fail to occur. Exercise caution, for example, in explaining outcomes and risks for cosmetic procedures. If a patient has a complicated problem or unrealistically high expectations, consider a referral for a second opinion or for management by a specialist.
8. Document, document, document
Documentation is a necessary, final step. It records the process that is vital to good patient care and it may be the only proof that a discussion took place. Legal case opinions shed little light on what represents adequate documentation. Implement a record-keeping strategy that suits your practice setting and style. Products or guides for this purpose are available commercially or through medical societies, malpractice carriers, legislative initiatives, and special interest groups.16 If you use a preset consent form, make sure it is not intimidating or confusing. Initiatives to improve health literacy suggest that literature, to be effective, should be written at the fifth grade level.17
Forms with boilerplate language that simply require a signature are inferior to documents that give details of the meaningful discussion that took place. Drawings or notes stating which family members were present or what questions were asked can demonstrate the particulars of the discussion for a specific patient. It is also useful to document secondary resources you used, suggested, or gave to the patient, such as models, diagrams, pamphlets, CDs, and DVDs.
Dr. B holds a busy walk-in clinic after-hours in her office. She sees Harry, a 27-year-old man for the first time. Harry is uninsured and presents with fatigue and a 3-month cough. He is a nonsmoker and believes he is tired because he has been working 2 jobs and has had 2 severe viral illnesses in the last few months. Many of his symptoms suggest reactive airways and post-viral cough. However, Dr. B is concerned about other diseases based on Harry’s general appearance, such as Hodgkin’s lymphoma. Dr. B is also anxious, because one of the clinic doctors has recently been sued for missing lung cancer in a young man. She wonders how to best proceed with treatment to suit Harry’s needs and avoid unnecessary liability.
Dr. B does not have the benefit of a long-term relationship with Harry and may feel inclined to suggest an aggressive investigation to avoid missing disease and subsequent litigation. However, establishing good rapport and communicating effectively would better serve them both. Dr. B’s challenge is to ensure that Harry understands that he most likely has a simple disease process, but that they need to consider more serious possibilities. Harry must also understand the consequences of poor follow-up and delayed investigation. Dr. B should explain that deciding how to proceed will be a shared process that leads to a medically sound and financially practical option.
As an example, Dr. B could suggest a course of therapy for reactive airways, such as a beta-2 agonist and inhaled corticosteroid. She should describe both the common and the serious side effects for each. She should also counsel Harry to go over his medications with the pharmacist.
Dr. B may advise a screening chest x-ray now or offer close follow-up and a trial of inhalers for a reasonable period. She should explain that a chest x-ray has diagnostic limitations and that Harry may need further investigations, such as a chest cT or referral. at this point, Harry is ready to share in the decision on how to proceed.
At the end of this discussion, Harry should be asked to “teach back” his understanding of the treatment plan. Dr. B should then document the salient points (at minimum: “cost concerns,” “follow-up necessary to rule out serious pathology,” “risks and benefits of medications discussed and advised to also discuss with pharmacist,” “chest x-ray advised and discussed other investigation options,” and “teach-back method used to confirm plan”).
CORRESPONDENCE Preethy D. Kaibara, MD, Esq, Shufeldt Law, 2550 N. Thunderbird Circle, Suite 303, Mesa, AZ 85215; [email protected]
• Let patients know that informed consent is an interactive process leading to mutual agreement, rather than a formality and forced choice. C
• Present all treatment options even if a patient’s insurance does not cover them all. C
• Discuss the advantages, disadvantages, and limitations of the tests you are ordering or recommending—particularly nonroutine lab work. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Once viewed as simply a legal preamble to treatment, informed consent today encompasses much more. An essential part of the ethical practice of medicine,1 it is also an opportunity to strengthen the doctor-patient relationship. Effective informed consent embodies the shift in primary care medicine to guide rather than dictate an individual’s health care decisions, often termed shared decision making.2 Furthermore, informed consent is increasingly relevant in today’s evolving legislative expectations3 and health care initiatives.4 For risk management, the physician has more direct control over the process of informed consent than most other areas pertaining to medical negligence. Thus, incorporating improvements to the process of informed consent is time well invested.
In this article I will discuss the components of informed consent and recommend practical steps to its effective delivery for mentally competent adult patients.
What informed consent requires
In the office setting, obtain informed consent before you start treatments or procedures, prescribe medications, or order most diagnostic tests. Informed consent requires that the patient understands the following:
- the material risks and benefits of a proposed treatment
- the reasonable alternative treatments
- the consequences of no treatment.
Physicians are not expected to disclose every risk to a patient. However, both common risks and uncommon but serious risks are considered material.5 In the extreme, a mentally competent patient may refuse lifesaving therapy if she or he understands the risk of doing so.6
The legal criteria that determine whether a physician satisfied the standard of care for informed consent vary from state to state. Some states test the physician’s conduct against what a “reasonable physician” should have disclosed. Other states apply what a “reasonable patient” would want to know, and some apply a combination of both. As such, legal cases provide little direction to physicians on how to decide what is material.7
Patients generally take 1 of 2 approaches when pursuing action against a physician that is related to informed consent.8
The first, and most common, cause of action is negligence. This occurs when a patient claims that the physician’s disclosures in the consent process were inadequate. The patient is then required to prove the elements of negligence: breach of standard of care, causation, and damages.
The second potential cause of action is battery, which is an unlawful touching. Proving battery is simpler because there are fewer elements to the claim. If a procedure or examination took place without the patient’s consent or was beyond the scope of the consent given, a battery action is possible, whether or not the outcome of a treatment was beneficial to the patient.
Now, on to the steps that can help improve the informed consent process.
1. Work on your rapport
The importance of good rapport between the patient and physician cannot be overemphasized. The level of rapport is a better predictor of the risk of litigation than the actual content of any particular discussion.9
A few tips to improve rapport. If you approach informed consent merely as a legal technicality, the tone you take in the discussion may reflect that attitude. Instead, enter into a consent discussion in such a manner that the patient understands it is an interactive process leading to mutual agreement rather than a formality. Should an adverse outcome occur, a patient who recalls feeling pressured may claim that not all the key information was presented. Don’t be dogmatic in making recommendations; scientific evidence and medical opinion can change with time. Hormone replacement therapy and cyclooxygenase-2 inhibitors are current examples that demonstrate the importance of allowing the patient to make a decision about therapy.
Effective communication reduces the likelihood of litigation.7 One model of more effective communication is the “teach back” approach,4 in which you identify the principal messages of the discussion and ask the patient to paraphrase them. This approach emphasizes the use of simple, clear language in layman’s terms, relying on your ability to explain rather than the patient’s ability to comprehend. Questions such as “Do you have any questions?” or “Do you understand?” are less effective than saying “I want to be sure we have the same understanding” or asking “Can you tell me in your own words?”4 (See “Putting informed consent principles into practice” by going to jfponline.com and scrolling to the end of this article.)
2. Discuss all treatment options—regardless of insurance coverage
Determining what should be disclosed as a material risk in the consent process can be challenging. It’s imperative to be familiar with the medical literature as well as the important risks and benefits of treatments. However, use statistics judiciously and meaningfully. Overusing statistical data can confuse and even alienate some patients. The goal is to achieve an understanding about whether a risk is relatively common or relatively rare, but serious.
Present all treatment options regardless of whether the patient’s insurance covers all of them.1 Consider a patient’s unique financial situation in the shared decision-making process.
Exhaustive lists of potential risks are impractical and, more important, are ineffective, as the risks have not been put into context for the patient. A list of routine risks is a good starting point and provides structure to the discussion. Then, by taking the patient’s point of view, identify important, patient-specific risks. Customizing the discussion for each individual is the key principle in the duty to inform.10 Common issues include how a side effect or adverse outcome might affect a person’s occupation, fertility, sexual function, appearance, etc. Other issues include the pain incurred, degree of rehabilitation, restrictions on lifestyle, etc.
3. Use the ABCDEF mnemonic
The following mnemonic is useful for guiding and documenting your discussion with the patient:
- Alternative therapies available
- Benefits of the therapy proposed
- Common but not devastating risks
- Devastating but not common risks
- Extra considerations specific to this patient
- Facial expressions, body language, and questions.
4. Decide how much medication information the patient needs
The learned intermediary doctrine is a legal concept whereby a pharmaceutical company is deemed to have discharged its responsibility to patients (in whole or in part) for side effects they have disclosed to physicians, commonly through the product monograph.11 To limit risks, it is prudent to use a limited number of first-line drugs in each class, rather than a lot of samples and new drugs, until you review monographs and the literature. As a final check on your duty to inform, encourage a patient to discuss with his or her pharmacist the drug you have prescribed, to further reduce inadvertent errors and side effects.
Decide how much information the patient needs. A recital of every risk in taking an antibiotic is untenable. However, certain drugs require more detailed discussion. Oral contraceptives, analgesics, and cardiovascular drugs are a few classes of medications that have infrequent, but serious, side effects. Generally, these risks are so devastating (eg, stroke associated with oral contraceptives) that lawsuits are common. Important information that is not directly health related includes occupational or driving limitations while a patient is taking a drug that alters mental status.
Ask patients to tell you about any supplements or alternative therapies they use. Many complementary treatments can have an effect on medical therapy.
If a patient asks about alternative medicine, disclose your level of training in the area and discuss candidly any known related medical issues. For example, a patient with neck pain may ask for an opinion about, or a referral for, chiropractic care. Discuss known risks, such as vertebral artery dissection, and be frank when you cannot endorse the therapy for lack of training or scientific evidence.
Discussing enrollment in a medication research trial increases a physician’s duties of disclosure before a patient decides to participate.12 You must convey that the therapy has unknown risks and may turn out to be harmful. Also, the sponsor of the study, the institutional review board, and government agencies (eg, the US Food and Drug Administration and the Department of Health and Human Services) may require discussion and documentation of specific risks.
5. Discuss how test results will be communicated
Laboratory or radiology investigations and their results introduce a unique set of issues. Particularly for nonroutine lab work, it’s prudent to discuss the advantages, disadvantages, and limitations of the test being ordered or recommended. These discussions can become the subject of suits when a patient receives a diagnosis and wonders in hindsight if his or her doctor missed the true diagnosis or should have been more aggressive in the investigation. Consider inviting your local laboratory or radiology group to make a presentation to keep you up-to-date on available options.
Obtaining informed consent provides a helpful segue to discussing how test results can best be shared with the patient. An all-too-common problem is that test results can become lost or misfiled. Describe your office policy on calling patients with results, and think about when it might be advisable for the patient to follow up with the office, to reduce error and liability—eg, cancer screenings, Pap tests, and biopsy results.
6. Keep a record of referrals
A patient generally has the right to refuse specialty treatment13 or referral to a specialist,14 once informed of the risks of delay or lack of treatment after making such a decision. If a patient still refuses referral, document the decision in case it results in a delayed diagnosis or an adverse outcome.
Generally, the specialist has the duty to inform the patient of the risks and benefits of the specialty treatment. However, it has been held that a primary care physician still bears some responsibility to assure the welfare of the patient in all phases of treatment.15 Thus, it is prudent to ensure that patients have not been lost to specialty follow-up. In a busy practice it is often difficult, of course, to keep track of the status of all referrals, and specialty offices differ in efforts to keep primary care physicians informed. Use the informed consent process to raise and discuss such issues. Encourage your patients to notify you or your staff if they have experienced a delay in care with a specialist you referred them to.
7. Avoid making guarantees about procedures
All procedures, including associated anesthesia, require a discussion of risks and benefits. If appropriate, also discuss available alternative procedures and your reasons for not recommending them. For example, a breast lump can be imaged, aspirated in the office, or surgically excised. All options need to be discussed and the course of action mutually agreed upon. A patient may not necessarily want the least invasive option.
Avoid assurances or guarantees regarding a specific outcome. Such guarantees can be the impetus for legal action (breach of warranty claim) should the promised outcome fail to occur. Exercise caution, for example, in explaining outcomes and risks for cosmetic procedures. If a patient has a complicated problem or unrealistically high expectations, consider a referral for a second opinion or for management by a specialist.
8. Document, document, document
Documentation is a necessary, final step. It records the process that is vital to good patient care and it may be the only proof that a discussion took place. Legal case opinions shed little light on what represents adequate documentation. Implement a record-keeping strategy that suits your practice setting and style. Products or guides for this purpose are available commercially or through medical societies, malpractice carriers, legislative initiatives, and special interest groups.16 If you use a preset consent form, make sure it is not intimidating or confusing. Initiatives to improve health literacy suggest that literature, to be effective, should be written at the fifth grade level.17
Forms with boilerplate language that simply require a signature are inferior to documents that give details of the meaningful discussion that took place. Drawings or notes stating which family members were present or what questions were asked can demonstrate the particulars of the discussion for a specific patient. It is also useful to document secondary resources you used, suggested, or gave to the patient, such as models, diagrams, pamphlets, CDs, and DVDs.
Dr. B holds a busy walk-in clinic after-hours in her office. She sees Harry, a 27-year-old man for the first time. Harry is uninsured and presents with fatigue and a 3-month cough. He is a nonsmoker and believes he is tired because he has been working 2 jobs and has had 2 severe viral illnesses in the last few months. Many of his symptoms suggest reactive airways and post-viral cough. However, Dr. B is concerned about other diseases based on Harry’s general appearance, such as Hodgkin’s lymphoma. Dr. B is also anxious, because one of the clinic doctors has recently been sued for missing lung cancer in a young man. She wonders how to best proceed with treatment to suit Harry’s needs and avoid unnecessary liability.
Dr. B does not have the benefit of a long-term relationship with Harry and may feel inclined to suggest an aggressive investigation to avoid missing disease and subsequent litigation. However, establishing good rapport and communicating effectively would better serve them both. Dr. B’s challenge is to ensure that Harry understands that he most likely has a simple disease process, but that they need to consider more serious possibilities. Harry must also understand the consequences of poor follow-up and delayed investigation. Dr. B should explain that deciding how to proceed will be a shared process that leads to a medically sound and financially practical option.
As an example, Dr. B could suggest a course of therapy for reactive airways, such as a beta-2 agonist and inhaled corticosteroid. She should describe both the common and the serious side effects for each. She should also counsel Harry to go over his medications with the pharmacist.
Dr. B may advise a screening chest x-ray now or offer close follow-up and a trial of inhalers for a reasonable period. She should explain that a chest x-ray has diagnostic limitations and that Harry may need further investigations, such as a chest cT or referral. at this point, Harry is ready to share in the decision on how to proceed.
At the end of this discussion, Harry should be asked to “teach back” his understanding of the treatment plan. Dr. B should then document the salient points (at minimum: “cost concerns,” “follow-up necessary to rule out serious pathology,” “risks and benefits of medications discussed and advised to also discuss with pharmacist,” “chest x-ray advised and discussed other investigation options,” and “teach-back method used to confirm plan”).
CORRESPONDENCE Preethy D. Kaibara, MD, Esq, Shufeldt Law, 2550 N. Thunderbird Circle, Suite 303, Mesa, AZ 85215; [email protected]
1. American Medical Association (AMA). Health and Ethics Policies of the AMA House of Delegates (Policy H-140.989 and H-160.998). Available at: http://www.ama-assn.org/ad-com/polfind/Hlth-Ethics.pdf. Accessed June 16, 1010.
2. Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285-294.
3. For example, The Patient Self-Determination Act 42 U.S.C.§§1395cc(a) et seq., as amended (2003).
4. National Quality Forum. Implementing a National Voluntary Consensus Standard for Informed Consent: A Users Guide for Healthcare Professionals. Washington, DC: National Quality Forum; 2005:13, 15, 27.
5. Salgo v Stanford University Board of Trustees, 154 Cal. App. 2d 560, 317 P. 2d 170 (1957).
6. Cruzan v Director, Missouri Department of Health, 497 U.S. 261 (1990).
7. Berg JW, Appelbaum PS, Lidz CW, et al. Informed Consent Legal Theory and Clinical Practice. 2nd ed. New York, NY: Oxford University Press; 2001:46-55.
8. 61 Am Jur 2d Physicians and Surgeons §151-152 pp 274-275.
9. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
10. Rozovsky FA. Consent to Treatment: A Practical Guide. 4th ed. Frederick, Md: Aspen Publishers; 2007:8-11.
11. Martin v Hacker, 83 NY 2d 1, N.E. 2d 1308 (1993).
12. Moore v The Regents of the University of California, 793 P.2d 479 (Cal 1990).
13. Moore v Preventative Medicine Group Inc., 178 Cal. App 3d 728, 223 Cal Rptr. 859 (Cal Ct. App. 1986).
14. Truman v Thomas, 27 Cal. 3d 285, 611 P. 2d 902, 165 Cal. Rptr. 308 (1980).
15. Prooth v Walsh, 432 N.Y.S. 2d 663 (Sup Ct. 1980).
16. Landro L. Consent forms that patients can understand. The Wall Street Journal. February 8, 2008. Available at: http://online.wsj.com/article/SB120224055435844931.html. Accessed February 10, 2008.
17. Safeer RS, Keenan ES. Health literacy: the gap between physicians and patients. Am Fam Phys. 2005;72:463-468.
1. American Medical Association (AMA). Health and Ethics Policies of the AMA House of Delegates (Policy H-140.989 and H-160.998). Available at: http://www.ama-assn.org/ad-com/polfind/Hlth-Ethics.pdf. Accessed June 16, 1010.
2. Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285-294.
3. For example, The Patient Self-Determination Act 42 U.S.C.§§1395cc(a) et seq., as amended (2003).
4. National Quality Forum. Implementing a National Voluntary Consensus Standard for Informed Consent: A Users Guide for Healthcare Professionals. Washington, DC: National Quality Forum; 2005:13, 15, 27.
5. Salgo v Stanford University Board of Trustees, 154 Cal. App. 2d 560, 317 P. 2d 170 (1957).
6. Cruzan v Director, Missouri Department of Health, 497 U.S. 261 (1990).
7. Berg JW, Appelbaum PS, Lidz CW, et al. Informed Consent Legal Theory and Clinical Practice. 2nd ed. New York, NY: Oxford University Press; 2001:46-55.
8. 61 Am Jur 2d Physicians and Surgeons §151-152 pp 274-275.
9. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
10. Rozovsky FA. Consent to Treatment: A Practical Guide. 4th ed. Frederick, Md: Aspen Publishers; 2007:8-11.
11. Martin v Hacker, 83 NY 2d 1, N.E. 2d 1308 (1993).
12. Moore v The Regents of the University of California, 793 P.2d 479 (Cal 1990).
13. Moore v Preventative Medicine Group Inc., 178 Cal. App 3d 728, 223 Cal Rptr. 859 (Cal Ct. App. 1986).
14. Truman v Thomas, 27 Cal. 3d 285, 611 P. 2d 902, 165 Cal. Rptr. 308 (1980).
15. Prooth v Walsh, 432 N.Y.S. 2d 663 (Sup Ct. 1980).
16. Landro L. Consent forms that patients can understand. The Wall Street Journal. February 8, 2008. Available at: http://online.wsj.com/article/SB120224055435844931.html. Accessed February 10, 2008.
17. Safeer RS, Keenan ES. Health literacy: the gap between physicians and patients. Am Fam Phys. 2005;72:463-468.
Postmenopausal osteoporosis: Another approach to management
Using the World Health Organization’s online Fracture Risk Assessment Tool (FRAX) may help you decide when to initiate treatment for patients with osteopenia. C
Consider using intravenous bisphosphonates as first-line therapy for women with postmenopausal osteoporosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
To prevent bone loss and fractures in postmenopausal osteoporosis, the best choice of medication is one a patient will actually take. Bisphosphonates are the standard of care for maintaining or increasing bone mass and reducing excessive bone turnover,1 and oral bisphosphonates have proven to be safe and effective in reducing osteoporotic fractures. However, numerous studies have shown that the effectiveness of oral bisphosphonates is compromised by poor patient compliance in taking the medication as directed and by poor persistence in continuing the medication over the long term.
Intravenous (IV) bisphosphonates are another option: They bypass the GI tract and thereby avoid the difficult requirements of oral dosing that many patients end up disregarding. And because IV administration occurs under medical supervision, it ensures persistence throughout the entire dosing interval. IV ibandronate 3 mg is administered every 3 months, and IV zoledronic acid 5 mg is administered once a year.
Osteoporosis and osteoporotic fractures are markedly underdiagnosed
Delmas and colleagues evaluated the underdetection of vertebral fractures in an international, multicenter prospective study of 2451 women 65 to 80 years of age who had not received a diagnosis of osteoporosis.2 Expert review of radiographic reports identified at least 1 mild vertebral fracture in 32% of the study population, but one-third of these cases had not been identified before the review.
Determine bone mineral density. Detection of osteoporosis depends in part on measuring bone mineral density (BMD) of the hip and spine by dual-energy x-ray absorptiometry scan. BMD correlates with bone strength and helps predict fracture risk. The World Health Organization has established definitions for bone integrity based on BMD (TABLE).3
One standard deviation below normal equals a loss of 10% to 15% of bone mass. A patient with a T-score of –2.5 has lost >25% of bone mass. Although patients with osteoporosis have the highest probability of fracture, studies have consistently found that patients with osteopenia can also sustain fractures. Therefore, assess other risk factors for bone loss (identified below) when evaluating a patient.4-6
A useful assessment tool. To identify patients at risk for fractures, the World Health Organization offers a Web-based Fracture Risk Assessment Tool (FRAX).7 This tool, which takes into account risk factors and a femoral neck T-score, helps predict the 10-year probability of a hip or other major osteoporotic fracture. FRAX recognizes the following risk factors: age, sex, weight, height, fracture history, parental history of a hip fracture, cigarette use, long-term use of glucocorticoids, rheumatoid arthritis, concomitant disorders known to cause secondary osteoporosis, and daily alcohol consumption. If a T-score is not available, a patient’s body mass index may be used to estimate fracture risk. This free tool is available at www.shef.ac.uk/FRAX. Fracture risk calculation may also help resolve questions about when to initiate treatment for patients with osteopenia.
TABLE
Defining bone integrity by bone mineral density
| Normal | BMD is within 1 SD of that of a young normal adult (T-score ≥–1.0) |
| Low bone mass (osteopenia) | BMD is between 1.0 and 2.5 SD below that of a young normal adult (T-score between –1.0 and –2.5) |
| Osteoporosis | BMD is at least 2.5 SD below that of a young normal adult (T-score ≤–2.5) |
| BMD, bone mineral density; SD, standard deviation. | |
| Source: WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series, No. 843. 1994.3 | |
Undertreatment of osteoporosis is also a significant problem
A study based on National Health and Nutrition Examination Survey data reported that fewer than 20% of women and men who had sustained an osteoporotic fracture or were at high risk for fracture were being treated with antiresorptive agents.8
National Osteoporosis Foundation (NOF) guidelines9 recommend considering treatment for postmenopausal women 50 years of age or older who exhibit the following:
- A hip or vertebral fracture
- T-score ≤–2.5 at the femoral neck, total hip, or spine after appropriate evaluation to exclude secondary causes
- Low bone mass and a 10-year probability of hip fracture ≥3%, or a 10-year probability of any major osteoporosis-related fracture ≥20%, based on the FRAX calculation.
The NOF Clinician’s Guide to Prevention and Treatment of Osteoporosis is available at http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf.
Also discuss with patients the importance of adequate supplementation with calcium and vitamin D. All patients with osteoporosis should consume at least 1200 mg of calcium and 800 to 1000 IU of vitamin D each day.9
Oral bisphosphonate therapies are effective …
Oral bisphosphonates (alendronate, risedronate, and ibandronate) effectively reduce fracture risk at various skeletal sites, and are generally safe and well tolerated. In a 3-year clinical trial involving postmenopausal women with low femoral neck BMD and at least 1 baseline vertebral fracture, alendronate 10 mg/d, compared with placebo, reduced the risk of vertebral fracture by 47% (8% vs 15%; absolute risk reduction [ARR], 7%) and hip fracture by 51% (1.1% vs 2.2%; ARR, 1.1%).10 Risedronate 5 mg/d has been shown to reduce the risk of vertebral fracture compared with placebo by up to 49% (18% vs 29%; ARR, 11%)11 and nonvertebral fracture by up to 39% (5.2% vs 8.4%; ARR, 3.2%)12 in similar populations. Oral ibandronate reduced the risk of vertebral fracture by 52% (4.7% vs 9.6%; ARR, 4.9%) at doses of 2.5 mg/d compared with placebo; this study was not powered to assess hip fracture reduction, and there was a similar number of nonvertebral osteoporotic fractures in both groups.13
… but patients have trouble with compliance
Rates of compliance (taking medication regularly and properly) at 1 year are poor, ranging from 30% to 55%.14-16 Studies show a strong link between poor compliance with oral bisphosphonates and increased fracture rates, as well as increased health care costs.15-20
Compliance problems associated with oral bisphosphonates may be attributed, in part, to their complex dosing requirements.14 Because these agents are poorly absorbed, patients must fast overnight before ingestion, and then avoid eating, drinking, or taking other medications for 30 to 60 minutes afterward. To reduce the potential for gastrointestinal (GI) irritation, patients must also maintain an upright position for at least 30 to 60 minutes after ingestion. Even when health care providers give complete instructions, between 25% and 50% of patients disregard at least 1 requirement.21,22
The complex dosing regimens with oral bisphosphonates may be particularly challenging for patients with cognitive deficits. Noncompliance also rises with an increase in the number of comorbid conditions, with an increase in the number of medications a patient takes unrelated to osteoporosis, and with older age.23
Studies examining persistence (the length of time a patient continues treatment) with osteoporosis therapy have confirmed that 32% to 55% of patients stop taking their oral medications for osteoporosis within 1 year.14,15,23,24 Several studies have reported that weekly dosing is associated with greater persistence than daily osteoporosis therapy.14 Monthly dosing may yield additional improvements. For example, an open-label, randomized trial found that patients who received once-monthly ibandronate and support measures showed greater persistence at 6 months, compared with those receiving weekly alendronate (56.6% vs 38.6%, P<.0001).24 Nevertheless, weekly and monthly regimens result in only modest improvements in persistence, and approximately 50% of all patients discontinue therapy by 6 months.14,24-26 The recent availability of IV bisphosphonates administered under medical supervision offers a potential advantage in increasing persistence.
What we know about the effectiveness of IV bisphosphonates
IV ibandronate 3 mg every 3 months and IV zoledronic acid 5 mg every 12 months are approved for the treatment of postmenopausal osteoporosis. Health care professionals can provide the ibandronate IV injection or zoledronic acid infusion in their office or refer patients to a local provider or infusion center.
Ibandronate. The effectiveness of IV ibandronate is based on clinical trials with daily oral ibandronate, such as the BONE (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) study (FIGURE 1).
The DIVA (Dosing IntraVenous Administration) study compared IV ibandronate 3 mg every 3 months (n=471) with daily oral dosing with ibandronate 2.5 mg (n=470) in women with postmenopausal osteoporosis.27 The IV regimen was significantly superior to daily oral dosing, with increases in mean lumbar spine BMD of 4.8% for the IV formulation and 3.8% for daily oral therapy (P<.001). Similar improvements in BMD occurred at other bone sites.
Zoledronic acid. The effectiveness of IV zoledronic acid, administered over a period lasting at least 15 minutes, was shown in the 3-year Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).28 An annual infusion of zoledronic acid 5 mg in postmenopausal women with osteoporosis (n=3875) compared with placebo (n=3861) reduced the risk of hip fractures, vertebral fractures, and nonvertebral fractures by 41% (1.4% vs 2.5%; ARR, 1.1%), 70% (3.3% vs 10.9%; ARR, 7.6%), and 25% (8% vs 10.7%; ARR, 2.7%), respectively (P≤.002) (FIGURE 2). The numbers of patients needed to treat (NNT) to prevent 1 fracture at 3 years were 91, 13, and 37 for hip, vertebral, and nonvertebral fractures, respectively. IV zoledronic acid also significantly increased BMD and reduced bone turnover markers.
Therapy underused after hip fracture. Most patients are not properly evaluated or treated for osteoporosis after a hip fracture. Zoledronic acid is the only bisphosphonate that has been specifically studied in this population to evaluate its effectiveness in preventing additional fractures. In the HORIZON-Recurrent Fracture Trial, patients were randomized to receive IV zoledronic acid 5 mg (n=1065) or placebo (n=1062) within 90 days after repair of a hip fracture and yearly thereafter.29 Compared with placebo, an annual infusion of IV zoledronic acid resulted in a 35% reduction in the rate of any new clinical fracture (13.9% vs 8.6%; P=.001; NNT to prevent 1 fracture at 3 years=19). BMD of the contralateral hip increased in the zoledronic acid group by 5.5% after 3 years compared with a 0.9% decrease in the placebo group (P<.001). In addition, there was a 28% reduction in all-cause mortality (9.6% for zoledronic acid vs 13.3% for placebo; P=.01).
This study demonstrated that a yearly infusion of zoledronic acid is effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture and that it may reduce all-cause mortality. These results support the need for careful discharge planning after a hip fracture to include appropriate treatment to prevent subsequent fractures.
FIGURE 1
Daily oral ibandronate (2.5 mg*) reduced risk of vertebral fractures in the BONE study (n=982)
*Ibandronate 2.5 mg tablets are not available on the market.
†P<.001 vs placebo.
‡No significant difference vs placebo.
BONE, oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe; RR, relative risk reduction.
Data from Chesnut CH II, et al. J Bone Miner Res. 2004.13
FIGURE 2
Intravenous zoledronic acid (5 mg, once yearly) reduced risk of fractures in the HORIZON-Pivotal Fracture Trial (n=7736)
*P≤.002 vs placebo.
†Hip fracture was not excluded from analysis of nonvertebral fracture.
HORIZON, Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly; RR, relative risk reduction.
Data from Black DM et al. N Engl J Med. 2007.28
GI issues and flu-like symptoms are among the side effects
Side effects of oral bisphosphonates include such GI problems as dyspepsia, nausea, and reflux. There is also a small risk of developing inflammation of the esophagus and gastric ulcers.
In some patients, IV bisphosphonates can cause transient flu-like symptoms (eg, myalgia, headache, pyrexia) within 3 days of administration.27-30 These symptoms are generally mild and last a few days, and they can be reduced by taking acetaminophen or ibuprofen for several days after the injection or infusion. Symptoms are uncommon with subsequent injections or infusions. Counsel patients about the possibility of these symptoms and how to manage them.
The safety of switching directly from weekly oral alendronate 70 mg to IV zoledronic acid 5 mg was evaluated in a 12-month randomized, double-blind study. The results showed that switching to zoledronic acid was well tolerated.31 The overall cost of IV annual zoledronic acid is similar to the annual cost of a branded oral bisphosphonate.
When to avoid bisphosphonates
Give no bisphosphonate, oral or IV, to a patient with significant renal impairment because the drugs are excreted renally. Be sure a patient’s calcium level is normal and that she is not vitamin D deficient. Due to the use of sunscreens and sun avoidance, vitamin D deficiency is common, even in sunny climates. Consider using vitamin D supplementation, as needed, before infusion of an IV bisphosphonate. And emphasize the importance of lifelong calcium and vitamin D supplementation to all patients with osteoporosis.
Osteonecrosis of the jaw (ONJ) in bisphosphonate-treated patients with osteoporosis is rare (<1 in 100,000 patient-years in non-cancer patients).32,33 Most cases of ONJ in this setting have occurred “in cancer patients treated with intravenous bisphosphonates undergoing dental procedures. Some cases have occurred in patients with postmenopausal osteoporosis treated with either oral or intravenous bisphosphonates.”34 A prescriber should perform a routine oral examination before initiating bisphosphonate treatment. Consider referring patients who have a history of concomitant risk factors (eg, cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene, pre-existing dental disease or infection, anemia, coagulopathy) for a dental examination and appropriate preventive dentistry.
While on treatment, patients with concomitant risk factors should avoid invasive dental procedures, if possible. If ONJ develops during bisphosphonate therapy, dental surgery may exacerbate the condition. If a dental procedure is unavoidable, no data are available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. The treating physician must rely on clinical judgment regarding the benefits/risks for an individual.34
Possible downside to long-term use. Two studies presented at the 2010 annual meeting of the American Academy of Orthopaedic Surgeons showed that long-term use of oral bisphosphonates may diminish bone quality while increasing bone quantity, perhaps increasing the risk of atypical femoral fractures. In one study of 111 postmenopausal women—61 who received bisphosphonate therapy and 50 non-bisphosphonate controls—the bisphosphonate group exhibited improved structural integrity early in the course of treatment, but those gains were diminished at 4 years.35
In the other study, bone biopsy samples taken from the lateral femur in 21 postmenopausal women with femoral fractures (12 who received bisphosphonate therapy for an average of 8.5 years and 9 non-bisphosphonate controls) indicated that bisphosphonate therapy reduced the heterogeneity of bone tissue properties.36
The Food and Drug Administration has said it is looking closely at all evidence, but that its review of data to date has not shown an unequivocal association between bisphosphonate use and increased risk of atypical femoral fractures.37
A new approach to the management of osteoporosis
To avoid noncompliance problems and the associated increase in fracture risk, consider IV bisphosphonates for first-line therapy in women with postmenopausal osteoporosis. The intermittent dosing regimens of IV bisphosphonates ensure 100% persistence throughout the dosing interval.
CORRESPONDENCE Raymond Cole, DO, Michigan State University College of Osteopathic Medicine, Osteoporosis Testing Center of Michigan, 107 Chicago Street, Brooklyn, MI 49230; [email protected]
1. Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:211-219.
2. Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT Study. J Bone Miner Res. 2005;20:557-563.
3. WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1994. WHO Technical Report Series, No. 843.
4. Siris ES, Chen Y-T, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
5. Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17:1404-1409.
6. Cranney A, Jamal SA, Tsang JF, et al. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575-580.
7. Kanis JA, Burlet N, Cooper C, et al. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399-428.
8. Gehlbach SH, Avrunin JS, Puleo E, et al. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805-810.
9. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed March 25, 2008.
10. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
11. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
12. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352.
13. Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249.
14. Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023-1031.
15. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271-277.
16. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013-1022.
17. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003-1008.
18. Briesacher BA, Andrade SE, Yood RA, et al. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882-887.
19. Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811-818.
20. Earnshaw SR, Graham CN, Ettinger B, et al. Cost-effectiveness of bisphosphonate therapies for women with postmenopausal osteoporosis: implications of improved persistence with less frequently administered oral bisphosphonates. Curr Med Res Opin. 2007;23:2517-2529.
21. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259-262.
22. Ettinger MP. Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237-2246.
23. Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414-2419.
24. Cooper A, Drake J, Brankin E. The PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896-905.
25. Weiss TW, Henderson SC, McHorney CA, et al. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Res Med Opin. 2007;23:2193-2203.
26. Penning-van Beest FJA, Goettsch WG, Erkens JA, et al. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236-242.
27. Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis. Arthritis Rheum. 2006;54:1838-1846.
28. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
29. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
30. Adami S, Felsenberg D, Christiansen C, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone. 2004;34:881-889.
31. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122-128.
32. Felsenberg D, Hoffmeister B, Amling M, et al. Kiefernekrosen nach hoch dosierter Bisphosphonattherapie. Dtsch Arztebl. 2006;103:3078-3081.
33. American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137:1144-1150.
34. Reclast [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; October 2009. Available at: http://www.pharma.us.novartis.com:80/product/pi/pdf/reclast.pdf. Accessed May 12, 2010.
35. Ding A. The structural effects of long-term bisphosphonate treatment leading to atypical hip fractures. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 10, 2010; New Orleans, La.
36. Gladnick B. The effects of long-term bisphosphonate use on bone quality. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11, 2010; New Orleans, La.
37. FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed April 20, 2010.
Using the World Health Organization’s online Fracture Risk Assessment Tool (FRAX) may help you decide when to initiate treatment for patients with osteopenia. C
Consider using intravenous bisphosphonates as first-line therapy for women with postmenopausal osteoporosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
To prevent bone loss and fractures in postmenopausal osteoporosis, the best choice of medication is one a patient will actually take. Bisphosphonates are the standard of care for maintaining or increasing bone mass and reducing excessive bone turnover,1 and oral bisphosphonates have proven to be safe and effective in reducing osteoporotic fractures. However, numerous studies have shown that the effectiveness of oral bisphosphonates is compromised by poor patient compliance in taking the medication as directed and by poor persistence in continuing the medication over the long term.
Intravenous (IV) bisphosphonates are another option: They bypass the GI tract and thereby avoid the difficult requirements of oral dosing that many patients end up disregarding. And because IV administration occurs under medical supervision, it ensures persistence throughout the entire dosing interval. IV ibandronate 3 mg is administered every 3 months, and IV zoledronic acid 5 mg is administered once a year.
Osteoporosis and osteoporotic fractures are markedly underdiagnosed
Delmas and colleagues evaluated the underdetection of vertebral fractures in an international, multicenter prospective study of 2451 women 65 to 80 years of age who had not received a diagnosis of osteoporosis.2 Expert review of radiographic reports identified at least 1 mild vertebral fracture in 32% of the study population, but one-third of these cases had not been identified before the review.
Determine bone mineral density. Detection of osteoporosis depends in part on measuring bone mineral density (BMD) of the hip and spine by dual-energy x-ray absorptiometry scan. BMD correlates with bone strength and helps predict fracture risk. The World Health Organization has established definitions for bone integrity based on BMD (TABLE).3
One standard deviation below normal equals a loss of 10% to 15% of bone mass. A patient with a T-score of –2.5 has lost >25% of bone mass. Although patients with osteoporosis have the highest probability of fracture, studies have consistently found that patients with osteopenia can also sustain fractures. Therefore, assess other risk factors for bone loss (identified below) when evaluating a patient.4-6
A useful assessment tool. To identify patients at risk for fractures, the World Health Organization offers a Web-based Fracture Risk Assessment Tool (FRAX).7 This tool, which takes into account risk factors and a femoral neck T-score, helps predict the 10-year probability of a hip or other major osteoporotic fracture. FRAX recognizes the following risk factors: age, sex, weight, height, fracture history, parental history of a hip fracture, cigarette use, long-term use of glucocorticoids, rheumatoid arthritis, concomitant disorders known to cause secondary osteoporosis, and daily alcohol consumption. If a T-score is not available, a patient’s body mass index may be used to estimate fracture risk. This free tool is available at www.shef.ac.uk/FRAX. Fracture risk calculation may also help resolve questions about when to initiate treatment for patients with osteopenia.
TABLE
Defining bone integrity by bone mineral density
| Normal | BMD is within 1 SD of that of a young normal adult (T-score ≥–1.0) |
| Low bone mass (osteopenia) | BMD is between 1.0 and 2.5 SD below that of a young normal adult (T-score between –1.0 and –2.5) |
| Osteoporosis | BMD is at least 2.5 SD below that of a young normal adult (T-score ≤–2.5) |
| BMD, bone mineral density; SD, standard deviation. | |
| Source: WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series, No. 843. 1994.3 | |
Undertreatment of osteoporosis is also a significant problem
A study based on National Health and Nutrition Examination Survey data reported that fewer than 20% of women and men who had sustained an osteoporotic fracture or were at high risk for fracture were being treated with antiresorptive agents.8
National Osteoporosis Foundation (NOF) guidelines9 recommend considering treatment for postmenopausal women 50 years of age or older who exhibit the following:
- A hip or vertebral fracture
- T-score ≤–2.5 at the femoral neck, total hip, or spine after appropriate evaluation to exclude secondary causes
- Low bone mass and a 10-year probability of hip fracture ≥3%, or a 10-year probability of any major osteoporosis-related fracture ≥20%, based on the FRAX calculation.
The NOF Clinician’s Guide to Prevention and Treatment of Osteoporosis is available at http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf.
Also discuss with patients the importance of adequate supplementation with calcium and vitamin D. All patients with osteoporosis should consume at least 1200 mg of calcium and 800 to 1000 IU of vitamin D each day.9
Oral bisphosphonate therapies are effective …
Oral bisphosphonates (alendronate, risedronate, and ibandronate) effectively reduce fracture risk at various skeletal sites, and are generally safe and well tolerated. In a 3-year clinical trial involving postmenopausal women with low femoral neck BMD and at least 1 baseline vertebral fracture, alendronate 10 mg/d, compared with placebo, reduced the risk of vertebral fracture by 47% (8% vs 15%; absolute risk reduction [ARR], 7%) and hip fracture by 51% (1.1% vs 2.2%; ARR, 1.1%).10 Risedronate 5 mg/d has been shown to reduce the risk of vertebral fracture compared with placebo by up to 49% (18% vs 29%; ARR, 11%)11 and nonvertebral fracture by up to 39% (5.2% vs 8.4%; ARR, 3.2%)12 in similar populations. Oral ibandronate reduced the risk of vertebral fracture by 52% (4.7% vs 9.6%; ARR, 4.9%) at doses of 2.5 mg/d compared with placebo; this study was not powered to assess hip fracture reduction, and there was a similar number of nonvertebral osteoporotic fractures in both groups.13
… but patients have trouble with compliance
Rates of compliance (taking medication regularly and properly) at 1 year are poor, ranging from 30% to 55%.14-16 Studies show a strong link between poor compliance with oral bisphosphonates and increased fracture rates, as well as increased health care costs.15-20
Compliance problems associated with oral bisphosphonates may be attributed, in part, to their complex dosing requirements.14 Because these agents are poorly absorbed, patients must fast overnight before ingestion, and then avoid eating, drinking, or taking other medications for 30 to 60 minutes afterward. To reduce the potential for gastrointestinal (GI) irritation, patients must also maintain an upright position for at least 30 to 60 minutes after ingestion. Even when health care providers give complete instructions, between 25% and 50% of patients disregard at least 1 requirement.21,22
The complex dosing regimens with oral bisphosphonates may be particularly challenging for patients with cognitive deficits. Noncompliance also rises with an increase in the number of comorbid conditions, with an increase in the number of medications a patient takes unrelated to osteoporosis, and with older age.23
Studies examining persistence (the length of time a patient continues treatment) with osteoporosis therapy have confirmed that 32% to 55% of patients stop taking their oral medications for osteoporosis within 1 year.14,15,23,24 Several studies have reported that weekly dosing is associated with greater persistence than daily osteoporosis therapy.14 Monthly dosing may yield additional improvements. For example, an open-label, randomized trial found that patients who received once-monthly ibandronate and support measures showed greater persistence at 6 months, compared with those receiving weekly alendronate (56.6% vs 38.6%, P<.0001).24 Nevertheless, weekly and monthly regimens result in only modest improvements in persistence, and approximately 50% of all patients discontinue therapy by 6 months.14,24-26 The recent availability of IV bisphosphonates administered under medical supervision offers a potential advantage in increasing persistence.
What we know about the effectiveness of IV bisphosphonates
IV ibandronate 3 mg every 3 months and IV zoledronic acid 5 mg every 12 months are approved for the treatment of postmenopausal osteoporosis. Health care professionals can provide the ibandronate IV injection or zoledronic acid infusion in their office or refer patients to a local provider or infusion center.
Ibandronate. The effectiveness of IV ibandronate is based on clinical trials with daily oral ibandronate, such as the BONE (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) study (FIGURE 1).
The DIVA (Dosing IntraVenous Administration) study compared IV ibandronate 3 mg every 3 months (n=471) with daily oral dosing with ibandronate 2.5 mg (n=470) in women with postmenopausal osteoporosis.27 The IV regimen was significantly superior to daily oral dosing, with increases in mean lumbar spine BMD of 4.8% for the IV formulation and 3.8% for daily oral therapy (P<.001). Similar improvements in BMD occurred at other bone sites.
Zoledronic acid. The effectiveness of IV zoledronic acid, administered over a period lasting at least 15 minutes, was shown in the 3-year Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).28 An annual infusion of zoledronic acid 5 mg in postmenopausal women with osteoporosis (n=3875) compared with placebo (n=3861) reduced the risk of hip fractures, vertebral fractures, and nonvertebral fractures by 41% (1.4% vs 2.5%; ARR, 1.1%), 70% (3.3% vs 10.9%; ARR, 7.6%), and 25% (8% vs 10.7%; ARR, 2.7%), respectively (P≤.002) (FIGURE 2). The numbers of patients needed to treat (NNT) to prevent 1 fracture at 3 years were 91, 13, and 37 for hip, vertebral, and nonvertebral fractures, respectively. IV zoledronic acid also significantly increased BMD and reduced bone turnover markers.
Therapy underused after hip fracture. Most patients are not properly evaluated or treated for osteoporosis after a hip fracture. Zoledronic acid is the only bisphosphonate that has been specifically studied in this population to evaluate its effectiveness in preventing additional fractures. In the HORIZON-Recurrent Fracture Trial, patients were randomized to receive IV zoledronic acid 5 mg (n=1065) or placebo (n=1062) within 90 days after repair of a hip fracture and yearly thereafter.29 Compared with placebo, an annual infusion of IV zoledronic acid resulted in a 35% reduction in the rate of any new clinical fracture (13.9% vs 8.6%; P=.001; NNT to prevent 1 fracture at 3 years=19). BMD of the contralateral hip increased in the zoledronic acid group by 5.5% after 3 years compared with a 0.9% decrease in the placebo group (P<.001). In addition, there was a 28% reduction in all-cause mortality (9.6% for zoledronic acid vs 13.3% for placebo; P=.01).
This study demonstrated that a yearly infusion of zoledronic acid is effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture and that it may reduce all-cause mortality. These results support the need for careful discharge planning after a hip fracture to include appropriate treatment to prevent subsequent fractures.
FIGURE 1
Daily oral ibandronate (2.5 mg*) reduced risk of vertebral fractures in the BONE study (n=982)
*Ibandronate 2.5 mg tablets are not available on the market.
†P<.001 vs placebo.
‡No significant difference vs placebo.
BONE, oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe; RR, relative risk reduction.
Data from Chesnut CH II, et al. J Bone Miner Res. 2004.13
FIGURE 2
Intravenous zoledronic acid (5 mg, once yearly) reduced risk of fractures in the HORIZON-Pivotal Fracture Trial (n=7736)
*P≤.002 vs placebo.
†Hip fracture was not excluded from analysis of nonvertebral fracture.
HORIZON, Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly; RR, relative risk reduction.
Data from Black DM et al. N Engl J Med. 2007.28
GI issues and flu-like symptoms are among the side effects
Side effects of oral bisphosphonates include such GI problems as dyspepsia, nausea, and reflux. There is also a small risk of developing inflammation of the esophagus and gastric ulcers.
In some patients, IV bisphosphonates can cause transient flu-like symptoms (eg, myalgia, headache, pyrexia) within 3 days of administration.27-30 These symptoms are generally mild and last a few days, and they can be reduced by taking acetaminophen or ibuprofen for several days after the injection or infusion. Symptoms are uncommon with subsequent injections or infusions. Counsel patients about the possibility of these symptoms and how to manage them.
The safety of switching directly from weekly oral alendronate 70 mg to IV zoledronic acid 5 mg was evaluated in a 12-month randomized, double-blind study. The results showed that switching to zoledronic acid was well tolerated.31 The overall cost of IV annual zoledronic acid is similar to the annual cost of a branded oral bisphosphonate.
When to avoid bisphosphonates
Give no bisphosphonate, oral or IV, to a patient with significant renal impairment because the drugs are excreted renally. Be sure a patient’s calcium level is normal and that she is not vitamin D deficient. Due to the use of sunscreens and sun avoidance, vitamin D deficiency is common, even in sunny climates. Consider using vitamin D supplementation, as needed, before infusion of an IV bisphosphonate. And emphasize the importance of lifelong calcium and vitamin D supplementation to all patients with osteoporosis.
Osteonecrosis of the jaw (ONJ) in bisphosphonate-treated patients with osteoporosis is rare (<1 in 100,000 patient-years in non-cancer patients).32,33 Most cases of ONJ in this setting have occurred “in cancer patients treated with intravenous bisphosphonates undergoing dental procedures. Some cases have occurred in patients with postmenopausal osteoporosis treated with either oral or intravenous bisphosphonates.”34 A prescriber should perform a routine oral examination before initiating bisphosphonate treatment. Consider referring patients who have a history of concomitant risk factors (eg, cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene, pre-existing dental disease or infection, anemia, coagulopathy) for a dental examination and appropriate preventive dentistry.
While on treatment, patients with concomitant risk factors should avoid invasive dental procedures, if possible. If ONJ develops during bisphosphonate therapy, dental surgery may exacerbate the condition. If a dental procedure is unavoidable, no data are available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. The treating physician must rely on clinical judgment regarding the benefits/risks for an individual.34
Possible downside to long-term use. Two studies presented at the 2010 annual meeting of the American Academy of Orthopaedic Surgeons showed that long-term use of oral bisphosphonates may diminish bone quality while increasing bone quantity, perhaps increasing the risk of atypical femoral fractures. In one study of 111 postmenopausal women—61 who received bisphosphonate therapy and 50 non-bisphosphonate controls—the bisphosphonate group exhibited improved structural integrity early in the course of treatment, but those gains were diminished at 4 years.35
In the other study, bone biopsy samples taken from the lateral femur in 21 postmenopausal women with femoral fractures (12 who received bisphosphonate therapy for an average of 8.5 years and 9 non-bisphosphonate controls) indicated that bisphosphonate therapy reduced the heterogeneity of bone tissue properties.36
The Food and Drug Administration has said it is looking closely at all evidence, but that its review of data to date has not shown an unequivocal association between bisphosphonate use and increased risk of atypical femoral fractures.37
A new approach to the management of osteoporosis
To avoid noncompliance problems and the associated increase in fracture risk, consider IV bisphosphonates for first-line therapy in women with postmenopausal osteoporosis. The intermittent dosing regimens of IV bisphosphonates ensure 100% persistence throughout the dosing interval.
CORRESPONDENCE Raymond Cole, DO, Michigan State University College of Osteopathic Medicine, Osteoporosis Testing Center of Michigan, 107 Chicago Street, Brooklyn, MI 49230; [email protected]
Using the World Health Organization’s online Fracture Risk Assessment Tool (FRAX) may help you decide when to initiate treatment for patients with osteopenia. C
Consider using intravenous bisphosphonates as first-line therapy for women with postmenopausal osteoporosis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
To prevent bone loss and fractures in postmenopausal osteoporosis, the best choice of medication is one a patient will actually take. Bisphosphonates are the standard of care for maintaining or increasing bone mass and reducing excessive bone turnover,1 and oral bisphosphonates have proven to be safe and effective in reducing osteoporotic fractures. However, numerous studies have shown that the effectiveness of oral bisphosphonates is compromised by poor patient compliance in taking the medication as directed and by poor persistence in continuing the medication over the long term.
Intravenous (IV) bisphosphonates are another option: They bypass the GI tract and thereby avoid the difficult requirements of oral dosing that many patients end up disregarding. And because IV administration occurs under medical supervision, it ensures persistence throughout the entire dosing interval. IV ibandronate 3 mg is administered every 3 months, and IV zoledronic acid 5 mg is administered once a year.
Osteoporosis and osteoporotic fractures are markedly underdiagnosed
Delmas and colleagues evaluated the underdetection of vertebral fractures in an international, multicenter prospective study of 2451 women 65 to 80 years of age who had not received a diagnosis of osteoporosis.2 Expert review of radiographic reports identified at least 1 mild vertebral fracture in 32% of the study population, but one-third of these cases had not been identified before the review.
Determine bone mineral density. Detection of osteoporosis depends in part on measuring bone mineral density (BMD) of the hip and spine by dual-energy x-ray absorptiometry scan. BMD correlates with bone strength and helps predict fracture risk. The World Health Organization has established definitions for bone integrity based on BMD (TABLE).3
One standard deviation below normal equals a loss of 10% to 15% of bone mass. A patient with a T-score of –2.5 has lost >25% of bone mass. Although patients with osteoporosis have the highest probability of fracture, studies have consistently found that patients with osteopenia can also sustain fractures. Therefore, assess other risk factors for bone loss (identified below) when evaluating a patient.4-6
A useful assessment tool. To identify patients at risk for fractures, the World Health Organization offers a Web-based Fracture Risk Assessment Tool (FRAX).7 This tool, which takes into account risk factors and a femoral neck T-score, helps predict the 10-year probability of a hip or other major osteoporotic fracture. FRAX recognizes the following risk factors: age, sex, weight, height, fracture history, parental history of a hip fracture, cigarette use, long-term use of glucocorticoids, rheumatoid arthritis, concomitant disorders known to cause secondary osteoporosis, and daily alcohol consumption. If a T-score is not available, a patient’s body mass index may be used to estimate fracture risk. This free tool is available at www.shef.ac.uk/FRAX. Fracture risk calculation may also help resolve questions about when to initiate treatment for patients with osteopenia.
TABLE
Defining bone integrity by bone mineral density
| Normal | BMD is within 1 SD of that of a young normal adult (T-score ≥–1.0) |
| Low bone mass (osteopenia) | BMD is between 1.0 and 2.5 SD below that of a young normal adult (T-score between –1.0 and –2.5) |
| Osteoporosis | BMD is at least 2.5 SD below that of a young normal adult (T-score ≤–2.5) |
| BMD, bone mineral density; SD, standard deviation. | |
| Source: WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. WHO Technical Report Series, No. 843. 1994.3 | |
Undertreatment of osteoporosis is also a significant problem
A study based on National Health and Nutrition Examination Survey data reported that fewer than 20% of women and men who had sustained an osteoporotic fracture or were at high risk for fracture were being treated with antiresorptive agents.8
National Osteoporosis Foundation (NOF) guidelines9 recommend considering treatment for postmenopausal women 50 years of age or older who exhibit the following:
- A hip or vertebral fracture
- T-score ≤–2.5 at the femoral neck, total hip, or spine after appropriate evaluation to exclude secondary causes
- Low bone mass and a 10-year probability of hip fracture ≥3%, or a 10-year probability of any major osteoporosis-related fracture ≥20%, based on the FRAX calculation.
The NOF Clinician’s Guide to Prevention and Treatment of Osteoporosis is available at http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf.
Also discuss with patients the importance of adequate supplementation with calcium and vitamin D. All patients with osteoporosis should consume at least 1200 mg of calcium and 800 to 1000 IU of vitamin D each day.9
Oral bisphosphonate therapies are effective …
Oral bisphosphonates (alendronate, risedronate, and ibandronate) effectively reduce fracture risk at various skeletal sites, and are generally safe and well tolerated. In a 3-year clinical trial involving postmenopausal women with low femoral neck BMD and at least 1 baseline vertebral fracture, alendronate 10 mg/d, compared with placebo, reduced the risk of vertebral fracture by 47% (8% vs 15%; absolute risk reduction [ARR], 7%) and hip fracture by 51% (1.1% vs 2.2%; ARR, 1.1%).10 Risedronate 5 mg/d has been shown to reduce the risk of vertebral fracture compared with placebo by up to 49% (18% vs 29%; ARR, 11%)11 and nonvertebral fracture by up to 39% (5.2% vs 8.4%; ARR, 3.2%)12 in similar populations. Oral ibandronate reduced the risk of vertebral fracture by 52% (4.7% vs 9.6%; ARR, 4.9%) at doses of 2.5 mg/d compared with placebo; this study was not powered to assess hip fracture reduction, and there was a similar number of nonvertebral osteoporotic fractures in both groups.13
… but patients have trouble with compliance
Rates of compliance (taking medication regularly and properly) at 1 year are poor, ranging from 30% to 55%.14-16 Studies show a strong link between poor compliance with oral bisphosphonates and increased fracture rates, as well as increased health care costs.15-20
Compliance problems associated with oral bisphosphonates may be attributed, in part, to their complex dosing requirements.14 Because these agents are poorly absorbed, patients must fast overnight before ingestion, and then avoid eating, drinking, or taking other medications for 30 to 60 minutes afterward. To reduce the potential for gastrointestinal (GI) irritation, patients must also maintain an upright position for at least 30 to 60 minutes after ingestion. Even when health care providers give complete instructions, between 25% and 50% of patients disregard at least 1 requirement.21,22
The complex dosing regimens with oral bisphosphonates may be particularly challenging for patients with cognitive deficits. Noncompliance also rises with an increase in the number of comorbid conditions, with an increase in the number of medications a patient takes unrelated to osteoporosis, and with older age.23
Studies examining persistence (the length of time a patient continues treatment) with osteoporosis therapy have confirmed that 32% to 55% of patients stop taking their oral medications for osteoporosis within 1 year.14,15,23,24 Several studies have reported that weekly dosing is associated with greater persistence than daily osteoporosis therapy.14 Monthly dosing may yield additional improvements. For example, an open-label, randomized trial found that patients who received once-monthly ibandronate and support measures showed greater persistence at 6 months, compared with those receiving weekly alendronate (56.6% vs 38.6%, P<.0001).24 Nevertheless, weekly and monthly regimens result in only modest improvements in persistence, and approximately 50% of all patients discontinue therapy by 6 months.14,24-26 The recent availability of IV bisphosphonates administered under medical supervision offers a potential advantage in increasing persistence.
What we know about the effectiveness of IV bisphosphonates
IV ibandronate 3 mg every 3 months and IV zoledronic acid 5 mg every 12 months are approved for the treatment of postmenopausal osteoporosis. Health care professionals can provide the ibandronate IV injection or zoledronic acid infusion in their office or refer patients to a local provider or infusion center.
Ibandronate. The effectiveness of IV ibandronate is based on clinical trials with daily oral ibandronate, such as the BONE (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) study (FIGURE 1).
The DIVA (Dosing IntraVenous Administration) study compared IV ibandronate 3 mg every 3 months (n=471) with daily oral dosing with ibandronate 2.5 mg (n=470) in women with postmenopausal osteoporosis.27 The IV regimen was significantly superior to daily oral dosing, with increases in mean lumbar spine BMD of 4.8% for the IV formulation and 3.8% for daily oral therapy (P<.001). Similar improvements in BMD occurred at other bone sites.
Zoledronic acid. The effectiveness of IV zoledronic acid, administered over a period lasting at least 15 minutes, was shown in the 3-year Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).28 An annual infusion of zoledronic acid 5 mg in postmenopausal women with osteoporosis (n=3875) compared with placebo (n=3861) reduced the risk of hip fractures, vertebral fractures, and nonvertebral fractures by 41% (1.4% vs 2.5%; ARR, 1.1%), 70% (3.3% vs 10.9%; ARR, 7.6%), and 25% (8% vs 10.7%; ARR, 2.7%), respectively (P≤.002) (FIGURE 2). The numbers of patients needed to treat (NNT) to prevent 1 fracture at 3 years were 91, 13, and 37 for hip, vertebral, and nonvertebral fractures, respectively. IV zoledronic acid also significantly increased BMD and reduced bone turnover markers.
Therapy underused after hip fracture. Most patients are not properly evaluated or treated for osteoporosis after a hip fracture. Zoledronic acid is the only bisphosphonate that has been specifically studied in this population to evaluate its effectiveness in preventing additional fractures. In the HORIZON-Recurrent Fracture Trial, patients were randomized to receive IV zoledronic acid 5 mg (n=1065) or placebo (n=1062) within 90 days after repair of a hip fracture and yearly thereafter.29 Compared with placebo, an annual infusion of IV zoledronic acid resulted in a 35% reduction in the rate of any new clinical fracture (13.9% vs 8.6%; P=.001; NNT to prevent 1 fracture at 3 years=19). BMD of the contralateral hip increased in the zoledronic acid group by 5.5% after 3 years compared with a 0.9% decrease in the placebo group (P<.001). In addition, there was a 28% reduction in all-cause mortality (9.6% for zoledronic acid vs 13.3% for placebo; P=.01).
This study demonstrated that a yearly infusion of zoledronic acid is effective in preventing subsequent clinical fractures in patients who have recently suffered a hip fracture and that it may reduce all-cause mortality. These results support the need for careful discharge planning after a hip fracture to include appropriate treatment to prevent subsequent fractures.
FIGURE 1
Daily oral ibandronate (2.5 mg*) reduced risk of vertebral fractures in the BONE study (n=982)
*Ibandronate 2.5 mg tablets are not available on the market.
†P<.001 vs placebo.
‡No significant difference vs placebo.
BONE, oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe; RR, relative risk reduction.
Data from Chesnut CH II, et al. J Bone Miner Res. 2004.13
FIGURE 2
Intravenous zoledronic acid (5 mg, once yearly) reduced risk of fractures in the HORIZON-Pivotal Fracture Trial (n=7736)
*P≤.002 vs placebo.
†Hip fracture was not excluded from analysis of nonvertebral fracture.
HORIZON, Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly; RR, relative risk reduction.
Data from Black DM et al. N Engl J Med. 2007.28
GI issues and flu-like symptoms are among the side effects
Side effects of oral bisphosphonates include such GI problems as dyspepsia, nausea, and reflux. There is also a small risk of developing inflammation of the esophagus and gastric ulcers.
In some patients, IV bisphosphonates can cause transient flu-like symptoms (eg, myalgia, headache, pyrexia) within 3 days of administration.27-30 These symptoms are generally mild and last a few days, and they can be reduced by taking acetaminophen or ibuprofen for several days after the injection or infusion. Symptoms are uncommon with subsequent injections or infusions. Counsel patients about the possibility of these symptoms and how to manage them.
The safety of switching directly from weekly oral alendronate 70 mg to IV zoledronic acid 5 mg was evaluated in a 12-month randomized, double-blind study. The results showed that switching to zoledronic acid was well tolerated.31 The overall cost of IV annual zoledronic acid is similar to the annual cost of a branded oral bisphosphonate.
When to avoid bisphosphonates
Give no bisphosphonate, oral or IV, to a patient with significant renal impairment because the drugs are excreted renally. Be sure a patient’s calcium level is normal and that she is not vitamin D deficient. Due to the use of sunscreens and sun avoidance, vitamin D deficiency is common, even in sunny climates. Consider using vitamin D supplementation, as needed, before infusion of an IV bisphosphonate. And emphasize the importance of lifelong calcium and vitamin D supplementation to all patients with osteoporosis.
Osteonecrosis of the jaw (ONJ) in bisphosphonate-treated patients with osteoporosis is rare (<1 in 100,000 patient-years in non-cancer patients).32,33 Most cases of ONJ in this setting have occurred “in cancer patients treated with intravenous bisphosphonates undergoing dental procedures. Some cases have occurred in patients with postmenopausal osteoporosis treated with either oral or intravenous bisphosphonates.”34 A prescriber should perform a routine oral examination before initiating bisphosphonate treatment. Consider referring patients who have a history of concomitant risk factors (eg, cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene, pre-existing dental disease or infection, anemia, coagulopathy) for a dental examination and appropriate preventive dentistry.
While on treatment, patients with concomitant risk factors should avoid invasive dental procedures, if possible. If ONJ develops during bisphosphonate therapy, dental surgery may exacerbate the condition. If a dental procedure is unavoidable, no data are available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. The treating physician must rely on clinical judgment regarding the benefits/risks for an individual.34
Possible downside to long-term use. Two studies presented at the 2010 annual meeting of the American Academy of Orthopaedic Surgeons showed that long-term use of oral bisphosphonates may diminish bone quality while increasing bone quantity, perhaps increasing the risk of atypical femoral fractures. In one study of 111 postmenopausal women—61 who received bisphosphonate therapy and 50 non-bisphosphonate controls—the bisphosphonate group exhibited improved structural integrity early in the course of treatment, but those gains were diminished at 4 years.35
In the other study, bone biopsy samples taken from the lateral femur in 21 postmenopausal women with femoral fractures (12 who received bisphosphonate therapy for an average of 8.5 years and 9 non-bisphosphonate controls) indicated that bisphosphonate therapy reduced the heterogeneity of bone tissue properties.36
The Food and Drug Administration has said it is looking closely at all evidence, but that its review of data to date has not shown an unequivocal association between bisphosphonate use and increased risk of atypical femoral fractures.37
A new approach to the management of osteoporosis
To avoid noncompliance problems and the associated increase in fracture risk, consider IV bisphosphonates for first-line therapy in women with postmenopausal osteoporosis. The intermittent dosing regimens of IV bisphosphonates ensure 100% persistence throughout the dosing interval.
CORRESPONDENCE Raymond Cole, DO, Michigan State University College of Osteopathic Medicine, Osteoporosis Testing Center of Michigan, 107 Chicago Street, Brooklyn, MI 49230; [email protected]
1. Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:211-219.
2. Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT Study. J Bone Miner Res. 2005;20:557-563.
3. WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1994. WHO Technical Report Series, No. 843.
4. Siris ES, Chen Y-T, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
5. Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17:1404-1409.
6. Cranney A, Jamal SA, Tsang JF, et al. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575-580.
7. Kanis JA, Burlet N, Cooper C, et al. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399-428.
8. Gehlbach SH, Avrunin JS, Puleo E, et al. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805-810.
9. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed March 25, 2008.
10. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
11. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
12. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352.
13. Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249.
14. Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023-1031.
15. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271-277.
16. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013-1022.
17. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003-1008.
18. Briesacher BA, Andrade SE, Yood RA, et al. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882-887.
19. Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811-818.
20. Earnshaw SR, Graham CN, Ettinger B, et al. Cost-effectiveness of bisphosphonate therapies for women with postmenopausal osteoporosis: implications of improved persistence with less frequently administered oral bisphosphonates. Curr Med Res Opin. 2007;23:2517-2529.
21. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259-262.
22. Ettinger MP. Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237-2246.
23. Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414-2419.
24. Cooper A, Drake J, Brankin E. The PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896-905.
25. Weiss TW, Henderson SC, McHorney CA, et al. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Res Med Opin. 2007;23:2193-2203.
26. Penning-van Beest FJA, Goettsch WG, Erkens JA, et al. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236-242.
27. Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis. Arthritis Rheum. 2006;54:1838-1846.
28. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
29. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
30. Adami S, Felsenberg D, Christiansen C, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone. 2004;34:881-889.
31. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122-128.
32. Felsenberg D, Hoffmeister B, Amling M, et al. Kiefernekrosen nach hoch dosierter Bisphosphonattherapie. Dtsch Arztebl. 2006;103:3078-3081.
33. American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137:1144-1150.
34. Reclast [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; October 2009. Available at: http://www.pharma.us.novartis.com:80/product/pi/pdf/reclast.pdf. Accessed May 12, 2010.
35. Ding A. The structural effects of long-term bisphosphonate treatment leading to atypical hip fractures. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 10, 2010; New Orleans, La.
36. Gladnick B. The effects of long-term bisphosphonate use on bone quality. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11, 2010; New Orleans, La.
37. FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed April 20, 2010.
1. Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:211-219.
2. Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT Study. J Bone Miner Res. 2005;20:557-563.
3. WHO Study Group on Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1994. WHO Technical Report Series, No. 843.
4. Siris ES, Chen Y-T, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
5. Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17:1404-1409.
6. Cranney A, Jamal SA, Tsang JF, et al. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177:575-580.
7. Kanis JA, Burlet N, Cooper C, et al. European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399-428.
8. Gehlbach SH, Avrunin JS, Puleo E, et al. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805-810.
9. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf. Accessed March 25, 2008.
10. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541.
11. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83-91.
12. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352.
13. Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249.
14. Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023-1031.
15. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18:271-277.
16. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013-1022.
17. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003-1008.
18. Briesacher BA, Andrade SE, Yood RA, et al. Consequences of poor compliance with bisphosphonates. Bone. 2007;41:882-887.
19. Rabenda V, Mertens R, Fabri V, et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int. 2008;19:811-818.
20. Earnshaw SR, Graham CN, Ettinger B, et al. Cost-effectiveness of bisphosphonate therapies for women with postmenopausal osteoporosis: implications of improved persistence with less frequently administered oral bisphosphonates. Curr Med Res Opin. 2007;23:2517-2529.
21. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003;14:259-262.
22. Ettinger MP. Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237-2246.
23. Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414-2419.
24. Cooper A, Drake J, Brankin E. The PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006;60:896-905.
25. Weiss TW, Henderson SC, McHorney CA, et al. Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refills. Curr Res Med Opin. 2007;23:2193-2203.
26. Penning-van Beest FJA, Goettsch WG, Erkens JA, et al. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006;28:236-242.
27. Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis. Arthritis Rheum. 2006;54:1838-1846.
28. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
29. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
30. Adami S, Felsenberg D, Christiansen C, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone. 2004;34:881-889.
31. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122-128.
32. Felsenberg D, Hoffmeister B, Amling M, et al. Kiefernekrosen nach hoch dosierter Bisphosphonattherapie. Dtsch Arztebl. 2006;103:3078-3081.
33. American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006;137:1144-1150.
34. Reclast [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; October 2009. Available at: http://www.pharma.us.novartis.com:80/product/pi/pdf/reclast.pdf. Accessed May 12, 2010.
35. Ding A. The structural effects of long-term bisphosphonate treatment leading to atypical hip fractures. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 10, 2010; New Orleans, La.
36. Gladnick B. The effects of long-term bisphosphonate use on bone quality. Presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 11, 2010; New Orleans, La.
37. FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed April 20, 2010.